Pedro López-Mendoza and Luis D. Miranda
Org. Biomol. Chem., 2020,18, 3487-3491
DOI:
10.1039/D0OB00136H,
Communication
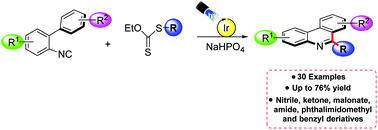
A photocatalytic xanthate-based radical addition/cyclization reaction cascade toward 2-biphenylisocyanides is described as a practical and modular approach to 6-alkylated phenanthridines. The use of xanthates as radical precursors allowed the synthesis of diversely 6-substituted phenanthridines. Electrophilic radicals derived from nitriles, aromatic and aliphatic ketones, malonates, and amide derivatives, as well as radicals derived from phthalimidomethyl and benzylic derivatives were successfully introduced. The reaction proceeds under mild conditions without a stoichiometric amount of oxidant. Thirty novel phenanthridine scaffolds were synthesized with yields ranging from 24 to 76%.