Emerging bioinspired hydrovoltaic electricity generators
Guangtao
Zan†
 a,
Shengyou
Li†
a,
Shengyou
Li†
 a,
Kaiying
Zhao†
a,
Kaiying
Zhao†
 a,
HoYeon
Kim
a,
HoYeon
Kim
 a,
EunAe
Shin
a,
EunAe
Shin
 ab,
Kyuho
Lee
ab,
Kyuho
Lee
 a,
Jihye
Jang
a,
Jihye
Jang
 a,
Gwanho
Kim
a,
Yeonji
Kim
a,
Wei
Jiang
a,
Gwanho
Kim
a,
Yeonji
Kim
a,
Wei
Jiang
 a,
Taebin
Kim
a,
Taebin
Kim
 a,
Woojoong
Kim
a and
Cheolmin
Park
a,
Woojoong
Kim
a and
Cheolmin
Park
 *ac
*ac
aDepartment of Materials Science and Engineering, Yonsei University, Seoul 03722, Republic of Korea. E-mail: cmpark@yonsei.ac.kr
bKorea Packaging Center, Korea Institute of Industrial Technology, Bucheon 14449, Republic of Korea
cPost-Silicon Semiconductor Institute, Korea Institute of Science and Technology, Seoul 02792, Republic of Korea
First published on 27th November 2024
Abstract
In recent years, hydrovoltaic electricity generators (HEGs) have garnered increasing interest and attention due to their unparalleled advantages. They typically operate through direct interactions between various nanomaterials/structures and different forms of water (e.g., moisture, liquid water, waves, and droplets) capable of transforming diverse energy forms into electricity, resulting in the development of four types of HEGs: moisture electricity generators (MEGs), evaporation electricity generators (EEGs), droplet electricity generators (DEGs), and reverse electrodialysis electricity generators (REGs). Consequently, a deep understanding of their interactions is crucial for the development of different types of high-performance HEGs. In this regard, the efficient utilization of water by natural organisms to achieve various complex life processes and functions provides inexhaustible and ingenious inspirations for fabricating superior HEGs, establishing this as a vibrant area of current research. In this review, we will comprehensively review the recent key advancements in the field of bioinspired HEGs. We begin by elucidating the concepts and relationships between HEGs and bioinspired design, followed by an explanation of the mechanisms behind the above four types of HEGs. Building on this foundation, we systematically summarize and discuss the current progress of HEG devices from three bioinspired perspectives: (1) elementary bioinspired materials for HEGs; (2) smart bioinspired structures for HEGs; and (3) living bioinspired devices for HEGs. In this review, we will also highlight various biological structures, functions, and processes that can inspire the design of HEGs. We conclude by summarizing the challenges in the bioinspired HEG field and providing insights into future prospects for this exciting research area.
Broader contextHydrovoltaic electricity generators (HEGs) represent a groundbreaking advancement in sustainable energy technology, offering a novel approach to harnessing the vast and largely untapped potential of water as an energy source. This emerging field bridges the gap between traditional hydroelectric power and the need for smaller, more versatile energy generation systems. By leveraging direct interactions between nanomaterials and various forms of water, HEGs open up new possibilities for energy harvesting that go beyond conventional hydroelectric systems. The bioinspired approach to HEG development marks a significant leap forward, drawing inspiration from nature's time-tested strategies for efficient water utilization. This biomimetic perspective not only enhances the performance of HEGs but also paves the way for more sustainable and environmentally friendly energy solutions. The integration of biological principles into HEG design—from elementary bioinspired materials to smart structures and even living devices—showcases the interdisciplinary nature of this field and its potential to revolutionize energy and environmental science. As global concerns about energy security and environmental impact continue to grow, the development of bioinspired HEGs offers a promising path towards addressing these challenges, potentially transforming our approach to clean energy generation and resource management in the years to come. |
1. Introduction
Amidst the escalating global energy crisis and environmental pollution, there has been rising interest in developing renewable, clean energy sources, notably solar, wind, and water.1–7 Among these, water, as one of the most abundant resources on Earth and a major carrier of energy, has drawn significant attention.8,9 The concept of harnessing energy from water has a long history; as early as 400 BC, waterwheels were used to convert the kinetic energy of flowing or falling water into useful mechanical energy. However, it was not until the 19th century, with the establishment and development of electromagnetic induction theory, that water began to be utilized to drive electromagnetic generators, converting mechanical energy into electrical energy (hydroelectric power). This form of energy capture, based on classical electrodynamics, is currently the most widely used form of energy utilization from water. However, the vast majority of energy contained in water remains undeveloped and unused. If effective energy conversion pathways could be developed to transform this vast energy into electricity, it would significantly enrich current energy production methods. The recent development of hydrovoltaic electricity generators (HEGs) has filled this gap.10–18Hydrovoltaic, similar to the logic of photovoltaic, where “hydro” refers to water and “voltaic” refers to voltage generation, makes the term self-explanatory. It can be defined as generating electricity through direct interactions between nanomaterials/structures and various forms of water, including flowing, waving, dripping, and evaporating water. Although research in this field began quite some time ago, various types of devices were given different names based on their specific characteristics. It wasn’t until 2018 that the term “hydrovoltaic” was introduced to encapsulate the common features of these devices. Since then, this term has gained wider usage in the scientific community.10 This represents a novel form of energy transformation, typically involving electrokinetic theory, which differs from the aforementioned hydroelectric power based on classical electrodynamics. Compared to large hydroelectric, complex wind, and photovoltaic generators, HEGs are typically smaller, portable, low-cost, and easily integrated to power wearable electronic devices, showing broad application prospects.
The development of the hydrovoltaic phenomenon, from its discovery to theoretical advancements and device innovation, has undergone a long and arduous journey (Fig. 1). In the early 19th century, phenomena such as electroosmosis and electrophoresis were discovered. In 1859, it was observed that when electrolytes flowed through narrow channels under pressure, a voltage was generated due to the interaction between water and solids. This phenomenon, known as the streaming potential, can be considered a precursor to hydrovoltaic electricity generation. Over the subsequent 100 years, the development of hydrovoltaic theory and related devices progressed slowly through persistent exploration and experimentation. In 1945, reverse electrodialysis was introduced. Based on this principle, osmotic energy generation was proposed in the 1970s, utilizing the free energy released from the thermodynamically reversible mixing of freshwater and seawater at a constant temperature.19 In the 1960s, researchers observed that inserting electrodes into living tree trunks could generate electricity, and similar studies expanded over the following decades.20 It wasn’t until the 21st century, with the advent of nanoscience and nanotechnology, that the field of hydrovoltaics experienced rapid development.21,22 This led to the emergence and breakthrough of various new types of HEGs, including moisture electricity generators (MEGs) based on moisture-induced ion gradient diffusion in 2015,23 water evaporation electricity generators (EEGs) based on streaming potential in 2017,24 droplet electricity generators (DEGs) capable of generating voltages exceeding 100 V in 2020,25 and reverse electrodialysis electricity generators (REGs) that can output 110 V at open circuit potential using stacked hydrogels.26 Despite these advancements, HEGs remain in the early stages of research, and their practical electricity generation performance still has significant room for improvement. Enhancing their efficiency and output is a key challenge currently being addressed. In this regard, bioinspired design is anticipated to provide effective solutions.
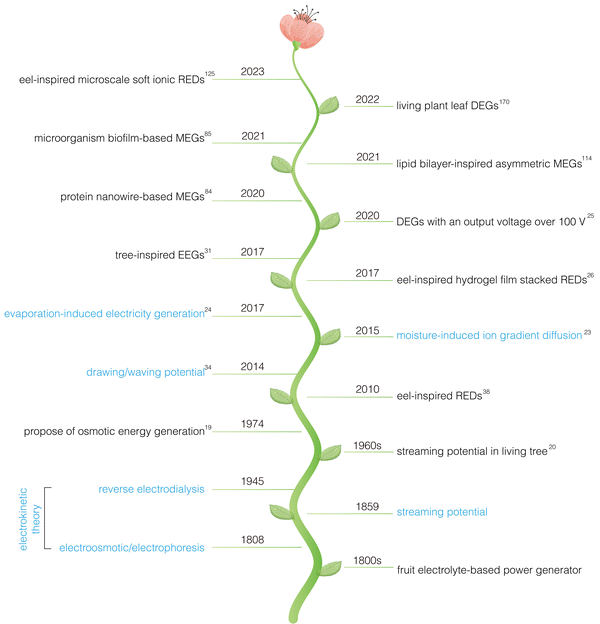 | ||
| Fig. 1 Timeline of bioinspired hydrovoltaic technology. Blue represents theories related to HEGs, while black represents landmark works on bioinspired HEGs. | ||
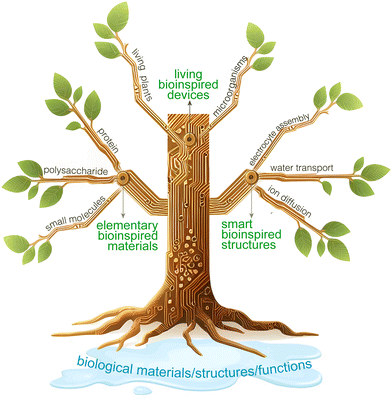
Water is not only a carrier of energy on Earth but also a source of life, crucial for the survival of organisms. Through millions of years of evolution, organisms have achieved a nearly perfect mastery of utilizing water and its contents. Organisms can use water and its substances in an extremely efficient way to build intricate biological structures and achieve complex life processes and functions.27–29 For example, trees rely on capillary action and transpirational pull, using the numerous vertical pores in their stems to transport water from roots to leaves; electric eels produce high voltages by controlling ion pumps in their tissue fluids across membranes. The key to developing high-performance HEGs lies in improving the interactions between nanomaterials/structures and water, paralleling the efficient water-utilization techniques of these organisms. It is foreseeable that marrying or integrating HEGs with the efficient water-utilization capabilities of organisms will herald a new peak in the development of this field, which we refer to as “bioinspired HEGs” (Fig. 2). We define bioinspired HEGs as those that expand and enhance the application and performance of hydrovoltaic electricity generation by mimicking or using biomaterial structures, physiological processes, and functions. Furthermore, we propose categorizing the development of the field as follows according to the complexity and intelligence of bioinspired design: (1) elementary bioinspired materials for HEGs; (2) smart bioinspired structures for HEGs; and (3) living bioinspired devices for HEGs.
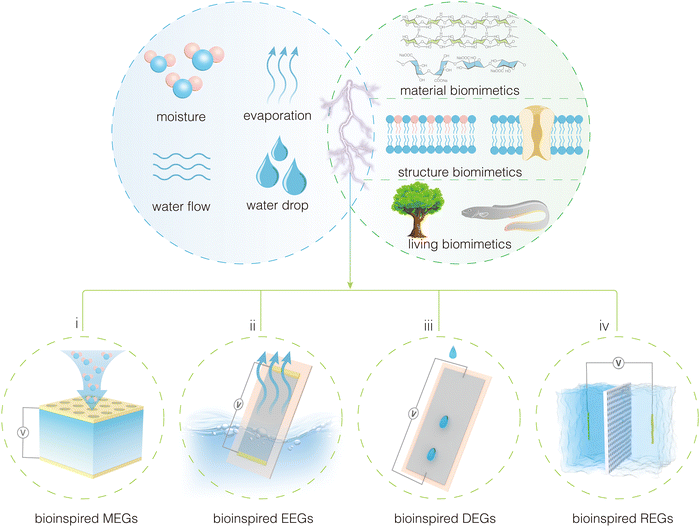 | ||
| Fig. 2 Different types of hydrovoltaic devices involved in the fusion of hydrovoltaic technology and bioinspired concepts. | ||
In this review, we will closely examine the four types of HEGs and the three levels of bioinspired intelligence (Fig. 3). First, we will clarify the concepts, classifications, and development history of hydrovoltaic devices. We will then discuss the water-utilization technologies of organisms and their application prospects in high-performance hydrovoltaic devices. Subsequently, we will elaborate on the working mechanisms of the four types of hydrovoltaic devices. Afterwards, we will comprehensively review the applications of bioinspired design in hydrovoltaic devices, systematically summarizing and reviewing developments based on the complexity and intelligence of bioinspired design. We conclude by summarizing the current state of intelligent HEGs and looking forward to the future development of this exciting research area.
2. Mechanism of various hydrovoltaic electricity generators (HEGs)
As mentioned above, the direct interaction between nanomaterials and different forms of water in HEGs leads to the generation of electricity (Fig. 4). To explore the interaction between nanomaterials and water in such devices, it is essential to study the solid–liquid interface, particularly the charge distribution and the electric double layer (EDL) at the interface. First, how do charges arise on the surface of a solid? In fact, any process that causes the separation of positive and negative charges on the surface of a solid will result in surface charging. For instance, solid materials may undergo ionization or hydrolysis reactions in a solution, or solid surfaces may selectively adsorb certain ions through hydrogen bonding or van der Waals forces. If the surface of the solid adsorbs positive ions, it becomes positively charged, and conversely, if it adsorbs negative ions, it becomes negatively charged.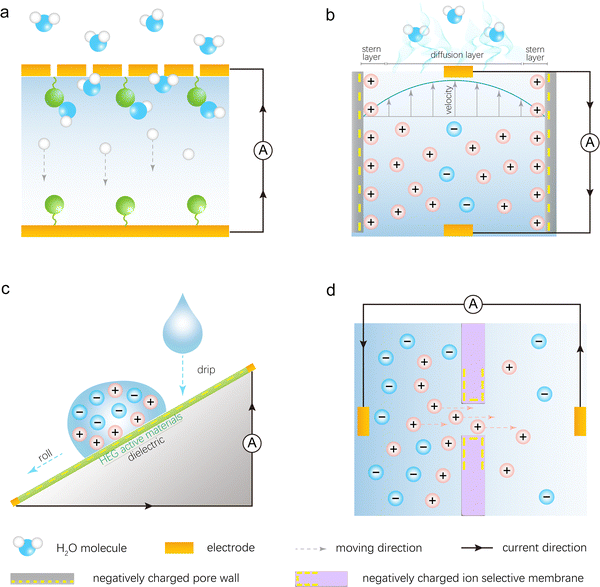 | ||
| Fig. 4 Electricity generation mechanisms of different types of HEGs: (a) MEGs, (b) EEGs, (c) DEGs, and (d) REGs. | ||
Once the solid surface is charged, it electrostatically attracts oppositely charged ions from the solution towards the surface. However, due to thermal motion (Brownian motion), some of the counterions do not remain on the surface but diffuse into the liquid near the surface, creating an ion concentration gradient. This gradient is characterized by a higher concentration of counterions and a lower concentration of co-ions as one approaches the surface. As illustrated in Fig. 4b, the layer of counterions tightly adsorbed onto the charged solid surface is known as the stern layer. Beyond the stern layer, counterions are more diffusely distributed, forming what is known as the diffuse layer. Part of the diffuse layer can move under the influence of external tangential stress, leading to the introduction of the concept of the shear plane, which separates the mobile fluid from the immobile fluid attached to the surface. The potential difference between the shear plane and the bulk solution is referred to as the zeta potential. The thickness from the shear plane to the outer side of the diffuse layer is regarded as the Debye length (λD), which is typically considered the thickness of the diffuse layer. Since the diffuse layer is much thicker than the stern layer, the Debye length is often used as an approximation for the thickness of the entire EDL. According to the equation for the Debye length, 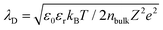 , where ε0 is the permittivity of free space, εr is the dielectric constant of the solution, kB is the Boltzmann constant, T is the absolute temperature, nbulk is the ion concentration, Z is the ionic charge state, and e is the elementary charge, it is evident that the Debye length is inversely proportional to the ion concentration in the solution.
, where ε0 is the permittivity of free space, εr is the dielectric constant of the solution, kB is the Boltzmann constant, T is the absolute temperature, nbulk is the ion concentration, Z is the ionic charge state, and e is the elementary charge, it is evident that the Debye length is inversely proportional to the ion concentration in the solution.
Next, we will elaborate on the electricity generation mechanisms of the four HEGs in detail, focusing on the ion–electron interactions.
2.1. Mechanism of MEGs
The ubiquitous moisture is a basic form of water, consisting of a mixture of water vapor and air. MEGs are devices that generate electricity in the presence of moisture. The Qu's team first proposed the mechanism of ion concentration gradient diffusion to explain the phenomenon of moisture-induced electricity generation in graphene oxide.23 As shown in Fig. 4a, this mechanism primarily involves three processes: the absorption of water molecules by the material, the ionization/hydrolysis of surface groups to produce ions and form an ion concentration gradient, and the asymmetric directional diffusion of these ions.There are generally two strategies for introducing ion concentration gradients. The first strategy involves directly constructing a gradient of functional groups within the power-generating material, while the second strategy entails directionally introducing moisture into the material. A typical example of the first strategy is a graphene oxide film with an oxygen-containing functional group gradient prepared through a moisture–electric annealing method (Fig. 4a).23 When this film absorbs water molecules from moisture, it prompts the ionization of oxygen-containing functional groups such as carboxyl groups on the nanosheets, producing freely mobile H+ ions and immobile –COO− groups attached to the carbon framework. The gradient of oxygen-containing functional groups within the film creates an ion concentration gradient, which induces the directional migration of H+ ions. A typical example of the second strategy is placing a polystyrene sulfonic acid (PSSA) membrane between two electrodes in a sandwich structure, with one electrode perforated and the other intact.30 Due to the different permeability capacities of the two electrodes for moisture, the amount of H+ ions produced by ionization at the two ends of the PSSA membrane varies, resulting in an ion concentration gradient (Fig. 2i). Similarly, the negatively charged –SO3− groups attached to the polymer backbone remain immobile, leading to the directional migration of H+ ions within the membrane. Both strategies achieve the separation of anions and cations and the directional movement of single-type ions, laying the foundation for electricity generation.
From the analysis above, it is evident that the generation of ions induced by moisture and their asymmetric directional diffusion significantly impact the power generation performance. Therefore, by regulating intrinsic factors of MEGs (such as the physicochemical properties of the material and device structure) and extrinsic factors (such as relative humidity (RH) and temperature), one can adjust the number of dissociated ions and their diffusion behavior, thereby enhancing the power generation performance. To be specific, ions are primarily generated through ionization and hydrolysis of surface functional groups on active materials under the influence of water molecules. Consequently, their quantity is influenced both by the intrinsic physicochemical properties of the material and the moisture availability in the surrounding humid environment. Typically, hydrophilic materials rich in readily ionizable functional groups and high-humidity conditions contribute to increased ion generation. Furthermore, the directional migration of ions is affected by humidity gradients across the material or differences in functional group concentration. Larger humidity or concentration gradients enhance the driving force for directional ion migration. Furthermore, different types of ions exhibit significantly varying migration rates due to differences in electric properties and charge amounts. The pore structure, quantity, and surface characteristics of the material itself also greatly influence the diffusion speed of carriers within it. Therefore, optimizing both the generation quantity and migration characteristics of carriers is crucial for enhancing power generation performance.
2.2. Mechanism of EEGs
Water evaporation is a spontaneous and continuous phase transition from liquid to gas that absorbs ambient heat. This ubiquitous phenomenon occurs constantly. However, direct energy harvesting from water evaporation has been limited due to the lack of efficient energy harvesting devices. In 2017, a breakthrough was made by the groups led by Guo and Zhou, who first proposed the use of carbon-based devices to directly harvest electrical energy from water evaporation, sparking rapid development in this field.24To illustrate the electricity generation process, the initially reported EEGs can be taken as an example.24,31 A typical device structure is shown in Fig. 2ii, where part of the device is immersed in water. The active material is a porous carbon black film deposited on an insulating substrate, with two multi-walled carbon nanotube (MWCNT) film electrodes deposited at either end as current collectors. Under ambient conditions, water is absorbed at the bottom of the device and flows upward through the carbon black film via capillary action. The natural evaporation process in the exposed carbon black film ensures sustained capillary flow. This process, which only consumes ambient heat, can generate a long-term open-circuit voltage of up to 1 V and a short-circuit current of 150 nA.
Studies indicate that evaporation-induced electricity generation is closely related to the traditional streaming potential discovered in 1859. Streaming potential is the electrical potential difference generated when an electrolyte solution flows through a stationary capillary or porous material under pressure, causing ions with charges opposite to the surface to be carried away, resulting in a streaming current and charge accumulation at the ends, thus creating an electric field (Fig. 4b). EEGs share similarities with this process: (1) the nanomaterials in EEGs are densely packed to form nanochannels; (2) the nanomaterials used for power generation are surface-charged in the solution; (3) capillary forces and continuous evaporation within the material ensure the steady flow of liquid through the nanochannels, akin to pressure-driven fluid flow. Consequently, streaming potential theory is widely applied to describe the power generation process in EEG devices.
In a classical streaming potential system, the streaming potential (V) and streaming current (I) can be expressed by the following equations:  ,
,  , where ε0 and εr denote the vacuum permittivity and the dielectric constant of the solution, respectively; σ represents the specific conductivity of the solution; η is the viscosity of the electrolyte solution; ΔP is the pressure difference across the channel; ζ is the zeta potential; and A and l are the cross-sectional area and length of the channel, respectively. According to these equations, the electrical generation property is directly proportional to the pressure difference (driving force) and inversely proportional to the solution viscosity, both of which directly affect the flow rate of the solution through the channel. This implies that the higher the flow rate of the electrolyte in the channel, the better the power generation performance may be. The flow rate can be enhanced by accelerating the evaporation rate through methods such as increasing ambient temperature, increasing air flow, or reducing humidity, all of which have been shown in previous studies to improve power generation performance. Additionally, the equations suggest that increasing the zeta potential benefits power generation performance, as a high zeta potential facilitates charge separation within the electrolyte.
, where ε0 and εr denote the vacuum permittivity and the dielectric constant of the solution, respectively; σ represents the specific conductivity of the solution; η is the viscosity of the electrolyte solution; ΔP is the pressure difference across the channel; ζ is the zeta potential; and A and l are the cross-sectional area and length of the channel, respectively. According to these equations, the electrical generation property is directly proportional to the pressure difference (driving force) and inversely proportional to the solution viscosity, both of which directly affect the flow rate of the solution through the channel. This implies that the higher the flow rate of the electrolyte in the channel, the better the power generation performance may be. The flow rate can be enhanced by accelerating the evaporation rate through methods such as increasing ambient temperature, increasing air flow, or reducing humidity, all of which have been shown in previous studies to improve power generation performance. Additionally, the equations suggest that increasing the zeta potential benefits power generation performance, as a high zeta potential facilitates charge separation within the electrolyte.
Furthermore, studies have demonstrated that EDL overlap is another effective strategy for enhancing power generation performance (Fig. 4b). When the electrolyte flows through a nanochannel, the static charges within the EDL flow along with the liquid, while counterions and co-ions in the bulk water outside the EDL move in the same direction. Since these ions carry opposite charges, their contributions to the total current will counterbalance each other. However, when EDL overlap occurs within the channel, ion selectivity arises: the channel contains a large number of counterions due to electrostatic interactions and is nearly devoid of co-ions. Under these conditions, only counterions with a single charge move with the liquid flow, resulting in the highest energy conversion efficiency. EDL overlap can be achieved by reducing the size of the channel or increasing the thickness of the EDL. The size of channels can be created by controlling the material fabrication process, while the EDL thickness can be modulated by adjusting the electrolyte concentration. According to the above Debye length formula, as the electrolyte concentration increases, the EDL thickness rapidly decreases, leading to a decline in power generation capability. Therefore, a low-concentration electrolyte is more likely to experience EDL overlap when the size of the channel is fixed, enabling more efficient energy conversion.
Additionally, as the understanding of EEGs continues to deepen, other mechanisms have been proposed, such as the ionovoltaic effect32 and the pseudostreaming mechanism.33 They simultaneously account for the coupled motion of ions and electrons at the solid–liquid interface, providing an effective supplement to the mechanisms of power generation.
