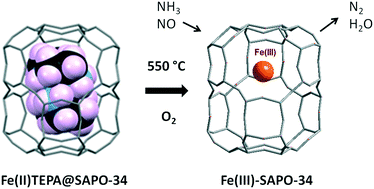
The use of transition metal cations complexed by polyamines as structure directing agents (SDAs) for silicoaluminophosphate (SAPO) zeotypes provides a route, via removal of the organic by calcination, to microporous solids with well-distributed, catalytically-active extra-framework cations and avoids the need for post-synthesis aqueous cation exchange. Iron(II) complexed with tetraethylenepentamine (TEPA) is found to be an effective SDA for SAPO-34, giving as-prepared solids where Fe2+–TEPA complexes reside within the cha cages, as indicated by Mössbauer, optical and X-ray absorption near edge spectroscopies. By contrast, when non-coordinating tetraethylammonium ions are used as the SDAs in Fe-SAPO-34 preparations, iron is included as octahedral Fe3+ within the framework. The complex-containing Fe-SAPO-34(TEPA) materials give a characteristic visible absorption band at 550 nm (and purple colouration) when dried in air that is attributed to oxygen chemisorption. Some other Fe2+ polyamine complexes (diethylenetriamine, triethylenetetramine and pentaethylenehexamine) show similar behaviour. After calcination in flowing oxygen at 550 °C, ‘one-pot’ Fe(TEPA) materials possess Fe3+ cations and a characteristic UV-visible spectrum: they also show appreciable activity in the selective catalytic reduction of NO with NH3.