Biologically synthesized Fe0-based nanoparticles and their application trends as catalysts in the treatment of chlorinated organic compounds: a review
Hong Son
Nguyen
 a,
Van Hoang
Nguyen
a,
Thanh Binh
Nguyen
b,
Trung Thien
Luong
a and
Ngoc Toan
Vu
*a
a,
Van Hoang
Nguyen
a,
Thanh Binh
Nguyen
b,
Trung Thien
Luong
a and
Ngoc Toan
Vu
*a
aInstitute of New Technology, 17, Hoang Sam, Nghia Do, Cau Giay, Hanoi, Vietnam. E-mail: vntoanchem@gmail.com
bCommander Chemical of Engineering, Son Tay, Hanoi, Vietnam
First published on 21st January 2025
Abstract
This review explores the advancements and trends in biologically synthesized Fe0-based nanoparticles (NPs) and their applications as catalysts in treating chlorinated organic compounds. The persistent nature and bioaccumulative characteristics of chlorinated organic compounds enable their accumulation in water, soil, and the food chain, leading to significant environmental and human health issues. The widespread presence of these toxic substances underscores the urgent need for effective treatment and remediation strategies. Biologically synthesized Fe0-based NPs are recognized for their considerable surface area, potent reduction properties, and environmental compatibility. These attributes render them a promising approach for the remediation of chlorinated compounds. This review categorizes synthesis methods into key groups: microorganisms, plant extracts, biological waste, and industrial–agricultural by-products. Recent studies highlight the promising applications of bio-NPs in environmental remediation, emphasizing their potential for sustainable and efficient treatment solutions. This analysis thoroughly examines current trends in the application and enhancement of nanoparticle activity, delineating various challenges and future prospects comprehensively. It offers well-defined research directions with high practical relevance, aiming to contribute to advancing knowledge and guiding future research endeavors in the field.
Environmental significanceChlorinated organic compounds pose significant environmental and health hazards due to their persistence, bioaccumulation, and toxic effects. Conventional treatment methods often fall short in effectively remediating these pollutants. Biologically synthesized Fe0-based nanoparticles offer a promising alternative, as they exhibit high reactivity, large surface area, and environmental compatibility. Their green synthesis using biological agents not only reduces the environmental impact associated with chemical synthesis methods but also enhances sustainability. This review highlights the potential of biogenic Fe0 nanoparticles as catalysts in the degradation of chlorinated organic compounds, contributing to advancing eco-friendly technologies for water and soil remediation. Their adoption could mark a substantial step forward in achieving cleaner and safer ecosystems. |
1. Introduction
In the era of modern technology, the remarkable advancement of nanotechnology has led to significant scientific progress, unveiling vast potential applications across various fields such as medicine, the environment, energy, and industry. Among these, the application of nanomaterials to address environmental pollution issues has become a hot topic, garnering substantial interest.1–3The issue of pollution and the inherent risks of chlorinated organic pollutants have posed a persistent challenge, becoming a global concern and drawing attention from scientists for decades.4–6 Control measures and numerous technologies are being researched, but they still face challenges in completely mitigating the destructive effects of these pollutants.6 Soil contamination with chlorinated organic chemicals is widespread, primarily resulting from industrial chemical waste or extensive pesticide use in agriculture.7 Meanwhile, the increasing presence of chlorinated organic compounds in aqueous environments has been acknowledged as a considerable threat to ecosystems. These substances' long-term persistence and bioaccumulation potential threaten the environment and human health.8 The application of metallic nanomaterials to remediate and eliminate chlorinated organic compounds has been extensively studied.9,10 Fe0-based nanomaterials (also known as zero-valent iron/nZVI) possess the ability to catalyze the conversion of chlorinated organic compounds through a common mechanism, namely the hydro dechlorination (HDC) reaction.11,12 Ševců et al.13 demonstrated that nZVI has the capacity to degrade two polychlorinated biphenyl (PCB) isomers, specifically PCB-28 and PCB-52, with their concentrations decreasing from 20.7 μg L−1 to 3.0–5.8 μg L−1 within 1 hour. Studies by Kim et al.14 and Dumestre et al.15 also highlighted nZVI's ability to degrade trichloroethylene (TCE) by over 99%. Similarly, Calderon et al.16 showed that polychlorinated dioxins and furans (PCDD/Fs) could be adsorbed onto the surface of nZVI, with total PCDD/F WHO-TEQ concentrations reaching up to 35 pg g−1. This process effectively cleaves the C–Cl bond, forming less harmful or non-toxic by-products17 (Fig. 1).
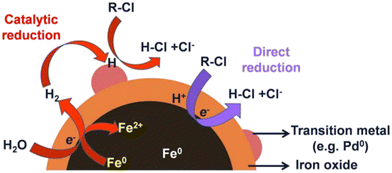 | ||
| Fig. 1 Mechanisms of reductive dechlorination by nZVI and nZVI/Pd.17 | ||
Typically, the synthesis of nanomaterials via physical (ball milling,18,19 laser ablation,20 mechanical grinding21) or chemical methods (using NaBH4 reducing agent22,23) is costly, time-consuming, and often generates highly toxic by-products, requiring high energy inputs and posing potential risks to human health.24–26 To address these limitations, many researchers have developed a synthesis method that is more biocompatible, rapid, and cost-effective. The biological synthesis of NPs, also known as “green synthesis”, offers an alternative and more environmentally friendly approach to nanomaterial fabrication.27,28 Green synthesis involves producing NPs without the use of hazardous or expensive chemicals.29 Instead, natural resources are employed to create NPs, resulting in more environmentally friendly and biocompatible products. NPs synthesized through this method are commonly referred to as bio-NPs.30,31 Recent studies have highlighted the potential of this approach for multi-faceted applications, particularly in the environmental field.
This review consolidates recent research on bio-synthesized Fe0-based NPs and their application in environmental pollution control, focusing on chlorinated organic compounds in water and soil. We will present some recent trends in nanotechnology fabrication, evaluate the advantages of green synthesis methods compared to traditional approaches, and discuss the future prospects of these materials in environmental applications.
2. Biological approach of NPs based on Fe0
NPs synthesized through biological approaches are commonly referred to as bio-NPs or bio-nanomaterials.30,31 Iron NPs are characterized by their large surface area and strong reduction capability, and they are considered relatively safe and environmentally friendly.32,33 Consequently, iron salts are frequently investigated in conjunction with biological agents to produce ‘green’ materials, minimizing negative environmental impacts and facilitating biodegradability. The trend in synthesizing Fe0-based nanomaterials biologically can be categorized into several groups: microorganisms, plant extracts, biological waste, and industrial–agricultural by-products.31,342.1. Using microorganisms
The use of microorganisms for the biological synthesis of NPs is an innovative approach, leveraging natural microbial resources. Microbial nano-biosynthesis presents numerous advantages, including environmental friendliness and sustainability.35,36 Furthermore, microbes provide enhanced control over the size, shape, and distribution of NPs because of their unique metabolic pathways and enzymatic systems.37 The synthesis usually occurs under mild conditions, such as ambient temperature and pressure, which minimizes the need for specialized equipment and reduces energy consumption.38 Besides, proteins and other biomolecules produced by microorganisms can serve as natural capping agents, stabilizing NPs and preventing their aggregation.39 However, microbial nanotechnology still faces several challenging limitations, including slow production rates,40 a limited variety of NPs that can be synthesized, the need for sterile conditions, and variability in the characteristics of the NPs produced.41 Moreover, the highly complex mechanism of biosynthesis of NPs is one of the limitations that has hindered the widespread research on this method. Fig. 2 summarizes some advantages and limitations of microbial nanoparticle synthesis.Microorganisms, which are microscopic in size, can be categorized into bacteria, fungi, yeasts, algae, and viruses.42,43 They are considered ‘nano-factories’ for nanoparticle synthesis due to their ability to accumulate and detoxify heavy metals through various types of reductive enzymes.42 In microbial synthesis of Fe0-based nanoparticles, a wide range of microorganisms are utilized, including bacteria, fungi, algae, and yeasts. These microorganisms share common characteristics beneficial for nanoparticle synthesis, such as the ability to produce reductive enzymes and extracellular metabolites that aid in metal reduction and stabilization.44 They also exhibit biocompatibility and environmental adaptability, allowing them to be effective and eco-friendly agents in synthesizing Fe0-based nanoparticles. Among these microorganisms, each group has unique attributes that further enhance the synthesis process. The suitability of Fe metal in the biosynthesis of NPs is attributed to its favorable properties, such as biocompatibility, availability, and the ability to facilitate various reduction and stabilization processes.45 Iron NPs (Fe-NPs) are of particular interest due to their magnetic properties, which can enhance the efficiency of catalytic processes and environmental applications. Furthermore, Fe is often used in microbial synthesis due to its relatively low cost and the ease with which microorganisms can mediate its reduction to nanoparticle form. In the study by Dlamini et al.,46 iron/copper (Fe/Cu) NPs, along with iron NPs (Fe-NPs) and copper NPs (Cu-NPs), were synthesized using a bioflocculant in an eco-friendly method. The Fe/Cu-NPs exhibited superior flocculation activity, achieving over 90% efficiency at a concentration of 0.2 mg mL−1, while an optimal concentration of 0.4 mg mL−1 was found for Fe-NPs. Additionally, these NPs demonstrated notable antimicrobial effects against both Gram-positive and Gram-negative bacteria. Safety tests indicated that the synthesized materials were non-toxic at low concentrations when tested on human embryonic (HEK293) and breast cancer (MCF7) cells, and they were also found to be biodegradable. In addition to bacteria, fungi, and yeasts are abundant and widely available natural sources that have attracted significant scientific interest. The synthesis of metallic NPs by fungi is facilitated by their ability to secrete molecules into the extracellular environment, reducing metal ions and stabilizing the resulting atom clusters. These processes are primarily driven by enzymes and other proteins. In the study by Alamilla-Martinez et al.,47 Fe-NPs were synthesized by Alternaria alternata MVSS-AH-5, which had been previously isolated. TEM analysis revealed that the nanoparticle sizes ranged from 20 to 80 nm. Rojas-Avelizapa et al.48 further demonstrated the ability of Alternaria alternata MVSS-AH-5 to produce siderophore organic acids. Siderophores are low-molecular-weight molecules known to be among the strongest Fe3+ chelators,49 with exceptionally high affinity. While their primary role is to scavenge iron, they also form complexes with other essential elements. Another study by Tsilo et al.50 explored the synthesis of Fe-NPs using a bioflocculant derived from the Pichia kudriavzevii yeast strain. These NPs were assessed for their flocculation and antimicrobial properties. The Fe-NPs showed significant antimicrobial activity against various Gram-positive and Gram-negative microorganisms. Another biological agent attracting scientific interest is algae, which are abundantly found in seaweed (Fig. 3a). Bensy et al.51 successfully synthesized iron NPs using a water extract from marine algae (Ulva lactuca) collected from the Tamilnadu coast, India. The green-synthesized NPs measured between 30–40 nm and exhibited strong biological activities. These NPs demonstrated anticancer effects against HeLa and Duke's Lymphoma of the Dog (DLD-1) cell lines and showed antimicrobial activity against S. aureus (24 ± 1 mm), E. coli (29 ± 1 mm), and S. typhimurium (31 ± 2 mm). Additional studies are summarized in Table 1.
 | ||
| Fig. 3 a) Nanoparticle synthesis using algae; b) intracellular biosynthesis of FeNPs in Pseudomonas putida;52 c) schematics of bacteria interaction with heavy metals and mechanisms of nanoparticle formation.53 | ||
| NPs | Species | Particles size | Characterization | Shape | Ref. |
|---|---|---|---|---|---|
| Bacteria | |||||
| Fe | Escherichia coli, Pseudomonas aeruginosa | 18 ± 2 nm | FESEM, EDX, TEM, and UV-vis | Spherical | Crespo et al., (2017)54 |
| Fe | SRB strain WCA1 | 21 nm | SEM–EDS, and XRD | Spherical | Das et al., 2017 (ref. 55) |
| Fe | Proteus mirabilis strain 10B | 11.7–60.8 nm | UV-vis, XRD, EDX, and TEM | Spherical | Zaki et al., 2019 (ref. 56) |
| Fe | Alcaligenes faecalis HCB2 | 50–60 nm | SEM, EDS, and FT-IR | Granular-like | Dlamini et al., 2020 (ref. 57) |
| Fe, Fe/Cu | Alcaligenes faecalis HCB2 | <100 nm | FT-IR, TGA, SEM, TEM, UV-vis, and XRD | Spherical | Dlamini et al., 2020 (ref. 46) |
| Fe | Pseudomonas putida | 1–4 nm | UV-vis, TEM, XRD, EDX, and VSM | Spherical | Zaki et al., 2021 (ref. 52) |
| Fungi | |||||
|---|---|---|---|---|---|
| Fe | Haemoglobin and myoglobin | 2–5 nm | SEM, TEM, EDX, and XRD | Crystals | Sayyad et al., 2012 (ref. 58) |
| Fe | Fusarium oxysporum | 20–40 nm | TEM and UV-vis | Spherical | Abdeen et al. 2013 (ref. 59) |
| Fe | Aspergillus oryzae | 10–24.6 nm | DLS, TEM, HR-TEM and EDS | Spherical | Tarafdar et al., 2013 (ref. 60) |
| Fe | Alternaria alternata | 5.4–12.1 nm | SEM, TEM, EDX, and UV-vis | Spherical | Yosri et al. 2015 (ref. 61) |
| Fe | Alternaria alternata | 20–80 nm | TEM and UV-vis | Semi-oval/spherical | Alamilla-Martínez et al., 2019 (ref. 47) |
| Fe | Neurospora crassa | 50 nm | SEM, EDX, XRD, FT-IR, and XPS | Granular | Li et al., 2020 (ref. 62) |
| Fe | Pleurotus florida | 100 nm | SEM and UV-vis | Spherical | Manikandan et al., 2021 (ref. 63) |
| Yeasts | |||||
|---|---|---|---|---|---|
| Fe | Pichia kudriavzevii | 58–79 nm | SEM–EDX, FT-IR, TEM, XRD, UV-vis, and TGA | Spherical | Tsilo et al., 2023 (ref. 50) |
| Algae | |||||
|---|---|---|---|---|---|
| nZVI | Chloroccum sp. | 20–50 nm | TEM, DLS, and FT-IR | Spherical | Subramaniyam et al., 2015 (ref. 64) |
| Fe | Dictyota dichotoma | 40–50 nm | SEM, FT-IR, and UV-vis | Cubic | Chandran et al., 2016 (ref. 65) |
| Fe | Chlorella sp. | 5–50 nm | FE-SEM and XPS | Spherical | Subramaniyam et al., 2016 (ref. 66) |
| nZVI | Blue green alga | 70–430 nm | SEM, UV-vis, and FT-IR | Agglomerated clusters | Karthika et al., 2019 (ref. 67) |
| Fe | Ulva lactuca | 20–40 nm | FT-IR and SEM | Spherical | Bensy et al., 2022 (ref. 51) |
Microorganisms have developed mechanisms for metal sequestration to transport ions and regulate their availability. In the simplest scenario, metal ion sequestration occurs through adsorption.53 The formation of NPs involves a complex mechanism, which Campaña et al.53 have described, outlining the interactions between bacteria and metal ions in nanoparticle formation (Fig. 3c). During adsorption, deprotonated functional groups on the cell surface, such as carboxyl, phosphonate, amine, and hydroxyl groups, resulting in a net negative charge. This attracts metal cations and leads to non-specific binding of the metal to the cell surface.68 The ultrastructural study of intracellular metal nanoparticle synthesis is often examined using Transmission Electron Microscopy (TEM). Nanoparticle formation is typically indicated by higher electron density, which appears darker in TEM images (Fig. 3b).
2.2. Plant extract
Plant extracts act as both reducing and stabilizing agents in nanoparticle synthesis. The key advantage of using plant extracts for metallic nanoparticle synthesis lies in their rapid reaction, mild conditions, and ability to prevent nanoparticle aggregation. The primary components in plant extracts include proteins, carbohydrates, vitamins, amino acids, flavonoids, alkaloids, terpenoids, ketones, tannins, aldehydes, amides, polysaccharides, polyphenols, carboxylic acids, and phenolic acids.69,70 Among these, polyphenols are crucial, functioning as chelating/reducing agents and as capping agents for the NPs. This protects the particles from oxidation and aggregation, enhancing their stability and longevity. The mechanism of chelate bond formation between metals and polyphenols, resulting in a protective coating over the metal core, was proposed by Changyuan Xiao et al. (2020),71 illustrated in Fig. 4. | ||
| Fig. 4 The chelation reaction may occur between epicatechin and Fe2+.71 | ||
Plants such as Camellia sinensis (green tea), Aloe vera, Azadirachta indica, and extracts from various fruits, roots, and barks have long been studied for their role as reducing and stabilizing agents in the synthesis of Fe-NPs (Fig. 5). Machado et al. (2013) investigated approximately 26 different plant species for their ability to produce extracts capable of reducing Fe(III) in aqueous solutions to form nZVI.72 This study demonstrated that oak leaves, pomegranate leaves, and green tea leaves yielded extracts with the richest compositions. TEM analysis confirmed that the nZVI particles produced had an average size of 10–20 nm. In Karavasilis's (2019) study, green tea, pomegranate leaf extracts, and gallic acid were employed to synthesize GT-nZVI, PG-nZVI, and GA-nZVI for treatment in aqueous solutions.73 The total polyphenol content (TPC) in the green tea and pomegranate extracts was relatively low, at 5.24 and 7.06 (g GAE L−1), respectively. The GT-nZVI and PG-nZVI NPs exhibited very small sizes, ranging from 2.45 nm to 19.7 nm. A more recent study by Xiyao Liu et al. (2024) highlighted the simplicity and high reproducibility of using plant-based Fe-NPs. TEM analysis revealed spherical Fe-NPs ranging from 24–52 nm synthesized using pruned tea leaf extract.74
In recent years, studies have emerged focusing on the green synthesis of bimetallic nanoparticles (BNPs). Combining two different metals exhibits multiple functions, enhanced selectivity, catalytic activity, and stability compared to monometallic NPs.75,76 Fe0-based BNPs utilize two metals, one in a zero-valent state (Fe0 → Fe2+) and a noble or transition metal (typically Pd, Pt, Ni, Cu, …), which is added to ZVI. This results in the formation of galvanic cells, accelerating the production of electrons and atomic hydrogen.77 A study on the one-step green synthesis of Fe/Ni BNPs using Eucalyptus leaf extract was conducted by Xiulan Weng et al. (2017).78 The research identified over eight compounds in the Eucalyptus leaf extract that could serve as reducing agents and protective coatings confirmed through GC-MS analysis. Ahmed K. Hassan's et al. (2022) synthesized Fe/Cu NPs using Ficus leaf extract as a catalyst for the degradation of pollutants via a Fenton-like mechanism.79 Another study by Inas A. Ahmed (2023) focused on the green synthesis of Fe/Cu NPs on an alginate limestone substrate (Fe/Cu/Alg-LS), which was utilized as an adsorbent to remove ciprofloxacin (CIP) and levofloxacin (LEV) from contaminated water. The maximum removal efficiencies for CIP (20 ppm) and LEV (10 ppm) were 97.3% and 100%, respectively. This material demonstrated significant potential as an adsorbent for water treatment applications.80 Additional studies on the green synthesis of Fe0-based NPs using plant extracts are listed in Table 2 as follow.
| NPs | Plant extract | Particles size | Functional group | Characterization | Shape | Ref. |
|---|---|---|---|---|---|---|
| Fe | 26 different tree species | 10–20 nm | NI | SEM and TEM | Spherical | Machado et al., 2013 (ref. 72) |
| Fe | Rosa damascene, Thymus vulgaris, and Urtica dioica | 100 nm | –OH stretch, C–H stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch, CH3 bend C stretch, CH3 bend |
SEM, FTIR and XRD | Different shapes | Fazlzadeh et al., 2017 (ref. 81) |
| Fe/Cu | Camellia sinensis | 60–120 nm | O–H stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch, C–O–C stretch O stretch, C–O–C stretch |
SEM, FTIR, and XRD | Spherical | Zhu et al. 2017 (ref. 82) |
| Fe/Ni | Eucalyptus | 20–50 nm | N–H stretch, C–H stretch, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretch, C–C stretch N stretch, C–C stretch |
FTIR, TGA, SEM, TEM, EDS, XRD and XPS | Sphericaland irregular | Weng et al. 2017 (ref. 78) |
| Fe/Ni | Punica granatum peel | 129 ± 7.23 nm | Phenolic and COOH functional groups | XRD, FE-SEM, FT-IR, and BET | Spherical | Ravikumar et al., 2018 (ref. 83) |
| AC-Fe/Zn | Citrus limon | 126 nm | O–H stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching in aromatic rings, C–CH3 deformation, Fe–O C stretching in aromatic rings, C–CH3 deformation, Fe–O |
FTIR, SEM, EDX, BET, and XRD | Spherical, agglomerated | Oruç et al. 2019 (ref. 84) |
| Fe/Pd | Eucalyptus | 30–60 nm | NI | TEM, EDS, XRD, and XPS | Spherical | Lin et al., 2020 (ref. 85) |
| Fe | Mangifera indica | 200–400 nm | C–H and CH2 bond vibration of aliphatic, C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic ring, Fe–O C aromatic ring, Fe–O |
SEM and FTIR | Spherical | Zulfikar et al., 2021 (ref. 86) |
| Fe/Ag | Salvia officinalis | 27 ± 7 nm | O–H stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch, C O stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch, C–H bending, C–O stretch C stretch, C–H bending, C–O stretch |
SEM, TEM, XRD, FTIR, EDX, and TGA | Nearly spherical | Malik et al. 2021 (ref. 87) |
| Fe/Ag | Gardenia jasminoides | 13 ± 6.3 nm | O–H stretch, C–H stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch, C–O stretch C stretch, C–O stretch |
TEM, UV-vis, and EDX | Spherical | Padilla-Cruz et al. 2021 (ref. 88) |
| Fe/Cu | Ixora finlaysoniana | 50–200 nm | C–H stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch, C–O–C C stretch, C–O–C |
UV-vis, FTIR, XRD, and SEM | Spherical | Younas et al. 2021 (ref. 89) |
| Fe | Camellia sinensis | NI | O–H stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the aromatic ring, C–O bond C of the aromatic ring, C–O bond |
UV-vis, FTIR, SEM and EDS | Amorphous forms | Eddy et al., 2022 (ref. 90) |
| Fe | Strychnos nux vomica (SN), Abrus precatorius (AP) | 3–32 nm (AP-Fe NPs), 2.1–5 nm (SN-Fe NPs) | O–H and N–H stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch, O–H bending, C–N stretch, N–H bending, Fe–C O stretch, O–H bending, C–N stretch, N–H bending, Fe–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, Fe–O stretch O, Fe–O stretch |
HR-TEM, UV-vis, and FTIR | Crystalline and amorphous forms | Puthukkara et al. 2022 (ref. 91) |
| Fe/Cu | Pomegranate peel | <100 nm | NI | NI | Irregularly spherical | Medina et al. 2020, Huang et al. 2023 (ref. 92 and 93) |
| Fe | Oolong tea, green tea, and black tea | 39.5–50.5 nm | NI | XRD, SEM, TEM, FTIR, BET, EDS | Spherical | Benković et al. 2023 (ref. 94) |
| Fe/Cu, Fe/Ag | Annona muricata | NI | NI | XRD, FTIR, UV-vis, FE-SEM, and EDX | NI | Raja Nandhini et al. 2023 (ref. 95) |
| nZVI-SCM | Tobacco leaf extract | <100 nm | O–H/N–H stretch, amide II band, amide I band, Fe–O | XPS, SEM, FT-IR and XRD | Quasi spherical | Shi et al. 2023 (ref. 96) |
| Fe | Pruned tea leaves | 17.9 nm | O–H stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic ring stretch, C C aromatic ring stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of polysaccharide and N–H stretch, Fe–O stretch O of polysaccharide and N–H stretch, Fe–O stretch |
XRD, FTIR, XPS, and TEM | Spherical | Liu et al., 2024 (ref. 74) |
| Fe/Cu, Fe/Ni | Camellia sinensis | 37–45 nm (Fe/Cu), 11–22 nm (Fe/Ni) | NI | SEM, TEM, XRD, EDS, and XPS | Spherical | Son et al. 2024 (ref. 97) |
| Fe/Mg | Azadirachta indica | 88 nm | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch, C O stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic, C–H bending C aromatic, C–H bending |
XRD, SEM, EDX and FTIR | Spherical | Qadir Ahmad et al. 2024 (ref. 98) |
Nano-scale Fe0-based NPs are the main nanomaterial used in environmental remediation processes. However, as with any remediation technique, the use of nanomaterials can also cause undesirable impacts on human health and the environment.99 Martins et al.100 conducted a comparative assessment of environmental and economic aspects involved in synthesizing nZVI via conventional methods using NaBH4versus approaches utilizing natural extracts. Their study applied life cycle assessment (LCA) as a strategy for ecodesign, focusing on the environmental performance of these two synthesis techniques and identifying critical stages in each. Findings demonstrated that the green synthesis method exhibited substantially lower environmental impacts than the conventional approach, with reductions of around 50% in the first scenario and about 62% in the second. In the green synthesis, the primary environmental burden stems from the extraction process, primarily influenced by electricity consumption. Conversely, the critical stage in the traditional method is associated with reactant usage, particularly the production of sodium borohydride. Economically, the conventional synthesis is significantly more costly, approximately eight times more than the green synthesis.
Although using plant extracts in nanoparticle synthesis offers environmental benefits, this method still presents several limitations that necessitate continued research. The biological reaction process inherently depends on natural compounds in the plant extracts, making it challenging to control the size and morphology of the NPs.101 Additionally, the purity of the resulting product is relatively low, as impurities from the plant extracts can negatively impact the catalytic activity and adsorption efficiency of the material.102 The varying composition of different plant extracts can also lead to inconsistencies in the structure and properties of the NPs,103 reducing their practical applications. Another drawback lies in reproducibility, as maintaining consistent conditions across multiple batches of synthesis with plant extracts is difficult, leading to variations in the final product. Nevertheless, considering the environmental impact of chemical synthesis, green methods present a promising, more sustainable alternative for future applications.
2.3. Agricultural and biowaste by-products
Biological and agricultural waste are abundant sources of phytochemicals, making them ideal for nanoparticle synthesis. These waste materials offer a sustainable and readily available resource for nanomaterial production while also helping to reduce environmental pollution.31 The synthesis process avoids the use of toxic chemicals, and the repurposing of waste plays a crucial role in promoting a circular economy. For example, one study demonstrated the synthesis of Fe-Zn activated carbon NPs from lemon pomace waste, which were applied as catalysts in water treatment by Fenton-like reaction.31,84 Similarly, waste silkworm cocoons, a by-product of the protein-rich silk industry, were used to synthesize Fe/Cu alloy NPs.104 Silkworm cocoon is a by-product of the silk industry. The cocoon is rich in protein, and its extract serves as a reducing and stabilizing agent. The protein extract acted as both the reducing and stabilizing agent. These NPs exhibited notable antimicrobial properties against selected bacterial strains and fungal pathogens, suggesting their potential use as protective agents in cement mortars. By inhibiting the growth of harmful organisms, they may also help prevent premature deterioration of the material. Palm waste was studied by Tesnim et al. for extraction and nanoparticle synthesis.105 Several other studies following this trend are presented in Table 3.| NPs | Precursor | Particles size | Functional group | Characterization | Shape | Ref. |
|---|---|---|---|---|---|---|
| ARH-nZVI | Ash rice husk | 10–15 nm | O–H from alcohol, H–O–H stretch, C–H bending, Si–O stretch | SEM, TEM, UV-vis, FTIR, and EDX | Spherical clusters | Dada et al., 2014 (ref. 106) |
| Fe | Waste tea | 98 nm | –OH groups stretch, –S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group stretch, –C O group stretch, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch C stretch |
SEM, EDX, FTIR and BSE | Slight clumping form | Gautam et al., 2018 (ref. 107) |
| Fe/Cu | Waste silkworm cocoon | 50–60 nm | C–N stretch, C–N triple bond stretch and C–H stretch | UV-vis, FE-SEM, EDAX, XRD, and FTIR | Spherical | Dhruval et al., 2020 (ref. 104) |
| nZVI | Green tea bio-waste | 100 nm | Silicate ions, Fe–O stretch | FTIR, SEM, XRD, and BET | Cubical | Kadhum et al., 2021 (ref. 108) |
| nZVI-LBC | Lemon residues | 50 nm | OH vibration stretch, C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the aromatic ring, Si–O, C–O of ester, FeOOH C of the aromatic ring, Si–O, C–O of ester, FeOOH |
FTIR, XRD, TEM, XPS, VSM, and BET | Spherical | El-Monaem et al., 2022 (ref. 109) |
| Fe | Onion, potato, tea and moringa | NI | O–H stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch, C O stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–O C, C–O |
FTIR, XRD, XRF, and EDX | NI | Ahmed et al., 2023 (ref. 110) |
| nZVI-NC | Palm waste extract | 91 nm | O–H stretch, C–C bonds in the phenolic groups, C–O–C stretch, Fe–O stretch | SEM, TEM, BET, FTIR, DLS and XRD | Spherical | Tesnim et al., 2023 (ref. 111) |
| nZVI | Palm petiole extract | 45–90 nm | O–H stretch, C–O, C–O–H, C–O–C, Fe–O stretch | UV-vis, SEM, TEM, XRD, FTIR, and BET | Spherical | Tesnim et al., 2024 (ref. 105) |
| GO–nZVI | Sugarcane bagasse | NI | NI | FESEM, HR-TEM, EDX, and XRD | NI | Jha et al., 2024 (ref. 112) |
2.4. Comparison of chemically and biologically synthesized Fe0-based NPs
Each approach has distinct advantages and limitations; chemically synthesized Fe0 nanoparticles offer precise control over particle size and high reaction efficiency, while biologically synthesized nanoparticles are more environmentally sustainable, utilizing natural biological agents. Although chemically synthesized nanoparticles have shown promising results, their safety is often compromised due to the use of toxic reagents in the synthesis process. For instance, sodium borohydride (NaBH4), a widely used reducing agent in metal NPs synthesis,22,113,114 has been classified as harmful if inhaled or absorbed through the skin.115 The use of such toxic chemicals is common in synthesis since they tend to drive reactions that are favorable in terms of kinetics or thermodynamics.34 While this can lead to high yield and reproducibility in metal NPs production, the chemical processes involved frequently contribute to an elevated toxicity profile of the final products, posing risks to both health and the environment. To address these issues, green nanotechnology prioritizes the use of environmentally benign agents and highlights biological materials as sustainable sources for reagents in nanoparticle synthesis. Several studies have been conducted to compare the effectiveness of these two synthesis methods. Ravikumar et al.116 report a comparative study of Cr(VI) removal using biologically synthesized nano zero-valent iron (BS-nZVI) using fresh neem leaves and chemically synthesized nZVI (CS-nZVI) using NaBH4, both immobilized in calcium alginate beads. Under the optimized conditions concentration of nZVI = 1000 mg L−1, contact time = ∼80 min, and initial concentration of Cr(VI) = 10 mg L−1, the Cr(VI) removal by the immobilized BS-nZVI and CS-nZVI alginate beads was 80.04% and 81.08%, respectively. A study by Hammad et al.117 demonstrated that iron nanoparticles synthesized using Chlorella vulgaris microalga extract achieved a particle size of 4.8 nm, whereas those synthesized chemically had a particle size of 9.07 nm. The adsorption data for methylene blue (MB) for both materials followed the Langmuir isotherm model, with maximum adsorption capacities (qmax) of 29.14 mg g−1 and 19.08 mg g−1, respectively. This study highlights the superior adsorption capacity of biologically synthesized nanoparticles. Additionally, several studies have provided specific assessments of the environmental impact of Fe0-based NPs synthesized via these two methods. Bhuvaneshwari et al.118 examined their effects on algae and crustaceans to evaluate potential toxicity and nutrient transfer within the aquatic food chain. By evaluating factors such as ROS generation, oxidative enzyme activity, membrane damage, and biouptake, the study reveals significant differences in toxicity between CS and BS-nZVI. Daphnia showed greater sensitivity to CS-nZVI, while algae exhibited toxicity differences only at higher nZVI concentrations.In conclusion, both chemically and biologically synthesized Fe0-based NPs offer distinct advantages and limitations. Chemically synthesized NPs provide better control over size and uniformity, often leading to higher catalytic efficiency; however, they involve toxic reagents that may pose environmental and health risks. In contrast, biologically synthesized NPs are produced through environmentally friendly processes using natural reducing agents, which often enhance their stability and compatibility in ecological applications. The choice between these synthesis methods should be guided by the specific requirements of the application, balancing factors such as environmental safety, cost, and performance.
3. Trends in the application of biological NPs based on Fe0 to treat chlorine organic compound
3.1. Targeted pollutant removal
| 2Fe0 + R–Cl + 3H2O → 2Fe2+ + R–H + 3OH− + H2 + Cl− |
Additionally, the incorporation of a second metal (commonly transition metals such as Pd, Pt, Ni, or Cu) into nZVI results in the formation of galvanic cells, which accelerate the production of atomic hydrogen. This atomic hydrogen is adsorbed onto the reducing catalyst, forming metal hydrides (M–H) that act to dehalogenate chlorinated organic compounds. The mechanism for the degradation of chlorinated organic compounds by the Fe/Me bimetallic catalytic system can be described in detail in the study by Liu et al.,122 as follows:
| Fe0 + 2H+ → Fe2+ + H2 |
| M (M = Pd, Ni, Cu, …) + H2 → M*2H· |
| M + RCl → M⋯R⋯Cl |
| M*2H· + M⋯R⋯Cl → R–H + H+ + Cl− + 2M |
In a pioneering study by Wang et al. (1997),123 the complete degradation of PCBs Aroclor 1254 was demonstrated using nZVI and Pd/Fe NPs synthesized via borohydride reduction. Since then, this method has become widely adopted for the synthesis of highly active Fe0-based NPs. Numerous studies have emerged aiming to enhance the application of nano Fe0 by modifying it with surfactants,124,125 anchoring nano particles onto substrates to form composites,126,127 combining it with other metals to create multi-metal systems,128–130 and developing biologically synthesized nano Fe0.
In recent years, the use of biologically synthesized nanomaterials for the treatment of Cl-POPs has garnered significant attention from the scientific community, with promising preliminary results indicating the potential of this approach. For instance, a 2019 study led by Yuanqiong Lin explored the green synthesis of Fe/Ni BNPs using Eucalyptus leaf extracts. These BNPs were applied for the simultaneous removal of triclosan (TCS) and Cu(II) from aqueous solutions, achieving notable removal efficiencies of 75.8% for TCS and 44.1% for Cu(II). This study demonstrated the versatility of Fe/Ni BNPs in treating complex pollutant systems under a variety of conditions.131 The mechanisms and effectiveness of this removal process are illustrated in Fig. 6. Another study highlighted Pomegranate peel extracts for the green synthesis of nZVI, which exhibited high efficacy in treating lindane (γ-HCH).132 In liquid medium, 0.1 g L−1 of FeNPs successfully degraded approximately 99% of lindane within 24 hours. Moreover, treatment with the FeNPs significantly reduced lindane's cytotoxicity, suggesting its breakdown into less harmful and simpler by-products. Within the group of Cl-POPs, compounds such as PCBs and tetrachlorodibenzo-p-dioxin (TCDD), particularly 2,3,7,8-TCDD, are regarded as highly toxic. These substances pose severe health risks, including links to cancer, immune system disorders, and reproductive issues.121 The study conducted by Tlčíková et al. presented a solution for removing PCBs from the environment by employing biologically synthesized iron NPs derived from plant matrices. This approach was coupled with the use of bacteria, specifically Stenotrophomonas maltophilia (SM) and Ochrobactrum anthropi (OA), both of which were isolated from a PCB-contaminated site, the Strážsky canal in Slovakia.133 This study also addresses the optimization of parameters for using nZVI in PCBs degradation, including reaction pH, oxygen requirements, and nZVI dosage. Experimental analyses revealed that sequential nanobiodegradation proved to be the most effective for PCBs degradation among the various approaches tested. Specifically, the combination of Fe-Bio from sage and Stenotrophomonas maltophilia (SM) resulted in a 75% degradation of PCBs, while Fe-Bio synthesized from Garcinia atroviridis (GA) at 0.3 g L−1, combined with Ochrobactrum anthropi (OA), achieved a 92% PCB degradation rate. Recently, Toan et al. proposed a technological solution combining green-synthesized BNPs (Fe/Cu and Fe/Ni) using Camellia sinensis with 11 microbial strains to reduce 2,3,7,8-TCDD concentrations in soil at the Phu Cat airport, Vietnam.134 Approximately 90% of the 2,3,7,8-TCDD was successfully treated in both laboratory and pilot-scale experiments. Table 4 summarizes several studies that highlight the potential of bio-nano Fe0-based systems for the treatment of Cl-POPs.
 | ||
| Fig. 6 Mechanism and efficiency of TCS and Cu(II) elimination by Fe/Ni BNPs.131 | ||
| NPs | Precursor | POPs | Condition | Removal efficiency | Ref. |
|---|---|---|---|---|---|
| Fe/Ni | Eucalyptus leaves | Triclosan | pH = 2 ÷ 6, [TCS] = 10 mg L−1, T = 303 K, [Fe/Ni] = 1.5 g L−1 | 75.8–96.6% | Lin et al., 2019 (ref. 131) |
| G-Fe/Co | Ginkgo biloba L. extract | Triclosan | pH = 6.92, 5% Co wt, [NPs] = 0.56 g L−1, t = 5 min | 62.7–92.8% | Gao et al., 2019 (ref. 135) |
| Fe | Euphorbia cochinchinensis leaves | 2,4-Dichlorophenol (2,4-DCP) | pH = 3 ÷ 6, [2,4-DCP] = 50 mg L−1, T = 303 K, rotative speed: 250 r min−1, [Fe NPs] = 1 g L−1 | >83.5% | Gan et al., 2018 (ref. 136) |
| Fe | Euphorbia cochinchinensis leaves | 2,4-DCP | pH = 6.8, [2,4-DCP] = 50 mg L−1, T = 313 K, rotative speed: 250 r min−1, [Fe NPs] = 1 g L−1 | 51.9% | Guo et al., 2016 (ref. 137) |
| Fe | Yeast extract | Dichlorvos | [Fe NPs] = 2000 mg L−1, VH2O2 = 1 mL | ∼93.6% | Mehrotra et al., 2017 (ref. 138) |
| Fe | Neem leaf extract | DDT | pH = 3, [DDT] = 500 ppm, t = 120 min | 88–92% | Pushkar et al., 2019 (ref. 139) |
| nZVI | Pomegranate peel extracts | Lindane | [Fe NPs] = 0.1 g L−1, t = 24 h | ∼99% | Ningthoujam et al., 2023 (ref. 132) |
| Fe | Camellia sinensis extracts | PCBs | Fe-BNPs combined with Ochrobactrum anthropi bacteria | 92% | Tlčíková et al., 2024 (ref. 133) |
| Fe/Cu, Fe/Ni | Camellia sinensis extracts | 2,3,7,8-TCDD | [TCDD] = 5000 ppt, t = 30 days, pH = 5–7, [NPs] ≥ 40 ppm, combined with 11 microbial strains | 90% | Toan et al., 2024 (ref. 134) |
In the study by Smuleac et al., a novel method was developed to prepare membranes incorporating reactive NPs (Fe and Fe/Pd) immobilized within a polymer film, specifically polyacrylic acid (PAA)-coated polyvinylidene fluoride (PVDF) membranes.145 Instead of the commonly used sodium borohydride, a non-toxic “green” reducing agent, green tea extract (Camellia sinensis), was employed for nanoparticle synthesis. These membrane-supported NPs were effectively utilized for the degradation of TCE, a prevalent and significant pollutant. The degradation rate of TCE increased linearly with the amount of Fe immobilized on the membrane, with a surface-normalized rate constant k(SA) of 0.005 L m−2 h−1. Incorporating a second catalytic metal, Pd, to form a bimetallic Fe/Pd system enhanced the k(SA) value to 0.008 L m−2 h−1. The authors suggested that dechlorination could be carried out in a convective mode using a pump-and-treat approach or in a batch mode, where the membrane-immobilized NPs could be injected underground. Qianwei Li et al.62 developed a straightforward fungal biomineralization method to synthesize nZVI with a distinct N-doped branching structure (Fig. 7). This innovative approach demonstrated exceptional stability and facilitated efficient degradation of carbon tetrachloride (CCl4) in aqueous solutions. The ureolytic fungus Neurospora crassa was cultured in a medium containing Fe2+ and urea, which precipitated iron carbonate biominerals. The resulting “iron coral” composite was characterized as a blend of zero-valent iron (Fe0), carbon iron (Fe1.91C0.09), and iron oxide (Fe3O4). The porous branching hyphal framework enhanced CCl4 capture, while the N-doped sites likely accelerated electron transfer between CCl4 and nZVI. Geochemical simulations supported the biomineral formation, and chemical analyses confirmed its significant efficacy in degrading CCl4. In actual field conditions, some commonly encountered contaminants, such as dichloromethane (DCM) and 1,2-dichloroethane (1,2-DCA), exhibit minimal reactivity with nZVI. Salom et al.146 explored the feasibility of integrating anaerobic dechlorinating bacteria, specifically Dehalobacterium and Dehalogenimonas, with nZVI as a combined treatment approach for detoxifying chlorinated methanes (e.g., chloroform-CF and DCM) and 1,2-DCA. The authors reported that the recovery of dechlorinating activity in Dehalobacterium and Dehalogenimonas indicates that combining nZVI with bioremediation techniques could be effective under field conditions, where dilution processes may mitigate the impact of potentially inhibitory soluble compounds. Additional studies on this topic are summarized in Table 5.
 | ||
| Fig. 7 Degradation of CCl4 by nZVI with a distinct N-doped branching structure.62 | ||
| NPs | Precursor | VOCs | Condition | Removal efficiency | Ref. |
|---|---|---|---|---|---|
| Fe, Fe/Pd | Green tea extract | TCE | pH = 7, t = 23 h, [TCE] = 30 mg L−1, 8 mg Fe (3% wt Pd) | ∼60% | Smuleac et al., 2011 (ref. 145) |
| nZVI | Green tea, black tea, oolong tea | Chlorobenzene (CB) | pH = 3, [GT-Fe NPs] = 0.6 g L−1, [CB] = 50 mg L−1, T = 313 K | 81% | Kuang et al., 2013 (ref. 147) |
| nZVI | Virginia creeper extract | VOCl |
t = 6 h, [nZVI] = 2500–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g dm−3 000 g dm−3 |
100% | Kozma et al., 2015 (ref. 148) |
| nZVI | Neurospora crassa | Carbon tetrachloride (CCl4) | — | ∼75% | Li et al., 2020 (ref. 62) |
| nZVI-PS | Tea polyphenol extract | 1,2-Dichlorobenzene | [1,2-DCB] = 28.6 mg kg−1, [nZVI] = 67.2 mg L−1, [PS] = 1.2 mmol L−1, pH = 7.5 | 61.3% | Yan et al., 2022 (ref. 149) |
| nZVI | Anaerobic dechlorinating bacteria | Chloroform, DCM, 1,2-dichloroethane | [nZVI] = 1 gL−1, pH = 7 | 100% | Salom et al., 2023 (ref. 146) |
According to Zhan et al.,150 the bioprecipitation synthesis of sulfidated nZVI particles can be considered a “green synthesis” process. However, using NaBH4 in the prior synthesis of nZVI particles introduces an expensive and toxic chemical, leading to potential secondary pollution for environmental. Therefore, we adopt the “semi-green” synthesis concept to describe this method. Studies on Fe0-based nanomaterials synthesized through semi-green processes have also demonstrated their effectiveness in treating POPs and VOCs. To remediate organic contaminants in natural waters and soils, ZVI-included biochar was synthesized via slow pyrolysis (Seok-Young Oh et al.151). The removal of 2,4-dinitrotoluene (DNT) and 2,4-DCP from water was achieved via sorption onto Fe0-embedded biochar. Results indicated that after 24 h, only 40% of DCP was removed from the solution. Regarding the sorbed amount, the total removal of DCP by Fe0 alone was just 23% in the same period. However, the removal efficiency of DCP increased with higher carbon content in the Fe0-embedded biochar. In another study, Junyi Huang et al.152 successfully synthesized an nZVI composite supported on zeolite through ion exchange and borohydride reduction methods, specifically to study its ability to activate persulfate (PS) for the degradation of TCE. The Z/nZVI composite exhibited excellent PS activation capacity (1.5 mM) and achieved 98.8% TCE degradation at pH = 7 within 120 min.
To assess the treatment efficiency of Fe0-based nanoparticles synthesized through chemical and biological methods, various studies have conducted comparative analyses on the removal of chlorinated organic compounds. Variations in synthesis techniques markedly influence reaction efficiency, degradation kinetics, and nanoparticle stability in pollutant exposure scenarios. Table 6 presents a comparison of treatment performances for different chlorinated organic compounds.
| NPs | Precursor | Pollutants | Concentration | Effects | Ref. |
|---|---|---|---|---|---|
| Fe/Ni | Eucalyptus leaves | Triclosan | [TCS] = 10 mg L−1, [Fe/Ni] = 1.5 g L−1 | 75.8–96.6% | Lin et al., 2019 (ref. 131) |
| Fe/Ni | NaBH4 | Triclosan | [TCS] = 10 mg L−1, [Fe/Ni] = 2.2 g L−1 | 63.9% | Ren et al., 2022 (ref. 153) |
| Fe | NaBH4 | Triclosan | [TCS] = 10 mg L−1, [Fe] = 2.0 g L−1 | 55.4% | |
| Fe | Euphorbia cochinchinensis leaves | 2,4-DCP | [2,4-DCP] = 50 mg L−1, [Fe NPs] = 1.0 g L−1 | >83.5% | Gan et al., 2018 (ref. 136) |
| S-nZVI | NaBH4 | 2,4-DCP | [2,4-DCP] = 20 mg L−1, S/nZVI = 0.129 (mole ratio), [S-nZVI] = 2.0 g L−1 | 91.9% | Yajun Li et al., 2022 (ref. 154) |
| Fe, Fe/Pd | Green tea extract | TCE | [TCE] = 30 mg L−1, [Fe] = 0.4 g L−1 (3% wt Pd) | ∼60% | Smuleac et al., 2011 (ref. 145) |
| Fe | NaBH4 | TCE | [TCE] = 0.15 mM, [Fe] = 5 g L−1 | >95% | Ahn et al., 2014 (ref. 155) |
| CaCO3@nZVI | KBH4 | TCE | [CaCO3@nZVI] = 0.24 g L−1, [TCE] = 25 mg L−1 | >80% | Zheng et al., 2023 (ref. 156) |
| Fe | Green tea, black tea, oolong tea | CB | [Fe] = 0.6 g L−1, [CB] = 50 mg L−1 | 81% | Kuang et al., 2013 (ref. 147) |
| Fe | NaBH4 | Hexachloro benzene (HCB) | [HCB] = 2.0–2.8 mg g−1, [Fe] = 1 mg g−1 | 80% | Wang et al., 2023 (ref. 157) |
The application of biogenic Fe0 NPs in the remediation of VOCs and POPs presents a promising and sustainable approach. These NPs offer significant advantages in terms of reactivity, environmental compatibility, and scalability. Beyond Cl-VOCs and Cl-POPs, Fe0-based nanoparticles have also been recognized as a suitable option for the treatment and degradation of other pollutants, such as antibiotics and per- and polyfluoroalkyl substances (PFAS). In general, the mechanism of antibiotic degradation by Fe0 mainly includes adsorption and reduction, while promoting the biodegradation of antibiotics by affecting the microbial community. Fe0 can also be combined with persulfates to degrade antibiotics through advanced oxidation processes.158 Specifically, the structure and chemical properties of Fe0 make it highly compatible with interactions with the resilient bonds in PFAS, resulting in high treatment efficiency.159 However, further practical and large-scale studies are needed to comprehensively assess the material's capabilities in complex environmental conditions.
While challenges remain, particularly in optimizing the synthesis and enhancing these nanomaterials' long-term stability and reactivity, their potential to mitigate environmental pollution, especially for persistent organic compounds, is undeniable. Continued research into the synergistic effects and biocompatibility of biogenic Fe0 NPs will be crucial in advancing their practical deployment in environmental cleanup efforts.
3.2. Enhanced catalytic activity
 | ||
| Fig. 8 Schematic diagram of organic pollutants degradation by nZVI/BC-AOPs.161 | ||
In Xiangyu Wang's study, a cost-effective, eco-friendly, robust, and efficient synthesis method was established for nZVI-based composites.162 The nZVI@chitin-modified ZSM-5 (nZVI@C-ZSM) composite was synthesized through a simple and green approach, where nZVI was loaded onto alkali-modified ZSM-5 molecular sieves, using chitin as a surfactant and binder. The nZVI@C-ZSM material has a surface area of approximately 52.22 m2 g−1 and a pore size of 18.48 nm. The presence of chitin on nZVI@C-ZSM results in more prominent surface wrinkles, while the coating of fine particles on nZVI@C-ZSM is significantly reduced due to the protective effect of chitosan. This composite demonstrated remarkable performance in the removal of tetracycline, achieving a removal efficiency of 97.72% within 60 min. Compared to pristine nZVI, the nZVI@C-ZSM composite exhibited twice the removal efficiency, which suggests that the composite significantly improved the dispersion and stability of nZVI. This improvement allowed for more reactive sites to generate reactive oxygen species (ROS), greatly enhancing the catalytic activity and durability while minimizing the risk of secondary pollution162 (Fig. 9).
 | ||
| Fig. 9 Proposed synergistic mechanism of the components in nZVI@C-ZSM.162 | ||
Bimetallization strategies lead to materials that combine two different metals exhibiting enhanced and modified properties with respect to a monometallic system. BNPs based on Fe0 offer significant advantages over their monometallic counterparts due to the synergistic effects arising from electronic, mechanical, and structural modifications.34 Wang et al.163 investigated the debromination of BDE-47 using six bimetallic Fe/M systems (M: Cu, Ni, Pd, Ag, Pt, Au). They found that the additive metals alone were inactive without Fe, highlighting iron's critical reductive role. Debromination rates followed the order: Fe/Pd > Fe/Ag > Fe/Cu > Fe/Ni > Fe/Au > Fe/Pt ≈ n-ZVI. Furthermore, each system exhibited distinct mechanisms-Fe/Ni, Fe/Pd, and Fe/Pt employed H-transfer, while Fe/Ag followed electron-transfer, and Fe/Cu and Fe/Au used both pathways, producing different final products. Fang et al.164 reported that S-nZVI particles were synthesized from steel pickling waste liquor via chemical deposition and were used to remove BDE209 in a water/tetrahydrofuran (4/6, v/v) solution. The crystalline structure of S-nZVI differed from conventional nZVI, though the BET surface area of S-nZVI remained comparable to that of nZVI. The authors concluded that, by considering both the cost and the importance of reclaiming iron resources, S-nZVI offered better compensation than other metals. S-nZVI has garnered increasing attention due to its simple production process, high reactivity toward a variety of contaminants, including TCE, diclofenac, cadmium, and chromate, and especially its selectivity in water pollution treatment. S-nZVI nanoparticles with an optimal S/Fe molar ratio demonstrate significantly higher contaminant removal efficiency than unmodified nZVI.165 Qu et al.166 successfully synthesized S-nZVI on a hydrophilic porous activated carbon substrate (S-nZVI@HPAC) using green tea extract as a reducing agent. The S-nZVI@HPAC with an atomic S/Fe ratio of 0.16 was found to enhance Pb(II) uptake through synergistic effects of electrostatic attraction, chemical precipitation, complexation, and reduction. Despite these advancements, S-nZVI synthesis remains predominantly reliant on chemical methods utilizing NaBH4 as a reducing agent. A series of studies167–170 indicate that chemically synthesized S-nZVI achieves high contaminant removal efficiencies, making it a viable method for practical applications, though potential contamination risks during production remain a consideration.
Synergistic effects significantly enhance the performance of materials by combining different components to achieve superior properties compared to individual elements (monometallic). These effects can lead to improved catalytic activity, increased stability, and greater efficiency in various applications. By leveraging the interactions between different substances, synergistic effects facilitate faster reaction rates, higher selectivity, and more effective treatment of complex pollutants. This approach optimizes material performance and supports the development of more sustainable and versatile technologies for diverse industrial and environmental applications.
 | ||
| Fig. 10 The potential of integrating polymers with green-synthesized nanomaterials in addressing environmental challenges.172 | ||
4. Challenges and future prospective
Although laboratory-scale studies have demonstrated potential, scaling up the biosynthesis of Fe0 NPs for real-world applications presents numerous challenges. Consistently controlling the size, shape, and reactivity of NPs at a larger scale remains difficult. The biosynthesis process also tends to vary depending on the biological agent used, which can lead to changes in the properties of the final product. Despite advancements, the precise mechanisms underlying interactions between NPs and chlorine compounds are not yet fully understood. Identifying the key factors influencing catalytic efficiency, especially within complex environmental matrices, is crucial for optimizing their performance. Consequently, there are still limited studies worldwide on practical applications and large-scale trials using biosynthesized Fe0 NPs to treat chlorinated organic compounds in the environment.Future research should focus on strategies to enhance the stability and longevity of Fe0-based NPs, refine biological agents to achieve desired synthesis efficiencies, and reduce the costs associated with nanoparticle synthesis and the treatment of chlorinated organic compounds and other pollutants. The reaction mechanisms, degradation pathways, and environmental factor impacts need further investigation through advanced modeling techniques to optimize catalytic performance under various conditions. Translating laboratory results to field applications and large-scale pilot studies is crucial to demonstrate the feasibility of biosynthesized Fe0 NPs. These studies will provide valuable insights into practical challenges such as particle dispersion, reactivity, and recovery in different environmental contexts. Developing a standardized technological process for applying biosynthesized Fe0-based NPs will also be a significant future prospect. Establishing a clear and scalable protocol for synthesizing, stabilizing, applying, and recovering these NPs under real-world conditions could bridge the gap between laboratory research and practical environmental solutions.
5. Conclusion
This review has highlighted the significant progress and emerging trends in developing and applying biologically synthesized Fe0-based NPs for treating chlorinated organic compounds in soil and water. The various synthesis methods, ranging from microbial, plant, and biological waste sources to industrial and agricultural by-products demonstrate the versatility and sustainability of biologically synthesized Fe0-based NPs. Recent advancements have shown promising results in enhancing the efficiency of these NPs in environmental remediation, emphasizing their potential for large-scale applications. In conclusion, biologically synthesized Fe0-based NPs represent a valuable tool in mitigating the environmental and health impacts of chlorinated organic compounds. By addressing the current gaps and pursuing targeted research, these NPs have the potential to contribute significantly to sustainable and effective environmental remediation strategies.Data availability
No new experimental data were generated or analyzed in this review article. All data discussed in this manuscript are derived from previously published sources, which are appropriately cited in the reference section. For any inquiries regarding data access or additional information, the corresponding author can be contacted.Author contributions
The authors collectively contributed to the conceptualization, data analysis, and writing of this manuscript. Specific roles included statistics, document collection, data collection, and manuscript revision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the scientific and technical assistance facilities at the Institute of New Technology/Vietnam.References
- P. Jangid and M. Prabhu Inbaraj, Applications of nanomaterials in wastewater treatment, Mater. Today: Proc., 2021, 43, 2877–2881 CAS.
- O. Adenuga and A. Oduroye, Nanotechnology Applications in Water Purification, 2024 Search PubMed.
- H. Lu, J. Wang, M. Stoller, T. Wang, Y. Bao and H. Hao, An Overview of Nanomaterials for Water and Wastewater Treatment, 2016, p. 4964828 Search PubMed.
- B. Kalandadze and L. Matchavariani, Soil Pollution, The Soils of Georgia, 2019, pp. 153–166 Search PubMed.
- T. Svensson, H. Kylin, M. Montelius, P. Sandén and D. Bastviken, Chlorine cycling and the fate of Cl in terrestrial environments, Environ. Sci. Pollut. Res., 2021, 28, 7691–7709 CrossRef CAS PubMed.
- A. Mandal, P. Senthil Kumar, C. S. Poorva, L. Srinivasa Raju, S. R. Balasubramani and G. Rangasamy, Research progress of persistent organic pollutants in water: classification, sources, potential risks, and treatment approaches, Water Pract. Technol., 2024, 19, 937–959 CrossRef.
- Z. Chen, Z. Chen, Y. Li, R. Zhang, Y. Liu, A. Hui, W. Cao, J. Liu, H. Bai and J. Song, A review on remediation of chlorinated organic contaminants in soils by thermal desorption, J. Ind. Eng. Chem., 2024, 133, 112–121 CrossRef CAS.
- M. Zaynab, M. Fatima, Y. Sharif, K. Sughra, M. Sajid, K. A. Khan, A. H. Sneharani and S. Li, Health and environmental effects of silent killers Organochlorine pesticides and polychlorinated biphenyl, J. King Saud Univ., Sci., 2021, 33, 101511 CrossRef.
- I. Z. Ahmad, A. Ahmad, H. Tabassum and M. Kuddus, in Handbook of Ecomaterials, ed. L. M. T. Martínez, O. V. Kharissova and B. I. Kharisov, Springer International Publishing, Cham, 2019, pp. 275–299 Search PubMed.
- H. Tabassum and I. Z. Ahmad, Applications of metallic nanomaterials for the treatment of water, Lett. Appl. Microbiol., 2022, 75, 731–743 CrossRef CAS PubMed.
- X. Chen, X. Yao, C. Yu, X. Su, C. Shen, C. Chen, R. Huang and X. Xu, Hydrodechlorination of polychlorinated biphenyls in contaminated soil from an e-waste recycling area, using nanoscale zerovalent iron and Pd/Fe bimetallic nanoparticles, Environ. Sci. Pollut. Res., 2014, 21, 5201–5210 CrossRef CAS PubMed.
- H. Gomes, G. Fan, L. Ottosen, C. Dias-Ferreira and A. Ribeiro, Nanoremediation Coupled to Electrokinetics for PCB Removal from Soil, Electrokinetics Across Disciplines and Continents, 2015, pp. 331–350 Search PubMed.
- A. Ševců, Y. S. El-Temsah, J. Filip, E. J. Joner, K. Bobčíková and M. Černík, Zero-valent iron particles for PCB degradation and an evaluation of their effects on bacteria, plants, and soil organisms, Environ. Sci. Pollut. Res., 2017, 24, 21191–21202 CrossRef PubMed.
- H. Kim, H. J. Hong, J. Jung, S. H. Kim and J. W. Yang, Degradation of trichloroethylene (TCE) by nanoscale zero-valent iron (nZVI) immobilized in alginate bead, J. Hazard. Mater., 2010, 176, 1038–1043 CrossRef CAS PubMed.
- A. Dumestre, A. Joubert, J. Dumont, M. Cardetti, P. Kvapil, J.-Y. Bottero, J. Rose, N. Kumar, L. Malleret, D. Kaifas, L. Chancerelle, F. Quiot, F. Rebischung, P. Roudier and P. Doumenq, TCE and CrVI source zone treatment by innovative formulation of NZVI, International UFZ-Deltares Conference on Groundwater-Soil-Systems and Water Resource Management, 2013, vol. 12 Search PubMed.
- B. Calderon, L. Lundin, I. Aracil and A. Fullana, Study of the presence of PCDDs/PCDFs on zero-valent iron nanoparticles, Chemosphere, 2017, 169, 361–368 CrossRef CAS PubMed.
- X. Zhao, W. Liu, Z. Cai, B. Han, T. Qian and D. Zhao, An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation, Water Res., 2016, 100, 245–266 CrossRef CAS PubMed.
- P. Wang, J. Hu, T. Liu, G. Han, W.-M. Ma and J. Li, New insights into ball-milled zero-valent iron composites for pollution remediation: An overview, J. Cleaner Prod., 2023, 385, 135513 CrossRef CAS.
- D. Ai, T. Wei, Y. Meng, X. Chen and B. Wang, Ball milling sulfur-doped nano zero-valent iron @biochar composite for the efficient removal of phosphorus from water: Performance and mechanisms, Bioresour. Technol., 2022, 357, 127316 CrossRef CAS PubMed.
- R. Lahoz, E. Natividad, Á. Mayoral, C. Rentenberger, D. Díaz-Fernández, E. J. Félix, L. Soriano, W. Kautek and O. Bomati-Miguel, Pursuit of optimal synthetic conditions for obtaining colloidal zero-valent iron nanoparticles by scanning pulsed laser ablation in liquids, J. Ind. Eng. Chem., 2020, 81, 340–351 CrossRef CAS.
- T. Zhang, Y. Zhao, S. Kang, H. Bai, W. Gu, D. Fang, S. Komarneni and Q. Zhang, Mechanical activation of zero-valent iron (ZVI) in the presence of CaCO3: Improved reactivity of ZVI for enhancing As(III) removal from water, J. Cleaner Prod., 2021, 286, 124926 CrossRef CAS.
- L. Yin, L. Liu, S. Lin, G. Owens and Z. Chen, Synthesis and characterization of Nanoscale Zero-Valent Iron (nZVI) as an adsorbent for the simultaneous removal of As(III) and As(V) from groundwater, J. Water Process Eng., 2022, 47, 102677 CrossRef.
- T. Pasinszki and M. Krebsz, Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects, Nanomaterials, 2020, 10, 917 CrossRef CAS PubMed.
- H. Pérez-Hernández, A. Pérez-Moreno, C. R. Sarabia-Castillo, S. García-Mayagoitia, G. Medina-Pérez, F. López-Valdez, R. G. Campos-Montiel, P. Jayanta-Kumar and F. Fernández-Luqueño, Ecological Drawbacks of Nanomaterials Produced on an Industrial Scale: Collateral Effect on Human and Environmental Health, Water, Air, Soil Pollut., 2021, 232, 435 CrossRef PubMed.
- A. Nyabadza, É. McCarthy, M. Makhesana, S. Heidarinassab, A. Plouze, M. Vazquez and D. Brabazon, A review of physical, chemical and biological synthesis methods of bimetallic nanoparticles and applications in sensing, water treatment, biomedicine, catalysis and hydrogen storage, Adv. Colloid Interface Sci., 2023, 321, 103010 CrossRef CAS PubMed.
- L. Xuan, Z. Ju, M. Skonieczna, P. K. Zhou and R. Huang, Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models, MedComm, 2023, 4, e327 CrossRef CAS PubMed.
- H. Singh, M. F. Desimone, S. Pandya, S. Jasani, N. George, M. Adnan, A. Aldarhami, A. S. Bazaid and S. A. Alderhami, Revisiting the Green Synthesis of Nanoparticles: Uncovering Influences of Plant Extracts as Reducing Agents for Enhanced Synthesis Efficiency and Its Biomedical Applications, Int. J. Nanomed., 2023, 18, 4727–4750 CrossRef CAS PubMed.
- C. Pandit, A. Roy, S. Ghotekar, A. Khusro, M. N. Islam, T. B. Emran, S. E. Lam, M. U. Khandaker and D. A. Bradley, Biological agents for synthesis of nanoparticles and their applications, J. King Saud Univ., Sci., 2022, 34, 101869 CrossRef.
- R. Álvarez-Chimal and J. Arenas-Alatorre, Green Synthesis of Nanoparticles: A Biological Approach, Advances in Green Chemistry, 2023, pp. 1–18 Search PubMed.
- A. Behera, B. Mittu, S. Padhi, N. Patra and J. Singh, Bimetallic nanoparticles: Green synthesis, applications, and future perspectives, Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems, 2020, vol. 25, pp. 639–682 Search PubMed.
- D. S. Idris and A. Roy, Synthesis of Bimetallic Nanoparticles and Applications-An Updated Review, Crystals, 2023, 13, 637–668 CrossRef CAS.
- K. Priya, V. Naveen, K. Kaur and A. K. Sidhu, Green Synthesis: An Eco-friendly Route for the Synthesis of Iron Oxide Nanoparticles, Front. Nanotechnol., 2021, 3, 655062 CrossRef.
- M. S. Samuel, S. Datta, N. Chandrasekar, R. Balaji, E. Selvarajan and S. Vuppala, Biogenic Synthesis of Iron Oxide Nanoparticles Using Enterococcus faecalis: Adsorption of Hexavalent Chromium from Aqueous Solution and In Vitro Cytotoxicity Analysis, Nanomaterials, 2021, 11, 3290 CrossRef CAS PubMed.
- M. Larrañaga-Tapia, B. Betancourt-Tovar, M. Videa, M. Antunes-Ricardo and J. L. Cholula-Díaz, Green synthesis trends and potential applications of bimetallic nanoparticles towards the sustainable development goals 2030, Nanoscale Adv., 2024, 6, 51–71 RSC.
- A. Punia, R. Pratap Singh, V. Singh and N. S. Chauhan, Environment sustainability with microbial nanotechnology, Environmental Applications of Microbial Nanotechnology, 2023, vol. 17, pp. 289–314 Search PubMed.
- O. Darwesh, M. Eida and I. Matter, Environmental Nanobiotechnology: Microbial-Mediated Nanoparticles for Sustainable Environment, Microbial Nanobiotechnology, 2021, pp. 145–164 Search PubMed.
- N. A. Singh, J. Narang, D. Garg, V. Jain, D. Payasi, S. Suleman and R. K. Swami, Nanoparticles synthesis via microorganisms and their prospective applications in agriculture, Plant Nano Biol., 2023, 5, 100047 CrossRef.
- B. Nural Yaman and B. İnan, in Methods in Microbiology, ed. P. A. Ramsland, A. Elbourne and V. Gurtler, Academic Press, 2024, vol. 54, pp. 243–271 Search PubMed.
- A. K. Sidhu, N. Verma and P. Kaushal, Role of Biogenic Capping Agents in the Synthesis of Metallic Nanoparticles and Evaluation of Their Therapeutic Potential, Front. Nanotechnol., 2022, 3, 1–17 CrossRef.
- X. Li, H. Xu, Z.-S. Chen and G. Chen, Biosynthesis of Nanoparticles by Microorganisms and Their Applications, J. Nanomater., 2011, 2011, 270974 Search PubMed.
- S. Enitan, F. S. Bwala, E. Adejumo, J.-U. Grace, S. Gupta, R. Adole, M. Dada, S. Udofia, A. Nwankiti, J. Nnaji, A. Idoko, R. Akele, B. Joshua, A. Adekunle Totoola and A. Okuneye, Leveraging on Microbial Nanotechnology for Drug Delivery and Targeting: Challenges and Prospects, Biomed. J. Sci. Tech. Res., 2024, 56, 47984–47995 Search PubMed.
- G. Gahlawat and A. R. Choudhury, A review on the biosynthesis of metal and metal salt nanoparticles by microbes, RSC Adv., 2019, 9, 12944–12967 RSC.
- K. H. Huynh, X. H. Pham, J. Kim, S. H. Lee, H. Chang, W. Y. Rho and B. H. Jun, Synthesis, Properties, and Biological Applications of Metallic Alloy Nanoparticles, Int. J. Mol. Sci., 2020, 21, 5174 CrossRef CAS PubMed.
- S. Iravani, et al., Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects, Int. Scholarly Res. Not., 2014, 2014, 359316 Search PubMed.
- A. Miri, H. Najafzadeh, M. Darroudi, M. J. Miri, M. A. J. Kouhbanani and M. Sarani, Iron Oxide Nanoparticles: Biosynthesis, Magnetic Behavior, Cytotoxic Effect, ChemistryOpen, 2021, 10, 327–333 CrossRef CAS PubMed.
- N. G. Dlamini, A. K. Basson and V. S. R. Pullabhotla, A Comparative Study between Bimetallic Iron@copper Nanoparticles with Iron and Copper Nanoparticles Synthesized Using a Bioflocculant: Their Applications and Biosafety, Processes, 2020, 8, 1125 CrossRef CAS.
- D. Alamilla-Martínez, N. Rojas-Avelizapa, I. Dominguez-Lopez, H. Pool and G. Ramirez, Biosynthesis of iron nanoparticles (FeNPs) by Alternaria alternata MVSS-AH-5 Biosíntesis de nanopartículas de hierro (FeNPs) por Alternaria alternata MVSS-AH-5, Mex. J. Biotechnol., 2019, 4, 1–14 CrossRef.
- N. Rojas-Avelizapa, J. Otamendi-Valdez and G. Ramirez, Metal leaching from a spent catalyst by Alternaria alternata, Mex. J. Biotechnol., 2017, 2, 221–231 Search PubMed.
- T. C. Johnstone and E. M. Nolan, Beyond iron: non-classical biological functions of bacterial siderophores, Dalton Trans., 2015, 44, 6320–6339 RSC.
- P. H. Tsilo, A. K. Basson, Z. G. Ntombela, N. G. Dlamini and R. V. S. R. Pullabhotla, Application of Iron Nanoparticles Synthesized from a Bioflocculant Produced by Yeast Strain Pichia kudriavzevii Obtained from Kombucha Tea SCOBY in the Treatment of Wastewater, Int. J. Mol. Sci., 2023, 24, 14731 CrossRef CAS PubMed.
- A. D. V. Bensy, G. J. Christobel, K. Muthusamy, A. Alfarhan and P. Anantharaman, Green synthesis of iron nanoparticles from Ulva lactuca and bactericidal activity against enteropathogens, J. King Saud Univ., Sci., 2022, 34, 101888 CrossRef.
- S. A. E.-F. Zaki, A. Kamal, N. A. Ashmawy and A. A. Shoeib, Nano-metals forming bacteria in Egypt. I. Synthesis, characterization and effect on some phytopathogenic bacteria in vitro, Sci. Rep., 2021, 11, 12876 CrossRef CAS PubMed.
- A. L. Campaña, A. Saragliadis, P. Mikheenko and D. Linke, Insights into the bacterial synthesis of metal nanoparticles, Frontiers in Nanotechnology, 2023, 5, 1–21 CrossRef.
- K. A. Crespo, J. L. Baronetti, M. A. Quinteros, P. L. Páez and M. G. Paraje, Intra- and Extracellular Biosynthesis and Characterization of Iron Nanoparticles from Prokaryotic Microorganisms with Anticoagulant Activity, Pharm. Res., 2017, 34, 591–598 CrossRef CAS PubMed.
- K. Das and S. Kerkar, Biosynthesis of iron nanoparticles by sulphate reducing bacteria and its application in remediating chromium from water, Int. J. Pharma Bio Sci., 2017, 8, 538–546 CAS.
- S. A. Zaki, M. M. Eltarahony and D. A. Abd-El-Haleem, Disinfection of water and wastewater by biosynthesized magnetite and zerovalent iron nanoparticles via NAP-NAR enzymes of Proteus mirabilis 10B, Environ. Sci. Pollut. Res., 2019, 26, 23661–23678 CrossRef CAS PubMed.
- N. G. Dlamini, A. K. Basson and R. V. Pullabhotla, Wastewater Treatment by a Polymeric Bioflocculant and Iron Nanoparticles Synthesized from a Bioflocculant, Polymer, 2020, 12, 1618 CAS.
- A. S. Sayyad, K. Balakrishnan, L. Ci, A. T. Kabbani, R. Vajtai and P. M. Ajayan, Synthesis of iron nanoparticles from hemoglobin and myoglobin, Nanotechnology, 2012, 23, 055602 CrossRef PubMed.
- S. Abdeen, R. Isaac, S. Geo, S. Sornalekshmi, A. Rose and P. K. Praseeth, Evaluation of Antimicrobial Activity of Biosynthesized Iron and Silver Nanoparticles Using the Fungi Fusarium Oxysporum and Actinomycetes SP. on Human Pathogens, Nano Biomed. Eng., 2013, 5, 39–45 CAS.
- J. Tarafdar and R. Raliya, Rapid, Low-Cost, and Ecofriendly Approach for Iron Nanoparticle Synthesis Using Aspergillus oryzae TFR9, J. Nanopart., 2013, 2013, 141274 Search PubMed.
- M. Yosri, A. Azzam, B. H. Amin and N. A. Safwat, Mycosynthesis of iron nanoparticles by Alternaria alternata and its antibacterial activity, Afr. J. Biotechnol., 2015, 14, 1234–1241 CrossRef.
- Q. Li, D. Liu, T. Wang, C. Chen and G. M. Gadd, Iron coral: Novel fungal biomineralization of nanoscale zerovalent iron composites for treatment of chlorinated pollutants, Chem. Eng. J., 2020, 402, 126263 CrossRef CAS.
- R. Ramasubbu and G. Manikandan, Biosynthesis of Iron Nanoparticles from Pleurotus florida and its Antimicrobial Activity against Selected Human Pathogens, Indian J. Pharm. Sci., 2021, 83, 45–51 Search PubMed.
- V. Subramaniyam, S. R. Subashchandrabose, P. Thavamani, M. Megharaj, Z. Chen and R. Naidu, Chlorococcum sp. MM11—a novel phyco-nanofactory for the synthesis of iron nanoparticles, J. Appl. Phycol., 2015, 27, 1861–1869 CrossRef CAS.
- M. Chandran and K. Ramesh, Bio-Synthesis of Iron Nanoparticles using the Brown Seaweed, Dictyota dicotoma, BioTechnol.:Indian J., 2016, 12, 112–121 Search PubMed.
- V. Subramaniyam, S. R. Subashchandrabose, V. Ganeshkumar, P. Thavamani, Z. Chen, R. Naidu and M. Megharaj, Cultivation of Chlorella on brewery wastewater and nano-particle biosynthesis by its biomass, Bioresour. Technol., 2016, 211, 698–703 CrossRef CAS PubMed.
- S. Karthika and A. Lakshmanan, Science and Research, The Green Synthesis and Characterization of Zero Valent Iron Nanoparticles using Azolla and Blue Green Algal Systems, Int. J. Agric. Sci. Res., 2019, 9, 1–20 Search PubMed.
- A. Saravanan, P. S. Kumar, S. Karishma, D.-V. N. Vo, S. Jeevanantham, P. R. Yaashikaa and C. S. George, A review on biosynthesis of metal nanoparticles and its environmental applications, Chemosphere, 2021, 264, 128580 CrossRef CAS PubMed.
- A. Lateef, S. Ojo, F. Bolaji, E. Gueguim-Kana and L. Beukes, Kolanut (Cola nitida) Mediated Synthesis of Silver–Gold Alloy Nanoparticles: Antifungal, Catalytic, Larvicidal and Thrombolytic Applications, J. Cluster Sci., 2016, 27, 1561–1577 CrossRef CAS.
- P. Ashishie, C. Anyama, A. Ayi, C. Oseghale, E. Adesuji and L. Hassan, Green synthesis of silver monometallic and copper-silver bimetallic nanoparticles using Kigelia africana fruit extract and evaluation of their antimicrobial activities, Int. J. Phys. Sci., 2018, 13, 24–32 CrossRef CAS.
- C. Xiao, H. Li, Y. Zhao, X. Zhang and X. Wang, Green synthesis of iron nanoparticle by tea extract (polyphenols) and its selective removal of cationic dyes, J. Environ. Manage., 2020, 275, 111262 CrossRef CAS PubMed.
- S. Machado, S. L. Pinto, J. P. Grosso, H. P. A. Nouws, J. T. Albergaria and C. Delerue-Matos, Green production of zero-valent iron nanoparticles using tree leaf extracts, Sci. Total Environ., 2013, 445–446, 1–8 CrossRef CAS PubMed.
- M. Karavasilis and C. Tsakiroglou, Synthesis of Aqueous Suspensions of Zero-Valent Iron Nanoparticles (nZVI) from Plant Extracts: Experimental Study and Numerical Modeling, Emerg. Sci. J., 2019, 3, 344–360 CrossRef.
- X. Liu, W. Zhao, Z. Huang, T.-H. Ko, Z. Song, H. Han and M. Yilmaz, Green synthesis of Fe-nanoparticles using pruned tea leaf extract: characterization and use for degradation of dyes from aqueous phase in Fenton-like systems, Water Reuse, 2024, 14, 160–176 CrossRef CAS.
- K. Shaheen, Z. Shah, A. Asad, T. Arshad, S. Khan and H. Suo, Synthesis, Characterization, and Multifunctional Applications of Cu-Fe and Ni-Fe Nanomaterials, ACS Omega, 2020, 5, 15992–16002 CrossRef CAS PubMed.
- X. Wu, Y. Wang, J. Xu and H. Wang, Cu/Fe Bimetallic Treatment Performance on Organophosphorus Pesticides, Front. Environ. Sci., 2022, 10, 915465 CrossRef.
- C. Settimi, D. Zingaretti, S. Sanna, I. Verginelli, I. Luisetto, A. Tebano and R. Baciocchi, Synthesis and Characterization of Zero-Valent Fe-Cu and Fe-Ni Bimetals for the Dehalogenation of Trichloroethylene Vapors, Sustainability, 2022, 14, 7760 CrossRef CAS.
- X. Weng, M. Guo, F. Luo and Z. Chen, One-step green synthesis of bimetallic Fe/Ni nanoparticles by eucalyptus leaf extract: Biomolecules identification, characterization and catalytic activity, Chem. Eng. J., 2017, 308, 904–911 CrossRef CAS.
- A. Hassan, M. Atiya and I. Luaibi, A Green Synthesis of Iron/Copper Nanoparticles as a Catalytic of Fenton-like Reactions for Removal of Orange G Dye, Baghdad Sci. J., 2022, 19, 1249 CrossRef.
- I. A. Ahmed, H. S. Hussein, Z. A. ALOthman, A. G. ALanazi, N. S. Alsaiari and A. Khalid, Green Synthesis of Fe–Cu Bimetallic Supported on Alginate-Limestone Nanocomposite for the Removal of Drugs from Contaminated Water, Polymers, 2023, 15, 1221 CrossRef CAS PubMed.
- M. Fazlzadeh, K. Rahmani, A. Zarei, H. Abdoallahzadeh, F. Nasiri and R. Khosravi, A novel green synthesis of zero valent iron nanoparticles (NZVI) using three plant extracts and their efficient application for removal of Cr(VI) from aqueous solutions, Adv. Powder Technol., 2017, 28, 122–130 CrossRef CAS.
- F. Zhu, S. Ma, T. Liu and X. Deng, Green Synthesis of Nano Zero-Valent Iron/Cu by Green Tea to Remove Hexavalent Chromium from Groundwater, J. Cleaner Prod., 2017, 174, 184–190 CrossRef.
- K. V. G. Ravikumar, S. Sudakaran, K. Ravichandran, M. Pulimi, N. Chandrasekaran and A. Mukherjee, Green synthesis of NiFe nano particles using Punica granatum peel extract for tetracycline removal, J. Cleaner Prod., 2018, 210, 767–776 CrossRef.
- Z. Oruç, M. Ergüt, D. Uzunoğlu and A. Özer, Green synthesis of biomass-derived activated carbon/Fe-Zn bimetallic nanoparticles from lemon (Citrus limon (L.) Burm. f.) wastes for heterogeneous Fenton-like decolorization of Reactive Red 2, J. Environ. Chem. Eng., 2019, 7, 103231 CrossRef.
- Y. Lin, X. Jin, N. Khan, G. Owens and Z. Chen, Efficient removal of As (III) by calcined green synthesized bimetallic Fe/Pd nanoparticles based on adsorption and oxidation, J. Cleaner Prod., 2020, 286, 124987 CrossRef.
- M. Zulfikar, R. Nadeem, T. Javed, M. I. Jilani and I. Javed, Green synthesis of Fe nanoparticles by using Mangifera indica extract and its application in photo-catalytic degradation of dyes, Water Sci. Technol., 2021, 83, 1739–1752 CrossRef CAS PubMed.
- M. A. Malik, A. A. Alshehri and R. Patel, Facile one-pot green synthesis of Ag–Fe bimetallic nanoparticles and their catalytic capability for 4-nitrophenol reduction, J. Mater. Res. Technol., 2021, 12, 455–470 CrossRef CAS.
- A. L. Padilla-Cruz, J. A. Garza-Cervantes, X. G. Vasto-Anzaldo, G. García-Rivas, A. León-Buitimea and J. R. Morones-Ramírez, Synthesis and design of Ag-Fe bimetallic nanoparticles as antimicrobial synergistic combination therapies against clinically relevant pathogens, Sci. Rep., 2021, 11, 5351 CrossRef CAS PubMed.
- U. Younas, S. Hassan, F. Ali, F. Hassan, Z. Saeed, M. Pervaiz, S. Khan, F. Tul Jannat, S. Bibi, A. Sadiqa, Z. Ali, S. Iqbal, A. Ghfar, M. Ouladsmane, M. Al-Anazy and S. Ali, Radical Scavenging and Catalytic Activity of Fe-Cu Bimetallic Nanoparticles Synthesized from Ixora finlaysoniana Extract, Coatings, 2021, 11, 813 CrossRef CAS.
- D. R. Eddy, D. Nursyamsiah, M. D. Permana, Solihudin, A. R. Noviyanti and I. Rahayu, Green Production of Zero-Valent Iron (ZVI) Using Tea-Leaf Extracts for Fenton Degradation of Mixed Rhodamine B and Methyl Orange Dyes, Materials, 2022, 15, 332 CrossRef CAS PubMed.
- P. A. R. Puthukkara, T. S. Jose and L. S. Dinoop, Green synthesis of iron nanoparticles for malachite green removal, Mater. Today Commun., 2022, 33, 104759 CrossRef.
- D. Medina, B. Saleh, A. Crua, A. Nieto-Argüello, D. Lomelí-Marroquín, L. Vélez-Escamilla, J. Cholula-Díaz, J.-M. Garcia-Martin and T. Webster, Bimetallic Nanoparticles for Biomedical Applications: A Review, Racing for the Surface, 2020, pp. 397–434 Search PubMed.
- G. Huang, M. Wang, X. Sun, H. Liu and F. Liu, Convenient green synthesis of Cu/Fe nanoparticles using pomegranate peel extracts and their performance for tetrabromobisphenol A removal, Environ. Sci. Pollut. Res., 2023, 30, 80817–80827 CrossRef CAS PubMed.
- M. Benković, D. Valinger, T. Jurina, J. Gajdoš Kljusurić and A. Jurinjak Tušek, Biocatalysis as a Green Approach for Synthesis of Iron Nanoparticles-Batch and Microflow Process Comparison, Catalysts, 2023, 13, 112 CrossRef.
- R. Raja Nandhini, H. Joy Prabu, E. Thaninayagam, R. R. Gopi, I. Johnson and A. Felix Sahayaraj, Green Synthesis of Silver/Iron(Ag/Fe) and Copper/Iron(Cu/Fe) Nanoparticles for Cytotoxic Investigation on Henrietta Lacks(HeLa) Cancer Cell, Proceedings of the International Symposium on Lightweight and Sustainable Polymeric Materials, Springer Nature Singapore, 2023, vol. 32, pp. 79–95 Search PubMed.
- G. Shi, S. Zeng, Y. Liu, J. Xiang, D. Deng, C. Wu, Q. Teng and H. Yang, Efficient heterogeneous Fenton-like degradation of methylene blue using green synthesized yeast supported iron nanoparticles, Ecotoxicol. Environ. Saf., 2023, 263, 115240 CrossRef CAS PubMed.
- H. Son, et al., Optimization of Fe/Ni, Fe/Cu bimetallic nanoparticle synthesis process utilizing concentrated Camellia sinensis extract solution and activity evaluation through methylene blue removal reaction, Nano Express, 2024, 5, 025026 CrossRef.
- A. Qadir Ahmad, N. Attique, R. Ali, W. Abbas, M. Nadeem, M. Junaid and N. Ul Ain, Green synthesis and characterization of Fe/Mg nanoparticles for their potential applications against aflatoxogenic A. flavus, Results Chem., 2024, 7, 101312 CrossRef CAS.
- C. Visentin, A. W. d. S. Trentin, A. B. Braun and A. Thomé, Lifecycle assessment of environmental and economic impacts of nano-iron synthesis process for application in contaminated site remediation, J. Cleaner Prod., 2019, 231, 307–319 CrossRef CAS.
- F. Martins, S. Machado, T. Albergaria and C. Delerue-Matos, LCA applied to nano scale zero valent iron synthesis, Int. J. Life Cycle Assess., 2017, 22, 707–714 CrossRef CAS.
- N. G. Dlamini, A. K. Basson and V. S. R. Pullabhotla, Synthesis and Characterization of Various Bimetallic Nanoparticles and Their Application, Appl. Nano, 2023, 4, 1–24 CrossRef.
- S. Ying, Z. Guan, P. C. Ofoegbu, P. Clubb, C. Rico, F. He and J. Hong, Green synthesis of nanoparticles: Current developments and limitations, Environ. Technol. Innovation, 2022, 26, 102336 CrossRef CAS.
- S. Saif, A. Tahir and Y. Chen, Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications, Nanomaterials, 2016, 6, 209 CrossRef PubMed.
- S. R. Dhruval, N. Pai, S. S. Dhanwant, B. Hussein, S. Nayak, C. V. Rao, A. Kumar and M. Janakaraj, Rapid synthesis of antimicrobial Fe/Cu alloy nanoparticles using Waste Silkworm Cocoon extract for cement mortar applications, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2020, 11, 025006 CAS.
- D. Tesnim, B. A. Hédi, D. Ridha and A. Cid-Samamed, Green low-cost synthesis of zero-valent iron nanoparticles from Palm Petiole Extract for Cr(VI) removal from water, Environ. Sci. Pollut. Res., 2024, 31, 44272–44288 CrossRef CAS PubMed.
- A. O. Dada, F. Adekola and E. Odebunmi, Synthesis and characterization of iron nanoparticles and its ash rice husk supported nanocomposite, Conference: Proceedings of the 1st African International Conference, 2014, pp. 138–149 Search PubMed.
- A. Gautam, S. Rawat, L. Verma, J. Singh, S. Sikarwar, B. C. Yadav and A. S. Kalamdhad, Green synthesis of iron nanoparticle from extract of waste tea: An application for phenol red removal from aqueous solution, Environ. Nanotechnol., Monit. Manage., 2018, 10, 377–387 Search PubMed.
- S. T. Kadhum, G. Y. Alkindi and T. M. Albayati, Eco friendly adsorbents for removal of phenol from aqueous solution employing nanoparticle zero-valent iron synthesized from modified green tea bio-waste and supported on silty clay, Chin. J. Chem. Eng., 2021, 36, 19–28 CrossRef CAS.
- E. El-Monaem, A. Omer, G. El-Subruiti, M. Mohy Eldin and A. Eltaweil, Zero-valent iron supported-lemon derived biochar for ultra-fast adsorption of methylene blue, Biomass Convers. Biorefin., 2022, 14, 3 Search PubMed.
- H. Ahmed, M. El-Khateeb, N. Reiad, M. Hefny and F. Abdel-Haleem, Green Synthesis of Magnetite Nanoparticles Using Waste Natural Materials and Its Application for Wastewater Treatment, Environ. Sci. Proc., 2023, 25, 99–110 Search PubMed.
- D. Tesnim, B. A. Hedi and J. Simal-Gandara, Sustainable and Green Synthesis of Iron Nanoparticles Supported on Natural Clays via Palm Waste Extract for Catalytic Oxidation of Crocein Orange G Mono Azoic Dye, ACS Omega, 2023, 8, 34364–34376 CrossRef CAS PubMed.
- S. C. Aditya Kumar Jha and J. K. Biswas, Green Synthesis of a Low-Cost GO-nZVI Composite from Solid Waste and Its Application for Photocatalytic Degradation of Antibiotic Residues in Aqueous Phase, CellPress, 2024, 111486 Search PubMed.
- J. Ye, Y. Wang, Q. Xu, H. Wu, J. Tong and J. Shi, Removal of Hexavalent Chromium From Wastewater by Cu/Fe Bimetallic Nanoparticles, Sci. Rep., 2020, 11, 10848 CrossRef PubMed.
- G. G. Valiyeva, I. Bavasso, L. Di Palma, S. R. Hajiyeva, M. A. Ramazanov and F. V. Hajiyeva, Synthesis of Fe/Ni Bimetallic Nanoparticles and Application to the Catalytic Removal of Nitrates from Water, Nanomaterials, 2019, 9, 1130 CrossRef CAS PubMed.
- E. N. L. Banfi, R. Riva, N. Stiasni, M. Hiersemann, T. Yamada and T. Tsubo, Encyclopedia of Reagents for Organic Synthesis (EROS), 2014, pp. 1–13 Search PubMed.
- K. V. G. Ravikumar, D. Kumar, A. Rajeshwari, G. M. Madhu, P. Mrudula, N. Chandrasekaran and A. Mukherjee, A comparative study with biologically and chemically synthesized nZVI: applications in Cr (VI) removal and ecotoxicity assessment using indigenous microorganisms from chromium-contaminated site, Environ. Sci. Pollut. Res., 2016, 23, 2613–2627 CrossRef CAS PubMed.
- D. M. Hammad and A. A. Asaad, A comparative study: biological and chemical synthesis of iron oxide nanoparticles and their affinity towards adsorption of methylene blue dye, Desalin. Water Treat., 2021, 224, 354–366 CrossRef CAS.
- M. Bhuvaneshwari, D. Kumar, R. Roy, S. Chakraborty, A. Parashar, A. Mukherjee, N. Chandrasekaran and A. Mukherjee, Toxicity, accumulation, and trophic transfer of chemically and biologically synthesized nano zero valent iron in a two species freshwater food chain, Aquat. Toxicol., 2017, 183, 63–75 CrossRef CAS PubMed.
- J. O. Ighalo, P. S. Yap, K. O. Iwuozor, C. O. Aniagor, T. Liu, K. Dulta, F. U. Iwuchukwu and S. Rangabhashiyam, Adsorption of persistent organic pollutants (POPs) from the aqueous environment by nano-adsorbents: A review, Environ. Res., 2022, 212, 113123 CrossRef CAS PubMed.
- L. Fitzgerald and D. S. Wikoff, Persistent Organic Pollutants, Encyclopedia of Toxicology, 3rd edn, 2014, pp. 820–825 Search PubMed.
- G. O'Sullivan and D. Megson, Brief Overview: Discovery, Regulation, Properties, and Fate of POPs, Environmental Forensics for Persistent Organic Pollutants, 2014, vol. 1, pp. 1–20 Search PubMed.
- W.-J. Liu, T.-T. Qian and H. Jiang, Bimetallic Fe nanoparticles: Recent advances in synthesis and application in catalytic elimination of environmental pollutants, Chem. Eng. J., 2014, 236, 448–463 CrossRef CAS.
- C.-B. Wang and W.-X. Zhang, Synthesizing Nanoscale Iron Particles for Rapid and Complete Dechlorination of TCE and PCBs, Environ. Sci. Technol., 1997, 31, 2154–2156 CrossRef CAS.
- H. Wu, W. Wei, C. Xu, Y. Meng, W. Bai, W. Yang and A. Lin, Polyethylene glycol-stabilized nano zero-valent iron supported by biochar for highly efficient removal of Cr(VI), Ecotoxicol. Environ. Saf., 2020, 188, 109902 CrossRef CAS PubMed.
- L. Zhao, Y. Zhao, B. Yang and H. Teng, Application of Carboxymethyl Cellulose–Stabilized Sulfidated Nano Zerovalent Iron for Removal of Cr(VI) in Simulated Groundwater, Water, Air, Soil Pollut., 2019, 230, 113 CrossRef.
- A. Shan, A. Idrees, W. Q. Zaman, Z. Abbas, M. Ali, M. S. U. Rehman, S. Hussain, M. Danish, X. Gu and S. Lyu, Synthesis of nZVI-Ni@BC composite as a stable catalyst to activate persulfate: Trichloroethylene degradation and insight mechanism, J. Environ. Chem. Eng., 2021, 9, 104808 CrossRef CAS.
- J. Guo, J. Jiang, Y. Chen, X. Wen, W. Chen, Y. Wang, L. Su and J. Cao, Synthesis of nZVI-BC composite for persulfate activation to degrade pyrene: Performance, correlative mechanisms and degradation pathways, Process Saf. Environ. Prot., 2022, 162, 733–745 CrossRef CAS.
- M.-B. Gholivand, A. R. Jalalvand, H. C. Goicoechea, G. Paimard and T. Skov, Surface exploration of a room-temperature ionic liquid-chitin composite film decorated with electrochemically deposited PdFeNi trimetallic alloy nanoparticles by pattern recognition: An elegant approach to developing a novel biotin biosensor, Talanta, 2015, 131, 249–258 CrossRef CAS PubMed.
- N. Basavegowda, K. Mishra and Y. R. Lee, Trimetallic FeAgPt alloy as a nanocatalyst for the reduction of 4-nitroaniline and decolorization of rhodamine B: A comparative study, J. Alloys Compd., 2017, 701, 456–464 CrossRef CAS.
- S. Dey, S. Nath, T. Alam Ansari, A. Biswas, F. Barman, S. Mukherjee, G. Gopal, A. Bhattacharyya, A. Mukherjee, R. Kundu and S. Paul, Application of green synthesized bimetallic nZVI-Cu nanoparticle as a sustainable alternative to chemical fertilizers to enhance growth and photosynthetic efficiency of rice seedlings, Plant Physiol. Biochem., 2023, 201, 107837 CrossRef CAS PubMed.
- Y. Lin, X. Jin, G. Owens and Z. Chen, Simultaneous removal of mixed contaminants triclosan and copper by green synthesized bimetallic iron/nickel nanoparticles, Sci. Total Environ., 2019, 695, 133878 CrossRef CAS PubMed.
- R. Ningthoujam, B. Sahoo, P. Ghosh, A. Shivani, P. Ganguli and S. Chaudhuri, Green production of zero-valent iron nanoparticles using pomegranate peel extracts and its use in lindane degradation, Nanotechnol. Environ. Eng., 2023, 8, 581–589 CrossRef CAS.
- M. Tlčíková, H. Horváthová, K. Dercová, M. Majčinová, M. Hurbanová, K. Turanská and Ľ. Jurkovič, Plant-Based Substrates for the Production of Iron Bionanoparticles (Fe-BNPs) and Application in PCB Degradation with Bacterial Strains, Processes, 2024, 12, 1695 CrossRef.
- V. N. Toan, et al., Results of research on technology to treat soil contaminated with toxic chemical/dioxins by combining nanomaterials with probiotics, International Conference Proceeding: Science and Technology on Treatment and Mitigation of the Post-war Toxic Chemicals/Dioxins' Harmful Effects on Humans and Environment in Viet Nam, 2024, pp. 274–288 Search PubMed.
- J.-F. Gao, Z.-L. Wu, W.-J. Duan and W.-Z. Zhang, Simultaneous adsorption and degradation of triclosan by Ginkgo biloba L. stabilized Fe/Co bimetallic nanoparticles, Sci. Total Environ., 2019, 662, 978–989 CrossRef CAS PubMed.
- L. Gan, B. Li, M. Guo, X. Weng, T. Wang and Z. Chen, Mechanism for removing 2,4-dichlorophenol via adsorption and Fenton-like oxidation using iron-based nanoparticles, Chemosphere, 2018, 206, 168–174 CrossRef CAS PubMed.
- M. Guo, X. Weng, T. Wang and Z. Chen, Biosynthesized iron-based nanoparticles used as a heterogeneous catalyst for the removal of 2, 4-dichlorophenol, Sep. Purif. Technol., 2016, 175, 222–228 CrossRef.
- N. Mehrotra, R. M. Tripathi, F. Zafar and M. P. Singh, Catalytic Degradation of Dichlorvos Using Biosynthesized Zero Valent Iron Nanoparticles, IEEE Trans. NanoBiosci., 2017, 16, 280–286 Search PubMed.
- B. Pushkar and P. Sevak, Adsorption Study of Green Synthesized Fe-Oxide Nanoparticle for DDT Removal, Int. J. Pharm. Sci. Rev. Res., 2019, 55, 84–90 CAS.
- B. Huang, C. Lei, C. Wei and G. Zeng, Chlorinated volatile organic compounds (Cl-VOCs) in environment - sources, potential human health impacts, and current remediation technologies, Environ. Int., 2014, 71, 118–138 CrossRef CAS PubMed.
- Y. Guo, M. Wen, G. Li and T. An, Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: a critical review, Appl. Catal., B, 2021, 281, 119447 CrossRef CAS.
- C. Yang, G. Miao, Y. Pi, Q. Xia, J. Wu, Z. Li and J. Xiao, Abatement of various types of VOCs by adsorption/catalytic oxidation: A review, Chem. Eng. J., 2019, 370, 1128–1153 CrossRef CAS.
- M. Li, B. Lu, Q.-F. Ke, Y.-J. Guo and Y.-P. Guo, Synergetic effect between adsorption and photodegradation on nanostructured TiO2/activated carbon fiber felt porous composites for toluene removal, J. Hazard. Mater., 2017, 333, 88–98 CrossRef CAS PubMed.
- K. W. Shah and W. Li, A Review on Catalytic Nanomaterials for Volatile Organic Compounds VOC Removal and Their Applications for Healthy Buildings, Nanomaterials, 2019, 9, 910 CrossRef CAS PubMed.
- V. Smuleac, R. Varma, S. Sikdar and D. Bhattacharyya, Green synthesis of Fe and Fe/Pd bimetallic nanoparticles in membranes for reductive degradation of chlorinated organics, J. Membr. Sci., 2011, 379, 131–137 CrossRef CAS PubMed.
- D. Salom, D. Fernández-Verdejo, J. Moral-Vico, X. Font and E. Marco-Urrea, Combining nanoscale zero-valent iron and anaerobic dechlorinating bacteria to degrade chlorinated methanes and 1,2-dichloroethane, Environ. Sci. Pollut. Res., 2023, 30, 45231–45243 CrossRef CAS PubMed.
- Y. Kuang, Q. Wang, Z. Chen, M. Megharaj and R. Naidu, Heterogeneous Fenton-like oxidation of monochlorobenzene using green synthesis of iron nanoparticles, J. Colloid Interface Sci., 2013, 410, 67–73 CrossRef CAS PubMed.
- G. Kozma, A. Rónavári, Z. Kónya and A. Kukovecz, Environmentally Benign Synthesis Methods of Zero-Valent Iron Nanoparticles, ACS Sustainable Chem. Eng., 2015, 4, 291–297 CrossRef.
- J. Yan, L. Hu, W. Gao, L. Yang, L. Qian, L. Han and M. Chen, Remediation of 1,2-dichlorobenzene contaminated soil by activated persulfate using green synthesized nanoscale zero valent iron: activation mechanism and degradation pathways, J. Soils Sediments, 2022, 22, 1135–1144 CrossRef CAS.
- J. Zhan, X. Yang, X. Zhang, Y. Wang, X. Cai and H. Chen, Bioprecipitation facilitates the green synthesis of sulfidated nanoscale zero-valent iron particles for highly selective dechlorination of trichloroethene, J. Environ. Chem. Eng., 2021, 9, 106050 CrossRef CAS.
- S.-Y. Oh, Y.-D. Seo and K.-S. Ryu, Reductive removal of 2,4-dinitrotoluene and 2,4-dichlorophenol with zero-valent iron-included biochar, Bioresour. Technol., 2016, 216, 1014–1021 CrossRef CAS PubMed.
- J. Huang, S. Yi, C. Zheng and I. M. C. Lo, Persulfate activation by natural zeolite supported nanoscale zero-valent iron for trichloroethylene degradation in groundwater, Sci. Total Environ., 2019, 684, 351–359 CrossRef CAS PubMed.
- G. Ren, Y. Shi, Y. Cai, L. Yuan, K. Wu, M. Ouyang and K. Zheng, Removal of triclosan from water by sepiolite supported bimetallic Fe/Ni nanoparticles, Environ. Technol., 2022, 43, 3319–3328 CrossRef CAS PubMed.
- Y. Li, Y. Zhang and J. Wang, Influence Factors on Removal of 2, 4-DCP by Sulfided Nanoscale Zerovalent Iron, E3S Web Conf., 2022, 350, 01012 CrossRef CAS.
- J.-Y. Ahn, C. Kim, K.-Y. Hwang, S.-C. Jun and I. Hwang, Field Study on Application of Reactive Zone Technology Using Zero-Valent Iron Nanoparticles for Remediation of TCE-Contaminated Groundwater, J. Soil Groundwater Environ., 2014, 19, 80–90 CrossRef.
- T. Zheng, D. Hou, N. Wu, M. Wang, N. Luo, H. Luo, W. Leng, P. Li and W. Wei, Synergistic effect and removal mechanism of trichloroethylene (TCE) by nano scale zero-valent iron (nZVI) supported on biological calcium carbonate (CaCO3), J. Environ. Chem. Eng., 2023, 11, 111573 CrossRef CAS.
- Q. Wang, Remediation of Aged Hexachlorobenzene Contaminated Soil by Nanoscale Zero-Valent Iron, Research Square, 2023, pp. 1–20 Search PubMed.
- W.-Z. Zhang, J.-F. Gao, W.-J. Duan, D. Zhang, J.-X. Jia and Y.-W. Wang, Sulfidated nanoscale zero-valent iron is an efficient material for the removal and regrowth inhibition of antibiotic resistance genes, Environ. Pollut., 2020, 263, 114508 CrossRef CAS PubMed.
- J. Fang, K. Xu, A. Liu, Y. Xue, L. Tie, Z. Deng, R. Qiu and W.-X. Zhang, Selective perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) adsorption by nanoscale zero-valent iron (nZVI): performance and mechanisms, Environ. Sci.: Nano, 2024, 11, 1915–1925 RSC.
- A. M. Abdelfatah, N. El-Maghrabi, A. E. D. Mahmoud and M. Fawzy, Synergetic effect of green synthesized reduced graphene oxide and nano-zero valent iron composite for the removal of doxycycline antibiotic from water, Sci. Rep., 2022, 12, 19372 CrossRef CAS PubMed.
- A. Chen, H. Wang, X. Zhan, K. Gong, W. Xie, W. Liang, W. Zhang and C. Peng, Applications and synergistic degradation mechanisms of nZVI-modified biochar for the remediation of organic polluted soil and water: A review, Sci. Total Environ., 2024, 911, 168548 CrossRef CAS PubMed.
- X. Wang, Y. Zheng, P. Ning, I. Lynch, Z. Guo, P. Zhang and L. Wu, Synergetic effect of green synthesized NZVI@Chitin-modified ZSM-5 for efficient oxidative degradation of tetracycline, Environ. Res., 2024, 258, 119360 CrossRef CAS PubMed.
- R. Wang, T. Tang, G. Lu, Z. Zheng, K. Huang, H. Li, X. Tao, H. Yin, Z. Shi, Z. Lin, F. Wu and Z. Dang, Mechanisms and pathways of debromination of polybrominated diphenyl ethers (PBDEs) in various nano-zerovalent iron-based bimetallic systems, Sci. Total Environ., 2019, 661, 18–26 CrossRef CAS PubMed.
- Z. Fang, X. Qiu, J. Chen and X. Qiu, Degradation of the polybrominated diphenyl ethers by nanoscale zero-valent metallic particles prepared from steel pickling waste liquor, Desalination, 2011, 267, 34–41 CrossRef CAS.
- Y. Su, G. Lowry, D. Jassby and Y. Zhang, Sulfide-Modified NZVI (S-NZVI): Synthesis, Characterization, and Reactivity, Nanoscale Zerovalent Iron Particles for Environmental Restoration, 2019, pp. 359–386 Search PubMed.
- J. Qu, Y. Liu, L. Cheng, Z. Jiang, G. Zhang, F. Deng, L. Wang, W. Han and Y. Zhang, Green synthesis of hydrophilic activated carbon supported sulfide nZVI for enhanced Pb(II) scavenging from water: Characterization, kinetics, isotherms and mechanisms, J. Hazard. Mater., 2021, 403, 123607 CrossRef CAS PubMed.
- Y. Su, G. V. Lowry, D. Jassby and Y. Zhang, Sulfide-Modified NZVI (S-NZVI): Synthesis, Characterization, and Reactivity, Nanoscale Zerovalent Iron Particles for Environmental Restoration: From Fundamental Science to Field Scale Engineering Applications, 2019, pp. 359–386 Search PubMed.
- M. Lei, J. Zhang, C. Liu, J. Guo and Y. Li, Synthesis of magnetic S-nZVI nanoparticle and its application for the effective removal of radioactive uranium from seawater, J. Radioanal. Nucl. Chem., 2024, 333, 6429–6440 CrossRef CAS.
- J. Xu, H. Li and G. V. Lowry, Sulfidized Nanoscale Zero-Valent Iron: Tuning the Properties of This Complex Material for Efficient Groundwater Remediation, Acc. Mater. Res., 2021, 2, 420–431 CrossRef CAS.
- D. Fan, G. O'Brien Johnson and P. G. Tratnyek, Sulfidation of Nano Zerovalent Iron (nZVI) for Improved Selectivity During In-Situ Chemical Reduction (ISCR), Environ. Sci. Technol., 2016, 50, 9558–9565 CrossRef CAS PubMed.
- S. Kazemi, A. Hosseingholian, S. D. Gohari, F. Feirahi, F. Moammeri, G. Mesbahian, Z. S. Moghaddam and Q. Ren, Recent advances in green synthesized nanoparticles: from production to application, Mater. Today Sustain., 2023, 24, 100500 Search PubMed.
- S. Jafarzadeh, M. Nooshkam, M. Zargar, F. Garavand, S. Ghosh, M. Hadidi and M. Forough, Green synthesis of nanomaterials for smart biopolymer packaging: challenges and outlooks, J. Nanostruct. Chem., 2024, 14, 113–136 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |