Open Access Article
Open Access ArticleMagnetically recoverable MFe12O19 nanoparticles as efficient and environmentally benign catalysts for gram-scale selective oxidation of olefins
Mouhsine
Laayati
abe,
Ayoub Abdelkader
Mekkaoui
 *b,
Ahsen Sare
Yalın
c,
Abdelhamid
El Boubekri
d,
Mohammed
Sajieddine
d,
Larbi
El Firdoussi
b,
Antonia
Neels
*b,
Ahsen Sare
Yalın
c,
Abdelhamid
El Boubekri
d,
Mohammed
Sajieddine
d,
Larbi
El Firdoussi
b,
Antonia
Neels
 e,
Önder
Metin
e,
Önder
Metin
 cf and
Soufiane
El Houssame
cf and
Soufiane
El Houssame
 *a
*a
aLaboratoire des Sciences des Matériaux, Mathématiques et Environnement, Université Sultan Moulay Slimane, Faculté Polydisciplinaire de Khouribga, BP 145, Khouribga 25000, Morocco. E-mail: hous_soufiane@hotmail.com
bCadi Ayyad University, UCA, Faculté des Sciences Semlalia Marrakech, Département de Chimie, Laboratoire de Chimie Moléculaire, Equipe de Chimie de Coordination et de Catalyse, BP 2390, Marrakech 40001, Morocco. E-mail: mekk.ayoub@gmail.com; a.mekkaoui@uca.ac.ma
cDepartment of Chemistry, College of Sciences, Koç University, 34450 Istanbul, Turkey
dLaboratoire Génie Energétique et Matériaux, Université Sultan Moulay Slimane, Faculté des Sciences et Techniques, BP 523, Béni Mellal 23000, Morocco
eCenter for X-Ray Analytics, Empa - Swiss Federal Laboratories for Materials Science and Technology, Überlandstrasse 129, 8600 Dübendorf, Switzerland
fKoç University Surface Science and Technology Center (KUYTAM), Koç University, Istanbul 34450, Turkey
First published on 25th March 2025
Abstract
Catalytic oxidation is an efficient route for synthesizing oxygenated compounds such as epoxides and aldehydes. However, developing cost-effective, environmentally friendly and selective gram-scale catalysts, in full agreement with circular economy and green chemistry principles, remains a significant challenge. Herein, we report on MFe12O19 magnetic nanoparticles (MNPs) as a novel, magnetically recoverable and selective catalyst for the oxidation of olefins. Three different MFe12O19 (M = Cu, Sn and Sr) MNPs were synthesized using the coprecipitation method and characterized by XRD, FTIR, Raman, XPS, SEM-EDX, TEM, BET, zeta potential and 57Fe Mössbauer spectroscopy. XRD analysis demonstrates that the patterns of SnFe12O19 and CuFe12O19 are totally different from those of the magnetoplumbite hexaferrite structure due to the confirmed coexistence by Rietveld refinements of SnO2–Fe2O3 and CuFe2O4–Fe2O3 as composite structures, respectively. In good agreement, Raman studies exclusively confirms the coexistence of MOx−Fe2O3 as a composite structure in MFe12O19, due to the presence of α-Fe2O3 and γ-Fe2O3 as intermediate phases during the formation process of the hexaferrite structure. Moreover, the XPS and Mössbauer results are consistent with the experimental evidence and spectroscopic characterizations. Subsequently, the catalytic activity of the as-synthesized MNPs was evaluated for the oxidation of styrene as a model olefinic substrate. Among the as-prepared MFe12O19 MNPs, the composite structure CuFe2O4–Fe2O3 in CuFe12O19 effectively enhances catalytic activity, selectivity and reusability due to the synergistic catalytic effect within a single magnetically recoverable nanostructure. Overall, MFe12O19 MNPs present a facile and greener approach using magnetically recyclable hexaferrites for selective catalytic oxidation reactions.
1. Introduction
Styrene oxide and benzaldehyde are important organic intermediates widely used in the industrial manufacture of fragrances, pharmaceuticals, and organic synthesis.1–4 Hence, the oxidation of olefins has been widely regarded as one of the most important routes to accessing epoxides and aldehydes.5 To date, both homogeneous and heterogeneous catalysts have been extensively reported for the oxidation of olefins.6–15 Catalysis plays an important role in green chemistry by enabling less polluting chemical processes and providing sustainable pathways to synthesize desired products.16 Heterogeneous catalysts are widely preferred over homogeneous ones owing to their recyclability and atom economy, which contribute to sustainability by reducing time- and energy-consuming catalytic processes in alignment with the circular economy. Particular attention has been given to the development of selective heterogenous catalysts for the oxidation of styrene to benzaldehyde or styrene oxide. Magnetic nanoparticles (MNPs) are especially attractive candidates for this reaction owing to their high activity, selectivity, and facile reusability.17 Moreover, MNPs exhibit numerous advantageous properties, including a large surface-to-volume ratio, good crystallinity, excellent thermal stability and homogeneous composition as heterogeneous bulk catalysts, all of which contribute to their high catalytic performance.17–20M-type hexaferrite, with a chemical formula of MFe12O19, is a class of promising chemically and thermally stable magnetic materials for a wide range of applications. Regarding their high magneto-crystalline anisotropy with a single easy magnetization axis, they are classified as hard magnets with high coercivity.21 They have been widely used as permanent magnets, recording media, photocatalysts and components in microwave, telecommunication, higher-frequency, and magnetooptical devices.22–29 Moreover, special attention has been given to magnetic materials based on barium, strontium, palladium and lead with unique properties.30 Previously, Ansari et al. reported on the synthesis of CuFe12O19 nanostructures by using sol–gel auto-combustion method and its magnetic properties.31 Recently, SnFe12O19 nanostructures were synthesized through ultrasonic irradiation and utilized in an environmental application.32 Based on the current understanding, the XRD patterns of these reported structures MFe12O19 (M = Cu or Sn) are not yet confirmed, and they are totally different from those of the magnetoplumbite hexaferrite structure. Hence, there is a lack of advanced spectroscopic investigations in the reported literature to accurately assess the structural properties of such MFe12O19 compounds. For this reason, Cu and Sn have been selected to study the obtained MFe12O19 compounds and compare them with the SrFe12O19 M-type hexaferrite.
In the last decades, owing to their magnetic properties, M-type hexaferrites have attracted the attention of scientists in various fields of catalytic applications. Recently, the SrFe12O19 M-type hexaferrite was reported as high catalytically active MNPs for the improvement of the catalytic epoxide ring-opening reaction with amines, as pristine33 or in hybrid graphene-derived nanocomposites,34 as well as in the synthesis of 2-amino-4,6-diphenylnicotinonitrile35 and 1,5-benzodiazepine derivatives.36 However, the presumed MFe12O19 (M = Cu or Sn) nanoparticles have only been reported in a few studies. According to the literature, CuFe12O19 MNPs and the grafted ones on CNT have only been reported for photocatalytic elimination of water contamination.37,38 To the best of our knowledge, CuFe12O19 and SnFe12O19 nanoparticles have not yet been used as heterogeneous catalysts for any application. In this regard, the growing potential of hexaferrite MNPs in catalysis motivates further study of their catalytic activity in alignment with green chemistry principles and circular economy.
In this study, we focus on the development of novel hexaferrite nanostructures as magnetically recoverable nanoparticles (MNPs) for catalytic applications, leveraging their magnetic properties, stability and high specific surface area. Various chemical methods, including sol–gel,31,39,40 salt melting process,41 sonochemical32,42 and coprecipitation,33,43 have previously been employed for the synthesis of M-type hexaferrites. Among these, the chemical coprecipitation method stands out due to its simplicity and precision in controlling the grain size, making it an excellent approach for preparing magnetic oxide nanoparticles. Herein, we present the synthesis of CuFe12O19, SnFe12O19 and SrFe12O19 NPs using a reproducible coprecipitation method. To the best of our knowledge, this is the first study to evaluate the catalytic performance of as-prepared MFe12O19 NPs in the oxidation of styrene derivatives, chosen as model substrates for olefin oxidation.
2. Experimental
2.1. Materials
All reagents and solvents were obtained from commercial suppliers (Aldrich and Acros), and used directly without further purification. As-synthesized nanoparticles (NPs) were characterized by X'Pert MPD Powder instrument from Malvern Panalytical with Cu Kα radiation (λCu = 1.5406 Å). A Bruker vertex 70 DTGSFTIR spectrophotometer was used to record the stretching vibrational frequencies of NPs in the range of 400–4000 cm−1. X-ray photoelectron spectra (XPS) were obtained using a Thermo Scientific K-Alpha spectrometer using an aluminum anode (Al Kα1/4 1468.3 eV). The binding energies were calibrated by referencing the C 1s signal at 284.4 eV. Morphology and topology of the materials were characterized by using a scanning electron microscope (SEM) (FEI, Quanta FEG 450). Transmission Mössbauer spectra were collected by using a conventional constant acceleration spectrometer with a 25 mCi source 57Co sealed in a Rh matrix at room temperature. In this configuration, the γ-ray direction is perpendicular to the powder plane. NORMOS DIST program was used to interpret the experimental spectra.44 All isomer shift values are reported with respect to that of the α-iron foil at room temperature. Transmission electron microscope (TEM) images were recorded on a 120 kV Hitachi HT7800 TEM instrument with EXALENS module capable of working at high-resolution (HR) mode in the magnification range of 10–600k×. BET measurements were conducted using the Micromeritics Tristar II automated gas sorption system. The zeta potential was measured using a Malvern Panalytical Zetasizer, after dispersing 0.1 g of nanopowders in 3 ml of solvent (acetonitrile; acetone or deionized water). Aliquot samples from the reaction mixture were monitored by gas chromatography (GC, Shimadzu) equipped with a flame ionization detector and nitrogen as the carrier gas. The GC conditions for the BP capillary columns (25 m × 0.25 mm, SGE) were set as follows: injector temperature at 250 °C; detector temperature at 250 °C and oven temperature initially set at 70 °C for 5 min, followed by a ramp of 3 °C min−1 until reaching 250 °C for 30 min. The column pressure was maintained at 20 kPa with a flow rate of 6.3 ml min−1, linear velocity of 53.1 cm s−1 and total flow of 138 ml min−1. The products were confirmed by injecting the reaction mixture into an ISQ LT single quadrupole mass spectrometer, operating in positive EI mode, and scannig a mass range of 50 to 400 m/z.2.2. Synthesis of the MFe12O19 nanoparticles
The magnetic MFe12O19 (M = Sr; Cu; or Sn) nanoparticles were synthesized by following a coprecipitation protocol.33,34 In a typical synthesis protocol, stoichiometric quantities of metal chlorides (SrCl2·6H2O; CuCl2; SnCl2·2H2O; FeCl3) were individually dissolved in 30 ml of deionized water to form homogeneous solutions. These solution were then mixed and stirred for 30 min at 80 °C. Next, pH of the reaction mixture was adjusted to 11–12 via dropwise addition of NaOH (1.5 M) under continuous stirring for 1.5–2 h at 80 °C, ensuring the homogeneity of the mixture. The precipitated CuFe12O19 and SnFe12O19 nanopowders were separated magnetically. In contrast, SnFe12O19 was separated via centrifugation due to the lack of its magnetization. The prepared MFe12O19 nanopowders were washed several times with deionized water to remove the excess salt and dried at 80 °C overnight. The obtained nanopowders were calcined at 900 °C for 8 h to obtain the pure hexaferrites.2.3. Catalytic studies
3. Results and discussion
3.1. MFe12O19 characterization
The structures of the as-synthesized MFe12O19 NPs (M = Cu, Sn and Sr) were first characterized by X-ray diffraction (XRD) (Fig. 1). While all the diffraction lines of CuFe12O19 MNPs match the reported ones in the literature,31 the diffraction patterns of CuFe2O4 (JCPDS: 77-0010) and Fe2O3 (JCPDS: 24-0072) reveal the coexistence of CuFe2O4–Fe2O3 as a composite structure in the as-synthesized CuFe12O19 MNPs. Accordingly, while the XRD pattern of SnFe12O19 also matched the reported literature,32 the crystalline phases of SnO2 (JCPDS: 72-1147) and Fe2O3 (JCPDS: 24-0072) indicate the coexistence of SnO2–Fe2O3 as possible composite structure. However, the XRD pattern of the as-prepared SrFe12O19 presents a very similar diffraction pattern as reported for the magnetoplumbite hexaferrite structure.34 In addition, the SrFe12O19 crystal phase clearly matches well with the known strontium hexaferrite crystal phase (JCPDS: 33-1340). No obvious peaks of metal oxides (SrO and Fe2O3) are observed, clearly indicating only the presence of the pure SrFe12O19 hexaferrite structure.Based on the XRD results, Rietveld refinement was carried out to accurately assess the observed structural differences of both compounds CuFe12O19 and SnFe12O19 compared to the magnetoplumbite M-type hexaferrite structure of the SrFe12O19 MNPs. The recorded and Rietveld refined XRD patterns of the as-presumed prepared hexaferrites are displayed in Fig. 2. Clearly, in SnFe12O19 and CuFe12O19, the refined XRD patterns exhibit a combination of two distinct parent phases. The refinement confirmed that CuFe12O19 exhibits a spinel structure with the Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group and hematite (Fe2O3) as the main phase (73.5%). However, the SnFe12O19 XRD pattern presents the hematite phase (92.5%) as the major component and the minor crystalline phase of SnO2 (7.5%). Table 1 presents the fitting parameters and crystallite sizes (D), using the most intense and single indexed reflection of all phases, as found in the as-prepared MFe12O19 NPs.
m space group and hematite (Fe2O3) as the main phase (73.5%). However, the SnFe12O19 XRD pattern presents the hematite phase (92.5%) as the major component and the minor crystalline phase of SnO2 (7.5%). Table 1 presents the fitting parameters and crystallite sizes (D), using the most intense and single indexed reflection of all phases, as found in the as-prepared MFe12O19 NPs.
| CuFe12O19 = {CuFe2O4–Fe2O3} | SnFe12O19 = {SnO2–Fe2O3} | SrFe12O19 | |||
|---|---|---|---|---|---|
| Phase | CuFe2O4 | Fe2O3 | SnO2 | Fe2O3 | SrFe12O19 |
| Crystal structure | Cubic spinel | Hexagonal | Tetragonal | Hexagonal | Hexagonal |
| Group space |
Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
P42/mnm |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
P63/mmc |
| Composition (%) | 23.8 | 76.2 | 7.5 | 92.5 | 100 |
| a (Å) | 8.42 | 5.033 | 4.735 | 5.037 | 5.882 |
| b (Å) | 8.42 | 5.033 | 4.735 | 5.037 | 5.882 |
| c (Å) | 8.42 | 13.741 | 3.179 | 13.748 | 23.051 |
| V (Å3) | 598.69 | 301.53 | 71.28 | 302.11 | 690.85 |
| D (nm) | 52.2 | 119.9 | 37.4 | 160.1 | 118.1 |
| R wp (%) | 8.09 | 2.55 | 3.77 | ||
| R p (%) | 4.61 | 1.99 | 2.62 | ||
| R exp (%) | 1.77 | 1.93 | 1.61 | ||
| χ 2 | 4.56 | 1.32 | 2.33 | ||
The Rietveld refinement study reveals good agreement between the experimental and calculated patterns. Moreover, based on what is currently known, we can assume that the structural properties of both CuFe12O19 and SnFe12O19 have never been reported with such experimental proof, which may explain the XRD outcomes compared to the magnetoplumbite structure due to the coexistence of SnO2–Fe2O3 and CuFe2O4–Fe2O3. Consequently, we can assume that they are not isostructural with the SrFe12O19 M-type hexaferrite.
The FTIR spectra of all synthesized MFe12O19 NPs present the characteristic peaks of metal–oxygen stretching vibrations at around 460–630 cm−1 (Fig. 3).34 However, the peaks around 3400 and 1600 cm−1 are attributed to the stretching and the bending vibrations, respectively, of the adsorbed water on the MFe12O19 NPs.32,38
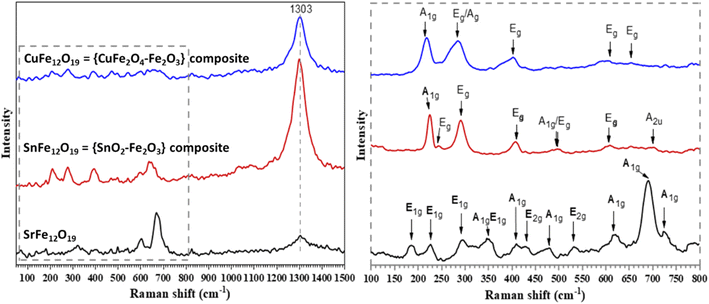
Fig. 4 shows the Raman spectra of the obtained MFe12O19 NPs. The Raman shifts observed for SrFe12O19 are attributed to the A1g, E1g, and E2g modes. The tetrahedral 4f1 and bipyramidal 2b sites of SrFe12O19 exhibit A1g vibrations of Fe–O bonds at 724 and 690 cm−1, respectively. Meanwhile, the octahedral 4f2, 2a and 12k sites exhibit the same A1g vibrations at 620 and 479 cm−1, respectively. At the octahedral 12k dominant site, the peak of the A1g vibrations was detected at 407 cm−1. The band at 347 cm−1 is attributed to an octahedra mode mix with A1g and E1g symmetries. While the E1g vibrations of the entire spinel block are shown at 185 cm−1, the E1g vibration modes are also seen at 295 and 225 cm−1. The bands at 534 and 432 cm−1 were both attributed to E2g modes.45,46 On the other hand, the Raman spectrum of SnFe12O19 reveals three Raman active modes (Fig. 4). While the observed peaks at 222 and 497 cm−1 are both attributed to A1g, the remaining four peaks at 243, 284, 407, and 609 cm−1 may be assigned to the Eg mode of α-Fe2O3 NPs.47 However, the peaks at 499 and 701 cm−1 are assigned to the Eg and A2u active modes of SnO2.47 In addition, the Raman spectrum of CuFe12O19 reveals peaks at 226, 228, 409, 611, and 656 cm−1, matching the Raman active modes of α-Fe2O3 NPs.48 Moreover, the broad peak around 1303 cm−1 observed in all samples, with a noticeable differentiation in intensity, could be attributed to the Raman mode of α-Fe2O3,49 which is in good agreement with the Rietveld study. Furthermore, the significant intensity of the α-Fe2O3 peak in the SnFe12O19 structure may explain its lack of magnetization. In conclusion, the Raman spectra support the recorded and Rietveld refined XRD patterns, especially those of CuFe12O19 and SnFe12O19, about a possible coexistence of MOx–Fe2O3 as a composite structure in the MFe12O19 composition. Indeed, it was reported that α-Fe2O3 and γ-Fe2O3 are intermediate phases during the formation process of the hexaferrite structure.49,50 Moreover, their existence as intermediate phases supports the formation mechanism of MFe12O19. Specifically, the γ-Fe2O3 phase is formed at low temperatures and incorporates cations into MFe2O4, which further reacts with γ-Fe2O3 to form the MFe12O19 phase. However, the α-Fe2O3 phase appears at higher temperatures, accompanied by the formation of the hexaferrite structure.
To understand the surface electronic and chemical configurations of the as-synthesized MFe12O19 NPs, XPS analyses were carried out (Fig. 5). The chemical environment and the oxidation state of Cu, Sr, Sn and Fe were studied through the Cu 2p, Sr 3d, Sn 3p, Sn 3d and Fe 2p regions (Fig. 5b and c). All the expected elements, Cu, Sr, Sn, Fe and O, were detected in the XPS survey spectra of MFe12O19 (Fig. 5a1–a3). The high-resolution XPS spectrum of the Cu 2p region in CuFe12O19 shows the characteristic peaks of the Cu 2p3/2 and Cu 2p1/2 core-levels at the binding energies (BEs) of 933.38 and 953.10 eV, as well as two additional peaks attributed to shakeup satellites at BEs of 940.49 and 961.78 eV (Fig. 5b1), respectively. Deconvolution of the Cu 2p XPS spectrum indicated the presence of Cu2+ of CuFe2O4 in the as-synthesized CuFe12O19.51 Meanwhile, the peaks located at BEs of 486.13 and 494.60 eV are attributed to 3d5/2 and 3d3/2 of Sn4+ (Fig. 5b3), respectively. In addition, the Sn 3d5/2 and Sn 3d3/2 core-levels are presented as a doublet with a spin–orbit split of ≈8.5 eV, which confirms the presence of SnO2 in the SnFe12O19 composite structure.52 However, the XPS spectrum of Sr 3d in SrFe12O19 exhibits two peaks at BEs of 133.38 and 134.98 eV, attributed to the characteristic doublets of Sr2+ (Sr 3d5/2 and Sr 3d3/2, respectively) (Fig. 5b3).53
 | ||
| Fig. 5 XPS survey (a1–3), high-resolution M (M = Cu 2p, Sr 3d, and Sn 3d) (b1–3), Fe 2p (c1–3) and O 1s spectra (d1–3) of CuFe12O19, SnFe12O19, and SrFe12O19, respectively. | ||
The high-resolution XPS spectra of the Fe 2p regions of the MFe12O19 NPs (CuFe12O19, SnFe12O19, and SrFe12O19) show similar two spin–orbit doublets at BEs of around 710 and 720 eV, corresponding to the Fe 2p3/2 and Fe 2p1/2 core-levels, as well as two additional peaks attributed to shakeup satellites at BEs of around 719 and 732 eV (Fig. 5c), respectively. Hence, the obtained 2p peaks and satellite peaks of Fe are in close agreement with the reported values for Fe3+ and the energy differences between the main 2p3/2 and 2p1/2 peaks (≈13.5 eV), confirming the electronic state of Fe3+.54 Moreover, this electronic state in the as-synthesized MFe12O19 NPs can be assigned to the contributions from Fe3+ in the octahedral and tetrahedral sites.54 However, the noticeable difference in XPS spectrum of Fe in SnFe12O19 is due to the appearance of Sn 3p3/2 at the BE of 725.61 eV, which is in the same region as the shakeup satellite (at BE of 718.98 eV).55 The deconvoluted spectra of O 1s show two peaks in the BE region of around 529–534 eV (Fig. 5d). While the peak at BE of around 530 eV is ascribed to the lattice oxygen in the metal–oxygen bond, the other one at BE of around 531.5 eV corresponds to the adsorbed O− or O22− species, which are associated with the intrinsic oxygen vacancies on the surface. However, the peak at BE of 530.04 eV for SrFe12O19 is shifted to BE of around 529.5 eV in both CuFe12O19 and SnFe12O19, which could be attributed to the characteristic peak of O2− in the hybrid oxide framework.56
57Fe Mössbauer spectroscopy is a highly effective technique for examining the oxidation state, local environment and magnetic characteristics of Fe atoms in the studied MFe12O19 NPs. Fig. 6 shows the room temperature Mössbauer spectra of CuFe12O19, SnFe12O19 and SrFe12O19 NPs. For CuFe12O19, the Mössbauer spectrum is well fitted using two sextets and one doublet, which confirms the presence of the CuFe2O4–Fe2O3 composite structure. While the two sextets are characteristic of the A and B sites of the Fe3+ ions present in the magnetically ordered structure of CuFe2O4 at the tetrahedral and octahedral sites, the doublet is associated with Fe3+ ions in the paramagnetic state of α-Fe2O3. However, the fitted experimental spectrum of SrFe12O19 reveals a superposition of five magnetically split spectral components (sextets) characteristic of five different atomic environments around the Fe nuclei in the SrFe12O19 M-type hexaferrite structure. Each one of these sextets is associated with a specific Fe3+ crystallographic site: one tetrahedral (4f1), one bipyramidal (2b) and three octahedral sites (12k, 4f2, and 2a). On the other hand, the spectrum of SnFe12O19 shows a doublet characteristic of the paramagnetic iron atoms, confirming the lack of magnetization of the as-synthesized SnFe12O19 nanoparticles due to the presence of the SnO2–Fe2O3 composite structure. Table 2 lists all of the hyperfine parameters, namely the percentage of area, isomer shift (IS), hyperfine field (Bhf) and quadrupole splitting (QS). It should also be noted that the Mössbauer results are consistent with the experimental evidence and the outcomes of the spectroscopic characterizations (XRD, FTIR, Raman and XPS).
| MFe12O19 | Component | Area (%) | IS (mm s−1) | B hf (T) | QS (mm s−1) | Site |
|---|---|---|---|---|---|---|
| CuFe12O19 = {CuFe2O4–Fe2O3} | Sextet-1 | 72.35 | 0.36 | 51.71 | — | Octahedral |
| Sextet-2 | 12.15 | 0.36 | 50.16 | — | Tetrahedral | |
| Doublet | 15.50 | 0.59 | — | 2.65 | — | |
| SrFe12O19 | Sextet-1 | 25.12 | 0.28 | 51.95 | — | 4f2 |
| Sextet-2 | 7.00 | 0.39 | 50.93 | — | 2a | |
| Sextet-3 | 10.96 | 0.26 | 49.13 | — | 4f1 | |
| Sextet-4 | 55.94 | 0.36 | 40.90 | — | 12k | |
| Sextet-5 | 0.98 | 0.38 | 34.95 | — | 2b | |
| SnFe12O19 = {SnO2–Fe2O3} | Doublet | 100 | 0.41 | — | 0.20 | — |
To investigate the morphology and topology of the as-prepared MFe12O19 nanoparticles, SEM images were recorded for all MFe12O19 NPs (Fig. 7). CuFe12O19 shows a uniform nanostructure with a homogeneous shape distribution and slight agglomerations (Fig. 7a1). However, SnFe12O19 reveals a slightly uniform distribution of crystallized NPs (Fig. 7b1). On the other hand, SrFe12O19 shows the formation of nano-sized grains and uniform particle shape distribution (Fig. 7c1). Moreover, all EDX elemental mapping images indicate that the main constituent elements of the synthesized MFe12O19 nanostructures are well-dispersed throughout the structure (Fig. 7a2–c2).
TEM analysis was performed to further investigate the shape and size of the as-synthesized MFe12O19 nanostructures (Fig. 8). The TEM images of CuFe12O19 reveal spherical nanoparticles with a size range of approximately 75–85 nm, and confirm the presence of tightly agglomerated NPs (Fig. 8a). The TEM images of SnFe12O19 reveal small platelets forming large spherical grains, with an average size of about 40–80 nm (Fig. 8b), while the TEM images of SrFe12O19 reveal interconnected spherical NPs with a size range of about 25–55 nm (Fig. 8c).
Fig. 9 shows the nitrogen adsorption–desorption isotherms of CuFe12O19, SnFe12O19 and SrFe12O19 NPs. According to the IUPAC classification, the isotherms of the MFe12O19 NPs may be assigned to type III, III and II for the CuFe12O19, SnFe12O19 and SrFe12O19 NPs, respectively. The pore size distributions (BJH model) of all MFe12O19 NPs are presented in the inset spectra in Fig. 9a–c. The BJH average pore diameters of CuFe12O19, SnFe12O19 and SrFe12O19 NPs are found to be 12.98, 14.95 and 4.89 nm, respectively. According to the IUPAC, these average values indicate that the as-synthesized MFe12O19 are classified as a mesoporous nanomaterial.57,58 Moreover, the BET surface area of the obtained NPs was found to be 1.87, 5.25 and 24.91 m2 g−1, respectively.
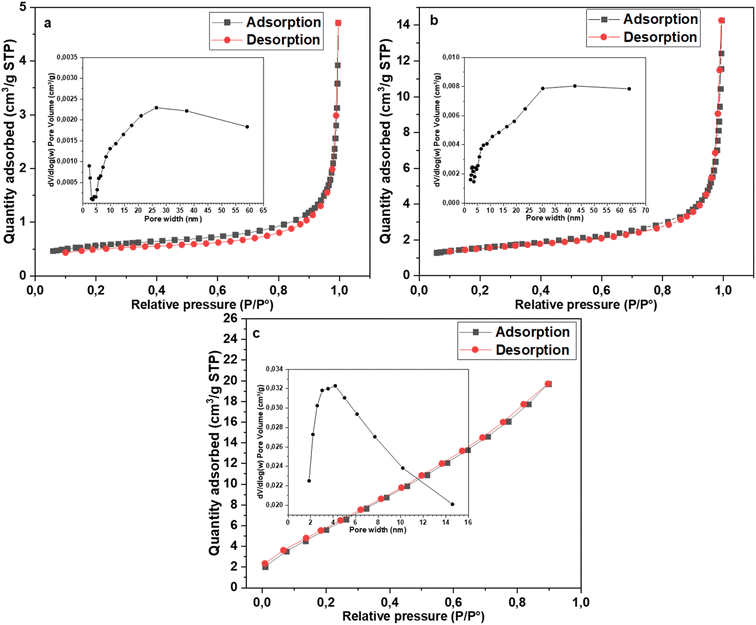 | ||
| Fig. 9 N2 adsorption–desorption isotherm and BJH pore diameter distribution of (a) CuFe12O19, (b) SnFe12O19 and (c) SrFe12O19. | ||
To evaluate the stability of the as-prepared MFe12O19 for potential catalytic applications, zeta potential (ZP) measurements were carried out on MFe12O19 NPs dispersed in a solvent (acetonitrile; acetone or deionized water). The CuFe12O19, SnFe12O19 and SrFe12O19 NPs present ZP values of −24.8, −16.9 and −20.6 mV, respectively. Accordingly, it was reported that NPs manifesting −25 > ZP > +25 mV usually indicate a high degree of stability.59 Indeed, the obtained surface charge values indicate that the prepared NPs exhibit stabilities ranging from good to a high degree of stability. In addition, the obtained ZP values of the synthesized MFe12O19 confirm the observed fluctuation in particle agglomeration (Cu > Sr > Sn) during the microscopic study (CuFe12O19 presents a threshold ZP value).
3.2. Catalytic studies
The catalytic activity of the as-synthesized MFe12O19 NPs were evaluated in the selective oxidation reaction of aromatic olefins. Hence, styrene was chosen as a model substrate to conduct the optimization study of the catalytic oxidation. Moreover, preliminary catalytic tests of MFe12O19 were focused on the selective formation of styrene oxide or benzaldehyde, while tBHP or H2O2 were used as the oxidizing agent (Scheme 1 and Table 3).| Reaction | Catalyst | T (°C) | Ox. agent | Solvent | Conversiond (%) | Yieldd (%) | |
|---|---|---|---|---|---|---|---|
| Benzaldehyde | Styrene oxide | ||||||
| a General reaction conditions: 1.92 mmol of styrene with 3 eq. of oxidizing agent and 2 ml of solvent for 24 h. b Condition 1: tBHP in acetonitrile at 60 °C. c Condition 2: H2O2 in acetone at 80 °C. d The conversion and yields were determined by GC using dodecane as the internal standard. | |||||||
| Condition 1 | — | 60 | tBHP | Acetonitrile | 8 | 7 | — |
| CuFe 12 O 19 | 60 | tBHP | Acetonitrile | 93 | 30 | 61 | |
| SnFe12O19 | 60 | tBHP | Acetonitrile | 20 | 11 | 8 | |
| SrFe12O19 | 60 | tBHP | Acetonitrile | 30 | 11 | 18 | |
| Condition 2 | — | 80 | H2O2 | Acetone | 4 | 4 | — |
| CuFe 12 O 19 | 80 | H 2 O 2 | Acetone | 100 | 80 | — | |
| SnFe12O19 | 80 | H2O2 | Acetone | 100 | 28 | — | |
| SrFe12O19 | 80 | H2O2 | Acetone | 72 | 40 | — | |
In the absence of a catalyst, the oxidation reaction gives only a conversion of up to 8% (Table 3). However, the CuFe12O19 nanoparticles exhibited the highest efficiency and selectivity in the presence of both oxidizing agents (tBHP or H2O2). While the use of H2O2 in acetone provides a high selectivity towards benzaldehyde (80%) in the presence of CuFe12O19, switching to tBHP in acetonitrile resulted in two times higher selectivity towards styrene oxide formation (61%) compared to benzaldehyde (30%). Therefore, both reaction conditions (1 and 2) were optimized for a better catalytic selectivity throughout the study of various parameters: the catalyst amount, reaction time, temperature, amount of oxidizing agent and solvent nature (Scheme 2).
To investigate the active site responsible for the obtained catalytic performance, we studied the catalytic activity of both CuFe2O4 and Fe2O3 compared to the as-prepared composite structure CuFe12O19 (Table 4). Indeed, the XRD pattern and Rietveld refinement of CuFe12O19 illustrate the presence of both CuFe2O4 and Fe2O3 phases as the composite nanostructure (CuFe12O19 = {CuFe2O4–Fe2O3} composite). Hence, the catalytic oxidation in the presence of CuFe12O19 reveals high conversion and good selectivity compared to CuFe2O4 or Fe2O3 for both reaction conditions. Consequently, the composite structure of the as-prepared CuFe12O19 NPs effectively enhances the catalytic activity and reusability of the developed nanocatalyst, owing to the synergistic catalytic effect in a single magnetically recoverable nanostructure.
| Oxidizing agent | Catalyst | Conversionb (%) | Yieldb (%) | |
|---|---|---|---|---|
| Benzaldehyde | Styrene oxide | |||
| a General reaction conditions: 1.92 mmol of styrene, 3 eq. of oxidizing agent (tBHP, acetonitrile at 60 °C or H2O2, acetone at 80 °C), catalyst (20 mg) and 2 ml of solvent for 24 h. b The conversion and yields were determined by GC using dodecane as the internal standard. | ||||
| tBHP | Fe2O3 | 86 | 34 | 39 |
| CuFe2O4 | 99 | 14 | 37 | |
| CuFe 12 O 19 | 93 | 30 | 61 | |
| H2O2 | Fe2O3 | 18 | 11 | Trace |
| CuFe2O4 | 5 | Trace | — | |
| CuFe 12 O 19 | 100 | 81 | — | |
3.3. Optimization of the CuFe12O19 catalyzed oxidation of styrene
| Oxidizing agent | Solvent | Conversionb (%) | Yieldb (%) | |
|---|---|---|---|---|
| Benzaldehyde | Styrene oxide | |||
| a General reaction conditions: 1.92 mmol of styrene, 3 eq. of oxidizing agent (tBHP at 60 °C or H2O2 at 80 °C), CuFe12O19 (20 mg) and 2 ml of solvent for 24 h. b The conversion and yields were determined by GC using dodecane as the internal standard. | ||||
| tBHP | None | 24 | 6 | 17 |
| H2O | 17 | 16 | — | |
| Methanol | 45 | 26 | 13 | |
| Ethanol | 18 | 14 | 3 | |
| Acetonitrile | 93 | 30 | 61 | |
| THF | 65 | 19 | 43 | |
| Acetone | 63 | 17 | 39 | |
| H2O2 | None | 5 | 5 | — |
| H2O | 4 | 4 | — | |
| Methanol | 82 | 10 | — | |
| Ethanol | 100 | 17 | — | |
| Acetonitrile | 22 | 20 | — | |
| THF | 79 | 45 | — | |
| Acetone | 100 | 81 | — | |
| Reaction | Temperature (°C) | Conversiond (%) | Yieldd (%) | |
|---|---|---|---|---|
| Benzaldehyde | Styrene oxide | |||
| a General reaction conditions: 1.92 mmol of styrene with 3 eq. of oxidizing agent, CuFe12O19 (20 mg) and 2 ml of solvent for 24 h. b Condition 1: tBHP in acetonitrile. c Condition 2: H2O2 in acetone. d The conversion and yields were determined by GC using dodecane as the internal standard. | ||||
| Condition 1b | 50 | 64 | 20 | 40 |
| 60 | 93 | 30 | 61 | |
| 70 | 100 | 32 | 59 | |
| 80 | 100 | 44 | 11 | |
| Condition 2c | 50 | 23 | 21 | — |
| 60 | 24 | 22 | — | |
| 70 | 52 | 50 | — | |
| 80 | 100 | 81 | — | |
| Reaction | Catalyst amount (mg) | Conversiond (%) | Yieldd (%) | |
|---|---|---|---|---|
| Benzaldehyde | Styrene oxide | |||
| a General reaction conditions: 1.92 mmol of styrene with 3 eq. of oxidizing agent, CuFe12O19 and 2 ml of solvent for 24 h. b Condition 1: tBHP in acetonitrile at 60 °C. c Condition 2: H2O2 in acetone at 80 °C. d The conversion and yields were determined by GC using dodecane as the internal standard. | ||||
| Condition 1b | 10 | 87 | 33 | 51 |
| 15 | 91 | 32 | 57 | |
| 20 | 93 | 30 | 61 | |
| 25 | 96 | 36 | 56 | |
| 30 | 85 | 33 | 51 | |
| Condition 2c | 10 | 85 | 68 | — |
| 15 | 88 | 70 | — | |
| 20 | 100 | 81 | — | |
| 25 | 100 | 67 | — | |
| 30 | 100 | 60 | — | |
| Reaction | Oxidizing agent/styrene | Conversiond (%) | Yieldd (%) | |
|---|---|---|---|---|
| Benzaldehyde | Styrene oxide | |||
| a General reaction conditions: 1.92 mmol of styrene, oxidizing agent, CuFe12O19 (20 mg) and 2 ml of solvent for 24 h. b Condition 1: tBHP in acetonitrile at 60 °C. c Condition 2: H2O2 in acetone at 80 °C. d The conversion and yields were determined by GC using dodecane as the internal standard. | ||||
| Condition 1b | 1 | 43 | 15 | 24 |
| 2 | 66 | 19 | 42 | |
| 3 | 93 | 30 | 61 | |
| 4 | 80 | 30 | 45 | |
| Condition 2c | 1 | 10 | 9 | — |
| 2 | 40 | 26 | — | |
| 3 | 100 | 81 | — | |
| 4 | 100 | 48 | — | |
| Reaction | Time (h) | Conversiond (%) | Yieldd (%) | |
|---|---|---|---|---|
| Benzaldehyde | Styrene oxide | |||
| a General reaction conditions: 1.92 mmol of styrene with 3 eq. of oxidizing agent, CuFe12O19 (20 mg) and 2 ml of solvent. b Condition 1: tBHP in acetonitrile at 60 °C. c Condition 2: H2O2 in acetone at 80 °C. d The conversion and yields were determined by GC using dodecane as the internal standard. | ||||
| Condition 1b | 3 | 28 | 12 | 12 |
| 6 | 48 | 20 | 21 | |
| 12 | 78 | 29 | 46 | |
| 15 | 83 | 30 | 50 | |
| 18 | 87 | 30 | 55 | |
| 24 | 93 | 30 | 61 | |
| Condition 2c | 3 | 9 | 8 | — |
| 6 | 40 | 36 | — | |
| 12 | 68 | 61 | — | |
| 15 | 77 | 67 | — | |
| 18 | 83 | 68 | — | |
| 24 | 100 | 81 | — | |
3.4. Heterogeneity test
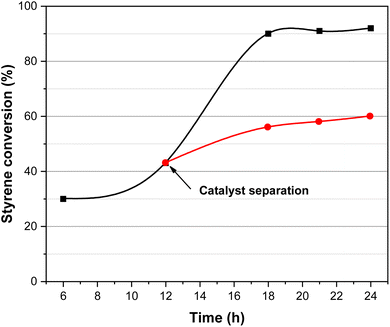
A hot-filtration test was performed to investigate the heterogeneity of the CuFe12O19 NPs catalytic system (Fig. 11). After 12 h of the catalytic oxidation of styrene, the catalyst was separated from the reaction mixture by an external magnetic field and the obtained filtrate was continually stirred under the same reaction conditions. The results indicated that no significant enhancement of the styrene conversion was observed even after completing 24 h of reaction time. This result clearly indicates that the CuFe12O19 nanoparticles act as a heterogeneous catalyst in the styrene oxidation.3.5. Recycling of catalyst
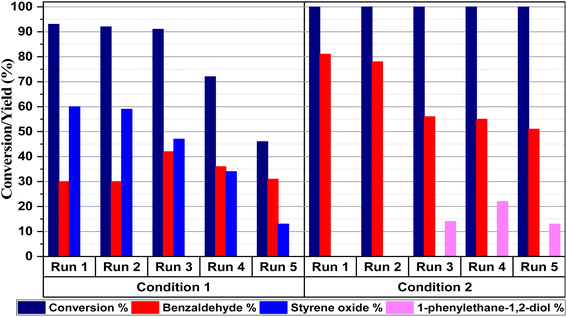
Since catalyst recyclability has great potential for practical applications, the reusability of magnetic CuFe12O19 NPs was evaluated in five consecutive cycles in the selective styrene oxidation reaction under gram-scale conditions (Fig. 12). In the presence of tBHP, the catalytic activity of CuFe12O19 NPs slightly decreases in terms of the styrene conversion and styrene oxide selectivity during the consecutive runs. Hence, the investigation of XRD analysis shows that the identity of the recovered CuFe12O19 catalyst remains like the fresh one (Fig. 13a). However, the SEM image shows agglomerated particles with a change in the surface morphology (Fig. 13b), which could explain the noticeable drop in the reaction conversion and selectivity. On the other hand, the selective catalytic reaction carried out in presence of H2O2 and CuFe12O19 exhibits a total and stable conversion within the five runs with a slight decrease in benzaldehyde selectivity over the runs. Furthermore, the XRD patterns and SEM images of the recycled CuFe12O19 catalyst present no obvious changes compared to the fresh one (Fig. 13a and c).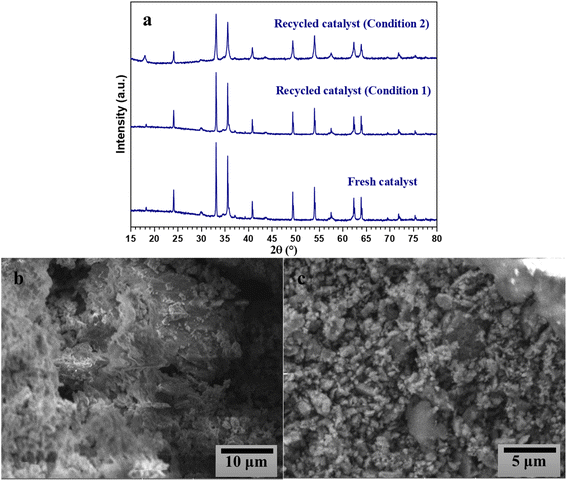 | ||
| Fig. 13 XRD patterns of the fresh and recycled CuFe12O19 (a), and SEM images of CuFe12O19 after five runs while using (b) tBHP or (c) H2O2. | ||
3.6. Catalytic oxidation of styrene derivatives
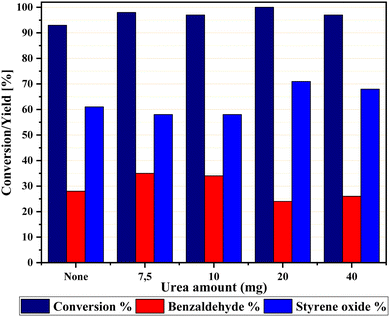
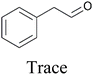
With the optimized reaction conditions (1 and 2) in hand, the scope and limitations of the as-developed catalytic system MFe12O19 were investigated in the catalytic oxidation of various aromatic olefins in the presence of CuFe12O19 NPs as catalysts (Table 10). All studied olefins were selectively converted to the corresponding oxygenated products in excellent to good yields.| Reaction | Substrate | Conversiond (%) | Yieldd (%) | ||
|---|---|---|---|---|---|
| a General reaction conditions: 1.92 mmol of styrene with 3 eq. of oxidizing agent, CuFe12O19 (20 mg) and 2 ml of solvent for 24 h. b Condition 1: tBHP in acetonitrile with 20 mg of urea at 60 °C. c Condition 2: H2O2 in acetone at 80 °C. d The conversion and yields were determined by GC using dodecane as the internal standard. | |||||
| Condition 1b |

|
100 |
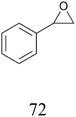
|

|
|

|
98 |

|

|

|
|

|
100 |

|
— | — | |

|
100 |

|

|

|
|
|
80 |
|
— | — | |
| Condition 2c |

|
100 |

|

|

|

|
71 |

|

|
— | |

|
80 |

|

|
— | |

|
58 |

|
|
— | |
3.7. Comparison of the catalytic performance of MNPs
To shed more light on the scope of CuFe12O19 NPs in organic synthesis as MNPs catalysts, the catalytic performance of other MNPs-based catalysts that have been tested in styrene catalytic oxidation so far is summarized in Table 11. Compared to the previous studies, CuFe12O19 exhibits a high catalytic conversion with good selectivity under both conditions (1 and 2). The higher efficiency of CuFe12O19 MNPs can be attributed to the superior catalytic role of Cu and its synergistic effect with Fe, in comparison to Sn and Sr in the studied MFe12O19 NPs. This can be explained by the synergy between CuFe2O4 and Fe2O3 during the oxidation process, where both Cu and Fe are involved in the coordination with the activated oxidizing agent (tBHP or H2O2). This could lead to the formation of peroxo or superoxo species, such as Fe(III)OO˙ and Cu(II)OO˙, complexes that may not be formed with Sn and Sr. Additionally, the possible synergistic intersection between Cu+/Cu2+ and Fe2+/Fe3+ redox pairs at the catalyst surface could also contribute to the observed catalytic performance.| Catalyst | Ox. agent | Conv. (%) | Select. SO (%) | Select. BA (%) | Reusability (conv. %![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) run) run) |
Ref. | |
|---|---|---|---|---|---|---|---|
| CoFe2O4 | tBHP | 81 | 76 | 23 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
17 | |
| α-Fe2O3 | tBHP | 73 | 77 | ND | — | 60 | |
| Au/L-Fe3O4 | tBHP | 76.1 | 70.1 | 27.5 | — | 61 | |
| Ag/KOH-γ-Fe2O3 | tBHP | 89.6 | 89.7 | 10.3 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
62 | |
| MFe 12 O 19 | CuFe 12 O 19 = {CuFe 2 O 4 –Fe 2 O 3 } | tBHP | 100 | 72 | 24 |
45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5 5
|
Present work |
| SnFe 12 O 19 = {SnO 2 –Fe 2 O 3 } | tBHP | 65 | 26 | 5 | — | ||
| SrFe 12 O 19 | tBHP | 30 | 11 | 18 | — | ||
| SrFe2O4 | H2O2 | 63.7 | ND | 32 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
20 | |
| BaFe2O4 | H2O2 | 45.1 | 9.5 | 88.5 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
63 | |
| CaFe2O4 | H2O2 | 37.9 | ND | 91.1 | — | 64 | |
| NiFe2O4 | H2O2 | 31.4 | ND | 55.6 | — | 18 | |
| MFe 12 O 19 | CuFe 12 O 19 = {CuFe 2 O 4 –Fe 2 O 3 } | H 2 O 2 | 100 | — | 82 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 5 5
|
Present work |
| SnFe 12 O 19 = {SnO 2 –Fe 2 O 3 } | H 2 O 2 | 100 | — | 28 | — | ||
| SrFe 12 O 19 | H 2 O 2 | 72 | — | 40 | — | ||
4. Conclusion
Magnetically separable MFe12O19 NPs were successfully synthesized using the coprecipitation method. As-prepared MFe12O19 (M = Cu, Sn and Sr) NPs exhibited nanoscale particle size, magnetic behavior, mesoporous structure and good recyclability. Advanced characterization studies revealed that SnFe12O19 and CuFe12O19 are not isostructural with the SrFe12O19 M-type hexaferrite, due to confirmed coexistence of SnO2–Fe2O3 and CuFe2O4–Fe2O3 as a composite structure, respectively. The magnetic MFe12O19 nanocatalysts were found to be highly efficient for the selective oxidation reaction of various styrene derivatives compared to other reported MNPs heterogeneous catalysts. Among the as-synthesized MFe12O19 NPs, CuFe12O19 was found to be the best catalyst either in the presence of tBHP or H2O2 as the oxidizing agent. The high catalytic activity and good selectivity of CuFe12O19 MNPs could be associated with the synergistic catalytic effect of CuFe2O4 and Fe2O3 in a single magnetically recoverable nanostructure, compared to SnFe12O19 and SrFe12O19. We can assume that the developed MFe12O19 MNPs present a facile and greener approach using magnetically recyclable nanostructures for the selective catalytic oxidation reaction of olefins.Data availability
The device types used for recording spectra and other analytical data that support the findings of this study are included in the manuscript.Conflicts of interest
The authors declare that they have no conflicts of interest.References
- N. Ma, Y. Yue, W. Hua and Z. Gao, Appl. Catal., A, 2003, 251, 39–47 CrossRef CAS.
- J. Liu, F. Wang, Z. Gu and X. Xu, Chem. Eng. J., 2009, 151, 319–323 CrossRef CAS.
- S. Tian, C. Peng, J. Dong, Q. Xu, Z. Chen, D. Zhai, Y. Wang, L. Gu, P. Hu, H. Duan, D. Wang and Y. Li, ACS Catal., 2021, 11, 4946–4954 CrossRef CAS.
- A. Aberkouks, A. A. Mekkaoui, B. Boualy, S. El Houssame, M. Ait Ali and L. El Firdoussi, Adv. Mater. Sci. Eng., 2018, 2018, 2716435 CrossRef.
- G. Grigoropoulou, J. H. Clark and J. A. Elings, Green Chem., 2003, 5, 1–7 RSC.
- A. Patel and S. Pathan, Ind. Eng. Chem. Res., 2012, 51, 732–740 CrossRef.
- C. M. Granadeiro, A. D. S. Barbosa, S. Ribeiro, I. C. M. S. Santos, B. De Castro, L. Cunha-Silva and S. S. Balula, Catal. Sci. Technol., 2014, 4, 1416–1425 RSC.
- T. A. G. Duarte, I. C. M. S. Santos, M. M. Q. Simões, M. G. P. M. S. Neves, A. M. V. Cavaleiro and J. A. S. Cavaleiro, Catal. Lett., 2014, 144, 104–111 CrossRef CAS.
- S. S. Balula, L. Cunha-Silva, I. C. M. S. Santos, A. C. Estrada, A. C. Fernandes, J. A. S. Cavaleiro, J. Pires, C. Freire and A. M. V. Cavaleiro, New J. Chem., 2013, 37, 2341–2350 RSC.
- A. Aberkouks, A. A. Mekkaoui, B. Boualy, S. E. L. Houssame, M. Ait Ali and L. El Firdoussi, Mater. Today: Proc., 2019, 13, 453–457 CAS.
- V. R. Choudhary, R. Jha and P. Jana, Catal. Commun., 2008, 10, 205–207 CrossRef CAS.
- I. W. Davies, L. Matty, D. L. Hughes and P. J. Reider, J. Am. Chem. Soc., 2001, 123, 10139–10140 CrossRef CAS PubMed.
- M. Nemanashi and R. Meijboom, Catal. Lett., 2013, 143, 324–332 CrossRef CAS.
- D. Yin, L. Qin, J. Liu, C. Li and Y. Jin, J. Mol. Catal. A:Chem., 2005, 240, 40–48 CAS.
- A. Aberkouks, A. A. Mekkaoui, M. Ait Ali, L. El Firdoussi and S. El Houssame, J. Chem., 2020, 2020, 1241952 Search PubMed.
- I. T. Horváth and P. T. Anastas, Chem. Rev., 2007, 107, 2167–2168 CrossRef.
- J. Liu, R. Meng, J. Li, P. Jian, L. Wang and R. Jian, Appl. Catal., B, 2019, 254, 214–222 CrossRef CAS.
- D. Guin, B. Baruwati and S. V. Manorama, J. Mol. Catal. A:Chem., 2005, 242, 26–31 CrossRef CAS.
- R. Ramanathan and S. Sugunan, Catal. Commun., 2007, 8, 1521–1526 CrossRef CAS.
- S. K. Pardeshi and R. Y. Pawar, J. Mol. Catal. A:Chem., 2011, 334, 35–43 CrossRef CAS.
- Ü. Özgür, Y. Alivov and H. Morkoç, J. Mater. Sci.: Mater. Electron., 2009, 20, 789–834 CrossRef.
- J. M. D. Coey, J. Alloys Compd., 2001, 326, 2–6 CrossRef CAS.
- P. E. Kazin, L. A. Trusov, D. D. Zaitsev, Y. D. Tretyakov and M. Jansen, J. Magn. Magn. Mater., 2008, 320, 1068–1072 CrossRef CAS.
- A. Morisako, T. Naka, K. Ito, A. Takizawa, M. Matsumoto and Y. K. Hong, J. Magn. Magn. Mater., 2002, 242–245, 304–310 CrossRef CAS.
- P. Hernández, C. De Francisco, J. M. Muñoz, J. Iñiguez, L. Torres and M. Zazo, J. Magn. Magn. Mater., 1996, 157–158, 123–124 CrossRef.
- Z. Jin, W. Tang, J. Zhang, H. Lin and Y. Du, J. Magn. Magn. Mater., 1998, 182, 231–237 CrossRef CAS.
- M. J. Iqbal, M. N. Ashiq, P. Hernandez-Gomez and J. M. Munoz, J. Magn. Magn. Mater., 2008, 320, 881–886 CrossRef CAS.
- B. T. Shirk and W. R. Buessem, J. Am. Ceram. Soc., 1970, 53, 192–196 CrossRef CAS.
- E. Alimohammadi, S. Sheibani and A. Ataie, Mater. Chem. Phys., 2022, 275, 125312 CrossRef CAS.
- R. C. Pullar, Prog. Mater. Sci., 2012, 57, 1191–1334 CrossRef CAS.
- F. Ansari, A. Sobhani and M. Salavati-Niasari, J. Magn. Magn. Mater., 2016, 401, 362–369 CrossRef CAS.
- A. Khoobi, M. Salavati-Niasari and O. Amiri, J. Alloys Compd., 2021, 858, 157745 CrossRef CAS.
- M. Laayati, A. Hasnaoui, N. Abdallah, S. Oubaassine, L. Fkhar, O. Mounkachi, S. El Houssame, M. Ait Ali and L. El Firdoussi, J. Chem., 2020, 1–10 Search PubMed.
- M. Laayati, A. A. Mekkaoui, L. Fkhar, M. Ait Ali, H. Anane, L. Bahsis, L. El Firdoussi and S. El Houssame, RSC Adv., 2022, 12, 11139–11154 RSC.
- Z. kheilkordi, G. Mohammadi Ziarani, S. Bahar and A. Badiei, J. Iran. Chem. Soc., 2019, 16, 365–372 CrossRef CAS.
- T. Ahmadi, G. Mohammadi Ziarani, S. M. Masoumian Hoseini, A. Badiei and M. M. Ranjbar, J. Iran. Chem. Soc., 2021, 18, 2047–2056 CrossRef CAS.
- F. Sanatkar, A. Khoobi and M. Salavati-Niasari, Environ. Sci. Pollut. Res., 2021, 28, 10791–10803 CrossRef CAS PubMed.
- M. Mahdiani, F. Soofivand, F. Ansari and M. Salavati-Niasari, J. Cleaner Prod., 2018, 176, 1185–1197 CrossRef CAS.
- P. C. A. Brito, R. F. Gomes, J. G. S. Duque and M. A. Macêdo, Phys. B Condens. Matter, 2006, 384, 91–93 CrossRef CAS.
- A. Ghasemi and A. Morisako, J. Magn. Magn. Mater., 2008, 320, 1167–1172 CrossRef CAS.
- T. Chin, S. L. Hsu and M. C. Deng, J. Magn. Magn. Mater., 1993, 120, 64–68 CrossRef CAS.
- P. Sivakumar, L. Shani, Y. Yeshurun, A. Shaulov and A. Gedanken, J. Mater. Sci.: Mater. Electron., 2016, 27, 5707–5714 CrossRef CAS.
- Z. F. Zi, Y. P. Sun, X. B. Zhu, Z. R. Yang, J. M. Dai and W. H. Song, J. Magn. Magn. Mater., 2008, 320, 2746–2751 CrossRef CAS.
- R. A. Brand, Normos Program, Internal Report, Angewandte Physic, Univertitat Duisburg, 1987 Search PubMed.
- S. Wang, D. Li, Y. Xiao, W. Dang and J. Feng, Russ. J. Phys. Chem. A, 2017, 91, 1981–1986 CrossRef CAS.
- M. A. P. Buzinaro, M. A. Macêdo, B. F. O. Costa and N. S. Ferreira, Ceram. Int., 2019, 45, 13571–13574 CrossRef CAS.
- J. Didari and A. Sadeghzadeh-Attar, J. Taiwan Inst. Chem. Eng., 2021, 119, 232–244 CrossRef CAS.
- T. P. Martin, R. Merlin, D. R. Huffman and M. Cardona, Solid State Commun., 1977, 22, 565–567 CrossRef CAS.
- N. Yang, H. Yang, J. Jia and X. Pang, J. Alloys Compd., 2007, 438, 263–267 CrossRef CAS.
- M. S. Chen, Z. X. Shen, X. Y. Liu and J. Wang, J. Mater. Res., 2000, 15, 483–487 CrossRef CAS.
- C. Akshhayya, M. K. Okla, A. A. Al-ghamdi, S. A. Al-amri, A. A. Alatar, M. A. Abdel-Maksoud, M. Aufy and S. S. Khan, J. Cluster Sci., 2023, 34, 2459–2469 CrossRef CAS.
- J. Li, X. Zhang, Y. Chen, Y. Li, Y. Huang, Z. Du and T. Li, Chin. Sci. Bull., 2005, 50, 1044–1047 CrossRef CAS.
- S. Katlakunta, S. Singh, S. Srinath, M. Bououdina, R. Sandhya and K. Praveena, Mater. Res. Bull., 2015, 63, 58–66 CrossRef CAS.
- M. Manikandan, K. S. Kumar, N. Aparnadevi and C. Venkateswaran, Phys. Status Solidi A, 2015, 212, 2179–2185 CrossRef CAS.
- F. Zan, N. Jabeen, W. Xiong, A. Hussain, Y. Wang and H. Xia, Nanotechnology, 2020, 31, 185402 CrossRef CAS PubMed.
- T. Xie, L. Xu, C. Liu and Y. Wang, Appl. Surf. Sci., 2018, 273, 684–691 CrossRef.
- B. D. Zdravkov, J. J. Čermák, M. Šefara and J. Janků, Cent. Eur. J. Chem., 2007, 5, 385–395 CAS.
- S. Polarz and B. Smarsly, J. Nanosci. Nanotechnol., 2002, 2, 581–612 CrossRef CAS.
- A. J. Shnoudeh, I. Hamad, R. W. Abdo, L. Qadumii, A. Y. Jaber, H. S. Surchi and S. Z. Alkelany, Biomater. Bionanotechnol., 2019, 527–612 CAS.
- R. A. Bepari, P. Bharali and B. K. Das, J. Saudi Chem. Soc., 2017, 21, S170–S178 CrossRef CAS.
- C. Huang, H. Zhang, Z. Sun, Y. Zhao, S. Chen, R. Tao and Z. Liu, J. Colloid Interface Sci., 2011, 364, 298–303 CrossRef CAS PubMed.
- Z. Pan, L. Hua, Y. Qiao, H. Yang, X. Zhao, B. Feng, W. Zhu and Z. Hou, Chin. J. Catal., 2011, 32, 428–435 CrossRef CAS.
- R. Y. Pawar and S. K. Pardeshi, Arabian J. Chem., 2018, 11, 282–290 CrossRef CAS.
- S. K. Pardeshi and R. Y. Pawar, Mater. Res. Bull., 2010, 45, 609–615 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |