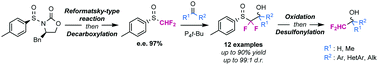Chloé Batisse, Armen Panossian, Gilles Hanquet and Frédéric R. Leroux
Chem. Commun., 2018,54, 10423-10426
DOI:
10.1039/C8CC05571H,
Communication
A new methodology to access enantiopure α,α-difluoromethyl alcohols is hereby being described. The strategy relies on the use of an enantiopure aryl α,α-difluoromethyl sulfoxide employed as chiral and removable auxiliary for the stereoselective difluoromethylation of carbonyl derivatives. The obtained α,α-difluoro-β-hydroxysulfoxides displayed unprecedented diastereomeric ratios.