HPMA nanomedicine: targeting cancer with precision
Sarita Rani
,
Vinay Kumar
,
Sofiya Tarannum
and
Umesh Gupta
 *
*
Nanopolymeric Drug Delivery Lab, Department of Pharmacy, School of Chemical Sciences and Pharmacy, Central University of Rajasthan, NH-8 Ajmer Jaipur Expressway, Bandarsindri, Ajmer, Rajasthan 305817, India. E-mail: umeshgupta175@gmail.com; umeshgupta@curaj.ac.in; Tel: +91-8003274082 Web: https://uguptalab.wixsite.com/nddl
First published on 20th June 2025
Abstract
Polymer nanotherapeutics have gained prominent attention in drug delivery systems. Polymers are widely explored tools to improve the solubility, stability, bioavailability, and prolonged circulation of therapeutic agents. Abraxane, Myocet, DaunoXome, and Doxil are some examples of successful polymeric nanocarriers approved for cancer treatment. Medicinal chemists have access to a vast array of nanomaterials that include polymeric nanoparticles (PNPs), polymeric micelles (PMCs), prodrugs, liposomes, and dendrimers. Polyethylene glycol (PEG), pHPMA (poly-N-2 hydroxypropyl methacrylamide), polyethylene, polystyrene, and other compounds have been extensively used for drug delivery. This review highlights the importance of pHPMA in nanodrug delivery. First, we review the chemical properties, pharmacology, and pharmacokinetics of pHPMA, followed by its synthetic routes of preparation. Second, we discuss pHPMA-based nanocarriers and their therapeutic efficacy in cancer. In addition, we present the clinical status and future prospects of pHPMA in combination with immunotherapy. We aim to provide comprehensive insights into the current pHPMA nanotherapeutics to facilitate future development.
1. Introduction
Cancer is a complex disease that is not only difficult to treat but also difficult to understand, especially its pathogenesis.1 The journey for cancer therapy started with nitrogen mustard, which is associated with severe toxicity. The mortality rate for different types of cancer is an indication to improve cancer therapy with less toxicity or side effects to the patients.2 Current therapeutic options suffer from certain drawbacks, especially off-targeting and severe side effects, premature drug release, lack of specificity, and multidrug resistance, that generate the need for progress and evolution of new strategies for cancer therapy and enhancing the survival rate. Hence, there is an unmet need to improve cancer chemotherapy for better outcomes.Polymeric nanotherapeutics are a promising approach for the effective delivery of anti-cancer drugs.3 Polymers have the potential to circumvent the drawbacks of traditional cancer chemotherapy via different nanocarriers, such as dendrimers, PNPs, PMCs, liposomes, hydrogels, and nanogels.4 They offer many advantages such as (i) optimized formulation by improving the solubility of hydrophobic drugs and stability, (ii) pre-mature drug release and degradation, (iii) enhanced retention time, (iv) selective/targeted drug delivery through ligands, (v) improved pharmacokinetics and bioavailability of drugs, (vi) enhanced cellular internalization, and (vii) improved drug distribution. All these properties promote better in vitro and in vivo delivery of therapeutics.5 Conjugation of ligands or targeting moieties with drugs, known as active targeting, ensures selective drug delivery.6 In passive targeting, owing to the leaky vasculature and defective lymphatic drainage in solid tumors, nanocarriers can preferentially penetrate and accumulate in the tumor. This phenomenon is known as the enhanced permeation retention effect (EPR).7 However, sustained drug release at the tumor site is generally based on stimuli (external or internal). External (light, temperature, and radiation) and internal (pH and redox) stimuli have been extensively studied.8,9 Drug release via enzymatic degradation depends on the type of bond formation (ester, azide, amide, etc.) between the polymer carrier and therapeutics.10
Abraxane (albumin-based PNP of paclitaxel) with improved solubility of paclitaxel for breast cancer, DaunoXome (daunorubicin liposome for Kaposi carcinoma) with less toxicity, and Doxil (liposomal doxorubicin for ovarian and Kaposi carcinoma)11,12 are used for cancer treatment. Additionally, polymeric micelle-based nanotherapeutics, such as NK-911, BIND-014, ThermoDOX and CPX, are in phase II/III clinical trials.13 Polymer therapeutics, such as white blood cell booster, Neulasta® (a modified PEG), and Copaxone® (glatiramer), are among the top ten-selling drugs globally.14 A recent significant and successful example of a nanotherapeutics is lipid formulation to deliver mRNA vaccine for SARS Covid-19.15–17
One class that is drawing much attention and discussed in this review is polymer-based therapeutics. Several PEG (polyethylene glycol) based formulations have been marketed since 2002, which include Neulasta.6 However, numerous hydrophilic polymers have been used for drug delivery in the past. Similarly, pHPMA (N-2-hydroxypropylmethacrylamide) as a hydrophilic polymer, has several advantages over other polymers: (i) enhances the blood circulation time of drugs and (ii) the high molecular weight (Mn) of polymers prevents the rapid clearance of the drug from the body while the free drug is eliminated fast by the kidney.10 The reticular endothelial system (RES) can detect polymeric carriers; however, surface modification by PEG, known as PEGylation, can avoid or delay this detection.18 However, PEGylation faces various challenges (briefly discussed in Section 2).
pHPMA was originally developed as a plasma expander.19,20 It is highly water-soluble, non-immunogenic, non-toxic in nature and has immense potential for drug delivery. The favorable chemical properties and its structure make pHPMA a choice for polymer–drug conjugation chemistry.21–23 Initially, the tetra-peptide GFLG linker was selected for the preparation of pHPMA–drug conjugates.24,25 The HPMA/pHPMA-based copolymeric conjugate and nanoparticulate system have been explored in the past for cancerous and non-cancerous (arthritis, tuberculosis, ophthalmic, dental, and central nervous system) disease therapy. HPMA in the monomer, diblock, triblock, and star-shaped forms has been used for the delivery of gemcitabine, cisplatin, paclitaxel (PCT), doxorubicin (DOX), and epirubicin. To date, some pHPMA–drug conjugates are in various phases of clinical trials, with the hope of achieving some positive results.26 This review provides insight into various pHPMA-based nanodrug delivery systems: PMC, PNPs, dendrimers, prodrugs, and liposomes. The review discusses the need for cancer nanotherapeutics and their working mechanism in the first section. The second section focuses on the pharmacology and pharmacokinetic aspects of pHPMA, as well as the synthetic routes and polymerization reactions. The third section presents recent updates on pHPMA-based nanotherapeutics, including PNPs, PMC, dendrimers, prodrugs, and liposomes, in cancer therapy. Finally, the fourth section focuses on the clinical status of pHPMA therapeutics, including future possibilities.
2. How does the nano drug delivery approach works?
Challenges in conventional drug delivery
Oral and intravenous are the two most popular routes of administration in cancer therapy. However, oral administration may show variable absorption rates in the gastrointestinal tract due to the presence of gastric acid, bile salts, and digestive enzymes, which can negatively affect drug absorption. This may result in low bioavailability and poor drug action. Tumor selective targeting usually fails due to the non-ideal and deprived distribution of the drug to the desired site. Additionally, the drug may be taken up by major organs of the body, such as the liver, spleen, and kidneys, which may cause severe toxicity.27 Hence, the conventional drug delivery system has some limitations and poor outcomes for patients which can be improved by nanocarriers. Considering this, intravenous administration of nanocarriers delivers the drug directly to the circulatory system. A targeting approach for cancer should be smart and intelligent enough to attack only the tumor cells and stop their invasion.Advantages of nanocarriers
Nanotechnology, coupled with polymers, enhances the stability and extends the circulation of drugs in the body. A beautifully said adage, “nano is big”, defines the significance of nanotherapeutics.28 The small size, optimized charge, improved solubility, controlled drug release, enhanced biodistribution, and pharmacokinetics are some key features of NDDS.29 However, the use of natural (chitosan, gelatin, and human serum albumin) and synthetic polymers (PLA (poly-L-lactide), PCL (polycaprolactone), and PLGA (poly-L-lactic acid)) aids the controlled release of drugs. These polymers are widely explored due to their unique properties, such as biodegradability, non-immunogenicity, non-toxicity, and controlled release behavior.30 Folkman and Long unveiled the “controlled release effect” in 1964 for the first time.31 Diffusion-controlled release, solvent-controlled release, degradation-controlled release, and pH-controlled release are the major types of release systems for nanoformulations.32Key factors influencing nano drug delivery
The size, shape, morphology, and surface charge have been shown to impact the interaction of nanocarriers with physiological barriers, as well as their ability to uptake macrophages and blood cells. Nanomaterials with a size range of 1–200 nm are considered suitable for drug delivery, as larger NPs could face clearance by the reticuloendothelial system (RES) or encounter difficulties in tumor penetration.33–35 The entry of NPs into the tumor depends on the pore size of tumor vessels and the type of solid tumor. The tumor environment is complex and composed of different cell types, such as tissue-associated macrophages (TAM), fibroblasts, and neutrophils etc. The ability to enter the tumor endothelium depends on the crossing of NPs through the basement membrane surrounding the tumor. NPs trapped in this membrane are generally transported by TAM to blood vessels. NPs of size less than 30 nm diffuse more easily in the tumor area, while larger-sized NPs are taken up by TAM, and therefore do not penetrate into tumor vessels.36,37 The liver is the largest RES organ, and the likelihood of a nanocarrier entering the liver through Kupffer cells is highly dependent on its size, surface charge, and shape.Regarding the surface charge, positively charged NPs induce protein aggregation while negatively charged NPs undergo RES uptake more easily; however, neutral charge NPs interact less with the RES and stay for a longer time in the circulatory system.38 It is reported in the literature that spherical-shaped NPs are internalized within 30 minutes and remain in blood circulation for a shorter period. Non-spherical NPs showed advantages over spherical NPs, as they were internalized for more than 22 h, which leads to a prolonged circulation time.39,40 The spherical-shaped nanocarriers were rapidly taken up by cancer cells. Drugs administered via the intravenous route enter the blood circulatory system and encounter some common barriers, such as the cell membrane, protein corona, and ECM. The protein corona effect can be reduced by shielding or coating polymers like PEG; however, the accelerated blood clearance (rapidly cleared from the body after subsequent administration that causes unexpected immunogenic response) and the PEG dilemma are the major drawbacks of PEGylation. Protein corona is a layer of proteins that forms on the surface of nanoparticles when they enter the intercellular environment and interact with biological fluids such as blood.41
Advantages and limitations of pHPMA over PEG
pHPMA lacks these shortcomings and has advantages over PEG. It can overcome the protein corona effect, immunogenicity, and PEG dilemma.42–44 The non-immunogenicity of pHPMA makes it an ideal candidate for polymer drug delivery. Similar to PEG, it is not recognized as a foreign particle by the human body, and the lack of anti-pHPMA antibody formation after administration did not result in toxicity. The pHPMA–drug conjugation enhances drug solubility, plasma half-life, and blood circulation. However, the pHPMA backbone is non-biodegradable; however, high molecular weight <40 kDa can be cleared renally. Thus, designing or synthesizing multiblock conjugates can modify the non-biodegradable property of pHPMA.12,13 The small-sized nanocarriers can enter tumor tissues through the vascular leakage of blood components and eventually get entrapped or retained in the interstitial space due to reduced lymphatic drainage. This phenomenon is known as the EPR (enhanced permeation) effect.45,46 Literature studies based on pHPMA–drug conjugates have shown higher penetration and uptake due to the EPR effect for anti-cancer agents.47–50 Briefly, the above-discussed parameters suggest that a deep knowledge of tumor physiology and type of drug carrier needs to be explored or critically evaluated before the administration of drugs via nanocarriers as the size, shape and charge affect drug delivery.3. pHPMA (Poly-N-2-hydroxypropyl methacrylamide): pharmacology and Pharmacokinetics
The pHPMA was first reported by Professor Jindrich Kopecek and his legendary team in the 1970's. The pHPMA renaissance was a result of the interaction between interdisciplinary scientists of Europe in the 1980s. Collaboration between R. Duncan at Keele University, UK, and J. Kopecek at the Institute of Macromolecular Chemistry, Prague, focused on the chemical and structural properties of pHPMA, biocompatibility, and interactions with blood components. N-Substituted methacrylamide was selected as the target because the α-substituted carbon and N-substituted amide bond are required for the hydrolytic stability of the side chains. They synthesized a series of compounds and selected HPMA for further research.pHPMA has been extensively studied as a copolymer for drug delivery in cancerous and non-cancerous diseases.51 The molecular weight (Mn) of the monomer chain of HPMA is 143.18 g mol−1, while the molecular mass of pHPMA usually depends on the size of the polymer chain and the molar mass of the drug. The Mn of pHPMA can be controlled by RAFT (reversible addition–fragmentation chain transfer) polymerization. Despite the large Mn of pHPMA–drug conjugates, their size is typically observed in the 5–100 nm range. The macromolecule assembly of pHPMA accumulates in the tumor area due to the EPR effect.52,53 The pHPMA backbone is non-biodegradable in nature; however, the renal threshold of pHPMA–drug conjugates is commonly observed at 40–45 kDa, which guarantees its renal elimination from the body. The pHPMA can be tuned into nano-assemblies such as PNPs, PMC, dendrimers, prodrugs, and liposomes. The elegant and smart chemistry of pHPMA makes it a choice for the synthesis of polymer–drug conjugates. Its chemical structure is comprised of a secondary alcohol, which is generally slightly less reactive;54 however, it can be reactive during radical polymerization reactions.55 The pHPMA–drug conjugates are synthesized via ATRP (atom transfer radical polymerization), RAFT (reversible addition–fragmentation chain transfer reaction), and NMP (nitroxide-mediated polymerization) reactions. The double-bonded carbon can undergo radical polymerization reactions and form several polymer–drug conjugates. Other than PK1 (HPMA–doxorubicin, FCE28068) and PK2 (HPMA–doxorubicin conjugate bearing additionally galactosamine FCE28069) to target liver cancer, paclitaxel-based (PNU166945), Cisplatin (AP5280 and AP5346) and pHPMA–GFLG–DOX (FCE 28068) and pHPMA–GFLG–DOX-galactosamine (FCE-28069) based polymeric conjugates are in clinical trials56–59 Insightful results have been obtained for pHPMA; the higher the Mn distribution with verifiable renal threshold, the more easily this polymer is removed from the body.60–62 Several chemotherapeutics were rejected due to their higher toxicity profile in humans; pHPMA is devoid of this toxicity. Hence, the toxicity and long-term biological behavior of NPs must be studied in detail through in vivo parameters for the approval of future nanomedicine.29 This copolymer, pHPMA, has several advantages, including being devoid of allergic reactions and the ABC (accelerated blood clearance) phenomenon, which is mainly noticed in PEG. However, it has some limitations and disadvantages, which are explained in the above paragraph and are further detailed in our previously reported review.63,64
4. Method of preparation of HPMA-based polymeric conjugates: synthetic routes, their limitations, and advantages
The synthesis of polymer–drug conjugates is a complex and tedious process. A chemist should carefully optimize the various conditions for a chemical reaction to proceed, such as the choice of solvent, moisture or dry environment, and impure reagents. The presence of byproducts, even after purification, is a major drawback. This is frequently observed in polymerization reactions, such as reversible addition–fragmentation chain transfer (RAFT), atom transfer radical polymerization (ATRP), and free radical polymerization (FRP). Therefore, tuning these conditions and simplifying the complex reaction into a simple one is useful in developing simple chemical reaction approaches.HPMA as a monomer can be conjugated with the targeting ligands or drugs of interest using coupling agents, as mentioned in some of the studies cited here. Researchers have utilized HPMA as a monomer and initiator using a DCC/DMAP coupling reaction. The prepared conjugates were purified by solvent crystallization and dialysis methods.65–71 However, more attempts have been made to synthesize pHPMA–drug conjugates through a polymer chain approach, specifically controlled radical polymerization reactions (CRP). Molecules with unsaturated homo or hetero-double bonds, such as diene, triene, cycloaliphatic, and vinyl compounds, can undergo radical polymerization that proceeds through a reversible chain-breaking process, i.e., chain transfer and chain termination. The multi-functionalities of pHPMA can be controlled by radical polymerization.70,71 In the CRP method, the polymer chain of HPMA assembles in a chain-growth fashion, forming a high Mn polymer by combining the monomers, which enables the tailoring of site-specific and desired targeting functionalities for different applications.72,73 The polymers prepared using these methods can be used for medical and industrial purposes. Before these robust polymerization reactions were invented, medicinal chemists had limited access to the then existing resources. The strict reaction conditions, such as the use of ultrapure chemical reagents and extremely dry conditions, are some limitations of CRP. In 1995, Professor Krzysztof Matyjaszewski from Carnegie Mellon University created history by discovering the first and most robust CRP methods. His contributions to polymer chemistry are renowned and credentialed. He prepared a polymer conjugate of mescaline via a dipeptide spacer and found that this high molecular-weight (HMW) polymeric carrier increased the in vivo residence time of mescaline in mice.74 ATRP, RAFT, and NMP are some CRP methods used for polymerization.75,76 Different approaches, such as RAFT, ATRP, and NMP, are exhaustively used to synthesize biomacromolecule-polymer conjugates (Fig. 1). Here, we focus on three approaches, which are also used for the preparation of pHPMA–drug conjugates and illustrate each one simultaneously.
4.1. NMP (nitroxide mediated polymerization)
NMP is the easiest method for radical polymerization. Solomon and his colleagues discovered the use of nitroxides and alkoxyamines to control the polymerization of monomers, such as styrene and acrylates, and disclosed it in a provisional patent granted in 1984. This thermal method is based on the reversible termination mechanism between the developing radical and the control agents. Alkoxyamines generate the propagating radical and with an increase in temperature, nitroxide can be regenerated by hemolytic cleavage. Therefore, an equilibrium is established between the activated and inactivated species. It is a widely used polymerization method that does not require a catalyst or an exchanger species.77 It is initiated by a two-component pathway based on the number of thermal initiators used, such as AIBN (2,2-azobisisobutyronitrile) and benzoyl peroxide (BPO). After the initiation step, the kinetics of polymerization is determined by the presence of excess nitroxide. The constant radical effect and activation–deactivation equilibrium determine the kinetics of polymerization. Excess free nitroxides shift the activation–deactivation equilibrium toward dormant species and reduce the polymerization rate of the reaction. However, thermal initiators used in NMP face difficulties in determining the efficiency of primary radicals that induce polymerization. This results in poorly reproducible polymerization kinetics and less reactive functional end groups. NMP can be used to construct biomaterials, photo-polymerization, lithium batteries, and organic electronics.784.2. ATRP (atom transfer radical polymerization)
In ATRP, the activation and deactivation of radicals involve an atomic transfer reaction. Transition metal complexes of Fe, Cu, Co, Ni, and Ru function as catalysts. It can be used to polymerize different monomers, such as styrene, acrylonitrile, methacrylate, methacrylic acid, and methacrylamide. The equilibrium constant for ATRP can be adjusted by choosing different initiators, ligands, and transition metals. The higher the equilibrium constant, the faster the rate of polymerization under mild reaction conditions.79 The main advantages of ATRP are (i) commonly available initiators like alkyl halides or compounds with weak halogen–heteroatom bonds, like sulfonyl halides. These initiators easily provide halogens to the polymers and are converted into the required functional groups further; (ii) environment-friendly reaction, (iii) can proceed in an aqueous homogenous system; (iv) economical, as it can occur in the presence of inexpensive solvents. ATRP also has some disadvantages, such as the use of transition metals in higher concentrations and quantities (0.1–1%), which are difficult to remove from the final product. However, this can be avoided by using highly efficient catalysts that can be recovered easily. Hence, ATRP is considered a widely accepted process for the synthesis of polymer-conjugates.4.3. RAFT (reversible-addition fragmentation transfer)
RAFT involves the formation of carbon–carbon bonds, where addition–fragmentation chain transfer agents act by a two-step mechanism. Compounds with C–X bonds are found to be reactive towards radical addition, where X is CH2 (methylene) or S (sulphur). It is used to introduce functionalities to the polymeric conjugates and allow cross-linking. RAFT agents can control the polymerization of monomers. The success of the RAFT method also depends on the structure of RAFT agents: thiocarbonylthio compounds such as dithioesters,73 dithiocarbamates,80,81 trithiocarbonates,82 and xanthates,83 and mediate polymerization via a reversible chain-transfer process. RAFT-polymerized pHPMA copolymers were synthesized in 2005 by Charles L. Mc Cormick84 and later became the most commonly explored approach for the synthesis of pHPMA–drug conjugates.85 These pHPMA–drug conjugation reactions have been reported here.20–24,48,49,51,56,57,615. HPMA/pHPMA: a potential drug carrier in cancer
HPMA is a biologically compatible, safe, linear, and non-immunogenic copolymer. Its functionalities are facile conjugation of drugs, therapeutic agents, proteins, and peptides that undergo degradation in response to various stimuli and are eliminated from the body. It eventually becomes hydrated due to its hydrophilicity, which prevents the deposition of proteins or macromolecules that are found abundantly in circulation.85 The conjugation or encapsulation of hydrophobic drugs with pHPMA increases their water solubility and pharmacokinetics and reduces the toxicity of drugs. Also, the pHPMA–drug conjugates exhibited prolonged circulation in the blood because of high Mn and were localized within the tumor through the EPR effect.86 Therefore, it is necessary that high molecular weight pHPMA must possess biologically degradable linkers within its backbone and can undergo elimination. This will allow passive targeting and easy removal of the carrier system.85Here, we discuss pHPMA-based nanotherapeutics, PMC, PNPs, dendrimers, prodrugs, and liposomes, along with their remarkable results and clinical translation. These nano-approaches have been selected here due to their advantages and potent therapeutic properties (Fig. 2). The pHPMA and pHPMA–drug conjugate-based nanomedicines are also detailed here.87–90 The PMC, PNPs, dendrimers, prodrug, and liposome approaches are discussed below.
 | ||
| Fig. 2 Main advantages of different nanosystems, PMCs, PNPs, dendrimers and prodrugs, in drug delivery using pHPMA as a copolymer. | ||
(a) Polymeric micelles (PMC)
Various chemotherapeutic agents are utilized in cancer chemotherapy. Unfortunately, their off-target effects, premature release, lack of specificity, multidrug resistance, and undesired therapeutic effectiveness are some drawbacks that necessitate solutions for a more effective delivery approach.90,91 One of the most promising solutions to tackle these critical issues is through surface-decorated polymeric micelle assemblies,91 composed of a hydrophilic corona and hydrophobic core92 to encapsulate both water-soluble and -insoluble drugs. The PMC shell prevents the enzymatic degradation of the therapeutic moiety in adverse environments.93 Therefore, PMCs are frequently explored in the design of nanoformulations with distinctive physicochemical features, easily modifiable functionalities, nanometric size distribution, and morphology.94 PMCs have gained huge attention in pharmaceutical sciences as drug carriers and for gene delivery.93,95Wei et al. developed a DOX-encapsulated PEG–pHPMA–lipoic acid-based PMC using PEG (Mn 5000 kg mol−1) and pHPMA (Mn 1.7, 4.1, and 5 kg mol−1) via the RAFT reaction. The PMC with superior DOX loading showed a sustained drug release profile. The in vitro cytotoxicity resulted in enhanced antitumor performance, with lower IC50 values, especially on HeLa and HepG2 cells. These reductive-assisted cross-linked micelles demonstrated better biocompatibility, excellent drug loading, and enhanced extracellular stability. Hence, the PMC of DOX acts as a promising platform for tumor-specific anticancer drugs.96
Filippov et al. reported the kinetics of pHPMA–DOX conjugates attached to different keto groups. The prepared nano-assembly facilitated the breakage of the pH-sensitive hydrazone bond to release DOX in the acidic microenvironment. Time-dependent SAXS/SANS measurements of PMC were performed to investigate pH variations that affect drug release and particle size. The size and morphology increase with the increase in cholesterol percentage; however, they are distributed well over the micellar surface.97
Shi et al. developed amphiphilic thermo-sensitive PMC of paclitaxel (PTX) and docetaxel (DTX) and then studied the effect of π–π stacking on stability and loading capacity. Aromatic groups (benzoyl chloride and 2-naphthoyl chloride) were introduced to prepare block copolymers of mPEG-Bz/Nt-pHPMA using PEG as initiator. PTX and DTX were loaded in different ratios using polymeric blocks and fabricated into PMC nano-assemblies. CMC (critical micelle concentration) and CMT (critical micelle temperature) of micelles decreased as the feed ratio of aromatic groups (Bz and Nt) increased. About 50% of PCT was released from PMC with aromatic groups (50 nm size) for up to 10 days, whereas non-aromatic micelles exhibited 90% drug release. Additionally, the PTX-loaded PMC showed increased stability and drug loading due to the π–π stacking of aromatic groups present in block copolymers, in addition to improved cytotoxicity compared to empty micelles (Table 1).98
| Nanoplatform | Drug | Cell lines | Size (nm) | PDI | Therapeutic effects |
|---|---|---|---|---|---|
| PMC | DOX | HEK-293 and A549112 | |||
| HepG2 and A54997 | — | — | |||
| EL4T106 | |||||
| PTX & DTX | B16F1099 | 50 | Enhanced stability | ||
| A549 and HeLa101 | 57–62 | 0.1 | pHPMA based micelles increased solubility of curcumin up to 2 mg mL−1 | ||
| Axitinib & DOX | 104 | 16–20 | Diblock micelles with pH-sensitive hydrazone bond showed potential to overcome multidrug resistance | ||
| Curcumin | Caco-2, OVCAR-3, and Molt-4102 | Improved solubility of curcumin and cellular uptake | |||
| Ritonavir | HeLa106 | pH-Sensitive drug release and inhibited tumor growth | |||
| HeLa108 | 50–100 | Fluorescent dye-conjugated micelles resulted in cellular toxicity on MDR-cancer cells | |||
| Curcumin | Neuro2A, EGI-1, TFK-1, SK-ChA-1, and Mz-ChA-1114 | 40–60 | ≤0.14 | Improved solubility and pharmacokinetics but no significant cytotoxicity in neuroblastoma cells due to low sensitivity of curcumin for Neuro2A cells | |
| PNPs | DOX + DTX | EL4T124 | Sustained drug release due to the formation of a hydrazone bond | ||
| PTX | HeLa125 | ≤100 | Excellent stability and controlled drug release | ||
| DOX | 4T1126 | 100 | Reduced side effects and toxicity of DOX to normal organs | ||
| HepG2, ADR134 | |||||
| GEM | 4T1127 | 100 | 1.05 | Enhanced accumulation of GEM in tumor areas and GFLG, enzyme-sensitive linker offered stability and prolonged circulation | |
| DOX | 4T1130 | 105 | Low toxicity, enzyme and pH sensitive drug release | ||
| Betulinic acid (BA) | HeLa, DLD-1 and HT-29131 | Higher cytotoxicity in cancer cells than free BA, enhanced drug accumulation in HT-29 xenograft model | |||
| DOX | 4T1133 | Increased plasma half-life, prolonged circulation | |||
| AT-1 and Walker-256134 | Uptake affected by MW, higher accumulation in low MW polymer conjugates NPs | ||||
| GA + DOX | HepG2/ADR135 | 18.61 | Increased ROS generation and mitochondria distribution | ||
| BTZ | MCF-7136 | 199.7 ± 1.32 | 0.19 | Biotinylated PNPs (targeted) showed enhanced uptake, improved pharmacokinetics, and cell cytotoxicity than non-biotinylated PNPs (non-targeted) | |
| Dendrimers | DOX | —149 | — | — | Improved plasma circulation and tumor accumulation |
| Pirarubicin | —150 | — | — | Released drug in acidic milieu that improved cytotoxicity of drug | |
| DOX | —151 | — | — | Higher IC50 values than free DOX, and linear polymer–DOX conjugate | |
| MTCP | KB cells and A549152 | — | — | Enhanced photo toxicity action for pHPMA-MTCP than unconjugated MTCP | |
| Prodrugs | PSA-peptide | —156 | — | — | Improved cytotoxicity against prostate cancer cells |
| AMD3465 (P-SS-AMD) | —157 | — | — | Efficient miRNA delivery | |
| GEM | —158 | — | — | More tumor inhibition and no metastasis for prodrugs | |
| DOX | 4T1159 | — | — | Tumor inhibition | |
| GEM + DOX | MCF-7160 | — | — | IC50 value 1.8–1.9 μM | |
| NFX + DOX | 4T1162 | ≤100 | — | Anti-metastasis and tumor inhibition | |
| Liposomes | DOX | —169 | 150–200 | Enhanced circulation time of DOX by 2.5 h | |
| —115 | 100 | Improved in vivo drug efficacy and prolonged circulation in Balb/c mice | |||
| AML-12, HepG2, RAW 264.7, HUVEC169 | Faster drug release at hyperthermic condition | ||||
Zhou et al. developed an amphiphilic conjugate of DOX with β-sitosterol and pHPMA through hydrazone linkage and then prepared micelles that quickly hydrolyzed at acidic pH. These micelles maintain their stability with minimal release of DOX at physiological pH/mouse plasma, and 80% of the drug was released at pH 5 (lyso/endosome). In vivo studies showed higher uptake of DOX in the H22 mouse xenograft model of hepatocarcinoma for cross-linked micelles compared to non-cross-linked micelles without toxicity to major organs, including the liver, kidney, spleen, heart, and brain.99
Najafi et al. explored the native chemical ligation approach to prepare core-crosslinked thermo-sensitive PMC. ABA triblocks (pHPMA–cysteine, pNIPAM–pHPMA–Cys–PEG–pNIPAM–pHPMA–Cys, pHPMA–ETSA, and pNIPAM–HPAM–ETSA–PEG–pNIPAM–HPAM) were synthesized by ATRP polymerization. A flower-shaped thermo-sensitive PMC of 50 nm showed spherical-shaped particles with dark patches of core/shell in cryo-TEM analysis. Cellular uptake studies were performed on A549 and HeLa cells using Alexa fluor 647 and C5-568, which showed significant internalization. However, no significant changes were observed in the cytotoxicity assay for HeLa cells.100
Okonogi and the group have studied the solubility issue of curcumin. Block copolymers of PEG–pHPMA with modified mono, di-lactate, and benzoyl groups were synthesized at different initiator concentrations (PEG–ABCPA). The TEM images of PEG–pHPMA PMC showed a difference in the surface morphology of curcumin-loaded and unloaded micelles (Fig. 3). The PMC of curcumin showed increased solubility up to 2 mg mL−1 due to the π–π stacking effect of aromatic groups with sustained release of curcumin up to 3 weeks. The anti-tumor effect of PMC was tested against three cancer cell lines (CaCo-2, OVCAR-3, and Molt-4), and IC50 was calculated to be 4–8 μg mL−1.68
 | ||
| Fig. 3 TEM images of unloaded PEG–pHPMA–DL micelles (A), CM loaded PEG–pHPMA–DL micelles (B), unloaded PEG–pHPMA–Bz–L micelles (C), CM loaded PEG–pHPMA–Bz–L (D), unloaded PEG–pHPMA–Bz micelles (E) and CM loaded PEG–pHPMA–Bz micelles (F). The small photograph in the right corner of each TEM image shows the visual appearance of each formulation. Reproduced from ref. 102 with permission from Elsevier, copyright [2021]. | ||
Further, Okonogi and group synthesized PEG-b-pHPMA conjugates using different monomers-to-initiators ratios; N-(2-hydroxypropyl)methacrylamide dilactate (pHPMA–DL) (0%), N-(2-benzoyloxypropyl)methacrylamide linked N-(2-hydroxypropyl)methacrylamide monolactate (pHPMA–BZ–ML) (25%), N-(2-benzoyloxypropyl)methacrylamide (pHPMA–BZ) (100%), and their molecular weights were determined using gas permeation chromatography and NMR spectroscopy. Then, curcumin was loaded into the prepared micelles using these synthesized polymer conjugates. The HPMA-based polymeric micelles increased curcumin solubility up to 2 mg mL−1. The stability of curcumin micelles was found to be directly proportional to the substituted aromatic chains. The incorporated aromatic groups ensure stability owing to π–π stacking interactions with curcumin.101,102
Hennink and co-workers performed tumor regression analysis studies using PTX-loaded PMC in two different triple-negative breast cancer models: A431 and MDA MB-231. PTX-loaded PEG–pHPMA–Bz PMC had excellent stability and increased solubility of the drug. This work was further continued by Shi et al. to study the effect of different feed ratios of PTX-loaded micelles on tumors. Micelles showed improved pharmacokinetics and biodistribution with two imaging agents (Cy-7 and Cy-5.5) compared to the marketed formulation of PTX (Taxol) and achieved 2000 times more drug accumulation at the tumor site. Xenograft model studies also suggested that the PTX-loaded micelles did not induce significant toxicity and ensured an excellent anti-tumor action.103
Haung et al. designed a pHPMA-based PMC and co-administered the angiogenesis-preventing drug axitinib (AXI) with DOX. The cross-linked micelle (DA-CM) released AXI and DOX at the tumor site and within lysosomes under various pH conditions. Noticeably, DA-CM showed significantly high localization within the tumor, enhanced cellular internalization, and extreme cytotoxicity against cancer cells. Conclusively, DA-CM prevented angiogenesis and suppressed cancer progression up to 88%.104
Braunova et al. synthesized amphiphilic di-block copolymers of pHPMA and lipophilic poly (propylene oxide) and prepared a PMC of DOX for better inhibition of multidrug resistance than the free drug. The DOX-encapsulated PMC inhibited MDR and caused stimuli-triggered drug release, enhanced pharmacokinetics, and modulated cellular uptake. Results suggested that di-block micelles with a pH-sensitive hydrazone bond demonstrated P-glycoprotein inhibition, improved pharmacokinetics, and increased uptake into cells and showed potential to overcome multidrug resistance.105
Koziolova and co-workers prepared ritonavir-loaded pHPMA micelles with controlled-release properties intended for tumor therapy. The micelles exhibited pH-sensitive drug release under a mild acidic microenvironment mimicking the conditions of cancerous cells. Surprisingly, ritonavir-loaded PMC substantially internalized into the HeLa cells more than free micelles. The anticancer efficacy of dual delivery resulted in the inhibition of tumor growth and proliferation factors, such as the cell nuclear antigen.106
Sponchioni et al. synthesized L18-MDP-loaded biodegradable NPs of PEGylated-pHPMA grafted with poly lactic acid (PLA). These amphiphilic block copolymers self-assemble into nano-micelle systems, which can be stabilized by their own photo cross-linking event. The PMC formulation exhibited carbohydrate-assisted binding and also induced the activation of dendritic cells.107
Machova and co-workers prepared ritonavir (RTV) loaded pHPMA nanoconstructs and studied their biological behavior via a clathrin-mediated pathway. A fluorescent dye was conjugated to RTV for enhanced cellular internalization, particularly in HeLa cells, and evaluated for mitochondrial dysfunction, resulting in reduced ATP production. Thus, the influence of mitochondrial mechanisms can have a significant impact on the ATP-dependent P-gp and cellular toxicity on MDR-assisted cancer cells.108
Bagheri et al. investigated the effects of the length of the copolymer, Mn, the ratio of hydrophilic or hydrophobic segments, and solvents on the stability of PEG–pHPMA–Bz PMC. It was observed that THF (tetrahydrofuran) and DMSO (dimethylsulfoxide) increased the size of micelles during nanoprecipitation. A higher Mn (18.5 KDa) PEG–pHPMA–Bz possessed a smaller size, whereas a lower Mn polymer (2.2 KDa) produced large-sized micelles. Hence, this study noted that the size of PMC could be tuned by the solvent, hydrophilic or hydrophobic content, the ratio of homo polymers, and Mn of copolymers.109
Biancacci et al. evaluated cyanine-7 (Cy-7) labeled core-crosslinked PMC of PEG–pHPMA-lactate for optical imaging, in vitro, and in vivo tumor analysis in mice. PEG–pHPMA-monolactate and PEG–pHPMA-dilactate of Mn 7.5 KDa were synthesized by free radical polymerization with the Cy-7 dye. Micro-computed tomography-fluorescence tomography (μCT-FLT) and 2D fluorescence reflectance imaging showed a remarkable amount of PMC in the kidneys and a low amount in other tissues, suggesting delayed clearance of PMC. A higher amount of drug accumulated in the tumor (65%), liver (64%), and spleen (72%) in the 4T1 TNBC model.110
Bobde et al. developed PEGylated–pHPMA PMC as nanocargos for the transport of DOX in breast cancer cells. The pHPMA conjugated with DOX via a pH-sensitive linker was prepared and characterized, followed by the preparation of PMC. DOX is released from PMC at a faster rate at acidic pH. Cell viability and uptake studies showed promising results for the prepared delivery systems against solid tumors.111
Wang et al. prepared biotin-decorated pHPMA-benzene conjugates by RAFT polymerization and prepared the PMC of PTX. The size of the self-assembled PMC was found to be less than 100 nm and accounted for 10% of the drug loading efficiency. The cell viability of PTX-loaded PMC was evaluated against HEK-293 and A549 cell lines. Non-biotin decorated PMC showed significantly lower internalization of the formulation than biotin-decorated PMC. Hence, the PMC of the biotin-pHPMA backbone is an appropriate nano-carrier for the delivery of paclitaxel.112
Sheybanifard et al. synthesized four polymeric conjugates of PEG–pHPMA-benzene (Bz) with Mn of 3, 5, 10, 17 KDa and then prepared PMC to study the effect of π–π stacking. The stability and in vitro (2D & 3D) tumor penetration of different ratios of loaded PTX (1.5, 3 and 5 mg) PMC were also explored. PMC was found to be stable over 35 days. PMC with a Mn of 17 KDa PMC showed more internalization in HepG2 than PC3. However, smaller-sized Cy3-labeled PMC (3 and 5 KDa Mn) showed a lower intensity fluorescence in the tumor spheroid than the 17 KDa PEG–pHPMA-benzene PMC.113
Similarly, Hennink and co-workers synthesized curcumin-loaded PEG–pHPMA–Bz PMC. Three polymeric conjugates of different Mn (5.2, 10, 17.1 KDa) were prepared, and the release pattern of curcumin was studied in plasma and PBS pH at 37 °C. PMC of high Mn PEG–pHPMA-benzene (17.1 KDa) released 22% of curcumin, while the low Mn conjugate PMC released 49% in 24 h. In vitro studies were performed on different cancer cell lines (Neuro2A, EGI-1, TFK-1, SK-ChA-1, and Mz- ChA-1), and the low anti-tumor effects could be due to the insensitivity of curcumin. The curcumin-loaded mPEG5kDa-b-p(HPMA–Bz) 17.1 kDa micelles resulted in improved solubility and pharmacokinetics; however, no significant cytotoxicity was observed in neuroblastoma cells, which may be due to the low sensitivity of curcumin for Neuro2A cells.114
Hennink et al. recently published a report where a pHPMA–Bz-based PMC was prepared using the solvent extraction method, and the impact on size was investigated.115
Stenzel et al. synthesized ellipticine-loaded PMCs of pHPMA–MMA (methyl methacrylate) of size 46 nm, PDI 0.33, and investigated its anti-cancer activity in U87MG cells. The 2D and 3D spheroid model studies showed increased uptake, which was directly translated to the toxicity profile of the drug.116
In the past decade, researchers have explored the π–π stacking effect to improve the stability of pHPMA–PEG-based PMC via the attachment of Bz/Nt (aromatic groups). Various advanced techniques, such as AF4 particle analyzer and dye-based cell biology assays, were used for characterization.109,113 Therefore, the focus of our study is also to conduct advanced research on PMC. We concluded from the above discussion that PMC prepared from high MW pHPMA resulted in a significant increase in solubility, stability, circulation, and tumor accumulation of DOX, PTX, ritonavir, and curcumin.
(b) Polymeric nanoparticles (PNPs)
Polymeric nanoparticles offer several advantageous features, including small size, stimuli-responsive behavior, sustained drug release, improved drug pharmacokinetics, and cellular uptake.117–120 They provide surface nanoengineering for drug moieties to facilitate cell entry and particularly cleave in the vicinity of tumors for drugs attached to pH-sensitive linkers.121 The prominence of PNPs causes greater blood residual circulation, which is subsequently prone to activate endosomal pH.122 PNPs modulate the bioavailability of drugs by reducing enzymatic and hydrolytic degradation and possess greater biocompatibility or cause lesser immunogenicity123 (Table 1). Notably, enhanced penetration and adequate release of drugs can be achieved by polymeric nanocarriers. Importantly, the nanometric size enables PNPs to enter the acidic microenvironment via enhanced permeability and retention effect (EPR).7 Therefore, PNPs are well-considered as a potential carrier for the administration of drugs.Ulbrich and coworkers encapsulated docetaxel (DTX) in PNPs assembled using the PBS/PBDL polyester core. DOX was covalently conjugated to pHPMA through hydrazone bonds, which eventually made the system pH-sensitive. The combination of drug NPs (DTX + DOX) effectively suppressed the progression of tumors in EL-4T mice more efficiently than single drug-loaded NPs.124
Jager et al. conjugated pH-sensitive water-insoluble poly[2-(diisopropylamino)ethyl methacrylate] (PDPA) with the protein-resistant water-soluble poly[N-(2-hydroxypropyl)methacrylamide] (pHPMA) and loaded PTX in the PNPs. From pH 6.8 to 6.5, the PDPA block acquires protons and becomes hydrophilic, resulting in the disassembling of NPs to release PTX in endosomes and lysosomes. The NPs were found to be highly cytotoxic against solid tumors. Aggregates with a particle size less than 100 nm showed excellent stability and drug release in an acidic tumor environment, possibly due to the advantage of the EPR effect.125
Yang et al. prepared and characterized a biodegradable polymeric conjugate of DOX using an enzyme-sensitive peptide. PNPs exhibited a high anti-proliferation effect in 4T1 tumor cells.126
Duan et al. synthesized amphiphilic pHPMA–GEM–GFLG stimuli-responsive NPs for breast cancer treatment. GFLG is an enzyme-sensitive peptide attached to pHPMA via RAFT. Later, near-infrared (NIR) dye Cy5.5 (optical imaging probe) was conjugated. Fluorescent imaging showed the accumulation and retention of GEM in tumor cells. In vivo studies revealed improved antitumor activity of GEM against 4T1 triple-negative breast cancer.127
Gong and group reported an enzyme and pH-sensitive pHPMA–DOX NP system that showed enhanced accumulation in inoculated breast tumors and resulted in an improved anti-cancer activity of DOX.128,129
Interestingly, in 2018, Chen et al. also reported enzyme and pH-sensitive dendritic NPs of DOX. The high (220 KDa) and low (≤40 KDa) molecular weight GFLG linker was conjugated with DOX via RAFT. The high MW, dendritic NPs showed a higher accumulation of DOX and were internalized via the endocytic pathway. DOX NPs showed potential against 4T1 tumor-bearing mice and exhibited lower toxicity in in vivo experiments than free DOX. Therefore, the dendritic NPs, in response to the intracellular enzymes and the acidic pH of tumor tissue, hold great promise in tumor-targeted therapy.130
Lomkova et al. prepared and characterized betulinic acid (BA) conjugated pHPMA copolymers via free radical polymerization and subsequently fabricated micellar systems for the sustained release of BA. BA-pHPMA conjugates with a spacer, methylated carboxyl groups, exhibited pH-sensitive release in vitro. The high molecular weight of the micellar conjugates improved drug uptake in solid tumors, attributed to the EPR effect. Micelles were cytostatic towards DLD-1, HT-29, as well as HeLa cells, and efficiently accumulated within HT-29 mice xenografts.131,132
Luo and coworkers prepared block copolymer NPs (b-NPs), where DOX was conjugated to pHPMA via the hydrazone linkage. The cross-linked conjugate transformed into cross-linked copolymer-NPs (cl-NPs), rich in disulfide bonds, which exhibited a prolonged circulation time and efficiently inhibited tumors in mice than b-NPs. NPs manifested pH-dependent liberation of DOX. Both NPs were equally cytotoxic toward A549 cell lines. However, cl-NPs demonstrated a remarkable improvement in half-life compared to b-NPs and free DOX. Thereby, the dual-susceptible cross-linked pHPMA copolymer–DOX conjugated systems that undergo biological degradation could be considered as a better option for treatment.133
Gundel et al. studied the cellular uptake of several pHPMA-derived polymeric constructs differing in molecular weight and size in two tumor models. The results suggested that the uptake is affected by the molecular structure and Mn of the polymers in different types of cancer cells. The accumulation of random copolymer was 5-fold greater than that of the homo polymer in AT1 cell lines, but in Walker-256, the intracellular accumulation of each pHPMA conjugate was almost similar and independent of the molecular composition or size.134
Liu et al. prepared pHPMA–based DOX nanoparticles for direct targeting of mitochondria. Glycyrrhetinic acid (GA) was covalently bonded with DOX (GA–DOX) and pHPMA to form P–DOX–GA and grafted on the exterior of the gelatin nanoparticles (GNPs). GNPs–P–DOX–GA enhanced intracellular uptake and mitochondrial distribution in HepG2/ADR cell lines, thereby improving the in vitro anticancer efficacy and in vivo anticancer properties on HepG2/ADR heterotopic tumor-bearing mice.135
Chen et al. reported the pHPMA–PEG-based NPs of DOX. The DOX attached via the hydrazone linkage was released at the acidic pH of tumor cells and induced cell death by decreasing the mitochondrial membrane potential and demonstrating anti-cancer potential against 4T1 tumor cells.136
Gupta and coworkers synthesized and characterized biotin-conjugated HPMA–PLA and prepared encapsulated BTZ NPs. Three conjugates, HPMA–biotin (HP-BT), HPMA–PLA–biotin (HPLA–BT), and HPMA–PLA (HPLA), were synthesized to study the effect of biotinylated and non-biotinylated polymeric conjugates in a nanoparticulate system. Biotin-attached PNPs significantly increased the cellular uptake of both BTZ-loaded HP–BT–PNPs and HPLA–BT–PNPs (199 nm size, PDI 0.196) and increased the cytotoxic effect of BTZ compared to non-biotinylated PNPs on MCF-7 cells.137
Gan and coworkers prepared PDLLA–pHPMA-based NPs via the solvent precipitation method and tested them against 4T1 cancer cells. The nano-assemblies showed potential anti-tumor activity with improved accelerated blood clearance (ABC) compared to PDLLA–PEG.138
Plichta et al. fabricated pHPMA-modified magnetic NPs conjugated with DOX via the hydrazone bond and potentially improved chemotherapy for glioblastoma.139
Conclusively, pHPMA-based PNPs were used to develop a stable nanoparticulate drug delivery system, yielding significant results and showing an effective anti-tumor effect in cancer cell lines and mouse models.
(c) Dendrimers
The attractive features, such as nanometric size, low polydispersity index, abundant surface functionalities, and more void space to encapsulate lipophilic drugs, make dendrimers an attractive nano-scaffold for drug delivery.140–144 Polyamidoamine (PAMAM) dendrimers are comprised of amine-terminated peripheral functionality that can be surface engineered with anionic groups, including PEG, carboxyl, and acetylation, facilitating conjugation or ligand attachment.145 The dendrimer-mediated nanoscale drug delivery provides a sufficient concentration of drug to the desired site, which also depends on passive targeting or the EPR effect.146,147 PAMAM dendrimers have emerged as promising candidates for drug delivery and are discussed below.Sadekar et al. performed a comparative study of three-dimensional (3D) PAMAM dendrimers and linear pHPMA to understand the effect of Mn, size measurement, and polymer nanostructure on the biodistribution profile in tumor-induced mice using compartmental pharmacokinetic analysis. The elimination clearance of pHPMA decreased more with the modulation in hydrodynamic size than with PAMAM dendrimers.148
Similarly, Ghandehari and coworkers compared the biodistribution of linear pHPMA and branched PAMAM–OH in an ovarian tumor model. In vitro cytotoxicity results revealed that nano-formulations showed enhanced anti-proliferation activity and internalization. It was noted that 7.0 G (generation) hydroxyl-terminated PAMAM dendrimers improved both plasma circulation and tumor accumulation.149 (Table 1).
Maeda and coworkers prepared pHPMA–pirarubicin and pHPMA–PAMAM conjugates via a pH-sensitive hydrazone spacer and compared the in vitro and in vivo effects. It was found that the PAMAM–pHPMA released the drug more rapidly in an acidic environment and showed a better anti-tumor effect in the mouse model as compared to pHPMA–pirarubicin.150
Kostkova and colleagues designed a star-like dendrimer grafted with DOX via a hydrazone bond by conjugating semi-telechelic pHPMA on a polyester-based multifunctional dendritic core. It was noted that the polymeric–DOX nanoconstructs showed significantly higher IC50 values than both free DOX and linear polymer–DOX conjugate.151
Ghandehari et al. designed nanoconstructs in which the photosensitizer MTCP (meso-tetra-4-carboxyphenyl porphyrin) was grafted to amine-terminated hyper-branched PAMAM. The comparative in vitro proliferation of pHPMA–MTCP and MTCP was studied for cytotoxicity in KB cells and A549 (Fig. 4). An enhanced phototoxicity action was noticed via higher cellular uptake for pHPMA–MTCP than unconjugated MTCP.152
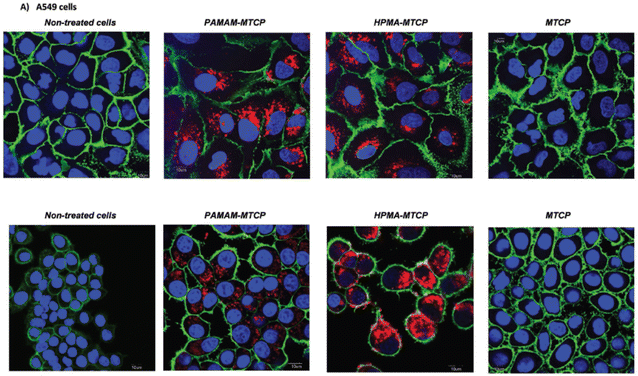 | ||
| Fig. 4 Cellular uptake study by confocal microscopy. Cells were grown on Lab-Tek Chambered Cover glass for 24 h. After incubation with drugs (MTCP 0.158 × 10−3 m, PAMAM-MTCP 20.61 × 10−6 m, pHPMA-MTCP 4.49 × 10−6 m) for 7 h, cell membranes were stained with Wheat germ agglutinin CF488A conjugate, and cell nuclei were stained with Hoechst according to the manufacturer’s protocol. Cells fixed with 4% paraformaldehyde solution in PBS. Red dots are represented to polymer conjugated MTCP. (A) Fluorescent images of fixed cells were taken under a confocal laser scanning microscope (Olympus FV1000-USA) on A549 cells. Reproduced from ref. 145 with permission from Wiley copyright [2021]. | ||
Pan et al. prepared and functionalized dendrimer-based pHPMA conjugates with a metabolism-disturbing molecule, dendronized pyro-pheophorbide polymer (DPP). The synthesized formulation showed potential anti-cancer activity by targeting the metabolism of tumor cells.153
PAMAM dendrimers with pHPMA have been widely explored. Here, we present a qualitative discussion of past research highlighting the importance of Mn and its effect on cellular uptake and in vivo pharmacokinetics of drugs using pHPMA as a carrier with different architectures.
(d) Prodrugs
Prodrugs are pharmacologically inactive compounds that can carry drugs in a single carrier through different linkers. Prodrugs become active once they undergo chemical or enzymatic modification and then release the active moiety to produce the desired action.154 The chemical linkage ester, amine, amide, oxime, etc., is a fantastic strategy to illustrate prodrug designing, and some of these bond-based prodrug nanosystems are under clinical trials. It can solve some issues such as hydrophobicity, stability, fast metabolism, cell membrane barriers, and toxic effects. It can be divided into 3 categories; (a) drug attached to a polymer to form a polymer–drug conjugate system, (b) small molecular weight drug linked to a small molecular weight polymer, (c) encapsulation of the drug into the nanoparticulate system.155Chandran et al. coupled pHPMA with prostate-specific antigen (PSA) via covalent linkage and developed a prodrug system that increased the solubility of peptide drugs. The enzymatically cleaved PSA is present in higher amounts at the extracellular sites of the tumor and shows better compatibility in cancer xenograft models.156
Oupicky and coworkers prepared pHPMA-based prodrugs for AMD3465 (P-SS-AMD) and antagonist CXCR4, which carried therapeutic miRNA. The cationic nature of P-SS-AMD formed polyplexes with miRNA, resulting in the efficient transfection of a downstream target ZEB-1 in cancer cells. The result shows that P-SS-AMD functions efficiently to deliver miRNA.157
Recently, Dai et al. have performed a one-pot synthesis to prepare dendritic-pHPMA–GEM, with a high Mn 168 KDa, via RAFT polymerization. More than 90% of GEM was released from stimuli-responsive prodrugs within 3 h with Cathepsin B. In the dendritic GEM-treated lung and liver, no metastasis was observed; however, metastasis was visible in pure GEM. Also, more tumor inhibition was observed with the dendritic prodrug than with free GEM in the in vivo studies.158
Denmeade et al. synthesized a prodrug of pHPMA with PSA-activated peptide against prostate cancer. The PSA-activated peptide was specifically cleaved in the extracellular matrix of the tumor, releasing cytotoxin and causing cytotoxicity in CWR22Rv1 cells.156
Luo and coworkers prepared a prodrug conjugate of DOX–pHPMA (Mn 95 KDa). Significant tumor inhibition was observed in the 4T1 tumor model, suggesting that it may be a good idea to deliver DOX by attaching GFLG (an enzyme-sensitive linker).159
Vinciguerra and co-workers attempted the fabrication of polymeric prodrug NPs with high drug-loading content for GEM and DOX. Two conjugates, GEM-Rhodamine (imaging agent) and DOX–aminoglutethimide, were prepared using a drug-initiated method and characterized. NPs of size 160 nm showed a significant cytotoxic effect on MCF-7 cells. High and low Mn DOX–aminoglutethimide showed IC50 values of 1.8–1.9 and 2.82 μM, respectively.160
In another recent study, the same group developed polymeric prodrug NPs for the delivery of GEM and lapatinib (LAP). Empty and dual-functioning prodrug NPs showed excellent stability and variable IC50 values from 150–300 nM.161
Luo et al. prepared and delivered nifuroxazide (NFX) and DOX pHPMA prodrugs, where pHPMA was conjugated with pH and enzyme-sensitive linkers OEGMA and GFLG, respectively. The PNP of NFX and DOX inhibited the growth of 4T1 cells. Anti-metastasis and excellent tumor inhibition activity were observed in mice after intravenous injection of PNPs. These results were further confirmed by immuno-histochemical studies by assessing the proteins (MMP) in cells.162
Recently, Cai et al. developed stimuli-responsive polymeric prodrugs of PTX and studied them by attaching two imaging agents: Cy 5.5 and gadolinium (Gd). In MRI imaging results, the prodrugs were taken up by tumor cells, as visible in the different images (Fig. 5), suggesting the effectiveness of the prodrug (IC50 values of Taxol and PTX-Gd-based NPs were 0.45 and 0.55 μg mL−1, respectively). The PTX-Gd-based prodrugs displayed more cytotoxic effects than pure PTX by disturbing the function of microtubules (Fig. 6). In conclusion, prodrugs remarkably increased the relaxation efficiency and enhanced contrast intensity compared to the Cy 5.5-pHPMA–PTX prodrug system.163
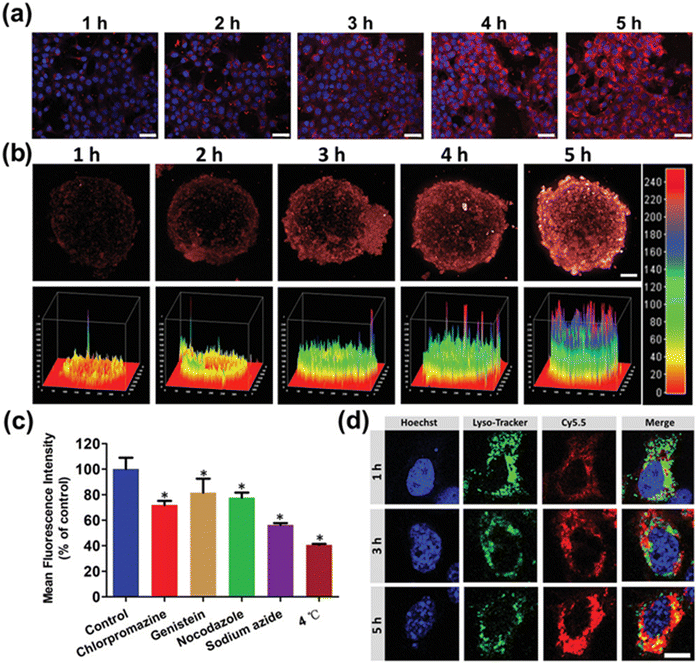 | ||
| Fig. 5 (a) CLSM images of 4T1 cells after treatment with Cy5.5-labeled BP–PTX–Gd NPs at different times. Hoechst 33342 (blue) for nuclei and fluorescence dye Cy5.5 (red) for BP–PTX–Gd NPs. The scale is 50 μm. (b) CLSM images of 4T1 tumor spheroids after treatment with Cy5.5-labeled PCT-Gd NPs at different times; red represents the fluorescence of Cy5.5. The scale is 100 μm. (c) Percentage of BP–PTX–Gd NPs taken up by 4T1 cells at different inhibitor conditions (*p < 0.01). (d) The lysosomal escape behavior of BP–PTX–Gd NPs at different times in 4T1 cells. Hoechst 33342 (blue) for nuclei, lysotracker (green) for lysosomal, and fluorescence dye Cy5.5 (red) for BP–PTX–Gd NPs. The scale is 10 μm. Reproduced from ref. 156 with permission from ACS, Advance Science, copyright [2021]. | ||
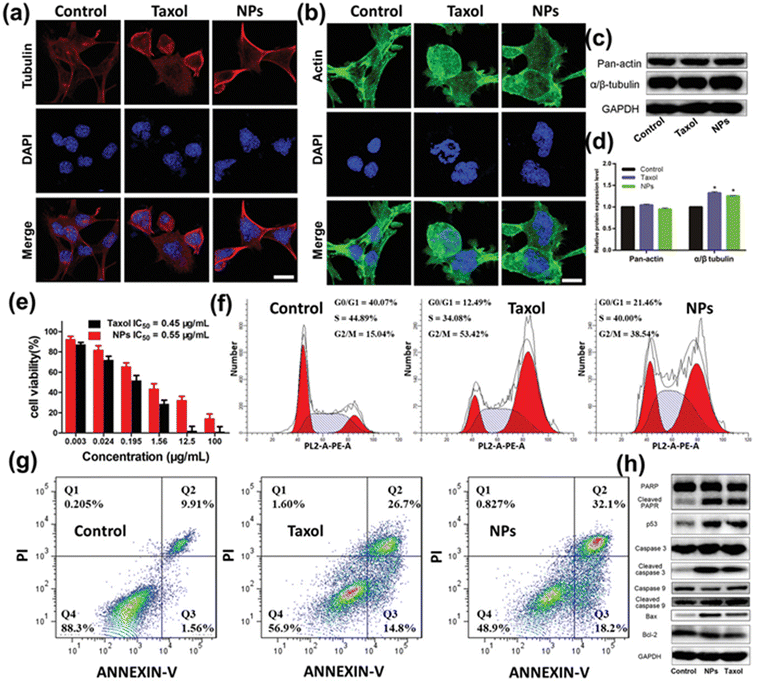 | ||
| Fig. 6 (a) Microtubule aggregation and (b) microfilament damage of 4T1 cells after treatment with BP–PTX–Gd NPs and Taxol for 24 h. Red fluorescence for the tubulin antibody, green for the actin antibody, and blue for nuclear staining with DAPI. The scale is 10 μm. (c) Western blot assay and (d) semi-quantitative analysis of α/β tubulin and pan-actin in the 4T1 tumor cells after incubation with Taxol and BP–PTX–Gd NPs for 24 h (*p < 0.01 compared with the control group). (e) Cytotoxicity of BP–PTX–Gd NPs and Taxol against 4T1 cells after incubation for 48 h (means ± SD, n = 5). (f) Cell cycle distribution and (g) apoptosis of 4T1 cells after treatment with Taxol and BP–PTX–Gd NPs for 24 h. (h) Western blot assay of Bcl-2, Bax, PARP, cleaved PARP, caspase-3, cleaved caspase-3, caspase-9, cleaved caspase-9, and p53 in the 4T1 tumor cells after incubation with Taxol and BP–PTX–Gd NPs for 24 h. Cells treated with PBS were used as the control. Reproduced from ref. 156 with permission from ACS, Advance Science, copyright [2021]. | ||
Luo and coworkers synthesized GSH-sensitive prodrugs and attached them to a photosensitizer (chlorin e6). Using photodynamic therapy, reactive oxygen species were generated that induced cell apoptosis and inhibited tumor growth.164
To summarize, prodrugs have advantages in drug delivery systems due to the active cleavage of drug moieties at the target site. Several pHPMA conjugates have been published in the literature, which are outside the scope of this review; therefore, readers are referred to the literature.4,7,51,165
(e) Liposomes
Liposomes are bilipid layer vesicles used for the delivery of drugs, proteins, genes, and bioactive agents. They comprise a hydrophilic head and lipophilic tail that can accommodate both water-soluble and lipid-soluble drugs in their core and periphery. Homogenization, rota evaporator, hydration, etc., are different methods for the preparation of liposomes. These methods result in unilamellar, oligolamellar, and multilamellar vesicles. However, the size of these vehicles can be reduced by the sonication and extrusion approach.166,167 The size, charge, and zeta potential of liposomes depends on selection of method of preparation. The Covid vaccine is a significant and successful achievement in mRNA delivery using lipid formulation.15–17 Ambisome, Myocet, Lipoplatin, Doxil, and DaunoXome are some commercial products that utilize liposomes. Some polymers, such as PEG, are widely used to improve the stability of lipid nanoparticles and liposomes. However, some unexpected immune reactions, such as accelerated blood clearance (ABC) and allergic reactions against PEGylated nanocarriers, have been observed in patients.168 HPMA has been explored for liposome formulations for drug delivery.169–171Torchilin and colleagues prepared pHPMA-based liposomes using cholesterol to study the effect of surface modification on DOX. The size of prepared liposomes was found to be in the range 150–200 nm. The 111In radiolabeled high molecular weight liposomes were highly taken up by endocytosis, thereby increasing the circulation time of DOX up to 2.5 hours.169
Next, Paasonen et al. synthesized pHPMA (mono/dilactate) and studied the temperature-dependent release of calcein. Cholesterol was used as a core to prepare liposomes, and the size was measured at different temperatures up to 42 °C. The pHPMA-based liposomes triggered the release of calcein above the cloud point. The study suggested that the cholesterol-incorporated pHPMA liposomes avoid drug leakage at 37 °C and could be a better option for drug delivery.170
Wang and coworkers reported thermosensitive liposomes for controlled release of DOX. They first synthesized thermo-sensitive polymeric conjugates of p-(NIPAM-r-HPMA) and p(HPMA-r-APMA) via RAFT and then extended the method to liposomes. The prepared lipo-assembly of size 100 nm was stable at 37 °C and showed burst release at 42 °C. DOX under hyperthermia conditions exhibited deep penetration in the tumor environment. The Cy7.5 labeled liposomes exhibited the best in vivo drug efficacy and prolonged circulation in Balb/c mice.115
Elk et al. also prepared cholesterol-conjugated HPMA–DOX liposomes and tested them against AML-12, HepG2 (both hepatocyte-derived cancer cells), RAW 264.7 (macrophages), and HUVEC (endothelial) cells. DOX showed rapid release under mild hyperthermia conditions; hence, it was considered to be a good candidate for the tumor stroma.171
6. Clinical translation or future prospects
In oncology, polymeric nanotherapeutics have manifested a trend towards improved cancer therapy. Nanomedicines are designed with different strategies and formulas; however, scaling up these processes is very challenging for the pharmaceutical industry. Fortunately, the multifunctional potential of nanomedicine makes them ideal for filling a clinical niche. Indeed, before the clinical translation of these products, some optimization and improvements are needed. To date, numerous polymer-based nanotherapeutics are available in the market for the treatment of cancerous and non-cancerous diseases.51,172,173 HPMA has gained exponential growth in the realm of polymer conjugation, offering precise control over polymer–drug conjugations. Plentiful literature is available on the pHPMA–drug conjugate nanomedicines.19,47–50 The standard protocols and dosage control may provide some profitable clinical translation of pHPMA as a potential carrier. Undeniably, HPMA opens opportunities for the development of novel therapeutics. Insightful results of HPMA nanotherapeutics have entered the clinical trials phase I/II (Table 2).26,60–62 Although numerous clinical trials of pHPMA-based conjugates are still ongoing, no product has been successfully commercialized. Thus, further research is required to develop HPMA-based nanotherapeutics with greater speed to firm up the clinical translation of drug delivery systems. Polymer-based drug delivery systems may aid in the scaling of high-stability therapeutic products that meet clinical demands.| Product | Drug | Platform | Status | Manufacturers |
|---|---|---|---|---|
| ProLindac (AP5346) | DACH–oxaliplatin | HPMA-polymeric nanoparticles | Phase II/III advanced | Access pharmaceuticals |
| Camptothecin | pHPMA | I | Pharmacia | |
| DOX | pHPMA | II | Pharmacia | |
| DOX–galactosamine | pHPMA based PMC | II | Pharmacia | |
| DOX–platinate | pHPMA | II/III | Access pharma | |
| Doxetaxel | mPEG5000-b-p(HPMAm-Lacn) | I | Cristal therapeutics | |
| PCT | ppHPMA | I | Pharmacia | |
| PK1 (FCE28068) | DOX | pHPMA | II | Pfizer |
| AP5280 | Platinum | pHPMA | II | Access pharmaceuticals |
7. Conclusion and future prospects
The promising goal of different nanotherapeutics is to achieve sufficient availability and on-demand release of drugs at the tumor site. This goal is still not fully accomplished due to the heterogeneity and complexity of tumors. Several PEG, pHPMA, and other polymeric nanoformulations are currently in clinical trials. Several research studies have been reported on HPMA, and we hope this copolymer will yield some fruitful results in the pharmaceutical industry. A few pHPMA–drug conjugates have been widely explored and are in different phases of clinical trials. It is anticipated that the insights presented in this review will inspire further exploration of pHPMA drug delivery systems. However, some basic questions related to drug delivery still remain. The connection between different physicochemical parameters of nanodrug delivery and tumor areas is a crucial factor in fighting against cancer. In the introduction section, we have provided insights into how the size, charge, and shape of nanoparticles influence drug accumulation in tumors. Small-sized (1–200 nm), non-spherical-shaped, and neutral-charged nanocarriers are considered to provide better rationale for effective drug delivery. However, the enhanced permeation retention (EPR) effect is crucial because it is useful for solid tumors and depends on interstitial pressure and solid stress. This stress could compress blood vessels and impede drug delivery. For an anticancer drug, it is not necessary for it to penetrate the center of a solid tumor to elicit anticancer action. Trastuzumab (Herceptin) antibody is a successful example of the EPR effect against HER2-positive breast cancer because it penetrates only into the vascularized area.174 However, successful examples are few. Doxil (Doxorubicin liposomes) does not benefit from the EPR effect for solid tumors; however, PEGylated–DOX liposomes accumulated in the tumor, yet overall outcomes were not satisfactory.175 A better design strategy, combination of drugs and EPR modulators, blood flow enhancers, and large animal tumor models could be a potential solution to this problem.Hence, there is an urgent need to consider the various issues of drug delivery systems using polymer carriers. Insights gained from the synthesis of pHPMA–drug conjugates and their use as a polymer for drug delivery further attracted the attention of scientists towards combination therapy and immunotherapy. Yang et al. designed an effective strategy to inhibit PD-1/PD-L1 based on polymer (pHPMA)-peptide against a non-immunogenic tumor. “Priming” tumors with a biodegradable pHPMA–epirubicin conjugate induced immunogenic cell death and enhanced tumor-specific CD8+ T cell response. This produced prolonged elimination of PD-L1, and these findings suggested that the pHPMA facilitated tumor targeting of immunogenic drugs and surface crosslinking of PD-L1 as a potential new therapeutic approach for long-term antitumor immunity.176 Further, Yang and team used a polymer-based chemo-immunotherapy for the MMTV-PyMT breast cancer model. Here, the combination of two polymer–drug conjugates induced immunogenic cell death (ICD) and disrupted PD-L1/PD-1 interactions. This strategy overcomes immunosuppression and indicates that polymer–drug conjugates could be a promising rationale for cancer immunotherapy.177 Therefore, a combination therapy of drugs with pHPMA conjugation along with a nanomedicine approach could be explored in detail in the future and may result in better outcomes for cancer immunotherapy.
Author contributions
Sarita Rani: Conten design, writing – review & editing. Vinay Kumar: Writing. Sofiya Tarannum: Writing. Umesh Gupta: Conceptualization, writing – review & editing.Conflicts of interest
The authors declare that they do not have any conflict of interest.Data availability
No primary research results, software or code has been included and no new data were generated or analysed as part of this review.Acknowledgements
The authors would like to acknowledge the financial support from the Indian Council of Medical Research (ICMR), India, for providing a Senior Research Fellowship (Award letter no. 45/16/2020-Nan/BMS) to Ms Sarita Rani and Department of Pharmacy, School of Chemical Sciences at the Central University of Rajasthan, Ajmer, Rajasthan.References
- G. S. P. A. B. Stein, Cell cycle and growth control: biomolecular regulation and cancer, John Wiley & Sons, 2004, ISBN 0-471-25071-6 Search PubMed.
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal and F. Bray, Ca-Cancer J. Clin., 2021, 71, 209–249 CrossRef PubMed.
- M. Ghezzi, S. Pescina, C. Padula, P. Santi, E. Del Favero, L. Cantù and S. Nicoli, J. Controlled Release, 2021, 332, 312–336 CrossRef CAS PubMed.
- A. C. Anselmo, S. Mitragotri and J. A. Paulson, Bioeng. Transl. Med., 2019, 4, e10143 CrossRef PubMed.
- J. K. Patra, G. Das, L. F. Fraceto, E. V. R. Campos, M. D. P. Rodriguez-Torres, L. S. Acosta-Torres, L. A. Diaz-Torres, R. Grillo, M. K. Swamy, S. Sharma, S. Habtemariam and H. S. Shin, J. Nanobiotechnol., 2018, 16, 1–33 CrossRef PubMed.
- M. Srinivasarao and P. S. Low, Chem. Rev., 2017, 117, 12133–12164 CrossRef CAS PubMed.
- H. Maeda, Adv. Enzyme Regul., 2001, 41, 189–207 CrossRef CAS PubMed.
- R. Cheng, F. Meng, C. Deng, H. A. Klok and Z. Zhong, Biomaterials, 2013, 34, 3647–3657 CrossRef CAS PubMed.
- C. I. C. Crucho, ChemMedChem, 2015, 10, 24–38 CrossRef CAS PubMed.
- P. T. Wong and S. K. Choi, Chem. Rev., 2015, 115, 3388–3432 CrossRef CAS PubMed.
- U. Bulbake, S. Doppalapudi, N. Kommineni and W. Khan, Pharmaceutics, 2017, 9, 12 CrossRef PubMed.
- P. Ma and R. J. Mumper, J. Nanomed. Nanotechnol., 2013, 4, 1000164 Search PubMed.
- R. Duncan, Nat. Rev. Drug Discovery, 2003, 2, 347–360 CrossRef CAS PubMed.
- R. Duncan, J. Controlled Release, 2014, 190, 371–380 CrossRef CAS PubMed.
- Y. Eygeris, S. Patel, A. Jozic, G. Sahay and G. Sahay, Nano Lett., 2020, 20, 4543–4549 CrossRef CAS PubMed.
- M. Cavaleri, H. Enzmann, S. Straus and E. Cooke, Lancet, 2021, 397, 355–357 CrossRef CAS PubMed.
- O. Schildgen, A. Monforte and B. Z. Igyártó, Microorganisms, 2022, 10, 1463 CrossRef PubMed.
- F. M. Veronese and G. Pasut, Drug Discovery Today, 2005, 10, 1451–1458 CrossRef CAS PubMed.
- L. Šprincl, J. Exner, O. Štěrba and J. Kopeček, J. Biomed. Mater. Res., 1976, 10, 953–963 CrossRef PubMed.
- J. Kopecek and H. Bazilova, Eur. Polym. J., 1973, 9, 7–14 CrossRef CAS.
- H. Krakovičová, T. Etrych and K. Ulbrich, Eur. J. Pharm. Sci., 2009, 37, 405–412 CrossRef PubMed.
- J. Kopeček and P. Kopečková, Adv. Drug Delivery Rev., 2010, 62, 122–149 CrossRef PubMed.
- T. Lammers, Adv. Drug Delivery Rev., 2010, 62, 203–230 CrossRef CAS PubMed.
- R. Duncan, J. B. Lloyd and J. Kopeček, Biochem. Biophys. Res. Commun., 1980, 94, 284–290 CrossRef CAS PubMed.
- J. Kopeček, P. Rejmanová and V. Chytrý, Die Makromol. Chemie., 1981, 182, 799–809 CrossRef.
- R. Duncan, S. Gac-Breton, R. Keane, R. Musila, Y. N. Sat, R. Satchi and F. Searle, J. Controlled Release, 2001, 74, 135–146 CrossRef CAS PubMed.
- Y. Dang and J. Guan, Smart Mater. Med., 2020, 1, 10–19 CrossRef PubMed.
- C. J. Cheng, G. T. Tietjen, J. K. Saucier-Sawyer and W. M. Saltzman, Nat. Rev. Drug Discovery, 2015, 14, 239–247 CrossRef CAS PubMed.
- W. H. De Jong and P. J. A. Borm, Int. J. Nanomed., 2008, 3, 133–149 CrossRef CAS PubMed.
- A. Kumari, S. K. Yadav and S. C. Yadav, Colloids Surf., B, 2010, 75, 1–18 CrossRef CAS PubMed.
- J. Folkman and D. M. Long, J. Surg. Res., 1964, 4, 139–142 CrossRef CAS PubMed.
- G. H. Son, B. J. Lee and C. W. Cho, J. Pharm. Invest., 2017, 47, 287–296 CrossRef CAS.
- E. S. Alia, S. M. Sharker, M. T. Islam, I. N. Khane, S. Shawf, M. A. Rahmang, S. J. Uddinh, M. C. Shillb, S. Rehmani, N. Dasj, S. Ahmadi, J. A. Shilpih, S. Tripathi, S. K. Mishram and M. S. Mubarak, Semin. Cancer Biol., 2021, 69, 52–68 CrossRef PubMed.
- L. Tang, T. M. Fan, L. B. Borst and J. Cheng, ACS Nano, 2012, 6, 3954–3966 CrossRef CAS PubMed.
- R. Toy, P. M. Peiris, K. B. Ghaghada and E. Karathanasis, Nanomedicine, 2014, 9, 121–134 CrossRef CAS PubMed.
- K. Cho, X. Wang, S. Nie, Z. Chen and D. M. Shin, Clin. Cancer Res., 2008, 14, 1310–1316 CrossRef CAS PubMed.
- W. C. Chan, BME Front., 2023, 4, 1–10 CrossRef PubMed.
- R. Zein, W. Sharrouf and K. Selting, J. Oncol., 2020, 5194780 CAS.
- J. A. Champion and S. Mitragotri, Pharm. Res., 2009, 26, 244–249 CrossRef CAS PubMed.
- C. He, Y. Hu, L. Yin, C. Tang and C. Yin, Biomaterials, 2010, 31, 3657–3666 CrossRef CAS PubMed.
- J. Milton Harris and R. B. Chess, Nat. Rev. Drug Discovery, 2003, 2, 214–221 CrossRef PubMed.
- T. Ishida, M. Harada, Y. W. Xin, M. Ichihara, K. Irimura and H. Kiwada, J. Controlled Release, 2005, 105, 305–317 CrossRef CAS PubMed.
- P. Laverman, M. G. Carstens, O. C. Boerman, E. T. M. Dams, W. J. G. Oyen, N. Van Rooijen, F. H. M. Corstens and G. Storm, J. Pharmacol. Exp. Ther., 2004, 10, 950–953 Search PubMed.
- E. T. M. Dams, P. Laverman, W. J. G. Oyen, G. Storm, G. L. Scherphof, J. W. M. van der Meer, F. H. M. Corstens and O. C. Boerman, J. Pharmacol. Exp. Ther., 2000, 293, 996–1001 CrossRef.
- R. Singh and J. W. Lillard, Exp. Mol. Pathol., 2009, 86, 215–223 CrossRef CAS PubMed.
- Y. Matsumura and H. Maeda, Cancer Res., 1986, 46, 6387–6392 CAS.
- E. Randárová, H. Nakamura, R. Islam, M. Studenovský, H. Mamoru, J. Fang, P. Chytil and T. Etrych, Acta Biomater., 2020, 106, 256–266 CrossRef PubMed.
- H. Nakamura, E. Koziolová, P. Chytil, T. Etrych, M. Haratake and H. Maeda, Mol. Pharmaceutics, 2019, 16, 3452–3459 CrossRef CAS PubMed.
- H. Nakamura, E. Koziolová, P. Chytil, K. Tsukigawa, J. Fang, M. Haratake, K. Ulbrich, T. Etrych and H. Maeda, Mol. Pharmaceutics, 2016, 13, 4106–4115 CrossRef CAS PubMed.
- R. Duncan, Y. N. Sat-Klopsch, A. M. Burger, M. C. Bibby, H. H. Fiebig and E. A. Sausville, Cancer Chemother. Pharmacol., 2013, 72, 417–427 CrossRef CAS PubMed.
- X. M. Liu, S. C. Miller and D. Wang, Adv. Drug Delivery Rev., 2010, 62, 258–271 CrossRef CAS PubMed.
- H. Maeda, Adv. Drug Delivery Rev., 2015, 91, 3–6 CrossRef CAS PubMed.
- Y. Noguchi, J. Wu, R. Duncan, J. Strohalm, K. Ulbrich, T. Akaike and H. Maeda, Jpn. J. Cancer Res., 1998, 89, 307–314 CrossRef CAS PubMed.
- M. Sponchioni, L. Morosi, M. Lupi and U. Capasso Palmiero, RSC Adv., 2017, 7, 50981–50992 RSC.
- M. H. Stenzel, Chem. Commun., 2008, 3486–3503 RSC.
- L. W. Seymour, D. R. Ferry, D. Anderson, S. Hesslewood, P. J. Julyan, R. Poyner, J. Doran, A. M. Young, S. Burtles and D. J. Kerr, J. Clin. Oncol., 2002, 20, 1668–1676 CrossRef CAS PubMed.
- P. A. Vasey, S. B. Kaye, R. Morrison, C. Twelves, P. Wilson, R. Duncan, A. H. Thomson, L. S. Murray, T. E. Hilditch, T. Murray, S. Burtles, D. Fraier, E. Frigerio and J. Cassidy, Clin. Cancer Res., 1999, 5, 83–94 CAS.
- J. M. Rademaker-Lakhai, C. Terret, S. B. Howell, C. M. Baud, R. F. De Boer, D. Pluim, J. H. Beijnen, J. H. M. Schellens and J. P. Droz, Clin. Cancer Res., 2004, 10, 3386–3395 CrossRef CAS PubMed.
- R. Duncan and M. Vicent, Adv Drug Delivery Rev., 2010, 62, 272–282 CrossRef CAS PubMed.
- L. W. Seymour, D. R. Ferry, D. J. Kerr, D. Rea, M. Whitlock, R. Poyner, C. Boivin, S. Hesselewood, C. Twelves, R. Blackie, A. Schatzlein, D. Jodrell, D. Bissett, H. Calvert, M. Lind, A. Robbins, S. Burtles, R. Duncan and J. Cassidy, Int. J. Oncol., 2009, 34, 1629–1636 CrossRef CAS PubMed.
- T. Etrych, V. Šubr, J. Strohalm, M. Šírová, B. Říhová and K. Ulbrich, J. Controlled Release, 2012, 164, 346–354 CrossRef CAS PubMed.
- M. Barz, A. Armiñán, F. Canal, F. Wolf, K. Koynov, H. Frey, R. Zentel and M. J. Vicent, J. Controlled Release, 2012, 163, 63–74 CrossRef CAS PubMed.
- P. Yin, W. Liang, B. Han, Y. Yang, D. Sun, X. Qu, Y. Hai and D. Luo, Small Methods, 2024, 8, 2301173 CrossRef PubMed.
- S. Rani and U. Gupta, Drug Discovery Today, 2020, 25, 997–1012 CrossRef CAS PubMed.
- L. Nuhn, M. Barz and R. Zentel, Macromol. Biosci., 2014, 14, 607–618 CrossRef CAS PubMed.
- S. Rani, A. Gothwal, P. K. Pandey, D. S. Chauhan, P. K. Pachouri, U. D. Gupta and U. Gupta, Pharm. Res., 2019, 36, 1–12 CrossRef CAS PubMed.
- Y. Bobde, S. Biswas and B. Ghosh, Eur. Polym. J., 2020, 139, 110018 CrossRef CAS.
- M. Najafi, N. Kordalivand, M. A. Moradi, J. Van Den Dikkenberg, R. Fokkink, H. Friedrich, N. A. J. M. Sommerdijk, M. Hembury and T. Vermonden, Biomacromolecules, 2018, 19, 3766–3775 CrossRef CAS PubMed.
- K. W. M. Boere, B. G. Soliman, D. T. S. Rijkers, W. E. Hennink and T. Vermonden, Macromolecules, 2014, 47, 2430–2438 CrossRef CAS.
- Y. Cui, W. Shan, M. Liu, L. Wu and Y. Huang, J. Mater. Chem. B, 2017, 5, 1302–1314 RSC.
- S. K. Filippov, P. Chytil, P. V. Konarev, M. Dyakonova, C. Papadakis, A. Zhigunov, J. Plestil, P. Stepanek, T. Etrych, K. Ulbrich and D. I. Svergun, Biomacromolecules, 2012, 13, 2594–2604 CrossRef CAS PubMed.
- V. Kumar, I. Khan and U. Gupta, Appl. Nanosci., 2020, 10, 4049–4062 CrossRef CAS.
- J. Chiefari, Y. K. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo and S. H. Thang, Macromolecules, 1998, 31, 5559–5562 CrossRef CAS.
- H. Jatzkewitz, Naturforscher, 1995, 10, 27–31 CrossRef.
- S. L. Baker, B. Kaupbayeva, S. Lathwal, S. R. Das, A. J. Russell and K. Matyjaszewski, Biomacromolecules, 2019, 20, 4272–4298 CrossRef CAS PubMed.
- K. Kapil, H. Murata, G. Szczepaniak, A. J. Russell and K. Matyjaszewski, ACS Macro Lett., 2024, 13, 461–467 CrossRef CAS PubMed.
- M. Dollin, A. R. Szkurhan and M. K. Georges, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 5487–5493 CrossRef CAS.
- H. R. Lamontagne and B. H. Lessard, ACS Appl. Polym. Mater., 2020, 2, 5327–5344 CrossRef CAS.
- G. Kim, C. Piao, J. Oh and M. Lee, Nanoscale, 2018, 10, 8503–8514 RSC.
- R. T. A. Mayadunne, E. Rizzardo, J. Chiefari, Y. K. Chong, G. Moad and S. H. Thang, Macromolecules, 1999, 32, 6977–6980 CrossRef CAS.
- L. Barner, J. F. Quinn, C. Barner-Kowollik, P. Vana and T. P. Davis, Eur. Polym. J., 2003, 39, 449–459 CrossRef CAS.
- R. T. A. Mayadunne, E. Rizzardo, J. Chiefari, J. Krstina, G. Moad, A. Postma and S. H. Thang, Macromolecules, 2000, 33, 243–245 CrossRef CAS.
- R. Francis and A. Ajayaghosh, Macromolecules, 2000, 33, 4699–4704 CrossRef CAS.
- C. W. Scales, Y. A. Vasilieva, A. J. Convertine, A. B. Lowe and C. L. McCormick, Biomacromolecules, 2005, 6, 1846–1850 CrossRef CAS PubMed.
- M. A. Bag and L. M. Valenzuela, Int. J. Mol. Sci., 2017, 18, 1422 CrossRef PubMed.
- H. Maeda, H. Nakamura and J. Fang, Adv. Drug Delivery Rev., 2013, 65, 71–79 CrossRef CAS PubMed.
- N. Nishiyama, Y. Matsumura and K. Kataoka, Cancer Sci., 2016, 107, 867–874 CrossRef CAS PubMed.
- P. Chytil, L. Kostka and T. Etrych, J. Pers. Med., 2021, 11, 115 CrossRef PubMed.
- S. Pytlíková, M. Pechar, P. Chytil, M. Studenovský, R. Pola, L. Kotrchová, R. Konefał, L. Čtveráčková, R. Laga, J. Pankrác, S. Gao, B. Jiang, K. Yang, J. Fang, M. Filipová and T. Etrych, Eur. Polym. J., 2024, 205, 112756 CrossRef.
- J. Yang and J. Kopeček, Curr. Opin. Colloid Interface Sci., 2017, 31, 30–42 CrossRef CAS PubMed.
- Y. Matsumura, Adv. Drug Delivery Rev., 2011, 63, 184–192 CrossRef CAS PubMed.
- A. Gothwal, I. Khan and U. Gupta, Pharm. Res., 2015, 33, 18–39 CrossRef PubMed.
- K. Miyata, R. J. Christie and K. Kataoka, React. Funct. Polym., 2011, 71, 227–234 CrossRef CAS.
- J. Gong, M. Chen, Y. Zheng, S. Wang and Y. Wang, J. Controlled Release, 2012, 159, 312–323 CrossRef CAS PubMed.
- K. Osada, R. J. Christie and K. Kataoka, J. R. Soc., Interface, 2009, 6, S3225–S6339 CrossRef PubMed.
- R. Wei, L. Cheng, M. Zheng, R. Cheng, F. Meng, C. Deng and Z. Zhong, Biomacromolecules, 2012, 13, 2429–2438 CrossRef CAS PubMed.
- S. K. Filippov, J. M. Franklin, P. V. Konarev, P. Chytil, T. Etrych, A. Bogomolova, M. Dyakonova, C. M. Papadakis, A. Radulescu, K. Ulbrich, P. Stepanek and D. I. Svergun, Biomacromolecules, 2013, 14, 4061–4070 CrossRef CAS PubMed.
- Y. Shi, M. J. Van Steenbergen, E. A. Teunissen, L. Novo, S. Gradmann, M. Baldus, C. F. Van Nostrum and W. E. Hennink, Biomacromolecules, 2013, 14, 1826–1837 CrossRef CAS PubMed.
- Z. Zhou, L. Li, Y. Yang, X. Xu and Y. Huang, Biomaterials, 2014, 35, 6622–6635 CrossRef CAS PubMed.
- O. Naksuriya, Y. Shi, C. F. Van Nostrum, S. Anuchapreeda, W. E. Hennink and S. Okonogi, Eur. J. Pharm. Biopharm., 2015, 94, 501–512 CrossRef CAS PubMed.
- L. Kostka, L. Kotrchová, V. Šubr, A. Libánská, C. A. Ferreira, I. Malátová, H. J. Lee, T. E. Barnhart, J. W. Engle, W. Cai, M. Šírová and T. Etrych, Biomaterials, 2020, 235, 119728 CrossRef CAS PubMed.
- S. Okonogi, O. Naksuriya, S. Charumanee and J. Sirithunyalug, Sci. Pharm., 2016, 84, 625–633 CrossRef CAS PubMed.
- Y. Shi, R. Van Der Meel, B. Theek, E. Oude Blenke, E. H. E. Pieters, M. H. A. M. Fens, J. Ehling, R. M. Schiffelers, G. Storm, C. F. Van Nostrum, T. Lammers and W. E. Hennink, ACS Nano, 2015, 9, 3740–3752 CrossRef CAS PubMed.
- X. Xu, L. Li, Z. Zhou, W. Sun and Y. Huang, Int. J. Pharm., 2016, 507, 50–60 CrossRef CAS PubMed.
- A. Braunová, L. Kostka, L. Sivák, L. Cuchalová, Z. Hvězdová, R. Laga, S. Filippov, P. Černoch, M. Pechar, O. Janoušková, M. Šírová and T. Etrych, J. Controlled Release, 2017, 245, 41–51 CrossRef PubMed.
- E. Koziolová, D. Machová, R. Pola, O. Janoušková, P. Chytil, R. Laga, S. K. Filippov, V. Šubr, T. Etrych and M. Pechar, J. Mater. Chem. B, 2016, 4, 7620–7629 RSC.
- M. Sponchioni, U. C. Palmiero and D. Moscatelli, Macromol. Chem. Phys., 2017, 218, 1700380 CrossRef.
- D. Machová, E. Koziolová, P. Chytil, K. Venclíková, T. Etrych and O. Janoušková, Eur. J. Pharm. Biopharm., 2018, 131, 141–150 CrossRef PubMed.
- M. Bagheri, J. Bresseleers, A. Varela-Moreira, O. Sandre, S. A. Meeuwissen, R. M. Schiffelers, J. M. Metselaar, C. F. Van Nostrum, J. C. M. Van Hest and W. E. Hennink, Langmuir, 2018, 34, 15495–15506 CrossRef CAS PubMed.
- I. Biancacci, Q. Sun, D. Möckel, F. Gremse, S. Rosenhain, F. Kiessling, M. Bartneck, Q. Hu, M. Thewissen, G. Storm, W. E. Hennink, Y. Shi, C. J. F. Rijcken, T. Lammers and A. M. Sofias, J. Controlled Release, 2020, 328, 805–816 CrossRef CAS PubMed.
- Y. Bobde, T. Patel, M. Paul, S. Biswas and B. Ghosh, Colloids Surf., B, 2021, 204, 111833 CrossRef CAS PubMed.
- Y. Wang, M. J. van Steenbergen, N. Beztsinna, Y. Shi, T. Lammers, C. F. van Nostrum and W. E. Hennink, J. Controlled Release, 2020, 328, 970–984 CrossRef CAS PubMed.
- M. Sheybanifard, N. Beztsinna, M. Bagheri, E. M. Buhl, J. Bresseleers, A. Varela-Moreira, Y. Shi, C. F. van Nostrum, G. van der Pluijm, G. Storm, W. E. Hennink, T. Lammers and J. M. Metselaar, Int. J. Pharm., 2020, 584, 119409 CrossRef CAS PubMed.
- M. Bagheri, M. H. Fens, T. G. Kleijn, R. B. Capomaccio, D. Mehn, P. M. Krawczyk, E. M. Scutigliani, A. Gurinov, M. Baldus, N. C. H. Van Kronenburg, R. J. Kok, M. Heger, C. F. Van Nostrum and W. E. Hennink, Mol. Pharmaceutics, 2021, 18, 1247–1263 CrossRef CAS PubMed.
- Y. Wang, D. M. E. Thies-Weesie, E. D. C. Bosman, M. J. van Steenbergen, J. van den Dikkenberg, Y. Shi, T. Lammers, C. F. van Nostrum and W. E. Hennink, J. Controlled Release, 2022, 343, 338–346 CrossRef CAS PubMed.
- R. M. Al-Nakashli, C. Cao, R. Raveendran, H. Lu, M. H. Stenzel, R. M. Al-Nakashli, C. Cao, R. Raveendran, M. H. Stenzel and H. Lu, Macromol. Chem. Phys., 2023, 224, 2200179 CrossRef CAS.
- B. Fortuni, T. Inose, M. Ricci, Y. Fujita, I. Van Zundert, A. Masuhara, E. Fron, H. Mizuno, L. Latterini, S. Rocha and H. Uji-i, Sci. Rep., 2019, 9, 1–13 CrossRef CAS PubMed.
- N. Karra and S. Benita, Curr. Drug Metab., 2011, 13, 22–41 CrossRef PubMed.
- L. Basile, R. Pignatello and C. Passirani, Curr. Drug Delivery, 2012, 9, 255–268 CrossRef CAS PubMed.
- R. Vivek, V. Nipun Babu, R. Thangam, K. S. Subramanian and S. Kannan, Colloids Surf., B, 2013, 111, 117–123 CrossRef CAS PubMed.
- J. D. Kingsley, H. Dou, J. Morehead, B. Rabinow, H. E. Gendelman and C. J. Destache, J. Neuroimmune Pharmacol., 2006, 1, 340–350 CrossRef PubMed.
- I. Denis, F. El Bahhaj, F. Collette, R. Delatouche, F. Gueugnon, D. Pouliquen, L. Pichavant, V. Héroguez, M. Grégoire, P. Bertrand and C. Blanquart, Biomacromolecules, 2014, 15, 4534–4543 CrossRef CAS PubMed.
- A. Gad, J. Kydd, B. Piel and P. Rai, Int. J. Nanomed. Nanosurgery, 2016, 2, 1–5 Search PubMed.
- K. Ulbrich, K. Holá, V. Šubr, A. Bakandritsos, J. Tuček and R. Zbořil, Chem. Rev., 2016, 116, 5338–5431 CrossRef CAS PubMed.
- E. Jäger, A. Jäger, P. Chytil, T. Etrych, B. Říhová, F. C. Giacomelli, P. Štěpánek and K. Ulbrich, J. Controlled Release, 2013, 165, 153–161 CrossRef PubMed.
- Y. Yang, D. Pan, K. Luo, L. Li and Z. Gu, Biomaterials, 2013, 34, 8430–8443 CrossRef CAS PubMed.
- Z. Duan, Y. Zhang, H. Zhu, L. Sun, H. Cai, B. Li, Q. Gong, Z. Gu and K. Luo, ACS Appl. Mater. Interfaces, 2017, 9, 3474–3486 CrossRef CAS PubMed.
- Z. Duan, Y. Zhang, H. Zhu, L. Sun, H. Cai, B. Li, Q. Gong, Z. Gu and K. Luo, ACS Appl. Mater. Interfaces, 2017, 9, 3474–3486 CrossRef CAS PubMed.
- X. Wei, Q. Luo, L. Sun, X. Li, H. Zhu, P. Guan, M. Wu, K. Luo and Q. Gong, ACS Appl. Mater. Interfaces, 2016, 8, 11765–11778 CrossRef CAS PubMed.
- K. Chen, S. Liao, S. Guo, H. Zhang, H. Cai, Q. Gong, Z. Gu and K. Luo, Sci. China Mater., 2018, 61, 1462–1474 CrossRef CAS.
- E. A. Lomkova, P. Chytil, O. Janoušková, T. Mueller, H. Lucas, S. K. Filippov, O. Trhlíková, P. A. Aleshunin, Y. A. Skorik, K. Ulbrich and T. Etrych, Biomacromolecules, 2016, 17, 3493–3507 CrossRef CAS PubMed.
- J. Wang, N. Li, L. Cao, C. Gao, Y. Zhang, Q. Shuai, J. Xie, K. Luo, J. Yang and Z. Gu, J. Mater. Chem. B, 2020, 8, 1157–1170 RSC.
- M. Tang, M. Zhou, Y. Huang, J. Zhong, Z. Zhou and K. Luo, Polym. Chem., 2017, 8, 2370–2380 RSC.
- D. Gündel, M. Allmeroth, S. Reime, R. Zentel and O. Thews, Int. J. Nanomed., 2017, 12, 5571–5584 CrossRef PubMed.
- Y. Liu, Z. Zhou, X. Lin, X. Xiong, R. Zhou, M. Zhou and Y. Huang, Biomacromolecules, 2019, 20, 3755–3766 CrossRef CAS PubMed.
- K. Chen, S. Liao, S. Guo, X. Zheng, B. Wang, Z. Duan, H. Zhang, Q. Gong and K. Luo, Chem. Eng. J., 2020, 391, 123543 CrossRef CAS.
- S. Rani, R. K. Sahoo, K. T. Nakhate, A. Ajazuddin and U. Gupta, Int. J. Pharm., 2020, 579, 119173 CrossRef CAS PubMed.
- N. Du, W. Guo, Q. Yu, S. Guan, L. Guo, T. Shen, H. Tang and Z. Gan, Polym. Chem., 2016, 7, 5719–5729 RSC.
- Z. Plichta, D. Horák, D. Mareková, K. Turnovcová, R. Kaiser and P. Jendelová, ChemMedChem, 2020, 15, 96–104 CrossRef CAS PubMed.
- R. K. Sahoo, V. Kumar, S. Batheja and U. Gupta, Nanomedicine-Based Approaches for the Treatment of Dementia, 2023, pp. 71–88 Search PubMed.
- H. Singh, S. Tarannum, R. K. Sahoo, V. Kumar and U. Gupta, Smart Polymeric Nano-Constructs in Drug Delivery: Concept, Design and Therapeutic Applications, 2023, pp. 289–328 Search PubMed.
- V. Kumar, R. K. Sahoo, T. Basak, T. Yasmin, U. Gupta and A. K. Goyal, Front. Biosci.-Landmark, 2021, 26, 518–536 CrossRef CAS PubMed.
- V. Kumar and U. Gupta, J. Drug Delivery Sci. Technol., 2023, 84, 104466 CrossRef CAS.
- V. Kumar, M. Rana, A. K. Sharma, S. Sinha, A. Ajazuddin and U. Gupta, J. Drug Delivery Sci. Technol., 2023, 89, 105058 CrossRef CAS.
- R. Esfand and D. A. Tomalia, Drug Discovery Today, 2001, 6, 427–436 CrossRef CAS PubMed.
- D. Luong, P. Kesharwani, R. Deshmukh, M. C. I. Mohd Amin, U. Gupta, K. Greish and A. K. Iyer, Acta Biomater., 2016, 43, 14–29 CrossRef CAS PubMed.
- U. Gupta, S. K. D. Dwivedi, H. K. Bid, R. Konwar and N. K. Jain, Int. J. Pharm., 2010, 393, 186–197 CrossRef PubMed.
- S. Sadekar, A. Ray, M. Janàt-Amsbury, C. M. Peterson and H. Ghandehari, Biomacromolecules, 2011, 12, 88–96 CrossRef CAS PubMed.
- S. Sadekar, O. Linares, G. J. Noh, D. Hubbard, A. Ray, M. Janát-Amsbury, C. M. Peterson, J. Facelli and H. Ghandehari, Drug Delivery Transl. Res., 2013, 3, 260–271 CrossRef CAS PubMed.
- H. Nakamura, E. Koziolová, T. Etrych, P. Chytil, J. Fang, K. Ulbrich and H. Maeda, Eur. J. Pharm. Biopharm., 2015, 90, 90–96 CrossRef CAS PubMed.
- H. Kostková, L. Schindler, L. Kotrchová, M. Kovář, M. Šírová, L. Kostka and T. Etrych, J. Nanomater., 2017, 2017, 8675435 Search PubMed.
- R. Mohammadpour, S. Safarian, B. Buckway and H. Ghandehari, Macromol. Biosci., 2017, 17, 1600333 CrossRef PubMed.
- D. Pan, X. Zheng, Q. Zhang, Z. Li, Z. Duan, W. Zheng, M. Gong, H. Zhu, H. Zhang, Q. Gong, Z. Gu and K. Luo, Adv. Mater., 2020, 32, 1907490 CrossRef CAS PubMed.
- C. Luo, J. Sun, B. Sun and Z. He, Trends Pharmacol. Sci., 2014, 35, 556–566 CrossRef CAS PubMed.
- J. Rautio, H. Kumpulainen, T. Heimbach, R. Oliyai, D. Oh, T. Järvinen and J. Savolainen, Nat. Rev. Drug Discovery, 2008, 7, 255–270 CrossRef CAS PubMed.
- S. S. Chandran, A. Nan, D. M. Rosen, H. Ghandehari and S. R. Denmeade, Mol. Cancer Ther., 2007, 6, 2928–2937 CrossRef CAS PubMed.
- Z. H. Peng, Y. Xie, Y. Wang, J. Li and D. Oupický, Mol. Pharmaceutics, 2017, 14, 1395–1404 CrossRef CAS PubMed.
- Y. Dai, X. Ma, Y. Zhang, K. Chen, J. Z. Tang, Q. Gong and K. Luo, Biomater. Sci., 2018, 6, 2979–2986 RSC.
- K. Chen, H. Cai, H. Zhang, H. Zhu, Z. Gu, Q. Gong and K. Luo, Acta Biomater., 2019, 84, 339–355 CrossRef CAS PubMed.
- D. Vinciguerra, S. Denis, J. Mougin, M. Jacobs, Y. Guillaneuf, S. Mura, P. Couvreur and J. Nicolas, J. Controlled Release, 2018, 286, 425–438 CrossRef CAS PubMed.
- D. Vinciguerra, M. Jacobs, S. Denis, J. Mougin, Y. Guillaneuf, G. Lazzari, C. Zhu, S. Mura, P. Couvreur and J. Nicolas, J. Controlled Release, 2019, 295, 223–236 CrossRef CAS PubMed.
- L. Luo, F. Xu, H. Peng, Y. Luo, X. Tian, G. Battaglia, H. Zhang, Q. Gong, Z. Gu and K. Luo, J. Controlled Release, 2020, 318, 124–135 CrossRef CAS PubMed.
- H. Cai, X. Dai, X. Wang, P. Tan, L. Gu, Q. Luo, X. Zheng, Z. Li, H. Zhu, H. Zhang, Z. Gu, Q. Gong, K. Luo, H. Cai, X. M. Wang, P. Tan, L. Gu, Q. Luo, X. L. Zheng, Z. Q. Li, H. Y. Zhu, Z. W. Gu, Q. Y. Gong, K. Luo, X. H. Dai and H. Zhang, Adv. Sci., 2020, 7, 1903243 CrossRef CAS PubMed.
- L. Luo, Y. Qi, H. Zhong, S. Jiang, H. Zhang, H. Cai, Y. Wu, Z. Gu, Q. Gong and K. Luo, Acta Pharm. Sin. B, 2022, 12, 424–436 CrossRef CAS PubMed.
- H. Tang, J. Zhang, J. Tang, Y. Shen, W. Guo, M. Zhou, R. Wang, N. Jiang, Z. Gan and Q. Yu, Biomacromolecules, 2018, 19, 2849–2862 CrossRef CAS PubMed.
- H. Nsairat, D. Khater, U. Sayed, F. Odeh, A. Al Bawab and W. Alshaer, Heliyon, 2022, 8, e09394 CrossRef CAS PubMed.
- D. Lombardo and M. A. Kiselev, Pharmaceutics, 2022, 14, 543 CrossRef CAS PubMed.
- R. Tenchov, J. M. Sasso and Q. A. Zhou, Bioconjugate Chem., 2023, 34, 941–960 CrossRef CAS PubMed.
- K. R. Whiteman, V. Subr, K. Ulbrich and V. P. Torchilin, J. Liposome Res., 2001, 11, 153–164 CrossRef CAS PubMed.
- L. Paasonen, B. Romberg, G. Storm, M. Yliperttula, A. Urtti and W. E. Hennink, Bioconjugate Chem., 2007, 18, 2131–2136 CrossRef CAS PubMed.
- Y. Mo, H. Du, B. Chen, D. Liu, Q. Yin, Y. Yan, Z. Wang, F. Wan, T. Qi, Y. Wang, Q. Zhang and Y. Wang, ACS Biomater. Sci. Eng., 2019, 5, 2316–2329 CrossRef CAS PubMed.
- J. Khandare and T. Minko, Prog. Polym. Sci., 2006, 31, 359–397 CrossRef CAS.
- X. Pang, H. L. Du, H. Q. Zhang, Y. J. Zhai and G. X. Zhai, Drug Discovery Today, 2013, 18, 1316–1322 CrossRef CAS PubMed.
- J. H. Baker, K. E. Lindquist, L. A. Huxham, A. H. Kyle, J. T. Sy and A. I. Minchinton, Clin. Cancer Res., 2008, 14, 2171–2179 CrossRef CAS PubMed.
- D. W. Northfelt, B. J. Dezube, J. A. Thommes, B. J. Miller, M. A. Fischl, A. F. Kien, L. D. Kaplan, C. Du Mond, R. D. Mamelok and D. H. Henry, J. Clin. Oncol., 1998, 16, 2445–2451 CrossRef CAS PubMed.
- L. Li, Y. Li, C. H. Yang, D. C. Radford, J. Wang, M. Janát-Amsbury and J. Kopecek, Adv. Funct. Mater., 2020, 30, 1–26, DOI:10.1002/adfm.201908961.
- L. Li, J. Wang, D. C. Radford, J. Kopeček and J. Yang, J. Controlled Release, 2021, 332, 652–659 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |





