Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Soil species sensitivity distributions for terrestrial risk assessment of silver nanomaterials: the influence of nanomaterial characteristics and soil type†
Sarah L.
Roberts
 *a,
Elise
Morel
a,
Richard K.
Cross
*a,
Elise
Morel
a,
Richard K.
Cross
 a,
David J.
Spurgeon
a,
David J.
Spurgeon
 a,
Marta
Baccaro
ab and
Elma
Lahive
a,
Marta
Baccaro
ab and
Elma
Lahive
 a
a
aUK Centre for Ecology and Hydrology, MacLean Building, Benson Lane, Wallingford, UK. E-mail: sarrob@ceh.ac.uk
bDivision of Toxicology, Wageningen University & Research, Stippeneng 4, 6708 WE Wageningen, The Netherlands
First published on 21st March 2025
Abstract
Silver nanomaterials (AgNMs) are released into the soil through various anthropogenic activities, including as biocides and in biosolid amendments. There is an abundance of toxicity data available for AgNMs and soil organisms, yet the assessment of their ecological risk and the influence of NM characteristics and exposure conditions on AgNM hazard in soils are not well elucidated. In this study, available soil ecotoxicology data for AgNMs and other Ag forms were collated from literature into a database. Using this database, species sensitivity distributions (SSDs) for soil biota were constructed. From these SSDs we calculated hazard concentrations for 50% of species (HC50) that would allow us to robustly compare effects on soil organisms soil or liquid media and to assess relationships to NM properties (coating) and major soil properties. For all AgNMs, the calculated HC50 value was 3.09 (1.74–5.21) mg kg−1 for studies conducted with soil dwelling species in soils and 0.70 (0.32–1.64) mg L−1 for liquid exposures. In comparison, the HC50 value for Ag salt (silver nitrate, AgNO3) was 2.74 (1.22–5.23) mg kg−1 for soil and 0.01 (0.01–0.03) mg L−1 for liquid-based exposures. At a detailed level, the Ag salt was more toxic than the NMs across most soil species and endpoints. Further analyses indicated that both NM surface coating and soil type influence AgNM toxicity. In soil exposures SSDs indicated similar effects across differently coated NM forms, however, in liquid-based assays both uncoated and PVP-coated AgNMs were more toxic to soil tested organisms than citrate-coated AgNMs. Soil cation exchange capacity (CEC) and organic carbon (OC) also influenced AgNM toxicity, with AgNMs being more toxic in soils with higher CEC and lower OC. Our study provides a data resource of toxicity data for soil species and the first hazard thresholds for risk assessment of AgNMs in soils and provides new insights into the factors driving AgNM hazard for soils species.
Environmental significanceSilver nanomaterials (AgNMs) are widely used in many consumer products and biocides. Their main pathway to the environment is through biosolids that are applied to agricultural lands, making them a risk to both freshwater and soil ecosystems. In comparison to freshwater ecosystems, the ecological risk of AgNMs to soil biota is understudied. Our study constructs the first species sensitivity distribution specifically for soils and gives insight into how both particle and soil properties can influence AgNM hazard to soil biota. |
Introduction
Silver nanomaterials (AgNMs) are one of the fastest growing classes of NMs,1 accounting for 15% of market share in 2022.2 Pathways for AgNMs to the soil environment include direct releases (e.g. as biocides) and biosolid application to land.3 Material flow analysis predicts that >90% of AgNMs in commercial products end up in wastewater treatment plants (WWTP).4 Within the WWTP system, the majority of these NMs partition to the sludge.5 In many countries, a significant proportion of the sludge is further treated to generate biosolids that are used as a soil amendment in agricultural and non-agricultural soils. Given the potential release of AgNMs to soil environments, the hazard of these materials to soil communities has been a subject of some concern, with a strong drive over the past decade to assess their toxicity to soil species. Yet, hazard thresholds for risk assessment of AgNMs in soils have not yet been determined and the relative importance of NM characteristics and soil type are not robustly established.Multiple studies investigating the effects of AgNM on soil organisms and processes have found that exposure can inhibit vital rates (e.g., survival, growth, reproduction) of soil invertebrates and plants, as well as altered or inhibited functioning of soil microbial communities and processes.6–11 It is widely proposed that the toxicity of AgNMs is largely driven by the release of Ag ions.12–14 However, ion release may only be partial, which means that comparison of exposures to similar ionic and NM silver masses will result in a lower toxicity for the partially dissolved AgNM form. Consistent with this, when compared with the toxicity of Ag salt, a meta-analysis of toxicity data for aquatic species showed that AgNMs were less toxic compared with the salt.15 Similarly, some soil toxicity studies have also shown the Ag salt to be more toxic than AgNMs,12,14 although this is not always the case.13
Within AgNM studies, there are multiple additional drivers that can lead to differences in toxicity for soil organisms. Processes linked to aging and transformations over time have been found to be important. For example, AgNM toxicity in soil was shown to increase with time as a result of the slow Ag ion release kinetics and relatively slow toxicokinetics of Ag uptake in, e.g., earthworm species.16,17 Not all NM “ageing” reactions, however, lead to an increase in toxic effect. Other NM transformation processes such as sulfidation have been shown to reduce the toxicity of AgNMs.18 In the case of aquatic species, SSDs constructed to compare the sensitivity of organisms to pristine and transformed (sulfidised) AgNM found the HC5 value for transformed AgNMs was >200-fold higher compared with that for the pristine AgNM.19 Whether differences between hazard thresholds would be similarly reflected for soil environments has yet to be tested.
NM physiochemical properties can also be important drivers of toxicity. Multiple toxicity studies have investigated the influence of NM properties such as size, shape and coating on AgNM toxicity, however, to date no consistent conclusions have been reached.20–22 For example, AgNM shape was found to be more important than size for driving AgNM toxicity to the nematode Caenorhabditis elegans,23 while another study showed the importance of size and surface coating for toxicity to earthworms.24 Although a meta-analysis using a species sensitivity distribution (SSD) approach for freshwater species found it difficult to establish the most important AgNMs driving NM hazard,25 there is some evidence of the potential importance of NM coating for aquatic effects.22,25,26 Some evidence of the importance of surface coating for toxicity to soil organisms supports this,27,28 however, the knowledge gap on the nature and extent of such effect remains.
Soil properties (e.g., pH, organic carbon, clay and sand content) are a further factor that can modify AgNM exposure and effects in soil ecosystems. Several studies have demonstrated that the soil properties (e.g., pH, organic matter content, cation exchange capacity) can influence the chemical speciation, mobility, bioavailability and ultimately toxicity of metals and metallic NMs.6,28–33 Although some studies have highlighted the importance of pH,28 the toxicity of AgNMs generally cannot be attributed to a single property and rather multiple soil chemistry parameters have been shown to influence bioavailability and toxicity.6,34 However, within this growing body of information, the scale and magnitude of such effects are not established.
In this study, we collate available literature data to establish a comprehensive database comprising of toxicity data from studies investigating Ag salt and AgNMs toxicity to soil organisms. A database of effect studies and SSDs are used to explore, 1) the differences in toxicity between AgNMs and Ag salt (silver nitrate, AgNO3), 2) the effect of AgNM particle properties (i.e., size, shape, coating) and AgNM ageing on toxicity, and finally 3) the influence of soil properties on AgNM toxicity. To maximise the available data, we collated data for both soil and liquid-based toxicity studies conducted with soil species. From the available data and derived SSDs, we sought to evaluate the hypotheses that, 1) soluble Ag is more toxic than AgNMs to soil organisms, 2) particle ageing through incubation in a natural environment or testing of artificially aged AgNMs (e.g., sulphidised AgNMs intended to simulate changes that may occur in treatment to generate biosolids) reduced toxicity compared to unaged AgNMs, and 3) both soil and particle properties are important drivers of AgNM toxicity.
Methods
Literature search and evaluation
A comprehensive literature search was conducted to collate available ecotoxicological data for soil species exposed to AgNMs and other Ag forms using Web of Science with the search terms: TOPIC (Nano* AND (toxic* OR effect*) AND (Ag OR silver)) AND ALL FIELDS (Arthropod*). More than one word was added in the search terms to define the organism group, for example using TOPIC (Nano* AND (toxic* OR effect*) AND (Ag OR silver)) AND ALL FIELDS (earthworm* OR enchy* OR Eisenia* OR Lumbric* OR Aporrectodea) AND ALL FIELDS (soil*). The term “soil*” was further added for plants, bacteria, enzymatic activity, insects, fungi and invertebrates. The search period was restricted to the period 2009 to 2021 representing the time since work on engineered nanomaterials was first published at any scale and the last full year available.Relevance screening was conducted on the collated literature. Firstly, titles and keywords were evaluated to identify papers likely to contain relevant toxicity data. From this evaluation, a total of 277 papers were identified for more detailed assessment. In the second step, these papers were assessed and prioritised based on containing the following 1) studies where three and more test concentrations plus a control were used in the ecotoxicity test, 2) reported toxicity values or toxicity data were reported in the text, graphs or tables, 3) whether the exposure was in soil or in a liquid based exposure medium was reported; 4) available characterisation information for the exposure medium were given. This second step prioritised a total of 165 papers. Ecotoxicity data and data around experimental design were gathered where from this refined list.
Data collection
The dose descriptors for toxicity endpoints collated from the literature studies were effect concentrations (ECx), inhibition concentrations (ICx), lethal concentrations (LCx), lowest observed effect concentrations (LOEC) and no observed effect concentrations (NOEC). From microbial studies, dose descriptors also included minimum inhibition concentration (MIC) and minimum bactericidal concentration (MBC).Alongside the ecotoxicity data from the study, information on the AgNM form used (i.e. powder or suspension), NM pre-exposure treatments (i.e., sonication and ageing in biosolids) as well as extrinsic AgNM properties (hydrodynamic diameter, polydispersity index and surface charge) were collected. Data for as-manufactured (pristine) AgNM and transformed (aged) material were also collected. Aged AgNMs represent a more environmentally relevant form of AgNM for soils. The available soil property information reported for the study were also collected – i.e. pH, organic carbon (OC), water holding capacity (WHC), cation exchange capacity (CEC) and sand, silt and clay content.
Normalising toxicity data to chronic NOEC
A range of different dose descriptors (LCx, ECx, NOEC, LOEC etc.) and test durations (hours to >100 days) were represented in the collated dataset. For the SSD analysis, it is important that the data derived from studies can be reasonably compared. To this end, a previously applied framework was applied to the dataset, whereby toxicity values expressed as different metrics and for different exposure times collected from studies are converted to chronic (long-term) no-effect concentrations (NOEC) using a defined set of assessment factors (AFs) (eqn (1)).35 AFs are widely used in ecotoxicology literature to harmonise toxicity values for ERA purposes. These AFs are applied depending on the dose descriptor and the duration of the assay associated with the toxicity value.| Chronic NOEC = (dose descriptor value/(AFeffect × AFduration)) | (1) |
| Dose descriptor value | AFeffect | Ref. |
|---|---|---|
| NOEC, LNOEC | 1 | 35 |
| <EC25/LC25/IC25, LOEC, MIC or MBC | 2 | |
| ≥EC25/LC25/IC25 | 10 |
| Taxonomic group | Exposure | Duration (h) | AFduration | Ref. |
|---|---|---|---|---|
| Soil microbes | Acute | ≤24 | 10 | 37, 38 |
| Chronic | >24 | 1 | 39, 40 | |
| Soil invertebrates: arachnid | Acute | <72 | 10 | (—) |
| Chronic | ≥72 | 1 | (—) | |
| Soil invertebrates: chromadorea | Acute | <72 | 10 | (—) |
| Chronic | ≥72 | 1 | (—) | |
| Soil invertebrates: clitellate | Acute | <672 | 10 | 41, 42 |
| Chronic | ≥672 | 1 | 41, 42 | |
| Soil invertebrates: Collembola | Acute | ≤336 | 10 | 43 |
| Chronic | >336 | 1 | 41, 42 | |
| Soil invertebrates: Crustacea | Acute | <672 | 10 | 41, 42 |
| Chronic | ≥672 | 1 | 41, 42 | |
| Soil invertebrates: Enchytraeidae | Acute | ≤336 | 10 | 43 |
| Chronic | >336 | 1 | 41, 42 | |
| Soil invertebrates: Malacostraca | Acute | <672 | 10 | 41, 42 |
| Chronic | ≥672 | 1 | 41, 42 | |
| Soil invertebrates: Tetranychidae | Acute | <72 | 10 | (—) |
| Chronic | ≥72 | 1 | (—) | |
| Terrestrial plants | Acute | ≤336 | 10 | 44 |
| Chronic | >336 | 1 | 44 |
Due to the limited use of AFs in soil ecotoxicology studies, relevant acute and chronic durations for soil microbes, invertebrates and plants are proposed here (Table 1). To apply AFs associated with test duration, standard test guidelines for acute and chronic studies were considered alongside the lifespan of the test species to establish appropriate AFs that were species dependent.
For soil microbes, acute toxicity duration was derived from rapid nitrification and respiration tests conducted over 24 hours (ref. 37 and 38) and chronic toxicity tests including nitrogen and carbon transformation tests.39,40 For soil invertebrates with lifespans >1 year (Clitellata, Malacostraca and Crustacea), acute and chronic duration thresholds were based on the Eisenia and Folsomia candida reproduction tests.41,42 For soil invertebrates with lifespans <1 year (Collembola and Enchytraeidae), acute toxicity duration was derived from earthworm acute toxicity testing measuring mortality endpoints over 336 hours.43 Chronic toxicity tests include Collembola and earthworm reproduction testing over 672 hours.41,42 For soil invertebrates with lifespans between ∼20–30 days (arachnid, chromadorea and Tetranychidae), acute and chronic duration thresholds were based on test species lifespans. For terrestrial plants, acute and chronic duration thresholds were set from the EPA ecological effects test guidelines for seedling emergence and seeding growth, which states that if effects are observed between day 7–14 then the test period should be extended.44
Developing species sensitivity distributions
The data used to construct SSDs covered a range of species with different life histories, life spans and life stages (embryo, juvenile, adult).36 For soil invertebrates and plants, most available data were for apical endpoints (i.e. survival, growth and reproduction). Non-apical endpoints were also included to maximise available data, particularly for soil microbial data.The SSD modelling used the geomean of the chronic NOEC values for each species across soil invertebrates, microbes and plants. Separate SSDs were constructed for soil and liquid based assays using the “ssdtools” package in R Studio. This SSD modelling approach uses maximum likelihood and model averaging to fit multiple distributions (log logistic, log normal, gamma) and calculates a weighted average HC50 and 95% confidence intervals. The HC50 represents the chemical concentration predicted to be hazardous to 50% of species in an ecosystem. The HC50 value has been chosen rather than the more traditional HC5 value, as the HC50 is a more robust metric to use for hazard potential. It is positioned in the centre of the modelled species sensitivity curve and thus gives the lowest uncertainty, making it more robust when comparing different SSD than using values in the tail of the SSD model (i.e., the HC5). However, due to their prevalent use in risk assessment to derive risk characterization ratios, HC5 values were also calculated. Separate SSDs were constructed for ungrouped AgNMs (pristine and aged together), pristine AgNMs, aged AgNMs and Ag salt (AgNO3) based on either soil or liquid based exposure for comparison. All SSDs were constructed using available data to generate species geomean values, and using the criteria of five or more species across soil taxonomic groups (≥3 taxonomic groups).
To assess how particle properties can influence AgNM toxicity, data on the intrinsic AgNM properties (primary particle size, shape, coating) were binned into categories. AgNMs were split into three size range categories (<20 nm, 20–50 nm and >50 nm), aligning with those from previous freshwater SSDs for AgNMs.25 The AgNM data was also grouped into five different shape categories: spheroidal, cuboid, irregular, wire and plate. The spheroidal category included spherical-shaped AgNMs, while irregular referred to angular shaped AgNMs (Table S1†). Finally, the AgNM data was also grouped by coating type: polymer-coated (acrylic, PVP and PVP-PEI), organic-coated (alkane, bovine serum albumin, carbon, chitosan, citrate, citric-tannic acid, gum arabic, oleic acid, paraffin wax, Tween and Tween-ammonium nitrate, NH4NO3), combination coatings (citrate-PVP, Tween-acrylic and Tween-NH4NO3-acrylic) and uncoated (Table S1†). Where coating was not reported the AgNM was assigned to the “uncoated” category.
SSDs were constructed for AgNM characteristics (size, shape, coating), however not all AgNM particle characteristic categories had enough data points to create an SSD. Soil property data (organic carbon (OC), pH, water holding capacity (WHC), cation exchange capacity (CEC), sand %, silt % and clay %) were collected and categorised by the upper and lower quartiles of the property ranges (Table S1†). SSDs were also constructed for soil properties that showed significant correlations with chronic NOEC toxicity values: namely, OC, CEC, pH and silt content. SSDs were then developed for the upper and lower quartile OC and CEC values across the soil types.
To assess if SSD curves are significantly different from each other, an F-test was performed using the sum of squares (SS) and number of observations (df) for both combined and individual SSD curves (eqn (2)).
| F = ((SScombined − SSseparate)/(dfcombined − dfseparate))/SSseparate/dfseparate | (2) |
Relationships between particle or soil properties and toxicity
To further understand the influence of soil properties on toxicity regression analyses were conducted for both Ag forms (AgNM and Ag salt) between the logged chronic NOEC values and logged soil properties (pH, OC, WHC, CEC and sand, silt and clay content) (Table S1†). The influence of AgNM particle properties on chronic NOEC values was also explored using ANOVA. Normality tests on the toxicity data were conducted using a Shapiro test and a factorial analysis of variance (ANOVA) was conducted to compare the mean chronic NOEC values across the AgNM size, shape and coating category groups for soil and liquid-based exposures. Tukey's HSD post hoc tests were then used to assess where the significance of differences between the NM property categories. ANOVA analyses were conducted in R using “afex”, “PMCMR” and “PMCMRplus” packages.Results
Data collation summary
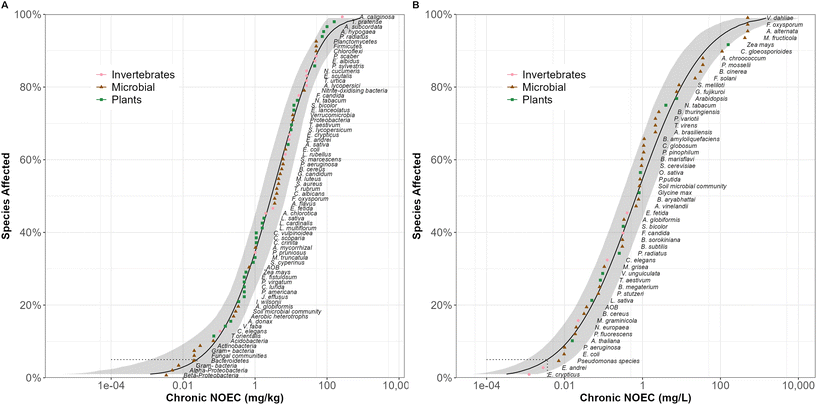
In total 2122 toxicity values were extracted from 139 soil AgNM ecotoxicology papers (1051 soil-based assays, 445 liquid-based assays). Most values were based on apical endpoints (e.g., mortality, growth, reproduction, development) for soil invertebrates and plants (Table S2†). The soil-based microbial endpoints were primarily for measures of nitrification, respiration and other soil functional endpoints (Table S2†). Microbial studies in liquid-based assays were based often on apical endpoints (microbial biomass change). The majority (>50%) of the toxicity data were for AgNMs <20 nm in size, spheroidal in shape and with some form of surface coating. The Tween-coated AgNMs refers almost exclusively to studies conducted with the Joint Research Centre (JRC) AgNM300K test material, which are coated in a polyoxyethylene glycerol trioleate and polyoxyethylene sorbitan mono-laurate “Tween” suspension.45 Sufficient toxicity data meeting the SSD criteria for model construction were available for AgNMs in the property groups: coating (Tween, citrate, PVP and uncoated), shape (spheroidal, irregular) and size (split in three categories: <20 nm, 20–50 nm, >50 nm), as well as for pristine and for aged AgNMs. Sufficient data were also available for Ag salt (AgNO3). However, there was insufficient data to construct separate SSDs based for the different physiochemical property groups for Ag2S NMs.SSDs constructed using all available Ag data
SSDs were generated for AgNMs (ungrouped = pristine and aged together, as well as pristine and aged separately) and the Ag salt (AgNO3), including all the available toxicity data for soil invertebrates, plants, microbial apical effects and microbial functions (Fig. 1 and S1†). HC50 values calculated from these AgNM SSDs were 3.06 (1.74–5.21) mg kg−1 for the studies in soil and 0.70 (0.32–1.64) mg L−1 for liquid-based exposures (Fig. 1, Table S3†). Assessment factors were applied to 75% and 91% of the toxicity values in the soil and liquid based assays respectively. HC5 values calculated from these AgNM SSDs were 0.03 (0.01–0.08) mg kg−1 for the studies in soil and 0.004 (0.001–0.01) mg L−1 for liquid-based exposures (Table S4†).Soil microbial species and associated enzyme responses were generally most sensitive to AgNM in the soil exposures. For Ag salt, HC50 values were comparable to those for AgNMs in soil exposures, 2.74 (1.22–5.23) mg kg−1 but lower than those for AgNMs when compared to the SSD based on liquid exposures, 0.01 (0.01–0.03) mg L−1 (Table S3†). However, the sensitivity of microbial endpoints was consistent between both Ag forms. Compared with pristine AgNMs, environmentally relevant AgNMs, presented a higher, although not significantly different, HC50 value (7.05 mg kg−1) (Fig. 3 and S2, Table S3†), (F ratio 0.06; p value = 0.81).
Insufficient data were available to create a separate SSD for the different effect endpoints available for the invertebrate, plant and microbial studies. Instead, to compare AgNM and Ag salt toxicity for different effect endpoints, we used the geomeans of the toxicity values calculated for the set of reported endpoint effects for the AgNM and Ag salt, expressing them as ratios. These comparisons indicated that Ag salt was typically more toxic than the NM form for 11 out of 14 effect endpoints (ratio <1) for invertebrates and microbial studies, and for all plant endpoints (Fig. 2, Table S5†). Exceptions to the general pattern were for microbial enzyme activity, microbial carbon transformation and invertebrate enzyme activity endpoints (Fig. 2, Table S5†). In liquid-based assays, the Ag salt was consistently more toxic than the NM across all effect endpoints (Table S5†). Generally, the calculated geomeans showed lower variance for Ag salt studies compared with AgNMs, in both soil and liquid assays, suggesting that AgNMs effect values vary more compared with those from for Ag salt studies, with nanomaterial property related effects being a potential cause (Table S5†).
