Intelligent soft wearable bioelectronics for neurological disorders
Dohyung
Kim†
a,
Junhyuk
Bang†
a,
Juho
Jeong
a,
Dae Lim
Koo
*b and
Seung Hwan
Ko
 *acd
*acd
aDepartment of Mechanical Engineering, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul, Korea. E-mail: maxko@snu.ac.kr
bDepartment of Neurology, Seoul Metropolitan Government - Seoul National University Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea. E-mail: koodaelim@snu.ac.kr
cInstitute of Engineering Research/Institute of Advanced Machinery and Design (SNU-IAMD), Seoul National University, 1 Gwanak-ro, Gwanak-gu, Korea
dInterdisciplinary Program in Bioengineering, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul, Korea
First published on 16th June 2025
Abstract
Using soft wearable electronics has emerged as an innovative neurological disorder monitoring and rehabilitation approach. Traditional diagnostic and treatment systems are hospital-centered, bulky, and unsuitable for long-term use, limiting their applicability in real-world settings. Recent advancements in materials, device design, and fabrication processes have enabled the development of stretchable, skin-conformal sensors, improving wearability, signal quality, and usability. This review discusses key design considerations for ensuring conformal integration with the human body, covering aspects from materials selection to structural engineering. Additionally, we explore recent research trends in soft electronics-based electrophysiological and physical activity sensors and the system integration challenges that must be addressed for clinical applications. Finally, we introduce emerging neurological disorder applications utilizing soft wearable electronics, highlighting their limitations and potential. By addressing these challenges, soft wearable electronics will advance continuous health monitoring, personalized rehabilitation, and next-generation neuroprosthetic systems.
Wider impactThis review highlights key advancements in soft wearable electronics for neurological disorders, focusing on innovations in materials, sensor design, and system integration. Recent soft wearable devices offer enhanced comfort and signal sensing, addressing the limitations of traditional hospital-based systems and enabling continuous real-time health tracking. These technologies have the potential to improve early diagnosis and support personalized rehabilitation. Moreover, they enhance accessibility, comfort, and effectiveness in neurological healthcare, paving the way for future innovations in digital health management. |
1. Introduction
Recent advances in medical and sensing technologies have enabled the simultaneous measurement of multiple physiological parameters, providing comprehensive analysis and accurate diagnosis.1,2 However, despite these advancements, most existing diagnostic and treatment methods remain hospital-centered, limiting their accessibility for continuous use in daily life. These systems are often bulky and require complex setups, making them impractical for long-term monitoring outside clinical settings. Such limitations are particularly problematic for neurological disorders, where symptoms can be unpredictable (e.g., epileptic seizures and Parkinsonian tremors).3 Continuous monitoring is crucial for managing these conditions effectively, yet hospital-based tests often fail to capture a patient's condition accurately. This underscores the urgent need for compact, user-friendly devices that enable real-time health status tracking.Compact, wearable electronic devices allow convenient acquisition of biosignals such as electrophysiological signals and joint or muscle movements.4 Although wearable devices may not yet achieve the full diagnostic accuracy of bulky medical equipment, they offer continuous monitoring in daily life. Technologies such as Zio Patch, Empatica Embrace, Muse EEG, and Delsys EMG have already been commercialized and are readily available on the market, bringing biosignal monitoring closer to everyday applications.
However, most wearable devices still employ rigid form factors, creating challenges in interfacing effectively with biological tissues.5 Significant areas for improvement remain, particularly regarding user comfort and device performance. Human skin is soft, curved, wrinkled, and constantly experiences deformation, making rigid devices unsuitable for seamless interfacing. Imperfect contact between rigid electrodes and skin leads to fluctuations in interfacial impedance, reducing signal quality and increasing artifacts over long-term measurements. To compensate for this, wearable devices typically require forced contact, leading to significant user discomfort. For instance, wearable patches often necessitate bandages or adhesive tapes, and electrodes for electrophysiology monitoring rely on medical gels to maintain stable sensing. These constraints severely limit the duration of continuous wearing, typically ranging from several hours to a few days, making long-term neural monitoring difficult.
Given these limitations, soft wearable devices hold great promise for practical, user-friendly real-world applications. Recent advances in materials, device design, and manufacturing processes enable more seamless, conformal interfacing with biological tissues.6 The transition from rigid to soft devices represents a fundamental convergence of materials science, mechanical engineering, electrical engineering, computer science, and medical engineering.
This review highlights recent research trends and technological advancements in soft wearable technology for neurological diseases (Fig. 1). First, we discuss critical factors for effective interfacing with biological tissues and examine technological progress. Next, we present advances in sensors designed for capturing various biosignals. Finally, we explore the practical applications of soft wearable electronics in managing and rehabilitating neurological diseases. Through this comprehensive review, we aim to clarify how soft wearable technologies differentiate themselves from traditional rigid-based systems and suggest future research directions and opportunities for technological development.
 | ||
| Fig. 1 Overview of soft wearable technology for neurological disorders. This schematic was created with https://BioRender.com. | ||
2. Design strategies for interfacing with the human body
Interfacing between electronic devices and biological tissues is essential for accurately and reliably capturing physiological and physical signals from the human body. In particular, electrodes for electrophysiological sensing, such as electroencephalogram (EEG), electromyogram (EMG), and electrocardiogram (ECG) must meet several key requirements to ensure high-quality and robust measurements over extended periods. These requirements include stretchability to accommodate dynamic movements, seamless adhesion to curved and deformable biological tissues, and long-term stability in diverse physical and chemical environments. However, achieving these properties simultaneously presents a significant challenge due to inherent trade-offs between mechanical and electrical characteristics. To overcome these limitations, extensive research has focused on developing advanced materials, form factors, and structural designs to balance these competing factors effectively. This section explores the key design considerations and recent advancements in soft electronic devices that enable stable and efficient interfacing with biological tissues.2.1. Materials for soft electronic devices
Soft electronic devices are widely utilized in medical and wearable applications due to their optimized interface with biological tissues. However, existing rigid electronic devices can cause tissue damage because of mechanical mismatches and may induce immune responses on the skin, leading to inflammation. To address these issues, materials used in electronic devices must possess excellent mechanical properties, ensuring long-term stability even in a dynamic environment. Additionally, these materials should exhibit high electrical performance, stretchability, and compatibility with fabrication processes to enable reliable functionality without compromising mechanical integrity (Table 1).| Conductivity (S cm−1) | Mechanical stability | Degradation | Compatible Process | Manufacturing Cost | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Stretchability (%) | Fatigue threshold | |||||||
| Metal thin film | Up to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Very low (<1%) | Weak | Cu oxidizes readily and Ag prone to sulfidation. | Physical vapor deposition | High | 7 | |
| Nano materials (1D and 2D) | Metal-based | Up to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Up to 400% | Medium | High surface-to-volume ratio promotes rapid surface oxidation | Inkjet printing, spray coating, and laser direct writing | Medium | 8 and 9 |
| Carbon-based | Up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Up to 200% | Medium | MXenes are susceptible to humidity, and induced oxidation. rGO may degrade under prolonged heat or alkaline pH. | Chemical vapor deposition, inkjet printing, spray coating, and laser direct writing | Medium | 10–12 | |
| Conducting polymer (PEDOT:PSS and PANI) | Up to 600 | Up to 30% | Weak | Highly hygroscopic and ionically conductive, performance varies with moisture and pH. | Inkjet printing, laser direct writing, and electropolymerization | Low | 13 and 14 | |
| Liquid metal | Up to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Up to 1000% | High | Surface oxidation occurs easily, yet preserves internal conductivity | Inkjet printing and laser direct writing | High | 15 and 16 | |
Thin film electrodes are currently the most used candidates because of their high conductivity and high-resolution patterning capabilities.17,18 Typically, thin film electrodes, a few hundred nanometers thick or less, exhibit excellent flexibility, allowing them to deform with their polymer substrates. However, thin film electrodes are inherently non-stretchable, which poses risks such as cracking and delamination.
From this perspective, nanomaterial-based electrodes are particularly promising for stretchable electronic devices because they can achieve high mechanical stability and electrical conductivity simultaneously. In particular, one-dimensional (1D) nanomaterials (e.g., carbon nanotubes (CNTs), Ag nanowires (AgNWs), and copper nanowires (CuNWs)) leverage their high aspect ratio. Even when the electrode is stretched, these nanomaterials can slide or rearrange while maintaining electrical connections.19–21 Meanwhile, two-dimensional (2D) carbon nanomaterials (e.g., graphene and MXenes) utilize their layered structures to gently slide their flakes under tension, maintaining continuous electrical pathways.22
To effectively implement these nanomaterials into soft electronic systems, scalable and reliable patterning techniques are required. Laser processing, inkjet printing, and roll-to-roll manufacturing are widely employed for the fabrication of soft electronic devices based on nanomaterials. These processes are considered based on factors such as resolution, patterning area scalability, production throughput, material compatibility, design flexibility, and cost. Laser processing offers a microscale resolution, enabling precise feature definition.23 However, it often entails higher cost due to material loss during processing and is limited in the patterning area by the scanning range of galvanometric mirrors or motion stages. Inkjet printing provides excellent compatibility with various nanomaterial inks and allows for multi-material integration through multi-nozzle configurations.24 Its non-contact deposition minimizes material waste, though maintaining patterning uniformity can be challenging depending on ink viscosity and surface tension.25 Both laser and inkjet systems support CAD-based digital control, offering high design flexibility and enabling customization tailored to specific applications and attachment sites in wearable electronics. Nevertheless, such flexibility and precision inherently trade off with large-scale manufacturability. To address this, roll-to-roll printing is a promising candidate for continuous, large-area fabrication. Roll-to-roll processes offer high reproducibility, efficient material utilization, and low per-unit production cost, with high patterning area scalability.26
Conducting polymers have recently gained significant attention in the field of soft electronics. Although their conductivity is lower than that of metal-based or nanomaterial-based electrodes, they offer excellent biocompatibility and low impedance, making them advantageous for long-term continuous measurement in wearable and implantable biosensors. The impedance at the electrode-skin interface can be expressed as eqn (1).
| Z(ω) = R/(1 + jωCR) | (1) |
Liquid metals (LMs), particularly gallium-based alloys, are emerging as promising candidates for next-generation soft electronic devices due to their excellent biocompatibility, high conductivity, and mechanical deformability.29 Unlike solid metal conductors, LMs remain liquid at room temperature, allowing them to conform to dynamic surfaces such as body tissues without causing mechanical damage, which makes them particularly suitable for bioelectronic applications. LMs exhibit high conductivity on the order of 106 S m−1, comparable to Cu and silver Ag. Additionally, their liquid state affords excellent stretchability and self-healing capabilities. Solid metal conductors may crack under external deformation, whereas LMs maintain electrical connectivity through their fluidity, making them highly suitable for stretchable electronic devices. However, achieving precise patterning is challenging due to LMs' high surface tension and fluidic characteristics. Additionally, leakage poses a significant challenge in certain applications. Various patterning techniques are being explored to address these challenges, including microfluidic injection, intermetallic bond-assisted patterning, and stencil printing.30
2.2. Biocompatibility
Nanomaterial-based electrodes are emerging as leading candidates for soft bioelectronics due to their exceptional mechanical robustness and flexibility. However, ensuring biocompatibility remains a critical challenge, often necessitating protective coatings. Metallic nanowires such as Ag NWs and Cu NWs face rapid degradation in biological environments, including bodily fluids, sweat, and blood. Such prolonged exposure often leads to rapid oxidation, significantly declining electrical conductivity with structural integrity.31 Moreover, these nanowires can release metal ions (Ag+, Cu2+) during oxidation, posing risks of cytotoxicity and adverse biological responses. To address these concerns, extensive research has focused on developing stable, biocompatible protective coatings, with Au-based coatings being the most extensively studied. A uniform Au coating prevents ion release from the underlying metal cores, enhancing long-term chemical stability and biocompatibility. However, depositing an Au layer involves the galvanic replacement reaction, which often oxidizes the underlying metals (Ag or Cu), resulting in non-uniform layer formation and nanowire degradation.Recent advances have introduced novel coating techniques to mitigate the galvanic replacement reaction. One such promising approach involves reducing Au3+ ions to Au+ using sodium sulfite (Na2SO3) before gentle deposition onto Ag surfaces (Fig. 2a), enabling the formation of uniform Au layers on AgNWs.32 Additional surface modifications are necessary for CuNWs, which are more prone to oxidation than Ag.33 By substituting hexadecylamine ligands with polyvinylpyrrolidone, researchers have enabled uniform penetration of Au ions into the CuNW surface. Furthermore, by employing mild alkaline conditions combined with Na2SO3 and diethylhydroxylamine, galvanic corrosion is significantly suppressed, facilitating the successful synthesis of highly conductive and biocompatible Cu@Au core–shell nanowires (Fig. 2b).
 | ||
| Fig. 2 Strategies for interfacing with the human body. (a) SEM image and HRTEM image of Ag@Au core–shell nanowires. Reproduced with permission.32 Copyright 2018 Springer Nature. (b) TEM image of The synthesized Cu@Au core–shell nanowire. Reproduced with permission.33 Copyright 2020 John Wiley and Sons. (c) Optical images and FEA results for the deformations of a 3D coil electrode. Reproduced with permission.34 Copyright 2017 Springer Nature. (d) Optical images of highly stretchable Kirigami electrodes. Reproduced with permission.35 Copyright 2019 American Chemical Society. (e) 10 μm thick and a 150 μm thick hydrogel film attached to the fingerprint replica. Reproduced with permission.36 Copyright 2022 John Wiley and Sons. (f) Conformal nanomesh on skin, and no signal coupling of the nanomesh. Reproduced with permission.37 Copyright 2022 Springer Nature. (g) SEM image of low-modulus hydrogel cross-section for enhancing toughness. Reproduced with permission.38 Copyright 2024 Elsevier. (h) High-resolution printing of nanomaterials on breathable nanofiber. Reproduced with permission.39 Copyright 2024 John Wiley and Sons. | ||
From a device-level perspective, all bioelectronic systems require encapsulation, which serves as a critical barrier to ensure biocompatibility, chemical stability, and long-term functionality.40 Effective encapsulation must not only shield internal components from complex biofluids while serving as a mechanical buffer and an electrical insulator.41,42 Accordingly, material selection should be tailored to the specific requirements of each device, balancing long-term biostability, mechanical performance, interfacial adhesion, and compatibility with integrated architectures in soft bioelectronic systems.43–47
Since biological tissues remain in continuous contact with device surfaces, inadequate encapsulation can result in the release of cytotoxic byproducts, trigger immune responses, or induce foreign-body reactions, ultimately compromising both device performance and tissue health.48 These immune responses can be initiated through several pathways. Mechanical mismatch between the material and tissue can cause chronic irritation.49 Chemical degradation of encapsulants may result in the release of reactive byproducts.50
To mitigate such biological risks, encapsulation materials must exhibit appropriate mechanical compliance, chemical inertness, resistance to enzymatic or hydrolytic degradation, and reliable interfacial stability. Silicone-based elastomers such as polydimethylsiloxane (PDMS) (Young's modulus: 1–3 MPa) and Ecoflex (∼0.2 MPa) are widely employed due to their softness, oxidative stability, and excellent long-term biocompatibility.51,52 However, their poor interfacial compatibility with other polymeric materials often increases processing complexity during device fabrication.53,54
Thermoplastic elastomers such as polyurethane (PU) and poly(styrene-ethylene-butylene-styrene) (SEBS) provide high stretchability and processing versatility, making them attractive for use in mechanically dynamic environments. However, under prolonged biological exposure, PU is susceptible to hydrolytic degradation, while SEBS is more prone to oxidative degradation.50,55 In addition, hydrogels and bio-derived polymers are being actively explored as next-generation encapsulation materials. Hydrogels provide tissue-like softness and exhibit resistance to nonspecific protein adsorption, both of which are beneficial for minimizing immune responses.56 Nonetheless, their inherent mechanical fragility and sensitivity to environmental factors such as dehydration, degradation, and poor electrical insulation remain key technical challenges. Bio-derived polymers such as silk fibroin and cellulose derivatives offer excellent biocompatibility and biodegradability.46
Biocompatibility of encapsulation materials and functional components must be evaluated not only at the material level but also at the device level, considering their long-term interaction with biological systems. In this regard, the ISO 10993 series provides a rigorous and standardized framework for biological evaluation, covering a wide range of tests including in vitro cytotoxicity (ISO 10993-5),57 skin sensitization (ISO 10993-10),58 systemic toxicity (ISO 10993-11),59 and chemical characterization of degradation (ISO 10993-13).60 These assessments are especially crucial for wearable devices that undergo prolonged skin contact or repeated mechanical deformation, which may increase the risk of material degradation or immune response.
2.3. Stretchability
Conventional rigid electronic devices cannot accommodate these large deformations, often resulting in electrode detachment, delamination, and signal distortion, leading to user discomfort.5 Therefore, achieving stretchability is crucial for ensuring long-term stability and reliable signal acquisition in soft wearable electronics.61 Different biological tissues, such as skin, muscles, and cardiac tissues, exhibit varying degrees of deformation; for example, skin continuously stretches, wrinkles, and contracts, with joints undergoing strains exceeding 30%.62 Stretchable electronics closely conform to the body's curvature and dynamic movements, significantly reducing interface impedance and enhancing signal fidelity.However, the most commonly used electrode materials, thin metal electrodes, are inherently non-stretchable. To address low stretchability of thin metal films, structural design strategies such as microcracked films, serpentine design, fractal designs, kirigami patterns, and mesh structures effectively distribute mechanical stress and enable stretching.63–67
A three-dimensional (3D) micro-coil network electrode design was proposed to enhance stretchability.34 The researchers utilized a self-assembly approach induced by compressive buckling to fabricate 3D micro-coil electrodes (Fig. 2c). First, they patterned a 2D serpentine structure using a multilayer polyimide (PI)/Au/Cr/PI film, selectively attaching it to a low-elasticity silicon-based substrate under biaxial prestrain. After applying and releasing the biaxial prestrain, the 2D structure transformed into a 3D helical shape due to compressive buckling, forming micro-coil electrodes with spring-like mechanical properties. These electrodes exhibited a 9.5-fold increase in stretchability compared to conventional 2D serpentine structures and enhanced mechanical adhesion due to their increased contact area with the substrate.
Such structural strategies are widely applicable beyond metal films, extending to intrinsically stretchable nanomaterials and conductive hydrogels.35,68 A kirigami-inspired structural design was introduced to enable electrodes with extremely high stretchability (over 400%) while maintaining high conductivity and transparency (Fig. 2d).35 AgNWs were embedded in ultrathin, colorless PI layers. To enhance biocompatibility, partially exposed silver surfaces were coated with gold through a galvanic replacement process. Laser ablation was then used to create well-defined kirigami patterns, enabling precise control over stretchability. These electrodes demonstrated excellent strain reversibility, sustaining stable electrical performance over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 repetitive strain cycles.
000 repetitive strain cycles.
Another unique structural approach involves auxetic patterning, utilizing a negative Poisson's ratio similar to that of human skin. Human tissues, such as joints and skin, expand simultaneously in multiple directions during movement. This behavior contrasts with conventional materials, which tend to contract in one direction when stretched in another. Auxetic substrates effectively accommodate bidirectional strain around joints such as knees and elbows, enhancing device adaptability.69 An auxetic stretchable substrate reinforced with high-rigidity glass fabric was developed to ensure mechanical stability.70 The substrate was then encapsulated with a soft and adhesive PDMS elastomer, providing a seamless interface. By optimizing the PDMS ratio (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and geometric pattern, the final substrate achieved a negative Poisson's ratio of −0.16, which closely mimics the deformation characteristics of wrist skin—devices based on this auxetic substrate maintained stable adhesion to the skin even after repeated deformations.
1) and geometric pattern, the final substrate achieved a negative Poisson's ratio of −0.16, which closely mimics the deformation characteristics of wrist skin—devices based on this auxetic substrate maintained stable adhesion to the skin even after repeated deformations.
Although structural approaches effectively enhance stretchability, they often lengthen electrode paths, which may compromise electrical performance. Moreover, unavoidable 3D buckling during deformation poses a risk of electrode detachment at interfaces. As a result, there is increasing demand for intrinsically stretchable materials. The stability and performance of percolation-based electrodes depend on factors such as nanomaterial composition, length, and density. Maintaining stable junction contacts between nanomaterials under mechanical deformation is essential due to their inherently high junction resistance.32,71 While simple mixing methods combining elastic polymers with conductive nanomaterials have been explored to strengthen junction contacts, they often suffer from irreversible resistance increases due to the intrinsic brittleness and fracture of nanomaterials under strain.32,71 A structured 3D composite network of elastic polymers and conductive nanomaterials has been developed to overcome this challenge. This network reinforces robust junction contacts, facilitates effective internal stress dissipation, and preserves electrical integrity under stretching.
A phase-separation strategy was employed to self-organize Ag NWs within a porous PU matrix, forming a structured percolation network.72 In their study, PU dissolved in tetrahydrofuran was mixed with ethanol-dispersed Ag NWs and drop-cast onto a substrate. During solvent evaporation, phase separation resulted in the formation of distinct PU-rich and PU-poor domains. Ag NWs spontaneously self-assembled within the PU-poor domains via the Pickering effect, forming a structured self-organized percolation network. This porous PU matrix effectively absorbed mechanical stress, acting as an energy-dissipative structure and dramatically enhancing stretchability. The resulting composite exhibited less than 10% resistance variation under 50% strain and maintained stable conductivity even at elongations up to 600%.
Combining elastic polymers represents a promising approach for developing stretchable conducting polymer composites with significantly improved mechanical properties. While conductive polymers exhibit excellent electrical properties for electrophysiological monitoring, they often suffer from poor mechanical strength and brittleness, making them prone to strain-induced damage. Composite materials incorporating conductive polymers into elastic matrices have been extensively explored to address these limitations.73–76
A method was developed to simultaneously enhance electrical conductivity and mechanical stretchability using a nanofibrous composite network by fabricating a highly conductive nanofibrous composite network composed of PEDOT:PSS and polyacrylic acid (PAA).75 Typically, conventional composites experience aggregation in PEDOT-rich domains, resulting in inferior properties. However, the researchers achieved a uniformly interconnected network through dimethyl sulfoxide treatment, attaining a high conductivity (247 S cm−1) and excellent stretchability (400% elongation).
Furthermore, achieving high-resolution patterning of stretchable conductive polymer composites via conventional fabrication methods remains a key challenge. The trade-off between mechanical strength and conductivity in conductive hydrogel composites was addressed through by carefully optimizing the ratios of conductive (PEDOT:PSS) and elastic (hydrophilic PU) phases.76 By establishing a bi-continuous network structure, they achieved a conductivity of over 11 S cm−1, a more than 400% stretchability, and an exceptional fracture toughness exceeding 3300 J m−2. Moreover, precise control over the composite's viscosity enabled compatibility with various microfabrication techniques, including 3D printing, micro-molding, spin coating, and electrospinning.
2.4. Adhesion
Biological tissues undergo continuous deformation, and poor electrode adhesion to the skin can significantly degrade bioelectrical signal quality and physical activity measurements. Incomplete adhesion introduces air gaps at the electrode–skin interface, increasing interfacial impedance and causing signal distortion. As a result, the recorded signal quality deteriorates, and noise levels increase. Thus, conformality is particularly important when measuring weak electrophysiological signals such as EEG and EMG. Conventionally, conductive gels have been applied between the electrode and skin to reduce interfacial impedance and maintain tight contact. However, conductive gels often cause discomfort to users and experience performance degradation as they dry over time. Therefore, wearable electronics must maintain strong and stable adhesion to skin and tissues for extended periods.77 This should be achieved without causing discomfort or performance degradation. Adhesive polymers such as PDMS:polyethylenimine ethoxylated composites, silbione, and polydopamine (PDA) have been investigated to address this.78–80 However, conventional polymer adhesives exhibit weakened adhesion when moisture or sweat accumulates between the skin and the adhesive layer. This issue is unavoidable because over 70% of the human body is water. Therefore, developing adhesion technologies that function reliably in moist and wet environments is crucial.A dry-crosslinking mechanism inspired by marine organisms like mussels and barnacles was developed to ensure strong adhesion in wet environments.81 They fabricated a dry double-sided tape by combining PAA-N-hydroxysuccinimide (NHS) ester polymers with biodegradable gelatin or chitosan-based polymers. Upon contact with biological tissues, the adhesive layer rapidly swells, efficiently removing interfacial water and forming temporary intermolecular bonds at the tissue interface. This is followed by covalent crosslinking between NHS ester groups on the adhesive surface and amine groups in biological tissues, leading to stronger and more stable adhesion. This adhesive demonstrated an interfacial toughness exceeding 710 J m−2 on wet porcine skin and maintained strong adhesion on various substrates, including silicon, PDMS, and PI. Furthermore, it exhibited shear and tensile strengths exceeding 120 kPa, confirming its robust adhesion across diverse environmental conditions.
Additionally, electrode structures designed for direct adhesion to wet biological surfaces have been developed, enhancing bioelectrical signal recording.82 An electrical bioadhesive interface using graphene oxide-poly(vinyl alcohol) (PVA) nanocomposite hydrogels was developed to enhance tissue adhesion. PAA and NHS ester groups were grafted onto the hydrogel to further enhance immediate and stable adhesion, forming rapid covalent crosslinks with biological tissue. This interface efficiently removes interfacial water via hydration and anisotropic swelling, rapidly achieving strong and stable adhesion.
In addition to material-based strategies, biomimetic approaches inspired by natural adhesion mechanisms in octopus suckers and geckos have demonstrated significant improvements in adhesion performance.83,84 A hybrid adhesion technology combining mechanical interlocking and hydrogen bonding was inspired by octopus suckers and tree frog pads.85 Their approach incorporated nanoporous polyacrylamide (PAAm) hydrogels into elastomeric micropatterned structures to maximize adhesion. The micropattern enhances mechanical interlocking, reinforcing the physical bond with tissues, while the embedded hydrogel facilitates hydrogen bonding, improving chemical affinity to biological surfaces. As a result, this combined structural and chemical bonding achieved adhesion strengths 2–3 times higher than conventional PDMS adhesives (up to 61 kPa) while maintaining adhesion performance under underwater conditions. Additionally, this hybrid adhesive enables residue-free detachment from surfaces and supports repeated use.
To achieve a conformal interface with the skin, both adhesive layer design and the mechanical properties of electronic devices are critically important.8,86,87 The interface energy can be expressed as eqn (2).87
Einterface = Edevice![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) bending + Eskin bending + Eskin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) elasticity + Eadhesion elasticity + Eadhesion | (2) |
A highly conformal electrode was fabricated by directly spray-coating an Ag@Au core–shell nanowire nanomesh onto the skin.37 This approach used partial PU to robustly secure the nanomesh without requiring additional substrate. This substrate-free structure enabled an ultrathin device (<10 μm), allowing the electrode to conform closely to skin movements. As a result, localized strains were confined to regions undergoing significant bending, substantially reducing motion artifacts and improving signal accuracy (Fig. 2f).
2.5. Softness
Softness is a key requirement for wearable electronics, directly influencing conformality, signal quality, and long-term wearing comfort. Conventional metal- and silicon-based devices have Young's modulus exceeding 1 GPa, whereas human soft tissue has a significantly lower modulus of approximately 0.1–1 MPa.5 This substantial mechanical mismatch can lead to device detachment in dynamic environments. Furthermore, localized stress concentration at the interface, caused by mechanical mismatch, increases friction between the device and skin, potentially triggering immune responses and even tissue rejection. Thus, ensuring biocompatibility requires materials with mechanical properties closely matching those of biological tissues.88 Highly soft materials are essential for enhancing accuracy and reliability in wearable bioelectronics.Various low-modulus materials have been explored to address these challenges, with hydrogels standing out for their exceptional flexibility and softness. However, conventional hydrogels exhibit low toughness and inadequate mechanical strength. To overcome this limitation, A chrysalis-inspired structure was created by treating the surface of a PVA-based hydrogel with sodium phytate.38 This approach retained the hydrogel's intrinsic softness while markedly improving its fracture strength (4.4 MPa), tensile strain (418%), and toughness (6.82 MJ m−3) (Fig. 2g). Notably, this hydrogel exhibited an elastic modulus of approximately 0.6 MPa, closely matching that of human soft tissues, thus achieving high mechanical compatibility. Furthermore, a novel nanocomposite integrating laser-induced graphene (LIG) with hydrogel was developed, offering low modulus, high stretchability, superior biocompatibility, and high-resolution electrode patterning for multimodal functionality.89 The researchers employed a PVA-phytic acid-honey (PPH) hydrogel to transfer LIG onto an ultrathin hydrogel film (1.0–1.5 μm). The PPH hydrogel prevents stress concentration with its low modulus (12.9 kPa). Despite the brittle nature of LIG, it establishes an out-of-plane conductive pathway, preserving conductivity even as cracks propagate.
2.6. Breathability
Soft wearable electronics must maintain close and stable contact with the skin over extended periods. Therefore, ensuring both comfort and durability for long-term stability is essential. However, conventional materials often exhibit low air and moisture permeability, resulting in device corrosion, sweat accumulation, and an increased risk of skin irritation, inflammation, and allergic reactions with prolonged use.90 To address these limitations, breathable electronic materials capable of efficiently dissipating sweat and moisture while maintaining high electrical performance are required. Recent research has focused on developing nanofiber-based porous electrodes, mesh-structured electrodes, substrate-free ultrathin graphene electrodes, and hydrogel-based ultrathin interfaces, contributing to enhanced long-term stability.36,90–92In particular, research has increasingly focused on patterning multiple nanomaterials onto breathable nanofiber membranes to enhance biological signal measurement. They proposed a new technique called nanowire direct local filtering.39 This method utilizes carbon paper beneath the substrate to exploit capillary action, enabling high-resolution patterning (Fig. 2h). The filtered nanowires are then fixed onto the substrate via laser processing, forming a conductive network. Thermoplastic PU membranes fabricated via Nanowire Direct Local Filtration exhibited breathability of 4015 g m−2 day−1, while PDMS/hexane-insulated regions achieved 505 g m−2 day−1, surpassing that of Tegaderm (432 g m−2 day−1), a commonly used medical adhesive. Additionally, Ag@Au nanowire patterns enabled the fabrication of electrodes with approximately 300 μm resolution while maintaining a sheet resistance of 1 Ω sq−1. Furthermore, a separate study developed breathable yet waterproof electrodes by sequentially coating fabric with PDA, MXenes, and PDMS.93 In this approach, MXenes imparted electrical conductivity, while PDMS functioned as an oxidation-resistant hydrophobic layer. Water vapor permeability tests indicated that the evaporation rate was 0.55 kg m−2 h−1 at 45 °C and remained at 0.49 kg m−2 h−1 even after 100 repeated deformations, confirming its long-term breathability. Moreover, liquid water remained on the fabric's surface while water vapor permeated through, ensuring both waterproofing and breathability. Increasing the number of MXene coating layers further enhanced conductivity, under optimal conditions achieving 126 S m−1.
In addition, an ultrathin hydrogel interface was developed to achieve both breathability and long-term wearability.36 The researchers employed a cold-lamination method to fabricate ultrathin hydrogel films (7 μm thick). This method involves injecting a hydrogel precursor (PAAm-sodium alginate) between polyethylene terephthalate films and rapidly passing them through two rollers, forming an ultrathin film. Subsequent ultraviolet curing induced gelation, creating a highly controlled thickness-adjustable and homogeneous hydrogel layer. Compared to conventional spin-coating or casting-molding methods, this approach produces thinner and more uniform hydrogel films. Moreover, the fabricated hydrogel film exhibits a high water vapor transmission rate, effectively preventing trans-epidermal water loss and minimizing skin irritation during prolonged wearing.
3. Sensor design for neurological disorder monitoring
3.1. Electrophysiological sensors
Electrophysiological signals include EEG, EMG, ECG, and electrooculogram (EOG), important biosignals that reflect various physiological activities in the human body. These signals are measured through electrodes attached to the skin's surface to assess the nervous system, cardiovascular system, muscles, and eye movements.EEG is a non-invasive technique that measures the brain's electrical activity through electrodes attached to the scalp's surface. EEG signals originate primarily from postsynaptic potentials, which allow us to record the electrical signals occurring in the brain.94 EEG signals play an important role in diagnosing and monitoring neurological diseases. For example, EEG is instrumental in predicting seizures, enabling the development of early warning systems.95 In Alzheimer's disease, frequency analysis of EEG can detect early signs of cognitive decline.96 Additionally, EEG-based gamma wave analysis can help explore the link between motor function decline and Parkinson's disease.97 The international standard for EEG measurements is a 10–20 electrode placement system, and electrode caps or nets are being explored for more convenient wearing.98 However, existing EEG systems suffer from poor contact in hairy areas and are uncomfortable to wear.99 Recent studies have explored various designs of soft wearable EEG sensors to address this issue, including forehead patches,35,100 scalp tattoos,101–103 and earbud-shaped devices (Fig. 3a–c).104–106
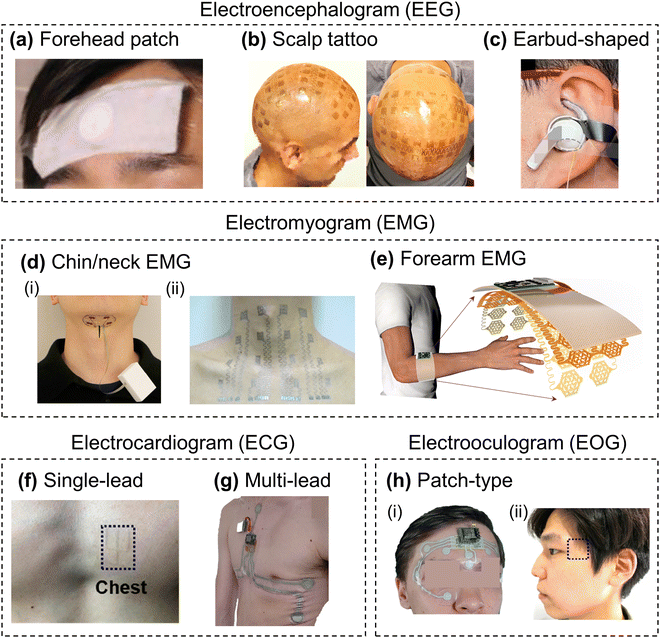 | ||
| Fig. 3 Soft wearable electrophysiological sensors and their form factors. (a) Forehead patch-type EEG sensor. Reproduced with permission.100 Copyright 2019 John Wiley and Sons. (b) Scalp tattoo-type EEG sensor. Reproduced with permission.103 Copyright 2019 Springer Nature. (c) Earbud-shaped EEG sensor. Reproduced with permission.106 Copyright 2023 Springer Nature (d) EMG sensing patches placed on the chin or neck. (i) Reproduced with permission.107 Copyright 2019 AAAS. (ii) Reproduced with permission.108 Copyright 2020 AAAS. (e) Multi-channel EMG array on the forearm. Reproduced with permission.109 Copyright 2023 Springer Nature. (f) Wearable single-lead ECG sensor. Reproduced with permission.35 Copyright 2019 American Chemical Society. (g) Wearable multi-lead ECG sensor. Reproduced with permission.110 Copyright 2022 John Wiley and Sons. (h) Patch-type EOG sensors. (i) Reproduced with permission.110 Copyright 2022 John Wiley and Sons. (ii) Reproduced with permission.35 Copyright 2019 American Chemical Society. | ||
EMG is a non-invasive approach for detecting the electrical signals generated by muscle activity to assess the function of the neuromuscular system. Muscles are activated through nerve impulses from motor neurons, which generate an action potential and cause the muscle fiber membrane to depolarize and repolarize.111 EMG captures these signals to analyze muscle activation patterns, fatigue, and dysfunction, while also serving as a valuable tool for identifying key biomarkers and contributing to the understanding of neurological disorders. For example, in patients with amyotrophic lateral sclerosis (ALS), EMG signals can be used to assess the degree of degeneration of motor neurons.112 In Parkinson's disease, they can quantitatively measure muscle stiffness and slowed movement.113 EMG electrodes should be placed between the innervation zone and the distal tendon, preferably on the muscle belly, aligned parallel to muscle fibers to ensure reliable signal quality.114 EMG signals result from the summation of motor unit action potentials, and different groups of muscle fibers are selectively activated depending on the movement and electrode placement.115 To analyze this more precisely, EMG arrays are used, which are useful for visualizing the firing patterns of motor units and changes in conduction velocity during specific movements. Patchable or tattooed wearable electrode arrays were attached to the skin where the muscles are located, allowing for comfortable multi-channel EMG measurements.39,109,116 Wearable EMG sensors placed on the chin or neck can monitor swallowing, vocal activity, and neck movements,107,108 while multi-channel EMG arrays on the forearm enable accurate recognition of hand gestures (Fig. 3d and e).39,109,116
An ECG is a non-invasive method used to record the electrical activity of the heart form the skin's surface. The heart generates electrical signals originating at the sinoatrial node and conducting along the atria and ventricles, creating a potential difference, which is recorded through electrodes attached to the skin.117 ECG is instrumental in monitoring cardiac autonomic function and detecting neurological diseases associated with heart rate variability (HRV). For example, Patients who have suffered a stroke often exhibit reduced HRV, which is linked to an increased risk of mortality and negative cardiovascular outcomes. ECG monitoring in these patients helps in assessing autonomic dysfunction and stratifying risk.118 Ictal bradycardia, a condition where seizures are accompanied by a significant decrease in the heart rate, can be detected through simultaneous EEG and ECG monitoring.119 Typically, a 12-lead ECG system is the standard, which includes limb leads and precordial leads.120 However, traditional ECG systems involve numerous wires, making them cumbersome to use. As a result, simpler single-lead electrodes are more commonly used in everyday applications. In laboratory settings, researchers have developed single-lead ECG sensors in the form of soft wearable patches that can be placed near the heart, offering greater convenience (Fig. 3f).35,121–123 Recent efforts have also focused on leveraging skin-conformal, multi-lead wearable ECG systems to enable more comprehensive and continuous cardiac monitoring in a user-friendly manner (Fig. 3g).110,124,125
EOG is an electrophysiological signal that detects eye movement by measuring the electrical potential difference between the cornea and retina, which changes as the eye moves.126 EOG is widely used to monitor eye movement abnormalities in neurological disorders, as abnormal saccadic patterns can be indicative of underlying neurodegenerative disorders, including Parkinson's disease, progressive supranuclear palsy, and cerebellar ataxia.127 EOG signals are typically recorded using two electrodes: one placed at the temples to capture horizontal movements and another above and below the eyes to measure vertical movements (Fig. 3h).35,110,128,129 Wearable EOG sensors can also be used to analyze blinking and eyeball movement.
Conventional Ag/AgCl wet electrode-based sensors offer high signal quality and low impedance, but are limited by the drying out of the electrolyte gel, which makes long-term use difficult and causes skin irritation. Another drawback is the complexity of wire connections, which makes them less wearable and requires professional attachment. To solve these problems, wearable sensors based on various soft electrodes have been developed in recent years.
Patch-type sensors are the most common form factor that are applied directly to the skin to measure signals and can utilize electrodes that are inherently soft or structurally stretchable and conformable. Conductive polymer-based electrodes are well suited for fabricating patchable electrophysiological sensors based on their low mechanical modulus and impedance. For example, a patch-like electrode with high stretchability and low impedance was fabricated by mixing PEDOT:PSS with waterborne PU and D-sorbitol.130 It showed lower impedance than Ag/AgCl electrodes and could reliably detect ECG, EMG, and EEG signals on both dry and wet skin, as well as during movement. LM-based electrodes can also be utilized as electrophysiological sensors due to their inherent stretchability, conformal contact, and high conductivity. Utilizing Ag-LM composites, a multi-electrode patch-type biosensor was designed for long-term monitoring of ECG and EMG.131 The electrodes exhibit lower skin-to-electrode impedance than Ag/AgCl clinical electrodes, and signal quality remains stable over long-term wearing. Stretchable and breathable patchable biosensors have also been fabricated via stamp-based LM activation patterning on electrospun nanofiber membranes containing semi-embedded LM particles, enabling the realization of EMG and ECG sensors with high mechanical stability and electrical performance.132
Serpentine, honeycomb, and kirigami-patterned electrodes leverage structural deformation to enhance stretchability and conformability, ensuring stable skin contact for robust electrophysiological sensing. For instance, long-term ECG monitoring systems have been realized by integrating ultrathin gold electrodes with serpentine patterns onto low-modulus elastomer substrates, allowing intimate skin adhesion without conductive gels.121 Serpentine-based filamentary conductive networks have also been applied to establish large-area, MRI-compatible epidermal electronic interfaces capable of reliable EEG, EMG, and EOG recordings.103 Likewise, honeycomb-structured electrodes utilize hexagonal patterns that rotate and deform under strain, effectively distributing mechanical stress and improving skin conformability. Mesh electrodes based on this design have shown stable surface electromyogram (sEMG) acquisition on complex facial contours such as the jawline.107 In addition, transparent kirigami-patterned electrodes fabricated with AgNWs have demonstrated high mechanical stability and stretchability while maintaining reliable electrophysiological signal capture.35 These studies collectively highlight how structural engineering strategies enhance the performance and durability of stretchable electrophysiological sensors.
Tattoo-like electrodes are an emerging class of epidermal sensors designed to seamlessly conform to the skin, providing stable, high-quality electrophysiological signal acquisition while remaining ultrathin and nearly imperceptible. Unlike conventional electrodes, tattoo-based sensors use conductive materials printed or transferred onto temporary tattoo paper, enabling intimate skin contact with minimal mechanical stress and motion artifacts. Their ultrathin nature ensures enhanced wearability, long-term usability, and compatibility with multimodal sensing applications. Inkjet-printed PEDOT:PSS tattoo electrodes have been developed to achieve low impedance and stable long-term EEG recordings.101 Tattoo-like dry electrodes integrated with earbud-based EEG devices have also been implemented, utilizing open-mesh structured connectors for improved interface performance.102 The ultrathin tattoo electrodes (900 nm thick) and connectors (2.8 μm thick) provided low impedance and minimized motion artifacts, enabling real-time brain activity monitoring even during movement. Graphene electronic tattoos (GETs) have been fabricated for imperceptible EOG monitoring by employing wet-transfer graphene on a polymer bilayer.129 The ultrathin GET sensors (350 nm thick) offered high optical transparency (85%) and stretchability up to 50%, ensuring minimal visibility while capturing precise eye movement signals. The GET-based EOG system demonstrated high sensitivity with an angular resolution of 4° of eye movement, enabling real-time wireless control of a quadcopter via eye gestures.
Microneedle-based patch sensors achieve lower impedance and long-term stable signal acquisition by penetrating the stratum corneum, unlike conventional surface electrodes that rely solely on skin contact. A PI-based flexible microneedle array electrode has been developed to eliminate the need for conductive gels, demonstrating stable long-term performance in polysomnography (PSG) by enabling continuous monitoring of EEG, EOG, EMG, and ECG signals over extended periods, including multi-night use.128 The PI-MNA electrodes exhibited an impedance of approximately 1/250 of standard clinical electrodes, resulting in higher SNR and reduced motion artifacts. Their ultrathin, flexible structure minimizes skin irritation while ensuring conformability and stable signal detection, making them a strong alternative to patch-type sensors.
In-ear sensors leverage the proximity of the ear canal to the central nervous system, enabling unobtrusive and stable electrophysiological signal acquisition. Unlike scalp EEG systems, which require conductive gels and extensive setup, in-ear bioelectronics offer a discreet and comfortable alternative with minimal motion artifacts, making them ideal for long-term monitoring. In-ear electrophysiological sensor systems using flexible silver-based electrodes have been developed to detect EEG and EOG signals.106 The electrode placement was optimized to enhance signal quality while minimizing interference, achieving an impedance of 386 kΩ at 50 Hz. The system incorporated a driven right-leg electrode configuration to suppress common-mode noise, ensuring stable recordings even during movement. Additionally, a bioelectronic sensor featuring electrothermal actuation has been implemented to conform to the shape of the ear canal, maintaining consistent skin contact for high-fidelity EEG acquisition.104 This system demonstrated 95% classification accuracy in a multi-target steady-state visual evoked potential task, highlighting its ability to reliably track visual attention. Additionally, its open design allowed natural auditory perception, enabling 84% accuracy in decoding auditory attention during real-world listening tasks.
3.2. Physical activity sensors
The body's physical movements and activities are closely linked to various neurological disorders. Joint movements, breathing, vocal cord vibration, and swallowing are important biomarkers for early detection of neurological disorders. For example, patients with Parkinson's disease may have gait abnormalities, while those with ALS or spinal muscular atrophy may have breathing difficulties due to weakness in the respiratory muscles.133–135 People with dystonia, particularly oromandibular dystonia, often experience impaired swallowing and chewing abnormalities caused by repetitive involuntary contractions of the masticatory, facial, and lingual muscles, potentially resulting in severe dysphagia and significant weight loss.136 Recent advances in wearable technology have led to the development of pressure, strain, triboelectric, and piezoelectric-based skin-worn sensors to precisely detect these physical activities.137–140 These sensors are attached to various body parts to enable real-time monitoring of movements and biosignals.A pressure-based motion monitoring film with high adhesion and flexibility has been developed to enable precise tracking of joint movements including the finger, wrist, elbow, and shoulder.142 The film was integrated with deep learning algorithms for real-time motion classification, enhancing virtual reality (VR) and augmented reality (AR) applications. A flexible pressure sensor based on MXene/polypyrrole@PDMS demonstrated high sensitivity and durability.143 When attached to joints such as the neck, elbow, finger, and knee, it effectively monitored fitness postures. A transparent, CNT-based stretchable strain sensor has also been implemented to conform seamlessly to the skin for high-precision detection of hand and finger motions.144 By integrating sensor data with visual information using a bioinspired learning architecture, the system achieved high-accuracy gesture recognition, demonstrating strong potential for HMIs. A triboelectric nanogenerator-based stretchable rubber sensor was explored as a self-powered motion detection system.145 This system was applied to the diaphragm and knee joints, capturing breathing patterns and limb motion, proving its potential in wearable health monitoring and motion tracking.
Pressure sensor array-based motion sensing enables movement tracking without direct sensor placement on individual joints. A flexible iontronic capacitive sensing array has been developed to wrap around the wrist and monitor pressure distribution resulting from skin and tendon deformations.146 By applying deep convolutional neural networks (CNNs), their system predicts multi-joint hand gestures based on learned pressure patterns rather than a direct joint attachment. Similarly, a hybrid-fabric capacitive pressure array was integrated into a wristband for handwriting recognition and gesture-based HMI applications (Fig. 4a).147 Thermally encapsulated PU fiber membranes ensure lightweight, breathable wearability, making them well-suited for long-term HMI applications. Moreover, some systems achieve motion tracking with a single or very few sensors. A deep-learned skin sensor was developed to decode complex joint movements from a single skin-mounted device (Fig. 4b).80 By leveraging laser-induced crack structures, this sensor captures minute deformations at the wrist and reconstructs multi-finger movements using deep neural networks, eliminating the need for multiple sensors placed on each joint. In addition, a substrate-less nanomesh artificial mechanoreceptor has been implemented to adhere directly to the finger and wrist, mimicking cutaneous proprioceptive receptors.37 This approach enables high-resolution joint movement tracking without requiring a dense sensor network, using meta-learning for user-independent motion recognition.
 | ||
| Fig. 4 Soft wearable physical activity sensors. (a) Wearable pressure sensor array for body motion monitoring. Reproduced with permission.147 Copyright 2025 Springer Nature. (b) Laser-induced crack-based strain sensor for decoding complex joint movements. Reproduced with permission.80 Copyright 2020 Springer Nature. (c) CNT–PDMS composite-based wearable sensor for detecting voice and swallowing. Reproduced with permission.148 Copyright 2018 Royal Society of Chemistry. (d) Pressure sensor integrated into a mask for respiration monitoring. Reproduced with permission.149 Copyright 2021 John Wiley and Sons. (e) Wearable temperature sensor placed on the philtrum for respiration tracking. Reproduced with permission.150 Copyright 2019 John Wiley and Sons. | ||
Unlike typical strain sensors, resistive vibration sensors must be able to recognize tremors with high sensitivity, which requires a high gauge factor (GF) based on graphene, metal nanoparticles, and nanocrack-based films.153 A strain sensor based on a CNT-PDMS composite with a network crack structure achieved a maximum GF of 87 and a sensing range of up to 100%, exhibiting high sensitivity to subtle vibrations (Fig. 4c).148 Researchers attached the sensor to the laryngeal prominence for real-time monitoring of voice and swallowing movements. An ultra-sensitive strain sensor based on a tile-like stacked structure of MXene–PANI nanocomposites has been developed, achieving a gauge factor of 2369.1.154 This sensor could detect minute deformations as small as 0.1538%, enabling precise monitoring of vocal cord vibrations. Beyond resistive sensors, other sensing mechanisms have been explored for wearable voice and swallowing monitoring. A piezoelectric acoustic sensor utilizing a ZnO thin film on an aluminum foil substrate demonstrated high precision in speech recognition (98%) and strong similarity to commercial microphones.155 Its flexibility and high fidelity make it suitable for continuous voice and respiratory sensing in wearable applications. Additionally, a capacitive strain sensor based on a MXene–PVA hydrogel was designed with high stretchability (1200%) and demonstrated sensitivity to drinking-induced epidermal movement, suggesting its applicability for swallowing monitoring.156 A triboelectric sensor composed of phyllosilicate–polysaccharide composite paper was further developed to track swallowing activity self-powered.157 The sensor successfully captured throat vibrations during swallowing, demonstrating its potential for non-invasive and continuous monitoring of dysphagia.
Furthermore, beyond mask-based implementations, wearable breath sensors can also be attached to the philtrum area, providing a more convenient method for real-time respiratory monitoring. For instance, a thin-film NiO-based temperature sensor has been applied to the philtrum to detect temperature fluctuations during inhalation and exhalation, particularly under physical activity (Fig. 4e).150 This demonstrated its effectiveness for continuous respiratory monitoring. Similarly, an Au-doped silicon nanomembrane temperature sensor, also placed on the philtrum, was designed for high-precision respiratory monitoring.161 In addition, a twistable and stretchable nasal patch was attached around the philtrum to measure both surface airflow and nasal cavity vibrations.162 This design allowed deeper respiratory monitoring by capturing surface airflow dynamics and internal breathing-induced micro-vibrations.
3.3. System integration
For the clinical application of soft bioelectronic sensors in neurological disorder monitoring, the sensor system must be implemented as a wearable platform capable of long-term, continuous measurement. This requires wireless data transmission and directly integrating hardware components such as analog filters, wireless communication modules, and microcontroller units (MCU) into the sensor. The sensor collects physiological signals and transmits them through stretchable interconnects and interfaces, ensuring stable electrical connectivity even under mechanical deformation. If necessary, the signals are processed through analog filters to enhance signal quality by reducing noise and artifacts. The processed signals are then relayed to a power-efficient MCU, digitized, and transmitted via wireless communication modules such as Bluetooth low energy (BLE) or Wi-Fi. This allows real-time data transmission to external devices, including smartphones or dedicated cloud servers, facilitating continuous monitoring and remote analysis of neurological conditions. However, direct soldering of passive components and integrated circuit chips onto soft electronics or connecting them to rigid module boards like flexible printed circuit boards (PCBs) can lead to stress concentration and mechanical failure due to the mismatch in the mechanical properties between soft and rigid components. To address this issue, research on stretchable interconnects and rigid-soft interface engineering has been actively pursued.Conventional soldering methods suffer from high stiffness and low elongation, leading to mechanical fatigue or cracking under repeated deformations. To overcome these limitations, stretchable soldering technologies based on LM have been proposed. A self-soldering mechanism employing small-molecule interfacial modulation has been developed, achieving over 1000% stretchability.163 This approach optimizes the interfacial interactions between LM and polymer matrices, effectively mitigating mechanical mismatches and enhancing long-term stability. Similarly, hybrid LM soldering techniques have been developed to improve both adhesion and stretchability by incorporating oxidized LM.164 This hybrid LM approach enhances adhesion at the bonding sites while maintaining high electrical conductivity, demonstrating stretchability of up to 1500%.
A biphasic gallium–indium (bGaIn) soldering technique has been developed, in which a mixture of liquid and crystalline solid phases enables a mechanically robust and highly conductive stretchable PCB.15 This method enhances durability compared to conventional solder pastes, maintaining electrical stability over 1000% strain. Additionally, a stretchable solder sticker has been designed to support a pick-and-place assembly process.165 This sticker enables room-temperature bonding, allowing stable electrical connections with a simple pressing motion, making it significantly simpler and more stretchable than traditional soldering techniques.
Beyond stretchable soldering, several strategies have been explored to engineer robust rigid–soft interfaces by modifying substrate stiffness, incorporating interlayers, and employing self-adhesive or mechanical interlocking methods. For example, a method for forming rigid zones within a soft substrate has been developed using localized chemical crosslinking, which minimizes stress concentration at the interface.166 This technique optimizes the mechanical strength of each region, providing a mechanically reliable interface. Furthermore, a gradient stiffness-programmed interface has been achieved by controlling localized phase transitions in hydrogels.167 Unlike abrupt stiffness transitions, which can lead to localized stress concentrations, this gradient approach gradually distributes stress, improving the mechanical durability of the rigid–soft interface.
Another approach involves using adhesive interposer layers for packaging rigid and soft components.168 This method enhances adhesion between materials with different stiffnesses while ensuring the long-term stability of the attached components. Additionally, mechanical interlocking strategies have been explored to enhance rigid–soft adhesion. In one approach, a soft substrate with a LM-based circuitry is bonded to a porous PI substrate using a thermoplastic adhesive, which, upon heating, forms a mechanical interlock.169 This results in significantly improved adhesion strength which is three times higher than those of conventional methods, and a high stretchability of approximately 700%. Moreover, a plug-and-play universal interface has been developed using a self-adhesive polymer matrix embedded with nanoparticles.170 This interface enables stable bonding between rigid modules and soft electrodes through simple pressing, achieving over three times the electrical stretchability and ten times the mechanical stretchability compared to conventional solder pastes.
Establishing a reliable and wearable power supply system is essential to enable long-term, autonomous operation of soft wearable bioelectronics. While miniaturized batteries are commonly used, increasing their capacity to support long-duration use often results in greater bulk, compromising comfort and wearability. To address this trade-off, recent efforts have explored self-powered strategies based on diverse wearable energy harvesters such as triboelectric nanogenerators,171,172 piezoelectric nanogenerators,173 thermoelectric generators,174–177 droplet-based electricity generators,177 photovoltaic cells,178 and sweat-based biofuel cells.172,179,180 These devices convert mechanical motion, body heat, light, or biochemical metabolites into electrical energy, and can be integrated with power management integrated circuits or a voltage regulator to provide a stable, continuous energy supply.171,174,177–179 When incorporated into a wearable sensor platform, such integrated systems enable long-term, untethered operation, minimizing the need for frequent recharging or bulky battery replacement and enhancing user comfort.171,172,176–179
NFC-based wireless charging in soft electronics offers a promising alternative to support battery-free or intermittently charged platforms. These systems utilize flexible or stretchable antennas based on materials such as AgNWs, Ag flakes, and LMs to enable efficient power transfer, even under mechanical deformation.72,181,182 In addition, these soft antenna technologies can support wireless data transmission, offering a soft form factor solution for both power delivery and communication in wearable bioelectronics.181–183
Soft energy storage systems, including soft batteries and supercapacitors, are essential components for achieving a fully compliant form factor in wearable bioelectronics. Recent developments feature strategies such as serpentine-framed lithium-ion cells,64 printed AgO–Zn184 and Ag2O–Zn185 batteries, and sweat-activated Mg–Ag/AgCl batteries,186 all tailored to enhance mechanical flexibility, resilience, and integration with soft platforms. In parallel, wearable supercapacitors utilizing conductive polymers, MXenes, metal–organic frameworks, and carbon nanomaterials have been engineered to operate via electric double-layer and pseudocapacitive mechanisms, offering high power density and mechanical adaptability.187 When combined with sensors and energy harvesters, such storage units have the potential to deliver stable and continuous power under dynamic conditions.
4. Applications for neurological disorders
In recent years, wearable sensors have been increasingly developed to monitor and support the rehabilitation of neurological disorders by continuously tracking patients' conditions through various electrophysiological and physical activity sensors (Fig. 5). Existing wearable systems, primarily available in commercial forms such as headsets, patches, and wristbands, provide critical data for early diagnosis, disease progression assessment, and rehabilitation.4 However, most of these devices remain rigid or semi-rigid, limiting user comfort and causing discomfort during prolonged use. The integration of soft wearable sensors is expected to enhance wearability, improve long-term stability, and enable more precise and continuous neurological monitoring. | ||
| Fig. 5 Timeline of key breakthroughs in wearable bioelectronics for neurological disorders.8,107,116,123,188–192 | ||
4.1. Sleep monitoring
Sleep disorders are strongly associated with neurological conditions and present in various forms, including insomnia, sleep apnea, rapid eye movement sleep behavior disorder (RBD), and periodic limb movement disorder.193 Additionally, disrupted sleep patterns are a key characteristic of neurodegenerative diseases such as Parkinson's and Alzheimer's diseases, making sleep monitoring essential for early diagnosis and disease progression assessment.194The gold standard for evaluating sleep quality has traditionally been PSG. PSG simultaneously measures multiple physiological signals, including EEG, EOG, EMG, ECG, respiratory patterns, and blood oxygen saturation (SpO2).195 This test is typically conducted in a clinical setting under medical supervision, requiring patients to wear multiple electrodes and sensors overnight. However, PSG is inherently bulky and complex, making it difficult to capture a patient's natural sleep patterns. Additionally, the laboratory environment may alter typical sleep behavior, creating a demand for portable and wearable alternatives that allow for sleep monitoring in real-world settings. Sleep is generally categorized into non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep.196 NREM sleep consists of three progressively deeper stages (N1, N2, and N3), while REM sleep is characterized by distinct neural activity and physiological responses. Each stage has specific neurophysiological characteristics, which can be identified using EEG, EOG, and EMG signals. For example, REM sleep is characterized by rapid eye movements (detected by EOG), bursts of sawtooth waves (2–4 Hz slow oscillations in the frontocentral EEG), and a marked reduction in muscle tone in EMG, although muscle atonia can be absent in RBD.197,198 These distinct electrophysiological signals allow for the classification of sleep stages and the detection of sleep disorders.
Soft wearable sleep monitoring devices offer a practical alternative to PSG by enabling non-intrusive, real-world measurement of biosignals. For example, a soft sternal patch has been developed for obstructive sleep apnea (OSA) detection and sleep stage monitoring (Fig. 6a).123 The wireless patch was designed for attachment to the sternum and could measure ECG, photoplethysmogram (PPG), seismocardiogram (SCG), and accelerometry signals. The soft material provided a skin-like mechanical property, allowing comfortable and effective home-based sleep monitoring compared to traditional PSG. The study implemented a residual CNN for automatic apnea/hypopnea detection and conducted clinical trials with nine participants, including four individuals diagnosed with OSA. The results demonstrated 100% sensitivity and 95% precision in OSA detection, showing strong potential for real-time, at-home monitoring of sleep disturbances. The system also analyzed the heart rate, HRV, respiration rate, SpO2, and body position to classify sleep stages and identify apnea events. Furthermore, an ultrathin, stretchable electronic patch was developed for wireless EEG, EOG, and EMG signal acquisition to facilitate automated sleep stage classification and OSA detection. A soft patch has also been developed for application to the forehead (EEG), periocular region (EOG), and chin (EMG), utilizing a skin-friendly Silbione material to ensure long-term wearability with minimal skin irritation (Fig. 6b).78 A BLE module enabled real-time wireless data transmission, while a CNN-based machine learning model was employed for automatic sleep stage classification. The sleep stage classification system was tested on eight subjects, achieving 82.43% accuracy (Cohen's kappa: 0.74) for manual scoring and 83.89% accuracy (Cohen's kappa: 0.76) for automated CNN-based scoring. For OSA detection, the system was evaluated on eight sleep apnea patients, achieving an 88.52% detection accuracy.
 | ||
| Fig. 6 Soft wearable sensor-based applications for neurological disorders. (a) Soft sternal patch for sleep stage monitoring and sleep apnea detection. Reproduced with permission.123 Copyright 2021 AAAS. (b) Face-mounted patch for sleep stage monitoring and sleep apnea detection. Reproduced with permission.78 Copyright 2023 AAAS. (c) Wearable patch for diagnosing swallowing disorders in stroke patients, integrating electromyogram and acoustic sensors. Reproduced with permission.188 Copyright 2024 John Wiley and Sons. (d) Skin-mounted patch for remote dysphagia monitoring. Reproduced with permission.107 Copyright 2019 AAAS. (e) Human–machine interface for quadruped robot control enabled by hand gesture recognition using a bioinspired somatosensory-visual fusion framework. Reproduced with permission.144 Copyright 2020 Springer Nature. (f) Hand motion recognition using substrate-less nanomesh mechanoreceptor. Reproduced with permission.37 Copyright 2022 Springer Nature. (g) Tattoo electrode-based wearable electroencephalogram sensor for brain–computer interface applications. Reproduced with permission.102 Copyright 2022 Springer Nature. | ||
On the other hand, the feasibility of a soft electrode array for sleep monitoring has been explored and validated against PSG.199 The system comprised a soft, dry electrode array and a compact data acquisition unit, integrating EEG electrodes on the forehead, EOG electrodes on the right facial area, and EMG electrodes on the chin. The recorded data were transmitted via Bluetooth and stored in a cloud-based system for offline analysis. Compared to PSG, the wearable system achieved an overall sleep stage classification agreement of Cohen's kappa = 0.688, with individual stage agreements of wake (k = 0.701), N1 (k = 0.224), N2 (k = 0.584), N3 (k = 0.410), and REM sleep (k = 0.723). Additionally, the system effectively detected REM sleep atonia with a sensitivity of 85.7%, demonstrating its potential for RBD detection in neurodegenerative disease patients. The study also compared laboratory-based PSG results with home-based monitoring, revealing that at-home recordings showed a significant reduction in wake after sleep onset, suggesting that home-based monitoring may more accurately reflect natural sleep patterns than PSG conducted in a clinical setting.
4.2. Monitoring motor impairment
Motion impairment is a prevalent symptom of various neurological diseases, including Parkinson's disease, MS, ALS, and stroke. These disorders lead to impairments in gait, balance, and posture, manifesting as muscle rigidity, tremors, postural instability, and gait disturbances such as freezing of gait and reduced step height.200 These symptoms significantly impact mobility, increasing fall risk and reducing overall quality of life. Therefore, quantitative assessment and real-time monitoring of motor function are essential for early diagnosis, progression assessment, and personalized treatment and rehabilitation plans.Traditionally, motor impairment has been assessed using clinical rating scales such as the Unified Parkinson's disease rating scale and the expanded disability status scale, as well as laboratory-based gait analysis.201–203 However, these methods require an office visit and are limited by their one-time measurement, which does not reflect the variability of motor symptoms in a patient's real life. Therefore, continuous and objective monitoring techniques utilizing wearable sensors have recently emerged as an important tool for the assessment and management of movement disorders.
Wearable sensor-based monitoring of movement disorders is emerging as a valuable tool for assessing the progression of neurological diseases with greater sensitivity and continuous data collection compared to traditional clinical evaluations. For example, wearable sensor-based monitoring systems for Parkinson's disease, such as PDMonitor® and Personal KinetiGraph (PKG), utilize sensors attached to the wrist, waist, and legs to quantitatively assess symptoms like tremors, motor fluctuations, and dyskinesia.204 In particular, PKG analyzes changes in motor function after medication intake, allowing for personalized treatment adjustments, improving patients’ quality of life, and optimizing therapy. Additionally, a study on patients with ALS developed a machine learning-based model that automatically analyzes daily movements using accelerometer sensors attached to the wrist and ankle.205 This model can detect disease progression faster than the conventional ALS Functional Rating Scale-Revised, reducing clinical trial sample sizes and facilitating more precise patient monitoring. For MS, wearable technology is primarily used to monitor gait patterns and activity levels.206 Devices such as the ActiGraph GT3X-BT measure physical activity to track functional changes in MS patients, while commercial wearables like Fitbit® are also applied in remote monitoring to assess motor function. Studies on MS patients have demonstrated that continuous monitoring with wearable sensors detects disease-related changes, supporting the development of telemedicine and personalized rehabilitation programs.
However, most existing wearable sensors are either rigid or semi-rigid, making it difficult to achieve proper adhesion to the body's curved surfaces and often causing discomfort during prolonged use. To address this limitation, recent advancements in soft electronics have led to the development of skin-conformal and stretchable sensors. These soft electronics-based wearable sensors offer greater flexibility and improved long-term wearability compared to conventional systems, enabling accurate monitoring of motor function by conforming to various body regions. In ALS, dysphagia is a critical symptom affecting bulbar muscle function, yet conventional preclinical evaluations primarily focus on limb function. To address this, a wireless wearable system was developed to monitor dysphagia-related muscle activity in ALS animal models, utilizing sEMG on the masseter and digastric muscles during natural eating behaviors.207 This system, incorporating a kirigami-based strain isolation mechanism, reduces motion artifacts and enables continuous, high-precision tracking of motor neuron denervation, serving as a non-invasive tool for assessing drug efficacy. In a clinical setting, a separate multimodal wearable system incorporating dual-channel EMG and an acoustic sensor was developed for the automatic diagnosis of swallowing disorders in stroke patients (Fig. 6c).188 By employing the CNN-long short-term memory (LSTM) hybrid algorithm, this system classified swallowing patterns and detected silent aspiration with 89.47% accuracy, demonstrating its potential as a non-invasive alternative to a videofluoroscopic swallow study, the current clinical gold standard. Additionally, a flexible submental sensor patch was introduced for remote dysphagia rehabilitation monitoring (Fig. 6d).107 This skin-mounted patch conforms to the curved submental area and enables simultaneous recording of muscle activity via sEMG electrodes and laryngeal movement via flexible strain gauge during swallowing. Designed for telerehabilitation applications, it facilitates long-term dysphagia management while improving treatment adherence and accessibility, particularly for a Parkinson's disease patient with dysphagia. These advancements highlight the growing role of wearable electronics in neurological disorder management, providing continuous, high-accuracy assessments that can enhance personalized treatment and rehabilitation strategies.
In addition, as discussed in the physical activity sensors section, soft electronics-based wearable systems offer precise detection of gait, posture, and tremors, making them valuable tools for long-term monitoring of motor function changes in patients with neurological disorders. With greater stretchability than conventional wearable devices and the use of skin-friendly materials for prolonged wearing, these systems are expected to play a crucial role in personalized treatment and rehabilitation strategies for neurological patients.
4.3. Aiding rehabilitation via a human–machine interface
Patients with neurological disorders, such as stroke, spinal cord injury, Parkinson's disease, and multiple sclerosis (MS) experience impairments in motor and sensory functions, necessitating continuous rehabilitation therapy.208 However, traditional rehabilitation approaches rely on hospital-based treatments, making real-time monitoring of a patient's recovery process challenging and limiting the implementation of personalized therapy. To address these limitations, HMI-based rehabilitation monitoring systems have gained attention, enabling precise and individualized rehabilitation by collecting and analyzing biological signals (EEG, EMG, and EOG) and movement data in real-time.209 These systems leverage inertial measurement units and pressure sensors to analyze gait patterns and balance,210 integrate VR/AR environments to enhance patient engagement,211 and combine brain–computer interface (BCI) technology to promote neuroplasticity,212 ultimately maximizing the effectiveness of rehabilitation therapy.Soft electronics-based HMI systems have significantly improved gesture recognition and motion tracking by integrating flexible sensors, multimodal data processing, and adaptive machine learning models. A high-density screen-printed sEMG electrode array on the forearm enabled real-time hand gesture recognition with 97.12% accuracy for 13 gestures.116 Using hyperdimensional computing for in-sensor learning, the system allows rapid adaptation to new gestures with minimal retraining, enhancing robustness for continuous rehabilitation monitoring. In addition, a stretchable single-walled CNT-based strain sensor has been developed and conformally attached to the knuckle joints to record finger bending and motion patterns.144 Using early-stage fusion of visual and somatosensory data through a bioinspired framework, the system achieved 100% recognition accuracy across 10 hand gesture categories and maintained robust performance under noisy and low-light conditions, enabling reliable control of a quadruped robot (Fig. 6e). A substrate-less nanomesh mechanoreceptor has also been developed, directly printed along the index finger and wrist to provide proprioceptive feedback for hand motion recognition (Fig. 6f).37 Utilizing a meta-learning framework with contrastive learning, the system rapidly adapted to new users and tasks. It classified motion commands, keyboard typing, and object interaction with 80% accuracy after only 20 training epochs, enabling efficient, user-independent adaptation. These soft electronics-based HMI systems’ continuous and precise motion tracking could support personalized rehabilitation strategies, real-time motor function monitoring, and improved patient engagement in neurological rehabilitation.
Soft wearable electronics-based BCI systems offer a more convenient and reliable method for brain signal acquisition, presenting the potential for future applications in neurological rehabilitation. A wireless, flexible EEG sensing system in an earbud form factor has been developed, utilizing tattoo-like dry electrodes attached to the forehead, temples, and mastoid region to measure EEG signals (Fig. 6g).102 This system enabled real-time detection of error-related potentials induced by AI misbehavior, achieving a single-trial classification accuracy of up to 83.81% using an LSTM-based deep learning classifier. Meanwhile, a skin-conformal, flexible scalp electronic system has been developed for mounting on the occipital lobe.213 This system utilized only two dry EEG channels to record steady-state visually evoked potentials and classify the EEG signals with an average classification accuracy of 94.54% and an information transfer rate of 122.1 ± 3.53 bits min−1 using a CNN-based classification model, demonstrating its feasibility for real-time neuroprosthetic applications, such as wheelchair control. These soft electronics-based BCI systems provide greater convenience than conventional gel-based EEG systems, enabling continuous and reliable EEG monitoring in everyday environments. This suggests their potential contribution to long-term monitoring and neurological rehabilitation for patients with neurological disorders. As technology advances, such systems could be crucial in personalized rehabilitation strategies and neuroplasticity-based interventions, ultimately supporting motor function assessment and adaptive rehabilitation training.
5. Toward clinical implementation
5.1. Clinical translations
Although soft wearable bioelectronic technologies have rapidly advanced toward applications in various neurological disorders, their clinical adoption remains limited. Compared to conventional commercial wearable devices, soft bioelectronic systems offer superior flexibility and biocompatibility. However, they still face substantial challenges in meeting the rigorous requirements of clinical environments such as long-term repeatability, reliability, biocompatibility under extended use, and inter-subject reproducibility. As a result, further validation is essential before these technologies can be widely integrated into clinical practice.Several essential requirements must first be addressed to enable the clinical translation of soft wearable bioelectronics. Devices must demonstrate consistent performance across diverse user conditions and environments and exhibit long-term biocompatibility without causing skin irritation or adverse reactions during extended use.5,214 In parallel, the ethical deployment of wearable technologies in healthcare and research must adhere to core principles such as data privacy, prevention of misuse, and equitable access, ensuring that such technologies are employed human-centered and non-discriminatory.215,216
Several soft bioelectronic devices have already advanced to the stage of clinical testing, demonstrating their practical potential for real-world applications. For example, a wireless multimodal system that simultaneously measures electromyographic and acoustic signals was evaluated against videofluoroscopic swallow studies in 33 healthy subjects and 30 post-stroke patients with dysphagia, achieving 89.47% accuracy in classifying swallow status.188 In another study, a flexible sensor attached beneath the chin was used to monitor muscle activity and laryngeal movement in a Parkinson's disease patient and a healthy control, showing signal quality comparable to that of conventional clinical equipment.107 A system designed to monitor both swallowing and respiration was also developed, utilizing flexible sensors attached to the neck and chest.217 This system was tested in 67 healthy adults and 4 patients with dysphagia and demonstrated comparable event detection accuracy to clinical instruments. Clinical validation has also expanded into sleep monitoring. A flexible patch mounted on the sternum, capable of recording ECG, PPG, and SCG signals, successfully detected apneic and hypopneic events in nine participants with 100% sensitivity and 95% precision.123 Furthermore, a face-mounted soft sensor platform measuring EEG, EOG, and EMG enabled sleep stage classification and OSA detection, achieving 88.5% diagnostic accuracy, comparable to PSG.78
Such clinical translation requires not only one-time device validation but also a multilayered consideration encompassing long-term data processing and analysis strategies, seamless integration into existing healthcare infrastructures, and alignment with relevant regulatory frameworks. All personal information collected during the course of patient care must not be disclosed to external parties or third parties without the patient's consent. Physiological signals acquired through wearable devices often reflect highly individualized biological and behavioral traits, introducing the risk of unintentionally disclosing sensitive personal information, including emotional or health-related states. These risks are particularly pronounced in telemedicine and BCIs, where continuous signal transmission and user profiling are involved. Strict compliance with international data protection regulations, such as the General Data Protection Regulation and the Health Insurance Portability and Accountability Act, is essential to mitigate such vulnerabilities.218,219 Furthermore, emerging approaches such as federated learning and edge machine learning offer technical solutions that process data locally on the device, thereby minimizing external transmission and substantially enhancing user privacy.220,221
5.2. Intelligent signal processing and analytics
One of the key strengths of soft wearable bioelectronics is their ability to acquire high-density biosignals continuously in real-time. The interpretation of such longitudinal data plays a critical role in diagnosing neurological disorders and designing effective rehabilitation therapies. Traditionally, biosignal processing has relied on simple filtering and offline postprocessing; however, the integration of artificial intelligence has significantly expanded the analytical capabilities of these systems. Recent advances now enable automated artifact removal, accurate disease prediction, and the development of personalized rehabilitation strategies based on complex physiological patterns.Deep learning-based signal denoising techniques have enabled the accurate reconstruction of EEG signals contaminated by physiological artifacts, such as eye movements, muscle contractions, and cardiac activity, with performance comparable to expert manual correction.222 Artifact removal using neural network architectures such as CNNs and LSTMs has demonstrated high denoising performance with reduced information loss compared to traditional methods like independent component analysis, while also facilitating automation of the preprocessing workflow.222,223
Furthermore, deep learning-based biosignal analysis techniques have been increasingly utilized for disease classification and early prediction across various neurological disorders. These approaches leverage the ability of neural networks to extract nonlinear, high-dimensional features automatically and to learn complex temporal dependencies in physiological time-series data. For example, classification models have been effectively applied to tasks such as sleep stage annotation and analysis of Parkinsonian motor symptoms, which often exhibit recurring or structured signal patterns.224–226 On the other hand, seizure prediction requires models that can capture rapid temporal changes and long-term dependencies in EEG signals.227 These deep learning-based approaches are increasingly regarded as a foundation for personalized diagnostic and therapeutic strategies in clinical neuroscience.
AI-based decoding of physiological signals such as EEG and EMG enables the quantitative assessment of motor intention, neuromuscular function, and rehabilitation prognosis in patients with neurological disorders.228–231 This capability forms the foundation for adaptive rehabilitation systems, which detect discrepancies between a patient's intended and actual movements and deliver iterative, personalized feedback to promote neuroplasticity and accelerate functional recovery.228 Such systems can be further enhanced by integrating peripheral actuation technologies, including functional electrical stimulation and exoskeletons, that respond quickly to detected motor intentions.229,230,232 These closed-loop frameworks have the potential to support autonomous rehabilitation, offering a scalable solution for the long-term management of motor impairments.
6. Conclusions
Soft wearable electronics have demonstrated significant potential in enabling real-time, continuous physiological signal acquisition for neurological disorder monitoring and rehabilitation. Unlike traditional rigid or semi-rigid sensors, soft electronics offer greater stretchability, conformal skin adhesion, and long-term wearability, making them well-suited for tracking disease progression and supporting personalized rehabilitation strategies. These advantages position soft wearable electronics as a promising alternative to conventional hospital-based assessment methods, often failing to capture dynamic changes in patients’ conditions.However, several challenges remain before widespread clinical adoption can be achieved. Most studies have yet to undergo large-scale clinical trials, and concerns regarding long-term stability, reproducibility, and manufacturing scalability must be addressed.
Despite significant advances in the development of novel material platforms for soft bioelectronics, including LMs, hydrogels, metallic nanostructures, and polymeric composites, several fundamental challenges remain unresolved. For example, LMs while offering high conductivity and mechanical deformability exist in a fluid state and are vulnerable to leakage when subjected to mechanical stress such as stretching, compression, or friction. Even with their high surface tension, they can infiltrate through microcracks, delamination sites, or pinholes in the encapsulation layer. Accumulated mechanical fatigue, surface oxidation, and limited long-term chemical stability in biological environments further intensify this issue. These limitations are particularly critical in wearable applications, where repeated mechanical stimulation makes long-term containment difficult. Once leakage occurs, electrical shorting occurs, leading to circuit failure and fluctuations in contact impedance, thereby compromising both signal quality and device reliability.
Hydrogel electrodes are highly dependent on environmental factors, as their water content and ionic conductivity vary in response to humidity, temperature, and contact with skin or body fluids. Over time, additional changes such as drying, excessive swelling, or polymer chain degradation can further increase impedance instability. Impedance drift in hydrogels can cause baseline shifts, increased noise, and signal interference during long-term monitoring, which significantly limits the diagnostic reliability and scalability of wearable healthcare systems.
In parallel, although many promising materials are actively being explored, comprehensive biological validation in accordance with ISO 10993 remains scarce in academic research. Most studies are limited to in vitro or benchtop-level evaluations, lacking standardized assessment of long-term degradation, immune responses, or systemic toxicity. Addressing this gap between laboratory-scale demonstration and clinical-level safety evaluation will be a critical step toward the regulatory approval and practical deployment of wearable bioelectronic devices.
From a system integration perspective, while AI-based data analytics play a pivotal role in neurological monitoring, the absence of standardized machine learning models and limited real-world validation impede clinical adoption. Additionally, integrating multimodal biosignals, particularly combining electrophysiological and physical activity data, faces significant algorithmic complexity due to modality heterogeneity. Differences in sampling rates, data formats, and temporal alignment often lead to information loss and increased computational burden.233 To effectively integrate heterogeneous modalities, it is essential to perform data preprocessing and normalization for temporal-spatial feature alignment while addressing incomplete or imbalanced data through imputation and augmentation strategies.234,235 Furthermore, optimizing the choice of fusion strategy by considering the level of modality interaction, tolerance to missing data, and computational constraints is essential for achieving robust and scalable multimodal representation learning.236 When effectively applied, these approaches can enhance high-dimensional representations of neurological states, supporting more accurate diagnosis and personalized monitoring.237,238
Future research should also focus on enhancing reproducibility across different users and environments, as well as optimizing wireless communication for remote healthcare applications. Strengthening collaboration among materials science, electronics, and medical research will be key to advancing soft wearable electronics toward clinical-grade neuro-monitoring and rehabilitation systems. As these challenges are progressively addressed, soft bioelectronics are expected to play a pivotal role in personalized neurorehabilitation, adaptive therapy, and next-generation BCI systems, ultimately transforming neurological healthcare and patient care.
Author contributions
D. K., J. B. and S. H. K. wrote the manuscript. All authors contributed to the discussion, reviewing, and editing of the manuscript.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was supported by the National Research Foundation of Korea (grant number RS-2025-11092968, RS-2023-00208052) and by Bio-MAX Institute.References
- V. L. Feigin, et al., Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015, Lancet Neurol., 2017, 16, 877–897 CrossRef.
- S. Singh, S. K. Gupta and P. K. Seth, Biomarkers for detection, prognosis and therapeutic assessment of neurological disorders, Rev. Neurosci., 2018, 29, 771–789 CrossRef.
- S. Bhat, U. R. Acharya, Y. Hagiwara, N. Dadmehr and H. Adeli, Parkinson's disease: Cause factors, measurable indicators, and early diagnosis, Comput. Biol. Med., 2018, 102, 234–241 CrossRef PubMed.
- J. Huang, et al., Clinical research on neurological and psychiatric diagnosis and monitoring using wearable devices: A literature review, Interdiscip. Med., 2024, 2, e20230037 CrossRef.
- Y. F. Luo, et al., Technology Roadmap for Flexible Sensors, ACS Nano, 2023, 17, 5211–5295 CrossRef CAS PubMed.
- T. R. Ray, et al., Bio-integrated wearable systems: a comprehensive review, Chem. Rev., 2019, 119, 5461–5533 CrossRef CAS.
- M. M. Shawrav, et al., Highly conductive and pure gold nanostructures grown by electron beam induced deposition, Sci. Rep., 2016, 6, 34003 CrossRef CAS.
- A. Miyamoto, et al., Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes, Nat. Nanotechnol., 2017, 12, 907–913 CrossRef CAS PubMed.
- P. Lee, et al., Highly stretchable and highly conductive metal electrode by very long metal nanowire percolation network, Adv. Mater., 2012, 24, 3326–3332 CrossRef CAS PubMed.
- N. Liu, et al., Ultratransparent and stretchable graphene electrodes, Sci. Adv., 2017, 3, e1700159 CrossRef PubMed.
- T. Habib, et al., Oxidation stability of TiCT MXene nanosheets in solvents and composite films, npj 2D Mater. Appl., 2019, 3, 8 CrossRef.
- H. Bi, et al., Ultrahigh humidity sensitivity of graphene oxide, Sci. Rep., 2013, 3, 2714 CrossRef PubMed.
- D. Won, et al., Digital selective transformation and patterning of highly conductive hydrogel bioelectronics by laser-induced phase separation, Sci. Adv., 2022, 8, eabo3209 CrossRef CAS PubMed.
- B. Lu, et al., Pure PEDOT:PSS hydrogels, Nat. Commun., 2017, 10, 1043 CrossRef PubMed.
- S. Liu, D. S. Shah and R. Kramer-Bottiglio, Highly stretchable multilayer electronic circuits using biphasic gallium-indium, Nat. Mater., 2021, 20, 851–858 CrossRef CAS PubMed.
- Y. Deng, et al., Stretchable liquid metal based biomedical devices, npj Flexible Electron., 2024, 8, 12 CrossRef CAS.
- S. P. Lacour, S. Wagner and Z. Suo, Stretchable conductors: thin gold films on silicone elastomer, MRS Online Proc. Libr., 2003, 795(U6), 9 Search PubMed.
- J. Viventi, et al., Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo, Nat. Neurosci., 2011, 14, 1599–1605 CrossRef CAS PubMed.
- S. K. Soni, B. Thomas and V. R. Kar, A comprehensive review on CNTs and CNT-reinforced composites: syntheses, characteristics and applications, Mater. Today Commun., 2020, 25, 101546 CrossRef CAS.
- S. Zhao, et al., Advancements in copper nanowires: synthesis, purification, assemblies, surface modification, and applications, Small, 2018, 14, 1800047 CrossRef.
- L. Zhang, et al., Recent progress for silver nanowires conducting film for flexible electronics, J. Nanostruct. Chem., 2021, 11, 323–341 CrossRef CAS PubMed.
- A. K. Katiyar, et al., 2D materials in flexible electronics: recent advances and future prospectives, Chem. Rev., 2023, 124, 318–419 CrossRef PubMed.
- Y. Kim, et al., Recent developments in selective laser processes for wearable devices, Bio-Des. Manuf., 2024, 7, 517–547 CrossRef.
- X. Du, et al., A review of inkjet printing technology for personalized-healthcare wearable devices, J. Mater. Chem. C, 2022, 10, 14091–14115 RSC.
- D. Lohse, Fundamental Fluid Dynamics Challenges in Inkjet Printing, Annu. Rev. Fluid Mech., 2022, 54, 349–382 CrossRef.
- N. Palavesam, et al., Roll-to-roll processing of film substrates for hybrid integrated flexible electronics, Flexible Printed Electron., 2018, 3, 014002 CrossRef.
- T. K. Das and S. Prusty, Review on conducting polymers and their applications, Polym.-Plast. Technol. Eng., 2012, 51, 1487–1500 CrossRef CAS.
- Y. Liu, et al., Soft and elastic hydrogel-based microelectronics for localized low-voltage neuromodulation, Nat. Biomed. Eng., 2019, 3, 58–68 CrossRef CAS PubMed.
- M. D. Dickey, et al., Eutectic gallium-indium (EGaIn): a liquid metal alloy for the formation of stable structures in microchannels at room temperature, Adv. Funct. Mater., 2008, 18, 1097–1104 CrossRef CAS.
- M. Kim, H. Lim and S. H. Ko, Liquid metal patterning and unique properties for next-generation soft electronics, Adv. Sci., 2023, 10, 2205795 CrossRef CAS PubMed.
- J. Bang, et al., Advances in protective layer-coating on metal nanowires with enhanced stability and their applications, Appl. Mater. Today, 2021, 22, 100909 CrossRef.
- S. Choi, et al., Highly conductive, stretchable and biocompatible Ag-Au core-sheath nanowire composite for wearable and implantable bioelectronics, Nat. Nanotechnol., 2013, 13, 1048–1056 CrossRef PubMed.
- D. Kim, et al., Biocompatible Cost-Effective Electrophysiological Monitoring with Oxidation-Free Cu-Au Core-Shell Nanowire, Adv. Mater. Technol., 2020, 5, 2000661 CrossRef CAS.
- K. I. Jang, et al., Self-assembled three dimensional network designs for soft electronics, Nat. Commun., 2017, 8, 1–10 CrossRef PubMed.
- P. Won, et al., Stretchable and Transparent Kirigami Conductor of Nanowire Percolation Network for Electronic Skin Applications, Nano Lett., 2019, 19, 6087–6096 CrossRef CAS PubMed.
- S. M. Cheng, et al., Ultrathin Hydrogel Films toward Breathable Skin-Integrated Electronics, Adv. Mater., 2023, 35, 2206793 CrossRef CAS PubMed.
- K. K. Kim, et al., A substrate-less nanomesh receptor with meta-learning for rapid hand task recognition, Nat. Electron., 2023, 6, 64–75 Search PubMed.
- Y. G. Cheng, et al., Chrysalis-inspired high-toughness low-modulus conductive hydrogel sensor for intelligent sensing, Chem. Eng. J., 2024, 498, 155475 CrossRef CAS.
- H. Yoon, et al., Adaptive Epidermal Bioelectronics by Highly Breathable and Stretchable Metal Nanowire Bioelectrodes on Electrospun Nanofiber Membrane, Adv. Funct. Mater., 2024, 34, 2313504 CrossRef CAS.
- M. Sang, K. Kim, J. Shin and K. J. Yu, Ultra-Thin Flexible Encapsulating Materials for Soft Bio-Integrated Electronics, Adv. Sci., 2022, 9, 2202980 CrossRef CAS PubMed.
- P. Le Floch, et al., 3D spatiotemporally scalable in vivo neural probes based on fluorinated elastomers, Nat. Nanotechnol., 2024, 19, 319–329 CrossRef CAS PubMed.
- J. H. Kwon, et al., Design of highly water resistant, impermeable, and flexible thin-film encapsulation based on inorganic/organic hybrid layers, ACS Appl. Mater. Interfaces, 2018, 11, 3251–3261 CrossRef PubMed.
- J. Chen, et al., Polydimethylsiloxane (PDMS)-Based Flexible Resistive Strain Sensors for Wearable Applications, Appl. Sci., 2018, 8, 345 CrossRef.
- M. Hassan, et al., Significance of Flexible Substrates for Wearable and Implantable Devices: Recent Advances and Perspectives, Adv. Mater. Technol., 2022, 7, 2100773 CrossRef.
- Q. J. Fu, et al., Emerging cellulose-derived materials: a promising platform for the design of flexible wearable sensors toward health and environment monitoring, Mater. Chem. Front., 2021, 5, 2051–2091 RSC.
- D. L. Wen, et al., Printed silk-fibroin-based triboelectric nanogenerators for multi-functional wearable sensing, Nano Energy, 2019, 66, 104123 CrossRef CAS.
- J. J. Liu, S. X. Qu, Z. G. Suo and W. Yang, Functional hydrogel coatings, Natl. Sci. Rev., 2021, 8, nwaa254 CrossRef CAS PubMed.
- A. Hogg, et al., Ultra-thin layer packaging for implantable electronic devices, J. Micromech. Microeng., 2013, 23, 075001 CrossRef CAS.
- R. Feiner and T. Dvir, Tissue–electronics interfaces: from implantable devices to engineered tissues, Nat. Rev. Mater., 2017, 3, 1–16 Search PubMed.
- J. P. Santerre, K. Woodhouse, G. Laroche and R. S. Labow, Understanding the biodegradation of polyurethanes: from classical implants to tissue engineering materials, Biomaterials, 2005, 26, 7457–7470 CrossRef CAS PubMed.
- D. R. Darby, Z. Cai, C. R. Mason and J. T. Pham, Modulus and adhesion of Sylgard 184, Solaris, and Ecoflex 00-30 silicone elastomers with varied mixing ratios, J. Appl. Polym. Sci., 2022, 139, e52412 CrossRef CAS.
- I. Miranda, et al., Properties and Applications of PDMS for Biomedical Engineering: A Review, J. Funct. Biomater., 2021, 13, 2 CrossRef PubMed.
- X. Ji, H. Wang, X. Ma, C. Hou and G. Ma, Progress in polydimethylsiloxane-modified waterborne polyurethanes, RSC Adv., 2017, 7, 34086–34095 RSC.
- A. Arkhangelskiy, A. Quaranta, L. Pancheri and D. Maniglio, Deposition of PEDOT:PSS via Atmospheric Pressure Plasma Torch, Adv. Eng. Mater., 2023, 25, 2301280 CrossRef CAS.
- N. S. Allen, et al., Degradation and stabilisation of styrene-ethylene-butadiene-styrene (SEBS) block copolymer, Polym. Degrad. Stab., 2021, 71, 113–122 CrossRef.
- R. Kouser, A. Vashist, M. Zafaryab, M. A. Rizvi and S. Ahmad, Biocompatible and mechanically robust nanocomposite hydrogels for potential applications in tissue engineering, Mater. Sci. Eng., C, 2018, 84, 168–179 CrossRef CAS PubMed.
- T. Araki, et al., Long-Term Implantable, Flexible, and Transparent Neural Interface Based on Ag/Au Core-Shell Nanowires, Adv. Healthcare Mater., 2019, 8, 1900130 CrossRef PubMed.
- R. Damalerio and M. Y. ChengDevelopment of Dry EEG Electrodes and Dry EEG Cap for Neuromonitoring. Elec Comp C, 841–846 ( 2020) Search PubMed.
- K. Stokes, in Implantable Neural Prostheses 2: Techniques and Engineering Approaches, Springer, 2009, pp. 1–26 Search PubMed.
- P. Oldroyd, J. Gurke and G. G. Malliaras, Stability of Thin Film Neuromodulation Electrodes under Accelerated Aging Conditions, Adv. Funct. Mater., 2022, 33, 2208881 CrossRef.
- Y. Liu, M. Pharr and G. A. Salvatore, Lab-on-Skin: A Review of Flexible and Stretchable Electronics for Wearable Health Monitoring, ACS Nano, 2017, 11, 9614–9635 CrossRef CAS PubMed.
- A. Chortos, J. Liu and Z. A. Bao, Pursuing prosthetic electronic skin, Nat. Mater., 2016, 15, 937–950 CrossRef CAS PubMed.
- N. Matsuhisa, et al., High-Transconductance Stretchable Transistors Achieved by Controlled Gold Microcrack Morphology, Adv. Electron. Mater., 2019, 5, 1900347 CrossRef.
- S. Xu, et al., Stretchable batteries with self-similar serpentine interconnects and integrated wireless recharging systems, Nat. Commun., 2013, 4, 1543 CrossRef PubMed.
- J. A. Fan, et al., Fractal design concepts for stretchable electronics, Nat. Commun., 2014, 5, 3266 CrossRef PubMed.
- H. Choi, et al., Highly Stretchable and Strain-Insensitive Liquid Metal based Elastic Kirigami Electrodes (LM-eKE), Adv. Funct. Mater., 2023, 33, 2301388 CrossRef CAS.
- P. Le Floch, et al., Stretchable Mesh Nanoelectronics for 3D Single-Cell Chronic Electrophysiology from Developing Brain Organoids, Adv. Mater., 2022, 34, 2106829 CrossRef CAS PubMed.
- D. Won, et al., Laser-induced wet stability and adhesion of pure conducting polymer hydrogels, Nat. Electron., 2014, 7, 475–486 CrossRef.
- Y. Jiang, et al., Auxetic Mechanical Metamaterials to Enhance Sensitivity of Stretchable Strain Sensors, Adv. Mater., 2018, 30, 1706589 CrossRef PubMed.
- H. S. Kang, et al., Skin-Tailored Adhesive Bio-Electrode with Tunable Poisson’s Ratio for Stable Electrophysiological Communication, Adv. Healthcare Mater., 2025, 14, 2404882 CrossRef CAS PubMed.
- D. Kim, et al., Highly stretchable and oxidation-resistive Cu nanowire heater for replication of the feeling of heat in a virtual world, J. Mater. Chem. A, 2020, 8, 8281–8291 RSC.
- Y. D. Xu, et al., Phase-separated porous nanocomposite with ultralow percolation threshold for wireless bioelectronics, Nat. Nanotechnol., 2024, 19, 1158–1167 CrossRef CAS PubMed.
- Y. W. Jiang, et al., Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics, Science, 2022, 375, 1411–1417 CrossRef CAS PubMed.
- P. Tan, et al., Solution-processable, soft, self-adhesive, and conductive polymer composites for soft electronics, Nat. Commun., 2022, 13, 358 CrossRef CAS PubMed.
- J. Y. Chong, et al., Highly conductive tissue-like hydrogel interface through template-directed assembly, Nat. Commun., 2023, 14, 2206 CrossRef CAS PubMed.
- T. Zhou, et al., 3D printable high-performance conducting polymer hydrogel for all-hydrogel bioelectronic interfaces, Nat. Mater., 2023, 22, 895–902 CrossRef CAS PubMed.
- X. C. He, et al., Adhesive tapes: From daily necessities to flexible smart electronics, Appl. Phys. Rev., 2023, 10, 011305 CAS.
- S. Kwon, et al., At-home wireless sleep monitoring patches for the clinical assessment of sleep quality and sleep apnea, Sci. Adv., 2023, 9, eadg9671 CrossRef CAS PubMed.
- R. Zhang, et al., Mussel-inspired cellulose nanofiber/poly(vinyl alcohol) hydrogels with robustness, self-adhesion and antimicrobial activity for strain sensors, Int. J. Biol. Macromol., 2023, 245, 125469 CrossRef CAS PubMed.
- K. K. Kim, et al., A deep-learned skin sensor decoding the epicentral human motions, Nat. Commun., 2020, 11, 2149 CrossRef CAS PubMed.
- H. Yuk, et al., Dry double-sided tape for adhesion of wet tissues and devices, Nature, 2019, 575, 169–174 CrossRef CAS PubMed.
- J. Deng, et al., Electrical bioadhesive interface for bioelectronics, Nat. Mater., 2021, 20, 229–236 CrossRef CAS PubMed.
- S. T. Frey, et al., Octopus-inspired adhesive skins for intelligent and rapidly switchable underwater adhesion, Sci. Adv., 2022, 8, eabq1905 CrossRef PubMed.
- T. Kim, J. Park, J. Sohn, D. Cho and S. Jeon, Bioinspired, Highly Stretchable, and Conductive Dry Adhesives Based on 1D-2D Hybrid Carbon Nanocomposites for All-in-One ECG Electrodes, ACS Nano, 2021, 10, 4770–4778 CrossRef PubMed.
- D. W. Kim, et al., Electrostatic-Mechanical Synergistic In Situ Multiscale Tissue Adhesion for Sustainable Residue-Free Bioelectronics Interfaces, Adv. Mater., 2022, 34, 2105338 CrossRef CAS.
- R. C. Webb, et al., Ultrathin conformal devices for precise and continuous thermal characterization of human skin (vol 12, pg 938, 2013), Nat. Mater., 2013, 12, 1078 CrossRef CAS.
- Y. Kim, et al., Chip-less wireless electronic skins by remote epitaxial freestanding compound semiconductors, Science, 2022, 377, 859–869 CrossRef CAS PubMed.
- S. P. Lacour, G. Courtine and J. Guck, Materials and technologies for soft implantable neuroprostheses, Nat. Rev. Mater., 2016, 1, 1–14 Search PubMed.
- Y. Y. Lu, et al., Stretchable graphene-hydrogel interfaces for wearable and implantable bioelectronics, Nat. Electron., 2024, 7, 51–65 CrossRef CAS.
- Y. Yang, et al., Breathable Electronic Skins for Daily Physiological Signal Monitoring, Nano-Micro Lett., 2022, 14, 161 CrossRef CAS PubMed.
- D. Kireev, J. Kampfe, A. Hall and D. Akinwande, Graphene electronic tattoos 2.0 with enhanced performance, breathability and robustness, npj 2D Mater. Appl., 2022, 6, 46 CrossRef CAS.
- T. R. Cui, et al., Multifunctional, breathable MXene-PU mesh electronic skin for wearable intelligent 12-lead ECG monitoring system, Chem. Eng. J., 2023, 455, 140690 CrossRef CAS.
- J. C. Luo, et al., Superhydrophobic and breathable smart MXene-based textile for multifunctional wearable sensing electronics, Chem. Eng. J., 2021, 406, 126898 CrossRef CAS.
- T. Kirschstein and R. Kohling, What is the source of the EEG?, Clin. EEG Neurosci., 2009, 40, 146–149 CrossRef PubMed.
- F. Mormann, R. G. Andrzejak, C. E. Elger and K. Lehnertz, Seizure prediction: the long and winding road, Brain, 2007, 130, 314–333 CrossRef PubMed.
- S. S. Poil, et al., Integrative EEG biomarkers predict progression to Alzheimer's disease at the MCI stage, Front. Aging Neurosci., 2013, 5, 58 Search PubMed.
- A. Guerra, et al., Enhancing Gamma Oscillations Restores Primary Motor Cortex Plasticity in Parkinson's Disease, J. Neurosci., 2020, 40, 4788–4796 CrossRef CAS PubMed.
- U. Herwig, P. Satrapi and C. Schonfeldt-Lecuona, Using the international 10-20 EEG system for positioning of transcranial magnetic stimulation, Brain Topogr., 2003, 16, 95–99 CrossRef PubMed.
- M. Soufineyestani, D. Dowling and A. Khan, Electroencephalography (EEG) Technology Applications and Available Devices, Appl. Sci., 2020, 10, 7453 CrossRef CAS.
- T. Araki, et al., Wireless Monitoring Using a Stretchable and Transparent Sensor Sheet Containing Metal Nanowires, Adv. Mater., 2020, 32, e1902684 CrossRef PubMed.
- L. M. Ferrari, U. Ismailov, J. M. Badier, F. Greco and E. Ismailova, Conducting polymer tattoo electrodes in clinical electro- and magneto-encephalography, npj Flexible Electron., 2020, 4, 4 CrossRef CAS.
- J. H. Shin, et al., Wearable EEG electronics for a Brain-AI Closed-Loop System to enhance autonomous machine decision-making, npj Flexible Electron., 2022, 6, 32 CrossRef.
- L. Tian, et al., Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring, Nat. Biomed. Eng., 2019, 3, 194–205 CrossRef PubMed.
- Z. Wang, et al., Conformal in-ear bioelectronics for visual and auditory brain-computer interfaces, Nat. Commun., 2023, 14, 4213 CrossRef CAS PubMed.
- Y. D. Alqurashi, et al., A novel in-ear sensor to determine sleep latency during the Multiple Sleep Latency Test in healthy adults with and without sleep restriction, Nat. Sci. Sleep, 2018, 10, 385–396 CrossRef PubMed.
- Y. Xu, et al., In-ear integrated sensor array for the continuous monitoring of brain activity and of lactate in sweat, Nat. Biomed. Eng., 2023, 7, 1307–1320 CrossRef PubMed.
- M. K. Kim, et al., Flexible submental sensor patch with remote monitoring controls for management of oropharyngeal swallowing disorders, Sci. Adv., 2019, 5, eaay3210 CrossRef PubMed.
- Y. Wang, et al., Electrically compensated, tattoo-like electrodes for epidermal electrophysiology at scale, Sci. Adv., 2020, 6, eabd0996 CrossRef CAS PubMed.
- H. Lee, et al., Stretchable array electromyography sensor with graph neural network for static and dynamic gestures recognition system, npj Flexible Electron., 2023, 7, 20 CrossRef.
- M. Reis Carneiro, C. Majidi and M. Tavakoli, Multi-Electrode Printed Bioelectronic Patches for Long-Term Electrophysiological Monitoring, Adv. Funct. Mater., 2022, 32, 2205956 CrossRef CAS.
- P. Konrad, The abc of emg, A practical introduction to kinesiological electromyography, 2006, vol. 1, pp. 30–35 Search PubMed.
- N. C. Joyce and G. T. Carter, Electrodiagnosis in persons with amyotrophic lateral sclerosis, PMR, 2013, 5, S89–S95 Search PubMed.
- J. A. Robichaud, et al., Variability of EMG patterns: a potential neurophysiological marker of Parkinson's disease?, Clin. Neurophysiol., 2009, 120, 390–397 CrossRef PubMed.
- L. Mesin, R. Merletti and A. Rainoldi, Surface EMG: the issue of electrode location, J Electromyogr. Kinesiol., 2009, 19, 719–726 CrossRef CAS PubMed.
- J. M. Zuniga, et al., The effects of electrode orientation on electromyographic amplitude and mean power frequency during cycle ergometry, J. Neurosci. Methods, 2009, 184, 256–262 CrossRef PubMed.
- A. Moin, et al., A wearable biosensing system with in-sensor adaptive machine learning for hand gesture recognition, Nat. Electron., 2020, 4, 54–63 CrossRef.
- R. J. Hariman, et al., Method for recording electrical activity of the sinoatrial node and automatic atrial foci during cardiac catheterization in human subjects, Am. J. Cardiol., 1980, 45, 775–781 CrossRef CAS PubMed.
- J. T. Korpelainen, K. A. Sotaniemi, H. V. Huikuri and V. V. Myllya, Abnormal heart rate variability as a manifestation of autonomic dysfunction in hemispheric brain infarction, Stroke, 1996, 27, 2059–2063 CrossRef CAS PubMed.
- G. R. Ghearing, T. M. Munger, A. S. Jaffe, E. E. Benarroch and J. W. Britton, Clinical cues for detecting ictal asystole, Clin. Auton. Res., 2007, 17, 221–226 CrossRef PubMed.
- S. P. Nelwan, J. A. Kors, S. H. Meij, J. H. van Bemmel and M. L. Simoons, Reconstruction of the 12-lead electrocardiogram from reduced lead sets, J. Electrocardiol., 2004, 37, 11–18 CrossRef PubMed.
- Y. S. Kim, et al., All-in-One, Wireless, Stretchable Hybrid Electronics for Smart, Connected, and Ambulatory Physiological Monitoring, Adv. Sci., 2019, 6, 1900939 CrossRef PubMed.
- H. Yang, et al., Adhesive Biocomposite Electrodes on Sweaty Skin for Long-Term Continuous Electrophysiological Monitoring, ACS Mater. Lett., 2020, 2, 478–484 CrossRef CAS.
- N. Zavanelli, et al., At-home wireless monitoring of acute hemodynamic disturbances to detect sleep apnea and sleep stages via a soft sternal patch, Sci. Adv., 2021, 7, eabl4146 CrossRef PubMed.
- Y. Deng, et al., Large-area stretchable dynamic 18-lead ECG monitoring patch integrated with deep learning for cardiovascular disease diagnosis, Cell Rep. Phys. Sci., 2024, 5, 102077 CrossRef CAS.
- D. Li, et al., Motion-unrestricted dynamic electrocardiogram system utilizing imperceptible electronics, Nat. Commun., 2025, 16, 3259 CrossRef CAS PubMed.
- R. Barea, L. Boquete, S. Ortega, E. López and J. M. Rodríguez-Ascariz, EOG-based eye movements codification for human computer interaction, Expert Syst. Appl., 2012, 39, 2677–2683 CrossRef.
- Y. Terao, H. Fukuda and O. Hikosaka, What do eye movements tell us about patients with neurological disorders? – An introduction to saccade recording in the clinical setting, Proc. Jpn. Acad., Ser. B, 2017, 93, 772–801 CrossRef PubMed.
- J. S. Li, et al., High-Performance Flexible Microneedle Array as a Low-Impedance Surface Biopotential Dry Electrode for Wearable Electrophysiological Recording and Polysomnography, Nano-Micro Lett., 2022, 14, 132 CrossRef CAS PubMed.
- S. K. Ameri, et al., Imperceptible electrooculography graphene sensor system for human-robot interface, npj 2D Mater. Appl., 2018, 2, 19 CrossRef.
- L. Zhang, et al., Fully organic compliant dry electrodes self-adhesive to skin for long-term motion-robust epidermal biopotential monitoring, Nat. Commun., 2020, 11, 4683 CrossRef CAS PubMed.
- M. Reis Carneiro, A. T. de Almeida, M. Tavakoli and C. Majidi, Recyclable Thin-Film Soft Electronics for Smart Packaging and E-Skins, Adv. Sci., 2023, 10, e2301673 CrossRef PubMed.
- S. Zheng, et al., Pressure-stamped stretchable electronics using a nanofibre membrane containing semi-embedded liquid metal particles, Nat. Electron., 2024, 7, 576–585 CrossRef CAS.
- P.-H. Chen, R.-L. Wang, D.-J. Liou and J.-S. Shaw, Gait Disorders in Parkinson's Disease: Assessment and Management, Int. J. Gerontol., 2013, 7, 189–193 CrossRef.
- S. Niedermeyer, M. Murn and P. J. Choi, Respiratory Failure in Amyotrophic Lateral Sclerosis, Chest, 2019, 155, 401–408 CrossRef PubMed.
- B. Fauroux, et al., Respiratory management of children with spinal muscular atrophy (SMA), Arch. Pediatr., 2020, 27, 7S29–27S34 CrossRef CAS PubMed.
- S. Papapetropoulos and C. Singer, Eating dysfunction associated with oromandibular dystonia: clinical characteristics and treatment considerations, Head Face Med., 2006, 2, 47 CrossRef PubMed.
- X. Wang, J. Yu, Y. Cui and W. Li, Research progress of flexible wearable pressure sensors, Sens. Actuators, A, 2021, 330, 112838 CrossRef CAS.
- A. Yadav, N. Yadav, Y. Wu, S. RamaKrishna and Z. Hongyu, Wearable strain sensors: state-of-the-art and future applications, Mater. Adv., 2023, 4, 1444–1459 RSC.
- R. L. Bulathsinghala, W. Ding and R. D. I. G. Dharmasena, Triboelectric nanogenerators for wearable sensing applications: A system level analysis, Nano Energy, 2023, 116, 108792 CrossRef CAS.
- Y. Wu, Y. Ma, H. Zheng and S. Ramakrishna, Piezoelectric materials for flexible and wearable electronics: A review, Mater. Des., 2021, 211, 110164 CrossRef CAS.
- H. Chen, et al., An AI-enabled self-sustaining sensing lower-limb motion detection system for HMI in the metaverse, Nano Energy, 2025, 136, 110724 CrossRef CAS.
- J. Ahn, et al., Skin-Conformal Motion Monitoring Film for Deep-Learning-Based Immersive Extended Reality, Adv. Funct. Mater., 2025, 2502568 CrossRef.
- H. Xia, et al., MXene/PPy@PDMS sponge-based flexible pressure sensor for human posture recognition with the assistance of a convolutional neural network in deep learning, Microsyst. Nanoeng., 2023, 9, 155 CrossRef CAS PubMed.
- M. Wang, et al., Gesture recognition using a bioinspired learning architecture that integrates visual data with somatosensory data from stretchable sensors, Nat. Electron., 2020, 3, 563–570 CrossRef.
- F. Yi, et al., Stretchable-Rubber-Based Triboelectric Nanogenerator and Its Application as Self-Powered Body Motion Sensors, Adv. Funct. Mater., 2015, 25, 3688–3696 CrossRef CAS.
- T. Wang, Y. Zhao and Q. Wang, A Flexible Iontronic Capacitive Sensing Array for Hand Gesture Recognition Using Deep Convolutional Neural Networks, Soft Robot., 2023, 10, 443–453 CrossRef PubMed.
- A. Cheng, et al., An intelligent hybrid-fabric wristband system enabled by thermal encapsulation for ergonomic human-machine interaction, Nat. Commun., 2025, 16, 591 CrossRef CAS PubMed.
- S. Wang, et al., Network cracks-based wearable strain sensors for subtle and large strain detection of human motions, J. Mater. Chem. C, 2018, 6, 5140–5147 RSC.
- J. Zhong, et al., Smart Face Mask Based on an Ultrathin Pressure Sensor for Wireless Monitoring of Breath Conditions, Adv. Mater., 2022, 34, e2107758 CrossRef PubMed.
- J. Shin, et al., Sensitive Wearable Temperature Sensor with Seamless Monolithic Integration, Adv. Mater., 2020, 32, e1905527 CrossRef PubMed.
- J. C. Rosenbek and M. S. Troche, Progressive neurologic disease and dysphagia (including Parkinson's disease, multiple sclerosis, amyotrophic lateral sclerosis, myasthenia gravis, post-polio syndrome), Principles of deglutition: a multidisciplinary text for swallowing and its disorders, pp. 395–409. 2013 Search PubMed.
- B. Patel, J. Legacy, K. W. Hegland, M. S. Okun and N. E. Herndon, A comprehensive review of the diagnosis and treatment of Parkinson's disease dysphagia and aspiration, Expert Rev. Gastroenterol. Hepatol., 2020, 14, 411–424 CrossRef CAS PubMed.
- J. H. Lee, K. H. Cho and K. Cho, Emerging Trends in Soft Electronics: Integrating Machine Intelligence with Soft Acoustic/Vibration Sensors, Adv. Mater., 2023, 35, 2209673 CrossRef CAS PubMed.
- M. Chao, et al., Wearable MXene nanocomposites-based strain sensor with tile-like stacked hierarchical microstructure for broad-range ultrasensitive sensing, Nano Energy, 2020, 78, 105187 CrossRef CAS.
- Q. Zhang, et al., Flexible multifunctional platform based on piezoelectric acoustics for human-machine interaction and environmental perception, Microsyst. Nanoeng., 2022, 8, 99 CrossRef PubMed.
- J. Zhang, et al., Highly Stretchable and Self-Healable MXene/Polyvinyl Alcohol Hydrogel Electrode for Wearable Capacitive Electronic Skin, Adv. Electron. Mater., 2019, 5, 1900285 CrossRef.
- Y. H. Liu, et al., All-natural phyllosilicate-polysaccharide triboelectric sensor for machine learning-assisted human motion prediction, npj Flexible Electron., 2023, 7, 21 CrossRef.
- T. Duning, et al., Brainstem involvement as a cause of central sleep apnea: pattern of microstructural cerebral damage in patients with cerebral microangiopathy, PLoS One, 2013, 8, e60304 CrossRef CAS PubMed.
- F. Racca, et al., Practical approach to respiratory emergencies in neurological diseases, Neurol. Sci., 2020, 41, 497–508 CrossRef PubMed.
- D. Kim, J. Lee, M. K. Park and S. H. Ko, Recent developments in wearable breath sensors for healthcare monitoring, Commun. Mater., 2024, 5, 41 CrossRef CAS.
- M. Sang, et al., Ultrahigh Sensitive Au-Doped Silicon Nanomembrane Based Wearable Sensor Arrays for Continuous Skin Temperature Monitoring with High Precision, Adv. Mater., 2022, 34, 2105865 CrossRef CAS PubMed.
- W. Hong, Twistable and Stretchable Nasal Patch for Monitoring Sleep-Related Breathing Disorders Based on a Stacking Ensemble Learning Model, ACS Appl. Mater. Interfaces, 2024, 16, 47337–47347 CrossRef CAS PubMed.
- L. Ai, et al., Tough soldering for stretchable electronics by small-molecule modulated interfacial assemblies, Nat. Commun., 2023, 14, 7723 CrossRef CAS PubMed.
- Q. N. Zhuang, et al., Permeable, three-dimensional integrated electronic skins with stretchable hybrid liquid metal solders, Nat. Electron., 2024, 7, 598–609 CrossRef CAS.
- M. Kim, J. J. Park, C. Cho and S. H. Ko, Liquid Metal based Stretchable Room Temperature Soldering Sticker Patch for Stretchable Electronics Integration, Adv. Funct. Mater., 2023, 33, 2303286 CrossRef CAS.
- X. Liu, et al., Stiffness and Interface Engineered Soft Electronics with Large-Scale Robust Deformability, Adv. Mater., 2024, 36, e2407886 CrossRef PubMed.
- M. Kim, et al., A Gradient Stiffness-Programmed Circuit Board by Spatially Controlled Phase-Transition of Supercooled Hydrogel for Stretchable Electronics Integration, Adv. Mater., 2024, 36, e2313344 CrossRef PubMed.
- Y. Shao, et al., A universal packaging substrate for mechanically stable assembly of stretchable electronics, Nat. Commun., 2024, 15, 6106 CrossRef CAS PubMed.
- L. Tang, H. Wang, J. Ren and X. Jiang, Highly robust soft-rigid connections via mechanical interlocking for assembling ultra-stretchable displays, npj Flexible Electron., 2024, 8, 50 CrossRef.
- Y. Jiang, et al., A universal interface for plug-and-play assembly of stretchable devices, Nature, 2023, 614, 456–462 CrossRef CAS PubMed.
- Y. Song, et al., Wireless battery-free wearable sweat sensor powered by human motion, Sci. Adv., 2020, 6, eaay9842 CrossRef CAS PubMed.
- L. Yin, et al., A self-sustainable wearable multi-modular E-textile bioenergy microgrid system, Nat. Commun., 2021, 12, 1542 CrossRef CAS PubMed.
- J. Kim, et al., Cost-effective and strongly integrated fabric-based wearable piezoelectric energy harvester, Nano Energy, 2020, 75, 104992 CrossRef CAS.
- B. Lee, et al., High-performance compliant thermoelectric generators with magnetically self-assembled soft heat conductors for self-powered wearable electronics, Nat. Commun., 2020, 11, 5948 CrossRef CAS PubMed.
- Y. Jung, S. Jeong, J. Ahn, J. Lee and S. H. Ko, High Efficiency Breathable Thermoelectric Skin Using Multimode Radiative Cooling/Solar Heating Assisted Large Thermal Gradient, Small, 2024, 20, 2304338 CrossRef CAS PubMed.
- Y. Jung, et al., Soft multi-modal thermoelectric skin for dual functionality of underwater energy harvesting and thermoregulation, Nano Energy, 2022, 95, 107002 CrossRef CAS.
- Y. Jung, et al., All Weather-Usable Wearable Dual Energy Harvester for Outdoor Sustainable Operation, SusMat, 2025, 5, e264 CrossRef CAS.
- J. Min, et al., An autonomous wearable biosensor powered by a perovskite solar cell, Nat. Electron., 2023, 6, 630–641 CrossRef CAS PubMed.
- Y. Yu, et al., Biofuel-powered soft electronic skin with multiplexed and wireless sensing for human-machine interfaces, Sci. Rob., 2020, 5, eaaz7946 CrossRef PubMed.
- A. J. Bandodkar, et al., Soft, stretchable, high power density electronic skin-based biofuel cells for scavenging energy from human sweat, Energy Environ. Sci., 2017, 10, 1581–1589 RSC.
- S. H. Kim, et al., Strain-invariant stretchable radio-frequency electronics, Nature, 2024, 629, 1047–1054 CrossRef CAS PubMed.
- R. Lin, et al., Digitally-embroidered liquid metal electronic textiles for wearable wireless systems, Nat. Commun., 2022, 13, 2190 CrossRef CAS PubMed.
- P. Escobedo, et al., Smart facemask for wireless CO(2) monitoring, Nat. Commun., 2022, 13, 72 CrossRef CAS PubMed.
- L. Yin, et al., High performance printed AgO–Zn rechargeable battery for flexible electronics, Joule, 2021, 5, 228–248 CrossRef CAS.
- L. Yin, et al., A stretchable epidermal sweat sensing platform with an integrated printed battery and electrochromic display, Nat. Electron., 2022, 5, 694–705 CrossRef.
- A. Bandodkar, et al., Sweat-activated biocompatible batteries for epidermal electronic and microfluidic systems, Nat. Electron., 2020, 3, 554–562 CrossRef CAS.
- L. Jiang, L. Yuan, W. Wang and Q. Zhang, Soft materials for wearable supercapacitors, Soft Sci., 2021, 1, 5 CAS.
- B. Shin, et al., Automatic Clinical Assessment of Swallowing Behavior and Diagnosis of Silent Aspiration Using Wireless Multimodal Wearable Electronics, Adv. Sci., 2024, 11, e2404211 CrossRef PubMed.
- T. Someya, et al., A large-area, flexible pressure sensor matrix with organic field-effect transistors for artificial skin applications, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 9966–9970 CrossRef CAS PubMed.
- T. Yamada, et al., A stretchable carbon nanotube strain sensor for human-motion detection, Nat. Nanotechnol., 2011, 6, 296–301 CrossRef CAS PubMed.
- D.-H. Kim, et al., Epidermal electronics, Science, 2011, 333, 838–843 CrossRef CAS PubMed.
- W. Gao, et al., Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis, Nature, 2016, 529, 509–514 CrossRef CAS PubMed.
- J. Remi, T. Pollmacher, K. Spiegelhalder, C. Trenkwalder and P. Young, Sleep-Related Disorders in Neurology and Psychiatry, Dtsch. Arztebl. Int., 2019, 116, 681–688 Search PubMed.
- S. M. Rothman and M. P. Mattson, Sleep disturbances in Alzheimer's and Parkinson's diseases, NeuroMol. Med., 2012, 14, 194–204 CrossRef CAS PubMed.
- B. Jafari and V. Mohsenin, Polysomnography, Clin. Chest Med., 2010, 31, 287–297 CrossRef PubMed.
- O. Le Bon, Relationships between REM and NREM in the NREM-REM sleep cycle: a review on competing concepts, Sleep Med., 2020, 70, 6–16 CrossRef PubMed.
- N. Cooray, et al., Detection of REM sleep behaviour disorder by automated polysomnography analysis, Clin. Neurophysiol., 2019, 130, 505–514 CrossRef PubMed.
- B. Frauscher, et al., Rapid Eye Movement Sleep Sawtooth Waves Are Associated with Widespread Cortical Activations, J. Neurosci., 2020, 40, 8900–8912 CrossRef CAS PubMed.
- S. Oz, et al., Monitoring sleep stages with a soft electrode array: Comparison against vPSG and home-based detection of REM sleep without atonia, J. Sleep Res., 2023, 32, e13909 CrossRef PubMed.
- J. Nonnekes, et al., Neurological disorders of gait, balance and posture: a sign-based approach, Nat. Rev. Neurol., 2018, 14, 183–189 CrossRef PubMed.
- Movement Disorder Society Task Force on Rating Scales for Parkinson's, Disease, The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations, Mov. Disord, 2003, 18, 738–750 CrossRef PubMed.
- J. F. Kurtzke, Historical and clinical perspectives of the expanded disability status scale, Neuroepidemiology, 2008, 31, 1–9 Search PubMed.
- I. Klopfer-Kramer, et al., Gait analysis - Available platforms for outcome assessment, Injury, 2020, 51(Suppl 2), S90–S96 CrossRef PubMed.
- C. Moreau, et al., Overview on wearable sensors for the management of Parkinson's disease, NPJ Parkinsons Dis., 2023, 9, 153 CrossRef PubMed.
- A. S. Gupta, S. Patel, A. Premasiri and F. Vieira, At-home wearables and machine learning sensitively capture disease progression in amyotrophic lateral sclerosis, Nat. Commun., 2023, 14, 5080 CrossRef CAS PubMed.
- S. Alexander, G. Peryer, E. Gray, F. Barkhof and J. Chataway, Wearable technologies to measure clinical outcomes in multiple sclerosis: A scoping review, Mult. Scler., 2021, 27, 1643–1656 CrossRef PubMed.
- B. Shin, et al., All-in-one wearable drug efficacy assessment systems for bulbar muscle function using amyotrophic lateral sclerosis animal models, Nat. Commun., 2024, 15, 6803 CrossRef CAS PubMed.
- C. Tefertiller, B. Pharo, N. Evans and P. Winchester, Efficacy of rehabilitation robotics for walking training in neurological disorders: a review, J. Rehabil. Res. Dev., 2011, 48, 387–416 CrossRef PubMed.
- D. Esposito, et al., Biosignal-Based Human-Machine Interfaces for Assistance and Rehabilitation: A Survey, Sensors, 2021, 21, 6863 CrossRef PubMed.
- J. Paulo, P. Peixoto and U. J. Nunes, ISR-AIWALKER: Robotic Walker for Intuitive and Safe Mobility Assistance and Gait Analysis, IEEE Trans. Hum.-Mach. Syst., 2017, 47, 1110–1122 Search PubMed.
- K. E. Laver, et al., Virtual reality for stroke rehabilitation, Cochrane Database Syst. Rev., 2017, 11, CD008349 Search PubMed.
- W. Wang, et al., Neural interface technology for rehabilitation: exploiting and promoting neuroplasticity, Phys. Med. Rehabil. Clin. North Am., 2010, 21, 157–178 CrossRef PubMed.
- M. Mahmood, et al., Fully portable and wireless universal brain–machine interfaces enabled by flexible scalp electronics and deep learning algorithm, Nat. Mach. Intell., 2019, 1, 412–422 CrossRef.
- D. Won, et al., Transparent electronics for wearable electronics application, Chem. Rev., 2023, 123, 9982–10078 CrossRef CAS PubMed.
- J. Tu and W. Gao, Ethical Considerations of Wearable Technologies in Human Research, Adv. Healthcare Mater., 2021, 10, e2100127 CrossRef PubMed.
- E. Capulli, et al., Ethical and legal implications of health monitoring wearable devices: A scoping review, Soc. Sci. Med., 2025, 370, 117685 CrossRef PubMed.
- Y. J. Kang, et al., Soft skin-interfaced mechano-acoustic sensors for real-time monitoring and patient feedback on respiratory and swallowing biomechanics, NPJ Digital Med., 2022, 5, 147 CrossRef PubMed.
- I. Ioannidou and N. Sklavos, On general data protection regulation vulnerabilities and privacy issues, for wearable devices and fitness tracking applications, Cryptography, 2021, 5, 29 CrossRef.
- S. Banerjee, T. Hemphill and P. Longstreet, Wearable devices and healthcare: Data sharing and privacy, Inf. Soc., 2018, 34, 49–57 CrossRef.
- T. Zhang, et al., Federated learning for the internet of things: Applications, challenges, and opportunities, IEEE Int. Things Mag., 2022, 5, 24–29 Search PubMed.
- M. Merenda, C. Porcaro and D. Iero, Edge Machine Learning for AI-Enabled IoT Devices: A Review, Sensors, 2020, 20, 2533 CrossRef PubMed.
- F. Lopes, et al., Automatic electroencephalogram artifact removal using deep convolutional neural networks, IEEE Access, 2021, 9, 149955–149970 Search PubMed.
- T. Gao, D. Chen, Y. Tang, Z. Ming and X. Li, EEG reconstruction with a dual-scale CNN-LSTM model for deep artifact removal, IEEE J. Biomed. Health Inf., 2022, 27, 1283–1294 Search PubMed.
- M. Perslev, et al., U-Sleep: resilient high-frequency sleep staging, NPJ Digital Med., 2021, 4, 72 CrossRef PubMed.
- S. Zhou, et al., Continuous sleep depth index annotation with deep learning yields novel digital biomarkers for sleep health, NPJ Digital Med., 2025, 8, 203 CrossRef PubMed.
- A. S. Chandrabhatla, I. J. Pomeraniec and A. Ksendzovsky, Co-evolution of machine learning and digital technologies to improve monitoring of Parkinson's disease motor symptoms, NPJ Digital Med., 2022, 5, 32 CrossRef PubMed.
- X. Zhang, X. Zhang, Q. Huang and F. Chen, A review of epilepsy detection and prediction methods based on EEG signal processing and deep learning, Front. Neurosci., 2024, 18, 1468967 CrossRef PubMed.
- H. Li, et al., A sequential learning model with GNN for EEG-EMG-based stroke rehabilitation BCI, Front. Neurosci., 2024, 17, 1125230 CrossRef PubMed.
- C. Fang, B. He, Y. Wang, J. Cao and S. Gao, EMG-Centered Multisensory Based Technologies for Pattern Recognition in Rehabilitation: State of the Art and Challenges, Biosensors, 2020, 10, 85 CrossRef PubMed.
- N. Kueper, S. K. Kim and E. A. Kirchner, Avoidance of specific calibration sessions in motor intention recognition for exoskeleton-supported rehabilitation through transfer learning on EEG data, Sci. Rep., 2024, 14, 16690 CrossRef CAS PubMed.
- P. J. Lin, et al., A Transferable Deep Learning Prognosis Model for Predicting Stroke Patients' Recovery in Different Rehabilitation Trainings, IEEE J. Biomed. Health Inf., 2022, 26, 6003–6011 Search PubMed.
- R. Mane, Z. Wu and D. Wang, Poststroke motor, cognitive and speech rehabilitation with brain-computer interface: a perspective review, Stroke Vasc. Neurol., 2022, 7, 541–549 CrossRef PubMed.
- D. Lahat, T. Adali and C. Jutten, Multimodal data fusion: an overview of methods, challenges, and prospects, Proc. IEEE, 2015, 103, 1449–1477 Search PubMed.
- Y. Zhang, S. Qiu and H. He, Multimodal motor imagery decoding method based on temporal spatial feature alignment and fusion, J. Neural Eng., 2023, 20, 026009 CrossRef PubMed.
- J. Duan, J. Xiong, Y. Li and W. Ding, Deep learning based multimodal biomedical data fusion: An overview and comparative review, Inf. Fusion, 2015, 102536 Search PubMed.
- M. Pawlowski, A. Wroblewska and S. Sysko-Romanczuk, Effective Techniques for Multimodal Data Fusion: A Comparative Analysis, Sensors, 2023, 23, 2381 CrossRef PubMed.
- G. K. Verma and U. S. Tiwary, Multimodal fusion framework: a multiresolution approach for emotion classification and recognition from physiological signals, NeuroImage, 2014, 102(Pt 1), 162–172 CrossRef PubMed.
- E. Debie, et al., Multimodal fusion for objective assessment of cognitive workload: A review, IEEE Trans. Cybern., 2019, 51, 1542–1555 Search PubMed.
Footnote |
| † These authors contributed equally: D. Kim, J. Bang. |
| This journal is © The Royal Society of Chemistry 2025 |




