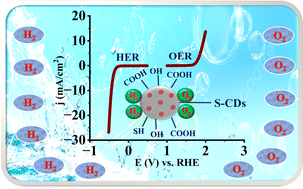
Herein, the synthesis of bifunctional, metal-free, and highly efficient sulphur-doped carbon dots (S-CDs) through a facile chemical method is demonstrated for electrocatalytic water splitting reactions, specifically targeting both the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER). S-CDs were characterized using thermogravimetric analysis (TGA), which demonstrated remarkable thermal stability up to 800 °C in Ar, and transmission electron microscopy (TEM) revealed a well-defined crystalline structure with a d-spacing of approximately 0.375 nm. The elemental composition confirmed using energy-dispersive analysis of X-ray (EDAX) showed the presence of carbon (15.91%), oxygen (62.49%), and sulphur (21.60%). X-ray diffraction (XRD) analysis exhibited a broad peak at 2θ = 24°, indicating the graphitic nature of the sulphur doping. Brunauer–Emmett–Teller (BET) surface area analysis revealed a large surface area of 160.49 m2 g−1 and a pore volume of 0.2209 cc g−1 for S-CDs. Fourier-transform infrared (FTIR) spectroscopy identified three distinct peaks corresponding to the functional groups associated with S-CDs. The electrocatalytic performance for the HER was characterized by a low overpotential of −0.64 V vs. RHE to achieve a current density of 10 mA cm−2, with a Tafel slope of 108 mV dec−1 in 1 M H2SO4. For the OER, S-CDs exhibited an overpotential of 1.94 V vs. RHE, with a Tafel slope of 21 mV dec−1 in 1 M KOH. These results demonstrate the potential of S-CDs as cost-effective and sustainable electrocatalysts for overall water splitting, and they hold promise as leading candidates for futuristic renewable energy applications.