Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photoresponsive polymers for carbodiimide-fueled transient hydrogels†
Ibrahim O.
Raji
,
Torin C.
Wilcox
,
C. Scott
Hartley
 and
Dominik
Konkolewicz
and
Dominik
Konkolewicz
 *
*
Department of Chemistry and Biochemistry, Miami University, 651 E High St, Oxford, OH, USA. E-mail: d.konkolewicz@miamiOH.edu
First published on 9th January 2025
Abstract
Light- and carbodiimide-responsive hydrogels were synthesized. An “AND” gate was developed using ortho-nitrobenzyl (ONB) protected carboxylic acids in the polymer backbone. Crosslinking was only realized in the presence of both UV stimulus to photocleave the ONB group and carbodiimide fuels to induce anhydride bonds. In the presence of water, the anhydride bonds eventually hydrolyze to carboxylic acids and the system returns to the solution state. The mechanical properties of the out-of-equilibrium hydrogels were investigated using oscillatory rheology to examine the effects of deprotection efficiency, carbodiimide concentration and chain architecture on the hydrogels’ moduli and decrosslinking time. Higher moduli and longer decrosslinking times were found with increased carbodiimide concentration and deprotection efficiency. These discoveries unveil new possibilities for photoresponsive chemically fueled soft materials.
Introduction
Dissipative self-assembly, driven by continuous energy input fueled by molecules like ATP, is predominant in nature.1,2 Consequently, chemically fueled, out-of-equilibrium systems have received significant attention in recent years because of their potential in mimicking natural biochemical systems.3,4 The spatiotemporal regulation of self-assembly in biological systems is enabled by continuous energy input, allowing for intricate processes like intracellular transport and cell division.5–8 Building on the work initiated by van Esch and Eelkema, significant efforts over the past decade have focused on the development of chemically fueled systems beyond biological systems.9–11 Recent studies have explored chemically fueled assembly in, for example, dynamic polymer networks,12 colloidal clusters,13 and responsive supramolecular structures.14–16 This marks a significant stride toward practical applications in not only drug delivery17 but also in customizable biosensing18 and wearable devices.19Of special interest are systems with components that respond to external stimuli.20–23 Among the different external stimuli used to influence polymer system properties, light uniquely provides exact, real-time control over spatiotemporal distribution.24 One common use of light is to activate or demask photoresponsive protecting groups (PPGs).25 PPGs use a photochemically sensitive moiety to protect a functional group, enabling a light-triggered approach to unveiling functional groups within soluble organic polymers, biohybrids, and bulk materials.25,26 Polymers with PPGs are useful in therapeutic delivery, offering precise control over the activation of prodrugs or the decomposition of bioactive nanoparticles.27–29 Among PPGs, the 2-nitrobenzyl moiety has received the most attention due to its potential to induce light-responsive properties through either bioconjugation,30 crosslinking,31–34etc., to develop photo-responsive polymers.25,35
Carbodiimide hydration coupled with the coupling of carboxylic acids into anhydrides has emerged as one of the most versatile chemically fueled systems.36 In the presence of water, the anhydrides undergo hydrolysis over time and revert to the carboxylic acid resting state. The use of carbodiimide-induced chemistry has been applied to transiently crosslinked networks undergoing a sol–gel–sol transition.37,38 The mechanical properties of anhydride-crosslinked hydrogels can be controlled using the fuel concentration, polymer composition, temperature and chain length.12,37,38 In addition, the modulus of a permanently crosslinked network with pendant carboxylic acid groups can be tuned by using EDC to form additional transient crosslinks, and by careful choice of transient network architectures, such as interpenetrated networks, which enables materials with remarkable fracture energies, resilience to compression, and improved deformation capabilities.39 Wang and colleagues have explored the chemistry of dissipative out-of-equilibrium systems using the temporary crosslinked network to develop intriguing functional writable, self-erasable and self-healing hydrogels with intricate medical applications.19 However, in the above examples the carboxylic acid is always exposed, making the materials immediately susceptible to reaction with the carbodiimides. Previous reports3,37,40,41 established the concept of transiently crosslinked networks based on aqueous polymers with pendant carboxylic acids being treated with carbodiimides to give anhydride-cross-linked hydrogels. No research has been reported on photoresponsive transiently anhydride-crosslinked hydrogels with PPG despite the responsive nature of PPGs. PPGs would allow an additional level of control, since non equilibrium materials properties would be controlled by both the demasking of the PPG, and the carbodiimide.
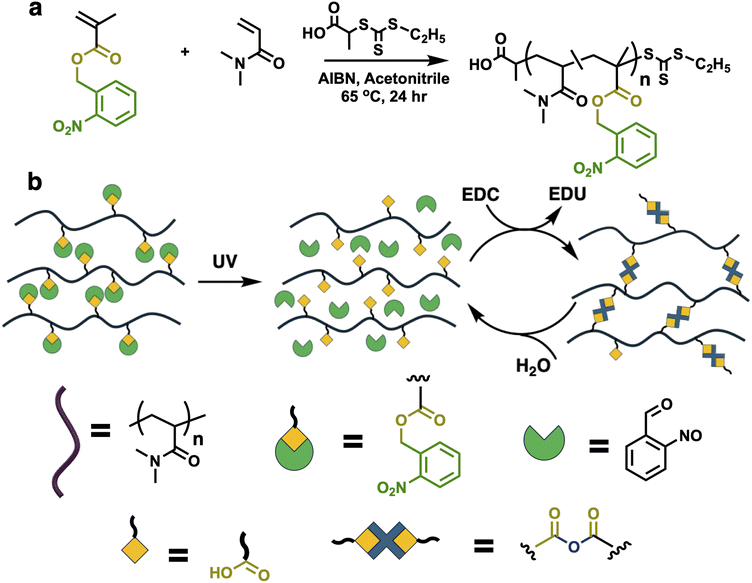
The present study expands this concept to attach a PPG to the carboxylic acid, which then requires UV deprotection before transient anhydride crosslinks can be formed upon addition of the carbodiimide 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC). The concept of photoprotection of the carboxylic acid, with its post-deprotection formation of carbodiimides in the presence of EDC, is shown in Scheme 1b. This work presents photosensitive carbodiimide-fueled transiently crosslinked polymer hydrogels bearing a UV-labile PPG. Upon deprotection, free carboxylic acids can be crosslinked using carbodiimide hydration resulting in transient anhydride-crosslinked hydrogels. The transient hydrogels’ mechanical properties are tied to the deprotection extent, fuel concentration and chain architecture. Therefore, anhydride crosslinks can only be formed when the photolabile protected group has been cleaved. This strategy of masked carboxylic groups with photosensitive moiety offers the possibility of photo-removal-on-demand chemically fueled hydrogels.
Results and discussion
Well-defined copolymers were synthesized using reversible addition–fragmentation chain transfer polymerization (RAFT), as shown in Scheme 1a. The copolymer included 2-nitrobenzylmethacrylate (ONBM) containing the photolabile-group-protected carboxylic acid and N,N-dimethylacrylamide as the core backbone forming monomer. The RAFT chain transfer agent was 2-(((ethylthio)-carbonothioyl)thio) propionic acid (PAETC) and the thermal initiator azobisisobutyronitrile (AIBN) were used for polymerization in acetonitrile as solvent. Using RAFT, the chain length and composition of the polymers were varied. The copolymers are denoted poly(ONBMx–DMAmy) where x and y represent the total number of repeating units of ONBM and DMAm respectively. The proposed polymers synthesized are poly(ONBM15–DMAm85), poly(ONBM15–DMAm135) and poly(ONBM30–DMAm170) yielding chain lengths of 100, 150 and 200 respectively. However, 1H NMR confirmed that in all cases the monomer conversion of the polymers was between 90–95% (Fig. S2†), resulting to chain length of 96 poly(ONBM15–DMAm81), 137 poly(ONBM15–DMAm122) and 185 poly(ONBM30–DMAm155), respectively. The chemical composition of the copolymers was carefully designed to enable solubility of the polymer in aqueous media irrespective of the polymer chain length. However, the quantity of the comonomer ONBM moiety used was necessary for solubility and to provide sufficient acid group required for anhydride crosslinks in the presence of EDC. Size exclusion chromatography (SEC) was used to determine the molecular weight dispersity of the polymers which was in the order of 1.25–1.35 (Fig. S3†).To test the photodeprotection, a polymer solution of poly(ONBM15–DMAM81) was prepared at 33 wt% polymer in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of water and N,N-dimethylformamide (DMF). The dissolved polymer was exposed to 360 nm UV irradiation with radiation intensity of 4.8 ± 0.5 mW cm−2 for 24 h. 1H NMR spectroscopy showed that the 2-nitrobenzyl moiety of the polymer was more than 95% cleaved (Fig S4d†). After deprotection the solution turned a red/brown color, attributed to the ONB byproduct.
1 mixture of water and N,N-dimethylformamide (DMF). The dissolved polymer was exposed to 360 nm UV irradiation with radiation intensity of 4.8 ± 0.5 mW cm−2 for 24 h. 1H NMR spectroscopy showed that the 2-nitrobenzyl moiety of the polymer was more than 95% cleaved (Fig S4d†). After deprotection the solution turned a red/brown color, attributed to the ONB byproduct.
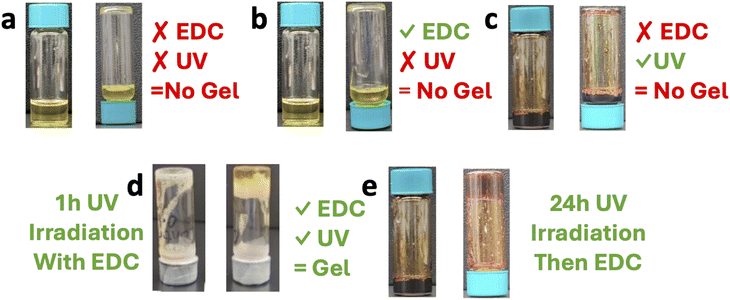
Three control experiments were conducted to validate the “AND” gate concept of the chemically and light responsive gelation. The first control involved protected polymer solution of poly(ONBM15–DMAM81) without EDC solution, Fig. 1a. As expected, no trace of gel was observed because there are no free carboxylic acids, nor any EDC to generate crosslinks. In Fig. 1b, the second control was the addition of EDC solution to protected polymer solution of poly(ONBM15–DMAM81). Predictably, there was no gelation observed because the polymer still has the PPG. The presence of the ONB group masks the potential site for EDC driven anhydride crosslinking. Lastly, the third control involved deprotected polymer solution of poly(ONBM15–DMAM81) in the absence of EDC solution, Fig. 1c. Unsurprisingly, no gel was formed, because in the absence of the carbodiimide, it was not possible to link the unmasked carboxylic acids to form anhydride.
In Fig. 1d, 1 M EDC solution was added to the protected polymer solution of poly(ONBM15–DMAM81), which was then exposed to UV 360 nm UV for 1 h leading to the formation of a soft gel. That is, after deprotection, the free carboxylic acids can be crosslinked with the carbodiimide. To create a stronger gel, full deprotection was obtained after 24 h of irradiation, followed by the addition of 1 M EDC solution to the deprotected polymer. 15 min after the addition of the EDC, a hydrogel was formed as depicted in the inverted vial test shown in Fig. 1e, consistent with the formation of an anhydride crosslinked hydrogel. In systems of Fig. 1d and e, the anhydrides eventually hydrolyzed leading to de-gelation and the system returned back to a polymer solution. Importantly, the anhydride-crosslinked network can be formed on repeated cycles with addition of EDC. This is consistent with previous works where transiently crosslinked polymer networks are developed, using EDC hydration to link carboxylic acids as anhydrides and the anhydrides hydrolyzing back to the polymer solution while forming repeated gels upon subsequent EDC addition.37,38 This indicates the responsiveness of the system and shows that the anhydride crosslinks can be achieved either deprotecting the polymer before adding EDC solution or deprotecting the polymer upon addition of EDC solution. Overall, the system functions as an AND gate, where both the UV and the EDC stimuli need to be present to induce the macroscopic crosslinking.18,36 It is important to note that the EDC fueled chemistry generates 1 EDU compound from every EDC consumed; however, prior work has shown that the EDU byproduct has minimal impact on the gelation.38
To investigate the mechanical behavior of the anhydride-crosslinked hydrogels, small amplitude oscillatory rheological time sweep experiments were conducted. This enables investigation of the mechanical properties’ evolution over time due to the non-equilibrium nature of the chemistry. Specifically, changes in the storage (G′) and loss (G′′) moduli, and therefore the de-gelation times of hydrogels, were examined under various crosslinking conditions. Two key parameters are the peak storage modulus (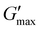 ) which is proportional to the crosslink density in the material,38,42 and the time needed to degel the network (tdegel) as evidenced by the crossover of G′ and G′′, giving G′ < G′′. The deprotected polymer solutions and EDC solutions were mixed at room temperature, and the mixture was injected onto the static Peltier plate of the rheometer. Since UV deprotection is critical to the AND gate and dual stimulus responsiveness of these materials, the effect of UV deprotection extent was explored on the anhydride formation and mechanical properties. Fig. 2 shows how the extent of UV deprotection of the masked acids is critically tied to the transient network properties.
) which is proportional to the crosslink density in the material,38,42 and the time needed to degel the network (tdegel) as evidenced by the crossover of G′ and G′′, giving G′ < G′′. The deprotected polymer solutions and EDC solutions were mixed at room temperature, and the mixture was injected onto the static Peltier plate of the rheometer. Since UV deprotection is critical to the AND gate and dual stimulus responsiveness of these materials, the effect of UV deprotection extent was explored on the anhydride formation and mechanical properties. Fig. 2 shows how the extent of UV deprotection of the masked acids is critically tied to the transient network properties.
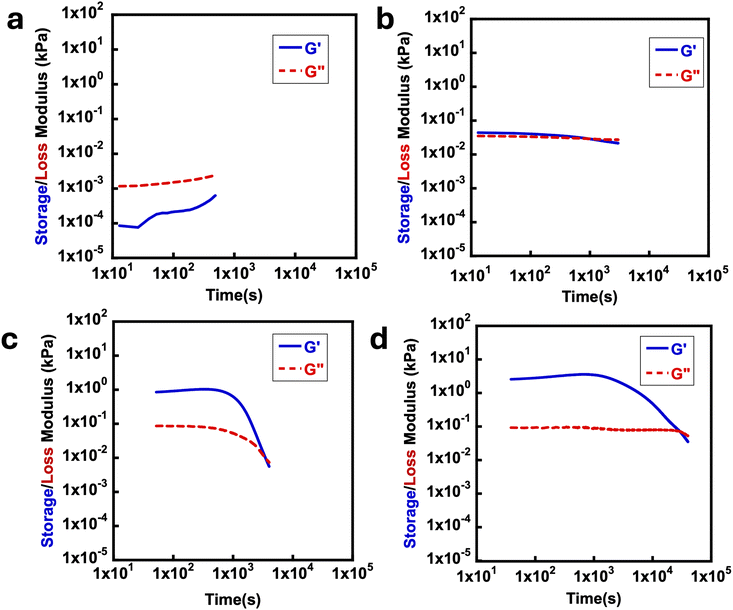
An aqueous polymer solution of poly(ONBM15–DMAM81) was prepared and exposed to UV light of 4.8 ± 0.5 mW cm−2 intensity at different times of 0 h, 8 h 16 h, and 24 h. At 0 h irradiation essentially all the ONB groups remained in place leading to no free carboxylic acid groups to form anhydrides upon EDC treatment. This gave very low moduli, which were not reliably measured on the rheometer. However, after 8 h UV irradiation, ∼15% deprotection of 2-nitrobenzyl was observed (Fig. S4b†) and the ∼15% deprotected polymer solution was reacted with 1 M EDC. As shown in Fig. 2a, the time sweep plot indicates that no gel was formed as the mixture remained a rheological liquid in which the G′′ is higher than G′ by about 1 order of magnitude. This could be attributed to insufficient free acids to form anhydrides due to ∼85% of the acids still being masked with the 2-nitrobenzyl group. Upon increasing the concentration of fuel from 1 M to 2 M EDC, a soft gel with G′ greater than G′′ was formed, 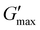 of about 0.05 kPa and tdegel of ∼15 min was formed in Fig. 2b. This shows that despite the low deprotection efficiency of 2-nitrobenzyl group, excess fuel can still potentially crosslink the available free acids to form anhydrides with a relatively short lifetime. Compared to 8 h irradiation time, 16 h of UV exposure showed about 33% deprotection efficiency (Fig. S4c†). This increased the formed crosslinks, with a higher
of about 0.05 kPa and tdegel of ∼15 min was formed in Fig. 2b. This shows that despite the low deprotection efficiency of 2-nitrobenzyl group, excess fuel can still potentially crosslink the available free acids to form anhydrides with a relatively short lifetime. Compared to 8 h irradiation time, 16 h of UV exposure showed about 33% deprotection efficiency (Fig. S4c†). This increased the formed crosslinks, with a higher 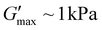 , with G′ significantly above G′′ until the tdegel of ∼50 min. This is attributed to the formation of more anhydride crosslinks as a result of more free acids in the system, unlike the conditions in Fig. 2b with lesser deprotection efficiency. Predictably, more than 95% of the ONB groups were successfully deprotected at 24 h UV irradiation (Fig. S4d†) which resulted in an even more stable gel compared to gels formed with less than 50% of deprotection efficiency. With
, with G′ significantly above G′′ until the tdegel of ∼50 min. This is attributed to the formation of more anhydride crosslinks as a result of more free acids in the system, unlike the conditions in Fig. 2b with lesser deprotection efficiency. Predictably, more than 95% of the ONB groups were successfully deprotected at 24 h UV irradiation (Fig. S4d†) which resulted in an even more stable gel compared to gels formed with less than 50% of deprotection efficiency. With 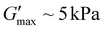 and tdegel ∼ 300 min, this is similar to previous gels formed through EDC hydration of carboxylic acids to form transiently crosslinked anhydrides.37,38
and tdegel ∼ 300 min, this is similar to previous gels formed through EDC hydration of carboxylic acids to form transiently crosslinked anhydrides.37,38
To test whether the fully deprotected polymers perform similarly to previously reported MAA-based systems, the mechanical properties of the fully deprotected polymers were investigated. In all cases the polymers were exposed to the UV source for 24 h. As seen in Fig. 3, time sweep experiments for each system showed the formation of rheological solids with G′ greater than G′′ which indicates the formation of gel materials due to the formation of anhydride bonds. Over time, the anhydride-based crosslinks hydrolyze, with all time sweep data in Fig. 3a–d showing a final crossover between G′ and G′′. The results in Fig. 3 are comparable with the EDC-fueled transiently crosslinked diacids leading to anhydride formation in previous studies.37,38 In Fig. 3a–d, the effect of EDC concentration is not only evident on 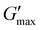 but also on the tdegel to occur in systems with poly(ONBM15–DMAM81) irradiated for 24 h under 360 nm UV. In Fig. 3a with 0.5 M of EDC fuel, there's a relatively lower modulus of
but also on the tdegel to occur in systems with poly(ONBM15–DMAM81) irradiated for 24 h under 360 nm UV. In Fig. 3a with 0.5 M of EDC fuel, there's a relatively lower modulus of 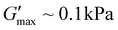 and shorter crossover time of tdegel ∼ 15 min compared to Fig. 3b–d with higher fuel concentration. Using a fuel concentration of 2.0 M gave
and shorter crossover time of tdegel ∼ 15 min compared to Fig. 3b–d with higher fuel concentration. Using a fuel concentration of 2.0 M gave 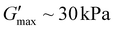 and a longer crossover time of tdegel ∼ 800 min. The relatively small increase in G′ after the crossover could be due to some solvent evaporation with the longer experimental timescale. With intermediate concentrations of EDC, the peak storage modulus and crossover time were intermediate between the extremes that occur at 0.5 M and 2.0 M. These findings showed that EDC fuels added to aqueous polymer solution with free diacids result in transiently crosslinked anhydrides which hydrolyze back to its original state over time. The properties of the gel can be fine-tuned with respect to the concentration of EDC-fuel added. However, as identified in the control experiments, this is strictly dependent on the prior deprotection of ONB group. The effect of polymer chain length on rheological properties of the materials was also investigated, looking at polymers with primary chain lengths of 100 with poly(ONBM15–DMAm81) and 150 with poly(ONBM15–DMAm122), keeping the same number of potential crosslinkers per chain. These ratios were carefully designed to accommodate the solubility of the polymer materials in aqueous solution as the ONB group reduced the solubility of the polymer in aqueous medium. As seen in Fig. 4, irrespective of the chain length and the ratio of the ONBM group to DMAm, all networks had G′ exceeds G′′ while G′ dominates for several minutes, indicating anhydride formation and a gel or solid material.
and a longer crossover time of tdegel ∼ 800 min. The relatively small increase in G′ after the crossover could be due to some solvent evaporation with the longer experimental timescale. With intermediate concentrations of EDC, the peak storage modulus and crossover time were intermediate between the extremes that occur at 0.5 M and 2.0 M. These findings showed that EDC fuels added to aqueous polymer solution with free diacids result in transiently crosslinked anhydrides which hydrolyze back to its original state over time. The properties of the gel can be fine-tuned with respect to the concentration of EDC-fuel added. However, as identified in the control experiments, this is strictly dependent on the prior deprotection of ONB group. The effect of polymer chain length on rheological properties of the materials was also investigated, looking at polymers with primary chain lengths of 100 with poly(ONBM15–DMAm81) and 150 with poly(ONBM15–DMAm122), keeping the same number of potential crosslinkers per chain. These ratios were carefully designed to accommodate the solubility of the polymer materials in aqueous solution as the ONB group reduced the solubility of the polymer in aqueous medium. As seen in Fig. 4, irrespective of the chain length and the ratio of the ONBM group to DMAm, all networks had G′ exceeds G′′ while G′ dominates for several minutes, indicating anhydride formation and a gel or solid material.
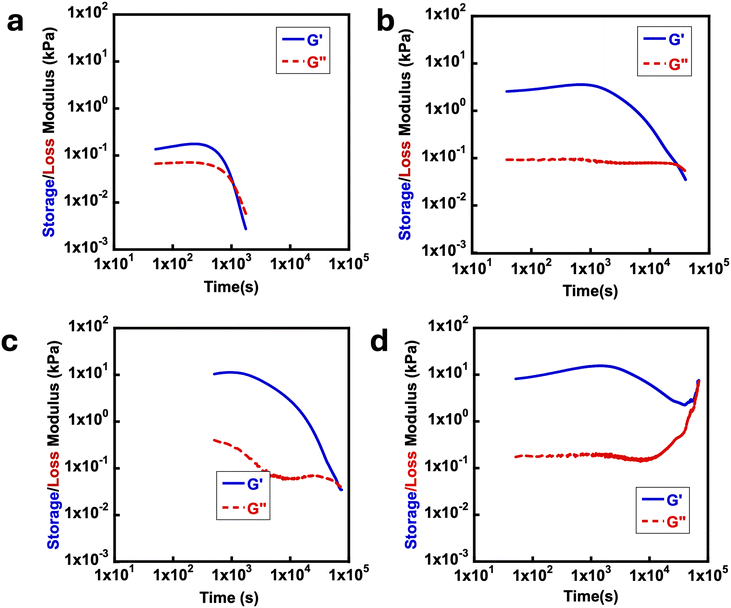 | ||
| Fig. 3 Typical rheology time sweeps at 15 °C using a frequency of 10 rad s−1 for deprotected poly(ONBM15–DMAm81) with (a) 0.5 M, (b) 1.0 M, (c) 1.5 M and, (d) 2.0 M concentrations of EDC. | ||
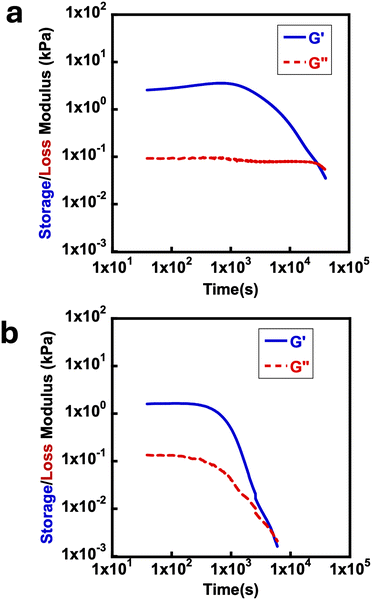 | ||
| Fig. 4 Typical rheology time sweeps at 15 °C using a frequency of 10 rad s−1 with 1 M EDC for deprotected (a) poly(ONBM15–DMAm81) and (b) poly(ONBM15–DMAm122). | ||
Poly(ONBM15–DMAm122) had a shorter tdegel at ∼100 min compared to tdegel ∼ 300 min for poly(ONBM15–DMAm81) as shown in Fig. 4. Similarly, poly(ONBM15–DMAm122) had a lower 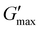 of ∼1 kPa compared to
of ∼1 kPa compared to 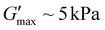 for poly(ONBM15–DMAm81), which could be because there is more space between the deprotected acid groups leading to less efficient crosslinking. Time sweep data in Fig. S6† showed that poly(ONBM30–DMAm155) had the longest transient network timescale of tdegel ∼ 1700 min. However, this material also had a slight increase in moduli at long times which could be due to unavoidable evaporation at these long timescales. The increase in tdegel as the chain length increases could be due to more efficient crosslinking due to longer polymer facilitating connections between chains. This trend is consistent with similar reports on the influence of chain length on the de-crosslinking times of anhydride hydrolysis.12,38
for poly(ONBM15–DMAm81), which could be because there is more space between the deprotected acid groups leading to less efficient crosslinking. Time sweep data in Fig. S6† showed that poly(ONBM30–DMAm155) had the longest transient network timescale of tdegel ∼ 1700 min. However, this material also had a slight increase in moduli at long times which could be due to unavoidable evaporation at these long timescales. The increase in tdegel as the chain length increases could be due to more efficient crosslinking due to longer polymer facilitating connections between chains. This trend is consistent with similar reports on the influence of chain length on the de-crosslinking times of anhydride hydrolysis.12,38
Conclusion
In conclusion, RAFT polymerization was used to synthesize photolabile copolymers of ONBM and DMAm with varied architecture. Photoresponsive carbodiimide-fueled and transiently anhydride crosslinked networks were synthesized based on the hydration of unmasked acids to anhydrides. This creates an “AND” gate where both UV and carbodiimide stimuli are needed to induce transient network formation. Oscillatory rheological tests were used to investigate the properties of the resulting hydrogels based on factors like EDC fuel concentration, UV deprotection efficiency and polymer chain length. Tuning EDC fuel concentration showed an increase in the peak G′ and degelation time as fuel concentration was increased. Also, higher deprotection efficiency significantly led to higher de-gelation time and increased the peak G′ while no gel was formed with protected polymer bearing 2-nitrobenzyl moiety. These findings are pertinent for potential applications where gelation time can be tuned by varying either fuel concentration, UV deprotection or polymer architecture. Future research exploring PPGs with different deprotection wavelength are anticipated to enable a broad range of tunability in mechanical performance for different photoresponsive chemically fueled transiently crosslinked polymer.Data availability
Data for this article, including polymer characterization and rheological data are available at Miami University Scholarly Commons at https://hdl.handle.net/2374.MIA/7004.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by United States Department of Energy, Office of Science, Basic Energy Sciences, under Award No. DE-SC0018645.References
- R. Moreno-Sánchez, A. Marín-Hernández, E. Saavedra, J. P. Pardo, S. J. Ralph and S. Rodríguez-Enríquez, Int. J. Biochem. Cell Biol., 2014, 50, 10–23 CrossRef PubMed.
- M. Bonora, S. Patergnani, A. Rimessi, E. de Marchi, J. M. Suski, A. Bononi, C. Giorgi, S. Marchi, S. Missiroli, F. Poletti, M. R. Wieckowski and P. Pinton, Purinergic Signalling, 2012, 8, 343–357 CrossRef CAS PubMed.
- B. Rieß, R. K. Grötsch and J. Boekhoven, Chem, 2020, 6, 552–578 Search PubMed.
- L. S. Kariyawasam, M. M. Hossain and C. S. Hartley, Angew. Chem., Int. Ed., 2021, 60, 12648–12658 CrossRef CAS PubMed.
- J. A. Knoblich, Nat. Rev. Mol. Cell Biol., 2010, 11, 849–860 CrossRef CAS PubMed.
- T. E. Gillies and C. Cabernard, Curr. Biol., 2011, 21, R599–R609 CrossRef CAS PubMed.
- A. A. Mironov and G. V. Beznoussenko, Front. Cell Dev. Biol., 2019, 7, 146 CrossRef PubMed.
- S. S. Mogre, A. I. Brown and E. F. Koslover, Phys. Biol., 2020, 17, 061003 CrossRef CAS PubMed.
- J. Boekhoven, A. M. Brizard, K. N. K. Kowlgi, G. J. M. Koper, R. Eelkema and J. H. van Esch, Angew. Chem., 2010, 122, 4935–4938 CrossRef.
- S. A. P. Van Rossum, M. Tena-Solsona, J. H. Van Esch, R. Eelkema and J. Boekhoven, Chem. Soc. Rev., 2017, 46, 5519–5535 RSC.
- E. J. Johnston, E. L. Rylott, E. Beynon, A. Lorenz, V. Chechik and N. C. Bruce, Science, 2015, 349, 1072–1075 CrossRef CAS PubMed.
- C. W. H. Rajawasam, C. Tran, M. Weeks, K. S. McCoy, R. Ross-Shannon, O. J. Dodo, J. L. Sparks, C. S. Hartley and D. Konkolewicz, J. Am. Chem. Soc., 2023, 145, 5553–5560 CrossRef CAS PubMed.
- B. G. P. Van Ravensteijn, W. E. Hendriksen, R. Eelkema, J. H. Van Esch and W. K. Kegel, J. Am. Chem. Soc., 2017, 139, 9763–9766 CrossRef CAS PubMed.
- A. Sorrenti, J. Leira-Iglesias, A. J. Markvoort, T. F. A. De Greef and T. M. Hermans, Chem. Soc. Rev., 2017, 46, 5476–5490 RSC.
- R. K. Grötsch and J. Boekhoven, in Self-Assembling Biomaterials: Molecular Design, Characterization and Application in Biology and Medicine, Elsevier, 2018, pp. 235–250 Search PubMed.
- J. Leira-Iglesias, A. Sorrenti, A. Sato, P. A. Dunne and T. M. Hermans, Chem. Commun., 2016, 52, 9009–9012 RSC.
- S. Bai, X. Niu, H. Wang, L. Wei, L. Liu, X. Liu, R. Eelkema, X. Guo, J. H. van Esch and Y. Wang, Chem. Eng. J., 2021, 414, 128877 CrossRef CAS.
- J. Heckel, S. Loescher, R. T. Mathers and A. Walther, Angew. Chem., Int. Ed., 2021, 60, 7117–7125 CrossRef CAS PubMed.
- M. Cheng, C. Qian, Y. Ding, Y. Chen, T. Xiao, X. Lu, J. Jiang and L. Wang, ACS Mater. Lett., 2020, 2, 425–429 CrossRef CAS.
- P. Chakma, L. H. Rodrigues Possarle, Z. A. Digby, B. Zhang, J. L. Sparks and D. Konkolewicz, Polym. Chem., 2017, 8, 6534–6543 RSC.
- N. Nath and A. Chilkoti, Adv. Mater., 2002, 14, 1243–1247 CrossRef CAS.
- P. Schattling, F. D. Jochum and P. Theato, Polym. Chem., 2014, 5, 25–36 RSC.
- M. Wei, Y. Gao, X. Li and M. J. Serpe, Polym. Chem., 2017, 8, 127–143 RSC.
- X. Hu, Z. Qureishi and S. W. Thomas, Chem. Mater., 2017, 29, 2951–2960 CrossRef CAS.
- N. De Alwis Watuthanthrige, P. N. Kurek and D. Konkolewicz, Polym. Chem., 2018, 9, 1557–1561 RSC.
- A. Bagheri, J. Yeow, H. Arandiyan, J. Xu, C. Boyer and M. Lim, Macromol. Rapid Commun., 2016, 37, 905–910 CrossRef CAS PubMed.
- S. K. Choi, M. Verma, J. Silpe, R. E. Moody, K. Tang, J. J. Hanson and J. R. Baker, Bioorg. Med. Chem., 2012, 20, 1281–1290 CrossRef CAS PubMed.
- Y. Li, Y. Zhang and W. Wang, Nano Res., 2018, 11, 5424–5438 CrossRef CAS.
- J. Liu, W. Kang and W. Wang, Photochem. Photobiol., 2022, 98, 288–302 CrossRef CAS PubMed.
- S. Kumar, D. Kumar, R. Ahirwar and P. Nahar, Anal. Bioanal. Chem., 2016, 408, 6945–6956 CrossRef CAS PubMed.
- V. Gaud, F. Rougé, Y. Gnanou and J. P. Desvergne, React. Funct. Polym., 2012, 72, 521–532 CrossRef CAS.
- G. Chang, Y. Zhao, B. Zhao, X. Yang, S. Zhang, C. Wang and Z. Wang, Anal. Chim. Acta, 2023, 1238, 340638 CrossRef CAS PubMed.
- M. Kim and H. Chung, Polym. Chem., 2017, 8, 6300–6308 RSC.
- V. V. Ramanan, J. S. Katz, M. Guvendiren, E. R. Cohen, R. A. Marklein and J. A. Burdick, J. Mater. Chem., 2010, 20, 8920–8926 RSC.
- H. Zhao, E. S. Sterner, E. B. Coughlin and P. Theato, Macromolecules, 2012, 45, 1723–1736 CrossRef CAS.
- M. Tena-Solsona, B. Rieß, R. K. Grötsch, F. C. Löhrer, C. Wanzke, B. Käsdorf, A. R. Bausch, P. Müller-Buschbaum, O. Lieleg and J. Boekhoven, Nat. Commun., 2017, 8, 15895 CrossRef CAS PubMed.
- B. Zhang, I. M. Jayalath, J. Ke, J. L. Sparks, C. S. Hartley and D. Konkolewicz, Chem. Commun., 2019, 55, 2086–2089 RSC.
- O. J. Dodo, L. Petit, C. W. H. Rajawasam, C. S. Hartley and D. Konkolewicz, Macromolecules, 2021, 54, 9860–9867 CrossRef CAS.
- C. W. H. Rajawasam, C. Tran, J. L. Sparks, W. H. Krueger, C. S. Hartley and D. Konkolewicz, Angew. Chem., Int. Ed., 2024, 63, e202400843 CrossRef CAS PubMed.
- L. S. Kariyawasam, M. M. Hossain and C. S. Hartley, Angew. Chem., Int. Ed., 2021, 60, 12648–12658 CrossRef CAS PubMed.
- X. Chen, M. A. Würbser and J. Boekhoven, Acc. Mater. Res., 2023, 4, 416–426 CrossRef CAS PubMed.
- R. T. Deam and S. F. Edwards, Philos. Trans. R. Soc., A, 1976, 280, 317–353 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed procedures of the polymerization reactions, deprotection, EDC fueled crosslinking, and characterization methods. Supplemental data of NMR spectra of ONBM monomer, polymer after precipitation, SEC traces of synthesized polymers, NMR of deprotection efficiency and supplemental time sweep data. See DOI: https://doi.org/10.1039/d4py01244e |
| This journal is © The Royal Society of Chemistry 2025 |