Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fundamental concepts of 3,4-alkoxythiophene-based polymeric systems, from synthesis to applications
Francisco A. Bravo-Plascencia
ab,
M. Paola Flores-Morales
 ab,
Alexander Kuhn
ab,
Alexander Kuhn
 c,
Gerardo Salinas
c,
Gerardo Salinas
 *c and
Bernardo A. Frontana-Uribe
*c and
Bernardo A. Frontana-Uribe
 *ab
*ab
aInstituto de Química, Universidad Nacional Autónoma de México, Circuito Exterior Ciudad Universitaria, CDMX, 04510, Mexico
bCentro Conjunto de Investigación en Química Sustentable UAEM-UNAM, Km 14.5 Carretera Toluca-Atlacomulco, Toluca, 5200, Mexico. E-mail: bafrontu@unam.mx
cUniv. Bordeaux, CNRS, Bordeaux INP, ISM, F-33607 Pessac, UMR 5255, France. E-mail: gerardo.salinassanchez@enscbp.fr
First published on 14th July 2025
Abstract
Conducting polymers are one of the most interesting materials due to their broad range of applications, from sensing and energy storage to organic actuators. Among the different conducting polymers, the family of poly-3,4-alkoxy thiophenes stands out due to its exceptional electrical, optical, and chemical properties. The electro-donating effect of the 3,4-alkoxy groups favors the polymerization reaction, provides stereoregularity, and increases the effective conjugation. With poly-3,4-ethylendioxythiophene (PEDOT) as its flagship, this family has gained increasing attention in multiple fields of science, ranging from bioanalysis and electrochromic systems to microelectronics. This review outlines the most recent developments in the study of 3,4-alkoxy thiophenes, from the synthetic routes of monomers to the fundamentals behind the polymerization and functionalization. Finally, conventional and unconventional applications of these materials in different fields of science, i.e., batteries and supercapacitors, electroanalysis, or optoelectronics, are discussed.
Introduction
Since the late 70s, when Shirakawa, MacDiarmid, and Heeger published the first work concerning synthesizing high-conductivity doped polyacetylene, conducting polymers (CPs) have gained considerable attention in the scientific community.1–3 From a materials science point of view, CPs have a unique combination of the electrical behavior of metals and the mechanical properties associated with organic polymers. As is well known, the semiconductor behavior of these polymers is based on the redox formation and mobility of charged species within the π-conjugated backbone.4–7 CPs have passed from a laboratory curiosity to a technological reality due to their numerous applications, ranging from sensing to optoelectronics and energy storage devices.8 Commonly, many π-conjugated monomers can be used to produce CPs, such as aniline, pyrrole, furan, or thiophene. In particular, thiophene-based polymers exhibit high chemical and electrochemical stability, which is attributed to the stabilizing effect of the positive charges by the sulfur heteroatom and the resonance effect within the conjugated oligomer.9 Furthermore, incorporating electron-donating substituents onto positions 3 and 4 of the thiophene ring favours the electropolymerization mechanism, stabilizes the formed radical cations, and minimizes α-β and β-β couplings of the oligomers. Consequently, poly-3,4-dialkoxythiophenes have become one of the most studied materials in the field of CPs, and numerous potential applications have been developed.10–12 The poly-3,4-dialkoxythiophene family has a good balance between conductivity, stability, and processability, making their materials very attractive among different families of CPs (Table 1). This review provides a complete overview of recent trends in the study and applications of poly-3,4-dialkoxythiophenes. At first, different synthetic routes for the efficient design of 3,4-dialkoxythiophene monomeric derivatives and the possible chemical modification of the conjugated ring are summarized. Afterwards, chemical and electrochemical polymerization methods of the monomers and the formation of copolymers are discussed. Finally, the current state of the art of selected applications of these π-conjugated systems is presented.| Polymer family | Conductivity (S cm−1) | Stability | Processability |
|---|---|---|---|
| Polyacetylene | 105 (Doped state) | Very poor | Very poor |
| Polpyrroles | 1–100 | Poor | Poor |
| Polyanilines | 1–100 | Fair | Moderate |
| Polythiophenes | 10−3–102 | Good | Poor |
| Poly-3,4-dialkoxythiophenes | 10−3–102 | Excellent | Excellent |
Synthesis of 3,4-alkoxy thiophene-based monomers
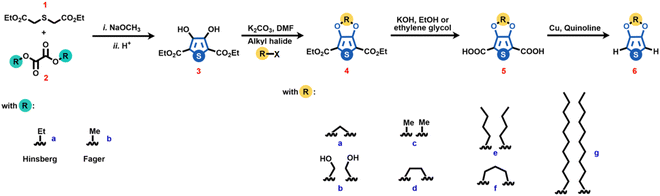
The most well-established methodology for synthesizing 3,4-dialkoxythiophenes is based on a four-step reaction route (Scheme 1). At first, a Hinsberg condensation between the thiodiglycolic acid ester (1) and an α-dicarbonyl (2a, b) in the presence of a strong base, i.e., sodium methoxide, generates the corresponding 3,4-dihydroxythiophene-2,5-dialkyl ether (3), which is the starting molecule for the synthesis of 3,4-alkoxythiophenes.13–15 After this, the Williamson etherification of (3) with alkyl halides in the presence of K2CO3 leads to O-alkylation at the 3,4-position, producing the 3,4-alkoxythiophene-2,5-dialkyl ether (4a-g).16 In the next step, 3,4-alkoxythiophene-2,5-dicarboxyl acid (5) is formed via a simple saponification reaction, and finally, the Cu-catalyzed proto decarboxylation in quinoline leads to the synthesis of the desired 3,4-alkoxythiophene (6).17 Although, as mentioned above, this is the most common methodology for synthesizing 3,4-alkoxythiophenes, multiple variations of each reaction step have been developed.18–20 This section summarizes different synthetic approaches explored to improve each reaction step involved in the synthetic route to obtain 3,4-alkoxy thiophenes.Thiophene moiety generation
Alternative chemical pathways to the previously described Hinsberg condensation have been developed. For example, Al-jumaili et al. modified the classic condensation protocol by using 2,2-thiodiacetonitrile (7) as the starting compound instead of thioglycolic acid, producing 3,4-ethylenedioxythiophene-2,5-dicarbonitrile (9) as an intermediate to access the corresponding aldehyde (10) as the final compound (Scheme 2a).21 Moreover, Hellberg et al. reported a one-step synthesis approach for the synthesis of 3,4-dimethoxy thiophene (12) based on a ring closure (addition–elimination) between 2,3-dimethoxy-1,3-butadiene (11) and sulphur dichloride (Scheme 2b).22 In an alternative approach, a two-step route for the synthesis of 3,4-ethylenedioxythiophene (EDOT) (6d) was developed based on the cyclization reaction of a diyne precursor (13), mediated by di(cyclopentadienyl)zirconium(IV) dichloride (Cp2ZrCl2) and using disulphuric dichloride as a sulphur source, to generate 2,5-bis(trimethylsilyl)-3,4-ethylenedioxythiophene (14) (Scheme 2c).23 Afterward, EDOT was obtained through a proto-desilylation in the presence of tetra-n-butylammonium fluoride. Finally, a radical-cascade procedure was proposed to synthesize EDOT, in which the thioacetic acid reacts with a diyne precursor (15) in the presence of AIBN acting as the initiator.24O-Alkylation of the thiophene ring from 3,4-positions
From a synthetic point of view, the O-alkylation of (3) or 3,4-dimethoxythiophene is a crucial reaction for synthesizing novel and more sophisticated 3,4-alkoxythiophenes. Three synthetic routes have been developed: the Mitsunobu reaction, a transetherification synthesis, and a double Williamson etherification. The Mitsunobu reaction is based on the use of diethyl azodicarboxylate (DEAD),25 dialkylazodicarboxylate (DIAD)26,27 or tetramethylazodicarboxamide (TMAD),28,29 acting as a dehydrogenating agent in the presence of tri-substituted phosphine and 1,2- or 1,3-diols (Scheme 3a). This approach has been used for the synthesis of multiple 3,4-alkoxythiophenes, from classic EDOT and 3,4-propylenedioxythiophene (ProDoT) derivatives to more sophisticated alkyl short and large ring-alkylene, benzyl, or crown ether-like thiophenes.Transetherification is a reaction between an ether and an alcohol, catalyzed by an acid, to interchange functional groups. Such a reaction has been extended to the O-alkylation of 3,4-dimethoxythiophene, acting as a starting compound.30 For example, Krishnamoorthy et al. used dibenzyl and substituted phenylene 1,3-diols to synthesize dibenzyl propylenedioxythiophene and 3,4-phenylenedioxythiophene derivatives (Scheme 4a).31,32 In addition, with this methodology, enantiomerically pure chiral EDOT derivatives have been designed (Scheme 4b).30,33 Furthermore, a double transetherification of the four positions of pentaerythritol with two 3,4-dimethoxythiophene molecules was reported to produce the spiro bipropylenedioxythiophene (19) (Scheme 4c).34 The same approach was used to synthesize bis-EDOT by using threitol as a bridge to connect two EDOT moieties.35 On the other hand, using the acid transetherification reaction, it is possible to produce interesting reactive 3,4-dialkoxythiophene intermediaries such as 3,4-diiodoethyloxythiophene. Roncali et al. exploited the reactivity of such a molecule to synthesize 3,4-vinylenedioxythiophene (VDOT) (23) (Scheme 4d).36 A continuous flow synthesis process of 3,4-propylenedioxythiophene derivatives is based on this methodology.37
The double Williamson etherification reaction has recently garnered considerable attention due to the potential O-alkylation of (3) with high steric hindrance substituents (Scheme 5a). The ring closure step is achieved using alkyl halides and trialkyl amines, acting as substituent moieties and a base-solvent.38 This approach was used to prepare ethylene, propylene, mono and di-toluene, phenyl-propene derivatives, para-, meta-, and ortho-xylene, and 3,4-substituted thiophenes (Scheme 5b).39,40 In particular, using xylene groups enables the synthesis of more sophisticated macrocycle alkoxythiophenes. Furthermore, Krompiec et al. extended the double Williamson etherification reaction to the synthesis of 3,4-phenylenedioxythiophene (PhEDOT) functionalized with electron-withdrawing groups via a double nucleophilic aromatic substitution of substituted benzenes in a one-pot reaction (Scheme 5c).41
Proto decarboxylation
The final monomers are obtained through the double proto-decarboxylation of (5), commonly carried out until a decade ago, using Cu-based catalysts in quinoline as the solvent (Scheme 1).42 Under these conditions, the complete conversion of electron-rich aromatic systems like 3,4-alcoxythiophenes is limited, leading to moderate yields. Besides, novel and environmentally friendly methodologies have been developed due to the toxicity of the reaction media and the time-consuming experimental procedure. For example, using different metal phthalocyanines (PC) of Cu, Zn, Co, Ni, Mn, Mg, Fe, and Ag as catalysts allows decarboxylation in aqueous media and at mild temperatures (Scheme 6).43 Recently, Cisneros-Pérez et al. developed a highly efficient, low-temperature, and toxic-solvent-free proto-decarboxylation reaction with Ag2CO3 as a catalyst and in a microwave reactor.44 This synthetic method presents a considerable improvement in yield and time (from hours to minutes) compared with decarboxylation based on Cu2Cr2O7 (Scheme 6).Chemical modification of the 2 and 5 positions
Interestingly, it is possible to fine-tune the physicochemical properties of 3,4-alkoxythiophenes by introducing additional π-conjugated groups within the aromatic structure of the monomer. For example, the formation of a Mannich-type base at one α-position of the thiophene ring allows the functionalization of positions 2 and 5 (Scheme 7a).45 However, the most straightforward alternative is via a direct C–H arylation of EDOT, catalyzed by clay-supported Pd.46 This approach has given access to multiple mono- and bis-arylated EDOT-based functional π-conjugated molecules.With a similar philosophy, donor–acceptor–donor monomers have been designed either by using a Pd(OAc)2-catalyzed direct heteroarylation of EDOT or via a Suzuki–Miyaura coupling synthetic pathway (Scheme 7b);47 recently, deep eutectic solvents have been employed for these couplings, thereby avoiding the use of toxic solvents.48 In this context, different methodologies involving metallic catalysts have been developed. For example, the mono-arylation of Grignard-type EDOT catalyzed by NiCl2 in 1,3-bis(diphenylphosphine)-propane allows the introduction of conjugated structures, such as para-phenylene, vinylene, furane, or thiophene (Scheme 7c).49 Furthermore, thioporphyrins were produced in a three-step synthetic pathway; at first, the reaction between the corresponding 3,4-diakoxythiophene and butyl-lithium to generate the organolithium compound, followed by a condensation with benzaldehyde, treated with boron trifluoride etherate, and finally the proper oxidation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (Scheme 7d).50,51 Nonetheless, generating a Mannich-type base at one α-position of the thiophene ring is a practical approach to overcome the use of metallic catalysts. Such an active group facilitates the efficient chemical coupling of para-phenylene–vinylene, thiophenylene–vinylene, substituted thiophene,52 and vinylene-linked N-ethyl-carbazole53 groups to the α-position of thiophene (Scheme 7e). Stolić et al. developed a synthetic route based on the double oxidation of the ether moiety present in the 3,4-ethylenedioxythiophene-2,5-diethyl ether with LiAlH4 and IBX to the corresponding aldehyde, followed by the formation of the benzimidazole rings (Scheme 7f).54
Ethylene and propylene bridge modification
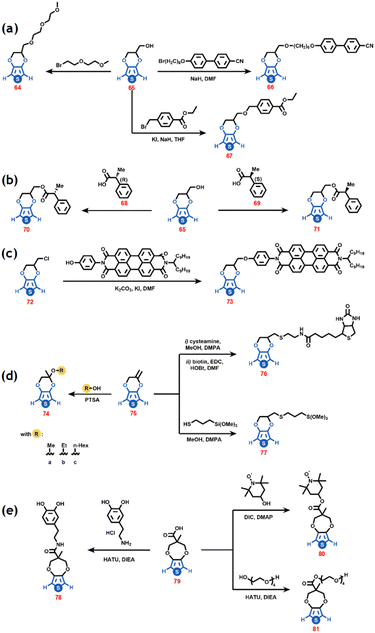
In addition to the above-mentioned α-α functionalization of the thiophene ring, it is possible to chemically modify the 3,4-ethylene or the 3,4-propylene groups. At first, through a Williamson etherification reaction, ethylene or propylene groups containing leaving groups, such as Cl and OH, are introduced to the monomeric unit.55 Afterward, it is possible to substitute the halogen atoms to functionalize 3,4-alkoxythiophene. Different pendant groups have been introduced to the 3,4-alkoxy structure, ranging from polyethers56 and long alkyl chains57 to bulky groups such as 4-(6-bromohexyl-oxy)-40-cyano biphenyl ether58 and perylenebisimide, hydroxymethyl- or chloromethyl-substituted EDOT (65 or 72) (Scheme 8a and 8b).59 For example, Dong et al. reported the successful anchoring of (R)– and (S)-2-phenyl propionic acid as pendant groups to Me-EDOT, where the obtained monomers retain the configuration of the initial chiral reactant (Scheme 8c).60 The incorporation of crown ether into compound 72 produced CP with enhanced properties.;61 after several synthetic steps it is possible to attach maleimide derivatives from the same starting compound to prepare human cell platforms.62A helpful alternative is using exomethylene-3,4-ethylenedioxythiophene (emEDOT) (75) as a versatile building block. This can be obtained by the reaction between the mixture of EDOT-CH2OH and ProDOT with para-toluenesulphony chloride to obtain the tosylate ester, and after crystallization, EDOT-CH2OpTS can be separated, and this at last, in the presence of potassium tert-butoxide, generates emEDOT.63 With this monomer as a starting unit, it is possible to functionalize the 3,4-ethylene group with many substituents (Scheme 8d). Finally, ProDOT carboxylic acid (ProDOT-COOH) (79) was synthesized by trans-etherification of 1,3-diol and 3,4-dimethoxy-thiophene under acidic conditions. From this starting monomer, it is possible to substitute the OH from the carboxylic group via a classic esterification reaction with different redox active moieties, i.e., dopamine, hydroxy-TEMPO, or tetra ethyleneglycol (Scheme 8e).64 The central sp3 carbon of ProDOT has been symmetrically functionalized to attach diverse substituents, for example azide, triazole,65 aliphatic alkyl ether chains,66 alkyl ester chains,67 glyme side chains,68,69 and disulphide70 among others. These monomers have shown excellent properties for use in technological applications.