Influence of NM properties on hazard thresholds
In soil-based assays, the smallest size class (<20 nm = 1.98 mg kg−1; 20–50 nm = 6.41 mg kg−1) had lower HC50 values for NMs compared to larger classes (>50 nm = 13.82 mg kg−1). However, no significant differences across size classes were found, i.e., the SSD models were not significantly different by F-test (Fig. S3; Table S3†), due to broader confidence intervals for larger classes (<20 nm = 1.27–3.21 mg kg−1; 20–50 nm = 2.48–14.08 mg kg−1; >50 nm = 2.84–55.78 mg kg−1). For the shape classes, there was overlap in the HC50 95% CI values for spheroidal (1.15–3.97 mg kg−1) and irregular (1.37–67.83 mg kg−1) shaped AgNMs (Fig. S3, Table S3†) indicating that shape did not have a significant effect on AgNM ecotoxicity. For coatings, SSDs were overlapping for citrate-PVP-, tween- and uncoated AgNMs (Fig. S3†) indicating no significant difference on ecotoxicity for these coating. The SSD for citrate-coated AgNMs (n = 7 studies) was, however, different from the distributions for the other AgNM coating groups, with a lower HC50 value (1.14 mg kg−1) (Fig. 3 and S3, Table S3†), and a significantly lower value compared with uncoated NMs (n = 22 studies) (F ratio = 11.2; p value <0.01). In liquid-based assays, SSD curves for smaller NMs (<50 nm) indicated a slightly lower HC50 (<20 nm = 0.51 mg L−1 and 20–50 nm = 0.14 mg L−1) compared with the larger size class (>50 nm) AgNMs (2.49 mg L−1) (Fig. S4†), although this difference was not significant (Fig. 3 and S4, Table S3†). Comparing the HC50 from each coating groups indicated similar values for PVP (n = 24 studies) and uncoated AgNMs (n = 24 studies). Both, however, had a significantly lower HC50 values compared with citrate-coated AgNMs (n = 10 studies) (citrate vs. PVP: F ratio = 23.5; p value <0.01; and citrate vs. uncoated: F ratio = 42.8; p value <0.01). For shape, sufficient species toxicity data were only available to generate a single SSD for spheroidal shaped AgNMs. Hence, no comparison to other shapes could be made (Fig. S4†).Considering AgNM size class, the chronic NOEC for the smallest size class (<20 nm mean = 14.2 mg kg−1 ± 52.4) was significantly lower than the 20–50 nm size class (46.9 mg kg−1 ± 153.2) (Fig. S5, Tables S6 and S7†) from the ANOVA analyses. Significant differences were also found between surface coating, with the chronic NOEC values PVP-coated AgNMs being significantly lower compared with uncoated AgNMs (Fig. S6 and S7, Tables S6 and S7†). In the case of shape, no significant differences were found, although factorial ANOVA indicated interactions between size-shape as well as size-coating (Fig. S5 and S8, Table S6†).
Influence of soil properties on hazard thresholds
SSD models for soil-based studies indicated that AgNMs were significantly more toxic in soils in the “low” OC category (HC50 95% CI for lower quartile values = 0.46–2.65 mg kg−1 from 40 studies and HC50 95% CI for upper quartile values = 6.21–14.16 mg kg−1 from 20 studies) (Fig. 3 and S9, Table S3†) (F ratio = 30.2; p value <0.01). Lower hazard thresholds were determined in soils with higher CEC (lower quartile values = 2.33–6.34 mg kg−1 from 64 studies and upper quartile values = 0.03–1.87 mg kg−1 from 10 studies) (F ratio = 16.0; p value <0.01). Soil pH did not influence the SSD curves with no significant difference between HC50 values (Fig. S9†). There were weakly significant relationships between soil properties and toxicity for both Ag salt and AgNMs (Fig. S10 and S11, Table S8†). Toxicity decreased with lower WHC, lower CEC and higher OC (Fig. S10 and S11, Table S8†) for both the Ag salt and AgNMs. For soil pH, there was also a weak but significant relationship between AgNM toxicity and pH, with lower toxicity at higher pH. In contrast there was a positive relationship between pH and toxicity for the Ag salt (Fig. S10 and S11, Table S8†).Discussion
Hazard thresholds for AgNMs
Reliable hazard thresholds are key to underpinning risk assessment but determining these needs robust data to be available for multiple species. For nanomaterials, there is a wealth of ecotoxicity data now available, in particular for AgNMs, which allows hazard thresholds to be derived. Currently the extent to which chemical form, NM properties or soil properties could influence NM hazards are not fully elucidated for soils. In this study, we established that AgNMs had higher hazard thresholds (i.e., lower toxicity) compared to Ag salts. This finding is in line with previous findings for studies of similar aim conducted for aquatic species.12–14The one previous attempt to construct an AgNM SSD for soil species was limited by the availability of ecotoxicity data (pre-2015). At the time, only data for 4 species across 2 taxonomic groups was reported.46 The HC5 value of 8.2 mg kg−1 [95% CIs: 4.3–12.5 mg kg−1] calculated by this early study is ∼300-fold higher than the HC5 concentration that we calculated from our expanded dataset (Table S4†). The data that underpins our SSDs for soil species cover studies across 3 trophic levels (microbes, invertebrates and terrestrial plants) for both AgNMs and Ag salts. The inclusion of microbial studies in our dataset will have influenced the lower HC5 value determined from our model (n.b. the Coll et al. (2016) study only included data for invertebrates and plants).46 The inclusion of microbial endpoints highlights the relatively high sensitivity of the group to AgNMs consistent with the known anti-bacterial mode of action of Ag generally and as NMs. For example, sensitivity of microbes to AgNMs was demonstrated in toxicity studies by Peyrot et al. (2014),31 who found that AgNMs were similar or more toxic than Ag salt to soil microbial enzyme activity endpoints.
Our analyses found similar HC50 values for both Ag forms (salt and NM) when exposed in soils. However, in liquid medium, the Ag salt was significantly more toxic. Individual soil toxicity studies have found Ag salt to be more toxic to soil species than AgNMs, e.g., for earthworms and Collembola for endpoints including gene expression, survival and reproduction.12,14,47 However, other studies, have found effects at similar concentrations for both Ag salt and Ag NMs, e.g. for nematodes.13 Although greater NM effects compared with salt can be observed in some cases, this effect appears to be species or exposure dependant, whereby NM and soil physiochemical characteristics as well as exposure conditions can influence hazard outcomes. Exposure will differ based on species traits such as feeding behaviour and physiology as these will influence the uptake of NMs and their potential to cause effects. For example, uptake via ingestion or across epithelium and through body surfaces and openings can result in varying exposure to NMs and Ag ions associated with different components of the soil (e.g. soil pore water versus solid soil matrix), thus influencing subsequent effects.48 Thus, the relative sensitivity of species will be linked not only with species traits, but also with Ag bioavailability in the soil.
Aquatic SSDs comparing pristine and transformed (sulfidised) AgNM have shown a significant difference in toxicity, with a ∼240-fold higher HC5 value for transformed AgNMs indicating their lower toxicity compared to pristine forms.19 Here we observed a similar difference, albeit less extreme, with pristine AgNMs being 3-fold more toxic than aged AgNMs (although the overlap of HC50 95% CIs indicate that this effect size difference was not statistically significant). The aged state (e.g., sulfidised) of AgNMs is likely to be the most environmentally relevant exposure form and so is important to consider within soil risk assessments.18 While not conclusive, our evidence points to aging having a small effect of toxicity, with this most likely being to reduce NM toxicity.
NM properties driving hazard thresholds
Particle properties such as size and surface coating can alter the surface area-to-size ratio, surface charge, aggregation state and stability of NMs.20–22 These characteristics can also affect transformation processes, such as the rate of ion release, which will influence bioavailability of NMs and toxicity to organisms.49 Individual toxicity studies have investigated NM physiochemical property-driven toxicity in soils,20–22 but consensus has not been reached on the key driving properties for NMs more generally. This is the first study we are aware of to use an SSD approach to investigate how NM properties influence hazard for soil species. Collecting sufficient information to construct separate property group SSDs was constrained by the available data yet we were able to successfully construct separate SSDs for NM size, shape and coating from the dataset. For both soil and liquid medium based assays, there was no effect of NM size or shape on the SSD outcome and similar HC50 values were obtained, suggesting that fate and bioavailability is similar across the different NMs. Size and shape have also been found not to influence hazard based on comparable SSDs constructed for aquatic species.25 NM coating on the other hand was found to influence hazard thresholds for soil and liquid based SSDs. In the case of soil-based SSD citrate-coated NMs had HC50 values in a lower range compared with the uncoated NMs or NMs with other coatings (PVP etc.). However, there was clearly substantial variance among the different studies collated in the dataset which means there is overlap of the HC50 confidence intervals and convergence of the SSD curves. The liquid media SSDs also showed a significant effect of coating on the hazard but was more pronounced compared with the soil-based SSD. Also, in contrast to the soils SSD, citrate-coated AgNMs were found to be less toxic compared with other coated or uncoated NMs. Previously constructed aquatic SSDs for AgNMs have likewise explored the influence of AgNM properties on SSD curves and similar to our findings from the liquid media-based SSD, hazard was coating dependent with coated particles showing higher toxicity compared with uncoated particles.25,26 Surface coatings generally act as a means of stabilising but will differ in their potential for degradation as well as their strength of interaction with the NM surface which will determine their persistence in the environment as well as their stability and toxicity.49,50 It has been proposed that pristine NMs with differing properties that enter the environment can subsequently converge and may be grouped into function fate groups based on common transformations and interactions (e.g. attachment to soil solid phase, particle heteroaggregation and chemical transformation including surface coating degradation).18,49 Dissolution is one of the principal parameters proposed to allow for NMs of different properties to be grouped in aquatic toxicity assessment.51 Several studies have investigated the role of dissolution in driving Ag NM bioavailability and toxicity in different soils.52,53 While some studies show clear relationships between dissolution and Ag uptake,53 others show that there can be uptake and toxicity without dissolution occurring.54,55 NM coating has also been shown to govern toxicity of Ag NM in aquatic studies55 with citrate showing higher dissolution rates and toxicity compared with PVP coated NMs.20,56 However, to conclusively link with dissolution across the wider dataset there needs to be more comprehensive measurement and reporting of dissolution in studies, within the timescales of the of bioassays. Thus, enhanced reporting of NM properties alongside toxicity data in studies would improve our ability to interpret the hazard outcomes for NMs with differing characteristics. It would elucidate the relative importance of NM coating for driving NM hazard in soils as well as examine to what extent to which functional fate groups converge hazard outcomes.Soil properties as a driver of AgNM toxicity
Soil properties are known to affect metal and NM bioavailability in soils.29,57,58 Regression analysis indicated that multiple soil properties (OC, WHC and CEC) influenced AgNMs and Ag salt in a similar way in soils. For both the AgNM and Ag salt, there was a significant positive relationship between chronic NOECs and % OC. This pattern can be linked to bioavailability through the adsorption of Ag ions to organic matter decreasing mobility and bioavailability,59 affecting biological responses in soil, e.g. AgNM effects on nitrification and enzyme activity.60 A study showing OC effects on AgNM toxicity was also able to link moderated toxicity with reduced NM dissolution in OC amended soil,31 with low OC linked to greater Ag ion bioavailability through altered oxidation rates of the AgNMs.24,61 Other studies have also highlighted the importance of grain size on the bioavailability of Ag ions, reporting increases in AgNM toxicity with higher sand content and lower clay content in soils.6 Clay is a high surface area sorbent. However, our study found no significant relationship between chronic NOEC values for AgNM and sand or clay content. The greater toxicity of both Ag forms seen under high CEC conditions was unexpected as it would be expected that higher binding under these conditions would result in lower bioavailability and so toxicity to soil organisms. However, soil properties will be interactive and correlate which means it can be difficult to tease apart the main driving property. For example, ion release under low pH conditions could contribute to toxicity although combined in a soil with higher OC can then counteract this through binding of available ions reducing toxicity.29 Other studies have also shown that effect values based on internal concentration in organisms rather than based on total soil concentrations can improve correlations between soil properties and effects.34,62 Effect concentrations based on internal concentrations are less commonly reported in literature but with more data being available and for a greater diversity of soils we would be better able elucidate what soil properties dominate NM fate but also how these properties interact to govern these fate process and consequently toxicity.Limitations of existing data and developed soil SSDs for AgNMs
The effects observed in toxicity studies can be driven by NM and test media characteristics as well as aspects of experimental design, such as the dispersion status of the NM during testing, exposure duration and the endpoint(s) measured. While some NM, soil and experimental features can have a clear effect on AgNM hazard individually, it is probable that interactions between these features also occur that will influence fate and behaviours, for example aggregation, dissolution, bioavailability.18 Previous work to develop SSD models for aquatic species have attempted to incorporate NM characteristics and types (i.e., powder or suspension), test pre-treatments (i.e., sonication) and test set-up conditions (i.e., pH) into developing models for ZnO and CuO NMs.63 This modelling indicated that interacting factors do influence NM stability and fate. Although our dataset was sufficient to allow SSDs to be constructed for some particle characteristics (size, shape and coating) groups, other such groups could not be modelled, indicated further work on these less well studied AgNM types is needed.In currently available soil ecotoxicology literature for AgNMs there is only limited in-exposure measurements (e.g., hydrodynamic diameter, polydispersity index and zeta potential) with values for such dynamic properties reported in <11% of the liquid medium toxicity values and <3% of the soil studies (Table S9†). For most studies (∼66%), pre-exposure measurements of these variables were available, however, these were generally only made in ultra-pure water, and not the test exposure medium (Table S9†). With more available in situ measurements (including dissolution) the fate and behaviours of AgNM characteristics could be explored in more detail. It is notable that this ecotoxicity dataset for AgNM is one of the richest available for all NMs, suggesting that studies taking a similar approach with other commonly produced NMs such as Al2O3, CeO2, iron oxide (Fe2O3), silicon dioxide (SiO2), TiO2 and ZnO would likely be more restricted.
Conclusion
SSDs were generated for AgNM and Ag salt for soil organisms using existing published ecotoxicological data. The available data allowed the first SSDs to be constructed that incorporate toxicity data from 3 soil taxonomic groups (microbes, invertebrates and plants) for up to 74 species. Microbial-based endpoints were most sensitive, consistent with the antibacterial (biocidal) properties of AgNMs. The available toxicity data allowed us to model multiple SSDs for AgNMs and Ag salt, including SSDs for specific NM property groups. Although it would be expected that size and shape would influence AgNM toxicity to soil species, this was not observed from our SSDs. It was, however, possible to identify coating as a driving factor of AgNM toxicity in soils. Grouping of nanoforms with different properties may be possible when considering soil hazards, at least within the diversity of properties that could be tested through distinct SSDs. However future studies with more in-exposure particle measurements would improve the relevance of the NM property data to the assessment of AgNM toxicity in soils. We found a 3-fold difference in toxicity between pristine and aged AgNMs, however, high variation in toxicity data meant that this effect was not significant, although it may warrant further study. Effects of coating type on AgNM toxicity were found, with citrate-coated AgNMs being significantly more toxic than uncoated NMs in soil exposures, whilst in liquid medium citrate-coated AgNMs were significantly less toxic than uncoated or PVP coated NMs. Significant differences were observed in AgNM toxicity from corresponding SSDs for CEC and OC as these soil characteristics can influence the release and bioavailability of Ag ions. Overall, our work provides a database (see ESI†) of soil Ag toxicity data which can be built upon with future ecotoxicological studies and SSDs derived hazard thresholds for AgNMs in soil.Data availability
The authors confirm that the data supporting the findings of this study are available within the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the “Simple, robust and cost-effective approaches to guide industry in the development of safer nanomaterials and nano-enabled products” (SabyNA) and the “Anticipating safety issues at the design stage of Nano product development” (ASINA) projects. These projects are financed by the Horizon 2020 EU's research in innovation funding programme (SabyNA project grant agreement ID: 862419). The SabyNA project provided input on AgNM300K and the ASINA project provided input on particle properties as drivers of toxicity.References
- L. Gutierrez, C. Aubry, M. Cornejo and J. P. Croue, Citrate-coated silver nanoparticles interactions with effluent organic matter: influence of capping agent and solution conditions, Langmuir, 2015, 31, 8865–8872, DOI:10.1021/acs.langmuir.5b02067.
- Nanomaterials Market Size, Growth, Trends Report 2023-2032, 2023, (https://www.precedenceresearch.com/sample/1752).
- J. Kyziol-Komosinska, A. Dzieniszewska and J. Czupioł, Behaviour of silver species in soil: Ag Nanoparticles vs. ionic Ag, Molecules, 2024, 29, 5531 CrossRef CAS PubMed , Available from: https://www.mdpi.com/1420-3049/29/23/5531.
- R. Ding, L. Li, Y. Pingjian, L. Luo, L. Li and Q. Wang, Assessing the environmental occurrence and risk of nano-silver in Hunan, China using probabilistic material flow modelling, Sci. Total Environ., 2019, 658, 1249–1255 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0048969718351258?via%3Dihub.
- R. Ma, C. Levard, J. D. Judy, J. M. Unrine, M. Durenkamp, B. Martin, B. Jefferson and V. L. Gregory, Fate of zinc oxide and silver nanoparticles in a pilot wastewater treatment plant and in processes biosolids, Environ. Sci. Technol., 2014, 48, 104–112, DOI:10.1021/es403646x.
- K. Schlich and K. Hund-Rinke, Influence of soil properties on the effect of silver nanomaterials on microbial activity in five soils, Environ. Pollut., 2015, 196, 321–330 CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0269749114004448?via%3Dihub.
- R. C. Bicho, T. Ribeiro, N. P. Rodrigues, J. J. Scott-Fordsmand and M. J. B. Amorim, Effects of Ag nanomaterials (NM300K) and Ag salt (AgNO3) can be discriminated in a full life cycle long term test with Enchytraeus crypticus, J. Hazard. Mater., 2016, 318, 608–614 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0304389416306720.
- A. H. Jesmer, J. R. Velicogna, D. M. Schwertfeger and J. I. Princz, The toxicity of silver to soil organisms exposed to silver nanoparticles and silver nitrate in biosolids-amended field soil, Environ. Toxicol. Chem., 2017, 36, 2756–2765 CrossRef CAS PubMed , Available from: https://academic.oup.com/etc/article/36/10/2756/7740422?login=true.
- A. D. Samarajeewa, J. R. Velicogna, J. I. Princz, R. M. Subasinghe, R. P. Scroggins and L. A. Beaudette, Effect of AgNPs on soil microbial growth, activity and community diversity in a sandy loam soil, Environ. Pollut., 2017, 220, 504–513 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0269749116315585?via%3Dihub.
- J. Huang, C. Cao, R. Li and W. Guan, Effects of Silver Nanoparticles on soil Ammonia-Oxidizing Microorganisms under temperatures of 25 and 5°C, Pedosphere, 2018, 28, 607–616 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S1002016018600360?via%3Dihub.
- K. Hund-Rinke, A. Hümmler, R. Schlinkert, F. Wege and G. Broll, Evaluation of microbial shifts caused by a silver nanomaterial: comparison of four test systems, Environ. Sci. Eur., 2019, 31, 1–13, DOI:10.1186/s12302-019-0268-z.
- S. I. L. Gomes, A. M. V. M. Soares, J. J. Scott-Fordsmand and M. J. B. Amorim, Mechanisms of response to silver nanoparticles on Enchytraeus albidus (Oligochaeta): Survival, reproduction and gene expression profile, J. Hazard. Mater., 2013, 254–255, 336–344 CrossRef CAS PubMed , https://www.sciencedirect.com/science/article/pii/S0304389413002604?via%3Dihub.
- J. M. Ahn, H. J. Eom, X. Yang, J. N. Meyer and J. Choi, Comparative toxicity of AgNPs on oxidative stress and DNA damage in the nematode, Caenorhabditis elegans, Chemosphere, 2014, 108, 343–352, DOI:10.1016/j.chemosphere.2014.01.078 , Epub 2014 Apr 13. PMID: 24726479.
- D. Hlavkova, M. Beklova, P. Kopel and B. Havelkova, Effects of AgNPs and ions exposure on the soil invertebrates folsomia candida and enchytraeus crypticus, Bull. Environ. Contam. Toxicol., 2020, 105, 244–249, DOI:10.1007/s00128-020-02909-7.
- D. A. Notter, D. M. Mitrano and B. Nowack, Are nanosized or dissolved metals more toxic in the environment? A meta-analysis, Environ. Toxicol. Chem., 2014, 33, 2733–2739 CrossRef CAS PubMed , Available from: https://academic.oup.com/etc/article/33/12/2733/7761356?login=true.
- M. Diez-Ortiz, E. Lahive, S. George, A. Ter Schure, C. A. M. Van Gestel, K. Jurkschat, C. Svendsen and D. J. Spurgeon, Short-term soil bioassays may not reveal the full toxicity potential for nanomaterials; bioavailability and toxicity of silver ions (AgNO3) and silver nanoparticles to earthworm Eisenia fetida in long-term aged soils, Environ. Pollut., 2015, 203, 191–198 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0269749115001633?via%3Dihub.
- F. C. F. Santos, R. A. Verweij, C. A. M. van Gestel and M. J. B. Amorim, Toxicokinetics and toxicodynamics of Ag nanomaterials (NM300K) in the soil environment-impact on Enchytraeus crypticus (Oligochaeta), Ecotoxicol. Environ. Saf., 2023, 252, 114599 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0147651323001033?via%3Dihub.
- C. Svendsen, L. W. Walker, M. Matzke, E. Lahive, S. Harrison, A. Crossley, B. Park, S. Lofts, I. Lynch, S. Vázquez-Campos, R. Kaegi, A. Gogos, C. Asbach, G. Cornelis, F. von der Kammer, N. W. van den Brink, C. Mays and D. J. Spurgeon, Key principles and operational practices for improved nanotechnology environmental exposure assessment, Nat. Nanotechnol., 2020, 15, 731–742 CrossRef CAS PubMed , Available from: https://www.nature.com/articles/s41565-020-0742-1.
- H. Hong, V. Adam and B. Nowack, Form-specific and probabilistic environmental risk assessment of 3 engineered nanomaterials (nano-Ag, nano-TiO2, and nano-ZnO) in European freshwaters, Environ. Toxicol. Chem., 2021, 40, 2629–2639 CAS , Available from: https://academic.oup.com/etc/article/40/9/2629/7734406?login=true.
- B. M. Angel, G. E. Batley, C. V. Jarolimek and N. J. Rogers, The impact of size on the fate and toxicity of nanoparticulate silver in aquatic systems, Chemosphere, 2013, 93, 359–365 CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0045653513007212?via%3Dihub.
- S. Schiavo, N. Duroudier, E. Bilbao, M. Mikolaczyk, J. Schäfer and M. P. Caiaraville, et al., Effects of PVP/PEI coated and uncoated silver NPs and PVP/PEI coating agent on three species of marine microalgae, Sci. Total Environ., 2017, 577, 45–53 CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0048969716322185?via%3Dihub.
- S. Temizel-Sekeryan and A. L. Hicks, Emerging investigator series: calculating size- and coating- dependent effect factors for silver nanoparticles to inform characterization factor development for usage in life cycle assessment, Environ. Sci.: Nano, 2020, 7, 2436 RSC , Available from: https://rsc.66557.net/en/content/articlelanding/2020/en/d0en00675k.
- J. Moon, J. I. Kwak and A. Youn-Joo, The effects of AgNM shape and size on toxicity to C.elegans in soil media, Chemosphere, 2019, 215, 50–56 CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0045653518318435?via%3Dihub.
- W. A. Shoults-Wilson, B. C. Reinsch, O. V. Tsyusko, P. M. Bertsch, G. V. Lowry and J. M. Unrine, Role of particle size and soil type in toxicity of AgNPs to earthworms, Soil Sci. Soc. Am. J., 2011, 75, 365–377, DOI:10.2136/sssaj2010.0127nps.
- G. Chen, W. J. G. M. Peijnenburg, Y. Xiao and M. G. Vijver, Developing species sensitivity distributions for metallic nanomaterials considering the characteristics of nanomaterials, experimental conditions, and different types of endpoints, Food Chem. Toxicol., 2018, 112, 563–570 CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0278691517301710?via%3Dihub.
- K. L. Garner, S. Suh, H. S. Lenihan and A. A. Keller, Species Sensitivity Distributions for Engineered Nanomaterials, Environ. Sci. Technol., 2015, 49, 5753–5759, DOI:10.1021/acs.est.5b00081.
- S. Makama, J. Piella, A. Undas, W. J. Dimmers, R. Peters, V. F. Puntes and N. W. van den Brink, Properties of silver nanoparticles influencing their uptake in and toxicity to the earthworm Lumbricus rubellus following exposure in soil, Environ. Pollut., 2016, 218, 870–878 CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0269749116307114?via%3Dihub.
- C. Schultz, E. Lahive, A. Lawlor, A. Crossley, V. Puntes, J. M. Unrine, C. Svendsen and D. J. Spurgeon, Influence of soil porewater properties on the fate and toxicity of AgNPs to C.elegans, Environ. Toxicol. Chem., 2018, 37, 2609–2618 CAS , Available from: https://academic.oup.com/etc/article/37/10/2609/7739413?login=true.
- E. Lahive, M. Matzke, C. Svendsen, D. J. Spurgeon, H. Pouran, H. Zhang, A. Lawlor, G. Pereira and S. Lofts, Soil properties influence the toxicity and availability of Zn from ZnO nanoparticles to earthworms, Environ. Pollut., 2023, 319, 120907 CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0269749122021224?via%3Dihub.
- A. J. Calder, C. O. Dimkpa, J. E. McLean, D. W. Britt, W. Johnson and A. J. Anderson, Soil components mitigate the antimicrobial effects of silver nanoparticles towards a beneficial soil bacterium, Pseudomonas chlororaphis O6, Sci. Total Environ., 2012, 429, 215–222 CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0048969712005815?via%3Dihub.
- C. Peyrot, K. J. Wilkinson, M. Desrosiers and S. Sauve, Effects of silver nanoparticles on soil enzyme activities with and without added organic matter, Environ. Toxicol. Chem., 2014, 33, 115–125 CrossRef CAS , Available from: https://academic.oup.com/etc/article/33/1/115/7761106?login=true.
- M. S. McKee, M. Engelke, X. Zhang, E. Lesnikov, J. Köser, T. Eickhorst and J. Filser, Collembola reproduction decreases with aging of silver nanoparticles in a sewage sludge treated soil, Front. Environ. Sci., 2017, 5, 1–9, DOI:10.3389/fenvs.2017.00019/full.
- A. L. Grün, W. Manz, Y. L. Kohl, F. Meier, S. Straskraba, C. Jost, R. Drexel and C. Emmerling, Impact of silver nanoparticles (AgNP) on soil microbial community depending on functionalization, concentration, exposure time, and soil texture, Environ. Sci. Eur., 2019, 31, 1–22, DOI:10.1186/s12302-019-0196-y.
- E. Topuz and C. A. M. van Gestel, The effect of soil properties on the toxicity and bioaccumulation of Ag nanoparticles and Ag ions in Enchytraeus crypticus, Ecotoxicol. Environ. Saf., 2017, 144, 330–337 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0147651317303688?via%3Dihub.
- H. Wigger, D. Kawecki, B. Nowack and V. Adam, Systematic consideration of parameter 727 uncertainty and variability in probabilistic species sensitivity distributions, Integr. Environ. Assess. Manage., 2020, 16, 211–222 Search PubMed , Available from: https://academic.oup.com/ieam/article/16/2/211/7726242?login=true.
- ECHA, Guidance on information requirements and chemical safety assessment. Chapter R.10: Characterisation of dose [concentration] response for environment, European chemicals agency, Helsinki, Finland, 2008, Available from: https://www.reach-clp-biozid-helpdesk.de/SharedDocs/Publikationen/EN/REACH/ECHA/IR-CSA/ECHA_Leitlinie_Guidance_on_information_requirements_and_chemical_safety_assessment_Chapter_R.10 Search PubMed.
- ISO 15685, Soil quality determination of potential nitrification and inhibition of nitrification. Rapid test by ammonium oxidation, 2012, Available from: https://www.iso.org/standard/53530.html.
- ISO 16072, Soil quality laboratory methods for determination of microbial soil respiration, 2002, Available from: https://www.iso.org/standard/32096.html.
- OECD Test No. 216, Soil microorganisms: nitrogen transformation test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris, 2000, Available from: https://www.oecd.org/content/dam/oecd/en/publications/reports/2000/01/test-no-216-soil-microorganisms-nitrogen-transformation-test_g1gh290f/9789264070226-en.pdf Search PubMed.
- OECD Test No. 217, Soil microorganisms: carbon transformation test, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris, 2000, Available from: https://www.oecd.org/en/publications/2000/01/test-no-217-soil-microorganisms-carbon-transformation-test_g1gh2911.html Search PubMed.
- ISO 11267, Soil quality Inhibition of reproduction of Collembola (Folsomia candida) by soil contaminants, 2023, Available from: https://www.iso.org/standard/79816.html.
- ISO 11268-2, Soil quality Effects of pollutants on earthworms. Part 2: Determination of effects on reproduction of Eisenia fetida/Eisenia andrei and other earthworm species, 2023, Available from: https://www.iso.org/standard/79045.html.
- OECD Test No. 207, Earthworm Acute Toxicity Tests, OECD Guidelines for the Testing of Chemicals, Section 2, OECD Publishing, Paris, 1984, Available from: https://www.oecd.org/content/dam/oecd/en/publications/reports/1984/04/test-no-207-earthworm-acute-toxicity-tests_g1gh28fd/9789264070042-en.pdf Search PubMed.
- EPA, Ecological effects test guidelines. Seedling emergence and seedling growth, 2012, Available from: https://www.epa.gov/test-guidelines-pesticides-and-toxic-substances/series-850-ecological-effects-test-guidelines.
- S. Comero, C. Klein, B. Stahlmecke, J. Romazoanov, T. Kuhlbusch, E. Van Doren, P. Wick, G. Locoro, W. Kördel, B. Gawlik, J. Mast, H. Krug, K. Hund-Rinke, S. Friedrichs, G. Maier, J. Werner and T. Linsinger, NM-series of representative manufactured nanomaterials NM-300 silver characterisation, stability, homogeneity, EUR 24693 EN, Publications Office of the European Union, Luxembourg, 2011, p. JRC60709.
- C. Coll, D. Notter, F. Gottschalk, T. Sun, C. Som and B. Nowack, Probabilistic environmental risk assessment of five nanomaterials (nano-TiO2, nano-Ag, nano-ZnO, CNT, and fullerenes), Nanotoxicology, 2016, 10, 436–444, DOI:10.3109/17435390.2015.1073812.
- S. I. L. Gomes, I. Zanoni, M. Blosi, A. Costa, D. Hristozov, J. J. Scott-Fordsmand and M. J. B. Amorim, Safe and sustainable by design Ag nanomaterial: A case study to evaluate the bio-reactivity in the environment using a soil model invertebrate, Sci. Total Environ., 2024, 927, 171860 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0048969724020035?via%3Dihub.
- N. W. Van den Brink, A. J. Kokoalj, P. V. Silva, E. Lahive, K. Norrfors, M. Baccaro, Z. Khodaparast, S. Loureiro, D. Drobne, G. Cornelis, S. Lofts, R. D. Handy, C. Svendsen, D. Spurgeon and C. A. M. van Gestel, Tools and rules for modelling uptake and bioaccumulation of nanomaterials in invertebrate organisms, Environ. Sci.: Nano, 2019, 6, 1985–2001 RSC , Available from: https://rsc.66557.net/en/content/articlelanding/2019/en/c8en01122b.
- D. J. Spurgeon, E. Lahive and C. L. Schultz, Nanomaterial transformations in the environment: effects on changing exposure forms on bioaccumulation and toxicity, Small, 2020, 16, 1–12, DOI:10.1002/smll.202000618.
- D. D. Varsou, L. J. A. Ellis, A. Afantitis, G. Melagraki and I. Lynch, Ecotoxicological read-across models for predicting acute toxicity of freshly dispersed versus medium-aged NMs to Daphnia magna, Chemosphere, 2021, 285, 131452 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S004565352101924X?via%3Dihub.
- R. K. Cross, D. Spurgeon, C. Svendsen, E. Lahive, S. Little, F. von der Kammer, F. Loosli, M. Matzke, T. F. Fernandes, V. Stone, W. J. G. M. Peijnenburg and E. A. J. Bleeker, An integrated approach to testing and assessment (IATA) to support grouping and read-across of nanomaterials in aquatic systems, Nano Today, 2024, 54, 102065 CrossRef CAS , Available from: https://www.sciencedirect.com/science/article/pii/S1748013223003146?via%3Dihub.
- Z. Khodaparast, C. A. M. van Gestel, A. G. Papadiamantis, S. F. Gonçalves, I. Lynch and S. Louriero, Toxicokinetics of silver nanoparticles in the mealworm Tenebrio molitor exposed via soil or food, Sci. Total Environ., 2021, 777, 146071 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0048969721011384.
- M. Baccaro, M. D. Montaño, X. Cui, A. Mackevica, I. Lynch, F. von der Kammer, R. W. Lodge, A. N. Khlobystov and N. W. van den Brink, Influence of dissolution on the uptake of biometallic nanoparticles Au@Ag-NPs in soil organism Eisenia fetida, Chemosphere, 2022, 302, 134909 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0045653522014023?via%3Dihub.
- C. L. Doolette, M. J. McLaughlin, J. K. Kirby and D. A. Navarro, Bioavailability of silver and silver sulfide nanoparticles to lettuce (Lactuca sativa): Effect of agricultural amendments on plant uptake, J. Hazard. Mater., 2015, 300, 788–795 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0304389415300030?via%3Dihub.
- D. Hernández-Moreno, M. Fernández-Díaz, I. Rucandio, J. M. Navas and M. L. Fernández-Cruz, Toxic effects of different coating-related functionalized nanoparticles on aquatic organisms, Toxics, 2024, 12, 142 CrossRef PubMed , Available from: https://www.mdpi.com/2305-6304/12/2/142.
- A. J. Kennedy, M. S. Hull, A. Bednar, J. D. Goss, J. C. Gunter, J. L. Bouldin, P. J. Vikesland and J. A. Steevens, Fractionating Nanosilver: Importance for determining toxicity to aquatic test organisms, Environ. Sci. Technol., 2010, 44, 9571–9577, DOI:10.1021/es1025382.
- K. Lock and C. R. Janssen, Influence of ageing on zinc bioavailability in soils, Environ. Pollut., 2003, 126, 371–374 CAS , Available from: https://www.sciencedirect.com/science/article/pii/S026974910300232X?via%3Dihub.
- H. Qiu and E. Smolders, Nanospecific phytotoxicity of CuO Nanoparticles in soils disappeared when bioavailability factors were considered, Environ. Sci. Technol., 2017, 51, 11976–11985, DOI:10.1021/acs.est.7b01892.
- C. Coutris, E. J. Joner and D. H. Oughton, Aging and soil organic matter content affect the fate of silver nanoparticles in soil, Sci. Total Environ., 2012, 420, 327–333 CAS , Available from: https://www.sciencedirect.com/science/article/pii/S0048969712000708?via%3Dihub.
- K. Hund-Rinke and K. Schlich, The potential benefits and limitations of different test procedures to determine the effects of Ag nanomaterials and AgNO3 on microbial nitrogen transformation in soil, Environ. Sci., 2014, 26, 1–12, DOI:10.1186/s12302-014-0028-z.
- G. Cornelis, C. D. M. Thomas, M. J. MacLaughlin, J. K. Kirby, D. G. Beak and D. Chittleborough, Retention and dissolution of engineered silver nanoparticles in natural soils, Soil Sci. Soc. Am. J., 2012, 76, 891–902, DOI:10.2136/sssaj2011.0360.
- J. J. Scott-Fordsman, J. Mariyadas and M. J. B. Amorim, Soil type dependent toxicity of AgNM300K can be predicted by internal concentrations in earthworms, Chemosphere, 2024, 364, 143079 CrossRef PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0045653524019763?via%3Dihub.
- N. Adam, C. Schmitt, L. De Bruyn, D. Knapen and R. Blust, Aquatic acute species sensitivity distributions of ZnO and CuO nanoparticles, Sci. Total Environ., 2015, 526, 233–242, DOI:10.1016/j.scitotenv.2015.04.064 , PMID: 25933293.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en01102c |
| This journal is © The Royal Society of Chemistry 2025 |