Domino 1,3-dipolar cycloaddition/ring-opening/ring-cleavage: synthesis of trisubstituted pyrrole and chiral dihydropyrrole-3-carbaldehydes†
Solai
Pandidurai
,
Maddala
Chinababu
and
Govindasamy
Sekar
 *
*
Department of Chemistry, Indian Institute of Technology Madras, Chennai-600036, India. E-mail: gsekar@iitm.ac.in
First published on 15th March 2025
Abstract
A unique approach has been developed to synthesize trisubstituted 1H-pyrrole-3-carbaldehydes using 4-methyl thiazolium salts, α,β-unsaturated aldehydes, and organocatalysts via a domino 1,3-dipolar cycloaddition/ring-opening/C–S and C–N bond cleavage reaction sequence. This methodology has been successfully extended for the asymmetric synthesis of enantioenriched trisubstituted-4,5-dihydro-1H-pyrrole-3-carbaldehydes employing chiral amine organocatalysts with high efficiency (up to 98% ee, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r.).
1 d.r.).
Poly-substituted 1H-pyrroles are essential building blocks in many synthetic chemists because of their diverse use in organic synthesis, bioactive molecules, natural products, and catalysis.1 There are several approaches to accessing diverse poly-substituted 1H-pyrroles in the literature.2 Due to their privileged structure, they can be used in drug discovery, such as antitumor, antibacterial, antiviral, and anti-inflammatory agents, anticancer drugs like veliparib, and antibacterial agents like selvamicin.3
Enantioselectively synthesized, highly substituted dihydropyrrole is an essential building block in many bioactive molecules and natural products.4 Various attractive methods have been designed to synthesize these heterocycles.5 For instance, cyclopropane ring-opening,6a 1,3-dipolar cycloaddition reactions,6b domino ring-opening cyclization (DROC),6c,15 intramolecular iminium ion cyclization,6d and intramolecular nucleophilic addition/rearrangement6e reactions have been reported in the literature. However, developing an efficient method for the synthesis of highly substituted chiral 4,5-dihydropyrroles from readily accessible starting materials using asymmetric organocatalysts in a greener and sustainable manner is highly warranted.7
Cycloaddition is an essential method for the synthesis of complex chiral molecules.8a In this regard, 1,3-dipolar cycloaddition8b,c using thiazolium azomethine ylides has been known for the past few decades, while less attention has been paid to its development towards asymmetric transformation.9 Over the past few decades, scientists have successfully developed a series of methods for synthesizing various achiral and racemic heterocyclic compounds using thiazolium salt with various unsaturated systems via 1,3-dipolar cycloaddition reactions (Scheme 1(i)).10 Very recently, our group developed the organocatalytic asymmetric synthesis of chiral heterocycles using benzothiazolium azomethine ylide (Scheme 1(ii)).11
Both thiazolium and benzothiazolium azomethine ylides are expected to have the same reactivity pattern with dipolarophiles to produce a 1,3-dipolar cycloadduct as a common intermediate.11 This cycloadduct further undergoes ring-opening/rearrangement, yielding various racemic and chiral N,S-heterocyclic compounds in the literature.11,12,15 However, the cycloadduct experiencing ring-opening followed by unprecedented C–S/C–N bond cleavage towards synthesizing highly substituted five-membered chiral and achiral heterocyclic compounds has not been reported. We present a novel reactivity of 4-methyl thiazolium azomethine ylide with α,β-unsaturated aldehydes, enabling the synthesis of trisubstituted 1H-pyrrole-3-carbaldehydes using amine organocatalysts. Furthermore, this approach has been extended to the enantioselective synthesis of highly enantioenriched trisubstituted 4,5-dihydro-1H-pyrrole-3-carbaldehydes using chiral amine organocatalyst (Scheme 1(iii)).
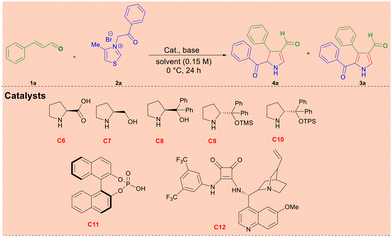
The initial reaction commenced with cinnamaldehyde 1a (0.3 mmol), 4-methyl thiazolium salt 2a (0.3 mmol), and racemic proline (20 mol%) with NEt3 as a base, and IPA (isopropyl alcohol) as a solvent at room temperature. This reaction provided an unexpected trisubstituted 1H-pyrrole 3a product with a 30% yield in 48 h. The reaction conditions were varied to increase the yield 3a with several parameters such as racemic secondary amine catalysts C1–C5, bases, and solvents. The results are summarized in Tables S1–S3 (ESI†).14 For the complete optimization studies, refer to ESI,† Page S3–S4. From the optimization, we found the best-optimized reaction conditions with α,β-unsaturated aldehyde 1a (1 equiv.), 4-methyl thiazolium salt 2a (1 equiv.), DMAP (2 equiv.), and catalyst C5 in EtOH (0.15 M) at room temperature for 48 h.
With the optimized reaction conditions in hand, the generality, and functional group tolerance of the domino reactions were investigated with various α,β-unsaturated aldehydes 1 and 4-methyl thiazolium salts 2 with electron-donating and electron-withdrawing groups, halogens, and bulky substituents. All the reactions furnished the desired products 3a–3o in good yields (Scheme 2).
The simple 4-methyl thiazolium salt gave the product 3a in 72% yield. The 4-methyl thiazolium salt, having the election donating methoxy group at the ortho position, gave product 3b in a moderate yield of 54% compared to the unsubstituted product 3a. The reason may be a steric hindrance to ortho-OMe substitution on the phenyl ring. Meanwhile, the halogen-substitution at the para positions of 4-methyl thiazolium salts delivered 3c–3e in good yields (Scheme 2). The electron-withdrawing groups such as –NO2, –CN, and –CF3 at the para positions of 4-methyl thiazolium salt led to the desired trisubstituted 1H-pyrrole products 3f–3h in 78–83% yields (Scheme 2). The bulky naphthyl group, well tolerated for this domino strategy, led to the product 3i in 81% yield.
α,β-Unsaturated aldehydes 1 containing an electron-donating methoxy group at the para position provided the desired product 3j in 71% yield. The halogen substitution at the para position furnished the desired product 3k in 74% yield. Surprisingly, the bromine substitution at the ortho position shows atropisomerism, confirmed by chiral HPLC analysis (Fig. S2 in ESI†),14 and afforded the desired product 3l in 75% yield. The electron-withdrawing group at the para position delivered the trisubstituted pyrrole product 3m in 80% yield. The substitution at the 3-position of α,β-unsaturated aldehydes such as the furan ring also offered the desired product 3n in 75% yield. Delightfully, alkyl substitution at the 3-position of α,β-unsaturated aldehyde also delivered the product 3o in 50% yield. The structure of compound 3h was unambiguously confirmed through single-crystal X-ray analysis, and a plausible reaction mechanism is provided in the ESI† (Page S13).14
We envisaged that if we could control the reaction rate of the domino synthesis of product 3, there is a possibility of stopping the reaction at trisubstituted-4,5-dihydro-1H-pyrroles. In this case, if we use a chiral amine catalyst, there is a possibility of making the trisubstituted-4,5-dihydro-1H-pyrroles in enantioenriched form by an enantioselective domino reaction. So, to slow down the rate of the reaction, the domino reaction was performed at 0 °C, in the presence of L-proline (20 mol%), and NEt3 (1 equiv.) in EtOH solvent, and the reaction afforded the desired product 3a in 35% yield, along with the expected chiral trisubstituted-4,5-dihydro-1H-pyrrole 4a 37% yield with 16% ee in >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r. (Scheme 3 and Table 1, entry 1).
1 d.r. (Scheme 3 and Table 1, entry 1).
| Entry | Base (equiv.) | Cat. (mol%) | Solvent (0.15 M) | Yield of 4ab | eec (%) | d.r.d | Yield of 3ab |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), 2a (0.3 mmol), base (1–2 equiv.), catalyst C6–C12 (10–20 mol%), solvent (0.15 M). b Isolated yield. c Enantiomeric excess was determined by chiral HPLC. d d.r. ratio was determined by 1H NMR using a crude reaction mixture. e The reaction was performed using 1 equivalent of DMAP base. f The reaction was performed using 10 mol% of the C9 catalyst. g The reaction was performed using 5 mol% of the C9 catalyst. nr = no reaction. | |||||||
| 1 | Net3 (1) | C6 (20) | EtOH | 37 | 16 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
35 |
| 2 | Net3 (1) | C7 (20) | EtOH | 36 | 10 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 |
| 3 | Net3 (1) | C8 (20) | EtOH | 35 | 15 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 |
| 4 | Net3 (1) | C9 (20) | EtOH | 40 | 16 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 |
| 5 | Net3 (1) | C10 (20) | EtOH | 30 | 12 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 |
| 6 | DMAP (1) | C9 (20) | EtOH | 50 | 65 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 |
| 7 | DMAP (2) | C9 (20) | EtOH | 48 | 85 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 |
| 8 | DMAP (2) | C9 (20) | MeOH | 48 | 48 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
12 |
| 9 | DMAP (2) | C9 (20) | H2O | nr | — | — | — |
| 10 | DMAP (2) | C9 (20) | 1,2-DCE | 10 | 94 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 |
| 11 | DMAP (2) | C9 (20) | Toluene | 25 | 94 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 |
| 12 | DMAP (2) | C9 (20) | THF | 30 | 96 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
30 |
| 13 | DMAP (2) | C9 (20) | Dry THF | 45 | 96 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15 |
| 14 | DMAP (2) | C9 (20) | Dry toluene | 60 | 96 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 |
| 15e | DMAP (1) | C9 (20) | Dry toluene | 45 | 90 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 |
| 16f | DMAP (2) | C9 (10) | Dry toluene | 40 | 60 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 |
| 17g | DMAP (2) | C9 (5) | Dry toluene | 30 | 20 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
35 |
| 18 | DMAP (2) | C11 (10) | Dry toluene | — | — | — | 60 |
| 19 | DMAP (2) | C12 (10) | Dry toluene | — | — | — | 65 |
Notably, the domino reaction was successfully controlled at the dihydropyrrole stage by lowering the reaction temperature to 0 °C and achieving the dihydropyrrole in an enantioselective manner. Inspired by the preliminary result, further optimization was done for the enantioselective formation of 4a and to minimize the formation of 3a. The domino reaction was optimized with various chiral catalysts, bases, and solvents, and the results are summarized in Table 1.14 Among the chiral catalysts, C6–C12 were screened to increase % ee (entries 1–5), and C9 was the best choice (entry 4). Then, the reaction was carried out with several bases (Table S5, ESI†)14 to improve the yield and % ee of 4a.
When DMAP was used as a base, the yield and % ee of product 4a increased to 50% and 65%, respectively (entry 6). In contrast, other bases failed to give better outcomes (Table S5, ESI†).14 Increasing DMAP equivalents into two resulted in 48% with 85% ee of the product (entry 7). Notably, when dry toluene was used as a solvent, the domino reaction provided a 60% yield of 4a with 96% ee (entry 14), and minimizing the formation of aromatic product 3a. When the quantity of DMAP was decreased by one equivalent, the yield of product 4a was reduced to 45% with 90% ee (entry 15). Reducing the catalyst loading to 10 mol%, the yield and % ee of product 4a were also reduced to 40% and 60%, respectively (entry 16).14 Also, the reaction was performed with other green catalysts, such as C11 and C12, which produced only racemic products (entries 18 and 19).
With the optimized reaction conditions in hand, the generality of the asymmetric domino reaction was investigated with various α,β-unsaturated aldehydes 1, and 4-methyl thiazolium salts 2 containing electron-donating groups, heterocycles, and bulky aryl groups, and the results are summarized in Scheme 4.
 | ||
Scheme 4 Substrate scope for chiral dihydro-1H-pyrrole-3-carbaldehydes. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) When this reaction was allowed for a longer time, aromatization took place to yield the racemic atropoisomeric product 3l. When this reaction was allowed for a longer time, aromatization took place to yield the racemic atropoisomeric product 3l. | ||
All the domino reactions took place smoothly via intermolecular 1,3-dipolar cycloaddition/intramolecular ring-opening/C–S/C–N bond cleavage to afford the chiral trisubstituted-4,5-dihydro-1H-pyrrole-3-carbaldehydes 4a–4r in good to excellent enantioselectivity (63–98% ee). Gratifyingly, 4-methyl thiazolium salts bearing an electron-donating group at the ortho, meta, and para positions exhibited good reactivity and enantioselectivity (4a–4d and 4r; 81–95% ee). The halogen substitution at the para positions provided the desired products 4e–4g in 60–65% yields with 96–98% ee. The 4-biphenyl and bulky substitution containing naphthyl 4-methyl thiazolium salts 4h and 4i delivered the desired chiral products in 57% and 52% yield with 90% ee and 87% ee. The decreasing yield of 4d, 4i, and 4r is due to steric hindrance with the thiazolium ring methyl group. As a result, the formation of the hydropyrrolo-thiazole cycloadduct intermediate is decreased, reducing the yield of the dihydropyrrole product.
To showcase the functional group tolerance of α,β-unsaturated aldehyde 1, the domino reaction proceeded with electron-donating groups, halogens, and heterocycle substituents. All the reactions underwent smoothly and yielded the enantioselective domino products 4j–4q in 63–96% ee (Scheme 4). The cinnamaldehyde-bearing electron-donating group at the para position delivered the desired products 4j, 4k, and 4p in good yields with 69–88% ee. The halogen substitution at cinnamaldehyde's para and ortho positions furnished 4l–4n and 4q in 79–96% ee. The reaction was also suitable for substituting at the 3-position of α,β-unsaturated aldehydes such as the furan ring and delivered the domino chiral product 4o in 63% ee.
Gram scale synthesis was performed to check the scalability of both domino methodologies.14 Also, several control experiments and mechanistic investigations were conducted to probe the reaction mechanism.14
Based on our control experiments and previous literature reports,11 a plausible reaction mechanism has been proposed in the ESI† (Page No. S13).14 The 4-methylthiazolium salt 2a will initially react with DMAP to yield azomethine ylides III. Subsequently, α,β-unsaturated aldehyde 1a in the presence of chiral catalyst C9 will provide iminium ion intermediate I. Intermediate I will react with azomethine ylide III to produce Michael adduct intermediate IVvia a 1,4-addition. The formation of intermediate IV is a chiral induction step through the 4-methyl thiazolium anion III approaching from the Si-face of iminium ion I, which is the favorable transition state.
The unfavorable transition state is a 4-methyl thiazolium anion III approaching from the Re-face of iminium ion I. According to our previous report, computational study11a shows the favorable and unfavorable diastereomeric transition in Scheme 5 (for a detailed, plausible reaction mechanism, see ESI,† Page S13).14
In conclusion, we have developed a new, unusual domino methodology for the synthesis of trisubstituted 1H-pyrrole-3-carbaldehydes and enantioenriched dihydro-1H-pyrrole-3-carbaldehydes via domino 1,3-dipolar cycloaddition/ring-opening/C–S and C–N bond-cleavage reactions of α,β-unsaturated aldehydes with 4-methyl thiazolium salt utilizing organocatalysts. The enantioselective synthesis was achieved with excellent enantio- and diastereoselectivity. We have performed various control experiments and mechanistic studies to confirm the product formation. HRMS analysis confirms that the formation of 1-mercaptopropane-2-one is a by-product. However, a detailed mechanistic investigation is in progress.
G. S. thanks DST-ANRF (CRG/2022/001739) and CSIR (02/0484/23/EMR-11) for financial support and DST-FIST for the instrumentation facility. S. P. thanks IIT Madras for the research fellowship. Dedicated to Prof. Vinod K. Singh (IIT Kanpur) on the occasion of his 65th birthday.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare the following competing financial interest(s): a patent is pending for both domino methodologies described herein (Indian patent application numbers: 202441067200 and 202441067175, May 05, 2024).13References
- D. Tzankova, S. Vladimirova, L. Peikova and M. Georgieva, J. Chem. Tech. Metall., 2018, 53, 451 CAS.
- (a) M. Thwin, B. Mahmoudi, O. A. Ivaschuk and Q. A. Yousif, RSC Adv., 2019, 9, 15966 CAS; (b) M. K. Hunjan, S. Panday, A. Gupta, J. Bhaumik, P. Das and J. K. Laha, Chem. Rec., 2021, 21, 715 CrossRef CAS PubMed.
- (a) H. Pourtaher, A. Hasaninejad and A. Iraji, Sci. Rep., 2022, 12, 15236 CrossRef CAS PubMed; (b) S. Boussios, P. Karihtala, M. Moschetta, C. Abson, A. Karathanasi, N. Zakynthinakis-Kyriakou, J. E. Ryan, M. Sheriff, E. Rassy and N. Pavlidis, Invest. New Drugs, 2020, 38, 181 CrossRef PubMed; (c) E. B. Van Arnam, A. C. Ruzzini, C. S. Sit, H. Horn, A. A. Pinto-Tomás, C. R. Currie and J. Clardy, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 12940 CrossRef CAS PubMed.
- (a) S. Castellano, H. D. G. Fiji, S. S. Kinderman, M. Watanabe, P. de Leon, F. Tamanoi and O. Kwon, J. Am. Chem. Soc., 2007, 129, 5843 CAS; (b) E. Fattorusso and O. Taglialatela-Scafati, Modern Alkaloids: Structure, Isolation, Synthesis and Biology, Wiley-VCH Verlag GmbH&Co. KGaA, Weinheim, 2008 Search PubMed.
- Studies about synthesis of dihydropyrroles: (a) A. Guđmundsson, K. P. J. Gustafson, B. K. Mai, V. Hobiger, F. Himo and J.-E. Bäckvall, ACS Catal., 2019, 9, 1733 Search PubMed; (b) D. Wang, Y. Fan, P. Yu and L. Désaubry, Chem. Commun., 2020, 56, 5584 CAS.
- (a) S. Yaragorla, R. Tangellapally and D. Arun, Eur. J. Org. Chem., 2024, e202400238 CAS; (b) F.-F. Tang, W.-L. Yang, X. Yu and W.-P. Deng, Catal. Sci. Technol., 2015, 5, 3568 CAS; (c) M. K. Ghorai and D. P. Tiwari, J. Org. Chem., 2013, 78, 2617 CAS; (d) J. Xiang, H. Xie, Z. Li, Q. Dang and X. Bai, Org. Lett., 2015, 17, 3818 CAS; (e) K. M. Wen Ting and Chen Zhanguo, Chin. J. Org. Chem., 2019, 39, 3162 Search PubMed.
- Recent studies on asymmetric catalysis and sustainable methodologies: (a) A. Garg, D. Rendina, H. Bendale, T. Akiyama and I. Ojima, Front. Chem., 2024, 12, 1398397 CAS; (b) A. Chaskar and R. Darade, Int. J. Creat. Res. Thoughts., 2022, 10, 2320 Search PubMed; (c) A. M. Koskinen, Asymmetric synthesis of natural products, John Wiley & Sons, 2022 Search PubMed.
- (a) X. Liu, H. Zheng, Y. Xia, L. Lin and X. Feng, Acc. Chem. Res., 2017, 50, 2621 CAS; (b) K. Wang, L. Yang, Y. Li, H. Li, Z. Liu, L. Ning, X. Liu and X. Feng, Angew. Chem., Int. Ed., 2023, 62, e202307249 CAS; (c) F. Zhang, Y. Zhou, H. Zhao, L. Chen, W. Cao and X. Feng, Precis. Chem., 2023, 1, 423 CrossRef CAS.
- J. L. García Ruano, A. Fraile, M. R. Martín, G. González and C. Fajardo, J. Org. Chem., 2008, 73, 8484 CrossRef PubMed.
- (a) T. Iwamura, M. Kobayashi, T. Ichikawa, H. Shimizu and T. Kataoka, J. Chem. Soc., Perkin Trans. 1, 1996, 629 RSC; (b) C. R. Berry, C. A. Zificsak, A. C. Gibbs and D. J. Hlasta, Org. Lett., 2007, 9, 4099 CAS; (c) Y.-M. Shen, P.-C. Lv, M.-Z. Zhang, H.-Q. Xiao, L.-P. Deng, H.-L. Zhu and C.-Z. Qi, Monatsh. Chem., 2011, 142, 521 CrossRef CAS; (d) V. A. Motornov, A. A. Tabolin and S. L. Ioffe, New J. Chem., 2022, 46, 4134 RSC.
- (a) S. Pandidurai, V. S. Kumar Choutipalli, V. Subramanian and G. Sekar, Org. Lett., 2024, 26, 2971 CrossRef CAS PubMed; (b) S. Pandidurai and G. Sekar, Org. Biomol. Chem., 2024, 22, 8119 RSC.
- (a) O. Tsuge, S. Kanemasa and S. Takenaka, Bull. Chem. Soc. Jpn., 1985, 58, 3320 CrossRef CAS; (b) X. Zhang, X. Liu, J. Zhang, D. Zhang, L. Lin and X. Feng, Org. Chem. Front., 2018, 5, 2126 CAS; (c) Z.-H. Wang, T. Zhang, L.-W. Shen, X. Yang, Y.-P. Zhang, Y. You, J.-Q. Zhao and W.-C. Yuan, Molecules, 2023, 28, 4410 CrossRef CAS PubMed.
- (a) S. Pandidurai, S. Kishor and G. Sekar, Indian Pat., 202441067200, 2024 Search PubMed; (b) S. Pandidurai and G. Sekar, Indian Pat., 202441067175, 2024 Search PubMed.
- See the ESI† for more details.
- The comparative study of ref. 6a and 12c with this study is included in the ESI,† Page 91.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, 1H, 13C{1H}, and 19F NMR spectra, and HPLC chromatogram (PDF). X-ray crystallography data for 3h. CCDC 2354829. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc06706a |
| This journal is © The Royal Society of Chemistry 2025 |