Chemical and electrochemical polymerization
As is well known, π-conjugated monomers are suitable starting units for generating conducting polymers. Commonly, this is achieved by following an oxidative radical polymerization mechanism, which involves the formation of highly reactive species, i.e., radical anions or dications. In this context, the oxidative dimerization and oligomerization pathway is the most studied polymerization mechanism in conducting polymers (Scheme 9a). At first, oxidation of the monomer takes place to form the corresponding radical cation, which dimerizes at the α-position of the conjugated ring, with the subsequent deprotonation step to recover aromaticity. Afterwards, the formed dimers undergo a series of consecutive oxidation and coupling-deprotonation steps, continuously increasing the oligomers' length. However, the coupling pathway followed by the formed oligomeric radical cations strongly depends on the substituents and their molecular position within the monomer. For example, for the simple thiophene, α-α-, α-β and β-β- oligomeric couplings can take place, leading to the formation of highly cross-linked films. Thus, as stated above, the electron-donating effect of the 3,4-alkoxy groups favours α-α-couplings, producing well-defined oligomeric structures. Since the polymerization conditions can fine-tune the electrical, electrochemical, mechanical, and optical properties of such materials, this section summarizes the recent advances in the chemical and electrochemical polymerization of 3,4-alkoxythiophenes and the possible post-functionalization of the obtained conjugated films. The reader can consult the reviews and books in this field for more details concerning the different oxidative and reductive polymerization mechanisms.4,8,71 | ||
| Scheme 9 (a) Oxidative dimerization and oligomerization mechanism of 3,4-alkoxy thiophenes and (b) long chain propagation catalyzed by solid-state redox processes. | ||
Homogeneous redox polymerization
The homogeneous oxidative redox polymerization takes advantage of the thermodynamically favored reduction of a redox initiator, i.e., FeCl3.72 Thus, in the previously mentioned mechanism, the oxidant dissociates into active species before the oligomerization reaction.73 Such highly reactive oxidants cause the continuous generation of the radical cation of the monomer and its subsequent oligomers, which, via the oxidative dimerization pathway, produce the corresponding CP. Among all the different π-conjugated systems, PEDOT produced with homogeneous redox polymerization is one of the few conducting polymers that have become an industrial reality.74 However, solutions of doped monomers and oligomers are highly unstable due to their different thermodynamic standard redox potentials. An interesting alternative to slowing down the oxidative process is to increase the pH slightly during homogeneous redox polymerization. Different approaches have been developed to produce highly conducting PEDOT films, ranging from the use of vapour phase polymerization (VPP)75 and high temperature-non-hydrolyzed oxidants (Fe2(SO4)3),76 to ultrasonic spray polymerization (USPo) (producing nanospheres of PEDOT)77 and organic solid Brønsted acids (p-toluenesulfonic acid), to generate α–α carbon bonds.78 The use of mechanochemistry with 3,4-alkoxythiohenes, Fe3+ salts as an oxidant and NaCl as an additive, allows the solvent-free synthesis of the corresponding polymers.79,80 Also, considerable efforts have been made to achieve homogeneous redox polymerization of alternative 3,4-alkoxythiophenes. For example, a family of soluble donor–acceptor ProDOT-based films has been reported. Such conjugated polymers were synthesized through a Knoevenagel polycondensation or a Yamamoto coupling polymerization.81 The solid-state polymerization of 3,4-ethylenedioxythiophene-methanol (EDTM) derivatives was carried out from the 2,5-dihalogenated monomers, which induces the solid-state radical polymerization at slightly higher temperatures (80 °C for 24 h),82 which is an alternative to Br2 oxidative polymerization using the same starting materials.83 Lu et al., designed 3,4-dialkoxythiphene-based copolymers using a palladium-catalyzed direct C–H arylation polymerization (DHAP). Such a methodology produces the linked dithiazole unit with the alkyl-substituted 3,4-dialkoxythiophene derivatives.84 Following this approach, copolymers of ProDOT and acyclic dioxythiophene (AcDOT) with PhEDOT were designed to increase the chemical stability of the starting monomers.85 Water-processable polythiophenes with conductivities up to 1000 Scm−1 useful for roll-to-roll processing were obtained in this way.86 An alternative for these Pd-based hetero couplings employing the indophenine reaction to incorporate an aromatic connector between thiophene moieties is gaining interest.87–89Furthermore, the homogeneous redox polymerization approach has been successfully coupled with templated substrates to generate sophisticated nanostructured conducting films. Such highly ordered surfaces present an increasing potentiality in microelectronics and photonic devices for energy storage and biological applications. For example, V2O5 nanofibers were used as sacrificial templates during the chemical synthesis of PEDOT nanofibers.90 Monodimensional (1-D) nanostructured PEDOT systems (tubes, thimbles, rods, and belts) were obtained via oxidative polymerization using Al2O3 membranes as a template.91 With the same philosophy, PEDOT: VDOT thin films were obtained by chemical oxidation using a sol–gel generated surface of di-Si(OEt)3 functionalized with a free radical initiator (AIBN), which acts as an anchor point for EDOT oligomerization.92 In addition to EDOT, the homogeneous redox polymerization of ProDOT-based monomers has been successfully carried out. In principle, these materials were used as model systems for optoelectronic applications; they have recently gained considerable interest in the study of solid-state electrochemical doping.68,93 Although homogeneous redox polymerization is so far the classic method for producing poly-3,4-alkoxythiophene-based devices, the main limitation is the poor control of the doping level within the oligomeric units, due to the high thermodynamic redox potential value of the required oxidants and the impurities that are frequently found in the polymer matrix. In this context, electrochemical polymerization is a powerful tool for overcoming and controlling the properties of the prepared materials.
Electrochemical polymerization
As stated above, electropolymerization offers several advantages over homogeneous polymerization, including morphology control and efficient fine-tuning of the doping level. This is commonly performed using a conventional three-electrode system composed of a working, counter, and reference electrode. In such a setup, the anodic reactions are triggered by controlling the thermodynamic (potentiodynamic or potentiostatic methods) or kinetic (galvanostatic method) parameters associated with the oxidation of the monomers. Thus, the oxidative dimerization pathway occurs at the electrode/electrolyte interface, followed by an electrostatic deposit of short-chain oligomers (nucleation).94 Afterwards, three-dimensional growth catalyzed by solid-state redox processes takes place. In addition, electrochemical methods can produce templated electrodes to design sophisticated and highly structured materials (Scheme 9b).95 Interestingly, the properties of the resulting polymers depend on the electrochemical technique used.96Once again, electropolymerization of EDOT is the most studied system since the produced polymer exhibits high electrochemical stability during multiple charge/discharge cycles.97 Furthermore, heterogeneous redox polymerization can be carried out in the presence of different doping ions, to fine-tune the electrochemical properties.98 Due to its efficient electropolymerization, EDOT has been used as a building block to design multiple functionalized conducting polymers. PEDOT films functionalized with cyanobiphenyl derivatives,99 sodium sulfinopropyl,100 electron acceptor groups such as perylenebisimide,59 9,10-anthroquinone (AQ), perylenetetracarboxylic diimide (PTCDI), and 11,11,12,12-tetracyano-9, 10-anthraquinodimethane (TCAQ)101 or biocompatible groups, i.e., biotin102 have been designed, among many others. An exciting alternative to facilitate the electropolymerization and minimize steric hindrance is to use dimeric and trimeric units as starting compounds.103–106 For example, the dimer and trimer of PheDOT present a higher reactivity than the monomer unit, which facilitates electropolymerization.107 Besides, starting from more conjugated monomers decreases the oxidation potential; thus, the redox properties of the CPs are enhanced. A classic example is thiophene, where bithiophene is always preferred to obtain PTh CPs due to the thiophene paradox.71 EDOT-aldehyde-bearing thiophene-EDOT units facilitate the incorporation of functional groups on the oligomeric backbone.108 Another alternative is the design of π-conjugated 3D systems, where EDOT groups are located strategically on the α and β positions of bithiophene to generate a 3D template unit (Fig. 1a).109 In addition, the electrochemical co-polymerization of EDOT with alternative conjugated groups has gained considerable attention.110 For example, bithiophene-alt-thiophene-S,S-dioxide generated by anodic coupling of 2,5-bis(2-thienyl)thiophene-S,S-dioxide substituted with EDOT exhibits peculiar magnetic and luminescent properties.111 The efficient coupling of EDOT electrochemically linked to thienopyrazine, cyclopentadithiophen-4-one or 4-dicyanomethylenecyclopentadithiophene produces low-gap thiophene-based materials.112 Electrochemical co-polymerization of ferrocene-derived EDOT with hydroxymethyl-EDOT and oligo(oxyethylene)di-EDOT leads to chemically and electrochemically stable polymeric films.113 In particular, the non-covalent intramolecular S–O interactions of EDOT promote planar conformations of the π-conjugated systems during electrochemical copolymerization. For example, sulphur–bromine interactions lead to a strengthened and rigid backbone in a co-polymer formed by EDOT and 3-bromothiophene.114 However, multiple efforts have been made to polymerize alternative 3,4-alkoxythiophene systems electrochemically. For example, electropolymerized derivatives of PhDOT modified with naphthylmethyl-, 1-naphthylmethyl-, and 9-anthracenylmethyl-groups, exhibit nanostructured surfaces, providing these materials with unconventional physicochemical properties (Fig. 1b).32
 | ||
| Fig. 1 (a) Chemical structure of a 3D template formed by four EDOT groups attached at the α and β positions of a bithiophenic structure (left) and potentiodynamic electropolymerization of the corresponding 3D oligomeric material obtained in 10 mM monomer 0.10 M Bu4NPF6/ACN solution. Reproduced from ref. 109 with permission from Elsevier, copyright 2008. (b) Chemical structure and SEM images of the top view of electrodeposited functionalized PhDOT modified with naphthylmethyl-, 1-naphthylmethyl-, and 9-anthracenylmethyl-groups. Reproduced from ref. 32 with permission from the American Chemical Society, copyright 2019. (c) Chemical structure of ordered 3D oligomeric networks formed by PDbDOT and electrochemical polymerization of DbDOT obtained in a 5 mM monomer 0.1 M TBAPF6/CH2Cl2 solution. Reproduced from ref. 118 with permission from Elsevier, copyright 2025. | ||
Recently, the electropolymerization of 3,4-ortho-xylendioxytiophene (XDOT) in organic media was carried out.115,116 Such a polymer exhibits a potentiodynamic mirror image associated with fast electron transfer, attributed to an in situ internal oligomeric order caused by the presence of a xylene unit. This polymeric internal arrangement leads to an improved insulating/conducting transition of PXDOT compared to PEDOT or bithiophene.117 Furthermore, it has been proposed that during the electropolymerization of 3,4-duren-bi-dioxythiophene (DbDOT) ordered 3D oligomeric networks could be generated (Fig. 1c).118 This is associated with the double thiophene core within the monomeric unit and the spatial conformation provided by the duren moiety. Poly-ProDOT derivatives containing different functional groups such as oxyphenyl methanol (-OPhCH2OH), oxybenzyl (–OBz), bromide (–Br), and tosyl (–OTs) have been synthesized and electropolymerized.119 A direct correlation was observed between the oxidation potential of the monomer and the electron-donating or electron-withdrawing character of the substituent at the ether bridge. The presence of these substituents leads to a more pronounced π–π intermolecular stacking, improving the charge transport.
Although electrodeposition of 3,4-alkoxythiophenes is carried out in non-aqueous media, mainly due to the monomer's low solubility in water, the possible formation of micellar structures employing a surfactant, i.e., sodium dodecyl sulfate (SDS) or Triton X-100, allows the electrochemical polymerization of EDOT in aqueous media.120,121 This approach has been extended to alternative 3,4-alkoxythiophene monomers such as hydroxyl-methylated-EDOT55 or 3,4-dimethoxythiophene.122 However, the functionalization of EDOT facilitates electropolymerization in aqueous media. For example, introducing hydroxymethyl groups significantly improved the polymerization and electroactivity of the polymer generated in aqueous media.123 Additionally, Sun et al. carried out the polymerization of 2′-aminomethyl-3,4-ethylenedioxythiophene hydrochloride (EDOT-MeNH2 HCl) in an aqueous solution, obtaining highly stable polymers with efficient electrochromic properties.124
In addition to organic and aqueous media, multiple efforts have been carried out to explore the possible electropolymerization in alternative and, so far, unconventional solvents, such as ionic liquids or liquid crystals. For example, the oxidative electropolymerization of EDOT in an aqueous solution of hydroxypropyl cellulose (HPC) acting as an inert chiral nematic liquid crystal medium led to the generation of chiral PEDOT with good electrochemical stability and reversible optoelectronic properties.125 With a similar philosophy, Matsushita et al. used a chiral nematic liquid crystal system to electrochemically polymerize EDOT and bis-EDOT on helical carbon and graphite films.126 Furthermore, it has been demonstrated that the use of deep eutectic solvents such as choline chloride-urea (reline) and choline chloride-ethylene glycol (ethaline) leads to changes in the morphology and electrochemical properties of PEDOT,127,128 which enable the possible use of these surfaces for sensing applications.
Finally, post-polymerization functionalization allows fine-tuning of the chemical, electrochemical, electrical, and optical properties of CPs. For example, poly-3-(x-haloalkyl) thiophenes were post-functionalized with tetrathiafulvalene (TTF) derivatives to improve their electrochemical properties. These materials exhibit a lower insulating/conducting transition onset potential and an improved ionic permeability compared with PEDOT.129 With a similar philosophy, Wei et al. used thiol–ene ‘‘click’’ chemistry to covalently attach alkyl-thiols to electrochemically deposited poly-(ProDOT-diene). The presence of such pendant groups modifies the surface chemistry of the CP, which is reflected in changes in its physico–chemical properties, i.e., capacitance or hydrophilicity.130 Electrochemically driven post-functionalization of a poly succinimidyl ester EDOT derivative and oligo(oxyethylene)diEDOT with ferrocene-labelled oligonucleotides (Fc-ODNs) has been carried out for the design of bioelectrochemical sensors.131 In another example, co-polymers of dihexyl-substituted EDOT (Hex2-EDOT) and azidomethyl-EDOT (N3-EDOT) have been electrochemically functionalized with an electron-accepting phthalimide substituent by “click chemistry”.132
Property control by physicochemical interactions
For decades, diverse effects of the 3,4-alkoxythiophenes have been recognized as an important issue to consider during polymer engineering, particularly in the case of conducting or semiconducting organic polymers. For example, the band gap of these materials depends on five energy contributions: the energy related to the degree of bond length alternation, the mean deviation from planarity, the aromatic resonance energy of the cycle, the inductive or mesomeric electronic effects of substitution, and the intermolecular or interchain coupling in the solid state.133 Recently, an excellent review addressing this last contribution has reminded us that this sometimes unattended aspect is important to consider for solid-state applications in these organic conductors.134 One of the most important interactions in the polymer chain is π–π stacking, which depends on the ability to form long-range planar structures. Here, non-covalent intra-chain interactions, which generate conformational locks of the polymer backbone, favour planar structures between adjacent monomers. For poly 3,4-alkoxythiophenes, noncovalent O⋯S interactions are present to control the planarity, whereas when nitrogenated hetero co-polymers are used, N⋯S interactions guide the planarity of the material (Fig. 2).135 | ||
| Fig. 2 Chemical and single-crystal structures of 3,4-alkoxythiophenes showing the O⋯S interactions in homo polymers and N⋯S interactions in heterocopolymers. Adapted from ref. 135 with permission from the American Chemical Society, copyright 2017. | ||
On the other hand, side–chain interactions of the polymers contribute to controlling the material's properties. For example, the presence of glycolated chains favours the processability of the poly-3,4-alkoxythiophenes in aqueous media with good electronic properties.86 In this example, a combination of O⋯S interactions of the central heterocyclic moiety with the polar chains was an innovative proposal to produce large CP surfaces. Large alkyl chains on the 3,4-alkoxythiophenes promote van der Waals interactions, which generate a large range order in the polymer. Examples can be found in those used for organic electrochemical transistors;136 a similar situation to that observed with 3-hexylthiophene used for organic solar cells.137 Electronic properties like conductivity, charge mobility, band gap, and optoelectronic properties, among many others, depend on these intra- and inter-chain interactions. Therefore, the hydrophilicity and hydrophobicity of the chains138 and their chemical structure (linear or branched)69,139 modulate the electronic properties. In addition to these, the doping level is a crucial parameter to consider. The magnitude of this parameter directly impacts the electrical, electrochemical, and optoelectronic response of the material. Commonly this can be fine-tuned by using the nature of the dopant (counter-ion), the side-chain structure, the polymerization method and post-polymerization treatments.4,8,140,141 More detailed information about the relationship between the polymer structure and properties can be found in specialized literature dealing with organic semiconductors or polymer engineering.142,143 Nonetheless, it is evident that the nature of the 3,4-alkoxy group controls the physicochemical properties of the polymer, thus guiding the selection of the possible application (Table 2).
| Monomer | Polymer conductivity (S cm−1) | Polymer anodic window | Absorption maxima | Applications | Reference |
|---|---|---|---|---|---|
| PEDOT (6d) PEDOT:PSS | 10−4 to 103 | −0.6 V to 0.3 V vs. Ag/AgCl | 600 nm | Solar cells, supercapacitors, transparent conductive electrodes, sensors, and drug delivery | 76, 115 and 275 |
| PXDOT (6m) | 100 mS (Conductance) | −0.3 V to 0.6 V vs. Ag/AgCl | Electroanalysis | 116 | |
| DbDOT (Fig. 1c) | 9 mS (Conductance) | −0.25 V to 0.5 V vs. Fc/Fc+ | 420 nm | 3-D nanostructured surfaces | 118 |
| ProDOT-OBn2 (16) | −0.1 V to −0.3 V vs. SCE | 578 nm, 632 nm | Electrochromism | 31 | |
| PheDOTs (17a–c) | 0.7 V to 1.1 V SCE | Hydrophobic nanostructured surfaces | 32 | ||
| Stereo- and regioregular PEDOTs (18a–j) | −1.0 V to -0.5 V vs. Fc/Fc+ | Enantioselective electroanalysis | 30 | ||
| Poly(spiroBiProDOT) (19) | −0.45 V to 0.85 V vs. Fc/Fc+ | 486 nm | Electrochromism and solar cells | 34 | |
| Poly-(2,2′,3,3′-tetrahydro-2,2′-bithieno[3,4-b][1,4] dioxine) (20) | 0.0 V to 1.0 V vs. Ag/AgCl | 481 nm | Electrochromism | 35 | |
| (E)-1,2-bis(2-(3,4 ethylenedioxy) thienyl)vinylene (49a) | 20 | −0.6 V to 0.4 V vs. Ag/Ag+ | 590 nm | Electrochromism | 49 |
| (E)-1,2-bis(2-(3,4 ethylenedioxy)thienyl)benzene (49b) | 2.0 | −0.2 V to 0.2 V vs. Ag/Ag+ | 495 nm | Electrochromism | 49 |
| 2,2′:5′,2′′-ter(3,4-ethylenedioxy)thiophene (49f) | 10 | −1.2 V to 0.9 V vs. Ag/Ag+ | 442 nm | Electrochromism | 49 |
| Hydroxymethyene-3,4-ethylenedioxylthiophene (65) | 18 to 65 | −0.6 V to 0.6 V vs. Ag/AgCl | 520 nm | Electroanalysis | 55 |
| 4-(6 oxymethylenedioxythiophene hexyloxy)-4′-cyano biphenyl) (66) | 0.03 | −0.6 V to 0.6 V vs. Ag/Ag+ | 600 nm to 660 nm | Electrochromism | 58 |
| (2,3-dihydrothieno[3,4-b][1,4]dioxin-2-yl)methyl 2-phenylpropanoate) (70 and 71) | −0.4 V to 0.5 V vs. Ag/AgCl | 627 nm | Electroanalysis | 60 | |
| Perylenetetracarboxylic-bisdiimide-EDOT (73) | −0.5 V to 0.75 V vs. Fc/Fc+ | 400 nm to 850 nm | Organic solar cells | 59 | |
| emEDOT (74) | 0.1 V to 0.8 V vs. Fc/Fc+ | Building block for CPs | 63 |
Applications of poly-3,4-alkoxythiophenes
Due to their outstanding physicochemical properties, poly-3,4-alkoxythiophenes have gained considerable attention in multiple applications. Most of these utilize the polymers' conductivity, their inherently large electroactive area, reversible insulating/conducting transition, or efficient color switching. It is essential to highlight that most reported systems use PEDOT-based polymers.144 A representative group of these materials is shown in Table 2, where it can be observed that properties such as conductivity, polymer anodic window, and UV maxima absorption can be manipulated to convenience with the modification of the lateral chains of the thiophenic core. The conductivity of the CP can be enhanced by generating hydroxyl moieties on the polymer surface through post-processing side chain removal, resulting in quasi-metal-like electron transport with conductivities of ca. 1200 S cm−1,68,145 thereby reviving its use. Thus, recently, novel and unexplored poly-3,4-alkoxythiophenes have begun to replace these well-known materials. This section describes the various applications of poly-3,4-dialkoxythiophenes over the past few years.Batteries and supercapacitors
Due to their relatively fast charge/discharge transition, conducting polymers, particularly 3,4-alkoxythiophenes, have become a promising alternative for the design of energy storage devices.146–149 For example, poly-(8-bis(2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)-3,3-dimethyl-3,4-dihydro-2H-thieno[3,4-b][1,4]dioxepine), exhibits a 3D porous network, which significantly enhances the capacitance performance of the material.150 However, problems concerning the ionic transport within the polymer matrix and mechanical and electrical stability hinder the use of such conjugated materials. Therefore, the possible use of conducting polymers as redox-active materials for high-capacity commercial devices remains challenging. Research in this field has focused on developing composites formed by a conducting matrix and alkoxythiophenes.151–154 Recently, the efficient conductivity of poly-3,4-alkoxythiophenes has become a promising alternative as anodic and cathodic binders to enable charge transfer through the electrode.155 For example, dihexyl-substituted poly-ProDOT (PProDOT-Hx2) was used as a cathodic binder, showing a five-fold increase in capacitance at high discharge rates compared to the classic insulating polyvinylidene fluoride.156 Such an enhancement in the performance of the cathode is associated with an open polymeric structure and increased solvation of the PProDOT-Hx2 chains, which improve the ionic conductivity. Furthermore, Thompson et al. demonstrated that using copolymers of PProDOT-Hx2 and oligoether-substituted poly-ProDOT makes it possible to fine-tune the ionic conductivity of the binder (Fig. 3a and b).157 An interesting alternative is using conducting polymers as redox-active materials and conducting electrodes. Such an approach has gained considerable attention in the design of all-organic batteries. For this, the 3,4-alkoxythiophene monomers are functionalized with a redox-active group, i.e., TEMPO or quinone-type moieties.158–163 In addition, by functionalizing the pendant quinone groups with electron-withdrawing substituents, an increase in the formal redox potential of the redox-active moiety has been observed. The synergy between the conductive properties of PEDOT and these active pendant groups makes these polymers a promising alternative for the design of high-voltage cathode materials for lithium-ion batteries.164 With a similar philosophy, a poly-3,4-alkoxythiophene cathode material, functionalized with a hydroquinone group as a redox group and pyridine moieties acting as proton acceptors, was designed (Fig. 3c).165 For this, a trimer formed by EDOT and ProDOT units was used as a conducting material, exhibiting highly reversible redox properties. When using this polymer as an organic cathode coupled to a lithium foil anode, efficient cycling with a high-capacity retention of 99% after 100 cycles and 98% after 200 cycles was achieved (Fig. 3d). Finally, an all-organic redox-polymer-based proton battery was designed.166 In this case, the EDOT-ProDOT-EDOT trimers were used as conducting materials. In contrast, the benzoquinone/hydroquinone (Q/QH2) and naphthoquinone/naphthohydroquinone (NQ/NQH2) systems act as cathodic and anodic redox groups, respectively (Fig. 3e). Such an organic battery exhibits good stability (around 85% after 500 cycles) and fast full-capacity charging (100 seconds) at low working temperatures (−24 °C) (Fig. 3f). Recently, a quinizarin-poly-3,4-alkoxythiophene was used as the cathode in an all-organic proton battery.167 Once again, in combination with NQ/NQH2-poly-3,4-alkoxythiophene, acting as anode, a good cycling stability of the battery (80% after 500 cycles) was obtained. With a similar philosophy, donor-node-acceptor ambipolar 3,4-alkoxythiophenes have been used for the design of wide-voltage and high-stability super capacitors.168,169 For this, EDOT and phthalic anhydride derivatives act as donor and acceptor units, respectively, whereas the linker moiety works as a separator, producing independent redox activity of the electroactive groups. The simultaneous p- or n-doping within the polymer increased the operating voltage of supercapacitors composed by this material. | ||
| Fig. 3 (a) Chemical structure of the copolymer of PProDOT-Hx2 and oligoether-substituted poly-ProDOT with possible use as a conductive cathode binder in lithium-ion batteries. (b) Electronic (left blue axis) and ionic (right red axis) conductivities of the copolymer series. Adapted from ref. 157 with permission from the American Chemical Society, copyright 2022. (c) Schematic illustration of the EDOT-ProDOT-EDOT trimer functionalized with a combined hydroquinone-trap pendant, with a representation of electropolymerization and the redox active group. (d) Voltage profile for galvanostatic charge and discharge for multiple cycles (indicated in the figure) of the proton acceptor poly-3,4-alkoxythiophene acting as the organic cathode. Adapted from ref. 165 with permission from the Royal Chemical Soceity, copyright 2020. (e) Chemical structures of the EDOT-ProDOT-EDOT trimers functionalized with NQ/NQH2 and Q/QH2 acting as anodic and cathodic redox groups, respectively. (f) Voltage profile for the first (cyan) and the 500th cycle (grey) of a battery working at room temperature (left plot) and 24 °C (right plot). Adapted from ref. 166 with permission from John Wiley and Sons, copyright 2020. | ||
Electrochromism
Optical transitions of π-conjugated polymers have gained considerable attention in electrochromic devices due to the possible generation of different optical absorption bands during the charging/discharging process. Such outstanding features of these materials enable the possible fine-tuning of the film coloration by controlling the applied potential. Among the different 3,4-alkoxy thiophenes, PEDOT is an electrochromic material due to its efficient optical transition between dark blue and slightly transparent.170,171 In its discharged state, PEDOT presents an optical absorption between 1.5 eV and 2 eV (dark blue), whereas, in its conducting state, the maximum absorption is at 0.6 eV (transparent in the visible region). However, different approaches have been used to improve the optical transition, extend the range of coloration, and improve the switching time. For example, by fine-tuning the chemical structure of the 3,4-alkoxy moiety, it is possible to improve the electrochromic properties of these materials. Reynolds's group studied the influence of linear and branched side chains on the optoelectronic properties of poly-3,4-alkylenedioxythiophenes.172 Changing the side chain structure can modify the color, switching time, electrochromic contrast, and switching stability. ProDOT-based polymers with oligoether or mono and di-substituted amide functional groups have been designed by a similar approach.172,173 Recently, Cumuru et al., designed multichromic metallopolymers of PEDOT and poly-ProDOT by functionalizing the ethylene group with bipyridine-based Ru(II) complexes.174,175 In this case, the wide range of color is attributed to the optical transition of the 3,4-alkoxythiophenes and the redox transitions of the metallic complexes.An alternative approach is to design donor–acceptor monomers by functionalizing the EDOT moiety in the α-position to combine the electrochromic properties of each conjugated unit. For example, different EDOT-para-phenylene-based polymers present a broad range of coloration, between red and blue, with fast transition times (0.9 s) and high coloration efficiency (305 C−1 cm2) (Fig. 4a and b).176 Liu et al. designed donor–acceptor EDOT-quinoxaline derivatives, which exhibit an optical transition between blue and transparent after electropolymerization.177 Furthermore, with such systems, fast transition times (≈0.5 s), high optical contrast (>50%), and coloration efficiency (427 C−1 cm2) were obtained. Finally, considerable efforts have been made to extend the range of coloration of different π-conjugated monomers by producing copolymers with EDOT. Wang et al. used direct (hetero)arylation polymerization to obtain 2,7- and 3,6- substituted carbazole-EDOT copolymers.178 The obtained materials present short response times (<1 s), high coloration efficiency (>370 C−1 cm2), and a range of coloration between yellow and dark blue. Thiophene-benzothiadiazole-EDOT copolymers, obtained by electropolymerization, exhibit good transition times (<1 s) during multiple double pulse potentiostatic cycling, however, with relatively low coloration efficiency (<100 C−1 cm2).179 A copolymer based on EDOT-benzothiadiazole and ProDOT was designed using a similar approach.180 The obtained polymer exhibited adsorption in almost the entire visible region in its neutral state and exhibited an enhanced optical contrast (>40%) compared with pristine ProDOT. Cyan, magenta and yellow colours were achieved by combining the electrochromic properties of ProDOT-based polymers with benzothiadiazole or N-octylcarbazole.181 The trichromatic electrochromic device reproduces the entire color spectrum of the visible region by fine-tuning the applied potential. Moreover, a low-power flexible electrochromic device was designed by immobilizing a co-polymer formed by a branched 3,4-dialkoxy thiophene and a pyrene-substituted EDOT on multi walled carbon nanotubes.182 This system exhibits short transition times (below 3 seconds) and long cyclability (above 15 k cycles).
