Open Access Article
Open Access ArticleA study of saponin-encapsulated ultrasound microbubbles Rb3NPs@MBs for atherosclerosis targeted treatment†
Chunting Zhong
 ,
Jianhua Bai,
Xiaoting Yang,
Yiran Ji,
Jiabao Huang,
Xiao Tan
,
Jianhua Bai,
Xiaoting Yang,
Yiran Ji,
Jiabao Huang,
Xiao Tan ,
Xiaoyu Chen,
LiJun Xing,
Bingxuan Xu,
Dianhuan Tan,
Yun Chen
,
Xiaoyu Chen,
LiJun Xing,
Bingxuan Xu,
Dianhuan Tan,
Yun Chen
 * and
Tingting Zheng
* and
Tingting Zheng
 *
*
Shenzhen Key Laboratory for Drug Addiction and Medication Safety, Department of Ultrasound, Institute of Ultrasonic Medicine, Peking University Shenzhen Hospital, Shenzhen Peking University-The Hong Kong University of Science and Technology Medical Center, Shenzhen, Guangdong Province 518036, P. R. China. E-mail: yunchen@sphmc.org; kyzs_018@126.com
First published on 7th May 2025
Abstract
Atherosclerosis remains a leading disease posing significant threats to human health and life. Oxidative stress plays a critical role in the initiation of early atherosclerosis. Ginsenoside Rb3 has been shown to exert potential therapeutic effects against atherosclerosis due to its antioxidant properties. However, its clinical utility remains constrained to the nanometer scale, offering insufficient targeting capability for atherosclerosis treatment. To address this limitation, we designed a novel Rb3-loaded microbubble system Rb3NPs@MBs. This microbubble system effectively encapsulates Rb3 nanoparticles and, via ultrasound-targeted microbubble destruction (UTMD), facilitates their targeted accumulation in the aortic arch of atherosclerotic mice. Subsequently, Rb3NPs@MBs reduce oxidative stress, attenuate endothelial cell apoptosis and foam cell formation, and ultimately diminish plaque development at the lesion site. This strategy holds promise as a therapeutic approach for atherosclerosis. These findings suggest that Rb3NPs@MBs represent a promising therapeutic strategy for atherosclerosis.
1. Introduction
Cardiovascular disease (CVD) remains the leading cause of mortality among non-communicable diseases globally, exhibiting a high incidence rate.1 Atherosclerosis (AS) is characterized by the formation of fibrofatty lesions within the arterial walls, leading to cardiovascular and cerebrovascular conditions such as coronary heart disease and stroke. AS serves as the pathological foundation for the onset and progression of CVD.2AS is a complex, multicellular, and staged disease that typically begins with apoptosis and dysfunction of endothelial cells.3 When vascular endothelial cells are subjected to abnormal shear stress within blood vessels, the intracellular response to this disturbance results in the upregulation and increased activity of vascular cell adhesion molecule-1 (VCAM-1), as well as increased permeability to lipoproteins, particularly to low intensity lipoprotein (LDL) in the circulation.4,5 LDL traverses the endothelial barrier and undergoes oxidation to form oxidized LDL (ox-LDL) within the intimal layer of blood vessels, where it accumulates.6 Concurrently, monocytes are recruited from the bloodstream into the intimal region, where they differentiate into macrophages.7,8 These macrophages take up ox-LDL and transform into foam cells, which accumulate and ultimately form plaques.9 In summary, AS is a fibrofatty lesion in the arterial wall mediated by oxidative stress and inflammation.10,11 At present, various symptomatic treatments are available for different clinical phenotypes of AS, including antithrombotic drugs, antilipemic agents, and anti-myocardial ischemia medications. Additionally, targeted therapies such as PCSK9 inhibitors and inflammatory factor monoclonal antibodies like Canakinumab have progressed to clinical trial stages.12–16
Ginseng, a traditional medicine in ancient China, is widely used for vascular protection in traditional medicine practices.17–19 Saponin Rb3, an extract derived from ginseng, has been investigated for its potential in treating AS due to its potent antioxidant effects.20 Rb3 can regulate energy metabolism and apoptosis in cardiomyocytes by activating the peroxisome proliferator-activated receptor alpha (PPARα) pathway, thereby enhancing cardiovascular function.21,22 However, existing studies are limited to the incorporation of Rb3 into nano-systems and the efficiency of targeted delivery is often suboptimal, particularly in regions with severe vascular plaques or specific localized areas.23,24 In order to solve the problem of drug targeting, this study employed ultrasound-targeted microbubble destruction (UTMD) technology.25–27 Typically, microbubbles—vacuole-like structures encapsulating a gas core—are utilized as ultrasound contrast agents.28 By modifying the components of microbubbles, such as replacing the core gas with therapeutic gases like oxygen or carbon monoxide,28,29 or by incorporating therapeutic agents (e.g., genes, proteins, and nanomedicines),30,31 therapeutic purposes can be achieved. UTMD facilitates the targeted explosion of microbubbles at specific sites under ultrasound guidance.32 The resultant physical effects include the opening of tight junctions between cells, increased tissue permeability, enhanced cellular uptake of drugs, and augmented drug delivery efficiency.33–40 As a non-invasive treatment modality,41 UTMD has been validated for applications such as opening the blood–brain barrier,42 enhancing the enhanced permeability and retention (EPR) effect in tumour therapy,43,44 and treating AS.45–48
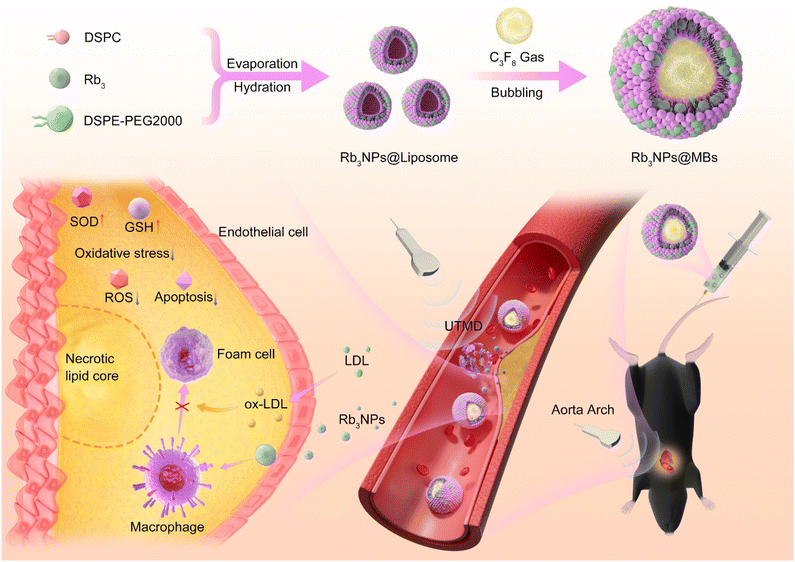
Therefore, in this study, a saponin-encapsulated microbubble system Rb3NPs@MBs was constructed and combined with UTMD, so that the drug could be enriched in the targeted region of AS mice under ultrasound guidance. Using this drug delivery system, we verified the feasibility of RB3NPs@MBs for reducing aortic plaques in AS mice and explored the related mechanism of RB3NPs@MBs for the treatment of AS.
2. Materials and methods
2.1. Materials
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (DSPE-PEG2000) and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) were purchased from Avanti (816-94-4 & 474922-77-5, USA). Saponin Rb3 was obtained from Chengdu Pufei (JOT-10260, China). Lipopolysaccharides (LPS) were obtained from Solarbio (L8880, China). Dulbecco's minimum essential medium (DMEM) and fetal bovine serum were purchased from Gibco (USA). Endothelial cell medium (ECM) was obtained from Sciencell (1001, USA). The mouse macrophage cell line (RAW264.7) was obtained from the Laboratory Center of Shenzhen University; the human umbilical vein endothelial cell line (HUVEC) was obtained from BeNa Culture Collection (BNCC378266, China).2.2. Preparation and characterization of Rb3NPs@MBs
DSPC and DSPE-PEG2000 were dissolved in chloroform at 7.90 mg ml−1 and 29.92 mg ml−1 respectively. Rb3 was dissolved in a solvent mixture of chloroform and methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at a concentration of 2 mg ml−1. To synthesize Rb3NPs@MBs, 272 μl of DSPC, 30 μl of DSPE-PEG2000, and 500 μl of Rb3 were combined in a round-bottom flask and subjected to rotary evaporation in a fume hood for 40 min to form a lipid film. The resultant lipid film was hydrated with 1 ml of phosphate-buffered saline (PBS) and ultrasonicated in a water bath maintained at 65 °C for 5–6 min. The hydrodynamic diameter of Rb3NPs@Lipos was measured using dynamic light scattering (DLS) with a Zetasizer Nano (Malvern, England), and the structural morphology was characterized by transmission electron microscopy (TEM).
1, v/v) at a concentration of 2 mg ml−1. To synthesize Rb3NPs@MBs, 272 μl of DSPC, 30 μl of DSPE-PEG2000, and 500 μl of Rb3 were combined in a round-bottom flask and subjected to rotary evaporation in a fume hood for 40 min to form a lipid film. The resultant lipid film was hydrated with 1 ml of phosphate-buffered saline (PBS) and ultrasonicated in a water bath maintained at 65 °C for 5–6 min. The hydrodynamic diameter of Rb3NPs@Lipos was measured using dynamic light scattering (DLS) with a Zetasizer Nano (Malvern, England), and the structural morphology was characterized by transmission electron microscopy (TEM).
Subsequently, the Rb3NPs@Lipos solution was transferred to a vial connected for gas exchange via syringes through rubber caps to produce Rb3NPs@MBs. Initially, the gas within the vial was evacuated by vacuuming for 5 min, followed by the introduction of perfluoropropane (C3F8) for 1 min to facilitate gas mixing. To ensure complete gas exchange, this procedure was repeated 3–5 times. Subsequently, additional C3F8 was added, and the mixture was agitated using a shaker for 45 seconds to form Rb3NPs@MBs. The size, particle size distribution, and concentration of Rb3NPs@MBs were quantified using a particle analyzer (AccuSizer 780AD, USA) prior to structural examination under an optical microscope.
Conventional microbubbles (MBs) were prepared similarly to Rb3NPs@MBs, excluding the addition of Rb3. After establishing a standard curve for Rb3 via ultraviolet (UV) spectrophotometry, uncoated and coated Rb3 were separated by centrifuging the Rb3NPs@MBs solution (5000 rpm for 10 min) in an ultrafiltration tube. The encapsulation efficiency and drug loading of Rb3NPs@MBs were subsequently determined using UV spectrophotometry.
To assess blood compatibility, the hemolysis rate (HR) of Rb3NPs@MBs after contact with blood was calculated. After the whole blood of fresh heparin sodium anticoagulated C57BL/6J mice was centrifuged and washed, the red blood cells were resuspended in a 2% concentration red blood cell suspension in PBS. According to the concentration gradient (10, 50, 100, and 1000 μg ml−1), Rb3NPs@MBs and the red blood cell suspension were gently mixed (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and each concentration was divided into two groups: the UTMD group (given ultrasound operation) and the free group (without ultrasound operation), and incubated for 1 h at 37 °C. NS and the red blood cell suspension (volume ratio 1
1), and each concentration was divided into two groups: the UTMD group (given ultrasound operation) and the free group (without ultrasound operation), and incubated for 1 h at 37 °C. NS and the red blood cell suspension (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were used as negative controls, and ddH2O and the red blood cell suspension (volume ratio 1
1) were used as negative controls, and ddH2O and the red blood cell suspension (volume ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
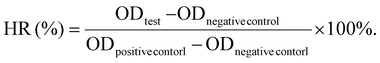
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were used as positive controls. The ultrasonic conditions were as follows: output frequency: 1.0 MHz; duty cycle: 20%; and pulse frequency: 100 Hz. The output sound intensity was 100 mW cm−2. The time was 1 min. At the end of the incubation, the mixture was centrifuged at 3000 rpm for 10 min and the supernatant was removed. The supernatant was transferred to a 96-well plate, and the absorbance value at the specific wavelength of 541 nm of hemoglobin was measured using a microplate reader. Each well was set up with three repeat wells. The HR was calculated using the following formula:
1) were used as positive controls. The ultrasonic conditions were as follows: output frequency: 1.0 MHz; duty cycle: 20%; and pulse frequency: 100 Hz. The output sound intensity was 100 mW cm−2. The time was 1 min. At the end of the incubation, the mixture was centrifuged at 3000 rpm for 10 min and the supernatant was removed. The supernatant was transferred to a 96-well plate, and the absorbance value at the specific wavelength of 541 nm of hemoglobin was measured using a microplate reader. Each well was set up with three repeat wells. The HR was calculated using the following formula:
2.3. Evaluation of the therapeutic effect in vitro
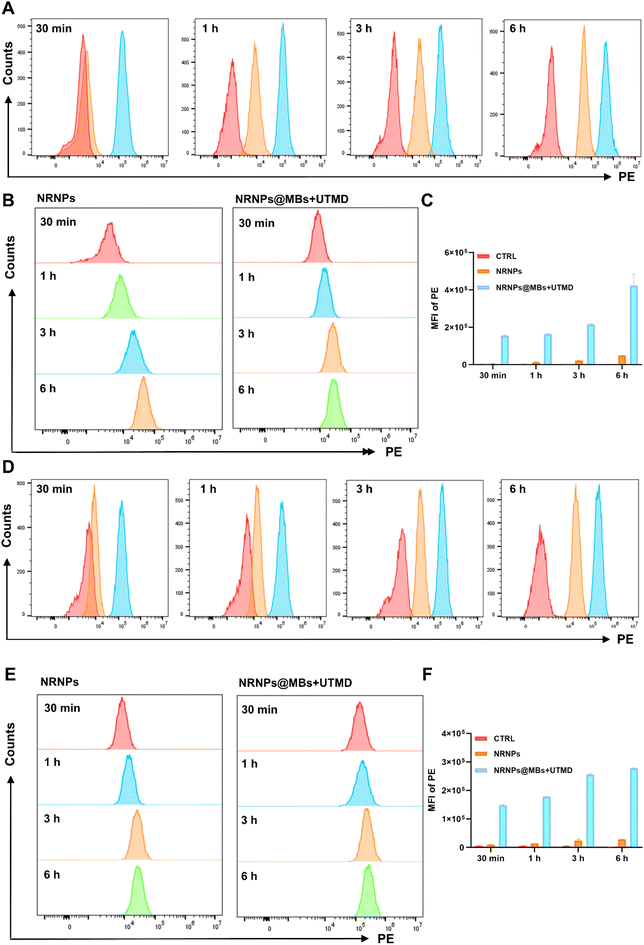
RAW264.7 cells were cultured in DMEM with 10% FBS and 1% penicillin/streptomycin (1% penicillin and 1% streptomycin). HUVEC cells were cultured in ECM with 10% FBS, 1% ECGs, and 1% penicillin/streptomycin (1% penicillin and 1% streptomycin). Cells were maintained in an incubator at 37 °C under a humidified atmosphere and 5% CO2.The NRNPs concentration, NRNPs@MBs preparation, and subsequent characterization of the NRNPs@MBs were the same as that in section 2.2. RAW264.7 cells and HUVEC cells in good growth condition were plated in 6-well plates, and 2 ml of 1 × 105 ml−1 cell suspension was added to each well and cultured for 24 h. The cells were divided into the control group, NRNPs group (10 μg ml−1), and NRNPs@MBs + UTMD (10 μg ml−1) group, and the UTMD parameters were the same as those in section 2.3.2. After treatment, the cells were collected at different time points (30 min, 1 h, 3 h, 6 h, 12 h, and 24 h), washed with PBS three times, and centrifuged at 1000 rpm for 5 min each time. After the last wash, the cells were resuspended in 500 μl PBS for flow cytometry, and the fluorescence channel was the PE channel.
2.4 Evaluation of the therapeutic effect in vivo
5–6 week old male C57BL/6J mice and ApoE−/− mice were purchased from Guangdong Yaokang Biological Technology (China). This study was approved by the Experimental Animal Center of Shenzhen PKU-HKUST Medical Center. All experimental procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and according to the institutional animal experimental ethical guidelines. ApoE−/− mice were fed a high-fat diet (1.25% cholesterol), C57BL/6J mice were fed a normal diet, and all mice were provided free access to water. ApoE−/− mice were fed for 12 weeks to establish an AS model.In order to further verify the targeting function of UTMD, another 6 mice were selected for the same operation. After Cy7.5@MBs was circulated in the mice for 1 hour, the mice were sacrificed and the aortic tissues were collected for frozen section, stained with DAPI, and confocal microscopy was used to observe the enrichment of Cy7.5 in the plaque at the targeted site of ultrasound.
Following the treatment period, the mice were subjected to a 12-hour fasting regimen before being euthanized and dissected. The serum biomarker detection was performed by Servicebio (Wuhan, China).
2.5 Statistical analysis
Data are indicated as mean ± SD. Paired t-test or ANOVA was carried out as indicated to establish the statistical differences. All the statistical computations were performed using the GraphPad Prism software. P-Values <0.05 were considered to indicate significant differences: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.3. Results
3.1 Characterization of Rb3NPs@Lipos and Rb3NPs@MBs
In this study, Rb3NPs@Lipos were prepared using the classical film hydration technique, and their particle size and zeta potential were determined by dynamic light scattering (DLS) (Fig. 1A & Fig. S1B†).49 The results showed that the hydrated particle size of Rb3NPs@Lipos was about 165.7 ± 21.52 nm. Transmission electron microscopy (TEM) results revealed that Rb3NPs@Lipos exhibited the classic circular and homogeneous liposomal morphology (Fig. 1B). These findings indicate that the prepared Rb3NPs@Lipos possess uniform size distribution and can be employed for subsequent microbubble fabrication.Following gas displacement of the Rb3NPs@Lipos solution, Rb3NPs@MBs were generated. The particle size and concentration of Rb3NPs@MBs were evaluated using a particle size analyser (PSS). To minimize the risk of vascular occlusion, the size of microbubbles should be below 10 μm, preferably near the diameter of erythrocytes (∼7.2 μm).50 As shown in Fig. 1C, Rb3NPs@MBs exhibited a uniform particle size of 1.70 ± 0.036 μm with a concentration of 2.1 × 109 ml−1, and a normally distributed particle size profile. Optical microscopy revealed that Rb3NPs@MBs exhibited the classical hollow spherical structure with uniform dispersion (Fig. 1D). The zeta potential of Rb3NPs@MBs was −2.909 ± 0.636 mV, bigger than that of Rb3NPs@Lipos (Fig. S1B†). Stability assessments over a 72 h period (Fig. S2†) showed that the concentration of Rb3NPs@MBs remained on the order of 1 × 108 mL−1, suggesting that the Rb3NPs@MBs system is stable.
Then, using an ultraviolet spectrophotometer, we found that the encapsulation efficiency of Rb3NPs@MBs was 88.48%, and the drug loading was 22.12%, respectively. In addition, to evaluate the compatibility of Rb3NPs@MBs in contact with blood, the hemolysis rate test results showed that Rb3NPs@MBs has excellent blood compatibility (HR% <5%) at concentrations ≤100 μg ml−1, and its ultrasonic response characteristics do not induce additional hemolytic damage, which meets the biosafety requirements of intravenous drug delivery systems (Fig. S3†). Then, using an ultraviolet spectrophotometer, we found the encapsulation efficiency of Rb3NPs@MBs to be 88.48% and the drug loading to be 22.12%, respectively. Furthermore, the assessments over a 72 h period (Fig. S2†) showed that the concentration of Rb3NPs@MBs remained on the order of 1 × 108 ml−1, suggesting that the Rb3NPs@MBs system is stable.
In summary, we successfully fabricated Rb3NPs@MBs with diameters of 1.70 μm, which demonstrated uniform size distribution, stability, and homogeneous dispersion.
3.2 Ultrasound enhanced cellular uptake
For cellular uptake experiments, cells were treated with 10 μg ml−1 NRNPs and 10 μg ml−1 NR@MBs, and then collected at 4 time points (30 min, 1 h, 3 h, and 6 h) to observe the cellular uptake of the dye. The results are shown in Fig. 2. Fig. 2A–C shows the cellular uptake results of RAW264.7 cells. It can be seen that UTMD promoted the uptake of NR by cells within 30 min–6 h after treatment, and the fluorescence intensity of cells was significantly stronger than that of the NRNPs group. Fig. 2D–F show the cellular uptake results of HUVEC cells. It can be seen that in 30 min–6 h, ultrasound was also observed to promote the absorption of NR in HUVEC cells.However, after careful analysis of the comparative data, we found that the fluorescence of the NRNPs group in RAW264.7 cells increased with time, but this phenomenon was not observed in HUVEC cells. Considering that the drug action time is usually 24 hours in in vitro experiments, in order to explore the reason for the fluorescence growth of RAW264.7 cells, we continued to set a longer time curve to observe the fluorescence uptake of cells, and the results are shown in Fig. S4.† The fluorescence uptake of RAW264.7 cells increased with time, and there was no statistically significant difference in fluorescence intensity between the NRNPs group and the NRNPs@MBs + UTMD group at 24 h.
In summary, UTMD promoted the uptake of fluorescence, and it can also be assumed that when NR was replaced by Rb3NPs, UTMD would also promote the uptake of the drug.
3.3 The therapeutic effect of Rb3NPs@MBs in vitro
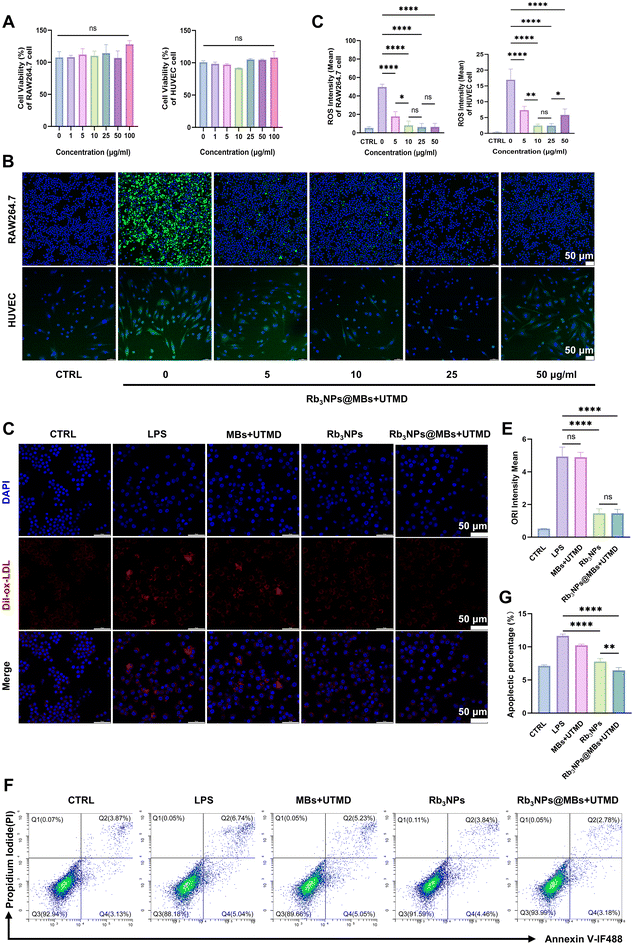
In order to elucidate the therapeutic effects of the prepared formulation on early AS events, RAW264.7 cells and HUVECs were employed in vitro. Additionally, a microbubble-only (MBs) group was included to confirm that any therapeutic benefits were attributable to Rb3NPs@MBs rather than the mechanical effects associated with microbubble destruction.First, a CCK-8 assay was performed to assess the impact of UTMD combined with Rb3NPs@MBs on cell viability. As a widely used assay reflecting proliferative capacity, the CCK-8 results revealed that the combination of UTMD and Rb3NPs@MBs at concentrations ranging from 1 to 100 μg ml−1 did not significantly affect RAW264.7 cells and HUVEC viability (Fig. 3A).
Subsequently, confocal microscopy was utilized to examine the intracellular ROS-scavenging capacity of Rb3NPs@MBs at various concentrations in order to determine the optimal concentration for in vitro experiments. Here, LPS was used to induce an inflammatory response, reflecting its well-established role as a cost-effective, highly reproducible stimulus for creating in vitro inflammatory disease models, including those relevant to AS.51Confocal images showed that when the treatment of Rb3NPs@MBs combined with UTMD was absent (0 μg ml−1, only LPS treatment), green fluorescence indicative of ROS was most pronounced. Among the 5–50 μg ml−1 Rb3NPs@MBs treatment groups, the 10 and 25 μg ml−1 conditions demonstrated the most substantial reduction in fluorescence (Fig. 3B), with subsequent ImageJ-based quantitative analysis corroborating that 10 and 25 μg ml−1 yielded optimal ROS-scavenging activity (Fig. 3C). Based on the principle of administering the minimum effective dose, 10 μg ml−1 was chosen for all subsequent in vitro experiments.
To investigate whether Rb3NPs@MBs could diminish ox-LDL uptake by RAW264.7 cells and thereby attenuate foam cell formation, cells were incubated with Dil-ox-LDL, an oxidized low-density lipoprotein labeled red fluorescence.52 Confocal microscopy and quantitative fluorescence analysis revealed that MBs combined with UTMD did not significantly reduce Dil-ox-LDL uptake compared to the LPS-only group. In contrast, both the Rb3NPs and the Rb3NPs@MBs + UTMD treatments exhibited a marked decrease in red fluorescence (Fig. 3D and E), indicating a significant inhibition of ox-LDL uptake and foam cell formation. Notably, no statistically significant difference was observed between the Rb3NPs and Rb3NPs@MBs + UTMD groups. Then, the effect of Rb3NPs@MBs on HUVEC apoptosis was examined. Flow cytometry demonstrated that both Rb3NPs and Rb3NPs@MBs + UTMD treatments reduced LPS-induced HUVEC apoptosis, with a more pronounced anti-apoptotic effect observed in the Rb3NPs@MBs + UTMD group (Fig. 3F and G). These findings suggest that the combination of Rb3NPs@MBs and UTMD effectively mitigates two hallmarks of early AS events—foam cell formation and endothelial cell apoptosis.
Moreover, the therapeutic benefit in HUVECs was superior when Rb3NPs were delivered via MBs combined with UTMD relative to Rb3NPs alone.
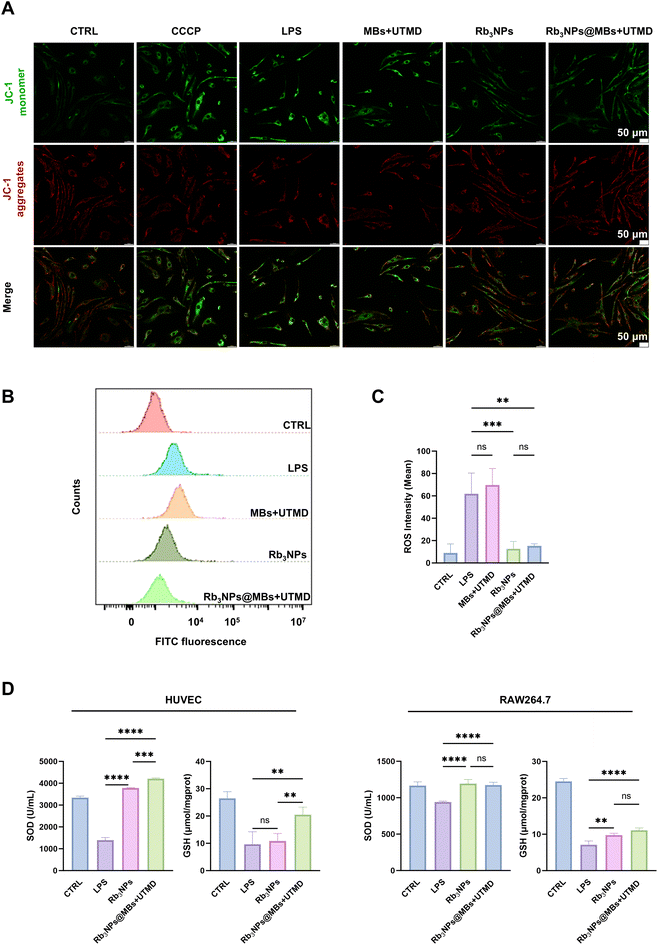
3.4 Rb3NPs@MBs reduce oxidative stress in cells
Numerous studies have demonstrated that oxidative stress plays a pivotal role in the pathogenesis of AS. As an inflammatory disease, the entire progression of AS is often accompanied by an imbalance between intracellular oxidative and antioxidant mechanisms. To determine whether Rb3NPs@MBs can modulate key cellular events through the reduction of oxidative stress, we performed a series of experiments to assess the oxidative stress levels in HUVECs and RAW264.7 cells.First, in HUVECs, we assessed mitochondrial membrane potential (ΔΨm) using JC-1 staining. Mitochondria constitute the primary center of cellular energy and metabolism, with the mitochondrial respiratory chain serving as a principal source of reactive oxygen species (ROS).53 The extent of mitochondrial mass is closely linked to ROS levels, and the decline in ΔΨm reflects mitochondrial impairment. Under conditions of high mitochondrial membrane potential, the JC-1 dye accumulates within the mitochondrial matrix, forming aggregates that exhibit red fluorescence. Conversely, when the membrane potential is low, JC-1 remains in its monomeric form and produces green fluorescence. In this study, we observed that the green fluorescence intensity in the LPS group was comparable to that of the CCCP (positive control) and MBs + UTMD groups, whereas the Rb3NPs@MBs + UTMD groups displayed markedly weaker green fluorescence (Fig. 4A). These findings indicate that Rb3NPs@MBs + UTMD effectively mitigated the LPS-induced reduction in mitochondrial membrane potential in HUVECs.
In RAW264.7 cells, intracellular ROS levels were quantified by flow cytometry. As shown in Fig. 4B and C, the MBs + UTMD treatment alone did not attenuate LPS-induced ROS generation, while both Rb3NPs and Rb3NPs@MBs + UTMD substantially reduced ROS levels. This result is consistent with our previous observations. Notably, no significant difference was detected between the Rb3NPs and Rb3NPs@MBs + UTMD groups in terms of ROS suppression.
Finally, we measured superoxide dismutase (SOD) and glutathione (GSH) levels in HUVECs and RAW264.7 cells using commercial assay kits. SOD is a major antioxidant enzyme responsible for catalyzing the dismutation of superoxide anion radicals (O2−) into oxygen and hydrogen peroxide (H2O2), while GSH is a key antioxidant that directly scavenges free radicals and participates in multiple antioxidant reactions. Intracellular antioxidant capacity can therefore be evaluated by measuring both SOD and GSH. In HUVECs, the Rb3NPs@MBs + UTMD group exhibited significantly higher SOD and GSH levels compared with the LPS group, and this effect exceeded that observed in the Rb3NPs group. Similarly, in RAW264.7 cells, Rb3NPs@MBs + UTMD markedly enhanced SOD and GSH content relative to the LPS group, although no statistically significant difference was found between the Rb3NPs and Rb3NPs@MBs + UTMD groups (Fig. 4D).
Collectively, these data suggest that Rb3NPs@MBs effectively mitigate oxidative stress in both RAW264.7 and HUVEC cells. However, whether the combined delivery of Rb3NPs@MBs and UTMD confers additional therapeutic benefits in RAW264.7 cells requires further investigation.
3.5 Biodistribution of microbubbles combined with UTMD in vivo
The effects of 2 weeks Rb3NPs@MBs + UTMD on liver and kidney biochemical indicators (ALT, AST, BUN, and CRE), blood cell count (white blood cell count, neutrophil count, lymphocyte count, and monocyte count) and important organs (heart, liver, spleen, lungs, and kidneys) of mice were not different from those of normal mice. We then set out to explore whether UTMD similarly promotes drug absorption in vivo (Fig. S7†). Ultrasound is a commonly used imaging modality, particularly valuable for vascular imaging. It enables the visualization of multidimensional parameters, such as hemorheology, vascular plaque formation, and vascular stenosis.54 Among various ultrasound-based techniques, UTMD has emerged as a promising drug delivery strategy, owing to its safety, noninvasive nature, ease of operation, and high targeting efficiency.To evaluate the in vivo targeting efficiency of UTMD and the distribution and metabolism of microbubbles, we substituted Rb3NPs with Cy7.5 (a fluorescent probe) and prepared Cy7.5@MBs following the same protocol and concentration used for Rb3NPs@MBs. The UTMD-induced disruption process of Cy7.5@MBs is depicted in Scheme 1, while the operational details are demonstrated in Video 1 of the ESI.† Briefly, after locating the aortic arch via ultrasound, Cy7.5@MBs were administered through the tail vein of AS mice. When the microbubbles had adequately filled the vessels and enhanced the ultrasound contrast, UTMD was applied at the aortic arch. One hour later, in vivo fluorescence imaging was performed (n = 3), and after 24 hours of circulation, the major organs were collected for ex vivo imaging (n = 3). As shown in Fig. 4A, no fluorescence was detected in the aortic arch for either the control or the Cy7.5 groups, whereas strong fluorescence signals were observed in the aortic arch of the Cy7.5@MBs + UTMD group. This conclusion was also verified by fluorescent section staining of the aortic arch (Fig. S8†). After 24 hours of circulation, fluorescence imaging of the major organs revealed that, in the Cy7.5 group, the liver exhibited the highest fluorescence intensity, whereas the Cy7.5@MBs + UTMD group showed minimal fluorescence in both the liver and the lungs (Fig. 4B).
Moreover, the overall fluorescence intensity in these organs was substantially lower than that in the Cy7.5 group. These findings suggest reduced hepatic retention of Cy7.5 in the Cy7.5@MBs + UTMD group, while residual fluorescence in the lungs confirms that microbubbles with small diameters can be metabolized via pulmonary clearance. Notably, the fluorescence indicated that UTMD successfully concentrated Cy7.5 at the ultrasound-targeted site and demonstrated the feasibility of this targeted delivery approach to overcome blood circulation.
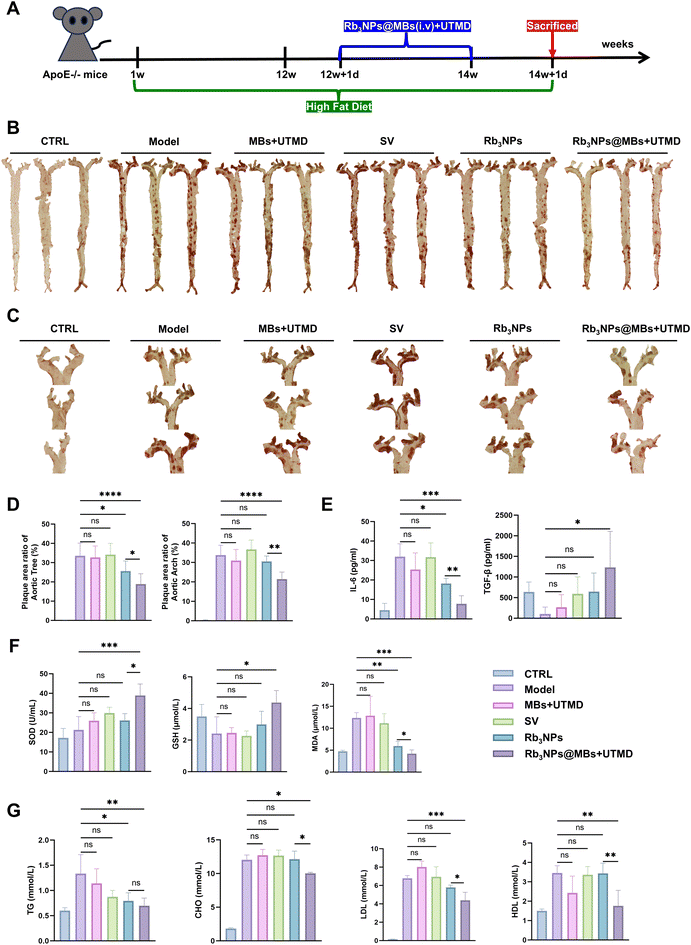
3.6 Rb3NPs@MBs in combination with UTMD targeted reduction of arterial plaques in AS mice
Apolipoprotein E knockout (ApoE−/−) mice, also referred to as APOE-deficient mice, are widely recognized as a classical model for investigating AS.55,56 Accordingly, five- to six-week-old ApoE−/− mice were maintained on a high-fat diet for 12 weeks to establish an AS model. In this study, simvastatin (SV) was selected as the positive control drug, given that statins remain the first-line therapy for AS. Additionally, to confirm that any therapeutic benefit did not result solely from the mechanical effects of MBs under ultrasound disruption, an MB-only group was included. Thus, C57BL/6 mice were assigned to the control group, whereas AS mice were allocated to the following groups, each containing eight animals: model, MBs + UTMD, SV, Rb3NPs, and Rb3NPs@MBs + UTMD. After 12 weeks of high-fat feeding, treatments were initiated, and following two weeks of intervention, the mice were euthanized for subsequent analyses (Fig. 6A).SOD and GSH are key intracellular antioxidants, whereas malondialdehyde (MDA), a lipid peroxidation byproduct, is an indicator of oxidative stress. Biochemical assays demonstrated that SOD and GSH concentrations in the Rb3NPs@MBs + UTMD group were significantly elevated compared with the model group, whereas MDA levels were markedly reduced, notably surpassing the reduction observed in the Rb3NPs group (Fig. 6F). These results indicate that Rb3NPs@MBs + UTMD ameliorates oxidative stress by increasing levels of the antioxidant factors SOD and GSH while decreasing MDA.
Next, to examine lipid profiles—a key reflection of abnormal lipid metabolism in AS—serum levels of triglycerides (TG), cholesterol (CHO), LDL, and HDL were measured. Levels of these lipids were significantly reduced in the Rb3NPs@MBs + UTMD group compared with the model group (Fig. 6G), confirming that Rb3NPs@MBs + UTMD can effectively modulate dyslipidaemia in AS.
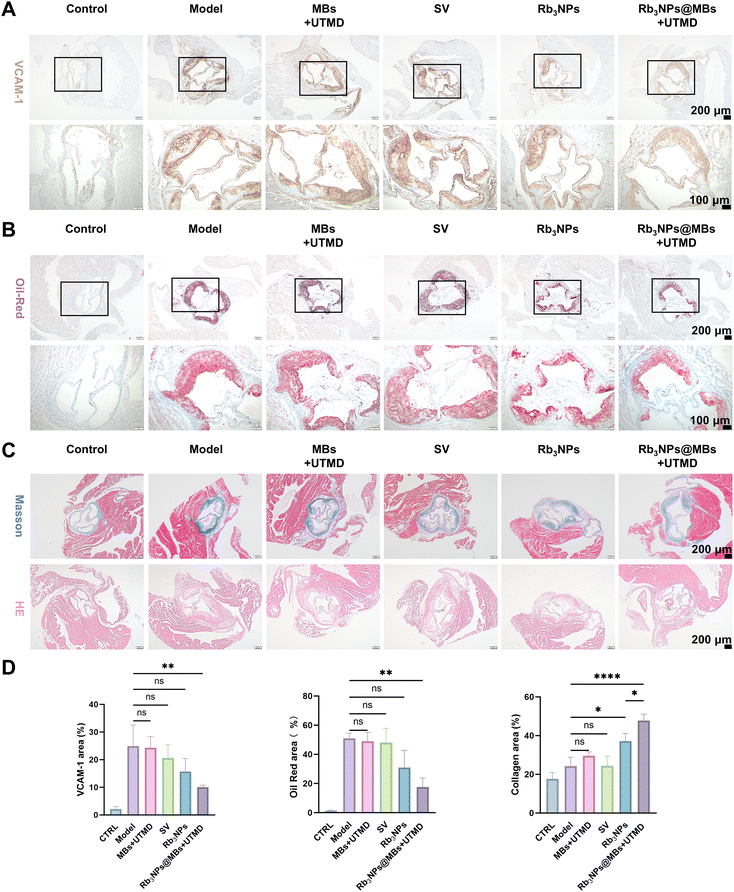
Collectively, these data demonstrate that Rb3NPs@MBs + UTMD successfully released the drug at the aortic arch targeted by ultrasound, thereby reducing local plaque burden, downregulating VCAM-1 expression, mitigating inflammation, and lowering both lipid and oxidative stress levels in treated mice.
4. Discussion and conclusions
In this study, we developed a novel ultrasound-visualized drug delivery system, termed Rb3NPs@MBs, using saponin Rb3. Both in vitro and in vivo experiments demonstrated that the combination of Rb3NPs@MBs with UTMD effectively alleviated arterial plaques in AS.Firstly, we successfully prepared microbubbles with a diameter of about 1.70 μm, and the Rb3NPs@MBs were uniformly dispersed with good properties. The results of cellular uptake indicate that the mechanism of UTMD promoting cellular uptake of drugs is feasible (Fig. 2). Then at the cellular level, a CCK-8 assay confirmed that Rb3NPs@MBs in conjunction with UTMD (1–100 μg ml−1) did not compromise cell viability in both HUVECs and RAW264.7 cells. Following this screening, 10 μg ml−1 was chosen as the optimal in vitro concentration based on confocal microscopy analyses (Fig. 3A–C). Subsequent experiments indicated that Rb3NPs@MBs reduced Dil-ox-LDL uptake in RAW264.7 cells, thereby inhibiting foam cell formation (Fig. 3D and E). In addition, flow cytometry revealed that Rb3NPs@MBs suppressed HUVEC apoptosis (Fig. 3F and G). To investigate whether these beneficial effects were mediated through modulation of oxidative stress, we evaluated the mitochondrial membrane potential (via JC-1 staining) in HUVECs, measured ROS levels in RAW264.7 cells, and quantified key antioxidant factors (SOD and GSH) (Fig. 4). The results showed that Rb3NPs@MBs preserved mitochondrial membrane potential in HUVECs, mitigated mitochondrial damage and ROS generation, and reduced ROS levels in RAW264.7 cells. Moreover, Rb3NPs@MBs therapy elevated both SOD and GSH in endothelial cells and macrophages. Thus, Rb3NPs@MBs inhibited early pathological events in AS, primarily by decreasing intracellular oxidative stress.
Because the aortic arch is predisposed to plaque formation (due to its angulation, increased branching, and abnormal shear forces),57 we selected it as the primary target for UTMD-mediated drug delivery in vivo. In vivo imaging of small animals confirmed that UTMD overcame blood flow constraints, facilitated traversal of the endothelial barrier, and effectively accumulated Cy7.5 in the targeted aortic arch (Fig. 5A). ApoE−/− mice were treated for two weeks. Notably, the MBs + UTMD and SV groups did not show significant differences in biochemical indices or plaque burden relative to untreated AS controls (model group). Although Rb3NPs alone reduced total plaque area in the aorta, its effect on the aortic arch, where the plaque was most advanced, remained limited. In contrast, Rb3NPs@MBs + UTMD not only effectively reduced the overall plaque burden in the aorta but also significantly diminished plaques in the targeted aortic arch (Fig. 6B–D). The combination therapy simultaneously lowered inflammatory markers, oxidative stress, and lipid levels in AS mice (Fig. 6E–G). Pathological assessments of aortic valve plaques demonstrated that Rb3NPs@MBs + UTMD reduced VCAM-1 expression, decreased lipid accumulation, increased plaque collagen content, and diminished plaque area, thereby corroborating its enhanced therapeutic efficacy (Fig. 7). Together, these results suggest that Rb3NPs@MBs with UTMD can effectively deliver the drug to the targeted aortic arch, reduce plaque formation, and improve pathological indices relevant to AS.
In vitro, we observed that there was no significant difference between the Rb3NPs@MBs + UTMD group and the Rb3NPs group in RAW264.7 cells. Combined with the results of cellular uptake, we believe that the mechanism of ultrasound promoting drug uptake in RAW264.7 cells is also applicable, and the therapeutic effect is not obvious mainly because the active uptake of Rb3NPs by cells also increases with time, accumulating to a level comparable to that of the Rb3NPs@MBs + UTMD group at 24 h. Therefore, the final intracellular drug concentration was not significantly different between the two groups. This phenomenon may be related to the high affinity of macrophages for fat-soluble drugs, and further research is needed in the future. However, in vitro models cannot fully replicate the complexity of in vivo pathophysiology, and our in vivo results confirmed that Rb3NPs@MBs combined with UTMD outperformed Rb3NPs alone.
In conclusion, we successfully constructed a targeted drug delivery system—Rb3NPs@MBs with UTMD—that demonstrated feasibility, efficacy, and safety in both in vitro and in vivo models. These findings highlight its potential for clinical application as a promising therapeutic strategy for AS. Ongoing research will aim to further optimize this system and evaluate its effectiveness in diverse AS models and clinical settings.
Author contributions
Manuscript drafting, data curation, investigation, and visualization: Chunting Zhong, Xiaoting Yang, Jianhua Bai, Yiran Ji, Xiao Tan, Xiaoyu Chen and Lijun Xing; methodology, validation, and project administration: Bingxuan Xu and Dianhuan Tan; in vitro experiments: Chunting Zhong and Jianhua Bai; in vivo imaging experiments: Chunting Zhong; in vivo experiments: Chunting Zhong, Xiaoting Yang, Jianhua Bai, Yiran Ji, Xiao Tan and Xiaoyu Chen; study design and conceptualization: Tingting Zheng; supplementary experimental data: Chunting Zhong, Jianhua Bai and Xiaoting Yang; resources and funding acquisition: Yun Chen and Tingting Zheng; supervision and manuscript editing: Tingting Zheng. All authors have approved the manuscript and agreed to its submission to Biomaterials Science.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the following: YC received financial support from grant no. KXCFZ202002011010487, JCYJ20210324131402008 and ZDSYS201504301045406. TZ received financial support from grant no. 2022A1515010986 and 2022A1515010296. All authors received financial support from grant no. SZSM202111011 and SZXK051.References
- V. Junaid, A. M. K. Minhas, M. Inam, C. Hinkamp, K. M. Talha, C. Meloche, S. Sheikh, A. Khoja, C. Krittanawong, E. M. Vaughan, D. K. Kalra, L. Slipczuk and S. S. Virani, Curr. Atheroscler. Rep., 2024, 27, 14 CrossRef CAS PubMed.
- P. Libby, J. E. Buring, L. Badimon, G. K. Hansson, J. Deanfield, M. S. Bittencourt, L. Tokgözoğlu and E. F. Lewis, Nat. Rev. Dis. Primers, 2019, 5, 56 CrossRef PubMed.
- L.-L. Bu, H.-H. Yuan, L.-L. Xie, M.-H. Guo, D.-F. Liao and X.-L. Zheng, Int. J. Mol. Sci., 2023, 24, 15160 CrossRef CAS PubMed.
- S. Jebari-Benslaiman, U. Galicia-García, A. Larrea-Sebal, J. R. Olaetxea, I. Alloza, K. Vandenbroeck, A. Benito-Vicente and C. Martín, Int. J. Mol. Sci., 2022, 23, 3346 CrossRef CAS PubMed.
- E. Niki, Free Radicals Biol. Med., 2018, 120, 425–440 CrossRef CAS PubMed.
- S. Zhong, L. Li, X. Shen, Q. Li, W. Xu, X. Wang, Y. Tao and H. Yin, Free Radicals Biol. Med., 2019, 144, 266–278 CrossRef CAS PubMed.
- Y. Li, M. Zhou, H. Li, C. Dai, L. Yin, C. Liu, Y. Li, E. Zhang, X. Dong, H. Ji and Q. Hu, Eur. Heart J., 2024, 45, 268–283 CrossRef PubMed.
- L. Zhu, Y. Zhong, M. Yan, S. Ni, X. Zhao, S. Wu, G. Wang, K. Zhang, Q. Chi, X. Qin, C. Li, X. Huang and W. Wu, ACS Appl. Mater. Interfaces, 2024, 16, 32027–32044 CrossRef CAS PubMed.
- P. Libby, Nature, 2021, 592, 524–533 CrossRef CAS PubMed.
- P. Marchio, S. Guerra-Ojeda, J. M. Vila, M. Aldasoro, V. M. Victor and M. D. Mauricio, Oxid. Med. Cell. Longevity, 2019, 2019, 1–32 CrossRef PubMed.
- H. Ait-Oufella and P. Libby, Arterioscler., Thromb., Vasc. Biol., 2024, 44, 1899–1905 CrossRef CAS PubMed.
- X. Zhang, F. Centurion, A. Misra, S. Patel and Z. Gu, Adv. Drug Delivery Rev., 2023, 194, 114709 CrossRef CAS PubMed.
- L. H. Opie, Trends Cardiovasc. Med., 2015, 25, 216–225 CrossRef CAS PubMed.
- C. P. Cannon, Eur. Heart J., 2019, 40, 3526–3528 CrossRef PubMed.
- A. Ajoolabady, D. Pratico, M. Mazidi, I. G. Davies, G. Y. H. Lip, N. Seidah, P. Libby, G. Kroemer and J. Ren, Metabolism, 2025, 163, 156064 CrossRef CAS PubMed.
- P. M. Ridker, B. M. Everett, T. Thuren, J. G. MacFadyen, W. H. Chang, C. Ballantyne, F. Fonseca, J. Nicolau, W. Koenig, S. D. Anker, J. J. P. Kastelein, J. H. Cornel, P. Pais, D. Pella, J. Genest, R. Cifkova, A. Lorenzatti, T. Forster, Z. Kobalava, L. Vida-Simiti, M. Flather, H. Shimokawa, H. Ogawa, M. Dellborg, P. R. F. Rossi, R. P. T. Troquay, P. Libby and R. J. Glynn, N. Engl. J. Med., 2017, 377, 1119–1131 CrossRef CAS PubMed.
- M. A. Potenza, M. Montagnani, L. Santacroce, I. A. Charitos and L. Bottalico, J. Ginseng Res., 2023, 47, 359–365 CrossRef PubMed.
- W. Fan, Y. Huang, H. Zheng, S. Li, Z. Li, L. Yuan, X. Cheng, C. He and J. Sun, Biomed. Pharmacother., 2020, 132, 110915 CrossRef CAS PubMed.
- J. Chen, X. Wei, Q. Zhang, Y. Wu, G. Xia, H. Xia, L. Wang, H. Shang and S. Lin, Acta Pharm. Sin. B, 2023, 13, 1919–1955 CrossRef CAS PubMed.
- W. Jin, C. Li, S. Yang, S. Song, W. Hou, Y. Song and Q. Du, Front. Pharmacol., 2023, 14, 1166898 CrossRef CAS PubMed.
- S.-J. Oh, Y. Oh, I. W. Ryu, K. Kim and C.-J. Lim, Biosci., Biotechnol., Biochem., 2016, 80, 95–103 CrossRef CAS PubMed.
- X. Chen, Q. Wang, M. Shao, L. Ma, D. Guo, Y. Wu, P. Gao, X. Wang, W. Li, C. Li and Y. Wang, Biomed. Pharmacother., 2019, 120, 109487 CrossRef CAS PubMed.
- T. Dai, W. He, C. Yao, X. Ma, W. Ren, Y. Mai and A. Wu, Biomater. Sci., 2020, 8, 3784–3799 RSC.
- Y. Zhang, H. Ji, O. Qiao, Z. Li, L. Pecoraro, X. Zhang, X. Han, W. Wang, X. Zhang, S. Man, J. Wang, X. Li, C. Liu, L. Huang and W. Gao, Biomed. Pharmacother., 2021, 139, 111630 CrossRef CAS PubMed.
- S. Lu, P. Zhao, Y. Deng and Y. Liu, Pharmaceutics, 2022, 14, 480 CrossRef CAS PubMed.
- W. He, X. Xing, X. Wang, D. Wu, W. Wu, J. Guo and S. Mitragotri, Adv. Funct. Mater., 2019, 11, 1855–1863 Search PubMed.
- S. Huo, P. Zhao, Z. Shi, M. Zou, X. Yang, E. Warszawik, M. Loznik, R. Göstl and A. Herrmann, Nat. Chem., 2021, 13, 131–139 CrossRef CAS PubMed.
- Q. Deng, J. Mi, J. Dong, Y. Chen, L. Chen, J. He and J. Zhou, ACS Nano, 2022, 17, 263–274 CrossRef PubMed.
- W. Guo, S. Huang, J. An, J. Zhang, F. Dong, J. Dang and J. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 50664–50676 CrossRef CAS PubMed.
- X. Wang, F. Li, J. Zhang, L. Guo, M. Shang, X. Sun, S. Xiao, D. Shi, D. Meng, Y. Zhao, C. Jiang and J. Li, J. Controlled Release, 2024, 367, 45–60 CrossRef CAS PubMed.
- H. Yang, Y. Sun, J. Wei, L. Xu, Y. Tang, L. Yang, X. Zhang and Y. Lu, Biomed. Pharmacother., 2019, 118, 109161 CrossRef CAS PubMed.
- A. Bouakaz and J. Escoffre, Adv. Drug Delivery Rev., 2024, 206, 115199 CrossRef CAS PubMed.
- W. Wei, Y. Wang, Z. Wang and X. Duan, TrAC, Trends Anal. Chem., 2023, 160, 116958 CrossRef CAS.
- Y. Yang, Q. Li, X. Guo, J. Tu and D. Zhang, Ultrason. Sonochem., 2020, 67, 105096 CrossRef CAS PubMed.
- S. Keller, M. Bruce and M. A. Averkiou, Ultrasound Med. Biol., 2019, 45, 833–845 CrossRef PubMed.
- S. M. Chowdhury, L. Abou-Elkacem, T. Lee, J. Dahl and A. M. Lutz, J. Controlled Release, 2020, 326, 75–90 CrossRef CAS PubMed.
- I. Lentacker, I. De Cock, R. Deckers, S. C. De Smedt and C. T. W. Moonen, Adv. Drug Delivery Rev., 2014, 72, 49–64 CrossRef CAS PubMed.
- I. De Cock, E. Zagato, K. Braeckmans, Y. Luan, N. de Jong, S. C. De Smedt and I. Lentacker, J. Controlled Release, 2015, 197, 20–28 CrossRef CAS PubMed.
- B. van Elburg, J. Deprez, M. van den Broek, S. C. De Smedt, M. Versluis, G. Lajoinie, I. Lentacker and T. Segers, J. Controlled Release, 2023, 363, 747–755 CrossRef CAS PubMed.
- K.-H. Song, A. C. Fan, J. T. Brlansky, T. Trudeau, A. Gutierrez-Hartmann, M. L. Calvisi and M. A. Borden, Theranostics, 2015, 5, 1419–1427 CrossRef CAS PubMed.
- M. A. O’Reilly, Science, 2024, 385, eadp7206 CrossRef PubMed.
- Z. Zhang, B. Xu, T. Lv, Y. Shi, M. Wang, D. Hu, A. Hu, P. Li, S. Lin, S. Zhang, R. Yao, L. Luo, L. Wang, Y. Zhang, Y. Han, H. Hu, X. Shuai, J. Shi, Y. Chen and T. Zheng, Adv. Ther., 2023, 6, 2300056 CrossRef CAS.
- L. Duan, L. Yang, J. Jin, F. Yang, D. Liu, K. Hu, Q. Wang, Y. Yue and N. Gu, Theranostics, 2020, 10, 462–483 CrossRef CAS PubMed.
- S. Liu, Y. Zhang, Y. Liu, W. Wang, S. Gao, W. Yuan, Z. Sun, L. Liu and C. Wang, Br. J. Cancer, 2022, 128, 715–725 CrossRef PubMed.
- X. Li, S. Guo, T. Xu, X. He, Y. Sun, X. Chen, S. Cao, X. Si, W. Liao, Y. Liao, Y. Han and J. Bin, Theranostics, 2020, 10, 2522–2537 CrossRef CAS PubMed.
- B. G. Brown and X.-Q. Zhao, J. Am. Coll. Cardiol., 2007, 49, 933–938 CrossRef PubMed.
- H. Yuan, H. Hu, J. Sun, M. Shi, H. Yu, C. Li, Y. U. Sun, Z. Yang and R. M. Hoffman, In Vivo, 2018, 32, 1025–1032 CrossRef CAS PubMed.
- F. Liu, Y. Mao, J. Yan, Y. Sun, Z. Xie, F. Li, F. Yan, H. Zhang and P. Zhang, Research, 2022, 2022, 9830627 CAS.
- J. Pazzi and A. B. Subramaniam, J. Colloid Interface Sci., 2024, 661, 1033–1045 CrossRef CAS PubMed.
- L. Fournier, T. de La Taille and C. Chauvierre, Biomaterials, 2023, 294, 122025 CrossRef CAS PubMed.
- Y. Wen, Y. Ji, S. Zhang, B. Xu, S. Sun, Y. Chen, X. Shuai and T. Zheng, Aging, 2024, 16, 10784–10798 CrossRef PubMed.
- Z. He, W. Chen, K. Hu, Y. Luo, W. Zeng, X. He, T. Li, J. Ouyang, Y. Li, L. Xie, Y. Zhang, Q. Xu, S. Yang, M. Guo, W. Zou, Y. Li, L. Huang, L. Chen, X. Zhang, Q. Saiding, R. Wang, M.-R. Zhang, N. Kong, T. Xie, X. Song and W. Tao, Nat. Nanotechnol., 2024, 19, 1386–1398 CrossRef CAS PubMed.
- Z.-C. Wang, K.-M. Niu, Y.-J. Wu, K.-R. Du, L.-W. Qi, Y.-B. Zhou and H.-J. Sun, Cell Death Dis., 2022, 13, 824 CrossRef CAS PubMed.
- R. Cannella, G. Pilato, M. Mazzola and T. V. Bartolotta, Radiol. Med., 2023, 128, 1023–1034 CrossRef PubMed.
- C. Lane-Donovan, W. M. Wong, M. S. Durakoglugil, C. R. Wasser, S. Jiang, X. Xian and J. Herz, J. Neurosci., 2016, 36, 10141–10150 CrossRef CAS PubMed.
- E. Asimakidou, E. N. Saipuljumri, C. H. Lo and J. Zeng, Neural Regener. Res., 2024, 20, 1069–1076 CrossRef PubMed.
- P. Libby and G. K. Hansson, Circ. Res., 2015, 116, 307–311 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S8. See DOI: https://doi.org/10.1039/d5bm00078e |
| This journal is © The Royal Society of Chemistry 2025 |