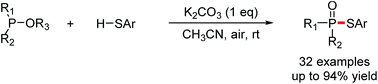Chunxiao Wen, Qian Chen, Yulin Huang, Xiaofeng Wang, Xinxing Yan, Jiekun Zeng, Yanping Huo and Kun Zhang
RSC Adv., 2017,7, 45416-45419
DOI:
10.1039/C7RA09057A,
Paper
Convenient, practical and economical phosphorylation of thiols has been achieved via halogen- and metal-free K2CO3-promoted aerobic oxidative cross-coupling of trialkyl phosphites, dimethyl phenylphosphonite, or methyl diphenylphosphinite with thiophenols using air as the oxidant at room temperature. This transformation provides a straightforward route to the construction of phosphorus–sulfur bonds with wide functional group compatibility, which affords phosphorothioates in up to 94% yield.