2.3. Mechanism of DEGs
Water droplets are abundant and carry significant energy, making droplet-based DEGs a subject of extensive research and interest. In 2014, Yin et al. discovered that the motion of water droplets on graphene sheets could generate a constant electrical signal of several millivolts, a phenomenon they termed “drawing potential”.34 In subsequent studies, the concept of droplet-based power generation was expanded. Through improvements in active materials and device structures, rolling droplets interacting with active materials could generate instantaneous electricity, significantly enhancing performance.Research has shown that the electricity generation mechanism of DEGs is closely related to the EDL. When a water droplet contacts a solid surface, an EDL is formed. This consists of a layer of ions adsorbed onto the surface due to electrochemical interactions, and a second layer of counter-ions attracted to the first layer by Coulomb forces. In a drawing potential system, for example, the electricity generation mechanism is based on the moving boundaries of the EDL (Fig. 4c). Specifically, the device comprises an NaCl droplet moving across graphene under drawing force. When the NaCl droplet contacts the graphene, Na+ ions are adsorbed onto the graphene surface while Cl− ions are repelled. As more hydrated Na+ ions are adsorbed, an increasing number of electrons accumulate on the graphene's surface, forming a pseudocapacitor. When the ionic droplet moves across the graphene surface, ions are adsorbed at the leading edge of the droplet, advancing the pseudocapacitor and drawing electrons along the graphene. Simultaneously, at the trailing edge of the droplet, ions are desorbed, discharging the pseudocapacitor and releasing electrons back to the graphene. This dynamic EDL creates a potential difference between the front and rear ends of the droplet, enabling continuous power generation as the droplet moves. Research indicates that the generated potential is proportional to the velocity and quantity of droplets. Additionally, the potential depends on the droplet's concentration and ion type, and it drops sharply with an increasing number of graphene layers. Overall, further exploration of suitable active materials and effective ion attraction to the interface is crucial for enhancing this type of DEG.
Additionally, introducing electret materials into DEG devices, coupled with different electrode arrangement designs, can yield performance-enhanced devices.35 These devices typically operate through an electrostatic induction mechanism and, unlike the “moving boundaries of EDL” mechanism, generally produce intermittent, periodic power. For instance, in a DEG composed of a polytetrafluoroethylene (PTFE) membrane fully covered by a Cu electrode at the bottom, the power generation mechanism is as follows: when a water droplet falls onto the PTFE surface, the PTFE film tends to become negatively charged while the water becomes positively charged, forming an EDL at the liquid–solid interface. The long-lasting negative charge retained in the PTFE film attracts counter-ions in each subsequent droplet, causing electrons to flow from the ground to the electrode during spreading and from the electrode to the ground during shrinking until electrical equilibrium is reached. Ultimately, this setup produces a periodic output. Overall, for these types of devices, further investigations into optimizing surface properties (such as enhancing surface negativity and hydrophobicity to improve interfacial charge separation efficiency) and innovative structural designs are essential for advancing DEG performance.
2.4. Mechanism of REGs
The osmotic pressure difference between river water and seawater represents a highly promising source of renewable energy. The concept of utilizing this difference for power generation was first proposed by Loeb in 1975. With advancements in nanotechnology and membrane science, the use of osmotic energy, or salinity gradient energy, has gained widespread attention and development. The reverse electrodialysis (RED) method is considered a promising solution for harvesting osmotic energy.36–38 Devices that harvest energy based on the RED principle are named REGs.As shown in Fig. 2iv, placing selective permeable membranes between two salt solutions of different concentrations and connecting two non-polarizable Ag/AgCl electrodes to an external circuit forms an REG. Within the salinity gradient environment of this REG system, ions in the high-concentration region naturally migrate towards the low-concentration region. When ions selectively diffuse through the permeable membranes, osmotic potential energy is released and captured and subsequently converted into electrical energy via the circuit (Fig. 4d). If the channel lacks ion selectivity for cations and anions, the net ion flux through the membrane will be zero due to the mutual cancellation of cations and anions, resulting in no current generation. This reveals two critical requirements for REG electricity generation: solutions with different salinities and a differential diffusion rate for cations and anions. Consequently, the channels on the selective permeable membranes are key determinants of the electricity generation performance.
To enhance the power output of REGs, it is essential to simultaneously improve the ion selectivity and permeability of the channels, necessitating the design and construction of channels with suitable structural and chemical properties. In charged nanochannels, the EDL effect attracts counterions and repels co-ions, promoting the preferential transport of ions with a charge opposite to that of the channel walls. Notably, when the channel radius reaches or is smaller than the Debye length, EDL overlap occurs, effectively excluding co-ions from the channel while primarily allowing counterions as charge carriers to diffuse within the channel, significantly increasing ion selectivity. While reducing the channel radius to the Debye length scale enhances ion selectivity, it may also reduce ion permeability. These two parameters often exhibit a trade-off relationship, wherein an increase in one often results in a decrease in the other. Overcoming this trade-off—achieving high efficiency in selective anion or cation transport while ensuring rapid ion passage—is crucial for optimizing osmotic energy harvesting. Therefore, the design of channel density, size, and charge characteristics should be comprehensively optimized to achieve maximum power density.
3. Elementary bioinspired materials for HEGs
Biomass forms the foundation of biological organisms, often exhibiting diverse chemical structures, functional group activities, and self-assembly properties. These characteristics allow biomass to participate extensively in physiological processes such as substance transport and electron transfer. This naturally raises the question: can these materials exhibit similar behaviors outside of biological systems to generate electricity? Extensive exploration has been conducted on this front. Given that this process involves numerous elementary materials and mimics fundamental biological processes, we term it elementary biomimetics. From a materials perspective, the biomass used in HEGs can be primarily categorized into polysaccharides, proteins, and natural small molecules. A detailed review of the progress in these three areas within the context of HEGs will be provided in the following section (Table 1).| Materials | Mechanism | Functional layers | Electrodes (top/bottom) | Area (cm−2) | Voltage (V) | Current (μA cm−2) | Power density (μW cm−2) | Condition | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Condition refers to the specific state and form of water used during testing. For MEGs, condition pertains to the relative humidity at which the device is tested. In the case of EEGs, it refers to the water source employed for evaporation, such as DI water or saltwater flow. For DEGs, condition denotes the composition of the liquid droplets. For REGs, it indicates the composition and concentration of the salt solution used to create the concentration gradient. | |||||||||
| Cellulose | MEG | CNF film | Au/Au | 0.25 | 0.25 | 0.11 | 0.2 | 50% RH | 39 |
| Cellulose | MEG | CNF aerogel | Pt/Pt | 1 | 0.11 | 0.023 | 0.63 × 10−3 | 99% RH | 40 |
| Cellulose | MEG | Quatern-CNFs/Tempo-CNFs | Pt/Pt | 3.14 | 0.115 | 0.0047 | — | 99% RH | 41 |
| Cellulose | MEG | Laser-induced CNF film | Cu/Ag | 0.09 | 0.65 | 550 | 357.3 | 90% RH | 42 |
| Cellulose | MEG | CNF/GO composite film | Ti/Ti | 3 | 0.286 | — | — | 81% RH | 43 |
| Cellulose | MEG | CNF/PSSA/MXene film | Ag/Ag | 1 | 0.3 | 1.2 | 0.073 | 80% RH | 44 |
| Cellulose | MEG | Aminated-CNF films | Al/Al | 2.25 | 4.2 | 0.008 | 0.033 | 85% RH | 45 |
| Cellulose | MEG | Bacterial cellulose films | Pt/Al | 15 | 0.935 | 500 | 404 | 40% RH | 46 |
| Cellulose | MEG | Cellulose acetate membranes | Ag/Ag | 3 | 0.3 | 0.08 | 0.0084 | 85% RH | 47 |
| Cellulose | MEG | CNF/COF aerogel | Stainless steel (SS)/SS | 0.3 | 0.55 | 0.34 | 0.212 | 100% RH | 48 |
| Cellulose | MEG | BG/NC bilayer | Au@SS/Au@SS | 0.25 | 1.17 | 2770 | 541 | 90% RH | 49 |
| Cellulose | MEG | Paper | Au/ITO | 1.5 | 0.25 | 0.01 | — | 70% RH | 50 |
| Cellulose | EEG | CNF/CNT fiber | Cu/Cu | — | 0.16 | — | 400 μW cm3 | DI water | 51 |
| Cellulose | EEG | Graphene oxide/deacetylated cellulose acetate film | Cu/Cu | 4 | 0.316 | 0.249 | — | DI water | 52 |
| Cellulose | EEG | Carbon black coated cellulose acetate microfiber | Fe/Fe | 5.18 | 0.3 | 19.3 | 0.88 | DI water | 53 |
| Cellulose | EEG | CNF/melanin film | Pt/Pt | 15 | 0.48 | 0.012 | 0.0058 | DI water | 54 |
| Cellulose | EEG | Cellulon paper | Carbon/carbon | 9 | 0.78 | 0.83 | 0.7 | 60% RH | 55 |
| Cellulose | REG | CNF/clay film | Both Ag/AgCl | 3 × 10−4 | 0.108 | 5.95 × 104 | 861 | 0.5 M/0.01 M NaCl | 56 |
| Cellulose | REG | CNF/WS2 film | Both Ag/AgCl | 3 × 10−4 | 0.055 | 1.46 × 104 | 199 | 0.5 M/0.01 M NaCl | 57 |
| Cellulose | REG | CNF/BN film | Both Ag/AgCl | 3 × 10−4 | 0.15 | 1.19 × 104 | 459 | 0.5 M/0.01 M KCl | 58 |
| Cellulose | REG | CNF/MXene film | Both Ag/AgCl | 3 × 10−4 | 0.175 | 1.21 × 104 | 530 | 0.5 M/0.01 M NaCl | 59 |
| Cellulose | REG | CNC/GO films | Both Ag/AgCl | 2 × 10−3 | 0.176 | 1.2 × 104 | 473 | 0.5 M/0.01 M KCl | 60 |
| Cellulose | REG | Aligned bacterial cellulose films | Both Ag/AgCl | 1.8 × 10−2 | 0.125 | 6.94 × 102 | 23 | 0.5 M/0.01 M NaCl | 61 |
| Cellulose | REG | BC nanofibers | Both Ag/AgCl | 3 × 10−4 | 0.133 | 4 × 103 | 72 | 0.5 M/0.01 M NaCl | 62 |
| Alginate | MEG | PVA/SA ionic gel | Al/PEDOT:PSS | 1 | 1.3 | 1310 | 110 | 80% RH | 63 |
| Alginate | MEG | SA/rGO/SiO2 composite film | Au/Au | 1 | 0.5 | 100 | 12 | 100% RH | 64 |
| Alginate | MEG | SA/PEDOT:PSS fiber | Fe/MWCNTs | 9.2 × 10−4 | 1.2 | 2.28 × 104 | 556 | 61% RH | 65 |
| Alginate | MEG | SA/MWCNT fiber | Ag/MWCNT | 1.38× 10−3 | 0.38 | 1.26 × 103 | 9.5 | 90% RH | 66 |
| Alginate | MEG | Nb2CTx/SA composite film | Cu–Ni/Cu–Ni | 0.25 | 0.52 | 6.4 | 0.5 | 91% RH | 67 |
| Alginate | MEG | SA/MWCNTs + SA bilayers | Al/Cu | 16 | 0.78 | 4.13 | 0.089 | 90% RH | 68 |
| Alginate | EEG | SA-ZnCl2 hydrogel | Ag/Ag | 0.1 | 0.16 | 14 | 0.48 | DI water | 69 |
| Chitosan | MEG | CS + CS/CNF + PVA/CNF tri-layers | Ni/Ni | 7.06 | 1.15 | 15.87 | 32.59 | 80% RH | 70 |
| Chitosan | MEG | CS-KGM aerogel fibers | CNT/Cu | 0.28 | 0.189 | 3.46 | 47.66 | 90% RH | 71 |
| Chitosan | REG | CS quaternary ammonium BC films | Both Ag/AgCl | 3 × 10−4 | 0.087 | 1.08 × 104 | 40 | 0.5 M/0.01 M NaCl | 72 |
| Chitosan | REG | CS/SA hydrogel | Both Ag/AgCl | 3 × 10−4 | 0.043 | 2.5 × 104 | 764 | 0.5 M/0.01 M KCl | 73 |
| Silk | MEG | SF film | Au/Au | 0.25 | 0.14 | 0.18 | 0.05 | 50% RH | 39 |
| Silk | MEG | Quatern-SF aerogel | Pt/Pt | 3.14 | 0.12 | — | — | 99% RH | 74 |
| Silk | MEG | Oxi-SF electrospun film | Au/Au | 4 | 0.28 | 0.0475 | — | 99% RH | 75 |
| Silk | MEG | SF/sericin film | Cu/Cu | 4 | 0.276 | 0.0175 | 1.3 × 10−3 | 95% RH | 76 |
| Silk | EEG | Nylon-66@SF film | Carbon/carbon | 4.5 | 4.82 | 0.029 | — | DI water | 77 |
| Silk | REG | SNF-IL membrane | Both Ag/AgCl | 10.75 | 0.112 | 40 | — | 100 mM/0.001 mM NaCl | 78 |
| Silk | REG | SNF/AAO film | Both Ag/AgCl | 3 × 10−4 | 0.058 | 1.04 × 104 | 286 | 0.5/0.01 M NaCl | 79 |
| Silk | REG | Ultrathin SF film | Both Ag/AgCl | 3 × 10−4 | 0.177 | 1.24 × 105 | 406 | 0.5/0.01 M NaCl | 80 |
| Protein | MEG | Gelatin films | Al/Cu | 0.04 | 0.71 | 7.77 | 5.5 | 80% RH | 81 |
| Protein | MEG | Milk whey films | Ti/ITO | 2.5 | 0.65 | 1.16 | 0.189 | 90% RH | 82 |
| Protein | MEG | Whey protein film | CNT/FTO | 0.1 | 1.45 | 113 | 11.6 | 40% RH | 83 |
| Protein | MEG | Protein nanowire film | Au/Au | 0.01 | 0.53 | 25 | 5 | 50% RH | 84 |
| Microbial | MEG | Whole-cell Geobacter sulfurreducens film | ITO/ITO | 1 | 0.3 | 0.3 | 0.052 | 75% RH | 85 |
| Microbial | MEG | G. sulfurreducens-PCA film | SS/ITO | 1 | 0.35 | 35.4 | 5.07 | 95% RH | 86 |
| Microbial | MEG | G. sulfurreducens-KN400 film | SS/ITO | 1 | 0.35 | 20 | 1.7 | 95% RH | 86 |
| Microbial | MEG | G. sulfurreducens-E.c film | SS/ITO | 1 | 0.31 | 5.1 | 0.52 | 95% RH | 86 |
| Microbial | MEG | G. sulfurreducens-S.o film | SS/ITO | 1 | 0.33 | 5 | 0.76 | 95% RH | 86 |
| Microbial | EEG | Geobacter sulfurreducens strain CL-1 film | Au/Au | 0.25 | 0.45 | 6 | 1 | DI water | 87 |
| Microbial | EEG | G. sulfurreducens-PCA film | Cu/Cu | 5 × 10−4 | 0.53 | 4.56 × 103 | 685.12 | DI water | 88 |
| Microbial | DEG | Geobacter sulfurreducens film | Carbon/carbon | 2 | 0.43 | — | — | DI water droplet | 89 |
| PA | MEG | PVA/PA ionic hydrogel | Cu/Ag | 0.25 | 0.8 | 240 | 35 | 85% RH | 90 |
| PA | MEG | PA-CPD films | LM/Ag | 0.5 | 0.8 | 1640 | 1312 | 85% RH | 91 |
| TA | MEG | PVA/TA-CNT hydrogel | Cu/Cu | 13.75 | 0.08 | — | — | — | 92 |
| Citric acid | MEG | Citric acid/paper | Cu/Cu | 3 | 0.275 | 7.6 | 2.1 | 73% RH | 93 |
3.1. Natural polysaccharide inspired HEGs
Natural polysaccharides are high-molecular-weight carbohydrates synthesized by living organisms in nature. They are composed of multiple monosaccharide units linked by glycosidic bonds. These polysaccharides are widely found in plants, animals, and microorganisms, serving various biological functions such as structural support, energy storage, and skin hydration.94 This section will review the research progress on the most representative polysaccharides and their derivatives in the context of HEGs: cellulose, sodium alginate, and chitosan.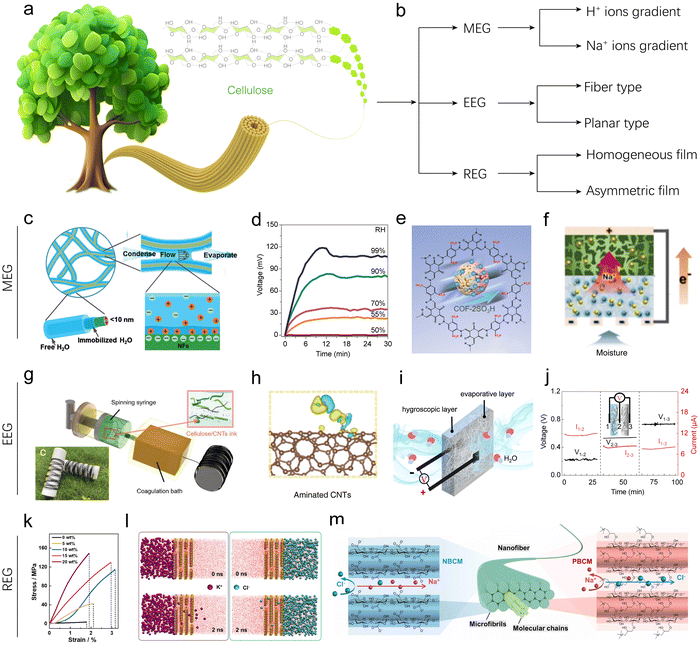 | ||
| Fig. 5 Cellulose-based HEGs. (a) Schematic illustration of cellulose structure and composition. (b) Classification of cellulose-based HEG devices. (c) Schematic illustration of hydrated channels around and between CNFs. (d) Voc of CNF-based MEGs at different RHs. Reproduced with permission.40 Copyright 2019, Wiley-VCH. (e) Schematic illustration of the COF-2SO3H structure. Reproduced with permission.48 Copyright 2024, American Chemical Society. (f) Schematic illustration of the transportation of Na+ in BGC–NC layers. Reproduced with permission.49 Copyright 2024, Royal Society of Chemistry. (g) Schematic of the fabrication of CNF/CNT fibers by a continuous wet-spinning method. (h) DFT simulation showing deformation charge density between aminated CNTs and H2O. Reproduced with permission.51 Copyright 2022, Wiley-VCH. (i) and (j) Schematic of the bilayer EEG, and voltage and current outputs of different component layers. Reproduced with permission.55 Copyright 2022, Springer Nature. (k) Tensile stress–strain curves of natural nanofluidics with different CNF contents. (l) MD simulations showing an ion permeation system in a natural nanofluidic channel at 0 ns and 2 ns at equilibrium. Reproduced with permission.56 Copyright 2024, Springer Nature. (m) Schematic illustration of nanochannel structures of ionized bacterial cellulose membranes. Reproduced with permission.62 Copyright 2022, Elsevier. | ||
Pure cellulose nanofibrils (CNFs), which were exfoliated mechanically from softwood via 2,2,6,6-tetramethylpiperidine-1-oxyl radical-mediated oxidation, were initially directly used as the active component/matrix for fabricating MEG devices. Due to the intrinsic hydrophilicity and charged states, CNFs can capture moisture from air and form hydrated nanochannels, analogous to ionic channels of cytomembranes (Fig. 5c). Li's group prepared a pure CNF aerogel for MEGs.40 They found that the CNF aerogel can generate a balance between water absorption and evaporation when exposed to moisture and produce an open-circuit voltage (Voc) of 0.11 V at 99% RH (Fig. 5d). Yao's group showed that the CNF assembled thin film can generate a spontaneous voltage output of 0.26 V attributed to the hierarchical nanopore channels besides its hygroscopic surface.39 The nanopores allow moisture to pass through and undergo dynamic adsorption–desorption exchange at the porous interface, resulting in surface charging. In the above studies, the device structures are typically designed such that the top can partially contact moisture, allowing for dynamic interaction, while the bottom is sealed. This configuration can generate a spontaneous and sustained charging gradient of H+ ions from the top to the bottom, enabling continuous electric output.
To improve the low performance of pristine cellulose-based MEG devices, various strategies have been developed, including surface modification,45 blending with other functional materials,43,44 bilayer designs,41,96 introducing new ionic gradients,42 and incorporating reactive components (Fig. 5e and f).49 Yuan's group prepared two types of surface-modified cellulose, namely oxidized regenerated cellulose (ORC) and aminated regenerated cellulose (ARC).45 The modified materials exhibited excellent hydrophilicity and abundant active sites, attributed to the plentiful functional groups and three-dimensional porous structure. They then assembled devices using these materials with aluminum foil electrodes. The aluminum foil electrodes had an asymmetric structure: the upper aluminum foil was perforated to allow moisture absorption, while the bottom aluminum foil was left intact to act as a seal. The ORC-based MEG can generate a Voc of 1.07 V at 85% RH, while the ARC-based MEG can produce a voltage as high as 4.20 V, which is about four times that of the ORC-based MEG. This significant increase in voltage is likely related to the greatly enhanced isoelectric points of cellulose after amino grafting. Although its voltage is high, its current is typically only tens of nanoamperes, which may limit its practical power output. Chen's group mixed COF materials with CNFs to prepare a COF/CNF aerogel for an MEG.48 The COF was strategically synthesized with a high density of accessible hydrophilic functional groups (e.g., –SO3H and –COOH), providing an excellent platform to hybridize with cellulose, which contains abundant hydroxyl groups, thereby synergistically enhancing the energy harvesting systems from moisture (Fig. 5e). As a result, the hybrid aerogel-based MEG achieves a continuous output voltage of approximately 0.55 V for over 5 hours in ambient environments.
The aforementioned strategies that enhance cellulose by modifying its inherent properties to influence hydrogen ion concentration gradients have indeed proven effective in improving performance. Introducing new ion species to create concentration gradients offers additional possibilities for performance enhancement. One approach involves artificially introducing Na+ ions to generate a concentration gradient for MEGs based on laser-induced graphitization of sodium chloride-impregnated CNF films, as reported by Jeon's group.42 They focused the laser beam of a CO2 laser engraver onto the top surface of the CNF film, where the focal temperature of the laser beam exceeded the boiling point of sodium chloride, causing it to evaporate. As the laser intensity gradually decreased from the top surface to the bottom surface of the film, the evaporation rate of sodium chloride correspondingly decreased, thereby artificially creating a concentration gradient of Na+ in the thickness direction. When this modified CNF film comes into contact with moisture, the dissociated ions migrate from the top to the bottom surfaces, generating an electric current. The maximum output voltage and current reached 0.65 V and 550 μA cm−2 at 90% RH, respectively. Subsequently, the research group further enhanced the sodium-ion gradient-driven MEG device by introducing reactive components (Berlin green, BG) that can interact with sodium ions (Fig. 5f). This not only maintained the sodium-ion concentration gradient but also introduced additional faradaic currents, achieving the highest reported current density for MEG devices. Specifically, the authors stacked a BG/graphene oxide/CNF (BGC) composite layer on top of a NaCl/CNF (NC) composite layer to form a bilayer MEG device. When the NC layer adsorbs moisture, it triggers the dissociation of sodium ions, which then diffuse towards the BGC layer driven by the ion concentration gradient, generating non-faradaic currents. As the sodium ions enter the BGC layer, they insert into the BG framework and reduce it to Prussian blue, thereby converting chemical energy into electrical energy and generating additional faradaic currents. The synergistic effects of these physical and electrochemical processes result in exceptional MEG performance. At 90% RH, the Voc and short-circuit current (Isc) reach 1.17 V and 2770 μA cm−2, respectively, making it the first MEG device to simultaneously achieve voltage and current outputs exceeding 1 V and 1 mA cm−2.
In addition to its extensive research in the field of MEGs, cellulose has also been applied in the field of EEGs. For typical EEG devices, the active material is often partially immersed in or in contact with water, necessitating that the material does not dissolve in water to maintain its electricity-generating function. Compared to most polysaccharides, cellulose's strong intermolecular hydrogen bonds and van der Waals forces between molecular chains result in tightly packed molecules that are resistant to water penetration and dissolution. This unique water-insoluble characteristic makes cellulose particularly suitable for use in EEGs.
