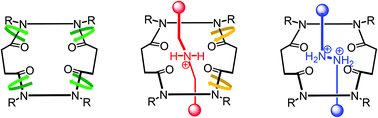
In this study, we synthesized the macrocyclic tetraamide 1, possessing four tertiary amide units, as a host for mono- and bis-ammonium ions, forming corresponding [2]pseudorotaxanes stabilized through hydrogen bonding between the components. [2]Rotaxanes comprising 1 as the macrocycle and mono- and bis-ammonium ions as the axle components were synthesized through imine bond formation. The tetraamide 1 exists as a mixture of rotamers in solution; in the [2]rotaxanes, however, the conformation of this component was controlled through intramolecular hydrogen bonding between the axle and macrocyclic components. In a nonpolar solvent (CDCl3), only one conformational isomer existed for each [2]rotaxane. On the other hand, in a polar solvent (DMSO-d6), the [2]rotaxane possessing a mono-ammonium ion in the axle was partially isomerized; only a single rotational isomer existed for the [2]rotaxane featuring a bis-ammonium ion in the axle, because of a complete set of hydrogen bonds, in this polar solvent.