Open Access Article
Open Access ArticleThe intersection of field-limited density of states and matter: nanophotonic control of fluorescence energy transfer†
Haley W.
Jones
 ab,
Yuriy
Bandera
ab and
Stephen H.
Foulger
ab,
Yuriy
Bandera
ab and
Stephen H.
Foulger
 *abc
*abc
aCenter for Optical Materials Science and Engineering Technologies (COMSET), Clemson University, Anderson, SC 29625, USA. E-mail: foulger@clemson.edu
bDepartment of Materials Science and Engineering, Clemson University, Clemson, SC 29634, USA
cDepartment of Bioengineering, Clemson University, Clemson, SC 29634, USA
First published on 25th November 2024
Abstract
Understanding the unresolved connection between a structured environment and Förster resonance energy transfer (FRET) is critical in the realm of quantum light–matter interactions, especially for quantum technology applications. This crucial topic was explored by copolymerizing three emitters capable of energy transfer within two nanoparticle series (n1 and n2) that self-assembled into a crystalline colloidal array. Upon excitation, sequential energy transfer between the copolymerized derivatives of anthracene, naphthalimide, and rhodamine B within n1 and n2 resulted in emission spanning the visible spectrum. Nanophotonic control over the photoluminescence of n1 and n2 assembled in an ordered structure was demonstrated by red-shifting the partial photonic bandgap of the ordered structure through the emission spectra of the copolymerized emitters, which was achieved by dilution with deionized water. Nanophotonic manipulation of the energy transfer between the two FRET pairs copolymerized within n1 and n2 was observed, revealing insights in the context of light–matter interactions. Specifically, nanophotonic control over photoluminescence, energy transfer efficiency, and decay kinetics was demonstrated by strategic placement of the partial photonic bandgap.
1 Introduction
Förster resonance energy transfer (FRET) is a well-known nonradiative energy transfer process that occurs between two quantum emitters, an excited-state donor and a ground-state acceptor.1 FRET is a resonant dipole–dipole interaction and is, therefore, the dominant energy transfer mechanism when emitters are within nanometer proximity to each other. FRET is a vital mechanism in photosynthesis2 and is the basis of some photovoltaic,3,4 lighting,5 and biomedical sensing6 applications. Systems that exploit FRET can be controlled by engineering the spectral characteristics of the emitters or the relative orientations of their dipole moments;1,7 however, FRET control by a structured environment remains a highly debated and relevant topic8–20 with the potential to revolutionize high-priority technologies, such as quantum computing and communications.21Nanophotonic control over quantum states of matter represents a pivotal area of research within the ongoing second quantum revolution, offering unprecedented capabilities in manipulating quantum phenomena at the nanoscale.21 While it is well established that a structured environment, such as a photonic crystal, can be utilized to control the spontaneous emission of light (i.e., radiative decay) of an emitter,22,23 the ability to control and manipulate the interactions between multiple emitters, such as the nonradiative transfer of energy between a donor/acceptor FRET pair, by these means remains unresolved. Andrew and Barnes' pioneering work on this subject suggested a linear relationship between the local density of optical states (LDOS) and the energy transfer rate between a donor/acceptor pair of emitters,8 which was later theoretically supported.9 In the past decade, additional support for the linear dependence of FRET on the LDOS has been reported in the literature.10–12 However, some reports provide evidence of contradicting relationships between FRET and the LDOS, including a quadratic dependence on the LDOS13 or complete independence.16,18–20
Photonic crystals describe a class of periodic dielectric materials that exhibit a photonic bandgap corresponding to wavelengths where propagation through the crystal is forbidden.24–26 Photonic crystals are commonly embedded with emitters and exploited for their control over the LDOS of the emitter. In the case of a photonic crystal exhibiting a complete photonic bandgap, the LDOS at the bandgap frequency range is significantly diminished in all directions. However, in the case of a photonic crystal exhibiting an incomplete, or partial, photonic bandgap, the LDOS at the bandgap frequency range is inhibited only in certain directions. This work focuses on photonic crystals composed of highly-ordered colloidal nanoparticles known as crystalline colloidal arrays (CCAs), which rely on the spontaneous self-assembly of electrically charged nanoparticles into three-dimensional periodic arrays driven by repulsive Coulombic interactions.27,28 The minimum energy configuration of the nanoparticles is a face-centered cubic (FCC) structure at the high particle concentrations employed in this work.29 While a CCA does not exhibit a complete photonic bandgap due to the low refractive index contrast between the nanoparticles and surrounding medium, a CCA possesses a partial photonic bandgap (i.e., stop-band) in the visible regime, which can be approximated by Bragg's Law.30 It is well-known that the stop-band of the CCA, which corresponds to the observed reflectance of the liquid system and is denoted as the rejection wavelength (λrw), can be dynamically shifted across the full visible spectrum by a change in interparticle spacing or refractive index.31 CCAs have been employed as precursors for various photoluminescent and optoelectronic materials32,33 and have been utilized for investigations of quantum light–matter interactions in nanostructured environments.34–39 Notably, control over nonradiative decay mechanisms, such as intersystem crossing, has been demonstrated in a sterically-packed colloidal crystal exhibiting a partial photonic bandgap.39 Specifically, the suppressed emission intensity of an embedded emitter by the photonic bandgap effect has been suggested to increase the likelihood of nonradiative decay, thereby facilitating direct manipulation of luminescence.39
Motivated by the unresolved dependence, if any, of FRET on the photonic bandgap effect, two polystyrene-based nanoparticle series (n1 and n2) copolymerized with three FRET-pairing emitters were synthesized. Notably, n2 was synthesized using 100× less emitter content than n1 such that the decay kinetics of each emitter could be investigated using the n2 nanoparticles. Both n1 and n2 spontaneously self-assembled into CCAs, allowing for precise control over the partial photonic bandgap frequency range of the liquid structures without chemically or geometrically altering the copolymerized emitters. Specifically, the partial photonic bandgap of the liquid structures could be red-shifted across the full visible spectrum by dilution with deionized water, which increased the interparticle spacing. In both nanoparticle series, an anthracene derivative (AMMA) was copolymerized to play the role of the initial donor in the FRET system. In addition to AMMA, naphthalimide and rhodamine B derivatives (NMMA and RMMA, respectively) were copolymerized within the nanoparticles, extending the emission to the red region of the visible spectrum through two sequential transfers of energy. Anthracene, naphthalimide, and rhodamine B derivatives were chosen due to their known FRET-pairing with each other.40 Upon nanoparticle excitation with ultraviolet (UV) light, the AMMA donor transfers energy to NMMA, which acts as an acceptor in the AMMA/NMMA FRET pair. Subsequently, NMMA acts as a donor and transfers energy to RMMA, which acts as an acceptor in the NMMA/RMMA FRET pair. Steady state photoluminescence (PL) measurements of n1 and n2 assembled into an ordered structure (OS) were collected as the λrw of each structure was shifted across the emission spectrum. Additionally, the decay kinetics of n2 were investigated as the λrw was shifted to overlap the emission attributed to each emitter copolymerized within the nanoparticles. Specifically, as the λrw coincided with the emission attributed to each emitter, the lifetime was monitored at the λrw as well as at the peaks attributed to the corresponding donor and/or acceptor emitters. In this way, the photonic bandgap effect on the decay kinetics of the donor and acceptor in each FRET pair was observed. Notably, the reference systems utilized to quantify the photonic bandgap effect on the steady state and time-resolved spectra of the liquid structures with various λrw positions were fabricated such that nanoparticle density and emitter content were constant for a precise assessment of only photonic effects.
2 Experimental
2.1 Reagents and solvents
Inhibitors were removed from styrene (99% Alfa Aesar), propargyl acrylate (96% Alfa Aesar), and divinylbenzene (80% Aldrich) by passing over alumina basic prior to use. All other commercial reagents were used without further purification. A Nanopure System supplied deionized (DI) water at a resistivity of ca. 18.2 MΩ cm. Anthracen-9-ylmethyl methacrylate (AMMA), 2-(1,3-dioxo-6-(piperidin-1-yl)-1H-benzo[de]isoquinolin-2(3H)-yl)ethyl methacrylate (NMMA), and N-(6-(diethylamino)-9-(2-((2-(methacryloyloxy)ethoxy)carbonyl)phenyl)-3H-xanthen-3-ylidene)-N-ethylethanaminium chloride (RMMA) were synthesized according to previously described methods.41 Additionally, N-(9-(2-((2-(2-(2-azidoethoxy)ethoxy)ethoxy)carbonyl)phenyl)-6-(diethylamino)9,9a-dihydro-3H-xanthen-3-ylidene-N-ethylethanaminium) (azRhod) was synthesized according to previously described methods.422.2 Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MWCO). The product was dialyzed against DI water for 1–2 weeks, with water changed once a day. The product was then stored in a Nalgene container with an excess of mixed bed ion-exchange resin (Bio-Rad AB-501-X8(D)). The hydrodynamic particle size and zeta potential of the n1 nanoparticles after dialysis was 125 ± 8 nm and −47.6 ± 1.8 mV, respectively.
000 MWCO). The product was dialyzed against DI water for 1–2 weeks, with water changed once a day. The product was then stored in a Nalgene container with an excess of mixed bed ion-exchange resin (Bio-Rad AB-501-X8(D)). The hydrodynamic particle size and zeta potential of the n1 nanoparticles after dialysis was 125 ± 8 nm and −47.6 ± 1.8 mV, respectively.
2.3 Nanoparticle characterization
A Coulter N4Plus Dynamic Light Scatter (DLS) system was used to measure the hydrodynamic diameter of the nanoparticles. A Brookhaven Instruments Corp. ZetaPlus Zeta Potential Analyzer was used to measured the zeta potential of the nanoparticles.2.4 Optical characterization
Absorbance spectra was collected using a PerkinElmer Lambda 900 UV-vis/NIR spectrophotometer (cf. Fig. S1 and S6, ESI†). Photoluminescence excitation (PLE) spectra, photoluminescence (PL) spectra, and fluorescence decay profiles via a time-correlated single photon counting (TCSPC) technique were collected using a Horiba Jobin-Yvon Fluorolog 3-222 Tau/TCSPC spectrofluorometer. PL spectra presented in Fig. 1 and 3 were collected using a 370 nm excitation, 2 nm entrance/exit slit width, and 0.5 second integration time. PL spectra integrated in Fig. 2 were collected using a 370 nm excitation, 2 nm entrance/exit slit width, and 0.2 second integration time. PL spectra presented in Fig. 4A–D were collected using a pulsed 370 nm NanoLED source with a short pass filter (Horiba UG1 Short Pass Filter), 2 nm exit slit width, and 0.5 second integration time. PLE/PL spectra presented in Fig. S2 (ESI†) were collected using emission/excitation wavelengths indicated, 2 nm entrance/exit slit width, and a 0.5 second integration time. PL spectra integrated in Fig. S3c (ESI†) were collected using a 366 nm excitation, 2 nm entrance/exit slit width, and a 0.2 second integration time. PL spectra integrated in Fig. S3f (ESI†) were collected using a 403 nm excitation, 2 nm entrance/exit slit width, and a 0.2 second integration time. PL spectra presented in Fig. S5 (ESI†) were collected using a 370 nm or 400 nm excitation, 2 nm entrance/exit slit width, and a 0.5 second integration time. PL spectra presented in Fig. S7 (ESI†) were collected using a 530 nm excitation, 2 nm entrance/exit slit width, and a 0.5 second integration time. PL spectra presented in Fig. S8 (ESI†) were collected using a 370 nm excitation, 2 nm entrance/exit slit width, and a 0.5 second integration time. A Horiba Jobin-Yvon NanoLED 370 nm pulsed light source with a short pass filter (Horiba UG1 Short Pass Filter) operating at a frequency of 1 MHz was utilized as the excitation source for all TCSPC measurements (cf.Fig. 4, Fig. S4, S9–S13 and Tables S1–S6, ESI†), which was run in reverse mode and using a TAC range of 100 ns, coaxial delay of 50 ns, and sync delay of 116 ns. A peak present of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 counts (P10k) and a bandpass width of 2 nm at the monitored wavelength was employed for all TCSPC measurements. Additionally, it was ensured that α < 2% for all samples. A Horiba Jobin-Yvon DAS6 fluorescence decay analysis software was utilized to fit fluorescence decay profiles and best fits were determined by the lowest χ2 value. An Ocean Optics bifurcated fiber optic bundle attached to an Ocean Optics USB2000 fiber coupled spectrometer, where the input arm of the fiber bundle was attached to a white light source (Ocean Optics LS-1-CAL) and the output arm was attached to the spectrometer, was utilized to collect reflectance spectra of the nanoparticles assembled in an ordered structure (OS) (cf.Fig. 1E–G and 3E–G). It should be noted that significant scattering of reflectance spectra in the blue region of the visible spectrum occurs (cf.Fig. 1E and 3E). The incident light for all reflectance spectra was normal to the sample surface at the [111] plane of the OS. A Canon Rebel Ti1 was utilized to collect photographs of the OS (cf.Fig. 2D and E), where ultraviolet (UV) excitation was supplied by a Spectroline B-100 black light lamp (long wave ultraviolet, 365 nm).
000 counts (P10k) and a bandpass width of 2 nm at the monitored wavelength was employed for all TCSPC measurements. Additionally, it was ensured that α < 2% for all samples. A Horiba Jobin-Yvon DAS6 fluorescence decay analysis software was utilized to fit fluorescence decay profiles and best fits were determined by the lowest χ2 value. An Ocean Optics bifurcated fiber optic bundle attached to an Ocean Optics USB2000 fiber coupled spectrometer, where the input arm of the fiber bundle was attached to a white light source (Ocean Optics LS-1-CAL) and the output arm was attached to the spectrometer, was utilized to collect reflectance spectra of the nanoparticles assembled in an ordered structure (OS) (cf.Fig. 1E–G and 3E–G). It should be noted that significant scattering of reflectance spectra in the blue region of the visible spectrum occurs (cf.Fig. 1E and 3E). The incident light for all reflectance spectra was normal to the sample surface at the [111] plane of the OS. A Canon Rebel Ti1 was utilized to collect photographs of the OS (cf.Fig. 2D and E), where ultraviolet (UV) excitation was supplied by a Spectroline B-100 black light lamp (long wave ultraviolet, 365 nm).
3 Results and discussion
Two series of polystyrene-based nanoparticles containing three copolymerized emitters, AMMA, NMMA, and RMMA, were synthesized using a general emulsion polymerization procedure (cf. Experimental). The photophysical properties of the individual emitters are presented in the ESI† (cf. Fig. S1–S6 and Table S1). Briefly, the AMMA emitter exhibits blue emission upon UV excitation. The absorption spectrum of the NMMA emitter has adequate overlap with the emission spectrum of AMMA such that, when in close proximity, energy transfer occurs between the donor AMMA and the acceptor NMMA. Subsequently, the absorption spectrum of the RMMA emitter has adequate overlap with the emission spectrum of NMMA such that, when in close proximity, energy transfer occurs between the donor NMMA and the acceptor RMMA.Series n1 was synthesized using 0.3% (w/w) of each emitter to styrene while a more emitter dilute series, n2, was synthesized using 100× less emitter content (0.003% (w/w) to styrene). Both n1 and n2 spontaneously self-assembled into a CCA due to their monodispersity and excellent colloidal stability (cf. ESI†). The incorporation and arrangement of the emitters in n1 was confirmed and assessed in the ESI† (cf. Fig. S6 and S7, ESI†). Due to the very small emitter content of n2, the absorbance spectrum of n2 in aniline did not reveal any discernible peaks such that the number of emitter molecules per particle could be estimated. Nonetheless, the incorporation of all three emitters is evident in the PL spectra of n2 discussed later (cf.Fig. 3 and Fig. S8, ESI†).
3.1 Nanophotonic manipulation of spectral properties and FRET efficiency
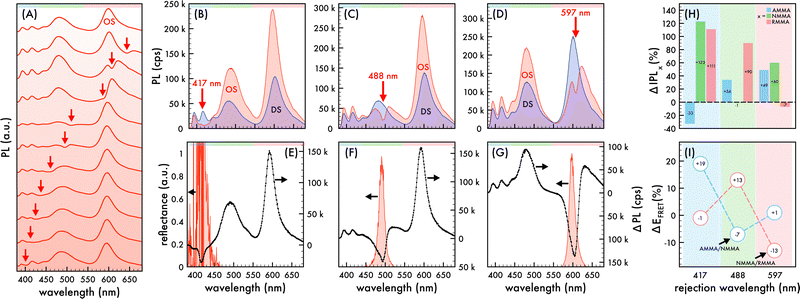
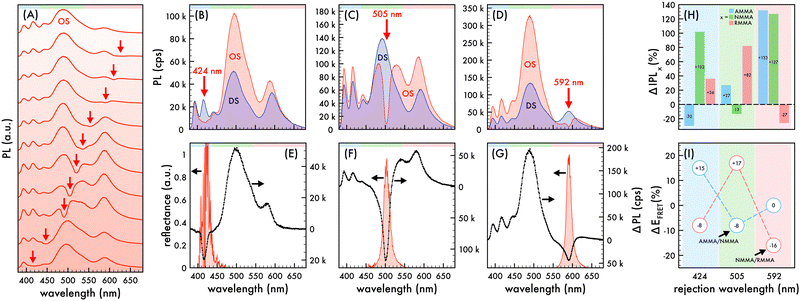
Nanophotonic manipulation of the PL spectra of n1 and n2 was explored by shifting the observed λrw of each nanoparticle series assembled into an ordered structure (OS) through their respective emission. It is important to note that due to the large quantity of emitters copolymerized in n1, the n1 OSs utilized for all PL measurements were composed of 1.36% (v/v) of n1 in “blank” polystyrene-based nanoparticles that contained no copolymerized emitters (cf. ESI†). Due to the size and stability similarities of n1 and the “blank” nanoparticles, it was assumed that there was no preferential ordering of the particles during mixing. Therefore, the probability of n1 nanoparticle proximity to other n1 nanoparticles within the OS lattice was determined by its volume fraction. At this n1 dilution, the probability that two n1 nanoparticles were located next to each other was 0.000185, or 1 in 5405. PL spectra of n1 and n2 assembled in an OS were obtained at the (111) face of the FCC crystal, which aligns parallel to the wall of a cuvette when the liquid arrays are placed within a cuvette.45,46 Thus, the observed λrw of the OS can be ascribed to the {111} stop-band. The interplanar spacing (d111) of each nanoparticle series assembled into an OS could be estimated using Bragg's law and the refractive index of the system.47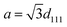 | (1) |
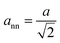 | (2) |
To explore the hypothesized phenomenon, the partial photonic bandgap effect on the PL characteristics of n1 and n2 and the energy transfer efficiency (EFRET) between the two FRET pairs copolymerized within n1 and n2 was investigated. To that end, the PL spectra of n1 and n2 assembled in an OS are presented in Fig. 1B–G and 3B–G, respectively, as the λrw of each liquid structure was shifted through the spectral regimes attributed to each copolymerized emitter. At each stop-band frequency, the PL spectra of the OS was compared to that of a disordered reference structure, referred to as the DS. Precisely comparable DSs were fabricated for each stop-band frequency of n1 and n2 assembled in an OS by adding a small amount of an ionic impurity (NaCl) to the OS.45 In this way, the long-range order of the nanoparticles in the OS was disrupted while maintaining particle density and emitter content for a controlled investigation of purely photonic effects.
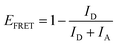 | (3) |
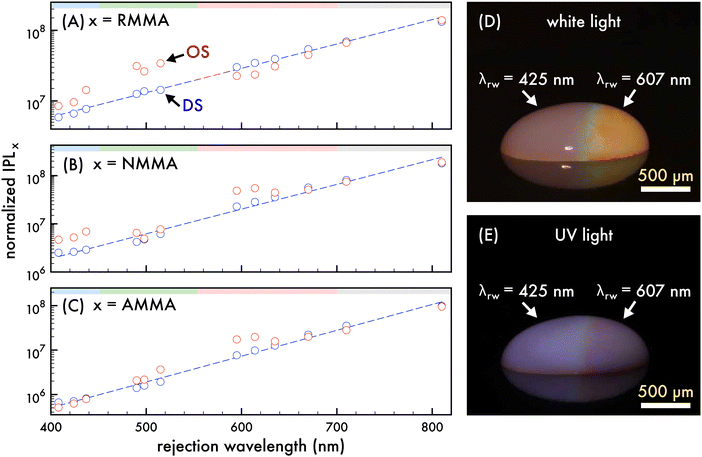
To ascertain that the spectral trends observed were a result of the photonic bandgap effect, the integrated PL spectra attributed to each copolymerized emitter in the n1 OS was compared to that of a corresponding DS as the λrw of the OS was shifted from ca. 400 nm to 800 nm (cf.Fig. 2A–C). In this case, a n1 DS was fabricated at the same initial particle density of the n1 OS and was diluted by deionized water similar to the OS such that the particle densities of the OS and DS were closely matched. The sample volume and optically sampled area of the OS and DS remained invariant through the dilution, resulting in a decreasing number of nanoparticles optically sampled with each dilution. Thus, the integrated PL was normalized by the number of nanoparticles in the optically sampled area at each dilution. The decreased nanoparticle density associated with each subsequent dilution resulted in decreased scattering from the colloid. Due to this, the integrated PL attributed to each copolymerized emitter in the DS exhibited an positive increment with decreasing particle density. Conversely, the integrated PL attributed to each copolymerized emitter in the OS did not exhibit this same trend and instead exhibited fluctuations of increased or decreased integrated PL intensity compared to that of the DS, depending on the stop-band frequency. Dips below the trendlines fit to the integrated PL of the emitters in the DS were observed in the integrated PL of the emitters in the OS as the λrw was shifted through the emission spectrum. When the λrw overlapped the emission of the donor (AMMA or NMMA), the emission of the respective acceptor (NMMA or RMMA) was enhanced. Conversely, when the λrw overlapped the emission of the acceptor (NMMA or RMMA), the emission of the respective donor (AMMA or NMMA) was enhanced. Additionally, when the λrw of the n1 OS was red-shifted past the emission range of the nanoparticles, the integrated PL attributed to each emitter in the OS and DS was closely matched, indicating no photonic bandgap effect on OS emission when the λrw was not coupled to the emission. The nanophotonic amplification and suppression of FRET between the copolymerized emitters in the n1 OS and resulting enhanced or inhibited PL emission are visually exemplified in Fig. 2D and E, where photographs of a droplet of the n1 OS under white light and UV excitation are presented. The λrw of the left half of the droplet is at 425 nm, exhibiting an enhanced total emission and a visually brighter optical output when excited with a UV source. The λrw of the right half of the droplet is at ca. 607 nm, resulting in a suppressed total emission and a visually dimmer optical output when excited with a UV source.
3.2 Nanophotonic manipulation of FRET in time-resolved fluorescence measurements
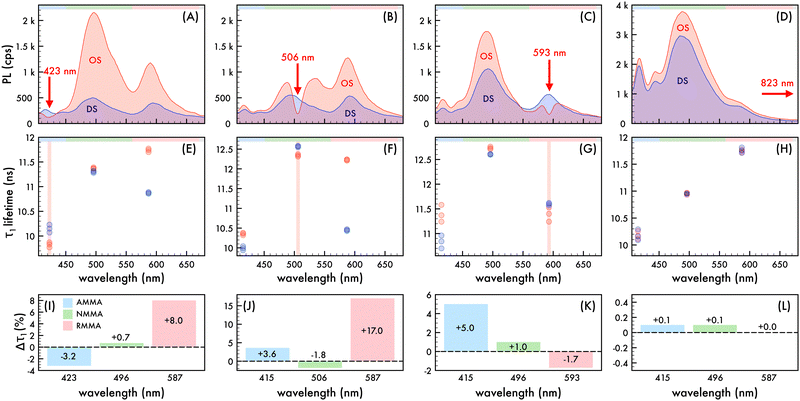
To ascertain that the trends observed in the steady-state measurements were indicative of decay process modulations, a time-correlated single photon counting (TCSPC) technique was utilized to evaluate the photonic bandgap effect on the fluorescence lifetime of the three emitters copolymerized within the nanoparticle building blocks of the n2 OS. It is important to note that fluorescence lifetime measurements were performed using an undiluted array of colloidal nanoparticles. Thus, the n2 nanoparticles with 100× less dye content than the n1 nanoparticles were utilized such that changes in decay kinetics of each emitter could be discerned. The measured fluorescence lifetime (τ), or total decay rate (Γt), is comprised of both radiative (Γr) and nonradiative (Γnr) decay components (cf.eqn (4)). It is well known through Fermi's golden rule that the rate of radiative decay is proportional to the LDOS at the emission frequency and, thus, it can be predicted that the radiative decay rate is decreased at the stop-band frequency.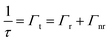 | (4) |
To explore the photonic bandgap modulation of decay kinetics, the lifetime of the n2 OS was monitored at wavelengths corresponding to AMMA, NMMA, and RMMA emission as the λrw was red-shifted across the entire emission spectrum (cf.Fig. 4). Precisely comparable reference DSs were fabricated for each λrw condition of the OS as previously described such that the nanoparticle density and emitter content were invariant for a controlled assessment of only photonic effects. All collected decay profiles of n2 in an OS and DS exhibited nonexponential behavior and were adequately modeled by assuming two or three decay components (τ1–3) (cf. Fig. S9–S12 and Tables S2–S5, ESI†), identified using a multi-exponential least-squares fitting procedure. The multi-exponential characteristics of the decay of each emitter are ascribed to different sub-ensembles of emitter molecules, each subject to varying nonradiative decay rates, in addition to contributions from the polystyrene-based host polymer. It was assumed that the greatest lifetimes with the largest contribution to the decay profile (τ1), which range from approximately 9.8 ns to 12.8 ns, were due to the copolymerized emitters. The components with shorter lifetimes (cf. Fig. S13, ESI†), which are less than ca. 8 ns, were ascribed to the host material (cf. Table S6, ESI†) and excluded from further analysis. The spectral properties of the n2 OS and corresponding DS as the λrw was shifted through the emission of the nanoparticles are presented in Fig. 4A–D. The average measured fluorescence lifetime attributed to each emitter at each λrw position is presented in Fig. 4E–H, where the average value was obtained from three lifetime acquisitions at different locations in the structure. Additionally, the highest and lowest observed lifetime at each wavelength is presented. When the λrw overlapped the n2 OS emission attributed to the AMMA donor at 423 nm (cf.Fig. 4I), the lifetime attributed to AMMA at 423 nm exhibited a −3.2% decrease, owing to the decreased probability of photon emission at the bandgap frequency. The lifetime attributed to the acceptor/donor NMMA and acceptor RMMA exhibited a +0.7% and +8.0% increase, respectively, owing to the enhanced probability of energy transfer between the AMMA/NMMA and NMMA/RMMA pairs through the cascaded energy transfer and, thus, enhanced probability of photon emission by NMMA and RMMA. Shifting the λrw of the n2 OS to overlap the emission attributed to NMMA at 506 nm (cf.Fig. 4J) resulted in a −1.8% decrease in lifetime attributed to the NMMA acceptor/donor. The lifetime attributed to the AMMA donor increased by +3.6%, owing to the increased probability of radiative emission from AMMA when energy transfer from AMMA to NMMA was suppressed. Additionally, the largest increase in RMMA lifetime was observed (+17.0%), attributed to the greater probability of NMMA to decay nonradiatively and transfer energy to RMMA, resulting in a greater probability of RMMA to emit a photon. Shifting the λrw of the n2 OS to overlap the emission attributed to RMMA at 593 nm (cf.Fig. 4K), the RMMA acceptor lifetime decreased by −1.7%. The lifetime of the AMMA donor and NMMA acceptor/donor exhibited an increase of +1.0% and +5.0%, respectively, owing to the suppressed energy transfer from AMMA and NMMA, resulting in a greater probability of AMMA and NMMA to emit a photon. Finally, by shifting the λrw of the n2 OS outside of the emission spectrum of the nanoparticles at 823 nm (cf.Fig. 4L), the lifetime of each emitter showed no significant change compared to that of the DS, indicating that the lifetime modulations were a result of the photonic bandgap effect.
Förster's rate equation (cf.eqn (5)) can be utilized to estimate the energy transfer rate (Γet) between a donor/acceptor pair, where r is the distance between the donor and acceptor, R0 is the Förster distance, and τd is the lifetime of the donor without the acceptor.
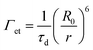 | (5) |
 | (6) |
4 Conclusions
These results present significant evidence that the nonradiative energy transfer between multiple interacting emitters can be manipulated and controlled by the photonic bandgap effect. Specifically, the direction and efficiency of FRET was modulated in two FRET pairs, exhibiting a cascaded energy transfer, that were copolymerized within two series of nanoparticles assembled into CCAs. As evidenced by steady-state PL and time-correlated fluorescence measurements, the probability and efficiency of FRET between a donor/acceptor pair was enhanced by coinciding the partial photonic bandgap with the donor emission. Conversely, the probability and efficiency of FRET between a donor/acceptor pair was suppressed by coinciding the partial photonic bandgap with the acceptor emission. Both cases exhibited significant modifications in the spectral properties of the nanoparticles, energy transfer efficiency of each FRET pair, and decay kinetics of the interacting emitters, emanating from the optical confinement of the emitters within a nanostructured environment and ability to manipulate their likelihood of decay by radiative and nonradiative pathways using the partial photonic bandgap of the CCA. These results support that the modified LDOS at the partial bandgap frequency enhances nonradiative processes, such as FRET, and a nanostructured environment, such as a CCA, can be exploited for strategic control over the quantum interactions between multiple emitters. These findings have profound implications for both advancing the understanding of basic quantum light–matter interactions and serving as a versatile tool across various quantum technologies, including computing, communication, and sensing applications.Author contributions
H. J. contributed to the conceptualization, methodology, investigation, data curation, visualization, writing – original draft, and writing – review & editing. Y. B. contributed to the resources and methodology. S. F. contributed to the conceptualization, methodology, data curation, visualization, writing – original draft, and writing – review & editing.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Gregg-Graniteville Foundation and the National Science Foundation (OIA-1632881) for financial support.References
- T. Forster, Ann. Phys., 1948, 437, 55–75 CrossRef.
- R. van Grondelle, J. P. Dekker, T. Gillbro and V. Sundstrom, Biochim. Biophys. Acta, 1994, 1187, 1–65 CrossRef.
- G. D. Scholes, G. R. Fleming, A. Olaya-Castro and R. van Grondelle, Nat. Chem., 2011, 3, 763–774 Search PubMed.
- S. Buhbut, S. Itzhakov, E. Tauber, M. Shalom, I. Hod, T. Geiger, Y. Garini, D. Oron and A. Zaban, ACS Nano, 2010, 4, 1293–1298 CrossRef CAS.
- M. A. Baldo, M. E. Thompson and S. R. Forrest, Nature, 2000, 403, 750–753 Search PubMed.
- I. L. Medintz, A. R. Clapp, H. Mattoussi, E. R. Goldman, B. Fisher and J. M. Mauro, Nat. Mater., 2003, 2, 630–638 CrossRef CAS PubMed.
- D. L. Andrews and J. Rodriguez, J. Chem. Phys., 2007, 127, 084509 Search PubMed.
- P. Andrew and W. L. Barnes, Science, 2000, 290, 785–788 Search PubMed.
- H. T. Dung, L. Knoll and D. G. Welsch, Phys. Rev. A: At., Mol., Opt. Phys., 2002, 65, 043813 Search PubMed.
- P. Ghenuche, J. de Torres, S. B. Moparthi, V. Grigoriev and J. Wenger, Nano Lett., 2014, 14, 4707–4714 CrossRef CAS PubMed.
- D. Weeraddana, M. Premaratne, S. D. Gunapala and D. L. Andrews, J. Chem. Phys., 2017, 147, 074117 Search PubMed.
- S. Patra, J.-B. Claude and J. Wenger, ACS Photonics, 2022, 9, 2109–2118 CrossRef CAS.
- T. Nakamura, M. Fujii, S. Miura, M. Inui and S. Hayashi, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 045302 CrossRef.
- B. Kolaric, K. Baert, M. Van der Auweraer, R. A. L. Vallee and K. Clays, Chem. Mater., 2007, 19, 5547–5552 CrossRef CAS.
- L. Gonzalez-Urbina, K. Baert, B. Kolaric, J. Perez-Moreno and K. Clays, Chem. Rev., 2012, 112, 2268–2285 CrossRef CAS PubMed.
- M. J. A. de Dood, J. Knoester, A. Tip and A. Polman, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 115102 CrossRef.
- F. T. Rabouw, S. A. den Hartog, T. Senden and A. Meijerink, Nat. Commun., 2014, 5, 3610 CrossRef.
- C. Blum, N. Zijlstra, A. Lagendijk, M. Wubs, A. P. Mosk, V. Subramaniam and W. L. Vos, Phys. Rev. Lett., 2012, 109, 203601 Search PubMed.
- M. Wubs and W. L. Vos, New J. Phys., 2016, 18, 053037 CrossRef.
- G. Rosolen, B. Maes, P. Y. Chen and Y. Sivan, Phys. Rev. B, 2020, 101, 155401 CrossRef CAS.
- B. Kolaric, B. Maes, K. Clays, T. Durt and Y. Caudano, Adv. Quantum Technol., 2018, 1, 1800001 CrossRef.
- K. H. Drexhage, J. Lumin., 1970, 1(2), 693–701 Search PubMed.
- W. L. Barnes, J. Mod. Opt., 1998, 45, 661–669 CrossRef CAS.
- T. Okubo, Prog. Polym. Sci., 1993, 18, 481–517 CrossRef CAS.
- E. Yablonovitch, Phys. Rev. Lett., 1987, 58, 2059–2062 CrossRef CAS PubMed.
- S. John, Phys. Rev. Lett., 1987, 58, 2486–2489 CrossRef CAS PubMed.
- P. A. Hiltner, Y. S. Papir and I. M. Krieger, J. Phys. Chem., 1971, 75, 1881–1886 CrossRef CAS.
- P. A. Hiltner and I. M. Krieger, J. Phys. Chem., 1969, 73, 2386 CrossRef CAS.
- N. Clark, A. Hurd and B. Ackerson, Nature, 1979, 281, 57–60 CrossRef CAS.
- P. Rundquist, P. Photinos, S. Jagannathan and S. Asher, J. Chem. Phys., 1989, 91, 4932–4941 CrossRef CAS.
- C. López, Adv. Mater., 2003, 15, 1679–1704 CrossRef.
- J. R. Lawrence, Y. Ying, P. Jiang and S. H. Foulger, Adv. Mater., 2006, 18, 300–303 CrossRef CAS.
- J. R. Lawrence, G.-H. Shim, P. Jiang, M. Han, Y. Ying and S. H. Foulger, Adv. Mater., 2005, 17, 2344–2349 CrossRef CAS.
- J. Martorell and N. M. Lawandy, Phys. Rev. Lett., 1990, 65, 1877–1880 CrossRef CAS PubMed.
- B. Tong, P. John, Y. Zhu, Y. Liu, S. Wong and W. Ware, J. Opt. Soc. Am. B, 1993, 10, 356–359 CrossRef CAS.
- M. Megens, J. E. G. J. Wijnhoven, A. Lagendijk and W. L. Vos, Phys. Rev. A: At., Mol., Opt. Phys., 1999, 59, 4727–4731 CrossRef CAS.
- K. Shibata, H. Kimura, A. Tsuchida and T. Okubo, Colloid Polym. Sci., 2006, 285, 127–133 CrossRef CAS.
- M. Khokhar, Priya and R. V. Nair, Phys. Rev. A, 2020, 102, 013502 CrossRef CAS.
- L. González-Urbina, J. Perez-Moreno, K. Clays and B. Kolaric, Mol. Phys., 2016, 114, 2248–2252 CrossRef.
- M. K. Burdette, Y. P. Bandera, G. M. Gray and S. H. Foulger, Adv. Opt. Mater., 2019, 7, 1801142 CrossRef.
- S. Mell, H. W. Jones, Y. P. Bandera and S. H. Foulger, Langmuir, 2022, 38, 10089–10097 CrossRef CAS PubMed.
- M. K. Burdette, H. W. Jones, Y. Bandera and S. H. Foulger, Opt. Mater. Express, 2019, 9, 1416–1429 CrossRef CAS.
- M. E. Woods, J. S. Dodge, I. M. Krieger and P. E. Pierce, J. Paint Technol., 1968, 40, 541 CAS.
- Y. S. Papir, M. E. Woods and I. M. Krieger, J. Paint Technol., 1970, 42, 571–578 CAS.
- Y. Monovoukas and A. P. Gast, J. Colloid Interface Sci., 1989, 128, 533–548 CrossRef CAS.
- Y. Monovoukas and A. P. Gast, Phase Transform., 1990, 21, 183 CrossRef CAS.
- S. H. Foulger, P. Jiang, A. Lattam, D. Smith and J. Ballato, Langmuir, 2001, 17, 6023–6026 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc03973d |
| This journal is © The Royal Society of Chemistry 2025 |