Open Access Article
Open Access ArticleControl and interplay of scaffold–biomolecule interactions applied to cartilage tissue engineering
Silouane
Dupuy†
ac,
Jérémy
Salvador†
 abc,
Marie
Morille
abc,
Marie
Morille
 a,
Danièle
Noël
c and
Emmanuel
Belamie
a,
Danièle
Noël
c and
Emmanuel
Belamie
 *ab
*ab
aICGM, University of Montpellier, CNRS, ENSCM, Montpellier, France. E-mail: Emmanuel.Belamie@ephe.psl.eu
bEPHE, PSL Research University, 75014 Paris, France
cIRMB, University of Montpellier, INSERM, Montpellier, France
First published on 25th February 2025
Abstract
Cartilage tissue engineering based on the combination of biomaterials, adult or stem cells and bioactive factors is a challenging approach for regenerative medicine with the aim of achieving the formation of a functional neotissue stable in the long term. Various 3D scaffolds have been developed to mimic the extracellular matrix environment and promote cartilage repair. In addition, bioactive factors have been extensively employed to induce and maintain the cartilage phenotype. However, the spatiotemporal control of bioactive factor release remains critical for maximizing the regenerative potential of multipotent cells, such as mesenchymal stromal cells (MSCs), and achieving efficient chondrogenesis and sustained tissue homeostasis, which are essential for the repair of hyaline cartilage. Despite advances, the effective delivery of bioactive factors is limited by challenges such as insufficient retention at the site of injury and the loss of therapeutic efficacy due to uncontrolled drug release. These limitations have prompted research on biomolecule–scaffold interactions to develop advanced delivery systems that provide sustained release and controlled bioavailability of biological factors, thereby improving therapeutic outcomes. This review focuses specifically on biomaterials (natural, hybrid and synthetic) and biomolecules (molecules, proteins, nucleic acids) of interest for cartilage engineering. Herein, we review in detail the approaches developed to maintain the biomolecules in scaffolds and control their release, based on their chemical nature and structure, through steric, non-covalent and/or covalent interactions, with a view to their application in cartilage repair.
I. Introduction
Articular cartilage lesions can significantly compromise a patient's quality of life due to pain and functional disability and represent a heavy burden for the healthcare economy worldwide. Joint injuries have many possible origins including degenerative diseases and traumatic events. Due to its avascular nature and low chondrocyte to extracellular matrix (ECM) ratio, damaged cartilage has a limited self-healing capability.1,2 Surgical techniques such as microfracture, implantation of autologous chondrocytes or mosaicplasty attempt to repair damaged cartilage. However, current methods do not enable optimal biophysical properties to be achieved; this results in accelerated matrix degradation and generally poor tissue quality in the long-term.3 Tissue engineering (TE) appears to be a promising solution to restore the structure and function of articular cartilage.4,5 It relies on the association of at least three different elements: cells, a supporting scaffold and biological factors.6 The ultimate objective of cartilage engineering is to generate a fully functional tissue produced by chondrocytes, the only mature cellular component of cartilage capable of secreting the ECM specific to hyaline cartilage.7,8Initial TE strategies have used chondrocytes combined with biofactors and a 3-dimensional construct to avoid chondrocyte dedifferentiation during the amplification phase in vitro.9,10 Besides, mesenchymal stromal cells (MSCs) are of particular interest owing to their ability to differentiate into chondrocytes under appropriate conditions.11,12 MSCs can be obtained from different sources including bone marrow, umbilical cords, adipose tissue and synovial membrane.13,14 However, bone marrow-derived MSCs represent the most promising source of MSCs used for cartilage engineering because of their superior chondrogenic potential.15,16 MSC-based therapies for cartilage engineering require the use of chondro-inductive biofactors including growth factors (GFs), peptides, or genetic material to help control or enhance cell differentiation and maintain the mature chondrocyte state, hence promoting cartilage repair. These factors differ in their mechanisms of action but also in their functions during the process of cartilage repair. It is therefore important to precisely control the kinetics of action of these biomolecules to avoid unwanted adverse effects. For these reasons, controlled levels and spatio-temporal release of these molecules are essential for promoting the formation of high-quality cartilage matrix, with the required biomechanical properties. To avoid repeated injections of biomolecules during the cartilage regeneration process, much work has been done to incorporate biomolecules into constructs and control their delivery to resident or implanted cells.
The physical and biochemical properties of scaffolds are critical for the success of cartilage repair. A biomaterial has to be biocompatible, biofunctional to promote cell adhesion and integration into the host tissue, and biodegradable. Scaffolds should also have appropriate biomechanical properties to withstand external forces resulting from joint motion. Therefore, the architecture of the scaffold plays a key role in maintaining its stability while allowing cell impregnation, cell–cell interactions and free circulation of nutrients and cell waste.5 Obviously, the chemical nature of the material forming the scaffold has a significant impact on the above properties.
In this article, we first provide an overview of the options for careful selection of the appropriate scaffold for cartilage TE. In the next part, we present the different biofactors currently used for cartilage TE and their regulatory role in both the differentiation of MSCs towards chondrocytes and the secretion of cartilage ECM by chondrocytes. Finally, we discuss the strategies used to functionalize scaffolds with biofactors and evaluate the impact of different approaches for the release kinetics of biofactors and their effects on chondrogenesis.
II. Scaffolds for cartilage tissue engineering: requirements and elaboration
II.1. Criteria
In the field of cartilage TE, scaffolds play a pivotal role in establishing an ideal microenvironment for promoting cell adhesion, migration, proliferation and/or differentiation. This essential function relies on several key parameters, such as appropriate architecture, controlled degradability, mechanical properties, and biocompatibility. In fact, the structural characteristics of scaffolds, including their porosity, permeability, and interconnectivity, exert a significant influence over the complex process of articular cartilage formation and subsequent tissue regeneration.17,18 Overall, the scaffold architecture must enable cell attachment and migration onto the scaffold while ensuring appropriate interconnectivity.19 Since scaffolds act as temporary supports for tissue development, their controlled degradation is critical for the effective formation and integration of newly generated cartilage tissue within the surrounding endogenous tissue. It is also essential that the by-products released during the degradation process are non-toxic and easily eliminated from the body.20 Ideally, scaffolds should exhibit intrinsic mechanical properties similar to those of the native cartilage tissue such as tensile strength, toughness and stiffness. These parameters are important for promoting integration and supporting continued tissue development after scaffold implantation into the knee joint.21II.2. Materials
Materials used for cartilage TE obviously must be biocompatible and meet specific requirements of the target application. Numerous reviews have thoroughly examined the diverse compositions, structures, fabrication techniques, and characteristics of existing biomaterials.22–25Briefly, biomaterials can include either natural or synthetic polymers as well as hybrid materials combining both (Table 1). Natural polymers such as collagen,26,27 gelatin,28,29 hyaluronic acid (HA),30,31 chitosan (CH),32,33 chondroitin sulfate (CS),34,35 fibrin36 and alginate37,38 are commonly employed in fabricating scaffolds for cartilage repair. Originating from natural sources, these materials exhibit high biocompatibility, bioactivity and possess properties close to those of native tissues, making them suitable candidates. Unfortunately, most natural materials exhibit rapid degradation, which may compromise scaffold integrity. Extracted and processed biopolymers also exhibit limited mechanical strength, which may hinder their ability to support cells and promote tissue repair. Moreover, processing natural polymers into scaffolds may be challenging due to their susceptibility to changes during processing, such as chain scission or protein denaturation.5
| Materials | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|
| Natural | Collagen | Biocompatibility | Low mechanical strength | 26 and 27 |
| Biodegradability | Low solubility | |||
| Low immunogenicity | Rapid biodegradation | |||
| Cell adhesion proliferation and differentiation | Difficult to handle | |||
| Gelatin | Biocompatibility | Low mechanical strength | 28 and 29 | |
| Biodegradability | Stability | |||
| Accessibility | Poor mechanism properties | |||
| Low solubility | ||||
| Hyaluronic acid (HA) | Biocompatibility | Cost | 30 and 31 | |
| Biodegradability | Low mechanical strength | |||
| Easy chemical modification | Fast degradation | |||
| Bioactivity | ||||
| Chitosan (CH) | Biocompatibility | Low mechanical strength | 32 and 33 | |
| Biodegradability | Low solubility | |||
| Cell adhesion, proliferation and differentiation | ||||
| Anti-microbial activity | ||||
| Fibrin | Biocompatibility | Cost | 36 | |
| Inexpensive | Low mechanical strength | |||
| Accessibility | Fast degradation | |||
| Cell adhesion and proliferation | ||||
| Alginate | Biocompatibility | Low mechanical strength | 37 and 38 | |
| Biodegradability | Limited strength | |||
| Accessibility | Difficult to handle | |||
| Bioactivity | ||||
| Synthetic | PLA | Biocompatibility | Poor cell adhesion | 39–42 |
| Thermostability | ||||
| Thermoplasticity | ||||
| Degradability | ||||
| PGA | Availability | Acid release upon degradation | 43 | |
| Easy processing | Poor cell adhesion | |||
| Biocompatibility | Fast degradation | |||
| Mechanical properties | ||||
| PLGA | Mechanical properties | Acid release upon degradation | 44 and 45 | |
| Controlled degradability | ||||
| PCL | Mechanical properties | Poor cell adhesion | 46 and 47 | |
| Biocompatibility | Poor hydrophobicity | |||
| Thermoplasticity | ||||
| Biodegradability | ||||
| PEG | Biocompatibility | Poor cell adhesion | 48 and 49 | |
| Biodegradability |
On the other hand, synthetic polymers have emerged as promising alternatives in TE, due to their tunable properties. Key synthetic polymers include polylactic acid (PLA),39–42 polyglycolic acid (PGA),43 poly-lactic-co-glycolic acid (PLGA),44,45 polycaprolactone (PCL)46,47 and polyethylene glycol (PEG).48,49 The chemical composition and structure of synthetic polymers make them scaffolds with highly customizable properties. In fact, they can be tailored to exhibit specific mechanical, physical and chemical properties such as stiffness, porosity, and degradation rate in order to meet the requirements of cartilage tissue repair. However, synthetic materials also exhibit some drawbacks, notably the lack of inherent bioactivity, which hinders cell adhesion, proliferation, and differentiation. Tissue integration and functionality are consequently affected because the biomaterial does not fully replicate the biochemical and biomechanical properties and cues of native cartilage tissue.50 Moreover, harmful acidic degradation products may compromise biocompatibility and trigger an inflammatory response.20
Obviously, the chemical nature of the material forming the supporting scaffold has a significant impact on its properties, and notably its ability to incorporate bioactive compounds. For instance, hydrophobic aliphatic polyesters (PLA, PGA, PLGA, PCL…) can be processed into porous scaffolds but with poorly hydrated solid walls, while hydrophilic polymers (collagen, gelatin, hyaluronic acid, PEG…) can form highly swollen hydrogels. Of note, the biophysical characteristics of the scaffolds resulting from their chemical composition can also be engineered to enhance or direct MSC differentiation. The roles of substrate features such as mechanical properties, porosity or topology have been reviewed elsewhere51,52 and are addressed in the present article only when directly related to biomolecule interactions with the scaffold and their release profiles.
II.3. Techniques
The desired scaffold architecture, mechanical properties and shapes can be achieved by selecting the appropriate scaffold fabrication method. A variety of fabrication methods enable the production of scaffolds in the form of three-dimensional membranes, hydrogels, microspheres, sponges, or their combinations, thereby providing versatility to meet the diverse requirements of cartilage TE.53 Hydrogels, in particular, have attracted considerable attention as scaffolds for cartilage TE due to their structural and functional resemblance to the ECM.54 Hydrogel formation involves the development of hydrophilic polymer networks through chemical cross-linking, physical gelation, or self-assembly processes. These networks are capable of absorbing water and swelling in aqueous solutions; this promotes the attachment, migration, differentiation, and proliferation of cells while effectively delivering growth factors and creating an appropriate microenvironment for nutrients.55,56 Scaffold fabrication techniques for cartilage TE include conventional and rapid prototyping (RP) methods, which have been described in many reviews (Table 2).57,58| Techniques | Advantages | Disadvantage | Ref. |
|---|---|---|---|
| Phase inversion | Versatility | Lack of pore interconnectivity control | 60 and 278 |
| Compatibility | Processing conditions | ||
| Repeatability | Applicability | ||
| Solvent casting and particle leaching | Easy processing | Cytotoxic solvent | 279 and 280 |
| Adjustable porosity | Low mechanical strength | ||
| Gas foaming | Inexpensive | Pore size distribution | 281 |
| Porosity control | Lack of pore interconnectivity control | ||
| Electrospinning | Large-scale production possibilities | Limited range of polymers | 282–284 |
| Repeatability | |||
| Easy process | |||
| Freeze-drying | Adjustable porosity and structure | Energy intensive and time consuming | 285 |
| Greater interconnectivity of the porous structure | Cytotoxic solvents | ||
| 3D bioprinting | High resolution | Inkjet viscosity | 286–288 |
| High throughput capability | |||
| Reproducibility | |||
| Easy to use |
Conventional methods for scaffold fabrication are often constrained by limitations in compatibility and repeatability, and frequently rely on manual intervention, making them unsuitable for large-scale application. Among these methods, phase inversion is commonly employed for membrane preparation, and usually divided into thermally induced phase separation (TIPS) and non-solvent induced phase separation (NIPS). TIPS utilizes temperature manipulation in liquid–liquid or liquid–solid systems to achieve membranes with varied porosities, whereas NIPS involves immersing a polymer solution in a non-solvent solution, resulting in membranes with different porosities and pore sizes.59 The TIPS technique has been specifically applied to fabricate PLLA scaffolds with finely tuned pore dimensions, mimicking natural conditions conducive to efficient chondrogenesis.60 Additional techniques, such as freeze-drying, can also improve scaffold porosity and pore size. Freeze-drying, or lyophilization, involves freezing polymer solutions followed by solvent sublimation under vacuum, producing scaffolds with interconnected porous structures.61 Electrospinning is another widely used method due to its simplicity, rapidity, cost-effectiveness, and ability to generate nonwoven scaffolds with high porosity and interconnectivity.62 Electrospun nanofiber-based scaffolds are expected to be good candidates for osteochondral and cartilage repair but their outcome is mostly limited by spinnability issues of the biomolecule-containing aqueous solutions. Coaxial electrospinning offers a promising alternative, as only the shell solution, producing the outer part of the fibre, must exhibit good spinnability. In this technique, drugs, proteins, or other bioactive substances are incorporated into the fibre core through coaxial flow, resulting in a core–sheath structure. These biphasic nanofibers, capable of controlled biomolecule release, have been extensively studied for biomedical applications, including osteochondral regeneration.63–65 However, despite its effectiveness, it often lacks the ability to precisely control scaffold architecture and mechanical properties. Nevertheless, developments in biomaterial design have shown the potential to overcome these limitations. For instance, electrospun scaffolds combining gelatin–chondroitin sulphate nanofibers with mechanically robust polycaprolactone (PCL) have been shown to successfully promote chondrogenesis without the need for differentiation media.66
In contrast, advanced RP techniques, such as 3D printing, including 3D bioprinting and selective laser sintering, allow for intricate scaffold design with precise spatial control, enabling the formation of complex structures layer by layer (LBL).67 3D bioprinting appears to be a promising approach for inserting biomolecules at desired 3D locations to build a scaffold with spatiotemporally controlled biomolecule release properties.68–70 The modification of scaffold characteristics depends on various factors such as the ink type, and other parameters such as the printing temperature, needle size, layer density, and extrusion rate.67,71,72
Additionally, the fabrication process must meet several critical criteria beyond its functionality, such as cost-effectiveness and scalability. Developing scalable manufacturing processes up to good manufacturing practice (GMP) standards is also crucial for ensuring the successful translation of TE strategies into clinical practice.