Fu's group developed a CNF/carbon nanotube (CNT) fiber via wet-spinning of their mixed solution for a fiber-type EEG device (Fig. 5g and h).51 CNFs serve as the primary matrix, providing lightweight, environmentally friendly, and hydrophilic properties. CNTs, with their abundant out-of-plane π-bonds, are chemically reactive, allowing substantial coupling with water molecules and facilitating charge separation. When one end of the fiber-type MEG contacts a water droplet, the macromolecular chain channels and spontaneous evaporation drive a continuous and efficient water flow along the fiber, sustaining power generation. Notably, three different types of CNTs were prepared—surfactant-treated CNTs, acidified CNTs, and aminated CNTs—through various surface modification methods to enhance the fiber's coupling capacity with water. Density functional theory (DFT) calculations indicated that acidified CNTs containing –COOH showed the lowest binding energy (−0.84 eV) with H2O among the modified products, exhibiting the best electricity generation performance. As a result, a constant output voltage of 160.4 mV and a short-circuit current of 171.8 nA were achieved in a single fiber, with volume power density reaching 0.4 mW cm−3. These fibers also demonstrated excellent knittability and design flexibility. By weaving 108 fibers in series or parallel into flexible fabrics, a maximum power supply of 1.2 V was obtained with only 10 minutes of charging time, capable of powering an electronic calculator. Guo's group presented a cellulose paper-based bilayer EEG designed for enhanced power generation from ambient humidity (Fig. 5i and j).55 The bilayer EEG consists of a layer of LiCl-loaded cellulose paper to facilitate moisture adsorption and a layer of carbon-black-loaded cellulose paper to promote water evaporation (Fig. 5i). When exposed to air, the LiCl in the hygroscopic layer rapidly absorbs moisture and partially dissociates into ions, creating both a water content gradient and an ion concentration gradient within the bilayer structure. Consequently, water and ions, including protons and lithium ions, flow directionally through the channels and micropores from the hygroscopic layer to the evaporative layer, generating voltage and current outputs. This design is highly ingenious, as it directly harvests moisture from the surrounding environment via the hygroscopic layer, eliminating the need for liquid water and making it suitable for flexible and wearable devices. More importantly, this structure can maintain stable voltage and current output for up to 10 days in the air.
Besides, cellulose is also explored for use in REGs. To enhance the performance of REGs, blending and surface modification of cellulose are two commonly used strategies. In terms of blending, various two-dimensional materials such as GO,97 MXenes, clay,56 boron nitride,58 and WS257 have been used to prepare composite membranes with cellulose. For example, an interlocking configuration of stacked montmorillonite nanosheets and intercalated CNFs was developed for a robust and efficient REG.56 In this device, CNFs acted as intercalators, where their intrinsic high mechanical strength enhanced the 2D nanofluidic membranes (Fig. 5k). Additionally, the formation of oxo-bridges between dense oxygen-containing groups on the CNF molecules and the 2D nanosheets further stabilized the membrane with an interlocking configuration. Molecular dynamics (MD) simulations demonstrated its ion (K+, Cl−) permeability and selectivity, which were consistent with experimental results (Fig. 5l). On the other hand, surface modification can increase the surface charge density of cellulose and alter the surface charging properties. Thus, various positively and negatively charged cellulose materials have been developed for high-performance REGs. For instance, Zhang's group developed a pair of CNF membranes with opposite surface charges and channel-like nanopores to improve selective ion transport (Fig. 5m).62 They achieved higher surface charge density for positively and negatively charged CNFs through a two-step oxidation and esterification reaction, respectively. Concurrently, a space-confined flattened extrusion process was used to create regular internal channel-like nanopores in the cellulose film. These structural features ensured less electrical imperfection and therefore higher ion selectivity, leading to REG systems with a high output power density of 0.72 W m−2.
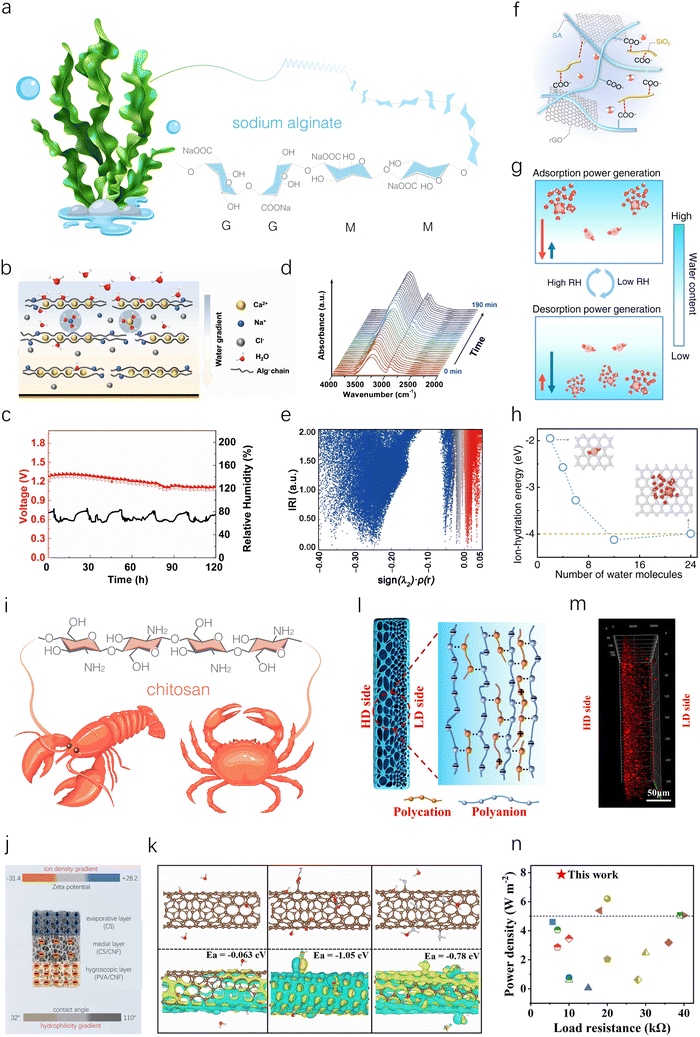 | ||
| Fig. 6 Sodium alginate- and chitosan-based HEGs. (a) Schematic illustration of the structure and composition of SA. (b) Schematic illustration of a supramolecular AlgCa/Na network within a PVA hydrogel, formed through replacing partial Na+ ions by Ca2+ ions as the egg-box crosslinked points. (c) The long-term Voc of an MEG device at ambient RH in an open environment. (d) In situ FTIR spectrum tracking versus time once the sample is exposed to the air of 65% RH. (e) Scatter maps between IRI (interaction region indicator) and sign(λ2)ρ of AlgCa-H2O. Reproduced with permission.63 Copyright 2024, Springer Nature. (f) Schematic illustration of a composite film composed of SA chains, SiO2 nanofibers, and rGO nanosheets. (g) Schematic illustration of the working principle during the moisture absorption–desorption cycle. (h) MD simulations showing ion hydration energy of hydrated Na+ ions with different numbers of H2O molecules. Reproduced with permission.64 Copyright 2022, Springer Nature. (i) Molecular structure of CS extracted from a shrimp or crab. (j) Schematic illustration of multi-layered aerogels. (k) DFT simulations of charge redistributions and binding energies of SWNTs, CSWNTs and NSWNTs. Reproduced with permission.70 Copyright 2023, Royal Society of Chemistry. (l) Schematic illustration of gradient structure in resulting hydrogel membranes. (m) Confocal laser scanning microscopy image of gradient CS/SA hydrogel membranes. (n) Power density comparison of gradient CS/SA hydrogel membranes with reported macroscopic scale nanofluidic membranes under mixing seawater and river water. Reproduced with permission.73 Copyright 2021, Wiley-VCH. | ||
Due to limited MEG properties of pure sodium alginate film, blending it with other functional materials is an effective strategy to enhance its power generation performance. For instance, a polyvinyl alcohol (PVA)-SA-based supramolecular hydrogel was prepared as an active material for an MEG (Fig. 6b).63 A single unit with top/bottom electrodes of Al and poly(3,4-ethylenedioxythiophene):poly (styrenesulfonate) (PEDOT:PSS) can generate a high voltage of 1.30 V, a current density of 1.31 mA cm−2, and a power density of 0.11 mW cm−2 (Fig. 6c). Such high electricity is mainly attributed to enhanced moisture absorption and remained water gradient to initiate ample ion transport within the hybrid hydrogel verified by in situ Fourier-transform infrared spectroscopy (FTIR) and DFT calculation (Fig. 6d and e). Due to its flexibility, light weight, high performance output and scalability, this MEG can be used in real life applications. For example, 3 × 3 MEG banks in series were shown to charge a smart watch. Furthermore, MEG banks were demonstrated to serve as gate voltage sources to fabricate a self-powered metal-oxide-semiconductor field effect transistor.
Using a similar blending strategy, a film composed of SA, SiO2 nanofibers, and reduced graphene oxide (rGO) was prepared through freeze-drying and chemical reduction for moisture absorption–desorption full-cycle power generation (Fig. 6f).64 SA, with its abundant –COONa groups, dissociates to release mobile Na+ ions during interaction with water molecules. SiO2 nanofibers help construct a hierarchical porous structure, promoting the transmission of water molecules and ions while providing mechanical stability in water. Meanwhile, rGO nanosheets assemble into a 3D conductive framework and adjust the electrical resistance. Research indicates that this material can spontaneously absorb moisture at high RH and desorb moisture at low RH, thereby generating cyclic electric output. Specifically, during water absorption, dissociated Na+ ions from the SA chain form an ion gradient from the top to the bottom side, thus generating an electric output. During water desorption, ion-hydration serves as the main driving force to generate a second electric output (Fig. 6g). This is attributed to the fact that hydrated Na+ ions combined with more water molecules possess a lower ion-hydration energy than those surrounded by fewer water molecules (Fig. 6h). Thanks to the synergistic structural design, a single device can produce a high voltage of ∼0.5 V and a current of 100 μA at 100% RH, provide an electric output of ∼0.5 V and ∼50 μA at 15 ± 5% RH, and deliver a maximum power density close to 120 mW m−2. To demonstrate the potential of this MEG for outdoor applications, a proof-of-concept self-powered road lamp is designed. It uses an MEG as a source of power supply and then stores continuous electrical energy in a capacitor, which is subsequently accumulated continuously to light the road lamp.
In addition to film-type MEGs, fiber-type MEGs based on SA have been been developed.65,66 A coaxial fiber composed of a MWCNT core and an SA/(PEDOT:PSS) shell was obtained through wet spinning technology.65 When constructing an MEG device using the coaxial fiber with an active Fe metal wire, the SA/(PEDOT:PSS) component interacts with moisture at the interface, providing a substantial amount of mobile ions. The asymmetric electrode structure formed by carbon and Fe creates a built-in electric field that accelerates ion movement. Consequently, the 1 cm long fiber MEG generated an output voltage of up to 1.2 V, an output current of 21.0 μA, and a power density of 5.56 W m−2 (RH = 61–64%). The combination of the flexible fiber structure and high MEG performance makes it highly competitive for use in wearable and sustainable energy-generating textiles and devices.
The positively charged nature of CS makes it highly compatible with various negatively charged materials, enhancing surface potential differences and accelerating ion diffusion, thereby improving the performance of MEGs. For instance, Deng's group developed a double-gradient-structured MEG device by sequentially stacking a hygroscopic layer (PVA/CNF aerogel) at the bottom, a medial layer (CS/CNF aerogel), and an evaporative layer (CS aerogel) at the top (Fig. 6j).70 The bottom PVA/CNF layer provides a continuous water supply during evaporation. The top CS aerogel layer, with its abundant positive charges, high adhesion, and surface modification-induced hydrophobicity, significantly enhances water evaporation efficiency. The medial CS/CNF aerogel layer effectively bonds the top and bottom layers. These three aerogel layers created a hydrophilicity gradient from bottom to top, ensuring the long-term operation of MEGs. To further establish an ion gradient, multiple single-walled carbon nanotubes (SWNTs) are assembled on the polymer surface of each layer, forming a biomimetic core–shell structure. Specifically, carboxylated SWNTs (CSWNTs) and aminated SWNTs (NSWNTs) were assembled onto the hygroscopic and evaporative layers, respectively. DFT simulations showed that the adsorption energies of SWNTs, CSWNTs, and NSWNTs were −0.063, −1.05, and −0.78 eV, respectively (Fig. 6k), indicating that CSWNTs and NSWNTs can enhance water molecule absorption and accelerate charge carrier release. Additionally, combined with the negatively charged CNF backbone and positively charged CS backbone, a double gradient is formed (from −30.2 to +27.6 mV). This double-gradient design enabled the MEG to achieve industry-leading energy density (165.23 mW h cm−2), power density (32.59 mW cm−2), and operational duration (120 h) by optimizing their working environment.
The positively charged characteristic of CS has been utilized to modify other materials for selective ion transport in REG applications. Wang's group developed positively charged chitosan quaternary ammonium bacterial cellulose membranes with adjustable charge density and nanochannel size through in situ culture.72 These were assembled into REG devices alongside negatively charged carboxymethyl bacterial cellulose membranes, achieving an output power density of 2.25 W m−2. Although these prepared asymmetric ionic membranes, composed of two different porous membranes, demonstrated significant advantages in REGs, issues with incompatible interfaces and low interfacial ionic transport efficiency have constrained their further development.
CS can also be used to achieve controlled preparation of gradient polyelectrolyte hydrogel membranes through electrostatic complexation with oppositely charged polysaccharides, facilitating unidirectional ion transport for high-performance REG. Jiang's group rapidly prepared an all-polysaccharide gradient polyelectrolyte hydrogel membrane within 30 seconds using a reaction-diffusion method with low-molecular-weight (LMW) CS and high-molecular-weight (HMW) SA.73 During fabrication, when the CS solution came into contact with the SA solution, a thin complex layer formed instantly at the interface due to strong electrostatic complexation. Subsequently, CS gradually diffused across the complex layer and interacted with SA, leading to the growth of the membrane's thickness. The side closer to the LMW CS solution (denoted as the LD side) reacted with more LMW CS, resulting in a higher density of electrostatic complexation compared to the side closer to the HMW SA solution (denoted as the HD side). This indicated that the LD side had a lower density of residual negative charge and a denser network structure (Fig. 6l). This observation was consistent with confocal laser scanning microscopy characterization of the membranes, which showed a gradient distribution of the positively charged rhodamine 6G (red fluorescence) (Fig. 6m). The gradient polyelectrolyte hydrogel membranes exhibited an ionic diode effect, facilitating unidirectional ion transport. Meanwhile, the anti-swelling 3D charged networks provided inherent high ion conductivity and excellent ion selectivity. Consequently, when mixing seawater and river water, the REG based on the all-polysaccharide gradient polyelectrolyte hydrogel membrane achieved a power density as high as 7.87 W m−2, significantly superior to previously reported REGs (Fig. 6n).
3.2. Natural protein-inspired HEGs
The aforementioned polysaccharides, composed of monosaccharide units linked by glycosidic bonds, form linear or branched polymer chains. These polysaccharides have relatively simple molecular structures, characterized by high repeatability and crystallinity, typically exhibiting one-dimensional or two-dimensional simple structures. In contrast, natural proteins, which are polymer chains composed of amino acids linked by peptide bonds, possess primary, secondary, tertiary, and quaternary structures. These complex molecular structures enable proteins to self-assemble into hierarchical structures, enhancing their interactions with water molecules. Additionally, the diverse side chains and functional groups in proteins, including carboxyl, amino, and thiol groups, result in complex surface charge distributions, providing good electrical conductivity and ion transport capabilities. Furthermore, protein materials can be functionalized through genetic engineering or chemical modifications, potentially enhancing their electricity generation performance and application potential. It is also noteworthy that natural protein materials typically exhibit excellent biocompatibility and biodegradability. These characteristics make proteins promising candidates for use in HEGs. To date, various proteins such as silk, gelatin, milk proteins, and microorganism biofilms have been explored for their potential applications in HEGs.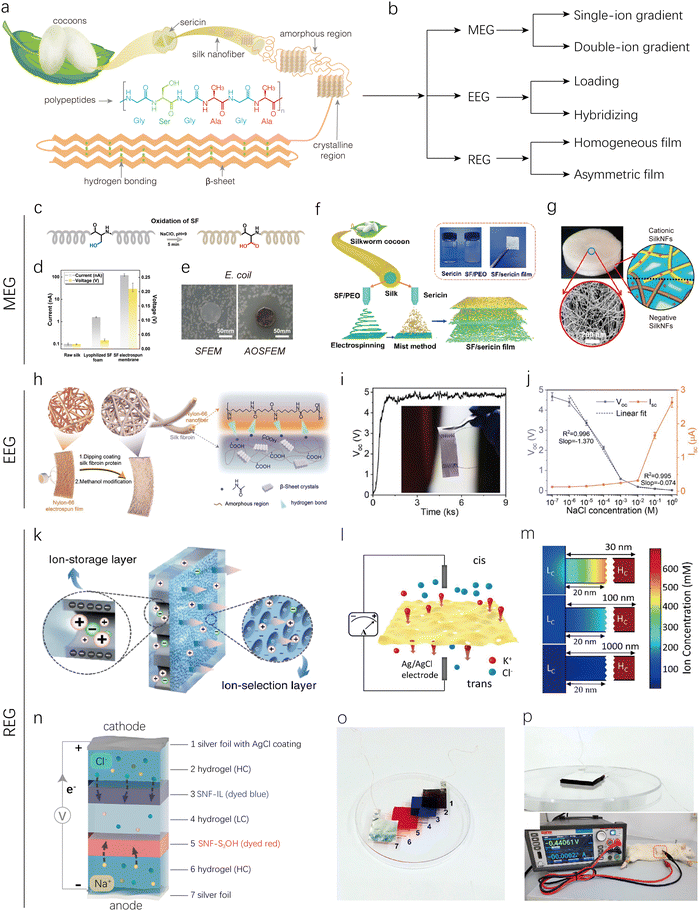 | ||
| Fig. 7 Silk-based HEGs. (a) Schematic illustration of hierarchical silk fiber. (b) Classification of SF-based MEG, EEG, and REG devices. (c) Preparation route of oxidized SF. (d) MEG properties for three different silk-based samples. (e) Inhibition zones of electrospun membranes containing pure SF (SFEM) and oxidized SF with AgNPs (AOSFEM) to E. coli. Reproduced with permission.75 Copyright 2021, Elsevier. (f) Schematic illustration of preparation of SF/sericin film. Reproduced with permission.76 Copyright 2024, American Chemical Society. (g) Structures of the SF bilayer aerogel. Reproduced with permission.74 Copyright 2020, American Chemical Society. (h) Schematic of structure and composition of SF@NNFs. (i) Output Voc of SF@NNFs based EEG. (j) Voc and Isc signal response of devices to NaCl solutions of different concentrations. Reproduced with permission.77 Copyright 2024, Wiley-VCH. (k) Structural schematic of AAO/SNF with AAO and SNF membranes working as an ion-storage layer and ion-selection layer, respectively. Reproduced with permission.79 Copyright 2019, Springer Nature. (l) Schematic illustration of an ultra-thin SF film based REG under a salinity gradient. (m) Numerical simulation of cation concentrations at the entrance of the low-concentration side. As the length increases, the ion concentration polarization phenomena become weaker. Reproduced with permission.80 Copyright 2019, American Chemical Society. (n) and (o) Schematic and picture of the miniaturized RED using SNF membranes as ion-exchange membranes and saline polyacrylamide hydrogels as solid electrolytes. (p) Picture of the miniaturized REG implanted beneath the skin of a Sprague-Dawley rat. Reproduced with permission.106 Copyright 2021, American Chemical Society. | ||
Pure SF film used directly for an MEG can produce a stable voltage of 0.15 V in the ambient environment.39 Research has shown that increasing the film thickness can enhance the voltage output from 0.1 to 0.26 V, due to the improved water absorption gradient in thicker SF films. However, the performance remains significantly suboptimal. To further enhance the performance of SF-based MEGs, Qu's group designed an SF-MEG device through electrospinning and in situ synthesis of Ag nanoparticles.75 The electrospun membrane containing oxidized SF can generate electricity due to the directional migration of charged ions in an asymmetric humid environment (Fig. 7c). The carboxyl content of the SF film increased after oxidation, resulting in an improved MEG output voltage of 0.22 V and a current density of 100 nA cm−2 (Fig. 7d). Additionally, since Ag+ ions can migrate as positively charged ions along with protons, increasing the concentration of positive ions, the output was further enhanced to 0.28 V and 190 nA cm−2. The addition of Ag+ also imparted potent antibacterial activity against the Gram-negative bacterium Escherichia coli (E. coli) (Fig. 7e), demonstrating its potential for applications in multifunctional wearable power generation.
SF's MEG properties can be enhanced by incorporating sericin, which contains over 70% polar side-chain amino acids, compared to SF's 29.5%. The high content of polar side-chain amino acids in sericin endows it with remarkable hygroscopic and humectant properties, making it a promising active component for MEGs. Fu's group prepared a gradient-structured sericin/SF/poly(ethylene oxide) (PEO) composite film by combining electrospinning and mist methods.76 Specifically, an SF/PEO film was first obtained through electrospinning, followed by the application of sericin via spraying onto the SF/PEO film. Due to sericin's adhesive properties, it adheres to the top layer of the SF film and seals the film's pores. As the misting process continues, sericin protein finds it increasingly difficult to reach the bottom of the film, resulting in a gradient distribution of sericin within the SF matrix (Fig. 7f). The SF/sericin-based MEG demonstrated maximum Voc and Isc of 276 mV and 70 nA, respectively at 95% RH.
Furthermore, asymmetric bilayers based on oppositely charged SFs were explored to regulate ion transport properties for MEGs. The cationic SF nanofibrils (SNFs), with an ultrathin thickness of approximately 4 nm and a high aspect ratio of up to 500, were successfully exfoliated from natural cocoon fibers via quaternization followed by mechanical homogenization by Li's group.74 These cationic SNFs, positively charged over a wide pH range of 2–12, could combine with negatively charged biological nanofibrils to produce asymmetric ionic membranes (Fig. 7g). Then a bilayer aerogel was assembled using the positively charged SFs and negatively charged SFs (obtained through NaClO oxidation and ultrasonic treatment) via freeze-drying. This bilayer aerogel was placed between two Pt mesh electrodes to construct a MEG. When exposed to moisture, the hydrated and positively charged nanofibrils acted as nanochannels for ion transport, creating an ion gradient, which achieved an optimal Voc of 121 mV at 99% RH.
In addition to MEGs, SFs have also been used as active materials for EEGs. Zhang's group reported a controllable nanochannel regulation strategy for high-performance, flexible EEGs by controlled dip-coating of SF on electrospun nylon-66 nanofiber (NNF) films (Fig. 7h).77 By adjusting the number of dip-coating cycles, the surface polarity of NNF is enhanced, with the zeta potential increasing from −10.9 to −38.4 mV, and the fiber size can be regulated with a precision of about 25 nm. The resulting flexible, free-standing EEG device based on SF-coated NNF exhibits a Voc of up to 4.82 V in deionized (DI) water at room temperature (Fig. 7i). The different concentrations of salt solutions affect the Debye length, resulting in variations in the corresponding Voc and Isc. Based on this, the ion sensing performance of the SF@NNF EEG was further investigated. As shown in Fig. 7j, the SF@NNF EEG device exhibited a high sensitivity of up to 1.37 V dec−1 in the NaCl concentration range of 10−6 to 10−3 M. Moreover, SF formed hydrogen bonds with nylon-66, firmly binding the NNFs, and after ethanol treatment, the SF secondary structure on the NNF surface transformed more into β-microcrystalline form. This transformation ensured that the SF@NNF films can withstand severe mechanical interference, including rubbing and stirring at 2000 rpm. Consequently, the fabricated flexible ion-sensitive EEG device was successfully applied in wearable human sweat electrolyte sensing and environmental monitoring of trace ions. For example, it was mounted onto a subject's chest, back, forehead, and forearm to measure perspiration through generated voltage variations. In addition to this, the SF@NNF EEG device has also been used to detect changes in salt concentration in seawater.
SFs possess abundant and tunable functional groups, which facilitate selective ion transport, garnering significant attention in the field of REGs. For example, Wen's group developed a hybrid membrane composed of a SF membrane and an anodic aluminum oxide membrane for REG devices (Fig. 7k).79 In this hybrid membrane, the SF membrane, acting as a screening layer with a condensed negative surface and nanochannels, dominates ion transport, while the anodic aluminum oxide membrane, serving as a supporting substrate, provides tunable channels and amphoteric groups. This configuration creates a nanofluidic membrane with asymmetric geometry and charge polarity, exhibiting low resistance, high power density (2.86 W m−2), and long-term stability. Additionally, freestanding ultrathin SF membranes have been utilized for high-performance REGs. Wen's group prepared self-supporting SF membranes with a thickness of only 10 nm per layer using a spin-coating method, which exhibited outstanding mechanical properties by modulating the crystallinity of β-sheets (Fig. 7l).80 The thickness of these membranes can be adjusted by controlling the number of spin-coating cycles. These ultrathin membranes demonstrated low resistance and high ion throughput. By optimizing the membrane thickness, the optimal value was found to be approximately 100 nm, maximizing permeability while maintaining effective selectivity, as supported by numerical simulations (Fig. 7m). Consequently, REG devices based on these optimized membranes achieved top-level osmotic energy conversion rates of up to 21.66 W m−2 (Fig. 7l). Furthermore, the design of bilayers based on the asymmetric structure of SFs has also been reported, frequently showing promising results in the field of REGs.
In addition, SFs have been further chemically modified to enhance ion selectivity for high-performance REGs. Liu's group transformed a SNF suspension into a 3D nanofiber network with densely packed nanochannels using vacuum filtration.106 The structure and surface charge properties of these nanochannels were optimized through additional chemical modifications. This process yielded SNF membranes with negatively charged sulfonic acid groups (SNF-SO3H) for cation selectivity and SNF membranes with positively charged alkyl-substituted imidazolium groups (SNF-IL) for anion selectivity. Both SNF-IL and SNF-SO3H exhibit ionic selectivity in low concentration ranges and regulate ion diffusion, resulting in efficient charge separation to generate electric power. A maximum power output of 0.59 mW m−2 was observed at an external load resistance of 66 kΩ. Interestingly, benefiting from SNF's good biocompatibility, 3T3 cells were well spread on both the SNF-IL and SNF-SO3H membranes and reached high confluence on day 7 after seeding, indicating excellent cytocompatibility. Subsequently, a flexible, miniaturized RED was assembled using silver foil, silver foil coated with AgCl, thin saline polyacrylamide hydrogels, and SNF membranes as the anode, cathode, solid electrolytes, and exchange membranes, respectively (Fig. 7n and o). This device was then subcutaneously implanted in the back region of a Sprague-Dawley rat (Fig. 7p). Measurements with a source meter instrument showed that the Voc of the implanted RED was 0.441 V, demonstrating the potential of SF-based REDs for in vivo applications.
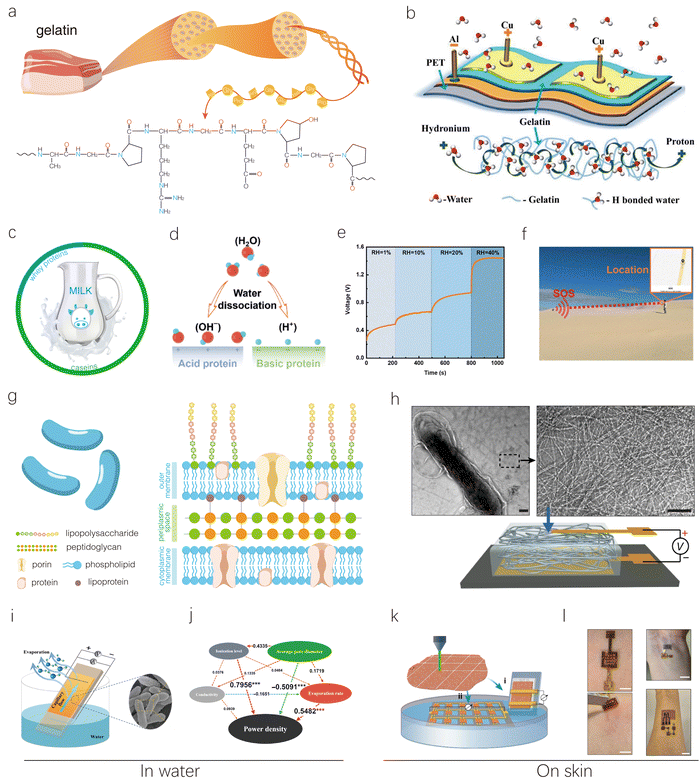 | ||
| Fig. 8 Other representative proteins including gelatin, milk, and bacterial biofilm-based HEGs. (a) Schematic illustration of the structure and composition of gelatin. (b) Schematic of a gelatin-based MEG (top) and a conducting path formed by hydrogen bonds with protein molecules (bottom). Reproduced with permission.81 Copyright 2020, American Chemical Society. (c) Schematic showing milk's main proteins and their contents. (d) Schematic illustration of electricity generation of modified acid and basic whey protein films exposed to moisture. (e) Output voltage of whey protein-based MEGs at different RH levels with a CNT top electrode and a FTO glass bottom electrode. (f) Operation photograph of a wireless location tracker powered by a 0.01 F capacitor charged by the whey protein-based MEG for immediate location information transmission. Reproduced with permission.83 Copyright 2023, Royal Society of Chemistry. (g) Schematic illustration of microbial cells and their cell wall structure. (h) TEM images of the purified nanowire network (right panel) produced from the microorganism Geobacter sulfurreducens (left panel) and MEG based on it. Scale bar, 100 nm. Reproduced with permission.84 Copyright 2020, Springer Nature. (i) Schematic of a G. sulfurreducens biofilm-based EEG with two Cu electrodes attached to the top and bottom of a biofilm surface. (j) Partial least squares path modeling showing the effects of various factors on power density of a G. sulfurreducens biofilm-based EEG. Reproduced with permission.88 Copyright 2022, AAAS. (k) Schematic of using laser-patterned biofilms to construct (i) a single device and (ii) an interconnected device array on a PDMS substrate, with a portion of the biofilm at one electrode immersed in water. (l) Biofilm-based EEG devices patched on the skin to power a skin-wearable strain sensor with one device and an electrochemical glucose sensor with three devices. Reproduced with permission.87 Copyright 2022, Springer Nature. | ||
Yuan's group utilized protein nanofibrils from milk whey β-lactoglobulin to construct MEG devices.82 The developed device can generate a Voc of up to 0.65 V, an Isc of 2.9 μA, and a maximum power density of 38.88 μW cm−2 at 90% RH. The superior performance of this device is closely related to the remarkable hydrophilicity, high ionization, and surface-to-volume ratio of protein nanofibrils. Their DFT calculations revealed that carboxyl groups were the favorable active sites in protein nanofibrils for binding water molecules. In another work, Chu's group fabricated functional layers using low-cost whey protein for MEGs, which contains abundant hydrophilic functional groups.83 The surface charges of whey protein were modified on a large scale by adjusting its pH value (Fig. 8d). Moreover, plasma treatment significantly enhanced the water absorption ability of the protein films from the environment for electricity generation. Consequently, the whey protein-based MEGs delivered an ultra-high Voc of 1.45 V, the highest value at room humidity, and a current density of 113 μA cm−2 at 40% RH (Fig. 8e). The high MEG performance at low humidity makes it suitable for powering electronics in deserts. As shown in Fig. 8f, four whey protein-based MEG units can operate normally in the desert at 26% RH, charging a 0.01 F capacitor to 3.5 V, thereby powering an electronic location tracker to send immediate location information to a monitor in the dry desert. This provides a facile approach for location tracking in urgent situations.
Yao's group has pioneered the use of bacterial components in the development of MEGs.84 They extracted nanometer-scale protein wires from the microbe Geobacter sulfurreducens and used them to construct a thin-film MEG device (Fig. 8h). The study demonstrated that this device can generate continuous electric power in an ambient environment, producing a sustained Voc of around 0.5 V across a 7-μm-thick film, with a current density of approximately 17 μA cm−2. Remarkably, it can operate for up to 1500 hours under ambient conditions. The driving force behind this energy generation is believed to be a self-maintained moisture gradient that forms within the film when exposed to ambient humidity.
Although these protein nanowires exhibit good MEG performance, the complex extraction process and low yield limit their further development. Consequently, researchers have begun exploring the potential of using inexpensive whole-cell microorganisms directly for MEG applications. Interestingly, devices based on whole-cell microorganisms can still function effectively in MEG applications, likely due to their intrinsic characteristics such as hydrophilicity, porous structure, and conductivity. Zhou's group prepared films using whole-cell Geobacter sulfurreducens and low-cost indium tin oxide as electrodes.86 The resulting MEG device produced a Voc of 0.3 V, a load current of 0.3 μA, and a power density of 2.5 μW cm−2. Remarkably, it continued to operate for over 2160 hours.
The application of microorganism biofilms in the MEG field has been successfully extended to the EEG domain. Zhou's group constructed a typical EEG device with one end immersed in water using a Geobacter sulfurreducens biofilm (Fig. 8i), which exhibited a maximum output power density of ∼685.12 μW cm−2, two orders of magnitude higher than that of previously reported analogous devices.85 However, its working mechanism is relatively complex, influenced by factors such as the water evaporation rate, ionization level, conductivity, and average pore diameter. Partial least squares path modeling analysis was conducted to probe the complex relationships among these factors (Fig. 8j).
In addition to the common in-water-type of EEGs, Yao's group demonstrated a wearable-type EEG that operates on the skin using Geobacter sulfurreducens microbial biofilms.87 The authors first prepared the device by laser-cutting microbial films and verified that the in-water-type device could function normally (Fig. 8k). They found that a single microbial sheet (∼40 μm thick) serving as the functional component in an electronic device continuously produced a power density of ∼1 μW cm−2, which is higher than that achieved with thicker engineered materials. Moreover, the microbial sheet-based device maintained normal electrical output even in test environments with ion concentrations higher than those in bodily fluids. This may be attributed to the unique material properties of the biofilm. Specifically, unlike many inorganic materials with uniform surface charge states, biofilms contain numerous amphiphilic groups. The resulting amphiphilic surface may help effectively repel both cations and anions, leading to reduced local ion strength or increased effective Debye length. Based on these results, on-skin EEGs were further developed (Fig. 8l). Devices worn on sweaty skin produced power comparable to that generated with saline solution. Even on non-sweaty skin, a significant amount of power was generated, indicating that the continuous low-level moisture secretion from the skin was sufficient to drive this hydrovoltaic electricity generation. The on-skin EEG device was demonstrated to power wearable sensors for monitoring various physiological signals, such as pulse and glucose levels in sweat.
3.3. Natural small molecule-inspired HEGs
Natural small molecules, such as phytic acid (PA), tannic acid (TA), and citric acid (CA), are used in HEGs due to their excellent hydrophilicity, abundance of functional groups, and ability to dissociate into a large number of protons when interacting with water (Fig. 9a). These small molecules, typically derived from natural sources, are environmentally friendly and exhibit good biocompatibility. Compared to natural polymers like polysaccharides and proteins, they often cannot form stable structures on their own and usually need to be combined with other materials. However, it is noteworthy that their small molecular size and simple structure allow for easier penetration and diffusion, which can potentially enhance the flexibility and responsiveness of the hydrovoltaic devices. | ||
| Fig. 9 Natural small molecule-based HEGs. (a) Molecular structures of representative natural small molecules. (b) Schematic of a PA-MEG device with asymmetric-moisture penetration layers. (c) 2D-FTIR correlation spectra in the 3700–3000 cm−1 wavenumber region: (left) synchronous and (right) asynchronous contour maps. (d) Long-term Voc output of a PA-MEG device in an open ambient environment. Reproduced with permission.90 Copyright 2022, Wiley-VCH. (e) Structural schematic of PA-CPDs and the corresponding MEG device based on them. (f) Voc and Isc of the PA-CPD-based MEG at 60% RH. (g) Schematic of integration of the PA-CPD-based MEG device. Reproduced with permission.91 Copyright 2023, Wiley-VCH. | ||
A PVA-PA ionic hydrogel was designed for a flexible and all-weather MEG by Tao's group.91 In this hydrogel, the combination of a hydrophilic PVA-PA network and hygroscopic glycerol endows the MEG with high moisture absorption capability. Notably, the PA with six esterified phosphoric acid groups enables significant proton dissociation and migration through sufficient hydration (Fig. 9b). As shown in Fig. 9c, 2D-FTIR analysis confirmed that when the dissociated protons from PA encounter the ample water clusters in the hydrogel, they form protonated water clusters (H3O+ or H+(H2O)n). These protonated water clusters facilitate the transport of protons, generating a larger diffusion current and internal potential. A single MEG device can deliver a stable Voc of about 0.8 V for more than 1000 hours in an open ambient environment with RH fluctuating from 60% to 90% at 22 °C, demonstrating outstanding operational stability (Fig. 9d). Furthermore, Ding's group reported a scalable MEG based on PA-carbonized polymer dots (CPDs) (Fig. 9e).91 Experimental results showed that CPDs with phosphate groups exhibit higher hygroscopicity and ionization ability compared to CDs with carboxyl and hydroxyl groups. Consequently, one device unit could generate a high Voc of 0.8 V and a record-high current density of 1640 μA cm−2 at 80% RH (Fig. 9f). Additionally, the moisture-induced HEGs could be easily integrated onto various flexible substrates, demonstrating notable scalability by offering a voltage of 210 V in series and a short-circuit current of 72 mA in parallel to power commercial electronics (Fig. 9g).
Overall, the properties of natural biomass-based HEGs have been extensively studied, owing to the abundance, accessibility, and low cost of such materials. In the process, a material-performance database (Table 1) has been naturally established. More importantly, some key structure–performance relationships for these materials have been identified and summarized. For example, materials with higher zeta potential tend to exhibit stronger charge separation ability, materials that can self-assemble to form more porous structures generally show faster water and ion transport, and materials capable of ionizing to release H+ ions (with these ions acting as carriers) often exhibit higher current output due to the rapid diffusion of hydrogen ions in water compared to other ions. These fundamental insights not only assist researchers in further modifying biomass materials (among others) to achieve enhanced HEG performance, driving multifunctionality and practicality in devices, but they also contribute to a deeper understanding of the interactions between water, ions, electrons, physiological activities, and biological systems. This knowledge may, in turn, play a positive role in advancing our understanding of life systems and even hold potential for future disease treatments.
4. Smart bioinspired structures for HEGs
In the aforementioned section, researchers have constructed numerous HEGs by mimicking the direct use of natural biomass materials and substances by living organisms. Through various regulation strategies, performance enhancement to a certain extent has been achieved in these devices. However, it is important to note that, compared to the sophisticated use of these materials by living organisms, the current research remains quite elementary. Living organisms often process biomass with higher precision to form advanced assembly structures, thereby enabling more efficient transport of substances/electrons and realizing more complex physiological processes. For example, the stems of plants exhibit intricate assembly structures with vertical channels and hierarchical pores by controlling the assembly of cellulose and other substances, significantly accelerating substance transport. Similarly, the electric eel generates high voltages of up to 600 V by assembling thousands of long and thin electrically active cells known as electrocytes in parallel stacks spanning the rear 80% of its body. The advanced assembly structures and complex physiological processes in living organisms provide researchers with a wealth of inspiration for the design of HEGs, aimed at further enhancing their power generation performance. The subsequent review is divided into four sections based on bio-inspired sources (Table 2).| Bioinspired source | Materials | Mechanism | Electrodes (top/bottom) | Area (cm−2) | Voltage (V) | Current density (μA cm−2) | Power density (μW cm−2) | Condition | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Fern roots and leaves | PVA/GO | MEG | — | 7 | 1.9 | 11.8 | 22.55 | 80% RH | 109 |
| Water transport | Cellulose nanofibril CMC/single-walled CNTs | MEG | Ag/Al | 4 | 0.668 | 1.6 | 0.871 | 90% RH | 110 |
| Moth eye | BMIMCl ionic hydrogel/MWCT/MXene/CsPbBr3/MWNT nanofibers | MEG | Au/Au | — | 0.4 | 64.2 | 11.8 | 40% RH | 111 |
| Porous structure of wood | Ionic balsa wood | MEG | Cu/Ag | 16 | 0.75 | 44.5 | — | 70% RH | 112 |
| Water absorption and transport | PSS/poly-ionic liquid membrane | MEG | Ag mesh/Ag mesh | — | 0.6 | 0.2 μA | 1.6 | 70–90% RH | 113 |
| Biological ion channels | CNF/GO | MEG | Ti/Ti | 3 | 0.28 | — | — | — | 43 |
| Asymmetrical lipid bilayer | PSSA/PDDA | MEG | Both carbon tape | — | 0.95 | 0.05 μA | 0.076 | 25% RH | 114 |
| Mitochondrial membrane | PSSA | MEG | Au/Au | 1 | 0.8 | 100 | 17 | 80% RH | 30 |
| Rice leaf & butterfly | PSSA/KC | MEG | Au/Au | 1 | 0.8 | 1600 | — | 70% RH | 115 |
| Willow branch | GO aerogel | EEG | Cu/Cu | — | 0.03 | 12 μA | — | DI water | 116 |
| Lotus | MXene/GQDS/Hydrogel | EEG | Pt/CNT | 0.3 | 0.8 | — | 45.6 | Fresh water | 117 |
| Water uptake and transpiration | Porous wood | EEG | Both Pt mesh | 1.5 | 0.55 | 4.67 | 0.62 | Alkaline electrolyte | 118 |
| Aligned structure of wood | Balsa wood sponge | EEG | Cu/carbon black | 8 | 0.5 | — | 27 | DI water | 119 |
| Aligned structure of wood | Balsa wood | EEG | Both carbon paste | 16 | 0.77 | 9.25 | 0.13 | DI water | 120 |
| Aligned structure of wood | Carbon black coated wood | EEG | Cu/Cu | 1 | 0.73 | 360 | — | DI water | 121 |
| Water transport | Wood | EEG | Pt/Pt | 4 | 1.1 | 80 | 6.75 | Wastewater | 122 |
| Water transport | Wood-based biochar | EEG | Pt/Pt | 6 | 0.42 | 0.087 | — | DI water | 123 |
| Human blood vessels | PCMVImTf2N poly (ionic liquid) membrane | REG | Both Ag/AgCl | 3 × 10−4 | 0.2 | 700 | 433 | 0.5 M/0.01 M NaCl | 124 |
| Eel's electrocyte assembly | Acrylamide hydrogel | REG | Both Ag/AgCl | 8.5 × 10−5 m2 | 0.17 | 1.6 μA | 2.7 | 2.5 M/0.015 M NaCl | 26 |
| Eel's electrocyte assembly | Agarose hydrogel/insulating lipid bilayers | REG | Both Ag/AgCl | — | 0.127 | 2.2 μA | 1300 W m−3 | 2 M/0.01 M CaCl2 | 125 |
| Eel's electrocyte assembly | Paper-acrylamide gel | REG | Both Ag/AgCl | 2.25 × 10−4 | 0.24 | — | 0.18 | 6 M/0.1 M LiCl | 126 |
| Eel's electrocyte assembly | GO/rGO ink | REG | Ag/Ag | 0.05 | 0.455 | 0.35 μA | 0.6 mW cm−3 | — | 127 |
| Eel's ion channel | SiO2 core-PANI shell fiber assembled aerogels | REG | Both Ag/AgCl | 3 × 10−4 | 0.127 | 6.93 × 104 | 3070 | 0.5 M/0.01 M NaCl | 128 |
| Eel's ion channel | BMP/GO membrane | REG | Both Ag/AgCl | 3.14 | 0.0146 | 0.764 | 1.51 | 1 M KCl/(0.1 M KCl + 0.9 M NaCl) | 129 |
| Eel's ion channel | DAC-Ti0.87O2 membrane | REG | Both Ag/AgCl | 3 × 10−4 | 0.136 | 6.1 × 104 | 1780 | 0.5 M/0.01 M NaCl | 130 |
| Eel's ion channel | ZIF-65 membrane | REG | Both Ag/AgCl | 7 × 10−2 | — | — | 100 | 0.5 M NaCl/0.005 M Ca(HCO3)2 | 131 |
| Eel's ion channel | Heterogeneous MXene-based membranes | REG | Both Ag/AgCl | 3 × 10−4 | 0.157 | 3.83 × 104 | 860 | 0.5 M/0.01 M NaCl | 132 |
| Eel's ion channel | TFP-TPA COF membrane | REG | Both Ag/AgCl | 3 × 10−4 | 0.0723 | 3.05 × 104 | 1860 | 3000 mM/1 mM KCl | 133 |
| Eel's ion channel | UiO-66-NH2/ZIF-8 film | REG | Both Ag/AgCl | 3 × 10−4 | 0.0902 | 6.55 × 104 | 920 | 0.5 M/0.01 M KCl | 134 |
| Cell membrane | UiO-66-NH2(PSS)/AAO | REG | Both Ag/AgCl | 3 × 10−4 | 0.09 | 19.64 | 2680 | 10 mM/1000 mM KBr | 135 |
| Cell membrane | Monovalent anion/cation exchange membrane | REG | Both Ag/AgCl | 3 × 10−2 | 0.097 | 1.16 × 104 | 830 | 4 M KCl/2 M MgSO4 | 136 |
4.1. Water transport-inspired HEGs
The capture and transport of water in HEGs are critical factors influencing their electricity generation performance. Plants' transpiration process offers abundant and valuable design inspiration for this purpose. Transpiration involves three stages: absorption, transport, and evaporation of water (Fig. 10a). During this process, water sequentially flows from the soil through root hairs, root xylem, stem xylem, leaf xylem, and stomata, eventually entering the atmosphere. The escape of water through the stomata creates negative pressure, driving continuous upward movement of water. To enhance the evaporation rate, plants have evolved the following intelligent structures: (1) root hairs with enlarged surface area: root hairs are elongated projections of root epidermal cells that significantly increase the root's surface area, thereby greatly enhancing the area available for water absorption. (2) Capillary vessels with surface adsorption and vertical channels: xylem vessels in the stem are vertically oriented and have tiny diameters, allowing water to move upward via capillary action; additionally, the cohesion between water molecules and the adhesion between water molecules and the vessel walls enable the formation of a continuous water column, accelerating transport. (3) Abundant and adjustable micro-stomata: according to the small pore diffusion law, the rate of molecular diffusion through small pores is proportional to the pore's circumference rather than its area. This is because, in very small pores, molecules diffuse more rapidly along the edges with minimal resistance. Although the stomata occupy only 0.5–1% of the leaf surface area, their high number and large cumulative circumference facilitate a high rate of water vapor diffusion. Additionally, various external environmental factors such as light, temperature, and humidity can interact with the aforementioned structures, further enhancing transpiration. In summary, this system, through the efficient absorption by root hairs, effective transport through the stem, and rapid evaporation from the leaves—sometimes aided by external environmental conditions—forms a highly coordinated water management mechanism. These insights provide a wealth of inspiration for designing various high-performance HEGs. | ||
| Fig. 10 Plant transpiration-inspired HEGs. (a) Biological structures in the tree transpiration process that can inspire HEG design. (b) Schematic of a delignified wood-based green EEG design with ionic power generation triggered by wastewater. Reproduced with permission.122 Copyright 2024, American Chemical Society. (c) Schematic of wood with precipitated nanofibrillated networks within the lumen and its abundance of functional groups. The final hydrovoltaic energy harvester is shown under operation. Reproduced with permission.118 Copyright 2023, Wiley-VCH. (d) Schematic of bilayer wood membranes with aligned ion nanochannels for spontaneous MEGs. Reproduced with permission.137 Copyright 2022, American Chemical Society. (e) and (f) Photograph of fabricated IEHVG inspired by lotus leaves, schematic illustration of the layered functionalization, and its properties. Reproduced with permission.117 Copyright 2024, Wiley-VCH. (g) Structure illustration of biomimetic IL-GO/GO@PVA aerogels. Reproduced with permission.109 Copyright 2023, Wiley-VCH. (h)–(k) Mac-fabric absorption-evaporation circulation structure consists of a moisture absorption layer [cellulose nonwoven (CNW)/LiCl], a transmission layer [polyacrylonitrile (PAN)], and an evaporation layer polyvinylidene difluoride [P(VDF-TrFE)] (h); the cone model is used for analyzing the Laplace pressure of the multilayer Mac-fabric and flow current induced by water flow in a single nanochannel (i); device properties (j); and large-scale application (k). Reproduced with permission.138 Copyright 2024, AAAS. | ||
Direct modification of tree stems is the most common strategy for preparing bioinspired structured HEGs. For example, grafting or oxidation of the channel walls can increase charge density, and filling the channels can create more interfaces for charge separation. The advantage of this strategy is that it retains and utilizes the highly ordered channel structure in natural wood, which is beneficial for rapid water transport. Zhou et al. used CA-modified beech wood as the active electrode and polyethylene terephthalate (PET) meshes coated with conductive carbon paste as the electrodes to construct a bioinspired EEG device.139 This single device achieved a Voc of 300 mV and an Isc of 10 μA, with a particularly effective improvement in current density compared to previous EEG devices. Additionally, in optimizing the EEG devices, the authors selected four different natural woods with varying pore sizes and densities to study EEG performance, including balsa, basswood, beech, and santalinus, whose pore sizes decrease and densities increase sequentially. The results showed that the beech-based EEG exhibited the best performance because there is a trade-off relationship between microchannel size and device performance. When the channel size is too large (as in balsa), the interface for charge separation is greatly reduced, resulting in lower device performance. Conversely, when the channel size is too small (as in santalinus), the hydrodynamic resistance increases, also leading to low current and voltage.
Using delignified wood as an active material for EEGs can provide more nanoscale ion transport channels and expose more hydrophilic functional groups of cellulose and hemicellulose on the wood surface, thus enhancing charge dissociation, as demonstrated by Zhang et al.122 Subsequently, the bottom of the delignified wood was placed in alkaline wastewater, and water droplets were intermittently added to the top. Using Pt mesh electrodes, an all-wood-based EEG device was constructed (Fig. 10b). This operation method ensures the continuous presence of different levels of functional group dissociation at both ends of the device, creating a persistent ionization gradient in the wood. As a result, the optimized device exhibited a Voc of up to 1.1 V, a very high Isc of 320 μA, and a power density of 6.75 μW cm−2. In contrast, if water droplets were not added to the top, the device only achieved a Voc of 0.2 V, demonstrating that the difference in alkalinity between the top and bottom of the wood leads to uneven dissociation of surface functional groups, and establishing an ion concentration gradient along the surface of the nanoscale channels is the key to achieving high performance. They also explored the direct use of various industrial wastewaters, such as aluminum smelting wastewater, printing wastewater, pulping black liquor, and silicate wastewater, for power generation. The results showed that the EEG's voltage in alkaline wastewater is significantly higher than in acidic wastewater, with the output voltage reaching approximately 1 V in pulping black liquor and stable power output observed over a test period of up to 11 hours. Interestingly, the EEG significantly reduced copper ion concentrations through electrosorption, achieving a certain level of heavy metal removal and highlighting its potential in sustainable energy and wastewater reuse.
Additionally, a cell wall nanoengineering strategy to produce a highly porous wood with a cellulosic network filling the channels through a green one-step treatment using sodium hydroxide was employed by Garemark et al., which was used as the active component in EEG devices.118 This treatment introduced more chemical functional groups and enhanced the cell wall permeability to water, while its specific surface area reached up to 180 m2 g−1, significantly higher than the specific surface area of native wood (1 m2 g−1). The resultant EEG device exhibited a Voc of approximately 140 mV in DI water, over tenfold higher than that of untreated wood (Fig. 10c). Subsequently, the authors immersed the treated wood in hydrochloric acid and then placed it in an alkali (pH 13.4) reservoir. As the pH difference between the wood samples and the reservoir increased, a maximum Voc of 1 V (when the wood pH = 1) and a power density of 1.35 μW cm−2 were achieved.
Furthermore, assembling modified bilayer wood to create concentration gradients of two ions is an effective way to enhance the power generation performance of the device. Cai et al. selected balsa wood for delignification treatment and applied chemical modifications of carboxylation and quaternization to obtain two types of modified wood (Fig. 10d).137 These were placed at the top and bottom, respectively, to create a MEG device that retains the wood's inherent aligned ion nanochannels. The delignified wood produces numerous micro/nano-scale voids, increasing its specific surface area from 2.5 m2 g−1 to 14.8 m2 g−1, which facilitates the penetration of modifiers and free ions into the cell walls. The carboxylated and quaternized woods contain abundant hydrophilic functional groups and exhibit spontaneous water absorption, which can release large amounts of H+ ions and Cl− ions, respectively, upon absorbing moisture from the air. Driven by the concentration gradient, the H+ ions and Cl− ions spontaneously diffuse to the opposite end, generating an electric current. More importantly, this asymmetric structure helps mitigate the negative effects of ion concentration polarization, thereby optimizing ion enrichment and ion consumption effects. As a result, a single MEG device can generate a Voc of 0.57 V under 85% RH. Its good power generation characteristics enable it to be directly used as a power source to drive commercial electronic devices, such as calculators and temperature and humidity sensors. Additionally, it can serve as a self-powered sensing device to detect human and environmental signals. For instance, it can be integrated into a standard medical mask to monitor real-time respiratory parameters such as breathing rate and depth, providing diagnostic insights into abnormal physiological conditions. Moreover, it can detect moisture changes near plant leaves, allowing for early warnings of gas leaks, weather changes, and forest fires.
In addition to directly modifying and assembling natural plant structures, some studies have used non-biomass materials to construct structures resembling plant tissues and organs for HEGs. Zhang et al. prepared a biomimetic partially reduced graphene oxide aerogel with aligned porous structures using an ice-templating method followed by mild thermal reduction for EEGs.116 This mimicked the xylem structure of trees, which has directional water transport capabilities. Compared to control samples with random porous structures, these biomimetic aligned porous aerogels increased the water pumping rate by 1.2 times. Furthermore, the EEG's Isc based on these biomimetic aerogels (12 μA) was approximately four times higher than that of the control samples. This study demonstrates that xylem-inspired structures can indeed enhance water transport rates, thereby improving the performance of HEGs.
Besides mimicking individual plant organs, the simulation of synergistic interaction of two plant organs has also been incorporated into HEGs. Chen et al. developed T-shaped EEGs by emulating the “stems and leaves of lotus”. This design efficiently utilizes sunlight to simultaneously generate water vapor and electricity directly from seawater, a feat typically challenging for conventional EEG devices (Fig. 10e and f).117 The device consists of three layers: the bottom layer is a chitosan/carboxymethyl cellulose hydrogel with a regular channel structure, prepared via an ice-templating method to mimic the rapid water transport function of stems and leaves. The middle layer comprises graphene quantum dot/MXene nanocomposites assembled on the hydrogel surface, serving as the active components for electricity generation and photothermal effects. The top layer features a gradient hydrophobic coating formed by controlling the spray deposition of an octadecyltrichlorosilane solution, which facilitates effective thermal gradient distribution along the water diffusion direction, enhancing power output. Thanks to the synergistic action of these distinct layers, the device overcomes the conflict between the thermodiffusion effect and streaming potential convective of hydrated ions in conventional HEGs, achieving a power generation of 45.6 μW cm−2 and a freshwater production rate of 2.4 kg m−2 h−1. Impressively, integrating multiple EEG units can simultaneously achieve a high voltage output of over 105 V and a freshwater production rate of 2.0 L m−2 h−1. This work demonstrates the potential of biomimetic design in developing high-performance solar-driven freshwater-electricity cogeneration systems, which could be promising for power and freshwater cogeneration on offshore platforms. Similarly, Zhao et al. constructed a bilayer membrane structure that mimics the root and stem structures of plants for MEGs.140 Specifically, the bottom layer is a hydroxypropyl cellulose-konjac glucomannan hygroscopic gel, which, like plant roots, has a strong ability to absorb moisture from the surrounding air, achieving a moisture uptake of 0.5 g g−1 even at 20% RH. The top layer is a polystyrene sulfonic acid/polyvinyl alcohol (PSSA-PVA) aerogel with vertically aligned channels, prepared using directional freeze-drying technology, which mimics plant stems by enabling rapid water transport. As a result, the MEG achieves a Voc up to 1.2 V and a Isc of 8 nA at 20% RH, whereas a disordered porous aerogel-based EEG only reaches 0.6 V and 0.09 nA, respectively. This biomimetic design allows the device to generate high voltages under extremely low-humidity conditions, making it suitable for power generation and wearable applications in desert and other low-humidity environments.
Mimicking the entire transpiration process involving multiple plant organs offers the potential to further enhance the HEG performance. In fact, the development of the first EEG was inspired by plant transpiration. Zhou et al. assembled an EEG device by growing carbon black sheets between two electrodes using a simple ethanol flame method.24 By immersing one end of the device in DI water and exposing the other end to air, they observed that an open-circuit voltage was generated at room temperature, gradually increasing to 1 V. Over an eight-day experiment, the device maintained a stable Voc of around 1 V, although the Isc was only about 150 nA. Recently, Zhang et al. have prepared biomimetic aerogels for high-performance MEGs by comprehensively mimicking the organs used by ferns for transpiration.109 They employed PVA dendritic colloids to simulate “roots” and “stems,” facilitating the collection and transport of water molecules. Simultaneously, GO sheets were used to mimic fern “leaves”, generating electricity through direct interaction with water (Fig. 10g). Based on this biomimetic structure, they incorporated nanofillers of GO and ionic liquid-modified GO with different charges onto the PVA dendritic colloids, creating an ultra-high ion density gradient within the aerogel. This gradient serves as an additional driving force for the directional diffusion of charged ions. The resulting MEG exhibited exceptional performance, including an ultra-high Voc of 1.9 V, an Isc of 82.5 μA, and a leading power density of 22.55 μW cm−2. This MEG also functions as electronic skin, capable of responding to changes in humidity, temperature, wind, and strain. It effectively monitors respiratory patterns, limb movements, and walking, while sensor arrays accurately detect the size and location of applied pressure, which highlights its multifunctional sensing potential across diverse scenarios.
By mimicking the transpiration mechanism of plants, Hu et al. also designed a sustainable moisture absorption-evaporation cycling fabric generator (Fig. 10h).138 This device features a three-layer structure with excellent unidirectional moisture conductivity. The bottom layer consists of LiCl-impregnated cellulose nonwoven (CNW) fabric, the middle layer is an electrospun polyacrylonitrile (PAN) membrane, and the top layer is poly(vinylidene fluoride-trifluoroethylene) [P(VDF-TrFE)]. These layers respectively mimic the water absorption by roots, water transport through stems, and water evaporation by leaves in plants. The bioinspired multilayer fabric is characterized by a gradually increasing hydrophobicity, decreasing pore size, and increasing surface electronegativity from the bottom to the top layer (Fig. 10i). This structure promotes the continuous high-speed movement of liquids and effective charge separation, enhancing power generation performance. The efficient cycle of water absorption at the bottom layer and water discharge at the top layer addresses the challenge of ion concentration gradient disappearance after continuous moisture absorption in MEG devices and the dependency on liquid water in EEG devices. It ensures continuous water flow through the negatively charged micro/nanochannels in the fabric for sustained power generation. As a result, a generator unit made from this multilayer fabric and equipped with carbon and aluminum electrodes (1 cm × 2 cm × 0.025 cm) can provide a Voc of 0.85 V for over one month under conditions of 40% RH and 20 °C (Fig. 10j). The peak power density is 0.144 W m−2 based on its apparent area, and the volume power density is 5.77 × 102 W m−3, demonstrating significant improvements in performance and sustainability compared to previous HEGs. Furthermore, the lightweight and flexible nature of the fabric makes it easy to integrate into various applications. Approximately 2000 fabric units (total area 0.384 m2) integrated into a camping tent can achieve a peak power output of 55.3 mW, directly powering commercial portable electronic devices such as mobile phones in outdoor environments (Fig. 10k), showcasing great application potential.
4.2. Ion diffusion-inspired HEGs
Ion diffusion plays a pivotal role in the functionality and performance of HEGs. Drawing inspiration from biological systems, researchers have devised innovative HEGs that harness the principles of ion transport observed in living organisms. This section will explore two primary categories of ion diffusion-inspired HEGs: bilipid-inspired HEGs and ion pump/ion channel-inspired HEGs.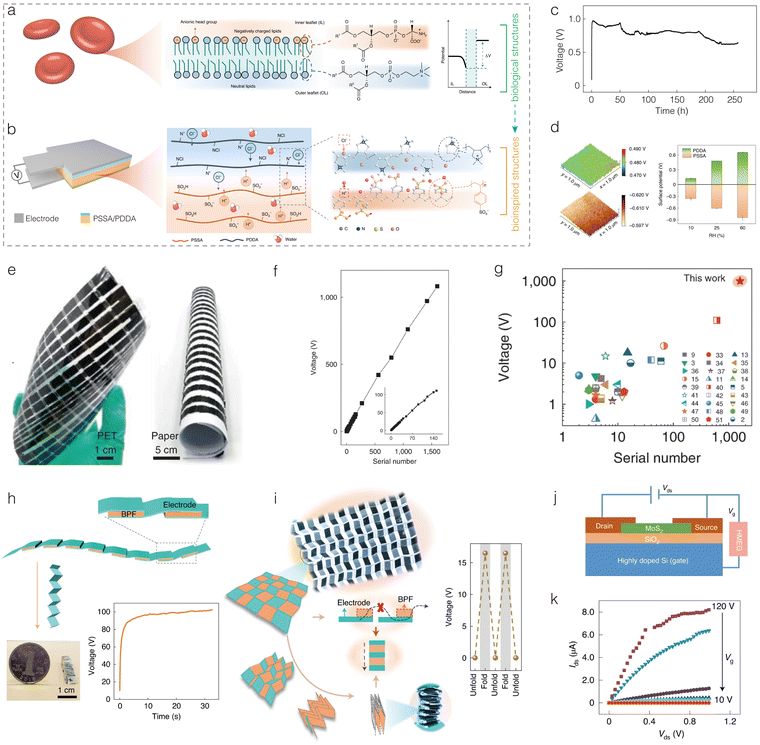 | ||
| Fig. 11 Asymmetrical bilipid-inspired HEGs. (a, b) Schematic of an asymmetrical lipid bilayer (a) and the corresponding moisture-enabled electric generation in a bilayer of polyelectrolyte film (BPF) (b) inspired by it. (c) Long-term (258 h) voltage output of a bilayer MEG device under atmospheric conditions of ∼15–30% RH and 25 ± 5 °C. (d) Relative surface potentials of the top PDDA layer and the bottom PSSA layer at 25% RH and 25 °C (left), and varying RH (right). (e) Photographs of flexible integrated devices on different substrates including polyethylene terephthalate (PET) and paper. (f) Plot of voltage output with different serial numbers of bilayer MEG units. Inset: Enlargement of serial numbers ranging from 1 to 140. (g) Systematic performance comparison of reported integrated devices based on a variety of materials including graphene-related materials, homogeneous polyelectrolytes and other carbon materials. (h) A small, compact MEG device (0.5 × 0.5 × 9.2 cm3) fabricated by origami assembly of 100 units. (i) Photographs and schematic of Miura-ori folding and voltage output of integrated MEGs under unfolding and folding states, indicating the responsive performance for its electricity generation. (j) Schematic of a self-powered FET. (k) Transfer curves of the MoS2 transistor powered by integrated MEGs at varying gate voltage. Reproduced with permission.114 Copyright 2021, Springer Nature. | ||
Inspired by the asymmetric structure of the lipid bilayer, Wang et al. developed a polyelectrolyte film biomimetic bilayer as the active component of MEGs achieving high voltage outputs.114 This biomimetic bilayer consists of two tightly bonded layers of polydiallyl dimethyl ammonium chloride (PDDA) film and a PSSA/PVA hybrid film (Fig. 11b). The primary role of PVA is to enhance the flexibility of the PSSA film. The bilayer can spontaneously absorb water molecules from the air, continuously dissociating Cl− ions from the PDDA layer and H+ ions from the PSSA layer. This naturally creates a concentration gradient of these ions between the two layers. Driven by this gradient, the negatively charged Cl− ions from the PDDA layer and the positively charged H+ ions from the PSSA layer diffuse towards each other, generating voltage and current. Thanks to the continuous absorption of water molecules and the design of dual-charge-carrier materials, the MEGs can sustainably output high voltage. Studies have shown that this MEG device can produce a Voc of up to 0.95 V and operate for over 250 hours under 25% RH and 25 °C, far exceeding the output voltage of other reported MEGs under similar low humidity conditions (Fig. 11c and d). As the humidity increases to 85% RH, the voltage can further increase to 1.38 V. However, it should be noted that the current density remains relatively low. This bilayer structure is easily scalable and can be assembled into flexible devices on various substrates, including PET and paper, using a sequentially aligned stacking strategy. The voltage output of the integrated device increases linearly with the number of series-connected units. Impressively, 1600 series-connected units generated a voltage of up to 1000 V under conditions of 25% RH and 25 °C, which is the highest voltage reported for integrated devices at that time (Fig. 11e–g). Moreover, the MEG unit can be miniaturized and made portable using origami techniques. By employing the Miura-ori folding method, a responsive power source was constructed that operates in a folded state and breaks when unfolded (Fig. 11h and i). As a self-powered source, this bilayer MEG can control the switching characteristics of MoS2 channels in field-effect transistors (FETs), demonstrating its potential for use in self-powered devices (Fig. 11j and k).
In the mitochondria, protons are pumped across the inner membrane using energy derived from the electron transport chain, creating a pH gradient and electrochemical potential. These protons then flow back into the inner mitochondrial membrane through adenosine triphosphate (ATP) synthase, driven by the concentration gradient. ATP synthase utilizes this potential energy to convert adenosine diphosphate (ADP) into ATP, achieving highly efficient energy conversion. The tightly coupled electron transfer and the resultant proton movement driven by the proton concentration gradient are the primary driving forces for the entire energy conversion process. Inspired by this mechanism, Xu et al. developed the first polymer-based MEGs using a freestanding PSSA polyelectrolyte membrane, directly cast from a commercial PSSA solution (18 wt% aqueous solution).30 In the electricity generation process, the side of the PSSA membrane exposed to moisture first releases mobile protons from the sulfonic acid groups. As water molecules gradually permeate and wet the membrane, the released protons migrate to the other side of the membrane, driven by the concentration gradient. This process closely mimics the proton movement along the concentration gradient back into the mitochondrial inner membrane. The results demonstrate that this bioinspired MEG device (only 1 × 1 cm2) exhibits excellent power generation performance, producing a Voc of up to ∼0.8 V and a high short-circuit current density of ∼0.1 mA cm−2.
In the field of REGs, various materials such as polyaniline (PANI),128 MXenes,132,141 and MOFs134 have been utilized to create channels with specific functionalities, which mimic the ion pump's selective and efficient ion transport properties to enhance the power generation performance of the devices. Inspired by the working principles of electric eel electrocytes, Zhang et al. developed nanofluidic cable fibers assembled into a 3D network aerogel for REGs (Fig. 12a and b).128 The basic assembly unit consists of a cable structure with a SiO2 core and a PANI shell, featuring mass transfer gaps between the core and shell (Fig. 12c). Since the PANI shell is positively charged, it repels cations and selectively allows Cl− anions to pass through. The Cl− anions traverse the PANI shell and achieve rapid nanofluidic transport within the overlapped EDL. Concurrently, the negatively charged SiO2 core repels these anions and facilitates their diffusion through the gap structures, thereby increasing the likelihood of anion proximity to the nanopore channels in the PANI tubular layer. Consequently, the fabricated aerogel material exhibits an integrated coupling effect of boosted ion propulsion and surface-charge-dominated selective ion transport. This effect can be further validated through MD simulations. Thanks to the bioinspired design, the REGs demonstrate a power density of up to 30.7 W m−2 under a 50-fold salinity gradient (Fig. 12d). Notably, the aerogel membranes can be easily scaled up. When multiple REG units were connected in series, the assembled 12-unit system produced a Voc of 1.52 V and an Isc of 20.79 μA, capable of powering a model ship for maritime navigation. This innovation paves the way for the next generation of blue energy transportation.
 | ||
| Fig. 12 Ion channel-inspired REGs. (a) Electricity generation mechanism of electric eels. Reproduced with permission.128 Copyright 2023, Wiley-VCH. (b) Concentration-gradient-driven energy harvesting in a fibrous aerogel. (c) and (d) Schematic of ions passing through the cable fiber and its side view. (e)–(h) Schematic of the structure and composition of an MXene-based ionic diode membrane and its REG properties. Reproduced with permission.132 Copyright 2022, Wiley-VCH. (i) I–V curves of the MOF-on-MOF membrane under two configurations of forward and reverse diffusion and drift directions. Reproduced with permission.134 Copyright 2023, American Chemical Society. (j) Schematic of molecular structure of a MOF-on-MOF membrane. (k) Simulated concentration map of the membrane internal space when ions diffuse from UiO-66-NH2(PSS) to the ZIF-8 section and in the opposite direction. (l) Basic device configuration for implementing the Donnan effect. Reproduced with permission.136 Copyright 2023, Wiley-VCH. (m) Structure of ZIF-65. Reproduced with permission.131 Copyright 2023, American Chemical Society. (n) Schematic showing the interaction process of BMP and K+ in the gap of GO nanosheets. (o) Mechanism of ion selectivity in biomimetic nanochannels and binding energy between the ion and the membrane at different stages. Reproduced with permission.129 Copyright 2024, Wiley-VCH. | ||
By mimicking the asymmetric structure of ion channels in electric eels, Ding et al. developed a self-supporting, flexible, bioinspired Ti3C2Tx MXene-based ionic diode membrane for REGs (Fig. 12e).132 This membrane was fabricated by sequentially vacuum-filtering negatively charged MXene (NCM) and positively charged MXene (PCM) solutions. The resulting asymmetric ion channel structure achieved rectified ion transport, with a rectification ratio as high as 15.4. Benefiting from this extraordinary ion rectification effect, the REGs could generate an ultrahigh power density of 8.6 W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m−2 by mixing synthetic seawater and river water. Under a 500-fold salinity gradient, the power density could further increase to 17.8 W m−2 (Fig. 12f–h). Tonnah et al. emulated the highly selective ion transport of ion channels by using a MOF-on-MOF heterogeneous membrane structure to enhance REG performance (Fig. 12i–k).134 Specifically, they deposited an imidazolate framework-8 (ZIF-8) layer on a UiO-66-NH2 membrane intercalated with PSS. The ZIF-8 layer, containing numerous angstrom-sized cavities, facilitated ion selectivity through size exclusion, while the PSS-intercalated UiO-66-NH2 film ensured cation permeability. Due to the synergistic effect of these two components, the REGs could achieve a power density of 40.01 W
m−2 by mixing synthetic seawater and river water. Under a 500-fold salinity gradient, the power density could further increase to 17.8 W m−2 (Fig. 12f–h). Tonnah et al. emulated the highly selective ion transport of ion channels by using a MOF-on-MOF heterogeneous membrane structure to enhance REG performance (Fig. 12i–k).134 Specifically, they deposited an imidazolate framework-8 (ZIF-8) layer on a UiO-66-NH2 membrane intercalated with PSS. The ZIF-8 layer, containing numerous angstrom-sized cavities, facilitated ion selectivity through size exclusion, while the PSS-intercalated UiO-66-NH2 film ensured cation permeability. Due to the synergistic effect of these two components, the REGs could achieve a power density of 40.01 W![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m−2 and a current density of 665 A
m−2 and a current density of 665 A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m−2 under a 500-fold salinity gradient.
m−2 under a 500-fold salinity gradient.
The aforementioned bioinspired REGs utilizing cation-/anion-selective membranes and solutions with different salinity have indeed effectively improved the power generation performance of REGs. However, they still fall short of replicating the actual biological power generation systems, such as those in electric eels. For example, most current REGs typically employ electrolyte solutions of different concentrations to construct the osmotic system, whereas electric eels actually use a system with similar electrolyte solutions, an iso-osmotic physiological fluid, meaning the total ion concentration of intracellular and extracellular fluids is similar. This iso-osmotic generator can effectively address the limitation of low carrier concentration in low-concentration solutions, potentially further enhancing the power generation performance of REGs. Kim et al. separated two ion-rich electrolytes containing ions of different valences and the same polarity using a monovalent cation exchange membrane or monovalent anion exchange membrane, generating a Donnan potential primarily driven by the monovalent ion gradient for high-performance osmotic energy harvesting (Fig. 12l).136 Unlike traditional ion gradient systems, this system produces a high ion current because the electrolytes on both sides of the membrane are ion-rich. Tests showed that this REG could generate a maximum power density of 3.8 W m−2 and a high current density of up to 100 A m−2, representing an 8.5-fold and 10-fold increase in power density and current density, respectively, compared to REG devices using a 100-fold KCl gradient. This device can also activate growing muscle cells through serial power increases, similar to electric eels, demonstrating the potential for ion-based artificial nervous systems. Using a similar strategy, Li et al. constructed artificial Na+ channels with zeolitic imidazolate framework-65 crystals, which selectively transport Na+ while almost completely blocking Ca2+ (Fig. 12m).131 By preventing major cations in river water from participating in ion diffusion, the effective concentration gradient between seawater and river water is significantly increased. Results showed that replacing the commonly used Na+-containing water with Ca2+-containing water to simulate river water with an ion charge concentration equivalent to that of Na+-containing seawater in the REG system increased the Voc generated by the artificial Na+ channels by approximately six times, and the power density increased by approximately 37 times, reaching 2221 W m−2. These findings open new pathways for developing high-performance REGs and may advance other applications based on bioinspired ion channels, such as neuromorphic information processing.
Although the aforementioned bioinspired iso-osmotic REGs show enhanced performance by selectively filtering the same-charge ions of different valencies through channels, they have not yet achieved true selectivity for different monovalent ions (such as K+ and Na+) similar to the ion channels of electric eels. Liu et al. addressed this challenge by confining the assembly of potassium ligands in the gaps between GO nanosheets, achieving highly efficient iso-osmotic REGs (Fig. 12n).129 1,1,1-Tris{[(2′-benzylaminoformyl)phenoxy]methyl}ethane (BMP) was chosen as the potassium-selective ligand. By combining it with GO through a two-step filtration process, they constructed a hybrid graphene oxide membrane with a K+ selectivity of up to 17.8 over Na+. MD simulations indicate that this selectivity is closely related to ion dehydration and transport facilitated by ion–membrane interactions (Fig. 12o). The assembled REGs exhibited a power density of 15.1 mW m−2 in iso-osmotic solutions (cK+:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (0.1cK+ + cNa+) = 10
(0.1cK+ + cNa+) = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10), which is 5.9 times higher than that in traditional salinity gradient solutions (cK+:
10), which is 5.9 times higher than that in traditional salinity gradient solutions (cK+:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1cK+ = 10
0.1cK+ = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The K+-selective iso-osmotic REGs present an attractive strategy for designing novel energy conversion systems. Moreover, by incorporating other types of ion-selective materials, it is possible to design and integrate independent, ion species-resolved multiple ionic iso-osmotic energy harvesting systems on demand.
1). The K+-selective iso-osmotic REGs present an attractive strategy for designing novel energy conversion systems. Moreover, by incorporating other types of ion-selective materials, it is possible to design and integrate independent, ion species-resolved multiple ionic iso-osmotic energy harvesting systems on demand.
4.3. Electrocyte assembly-inspired HEGs
In the aforementioned sections, we focused on how various bio-inspired strategies can enhance the performance of individual devices. It is evident that the performance of single devices is often insufficient for practical applications, necessitating their assembly in series and parallel configurations. In previous reports, the assembly of such devices is often bulky and rigid, significantly reducing the energy density and hindering their application in multifunctional devices, such as wearable and implantable technologies. In this context, the natural evolution of the electric eel's electrocyte arrangement offers valuable insights into the compact, portable, and flexible assembly of HEGs, paving the way for their application in wearable and implantable devices.26,125,142,143The electric eel possesses specialized organs known as electric organs, which are composed of thousands of elongated electrocytes. These electrocytes are stacked in parallel arrangements, and during discharge, the ionic gradients they generate are connected in series, producing voltages of up to 600 V. Additionally, multiple stacks of these electrocytes are connected in parallel within the eel's body, enabling the generation of peak currents up to 1 A. The electrocytes' power generation mechanism involves distinct anterior and posterior membranes (Fig. 13a).26 The posterior membrane is densely packed with nerve-controlled voltage-gated sodium channels, while the anterior membrane, which is not nerve-controlled, features papillae to increase surface area. In the resting state, potassium channels in both membranes are open, allowing potassium ions to flow across the membranes, generating equal and opposite transmembrane potentials of −85 mV, resulting in a net transmembrane potential of zero. During discharge, the sodium channels in the posterior membrane open, while the potassium channels close in response to nerve signals. This causes sodium ions to flow into the cells, generating an action potential of +65 mV. Consequently, the total transcellular potential reaches 150 mV (Fig. 13b).
 | ||
Fig. 13 Electric eel's electrocyte assembly-inspired HEGs. (a) Electrocytes assembly within electric organs of Electrophorus electricus. (b) and (c) Voltage generation mechanism in the artificial electric organ, and artificial electric organ in its printed implementation. (d) Photographs of large complementary arrays of printed hydrogel lenses combining to form a continuous series of 2449 gels with serpentine geometry. (e) Schematic and photographs of an artificial electric organ with Miura-ori folding design. Reproduced with permission.26 Copyright 2017, Springer Nature. (f)–(h) Fabrication process of a power unit through depositing hydrogel droplets. (i) and (j) Template-assisted preparation of a large-scale patterned power source network. (k) Bright-field image of a mould with multiple droplet hexagons with a volume of about 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nL for each droplet. Scale bar, 600 nL for each droplet. Scale bar, 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μm. (l) Zoom-in of a single hexagonal layer. Scale bar, 200 μm. (l) Zoom-in of a single hexagonal layer. Scale bar, 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μm. (m) Stacks of 7 and 28 power units. Scale bar, 600 μm. (n) and (o) A chain of 20 power units formed by droplet self-assembly through four-step sequential deposition into a spiral mould, scale bar, 1.2 μm. (m) Stacks of 7 and 28 power units. Scale bar, 600 μm. (n) and (o) A chain of 20 power units formed by droplet self-assembly through four-step sequential deposition into a spiral mould, scale bar, 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mm. (p) and (q) Schematic of neuronal modulation induced by the ionic droplet device. Reproduced with permission.125 Copyright 2017, Springer Nature. mm. (p) and (q) Schematic of neuronal modulation induced by the ionic droplet device. Reproduced with permission.125 Copyright 2017, Springer Nature. | ||
Inspired by the assembly of electric organ cells in the electric eel, Schroeder et al. developed a soft power source from stacked hydrogels.26 They used four hydrogel components to mimic the four main parts of the electric cells: specifically, a red hydrogel, composed of neutral monomers polymerized with a sodium chloride solution, to mimic the extracellular salt chamber; a blue hydrogel, also composed of neutral monomers, with a dilute sodium chloride solution to simulate the intracellular salt chamber; a green hydrogel, made from negatively charged monomers with cation selectivity, to mimic the cell membrane; and a yellow hydrogel, composed of positively charged monomers with anion selectivity, to replicate the cell's front membrane. These four basic hydrogel units, arranged in the sequence red–green–blue–yellow–red, form an ionic conduction pathway (Fig. 13c). Based on the principle of reverse electrodialysis, this configuration can produce a Voc of 130–185 mV, similar to the potential generated by a single electrocyte. Building on this, they used surface printing technology to print two complementary gel pattern arrays: one pattern involved printing the precursor solution of the ion-selective membrane on a polyester substrate, and the complementary pattern was printed with the precursor solution of the salt chamber on a second substrate (Fig. 13d). When these patterned gels were cured and overlaid, the hydrogel array immediately formed a serpentine ionic conduction pathway, with a combined potential of up to 110 V. The physical separation of the two hydrogel pattern arrays achieved ion gradient dominance without the need for energy consumption, unlike the electric cells. However, compared to the electric organ of the electric eel, the larger thickness and smaller contact area of these hydrogel units resulted in a significant internal resistance of the assembled REG device (with each hydrogel power unit having a resistance as high as 115 kΩ) and limited power output (with 2449 hydrogel power units generating less than 50 μW). To improve performance, they designed flat hydrogel films with patterns on a substrate, using the folding strategy initially developed for deploying solar panels in space. This Miura-ori folding technique stacks a series of thin films with a large contact area in a single synchronized and self-registered motion (Fig. 13e). This folding technique reduced the resistance by 40 times compared to the previous serpentine arrangement, significantly enhancing the maximum power density to 27 mW m−2. However, this remains two to three orders of magnitude lower than the performance of the electric eel's electrocytes.
By reducing the volume of individual droplet units, the overall size of the assembled device can be further minimized, significantly enhancing the power density. Zhang et al. significantly reduced the volume of unit generators by depositing lipid-supported networks of nanoliter hydrogel droplets, achieving a 105-fold reduction compared to the above work.125 This resulted in an astonishing 680-fold increase in power density, reaching 1300 W m−3. The authors simulated the overall layout and mechanism of eel electric organs by sequentially combining five nanoliter aqueous pre-gel (agarose) droplets. In each unit, the sequence of droplets was: high salt (e.g., CaCl2, KCl, or NaCl), cation-selective, low salt, anion-selective, and another high salt droplet (Fig. 13f). These droplets were deposited in lipid-containing oil using an electronic microinjector. Initially surrounded by a monolayer of lipids, the droplets formed droplet interface bilayers (DIB) upon contact within seconds, creating a stable unsupported structure (Fig. 13g). To activate the power source, the assembled droplets were moved into lipid-free oil to remove the lipids and disassemble the DIB (Fig. 13h). The droplets were then gelled at 4 °C, forming a continuous hydrogel structure for power generation. To construct larger droplet networks for increased power output, the authors employed a template method, depositing multiple droplets into molds to produce power units with pre-designed patterns. In each mold, seven droplets (4 nl each) spontaneously assembled into a hexagonal “flower-like” pattern within seconds (Fig. 13i and j). Five such self-assembled hexagonal arrays were then stacked into deeper cylindrical molds to create a larger power network (Fig. 13k–m). Additionally, the droplets could be arranged in series within a spiral mold, forming a chain structure with a series of 20 power units, which was sufficient to light up a red LED (Fig. 13n and o). This miniaturized power-generating device can be designed to connect with neurons, creating conductive pathways and modulating neuronal network activity (Fig. 13p and q). This showcases their potential as implantable power sources with significant application prospects.
4.4. Other biological structure-inspired HEGs
The aforementioned biological structures have been widely studied as sources of inspiration for HEGs, leading to significant advancements. Additionally, other bioinspired structures have also contributed to the design of HEGs from different perspectives, enhancing the overall performance of these devices. For example, the light-capturing structures of moth eyes and butterfly wings have inspired the development of HEGs that efficiently capture and utilize light; the brick-and-mortar structure found in nacre has inspired the construction of highly stable HEGs.Inspired by the high light absorption efficiency of bean hawk moth eyes, Sun and colleagues introduced similar light-capturing structures into EEGs.111 These bioinspired structures significantly improved the water evaporation rate at the middle and top layers due to increased photothermal conversion efficiency, thereby boosting power generation performance of EEGs. Moth eyes typically consist of a hexagonal array of cone-like pillars, which almost entirely eliminate reflection of sunlight, thus exhibiting excellent light-capturing capabilities (Fig. 14a). The authors employed a unique 3D templating method to fabricate a periodic concave–convex structure that mimics the structure and function of moth eyes, using it as the middle layer of the EEG device (Fig. 14b). Further tests revealed that this bioinspired middle layer achieved an outstanding light absorption efficiency of 96.7% and an impressive water evaporation rate of 2.78 kg m−2 h−1 under 1 sun illumination. The EEG device was then constructed through layered self-assembly. Its bottom layer consisted of a porous ionic hydrogel for efficient water supply, while the bioinspired middle layer incorporated MWCNTs, MXenes, ionic hydrogels, and a CsPbBr3-type perovskite to enhance light absorption and power generation. The top layer, composed of a nanofiber layer of MWCNTs covering the bioinspired middle layer, facilitated the primary photothermal conversion and power generation processes (Fig. 14c). Under simulated sunlight, this bioinspired EEG device demonstrated a Voc of 432 mV, a short-circuit current density of up to 64.2 μA cm−2, and an impressive continuous power density of 11.8 μW cm−2. These voltage and current values were significantly enhanced, showing a threefold increase compared to devices without the light-capturing structure, thereby confirming the effectiveness of the bioinspired light-capturing structure (Fig. 14d).
 | ||
| Fig. 14 Other representative HEGs with bioinspired structures. (a) Structure of moth eyes. (b) Schematic of water flowing and evaporation path in the bioinspired EEG. (c) and (d) Schematic diagram of the multi-layer biomimetic EEG (c), and its power density comparison with other solar-driven EEGs (d). Reproduced with permission.111 Copyright 2022, Springer Nature. (e) The bioinspired MEG with interfacial microgrooves for moisture-harvesting and light-collection. (f) The top view and cross-sectional illustration of MEG devices with (i) a planar top electrode and (ii) a top bioinspired electrode. (g) Detailed structural parameters for 3D finite-difference time-domain simulation and the absorption field density distribution profile of MEGs with or without bioinspired microgrooves at 600 nm. Reproduced with permission.115 Copyright 2023, Royal Society of Chemistry. (h) Capillary vessel-inspired PIL membrane for a high-performance REG. (i) Schematic of the nanochannel formation mechanism in the PIL membrane using two polyelectrolytes, and the change in the contact angle. Reproduced with permission.124 Copyright 2022, Elsevier. | ||
Introducing coupled bioinspired structures into HEGs can endow devices with multiple enhanced functionalities, thereby improving their power generation performance. Bai et al. designed high-performance MEGs by mimicking the microgroove structures of rice leaves and butterfly wings, which simultaneously enhance moisture-harvesting and light-trapping capabilities. The surface of rice leaves features abundant aligned grooves, resembling microscopic capillaries, which attract and guide liquids through capillary action. The aligned structure of these grooves allows water molecules to efficiently move over long distances, covering a larger surface area. Similarly, the surface of butterfly wings has orderly arranged periodic microgrooves that create micro-vortices as liquid passes through, increasing the interaction between the liquid and the surface (Fig. 14e). Additionally, some butterfly wings possess anti-reflective properties that manipulate incident light propagation, naturally extending the optical path, reducing reflection loss, and enhancing light absorption.
By integrating these bioinspired designs, the authors proposed a self-induced imprinting strategy to construct bioinspired interface structures with microgrooves, combining water molecule coalescence, migration, and light capturing functionalities. They used a perforated Au-coated commercial digital video disc as a template to imprint microgrooves on the surface of the power-generating layer PSSA and kuromanin (chloride) (PSSA-Kc). This was then assembled with a bottom Au electrode to form an MEG device (Fig. 14f). During power generation, PSSA spontaneously dissociates upon moisture absorption, releasing mobile hydrogen ions that generate a current as they migrate from the top to the bottom. Additionally, Kc can harvest photons to generate nonequilibrium carriers upon illumination. When incident light accompanies moisture, the electric field induced by moisture facilitates the transfer of photogenerated electrons, supplementing negative charge. Simultaneously, the migration and redistribution of H+ ions in PSSA during moisture permeability affect the energy barrier of electron–hole recombination in Kc, thus efficiently improving electron–hole separation for carrier supplementation (Fig. 14g). Thanks to this bioinspired design, the MEG placed in a sealed container with controlled RH and light intensity showed impressive power generation performance. Under optimized conditions, it achieved a Voc of up to 0.8 V, a substantial Isc density of 1.6 mA cm−2, and a power density of 88 μW cm−2, maintaining stable operation for 180 hours. In contrast, an MEG device with a planar top electrode only achieved a short-circuit current density of 0.3 mA cm−2. The authors also demonstrated that this self-induced imprinting strategy could be widely applied to bioinspired MEGs based on other power-generating layers (such as carbon materials, chitosan, and gelatin), all showing significant performance enhancements. This underscores the versatility and effectiveness of the coupled bioinspired design for HEGs.
The hierarchical blood vessel structure in living organisms facilitates rapid and efficient mass transport and exchange, providing inspiration for designing high-performance REGs. This efficiency can be explained by Murray's Law, which optimizes the dimensions of vessels or other transport channels to minimize energy consumption and maximize transport efficiency.144 For instance, in the arterial system of mammals, arteries branch into smaller arterioles and eventually into tiny capillaries, minimizing the energy required for blood flow while optimizing the delivery of oxygen and nutrients (Fig. 14h). Murray's Law is not only applicable to the vascular systems of mammals but also to the vascular systems of plants, the respiratory system, and the nervous system of mammals. Inspired by this principle, Hu et al. developed bioinspired poly(ionic liquid) (PIL) membranes by employing ion cross-linking between two polyelectrolytes with different charges (Fig. 14i).124 This approach allowed for the reconstruction of the membrane structure and the formation of nanochannels, resulting in a membrane that mimics the hierarchical blood vessel structure. Additionally, due to the electrostatic cross-linking occurring during preparation, the fabricated membrane exhibited high chemical stability, functioning effectively in acidic environments and various solvents. The positive functional groups on the surface of the nanochannels provided a high-speed pathway for anion transport, while the interconnected nanoporous network laid the foundation for efficient osmotic energy conversion under various operating conditions. When utilized as an REG and tested under laboratory conditions using artificial seawater and river water, the membrane achieved a maximum power density of 4.33 W m−2. This demonstrates the potential of bioinspired designs for enhancing the performance of energy conversion devices.
Inspired by the “brick-and-mortar” structure that enhances the mechanical properties of nacre, Chen et al. developed a self-supporting graphene oxide/aramid nanofiber (GO/ANF) membrane for high-stability REGs.145 They used hard GO to mimic the “bricks” and soft ANFs to mimic the “mortar,” achieving a nacre-like structural stability. In this biomimetic design, one-dimensional ANFs serve as cross-linking agents to connect two-dimensional GO nanosheets. The oxygen-containing groups on the GO nanosheets and the abundant amide groups on the ANFs form hydrogen bonds, preventing membrane expansion and stabilizing interlayer spacing during the REG process. This design ensures excellent structural stability for over a month in both acidic and alkaline salt solutions. More importantly, the biomimetic structure provides highly stable interlayer spacing, ensuring precise ion-selective transport. As a result, it generates a stable high net current and increases the osmotic power density of artificial river water and seawater to 5.06 W m−2.
5. Living bioinspired devices for HEGs
Electrons are omnipresent within biological systems, with each cell essentially acting as a micro-powerhouse. Critical life processes, including photosynthesis, respiration, and neural transmission, all involve the generation and transfer of electrons. Living hydrovoltaic technology involves using living organisms as the primary medium for electricity generation. This technique leverages the direct interactions between living organisms and various forms of water to convert the energy contained within water into electrical energy, utilizing electrochemical methods, the physiological processes of the living organisms, and external environmental regulation. While a variety of plants, animals, and microorganisms have the potential to be utilized for living HEGs, research on living animals and microorganisms is relatively limited compared to plants.146,147 This is due to the higher requirements for their living environments, greater complexity in operation and maintenance, and potential ethical and safety issues. Therefore, this section will focus on the use of living plants for HEGs.Plants possess characteristics such as long lifespan, wide distribution, diverse species, and strong environmental adaptability. Utilizing plants for large-scale power generation introduces a novel form of clean and sustainable energy production. To date, various techniques have been developed to harness electricity from plants. For example, piezoelectric and triboelectric devices convert the movement and vibrations of plant organs into electrical energy;148,149 plant microbial fuel cells can transform the chemical energy of organic substances secreted by plant roots into electricity through microbial metabolism;150 by inserting two different reactive metal electrodes into plants and using plant sap as the electrolyte solution, plant-based primary batteries can convert the chemical energy of the metal electrodes into electrical energy.151 However, these power generation technologies do not fall under the category of hydrovoltaic electricity generation, as they do not directly relate to the electricity generated by the plants themselves. So, does electrical generation and conduction occur within living plants? The study of living plants generating electrical currents dates back to 1873 when Burdon-Sanderson observed action potentials (AP) in the living leaf of Dionaea muscipula upon mechanical stimulation (Fig. 15a).152,153 Later, in 1935, Houwinck discovered that plants produce variation potentials (VP) in response to damaging external stimuli.154,155 These signals in plants are essentially physiological responses to external stimuli and are short-term electrical signals, making them difficult to collect and utilize effectively. In 1920s, researchers observed a lasting potential difference generated between electrodes inserted into plants.156,157 This electrical signal is directly related to the flow of water within the plant and the structure of the living plant itself, sparking extensive research into plant-based power generation (Fig. 15b–d).158,159 In the following sections, a detailed review of this stable and enduring phenomenon of hydrovoltaic electricity generation in living plants will be presented.
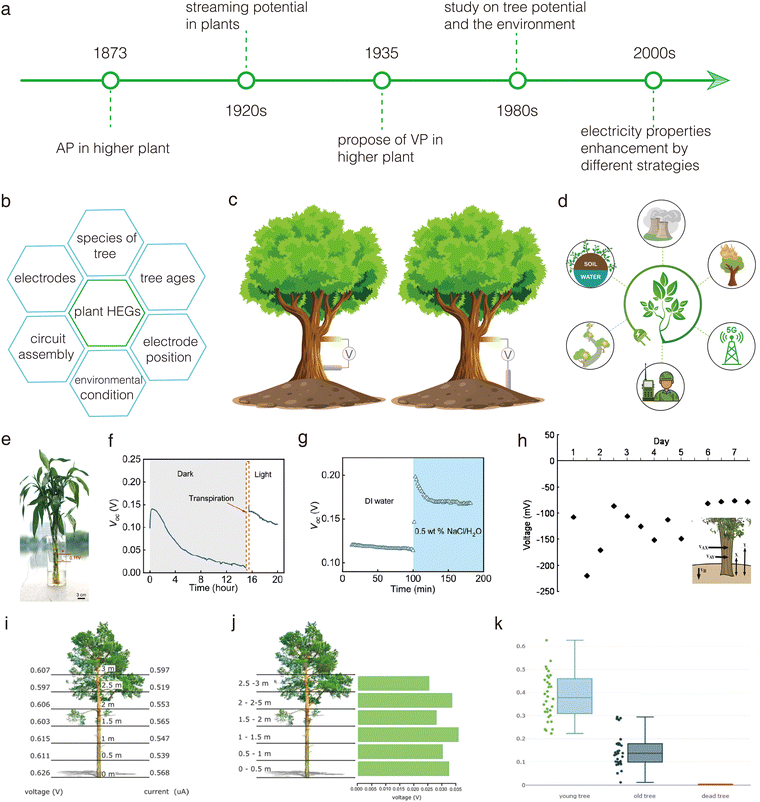 | ||
| Fig. 15 Living plant-based HEGs. (a) Evolution timeline of living plant-based HEGs. (b) Factors influencing the performance of living plant-based HEGs. (c) Two typical testing methods for electrical properties of living plants. (d) Potential applications of living plant-based HEGs. (e)–(g) The impact of light and saline water on power generation in living lucky bamboo. Reproduced with permission.138 Copyright 2024, AAAS. (h) Long-term output voltage of a living bigleaf maple tree with a load of 100 kΩ. Reproduced with permission.158 Copyright 2010, IEEE. (i)–(k) Influence of measurement type, electrode placement, and tree age on electrical properties of a living pine tree. Reproduced with permission.160 Copyright 2020, Taylor & Francis. | ||
As early as 1963, Fensom et al. conducted a 15-month measurement of the electrical potential in maple tree trunks, discovering that the output potential exhibited clear diurnal and annual rhythms.20 This rhythmic variation in potential was believed to be associated with streaming potential caused by sap flow in the stems. Subsequently, the electrical potentials of various living plants, such as chestnut trees,161 willow stems,162 populus nigra,159 fruit trees,163–165 and olive trees,166 were tested. Studies also examined the effects of different environmental factors, such as soil moisture, light, humidity, and temperature, on plant sap flow and electrical potential. These investigations revealed the widespread phenomenon of rhythmic changes in plant electrical potential, laying a solid foundation for the development and potential applications of electricity generation using living plants (Fig. 15d).
To enhance the output performance of living plant-based HEGs, extensive research has been conducted on various influencing factors, including tree species and ages, electrode components and positions, and environmental conditions (Fig. 15b).160,167,168 Among these factors, the placement of electrodes is particularly significant. Currently, there are two main electrode placement methods: one involves inserting two electrodes at different positions along the plant stem, while the other involves inserting one electrode into the plant stem and the other into the soil (Fig. 15c). Typically, the latter method yields higher electrical output performance compared to the former (Fig. 15e–k). This difference is likely because the electrical performance in the first method is primarily related to streaming potential, whereas the second method involves more complex interactions. The performance in the latter method is influenced by a combination of physical, chemical, and physiological processes, including fluid flow, soil pH, and plant physiological activities.
The impact of environmental conditions on the electricity generation performance of living plants was investigated in a configuration with two electrodes inserted in plant stems. Panchapakesan et al. inserted two Ag/AgCl electrodes into the stems of six- to eight-week-old tobacco plants, with one electrode placed near the root and the other near the shoot, approximately 70–80 cm apart.169 This setup formed a circuit to collect the streaming potential. They observed that during the daytime, when water was abundant, the plants generated a higher streaming potential, reaching up to 60 mV. Hu et al. investigated the electricity generation of living lucky bamboo during the evaporation process and examined the effects of light, plant size, and salt solutions on plant-based electricity generation (Fig. 15e–g).138 They inserted two gold-plated needle electrodes into the root and upper stem to construct a circuit (Fig. 15e). The results showed that in a dark room, the output voltage of plants soaked in DI water gradually decreased from 0.1 V to 0.01 V over 15 hours (Fig. 15f). However, when the plants were moved to a sunny room, the output voltage rapidly increased to 0.15 V, highlighting the significant role of transpiration in electricity generation. The study of electricity generation in plants of different sizes revealed that smaller plants exhibited the highest output voltage. This phenomenon is likely related to the pore structure in the stems of plants at different growth stages. Additionally, they found that when the plant roots were soaked in a 0.5 wt% NaCl solution, the increased concentration of inorganic salts in the water raised the ion concentration in the plant stems, resulting in a rapid increase in output voltage to 0.2 V (Fig. 15g). The authors concluded that the electricity generation during plant transpiration is due to charge separation and directional movement of water caused by the xylem, which is rich in negatively charged cellulose fibers.
A living bigleaf maple tree-based HEG with two electrodes respectively inserted into plant stem and soil was fabricated to generate micro-electricity to power integrated circuits by Carlton et al. (Fig. 15h).158 They found that this circuit could provide long-term power to a 100 kΩ load for a week, producing voltages between 70 and 250 mV. Although the generated voltage was not high, it was sufficient to power the designed integrated circuits and achieve simple functions. The integrated circuit comprised two parts. The first circuit, built with 130-nm technology, created a stable 1.1 V supply from input voltages as low as 20 mV, enabling it to generate a usable voltage level for standard circuits. The second circuit, fabricated with 90-nm technology, was a timer operating at 0.045 Hz, suitable for timekeeping in standalone sensor network nodes. This study directly demonstrates that electricity generated by living plants can serve as a sustainable green power source with significant application potential.
A comparative study on the influence of two different circuit configurations on the electricity generation performance of living pine trees was conducted by Zapata et al.160 They found that the measurement type had a significant impact on the output voltage and current. Specifically, the circuit with one electrode inserted into the soil and the other into the stem produced an average voltage and current of 0.626 V and 0.597 μA, respectively (Fig. 15i). In contrast, the circuit with both electrodes inserted into the stem yielded an average voltage and current of 0.036 V and 0.471 μA, respectively (Fig. 15j). During the study, they also observed that the placement of the electrodes (height and orientation) did not significantly affect the output voltage and current. Furthermore, they investigated the influence of tree age on the electrical output. The results indicated that tree maturity directly affects the output voltage in circuits with electrodes inserted into the stem and soil (Fig. 15k). Young trees exhibited an average voltage of 0.383 V, with a maximum of 0.626 V. Mature trees showed significantly lower values, with an average voltage of 0.150 V and a maximum of 0.295 V. In stark contrast, dead trees produced a voltage of 0 V, clearly demonstrating the critical role of physiological activity in living organism-based HEGs.
Besides the roots and stems of living plants, the leaves of living plants can interact with water droplets to form DEGs for electricity generation.170,171 Armiento et al. constructed a DEG circuit using only exposed living plant leaves and a single electrode inserted into the stem, without any additional components (Fig. 16a).170 Each droplet impacting the leaf surface generated characteristic electrical signals in the tissue, resulting in electricity generation. Two plants, A. macrorrhiza and C. antiquorum, were selected due to their similar macroscopic leaf size and shape, but significantly different leaf microstructures, which made the leaves hydrophilic and superhydrophobic, respectively. The results showed that droplets on superhydrophobic leaves had a significantly higher electricity generation performance compared to hydrophilic leaves (Fig. 16b and c). This was attributed to the inherent nanostructures and physicochemical properties of the epicuticular wax layer on superhydrophobic leaves, which facilitated better charge induction from water droplets interacting with the leaf surface (Fig. 16d). It was found that the physicochemical properties of the epicuticular wax layer played a more crucial role in high electricity generation performance than the nanostructures themselves. The influence of droplet characteristics on electricity generation performance was also investigated. The ion strength (i.e., salt concentration) and pH of the droplets had significant effects on electricity generation (Fig. 16e and f). As the salt concentration in the droplets increased, the output current of the DEG initially increased and then decreased. This was likely due to enhanced conductivity with increasing ion concentration, which improved output current, while excessively high salt concentrations might have led to charge shielding effects, reducing performance. Alkaline pH conditions reduced current output, possibly due to the damage caused to the epicuticular wax layer under such conditions. The generality of these phenomena was confirmed by examining a wider range of superhydrophobic and hydrophilic plant leaves (Fig. 16g). These works provide new avenues for the development of living plant HEGs and also offer inspiration for the design of bioinspired artificial or biohybrid energy harvesters and sensors.
 | ||
| Fig. 16 Living plant leaf-based DEGs. (a) Schematic of living leaf-based DEGs. (b) and (c) DEG power generation properties with two different leaves. (d) Mechanism of living leaf-based DEGs. (e) and (f) Output current of a C. antiquorum leaf-based DEG at different ion concentrations and pH levels. (g) Output current of different living plant leaf-based DEGs with varying hydrophilic and hydrophobic properties. Reproduced with permission.170 Copyright 2022, Springer Nature. | ||
Overall, living plant-based electricity generation has garnered considerable attention and research. However, due to the complexity of living systems, progress in this area has been slow and challenging. It is important to recognize that research on living plant-based HEGs is of significant importance. Such in-depth studies can reveal fundamental scientific questions, such as the relationships between ions, water, electricity, biological structures, and life activities. Additionally, these studies can inspire the development of more efficient power generation devices. In terms of device types, current living-plant-based HEGs have primarily focused on EEG and DEG-type devices, with other types yet to be designed. Regarding power generation performance, individual devices typically produce voltages below 0.25 V, with current still in the nA range, indicating relatively low power output. Nevertheless, the vast diversity and widespread distribution of plants offer a promising solution. By connecting living power units in series and parallel, it is possible to enhance power output significantly, making them potentially useful for rural and remote network expansion and military applications (Fig. 15d). Furthermore, due to the sensitivity of living plants' electrical properties to environmental changes, they hold vast potential for applications such as environmental monitoring and forest fire monitoring.
6. Summary and perspectives
In this review, we comprehensively summarize the application of bioinspired strategies in various types of HEGs – from elementary bioinspired materials to smart bioinspired structures and living bioinspired devices. In the process, we systematically trace the development history of bioinspired HEGs, clarify the origins of HEG research, summarize various bioinspired strategies that enhance HEG performance, and provide a detailed description of their applications as power sources and self-powered sensors. Overall, although the device performance of HEGs has been effectively improved, it must be acknowledged that the current stage of HEG development is still in its infancy (Table 3). Compared to other green electricity generators, such as photovoltaic devices and triboelectric nanogenerators, the power generation performance of HEGs is still very low, which significantly limits their practical applications (Fig. 17a). Their limited output power density and not yet fully understood mechanisms continue to restrict their practical applications.| Items | MEGs | EEGs | DEGs | REGs |
|---|---|---|---|---|
| Water form | Moisture | Liquid water | Droplet | Salt water of different concentrations |
| Mechanism | Ion gradient diffusion | Streaming potential | Dynamic EDL | Ion selective permeability |
| Voltage | 0.1–4.2 V | 0.03–4.82 V | 0.04–0.22 V | 0.01–0.43 V |
| Current density | 0.004–2.28 × 104 μA cm−2 | 0.08–4.56 × 103 μA cm−2 | 0.1–2.5 nA cm−2 | 694–1.24 × 105 μA cm−2 |
| Working mode | Continuous/intermittent | Continuous | Intermittent | Continuous |
| Stability | High | Very high | Moderate | Moderate |
| Material cost | Low | Low | Low | High |
| Environmental adaptability | High | Moderate | High | Poor |
 | ||
| Fig. 17 Comparison of electrical performance of bioinspired HEGs. (a) Comparison of the performance of bioinspired HEGs with reported photovoltaic and TENG devices.172,173 (b) Impact of different bioinspired strategies on the performance of HEG devices. (c) Comparison of the performance of various types of bioinspired HEGs. | ||
Biomimetic strategies have shown effectiveness and great potential across four different types of HEGs, resulting in noticeable improvements in the overall performance of the devices. Significant effort from researchers is needed to further develop this area and achieve practical applications in the near future. A longitudinal comparison of these strategies reveals that elementary bioinspired materials are the most studied, followed by smart bioinspired structures, with living bioinspired devices being the least researched. This is understandable, as increasing the level of biomimicry requires a deeper understanding of complex biological processes, making the design of high-performance HEGs inspired by advanced biological processes more challenging. In terms of performance, as shown in Fig. 17b, bioinspired structures in HEGs often appear in the upper middle region of the chart. This indicates that advanced biomimetic structures tend to enhance the overall performance of the devices compared to using elementary materials alone. When comparing the four types of biomimetic HEG devices, MEG devices are currently the most researched, followed by EEG and REG devices, with DEG devices being the least studied. In terms of output voltage and current density, biomimetic MEG devices generally achieve the highest voltage, while REG devices demonstrate the highest current density, with EEG devices falling in between (Fig. 17c). From the perspective of working modes, the intermittent mode may be less efficient in power generation compared to the continuous mode, but it offers the potential for specific functional designs, such as in self-powered artificial synaptic devices (Table 3). MEGs can be designed to operate in either continuous or intermittent mode, providing greater flexibility and diversity in design and functionality. Additionally, bioinspired HEGs generally have lower material costs. However, REGs, which often require precise control of numerous micro- and nanopores, may necessitate the use of specialized materials, potentially increasing the overall cost.
Based on the summarized content, we propose several potential future directions for the field of bioinspired HEGs to accelerate its rapid development:
(1) Elementary bioinspired material-based HEGs
There are still numerous biomass materials and substances that remain unexplored. Comprehensive research on their performance in HEGs to establish a suitable material-performance database is crucial. This database would provide broad guidance for discovering and utilizing the properties of biomass to construct high-performance HEGs.(2) Smart bioinspired structure-based HEGs
Although many key structures in biological electricity generation have been identified and their mechanisms revealed, their application in constructing HEGs is still limited. This is mainly due to the complexity and challenges in mimicking these bioinspired structures. By deepening our understanding of the nature of bioinspired structures and developing new synthesis methods, progress in this area could be significantly advanced.(3) Living bioinspired-based HEGs
Living organisms contain abundant energy sources, yet this field has seen slow development over centuries, likely due to the complexity of living systems. Additionally, connecting electrodes to living organisms may disrupt their normal physiological processes, and biological systems might reject foreign electrodes, leading to signal passivation. More research is needed to address these issues in detail. Moreover, the current output performance of such devices is relatively low. To improve electricity generation without affecting the normal life processes of living plants, strategies such as plant modification and enhancement through irrigation or injection should be considered. Furthermore, practical power generation could be achieved by integrating living plant-based device units through optimized circuit design and configuration.(4) Understanding biological power generation processes
The foundation of bioinspired HEGs lies in the power generation processes observed in living organisms. Investigating more biological power generation processes and deepening our understanding of the water-material/structure-power generation mechanisms in living systems can provide abundant inspiration for constructing high-performance HEGs.(5) Clarifying the intrinsic performance of HEGs
In MEGs, non-inert metal electrodes such as Al and Zn are sometimes used as current collectors, which may inadvertently introduce battery-like reactions. These reactions can enhance the output performance of MEG devices, making it challenging to accurately assess their intrinsic performance. Therefore, it is recommended that standardized testing protocols should be gradually established to obtain the intrinsic performance of materials and devices in HEGs. For instance, using symmetric inert electrodes could help better evaluate the intrinsic activity of HEG devices, thereby promoting healthy and sustainable development of this field.(6) Application scenarios for different types of bioinspired HEGs
Given the varying operating environments and output performance of different types of bioinspired HEGs, finding suitable application scenarios for each type is essential. For example, bioinspired MEGs, free from the limitations of liquid water and possessing good biocompatibility, can be well-suited for wearable applications. Living tree-based devices, through series and parallel configurations, can be utilized for forest environment monitoring and remote area signal transmission.(7) Energy storage and reuse
Considering the instability in the voltage and current output of bioinspired HEGs, efficient storage of this small-scale energy for reuse is a crucial issue. Research into the collaborative design of various energy storage devices with HEGs to improve the efficiency of energy conversion, storage, and utilization is warranted.(8) Coupling with other energy harvesting devices
The performance of individual bioinspired HEG devices is currently relatively low. Coupling HEGs with other energy harvesting devices, such as triboelectric devices, thermoelectric devices, and photovoltaic devices, could enable more efficient environmental energy conversion. This approach holds promise for designing high-performance energy harvesting devices.According to recent reports, HEGs exhibit relatively high theoretical energy conversion efficiencies—for instance, up to 50% for REGs and approximately 12% for EEGs.174,175 While current device efficiencies and power densities have yet to meet the thresholds required for direct practical applications, it is anticipated that, through the continued exploration of the above strategies, the power generation performance of bioinspired HEGs will improve substantially, thereby establishing a solid foundation for future practical deployment. More importantly, unlike large hydropower generators that convert the potential energy of water into high-power electricity through electromagnetic induction, HEGs generate electricity via direct ion–electron interactions between active materials and various forms of water. This distinct mechanism provides a crucial foundation for the development of miniaturized, portable, wearable, implantable, and self-powered electronic devices, greatly expanding their potential for multifunctional applications. In particular, the bioinspired design of HEGs endows them with enhanced biocompatibility and adaptability to the human body, making them especially suited for scenarios involving direct human interaction, such as wearable and implantable devices. Thus, it is evident that HEGs and traditional hydropower generators serve more as complementary technologies rather than competing replacements.
Furthermore, bioinspired HEGs, compared to other green energy technologies such as photovoltaic solar cells, thermoelectric generators, and triboelectric generators, are relative newcomers. They exist in a parallel development path rather than a purely competitive one because their energy sources differ, with each technology occupying an essential role in green and sustainable energy systems. Notably, HEGs stand out due to their energy carrier—water—which is diverse, ubiquitous, and unrestricted by time or location. As a result, not only are these technologies unlikely to replace one another, but HEGs also have the potential to be coupled with other technologies in the future, enabling the development of highly efficient, multifunctional, and versatile power generation systems.
Looking ahead, bioinspired HEGs, with their biomimetic characteristics, may be endowed with even greater intelligence. By coupling with advanced technologies like neuromorphic computing systems or artificial intelligence, HEGs could enable complex computational capabilities in hydrovoltaic systems. Specifically, by designing ubiquitous living plants as individual hydrovoltaic units and integrating these units into an advanced “Internet of Hydrovoltaics,” it may be possible to achieve real-time monitoring of geographically distributed information and sophisticated human–environment–machine interactions in the future.
Author contributions
Guangtao Zan: conceptualization, methodology, visualization, formal analysis, writing – original draft; Shengyou Li: conceptualization, investigation, writing – original draft; Kaiying Zhao: methodology, writing – original draft; HoYeon Kim: software, visualization; Eun Ae Shin: investigation, visualization; Kyuho Lee: visualization; Jihye Jang: investigation; Gwanho Kim: visualization; Yeonji Kim: writing – reviewing and editing; Wei Jiang: writing – reviewing and editing; Taebin Kim: writing – reviewing and editing; Woojoong Kim: writing – reviewing and editing; and Cheolmin Park: conceptualization, funding acquisition, resources, supervision, writing – reviewing and editing.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF), funded by the Korean Government (MEST) (RS-2023-00208577), the Creative Materials Discovery Program and the Pioneer Research Center Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (NRF-2022M3C1A3081211), the Nano & Material Technology Development Program through the National Research Foundation of Korea (NRF), funded by Ministry of Science and ICT (RS-2024-00416938), and the Open Resource Research Program of the Korea Institute of Science and Technology (2E32961).References
- Z. L. Wang and J. H. Song, Science, 2006, 312, 242–246 CrossRef CAS.
- Q. Yang, S. Yang, P. Qiu, L. Peng, T. Wei, Z. Zhang, X. Shi and L. Chen, Science, 2022, 377, 854–858 CrossRef CAS.
- S. M. Park, M. Wei, N. Lempesis, W. Yu, T. Hossain, L. Agosta, V. Carnevali, H. R. Atapattu, P. Serles, F. T. Eickemeyer, H. Shin, M. Vafaie, D. Choi, K. Darabi, E. D. Jung, Y. Yang, D. Bin Kim, S. M. Zakeeruddin, B. Chen, A. Amassian, T. Filleter, M. G. Kanatzidis, K. R. Graham, L. Xiao, U. Rothlisberger, M. Gratzel and E. H. Sargent, Nature, 2023, 624, 289–294 CrossRef CAS.
- G. Zan, S. Li, P. Chen, K. Dong, Q. Wu and T. Wu, ACS Cent. Sci., 2024, 10, 1283–1294 CrossRef CAS PubMed.
- S. Li, K. Zhao, E. Shin, G. Kim and G. Zan, Clean Energy Sci. Technol., 2024, 2, 140 CrossRef.
- S. Pu, G. Zan, H. Zhou, K. Dong, X. Mao, Q. Wu and T. Wu, Angew. Chem., Int. Ed., 2024, e202417589 Search PubMed.
- H. Kim, G. Zan, Y. Seo, S. Lee and C. Park, Adv. Funct. Mater., 2024, 34, 2308703 CrossRef CAS.
- J. Xu, P. Wang, Z. Bai, H. Cheng, R. Wang, L. Qu and T. Li, Nat. Rev. Mater., 2024, 1–16 Search PubMed.
- P. Poredos and R. Wang, Science, 2023, 380, 458–459 CrossRef CAS PubMed.
- Z. Zhang, X. Li, J. Yin, Y. Xu, W. Fei, M. Xue, Q. Wang, J. Zhou and W. Guo, Nat. Nanotechnol., 2018, 13, 1109–1119 CrossRef CAS.
- D. Shen, W. W. Duley, P. Peng, M. Xiao, J. Feng, L. Liu, G. Zou and Y. N. Zhou, Adv. Mater., 2020, 32, 2003722 CrossRef.
- J. Yin, J. Zhou, S. Fang and W. Guo, Joule, 2020, 4, 1852–1855 CrossRef.
- X. Wang, F. Lin, X. Wang, S. Fang, J. Tan, W. Chu, R. Rong, J. Yin, Z. Zhang and Y. Liu, Chem. Soc. Rev., 2022, 51, 4902–4927 RSC.
- Y. Jin, C. Wu, P. Sun, M. Wang, M. Cui, C. Zhang and Z. Wang, Droplet, 2022, 1, 92–109 CrossRef.
- H. Lim, M. S. Kim, Y. Cho, J. Ahn, S. Ahn, J. S. Nam, J. Bae, T. G. Yun and I. D. Kim, Adv. Mater., 2024, 36, 2301080 CrossRef CAS PubMed.
- T. Xu, X. Ding, H. Cheng, G. Han and L. Qu, Adv. Mater., 2024, 36, 2209661 CrossRef CAS PubMed.
- Z. Liu, C. Liu, Z. Chen, H. Huang, Y. Liu, L. Xue, J. Sun, X. Wang, P. Xiong and J. Zhu, Exploration, 2023, 3, 20220061 CrossRef.
- J. Wang, X. Cao, X. Cui, H. Wang, H. Zhang, K. Wang, X. Li, Z. Li and Y. Zhou, Adv. Mater., 2024, 2311151 CrossRef CAS.
- R. S. Norman, Science, 1974, 186, 350–352 CrossRef CAS.
- D. S. Fensom, Can. J. Bot., 1963, 41, 831–851 CrossRef CAS.
- G. Kim, J. W. Lee, K. Zhao, T. Kim, W. Kim, J. W. Oh, K. Lee, J. Jang, G. Zan and J. W. Park, Energy Environ. Sci., 2024, 17, 134–148 RSC.
- K. Zhao, J. W. Lee, Z. G. Yu, W. Jiang, J. W. Oh, G. Kim, H. Han, Y. Kim, K. Lee and S. Lee, ACS Nano, 2023, 17, 5472–5485 CrossRef CAS PubMed.
- F. Zhao, H. Cheng, Z. Zhang, L. Jiang and L. Qu, Adv. Mater., 2015, 27, 4351–4357 CrossRef CAS PubMed.
- G. Xue, Y. Xu, T. Ding, J. Li, J. Yin, W. Fei, Y. Cao, J. Yu, L. Yuan and L. Gong, Nat. Nanotechnol., 2017, 12, 317–321 CrossRef CAS.
- W. Xu, H. Zheng, Y. Liu, X. Zhou, C. Zhang, Y. Song, X. Deng, M. Leung, Z. Yang, R. X. Xu, Z. L. Wang, X. C. Zeng and Z. Wang, Nature, 2020, 578, 392 CrossRef CAS PubMed.
- T. B. Schroeder, A. Guha, A. Lamoureux, G. VanRenterghem, D. Sept, M. Shtein, J. Yang and M. Mayer, Nature, 2017, 552, 214–218 CrossRef CAS PubMed.
- B. Zhang, W. Xu, L. Peng, Y. Li, W. Zhang and Z. Wang, Nat. Rev. Electr. Eng., 2024, 1, 218–233 CrossRef.
- G. Zan and Q. Wu, Adv. Mater., 2016, 28, 2099–2147 CrossRef CAS PubMed.
- K. Wang, W. Xu, W. Zhang, X. Wang, X. Yang, J. Li, H. Zhang, J. Li and Z. Wang, Nano Res. Energy, 2023, 2, e9120042 CrossRef.
- T. Xu, X. Ding, Y. Huang, C. Shao, L. Song, X. Gao, Z. Zhang and L. Qu, Energy Environ. Sci., 2019, 12, 972–978 RSC.
- T. Ding, K. Liu, J. Li, G. Xue, Q. Chen, L. Huang, B. Hu and J. Zhou, Adv. Funct. Mater., 2017, 27, 1700551 CrossRef.
- S. G. Yoon, Y. Yang, J. Yoo, H. Jin, W. H. Lee, J. Park and Y. S. Kim, ACS Appl. Electron. Mater., 2019, 1, 1746–1751 CrossRef CAS.
- T. G. Yun, J. Bae, A. Rothschild and I. Kim, ACS Nano, 2019, 13, 12703–12709 CrossRef CAS PubMed.
- J. Yin, X. Li, J. Yu, Z. Zhang, J. Zhou and W. Guo, Nat. Nanotechnol., 2014, 9, 378–383 CrossRef CAS.
- L. Li, X. Wang, W. Deng, J. Yin, X. Li and W. Guo, Droplet, 2023, 2, e77 CrossRef.
- Z. Zhang, L. Wen and L. Jiang, Nat. Rev. Mater., 2021, 6, 622–639 CrossRef CAS.
- Y. Zhou and L. Jiang, Joule, 2020, 4, 2244–2248 CrossRef.
- W. Guo, L. Cao, J. Xia, F. Q. Nie, W. Ma, J. Xue, Y. Song, D. Zhu, Y. Wang and L. Jiang, Adv. Funct. Mater., 2010, 20, 1339–1344 CrossRef CAS.
- X. Liu, H. Gao, L. Sun and J. Yao, Adv. Mater., 2024, 36, 2300748 CrossRef CAS.
- M. Li, L. Zong, W. Yang, X. Li, J. You, X. Wu, Z. Li and C. Li, Adv. Funct. Mater., 2019, 29, 1901798 CrossRef.
- W. Yang, X. Li, X. Han, W. Zhang, Z. Wang, X. Ma, M. Li and C. Li, Nano Energy, 2020, 71, 104610–104617 CrossRef CAS.
- J. Eun and S. Jeon, Nano Energy, 2022, 92, 106772–106779 CrossRef CAS.
- Z. Li, J. Wang, L. Dai, X. Sun, M. An, C. Duan, J. Li and Y. Ni, ACS Appl. Mater. Interfaces, 2020, 12, 55205–55214 CrossRef CAS.
- P. Li, N. Su, Z. Wang and J. Qiu, ACS Nano, 2021, 15, 16811–16818 CrossRef CAS.
- L. Huang, Y. Zhang, X. Song, D. Li, X. Chen and Q. Yuan, Nano Energy, 2023, 118, 108973–108984 CrossRef CAS.
- Y. J. Yun, O. J. Yoon, D. I. Son and Y. Jun, Nano Energy, 2023, 118, 108934 CrossRef CAS.
- Q. Lyu, B. Peng, Z. Xie, S. Du, L. Zhang and J. Zhu, ACS Appl. Mater. Interfaces, 2020, 12, 57373–57381 CrossRef CAS.
- X. Xie, X. Wang, Y. Zhang, L. Fang, J. Feng, S. Liu, D. Yu, F. Zhu and X. Chen, ACS Appl. Mater. Interfaces, 2024, 16, 3279–3288 CrossRef CAS PubMed.
- M. Song, D. Kim, H. Lee, H. Han and S. Jeon, Energy Environ. Sci., 2024, 17, 5421–5428 RSC.
- X. Gao, T. Xu, C. Shao, Y. Han, B. Lu, Z. Zhang and L. Qu, J. Mater. Chem. A, 2019, 7, 20574–20578 RSC.
- J. Chen, Y. Li, Y. Zhang, D. Ye, C. Lei, K. Wu and Q. Fu, Adv. Funct. Mater., 2022, 32, 2203666 CrossRef CAS.
- F. Wang, Y. Zhang, J. Shi, L. Sun, A. Ullah, C. Zhu and I. S. Kim, ACS Sustainable Chem. Eng., 2023, 11, 9792–9803 CrossRef CAS.
- J. Youm, S. Lee, I. Cho, D. Jeong, J. Bang, H. Park and M. Kim, Surf. Interfaces, 2023, 38, 102853 CrossRef CAS.
- W. Yang, P. Xiao, F. Ni, C. Zhang, J. Gu, S. Kuo, Q. Liu and T. Chen, Nano Energy, 2022, 97, 107180 CrossRef CAS.
- J. Tan, S. Fang, Z. Zhang, J. Yin, L. Li, X. Wang and W. Guo, Nat. Commun., 2022, 13, 3643 CrossRef CAS.
- J. Tang, Y. Wang, H. Yang, Q. Zhang, C. Wang, L. Li, Z. Zheng, Y. Jin, H. Wang and Y. Gu, Nat. Commun., 2024, 15, 3649 CrossRef CAS PubMed.
- Z. Gao, J. Zhang, M. Ahmad, B. Jiang, Z. Sun, S. Wang and Y. Jin, Carbohydr. Polym., 2022, 296, 119847 CrossRef CAS PubMed.
- X. Jia, M. Zhang, Y. Zhang, Y. Fu, N. Sheng, S. Chen, H. Wang and Y. Du, Nano Lett., 2024, 24, 2218–2225 CrossRef CAS PubMed.
- B. Wang, J. Li, Z. Wu, N. Sheng, M. Zhang, Z. Han, M. Jin, J. Li, X. Lv, K. Ou, H. Wang and S. Chen, Nano Energy, 2022, 102, 107702 CrossRef CAS.
- W. Zhao, Y. Wang, M. Han, J. Xu, L. Han and K. C. Tam, Nano Energy, 2022, 98, 107291 CrossRef CAS.
- Z. Wu, P. Ji, B. Wang, N. Sheng, M. Zhang, S. Chen and H. Wang, Nano Energy, 2021, 80, 105554 CrossRef CAS.
- M. Zhang, N. Sheng, Q. Song, H. Zhang, S. Chen, H. Wang and K. Zhang, Nano Energy, 2022, 103, 107786 CrossRef CAS.
- S. Yang, L. Zhang, J. Mao, J. Guo, Y. Chai, J. Hao, W. Chen and X. Tao, Nat. Commun., 2024, 15, 3329 CrossRef CAS PubMed.
- H. Wang, T. He, X. Hao, Y. Huang, H. Yao, F. Liu, H. Cheng and L. Qu, Nat. Commun., 2022, 13, 2524 CrossRef CAS PubMed.
- R. Zhang, M. Qu, H. Wang, S. Li, Y. Song, P. Tang and Y. Bin, J. Mater. Chem. A, 2023, 11, 3616–3624 RSC.
- R. Zhang, H. Wang, M. Qu, S. Li, Y. Ma, X. Li, P. Tang and Y. Bin, Chem. Eng. J., 2023, 473, 145325 CrossRef.
- Q. Zhao, Y. Jiang, Z. Duan, Z. Yuan, J. Zha, Z. Wu, Q. Huang, Z. Zhou, H. Li and F. He, Chem. Eng. J., 2022, 438, 135588 CrossRef CAS.
- R. Zhang, M. Qu, H. Wang, M. Fan, Q. Chen, P. Tang and Y. Bin, React. Funct. Polym., 2022, 181, 105421 CrossRef CAS.
- J. Pei, G. Chen, Z. Li, Z. Zhou, A. Chen, S. Xie and X. Jiang, Ind. Eng. Chem. Res., 2023, 62, 21666–21672 CrossRef CAS.
- X. Zhang, Z. Dai, J. Chen, X. Chen, X. Lin, S. Yang, K. Wu, Q. Fu and H. Deng, Energy Environ. Sci., 2023, 16, 3600–3611 RSC.
- Y. Song, R. Zhang, M. Qu, R. Zheng, Q. Zhao, P. Tang, Y. Bin and H. Wang, React. Funct. Polym., 2024, 195, 105806 CrossRef CAS.
- Z. Wu, T. Zhang, B. Wang, P. Ji, N. Sheng, M. Zhang, Q. Liang, S. Chen and H. Wang, Nano Energy, 2021, 88, 106275 CrossRef CAS.
- G. Bian, N. Pan, Z. Luan, X. Sui, W. Fan, Y. Xia, K. Sui and L. Jiang, Angew. Chem., 2021, 133, 20456–20462 CrossRef.
- W. Yang, L. Lv, X. Li, X. Han, M. Li and C. Li, ACS Nano, 2020, 14, 10600–10607 CrossRef CAS.
- Z. Wang, J. Li, C. Shao, X. Lin, Y. N. Yang, N. Chen, Y. Wang and L. Qu, Nano Energy, 2021, 90, 106529 CrossRef CAS.
- H. He, J. Zhang, J. Pan, Z. Wang, M. Deng, X. Liu and F. Fu, ACS Appl. Energy Mater., 2024, 7, 2980–2988 CrossRef CAS.
- C. Ge, Y. Wang, M. Wang, Z. Zheng, S. Wang, Y. Kong, Q. Gao, M. Liu, F. Sun and L. Li, Adv. Mater., 2024, 36, 2310260 CrossRef CAS.
- W. Xin, H. Xiao, X. Kong, J. Chen, L. Yang, B. Niu, Y. Qian, Y. Teng, L. Jiang and L. Wen, ACS Nano, 2020, 14, 9701–9710 CrossRef CAS PubMed.
- W. Xin, Z. Zhang, X. Huang, Y. Hu, T. Zhou, C. Zhu, X. Kong, L. Jiang and L. Wen, Nat. Commun., 2019, 10, 3876 CrossRef.
- J. Chen, W. Xin, X. Kong, Y. Qian, X. Zhao, W. Chen, Y. Sun, Y. Wu, L. Jiang and L. Wen, ACS Energy Lett., 2019, 5, 742–748 CrossRef.
- S. Mandal, S. Roy, A. Mandal, T. Ghoshal, G. Das, A. Singh and D. K. Goswami, ACS Appl. Electron. Mater., 2020, 2, 780–789 CrossRef CAS.
- J. Liu, L. Huang, W. He, X. Cai, Y. Wang, L. Zhou and Y. Yuan, Nano Energy, 2022, 102, 107709 CrossRef CAS.
- R. Zhu, Y. Zhu, L. Hu, P. Guan, D. Su, S. Zhang, C. Liu, Z. Feng, G. Hu and F. Chen, Energy Environ. Sci., 2023, 16, 2338–2345 RSC.
- X. Liu, H. Gao, J. E. Ward, X. Liu, B. Yin, T. Fu, J. Chen, D. R. Lovley and J. Yao, Nature, 2020, 578, 550–554 CrossRef CAS PubMed.
- G. Ren, Z. Wang, B. Zhang, X. Liu, J. Ye, Q. Hu and S. Zhou, Nano Energy, 2021, 89, 106361 CrossRef CAS.
- G. Ren, Q. Hu, J. Ye, X. Liu, S. Zhou and Z. He, Chem. Eng. J., 2022, 441, 135921 CrossRef CAS.
- X. Liu, T. Ueki, H. Gao, T. L. Woodard, K. P. Nevin, T. Fu, S. Fu, L. Sun, D. R. Lovley and J. Yao, Nat. Commun., 2022, 13, 4369 CrossRef CAS.
- Q. Hu, Y. Ma, G. Ren, B. Zhang and S. Zhou, Sci. Adv., 2022, 8, m8047 CrossRef.
- Y. Ma, G. Ren, Y. Qiu, S. Zhou and Q. Hu, SCIENTIA SINICA Technologica, 2022, 52, 1669–1678 CrossRef.
- S. Yang, X. Tao, W. Chen, J. Mao, H. Luo, S. Lin, L. Zhang and J. Hao, Adv. Mater., 2022, 34, 2200693 CrossRef CAS PubMed.
- Q. Li, Y. Qin, D. Cheng, M. Cheng, H. Zhao, L. Li, S. Qu, J. Tan and J. Ding, Adv. Funct. Mater., 2023, 33, 2211013 CrossRef CAS.
- P. He, J. Wu, X. Pan, L. Chen, K. Liu, H. Gao, H. Wu, S. Cao, L. Huang and Y. Ni, J. Mater. Chem. A, 2020, 8, 3109–3118 RSC.
- L. Yang, L. Zhang and D. Sun, ACS Appl. Mater. Interfaces, 2022, 14, 53615–53626 CrossRef CAS PubMed.
- F. Ni, P. Xiao, C. Zhang and T. Chen, Matter, 2022, 5, 2624–2658 CrossRef CAS.
- D. Zhao, Y. Zhu, W. Cheng, W. Chen, Y. Wu and H. Yu, Adv. Mater., 2021, 33, e2000619 CrossRef PubMed.
- E. Shin, G. Kim, K. Zhao, G. Zan, H. Kim, S. Li, J. Lee, D. Kang, J. W. Oh and J. Jung, Energy Environ. Sci., 2024, 17, 7165–7181 RSC.
- X. Zhang, M. Li, F. Zhang, Q. Li, J. Xiao, Q. Lin and G. Qing, Small, 2023, 19, 2304603 CrossRef CAS.
- G. Zan, W. Jiang, H. Kim, K. Zhao, S. Li, K. Lee, J. Jang, G. Kim, E. Shin, W. Kim, J. W. Oh, Y. Kim, J. W. Park, T. Kim, S. Lee, J. Oh, J. Shin, H. J. Kim and C. Park, Nat. Commun., 2024, 15, 10056 CrossRef CAS PubMed.
- R. Xiong, A. M. Grant, R. Ma, S. Zhang and V. V. Tsukruk, Mater. Sci. Eng., R, 2018, 125, 1–41 CrossRef.
- S. Li, A. Liu, W. Qiu, Y. Wang, G. Liu, J. Liu, Y. Shi, Y. Li, J. Li, W. Cai, C. Park, M. Ye and W. Guo, ACS Nano, 2024, 18, 4579–4589 CrossRef CAS.
- L. Shengyou, L. Jiarong, W. Hao, L. Xiangyang and G. Wenxi, Acta. Phys. Sin., 2020, 69, 178703 CrossRef.
- S. Li, G. Liu, H. Wen, G. Liu, H. Wang, M. Ye, Y. Yang, W. Guo and Y. Liu, Adv. Funct. Mater., 2022, 32, 2111747 CrossRef CAS.
- Y. Wang, K. Qu, S. Li, J. Zheng, W. Qiu, F. Ye, Z. Xiao, Q. Xu, J. Xu and W. Guo, Chem. Eng. J., 2023, 469, 143920 CrossRef CAS.
- J. Liu, J. Chen, F. Dai, J. Zhao, S. Li, Y. Shi, W. Li, L. Geng, M. Ye and X. Chen, Nano Energy, 2022, 103, 107764 CrossRef CAS.
- S. Li, K. Zhao, G. Zan, G. Kim, J. Oh, W. Jiang, E. Shin, W. Kim, T. Kim, J. Jang, H. Kim, J. W. Park, K. Lee and C. Park, Device, 2025, 3, 100561 Search PubMed.
- Z. Lin, Z. Meng, H. Miao, R. Wu, W. Qiu, N. Lin and X. Y. Liu, ACS Nano, 2021, 15, 5649–5660 CrossRef CAS PubMed.
- S. Asim, T. A. Tabish, U. Liaqat, I. T. Ozbolat and M. Rizwan, Adv. Healthcare Mater., 2023, 12, 2203148 CrossRef CAS PubMed.
- X. Wu, J. J. Hu and J. Yoon, Angew. Chem., 2024, 136, e202400249 CrossRef.
- X. Zhang, M. Wang, Y. Wu, X. Chen, K. Wu, Q. Fu and H. Deng, Adv. Funct. Mater., 2023, 33, 2210027 CrossRef CAS.
- H. Zhong, S. Wang, Z. Wang and J. Jiang, Chem. Eng. J., 2024, 486, 150203 CrossRef CAS.
- Z. Sun, C. Han, S. Gao, Z. Li, M. Jing, H. Yu and Z. Wang, Nat. Commun., 2022, 13, 5077 CrossRef CAS.
- Y. Li, J. Cui, H. Shen, C. Liu, P. Wu, Z. Qian, Y. Duan and D. Liu, Nano Energy, 2022, 96, 107065 CrossRef CAS.
- M. Wang, Y. Wang, Y. Han, H. Dong, F. Huo and H. He, Nano Energy, 2024, 123, 109376 CrossRef CAS.
- H. Wang, Y. Sun, T. He, Y. Huang, H. Cheng, C. Li, D. Xie, P. Yang, Y. Zhang and L. Qu, Nat. Nanotechnol., 2021, 16, 811–819 CrossRef CAS PubMed.
- J. Bai, Q. Liao, H. Yao, T. Guang, T. He, H. Cheng and L. Qu, Energy Environ. Sci., 2023, 16, 3088–3097 RSC.
- J. Zhang, P. Cui, J. Wang, Y. Ge, H. Meng, C. Feng, H. Liu, G. Cheng and Z. Du, Adv. Mater. Technol., 2023, 8, 2300370 CrossRef CAS.
- Y. Chen, J. He, C. Ye and S. Tang, Adv. Energy Mater., 2024, 14, 2400529 CrossRef CAS.
- J. Garemark, F. Ram, L. Liu, I. Sapouna, M. F. Cortes Ruiz, P. T. Larsson and Y. Li, Adv. Funct. Mater., 2023, 33, 2208933 CrossRef CAS.
- K. Zhang, L. Cai, A. Nilghaz, G. Chen, X. Wan and J. Tian, Nano Energy, 2022, 98, 107288 CrossRef CAS.
- J. Lin, Z. Zhang, X. Lin, X. Cai, S. Fu, X. Fang, Y. Ding, X. Wang, G. Sèbe and G. Zhou, Adv. Funct. Mater., 2024, 34, 2314231 CrossRef CAS.
- T. Zhang, X. Han, Y. Peng, H. Yu and J. Pu, Polymers, 2024, 16, 260 CrossRef CAS.
- K. Zhang, X. Li, C. Yan, R. Shi, Z. Fang, S. Zhou, R. Cao and J. Tian, ACS Nano, 2024, 18, 10259–10269 CrossRef CAS.
- X. Li, K. Zhang, A. Nilghaz, G. Chen and J. Tian, Nano Energy, 2023, 112, 108491 CrossRef CAS.
- Y. Hu, Y. Teng, Y. Sun, P. Liu, L. Fu, L. Yang, X. Kong, Q. Zhao, L. Jiang and L. Wen, Nano Energy, 2022, 97, 107170 CrossRef CAS.
- Y. Zhang, J. Riexinger, X. Yang, E. Mikhailova, Y. Jin, L. Zhou and H. Bayley, Nature, 2023, 620, 1001–1006 CrossRef CAS PubMed.
- A. Guha, T. J. Kalkus, T. B. H. Schroeder, O. G. Willis, C. Rader, A. Ianiro and M. Mayer, Adv. Mater., 2021, 33, 210175731 CrossRef.
- L. Yang, F. Yang, X. Liu, K. Li, Y. Zhou, Y. Wang, T. Yu, M. Zhong, X. Xu, L. Zhang, W. Shen and D. Wei, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e452948904 Search PubMed.
- F. Zhang, J. Yu, Y. Si and B. Ding, Adv. Mater., 2023, 35, 2302511 CrossRef CAS PubMed.
- B. Y. Liu, Y. H. Zhang, Y. Qian, D. Quan, M. J. Jia, X. Y. Jin, M. Zhou, X. Y. Kong and L. Jiang, Angew. Chem., Int. Ed., 2024, 63, e202317361 CrossRef CAS PubMed.
- C. Liu, C. Ye, T. Zhang, J. Tang, K. Mao, L. Chen, L. Xue, J. Sun, W. Zhang and X. Wang, Angew. Chem., 2024, 136, e202315947 CrossRef.
- Q. Li, K. Zhou, B. Zhu, X. Liu, J. Lao, J. Gao and L. Jiang, J. Am. Chem. Soc., 2023, 145, 28038–28048 CrossRef CAS.
- L. Ding, M. Zheng, D. Xiao, Z. Zhao, J. Xue, S. Zhang, J. Caro and H. Wang, Angew. Chem., Int. Ed., 2022, 61, e202206152 CrossRef CAS PubMed.
- M. Gao, M. Zheng, A. F. EL-Mahdy, C. Chang, Y. Su, W. Hung, S. Kuo and L. Yeh, Nano Energy, 2023, 105, 108007 CrossRef CAS.
- R. K. Tonnah, M. Chai, M. Abdollahzadeh, H. Xiao, M. Mohammad, E. Hosseini, M. Zakertabrizi, D. Jarrahbashi, A. Asadi and A. Razmjou, ACS Nano, 2023, 17, 12445–12457 CrossRef CAS PubMed.
- Y. Liu, L. Yeh, M. Zheng and K. C. Wu, Sci. Adv., 2021, 7, e9924 CrossRef.
- J. S. Kim, J. Kim, J. Ahn, S. Chung and C. S. Han, Adv. Sci., 2023, 10, 2301037 CrossRef CAS.
- T. Cai, L. Lan, B. Peng, C. Zhang, S. Dai, C. Zhang, J. Ping and Y. Ying, Nano Lett., 2022, 22, 6476–6483 CrossRef CAS PubMed.
- Y. Hu, W. Yang, W. Wei, Z. Sun, B. Wu, K. Li, Y. Li, Q. Zhang, R. Xiao and C. Hou, Sci. Adv., 2024, 10, k4620 CrossRef.
- X. Zhou, W. Zhang, C. Zhang, Y. Tan, J. Guo, Z. Sun and X. Deng, ACS Appl. Mater. Interfaces, 2020, 12, 11232–11239 CrossRef CAS.
- K. Zhao, S. Li, G. Zan, G. Kim, W. Jiang, J. W. Park, J. Yoon, J. H. Oh, J. Jang and S. Lee, Nano Energy, 2024, 126, 109645 CrossRef CAS.
- X. Li, J. Lao, G. Li, J. Song and J. Luo, Mater. Chem. Front., 2020, 4, 3361–3367 RSC.
- P. He, J. Yue, Z. Qiu, Z. Meng, J. He and D. Li, Nat. Commun., 2024, 15, 5261 CrossRef CAS PubMed.
- C. C. Sproncken, P. Liu, J. Monney, W. S. Fall, C. Pierucci, P. B. Scholten, B. Van Bueren, M. Penedo, G. E. Fantner and H. H. Wensink, Nature, 2024, 630, 866–871 CrossRef CAS PubMed.
- B. Zhou, Q. Cheng, Z. Chen, Z. Chen, D. Liang, E. A. Munro, G. Yun, Y. Kawai, J. Chen and T. Bhowmick, Nat. Commun., 2024, 15, 3652 CrossRef CAS.
- J. Chen, W. Xin, W. Chen, X. Zhao, Y. Qian, X. Kong, L. Jiang and L. Wen, ACS Cent. Sci., 2021, 7, 1486–1492 CrossRef CAS.
- J. Milanezi, J. P. C. Da Costa, E. P. de Freitas, J. A. Gomes and R. Schmitt, presented in part at the 2014 International Conference on Renewable Energy Research and Application (ICRERA)2014.
- G. Ren, Q. Hu, J. Ye, A. Hu, J. Lu and S. Zhou, Research, 2022, 2022, 9873203 CrossRef CAS PubMed.
- L. Lan, J. Xiong, D. Gao, Y. Li, J. Chen, J. Lv, J. Ping, Y. Ying and P. S. Lee, ACS Nano, 2021, 15, 5307–5315 CrossRef CAS PubMed.
- V. Slabov, S. Kopyl, M. P. S. Dos Santos and A. L. Kholkin, Nano-Micro Lett., 2020, 12, 42 CrossRef CAS.
- M. Guadalupe Salinas-Juarez, P. Roquero and M. Del Carmen Duran-Dominguez-de-Bazua, Bioelectrochemistry, 2016, 112, 145–152 CrossRef.
- H. Guesmi, R. Ajjel, T. Alqahtani and S. Algarni, Int. J. Sustainable Eng., 2022, 15, 253–265 Search PubMed.
- M. A. Mudrilov, M. M. Ladeynova, D. V. Kuznetsova and V. A. Vodeneev, Biochemistry, 2023, 88, 1467–1487 CAS.
- J. S. Burdon-Sanderson, Proc. R. Soc. London, 1873, 21, 495–496 CrossRef.
- J. Fromm and S. Lautner, Plant, Cell Environ., 2007, 30, 249–257 CrossRef CAS PubMed.
- A. L. Houwink, Recl. Trav. Bot. Neerl., 1935, 32, 51–91 Search PubMed.
- A. Q. Ansari and D. Bowling, New Phytol., 1972, 71, 111–117 CrossRef.
- S. Gelfan, Science, 1928, 67, 589–590 CrossRef CAS PubMed.
- C. Himes, E. Carlson, R. J. Ricchiuti, B. P. Otis and B. A. Parviz, IEEE Trans. Nanotechnol., 2010, 9, 2–5 Search PubMed.
- D. Gibert, J. Le Mouel, L. Lambs, F. Nicollin and F. Perrier, Plant Sci., 2006, 171, 572–584 CrossRef CAS.
- R. Zapata, J. Oliver-Villanueva, L. Lemus-Zuniga, J. E. Luzuriaga, M. A. Mateo Pla and J. F. Urchueguia, Plant Signaling Behav., 2020, 15, 1795580 CrossRef.
- P. Morat, J. L. Lemouel and A. Granier, C. R. Acad. Sci., Ser. III, 1994, 317, 98–101 Search PubMed.
- W. Gindl, H. G. Löppert and R. Wimmer, Phyton, 1999, 39, 217–224 Search PubMed.
- L. A. Gurovich and P. Hermosilla, J. Plant Physiol., 2009, 166, 290–300 CrossRef CAS.
- L. Rios-Rojas, D. Morales-Moraga, J. A. Alcalde and L. A. Gurovich, Plant Signaling Behav., 2015, 10, e976487 CrossRef.
- Z. Hao, W. Li and X. Hao, J. Exp. Bot., 2021, 72, 1321–1335 CrossRef CAS.
- D. Comparini, E. Masi, C. Pandolfi, L. Sabbatini, M. Dolfi, S. Morosi and S. Mancuso, Agric. Water Manage., 2020, 234, 106109 CrossRef.
- Z. Hao, G. Wang, W. Li, J. Zhang and J. Kan, PLoS One, 2015, 10, e136639 Search PubMed.
- Z. Hao, K. Liu, W. Li, J. Zhang, J. Kan and X. Hao, J. Renewable Sustainable Energy, 2018, 10, 43101 CrossRef.
- R. Panchapakesan and K. W. Oh, ASME Int. Mech. Eng. Congr. Expo., 2009, 43857, 341–342 Search PubMed.
- S. Armiento, C. Filippeschi, F. Meder and B. Mazzolai, Commun. Mater., 2022, 3, 79 CrossRef.
- H. Wu, Z. Chen, G. Xu, J. Xu, Z. Wang and Y. Zi, ACS Appl. Mater. Interfaces, 2020, 12, 56060–56067 CrossRef CAS.
- O. Almora, D. Baran, G. C. Bazan, C. Berger, C. I. Cabrera, K. R. Catchpole, S. Erten-Ela, F. Guo, J. Hauch, A. W. Y. Ho-Baillie, T. J. Jacobsson, R. A. J. Janssen, T. Kirchartz, N. Kopidakis, Y. Li, M. A. Loi, R. R. Lunt, X. Mathew, M. D. McGehee, J. Min, D. B. Mitzi, M. K. Nazeeruddin, J. Nelson, A. F. Nogueira, U. W. Paetzold, N. Park, B. P. Rand, U. Rau, H. J. Snaith, E. Unger, L. Vaillant-Roca, H. Yip and C. J. Brabec, Adv. Energy Mater., 2021, 11, 2102526 CrossRef CAS.
- R. Walden, C. Kumar, D. M. Mulvihill and S. C. Pillai, Chem. Eng. J. Adv., 2022, 9, 100237 CrossRef.
- F. H. Van der Heyden, D. J. Bonthuis, D. Stein, C. Meyer and C. Dekker, Nano Lett., 2006, 6, 2232–2237 CrossRef CAS PubMed.
- Q. Ren, Q. Cui, K. Chen, J. Xie and P. Wang, Desalination, 2022, 535, 115802 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |