Doped-graphdiyne: synthesis, theoretical prediction and application for electrochemical energy storage
Ziqi Chen†
a,
Deyi Zhang†
b,
Ze Yang
*a,
Yan Xu
*a,
Xuqi Wanga,
Hao Huanga,
Fangcheng Qiu
c and
Changshui Huang
 *b
*b
aShandong Key Laboratory of Integrated Multi-Energy Systems for High Efficiency and Intelligent Operation, College of Energy Storage Technology, Shandong University of Science and Technology, Qingdao 266590, China. E-mail: yangze@sdust.edu.cn; xuyan2020@sdust.edu.cn
bBeijing National Laboratory for Molecular Sciences (BNLMS), CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: huangcs@iccas.ac.cn
cElectric Power Research Institute of Yunnan Power Grid Co., Ltd, Kunming, 650011, China
First published on 3rd July 2025
Abstract
Graphdiyne (GDY), an emerging carbon allotrope with sp–sp2 hybridized networks, possesses a distinctive hierarchical architecture combining two-dimensional planar conjugation with three-dimensional porous frameworks. This unique configuration, characterized by abundant acetylene linkages and uniformly distributed nanopores, provides exceptional advantages for metal ion intercalation kinetics and heteroatomic integration. However, the material's development is constrained by morphological homogeneity and insufficient defect density. To expand the functional versatility of GDY-based systems and engineer enhanced storage capacities through defect engineering, strategic heteroatom doping has emerged as a pivotal modification strategy. Recent advancements in GDY functionalization have demonstrated remarkable progress in tailoring its electrochemical properties via atomic-scale modifications. This review systematically analyzes contemporary synthetic approaches for heteroatom incorporation in GDY matrices, including single-element doping, functional group grafting, and heteroatomic anchoring techniques. Furthermore, we critically evaluate theoretical simulations elucidating doping mechanisms and summarize cutting-edge applications in metal-ion battery systems. Through comprehensive discussion of structure–property relationships in doped GDY electrodes, this work aims to stimulate innovative designs of advanced carbon architectures for next-generation energy storage technologies
1. Introduction
The rapid development of energy storage technologies and explosive demand in the energy market have led to the wide application of novel batteries in electric vehicles, advanced power grids, and 3C consumer electronics. Particularly, the boom in the demand for high-performance batteries has significantly accelerated the development of carbon-based anode materials, including natural graphite (Gr), artificial graphite, soft carbon, and hard carbon.1–4 Carbon-based materials exhibit exceptional metal ion storage capacity, high ionic mobility, excellent electronic conductivity, and robust chemical and thermodynamic stability, which are critical factors for battery anodes.5–9 The development of carbon-based anode materials represents a major breakthrough in the development of electrochemical energy storage systems. For example, the development of Gr has facilitated the rapid commercialization of lithium ion batteries (LIBs),10,11 whereas the utilization of hard carbon anodes provides a material base for the rapid development of sodium ion batteries (SIBs).12 However, the low theoretical capacity of Gr (<372 mAh g−1) significantly limits the energy density of batteries.13 Consequently, the development of anode materials with higher theoretical capacities has become a central focus of current research. GDY, a new carbon allotrope, is composed of a large number of conjugated acetylene bonds and uniform cavities,14,15 constructing a unique 2D planar structure coupled with a 3D porous architecture.16 Metal ions such as lithium (Li), sodium (Na), and potassium (K) can not only be stored and transported between layers but can also be absorbed and diffused vertically to the carbon skeleton plane due to the large cavities.17–19 For the hierarchical porous structure, distinctive chemical modifiability, abundant sp-hybridized carbon atoms, and ample ion storage sites and transport passageways, GDY demonstrates significant advantages and immense potential as an anode material for metal ion batteries, which have increasingly attracted the attention of researchers and have led to extensive research into energy storage applications.20–26Hetero-structured materials offer excellent properties that cannot be achieved with conventional homogeneous materials. Exploring carbon-based heterostructures with high ion storage and transfer capabilities and superior electrochemical properties remains a primary strategy for the development of high-performance energy storage devices.18,27 GDY, with the natural advantages of uniform and highly dispersed actives, can facilitate the construction of highly active interfaces and significantly improve the electrochemical properties through doping heteroatoms, such as nitrogen (N),28–30 sulfur (S),31,32 fluorine (F),33–35 and phosphorus (P).36 Doping heteroatoms can enhance the theoretical storage capacity of metal ions, improve electrolyte wettability, and facilitate metal ion migration, thereby significantly improving the electrochemical performance of GDY-based electrode materials. For instance, N-doped GDY (N-GDY) exhibits a theoretical Li storage capacity of 1965 mAh g−1,30 while P-doped GDY (P-GDY)36,37 and F-doped GDY (F-GDY) have delivered the value of 1929 mAh g−1 and 1867 mAh g−1,33,38 respectively. The theoretical capacities of doped GDYs are much higher than that of pristine GDY with a value of 744 mAh g−1.39 Meanwhile, the introduction of foreign atoms can transform the triangular cavity structure of GDY into a larger hexagonal configuration. This structural modification is more favorable for the migration and diffusion of metal ions. The migration energy barrier of Na in hydrogen-substituted graphyne (H-GY) is 0.22 eV, that of Li ion in N-GY is 0.08–0.11 eV, which is obviously lower than the value of GY (2.26 eV). Actually, HsGDY,40 N-GDY30 and F-GDY33 based electrodes in LIBs can achieve high reversible capacities of 1050, 1300, and 1700 mAh g−1, respectively, all of which are higher than that of GDY. This indicates that the electrochemical properties of pristine GDY can be effectively regulated by doping with different heteroatoms28,41–44 (Fig. 1).
In recent years, extensive efforts have been made on the heteroatom doping of GDY and its applications in metal ion batteries, leading to significant advancements in this field. To follow recent advances, broaden academic perspectives, and promote interest in the design, synthesis, understanding, and energy storage applications of high-performance GDY-based electrode materials, this review focuses on recent reports on heteroatom-doped GDY electrode materials, including synthetic approaches for introducing heteroatoms, theoretical investigations, and applications in metal ion batteries. Particular emphasis is placed on understanding how heteroatom doping influences the energy storage properties of materials and exploring the potential of doped GDY-based anode materials for metal ion batteries. Firstly, the strategies for introducing foreign atoms into GDY are summarized, focusing on approaches such as pre-doping and post-modification. Secondly, theoretical studies on heteroatom doped GDY electrodes and their applications in metal ion storage are reviewed, including the storage sites, binding energies, theoretical storage capacities, ionic transport passageways, and diffusion energy barriers. Thirdly, the research progress in heteroatom-doped GDY materials for metal ion batteries is systematically described and summarized. Finally, this review highlights the key challenges, pressing issues, and prospects for the development of GDY-based materials; provides scientific guidelines and practical research insights for future investigations; and reiterates the necessity and significance of studying GDY and its derivatives.
2. Synthesis strategies for doped GDY
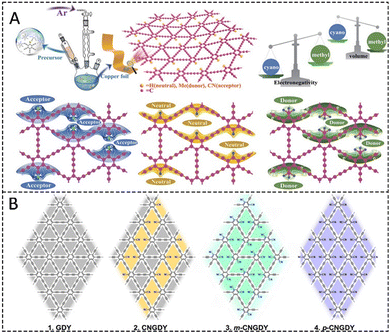
Several doping methods for GDY have been reported since GDY was first prepared by Prof. Li in 2010.16 These distinct methods enable the preparation of GDY with various properties and structures tailored to specific applications. The doping methods of GDY can be broadly categorized into single-element substitution (H,40,45–48 F,33,47,49,50 N,29,30,41,51 S,52–54 P,36 B55 etc.), functional group substitution (–CH3,56–58 –CN,59–61 aromatic hydrocarbon62,63 etc.), anchoring of atoms or functional groups.24,31,64 By introducing foreign atoms and functional groups through different synthetic techniques, the energy storage capacity of GDY can be further enhanced, and these strategies offer valuable insights and guidelines for the development of advanced GDY-based materials.44,652.1. Single element doping methods
 | ||
| Fig. 2 (A) Schematic diagram of the synthesis of HsGDY and the minimal repeat unit of HsGDY.40 (B) Fabrication of the Li@HGDY based anode. HGDY aerogel was synthesized first, then sparked HGDY aerogel, and finally molten lithium was injected into the aerogel in an argon-filled glove box using the HGDY aerogel as a frame.45 (C) HsGDY and digital image showing the color evolution of Zn plate before and after HsGDY growth.48 | ||
 | ||
| Fig. 3 (A) Schematic representation of the synthesis and structure of F-GDY.33 (B) Illustration of three properties of F-GDY as an electrode material for KIB applications.49 (C) The schematic diagram of preparation for H1F1-GDY, the ball-and-stick model of two precursors and the advantages of doped H and F atoms.47 | ||
| Carbon allotrope | Theoretical capacity (mAh g−1) | Specie | Ref. |
|---|---|---|---|
| F-GDY | 1867 | C24F6Li28 | 33 |
| PYGDY | 1630 | C22N2H4Li18 | 29 |
| PMGDY | 1799 | C20N4H2Li20 | 29 |
| TA-GDY | 1965 | C18N6Li22 | 30 |
| GDY | 744 | C6Li2 | 96 |
| H-GDY | 2553 | C24H6Li28 | 40 |
| Cl-GDY | 1284 | C24Cl6Li24 | 87 |
| Me-GDY | 1701 | C30H18Li22 | 59 |
| CNGDY | 2090 | C34N2Li34 | 59 |
| HGDY | 1528 | C32H2Li22 | 59 |
| MeGDY | 1554 | C34H6Li24 | 59 |
| P-GDY | 1929 | C32PO4H2Li52 | 37 |
| GY | 2233 | C12Li12 | 51 |
| H-GY | 1932 | C18H6Li16 | 102 |
| PY-GY | 2154 | C16N2H4Li18 | 51 |
| PM-GY | 2372 | C14N4H2Li20 | 51 |
| F-GDY | 1200 | C24F6K18 | 49 |
| GDY | 620 | C36K10 | 103 |
| TA-GY | 2586 | C12N6Li22 | 51 |
| 3DP-GDY | 2129 | Ca6PC10 | 104 |
| 3DP-GDY | 1064 | K6PC10 | 104 |
| H/F-GDY | 2050 | Li28C24H3F3 | 47 |
| S-GDY | 1684 | — | 37 |
| Ge-GDY | 2340 | — | 70 |
| HsGDY | 1375 | — | 70 |
| 3D-SGDY | 913.8 | C24S2Na12 | 20 |
| 3D-SGDY | 913.8 | C24S2Li12 | 20 |
| 3D-SGDY | 1826.4 | C24S2Ca12 | 20 |
To combine the common advantages of H and F elements,47 they also mixed two monomers of trifluoro triacetylene and triacetylene according to the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to synthesize H and F Co-doped GDY through a similar coupling reaction as shown in Fig. 3C. This material not only enhances the cyclic stability through a strong C–F bond, but the introduction of H effectively improves the storage capacity of LIBs, and an ultra-high capacity of 2050 mAh g−1 is reached at 50 mA g−1. The co-doped system (H1F1-GDY) demonstrates the viability of the “multi-element synergy” strategy. In future research, we may explore F/N or F/P co-doping, which, when combined with the interfacial stability of fluorine and the pseudo-capacitance contribution of nitrogen or phosphorus, has the potential to surpass the performance limitations associated with single-element doping. In addition, F-GDY has recently demonstrated a great breakthrough in the application of solid-state electrolyte, as the nanofiller of solid-state electrolyte film not only facilitates the promotion of Li ion migration, but also effectively inhibits the growth of lithium dendrites.75 These designs provide a great deal of inspiration. The introduction of F atoms not only enhances the structural stability but also increases the structural defects and active sites of GDY to some extent. F doping breaks through the energy storage bottleneck of GDY through the dual path of “structural pore expansion + interfacial stabilization”, which is a functionalization strategy with both theoretical and applied values. It is imperative to concentrate on addressing the issue of controllable preparation and to investigate its comprehensive application in solid-state batteries and other high-security systems.
1 to synthesize H and F Co-doped GDY through a similar coupling reaction as shown in Fig. 3C. This material not only enhances the cyclic stability through a strong C–F bond, but the introduction of H effectively improves the storage capacity of LIBs, and an ultra-high capacity of 2050 mAh g−1 is reached at 50 mA g−1. The co-doped system (H1F1-GDY) demonstrates the viability of the “multi-element synergy” strategy. In future research, we may explore F/N or F/P co-doping, which, when combined with the interfacial stability of fluorine and the pseudo-capacitance contribution of nitrogen or phosphorus, has the potential to surpass the performance limitations associated with single-element doping. In addition, F-GDY has recently demonstrated a great breakthrough in the application of solid-state electrolyte, as the nanofiller of solid-state electrolyte film not only facilitates the promotion of Li ion migration, but also effectively inhibits the growth of lithium dendrites.75 These designs provide a great deal of inspiration. The introduction of F atoms not only enhances the structural stability but also increases the structural defects and active sites of GDY to some extent. F doping breaks through the energy storage bottleneck of GDY through the dual path of “structural pore expansion + interfacial stabilization”, which is a functionalization strategy with both theoretical and applied values. It is imperative to concentrate on addressing the issue of controllable preparation and to investigate its comprehensive application in solid-state batteries and other high-security systems.
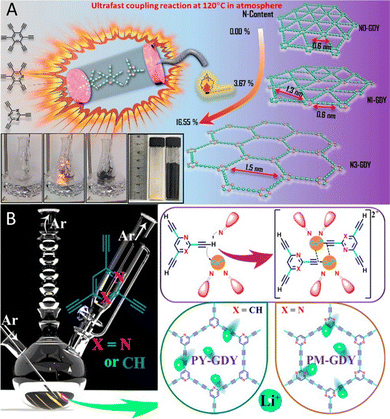 | ||
| Fig. 4 (A) Schematic illustration for experimental setup and reaction processes to prepare N substituted GDY.41 (B) Schematic diagrams of the structures and reaction setup of PY-GDY and PM-GDY.29 | ||
Zou et al. taking the “local electronic structure” as a key breakthrough, combined ab initio molecular dynamics (AIMD) calculations with experimental analysis of various TM@NGDY systems.81 Their results demonstrated that N-GDY can improve the catalytic performance and stability of the catalyst, suggesting a promising strategy for the design of related catalysts. This indicates that N doping can be an effective method for optimizing the storage performance of GDY-based electrode materials. Both experimental and theoretical evidence has shown that the introduction of N positively influences the energy storage applications of GDY. Effectively and economically controlling the N content of GDY can regulate the micropores and surface area of GDY, thus regulating the chemical properties of GDY. N doping improves the charge concentration, band gap, and Li storage capacity of GDY. As a result, N doping is widely utilized in GDY materials, opening new possibilities for the development of high-performance carbon materials in the fields of batteries and electrocatalysis.
Although both N-GDY and F-GDY significantly enhance capacity through heteroatom doping (1965 vs. 1867 mAh g−1), the difference in performance reveals the universality and specificity of the doping mechanism: nitrogen atoms enhance multiplicative performance by introducing pseudocapacitive contributions (32% of capacity), while F atoms enhance cycle life by stabilizing the SEI film (44% reduction in impedance) to extend the cycle life. Nitrogen-doped GDY, as one of the most intensively researched doping systems, has a value that goes far beyond the traditional role of “capacity enhancer”. The core advantage of nitrogen doping stems from its reconstruction of the electronic structure of GDY, but the existing studies have focused too much on the static doping effect and neglected the evolution of nitrogen activity under dynamic working conditions. Studies have shown that the nitrogen doping concentration and performance are not linear, so a single doping strategy is difficult to face the complex needs of energy storage, the future need to establish a “dynamic doping – real-time response – closed-loop optimization” of the new paradigm, the nitrogen atoms from the “passive dopant site” into a “smart functional unit”, the nitrogen atom from the “passive doping site” to the “smart functional unit”. In the future, it is imperative to establish a novel paradigm characterized by “dynamic doping – real-time response – closed-loop optimization.” This approach aims to transform nitrogen atoms from “passive doping sites” into “intelligent functional units,” thereby fully realizing the revolutionary potential of N-GDY in next-generation energy storage systems.
 | ||
| Fig. 6 (A) Schematic representation of the experimental setup, and the 2D structure of B-GDY (the inset photograph shows the as-prepared B-GDY on the Cu foil).86 (B) Proposed preparation mechanism and structure of monolayer P-GDY with P atoms replacing the benzene ring as the center.36 (C) Schematic diagram of the synthesis of the Cl-GDY.87 | ||
2.2. Functional group substitution methods
In recent years, researchers have developed structural modification strategies using covalent groups to tune the electronic structure and related properties of the carbon isomers. Compared with single atoms, the structure of functional groups is more variable, and introducing the characteristic differences of functional groups can be an important positive factor in realizing systematic tuning of the properties of GDY parts. Properties such as the energy gap, morphology, and adsorption capacity of 3D can be modulated by the introduction of electron withdrawing or donating groups, and differences in the pushing and pulling of electrons and the sizes of the groups play a key role in modulating the properties of GDY derivatives. The introduction of the groups reduced the band gap and increased the electrical conductivity of the GDY network, affected the aggregation of GDY, and increased the number of micropores and the specific surface area. The study of the relationship between the functionality of groups and the properties of carbon materials not only facilitates the preparation of new carbon materials with excellent properties but also opens a new platform for theoretical and experimental studies of carbon materials.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bonding strengthens the ion adsorption energy through dipole–ion interactions. However, the introduction of CN may expose GDY to the risk of structural embrittlement, and an imbalance in the sp2/sp hybridization ratio may trigger interlayer slippage and reduce mechanical stability, and we also hope that subsequent studies will focus on this issue.
N bonding strengthens the ion adsorption energy through dipole–ion interactions. However, the introduction of CN may expose GDY to the risk of structural embrittlement, and an imbalance in the sp2/sp hybridization ratio may trigger interlayer slippage and reduce mechanical stability, and we also hope that subsequent studies will focus on this issue.
 | ||
| Fig. 8 (A) Schematic of Me-GDY synthesis.58 (B) Schematic diagram of one-step preparation of Me-GDY nano thorn arrays.57 | ||
Methyl group substitution, as an important strategy for GDY functionalization, exhibits a unique potential for energy storage performance modulation by introducing a dual mechanism of spatial site resistance effect and electronic fine-tuning. The current study shows that methyl substitution can significantly enlarge the GDY layer spacing (0.41 nm vs. 0.36 nm in the original GDY), provide more active sites for lithium-ion embedding, and enhance the cycling stability (only 3.2% decrease in contact angle for 800 days). However, there is an inherent “structure-function” contradiction in this strategy: while the sp3 hybridization of methyl groups alleviates the spatial limitation of ion diffusion, it may sacrifice the intrinsic electrical conductivity, resulting in limited high-magnification performance. In addition, the cross-scale synergistic effect of methyl substitution and heteroatom doping has not been fully explored, and the electron-donating effect of methyl may neutralize the strong polar interface of F doping, which may provide a new paradigm for the design of electrodes for high-power batteries.
 | ||
| Fig. 9 (A) Schematic of Ben-GDY synthesis.62 (B) Synthetic diagram of TPyB and TPB and schematic illustration for the preparation of Py-GDY, Py-Ph-GDY, Ph-GDY.63 | ||
Current research shows that benzene ring substitution can guide the directional growth of two-dimensional layered structures through supramolecular effects to enhance the specific surface area, but the overly dense stacking of aryl rings may sacrifice the ion transport channels, leading to a decrease in the ion diffusion coefficient. Future studies need to explore the cross-scale synergy between aryl ring substitution and porous structures, such as embedding mesoporous carbon nanorods (3–5 nm) in the benzene ring-substituted GDY skeleton to construct a hierarchical “microporous adsorption–mesoporous transport” channel.
2.3. Heteroatoms anchoring methods
Beyond conventional structural substitution strategies, the functionalization of GDY can be achieved through direct anchoring of heteroatoms or functional groups onto its native carbon skeleton via post-synthetic modification approaches. This methodology offers distinct advantages over precursor-mediated pre-design techniques, particularly in operational simplicity and processing efficiency. The coordination environment of heteroatoms (e.g., N, S, P) within GDY can be precisely engineered by modulating dopant precursors or synthetic protocols, enabling tailored electronic structure modifications. The post-modification method has fewer restrictions on raw materials, relatively simple and rapid synthesis, easy control of the heteroatom doping amount, and a wider range of doping sources. These characteristics position post-modification as a versatile platform for creating defect-engineered GDY architectures with programmable electrochemical properties.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and P–C bonds in the P-GDY material, forming a porous structure with richer layers and a large number of disordered heteroatom defects and active sites, thus providing more storage sites and corresponding ion transmission paths for Li.
O, and P–C bonds in the P-GDY material, forming a porous structure with richer layers and a large number of disordered heteroatom defects and active sites, thus providing more storage sites and corresponding ion transmission paths for Li.
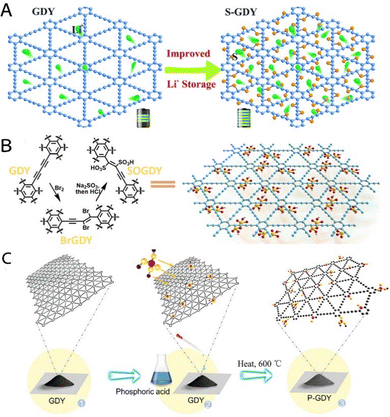 | ||
| Fig. 10 (A) Illustration of the proposed diffusion of Li ions in GDY and S-GDY.25 (B) Schematic representation of SOGDY preparation.24 (C) The preparation of P-GDY by a simple thermal synthesis method. Phosphoric acid was sufficiently infiltrated into the bulk GDY, and the mixture was subsequently transferred to a tube furnace and calcined for 3 h.37 | ||
Anchoring strategies for non-metals immobilizing non-metallic species (such as S, P, B, etc.) to GDY backbones via chemical bonding or physical adsorption provide a unique pathway to modulate their electronic structure and energy storage properties, but their potential is limited by the lack of knowledge of dynamic interfacial behaviors and multi-scale synergistic effects in the current studies. Its static anchoring model neglects two key issues. On the one hand, the chemical state evolution of non-metallic species during cycling may lead to interfacial reconfiguration, triggering irreversible capacity decay. On the other hand, the uneven spatial distribution of anchoring sites exacerbates the anisotropy of ion transport. Future research should focus on integrating in situ characterization with multi-scale simulation to elucidate the dynamic mechanisms of bond breaking and reorganization during the anchoring process. This approach aims to facilitate a paradigm shift in non-metallic anchoring from “empirical modification” to “precise interface engineering”.
 | ||
| Fig. 11 (A) Ni single atoms and clusters are anchored to the HsGDY support.45 (B) The synthesize route of IV-GDYFO includes controllable growth of GDY vertical nanosheet array, in situ nucleation and growth of ferroferric oxide on GDY (GDY-FO) and selective removing of Al from ferroferric oxide to prepare Fe vacancies.91 (C) Schematic illustration of the synthesis strategy and reversible plating/stripping processes of the Zn/GDY atomic electrodes.86 (D) Illustrates the preparation process for making the single-atom Co embedded in ultrathin GDY nanosheets a superior host for sulfur (S/Co-GDY).31 (E) Schematic illustration of the preparation of the Co–N-GDY synthesis steps: (1) adsorption of melamine and Co(NO3)2·6H2O onto GDY nanosheets; (2) high-temperature calcination of the mixture at 400 and 900 °C in Ar atmosphere.64 | ||
Anchoring strategies for metals provide innovative paths for synergistic enhancement of electrochemical activity and stability. However, there are two core contradictions in the existing strategies: First, although high metal loading can increase the density of active sites, cluster aggregation leads to a sudden drop in atomic utilization; second, the dynamic reconfiguration of the metal-carrier interfaces during cycling (e.g., the fluctuation of the valence state of Fe3+ ↔ Fe2+ valence fluctuations) at the metal-carrier interface during the cycling process is not adequately captured by in situ characterization techniques, leading to ambiguous mechanisms of performance decay. In recent years, researchers have used machine-learning as a cutting-edge way to screen new materials in a way that minimizes cost to evaluate the target material in a short period of time, and Zheng et al. used this method to screen the cathode material of an aluminum-ion battery, which has inspired the exploration of other materials.93 In addition, the potential of multi-metal synergistic anchoring has not yet been explored, and future research needs to integrate in situ synchrotron radiation (e.g., XANES to track metal valence evolution) with machine-learning-driven cross-scale simulations to construct a quantitative correlation framework of “anchoring configuration-interface dynamics-lifetime decay”, and ultimately realize the transition from “static modification” to “lifetimes” of metal anchoring. This will ultimately realize the paradigm upgrade of metal anchoring from “static modification” to “intelligent response”, and open up a new dimension of atomistic precision control for the electrode design of the next-generation high-energy-density batteries.
3. Theoretical calculation
3.1. Binding energy and theoretical capacity
Compared to Gr, GDY comprises both sp and sp2 hybridized carbon atoms. It featured abundant conjugated acetylene bonds and uniformly distributed surface cavities. This unique structural configuration offers numerous storage sites for the metal ions. Research indicates that sp hybridization offers a greater density of storage sites than sp2 hybridization. For instance, Gr, which is composed solely of sp2 carbon atoms, has a theoretical capacity of 372 mAh g−1, whereas carbon made entirely of sp-hybridized atoms can achieve a theoretical capacity of approximately 4840 mAh g−1.94 Consequently, the theoretical capacity of GDY (744 mAh g−1) significantly exceeds that of Gr (372 mAh g−1).95 Numerous studies utilizing density functional theory (DFT) and first-principles calculations have explored the adsorption and diffusion mechanisms of metal ions in GDY, elucidating the dynamics and energetics associated with Li-ion insertion and transport. The adsorption and storage mechanisms of Li ions in conventional GDY are among the earliest aspects studied. The distinctive atomic configuration of GDY promotes Li-ion diffusion and significantly enhances its storage capacity. This study proposes that Li ions predominantly occupy GDY by positioning three Li atoms above the center of its triangular cavities.96 Notably, Li+ exhibit dual storage mechanisms in GDY, with intercalation dynamics predominating within the interlamellar spaces rather than being restricted to surface confinement mechanisms. According to this storage method, the Li storage capacity of single-layer GDY is LiC3, and the specific capacity of the high-capacity Li storage (744 mAh g−1) in the form of LiC3 is twice that of Gr.In reality, other storage sites for Li ions exist in GDY, and the maximum storage capacity far exceeds this value. Experimental studies have confirmed a storage capacity of 1388 mAh g−1 at a current density of 100 mA g−1.97 The high mobility and superior Li storage capacity of GDY make it an ideal candidate for Li battery anode materials. During Li storage, metal ions are widely believed to occupy GDY via electrostatic adsorption, coordination bonding, and related interactions.69 The preferred adsorption sites are typically situated near alkyne bond with elevated electron density, along with regions above the benzene rings and triangular cavities. Yang's previous work has proved that most of the inserted Na ions are adsorbed near the acetylene bond in the triangular plane cavity, and some Na+ can be adsorbed on the edge and surface of GDY.98 Further research on the substitution of GDY by different atoms, such as N, H, and F, shows that the introduction of different atoms can effectively adjust the carbon skeleton of GDY and further adjust the electronic configuration, thus affecting its ion storage performance. According to previous reports, in H-containing carbon,99 Li atoms tend to bind to near H atoms, so researchers are inspired to introduce aromatic H groups (Ar–H) on benzene rings to generate HsGDY.40 The introduction of H causes the hole of GDY to expand from a triangular to a larger hexagonal structure, which provides more binding sites for Li ion storage, except for typical adsorption on benzene rings and diacetylene bonds. Because of the lower binding energy (approximately 0.7–1.5 V) adsorbed near the H atom, the theoretical storage capacity of Li is calculated to be 2553 mAh g−1 according to first principles, and the reversible capacity can reach 1050 mAh g−1 when applied to the flexible electrode of LIBs. In addition, as shown in Fig. 12A,69 GY and GDY were incorporated into the interlayer structures, and the ability of the acetylene bond of HsGDY to capture Li ions in the Li–S battery was confirmed by theoretical calculations and characterization tests (Fig. 12A calculates the adsorption energy of various LiPSs in HsGDY). Yang et al. used aromatic rings instead of benzene rings to introduce N atoms into GDY to generate three types of GDY with different N content,29,30 found that the theoretical capacity of GDY is directly related to the N content. The higher the N content, the higher is the theoretical capacity of GDY. The storage capacity of TA-GDY is 1965 mAh g−1, which is significantly higher than that of PY-GDY (1630 mAh g−1) and PM-GDY (1799 mAh g−1).29 Fig. 12B calculates the binding energy of Li at different adsorption sites.29 By comparison, we found that the adsorption energy of Li near the N atom is greater than that at the position of the N atom, which shows that the Li atom adsorbed near N is more stable than that farther away, so it is not difficult to explain the significant improvement in the Li storage effect of GDY by N introduction. In the periodic table of elements, N and P belong to the same main family, therefore, their doping properties are similar. When P is doped into carbon materials, a large number of P–O groups are introduced,100,101 which is beneficial for forming a thin and dense solid electrolyte interface (SEI) layer on the carbon surface, thus improving its electrochemical performance. In addition, the doped P atoms in the material usually present an sp3 orbital configuration, which may lead to many distortions, open edges, and wrinkled morphologies, thus providing more active sites.105,106 These properties of P-doping are beneficial for the storage of alkali metal atoms and transfer of relative ions. The theoretical capacity of P-GDY synthesized by Huang's team using phosphoric acid as a P source is up to 1929 mAh g−1, and the reversible capacity of P-GDY is more than twice that of GDY under the same conditions as the electrode.37 For the introduction of F element, the high energy and power density of F-GDY are achieved through the storage of Li in C–F semi-ionic bonds and micropores.13,33 Stable K18-C24F6 complexes are calculated under the premise of ensuring the most stable binding energy and maximizing Li storage. The binding energy of each Li atom in this configuration is 0.91 eV and the storage capacity is up to 1200 mAh g−1, as shown in Fig. 12C.33 This is not only attributed to its unique chemical structure and hierarchical pore structure, but also to the fact that the F in F-GDY has special contact with the electrolyte, which reduces the interface resistance, facilitates ion transport, and stabilizes the F-GDY electrode. A large number of experiments have proved that the introduction of heteroatoms is a good way to increase the storage sites and capacity of GDY.
 | ||
| Fig. 12 (A) Theoretical simulations of Li2Sn (n = 2, 4, 6, 8) molecules adsorbed on sp2 and sp hybridized carbon of HsGDY.69 (B) Geometries and calculated Eb of optimized Li-C22N2H4 and theoretical capacities of optimized Li18-C22N2H4.29 (C) The top view and cross-section view of the K18-C24F6.33 | ||
3.2. Migration path and diffusion barrier
GDY possesses a uniformly distributed 2D atomic layer structure composed of 18 carbon atoms, forming numerous 3D porous channels within the material. These channels facilitate the diffusion of Li ions into the interior of GDY. Similar to Gr, GDY exhibited a large interlayer spacing (0.365 nm). Its 2D structure, rich in carbon–carbon triple bonds, provides electron-dense regions that promote the migration of metal ions between layers, while influencing its conductivity and electronic structure. Unlike Gr and GY, GDY's unique 3D porous channels and 2D atomic layer configuration enable Li ions to diffuse both in-plane and out-of-plane, as illustrated in Fig. 13A.107 First-principles calculations by Zhang et al. revealed that enabling 3D Li diffusion in GDY requires overcoming an energy barrier of only 0.53–0.7 eV.108 Specifically, the energy barrier for continuous in-plane Li diffusion on a single GDY layer is moderate, at 0.51–0.52 eV,107 comparable to that of Gr. The vertical diffusion energy barrier, however, is significantly lower at approximately 0.35 eV, which is easily overcome under experimental conditions. For GDY/Gr anode in LIBs (Fig. 13B), the diffusion steps process of Li ions includes through the GDY interlayers of GDY (A → B), inside the Gr (D), and along the Gr/GDY heterojunction interface (B → C).109 Notably, the interlayer diffusion energy barrier of GDY (0.30 eV) is lower than that of Gr (0.33 eV), suggesting that GDY enhances Li ion diffusion. Furthermore, under the influence of an internal electric field, the Li ion diffusion barrier at the GDY/Gr interface was significantly reduced. This reduction in the energy barrier (A → B → C) accelerated the transport dynamics at the GDY and heterojunction interfaces. In addition to Li-ion diffusion, GDY's carbon skeleton contains numerous homogeneous cavities with relatively large pore diameters. These cavities allow alkali metal ions (Li, Na, and K) to migrate not only between layers, as seen in Gr, but also vertically through the carbon plane via the pore diameters, further supporting efficient ion transport. This phenomenon is evident from the A → B → C diffusion pathway and its associated energy changes, as depicted in Fig. 13C.49 In addition, several researchers have reported the effects of heteroatoms doping on metal ion storage capability of GDY. The introduction of N atoms can enlarge the pores of GDY.17 Since the diameter of Na ion is larger than that of Li ion; this change significantly improves the Na storage capacity of GDY. The GDY prepared by substituting the benzene ring with B exhibited similar properties. For sites 1–5, the change in the energy barrier, as shown in Fig. 13D, indicates that the introduction of B atoms significantly reduced the diffusion barrier of GDY. AB-stacked GDY was chosen for modelling due to its high stability.103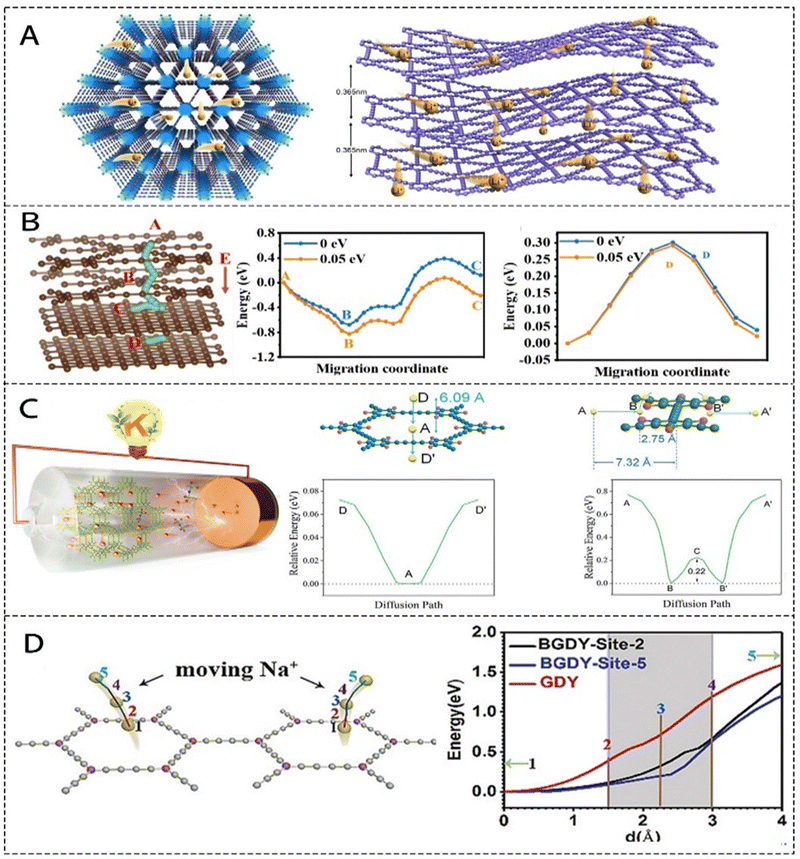 | ||
| Fig. 13 (A) Top and side views of Li diffusion in GDY.104 (B) Li-ion transport pathway in GDY/Gr and the calculation of Li-ion diffusion energy barriers in GDY and GDY/Gr.106 (C) Superior K storage in KIB based on F-GDY films and the energy barriers corresponding to the two diffusion pathways are illustrated.49 (D) The energy potential curves along the trajectories for the Na atom transfer from the plane of B-GDY through the sites 2 and 5.107 | ||
4. Applications of doped GDYs in rechargeable batteries
The development of novel electrode materials is crucial for advancing the performance of secondary batteries, particularly in terms of improving the storage capacity and charge–discharge rates.110 Among these, 2D planar carbon materials, especially the recently emerging GDY, have garnered significant attention owing to their distinctive physical and chemical properties, such as tunable inherent band gaps and highly conjugated porous structures. GDY is widely regarded as a promising material because of its excellent conductivity, expansive specific surface area, and unique structural characteristics, which collectively suggest its broad application prospects in the field of rechargeable batteries. One of GDY's key advantages lies in its abundant 3D pores and larger interlayer spacing compared to traditional Gr. These features create ideal conditions for the rapid migration of metal ions within the material.91,111 This property positions GDY as a potential candidate to replace Gr as a next-generation electrode material for rechargeable batteries. Over the past few years, extensive research has been conducted to explore the applicability of GDY to energy storage systems. Such studies have highlighted its superior ion-transport properties and structural stability, reinforcing its potential as an innovative electrode material. As research continues to uncover its full capabilities, GDY is expected to play a pivotal role in revolutionizing secondary battery technologies.4.1. Applications in Li-ion batteries
LIBs are widely employed in portable electronic devices and new energy vehicles. The performance of the anode materials plays a crucial role in determining the capacity and charging rate of these batteries.112–114 The theoretical capacity of Gr as a commercial anode material is only 372 mAh g−1, which underscores the urgency of exploring new high-performance anode materials for LIBs.52 GDY thin films have emerged as promising candidates for LIBs anodes owing to their high specific Li-ion capacity, extended cycle life, and remarkable stability.113,115,116 In 2014, researchers explored the Li-ion storage performance of multilayer GDY grown on copper foil for the first time.96 GDY films of three different thicknesses were prepared and directly used as anode materials. The GDY-based anode demonstrated superior cycling performance. At a current density of 2 A g−1, the specific capacity remained 420 mAh g−1 after 1000 cycles.107 These studies revealed that the porous nanoparticle-aggregated structure of GDY confers excellent battery performance. Building upon these results, researchers have attempted to modify GDY by introducing heteroatoms to fine-tune its structure and enhance its electrochemical activity.51 Zhao synthesized N-substituted GDY (PyN-GDY), and the lithium-ion capacitors (LICs) utilizing PyN-GDY retained a specific capacity exceeding 500 mAh g−1 even at a current density of 5 A g−1.42 In 2018, Yang et al. prepared TA-GDY with a uniform distribution of N atoms (Fig. 14A).30 TA-GDY-based electrodes achieved a highly stable specific capacity of 1467 mA h g−1 at a current density of 50 mA g−1 and a specific capacity of 730 mA h g−1 at a current density as high as 5 A g−1. The increase in capacity is mainly attributed to the high N content, which provides a large number of active heteroatom sites and enlarges the interlayer spacing of GDY, allowing for better transport of ions between the layers. | ||
| Fig. 14 (A) Schematic structure of GDY as an anode for lithium-ion batteries and the rate performance of TA-GDY in LIBs at different current densities.30 (B) The mechanism of Li storage and the cycle performance of flexible electrode at the current density of 0.1 A g−1.40 (C) The illustration of Li storage mechanism in F-GDY and the cycle performance of F-GDY at 50 mA g−1, 100 mA g−1.33 | ||
As shown in Fig. 14B, He et al. first prepared HsGDY and used it as an anode for LIBs.40 At a current density of 0.1 A g−1, its volumetric capacity is 1447 mAh cm−3, and at a current density of 5 A g−1, it can even achieve 815 mAh cm−3. Recently, they combined HsGDY and Cu2O to create a hybrid HsGDY/Cu2O-quantum dots (QDs) electrode material system.70 The reversible capacity of the HsGDY/Cu2O-QD heterostructure can achieve 1200 mAh g−1 at the current density of 50 mA g−1. The electrode also exhibited a long cycle stability of more than 8000 cycles. The molecular structure of HsGDY changed considerably compared to GDY, the larger pore size is favorable for lowering the diffusion energy barrier of the particles, and Li can be bonded to the vacancies of the H atoms, which increases the storage capacity of the material. The high strength of the C–F bond enhanced the stability of the material. Thus, F-GDY (Fig. 14C) exhibits exceptionally cycle stability (9000 cycles).33 In addition, it has been experimentally demonstrated that Cl-GDY anode can reach 1150 mAh g−1 at a current density of 0.05 A g−1,87 S-GDY anode can reach 920 mAh g−1 at a current density of 0.1 A g−1.85 The commonality of the introduction of heteroatoms is that it greatly increases the number of electrochemically active sites and improves the capacity of GDYs as electrodes for LIBs. Moreover, different heteroatoms or doping amounts can cause different structural and chemical changes, which opens up the possibility of exploring more efficient LIBs anode materials. The performance breakthrough of GDY-based electrode materials in LIBs has always been centered on the triangular equilibrium of “high capacity-fast kinetics-long lifetime”, and there is still a significant blind spot in the knowledge of the synergistic mechanism of the three in the existing studies. Although N-GDY achieves an ultra-high capacity of 1965 mAh g−1 by introducing a pyridine nitrogen site, and F-GDY achieves a lifetime of 9000 cycles by virtue of the interfacial stability of C–F half-ionic bonding, most of these achievements are based on the optimization of a single performance index, ignoring the multi-scale coupling effect. For example, there is an inherent contradiction between the dense active sites required for high capacity and the open pore structure dependent on fast kinetics, and it is difficult to accurately regulate the spatial distribution of the two by the traditional “trial-and-error” synthesis strategy.
4.2. Applications in Na-ion batteries
Due to the abundance of Na on earth, SIBs are strong candidates for next-generation battery systems and large-scale energy storage devices.117 Li+ and Na+ are similar in size, and GDY's transmission ability for Na+ is similar to that for Li+. Therefore, researchers have proposed the application of trying to apply GDY to SIBs anodes. In order to reduce experimental costs and provide a feasible scheme for the experiment, some researchers took the lead in studying the energetics and kinetics of Na ions in GDY by theoretical calculations. First-principles calculations have shown that the unique atomic hollow structure formed by sp and sp2 hybridized carbon atoms in GDY not only increases the storage capacity of Na, but also accelerates Na diffusion, and the stable configuration of the maximum Na storage capacity in GDY is NaC3, which is much larger than that of Gr's NaC12.118,119 Zhang et al. were the first to attempt GDY in SIBs. They used bulk GDY powder as the anode in SIBs and found that the batteries had relatively good data.98 Zhang directly assembled GDY grown on Cu foils as an anode for SIBs,98 obtaining higher energy and power performances as well as stable cycling capabilities, even at a current density of 1 A g−1, it still maintained 211 mA h g−1 after 1000 cycles. Such an attempt provides a paradigm for the application of GDY-based materials in SIBs.Recently, Xu et al. generated MoS2@GDY composites by growing molybdenum disulfide (MoS2) on the surface of GDY substrates for the first time (Fig. 15A).120 It was found that the discharge capacity of the MoS2@GDY anode can reach up to 328 mAh g−1 at 1000 mA g−1. After bringing the test current back to 200 mA g−1, the capacity retention rate remained as high as 368 mAh g−1. After bringing the test current back to 200 mA g−1, the capacity retention was 93%, demonstrating superior multiplicity characteristics. The GDY in this experiment acts as an effective conductive substrate to prevent the host material from agglomerating during electrochemical processes and provides a new design for fabricating efficient electrode materials for future energy storage systems. Wang directly synthesized B-GDY with a full sp carbon structure, the appropriate synergistic stabilization of sodium atoms by B atoms and the abundance of sp carbons in B-GDY provide more storage sites for the intercalated Na atoms (Fig. 15B).55 In addition, the well-distributed molecular pore size of B-GDY will also effectively reduce the spatial site resistance of Na ions in the vertical direction throughout the molecular plane of B-GDY. The excellent performance of B-GDY based anodes in SIBs confirms these observations. It can be maintained at 180 mAh g−1 after even 4000 cycles at a current density of 5 A g−1. Besides, the characteristics of the HsGDY material make it favorable for storing not only Li but also Na, it also performs well in SIBs.40 As shown in Fig. 15C, even at a current density of 1 A g−1, a reversible capacity of 360 mAh g−1 was maintained after 1000 cycles. The reversible capacity was 220 mAh g−1 for the electrode at 5 A g−1, benefiting from good conductivity.
 | ||
| Fig. 15 (A) The fabricated sodium-ion battery and rate capability of MoS2@GDY at various current densities from 200 to 1000 mA g−1.120 (B) Schematic diagram of B-GDY and the cycle performance of the flexible electrode at 5 A g−1.55 (C) Illustration of Li and Na ion diffusion in HsGDY and the rate performance of the flexible electrode for SIBs.40 | ||
Although the application of GDY-based electrodes in SIBs shows theoretical potential, their practical performance is still limited by the dual challenges of sodium ion kinetic properties and material structural suitability. The current study shows that B-GDY can achieve a high multiplicity performance of 180 mAh g−1 under 5 A g−1 through the strong interaction of electron-deficient boron sites with Na+, but its capacity is only 1/10 of that of the lithium-ion system, highlighting the intrinsic disadvantage of the sodium-ion de-embedding kinetics. Existing studies overly rely on the Li+ design paradigm, ignoring the specificity of the sodium ion size and solvated structure. For example, conventional pore design (0.36–0.52 nm), while accommodating bare Na+, is difficult to adapt to solvated ion embedding, resulting in a significantly lower actual capacity than the theoretical value. In the future, we should jump out of the framework of “lithium imitation”, focus on the unique “high polarization-weak adsorption” characteristics of Na+, and build a “pore – interface – body-phase” multilevel control system to promote GDY-based SIBs from “potential materials” to “practical devices” leap.
4.3. Applications in K-ion batteries
Similar to SIBs, KIBs are low in cost and rich in resources (1.5% of the Earth's crust), and they have attracted attention because of their low redox potential and high energy density.121–123 In recent years, some progress has been made in the research on GDY and its derivatives in the anodes of KIBs. Adjusting the structure of GDY to improve its performance in KIBs has become a research focus. Several theoretical studies have predicted the potential application of GDYs in potassium ion batteries (KIBs),104,124 revealed significant information about the electronic structure and ion transport mechanism of GDYs, provided theoretical guidance for the design of more efficient GDY anodes. Liu's team used GDYs in KIBs for the first time, and Fig. 16A shows the synthesis of the pristine GDYs as well as the potassium ion storage properties of the GDYs.103 The experiments show that the prepared GDY framework indeed harvests excellent electrochemical performance as a KIBs anode, achieving high specific capacity (≈505 mAh g−1 at 50 mA g−1), outstanding rate performance (≈150 mAh g−1 at 5000 mA g−1), and favorable cycling stability. GDY doping is also an important way to improve the electrochemical performance of KIBs. F-GDY in the anode of KIBs showed a capacity of 320 mAh g−1 and 120 mAh g−1 at 50 mA g−1 and 1000 mA g−1, respectively, after 1800 cycles. As shown in Fig. 16B, when the current density was restored to 50 mA g−1, the reversible capacity returned to 307 mAh g−1, indicating interfacial stability and superior rate performance.49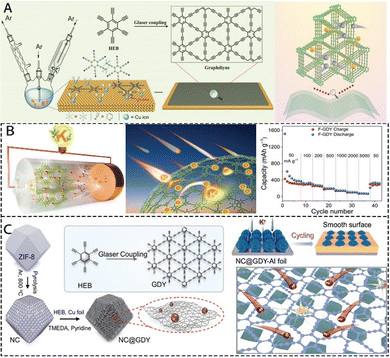 | ||
| Fig. 16 (A) Synthetic procedure for pristine GDY and K-ion storage features of GDY.103 (B) Illustration of K atom diffusion in the F-GDY film and the rate of performance of F-GDY in KIBs at different current densities.49 (C) Schematic illustration of the synthetic steps of NC@GDY and the K affinity features of NC@GDY.126 | ||
The assembly of GDY with other nanomaterials or carbon materials can improve its electrochemical performance as an electrode material for KIBs. The sandwich structure of GDY/Gr/GDY was used as a cathode for KIBs with a higher capacity output and cycling stability than that of GDY, which demonstrates the good potential of GDY in KIBs.125 As shown in Fig. 16C, by directly growing GDY nanosheets with Cu QDs on NC polyhedral templates (NC@GDY),126 the NC@GDY-modified Al collector with K properties inhibits the growth of dendrites and prolongs the service life of K anodes. This kind of assembled material also provides us with new ideas, perhaps assembling doped GDYs with other materials can show more surprising properties. The above studies show the potential application of GDYs in KIBs, but current research on doped GDY storage K still needs to be deepened, and it is believed that more GDYs with excellent properties will appear as KIBs electrodes in the near future. The development of potassium ion batteries (KIBs), as a complementary system to lithium/sodium ion batteries, is limited by the slow dynamics and electrode structural degradation caused by the large size of potassium ions (1.38 Å), while the flexible skeleton and adjustable pore structure of GDY provide a unique opportunity to solve this challenge. Research on GDY-based KIBs needs to shift from “passive structural adaptation” to “dynamic interface engineering”, integrating in situ mechanical characterization, multiphysics simulation and artificial intelligence-driven material design, in order to break the bottleneck of balancing energy storage density and lifetime, and to promote the KIBs from the laboratory to large-scale applications.
4.4. Applications in other batteries
In addition to LIBs, SIBs, and KIBs, GDYs also have very good applications in other batteries, such as aqueous zinc-ion batteries (ZIBs),127 zinc–air batteries (ZABs),128 Li–S batteries,129 and dual-ion batteries (DIBs), etc. GDYs have been used as the electrode catalyst in ZABs to solve the problem that it is difficult to maintain the activity and stability of the catalyst at the same time. Fig. 17A shows the synthesis of IrOx@GDY@CC electrode,130 where the researchers loaded uniformly distributed IrOx on GDY nanosheets using CC as the vehicle. The aqueous ZAB assembled with the IrOx@GDY@CC electrodes had a durability and operational stability of up to 3800 h. The high-capacity solid ZAB was cut into several small pieces and bonded to maintain normal operation with high stability, recyclability, and safety. | ||
| Fig. 17 (A) Schematic illustration of the IrOx@GDY@CC electrode preparation.130 (B) Schematic diagram of the ORR process on PyN-GDY and rechargeability cycling tests of ZABs using PyN-GDY or Pt/C as a cathode at 2 mA cm−2.28 (C) Proposed schematic diagrams of the Li ion intercalation and deintercalation process in the Thi-Dy-modified Al foil for the DIB cell configuration, cycle performance of the Al and Thi-Dy-modified Al foil-based cells at 100 mA g−1.132 (D) Design principle of e-TDYP@Zn and cycling performance at a current density of 2 A g−1 for e-TDYP@Zn full cells.53 | ||
GDY, as an electron reservoir, regulated the valence of Ir in the OER process by maintaining Ir3+ and Ir4+ in dynamic equilibrium to achieve long-term catalytic activity. Fu fabricated a solid magnesium water battery (SMB) based on GDY nanosheet arrays (GDY/MS).131 The distinctive structure, along with the exceptional catalytic and semiconducting properties of GDY/MS, facilitates superior performance in the capture and transfer of water molecules, the catalysis of HER, and the utilization of solar energy. Furthermore, the catalytic effect of PyN-GDY (Fig. 17B) was demonstrated in ZAB.28 PyN-GDY showed good cyclic stability compared to Pt/C. DFT calculations confirmed that the alkyne carbon closest to the N atom is the most likely active site of PyN-GDY. Thienyl rings, due to the high electrical conductivity, film-forming ability, chemical stability, and various formability properties, was introduced to prepare Thi-Dy (Fig. 17C),132 which can be applied as a protective layer on Al foils for DIBs, delaying the pulverization and huge volume expansion of the Al–Li alloy during long cycling, and improving the cycling stability of the DIBs. A similar structure has also been used in ZIBs for the anodic protection of Zn metal (Fig. 17D),53 compared with bare Zn, the electrode with the protective layer exhibited significant cycling stability, and the electron-rich thiophdiyne interphase (e-TDYP)-modified Zn anode achieved a low polarization voltage and long-term reversible plating/stripping over 1000 h at 5 mA cm−2. The e-TDYP@Zn full cell demonstrates a capacity retention of approximately 90.3% after 6000 cycles, in contrast to the bare Zn full cell, which exhibits a capacity retention of only 56.8%. GDY has demonstrated significant potential for use in battery applications, including roles as battery electrodes, electrode protection, and electrode catalysts. The introduction of heteroatoms not only increased the number of structure types of GDYs but also met the application requirements in practice. With its tunable electronic structure and unique ion transport channels, GDY shows potential to outperform conventional carbon materials in emerging systems such as Li–S, ZIBs, ZABs, and DIBs. The core value lies in the ability of multilevel interfacial modulation: Thi-Dy acts as a protective layer for aluminum foils to inhibit Al–Li alloy chalking in DIBs by enhancing the toughness of SEIs; IrOx@GDY@CC electrode utilizes the “electron pool effect” of GDY to stabilize Ir3+/Ir4+ dynamic equilibrium to realize the ultra-long durability of ZABs catalysts of 6000 hours. These applications highlight GDY's shift in role from passive electrode material to active interface engineer.
5. Conclusion and perspectives
GDY and its derivatives have excellent intrinsic properties, including superb storage capacity, high electronic conductivity and easy synthesis. These properties make GDY promising to replace conventional hard carbon as an advanced anode material in metal-ion batteries, thus driving the rapid development of this field. In all battery applications, electrode materials with heterogeneous structures are considered to be the key to tackle future energy challenges and achieve breakthroughs in high capacity and stability energy storage systems. This review systematically summarizes and analyzes the recent advances in the synthesis of GDY and its derivatives and their applications in rechargeable batteries. First, the preparation methods of doped GDY, including the doping of various single elements (N, F, P) and functional groups (cyano, methyl, etc.), are elucidated. Subsequently, the applications of GDY materials as battery anodes were evaluated by in-depth analysis of their molecular structures, electrochemical properties, and electronic configurations. In addition, a comprehensive review of the applications of various GDY-based materials in LIBs, SIBs, KIBs and others is also presented. According to the above reports, GDY heterostructures exhibit irreplaceable advantages in electrochemical energy storage: (1) high theoretical capacity with fast kinetics: doping engineering (e.g., N/F atom substitution) significantly enhances the metal ion storage capacity of GDY (e.g., Li capacity of N-GDY up to 1965 mAh g−1), and at the same time optimizes the multiplicity by lowering the diffusion energy barrier performance; (2) structural tunability and multifunctionality: through heteroatom or functional group modification, the electronic structure (energy band gap, charge distribution) and pore size (0.36–0.52 nm) of GDY can be precisely tuned to fit different ions (Li+/Na+/K+) storage requirements; (3) enhanced interfacial stability: doping-induced strong chemical bonds (e.g., C–F bonds) and uniform active site distribution effectively inhibit electrode/electrolyte interfacial side-reactions and prolong the cycling life (e.g., capacity retention of F-GDY is >80% after 9000 cycles). These studies have significantly contributed to the understanding of the application of GDY in energy storage devices, especially in rechargeable batteries.Despite the impressive progress in GDY-based materials, research on doped GDY is still in its early stages, and several challenges must be addressed to unlock its full potential. In terms of synthesis, current doping methods rely heavily on coupling reactions with precursors containing heteroatoms or functional groups, which pose challenges for large-scale production. The development of scalable and cost-effective synthesis techniques, such as chemical vapor deposition or template-assisted methods, is crucial for industrial applications. Secondly, the current distribution of dopant atoms or groups still suffers from inhomogeneity, and advanced doping strategies, including atomically sophisticated techniques (such as photochemical modification or atomic layer deposition), are required to optimize the properties of GDY-based materials. Thirdly, exploring new dopant types and multi-element co-doping methods could further improve the electrochemical performance of GDY in a wider range of energy storage systems. The following key issues remain for the application of doped GDYs in rechargeable batteries: (1) the synthesis strategies should be optimized for high efficiency, low cost, and large-scale production; (2) development of multiple atom co-doping strategies to further optimize the electronic structure and ion transport paths, the theoretical capacity of H/F-GDY of 2050 mAh g−1 demonstrates the potential of composite doping for synergistic effects; (3) development of interfacial engineering (such as solid electrolyte coatings) and heterostructures (such as GDY/MoS2 or GDY/graphene) to mitigate problems related to volume expansion and solid electrolyte phase evolution. It is worth noting that in the synthesis of GDY heterogeneous materials, machine learning is becoming a key tool to break through the bottleneck of the traditional trial-and-error method. Based on existing experimental data, the neural network can accurately predict the changes in layer spacing and electronic structure evolution under different doping strategies (e.g., N/F co-doping). More importantly, the active learning algorithm can lock the optimal synthesis window step-by-step through iterative feedback (automatically updating the model with each round of experimental data), which can shorten the process optimization, which has been traditionally required for hundreds of attempts, to 10–15 rounds of experiments. In the future, hybrid modelling incorporating first-principles calculations and deep learning potential functions is expected to enable the synthesis of GDY with controlled atomic precision, thereby providing a new paradigm for heterostructure design.
In the future, the integration of computational materials science, in situ characterization techniques (such as in situ TEM, XRD), and artificial intelligence is anticipated to play a pivotal role in expediting the discovery and optimization of GDY-based materials. Furthermore, investigating the application of GDY in emerging fields such as flexible electronics, catalysis, and sensors is expected to infuse new vitality into the development of GDY materials. In conclusion, GDY and its derivatives hold significant potential for transforming energy storage technologies. By addressing current challenges and leveraging interdisciplinary collaborations, the full potential of GDY can be realized, thereby paving the way for the next generation of high-performance energy storage systems. It is anticipated that ongoing research efforts will unveil new possibilities for GDY and drive innovation to meet global energy demands and achieve sustainable development goals.
Author contributions
Ziqi Chen: conceptualization, investigation, data curation, writing – original draft. Deyi Zhang: methodology, formal analysis. Ze Yang: supervision, funding acquisition, writing – review & editing. Yan Xu: resources, visualization, writing – review and editing. Xiqi Wang: formatting, data collection investigation (theoretical calculations). Hao Huang: data collection, software (mapping). Fangcheng Qiu: formal analysis. Changshui Huang: supervision, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
This manuscript is a review article and does not report new primary experimental data. No primary research results, software or code have been included and no new data were generated or analysed as part of this review. All data analysed or discussed in this review, including those presented in figures and tables, are derived from previously published studies. The original sources of these data are fully cited within the reference list of this article. Where applicable, specific identifiers (such as DOIs) for key datasets referenced in this review are provided in the relevant figure captions, table footnotes, or directly within the discussion. No new datasets were generated or analysed in the preparation of this review. Computational models and parameters discussed are described in detail within the cited original publications.Acknowledgements
The authors acknowledge the support from the National Key Research and Development Project of China (2022YFA1204500, 2022YFA1204501), the strategic priority research program of the Chinese Academy of Sciences (XDB0520200), the Basic Science Center Project of the National Natural Science Foundation of China (22388101), the Shandong Province Natural Science Foundation (ZR2024ME026, ZR2024QC122, ZR2021QB107), the Qingdao Natural Science Foundation (24-4-4-zrjj-177-jch), and the Science and Technology Projects of China Southern Power Grid (YNKJXM20240030, YNKJXM20240033).Notes and references
- W. Zhao, C. Zhao, H. Wu, L. Li and C. Zhang, J. Energy Storage, 2024, 81, 110409 CrossRef.
- Q. Zhang, Y. Chu, J. Wu, P. Dong, Q. Deng, C. Chen, K. Huang, C. Yang and J. Lu, Adv. Energy Mater., 2024, 14, 2303764 CrossRef CAS.
- Z. Huang, H. Lyu, L. C. Greenburg, Y. Cui and Z. Bao, Nat. Energy, 2025 DOI:10.1038/s41560-025-01767-z.
- S. Wang, F. Vallejos-Burgos, A. Furuse, H. Otsuka, M. Nagae, Y. Kawamata, T. Ohba, H. Kanoh, K. Urita, H. Notohara, I. Moriguchi, H. Tanaka, J. P. Marco-Lozar, J. Silvestre-Albero, T. Hayashi and K. Kaneko, Nat. Energy, 2025 DOI:10.1038/s41560-025-01783-z.
- Z. Jia, Y. Li, Z. Zuo, H. Liu, C. Huang and Y. Li, Acc. Chem. Res., 2017, 50, 2470–2478 CrossRef CAS PubMed.
- K. Gong, F. Du, Z. Xia, M. Durstock and L. Dai, Science, 2009, 323, 760–764 CrossRef CAS PubMed.
- J. Zhang and X. L. Feng, Joule, 2018, 2, 1396–1398 CrossRef.
- D. H. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo and J. Nakamura, Science, 2016, 351, 361–365 CrossRef CAS.
- Y. Dong, C. Xu, Y. Fu, H. Zhao and Y. Lei, Energy Mater., 2025, 5, 500039 CAS.
- W. Cai, Y.-X. Yao, G.-L. Zhu, C. Yan, L.-L. Jiang, C. He, J.-Q. Huang and Q. Zhang, Chem. Soc. Rev., 2020, 49, 3806–3833 RSC.
- N. Nasajpour-Esfahani, H. Garmestani, M. Bagheritabar, D. J. Jasim, D. Toghraie, S. Dadkhah and H. Firoozeh, Renewable Sustainable Energy Rev., 2024, 203, 114783 CrossRef CAS.
- X. Fan, X. Kong, P. Zhang and J. Wang, Energy Storage Mater., 2024, 69, 103386 CrossRef.
- H. Huang, B. Liu, D. Wang, R. L. Cui, X. H. Guo, Y. Li, S. W. Zuo, Z. Yin, H. H. Wang, J. Zhang, H. Yuan, L. R. Zheng and B. Y. Sun, Nano Res., 2022, 15, 573–580 CrossRef CAS.
- Y. Li and Y. Li, Acta Polym. Sin., 2015, 2(2), 147–165 Search PubMed.
- Z. Wang, N. Yang, R. Yu and D. Wang, J. Chin. Ceram. Soc., 2024, 2, 390–404 Search PubMed.
- G. Li, Y. Li, H. Liu, Y. Guo, Y. Li and D. Zhu, Chem. Commun., 2010, 46, 3256–3258 RSC.
- Z. Yang, D. Zhang, K. Wang, J. He, J. Li and C. Huang, Nano Today, 2022, 46, 101588 CrossRef CAS.
- Z. Zheng, Y. Xue and Y. Li, Trends Chem., 2022, 4, 754–768 CrossRef CAS.
- B. Liu, L. Xu, Y. Zhao, J. Du, N. Yang and D. Wang, J. Mater. Chem. A, 2021, 9, 19298–19316 RSC.
- I. Muhammad, S. Ahmed, H. Cao, Z. Yao, D. Khan, A. Mahmood, T. Hussain, X.-G. Xiong, R. Ahuja and Y.-G. Wang, Mater. Today Chem., 2023, 34, 101756 CrossRef CAS.
- E. Germán, A. Alvarez-Yenes, J. A. Alonso and M. J. López, Appl. Surf. Sci., 2021, 548, 149270 CrossRef.
- W. Zeng, Y. Zhang, X. Liu, L. Qi, W. Kang, L. Fang and M. Zhou, Appl. Surf. Sci., 2020, 523, 146468 CrossRef CAS.
- Y. Zhao, N. Yang, C. Wang, L. Song, R. Yu and D. Wang, APL Mater., 2021, 9, 071102 CrossRef CAS.
- Y. Kong, X. Qiu, Y. Xue, G. Li, L. Qi, W. Yang, T. Liu and Y. Li, J. Am. Chem. Soc., 2024, 146, 23764–23774 CrossRef CAS PubMed.
- Z. Yang, W. Cui, K. Wang, Y. Song, F. Zhao, N. Wang, Y. Long, H. Wang and C. Huang, Chem. Eng. J., 2019, 25, 5643–5647 CAS.
- F. He and Y. Li, CCS Chem., 2023, 5, 72–94 CrossRef CAS.
- Q. Wang, Z. Yan, Y. Hu, Q. Zhang, X.-Y. Kong, Y. Qian, H. Ling, Z.-H. Zhang, T. Li, X. Li, L. Kang, L. Yang, L. Jiang, Z. Zhang and L. Wen, J. Am. Chem. Soc., 2025, 147, 14595–14604 CrossRef CAS PubMed.
- Q. Lv, N. Wang, W. Si, Z. Hou, X. Li, X. Wang, F. Zhao, Z. Yang, Y. Zhang and C. Huang, Appl. Catal., B, 2020, 261, 118234 CrossRef CAS.
- Z. Yang, X. Shen, N. Wang, J. He, X. Li, X. Wang, Z. Hou, K. Wang, J. Gao, T. Jiu and C. Huang, ACS Appl. Mater. Interfaces, 2019, 11, 2608–2617 CrossRef CAS PubMed.
- Z. Yang, R. Liu, N. Wang, J. He, K. Wang, X. Li, X. Shen, X. Wang, Q. Lv, M. Zhang, J. Luo, T. Jiu, Z. Hou and C. Huang, Carbon, 2018, 137, 442–450 CrossRef CAS.
- S. Zhang, Y. Kong, Y. Gu, R. Bai, M. Li, S. Zhao, M. Ma, Z. Li, L. Zeng, D. Qiu, Q. Zhang, M. Luo, L. Gu, Y. Yu, S. Guo and J. Zhang, J. Am. Chem. Soc., 2024, 146, 4433–4443 CrossRef CAS PubMed.
- H. Du, Z. Zhang, J. He, Z. Cui, J. Chai, J. Ma, Z. Yang, C. Huang and G. Cui, Small, 2017, 13, 1702277 CrossRef.
- J. He, N. Wang, Z. Yang, X. Shen, K. Wang, C. Huang, Y. Yi, Z. Tu and Y. Li, Energy Environ. Sci., 2018, 11, 2893–2903 RSC.
- J. Zhang, D. Ding, Q. Fang, J. Cheng, H. Xiao and B. Wang, Angew. Chem., Int. Ed., 2025, 64, e202420892 CrossRef CAS.
- X. Wei, D. He, Y. N. Yang, Z. Geng, M. Shi, Z. Jia, J. Wang, T. Zhao and N. Chen, Adv. Mater., 2025, 37, e2419706 CrossRef PubMed.
- Y. Wang, J. An, L. Qi, Y. Xue, G. Li, Q. Lyu, W. Yang and Y. Li, J. Am. Chem. Soc., 2022, 145, 864–872 CrossRef PubMed.
- X. Shen, X. Li, F. Zhao, N. Wang, C. Xie, J. He, W. Si, Y. Yi, Z. Yang, X. Li, F. Lu and C. Huang, 2D Mater., 2019, 6, 035020 CrossRef CAS.
- C. Kong, Y. Hu, F. Bai, H. Zhang and R. Jia, Appl. Surf. Sci., 2022, 595, 153543 CrossRef CAS.
- B. Jang, J. Koo, M. Park, H. Lee, J. Nam, Y. Kwon and H. Lee, Appl. Phys. Lett., 2013, 103, 263904 CrossRef.
- J. He, N. Wang, Z. Cui, H. Du, L. Fu, C. Huang, Z. Yang, X. Shen, Y. Yi, Z. Tu and Y. Li, Nat. Commun., 2017, 8, 1172 CrossRef PubMed.
- H. Shang, Z. C. Zuo, H. Y. Zheng, K. Li, Z. Y. Tu, Y. P. Yi, H. B. Liu, Y. J. Li and Y. L. Li, Nano Energy, 2018, 44, 144–154 CrossRef CAS.
- F. Zhao, N. Wang, K. Wang, X. Li, Z. Yang, W. Si, Q. Sun and C. Huang, 2D Mater., 2021, 8, 044013 CrossRef CAS.
- X. Li, X. Li, Q. Sun, J. He, Z. Yang, J. Xiao and C. Huang, Acta Phys.-Chim. Sin., 2022, 2206029 Search PubMed.
- L. Gao, Z. Yang, X. Li and C. Huang, Chem. – Asian J., 2021, 16, 2185–2194 CrossRef CAS PubMed.
- L. C. Greenburg, X. Gao, P. Zhang, X. Zheng, J. Wang, R. A. Vila and Y. Cui, Nano Lett., 2023, 23, 5967–5974 CrossRef CAS PubMed.
- X. Gao, X. Zheng, Y. Ye, H. K. Lee, P. Zhang, A. Cui, X. Xiao, Y. Yang and Y. Cui, Nano Lett., 2024, 24, 3044–3050 CrossRef CAS PubMed.
- T. Lu, J. He, R. Li, K. Wang, Z. Yang, X. Shen, Y. Li, J. Xiao and C. Huang, Energy Storage Mater., 2020, 29, 131–139 CrossRef.
- Q. Yang, Y. Guo, B. Yan, C. Wang, Z. Liu, Z. Huang, Y. Wang, Y. Li, H. Li, L. Song, J. Fan and C. Zhi, Adv. Mater., 2020, 32, 2001755 CrossRef CAS PubMed.
- J. He, T. Lu, K. Wang, X. Wang, X. Li, X. Shen, J. Gao, W. Si, Z. Yang and C. Huang, Adv. Funct. Mater., 2021, 31, 2005933 CrossRef CAS.
- K. M. Tran, J. Shim, H.-K. Lee, S. Seo, S. Haldar and H. Lee, ACS Appl. Mater. Interfaces, 2023, 15, 56084–56094 CrossRef CAS PubMed.
- Z. Yang, Y. Song, X. Ren, C. Zhang, X. Hu, X. Li, K. Wang, J. Li and C. Huang, Carbon, 2021, 182, 413–421 CrossRef CAS.
- K. Wang, X. Li, J. Gao, Q. Sun, Z. Yang, J. He, S. Cui and C. Huang, Adv. Funct. Mater., 2021, 31, 2009917 CrossRef CAS.
- K. Wang, X. Li, Y. Xie, J. He, Z. Yang, X. Shen, N. Wang and C. Huang, ACS Appl. Mater. Interfaces, 2019, 11, 23990–23999 CrossRef CAS PubMed.
- Q. Pan, S. Chen, C. Wu, Z. Zhang, Z. Li and Y. Zhao, ACS Appl. Mater. Interfaces, 2019, 11, 46070–46076 CrossRef CAS.
- N. Wang, X. Li, Z. Tu, F. Zhao, J. He, Z. Guan, C. Huang, Y. Yi and Y. Li, Angew. Chem., Int. Ed., 2018, 57, 3968–3973 CrossRef CAS PubMed.
- X. Li, N. Wang, J. He, Z. Yang, F. Zhao, K. Wang and C. Huang, Small, 2020, 16, e1907013 CrossRef PubMed.
- X. Li, Y. Li, M. Zhang, Z. Yang, K. Wang and C. Huang, Nano Energy, 2021, 90, 106571 CrossRef CAS.
- X. Li, N. Wang, J. He, Z. Yang, Z. Tu, F. Zhao, K. Wang, Y. Yi and C. Huang, Carbon, 2020, 162, 579–585 CrossRef CAS.
- C. Xie, X. Hu, Z. Guan, X. Li, F. Zhao, Y. Song, Y. Li, X. Li, N. Wang and C. Huang, Angew. Chem., Int. Ed., 2020, 59, 13542–13546 CrossRef CAS PubMed.
- H. Liu, H. Zou, D. Wang, C. Wang, F. Li, H. Dai, T. Song, M. Wang, Y. Ji and L. Duan, Angew. Chem., Int. Ed., 2023, 62, e202216739 CrossRef CAS PubMed.
- L. Gao, S. Wang, F. Wang, Z. Yang, X. Li, J. Gao, D. Fazzi, X. Ye, X. Wang and C. Huang, ACS Nano, 2024, 18, 30368–30377 CrossRef CAS PubMed.
- W. Zhou, H. Shen, C. Wu, Z. Tu, F. He, Y. Gu, Y. Xue, Y. Zhao, Y. Yi, Y. Li and Y. Li, J. Am. Chem. Soc., 2018, 141, 48–52 CrossRef PubMed.
- H. Dai, H. Zou, T. Song, L. Gao, S. Wei, H. Liu, H. Xiong, C. Huang and L. Duan, Inorg. Chem. Front., 2023, 10, 2189–2196 RSC.
- X. Wang, Z. Yang, W. Si, X. Shen, X. Li, R. Li, Q. Lv, N. Wang and C. Huang, Carbon, 2019, 147, 9–18 CrossRef CAS.
- W. Si, Z. Yang, X. Wang, Q. Lv, F. Zhao, X. Li, J. He, Y. Long, J. Gao and C. Huang, ChemSusChem, 2019, 12, 173–178 CrossRef CAS PubMed.
- Y. Liu, Z. Chen, C. Lai, X. Li, Z. Qu, C. Li, M. Peng, H. Fan, F. Ding and L. Zhang, Angew. Chem., Int. Ed., 2025, 64, e202422089 CrossRef CAS PubMed.
- J. Zhang, G. Qi, J. Cheng, P. Ratajczak, Z. Wang, F. Beguin and B. Wang, ACS Sustainable Chem. Eng., 2023, 11, 16185–16193 CrossRef CAS.
- J. Xiang, Y. Qu, Y. Zeng, S. Hu, H. Xu, H. Xia, M. Ji, L. Duan and F. Lu, Energy Mater. Adv., 2023, 4, 0054 CrossRef CAS.
- S. Kong, D. Cai, G. Li, X. Xu, S. Zhou, X. Ding, Y. Zhang, S. Yang, X. Zhou, H. Nie, S. Huang, P. Peng and Z. Yang, Nanoscale, 2021, 13, 3817–3826 RSC.
- J. He, X. Li, Z. Yang, D. Zhang, T. Lu, W. Liu, Q. Liu, K. Wang and C. Huang, ACS Appl. Mater. Interfaces, 2024, 16, 18008–18018 CrossRef CAS.
- L. Wang, Y. Wan, Y. Ding, S. Wu, Y. Zhang, X. Zhang, G. Zhang, Y. Xiong, X. Wu, J. Yang and H. Xu, Adv. Mater., 2017, 29, 1702428 CrossRef PubMed.
- W. Feng, P. Long, Y. Feng and Y. Li, Adv. Sci., 2016, 3, 1500413 CrossRef PubMed.
- N. Tsuyoshi, J. Fluorine Chem., 2013, 149, 104–111 CrossRef.
- S. S. Zhang, J. Power Sources, 2006, 162, 1379–1394 CrossRef CAS.
- K. Wang, C. Jiang, L. Zhang, Z. Yang, C. Zhang and N. Wang, Small, 2025, 21, e2412204 CrossRef PubMed.
- J. Hou, D. Wang, M. Chao, L. Zhang, H. Liu and Y. Zhao, Chem. Commun., 2024, 60, 1908–1911 RSC.
- H. Shang, Y. Gu, Y. Wang and Z. Zuo, Chem. Eng. J., 2020, 26, 5434–5440 CAS.
- S. Zhang, H. Du, J. He, C. Huang, H. Liu, G. Cui and Y. Li, ACS Appl. Mater. Interfaces, 2016, 8, 8467–8473 CrossRef CAS PubMed.
- T. Lu, X. Hu, J. He, R. Li, J. Gao, Q. Lv, Z. Yang, S. Cui and C. Huang, Nano Energy, 2021, 85, 106024 CrossRef CAS.
- Q. Lv, W. Si, Z. Yang, N. Wang, Z. Tu, Y. Yi, C. Huang, L. Jiang, M. Zhang, J. He and Y. Long, ACS Appl. Mater. Interfaces, 2017, 9, 29744–29752 CrossRef CAS.
- M. Zou, J. Yang, X. Yue, Y. Yuan, Z. Che, M. Li, B. Li, J. Cui, W. Hu, S. Wang, J. Jiang and C. Jia, J. Phys. Chem. Lett., 2023, 14, 9624–9632 CrossRef CAS PubMed.
- J. Sheng, L. Yang, Y.-E. Zhu, F. Li, Y. Zhang and Z. Zhou, J. Mater. Chem. A, 2017, 5, 19745–19751 RSC.
- F. Dang, W. Zhao, P. Yang, H. Wu and Y. Liu, J. Appl. Electrochem., 2020, 50, 463–473 CrossRef CAS.
- J. Zhang, Q. Bai, X. Bi, C. Zhang, M. Shi, W. W. Yu, F. Du, L. Wang, Z. Wang, Z. Zhu and N. Sui, Nano Today, 2022, 43, 101429 CrossRef CAS.
- F. Kong, Y. Yue, Q. Li and S. Ren, Nanomaterials, 2021, 11, 1161 CrossRef CAS PubMed.
- X. Luan, L. Qi, Z. Zheng, Y. Gao, Y. Xue and Y. Li, Angew. Chem., Int. Ed., 2023, 62, e202215968 CrossRef CAS PubMed.
- N. Wang, J. He, Z. Tu, Z. Yang, F. Zhao, X. Li, C. Huang, K. Wang, T. Jiu, Y. Yi and Y. Li, Angew. Chem., Int. Ed., 2017, 56, 10740–10745 CrossRef CAS.
- F. Wang, Z. Zuo, L. Li, K. Li, F. He, Z. Jiang and Y. Li, Angew. Chem., Int. Ed., 2019, 58, 15010–15015 CrossRef CAS.
- H. Sun, H. Liu and S. Ren, J. Funct. Polym., 2024, 37, 473–480 CAS.
- Y. Kong, Y. Wang, Y. Xue, L. Qi, W. Yang, G. Li, T. Liu and Y. Li, J. Am. Chem. Soc., 2025, 147, 14219–14230 CrossRef CAS.
- J. Gao, X. Yan, C. Huang, Z. Zhang, X. Fu, Q. Chang, F. He, M. Li and Y. Li, Angew. Chem., Int. Ed., 2023, 62, e202307874 CrossRef CAS.
- Q. Sun, J. He, L. Gao, T. Lu, X. Ma and C. Huang, Chin. J. Chem., 2022, 40, 872–880 CrossRef CAS.
- L. Zheng, R. Liu, C. Zhang, Y. Shi, J. Man, Y. Wang, L. Chang, M. Cai, Z. Yang and H. Du, Appl. Energy, 2024, 376, 124182 CrossRef CAS.
- M. Winter, J. O. Besenhard, M. E. Spahr and P. Novak, Adv. Mater., 1998, 10, 725–763 CrossRef CAS.
- V. Manev, I. Naidenov, B. Puresheva, P. Zlatilova and G. Pistoia, J. Power Sources, 1995, 55, 211–215 CrossRef CAS.
- C. Huang, S. Zhang, H. Liu, Y. Li, G. Cui and Y. Li, Nano Energy, 2015, 11, 481–489 CrossRef CAS.
- H. Shang, Z. Zuo, L. Li, F. Wang, H. Liu, Y. Li and Y. Li, Angew. Chem., Int. Ed., 2018, 57, 774–778 CrossRef CAS PubMed.
- S. Zhang, J. He, J. Zheng, C. Huang, Q. Lv, K. Wang, N. Wang and Z. Lan, J. Mater. Chem. A, 2017, 5, 2045–2051 RSC.
- J. R. Dahn, T. Zheng, Y. Liu and J. S. Xue, Science, 1995, 270, 590–593 CrossRef CAS.
- M.-J. Kim, I.-Y. Jeon, J.-M. Seo, L. Dai and J.-B. Baek, ACS Nano, 2014, 8, 2820–2825 CrossRef CAS PubMed.
- S. Some, I. Shackery, S. J. Kim and S. C. Jun, Chem. Eng. J., 2015, 21, 15480–15485 CAS.
- X. Ren, X. D. Li, Z. Yang, X. Wang, J. J. He, K. Wang, J. G. Yin, J. Z. Li and C. S. Huang, ACS Sustainable Chem. Eng., 2020, 8, 2614–2621 CrossRef CAS.
- Y. Yi, J. Li, W. Zhao, Z. Zeng, C. Lu, H. Ren, J. Sun, J. Zhang and Z. Liu, Adv. Funct. Mater., 2020, 30, 2003039 CrossRef CAS.
- I. Muhammad, U. Younis, W. Wu, H. Xie, A. Khaliq and Q. Sun, J. Power Sources, 2020, 480, 228876 CrossRef CAS.
- C. Marino, M. El Kazzi, E. J. Berg, M. He and C. Villevieille, Chem. Mater., 2017, 29, 7151–7158 CrossRef CAS.
- H. Tao, S. Du, F. Zhang, L. Xiong, Y. Zhang, H. Ma and X. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 34245–34253 CrossRef CAS PubMed.
- H. Du, H. Yang, C. Huang, J. He, H. Liu and Y. Li, Nano Energy, 2016, 22, 615–622 CrossRef CAS.
- H. Y. Zhang, M. W. Zhao, X. J. He, Z. H. Wang, X. J. Zhang and X. D. Liu, J. Phys. Chem. C, 2011, 115, 8845–8850 CrossRef CAS.
- J. An, F. Wang, J.-Y. Yang, G. Li and Y. Li, CCS Chem., 2024, 6, 110–124 CrossRef CAS.
- M. Salavati and T. Rabczuk, Comput. Mater. Sci., 2019, 169, 109093 CrossRef CAS.
- Z. Zhao, S. Das, G. Xing, P. Fayon, P. Heasman, M. Jay, S. Bailey, C. Lambert, H. Yamada, T. Wakihara, A. Trewin, T. Ben, S. Qiu and V. Valtchev, Angew. Chem., Int. Ed., 2018, 57, 11952–11956 CrossRef CAS PubMed.
- X. Chen, X. Jiang and N. Yang, Small, 2022, 18, e2201135 CrossRef.
- N. Wang, J. He, K. Wang, Y. Zhao, T. Jiu, C. Huang and Y. Li, Adv. Mater., 2019, 31, e1803202 CrossRef PubMed.
- F. Wang, J. An, H. Shen, Z. Wang, G. Li and Y. Li, Angew. Chem., Int. Ed., 2023, 62, e202216397 CrossRef CAS PubMed.
- J. He, G. Hu, J. Chen, X. Wu and Y. Li, Chem. Eng. J., 2025, 511, 161941 CrossRef CAS.
- K. Wang, C. Jiang, L. Zhang, R. Li, Z. Yang, C. Zhang and N. Wang, Nano Today, 2025, 64, 102803 CrossRef.
- T. Jin, X. Ji, P. F. Wang, K. Zhu, J. Zhang, L. Cao, L. Chen, C. Cui, T. Deng, S. Liu, N. Piao, Y. Liu, C. Shen, K. Xie, L. Jiao and C. Wang, Angew. Chem., Int. Ed., 2021, 60, 11943–11948 CrossRef CAS PubMed.
- Z. Xu, X. Lv, J. Li, J. Chen and Q. Liu, RSC Adv., 2016, 6, 25594–25600 RSC.
- H. Huang, K. Li, X. Fan, D. J. Singh and W. T. Zheng, J. Mater. Chem. A, 2019, 7, 25609–25618 RSC.
- J. Xu, Q. Liu, Z. Dong, L. Wang, X. Xie, Y. Jiang, Z. Wei, Y. Gao, Y. Zhang and K. Huang, ACS Appl. Mater. Interfaces, 2021, 13, 54974–54980 CrossRef CAS PubMed.
- T. Hosaka, K. Kubota, A. S. Hameed and S. Komaba, Chem. Rev., 2020, 120, 6358–6466 CrossRef CAS PubMed.
- A. J. Naylor, M. Carboni, M. Valvo and R. Younesi, ACS Appl. Mater. Interfaces, 2019, 11, 45636–45645 CrossRef CAS.
- S. Dhir, S. Wheeler, I. Capone and M. Pasta, Chem, 2020, 6, 2442–2460 CAS.
- Y. Ma, X. Fan, D. J. Singh and W. T. Zheng, J. Mater. Chem. A, 2021, 9, 12320–12330 RSC.
- J. Li, Y. Yi, X. Zuo, B. Hu, Z. Xiao, R. Lian, Y. Kong, L. Tong, R. Shao, J. Sun and J. Zhang, ACS Nano, 2022, 16, 3163–3172 CrossRef CAS.
- Y. Yi, J. Li, Z. Gao, W. Liu, Y. Zhao, M. Wang, W. Zhao, Y. Han, J. Sun and J. Zhang, Adv. Mater., 2022, 34, e2202685 CrossRef PubMed.
- J. Li, Y. Chen, F. Wang, J. Guo, F. He and H. Liu, Chem. Res. Chin. Univ., 2021, 37, 1301–1308 CrossRef CAS.
- M. Huo, J. Sun, W. Liu, Q. Li, J. Chang and Z. Xing, Sci. China Mater., 2024, 67, 4005–4012 CrossRef CAS.
- Z. Wang, C. Song, H. Shen, S. Ma, G. Li and Y. Li, Adv. Mater., 2024, 36, 2307786 CrossRef CAS PubMed.
- Q. Chang, X. Fu, J. Gao, Z. Zhang, Y. Wang, C. Huang and Y. Li, CCS Chem., 2024, 7, 1–14 Search PubMed.
- X. Fu, F. He, J. Gao, X. Yan, Q. Chang, Z. Zhang, C. Huang and Y. Li, J. Am. Chem. Soc., 2022, 145, 2759–2764 CrossRef PubMed.
- X. Liu, W. Zhang, Y. Liu, X. Li, D. Zhang, K. Wang, L. Liu and C. Huang, Energy Environ. Sci., 2024, 17, 9538–9547 RSC.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |