Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Stable staining of microplastics using conjugated polymer nanoparticles†
H.
Peace
 *a,
M.
Broadsmith
*a,
M.
Broadsmith
 a,
D.
O'Callaghan
a,
D.
Kaloudis
b,
R. L.
Coppock
c and
M.
Green
a,
D.
O'Callaghan
a,
D.
Kaloudis
b,
R. L.
Coppock
c and
M.
Green
 *d
*d
aStream Bio Ltd, Alderley Park, Nether Alderley, Cheshire SK10 4TG, UK. E-mail: hannah.peace@streambio.co.uk
bPML Applications Ltd., Prospect Place, Plymouth, PL1 3DH, UK
cPlymouth Marine Laboratory, Prospect Place, Plymouth, PL1 3DH, UK
dDepartment of Physics, King's College London, Strand, London WC2R 2LS, UK. E-mail: mark.a.green@kcl.ac.uk
First published on 26th February 2025
Abstract
Microplastics are a recognised global pollutant, contaminating almost all aspects of our daily life, the detection of which primarily relies on visual inspection. The use of a stable and consistent imaging agent is therefore of paramount importance. In this report, we describe the use of conjugated polymer nanoparticles as staining agents, which can, in specific cases, provide a positive stain up to 30 months after initial marking.
Environmental significanceThe peer reviewed literature highlights the importance and urgency required in addressing issues associated with microplastic pollution. We also highlight the limitations of the existing techniques for visual inspection, the initial step in detecting microplastics. This study concludes by presenting a new tool in the optical detection of microplastics, notably highlighting that the materials described can be used to image the polystyrene pollutants for at least 2.5 years after initial staining, thus presenting a significantly superior analytical tool to existing staining materials. |
Plastic waste is a global issue. According to Geyer et al. 8.3 billion metric tons of total plastic have been produced to date, and of the 6300 metric tons of plastic waste produced since 2015, under 10% has been recycled whilst 79% accumulated in the environment (including land fill) and 12% was incinerated.1 Left unchecked and unregulated, plastic waste in the environment will hit 12
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons by 2050.1 Although not all waste plastics are classified as such, microplastics are often the product of chemical and photochemical action on waste plastics and have been identified and recognised as a major pollutant since 2004.2 Microplastics have since been detected on Mount Everest,3 in the Mariana Trench,4 the Arctic,5 in the human reproductive system,6 in the human brain7 and have been implicated in the alteration of the immune response,8 gene function, neurological issues and cancer.9 In a study on the American diet, it was estimated that an individual could consume up to 121
000 metric tons by 2050.1 Although not all waste plastics are classified as such, microplastics are often the product of chemical and photochemical action on waste plastics and have been identified and recognised as a major pollutant since 2004.2 Microplastics have since been detected on Mount Everest,3 in the Mariana Trench,4 the Arctic,5 in the human reproductive system,6 in the human brain7 and have been implicated in the alteration of the immune response,8 gene function, neurological issues and cancer.9 In a study on the American diet, it was estimated that an individual could consume up to 121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 microparticles per year (depending on numerous variables) and that this is likely to be an underestimation.10 In response to the emergence of microplastics, the European Commission has restricted the use of microplastics from October 2023 (Commission Regulation 2023/2055) in everyday products, defining microplastics as insoluble, organic, synthetic polymer particles smaller than 5 mm that are resistant to degradation.11 Notably, California addressed the issue of microplastics in drinking water as early as 2018 through Senate Bill 1422,12 and the United Nations has explored the problem and produced a key report in 2021.13
000 microparticles per year (depending on numerous variables) and that this is likely to be an underestimation.10 In response to the emergence of microplastics, the European Commission has restricted the use of microplastics from October 2023 (Commission Regulation 2023/2055) in everyday products, defining microplastics as insoluble, organic, synthetic polymer particles smaller than 5 mm that are resistant to degradation.11 Notably, California addressed the issue of microplastics in drinking water as early as 2018 through Senate Bill 1422,12 and the United Nations has explored the problem and produced a key report in 2021.13
A major issue surrounding microplastics is their identification and analysis. Their polydispersity, shape, size, differing chemical composition and types alongside their location and subsequent purification and isolation methods present a notable challenge for their routine detection and identification. Thermal degradation techniques such as gas chromatography/mass spectrometry (GC/MS) can be effective, as can vibrational spectroscopic methods such as Raman and infra-red spectroscopies, however, these require significant capital outlay and experience in analysing the results.14 For microplastic particles towards the higher end of the defined size range, optical detection methods,15 notably visual sorting, after dye staining, is a standard and accepted method for detecting microplastics. Such methods have numerous attractive advantages, such as simplicity and rapid time scales, and can make microplastic identification routine. As a result, 79% of all analysis uses manual counting via optical microscopy.16
One such staining agent is Nile Red, a luminescent organic molecular material commonly used as a lipid imaging dye in biology. Application of Nile Red to the analysis of microplastics have, for example, resulted in effective protocols for the detection of particulate polyethylene, polypropylene, polystyrene and nylon-6, in the size range 10–1000 μm.17,18 Like the majority of simple organic molecules used as imaging agents in biology, Nile Red exhibits limited stability, the propensity to precipitate and inconsistent optical properties such as the lack of emission in polar solvents, whilst certain microplastics, such as fibres cannot easily be stained with the dye. Analogous to biological imaging, better imaging agents are desirable for the detection of microplastics, and conjugated polymer particles (CPNs) offer an attractive alternative. Conjugated polymer nanoparticles are self-assembled nanostructures composed of strands of molecular light emitting polymers, similar in chemistry to the light emitting materials in OLED displays and were developed as biological labels due to their water solubility, stable bright emission (essential for detecting low level disease states), organic non-toxic character, which can be engineered to decompose and hence be cleared from biological systems.19 The stable optical properties, non-cytotoxic nature and commercial availability make CPNs ideal labels for the visual detection of microplastics.
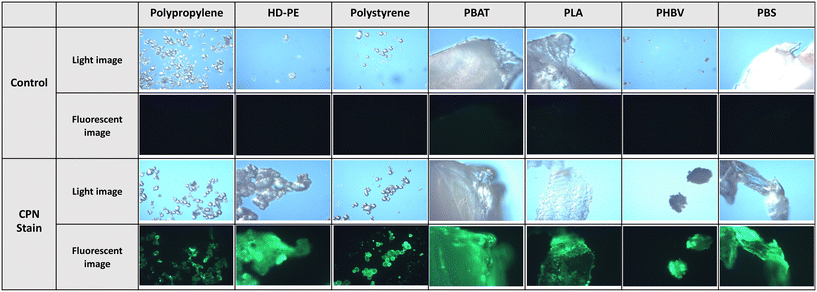
In this study, optical detection of microplastics was explored using a Nikon Eclipse TS100 optical fluorescent microscope at ×10 magnification, coupled with a commercial illuminator (pE-200) with variable irradiance intensities. Incubation of CPNs (Stream Bio, CPN 530 but with no surface passivation) with a variety of virgin conventional plastics showed positive staining as observed through fluorescent microscopy, as described in the ESI.† Polypropylene, high-density polyethylene (HD-PE) and polystyrene were initially chosen for their ubiquity as microplastics, and all three samples were clearly visible through luminescent imaging after staining with CPNs (Fig. 1) overnight and excited at 470 nm with 3% irradiance intensity on the illuminator. Likewise, incubation of CPNs with virgin biodegradable plastics, notably polybutylene adipate terephthalate (PBAT), polylactic acid (PLA), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) and polybutylene succinate (PBS) all gave similar, positive staining after incubation for the same time period using the same excitation power. Clothing microfibres are a known source of microplastics from repeated washing, notably, from polyester and acrylic fabrics. Overnight incubation of acrylic and polyester fibres with CPNs, (and imaged with 3% excitation intensity) again gave positive stains, whilst incubation of cotton, a non-oil-based fabric that does not shed microplastics, gave a negative result. Incubation of a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 blend of cotton and polyester gave a weak positive result (Fig. 2). The nature of the coordination of the CPNs to the microplastics and fibres is, as-yet, unresolved. We further note that in the case of Nile Red staining microplastics, van der Waals and dipole interactions were stated to be the mode of coordination.17
50 blend of cotton and polyester gave a weak positive result (Fig. 2). The nature of the coordination of the CPNs to the microplastics and fibres is, as-yet, unresolved. We further note that in the case of Nile Red staining microplastics, van der Waals and dipole interactions were stated to be the mode of coordination.17
Although overnight incubation is ideal, a rapid staining procedure for microplastics is desirable in certain conditions, so a 30-minute incubation protocol (with imaging irradiance intensity increased to 15%) was explored. The resultant stained conventional microplastics could be imaged easily, as shown in ESI† Fig. S1, with little visible difference when compared to the microplastics imaged using the overnight staining and lower irradiance method. Staining and imaging the virgin biodegradable plastics using the shorter time protocol gave similar positive results, except for PHBV, which, whilst visible, was significantly reduced (ESI† Fig. S2). Imaging clothing fibres after a 30-minute incubation period required a significantly higher irradiance intensity of 75%, with acrylic the most visible (yet less visible than the fibres incubated for the longer period). In contrast polyester was stained significantly less (ESI† Fig. S3).
One of the key characteristics of conjugated polymer nanoparticles, alongside their brightness, is their enhanced optical stability, in stark contrast to most luminescent organic dyes. The samples which were initially imaged using the overnight staining protocol (and 3% irradiance intensity) were stored in ambient conditions for 30 months and then re-examined. Stained polypropylene could not be imaged after 30-month storage; however, stained HD-PE was still visible (although not as bright as when imaged initially) when imaged with the excitation radiance increased from 3% to 7% (ESI† Fig. S4). Stained polystyrene was clearly visible after 30 months storage when imaged with the same radiance (3%), with a slight reduction in brightness. Increasing the radiance to 7% resulted in almost indistinguishable imaging when compared to the initial images (ESI† Fig. S5). Imaging of the virgin biodegradable microplastics 30 months after staining gave similar results. Imaging of stained PLA using 7% irradiance gave extremely weak emission, and PBAT was visible yet significantly weaker than the original sample which used 3% irradiance (ESI† Fig. S6). Similarly, stained PHBV gave no visible images after 30 months storage, whereas PBS showed weaker, yet visible images using 7% irradiance (ESI† Fig. S7). Stained clothing fibres gave no appreciable signals after 30 months storage and excitation at 7% irradiance (ESI† Fig. S8).
To initially determine the suitability of the staining agent in ‘real life’ samples obtained from the environment, CPNs were used to image microplastic contamination in mussel faeces, as mussels have long been used to monitor marine pollution due to their general location, ease of harvesting, and resilience to salinity and pollutants. There have been several reports on finding microplastics in mussels.20–22 In our preliminary work, harvested mussel faeces were incubated and imaged with CPNs in a similar manner to the lab-based microplastic samples described above (30 minutes incubation, 75% irradiance). As shown in Fig. 3, a positive stain was recorded consistent with the presence of microplastics, and a control experiment confirmed the absence of a natural background emission. Further work is needed to confirm the identity of the materials, but it is noted that optical detection of microplastics is often used in conjunction with other methods mentioned earlier, such as mass spectrometry.
In conclusion, conjugated polymer nanoparticles have been identified as effective optical staining materials for the visual identification of microplastics. The well-documented issues with photobleaching and instability of Nile Red, for example, may lead to underestimating the amount of microplastics in a sample, whereas the prolonged stable emission from CPNs will result in a more accurate visual assessment.
It is noteworthy that CPNs were used to image a range of microplastic and clothing fibres, giving a negative result with natural fibres that do not produce microplastics. Of note is the enhanced stability afforded by the labels, notably in imaging polystyrene, which was clearly still detectable after 2.5 years of ambient storage after initial staining. The efficiency of the particles was then demonstrated by their application in a rapid detection of microplastics in a real-life environmental sample (mussel faeces).
Data availability
All data is available from the corresponding authors upon reasonable request.Conflicts of interest
MG acknowledges equity in Stream Bio and a non-paid role as Director of Research.Acknowledgements
MG acknowledges the EPSRC for funding through grant EP/X014495/1. RLC acknowledges funding from NERC (Grant NE/V007351/1) and March Marine Initiative.References
- R. Geyer, J. R. Jambeck and K. Lavender Law, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3, e170782 CrossRef PubMed.
- R. C. Thompson, Y. Olsen, R. P. Mitchell, A. Davis, S. J. Rowland, A. W. G. John, D. McGonigle and A. E. Russell, Lost at sea: Where is all the plastics?, Science, 2004, 304, 838 CrossRef CAS PubMed.
- I. E. Napper, B. F. R. Davies, H. Clifford, S. Elvin, H. J. Koldewey, P. A. Mayewski, K. R. Miner, M. Potocki, A. C. Elmore, A. P. Gajurel and R. C. Thompson, Reaching new heights in plastic pollution – preliminary findings of microplastics on Mount Everest, One Earth, 2020, 3, 621 CrossRef.
- X. Peng, M. Chen, S. Chen, S. Dasgupta, H. Xu, K. Ta, M. Du, J. Li, Z. Guo and S. Bai, Microplastics contaminate the deepest part of the world's ocean, Geochem. Perspect. Lett., 2018, 9, 1 Search PubMed.
- M. Bergmann, F. Collard, J. Fabres, G. W. Gabrielsen, J. F. Provencher, C. M. Rochman, E. van Sebille and M. B. Tekman, Plastic pollution in the Arctic, Nat. Rev. Earth Environ., 2022, 3, 323 CrossRef CAS.
- C. J. Hu, M. A. Garcia, A. Nihart, R. Liu, L. Yin, N. Adolphi, D. F. Gallego, H. Kang, M. J. Campen and X. Yu, Microplastic presence in dog and human testis and its potential association with sperm count and weights of testis and epididymis, Toxicol. Sci., 2024, 200, 235 CrossRef CAS PubMed.
- A. J. Nihart, M. A. Garcia, E. El Hayek, R. Liu, M. Olewine, J. D. Kingston, E. F. Castillo, R. R. Gullapalli, T. Howard, B. Bleske, J. Scott, J. Gonzalaez-Estrella, J. M. Gross, M. Spilde, N. L. Adolphi, D. F. Gallego, H. S. Jarrell, G. Dvorscak, M. E. Zuluaga-Ruiz, A. B. West and M. J. Campen, Bioaccumulation of microplastics in decedent human brains, Nat. Med., 2025 DOI:10.1038/s41591-024-03453-1.
- L. Zhi, Z. Li, Z. Su and J. Wang, Immunotoxicity of microplastics: carrying pathogens and destroying the immune system, TrAC, Trends Anal. Chem., 2024, 177, 117817 CrossRef CAS.
- Y. Li, L. Tao, Q. Wang, F. Wang, G. Li and M. Song, Potential health impact of microplastics: A review of environmental distribution, human exposure and toxic effects, Environ. Health, 2023, 1, 249 CAS.
- K. D. Cox, G. A. Covernton, H. L. Davies, J. F. Dower, F. Juanes and S. E. Dudas, Human consumption of microplastics, Environ. Sci. Technol., 2019, 53, 7068 CrossRef CAS PubMed.
- https://single-market-economy.ec.europa.eu/commission-regulation-eu-20232055-restriction-microplastics-intentionally-added-products_en, (last accessed 28.11.24).
- https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201720180SB1422, (last accessed 28.11.24).
- https://www.unep.org/resources/pollution-solution-global-assessment-marine-litter-and-plastic-pollution, (last accessed 28.11.24).
- N. P. Ivleva, Chemical analysis of microplastics and nanoplastics: Challenges, advanced methods, and perspectives, Chem. Rev., 2021, 121, 11886 CrossRef CAS PubMed.
- J. Lukose, M. Sunil, E. K. Westhead, S. Chidangil and S. Kumar, Gaining traction of optical modalitites in the detection of microplastics, Curr. Opin. Chem. Eng., 2025, 47, 101086 CrossRef.
- M. T. Sturm, H. Horn and K. Schuhen, The potential of fluorescent dyes - comparative study of Nle red and three derivatives for the detection of microplastics, Anal. Bioanal. Chem., 2021, 413, 1059 CrossRef CAS PubMed.
- T. Maes, R. Jessop, N. Wellner, K. Haupt and A. G. Mayes, A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red, Sci. Rep., 2017, 7, 44501 CrossRef CAS PubMed.
- G. Erni-Cassola, M. I. Gibson, R. C. Thompson and J. A. Christie-Oleza, Lost, but found with Nile Red: A novel method for detecting and quantifying small microplastics (1 mm to 20 μm) in environmental samples, Environ. Sci. Technol., 2017, 51, 13641 CrossRef CAS PubMed.
- L. R. MacFarlane, H. Shaikh, J. D. Garcia-Hernandez, M. Vespa, T. Fukui and I. Manners, Functional nanoparticles through π-conjugated polymer self-assembly, Nat. Rev. Mater., 2021, 6, 7 CrossRef CAS.
- S. Fraissinet, G. E. De Benedetto, C. Malitesta, R. Holzinger and D. Materic, Microplastics and nanoplastics size distribution in farmed mussel tissues, Commun. Earth Environ., 2024, 5, 128 CrossRef.
- J. Li, C. Green, A. Reynolds, H. Shi and J. M. Rotchell, Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdon, Environ. Pollut., 2018, 241, 35 CrossRef CAS PubMed.
- M. Cole, Y. Artioli, R. Coppock, G. Galli, R. Saad, R. Torres, T. Vance, A. Yunnie and P. K. Lindeque, Mussel power: Scoping a nature-based solution to microplastic debris, J. Hazard. Mater., 2023, 453, 131392 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5en00026b |
| This journal is © The Royal Society of Chemistry 2025 |