Nanozymes: recent advances for sustainable agricultural development
Runxin Houab,
Na Yin *ab,
Yinghui Wang
*ab,
Yinghui Wang *ab,
Shuyan Song
*ab,
Shuyan Song ab and
Hongjie Zhang
ab and
Hongjie Zhang abc
abc
aChangchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, Jilin 130022, P. R. China. E-mail: yinna@ciac.ac.cn; yhwang@ciac.ac.cn
bUniversity of Science and Technology of China, Hefei, Anhui 230026, P. R. China
cDepartment of Chemistry, Tsinghua University, Beijing 100084, P. R. China
First published on 3rd July 2025
Abstract
Agricultural production is currently facing numerous abiotic and biotic stresses. To mitigate the impacts of these stresses on crop yields, conventional agrochemicals have been widely employed to support farming practices. However, these chemicals exhibit limited functionality and are prone to overuse and residue accumulation on agricultural products, leading to environmental concerns such as pollution and bioaccumulation, which may hinder the development of sustainable agriculture. These drawbacks restrict their broader application in future sustainable agricultural systems. Notably, nanozymes possess unique advantages, including enhanced stability, tunable catalytic activity, and functional versatility. They exhibit significant potential in optimizing plant growth environments, mitigating stress conditions and enhancing crop stress resistance. As a promising alternative, nanozymes demonstrate the capability to address the limitations of conventional agrochemicals while advancing sustainable agricultural practices. Building on this progress, this review first explores the essential properties required for nanozyme applications in agriculture. It further categorizes nanozymes based on their diverse catalytic activities and discusses their roles in sustainable agricultural practices. Additionally, this review addresses current challenges in the field and proposes future directions for nanozyme-based agrochemicals. The goal is to deepen readers’ understanding of recent advances in agricultural nanozymes and stimulate broader scientific interest to explore their potential to advance sustainable agriculture. It is also hoped to provide some constructive inspirations for subsequent scientific research.
1. Introduction
Agricultural production is facing various abiotic and biotic stresses (such as pathogen infection, extreme temperatures, drought and heavy metal exposure). These stressors will affect the homeostasis of reactive oxygen species (ROS) in plant systems, leading to the overproduction of ROS in plants, including hydrogen peroxide (H2O2), hydroxyl radicals (˙OH) and superoxide radicals (O2˙−). These ROS predominantly accumulate in plant cellular organelles, notably mitochondria and chloroplasts. Such ROS imbalance can critically impair the redox equilibrium, with severe cases manifesting as significant inhibition of plant growth and developmental processes, ultimately leading to substantial reductions in crop yield.1–4 Conventional agrochemicals have been extensively employed to enhance agricultural productivity, yet their unsustainability poses significant challenges to the advancement of sustainable agricultural systems. These chemical inputs are prone to trigger cascading ecological disturbances, including overapplication scenarios, bioaccumulation phenomena, resistance development in target organisms, and persistent environmental contamination. Such multifaceted limitations have substantially constrained their broad-spectrum applicability within modern sustainable agricultural paradigms.5–7Nanotechnology and nanomaterials (NMs) currently represent the most promising approaches reported to address these issues, with nanotechnology playing a pivotal role in the development of sustainable agriculture and precision agriculture. In recent years, driven by continuous advancements in nanotechnology-enabled pesticide applications, a new generation of nano-pesticides has emerged. These innovative formulations exhibit enhanced characteristics including higher biocompatibility, environmental friendliness, superior stability, and stimuli-responsive targeting capabilities (intelligent responsiveness).8–11 Nano-pesticides refer to organic or inorganic chemical agents prepared through nanotechnology for treating or preventing pests, bacteria, fungi, and plant diseases. They can be categorized into two primary classes:12,13 the first class comprises pesticide delivery systems utilizing NMs as encapsulation vectors, including metal–organic frameworks (MOFs) as representative organic/inorganic hybrid materials. These systems focus on stimuli-responsive nano-carriers capable of achieving targeted release of active ingredients, which can encapsulate conventional pesticides for delivery to plant infection sites. Current advancements have led to various intelligent-responsive nano-carriers for sustained pesticide release.14–16 While these smart nano-carriers have realized on-demand pesticide release with enhanced spatial and temporal resolution compared to traditional controlled-release systems, their core functional components remain conventional pesticides. Consequently, they cannot circumvent limitations of traditional pesticides, such as growth resistance from prolonged usage. Furthermore, environmental contamination caused by pesticide runoff persists as a critical challenge. The second category of nano-pesticides encompasses NMs possessing inherent pest-resistant and disease-suppressing functionalities, such as metal-based nanomaterials and carbon-based NMs. These materials exhibit therapeutic or defensive properties for plants, which can effectively mitigate the issues in the first category.9,17 Notably, emerging nanozymes capable of mimicking natural enzymatic activities have garnered significant attention. Some of them have excellent properties such as small size, high biocompatibility and excellent stability, and have great potential in addressing the problems of agricultural production such as alleviating oxidative stress in plants and in promoting crop growth.18–20
In response to the imperatives of sustainable agriculture, researchers have dedicated research efforts to developing agricultural nanozymes. In 2004, Manea et al. first designated gold NPs (Au NPs) with ribonuclease-like activity as “nanozymes”.21,22 Subsequently, Yan and colleagues in 2007 first reported that Fe3O4 NPs exhibit peroxidase-like catalytic properties, marking the advent of a new era in nanozyme research.23 Nanozymes are synthetic enzymes with enzyme-like nanocatalytic materials that can simulate the activity of natural enzymes. Compared with traditional natural enzymes, nanozymes have higher stability, persistence, tolerance and multi-enzymatic activity, so they are widely used in agriculture, environmental protection, biomedicine and other fields.24–26 Nowadays, many relevant studies have reported that the enzyme-like activity of nanozymes can improve the growth environment of plants, help alleviate various biotic and abiotic stresses faced by crops, and further promote the growth of crops.27–29 According to their different mimetic enzyme activities, nanozymes can be divided into two categories: (1) the oxidoreductase family, including peroxidase (POD), laccase (LAC), catalase (CAT), oxidases (OXD) and superoxide dismutase (SOD); (2) the hydrolase family, including esterase, urease, protease, and nuclease.18,29
It is evident that there are relatively few review articles on the role of nanozymes in promoting sustainable agricultural development. Based on this, this work firstly discusses the characteristics of nanozymes when they are applied in agriculture; and then summarizes the classification of nanozymes based on their different catalytic activities, such as SOD-like, OXD-like, POD-like, CAT-like, LAC-like, and multi-enzymatic activities, etc.; while discussing their applications in sustainable agriculture. Finally, some problems existing in the current research are pointed out, and suggestions are made for the future development direction of nanozyme pesticides. The aim is to deepen the understanding of the latest achievements in agricultural nanozymes, so as to draw more and more researchers’ attention to explore the great potential of nanozymes in promoting sustainable agriculture. Meanwhile, it is hoped to provide some constructive inspiration for the subsequent scientific research.
2. Physicochemical properties of agricultural nanozymes
Nanozymes, as a class of nanoscale materials with enzyme-like activities, integrate some of the advantages of enzymes and NMs. They exhibit excellent stability in various external environments, as well as highly efficient and tunable catalytic activities. With the continuous development of synthesis technologies, researchers have gained a deeper understanding of the physicochemical properties of nanozymes, such as their morphology, size, and structure. They are also able to customize nanozymes according to specific requirements to meet diverse application needs. The physicochemical properties of nanozymes largely determine their application potential and possible impacts on agricultural ecosystems. Moreover, these properties may be directly related to their efficacy, stability, and impacts on the environment and humans. This inspires us that in order to better exploit their application value and contribute to the sustainable development of agriculture, it is necessary to conduct in-depth investigations into their basic physicochemical properties to prepare more efficient and controllable agricultural nanozymes. In the following, we will introduce the basic physicochemical properties of nanozymes from the aspects of their special structure, particle size and surface charge characteristics, stability, and biocompatibility.2.1 Special structure
Creating a specific nanozyme structure helps to enhance its catalytic function. Nanozymes with different structural characteristics usually exhibit different efficacies.30–32 For example, in metal-based nanozymes, the different surface valence states of the metal active centers play a major role in their catalytic behavior. They have a catalytic mechanism closer to that of natural enzymes, promoting electron transfer during the catalytic process.33 The excellent catalytic performance of metal-based nanozymes can generally be improved through the following approaches: (1) structural modulation of metal active centers; (2) synergistic effects among multimetallic nanozymes; (3) interface engineering to form heterojunction structures; and (4) design of materials with specific surface morphologies and crystallographic facets. Among non-metal nanozymes, carbon-based nanozymes usually have good biocompatibility and multiple enzyme-like activities due to the unique electronic and geometric structures of the nanocarbon materials at their active centers.34,35 In addition, nanozymes with a morphology featuring a higher surface area and abundant pores can display better catalytic performance.36,37 The morphology of carbon-based nanozymes constitutes a critical factor influencing their performance, where nanostructures with higher surface areas and abundant porosity exhibit enhanced catalytic properties. Subsequent sections therefore systematically summarize the design strategies and characteristics of zero-dimensional (0D), one-dimensional (1D), and two-dimensional (2D) carbon-based nanozymes.Metal-based nanozymes prepared from metal materials possess unique metal active centers. Their distinctive electronic structures enhance the efficiency of electron transfer and catalytic substrate adsorption. Nanozymes with different metal active centers also exhibit differences in catalytic activity. A research study has integrated four noble-metal porphyrins (Ir, Ru, Pt, and Pd) into a Zr-based metal–organic framework (MxP).38 Due to the strong interaction between Zr(IV) and carboxylate linkers, nanozymes with a robust structure were obtained. The porphyrins in the nanozymes are anchored at the porphyrin center through atomic metal–N coordination, which can mimic the active sites of natural peroxidases. Among them, MIrP shows higher POD-like activity (685.61 U mg−1) than horseradish peroxidase (HRP). The excellent catalytic activity of MIrP stems from its strong H2O2 adsorption capacity and low energy threshold. Moreover, compared with traditional MFeP, its unique structural design endows it with better stability at room temperature and in high-concentration H2O2 environments.
In addition, multi-metal nanozymes are composite systems composed of multiple metal ions. The synergistic effect between multiple metals promotes the electron transfer or transport of the composite system, thus the system exhibits unique catalytic activity. A novel ultra-small high-entropy alloy NP (US-HEANP) has been reported in recent studies.31 This nanozyme, composed of five noble metals (Ir, Pt, Ru, Pd and Rh), was fabricated via a metal–ligand cross-linking strategy. The unique multi-metallic composition and ultra-small size endow it with exceptional nanozyme performance. Specifically, the high-entropy alloy structure exhibits distinct high-entropy effects and lattice distortion characteristics, which synergistically modulate the electronic structure and adsorption energy of the catalyst, thereby optimizing its catalytic activity. Experiments have shown that US-HEANP exhibit POD-like activity and can catalyze endogenous H2O2 to generate highly toxic ˙OH. The calculated Michaelis constant (Km) values of US-HEANP for H2O2 and TMB are 4.094 mM and 34.40 μM, respectively, and the maximum reaction velocity (Vmax) values are 1.882 × 10−7 M s−1 and 2.457 × 10−7 M s−1 respectively, indicating good catalytic activity. In addition, another research study adopted a reverse oxide/alloy structure. Co7Fe3 was used as the alloy core, which was encapsulated by ZnO and then stabilized with a carbon layer.39 Finally, a nanozyme Co7Fe3/ZnO@C with multi-enzyme activity was designed. This structural design significantly enhances the enzyme-like activity of the nanozyme by optimizing the electronic structure and transmission path. By mimicking multiple natural enzymes (SOD, CAT, and POD) and directly scavenging ˙OH, it can regulate ROS balance to protect cells and tissues from oxidative damage.
Furthermore, through rational adjustment of metal components and optimizing the valence states of metal active sites, the catalytic performance of active centers can be further enhanced. Zhang et al. successfully developed a symbiotic nanozyme catalytic system with CAT-like and SOD-like enzymatic activities by controlling metal active sites through electron transfer and constraint anchoring, modulating vacancy concentrations, and enhancing electron transport.40 The nanozyme exhibited increased Ce3+/Ce4+ and Mn3+/Mn2+ ratios of 0.229 and 2.043, respectively, which were higher than those of the control samples (Fig. 1(A)). In chemical environments, it demonstrated extremely high affinity for H2O2 (Km = 7.194 mM) and a Vmax value of 5.863 μM s−1, significantly surpassing those of other Ce-based and Mn-based nanozymes (Fig. 1(B)). This demonstrates its superior CAT-like enzymatic activity. Furthermore, they validated the SOD-like activity of the nanozyme using the nitroblue tetrazolium chloride (NBT) colorimetric assay. As shown in the experimental data of Fig. 1(C), the system exhibited strong absorption at 580 nm after NBT addition, with the CeOx/Mn3O4 treatment group displaying a significantly greater reduction in the 589 nm absorption peak compared to controls. Notably, a 20 μg mL−1 nanozyme solution exhibited 82.88% O2˙− scavenging efficiency at room temperature. Additionally, Fig. 1(D) reveals that the SOD-like activity of CeOx/Mn3O4 (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 176.05 U mg−1) was significantly higher than that of other control groups. In this system, CeOx (bacteria-shaped) nanoclusters are firmly anchored on the octahedral Mn3O4 (root-shaped) nanocarriers to form a biomimetic nanozyme system (CeOx/Mn3O4) with multiple active sites (Fig. 1(E)). As shown in Fig. 1(F), the tightly coupled restricted anchoring facilitates electron transfer, and the increase in vacancies promotes electron transport. These two factors effectively improve the catalytic activity of the system, providing insights for the design of nanozymes with high catalytic activity.
176.05 U mg−1) was significantly higher than that of other control groups. In this system, CeOx (bacteria-shaped) nanoclusters are firmly anchored on the octahedral Mn3O4 (root-shaped) nanocarriers to form a biomimetic nanozyme system (CeOx/Mn3O4) with multiple active sites (Fig. 1(E)). As shown in Fig. 1(F), the tightly coupled restricted anchoring facilitates electron transfer, and the increase in vacancies promotes electron transport. These two factors effectively improve the catalytic activity of the system, providing insights for the design of nanozymes with high catalytic activity.
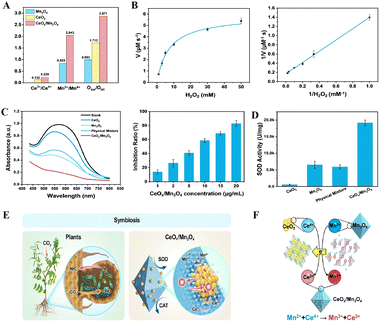 | ||
| Fig. 1 (A) The ratios of Ce3+/Ce4+, Mn3+/Mn2+, and Osur/Olat in Mn3O4, CeO2 and CeOx/Mn3O4 were obtained using XPS. (B) Catalytic kinetics of CeOx/Mn3O4 CAT-like activity: Michaelis–Menten curves obtained by varying H2O2 concentrations (left); corresponding Lineweaver–Burk plot with H2O2 as the substrate (right). (C) SOD-like activity simulation of CeOx/Mn3O4: UV absorption spectra of SOD-like activity in nanozyme catalytic systems measured by the NBT staining method (left); inhibition rate of O2˙− by different concentrations of CeOx/Mn3O4 at room temperature (right). (D) CeOx/Mn3O4 exhibited superior SOD-like activity compared with other control groups. (E) Corresponding schematic illustration of rhizobium-like CeOx nanoclusters confined and anchored on root-like Mn3O4 octahedral nanocarriers, leading to lattice interweaving and electron transfer from Mn2+ to Ce4+. (F) Diagram of electron transfer between Mn and Ce atoms.40 Copyright 2024, John Wiley and Sons. | ||
A heterojunction is a structure formed by the interfacial bonding of two or more distinct materials (typically with different band gaps). Its unique structural configuration often endows materials with distinctive physical and chemical properties. The introduction of chemical bond coupling and band structure modulation at the material interface can significantly enhance the catalytic performance of nanozymes.41–43 As shown in Fig. 2(A), a research study has obtained a Ni/CoMoO4 heterostructure nanozyme through interface engineering by introducing a large number of Ni–O–Co bonds.44 Compared with CoMoO4, it exhibits a larger surface area and more abundant pores, which provide sufficient space for active sites and promote substrate adsorption. During the reaction process, electrons are transferred from Ni0 to Co3+, and Co3+ is converted into Co2+, which promotes the reaction kinetics and is beneficial to improving the catalytic activity. In this Ni/CoMoO4 heterostructure with strong Ni–O–Co bonds, the strong electronic interaction (Ni–O–Co bonds) between Ni and CoMoO4 promotes charge transfer and reduces the reaction energy barrier. Fig. 2(B) illustrates the catalytic mechanisms of the heterojunction nanozyme for OXD-like (left) and POD-like (right) enzymatic activities. Ni/CoMoO4 with OXD-like activity promotes the generation of O2˙− and oxygen vacancies (OVs), thereby further oxidizing TMB. Briefly, the POD-like catalytic process of Ni/CoMoO4 involves the following steps: (1) substrate adsorption: H2O2 binds to the active sites; (2) electron transfer: electrons from the Ni0–O–Co3+ bonds in Ni/CoMoO4 are transferred to H2O2, accelerating the critical reaction step (1/2H2O2 → ˙OH); and (3) TMB adsorption: lone pair electrons from amino groups of TMB interact with Ni2+–O–Co2+, restoring Ni2+–O–Co2+ to the initial Ni0–O–Co3+ state. This facilitates electron transfer from Ni0–O–Co3+ to H2O2. It not only breaks the linear relationship between the active sites and reaction intermediates in traditional nanozymes but also significantly enhances the dual-enzyme-like activity.
 | ||
| Fig. 2 (A) Schematic diagram of Ni/CoMoO4 synthesis through interfacial engineering. (B) Study on the enzyme-like catalytic mechanism of Ni/CoMoO4: OXD-like enzyme (left) and POD-like enzyme (right).44 Copyright 2022, ACS. (C) Investigation of antioxidant enzyme activity differences in Pd nanocrystals with distinct surface facets using ESR experiments: CAT-like activity (left, samples containing 10 mM H2O2, 0.1 mM 15N-PDT, with 25 μg mL−1 Pd naonocubes/octahedrons and without (control) vs. 10 U mL−1 CAT); SOD-like activity (right, O2˙− in the Xan/XOD generating system, without (control) and with 25 μg mL−1 Pd nanocrystals vs. 1 U mL−1 SOD).45 Copyright 2016, ACS. | ||
Besides, the regulation of the catalytic activity of nanozymes is also closely related to their surface morphology and exposed crystal planes. Chen et al. compared the enzyme-like activities of Pd nanocubes and Pd octahedrons and found a close relationship between the surface energy of Pd nanocrystals and their antioxidant enzyme-like activities.45 Compared with Pd nanocubes with a higher-energy {100} facet, Pd octahedrons with a lower-energy {111} facet exhibit higher SOD-CAT-like antioxidant activity. They investigated the differences in antioxidant enzyme-like activities between two surface-faceted Pd nanocrystals using electron spin resonance (ESR) (Fig. 2(C)). Notably, the dissolved oxygen rate of Pd octahedrons increased more rapidly than that of Pd nanocubes, indicating that Pd octahedrons exhibits higher CAT-like activity compared to cubic Pd. Similarly, Pd octahedrons demonstrated superior scavenging activity toward O2˙− radicals relative to nanocubic Pd. Meanwhile, the results of theoretical computational simulations show that Pd cubes with {111} facets exhibit greater H2O2-scavenging activity and O2˙−-scavenging activity, which is consistent with the experimental observations. This indicates the potential influence of the morphology (surface energy) of nanozymes on their activity.
Non-metallic nanozymes mainly refer to NMs based on carbon structures. Compared with certain metal-based nanozymes, carbon-based nanozymes eliminate the risk of metal ion leaching and exhibit superior environmental compatibility, thereby offering greater potential for sustainable agricultural development. At appropriate concentrations, these nanozymes can penetrate into plants and affect their metabolic processes. As a result, they have a positive impact on plant growth, showing great potential in crop treatment.46,47 The shape of carbon-based nanozymes influences their relevant effects in plant therapy. A recent report compared the priming effects of tubular single-walled carbon nanotubes (SWNTs) and graphene oxide (GO) on the phytopathogen Pseudomonas syringae pv tomato DC3000. Fig. 3(A) displays the experimental treatment process, where plant leaves were treated with different conditions: water, 1 mg L−1 or 10 mg L−1 SWNTs, or GO. Compared with GO, plants treated with SWNTs reduced the pathogen load by 56% when resisting the plant pathogen, showing a stronger immune response (Fig. 3(B)). Moreover, at a concentration of 10 mg L−1, SWNTs exhibited greater potential in promoting plant growth. The plate counting assay in Fig. 3(C) further validated the results related to plant growth promotion. As shown in Fig. 3(D), EDF-HSI imaging is utilized for visualization, demonstrating that SWNTs were only detectable at a depth of 0.8 cm. The reason for this result is that the tubular structure of SWNTs enables them to be transported farther within the plant, triggering a more intense wound response. However, coating SWNTs with bovine serum albumin weakens this response.48 The sharp edges and special morphologies of carbon NMs facilitate their penetration of cell membranes and cell walls. Moreover, carbon NMs have the potential to mimic relevant mechanical stresses. Therefore, the immune signals they induce are similar to the wound responses typically triggered by mechanical stimuli. This indicates that plants treated with carbon NMs may exhibit stronger resistance to certain plant pathogens.
 | ||
| Fig. 3 (A) Schematic diagram of 4–5-week-old plants subjected to different treatments and bacterial infection. (B) Transcriptional level analysis of oprF gene by qPCR at 24 hours post-infection. (C) Corresponding plate counting analysis. (D) Visualization of NM transportation in leaves using EDF-HSI imaging technology (red pixels represent NM distribution identified by hyperspectral features; blue pixels indicate the presence of NMs).48 Copyright 2024, ACS. | ||
Based on this, Table 1 presents the properties of carbon-based nanozymes with varied shapes and dimensionalities. 0 D carbon NMs (carbon quantum dots and fullerenes) have a relatively high specific surface area and high surface energy. They exhibit excellent substrate affinity and enzyme-like activity. The easily modifiable nano-fullerenes have a highly symmetric spherical structure and can act as electron acceptors, playing a role similar to SOD.30,49 1D CNTs possess a large specific surface area, high strength, and excellent physical and chemical properties (good electronic tunability, high anti-aggregation ability, and electrical conductivity).50,51 Li's team treated rice seedlings with low-concentration SWCNTs and multi-walled carbon nanotubes (MWCNTs). The results showed that the activities of SOD and POD enzymes in the rice seedlings increased, and the levels of O2˙− and H2O2 slightly increased. It promoted leaf growth and seedling development, accelerating the growth of rice seedlings.52 Compared to the SWCNT-treated group, seedlings subjected to MWCNT treatment exhibited a more pronounced increase in both O2˙− and H2O2 levels. Through multi-dimensional physiological impact studies, it has been demonstrated that under low-concentration CNT treatment, there may be a relationship between reactive oxygen species and plant hormones that promotes the growth of rice seedlings. 2D carbon materials such as graphene have relatively large lateral dimensions. Their unique geometric structures can provide more pores or defects, which is conducive to creating more active sites.53 GO is one of the important derivatives of graphene. The carboxyl, hydroxyl, epoxy and carbonyl functional groups contained in it endow GO with high water-dispersibility. Therefore, GO has been widely used in many fields. Among them, the applications of GO in the field of crop stress resistance mainly include resistance to drought stress and saline–alkali stress.54,55
| Type | Material | Structure properties | Advantage | Enzyme-like activity | Ref. |
|---|---|---|---|---|---|
| 0D | Carbon dots (CDs) and carbon quantum dots (CQDs) | Sp2-bonded carbon structures | Superior enzyme-like activity and substrate affinity | SOD, CAT, POD, and OXD | 30 |
| Rich in oxygen-containing functional groups | High charge transfer efficiency | ||||
| High surface energy and specific surface area | Facile surface functionalization | ||||
| Robust biocompatibility | |||||
| Fullerenes | Highly symmetric hollow cage-like structures | Superior photodynamic activity | SOD and POD | 49 | |
| Delocalized π-double bond structures | Superior enzyme-like activity and substrate affinity | ||||
| High surface energy and specific surface area | High charge transfer efficiency | ||||
| 1D | CNTs | Single-atom-thick 2D hexagonal carbon lattices | Good electronic tunability | POD and OXD | 50 and 51 |
| Large lateral dimensions | High anti-aggregation capability and electrical conductivity | ||||
| Unique geometric structures providing more defects or voids | Superior anti-aggregation capability | ||||
| High surface area | Superior enzyme-like activity | ||||
| Robust biocompatibility | |||||
| 2D | Graphene | Single-atom-thick 2D hexagonal carbon lattice | Easily modifiable surface functional groups | SOD and POD | 48 and 52 |
| Large lateral dimensions | Abundant catalytic sites | ||||
| Unique geometric structure enabling more defects/voids | Superior enzyme-like activity | ||||
| High surface area | |||||
| Transition-metal carbides/nitrides (MXenes) | Layered structure with regular geometric shapes | High electrical conductivity and hydrophilicity | POD, OXD, SOD, and CAT | 53–55 | |
| High surface area and abundant surface functional groups | Adjustable surface termination | ||||
| Controlled thickness with high mechanical strength |
To meet the requirements of different application scenarios, contribute to the sustainable development of agriculture, enhance crop stress resistance and yield, it is crucial to design nanozymes with special structures.
2.2 Particle size and surface charge characteristics
The size effect of nano-pesticides refers to the significant changes in their physicochemical properties and application performance compared with traditional pesticides due to their particle sizes being within the nanoscale range. Through the size effect, the number of active sites on the surface of NMs per unit mass can be adjusted, thereby enhancing or reducing the catalytic activity of nanozymes.56,57 The particle size of nanozymes plays a decisive role in their effective penetration and performance in plants.Generally speaking, nanozymes with smaller sizes have a larger specific surface area, and the proportion of surface atoms is relatively high. This makes them more likely to interact with the surrounding medium or dispersant, thus achieving better dispersibility. Moreover, they have more active sites to bind with substrates, thereby enhancing the catalytic activity.58,59 A study conducted by Yan's group concluded that 30 nm Fe3O4 NPs exhibit stronger POD-like activity than 150 nm and 300 nm Fe3O4 NPs.23 This difference in activity is closely related to the size of the nanocrystals, which reflects the influence of the size effect on catalytic activity. In addition, a recent study on the foliar application of iron oxide nanozymes on soybean leaves further clarified the relationship between size and effects, providing insights for the precise design and application of nanozymes.60 The experimental results showed that foliar application of γ-Fe2O3 nanozymes with different particle sizes had the significant impact on soybean growth, nitrogen-fixation ability, yield, and nutritional quality. This impact was clearly size-dependent. As shown in Fig. 4(A), treatment with 30 and 50 mg L−1 S–Fe2O3 NMs resulted in 55.4% and 38.7% increases in fresh shoot biomass of soybean, respectively, which were 2.0-fold and 1.4-fold higher than those in the commercial iron fertilizer group (160 mg L−1 EDTA–Fe). Notably, Fig. 4(B) reveals that foliar application of 30 mg L−1 γ-Fe2O3 NMs enhanced shoot biomass in the order of S–Fe2O3 NMs > M-Fe2O3 NMs > L-Fe2O3 NMs, demonstrating a distinct size-dependent effect. In promoting soybean growth, enhancing yield, and improving seed quality, S–Fe2O3 NMs demonstrated greater advantages (Fig. 4(C)). After six applications of S–Fe2O3 NMs, the grain dry weight per soybean plant increased by 35.3%, which was significantly higher than that of the EDTA–Fe treatment group. Its effect was superior to that of the commercially available iron fertilizer (EDTA–Fe). It showed greater advantages in promoting soybean growth, increasing yield, and improving seed quality. This further reveals the crucial role of particle size in plant growth promotion.
 | ||
| Fig. 4 (A) Fresh biomass of soybean shoots and roots after foliar application of 160 mg L−1 EDTA–Fe and varying concentrations (10, 30, 50, and 100 mg L−1) of S–Fe2O3 NMs. (B) Fresh biomass of soybean shoots and roots after foliar application of 160 mg L−1 EDTA–Fe and 30 mg L−1 γ-Fe2O3 NMs with different sizes. (C) Yield of soybean plants after foliar application of 160 mg L−1 EDTA–Fe and 30 mg L−1 S–Fe2O3 NMs (3 or 6 applications).60 Copyright 2022, ACS. (D) High spatiotemporal resolution imaging of NPs in plants and their interaction model with leaves.62 Copyright 2020, ACS. (E) Reconstructed nano-CT images of Arabidopsis root tips; hyperspectral mapping visualization of Au NPs in root tips; and schematic diagram of distribution and translocation of positively or negatively charged Au NPs in roots.63 Copyright 2017, ACS. | ||
However, nanozyme dimensions are not universally optimized by minimizing size. Excessively small NPs may induce excessive accumulation within plant cells, potentially disrupting normal cellular metabolic processes.30,61 In plant applications, the pore sizes of cell walls and stoma may vary significantly across different plant species. Consequently, practical implementations require tailored design of nanozymes with species-specific dimensions and surface charge characteristics. A study demonstrated that the effective delivery of NPs to guard cells, extracellular spaces, and chloroplasts is dependent on their size, charge, and the specific plant species involved.62 They discovered that hydrophilic NPs with hydrodynamic diameters below 20 nm and 11 nm, respectively, and a positive surface charge (>15 mV), exhibited the highest foliar delivery efficiency into guard cells, extracellular spaces, and chloroplasts of cotton and maize. The enhanced affinity between positively charged NPs and the negatively charged plant cell walls, coupled with the negative transmembrane potential of cell membranes (Fig. 4(D)) likely explains their superior foliar delivery efficiency.
However, conflicting perspectives exist regarding the role of NP surface charge. One study demonstrated that negatively charged AuNPs (approximately 12 nm, −32 mV) were almost entirely distributed within the roots of Arabidopsis thaliana, predominantly localized in the intercellular spaces and near the cell walls. In contrast, positively charged AuNPs (approximately 2 nm, +46 mV) were largely entrapped as large aggregates in the outer mucilage layer of the root cap, and thus no internal root penetration was observed (Fig. 4(E)).63 Another study investigated the transport efficiency and pathways of negatively and positively charged CDs in plants. The results demonstrated that negatively charged CDs displayed higher transport efficiency in cucumber and cotton roots compared to positively charged counterparts. And they were more effectively translocated from roots to leaves. This phenomenon might be attributed to the predominant transport of negatively charged CDs via symplastic and apoplastic pathways, whereas positively charged CDs primarily utilized the apoplastic route.64 These divergent findings may arise from differences in plant species, growth stages, and variations in synthesized NMs.
In summary, researchers should prioritize comprehensive considerations of the size-dependent effects and surface charge characteristics of agronanozymes in agricultural applications. A critical objective is to establish an optimal size range that ensures not only efficient cellular internalization but also maintains high catalytic activity and biocompatibility.
2.3 Stability
Superior stability serves as the foundation for nanozymes to exhibit high catalytic efficiency, enabling them to function persistently within plant tissues or soil environments. This durability allows nanozymes to mediate critical biological processes, such as nutrient assimilation and metabolic regulation in plants. By optimizing their size, morphology, and surface modifications, highly stable nanozymes can minimize denaturation-induced deactivation while precisely tuning their catalytic properties to meet specific demands across diverse application scenarios.65,66 The intrinsic properties of nanozymes significantly influence stability. Excessively small dimensions may induce stability degradation, as the heightened surface energy associated with nanoscale materials can lead to aggregation or oxidative damage, thereby compromising long-term structural integrity. Concurrently, environmental conditions (external factors), including pH, temperature, and ionic strength, exert varying degrees of influence on nanozyme stability. Robust environmental tolerance enables nanozymes to maintain high catalytic activity under dynamic conditions, resisting structural alterations, aggregation, or deactivation. This dual resilience ensures prolonged operational lifespan and enhanced stability in practical applications.56,67 In addition, for some metal-based nanozymes, poor stability may lead to the leaching of heavy metal ions, which may in turn impact the environment. Therefore, to achieve better sustainability, surface modification methods such as physical barrier formation (anchoring a metal with a porous structure),68 chemical anchoring,69,70 core–shell structure formation,71 and ligand engineering72 can be considered to enhance the stability of metal-based nanozymes and reduce the risk of spillover of metal active centers. In a recent research study, a ZIF-8-pPt nanozyme was constructed, whose catalytic activity is 18 times that of traditional monodispersed Pt particles.73 Experiments show that the ZIF-8-pPt nanozyme exhibits good stability. It maintains stable catalytic activity in the temperature range of 20–50 °C and has a wider pH tolerance range than natural HRP (Fig. 5(A)). Moreover, the structure of this nanozyme does not change significantly after multiple catalytic reactions, indicating excellent reusability and long-term stability. This stability primarily originates from the physical confinement effect, where the aggregation and migration of Pt nanoclusters are suppressed through the pore-channel encapsulation of ZIF-8, while the weak chemical anchoring within the framework also plays an auxiliary role in stabilizing the active sites. The Pt nanoclusters are uniformly distributed in the ZIF-8 framework and tightly bound to it. This structure enhances the catalytic activity and improves the overall stability of the nanozyme. As a MOF material, ZIF-8 possesses a highly ordered porous structure and excellent chemical stability. ZIF-8 provides a stable microenvironment for the Pt nanoclusters, effectively preventing their aggregation and deactivation through uniform distribution within the framework and strong interfacial interactions. Additionally, a study has demonstrated that structural optimization at the single-atom level can reduce stability limitations. They have demonstrated the design of a 3D biomimetic Pt–NC SAzyme by encapsulating platinum 2,4-pentanedionate within ZIF-8 molecular cages followed by pyrolysis.74 In contrast to the physical confinement effect in ZIF-8-pPt, the Pt–NC SAzyme achieves atomic-level chemical anchoring through high-temperature pyrolysis-induced Pt–N4 coordination bonds, thereby ensuring stable retention of single atoms under extreme conditions and during multiple cycles. DFT calculations revealed that the 3D porous structure of Pt–NC SAzyme enables a lower free energy barrier and shorter reaction pathway during the catalytic decomposition of H2O2. This 3D mass transport channels not only enhance catalytic activity but also reduce the risk of active site poisoning by minimizing intermediate residence time, thereby improving structural stability. As shown in Fig. 5(B), the Pt–NC SAzyme exhibited higher affinity for H2O2, with a significantly lower Km value compared to those of Pt NPs and the NC group. Furthermore, the CAT-like specific activity (SA) further confirmed the superior catalytic activity of the Pt–NC SAzyme. In Fig. 5(C), the SA of the Pt–NC SAzyme (435.4 U mg−1) was significantly higher than those of the Pt NP group (207.8 U mg−1) and the NC group (34.1 U mg−1). The low activity of nitrogen-doped carbon nanozymes (NC) underscores the necessity of single-atom sites. The aforementioned research demonstrates that 3D biomimetic structural optimization through a progressive design strategy transitioning from physical confinement of NPs to chemical bonding of single atoms significantly enhances the catalytic stability, providing a theoretical foundation for precise regulation of active sites and improvement of catalytic stability. | ||
| Fig. 5 (A) Temperature-dependent catalytic activity profiles of HRP and synthetic nanozymes.73 Copyright 2024, John Wiley and Sons. (B) CAT-like activity investigation of Pt–NC SAzyme, NC, and Pt NPs (substrate: H2O2): Michaelis–Menten kinetic analysis (left) and Lineweaver–Burk plot (right). (C) Comparative analysis of specific activity (SA) among Pt–NC SAzyme, NC, and Pt NPs.74 Copyright 2024, John Wiley and Sons. | ||
2.4 Biocompatibility
As plants constitute integral components of ecosystems, the application of nanozyme-based pesticides may impose potential impacts on cohabiting organisms within the same ecological matrix, such as rhizosphere microbiota. Alterations in microbial diversity and metabolic activity could disrupt plant-microbe symbiosis, root-mediated nutrient cycling, and soil fertility, ultimately jeopardizing crop health and in severe cases, it may destabilize ecological equilibrium.75,76 Therefore, high biocompatibility is one of the properties that must be possessed by nanozymes for agricultural use. It ensures that nanozymes can promote the healthy growth of plants with less negative effects. Li et al. investigated the impact of foliar application of Salvia miltiorrhiza-derived CDs on lettuce resilience under salt stress.77 The related mechanism is illustrated in Fig. 6(A). CDs mimic SOD enzyme activity and exhibit excellent ROS-scavenging capacity. CDs catalyze the conversion of O2˙− to H2O2, and the generated H2O2 is subsequently transformed into ˙OH via Fenton reactions. CDs with potent ˙OH-scavenging capability reduce them to H2O2. Cytotoxicity assays confirmed the excellent biocompatibility of CDs (Fig. 6(C)). In vivo, CDs effectively translocated into plant cells and scavenged intracellular ROS (Fig. 6(B)), thereby alleviating salt stress in Italian lettuce. This resulted in a significant increase in both root and leaf biomass of salt-stressed lettuce plants (Fig. 6(D)).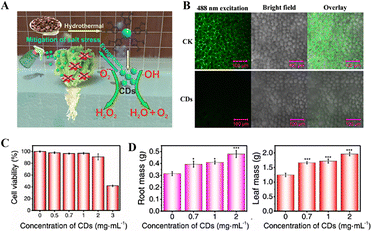 | ||
| Fig. 6 (A) Schematic illustration of the mechanism by which CDs alleviate oxidative damage in salt-stressed Italian lettuce. (B) Confocal imaging of ROS levels in mesophyll cells of Italian lettuce under different treatments: TES buffer (CK) and 2.0 mg mL−1 CDs-180 infiltrated leaves, visualized by DCFH2-DA staining. (C) Cytotoxicity evaluation of CDs-180 via CCK8 assay. (D) Effects of varying concentrations of CDs-180 on root dry weight (left) and leaf dry weight (right) in salt-stressed Italian lettuce seedlings.77 Copyright 2021, ACS. | ||
In parallel, some metal-based nanozymes have shown potential in biopharmaceutical and environmental applications due to their low biotoxicity, efficient bioaccumulation in tissues, and moderate biocompatibility.78–80 Notably, Fe3O4 NPs were found to be predominantly absorbed by barley roots, significantly enhancing plant growth at elevated concentrations without phytotoxicity, thereby validating their biocompatibility.81 To engineer nanozymes with superior biosafety, priority should be given to plant-essential elements (e.g., Fe, Zn) or low-toxicity metals (e.g., TiO2), while avoiding heavy metals during synthesis. Given the potential ecological risks posed by high-concentration nanozymes to plants and other organisms.82 Field trials should be conducted to establish safe and effective concentration thresholds.
3. Nanozymes capable of mimicking enzyme activity: classification
Nanozymes, used in sustainable agriculture, are mainly used to alleviate various stresses faced by crops by mimicking oxidoreductase activity. They can regulate the ROS balance of plants and increase their resistance. Nanozymes have significant catalytic activity and environmental tolerance and are considered as promising alternatives to natural enzymes. In this study, we comprehensively discuss various types of nanozymes for sustainable agriculture, including superoxide dismutase-like (SOD-like), oxidase-like (OXD-like), peroxidase-like (POD-like), catalase-like (CAT-like), laccase-like (LAC-like). The following research work on nanozymes with different mimetic enzyme activities is presented with the aim of providing a reference when selecting the appropriate nanozymes for different agricultural applications.3.1 The oxidoreductase family
 | (1) |
SOD-like nanozymes have natural SOD-like activities that can alleviate excessive accumulation of ROS (O2˙−) through adsorption activation and electron transfer. They are important artificial enzymes that help plants to regulate the balance of ROS and improve stress tolerance.83,84 Recently, various types of nanozymes with SOD-like activity have emerged. For example, Yu et al. developed a multiscale laminated nanozyme with a honeycomb-like morphology (MnO2@GO) following the synthetic strategy outlined in Fig. 7(A).85 The catalytically active δ-MnO2 nanosheets were uniformly distributed on the GO surface. MnO2@GO is characterized by a large specific surface area and abundant active sites. Its unique honeycomb-like multiscale laminated structure provides a confined space for the adsorption of O2˙− and catalytic reactions. Consequently, it is endowed with excellent SOD-like activity. As demonstrated by the WST-8 colorimetric assay in Fig. 7(B), the absorption peak at 450 nm (of WST-8 formazan) significantly decreased upon the addition of the nanozymes, corresponding to a SOD-specific activity of 161 U mg−1 (Fig. 7(C)). Furthermore, MnO2@GO exhibited a 95.5% specific inhibition rate against O2⋅- (Fig. 7(D)), which was 3-fold and 17-fold higher than those of the GO group and MnO2 group, respectively. These results demonstrate the robust antioxidant performance of MnO2@GO and propose a novel strategy for developing high-efficiency SOD nanozymes.
 | ||
| Fig. 7 (A) Synthesis strategy and honeycomb-like morphology schematic of MnO2@GO nanozymes. (B) UV-Vis absorption spectra of SOD-like activity in MnO2@GO nanozymes (0.5 mg mL−1) measured by WTS-8 colorimetric assay. (C) Determination of SOD-like specific activity in MnO2@GO. (D) Comparative analysis of SOD activity among MnO2, GO, and MnO2@GO.85 Copyright 2023, Springer Nature. (E) Catalytic cycle Gibbs free energy profiles of hydroxyl-free (left) and hydroxyl-containing (right) CDs SOD nanozymes for SOD-like activity. (F) Quantitative analysis of SOD-like activity in CDs (prepared from activated charcoal, graphite powder, and carbon black) using the SOD assay kit (WST-1).86 Copyright 2023, Springer Nature. (G) Determination of SOD-like activity in CDs by the WST-1 assay. (H) Comparative analysis of SOD-like activity between CDs-PS (passivated with carboxyl, hydroxyl, and amino groups) and CDs-PS-Hy (passivated with hydroxyl and amino groups).87 Copyright 2023, John Wiley and Sons. | ||
Recently, many CDs with enzyme-like activities have been reported by researchers. One study has also developed a carbon dot nanozyme with SOD activity (CDs SOD nanozyme).86 By comparing the SOD-like enzymatic activities of CDs SOD nanozymes with and without hydroxyl groups, they validated the SOD-like catalytic mechanism of the CDs SOD nanozyme. Experimental results revealed that the activity of the CDs SOD nanozyme primarily depends on the binding of hydroxyl and carboxyl groups on its surface to superoxide anions, as well as electron transfer between carbonyl groups and π-systems. Computational analysis further demonstrated that the hydroxyl-containing CD nanozyme exhibited a significantly lower binding energy (−0.65 eV) with HO2˙ radicals compared to the hydroxyl-free counterpart (−0.54 eV), indicating an enhanced ability of the hydroxylated CDs nanozyme to scavenge HO2˙ radicals (Fig. 7(E)). Additionally, Fig. 7(F) shows that CDs derived from activated carbon displayed ultrahigh SOD-like activity (1.1 × 10 U mg−1), providing a mechanistic foundation for their role in mitigating oxidative damage. In addition, another study used CDs as the matrix. By regulating the sp2/sp3 hybrid structure of the carbon core and surface functional groups, catalytic activity was improved.87 This work complements the aforementioned study: both investigations confirm that the π-conjugated system serves as the central mediator for electron transfer. However, the former emphasizes the electrostatic interactions of hydroxyl/carboxyl groups, while the latter enhances the binding between CDs and O2˙− through the introduction of oxygen/nitrogen-containing functional groups. Optimizing the surface charge distribution of CDs promoted their electrostatic interaction with negatively charged O2˙−. The experimental results showed that their SOD-like activity exceeded 4000 U mg−1 (Fig. 7(G)). Furthermore, they demonstrated the influence of carboxyl and hydroxyl groups on the SOD-like activity of CDs through surface modification and passivation using 1,3-propanesulfonate (PS) and 4-sulfophenyl isothiocyanate sodium salt (SPI), respectively. As shown in Fig. 7(H), the SOD-like activity of CDs-PS (563 U mg−1) was significantly lower than that of CDs-SPI (1147 U mg−1). Notably, CDs-PS-Hy (883 U mg−1) exhibited higher SOD-like activity than CDs-PS but still underperformed compared to CDs-SPI. These results confirm that carboxyl and hydroxyl groups enhance the SOD-like activity of CDs. These two studies collectively demonstrate that the synergistic interaction between hydroxyl and carboxyl groups constitutes a universal core mechanism underlying the SOD activity of CDs. The former investigation (PS/SPI experiments) primarily validated the essentiality of hydroxyl/carboxyl groups, while the latter (binding energy calculations) quantitatively elucidated the interaction strength between functional groups and free radicals, thereby providing multi-scale insights into mechanistic elucidation.
 | ||
| Fig. 8 (A) Schematic diagram of the catalytic mechanism of IOPM. (B) Validation of OXD-like activity in IOPM by UV-Vis spectroscopy with TMB as the substrate. (C) Steady-state kinetic analysis of IOPM.89 Copyright 2024, Elsevier. (D) EPR spectra of nanowires.90 Copyright 2024, Elsevier. (E) Schematic illustration of the catalytic mechanism of hybrid NMs.91 Copyright 2024, Elsevier. | ||
 | (2) |
POD-like nanozymes catalyze substrate oxidation through the decomposition of H2O2 to produce ROS, electron transfer, or cascade reactions with synergistic effects.92–94 To date, an increasing number of nanozymes capable of mimicking both the catalytic activity and structural features of natural POD enzymes have been reported. For example, Li's group, through the precise geometric and electronic structure design.95 A geometry similar to that of natural peroxidases was constructed through precise phosphorus (P) and nitrogen (N) coordination. As illustrated in Fig. 9(A), using ZIF-8 as the carbon and nitrogen precursor, the metal active centers were first anchored by precursor P while modulating the local coordination structure. Subsequently, a polymerization reaction was performed to coat the ZIF-8 surface with Fe ions and monomers of poly-(cyclotriphosphazene-co-4,4′-diaminodiphenylether) (PZM), forming a Fe/ZIF-8@PZM core–shell composite. Finally, pyrolysis of the composite yielded the FeN3P-SAzyme. Experimental results demonstrated that FeN3P-SAzyme exhibits significantly higher catalytic activity (316 U mg−1), surpassing those of Fe3O4 nanozymes (9.12 U mg−1) and P-free FeN4-SAzyme (33.8 U mg−1) by approximately 30-fold and 10-fold, respectively (Fig. 9(B)). Kinetic parameter studies (Fig. 9(C)) revealed that FeN3P-SAzyme achieves a catalytic efficiency (Kcat/Km = 1.40 × 108 M−1 min−1) comparable to that of the natural HRP enzyme (Kcat/Km = 1.15 × 107 M−1 min−1). This indicates that the engineered structure successfully mimics both the catalytic activity and kinetic properties of natural POD by replicating the metal active sites of native enzymes. In contrast to the single-atom design of FeN3P-SAzyme, another study has developed a biomimetic sulfur–iron–heme nanozyme (HCFe) with selective peroxidase POD-like activity.96 Through a supramolecular self-assembly approach, they integrated heme and [Fe–S] structures by leveraging π–π stacking interactions between Fmoc-modified L-cysteine and heme (Fig. 9(D)). Comparative analysis of the nanozyme's POD-like and CAT-like activities under pH = 6.5 (tumor microenvironment) and pH = 7.4 (physiological pH) conditions revealed a pH-responsive activation state. Specifically, the HCFe nanozyme exhibited no CAT-like activity, while its POD-like activity remained inactive at pH = 7.4 but became enzymatically active at pH = 6.5 (Fig. 9(E) and (F)). These findings highlight the critical role of sulfur coordination in the active center in governing catalytic selectivity, which serves as a key factor enabling the mimicry of the native POD enzymatic mechanism. This design strategy shares with FeN3P-SAzyme a common foundation in coordination environment engineering for nanozyme development, yet demonstrates complementary approaches: the former achieves targeted catalysis through sulfur coordination-mediated CAT activity suppression, while the latter directly enhances POD activity via phosphorus coordination. Both strategies fundamentally rely on coordination environment modulation as their core mechanism, but undergo optimization for distinct application scenarios.
 | ||
| Fig. 9 (A) Illustration of the preparation process of FeN3P-SAzyme. (B) Comparative analysis of specific activity (U mg−1) among Fe3O4, FeN4-SAzyme and FeN3P-SAzyme nanozymes. (C) Kinetic parameters of Fe-doped active sites in HRP enzyme, Fe3O4, FeN4-SAzyme and FeN3P-SAzyme nanozymes.95 Copyright 2021, Springer Nature. (D) Schematic diagram of biomimetic sulfur–iron–heme nanozyme synthesis. (E) Investigation of POD-like activity: reaction time curves of the TMB colorimetric reaction catalyzed by HCFe nanozymes under varying pH conditions. (F) Study on CAT-like activity: dissolved O2 production from H2O2 decomposition catalyzed by HCFe nanozymes at different pH values.96 Copyright 2024, Springer Nature. | ||
To further expand the dimensions of material design, Feng and colleagues developed a novel transition metal high-entropy nanozyme (HEzyme) by leveraging the structural entropy effect.97 They utilized five transition metals, namely Mn, Fe, Co, Ni, and Cu, and broke through the component limitations of traditional single-metal or low-entropy alloys through multi-metal synergy. First, a uniform atomic distribution was achieved via the high-entropy effect. The diverse surface-active sites formed (such as metal–oxygen bonds and vacancies) could mimic the multi-active centers of natural enzymes. Then, the d-orbital coupling of different metals (e.g., the strong oxidizing property of Fe, the electron-transfer ability of Co, and the adsorption characteristics of Cu) was exploited to synergistically optimize the electron density and band structure, promoting HEzyme to decompose H2O2 into ˙OH radicals. Experiments showed that the Kcat/Km value of HEzyme was close to that of natural HRP, indicating that its POD-like activity was comparable to that of HRP. Moreover, its catalytic efficiency and substrate affinity were significantly superior to those of traditional nanozymes. They achieved highly efficient POD-like activity through their unique surface atomic configuration and multisite orbital-coupling properties.
 | (3) |
CAT-like nanozymes can decompose H2O2 through adsorption-activated and redox reactions. Many NMs show CAT-like activity that catalyzes the conversion of H2O2 to H2O and O2.
For example, as illustrated in Fig. 10(A), a nanocomposite ZnO@PDA–Mn was synthesized by coating zinc oxide NPs (ZnO) with polydopamine (PDA) and manganese (Mn). The study monitored the O2 generation capacity of 100 μg mL−1 ZnO@PDA–Mn under varying H2O2 concentrations, generating a Michaelis–Menten curve (Fig. 10(B)). Subsequent Lineweaver–Burk fitting (Fig. 10(C)) determined the kinetic parameters of ZnO@PDA–Mn, with a Km of 116.3 mM and a Vmax of 6.03 mg (L min)−1, demonstrating its exceptional CAT-like activity in decomposing H2O2 into H2O and O2.99
 | ||
| Fig. 10 (A) Synthesis strategy of ZnO@PDA–Mn. (B) Steady-state kinetic analysis of CAT-like activity in ZnO@PDA–Mn with varying concentrations of H2O2. (C) Lineweaver–Burk plot of CAT-like activity in ZnO@PDA–Mn at 25 °C (substrate: H2O2).99 Copyright 2024, RSC. (D) Synthesis strategy of Co–N3PS SAzyme. (E) CAT-like specific activity (U μmol−1 Co atom) of Co–N3PS SAzyme.100 Copyright 2023, John Wiley and Sons. | ||
In addition, another study has synthesized an N, P, and S uniformly co-doped hollow carbon (NPS-HC) framework using poly (cyclotriphospazene-co-4,4′-sulfonyldiphenol) (PZS) and ZIF-8 as dual templates. Cobalt precursors were subsequently introduced to occupy the anchoring sites of the NPS-HC framework (denoted as Co2+/NPS-HC), successfully constructing a cobalt single-atom nanozyme with Co–N3PS active centers (Fig. 10(D)).100 By optimizing the coordination micro-environment of cobalt atoms (sulfur and phosphorus doping to regulate the electronic structure) and the reaction pathway (reducing the energy barrier for H2O2 decomposition) it precisely mimics the highly efficient catalytic mechanism of the natural CAT enzyme. Experimental results demonstrated that at pH = 10.8 (Fig. 10(E)), the Co–N3PS SAzyme exhibited a CAT-like activity of 7046 U μmol−1 Co atom, significantly surpassing the specific activity of native CAT (734.1 U μmol−1 Co atom). This highlights the Co–N3PS SAzyme's exceptional enzyme-mimicking performance, characterized by ultrahigh catalytic efficiency (approaching kinetic parameters of natural enzymes), substrate specificity (selective decomposition of H2O2 to H2O and O2), and structural stability (anchoring single-quantitative relationship between the active center configuration and performance has been revealed, providing a new “atom-level biomimetic” paradigm for the design of highly efficient artificial enzymes. Both aforementioned studies have overcome the limitations of conventional materials through biomimetic design (ZnO@PDA–Mn mimicking the Mn active site of CAT, and Co–N3PS simulating the cobalt porphyrin structure of CAT): the former elucidated the quantitative correlation between the Co–N3PS configuration and catalytic activity at the atomic scale. The latter achieved functional integration of antibacterial and antioxidant properties via a core–shell structure. Collectively, they validate that “modulating the microenvironment of active centers” constitutes the central strategy for enhancing nanozyme performance.
The mechanism by which the nanozymes mimic the activity of LAC is largely dependent on their unique catalytic center and electron transfer mechanism. LAC-like nanozymes usually promote electron transfer by mimicking the active center structure of natural LAC and using surface metal ions as the active site to coordinate with the substrate molecules (the electron transfer mechanism includes: substrate binding, electron transfer and oxygen reduction). This mimics the catalytic process of oxygen reduction to water by natural LAC through a single electron transfer mechanism.103 Wang et al. designed a novel species of biomimetic LAC-like nanozymes (CH–Cu). CH–Cu nanozymes were synthesized by liganding a cysteine–histidine dipeptide with copper ions (Fig. 12(A)).104 This design demonstrates high structural similarity to the T1 copper active center of natural LAC, while effectively addressing the environmental sensitivity of native LAC through implementation of a rigid coordination architecture. Experimental results revealed that at varying substrate concentrations, the CH–Cu nanozyme exhibited Michaelis–Menten kinetic parameters of Km and Vmax = 0.42 mM and 7.32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
× ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−3 mM min−1. This demonstrates not only substrate affinity comparable to natural LAC (Km = 0.41 mM), but also superior catalytic efficiency (Vmax = 6.41
10−3 mM min−1. This demonstrates not only substrate affinity comparable to natural LAC (Km = 0.41 mM), but also superior catalytic efficiency (Vmax = 6.41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
× ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−3 mM min−1). This performance advantage originates from the dipeptide ligands’ optimization of electron transfer pathways at the copper active centers. In addition, the CH–Cu nanozymes showed remarkable stability under the conditions of extreme pH, high temperature, long-term storage, and high-salt environments, and their performance was superior to that of natural LAC. For instance, under aqueous storage conditions at 25 °C, LAC exhibited a gradual decline in catalytic activity and became completely deactivated by Day 9 (Fig. 12(B)). In contrast, the CH–Cu nanozymes retained 72% of their catalytic activity throughout the 20-day storage period. The developed CH–Cu nanozymes integrate high catalytic activity with exceptional stability, demonstrating significant potential to address the environmental tolerance limitations and recovery challenges inherent in natural LAC systems. In another study, two multivalent cerium-based metal–organic framework (Ce-MOF) materials (Ce-UiO-66 and Ce-MOF-808) have been designed to mimic the catalytic function of LAC.105 These materials mimic the electron transfer mechanism of natural LAC by replacing traditional copper clusters with multivalent cerium (Ce3+/Ce4+) active centers, thereby broadening the metal selection scope for LAC mimicry. They selected 2,4-DP as a model substrate, demonstrating that the porous structure and high specific surface area of Ce-MOFs provide abundant active sites for substrate adsorption and catalytic reactions, thereby effectively enhancing their catalytic performance. Experimental results revealed that in the presence of LAC or Ce-MOFs, 2,4-DP was oxidized into quinone intermediates, which subsequently reacted with 4-AP to generate colored products detectable at UV510 nm (Fig. 12(C)). After 45 minutes of reaction, Ce-UiO-66 and Ce-MOF-808 produced significantly higher amounts of colored products compared to native LAC at equivalent mass concentrations, demonstrating their exceptional LAC-mimicking activity. In addition, Li's research team further integrated catalysis and separation technologies to design the GA–Cu nanozyme, utilizing Cu2+ as active centers with 2-aminoimidazole and glutathione (GSH) as ligands (Fig. 12(D)).106 Compared to the dipeptide coordination in CH–Cu, the –SH group of GSH dynamically regulates the Cu2+/Cu+ redox states, while the electron bridge constructed by imidazole groups reduces the Km value to 0.18 mM, demonstrating the effect of precise modulation of ligands on catalytic performance. Catalytic kinetics studies revealed (Fig. 12(E)) that GA–Cu exhibits a Vmax of 7.53 × 10−3 mM min−1, with its singlet oxygen (1O2)-dominated catalytic mechanism (validated by ESR) contrasting with the substrate direct oxidation pathways of CH–Cu and Ce-MOFs.
10−3 mM min−1). This performance advantage originates from the dipeptide ligands’ optimization of electron transfer pathways at the copper active centers. In addition, the CH–Cu nanozymes showed remarkable stability under the conditions of extreme pH, high temperature, long-term storage, and high-salt environments, and their performance was superior to that of natural LAC. For instance, under aqueous storage conditions at 25 °C, LAC exhibited a gradual decline in catalytic activity and became completely deactivated by Day 9 (Fig. 12(B)). In contrast, the CH–Cu nanozymes retained 72% of their catalytic activity throughout the 20-day storage period. The developed CH–Cu nanozymes integrate high catalytic activity with exceptional stability, demonstrating significant potential to address the environmental tolerance limitations and recovery challenges inherent in natural LAC systems. In another study, two multivalent cerium-based metal–organic framework (Ce-MOF) materials (Ce-UiO-66 and Ce-MOF-808) have been designed to mimic the catalytic function of LAC.105 These materials mimic the electron transfer mechanism of natural LAC by replacing traditional copper clusters with multivalent cerium (Ce3+/Ce4+) active centers, thereby broadening the metal selection scope for LAC mimicry. They selected 2,4-DP as a model substrate, demonstrating that the porous structure and high specific surface area of Ce-MOFs provide abundant active sites for substrate adsorption and catalytic reactions, thereby effectively enhancing their catalytic performance. Experimental results revealed that in the presence of LAC or Ce-MOFs, 2,4-DP was oxidized into quinone intermediates, which subsequently reacted with 4-AP to generate colored products detectable at UV510 nm (Fig. 12(C)). After 45 minutes of reaction, Ce-UiO-66 and Ce-MOF-808 produced significantly higher amounts of colored products compared to native LAC at equivalent mass concentrations, demonstrating their exceptional LAC-mimicking activity. In addition, Li's research team further integrated catalysis and separation technologies to design the GA–Cu nanozyme, utilizing Cu2+ as active centers with 2-aminoimidazole and glutathione (GSH) as ligands (Fig. 12(D)).106 Compared to the dipeptide coordination in CH–Cu, the –SH group of GSH dynamically regulates the Cu2+/Cu+ redox states, while the electron bridge constructed by imidazole groups reduces the Km value to 0.18 mM, demonstrating the effect of precise modulation of ligands on catalytic performance. Catalytic kinetics studies revealed (Fig. 12(E)) that GA–Cu exhibits a Vmax of 7.53 × 10−3 mM min−1, with its singlet oxygen (1O2)-dominated catalytic mechanism (validated by ESR) contrasting with the substrate direct oxidation pathways of CH–Cu and Ce-MOFs.
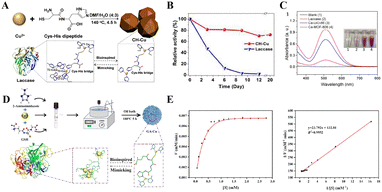 | ||
| Fig. 12 (A) Schematic diagram of the synthesis process and biomimetic structure of CH–Cu nanozymes. (B) Stability comparison between LAC and CH–Cu at identical mass concentrations and varying storage durations.104 Copyright 2019, Elsevier. (C) UV spectra of the supernatant from the chromogenic reaction mixture and the corresponding photographs of the mixtures.105 Copyright 2022, Elsevier. (D) Schematic illustration of the biomimetic design and synthesis of GA–Cu nanozymes. (E) Michaelis–Menten curve (left) and the corresponding Lineweaver–Burk double-reciprocal plot (right) for 2,4-DP catalysis by GA–Cu nanozymes.106 Copyright 2024, Elsevier. | ||
3.2 The hydrolase family
Hydrolases are a class of biocatalysts that mediate hydrolytic reactions. This category encompasses lipases catalyzing ester bond cleavage, proteases facilitating amide/peptide bond scission, glycoside hydrolases (GHs) mediating glycosidic bond hydrolysis, among others.107–109 Hydrolases play pivotal roles throughout important developmental stages of plants. During the seed germination phase, they degrade starch into glucose within seeds to provide energy reserves for sprouting, while simultaneously mobilizing storage proteins to support seedling establishment. In vegetative and reproductive growth phases, these enzymes assist plants in mitigating biotic and abiotic stresses, such as pathogen invasion and xenobiotic toxins, by degrading pathogen cell wall components (e.g., chitinases) and eliciting plant immune responses.110–113 The general catalytic mechanism of natural hydrolases in hydrolytic reactions (exemplified by proteases) involves three sequential stages: (1) substrate binding, where catalytic residues specifically recognize and bind amino acid residues of the substrate via complementary electrostatic and hydrogen-bonding interactions; (2) nucleophilic attack on the carbonyl carbon of the peptide bond by the active-site nucleophile (e.g., serine hydroxyl or cysteine thiol), accompanied by concerted acid–base catalysis to mediate proton transfer and charge redistribution, culminating in peptide bond scission and water molecule release; and (3) product dissociation, wherein the enzyme releases hydrolyzed products from its active site, regenerating catalytic competency for subsequent substrate turnover.114Inspired by the catalytic mechanisms of natural hydrolases, researchers have developed diverse nanozymes with hydrolase-mimicking activities.115–117 These nanozymes are primarily engineered through two strategies: biomimetic design and emulation of natural enzymatic catalytic pathways.118,119 For instance, Yu and colleagues uncovered the pan-glycoside hydrolase-like (p-GHs-like) activity of copper compound NPs (Cu3P and Cu2O NPs), which exhibit bond cleavage precision, anomeric carbon configuration selectivity, and catalytic attack modes analogous to natural GHs.120 As illustrated in Fig. 13(A), these systems structurally replicate the two critical catalytic residues responsible for hydrolysis in natural GHs. Building upon the catalytic mechanisms of natural enzymes, they proposed two theoretically feasible pathways (path I and path II) for the NP-catalyzed hydrolysis of the artificial substrate PNPG (p-nitrophenyl-β-D-glucopyranoside). In both pathways, oxygen atoms of the NPs act as nucleophilic agents to attack the anomeric carbon of the glycosidic bond, while surface-adsorbed protons or copper atoms exhibit binding affinity toward the glycosidic oxygen. DFT calculations elucidated the reaction trajectories (Fig. 13(B)), revealing path I as the thermodynamically favored pathway. However, path II is an exothermic process that is thermodynamically unfavorable for the reaction progression. This mechanistic parallelism strongly supports the existence of catalytic sites on the NPs’ surface mirroring those of natural enzymes. Furthermore, building upon this biomimetic catalytic rationale, another study achieved a breakthrough by designing a molecularly imprinted polymer nanozyme (NP-Zn-6b) through biomimicking the active center structure of natural carbonic anhydrase.121 This bioinspired design primarily involves two structural strategies: (1) incorporation of Zn2+ as catalytic centers, and (2) construction of substrate-selective rigid cavities via molecular imprinting and post-modification, which precisely position coordinating groups (e.g., histidine-like moieties) around Zn2+ to replicate the enzymatic microenvironment. The Zn2+ ions synergize with proximal basic residues to activate H2O, generating nucleophilic hydroxide ions for ester bond hydrolysis – a mechanism analogous to that of carbonic anhydrase. They demonstrated that the bioinspired nanozyme efficiently hydrolyzes unactivated alkyl esters under mild conditions (40 °C, neutral pH = 7). Experimental results revealed that NP-Zn-6b exhibits significantly higher activity in the hydrolysis of para-nitrophenyl hexanoate (PNPH) compared to monofunctional catalysts lacking Zn or basic groups. Notably, NP-Zn-6b displayed a distinct activity maximum at pH = 7 (Fig. 13(C)). Intriguingly, NP-Zn-6b also hydrolyzes a range of esters beyond PNPH in pH 7 buffer (Fig. 13(D)). The reactivity hierarchy suggests that the synthetic NP-Zn-6b esterase readily discriminates substrates based on positional shifts or additions of methyl groups, modulated by structural variations influencing substrate binding. This work transcends the conventional paradigm of traditional chemical catalysis reliant on strong acids/bases, pioneering the construction of an artificial enzyme system that integrates metal–base synergistic catalysis with substrate-selective recognition.
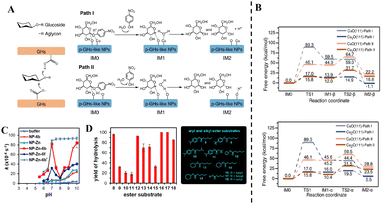 | ||
| Fig. 13 (A) Two possible attack pathways (path I and path II) for PNPG hydrolysis catalyzed by Cu2O and CuO NPs. (B) Energy profile diagrams of β-conformation (top) and α-conformation (bottom) glucose generated by Cu2O and CuO NPs via path I and path II, respectively.120 Copyright 2023, Elsevier. (C) pH-Dependent reaction rate curves of PNPH hydrolysis catalyzed by NP-Zn-6b compared with other catalysts. (D) Yield of 8–18 ester hydrolysis catalyzed by NP-Zn-6b in 25 mM HEPES buffer (pH = 7.0) at 40 °C after 4 hours.121 Copyright 2022, Elsevier. | ||
3.3 Multienzymatic activity
With the rapid development of nanozyme technology and the increasing demand for catalysis, single-functional nanozymes have exhibited certain limitations. Multienzymatic nanozymes, due to their potential synergistic or cascade effects, often demonstrate superior catalytic efficiency compared to single-enzyme-like nanozymes.122,123 In addition, plants in agricultural production often suffer from various stress conditions such as drought, high-temperature, low-temperature, pests and diseases. Nanozymes with multienzymatic activities can mimic the functions of multiple natural enzymes and enhance the stress resistance of plants through synergistic effects. Therefore, there is an urgent need to develop new NMs with multifunctional catalytic properties. A study developed ultrasmall calcium hexacyanoferrate(III) nanozymes (CaHF NPs) with quadruple enzyme-mimetic activities.124 Experimental studies demonstrated that the SOD-like activity of CaHF NPs is concentration-dependent, achieving a 99% suppression rate of O2˙− at a concentration of 320 μg mL−1 (Fig. 14(A)). Subsequent detection of NADPH depletion (via an absorption peak at 340 nm) revealed a sharp decline in NADPH absorbance, thereby confirming the GPx-like activity of CaHF NPs. Additionally, the TMB chromogenic reaction revealed that the maximum absorbance of ox-TMB at 652 nm decreased with increasing concentrations of CaHF NPs, reflecting their POD-like enzymatic activity. The CAT-like activity of CaHF NPs was determined by monitoring O2 generation via a dissolved oxygen meter during the catalytic decomposition of H2O2, where a significant increase in O2 production was observed within the first minute after adding CaHF NPs. The CaHF NPs with the aforementioned quadruple enzyme-mimetic activities exhibit broad-spectrum scavenging of multiple reactive oxygen and nitrogen species (RONS), including H2O2, O2, O2˙−, ˙OH, ONOO− and NO, through a synergistic antioxidant mechanism, thus demonstrating potential in disrupting the vicious cycle of oxidative stress. Another study designed an ultrasound-enhanced nanozyme named ACPCAH.125 This material is co-loaded with phosphorus-doped graphitic carbon nitride nanosheets, ultra-small gold NPs, and Cu1.6O NPs, and is wrapped with L-arginine and hyaluronic acid (Fig. 14(B)). Similar to CaHF NPs, ACPCAH achieves multi-enzymatic activities (SOD, CAT, GOx, POD, and NOS) by mimicking the active centers of natural enzymes. However, its innovation lies in the introduction of an ultrasound-responsive mechanism: dynamically enhancing catalytic efficiency through piezoelectric and sonothermal effects, which addresses the issue of limited activity in traditional nanozymes within complex wound microenvironments. Experimental results demonstrated that under ultrasound assistance, the catalytic activity was significantly enhanced, leading to effective scavenging of ROS. Fig. 14(C) and (D) evaluate the ultrasound-augmented POD-like and GOx-like activities using the TMB chromogenic assay. A pronounced 40% increase in absorbance (indicative of POD-like activity) was observed for ACPCAH under sonication. The Vmax values for H2O2 and TMB under ultrasound treatment reached 8.27 × 10−8 M s−1 and 10.5 × 10−8 M s−1, respectively, representing 25% and 69% enhancements compared to non-sonicated reactions. Additionally, the ultrasound-treated system exhibited a 16% improvement in the glucose consumption rate (Vmax = 6.77 × 10−8 M s−1) for its GOx-like activity relative to the control group. | ||
| Fig. 14 (A) Study on enzyme-mimetic activities of CaHF NPs (from left to right: SOD-like, GPx-like, POD-like, and CAT-like activities).124 Copyright 2023, Elsevier. (B) Schematic illustration of the design and synthesis strategy for ultrasound-augmented ACPCAH nanozymes. (C) POD-like activities of different treatment groups under conditions with or without ultrasound treatment. (D) GOx-like activities of different treatment groups under conditions with or without ultrasound treatment.125 Copyright 2023, ACS. | ||
4. Nanozymes: applications in phytotherapy
Under physiological conditions, the generation and scavenging of ROS in plants constitute a dynamic equilibrium process (ROS homeostasis).126 Low-level ROS play beneficial roles as signaling molecules in growth, development, and defense responses—for instance, activating stomatal closure mechanisms in guard cells to combat environmental drought and biotic stresses.127,128 However, under stress conditions, the redox equilibrium in plants may be disrupted. Excessively accumulated ROS (O2˙−, H2O2, and ˙OH) exhibit potent oxidative capacity, leading to membrane lipid peroxidation, protein denaturation, DNA damage, and other cellular component impairments, which can severely inhibit plant growth.129 The development of materials capable of modulating ROS homeostasis in plant systems is therefore imperative. Extensive studies have demonstrated that certain high-performance nanozymes can internalize into plant cells and enhance the expression of antioxidant enzymes (e.g., SOD, CAT) and non-enzymatic antioxidants (e.g., glutathione, ascorbate). Application of nanozymes under stress conditions achieves dual synergies: by not only augmenting intrinsic ROS-scavenging capacity but also enzymatically modulating ROS levels to enhance stress tolerance, thereby mitigating stress-induced physiological damage and yield losses.130,131 This approach can significantly reduce the application of synthetic chemical pesticides and fertilizers, decreasing their pollution to soil, water bodies, and the atmosphere. Accordingly, Table 2 catalogs diverse agricultural nanozyme materials, detailing their chemical compositions, enzymes-like activities, target organisms, and mitigation mechanisms against biotic stresses (e.g., pathogens/pests) and abiotic stresses (e.g., extreme weather, soil contamination).| Type | Application | Nanozyme | Enzymes-like activity | Strategies for mitigating stresses | Application target | Ref. |
|---|---|---|---|---|---|---|
| Biotic stresses | Plant antiviral agent | Cu1.96S NPs | Protease | 1. Chirality-dependent selective recognition for the QANPTTA epitope | Tobacco mosaic virus | 138 |
| 2. Photo-triggered site-specific proteolysis | ||||||
| 3. Virion structural disruption | ||||||
| Pesticide | MON@CeO2 | SOD | 1. SOD-like activity for ROS scavenging | Nilaparvata lugens, Sogatella furcifera | 139 | |
| 2. Downregulation of ROS-dependent P450 gene expression | ||||||
| 3. Reduction of detoxification enzyme activities | ||||||
| 4. Overcoming pesticide resistance | ||||||
| Abiotic stresses | Drought stress | Ag NPs | Catalyze ROS generation | 1. ROS signal-triggered defense priming | Maize seed | 148 |
| 2. Root hair morphological remodeling | ||||||
| 3. Transcriptional reprogramming of defense pathways | ||||||
| Fe-based NPs | POD | 1. Environmentally friendly ROS homeostasis | ||||
| 2. Enhancement of osmotic adjustment capacity | ||||||
| 3. Augmentation of antioxidant enzyme activities | ||||||
| Yttrium doping-stabilized γ-Fe2O3 NPs | CAT and POD | 1. Nanozyme-mediated ROS scavenging | Brassica napus | 149 | ||
| 2. Suppression of lipid peroxidation | ||||||
| 3. Enhanced growth and photosynthetic capacity | ||||||
| Salinty stress | Ag NPs | POD | 1. Nanozyme-catalyzed ROS generation (specifically ˙OH) | Rice seeds (Oryza sativa L.) | 153 | |
| 2. Metabolic reprogramming accelerates biosynthesis and energy supply | ||||||
| 3. Activation of defense response genes and stress signaling molecules | ||||||
| CDzymes | SOD and CAT | 1. Scavenging ROS/RNS and free radicals | Pisum sativum Linn, Eucommia | 154 | ||
| 2. Regulating antioxidant enzymes | ||||||
| 3. Protecting key biomolecules | ||||||
| Mn3O4 | SOD and CAT | 1. Boosting endogenous antioxidant metabolites | Cucumber (Cucumis sativus) | 155 | ||
| 2. Reducing oxidative damage and lipid peroxidation | ||||||
| 3. Accumulating osmoprotectants | ||||||
| 4. Modulating stress-responsive metabolic pathways | ||||||
| V4AlC3 nanosheets | SOD, CAT and POD | 1. Activation of antioxidant system | ||||
| 2. Phytohormone signaling modulation | Pea seed | 156 | ||||
| 3. Enhancement of energy metabolism | ||||||
| 4. Phytohormone signaling modulation | ||||||
| CeO2 NMs | Antioxidative enzyme | 1. Nanozyme-mediated ROS scavenging | Maize (Zea may L.) | 157 | ||
| 2. Maintenance of Na+/K+ homeostasis | ||||||
| 3. Enhancement of photosynthetic efficiency | ||||||
| 4. Down-regulation of lignin synthesis genes to accelerate cell elongation | ||||||
| Heavy metal stress | CaHCF NPs | SOD, CAT, POD, GPX, TPX and APX | 1. Multi-enzyme activities for direct ROS scavenging | Arabidopsis, Tomato | 27 | |
| 2. Ion exchange to reduce Cd2+ uptake and release Ca2+ | ||||||
| 3. Activation of endogenous antioxidants and gene regulation | ||||||
| 4. Restoration of photosynthesis and growth promotion | ||||||
| Other stresses | Cold stress (10 °C) | Yellow light CDs (YCDs) nanozyme | CAT | 1. Selective photosynthetic activation mechanism | Kale-type overwintering (oilseed rape) | 164 |
| 2. Multi-enzyme activities for direct ROS scavenging | ||||||
| 3. Antioxidant Enzyme system enhancement mechanism | ||||||
| 4. Root proliferation promotion mechanism (synergistic effect) | ||||||
| IMI stress | CDs | SOD and POD | 1. Cascade nanozyme activities scavenging ROS | Italian lettuce (Lactuca sativa) | 167 | |
| 2. Detoxification gene regulation for pesticide degradation | ||||||
| 3. Hormonal signaling pathway activation | ||||||
| 4. Nutritional quality and element homeostasis improvement | ||||||
| Arsenic stress | Fe-CDs | SOD, CAT and POD | 1. Multi-enzyme activities for direct ROS scavenging | Italian lettuce | 168 | |
| 2. Suppression of arsenic uptake and enhancement of plant stress resistance | ||||||
| 3. Targeted regulation of ROS homeostasis |
4.1 Responding to biotic stress
Various biotic stresses faced by plants, such as pathogenic bacterial infections and pest infestations, are important factors affecting crop production. Although conventional pesticides (for instance, insecticides and antimicrobials) can mitigate these biological infestations to increase crop yields. However, it also poses potential hazards to the environment and living organisms. Excessive application of xenobiotic agrochemicals compromises critical phyto-developmental processes through multilevel perturbations in physiological homeostasis and biochemical cascades, ultimately jeopardizing the sustainability paradigm in agricultural systems.132–134 Nanozymes can augment the plant immune system by triggering the activation of defense-related genes and proteins, thereby bolstering the plant's resistance against pathogenic infections and pest infestations. This mechanism ultimately leads to a reduced reliance on conventional pesticides, effectively mitigating environmental contamination and ecological disruption caused by pesticide usage.135–137Bacteria, fungi, and insects are the primary pathogens responsible for plant diseases. Certain nanozymes can enhance plant antiviral resistance by scavenging ROS generated during pathogen infection, maintaining ROS homeostasis, and activating the plant's intrinsic defense mechanisms, thereby reducing viral damage. Others function as plant antiviral agents by directly inhibiting viral activity through protease-like catalytic activity. A study developed a novel plant antiviral agent (chiral 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm Cu1.96S NPs) composed of copper sulfide NPs (3 ± 0.5 nm in diameter) and D-penicillamine as surface ligands (Fig. 15(A)).138 This antiviral agent exhibits protease-like enzymatic activity, enabling specific recognition and binding to the Gln99-Ala105 fragment within the capsid protein of Tobacco Mosaic Virus (TMV). The nanozyme demonstrates high binding affinity (via a supramolecular bonding network) to the Gln99–Ala105 region of the viral capsid, while showing 3000–10
nm Cu1.96S NPs) composed of copper sulfide NPs (3 ± 0.5 nm in diameter) and D-penicillamine as surface ligands (Fig. 15(A)).138 This antiviral agent exhibits protease-like enzymatic activity, enabling specific recognition and binding to the Gln99-Ala105 fragment within the capsid protein of Tobacco Mosaic Virus (TMV). The nanozyme demonstrates high binding affinity (via a supramolecular bonding network) to the Gln99–Ala105 region of the viral capsid, while showing 3000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold lower affinity to capsid proteins of other viruses. In addition, under green light irradiation, the antiviral agent hydrolyzes the amide bond between Asn101 and Pro102 in a polarization-dependent manner. As demonstrated in Fig. 15(B), the inhibitory efficacy against viral infection reached 98.7% in protoplasts and 92.6% in whole plants at 7 days post-treatment. The amount of viral nucleic acid is significantly reduced without triggering an allergic reaction in plants. Subsequently, the copper distribution in the treated plants was analyzed, and it was found that the copper content did not increase significantly, indicating that the antiviral agent has no significant impact on the environment. Circularly polarized light can significantly enhance the catalytic efficiency of chiral nanozymes. This photocatalytic-driven proteolytic cleavage provides an important reference for the development of novel photo-activated antiviral agents against plant viruses.
000-fold lower affinity to capsid proteins of other viruses. In addition, under green light irradiation, the antiviral agent hydrolyzes the amide bond between Asn101 and Pro102 in a polarization-dependent manner. As demonstrated in Fig. 15(B), the inhibitory efficacy against viral infection reached 98.7% in protoplasts and 92.6% in whole plants at 7 days post-treatment. The amount of viral nucleic acid is significantly reduced without triggering an allergic reaction in plants. Subsequently, the copper distribution in the treated plants was analyzed, and it was found that the copper content did not increase significantly, indicating that the antiviral agent has no significant impact on the environment. Circularly polarized light can significantly enhance the catalytic efficiency of chiral nanozymes. This photocatalytic-driven proteolytic cleavage provides an important reference for the development of novel photo-activated antiviral agents against plant viruses.
 | ||
Fig. 15 (A) Design and synthesis strategy of chiral 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm Cu1.96S NPs. (B) Contents of protoplast and plant viral nucleic acid extracts under different treatments.138 Copyright 2022, Springer Nature. (C) SOD-like activity of MON@CeO2 evaluated using a SOD detection kit. (D) Relative ROS intensity in N. lugens (JXF and NR) fed with rice seedlings treated with MON@CeO2 (100 mg L−1) for 24 h. (E) Effect of MON@CeO2 on the expression levels of detoxification enzyme cytochrome P450 genes in N. lugens (JXF). (F) Effect of MON@CeO2 on the expression levels of detoxification enzyme cytochrome P450 genes in N. lugens (NR) (JXF: field strains; NR: nitenpyram-resistant strains).139 Copyright 2022, Elsevier. nm Cu1.96S NPs. (B) Contents of protoplast and plant viral nucleic acid extracts under different treatments.138 Copyright 2022, Springer Nature. (C) SOD-like activity of MON@CeO2 evaluated using a SOD detection kit. (D) Relative ROS intensity in N. lugens (JXF and NR) fed with rice seedlings treated with MON@CeO2 (100 mg L−1) for 24 h. (E) Effect of MON@CeO2 on the expression levels of detoxification enzyme cytochrome P450 genes in N. lugens (JXF). (F) Effect of MON@CeO2 on the expression levels of detoxification enzyme cytochrome P450 genes in N. lugens (NR) (JXF: field strains; NR: nitenpyram-resistant strains).139 Copyright 2022, Elsevier. | ||
In addition, another study developed a SOD-like nano-pesticide through redox-driven modulation of catalytic activity. Zeng et al. fabricated a nano-hybrid material, MON@CeO2, by embedding CeO2 within mesoporous organosilica NPs (MONs).139 MONs served as templates with excellent biocompatibility and monodispersity, effectively preventing aggregation of CeO2 NPs. This strategy shares conceptual similarities with the steric hindrance effect induced by D-penicillamine ligands in Cu1.96S NPs, as both approaches employ interface engineering to optimize the exposure of catalytic sites. Experimental results demonstrated that MON@CeO2 scavenged ROS in pests by mimicking SOD activity (Fig. 15(C)). The system effectively scavenges ROS within pest organisms (Fig. 15(D)). In contrast to the molecular recognition mechanism of Cu1.96S NPs targeting viral capsids, MON@CeO2 achieves broad-spectrum synergistic effects through systemic modulation of the ROS-P450 signaling pathway. This mechanism effectively suppresses ROS-dependent cytochrome P450 gene expression in both nitenpyram-resistant N. lugens (NR) and field-collected N. lugens (JXF) (Fig. 15(E) and (F)). As P450 genes play a pivotal role in pesticide metabolism in pests, their downregulation reduced detoxification enzyme levels, consequently increasing pest susceptibility to insecticides. This mechanism holds significant implications for prolonging insecticide efficacy, optimizing dosage regimes, and enhancing pest control sustainability.
4.2 Mitigation of abiotic stresses
Abiotic stresses faced by plants, in the form of drought, high temperature, high salinity, cold, nutrient deficiencies, heavy metals, etc., are the main cause of reduced crop yields globally. Plants subjected to abiotic stress undergo changes at the morphological, physicochemical, and molecular levels, and their growth and developmental conditions and productivity are adversely affected to some extent.3,4 Excessive use of conventional pesticides also produces abiotic stress in plants. Pesticide stress affects plant health and ecosystem sustainability through mechanisms such as inducing the production of ROS in plants.140,141 It is worth noting that nanozymes, which are potential alternatives to conventional pesticides, are able to help plants to maintain cellular stability and normal metabolic functions in abiotic stress environments, such as drought, salinity, and heavy metals. For example, nanozymes can increase the activity of antioxidant enzymes in plant cells and scavenge excess ROS generated by environmental stresses.27 It can ensure plant growth and development in harsh environments, reducing crop yield reduction due to natural disasters, and enhancing plant resilience to abiotic stresses. | ||
| Fig. 16 (A) Effect of AgNP seed priming on the maize germination rate under drought stress. (B) Impact of AgNP seed priming on maize seedling vigor under drought stress. (C) Influence of Fe2O3 and Fe3O4 NP seed priming on the maize seedling vigor index under different abiotic stresses. (D) Effects of Fe2O3 and Fe3O4 NP seed priming on shoot and root length of maize seedlings under different abiotic stresses.148 Copyright 2023, ACS. (E) Phenotypic images of plants rehydrated after 5-day drought stress (from left to right: control group irrigated with nutrient solution; nutrient solution with 0.8 mg mL−1 ION; nutrient solution with 2 mg mL−1 ION). (F) The H2O2 and fresh weight (G) of plants irrigated with nutrient solution containing or lacking ION after 5-day drought stress.149 Copyright 2017, Springer Nature. | ||
Building on this foundation, researchers have developed Yttrium doping-stabilized γ-Fe2O3 NPs (IONs) to meet the persistent stress resistance requirements of crops during their growth phase. This material functionally integrates the catalytic properties of iron-based nanomaterials and stability optimization strategies from prior studies, ensuring sustained efficacy throughout plant developmental stages.149 These Y-doped γ-Fe2O3 NPs exhibit dual CAT-like and POD-like activities for efficient ROS scavenging. Different irrigation treatments were applied to drought-stressed plants, and phenotypic imaging revealed that ION treatment alleviated drought stress impacts on the plants (Fig. 16(E)). Experimental data (Fig. 16(F)) confirm that NP irrigation under drought stress significantly reduces leaf H2O2 accumulation (by 38.2%) and MDA production (by 27.5%), while markedly increasing fresh biomass (21.3%) (Fig. 16(G)), leaf length (18.7%), and chlorophyll SPAD values (34.6%). These collectively enhance the drought resilience and rehydration recovery of Brassica napus. This dual-functional strategy synergizes nanozyme-mediated ROS regulation with iron nutrient supplementation, simultaneously boosting crop stress tolerance and reducing reliance on conventional fertilizers, thereby mitigating environmental contamination risks.
 | ||
| Fig. 17 (A) Phenotypic images of cucumber plants under different treatments (blank control, NaCl, NaCl + Mn3O4 nanozymes). In the NaCl + Mn3O4 nanozyme group, foliar application of Mn3O4 nanozymes was initiated at the three-week-old stage and continued daily for one week. (B) Fresh biomass of cucumber tissues under different treatments.154 Copyright 2020, RSC. (C) Phenotypic photographs of pea seeds after 9 days of different treatments (blank control, NaCl, NaCl + V4AlC3 nanosheets). (D) ROS fluorescence intensity in pea seeds under different treatments detected by DCFH-DA. (E) Application of V4AlC3 nanosheets significantly promoted the recovery of germination rate and radicle length in pea seeds subjected to salt stress (200 mM NaCl treatment) (F).156 Copyright 2024, ACS. (G)–(J) Growth parameters (fresh biomass (G), dry biomass (H), and STI (I)) and phenotypic images (J) of maize under salt stress after foliar application of CeO2 NMs at different concentrations (1, 5, 10, 20, and 50 mg L−1). (K) Images of maize ears and corn kernels (L) after foliar application of 10 mg L−1 CeO2 NMs to salt-stressed maize plants.157 Copyright 2022, Elsevier. | ||
CeO2 NPs, as rare earth nanomaterials with exceptional catalytic properties, can mimic natural SOD enzymatic activity. In plants, they serve as an efficient ROS scavenger, exerting SOD-like effects while enhancing salt tolerance.152 Their SOD-like activity primarily stems from the redox cycling between Ce3+ and Ce4+, which facilitates oxygen release and electron transfer to form oxygen vacancies or defects. These oxygen vacancies enable the storage and release of oxygen, providing additional active sites that promote electron transfer and reactant adsorption, thereby amplifying catalytic efficiency under stress conditions.157 The research team led by Wang reported the mechanism by which CeO2 NMs alleviate salinity stress in maize.158 Phenotypic and biomass experimental results (Fig. 17(G), (H) and (J)) revealed that foliar application of 10, 20, and 50 mg L−1 CeO2 NMs significantly alleviated salt stress-induced growth inhibition in maize after one week of treatment. Concurrently, the salt tolerance index (STI) was markedly enhanced by 69.5%, 69.1%, and 86.8%, respectively (Fig. 17(I)). Furthermore, as shown in Fig. 17(K) and (L), salt stress adversely affected the development and maturation of maize ears and corn kernels, while 10 mg L−1 CeO2 NMs significantly mitigated these adverse effects. Transcriptomic analysis revealed that CeO2 NMs scavenged ROS through their intrinsic antioxidant enzymatic properties. Post-application, antioxidant defense system-related genes were restored to normal control levels. Additionally, while optimizing Na+/K+ homeostasis, CeO2 NMs increased carbon sources in root exudates to enhance rhizobacterial richness and diversity, elevating the abundance of halotolerant plant growth-promoting rhizobacteria (HT-PGPR). Compared to traditional fertilizers, CeO2 NMs demonstrate both high efficiency and cost-effectiveness.
 | ||
| Fig. 18 (A) NBT assay for SOD-like activity of CaHCF NPs. (B) Photograph of the reaction between H2O2 and CaHCF NPs with CAT-like activity. (C) Amount of O2 generated by CaHCF NPs reacting with H2O2 at different concentrations (0, 1, 3, and 5 M). (D) POD-like activity of CaHCF NPs. (E) Absorbance–time curves of CaHCF NPs in H2O2 solutions at varying concentrations (10, 20, 30, 40, and 50 mM). (F) Michaelis–Menten kinetics (left) and the Lineweaver–Burk plot (right) for the POD-like activity of CaHCF NPs. (G) GPx-like activity, TPX-like activity (substrate: DTNB), and APX-like activity of CaHCF NPs (from left to right). (H) ESR spectra of CaHCF NPs (˙OH radical elimination using DMPO as a trapping agent). (I) Phenotypic photographs of Cd-stressed tomato seedlings alleviated by foliar application of CaHCF NPs (1-month-old seedlings treated with Cd stress and CaHCF NPs applied every two days for 18 days). (J) Phenotypic photographs of Cd-stressed tomato seedlings alleviated by soil irrigation with CaHCF NPs (15-day-old seedlings treated with Cd stress and CaHCF NPs via soil irrigation for 30 days).27 Copyright 2024, John Wiley and Sons. | ||
 | ||
| Fig. 19 (A) Apparent morphology and root microscopic images of rapeseed under different treatments (CK, CDs, and YCDs). (B) Evaluation of fresh weight (B) and increment (C) of shoots and roots in rapeseed under cold stress (10 °C) after 27 days of different treatments. (D) Evaluation of dry weight (D) and increment (E) of shoots and roots in rapeseed seedlings.164 Copyright 2025, Elsevier. (F) Phenotypic images of lettuce plants subjected to IMI stress under different treatments (H2O or CDs) over 15 days.167 Copyright 2025, Elsevier. (G) Steady-state kinetic analysis of Fe-CDs (left: substrate TMB; right: substrate H2O2). (H) Schematic illustration of the comparative analysis of O2˙− scavenging activity (SOD-like) between Fe-CDs and CDs (left); quantitative OH− scavenging capacity of CDs and Fe-CDs via UV-vis method (right).168 Copyright 2025, Elsevier. | ||
Additionally, as previously mentioned, crops are susceptible to biotic stresses such as pest infestations. Traditional agriculture typically addresses insect problems through the application of pesticides like insecticides. However, to maintain efficacy, conventional pesticides may be prone to over-application and environmental accumulation of residues, potentially causing adverse impacts on crops and ecosystems.165,166 Therefore, reducing pesticide residues has become a critical issue. A study synthesized CDs with cascade nanozyme activity, which effectively alleviated imidacloprid (IMI)-induced phytotoxicity in lettuce under pesticide stress.167 CDs exhibit activities similar to those of SOD and POD. Through surface modification and analysis, it was confirmed that their SOD-like activity depends on the binding of –NH2, –COOH, and –OH groups to O2˙−, while the POD-like activity depends on the –COOH and CO groups. Under IMI stress, CDs can enhance various defense systems in lettuce and reduce the toxicity level of ROS. Specifically, the levels of O2˙−, H2O2, and MDA were reduced by 26.77%, 48.52%, and 13.10%, respectively. Meanwhile, the nano-enzyme upregulated the expression of detoxification genes, resulting in a reduction in IMI residues in lettuce. Fig. 19(F) displays phenotypic images of lettuce plants subjected to high-concentration IMI stress following the application of 25 μg mL−1 CDs. Compared to the control group, treatment with significantly alleviated developmental stunting and incomplete leaf expansion in IMI-stressed lettuce plants, restoring normal growth in the impaired specimens. Additionally, after CD treatment, the acceptable daily intake of IMI in lettuce was far lower than the reference dose, even being less than 18.0% under high-concentration IMI exposure. These results indicate that CDs have great potential in reducing pesticide residues and improving food safety.
Arsenic, as a highly toxic nonmetallic element, typically enters the environment through industrial emissions and agricultural fertilizers. Soil-accumulated arsenic generally enters food chains through plant uptake. Severe arsenic stress induces excessive ROS accumulation in plants, leading to reduced crop yields, quality deterioration, and potential human health risks through contaminated agricultural products. Therefore, developing effective strategies for arsenic pollution remediation and toxicity mitigation becomes imperative. Xie et al. synthesized iron-doped carbon dot (Fe-CD) nanozymes via a one-step hydrothermal method (average diameter = 2.01 nm; zeta potential = −34 mV).168 By utilizing Fe3+ ions as catalytic active sites, they optimized the electronic structure and surface functional groups of carbon dots through a metal-doping strategy. Experimental results demonstrated that Fe-CDs exhibit superior multienzymatic activities, including POD-like, CAT-like, and SOD-like functionalities. As shown in Fig. 19(G), the Km and Vmax values for TMB and H2O2 were determined to be 8.56 mM and 2.04 × 10−5 M s−1, and 6.72 mM and 2.82 × 10−5 M s−1, respectively, demonstrating superior catalytic performance compared to other iron-based materials. In addition, Fe-CDs exhibited superior CAT-like and SOD activities, along with higher scavenging capacities for O2˙− (64.4% vs. 42.8%) and ˙OH (69.6% vs. 16.8%), compared to conventional CDs (Fig. 19(H)). These properties substantiate the effectiveness of Fe-CDs (applied at a spraying concentration of 300 mg L−1) in mitigating arsenic toxicity in lettuce: not only effectively scavenging ROS under arsenic stress but also providing nutritional support for lettuce growth and development. This offers potential solutions for agricultural heavy metal contamination remediation and crop stress resistance enhancement. Additionally, Wang and colleagues engineered CeO2 NPs with an irregular morphology (average particle size = 32.22 ± 7.57 nm, zeta potential = 16.47 ± 2.10 mV).169 Their research demonstrated that these NPs exhibited remarkable enzyme-like activities, effectively alleviating As toxicity in pakchoi (Brassica chinensis L.). Foliar application of CeO2 NPs with SOD-like and CAT-like activities significantly scavenged As-induced ROS overaccumulation in pak choi under As stress. Concurrently, the antioxidant defense system was modulated, resulting in substantial mitigation of phytotoxic effects. Additionally, CeO2 NPs enhanced plant antioxidant capacity by modulating the GSH/oxidized glutathione (GSSG) ratio. Notably, foliar application of CeO2 NPs not only reduced arsenic accumulation in pak choi (thereby minimizing its transfer through the food chain) but also substantially decreased human arsenic exposure risks via dietary intake of pak choi and snails. CeO2 NPs also exhibited negligible self-translocation within the food chain, demonstrating exceptional biosafety.
5. Challenges and opportunities for agricultural nanozymes
With the advancement of agricultural modernization, nanozymes, as emerging materials, show great application prospects in the field of agriculture and are expected to provide new solutions for the problems in sustainable development of agriculture. Nanozymes play a key role in enhancing the adaptability of crops to adversity by regulating the physiological mechanisms of crops and enhancing their tolerance to adversities such as drought, salinity, and heavy metal pollution, thereby improving crop yield and quality. However, as with any emerging technology, nanozymes are facing both unprecedented opportunities and a series of challenges in agricultural applications.5.1 Biosafety and toxicity issues
While considering the effects of nanozymes on plants, it is also important to consider their effects on the ecosystems in which the plants are found. The release of nanozymes in the environment may cause toxic effects on non-target organisms (such as beneficial microorganisms in the soil, insects, etc.), which may disrupt the ecological balance in severe cases. The metabolic pathways, accumulation patterns, and long-term toxic effects of nanozymes in plants are not well understood. Therefore, in-depth studies are needed to ensure that there is no or as little impact as possible on the ecosystems in which the plants live. In addition to this, greener and more environmentally friendly synthesis methods for nanozymes should be considered in order to better respond to sustainable development strategies in agriculture.5.2 Stability and effectiveness issues
The activity and stability of nanozymes are susceptible to environmental factors such as humidity, temperature, and soil pH, which may compromise their stability in practical applications. Furthermore, although nanozymes can enhance plant stress resistance to some extent, the long-term durability of their stress-resistant effects under prolonged and complex stress conditions requires further improvement. Therefore, to better meet the diversified demands of agricultural production in complex ecological environments, researchers need to further investigate methods to enhance the stability and long-term effectiveness of nanozymes. This requires strengthening fundamental research on nanozymes, deepening the understanding of their mechanisms of action, and ensuring their stable functionality throughout the entire crop growth cycle.5.3 Resistance and environmental accumulation
Similar to conventional pesticides, repeated use of nanozymes may lead to the development of resistance in harmful organisms, limiting their long-term application. Therefore, effective strategies are needed to delay or avoid the development of resistance. The unique physicochemical properties of nanozymes confer high catalytic activity, but this may make them susceptible to accumulation in environmental media, such as soil and water, and potentially harmful to the environment. The cumulative behavior of nanozymes in the environment and their long-term effects on the ecosystem are not yet clear. Therefore, in order to assess the risk of environmental accumulation of nanozymes, it is important to carry out long-term environmental monitoring and research.5.4 Development of multifunctional nanozymes
By combining unique physicochemical properties and mimicking the catalytic activity of enzymes, nanozymes will provide a variety of simple but efficient and multifunctional platforms. In the future, it is important to consider the construction of nanozymes with multienzymatic characteristics and multifunctionality. They are expected to revolutionize the traditional agricultural production model from multiple dimensions and provide innovative solutions to cope with the complex and changing demands of agricultural production.5.5 Real-world agricultural production
Although many research papers on the use of nanozymes for crop treatment have been reported, most of these studies were conducted in laboratory cultivation or sowing stage experiments. At present, there is a lack of research papers on the entire growth cycle of plants, and there is also a shortage of relevant research papers on application experiments in real fields. Therefore, in the future, researchers should consider narrowing the gap between experiments and real-world production. They should take into account various factors such as cost-effectiveness in agricultural practice and the complex real-world environment to better apply nanozyme materials to actual agricultural production.Author contributions
N. Yin and Y. Wang proposed and supervised the project. R. Hou wrote the manuscript with comments from N. Yin, Y. Wang, S. Song and H. Zhang. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This work was supported by the financial aid from the National Key Research and Development Program of China (2020YFE0204500), the Basic Science Center Project of the National Natural Science Foundation of China (22388101), the National Natural Science Foundation of China (grant no. 22020102003, U23A20581, 52272169, 22393932, 52402352 and 52022094), the Program of Science and Technology Development Plan of Jilin Province of China (no. 20230508071RC) and the Youth Innovation Promotion Association of Chinese Academy of Sciences (grant Y2023067).References
- J.-K. Zhu, Cell, 2016, 167, 313–324 CrossRef CAS PubMed.
- R. Mittler, S. I. Zandalinas, Y. Fichman and F. Van Breusegem, Nat. Rev. Mol. Cell Biol., 2022, 23, 663–679 CrossRef CAS PubMed.
- R. Waadt, C. A. Seller, P.-K. Hsu, Y. Takahashi, S. Munemasa and J. I. Schroeder, Nat. Rev. Mol. Cell Biol., 2022, 23, 680–694 CrossRef CAS PubMed.
- H. Zhang, J. Zhu, Z. Gong and J.-K. Zhu, Nat. Rev. Genet., 2021, 23, 104–119 CrossRef PubMed.
- B. D. Collins and G. M. Stock, Nat. Geosci., 2016, 9, 395–400 CrossRef CAS.
- A. M. Milner and I. L. Boyd, Science, 2017, 357, 1232–1234 CrossRef CAS PubMed.
- A. E. Larsen, F. Noack and L. C. Powers, Science, 2024, 383, 1308 CrossRef PubMed.
- M. Kah, R. S. Kookana, A. Gogos and T. D. Bucheli, Nat. Nanotechnol., 2018, 13, 677–684 CrossRef CAS PubMed.
- D. Wang, N. B. Saleh, A. Byro, R. Zepp, E. Sahle-Demessie, T. P. Luxton, K. T. Ho, R. M. Burgess, M. Flury, J. C. White and C. Su, Nat. Nanotechnol., 2022, 17, 347–360 CrossRef CAS PubMed.
- N. Kandhol, V. P. Singh, S. Pandey, S. Sharma, L. Zhao, F. J. Corpas, Z.-H. Chen, J. C. White and D. K. Tripathi, Trends Plant Sci., 2024, 29, 1310–1318 CrossRef CAS PubMed.
- H. Chhipa, Environ. Chem. Lett., 2016, 15, 15–22 CrossRef.
- S.-Y. Kwak, T. T. S. Lew, C. J. Sweeney, V. B. Koman, M. H. Wong, K. Bohmert-Tatarev, K. D. Snell, J. S. Seo, N.-H. Chua and M. S. Strano, Nat. Nanotechnol., 2019, 14, 447–455 CrossRef CAS PubMed.
- H. Karimi-Maleh, M. Ghalkhani, Z. Saberi Dehkordi, M. Mohsenpour Tehran, J. Singh, Y. Wen, M. Baghayeri, J. Rouhi, L. Fu and S. Rajendran, J. Ind. Eng. Chem., 2024, 129, 105–123 CrossRef CAS.
- M. Kah, L. J. Johnston, R. S. Kookana, W. Bruce, A. Haase, V. Ritz, J. Dinglasan, S. Doak, H. Garelick and V. Gubala, Nat. Nanotechnol., 2021, 16, 955–964 CrossRef CAS PubMed.
- R. Wang, F. x Luan, Z. s Wang, X. c Liu, X. g Zhang, K. Wang, W. Mu, F. Liu and D. x Zhang, Adv. Mater., 2024, 36, 2409839 CrossRef CAS PubMed.
- A. Yayci, T. Sassmann, A. Boes, F. Jakob, A. Töpel, A. Loreth, C. Rauch, A. Pich and U. Schwaneberg, Angew. Chem., Int. Ed., 2024, 63, e202319832 CrossRef CAS PubMed.
- M. Kah, N. Tufenkji and J. C. White, Nat. Nanotechnol., 2019, 14, 532–540 CrossRef CAS PubMed.
- Y. Wang, X. Jia, S. An, W. Yin, J. Huang and X. Jiang, Adv. Mater., 2023, 36, 2301810 CrossRef PubMed.
- T. Ahmed, M. Noman, H. Jiang, M. Shahid, C. Ma, Z. Wu, M. M. Nazir, M. A. Ali, J. C. White, J. Chen and B. Li, Nano Today, 2022, 45, 101547 CrossRef CAS.
- Z. Xiao, N. Fan, W. Zhu, H.-L. Qian, X.-P. Yan, Z. Wang and S. Rasmann, ACS Nano, 2023, 17, 3107–3118 CrossRef CAS PubMed.
- D. Jiang, D. Ni, Z. T. Rosenkrans, P. Huang, X. Yan and W. Cai, Chem. Soc. Rev., 2019, 48, 3683–3704 RSC.
- F. Manea, F. B. Houillon, L. Pasquato and P. Scrimin, Angew. Chem., Int. Ed., 2004, 43, 6165–6169 CrossRef CAS PubMed.
- L. Gao, J. Zhuang, L. Nie, J. Zhang, Y. Zhang, N. Gu, T. Wang, J. Feng, D. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- M. Zandieh and J. Liu, Adv. Mater., 2023, 36, 2211041 CrossRef PubMed.
- L. Mei, S. Zhu, Y. Liu, W. Yin, Z. Gu and Y. Zhao, Chem. Eng. J., 2021, 418, 129431 CrossRef CAS.
- H. Wei, L. Gao, K. Fan, J. Liu, J. He, X. Qu, S. Dong, E. Wang and X. Yan, Nano Today, 2021, 40, 101269 CrossRef CAS.
- X. Shen, Z. Yang, X. Dai, W. Feng, P. Li and Y. Chen, Adv. Mater., 2024, 36, 2402745 CrossRef CAS PubMed.
- H. Wu, N. Tito and J. P. Giraldo, ACS Nano, 2017, 11, 11283–11297 CrossRef CAS PubMed.
- Y. Liu, D. Liu, X. Han, Z. Chen, M. Li, L. Jiang and J. Zeng, ACS Nano, 2024, 18, 31188–31203 CrossRef CAS PubMed.
- Z. Wang, R. Zhang, X. Yan and K. Fan, Mater. Today, 2020, 41, 81–119 CrossRef CAS.
- Y. Ai, M. Q. He, H. Sun, X. Jia, L. Wu, X. Zhang, H. B. Sun and Q. Liang, Adv. Mater., 2023, 35, 2302335 CrossRef CAS PubMed.
- H. Fan, R. Zhang, K. Fan, L. Gao and X. Yan, ACS Nano, 2024, 18, 2533–2540 CrossRef CAS PubMed.
- L. Li, S. J. Cao, Z. H. Wu, R. Q. Guo, L. Xie, L. Y. Wang, Y. J. Tang, Q. Li, X. L. Luo, L. Ma, C. Cheng and L. Qiu, Adv. Mater., 2022, 34, 2108646 CrossRef CAS PubMed.
- H. Ding, B. Hu, B. Zhang, H. Zhang, X. Yan, G. Nie and M. Liang, Nano Res., 2020, 14, 570–583 CrossRef.
- L. Huang, X. He, J. Hu, C. Qin, C. Huang, Y. Tang, F. Zhong, X. Kong and X. Wei, ACS Nano, 2024, 18, 33105–33118 CrossRef CAS PubMed.
- N. Singh, M. A. Savanur, S. Srivastava, P. D’Silva and G. Mugesh, Angew. Chem., Int. Ed., 2017, 56, 14267–14271 CrossRef CAS PubMed.
- G. Fang, W. F. Li, X. M. Shen, J. M. Perez-Aguilar, Y. Chong, X. F. Gao, Z. F. Chai, C. Y. Chen, C. C. Ge and R. H. Zhou, Nat. Commun., 2018, 9, 129 CrossRef PubMed.
- D. Wang, J. Wang, X. J. Gao, H. Ding, M. Yang, Z. He, J. Xie, Z. Zhang, H. Huang, G. Nie, X. Yan and K. Fan, Adv. Mater., 2023, 36, 2310033 CrossRef PubMed.
- Y. Zhao, S. Zhao, Y. Du, Z. Gao, Y. Li, H. Ma, H. Li, X. Ren, Q. Fan, D. Wu and Q. Wei, Adv. Mater., 2025, 37, 2418731 CrossRef CAS PubMed.
- J. Zhang, Z. Wang, X. Lin, X. Gao, Q. Wang, R. Huang, Y. Ruan, H. Xu, L. Tian, C. Ling, R. Shi, S. Xu, K. Chen and Y. Wu, Angew. Chem., Int. Ed., 2024, 64, e202416686 CrossRef PubMed.
- Y. Lin, X. Ren, F. Xuan and Z. Liu, Adv. Compos. Hybrid Mater., 2024, 8, 68 CrossRef.
- Q. Qiao, Z. Liu, F. Hu, Z. Xu, Y. Kuang and C. Li, Adv. Funct. Mater., 2024, 35, 2414837 CrossRef.
- J. Liu, S. Dong, S. Gai, S. Li, Y. Dong, C. Yu, F. He and P. Yang, ACS Nano, 2024, 18, 23579–23598 CrossRef CAS PubMed.
- Y. Dang, G. Wang, G. Su, Z. Lu, Y. Wang, T. Liu, X. Pu, X. Wang, C. Wu, C. Song, Q. Zhao, H. Rao and M. Sun, ACS Nano, 2022, 16, 4536–4550 CrossRef CAS PubMed.
- C. Ge, G. Fang, X. Shen, Y. Chong, W. G. Wamer, X. Gao, Z. Chai, C. Chen and J.-J. Yin, ACS Nano, 2016, 10, 10436–10445 CrossRef CAS PubMed.
- T. R. Shojaei, M. A. M. Salleh, M. Tabatabaei, H. Mobli, M. Aghbashlo, S. A. Rashid and T. Tan, Synthesis, Technology and Applications of Carbon Nanomaterials, 2019, pp. 247–277 DOI:10.1016/b978-0-12-815757-2.00011-5.
- K. A. Abd-Elsalam, Carbon Nanomaterials for Agri-Food and Environmental Applications, 2020, pp. 1–18 DOI:10.1016/b978-0-12-819786-8.00001-3.
- Y. Cui, K. Wang and C. Zhang, ACS Nano, 2024, 18, 10829–10839 CrossRef CAS PubMed.
- Y. Sun, B. Xu, X. Pan, H. Wang, Q. Wu, S. Li, B. Jiang and H. Liu, Coord. Chem. Rev., 2023, 475, 214896 CrossRef CAS.
- V. R. Raphey, T. K. Henna, K. P. Nivitha, P. Mufeedha, C. Sabu and K. Pramod, Mater. Sci. Eng., C, 2019, 100, 616–630 CrossRef CAS PubMed.
- M. Safdar, W. Kim, S. Park, Y. Gwon, Y.-O. Kim and J. Kim, J. Nanobiotechnol., 2022, 20, 275 CrossRef CAS PubMed.
- H. Hou, X. Zheng, H. Zhang, M. Yue, Y. Hu, H. Zhou, Q. Wang, C. Xie, P. Wang and L. Li, IEEE Trans. NanoBiosci., 2017, 16(7), 563–570 Search PubMed.
- X. Li, S. Sun, S. Guo and X. Hu, Environ. Sci. Technol., 2021, 55, 9938–9948 CrossRef CAS PubMed.
- P. Zhang, Z. Guo, W. Luo, F. A. Monikh, C. Xie, E. Valsami-Jones, I. Lynch and Z. Zhang, Environ. Sci. Technol., 2020, 54, 3181–3190 CrossRef CAS PubMed.
- L. Duo, Y. Wang and S. Zhao, Ecotoxicol. Environ. Saf., 2022, 229, 113076 CrossRef CAS PubMed.
- Z. Chen, Y. Yu, Y. Gao and Z. Zhu, ACS Nano, 2023, 17, 13062–13080 CrossRef CAS PubMed.
- H. Fan, J. Zheng, J. Xie, J. Liu, X. Gao, X. Yan, K. Fan and L. Gao, Adv. Mater., 2023, 36, 2300387 CrossRef PubMed.
- Y. Huang, J. Ren and X. Qu, Chem. Rev., 2019, 119, 4357–4412 CrossRef CAS PubMed.
- B. Seong, J. Kim, W. Kim, S. H. Lee, X.-H. Pham and B.-H. Jun, Int. J. Mol. Sci., 2021, 22, 10382 CrossRef CAS PubMed.
- X. Cao, L. Yue, C. Wang, X. Luo, C. Zhang, X. Zhao, F. Wu, J. C. White, Z. Wang and B. Xing, ACS Nano, 2022, 16, 1170–1181 CrossRef CAS PubMed.
- Y. Huang, S. Peng, Y. Liu, G. Feng, Z. Ding, B. Xiang, L. Zheng, H. Cheng, S. Liu, H. Yao and J. Fang, J. Agric. Food Chem., 2024, 72, 23008–23023 CrossRef CAS PubMed.
- P. Hu, J. An, M. M. Faulkner, H. Wu, Z. Li, X. Tian and J. P. Giraldo, ACS Nano, 2020, 14, 7970–7986 CrossRef CAS PubMed.
- A. Avellan, F. Schwab, A. Masion, P. Chaurand, D. Borschneck, V. Vidal, J. Rose, C. Santaella and C. Levard, Environ. Sci. Technol., 2017, 51, 8682–8691 CrossRef CAS PubMed.
- L. Chen, L. Zhu, H. Cheng, W. Xu, G. Li, Y. Zhang, J. Gu, L. Chen, Z. Xie, Z. Li and H. Wu, ACS Nano, 2024, 18, 23154–23167 CrossRef CAS PubMed.
- J. Ma, Y. Zhou, J. Li, Z. Song and H. Han, J. Nanobiotechnol., 2022, 20, 168 CrossRef CAS PubMed.
- M. Li, L. Gao, J. C. White, C. L. Haynes, T. L. O’Keefe, Y. Rui, S. Ullah, Z. Guo, I. Lynch and P. Zhang, Nat. Nanotechnol., 2023, 18, 688–691 CrossRef CAS PubMed.
- L. Gao, H. Wei, S. Dong and X. Yan, Adv. Mater., 2024, 36, 2305249 CrossRef CAS PubMed.
- S. Deng, Y. Hao, L. Yang, T. Yu, X. Wang, H. Liu, Y. Liu and M. Xie, Biosens. Bioelectron., 2025, 278, 117339 CrossRef CAS PubMed.
- J. Zhang, Z. Wang, X. Lin, X. Gao, Q. Wang, R. Huang, Y. Ruan, H. Xu, L. Tian, C. Ling, R. Shi, S. Xu, K. Chen and Y. Wu, Angew. Chem., Int. Ed., 2024, 64, e202416686 CrossRef PubMed.
- M. Chen, A. Qileng, S. Chen, H. Huang, Z. Xu, W. Liu and Y. Liu, Adv. Funct. Mater., 2024, 34, 2423643 Search PubMed.
- Y. Shen, R. Xing, X. Xu, Y. Wang, R. Huang, R. Su, M. D. Dickey, W. Qi and J. Kong, Matter, 2025, 8, 102159 CrossRef.
- H. Shan, J. Shi, T. Chen, Y. Cao, Q. Yao, H. An, Z. Yang, Z. Wu, Z. Jiang and J. Xie, ACS Nano, 2023, 17, 2368–2377 CrossRef CAS PubMed.
- Z. Yu, Z. Xu, R. Zeng, M. Xu, M. Zou, D. Huang, Z. Weng and D. Tang, Angew. Chem., Int. Ed., 2024, 64, e202420200 CrossRef PubMed.
- M. Chen, A. Qileng, S. Chen, H. Huang, Z. Xu, W. Liu and Y. Liu, Adv. Funct. Mater., 2024, 34, 2402552 CrossRef CAS.
- M. Jabran, M. A. Ali, S. Muzammil, A. Zahoor, F. Ali, S. Hussain, G. Muhae-Ud-Din, M. Ijaz and L. Gao, Chem. Biol. Technol. Agric., 2024, 11, 75 CrossRef.
- Y. Hao, C. Ma, Z. Zhang, Y. Song, W. Cao, J. Guo, G. Zhou, Y. Rui, L. Liu and B. Xing, Environ. Pollut., 2018, 232, 123–136 CrossRef CAS PubMed.
- Y. Li, W. Li, X. Yang, Y. Kang, H. Zhang, Y. Liu and B. Lei, ACS Appl. Nano Mater., 2020, 4, 113–120 CrossRef.
- D. Li, D. Dai, G. Xiong, S. Lan and C. Zhang, Small, 2022, 19, 2205870 CrossRef PubMed.
- Q. Liu, A. Zhang, R. Wang, Q. Zhang and D. Cui, Nano-Micro Lett., 2021, 13, 154 CrossRef CAS PubMed.
- H. Bidi, H. Fallah, Y. Niknejad and D. Barari Tari, Plant Physiol. Biochem., 2021, 163, 348–357 CrossRef CAS PubMed.
- H. Tombuloglu, Y. Slimani, G. Tombuloglu, M. Almessiere and A. Baykal, Chemosphere, 2019, 226, 110–122 CrossRef CAS PubMed.
- M. Shen, W. Liu, A. Zeb, J. Lian, J. Wu and M. Lin, J. Environ. Manage., 2022, 306, 114454 CrossRef CAS PubMed.
- Z. Wang, J. Wu, J.-J. Zheng, X. Shen, L. Yan, H. Wei, X. Gao and Y. Zhao, Nat. Commun., 2021, 12, 6866 CrossRef CAS PubMed.
- M. Rizwan, S. Ali, M. Zia ur Rehman, M. Adrees, M. Arshad, M. F. Qayyum, L. Ali, A. Hussain, S. A. S. Chatha and M. Imran, Environ. Pollut., 2019, 248, 358–367 CrossRef CAS PubMed.
- Y. Yu, Y. Zhang, Y. Wang, W. Chen, Z. Guo, N. Song and M. Liang, Nano Res., 2023, 16, 10763–10769 CrossRef CAS.
- W. Gao, J. He, L. Chen, X. Meng, Y. Ma, L. Cheng, K. Tu, X. Gao, C. Liu, M. Zhang, K. Fan, D.-W. Pang and X. Yan, Nat. Commun., 2023, 14, 160 CrossRef CAS PubMed.
- C. Liu, W. Fan, W. X. Cheng, Y. Gu, Y. Chen, W. Zhou, X. F. Yu, M. Chen, M. Zhu, K. Fan and Q. Y. Luo, Adv. Funct. Mater., 2023, 33, 2213856 CrossRef CAS.
- Z. Wang, X. Shen and X. Gao, J. Phys. Chem. C, 2021, 125, 23098–23104 CrossRef CAS.
- Y. Gao, X. Gao, H. Hou, Z. Qu, H. Li, B. Du and P. Tang, Sens. Actuators, B, 2024, 404, 135280 CrossRef CAS.
- Z. Lin, T. Dong, L. Niu, X. Zhang, M. Wang, X. Liu, Y. Cai and A. Liu, Chem. Eng. J., 2024, 482, 148775 CrossRef CAS.
- F. Zhang, Z. Chen, J. Liu, C. Li, B. Jiang, J. Shen, Y. Liu and Z. Xu, Chem. Eng. J., 2024, 492, 152328 CrossRef CAS.
- B. Jiang, D. Duan, L. Gao, M. Zhou, K. Fan, Y. Tang, J. Xi, Y. Bi, Z. Tong, G. F. Gao, N. Xie, A. Tang, G. Nie, M. Liang and X. Yan, Nat. Protoc., 2018, 13, 1506–1520 CrossRef CAS PubMed.
- J.-J. Zheng, F. Zhu, N. Song, F. Deng, Q. Chen, C. Chen, J. He, X. Gao and M. Liang, Nat. Protoc., 2024, 19, 3470–3488 CrossRef CAS PubMed.
- Z. Li, B. Ding, J. Li, H. Chen, J. Zhang, J. Tan, X. Ma, D. Han, P. A. Ma and J. Lin, Angew. Chem., Int. Ed., 2024, 64, e202413661 CrossRef PubMed.
- S. Ji, B. Jiang, H. Hao, Y. Chen, J. Dong, Y. Mao, Z. Zhang, R. Gao, W. Chen, R. Zhang, Q. Liang, H. Li, S. Liu, Y. Wang, Q. Zhang, L. Gu, D. Duan, M. Liang, D. Wang, X. Yan and Y. Li, Nat. Catal., 2021, 4, 407–417 CrossRef CAS.
- S. Zhang, X. J. Gao, Y. Ma, K. Song, M. Ge, S. Ma, L. Zhang, Y. Yuan, W. Jiang, Z. Wu, L. Gao, X. Yan and B. Jiang, Nat. Commun., 2024, 15, 10605 CrossRef CAS PubMed.
- J. Feng, X. Yang, T. Du, L. Zhang, P. Zhang, J. Zhuo, L. Luo, H. Sun, Y. Han, L. Liu, Y. Shen, J. Wang and W. Zhang, Adv. Sci., 2023, 10, 2303078 CrossRef CAS PubMed.
- G. Noctor, J.-P. Reichheld and C. H. Foyer, Semin. Cell Dev. Biol., 2018, 80, 3–12 CrossRef CAS PubMed.
- Z. Ding, Q. Song, G. Wang, Z. Zhong, G. Zhong, H. Li, Y. Chen, X. Zhou, L. Liu and S. Yang, RSC Adv., 2024, 14, 17571–17582 RSC.
- Y. Chen, B. Jiang, H. Hao, H. Li, C. Qiu, X. Liang, Q. Qu, Z. Zhang, R. Gao, D. Duan, S. Ji, D. Wang and M. Liang, Angew. Chem., Int. Ed., 2023, 62, e202301879 CrossRef CAS PubMed.
- S. M. Jones and E. I. Solomon, Cell. Mol. Life Sci., 2015, 72, 869–883 CrossRef CAS PubMed.
- R. G. Hadt, S. I. Gorelsky and E. I. Solomon, J. Am. Chem. Soc., 2014, 136, 15034–15045 CrossRef CAS PubMed.
- X. Zhang, D. Wu, Y. Wu and G. Li, Biosens. Bioelectron., 2021, 172, 112776 CrossRef CAS PubMed.
- J. Wang, R. Huang, W. Qi, R. Su, B. P. Binks and Z. He, Appl. Catal., B, 2019, 254, 452–462 CrossRef CAS.
- S. Liang, X.-L. Wu, J. Xiong, X. Yuan, S.-L. Liu, M.-H. Zong and W.-Y. Lou, Chem. Eng. J., 2022, 450, 138220 CrossRef CAS.
- Y. Wang, D. Wang, Q. Mu, Y. Zhai, J. Hai, Y. Ji and R. Li, Chem. Eng. J., 2024, 502, 158021 CrossRef CAS.
- P. Villeneuve, J. M. Muderhwa, J. Graille and M. J. Haas, J. Mol. Catal. B: Enzym., 2000, 9, 113–148 CrossRef CAS.
- Y. M. Soh, I. F. Davidson, S. Zamuner, J. Basquin, F. P. Bock, M. Taschner, J. W. Veening, P. De Los Rios, J. M. Peters and S. Gruber, Science, 2019, 366, 1129–1133 CrossRef CAS PubMed.
- C. C. Akoh, G.-C. Lee, Y.-C. Liaw, T.-H. Huang and J.-F. Shaw, Prog. Lipid Res., 2004, 43, 534–552 CrossRef CAS PubMed.
- B. O’Leary, G. M. Preston and L. J. Sweetlove, Mol. Plant, 2014, 7, 231–243 CrossRef PubMed.
- Y.-Q. Gao, P. Jimenez-Sandoval, S. Tiwari, S. Stolz, J. Wang, G. Glauser, J. Santiago and E. E. Farmer, Cell, 2023, 186, 1337–1351 CrossRef CAS PubMed.
- Y. Sun and R. P. Jarvis, Annu. Rev. Plant Biol., 2023, 74, 259–283 CrossRef CAS PubMed.
- W. Vandenende, B. Deconinck and A. Vanlaere, Trends Plant Sci., 2004, 9, 523–528 CrossRef CAS PubMed.
- T. Klein, U. Eckhard, A. Dufour, N. Solis and C. M. Overall, Chem. Rev., 2018, 118, 261–292 CrossRef PubMed.
- A. A. Vernekar, T. Das and G. Mugesh, Angew. Chem., Int. Ed., 2015, 55, 1412–1416 CrossRef PubMed.
- R. Walther, A. K. Winther, A. S. Fruergaard, W. van den Akker, L. Sørensen, S. M. Nielsen, M. T. Jarlstad Olesen, Y. Dai, H. S. Jeppesen, P. Lamagni, A. Savateev, S. L. Pedersen, C. K. Frich, C. Vigier-Carrière, N. Lock, M. Singh, V. Bansal, R. L. Meyer and A. N. Zelikin, Angew. Chem., Int. Ed., 2018, 58, 278–282 CrossRef PubMed.
- Y. Lin, J. Ren and X. Qu, Adv. Mater., 2014, 26, 4200–4217 CrossRef CAS PubMed.
- T. Chen, Y. Lu, X. Xiong, M. Qiu, Y. Peng and Z. Xu, Adv. Colloid Interface Sci., 2024, 323, 103072 CrossRef CAS PubMed.
- Z. Zhang, Z. Chen, Y. Zhang, Z. Wang, D. Chen, J. Liu and Z. Zhu, Coord. Chem. Rev., 2025, 524, 216340 CrossRef CAS.
- Z. Yu, J. Chen, D. Chao, X. Sun, L. Liu and S. Dong, Appl. Catal., B, 2023, 330, 122639 CrossRef CAS.
- M. D. Arifuzzaman, I. Bose, F. Bahrami and Y. Zhao, Chem Catal., 2022, 2, 2049–2065 CAS.
- J. Sheng, Y. Wu, H. Ding, K. Feng, Y. Shen, Y. Zhang and N. Gu, Adv. Mater., 2023, 36, 2211210 CrossRef PubMed.
- Y. Jiang, Z. Chen, N. Sui and Z. Zhu, J. Am. Chem. Soc., 2024, 146, 7565–7574 CrossRef CAS PubMed.
- X. Ye, L. Xia, H. Yang, J. Xu, T. Liu, L. Wang, S. Zhang, Y. Chen, D. Du and W. Feng, Mater. Today, 2023, 68, 148–163 CrossRef CAS.
- L. Shang, Y. Yu, Y. Jiang, X. Liu, N. Sui, D. Yang and Z. Zhu, ACS Nano, 2023, 17, 15962–15977 CrossRef CAS PubMed.
- X. Huang, S. Chen, W. Li, L. Tang, Y. Zhang, N. Yang, Y. Zou, X. Zhai, N. Xiao, W. Liu, P. Li and C. Xu, Nat. Chem. Biol., 2021, 17, 549–557 CrossRef CAS PubMed.
- K. Sawinski, S. Mersmann, S. Robatzek and M. Böhmer, Mol. Plant-Microbe Interact., 2013, 26, 626–632 CrossRef CAS PubMed.
- Z. Chen, J. Niu, Z. Guo, X. Sui, N. Xu, H. A. Kareem, M. U. Hassan, M. Yan, Q. Zhang, J. Cui, J. Kang, Z. Wang, F. Mi, D. Karagić and Q. Wang, Environ. Sci.-Nano, 2021, 8, 2731–2748 RSC.
- B. Wu, F. Qi and Y. Liang, Trends Plant Sci., 2023, 28, 1124–1131 CrossRef CAS PubMed.
- L. Zhao, T. Bai, H. Wei, J. L. Gardea-Torresdey, A. Keller and J. C. White, Nat. Food, 2022, 3, 829–836 CrossRef PubMed.
- V. Demidchik, Environ. Exp. Bot., 2015, 109, 212–228 CrossRef CAS.
- Y. J. Jia, L. Kang, Y. L. Wu, C. R. Zhou, D. Li, J. Q. Li and C. P. Pan, J. Agric. Food Chem., 2023, 71, 13595–13611 CrossRef CAS PubMed.
- F. Liu, G. Zhang, C. L. Zhang, W. L. Zhou, X. J. Xu, Q. Y. Shou, F. Yuan, Q. Li, H. J. Huang, J. H. Hu, W. J. Jiang, J. M. Qin, W. G. Ye and P. L. Dai, Environ. Res., 2023, 219, 115097 CrossRef CAS PubMed.
- J. Prathiksha, R. K. Narasimhamurthy, H. S. Dsouza and K. D. Mumbrekar, Mol. Biol. Rep., 2023, 50, 5465–5479 CrossRef CAS PubMed.
- Y. Wang, Y. Liang, X. Ma, Y. Dong, M. Klakong, A. Wang, M. Chen, T. Fan, X. Xu, P. Xiao and W. Ding, Ind. Crops Prod., 2024, 220, 119269 CrossRef CAS.
- S. Li, Y. Long, G. Deng, Y. Men, F. Lu, Z. Wang, J. Li and H. Han, Environ. Sci.-Nano, 2025, 12, 701–715 RSC.
- C. Li, Y. Han, T. Gao, J. Zhang, D.-X. Xu and Y. Wāng, Environ. Chem. Lett., 2022, 21, 1141–1176 CrossRef.
- R. Gao, L. Xu, M. Sun, M. Xu, C. Hao, X. Guo, F. M. Colombari, X. Zheng, P. Král, A. F. de Moura, C. Xu, J. Yang, N. A. Kotov and H. Kuang, Nat. Catal., 2022, 5, 694–707 CrossRef CAS.
- Q. Zeng, C. Yu, X. Chang, Y. Wan, Y. Ba, C. Li, H. Lv, Z. Guo, T. Cai, Z. Ren, Y. Qin, Y. Zhang, K. Ma, J. Li, S. He and H. Wan, Chem. Eng. J., 2022, 446, 137074 CrossRef.
- M. Jakl, S. Ćavar Zeljković, I. Kovač, K. Bělonožníková and J. Jaklová Dytrtová, Chemosphere, 2021, 277, 130242 CrossRef CAS PubMed.
- P. Singh and S. M. Prasad, Sci. Total Environ., 2018, 630, 839–848 CrossRef CAS PubMed.
- Y. Song and C. H. Haney, Nat. Plants, 2021, 7, 994–995 CrossRef PubMed.
- M. Rezayian, V. Niknam and M. Arabloo, Sci. Rep., 2023, 13, 9628 CrossRef CAS PubMed.
- S. Shirvani-Naghani, S. Fallah, L. R. Pokhrel and A. Rostamnejadi, Sci. Rep., 2024, 14, 27898 CrossRef CAS PubMed.
- Q. Chen, X. Cao, X. Nie, Y. Li, T. Liang and L. Ci, J. Hazard. Mater., 2022, 423, 127260 CrossRef CAS PubMed.
- J. Liu, S. Z. Qiao, Q. H. Hu and G. Q. Lu, Small, 2011, 7, 425–443 CrossRef CAS PubMed.
- M. Zia-ur-Rehman, A. Naeem, H. Khalid, M. Rizwan, S. Ali and M. Azhar, Nanomaterials in Plants, Algae, and Microorganisms, 2018, pp. 221–238 DOI:10.1016/b978-0-12-811487-2.00010-4.
- S. Chen, H. Liu, Z. Yangzong, J. L. Gardea-Torresdey, J. C. White and L. Zhao, Environ. Sci. Technol., 2023, 57, 19932–19941 CrossRef CAS PubMed.
- N. G. M. Palmqvist, G. A. Seisenbaeva, P. Svedlindh and V. G. Kessler, Nanoscale Res. Lett., 2017, 12, 631 CrossRef PubMed.
- M. S. Haydar, S. Ali, P. Mandal, D. Roy, M. N. Roy, S. Kundu, S. Kundu and C. Choudhuri, Sci. Rep., 2023, 13, 11040 CrossRef CAS PubMed.
- E. van Zelm, Y. Zhang and C. Testerink, Annu. Rev. Plant Biol., 2020, 71, 403–433 CrossRef CAS PubMed.
- Z. Li, L. Zhu, F. Zhao, J. Li, X. Zhang, X. Kong, H. Wu and Z. Zhang, Front. Plant Sci., 2022, 13, 843994 CrossRef PubMed.
- X. Yan, S. Chen, Z. Pan, W. Zhao, Y. Rui and L. Zhao, ACS Nano, 2022, 17, 492–504 CrossRef PubMed.
- J. Gong, Q. Liu, L. Cai, Q. Yang, Y. Tong, X. Chen, S. Kotha, X. Mao and W. He, ACS Sustainable Chem. Eng., 2023, 11, 4237–4247 CrossRef CAS.
- L. Lu, M. Huang, Y. Huang, P. F. X. Corvini, R. Ji and L. Zhao, Environ. Sci.-Nano, 2020, 7, 1692–1703 RSC.
- H. Liu, C. Li, L. Cai, X. Zhang, J. Si, Y. Tong, L. Wang, Z. Xu and W. He, J. Agric. Food Chem., 2024, 72, 24311–24324 CrossRef CAS PubMed.
- H. Zhao, R. Zhang, X. Yan and K. Fan, J. Mater. Chem. B, 2021, 9, 6939–6957 RSC.
- Y. Liu, X. Cao, L. Yue, C. Wang, M. Tao, Z. Wang and B. Xing, Environ. Pollut., 2022, 299, 118900 CrossRef CAS PubMed.
- D. Feng, R. Wang, X. Sun, L. N. Liu, P. Liu, J. Tang, C. Zhang and H. Liu, Sci. Total Environ., 2023, 897, 165397 CrossRef CAS PubMed.
- B. B. Abdallah, X. Zhang, I. Andreu, B. D. Gates, R. El Mokni, S. Rubino, A. Landoulsi and A. Chatti, J. Hazard. Mater., 2020, 386, 121644 CrossRef CAS PubMed.
- M. Wang, C. Mu, X. Lin, W. Ma, H. Wu, D. Si, C. Ge, C. Cheng, L. Zhao, H. Li and D. Zhou, Environ. Sci. Technol., 2024, 58, 6900–6912 CrossRef CAS PubMed.
- C. J. Weiser, Science, 1970, 169, 1269–1278 CrossRef CAS PubMed.
- H. Wu, X. Wan, J. Niu, Y. Cao, S. Wang, Y. Zhang, Y. Guo, H. Xu, X. Xue, J. Yao, C. Zhu, Y. Li, Q. Li, T. Lu, H. Yu and W. Jiang, J. Nanobiotechnol., 2024, 22, 268 CrossRef CAS PubMed.
- Z. Ding, W. Xu, Y. Feng, Y. Li, X. Ma, Z. Zhou, K. Chen, A. A. Shah, Q. Wang, X. Ren, J. Liang, Y. Huang, Q. Xiao and Z. Zhang, Chem. Eng. J., 2025, 508, 160428 CrossRef CAS.
- Q. Shan, M. Liu, R. Li, Q. Shi, Y. Li and B. Gong, Sci. Total Environ., 2022, 835, 155404 CrossRef CAS PubMed.
- M. Yang, Y. Wang, G. Yang, Y. Wang, F. Liu and C. Chen, Trends Food Sci. Technol., 2024, 144, 104340 CrossRef CAS.
- F. Chen, Z. Zhou, N. Yang, Q. Jiang, X. Zhang, H. Zhang, Y. Zheng, W. Li and B. Lei, Food Chem., 2025, 464, 141926 CrossRef CAS PubMed.
- M. Xie, F. Li, Y. Li, K. Qian, Y. Liang, B. Lei, Y. Liu, J. Cui and Y. Xiao, Chem. Eng. J., 2025, 506, 159956 CrossRef CAS.
- Y. Wang, C. Ma, F. Dang, L. Zhao, D. Zhou and X. Gu, J. Hazard. Mater., 2024, 462, 132770 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |

