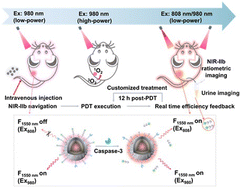
Customized cancer therapy relies on timely therapeutic effect evaluation to provide prescription adjustment for individual cases. However, currently reported therapeutic reagents are rarely integrated with imaging probes for self-evaluation of effects. Contrast imaging agents to measure tumor size changes must be administrated separately after therapy, complicating the therapeutic process and delaying reporting time. Herein, we design a customized therapy platform (LNPs-RB/Pep/cRGD) by conjugating lanthanide nanoparticles (LNPs) with the photosensitizer rose bengal, a caspase-3 substrate peptide (with Cy7.5 labelled at the terminal), and the tumor-targeting molecule cRGD. LNPs exhibit NIR-IIb downconversion luminescence under 980 nm/808 nm excitations for in vivo imaging, and visible upconversion luminescence under high-power 980 nm excitation for photodynamic therapy (PDT). By sequentially programming NIR excitation wavelength and power, NIR-IIb-imaging guided PDT and real-time cancer cell apoptosis imaging are achieved as therapeutic efficiency feedback. PDT induces cell apoptosis, generating caspase-3, which cleaves Cy7.5-containing peptide fragments from LNPs. This process corresponds to a recovery in vivo of NIR-IIb ratiometric imaging at 808 nm versus 980 nm excitation. The cleaved Cy7.5-containing peptide fragment is cleared into urine for NIR imaging. Both cell apoptosis imaging processes are completed 12 h after PDT, which is 7 days earlier than tumor size measurement. Therefore, customized therapy is achieved by timely adjusting PDT dosage, enhancing therapeutic efficacy.