Recent developments in lead-free bismuth-based halide perovskite nanomaterials for heterogeneous photocatalysis under visible light
Zehong
Wu
 abc,
Harun
Tüysüz
abc,
Harun
Tüysüz
 d,
Flemming
Besenbacher
d,
Flemming
Besenbacher
 e,
Yitao
Dai
e,
Yitao
Dai
 *abc and
Yujie
Xiong
*abc and
Yujie
Xiong
 *ab
*ab
aHefei National Laboratory for Physical Sciences at the Microscale, School of Chemistry and Materials Science, University of Science and Technology of China, Hefei, Anhui 230026, China. E-mail: yitaodai@ustc.edu.cn; yjxiong@ustc.edu.cn
bKey Laboratory of Precision and Intelligent Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, China
cSuzhou Institute for Advanced Research, University of Science and Technology of China, Suzhou, Jiangsu 215123, China
dMax-Planck-Institut für Kohlenforschung, Mülheim an der Ruhr 45470, Germany
eInterdisciplinary Nanoscience Center (iNANO), Aarhus University, DK-8000 Aarhus C, Denmark
First published on 16th February 2023
Abstract
Halide perovskite materials, especially lead-based perovskites, have been widely used for optoelectronic and catalytic applications. However, the high toxicity of the lead element is a major concern that directs the research work toward lead-free halide perovskites, which could utilize bismuth as a promising candidate. Until now, the replacement of lead by bismuth in perovskites has been well studied by designing bismuth-based halide perovskite (BHP) nanomaterials with versatile physical–chemical properties, which are emerging in various application fields, especially heterogeneous photocatalysis. In this mini-review, we present a brief overview of recent progress in BHP nanomaterials for photocatalysis under visible light. The synthesis and physical–chemical properties of BHP nanomaterials have been comprehensively summarized, including zero-dimensional, two-dimensional nanostructures and hetero-architectures. Later, we introduce the photocatalytic applications of these novel BHP nanomaterials with visible-light response, improved charge separation/transport and unique catalytic sites. Due to advanced nano-morphologies, a well-designed electronic structure and an engineered surface chemical micro-environment, BHP nanomaterials demonstrate enhanced photocatalytic performance for hydrogen generation, CO2 reduction, organic synthesis and pollutant removal. Finally, the challenges and future research directions of BHP nanomaterials for photocatalysis are discussed.
1. Introduction
Since Fujishima first reported TiO2 photocatalysis for water splitting in 1972,1 heterogeneous photocatalysis driven by semiconductor-based photocatalysts has attracted more and more attention due to its direct use of sustainable solar energy and mild reaction conditions.2 In recent years, heterogeneous photocatalysis has been employed in various applications, such as photocatalytic H2 generation,3,4 CO2 reduction,5,6 decontamination of heavy metals and organic pollutants,7,8 and organic synthesis.9–12 In heterogeneous photocatalysis, the physical–chemical properties of photocatalytic materials, including elemental compositions, geometric/electronic structures and surface chemical environment, play key roles in promoting photocatalytic performance.13–17 However, conventional photocatalysts such as TiO2 and ZnO can only absorb ultraviolet light (accounting for only 5% of sunlight), which has severely limited the widespread usage of heterogeneous photocatalysis. In contrast, metal halide perovskites (MHPs) can exhibit desired visible or even near-infrared light absorption with narrow bandgaps, which can be facilely regulated by their versatile element compositions (different metal and halide species).18–23 Moreover, the low-cost and facile synthesis of nanostructured MHPs with excellent electronic properties, such as long diffusion lengths of charge carriers (up to micrometers) and ultralow bulk defect density (∼1010 cm−3 for single crystals), renders them intriguing for employment in the optoelectronic fields.24–29 These advantages offer MHPs as promising photocatalytic materials with better light-harvesting capabilities, improved charge separation/transport and suitable catalytic sites on surface, which may lead to more efficient energy conversion from solar to chemical energy.30 MHPs were first discovered in 1893,31 but their research was not revived until Kojima et al. applied halide perovskites to solar cells in 2009.32 Subsequently, lead-based halide perovskites have prevailed in the fabrication of semiconductor-based optoelectronic devices33 and photocatalytic systems34 mainly due to high bandgap tunability, outstanding charge mobility and suitable redox capabilities. Unfortunately, the high toxicity and bioavailability of Pb as a heavy metal severely hinder the commercial applications of lead-containing perovskites due to the high risk of polluting the environment and damaging human health.35,36In order to address the concerns about the environmental impact of MHPs, researchers are trying to replace the heavy metal (Pb) in halide perovskites with other more inert candidates such as Sn and Bi metals, etc.37–43 However, the addition of Sn2+ always demonstrates undesired instability because of susceptibility to oxidation.44 As the isoelectric ion of Pb2+, Bi3+ is expected to be a suitable candidate to replace Pb2+; this has been reported in several successful cases such as bismuth-based halide perovskites with high stability.45–47 For example, Cs3Bi2X9 (X = Cl, Br and I) nanocrystals have been prepared, via a simple antisolvent method, which exhibited blue emission with a photoluminescence efficiency of 0.2% and quite high stability towards air exposure for over 30 days.48 Moreover, higher Lewis acidity of Bi3+ over Pb2+ gives the surface of BHP stronger acidic sites, thus enhancing the photocatalytic efficiency of BHP for acid-catalyzed organic reactions such as the ring-opening reaction of epoxides.49 For several important photocatalytic reactions (e.g., H2 evolution,50 CO2 reduction51 and H2O2 synthesis52), dozens of photocatalysts such as g-C3N4,53 ZnIn2S4,54 and Bi2WO655 have been well explored with satisfactory performance. In contrast, BHP photocatalysts, which possess more tunable bandgaps (varying from 1.8 to 3.1 eV) and diverse elemental compositions (e.g., different halides and Ag/Cu atoms), need further research to investigate their photocatalytic applications in various reactions.
So far, there exist several reviews on the development of MHP-based photocatalysts including their composites in combination with other semiconductors.34,56–62 Most of them discuss the synthesis, optical properties and photocatalytic applications of lead-containing MHPs. Some other reviews involving lead-free MHPs have mainly focused on their photoelectric properties and application in optoelectronic devices (e.g., solar cells, light-emitting diodes, and photodetectors) and photocatalysis.42,43,63–71 However, a dedicated review on the comprehensive analysis of BHP-based photocatalysts is still unavailable. Accordingly, this minireview summarizes the synthesis methods and physical–chemical properties of BHPs, with a focus on the fabrication of BHP nanomaterials with various micro-morphologies, band structures and surface chemical environment. Furthermore, we discuss recent applications of BHPs in heterogeneous photocatalysis, such as photocatalytic H2 generation, CO2 reduction, organic transformations, and degradation of organic dyes. Finally, an outlook on the rational design and synthesis of novel BHPs with more diverse nanostructures, satisfactory quantum yields, and stability is presented.
2. Photocatalytic principles and fundamental structures, compositions of BHPs
In photocatalytic reactions, the main roles of semiconductor-based photocatalysts involve light absorption with the generation of photoinduced electron–hole pairs and the transport of charge carriers to the surface for driving target redox reactions. The catalytic reaction processes usually require the existence of active sites on the surface to activate reactants after capturing charges. In detail, the fundamental steps occur as follows in the whole photocatalytic process (Fig. 1a):11 (1) the semiconductor-based photocatalyst is excited by light, and photogenerated electrons jump to the conduction band (CB) from the valence band (VB), which accepts the left holes (step 1). This process happens quite rapidly at a femtosecond level (∼10−15 s). (2) Subsequently, the electrons and holes are transferred to the photocatalyst's surface (steps 2 and 3). This charge diffusion occurs quickly as well, at a nanosecond scale (∼10−9 s). (3) Finally, the electrons and holes approaching the surface are utilized by catalytically active sites (e.g., co-catalysts) to initiate the desired redox reactions (steps 4 and 5), which is a quite slow process requiring microseconds to milliseconds (10−6–10−3 s) in contrast to the above steps.72 In addition, electron–hole pairs might recombine with each other, dissipating energy as fluorescence or heat in nanoseconds (step 6). | ||
| Fig. 1 (a) Fundamental working principles of a semiconductor-based photocatalyst. Reproduced with permission from ref. 11. Copyright 2022 Tsinghua University Press. (b) The typical crystal structure of MHPs. Reproduced with permission from ref. 73. Copyright 2020 MDPI. (c) The typical crystal structure of Cs3Bi2Br9. Reproduced with permission from ref. 77. Copyright 2016 American Chemical Society. | ||
According to the above-mentioned photocatalytic principles, BHP could be an ideal photocatalyst due to its visible spectral tunability, long diffusion lifetime of carriers, and flexible surface chemical environment.65 Generally, the crystal structure of a typical halide perovskite, ABX3, consists of corner-shared [BX6] octahedra with A+ ions occupying the cuboctahedra cavities (Fig. 1b).73 A-site ion is usually a monovalent cation (e.g., organic methylammonium (MA), formamidinium (FA), or inorganic Rb+/Cs+/Ag+/Cu+), while B-site ion typically corresponds to a divalent metal cation (such as Pb2+, Ge2+ or Sn2+).69 The X-site ion indicates a halide anion (e.g., Cl−, Br−, or I−), which always strongly affects the VB position of MHP.74,75 In addition, the value of Goldschmidt tolerance factor t ( with R as the ionic radius of related ions) should locate between 0.8 and 1.1 to form a stable MHP.76 In comparison, the replacement of divalent ions by trivalent Bi3+ ions leads to the structural derivation with a typical formula of A3Bi2X9 for BHP materials, where only two-thirds of the octahedral center positions are completely occupied by Bi3+ in a two-dimensional layered perovskite structure (Fig. 1c).77 Furthermore, the introduction of another monovalent cation can produce a halide double perovskite with a general formula of A2M(I)BiX6 (e.g., Cs2AgBiBr6 or Cs2CuBiBr6), which demonstrates a three-dimensional perovskite structure analogous to ABX3.78–81 Besides, all-inorganic BHPs (e.g., Cs3Bi2Br9) with inorganic A-site cations can exhibit improved thermal and chemical stability compared with their organic–inorganic hybrid counterparts (e.g., MA3Bi2Br9).82,83
with R as the ionic radius of related ions) should locate between 0.8 and 1.1 to form a stable MHP.76 In comparison, the replacement of divalent ions by trivalent Bi3+ ions leads to the structural derivation with a typical formula of A3Bi2X9 for BHP materials, where only two-thirds of the octahedral center positions are completely occupied by Bi3+ in a two-dimensional layered perovskite structure (Fig. 1c).77 Furthermore, the introduction of another monovalent cation can produce a halide double perovskite with a general formula of A2M(I)BiX6 (e.g., Cs2AgBiBr6 or Cs2CuBiBr6), which demonstrates a three-dimensional perovskite structure analogous to ABX3.78–81 Besides, all-inorganic BHPs (e.g., Cs3Bi2Br9) with inorganic A-site cations can exhibit improved thermal and chemical stability compared with their organic–inorganic hybrid counterparts (e.g., MA3Bi2Br9).82,83
3. BHP photocatalysts: synthesis and properties
The physical–chemical properties of BHP nanomaterials are significant for their optoelectronic applications, including photocatalysis. These indispensable properties can be well regulated by the preparation protocols, which can precisely vary the micro-morphologies and elemental compositions of BHPs. In this section, the synthesis methods and properties of BHP nanomaterials in different dimensions will be introduced, including zero-dimensional (0D),82,84–86 two-dimensional (2D) nanostructures87,143 and hetero-architectures.42,88,89 Notably, as far as we know, there are still few reports on 1D and 3D BHP nanomaterials, which can be regarded as promising research directions on material synthesis. Table 1 summarizes the typical BHP nanomaterials with versatile components (organic–inorganic hybrid perovskite or all-inorganic perovskite) based on synthesis approaches (including antisolvent recrystallization, hot-injection, solvothermal, rapid quenching, self-template-oriented method and other physical methods), nano-morphologies and optical properties. Additionally, it should be noted the dimension mentioned below relates to the morphological structure, not the crystal structure.| Dimension | Elemental compositions | Synthesis methods | Morphology | Band gap | Emission property | Ref. |
|---|---|---|---|---|---|---|
| Notes: NC short for nanocrystal; NP short for nanoparticle; PL short for photoluminescence; FWHM short for full width at the half maximum; PLQY short for photoluminescence quantum yield; τave means average lifetime. | ||||||
| 0D | MA3Bi2Cl9 | Anion exchange from MA3Bi2Br9 | QDs (3.05 nm diameter) | Not mentioned | PL peak = 360 nm; FWHM = 50 nm; PLQY = 15% | 84 |
| MA3Bi2Br9 | Anti-solvent re-precipitation | PL peak = 423 nm; FWHM = 62 nm; PLQY = 12% | ||||
| MA3Bi2I9 | Anion exchange from MA3Bi2Br9 | PL peak = 540 nm; FWHM = 91 nm; PLQY = 0.03% | ||||
| MA3Bi2I9 | Ultrasonication from bulk crystal | 7.6 nm NC | 2.4 eV | PL peak = 558 nm; τave = 0.64 ns | 90 | |
| Cs3Bi2I9 | 6.2 nm NC | 2.5 eV | PL peak = 578 nm; τave = 0.89 ns | |||
| Rb3Bi2I9 | 12.6 nm NC | 2.4 eV | PL peak = 575 nm; τave = 1.19 ns | |||
| Cs3BiCl6 | Hot-injection method | Cubic NCs (9.8 ± 1.3 nm edge lengths) | Not mentioned | PL peak = 391 nm; FWHM = 60 nm | 91 | |
| Cs3BiBr6 | cubic NCs (10.9 ± 1.5 nm edge lengths) | |||||
| Cs3BiBr6 | Anti-solvent re-precipitation | Branch-like NCs (1.6 nm diameter) | 2.89 eV | PL peak = 438 nm; FWHM = 92.1 nm; PLQY = 22% | 92 | |
| Cs3Bi2Br9 | Anti-solvent re-precipitation | Quasi-spherical NCs (6 nm diameter) | Not mentioned | PL peak = 460 nm; FWHM = 45 nm; PLQE = 4.5% | 48 | |
| Cs3Bi2I9 | Hot-injection method | Polydisperse NCs (39.5 ± 9.3 nm diameter) | Absorbance maximum at 489 nm | Unclear | 93 | |
| Solvothermal | Hexagonal prisms NCs | 2.10 eV | Not mentioned | 94 | ||
| Cs2AgBiCl6 | Anti-solvent re-precipitation | Quasi-sphere NCs (5.0 nm diameter) | Absorbance peak at 367 nm | PL peak = 395 nm; FWHM = 68 nm; PLQE = 6.7% | 95 | |
| Cs2AgBiBr6 | Not mentioned | Absorbance peak at 440 nm | PL peak = 465 nm; FWHM = 82 nm; PLQE = 0.7% | |||
| Cs2AgBiI6 | Not mentioned | Absorbance peak at 500 nm | PL peak = 575 nm; FWHM = 69 nm; PLQE < 0.1% | |||
| Cs2AgBiBr6 | Hot-injection method | Cubic NCs (9.5 nm average size) | 2.52 eV | PL peak = 625 nm; τave = 7.5 ns | 96 | |
| Rb7Bi3Cl16 | Anti-solvent re-precipitation | Quasi-spherical NCs (1.85 ± 0.8 nm diameter) | 3.27 eV | PL peak = 437 nm; FWHM = 93 nm; PLQY = 28.43%; τave = 5.17 ns | 97 | |
| Cs2AgSb1−yBiyX6 (X = Br, Cl; 0 ≤ y≤1) | Hot-injection method | Cube-shaped NCs (∼10 nm diameter) | Absorption peak at ∼430 nm | PL peak = 478 nm and 610 nm | 98 | |
| 2D | (PEG6-NH3+)nCs3−nBi2Br9 | Fast cooling | Nanosheets (360 ± 94 nm lateral width; 5.1 ± 1.1 nm thickness) | 2.7 eV | Not mentioned | 99 |
| Cs3Bi2Cl3I6 | Hot-injection then quenching | Nanosheets (∼5 μm lateral width; 2–4 nm thickness) | 2.04 eV | PL peak = 496 nm; FWHM = 13 nm; τave = 6.693 ns | 100 | |
| Cs3Bi2Br9 | Hot-injection then quenching | Parallelogram nanoplates (of 60–250 nm side length; 9 nm thickness) | Absorption peak at 380![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm nm |
PL peak = 427 nm and ∼455 nm; FWHM = 39 nm; PLQY = 0.54% | 101 | |
| Fast cooling in acid solution | Nanoplates (2–40 μm basal plane size; 100–500 nm thickness) | 2.64 eV | PL peak = 480 nm | 87 | ||
| Cs3Bi2I9 | Spin-coat and annealing | Hexagonal nanosheets (∼15 μm lateral size; 57 nm thickness) | 1.99 eV | PL peak = 642 nm; FWHM = 100 nm | 102 | |
| Hot-injection then quenching | Nanodiscs (30–55 nm lateral width; 2–6 nm thickness) | 1.97 eV | PL peak = 580 nm | 103 | ||
| Self-template-oriented method | Nanosheets (200 nm lateral size; 6–8 nm thickness) | 1.90 eV | Not mentioned | 104 | ||
| Cs2AgBiBr6 | Hot-injection then quenching | Nanoplates (200 nm lateral size; 3–5 nm thickness) | 2.01 eV | PL peak = 630 nm | 105 | |
| Cs2AgBiBr6 | Solvothermal-quenching | Rectangular nanoplates (180 ± 130 nm edge length; 3.6–6 nm thickness) | 2.06 eV | PL peak = ∼640 nm | 106 | |
| Heterojunction | MA3Bi2I9/DMA3BiI6 | In situ growth | Rod-like DMA3BiI6 on sheet-like MA3Bi2I9 | 1.99 eV | PL peak = ∼650 nm | 88 |
| Cs3Bi2Br9/Ti3C2Tx | In situ growth | Cs3Bi2Br9 NCs (6 nm average size) on monolayer Ti3C2Tx nanosheets | 2.47 eV | PL peak = ∼500 nm; τave = ∼0.04 ns | 107 | |
| Cs3Bi2I9/Bi2WO6 | in situ growth | Cs3Bi2I9 NCs (∼7 nm average size) on Bi2WO6 nanosheets (50–100 nm size; 2.5 nm thickness) | Cs3Bi2I9: 1.93 eV; Bi2WO6: 2.69 eV | Not mentioned | 42 | |
Cs3Bi2I9/CeO2-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Electrostatic assembly | Cs3Bi2I9 nanosheets (200 nm lateral size; 6–8 nm thickness) on CeO2 nanosheets (20–30 nm transverse size; 1.5 nm thickness) | Cs3Bi2I9: 1.90 eV; CeO2: 2.82 eV | Not mentioned | 104 | |
| Cs3Bi2Br9/d-BiOBr | In situ growth | Cs3Bi2Br9 nanodots (12.1 nm average size) on BiOBr nanosheets (4.5 nm thickness) | Cs3Bi2Br9: 2.68 eV; d-BiOBr: 2.77eV | PL peak = 467 nm; τave = 11.5 ns | 108 | |
| Cs3Bi2Br9/M-TiO2 | In situ growth | Cs3Bi2Br9 or Cs2AgBiBr6 nanodots (3.9–9.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm) on spherical mesoporous TiO2 (15.8 nm pore size) nm) on spherical mesoporous TiO2 (15.8 nm pore size) |
Cs3Bi2Br9: 2.59 eV; | PL peak = ∼470 nm; τave = 11.2 ns | 109 | |
| Cs2AgBiBr6/M-TiO2 | Cs2AgBiBr6: 2.22 eV; M-TiO2: 3.15 eV | PL peak = ∼640 nm; τave = 11.3 ns | ||||
| Cs3Bi2I9/g-C3N4 | Ultrasonic mixing | Cs3Bi2I9 particles (45–50 nm) anchoring to the g-C3N4 sheets | Cs3Bi2I9: 1.88 eV; g-C3N4: 2.68 eV | Not mentioned | 110 | |
| Cs3Bi2Cl9 QD /(BiO)2CO3 | Ultrasonic mixing | Cs3Bi2Cl9 nanoparticles (2.59 ± 0.6 nm particle size) on rose-like (BiO)2CO3 | Cs3Bi2Cl9: 3.05 eV; (BiO)2CO3: 3.35 eV | Not mentioned | 111 | |
| Cs3Bi2Br9 QDs/In4SnS8 | In situ growth | Cs3Bi2Br9 QDs (5.6 nm diameter) on In4SnS8 nanoflower (2–4 nm layer thickness) | Cs3Bi2Br9: 2.94 eV; In4SnS8: 2.36 eV | PL peak = ∼500 nm; τave = 4.87 ns | 112 | |
| Cs3Bi2Br9/CdS | In situ growth | Cs3Bi2Br9 particles on CdS nanorods | Cs3Bi2Br9: 2.65 eV; CdS: 2.44eV | Not mentioned | 113 | |
| Cs2AgBiBr6/Ti3C2Tx | Ultrasonic mixing | Cs2AgBiBr6 NCs (32.2 nm average size) on Ti3C2Tx nanosheets | Absorption peak at ∼435 nm | PL peak = ∼660 nm | 114 | |
| Cs2AgBiBr6@g-C3N4 | In situ growth | g-C3N4 shells (2–3 nm thickness) on Cs2AgBiBr6 particles | Absorption edge around 650 nm | PL peak = 645 nm | 89 | |
| Cs2AgBiBr6 /Sr2FeNbO6 | Electrostatic assembly | Cs2AgBiBr6 NPs (20–50 nm particle size) on polyhedral-like Sr2FeNbO6 NPs (200–500 nm particle size) | Cs2AgBiBr6: 2.17 eV; Sr2FeNbO6: 2.04 eV | PL peak = ∼610 nm; τave = 3.14 ns | 115 | |
| Cs2AgBiBr6/Bi2WO6 | In situ growth | Cubic Cs2AgBiBr6 NCs (average size: 10 nm) on Bi2WO6 nanosheets (40–100 nm lateral size; 3 nm thickness) | Cs2AgBiBr6: 2.04 eV; Bi2WO6: 2.48 eV | PL peak = ∼575 nm; τave = 5.39 ns | 116 | |
3.1 0D BHPs
0D materials refer to the microstructure size in a nanoscale range (1–100 nm) in three dimensions, mainly including very small quantum dots (QDs) and particles. 0D perovskite materials usually show superiority in optoelectronic properties that benefit from their particular size and structure. The typical 0D BHPs can be prepared by various approaches such as ultrasonication, ligand-assisted antisolvent precipitation, hot-injection, and ion-exchange process.19,85,90,96 Ultrasonication from bulk crystals can provide a direct and simple method to obtain BHP nanocrystals. For example, S. Bhosale et al. applied an ultrasonic top-down method with the assistance of ligands to produce Rb3Bi2I9, Cs3Bi2I9, and MA3Bi2I9 BHPs at a nanometer scale.90 However, this ultrasonic process takes a long time (3–5 days) for preparation and fails to precisely modulate the nano-morphologies of BHPs. In contrast, antisolvent recrystallization, with a much shorter process time and better control of nanostructures, has attracted more attention for the synthesis of 0D BHP nanomaterials. Generally, in an antisolvent recrystallization process, the precursor (e.g., bismuth bromide) is first dissolved in a suitable solvent (e.g., DMSO), which is subsequently dropped into an anti-solvent to precipitate quantum dot crystals. In this process, some surfactants such as octylamine (OLA) and oleic acid (OA) are added as ligands to control the crystallization rate and stabilize the formed colloidal solution with a narrow particle-size distribution.For instance, Tang and co-workers prepared a stable colloidal solution containing MA3Bi2Br9 QDs by dissolving MABr and BiBr3 in dimethylformamide (DMF) and ethyl acetate with n-octylamine to form the precursor solution, which was dropwise added to the antisolvent octane with oleic acid under vigorous stirring.84 After centrifuging at 8000 rpm for 10 min, a clear pale-yellow colloidal solution was obtained with stably dispersed MA3Bi2Br9 QDs, whose average size in diameter was as small as 3.05 nm (Fig. 2a and b). Besides, high-resolution transmission electron microscopy (HRTEM) and X-ray diffraction (XRD) results revealed the trigonal crystal structure of this 0D BHP nanomaterial (Fig. 2d and e). Moreover, the photoluminescence quantum yield (PLQY) of MA3Bi2Br9 QDs could reach 12% with the emission peak located at 423 nm (Fig. 2f). Based on the time-resolved PL decay profile, MA3Bi2Br9 QDs exhibited excitation radiative recombination as the dominant path for fluorescent decay with a short-lived PL lifetime of 1.96 ns (Fig. 2g). Meanwhile, the PL emission peaks of MA3Bi2X9 QDs can be easily tuned from 360 to 540 nm via varying the halide ions from Cl to I. Analogous to hybrid organic–inorganic BHPs, all-inorganic counterparts can be facilely prepared by this antisolvent method as well.48 Without the usage of ligands, the formed Cs3Bi2X9 (X = Cl, Br and I) nanocrystals demonstrated a quasi-spherical shape with an average size of 6 nm. The photoluminescent emission of Cs3Bi2X9 nanocrystals was tuned from 400 to 560 nm by the facile change of halide anions, which was unfortunately accompanied by a quite low PLQY of 0.2%. Impressively, Lou et al. reported a dramatic improvement of PLQY up to 22% after covering the surface of Cs3Bi2Br9 QDs with octylammonium bromide and oleic acid, which also rendered the Cl counterpart a high PLQY of 62%.85 This promotion in optical properties was mainly attributed to the passivation of surface trap-states through steady ligand binding. In addition, the ligands on the surface can serve as a protective shell to enhance thermal stability with unchanged PLQY after heating Cs3Bi2Br9 QDs at 180 °C for 1 h. Basically, the elemental variation of halide anions in BHP QDs efficiently tunes the range of light absorption bands, while the surface chemical environment (e.g., with or without ligands) significantly affects the intensity of photoluminescence.82
 | ||
| Fig. 2 Properties of MA3Bi2Br9 QDs. (a) TEM image. (b) Typical optical image of a colloidal MA3Bi2Br9 solution. (c) Colloidal MA3Bi2Br9 solution under 365 nm UV light. (d) HRTEM image of a typical QD. The inset in the bottom right corner is the corresponding fast Fourier transform (FFT) image. (e) XRD patterns. (f) Absorption and PL spectra. (g) Time-resolved PL decay and fitting curve of a typical QD sample. Reproduced with permission from ref. 84. Copyright 2016 Wiley-VCH. | ||
Moreover, the Cs3Bi2X9 QD nanomaterials obtained by the antisolvent method can demonstrate halogen-associated catalytically active sites, successfully reducing CO2 to CO under visible light.86 In contrast to their Cl counterpart, the QDs with Br anions presented a narrower bandgap (2.62 eV), promoted charge separation/transport, and lower activation energy (1.84 eV) for the formation of COOH− intermediate. These superior physical–chemical properties of Cs3Bi2Br9 QDs boosted the photocatalytic performance with a CO yield of 134.76 μmol g−1 and selectivity of 98.7%. In addition, another useful hot-injection method assisted with metal ion insertion led to the fabrication of Cs3Bi2X9 (X = Cl or Br) nanocrystals with an average size of 11.8–13.3 nm, which involved the fast transformation (∼5 min) from Cs2BiX6 nanocrystals in the presence of additional BiX3 salts.91 Furthermore, Zhou et al. reported the synthesis of Cs2AgBiBr6 cubic nanocrystals (the size of 9.5 nm) for the first time using a similar hot-injection method, where the Cs-oleate solution was injected into the 1-octadecene solvent containing BiBr3, AgNO3 and ligands (e.g., OLA and OA) at 200 °C.96 The as-prepared Cs2AgBiBr6 nanocrystals exhibited visible light absorption with a bandgap of 2.52 eV, indicating a blue shift in comparison with the bulk phase (1.95 eV) because of the quantum confinement effect in nanocrystals.48 Meanwhile, the novel colloidal nanocrystals of Cs2AgBiI6, which are difficult to synthesize by direct methods, were successfully prepared via a post-ion-exchange approach.117 Briefly, the exchange reaction between Cs2AgBiBr6 nanocrystals and trimethylsilyl iodide (TMSI) at room temperature in toluene for minutes produced the pure Cs2AgBiI6 phase. Different amounts of added TMSI reagent resulted in the generation of alloyed Cs2AgBiBr6−yIy (0 ≤ y ≤ 6), not the physical mixtures of Br and I counterparts. More I− species gradually reduced the bandgap from 2.33 eV for Cs2AgBiBr6 to 1.75 eV for pure iodide, demonstrating the red shift of optical absorption edge from 500 to ∼700 nm. TEM data clearly showed the slight change in particle size from 9.1 nm of Cs2AgBiBr6 to 9.9 nm of Cs2AgBiI6 nanocrystals. The analogous Cl to Br exchange occurred in the presence of Cs2AgBiCl6 nanocrystals and trimethylsilyl bromide. Notably, the anion redistribution could take place even between different nanocrystals, where the mixed solution of Cs2AgBiCl6 and Cs2AgBiBr6 led to the formation of an alloyed composition Cs2AgBiCl6−yBry after several hours. However, these formed nanocrystals crashed out of the solution after exposure to light and air for a few days, which may be connected with the decomposition in the presence of water. On the whole, this anion-exchange method not only developed novel BHP materials with mixed halides that may be hard to prepare by direct-synthesis methods (e.g., iodide ones), but also preserved the nanostructures of BHP precursors.118 In addition to the anion-exchange, by adding other cations (Mn2+,Yb3+,etc.) into precursors, Mn/Yb-doped BHPs could be synthesized, which exhibited superior stability.119,120
So far, 0D BHP nanomaterials have been successfully synthesized by various methods. Compared with the complicated hot-injection and solvothermal method, antisolvent recrystallization can present a few advantages, such as mild synthesis conditions, large-scale preparation, and broad scope for different precursors. Furthermore, the electronic structure and physicochemical properties of 0D BHP nanomaterials can be precisely regulated by tuning the metal ions and halide species. However, the stability of 0D BHP nanomaterials with small sizes in aqueous phase or polar solvents still requires substantial improvement for practical applications.
3.2 2D BHPs
The reported cases have demonstrated that 2D perovskite nanosheets have better stability,87,121 abundant active sites,122 and shorter charge transfer distance from the bulk to the surface of photocatalysts.123,124 The tunable thickness and preferential exposure of active crystal facets could bring 2D BHP nanomaterials improved stability and excellent photocatalytic activity. Compared with the bulk crystal materials with irregular shapes, the shorter charge transfer distance endows 2D BHP nanomaterials with faster charge transport and promotes charge separation. Generally, 2D BHP nanomaterials can be obtained by controlled crystallization and mechanical exfoliation processes. Through the regulation of synthesis parameters, the thickness of 2D BHP nanosheets can be precisely tuned from 2 to 500 nm. However, the synthesis of ultrathin BHP nanomaterials with atomic monolayers is still unavailable.Recently, 2D BHP materials have been successfully synthesized by controlled crystal growth processes. For instance, Dai et al. provided a facile and rapid synthetic protocol to prepare Cs3Bi2Br9 nanoplatelet crystals in one minute.87 They used ethyl acetoacetate (EA) as the structure-directing agent, which selectively interacted with bismuth atoms via two carbonyl groups to control the crystal growth in an acidic solution. In detail, the halide precursors (CsBr and BiBr3) and EA were first dissolved in a dilute H2SO4 solution. Then, the clear acidic solution was placed in liquid nitrogen for rapid cooling under vigorous stirring for ∼1 min (Fig. 3a). The hexagonal 2D BHP nanoplatelets with a basal size of 2–10 μm and a thickness of ∼100 nm could be fabricated. The size of the formed 2D materials varied from 100 to 500 nm by adjusting the concentrations of BHP precursors in diluted H2SO4 (Fig. 3b–f). The elemental mapping results from the nanoplatelet sample showed a quite homogeneous distribution of Cs, Bi, and Br elements in all regions (Fig. 3g–i). Furthermore, the quantitative analysis of elemental content based on energy dispersive X-ray spectroscopy (EDX) revealed that the ratio of Cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Bi
Bi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Br corresponded to 3.2
Br corresponded to 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0
2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.9, matching well with the theoretical value (Fig. 3j). As shown in Fig. 3k, the intensity of photoluminescence peak (around 480 nm) correlates well with the thickness of Cs3Bi2Br9 nanoplatelets. The larger and thicker microcrystals with fewer trap states exhibit weaker photoluminescence. Thus, the appropriate thickness affords Cs3Bi2Br9 nanoplatelets the suppressed radiative recombination behavior of charge carriers and abundant active sites for the activation of C–H bonds. Due to the rigid framework of these platelet microcrystals with fewer defects and suppressed charge recombination, the Cs3Bi2Br9 nanoplatelets demonstrated satisfactory photocatalytic performance of toluene oxidation to benzaldehyde with decent selectivity (≥88%) and stability (no deactivation after 36 h).
8.9, matching well with the theoretical value (Fig. 3j). As shown in Fig. 3k, the intensity of photoluminescence peak (around 480 nm) correlates well with the thickness of Cs3Bi2Br9 nanoplatelets. The larger and thicker microcrystals with fewer trap states exhibit weaker photoluminescence. Thus, the appropriate thickness affords Cs3Bi2Br9 nanoplatelets the suppressed radiative recombination behavior of charge carriers and abundant active sites for the activation of C–H bonds. Due to the rigid framework of these platelet microcrystals with fewer defects and suppressed charge recombination, the Cs3Bi2Br9 nanoplatelets demonstrated satisfactory photocatalytic performance of toluene oxidation to benzaldehyde with decent selectivity (≥88%) and stability (no deactivation after 36 h).
 | ||
| Fig. 3 (a) The synthesis procedure for Cs3Bi2Br9 nanoplatelet microcrystals grown in dilute H2SO4 acidic solution with EA as directing agent via rapid cooling down in a liquid nitrogen bath for ∼1 min. (b) Scanning electron microscope (SEM) image of the irregular crystals grown without the addition of EA. (c–f) SEM images of the platelet crystals grown in the presence of EA with different perovskite concentrations. (g–i) Scanning transmission electron microscopy (STEM)-based elemental mapping of the thinnest platelets. (j) SEM-EDX-based elemental analysis of the thinnest nanoplatelets. (k) Steady-state PL spectra of the Cs3Bi2Br9 platelets synthesized with different perovskite concentrations (10, 8, 6, and 4 g L−1). Reproduced with permission from ref. 87. Copyright 2021 Wiley-VCH. | ||
Besides, given the important influence of temperature on the crystallization process, synthesis in a high-temperature environment (hot-injection or solvothermal method) followed by fast quenching in ice water becomes a distinctive method to fabricate 2D BHP nanosheets. For example, Kundu et al. reported the synthesis of 2D layered Cs3Bi2I6Cl3 by a hot-injection method followed by fast quenching.100 They demonstrated that during the reaction temperature increase from 120 °C to 180 °C, the initially formed nanocrystals (Fig. 4a) transformed to nanosheets (with the lateral size of ∼5 μm and thickness of 2–4 nm) completely (Fig. 4b). In comparison with the bulk sample, the Cs3Bi2I6Cl3 nanosheets demonstrated a narrow and blue-shifted band-edge emission (peak at 520 nm) with a small Stokes shift (0.12 eV) (Fig. 4c–f). This may come from quantum confinement effect appearing on the unique 2D structure. Coincidentally, Huang et al. reported the effect of precursor concentrations on tuning the morphology of Cs2AgBiBr6.105 By increasing precursor concentrations, they successfully controlled the morphology varying from 0D nanocubes to 2D nanosheets, which demonstrated the thickness of 3–5 nm and lateral size of ∼200 nm. The 2D Cs2AgBiBr6 nanosheets exhibited a strong absorption peak at ∼430 nm with the PL emission located at ∼630 nm. These results illustrated that high temperature and concentration may accelerate the diffusion of reactant and weaken the ligand–surface binding, which contribute to the fabrication of 2D nanosheets.
 | ||
| Fig. 4 (a) TEM image of Cs3Bi2I6Cl3 nanocrystals synthesized in 120 °C. (b) TEM image of Cs3Bi2I6Cl3 nanosheets synthesized in 180 °C. (c) Solid-state electronic absorption (α/S) (black) and PL (red) spectra of Cs3Bi2I6Cl3 bulk powders. (d–f) UV/Vis absorption (black) and PL (red) spectra of (d) Cs3Bi2I6Cl3 NCs, (e) Cs3Bi2I6Cl3 NCs-NSs, and (f) Cs3Bi2I6Cl3 NSs measured in solution phase. Inset in (d) is the photograph of NCs solution in the absence (left) and presence (right) of UV light. Reproduced with permission from ref. 100. Copyright 2021 Wiley-VCH. | ||
In addition, Ji et al. prepared Cs3Bi2I9 hexagonal nanoplates by an ultrasound-based method, which exfoliated bulk BHP samples obtained from the antisolvent recrystallization process.143 However, this physical exfoliation method failed to precisely control the thickness of the above Cs3Bi2I9 nanoplates. Similarly, Qi et al. adopted the spin-coating and annealing approach to fabricate the film-like Cs3Bi2I9 with a hexagonal shape (lateral size of ∼15 μm and thickness of 57 nm). The 2D BHP material can also be prepared by using a suitable structural template. For instance, Feng et al. synthesized 2D lead-free halide perovskite (Cs3Bi2I9) nanosheets by a self-templating method with BiOI/Bi2O2.7 nanosheets as the scaffold.104 With the presence of HI and CsI in an alcohol solution, BiOI/Bi2O2.7 nanosheets with a thickness of 6 nm were gradually eroded by HI to release Bi3+ ions for the nucleation of Cs3Bi2I9. Simultaneously, the nanostructure of BiOI/Bi2O2.7 was reprinted after its complete transformation to pure Cs3Bi2I9 nanosheets with a thickness of around 6–8 nm. However, this self-template-oriented approach requires the complicated preparation of sacrificial materials and fails to control the lateral dimension of 2D nanosheets because of the vigorous corrosion process.
Overall, the synthesis of 2D BHP nanomaterials requires careful control of crystallization conditions with complicated processes (e.g., high-temperature step plus rapid cooling). To precisely regulate the thickness and lateral size of 2D nanosheets, organic ligands or template materials are always essential to direct the growth orientation of BHP crystals. However, atomic ultra-thin 2D BHP nanomaterials are still unavailable. Besides, a more effective and simple synthesis method is needed to avoid the usage of ligands for the construction of a clean perovskite surface.
3.3 BHP heterostructures
To promote the charge separation with a longer lifetime and facilitate the redox reactions on the surface, the construction of heterojunction-based photocatalysts (such as type II, Z-scheme and Schottky junctions)42,125–127 is considered as one promising protocol for improved photocatalytic efficiencies.60 The main methods used to assemble BHP heterojunctions include in situ growth, electrostatic assembly and ultrasound mixing. Among them, the in situ growth method is the most common and effective method. For example, Tang et al. reported the synthesis of air-stable MA3Bi2I9/DMA3BiI6 perovskite heterojunctions via a solvothermal in situ growth strategy.88 During the solvothermal process, the added DMF co-solvent underwent hydrolysis to produce dimethylamine, which was easily protonated to form [(CH3)2NH2]+ ions (DMA+) in the acidic environment. Thus, the rod-like DMA3BiI6in situ grew on the surface of sheet-like MA3Bi2I9, which was firstly formed due to the fast reaction between CH3NH2I and Bi3+ ions at room temperature before the solvothermal treatment. Besides, the state and composition of the final heterojunction product depended on the reactant concentration and reaction temperature. As expected, the optical absorption of this composite presented a combination of light responses from the respective pure phases. Correspondingly, the bandgap of the MA3Bi2I9/DMA3BiI6 composite should lie between that of the pure phases, which does not agree with the conclusion in the paper that the heterojunction had the smallest bandgap of 1.99 eV. Furthermore, the as-prepared type II MA3Bi2I9/DMA3BiI6 heterojunction accelerated the diffusion of charge carriers with improved charge mobilities, offering an extended diffusion lifetime of ∼38 ns and a lower charge transfer resistance of 1.15 kΩ cm2. Eventually, the MA3Bi2I9/DMA3BiI6 heterojunction exhibited promoted photocatalytic H2 evaluation (rate of 198.2 μmol h−1 g−1) without any co-catalyst under visible light irradiation.On the other hand, the formation of a direct Z-type heterojunction by 2D/2D contact can be another effective route to fabricate advanced photocatalysts with improved photocatalytic performance.128 Firstly, the large interfacial contact areas, abundant charge transfer channels and shortened charge transfer distance of 2D/2D heterojunctions benefit the rapid transfer and separation of photogenerated charge carriers. Secondly, the Z-type heterojunction can maximally maintain the redox ability of each component.128 As shown in Fig. 5a, Feng et al. constructed a Z-type heterojunction of Cs3Bi2I9/CeO2 by simple electrostatic assembly (Fig. 5a).104 The Cs3Bi2I9 and CeO2 nanosheets were fully dispersed in n-hexane with ultrasonic mixing and continuous stirring. Due to the attraction of the surface electrostatic charges, these two components were successfully assembled into the heterojunctions. TEM and elemental mapping analysis clearly indicated the close contact between Cs3Bi2I9 and CeO2 nanosheets (Fig. 5b–h). Furthermore, X-ray photoelectron spectroscopy (XPS) tests under light irradiation clearly showed the positive shifts (0.1–0.29 eV) of the binding energies from Ce 3d and O 1s, while two new signals appeared at lower energy positions for Bi 4f (Fig. 5i–k). This suggests the efficient electron transfer behavior from CeO2 to Cs3Bi2I9 nanosheets due to the presence of Z-scheme heterojunctions. Moreover, based on the results from electron paramagnetic resonance (ESR) tests, most holes were transferred from Cs3Bi2I9 to accumulate on the CeO2 phase with the strengthened DMPO-OH˙ signal by oxidation water (Fig. 5l). Therefore, the Z-scheme heterojunction of Cs3Bi2I9/CeO2 can provide powerful electronic modulation to separate charges with enhanced redox capabilities, consequently showing excellent activity for CO2 photoreduction with a high electron consumption rate of 877 μmol g−1.
 | ||
Fig. 5 (a) The preparation of Cs3Bi2I9 nanosheets and the self-assembly between Cs3Bi2I9 and CeO2 nanosheets. (b) HRTEM image of Cs3Bi2I9/CeO2-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 assembly. (c–h) High-angle annular dark field (HAADF) image and elemental mapping images of Cs3Bi2I9/CeO2-3 1 assembly. (c–h) High-angle annular dark field (HAADF) image and elemental mapping images of Cs3Bi2I9/CeO2-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. (i–k) High-resolution XPS spectra of (i) Ce 3d, (j) O 1s and (k) Bi 4f for Cs3Bi2I9/CeO2-3 1. (i–k) High-resolution XPS spectra of (i) Ce 3d, (j) O 1s and (k) Bi 4f for Cs3Bi2I9/CeO2-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under dark and light excitation. (l) ESR spectra of Cs3Bi2I9, CeO2 and Cs3Bi2I9/CeO2-3 1 under dark and light excitation. (l) ESR spectra of Cs3Bi2I9, CeO2 and Cs3Bi2I9/CeO2-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with DMPO as the radical trap. Reproduced with permission from ref. 104. Copyright 2021 Science Press and Dalian Institute of Chemical Physics, Chinese Academy of Sciences. 1 with DMPO as the radical trap. Reproduced with permission from ref. 104. Copyright 2021 Science Press and Dalian Institute of Chemical Physics, Chinese Academy of Sciences. | ||
On the whole, several preparation methods such as ultrasonic mixing, electrostatic assembly, and in situ growth have been applied to fabricate BHP heterojunctions.42,104,110,114 Compared with pristine BHP materials, the combination of BHP with other semiconductors (such as TiO2, C3N4, CdS, Sr2FeNbO6) can engineer band structures with Z-scheme or type-II heterojunctions. The charge diffusion path and electron–hole recombination are thus optimized in these composites, which can dramatically promote their photocatalytic performance.89,109,113,115
4. Applications of BHP photocatalysts
Compared with homogeneous photocatalysis, heterogeneous photocatalysis demonstrates the following merits:129 (1) facile separation and recovery of solid-state photocatalysts. (2) Lower costs in the preparation of photocatalysts. Cheap inorganic compounds or organic precursors are adopted in the heterogeneous cases, while expensive metal complexes are usually required in homogeneous photocatalysis. (3) Potential for large-scale and continuous production modes via fixed-bed reactors.130 Given the advantages of a high light absorption coefficient, tunable band structures, versatile surface chemical environment and good stability, BHP nanomaterials can serve as promising heterogeneous photocatalysts with visible-light response for various significant reactions. In recent years, several BHP nanomaterials have been applied for diverse photocatalytic reactions under visible light, including H2 generation,88,103,131 CO2 reduction,42,104,132 organic synthesis,49,87,133 pollutant degradation134 and photoinduced polymerization.135,1364.1 H2 generation
Photocatalytic H2 generation has received great interest for the green production of hydrogen fuel and the storage of sustainable solar energy.137,138 Recently, BHP photocatalysts have been explored for hydrogen production with decent activity under visible light illumination.45 Since BHP materials are easily decomposed in water, most of the photocatalytic hydrogen generation tests using BHP materials were carried out in acidic HX (X = Br, I) solutions(a few in ethanol or aqueous solution). The presence of halide ions from HX acids can suppress the reaction equilibrium of BHP decomposition and enhance the stability of BHP materials. Table 2 summarizes photocatalytic H2 production performance with different reaction conditions over BHP photocatalysts.| Catalyst | Solution | Irradiation conditions | Performance (μmol g−1 h−1) | Stability (h) | Ref. |
|---|---|---|---|---|---|
| PtIx/[(CH3)2NH2]3[BiI6] | H3PO2/HI = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
425 nm LED Lamp | 46.625 | >100 | 139 |
| Pt/MA3Bi2I9 | Aqueous HI solution | 300 W Xe-lamp (λ ≥ 400 nm) | 169.21 | >70 | 45 |
| Pt/MA3Bi2Cl8.8I0.2 | Saturated HCl/HI solution/H3PO2 | 300 W Xe-lamp (λ ≥ 420 nm; 100 mW cm−2) | 341 ± 61.7 | 5 | 140 |
| Pt-DA3BiI6 | HI and DAI solution | LED lamp | 216 | 5 | 141 |
| Pt/Cs3Bi2I9 nanodiscs | Aqueous HI solution/H3PO2 | AM 1.5G (150 mW cm−2) | 225 | 8 | 103 |
| Pt/Cs3Bi0.6Sb1.4I9 | Aqueous HI solution | AM 1.5G (100 mW cm−2) | 926 | 50 | 110 |
| Pt/Cs3Bi2I9/g-C3N4 | MeOH aqueous solution | 450 W Xe-lamp | 920.76 | 6 | 110 |
| Pt/Cs3Bi2Br9/g-C3N4 | Distilled water containing 10% triethanolamine | 1500 W Xenon lamp (300–800 nm; 500 W m−2) | 1050 | 6 | 142 |
| Cs3Bi2I9 nanosheets | Ethanol solution | 300 W Xe lamp (790 mW cm−2) | 2157.8 | 2 | 143 |
| Cs3Bi2I9 hexagonal prisms NCs | HI/ethyl acetate | AM1.5G (100 mW cm−2) | 1504.5 | 8 | 94 |
| MA3Bi2I9/DMA3BiI6 | Aqueous HI solution | 300 W Xe-lamp (λ ≥ 420 nm; 100 mW cm−2) | 198.4 | 100 | 88 |
| Cs2AgBiBr6/RGO | Saturated HBr and H3PO2 solution | 300 W Xe-lamp (λ ≥ 420 nm) | 48.9 | 120 | 131 |
| Cs2AgBiBr6/nitrogen-doped carbon (N–C) | Aqueous HBr solution | 300 W Xe-lamp (λ ≥ 420 nm; 100 mW cm−2) | 380 | 24 | 144 |
Guo et al. prepared an organo-inorganic hybrid perovskite material (CH3NH3)3Bi2I9 by a simple hydrothermal method, which presented a photocatalytic hydrogen production rate of 12.19 μmol g−1 h−1 in HI solution.45 After platinum was deposited as a co-catalyst, the rate for H2 evolution approached 169.21 μmol g−1 h−1 due to the improved charge separation and more active sites. Obviously, the addition of platinum gives an outstanding improvement in photocatalytic H2 generation rates.45,110,139,140,142,145 However, the natural scarcity and high price of noble metals limit their wide application. Therefore, designing high-performance BHPs for photocatalytic H2 generation without noble metals is challenging. Modulating the nanostructures of BHPs to improve photocatalytic H2 evolution performance may be a promising approach. Ji et al. applied Cs3Bi2X9 nanosheets to photocatalytic H2 evolution in ethanol solutions.143 The Cs3Bi2I9 nanosheets with a coplanar octahedral structure afford the shortest Bi–Bi distance, which is beneficial for carrier transport, significantly enhancing the photocatalytic H2 evolution rate (2157.8 μmol h−1 g−1) (Fig. 6a). The photocurrent response of the photocatalysts (Fig. 6b) and recycling experiments illustrated that the Cs3Bi2I9 nanosheets could maintain photocatalytic activity for several cycles with the satisfactory stability. In a similar way, Li et al. fabricated ordered BHPs Cs3Bi2I9 hexagonal prisms with well-defined (100) and (006) facets(Fig. 6c).94 Photogenerated holes and electrons in the nanocrystals were spatially separated to (100) and (006) facets, respectively, owing to a built-in electric field between the crystalline facets of ∼130 meV in these hexagonal prisms (Fig. 6d). Thus, Cs3Bi2I9 hexagonal prisms showed outstanding activity for photocatalytic H2 generation (1504.5 μmol h−1 g−1) by HI splitting. Obviously, the well-designed nanostructure of Cs3Bi2I9 crystals with the prism morphology could orientate the charge diffusion direction to improve the separation of electron–hole pairs, consequently resulting in the enhanced H2 evolution. Although this Cs3Bi2I9 photocatalyst showed stability over 8 h irradiation, some tiny solid iodine (I2) particles were generated on the (100) facets (Fig. 6e), which may have a negative impact on the photocatalyst over a longer time test by covering active sites and blocking light absorption. These works demonstrated that it is possible to fabricate 0D or 2D high-efficiency BHP-based photocatalysts by regulation of geometric structures (constructing nanocrystals, or nanosheets, or prisms) and surface chemical micro-environment (decorating co-catalysts).
 | ||
| Fig. 6 (a) Schematic mechanism of H2 evolution by Cs3Bi2X9 nanosheets in ethanol. (b) Transient photocurrent responses of the photocatalysts. Reproduced with permission from ref. 143. Copyright 2022 Wiley-VCH. (c) Morphology and crystalline structure of Cs3Bi2I9 hexagonal prisms. (d) H2 production process and anisotropic charge transfer to the different redox facets. (e) SEM image of the photocatalysts after reaction for 8 h. Reproduced with permission from ref. 94. Copyright 2022 American Chemical Society. | ||
On the other hand, the photocatalytic performance of BHPs may be improved by combining them with other semiconductors to establish nanocomposites, which offer improved charge diffusion and diverse surface chemical environments.60 For example, Wang et al. synthesized a composite consisting of Cs2AgBiBr6 (CABB) and reduced graphene oxide (RGO) for photocatalytic hydrogen production in a saturated HBr solution (Fig. 7a).131 The CABB/RGO composite presented higher photocatalytic activity than pristine CABB due to the contribution from RGO with promoted charge separation/transport. Under visible light irradiation, the hydrogen production rate of the CABB/RGO photocatalyst reached up to 489 μmol g−1 within 10 h when the loading of RGO was 2.5% (Fig. 7b). Meanwhile, CABB/2.5% RGO remained stable after consecutive irradiation of 120 hours (Fig. 7c). Based on the mechanistic study, firstly CABB generated electrons and holes as the light absorber under visible light irradiation. Then, the photogenerated electrons could transfer to conductive RGO through the M–O–C bonds, which was suggested by Fourier transform infrared (FTIR) spectra of as-prepared samples (Fig. 7d). Subsequently, the electrons reduced H+ to H2 at the active sites of RGO. Meanwhile, Br− was oxidized to Br3− by the holes on the surface of CABB nanoparticles. Besides, the XPS measurement indicated that the chemical environment of bismuth species on the catalyst surface was changed after 12 cycles of photocatalytic H2 evolution tests (Fig. 7e). These characterization data may suggest the recrystallization of CABB or the generation of other unclear bismuth species as active sites during photocatalysis. It should be noted that the nature of active sites on RGO for H2 evolution is mysterious as well. These active sites play key roles since they not only capture the electrons from CABB but also provide the adsorption sites for hydrogen atoms. In addition to pure carbon materials, some nitrogen-containing carbon materials (C3N4 or N–C) have been combined with BHPs to promote photocatalytic H2 evolution as well.110,144
 | ||
| Fig. 7 (a) The photocatalytic H2 generation over Cs2AgBiBr6/RGO (CABB/RGO) under visible light irradiation. (b) H2 evolution activities of as-prepared catalysts, including CABB /xRGO composites with different contents of RGO, Pt/CABB, the pristine CABB and RGO. (c) The cycling tests of CABB/2.5%RGO for photocatalytic H2 generation. (d) FTIR spectra of CABB/xRGO composites, CABB, and GO. (e) Bi 4f of CABB/2.5%RGO before and after 12 cycles of photocatalytic H2 evolution tests. Reproduced with permission from ref. 131. Copyright 2019 Elsevier. | ||
Even though BHPs have exhibited photocatalytic activity in hydrogen evolution under visible light, the essential presence of the HX acidic environment limits their practical applications due to the corrosive nature and high cost of HX species. More research work should be performed to design robust BHP photocatalytic systems. For example, the core–shell nanomaterials with protective and active shells encapsulating BHP cores may drive the whole water splitting under neutral conditions.14,146
4.2 CO2 reduction
Reducing atmospheric carbon dioxide levels is significant for environmental remediation to achieve ambitious climate goals such as net-zero greenhouse gas emissions by 2050.147 Among diverse technologies for CO2 reduction, solar-driven photocatalytic CO2 reduction has gained much attention due to the clean and sustainable process of photocatalysis with the production of valued chemicals (e.g., CH4, CH3OH, or HCOOH).15Table 3 summarizes recent studies about BHP photocatalysts for CO2 reduction, including liquid-phase and gas–solid reaction systems. Recently, BHP materials have been employed as promising photocatalysts for CO2 reduction. In the photocatalytic reduction of CO2 to CO at the gas–solid interface with H2O as a hydrogen source, a series of A3Bi2X9 (A = Cs, Rb, MA; X = Br, I) nanocrystals was systematically investigated.90,132 The photocatalytic activities of CO2 reduction over various BHP photocatalysts under visible light irradiation demonstrated a trend of Cs3Bi2I9 > Rb3Bi2I9 > MA3Bi2I9 » TiO2.90 The poor activity from TiO2 mainly came from its wide bandgap without visible-light response. For the case of organic–inorganic BHP of MA3Bi2I9, its self-oxidation of MA species by holes and the restricted charge transport retarded CO2 photoreduction. In contrast, Cs3Bi2I9 presented much higher activities with a methane yield of 14.9 μmol g−1 and CO yield of 77.6 μmol g−1. In combination with the superior charge transfer in Cs3Bi2I9, the BiI6 units as Lewis acid sites could form complexes with CO2 to offer the chemisorption of CO2, synergistically resulting in the high photocatalytic activity for CO2 reduction. Furthermore, the modification of halide species of Cs3Bi2X9 (X = Br, I) could tune the activity and selectivity for CO2 photoreduction. As a result, Cs3Bi2(Br0.5I0.5)9 NCs exhibited the optimal activity with 54 μmol g−1 of CO yield and 100% CO selectivity under visible light.132 This selective photocatalytic performance mainly originated in the suitable band structure of Cs3Bi2(Br0.5I0.5)9 with a broad light absorption range, which led to the generation of abundant photoinduced charges with suitable redox capability.| Catalyst | Reaction solution | Irradiation conditions | Performance (μmol g−1 h−1) | Ref. | |
|---|---|---|---|---|---|
| CO | CH4 | ||||
| Cs3Bi2Br9 QDs | Gas–solid reaction system | AM 1.5G | 26.95 | — | 86 |
| Cs3Bi2(Br0.5I0.5)9 NCs | Gas–solid reaction system | 300 W Xe lamp (λ ≥ 420 nm) | 18 | — | 132 |
| Cs2NaBiCl6 porous microsphere | Gas–solid reaction system | 300 W Xe lamp | 30.22 | 1.12 | 148 |
| Cs2AgBiBr6 NCs (washed) | Ethyl acetate | AM 1.5G (150 mW cm−2) | 2.35 | 1.6 | 96 |
| Cs2AgBiBr6 NPs | Ethyl acetate | a 405 nm laser diode | 1 | 0.67 | 106 |
| Cs2AgBiI6 NCs | Gas–solid reaction system | 300 W Xe lamp (λ ≥ 420 nm) | 6.3 | — | 149 |
| Cs3Bi2I9/Bi2WO6 | Gas–solid reaction system | Xe lamp (100 mW cm−2) | 7.3 | — | 42 |
| Cs2AgBiBr6@g-C3N4 | Ethyl acetate/methanol | Xenon lamp (AM 1.5G; 150 mW cm−2) | 0.6 | 1.5 | 89 |
| Cs3Bi2Br9/MCM-41 | Gas–solid reaction system | 300 W Xe lamp (350 mW cm−2) | 17.24 | — | 150 |
| Cs3Bi2I9/CeO2 | Gas–solid reaction system | 300 W Xe lamp (200 mW cm−2) | 15 | 5 | 104 |
| Cs2AgBiBr6/Sr2FeNbO6 | Ethyl acetate/H2O | 300 W Xe lamp (λ ≥ 420 nm) | 50.00 | 8.12 | 115 |
| Cs2AgBiBr6-Cu-RGO | Gas–solid reaction system | 300 W Xe lamp (AM-1.5G) | 1.9 | 10.7 | 151 |
| TiO2/Cs3Bi2Br9 nanodots | Isopropanol | 300 W Xe lamp (λ = 200–1100 nm; 70 mW cm−2) | 4.54 | 24.2 | 109 |
| TiO2/Cs2AgBiBr6 nanodots | 4.19 | 32.9 | |||
| Cs3Bi2Br9 QDs/In4SnS8 | Gas–solid reaction system | 300 W argon lamp (λ > 420 nm) | 9.55 | — | 112 |
| Cs2AgBiBr6/Ti3C2Tx | Gas–solid reaction system | Xe lamp (λ > 400 nm; 150 mW cm−2) | 11.1 | 1.3 | 114 |
| Cs2AgBiBr6/Bi2WO6 | Ethyl acetate/isopropanol | 300 W Xe lamp (AM-1.5G; 100 mW cm−2) | 42.19 | 0.41 | 116 |
Besides, double perovskite-based nanomaterials have also been applied in CO2 photoreduction. In 2018, Zhou et al. reported that Cs2AgBiBr6 photocatalysts prepared by a hot-injection method converted CO2 into CO and CH4 under simulated sunlight with an electron consumption of 105 μmol g−1.96 After washing away the ligands on the surface, the photocatalytic performance was further promoted with a quantum efficiency of 0.028% at 398 nm due to the removal of ligand shells, which may dampen the transfer of charges from photocatalysts to reactants. However, the solvent of this photocatalytic system was an organic compound (i.e., ethyl acetate), which may be the real source for the production of CH4 or CO under light irradiation in the presence of perovskite photocatalysts.90 Thus, the isotopic labeling test is required by use of 13CO2 to evidence the occurrence of CO2 photoreduction if there are other organic species in the system. Later, the Cs2AgBiI6 nanocrystals synthesized by the antisolvent recrystallization also exhibited highly selective CO2 photoreduction with a CO yield of 18.9 μmol g−1 and 100% CO selectivity.149 The usage of organic toluene as a solvent for CO2 reduction may introduce carbonaceous contamination as well.
The fabrication of heterojunctions via combining BHPs with other semiconductors can further improve the catalytic activity of CO2 photoreduction through promoted charge separation/transport. Liu et al. designed the in situ growth of Cs3Bi2I9 crystals on the surface of BiWO6 nanosheets to construct a Z-type heterojunction.42 Because of the shared bismuth atoms at the interface between BiWO6 and Cs3Bi2I9, the formed strong interaction could promote the interfacial charge transfer (Fig. 8a). As a result, the CO2-to-CO conversion rate of Cs3Bi2I9/Bi2WO6 heterojunction incredibly grew up to 66 μmol g−1 (Fig. 8b), which was more than four times higher than that of pristine Cs3Bi2I9 nanocrystals or their physical mixture (Cs3Bi2I9·Bi2WO6). As shown in Fig. 8c, the Cs3Bi2I9/Bi2WO6 photocatalyst could retain photocatalytic performance over three cycles. However, the increase in CO production rate was non-linear, which might be caused by the adsorption of some organic products on the surface of heterojunctions. Moreover, the photoinduced electrons from Bi2WO6 could effectively transfer to Cs3Bi2I9 in the composite, which was evidenced by the negative shifts (0.2–0.35 eV) of binding energies from Cs 3d and I 3d and the positive shifts in W 4f and O 1s spectra based on in situ XPS measurements (Fig. 8d–g). This charge transportation matched well with the proposed Z-type heterojunction mechanism, bringing enhanced charge separation for superior photocatalytic properties. Similarly, Sun et al. reported halide perovskites CBB (Cs3Bi2Br9), and CABB (Cs2AgBiBr6) grown in situ in a mesoporous titania (M-Ti) framework for efficient CO2 reduction reaction.109 The combination of BHP and M-Ti led to the remarkable CH4 production rates of 32.9 μmol g−1 h−1 and selectivity of 88.7% (Fig. 9a and d). This is the highest photoreduction rate of CO2 to CH4 for BHP nanomaterials among the known reports so far. The photocatalytic activity of these heterojunctions hardly decreased after five cycles, and the production rates of CO and CH4 remained constant (Fig. 9b, c, e and f). Moreover, no significant changes were observed in other characterizations (XRD, TEM, EDX mapping), indicating the high stability of BHP@M-Ti heterojunctions. In situ XPS (Fig. 9g–i) revealed the interfacial charge-transfer pathway of CBB@M-Ti under light. This result proved that the inner surface built-in electric field between the perovskite nanodots and mesoporous titania channels can efficiently promote photoinduced charge transfer (Fig. 9j). Moreover, the micromorphology of the titania frameworks with mesopores (specific surface area of 39.4 m2 g−1, pore size of 13.6 nm, and pore volume of 0.134 cm3 g−1) gave it excellent CO2 enrichment ability (with a CO2 uptake of 3.4 cm3 g−1), which benefits photocatalytic CO2 reduction with higher reactant concentrations. Meanwhile, the electron-rich perovskite phase with the nanodot shape can afford abundant active centers to smoothly transfer plenty of electrons for CO2 reduction. Thus, the combination of these nanostructures gives rise to the high methane production rate. Additionally, combination with other semiconductors (e.g., C3N4, Sr2FeNbO6, In4SnS8, Ti3C2Tx) also promoted the CO2 conversion rates in photoreduction.89,112,114,115 There is no doubt that the construction of heterojunctions is a feasible and effective way to improve photocatalytic performance by modulating charge transport.
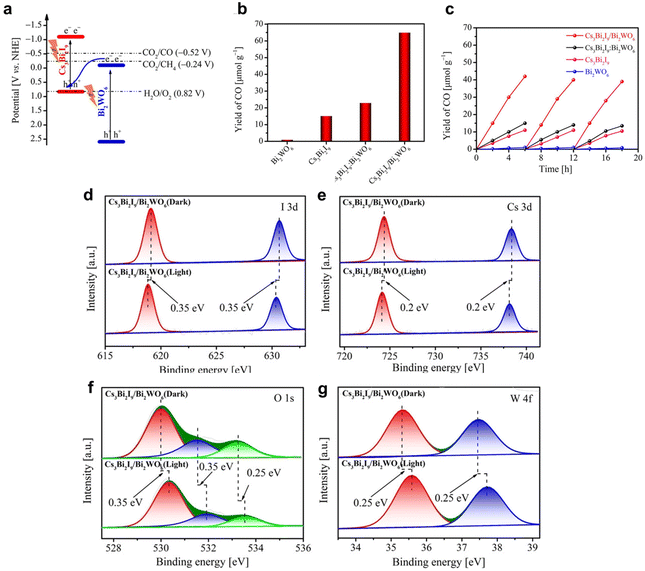 | ||
| Fig. 8 (a) The band structures for Cs3Bi2I9 and Bi2WO6. (b) The yield of CO generated from photocatalytic CO2 reduction after 9 h of irradiation. (c) Recycling stability tests of as-prepared samples with three cycles. (d–g) High-resolution XPS spectra of Cs3Bi2I9/Bi2WO6 in the dark or under 300 W Xe lamp irradiation with a 400 nm filter: (d) I 3d, (e) Cs 3d, (f) O 1s, and (g) W 4f. Reproduced with permission from ref. 42. Copyright 2021 Wiley-VCH. | ||
 | ||
Fig. 9 Photocatalytic yield of CH4 and CO in five hours over (a) X% Cs2AgBiBr6@M-Ti and (d) X% Cs3Bi2Br9@M-Ti. Time-dependent production of CH4 and CO over (b) 8.47% Cs2AgBiBr6 @M-Ti and (e)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.12% Cs3Bi2Br9@M-Ti. Cycling photocatalysis performance of (c) 8.47% Cs2AgBiBr6@M-Ti and (f) 8.12% Cs3Bi2Br9@M-Ti. High-resolution XPS spectra of Ti 2p (g), O 1s (h), and Cs 3d (i) of 8.12% CBB@M-Ti. (j) Charge transfer mechanism of perovskite@M-Ti under illumination (CBB short for Cs3Bi2Br9). Reproduced with permission from ref. 109. Copyright 2022 Wiley-VCH. 8.12% Cs3Bi2Br9@M-Ti. Cycling photocatalysis performance of (c) 8.47% Cs2AgBiBr6@M-Ti and (f) 8.12% Cs3Bi2Br9@M-Ti. High-resolution XPS spectra of Ti 2p (g), O 1s (h), and Cs 3d (i) of 8.12% CBB@M-Ti. (j) Charge transfer mechanism of perovskite@M-Ti under illumination (CBB short for Cs3Bi2Br9). Reproduced with permission from ref. 109. Copyright 2022 Wiley-VCH. | ||
On the whole, the applications of several BHP photocatalysts in CO2 photoreduction have been well illustrated with measurable activity and high stability. However, the low efficiency and poor selectivity toward valued hydrocarbons (e.g., CH4 and C2H6) in CO2 photoreduction should be improved via constructing more efficient perovskite photocatalysts. Moreover, the transformation of CO2 to more valuable oxygenated chemicals such as methanol and formic acid by use of BHP photocatalysts is still unavailable, which requires more investigation. Considering low-level activity in CO2 photoreduction, another significant concern is how to acquire reliable data with accurate and strict operation during the evaluation of photocatalytic systems. Avoiding the usage of organic solvents/additives, ensuring the cleanliness of photocatalysts, and conducting isotopic labeling tests may be good options to identify whether external carbonaceous contamination exists.
4.3 Organic synthesis
In the past three years, BHP materials have provided promising applications in heterogeneous photocatalytic organic synthesis. Table 4 summarizes recent photocatalytic organic reactions over BHPs materials, including ring-opening reactions, oxidizing reaction and C–H bond activation. In 2019, Dai et al. reported that Cs3Bi2Br9 nanoparticles could catalyze ring-opening alcoholysis reactions of various epoxides under visible light, which is the first case of employing BHP photocatalysts for organic synthesis.49 This photocatalytic system not only avoided the utilization of strong acids but also exhibited high activity and good selectivity for epoxide alcoholysis. Moreover, the surface acidity characterization and control experiments have demonstrated that the superior catalytic properties of this BHP photocatalyst originated from suitable acidic sites, which are absent in lead-based perovskites. These acidic sites could activate the epoxides, facilitating further ring-opening reactions.| Catalyst | Solution | Reaction types | Light source | Performance | Ref. |
|---|---|---|---|---|---|
| Cs3Bi2Br9 | Isopropanol | Ring-opening reactions | 300 W Xe-lamp (λ ≥ 420 nm) | 1333 μmol g−1 h−1 | 49 |
| Cs2AgBiBr6 | Acetonitrile | Oxidation of vanillyl alcohol | White light LED lamp | Conversion: 95% | 152 |
| Cs3(BixSb1−x)2Br9 | n-Hexane | Oxidation of thioanisole | 300 W Xe-lamp (λ ≥ 420 nm) | Conversion: 95%; selectivity: 99% | 153 |
| Cs3Bi2I9/TiO2 | Aqueous solution | Oxidation of MeOH | M455L3 (blue LED 455 nm) | 105 μmol h−1 | 154 |
| Cs3Bi2Br9/SBA-15 | Toluene | C–H bond activation | 300 W Xe-lamp (λ ≥ 420 nm) | 12.6 mmol gcat−1 h−1 (90% selectivity) | 133 |
| Cs3SbxBi2−xBr9 (0 ≤ x ≤ 2) | Acetonitrile/toluene | C–H bond activation | White light LED lamp | ∼26 μmol h−1 | 155 |
| Cs3Bi2Br9/TiO2 | Isopropanol | C–H bond activation | 300 W Xe-lamp (λ ≥ 420 nm) | 1465 μmol g−1 h−1 | 156 |
| Cs3Bi2Br9/CdS | Toluene | C–H bond activation | 300 W Xe-lamp (λ ≥ 420 nm) | 6.79 mmol g−1 h−1 | 113 |
| Cs3Bi2Br9/Ti3C2Tx | Toluene | C–H bond activation | 5 W LED | 2121μmol g−1 h−1 (100% selectivity) | 107 |
| Cs3Bi2Br9/d-BiOBr | Toluene | C–H bond activation | 300 W Xe-lamp (λ ≥ 420 nm) | 7.24 mmol g−1 h−1 | 108 |
| Cs3Bi2Br9/g-C3N4 | Toluene | C–H bond activation | 300 W Xe-lamp (λ ≥ 400 nm, 500 mW cm−2) | 4.53 mmol g−1 h−1 | 157 |
Moreover, in photocatalytic selective oxidation reactions, BHPs have the capability to activate inert C(sp3)–H bonds. Dai et al. designed a confined growth strategy by use of the mesoporous channels of SBA-15 silica, leading to the successful loading of uniform and highly dispersed small Cs3Bi2Br9 nanoparticles (2–5 nm).133 This Cs3Bi2Br9/SBA-15-supported photocatalyst could efficiently oxidize hydrocarbons (C5–C16, including aromatic and aliphatic alkanes) under visible light with a conversion rate up to 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 μmol gcat−1 h−1 and excellent selectivity towards aldehydes/ketones (>99%) (Fig. 10a–c). In comparison with the bulk sample, Cs3Bi2Br9 nanoparticles with small size can generate rich under-coordinated bismuth sites on the surface (BiBr3 and BiBi5 motifs), which promote the adsorption of hydrocarbons via electronic interactions to facilitate the activation of C–H bonds. Furthermore, the smaller perovskite nanoparticles can exhibit shorter diffusion distance of charges in excited states to suppress the charge recombination, boosting the final photocatalytic performance. As the reaction proceeded, the water produced led to the formation of BiOBr, which deactivated the catalyst with totally inhibited photocatalytic performance after 8 h irradiation (Fig. 10c). This deactivation of the photocatalysts can be figured out after the addition of anhydrous Na2SO4 (Fig. 10c), which can adsorb water to control the reaction environment with a low-level water content. Another effective strategy for enhancing stability is the fabrication of 2D Cs3Bi2Br9 nanoplatelets (a basal size of 2–10 μm and a thickness of ∼100 nm),87 which exhibit more grid crystal framework and fewer surface defects in comparison with the fragile 0D perovskite nanoparticles.
900 μmol gcat−1 h−1 and excellent selectivity towards aldehydes/ketones (>99%) (Fig. 10a–c). In comparison with the bulk sample, Cs3Bi2Br9 nanoparticles with small size can generate rich under-coordinated bismuth sites on the surface (BiBr3 and BiBi5 motifs), which promote the adsorption of hydrocarbons via electronic interactions to facilitate the activation of C–H bonds. Furthermore, the smaller perovskite nanoparticles can exhibit shorter diffusion distance of charges in excited states to suppress the charge recombination, boosting the final photocatalytic performance. As the reaction proceeded, the water produced led to the formation of BiOBr, which deactivated the catalyst with totally inhibited photocatalytic performance after 8 h irradiation (Fig. 10c). This deactivation of the photocatalysts can be figured out after the addition of anhydrous Na2SO4 (Fig. 10c), which can adsorb water to control the reaction environment with a low-level water content. Another effective strategy for enhancing stability is the fabrication of 2D Cs3Bi2Br9 nanoplatelets (a basal size of 2–10 μm and a thickness of ∼100 nm),87 which exhibit more grid crystal framework and fewer surface defects in comparison with the fragile 0D perovskite nanoparticles.
 | ||
| Fig. 10 (a) The reaction formula of photocatalytic toluene oxidation. (b) The toluene conversion rate over different photocatalysts. (c) The time dependence of toluene photooxidation over 10 wt% Cs3Bi2Br9/SBA-15. (d) Control tests in the anaerobic condition or with different scavengers. (e) In situ DRIFTs spectra of Cs3Bi2Br9/SBA-15 sample exposed to the gas mixture of O2 and toluene under light irradiation. (f) Possible reaction pathways for the photooxidation of toluene, involving the concerted proton–electron transfer process. (g) The band structure of Cs3Bi2Br9 nanoparticles, including the proposed redox reaction paths by use of electrons and holes. (h) The possible photocatalytic mechanism of the hydrocarbon oxidation over Cs3Bi2Br9 nanoparticles under visible light irradiation in air. The blue elliptic mark indicates the production of a benzyl radical intermediate after cleaving the C–H bond. Reproduced with permission from ref. 133. Copyright 2020 Wiley-VCH. | ||
Based on the control tests with different scavengers (Fig. 10d) and in situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTs) (Fig. 10e), the reaction pathway has been proposed with under-coordinated bismuth metal sites and Br anions playing the key roles (Fig. 10f). Under visible light irradiation, the photoinduced electrons and holes were generated from Cs3Bi2Br9 nanoparticles. Subsequently, the holes cleaved C–H bonds to produce alkyl radical intermediates (R˙), which further formed ROOH intermediates in the presence of electrons and O2 (Fig. 10g). Moreover, the oxidation sites (Br atoms) on the perovskite surface were in close proximity to the H atoms of hydrocarbon substrates (2.9 Å) due to their unique adsorption at bismuth metal sites, promoting the subsequent selective oxidation of the C(sp3)–H bonds (Fig. 10h).
Later, Shi et al. designed and fabricated an antimony-doped BHP Cs3SbxBi2−xBr9 (0 ≤ x ≤ 2).155 The addition of Sb broke the local symmetry and made the charges more dispersed, which facilitated the separation and transfer of charges. The simultaneous asymmetric charge distribution enhanced the adsorption of toluene. It effectively reduced the activation energy barrier of C–H bond, which improved the reaction efficiency. Besides, Estrada-Pomares et al. reported that Cs2AgBiBr6 and Cs3Bi2Br9 were able to efficiently and stably oxidize vanillyl alcohol to vanillin under visible light with a conversion rate of 95%.152 Mechanistic investigations have shown that photogenerated holes and superoxide radicals from the reduction of O2 by electrons mainly contributed to the oxidation of vanillyl alcohol. In addition, some composite materials such as Cs3Bi2Br9-TiO2 or -BiOBr or -CdS or -Ti3C2Tx or -C3N4 have been successfully applied in C–H bond activation with improved photocatalytic performance, especially for the oxidation of toluene.107,108,113,156,157
The complexity and diversity of photocatalytic reaction mechanisms bring the research of BHP photocatalysts in organic synthesis huge challenges and difficulties. More efficient, robust and well-matched BHP photocatalyst nanomaterials should be designed and prepared to drive significant organic transformations under visible light, which can generate value-added products in a green and sustainable way. Even though the photocatalytic organic synthesis reactions over BHP photocatalysts are still in their infancy at the present point, the BHP-based photocatalytic systems have shown highly promising potential, playing important roles in the future artificial photosynthesis field.
4.4 Organic pollutant degradation
The photocatalytic advanced oxidation process for the purification of wastewater with various organic contaminants has attracted increasing attention,115 and presents a few merits such as mild reaction conditions, powerful oxidation ability and low-cost treatment.158 Recently, BHPs have been reported as remarkable photocatalysts in the treatment of wastewater and air pollutants (such as NO).111,159 For example, the (CH3NH3)3Bi2I9 nanomaterial has been applied in cleaning simulated industrial wastewater with a high photodegradation activity for both fluorescent dye (rhodamine B) and thiazine dye (methylene blue) under visible light.134 However, the aqueous environment can lead to the hydrolysis of (CH3NH3)3Bi2I9 easily. Thus, the real photocatalytic species in this work is still uncertain, since the formed BiOI phase from hydrolysis of BHP can present decent activity for the photodegradation of dyes as well.160Impressively, the Cs2AgBiBr6 double perovskite material exhibited excellent activity (almost complete degradation) toward a few organic dyes, including rhodamine B (RhB), rhodamine 110 (RH110), methyl red (MR), and methyl orange (MO) (Fig. 11a).47 The excellent activities were probably ascribable to the unique surface Ag or Bi sites on Cs2AgBiBr6, facilitating the activation of O2 or dyes. Furthermore, the photocatalytic degradation activity could be dramatically promoted with the deposition of Pt or Au as co-catalysts (Fig. 11b). The stability of Cs2AgBiBr6 photocatalysts could be maintained within 5 cycles of reactions, which is consistent with the photocurrent response (Fig. 11c). However, if more cycling tests were performed, the produced water might lead to partial degradation of Cs2AgBiBr6via hydrolysis, which would inevitably lead to deactivation of perovskites. As shown in Fig. 11d, the proposed photodegradation mechanism revealed that the dye molecules underwent a complete mineralization process, where the main active species came from the superoxide radical (˙O2−). Additionally, the solvent using ethanol instead of water ensured the high temporary stability of Cs2AgBiBr6 photocatalysts. Otherwise, the common hydrolysis issue of BHPs would take place as well. The presence of alcohol solvent may lead to the undesired oxidation of ethanol to aldehydes, suggesting the waste of photoinduced charges. Besides, the products from the mineralization of dyes include water, which indicates the inevitable existence of a hydrolysis problem if abundant dyes are decomposed. In 2020, Bresolin et al. confirmed the advantage of composited heterojunctions in photocatalytic degradation.110 They fabricated Cs3Bi2I9/g-C3N4 heterojunctions through a facile electrostatic assembly method. The Cs3Bi2I9/g-C3N4 composites showed the better stability and an outstanding yield for photocatalytic degradation of organic compound (RhB, MB, MO) in water solution under visible light irradiation.
 | ||
| Fig. 11 (a) Kinetic curves of the RhB (pink curve), Rh110 (green curve), MR (red curve), and MO (orange curve) degradation fitted using a pseudo-zeroth-order reaction model. (b) Plots of C/C0versus the irradiation time for the photodegradation of RhB in the presence of Cs2AgBiBr6, Cs2AgBiBr6-Pt, and Cs2AgBiBr6-Au. (c) Photocurrent response of Cs2AgBiBr6, Cs2AgBiBr6-Pt, and Cs2AgBiBr6-Au. (d) The proposed photocatalytic degradational mechanism of dyes by Cs2AgBiBr6. Reproduced with permission from ref. 47. Copyright 2019 Wiley-VCH. | ||
On the whole, these research works illustrate the broad prospects and significance of BHP nanomaterials in photocatalytic organic pollution degradation. However, the photocatalytic activities of BHP photocatalysts in practical industrial wastewater with quite complicated composition are still unclear, which deserves more thorough investigations, especially in the neutral or basic aqueous solutions. Besides, the photocatalytic degradation of persistent organic pollutant (POPs) and plastic polymers has already been realized by some bismuth-based photocatalysts (such as BiOBr/Fe3O4 nanocomposites161 and BiOCl nanoflowers162) with good activity. The decent remediation performance mainly comes from the generation of powerful hydroxyl radicals or holes by these bismuth-based nanomaterials under visible light irradiation. Thus, BHPs with bismuth as the metal center, possessing diverse compositions and electronic structures, may be promising candidates for the photocatalytic degradation of POPs and polymers. This deserves more scientific investigations in the future.
4.5 Other photocatalytic reaction
Photocatalytic approaches have been applied in various fields, where photoinduced polymerization has attracted much attention due to the mild conditions, low cost and high efficiency.163 Some frequently used photocatalysts always involve expensive transition metal compounds and toxic organic initiators, leading to burdens on the environment and the economy. Recently, some BHP nanomaterials-based photocatalytic systems have been successfully designed for photoinduced polymerization.135,136Zhu et al. have in situ embedded MA3Bi2Br9 NCs in the hydrophobic COFs matrix for successful photocatalytic applications in both water and organic solvents.135 Firstly, BiBr3 was dissolved in TAPT-DMTA COF precursor solution. After the addition of MABr solution, MA3Bi2Br9 was generated in situ on the COFs (Fig. 12a and b). This nanocomposite demonstrated outstanding photocatalytic performance for photopolymerization in both organic and aqueous phases, yielding oligo-(ethylene glycol) methyl ether acrylate (OEGMEA) polymer with a Mn of 22.7 kDa and Đ of 1.27 (Fig. 12c). The added co-initiator MPA (or carboxylic acids) was activated by the hole generated from MA3Bi2Br9 under visible light to produce thiyl or alkyl free radicals. These active radicals could initiate chain polymerization though the chain transfer process. However, these polymerizations were difficult to control in a precise way. Moreover, the absence of COFs led to no activity towards polymerization, since the MA3Bi2Br9 perovskites were quickly degraded into white BiOBr phase in water. Thus, the water-resisting COF porous shells can efficiently protect perovskite nanocrystals from attack by H2O molecules, resulting in smooth photoinduced polymerization. Besides, this protection effect can ensure the reusability of MA3Bi2Br9-COFs nanocomposites for at least four cycles without an obvious decease in the final conversion of monomers. On the whole, both the powerful oxidation ability from photoinduced holes and hydrophobic encapsulation of COF shells in the MA3Bi2Br9-COF system can lead to photopolymerization with excellent performance and stability in water and organic phases.
 | ||
| Fig. 12 (a) The in situ synthetic protocol for MA3Bi2Br9-COFs nanocomposites. (b) The HRTEM image indicating the formation of MA3Bi2Br9 NCs-COFs nanocomposites; inserted graph: FFT pattern of the selected area. (c) Partial results of photoinduced polymerization reactions using MA3Bi2Br9-COFs nanocomposites in water. Reproduced with permission from ref. 135. Copyright 2022 American Chemical Society. | ||
Additionally, some other important photocatalytic reactions such as O2 evolution and H2O2 generation have been well investigated by the use of efficient photocatalysts.52,164 Although the application of BHP nanomaterials on these reactions is still unclear, the establishment of BHP-based photocatalytic systems with high stability in water phase and essential active sites could be promising candidates.
5. Conclusion and outlook
In conclusion, the recent progress in the synthesis and photocatalytic applications of BHP nanomaterials with diverse structures and their physical–chemical properties has been reviewed. The preparation of BHP nanomaterials with different dimensions in morphology (0D, 2D and hetero-structures) can utilize several chemical wet approaches, including antisolvent, hot-injection, ion-exchange reactions, physical exfoliation and rapid cooling recrystallization. The fabricated BHP nanomaterials can present diverse nanostructures, such as 0D quantum dots, 2D nanosheets and hetero-junctions (e.g., Z-type 2D Cs3Bi2I9/2D CeO2). Moreover, the changes of halide anions or the introduction of Ag/Cu heteroatoms can facilely regulate the band structures with bandgaps varying from 1.8 to 3.1 eV, which can offer tunable optoelectronic properties. Besides, the construction of heterojunctions (e.g., Schottky, type II, and Z-scheme junctions) by combining BHPs with other semiconductors or metals results in improved charge separation/transport and rich catalytically active sites on the surface, which help to drive desired redox reactions with higher efficiencies. Regarding photocatalytic applications, the established BHP photocatalysts have shown decent photocatalytic activity and stability in several reactions, including H2 generation, CO2 reduction, organic synthesis and pollutant degradation. However, the quantum yields and selectivity control over BHP-based photocatalytic systems still locate at a low level. Thus, it is urgent to design and prepare more efficient BHP photocatalysts with aligned active sites on the surface and promoted charge transport/separation. Another main issue lies in the stability of BHP nanomaterials in the environment containing H2O molecules, which severely hinders their application for pure water splitting or organic oxidations in aerobic conditions. Regarding the photocatalytic mechanisms over BHP photocatalysts, the nature of real active sites, the key reaction paths containing rate-determining steps, and the whole solar-to-chemical energy process are still unclear for most reported photocatalytic systems.Finally, in our opinion, future research directions for BHPs in photocatalysis may involve: (1) the fabrication of novel BHPs with diverse nanostructures and elemental compositions. For instance, 1D linear or 3D porous nanomaterials like inverse-opal crystals are still unreported. The replacement of A or B-site atoms by other species, such as Cu2AgBiX6 and Cs2InBiX6, may provide unique catalytically active sites; (2) the surface engineering on BHP photocatalysts. For example, the creation of bismuth or halide vacancy sites could vary the optical properties and surface chemical environment. The doping of heteroatoms (e.g., Mn, Eu, Sn, Sb, and In) on the surface may modulate the electronic structures with suitable sub-states and offer diverse adsorption sites for certain reactants. Besides, the surface environment of BHPs can be modified as well by bridging organic ligands, or depositing co-catalysts such as metal nanoparticles or metal complexes; (3) the establishment of effective heterojunctions. By introducing other semiconductors or plasmonic metals, the nanocomposites may present promoted photocatalytic properties with improved charge diffusion and desired active sites. Furthermore, the nanostructures of these composites are crucial for their physical–chemical properties as well. For instance, the core–shell architecture with inert shells (e.g., TiO2, CdS, and metal–organic frameworks) toward water may provide high moisture stability; (4) discovering more matched redox reactions in the field of photocatalytic organic synthesis. Through an in-depth understanding of the surface chemical environment in BHP photocatalysts, more organic reactions with high economy should be screened out in a rational and efficient way. For the organic reactions easily and safely realized by conventional thermal catalytic methods (e.g., reaction temperature Tr < 150 °C and ambient pressure) with high activities and selectivity, they are not the most desired target reactions. In contrast, challenging reactions always require harsh reaction conditions (e.g., Tr ≥ 350 °C, reaction pressure Pr ≥ 5 MPa), which should be targeted, such as the direct dehydrogenation of butane (usually Tr ≥ 600 °C). (5) Theoretical predications via artificial intelligence (AI). Powerful AI techniques such as machine learning and deep learning could build the material models to predict the optimal composition in BHPs by a high-throughput computational approach. This not only provides diverse design pathways, but also saves abundant time for researchers. This may guide the fabrication of next-generation BHPs with high stability and activity for photocatalysis.
Conflicts of interest
The authors declare no potential conflicts of interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (No. 22205230, 21725102 and 91961106), Youth Fund Project of Natural Science Foundation of Jiangsu Province (No. BK20220287), Gusu Innovation and Entrepreneurship Leading Talents Program (No. ZXL2022468), startup grant from the University of Science and Technology of China (USTC) (No. KY2260080010). The authors thank Xuesen Liu (Intermediate Engineer) and Zhibin Li (Intermediate Engineer) from Failure Analysis Laboratory company (FALAB) for the thorough discussions during the halide perovskite project cooperation.References
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS.
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- Y. Dai, Q. Bu, R. Sooriyagoda, P. Tavadze, O. Pavlic, T. Lim, Y. Shen, A. Mamakhel, X. Wang, Y. Li, H. Niemantsverdriet, B. B. Iversen, F. Besenbacher, T. Xie, J. P. Lewis, A. D. Bristow, N. Lock and R. Su, J. Phys. Chem. Lett., 2019, 10, 5381–5386 CrossRef CAS PubMed.
- Y. Liu, X. Zhang, L. Lu, J. Ye, J. Wang, X. Li, X. Bai and W. Wang, Chin. Chem. Lett., 2022, 33, 1271–1274 CrossRef CAS.
- X. Li, Y. Sun, J. Xu, Y. Shao, J. Wu, X. Xu, Y. Pan, H. Ju, J. Zhu and Y. Xie, Nat. Energy, 2019, 4, 690–699 CrossRef CAS.
- F. Xu, K. Meng, B. Cheng, S. Wang, J. Xu and J. Yu, Nat. Commun., 2020, 11, 4613 CrossRef CAS.
- L. Shi, Y. Shi, S. Zhuo, C. Zhang, Y. Aldrees, S. Aleid and P. Wang, Nano Energy, 2019, 60, 222–230 CrossRef CAS.
- R. Yang, S. Zhong, L. Zhang and B. Liu, Sep. Purif. Technol., 2020, 235, 116270 CrossRef CAS.
- H. Cheng and W. Xu, Org. Biomol. Chem., 2019, 17, 9977–9989 RSC.
- S. Gisbertz and B. Pieber, ChemPhotoChem, 2020, 4, 456–475 CrossRef CAS.
- Y. Dai and Y. Xiong, Nano Res. Energy, 2022, 1, e9120006 CrossRef.
- H. Tan, P. Kong, R. Zhang, M. Gao, M. Liu, X. Gu, W. Liu and Z. Zheng, The Innovation, 2021, 2, 100089 CrossRef CAS PubMed.
- H. L. Tan, F. F. Abdi and Y. H. Ng, Chem. Soc. Rev., 2019, 48, 1255–1271 RSC.
- N. Zhang, S. Liu and Y.-J. Xu, Nanoscale, 2012, 4, 2227–2238 RSC.
- S. C. Shit, I. Shown, R. Paul, K.-H. Chen, J. Mondal and L.-C. Chen, Nanoscale, 2020, 12, 23301–23332 RSC.
- J. Lee, L.-L. Tan and S.-P. Chai, Nanoscale, 2021, 13, 7011–7033 RSC.
- G. Zhao and X. Xu, Nanoscale, 2021, 13, 10649–10667 RSC.
- G. Xing, N. Mathews, S. S. Lim, N. Yantara, X. Liu, D. Sabba, M. Grätzel, S. Mhaisalkar and T. C. Sum, Nat. Mater., 2014, 13, 476–480 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- F. Zhang, H. Zhong, C. Chen, X.-G. Wu, X. Hu, H. Huang, J. Han, B. Zou and Y. Dong, ACS Nano, 2015, 9, 4533–4542 CrossRef CAS PubMed.
- Q. Zhang, R. Su, X. Liu, J. Xing, T. C. Sum and Q. Xiong, Adv. Funct. Mater., 2016, 26, 6238–6245 CrossRef CAS.
- W.-L. Hong, Y.-C. Huang, C.-Y. Chang, Z.-C. Zhang, H.-R. Tsai, N.-Y. Chang and Y.-C. Chao, Adv. Mater., 2016, 28, 8029–8036 CrossRef CAS.
- Y. Fu, H. Zhu, A. W. Schrader, D. Liang, Q. Ding, P. Joshi, L. Hwang, X. Y. Zhu and S. Jin, Nano Lett., 2016, 16, 1000–1008 CrossRef CAS.
- S. D. Stranks, G. E. Eperon, G. Grancini, C. Menelaou, M. J. P. Alcocer, T. Leijtens, L. M. Herz, A. Petrozza and H. J. Snaith, Science, 2013, 342, 341–344 CrossRef CAS PubMed.
- C. Wehrenfennig, G. E. Eperon, M. B. Johnston, H. J. Snaith and L. M. Herz, Adv. Mater., 2014, 26, 1584–1589 CrossRef CAS.
- D. Shi, V. Adinolfi, R. Comin, M. Yuan, E. Alarousu, A. Buin, Y. Chen, S. Hoogland, A. Rothenberger, K. Katsiev, Y. Losovyj, X. Zhang, P. A. Dowben, O. F. Mohammed, E. H. Sargent and O. M. Bakr, Science, 2015, 347, 519–522 CrossRef CAS.
- T. M. Brenner, D. A. Egger, L. Kronik, G. Hodes and D. Cahen, Nat. Rev. Mater., 2016, 1, 15007 CrossRef CAS.
- S.-T. Ha, R. Su, J. Xing, Q. Zhang and Q. Xiong, Chem. Sci., 2017, 8, 2522–2536 RSC.
- K. Chen, S. Schünemann, S. Song and H. Tüysüz, Chem. Soc. Rev., 2018, 47, 7045–7077 RSC.
- J. Yuan, H. Liu, S. Wang and X. Li, Nanoscale, 2021, 13, 10281–10304 RSC.
- H. L. Wells, Am. J. Sci., 1893, 45, 121 CrossRef.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS.
- J. Sun, J. Wu, X. Tong, F. Lin, Y. Wang and Z. M. Wang, Adv. Sci., 2018, 5, 1700780 CrossRef PubMed.
- P. Chen, W.-J. Ong, Z. Shi, X. Zhao and N. Li, Adv. Funct. Mater., 2020, 30, 1909667 CrossRef CAS.
- M. Ren, X. Qian, Y. Chen, T. Wang and Y. Zhao, J. Hazard. Mater., 2022, 426, 127848 CrossRef CAS PubMed.
- J. Li, H.-L. Cao, W.-B. Jiao, Q. Wang, M. Wei, I. Cantone, J. Lü and A. Abate, Nat. Commun., 2020, 11, 310 CrossRef CAS.
- M. Lyu, J.-H. Yun, P. Chen, M. Hao and L. Wang, Adv. Energy Mater., 2017, 7, 1602512 CrossRef.
- Q. Fan, G. V. Biesold-McGee, J. Ma, Q. Xu, S. Pan, J. Peng and Z. Lin, Angew. Chem., Int. Ed., 2020, 59, 1030–1046 CrossRef CAS.
- J. Li, J. Duan, X. Yang, Y. Duan, P. Yang and Q. Tang, Nano Energy, 2021, 80, 105526 CrossRef CAS.
- H. Fu, Sol. Energy Mater. Sol. Cells, 2019, 193, 107–132 CrossRef CAS.
- M. Wang, W. Wang, B. Ma, W. Shen, L. Liu, K. Cao, S. Chen and W. Huang, Nano-Micro Lett., 2021, 13, 62 CrossRef.
- Z.-L. Liu, R.-R. Liu, Y.-F. Mu, Y.-X. Feng, G.-X. Dong, M. Zhang and T.-B. Lu, Sol. RRL, 2021, 5, 2000691 CrossRef CAS.
- H. Tang, Y. Xu, X. Hu, Q. Hu, T. Chen, W. Jiang, L. Wang and W. Jiang, Adv. Sci., 2021, 8, 2004118 CrossRef CAS.
- J. Pascual, G. Nasti, M. H. Aldamasy, J. A. Smith, M. Flatken, N. Phung, D. Di Girolamo, S.-H. Turren-Cruz, M. Li, A. Dallmann, R. Avolio and A. Abate, Mater. Adv., 2020, 1, 1066–1070 RSC.
- Y. Guo, G. Liu, Z. Li, Y. Lou, J. Chen and Y. Zhao, ACS Sustainable Chem. Eng., 2019, 7, 15080–15085 CrossRef CAS.
- G.-N. Liu, R.-Y. Zhao, B. Xu, Y. Sun, X.-M. Jiang, X. Hu and C. Li, ACS Appl. Mater. Interfaces, 2020, 12, 54694–54702 CrossRef CAS.
- Z. Zhang, Y. Liang, H. Huang, X. Liu, Q. Li, L. Chen and D. Xu, Angew. Chem., Int. Ed., 2019, 58, 7263–7267 CrossRef CAS PubMed.
- B. Yang, J. Chen, F. Hong, X. Mao, K. Zheng, S. Yang, Y. Li, T. Pullerits, W. Deng and K. Han, Angew. Chem., Int. Ed., 2017, 56, 12471–12475 CrossRef CAS.
- Y. Dai and H. Tüysüz, ChemSusChem, 2019, 12, 2587–2592 CrossRef CAS PubMed.
- X. Li, J. Hu, T. Yang, X. Yang, J. Qu and C. M. Li, Nano Energy, 2022, 92, 106714 CrossRef CAS.
- C. Chen, J. Hu, X. Yang, T. Yang, J. Qu, C. Guo and C. M. Li, ACS Appl. Mater. Interfaces, 2021, 13, 20162–20173 CrossRef CAS.
- J. Hu, C. Chen, H. Yang, F. Yang, J. Qu, X. Yang, W. Sun, L. Dai and C. M. Li, Appl. Catal., B, 2022, 317, 121723 CrossRef CAS.
- J. Hu, C. Chen, T. Hu, J. Li, H. Lu, Y. Zheng, X. Yang, C. Guo and C. M. Li, J. Mater. Chem. A, 2020, 8, 19484–19492 RSC.
- J. Hu, C. Chen, Y. Zheng, G. Zhang, C. Guo and C. M. Li, Small, 2020, 16, 2002988 CrossRef CAS.
- J. Hu, D. Chen, Z. Mo, N. Li, Q. Xu, H. Li, J. He, H. Xu and J. Lu, Angew. Chem., Int. Ed., 2019, 58, 2073–2077 CrossRef CAS PubMed.
- C. B. Hiragond, N. S. Powar and S.-I. In, Nanomaterials, 2020, 10, 2569 CrossRef CAS PubMed.
- C. Han, X. Zhu, J. S. Martin, Y. Lin, S. Spears and Y. Yan, ChemSusChem, 2020, 13, 4005–4025 CrossRef CAS PubMed.
- J. Chen, C. Dong, H. Idriss, O. F. Mohammed and O. M. Bakr, Adv. Energy Mater., 2020, 10, 1902433 CrossRef CAS.
- J. Luo, W. Zhang, H. Yang, Q. Fan, F. Xiong, S. Liu, D.-S. Li and B. Liu, EcoMat, 2021, 3, e12079 CrossRef CAS.
- H. Huang, D. Verhaeghe, B. Weng, B. Ghosh, H. Zhang, J. Hofkens, J. A. Steele and M. B. J. Roeffaers, Angew. Chem., Int. Ed., 2022, 61, e202203261 CAS.
- K. Ren, S. Yue, C. Li, Z. Fang, K. A. M. Gasem, J. Leszczynski, S. Qu, Z. Wang and M. Fan, J. Mater. Chem. A, 2022, 10, 407–429 RSC.
- Y. Lin, J. Guo, J. San Martin, C. Han, R. Martinez and Y. Yan, Chem. – Eur. J., 2020, 26, 13118–13136 CrossRef CAS PubMed.
- Y. Zhang, Y. Ma, Y. Wang, X. Zhang, C. Zuo, L. Shen and L. Ding, Adv. Mater., 2021, 33, 2006691 CrossRef CAS.
- L. Zhang, K. Wang and B. Zou, ChemSusChem, 2019, 12, 1612–1630 CrossRef CAS PubMed.
- S. Attique, N. Ali, S. Ali, R. Khatoon, N. Li, A. Khesro, S. Rauf, S. Yang and H. Wu, Adv. Sci., 2020, 7, 1903143 CrossRef CAS.
- C. Wu, Q. Zhang, G. Liu, Z. Zhang, D. Wang, B. Qu, Z. Chen and L. Xiao, Adv. Energy Mater., 2020, 10, 1902496 CrossRef CAS.
- F. Ünlü, M. Deo, S. Mathur, T. Kirchartz and A. Kulkarni, J. Phys. D: Appl. Phys., 2021, 55, 113002 CrossRef.
- P. Cheng, K. Han and J. Chen, ACS Mater. Lett., 2023, 5, 60–78 CrossRef CAS.
- M. M. Byranvand, C. Otero-Martínez, J. Ye, W. Zuo, L. Manna, M. Saliba, R. L. Z. Hoye and L. Polavarapu, Adv. Opt. Mater., 2022, 10, 2200423 CrossRef CAS.
- Y. Tang, C. H. Mak, G. Jia, K.-C. Cheng, J.-J. Kai, C.-W. Hsieh, F. Meng, W. Niu, F.-F. Li, H.-H. Shen, X. Zhu, H. M. Chen and H.-Y. Hsu, J. Mater. Chem. A, 2022, 10, 12296–12316 RSC.
- J. Wu, J. Chen, J. Gao, Z. Chen, L. Li and W. Wang, Energy Fuels, 2022, 36, 14613–14624 CrossRef CAS.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- B.-M. Bresolin, Y. Park and D. W. Bahnemann, Catalysts, 2020, 10, 709 CrossRef CAS.
- S. Meloni, G. Palermo, N. Ashari-Astani, M. Grätzel and U. Rothlisberger, J. Mater. Chem. A, 2016, 4, 15997–16002 RSC.
- L. N. Quan, B. P. Rand, R. H. Friend, S. G. Mhaisalkar, T.-W. Lee and E. H. Sargent, Chem. Rev., 2019, 119, 7444–7477 CrossRef CAS PubMed.
- J. Shamsi, A. S. Urban, M. Imran, L. De Trizio and L. Manna, Chem. Rev., 2019, 119, 3296–3348 CrossRef CAS PubMed.
- K. K. Bass, L. Estergreen, C. N. Savory, J. Buckeridge, D. O. Scanlon, P. I. Djurovich, S. E. Bradforth, M. E. Thompson and B. C. Melot, Inorg. Chem., 2017, 56, 42–45 CrossRef CAS PubMed.
- M. Usman and Y. Qingfeng, Crystals, 2020, 10, 62 CrossRef CAS.
- X.-G. Zhao, D. Yang, J.-C. Ren, Y. Sun, Z. Xiao and L. Zhang, Joule, 2018, 2, 1662–1673 CrossRef CAS.
- H.-J. Feng, W. Deng, K. Yang, J. Huang and X. C. Zeng, J. Phys. Chem. C, 2017, 121, 4471–4480 CrossRef CAS.
- A. J. Kale, R. Chaurasiya and A. Dixit, ACS Appl. Energy Mater., 2022, 5, 10427–10445 CrossRef CAS.
- M. Y. Leng, Y. Yang, K. Zeng, Z. W. Chen, Z. F. Tan, S. R. Li, J. H. Li, B. Xu, D. B. Li, M. P. Hautzinger, Y. P. Fu, T. Y. Zhai, L. Xu, G. D. Niu, S. Jin and J. Tang, Adv. Funct. Mater., 2018, 28, 1704446 CrossRef.
- B. Li, L. Fu, S. Li, H. Li, L. Pan, L. Wang, B. Chang and L. Yin, J. Mater. Chem. A, 2019, 7, 20494–20518 RSC.
- M. Leng, Z. Chen, Y. Yang, Z. Li, K. Zeng, K. Li, G. Niu, Y. He, Q. Zhou and J. Tang, Angew. Chem., Int. Ed., 2016, 55, 15012–15016 CrossRef CAS.
- Y. Lou, M. Fang, J. Chen and Y. Zhao, Chem. Commun., 2018, 54, 3779–3782 RSC.
- J. Sheng, Y. He, J. Li, C. Yuan, H. Huang, S. Wang, Y. Sun, Z. Wang and F. Dong, ACS Nano, 2020, 14, 13103–13114 CrossRef CAS PubMed.
- Y. Dai and H. Tüysüz, Sol. RRL, 2021, 5, 2100265 CrossRef CAS.
- Y. Tang, C. H. Mak, R. Liu, Z. Wang, L. Ji, H. Song, C. Tan, F. Barrière and H.-Y. Hsu, Adv. Funct. Mater., 2020, 30, 2006919 CrossRef CAS.
- Y. Wang, H. Huang, Z. Zhang, C. Wang, Y. Yang, Q. Li and D. Xu, Appl. Catal., B, 2021, 282, 119570 CrossRef CAS.
- S. S. Bhosale, A. K. Kharade, E. Jokar, A. Fathi, S.-M. Chang and E. W.-G. Diau, J. Am. Chem. Soc., 2019, 141, 20434–20442 CrossRef CAS PubMed.
- H. Yang, T. Cai, E. Liu, K. Hills-Kimball, J. Gao and O. Chen, Nano Res., 2019, 13, 282–291 CrossRef.
- Y. Tang, L. Gomez, M. van der Laan, D. Timmerman, V. Sebastian, C.-C. Huang, T. Gregorkiewicz and P. Schall, J. Mater. Chem. C, 2021, 9, 158–163 RSC.
- R. D. Nelson, K. Santra, Y. Wang, A. Hadi, J. W. Petrich and M. G. Panthani, Chem. Commun., 2018, 54, 3640–3643 RSC.
- M. Li, S. Xu, L. Wu, H. Tang, B. Zhou, J. Xu, Q. Yang, T. Zhou, Y. Qiu, G. Chen, G. I. N. Waterhouse and K. Yan, ACS Energy Lett., 2022, 7, 3370–3377 CrossRef CAS.
- B. Yang, J. Chen, S. Yang, F. Hong, L. Sun, P. Han, T. Pullerits, W. Deng and K. Han, Angew. Chem., Int. Ed., 2018, 57, 5359–5363 CrossRef CAS PubMed.
- L. Zhou, Y.-F. Xu, B.-X. Chen, D.-B. Kuang and C.-Y. Su, Small, 2018, 14, 1703762 CrossRef PubMed.
- J.-L. Xie, Z.-Q. Huang, B. Wang, W.-J. Chen, W.-X. Lu, X. Liu and J.-L. Song, Nanoscale, 2019, 11, 6719–6726 RSC.
- B. Yang, F. Hong, J. Chen, Y. Tang, L. Yang, Y. Sang, X. Xia, J. Guo, H. He, S. Yang, W. Deng and K. Han, Angew. Chem., Int. Ed., 2019, 58, 2278–2283 CrossRef CAS PubMed.
- J. T. Lee, S. Seifert and R. Sardar, Chem. Mater., 2021, 33, 5917–5925 CrossRef CAS.
- K. Kundu, P. Acharyya, K. Maji, R. Sasmal, S. S. Agasti and K. Biswas, Angew. Chem., Int. Ed., 2020, 59, 13093–13100 CrossRef CAS PubMed.
- L. Lian, G. Zhai, F. Cheng, Y. Xia, M. Zheng, J. Ke, M. Gao, H. Liu, D. Zhang, L. Li, J. Gao, J. Tang and J. Zhang, CrystEngComm, 2018, 20, 7473–7478 RSC.
- Z. Qi, X. Fu, T. Yang, D. Li, P. Fan, H. Li, F. Jiang, L. Li, Z. Luo, X. Zhuang and A. Pan, Nano Res., 2019, 12, 1894–1899 CrossRef CAS.
- S. P. Chaudhary, S. Bhattacharjee, V. Hazra, S. Shyamal, N. Pradhan and S. Bhattacharyya, Nanoscale, 2022, 14, 4281–4291 RSC.
- Y.-X. Feng, G.-X. Dong, K. Su, Z.-L. Liu, W. Zhang, M. Zhang and T.-B. Lu, J. Energy Chem., 2022, 69, 348–355 CrossRef CAS.
- J. Huang, S. Zou, J. Lin, Z. Liu and M. Qi, Nano Res., 2021, 14, 4079–4086 CrossRef CAS.
- Z. Liu, H. Yang, J. Wang, Y. Yuan, K. Hills-Kimball, T. Cai, P. Wang, A. Tang and O. Chen, Nano Lett., 2021, 21, 1620–1627 CrossRef CAS PubMed.
- Q. Li, T. Song, Y. Zhang, Q. Wang and Y. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 27323–27333 CrossRef CAS PubMed.
- Z. J. Bai, S. Tian, T. Q. Zeng, L. Chen, B. H. Wang, B. Hu, X. Wang, W. Zhou, J. B. Pan, S. Shen, J. K. Guo, T. L. Xie, Y. J. Li, C. T. Au and S. F. Yin, ACS Catal., 2022, 12, 15157–15167 CrossRef CAS.
- Q. M. Sun, J. J. Xu, F. F. Tao, W. Ye, C. Zhou, J. H. He and J. M. Lu, Angew. Chem., Int. Ed., 2022, 61, e202200872 CAS.
- B. M. Bresolin, P. Sgarbossa, D. W. Bahnemann and M. Sillanpaa, Sep. Purif. Technol., 2020, 251, 117320 CrossRef CAS.
- G. Zhang, C. Yuan, X. Li, L. Yang, W. Yang, R. Fang, Y. Sun, J. Sheng and F. Dong, Chem. Eng. J., 2022, 430, 132974 CrossRef CAS.
- Z. Zhang, M. Wang, Z. Chi, W. Li, H. Yu, N. Yang and H. Yu, Appl. Catal., B, 2022, 313, 121426 CrossRef CAS.
- Y. Yang, Z. Chen, H. Huang, Y. Liu, J. Zou, S. Shen, J. Yan, J. Zhang, Z. Zhuang, Z. Luo, C. Yang, Y. Yu and Z. Zou, Appl. Catal., B, 2023, 323, 122146 CrossRef CAS.
- Z. P. Zhang, B. Z. Wang, H. B. Zhao, J. F. Liao, Z. C. Zhou, T. H. Liu, B. C. He, Q. Wei, S. Chen, H. Y. Chen, D. B. Kuang, Y. Li and G. C. Xing, Appl. Catal., B, 2022, 312, 121358 CrossRef CAS.
- A. Mahmoud Idris, S. Zheng, L. Wu, S. Zhou, H. Lin, Z. Chen, L. Xu, J. Wang and Z. Li, Chem. Eng. J., 2022, 446, 137197 CrossRef CAS.
- L. Wu, S. Zheng, H. Lin, S. Zhou, A. Mahmoud Idris, J. Wang, S. Li and Z. Li, J. Colloid Interface Sci., 2023, 629, 233–242 CrossRef CAS PubMed.
- S. E. Creutz, E. N. Crites, M. C. De Siena and D. R. Gamelin, Nano Lett., 2018, 18, 1118–1123 CrossRef CAS PubMed.
- K. Chen, X. Deng, R. Goddard and H. Tüysüz, Chem. Mater., 2016, 28, 5530–5537 CrossRef CAS.
- N. Chen, T. Cai, W. Li, K. Hills-Kimball, H. Yang, M. Que, Y. Nagaoka, Z. Liu, D. Yang, A. Dong, C.-Y. Xu, R. Zia and O. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 16855–16863 CrossRef CAS PubMed.
- M. Liu, H. Ali-Loytty, A. Hiltunen, E. Sarlin, S. Qudsia, J.-H. Smatt, M. Valden and P. Vivo, Small, 2021, 17, 2100101 CrossRef CAS PubMed.
- Z. Shi, Z. Cao, X. Sun, Y. Jia, D. Li, L. Cavallo and U. Schwingenschlögl, Small, 2019, 15, 1900462 CrossRef PubMed.
- Q. Han, X. Bai, Z. Man, H. He, L. Li, J. Hu, A. Alsaedi, T. Hayat, Z. Yu, W. Zhang, J. Wang, Y. Zhou and Z. Zou, J. Am. Chem. Soc., 2019, 141, 4209–4213 CrossRef CAS PubMed.
- Q. Liu, Y. Zhou, J. Kou, X. Chen, Z. Tian, J. Gao, S. Yan and Z. Zou, J. Am. Chem. Soc., 2010, 132, 14385–14387 CrossRef CAS PubMed.
- L.-Y. Wu, M.-R. Zhang, Y.-X. Feng, W. Zhang, M. Zhang and T.-B. Lu, Sol. RRL, 2021, 5, 2100263 CrossRef CAS.
- R. Marschall, Adv. Funct. Mater., 2014, 24, 2421–2440 CrossRef CAS.
- J. Low, C. Jiang, B. Cheng, S. Wageh, A. A. Al-Ghamdi and J. Yu, Small Methods, 2017, 1, 1700080 CrossRef.
- M. Ma, H. Wang and H. Liu, Chin. Chem. Lett., 2021, 32, 3613–3618 CrossRef CAS.
- X. Liu, Q. Zhang and D. Ma, Sol. RRL, 2020, 5, 2000397 CrossRef.
- X. Lang, X. Chen and J. Zhao, Chem. Soc. Rev., 2014, 43, 473–486 RSC.
- C. Yang, R. Li, K. A. I. Zhang, W. Lin, K. Landfester and X. Wang, Nat. Commun., 2020, 11, 1239 CrossRef CAS PubMed.
- T. Wang, D. Yue, X. Li and Y. Zhao, Appl. Catal., B, 2020, 268, 118399 CrossRef CAS.
- D. Wu, X. Zhao, Y. Huang, J. Lai, J. Yang, C. Tian, P. He, Q. Huang and X. Tang, J. Phys. Chem. C, 2021, 125, 18328–18333 CrossRef CAS.
- Y. Dai, C. Poidevin, C. Ochoa-Hernandez, A. A. Auer and H. Tüysüz, Angew. Chem., Int. Ed., 2020, 59, 5788–5796 CrossRef CAS PubMed.
- B.-M. Bresolin, S. B. Hammouda and M. Sillanpää, J. Photochem. Photobiol., A, 2019, 376, 116–126 CrossRef CAS.
- Y. F. Zhu, Y. F. Liu, Q. Ai, G. H. Gao, L. Yuan, Q. Y. Fang, X. Y. Tian, X. Zhang, E. Egap, P. M. Ajayan and J. Lou, ACS Mater. Lett., 2022, 4, 464–471 CrossRef CAS.
- S. W. Zhao, S. H. Jin, H. M. Liu, Y. X. Su, W. Liu, S. F. Li, C. C. She, H. N. Li and K. Chen, ChemCatChem, 2022, 14, e202101539 CAS.
- S. Park, W. J. Chang, C. W. Lee, S. Park, H.-Y. Ahn and K. T. Nam, Nat. Energy, 2016, 2, 16185 CrossRef.
- H. Nishiyama, T. Yamada, M. Nakabayashi, Y. Maehara, M. Yamaguchi, Y. Kuromiya, Y. Nagatsuma, H. Tokudome, S. Akiyama, T. Watanabe, R. Narushima, S. Okunaka, N. Shibata, T. Takata, T. Hisatomi and K. Domen, Nature, 2021, 598, 304–307 CrossRef CAS PubMed.
- H. Zhao, Y. Li, B. Zhang, T. Xu and C. Wang, Nano Energy, 2018, 50, 665–674 CrossRef CAS.
- Y. Tang, C. H. Mak, C. Wang, Y. Fu, F. F. Li, G. Jia, C. W. Hsieh, H. H. Shen, J. C. Colmenares, H. Song, M. Yuan, Y. Chen and H. Y. Hsu, Small Methods, 2022, 6, e2200326 CrossRef PubMed.
- H. Zhao, K. Chordiya, P. Leukkunen, A. Popov, M. Upadhyay Kahaly, K. Kordas and S. Ojala, Nano Res., 2020, 14, 1116–1125 CrossRef.
- L. Romani, A. Speltini, C. N. Dibenedetto, A. Listorti, F. Ambrosio, E. Mosconi, A. Simbula, M. Saba, A. Profumo, P. Quadrelli, F. De Angelis and L. Malavasi, Adv. Funct. Mater., 2021, 31, 2104428 CrossRef CAS.
- Y. Ji, M. She, X. Bai, E. Liu, W. Xue, Z. Zhang, K. Wan, P. Liu, S. Zhang and J. Li, Adv. Funct. Mater., 2022, 32, 2201721 CrossRef CAS.
- Y. Jiang, K. Li, X. Wu, M. Zhu, H. Zhang, K. Zhang, Y. Wang, K. P. Loh, Y. Shi and Q.-H. Xu, ACS Appl. Mater. Interfaces, 2021, 13, 10037–10046 CrossRef CAS PubMed.
- G. Chen, P. Wang, Y. Wu, Q. Zhang, Q. Wu, Z. Wang, Z. Zheng, Y. Liu, Y. Dai and B. Huang, Adv. Mater., 2020, 32, e2001344 CrossRef PubMed.
- C. Zhang, J. Chen, L. Kong, L. Wang, S. Wang, W. Chen, R. Mao, L. Turyanska, G. Jia and X. Yang, Adv. Funct. Mater., 2021, 31, 2100438 CrossRef CAS.
- S. M. S. U. Eskander and S. Fankhauser, Nat. Clim. Change, 2020, 10, 750–756 CrossRef CAS.
- J. Pi, X. Jia, Z. Long, S. Yang, H. Wu, D. Zhou, Q. Wang, H. Zheng, Y. Yang, J. Zhang and J. Qiu, Adv. Energy Mater., 2022, 12, 2202074 CrossRef CAS.
- D. Wu, X. Zhao, Y. Huang, J. Lai, H. Li, J. Yang, C. Tian, P. He, Q. Huang and X. Tang, Chem. Mater., 2021, 33, 4971–4976 CrossRef CAS.
- Z. H. Cui, P. Wang, Y. Q. Wu, X. L. Liu, G. Q. Chen, P. Gao, Q. Q. Zhang, Z. Y. Wang, Z. K. Zheng, H. F. Cheng, Y. Y. Liu, Y. Dai and B. B. Huang, Appl. Catal., B, 2022, 310, 121375 CrossRef CAS.
- S. Kumar, I. Hassan, M. Regue, S. Gonzalez-Carrero, E. Rattner, M. A. Isaacs and S. Eslava, J. Mater. Chem. A, 2021, 9, 12179–12187 RSC.
- J. Estrada-Pomares, S. Ramos-Terrón, G. Lasarte-Aragonés, R. Lucena, S. Cárdenas, D. Rodríguez-Padrón, R. Luque and G. de Miguel, J. Mater. Chem. A, 2022, 10, 11298–11305 RSC.
- Z. H. Cui, Y. Q. Wu, S. H. Zhang, H. Fu, G. Q. Chen, Z. Z. Lou, X. L. Liu, Q. Q. Zhang, Z. Y. Wang, Z. K. Zheng, H. F. Cheng, Y. Y. Liu, Y. Dai, B. B. Huang and P. Wang, Chem. Eng. J., 2023, 451, 138927 CrossRef CAS.
- B. M. Bresolin, N. O. Balayeva, L. I. Granone, R. Dillert, D. W. Bahnemann and M. Sillanpaa, Sol. Energy Mater. Sol. Cells, 2020, 204, 110214 CrossRef CAS.
- M. Shi, H. Zhou, W. Tian, B. Yang, S. Yang, K. Han, R. Li and C. Li, Cell Rep. Phys. Sci., 2021, 2, 100656 CrossRef CAS.
- Q. Sun, W. Ye, J. Wei, L. Li, J. Wang, J.-H. He and J.-M. Lu, J. Alloys Compd., 2022, 893, 162326 CrossRef CAS.
- Z.-J. Bai, Y. Mao, B.-H. Wang, L. Chen, S. Tian, B. Hu, Y.-J. Li, C.-T. Au and S.-F. Yin, Nano Res., 2022 DOI:10.1007/s12274-022-4835-z.
- Z. Wang, M. Liu, F. Xiao, G. Postole, H. Zhao and G. Zhao, Chin. Chem. Lett., 2022, 33, 653–662 CrossRef CAS.
- D. Wu, Y. Tao, Y. Huang, B. Huo, X. Zhao, J. Yang, X. Jiang, Q. Huang, F. Dong and X. Tang, J. Catal., 2021, 397, 27–35 CrossRef CAS.
- X. Wang, S. Yang, H. Li, W. Zhao, C. Sun and H. He, RSC Adv., 2014, 4, 42530–42537 RSC.
- L. Cao, D. Ma, Z. Zhou, C. Xu, C. Cao, P. Zhao and Q. Huang, Chem. Eng. J., 2019, 368, 212–222 CrossRef CAS.
- B. Sarwan, A. D. Acharya, S. Kaur and B. Pare, Eur. Polym. J., 2020, 134, 109793 CrossRef CAS.
- C. Aydogan, G. Yilmaz, A. Shegiwal, D. M. Haddleton and Y. Yagci, Angew. Chem., Int. Ed., 2022, 61, e202117377 CrossRef CAS PubMed.
- C. Ye, J.-X. Li, Z.-J. Li, X.-B. Li, X.-B. Fan, L.-P. Zhang, B. Chen, C.-H. Tung and L.-Z. Wu, ACS Catal., 2015, 5, 6973–6979 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |





