Tristan Chidley and Graham K. Murphy
Org. Biomol. Chem., 2018,16, 8486-8490
DOI:
10.1039/C8OB02636J,
Communication
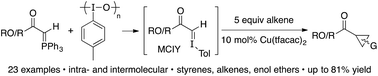
Reacting Wittig reagents and the hypervalent iodine reagent iodosotoluene, in the presence of 10 mol% Cu(tfacac)2 and 5 equiv. of alkene, results in a novel cyclopropanation reaction. The reagent combination is believed to generate a transient monocarbonyl iodonium ylide (MCIY) in situ, which can be intercepted by the copper catalyst to give a metallocarbene. Both ester and ketone derived phosphoranes can be used, as can styrenyl and non-styrenyl alkenes, which provides cyclopropanes in yields up to 81%.