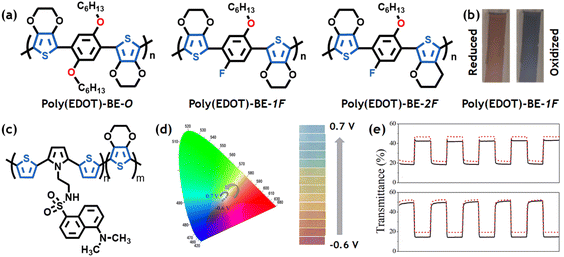 | ||
| Fig. 4 (a) Chemical structures of different EDOT-para-phenylene-based polymers: poly(EDOT-BE-O), poly(EDOT-BE-1F), and poly(EDOT-BE-2F). (b) Optical pictures of the charged and discharged state of poly(EDOT-BE-1F). Adapted from ref. 176 with permission from Elsevier, copyright 2022. (c) Chemical structure of a copolymer formed by an N-dansyl substituted SNS derivative and EDOT. (d) Calculated color trajectory in the CIE 1931 color space (left) and optical images of the rainbow-like multi-electrochromic behavior of the copolymer at different oxidation potentials (indicated in the figure) (right). (e) Transmittance transients at λ = 560 nm (top) and 990 nm (bottom) obtained during redox cycling. Adapted from ref. 190 with permission from Elsevier, copyright 2020. | ||
Recently, coupling the outstanding electrochromic properties of 2,5-dithienyl-N-substituted-pyrrole (SNS) and EDOT has gained considerable attention. Poly-SNS derivatives present well-defined redox processes, low switching times, and a broad range of coloration.183,184 In particular, N-substituted SNS-EDOT copolymers exhibit multi-electrochromic behaviors with fast switching times and good coloration efficiencies.185–188 For example, poly(EDOT-co-1-(3,5-bis(trifluoromethyl)phenyl)– 2,5-di(thiophen-2-yl)-1H-pyrrole) presents an optical contrast of 41% with a coloration efficiency of 258 C−1 cm2 and 1.4 s transition time.189 Ribeiro et al. recently obtained a rainbow-like multi-electrochromic copolymer by coupling an N-dansyl substituted SNS derivative and EDOT (Fig. 4c and d).190 This polymer presents a multi-chromatic contrast at 560 and 990 nm of 25 and 35, respectively, with a coloration efficiency between 110 and 180 C−1 cm2 and fast switching time (<1.0 s) during redox cycling (Fig. 4e). Nicolini et al. demonstrated that the inclusion of EDOT improves the kinetics of electropolymerization of an N-aniline substituted di-SNS derivative, producing a well-ordered cross-linked polymer matrix, which leads to an enhanced electron transfer rate during the doping/dedoping process.191 Furthermore, a multi-electrochromic behavior, from purple to light blue, was observed as a function of the applied potential.
Organic solar cells
Due to their outstanding optoelectronic properties, conducting polymers have become classic materials for the design of photovoltaic devices such as dye-sensitized solar cells (DSSCs), organic solar cells (OSCs), and perovskite solar cells (PSCs). Electropolymerized π-conjugated polymers are commonly used in solar cells as anodic/cathodic interlayers192,193 or hole-/electron-transporting layers.194,195 In such applications, PEDOT doped with PSS is the most used conducting polymer due to its unique electric and optical properties in synergy with its aqueous solution-processability.196–201 However, multiple efforts have been carried out to improve the performance of such devices. For example, it has been demonstrated that electrochemical polymerization allows fine-tuning of the thickness and morphology of the film, which impacts the performance of photovoltaic devices.202,203 Alternatively, a synergetic effect between the alkoxy thiophene's chemical structure and these systems' performance has been extensively studied.204–207 For example, poly-spirobipropylenedioxythiophene (poly-spiroBiProDOT) has been used as a counter electrode as an alternative of electrocatalytic platinum in solar cells.208 Due to its ordered thiophenic structure, poly-spiroBiProDOT exhibits better charge transfer than PEDOT, which increases the photocurrent density and as a consequence, the cell efficiency. Reynolds et al. studied the impact of the placement, design and branching point of side chains present in dialkoxy-functionalized thiophenes on the photostability of conducting polymers.209 An enhanced photostability was obtained for non-branched dialoxy-thiophenes in comparison with branched and cross-linked alkyl substituents.Electropolymerized PEDOT functionalized with C60 fullerene has been used as a selective contact and transparent electron-conducting layer in perovskite solar cells. The fullerene moiety acts as a strong electron acceptor, translating into a four-fold improved energy conversion efficiency compared to the device with pristine PEDOT.210 Kuway et al. used a core of alkoxy-substituted thiophene derivatives, connected to fluorinated 1,1-dicyanomethylene-3-indanone (DFIC) terminals for the design of organic solar cells based on near-infrared (NIR) (Fig. 5a).211 In particular, the presence of a non-cyclic alkoxy thiophene core led to a blue-shifted absorption and an elevated LUMO level; this resulted in a good conversion efficiency (9.83%), with a low energy loss of 0.47 eV, which is relatively low in comparison with that of alternative NIR based organic solar cells (Fig. 5b). In addition to this approach, the study of the nanostructure of the electrode surface impacting the performance of photovoltaic devices has been conducted. For example, organic photovoltaic devices based on PEDOT:PSS deposited on copper nanowires exhibit an outstanding power conversion efficiency of 17.6%.212 With a similar philosophy, TiO2 nanorod arrays (NRAs), modified electrochemically with PEDOT or PProDOT, present an efficient photocurrent performance (Fig. 5c and d).213 The three-dimensional porous network structure of the TiO2 nanorod arrays increases the contact surface during electropolymerization, which leads to a larger p–n heterojunction. Moreover, by fine-tuning the polymeric nanofibril geometry it is possible to enhance the efficiency of organic solar cells.214,215 The incorporation of 3,4-alkoxythiophene with different alkyl side chain lengths on the polymeric donor layer regulates the lamellar stacking, producing fibril structures with different diameters (from 12 nm to 28 nm). The formation of such structures promotes fibrillary charge transport which is reflected in enhanced power conversion efficiencies (above 18%).
 | ||
| Fig. 5 (a) Chemical structures of alkoxy substituted thiophene derivatives connected to fluorinated 1,1-dicyanomethylene-3-indanone groups: C6OT-4F, C6EDOT-4F, and PDOT-4F. (b) Energy level diagram of PTB7-Th and its comparison with the corresponding electron ring acceptors. Adapted from ref. 211 with permission from Elsevier, copyright 2022. (c) SEM images of the top view of TiO2 NRAs (left) PEDOT/TiO2-NRAs (center) and PProDOT/TiO2-NRAs (right). (d) I–V curves of TiO2-NRAs, PEDOT/TiO2-NRAs and PProDOT/TiO2-NRAs illuminated by 365 nm UV light. Adapted from ref. 223 with permission from Elsevier, copyright 2021. | ||
Electroanalytical applications
In the field of electroanalysis, electrogenerated CPs are interesting materials due to their large intrinsic contact area, intrinsic porosity, efficient conductivity, and wide electroactive window.94 Furthermore, by fine-tuning the chemical structure of the polymer, it is possible to produce an intrinsic surface enrichment caused by physicochemical interactions between the desired analyte and the heteroatoms within the monomeric structure. The synergy between these ingredients leads to a considerable increase in sensitivity and selectivity compared to classic electrodes. In this context, 3,4-alkoxy thiophenes have been widely used to analyze different organic and inorganic probes efficiently. For example, PEDOT and substituted-PEDOT modified electrodes exhibit relatively good detection limits, in the range between ppb and ppm, for multiple metal ions, e.g., Na+, Hg2+, Zn2+, Pb2+, As3+, Cu2+, Cd2+, Ag+ and Ca2+.216–220 More recently, highly ordered macroporous electrodes made of PXDOT were designed. With their unique design, these electrodes could efficiently quantify Cu2+ in commercial alcoholic spirituous beverages (Fig. 6a).221 The formation of porous structures within CPs improves the ion transport inside the polymer matrix. It increases the contact surface, causing an enhancement of the sensitivity and the response time of the electrodes. In the past decade, the use of 3,4-alkoxythiophenes has also been extended to chiral electroanalysis. Enantioselective polymers were designed by introducing chiral moieties such as hyaluronic acid and anionic collagen as counter ions during electropolymerization or by carrying out the electrochemical deposition in a chiral solid phase, such as hydroxypropyl cellulose (HPC). These materials have been used for the enantioselective discrimination of (R)–(−)– and (S)–(+)-mandelic acid in aqueous solution.222 With a similar philosophy, Dong et al. designed enantioselective electrochemical sensors through two different EDOT derivatives: (2R)–(2,3-dihydrothieno[3,4-b][1,4]dioxin-2-yl)methanol ((R)-EDTM) and (2S)–(2,3-dihydrothieno[3,4-b][1,4]dioxin-2-yl)methanol ((S)-EDTM) for the efficient recognition of D- and L-3,4-dihydroxyphenylalanine (DOPA) (Fig. 6b).223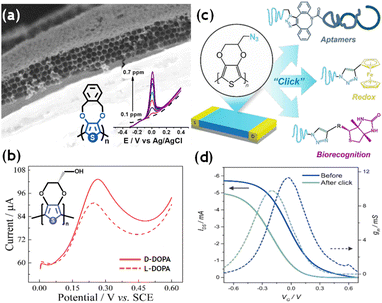 | ||
| Fig. 6 (a) SEM image of the cross-section of a highly ordered macroporous PXDOT electrode. Insets show a square wave anodic stripping voltammetry obtained during the successive addition of Cu2+ in a 10% diluted mezcal solution. Adapted from ref. 221 with permission from the American Chemical Society, copyright 2018. (b) Differential pulse voltammetry curves of (S)-PEDTM modified electrodes in a solution containing D- or L-DOPA. Adapted from ref. 223 with permission from The American Chemical Society, copyright 2017. (c) Chemical structure of the poly-azidomethyl-EDOT (PEDOT-N3) deposited between the drain and source electrodes to generate OECTs, and the schematic representation of the possible click functionalization with redox probes and biorecognition elements. (d) Threshold voltage and maximum transconductance for a PEDOT-N3 OECT before and after the click reaction with ethynyl-ferrocene. Adapted from ref. 239 with permission from The American Chemical Society, copyright 2022. | ||
The various efforts to develop novel and straightforward electroanalytical applications based on poly (3,4-alkoxythiophene) systems have also led to their use in organic electrochemical transistors (OECTs).224–226 These devices consist of an organic channel, a gate, and reference electrodes. Applying a voltage between the gate and channel electrodes (gate voltage, VG) induces a current flow across the organic transistor (drain current, ID) limited by the exchange of ions between the electrolyte and the channel. Thus, in theory, the OECTs transform relatively small VG changes into large ID variations through the π-conjugated polymer located in the channel. The reader is invited to consult other publications for a more detailed description of this technique.227,228 In particular, the organic conductor must present easy ionic exchange, fast and reversible charge/discharge transitions, and efficient charge storage operation (high volumetric capacitance). Among all the different types of conducting polymers, poly (3,4-alkoxythiophenes), particularly PEDOT, fulfills these requirements. Since the efficiency of the OECT is intimately related to the mixed conductivity of the polymer, it is possible to improve the performance of the device by fine-tuning either the structure of the doping counter ion229,230 or the π-conjugated polymer.231–233 For example, it has been demonstrated that by functionalizing EDOT and ProDOT with polar side chains, it is possible to enhance the ion and electron mobility within the polymeric matrix.234,235 OECTs designed with these modified alkoxythiophenes exhibit efficient hole mobility and volumetric capacitance with high stability (up to 500 cycles). With the same philosophy, one can selectively target chemical analytes of interest. Cloutet et al. designed a cation-selective polyelectrolyte, poly-4-styrene sulfonyl (phenyl-18-crown-6)imide.236 The crown ether moiety is a cation scavenger targeting K+ ions, conferring the PEDOT: P(STFSIco-S(18-crown-6)) films with cation selectivity. With a similar approach, copolymers of EDOT and crown ether-modified thiophenes allow the design of ion-selective OECTs, where the electric readout signal is a function of the type of cation (i.e., Na+ and K+) and its concentration.237 Recently, an EDOT-based trimer, modified with a dipicolylamine (DPA) group, was electropolymerized and used to build OECTs for the selective detection and quantification of Zn2+ cations.238 Finally, Knoll et al. proposed a “clickable” OECT, based on azidomethyl-EDOT (Fig. 6c) or ethynyl-EDOT.239,240 The obtained transistors present lower threshold voltages than alternative PEDOT-based devices, with maximum transconductance voltage values close to 0 V (Fig. 6d). More importantly, the azide and ethynyl moieties enable the functionalization of the obtained transistor with alkyne-bearing molecules, such as redox probes, biorecognition elements, and gelatin macrostructures, thereby expanding the capabilities of the OECT to alternative transistor-based biosensors and enhancing biocompatibility for cell interfacing.
Unconventional approaches
Finally, in recent years, attempts have been made to couple the outstanding properties of 3,4-alkoxythiophenes with bipolar electrochemistry (BE). Briefly, BE is an interesting alternative to induce wireless asymmetric polarization along a conducting object, or a bipolar electrode (BPE), present in an electrolyte, but without physical connection to an electrode.241–243 This can be achieved by applying a high enough electric field (ε), which produces a polarization potential difference (ΔV) between the extremities of the BPE (Fig. 7a). In the presence of electroactive species, it is possible to trigger redox reactions at each side of the BPE when the ΔV overcomes the reactions' thermodynamic threshold potential (ΔVmin). This approach has gained considerable attention in applications ranging from electroanalysis to electrosynthesis.244–249 For a more detailed description of this rather unconventional electrochemical concept, it is suggested that the reader consult specialized reviews.241–247 | ||
| Fig. 7 (a) Schematic illustration of the fundamental concept of bipolar electrochemistry (BE) with a representation of the induction of redox reactions at both extremities of the bipolar electrode (BPE). (b) Schematic illustration of the modified IDME BPE, indicating the associated chemical reactions. The yellow and purple parts represent the interdigitated structure and PXDOT, respectively. (c) Normalized resistance plot as a function of the applied electric field for evaluating HER activity of Pt-, Au- and Ni/Au-PXDOT IDME BPEs (blue, red, and green dots, respectively). Adapted from ref. 261 with permission from Elsevier, copyright 2023. | ||
Bipolar electrochemistry has been used as a powerful tool to modify conducting substrates with poly-3,4-alkoxythiophenes asymmetrically. Inagi's group has extensively studied the wireless electropolymerization of PEDOT employing this method. In brief, by applying an appropriate electric field, the oxidation of EDOT and the reduction of a sacrificial compound, i.e. quinone, occur at the anodic and cathodic extremities of the BPE, respectively. Furthermore, fine-tuning of the experimental setup enables the possible electrosynthesis of well-defined macroscopic polymers. For example, PEDOT microfiber networks have been obtained through alternating current bipolar electropolymerization, which involves controlling the frequency, type of solvent, and electrolyte.250,251 Similarly, Pt-PEDOT Janus wires, in-plane PEDOT films, and fibers were obtained.252–254 PEDOT's template-free perpendicular growth was obtained using a bipolar electrochemical setup. In this case, the spatial distribution of the feeder electrodes, coupled with alternating current polymerization, enables the formation of polymeric fiber arrays.255 This approach enables the generation of polymeric films with anisotropic electrical behaviour due to the formation of conductivity and composition gradients along the films.254,256 Förster et al. demonstrated the possible region-selective deposition of PEDOT by taking advantage of an array of multiple feeder electrodes with different spatial distributions.257,258 This approach enables the efficient mapping of the electric field distribution across the BPE by altering the coloration or thickness of the deposited conducting polymer.259
Besides, the potential of BE for the asymmetric functionalization of conductive objects has been recently explored. For example, free-standing PEDOT wires have been used as a BPE to design light-emitting self-propelled devices.260 In this approach, the electrogenerated chemiluminescence of luminol occurs at the anodic side of the CP wire, whereas at the cathodic side, hydrogen peroxide reduction occurs. The generation of oxygen triggers the self-propulsion of such objects due to the intrinsic breaking of symmetry. Finally, the synergy between the conducting/insulating transition of 3,4-alkoxythiophenes and BE was used to design a wireless method to evaluate the electrocatalytic activity of metallic surfaces towards hydrogen and oxygen evolution reactions.261 In this work, a PXDOT-modified interdigitated microelectrode (IDME) was used as a bipolar electrode, where the insulating/conducting transition of the polymer acts as a variable-resistance switch. In contrast, water splitting takes place on the opposite side of the BPE (Fig. 7b). The electrocatalytic activity of Pt, Au, and Ni was expressed in terms of the half-wave electric field, showing good agreement with the characteristic trend of these metals toward water-splitting reactions obtained using more traditional electrochemical tools, such as cyclic voltammetry (Fig. 7c).
Conclusions
The remarkable properties of poly-(3,4-alkoxythiophenes) compared to other families of conducting polymers have triggered intense research over the last few decades and can be considered a first-choice material for developing new applications. They offer a balance between electrical conductivity, stability, mechanical properties, and tunable chemical properties. The synthesis of the corresponding monomers produces custom-made materials with specific physical and chemical properties. This task can be achieved through diverse synthetic pathways, which are part of molecular and polymer engineering, generating significant molecular diversity to explore the most suitable material for a specific application. Such fine-tuning of these physicochemical features enabled an understanding of the relationship between the structure and response properties, a knowledge development highly appreciated by the materials chemistry community. This control is achieved by selecting adequate polymer properties that are reflected in the macroscopic behavior, such as inter- and intra-chain interactions, band gap, and doping level. Among the emerging and recent technologies, organic batteries and capacitors benefit from their reversible redox capacity, which requires fast kinetics for charge and discharge, as well as electrochemical and chemical stability, to continue progress in this field. Applications in organic electronics primarily benefit from the conductive properties of these materials, which are controlled by the doping level and band gap. As an example of future directions, post-processing the lateral chain to expose hydroxyl groups resulted in an increase in the conductivity of the PEDOT derivative to values close to 700 S cm−1 through a simple chemical process; with an additional thermal step, the conductivity reaches values averaging 1100 S cm−1.145 A key consideration is the potential recyclability-degradability of these materials,262,263 which would facilitate the use of sustainable materials in applications. This review demonstrates that imagination is the only limit in the field of conducting polymers, and many interesting and novel materials based on 3,4-alkoxythiophenes will undoubtedly continue to be developed in the future. Emerging applications such as biochemical sensing,264,265 imaging,266,267 wearable devices268,269 or electrocatalysis,270–272 nowadays use this polymer family. Moreover, the synergetic effect between wireless electrochemistry and the properties of poly-3,4-alkoxytiophenes has paved the way for novel applications in cell stimulation273 and drug delivery.274 Strategies to increase the amount of charge carriers, without suffering from chemical or electrochemical degradation, or an increase in processability, require the exploration of new substituents and strategies to achieve a long-range order of chains, which is forecast to be very promising for future members of the poly-(3,4-alkoxythiophenes).Data availability
No new data were obtained and the contained information comes from available bibliographical references.Author contributions
F. A. B.-P.: investigation, data curation & writing – original draft; M. P. F.-M.: investigation, data curation & writing – original draft; A. K.: review & editing; G. S.: conceptualization, methodology, supervision, validation, writing – reviewing & editing and B. A. F.-U.: conceptualization, funding acquisition, supervision, validation, writing – reviewing & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank M. E. Citlalit Martínez-Soto for the technical support. This research was conducted with the support of the CONAHCYT project A1-S-18230 and the project PAPIIT-DGAPA UNAM IV20222. Support by the ANR project CHIRASENSEO (ANR-23-CE42-0004-01) is also acknowledged.References
- H. Shirakawa, A. McDiarmid and A. Heeger, Chem. Commun., 2003, 4, 1–4 RSC.
- X. Guo and A. Facchetti, Nat. Mater., 2020, 19, 922–928 CrossRef CAS PubMed.
- T. M. Swager, Macromolecules, 2017, 50, 4867–4886 CrossRef CAS.
- J. Heinze, B. A. Frontana-Uribe and S. Ludwigs, Chem. Rev., 2010, 110, 4724–4771 CrossRef CAS PubMed.
- R. Shomura, K. Sugiyasu, T. Yasuda, A. Sato and M. Takeuchi, Macromolecules, 2012, 45, 3759–3771 CrossRef CAS.
- G. Pace, I. Bargigia, Y. Y. Noh, C. Silva and M. Caironi, Nat. Commun., 2019, 10, 5226 CrossRef PubMed.
- G. Salinas and B. A. Frontana-Uribe, Chemelectrochem, 2019, 6, 4105–4117 CrossRef CAS.
- J. G. Ibanez, M. E. Rincon, S. Gutierrez-Granados, M. Chahma, O. A. Jaramillo-Quintero and B. A. Frontana-Uribe, Chem. Rev., 2018, 118, 4731–4816 CrossRef CAS PubMed.
- G. Barbarella, M. Melucci and G. Sotgiu, Adv. Mater., 2005, 17, 1581–1593 CrossRef CAS.
- L. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik and J. R. Reynolds, Adv. Mater., 2000, 12, 481–494 CrossRef CAS.
- L. Groenendaal, G. Zotti, P. H. Aubert, S. M. Waybright and J. R. Reynolds, Adv. Mater., 2003, 15, 855–879 CrossRef CAS.
- Y. Wang, J. Phys.:Conf. Ser., 2009, 152, 012023 CrossRef.
- O. Hinsberg, Ber. Dtsch. Chem. Ges., 1910, 43, 901–906 CrossRef CAS.
- E. W. Fager, J. Am. Chem. Soc., 1945, 67, 2217–2218 CrossRef CAS.
- D. J. Chadwick, J. Chambers, G. D. Meakins and R. L. Snowden, J. Chem. Soc., Perkin Trans., 1972, 1, 2079–2081 RSC.
- V. N. Gogte, L. G. Shah, B. D. Tilak, K. N. Gadekar and M. B. Sahasrabudhe, Tetrahedron, 1967, 23, 2437–2441 CrossRef CAS PubMed.
- M. Coffey, B. R. McKellar, B. A. Reinhardt, T. Nijakowski and W. A. Feld, Synth. Commun., 1996, 26, 2205–2212 CrossRef CAS.
- F. Dallacker and V. Mues, Chem. Ber., 1975, 108, 569–575 CrossRef CAS.
- F. Dallacker and V. Mues, Chem. Ber., 1975, 108, 576–581 CrossRef CAS.
- A. Merz, C. Rehm and J. fur Prakt, J. Praktische Chem., Chemiker-Zeitung, 1996, 338, 672–674 CrossRef CAS.
- M. A. Al-jumaili and S. Woodward, Tetrahedron, 2017, 73, 5847–5852 CrossRef CAS.
- F. Von Kieseritzky, F. Allared, E. Dahlstedt and J. Hellberg, Tetrahedron Lett., 2004, 45, 6049–6050 CrossRef CAS.
- S. Das, P. K. Dutta, S. Panda and S. S. Zade, J. Org. Chem., 2010, 75, 4868–4871 CrossRef CAS PubMed.
- A. R. Agrawal, N. R. Kumar, S. Debnath, S. Das, C. Kumar and S. S. Zade, Org. Lett., 2018, 20, 4728–4731 CrossRef CAS PubMed.
- K. Zong, L. Madrigal and J. R. Reynolds, Chem. Commun., 2002, 2498–2499 RSC.
- D. Caras-Quintero and P. Bauerle, Chem. Commun., 2002, 2690–2691 RSC.
- L. Zuo, F. L. Qing, W. D. Meng, X. Huang, S. Zhang, Q. Wu and J. Fluor, Chem, 2004, 125, 1441–1446 CAS.
- T. Tsunoda, Y. Yamamiya, Y. Kawamura and S. Itô, Tetrahedron Lett., 1995, 36, 2529–2530 CrossRef CAS.
- Z. Xu, J. H. Kang, F. Wang, S. M. Paek, S. J. Hwang, Y. Kim, S. J. Kim, J. H. Choy and J. Yoon, Tetrahedron Lett., 2011, 52, 2823–2825 CrossRef CAS.
- D. Caras-Quintero and P. Bäuerle, Chem. Commun., 2004, 4, 926–927 RSC.
- K. Krishnamoorthy, A. V. Ambade, M. Kanungo, A. Q. Contractor and A. Kumar, J. Mater. Chem., 2001, 11, 2909–2911 RSC.
- T. Darmanin, E. L. Klimareva, I. Schewtschenko, F. Guittard and I. F. Perepichka, Macromolecules, 2019, 52, 8088–8102 CrossRef CAS.
- A. Martinelli, A. Nitti, R. Po and D. Pasini, J. Org. Chem., 2024, 89, 4237–4243 CrossRef CAS PubMed.
- B. D. Reeves, B. C. Thompson, K. A. Abboud, B. E. Smart and J. R. Reynolds, Adv. Mater., 2002, 14, 717–719 CrossRef CAS.
- J. Arias-Pardilla, P. A. Giménez-Gómez, A. De La Peña, J. L. Segura and T. F. Otero, J. Mater. Chem., 2012, 22, 4944–4952 RSC.
- P. Leriche, P. Blanchard, P. Frère, E. Levillain, G. Mabon and J. Roncali, Chem. Commun., 2006, 275–277 RSC.
- D. L. Tarange, N. Nayak and A. Kumar, Org. Process Res. Dev., 2023, 27, 358–366 CrossRef CAS.
- B. A. Frontana-Uribe and J. Heinze, Tetrahedron Lett., 2006, 47, 4635–4640 CrossRef CAS.
- E. V. Ganin, S. S. Basok, A. A. Yavolovskii, M. M. Botoshansky and M. S. Fonari, CrystEngComm, 2011, 13, 674–683 RSC.
- P. A. Cisneros-Pérez, B. A. Frontana-Uribe, D. Martínez-Otero and E. Cuevas-Yáñez, Tetrahedron Lett., 2016, 57, 5089–5093 CrossRef.
- M. P. Krompiec, S. N. Baxter, E. L. Klimareva, D. S. Yufit, D. G. Congrave, T. K. Britten and I. F. Perepichka, J. Mater. Chem. C, 2018, 6, 3743–3756 RSC.
- A. Kumar, D. M. Walsh, M. C. Morvant, F. Piroux, K. A. Abboud and J. R. Reynolds, Chem. Mater., 1998, 10, 896–902 CrossRef CAS.
- J. Zhao, X. Gou, C. Hua and L. Wang, Chin. J. Catal., 2012, 33, 1262–1265 CrossRef CAS.
- P. A. Cisneros-Pérez, D. Martínez-Otero, E. Cuevas-Yánez and B. A. Uribe-Frontana, Synth. Commun., 2014, 44, 222–230 CrossRef.
- J. M. Barker, P. R. Huddleston and M. L. Wood, Synth. Commun., 1975, 5, 59–64 CrossRef CAS.
- S. Sreekumar, S. Xavier, A. Govindan, R. E. Thampikannu, K. Vellayan and B. González, Res. Chem. Intermed., 2020, 46, 4529–4542 CrossRef CAS.
- P. M. Dicarmine, T. B. Schon, T. M. Mccormick, P. P. Klein and D. S. Seferos, J. Phys. Chem. C, 2014, 118, 8295–8307 CrossRef CAS.
- F. D'Amico, C. Papucci, D. Franchi, G. Reginato, M. Calamante, L. Zani, A. Dessi and A. Mordini, ACS Sustainable Chem. Eng., 2022, 10, 3037–3047 CrossRef.
- G. A. Sotzing, J. R. Reynolds and P. J. Steel, Chem. Mater., 1996, 8, 882–889 CrossRef CAS.
- A. Ulman and J. Manassen, J. Chem. Soc. Perkin Trans., 1979, 1, 1066–1069 RSC.
- N. Agarwal, C. H. Hung and M. Ravikanth, Tetrahedron, 2004, 60, 10671–10680 CrossRef CAS.
- A. K. Mohanakrishnan, A. Hucke, M. A. Lyon, M. V. Lakshmikantham and M. P. Cava, Tetrahedron, 1999, 55, 11745–11754 CrossRef CAS.
- K. Wang, T. Zhang, Y. Hu, W. Yang and Y. Shi, Electrochim. Acta, 2014, 130, 46–51 CrossRef CAS.
- I. Stolić, K. Miskovic, A. Magdaleno, A. M. Silber, I. Piantanida, M. Bajic and L. Glavas-Obravac, Bioorg. Med. Chem., 2009, 17, 2544–2554 CrossRef PubMed.
- Y. Lu, Y. P. Wen, B. Y. Lu, X. M. Duan, J. K. Xu, L. Zhang and Y. Huang, Chin. J. Polym. Sci., 2012, 30, 824–836 CrossRef CAS.
- S. Akoudad and J. Roncali, Electrochem. Commun., 2000, 2, 72–76 CrossRef CAS.
- I. F. Perepichka, E. Levillain and J. Roncali, J. Mater. Chem., 2004, 14, 1679–1681 RSC.
- K. Krishnamoorthy, M. Kanungo, A. Q. Contractor and A. Kumar, Synth. Met., 2001, 124, 471–475 CrossRef CAS.
- J. L. Segura, R. Gómez, E. Reinold and P. Bäuerle, Org. Lett., 2005, 7, 2345–2348 CrossRef CAS PubMed.
- L. Dong, B. Lu, X. Duan, J. Xu, D. Hu, K. Zhang, X. Zhu, H. Sun, S. Ming, Z. Wang and S. Zhen, J. Polym. Sci. Part A Polym. Chem., 2015, 53, 2238–2251 CrossRef CAS.
- C. J. Kousseff, F. E. Taifakou, W. G. Neal, M. Palma and C. B. Nielsen, J. Polym. Sci., 2022, 60, 504–516 CrossRef CAS.
- P. Sitarik, S. S. Nagane, S. Chhatre, Y. Wu, Q. Baugh and D. C. Martin, Mater. Adv., 2022, 3, 6037–6049 RSC.
- M. Sassi, L. Mascheroni, R. Ruffo, M. M. Salamone, G. A. Pagani, C. M. Mari, G. D'Orazio, B. La Ferla and L. Beverina, Org. Lett., 2013, 15, 3502–3505 CrossRef CAS PubMed.
- D. Mantione, N. Casado, A. Sanchez-Sanchez, H. Sardon and D. Mecerreyes, J. Polym. Sci. Part A Polym. Chem., 2017, 55, 2721–2724 CrossRef CAS.
- J. Kang, K. Zong and Y. S. Lee, React. Funct. Polym., 2024, 205, 106063 CrossRef CAS.
- N. Nayak, K. Arora, S. Shirke, S. Shriwardhankar and A. Kumar, Eur. Polym. J., 2022, 178, 111436 CrossRef CAS.
- B. T. Ditullio, L. R. Savagian, O. Bardagot, M. De Keersmaecker, A. M. Österholm, N. Banerji and J. R. Reynolds, J. Am. Chem. Soc., 2023, 145, 122–134 CrossRef CAS PubMed.
- J. F. Ponder Jr, S. A. Gregory, A. Atassi, A. K. Menon, A. W. Lang, L. R. Savagian, J. R. Reynolds and S. K. Yee, J. Am. Chem. Soc., 2022, 144, 1351–1360 CrossRef PubMed.
- M. M. Durbin, A. H. Balzer, J. R. Reynolds, E. L. Ratcliff, N. Stingelin and A. M. Österholm, Chem. Mater., 2024, 36, 2634–2641 CrossRef CAS PubMed.
- W. Wu, S. Yu, D. S. Forbes, H. Jiang, M. Ahmed and J. Mei, J. Am. Chem. Soc., 2024, 146, 578–585 CrossRef CAS PubMed.
- S. Cosnier and A. Karyakin, Electropolymerization: Concepts, Materials and Applications, Wiley-VCH, Weinheim, Germany, 2010 Search PubMed.
- K. A. Kurdi, S. A. Gregory, M. P. Gordon, J. F. Ponder Jr, A. Atassi, J. M. Rinehart, A. L. Jones, J. J. Urban, J. R. Reynolds, S. Barlow, S. R. Marder and S. K. Yee, ACS Appl. Mater. Interfaces, 2022, 14, 29039–29051 CrossRef PubMed.
- P. Sakunpongpitiporn, K. Phasuksom, N. Paradee and A. Sirivat, RSC Adv., 2019, 9, 6363–6378 RSC.
- W. Lövenich, Polym. Sci., Ser. C, 2014, 56, 135–143 CrossRef.
- B. Winther-Jensen and K. West, Macromolecules, 2004, 37, 4538–4543 CrossRef CAS.
- C. Jiang, G. Chen and X. Wang, Synth. Met., 2012, 162, 21–22 Search PubMed.
- Y. Zhang and K. S. Suslick, Chem. Mater., 2015, 27, 7559–7563 CrossRef CAS.
- S. Zhang, W. Zhang, G. Zhang, Y. Bai, S. Chen, J. Xu, Z. Yu and K. Sun, Mater. Lett., 2018, 222, 105–108 CrossRef CAS.
- T. Zubair, R. S. Ramos, A. Morales and R. M. Pankow, Polym. Chem., 2025, 16, 1188–1196 RSC.
- A. Kumar, R. Singh, S. P. Gopinathan and A. Kumar, Chem. Commun., 2012, 48, 4905–4907 RSC.
- B. C. Thompson, Y. G. Kim, T. D. McCarley and J. R. Reynolds, J. Am. Chem. Soc., 2006, 128, 12714–12725 CrossRef CAS PubMed.
- N. Gulprasertrat, J. Chapromma, T. Aree and Y. Sritana-Anant, J. Appl. Polym. Sci., 2015, 132, 42233 CrossRef.
- D. Bhardwaj, S. Gupta, A. Mishra, S. Singhal, Shahjad, M. Balkhandia, R. Sharma and A. Patra, J. Polym. Res., 2023, 30, 53 CrossRef CAS.
- W. Lu, J. Kuwabara, M. Kuramochi and T. Kanbara, J. Polym. Sci. Part A Polym. Chem., 2015, 53, 1396–1402 CrossRef CAS.
- J. F. Ponder, B. Schmatz, J. L. Hernandez and J. R. Reynolds, J. Mater. Chem. C, 2018, 6, 1064–1070 RSC.
- C. Beaumont, T. Lemieux, S. Aivali, M. H. Sangachin, A. Gasonoo, T. M. St-Pierre, M. Belanger, S. Beaupre, G. C. Welch and M. Leclerc, ACS Macro Lett., 2024, 13, 1133–1138 CrossRef CAS PubMed.
- J. F. Berbigier, R. M. D'Souza, R. N. Bennett and T. L. Kelly, ACS Appl. Polym. Mater., 2023, 5, 5687–5695 CrossRef.
- J. F. Berbigier and T. L. Kelly, ACS Appl. Polym. Mater., 2024, 6, 8318–8325 CrossRef.
- Y. Choi, Y. Kim, Y. Moon, K. Hwang, J. Hong, Y. Kim and D. Y. Kim, Macromolecules, 2023, 56, 3324–3333 CrossRef CAS.
- X. Zhang, A. G. MacDiarmid and S. K. Manohar, Chem. Commun., 2005, 5328–5330 RSC.
- M. G. Han and S. H. Foulger, Chem. Commun., 2005, 1, 3092–3094 RSC.
- A. G. Sadekar, D. Mohite, S. Mulik, N. Chandrasekaran, C. Sotiriou-Leventis and N. Leventis, J. Mater. Chem., 2012, 22, 100–108 RSC.
- I. Bargigia, L. R. Savagian, A. M. Österholm, J. R. Reynolds and C. Silva, J. Am. Chem. Soc., 2021, 143, 294–308 CrossRef CAS PubMed.
- G. Salinas and B. A. Frontana-Uribe, Electrochem, 2022, 3, 492–506 CrossRef CAS.
- R. Xiao, S. I. Cho, R. Liu and S. B. Lee, J. Am. Chem. Soc., 2007, 129, 4438–4489 Search PubMed.
- C. B. Tran, T. F. Otero, J. Travas-Sejdic, Q. B. Le and R. Kiefer, Synth. Met., 2023, 299, 117466 CrossRef CAS.
- M. Dietrich, J. Heinze, G. Heywang and F. Jonas, J. Electroanal. Chem., 1994, 369, 87–92 CrossRef CAS.
- H. Yamato, K. I. Kai, M. Ohwa, T. Asakura, T. Koshiba and W. Wernet, Synth. Met., 1996, 83, 125–130 CrossRef CAS.
- K. Krishnamoorthy, M. Kanungo, A. Q. Contractor and A. Kumar, Synth. Met., 2001, 124, 471–475 CrossRef CAS.
- K. Krishnamoorthy, M. Kanungo, A. V. Ambade, A. Q. Contractor and A. Kumar, Synth. Met., 2001, 125, 441–444 CrossRef.
- J. L. Segura, R. Gómez, R. Blanco, E. Reinold and P. Bäuerle, Chem. Mater., 2006, 18, 2834–2847 CrossRef CAS.
- F. Mouffouk and S. J. Higgins, Electrochem. Commun., 2006, 8, 15–20 CrossRef CAS.
- L. Vallan, E. Istif, I. J. Gomez, N. Alegret and D. Mantione, Polymers, 2021, 13, 1977 CrossRef CAS PubMed.
- I. F. Perepichka, M. Besbes, E. Levillain, M. Salle and J. Roncali, Chem. Mater., 2002, 14, 449–457 CrossRef CAS.
- D. Mantione, E. Istif, G. Dufil, L. Vallan, D. Parker, C. Brochon, E. Cloutet, G. Haziioannou, M. Berggren, E. Stavrinidou and E. Pavlopoulou, ACS Appl. Electron. Mater., 2020, 2, 4065–4071 CrossRef CAS.
- V. Agrawal, D. Shahjad, D. Bhardwaj, R. Bhargav, G. D. Sharma, R. K. Bhardwaj, A. Patra and S. Chand, Electrochim. Acta, 2016, 192, 52–60 CrossRef CAS.
- I. F. Perepichka, S. Roquet, P. Leriche, J. M. Raimimdo, P. Frère and J. Roncali, Chem. –Eur. J., 2006, 12, 2960–2966 CrossRef CAS PubMed.
- E. Istif, D. Mantione, L. Vallan, G. Haziioannou, C. Brochon, E. Cloutet and E. Pavlopoulou, ACS Appl. Mater. Interfaces, 2020, 12, 8695–8703 CrossRef CAS PubMed.
- F. Piron, P. Leriche, G. Mabon, I. Grosu and J. Roncali, Electrochem. Commun., 2008, 10, 1427–1430 CrossRef CAS.
- N. Hergué and P. Frère, Org. Biomol. Chem., 2007, 5, 3442–3449 RSC.
- A. Berlin, G. Zotti, S. Zecchin, G. Schiavon, M. Cocchi, D. Virgili and C. Sabatini, J. Mater. Chem., 2003, 13, 27–33 RSC.
- A. Berlin, G. Zotti, S. Zecchin, G. Schiavon, B. Vercelli and A. Zanelli, Chem. Mater., 2004, 16, 3667–3676 CrossRef CAS.
- H. Brisset, A. E. Navarro, C. Moustrou, I. F. Perepichka and J. Roncali, Electrochem. Commun., 2004, 6, 249–253 CrossRef CAS.
- N. Hergué, P. Leriche, P. Blanchard, M. Allain, N. Gallego-Planas, P. Frere and J. Roncali, New J. Chem., 2008, 32, 932–936 RSC.
- G. Salinas, J. G. Ibanez, R. Vasquez-Medrano and B. A. Frontana-Uribe, Synth. Met., 2018, 237, 65–72 CrossRef CAS.
- G. Salinas, J. A. Del-Oso, P. J. Espinoza-Montero, J. Heinze and B. A. Frontana-Uribe, Synth. Met., 2018, 245, 135–143 CrossRef CAS.
- T. Nicolini, A. Villarroel Marquez, B. Goudeau, A. Kuhn and G. Salinas, J. Phys. Chem. Lett., 2021, 21, 10422–10428 CrossRef PubMed.
- F. A. Bravo-Plascencia, M. P. Flores-Morales, G. Salinas and B. A. Frontana-Uribe, Electrochim. Acta, 2025, 511, 145375 CrossRef CAS.
- T. Karazehir, B. Sarac, H. D. Gilsing, S. Gumrukcu, J. Eckert and A. S. Sarac, Mol. Syst. Des. Eng., 2021, 6, 214 RSC.
- R. Schweiss, J. F. Lübben, D. Johannsmann and W. Knoll, Electrochim. Acta, 2005, 50, 2849–2856 CrossRef CAS.
- X. Du and Z. Wang, Electrochim. Acta, 2003, 48, 1713–1717 CrossRef CAS.
- M. Fall, L. Assogba, J. J. Aaron and M. M. Dieng, Synth. Met., 2001, 123, 365–372 CrossRef CAS.
- S. Akoudad and J. Roncali, Electrochem. Commun., 2000, 2, 72–76 CrossRef CAS.
- H. Sun, L. Zhang, L. Dong, X. Zhu, S. Ming, Y. Zhang, H. Xing, X. Duan and J. Xu, Synth. Met., 2016, 211, 147–154 CrossRef CAS.
- H. Goto and K. Akagi, Chem. Mater., 2006, 18, 255–262 CrossRef CAS.
- S. Matsushita, B. Yan, S. Yamamoto, Y. S. Jeong and K. Akagi, Angew. Chem., Int. Ed., 2014, 53, 1659–1663 CrossRef CAS PubMed.
- K. P. Prathish, R. C. Carvalho and C. M. A. Brett, Electrochem. Commun., 2014, 44, 8–11 CrossRef CAS.
- K. P. Prathish, R. C. Carvalho and C. M. A. Brett, Electrochim. Acta, 2016, 187, 704–713 CrossRef CAS.
- M. Besbes, G. Trippe, E. Leviallain, M. Mazari, F. LeDerf, I. F. Perepichka, A. Derdour, A. Gorgues, M. Salle and J. Roncali, Adv. Mater., 2001, 13, 1249–1252 CrossRef CAS.
- B. Wei, L. Ouyang, J. Liu and D. C. Martin, J. Mater. Chem. B, 2015, 3, 5028–5034 RSC.
- A. E. Navarro, F. Fages, C. Moustrou, H. Brisset, N. Spinelli, C. Chaix and B. Mandrand, Tetrahedron, 2005, 61, 3947–3952 CrossRef CAS.
- H. B. Bu, G. Gotz, E. Reinold, A. Vogt, R. Azumi, J. L. Segura and P. Bauerle, Chem. Commun., 2012, 48, 2677–2679 RSC.
- J. Roncali, Chem. Rev., 1997, 97, 173–206 CrossRef CAS PubMed.
- J. Cameron, A. L. Kanibolotsky and P. J. Skabara, Adv. Mater., 2024, 36, 2302259 CrossRef CAS PubMed.
- H. Huang, L. Yang, A. Facchetti and T. J. Marks, Chem. Rev., 2017, 117, 10291–10318 CrossRef CAS PubMed.
- A. Giovannitti, D. T. Sbircea, S. Inal, C. B. Nilsen, E. Bandiello, D. A. Hanifi, M. Sessolo, G. M. Malliaras, I. McCulloch and J. Rivnay, Proc. Natl. Acad. Sci. U. S. A, 2016, 113, 12017–12022 CrossRef CAS PubMed.
- M. Casalegno, A. Famulari and S. V. Meille, Macromolecules, 2022, 55, 2398–2412 CrossRef CAS.
- J. M. Rinehart, Z. Xu, Z. Wang, A. M. Österholm, L. Q. Flagg, L. J. Richter, C. R. Snyder, Y. Diao and J. R. Reynolds, Chem. Mater., 2025, 37(13), 4593–4606 CrossRef PubMed.
- M. A. Ochieng, J. F. Ponder Jr and J. R. Reynolds, Polym. Chem., 2020, 11, 2173–2181 RSC.
- K. Namsheer and C. S. Rout, RSC Adv., 2021, 11, 5659–5697 RSC.
- I. E. Jacobs, Y. Lin, Y. Huang, X. Ren, D. Simatos, C. Chen, D. The, M. Statz, L. Lai, P. A. Finn, W. G. Neal, G. D'Avino, V. Lemaur, S. Fratini, D. Beljonne, J. Strzalka, C. B. Nielsen, S. Barlow, S. R. Marder, I. McCulloch and H. Sirringhaus, Adv. Mater., 2022, 34, 2102988 CrossRef CAS PubMed.
- Semiconducting Polymers: Chemistry, Physics and Engineering, ed. G. Hadziioannou and P. F. Van Hutten, Wiley-VCH Verlag GmbH, Weinheim, Germany, 1999 Search PubMed.
- R. K. Gupta. Conducting Polymers. Boca Raton (FL), CRC Press, 2022 Search PubMed.
- L. V. Kayser and D. J. Lipomi, Adv. Mater., 2019, 31, 1806133 CrossRef PubMed.
- J. F. Ponder Jr, S. A. Gregory, A. Atassi, A. A. Advincula, J. M. Rinehart, G. Freychet, G. M. Su, S. K. Yee and J. R. Reynolds, Angew. Chem., Int. Ed., 2023, 62, e202211600 CrossRef PubMed.
- A. M. Bryan, L. M. Santino, Y. Lu, S. Acharya and J. M. D'Arcy, Chem. Mater., 2016, 28, 5989–5998 CrossRef CAS.
- X. Wang, J. Zhou and W. Tang, Mater. Horiz., 2021, 8, 2373–2386 RSC.
- D. Yuan, B. li, J. Cheng, Q. Guan, Z. Wang, W. Ni, C. Li, H. Liu and B. Wang, J. Mater. Chem. A, 2016, 4, 11616–11624 RSC.
- B. Li, J. Cheng, Z. Wang, Y. li, W. Ni and B. Wang, J. Power Sources, 2018, 376, 117–124 CrossRef CAS.
- Y. Zhang, H. Zhang, S. Ming, P. Lin, R. Yu and T. Xu, ACS Appl. Mater. Interfaces, 2024, 16, 22571–22579 CrossRef CAS PubMed.
- H. Wang, H. Yang, Y. Diao, Y. Lu, K. Chrulski and J. M. D'Arcy, ACS Nano, 2021, 15, 7799–7810 CrossRef CAS PubMed.
- T. Karazehir, B. Sarac, H. D. Gilsing, J. Eckert and A. S. Sarac, J. Electrochem. Soc., 2020, 167, 070543 CrossRef.
- Y. Liu, T. Abdiryim, R. Jamal, X. Liu, N. Fan, M. Niyaz and Y. Zhang, Appl. Surf. Sci., 2023, 608, 154989 CrossRef CAS.
- I. Sahalianov, M. G. Say, O. S. Abdullaeva, F. Ahmed, E. Glowacki, I. Engquist, M. Berggren and I. Zozoulenko, ACS Appl. Energy Mater., 2021, 4, 8629–8640 CrossRef CAS.
- C. Strietzel, K. Oka, M. Strømme, R. Emanuelsson and M. Sjödin, ACS Appl. Mater. Interfaces, 2021, 13, 5349–5356 CrossRef CAS PubMed.
- P. Das, B. Zayat, Q. Wei, C. Z. Salamat, I. B. Magdau, R. Elizalde-Segovia, D. Rawlings, D. Lee, G. Pace, A. Irshad, L. Ye, A. Schmitt, R. A. Segalman, T. F. Miller, S. H. Tolbert, B. S. Dunn, S. R. Narayan and B. C. Thompson, Chem. Mater., 2020, 32, 9176–9189 CrossRef CAS.
- P. Das, R. Elizalde-Segovia, B. Zayat, C. Z. Salamat, G. Pace, K. Zhai, R. C. Vincent, B. S. Dunn, R. A. Selgalman, S. H. Tolbert, S. R. Narayan and B. C. Thompson, Chem. Mater., 2022, 34, 2672–2686 CrossRef CAS.
- K. Oka, R. Löfgren, R. Emanuelsson, H. Nishide, K. Oyaizu, M. Strømme and M. Sjödin, Chemelectrochem, 2020, 7, 3336–3340 CrossRef CAS.
- K. Oka, C. Strietzel, R. Emanuelsson, H. Nishide, K. Oyaizu, M. Strømme and M. Sjödin, ChemSusChem, 2020, 13, 2280–2285 CrossRef CAS PubMed.
- N. Casado, G. Hernandez, A. Veloso, S. Devaraj, D. Mecerreyes and M. Armand, ACS Macro Lett., 2016, 5, 59–64 CrossRef CAS PubMed.
- P. O. Schwartz, M. Pejic, M. Wachtler and P. Bäuerle, Synth. Met., 2018, 243, 51–57 CrossRef CAS.
- H. Wang, R. Emanuelsson, H. Liu, F. Mamedov, M. Strømme and M. Sjödin, Electrochim. Acta, 2021, 391, 138901 CrossRef CAS.
- K. Oka, C. Strietzel, R. Emanuelsson, H. Nishide, K. Oyaizu, M. Strømme and M. Sjödin, Electrochem. Commun., 2019, 105, 106489 CrossRef CAS.
- H. Wang, R. Emanuelsson, H. Liu, K. Edström, F. Mamedov, M. Strømme and M. Sjödin, ACS Appl. Energy Mater., 2019, 2, 7162–7170 CrossRef CAS.
- L. Åkerlund, R. Emanuelsson, G. Hernandez, M. Strømme and M. Sjödin, J. Mater. Chem. A, 2020, 8, 12114–12123 RSC.
- C. Strietzel, M. Sterby, H. Huang, M. Strømme, R. Emanuelsson and M. Sjödin, Angew. Chem., Int. Ed., 2020, 59, 9631–9638 CrossRef CAS PubMed.
- H. Huang, R. Emanuelsson, C. Karlsson, P. Jannasch, M. Strømme and M. Sjödin, ACS Appl. Mater. Interfaces, 2021, 13, 19099–19108 CrossRef PubMed.
- W. Hou, D. Tang, B. han, Y. Chen, C. Wang, Y. Liu, W. Chi, J. Chen, Y. Zhu, M. Ouyang, J. Liu and C. Zhang, Chem. Eng. J., 2024, 497, 154901 CrossRef CAS.
- J. Liu, W. Hou, D. Tang, Y. Gao, S. Yao, L. Jiang, Z. Zhang, M. Ouyang and C. Zhang, ACS Sustainable Chem. Eng., 2022, 10, 15978–15986 CrossRef CAS.
- S. Sinha, R. Daniels, O. Yassin, M. Baczkowski, M. Tefferi, A. Deshmukh, Y. Cao and G. Sotzing, Adv. Meter. Technol., 2022, 7, 2100548 CrossRef CAS.
- X. Xie, J. Yu, Z. Li, Z. Wu and S. Chen, New J. Chem., 2022, 46, 21167 RSC.
- A. A. Advincula, A. L. Jones, K. J. Thorley, A. M. Österholm, J. F. Ponder Jr and J. R. Reynolds, Chem. Mater., 2022, 34, 4633–4645 CrossRef CAS.
- K. Perera, Z. Yi, L. You, Z. Ke and J. Mei, Polym. Chem., 2020, 11, 508 RSC.
- N. Guven, H. Sultanova, B. Ozer, B. Yucel and P. Camurlu, Electrochim. Acta, 2020, 329, 135134 CrossRef CAS.
- N. Guven, B. Yucel, H. Sultanova and P. Camurlu, Dyes Pigm., 2022, 205, 110526 CrossRef CAS.
- S. Ming, S. Zhen, H. Zhang, X. Han, Y. Zhang, J. Xu and J. Zhao, Eur. Polym. J., 2022, 163, 110938 CrossRef CAS.
- H. Chen, W. Wang, J. Zhu, J. Han and J. Liu, Polymer, 2022, 240, 124485 CrossRef CAS.
- H. Chen, K. Lin, H. Liang, J. Tan, D. Zhou, X. Zhang, F. Liu and Y. Wang, Synth. Met., 2022, 291, 117200 CrossRef CAS.
- S. Sanchita, P. Yadav, S. Naqvi, S. Gupta and A. Patra, ACS Omega, 2019, 4, 3484–3492 CrossRef.
- E. G. C. Ergun and B. B. Carbas, Polymer, 2022, 256, 125250 CrossRef.
- C. Ernest, S. Fagour, X. Sallenave, P. H. Aubert and F. Vidal, Adv. Mater. Technol., 2024, 9, 2301654 CrossRef CAS.
- R. R. Ferreira, D. Mosca, T. Moreira, V. C. Wakchaure, G. Romano, A. Stopin, C. Pinheiro, A. M. T. Luci, L. M. A. Perdigao, G. Constantini, H. Amenitsch, C. A. T. Laia, A. J. Parola, L. Maggini and D. Bonifazi, ACS Appl. Eng. Mater., 2024, 2, 2640–2650 CrossRef CAS PubMed.
- G. Salinas, A. A. Villegas-Barron, J. Tadeo-Leon and B. A. Frontana-Uribe, Electrochim. Acta, 2023, 439, 141673 CrossRef CAS.
- B. B. Carbas and E. G. C. Ergun, Eur. Polym. J., 2022, 175, 111363 CrossRef.
- P. Camurlu, C. Gültekin and Z. Bicil, Electrochim. Acta, 2012, 61, 50–56 CrossRef CAS.
- T. Soganci, H. C. Soyleyici and M. Ak, Phys. Chem. Chem. Phys., 2016, 18, 14401–14407 RSC.
- T. Soganci, S. Soyleyici, H. C. Soyleyici and M. Ak, J. Electrochem. Soc., 2017, 164, H11–H20 CrossRef CAS.
- E. G. C. Ergun and B. B. Carbas, Mater. Today Commun., 2022, 32, 103888 CrossRef.
- E. Tutuncu, M. I. Ozkut, B. Balci, H. Berk and A. Cihaner, Eur. Polym. J., 2019, 110, 233–239 CrossRef CAS.
- J. L. Neto, L. P. A. da Silva, J. B. da Silva, A. J. C. da Silva, D. J. P. Lima and A. S. Ribeiro, Synth. Met., 2020, 269, 116545 CrossRef CAS.
- T. Nicolini, B. A. Frontana-Uribe, A. Kuhn and G. Salinas, Chemelectrochem, 2023, 10, e202300346 CrossRef CAS.
- D. M. Montoya, E. Perez-Gutierrez, O. Barbosa-Garcia, W. Bernal, J. L. Maldonado, M. J. Percino, M. A. Meneses and M. Ceron, Sol. Energy, 2020, 195, 610–617 CrossRef CAS.
- E. Marchini, M. Orlandi, N. Bazzanella, R. Boaretto, V. Cristino, A. Miotello, S. Caramori and S. Carli, ACS Omega, 2022, 7, 29181–29194 CrossRef CAS PubMed.
- C. Anrango-Camacho, K. Pavon-Ipiales, B. A. Frontana-Uribe and A. Palma-Cando, Nanomaterials, 2022, 12, 443 CrossRef CAS PubMed.
- J. Y. Shao and Y. W. Zhong, ACS Sustainable Chem. Eng., 2022, 10, 13555–13567 CrossRef CAS.
- Y. Jiang, T. Liu and Y. Zhou, Adv. Funct. Mater., 2020, 30, 2006213 CrossRef CAS.
- M. Zhu, K. Meng, C. Xu, J. Zhang and G. Ni, Org. Electron., 2020, 78, 105585 CrossRef CAS.
- E. Marchini, S. Caramori, C. A. Bignozzi and S. Carli, Appl. Sci., 2021, 11, 3795 CrossRef CAS.
- R. Bhargav, D. Bhardwaj, Shahjad, A. Patra and S. Chand, ChemistrySelect, 2016, 1, 1347–1352 CrossRef CAS.
- S. Sandrez, Z. Molenda, C. Guyot, O. Renault, J. P. Barnes, L. Hirsch, T. Maindron and G. Wantz, Adv. Electron. Mater., 2021, 7, 2100394 CrossRef CAS.
- A. Gasonoo, C. Beaumont, A. Hoff, C. Xu, P. Egberts, M. Pahlevani, M. Leclerc and G. C. Welch, Chem. Mater., 2023, 35, 9102–9110 CrossRef CAS.
- J. A. Del-Oso, B. A. Frontana-Uribe, J. L. Maldonado, M. Rivera, M. Tapia-Tapia and G. Roa-Morales, J. Solid State Electrochem., 2018, 22, 2025–2037 CrossRef CAS.
- E. Nasybulin, S. Wei, M. Cox, I. Kymissis and K. Levon, J. Phys. Chem. C, 2011, 115, 4307–4314 CrossRef CAS.
- S. Lacher, N. Obata, S. C. Luo, Y. Matsuo, B. Zhu, H. H. Yu and E. Nakamura, ACS Appl. Mater. Interfaces, 2012, 4, 3396–3404 CrossRef CAS PubMed.
- J. Marques dos Santos, M. Neophytou, A. Wiles, C. T. Howells, R. S. Ashraf, I. McCulloch and G. Cooke, Dyes Pigm., 2021, 188, 109152 CrossRef CAS.
- A. Mishra, S. Gupta and A. Patra, J. Polym. Sci., 2022, 60, 975–984 CrossRef CAS.
- Q. Mu, L. Feng, Z. Li, K. Fan, Q. Li, Z. Wei, Y. Cheng and B. Xu, Sol. RRL, 2024, 8, 2400486 CrossRef CAS.
- M. C. Sil, H. D. Chang, J. J. Jhan and C. M. Chen, J. Mater. Chem. C, 2021, 9, 12094 RSC.
- D. E. Shen, A. W. lang, G. S. Collider, A. M. Österholm, E. M. Smith, A. L. Tomlinson and J. R. Reynolds, Chem. Mater., 2022, 34, 1041–1051 CrossRef CAS.
- M. B. Suarez, C. Aranda, L. Macor, J. Durantini, D. A. Heredia, E. N. Durantini, L. Otero, A. Guerrero and M. Gervaldo, Electrochim. Acta, 2018, 292, 697–706 CrossRef CAS.
- D. Luo, Y. Zhang, L. Li, C. Shan, Q. Liu, Z. Wang, W. C. H. Choy and A. K. K. Kyaw, Mater. Today Energy, 2022, 24, 100938 CrossRef CAS.
- M. A. Saeed, S. H. Kim, K. Baek, J. K. Hyun, S. Y. Lee and J. W. Shim, Appl. Surf. Sci., 2021, 567, 150852 CrossRef.
- Y. Che, H. Zhang, T. Abdiryim, R. Jamal, A. Kadir, Z. Helil and H. Lui, Opt. Mater., 2021, 122, 111805 CrossRef CAS.
- C. Liu, Y. Fu, J. Zhou, L. Wang, C. Guo, J. Cheng, W. Sun, C. Chen, J. Zhou, D. Liu, W. Li and T. Wang, Adv. Mater., 2024, 36, 2308608 CrossRef CAS PubMed.
- J. Zhou, L. Wang, C. Liu, C. Guo, C. Chen, Y. Sun, Y. Yang, J. Cheng, Z. Gan, Z. Chen, W. Sun, J. Zhou, W. Xia, D. Liu, W. Li and T. Wang, J. Am. Chem. Soc., 2024, 146, 34998–35006 CrossRef CAS PubMed.
- G. Salinas, A. Villarroel Marquez, M. Idir, S. Shinde, B. A. Frontana-Uribe, M. Raoux, J. Lang, E. Cloutet and A. Kuhn, Chemelectrochem, 2020, 7, 2826–2830 CrossRef CAS.
- M. Vazquez, J. Bobaka, M. Luostarinen, K. Rissanen, A. Lewenstam and A. Ivaska, J. Solid State Electrochem., 2005, 9, 312–319 CrossRef CAS.
- T. Han, Z. Mousavi, U. Mattinen and J. Bobacka, J. Solid State Electrochem., 2020, 24, 2975–2983 CrossRef CAS.
- P. Manisankar, C. Vedhi, G. Selvanathan and P. Arumugam, Microchim. Acta, 2008, 163, 289–295 CrossRef CAS.
- J. M. S. Alshawi, M. Q. Mohammed, H. F. Alesary, H. K. Ismail and S. Barton, ACS Omega, 2022, 7, 20405–20419 CrossRef CAS PubMed.
- G. Salinas, B. A. Frontana-Uribe, S. Reculusa, P. Garrigue and A. Kuhn, Anal. Chem., 2018, 90, 11770–11774 CrossRef CAS PubMed.
- Y. Sulaiman and R. Kataky, Analyst, 2012, 137, 2386–2393 RSC.
- L. Dong, Y. Zhang, X. Duan, X. Zhu, H. Sun and J. Xu, Anal. Chem., 2017, 89, 9695–9702 CrossRef CAS PubMed.
- M. Abarkan, A. Pirog, D. Mafilaza, G. Pathak, G. N'Kaoua, E. Puginier, R. O'Connor, M. Raoux, M. J. Donahue, S. Renaud and J. Lang, Adv. Sci., 2022, 9, 2105211 CrossRef CAS PubMed.
- Y. Liang, A. Offenhäusser, S. Ingebrandt and D. Mayer, Adv. Healthcare Mater., 2021, 10, 2100061 CrossRef CAS PubMed.
- A. A. Yazza, P. Blondeau and F. J. Andrade, ACS Appl. Electron. Mater., 2021, 3, 1886–1895 CrossRef.
- B. D. Paulsen, K. Tybrandt, E. Stavrinidou and J. Rivnay, Nat. Mater., 2020, 19, 13–26 CrossRef CAS PubMed.
- A. Villarroel Marquez, N. McEvoy and A. Pakdel, Molecules, 2020, 25, 5288 CrossRef PubMed.
- G. E. Fenoy, C. von Bilderling, W. Knoll, O. Azzaroni and W. A. Marmisolle, Adv. Electron. Mater., 2021, 7, 2100059 CrossRef CAS.
- S. Carli, M. Di Lauro, M. Bianchi, M. Murgia, A. De Salvo, M. Prato, L. Fadiga and F. Biscarini, ACS Appl. Mater. Interfaces, 2020, 12, 29807–29817 CAS.
- N. Wang, L. Xie, H. Ling, V. Piradi, L. Li, X. Wang, X. Zhu and F. Yan, J. Mater. Chem. C, 2021, 9, 4260 RSC.
- J. Hopkins, K. Fidanovski, L. Travaglini, D. Ta, J. Hook, P. Wagner, K. Wagner, A. Lauto, C. Cazorla, D. Officer and D. A. Mawad, Chem. Mater., 2022, 34, 140–151 CrossRef CAS.
- J. Wu, M. Gu, L. Travaglini, A. Lauto, D. Ta, P. Wagner, K. Wagner, E. Zeglio, A. Savva, D. Officer and D. Mawad, ACS Appl. Mater. Interfaces, 2024, 16, 28969–28979 CrossRef CAS PubMed.
- A. L. Jones, M. De Keersmaecker, L. R. Savagian, B. T. DiTullio, I. Pelse and J. R. Reynolds, Adv. Funct. Mater., 2021, 31, 2102688 CrossRef CAS.
- L. R. Savagian, A. M. Österholm, J. F. Ponder Jr, K. J. Barth, J. Rivnay and J. R. Reynolds, Adv. Mater., 2018, 30, 1804647 CrossRef PubMed.
- A. Villarroel Marquez, G. Salinas, M. Abarkan, M. Idir, C. Brochon, G. Hadziioannou, M. Raoux, A. Kuhn, J. Lang and E. Cloutet, Macromol. Rapid Commun., 2020, 41, 2000134 CrossRef CAS PubMed.
- S. Wustoni, C. Combe, D. Ohayon, M. H. Akhtar, I. McCulloch and S. Inal, Adv. Funct. Mater., 2019, 29, 1904403 CrossRef CAS.
- T. Nicolini, S. Shinde, R. El-Attar, G. Salinas, D. Thuau, M. Abbas, M. Raoux, J. Lang, E. Cloutet and A. Kuhn, Adv. Mater. Interfaces, 2024, 2400127 CrossRef CAS.
- G. E. Fenoy, R. Hasler, F. Quartinello, W. A. Marmisolle, C. Lorenz, O. Azzaroni, P. Bäuerle and W. Knoll, JACS Au, 2022, 2, 2778–2790 CrossRef CAS PubMed.
- G. E. Fenoy, C. Lorenz, M. A. Pasquale, W. A. Marmisolle, P. Bäuerle, O. Azzaroni and W. Knoll, Chem. Mater., 2024, 36, 7207–7221 CrossRef CAS.
- L. Bouffier, D. Zigah, N. Sojic and A. Kuhn, in Encyclopedia of Electrochemistry, ed. A. Bard, Wiley-VCH, Weinheim, Germany, 2022 Search PubMed.
- G. Salinas, S. Arnaboldi, L. Bouffier and A. Kuhn, Chemelectrochem, 2022, 9, e202101234 CrossRef CAS.
- S. E. Fosdick, K. N. Knust, K. Scida and R. M. Crooks, Angew. Chem., Int. Ed., 2013, 52, 2–21 CrossRef PubMed.
- K. L. Rahn and R. K. Anand, Anal. Chem., 2021, 93, 103–123 CrossRef CAS PubMed.
- N. Shida, Y. Zhou and S. Inagi, Acc. Chem. Res., 2019, 52, 2598–2608 CrossRef CAS PubMed.
- E. O. Bortnikov and S. N. Semenov, Curr. Opin. Electrochem., 2022, 35, 101050 CrossRef CAS.
- G. Salinas and A. Kuhn, Curr. Opin. Electrochem., 2025, 49, 101612 CrossRef CAS.
- Z. Qi, S. You and N. Ren, Electrochim. Acta, 2017, 229, 96–101 CrossRef CAS.
- L. Bouffier, D. Zigah, N. Sojic and A. Kuhn, Annu. Rev. Anal. Chem., 2021, 14, 65–86 CrossRef CAS PubMed.
- Y. Koizumi, N. Shida, M. Ohira, H. Nishiyama, I. Tomita and S. Inagi, Nat. Commun., 2016, 7, 10404 CrossRef CAS PubMed.
- M. Ohira, Y. Koizumi, H. Nishiyama, I. Tomita and S. Inagi, Polym. J., 2017, 49, 163–167 CrossRef CAS.
- Y. Koizumi, M. Ohira, T. Watanabe, H. Nishiyama, I. Tomita and S. Inagi, Lagnmuir, 2018, 34, 7598–7603 CrossRef CAS PubMed.
- T. Watanabe, M. Ohira, Y. Koizumi, H. Nishiyama, I. Tomita and S. Inagi, ACS Macro Lett., 2018, 7, 551–555 CrossRef CAS PubMed.
- N. Shida, T. Watanabe, I. Tomita and S. Inagi, Synth. Met., 2020, 266, 116439 CrossRef CAS.
- Y. Zhou, N. Shida, Y. Koizumi, T. Watanabe, H. Nishiyama, I. Tomita and S. Inagi, J. Mater. Chem. C, 2019, 7, 14745 RSC.
- E. Villani, Y. Zhang, Z. Chen, Y. Zhou, M. Konishi, I. Tomita and S. inagi, ACS Appl. Polym. Mater., 2023, 5, 6186–6198 CrossRef CAS.
- E. Villani, N. Shida and S. Inagi, Electrochim. Acta, 2021, 389, 138718 CrossRef CAS.
- A. Brady, M. Wagner and R. J. Forster, Chem. Commun., 2024, 60, 13000 RSC.
- M. Wagner, A. Brady, O. F. Doyle and R. J. Forster, Chemelectrochem, 2025, 12, e202400506 CrossRef CAS.
- A. Brady and R. J. Forster, Anal. Chem., 2025, 97, 410–418 CrossRef CAS PubMed.
- T. Nicolini, Y. Boukarkour, S. Reculusa, N. Sojic, A. Kuhn and G. Salinas, Electrochim. Acta, 2023, 458, 142506 CrossRef CAS.
- H. Jin, K. Kim, S. Park, J. H. Rhee, H. Ahn, D. J. Kim, K. Kim, J. H. Noh, T. S. Kim, E. Y. Shin and H. J. Son, Adv. Funct. Mater., 2023, 33, 2304930 CrossRef CAS.
- N. Nozaki, A. Uva, H. Matsumoto, H. Tran and M. Ashizawa, RSC Appl. Polym., 2024, 2, 163–171 RSC.
- D. Mantione, I. del Agua, A. Sanchez-Sanchez and D. Mecerreyes, Polymers, 2017, 9, 354 CrossRef PubMed.
- A. Emin, A. Ding, S. Ali, M. Chhattal, S. Ali, A. Parkash and Q. Li, Microchem. J., 2024, 207, 111972 CrossRef CAS.
- J. Gao, K. Yu, Q. Luo, M. Deng, X. Hou, W. Wang, X. Zeng, X. Xiong, Y. He, X. Hong and Y. Xiao, J. Med. Chem., 2024, 67, 12428–12438 CrossRef CAS PubMed.
- J. Gao, H. Zhu, H. Cai, Q. Ding, X. Xiong, T. Zhang, B. Chen, W. Wang, X. Ju, J. Huang, X. Zeng, J. S. Kim and X. Hong, Chem. Eng. J., 2025, 503, 158455 CrossRef CAS.
- F. Tian, J. Yu, W. Wang, D. Zhano, J. Cao, Q. Zhano, F. Wang, H. yanhg, Z. Wu, J. Xu and B. Lu, J. Colloid Interface Sci., 2023, 638, 339–348 CrossRef CAS PubMed.
- D. Liu, C. Huyan, Z. Wang, Z. Guo, X. Zhang, H. Torun, D. Mulvihill, B. B. Xu and F. Chen, Mater. Horiz., 2023, 10, 2800–2823 RSC.
- H. Wang, X. liu, C. Ling, Z. Shen and M. Li, React. Funct. Polym., 2025, 214, 106300 CrossRef CAS.
- Y. Zhang, R. Jamal, S. Xie, A. Abdurexit, T. Abdiryim, Y. Zhang, Y. Song and Y. Liu, J. Colloid Interface Sci., 2024, 659, 235–247 CrossRef CAS PubMed.
- B. Nemutlu, E. F. Eker, A. M. Önal, E. G. C. Ergun and C. Tanyeli, ACS Omega, 2025, 10, 29442–29451 CrossRef PubMed.
- A. C. Da Silva, X. Hu, V. H. Paschoal, N. Hagis, A. J. Zajac, M. C. C. Ribeiro and I. R. Minev, Commun. Mater., 2025, 6, 28 CrossRef CAS.
- C. Qin, Z. Yue, X. F. Huang, R. J. Forster, G. G. Wallace and J. Chen, Appl. Mater. Today, 2022, 27, 101481 CrossRef.
- T. A. Skotheim and J. Reynolds ed. Handbook of Conducting Polymers, CRC Press, Boca Raton, USA, 3rd edn, 2007 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |