Open Access Article
Open Access ArticleDetection and degradation of microplastics in the environment: a review
Saba Yousafzai a,
Mujahid Farid*a,
Muhammad Zubair
a,
Mujahid Farid*a,
Muhammad Zubair *b,
Nafeesa Naeem
*b,
Nafeesa Naeem b,
Wardha Zafarb,
Zaki ul Zaman Asama,
Sheharyaar Faridc and
Shafaqat Alid
b,
Wardha Zafarb,
Zaki ul Zaman Asama,
Sheharyaar Faridc and
Shafaqat Alid
aDepartment of Environmental Sciences, University of Gujrat, Hafiz Hayat Campus, Gujrat, 50700, Pakistan. E-mail: mujahid.farid@uog.edu.pk
bDepartment of Chemistry, University of Gujrat, Hafiz Hayat Campus, Gujrat, 50700, Pakistan. E-mail: muhammad.zubair@uog.edu.pk
cEarth and Ocean Sciences, Schools of Natural Sciences and Ryan Institute, University of Galway, Ireland
dDepartment of Environmental Sciences, Government College University, Faisalabad, 38000, Pakistan
First published on 30th June 2025
Abstract
Microplastics (MPs) are a growing environmental concern due to their persistence in the environment and potential negative impacts on human health and the ecosystem. Their widespread presence across terrestrial, aquatic, and atmospheric compartments has prompted an urgent need for improved detection techniques and effective degradation strategies. This review provides an integrated overview of recent advancements in the identification and removal of MPs, with a focus on both analytical and remediation technologies. Progress in spectroscopic, thermal, and imaging-based methods has enabled more precise detection, quantification, and characterization of MPs, particularly at the nano-scale. Simultaneously, a variety of degradation strategies have been developed to mitigate the environmental burden of MPs. These are broadly categorized into physical, chemical, and biological approaches. Physical methods include mechanical removal and thermal processes such as pyrolysis and thermal oxidation. Chemical degradation involves advanced oxidation processes (AOPs) and photocatalysis using semiconductors like titanium dioxide (TiO2) to accelerate polymer breakdown under light exposure. Among biological approaches, enzymatic and microbial degradation have shown promising results. Enzymes such as PETase, MHETase, cutinases, lipases, and cellulases catalyze the hydrolysis of ester and amide bonds in synthetic polymers, offering selective and environmentally benign pathways for microplastic decomposition. The review further explores the implications of microplastic accumulation, including bioaccumulation and oxidative stress in organisms, and discusses the limitations and challenges of current technologies. Emphasis is placed on integrating detection with degradation strategies to achieve sustainable, scalable, and interdisciplinary solutions. By highlighting the latest scientific advancements, this review aims to guide future research directions and support the development of effective policy and management frameworks for mitigating microplastic pollution.
Environmental significanceMicroplastic pollution has emerged as a critical environmental challenge, with pervasive distribution across terrestrial, freshwater, and marine ecosystems. These microscopic plastic fragments originate from various anthropogenic sources, including industrial processes, synthetic textiles, and the degradation of larger plastic debris. Due to their persistence, bioaccumulative potential, and capacity to adsorb hazardous contaminants, microplastics pose a substantial risk to ecological integrity and human health. Their ingestion by aquatic organisms can lead to trophic transfer, bioamplification, and physiological disruptions, further exacerbating ecosystem imbalances. Consequently, the development of efficient detection methods and sustainable degradation strategies is imperative to mitigate their environmental impact. This review critically evaluates recent advancements in microplastic detection techniques, including spectroscopic, chromatographic, and imaging-based approaches, alongside emerging degradation strategies such as bioremediation, photodegradation, and advanced oxidation processes. Understanding these mechanisms is essential for formulating effective policies and technological interventions aimed at reducing microplastic contamination and preserving environmental sustainability. |
1. Introduction
Microplastics, minute plastic particles measuring less than 5 mm, have emerged as a pervasive and intricate environmental concern, representing a global challenge that transcends geographical boundaries and ecosystems.1 Originating from the fragmentation of larger plastic items, industrial processes, and the breakdown of synthetic materials, these microscopic pollutants have infiltrated terrestrial, freshwater, and marine environments, raising critical questions about their occurrence, fate, and overarching environmental impacts.2,3 But marine habitats, encompassing shorelines, open oceans, and deep seas, are primary areas of focus for microplastics (MPs) research. Currently, over 96% of studies on MPs are related to marine systems. It is estimated that around 4.8 to 12.7 million tons of plastic waste are dumped into the ocean each year from terrestrial sources.4 Collectively 98% of primary MPs stems from land-based activities and only 2% are come from activities directly related to the ocean. Rivers play a significant role in conveying microplastics from inland areas to the oceans.5 The ubiquity of microplastics underscores their resilience and the complex pathways through which they enter ecosystems.6 Their presence has been documented in diverse environmental matrices, including soils, sediments, and aquatic systems, necessitating a comprehensive review to synthesize the current state of knowledge and address critical gaps in understanding.7,8 Microplastics can trace their origins to a myriad of sources, from the wear and tear of car tyres to the disintegration of plastic packaging and the breakdown of synthetic fibers from textiles.9–11Globally, in the present era, the excessive use of plastic materials and their disposal is a threat for the environment. Following their introduction into the environment, plastic waste endures a gradual degradation process, yielding numerous smaller plastic particles via interplay of physical, chemical, and biological mechanisms.12 This microplastic pollution is increasingly recognized as a significant global environmental concern.13
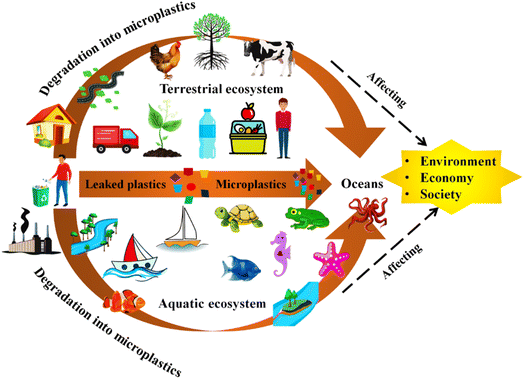
Approximately, 280 million tons of microplastics entering into our environment through different sources and their concentration increases with the passage of time.14 However, locating the precise source of microplastics detected in the environment is challenging, if not unattainable.15 Major sources of MPs include domestic sewage, beads, fibers from clothing and personal care products, fertilizer,16 biosolids,17,18 vinyl mulch heavily used in agricultural activities,19,20 illegal waste dumping and littering,21 dispersion from the landfills, water flooding, irrigation with wastewater, tyre abrasion and transportation of atmospheric particles22,23 (Fig. 1).
2. Microplastics
Predominantly microplastics originate from the degradation and fragmentation of larger plastic objects, which occurs through thermal, oxidative, and microbial processes. In fragmentation process, which is carried out through mechanical, chemical and biological processes that convert it into small-size particles.25,26 Many studies conducted by scientist considered that ‘Microplastics’ are emerging pollutants based on their size (5 mm).27,28 Microplastics, the fastest-growing source of aquatic environmental pollution, is becoming a major challenge for the world community. Many of the world scientists working in the area of the environment have reported that less than 10% of plastic products are recyclable and reused again whereas remaining released into the environment to be converted into challenging MPs.29–31According to an estimate, more than 80% of plastic enters into the oceans to make aquatic pollution from land which is freely disposed of on land without any control.32 In terrestrial ecosystems, the contamination of MPs might be 4–23 folds higher than in the oceans,16 whereas soil has more capacity to accumulate plastic debris than aquatic ecosystems.33 Plastic pollution is caused by smaller size particles (1 to <1000 mm) which are unpredictable, persistent and abundantly found in terrestrial, freshwater and marine ecosystems.34 Generally, it takes decades to centuries to completely degrade in a natural environment due to high stability and durability of plastic materials. As a result, it is crucial to focus on the pollution of MPs in environment as a serious issue (Fig. 2).
Plastic particles of sizes ranging between 1.0 nm to 1.0 μm, 1–5 mm, 5–25 mm and greater than 25 mm, were categorized as nano, micro, meso and macro plastics, respectively. The particulates of microplastics vary in composition, colour, density and dimension, and are classified into various categories. Considering their usage and source, microplastics (MPs) are classified into primary and secondary. Among various types of plastics, the most significant is non-fiber plastics production which includes polypropylene (PP), polyvinylchloride (PVC), polyethylene terephthalate (PET) and polyethylene (PE).29,35 Microplastic particles are known for having a high capacity of adsorption and desorption of organic pollutants.36
Primary microplastics are microscopically formed and present in personal care products such as scrubbers, toothpaste and other cosmetics.37 These kinds of microplastics are skin care product, and contains various naturally used cosmetics including almonds, walnuts and oatmeal.38 Small plastic particulates, typically about 0.25 mm in size, are widely utilized in industrial abrasives as shot-blasting agents and beauty products. Microplastic particulates having dimensions like powders and granules are commonly used in a wider variety of applications.28,39 Microplastic particulates display variable sizes, such as different-sized granules in the same product. Primary microplastics are released directly into the environment from sewage and domestic factories. Plastic pellets from the manufacturing industries, plastic fluff or powder used to produce plastic goods, plastic resin flakes, commercial cleaning abrasives and scrubbers,2 together with volatile particulate pollutants including nano SiO2 and Fe3O4 from printing toners and micro-polyester, are the possible origins of primary microplastics.40
Microplastic beads are also concluded as constituents in skin-cleansing personal care products, in face and hand scrubs, and for improving the viscosity of toothpaste.41 Gradual environmental degradation and high demand for plastic materials have become a major threat worldwide. Microplastic beads from skin care products will be carried through the sewage system with wastewater and will not be efficiently eradicated by sewage and therefore concentrate in the ecosystem.42 Besides the volatile release of primary microplastics, large fragments of plastics can gradually become fragile under the influence of heat and UV and then broken down into smaller particles by mechanical forces such as ocean currents and winds.43 The majority of microplastics produced in the aquatic environment are caused by the breakdown of larger plastics resulting in secondary microplastics.44 Degradation of large-sized plastics depends on the amount of UV rays and temperature.45 The sources of primary and secondary microplastics (MPs) have grown from different pathways of plastic fragmentation as illustrated in Fig. 3.
Along with airborne fragmentation, many other materials disintegrate during use, creating micro-sized particles in the air such as fibers released from clothes during washing.46 Fabricated clothes comprising microplastics as fibers shed approximately 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fibers from six kg of fabric in one wash.47 Microplastic pellets which are used as feedstock in industries for plastic products are also a cause of microplastics introduced to the environment. In the therapeutic area of research, microplastics are utilized in pharmaceutical and dental carriers entering the environment by wastewater. The low perceptibility and compact size of primary microplastics make them interesting to isolate from the aquatic environment.48
000 fibers from six kg of fabric in one wash.47 Microplastic pellets which are used as feedstock in industries for plastic products are also a cause of microplastics introduced to the environment. In the therapeutic area of research, microplastics are utilized in pharmaceutical and dental carriers entering the environment by wastewater. The low perceptibility and compact size of primary microplastics make them interesting to isolate from the aquatic environment.48
Secondary microplastics are produced from microbial fragmentation of already existing large plastics subsequently into macro-, micro- and nano-sizes, and incremental disintegration by wave abrasion, and UV light. Some other mechanical actions such as intense weathering result in the fragmentation of plastics, thus mounting secondary microplastics in the aqueous system greater than the primary microplastics.49
Before settling into the environment because of weathering, like ultraviolet radiation from sunlight, hydrolysis, biodegradation, photo-degradation, wave action and exposure to wind abrasion are the potential paths to produce secondary microplastics.50 The chemical structure of some major types of plastic found in microplastics (MPs) is illustrated in Fig. 4.
Likewise, the degradation process (Fig. 5), which highlights ways to create secondary microplastics, produced from the slow fragmentation of plastics in water, contains three procedures such as bio-deterioration, assimilation and bio-fragmentation presenting emphasized, impactful microplastic generation routes.51
Microplastics have the ability to adsorb and concentrate various chemical pollutants from the surrounding environment, including persistent organic pollutants (POPs) and heavy metals. When ingested, these chemical-laden microplastics may release toxins, leading to harmful physiological effects in organisms, disrupting endocrine systems, and causing developmental abnormalities.52 Microplastics can cause physical harm to organisms at the cellular and tissue levels. Their abrasive nature can lead to internal injuries and inflammation. Behavioral changes, such as altered feeding patterns, reproductive disruptions, and reduced foraging efficiency, have been observed in organisms exposed to microplastics (Fig. 6).53
Microplastics are often mistaken for food by marine and terrestrial organisms. Ingestion can lead to physical harm, blockage of digestive tracts, and malnutrition. Bioaccumulation of microplastics can occur as they move up the food chain, potentially reaching concentrations that may have adverse effects on higher trophic levels, including humans.54 Microplastics can accumulate in soil through various pathways, including the application of plastic mulches, sewage sludge, and the breakdown of plastic debris.55 Soil-dwelling organisms may be exposed to microplastics, impacting soil health and potentially influencing plant–microbe interactions. The marine environment is particularly vulnerable to microplastic pollution.56 Floating microplastics can absorb sunlight and affect ocean temperature, potentially disrupting marine ecosystems. Microplastics in the ocean can interact with marine organisms, such as corals, affecting their health and contributing to the overall degradation of marine habitats57 (Fig. 7).
The environmental persistence of microplastics is a significant concern. These particles are resistant to natural degradation processes, leading to their accumulation in the environment over extended periods. In marine ecosystems, microplastics have been found to interfere with the feeding behavior, growth, and reproductive health of various aquatic organisms.58,59 Moreover, their ability to adsorb and transport hazardous chemicals, such as persistent organic pollutants and heavy metals, exacerbates their ecological risks.60,61
Human exposure to microplastics is an emerging area of concern. Studies have reported the presence of microplastics in human tissues, including the liver, kidneys, and even the brain, raising questions about their potential health implications.62,63 The ingestion and inhalation of microplastics may lead to inflammatory responses, oxidative stress, and other adverse health effects.11,53
Despite the growing body of research on microplastics, significant knowledge gaps remain, particularly concerning their sources, transport mechanisms, and long-term effects on both ecosystems and human health. This review provides the latest research on microplastics, focusing on the crucial aspects of detection and degradation. By providing a comprehensive overview of current methodologies and emerging strategies, this review aims to guide future research endeavors and inform the development of effective solutions to mitigate the environmental impact of microplastics.
3. Detection of microplastics in environment
Quick separation and analysis of compositions of microplastics from terrestrial and aquatic systems have become high on the research agenda. By means of visual and combined analytical and visual techniques, a lot of research work was done for the identification and quantification of microplastics. Generally, the identification of MPs can be achieved by using two analytical state-of-the-art techniques: chemical characterization (spectroscopic) following physical characterization (microscopy) for the confirmation of plastics.64 Four basic stages, including density separation, filtration, sieving, and visual categorization of microplastics, are mandatory prior to identification. These 4 preliminary procedures can easily classify the morphological characteristics (color, size and shape) of large-sized fragments of microplastics.65Additionally, fluorescence as well as density separations offer a simple and sensitive technique to characterize the most frequent fragments of plastic polymers in marine sediments.66 In addition, multiple techniques were used resulting in a consensus, which may be the best information for detection methods, as demonstrated by the high precision.67 Therefore, methods that can be used for the identification of these microplastics and their smaller-size plastic particulates are significant for the process of identification, and well-known methods are detailed below.
3.1 Visual detection of MPs
An optical or naked-eye microscope with 10–50× magnification objectives is used for visual identification. Sometimes, software for image analysis such as Olympus and Histolab Stream has been used with the microscope.68 Visual identification has been used in the majority (almost 79%) to characterize microplastics for the reason that it is feasible and worthwhile to use. But this technique is not suitable for identifying MPs as non-plastic particle particulates like paint chips, fly/coil ash, viscose rayon, keratin and cellulose, which can hamper the method, leading to false positives and this method faces problems in detecting translucent particles and particles below 100 μm in size. Sequentially, for the optimization of methods for the digestion of cigarette filters, cotton clothing fibers and human hair, the staining method (rapid screening of microplastics done by using Nile Red dye) and wet peroxide oxidation (WPO) can be used.69Another specialized technique consists of an advanced digestion step that combines nitric acid (HNO3) and sodium hydroxide to digest all organic material in 1 hour, with a separate separation step that separates the minerals using sodium iodide (NaI) for reduction of residues in samples if required. With the exception of polyamide, this technique presented a recovery rate of 95% for microplastics and all investigated forms of polymers were obtained with only slight variations in color, size and weight.70 Also, if the scanning electron microscope (SEM) is included, which envisions the surface characteristic features of the particles, it will be a convenient task for research purposes. By scanning the material surface with an intense beam of electrons, this technique provides high-quality pictures. Additionally, particle discrimination is possible because of finer sample images (>0.5 nm). Still, the SEM fails to determine the composition of polymers.
It has been reported in the literature that the percentage of plastic particulates which were detected by stereomicroscopy, and then analyzed by different other methods, is around 20 to 70% of the total plastic particles, in the case of transparent plastic particles.72 Additionally, the stereomicroscope is insufficient to identify natural and synthetic fibers.73,74 The identification of microplastics using a stereomicroscope depends on their physical form. This is the foremost rapid inspection technique that permits fast characterization of color, size and shape of the plastic particles which will be additionally analyzed by some other approaches. Therefore, there is a requirement to couple the stereomicroscopy with other methods like spectroscopy.
The restriction in the visual scrutiny of microplastics is exhibited by the chemical additives added in the synthetic procedure that have also impact on the fluorescent properties.77 For instance, additives can also display fluorescent properties and affect the measurements by fluorescence microscopy.78 Consequently, it is essential to remove these impurities as much as possible with suitable pretreatment approaches.79 The rinsing of surfaces with oxidants such as hydrogen peroxide or acids, and digestion by enzymes is generally used.80 These pretreatment approaches can only eliminate contaminants or surface impurities, but they do not have the ability to decrease the promising inferences from the chemicals confined within the microplastics.
Conventional SEM is used in many research works to visualize microplastics in various matrices such as sand,81 sediments,82 mussels83 and sewage sludge.84 Field emission scanning electron microscopy (FE-SEM) is used as an alternative that operates at low voltage and provides high-magnification and high-resolution images of microplastic samples without special treatment for MPs prior to observation. This is a feasible and fast method because the microplastic is not covered with carbon or any metal, but the pieces of microplastic are placed directly on the aluminum stub of the carbon tape.
3.2 Analytical detection of MPs
Along with Raman spectroscopy, FT-IR is extensively practiced for the characterization of microplastics.86 In each research work, microplastic samples are excited to produce specific vibrations that yield a spectrum within the fingerprint range. This FT-IR spectrum details the nature of the microplastic, which can be detected by comparison with reported standard spectra. Large-sized particles greater than 500 nm can be examined using Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR), but smaller-sized particles require micro-FTIR (μ-FTIR) which requires concurrent imaging, and recording, and provides definite spectra. This can be achieved in both reflection and ATR mode.
The types of ingested plastic polymers have been identified in sea turtles by definitive ATR-FTIR validation.87 Attenuated total reflectance (ATR) provides high-resolution FT-IR spectra but requires an infrared-transparent substrate. The lateral resolution is confined to a diffraction limit and microplastics smaller than 20 μm cannot be detected. Micro-FTIR has been generally used in the field of microplastics for their determination and characterization in food, surface water,88 marine organisms and sediment.89 This method allows for obtaining infrared signals at a high spatial resolution, also valuable for the analysis of complex microplastics. Micro-FTIR analysis has been performed on MP contamination in surface sediments from 28 positions in Seychelles Bay, classifying eight types of polymers like polyurethane (PU), polymethyl methacrylate (PMMA), polystyrene (PS), polyethylene terephthalate (PET), polyamides (PA), polypropylene (PP), polyethylene (PE) and rayon.90
DSC is a valuable method to analyze polymeric materials, but it needs to be known as standard materials. Certainly, it is generally practiced to identify primary microplastics like polyethylene (PE) microbeads having well-defined characteristic features.92 However, the utilization of DSC to analyze plastics like acrylonitrile butadiene (ABS), polycarbonate (PC), polystyrene (PS) and polymethyl methacrylate (PMMA) among others, has restrictions because these plastics have long-ranged melting points. Additionally, the quantitation of the mass of microplastics present in the environment was also described in the literature.93 Amongst other limitations, DSC outcomes lack particularity when analyzing a combination of microplastics with an overlap of melting peaks or closely spaced melting points.
In TGA, mass loss is determined, while the disintegration of polymer-based materials starts normally by changing the enthalpy. Variations in enthalpy cannot be spotted in thermogravimetric analysis but can be detected through DSC measurements. Consequently, a mixture of two techniques is recommended to analyze microplastics.94 Various microplastics can be identified, but this detection is not possible for other types of polymers because of their overlapped phase transition signals.
Pyrolysis gas chromatography-mass spectrometry (Py-GC-MS) is one of the analytical techniques that has been effectively applicable to analyze plastic materials. It is perfect for the simultaneous identification as well as quantification of the most commonly found microplastics in complex samples. The semi-quantitative scheming for every single form of existing plastics, mainly for minute concentrations such as parts per billion (ppb) or lower than ppb, signifies the precise identification of the polymeric materials in the animal and environmental microplastics and the external calibration curve (Table 1).
| Detection method | Principle | Detected material | Minimum size | Advantages | Limitation | Ref. |
|---|---|---|---|---|---|---|
| Focal plane array-based micro-Fourier-transform infrared spectroscopy | Employs focal plane array detectors to conduct infrared imaging, enabling the identification of microplastics concentrated on membrane filters through their distinct spectral signatures | Microplastics of the polymer polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS), polyurethane (PUR), polyethylene terephthalate (PET), other polyesters (PES) and co-polyamide (PAs) | Detection of particles down to 20 μm | High lateral resolution, faster than traditional chemical mapping, and suitable for standard procedures | Requires further automation for fully automated analysis; initial setup may need specialized equipment and expertise | 95 |
| Raman spectroscopy | Identify microplastics by detecting molecular vibrations when exposed to laser light, providing a molecular fingerprint | All types of microplastics | Microplastics including those as small as 2–3 μm in plankton | Can identify extremely small microplastics, perform well in wet samples, generate spatial chemical images, complementary to FTIR | Weak signal, fluorescence interference, influenced by color, additives, and attached contaminants. Real-time monitoring is a significant challenge | 96 and 97 |
| Stimulated Raman scattering (SRS) | Uses two laser beams to generate a signal when their photon energy difference matches a vibrational state of molecules in the sample | PET = polyethylene terephthalate; PS = polystyrene; PP = polypropylene; PE = polyethylene | Very fast mapping speeds, significantly reduced signal acquisition time per pixel, suitable for high-throughput screening, non-invasive | 98 | ||
| Nylon | ||||||
| Pyrolysis-gas chromatography-mass spectrometry (Pyro-GC-MS) | Pyrolyzes single polymer particles under inert conditions, cryo-traps and separates thermal degradation products using a chromatographic column, identifies products via mass spectrometry | PE, PP, PET, PS PA | Suitable for single particle analysis, provides chemical identification of polymers, relatively low sample amount required (0.1–0.5 mg) | Time-consuming pre-selection of particles necessary, Prone to contamination during pyrolysis, limited by the sample amount (0.1–0.5 mg) | 99 | |
| Pyrolysis-gas chromatography-mass spectrometry (Pyro-GC-MS) | Pyrolyzes single polymer particles under inert conditions, cryo-traps and separates thermal degradation products using a chromatographic column, identifies products via mass spectrometry | PE, PP, PET, PS PA | Suitable for single particle analysis, provides chemical identification of polymers, relatively low sample amount required (0.1–0.5 mg) | Time-consuming pre-selection of particles necessary, Prone to contamination during pyrolysis, limited by the sample amount (0.1–0.5 mg) | 99 | |
| Liquid chromatography | Uses solvents and size exclusion chromatography for polymer characterization | All types of microplastics | Analyzes high masses, improves representativeness | Destructive, limited to chemical composition, requires large sample amounts | 79 | |
| X-ray fluorescence (XRF) spectroscopy | Excitation of sample with X-rays, resulting in emission of characteristic fluorescent X-rays, which are detected and analyzed to determine elemental composition | Beached microplastics (including Br, Cd, Cl, Cr, Cu, Fe, Pb, and Zn) | A few millimeters to a few micrometers | Rapid and non-destructive analysis, capable of characterizing elemental composition of synthetic polymers | Limited spatial resolution, potential interferences from sample matrix | 100 |
| Scanning electron microscopy (SEM) with energy-dispersive X-ray microanalysis | Microscopic imaging of microplastic particles coupled with energy-dispersive X-ray microanalysis to identify inorganic plastic additives (IPAs) | Inorganic plastic additives (IPAs) | Microscopic level | High-resolution imaging of microplastic particles, detection and identification of inorganic plastic additives (IPAs) | Limited to identification of inorganic plastic additives (IPAs), time-consuming analysis process | 101 |
| Pressurized fluid extraction | Utilizes solvents at subcritical temperature and pressure conditions to extract microplastics from environmental samples | All types of microplastics | Submicron | Provides a simple analytical method for quantifying common microplastics in a range of environmental samples | Requires optimization of PFE conditions, potential variability in extraction efficiency | 102 |
The thermal analysis serves as an alternate technique to spectroscopy to identify some polymers. However, it is a damaging method that prevents the analysis of microplastics with some other resulting approaches. Therefore, it is detrimental to utilize these types of analytical approaches to identify microplastics, but these approaches may facilitate the initial examination of large samples and subsequent investigation by spectroscopic methods.103
4. Degradation pathways
Microplastics (MPs) can be cleaned using traditional cleaning techniques.104 For instance, removing waste plastics from the beach, such as packaging, bags, and mess containers, can reduce the amount of plastic that enters rivers and the ocean, which helps to prevent the creation of SMPs. Green technologies to dispose of the gathered plastics are still lacking, though. The conventional approaches to treating plastic waste, such as landfilling or burning, are not the best ways to address the problem of MP contamination. The safe treatment of plastic garbage through incineration is quite effective, but it carries a danger of producing greenhouse gases such as carbon dioxide (CO2), methane, and carbon monoxide (CO).105,106 Today, several innovative strategies were proposed to lessen the pressing threat posed by MPs.Since microplastics have a larger surface-to-volume ratio than macroplastic detritus, they degrade through the same mechanisms more quickly. Smaller counterparts of the original microplastic particles, known as nanoplastics, will eventually replace them.107–111 Mechanical (abiotic), chemical (abiotic), and biological (biotic) degradation are the three basic categories that are taken into consideration, and in most cases, substantial particle degradation occurs when these processes are interconnected112 (Fig. 8).
4.1 Mechanical degradation
MPs subjected to mechanical abrasion result in particles with low sharpness of particle edges, resembling the morphology of natural sediment grains subjected to repeated abrasion in high energy conditions or long transit lengths. Conchoidal fractures and grooves on surfaces have been proven to be indicators of mechanical weathering in studies using scanning electron microscopy (SEM).113–118 As a result, beaches are the best places in nature for abrasion of microplastic debris to be present.118 Under simulated beach and offshore circumstances, polyethylene (PE) films were experimentally investigated. According to Kalogerakis et al. (2017), exposure to sunlight and mechanical stress increased the rate of weathering. The latter involves filling bottles with sand and inserting plastic strips before rotating the bottles continuously for 24 hours. The plastic lost 14% of its weight, which was represented by tiny particles that couldn't be seen with the naked eye called microplastics. This experiment demonstrates that polymer deterioration can happen as a result of mechanical abrasion alone.119In a study by Cooper and Corcoran (2010), plastic debris collected from the beaches of Kauai, Hawaii, exhibited surface features indicative of mechanical degradation, such as fractures, pits, and grooves. Scanning Electron Microscopy (SEM) analyses revealed that these physical alterations, resulting from wave action and sand abrasion, contributed to the embrittlement and subsequent fragmentation of polyethylene (PE) and polypropylene (PP) materials.114
Additional laboratory studies have measured the mechanical degradation rates under controlled conditions. As an example, in mesocosm experiments that mimic marine conditions, polyethylene films with mild mechanical stress following five months of weathering had a weight loss of around 13.9% to 16.7%, suggesting major fragmentation to the micro-sized particles.119
Recent research has also investigated the cumulative effects of photo-aging and mechanical stirring on plastic fragmentation. Haremaki et al. (2025) illustrated that photo-aged polypropylene (PP) samples, under water stirring, broke down into microplastics with sizes from 1 to 30 micrometers. The fragment size distribution shifted from exponential to power-law function as the stirring time increased, indicating an increasing fragmentation mechanism controlled by mechanical forces.120
Additionally, the synergistic action of ultraviolet (UV) radiation and mechanical abrasion has been demonstrated to enhance the degradation of different polymers. Under laboratory tests mimicking beach conditions, low-density polyethylene (LDPE), PP, and expanded polystyrene (EPS) pellets subjected to UV radiation followed by mechanical abrasion yielded considerably greater amounts of microplastic fragments than mechanical abrasion alone. For instance, PP pellets exposed to 12 months of UV and two months of mechanical abrasion produced around 6084 ± 1061 particles per pellet, while mechanical abrasion alone without initial UV exposure produced merely 10.7 ± 0.7 particles per pellet.121
These results highlight the significance of mechanical degradation as a dominant pathway for microplastic production in the environment. Quantifying the rates and mechanisms of mechanical fragmentation is essential in evaluating the environmental fate of plastic litter and designing efficient mitigation strategies.
4.2 Chemical degradation
The degree of chemical degradation of microplastics varies based on the kind of polymer, the presence of additives (UV stabilizers), as well as the depositional environment and medium.122–124 For instance, it is anticipated that microplastics on beach surfaces may absorb more UV radiation than particles suspended in the water column or buried in benthic soil.125–127A more brittle polymer that is susceptible to mechanical abrasion and/or biodegradation is produced as a result of the weight reduction. The hydrolysis of aromatic polyesters, like PET, can result in shorter chains and the formation of terephthalic acid and ethylene glycol in environments where biodegradation, abrasion and photooxidation are not possible. These environments include landfills and the ocean floor.112
Both chemical and surface signs are present showing that chemical weathering activities have taken place. Microplastics' physical characteristics fluctuate with time, causing color changes, mechanical property loss that causes surface fissures and embrittlement, and molecular weight fluctuations.128 Wider fractures produced by crazing result in a polymer that is more brittle and is linked to a reduction in tensile strength. Over-oxidation of the phenolic chemicals found in the polymer causes yellowing or discoloration.129,130
4.3 Degradation of MPs via advanced oxidation process
Chemical-catalytic degradation of MPs through advanced oxidation processes (AOPs) is a relatively new field of study, and in the past few years, there has been a notable interest in this area because of the environmental persistence of MPs.131 AOPs are a collection of chemical treatments that employ reactive species, including hydroxyl radicals (OH˙), to oxidize pollutants in wastewater and water. UV/H2O2, Fenton, and ozonation are some of the most common AOPs. These treatment processes are efficient in degrading various types of pollutants such as polychlorinated biphenyls (PCBs), polyaromatic hydrocarbons (PAHs), and polycyclic musk compounds (PCMs).104,132Over the past few years, there has been a study of using AOPs to degrade MPs, specifically focusing on polyethylene (PE) and polypropylene (PP), which are the most frequently utilized polymers in consumer products. It has been found through studies that AOPs are able to degrade MPs effectively, with total mineralization being recorded in some instances. For instance, in a study by Li et al. (2018), UV/H2O2 was employed to degrade PE films and determined that complete mineralization was achieved, with the major products being CO2 and H2O.133 Another study by Xiong et al. (2019) employed ozonation to degrade PP beads and determined that the process led to the production of carboxylic acids, which are degradation intermediates of PP.134 Nonetheless, not all studies have established that AOPs are efficient in degrading MPs. For instance, Wang et al. (2019) reported that Fenton oxidation did not lead to complete PE film degradation, and that the process led to the generation of smaller particles instead of complete mineralization.135,136
Furthermore, AOPs can also generate by-products such as hydroxyl radicals, superoxide radicals, and hydrogen peroxide, which are toxic to aquatic life, and therefore the use of AOPs in the degradation of MPs should be judicious. AOPs, especially the Fenton reaction, have been utilized to oxidize many different MPs.137
In another study by Liu et al. (2024), ultrahigh-molecular-weight polyethylene (UHMWPE), low-density polyethylene (LDPE), high-density polyethylene (HDPE), polystyrene (PS), polyvinyl chloride (PVC), polypropylene (PP), and polyethylene terephthalate (PET) MPs underwent a thermal Fenton reaction at 140 °C.138 Treatment consisted of 4 mM FeSO4 and 200 mM H2O2, where appreciable weight loss was observed for all the types of polymers, which is an indication of effective degradation. For example, UHMWPE MPs showed about a 30% weight loss following treatment.137 AOPs employ reactive oxygen species (ROS) like hydroxyl radicals (˙OH) to non-selectively oxidize organic pollutants. For example, a study proved that the treatment of polystyrene (PS) microplastics with a mixture of hydrogen peroxide (H2O2) and ultraviolet (UV) light led to a 95.9% weight loss in 16 hours, with a mineralization efficiency of 75.6% after 12 hours. Scanning Electron Microscopy (SEM) analyses revealed extensive surface erosion and fragmentation of the PS particles.138
In conclusion, chemical-catalytic degradation of MPs through AOPs is a promising study that can possibly degrade MPs efficiently. Nevertheless, more studies have to be carried out to develop the conditions in AOPs to fully mineralize MPs as well as avoid the formation of by-products harmful to aquatic animals.139
4.4 Degradation of MPs via photocatalysis
Chemical-catalytic degradation of microplastics (MPs) via photocatalysis is an emerging research area that has gained significant attention in recent years due to the persistence of MPs in the environment.140 Photocatalysis is a technique whereby a semiconductor material, for example, titanium dioxide (TiO2), serves as a catalyst to break down contaminants under the influence of light. The mechanism entails the absorption of light by the semiconductor, producing electron–hole pairs. The holes may subsequently react with oxygen or water to produce hydroxyl radicals (OH˙), which are extremely reactive and can break down contaminants.141One of the greatest challenges in photocatalytic degradation of MPs is the poor light absorption of MPs, leading to low photocatalysis efficiency. To address this, scientists have been investigating the use of various photocatalysts, including zinc oxide (ZnO), or the combination of MPs with other materials to enhance their light absorption. Besides, photocatalysis can also generate by-products like hydroxyl radicals, superoxide radicals, and hydrogen peroxide that are harmful to aquatic life. Therefore, the use of photocatalysis in MP degradation should be carried out with caution.142
Photocatalysis involves light-activated catalysts to produce reactive species that degrade polymer chains. TiO2 nanoparticles are among the most investigated photocatalysts. Zandieh et al. (2024) reported that LDPE films coated with 10 wt% TiO2 and exposed to UV light (36 W at 315 nm) for 360 hours achieved a weight loss of 78%.143 Similarly, PS microspheres subjected to UV light (256 W at 254 nm) for 12 hours showed a 98% weight loss.143
Other nanomaterials, such as ZnO, MnO2, and Cu2O, have also demonstrated photocatalytic activity. For example, PE microplastics treated with a GO/TiO2 composite under UV light (72 W at 350 nm) for 8 hours exhibited a 50% weight loss.143
Photocatalytic degradation employs semiconductors like titanium dioxide (TiO2) activated by UV or visible light to generate ROS, facilitating polymer breakdown. In one study, polyethylene (PE) microplastics exposed to TiO2 under UV irradiation exhibited a degradation rate constant of 2.6 × 10−7 h−1 for particles sized between 10 and 150 nm. The degradation efficiency was influenced by factors such as particle size, light intensity, and catalyst concentration (Table 2).144
| Photocatalysts used | Polymer | Degradation (%) | Time (h) | Irradiation source | Ref. |
|---|---|---|---|---|---|
| ZnO NRs (ZnO NRs) | Polypropylene | 65 | 360 | Visible light | 145 |
| Polypyrrole/TiO2 (PPy/TiO2) | Polyethylene | 35.4 | 240 | Sunlight | 146 |
| Ag/TiO2/RGO | Polyethylene | 76 | 4 | UV light | 147 |
| ZnO NRs | Low density polyethylene | 16–38 | >100 | UV-vis | 148 |
| TiO2/ZnO | Polyamide microfibers | 97 | <100 | UV | 149 |
| TiO2 | PE | 86 | 300 | UV | 150 |
| C,N–TiO2/SiO2 | PET | 16 | UV-vis | 151 | |
| Graphene oxide-based metal oxide | Polyethylene | 35–50 | 20 | Ultraviolet light | 152 |
| BiOI–Fe3O4 | Polystyrene | 64 | 8 | Visible light | 153 |
4.5 Biological degradation of MPs
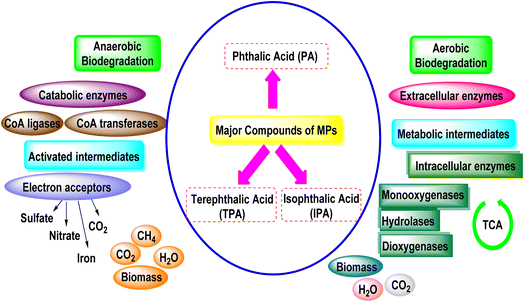
Biodegradation is the process through which plastic waste is digested by microorganisms to produce smaller products, according to Pathak et al. (2017).154 The following processes are used to carry out this process: colonization, bio-fragmentation, assimilation, and mineralization. When a microplastic particle's surface comes into touch with a body of ambient water, a conditioning film forms over the piece, which is necessary for biofilms to form.155 According to Rummel et al. (2017), the chemical composition of the film has a major role in determining the kinds of organisms that sorb onto it.156Exoenzymes are released by organisms once the microplastic surface has been colonized, and they cause the polymer to break down into oligomers, dimers, or monomers.157 The term “plastisphere”158 used to refer to the colonized surface area of microplastics is often used to refer to a microbial population forming in a biofilm.158 A wide variety of microorganisms, including bacteria, algae, fungi, and bryozoa, can be found in the plastisphere.158,159 Zalerion maritimum has been shown to degrade PE by Paço et al. (2017).160 Assimilation can only begin once the original plastic particle has been broken up into pieces small enough for the molecules to pass the cell walls of microorganisms. The molecules can be employed as sources of energy and carbon once assimilation has occurred. This last stage, called mineralization, results in the production of CO2, H2, and CH4. Additionally, this final stage completes the carbon biogeochemical cycle.157 Carbon dioxide, H2O, and CH4 can be produced during aerobic or anaerobic biodegradation, respectively. In landfill environments, anaerobic conditions can occur naturally, and Zhang et al. (2022) have described the five stages of deterioration that take place there. The authors demonstrate through their analysis of published studies that the biodegradation rates of the most prevalent environmental polymers (polyolefins) are significantly lower in landfills than those of their biodegradable equivalents. Therefore, aerobic biodegradation is more productive.161 Environmental factors, including the climate, salinity, amount of light, and atmospheric contaminants, may have an impact on biodegradation when aerobic conditions are present.162
The degradation of microplastic waste can be slowed down by colonization even though biological activity can help to break down microplastics in the environment. According to research by Long et al. (2015) and Rummel et al. (2017),156 colonizing microbes can make microplastic particles denser, which causes them to sink through the water column or shift their vertical orientation.163
4.6 Pathway of microplastics biodegradation
A number of processes are involved in the microbial biodegradation of MPs, including: (1) initial degradation of polymers in smaller particles from large polymeric structures; (2) polymer degradation to oligomer, dimer, and monomer; and (3) mineralization of MPs by microbial biomass.164 The complete mineralization of MPs into carbon dioxide is depicted in Fig. 9, along with the transformation of the intermediates that were created into a source of energy and biomass production. The hydrophilicity of plastic polymers is increased by microorganisms' extracellular enzymes, which include esterase, lipase, lignin peroxides, laccase, and manganese peroxides. Examples of hydrolases include lipases, esterases, poly(3-hydroxybutyrate) depolymerases, and cutinases (Fig. 9). These enzymes may attach certain sensitive bonds to the side chains of polymers or chemical groups to the polymer chain to facilitate chain cleavage during biodegradation. Due to their size, they are not likely to diffuse into the polymer structure; therefore, the degradation may take place on the surface, leading to cracks.165Studies have shown that a wide variety of microorganisms are capable of degrading MPs, including bacteria, fungi, and algae. For instance, Besseling et al. (2018) discovered that bacteria belonging to the genus Pseudomonas and Sphingomonas could degrade PE.169 Chen et al. (2019) discovered that fungi belonging to the genus Aspergillus and Penicillium could degrade PP.170 The conditions under which microorganisms degrade MPs also affect the efficiency of degradation. For instance, researchers have established that the availability of nutrients like nitrogen and phosphorus can promote degradation of MPs by microorganisms. Not all studies, however, have reported that microorganisms degrade MPs. For instance, Wang et al. (2019) conducted a study in which they detected that degradation of PE films by bacteria was extremely slow and that the process led to the generation of small particles and not total mineralization.171 Moreover, micro-biological degradation of MPs can also generate by-products that are toxic to aquatic life. Therefore, use of micro-biological degradation of MPs should be carried out cautiously and with further studies to realize the possible effects on aquatic animals.172
In a typical process of biodegradation, polymers are subjected to the microbial community after initially permitting microorganisms to adhere to their surfaces. Subsequently, the polymer was subjected to extracellular enzymes, which resulted in the polymer interacting with them and starting to disintegrate into lower molecular-weight polymers.173 In conclusion, micro-biological degradation of MPs is a promising area of research that can potentially degrade MPs effectively. Experiments have indicated that a broad range of microorganisms can degrade MPs, but the conditions under which microorganisms degrade MPs also influence the efficiency of degradation (Fig. 10). More research is required to determine the best conditions for micro-biological degradation of MPs and to reduce the formation of by-products that are toxic to aquatic life (Table 3).174
| Microorganism | Polymer | Degradation (%) | Time (days) | Ref. |
|---|---|---|---|---|
| Aspergillus niger | Polyethylene (PE) | ∼40 | 90 | 157 |
| Achatina fulica land snail | Polystyrene (PS) | 31 | 28 | 175 |
| Arthrobacter | PE | 12 | — | 176 |
| Pseudomonas | PE | 15 | — | 176 |
| Bacillus sp. and Paenibacillus sp. | PE | 15 | 60 | 177 |
| Bacillus cereus | PE | 2 | 40 | 178 |
| PET | 7 | |||
| PS | 7 | |||
| Bacillus gottheilii | PE | 6 | 40 | 178 |
| PET | 3 | |||
| PP | 4 | |||
| PS | 6 | |||
| Ideonella sakaiensis | PET | ∼75 | 60 | 179 |
| Pseudomonas putida | PS | ∼30 | 56 | 180 |
| Bacillus subtilis | Polyurethane (PU) | ∼50 | 60 | 181 |
| Rhizopus arrhizus | Polyvinyl chloride (PVC) | ∼33 | 60 | 182 |
| Penicillium simplicissimum | Low-density polyethylene (LDPE) | ∼25 | 90 | 183 |
| Streptomyces sp. | Polycaprolactone (PCL) | ∼85 | 30 | 184 |
| Phanerochaete chrysosporium | PE | ∼20 | 60 | 185 |
| Thermobifida fusca | PET | ∼70 | 60 | 186 |
| Alcaligenes faecalis | Polylactic acid (PLA) | ∼6 | 40 | 187 |
The enzymes that are commonly used for the degradation of MPs include lipases, esterases, and cellulases. Studies have shown that a wide variety of enzymes are capable of degrading MPs. For example, Yang et al. (2018) found that a lipase from the fungus Rhizopus oryzae was able to degrade PE.188 Another study by Li et al. (2017)89 found that a cellulase from the bacterium Bacillus subtilis was able to degrade PP.189 The conditions under which enzymes degrade MPs also have an impact on the efficiency of degradation. For example, studies have shown that the presence of surfactants can enhance the degradation of MPs by enzymes. Additionally, the pH and temperature of the reaction also play a role in the efficiency of enzyme-mediated degradation of MPs. However, this method's PET conversion rate is still insufficient to keep up with the rate at which plastic trash is produced, and the enzymes are now too expensive (Fig. 11). Inspired by the LCC enzyme's strong potential for destroying long-lasting semi-aromatic polyesters like PET.190
Enzymatic degradation of MPs offers a sustainable and environmentally benign approach to mitigating plastic pollution. This process involves specific enzymes that catalyze the hydrolysis of polymer chains, leading to the breakdown of complex plastics into monomers or oligomers. Key enzymes in this context include hydrolases, esterases, lipases, and cutinases, each exhibiting distinct mechanisms of action and substrate specificities. Hydrolases (EC 3.1.1) encompass a broad class of enzymes that catalyze the cleavage of ester bonds through the addition of water molecules. In the context of polyethylene terephthalate (PET) degradation, hydrolases such as PETase and MHETase have been identified. PETase initiates the hydrolysis of PET into mono(2-hydroxyethyl) terephthalate (MHET), which is subsequently degraded by MHETase into terephthalic acid (TPA) and ethylene glycol (EG).191 Cutinases (EC 3.1.1.74) are serine esterases that naturally degrade cutin, a structural component of plant cuticles. These enzymes possess an exposed active site, allowing them to interact with a variety of hydrophobic substrates, including synthetic polyesters like PET. Cutinases catalyze the hydrolysis of ester bonds within the polymer backbone, leading to the formation of monomers such as TPA and EG. Notably, cutinases from Fusarium solani and Humicola insolens have demonstrated significant PET-degrading capabilities.192–194 Esterases (EC 3.1.1.1) preferentially hydrolyze short-chain aliphatic esters and have been implicated in the degradation of various biodegradable plastics. For instance, esterases from Comamonas acidovorans have been shown to degrade low molecular weight polylactic acid (PLA), while those from Aspergillus flavus and Aspergillus tubingensis can degrade polybutylene succinate (PBS). The enzymatic action involves the cleavage of ester bonds, resulting in the formation of monomers or oligomers that can be further assimilated by microorganisms.195,196 Lipases (EC 3.1.1.3) catalyze the hydrolysis of long-chain triglycerides and have been employed in the degradation of various synthetic polymers. Lipases from Candida antarctica (CALB) and Rhizopus delemar have demonstrated efficacy in degrading polymers such as PET and polycaprolactone (PCL). The mechanism involves the cleavage of ester bonds within the polymer matrix, leading to the release of monomeric units.191 The enzymatic degradation process typically follows a surface erosion mechanism, where enzymes adsorb onto the polymer surface and catalyze the hydrolysis of accessible ester bonds. This results in the gradual breakdown of the polymer into smaller fragments, which can then be further degraded or assimilated by microorganisms.197 Factors influencing the efficiency of enzymatic degradation include the crystallinity of the polymer, surface area, and the presence of additives or contaminants.195 Combining different enzymes can enhance the degradation efficiency of complex polymers. For example, the sequential application of cutinase and lipase has been shown to result in a more complete depolymerization of PET, with cutinase initiating the breakdown of the polymer and lipase further degrading the resulting oligomers.191
The enzymatic degradation of microplastics has emerged as a highly promising and increasingly researched strategy within the broader framework of sustainable plastic remediation.198 Enzymes, particularly those derived from microbial sources, offer a selective, efficient, and environmentally benign approach to breaking down synthetic polymers into simpler, non-toxic compounds. Among the most studied are PET-hydrolyzing enzymes such as PETase and MHETase, originally identified in Ideonella sakaiensis, which catalyze the depolymerization of polyethylene terephthalate (PET) into its monomeric constituents.199
Recent advances have extended to the engineering of mutant variants with enhanced thermal stability, catalytic efficiency, and substrate specificity, enabling faster degradation under environmentally relevant conditions. Moreover, enzymes like cutinases, lipases, laccases, and peroxidases have shown activity against a broader range of polymers, including polyethylene (PE), polypropylene (PP), and polyurethane (PU), although their mechanisms and degradation pathways remain under active investigation.200
Enzymatic strategies are also being integrated with pretreatment technologies, such as mechanical shredding, photooxidation, or chemical modification, to enhance polymer surface accessibility and increase enzymatic affinity. Immobilization techniques, such as the anchoring of enzymes onto solid supports or nanoparticles, further improve their reusability and operational stability, making them suitable for incorporation into wastewater treatment and remediation systems.201
However, challenges remain in scaling up enzymatic degradation for field applications, including enzyme production costs, environmental robustness, and reaction kinetics. As such, ongoing research is focused on metagenomic screening, synthetic biology, and directed evolution to discover and optimize novel enzymatic systems capable of degrading a wider spectrum of microplastic types under diverse environmental conditions.202
The growing body of literature underscores the transformative potential of enzyme-based technologies in microplastic mitigation. These biocatalytic approaches not only support the degradation of existing pollutants but also provide insights into the design of next-generation biodegradable plastics and circular economy models.203
In summary, enzymatic degradation of MPs is a promising research area that has the potential to effectively degrade MPs. Studies have shown that a wide variety of enzymes are capable of degrading MPs, however, the conditions under which enzymes degrade MPs also have an impact on the efficiency of degradation. Further research is needed to understand the optimal conditions for enzymatic degradation of MPs and to minimize the production of by-products that can be toxic to aquatic organisms.
5. Current solutions for microplastic pollution
The solutions can be further grouped into three broad categories: source control, cleanup and treatment, and public awareness and education.204 Source control measures try to prevent the amount of microplastics entering the environment in the very first place. This may include steps like the prohibition or the imposition of taxation on single-use plastics, encouragement of the usage of biodegradable alternatives, and regulations in order to govern the release of microplastics from industries such as textiles and personal care products.204Treatment and cleanup methods target the elimination of microplastics from the environment after their release. Treatments involve physical extraction methods, such as beach cleaning and utilization of specialized boats or equipment to extract microplastics from the surface of the ocean. In addition, technologies that will extract microplastics from water are being designed, for example, membranes and filtration devices, and advanced oxidation processes (AOPs) are also under investigation to break down the plastics.205 Education and public awareness solutions seek to make the issue of microplastic pollution more known and raise consciousness among people and institutions to act to minimize their use of plastics and disposed them in an appropriate manner. These measures comprise educational campaigns, public education campaigns, and the creation of environmentally friendly consumer products.
In general, three different categories-containment, mitigation, and separation of remedies have been put up to reduce microplastic pollution.204 All of them aim to stop the spread of microplastics into the environment by taking action at crucial sources. Our current focus is on separation, which is a method of removing microplastics from wastewater during processing. Recycling and landfilling are the main methods of containment.206 The majority of plastic items require physical segregation in a landfill for effective disposal at the end of their useful lives. In order to confine the secondary microplastics that break off plastic debris, well-maintained landfills are built to reduce leaching. However, new research indicates that landfill runoff is high in microplastics206 and pollutes neighboring soil and natural water, spreading microplastic pollution. Furthermore, poor waste disposal and microplastic contamination are decreased by tougher littering legislation. In addition to these laws, several governments have supported educational initiatives to promote proper garbage disposal methods including recycling and using trash cans.207,208
Efforts to mitigate microplastic pollution encompass a wide range of strategies, including both conventional and emerging approaches. Traditional methods largely involve physical removal techniques such as filtration, sedimentation, and coagulation in wastewater treatment plants. While these approaches are effective to a degree, they often fail to capture the smallest microplastic particles, particularly those below 10 micrometers in size. Additionally, end-of-pipe solutions are limited in scope and do not address the upstream sources of microplastics.205,209
In response to these limitations, research has increasingly focused on innovative and multidisciplinary strategies. One promising avenue involves the development of advanced filtration materials, such as nanofiber membranes and bio-based filters, which exhibit enhanced selectivity and adsorption capacity for micro- and nanoplastics.210 Magnetic separation techniques, using functionalized magnetic nanoparticles, are also under investigation for their potential to capture microplastics from aqueous media with high efficiency.211
Biotechnological approaches represent another frontier in this field. Certain strains of bacteria and fungi have shown potential in biodegrading synthetic polymers into less harmful or inert compounds. For instance, species like Ideonella sakaiensis produce enzymes such as PETase that can depolymerize polyethylene terephthalate (PET) into environmentally benign products.179 Genetic engineering is being explored to enhance the efficiency and substrate specificity of these enzymatic systems.
Photocatalytic degradation, utilizing materials like titanium dioxide (TiO2) and doped zinc oxide (ZnO), has emerged as a promising method for breaking down microplastics under light irradiation. These catalysts generate reactive oxygen species (ROS) capable of fragmenting plastic polymers. Such approaches are being tailored for application in wastewater treatment systems and environmental remediation technologies.149,212
Policy innovations and behavioral interventions are increasingly recognized as essential complements to technical solutions. Regulatory measures restricting the use of microbeads in cosmetics and controlling plastic waste discharge from industrial sources have demonstrated measurable benefits. Public awareness campaigns and citizen science initiatives further contribute to pollution reduction by promoting responsible consumer behavior and waste management practices.213
Furthermore, machine learning and artificial intelligence are being integrated into monitoring systems for real-time detection and quantification of microplastics in various environmental compartments. These digital tools facilitate data-driven decision-making and the optimization of mitigation strategies.214
In summary, while conventional technologies play a critical role in managing microplastic contamination, the incorporation of advanced materials, biological degradation, catalytic processes, and digital innovation offers a more holistic and potentially transformative approach. Continued interdisciplinary research and international cooperation are pivotal to the successful deployment of these next-generation solutions in both developed and developing contexts.
6. Conclusions and future perspectives
In conclusion, MPs are a growing environmental concern due to their persistence in the environment and potential impacts on wildlife and human health. The degradation of MPs is a complex process that is influenced by a variety of factors, including the type of polymer, the size and shape of the particles, and the environmental conditions. The current research on the degradation of microplastics suggests that it can take hundreds to thousands of years for these particles to fully degrade and that they may persist in the environment for even longer. Future perspectives on the degradation of microplastics include the development of new technologies and methods for breaking down these particles more quickly and efficiently. This may include the use of enzymes, bacteria, or other microorganisms that can degrade plastics, as well as the development of new polymer materials that are more easily biodegradable. Additionally, there is a need for more research on the potential impacts of microplastics on human health, as well as the development of effective policy and management strategies to reduce the amount of microplastics entering the environment.To fully realize the potential of these strategies, future research must adopt a multidisciplinary approach that bridges material science, molecular biology, toxicology, and environmental engineering. Special emphasis should be placed on elucidating the mechanistic pathways of bioaccumulation and oxidative stress, evaluating the long-term impacts on human and ecological health, and accelerating the development of scalable and economically viable remediation technologies.
Furthermore, integration of smart monitoring systems, supported by AI and machine learning, and the implementation of robust policy measures will be critical in reducing the influx of microplastics into the environment. By synthesizing scientific innovation with regulatory and behavioral change, a holistic and adaptive framework can be established to not only reduce existing pollution but also prevent future accumulation, guiding sustainable environmental stewardship for generations to come.
Data availability
All the data is provided in the manuscript file.Author contributions
SY and MF designed the main idea and content of the article, including a write-up of different sections. MZ, NN, WZ, SF and ZA collected the data and wrote different portions of the article. NN, WZ and SF designed the graphical representation of data abstracted from different articles. SA critically reviewed the article. All the authors read and approved the final manuscript.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
The authors are thankful to the University of Gujrat, Pakistan and the Higher Education Commission of Pakistan for providing support in the interpretation of data and writing the manuscript. The present study was supported by the HEC-funded project 2017/HEC/NRPU/8996. The authors are highly thankful to the Higher Education Commission of Pakistan, the University of Gujrat, Pakistan and GC University Faisalabad, Pakistan, for providing access to literature.References
- R. C. Hale, M. E. Seeley, M. J. La Guardia, L. Mai and E. Y. Zeng, A global perspective on microplastics, J. Geophys. Res.:Oceans, 2020, 125, e2018JC014719 CrossRef.
- A. L. Andrady, Microplastics in the marine environment, Mar. Pollut. Bull., 2011, 62, 1596–1605 CrossRef CAS PubMed.
- K. D. Cox, G. A. Covernton, H. L. Davies, J. F. Dower, F. Juanes and S. E. Dudas, Human consumption of microplastics, Environ. Sci. Technol., 2019, 53, 7068–7074 CrossRef CAS.
- C. Xu, B. Zhang, C. Gu, C. Shen, S. Yin, M. Aamir and F. Li, Are we underestimating the sources of microplastic pollution in terrestrial environment?, J. Hazard. Mater., 2020, 400, 123228 CrossRef CAS PubMed.
- J. Boucher and D. Friot, Primary Microplastics in the Oceans: a Global Evaluation of Sources, IUCN, Gland, Switzerland, 2017 Search PubMed.
- A. B. Silva, A. S. Bastos, C. I. Justino, J. P. da Costa, A. C. Duarte and T. A. Rocha-Santos, Microplastics in the environment: Challenges in analytical chemistry-A review, Anal. Chim. Acta, 2018, 1017, 1–19 CrossRef CAS PubMed.
- C. M. Rochman, Microplastics research—from sink to source, Science, 2018, 360, 28–29 CrossRef CAS PubMed.
- A. A. Horton and S. J. Dixon, Microplastics: An introduction to environmental transport processes, Wiley Interdiscip. Rev.:Water, 2018, 5, e1268 CrossRef.
- X. Lim, Microplastics are everywhere—but are they harmful, Nature, 2021, 593, 22–25 CrossRef CAS PubMed.
- Z. Akdogan and B. Guven, Microplastics in the environment: A critical review of current understanding and identification of future research needs, Environ. Pollut., 2019, 254, 113011 CrossRef CAS PubMed.
- J. C. Prata, J. P. Da Costa, I. Lopes, A. C. Duarte and T. Rocha-Santos, Environmental exposure to microplastics: An overview on possible human health effects, Sci. Total Environ., 2020, 702, 134455 CrossRef CAS PubMed.
- P. Facts, An Analysis of European Plastics Production, Demand and Waste Data, Plastics Europe, 2019 Search PubMed.
- K. Zhang, A. H. Hamidian, A. Tubić, Y. Zhang, J. K. Fang, C. Wu and P. K. Lam, Understanding plastic degradation and microplastic formation in the environment: A review, Environ. Pollut., 2021, 274, 116554 CrossRef CAS PubMed.
- A. A. de Souza Machado, W. Kloas, C. Zarfl, S. Hempel and M. C. Rillig, Microplastics as an emerging threat to terrestrial ecosystems, Global Change Biol., 2018, 24, 1405–1416 CrossRef PubMed.
- L. An, Q. Liu, Y. Deng, W. Wu, Y. Gao and W. Ling, Sources of microplastic in the environment, Microplastics in Terrestrial Environments: Emerging Contaminants and Major Challenges, 2020, pp. 143–159 Search PubMed.
- A. A. Horton, A. Walton, D. J. Spurgeon, E. Lahive and C. Svendsen, Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities, Sci. Total Environ., 2017, 586, 127–141 CrossRef CAS.
- J. Talvitie, A. Mikola, O. Setälä, M. Heinonen and A. Koistinen, How well is microlitter purified from wastewater?–A detailed study on the stepwise removal of microlitter in a tertiary level wastewater treatment plant, Water Res., 2017, 109, 164–172 CrossRef CAS PubMed.
- S. Ziajahromi, P. A. Neale, L. Rintoul and F. D. Leusch, Wastewater treatment plants as a pathway for microplastics: development of a new approach to sample wastewater-based microplastics, Water Res., 2017, 112, 93–99 CrossRef CAS.
- H. Y. Sintim and M. Flury, Is biodegradable plastic mulch the solution to agriculture’s plastic problem?, Environ. Sci. Technol., 2017, 51(3), 1068–1069 CrossRef CAS PubMed.
- M. Pfister and S. Saha, Effects of biochar and fertilizer management on sunflower (Helianthus annuus L.) feedstock and soil properties, Arch. Agron. Soil Sci., 2017, 63, 651–662 CrossRef CAS.
- M. Bläsing and W. Amelung, Plastics in soil: Analytical methods and possible sources, Sci. Total Environ., 2018, 612, 422–435 CrossRef PubMed.
- M.-J. Foitzik, H.-J. Unrau, F. Gauterin, J. Dörnhöfer and T. Koch, Investigation of ultra fine particulate matter emission of rubber tires, Wear, 2018, 394, 87–95 CrossRef.
- S. Wagner, T. Hüffer, P. Klöckner, M. Wehrhahn, T. Hofmann and T. Reemtsma, Tire wear particles in the aquatic environment-a review on generation, analysis, occurrence, fate and effects, Water Res., 2018, 139, 83–100 CrossRef CAS PubMed.
- M. Carus, A. Eder, L. Dammer, H. Korte, L. Scholz, R. Essel, E. Breitmayer and M. Barth, Wood-Plastic Composites (WPC) and Natural Fibre Composites (NFC), Nova-Institute, Hürth, Germany, 2015, vol. 16 Search PubMed.
- Y. Cong, F. Jin, M. Tian, J. Wang, H. Shi, Y. Wang and J. Mu, Ingestion, egestion and post-exposure effects of polystyrene microspheres on marine medaka (Oryzias melastigma), Chemosphere, 2019, 228, 93–100 CrossRef CAS.
- B. Xu, F. Liu, P. C. Brookes and J. Xu, Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter, Environ. Pollut., 2018, 240, 87–94 CrossRef CAS PubMed.
- R. M. Blair, S. Waldron, V. Phoenix and C. Gauchotte-Lindsay, Micro-and nanoplastic pollution of freshwater and wastewater treatment systems, Springer Science Reviews, 2017, 5, 19–30 CrossRef.
- S. Sharma and S. Chatterjee, Microplastic pollution, a threat to marine ecosystem and human health: a short review, Environ. Sci. Pollut. Res., 2017, 24, 21530–21547 CrossRef PubMed.
- M. Mcnutt, Production, use, and fate of all plastics ever made, Sci. Adv., 2017, 3(7), e1700782 CrossRef PubMed.
- O. S. Alimi, J. Farner Budarz, L. M. Hernandez and N. Tufenkji, Microplastics and nanoplastics in aquatic environments: aggregation, deposition, and enhanced contaminant transport, Environ. Sci. Technol., 2018, 52, 1704–1724 CrossRef CAS PubMed.
- A. Malizia and A. C. Monmany-Garzia, Terrestrial ecologists should stop ignoring plastic pollution in the Anthropocene time, Sci. Total Environ., 2019, 668, 1025–1029 CrossRef CAS PubMed.
- M. Smith, D. C. Love, C. M. Rochman and R. A. Neff, Microplastics in seafood and the implications for human health, Curr. Environ. Health Rep., 2018, 5, 375–386 CrossRef CAS.
- L. Nizzetto, M. Futter and S. Langaas, Are agricultural soils dumps for microplastics of urban origin?, Environ. Sci. Technol., 2016, 50(20), 10777–10779 CrossRef CAS PubMed.
- N. Hartmann, T. Hüffer, R. Thompson, M. Hassellöv, A. Verschoor, A. Daugaard, S. Rist, T. Karlsson, N. Brennholt, M. Cole, M. P. Herrling, M. C. Hess, N. P. Ivleva and A. L. Lusher, Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris, Environ. Sci. Technol., 2019, 53, 1039–1047 CrossRef CAS PubMed.
- L. A. Amaral-Zettler, E. R. Zettler and T. J. Mincer, Ecology of the plastisphere, Nat. Rev. Microbiol., 2020, 18, 139–151 CrossRef CAS PubMed.
- W. Wang and J. Wang, Different partition of polycyclic aromatic hydrocarbon on environmental particulates in freshwater: microplastics in comparison to natural sediment, Ecotoxicol. Environ. Saf., 2018, 147, 648–655 CrossRef CAS PubMed.
- H. S. Auta, C. Emenike and S. H. Fauziah, Distribution and importance of microplastics in the marine environment: a review of the sources, fate, effects, and potential solutions, Environ. Int., 2017, 102, 165–176 CrossRef CAS PubMed.
- I. E. Napper, A. Bakir, S. J. Rowland and R. C. Thompson, Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics, Mar. Pollut. Bull., 2015, 99, 178–185 CrossRef CAS PubMed.
- E.-L. Ng, E. H. Lwanga, S. M. Eldridge, P. Johnston, H.-W. Hu, V. Geissen and D. Chen, An overview of microplastic and nanoplastic pollution in agroecosystems, Sci. Total Environ., 2018, 627, 1377–1388 CrossRef CAS PubMed.
- R. Jujun, L. Jia and X. Zhenming, Improvements of the recovery line of waste toner cartridges on environmental and safety performances, Environ. Sci. Technol., 2013, 47, 6457–6462 CrossRef PubMed.
- L. Anagnosti, A. Varvaresou, P. Pavlou, E. Protopapa and V. Carayanni, Worldwide actions against plastic pollution from microbeads and microplastics in cosmetics focusing on European policies. Has the issue been handled effectively?, Mar. Pollut. Bull., 2021, 162, 111883 CrossRef CAS PubMed.
- G. Kalčíková, B. Alič, T. Skalar, M. Bundschuh and A. Ž. Gotvajn, Wastewater treatment plant effluents as source of cosmetic polyethylene microbeads to freshwater, Chemosphere, 2017, 188, 25–31 CrossRef PubMed.
- R. C. Thompson, Microplastics in the marine environment: sources, consequences and solutions, Marine Anthropogenic Litter, 2015, pp. 185–200 Search PubMed.
- C. L. Waller, H. J. Griffiths, C. M. Waluda, S. E. Thorpe, I. Loaiza, B. Moreno, C. O. Pacherres and K. A. Hughes, Microplastics in the Antarctic marine system: an emerging area of research, Sci. Total Environ., 2017, 598, 220–227 CrossRef CAS PubMed.
- W. Li, H. Tse and L. Fok, Occurrence of microplastics and its pollution in the environment: a review, Sci. Total Environ., 2016, 566–567 CAS.
- F. De Falco, E. Di Pace, M. Cocca and M. Avella, The contribution of washing processes of synthetic clothes to microplastic pollution, Sci. Rep., 2019, 9, 6633 CrossRef PubMed.
- I. E. Napper and R. C. Thompson, Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions, Mar. Pollut. Bull., 2016, 112, 39–45 CrossRef CAS PubMed.
- J. P. Frias and R. Nash, Microplastics: Finding a consensus on the definition, Mar. Pollut. Bull., 2019, 138, 145–147 CrossRef CAS PubMed.
- I. L. Nerland, C. Halsband, I. Allan and K. V. Thomas, Microplastics in Marine Environments: Occurrence, Distribution and Effects, 2014 Search PubMed.
- Y. Picó and D. Barceló, Analysis and prevention of microplastics pollution in water: current perspectives and future directions, ACS Omega, 2019, 4, 6709–6719 CrossRef PubMed.
- S. M. Emadian, T. T. Onay and B. Demirel, Biodegradation of bioplastics in natural environments, Waste Manag., 2017, 59, 526–536 CrossRef CAS PubMed.
- C. Li, R. Busquets and L. C. Campos, Assessment of microplastics in freshwater systems: A review, Sci. Total Environ., 2020, 707, 135578 CrossRef CAS PubMed.
- A. D. Vethaak and J. Legler, Microplastics and human health, Science, 2021, 371, 672–674 CrossRef CAS PubMed.
- K. Blackburn and D. Green, The potential effects of microplastics on human health: What is known and what is unknown, Ambio, 2022, 51, 518–530 CrossRef PubMed.
- S. S. Sana, L. K. Dogiparthi, L. Gangadhar, A. Chakravorty and N. Abhishek, Effects of microplastics and nanoplastics on marine environment and human health, Environ. Sci. Pollut. Res., 2020, 27, 44743–44756 CrossRef CAS PubMed.
- M. S. Bhuyan, Effects of microplastics on fish and in human health, Front. Environ. Sci., 2022, 10, 250 Search PubMed.
- C. Pironti, M. Ricciardi, O. Motta, Y. Miele, A. Proto and L. Montano, Microplastics in the environment: intake through the food web, human exposure and toxicological effects, Toxics, 2021, 9, 224 CrossRef CAS PubMed.
- Y. Chae and Y.-J. An, Effects of micro-and nanoplastics on aquatic ecosystems: Current research trends and perspectives, Mar. Pollut. Bull., 2017, 124, 624–632 CrossRef CAS PubMed.
- S. L. Wright, R. C. Thompson and T. S. Galloway, The physical impacts of microplastics on marine organisms: a review, Environ. Pollut., 2013, 178, 483–492 CrossRef CAS PubMed.
- A. A. Koelmans, A. Bakir, G. A. Burton and C. R. Janssen, Microplastic as a vector for chemicals in the aquatic environment: critical review and model-supported reinterpretation of empirical studies, Environ. Sci. Technol., 2016, 50, 3315–3326 CrossRef CAS PubMed.
- J. Brahney, N. Mahowald, M. Prank, G. Cornwell, Z. Klimont, H. Matsui and K. A. Prather, Constraining the atmospheric limb of the plastic cycle, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2020719118 CrossRef CAS PubMed.
- H. A. Leslie, M. J. Van Velzen, S. H. Brandsma, A. D. Vethaak, J. J. Garcia-Vallejo and M. H. Lamoree, Discovery and quantification of plastic particle pollution in human blood, Environ. Int., 2022, 163, 107199 CrossRef CAS PubMed.
- L. C. Jenner, J. M. Rotchell, R. T. Bennett, M. Cowen, V. Tentzeris and L. R. Sadofsky, Detection of microplastics in human lung tissue using μFTIR spectroscopy, Sci. Total Environ., 2022, 831, 154907 CrossRef CAS PubMed.
- W. J. Shim, S. Hee Hong and S. Eo Eo, Identification methods in microplastic analysis, a review, Anal. Methods, 2017, 9, 1384–1391 RSC.
- V. Hidalgo-Ruz, L. Gutow, R. C. Thompson and M. Thiel, Microplastics in the marine environment: a review of the methods used for identification and quantification, Environ. Sci. Technol., 2012, 46, 3060–3075 CrossRef CAS PubMed.
- T. Maes, R. Jessop, N. Wellner, K. Haupt and A. Mayes, A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red, Sci. Rep., 2017, 7, 44501–44508 CrossRef CAS PubMed.
- J. Wagner, Z.-M. Wang, S. Ghosal, C. Rochman, M. Gassel and S. Wall, Novel method for the extraction and identification of microplastics in ocean trawl and fish gut matrices, Anal. Methods, 2017, 9, 1479–1490 RSC.
- D. Elkhatib and V. Oyanedel-Craver, A critical review of extraction and identification methods of microplastics in wastewater and drinking water, Environ. Sci. Technol., 2020, 54, 7037–7049 CrossRef CAS PubMed.
- G. Erni-Cassola, M. I. Gibson, R. C. Thompson and J. A. Christie-Oleza, Lost, but found with Nile red: a novel method for detecting and quantifying small microplastics (1 mm to 20 μm) in environmental samples, Environ. Sci. Technol., 2017, 51, 13641–13648 CrossRef CAS PubMed.
- S. Roch and A. Brinker, Rapid and efficient method for the detection of microplastic in the gastrointestinal tract of fishes, Environ. Sci. Technol., 2017, 51, 4522–4530 CrossRef CAS PubMed.
- Y. K. Song, S. H. Hong, M. Jang, G. M. Han, M. Rani, J. Lee and W. J. Shim, A comparison of microscopic and spectroscopic identification methods for analysis of microplastics in environmental samples, Mar. Pollut. Bull., 2015, 93, 202–209 CrossRef CAS PubMed.
- M. Eriksen, S. Mason, S. Wilson, C. Box, A. Zellers, W. Edwards, H. Farley and S. Amato, Microplastic pollution in the surface waters of the Laurentian Great Lakes, Mar. Pollut. Bull., 2013, 77, 177–182 CrossRef CAS PubMed.
- M. A. Browne, T. S. Galloway and R. C. Thompson, Spatial patterns of plastic debris along estuarine shorelines, Environ. Sci. Technol., 2010, 44, 3404–3409 CrossRef CAS PubMed.
- A. L. Lusher, M. Mchugh and R. C. Thompson, Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel, Mar. Pollut. Bull., 2013, 67, 94–99 CrossRef CAS PubMed.
- A. I. Catarino, A. Frutos and T. B. Henry, Use of fluorescent-labelled nanoplastics (NPs) to demonstrate NP absorption is inconclusive without adequate controls, Sci. Total Environ., 2019, 670, 915–920 CrossRef CAS.
- C. Schür, S. Rist, A. Baun, P. Mayer, N. B. Hartmann and M. Wagner, When fluorescence is not a particle: the tissue translocation of microplastics in Daphnia magna seems an artifact, Environ. Toxicol. Chem., 2019, 38, 1495–1503 CrossRef PubMed.
- A. Piruska, I. Nikcevic, S. H. Lee, C. Ahn, W. R. Heineman, P. A. Limbach and C. J. Seliskar, The autofluorescence of plastic materials and chips measured under laser irradiation, Lab Chip, 2005, 5, 1348–1354 RSC.
- Y. K. Lee, K. R. Murphy and J. Hur, Fluorescence signatures of dissolved organic matter leached from microplastics: polymers and additives, Environ. Sci. Technol., 2020, 54, 11905–11914 CrossRef CAS PubMed.
- A. M. Elert, R. Becker, E. Duemichen, P. Eisentraut, J. Falkenhagen, H. Sturm and U. Braun, Comparison of different methods for MP detection: what can we learn from them, and why asking the right question before measurements matters?, Environ. Pollut., 2017, 231, 1256–1264 CrossRef CAS PubMed.
- M. G. Löder, H. K. Imhof, M. Ladehoff, L. A. Löschel, C. Lorenz, S. Mintenig, S. Piehl, S. Primpke, I. Schrank and C. Laforsch, Enzymatic purification of microplastics in environmental samples, Environ. Sci. Technol., 2017, 51, 14283–14292 CrossRef PubMed.
- M. Tiwari, T. Rathod, P. Ajmal, R. Bhangare and S. Sahu, Distribution and characterization of microplastics in beach sand from three different Indian coastal environments, Mar. Pollut. Bull., 2019, 140, 262–273 CrossRef CAS PubMed.
- J. G. B. Neto, F. L. Rodrigues, I. Ortega, L. d. S. Rodrigues, A. L. Lacerda, J. L. Coletto, F. Kessler, L. G. Cardoso, L. Madureira and M. C. Proietti, Ingestion of plastic debris by commercially important marine fish in southeast-south Brazil, Environ. Pollut., 2020, 267, 115508 CrossRef CAS PubMed.
- J. Li, C. Green, A. Reynolds, H. Shi and J. M. Rotchell, Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom, Environ. Pollut., 2018, 241, 35–44 CrossRef CAS PubMed.
- A. M. Mahon, B. O'Connell, M. G. Healy, I. O'Connor, R. Officer, R. Nash and L. Morrison, Microplastics in sewage sludge: effects of treatment, Environ. Sci. Technol., 2017, 51, 810–818 CrossRef CAS PubMed.
- L. Van Cauwenberghe, A. Vanreusel, J. Mees and C. R. Janssen, Microplastic pollution in deep-sea sediments, Environ. Pollut., 2013, 182, 495–499 CrossRef CAS PubMed.
- F. Corami, B. Rosso, B. Bravo, A. Gambaro and C. Barbante, A novel method for purification, quantitative analysis and characterization of microplastic fibers using Micro-FTIR, Chemosphere, 2020, 238, 124564 CrossRef CAS PubMed.
- M. R. Jung, F. D. Horgen, S. V. Orski, V. Rodriguez, K. L. Beers, G. H. Balazs, T. T. Jones, T. M. Work, K. C. Brignac and S.-J. Royer, Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms, Mar. Pollut. Bull., 2018, 127, 704–716 CrossRef CAS PubMed.
- P. Wu, J. Huang, Y. Zheng, Y. Yang, Y. Zhang, F. He, H. Chen, G. Quan, J. Yan and T. Li, Environmental occurrences, fate, and impacts of microplastics, Ecotoxicol. Environ. Saf., 2019, 184, 109612 CrossRef CAS PubMed.
- G. Peng, B. Zhu, D. Yang, L. Su, H. Shi and D. Li, Microplastics in sediments of the Changjiang Estuary, China, Environ. Pollut., 2017, 225, 283–290 CrossRef CAS.
- D. Zhang, X. Liu, W. Huang, J. Li, C. Wang, D. Zhang and C. Zhang, Microplastic pollution in deep-sea sediments and organisms of the Western Pacific Ocean, Environ. Pollut., 2020, 259, 113948 CrossRef CAS PubMed.
- M. Majewsky, H. Bitter, E. Eiche and H. Horn, Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC), Sci. Total Environ., 2016, 568, 507–511 CrossRef CAS PubMed.
- R. A. Castañeda, S. Avlijas, M. A. Simard and A. Ricciardi, Microplastic pollution in St. Lawrence river sediments, Can. J. Fish. Aquat. Sci., 2014, 71, 1767–1771 CrossRef.
- W. J. Shim, Y. K. Song, S. H. Hong and M. Jang, Identification and quantification of microplastics using Nile Red staining, Mar. Pollut. Bull., 2016, 113, 469–476 CrossRef CAS PubMed.
- J. Golebiewski and A. Galeski, Thermal stability of nanoclay polypropylene composites by simultaneous DSC and TGA, Compos. Sci. Technol., 2007, 67, 3442–3447 CrossRef CAS.
- M. G. J. Löder, M. Kuczera, S. Mintenig, C. Lorenz and G. Gerdts, Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples, Environ. Chem., 2015, 12, 563–581 CrossRef.
- C. F. Araujo, M. M. Nolasco, A. M. Ribeiro and P. J. Ribeiro-Claro, Identification of microplastics using Raman spectroscopy: Latest developments and future prospects, Water Res., 2018, 142, 426–440 CrossRef CAS PubMed.
- Q. Qiu, Z. Tan, J. Wang, J. Peng, M. Li and Z. Zhan, Extraction, enumeration and identification methods for monitoring microplastics in the environment, Estuarine, Coastal Shelf Sci., 2016, 176, 102–109 CrossRef CAS.
- L. Zada, H. A. Leslie, A. D. Vethaak, G. H. Tinnevelt, J. J. Jansen, J. F. de Boer and F. Ariese, Fast microplastics identification with stimulated Raman scattering microscopy, J. Raman Spectrosc., 2018, 49, 1136–1144 CrossRef CAS.
- E. Dümichen, P. Eisentraut, C. G. Bannick, A.-K. Barthel, R. Senz and U. Braun, Fast identification of microplastics in complex environmental samples by a thermal degradation method, Chemosphere, 2017, 174, 572–584 CrossRef PubMed.
- A. Turner, In situ elemental characterisation of marine microplastics by portable XRF, Mar. Pollut. Bull., 2017, 124, 286–291 CrossRef CAS PubMed.
- E. Fries, J. H. Dekiff, J. Willmeyer, M.-T. Nuelle, M. Ebert and D. Remy, Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy, Environ. Sci.: Processes Impacts, 2013, 15, 1949–1956 RSC.
- S. Fuller and A. Gautam, A procedure for measuring microplastics using pressurized fluid extraction, Environ. Sci. Technol., 2016, 50, 5774–5780 CrossRef CAS PubMed.
- S. Mariano, S. Tacconi, M. Fidaleo, M. Rossi and L. Dini, Micro and nanoplastics identification: classic methods and innovative detection techniques, Frontiers in Toxicology, 2021, 3, 636640 CrossRef PubMed.
- J. Chen, J. Wu, P. C. Sherrell, J. Chen, H. Wang, W. x. Zhang and J. Yang, How to Build a Microplastics-Free Environment: Strategies for Microplastics Degradation and Plastics Recycling, Adv. Sci., 2022, 9, 2103764 CrossRef PubMed.
- A. S. A. Manap, Y. K. Lum, L. H. Ong, Y.-Q. Tang, L. T. Gew and A. Y. Y. Chia, Perspective approaches on melanogenesis inhibition, Dermatol. Sin., 2021, 39, 1–12 CrossRef.
- M. Urso and M. Pumera, Nano/microplastics capture and degradation by autonomous nano/microrobots: a perspective, Adv. Funct. Mater., 2022, 32, 2112120 CrossRef CAS.
- M. Enfrin, J. Lee, Y. Gibert, F. Basheer, L. Kong and L. F. Dumée, Release of hazardous nanoplastic contaminants due to microplastics fragmentation under shear stress forces, J. Hazard. Mater., 2020, 384, 121393 CrossRef CAS PubMed.
- Y. Yang, W. Liu, Z. Zhang, H.-P. Grossart and G. M. Gadd, Microplastics provide new microbial niches in aquatic environments, Appl. Microbiol. Biotechnol., 2020, 104, 6501–6511 CrossRef CAS PubMed.
- X. Guo and J. Wang, The chemical behaviors of microplastics in marine environment: A review, Mar. Pollut. Bull., 2019, 142, 1–14 CrossRef CAS PubMed.
- F. Wang, C. S. Wong, D. Chen, X. Lu, F. Wang and E. Y. Zeng, Interaction of toxic chemicals with microplastics: a critical review, Water Res., 2018, 139, 208–219 CrossRef CAS PubMed.
- S.-J. Royer, S. Ferrón, S. T. Wilson and D. M. Karl, Production of methane and ethylene from plastic in the environment, PLoS One, 2018, 13, e0200574 CrossRef PubMed.
- A. Chamas, H. Moon, J. Zheng, Y. Qiu, T. Tabassum, J. H. Jang, M. Abu-Omar, S. L. Scott and S. Suh, Degradation rates of plastics in the environment, ACS Sustain. Chem. Eng., 2020, 8, 3494–3511 CrossRef CAS.
- M. Zbyszewski and P. L. Corcoran, Distribution and degradation of fresh water plastic particles along the beaches of Lake Huron, Canada, Water, Air, Soil Pollut., 2011, 220, 365–372 CrossRef CAS.
- D. A. Cooper and P. L. Corcoran, Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii, Mar. Pollut. Bull., 2010, 60, 650–654 CrossRef CAS PubMed.
- F. Ning, W. Cong, Y. Hu and H. Wang, Additive manufacturing of carbon fiber-reinforced plastic composites using fused deposition modeling: Effects of process parameters on tensile properties, J. Compos. Mater., 2017, 51, 451–462 CrossRef CAS.
- Q. Zhou, H. Zhang, C. Fu, Y. Zhou, Z. Dai, Y. Li, C. Tu and Y. Luo, The distribution and morphology of microplastics in coastal soils adjacent to the Bohai Sea and the Yellow Sea, Geoderma, 2018, 322, 201–208 CrossRef CAS.
- L. Lear, D. Padfield, T. Dowsett, M. Jones, S. Kay, A. Hayward and M. Vos, Bacterial colonisation dynamics of household plastics in a coastal environment, Sci. Total Environ., 2022, 838, 156199 CrossRef CAS PubMed.
- P. L. Corcoran, M. C. Biesinger and M. Grifi, Plastics and beaches: a degrading relationship, Mar. Pollut. Bull., 2009, 58, 80–84 CrossRef CAS PubMed.
- N. Kalogerakis, K. Karkanorachaki, G. C. Kalogerakis, E. I. Triantafyllidi, A. D. Gotsis, P. Partsinevelos and F. Fava, Microplastics generation: onset of fragmentation of polyethylene films in marine environment mesocosms, Front. Mar. Sci., 2017, 4, 84 Search PubMed.
- K. Haremaki, T. Kida, Y. Koide, T. Uneyama, Y. Masubuchi and T. Ishida, Effects of Stirring Time on Formation of Microplastics Fragmented from Photo-aged Polypropylene, arXiv, 2025, preprint, arXiv:2503.21373, DOI:10.48550/arXiv.2503.21373.
- M. G. Mazzotta, C. M. Reddy and C. P. Ward, Rapid degradation of cellulose diacetate by marine microbes, Environ. Sci. Technol. Lett., 2021, 9, 37–41 CrossRef.
- B. Gewert, M. M. Plassmann and M. MacLeod, Pathways for degradation of plastic polymers floating in the marine environment, Environ. Sci.: Processes Impacts, 2015, 17, 1513–1521 RSC.
- P. Sidebotham, M. Brandon, S. Bailey, P. Belderson, J. Dodsworth, J. Garstang, E. Harrison, A. Retzer and P. Sorensen, Pathways to Harm, Pathways to Protection: a Triennial Analysis of Serious Case Reviews 2011 to 2014, 2016 Search PubMed.
- B. Song, G. Zeng, J. Gong, J. Liang, P. Xu, Z. Liu, Y. Zhang, C. Zhang, M. Cheng and Y. Liu, Evaluation methods for assessing effectiveness of in situ remediation of soil and sediment contaminated with organic pollutants and heavy metals, Environ. Int., 2017, 105, 43–55 CrossRef CAS PubMed.
- K. K. Leonas and R. W. Gorden, An accelerated laboratory study evaluating the disintegration rates of plastic films in simulated aquatic environments, J. Environ. Polym. Degrad., 1993, 1, 45–51 CrossRef CAS.
- J. E. Weinstein, B. K. Crocker and A. D. Gray, From macroplastic to microplastic: Degradation of high-density polyethylene, polypropylene, and polystyrene in a salt marsh habitat, Environ. Toxicol. Chem., 2016, 35, 1632–1640 CrossRef CAS PubMed.
- G. R. Oxnard, Y. Hu, K. F. Mileham, H. Husain, D. B. Costa, P. Tracy, N. Feeney, L. M. Sholl, S. E. Dahlberg and A. J. Redig, Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M–positive lung cancer and acquired resistance to osimertinib, JAMA Oncol., 2018, 4, 1527–1534 CrossRef PubMed.
- I. Yakimets, D. Lai and M. Guigon, Effect of photo-oxidation cracks on behaviour of thick polypropylene samples, Polym. Degrad. Stab., 2004, 86, 59–67 CrossRef CAS.
- A. Ter Halle, L. Jeanneau, M. Martignac, E. Jardé, B. Pedrono, L. Brach and J. Gigault, Nanoplastic in the North Atlantic subtropical gyre, Environ. Sci. Technol., 2017, 51, 13689–13697 CrossRef CAS PubMed.
- R. A. Naik, L. S. Rowles III, A. I. Hossain, M. Yen, R. M. Aldossary, O. G. Apul, J. Conkle and N. B. Saleh, Microplastic particle versus fiber generation during photo-transformation in simulated seawater, Sci. Total Environ., 2020, 736, 139690 CrossRef CAS PubMed.
- A. Buthiyappan, A. R. Abdul Aziz and W. M. A. Wan Daud, Recent advances and prospects of catalytic advanced oxidation process in treating textile effluents, Rev. Chem. Eng., 2016, 32, 1–47 CAS.
- Y. Deng and R. Zhao, Advanced oxidation processes (AOPs) in wastewater treatment, Curr. Pollut. Rep., 2015, 1, 167–176 CrossRef CAS.
- J. Li, H. Liu and J. P. Chen, Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection, Water Res., 2018, 137, 362–374 CrossRef CAS PubMed.
- X. Xiong, C. Wu, J. J. Elser, Z. Mei and Y. Hao, Occurrence and fate of microplastic debris in middle and lower reaches of the Yangtze River–from inland to the sea, Sci. Total Environ., 2019, 659, 66–73 CrossRef CAS PubMed.
- J. Wang, X. Liu, Y. Li, T. Powell, X. Wang, G. Wang and P. Zhang, Microplastics as contaminants in the soil environment: A mini-review, Sci. Total Environ., 2019, 691, 848–857 CrossRef CAS.
- Y. Huang, Y. Zhao, J. Wang, M. Zhang, W. Jia and X. Qin, LDPE microplastic films alter microbial community composition and enzymatic activities in soil, Environ. Pollut., 2019, 254, 112983 CrossRef CAS PubMed.
- K. Hu, P. Zhou, Y. Yang, T. Hall, G. Nie, Y. Yao, X. Duan and S. Wang, Degradation of microplastics by a thermal Fenton reaction, ACS ES&T Eng., 2021, 2, 110–120 Search PubMed.
- S. Guo, D. Feng, Y. Li, L. Liu and J. Tang, Innovations in chemical degradation technologies for the removal of micro/nano-plastics in water: A comprehensive review, Ecotoxicol. Environ. Saf., 2024, 271, 115979 CrossRef CAS PubMed.
- J. Hu, F. Y. Lim and J. Hu, Characteristics and Behaviors of Microplastics undergoing Photoaging and Advanced Oxidation Processes (AOPs) Initiated Aging, Water Res., 2023, 119628 CrossRef CAS PubMed.
- E. B. Esfahani, F. A. Zeidabadi, S. Zhang and M. Mohseni, Photo-chemical/catalytic Oxidative/Reductive Decomposition of Per-and Polyfluoroalkyl Substances (PFAS), Decomposition Mechanisms and Effects of Key Factors: A Review, Environ. Sci.:Water Res. Technol., 2022, 8(4), 698–728 RSC.
- D. Xiaoqiang, X. Zhoufeng, G. Yaqiong and D. Yong, Polyoxometalate-based catalysts for photocatalytic, chemical catalytic and electrocatalytic water oxidation, Int. J. Hydrogen Energy, 2017, 42, 24169–24175 CrossRef.
- Z. Chen, X. Liu, W. Wei, H. Chen and B.-J. Ni, Removal of microplastics and nanoplastics from urban waters: Separation and degradation, Water Res., 2022, 221, 118820 CrossRef CAS PubMed.
- M. Zandieh, E. Griffiths, A. Waldie, S. Li, J. Honek, F. Rezanezhad and J. Liu, Catalytic and biocatalytic degradation of microplastics, Exploration, 2024, 4(3), 20230018 CrossRef CAS PubMed.
- P. Pfohl, M. Wagner, L. Meyer, P. Domercq, A. Praetorius, T. Hüffer, T. Hofmann and W. Wohlleben, Environmental degradation of microplastics: how to measure fragmentation rates to secondary micro-and nanoplastic fragments and dissociation into dissolved organics, Environ. Sci. Technol., 2022, 56, 11323–11334 CrossRef CAS.
- A. Uheida, H. G. Mejía, M. Abdel-Rehim, W. Hamd and J. Dutta, Visible light photocatalytic degradation of polypropylene microplastics in a continuous water flow system, J. Hazard. Mater., 2021, 406, 124299 CrossRef CAS.
- A. Bratovcic, Degradation of micro-and nano-plastics by photocatalytic methods, J. Nanosci. Nanotechnol. Appl., 2019, 3, 206 Search PubMed.
- M. H. Fadli, M. Ibadurrohman and S. Slamet, Microplastic pollutant degradation in water using modified TiO2 photocatalyst under UV-irradiation, in IOP conference series: materials science and engineering, IOP Publishing, 2021, vol. 1011(1), p. 012055 Search PubMed.
- T. S. Tofa, Degradation of Microplastic Residuals in Water by Visible Light Photocatalysis, 2018 Search PubMed.
- Y. He, A. U. Rehman, M. Xu, C. A. Not, A. M. Ng and A. B. Djurišić, Photocatalytic degradation of different types of microplastics by TiOx/ZnO tetrapod photocatalysts, Heliyon, 2023, 9(11), e22562 CrossRef CAS PubMed.
- D. A. Maulana and M. Ibadurrohman, Synthesis of nano-composite Ag/TiO2 for polyethylene microplastic degradation applications, in IOP conference series: materials science and engineering, IOP Publishing, 2021, vol. 1011(1), p. 012054 Search PubMed.
- M. C. Ariza-Tarazona, C. Siligardi, H. A. Carreón-López, J. E. Valdéz-Cerda, P. Pozzi, G. Kaushik, J. F. Villarreal-Chiu and E. I. Cedillo-González, Low environmental impact remediation of microplastics: Visible-light photocatalytic degradation of PET microplastics using bio-inspired C, N-TiO2/SiO2 photocatalysts, Mar. Pollut. Bull., 2023, 193, 115206 CrossRef CAS PubMed.
- I. Uogintė, S. Pleskytė, M. Skapas, S. Stanionytė and G. Lujanienė, Degradation and optimization of microplastic in aqueous solutions with graphene oxide-based nanomaterials, Int. J. Environ. Sci. Technol., 2023, 20, 9693–9706 CrossRef.
- K. Khairudin, N. F. A. Bakar and M. S. Osman, Magnetically recyclable flake-like BiOI-Fe3O4 microswimmers for fast and efficient degradation of microplastics, J. Environ. Chem. Eng., 2022, 10, 108275 CrossRef CAS.
- P. Bhatt, V. M. Pathak, S. Joshi, T. S. Bisht, K. Singh and D. Chandra, in Smart Bioremediation Technologies, Elsevier, 2019, pp. 205–215 Search PubMed.
- V. M. Pathak, Review on the current status of polymer degradation: a microbial approach, Bioresources and Bioprocessing, 2017, 4, 1–31 CrossRef.
- C. D. Rummel, A. Jahnke, E. Gorokhova, D. Kühnel and M. Schmitt-Jansen, Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment, Environ. Sci. Technol. Lett., 2017, 4, 258–267 CrossRef CAS.
- A. A. Shah, F. Hasan, A. Hameed and S. Ahmed, Biological degradation of plastics: a comprehensive review, Biotechnol. Adv., 2008, 26, 246–265 CrossRef CAS PubMed.
- E. R. Zettler, T. J. Mincer and L. A. Amaral-Zettler, Life in the “plastisphere”: microbial communities on plastic marine debris, Environ. Sci. Technol., 2013, 47, 7137–7146 CrossRef CAS PubMed.
- S. Krause, M. Molari, E. Gorb, S. Gorb, E. Kossel and M. Haeckel, Persistence of plastic debris and its colonization by bacterial communities after two decades on the abyssal seafloor, Sci. Rep., 2020, 10, 9484 CrossRef CAS PubMed.
- A. Paço, K. Duarte, J. P. da Costa, P. S. Santos, R. Pereira, M. Pereira, A. C. Freitas, A. C. Duarte and T. A. Rocha-Santos, Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum, Sci. Total Environ., 2017, 586, 10–15 CrossRef PubMed.
- L. Liu, M. Xu, Y. Ye and B. Zhang, On the degradation of (micro) plastics: Degradation methods, influencing factors, environmental impacts, Sci. Total Environ., 2022, 806, 151312 CrossRef CAS PubMed.
- A. K. Urbanek, W. Rymowicz and A. M. Mirończuk, Degradation of plastics and plastic-degrading bacteria in cold marine habitats, Appl. Microbiol. Biotechnol., 2018, 102, 7669–7678 CrossRef CAS PubMed.
- M. Long, B. Moriceau, M. Gallinari, C. Lambert, A. Huvet, J. Raffray and P. Soudant, Interactions between microplastics and phytoplankton aggregates: impact on their respective fates, Mar. Chem., 2015, 175, 39–46 CrossRef CAS.
- R. M. Blair Espinoza, Microplastics in wastewater treatment systems and receiving waters, Doctoral dissertation, University of Glasgow, 2019.
- A. Baig, M. Zubair, M. N. Zafar, M. Farid, M. F. Nazar and S. H. Sumrra, in Biodegradation and Biodeterioration at the Nanoscale, Elsevier, 2022, pp. 239–259 Search PubMed.
- C. M. Rochman, The complex mixture, fate and toxicity of chemicals associated with plastic debris in the marine environment, Marine Anthropogenic Litter, 2015, pp. 117–140 Search PubMed.
- G. Zhang, C. Liu, C. Xiao, R. Xie, B. Ming, P. Hou, G. Liu, W. Xu, D. Shen and K. Wang, Optimizing water use efficiency and economic return of super high yield spring maize under drip irrigation and plastic mulching in arid areas of China, Field Crops Res., 2017, 211, 137–146 CrossRef.
- R. Wei and N. Wierckx, Microbial Degradation of Plastics, Front. Microbiol., 2021, 12, 635621 CrossRef PubMed.
- M. Kooi, E. Besseling, C. Kroeze, A. P. Van Wezel and A. A. Koelmans, Modeling the fate and transport of plastic debris in freshwaters: review and guidance, Freshwater Microplastics: Emerging Environmental Contaminants?, 2018, pp. 125–152 Search PubMed.
- H. Gao, C. Yan, Q. Liu, W. Ding, B. Chen and Z. Li, Effects of plastic mulching and plastic residue on agricultural production: A meta-analysis, Sci. Total Environ., 2019, 651, 484–492 CrossRef CAS PubMed.
- Y. Li, X. Wang, W. Fu, X. Xia, C. Liu, J. Min, W. Zhang and J. C. Crittenden, Interactions between nano/micro plastics and suspended sediment in water: Implications on aggregation and settling, Water Res., 2019, 161, 486–495 CrossRef CAS PubMed.
- S. S. Ali, T. Elsamahy, D. Zhu and J. Sun, Biodegradability of polyethylene by efficient bacteria from the guts of plastic-eating waxworms and investigation of its degradation mechanism, J. Hazard. Mater., 2023, 443, 130287 CrossRef CAS PubMed.
- K. Kaur, S. Sharma, N. Shree and R. Mehrotra, Recent Advancements and Mechanism of Plastics Biodegradation Promoted by Bacteria: A Key for Sustainable Remediation for Plastic Wastes, Biosci. Biotechnol. Res. Asia, 2023, 20, 1–12 Search PubMed.
- S. Hakvåg, O. G. Brakstad, S. Kubowicz and A. M. Booth, in Biodegradability of Conventional Plastics, Elsevier, 2023, pp. 17–45 Search PubMed.
- J. Yuan, J. Ma, Y. Sun, T. Zhou, Y. Zhao and F. Yu, Microbial degradation and other environmental aspects of microplastics/plastics, Sci. Total Environ., 2020, 715, 136968 CrossRef CAS PubMed.
- M. Wróbel, S. Szymańska, T. Kowalkowski and K. Hrynkiewicz, Selection of microorganisms capable of polyethylene (PE) and polypropylene (PP) degradation, Microbiol. Res., 2023, 267, 127251 CrossRef PubMed.
- S. Y. Park and C. G. Kim, Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site, Chemosphere, 2019, 222, 527–533 CrossRef CAS PubMed.
- H. Auta, C. Emenike and S. Fauziah, Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation, Environ. Pollut., 2017, 231, 1552–1559 CrossRef CAS PubMed.
- S. Yoshida, K. Hiraga, T. Takehana, I. Taniguchi, H. Yamaji, Y. Maeda, K. Toyohara, K. Miyamoto, Y. Kimura and K. Oda, A bacterium that degrades and assimilates poly (ethylene terephthalate), Science, 2016, 351, 1196–1199 CrossRef CAS PubMed.
- N. Tkachuk and L. Zelena, The impact of bacteria of the genus Bacillus upon the biodamage/biodegradation of some metals and extensively used petroleum-based plastics, Corros. Mater. Degrad., 2021, 2, 531–553 CrossRef.
- G. T. Howard, Biodegradation of polyurethane: a review, Int. Biodeterior. Biodegrad., 2002, 49, 245–252 CrossRef CAS.
- N. Mohanan, Z. Montazer, P. K. Sharma and D. B. Levin, Microbial and enzymatic degradation of synthetic plastics, Front. Microbiol., 2020, 11, 580709 CrossRef PubMed.
- R. Tiwari, N. Azad, D. Dutta, B. R. Yadav and S. Kumar, A critical review and future perspective of plastic waste recycling, Sci. Total Environ., 2023, 881, 163433 CrossRef CAS PubMed.
- Y. Tokiwa and B. P. Calabia, Review degradation of microbial polyesters, Biotechnol. Lett., 2004, 26, 1181–1189 CrossRef CAS PubMed.
- B. M. Kyaw, R. Champakalakshmi, M. K. Sakharkar, C. S. Lim and K. R. Sakharkar, Biodegradation of low density polythene (LDPE) by Pseudomonas species, Indian J. Microbiol., 2012, 52, 411–419 CrossRef CAS PubMed.
- R.-J. Müller, I. Kleeberg and W.-D. Deckwer, Biodegradation of polyesters containing aromatic constituents, J. Biotechnol., 2001, 86, 87–95 CrossRef.
- Y. Tokiwa and B. P. Calabia, Biodegradability and biodegradation of poly (lactide), Appl. Microbiol. Biotechnol., 2006, 72, 244–251 CrossRef CAS PubMed.
- Y. Qi, X. Yang, A. M. Pelaez, E. H. Lwanga, N. Beriot, H. Gertsen, P. Garbeva and V. Geissen, Macro-and micro-plastics in soil-plant system: effects of plastic mulch film residues on wheat (Triticum aestivum) growth, Sci. Total Environ., 2018, 645, 1048–1056 CrossRef CAS PubMed.
- P. Li, X. Wang, M. Su, X. Zou, L. Duan and H. Zhang, Characteristics of plastic pollution in the environment: a review, Bull. Environ. Contam. Toxicol., 2021, 107, 577–584 CrossRef CAS PubMed.
- H. Arshad, S. A. Sulaiman, Z. Hussain, Y. Naz and F. Basrawi, Microwave assisted pyrolysis of plastic waste for production of fuels: a review, in MATEC Web of conferences, EDP Sciences, 2017, vol. 131, p. 02005 Search PubMed.
- M. E. E. Temporiti, L. Nicola, E. Nielsen and S. Tosi, Fungal enzymes involved in plastics biodegradation, Microorganisms, 2022, 10, 1180 CrossRef CAS.
- A. Maurya, A. Bhattacharya and S. K. Khare, Enzymatic remediation of polyethylene terephthalate (PET)–based polymers for effective management of plastic wastes: an overview, Front. Bioeng. Biotechnol., 2020, 8, 602325 CrossRef.
- A. Satta, G. Zampieri, G. Loprete, S. Campanaro, L. Treu and E. Bergantino, Metabolic and enzymatic engineering strategies for polyethylene terephthalate degradation and valorization, Rev. Environ. Sci. Bio/Technol., 2024, 23, 351–383 CrossRef CAS.
- M. Srikanth, T. Sandeep, K. Sucharitha and S. Godi, Biodegradation of plastic polymers by fungi: a brief review, Bioresources and Bioprocessing, 2022, 9, 42 CrossRef PubMed.
- A. Rosato, A. Romano, G. Totaro, A. Celli, F. Fava, G. Zanaroli and L. Sisti, Enzymatic degradation of the most common aliphatic bio-polyesters and evaluation of the mechanisms involved: an extended study, Polymers, 2022, 14, 1850 CrossRef CAS PubMed.
- M. A. Akram, R. Savitha, G. K. Kinsella, K. Nolan, B. J. Ryan and G. T. Henehan, Microbial and Enzymatic Biodegradation of Plastic Waste for a Circular Economy, Appl. Sci., 2024, 14, 11942 CrossRef CAS.
- L. Wu, Y. Wang, X. Zhao, H. Mao and Z. Gu, Investigating the biodegradation mechanism of poly (trimethylene carbonate): macrophage-mediated erosion by secreting lipase, Biomacromolecules, 2023, 24, 921–928 CrossRef CAS PubMed.
- H. Mndlovu, P. Kumar, L. C. du Toit and Y. E. Choonara, A review of bio material degradation assessment approaches employed in the biomedical field, npj Mater. Degrad., 2024, 8, 66 CrossRef CAS.
- M. I. Penas, M. Criado-Gonzalez, A. M. de Ilarduya, A. Flores, J.-M. Raquez, R. Mincheva, A. J. Müller and R. Hernández, Tunable enzymatic biodegradation of poly (butylene succinate): biobased coatings and self-degradable films, Polym. Degrad. Stab., 2023, 211, 110341 CrossRef CAS.
- Y. Wang, R.-J. van Putten, A. Tietema, J. R. Parsons and G.-J. M. Gruter, Polyester biodegradability: importance and potential for optimisation, Green Chem., 2024, 26, 3698–3716 RSC.
- B. K. H. Lim and E. San Thian, Biodegradation of polymers in managing plastic waste—A review, Sci. Total Environ., 2022, 813, 151880 CrossRef CAS PubMed.
- Z. Lin, T. Jin, T. Zou, L. Xu, B. Xi, D. Xu, J. He, L. Xiong, C. Tang and J. Peng, Current progress on plastic/microplastic degradation: Fact influences and mechanism, Environ. Pollut., 2022, 304, 119159 CrossRef CAS PubMed.
- A. Bher, P. C. Mayekar, R. A. Auras and C. E. Schvezov, Biodegradation of biodegradable polymers in mesophilic aerobic environments, Int. J. Mol. Sci., 2022, 23, 12165 CrossRef CAS PubMed.
- Y. Hu, M. Gong, J. Wang and A. Bassi, Current research trends on microplastic pollution from wastewater systems: a critical review, Rev. Environ. Sci. Bio/Technol., 2019, 18, 207–230 CrossRef.
- J. C. Prata, A. L. P. Silva, J. P. Da Costa, C. Mouneyrac, T. R. Walker, A. C. Duarte and T. Rocha-Santos, Solutions and integrated strategies for the control and mitigation of plastic and microplastic pollution, Int. J. Environ. Res. Public Health, 2019, 16, 2411 CrossRef PubMed.
- G. Reimonn, T. Lu, N. Gandhi and C. Wan-Ting, Review of microplastic pollution in the environment and emerging recycling solutions, J. Renewable Mater., 2019, 7, 1251 CAS.
- E. B. Jadhav, M. S. Sankhla, R. A. Bhat and D. Bhagat, Microplastics from food packaging: An overview of human consumption, health threats, and alternative solutions, Environ. Nanotechnol., Monit. Manage., 2021, 16, 100608 CAS.
- V. P. Ranjan, A. Joseph and S. Goel, Microplastics and other harmful substances released from disposable paper cups into hot water, J. Hazard. Mater., 2021, 404, 124118 CrossRef CAS PubMed.
- E. McGuinty and T. R. Walker, in Plastic Pollution in the Global Ocean, 2023, pp. 375–398 Search PubMed.
- P. U. Iyare, S. K. Ouki and T. Bond, Microplastics removal in wastewater treatment plants: a critical review, Environ. Sci.:Water Res. Technol., 2020, 6, 2664–2675 RSC.
- S. Vohl, M. Kristl and J. Stergar, Harnessing magnetic nanoparticles for the effective removal of micro-and nanoplastics: A critical review, Nanomaterials, 2024, 14, 1179 CrossRef CAS PubMed.
- M.-K. Nguyen, in Plastic Degradation and Conversion by Photocatalysis (Volume 2): from Waste to Wealth, ACS Publications, 2024, pp. 249–265 Search PubMed.
- D. M. Mitrano and W. Wohlleben, Microplastic regulation should be more precise to incentivize both innovation and environmental safety, Nat. Commun., 2020, 11, 5324 CrossRef CAS PubMed.
- J. P. Da Costa, C. Mouneyrac, M. Costa, A. C. Duarte and T. Rocha-Santos, The role of legislation, regulatory initiatives and guidelines on the control of plastic pollution, Front. Environ. Sci., 2020, 8, 104 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |