Open Access Article
Open Access ArticleRecent advances in emerging nanozymes with aggregation-induced emission
Xin
Li
a,
Zhao
Wang
a,
Jing
He
a,
Haitham
Al-Mashriqi
a,
Jia
Chen
 *a and
Hongdeng
Qiu
*a and
Hongdeng
Qiu
 *ab
*ab
aResearch Center for Natural Medicine and Chemical Metrology, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China. E-mail: jiachen@licp.cas.cn; hdqiu@licp.cas.cn
bKey Laboratory of Rare Earths, Ganjiang Innovation Academy, Chinese Academy of Sciences, Ganzhou 341119, China
First published on 10th October 2024
Abstract
AIE luminogens (AIEgens) are a class of unique fluorescent molecules that exhibit significantly enhanced luminescence properties and excellent photostability in the aggregated state. Recently, it has been found that some AIEgens can produce reactive oxygen species, which means that they may have potential enzyme-like activities and are thus termed “AIEzymes”. Consequently, the discovery and design of novel AIEgens with enzyme-like properties have emerged as a new and exciting research direction. Additionally, AIEgens can enhance the catalytic efficiency of traditional nanozymes by direct combination, thereby endowing the nanozymes with multifunctionality. In this regard, nanozymes with aggregation-induced emission (AIE) properties, which represents a win–win integration, not only take full advantage of the low cost and stability of nanozymes, but also incorporate the excellent biocompatibility and fluorescence properties of AIEgens. These synergistic compounds bring about new opportunities for various applications, making AIEzymes of interest in biomedical research, food analysis, environmental monitoring, and especially imaging-guided diagnostics. This review will provide an overview of the latest strategies and achievements in the rational design and preparation of AIEzymes, as well as current research trends, future challenges and prospective solutions. We expect that this work will encourage and motivate more people to study and explore AIEzymes to further promote their applications in various fields.
1. Introduction
In recent years, researchers have discovered a class of special optical materials that exhibit significantly enhanced fluorescence in the aggregated state, a phenomenon known as aggregation-induced emission (AIE).1–3 This discovery not only showed the luminous features of the new compounds, but also confirmed Stokes' 1853 results with platinocyanides.4 The mechanism behind the AIE phenomenon is primarily attributed to the restriction of intramolecular motions, including the restriction of intramolecular rotations and vibrations.5–7 This phenomenon makes AIE luminogens (AIEgens) highly fluorescent with high quantum yields and excellent photostability in the aggregated and solid states, making them particularly suitable for high-quality fluorescence imaging and long-term tracking.8,9 Moreover, studies have revealed that specific AIEgens have the ability to generate reactive oxygen species (ROS) with high efficiency,10,11 which are known to be toxic to cancer cells, pathogens, and certain bacterial species.12 This unique property of AIEgens reveals their potential for a wide range of applications.In the field of catalysis, enzymes play a pivotal role due to their ability to significantly accelerate chemical reactions.13,14 However, although the use of enzymes expanded in the early 20th century, their inherent instability and limitations within specific reaction environments gradually became apparent.15,16 Initially, research switched to the development of artificial enzymes, which are made from tiny organic molecules such as cyclodextrins and used to enhance or replicate the functions of natural enzymes.17–22 In recent years, with the rise of nanotechnology, the concept of nanozymes has garnered significant attention since 2007, when Yan et al. initially identified that Fe3O4 nanoparticles exhibit peroxidase-like activity.23 This groundbreaking finding demonstrated that some nanomaterials possess both physicochemical properties and distinctive enzyme-like catalytic capabilities.24,25 In the last 17 years of research, metals, metal oxides, and carbon compounds have been shown to possess catalytic activities similar to peroxidase (POD), catalase (CAT), oxidase (OXD), and superoxide dismutase (SOD).26–31 It has been reported in the literature that nanozymes possess properties such as high stability, low cost, easy preparation and easy modification. In addition, as the selectivity problem has been gradually overcome and improved, nanozymes have been tailored to specific substrates or environmental conditions, endowing them with a high degree of versatility in various applications.32–37 Besides, nanozymes stand out from natural enzymes due to their possession of physical and chemical properties unique to nanomaterials, endowing them with bifunctional or multifunctional characteristics.38–41 Recently, the integration of nanozyme catalytic activity with other physicochemical properties has become a major hotspot.42–45 This synergistic approach aids in discovering new functions of nanozymes and broadening their applications.46–48 If nanozymes are endowed with AIE properties, it will open up a new field of great significance.
Nanozymes with aggregation-induced luminescence (AIE) properties, known as AIEzymes, combine the catalytic activity of nanozymes with the optical properties of AIE materials. This fusion not only retains the traditional advantages of nanozymes, such as their design flexibility, ease of preparation and regulation, and excellent stability, but also gains additional properties from AIEgens, including low background noise, good photostability, a substantial Stokes shift, and favorable biocompatibility, forming a powerful combination. This synergy has led to a thriving research direction. Specifically, AIEzymes have broad applications in the field of biomedicine. Their unique AIE properties enable them to exhibit low background noise and good photostability in biological systems, aiding in the real-time tracking and monitoring of biomarkers. Furthermore, AIEzymes can produce ROS in response to light, killing cancer cells or pathogens in a way similar to enzyme catalysis, providing novel approaches to cancer treatment and infection management. Additionally, AIEzymes demonstrate great potential in the field of sensing and analysis. Their high sensitivity and selectivity allow them to detect trace amounts of biomolecules and chemical pollutants, providing effective tools for environmental monitoring and food safety analysis.49,50
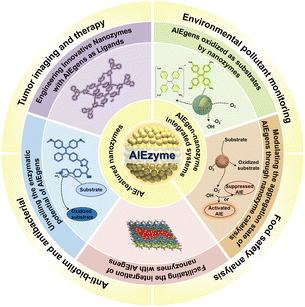
To date, luminescent materials with AIE properties have received great attention in various research fields.51,52 Despite comprehensive reviews on the combination of AIE luminogens and their applications in the fields of nanomedicine and photodynamic therapy (PDT),53,54 research into the enzyme-like activities of AIE materials remains lacking in systematic and in-depth exploration. This research gap has limited our comprehensive understanding of the potential applications of AIE materials and created obstacles in optimizing their practical use. Currently, there is a lack of a comprehensive review on the progress of nanozymes with AIE properties; hence, the publication of this review is both crucial and urgently needed. This work summarizes two research strategies for designing new AIEzymes (Fig. 1): first, the synthesis of novel nanozymes utilizing the enzyme-like activity of AIE luminogens, and second, the regulation of the aggregation state of AIE luminogens through nanozyme catalysis to construct functional integrated systems, providing a comprehensive view of the latest research trends in AIEzymes. Additionally, this review thoroughly introduces the application of AIEzymes in key areas such as biomedical diagnostics, treatment, food safety analysis, and environmental monitoring, revealing their vast potential in various industries. We hope that this review will become a valuable resource for researchers in related fields, inspiring new research ideas and providing theoretical foundations and practical guidance.
2. Synthesizing AIE-featured nanozymes
2.1 Unveiling the enzymatic potential of AIEgens
In recent years, as aggregation-induced emission materials have been widely applied, their ability to enhance emission in the aggregated state and generate ROS has garnered increasing attention from researchers.55 These materials have demonstrated significant potential in areas such as antimicrobial therapy, tumor imaging, photodynamic therapy, and biomedical engineering.56–58 However, the enzyme-like properties of AIEgens have not been sufficiently explored. Recently, Liu and his team have opened up a new research path by designing and synthesizing a unique AIE molecule, named “AIEzyme” (Fig. 2A). Under excitation, this AIEzyme is not only highly emissive, but also efficiently generates ROS, demonstrating enzyme-like activity.59 As shown in Fig. 2B, luminol (LUM) produces significant chemiluminescence (CL) in the presence of AIEzyme without relying on H2O2. Through electron spin resonance (ESR) technology, the study determined that AIEzyme primarily produces singlet oxygen (1O2) as the ROS under light excitation, rather than hydroxyl radicals (OH˙) or superoxide anions (O2˙−). Under alkaline conditions, luminol is catalyzed by 1O2 generated by AIEzyme to form excited state molecules, 3-APA*, which emit light. Although the emission spectrum of luminol overlaps with the absorption spectrum of AIE, the large distance between the two molecules prevents the efficient excitation of AIE's fluorescence through the chemiexcitation resonance energy transfer (CRET) mechanism. Instead, the luminol's light emission provides energy to AIEzyme, allowing for the continuous generation of ROS, which further catalyzes the emission of luminol, establishing a self-sustaining mechanism of persistent chemiluminescence. In summary, AIEzyme catalyzes the luminescence of luminol with ROS generated by extra light excitation and continues to catalyze dissolved oxygen to produce ROS after light irradiation stops, ultimately leading to the sustained chemiluminescence of luminol. Despite the significant advantages of these afterflow luminescence systems, such as no need for H2O2, good stability, and long sustained luminescence time, the variety of AIEgens with enzyme-like catalytic activity remains limited, restricting their potential for broader applications. Especially in complex biological systems or specific environments, existing AIEgens fail to meet diverse catalytic needs. Therefore, future research should focus not only on synthesizing more AIEgens with enzyme-like activity, but also on exploring the structure–function relationships of these molecules to develop more specific and highly reactive AIEgens suited for various applications. In conclusion, research on unveiling the enzymatic potential of AIEgens is still in its early stages, with immense potential yet to be fully tapped. By expanding the range of AIEgens and optimizing their structures, this new kind of AIEzyme is expected to see broader applications in bioimaging, diagnostics, and sensing in the future.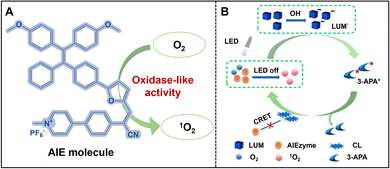 | ||
| Fig. 2 (A) Illustration of AIEgens with enzyme-like activity; (B) possible mechanism for the sustained chemiluminescence of the AIEzyme/LUM system. Reproduced from ref. 59 with permission from American Chemical Society, copyright 2023. | ||
2.2 Engineering innovative nanozymes with AIEgens as ligands
In recent years, research on nanozymes has gained significant attention, particularly in the development of micro- and nano-scale structures with enzyme-like functions using various materials. Researchers have successfully synthesized a range of materials with enzymatic activities by employing metals, metal oxides,60,61 carbon nanomaterials,28,62 polymers,63,64 and metal–organic frameworks.65,66 Despite their potential applications, these materials face technical challenges such as low specificity, a scarcity of active sites, and complex synthesis processes. Consequently, scientists have turned to supramolecular chemistry, aiming to mimic key characteristics of natural enzymes, such as amphiphilicity and precise control over conformational changes, to develop biomimetic nanozymes that enhance substrate recognition and catalytic activity.67,68For example, combining metal-based supramolecular systems with AIEgens can endow nanozymes with unique spectral properties. In this context, Zhang and colleagues made a breakthrough by incorporating AIEgens as ligands in the synthesis of novel nanozymes, such as organic molecular cages (OMCs) like TPE-Cages. These cage structures not only exhibited exceptional luminescence properties but also showed potential applications in gas detection, storage, and biological imaging.69,70 Additionally, Zhang's team introduced ZnDPA (Zn coordinated dipicolylamine) units into the TPE-Cages, creating a positively charged supramolecular cage, ZnDPA-TPE-Cage.71 As shown in Fig. 3A, this structure not only enhances the AIE characteristics but also demonstrates outstanding oxidase-like activity, generating ROS under light conditions to selectively recognize and eradicate Gram-positive bacteria. This research has opened new avenues for integrating AIEgens with enzyme-like functions. Nevertheless, there are limitations to the broad application of these materials. For instance, although ZnDPA-TPE-Cage has demonstrated the ability to produce ROS under light exposure, effectively targeting Gram-positive bacteria for recognition and eradication, its efficacy against a wider range of bacterial species and other microorganisms is yet to be fully explored. Additionally, the potential for broad-spectrum antimicrobial activity may require further investigation.
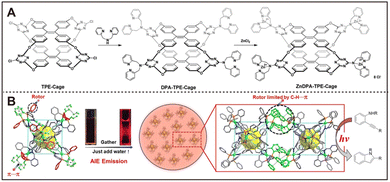 | ||
| Fig. 3 (A) Process for the preparation of novel organic molecular cages with oxidase properties and AIE properties. Reproduced from ref. 71 with permission from Royal Society of Chemistry, copyright 2021. (B) A Pt(II)-based molecular cage with aggregation-induced emission for enzymatic photocyclization of alkynylaniline. Reproduced from ref. 72 with permission from John Wiley and Sons, copyright 2022. | ||
In addition, although the above-mentioned materials can utilize the ROS-generating properties of AIEgens to achieve synergistic catalysis, the catalytic activity of the existing AIEzymes still needs to be further enhanced. Duan and colleagues have proposed an innovative strategy by incorporating triphenylphosphine ligands to construct AIE-active platinum-based cages, which successfully activate amino and alkyne groups through π-acidic Pt(I) complexes to facilitate photocyclization reactions.72Fig. 3B illustrates how the AIE-active cage activates amino groups via photoinduced electron transfer (PET) in the AIE excited state, while simultaneously activating alkyne groups through π-acidic Pt(I) complex formation, thus promoting the photocyclization reaction. Notably, the presence of water enhances the luminescence of AIE, increases the material's substrate affinity, and improves photocatalytic efficiency. This research, by integrating AIE fluorophores with π-acidic active sites, addresses the solubility issues of metal–organic cages in aqueous media and points to a new direction for developing more efficient enzyme mimics. Furthermore, Rana et al. developed a Pt(II)-centric supramolecular composite, named NanoPtA, which exhibits enhanced AIE in aqueous media. This complex demonstrates exceptional light driven oxidase-like activity and has the potential to degrade environmental pollutants.73 However, before practical applications, these composite materials must undergo evaluation regarding their stability, toxicity, cost, and feasibility for large-scale production.
2.3 Facilitating the integration of nanozymes with AIEgens
In recent years, the integration of AIEgens and nanozymes has increasingly become a hot topic in scientific research, primarily because this combination can produce synergistic effects in the biomedical field, significantly enhancing the efficacy of diagnosis and treatment. AIEgens, as an emerging class of luminescent molecules, are capable of emitting intense fluorescence in an aggregated state, a property that has shown broad application prospects in many fields. Nanozymes, on the other hand, are nanomaterials with enzyme-like activity that boast good stability, high catalytic efficiency, and ease of modification. Therefore, integrating AIEgens with nanozymes not only enhances the optical properties of AIEgens but also endows nanozymes with additional functionalities, thereby providing more efficient solutions in areas such as biosensing, environmental monitoring, and biological medicine (Fig. 4A).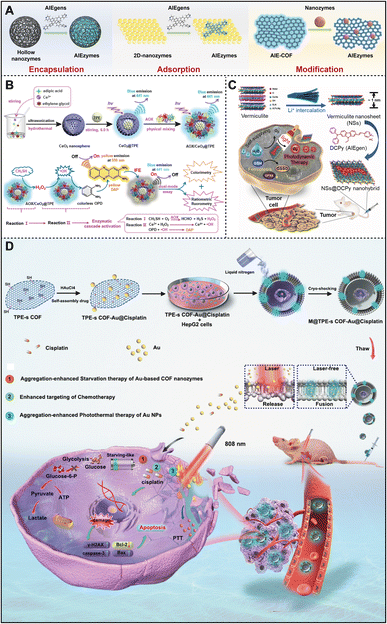 | ||
| Fig. 4 (A) Schematic diagram of several strategies for integrating nanozymes with AIEgens. (B) Dual enzyme cascade catalytic system AOX/CeO2@TPE + OPD that realizes ratiometric fluorescence–colorimetric dual-mode detection of CH3SH. Reproduced from ref. 74 with permission from American Chemical Society, copyright 2023. (C) NSs@DCPy nanohybrid and their application in oxygen self-contained photodynamic therapy. Reproduced from ref. 75 with permission from Elsevier, copyright 2022. (D) An AIEgen-based COF nanozyme for tumor cell-specific imaging and therapy. Reproduced from ref. 76 with permission from Elsevier, copyright 2023. | ||
One simple and effective approach to integrating these two components is encapsulating AIEgens within the cavity of nanozymes. This results in composites with several benefits: (1) protection of AIEgens. Encapsulation shields AIEgens from degradation by enzymes or other biomolecules, thus improving their stability and effectiveness in the body. (2) Targeted delivery. The strategic design of nanozymes enables targeted delivery to specific cells or tissues, facilitating the localized activation of AIEgens and allowing for precise imaging or therapy. (3) Synergistic effects. AIEgens with ROS-generating capabilities can enhance the catalytic functions of nanozymes, amplifying their overall effect. In this regard, various inorganic hollow spheres, including metals77 and metal oxides,74,78 have been engineered to encapsulate AIEgens.
Among them, mesoporous CeO2 nanospheres are considered as important candidates for the design of multifunctional and intelligent optical/electro-sensing platforms due to their excellent stability, good biocompatibility, and loading capacity.79 Driven by this, Wu et al. synthesized mesoporous cerium oxide nanospheres using hydrothermal techniques and embedded TPE into hollow CeO2 nanospheres to synthesize a novel blue fluorescent nanozyme CeO2@TPE with high peroxidase activity.74Fig. 4B illustrates the design of a dual-enzyme cascade fluorescence–colorimetric dual-mode system, AOX/CeO2@TPE + OPD, which is used for the detection of CH3SH in the presence of ethanol oxidase (AOX) and o-phenylenediamine (OPD). In the absence of CH3SH, AOX/CeO2@TPE emitted blue fluorescence at 441 nm. However, in the presence of CH3SH, AOX is activated to produce H2O2, which then triggers the catalytic activity of CeO2@TPE, generating ·OH. This results in the oxidation of non-fluorescent OPD to 2,3-diaminophenazine (DAP), emitting yellow fluorescence at 550 nm. Simultaneously, the internal filtering effect (IFE) led to a gradual reduction in the blue fluorescence of AOX/CeO2@TPE at 441 nm, yielding a characteristic ratio fluorescence response. Moreover, the enzyme cascade exhibited an increase in absorbance at 425 nm due to the transformation of colorless OPD to dark yellow DAP, visible to the naked eye, in the presence of CH3SH. Therefore, this dual-mode strategy, which combines ratiometric fluorescence and colorimetry, provides an effective means for the detection of CH3SH. By integrating smartphone technology, this method makes it possible to reliably and accurately detect CH3SH in field environmental samples. Although this study highlights the potential of integrating nanozymes and AIEgens to create efficient field sensors for environmental monitoring and pollution source analysis, further research is needed to validate and optimize their performance for various environmental monitoring and pollution source analysis tasks under different conditions.
Besides encapsulation, adsorbing or modifying AIEgens onto the surface of nanozymes is another common integration strategy. As depicted in Fig. 4C, Tang and colleagues drew inspiration from bonsai to synthesize ultra-thin nanosheets (NSs) as a “potting soil” from vermiculite, and subsequently “planted” DCPy (an AIEgen) onto the NSs' surface via electrostatic attraction, creating an AIE-vermiculite nano-hybrid photosensitizer (NSs@DCPy) for PDT.75 When NSs@DCPy is absorbed by hypoxic tumors and exposed to light radiation, NSs@DCPy, as an enzyme mimic, not only produces 1O2 and ·OH, but also catalyzes H2O2 to O2, thus providing “nutrients” for AIEgens to alleviate hypoxia and significantly improving the efficacy of photodynamic therapy. Remarkably, NSs@DCPy also demonstrated glutathione oxidase activity, capable of inducing cell ferroptosis. Hence, this study introduces an AIEgen-vermiculite nanohybrid platform that enables “self-sufficient” photodynamic cancer therapy, augmented by ferroptosis and oxygen generation. Liao et al. utilized bioorthogonal reactions to modify AIEgens onto the multifunctional Zn@MOF surface, yielding a nanozyme (Zn@MOF-TPD) with AIE properties, and applied this to enhance recovery after spinal cord injury.80 However, despite the great potential of these integration strategies, there are still some challenges in practical applications. For example, further research needs to address whether the production process for adsorbing AIEgens onto nanozymes is simple and cost-effective and whether these conjugates are able to maintain stable adsorption and avoid detachment or degradation in the complex physiological environments in living organisms.
Meanwhile, AIEgen-based covalent organic frameworks (COFs) offer new avenues for the integration of nanozymes.81,82 Utilizing their substantial specific surface area, the integration of AIEgens and nanozymes can also be achieved by modifying the nanozymes onto the surface of AIEgen-based COFs.76,83Fig. 4D illustrates a bionic multifunctional COF nanozyme reported by the Zhang group, which consists of an AIEgen-based COF (TPE-s COF) and gold nanoparticles (Au NPs) immobilized on its surface.76 This nanozyme was co-cultured with HepG2 cells, leading to lipophilic TPE-s COF-Au@cisplatin fusion with the cell membrane. Subsequently, an inactivated nanozyme (M@TPE-s COF-Au@cisplatin) was prepared by the freezing-shaking method and endocytosed by HepG2 cells, losing its proliferation and pathogenicity. After that, laser irradiation triggered the release of TPE-s COF-Au nanozymes and cisplatin for photothermal and drug therapy, respectively. This research introduces a novel strategy for synthesizing tumor-derived fluorescent TPE-s COF-Au nanozymes for efficient, synergistic, and targeted chemotherapy–photothermal combination therapy in hepatocellular carcinoma (HCC). Overall, there are several key factors that require special attention when integrating nanozymes with AIEgens by surface modification or adsorption: (1) the binding density must be finely tuned to avoid hindering the nanozyme's active sites and impacting its catalytic performance. Hence, optimizing the binding density is vital. (2) AIEgens' aggregation-induced emission is central to high-sensitivity detection and treatment. Ensuring that the AIEgens aggregate appropriately on the nanozyme surface for efficient luminescence is paramount. (3) The stability of the modified layer is essential for the long-term use of nanozyme-AIEgen integrations. The modified layer needs to be stable enough to resist various environmental conditions, such as pH changes and temperature fluctuations.
Additionally, co-loading AIEgens and nanozymes onto other carriers can further enhance their performance. For example, Luo et al. developed a chloramphenicol (CAP) sensor that combines the properties of aggregation-induced electrochemiluminescence (AI-ECL) and nanozymes, with the AI-ECL serving as the initial signal generator, while the nanozymes amplify the signal.84 As depicted in Fig. 5A, a COF material (COF-AI-ECL) containing AI-ECL groups and Co3O4 nanozymes was synthesized respectively. This material was then crosslinked and immobilized on a gold electrode. Subsequently, molecularly imprinted polymers (MIPs) were formed on the electrode surface using CAP as a template. The inclusion of COF-AI-ECL expanded the MIP's polymerization area, enhancing the MIP's CAP-imprinting effect and adsorption capacity. Additionally, COF-AI-ECL produced a robust and stable ECL signal, while Co3O4 nanozymes amplified the ECL signal in the presence of H2O2. This combination, along with the MIP's selectivity for CAP, effectively suppressed non-specific ECL signals. Consequently, the sensor not only facilitated the sensitive detection of CAP but also provided a model for detecting other analytes. By the same way, they developed a molecularly imprinted ciprofloxacin (CFX) electrochemiluminescence sensor with significantly improved detection sensitivity by utilizing COF-AIECL as a signaling element and Fe3O4@Pt NP nanozymes for signal amplification.87 However, the primary limitations of these kinds of MIP sensors are their small imprint capacity and insufficient membrane stability, which require resolution in future research.
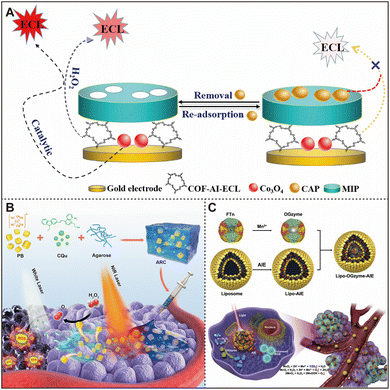 | ||
| Fig. 5 (A) Novel chloramphenicol sensors based on aggregation-induced electrochemiluminescence and nanozyme signal amplification. Reproduced from ref. 84 with permission from Elsevier, copyright 2021. (B) An injectable nanozyme hydrogel was used as an AIEgen reservoir and release controller for multiple rounds of tumor therapy. Reproduced from ref. 85 with permission from Elsevier, copyright 2021. (C) Preparation process and therapeutic mechanism of Lipo-OGzyme-AIE. Reproduced from ref. 86 with permission from Elsevier, copyright 2019. | ||
In addition to inorganic carriers, hydrogels have become an ideal choice for loading AIEgens and nanozymes due to their high biocompatibility and photo-controlled reversible phase transition properties.88 Tang et al. developed an injectable nanozyme hydrogel by encapsulating Prussian blue (PB) nanoparticles and AIEgens (CQu) within a hydrogel, serving as an AIEgen reservoir and release controller (ARC).85 As depicted in Fig. 5B, PB NPs convert NIR laser light into heat, triggering hydrogel degradation and the release of CQu. PB nanozymes can then catalyze the decomposition of endogenous hydrogen peroxide to produce O2. Under light irradiation, AIEgens generate high levels of ROS and sufficient O2 to induce cytotoxicity in tumor cells. Due to the hydrogel's extended residence time in the tumor (at least 48 hours), multiple treatment cycles can be achieved with a single injection. This study represents the first use of hydrogels for delivering AIEgens, and the developed ARC system provides a new clinical development tool for therapeutic strategies for cancer patients. However, despite the promising therapeutic potential of this carrier, the storage conditions of hydrogels and their long-term biocompatibility when remaining in the body need to be further evaluated.
Despite the superior properties of the above-mentioned inorganic carriers, their biocompatibility and targeting in biological systems still need to be improved. Consequently, bionic carriers are increasingly becoming a focus for the integration of nanozymes and AIEgens. Huang et al. synthesized manganese dioxide nanoparticles within the hollow cavities of ferritin nanocages (FTn) in a bioinspired manner, creating a hypoxia-tropic nanozyme as an oxygen generator (OGzyme). They further developed a reaction cascade therapeutic nanosystem by integrating the PDT potential of OGzymes with AIEgens.86 As shown in Fig. 5C, AIEgens were encapsulated within phospholipid bilayers, while OGzymes were placed inside the cavity (lipo-OGzyme-AIE). Encapsulating OGzymes within liposomes facilitated their efficient uptake by tumor cells. The inclusion of AIE molecules aided in the delivery of therapeutic substances to the tumor site and their aggregation post-liposome degradation, supporting PDT and enabling multimodal imaging. This proof-of-concept study underscores the multifunctional benefits of biomimetic nanozymes in tumor therapy and validates their potential of modulating tumor hypoxia as a therapeutic approach. Integrating nanozymes with AIEgens on biocarriers can achieve selective treatment of specific cells through targeting, improving the precision of treatment. However, the high production costs of biomimetic carriers, limited loading capacity, and strict conditions required for storage and transportation all limit their feasibility for widespread application.
3. Constructing nanozyme-AIEgen integrated systems
3.1 Modulating the aggregation state of AIEgens through nanozyme catalysis
The interaction between nanozymes and AIEgens can be complex and multifaceted. On the one hand, the structure of AIEgens may change during the nanozyme-mediated catalytic process, thus altering their aggregation state and leading to changes in luminescence properties.89 For instance, Xia et al. developed a Tyr-based TPE derivative, named TT, where TPE functions as the luminescent core, and two tyrosine residues serve as enzyme-like active sites and emission mediators.90 As presented in Fig. 6A, in inflammatory cells with high levels of H2O2 and overexpressed myeloperoxidase (MPO), the TT probe self-assembles through tyrosine cross-linking, forming hydrophobic aggregates that activate AIE, enabling differentiation between inflammatory and normal cells. Furthermore, they integrated enzyme-responsive AIEgens with linkage properties into nano-channels.93 When cervical cancer cells (HeLa) release H2O2, Tyr-containing AIEgens (TT) cross-link within the nano-channels under enzyme-like catalytic modulation, blocking the channels and modifying the ionic current signal. Combined with the fluorescence signal generated by TT aggregation, it successfully achieved in situ dual-signal detection of H2O2. Although this method has demonstrated significant advantages in dual-mode detection, the complex enzyme-responsive process and multi-step self-assembly mechanism may limit its simplicity and operability in practical applications.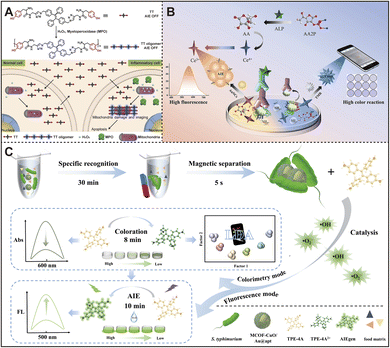 | ||
| Fig. 6 (A) Enzyme-motivated cross-linking aggregation of AIEgens for selective visualization and inhibition of inflammatory cells for precise detection and treatment. Reproduced from ref. 90 with permission from John Wiley and Sons, copyright 2018. (B) Dual-mode ochratoxin A immunoassay based on Ce4+-induced oxidation of TMB and Ce3+-induced aggregation of AuNCs. Reproduced from ref. 91 with permission from Elsevier, copyright 2022. (C) Colorimetric/fluorescence dual-mode detection of S. typhimurium based on MCOF-CuO/Au@apt multifunctional nanozymes catalyzing the oxidation of AIEgens (TPE-4A). Reproduced from ref. 92 with permission from Elsevier, copyright 2024. | ||
On the other hand, inorganic materials such as gold nanoclusters (AuNCs) have also attracted widespread attention in the development of AIEgens due to their exceptional fluorescence characteristics, biocompatibility, ease of synthesis, high quantum yield, and robust colloidal stability.91,94–97 Recently, Zhou et al. devised a dual-signal immunoassay for ochratoxin A (OTA) detection, leveraging Ce4+-induced oxidation of TMB and Ce3+-induced aggregation and emission enhancement of AuNCs.91 In this method, as shown in Fig. 6B, alkaline phosphatase (ALP) catalyzes the dephosphorylation of L-ascorbic acid-2-phosphate (AAP) to produce ascorbic acid (AA), which reduces Ce4+ to Ce3+, inducing an AIE effect on AuNCs and enhancing their fluorescence. Simultaneously, unreacted Ce4+ oxidizes TMB to form blue oxTMB. This multi-signal detection strategy leverages the AIE effect of AuNCs and the enzymatic properties of Ce4+, significantly enhancing the sensitivity of detection. However, it is important to note whether the aggregation and luminescence enhancement of AuNCs are susceptible to potential interferences.
In addition, the catalytic products of nanozymes, especially certain fluorescent products, may also quench the luminescence of AIEgens through the internal filtering effect (IFE).98,99 Li's group found that hemin/G-quadruplex with peroxidase-like properties can catalyze the generation of fluorescent dopachrome from dopamine, which quenches the fluorescence of AIEgens (TPE-BTD) through the IFE.100 Based on this, they proposed a TPE-BTD/dopamine system for the sensitive detection of various biomarkers, including H2O2 and G-quadruplex DNA. Eventually, a paper-based biosensor was developed, utilizing TPE-BTD as an emitter. The TPE-BTD has excellent emission characteristics that enable the sensor to detect a wide range of biomarkers in a reliable, device-free manner for visual identification. This development establishes a new platform for the practical implementation and advancement of biosensing technology. Although this development provides a new platform for device-free detection, given that a variety of substances produced during the nanozyme catalytic process can affect the luminescence of AIEgens, identifying the specific factors leading to the quenching of AIEgens in practical applications may become complex and challenging. Overall, the interaction between nanozymes and AIEgens offers a promising platform for multi-signal detection and biosensing. However, its practical application still faces several challenges. Future research should focus on enhancing the stability, sensitivity, and operability of this system within complex practical environments.
3.2 Utilizing AIEgens as substrates oxidized by nanozymes
AIEgens can be used as enzymatic substrates for nanozymes that undergo a chemical reaction that results in the breaking or formation of bonds within the AIEgens, thereby affecting the structure and luminescence properties of the AIEgens. This property can be used to develop biosensors to monitor specific analytes by detecting changes in luminescence when AIEgens react with nanozymes. Tang et al. were the first to propose and validate this concept. They developed several novel AIEgens to replace color-developing reagents in traditional ELISA experiments.101 When H2O2 is present, horseradish peroxidase (HRP) catalyzes the oxidation of TPE-4A, enhancing its light-absorbing properties. At the end of the reaction, a small amount of deionized water is added to allow unreacted AIEgens to precipitate and emit light due to the restriction of intramolecular motion (RIM).Adhering to this approach, TPE-4A can serve as a substrate for enzymatic reactions, generating color/fluorescence signals through nanozymatic catalytic oxidation.92,102 For instance, Wang et al. engineered a multifunctional nanohybrid, a magnetic covalent organic framework (MCOF) functionalized with aptamers and supported by copper oxide and gold nanoparticles (MCOF-CuO/Au@apt), which exhibits strong magnetic separation capabilities, high oxidase-mimicking activity towards TPE-4A, and high recognition abilities. This system was employed for colorimetric/fluorescent dual-mode sensing of Salmonella typhimurium (S. typhimurium).92 As illustrated in Fig. 6C, the nanohybrids' oxidase mimetic activity diminished when bound to MCOF-CuO/Au@apt S. typhimurium, resulting in reduced colorimetric and fluorescent signals due to TPE-4A oxidation, which led to the establishment of a dual-mode detection method. The results showed that the detection ranges for S. typhimurium were 102–106 CFU mL−1 and 101–106 CFU mL−1 in colorimetric and fluorescence detection modes, respectively, with limits of 7.6 CFU mL−1 and 2.1 CFU mL−1. In addition, by combining a smartphone and an analytical system, on-site portable detection was finally realized, while the method was also validated for reagent sample detection. The use of TPE-4A, which possesses catalytic color development and aggregation-induced emission characteristics, as a signal output instead of conventional display reagents brings about benefits such as reduced background noise and dual-reading capability. These advantages help to improve the accuracy of analysis and broaden the application areas of biosensing technology. Nevertheless, this approach faces several technical challenges. For instance, while TPE-4A reduces background noise through catalytic color development and aggregation-induced emission, its chemical stability in complex biological systems remains uncertain. Additionally, although this study demonstrated the system's efficacy under laboratory conditions, its application to real biological samples may introduce potential errors and limitations that must be addressed.
4. Applications of nanozymes with AIE
4.1 Tumor imaging and therapy
During tumor development, hypoxia is a key pathological feature that promotes tumor growth and invasion while also diminishing the effectiveness of treatments like PDT. PDT relies on oxygen to generate ROS capable of killing tumor cells, but low oxygen levels in the tumor microenvironment (TME) hinder this process. To address this challenge, nanozymes have been proposed as a new strategy. These nanozymes, particularly those with catalase-like activity, can decompose hydrogen peroxide under acidic conditions to release oxygen, alleviating tumor hypoxia and enhancing PDT efficacy. AIEgens are well-known for their unique fluorescence and photosensitive properties and can be engineered to efficiently produce ROS under specific conditions. When combined with the oxygen-producing capabilities of nanozymes, they create an ideal platform for tumor therapy. This composite material has shown significant synergistic effects in cancer treatment, offering precise tumor imaging and enhanced PDT efficacy through the oxygen generated by nanozymes, making tumor treatment more effective.80,85,86In this regard, Tang et al. developed platelet-mimicking MnO2 nanozyme-AIEgen composites (PMD) for the interventional PDT of hypoxic tumors.103 As shown in Fig. 7A, these biomimetic nanoparticles demonstrated robust stability and tumor targeting capabilities. Additionally, their catalase-like activity enables oxygen generation in the tumor microenvironment. Significantly, interventional PDT using optical fibers in the peritoneal cavity of orthotopic colon tumors in mice achieved favorable photodynamic therapeutic outcomes. The combination of AIEgen-based nanozymes with biomimetic cell membrane coatings represents an optimal therapeutic platform for targeted antitumor photodynamic therapy. Meanwhile, Zhang et al. constructed a tumor exosome-loaded CuSAZ/AIEgen cascade catalytic system (CCS) for sustained production of hydroxyl radicals and effective tumor treatment.104 The CCS fulfills the need for enhanced and sustained ·OH generation, addressing the issue of insufficient hydrogen peroxide in single-atom nanozyme (SAZ)-catalyzed therapies, and holds promise for clinical applications. Overall, AIEzymes provide new ideas and research directions for the future development of tumor therapy.
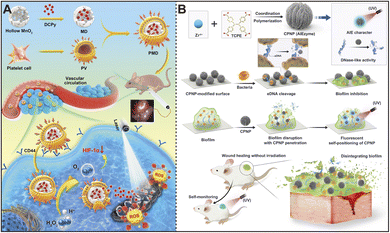 | ||
| Fig. 7 (A) Principles of interventional photodynamic therapy for colon cancer using AIEgen-based bionic nanozymes. Reproduced from ref. 103 with permission from American Chemical Society, copyright 2022. (B) Coordination polymer nanoparticles (CPNPs) with AIEzyme properties are employed in combating biofilms. Reproduced from ref. 12 with permission from John Wiley and Sons, copyright 2023. | ||
In order to enhance the therapeutic effect, researchers have adopted a multi-therapy synergistic strategy, which achieves a significant increase in therapeutic efficacy by combining two or more different therapeutic modalities. For example, Xia et al. developed a GSH-responsive MnO2 nanosheet combining an AIE-active photosensitizer and a DNAzyme.105 This composite is capable of not only killing tumor cells through PDT, but also intervening in tumor cells at the gene level through gene therapy, thus achieving dual therapy and significantly improving the therapeutic effect. Similarly, Zhang and colleagues used UiO-66 as a carrier, loaded AIEgens to form A-NUiO, and further encapsulated it in copper-doped hydrated ZIF-8 (ZIF-Cu) to create A-NUiO@DCDA@ZIF-Cu. This material can efficiently accumulate in the tumor microenvironment and exhibit fluorescence imaging capabilities, along with the combined effects of PDT and chemodynamic therapy (CDT), providing a new strategy for cancer treatment.106 Although the multiple therapies introduced above theoretically demonstrate promise in enhancing treatment outcomes, their therapeutic specificity and potential side effects still require thorough research to ensure the safety and effectiveness of their clinical applications.
As a result, the integration of AIEgens and nanozymes offers a novel approach to tumor imaging and therapy, particularly in enhancing the efficacy and targeting of PDT. This combination has demonstrated significant potential. However, ensuring the clinical safety and effectiveness of these technologies remains a key focus for future research.
4.2 Anti-biofilm and antibacterial applications
In addition to their application in tumor treatment, AIEzymes demonstrate significant potential in combating biofilms and bacterial infections.107–109 These nanomaterials harness their unique optical and catalytic properties to decompose hydrogen peroxide, producing oxygen in the process.110,111 This helps alleviate the hypoxic conditions typically found within bacterial biofilms or infected areas, which often reduce the effectiveness of conventional antibiotics.112 The oxygen generated by AIEzymes not only enhances the potency of antibacterial agents but also activates oxygen-dependent bactericidal mechanisms, further improving their ability to break down stubborn biofilms. Moreover, the high photostability and low background noise of AIEzymes enable precise real-time monitoring in complex biological environments, making them a promising tool for targeted antibacterial therapy.With this regard, Liu and his team further advanced the application of AIEzymes in bacterial detection by developing a chemiluminescence-based system that does not require hydrogen peroxide or an external light source.59 This research not only holds significance for antibacterial therapy but also broadens the practical applications of AIEzymes. The newly developed AIEzyme can catalyze luminol (LUM) to emit blue luminescence at 425 nm in the absence of hydrogen peroxide (H2O2). Upon incubation with E. coli and lysozymes, the adenosine triphosphate (ATP) released from bacterial lysis facilitates the dissolution of ZIF-8 nanoparticles. This dissolution, in turn, releases fluorescent molecules (FL), which leads to an enhancement of the fluorescence signal. Additionally, through the mechanism of chemiluminescence resonance energy transfer (CRET), the chemiluminescence of LUM is diminished, enabling ratiometric bacterial detection. This system boasts long-lasting luminescence, making it particularly suitable for point-of-care testing (POCT), while also improving detection accuracy and ease of use. Notably, compared to traditional single-signal POCT systems, this ratiometric detection method exhibits greater resistance to interference and enhanced visual detection sensitivity. Moreover, the system leverages smartphones as signal readers and analyzers, facilitating on-site quantitative bacterial detection. This innovative detection approach offers new perspectives for the future development of biosensing technologies.
In addition to their various applications, these multifunctional AIEzymes have demonstrated novel antibacterial mechanisms, particularly against resistant biofilms and pathogens. For instance, some nanozymes mimic the activity of DNA-cleaving enzymes.113,114 Han's research team developed an AIE nanomaterial with DNase-like properties, specifically utilizing zirconium-based coordination polymer nanoparticles.12 These nanoparticles possess key advantages such as low activation energy, robust structure, and stable fluorescence. As depicted in Fig. 7B, AIEzymes effectively hydrolyze extracellular DNA within biofilms, disrupting their structure and exhibiting significant penetration capabilities. Remarkably, a single dose of this AIEzyme was sufficient to accelerate the healing of wounds infected by superbugs. This study introduces a method of endowing AIEgens with DNase-like activity and anti-biofilm effects, significantly enhancing their therapeutic value. Moreover, the inherent AIE properties of these nanozymes facilitate easy visualization, enabling researchers and clinicians to track their location and persistence in the wound healing process. This dual functionality, both as a treatment and a diagnostic tool, makes AIEzymes a promising innovation in combating drug-resistant infections and promoting tissue regeneration.
4.3 Food safety analysis
Advancing precise detection methods is crucial for ensuring food safety and protecting consumer health. Sheng's team has made significant strides in creating AIEgen-nanozyme composites and is actively exploring their potential in food analysis. They coated TPE with PtPd NPs to create a fluorescent nanozyme (PtPd NPs@TPE) with oxidase-like activity.77 This nanozyme can monitor putrescine (PUT) through ratio fluorescence, enabling accurate assessment of the freshness and authenticity of soy products. In their design, PUT acted as an antioxidant that inhibited the PtPd NPs@TPE-catalysed oxidation of OPD, resulting in a reduction of the oxidation product DAP, which led to a decrease in the yellow fluorescence of DAP at 552 nm and a brightening of the blue fluorescence of PtPd NPs@TPE at 442 nm. Building on this principle, they further developed a ratio fluorescence strategy in collaboration with smartphone sensors, which has yielded promising results in combating food preservation fraud in soy products.Recently, they developed a dual-mode biosensor that relies on smartphone readout processing for the detection of the bioactive component hypoxanthine (Hx) in food.115 As shown in Fig. 8A, the core of this sensor is a multi-enzyme cascade catalytic system that includes amorphous Fe-doped phosphotungstate nanozyme Fe-Phos@TPE, xanthine oxidase (XOD), and a dual chromogenic substrate OPD. When Hx is present, XOD catalyzes the production of H2O2, activating the peroxidase activity of Fe-Phos@TPE to generate hydroxyl radicals, which further oxidize non-fluorescent OPD to form yellow fluorescent DAP. During this process, the intrinsic blue fluorescence of Fe-Phos@TPE at 440 nm is attenuated due to the internal filter effect of DAP, enabling ratiometric fluorescence analysis. Additionally, OPD is transformed into dark yellow DAP with enhanced absorbance at 435 nm, enabling a colorimetric analysis. By leveraging smartphones, the sensor is capable of outputting Hx-dependent ratiometric fluorescence and colorimetric images, achieving simple, rapid, portable, and on-site intelligent detection. Moreover, the nanozyme Fe-Phos@TPE and XOD in this system exhibit optimal catalytic activity at near-neutral pH 6.5, avoiding the potential effects of acidic conditions on food components, making this method more advantageous in practical applications.
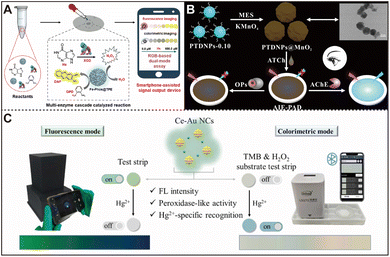 | ||
| Fig. 8 (A) Diagram of the smartphone-assisted Hx-activated multienzyme cascade biosensor for on-site visual fluorescence and colorimetric dual-mode detection. Reproduced from ref. 115 with permission from Elsevier, copyright 2024. (B) Scheme of PTDNPs@MnO2 preparation and its application in an AIE-PAD for AChE and OP sensing. Reproduced from ref. 78 with permission from Elsevier, copyright 2021. (C) Illustration of Ce-Au NC fabrication and use in a fluorescence and colorimetric dual-mode paper-based sensor for Hg2+ detection. Reproduced from ref. 116 with permission from Elsevier, copyright 2024. | ||
Despite the sensor system demonstrating good detection performance, it still faces technical challenges. For instance, while Fe-Phos@TPE and XOD exhibit optimal catalytic activity at near-neutral pH 6.5, avoiding potential damage to food components from acidic conditions, the stability and anti-interference capabilities of the system in complex food matrices still need to be further validated. Moreover, the sensor relies on a multi-enzyme catalytic system, which increases operational complexity and may pose limitations on its application. Therefore, future research should aim to further simplify the system design while ensuring the detection sensitivity and specificity, in order to enhance its practicality and operability.
4.4 Environmental pollutant monitoring
The widespread use of pesticides has played a crucial role in boosting crop yields, but their misuse poses a severe threat to the environment. Therefore, the rapid and accurate detection of pesticide residues has become an urgent issue that needs to be addressed. In recent years, some researchers have actively developed sensing technologies based on AIEzymes, aiming to achieve efficient detection of pesticide residues.117 Chi et al. developed a novel fluorescence-based paper analyzer device (PAD) for intuitive, instrumentation-free detection of organophosphorus pesticides (OPs) by combining the brightness emission properties of AIEgens in the aggerate state, the specific response of MnO2 to thiol compounds, and the difference in the fluorescence quenching effect between MnO2 and Mn2+.78 As shown in Fig. 8B, the core and shell of the nanoparticle are AIE nanoparticles PTDNPs-0.10 and MnO2, respectively, which form a PTDNPs@MnO2 composite with high stability and can be dropwise-added to cellulose paper to construct the AIE-PAD. The principle of the assay is that the OP-treated acetylcholinesterase (AChE) prevents the production of thiocholine, which reduces MnO2 to Mn2+, which in turn changes the signal output. As a result, the technique can intuitively detect OPs without any equipment, with a detection limit of 1.60 ng mL−1. This study illustrates the development of advanced sensors using nanozyme-AIEgen core–shell materials and the potential to replace complex, costly solution-phase sensors with PADs, offering a new direction for in situ OP detection.Building on this foundation, Han et al. further developed a bifunctional AIEzyme that combines the organophosphorus hydrolase (OPH)-like catalytic properties and fluorescence properties of AIE materials for the detection of nerve agents and organophosphorus pesticides.118 This AIEzyme capable of fluorescence and colorimetric detection without ROS production ensures system stability and enables specific, self-reporting detection. This dual-mode method enhances efficiency and detection scope, advancing AIEzymes for environmental monitoring. Separately, Feng's team synthesized a bright red-orange fluorescent probe, CeO2@PDA@AuNCs-MIPs, with high phosphatase-like activity for methyl paraoxon detection.119 Its hollow structure and MIPs enhance activity and specificity, while Ce(III) boosts fluorescence by inducing the AIE of AuNCs, offering exceptional analytical performance suitable for on-site testing. This nanozyme-based fluorescent assay demonstrates exceptional analytical performance with a wide linear range and a low detection limit, making it suitable for on-site testing and showcasing its promising practical application capabilities.
Similarly, heavy metal pollution, especially Hg2+, poses a serious threat to the natural environment and human health. Despite the potential of gold nanoclusters (Au NCs) as fluorescent probes or chromogenic nanozymes in Hg2+ detection, the development of fluorescent nanozymes with integrated multifunctionality remains a technical challenge. In this regard, You et al. innovatively reported Ce-alloyed gold nanoclusters (Ce-Au NCs), which combine three functions: intense fluorescence emission, excellent peroxidase activity, and highly specific recognition of Hg2+.116 Using Ce-Au NCs, they developed a portable dual-mode sensing device that combines fluorescence and colorimetric detection techniques, which is suitable for rapid and intuitive analysis of Hg2+ in the field. As shown in Fig. 8C, in the presence of Hg2+, the quenching of the fluorescence signal and the change in the color of the paper-based chip (from green to black) provide dual validation of the detection, which significantly improves the reliability and accuracy of the assay. The study introduces a simple synthesis method for multifunctional fluorescent nanozymes and presents the significant potential of the resulting portable detection device for rapid environmental heavy metal ion analysis, inspiring further research and assay diversification. However, their stability in complex environments, detection sensitivity, and cost-effectiveness still need to be further optimized.
5. Summary and prospects
Since nanozymes and AIE were first reported, their development in their respective fields has been remarkable. The combination of these technologies has resulted in a diverse array of novel applications, leveraging the complementary strengths of nanozymes and AIE materials. Nanozymes with AIE properties not only exhibit the advantages of nanozymes, such as low cost, stability, and ease of regulation, but also offer excellent biocompatibility, superior fluorescence properties, and simple preparation and modification of AIEgens. These properties help endow the nanozymes with more functionalities, improve the efficiency of photodynamic therapy and photothermal therapy, enable efficient photoacoustic imaging, and facilitate multimodal analytical sensing. To date, significant breakthroughs and developments have been made in nanozymes with AIE properties. However, there are still some challenges and opportunities that need to be addressed:(1) Relatively few AIE molecules with enzyme-like properties. In recent years, AIEgens with high luminescence and efficient generation of ROS under light excitation have found extensive application in antimicrobial, tumor imaging, photodynamic therapy, and biomedical engineering. In the study by Liu et al., a novel AIE molecule was designed, synthesized, and evaluated for its enzyme-like properties.59 This type of AIEzyme is simple in structure, easy to synthesize, and highly designable, and has great potential in practical applications. However, the discovery and utilization of AIEgens with enzyme-like properties are limited, largely due to the complexity and subtlety of natural enzyme catalysis, which poses challenges in the design and synthesis of AIEgens. The development of new AIE molecules with enzyme-like activities necessitates a profound understanding of AIE and enzyme catalysis mechanisms, as well as innovative molecular design and synthesis approaches. Therefore, future research should focus on a deep understanding of the molecular mechanisms of AIE and enzyme catalysis processes and the design and synthesis of AIE molecules with stronger enzyme activity.
(2) The mechanism of the interaction between nanozymes and AIEgens is uncertain. AIEgens usually exhibit enhanced luminescence properties in the aggregated state, which may be intrinsically related to the catalytic activity of many nanozymes. When integrating nanozymes with AIEgens, the mechanism of their interaction is a complex issue that has not been fully elucidated in current studies. Most of the current AIEzyme compounds are obtained by some simple methods such as direct encapsulation, surface modification, and co-loading on other carriers. Although the AIEzyme compounds thus obtained can exhibit their respective characteristics, different interactions and spatial arrangements will affect the catalytic activity and AIE properties of the materials. Therefore, future research needs to further explore the relationship between the structure and performance of AIEzymes and use them to guide the design of novel AIEzymes with multifunctionality in the future.
(3) The resolution of AIEzymes needs enhancement. The fluorescence color emitted by most existing AIEzymes is dominated by blue and green, which has limitations in imaging applications, especially in the analysis of deep tissues in vivo. Consequently, expanding the fluorescence wavelength range of AIEzymes into the near-infrared (NIR) spectrum is expected to address these limitations. NIR-emitting AIEzymes, with their superior tissue penetration and reduced cellular background interference, hold promise as practical and high-resolution tools for in situ living cell imaging.
(4) Practical application challenges with false positives. Prior to activation, AIEzymes may exhibit self-aggregation and be susceptible to environmental factors, potentially leading to unintended interfering signals and elevated background noise, thereby diminishing detection sensitivity. To address this issue, it is necessary to consider more comprehensively the influence of environmental factors on the detection results and to design AIEzymes capable of avoiding self-aggregation prior to enzyme activation. With such a strategy, when applying AIEzymes for imaging or detection, false-positive signals can be effectively reduced, the specificity and sensitivity of detection can be improved, and more reliable results can be obtained.
(5) Biocompatibility and toxicity assessment. The biocompatibility and potential toxicity of AIEzymes are issues that need to be carefully considered when applying them in the fields of medical diagnosis, cellular imaging and biosensing. Current studies have shown that most AIEzymes show good biocompatibility during assays, but their long-term presence in the cellular microenvironment, degradation after participation in cellular metabolism, and possible cytotoxicity are still not fully understood. Therefore, future research should establish more comprehensive animal models for long-term biosafety assessments to ensure their safety in clinical applications.
(6) Continuously expanding the range of applications. Currently, AIEzymes reported in the literature are predominantly employed in biosensing, disease diagnosis, and antibacterial applications, using their high sensitivity, selectivity, and visual tracking capabilities. However, their application potential extends far beyond these areas. Research indicates growing interest in employing AIEzymes for environmental and food safety assessments, such as detecting pollutants and monitoring harmful substances in food. In addition, with advances in materials science and nanotechnology, AIEzymes may be further optimized for a wider range of applications. It is foreseeable that in the future, AIEzymes will show their unique value and potential in more fields and become a new focus of research and application.
Overall, this review outlines effective approaches to integrating nanozymes with AIE, surveys the latest advances in this research field, analyzes the current problems faced by AIEzymes, and discusses possible strategies for their solution. At the same time, we also predict the future development trend. By summarizing and analyzing the latest research results in this field, we help researchers understand the potentials and limitations of AIEzymes, and at the same time provide guidance for the rational design and development of AIEzymes for biosensing, disease diagnosis, and disease therapy. We expect that this review will stimulate the interest of more researchers and attract them to join this promising field, in order to promote the wide application of AIEzyme technology in clinical therapy and practical applications.
Abbreviation
| AA | Ascorbic acid |
| AAP | Ascorbic acid-2-phosphate |
| AChE | Acetylcholinesterase |
| AIE | Aggregation-induced emission |
| AI-ECL | Aggregation-induced electrochemiluminescence |
| AIEgens | AIE luminogens |
| ALP | Alkaline phosphatase |
| AOX | Ethanol oxidase |
| ARC | AIEgen reservoir and release controller |
| ATP | Adenosine triphosphate |
| CAP | Chloramphenicol |
| CAT | Catalase |
| CCS | Cascade catalytic system |
| CDT | Chemodynamic therapy |
| CFX | Ciprofloxacin |
| CL | Chemiluminescence |
| COFs | Covalent organic frameworks |
| CRET | Chemiluminescence resonance energy transfer |
| DAP | 2,3-diaminophenazine |
| ESR | Electron spin resonance |
| HCC | Hepatocellular carcinoma |
| HRP | Horseradish peroxidase |
| IFE | Internal filtering effect |
| LUM | Luminol |
| MCOF | Magnetic covalent organic framework |
| MIPs | Molecularly imprinted polymers |
| MPO | Myeloperoxidase |
| NSs | Nanosheets |
| OMCs | Organic molecular cages |
| OPD | O-phenylenediamine |
| OPs | Organophosphorus pesticides |
| OTA | Ochratoxin A |
| OXD | Oxidase |
| PB | Prussian blue |
| PDT | Photodynamic therapy |
| PET | Photoinduced electron transfer |
| POCT | Point-of-care testing |
| POD | Peroxidase |
| RIM | Intramolecular motion |
| ROS | Reactive oxygen species |
| SAZ | Single-atom nanozymes |
| SOD | Superoxide dismutase |
| TME | Tumor microenvironment |
| TPE | Tetraphenylethylene |
| ZnDPA | Zn coordinated dipicolylamine |
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
All of the authors contributed to the manuscript preparation. X. L., J. H. and J. C. conceived the outline of the manuscript. X. L. wrote the original draft of the manuscript. Z. W., H. M. and H. Q. discussed and helped revise the manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors appreciate the support from the National Natural Science Foundation of China (22404170), the CAS Special Research Assistantship Program (E30480YZ), the Cooperation Fund of Lanzhou Institute of Chemical Physics Young Scientists Collaborative Innovation Alliance Program (HZJJ23-4), and the Gansu Science and Technology Program (24JRRA073).References
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741, 10.1039/B105159H
.
- H. Wang, Q. Li, P. Alam, H. Bai, V. Bhalla, M. R. Bryce, M. Cao, C. Chen, S. Chen, X. Chen, Y. Chen, Z. Chen, D. Dang, D. Ding, S. Ding, Y. Duo, M. Gao, W. He, X. He, X. Hong, Y. Hong, J. J. Hu, R. Hu, X. Huang, T. D. James, X. Jiang, G. I. Konishi, R. T. K. Kwok, J. W. Y. Lam, C. Li, H. Li, K. Li, N. Li, W. J. Li, Y. Li, X. J. Liang, Y. Liang, B. Liu, G. Liu, X. Liu, X. Lou, X. Y. Lou, L. Luo, P. R. McGonigal, Z. W. Mao, G. Niu, T. C. Owyong, A. Pucci, J. Qian, A. Qin, Z. Qiu, A. L. Rogach, B. Situ, K. Tanaka, Y. Tang, B. Wang, D. Wang, J. Wang, W. Wang, W. X. Wang, W. J. Wang, X. Wang, Y. F. Wang, S. Wu, Y. Wu, Y. Xiong, R. Xu, C. Yan, S. Yan, H. B. Yang, L. L. Yang, M. Yang, Y. W. Yang, J. Yoon, S. Q. Zang, J. Zhang, P. Zhang, T. Zhang, X. Zhang, X. Zhang, N. Zhao, Z. Zhao, J. Zheng, L. Zheng, Z. Zheng, M. Q. Zhu, W. H. Zhu, H. Zou and B. Z. Tang, ACS Nano, 2023, 17, 14347–14405 CrossRef CAS
.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS
.
- G. G. Stokes, Philos. Trans. R. Soc. London, 1997, 143, 385–396 Search PubMed
.
- A. Shao, Y. Xie, S. Zhu, Z. Guo, S. Zhu, J. Guo, P. Shi, T. D. James, H. Tian and W. H. Zhu, Angew. Chem., Int. Ed., 2015, 54, 7275–7280 CrossRef CAS PubMed
.
- H. Lu, Y. Zheng, X. Zhao, L. Wang, S. Ma, X. Han, B. Xu, W. Tian and H. Gao, Angew. Chem., Int. Ed., 2016, 55, 155–159 CrossRef CAS PubMed
.
- Y. Chen, W. Zhang, Z. Zhao, Y. Cai, J. Gong, R. T. K. Kwok, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams and B. Z. Tang, Angew. Chem., Int. Ed., 2018, 57, 5011–5015 CrossRef CAS PubMed
.
- C. Zhu, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, ACS Appl. Bio Mater., 2018, 1, 1768–1786 CrossRef CAS PubMed
.
- S. Guo, H. Tang, Y. Zhang, Z. Wang and S. C. Tan, BMEMat, 2024, e12076 CrossRef
.
- H. Yu, B. Chen, H. Huang, Z. He, J. Sun, G. Wang, X. Gu and B. Z. Tang, Biosensors, 2022, 12, 348 CrossRef CAS PubMed
.
- R. Qu, X. Zhen and X. Jiang, CCS Chem., 2021, 4, 401–419 CrossRef
.
- L. Han, Y. Zhang, B. Huang, X. Bian and B. Z. Tang, Aggregate, 2023, 4, e360 CrossRef CAS
.
- J. P. Richard, Biochemistry, 2013, 52, 2009–2011 CrossRef CAS PubMed
.
- R. Breslow and L. E. Overman, J. Am. Chem. Soc., 1970, 92, 1075–1077 CrossRef CAS PubMed
.
- F. Manea, F. B. Houillon, L. Pasquato and P. Scrimin, Angew. Chem., Int. Ed., 2004, 43, 6165–6169 CrossRef CAS
.
- G. Tang, J. He, J. Liu, X. Yan and K. Fan, Exploration, 2021, 1, 75–89 CrossRef
.
- B. Hauer, ACS Catal., 2020, 10, 8418–8427 CrossRef CAS
.
- D. Yi, T. Bayer, C. P. S. Badenhorst, S. Wu, M. Doerr, M. Höhne and U. T. Bornscheuer, Chem. Soc. Rev., 2021, 50, 8003–8049 RSC
.
- Y. Murakami, J. I. Kikuchi, Y. Hisaeda and O. Hayashida, Chem. Rev., 1996, 96, 721–758 CrossRef CAS
.
- C. M. Pedersen and M. Bols, Org. Synth. Mol. Eng., 2013, 305–332 Search PubMed
.
- H. Wang, K. Wan and X. Shi, Adv. Mater., 2019, 31, 1805368 CrossRef CAS
.
- R. Breslow, Acc. Chem. Res., 1995, 28, 146–153 CrossRef CAS
.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS
.
- K. Fan, L. Gao, H. Wei, B. Jiang, D. Wang, R. Zhang, J. He, X. Meng, Z. Wang, H. Fan, T. Wen, D. Duan, L. Chen, W. Jiang, Y. Lu, B. Jiang, Y. Wei, W. Li, Y. Yuan, H. Dong, L. Zhang, C. Hong, Z. Zhang, M. Cheng, X. Geng, T. Hou, Y. Hou, J. Li, G. Tang, Y. Zhao, H. Zhao, S. Zhang, J. Xie, Z. Zhou, J. Ren, X. Huang, X. Gao, M. Liang, Y. Zhang, H. Xu, X. Qu and X. Yan, Prog. Chem., 2023, 35, 1–87 CAS
.
- M. Zandieh and J. Liu, ACS Nano, 2021, 15, 15645–15655 CrossRef CAS PubMed
.
- J. Qin, N. Guo, J. Yang and J. Wei, Food Chem., 2024, 447, 139019 CrossRef CAS PubMed
.
- I. Chandio, Y. Ai, L. Wu and Q. Liang, Nano Res., 2024, 17, 39–64 CrossRef
.
- H. Sun, Y. Zhou, J. Ren and X. Qu, Angew. Chem., Int. Ed., 2018, 57, 9224–9237 CrossRef CAS PubMed
.
- J. Chen, Y. Liu, Z. Long, Y. Li and H. Qiu, Chin. Chem. Lett., 2024, 35, 109463 CrossRef CAS
.
- S. Luo, M. Sha, F. Tian, X. Li, L. Fu, Y. Gu, L. L. Qu, G. H. Yang and C. Zhu, Chin. Chem. Lett., 2022, 33, 344–348 CrossRef CAS
.
- X. Chen, L. Zhao, K. Wu, H. Yang, Q. Zhou, Y. Xu, Y. Zheng, Y. Shen, S. Liu and Y. Zhang, Chem. Sci., 2021, 12, 8865–8871 RSC
.
- X. Lu, S. Gao, H. Lin, H. Tian, D. Xu and J. Shi, Natl. Sci. Rev., 2022, 9, nwac022 CrossRef CAS
.
- X. Cao, C. Zhu, Q. Hong, X. Chen, K. Wang, Y. Shen, S. Liu and Y. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202302463 CrossRef CAS
.
- S. Cao, Z. Zhao, Y. Zheng, Z. Wu, T. Ma, B. Zhu, C. Yang, X. Xiang, L. Ma, X. Han, Y. Wang, Q. Guo, L. Qiu and C. Cheng, Adv. Mater., 2022, 34, 2200255 CrossRef CAS PubMed
.
- Y. Xu, Z. Zhou, N. Deng, K. Fu, C. Zhu, Q. Hong, Y. Shen, S. Liu and Y. Zhang, Sci. China: Chem., 2023, 66, 1318–1335 CrossRef CAS
.
- S. V. Somerville, Q. Li, J. Wordsworth, S. Jamali, M. R. Eskandarian, R. D. Tilley and J. J. Gooding, Adv. Mater., 2024, 36, 2211288 CrossRef CAS PubMed
.
- X. Li, H. Zhu, P. Liu, M. Wang, J. Pan, F. Qiu, L. Ni and X. Niu, TrAC, Trends Anal. Chem., 2021, 143, 116379 CrossRef CAS
.
- Y. Liu, X. Wei, J. Chen, Y. L. Yu, J. H. Wang and H. Qiu, Anal. Chem., 2022, 94, 5970–5979 CrossRef CAS PubMed
.
- J. Hao, C. Zhang, C. Feng, Q. Wang, Z. Y. Liu, Y. Li, J. Mu, E. C. Yang and Y. Wang, Chin. Chem. Lett., 2023, 34, 107650 CrossRef CAS
.
- Y. Wu, W. Chen, C. Wang and D. Xing, Chin. Chem. Lett., 2024, 35, 109096 CrossRef CAS
.
- Y. Xu, Y. Ma, X. Chen, K. Wu, K. Wang, Y. Shen, S. Liu, X. J. Gao and Y. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202408935 CrossRef CAS
.
- H. Wei and E. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC
.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC
.
- L. Yang, H. Guo, T. Hou, B. An and F. Li, Chin. Chem. Lett., 2023, 34, 107607 CrossRef CAS
.
- M. Cui, B. Xu and L. Wang, BMEMat, 2024, 2, e12043 CrossRef
.
- X. Li, J. Liu, J. Chen, H. Qiu and X. Niu, Sens. Diagn., 2023, 2, 307–319 RSC
.
- Y. Ai, Z. N. Hu, X. Liang, H. B. Sun, H. Xin and Q. Liang, Adv. Funct. Mater., 2022, 32, 2110432 CrossRef CAS
.
- R. Liu, F. Shi, Y. Xia, H. Zhu, J. Cao, K. Peng, C. Ren, J. Li and Z. Yang, Chin. Chem. Lett., 2024, 35, 109664 CrossRef CAS
.
- D. Wang, M. M. S. Lee, W. Xu, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Theranostics, 2018, 8, 4925–4956 CrossRef CAS PubMed
.
- Y. Zhang, Y. Huang, R. Miao and H. Chen, Small Struct., 2023, 4, 2300157 CrossRef CAS
.
- Y. Yao, Y. Zhang, C. Yan, W. H. Zhu and Z. Guo, Chem. Sci., 2021, 12, 9885–9894 RSC
.
- C. Liu, J. C. Yang, J. W. Y. Lam, H. T. Feng and B. Z. Tang, Chem. Sci., 2022, 13, 611–632 RSC
.
- Z. Li, B. Z. Tang and D. Wang, Adv. Mater., 2024, 2406047 CrossRef CAS
.
- S. Liu, G. Feng, B. Z. Tang and B. Liu, Chem. Sci., 2021, 12, 6488–6506 RSC
.
- H. Wang, X. Wang, P. Li, M. Dong, S. Q. Yao and B. Tang, Chem. Sci., 2021, 12, 11620–11646 RSC
.
- Q. Wan, R. Zhang, Z. Zhuang, Y. Li, Y. Huang, Z. Wang, W. Zhang, J. Hou and B. Z. Tang, Adv. Funct. Mater., 2020, 30, 2002057 CrossRef CAS
.
- T. Zhou, R. Hu, L. Wang, Y. Qiu, G. Zhang, Q. Deng, H. Zhang, P. Yin, B. Situ, C. Zhan, A. Qin and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 9952–9956 CrossRef CAS PubMed
.
- X. He, Y. Yang, Y. Guo, S. Lu, Y. Du, J. J. Li, X. Zhang, N. L. C. Leung, Z. Zhao, G. Niu, S. Yang, Z. Weng, R. T. K. Kwok, J. W. Y. Lam, G. Xie and B. Z. Tang, J. Am. Chem. Soc., 2020, 142, 3959–3969 CrossRef CAS
.
- H. Wu, Y. Fang, L. Tian, X. Liu, X. Zhou, X. Chen, H. Gao, H. Qin and Y. Liu, ACS Sensors, 2023, 8, 3205–3214 CrossRef CAS
.
- X. Li, B. Liu, K. Ye, L. Ni, X. Xu, F. Qiu, J. Pan and X. Niu, Sens. Actuators, B, 2019, 297, 126822 CrossRef CAS
.
- X. Li, B. Liu, Z. Hu, P. Liu, K. Ye, J. Pan and X. Niu, Environ. Res., 2020, 189, 109921 CrossRef CAS PubMed
.
- K. Fan, J. Xi, L. Fan, P. Wang, C. Zhu, Y. Tang, X. Xu, M. Liang, B. Jiang, X. Yan and L. Gao, Nat. Commun., 2018, 9, 1440 CrossRef
.
- Y. Jiang, X. Zhao, J. Huang, J. Li, P. K. Upputuri, H. Sun, X. Han, M. Pramanik, Y. Miao, H. Duan, K. Pu and R. Zhang, Nat. Commun., 2020, 11, 1857 CrossRef CAS PubMed
.
- Y. Hao, Y. Chen, X. He, Y. Yu, R. Han, Y. Li, C. Yang, D. Hu and Z. Qian, Adv. Sci., 2020, 7, 2001853 CrossRef CAS
.
- X. Li, X. Niu, P. Liu, X. Xu, D. Du and Y. Lin, Sens. Actuators, B, 2020, 321, 128546 CrossRef CAS
.
- X. Li, P. Liu, X. Niu, K. Ye, L. Ni, D. Du, J. Pan and Y. Lin, Nanoscale, 2020, 12, 19383–19389 RSC
.
- J. Han, H. Gong, X. Ren and X. Yan, Nano Today, 2021, 41, 101295 CrossRef CAS
.
- S. Abedanzadeh, Z. Moosavi-Movahedi, N. Sheibani and A. A. Moosavi-Movahedi, Biochem. Eng. J., 2022, 183, 108463 CrossRef CAS
.
- Z. Wang, X. He, T. Yong, Y. Miao, C. Zhang and B. Zhong Tang, J. Am. Chem. Soc., 2020, 142, 512–519 CrossRef CAS PubMed
.
- C. Zhang, Z. Wang, L. Tan, T. L. Zhai, S. Wang, B. Tan, Y. S. Zheng, X. L. Yang and H. B. Xu, Angew. Chem., Int. Ed., 2015, 54, 9244–9248 CrossRef CAS
.
- Z. Wang, B. B. Yang, Z. J. Fang, Q. Ou, H. Ma, Q. P. Zhang, Y. L. Sun and C. Zhang, Chem. Commun., 2021, 57, 11541–11544 RSC
.
- Z. Wei, X. Jing, Y. Yang, J. Yuan, M. Liu, C. He and C. Duan, Angew. Chem., Int. Ed., 2023, 62, e202214577 CrossRef CAS PubMed
.
- R. Kapila, B. Sen, A. Kamra, S. Chandran and S. Rana, Nanoscale, 2023, 15, 14809–14821 RSC
.
- Y. Shen, Y. Wei, X. Gao, C. Nie, J. Wang and Y. Wu, Environ. Sci. Technol., 2023, 57, 1680–1691 CrossRef CAS PubMed
.
- X. Yu, Y. C. Zhang, X. Yang, Z. Huang, T. Zhang, L. Yang, W. Meng, X. Liu, P. Gong, A. Forni, Z. Zheng, B. Liu, P. Zhang, L. Cai and B. Z. Tang, Nano Today, 2022, 44, 101477 CrossRef CAS
.
- S. Zhou, T. Tian, T. Meng, J. Wu, D. Hu, Q. Liao, J. Zhuang, H. Wang and G. Zhang, iScience, 2023, 26, 107348 CrossRef CAS PubMed
.
- P. Nie, X. Gao, X. Yang, Y. Zhang, H. Lu, H. Wang, Z. Zheng and Y. Shen, Food Chem., 2024, 439, 138122 CrossRef CAS PubMed
.
- J. Chen, X. Chen, P. Wang, S. Liu and Z. Chi, J. Hazard. Mater., 2021, 413, 125306 CrossRef CAS
.
- Z. Gao, Y. Li, C. Zhang, S. Zhang, Y. Jia, F. Li, H. Ding, X. Li, Z. Chen and Q. Wei, ACS Appl. Mater. Interfaces, 2019, 11, 12335–12341 CrossRef CAS PubMed
.
- J. Zheng, T. Chen, K. Wang, C. Peng, M. Zhao, Q. Xie, B. Li, H. Lin, Z. Zhao, Z. Ji, B. Z. Tang and Y. Liao, ACS Nano, 2024, 18, 2355–2369 CrossRef CAS PubMed
.
- S. Dalapati, E. Jin, M. Addicoat, T. Heine and D. Jiang, J. Am. Chem. Soc., 2016, 138, 5797–5800 CrossRef CAS PubMed
.
- H. Ding, J. Li, G. Xie, G. Lin, R. Chen, Z. Peng, C. Yang, B. Wang, J. Sun and C. Wang, Nat. Commun., 2018, 9, 5234 CrossRef CAS PubMed
.
- J. You, F. Yuan, S. Cheng, Q. Kong, Y. Jiang, X. Luo, Y. Xian and C. Zhang, Chem. Mater., 2022, 34, 7078–7089 CrossRef CAS
.
- S. Li, X. Ma, C. Pang, M. Wang, G. Yin, Z. Xu, J. Li and J. Luo, Biosens. Bioelectron., 2021, 176, 112944 CrossRef CAS
.
- D. Zhu, Z. Zheng, G. Luo, M. Suo, X. Li, Y. Duo and B. Z. Tang, Nano Today, 2021, 37, 101091 CrossRef CAS
.
- F. Gao, J. Wu, H. Gao, X. Hu, L. Liu, A. C. Midgley, Q. Liu, Z. Sun, Y. Liu, D. Ding, Y. Wang, D. Kong and X. Huang, Biomaterials, 2020, 230, 119635 CrossRef CAS PubMed
.
- S. Li, C. Pang, X. Ma, Y. Wu, M. Wang, Z. Xu and J. Luo, Microchem. J., 2022, 178, 107345 CrossRef CAS
.
- M. Qiu, D. Wang, W. Liang, L. Liu, Y. Zhang, X. Chen, D. K. Sang, C. Xing, Z. Li, B. Dong, F. Xing, D. Fan, S. Bao, H. Zhang and Y. Cao, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 501–506 CrossRef CAS
.
- L. Dai, W. Mao, L. Hu, J. Song, Y. Zhang, T. Huang and M. Wang, Dyes Pigm., 2022, 204, 110436 CrossRef CAS
.
- Y. Cheng, J. Dai, C. Sun, R. Liu, T. Zhai, X. Lou and F. Xia, Angew. Chem., Int. Ed., 2018, 57, 3123–3127 CrossRef CAS
.
- W. Chen, X. Zhang, Q. Zhang, G. Zhang, S. Wu, H. Yang and Y. Zhou, Anal. Chim. Acta, 2022, 1231, 340445 CrossRef CAS PubMed
.
- H. Li, H. Xu, X. Shi, C. Zhao, J. Li and J. Wang, Food Chem., 2024, 139220 CrossRef CAS PubMed
.
- X. Lou, Y. Song, R. Liu, Y. Cheng, J. Dai, Q. Chen, P. Gao, Z. Zhao and F. Xia, Small Methods, 2020, 4, 1900432 CrossRef CAS
.
- H. P. Peng, M. L. Jian, Z. N. Huang, W. J. Wang, H. H. Deng, W. H. Wu, A. L. Liu, X. H. Xia and W. Chen, Biosens. Bioelectron., 2018, 105, 71–76 CrossRef CAS
.
- Y. Wu, Y. Gao and J. Du, Talanta, 2019, 197, 599–604 CrossRef CAS PubMed
.
- Q. Li, Y. Gao and S. H. Liu, Anal. Bioanal. Chem., 2024, 416, 1179–1188 CrossRef CAS
.
- M. Tian, C. Lin, Y. Lin, F. Luo, B. Qiu, J. Wang, Z. Lin and L. Wang, ACS Appl. Nano Mater., 2024, 7, 5620–5627 CrossRef CAS
.
- H. Tan and Y. Li, Microchim. Acta, 2021, 188, 254 CrossRef CAS PubMed
.
- Y. Yang, J. Liu, W. Li, Y. Zheng and W. Xu, Talanta, 2024, 277, 126345 CrossRef CAS PubMed
.
- H. Li, H. Lin, W. Lv, P. Gai and F. Li, Biosens. Bioelectron., 2020, 165, 112336 CrossRef CAS PubMed
.
- J. Shen, B. Situ, X. Du, Z. Wang, R. Hu, B. Li, A. Qin and B. Z. Tang, ACS Sensors, 2022, 7, 766–774 CrossRef CAS
.
- H. Li, H. Xu, S. Yao, S. Wei, X. Shi, C. Zhao, J. Li and J. Wang, Talanta, 2024, 270, 125505 CrossRef CAS PubMed
.
- Y. Duo, M. Suo, D. Zhu, Z. Li, Z. Zheng and B. Z. Tang, ACS Appl. Mater. Interfaces, 2022, 14, 26394–26403 CrossRef CAS
.
- C. Huang, T. Zhang, Y. Li, M. Lyu, M. Suo, L. Xia, L. Liu, B. Tang and Q. Zhang, Chem. Eng. J., 2022, 446, 136381 CrossRef CAS
.
- X. Wang, J. Dai, X. Wang, Q. Hu, K. Huang, Z. Zhao, X. Lou and F. Xia, Talanta, 2019, 202, 591–599 CrossRef CAS PubMed
.
- M. J. Dong, W. Li, Q. Xiang, Y. Tan, X. Xing, C. Wu, H. Dong and X. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 29599–29612 CrossRef CAS PubMed
.
- N. Song, Y. Yu, Y. Zhang, Z. Wang, Z. Guo, J. Zhang, C. Zhang and M. Liang, Adv. Mater., 2024, 36, 2210455 CrossRef CAS
.
- X. Dai, H. Liu, B. Cai, Y. Liu, K. Song, J. Chen, S. Q. Ni, L. Kong and J. Zhan, Small, 2023, 19, 2303901 CrossRef CAS
.
- M. M. S. Lee, E. Y. Yu, J. H. C. Chau, J. W. Y. Lam, R. T. K. Kwok and B. Z. Tang, Adv. Mater., 2024, 2407707 CrossRef
.
- F. Gao, T. Shao, Y. Yu, Y. Xiong and L. Yang, Nat. Commun., 2021, 12, 745 CrossRef CAS
.
- M. Yang, Z. Wang, M. Su, S. Zhu, Y. Xie and B. Ying, ACS Appl. Mater. Interfaces, 2024, 16, 44361–44375 CrossRef CAS
.
- F. Feng, X. Zhang, B. Mu, P. Wang, Z. Chen, J. Zhang, H. Zhang, J. Zhuang, L. Zhao, Q. An and Y. Zhang, ACS Appl. Nano Mater., 2022, 5, 16720–16730 CrossRef CAS
.
- R. Fang and J. Liu, J. Mater. Chem. B, 2020, 8, 7135–7142 RSC
.
- H. Hu, X. Kang, Z. Shan, X. Yang, W. Bing, L. Wu, H. Ge and H. Ji, Nanoscale, 2022, 14, 2676–2685 Search PubMed
.
- G. Wu, J. Luo, C. Du, Z. Zheng, Y. Zhang, P. Luo, Y. Wu and Y. Shen, Food Chem., 2024, 450, 139242 CrossRef CAS PubMed
.
- L. Luo, J. Li, X. Bi, P. Jiang, L. Li, G. Qiao and T. You, J. Hazard. Mater., 2024, 476, 134967 Search PubMed
.
- H. Zhu, B. Liu, J. Pan, L. Xu, J. Liu, P. Hu, D. Du, Y. Lin and X. Niu, Biosens. Bioelectron., 2025, 267, 116756 Search PubMed
.
- X. Guo, Y. Zhang, B. Huang and L. Han, ChemRxiv, 2024, DOI:10.26434/chemrxiv-2024-9z92k.
- X. Zhang, N. Hao, S. Liu, K. Wei, C. Ma, J. Pan and S. Feng, Talanta, 2024, 277, 126434 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2025 |