Open Access Article
Open Access ArticleIonic liquids in polymer technology
Rebeca
Salas
a,
Rocio
Villa
*a,
Francisco
Velasco
a,
Francisco G.
Cirujano
 b,
Susana
Nieto
a,
Nuria
Martin
b,
Eduardo
Garcia-Verdugo
b,
Susana
Nieto
a,
Nuria
Martin
b,
Eduardo
Garcia-Verdugo
 b,
Jairton
Dupont
b,
Jairton
Dupont
 a and
Pedro
Lozano
a and
Pedro
Lozano
 *a
*a
aDepartamento de Bioquímica y Biología Molecular B e Inmunología, Facultad de Química, Universidad de Murcia, Campus de Espinardo, 30100 Murcia, Spain. E-mail: rocio.villa@um.es; plozanor@um.es
bDepartment of Inorganic and Organic Chemistry, Universitat Jaume I, Av. Vicent Sos Baynat, s/n, 12006, Castelló de la Plana, Castelló, Spain
First published on 13th December 2024
Abstract
Modern society is heavily dependent on advancements in polymer science, however, there are increasing environmental concerns about plastics waste that demand sustainable solutions. Ionic Liquids (ILs) have emerged as non-innocent solvents that demonstrate significant potential in polymer chemistry for both synthesis and depolymerization. This review highlights recent advancements in IL-based functional polymers, with particular focus on their applications in separation, energy storage, fire resistance, recycling, and biomedicine. Furthermore, the role of IL-driven polymer media in the research and development of polymer synthesis and degradation technologies is evaluated. Overall, ILs have emerged as key tools in advancing polymer technology, enabling the obtention of new valuable materials and supporting eco-friendly practices.
1. Introduction
In contemporary society, polymeric materials, commonly referred to as “plastics”, play a crucial role in modern civilization.1 These materials are not new, they have been used unknowingly for many years. The term “polymer” is derived from the classical Greek roots “poly”, meaning “many”, and “meres”, meaning “parts”. This implies that polymers are composed of numerous small molecules, named monomers, linked together to form long chains, which typically results in molecules of high molecular weight, ranging from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 to 1
000 g mol−1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.2
000 g mol−1.2
The modern understanding of polymers was introduced by Nobel laureate H. Staudinger in the 1920s, who established the basics for the development of synthetic materials.3 Before this breakthrough, materials such as steel, glass, wood, and brick were used for construction, whereas agricultural products like cotton and jute were used for fabric manufacture. The introduction of polymers has effectively addressed the growing demand for manufactured products. Nowadays, polymer-based products are ubiquitous from the synthetic fibbers that make our clothes to Teflon-coated cookware.2
Polymers are broadly categorized into natural and synthetic, based on the chemical reaction between the monomers that form the polymer chain. If the reaction process happens naturally, it is considered a natural polymer, whereas if the process is man-made, it is a synthetic polymer. Natural polymers are obtained from organic sources and include cellulose and starch present in vegetables, rubber from plant latex, and animal proteins. Synthetic polymers are industrially manufactured, with common examples including polyamide (PA), poly(vinyl chloride) (PVC), polyurethane (PU), and polyethylene terephthalate (PET).4 Both natural and synthetic polymers are indispensable in several fields, including food,5 pharmaceuticals and medicine,6 transportation,7 construction,8 textiles,9 and even oil recovery.10 Consequently, in our increasingly technological society, the presence of polymers, both natural and synthetic, plays a key role in enhancing human comfort and quality of life.
Over the last century, the polymer industry has shown a remarkable growth, surpassing the combined scale of the copper, steel, and aluminium industries.11 Hence, it is not surprising that the study of polymeric molecules is one of the fastest-growing fields of science. Indeed, the global plastic production rose to 400.3 Mt in 2023, accompanied by 29.5 Mt of plastic waste, according to Plastics Europe.12 By 2030, plastic production is projected to reach 700 Mt, equivalent to 80 kg of plastic per human being, while plastic waste will reach 460 Mt annually.13 However, 98% of polymers are still derived from fossil fuels, and current disposal methods (e.g. incineration, landfilling) are inefficient, non-sustainable, and significantly contribute to CO2 emissions.14,15
This underscores the pressing need for advancements in polymer technology. Several challenges remain, from optimizing polymer synthesis under the efficient and economical conditions to improving depolymerization methods, several challenges remain. First, there is a need for alternative solvents to replace conventional organic solvents in polymerization processes. In the realm of radical polymerization, precise control over key stages such as initiation, propagation, and termination via factors such as viscosity and polarity are paramount for shaping the ultimate polymer structure and its associated properties, including molecular weight, dispersity, and order. Moreover, the exploration of alternatives to radical polymerization, such as ionic polymerization utilizing environmentally sustainable solvents like Ionic Liquids (ILs), is currently underway. The research involves investigating the solubility of the polymer and/or polymerization catalysts in both organic solvents and alternative (green) solvents, such as ILs. Furthermore, the focus is on tuning catalytic performance by using alternative solvents as ligands for the organometallic catalysts.16 Secondly, the development of high-performance polymers is crucial for advancing many cutting-edge technologies. Polymers are increasingly used in applications related to energy in the form of electricity and/or light conduction/storage, to separation/adsorption (i.e. mass transport of neutral compounds or charged ions). In this context, novel organic compounds and inorganic materials are currently available for the modification of polymers, boosting their electrochemical and photochemical properties when used as electrodes. This has direct applications in solar panels, capacitors, batteries, fuel cells and catalytic converters, among others.
Our modern society is driven by an unsustainable consumption model characterized by a linear product life cycle (i.e. take-make-use-waste). Fortunately, significant efforts are underway to develop new green approaches.17,18 For instance, sustainable viewpoints such as the “3R rules”—recover, reuse, and recycle—emerged in the 1970s, promoting a circular economy model.19,20 In this context, the waste hierarchy (Fig. 1) encourages a change of mindset, prioritizing resource use and incorporating waste as a valuable starting material. Regarding polymers, the goal of the waste hierarchy is minimizing the ecological and carbon footprint of plastics by implementing the highest-level approach on a pyramid of decreasingly favourable choices. At the top strategy of the hierarchy, the most effective strategy is to refuse the product, followed by reducing the amount of plastic used, reusing, recycling, and recovering the product. The least desirable strategies involve disposing of plastic in a landfill or in a mismanaged way.
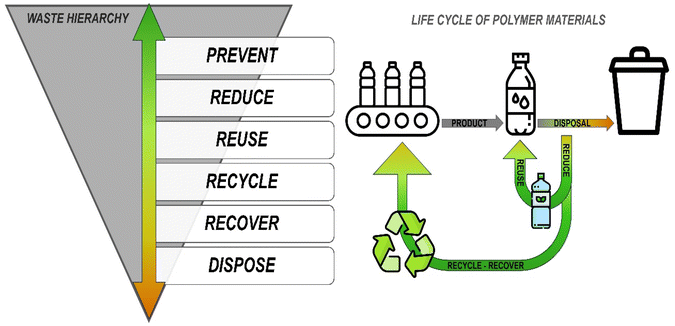 | ||
| Fig. 1 Synergistic coupling between the levels of the waste hierarchy and the life cycle of polymer materials, to achieve a circular economy model. | ||
Thus, replacing fossil-derived plastics with those based on renewable resources, alongside using renewable energy in their production, represents a promising strategy to reduce the carbon footprint of plastics. Furthermore, viewing end-of-life plastics as valuable resources for producing new products highlights an important opportunity in creating a sustainable plastics economy. These challenges raise the question: how can chemistry contribute to and support a circular economy.21
The concept of Green Chemistry emerged in the 1990s, driven by the need for environmentally friendly chemical processes. Green Chemistry is founded on scientific principles that encourage a guiding philosophy based on scientific approaches that initiate clean products and processes by the efficient use of non-toxic, renewable raw materials and their selective transformation through (bio)catalysis, maximizing atom economy, avoiding chemical derivatives, eliminating waste, avoiding the use of toxic and hazardous reagents, and using safer solvents in the manufacture and application of chemical products, as summarized by Paul T. Anastas and John C. Warner with the Twelve Principles of Green Chemistry.22 To achieve the goal of “plastics circularity”, the next challenges in polymer science involve the development of alternative methods for polymer synthesis and more efficient polymer recycling technologies.23,24
In the search for alternative environmentally benign solvents that can be easily recovered and reused, ILs have emerged as a green option with potential for use in a broad range of chemical processes. Indeed, the use of ILs is a paradigmatic example of green chemistry.25,26 Nowadays, ILs are widely recognized for their extensive list of genuine properties, such as negligible vapor pressure, high thermal stability, tuneable viscosity, and miscibility with both water and organic solvents.27 These salts, composed exclusively of ions, are liquid at temperatures below 100 °C and generally consist of an organic cation (e.g. imidazolium, pyrrolidinium, tetraalkyl phosphonium) and an inorganic or organic anion (e.g. tetrafluoroborate, hexafluorophosphate, bromide, acetate). The variety of possible cation–anion pair combinations results in a wide range of ILs with different properties (e.g. polarity, hydrophobicity, viscosity, among others), which are key features for developing integral green chemical processes.28 Thus, ILs are often referred to as “tailor-made solvents” due to their versatility and customizable nature (Fig. 2). This adaptability makes ILs valuable in several scientific fields, including polymer chemistry, biomass processing, catalysis, and energy storage. In addition, their properties align with the principles of Green Chemistry, offering an environmentally friendly alternative to traditional solvents. Through the reduction of hazardous waste, enhancement of atom economy, and facilitation of more efficient chemical transformations, ILs offer a sustainable route to realizing a circular plastics economy and advancing eco-friendly industrial processes.
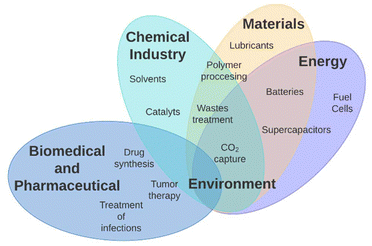 | ||
| Fig. 2 Applications of ionic liquids and their interrelations across various sectors, including the chemical industry, energy, materials, and medical fields. | ||
Table 1 shows the key differences between the physical properties of ILs and conventional organic solvents, which make ILs more suitable for green applications. These properties typically include characteristics such as vapor pressure, thermal stability, polarity, and miscibility, all of which are crucial in determining their suitability for different chemical reactions and applications. By integrating these sustainable solvents into industrial processes, the chemical industry can significantly reduce its environmental footprint and promote a more sustainable future.
| Organic solvents | ILs | |
|---|---|---|
| Viscosity | Low | High |
| Density | Low | High |
| Vapor pressure | High | Low |
| Electrical conductivity | Low | High |
| Thermal conductivity | Low | High |
| Refractive index | Low | High |
The growing interest in ILs in chemistry is evident from the dramatic increase in publications over the past two decades. ILs have been applied in material science for designing functional polymers, such as electromaterials with controlled ionic conductivity29 and in analytical chemistry for extraction and separation processes, such as membranes with controlled porosity.30,31 Current research includes the covalent immobilization of ILs onto silica materials and the attachment of ILs onto polymers, creating materials that can act as low-polarity phases for non-polar compounds and, conversely, for compounds with strong proton-donor groups.32 Additionally, ILs have been applied as electrolytes due to their high stability and mechanical durability, contributing to the development of smart light-controllable conductivity materials.33 Certain ILs have also been used to reduce the flammability of polymers and increase their flame retardancy.34 Notably, ILs have been used for the pretreatment of natural fillers before the fabrication of polymer biocomposites, improving their physical–mechanical properties.35 Thus, the genuine physical–chemical properties of ILs, such as porosity, charge, reactivity, can be modulated, offering several advantages in different applications such as separation, extraction, (bio)catalysis (as summarized in Table 2).17
| Structural | Electrical | Photo | Thermal | |
|---|---|---|---|---|
| Mechanical | Shaping/packaging | — | — | Isolation/conduction |
| Pore | Separation | — | — | Catalysis |
| Charge ion | — | Conduction/storage | Conduction/storage | — |
| Reactive site | — | Catalysis | Catalysis | Catalysis |
As an example of the properties and potential applications of poly(ionic liquids) (PILs), a recyclable bifunctional acid catalyst based on PILs combined with a magnetic Fe3O4–SiO2 carrier was developed. This catalyst exhibited a mesoporous structure, strong superparamagnetic properties, high hydrophobicity, and exceptional stability. It achieved one-pot conversion of Euphorbia lathyris L. oil, a low-quality feedstock, into biodiesel with an impressive yield of 98.1% after just 6 hours at 102 °C. The process incorporated a biomass-derived co-solvent, tetrahydrofuran (THF), which reduced methanol consumption and improved mass transfer. The catalyst demonstrated excellent reusability, maintaining a biodiesel yield of 91.2% after five cycles.36 This approach has not been limited to ILs, but also involves other sustainable alternatives (i.e. deep eutectic solvents, DES). For example, when used as co-solvents, DESs can modify phase distribution in the reaction mixture, enhancing catalyst recovery and simplifying the separation process.37
Another unique feature of ILs is their exceptional suitability as reaction media for chemical transformations, providing remarkable activity and stability to (bio)catalysts (e.g. enzymes, metal nanoparticles).25,38,39 Additionally, ILs show a superior capability to dissolve polar and nonpolar organic, inorganic, and polymeric compounds. Notably, the IL 1-butyl-3-methylimidazolium chloride, [Bmim][Cl], exhibits an exceptional ability to dissolve recalcitrant polymers, both natural (like cellulose) and synthetic (like PU), opening new opportunities for developing industrial and sustainable strategies to treat and recycle large amounts of waste.40,41 Another key parameter to be considered when selecting an IL is the intrinsic properties of the polymer to be processed, both in terms of synthesis and degradation. The suitability of these tuneable solvents as an optimal reaction medium depends on their compatibility and interactions with the polymer (e.g. hydrophobicity, hydrophilicity, miscibility, melting point, etc.), with gas/liquid–solid mass transfer limitation and further separation protocols as main targets to overcome.42
Generally, stability in ILs involves thermal, chemical, electrochemical, and radiolytic properties, all of which play key roles depending on the application.43 While some ILs (e.g. [Emim][NTf2]), exhibit thermal stability with decomposition at temperatures higher than 350 °C, their stability can be compromised under specific conditions such as prolonged high-temperature operation. Despite their high thermal stability, imidazolium-based ILs, face chemical stability issues like ring-opening, C–N bond cleavage, and reactions with biomass, leading to decomposition or by-products. Electrochemically, the stability of ILs is defined by their electrochemical window, which can narrow significantly in the presence of water or oxygen, reducing their suitability in demanding environments. Radiolytic stability is another concern for applications involving ionizing radiation, where ILs can degrade, producing radicals that further compromise their performance. Moreover, ILs have a hygroscopic behaviour, and moisture uptake can influence their physical and chemical properties, such as viscosity and conductivity, potentially inducing hydrolysis or affecting stability. Such moisture sensitivity necessitates careful design of processes to limit IL contact with ambient humidity.44,45
Ionic liquids (ILs) can exhibit toxic effects (i.e. cytotoxicity, neurotoxicity, ecotoxicity) affecting their potential for safe industrial use. Although some ILs show low toxicity and good biocompatibility, concerns remain about long-term harmful effects. Comprehensive toxicological and biodegradability assessments are necessary to support the safe use of ILs in industry. Furthermore, ILs are typically more expensive than VOCs, posing a challenge for large-scale industrial application although their full recovery and reuse is their main advantage. To enable direct industrial applications, it is essential to develop cost-effective, eco-friendly raw materials and highly reusable ILs.43 In this context, it is also important to mention the use of sustainable alternatives, such as DES, supercritical fluids (SCFs), and perfluorinated solvents (PFSs), which have also potential for advancing the development of chemical processes.46
DES are composed of hydrogen bond donors and acceptors, offering similar properties to ILs (e.g. high thermal stability, low volatility, and non-flammability, etc.).47 These tailored solvents are useful in different fields, such as catalysis, gas capture, nanotechnology, and enzyme stabilization.48–51 However, one of the main drawbacks convers both their typically high viscosity, which can difficult practical applications, and the remaining question about their toxicity for the environment.52
Alternatively, SCF is state of matter reached at pressure and temperature above their critical values, offering unique behaviour of gas-like viscosity and diffusivity, and liquid-like density and solvating properties, which make them effective solvents in many applications.46,53–55 However, the high cost of equipment and energy expense remains a major drawback in implementing SCF in industry.56 On the other hand, PFSs are suitable alternatives to design non-aqueous biphasic systems as a function of their temperature-dependent miscibility with organic solvents.57 Yet, their volatility and the need to combine them with organic solvents for product separation present challenges in developing sustainable, environmentally friendly chemical processes.58 To summarize, the main advantages and disadvantages of the above reported green solvents are summarizes in Table 3.
| Green solvent | Advantages | Disadvantages |
|---|---|---|
| ILs | - Negligible vapor pressure | - High-cost production |
| - High thermal and chemical stability | - Biodegradability issues | |
| - Fully recoverable and reusable | ||
| DESs | - Low toxicity | - High viscosity |
| - Cost-effective preparation | - Limited solubility for some compounds | |
| SCFs | - Suitable solvating power | - High pressure equipment required |
| - Easily separable from products | - High-energy costs | |
| PFSs | - Chemically inert | - Toxicity |
| - Reusable and low boiling points for easy recovery | - Need to combine with volatile organic solvents |
This comprehensive review seeks to vividly illustrate the tremendous potential of ILs in revolutionizing the polymer industry toward sustainability. It will delve into the intricate development of IL-based functional polymers and the intricate utilization of IL-based reaction systems through several carefully selected examples.
2. IL-driven polymer synthesis
This section explores the application of ILs as reaction media and/or catalysts in polymer synthesis without incorporating the IL into the final polymer product. This strategy provides a greener alternative to conventional organic solvents, such as hydrocarbons. Different examples revealed how ILs regulate the polymerization process through interactions with monomers, intermediates, or catalysts.59 ILs sever to not only control polymerization, but also enhance the environmental sustainability of polymer synthesis protocols by functioning as low vapor pressure and fully recyclable solvents.The first report of a polymer synthesis using a polymerization catalyst in an IL solvent was the production of polyethylene (PE) with moderate yields.60 In this system, AlCl3 and the [Emim][Cl] IL were employed in combination with AlEthylCl2 and TiCl4 or Cp2TiCl2 in a Ziegler–Natta-type catalysis.61 The introduction of ILs significantly enhanced the rate of the polymerization kinetics, resulting in higher polymer yields and increased molecular weight of the polymer.
ILs have also been used to control radical polymerization processes, including polymers such as methyl methacrylate (MMA), which was obtained in higher yields and molecular weight when using 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim][PF6]) as the solvent.62 The reduction in the termination rate was mainly attributed to diffusion limitations caused by the high viscosity of the IL medium. It is worth mentioning that the physical properties of the polymer obtained in the IL, such as glass transition temperatures and microstructures, were comparable to those obtained in bulk or in benzene solvent.
Advanced polymerization techniques, such as pulsed laser polymerization of MMA have also been studied in similar ILs.63 The propagation and termination steps in these systems were significantly influenced. The former was impacted by changes in charge transfer energies associated with the high polarity of the IL, while the latter was affected by the high viscosity of the IL. However, more sustainable alternatives to [Bmim][PF6] have been identified. These alternatives avoid the production of hydrofluoric acid upon hydrolysis and incorporate fewer toxic components, including imidazolium, pyridinium, and alkyl ammonium salts, as well as non-toxic metals within (e.g. Fe) their structure. These greener ILs have been used in the polymerization of alkyl methacrylates, styrene, and other polymers.64
Additionally, ILs have been employed as both solvents and catalysts in the synthesis of important polymers such as polycarbonates and polyamides, through a strategy of ring-opening polymerization of carbonates or ε-caprolactone. On the one hand, acid ILs facilitated the ring-opening polymerization of ethylene carbonate, enabling the polymerization to occur at lower temperatures and minimizing the formation of byproducts associated with decarboxylation at higher temperatures.65 On the other hand, the rapid microwave-assisted polymerization of ε-caprolactone was initiated using imidazolium IL alcohols intercalated in layered double hydroxides, resulting in the formation of block copolymers.
Furthermore, it is important to highlight the utilization of biomass and CO2 feedstocks, in conjunction with biomass-derived ILs as solvents, for the environmentally friendly production of polymers and biopolymers. These processes offer sustainable substitutes for conventional petroleum-derived polymers. For instance, tetraethylammonium lactate ([TEA][Lac]) has been used for the efficient synthesis of novel optical poly(isosorbide thioethers).66 The presence of hydroxyl anions in the IL enhanced the proton-accepting ability, while the cations were functionalized with dual activation sites, effectively lowering the activation energy of nucleophilic hydrothiolation through anion–cation synergistic IL catalysis. Alternatively, another type of IL containing acylamido anions favoured the melt polycondensation of isosorbide (ISB) and dimethyl carbonate (DMC) to yield a bio-based isosorbide polycarbonate.67 In this case, the IL cations and acylamido anions interact with the hydroxyl (–OH) groups, promoting the polymerization with a preference for endo-OH selectivity. In the upcoming section, we will explore the integration of IL monomers (e.g. PILs) during or after the polymerization process.
3. IL-based functional polymers
Functional polymers have emerged as a versatile class of materials capable of addressing the needs of specific sectors such as energy storage, biomedicine, and environmental separation processes. These specialized polymers exhibit a wide range of properties, including chemical reactivity, photosensitivity, electrical conductivity, catalytic activity, biocompatibility, and pharmacological effects.68The integration of IL technology has resulted in substantial progress in polymer science for the synthesis and modification of polymers.69 ILs have the capability to serve as a medium for polymerization or as a solvent for dissolving or dispersing polymers, thereby facilitating further chemical transformations or material processing. Additionally, they can be assimilated into polymers through derivatized monomers, post-polymerization reactions, or by forming polymer-IL composites.70,71
One interesting approach is the incorporation of ILs into polymer backbones to create supported ILs (SILPs). SILPs combine the advantages of both ILs and solid supports. They can be prepared through simple impregnation (coating) of the IL onto various porous and nonporous supports or through grafting (covalent attachment).72 Another approach entails synthesizing polymers by polymerizing ILs as monomers, resulting in poly(ionic liquids)s (PILs).29 PILs combine the unique properties of both ILs and polymers, providing tuneable physical–chemical characteristics alongside enhanced mechanical stability and dimensional control. Additionally, PILs can exhibit hierarchical assembly, forming organized morphologies such as micelles and vesicles.73,74
IL-based gels can also be synthesized via IL polymerization. In this regard, P. C. Marr and A. C. Marr provided a comprehensive review classifying IL-based gels based on their interactions, physical and mechanical properties, solvent properties, and solid properties. This review also explored the potential roles of IL-based gels in advancing sustainability.75 In this context, ionogels—hybrid materials that combine the properties of ILs with organic components (e.g. low molecular weight gelators, (bio)polymers) or inorganic components (e.g. inorganic polymers)—exemplify such innovative materials.76
3.1. Separation polymers
The escalating levels of CO2 concentration, oily wastewater, freshwater scarcity, and water pollution have propelled the development of separation membranes, particularly polymer variants. These membranes exhibit exceptional features, including high efficiency, flux, and selectivity, along with superior antifouling capabilities and robust chemical stability.77Polymer membranes are widely employed across a range of separation applications. For instance, Zhang et al.78 developed salt-responsive polyether sulfone (PES) membranes (solid phase) integrated with anion-sensitive PIL gels. This approach used the IL methacrylatoethyl trimethyl ammonium chloride ([MTA][Cl]) as a functional monomer. These membranes demonstrated sensitivity to various anion species (i.e. [PF6], [BF4], [SCN]) and their respective strengths. Precise control of membrane permeability was achieved by modifying the counter anions of the PIL, resulting in an anion-sensitive activation behaviour. Such membranes hold potential for chemical and biomedical purification applications that require ion recognition (Fig. 3).
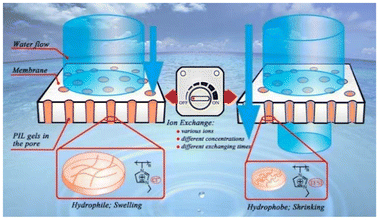 | ||
| Fig. 3 Salt-responsive PES membranes integrated with anion-sensitive poly(ionic liquid) (PIL) gels prepared with methacrylatoethyl trimethyl ammonium chloride ([MTA][Cl]). Reproduced from ref. 78 with permission from American Chemical Society, copyright 2017. | ||
Rynkowska et al.79 developed a membrane containing an interpenetrating network of cellulose acetate propionate (CAP) and a reactive IL, 1-methyl-3-(1,3-diethoxy-1,3-dioxopropan-2-yl)-1H-imidazolium bromide. The designed PIL system resulted in a significant improvement in the separation factor compared to pure CAP membranes. The separation factor (β) increased from approximately 10 for pure CAP membranes to around 380 for the new PIL/CAP membranes when contacting an azeotropic composition of water–isopropanol mixture (i.e. 12 wt% water). ILs have also been used in combination with membrane processes for volatile organic compounds (VOCs) and CO2 separation.80 Jebur et al.81 investigated the effectiveness of a polytetrafluoroethylene/imidazolium (PTFE/imidazolium) membrane to separate aromatic compounds from an organic medium. The order of separation efficiency was determined to be divinylbenzene > styrene > toluene, owing to their respective affinities with the IL. The durability of this membrane was assessed over a period of 60 days.
On the other hand, Chen et al.82 developed polymer/IL blend membranes based on polyvinylidene fluoride (PVDF) and 1-ethyl-3-methylimidazolium tetracyanoborate ([Emim][B(CN)4]). A direct correlation between CO2 permeability and IL content has been stablished and demonstrated. This correlation has been attributed to CO2 diffusivity and solubility. However, as the IL content increased, the mechanical properties of the composite membrane (e.g. tensile strength, elongation at break) declined.
Significant progress has been achieved in the incorporation of ILs into polymers. Although the liquid state of ILs is lost upon polymerization, their distinctive properties, including polarity and low volatility stemming from non-polar and ionic interactions, are effectively retained.32 As a result, these new materials have been successfully used as sorbents in solid-phase extraction (SPE).83,84 For example, Chen et al.85 immobilized 1-methyl-3-methylimidazolium chloride ([Mmim][Cl]) onto a PVC surface, obtaining 1-chlorovinyl-3-methylimidazolium chloride as a sorbent. This PVC/[Mmim][Cl] was successfully used to separate and preconcentrate Cr(VI) from water samples and demonstrated stability over time after 300 extractions with no significant differences. Moreover, different studies with other cations (e.g. Na+, Mg2+, Ni2+) and anions (e.g. Cl−, I−, SO42−) indicated no interferences with the extraction of Cr(VI).
Recently, ILs have been successfully used for the extraction of micro- and nanoplastics from water. For example, model polystyrene beads with diameters of 0.1, 0.2, 0.3, 0.5, and 1.0 μm were efficiently transferred from water to an IL phase using liquid–liquid extraction. Three ILs demonstrated exceptional performance, achieving nearly complete removal of nanoplastic pollutants, with a mean extraction efficiency of 98.4%. Notably, this method proved even more effective in extracting these nanoplastics from saline water, reaching an extraction efficiency of 99.8%. This is a significant finding, given that only 2.5% of the Earth's water is freshwater, highlighting the method's potential for broader environmental applications.86
Additionally, ILs offer valuable applications as stationary phases in gas chromatography, broadening both the selectivity range and temperature-operating range of the columns. In fact, it has been observed that columns prepared with a partially crosslinked mixture of 1-vinyl-3-nonylimidazolium bis(trifluoromethyl sulfonyl)imide ([VNim][NTf2]), 1,9-di(3-vinylimidazolium)nonane bis(trifluoromethyl sulfonyl)imide ([D-Vim][NTf2]), and a poly(dimethyl diphenyl siloxane) (PDMS) in equal proportions were more effective for separation of essential oils containing compounds than columns coated with poly(ethylene glycol) or 1,9-di(vinylimidazolium)nonane.87
3.2. Energy store and generation (release) polymers
One of the significant challenges of this century is the imminent energy shortage resulting from the depletion of fossil fuel resources. Consequently, it is imperative to focus on the development of new sustainable energy sources.88 Energy storage technologies, characterized by supercapacitors and batteries, are a viable alternative.89 Despite notable advances in electrode materials, the energy density of supercapacitors and the power density of batteries remain limited by charge storage mechanisms. Therefore, it is necessary either to develop novel devices with alternative and efficient charge storage mechanisms or to selectively engineer materials with superior properties.90,91Supercapacitors generally exhibit higher power-to-mass ratios compared to batteries, allowing them to store more energy per unit mass. Both supercapacitors and batteries rely on a conductive medium or solvent capable of transporting ions between their electrodes. ILs combined with polymers can outperform traditional organic solvents due to their ability to improve ionic transport. This is attributed to the multiple possible combinations of cations and anions, alongside their optimal integration with polymer matrices.
ILs can be used as electrolyte salts, electrolyte additives, and solvents for energy storage devices, owing to their numerous advantages,92 such as being thermally stable electrolytes.93 Furthermore, the viscosity and ionic conductivity can be altered by adjusting the size and charge of the organic cation of the IL. This modification influences the Lewis acid–base interactions between the cations and anions of the IL. Importantly, ILs can act as effective plasticizers, providing flexibility to the polymer, while also providing conductivity to the polymer by dissolving conductive cations derived from metal salts. Therefore, ILs are considered ideal electrolytes for electrochemical devices, such as supercapacitors and batteries, which require alternatives to traditional hazardous solvents. When utilized as high-voltage electrolytes in supercapacitors, ILs represent a promising strategy to enhance energy density.
Lithium-ion capacitors (LICs) are a type of energy storage device that combines a lithium-ion battery-type electrode with a capacitive electrode. This unique design allows LICs to harness the advantages of both supercapacitors and lithium-ion batteries. However, these innovative devices are not without their challenges. One of the primary concerns with LICs is their safety, particularly related to issues such as electrolyte leakage and limited voltage ranges. This is largely due to the electrolyte used in these systems, which is essential for facilitating the transport of lithium ions. Unfortunately, this electrolyte often contains volatile and flammable solvents, posing a significant risk of fire and explosion.
A potential solution involves the development of solid-state LICs using ionogel electrolytes, which consist of polymer matrices infused with ILs.94 For example, Zhang et al.95 designed a solid-state LIC using a PVDF-HFP/[Li][NTf2]/([Emim][BF4]) (polyvinylidene fluoride-hexafluoropropylene/lithium bis(trifluoromethylsulfonyl)imide/1-ethyl-3-methylimidazolium tetrafluoroborate) ionogel electrolyte characterized by an operation window of 4 V. However, it should be considered that larger voltage windows are often correlate to higher viscosity materials, which can hinder mass transport and elevate oxidation potentials. The resulting LIC achieved an energy density of 101 W h kg−1 (83% cycling stability after 8000 cycles) and a power density of 24 kW kg−1 at 60 °C. These results demonstrate that LIC devices based on ILs have higher capacitive energy storage contributions compared to those based on organic electrolytes, which could be explained by the relatively high diffusion rates of IL ions compared to lithium ions.
IL-based polymer electrolytes are promising candidates for solid-state electrolytes for battery applications.96 Lithium-containing ILs incorporated into polymers via gelation form conducting polymer gels, also known as “ion-gels”. The introduction of ILs serves to heighten the conductivity of polymers at temperatures that fall below the polymer's melting point. This enhancement eases the movement of lithium ions between oxide and carbon electrodes. Indeed, terms like “gel polymer electrolyte”, “ion gel”, “rubbery electrolyte”, “plasticized electrolyte”, or “solid (hybrid) polymer electrolyte” refer to combinations of ILs and polymers used for ion conduction applications. Particularly, protic ILs represent an interesting class of proton conductors for fuel cells or charge carriers in batteries, especially when integrated with polymers to form composites for membrane fabrication, enabling unprecedented practical applications.97
Ionic polymeric gels can be prepared by confining of ILs within polymer matrices, which leads to the gelation of the IL and reduces ion leaching from the polymer structure. For example, when Li salts or organic ILs are mixed with polymers, they act as both charge carriers and plasticizers within the gel. These IL–polymers, or “polymer-in-salt” electrolytes, can achieve ionic conductivities in the range of 10−4 to 10−3 S cm−1 at room temperature. In contrast, conventional polymer electrolytes, or “salt-in-polymer” electrolytes, typically have lower ionic conductivities, ranging from 10−6 to 10−4 S cm−1 S cm−1 at room temperature.98
Fig. 4 illustrates the movement of lithium ions within a polymer matrix, facilitated by interactions between lithium and oxygen atoms in the polyethylene oxide (PEO) (Fig. 4a and b). The movement of ions is significantly influenced by changes in temperature, with higher temperatures leading to increased ion transport. IL–polymer interactions may also occur in ternary polymer electrolytes, such as those containing imidazolium cations and PEO, enhancing ion and polymer mobility compared to traditional systems (Fig. 4a) Additionally, polymers like polyvinylidene fluoride (PVDF) have the ability of forming ionic conducting channels filled with lithium-containing ILs (Fig. 4d).96
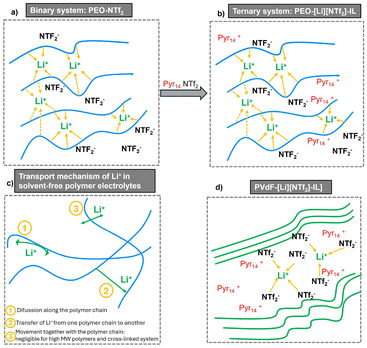 | ||
| Fig. 4 (a) Li+-ion coordination and (c) transport mechanisms in PEO-based, binary polymer electrolytes. Li+-ion coordination in ternary polymer electrolytes containing (b) active polymers (e.g. PEO) and (d) inactive polymers (e.g. PVdF). Adapted from ref. 96 with permission from John Wiley & Sons, copyright 2015. | ||
As summarized in Table 4, comparing traditional liquid electrolytes with solid electrolytes reveals key differences in safety, processability, ionic conductivity, and mechanical properties. Traditional liquid electrolytes (e.g. lithium salts), provide high ionic conductivity due to charge separation and dissociation but pose significant safety risks; they are flammable, toxic, and corrosive, raising concerns in battery applications. In contrast, organic ionic-liquid-like polymers (e.g. PEO-[Li][NTf2]), offer greater flexibility and reduced flammability, making them promising for flexible and semi-solid battery designs. However, they generally exhibit lower ionic conductivity. On the other hand, inorganic ceramic electrolytes excel in both conductivity and safety. Yet, they are characterized by rigidity, which can make shaping and processing challenging. Additionally, solid ceramics require elevated temperatures (over 300 °C) for effective ion diffusivity, while polymer-based electrolytes demonstrate tuneable conductivity at lower temperatures (below 150 °C). Overall, the selection of electrolyte type hinges on balancing safety, performance, and manufacturing considerations, as liquid electrolytes remain dominant in commercial batteries due to their established performance and cost-effectiveness. Alternatively, solid and polymer electrolytes are gaining attention for their potential to address the safety limitations of liquid-based systems.
| Solid ceramic electrolyte | Polymer/IL-based electrolyte | Liquid electrolytes | |
|---|---|---|---|
| Safety/cost | High stability, low flexibility (rigid and brittle)/poor shaping. Processability | Large-scale/low-cost manufacturing | Flammable, leaked, toxic, corrosive |
| Vapor pressure | Negligible | High (proportional to the ion size and cation–anion interactions) | Low, volatile, especially organic liquids |
| Ionic conductivity | High temperature needed (and defects/grain boundaries/interfaces) for ion diffusivity in the solid phase (>300 °C); high electrochemical stability | Tuneable at lower temperatures (<150 °C) | Charge separation/dissociation |
| Used as dielectrics or capacitors | Increase with temperature, charge carriers (valency and radius), polymer host viscosity/density, defects, etc. | High ion diffusivity/mobility in the liquid phase (solvent), resulting in high conductivity even/ion transport through the solvent at RT |
While PEO is the most widely used polymer for embedding conductive ILs, other polymers have also been investigated. For instance, compatible polyethers and polyvinyls, including poly(methyl methacrylate) (PMMA) and poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP), can be prepared through in situ polymerization, solvent casting or hot pressing, among other methods.99
Alternatively, pyrrolidinium cations can be incorporated into polymeric ILs, and anions exchanged for bis(trifluoromethylsulfonyl)imide, [NTf2]; hexafluoro phosphate, [PF6]; tetrafluoroborate [BF4]; or dodecyl benzenesulfonic acid [DBSA], counter-anions. These anions remain mobile, as they are not bound to the polymer structure, enabling ion conduction. The ionic conductivity of such polymer electrolytes at room temperature ranges from 3.15 × 10−6 to 9.8 × 10−6 S cm−1, increasing to between 3 × 10−5 and 1.8 × 10−4 S cm−1 at 60 °C. Among these materials, the poly(diallyldimethylammonium) [NTf2] and IL ([Pyrr14][NTf2]) membrane stand out, showing no phase separation or IL segregation, with a conductivity of 1.5 × 10−3 S cm−1 at 60 °C.100
Furthermore, the incorporation of 1-ethyl-3-methyl imidazolium bis(trifluoromethane sulfone)imide ([Emim][NTf2]) and hexyltrimethylammonium bis(trifluoromethane sulfone)imide ([Htma][NTf2]), both containing lithium cations, into a branched poly(ethylene oxide)/poly(methyl methacrylate) (PEO-PMA) matrix, resulted in conductivities of approximately 2 × 10−4 S cm−1.101 The combination of PEO with [Li][NTf2] and [Emim][NTf2] achieved an ionic conductivity of approximately 2.08 × 10−4 S cm−1 at 30 °C.102
A novel form of chemically crosslinked liquid crystalline nanocomposite ionogel electrolyte based on PILs was synthesized by photopolymerization of ionogels containing charged halloysite nanotubes (HNTs) and IL monomers (i.e. 1-vinyl-3-butylimidazolium tetrafluoroborate ([VBim][BF4]), 1-vinyl-3-butylimidazolium bis(trifluoromethylsulfonyl)imide ([VBim][NTf2]), 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim][BF4]), 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Bmim][NTf2])). The resulting materials were composed of aligned HNTs connected by chemically crosslinked PIL chains and exhibited high mechanical stability at temperatures of up to 200 °C and high ionic conductivity (up to 6 mS cm−1) at room temperature. Flexible solid-state supercapacitor devices based on these nanocomposite ionogels demonstrated high and temperature-dependent specific capacitance (175.8 F g−1 at 1 A g−1), along with outstanding cycling stability (96.1% after 5000 cycles).103
Moreover, Yao et al.104 developed a LICs using a PVDF-HFP/[Li][NTf2]/([Emim][NTf2]) (polyvinylidene fluoride-hexa fluoropropylene/lithium bis(trifluoromethylsulfonyl)imide/1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide) ionogel membrane that displayed superior comprehensive electrochemical performance. By incorporating SiO2 as an inorganic filler to enhance the thermodynamic properties, the resulting LIC achieved an energy density of 147.2 W h kg−1 (93.7% cycling stability after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) and a maximum capacitance of 68.3 F g−1 at 1 A g−1 and 60 °C.
000 cycles) and a maximum capacitance of 68.3 F g−1 at 1 A g−1 and 60 °C.
Other significant studies have explored the use of PIL-based gel electrolytes to obtain pseudo-supercapacitors, as demonstrated by Ponkratov et al.105 Their system involved synthesizing two novel families of cationic PILs. PIL1, obtained by copolymerization of 1-[2-(2-(2-(methacryloyloxy)ethoxy)ethoxy)ethyl]-3-methylimidazolium bis(trifluoromethyl sulfonyl)imide with poly(ethylene glycol) methyl ether methacrylate, and PIL2, formed via polymer modification of poly(1-methyl-3-[oxiran-2-ylmethyl]-1-imidazole-3-ium-co-ethylene oxide) bis(trifluoromethyl sulfonyl)imide. PIL1 was used as an electrolyte due to its high ionic conductivity, while PIL2 was suitable for producing thin electrode films. These PILs were applied for fabricating all-polymer pseudo-supercapacitors, PPy + PIL2/PIL1/PPy + PIL2, which showcased optimal characteristics in specific capacitance (2.8 and 7.0 F g−1 at 30 mV s−1 scan rate and 25 and 70 °C, respectively), energy (1.39 W h kg−1) and power (286 W h kg−1 at 70 °C and 30 mV s−1 scan rate). This flexible device underwent 1000 cycles with 96% capacitance retention at a voltage up to 1.2 V at 25 °C. These experiments underscored the feasibility of developing pseudo-supercapacitors using only polymeric materials.
Polymers and ILs have also been applied in battery technology advances. For instance, Hu et al.106 designed PIL-based nanofibers, specifically poly(pyrrole)@poly(diallyldimethylammonium) bis(trifluoromethane sulfonyl) imide-polyacrylonitrile (PPy@PIL-PAN), which were used as a interlayer for Li–S batteries (Fig. 5). The results showed that the PIL framework could selectively adsorb polysulphide species in the electrolytes, contributing to inhibition decrease and sulphur electrochemistry stabilization.
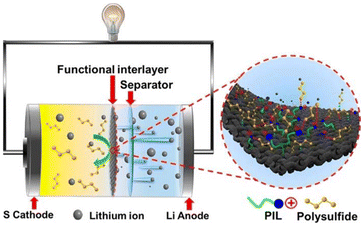 | ||
| Fig. 5 Schematic illustration of a Li–S battery with a PPy@PIL-PAN interlayer. Reproduced from ref. 106 with permission from American Chemical Society, copyright 2020. | ||
Alternatively, to address the modern energy crisis, it is crucial to produce energy via renewable resources, like geothermal, solar, wind, hydropower, and fuel cells Among the various types of fuel cells, proton exchange membrane fuel cells (PEMFCs) offer a viable electrochemical solution for converting chemical energy into electricity. Polymer electrolyte membranes (PEMs) transport hydrogen ions from the anode to the cathode, crucial for the functioning of PEMFCs (Fig. 6).107,108 IL-based composite membranes as PEMs provide a promising alternative for use in a broad temperature range. Thus, helping to overcome the limitations of traditional perfluoro-sulfonated polymers (PSPs), which are highly dependent on humidity and temperature. The use of IL-based PEMs in fuel cell stacks has been particularly noted for their high power density and low contamination levels.109
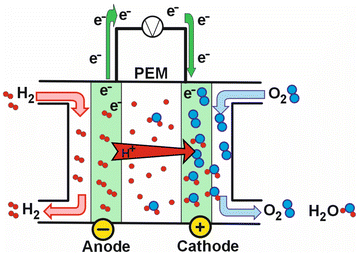 | ||
| Fig. 6 Schematic representation of a proton exchange membrane fuel cell.109 | ||
Several review articles have discussed different aspects of IL applications in PEMFCs. Zhang et al.110 discussed the use of ILs in modifying platinum/carbon electrocatalysts for PEMFC cathodes, focusing on the impact of IL content, dispersing solvents, and adsorption time. Alternatively, Rosli et al.111 reviewed biopolymer-based PEMs for fuel cells, highlighting the role of ILs as environmentally friendly additives that enhance performance. Ebrahimi et al.112 investigated the development of functionalized IL-based membranes with various ion exchange groups for use in PEMFC applications at medium and high temperatures. Alashkar et al.113 reviewed the preparation and application of PIL-based composite membranes in PEMFCs, providing comparative insights into the common polymers used in these composite membranes. Hence, advancements in IL-based membranes represent a significant step forward in developing PEMs that operate efficiently over a wide temperature range, thereby enhancing the viability and performance of PEMFCs for a variety of applications.
The crucial aspect is to achieve a combination of a high ionic conductivity and durable mechanical and thermal stability when considering its potential application as lithium anodes. This has been documented for polyimide and polybenzimidazole, which are highly stable polymers in terms of both thermal and mechanical properties. These polymers can be modified with sulfonated acid groups (similar to Nafion) and polymeric ILs (PILs).
3.3. Fire-resistant polymers
The flammability properties of polymers are crucial for ensuring the safety for both humans and the environment. Some polymers ignite and burn rapidly when exposed to heat, releasing toxic gases, smoke, and heat. The flammability characteristics are impacted by a range of factors, including chemical structure, molecular weight, composition, and the presence of fillers or additives. Flammability is commonly measured using various standard tests, such as the Limiting Oxygen Index (LOI)114 and the vertical burning test.115Reducing the flammability of polymers can be achieved through several methods, such as the addition of flame retardants, altering the polymer composition, or modifying the processing conditions. Flame retardants work by either reducing the release of flammable gases during combustion or by forming a protective char layer on the surface of the material, hindering flame propagation.34
Significant advancements have been made in the flammability reduction of synthetic polymers, with numerous studies focusing on polymers combined with ILs. For instance, a key is of research has been enhancing the flame retardancy of PU, especially polyurethane foams (PUFs). For example, PUF with enhanced flame retardancy was obtained by incorporating a room-temperature IL, namely tetrabutyl phosphonium thiocyanate ([P4444][SCN]), which contained flame-retardant phosphorus and sulfur elements. This new PUF, containing only 3.4 wt% of the IL, successfully passed the vertical burning standard test and exhibited a LOI value of 23.7% without compromising thermal stability or mechanical performance. Additionally, in the cone calorimeter test, the peak heat release rate of the obtained PUF/([P4444][SCN]) decreased by 40.5% compared to pure PUF, highlighting the potential of phosphorus–sulphur ILs as effective flame retardants for fire-safe polymeric foams.116
Significant advancements have been made in the flammability reduction of synthetic polymers, with numerous studies focusing on polymers combined with ILs. In fact, a key focus of research has been improving the flame retardancy of PU, especially polyurethane foams (PUFs). For example, PUF with enhanced flame retardancy was obtained by incorporating a room-temperature IL, namely tetrabutyl phosphonium.
Rigid polyurethane foams (RPUFs) commonly used for thermal and sound insulation, have also been studied for flame-retardants applications. For instance, Chen et al.117 proposed a synergistic flame-retardant combining 3-(N-diphenyl phosphate) amino propyl triethoxy silane (DPES) and 1-butyl-3-methyl-imidazole phosphate ([Bmim][PO4]) modified expandable graphite (IL-EG). Flame-retardant RPUFs compounded with IL-EG/DPES (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) showed uniform pore size, high compressive strength, and excellent flame-retardant performance. This performance improvement was attributed to the interwoven hydrogen bonds between IL-EG and DPES, as well as the new synergistic flame-retardant coating on the RPUF surface, which effectively restricted the transfer of gas or heat into the PU matrix, as can be seen in Fig. 7.
1, w/w) showed uniform pore size, high compressive strength, and excellent flame-retardant performance. This performance improvement was attributed to the interwoven hydrogen bonds between IL-EG and DPES, as well as the new synergistic flame-retardant coating on the RPUF surface, which effectively restricted the transfer of gas or heat into the PU matrix, as can be seen in Fig. 7.
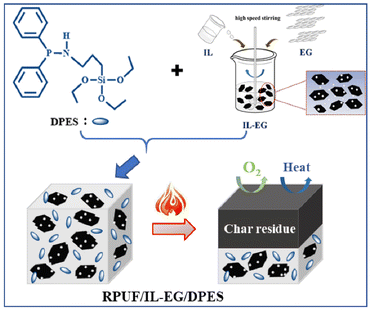 | ||
| Fig. 7 Schematic representation of the synthesis and burning mechanism of flame-retardant RPUFs compounded with IL-EG/DPES. Reproduced from ref. 117 with permission from Multidisciplinary Digital Publishing Institute, copyright 2020. | ||
Thermoplastic polyurethane (TPU) has also been investigated for its flame-retardant properties. In these studies, the IL 1-aminoethyl-3-methylimidazolium hexafluorophosphate ([AEmim][PF6]) was used as an additive in the synthesis of TPU. The resulting polymer showed improved thermal properties than pure TPU, with a 32.9% reduction of the peak heat release rate and a lower total heat release. These results highlight the potential of environmentally friendly green flame retardants in enhancing the safety of polymer materials.118
Chen et al.119 investigated the synergistic flame-retardant effects and smoke suppression properties of the [Emim][PF6] and aluminum hypophosphite (AHP). The most effective flame-retardant effects were obtained with a 0.0625 wt% content of [Emim][PF6] and a 19.9 wt% content of AHP, which increased the LOI value by 35.7%. Modified molecular sieve (MMS) by 1-((ethoxycarbonyl)methyl)-3-methylimidazolium hexafluorophosphate ([ECmim][PF6]) added to TPU also showed improved flame performance due to the formation of a char layer. This resulted in a reduced peak heat release rate and total heat release (48.4% and 32.5% lower, respectively) compared to pure TPU.120
Polymer composites, which combine two or more distinct materials, have also been studied for their flammability properties. For example, PVC was combined with activated carbon/phosphomolybdate 1-butyl-3-methylimidazolium (AC/PIL) to improve both flame retardancy and mechanical properties of PVC. AC/PIL triggered PVC crosslinking, leading to the formation of a dense char residue during thermal degradation, which prevented smoke generation and decreased heat transfer, thus protecting the matrix. AC/PIL-PVC exhibited a lower peak heat release rate and peak smoke production rate, 45.7% and 75.2% lower, respectively, compared to pure PVC.121
Other investigations have focused on functionalizing textile materials with ILs to improve their flame resistance and water repellence. In this context, Latifi et al.122 developed polymer/IL pastes based on polyacrylate, PU and latex mixed with different ILs, specifically N-hexylpyridinium ([HPy][PF6]) or hexafluorophosphate N-hexyl N,N,N tributyl ammonium ([N6444][PF6]). Fabrics coated with 2% of these pastes remained unburned even after 20 seconds of flame exposure, with high residue percentages (between 92% and 98%), as determined by the vertical flame test according to ISO 6940:2004. However, washing fastness tests according to ISO 105-C06:2010 indicated that the flame-retardant properties diminished after 12 wash cycles due to the removal of the IL-based [PF6] from the fabric surface. Despite this, the proposed treatment provided significant improvements in fire resistance and water–repellent properties.
3.4. Biomedical applications
ILs have demonstrated promising potential in a wide array of biomedical applications. These include their use in drug delivery systems, cancer therapy treatments, tissue engineering techniques, as well as in the development of antimicrobial and antifungal agents. Furthermore, ILs have also shown promise in the field of biosensing.123,124In this context, PILs have also been applied for proteomics. For example, a method for analysing of flavonoids in urine samples employed PIL-graphene oxide-grafted silica, providing a green and cost-effective approach.125 Additionally, imidazolium cations attached to cellulose aerogel facilitated the selective separation of BSA from serum by interacting with oxytetracycline through ion-exchange, reducing the detection limit and improving sample pretreatment efficiency.126,127
Moreover, functionalized magnetic nanospheres (Fe3O4@PCL-PILs), synthesized by grafting 1-vinylimidazolium-2-carboxylate bromide ([VimCOOH][Br]) onto ε-caprolactone (PCL), were used to separate Immunoglobulin G from human whole blood. These nanospheres exhibited zwitterionic behaviour and a negatively charged surface, achieving an adsorption capacity for Immunoglobulin G of 1136.4 mg g−1, with an 80.5% recovery using 0.5% NH3/H2O (v/v) as the stripping reagent.128
IL-based gels also hold promise for infection treatment and haemostasis due to their antibacterial properties. These properties arise from electrostatic interactions between the phosphate groups on bacterial cell membranes and the positively charged IL moieties. These interactions increase cell membrane permeability, leading to leakage of cytoplasmic content. Additionally, the incorporation of hydrophobic alkyl chains of ILs into bacterial lipid membranes can cause membrane perforation and cell death. Introducing ILs into dressings via covalent bonds provides long-lasting antibacterial effects without releasing ILs into the body, making this approach particularly appealing.129 For example, Wu et al.130 designed PIL hydrogels based on 1-vinyl-3-butylimidazolium bromide ([VBim][Br]), which remained active against Escherichia coli after up to five sterilization cycles at 120 °C and UV irradiation. Furthermore, conductive PIL hydrogels (PIL-KGM), composed of 1-vinyl-3-(aminopropyl)imidazolium tetrafluoroborate ([VAPim][BF4]) and konjac glucomannan (KGM), exhibited antibacterial activity and promoted angiogenesis and epidermal remodelling in a full-thickness skin defect model due to their electrical conductivity.131
IL-based gels can significantly enhance drug penetration into targeted tissues, making them valuable for tumour treatment. Stimuli-responsive hydrogels show promise as carriers for targeted drug delivery, minimizing systemic off-target side effects.132 An example is the polyvinyl alcohol (PVA)-based hydrogel developed by Kuddushi et al.,133 which incorporates an ester-functionalized morpholinium-based IL, 1-hexadecyl-3-ethylmorpholinium bromide ([C16C2Mor][Br]). This hydrogel formed a three-dimensional network with PVA through hydrophobic interactions, enabling Doxorubicin (DOX) loading and response to intracellular biological stimuli such as temperature and acidic pH in cancerous cells (Fig. 8). The hydrogel achieved 82.3% DOX release at 37 °C and pH 5.0 after 50 hours, compared to 53% at pH 7.4. At 25 °C, DOX release was 64.0% at pH 5.0 and 22.3% at pH 7.4. Enhanced release at acidic pH is due to protonation of the hydrogel, increasing water molecule interaction through hydrogen bonding and forming larger aggregates. Temperature and pH inversely affected the size of these aggregates, allowing intelligent DOX release.
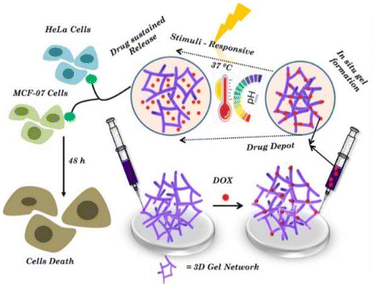 | ||
| Fig. 8 Scheme of responsive drug release from a PVA functionalized IL-based hydrogel for Doxorubicin (DOX) release against tumour cells. Reproduced from ref. 133 with permission from American Chemical Society, copyright 2020. | ||
In a radically different application, a polymer electrolyte-enabled biocompatible magnesium–air battery device was developed to use in miniaturized implanted medical devices (IMDs). The anode is made from a bioresorbable magnesium alloy, while the cathode uses biocompatible polypyrrole doped with para(toluene sulfonic acid). Interestedly, the electrolyte combines the biopolymer chitosan with the IL choline nitrate ([Ch][NO3]), ensuring full biocompatibility. This compact battery delivers a maximum volumetric power density of 3.9 W L−1, making it suitable for powering devices such as cardiac pacemakers and biomonitoring systems. This innovation could pave the way for the next generation of implantable power sources, offering a safer and more efficient solution for medical applications.134
4. IL-driven depolymerization
Plastics are essential materials in our daily lives due to their favourable characteristics, such as lightweight, durability, and resistance to degradation. However, these merits pose substantial challenges for waste management as these products reach the end of their useful life.18,135 Among various plastic recycling methods, chemical recycling stands out within the circular economy framework. This approach breaks down plastic wastes into valuable monomeric compounds136 by cleaving their molecular structures through nucleophilic attacks on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, which facilitates the breaking of C–O or C–N bonds.137,138 Common chemical recycling methods include alcoholysis (glycolysis and methanolysis), hydrolysis, and aminolysis (Fig. 9).139 The depolymerization of plastics can be facilitated by various catalysts, such as homogeneous, heterogeneous, and biocatalysts.140,141
O bonds, which facilitates the breaking of C–O or C–N bonds.137,138 Common chemical recycling methods include alcoholysis (glycolysis and methanolysis), hydrolysis, and aminolysis (Fig. 9).139 The depolymerization of plastics can be facilitated by various catalysts, such as homogeneous, heterogeneous, and biocatalysts.140,141
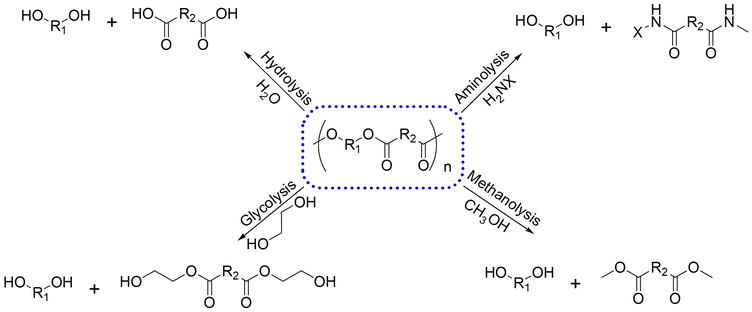 | ||
| Fig. 9 General schema of the solvolysis of polymers. Adapted from ref. 139 with permission from European Chemical Societies Publishing, copyright 2023. | ||
Chemical recycling processes often necessitate the use of harsh conditions, which can present significant challenges. These challenges encompass the need to separate liquid cleavage agents from by-products, ensuring optimal contact between the agent and solid polymer, and recovering dissolved catalysts. These complexities can significantly impede the industrial application of chemical recycling.142
In contrast, biocatalytic processes offer unique advantages due to the properties of enzymes—such as renewability, recoverability, biodegradability, and specificity—compared to precious metal catalysts. Enzymes can function as isolated catalysts (free or immobilized) or within whole cells overexpressing enzymes. Biocatalysts can operate in traditional aqueous environments or non-conventional media, where bulk water is absent. These non-conventional systems include solvent-free processes (where the substrate acts as the medium), micro-aqueous solutions (with minimal water content), supercritical fluids, or neoteric solvents like ILs and deep eutectic solvents (DES), which combine biocatalysis with non-aqueous systems.143,144 Despite these advantages, enzymes face challenges in hydrolysing C–C bonds present in the backbone of certain plastics.17,23 Moreover, enzymatic depolymerization is hindered by factors such as high molecular weight, crystallinity, hydrophobicity, and limited chain mobility, making biodegradation often inefficient and time-consuming, especially for polyolefins.145
Nevertheless, ILs possess unique physical–chemical properties that enhance their role in catalysis. These include low vapor pressure, non-flammability, high thermal and chemical stability, and the ability to enhance the activity and stability of (bio)catalysts. ILs can be fully recycled and reused multiple times without losing effectiveness. They also act as tuneable reaction media, modulating electronic and geometric effects, control reactant and product miscibility of reactants, and adjusting residence times of species. Furthermore, ILs can stabilize ionic and radical species, which further enhances their ability regulate catalytic processes.76
4.1. Cellulose
ILs have been widely applied in the conversion and characterization of biomass. Dissolving biomass, either partially or completely, is a crucial initial step for its efficient exploitation. However, the complex and rigid structure of biomass makes its processing challenging. ILs have emerged as valuable solvents for both chemical modification and dissolution of biomass and its components.146Cellulose, the most abundant biopolymer in the world, has been a major focus of research. The discovery of cellulose dissolution in ILs spurred the development of new processing technologies, methods for cellulose functionalization, and novel cellulose-based materials such as blends, composites, fibbers, and ion gels. ILs can dissolve cellulose by disrupting its strong hydrogen bonding network, enabling homogeneous chemical reactions that allow for the derivatization of cellulose into various functionalized products, including cellulose acetates, butyrates, phthalates, and benzoates. These derivatives have found applications in diverse fields (i.e. medicine, agriculture, and textiles). In addition to esterification and etherification reactions, the modification of the hydroxyl groups of cellulose has also led to the successful incorporation of a variety of functional groups, such as silylates, sulphates, and amines, expanding the range of potential applications. Furthermore, polymer grafting techniques, like ring-opening polymerization and RAFT (Reversible Addition–Fragmentation Chain Transfer) polymerization, have enhanced the properties of cellulose, imparting characteristics such as temperature responsiveness, hydrophobicity, and ion exchange capacity. These advancements position cellulose as a promising, sustainable alternative to petroleum-based materials. Despite challenges related to processing and large-scale fractionation, ongoing research into the use of ionic liquids for cellulose modification holds promise for the development of high-performance, environmentally friendly cellulosic materials.147–149
In biological biomass conversion, pretreatment plays a key role in reducing biomass recalcitrance and enhancing cellulose accessibility to enzymes. One notable sustainable strategy involved treating cellulose with [Bmim][Cl] for 1 hour at 115 °C, resulting in an amorphous cellulose solution via antisolvent precipitation with an IL recovery yield of up to 100% (Table 5, entry 1). Subsequent enzymatic hydrolysis of the obtained pretreated cellulose yielded glucose with up to 100% efficiency (after 4 hours at 50 °C) by the combined action of both cellulase and cellobiase enzymes. The ability of [Bmim][Cl] to dissolve cellulose remained unchanged after 5 cycles and the obtained glucose units could then be transformed into bioethanol through fermentation.40
| Entry | Polymer | IL/other reagents | Reaction conditions | Relevant properties | Proposed application | Ref. |
|---|---|---|---|---|---|---|
| Abbreviations: polyamide (PA), polylactic acid (PLA), poly(bisphenol A carbonate) (PC), polyethylene terephthalate (PET), polyurethane (PU), methyl lactate (ML), bisphenol A (BPA), bis(hydroxyethyl)terephthalate (BHET), terephthalatic acid (TPA), dimethyl terephthalate (DMT), bis(2-hydroxyethyl) terephthala-mide (BHETA), N1,N1,N4,N4-tetrakis (2-hydroxyethyl) terephthalamide (THETA), ethanolamine (EA), diethanolamine (DEA), ethylene glycol (EG), 4-dimethylaminopyridine (DMAP), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), 1-butyl-3-methylimidazolium chloride ([Bmim][Cl]), 1,3,5-trimethyl-1,3,5-triazapentane tetrafluoroborate ([TMPA][NTf2]), 1,5-diazabicyclo[4.3.0]non-5-ene acetate ([HDBN][OAc]), 1-hexyl-3-methylimidazolium trifluoromethanesulfonate ([Hmim][TfO]), 1,3-dimethylimidazolium methylsulfate ([Mmim][MeSO4]), 1-butyl-3-methylimidazolium methylsulfate ([Bmim][MeSO4]), 1-butyl-3-methylimidazolium acetate ([Bmim][OAc]), monoethanolammonium acetate ([MEA][OAc]), N-methyl-N-propylpiperidinium bis(trifluoromethane sulfonyl)imide ([Pip31][NTf2]), 1-ethyl-3-methylimidazolium tetrafluoroborate ([Emim][BF4]), 1-methyl-3-(3-sulfopropyl)-imidazolium hydrogen sulfate ([SPmim][HSO4]), 1-butyl-3-methylimidazolium tetrachloroferrate ([Bmim][FeCl4]), 1,8-diazabicyclo[5.4.0]undec-7-ene acetate ([HDBU][OAc]), 1,8-diazabicyclo[5.4.0]undec-7-ene succinate ([HDBU][Suc]), 1-butyl-3-methylimidazolium bromide ([Bmim][Br]), 1-butyl-3-vinylimidazolium bis[(trifluoromethyl)sulfonyl]imide ([BVim][NTf2]), cholinium glycinate ([Ch][Gly]), 1-hexyl-3-methylimidazolium trichlorozincate ([Hmim][ZnCl3]), 1-hexyl-3-methylimidazolium trichlorocobaltate ([Hmim][CoCl3]), 1-octyl-3-methylimidazolium bromide ([Omim][Br]), 1-vinyl-3-ethylimidazolium acetate ([VEim][OAc]); choline chloride ([Ch][Cl]). | ||||||
| 1 | Cellulose | [Bmim][Cl] | 115 °C, 1 h | - IL-based pretreatment step-Amorphous cellulose solution with reduced recalcitrance, adequate for enzymatic hydrolysis | Valorisation of cellulose to obtain bioethanol | 40 |
| 2 | Cellulose | [N3111][NTf2], HCl, LiCl | 100 °C, 15 min, MW | - Product recuperation by an acetone–isopropanol treatment | Biomass valorisation | 150 |
| 3 | Cellulose | [HDBN][OAc] | 80 °C, 90 min | - Cellulose can be shaped into fibbers | Production of high-quality man-made textile fibbers | 153 |
| - Technology effective for waste materials | ||||||
| 4 | Lignin | [Hmim][CF3SO3], [Mmim][MeSO4], [Bmim][MeSO4] | 60 °C, 1 h | - IL-based pretreatment step | Biomass valorisation | 155 |
| - Reduced lignin recalcitrance | ||||||
| 5 | Lignin | [Bmim][OAc] | 110 °C, 30 min | - IL-based pretreatment step | Valorisation of sugarcane bagasse | 156 |
| - Reduced lignin recalcitrance, adequate for enzymatic hydrolysis | ||||||
| 6 | Lignocellulose | [MEA][OAc] | 150 °C, 2 h | - IL-based pretreatment step | Valorisation of lignocellulose to obtain bioethanol | 157 |
| - Reduced lignocellulose recalcitrance, adequate for enzymatic hydrolysis | ||||||
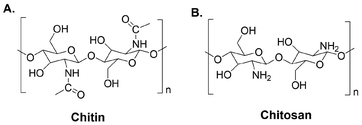
| 7 | Chitosan/chitin | [Bmim][Cl] | 110 °C, 5 h | - IL as suitable solvent | Functionalized synthetic polymers for capturing and releasing CO2 | 164 |
| - Disruption of the crystalline domains of both the polymers | ||||||
| 8 | Chitin from “biowastes” | [NH3(CH2)2OH][OAc] or [NH3OH][OAc] | 90 °C, 2 h | - IL for chitin extraction from “biowastes” | Demineralize and remove proteins from shrimp shells in an efficient one-pot pulping process (>80% chitin extraction) | 166 |
| 9 | Chitin from “biowastes” | [NH4][HCO2] | 150 °C 4 h | - IL for chitin extraction from “biowastes” | Chitin isolation and demineralization | 167 |
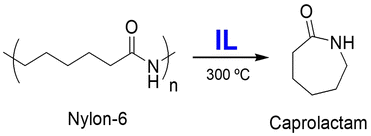
| 10 | PA | [Pip31][NTf2], DMAP | 300 °C, 6 h | - IL as catalyst | Depolymerization of PA to recover monomeric caprolactam | 168 |
| - Caprolactam yield of 86% | ||||||
| 11 | PA | [Emim][BF4], DMAP | 300 °C, 1 h, MW | - IL as catalyst | Depolymerization of PA to recover monomeric caprolactam | 169 |
| - Caprolactam yield of 55% | ||||||
| - Product recuperation by liquid–liquid extraction | ||||||
| 12 | PLA | [Bmim][OAc], | 115 °C, 3 h | - IL as catalyst | Methanolysis of PLA to obtain monomeric ML | 171 |
| [HSO3pmim] [HSO4], CH3OH | - PLA conversion of up to 100% | |||||
| - ML yield of 92% ([Bmim] [OAc]) and 88.7% ([HSO3−pmim][HSO4]) | ||||||
| 13 | PLA | [Bmim][OAc], H2O | 130 °C, 2 h | - IL catalyst, recycled for 5 times recycled for 7 times | Hydrolysis of PLA to obtain monomeric ML that can be conversed to calcium lactate | 172 |
| - PLA conversion of 93.9% | ||||||
| - Calcium lactate yield of 76.1% | ||||||
| 14 | PLA | [Bmim][FeCl4], CH3OH | 120 °C, 3 h | - IL catalyst, recycled for 6 times | Methanolysis of PLA to obtain monomeric ML | 173 |
| - PLA conversion of 99.3% | ||||||
| - ML yield of 94.6% | ||||||
| 15 | PLA | 2[Bmim][OAc]–Zn(OAc)2, CH3OH | 110 °C, 2 h | - IL catalyst, recycled for 5 times | Methanolysis of PLA to obtain monomeric ML | 174 |
| - PLA conversion of 97% | ||||||
| - ML yield of 92% | ||||||
| 16 | PLA | [HDBU][OAc], CH3OH | 100 °C, 5 h | - IL as catalyst, recycled for 5 times | Methanolysis of PLA to obtain monomeric ML | 175 |
| - PLA conversion of up to 100% | ||||||
| - ML yield of 91% | ||||||
| 17 | PC | [Bmim][OAc], CH3OH | 90 °C, 2.5 h | - IL catalyst, recycled for 6 times | Methanolysis of PC to obtain monomeric BPA | 176 |
| - PC conversion of up to 100% | ||||||
| - BPA yield of up to 95% | ||||||
| 18 | PC | [HDBU][OAc], CH3OH | 120 °C, 1 h | - IL catalyst, recycled for 6 times | Methanolysis of PC to obtain monomeric BPA | 177 |
| - PC conversion of up to 100% | ||||||
| - BPA yield of up to 95% | ||||||
| 19 | PC | [HDBU][Suc], CH3OH | 70 °C, 2 h | - IL catalyst, recycled for 6 times | Methanolysis of PC to obtain monomeric BPA | 178 |
| - BPA yield of 96% | ||||||
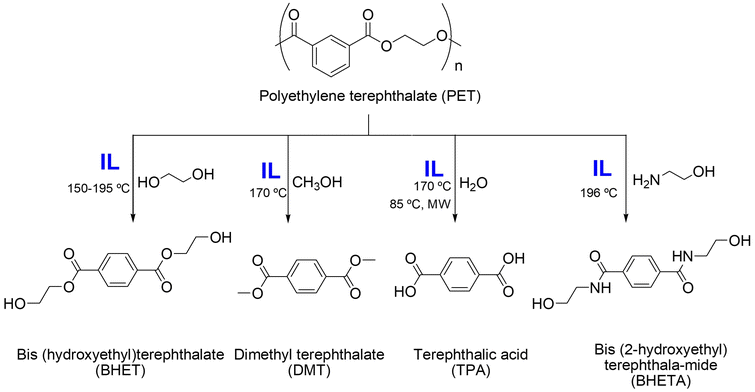
| 20 | PET | [Bmim][Br], EG | 180 °C, 8 h | - IL catalyst, recycled for 8 times | Glycolysis of PET to obtain monomeric BHET | 181 |
| - PET conversion of up to 100% | ||||||
| 21 | PET | [C6TMG][Cl]/2ZnCl2, EG | 195 °C, 70 min | - IL catalyst, recycled for 6 times | Glycolysis of PET to obtain monomeric BHET | 182 |
| - PET conversion of up to 100% | ||||||
| - BHET yield of 92.7% | ||||||
| 22 | PET | [BVim][NTf2]–Zn2+, EG | 195 °C, 2 h | - IL catalyst, recycled for 5 times | Glycolysis of PET to obtain monomeric BHET | 183 |
| - PET conversion of up to 95.4% | ||||||
| - BHET yield of 77.8% | ||||||
| 23 | PET | [Ch][Gly], EG | 150 °C, 6 h | - IL as catalyst | Glycolysis of PET to obtain monomeric BHET | 184 |
| - Technology effective for waste materials | ||||||
| - PET conversion of 85% | ||||||
| - BHET yield of 51% | ||||||
| 24 | PET | [Hmim][ZnCl3], [Hmim][CoCl3], EG | 190 °C, 2 h | - IL as catalyst | Glycolysis of PET to obtain monomeric BHET | 185 |
| - PET conversion of up to 100% | ||||||
| - BHET yield of 87.1% | ||||||
| 25 | PET | rGO/[TESPmim][CoCl4], EG | 190 °C, 3 h | - IL as catalyst | Glycolysis of PET to obtain monomeric BHET | 186 |
| - PET conversion of up to 100% | ||||||
| - BHET yield of 95.2% | ||||||
| 26 | PET | [Bmim][Cl], [HSO3−pmim][HSO4], H2O | 170 °C, 4.5 h | - IL as catalyst | Hydrolysis of PET to obtain monomeric TPA | 187 |
| - PET conversion of up to 100% | ||||||
| - TPA yield of 95.2% | ||||||
| 27 | PET | [Omim][Br], NaOH | 85 °C, 2.2 h, MW | - IL as catalyst | Hydrolysis of PET to obtain monomeric TPA | 188 |
| - TPA yield of 97.5% | ||||||
| 28 | PET | [VElm][OAc]–Zn2+, CH3OH | 170 °C, 70 min | - IL-Zn2+ catalyst, recycled for 6 times | Methanolysis of PET to obtain monomeric DMT | 189 |
| - PET conversion of up to 100% | ||||||
| - DMT yield of 89.1% | ||||||
| 29 | PET | [Hmim][TfO], EA, hydrazine hydrate | 196 °C, 30 min | - IL as catalyst | Aminolysis of PET to obtain monomeric BHETA | 190 |
| - BHETA yield of 84% | ||||||
| 30 | PET | [Ch][Cl]/ZnCl2, DEA | 30 min | - IL as catalyst | Aminolysis of PET to obtain monomeric THETA, BHETA, TPA | 191 |
| [Ch][Cl]/ZnCl2, DEA | - THETA yield of 82% | |||||
| [Ch][Cl]/ZnCl2, EA | - TPA yield of 83% | |||||
| 31 | Poly(succinate) (PSS) | [HDBU][Suc], H2O | 130 °C, 12 h | - IL-based media | Aminolysis of PSS to obtain succinimide derivatives and corresponding diols | 192 |
| - Stable for at least 4 cycles | ||||||
| - H2O enhanced HBs formation | ||||||
| - 80% PSS depolymerization yield | ||||||
| 32 | Polyglicolic acid (PGA) | Hydroxyl carboxylate anion-based ILs | 40–120 °C, 6–12 h | - IL-based media | Transformation of PGA into chemicals such as glycolic acid, alkyl glycolates, etc. | 193 |
| - Activity kept after 5 runs | ||||||
| - One-pot decomposition proved | ||||||
| 33 | PU | [Bmim][Cl], H2O, DBU | 95 °C, 4 h | - IL-based media | Hydrolysis of PU to obtain monomeric polyols | 41 |
| 34 | PU | [Ch][Cl]/urea | 170 °C, 8 h | - IL-based media | Hydrolysis of polycarbonate-based PU to obtain monomeric polycarbonate diol | 196 |
| - PU conversion of up to 100% | ||||||
| - Polycarbonate diol yield of 57.4% | ||||||
| 35 | PU | [Bmim][NTf2], solketal, H2O, lipase and urease | 60 °C, 48 h | - IL-based media | Enzymatic hydrolysis of a PU model system to obtain monomeric toluene diamine | 39 |
| - Biocatalysis | ||||||
| - Catalysis with 5% water | ||||||
Research has also focused on depolymerizing cellulose into glucose using IL-based media. For example, Kamimura et al.150 demonstrated effective conversion of cellulose to glucose using hydrophobic ILs such as 1,3,5-trimethyl-1,3,5-triazapentane tetrafluoroborate ([TMPA][NTf2]), in the presence of HCl and LiCl under microwave irradiation at 100 °C for 15 minutes, achieving glucose yields of up to 51% (Table 5, entry 2). The ILs were successfully recovered and reused at least five times without compromising the glucose yield, and glucose separation was easily achieved using an acetone–isopropanol treatment. Various ILs containing the [NTf2] anion (i.e. [Bmim][NTf2], [Emim][NTf2], [Pip31][NTf2], [Pyrr31][NTf2], [Pyrr41][NTf2], [N3111][NTf2], [N4111][NTf2]), have proven effective for this conversion (Fig. 10).
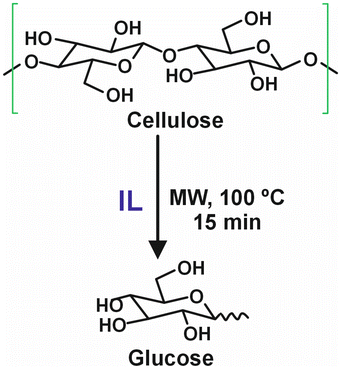 | ||
| Fig. 10 Degradation of cellulose to produce glucose in IL-based media.150 | ||
After being extracted from natural sources, regenerated cellulose fibbers can be transformed and used in a variety of shapes (e.g. filament or staple fibbers, yarns, fabric, nonwovens, membranes, etc.) and applications.151 As a leading example of a fully sustainable industrial process for converting cellulose into fibbers that can be made into durable fabrics, a method developed by researchers from Aalto University led by Professor Herbert Sixta, has been successfully implemented by the Finnish company IONCELL (Fig. 11).152 This process, known as Ioncell-F technology, has been employed to produce high-quality man-made cellulosic fibbers using the dry-jet wet spinning technique. Such has been the success and impact of this technology that Finland's First Lady, Jenni Haukio, wore one of the company's designs at the 2018 Independence Day celebration.
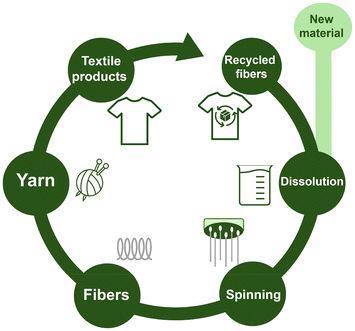 | ||
| Fig. 11 IONCELL production path.152 | ||
A key component of this technology is the use of the IL 1,5-diazabicyclo[4.3.0]non-5-ene acetate ([HDBN][OAc]) for dissolving cellulose at moderate conditions (80 °C, 90 minutes). This allows cellulose to be shaped into continuous filaments, which are then solidified through coagulation in cold water. The resulting fibbers can be spun into yarn via ring spinning technology, demonstrating excellent performance during knitting and weaving processes (Table 5, entry 3). Notably, this technology has also been successfully applied to transform waste materials, such as newsprint and waste cotton, into high-quality textile fibbers. This showcases the versatility and sustainability of the Ioncell-F process in producing man-made cellulosic fibbers from diverse sources.153
4.2. Lignin
Conversely, lignin represents the largest non-carbohydrate fraction of biomass, and significantly contributes to its recalcitrance due to its physical and chemical barriers. Therefore, isolating and dissolving lignin are critical steps in biomass valorisation. For instance, Pu et al.154 pioneered the study of lignin dissolution in selected aprotic ILs, discovering that up to 20 wt% of lignin could be dissolved after 1 hour at 60 °C in ILs such as 1-hexyl-3-methylimidazolium trifluoromethanesulfonate ([Hmim][CF3SO3]), 1-methyl-3-methylimidazolium methylsulfate ([Mmim][MeSO4]), and 1-butyl-3-methylimidazolium methylsulfate ([Bmim][MeSO4]) (Table 5, entry 4). Lignin solubility primarily depended on the with large non-coordinating anions (i.e. [BF4], [PF6]) were found to be unsuitable as lignin solvents. Further studies on the effect of the anionic and cationic components of ILs on lignin solubility revealed that the anion must possess a minimum hydrogen bonding basicity for effective lignin dissolution, and the cation type significantly influences lignin solubility.155Lignin solubility primarily depended on the nature of the anion in 1-butyl-3-methylimidazolium salts, with solubility in the order of [MeSO4] > [Cl] ≈ [Br] > [PF6], while ILs with large non-coordinating anions (i.e. [BF4], [PF6]) were found to be unsuitable as lignin solvents. Further studies on the effect of the anionic and cationic components of ILs on lignin solubility revealed that the anion must possess a minimum hydrogen bonding basicity for effective lignin dissolution, and the cation type significantly influences lignin solubility.
Hashmi et al.156 evaluated the valorisation of sugarcane bagasse through a two-step strategy: pretreatment with 1-butyl-3-methylimidazolium acetate ([Bmim][OAc]) at 110 °C for 30 minutes (Table 5, entry 5) followed by enzymatic saccharification using a mixture of commercial cellulases and β-glucosidase. IL-pretreated sugarcane bagasse exhibited reduced lignin content, decreased cellulose crystallinity, and enhanced digestibility of glucan and xylan compared to bagasse auto-hydrolysed at 205 °C for 6 minutes. Digestibility rates for glucan and xylan were significantly higher with IL-pretreated bagasse (97.4% and 98.6%, respectively) compared to auto-hydrolysed biomass (62.1% and 57.5%, respectively), demonstrating improved hydrolysis efficiency (Fig. 12).
 | ||
| Fig. 12 IL-pretreated Sugarcane Bagasse to decrease the lignin content, reduced cellulose crystallinity, followed by enzymatic saccharification using cellulases and glucosidases. Adapted from ref. 156 with permission from Elsevier, copyright 2017. | ||
Similarly, Nakasu et al.157 proposed a strategy using PILs as effective pretreatment agents for lignocellulosic biomass to produce second-generation ethanol. Their approach involved pretreating sugarcane bagasse with monoethanolammonium acetate ([MEA][OAc]) at 150 °C for 2 hours, achieving up to 60 wt% lignin solubilization without carbohydrate losses (Table 5, entry 6). Subsequent saccharification with cellulases and hemicellulases (5.8 wt%) yielded ethanol with a conversion efficiency of up to 66%.
4.3. Chitin and chitosan
Along with cellulose and lignin, chitin is one of the most abundant biopolymers in nature. It is found in the exoskeletons of crustaceans, insects and fungi,158 making this polysaccharide the second most abundant in nature after cellulose.159 Regarding the molecular structure of chitin, it is composed of successive units of 2-(acetylamino)-2-deoxy-D-glucose, so it can be described as a modified form of cellulose, where the hydroxyl group present in each monomer is substituted by an acetylamine group (Fig. 13A). Comparatively, chitosan is a natural biomaterial derived from the partial or complete deacetylation of chitin (Fig. 13B). Both chitin and chitosan have been extensively studied for their various applications and properties.Furthermore, these two polysaccharides are biodegradable with significant cytocompatibility, as well as antibacterial, antioxidant, analgesic and chelating activities.160 Thus, chitin and chitosan are considered as potential compounds for future in the biomedical research, with potential applications in drug delivery, tissue engineering biomaterials, wound healing materials, excipients in cosmetics, coating films in pharmaceuticals, etc.161 However, the traditional extraction from crustacean biomass, typically involves pulping processes, which involves the use of harsh chemicals (e.g. HCl, NaOH, organic solvents, oxidants, etc.) to accomplish different steps such as demineralization, deproteinization and decolouration.162 As a result of all these consecutive procedures, the final polymers obtained presents inconsistent characteristics and low quality, leading to less-favourable industrial products.
Alternatively, ILs have recently gained attention as versatile solvents for the isolation and processing of chitin and chitosan. These non-traditional solvents not only possess the capability to dissolve the polysaccharides but also function as reaction media. As a result, IL technologies have paved the way for the creation of custom 2D or 3D-based matrices from these biopolymers, thus introducing exciting new prospects for material design.
The first study regarding the solubility of chitin in different imidazolium-based ILs (i.e. [Bmim][Cl], [Bmim][OAc]) was published in 2001.163 In 2006, Xie et al.164 expanded on this work by dissolving chitin and chitosan in [Bmim][Cl] to obtain either chitin/IL or chitosan/IL solutions. The strategy involved heating in an oil bath the pure chitin/chitosan powder in [Bmim][Cl] at 110 °C in 1 wt% portion, adding a portion every 30 minutes until reaching a 10 wt% solution after 5 hours (Table 5, entry 7). Finally, both biopolymers were regenerated after the addition of methanol or water, and the analysis via wide-angle X-ray diffraction (WAXD) revealed complete and partial disruption of the crystalline domains of the chitin and chitosan, respectively. The presence of water in the IL significantly reduces cellulose solubility due to competitive hydrogen bonding with cellulose microfibrils, which hinders the dissolution process. The rate of cellulose dissolution decreases with lower temperatures, higher cellulose concentrations, and the addition of DMSO. Studies show that, in [Emim][OAc], the cellulose I lattice expands and distorts before complete dissolution. During precipitation, partially dissolved cellulose forms a less ordered intermediate structure, while fully dissolved cellulose results in a mixture of cellulose II and amorphous cellulose.165
More recently, research has shifted towards extraction chitin from “biowaste” feedstocks such as shrimp shells, fungi or even spider crab shells (Fig. 14). For example, in 2016, Shamshina et al.166 pioneered the employment of different ammonium-based ILs, such as 2-hydroxyethylammonium acetate ([NH3(CH2)2OH][OAc]) or hydroxylammonium acetate ([NH3OH][OAc]) (Table 5, entry 8) for chitin isolation from shrimp shells. This chemical pulping process, carried out at 90 °C for 2 hours with a 10 wt% biomass load, resulted in chitin yields of 22% and 40% from “processed” and “raw” shrimp shells, respectively. The extracted chitin had a purity of 83% from “processed” shells and 76% from “raw” shells, comparable to the commercial-grade chitin purity of 81%.
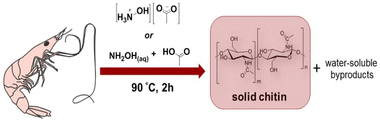 | ||
| Fig. 14 Scheme of the extraction of chitin from “biowaste” feedstock. Reproduced from ref. 166 with permission from American Chemical Society, copyright 2016. | ||
Similarly, Campalani et al.167 carried out a sustainable extraction of chitin from spider crab shells using ammonium acetate ([NH4][OAc]) and ammonium formate ([NH4][HCO2]) as solvents. Two strategies were evaluated: (1) using ILs as solid salts at 130 °C for 2 hours, and (2) preparing ILs in situ by sequentially adding acids (e.g. acetic acid) and bases (e.g. ammonia), or vice versa, at 100 °C for 2 hours. The best results were obtained using ammonium formate prepared in situ from aqueous ammonia and formic acid at 150 °C for 4 hours. This method yielded chitin with a 17 wt% yield and minimal mineral content (Table 5, entry 9).
4.4. Depolymerization of polyamide (PA)
Polyamides (PAs) are a class of crystalline polymers, commonly represented by two commercial products: nylon-6 and nylon-6,6. These materials are widely used across various industries, including electronics, electrical, automobiles, packaging, and construction. However, as the amount of PA waste continues to increase, recycling and disposal have become critical challenges.Kamimura and Yamamoto first studied the depolymerization of nylon-6 in ILs to recover monomeric caprolactam (Fig. 15). They evaluated the efficiency of different ILs, including N-methyl-N-propylpiperidinium bis(trifluoromethane sulfonyl)imide ([Pip31][NTf2]), 1-ethyl-3-methylimidazolium tetrafluoroborate ([Emim][BF4]), N-trimethyl-N-propylammonium bis(trifluoromethanesulfonyl)imide ([N3111][NTf2]), and [Bmim][NTf2], in the presence of 4-dimethylaminopyridine (DMAP) to yield caprolactam.
The most promising results were obtained with the combination of [Pip31][NTf2] and DMAP after 6 hours of reaction at 300 °C, resulting in a caprolactam yield of 86%. The product was purified by extraction or distillation to isolate the monomer and recover the IL (Table 5, entry 10).168 Depolymerization of PA was performed in the hydrophilic IL [Emim][BF4] under microwave irradiation at 300 °C for 1 hour to enhance reaction performance. The addition of 10 wt% DMAP as a catalyst significantly improved the depolymerization efficiency, increasing the yield of monomeric caprolactam to 55%.
This strategy provided a method to avoid direct distillation, which is energy-intensive for separating caprolactam from ILs. Caprolactam was isolated by liquid–liquid extraction using a CH2Cl2–water system, allowing the ILs recovery for further reuse (Table 5, entry 11).169
4.5. Depolymerization of polylactic acids (PLA)
Polylactic acid (PLA) is a representative biodegradable polymer that is expected to serve as a sustainable alternative to some petroleum-based materials. Notably, the properties of PLA products are significantly influenced by the quality of the lactide monomer, a crucial precursor in PLA production.170 The most common depolymerization methods for PLA are hydrolysis and methanolysis. Methanolytic depolymerization of PLA yields methyl lactate (ML), while hydrolysis of PLA produces lactic acid (LA), which is highly soluble in water (Fig. 16). Song et al.171 were the first to depolymerize PLA using [Bmim][OAc] and 1-methyl-3-(3-sulfopropyl)-imidazolium hydrogen sulfate ([SPmim][HSO4]) as catalysts. PLA conversions were of up to 100% and ML yields were 92% and 88.7%, respectively, after 3 hours at 115 °C (Table 5, entry 12). Besides methanolysis, Song et al.172 studied the depolymerization of PLA by hydrolysis using ILs as catalysts. Among the various ILs tested, [Bmim][OAc] exhibited the highest catalytic activity for the conversion of PLA and the production of calcium lactate (obtained after addition of calcium carbonate to the produced LA), achieving 93.9% and 76.1%, respectively, after 2 hours at 130 °C. A study on the reusability of the IL showed that it could be reused up to 7 times with no noticeable decrease in the hydrolysis conversion of PLA and the yield of calcium lactate (Table 5, entry 13).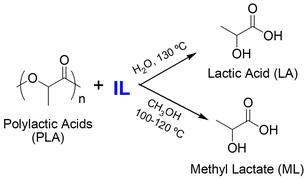 | ||
| Fig. 16 Depolymerization of PLA to produce methyl lactate (ML) or lactic acid (LA), from methanolysis or hydrolysis, respectively, in IL-based media. | ||
Liu et al.173 studied the methanolysis of PLA using a Lewis acidic IL (LAIL), 1-butyl-3-methylimidazolium tetrachloroferrate ([Bmim][FeCl4]), as a catalyst. The results showed a PLA conversion and ML yield of 99.3% and 94.6%, respectively, under the following optimized conditions: 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3OH/LA (mol/mol) and 0.0025
1 CH3OH/LA (mol/mol) and 0.0025![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [Bmim][FeCl4]/LA (mol/mol), reaction time 3 hours, and reaction temperature 120 °C. The reusability of the IL was investigated, and the results showed that it could be recycled up to 6 times with no apparent decrease in PLA conversion and methyl lactate yield (Table 5, entry 14).
1 [Bmim][FeCl4]/LA (mol/mol), reaction time 3 hours, and reaction temperature 120 °C. The reusability of the IL was investigated, and the results showed that it could be recycled up to 6 times with no apparent decrease in PLA conversion and methyl lactate yield (Table 5, entry 14).
Song et al.174 demonstrated the efficiency of a Zn-acetate-containing IL, 1-butyl-3-methylimidazolium acetate-promoted zinc acetate (2([Bmim][OAc])–Zn(OAc)2), for the methanolysis of PLA. PLA (4.0 g) was depolymerized in a medium based on Zn-acetate-containing IL (0.04 g) as catalyst and CH3OH (CH3OH/PLA, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mol/mol) as breaking agent at 110 °C for 2 hours, achieving a PLA conversion and ML yield of 97% and 92%, respectively. Compared with conventional ILs and other traditional catalysts, the Zn-acetate-containing IL exhibited excellent catalytic performance and required a smaller dosage. The IL could be reused up to 5 times (Table 5, entry 15).
1, mol/mol) as breaking agent at 110 °C for 2 hours, achieving a PLA conversion and ML yield of 97% and 92%, respectively. Compared with conventional ILs and other traditional catalysts, the Zn-acetate-containing IL exhibited excellent catalytic performance and required a smaller dosage. The IL could be reused up to 5 times (Table 5, entry 15).
Liu et al.175 synthesized a variety of DBU-based ILs and used them as solvents and catalysts for the methanolysis of PLA to yield lactate esters. The most outstanding IL was [HDBU][OAc], which comprised a 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) cation and an acetate anion. PLA was completely depolymerized with 5 mol% catalyst after 5 hours at 100 °C, yielding 91% ML (Table 5, entry 16). Moreover, [HDBU][OAc] showed excellent recyclability and structural stability for up to 6 times, making it a promising green and efficient platform for the direct and selective alcoholysis of PLA.
4.6. Depolymerization of poly(bisphenol A carbonate) (PC)
Poly(bisphenol A carbonate) (PC) is another important plastic with widespread applications in various industries due to its rigidity, stability, and flame resistance properties. PC depolymerization can be accomplished using several chemical processes, such as methanolysis and hydrolysis. Methanolytic depolymerization of PC yields bisphenol A (BPA), while hydrolysis of PC produces BPA and dimethyl carbonate (DMC) (Fig. 17). As a representative example, the depolymerization of PC was studied using a reaction system of [Bmim][OAc] as catalyst ([Bmim][OAc]/PC, 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) and methanol as breaking agent (CH3OH/PC, 0.75
1, w/w) and methanol as breaking agent (CH3OH/PC, 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w). The methanolysis results showed that the conversion of PC was nearly 100%, and the yield of BPA was over 95% after 2.5 hours at 90 °C. Moreover, the IL could be reused up to 6 times with no apparent decrease in PC conversion and BPA yield (Table 5, entry 17).176
1, w/w). The methanolysis results showed that the conversion of PC was nearly 100%, and the yield of BPA was over 95% after 2.5 hours at 90 °C. Moreover, the IL could be reused up to 6 times with no apparent decrease in PC conversion and BPA yield (Table 5, entry 17).176
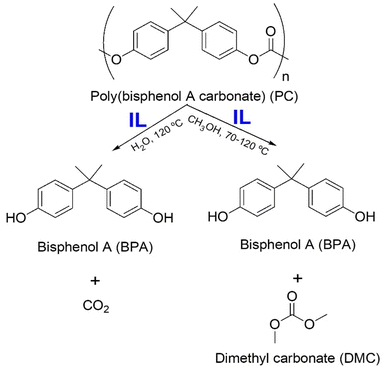 | ||
| Fig. 17 Depolymerization of PC to produce bisphenol A (BPA) or BPA and dimethyl carbonate (DMC), from methanolysis or hydrolysis, respectively, in IL-based media. | ||
Liu et al.177 developed an efficient protocol using different DBU-based synthesized ILs, determining that ILs facilitate depolymerization. While studying the methanolysis or hydrolysis of PC in the presence of ILs, they were able to recover DMC and BPA without using any other acid or base catalyst and under moderate conditions with up to 100% yield. The best results were obtained using [HDBU][OAc] (0.8 mol% with respect to reactants) and CH3OH (PC/CH3OH, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mol/mol) for the depolymerization of 15.7 mmol PC after 1 hour at 120 °C, achieving 100% PC conversion and 99% BPA yield. The catalytic IL showed excellent recyclability and could be reused for up to 6 cycles without any loss of catalytic activity (Table 5, entry 18).
1, mol/mol) for the depolymerization of 15.7 mmol PC after 1 hour at 120 °C, achieving 100% PC conversion and 99% BPA yield. The catalytic IL showed excellent recyclability and could be reused for up to 6 cycles without any loss of catalytic activity (Table 5, entry 18).
Recently, Liu et al.178 studied succinimide-based ILs (SIILs) as environmentally benign solvents and catalysts for the depolymerization of PC to BPA. Among the tested SIILs, [HDBU][Suc] displayed excellent efficiency and was able to carry out the methanolysis of PC in reaction systems with molar ratios [HDBU][Suc] to PC of 0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and CH3OH to PC of 6
1 and CH3OH to PC of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, at a reaction temperature of 70 °C for 2 hours, obtaining a BPA yield of 96%. It was found that after the depolymerization procedure, [HDBU][Suc] could be reused six times without any loss of catalytic activity (Table 5, entry 19).
1, at a reaction temperature of 70 °C for 2 hours, obtaining a BPA yield of 96%. It was found that after the depolymerization procedure, [HDBU][Suc] could be reused six times without any loss of catalytic activity (Table 5, entry 19).
4.7. Depolymerization of polyethylene terephthalate (PET)
Polyethylene terephthalate (PET) is one of the most extensively used commodity-grade thermoplastics due to its high mechanical strength, good barrier properties, and high optical clarity. It is commonly used in packaging and fibbers for clothing. PET is manufactured via polycondensation of dimethyl terephthalate (DMT) and/or terephthalic acid (TPA) with ethylene glycol (EG).179 Depending on the choice of nucleophiles (such as water, methanol, EG, or ethanolamine), the resulting products from PET depolymerization can be the monomers TPA, DMT, bis(hydroxyethyl)terephthalate (BHET), or bis (2-hydroxyethyl) terephthalamide (BHETA), as shown in Fig. 18.180Several studies have advanced on the recycling of PET, using ILs for the depolymerization step. The most prevalent method for PET depolymerization is glycolysis. In 2009, Wang and co-workers were the first to describe the use of ILs as catalysts in PET glycolysis. They observed that 1-butyl-3-methylimidazolium bromide ([Bmim][Br]) was the most efficient catalyst based on PET conversion and ease of preparation, achieving almost 100% PET conversion at 180 °C after 8 hours. Additionally, they concluded that the ILs could be reused at least eight times without compromising the conversion rate (Table 5, entry 20).181 In more recent studies, this group explored PET glycolysis using different synthesized 1,1,3,3-tetramethylguanidine (TMG) and metal salt-based ILs, such as [TMG][Cl]/ZnCl2, [TMG][Cl]/CoCl2, [TMG][Cl]/FeCl3, and [CnTMG][Cl]/ZnCl2 (n = 2, 4, 6, 8). They found that catalysts containing Zn(II) and alkylated tetramethylguanidinium chloride derivatives were more efficient for the depolymerization. In fact, using [C6TMG][Cl]/2ZnCl2 as catalyst in a reaction medium of PET/EG/IL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, w/w/w) at atmospheric pressure and 195 °C for 70 minutes led to the most promising performance for PET degradation, with a PET conversion and BHET yield of 100% and 92.7%, respectively. The reusability of residual EG and [C6TMG][Cl]/2(ZnCl2) was studied, showing that the catalyst could be reused up to six times without a noticeable change in reaction performance (Table 5, entry 21).182 Comparatively, the glycolysis of PET was investigated using a PIL based on 1-butyl-3-vinylimidazolium bis[(trifluoromethyl)sulfonyl]imide ([BVim][NTf2]) with immobilized metal ions, named PIL-Zn2+. PET conversion reached 95.4%, and the product BHET yield reached 77.8% using PIL-Zn2+ as a catalyst at 195 °C for 2 hours. After the reaction, the polymer IL-metal ion catalyst could be recovered at room temperature by simple filtration, and both the metal ion content in the polymer IL-metal ion complex and the catalytic performance of the synthesized catalysts did not decrease significantly after the catalyst was reused five times (Table 5, entry 22).183
0.1, w/w/w) at atmospheric pressure and 195 °C for 70 minutes led to the most promising performance for PET degradation, with a PET conversion and BHET yield of 100% and 92.7%, respectively. The reusability of residual EG and [C6TMG][Cl]/2(ZnCl2) was studied, showing that the catalyst could be reused up to six times without a noticeable change in reaction performance (Table 5, entry 21).182 Comparatively, the glycolysis of PET was investigated using a PIL based on 1-butyl-3-vinylimidazolium bis[(trifluoromethyl)sulfonyl]imide ([BVim][NTf2]) with immobilized metal ions, named PIL-Zn2+. PET conversion reached 95.4%, and the product BHET yield reached 77.8% using PIL-Zn2+ as a catalyst at 195 °C for 2 hours. After the reaction, the polymer IL-metal ion catalyst could be recovered at room temperature by simple filtration, and both the metal ion content in the polymer IL-metal ion complex and the catalytic performance of the synthesized catalysts did not decrease significantly after the catalyst was reused five times (Table 5, entry 22).183
Marullo et al.184 designed a glycolysis strategy using cholinium glycinate ([Ch][Gly]) as a catalyst in reactions at 150 °C for 6 hours, starting from PET derived from a clear water bottle. The results showed conversions of 85% and a yield of 51% (Table 5, entry 23). Chen et al.185 studied different 1-hexyl-3-methylimidazolium ([Hmim]) halometallates, such as [Hmim][ZnCl3], [Hmim][CoCl3], [Hmim][FeCl4] and [Hmim][CuCl3], as LAILs catalysts for PET degradation. The glycolysis reactions were carried out under atmospheric pressure at 190 °C for 2 hours. A synergistic effect was found when equimolar proportions of [Hmim][ZnCl3] and [Hmim][CoCl3] were mixed, obtaining a PET conversion of up to 100% and BHET yield of 87.1% (Table 5, entry 24). The glycolysis synergy came from the balance between the high reactivity offered by [Hmim][ZnCl3] and the high selectivity of [Hmim][CoCl3].
Additionally, PET glycolysis was achieved using a strategy based on a cobalt-based IL grafted on a graphene surface (rGO/[TESPmim][CoCl4]) as catalyst. The depolymerization of 1 g PET was carried out with 0.15 g catalyst and 14 g EG medium, incubated at 190 °C for 3 hours, resulting in a PET conversion and BHET yield of 100% and 95.2%, respectively. The catalyst demonstrated unchanged performance for five consecutive cycles (Table 5, entry 25).186
The hydrolysis of PET has also been investigated. For example, Liu et al.187 proposed a solvent mixture of water (4 g) and [Bmim][Cl] (6 g) that could depolymerizate 3 g of PET at 170 °C after 4.5 hours in the presence of 0.6 g of [HSO3pmim][HSO4] as a catalyst, achieving a PET conversion of 100% and a TPA yield of 88.7%. The IL could be reused eight times without an obvious decrease in the conversion of PET and the yield of TPA (Table 5, entry 26).
Hu et al.188 proposed a method for the hydrolysis of PET. The study optimized the process parameters as follows: 100 g PET, 2.7 g 1-octyl-3-methylimidazolium bromide ([Omim][Br]), 260 mL NaOH (15% w), 85 °C using microwave irradiation, and 2.2 hours. The depolymerization of PET occurred by an alkaline hydrolysis mechanism (saponification-neutralization), where PET was first hydrolysed in NaOH to yield TPA-Na2 and EG, and then TPA-Na2 was acidified to yield TPA. Under these conditions, the yield of TPA was 97.5%, demonstrating the viability of this method (Table 5, entry 27). Other approaches are based on methanolysis reaction systems. For example, a PET depolymerization strategy was demonstrated using a PIL based on 1-vinyl-3-ethylimidazolium acetate ([VEim][OAc]) and Zn(II), named PIL-Zn2+. When reactions were conducted with a mass ratio of methanol to PET of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the amount of PIL-Zn2+ was 2% of PET mass, the results showed 100% PET conversion and an 89.1% DMT yield through methanolysis at 170 °C for 60 minutes. Additionally, the PIL catalyst could be recycled up to six times without significant performance loss (Table 5, entry 28).189
1 and the amount of PIL-Zn2+ was 2% of PET mass, the results showed 100% PET conversion and an 89.1% DMT yield through methanolysis at 170 °C for 60 minutes. Additionally, the PIL catalyst could be recycled up to six times without significant performance loss (Table 5, entry 28).189
Palekar et al.190 carried out the aminolytic depolymerization of PET bottle wastes with ethanolamine and hydrazine hydrate under atmospheric conditions in the presence of 1-hexyl-3-methylimidazolium trifluoromethanesulfonate ([Hmim][TfO]). The results showed a BHETA yield of 84% after 30 minutes at 196 °C (Table 5, entry 29).
Musale et al.191 studied the depolymerization of PET bottle wastes using diethanolamine (DEA) or ethanolamine (EA) as breaking agents and synthesized DES, [Ch][Cl]/ZnCl2 and [Ch][Cl]/urea, as catalysts. The aminolysis of PET was divided into three reaction types, each resulting in a specific product: N1,N1,N4,N4-tetrakis (2-hydroxyethyl)-terephthalamide (THETA), TPA, and BHETA. THETA was obtained under conditions of PET (3 g), DEA (9.85 g), and DES catalyst (1–8% w) for a duration of up to 30 minutes. TPA was obtained under similar conditions. BHETA was obtained under conditions of PET (3 g), EA (5.73 g), and DES catalyst (1–8% w) for a duration of up to 30 minutes. The effect of different catalysts on the yield of each aminolysis product of PET was studied, revealing that [Ch][Cl]/ZnCl2 had the best performance among all aminolysis catalysts, with yields of THETA, TPA, and BHETA being 82%, 83%, and 95%, respectively (Table 5, entry 30).
On the other hand, polyester upcycling strategies are gaining recognition as viable and efficient processes with significant industrial-scale potential, driven by their inherent profitability. These methods are proving to be more economically attractive than traditional recycling, resulting in a growing commercial and scientific interest worldwide. In this context, Wu et al.192 developed an approach to upcycle poly(succinates) (PSS) into N-substituted succinimides via aminolysis, leveraging succinimide anion-based ILs (e.g., [HDBU][Suc]) properties. It was demonstrated how the presence of H2O enables the formation of hydrogen bonds between [HDBU][Suc] and ester, amino and amide groups, enhancing the catalysis at 130 °C of PSS aminolysis within the electroneutral microenvironment confined by the cation and anion (Table 5, entry 31). Comparatively, Zhao et al.193 designed a strategy to upgrade polyesters (e.g., polyglycolic acid) (PGA) over hydroxyl carboxylate anion-based ILs (e.g. 1-ethyl-3methylimidazolium glycolate, [Emim][Gac]) (Table 5, entry 32). This approach was based on the ability of these anions to activate the ester group through hydrogen bonding and subsequently decompose biopolyesters via autocatalyzed-transesterification, leading to hydroxyl carboxylate anion-based intermediates. These reactive and unstable intermediates further react with different nucleophilic agents to obtain the corresponding acids, esters, and/or amides at mild operating temperatures (from 40 to 120 °C).
4.8. Depolymerization of polyurethane (PU)
Polyurethane (PU) is another plastic product with a significant environmental impact, with production reaching 23.8 million metric tons in 2022 and increasing at a rate of 4.5% per year. In the European Union alone, over 40 million mattresses are discarded each year—enough to form a pile 904 times higher than Mount Everest. This amount increases by post-production wastes (e.g. mattress trimmings) up to 10% of total PUF production. Many PU types are thermosets with covalent crosslinking, making recycling extremely challenging. As a result, PU waste is mainly treated by environmentally unfriendly methods, with up to 45% ending up in landfills and 33% incinerated. Only about 5% of PU is mechanically recycled, underscoring the need for more sustainable waste management solutions.194 A potential solution involves the application of IL technologies due to their diverse physical–chemical properties, such as high solvent capacity for dissolving a wide range of compounds and their suitability as reaction media, even generating favourable synergistic effects on the basic strength of several organo-catalysts compared to classical molecular solvents.195 A depolymerization method for PU wastes was proposed based on the synergy of combining imidazolium-based ILs with bicyclic amidines catalysts. For example, a [Bmim][Cl]/DBU/water 67/13/20 (% w) system was tested for the hydrolysis of PUF wastes (25% w, with respect to reaction media) after 4 hours at 95 °C (Table 5, entry 33). The results showed the cleavage of urethane bonds, releasing aromatic amines, polyols, and carbon dioxide (Fig. 19).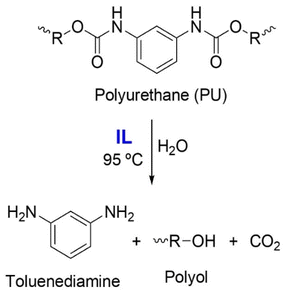 | ||
| Fig. 19 Depolymerization of PU to produce releasing aromatic amines, polyols, and carbon dioxide, in IL-based media. | ||
Furthermore, this technology facilitates the straightforward separation of polyols by a washing/centrifugation approach, which could be successfully reused and reintroduced in the production chain for the preparation of new polymer materials.41
On the other hand, Zhang et al.196 proposed a DES system of [Ch][Cl]/urea for the controlled degradation of polycarbonate-based PU under mild conditions (170 °C, 8 hours, 1 atm). The degradation rate of PU and the yield of polycarbonate diol (PCDL) reached 100% and 57.4%, respectively (Table 5, entry 34).
Recently, the suitability of different enzymes (i.e. lipase, urease, protease) have been demonstrated as high performance as biocatalysts for the hydrolysis toluene-based urethane model compounds (e.g. bis(2-methoxyethyl) (4-methyl-1,3-phenylene)dicarbamate, and bis(2-methoxyethyl) (2-methyl-1,3-phenylene)dicarbamate) by using ILs as reaction media (e.g. [Bmim][NTf2]) as can be seen in Fig. 20. It was observed that most of the enzymes were unable to catalyse the hydrolysis of the urethane bonds in pure water due to the insolubility of the substrates in this medium. The enzymatic activity clearly improved (up to 31.6 mU mg−1, urease test) in solketal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (90
water (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) reaction media. Furthermore, when hydrophobic ILs were used as reaction medium, the urease activity increased by more than twice (74.1 mU mg−1). The highest specific activity for the hydrolysis of these urethane compounds were obtained by combining lipase and urease enzymes in a IL
10, v/v) reaction media. Furthermore, when hydrophobic ILs were used as reaction medium, the urease activity increased by more than twice (74.1 mU mg−1). The highest specific activity for the hydrolysis of these urethane compounds were obtained by combining lipase and urease enzymes in a IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) solketal
solketal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (70
water (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v/v) reaction medium for 48 hours at 60 °C (Table 5, entry 35).39
5, v/v/v) reaction medium for 48 hours at 60 °C (Table 5, entry 35).39
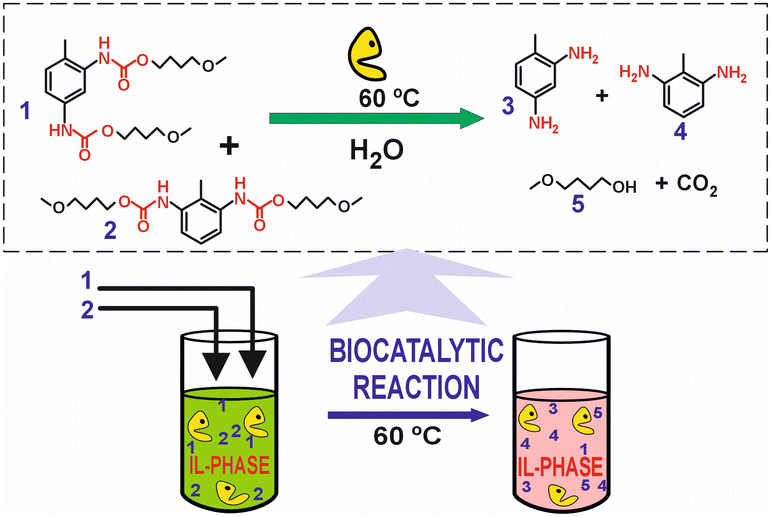 | ||
| Fig. 20 Enzymatic hydrolysis of bis(2-methoxyethyl) (4-methyl-1,3-phenylene)dicarbamate (1), and bis(2-methoxyethyl) (2-methyl-1,3-phenylene)dicarbamate (2) in several reaction media, obtaining the corresponding 2,4-toluene (3) and 2,6-toluene diamine (4), and EGME (5) products.39 | ||
These results demonstrated a promising, sustainable alternative to traditional PU recycling methods, which are environmentally harmful. Through the utilization of enzymes such as lipase, urease, and protease in ILs, this method amplifies enzymatic activity, resulting in the effective degradation of PU materials. This synergy aligns with green chemistry principles, enabling the recovery of valuable degradation products for reintegration into production, thus supporting a circular economy. Future research should focus on optimizing and scaling up these processes for industrial use, highlighting the potential of biocatalysis to drive sustainable waste management practices.
4.9. Application, prospects and development of IL-based depolymerization technologies
Table 5 summarizes some of the most relevant applications of ILs in depolymerization technologies. As described throughout the text, ILs offer interesting opportunities to improve polymer degradation processes, making them more efficient and sustainable. Their unique properties (i.e. solvent capacity, recyclability, and thermal stability), reveal ILs as transformative tools for addressing challenges in recycling and upcycling, summarized as follows:1. Enhancing Polymer Recycling. Recycling is essential to address the global plastic waste crisis. Traditional methods often require high energy input and produce unwanted by-products. ILs have shown exceptional performance in recycling various polymers. For example:
- PET and PLA Recycling: ILs like [Bmim][OAc] achieve up to 100% depolymerization efficiency for PET and PLA, with high monomer yields and excellent recyclability, reducing waste and energy consumption (sections 4.5 and 4.7).
- PC Recycling: ILs like [HDBU][OAc] have been used to depolymerize PC into monomers such as BPA with up to 100% efficiency under mild conditions. These ILs are reusable, further reducing the environmental impact of the process (section 4.6).
These examples highlight how ILs contribute to achieving a circular economy by recovering valuable materials from polymer and biomass waste.
2. Upcycling and Circular Economy. Beyond recycling, ILs also enable upcycling processes, turning waste into valuable resources. For instance:
- Biomass Conversion: ILs facilitate cellulose pretreatment, reducing its crystallinity and enabling enzymatic hydrolysis into glucose, which can be further converted into biofuels (section 4.1).
- Innovative Applications: Processes like the Ioncell-F technology use ILs to transform waste materials such as old textiles into durable, high-quality fibbers, showcasing their potential for sustainable material production (section 4.1).
3. Addressing Recycling Challenges. Despite their advantages, challenges remain. ILs need to be made cost-effective and scalable for industrial adoption. Task-specific ILs, designed for polymers like PET, PU or polyesters, could further expand their applications (sections 4.8 and 4.9). Research should also explore combining ILs with other catalysts, such as enzymes or metal complexes, to optimize their efficiency.
4. Expanding Applications Across Industries. The benefits of ILs extend beyond polymer recycling:
- Electronic and Biopolymer Waste: ILs show potential in processing complex waste types, such as electronic components or biodegradable plastics (sections 4.7 and 4.8).
- Medical Waste Management: ILs are being explored for processing biopolymer-based medical waste, such as PLA used in sutures or implants. Their ability to depolymerize such materials under mild conditions could facilitate the recovery of monomers like lactic acid for reuse, aligning with sustainable practices in healthcare (section 4.5).
Therefore, ILs have demonstrated their potential to transform polymer degradation by offering efficient, sustainable alternatives to traditional methods. Their adaptability and effectiveness across a range of processes make them valuable tools for advancing recycling and upcycling technologies, contributing to a greener and more sustainable future.
5. Conclusions and outlook
The growing environmental concerns related with traditional polymer production and disposal demand the search for sustainable alternatives. Ionic Liquids (ILs) have emerged as transformative agents in polymer science, offering innovative solutions both as functional components within polymers and as eco-friendly reaction media. ILs exhibit genuine properties such as negligible vapor pressure, high thermal stability, and tuneable viscosity, making them ideal candidates for advancing green chemistry. Their ability to dissolve a wide range of compounds, including recalcitrant polymers, positions them as valuable solvents for polymer synthesis, modification, and recycling. ILs facilitate efficient polymerization and can be integrated into polymers to form functional materials with specialized characteristics for applications in energy, biomedicine, and environmental remediation.The integration of ILs into polymer chemistry has led to significant advancements in various fields. Supported Ionic Liquid Phases (SILPs) and Poly(ionic liquid)s (PILs) represent novel materials that leverage the beneficial properties of ILs and polymers, enhancing their application potential in energy storage, biomedical materials, and environmental separation processes. Additionally, IL-based gels and ionogels further exemplify the innovative materials developed through IL technology.
Moreover, ILs play a pivotal role in the realm of chemical recycling by providing sustainable and environmentally friendly methods for breaking down polymers. Their unique ability to stabilize (bio)catalysts and streamline the depolymerization processes underscores their significant potential in effectively addressing the challenges associated with plastic waste management. Through the promotion of chemical recycling, ILs contribute to the circular economy model, thereby converting end-of-life plastics into valuable resources and diminishing the reliance on fossil fuels.
Nevertheless, the industrial implementation of ILs in waste treatment and recycling faces significant challenges, primarily due to the high cost of these components in relation to the low economic value of the final products. This issue remains underdeveloped for this emerging technology, even though ILs are widely valued for their genuine properties. Additionally, concerns regarding their environmental sustainability persist, specifically issues related to toxicity, biocompatibility, biodegradability, which may hinder their classification as truly green solvents. While strategies to enhance the “greenness” of ILs, such as using inexpensive, biodegradable, and abundant raw materials, are being explored, substantial research and advancements are still required to transition ILs from the laboratory to an industrial scale.
DES offer advantages similar to ILs, such as low toxicity and environmental safety, while being easier and more cost-effective to prepare. However, their higher viscosity and limited solubility can restrict their versatility. Supercritical fluids provide excellent solvating power and easy product recovery, but require high-pressure equipment and significant energy, making them less economically feasible than ILs. Perfluorinated solvents are chemically inert, non-reactive, and easy to recover, with lower toxicity than ILs. However, their environmental persistence and need for organic solvent combinations pose sustainability challenges. The choice of solvent for environmentally friendly processes depends on the applications, since these alternatives offering viable solutions to traditional volatile organic solvents.
In conclusion, ILs are pivotal in advancing the polymer industry toward sustainability. Their dual role as functional components and reaction media fosters the development of new materials and processes aligned with green chemistry and circular economy principles. As research continues to explore the full potential of ILs, their application in polymer science is set to play a crucial role in shaping a more sustainable future. The modular supramolecular framework of ILs provides a vital platform for integrating polymer chemistry into the circular economy, enabling greener and more sustainable technologies.
Abbreviation
| AC | Activated carbon |
| AHP | Aluminium hypophosphite |
| BHET | Bis(hydroxyethyl) terephthalate |
| BHETA | Bis(2-hydroxyethyl) terephthalamide |
| BPA | Bisphenol A |
| CAP | Cellulose acetate propionate |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| DEA | Diethanolamine |
| DES | Deep Eutectic Solvent |
| DMAP | 4-Dimethylaminopyridine |
| DMC | Dimethyl carbonate |
| DMT | Dimethyl terephthalate |
| DOX | Doxorubicin |
| DPES | 3-(N-Diphenyl phosphate) amino propyl triethoxy silane |
| EA | Ethanolamine |
| EG | Ethylene glycol |
| HNT | Halloysite nanotube |
| IL | Ionic liquid |
| IMD | Implanted medical device |
| KGM | Konjac glucomannan |
| LA | Lactic acid |
| LAIL | Lewis acidic ionic liquid |
| LIC | Lithium-ion capacitor |
| LOI | Limiting oxygen index |
| ML | Methyl lactate |
| MMA | Methyl methacrylate |
| MMS | Modified molecular sieve |
| PA | Polyamide |
| PC | Poly(bisphenol A carbonate) |
| PCDL | Polycarbonate diol |
| PCL | ε-Caprolactone |
| PDMS | Poly(dimethyl diphenyl siloxane) |
| PEMFC | Proton exchange membrane fuel cell |
| PEM | Polymer electrolyte membrane |
| PEO | Polyethylene oxide |
| PES | Polyether sulfone |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| PFS | Perfluorinated solvents |
| PGA | Polyglycolic acid |
| PIL | Poly(ionic liquid) |
| PLA | Polylactic acid |
| PSP | Perfluoro-sulfonated polymer |
| PSS | Poly(succinates) |
| PTFE | Polytetrafluoroethylene |
| PU | Polyurethane |
| PUF | Polyurethane foam |
| PVA | Polyvinyl alcohol |
| PVC | Poly(vinyl chloride) |
| PVDF | Polyvinylidene fluoride |
| PVDF-HFP | Polyvinylidene fluoride-hexa fluoropropylene |
| RPUF | Rigid polyurethane foam |
| SCF | Supercritical fluid |
| SILP | Supported ionic liquid phase |
| SPE | Solid-phase extraction |
| THETA | N 1,N1,N4,N4-Tetrakis (2-hydroxyethyl)-terephthalamide |
| TMG | 1,1,3,3-Tetramethylguanidine |
| TPA | Terephthalic acid |
| TPU | Thermoplastic polyurethane |
| VOC | Volatile organic compound |
| [N6444][PF6] | Hexafluorophosphate N-hexyl-N,N,N-tributylammonium |
| [AEmim][PF6] | 1-Aminoethyl-3-methylimidazolium hexafluorophosphate |
| [Bmim][BF4] | 1-Butyl-3-methylimidazolium tetrafluoroborate |
| [Bmim][Br] | 1-Butyl-3-methylimidazolium bromide |
| [Bmim][Cl] | 1-Butyl-3-methylimidazolium chloride |
| [Bmim][FeCl4] | 1-Butyl-3-methylimidazolium tetrachloroferrate |
| [Bmim][MeSO4] | 1-Butyl-3-methylimidazolium methylsulfate |
| [Bmim][NTf2] | 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [Bmim][OAc] | 1-Butyl-3-methylimidazolium acetate |
| [Bmim][PF6] | 1-Butyl-3-methylimidazolium hexafluorophosphate |
| [Bmim][PO4] | 1-Butyl-3-methyl-imidazole phosphate |
| [Bmim][NTf2] | 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [BVim][NTf2] | 1-Butyl-3-vinylimidazolium bis[(trifluoromethyl)sulfonyl]imide |
| [C16C2Mor][Br] | 1-Hexadecyl-3-ethylmorpholinium bromide |
| [Ch][Cl]/urea | DES: choline chloride and urea. |
| [Ch][Cl]/ZnCl2 | DES: choline chloride and zinc chloride |
| [Ch][Gly] | Cholinium glycinate |
| [Ch][NO3] | Choline nitrate |
| [CnTMG][Cl]/ZnCl2 | IL prepared from a mixture of alkylated tetramethylguanidinium chloride and zinc chloride |
| [D-VIm][NTf2] | 1,9-Di(3-vinylimidazolium)nonane bis(trifluoromethyl sulfonyl)imide |
| [Emim][B(CN)4] | 1-Ethyl-3-methylimidazolium tetracyanoborate |
| [Emim][BF4] | 1-Ethyl-3-methylimidazolium tetrafluoroborate |
| [Emim][Cl] | 1-Ethyl-3-methylimidazolium chloride |
| [Emim][Gac] | 1-Ethyl-3-methylimidazolium glycolate |
| [Emim][NTf2] | 1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| [Emim][PF6] | 1-Ethyl-3-methylimidazolium hexafluorophosphate |
| [ECmim][PF6] | 1-((Ethoxycarbonyl)methyl)-3-methylimidazolium hexafluorophosphate |
| [HDBN][OAc] | IL prepared from a mixture of 1,5-diazabicyclo[4.3.0]non-5-ene and acetate |
| [HDBU][OAc] | IL prepared from a mixture of 1,8-diazabicyclo[5.4.0]undec-7-ene and acetate |
| [HDBU][Suc] | IL prepared from a mixture of 1,8-diazabicyclo[5.4.0]undec-7-ene and succinate |
| [Hmim][CoCl3] | 1-Hexyl-3-methylimidazolium trichlorocobaltate |
| [Hmim][CuCl3] | 1-Hexyl-3-methylimidazolium trichlorocuprate |
| [Hmim][FeCl4] | 1-Hexyl-3-methylimidazolium tetrachloroferrate |
| [Hmim][TfO] | 1-Hexyl-3-methylimidazolium trifluoromethanesulfonate |
| [Hmim][ZnCl3] | 1-Hexyl-3-methylimidazolium trichlorozincate |
| [HPy][PF6] | N-Hexylpyridinium hexafluorophosphate |
| [SPmim] [HSO4] | 1-Methyl-3-(3-sulfopropyl)-imidazolium hydrogen sulfate |
| [Li][NTf2] | Lithium bis(trifluoromethylsulfonyl)imide |
| [MEA][OAc] | Monoethanolammonium acetate |
| [Mmim][MeSO4] | 1,3-Dimethylimidazolium methylsulfate |
| [MTA][Cl] | Methacrylatoethyl trimethyl ammonium chloride |
| [Mmim][Cl] | 1-Methyl-3-methylimidazolium chloride |
| [N3111][NTf2] | N,N,N-Trimethyl-N-propylammonium bis(trifluoromethylsulfonyl)imide |
| [N4111][NTf2] | N,N,N-Trimethyl-N-butylammonium bis(trifluoromethylsulfonyl)imide |
| [Omim][Br] | 1-Octyl-3-methylimidazolium bromide |
| [Pyrr31][NTf2] | 1-Propyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide |
| [Pyrr41][NTf2] | 1-Butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide |
| [P4444][SCN] | Tetrabutyl phosphonium thiocyanate |
| [Pip31][NTf2] | N-Methyl-N-propylpiperidinium bis(trifluoromethane sulfonyl)imide |
| [TEA][Lac] | Tetraethylammonium lactate |
| [TMG][Cl]/CoCl2 | IL prepared from a mixture of tetramethyl guanidinium chloride and cobalt chloride |
| [TMG][Cl]/FeCl3 | IL prepared from a mixture of tetramethyl guanidinium chloride and iron(III) chloride |
| [TMG][Cl]/ZnCl2 | IL prepared from a mixture of tetramethyl guanidinium chloride and zinc chloride |
| [TMPA][BF4] | 1,3,5-Trimethyl-1,3,5-triazapentane tetrafluoroborate |
| [VAPim][BF4] | 1-Vinyl-3-(aminopropyl)imidazolium tetrafluoroborate |
| [VBim][BF4] | 1-Vinyl-3-butylimidazolium tetrafluoroborate |
| [VBim][Br] | 1-Vinyl-3-butylimidazolium bromide |
| [VBim][NTf2] | 1-Vinyl-3-butylimidazolium bis(trifluoromethylsulfonyl)imide |
| [VEim][OAc] | 1-Vinyl-3-ethylimidazolium acetate |
| [VimCOOH][Br] | 1-Vinylimidazolium-2-carboxylate bromide |
| [VNim][NTf2] | 1-Vinyl-3-nonylimidazolium bis(trifluoromethyl sulfonyl)imide |
| (2[Bmim][OAc]-Zn(OAc)2) | IL prepared from a mixture of 1-butyl-3-methylimidazolium acetate and zinc acetate |
| (Fe3O4@PCL-PILs) | Magnetite, 1-vinylimidazolium-2-carboxylate bromide and ε-caprolactone nanospheres |
| (PPy@PIL-PAN) | Poly(pyrrole), poly(diallyldimethylammonium) bis(trifluoromethane sulfonyl) imide and polyacrylonitrile nanofibers |
| (PPy + PIL2/PIL1/PPy + PIL2) | Poly(pyrrole), 1-[2-(2-(2-(methacryloyloxy)ethoxy)ethoxy)ethyl]-3-methylimidazolium bis(trifluoromethyl sulfonyl)imide with poly(ethylene glycol) methyl ether methacrylate (PIL1), and poly(1-methyl-3-[oxiran-2-ylmethyl]-1-imidazole-3-ium-co-ethylene oxide) bis(trifluoromethyl sulfonyl)imide (PIL2) pseudo-supercapacitor |
| (PVDF-HFP/[Li][NTf2]/([Emim][BF4])) | Polyvinylidene fluoride-hexafluoropropylene, lithium bis(trifluoromethylsulfonyl)imide and 1-ethyl-3-methylimidazolium tetrafluoroborate LIC |
| (PVDF-HFP/[Li][NTf2]/([Emim][NTf2])) | Polyvinylidene fluoride-hexafluoropropylene, lithium bis(trifluoromethylsulfonyl)imide and 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide LIC |
| (rGO/[TESPmim] [CoCl4]) | Reduced graphene oxide (rGO), 1-(triethoxysilyl) propyl-3-methylimidazolium chloride and cobalt chloride composite |
Author contributions
R. Salas: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing – original draft, writing – review & editing; R. Villa: conceptualization, data curation, formal analysis, investigation, methodology, writing – original draft, writing – review & editing. F. Velasco: data curation, formal analysis, investigation, validation, visualization, writing – original draft, writing – review & editing. F. G. Cirujano: data curation, investigation, writing – review & editing; S. Nieto: data curation, investigation, writing – review & editing. N. Martin: data curation, formal analysis, investigation, writing – review & editing; E. Garcia-Verdugo: formal analysis, funding acquisition, investigation, methodology, writing – review & editing; J. Dupont: conceptualization, data curation, investigation, writing – review & editing; P. Lozano: conceptualization, formal analysis, funding acquisition, investigation, methodology, writing – review & editing.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the funding partially supported by MICINN-FEDER-AEI 10.13039/501100011033 (PID2021-124695OB-C21/C22 and PDC2022-133313-C21/C22), MICINN-European Union Next Generation EU-PRTR (TED2021-129626B-C21/C22), and Fundación SENECA CARM (21884/PI/22) grants. J. D. is a fellow of the “Maria Zambrano program” at the University of Murcia (Spain).References
- The New Plastics Economy: Rethinking the future of plastics. World Economic Forum, Ellen MacArthur Foundation and McKinsey & Company, 2016, https://www.ellenmacarthurfoundation.org/the-new-plastics-economy-rethinking-the-future-of-plastics-and-catalysing, (accessed 13 May 2024).
- H. Namazi, BioImpacts, 2017, 7, 73–74, DOI:10.15171/bi.2017.09.
- Hermann Staudinger – Nobel Lecture. NobelPrize.org. Nobel Prize Outreach AB 2024. https://www.nobelprize.org/prizes/chemistry/1953/staudinger/lecture/, (accessed 16 May 2024).
- M. T. Islam, M. M. Rahman and N. Mazumder, in Frontiers of Textile Materials: Polymers, Nanomaterials, Enzymes, and Advanced Modification Techniques, ed. M. Shabbir, S. Ahmed and J. N. Sheikh, Scrivener Publishing LLC, 1st edn, 2020, ch. 2, pp. 13–59. DOI:10.1002/9781119620396.ch2. ISBN: 978-1-119-62037-2.
- (a) J. A. Barbosa-Nunez, H. Espinosa-Andrews, A. A. V. Cardona and J. N. Haro-Gonzalez, J. Future Foods, 2024, 5, 36–49, DOI:10.1016/j.jfutfo.2024.01.003; (b) X. N. Lu, S. Qian, X. H. Wu, T. T. Lan, H. Zhang and J. S. Liu, Int. J. Biol. Macromol., 2024, 265, 130987, DOI:10.1016/j.ijbiomac.2024.130987.
- (a) K. Rojas, M. G. Verdugo-Molinares and A. A. V. Cardona, Food Chem. Adv., 2024, 4, 100619, DOI:10.1016/j.focha.2024.100619; (b) C. S. Dong, M. Petrovic and I. J. Davies, Ann. 3D Print. Med., 2024, 14, 100149, DOI:10.1016/j.stlm.2024.100149; (c) P. Sanjarnia, M. L. Picchio, A. N. P. Solis, K. Schuhladen, P. M. Fliss, N. Politakos, L. Metterhausen, M. Calderon and E. R. Osorio-Blanco, Adv. Drug Delivery Rev., 2024, 207, 115217, DOI:10.1016/j.addr.2024.115217.
- H. Wang, H. P. Li, C. K. Lee, N. S. M. Nanyan and G. S. Tay, Int. J. Biol. Macromol., 2024, 261, 129536, DOI:10.1016/j.ijbiomac.2024.129536.
- F. Y. Su, T. S. He, Z. M. He, Q. H. Yu and H. Y. Wang, Molecules, 2024, 29, 1260, DOI:10.3390/molecules29061260.
- J. R. Ghonia, N. G. Savani, V. Prajapati and B. Z. Dholakiya, J. Polym. Res., 2024, 31, 95, DOI:10.1007/s10965-024-03941-5.
- M. R. Krishnan, H. Omar, A. Almohsin and E. H. Alsharaeh, Polym. Bull., 2024, 81, 3883–3933, DOI:10.1007/s00289-023-04934-y.
- N. Karak, in Fundamentals of Polymers: Raw Materials to Finish Products, PHI Learning Pvt Ltd, 2009. ISBN: 978-81-203-3877-7 Search PubMed.
- (a) Plastics – the fast Facts 2023. Plastics Europe, https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed 13 May 2024); (b) Plastics – the Facts 2022. Plastics Europe, https://plasticseurope.org/wp-content/uploads/2022/10/PE-PLASTICS-THE-FACTS_V7-Tue_19-10-1.pdf (accessed 13 May 2024).
- (a) K. Zheng, Y. Wu, Z. X. Hu, S. M. Wang, X. C. Jiao, J. C. Zhu, Y. F. Sun and Y. Xie, Chem. Soc. Rev., 2023, 52, 8–29, 10.1039/d2cs00688j; (b) H. Sardon and A. P. Dove, Science, 2018, 360, 380–381, DOI:10.1126/science.aat4997; (c) P. F. Britt, G. W. Coates, K. I. Winey, J. Byers, E. Chen, B. Coughlin, C. Ellison, J. Garcia, A. Goldman, J. Guzman, J. Hartwig, B. Helms, G. Huber, C. Jenks, J. Martin, M. McCann, S. Miller, H. O'Neill, A. Sadow, S. Scott, L. Sita, D. Vlachos and R. Waymouth, Report of the Basic Energy Sciences Roundtable on Chemical Upcycling of polymers, United States, 2019. DOI:10.2172/1616517.
- S. R. Nicholson, N. A. Rorrer, A. C. Carpenter and G. T. Beckham, Joule, 2021, 5, 673–686, DOI:10.1016/j.joule.2020.12.027.
- C. Y. Wang, Y. Liu, W. Q. Chen, B. Zhu, S. Qu and M. Xu, J. Ind. Ecol., 2021, 25, 1300–1317, DOI:10.1111/jiec.13125.
- P. Kubisa, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 4675–4683, DOI:10.1002/pola.20971.
- (a) P. Lozano and E. Garcia-Verdugo, Green Chem., 2023, 25, 7041–7057, 10.1039/D3GC01878D; (b) R. Villa, E. Alvarez, R. Porcar, E. Garcia-Verdugo, S. V. Luis and P. Lozano, Green Chem., 2019, 21, 6527–6544, 10.1039/C9GC02553G.
- L. D. Ellis, N. A. Rorrer, K. P. Sullivan, M. Otto, J. E. McGeehan, Y. Roman-Leshkov, N. Wierckx and G. T. Beckham, Nat. Catal., 2021, 4, 539–556, DOI:10.1038/s41929-021-00648-4.
- M. Krasnodebski, Stud. Hist. Philos. Sci., 2022, 96, 112–120, DOI:10.1016/j.shpsa.2022.09.004.
- E. Nikolaivits, B. Pantelic, M. Azeem, G. Taxeidis, R. Babu, E. Topakas, M. B. Fournet and J. Nikodinovic-Runic, Front. Bioeng. Biotechnol., 2021, 9, 696040, DOI:10.3389/fbioe.2021.696040.
- (a) H. Mutlu and L. Barner, Macromol. Chem. Phys., 2022, 223, 2200111, DOI:10.1002/macp.202200111; (b) Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. OJ L 150, 2018, p. 109–140. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32018L0851, (accessed 8 June 2024).
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998. DOI:10.1093/oso/9780198506980.001.0001. ISBN: 9780198506980.
- V. Tournier, S. Duquesne, F. Glillamot, H. Cramail, I. Andre, D. Taton and A. Marty, Chem. Rev., 2023, 123, 5612–5701, DOI:10.1021/acs.chemrev.2c00644.
- V. G. Zuin and K. Kummerer, Nat. Rev. Mater., 2022, 7, 76–78, DOI:10.1038/s41578-022-00415-2.
- S. Nieto, E. Alvarez-Gonzalez, J. M. Bernal, A. Donaire and P. Lozano, in Sustainable Catalysis in Ionic Liquids, 2019, pp. 203–230. ISBN: 978-1-138-55370-5 Search PubMed.
- T. Christoff-Tempesta and T. H. Epps, ACS Macro Lett., 2023, 12, 1058–1070, DOI:10.1021/acsmacrolett.3c00276.
- (a) J. Dupont, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667–3692, DOI:10.1021/cr010338r; (b) P. Migowski, P. Lozano and J. Dupont, Green Chem., 2023, 25, 1237–1260, 10.1039/d2gc04749g.
- A. Dhakshinamoorthy, A. M. Asiri, M. Alvaro and H. Garcia, Green Chem., 2018, 20, 86–107, 10.1039/C7GC02260C.
- D. R. MacFarlane, M. Forsyth, P. C. Howlett, M. Kar, S. Passerini, J. M. Pringle, H. Ohno, M. Watanabe, F. Yan, W. Zheng, S. Zhang and J. Zhang, Nat. Rev. Mater., 2016, 1, 15005, DOI:10.1038/natrevmats.2015.5.
- P. Sun and D. W. Armstrong, Anal. Chim. Acta, 2010, 661, 1–16, DOI:10.1016/j.aca.2009.12.007.
- D. Han and K. H. Row, Molecules, 2010, 15, 2405–2426, DOI:10.3390/molecules15042405.
- E. Wanigasekara, S. Perera, J. A. Crank, L. Sidisky, R. Shirey, A. Berthod and D. W. Armstrong, Anal. Bioanal. Chem., 2010, 396, 511–524, DOI:10.1007/s00216-009-3254-2.
- H. Nie, N. S. Schauser, N. D. Dolinski, J. Hu, C. J. Hawker, R. A. Segalman and J. R. de Alaniz, Angew. Chem., Int. Ed., 2020, 59, 5123–5128, DOI:10.1002/anie.201912921.
- A. A. Shamsuri, M. Z. M. Yusoff, K. Abdan and S. N. A. M. Jamil, e-Polymers, 2023, 23, 20230060–20230075, DOI:10.1515/epoly-2023-0060.
- A. A. Shamsuri, S. N. A. M. Jamil and K. Abdan, e-Polymers, 2022, 22, 809–820, DOI:10.1515/epoly-2022-0074.
- H. Wang, H. Zhou, Q. Yan, X. Wu and H. Zhang, Energy Convers. Manage., 2023, 297, 117758, DOI:10.1016/j.enconman.2023.117758.
- L. J. He, L. Chen, B. H. Zheng, H. Zhou, H. Wang, H. Li, H. Zhang, C. C. Xu and S. Yang, Green Chem., 2023, 25, 7410–7440, 10.1039/D3GC02816J.
- C. Chiappe and D. Pieraccini, J. Phys. Org. Chem., 2005, 18, 275–297, DOI:10.1002/poc.863.
- R. Salas, R. Villa, S. Cano, S. Nieto, E. Garcia-Verdugo and P. Lozano, Catal. Today, 2024, 430, 114516–114523, DOI:10.1016/j.cattod.2024.114516.
- (a) P. Lozano, B. Bernal, I. Recio and M. P. Belleville, Green Chem., 2012, 14, 2631–2637, 10.1039/C2GC35905G; (b) P. Lozano, J. M. Bernal, R. Piamtongkam, D. Fetzer and M. Vaultier, ChemSusChem, 2010, 3, 1359–1363, DOI:10.1002/cssc.201000244.
- P. Lozano, R. Villa, R. Salas, E. Garcia-Verdugo and M. Macia. Patents PCT/ES2023/070452, WO2024/013423A1, 18 January 2024 Search PubMed.
- (a) A. Kamimura, T. Kawamoto and K. Fujii, Chem. Rec., 2023, 23, e202200269, DOI:10.1002/tcr.202200269; (b) Y. Qiao, W. Ma, N. Theyssen, C. Chen and Z. Hou, Chem. Rev., 2017, 117, 6881–6928, DOI:10.1021/acs.chemrev.6b00652; (c) C. Dai, J. Zhang, C. Huang and Z. Lei, Chem. Rev., 2017, 117, 6929–6983, DOI:10.1021/acs.chemrev.7b00030; (d) S. P. M. Ventura, F. A. e Silva, M. V. Quental, D. Mondal, M. G. Freire and J. A. P. Coutinho, Chem. Rev., 2017, 117, 6984–7052, DOI:10.1021/acs.chemrev.6b00550.
- Y. Chen and T. C. Mu, Green Chem. Eng., 2021, 2, 174–186, DOI:10.1016/j.gce.2021.01.004.
- B. S. Wang, L. Qin, T. C. Mu, Z. M. Xue and G. H. Gao, Chem. Rev., 2017, 117, 7113–7131, DOI:10.1021/acs.chemrev.6b00594.
- Z. M. Xue, L. Qin, J. Y. Jiang, T. C. Mu and G. H. Gao, Phys. Chem. Chem. Phys., 2018, 20, 8382–8402, 10.1039/C7CP07483B.
- (a) P. Lozano, Green Chem., 2010, 12, 555–569, 10.1039/B919088K; (b) P. Lozano and E. Garcia-Verdugo, in Ionic Liquids in Biotransformations and Organocatalysis, ed. P. Dominguez de Maria, Wiley, 2012, ch. 4, pp. 67–95. DOI:10.1002/9781118158753.ch4.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232–1285, DOI:10.1021/acs.chemrev.0c00385.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071, DOI:10.1021/sc500096j.
- F. M. Perna, P. Vitale and V. Capriati, Curr. Opin. Green Sustainable Chem., 2020, 21, 27–33, DOI:10.1016/j.cogsc.2019.09.004.
- S. Hong, H. L. Lian, X. Sun, D. Pan, A. Carranza, J. A. Pojman and J. D. Mota-Morales, RSC Adv., 2016, 6, 89599–89608, 10.1039/C6RA18290A.
- Y. Liu, J. B. Friesen, J. B. McAlpine, D. C. Lankin, S. N. Chen and G. F. Pauli, J. Nat. Prod., 2018, 81, 679–690, DOI:10.1021/acs.jnatprod.7b00945.
- D. J. G. P. van Osch, C. H. J. T. Dietz, S. E. E. Warrag and M. C. Kroon, ACS Sustainable Chem. Eng., 2020, 8, 10591–10612, DOI:10.1021/acssuschemeng.0c00559.
- T. R. Sekharan, O. Katari, S. N. R. Rahman, D. M. Pawde, A. Goswami, R. M. Chandira and T. Shunmugaperumal, Drug Discovery Today, 2021, 26, 1702–1711, DOI:10.1016/j.drudis.2021.03.005.
- Z. Knez, M. Pantic, D. Cor, Z. Novak and M. K. Hrncic, Chem. Eng. Process., 2019, 141, 107532, DOI:10.1016/j.cep.2019.107532.
- Z. Knez, E. Markocic, M. Leitgeb, M. Primozic, M. K. Hrncic and M. Skerget, Energy, 2014, 77, 235–243, DOI:10.1016/j.energy.2014.07.044.
- K. Broeckhoven, Trends Anal. Chem., 2022, 146, 116489, DOI:10.1016/j.trac.2021.116489.
- B. Betzemeier and P. Knochel, in Modern Solvents in Organic Synthesis. Topics in Current Chemistry, ed. P. Knochel, Springer, Berlin, Heidelberg, 1999, vol. 206, pp. 60–78. DOI:10.1007/3-540-48664-X_3. ISBN: 978-3-540-66213-6.
- I. T. Horvath and J. Rabai, Science, 1994, 266, 72–75, DOI:10.1126/science.266.5182.72.
- N. K. Singha, K. Hong and J. W. Mays, in Polymerized Ionic Liquids, ed. A. Eftekhari, The Royal Society of Chemistry, 2017, pp. 1–22. 10.1039/9781788010535-00001. ISBN: 978-1-78801-053-5.
- R. T. Carlin, R. A. Osteryoung, J. S. Wilkes and J. Rovang, J. Inorg. Chem., 1990, 29, 3003–3009, DOI:10.1021/ic00341a030.
- R. T. Carlin and J. S. Wilkes, J. Mol. Catal., 1990, 63, 125–129, DOI:10.1016/0304-5102(90)85135-5.
- K. L. Hong, H. W. Zhang, J. W. Mays, A. N. Visser, C. S. Brazel, J. D. Holbrey, W. M. Reichert and R. D. Rogers, Chem. Commun., 2002, 13, 1368–1369, 10.1039/B204319J.
- (a) S. Harrisson, S. R. Mackenzie and D. M. Haddleton, Chem. Commun., 2002, 23, 2850–2851, 10.1039/B209479G; (b) S. Harrison, S. R. MacKenzie and D. M. Haddleton, Macromolecules, 2003, 36, 5072–5075, DOI:10.1021/ma034447e.
- T. S. Rodrigues, F. Machado, P. M. Lalli, M. N. Eberlin and B. A. D. Neto, Catal. Commun., 2015, 63, 66–73, DOI:10.1016/j.catcom.2014.11.002.
- J. Kadokawa, Y. Iwasaki and H. Tagaya, Macromol. Rapid Commun., 2002, 23, 757–760, DOI:10.1002/1521-3927(20020901)23:13<757::AID-MARC757>3.0.CO;2-0.
- H. Wang, F. Xu, W. L. Ding, S. L. Wang, S. J. Zeng, Z. C. Zhang, X. P. Zhang and S. J. Zhang, Chem. Eng. J., 2024, 485, 149715, DOI:10.1016/j.cej.2024.149715.
- W. J. Fang, F. Xu, Y. Q. Zhang, H. Wang, Z. C. Zhang, Z. F. Yang, W. W. Wang, H. Y. He and Y. Luo, Catal. Sci. Technol., 2022, 12, 1756–1765, 10.1039/D1CY01824H.
- K. J. Wang, K. Amin, Z. S. An, Z. X. Cai, H. Chen, H. Z. Chen, Y. P. Dong, X. Feng, W. Q. Fu, J. B. Gu, Y. C. Han, D. D. Hu, R. R. Hu, D. Huang, F. Huang, F. H. Huang, Y. Z. Huang, J. Jin, X. Jin, Q. Q. Li, T. F. Li, Z. Li, Z. B. Li, J. G. Liu, J. Liu, S. Y. Liu, H. S. Peng, A. J. Qin, X. Qing, Y. Q. Shen, J. B. Shi, X. M. Sun, B. Tong, B. Wang, H. Wang, L. X. Wang, S. Wang, Z. X. Wei, T. Xie, C. Y. Xu, H. P. Xu, Z. K. Xu, B. Yang, Y. L. Yu, X. Zeng, X. W. Zhan, G. Z. Zhang, J. Zhang, M. Q. Zhang, X. Z. Zhang, X. Zhang, Y. Zhang, Y. Y. Zhang, C. S. Zhao, W. F. Zhao, Y. F. Zhou, Z. X. Zhou, J. T. Zhu, X. Y. Zhu and B. Z. Tang, Mater. Chem. Front., 2020, 4, 1803–1915, 10.1039/d0qm00025f.
- V. Strehmel, Chem. Ing. Tech., 2011, 83, 1443–1453, DOI:10.1002/cite.201100058.
- N. Winterton, J. Mater. Chem., 2006, 16, 4281–4293, 10.1039/B610143G.
- Y. F. Yuan, J. M. Zhang, B. Q. Zhang, J. J. Liu, Y. Zhou, M. X. Du, L. X. Han, K. J. Xu, X. Qiao and C. Y. Liu, Phys. Chem. Chem. Phys., 2021, 23, 21893–21900, 10.1039/D1CP03193G.
- S. Y. Zhang, Q. Zhuang, M. Zhang, H. Wang, Z. M. Gao, J. K. Sun and J. Y. Yuan, Chem. Soc. Rev., 2020, 49, 1726–1755, 10.1039/C8CS00938D.
- A. S. Shaplov, R. Marcilla and D. Mecerreyes, Electrochim. Acta, 2015, 175, 18–34, DOI:10.1016/j.electacta.2015.03.038.
- W. Y. Zhang, Z. Kochovski, Y. Lu, B. V. K. J. Schmidt, M. Antonietti and J. Y. Yuan, ACS Nano, 2016, 10, 7731–7737, DOI:10.1021/acsnano.6b03135.
- P. C. Marr and A. C. Marr, Green Chem., 2015, 18, 105–128, 10.1039/C5GC02277K.
- J. Dupont, B. C. Leal, P. Lozano, A. L. Monteiro, P. Migowski and J. D. Scholten, Chem. Rev., 2024, 124, 5227–5420, DOI:10.1021/acs.chemrev.3c00379.
- (a) R. van Reis and A. Zydney, Curr. Opin. Biotechnol., 2001, 12, 208–211, DOI:10.1016/S0958-1669(00)00201-9; (b) X. Q. Cheng, Z. X. Wang, X. Jiang, T. X. Li, C. H. Lau, Z. H. Guo, J. Ma and L. Shao, Prog. Mater. Sci., 2018, 92, 258–283, DOI:10.1016/j.pmatsci.2017.10.006.
- X. Zhang, S. Xu, J. K. Zhou, W. F. Zhao, S. D. Sun and C. S. Zhao, ACS Appl. Mater. Interfaces, 2017, 9, 32237–32247, DOI:10.1021/acsami.7b08740.
- E. Rynkowska, K. Fatyeyeva, J. Kujawa, K. Dzieszkowski, A. Wolan and W. Kujawski, Polymers, 2018, 10, 86, DOI:10.3390/polym10010086.
- X. R. Yan, S. Anguille, M. Bendahan and P. Moulin, Sep. Purif. Technol., 2019, 222, 230–253, DOI:10.1016/j.seppur.2019.03.103.
- M. Jebur, A. Sengupta, Y. H. Chiao, M. Kamaz, X. H. Qian and R. Wickramasinghe, J. Membr. Sci., 2018, 556, 1–11, DOI:10.1016/j.memsci.2018.03.064.
- H. Z. Chen, P. Li and T. S. Chung, Int. J. Hydrogen Energy, 2012, 37, 11796–11804, DOI:10.1016/j.ijhydene.2012.05.111.
- W. Bi, M. Tian and K. H. Row, J. Sep. Sci., 2010, 33, 1739–1745, DOI:10.1002/jssc.200900835.
- M. Tian, H. Yan and K. H. Row, Anal. Lett., 2010, 43, 110–118, DOI:10.1080/00032710903276554.
- M. L. Chen, Y. N. Zhao, D. W. Zhang, Y. Tian and J. H. Wang, J. Anal. At. Spectrom., 2010, 25, 1688–1694, 10.1039/C0JA00026D.
- P. Ishtaweera, C. L. Ray, W. Filley, G. Cobb and G. A. Baker, ACS Appl. Eng. Mater., 2024, 2, 1460–1466, DOI:10.1021/acsaenm.4c00159.
- M. L. Qi and D. W. Armstrong, Anal. Bioanal. Chem., 2007, 388, 889–899, DOI:10.1007/s00216-007-1290-3.
- M. R. Hossain, S. Singh, G. D. Sharma, S. A. Apostu and P. Bansal, Energy Policy, 2023, 174, 113469, DOI:10.1016/j.enpol.2023.113469.
- J. N. Wang and W. Azam, Geosci. Front., 2024, 15, 101757, DOI:10.1016/j.gsf.2023.101757.
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210–1211, DOI:10.1126/science.1249625.
- P. Jezowski, O. Crosnier, E. Deunf, P. Poizot, F. Beguin and T. Brousse, Nat. Mater., 2018, 17, 167–173, DOI:10.1038/nmat5029.
- S. S. Pan, M. Yao, J. H. Zhang, B. S. Li, C. X. Xing, X. L. Song, P. P. Su and H. T. Zhang, Front. Chem., 2020, 8, 261, DOI:10.3389/fchem.2020.00261.
- S. Pan, M. Yao, J. Zhang, B. Li, C. Xing, X. Song, P. Su and H. Zhang, Front. Chem., 2020, 8, 261, DOI:10.3389/fchem.2020.00261.
- L. X. Yuan, J. K. Feng, X. P. Ai, Y. L. Cao, S. L. Chen and H. X. Yang, Electrochem. Commun., 2006, 8, 610–614, DOI:10.1016/j.elecom.2006.02.007.
- J. H. Zhang, H. T. Zhang, Y. Q. Zhang, J. W. Zhang, H. Y. He, X. X. Zhang, J. J. Shim and S. J. Zhang, Electrochim. Acta, 2019, 313, 532–543, DOI:10.1016/j.electacta.2019.04.160.
- I. Osada, H. de Vries, B. Scrosati and S. Passerini, Angew. Chem., Int. Ed., 2016, 55, 500–513, DOI:10.1002/anie.201504971.
- M. Watanabe, M. L. Thomas, S. G. Zhang, K. Ueno, T. Yasuda and K. Dokko, Chem. Rev., 2017, 117, 7190–7239, DOI:10.1021/acs.chemrev.6b00504.
- (a) C. A. Angell, C. Liu and E. Sanchez, Nature, 1993, 362, 137–139, DOI:10.1038/362137a0; (b) M. Watanabe, S. Yamada, K. Sanui and N. Ogata, J. Chem. Soc., Chem. Commun., 1993, 11, 929–931, 10.1039/C39930000929.
- (a) J. Fuller, A. C. Breda and R. T. Carlin, J. Electroanal. Chem., 1998, 459, 29–34, DOI:10.1016/S0022-0728(98)00285-X; (b) J. H. Shin, W. A. Henderson and S. Passerini, Electrochem. Solid-State Lett., 2005, 8, A125–A127, DOI:10.1149/1.1850387; (c) M. A. Susan, T. Kaneko, A. Noda and M. Watanabe, J. Am. Chem. Soc., 2005, 127, 4976–4983, DOI:10.1021/ja045155b.
- A. L. Pont, R. Marcilla, I. De Meatza, H. Grande and D. Mecerreyes, J. Power Sources, 2009, 188, 558–563, DOI:10.1016/j.jpowsour.2008.11.115.
- M. Egashira, H. Todo, N. Yoshimoto and M. Morita, J. Power Sources, 2008, 178, 729–735, DOI:10.1016/j.jpowsour.2007.10.063.
- L. Balo, Shalu, H. Gupta, V. K. Singh and R. K. Singh, Electrochim. Acta, 2017, 230, 123–131, DOI:10.1016/j.electacta.2017.01.177.
- H. Li, Z. Q. Feng, K. Zhao, Z. H. Wang, J. H. Liu, J. Liu and H. Z. Song, Nanoscale, 2019, 11, 3689–3700, 10.1039/c8nr09030k.
- M. Yao, A. Liu, C. X. Xing, B. S. Li, S. S. Pan, J. H. Zhang, P. P. Su and H. T. Zhang, Chem. Eng. J., 2020, 394, 124883, DOI:10.1016/j.cej.2020.124883.
- D. O. Ponkratov, E. I. Lozinskaya, P. S. Vlasov, P. H. Aubert, C. Plesse, F. Vidal, Y. S. Vygodskii and A. S. Shaplov, Electrochim. Acta, 2018, 281, 777–788, DOI:10.1016/j.electacta.2018.05.191.
- Y. Hu, J. Pan, Q. Li, Y. Y. Ren, H. J. Qi, J. N. Guo, Z. Sun and F. Yan, ACS Sustainable Chem. Eng., 2020, 8, 11396–11403, DOI:10.1021/acssuschemeng.0c03754.
- M. Muthukumar, N. Rengarajan, B. Velliyangiri, M. A. Omprakas, C. B. Rohit and U. K. Raja, Mater. Today: Proc., 2021, 45, 1181–1187, DOI:10.1016/j.matpr.2020.03.679.
- R. Haider, Y. C. Wen, Z. F. Ma, D. P. Wilkinson, L. Zhang, X. X. Yuan, S. Q. Song and J. J. Zhang, Chem. Soc. Rev., 2021, 50, 1138–1187, 10.1039/D0CS00296H.
- G. Q. Li, W. Kujawski and E. Rynkowska, Rev. Chem. Eng., 2022, 38, 327–346, DOI:10.1515/revce-2019-0079.
- H. X. Zhang, J. Y. Liang, B. W. Xia, Y. Li and S. Du, Front. Chem. Sci. Eng., 2019, 13, 695–701, DOI:10.1007/s11705-019-1838-8.
- N. A. H. Rosli, K. S. Loh, W. Y. Wong, R. M. Yunus, T. K. Lee, A. Ahmad and S. T. Chong, Int. J. Mol. Sci., 2020, 21, 632, DOI:10.3390/ijms21020632.
- M. Ebrahimi, W. Kujawski, K. Fatyeyeva and J. Kujawa, Int. J. Mol. Sci., 2021, 22, 5430, DOI:10.3390/ijms22115430.
- A. Alashkar, A. Al-Othman, M. Tawalbeh and M. Qasim, Membranes, 2022, 12, 178, DOI:10.3390/membranes12020178.
- (a) ASTM International, ASTM D2863-19 Standard Test Method for Measuring the Minimum Oxygen Concentration to Support Candle-Like Combustion of Plastics (Oxygen Index), ASTM International, West Conshohocken, PA, 2019. DOI:10.1520/D2863-19; (b) International Organization for Standardization, ISO 4589-2:2017 Plastics - Determination of Burning Behaviour by Oxygen Index, ISO, Geneva, 2017. ISO 4589-2:2017 Search PubMed.
- Underwriters Laboratories, UL 94 Standard for Tests for Flammability of Plastic Materials for Parts in Devices and Appliances, Underwriters Laboratories, Northbrook, IL, 2013. DOI:10.1002/fam.937.
- (a) H. H. Zhou, S. Tan, C. H. Wang and Y. Wu, Polym. Degrad. Stab., 2022, 195, 109789, DOI:10.1016/j.polymdegradstab.2021.109789; (b) T. Boinowitz, R. Borgogelli and W. Gower, J. Cell. Plast., 2004, 40, 299–313, DOI:10.1177/0021955X04045183.
- Y. J. Chen, Y. F. Luo, X. H. Guo, L. J. Chen and D. M. Jia, Materials, 2020, 13, 3095, DOI:10.3390/ma13143095.
- C. M. Jiao, Y. L. Zhang, S. X. Li and X. L. Chen, J. Therm. Anal. Calorim., 2021, 145, 173–184, DOI:10.1007/s10973-020-09671-2.
- X. L. Chen, C. Y. Ma and C. M. Jiao, RSC Adv., 2016, 6, 67409–67417, 10.1039/C6RA14094G.
- T. T. Luo, C. M. Jiao, X. L. Chen and H. Z. Jiang, J. Therm. Anal. Calorim., 2022, 147, 4141–4150, DOI:10.1007/s10973-021-10840-0.
- W. W. Zhang, H. J. Wu, W. H. Meng, M. J. Zhang, W. Y. Xie, G. Bian and H. Q. Qu, Mater. Res. Express, 2019, 6, 075303, DOI:10.1088/2053-1591/ab1217.
- S. Latifi, A. Boukhriss, S. Saoiabi, A. Saoiabi and S. Gmouh, Polym. Bull., 2023, 80, 9253–9274, DOI:10.1007/s00289-022-04513-7.
- K. S. Egorova, E. G. Gordeev and V. P. Ananikov, Chem. Rev., 2017, 117, 7132–7189, DOI:10.1021/acs.chemrev.6b00562.
- D. M. Correia, L. C. Fernandes, M. M. Fernandes, B. Hermenegildo, R. M. Meira, C. Ribeiro, S. Ribeiro, J. Reguera and S. Lanceros-Mendez, Nanomaterials, 2021, 11, 2401, DOI:10.3390/nano11092401.
- X. D. Hou, S. J. Liu, P. P. Zhou, J. Li, X. Liu, L. C. Wang and Y. Guo, J. Chromatogr. A, 2016, 1456, 10–18, DOI:10.1016/j.chroma.2016.05.096.
- L. W. Qian, M. X. Yang, H. N. Chen, Y. Xu, S. F. Zhang, Q. S. Zhou, B. He, Y. Bai and W. Q. Song, Carbohydr. Polym., 2019, 218, 154–162, DOI:10.1016/j.carbpol.2019.04.081.
- T. A. Ferreira, J. F. Flores-Aguilar, E. M. Santos, J. A. Rodriguez and I. S. Ibarra, Molecules, 2019, 24, 430, DOI:10.3390/molecules24030430.
- Z. Y. Guo, X. Hai, Y. T. Wang, Y. Shu, X. W. Chen and J. H. Wang, Biomacromolecules, 2018, 19, 53–61, DOI:10.1021/acs.biomac.7b01231.
- N. Nikfarjam, M. Ghomi, T. Agarwal, M. Hassanpour, E. Sharifi, D. Khorsandi, M. A. Khan, F. Rossi, A. Rossetti, E. N. Zare, N. Rabiee, D. Afshar, M. Vosough, T. K. Maiti, V. Mattoli, E. Lichtfouse, F. R. Tay and P. Makvandi, Adv. Funct. Mater., 2021, 31, 2104148, DOI:10.1002/adfm.202104148.
- Y. X. Wu, J. H. Wang, L. Li, X. Fei, L. Q. Xu, Y. Wang, J. Tian and Y. Li, J. Colloid Interface Sci., 2021, 584, 484–494, DOI:10.1016/j.jcis.2020.09.105.
- P. Liu, K. Jin, W. L. Wong, Y. Y. Wang, T. Liang, M. He, H. Y. Li, C. F. Lu, X. Tang, Y. G. Zong and C. Y. Li, Chem. Eng. J., 2021, 415, 129025, DOI:10.1016/j.cej.2021.129025.
- (a) W. J. Shen, X. B. Chen, J. B. Luan, D. N. Wang, L. Yu and J. D. Ding, ACS Appl. Mater. Interfaces, 2017, 9, 40031–40046, DOI:10.1021/acsami.7b11998; (b) Y. H. Zheng, Y. L. Cheng, J. J. Chen, J. X. Ding, M. Q. Li, C. Li, J. C. Wang and X. S. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 3487–3496, DOI:10.1021/acsami.6b15245.
- M. Kuddushi, D. Ray, V. Aswal, C. Hoskins and N. Malek, ACS Appl. Bio Mater., 2020, 3, 4883–4894, DOI:10.1021/acsabm.0c00393.
- X. T. Jia, Y. Yang, C. Y. Wang, C. Zhao, R. Vijayaraghavan, D. R. MacFarlane, M. Forsyth and G. G. Wallace, ACS Appl. Mater. Interfaces, 2014, 6, 21110–21117, DOI:10.1021/am505985z.
- K. N. Ganesh, D. Q. Zhang, S. J. Miller, K. Rossen, P. J. Chirik, M. C. Kozlowski, J. B. Zimmerman, B. W. Brooks, P. E. Savage, D. T. Allen and A. M. Voutchkova-Kostal, Environ. Sci. Technol., 2021, 55, 8459–8463, DOI:10.1021/acs.est.1c03762.
- X. Chen, Y. D. Wang and L. Zhang, ChemSusChem, 2021, 14, 4137–4151, DOI:10.1002/cssc.202100868.
- H. Chen, K. Wan, Y. Y. Zhang and Y. Q. Wang, ChemSusChem, 2021, 14, 4123–4136, DOI:10.1002/cssc.202100652.
- (a) E. Barnard, J. J. R. Arias and W. Thielemans, Green Chem., 2021, 23, 3765–3789, 10.1039/d1gc00887k; (b) Z. Aayanifard, A. Khan, M. Naveed, J. Schager and M. Rabnawaz, Polymer, 2023, 265, 125585, DOI:10.1016/j.polymer.2022.125585; (c) N. Yan, Science, 2022, 378, 132–133, DOI:10.1126/science.ade5658.
- A. Chaudhary and R. Srivastava, ChemistrySelect, 2023, 8, e202301709, DOI:10.1002/slct.202301709.
- E. M. Krall, T. W. Klein, R. J. Andersen, A. J. Nett, R. W. Glasgow, D. S. Reader, B. C. Dauphinais, S. P. Mc Ilrath, A. A. Fischer, M. J. Carney, D. J. Hudson and N. J. Robertson, Chem. Commun., 2014, 50, 4884–4887, 10.1039/c4cc00541d.
- C. Y. Wang and O. El-Sepelgy, Curr. Opin. Green Sustainable Chem., 2021, 32, 100547, DOI:10.1016/j.cogsc.2021.100547.
- I. Vollmer, M. J. F. Jenks, M. C. P. Roelands, R. J. White, T. van Harmelen, P. de Wild, G. P. van der Laan, F. Meirer, J. T. F. Keurentjes and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2020, 59, 15402–15423, DOI:10.1002/anie.201915651.
- R. A. Sheldon and D. Brady, ChemSusChem, 2022, 15, e202102628, DOI:10.1002/cssc.202102628.
- M. M. C. H. van Schie, J. D. Sporing, M. Bocola, P. D. de Maria and D. Rother, Green Chem., 2021, 23, 3191–3206, 10.1039/D1GC00561H.
- R. Koshti, L. Mehta and N. Samarth, J. Polym. Environ., 2018, 26, 3520–3529, DOI:10.1007/s10924-018-1214-7.
- C. G. Yoo, Y. Q. Pu and A. J. Ragauskas, Curr. Opin. Green Sustainable Chem., 2017, 5, 5–11, DOI:10.1016/j.cogsc.2017.03.003.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975, DOI:10.1021/ja025790m.
- M. Isik, H. Sardon and D. Mecerreyes, Int. J. Mol. Sci., 2014, 15, 11922–11940, DOI:10.3390/ijms150711922.
- J. M. Zhang, J. Wu, J. Yu, X. Y. Zhang, J. S. He and J. Zhang, Mater. Chem. Front., 2017, 1, 1273–1290, 10.1039/C6QM00348F.
- A. Kamimura, T. Okagawa, N. Oyama, T. Otsuka and M. Yoshimoto, Green Chem., 2012, 14, 2816–2820, 10.1039/c2gc35811e.
- B. Azimi, H. Maleki, V. Gigante, R. Bagherzadeh, A. Mezzetta, M. Milazzo, L. Guazzelli, P. Cinelli, A. Lazzeri and S. Danti, Cellulose, 2022, 29, 3079–3129, DOI:10.1007/s10570-022-04473-1.
- (a) https://ioncell.fi/ ; (b) H. Sixta, M. Hummel, K. Le Boulch, I. A. Kilpelainen, A. W. T. King, J. K. J. Helminen and S. Hellsten, PCT/FI2018/050070, WO2018/138416A1, 2018 Search PubMed.
- (a) A. Michud, M. Tanttu, S. Asaadi, Y. B. Ma, E. Netti, P. Kaariainen, A. Persson, A. Berntsson, M. Hummel and H. Sixta, Text. Res. J., 2016, 86, 543–552, DOI:10.1177/0040517515591774; (b) Y. B. Ma, M. Hummel, I. Kontro and H. Sixta, Green Chem., 2018, 20, 160–169, 10.1039/C7GC02896B; (c) S. Asaadi, M. Hummel, S. Hellsten, T. Harkasalmi, Y. B. Ma, A. Michud and H. Sixta, ChemSusChem, 2016, 9, 3250–3258, DOI:10.1002/cssc.201600680.
- Y. Q. Pu, N. Jiang and A. J. Ragauskas, J. Wood Chem. Technol., 2007, 27, 23–33, DOI:10.1080/02773810701282330.
- W. E. S. Hart, J. B. Harper and L. Aldous, Green Chem., 2015, 17, 214–218, 10.1039/C4GC01888E.
- M. Hashmi, Q. N. Sun, J. M. Tao, T. Wells, A. A. Shah, N. Labbe and A. J. Ragauskas, Bioresour. Technol., 2017, 224, 714–720, DOI:10.1016/j.biortech.2016.10.089.
- P. Y. S. Nakasu, T. C. Pin, J. P. Hallett, S. C. Rabelo and A. C. Costa, Renewable Energy, 2021, 172, 816–828, DOI:10.1016/j.renene.2021.03.004.
- A. Samir, F. H. Ashour, A. A. Abdel Hakim and M. Bassyouni, npj Mater. Degrad., 2022, 6, 68, DOI:10.1038/s41529-022-00277-7.
- I. Younes and M. Rinaudo, Mar. Drugs, 2015, 13, 1133–1174, DOI:10.3390/md13031133.
- S. S. Silva, J. F. Mano and R. L. Reis, Green Chem., 2017, 19, 1208–2020, 10.1039/C6GC02827F.
- L. X. Mu, L. Q. Wu, S. Q. Wu, Q. F. Ye and Z. B. Zhong, Carbohydr. Polym., 2024, 343, 122233, DOI:10.1016/j.carbpol.2024.122233.
- E. Kohr, in Chitin: Fulfilling a Biomaterials Promise, Elsevier, Amsterdam, 2001, ch. 5, 63–72. DOI:10.1016/B978-008044018-7/50005-1.
- R. Sulthan, A. Reghunadhan and S. Sambhudevan, J. Mol. Liq., 2023, 380, 121794, DOI:10.1016/j.molliq.2023.121794.
- H. B. Xie, S. B. Zhang and S. H. Li, Green Chem., 2006, 8, 630–633, 10.1039/B517297G.
- G. Cheng, P. Varanasi, R. Arora, V. Stavila, B. A. Simmons, M. I. S. Kent and S. Singh, J. Phys. Chem. B, 2012, 116, 10049–10054, DOI:10.1021/jp304538v.
- J. L. Shamshina, P. S. Barber, G. Gurau, C. S. Griggs and R. D. Rogers, ACS Sustainable Chem. Eng., 2016, 4, 6072–6081, DOI:10.1021/acssuschemeng.6b01434.
- C. Campalani, I. Bertuol, C. Bersani, R. Calmanti, S. Filonenko, D. Rodriguez-Padron, M. Selva and A. Perosa, Carbohydr. Polym., 2024, 345, 122565, DOI:10.1016/j.carbpol.2024.122565.
- (a) A. Kamimura and S. Yamamoto, Org. Lett., 2007, 9, 2533, DOI:10.1021/ol070886c; (b) A. Kamimura and S. Yamamoto, Polym. Adv. Technol., 2008, 19, 1391, DOI:10.1002/pat.1199; (c) S. Yamamoto and A. Kamimura, Chem. Lett., 2009, 38, 1016–1017, DOI:10.1246/cl.2009.1016.
- A. Kamimura, Y. Shiramatsu and T. Kawamoto, Green Energy Environ., 2019, 4, 16–170, DOI:10.1016/j.gee.2019.01.002.
- B. Jiang, X. W. Tantai, L. H. Zhang, L. Hao, Y. L. Sun, L. Deng and Z. Q. Shi, RSC Adv., 2015, 5, 50747, 10.1039/C5RA05073A.
- X. Y. Song, X. J. Zhang, H. Wang, F. S. Liu, S. T. Yu and S. W. Liu, Polym. Degrad. Stab., 2013, 98, 2760–2764, DOI:10.1016/j.polymdegradstab.2013.10.012.
- X. Y. Song, H. Wang, X. Q. Yang, F. S. Liu, S. T. Yu and S. W. Liu, Polym. Degrad. Stab., 2014, 110, 65–70, DOI:10.1016/j.polymdegradstab.2014.08.020.
- H. Q. Liu, R. Y. Zhao, X. Y. Song, F. S. Liu, S. T. Yu, S. W. Liu and X. P. Ge, Catal. Lett., 2017, 147, 2298–2305, DOI:10.1007/s10562-017-2138-x.
- X. Y. Song, Z. Q. Bian, Y. H. Hui, H. Wang, F. S. Liu and S. T. Yu, Polym. Degrad. Stab., 2019, 168, 108937, DOI:10.1016/j.polymdegradstab.2019.108937.
- F. S. Liu, J. Guo, P. H. Zhao, Y. Q. Gu, J. Gao and M. S. Liu, Polym. Degrad. Stab., 2019, 167, 124–129, DOI:10.1016/j.polymdegradstab.2019.06.028.
- F. S. Liu, L. Li, S. T. Yu, Z. G. Lv and X. P. Ge, J. Hazard. Mater., 2011, 189, 249, DOI:10.1016/j.jhazmat.2011.02.032.
- M. S. Liu, J. Guo, Y. Q. Gu, J. Gao, F. S. Liu and S. T. Yu, ACS Sustainable Chem. Eng., 2018, 6, 13114–13121, DOI:10.1021/acssuschemeng.8b02650.
- F. S. Liu, J. Guo, P. H. Zhao, M. K. Jia, M. S. Liu and J. Gao, Polym. Degrad. Stab., 2019, 169, 108996, DOI:10.1016/j.polymdegradstab.2019.108996.
- A. B. Raheem, Z. Z. Noor, A. Hassan, M. K. Abd Hamid, S. A. Samsudin and A. H. Sabeen, J. Cleaner Prod., 2019, 225, 1052–1064, DOI:10.1016/j.jclepro.2019.04.019.
- N. George and T. Kurian, Ind. Eng. Chem. Res., 2014, 53, 14185, DOI:10.1021/ie501995m.
- H. Wang, Z. X. Li, Y. Q. Liu, X. P. Zhang and S. J. Zhang, Green Chem., 2009, 11, 1568–1575, 10.1039/b906831g.
- T. L. Wang, X. Gong, C. C. Shen, G. R. Yu and X. C. Chen, Polym. Degrad. Stab., 2021, 190, 109601, DOI:10.1016/j.polymdegradstab.2021.109601.
- T. L. Wang, C. C. Shen, G. R. Yu and X. C. Chen, Polym. Degrad. Stab., 2021, 194, 109751, DOI:10.1016/j.polymdegradstab.2021.109751.
- S. Marullo, C. Rizzo, N. T. Dintcheva and F. D'Anna, ACS Sustainable Chem. Eng., 2021, 9, 15157–15165, DOI:10.1021/acssuschemeng.1c04060.
- S. J. Chen, W. H. Shi, H. D. Cheng, H. Zhang, Z. W. Zhang and C. N. Fu, J. Therm. Anal. Calorim., 2021, 143, 3489–3497, DOI:10.1007/s10973-020-10331-8.
- S. Najafi-Shoa, M. Barikani, M. Ehsani and M. Ghaffari, Polym. Degrad. Stab., 2021, 192, 109691, DOI:10.1016/j.polymdegradstab.2021.109691.
- F. S. Liu, X. Cui, S. T. Yu, Z. Li and X. P. Ge, J. Appl. Polym. Sci., 2009, 114, 3561–3565, DOI:10.1002/app.30981.
- H. B. Hu, Y. Wu and Z. M. Zhu, J. Polym. Environ., 2018, 26, 375–382, DOI:10.1007/s10924-017-0952-2.
- Z. Q. Jiang, D. X. Yan, J. Y. Xin, F. Li, M. Q. Guo, Q. Zhou, J. L. Xu, Y. F. Hu and X. M. Lu, Polym. Degrad. Stab., 2022, 199, 109905, DOI:10.1016/j.polymdegradstab.2022.109905.
- V. S. Palekar, R. V. Shah and S. R. Shukla, J. Appl. Polym. Sci., 2012, 126, 1174, DOI:10.1002/app.36878.
- R. M. Musale and S. R. Shukla, Int. J. Plast. Technol., 2016, 20, 106–120, DOI:10.1007/s12588-016-9134-7.
- F. T. Wu, Y. P. Wang, Y. F. Zhao, S. J. Zeng, Z. P. Wang, M. H. Tang, W. Zeng, Y. Wang, X. Q. Chang, J. F. Xiang, Z. B. Xie, B. X. Han and Z. M. Liu, Nat. Commun., 2024, 15, 712, DOI:10.1038/s41467-024-44892-1.
- Y. Z. Zhao, H. Zhang, F. T. Wu, R. X. Li, M. H. Tang, Y. S. Wang, W. Zeng, B. X. Han and Z. M. Liu, Chem. Sci., 2024, 15, 10892–10899, 10.1039/D4SC02533D.
- (a) J. Chow, P. Perez-Garcia, R. Dierkes and W. R. Streit, Microb. Biotechnol., 2023, 16, 195–217, DOI:10.1111/1751-7915.14135; (b) EUROPUR (European Association of Flexible Polyurethane Foam Blocks Manufacturers), https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://europur.org/wp-content/uploads/2021/10/EoL-Brochure-2021-EUROPUR.pdf&ved=2ahUKEwiqj9mUnp-HAxXbQ_EDHWLhAuAQFnoECBEQAQ&usg=AOvVaw1hAzs0br4N0iC570WQwUCm, (accessed 11 July 2024).
- C. Mao, Z. Wang, P. J. Ji and J. P. Cheng, J. Org. Chem., 2015, 80, 8384–8389, DOI:10.1021/acs.joc.5b01200.
- H. Zhang, X. J. Cui, H. L. Wang, Y. Q. Wang, Y. H. Zhao, H. Ma, L. Chai, Y. X. Wang, X. L. Hou and T. S. Deng, Polym. Degrad. Stab., 2020, 181, 109342, DOI:10.1016/j.polymdegradstab.2020.109342.
| This journal is © The Royal Society of Chemistry 2025 |