III. Biomolecules for cartilage tissue engineering
Several biomolecules, including but not restricted to, growth factors, peptides, genetic material and small molecules, are described to mediate cellular proliferation, migration, and differentiation. These factors can interact with target cells and trigger a series of specific cellular activities. Here, we focus on the main biomolecules involved in the development of cartilage and describe their role in regulating the processes of chondrogenesis and cartilage homeostasis (Table 3).| Biomolecules | Type of biomolecule | Desired effect on MSC chondrogenic differentiation | Adverse effects on differentiation | Ref. |
|---|---|---|---|---|
| Kartogenin | Small molecule | Induces chondrogenesis/enhances matrix production | — | 74, 76 and 289–293 |
| Curcumin | Small molecule | Induces chondrogenesis | — | 85 and 86 |
| Glucosamine | Small molecule | Induces chondrogenesis | — | 87 and 88 |
| Icariin | Small molecule | Induces chondrogenesis/inhibits hypertrophic differentiation | — | 89–91 |
| Melatonin | Small molecule | Induces chondrogenesis | — | 96 and 98 |
| Ascorbic acid | Small molecule | Chondrocyte growth/enhances cartilage matrix production | — | 101 and 102 |
| TGF-β1 and 3 | Growth factor | Enhances proliferation/inhibits migration/induces differentiation/maintains articular chondrocytes | Promotes hypertrophic differentiation | 104, 105, 253, 262, 275, 289 and 294–298 |
| BMP-2 | Growth factor | Enhances matrix production | Promotes hypertrophic differentiation | 299 and 300 |
| Induces osteogenic differentiation | ||||
| BMP-4 | Growth factor | Induces chondrogenesis/maintains chrondrocyte phenotype/enhances matrix production/inhibits hypertrophic differentiation | — | 115 |
| BMP-6 | Growth factor | Induces chondrogenesis/enhances matrix production/inhibits hypertrophic differentiation | Promotes hypertrophic differentiation | 301 and 302 |
| BMP-7 | Growth factor | Induces chondrogenesis/enhances matrix production/inhibits hypertrophic differentiation | — | 299, 303 and 304 |
| IGF-1 | Growth factor | Induces chondrogenesis | — | 120 |
| IGF-2 | Growth factor | Primes chondrogenic differentiation | — | 122 |
| FGF-2 (bFGF) | Growth factor | Maintains chondrogenic potential/enhances matrix production | Promotes fibrocartilage formation | 126–131 |
| PDGF | Growth factor | Exerts chemotactic effects/induces proliferation/induces chondrogenesis | — | 298, 305 and 306 |
| PTHrP | Protein | Inhibits hypertrophic differentiation | — | 139–141 and 307 |
| Peptides | Amino acid sequence | Recruit endogenous stromal cells | — | 147 and 150 |
| Nucleic acid | miRNA | Potential interest for all cartilage engineering steps | — | 153, 158, 159, 308 and 309 |
| siRNA | ||||
| Hypoxia-mimicking agents | Small molecules | Induces chondrogenesis/inhibits hypertrophic differentiation | — | 169, 170 and 310 |
III.1 Small molecules
III.2. Growth factors and other proteins
 | ||
| Fig. 1 Signalling pathways and proteins involved in the chondrogenic differentiation of mesenchymal stromal cells. Reproduced from ref. 112 with permission from Springer Nature, copyright 2022. | ||
III.3. Peptides
More recently, the use of peptides that can induce cellular responses such as cell recruitment, tissue integration and differentiation has been investigated in cartilage TE.145–147 Technological advances have reduced synthesis costs, making research and potential applications more accessible. For instance, the peptide “HAVDI” from the N-cadherin sequence mimics the cell–cell interaction signal, which is key to facilitating MSC condensation, the initial step of chondrogenesis.148 This interaction enhances the chondrogenic potential of MSCs encapsulated in HA hydrogels.149 Another approach relies on the use of chemoattractive peptides to recruit endogenous MSCs. Although this strategy aims to increase neotissue integration rather than cartilage engineering, it shows great potential for cartilage repair. Indeed, scaffolds prepared from porcine acellular cartilage matrix functionalized with BMHP (bone marrow homing peptide) enhanced cartilage formation in full-thickness cartilage defects of rabbits.150 After a six-month period, defects were filled with neocartilage tissue that exhibited a smooth surface similar to native tissue. A similar approach used an injectable hydrogel functionalized with KLPP peptide to facilitate the simultaneous recruitment of endogenous MSCs promoting interface integration and improving cartilage repair.147III.4. Nucleic acids
Nucleic acid-based strategies rely on the modulation of transcription factors or regulatory molecules in transfected cells in vitro or in endogenous cartilage tissue in ex vivo strategies.144,151,152 A better understanding of MSC biology has led to the discovery of numerous nucleic acid molecules capable of influencing the chondrogenic differentiation of MSCs, as a complementary and powerful strategy for cartilage TE.153–157 Due to their diversity, nucleic acid-based therapies can target the entire differentiation process, from its induction to the maintenance of the quiescent stage of chondrocytes and the inhibition of hypertrophy.158–160 As an example, using RNA interference (RNAi) tools, the specific suppression of anti-chondrogenic factors could represent a promising approach for MSC-based cartilage repair. Recently, the potential of siRNAs targeting the RUNX2 gene to inhibit the expression of hypertrophic markers after the chondrogenic differentiation of MSCs was demonstrated.155,161 A siRNA targeting sonic hedgehog (SHH) can significantly attenuate cartilage degeneration and decrease the OA score in rat models.162 Other studies have reported the potential of microRNAs (miRNAs) for regulating the expression of genes involved in cartilage synthesis and homeostasis.163–166 MiRNAs are short, single-stranded RNA molecules 18 to 24 nucleotides long. They function as transcriptional repressors by binding to the untranslated region (UTR) of target messenger RNA (mRNA), decreasing the expression of target genes. Over 30 miRNAs present in human joint tissue are implicated in regulating cartilage homeostasis and OA development. Among these, miR-140 has attracted considerable interest and multiple miR140 targets have been identified and described.166 Notably, the inhibitory effect of miR-140 in chondrocyte hypertrophy has been shown to occur through the inhibition of histone deacetylase (HDAC)-4 and SMAD1.167,168III.5. Hypoxia-mimicking molecules
It is well recognized that the chondrogenic differentiation of MSCs can be achieved by maintaining the cells under hypoxic conditions, thus simulating the native environment of articular cartilage.169 Additionally, hypoxic culture conditions have been shown to suppress the expression of markers associated with endochondral ossification through the activation of the PI3K/Akt/FoxO pathway.170 Secretion of the ECM was found to be enhanced when using human articular chondrocytes pretreated with hypoxia prior to encapsulation in alginate hydrogels and implantation in a nude mouse model.171Hypoxia induces the expression and stabilization of hypoxia-inducible factor-1α (HIF-1α), a key regulator of the hypoxic response that plays a critical role in chondrocyte differentiation and survival in vivo. Under normoxia conditions, HIF-1α is hydroxylated by prolyl-hydroxylase domain enzymes (PHDs) and the factor inhibiting HIF (FIH) hydroxylase, resulting in immediate ubiquitination and subsequent proteasomal degradation of the subunit. In a low-oxygen environment, the activities of PHDs and FIH are inhibited. HIF-1α accumulates in cells and binds to HIF-1β to form HIF-1, which then binds to HRE to participate in multiple signalling pathways by regulating the transcription of hundreds of genes, including those specific to cartilage. Currently, HIF signalling pathways mainly include PI3K-Akt/HIF-1α, SENP1/HIF-1α, HIF-1α/BNIP3, and MAPK/HIF-1α172 (Fig. 1). Instead of hypoxia incubators or chambers, several hypoxia-mimetic agents have been employed to induce hypoxia. These molecules are not widely studied but their low cost and ease of use make them a promising class of potential tools for use in cartilage TE.173
As described above, many biomolecules (Table 3) can be used to regulate cellular activity at different stages of chondrogenesis, depending on their function or the time of application. However, most of these active molecules have short-term action due to their rapid elimination or degradation after delivery. Therefore, they must be protected before being released in a controlled manner. Scaffold engineering to precisely fine-tune the spatiotemporal release of biomolecules is therefore being investigated to better control cell behaviour and in fine, improve cartilage TE efficacy.113
IV. Interactions and mechanisms involved in biomolecule delivery from scaffolds
IV.1. Non-covalent interactions
As the field of cartilage TE has rapidly expanded, many three-dimensional and porous scaffolds are considered to be active biomolecule-release systems. Typically, active molecules entrapped in polymer materials are released into the surrounding medium due to the combination of diffusion of the molecule through the matrix and matrix erosion. The proportion of these two contributions depends on the nature of the polymers, the hydration level of the matrix, its porosity and interactions, specific or not, between the active compounds and the supporting materials. Regarding diffusion, in the absence of a covalent bond between the biomolecules and the matrix, the retention rates and release kinetics mostly result from weak interactions and steric hindrance (Fig. 2).To control the release of siRNA, siRNA lipoplexes encapsulated in a gellan gum hydrogel exhibited prolonged release over 60 days, while naked siRNA was released from the hydrogel within 48 h.182 Similarly, the impact of steric interactions was evaluated in the case of siRNA nanoparticles (si-NPs) loaded in a porous biodegradable polyester–polyurethane (PUR) scaffold.183 The si-NPs were formulated with a defined charge ratio between positively charged tertiary amines on the DMAEMA block of the polymer and negatively charged phosphate groups on the siRNA backbone (Fig. 3). The diffusion and release kinetics of encapsulated siRNAs and free siRNAs were compared. The release data demonstrated cumulative release of si-NPs approaching 80% over 21 days, which was considerably slower than naked siRNAs completely released in three days. The release rates of naked and complexed siRNAs scaled with their hydrodynamic diameters and their diffusivity throughout the PUR matrix. This property is particularly useful for gene transfer strategies, since NPs preserve nucleic acid integrity and serve as a vector that enables genetic material to cross the cell membrane.153,184,185 Recently, we demonstrated that it was possible to obtain different siRNA diffusion profiles from a collagen hydrogel, depending on the size of the vector used.186 Interestingly, the inhibition profiles of the target gene Runx2 over time was correlated with the release kinetics, and hence with the size of the siRNA vector.
 | ||
| Fig. 3 Sustained local delivery of siRNA from an injectable scaffold. (a) Chemical structure of polyurethane precursors. Lysine triisocyanate reacts with the –OH groups of the polyol to form urethane bonds and creates the PUR network. (b) Chemical structure of the micelle-forming, pH-responsive diblock copolymer used for siRNA packaging and intracellular delivery. The homo-2-(diethylamino)ethyl methacrylate (DMAEMA) block was designed for siRNA condensation due to the positive charge on the tertiary amines. The other block is pH-responsive and tuned for endosomal escape due to micelle destabilization and endosomolytic activity triggered by protonation of 2-propyl acrylic acid (PAA) and DMAEMA. (c) Naked siRNA is rapidly released with an initial burst of over 60% after 12 h and is entirely released after 3 days. Si-NPs exhibit slower kinetics with a burst release of less than 20% during the first 12 h, followed by sustained release reaching 80% after 21 days. The Weibull empirical model equation best-fit was determined and is overlaid here for each data set. Reproduced from ref. 183 with permission from Elsevier, copyright 2012. | ||
A multitude of factors can affect the rate of scaffold degradation in vivo, including the surrounding environment, the composition and structure of the biomaterial, and the physical loading to which the scaffold is subjected.189–191 Indeed, the degradation of a polymer scaffold involves chain cleavage processes induced for instance by hydrolysis, oxidation or photodegradation.190In vivo, scaffold degradation takes place in an aqueous biological environment where hydrolysis plays an important role, often promoted by enzymatic activities (proteases, esterases…). In the context of cartilage TE, the degradation of the natural polymer scaffold, comprised of collagen for instance, may be accelerated by matrix metalloproteases (MMPs) secreted by chondrocytes resulting in accelerated or triggered release of biomolecules.192 As an example, micelles containing miR-140 entrapped in MMP-sensitive microparticles composed of gelatin methacryloyl hydrogel have been developed.193 In the presence of MMPs, complete release of miR-140 from the microparticles was achieved after 5 days, compared to more than 14 days in the absence of MMPs (Fig. 5). The authors exploited the presence of large amounts of MMPs in the OA joint capsule to degrade the scaffold, thus releasing the entrapped micelles loaded with miR-140. After in vivo injection, a notable reduction in osteophyte formation and OARSI score was demonstrated in a DMM-induced OA model. The group that received the micelles containing miR-140 entrapped in MMP-sensitive microparticles demonstrated the most favourable outcome with regard to GAG level, indicating optimal retention of the cartilage thickness. In addition, the expression of COL2 was the highest while MMP13 expression exhibited an inverse correlation among this group. These findings collectively suggest that these MMP-sensitive microparticles have the potential to delay the degeneration of articular cartilage and the progression of OA.
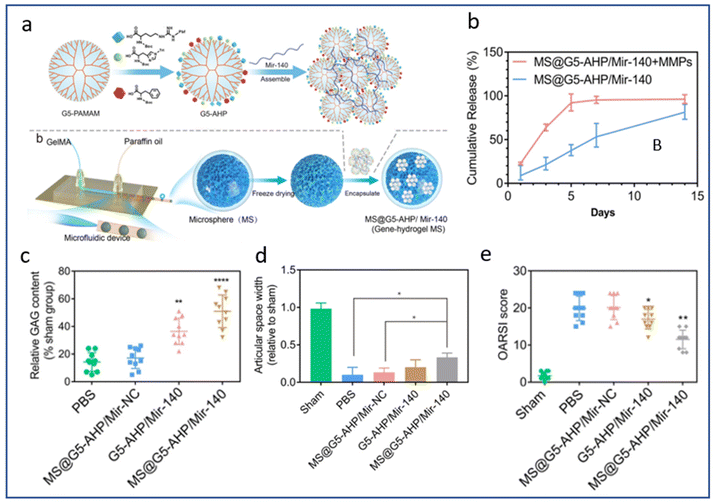 | ||
| Fig. 5 MMPs triggered mir-140 release for local treatment of osteoarthritis. (a) Synthesis of the multifunctional gene vector: arginine (A), histidine (H), and phenylalanine (P)-modified generation 5 (G5) polyamidoamine (G5-AHP), G5-AHP/miR-140 and G5-AHP/miR-140 after immobilization in gene–hydrogel microspheres (MS@G5-AHP/miR-140). (b) Cumulative release curve of MS@G5-AHP/miR-140. (c) Relative glycosaminoglycan (GAG) content after 12 weeks. (d) Articular space width in the medial compartment of the mouse knee joints 12 weeks after surgery. (e) OARSI score for each group after 12 weeks. Reproduced from ref. 193 with permission from Springer Nature, copyright 2022. | ||
Similarly, MMP-13-responsive hydrogel microspheres were used to control the release of celecoxib-loaded liposomes in the context of OA.194 Compared to microspheres immersed in hyaluronidase only, the drug release efficiency reached 82% on day 5 after immersion in the solution containing MMP13 and hyaluronidase, indicating a significantly accelerated release of celecoxib in the presence of MMP13 (Fig. 6b). After inducing OA in rats via ACL transection and partial medial meniscectomy, intra-articular injection of these microspheres yielded a significant reduction in cartilage degeneration,194 as shown by a lower OA score, larger joint space width and reduced osteophyte formation.
 | ||
| Fig. 6 MMP13-responsive hydrogel microspheres for precise delivery of celecoxib. (a) Schematic representation of the responsive release of celecoxib from hydrogel microspheres induced by MMP13. (b) Drug release profiles of celecoxib in HAase, MMP13 and MMP13/HAase solutions. (c) Measurement of joint space width of the lateral knee joint compartment evaluated from X-rays. (d) Relative osteophyte volume measured by micro-CT. (e) OARSI scores determined on histological sections of knee joints. Reproduced from ref. 194 with permission from Elsevier, copyright 2024. | ||
The rate of degradation also depends on the composition and structure of the biomaterial. For example, the release of curcumin from a gelatin scaffold can be prolonged by adding silk fibers to the scaffold.195 Another study showed that the degradation rate of a scaffold made of PLGA and PLA nanofibers could be adapted by varying the PLGA/PLA ratio: the higher the PLGA/PLA ratio, the faster the scaffold degraded.196 Finally, cartilage repair is also correlated with mechanical loading applied to the scaffold. Higher shear stresses resulted in early and fast release of sirolimus, with high cumulative drug release.197
However, achieving precise control of biomolecule delivery solely by adjusting scaffold biodegradation remains limited. One reason is that, depending on the application, it might be required that the active molecule be released faster than the scaffold degradation rate, which should be consistent with the rate of neo-tissue formation. In contrast, diffusion out of a highly solvated hydrogel is often very fast, hence retention of the active compounds through weak interactions or reversible covalent attachment to the matrix is needed. Different strategies have been explored to achieve optimal delivery profiles of single or multiple drugs.198 A comprehensive understanding of the interactions and release mechanisms underlying the drug delivery kinetics is key to providing new insights into cartilage repair.
Several natural macromolecules—abundant in the connective tissues of vertebrates (collagen, heparin, or hyaluronic acid) and largely used to elaborate scaffolds for TE—exhibit attractive interactions with diffusing active biomolecules. Collagen is the main component of the cartilage ECM and among the most used material for the construction of scaffolds in TE. Some GFs, including TGF-β1, bFGF and BMP-2, are demonstrated to naturally possess a strong affinity for collagen and can bind to collagen-based scaffolds via ionic interactions. However, the release profiles usually feature an initial burst, which makes this approach unsuitable for controlled release.199,200 One possibility to enhance their binding to the scaffold and slow down their release is to engineer recombinant GF by adding a collagen binding domain (CBD) at one terminus.201,202 Indeed, recombinant PTHrP expressed as a fusion with a CBD heptapeptide displayed a higher collagen-binding capacity than that of the native PTHrP, with a dissociation constant two times lower.203 Applied to cartilage TE, this engineered peptide allowed for sustained release from a collagen scaffold and a prolonged effect over several days. Conversely, peptides with a strong affinity for specific regions of GFs can be designed and bound to the scaffolds to control the GF presentation and activity.204 The modification of scaffolds with such peptides resulted in higher retention, less dissemination and better controlled release, thus reducing the amount of GFs required and providing a more cost-effective approach for TE applications.205–207 Several TGFβ1-binding peptides, including HSNGLPL, have been used to functionalize biopolymer scaffolds, with in vitro and in vivo applications.208–211 Similarly, the incorporation of heparin into scaffolds is widely used for drug delivery purposes.193,196,212–215 Indeed, positively charged amino acids of GFs can interact with GAGs through electrostatic interactions, particularly with the sulphate groups of heparan sulphate proteoglycans (HSPGs), which are widely produced in the ECM of tissues.213,216–220 For instance, heparin was trapped as a semi-interpenetrated polymer within a crosslinked network or covalently bound to a polymer backbone by grafting tyramine, methacrylate, thiols or maleimide moieties to the heparin chains.221–225 Once trapped in the network of polymers or grafted onto the scaffold, electrostatic interactions established between heparin and GFs such as TGF-β or bFGF slowed down their release. Recently, heparin covalently conjugated to a hyaluronan hydrogel was shown to achieve the sustained release of 60% TGF-β1 after two weeks, whereas in the absence of heparin, 97% of TGF-β1 was released over the same period.226 The system demonstrated remarkable efficacy for promoting chondrogenesis, as shown by 19- and 32-fold increases in aggrecan and type 2 collagen expression, respectively, after 21 days of differentiation when heparin was covalently conjugated to a hyaluronan hydrogel (Fig. 8).
 | ||
| Fig. 8 Injectable heparin-conjugated scaffold for local delivery of TGF-β1. (A) Schematic of the study design. (B) Cumulative TGF-β1 release from hyaluronan gel (HA-TG), hyaluronan gel + heparin–glutamine (HA-TG/Hep-TG) or hyaluronan gel + heparin (HA-TG/Hep). Gene expression after 21 days of differentiation for (C) aggrecan and (D) collagen type II. Reproduced from ref. 226 with permission from Elsevier, copyright 2019. | ||
Interestingly, sulphated alginate, which is used in cartilage TE for its beneficial effects on chondrocyte proliferation and phenotype maintenance, can also act as a GAG analogue and interact with most GFs, thereby extending their therapeutic effect.227–231 This dual property of alginate was used to form a macro-porous alginate scaffold, where uronic acid units were sulphated to mimic heparin, and successfully loaded with TGF-β1 to enhance MSC chondrogenesis.232 Finally, electrostatic interactions can also be involved, in particular for gene therapy applications using non-viral vectors for gene transfer.1,153,233,234 Naked nucleic acids bear a negative charge, while the vectors obtained by complexation with cationic polymers or lipids exhibit a net positive charge.183,235 For example, the incorporation of siRNA into positively charged copolymer micelles significantly slowed down the release from injectable polyurethane; this could be attributed in part to attractive electrostatic interactions between the vector and the scaffold matrix.183
Hydrophobic interactions also largely participate in the retention of biomolecules in a scaffold. As an example, gelatin or gelatin–silk fibroin microspheres have been used for the sustained release of curcumin adsorbed into the microspheres for the treatment of OA.195 The slower release rate with gelatin–silk fibroin microspheres than gelatin microspheres was attributed to hydrophobic interactions between curcumin and the hydrophobic domains of the silk fibroin, along with the lower degradation rate compared to that of pure gelatin microspheres. It is also possible to alter the interactions between proteins and scaffolds to control the biomolecule release kinetics. For example, PLGA microspheres have been used as pharmacologically active microcarriers for the delivery of TGF-β3 to promote the differentiation of MSCs into chondrocytes.236 In this study, PLGA microspheres were modified with poloxamer P188, resulting in a more hydrophilic scaffold and therefore allowing for greater and sustained release of TGF-β3, reaching 70% on day 30. These PLGA microspheres modified with poloxamer P188, exerted a superior effect on chondrogenic differentiation compared to unmodified PLGA-TGF-β3.
IV.2. Covalent interactions
Covalent binding of biomolecules to scaffolds is commonly used to increase retention rates and significantly reduce the initial burst release often observed with non-covalent methods.237 Biomolecules can be directly grafted to the materials or attached via a linker. The immobilization strategies may involve existing chemical functions present on the scaffolds and biomolecules or require previous activation. Because biomolecules are usually not soluble or will be denatured in organic solvents, their bioconjugation requires the use of aqueous-based chemistry. To address these needs, various activation strategies are being developed, and the main biocompatible chemical immobilization systems adapted to cartilage TE are presented below (see also Table 4) (Fig. 9).| Interaction type | Entrapment mechanism | Biomolecules | Ref. |
|---|---|---|---|
| Non-covalent interactions | Weak interactions | TGFβ1-2-3/PTHrP/bFGF/BMP-2/curcumin/chondroitin sulphate/nucleic acid | 173, 179–182 and 188–293 |
| Steric entrapment | TGFβ1-2-3/IGF/bFGF/PTHrP/BMPs/kartogenin/chondroitin sulphate/curcumin/nucleic acid | 151–153, 156 and 164–169 | |
| Covalent interactions | Crosslinking | TGF-β1-3/BMP-2/BMP-4/kartogenin | 207–210 and 212 |
| Photoimmobilization | BMP-2/PDGF | 95 and 217–221 | |
| Click chemistry | TGF-β1/BMP-2 | 227–228 | |
| Multi-scaffolding system | Combination of several techniques | TGF-β1-2-3/BMP-7/kartogenin/curcumin | 237–246 and 253–255 |
 | ||
| Fig. 10 Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles. Cumulative release of KGN from (a) kartogenin-conjugated chitosan microparticles (CHI-KGN MPs) and (b) nanoparticles (CHI-KGN NPs). (c) Safranin-O and Alcian-blue staining of cell micropellets cultured with or without kartogenin, CHI-KGN MPs or CHI-KGN NPs after 3 weeks. (d) OARSI scores from histological sections of medial tibial plateaus of rats injected with vehicle, KGN or implanted with the CHI-KGN MPs or CHI-KGN NPs after 14 weeks. Reproduced from ref. 244 with permission from Elsevier, copyright 2014. | ||
However, immobilization on the scaffold and the various necessary steps required can affect the bioactivity of the drug. Carbodiimide coupling can occur with the amine groups present in the lysine residues or N-terminus of GF, as well as the carboxylic groups present in the aspartate or glutamate residues or C-terminus. This lack of selectivity may result in some bioactive functional groups being involved in grafting bond formation, potentially resulting in a loss of GF bioactivity.204 Similarly, the presence of numerous functional groups leads to the random immobilisation of GF, thereby affecting the accessibility of ligands to the corresponding cell receptors.205
Another major click chemistry reaction that is attracting a great deal of interest in the biomedical field is strain-promoted azide–alkyne cycloaddition (SPAAC). For example, alkyne groups were added to BMP-2 and azides were added to a methoxy polyethylene glycol–polycaprolactone to facilitate attachment and in fine improve cell differentiation.251 In another example, hydroxyethyl cellulose (HEC) was modified to make it amenable for biorthogonal click chemistry. This largely available modified natural polymer has been considered for cartilage TE but it lacks reactive functional groups. A method based on an initial esterification step with citric acid to introduce carboxylic functions (handles), which are used to introduce either azide or alkyne (DBCO) moieties, was developed. Alkyne- and azide-modified HEC are then mixed and reacted through SPAAC to form a biocompatible and biorthogonal HEC scaffold.252 The reactivity introduced into HEC can be exploited to immobilize biomolecules, for instance, N-terminal azide-modified GF. However, poorly controlled covalent modifications can negatively affect the activity of biomolecules. Therefore, the immobilization strategy should be appropriate for the chemistry of biomaterials, the availability of reactive groups in the biomolecule structure and the location of the reactive groups relative to the receptor binding domain.253,254
IV.3. Multi-scaffolding system
Hydrogels are efficient biomaterials for TE, as already widely described, but are limited for the long-term delivery of biomolecules mainly due to the lack of strong interactions to prevent or slow down the release of molecules.261,263–265 To overcome this limitation, drug-loaded MPs or NPs can be incorporated into the hydrogels. Such composite systems, owing to the wide range of chemical structures and properties available for the polymers or inorganic materials, are a strong alternative for the localized entrapment of bioactive cargo, and their controlled and sequential release in cartilage TE266 (Fig. 11).MPs and NPs display high surface area to volume ratios, small dimensions, high drug encapsulation efficiencies and the capacity to quickly respond to surrounding environmental stimuli, such as temperature, pH, magnetic fields or ultrasound.267–269 In this field, the PLGA polymer is widely used due to its controllable degradation profile, ease of manufacture and FDA approval for drug delivery in clinical applications.270 Recently, an injectable hydrogel system (Col-Apt@KGN MPs), which is a collagen-based scaffold containing aptamer 19S (Apt19S) and PLGA-based MPs encapsulating KGN, was described to enable the sequential release of Apt19S and KGN271 (Fig. 12). Apt19S was rapidly released from the hydrogel within 6 days, while KGN was slowly released for 33 days via the degradation of PLGA MPs. Apt19S enabled the recruitment of endogenous MSCs and KGN promoted their chondrogenic differentiation and cartilage-specific ECM secretion, as confirmed by higher production of GAGs in the Col@KGN MP and Col-Apt@KGN MP groups after 14 and 21 days. In a rabbit model of full-thickness cartilage defects, the Col-Apt@KGN MP group demonstrated the most effective repair, with the regenerated tissue showing a smooth surface and uniform integration into the surrounding healthy cartilage and an ICRS score significantly superior to that of the other groups after 14 weeks.
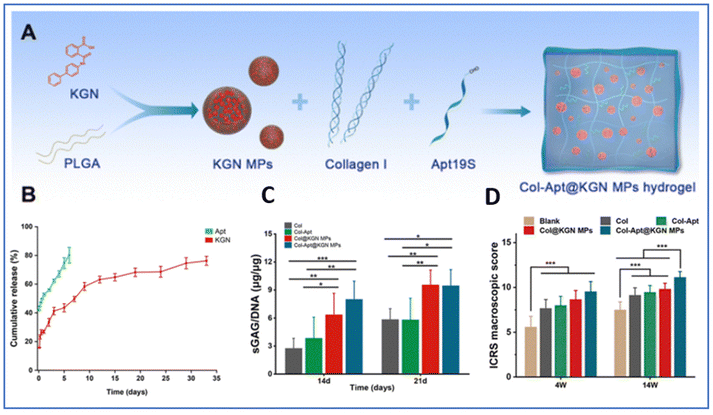 | ||
| Fig. 12 Spatiotemporal sequential releasing hydrogel for cartilage regeneration. (A) Fabrication of a collagen hydrogel incorporating I-Apt19S and KGN-loaded PLGA microspheres (Col-Apt@KGN MPs). (B) Cumulative drug release over 33 days in vitro. (C) Quantitative determination of the sGAG/DNA ratio after 14 days and 21 days. (D) ICRS macroscopic score evaluating cartilage repair. Reproduced from ref. 271 with permission from RSC, copyright 2008. | ||
Another composite scaffold, consisting of an injectable chitosan/silk fibroin hydrogel and PLGA MPs loaded respectively with SDF-1 and KGN, was successfully used to achieve the sequential release of these two biomolecules for cartilage TE.272 The authors suggest that the burst release of SDF-1 (ca. 40% after 24 h) accounts for the recruitment of endogenous MSCs to the defect area. The slower and sustained release of KGN promoted the differentiation of MSCs into chondrocytes, hence favouring cartilage repair. This approach gave interesting results in vivo, after creating surgical lesions on rabbit knees.
Finally, polyionic complex NPs loaded with TGF-β2 were encapsulated into an alginate hydrogel impregnated with BMP-7. This resulted in the sequential delivery of biological factors; BMP-7 was released faster than TGF-β2, with 80% and 30% of the GF, respectively, being released after 21 days of incubation.273 Since the molecular weight of the polymer that forms the particles could affect the release kinetics of the encapsulated drug, this property was also evaluated to tune the release of different active compounds. In vitro, TGFβ3 loaded into low-molecular-weight PLGA (10 kDa)-based MPs exhibited sustained release over 28 days, reaching approximately 96% of the initial dose.274 In contrast, lenvatinib, an anti-angiogenic small molecule, was released at a much slower rate from high-molecular-weight PLGA (100 kDa)-based MPs, with only 40% being released after 56 days. Although TGFβ3 and lenvatinib have distinct chemical natures, their dual release from 10 kDa and 100 kDa PLGA MPs resulted in the significant downregulation of osteogenesis-related genes (BMP2, RUNX2, OPN, OCN, ALP) being observed after 56 days.
Processing and 3D structures, as described in section II.3, offer versatile strategies for manipulating drug loading and release profiles from scaffolds.139,275 Layer-by-layer assembly is commonly used to construct porous scaffolds with good performance in avoiding GF loss of function, while achieving a high sequestration rate under mild aqueous conditions and controlled delivery. A method for the 3D printing of hydrogels with core–shell capsules sensitive to external stimuli was developed for the on-demand release of biomolecules.276 The capsules consisted of an aqueous core, which could be formulated to maintain the activity of payload biomolecules—here, the horse peroxidase (HRP) protein was used as a proof-of-concept—and a PLGA shell that sterically held the molecules inside the capsules. The shell is loaded with plasmonic gold nanorods (AuNR) that selectively disrupt the capsules when irradiated with a laser at a specific wavelength, therefore triggering the release of HRP with high spatiotemporal control.276 Similarly, TGF-β1-embedding core–shell nanospheres were fabricated via co-axial electrospraying of the GF along with PLGA and then mixed to form a bioink composed of 10% gelatin methacrylate (GelMA), 5% polyethylene glycol diacrylate (PEGDA) and a biocompatible photoinitiator.277 The MSC-laden constructs were 3D bioprinted by stereolithography and the sustained release of TGF-β1 for up to 21 days significantly improved the chondrogenic differentiation of encapsulated MSCs.
V. Challenges and future perspectives
Despite considerable advances being made in optimising scaffold design and association with bioactive molecules, clinical translation has achieved only limited success in the treatment of articular cartilage defects. While pre-clinical experiments in small and large animal models have yielded promising results, further investigation is necessary to assess the clinical safety, reliability, and efficacy of the TE strategy. With regard to the controlled release of drugs over space and time, the majority of research revolves around conditions simulated in vitro. However, there is a lack of clear evidence regarding the actual dosage and kinetics of growth factor release in vivo. It is necessary to create reliable assessment tools that can noninvasively track growth factor delivery after implantation. Real-time monitoring in living organisms represents one of the main challenges that requires urgent consideration, in particular for cartilage TE.A further issue that requires attention is the occurrence of hypertrophic or fibrotic neotissue over time following scaffold implantation in cartilage repair strategies. It is of paramount importance to prevent hypertrophy or fibrosis to enable appropriate integration between the implant and surrounding endogenous tissues. To address this challenge, the incorporation of anti-hypertrophic or anti-fibrotic cues may enhance the stability and longevity of engineered cartilage, thereby advancing the field closer to developing functional cartilage repair therapies. It is therefore crucial to gain a deeper understanding of the precise timing and dosage for their application and to control their release from scaffolds. Concurrently, cell source modulation, genetic engineering and optimisation of culture conditions will be pivotal factors in the translation of TE approaches for clinical success.
The microenvironment-responsive release approach is emerging as a promising solution for controlling the timing of molecule release. The use of cleavable linkers, such as those sensitive to pH or proteases, represents a significant opportunity to selectively release active molecules in response to changes in the nearby tissue environment, thereby controlling the temporal and spatial availability of specific factors for optimized tissue regeneration. Future strategies will undoubtedly benefit from evolving advances in monitoring, fabrication techniques and novel strategic pairings of biomolecules.
VI. Conclusion
Cartilage TE seeks to generate neo-tissue that mimics the physiological function of native cartilage, offering a viable solution for cartilage repair. However, existing strategies often result in the deposition of an ECM with suboptimal biomechanical properties that degrade over time, poor integration into the host tissue or tissue fibrosis. To enhance cartilage regeneration using scaffolds containing MSCs or chondrocytes, various biomolecules have been incorporated into different types of biomaterials in order to boost their biological activity and reduce the need for repeated injections. These biofactors play crucial roles in differentiation processes or cartilage homeostasis and typically require sustained release due to their rapid clearance in vivo. Moreover, the kinetics of their release must be tailored to the specific biofactor. Recent advances have successfully employed scaffolds as biomolecule reservoirs, ensuring prolonged release of active molecules over weeks or even months, with promising outcomes for cartilage engineering. Innovations in biomaterials chemistry have further improved the control and retention of biomolecules within scaffolds, preserving their bioactivity. However, the majority of controlled release research is conducted in vitro, under simulated conditions, and the absence of real-time monitoring in living organisms remains a major limitation, hindering further progress in the field. It is clear that future strategies will greatly benefit from the integration of advanced monitoring technologies, innovative fabrication techniques, and the development of novel agent combinations. Additionally, cleavable linkers, responsive to pH or proteases, offer a promising approach for the selective release of active molecules in response to dynamic changes in the local tissue environment, representing a critical avenue for future research and application in cartilage TE.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.References
- A. Saraf and A. G. Mikos, Gene delivery strategies for cartilage tissue engineering, Adv. Drug Delivery Rev., 2006, 58(4), 592–603 CrossRef CAS PubMed.
- T. A. Ahmed and M. T. Hincke, Strategies for articular cartilage lesion repair and functional restoration, Tissue Eng., Part B, 2010, 16(3), 305–329 CrossRef CAS PubMed.
- J. E. Browne and T. P. Branch, Surgical alternatives for treatment of articular cartilage lesions, J. Am. Acad. Orthop. Surg., 2000, 8(3), 180–189 CrossRef CAS PubMed.
- E. B. Hunziker, Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects, Osteoarthritis Cartilage, 2002, 10(6), 432–463 CrossRef CAS PubMed.
- A. R. Armiento, M. J. Stoddart, M. Alini and D. Eglin, Biomaterials for articular cartilage tissue engineering: Learning from biology, Acta Biomater., 2018, 65, 1–20 CrossRef CAS PubMed.
- J. B. Costa, H. Pereira, J. Espregueira-Mendes, G. Khang, J. M. Oliveira and R. L. Reis, Tissue engineering in orthopaedic sports medicine: current concepts, J. ISAKOS, 2017, 2(2), 60–66 CrossRef.
- J. A. Buckwalter, H. J. Mankin and A. J. Grodzinsky, Articular cartilage and osteoarthritis, Instr. Course Lect., 2005, 54, 465–480 Search PubMed.
- J. A. Buckwalter, S. L. Woo, V. M. Goldberg, E. C. Hadley, F. Booth and T. R. Oegema, et al., Soft-tissue aging and musculoskeletal function, J. Bone Joint Surg. Am., 1993, 75(10), 1533–1548 CrossRef CAS PubMed.
- M. W. Kessler, G. Ackerman, J. S. Dines and D. Grande, Emerging technologies and fourth generation issues in cartilage repair, Sports Med. Arthrosc. Rev., 2008, 16(4), 246–254 CrossRef PubMed.
- E. Perrier-Groult, M. Pasdeloup, M. Malbouyres, P. Galéra and F. Mallein-Gerin, Control of collagen production in mouse chondrocytes by using a combination of bone morphogenetic protein-2 and small interfering RNA targeting Col1a1 for hydrogel-based tissue-engineered cartilage, Tissue Eng., Part C, 2013, 19(8), 652–664 CrossRef CAS PubMed.
- A. J. Rosenbaum, D. A. Grande and J. S. Dines, The use of mesenchymal stem cells in tissue engineering: A global assessment, Organogenesis, 2008, 4(1), 23–27 CrossRef PubMed.
- H. Nejadnik, J. H. Hui, E. P. F. Choong, B. C. Tai and E. H. Lee, Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study, Am. J. Sports Med., 2010, 38(6), 1110–1116 CrossRef PubMed.
- I. Uzieliene, E. Bagdonas, K. Hoshi, T. Sakamoto, A. Hikita and Z. Tachtamisevaite, et al., Different phenotypes and chondrogenic responses of human menstrual blood and bone marrow mesenchymal stem cells to activin A and TGF-beta3, Stem Cell Res. Ther., 2021, 12(1), 251 CrossRef CAS PubMed.
- M. Maumus, J. A. Peyrafitte, R. D'Angelo, C. Fournier-Wirth, A. Bouloumie and L. Casteilla, et al., Native human adipose stromal cells: localization, morphology and phenotype, Int. J. Obes., 2011, 35(9), 1141–1153 CrossRef CAS PubMed.
- T. M. Liu, M. Martina, D. W. Hutmacher, J. H. Hui, E. H. Lee and B. Lim, Identification of common pathways mediating differentiation of bone marrow- and adipose tissue-derived human mesenchymal stem cells into three mesenchymal lineages, Stem Cells, 2007, 25(3), 750–760 CrossRef CAS PubMed.
- T. Hennig, H. Lorenz, A. Thiel, K. Goetzke, A. Dickhut and F. Geiger, et al., Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6, J. Cell Physiol., 2007, 211(3), 682–691 CrossRef CAS PubMed.
- Q. L. Loh and C. Choong, Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size, Tissue Eng., Part B, 2013, 19(6), 485–502 CrossRef CAS PubMed.
- I. Bružauskaitė, D. Bironaitė, E. Bagdonas and E. Bernotienė, Scaffolds and cells for tissue regeneration: different scaffold pore sizes-different cell effects, Cytotechnology, 2016, 68(3), 355–369 CrossRef PubMed.
- T. Takahashi, T. Ogasawara, Y. Asawa, Y. Mori, E. Uchinuma and T. Takato, et al., Three-dimensional microenvironments retain chondrocyte phenotypes during proliferation culture, Tissue Eng., 2007, 13(7), 1583–1592 CrossRef CAS PubMed.
- X. Zhang, Y. Wu, Z. Pan, H. Sun, J. Wang and D. Yu, et al., The effects of lactate and acid on articular chondrocytes function: Implications for polymeric cartilage scaffold design, Acta Biomater., 2016, 42, 329–340 CrossRef CAS PubMed.
- Y. G. Koh, J. A. Lee, Y. S. Kim, H. Y. Lee, H. J. Kim and K. T. Kang, Optimal mechanical properties of a scaffold for cartilage regeneration using finite element analysis, J. Tissue Eng., 2019, 10, 2041731419832133 CrossRef PubMed.
- F. J. O'Brien, Biomaterials & scaffolds for tissue engineering, Mater. Today, 2011, 14(3), 88–95 CrossRef.
- F. T. Moutos and F. Guilak, Composite scaffolds for cartilage tissue engineering, Biorheology, 2008, 45(3–4), 501–512 Search PubMed.
- M. Wasyłeczko, W. Sikorska and A. Chwojnowski, Review of Synthetic and Hybrid Scaffolds in Cartilage Tissue Engineering, Membranes, 2020, 10(11), 348 CrossRef PubMed.
- M. Krishani, W. Y. Shin, H. Suhaimi and N. S. Sambudi, Development of Scaffolds from Bio-Based Natural Materials for Tissue Regeneration Applications: A Review, Gels, 2023, 9(2), 100 CrossRef CAS PubMed.
- D. C. Crawford, C. M. Heveran, W. D. Cannon, L. F. Foo and H. G. Potter, An autologous cartilage tissue implant NeoCart for treatment of grade III chondral injury to the distal femur: prospective clinical safety trial at 2 years, Am. J. Sports Med., 2009, 37(7), 1334–1343 CrossRef PubMed.
- V. Irawan, T. C. Sung, A. Higuchi and T. Ikoma, Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development, Tissue Eng. Regener. Med., 2018, 15(6), 673–697 CrossRef CAS PubMed.
- I. Lukin, I. Erezuma, L. Maeso, J. Zarate, M. F. Desimone and T. H. Al-Tel, et al., Progress in Gelatin as Biomaterial for Tissue Engineering, Pharmaceutics, 2022, 14(6), 1177 CrossRef CAS PubMed.
- H. W. Liu, W. T. Su, C. Y. Liu and C. C. Huang, Highly Organized Porous Gelatin-Based Scaffold by Microfluidic 3D-Foaming Technology and Dynamic Culture for Cartilage Tissue Engineering, Int. J. Mol. Sci., 2022, 23(15), 8449 CrossRef CAS PubMed.
- E. Arslan, M. Sardan Ekiz, C. Eren Cimenci, N. Can, M. H. Gemci and H. Ozkan, et al., Protective therapeutic effects of peptide nanofiber and hyaluronic acid hybrid membrane in in vivo osteoarthritis model, Acta Biomater., 2018, 73, 263–274 CrossRef CAS PubMed.
- M. Wang, Z. Deng, Y. Guo and P. Xu, Designing functional hyaluronic acid-based hydrogels for cartilage tissue engineering, Mater. Today Bio, 2022, 17, 100495 CrossRef CAS PubMed.
- C. E. Garcia Garcia, B. Lardy, F. Bossard, F. A. Soltero Martìnez and M. Rinaudo, Chitosan based biomaterials for cartilage tissue engineering: Chondrocyte adhesion and proliferation, Food Hydrocolloids Health, 2021, 1, 100018 CrossRef CAS.
- A. Sadeghianmaryan, S. Naghieh, H. Alizadeh Sardroud, Z. Yazdanpanah, Y. Afzal Soltani and J. Sernaglia, et al., Extrusion-based printing of chitosan scaffolds and their in vitro characterization for cartilage tissue engineering, Int. J. Biol. Macromol., 2020, 164, 3179–3192 CrossRef CAS PubMed.
- S. Varghese, N. S. Hwang, A. C. Canver, P. Theprungsirikul, D. W. Lin and J. Elisseeff, Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells, Matrix Biol., 2008, 27(1), 12–21 CrossRef CAS PubMed.
- Y. Chen, W. Xu, M. Shafiq, D. Song, X. Xie and Z. Yuan, et al., Chondroitin sulfate cross-linked three-dimensional tailored electrospun scaffolds for cartilage regeneration, Biomater. Adv., 2022, 134, 112643 CrossRef CAS PubMed.
- A. B. Bonhome-Espinosa, F. Campos, D. Durand-Herrera, J. D. Sánchez-López, S. Schaub and J. D. G. Durán, et al., In vitro characterization of a novel magnetic fibrin-agarose hydrogel for cartilage tissue engineering, J. Mech. Behav. Biomed. Mater., 2020, 104, 103619 CrossRef CAS PubMed.
- M. Farokhi, F. Jonidi Shariatzadeh, A. Solouk and H. Mirzadeh, Alginate Based Scaffolds for Cartilage Tissue Engineering: A Review, Int. J. Polym. Mater. Polym. Biomater., 2020, 69(4), 230–247 CrossRef CAS.
- C. C. Huang, Characteristics and Preparation of Designed Alginate-Based Composite Scaffold Membranes with Decellularized Fibrous Micro-Scaffold Structures from Porcine Skin, Polymers, 2021, 13(20), 3464 CrossRef CAS PubMed.
- U. Urtaza, O. Guaresti, I. Gorroñogoitia, A. Zubiarrain-Laserna, E. Muiños-López and F. Granero-Moltó, et al., 3D printed bioresorbable scaffolds for articular cartilage tissue engineering: a comparative study between neat polycaprolactone (PCL) and poly(lactide-b-ethylene glycol) (PLA-PEG) block copolymer, Biomed. Mater., 2022, 17(4), 045028 CrossRef CAS PubMed.
- D. H. Rosenzweig, E. Carelli, T. Steffen, P. Jarzem and L. Haglund, 3D-Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration, Int. J. Mol. Sci., 2015, 16(7), 15118–15135 CrossRef CAS PubMed.
- M. S. Singhvi, S. S. Zinjarde and D. V. Gokhale, Polylactic acid: synthesis and biomedical applications, J. Appl. Microbiol., 2019, 127(6), 1612–1626 CrossRef CAS PubMed.
- D. da Silva, M. Kaduri, M. Poley, O. Adir, N. Krinsky and J. Shainsky-Roitman, et al., Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems, Chem. Eng. J., 1996. 2018, 340, 9–14 Search PubMed.
- K. Budak, O. Sogut and U. Aydemir Sezer, A review on synthesis and biomedical applications of polyglycolic acid, J. Polym. Res., 2020, 27(8), 208 CrossRef CAS.
- M. Morille, K. Toupet, C. N. Montero-Menei, C. Jorgensen and D. Noël, PLGA-based microcarriers induce mesenchymal stem cell chondrogenesis and stimulate cartilage repair in osteoarthritis, Biomaterials, 2016, 88, 60–69 CrossRef CAS PubMed.
- N. R. Eviana Putri, X. Wang, Y. Chen, X. Li, N. Kawazoe and G. Chen, Preparation of PLGA-collagen hybrid scaffolds with controlled pore structures for cartilage tissue engineering, Prog. Nat. Sci.: Mater. Int., 2020, 30(5), 642–650 CrossRef CAS.
- J. K. Venkatesan, W. Meng, A. Rey-Rico, G. Schmitt, S. Speicher-Mentges and C. Falentin-Daudré, et al., Enhanced Chondrogenic Differentiation Activities in Human Bone Marrow Aspirates via sox9 Overexpression Mediated by pNaSS-Grafted PCL Film-Guided rAAV Gene Transfer, Pharmaceutics, 2020, 12(3), 280 CrossRef CAS PubMed.
- K. Theodoridis, E. Aggelidou, M. Manthou, E. Demiri, A. Bakopoulou and A. Kritis, Assessment of cartilage regeneration on 3D collagen-polycaprolactone scaffolds: Evaluation of growth media in static and in perfusion bioreactor dynamic culture, Colloids Surf., B, 2019, 183, 110403 CrossRef CAS PubMed.
- K. Riewruja, A. M. Aguglia, S. Hines, M. J. Makarcyzk, S. Honsawek and H. Lin, PEG Reinforced Scaffold Promotes Uniform Distribution of Human MSC-Created Cartilage Matrix, Gels, 2022, 8(12), 794 CrossRef CAS PubMed.
- A. Cheng, Z. Schwartz, A. Kahn, X. Li, Z. Shao and M. Sun, et al., Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration, Tissue Eng., Part B, 2019, 25(1), 14–29 CrossRef PubMed.
- M. Setayeshmehr, E. Esfandiari, M. Rafieinia, B. Hashemibeni, A. Taheri-Kafrani and A. Samadikuchaksaraei, et al., Hybrid and Composite Scaffolds Based on Extracellular Matrices for Cartilage Tissue Engineering, Tissue Eng., Part B, 2019, 25(3), 202–224 CrossRef CAS PubMed.
- Z. Ge, C. Li, B. C. Heng, G. Cao and Z. Yang, Functional biomaterials for cartilage regeneration, J. Biomed. Mater. Res., Part A, 2012, 100(9), 2526–2536 CrossRef PubMed.
- J. K. Leach and J. Whitehead, Materials-Directed Differentiation of Mesenchymal Stem Cells for Tissue Engineering and Regeneration, ACS Biomater. Sci. Eng., 2018, 4(4), 1115–1127 CrossRef CAS PubMed.
- B. D. Ulery, L. S. Nair and C. T. Laurencin, Biomedical applications of biodegradable polymers, J. Polym. Sci., Part B: Polym. Phys., 2011, 49(12), 832–864 CrossRef CAS PubMed.
- S. V. Vlierberghe, E. Schacht and P. Dubruel, Reversible gelatin-based hydrogels: Finetuning of material properties, Eur. Polym. J., 2011, 47(5), 1039–1047 CrossRef.
- K. Kabiri, H. Omidian, S. A. Hashemi and M. J. Zohuriaan-Mehr, Synthesis of fast-swelling superabsorbent hydrogels: effect of crosslinker type and concentration on porosity and absorption rate, Eur. Polym. J., 2003, 39(7), 1341–1348 CrossRef CAS.
- A. C. Daly, L. Riley, T. Segura and J. A. Burdick, Hydrogel microparticles for biomedical applications, Nat. Rev. Mater., 2020, 5(1), 20–43 CrossRef CAS PubMed.
- A. K. Badekila, S. Kini and A. K. Jaiswal, Fabrication techniques of biomimetic scaffolds in three-dimensional cell culture: A review, J. Cell Physiol., 2021, 236(2), 741–762 CrossRef CAS PubMed.
- A. Kumar and A. Jacob, Techniques in scaffold fabrication process for tissue engineering applications: A review, J. Appl. Biol. Biotechnol., 2022, 10(3), 163–176 CrossRef CAS.
- E. Aram and S. Mehdipour-Ataei, A review on the micro- and nanoporous polymeric foams: Preparation and properties, Int. J. Polym. Mater. Polym. Biomater., 2016, 65(7), 358–375 CrossRef CAS.
- G. Conoscenti, T. Schneider, K. Stoelzel, F. Carfì Pavia, V. Brucato and C. Goegele, et al., PLLA scaffolds produced by thermally induced phase separation (TIPS) allow human chondrocyte growth and extracellular matrix formation dependent on pore size, Mater. Sci. Eng., C, 2017, 80, 449–459 CrossRef CAS PubMed.
- T. Lu, Y. Li and T. Chen, Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering, Int. J. Nanomed., 2013, 8, 337–350 CrossRef PubMed.
- D. Li and Y. Xia, Electrospinning of Nanofibers: Reinventing the Wheel?, Adv. Mater., 2004, 16(14), 1151–1170 CrossRef CAS.
- K. Ye, H. Kuang, Z. You, Y. Morsi and X. Mo, Electrospun Nanofibers for Tissue Engineering with Drug Loading and Release, Pharmaceutics, 2019, 11(4), 182 CrossRef CAS PubMed.
- E. Amler, E. Filová, M. Buzgo, E. Prosecká, M. Rampichová and A. Nečas, et al., Functionalized nanofibers as drug-delivery systems for osteochondral regeneration, Nanomedicine, 2014, 9, 1083–1094 CrossRef CAS PubMed.
- M. Rafiei, E. Jooybar, M. J. Abdekhodaie and M. Alvi, Construction of 3D fibrous PCL scaffolds by coaxial electrospinning for protein delivery, Mater. Sci. Eng., C, 2020, 113, 110913 CrossRef CAS PubMed.
- F. Sharifi, S. Irani, G. Azadegan, M. Pezeshki-Modaress, M. Zandi and M. Saeed, Co-electrospun gelatin-chondroitin sulfate/polycaprolactone nanofibrous scaffolds for cartilage tissue engineering, Bioact. Carbohydr. Diet. Fibre, 2020, 22, 100215 CrossRef CAS.
- A. Zaszczyńska, M. Moczulska-Heljak, A. Gradys and P. Sajkiewicz, Advances in 3D Printing for Tissue Engineering, Materials, 2021, 14(12), 3149 CrossRef PubMed.
- G. Gao and X. Cui, Three-dimensional bioprinting in tissue engineering and regenerative medicine, Biotechnol. Lett., 2016, 38(2), 203–211 CrossRef CAS PubMed.
- S. M. Bittner, J. L. Guo and A. G. Mikos, Spatiotemporal Control of Growth Factors in Three-Dimensional Printed Scaffolds, Bioprinting, 2018, 12, e00032 CrossRef PubMed.
- Y. Wang, C. Ling, J. Chen, H. Liu, Q. Mo and W. Zhang, et al., 3D-printed composite scaffold with gradient structure and programmed biomolecule delivery to guide stem cell behavior for osteochondral regeneration, Biomater. Adv., 2022, 140, 213067 CrossRef CAS PubMed.
- B. G. Pavan Kalyan and L. Kumar, 3D Printing: Applications in Tissue Engineering, Medical Devices, and Drug Delivery, AAPS PharmSciTech, 2022, 23(4), 92 CrossRef CAS PubMed.
- M. Dufaud, L. Solé, M. Maumus, M. Simon, E. Perrier-Groult and G. Subra, et al., 3D bioprinting of articular cartilage: Recent advances and perspectives, Bioprinting, 2022, 28, e00253 CrossRef CAS.
- K. Johnson, S. Zhu, M. S. Tremblay, J. N. Payette, J. Wang and L. C. Bouchez, et al., A stem cell-based approach to cartilage repair, Science, 2012, 336(6082), 717–721 CrossRef CAS PubMed.
- G. Cai, W. Liu, Y. He, J. Huang, L. Duan and J. Xiong, et al., Recent advances in kartogenin for cartilage regeneration, J. Drug Targeting, 2019, 27(1), 28–32 CrossRef CAS PubMed.
- M. Wu, C. Li, G. Zhu, Y. Wang, J. Jules and Y. Lu, et al., Deletion of Core-binding factor β (Cbfβ) in mesenchymal progenitor cells provides new insights into Cbfβ/Runxs complex function in cartilage and bone development, Bone, 2014, 65, 49–59 CrossRef CAS PubMed.
- X. Li, J. Ding, Z. Zhang, M. Yang, J. Yu and J. Wang, et al., Kartogenin-Incorporated Thermogel Supports Stem Cells for Significant Cartilage Regeneration, ACS Appl. Mater. Interfaces, 2016, 8(8), 5148–5159 CrossRef CAS PubMed.
- H. Yin, J. Wang, Z. Gu, W. Feng, M. Gao and Y. Wu, et al., Evaluation of the potential of kartogenin encapsulated poly(L-lactic acid-co-caprolactone)/collagen nanofibers for tracheal cartilage regeneration, J. Biomater. Appl., 2017, 32(3), 331–341 CrossRef CAS PubMed.
- D. Shi, X. Xu, Y. Ye, K. Song, Y. Cheng and J. Di, et al., Photo-Cross-Linked Scaffold with Kartogenin-Encapsulated Nanoparticles for Cartilage Regeneration, ACS Nano, 2016, 10(1), 1292–1299 CrossRef CAS PubMed.
- W. Zhang, G. Tang, B. Zhu, M. Yan, F. Yu and X. Wang, et al., Fabrication of an injectable hydrogel scaffold embedding kartogenin-encapsulated PLGA microsphere with long-term drug release to promote chondrogenesis, React. Funct. Polym., 2024, 196, 105821 CrossRef CAS.
- Y. Zhao, X. Zhao, R. Zhang, Y. Huang, Y. Li and M. Shan, et al., Cartilage Extracellular Matrix Scaffold With Kartogenin-Encapsulated PLGA Microspheres for Cartilage Regeneration, Front. Bioeng. Biotechnol., 2020, 8, 600103 CrossRef PubMed.
- S. Shishodia, T. Singh and M. M. Chaturvedi, Modulation of transcription factors by curcumin, Adv. Exp. Med. Biol., 2007, 595, 127–148 CrossRef PubMed.
- B. Chandran and A. Goel, A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis, Phytother. Res., 2012, 26(11), 1719–1725 CrossRef CAS PubMed.
- T. Masuda, K. Hidaka, A. Shinohara, T. Maekawa, Y. Takeda and H. Yamaguchi, Chemical studies on antioxidant mechanism of curcuminoid: analysis of radical reaction products from curcumin, J. Agric. Food Chem., 1999, 47(1), 71–77 CrossRef CAS PubMed.
- D. K. Kim, J. I. Kim, B. R. Sim and G. Khang, Bioengineered porous composite curcumin/silk scaffolds for cartilage regeneration, Mater. Sci. Eng., C, 2017, 78, 571–578 CrossRef CAS PubMed.
- C. Buhrmann, A. Mobasheri, U. Matis and M. Shakibaei, Curcumin mediated suppression of nuclear factor-kappaB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment, Arthritis Res. Ther., 2010, 12(4), 127 CrossRef PubMed.
- A. M. Gorabi, N. Kiaie, S. Hajighasemi, T. Jamialahmadi, M. Majeed and A. Sahebkar, The Effect of Curcumin on the Differentiation of Mesenchymal Stem Cells into Mesodermal Lineage, Molecules, 2019, 24(22), 4029 CrossRef CAS PubMed.
- J. Salazar, L. Bello, M. Chávez, R. Añez, J. Rojas and V. Bermúdez, Glucosamine for Osteoarthritis: Biological Effects, Clinical Efficacy, and Safety on Glucose Metabolism, Arthritis, 2014, 2014, e432463 CrossRef PubMed.
- I. Nagaoka, M. Igarashi and K. Sakamoto, Biological Activities of Glucosamine and Its Related Substances, in Advances in Food and Nutrition Research [Internet], ed. S. K. Kim, Academic Press, 2012, ch. 22, pp. 337–352. (Marine Medicinal Foods; vol. 65). Available from: https://www.sciencedirect.com/science/article/pii/B9780124160033000226 [cited 2024 May 30] Search PubMed.
- C. Li, Q. Li, Q. Mei and T. Lu, Pharmacological effects and pharmacokinetic properties of icariin, the major bioactive component in Herba Epimedii, Life Sci., 2015, 126, 57–68 CrossRef CAS PubMed.
- D. Li, T. Yuan, X. Zhang, Y. Xiao, R. Wang and Y. Fan, et al., Icariin: a potential promoting compound for cartilage tissue engineering, Osteoarthritis Cartilage, 2012, 20(12), 1647–1656 CrossRef CAS PubMed.
- Z. C. Wang, H. J. Sun, K. H. Li, C. Fu and M. Z. Liu, Icariin promotes directed chondrogenic differentiation of bone marrow mesenchymal stem cells but not hypertrophy in vitro, Exp. Ther. Med., 2014, 8(5), 1528–1534 CrossRef CAS PubMed.
- L. Zhang, X. Zhang, K. F. Li, D. X. Li, Y. M. Xiao and Y. J. Fan, et al., Icariin promotes extracellular matrix synthesis and gene expression of chondrocytes in vitro, Phytother. Res., 2012, 26(9), 1385–1392 CrossRef CAS PubMed.
- Y. Luo, Y. Zhang and Y. Huang, Icariin Reduces Cartilage Degeneration in a Mouse Model of Osteoarthritis and is Associated with the Changes in Expression of Indian Hedgehog and Parathyroid Hormone-Related Protein, Med. Sci. Monit., 2018, 24, 6695–6706 CrossRef CAS PubMed.
- C. C. Wei, D. Q. Ping, F. T. You, C. Y. Qiang and C. Tao, Icariin Prevents Cartilage and Bone Degradation in Experimental Models of Arthritis, Mediators Inflammation, 2016, 2016, 9529630 Search PubMed.
- M. Pei, F. He, L. Wei and A. Rawson, Melatonin enhances cartilage matrix synthesis by porcine articular chondrocytes, J. Pineal Res., 2009, 46(2), 181–187 CrossRef CAS PubMed.
- W. Gao, M. Lin, A. Liang, L. Zhang, C. Chen and G. Liang, et al., Melatonin enhances chondrogenic differentiation of human mesenchymal stem cells, J. Pineal Res., 2014, 56(1), 62–70 CrossRef CAS PubMed.
- T. Li, B. Liu, K. Chen, Y. Lou, Y. Jiang and D. Zhang, Small molecule compounds promote the proliferation of chondrocytes and chondrogenic differentiation of stem cells in cartilage tissue engineering, Biomed. Pharmacother., 2020, 131, 110652 CrossRef CAS PubMed.
- Y. Zhang, T. Liu, H. Yang, F. He and X. Zhu, Melatonin: A novel candidate for the treatment of osteoarthritis, Ageing Res. Rev., 2022, 78, 101635 CrossRef CAS PubMed.
- Z. Wu, X. Qiu, B. Gao, C. Lian, Y. Peng and A. Liang, et al., Melatonin-mediated miR-526b-3p and miR-590-5p upregulation promotes chondrogenic differentiation of human mesenchymal stem cells, J. Pineal Res., 2018, 65(1), e12483 CrossRef PubMed.
- Z. Zhang, J. Lin, N. Tian, Y. Wu, Y. Zhou and C. Wang, et al., Melatonin protects vertebral endplate chondrocytes against apoptosis and calcification via the Sirt1-autophagy pathway, J. Cell. Mol. Med., 2019, 23(1), 177–193 CrossRef CAS PubMed.
- A. J. Theruvath, E. E. Mahmoud, W. Wu, H. Nejadnik, L. Kiru and T. Liang, et al., Ascorbic Acid and Iron Supplement Treatment Improves Stem Cell-Mediated Cartilage Regeneration in a Minipig Model, Am. J. Sports Med., 2021, 49(7), 1861–1870 CrossRef PubMed.
- S. Murad, D. Grove, K. A. Lindberg, G. Reynolds, A. Sivarajah and S. R. Pinnell, Regulation of collagen synthesis by ascorbic acid, Proc. Natl. Acad. Sci. U. S. A., 1981, 78(5), 2879–2882 CrossRef CAS PubMed.
- Y. Sato, H. Mera, D. Takahashi, T. Majima, N. Iwasaki and S. Wakitani, et al., Synergistic effect of ascorbic acid and collagen addition on the increase in type 2 collagen accumulation in cartilage-like MSC sheet, Cytotechnology, 2017, 69(3), 405–416 CrossRef CAS PubMed.
- T. F. Li, R. J. O'Keefe and D. Chen, TGF-beta signaling in chondrocytes, Frontiers in bioscience : a journal and virtual library 10, 2005, pp. 681–688 Search PubMed.
- W. Wang, D. Rigueur and K. M. Lyons, TGFbeta signaling in cartilage development and maintenance, Birth Defects Res., Part C, 2014, 102(1), 37–51 CrossRef CAS PubMed.
- P. M. Kraan, E. N. B. Davidson, A. Blom and W. B. Berg, TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: modulation and integration of signaling pathways through receptor-Smads, Osteoarthritis Cartilage, 2009, 17(12), 1539–1545 CrossRef PubMed.
- A. Weiss and L. Attisano, The TGFbeta superfamily signaling pathway, Wiley interdisciplinary reviews, Dev. Biol., 2013, 2(1), 47–63 CAS.
- A. P. Hinck, Structural studies of the TGF-betas and their receptors - insights into evolution of the TGF-beta superfamily, FEBS Lett., 2012, 586(14), 1860–1870 CrossRef CAS PubMed.
- D. Horbelt, A. Denkis and P. Knaus, A portrait of Transforming Growth Factor beta superfamily signalling: Background matters, Int. J. Biochem. Cell Biol., 2012, 44(3), 469–474 CrossRef CAS PubMed.
- M. B. Mueller, M. Fischer, J. Zellner, A. Berner, T. Dienstknecht and L. Prantl, et al., Hypertrophy in Mesenchymal Stem Cell Chondrogenesis: Effect of TGF-β Isoforms and Chondrogenic Conditioning, Cells Tissues Organs, 2010, 192(3), 158–166 CrossRef CAS PubMed.
- A. T. Mehlhorn, H. Schmal, S. Kaiser, G. Lepski, G. Finkenzeller and G. B. Stark, et al., Mesenchymal Stem Cells Maintain TGF-β-Mediated Chondrogenic Phenotype in Alginate Bead Culture, Tissue Eng., 2006, 12(6), 1393–1403 CrossRef CAS PubMed.
- X. Yang, S. Tian, L. Fan, R. Niu, M. Yan and S. Chen, et al., Integrated regulation of chondrogenic differentiation in mesenchymal stem cells and differentiation of cancer cells, Cancer Cell Int., 2022, 22(1), 169 CrossRef CAS PubMed.
- Z. H. Deng, Y. S. Li, X. Gao, G. H. Lei and J. Huard, Bone morphogenetic proteins for articular cartilage regeneration, Osteoarthritis Cartilage, 2018, 26(9), 1153–1161 CrossRef CAS PubMed.
- A. F. Steinert, B. Proffen, M. Kunz, C. Hendrich, S. C. Ghivizzani and U. Noth, et al., Hypertrophy is induced during the in vitro chondrogenic differentiation of human mesenchymal stem cells by bone morphogenetic protein-2 and bone morphogenetic protein-4 gene transfer, Arthritis Res. Ther., 2009, 11(5), 148 CrossRef PubMed.
- A. Steinert, M. Weber, A. Dimmler, C. Julius, N. Schütze and U. Nöth, et al., Chondrogenic differentiation of mesenchymal progenitor cells encapsulated in ultrahigh-viscosity alginate, J. Orthop. Res., 2003, 21(6), 1090–1097 CrossRef CAS PubMed.
- N. D. Miljkovic, G. M. Cooper and K. G. Marra, Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells, Osteoarthritis Cartilage, 2008, 16(10), 1121–1130 CrossRef CAS PubMed.
- Y. Lópiz-Morales, A. Abarrategi, V. Ramos, C. Moreno-Vicente, L. López-Durán and J. L. López-Lacomba, et al., In vivo comparison of the effects of rhBMP-2 and rhBMP-4 in osteochondral tissue regeneration, Eur. Cells Mater., 2010, 20, 367–378 CrossRef PubMed.
- P. H. Liebesny, K. Mroszczyk, H. Zlotnick, H. H. Hung, E. Frank and B. Kurz, et al., Enzyme Pretreatment plus Locally Delivered HB-IGF-1 Stimulate Integrative Cartilage Repair In Vitro, Tissue engineering, Part A, 2019, 25(17–18), 1191–1201 CAS.
- L. Chen, J. Liu, M. Guan, T. Zhou, X. Duan and Z. Xiang, Growth Factor and Its Polymer Scaffold-Based Delivery System for Cartilage Tissue Engineering, Int. J. Nanomed., 2020, 15, 6097–6111 CrossRef CAS PubMed.
- Q. Zhou, B. Li, J. Zhao, W. Pan, J. Xu and S. Chen, IGF-I induces adipose derived mesenchymal cell chondrogenic differentiation in vitro and enhances chondrogenesis in vivo, In vitro cellular & developmental biology, Animal, 2016, 52(3), 356–364 CAS.
- C. Wen, L. Xu, X. Xu, D. Wang, Y. Liang and L. Duan, Insulin-like growth factor-1 in articular cartilage repair for osteoarthritis treatment, Arthritis Res. Ther., 2021, 23(1), 277 CrossRef CAS PubMed.
- A. M. Handorf and W. J. Li, Fibroblast growth factor-2 primes human mesenchymal stem cells for enhanced chondrogenesis, PLoS One, 2011, 6(7), 22887 CrossRef PubMed.
- T. I. Morales, The quantitative and functional relation between insulin-like growth factor-I (IGF) and IGF-binding proteins during human osteoarthritis, J. Orthop. Res., 2008, 26(4), 465–474 CrossRef CAS PubMed.
- L. A. Fortier, J. U. Barker, E. J. Strauss, T. M. McCarrel and B. J. Cole, The role of growth factors in cartilage repair, Clin. Orthop., 2011, 469(10), 2706–2715 CrossRef PubMed.
- M. B. Ellman, H. S. An, P. Muddasani and H. J. Im, Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis, Gene, 2008, 420(1), 82–89 CrossRef CAS PubMed.
- I. Martin, G. Vunjak-Novakovic, J. Yang, R. Langer and L. E. Freed, Mammalian chondrocytes expanded in the presence of fibroblast growth factor 2 maintain the ability to differentiate and regenerate three-dimensional cartilaginous tissue, Exp. Cell Res., 1999, 253(2), 681–688 CrossRef CAS PubMed.
- D. Correa, R. A. Somoza, P. Lin, S. Greenberg, E. Rom and L. Duesler, et al., Sequential exposure to fibroblast growth factors (FGF) 2, 9 and 18 enhances hMSC chondrogenic differentiation, Osteoarthritis Cartilage, 2015, 23(3), 443–453 CrossRef CAS PubMed.
- L. A. Solchaga, K. Penick, V. M. Goldberg, A. I. Caplan and J. F. Welter, Fibroblast growth factor-2 enhances proliferation and delays loss of chondrogenic potential in human adult bone-marrow-derived mesenchymal stem cells, Tissue engineering, Part A, 2010, 16(3), 1009–1019 Search PubMed.
- R. F. Loeser, S. Chubinskaya, C. Pacione and H. J. Im, Basic fibroblast growth factor inhibits the anabolic activity of insulin-like growth factor 1 and osteogenic protein 1 in adult human articular chondrocytes, Arthritis Rheum., 2005, 52(12), 3910–3917 CrossRef CAS PubMed.
- D. Yan, D. Chen and H. J. Im, Fibroblast growth factor-2 promotes catabolism via FGFR1-Ras-Raf-MEK1/2-ERK1/2 axis that coordinates with the PKCdelta pathway in human articular chondrocytes, J. Cell. Biochem., 2012, 113(9), 2856–2865 CrossRef CAS PubMed.
- K. Stewart, M. Pabbruwe, S. Dickinson, T. Sims, A. P. Hollander and J. B. Chaudhuri, The effect of growth factor treatment on meniscal chondrocyte proliferation and differentiation on polyglycolic acid scaffolds, Tissue Eng., 2007, 13(2), 271–280 CrossRef CAS PubMed.
- H. Maehara, S. Sotome, T. Yoshii, I. Torigoe, Y. Kawasaki and Y. Sugata, et al., Repair of large osteochondral defects in rabbits using porous hydroxyapatite/collagen (HAp/Col) and fibroblast growth factor-2 (FGF-2, J. Orthop. Res., 2010, 28(5), 677–686 CrossRef CAS PubMed.
- K. Kieswetter, Z. Schwartz, M. Alderete, D. D. Dean and B. D. Boyan, Platelet derived growth factor stimulates chondrocyte proliferation but prevents endochondral maturation, Endocrine, 1997, 6(3), 257–264 CrossRef CAS PubMed.
- B. Westermark, L. Claesson-Welsh and C. H. Heldin, Structural and functional aspects of platelet-derived growth factor and its receptors, Ciba Found. Symp., 1990, 150, 6–14 CAS , 14–22.
- S. R. Coughlin, M. A. Moskowitz, B. R. Zetter, H. N. Antoniades and L. Levine, Platelet-dependent stimulation of prostacyclin synthesis by platelet-derived growth factor, Nature, 1980, 288(5791), 600–602 CrossRef CAS PubMed.
- J. Fiedler, N. Etzel and R. E. Brenner, To go or not to go: Migration of human mesenchymal progenitor cells stimulated by isoforms of PDGF, J. Cell. Biochem., 2004, 93(5), 990–998 CrossRef CAS PubMed.
- R. E. Toribio, H. A. Brown, C. M. Novince, B. Marlow, K. Hernon, L. G. Lanigan, B. E. Hildreth III, J. L. Werbeck, S. T. Shu, G. Lorch, M. Carlton, J. Foley, P. Boyaka, L. K. McCauley and T. J. Rosol, The midregion, nuclear localization sequence, and C terminus of PTHrP regulate skeletal development, hematopoiesis, and survival in mice, FASEB J., 2010, 24(6), 1947–1957 CrossRef CAS PubMed.
- W. M. Philbrick, J. J. Wysolmerski, S. Galbraith, E. Holt, J. J. Orloff and K. H. Yang, et al., Defining the roles of parathyroid hormone-related protein in normal physiology, Physiol. Rev., 1996, 76(1), 127–173 CrossRef CAS PubMed.
- M. A. Szychlinska, U. D'Amora, S. Ravalli, L. Ambrosio, M. Rosa and G. Musumeci, Functional Biomolecule Delivery Systems and Bioengineering in Cartilage Regeneration, Curr. Pharm. Biotechnol., 2019, 20(1), 32–46 CAS.
- W. Zhang, J. Chen, S. Zhang and H. W. Ouyang, Inhibitory function of parathyroid hormone-related protein on chondrocyte hypertrophy: the implication for articular cartilage repair, Arthritis Res. Ther., 2012, 14(4), 221 CrossRef CAS PubMed.
- Y. J. Kim, H. J. Kim and G. I. Im, PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs, Biochem. Biophys. Res. Commun., 2008, 373(1), 104–108 CrossRef CAS PubMed.
- T. F. Li, Y. Dong, A. M. Ionescu, R. N. Rosier, M. J. Zuscik and E. M. Schwarz, et al., Parathyroid hormone-related peptide (PTHrP) inhibits Runx2 expression through the PKA signaling pathway, Exp. Cell Res., 2004, 299(1), 128–136 CrossRef CAS PubMed.
- B. Yu, X. Zhao, C. Yang, J. Crane, L. Xian and W. Lu, et al., Parathyroid hormone induces differentiation of mesenchymal stromal/stem cells by enhancing bone morphogenetic protein signaling, J. Bone Miner. Res., 2012, 27(9), 2001–2014 CrossRef CAS PubMed.
- A. Casado-Diaz, R. Santiago-Mora and J. M. Quesada, The N- and C-terminal domains of parathyroid hormone-related protein affect differently the osteogenic and adipogenic potential of human mesenchymal stem cells, Exp. Mol. Med., 2010, 42(2), 87–98 CrossRef CAS PubMed.
- X. Lin, P. O. Zamora, S. Albright, J. D. Glass and L. A. Peña, Multidomain synthetic peptide B2A2 synergistically enhances BMP-2 in vitro, J. Bone Miner. Res., 2005, 20(4), 693–703 CrossRef CAS PubMed.
- R. Li, J. Xu, D. S. H. Wong, J. Li, P. Zhao and L. Bian, Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/β-catenin signaling, Biomaterials, 2017, 145, 33–43 CrossRef CAS PubMed.
- X. Lv, C. Sun, B. Hu, S. Chen, Z. Wang and Q. Wu, et al., Simultaneous Recruitment of Stem Cells and Chondrocytes Induced by a Functionalized Self-Assembling Peptide Hydrogel Improves Endogenous Cartilage Regeneration, Front. Cell Dev. Biol., 2020, 8 DOI:10.3389/fcell.2020.00864.
- A. Woods, G. Wang, H. Dupuis, Z. Shao and F. Beier, Rac1 signaling stimulates N-cadherin expression, mesenchymal condensation, and chondrogenesis, J. Biol. Chem., 2007, 282(32), 23500–23508 CrossRef CAS PubMed.
- L. Bian, M. Guvendiren, R. L. Mauck and J. A. Burdick, Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(25), 10117–10122 CrossRef CAS PubMed.
- J. Lu, X. Shen, X. Sun, H. Yin, S. Yang and C. Lu, et al., Increased recruitment of endogenous stem cells and chondrogenic differentiation by a composite scaffold containing bone marrow homing peptide for cartilage regeneration, Theranostics, 2018, 8(18), 5039–5058 CrossRef CAS PubMed.
- X. Guo, Q. Zheng, S. Yang, Z. Shao, Q. Yuan and Z. Pan, et al., Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor beta1 gene, Biomed. Mater., 2006, 1(4), 206–215 CrossRef CAS PubMed.
- G. Kaul, M. Cucchiarini, D. Arntzen, D. Zurakowski, M. D. Menger and D. Kohn, et al., Local stimulation of articular cartilage repair by transplantation of encapsulated chondrocytes overexpressing human fibroblast growth factor 2 (FGF-2) in vivo, J. Gene Med., 2006, 8(1), 100–111 CrossRef CAS PubMed.
- S. Raisin, E. Belamie and M. Morille, Non-viral gene activated matrices for mesenchymal stem cells based tissue engineering of bone and cartilage, Biomaterials, 2016, 104, 223–237 CrossRef CAS PubMed.
- O. Bleiziffer, E. Eriksson, F. Yao, R. E. Horch and U. Kneser, Gene transfer strategies in tissue engineering, J. Cell. Mol. Med., 2007, 11(2), 206–223 CrossRef CAS PubMed.
- G. Lisignoli, C. Manferdini, E. Lambertini, N. Zini, M. Angelozzi and E. Gabusi, et al., Chondrogenic potential of Slug-depleted human mesenchymal stem cells, Tissue engineering, Part A, 2014, 20(19–20), 2795–2805 CAS.
- S. Y. Jeon, J. S. Park, H. N. Yang, H. J. Lim, S. W. Yi and H. Park, et al., Co-delivery of Cbfa-1-targeting siRNA and SOX9 protein using PLGA nanoparticles to induce chondrogenesis of human mesenchymal stem cells, Biomaterials, 2014, 35(28), 8236–8248 CrossRef CAS PubMed.
- D. Guerit, J. M. Brondello, P. Chuchana, D. Philipot, K. Toupet and C. Bony, et al., FOXO3A regulation by miRNA-29a Controls chondrogenic differentiation of mesenchymal stem cells and cartilage formation, Stem Cells Dev., 2014, 23(11), 1195–1205 CrossRef CAS PubMed.
- O. Ham, B. W. Song, S. Y. Lee, E. Choi, M. J. Cha and C. Y. Lee, et al., The role of microRNA-23b in the differentiation of MSC into chondrocyte by targeting protein kinase A signaling, Biomaterials, 2012, 33(18), 4500–4507 CrossRef CAS PubMed.
- S. Lv, J. Xu, L. Chen, H. Wu, W. Feng and Y. Zheng, et al., MicroRNA-27b targets CBFB to inhibit differentiation of human bone marrow mesenchymal stem cells into hypertrophic chondrocytes, Stem Cell Res. Ther., 2020, 11(1), 392 CrossRef CAS PubMed.
- I. Uzieliene, U. Kalvaityte, E. Bernotiene and A. Mobasheri, Non-viral Gene Therapy for Osteoarthritis, Front. Bioeng. Biotechnol., 2020, 8, 618399 CrossRef PubMed.
- J. Xu, J. Li, S. Lin, T. Wu, H. Huang and K. Zhang, et al., Nanocarrier-Mediated Codelivery of Small Molecular Drugs and siRNA to Enhance Chondrogenic Differentiation and Suppress Hypertrophy of Human Mesenchymal Stem Cells, Adv. Funct. Mater., 2016, 26(15), 2463–2472 CrossRef CAS.
- S. Wang, X. Wei, X. Sun, C. Chen, J. Zhou and G. Zhang, et al., A novel therapeutic strategy for cartilage diseases based on lipid nanoparticle-RNAi delivery system, Int. J. Nanomed., 2018, 13, 617–631 CrossRef CAS PubMed.
- G. Gibson and H. Asahara, microRNAs and cartilage, J. Orthop. Res., 2013, 31(9), 1333–1344 CrossRef CAS PubMed.
- E. Hong and A. H. Reddi, MicroRNAs in chondrogenesis, articular cartilage, and osteoarthritis: implications for tissue engineering, Tissue Eng., Part B, 2012, 18(6), 445–453 CrossRef CAS PubMed.
- H. Asahara, Current Status and Strategy of microRNA Research for Cartilage Development and Osteoarthritis Pathogenesis, J. Bone Metab., 2016, 23(3), 121–127 CrossRef PubMed.
- L. Duan, Y. Liang, X. Xu, Y. Xiao and D. Wang, Recent progress on the role of miR-140 in cartilage matrix remodelling and its implications for osteoarthritis treatment, Arthritis Res. Ther., 2020, 22(1), 194 CrossRef PubMed.
- Y. Sun and B. Qin, Long noncoding RNA MALAT1 regulates HDAC4-mediated proliferation and apoptosis via decoying of miR-140–5p in osteosarcoma cells, Cancer Med., 2018, 7(9), 4584–4597 CrossRef CAS PubMed.
- C. Li, Q. Hu, Z. Chen, B. Shen, J. Yang and P. Kang, et al., MicroRNA-140 Suppresses Human Chondrocytes Hypertrophy by Targeting SMAD1 and Controlling the Bone Morphogenetic Protein Pathway in Osteoarthritis, Am. J. Med. Sci., 2018, 355(5), 477–487 CrossRef PubMed.
- C. L. Murphy, B. L. Thoms, R. J. Vaghjiani and J. E. Lafont, Hypoxia. HIF-mediated articular chondrocyte function: prospects for cartilage repair, Arthritis Res. Ther., 2009, 11(1), 213 CrossRef PubMed.
- H. H. Lee, C. C. Chang, M. J. Shieh, J. P. Wang, Y. T. Chen and T. H. Young, et al., Hypoxia Enhances Chondrogenesis and Prevents Terminal Differentiation through PI3K/Akt/FoxO Dependent Anti-Apoptotic Effect, Sci. Rep., 2013, 3(1), 2683 CrossRef PubMed.
- E. Duval, C. Baugé, R. Andriamanalijaona, H. Bénateau, S. Leclercq and S. Dutoit, et al., Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering, Biomaterials, 2012, 33(26), 6042–6051 CrossRef CAS PubMed.
- N. Alijani, B. Johari, M. Moradi and M. Kadivar, A review on transcriptional regulation responses to hypoxia in mesenchymal stem cells, Cell Biol. Int., 2020, 44(1), 14–26 CrossRef CAS PubMed.
- A. Nowak-Stępniowska, P. N. Osuchowska, H. Fiedorowicz and E. A. Trafny, Insight in Hypoxia-Mimetic Agents as Potential Tools for Mesenchymal Stem Cell Priming in Regenerative Medicine, Stem Cells Int., 2022, 2022, 8775591 Search PubMed.
- D. K. Taheem, D. A. Foyt, S. Loaiza, S. A. Ferreira, D. Ilic and H. W. Auner, et al., Differential Regulation of Human Bone Marrow Mesenchymal Stromal Cell Chondrogenesis by Hypoxia Inducible Factor-1α Hydroxylase Inhibitors, Stem Cells, 2018, 36(9), 1380–1392 CrossRef CAS PubMed.
- L. Chen, X. Huang, H. Chen, D. Bao, X. Su and L. Wei, et al., Hypoxia-mimicking scaffolds with controlled release of DMOG and PTHrP to promote cartilage regeneration via the HIF-1α/YAP signaling pathway, Int. J. Biol. Macromol., 2023, 226, 716–729 CrossRef CAS PubMed.
- K. Bajbouj, J. Shafarin and M. Hamad, High-Dose Deferoxamine Treatment Disrupts Intracellular Iron Homeostasis, Reduces Growth, and Induces Apoptosis in Metastatic and Nonmetastatic Breast Cancer Cell Lines, Technol. Cancer Res. Treat., 2018, 17, 1533033818764470 CrossRef CAS PubMed.
- C. Gao, W. Dai, X. Wang, L. Zhang, Y. Wang and Y. Huang, et al., Magnesium Gradient-Based Hierarchical Scaffold for Dual-Lineage Regeneration of Osteochondral Defect, Adv. Funct. Mater., 2023, 33(43), 2304829 CrossRef CAS.
- J. Li and H. M. Wang, Effects of cobalt chloride on phenotypes of normal human saphenous vein smooth muscle cells, Int. J. Clin. Exp. Med., 2014, 7(12), 4933–4941 Search PubMed.
- S. Gao, J. Zhou, Y. Zhao, P. Toselli and W. Li, Hypoxia-response element (HRE)-directed transcriptional regulation of the rat lysyl oxidase gene in response to cobalt and cadmium, Toxicol. Sci., 2013, 132(2), 379–389 CrossRef CAS PubMed.
- S. Focaroli, G. Teti, V. Salvatore, I. Orienti and M. Falconi, Calcium/Cobalt Alginate Beads as Functional Scaffolds for Cartilage Tissue Engineering, Stem Cells Int., 2016, 2016, 2030478 CrossRef PubMed.
- T. A. Holland, Y. Tabata and A. G. Mikos, Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering, J. Controlled Release, 2005, 101(1–3), 111–125 CrossRef CAS PubMed.
- A. Nalbadis, M. L. Trutschel, H. Lucas, J. Luetzkendorf, A. Meister and K. Mader, Selection and Incorporation of siRNA Carrying Non-Viral Vector for Sustained Delivery from Gellan Gum Hydrogels, Pharmaceutics, 2021, 13(10), 1546 CrossRef CAS PubMed.
- C. E. Nelson, M. K. Gupta, E. J. Adolph, J. M. Shannon, S. A. Guelcher and C. L. Duvall, Sustained local delivery of siRNA from an injectable scaffold, Biomaterials, 2012, 33(4), 1154–1161 CrossRef CAS PubMed.
- L. Zhou, L. E. Rubin, C. Liu and Y. Chen, Short interfering RNA (siRNA)-Based Therapeutics for Cartilage Diseases, Regener. Eng. Transl. Med., 2020, 7(3), 283–290 CrossRef PubMed.
- M. Chen, S. Gao, M. Dong, J. Song, C. Yang and K. A. Howard, et al., Chitosan/siRNA nanoparticles encapsulated in PLGA nanofibers for siRNA delivery, ACS Nano, 2012, 6(6), 4835–4844 CrossRef CAS PubMed.
- J. Salvador, J. Berthelot, C. Bony, B. Robin, J. L. K. Him and D. Noël, et al., Size-tunable lipid vectors for controlled local delivery of siRNA from gene activated matrix, Acta Biomater., 2022, 153, 97–107 CrossRef CAS PubMed.
- S. Yang, K. F. Leong, Z. Du and C. K. Chua, The design of scaffolds for use in tissue engineering, Part I. Tradit Factors, Tissue Eng., 2001, 7(6), 679–689 CrossRef CAS PubMed.
- D. Howard, L. D. Buttery, K. M. Shakesheff and S. J. Roberts, Tissue engineering: strategies, stem cells and scaffolds, J. Anat., 2008, 213(1), 66–72 CrossRef CAS PubMed.
- M. I. Echeverria Molina, K. G. Malollari and K. Komvopoulos, Design Challenges in Polymeric Scaffolds for Tissue Engineering, Front. Bioeng. Biotechnol., 2021, 9 DOI:10.3389/fbioe.2021.617141.
- H. Zhang, L. Zhou and W. Zhang, Control of scaffold degradation in tissue engineering: a review, Tissue Eng., Part B, 2014, 20(5), 492–502 CrossRef CAS PubMed.
- J. Chi, M. Wang, J. Chen, L. Hu, Z. Chen and L. J. Backman, et al., Topographic Orientation of Scaffolds for Tissue Regeneration: Recent Advances in Biomaterial Design and Applications, Biomimetics, Basel, Switzerland, 2022, vol. 7 Search PubMed.
- H. Akkiraju and A. Nohe, Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration, J. Dev. Biol., 2015, 3(4), 177–192 CrossRef PubMed.
- B. Li, F. Wang, F. Hu, T. Ding, P. Huang and X. Xu, et al., Injectable “nano-micron” combined gene-hydrogel microspheres for local treatment of osteoarthritis, NPG Asia Mater., 2022, 14(1), 1 CrossRef CAS.
- H. Xiang, C. Zhang, Y. Xiong, Y. Wang, C. Pu and J. He, et al., MMP13-responsive hydrogel microspheres for osteoarthritis treatment by precise delivery of celecoxib, Mater. Des., 2024, 241, 112966 CrossRef CAS.
- J. Ratanavaraporn, K. Soontornvipart, S. Shuangshoti, S. Shuangshoti and S. Damrongsakkul, Localized delivery of curcumin from injectable gelatin/Thai silk fibroin microspheres for anti-inflammatory treatment of osteoarthritis in a rat model, Inflammopharmacology, 2017, 25(2), 211–221 CrossRef CAS PubMed.
- H. Liu, S. Wang and N. Qi, Controllable structure, properties, and degradation of the electrospun PLGA/PLA-blended nanofibrous scaffolds, J. Appl. Polym. Sci., 2012, 125(S2), 468–476 Search PubMed.
- Q. Zheng, Z. Chu, X. Li, H. Kang, X. Yang and Y. Fan, The Effect of Fluid Shear Stress on the In Vitro Release Kinetics of Sirolimus from PLGA Films, Polymers, 2017, 9(11), 618 CrossRef PubMed.
- T. Zhao, X. Li, H. Li, H. Deng, J. Li and Z. Yang, et al., Advancing drug delivery to articular cartilage: From single to multiple strategies, Acta Pharm. Sin. B, 2023, 13(10), 4127–4148 CrossRef CAS PubMed.
- J. H. Lee, P. Y. Kim, Y. C. Pyun, J. Park, T. W. Kang and J. S. Seo, et al., Cartilage regeneration using transforming growth factor-beta 3-loaded injectable crosslinked hyaluronic acid hydrogel, Biomater. Sci., 2024, 12(2), 479–494 RSC.
- X. Sun, J. Wang, Y. Wang, C. Huang, C. Yang and M. Chen, et al., Scaffold with Orientated Microtubule Structure Containing Polylysine-Heparin Sodium Nanoparticles for the Controlled Release of TGF-β1 in Cartilage Tissue Engineering, ACS Appl. Bio Mater., 2018, 1(6), 2030–2040 CrossRef CAS PubMed.
- C. Addi, F. Murschel and G. De Crescenzo, Design and Use of Chimeric Proteins Containing a Collagen-Binding Domain for Wound Healing and Bone Regeneration, Tissue Eng., Part B, 2017, 23(2), 163–182 CrossRef CAS PubMed.
- N. Nishi, O. Matsushita, K. Yuube, H. Miyanaka, A. Okabe and F. Wada, Collagen-binding growth factors: Production and characterization of functional fusion proteins having a collagen-binding domain, Proc. Natl. Acad. Sci. U. S. A., 1998, 95(12), 7018–7023 CrossRef CAS PubMed.
- X. C. Wu, B. O. Huang, J. Wang, C. Q. Li and Y. U. E. Zhou, Collagen-targeting parathyroid hormone-related peptide promotes collagen binding and in vitro chondrogenesis in bone marrow-derived MSCs, Int. J. Mol. Med., 2013, 31(2), 430–436 CrossRef CAS PubMed.
- T. Nakaji-Hirabayashi, K. Kato, Y. Arima and H. Iwata, Oriented immobilization of epidermal growth factor onto culture substrates for the selective expansion of neural stem cells, Biomaterials, 2007, 28(24), 3517–3529 CrossRef CAS PubMed.
- K. S. Masters, Covalent Growth Factor Immobilization Strategies for Tissue Repair and Regeneration, Macromol. Biosci., 2011, 11(9), 1149–1163 CrossRef CAS PubMed.
- K. B. Seims, N. K. Hunt and L. W. Chow, Strategies to Control or Mimic Growth Factor Activity for Bone, Cartilage, and Osteochondral Tissue Engineering, Bioconjugate Chem., 2021, 32(5), 861–878 CrossRef CAS PubMed.
- D. Enriquez-Ochoa, P. Robles-Ovalle, K. Mayolo-Deloisa and M. E. G. Brunck, Immobilization of Growth Factors for Cell Therapy Manufacturing, Front. Bioeng. Biotechnol., 2020, 8, 620 CrossRef PubMed.
- R. N. Shah, N. A. Shah, M. M. D. R. Lim, C. Hsieh, G. Nuber and S. I. Stupp, Supramolecular design of self-assembling nanofibers for cartilage regeneration, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(8), 3293–3298 CrossRef CAS PubMed.
- J. Chen, Y. Li, B. Wang, J. Yang, B. C. Heng and Z. Yang, et al., TGF-beta1 affinity peptides incorporated within a chitosan sponge scaffold can significantly enhance cartilage regeneration, J. Mater. Chem. B, 2018, 6(4), 675–687 RSC.
- J. A. Lewis, R. Freeman, J. K. Carrow, T. D. Clemons, L. C. Palmer and S. I. Stupp, Transforming Growth Factor β-1 Binding by Peptide Amphiphile Hydrogels, ACS Biomater. Sci. Eng., 2020, 6(8), 4551–4560 CrossRef CAS PubMed.
- M. Xiao, J. Xiao, G. Wu, Y. Ke, L. Fang and C. Deng, et al., Anchoring TGF-beta1 on biomaterial surface via affinitive interactions: Effects on spatial structures and bioactivity, Colloids Surf., B, 2018, 166, 254–261 CrossRef CAS PubMed.
- W. J. King and P. H. Krebsbach, Growth factor delivery: how surface interactions modulate release in vitro and in vivo, Adv. Drug Delivery Rev., 2012, 64(12), 1239–1256 CrossRef CAS PubMed.
- D. Hachim, T. E. Whittaker, H. Kim and M. M. Stevens, Glycosaminoglycan-based biomaterials for growth factor and cytokine delivery: Making the right choices, J. Controlled Release, 2019, 313, 131–147 CrossRef CAS PubMed.
- Y. Liang and K. L. Kiick, Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications, Acta Biomater., 2014, 10(4), 1588–1600 CrossRef CAS PubMed.
- Y. K. Joung, J. W. Bae and K. D. Park, Controlled release of heparin-binding growth factors using heparin-containing particulate systems for tissue regeneration, Expert Opin. Drug Delivery, 2008, 5(11), 1173–1184 CrossRef CAS PubMed.
- A. Ori, M. C. Wilkinson and D. G. Fernig, The heparanome and regulation of cell function: structures, functions and challenges, Front. Biosci., 2008, 13, 4309–4338 CrossRef CAS PubMed.
- J. Fang, Y. Dong, N. Salamat-Miller and C. R. Middaugh, DB-PABP: a database of polyanion-binding proteins, Nucleic Acids Res., 2008, 36(Database issue), 303–306 Search PubMed.
- A. G. Bajpayee and A. J. Grodzinsky, Cartilage-targeting drug delivery: can electrostatic interactions help?, Nature reviews, Rheumatology, 2017, 13(3), 183–193 CAS.
- V. Adibnia, M. Mirbagheri, S. Salimi, G. Crescenzo and X. Banquy, Nonspecific interactions in biomedical applications, Curr. Opin. Colloid Interface Sci., 2020, 47, 70–83 CrossRef CAS.
- A. Ori, P. Free, J. Courty, M. C. Wilkinson and D. G. Fernig, Identification of heparin-binding sites in proteins by selective labeling, Mol. Cell. Proteomics, 2009, 8(10), 2256–2265 CrossRef CAS PubMed.
- S. E. Sakiyama-Elbert and J. A. Hubbell, Development of fibrin derivatives for controlled release of heparin-binding growth factors, J. Controlled Release, 2000, 65(3), 389–402 CrossRef CAS PubMed.
- M. Lyon, G. Rushton and J. T. Gallagher, The interaction of the transforming growth factor-betas with heparin/heparan sulfate is isoform-specific, J. Biol. Chem., 1997, 272(29), 18000–18006 CrossRef CAS PubMed.
- R. Jin, L. S. M. Teixeira, P. J. Dijkstra, C. A. Blitterswijk, M. Karperien and J. Feijen, Chondrogenesis in injectable enzymatically crosslinked heparin/dextran hydrogels, J. Controlled Release, 2011, 152(1), 186–195 CrossRef CAS PubMed.
- G. C. J. Brown, K. S. Lim, B. L. Farrugia, G. J. Hooper and T. B. F. Woodfield, Covalent Incorporation of Heparin Improves Chondrogenesis in Photocurable Gelatin-Methacryloyl Hydrogels, Macromol. Biosci., 2017, 17, 1700158 CrossRef PubMed.
- E. Hesse, U. Freudenberg, T. Niemietz, C. Greth, M. Weisser and S. Hagmann, et al., Peptide-functionalized starPEG/heparin hydrogels direct mitogenicity, cell morphology and cartilage matrix distribution in vitro and in vivo, J. Tissue Eng. Regener. Med., 2018, 12(1), 229–239 CrossRef CAS PubMed.
- C. Levinson, M. Lee, L. A. Applegate and M. Zenobi-Wong, An injectable heparin-conjugated hyaluronan scaffold for local delivery of transforming growth factor beta1 promotes successful chondrogenesis, Acta Biomater., 2019, 99, 168–180 CrossRef CAS PubMed.
- M. Muller, E. Ozturk, O. Arlov, P. Gatenholm and M. Zenobi-Wong, Alginate Sulfate-Nanocellulose Bioinks for Cartilage Bioprinting Applications, Ann. Biomed. Eng., 2017, 45(1), 210–223 CrossRef PubMed.
- R. Mhanna, A. Kashyap, G. Palazzolo, Q. Vallmajo-Martin, J. Becher and S. Moller, et al., Chondrocyte culture in three dimensional alginate sulfate hydrogels promotes proliferation while maintaining expression of chondrogenic markers, Tissue engineering, Part A, 2014, 20(9–10), 1454–1464 CAS.
- O. Arlov and G. Skjak-Braek, Sulfated Alginates as Heparin Analogues: A Review of Chemical and Functional Properties, Molecules, 2017, 22(5), 778 CrossRef PubMed.
- M. Gionet-Gonzales, A. Casella, D. Diloretto, C. Ginnell, K. H. Griffin and A. Bigot, et al., Sulfated Alginate Hydrogels Prolong the Therapeutic Potential of MSC Spheroids by Sequestering the Secretome, Adv. Healthcare Mater., 2021, 10(21), e2101048 CrossRef PubMed.
- Ø. Arlov and G. Skjåk-Bræk, Sulfated Alginates as Heparin Analogues: A Review of Chemical and Functional Properties, Molecules, 2017, 22(5), 778 CrossRef PubMed.
- T. Re'em, Y. Kaminer-Israeli, E. Ruvinov and S. Cohen, Chondrogenesis of hMSC in affinity-bound TGF-beta scaffolds, Biomaterials, 2012, 33(3), 751–761 CrossRef PubMed.
- M. Cucchiarini and H. Madry, Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair, Nature reviews, Rheumatology, 2019, 15(1), 18–29 Search PubMed.
- S. Elsler, S. Schetting, G. Schmitt, D. Kohn, H. Madry and M. Cucchiarini, Effective, safe nonviral gene transfer to preserve the chondrogenic differentiation potential of human mesenchymal stem cells, J. Gene Med., 2012, 14(7), 501–511 CrossRef CAS PubMed.
- M. D. Krebs and E. Alsberg, Localized, targeted, and sustained siRNA delivery, Chemistry, 2011, 17(11), 3054–3062 CrossRef CAS PubMed.
- M. Morille, T. Van-Thanh, X. Garric, J. Cayon, J. Coudane and D. Noël, et al., New PLGA-P188-PLGA matrix enhances TGF-β3 release from pharmacologically active microcarriers and promotes chondrogenesis of mesenchymal stem cells, J. Controlled Release, 2013, 170(1), 99–110 CrossRef CAS PubMed.
- R. Budiraharjo, K. G. Neoh and E. T. Kang, Enhancing bioactivity of chitosan film for osteogenesis and wound healing by covalent immobilization of BMP-2 or FGF-2, J. Biomater. Sci., Polym. Ed., 2013, 24(6), 645–662 CrossRef CAS PubMed.
- Z. Wang, Z. Wang, W. W. Lu, W. Zhen, D. Yang and S. Peng, Novel biomaterial strategies for controlled growth factor delivery for biomedical applications, NPG Asia Mater., 2017, 9(10), 435–435 CrossRef.
- C. H. Chou, W. T. Cheng, C. C. Lin, C. H. Chang, C. C. Tsai and F. H. Lin, TGF-beta1 immobilized tri-co-polymer for articular cartilage tissue engineering, J. Biomed. Mater. Res., Part B, 2006, 77(2), 338–348 CrossRef PubMed.
- H. Fan, H. Tao, Y. Wu, Y. Hu, Y. Yan and Z. Luo, TGF-beta3 immobilized PLGA-gelatin/chondroitin sulfate/hyaluronic acid hybrid scaffold for cartilage regeneration, J. Biomed. Mater. Res., Part A, 2010, 95(4), 982–992 CrossRef PubMed.
- S. B. Gunnoo and A. Madder, Bioconjugation – using selective chemistry to enhance the properties of proteins and peptides as therapeutics and carriers, Org. Biomol. Chem., 2016, 14(34), 8002–8013 RSC.
- Z. Yang, F. Cao, H. Li, S. He, T. Zhao and H. Deng, et al., Microenvironmentally optimized 3D-printed TGFβ-functionalized scaffolds facilitate endogenous cartilage regeneration in sheep, Acta Biomater., 2022, 150, 181–198 CrossRef CAS PubMed.
- M. Motoyama, M. Deie, A. Kanaya, M. Nishimori, A. Miyamoto and S. Yanada, et al., In vitro cartilage formation using TGF-beta-immobilized magnetic beads and mesenchymal stem cell-magnetic bead complexes under magnetic field conditions, J. Biomed. Mater. Res., Part A, 2010, 92(1), 196–204 CrossRef PubMed.
- M. L. Kang, J. Y. Ko, J. E. Kim and G. I. Im, Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration, Biomaterials, 2014, 35(37), 9984–9994 CrossRef CAS PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Click Chemistry: Diverse Chemical Function from a Few Good Reactions, Angew. Chem., Int. Ed., 2001, 40(11), 2004–2021 CrossRef CAS PubMed.
- C. Barner-Kowollik, F. E. Prez, P. Espeel, C. J. Hawker, T. Junkers and H. Schlaad, et al., Clicking” polymers or just efficient linking: what is the difference?, Angew. Chem., Int. Ed., 2011, 50(1), 60–62 CrossRef CAS PubMed.
- M. A. Azagarsamy and K. S. Anseth, Bioorthogonal Click Chemistry: An Indispensable Tool to Create Multifaceted Cell Culture Scaffolds, ACS Macro Lett., 2013, 2(1), 5–9 CrossRef CAS PubMed.
- E. S. Place, R. Nair, H. N. Chia, G. Szulgit, E. H. Lim and M. M. Stevens, Latent TGF-beta hydrogels for cartilage tissue engineering, Adv. Healthcare Mater., 2012, 1(4), 480–484 CrossRef CAS PubMed.
- J. D. McCall, J. E. Luoma and K. S. Anseth, Covalently tethered transforming growth factor beta in PEG hydrogels promotes chondrogenic differentiation of encapsulated human mesenchymal stem cells, Drug Delivery Transl. Res., 2012, 2(5), 305–312 CrossRef CAS PubMed.
- T. Bock, V. Schill, M. Krahnke, A. F. Steinert, J. Tessmar and T. Blunk, et al., TGF-beta1-Modified Hyaluronic Acid/Poly(glycidol) Hydrogels for Chondrogenic Differentiation of Human Mesenchymal Stromal Cells, Macromol. Biosci., 2018, 18(7), 1700390 CrossRef PubMed.
- S. H. Park, J. S. Kwon, B. S. Lee, J. H. Park, B. K. Lee and J. H. Yun, et al., BMP2-modified injectable hydrogel for osteogenic differentiation of human periodontal ligament stem cells, Sci. Rep., 2017, 7(1), 6603 CrossRef PubMed.
- M. Nouri-Felekori, N. Nezafati, M. Moraveji, S. Hesaraki and T. Ramezani, Bioorthogonal hydroxyethyl cellulose-based scaffold crosslinked via click chemistry for cartilage tissue engineering applications, Int. J. Biol. Macromol., 2021, 183, 2030–2043 CrossRef CAS PubMed.
- J. J. Patel, C. L. Flanagan and S. J. Hollister, Bone Morphogenetic Protein-2 Adsorption onto Poly-ε-caprolactone Better Preserves Bioactivity In Vitro and Produces More Bone In Vivo than Conjugation Under Clinically Relevant Loading Scenarios, Tissue Eng., Part C, 2015, 21(5), 489–498 CrossRef CAS PubMed.
- H. Lee, S. Lim, M. S. Birajdar, S. H. Lee and H. Park, Fabrication of FGF-2 immobilized electrospun gelatin nanofibers for tissue engineering, Int. J. Biol. Macromol., 2016, 93(Pt B), 1559–1566 CrossRef CAS PubMed.
- E. K. Riga, J. S. Saar, R. Erath, M. Hechenbichler and K. Lienkamp, On the Limits of Benzophenone as Cross-Linker for Surface-Attached Polymer Hydrogels, Polymers, 2017, 9(12), 686 CrossRef PubMed.
- D. W. Ham, E. C. Jang, T. I. Son, T. J. Lee and K. S. Song, Photo-immobilization of bone morphogenetic protein-2 using azidophenyl gelatin on a collagen sheet enhances osteogenesis in a rat calvarial defect model, J. Ind. Eng. Chem., 2016, 40, 177–184 CrossRef CAS.
- C. D. Spicer and E. T. Pashuck, Achiev Control Biomol-Biomater Conjug, Chem. Rev., 2018, 118(16), 7702–7743 CrossRef CAS PubMed.
- J. H. Jeong, E. H. Kim, G. D. Han, J. W. Nah, Y. Ito and T. I. Son, BMP-2 immobilization by phosphonated UV-curable low-molecular-weight chitosan derivative on the surface of titanium, J. Ind. Eng. Chem., 2016, 34, 33–40 CrossRef CAS.
- J. M. Banks, L. C. Mozdzen, B. A. C. Harley and R. C. Bailey, The combined effects of matrix stiffness and growth factor immobilization on the bioactivity and differentiation capabilities of adipose-derived stem cells, Biomaterials, 2014, 35(32), 8951–8959 CrossRef CAS PubMed.
- M. B. Schmidt, E. H. Chen and S. E. Lynch, A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair, Osteoarthritis Cartilage, 2006, 14(5), 403–412 CrossRef CAS PubMed.
- J. Li, G. Chen, X. Xu, P. Abdou, Q. Jiang and D. Shi, et al., Advances of injectable hydrogel-based scaffolds for cartilage regeneration, Regener. Biomater., 2019, 6(3), 129–140 CrossRef CAS PubMed.
- B. K. Mann, R. H. Schmedlen and J. L. West, Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells, Biomaterials, 2001, 22(5), 439–444 CrossRef CAS PubMed.
- M. Liu, X. Zeng, C. Ma, H. Yi, Z. Ali and X. Mou, et al., Injectable hydrogels for cartilage and bone tissue engineering, Bone Res., 2017, 5, 17014 CrossRef CAS PubMed.
- O. Wichterle and D. Lím, Hydrophilic Gels for Biological Use, Nature, 1960, 185(4706), 117–118 CrossRef.
- J. L. Drury and D. J. Mooney, Hydrogels for tissue engineering: scaffold design variables and applications, Biomaterials, 2003, 24(24), 4337–4351 CrossRef CAS PubMed.
- H. Carrelo, P. I. P. Soares, J. P. Borges and M. T. Cidade, Injectable Composite Systems Based on Microparticles in Hydrogels for Bioactive Cargo Controlled Delivery, Gels, 2021, 7(3), 147 CrossRef CAS PubMed.
- V. E. Santo, M. E. Gomes, J. F. Mano and R. L. Reis, Controlled release strategies for bone, cartilage, and osteochondral engineering–Part II: challenges on the evolution from single to multiple bioactive factor delivery, Tissue Eng., Part B, 2013, 19(4), 327–352 CrossRef CAS PubMed.
- N. Butoescu, C. A. Seemayer, M. Foti, O. Jordan and E. Doelker, Dexamethasone-containing PLGA superparamagnetic microparticles as carriers for the local treatment of arthritis, Biomaterials, 2009, 30(9), 1772–1780 CrossRef CAS PubMed.
- E. Horisawa, K. Kubota, I. Tuboi, K. Sato, H. Yamamoto and H. Takeuchi, et al., Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium, Pharm. Res., 2002, 19(2), 132–139 CrossRef CAS PubMed.
- J. M. Lu, X. Wang, C. Marin-Muller, H. Wang, P. H. Lin and Q. Yao, et al., Current advances in research and clinical applications of PLGA-based nanotechnology, Expert Rev. Mol. Diagn., 2009, 9(4), 325–341 CrossRef CAS PubMed.
- W. Dai, Q. Liu, S. Li, Y. Gao, C. Feng and L. Guo, et al., Functional injectable hydrogel with spatiotemporal sequential release for recruitment of endogenous stem cells and in situ cartilage regeneration, J. Mater. Chem. B, 2023, 11(18), 4050–4064 RSC.
- Y. Dong, Y. Liu, Y. Chen, X. Sun, L. Zhang and Z. Zhang, et al., Spatiotemporal regulation of endogenous MSCs using a functional injectable hydrogel system for cartilage regeneration, NPG Asia Mater., 2021, 13(1), 71 CrossRef CAS.
- S. M. Lim, S. H. Oh, H. H. Lee, S. H. Yuk, G. I. Im and J. H. Lee, Dual growth factor-releasing nanoparticle/hydrogel system for cartilage tissue engineering, J. Mater. Sci. Mater. Med., 2010, 21(9), 2593–2600 CrossRef CAS PubMed.
- X. Ran, Q. Wang, Y. Sun, Q. Pan, H. Chen and W. Ren, et al., Dual microparticles programmed delivery system regulating stem cell-based cartilage regeneration by cartilage-specific matrix hydrogels, Composites, Part B, 2024, 272, 111221 CrossRef CAS.
- B. Wang, F. Chariyev-Prinz, R. Burdis, K. Eichholz and D. J. Kelly, Additive manufacturing of cartilage-mimetic scaffolds as off-the-shelf implants for joint regeneration, Biofabrication, 2022, 14(2), 024101 CrossRef PubMed.
- M. K. Gupta, F. Meng, B. N. Johnson, Y. L. Kong, L. Tian and Y. W. Yeh, et al., 3D Printed Programmable Release Capsules, Nano Lett., 2015, 15(8), 5321–5329 CrossRef CAS PubMed.
- W. Zhu, H. Cui, B. Boualam, F. Masood, E. Flynn and R. D. Rao, et al., 3D bioprinting mesenchymal stem cell-laden construct with core-shell nanospheres for cartilage tissue engineering, Nanotechnology, 2018, 29(18), 185101 CrossRef PubMed.
- G. A. Mannella, G. Conoscenti, F. Carfì Pavia, V. La Carrubba and V. Brucato, Preparation of polymeric foams with a pore size gradient via Thermally Induced Phase Separation (TIPS), Mater. Lett., 2015, 160, 31–33 CrossRef CAS.
- A. Prasad, M. R. Sankar and V. Katiyar, State of Art on Solvent Casting Particulate Leaching Method for Orthopedic ScaffoldsFabrication, Mater. Today: Proc., 2017, 4(2, Part A), 898–907 Search PubMed.
- T. V. Plisko, A. V. Penkova, K. S. Burts, A. V. Bildyukevich, M. E. Dmitrenko and G. B. Melnikova, et al., Effect of Pluronic F127 on porous and dense membrane structure formation via non-solvent induced and evaporation induced phase separation, J. Membr. Sci., 2019, 580, 336–349 CrossRef CAS.
- Y. Chen, Z. Jia, M. Shafiq, X. Xie, X. Xiao and R. Castro, et al., Gas foaming of electrospun poly(L-lactide-co-caprolactone)/silk fibroin nanofiber scaffolds to promote cellular infiltration and tissue regeneration, Colloids Surf., B, 2021, 201, 111637 CrossRef CAS PubMed.
- A. F. Girão, Â. Semitela, G. Ramalho, A. Completo and P. A. A. P. Marques, Mimicking nature: Fabrication of 3D anisotropic electrospun polycaprolactone scaffolds for cartilage tissue engineering applications, Composites, Part B, 2018, 154, 99–107 CrossRef.
- Y. Zhou, J. Chyu and M. Zumwalt, Recent Progress of Fabrication of Cell Scaffold by Electrospinning Technique for Articular Cartilage Tissue Engineering, Int. J. Biomater., 2018, 2018, 1953636 Search PubMed.
- Â. Semitela, A. F. Girão, C. Fernandes, G. Ramalho, I. Bdikin and A. Completo, et al., Electrospinning of bioactive polycaprolactone-gelatin nanofibres with increased pore size for cartilage tissue engineering applications, J. Biomater. Appl., 2020, 35(4–5), 471–484 CrossRef PubMed.
- A. Eltom, G. Zhong and A. Muhammad, Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review, Adv. Mater. Sci. Eng., 2019, 2019, e3429527 Search PubMed.
- J. Huang, J. Xiong, D. Wang, J. Zhang, L. Yang and S. Sun, et al., 3D Bioprinting of Hydrogels for Cartilage Tissue Engineering, Gels, 2021, 7(3), 144 CrossRef CAS PubMed.
- M. Li, D. Sun, J. Zhang, Y. Wang, Q. Wei and Y. Wang, Application and development of 3D bioprinting in cartilage tissue engineering, Biomater. Sci., 2022, 10(19), 5430–5458 RSC.
- J. Zhou, Q. Li, Z. Tian, Q. Yao and M. Zhang, Recent advances in 3D bioprinted cartilage-mimicking constructs for applications in tissue engineering, Mater. Today Bio, 2023, 23, 100870 CrossRef CAS PubMed.
- P. Baei, H. Daemi, F. Mostafaei, F. A. Sayahpour, H. Baharvand and M. B. Eslaminejad, A tough polysaccharide-based cell-laden double-network hydrogel promotes articular cartilage tissue regeneration in rabbits, Chem. Eng. J., 2021, 418, 129277 CrossRef CAS.
- X. Xu, Y. J. Liang, X. F. Li, K. Ouyang, M. Y. Wang and T. Cao, et al., Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration, Biomaterials, 2021, 269, 120539 CrossRef CAS PubMed.
- C. Y. Chen, C. C. Li, C. J. Ke, J. S. Sun and F. H. Lin, Kartogenin Enhances Chondrogenic Differentiation of MSCs in 3D Tri-Copolymer Scaffolds and the Self-Designed Bioreactor System, Biomolecules, 2021, 11(1), 115 CrossRef CAS PubMed.
- M. Rezvaninia, N. Baheiraei and F. Bagheri, Effects of kartogenin/PLGA nanoparticles on silk scaffold properties and stem cell fate, Bioinspired, Biomimetic Nanobiomater., 2021, 10(2), 45–53 CrossRef.
- J. J. Wei, P. Ran, Q. Y. Li, J. F. Lu, L. Zhao and Y. Liu, et al., Hierarchically structured injectable hydrogels with loaded cell spheroids for cartilage repairing and osteoarthritis treatment, Chem. Eng. J., 2022, 430, 132211 CrossRef CAS.
- M. S. Bozhokin, S. A. Bozhkova, G. I. Netylko, D. G. Nakonechny, Y. A. Nashchekina and M. I. Blinova, et al., Experimental Replacement of the Surface Defect of Rat Hyaline Cartilage by a Cell-Engineered Construct, Regener. Eng. Transl. Med., 2021, 7(2), 184–193 CrossRef CAS.
- B. Wang, P. J. Diaz-Payno, D. C. Browe, F. E. Freeman, J. Nulty and R. Burdis, et al., Affinity-bound growth factor within sulfated interpenetrating network bioinks for bioprinting cartilaginous tissues, Acta Biomater., 2021, 128, 130–142 CrossRef CAS PubMed.
- A. B. Bello, Y. Kim, S. Park, M. S. Muttigi, J. Kim and H. Park, et al., Matrilin3/TGF beta 3 gelatin microparticles promote chondrogenesis, prevent hypertrophy, and induce paracrine release in MSC spheroid for disc regeneration, npj Regener. Med., 2021, 6(1), 50 CrossRef CAS PubMed.
- E. P. Lamparelli, J. Lovecchio, M. C. Ciardulli, V. Giudice, T. P. Dale and C. Selleri, et al., Della Porta. Chondrogenic Commit Hum Bone Marrow Mesenchymal Stem Cells Perfus Collagen Hydrogel Funct HTGF-Beta 1-Releas PLGA Microcarrier, Pharmaceutics, 2021, 13(3), 399 CrossRef CAS PubMed.
- M. H. Hagmeijer, J. V. Korpershoek, J. F. Crispim, L. T. Chen, P. Jonkheijm and A. J. Krych, et al., The regenerative effect of different growth factors and platelet lysate on meniscus cells and mesenchymal stromal cells and proof of concept with a functionalized meniscus implant, J. Tissue Eng. Regener. Med., 2021, 15(7), 648–659 CrossRef CAS PubMed.
- M. Knippenberg, M. N. Helder, B. Z. Doulabi, P. I. Wuisman and J. Klein-Nulend, Osteogenesis versus chondrogenesis by BMP-2 and BMP-7 in adipose stem cells, Biochem. Biophys. Res. Commun., 2006, 342(3), 902–908 CrossRef CAS PubMed.
- S. Claus, E. Aubert-Foucher, M. Demoor, B. Camuzeaux, A. Paumier and M. Piperno, et al., Chronic exposure of bone morphogenetic protein-2 favors chondrogenic expression in human articular chondrocytes amplified in monolayer cultures, J. Cell. Biochem., 2010, 111(6), 1642–1651 CrossRef CAS PubMed.
- I. Sekiya, D. C. Colter and D. J. Prockop, BMP-6 enhances chondrogenesis in a subpopulation of human marrow stromal cells, Biochem. Biophys. Res. Commun., 2001, 284(2), 411–418 CrossRef CAS PubMed.
- B. T. Estes, A. W. Wu and F. Guilak, Potent induction of chondrocytic differentiation of human adipose-derived adult stem cells by bone morphogenetic protein 6, Arthritis Rheum., 2006, 54(4), 1222–1232 CrossRef CAS PubMed.
- Y. Z. Jiang, Y. Y. Qi, X. H. Zou, L. L. Wang and H. W. Ouyang, Comparison the Effects of BMP-4 and BMP-7 on Articular Cartilage Repair with Bone Marrow Mesenchymal Stem Cells, Springer, Berlin, Heidelberg, 2009 Search PubMed.
- M. M. Caron, P. J. Emans, A. Cremers, D. A. Surtel, M. M. Coolsen and L. W. Rhijn, et al., Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by BMP-7, Osteoarthritis Cartilage, 2013, 21(4), 604–613 CrossRef CAS PubMed.
- C. Betsholtz, M. Nistér, F. Rorsman, C. H. Heldin and B. Westermark, Structural and functional aspects of platelet-derived growth factor and its role in the pathogenesis of glioblastoma, Mol. Chem. Neuropathol., 1989, 10(1), 27–36 CrossRef CAS PubMed.
- M. Uomizu, T. Muneta, M. Ojima, I. Sekiya, H. Koga and K. Tsuji, PDGF-induced proliferation and differentiation of synovial mesenchymal stem cells is mediated by the PI3K-PKB/Akt pathway, J. Med. Dent. Sci., 2018, 65, 73–82 Search PubMed.
- M. Anderson-Baron, Y. Liang, M. Kunze, A. Mulet-Sierra, M. Osswald and K. Ansari, et al., Suppression of Hypertrophy During in vitro Chondrogenesis of Cocultures of Human Mesenchymal Stem Cells and Nasal Chondrocytes Correlates With Lack of in vivo Calcification and Vascular Invasion, Front. Bioeng. Biotechnol., 2021, 8 DOI:10.3389/fbioe.2020.572356.
- L. Y. Chan, C. C. Chang, P. L. Lai, T. Maeda, H. C. Hsu and C. Y. Lin, et al., Cre/LoxP Genetic Recombination Sustains Cartilage Anabolic Factor Expression in Hyaluronan Encapsulated MSCs Alleviates Intervertebral Disc Degeneration, Biomedicines, 2022, 10(3), 555 CrossRef CAS PubMed.
- D. J. Vail, R. A. Somoza and A. I. Caplan, MicroRNA Regulation of Bone Marrow Mesenchymal Stem Cell Chondrogenesis: Toward Articular Cartilage, Tissue Eng., Part A, 2022, 28(5–6), 254–269 CrossRef CAS PubMed.
- T. Gómez-Leduc, M. Desancé, M. Hervieu, F. Legendre, D. Ollitrault and C. De Vienne, et al., Hypoxia Is a Critical Parameter for Chondrogenic Differentiation of Human Umbilical Cord Blood Mesenchymal Stem Cells in Type I/III Collagen Sponges, Int. J. Mol. Sci., 2017, 18(9), 1933 CrossRef PubMed.
Footnote |
| † Both authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |