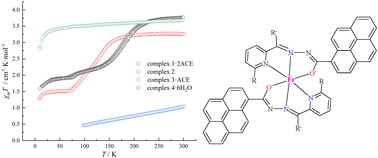
Multifunctional magnetic materials have broad application prospects in molecular switches and information storage. In this study, four mononuclear Fe(II) complexes are synthesized using a series of pyrenylhydrazone ligands HL1–4. Two deprotonated ligands are coordinated to the iron(II) ions in an enolic form, leading to neutral complexes FeII(Lx)2·xsol with a FeIIN4O2 octahedral coordination environment. Magnetic measurements suggest that complex Fe(L1)2·2ACE (1·2ACE, ACE = acetone) is mainly low spin below 300 K and complex Fe(L3)2·ACE (3·ACE) is high spin, whereas complexes Fe(L2)2 (2) and Fe(L4)2·6H2O (4·6H2O) exhibit gradual spin crossover behavior. The spin states of complexes 1–4 are confirmed by single-crystal X-ray diffraction analysis. The substituent effect on the magnetic properties of the complexes is significant in this system. Temperature-dependent fluorescence emission spectra show the coexistence but no coupling effect of spin crossover and fluorescence for complexes 2 and 4·6H2O.