Tailoring the structural and electro-optical properties of avisible-light emitting BaZrO3 photocatalyst: integrating DFT and comprehensive experimental analysis†
Shubha
Dubey
 *a,
Vipin
Kumar
*a,
Vipin
Kumar
 *bc,
Kumud
Dubey
a,
Chinmay
Sahu
a,
Anchit
Modi
d,
U. K.
Gautam
e,
R. K.
Sharma
e,
Fozia Z.
Haque
*bc,
Kumud
Dubey
a,
Chinmay
Sahu
a,
Anchit
Modi
d,
U. K.
Gautam
e,
R. K.
Sharma
e,
Fozia Z.
Haque
 f,
Gitanjali
Pagare
g and
N. K.
Gaur
a
f,
Gitanjali
Pagare
g and
N. K.
Gaur
a
aDepartment of Physics, Barkatullah University, Bhopal, 462 026, India. E-mail: shubha.dubey4@gmail.com
bDepartment of Physical Electronics, School of Electrical Engineering, Tel Aviv University, Tel Aviv 699 780, Israel. E-mail: vipinkumar@mail.tau.ac.il; vipinkumar0247@gmail.com
cThe Sackler Center for Computational Molecular and Materials Science, Tel Aviv University, Tel Aviv 699 780, Israel
dDepartment of Basic Sciences, IITM, IES University, Bhopal, 462 044, India
eTechnical Physics Division, Bhabha Atomic Research Centre, Mumbai, 400 085, India
fDepartment of Physics, Maulana Azad National Institute of Technology, Bhopal, 462 003, India
gDepartment of Physics, Sarojini Naidu Govt. Girls PG Autonomous College, Bhopal, 462016, India
First published on 27th August 2024
Abstract
In the present work, the synthesis of BaZrO3 nano-ceramics is explored through flash combustion utilizing glycine as a fuel. The resulting nanoparticles exhibit a cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group and a spherical morphology with an average size of 45.31 nm. XRD and EDAX verify the integrity of the phase. FTIR and Raman spectroscopy is used to analyze the molecular bonds and their vibrations, while XPS reveals surface compositions and oxidation states. The electro-optical properties of BaZrO3 are explored through UV-Vis spectroscopy and electronic band structure analysis. The Tauc plot displays a pair of band gaps, with values of 3.08 eV and 3.84 eV, corresponding to indirect and direct characteristics. BaZrO3 demonstrates photocatalytic potential with a degradation efficiency of approximately 36.41% for rhodamine B under visible light. Electronic band structure analysis reveals an indirect band gap of 3.05 eV in BaZrO3. The Bader analysis emphasizes the pronounced covalent characteristics present in the Zr–O bond. Photoluminescence spectra exhibit electronic transitions with a peak observed at 420.57 nm (∼2.94 eV), suggesting activity within the violet light spectrum. The CIE chromaticity coordinates imply prospective uses in the manufacture of violet-blue LEDs. These findings underscore the tailored properties of BaZrO3 nano-ceramics, showcasing their versatility for various applications, notably in advanced optoelectronic devices.
m space group and a spherical morphology with an average size of 45.31 nm. XRD and EDAX verify the integrity of the phase. FTIR and Raman spectroscopy is used to analyze the molecular bonds and their vibrations, while XPS reveals surface compositions and oxidation states. The electro-optical properties of BaZrO3 are explored through UV-Vis spectroscopy and electronic band structure analysis. The Tauc plot displays a pair of band gaps, with values of 3.08 eV and 3.84 eV, corresponding to indirect and direct characteristics. BaZrO3 demonstrates photocatalytic potential with a degradation efficiency of approximately 36.41% for rhodamine B under visible light. Electronic band structure analysis reveals an indirect band gap of 3.05 eV in BaZrO3. The Bader analysis emphasizes the pronounced covalent characteristics present in the Zr–O bond. Photoluminescence spectra exhibit electronic transitions with a peak observed at 420.57 nm (∼2.94 eV), suggesting activity within the violet light spectrum. The CIE chromaticity coordinates imply prospective uses in the manufacture of violet-blue LEDs. These findings underscore the tailored properties of BaZrO3 nano-ceramics, showcasing their versatility for various applications, notably in advanced optoelectronic devices.
1. Introduction
The modification of source materials across atomic and molecular levels has enabled the development of a wide range of nanoscale materials, allowing for the synthesis of metal–oxide nanoparticles with improved characteristics.1 The unique properties and potential applications of metal–oxide nanoparticles have sparked curiosity, leading to increased interest in various fields such as health, electronics, and energy.2 Moreover, the multifaceted applications and distinct characteristics exhibited by transition metal semiconductors in the realms of optoelectronics and energy sectors have garnered considerable interest across a spectrum of fields. Within this category of nanoparticles, transition metal semiconductors stand out prominently, fulfilling pivotal functions in magnetic storage media, catalytic processes, and gas detection systems. Additionally, metal–oxide nanoparticles hold promise for applications in electronic devices, solid oxide fuel cells, and optical materials. Research into metal oxide nanoparticles is ongoing, driven by their unique material properties and their potential utility in various domains.3 Metal oxides with the structure of a perovskite are frequently used in chemical sensors, solid oxide fuel cells, thermoelectric devices, and oxygen-permeable membranes, among other applications.4,5 Perovskites indicated by the chemical formula XYO3 (where X denotes alkaline earth and Y signifies 3d transition metals) demonstrate outstanding properties as both structural and electronic ceramics.6 When appropriately modified with dopants, these substances demonstrate conductivity in either ionic or electronic forms. Typically, in XYO3 compounds, the valence band (VB) near the Fermi energy level (Ef) is typically formed by the 2p orbitals of oxygen atoms. At the same time, the formation of the conduction band involves the contribution of d orbitals from the Y atom. The level of interaction between Y and O is contingent upon the electronegativity of the Y atom. As a result, the characteristics of the Y-site component have a significant impact on the photocatalytic capabilities of perovskite materials.Among the several perovskite nanoparticle varieties, attention has shifted to BaZrO3 nanoparticles due to their ability to emit visible light, making them useful in optical displays and devices.7 BaZrO3 nanoparticles have particular appeal in the realm of optoelectronics owing to their robust efficiency and stability across diverse operational conditions. Furthermore, nanomaterials such as BaZrO3 have unique properties that surpass many standard bulk materials in terms of efficiency and resilience.8,9 Additionally, studies suggest that the BaZrO3 nanoparticles can be used in catalysis, since they have catalytic properties across a wide range of chemical reactions. These nanoparticles not only possess optical prowess but also remarkable traits, such as a simple cubic perovskite structure, demonstrating high chemical stability, exceptional mechanical strength, and resilience to extreme temperatures. These features broaden their utility in fabricating heat-resistant composites. Recent research has unveiled the potential of BaZrO3 nanoparticles as proton conductors.10,11 Zhu and fellow researchers conducted a study aimed at accelerating proton conduction in yttrium (Y)-doped barium zirconate (BaZrO3) through the manipulation of oxygen vacancies. This approach involves engineering these vacancies, primarily achieved by introducing calcium (Ca) doping, with the goal of enhancing proton diffusion within the material.12 The functionality of semiconductor devices, encompassing photon absorption or emission, solid-state lighting, photovoltaic cells, detectors, displays, sensors, lasers, and photocatalysts, is substantially impacted by the bandgap.13 The BaZrO3 perovskite stands as a promising material across diverse applications, ranging from fuel cells to solar photovoltaics, owing to its notable oxide-ion conductivity.14
The bandgap, a fundamental property of semiconductor materials, profoundly influences the functionality of semiconductor devices by imparting unique electrical characteristics essential for modern electronics. It defines the energy distinction between the valence band, where electrons are bound to specific atoms, and the conduction band, housing free electrons capable of mobility within the material, consequently dictating the material's electrical conductivity and optical properties and requiring precise control and modification. Nevertheless, BaZrO3's broad bandgap limits its photoactivity and ability to absorb photons in the visible light spectrum.15 Consequently, modifying BaZrO3's bandgap has become a key area of research to enhance its effectiveness in photovoltaic and related applications. For example, Patra et al. achieved significant improvements in photocatalytic performance by modifying BaZrO3 with carbon dots, demonstrating superior efficiency with an optimal CD loading.6 Various methods have been employed to synthesize BaZrO3 in different sizes and shapes, such as polyhedra, nanocubes, nanowires, decaoctahedra, and monodispersed particles.16,17 These efforts have enhanced its optical, ferroelectric, and electronic properties. Common synthesis techniques include the solid-state reaction (SSR), one-step auto-combustion, sol–gel, precipitation, hydrothermal, and green synthesis techniques, which are widely used to produce BaZrO3 nanoparticles.7,9 Modifying the bandgap of BaZrO3 often involves doping and alloying with specific elements, introducing impurity energy levels into its band structure. This precise synthesis not only affects the size, shape, and composition but also unlocks potential applications in catalysis and microwave technology.18,19 Efforts to enhance visible light absorption in these wide bandgap materials include adjusting their band positions with alternative elements or modifying anions to create oxygen-related vacancies in the lattice structure. Ullah et al. used DFT calculations to analyze the properties of pristine and Cd-substituted BaZrO3, finding that Cd-substitution significantly enhanced optical performance, making it more efficient for optoelectronic devices. Both pure and Cd-substituted BaZrO3 showed potential for efficient overall water splitting, indicating their suitability for green energy applications and solar cells.20 Kayathiri et al.'s green synthesis narrowed BaZrO3's bandgap to 4.01 eV through plant chemical substitution, improving its properties. They reported an 84.1% maximum degradation efficiency of CR dye after 90 minutes of light irradiation.9
However, diverging from traditional doping approaches, an alternative method has been employed in this case, involving modified synthesis techniques, optimized sintering temperatures, and the creation of oxygen vacancies within the lattice.21 High-temperature synthesis techniques offer a means to fine-tune semiconductor bandgaps by tailoring interfacial energetics, adjusting valence and conduction band edges, and inducing surface oxygen vacancies. Surface oxygen voids function as traps for optically generated charge carriers, lessening the likelihood of electron–hole recombination, while bulk oxygen vacancies serve as centers for the recombination of photogenerated charges, significantly impacting photocatalysis.22 In addition, the material experiences the development of mid-gap energy levels as a consequence of these trap states, enabling precise adjustments to the electronic structure. The bandgap values achieved through various synthesis techniques are summarized in Table 1 for a comprehensive comparison.
| S. No. | Composition | Synthesis technique | Sintering temperature | Band gap (eV) | Particle size (nm) | Morphology | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | BaZr1−xCexO3 (x = 0.00–0.04) | Modified hydrothermal method | 100 °C | 2.37–2.14 | ∼150–200 | Hollow spheres | 6 |
| 2 | BaZrO3 | CHM approach | 50 °C | — | 50–240 | Nano cubes | 23 |
| 3 | BaZrO3 | Co precipitation method | 110 °C | 4.87 | 200 | Spheres | 24 |
| 4 | BaZrO3 | Chemically synthesized | 400 °C | 4.28 | 16 | Non-uniform nanoparticles | 9 |
| 5 | BaZrO3 | Green synthesized | 400 °C | 5.3 | 11 | Non-uniform nanoparticles | 9 |
| 6 | BaZrO3 | Pechini-type process | 1100 °C | 4.8 | — | — | 25 |
Our study introduces a novel approach to synthesizing and characterizing BaZrO3 that significantly advances the current understanding and potential applications of this material. We have developed an innovative synthesis method that allows for precise control over both particle size and morphology, addressing scalability and uniformity challenges prevalent in conventional techniques. Moreover, our thorough investigation into impurity phases, employing advanced techniques such as Raman spectroscopy and XPS, provides a deeper understanding of the synthesis process and its implications for materials properties, a level of analysis rarely explored in the existing literature. Crucially, we have demonstrated the capability to finely tune the bandgap of BaZrO3 through careful adjustment of synthesis parameters, essential for optimizing performance in optoelectronic devices. Departing from traditional doping strategies, our approach involves modified synthesis techniques and optimized sintering temperatures to deliberately introduce oxygen vacancies within the lattice, thereby enhancing semiconductor properties. This comprehensive synthesis and characterization approach not only advances fundamental knowledge but also positions BaZrO3 for innovative applications in fields ranging from electronics to catalysis.
Presently, the aim is to tailor the band gap of barium zirconate using suitable synthesis techniques to explore its potential as a photocatalyst for degrading rhodamine B dye and perform a detailed structural, electronic and optical analysis by means of different spectroscopic techniques. Density functional theory calculations, utilizing the projector augmented wave (PAW) method, are employed to confirm the origin of the optical band gap. Bader charge analysis is also performed in order to achieve a quantitative assessment of the bonding characteristics in the BaZrO3 ceramic.
2. Experimental and computational details
2.1 Synthesis
High-purity barium nitrate [Ba(NO3)2] and zirconium dioxide [ZrO2] nanoparticles were used as an oxidizer, while glycine [C2H5NO2] was used as a fuel in a proportion of 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.22 for a one-step facile flash combustion synthesis. The proportional quantities of these materials were determined based on the following chemical equation:
0.22 for a one-step facile flash combustion synthesis. The proportional quantities of these materials were determined based on the following chemical equation: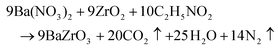 | (1) |
The main steps for the one-step facile flash combustion synthesis are schematically illustrated in Fig. 1. The appropriate amount of oxidizers and fuel was taken in a 100 ml beaker and mixed together to form a homogeneous mixture. A sufficient amount of glycine solution was added to ensure that the total number of reducing and oxidizing valences was equal, resulting in an equivalence ratio (oxidizing valences/reducing valences) of one. This served to maximize the release of heat due to the exothermic reaction.
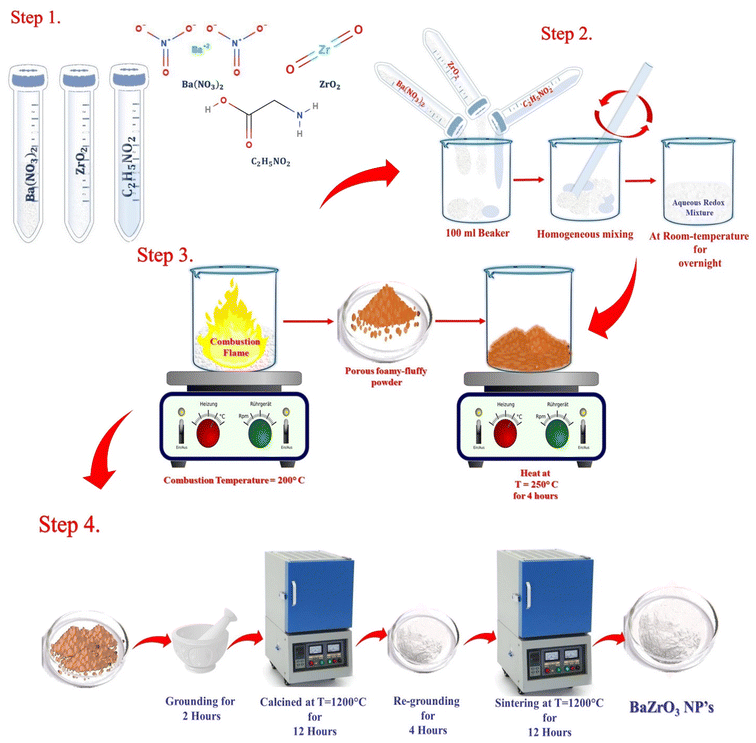 | ||
| Fig. 1 Schematic diagram of the synthesis of BaZrO3 nanoparticles via glycine-assisted combustion techniques. | ||
The obtained homogeneous mixture was then aged overnight at room temperature to form a xerogel. Subsequently, the xerogel was placed on a hotplate and maintained at a temperature of around 200 °C. During this stage, the xerogel underwent dehydration and self-propagating combustion within a few seconds, accompanied by a flash of light. This combustion reaction released CO2 and N2 gases and formed a porous foamy-fluffy powder due to the exothermic reaction between the oxidizer and fuel. The resulting porous foamy-fluffy powder of BaZrO3 was reheated at 250 °C for 4 hours. Afterward, the fluffy powder was ground for 2 hours and calcined at 1200 °C in air for 12 hours using a tubular furnace. Finally, the BaZrO3 powder was reground and sintered at 1200 °C under the same conditions and for the same duration to enhance its crystallinity.
2.2 Characterization
The determination of the phase composition and crystal structure of the prepared BaZrO3 nanoparticle is accomplished using powder X-ray diffraction (PXRD) with CuKα radiation (λ = 1.54056 Å) on the Bruker D8 Advance instrument. FullProf software was used for diffractogram analysis, utilizing the pseudo-Voigt function to refine peak profiles. Characterization of the sample size, surface morphology, and elemental composition was carried out using a “Carl Zeiss Supra 55” FESEM, combined with energy X-ray dispersive spectroscopy (EDS). The standard KBr pellet technique was used to record the Fourier transform infrared (FT-IR) absorption spectrum, using a PerkinElmer FT-IR spectrometer (Spectrum 1000, Japan). For Raman spectra, the LaBRAM HR Raman spectrometer from Horiba (France SAS), utilizing a 633 nm He–Ne laser source with 100% power, was employed. X-ray photoelectron spectroscopy (XPS) was performed at the Indus-2 synchrotron facility RRCAT Indore, operating in the hard X-ray region and utilizing a Photoemission Electron Spectrometer (PES), BL-14, powered by a 1.5 T bending magnet source. This synchrotron source is equipped with a double-crystal monochromator [Si (111)] featuring an excitation energy of 4.065 keV. The system is further enhanced with a hemispherical analyzer and detector system (Phoibos 225, Specs make). Throughout the experiments, the pressure in the experimental station was maintained at 5 × 10−9 mbar.To address surface charging effects, all peaks were referenced to the C 1s spectrum (284.77 eV) in the analysis. The XPS core level spectral data underwent analysis and quantification using XPSPEAK 4.1 software, and the Shirley background method was applied to subtract the background from all XPS spectra. All measurements were conducted at room temperature.
Polarization measurements were performed using ferroelectric hysteresis loops (M/s Radiant Technology, USA) at 20 kHz. The optical band study of synthesized BaZrO3 nanoparticles was carried out using a PerkinElmer Lambda 950 UV-visible spectrophotometer across a wavelength range of 200 to 800 nm. Photocatalytic activity testing of the BaZrO3 catalyst for RhB (10 mg L−1) degradation was conducted under UV light using a 150 W Xenon lamp housed within a column-shaped stainless-steel cabinet (λmax ∼400 nm). Room temperature photoluminescence excitation spectra were acquired using an F-7000 Hitachi fluorescence spectrophotometer, utilizing a Xenon lamp employed as the excitation source for emission.
2.3 Computational details
In this study, electronic and geometrical calculations based on density-functional theory (DFT) were conducted using the Vienna ab initio simulation package (VASP).26 The projector-augmented wave (PAW) method was employed to consider both ionic core effects and electron–electron interactions.27 The Perdew–Burke–Ernzerhof (PBE) functional within the generalized gradient approximation (GGA) handled the exchange–correlation potential.28 To ensure precision in both geometrical and electronic properties, a kinetic cut-off energy of 550 eV was specified. Optimization and partial density of states analysis utilized a 15 × 15 × 15 dense k-point mesh employing the Monkhorst–Pack (MP) system.3. Results and discussion
3.1 Crystal structure and phase composition analysis
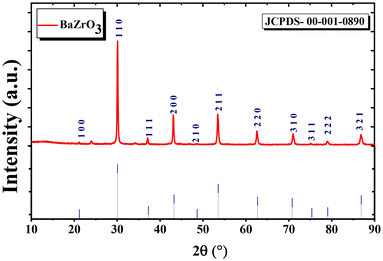
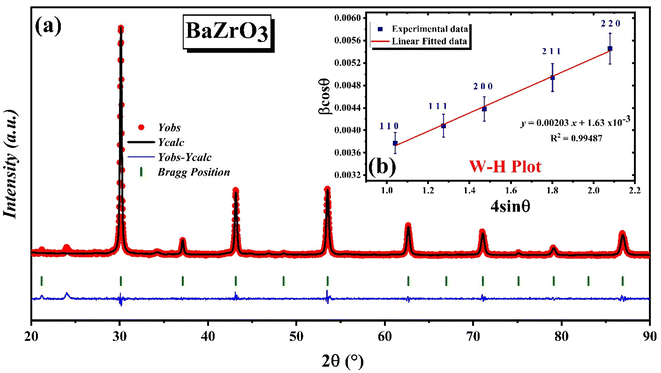
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (no. 221) space group is presented. The fitting resulted in a final difference and a profile fit. The XRD profile reveals three prominent peaks at 30°, 43°, and 53° with Miller indices (1 1 0), (2 0 0), and (2 1 1), respectively. These observations suggest that the crystal structure is predominantly oriented in the (1 1 0), (2 0 0), and (2 1 1) directions. The calculated pattern and the observed one exhibit a strong correspondence with characteristics atomic parameters after Rietveld refinement are presented in Table 2. The low values of various R-factors such as Rexp, Rbragg, Rp, RF, Rwp, and χ2, along with GOF, support the justification for the refined model, indicating a well-aligned agreement between the experimental data and the refined model.
m (no. 221) space group is presented. The fitting resulted in a final difference and a profile fit. The XRD profile reveals three prominent peaks at 30°, 43°, and 53° with Miller indices (1 1 0), (2 0 0), and (2 1 1), respectively. These observations suggest that the crystal structure is predominantly oriented in the (1 1 0), (2 0 0), and (2 1 1) directions. The calculated pattern and the observed one exhibit a strong correspondence with characteristics atomic parameters after Rietveld refinement are presented in Table 2. The low values of various R-factors such as Rexp, Rbragg, Rp, RF, Rwp, and χ2, along with GOF, support the justification for the refined model, indicating a well-aligned agreement between the experimental data and the refined model.
| Phase | BaZrO 3 | ||||
| Structural & lattice parameters | |||||
| Space group | Pm3m (221) | ||||
| a (Å) | 4.18990 | ||||
| Atoms | Atom | Site/Sym. | x | y | Z |
| Ba | 1b/m![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
0.5 | 0.5 | 0.5 | |
| Zr | 1a/m![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
0.0 | 0.0 | 0.0 | |
| O | 3d/4/mm·m | 0.5 | 0.0 | 0.0 | |
| V (Å 3 ) | 73.5500 | ||||
| D (nm) | 70.2900 | ||||
| ε (×10 −3 ) | 1.6200 | ||||
| 〈d Ba–O 〉 (Å) | 2.96270 | ||||
| 〈d Zr–O 〉 (Å) | 2.09494 | ||||
| Reliability factors | R p | 14.70 | |||
| R wp | 14.70 | ||||
| R exp | 13.94 | ||||
| R Bragg | 1.805 | ||||
| R f | 1.930 | ||||
| χ 2 | 1.120 | ||||
| GOF | 1.054 | ||||
| Elemental composition for BaZrO 3 | |||||
| Element | Weight % | Atomic % | Error % | ||
| C K | 17.1 | 45.3 | 11.4 | ||
| O K | 18.7 | 37.2 | 9.6 | ||
| Zr L | 22.2 | 7.8 | 5.5 | ||
| Ba L | 42.0 | 9.7 | 7.8 | ||
| Total | 100.00 | 100.00 | 34.3 | ||
| Effective Bader charges | |||||
| Atom | Effective Bader charge (e) | ||||
| Ba 2+ | 1.5229 | ||||
| Zr 4+ | 2.4952 | ||||
| O 2− | −1.3394 | ||||
The Williamson–Hall plot method31 was employed to determine the crystallite sizes of the synthesized samples through the following relationship:
β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 4ε θ = 4ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ + kλ/D θ + kλ/D | (2) |
In Fig. S1(a),† we depict the crystallographic representation of BaZrO3 nanoparticles, emphasizing their perovskite nature characterized by a robust cubic symmetry. In this crystalline arrangement, the central positions are occupied by Ba atoms, with Zr atoms positioned at the unit cell's vertices. Furthermore, oxygen atoms are situated at the midpoint of the edges. Notably, the Ba cations exhibit a 12-fold octahedral coordination, while the Zr cations display a 6-fold octahedral coordination, as illustrated in Fig. S1(b) and S1(c),† respectively, (ESI†).
The analysis of BaZrO3 charge density plots offers crucial insights into its electronic structure and bonding traits. An in-depth examination of the electron density distribution within the unit cell involved conducting a detailed study that mapped electron density in the 110 plane using the GFourier program,32 which is a component of the FullProf package. This method generated two-dimensional Fourier maps, effectively illustrating how electron density envelops each atom in the compound's elements.
Typically depicted with contour lines, these maps offer a clear and intuitive view of electron density distribution. Shown in Fig. S1(d and e),† the color scale in these maps illustrates varying electron density levels. Areas of higher electron density appear in shades of red, while lower density regions are depicted in violet. Significantly, there is a notable elevation in electron density for all atoms except oxygen and the interstitial region. This visual representation effectively illustrates unique electron density patterns for various atoms in the compound, unveiling localized electron distribution and highlighting significant density regions within the crystal structure. From the Pauling electronegativity scale,33 the Ba–O and Zr–O bonds exhibit a more ionic character. Moreover, predominant charge transfer occurs from other atomic species towards the oxygen atoms. Additionally, the Ba–O bond appears more ionic compared to the Zr–O bond.
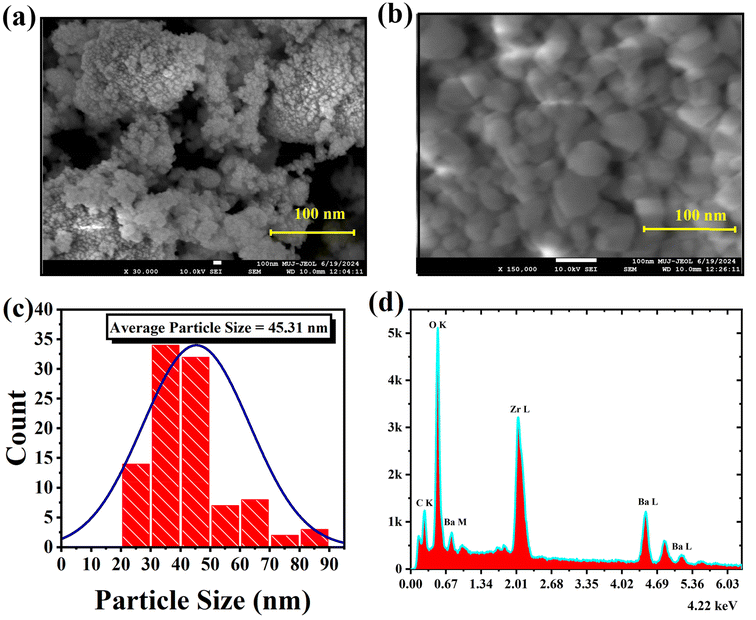 | ||
| Fig. 4 (a and b) Surface morphology micrographs, (c) average particle size distribution of BaZrO3, and (d) EDAX spectra. | ||
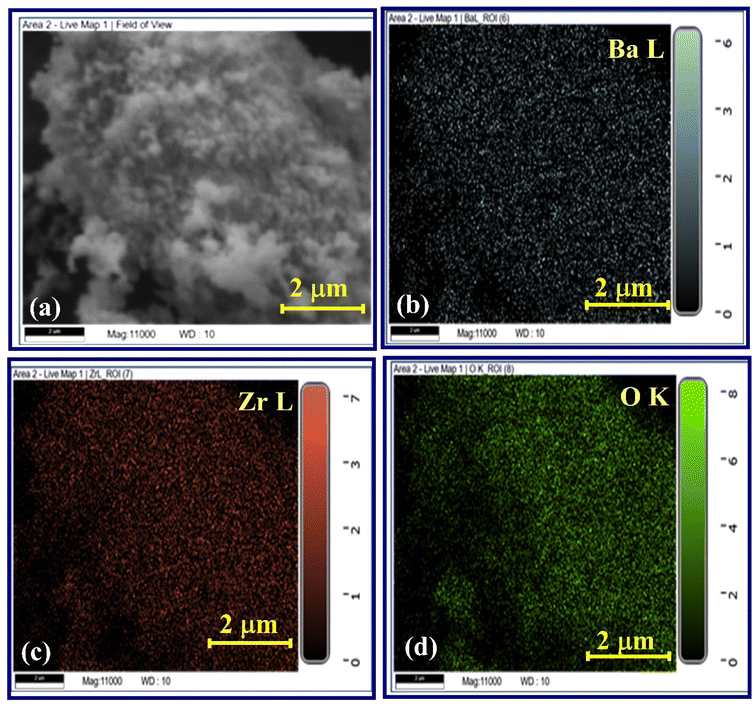
Fig. 4(c) presents the distribution of average particle sizes, with a frequency plot illustrating the size distribution derived from measurements of a substantial quantity of BaZrO3 particles. The particle size distribution conforms to the characteristics of a normal distribution function. Notably, the calculated average particle size of BaZrO3 stands at approximately 45.31 nm. Previously, BaZrO3 powder was synthesized using sol–gel auto-combustion in a pre-heated furnace, exploring the influence of pH variation on citrate nitrate sol-gels and resulting in nanoparticles averaging 33.3 nm in size.36 In order to establish the elemental composition of BaZrO3, Energy Dispersive X-ray Analysis (EDAX) was performed under precisely controlled temperature conditions. The EDAX spectrum depicted in Fig. 4(d) distinctly exhibits the detection of Ba, Zr, and O elements, thus confirming the absence of any significant loss of essential elements during the sintering process, accounting for experimental uncertainties. The elemental composition typical of BaZrO3, involving atomic percentage, is presented in Table 2. This meticulous analytical evaluation reinforces both the consistency in cationic composition within BaZrO3 and the non-existence of secondary phases within the structure of BaZrO3. The observation reveals a Ba/Zr ratio of 1.24, nearly approaching unity, signifying the integration of these elements into the structure and providing confirmation of the absence of any remaining oxides.37
Furthermore, for a thorough comprehension of the elemental composition and distribution within the synthesized nanoparticles, we have incorporated the elemental distribution graphs derived from EDS analysis as depicted in Fig. S2.† The EDS mapping data, shown in Fig. 5(a–d), highlights the following: Fig. 5(a) identifies the region where the EDS mapping was conducted, while Fig. 5(b–d) respectively displays the presence of Ba, Zr, and O in the synthesized sample. Importantly, no other detectable impurities were found within the resolution limit of the EDS measurement.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m phase. Fig. S3† illustrates phonon dispersion relations along the principal symmetry directions of the Brillouin zone. A noteworthy aspect of the phonon spectra is the lowest-frequency phonon branch, which demonstrates negative phonon frequencies at the M-points within the Brillouin zone. The unstable phonon mode at the M-point, which was not observed by Perrichon and coworkers38 or Bilic et al.,39 was detected in other calculations reported by Helal et al.40 and Akbarzadeh,41 which also employed the DFT method. The occurrence of phonon instability at the M-point is contingent upon the selection of the exchange–correlation potential and pseudopotential quality used to depict the interaction between the core and the valence electrons.42
m phase. Fig. S3† illustrates phonon dispersion relations along the principal symmetry directions of the Brillouin zone. A noteworthy aspect of the phonon spectra is the lowest-frequency phonon branch, which demonstrates negative phonon frequencies at the M-points within the Brillouin zone. The unstable phonon mode at the M-point, which was not observed by Perrichon and coworkers38 or Bilic et al.,39 was detected in other calculations reported by Helal et al.40 and Akbarzadeh,41 which also employed the DFT method. The occurrence of phonon instability at the M-point is contingent upon the selection of the exchange–correlation potential and pseudopotential quality used to depict the interaction between the core and the valence electrons.42
Imaginary frequencies, denoted by negative phonon frequencies, signify a breakdown in crystal symmetry. Anharmonic phonon interactions can also contribute to these negative frequencies, potentially triggering a structural phase transition in BaZrO3 under specific conditions. It is crucial to emphasize that the computed phonon dispersion spectra correspond to conditions of absolute zero temperature and zero pressure. Variations in temperature or pressure can significantly alter the situation. Thus, it can be inferred that BaZrO3 displays dynamic instability at low temperatures and zero pressure. However, this scenario may change under pressure, as evidenced by the previously documented pressure-induced phase transition in BaZrO3 crystals.42–44
Regarding lattice dynamics, this phase transformation corresponds to lattice instability at the Brillouin zone boundary points. The lattice instability identified in the present calculations aligns closely with earlier studies that employed a similar approach.
Raman spectroscopy stands as a remarkably sensitive and robust technique to investigate crystal structures through the analysis of their vibrational bands. By employing group theory analysis, one can ascertain the count of allowed vibrational modes in a specific crystal system. The factor group analysis for pertinent space groups is presented in Table 3.
| Atoms | Site | Symmetry | X | Y | Z | Site species | Irreducible representation |
|---|---|---|---|---|---|---|---|
Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group (Oh1) m space group (Oh1) |
|||||||
| Ba2+ | 1b | O h | 0.5 | 0.5 | 0.5 | F 1u | F 1u |
| Zr4+ | 1a | O h | 0.0 | 0.0 | 0.0 | F 1u | F 1u |
| O2− | 3d | D 4h | 0.5 | 0.0 | 0.0 | A 2u | F 1u |
| E u | F 1u + F2u | ||||||
| Γ Total = 4F1u + F2u | Γ Acaustic = F2u | ||||||
| No Raman active modes | Γ IR = 4F1u | ||||||
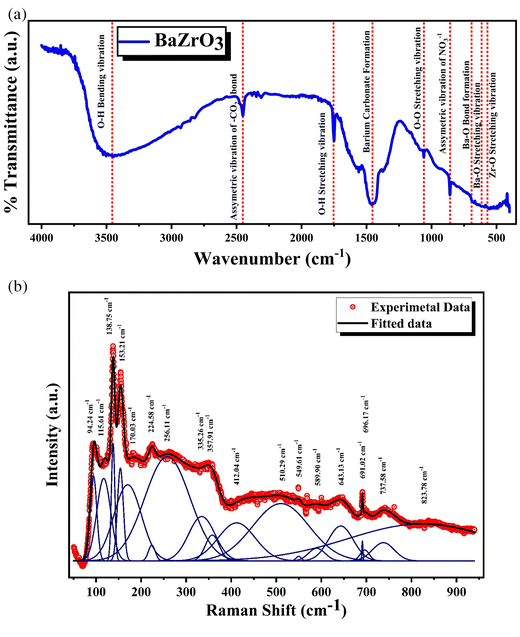
Group theoretical studies suggest that perovskite materials with a pure cubic crystal structure do not exhibit first-order Raman active phonon modes in vibrational spectra at room temperature, resulting in the absence of a Raman spectrum.55 However, experimental studies on BaZrO3 samples contradict this, revealing the presence of some Raman active modes or bands (Fig. 6(b)). The literature provides two explanations for the Raman spectrum of simple cubic perovskites. The primary explanation involves second-order scattering, where two phonons with opposite parallel wave vectors undergo allowed transitions, resulting in a low-intensity peak. The less supported explanation attributes the spectrum to the breaking of local symmetry caused by tilt and structural disorder, allowing deviation from the selection rule, and producing active modes in Raman.56 The local symmetry of barium zirconate is influenced by octahedral tilt, oxygen octahedral distortion, or structural instability. Therefore, the observed peaks in BaZrO3 are likely a result of second-order scattering.57,58
Raman active modes are further categorized into two optical components: longitudinal (LO) and transverse (TO). However, in the case of BaZrO3, a perovskite that maintains its cubic phase across all temperatures, it also lacks first-order Raman active modes due to its symmetry constraints. Nevertheless, it exhibits a remarkably intense Raman spectrum, characterized by distinct and well-defined features. This intriguing spectral behavior is likely due to its second-order nature.59Fig. 6(b) depicts the room temperature Raman spectra of the BaZrO3 ceramic. The spectra exhibit a comparable signature, bearing resemblance in their main features to the few spectra reported in the literature.55,56,60–62 The three polar modes in cubic perovskites are commonly referred to as the “Slater” mode, involving an anti-phase motion of the B cation concerning the oxygen octahedra, the “Axe” mode, which entails an anti-phase motion of the apical oxygens with respect to the other oxygen atoms within the octahedra, and the “Last” mode, characterized by an anti-phase motion of the A cation and the ZrO6 octahedra.63 These labels hold particular significance in the context of ferroelectric transitions.63
The Raman spectra of BaZrO3 exhibit distinct bands at approximately 138.75, 357.91, and 691.02 cm−1, which can be attributed to different zone center modes. Specifically, the 138.75 cm−1 band corresponds to the last mode, representing the displacement of Ba relative to the ZrO6 octahedra.64 The 357.91 cm−1 band corresponds to the Slater mode, signifying out-of-plane displacements of Zr in relation to in-plane oxygen atoms. Finally, the 691.02 cm−1 band corresponds to the Axe mode, indicating the displacement of apical oxygens with respect to in-plane oxygens. Additionally, the Raman mode observed at 94.24 cm−1 is associated with the anti-parallel motion of Ba–O. Two first order transverse optical Raman modes, TO2 and TO4 are observed at 170.03 and 549.61 cm−1. Second-order modes are identified at frequencies of approximately 224.58, 412.04 and 589.90 cm−1. Furthermore, three bands are observed at approximately 115.61, 224.58, and 510.29 cm−1, all representing f1u modes. The band at 153 cm−1 aligns with the presence of BaCO3, which may also leave a distinctive mark in XRD analysis. However, we would like to emphasize that the presence of BaCO3 is expected in our synthesis process, and its contribution to the spectra is consistent with its known presence as a precursor. This observation aligns well with previously reported data.55 The mode at 115.61 cm−1 is associated with cation-ZrO3 lattice vibrations, while the mode at 224.58 cm−1 corresponds to O–Zr–O bending vibrations and the mode at 510.29 cm−1 represents Zr–O stretching vibrations. Notably, the last two modes (224.58 and 510.29 cm−1) are independent of the Ba cation. Additionally, the vibrational modes observed at approximately 696.17 cm−1 are associated with distortions occurring at the octahedral site of zirconium within the BaZrO3 unit cell.55–64
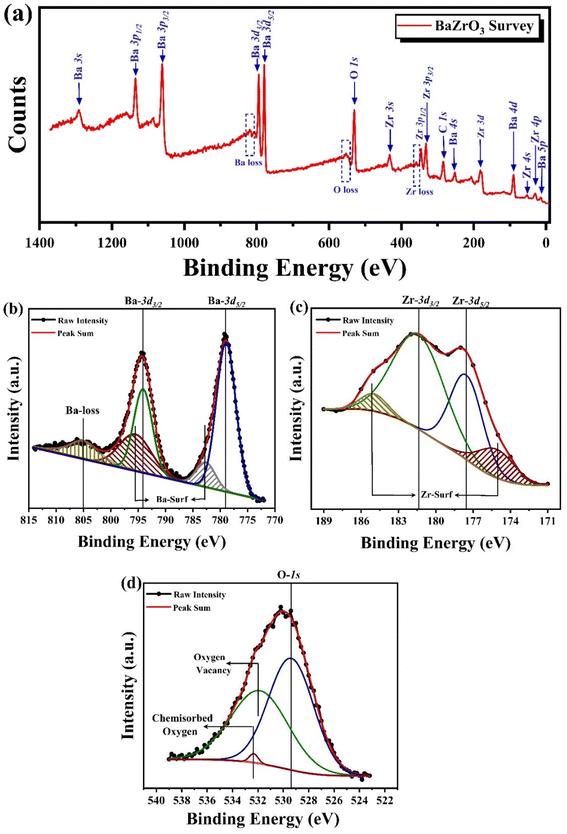 | ||
| Fig. 7 (a) XPS survey spectra of BaZrO3 nanoparticles (b) XPS spectra of Ba-3d element, (c) XPS spectra of Zr-3d element, and (d) XPS spectra of O-1s element. | ||
| Atom | Orbital | Binding energy | FWHM | Area | Peak specification |
|---|---|---|---|---|---|
| Ba2+ | 3d | 778.902 | 4.207 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 138.09 138.09 |
3d5/2 |
| 782.628 | 4.074 | 7550.826 | Surface | ||
| 794.083 | 3.912 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 517.36 517.36 |
3d3/2 | ||
| 795.418 | 6.845 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 014.05 014.05 |
Surface | ||
| 805.093 | 6.866 | 7622.065 | Ba-Loss | ||
| Zr4+ | 3p | 175.11 | 3.14 | 1703.944 | Surface |
| 177.54 | 3.06 | 4602.614 | 3d5/2 | ||
| 181.36 | 4.85 | 7983.784 | 3d3/2 | ||
| 184.98 | 2.15 | 773.7342 | Surface | ||
| O2− | 1s | 529.374 | 4.174 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 391.94 391.94 |
Lattice oxygen |
| 531.850 | 5.306 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 481.81 481.81 |
O2− vacancy | ||
| 532.330 | 0.792 | 306.157 | Chemisorbed oxygen |
In Fig. 7(c), the HR-XPS spectrum for the Zr 3d orbital is presented. The Zr 3d peaks can be primarily deconvoluted into two components: those around 177.542 eV correspond to Zr 3d5/2, and those at approximately 181.361 eV are attributed to Zr 3d3/2, indicating Zr4+ oxidation states, consistent with the existing literature.68 The observed spin–orbit splitting, i.e., the difference between the binding energy of Zr 3d3/2 and Zr 3d5/2, doublets, (i.e. ΔEBE = BE3d3/2 − BE3d5/2) measures 3.819 eV, aligning with previous findings.70 The presence of spin–orbit split components in the core-level spectra of Ba 3d and Zr 3d, with separations of 15.193 and 3.819 eV, respectively, confirms the +2 and +4 oxidation states of Ba and Zr in the BaZrO3 ceramic.71,72 Peaks observed at 175.110 and 184.984 eV are attributed to oxidized surface species of zirconium.67
Fig. 7(d) presents the high-resolution XPS spectra of oxygen 1s. For pristine BaZrO3, the spectra can be deconvoluted into three peaks at 529.374, 531.850, and 532.330 eV, respectively.73 The characteristic peak of lattice oxygen is evident in the O 1s core-level spectra at 529.374 eV,63 while the peak at 531.850 eV is possibly associated with oxygen vacancies,74 and the peak at 532.330 eV arises due to surface hydroxyl groups.75
3.2 Ferroelectric PE loop
Polarization versus electric field (P–E) hysteresis loops were employed for the examination of the ferroelectric characteristics of BaZrO3 ceramics. Fig. S4† presents the P–E hysteresis curves for the BaZrO3 ceramic at room temperature, measured at a frequency of 50 Hz. These curves demonstrate a distinctive saturated loop to a certain extent, as evidenced by the presence of leakage within the P–E hysteresis loop. This behavior can be attributed to the presence of pinning defects and the accumulation of free charges at both the grain and grain boundaries.31 The magnitude of ferroelectric parameters, such as remanent polarization and coercive field, is derived from the P–E loop. Notably, they exhibit a remanent polarization (Pr) of 0.068 μC cm−2, saturation polarization (Ps) of 0.11789 μC cm−2 and a coercive field (Ec) of 9.39 kV cm−1.The squareness of the ferroelectric ceramic can be determined utilizing the Haertling and Zimmer correlation.
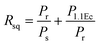 | (3) |
In this context, Rsq represents the squareness parameter of the hysteresis loop. Pr denotes the remanent polarization, Ps indicates the saturated polarization at a finite field strength below dielectric breakdown, and P1.1Ec stands for the polarization at a field equal to 1.1Ec. According to the literature, the ideal square loop (Rsq) of a ceramic equals 2.00. For BaZrO3, the estimated Rsq value is approximately 1.80.
3.3 UV-Vis spectroscopy analysis
The bandgap of a semiconductor, a critical parameter in materials science, signifies the energy difference between its valence and conduction bands, determining its electrical conductivity characteristics. A narrower bandgap denotes a better conductor and researchers harness specific bandgap values to tailor semiconductor materials for precise electronic applications like diodes and transistors. Moreover, the bandgap significantly influences a semiconductor's thermal properties, impacting its efficiency in thermoelectric devices used for heat-to-electricity conversion, making it a pivotal factor in materials selection and device design.In recent research, the optical properties of BaZrO3 have garnered considerable attention due to its optical band gap, which has been determined to be approximately 4.8 eV using UV-visible spectroscopy.70,71,73 The optical band gap serves as a critical parameter denoting the energy threshold required for photons to be absorbed by the material. It is noteworthy that this optical band gap tends to be somewhat narrower than the corresponding electrical band gap, a characteristic observed in various perovskite oxide materials. This unique feature has stimulated the exploration of BaZrO3's potential in a range of optical applications, particularly in the visible and ultraviolet (UV) spectral regions. The material's substantial band gap, which falls within the range of 3.8 to 7.0 eV, underscores its versatility and suitability for diverse photon-based technologies, making it an attractive candidate for future optoelectronic and photonic devices.15,30,76
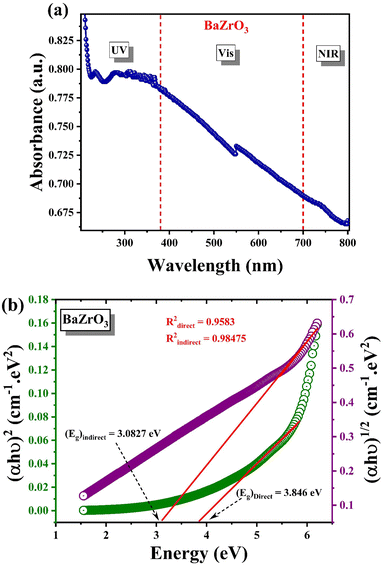
The efficiency of a photocatalyst relies significantly on its light absorption capability, crucial for initiating photogenerated charge carriers within the material. Fig. 8(a), describes the absorbance (A) versus the wavelength (λ) of BaZrO3 in three radiation domains: UV (ultra-violet), Vis (visible) and NIR (near infrared). The absorption band is observed in the UV-region in the wavelength range of 200–800 nm.
Fig. 8(a) illustrates that BaZrO3 exhibits its primary absorption peak at 230 nm, attributed to band-to-band transition. Additionally, a band tail extending beyond 400 nm is evident, indicating the presence of disordered states, defect states, or oxygen vacancies within the compound. Such impurities or defect-state transitions typically manifest as band tails in semiconductor absorption spectra.6 The occurrence of absorption bands in the UV-VIS range indicates the capability of BaZrO3 to absorb UV and visible light. This characteristic renders them suitable for applications such as UV-VIS light absorption, the development of photovoltaic solar cells, and the creation of photocatalytic materials.77 Semiconductors in photovoltaic solar cells can capture energy from both UV and visible light, enabling the generation of electricity. In photocatalysis, these semiconductors can also promote chemical reactions upon exposure to either UV or visible light.78 The absorbance-wavelength spectra enabled the determination of an optical bandgap energy value that corresponds to the absorption band observed in the UV-Vis region. The determination of the bandgap energy for the BaZrO3 sample under study can be accomplished using Tauc's law31,32
| αhν = β(hν − Eg)n | (4) |
In this equation, ‘hν’ represents the photon energy of the substance and ‘β’ is a constant. In this particular scenario, two distinct optical transitions were identified: one with ‘n’ equal to 1/2, indicative of a direct transition type, and another with ‘n’ equal to 2, signifying an indirect transition. The determination of the band gap energy can be accomplished by applying the Tauc expression provided below.
| (αhν)1/n = β(hν − Eg) | (5) |
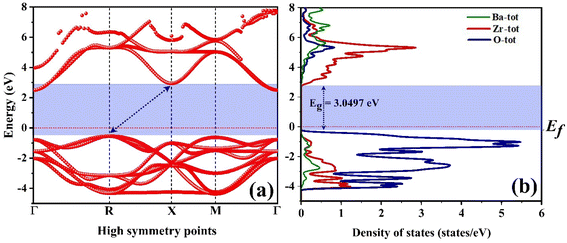
Fig. 8(b) illustrates the plots of (αhν)1/2 and (αhν)2 as a function of varying photon energy (hν) for the BaZrO3 compound. By linearly extrapolating the curves shown in Fig. 8(b) to zero hν, the determined values for the indirect and direct band gap energies are found to be (Eg)indirect = 3.0827 eV and (Eg)Direct = 3.846 eV, respectively. The observed band gap energy (Eg) for the sample produced in this study is lower than that reported in previous research. This reduction in the band gap is attributed to the high sintering temperature and is indicative of the quantum confinement effect.79 Moreover, upon comparing our findings with theoretical values, we find a good match, confirming that the band gap energy has indeed decreased. Reducing the bandgap value can boost the rate of electron–hole pair generation on the surface of the nanocatalyst, leading to an overall improvement in photocatalytic activity.21,22
The determination of the refractive index in BaZrO3 is accomplished using a contemporary empirical linear relationship between the energy band gap and refractive index, as proposed by Paswan80et al.
| n = 4.084 − 0.62Eg | (6) |
Subsequently, Fresnel's equation is utilized to compute the corresponding reflectance value.
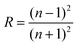 | (7) |
The determined value for ‘n’ in the case of BaZrO3 is 2.17, while the computed value for ‘R’ stands at 0.1375.
3.4 Photocatalytic degradation
Maximizing the semiconductor's absorption of incident sunlight stands as a powerful method to elevate the catalytic prowess of a photocatalytic material.81,82 Under consistent reaction parameters, the efficacy of a BaZrO3 catalyst was examined in conjunction with an initial RhB concentration set at 10 mg L−1 (10ppm). During experimentation, it was observed that elevating the initial dye concentration up to an optimal threshold corresponded to an escalation in RhB degradation. Nevertheless, exceeding this optimal concentration resulted in a decline in RhB degradation. Boczkaj et al. reported the highest degradation of RhB, reaching 37.3%, which occurred at an initial concentration of 10 ppm, while the lowest degradation, at 16%, was recorded at a concentration of 6 ppm.83 Arti et al.84 investigated the photocatalytic behavior of Dy3+-doped BaZrO3 nanopowders, highlighting significant enhancements in photocatalytic, photoluminescence, and fingerprint properties. Their findings revealed that optimal degradation was achieved within the first 180 minutes, after which no further degradation was observed. The kinetic analysis indicated that the degradation process followed zero-order kinetics, with a rate constant of 0.015 and an R2 value of 0.98, suggesting a strong correlation with the prepared nanopowder. In a related study, Jana et al. conducted an in-depth exploration of doped double perovskites, such as K2Ta2O6 and Ba2TiMoO6, uncovering their potential to significantly enhance the photocatalytic degradation rates of pollutants like methylene blue. The study elucidated the critical role of hydroxyl radicals as the primary reactive species driving these degradation processes.85 As per the literature survey, Yi et al.102 also observed analogous findings, noting a rise in the degradation rate from 0.00117 to 0.00136 min−1 with variations in the initial RhB concentration, ranging from 20 to 40 μmol L−1. This reveals that the extent of degradation depends on the generation of hydroxyl radicals and the accessibility of contaminants for reaction. Consequently, the role played by the initial concentration of the RhB dye emerges as a critical parameter for optimizing degradation processes. The previous literature presents a compilation of results concerning the application of BaZrO3 with diverse dyes, systematically recorded in a table format. Table 5 outlines the overall degradation efficiency of different industrial dyes using the BaZrO3 catalyst.| S. No. | Photocatalyst | Illumination | Synthesis method | Contaminant | Results | Ref. |
|---|---|---|---|---|---|---|
| 1. | BaZrO3 | UV radiation | Green synthesis | CR dye | Degradation efficiency, 84.1% in 90 min, k = 0.0175 min−1 | Kayathiri et al., (2022)9 |
| 2. | BaZrO3:W 15% | UV–A | Hydrothermal synthesis | Levofloxacin | Degradation efficiency 93.4% and k = 1.27 × 10−2 min−1 | Gulen et al. (2021)64 |
| 3. | BaZrO3:W 5% | Visible light | Hydrothermal synthesis | Tetracycline | Degradation efficiency 94.9% and k = 1.01 × 10−2 min−1 | Gulen et al. (2021)64 |
| 4. | BaZrO3:Dy3+ | UV illumination | Hydrothermal synthesis | Methylene Blue | Degradation efficiency 78.66%, in 180 min and k = 0.015 min−1 | Aarti et al. (2024)84 |
| 5. | 3% carbon dots modified BaZrO3 | UV radiation | Facile carbonation process via the hydrothermal route | Methylene Blue | Degradation efficiency ∼90% | Patra et al. (2018)6 |
The photocatalytic activity of the synthesized catalyst was examined by assessing its ability to degrade rhodamine B under visible light irradiation (λ = 400 nm) using a 150 W Xe lamp. Under visible light irradiation, the color of the RhB/BaZrO3 suspension changes and the absorption gradually decreases, indicating that the ethyl groups were removed and thus, RhB was degraded. When exposed to visible light, BaZrO3 becomes activated and generates photo-induced electron–hole (e−/h+) pairs. Following this, photo-induced electrons rapidly migrate from BaZrO3 to RhB. As a widely recognized phenomenon, these photo-induced electrons efficiently catalyze the reduction of O2 molecules adsorbed on the surface of RhB/BaZrO3, yielding 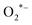 radicals that play a pivotal role as key active species in the degradation process.86 In addition, the photo-induced h+ can oxidize RhB or react with H2O to generate OH* radicals. The generated OH˙ and
radicals that play a pivotal role as key active species in the degradation process.86 In addition, the photo-induced h+ can oxidize RhB or react with H2O to generate OH* radicals. The generated OH˙ and 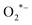 radicals can then oxidize RhB. The photo-degradation mechanism of RhB by BaZrO3 can be described by the following reactions:87
radicals can then oxidize RhB. The photo-degradation mechanism of RhB by BaZrO3 can be described by the following reactions:87
| BaZrO3 + hν → eCB−(BaZrO3) + h+VB(BaZrO3) | (8) |
| h+VB(BaZrO3) + H2O → OH* + H+ | (9) |
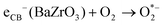 | (10) |
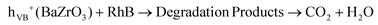 | (11) |
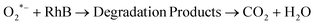 | (12) |
| OH* + RhB → Degradation Products → CO2 + H2O | (13) |
| h+ + e− → (e−,h+) (negligible recombination) | (14) |
Fig. 9(a) displays the temporal UV-vis spectral changes of RhB aqueous solution during photocatalytic degradation reactions. Under visible light irradiation (λ = 400 nm) in the presence of BaZrO3, the primary absorbance peaking at 552 nm significantly decreased with irradiation time, resulting in approximately 35 to 38% degradation after 40 minutes of irradiation, as depicted in Fig. 9(a). The efficiencies of the photocatalytic degradation under visible light irradiation are illustrated in Fig. 9(b), where C represents the absorption of RhB at a wavelength of 552 nm, and C0 signifies the absorption after adsorption equilibrium on BaZrO3 before irradiation.
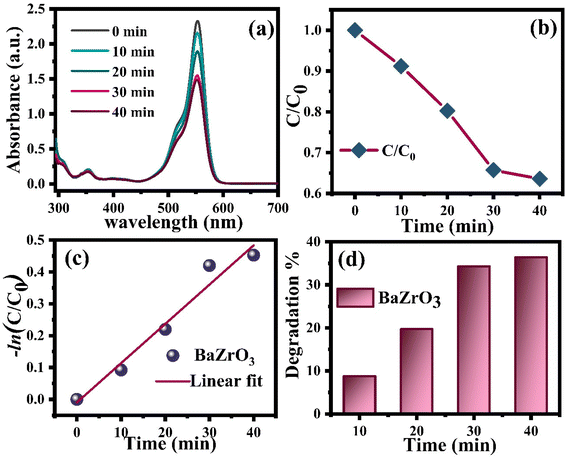 | ||
| Fig. 9 (a) Degradation absorbance spectra of RhB dye using BaZrO3 nanoparticles, and (b) and (c) degradation rate. (d) Efficiency of dye for degradation under visible light. | ||
The BaZrO3 photocatalyst undergoes energy absorption upon illumination with light. Upon reaching or surpassing the energy band-gap of BaZrO3, the transfer of electrons (e−) occurs from the valence band to the conduction band. The transitions lead to the creation of holes (h+) in the valence band, and the resulting electron–hole pairs (e− and h+) generated by the photo-excitation process then migrate towards the surface of BaZrO3. However, the energy transfer induced by the photo-excitation is directed towards the adsorbed species, where the electrons are responsible for reducing the oxygen (O2) molecule to the oxygen-radicals (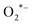 ), which are further converted into hydroxyl-radicals (OH*). Meanwhile, the holes produced by the photo-excitation process oxidize the hydroxyl (H2O) molecule to generate the hydroxyl-radicals (OH*).88 The resulting hydroxyl-radicals (OH*) are effective at decomposing RhB. The value of k is reported to be ∼0.0123 min−1, as shown in Fig. 9(c). The degradation efficiency of BaZrO3 reached ∼36.41% when exposed to UV-Visible light for a duration of ∼40 minutes, as recorded in Fig. 9(d).
), which are further converted into hydroxyl-radicals (OH*). Meanwhile, the holes produced by the photo-excitation process oxidize the hydroxyl (H2O) molecule to generate the hydroxyl-radicals (OH*).88 The resulting hydroxyl-radicals (OH*) are effective at decomposing RhB. The value of k is reported to be ∼0.0123 min−1, as shown in Fig. 9(c). The degradation efficiency of BaZrO3 reached ∼36.41% when exposed to UV-Visible light for a duration of ∼40 minutes, as recorded in Fig. 9(d).
3.5 DFT analysis
From Fig. 10(a), it is seen that the minimum conduction band of BaZrO3 mainly consists of the Zr 4d orbital and a minor hybridization of Ba 4d orbital in the energy region from 4 to 6 eV. The maximum valence band consists of the hybridization of O 2p and Zr 4p orbitals in the energy region from −0.2044 eV to −4.0680 eV. Indeed, the s and p states of the zirconium atom, as well as the s state of the barium atom, exert a partial influence on these valence bands, and their presence can be observed. At energy levels surpassing the Fermi energy, which corresponds to the conduction band, there exist vacant states, including the unoccupied 4d states of the zirconium atom and the 6s and 5d states of the barium atom.91,92
The symmetrical pattern of density of the states indicates the existence of non-magnetism in BaZrO3. As shown in Fig. 10(b), the conduction band of BaZrO3 is mainly composed of Zr 4d states while the valence band is strongly dominated by O 2p.
In order to investigate the contribution of various atomic orbitals (s, p, and d orbitals) of BaZrO3, the projected density of states (PDOS) ranging from −5 eV below the top of the VB to 10 eV above the top of the VB band has been computed and illustrated in Fig. S5.† It represents the main orbital that influences the gap state. In the VB main role is played by O 2p (px, py, and pz) orbitals within a range of −0.3 eV to −4.3 eV due to the admixture of Zr (4d) and Ba (5p, 6s) states just below the Fermi level, which can be seen in Fig. S5(a and b).† This is expected due to the large electronegativity of oxygen (3.44 eV) as compared to Ba (0.89 eV) and Zr (1.33) (Pauling scale). It is also to be noted that the formation of the conduction band maximum (CBM) is dominated by the d-orbital contribution of the Zr atoms except for a small contribution of the p-orbital of O atoms (especially pz orbital). To explore further the orbital contribution to the conduction bands, PDOS for d-orbitals of Zr atoms have also been analyzed and are presented in Fig. S5(b).† The conduction band minima is mainly contributed by the dxz orbital of Zr(4d) atom with minor contribution from dxy and dyz orbitals within the energy range from 2.86 to 8.17 eV.
Significantly, our results are in agreement with previously reported data in the scientific literature.101 The Bader analysis underscores a greater covalent nature in the Zr–O bonds as opposed to the Ba–O bonds. This observation becomes apparent when considering that the effective Bader charge of Ba represents 76.145% of its ionic threshold, while Zr's effective Bader charge falls significantly shorter at 62.38% of its ionic limit. Hence, the Bader analysis highlights that within BaZrO3, the Zr–O bond demonstrates a reduced ionic quality and a heightened covalent aspect when compared to the Ba–O bond.
3.6 Photoluminescence spectroscopy analysis
In the context of BaZrO3 nano-crystallites studied here, structural defects like the presence of Ba, Zr, and oxygen interstitials, as well as vacancies, give rise to a variety of radiative transitions. These transitions involve electrons located in either the conduction band or trapping levels interacting with holes situated in either the valence band or trapping levels.31,35 For a more thorough comprehension of the emission phenomenon, photo-luminescence analysis stands as a frequently utilized and indispensable investigative technique. Fig. 11(a) provides a schematic representation of the photoluminescence excitation.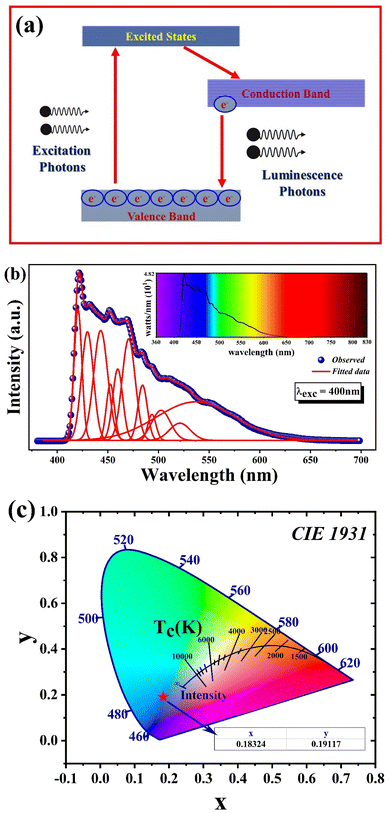 | ||
| Fig. 11 (a) Photoluminescence mechanism. (b) Photo luminescence spectra at the excitation wavelength λexc = 400 nm. (c). CIE diagram of BaZrO3 nanoparticles. | ||
The room temperature photoluminescence spectra of BaZrO3 nanoparticles, synthesized through a straightforward one-step flash combustion method, employing an excitation wavelength of 400 nm is showcased in Fig. 11(b). When subjected to this 400 nm excitation, the spectra manifest a prominent and expansive range of violet-blue emissions. Specifically, the violet emission peaks are located at 420.57 nm and 429.90 nm, while the indigo emission peaks are discernible at 442.98 nm, 452.49 nm and 459.82 nm and the blue emission peaks are discernible at 470.6 nm, 484.32 nm and 493.60 nm. The peaks at 502.63 nm, 521.17 nm and 536.96 nm correspond to the Green region.
The photoluminescence (PL) emission spectra depicted in Fig. 11(b) demonstrate a continuous spectrum of emissions, owing to the involvement of multiple components. To assess each component individually, it is imperative to deconvolute the spectra. Street and Bevingt proposed that these profiles could be separated using a Lorentzian line shape function. In our current study, we employed the Gaussian line shape function to deconvolute the PL spectra, effectively utilizing the Lorentzian line shape to fit the emission peaks. Emission peaks at 420.57 nm (2.94 eV), 429.90 nm (2.88 eV), 442.98 nm (2.79 eV), 452.49 nm (2.74 eV), 459.82 nm (2.69 eV), 470.60 nm (2.63 eV), 484.32 nm (2.56 eV), 493.60 (2.51 eV), 502.63 nm (521.17 eV) and 536.96 nm (2.30 eV) nm were observed. The presence of these emission bands signifies the existence of seven strong and distinct electronic transitions within the visible spectrum, spanning from violet to yellow wavelengths.19,20 Nevertheless, it is worth noting that the most prominent emission peak, which appears at approximately 420.57 nm (equivalent to 2.94 eV), displays the highest intensity among all the observed emissions. This emission peak suggests that BaZrO3 exhibits heightened activity in the violet light region when subjected to visible light irradiation.90,91 The radiative defect peaks are observed at 420.57 and 429.90 nm. Size-dependent excitonic transition is responsible for the emission peak at 470.60 nm.76 The peaks at 502.63, 521.17, and 536.96 nm might have been originated from vacancies or point defects which are either extrinsic or intrinsic that form recombination centers. The presence of recombination sites and defects can also play a role in influencing the process.30,101
The Commission International de I’ Eclairage (CIE) chromaticity coordinates are crucial factors for evaluating the luminescence properties of phosphors.31 The CIE 1931 x–y chromaticity diagram of BaZrO3 nanophosphors were presented in Fig. 11(c). According to PL spectra, the CIE chromaticity coordinates (x, y) of BaZrO3 are (0.18324, 0.19117). The red star in Fig. 11(c) represented the location of the CIE chromaticity coordinate of BaZrO3 under excitation wavelength 400 nm. These coordinates are situated in the violet-blue region, indicating the dominant color of the emitted light, which further verifies the potential application of BaZrO3 nanophosphors in the production of intense violet-bluish LEDs.
4. Conclusion
This study introduces a comprehensive investigation into refining the structural and electro-optical properties of BaZrO3, a visible light-emitting photocatalyst. Using a single-step flash combustion method with glycine as the fuel, we synthesized BaZrO3 nano-ceramics, confirmed in the cubic Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (221) space group with a crystallite size of 70.29 nm. Charge density plots revealed a charge transfer mechanism indicating the more ionic nature of the Ba–O bond compared to that of Zr–O. The surface morphology showed uniform particle dispersion with an average size of 45.31 nm. Negative phonon frequencies suggested potential structural phase transitions, and FTIR supported sample purity. Raman spectroscopy indicated second-order scattering, while XPS provided insights into surface compositions and oxidation states. UV-Vis spectroscopy showed a reduced bandgap energy of 3.08 eV, enhancing photocatalytic potential, with a degradation efficiency of 36.41% for rhodamine B under visible light. Photo-luminescence spectra displayed intense violet emission at 420.57 nm. Combining DFT simulations with extensive experimentation, this study highlights BaZrO3's tailored properties, and promising applications in photocatalysis and violet-bluish LEDs. Further research could amplify BaZrO3's capabilities.
m (221) space group with a crystallite size of 70.29 nm. Charge density plots revealed a charge transfer mechanism indicating the more ionic nature of the Ba–O bond compared to that of Zr–O. The surface morphology showed uniform particle dispersion with an average size of 45.31 nm. Negative phonon frequencies suggested potential structural phase transitions, and FTIR supported sample purity. Raman spectroscopy indicated second-order scattering, while XPS provided insights into surface compositions and oxidation states. UV-Vis spectroscopy showed a reduced bandgap energy of 3.08 eV, enhancing photocatalytic potential, with a degradation efficiency of 36.41% for rhodamine B under visible light. Photo-luminescence spectra displayed intense violet emission at 420.57 nm. Combining DFT simulations with extensive experimentation, this study highlights BaZrO3's tailored properties, and promising applications in photocatalysis and violet-bluish LEDs. Further research could amplify BaZrO3's capabilities.
Our study presents a novel synthesis method for BaZrO3, enabling precise control over particle size and morphology while addressing scalability challenges. Using advanced techniques like UV-spectroscopy and DFT analysis, we demonstrated the ability to fine-tune the bandgap, significantly enhancing BaZrO3's potential applications in electronics and catalysis.
Author contributions
SD contributed to the conceptualization, investigation, writing original draft and data curation, VK contributed to DFT investigation and reviewing and editing, KD and CS contributed to investigation, AM contributed to synthesis, UKG and RKS contributed to XPS measurement and validation, GP contributed to methodology, supervision and reviewing, FZH contributed to the resources and validation, and NKG contributed to the supervision and reviewing of the manuscript.Data availability
The datasets produced and/or scrutinized throughout the present study can be obtained by reaching out to the corresponding author upon a reasonable request.Conflicts of interest
We declare that we have no competing interests.Acknowledgements
The authors extend their gratitude to Barkatullah University, Bhopal, whose generous financial support facilitated this research. Additionally, they acknowledge the University Grants Commission (UGC) and the Department of Science and Technology (DST), New Delhi, for their invaluable assistance through the UGC DSA-III and DST-FIST Phase-II programs. Special thanks go to Dr Vasant Sathe (Raman spectroscopy), Dr Mukul Gupta (XRD), and Dr V. R. Reddy (Ferroelectric measurement) of UGC-CSR Indore for providing measurement facilities. The authors are also grateful to Prof. Bharat Modhera of MANIT, Bhopal, for conducting FTIR measurements and to Dr Che Parvathiraja for assistance with photocatalytic measurements. The authors express their gratitude to SAIF, Manipal University Jaipur, and Mr Vinay Kumar Sharma for his assistance and for providing the FESEM and EDS mapping facilities. VK wishes to acknowledge the computational facility support from the Sackler Center for Computational Molecular and Materials Science in Tel-Aviv University, Israel. CS acknowledges support from INSPIRE (ref. no. DST/INSPIRE/03/2022/003651), DST New-Delhi.References
- N. Baig, I. Kammakakam, W. Falath and I. Kammakakam, Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges, Mater. Adv., Royal Society of Chemistry, 2021, vol. 2, pp. 1821–1871 Search PubMed.
- A. Tarafder, K. Annapurna, R. Saikia Chaliha, V. S. Tiwari, P. K. Gupta and B. Karmakar, Structure, dielectric and optical properties of Nd3+-doped LiTaO3 transparent ferroelectric glass-ceramic nanocomposites, J. Alloys Compd., 2010, 489(1), 281–288 CrossRef CAS.
- I. H. Lone, J. Aslam, N. R. E. Radwan, A. H. Bashal, A. F. A. Ajlouni and A. Akhter, Multiferroic ABO3 Transition Metal Oxides: a Rare Interaction of Ferroelectricity and Magnetism, Nanoscale Res. Lett., Springer, New York LLC, 2019, vol. 14 Search PubMed.
- A. T. Mulder, N. A. Benedek, J. M. Rondinelli and C. J. Fennie, Turning ABO3 antiferroelectrics into ferroelectrics: Design rules for practical rotation-driven ferroelectricity in double perovskites and A3B2O7 Ruddlesden-popper compounds, Adv. Funct. Mater., 2013, 23(38), 4810–4820 CrossRef CAS.
- H. Labrim, Y. Selmani, S. Ziti, S. Idrissi, R. El Bouayadi and D. Zejli, et al., Study of the perovskites CaZrO3-xSx (x=0, 1, 2 and 3) for photovoltaic applications, Solid State Commun., 2023, 363, 115105 CrossRef CAS.
- A. S. Patra, M. S. Chauhan, S. Keene, G. Gogoi, K. A. Reddy and S. Ardo, et al., Combined Experimental and Theoretical Insights into the Synergistic Effect of Cerium Doping and Oxygen Vacancies in BaZrO 3-δ Hollow Nanospheres for Efficient Photocatalytic Hydrogen Production, J. Phys. Chem. C, 2019, 123(1), 233–249 CrossRef CAS.
- Y. Parganiha, J. Kaur, V. Dubey, R. Shrivastava and S. J. Dhoble, Synthesis and luminescence study of BaZrO3:Eu3+ phosphor, Superlattices Microstruct., 2015, 88, 262–270 CrossRef CAS.
- G. Chen, F. Yu, X. Hou, J. Liu, Y. Yang and E. Wang, et al., BaZrO3 refractory crucibles for vacuum induction melting of industrial Zr-based bulk metallic glass master alloys with Y addition, J. Eur. Ceram. Soc., 2022, 42(8), 3644–3651 CrossRef CAS.
- C. Kayathiri, A. R. Balu, M. Suganya, G. Vinitha, Z. Delci and S. Balamurugan, et al. BaZrO3 perovskite – A UV light mediated congo red dye deactivator catalyst with good optical switching and antimicrobial abilities green synthesized using Moringa oleifera leaf extract, Mater. Sci. Eng., B, 2022, 278, 115636 CrossRef CAS.
- H. Niu, Y. Jing, Y. Sun, L. Guo, N. R. Aluru and W. Li, et al., On the anomalous diffusion of proton in Y-doped BaZrO3 perovskite oxide, Solid State Ionics, 2022, 376, 115859 CrossRef CAS.
- H. Uehara, A. Ishii, I. Oikawa and H. Takamura, Preparation and mixed proton-hole conductivity of barium zirconate doped with scandium and cobalt, Int. J. Hydrogen Energy, 2022, 47(8), 5577–5584 CrossRef CAS.
- K. Zhu, N. Shi, L. Zhang, D. Huan, X. Li and X. Zhang, et al., Engineering oxygen vacancy to accelerate proton conduction in Y-doped BaZrO3, Ceram. Int., 2023, 49(9), 13321–13329 CrossRef CAS.
- A. K. Arora, V. S. Jaswal, K. Singh and R. Singh, Applications of metal/mixed metal oxides as photocatalyst: A review, Orient. J. Chem., Oriental Scientific Publishing Company, 2016, vol. 32, pp. 2035–2042 Search PubMed.
- H. Dai, H. Kou, H. Wang and L. Bi, Electrochemical performance of protonic ceramic fuel cells with stable BaZrO3-based electrolyte: A mini-review, Electrochem. Commun., 2018, 96, 11–15 CrossRef CAS.
- S. Akhtar, S. M. Alay-e-Abbas, J. Batool, W. Zulfiqar, A. Laref and G. Abbas, et al., Investigation of structural, electronic and optical properties of (V + P)-doped BaZrO3 for photocatalytic applications using density functional theory, J. Phys. Chem. Solids, 2020, 147, 109662 CrossRef CAS.
- M. L. Moreira, J. Andrés, J. A. Varela and E. Longo, Synthesis of fine micro-sized BaZrO3 powders based on a decaoctahedron shape by the microwave-assisted hydrothermal method, Cryst. Growth Des., 2009, 9(2), 833–839 CrossRef CAS.
- P. Charoonsuk, R. Baitahe, W. Vittayakorn, N. Atiwongsangthong, R. Muanghua and P. Seeharaj, et al. Synthesis of monodispersed perovskite barium zirconate (BaZrO3) by the sonochemical method, Ferroelectrics, 2013, 453(1), 54–61 CrossRef CAS.
- A. Nyabadza, É. McCarthy, M. Makhesana, S. Heidarinassab, A. Plouze and M. Vazquez, et al., A review of physical, chemical and biological synthesis methods of bimetallic nanoparticles and applications in sensing, water treatment, biomedicine, catalysis and hydrogen storage, Adv. Colloid Int. Sci., Elsevier B.V., 2023, vol. 321 Search PubMed.
- B. R. Cuenya, Synthesis and catalytic properties of metal nanoparticles: Size, shape, support, composition, and oxidation state effects, Thin Solid Films, 2010, 518(12), 3127–3150 CrossRef CAS.
- H. M. N. Ullah, M. Rizwan, S. S. Ali, Z. Usman and C. Cao, A DFT study of optical, elastic, mechanical, and overall water-splitting photocatalytic properties of pristine and Cd substituted BaZrO3: A lead free environment friendly material, Mater. Sci. Eng., B, 2022, 286, 116041 CrossRef CAS.
- B. B. Adormaa, W. K. Darkwah and Y. Ao, Oxygen vacancies of the TiO2 nano-based composite photocatalysts in visible light responsive photocatalysis, RSC Adv., Royal Society of Chemistry, 2018, vol. 8, pp. 33551–33563 Search PubMed.
- D. Glass, R. Quesada-Cabrera, S. Bardey, P. Promdet, R. Sapienza and V. Keller, et al., Probing the Role of Atomic Defects in Photocatalytic Systems through Photoinduced Enhanced Raman Scattering, ACS Energy Lett., 2021, 6(12), 4273–4281 CrossRef CAS.
- K. Nakashima, I. Fujii and S. Wada, Preparation of BaZrO 3 cubes by composite-hydroxide-mediated approach at low temperature, J. Ceram. Soc. Jpn., 2011, 119, 532–534 CrossRef CAS.
- J. Thomas, P. K. Anitha, T. Thomas and N. Thomas, BaZrO3 based non enzymatic single component single step ceramic electrochemical sensor for the picomolar detection of dopamine, Ceram. Int., 2022, 48(5), 7168–7182 CrossRef CAS.
- Y. Yuan, Z. Zhao, J. Zheng, M. Yang, L. Qiu and Z. Li, et al., Polymerizable complex synthesis of BaZr1-xSnxO 3 photocatalysts: Role of Sn4+ in the band structure and their photocatalytic water splitting activities, J. Mater. Chem., 2010, 20(32), 6772–6779 RSC.
- G. Kresse and J. Furthmüller, Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set, Comput. Mater. Sci., 1996, 6(1), 15–50 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77(18), 3865–3868 CrossRef CAS PubMed.
- T. Thananatthanachon, Synthesis and Characterization of a Perovskite Barium Zirconate (BaZrO3): An Experiment for an Advanced Inorganic Chemistry Laboratory, J. Chem. Educ., 2016, 93(6), 1120–1123 CrossRef CAS.
- S. Parida, S. K. Rout, L. S. Cavalcante, E. Sinha, M. S. Li and V. Subramanian, et al., Structural refinement, optical and microwave dielectric properties of BaZrO3, Ceram. Int., 2012, 38(3), 2129–2138 CrossRef CAS.
- S. Dubey, K. Dubey, V. Sahu, A. Modi, G. Pagare and F. Z. Haque, et al., Exploring structural, vibrational, optical and photoluminescence characteristic of tetragonal-tungsten bronze Ba4Bi2Fe2Nb8O30 compound, J. Mater. Sci.: Mater. Electron., 2023, 34(36), 2312 CrossRef CAS.
- K. Dubey, S. Dubey, A. Modi, R. K. Sharma, S. Chakravarty and C. Parvathiraja, et al., Purple-blue luminescence and magnetic properties of visible-light active novel BiMn2O5 photocatalyst by ultrasonication assisted sol–gel method, Appl. Phys. A: Mater. Sci. Process., 2023, 129(10), 715 CrossRef CAS.
- S. Dubey, J. A. Abraham, K. Dubey, V. Sahu, A. Modi and G. Pagare, et al., DFT study of RhTiP half Heusler semiconductor: Revealing its mechanical, optoelectronic, and thermoelectric properties, Phys. B, 2024, 672, 415452 CrossRef CAS.
- Y. Parganiha, J. Kaur, V. Dubey, R. Shrivastava and S. J. Dhoble, Synthesis and luminescence study of BaZrO3:Eu3+ phosphor, Superlattices Microstruct., 2015, 88, 262–270 CrossRef CAS.
- A. K. Kunti, N. Patra, R. A. Harris, S. K. Sharma, D. Bhattacharyya and S. N. Jha, et al., Local Structure and Spectroscopic Properties of Eu3+-Doped BaZrO3, Inorg. Chem., 2019, 58(5), 3073–3089 CrossRef CAS PubMed.
- A. Babu, D. Tirumalarao, S. Das, V. Dixit, S. P. Sruthy and V. Vijayan, et al., Effect of pH variation on citrate nitrate sol-gels obtained from auto-combustion method: Synthesis, calculations and characterisations of extremely dense BaZrO3 ceramic, Open Ceram., 2022, 12, 100303 CrossRef CAS.
- L. A. Morales, G. Sierra-Gallego, C. A. Barrero and O. Arnache, Relative recoilless F-factors in REFeO3 (RE = rare-earth La, Pr, Nd and Sm) orthoferrites synthesized by self-combustion method, Mater. Sci. Eng., B, 2016, 211, 94–100 CrossRef CAS.
- C. Zhu, K. Xia, G. R. Qian, C. L. Lu, W. Z. Luo and K. F. Wang, et al. Hydrostatic pressure induced structural instability and dielectric property of cubic BaZrO3, J. Appl. Phys., 2009, 105(4), 044110 CrossRef.
- A. Perrichon, E. J. Granhed, G. Romanelli, A. Piovano, A. Lindman and P. Hyldgaard, et al., Unraveling the ground-state structure of BaZrO3 by neutron scattering experiments and first-principles calculations, Chem. Mater., 2020, 32(7), 2824–2835 CrossRef CAS.
- A. Bilić and J. D. Gale, Ground state structure of BaZrO3: A comparative first-principles study, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79(17), 174107 CrossRef.
- M. A. Helal and S. Kojima, Structural instability and phase transition in BaZrO3 single crystals: Brillouin scattering and DFT study, Mater. Sci. Eng., B, 2021, 271, 115314 CrossRef CAS.
- A. R. Akbarzadeh, I. Kornev, C. Malibert, L. Bellaiche and J. M. Kiat, Combined theoretical and experimental study of the low-temperature properties of BaZrO3, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 205104 CrossRef.
- C. Toulouse, D. Amoroso, C. Xin, P. Veber, M. C. Hatnean and G. Balakrishnan, et al., Lattice dynamics and Raman spectrum of BaZrO3 single crystals, Phys. Rev. B, 2019, 100, 134102 CrossRef CAS.
- J. Zheng, D. Shi, Y. Yang, C. Lin, H. Huang and R. Guo, et al., Anharmonicity-induced phonon hardening and phonon transport enhancement in crystalline perovskite BaZrO3, Phys. Rev. B, 2022, 105, 224303 CrossRef CAS.
- C. Ostos, L. Mestres, M. L. Martínez-Sarrión, J. E. García, A. Albareda and R. Perez, Synthesis and characterization of A-site deficient rare-earth doped BaZrxTi1-xO3 perovskite-type compounds, Solid State Sci., 2009, 11(5), 1016–1022 CrossRef CAS.
- H. P. Kumar, C. Vijayakumar, C. N. George, S. Solomon, R. Jose and J. K. Thomas, et al., Characterization and sintering of BaZrO3 nanoparticles synthesized through a single-step combustion process, J. Alloys Compd., 2008, 458(1–2), 528–531 CrossRef CAS.
- P. P. Khirade, A. B. Shinde, A. Raut, S. D. Birajdar and K. M. Jadhav, Investigations on the synthesis, structural and microstructural characterizations of Ba1-xSrxZrO3 nanoceramics, Ferroelectrics, 2016, 504(1), 216–229 CrossRef CAS.
- E. Sundharam, A. K. S. Jeevaraj and C. Chinnusamy, Effect of ultrasonication on the synthesis of barium oxide nanoparticles, J. Bionanosci., 2017, 11(4), 310–314 CrossRef CAS.
- M. A. Ansari and N. Jahan, Structural and Optical Properties of BaO Nanoparticles Synthesized by Facile Co-precipitation Method, Mater. Highlights, 2021, 2(1–2), 23 CrossRef.
- C. W. Kee, Assignment of O-O and Mo=O Stretching Frequencies of Molybdenum/Tungsten Complexes Revisited, J. Chem., 2015, 439270, 1–10 Search PubMed.
- C. C. Li, S. J. Chang, J. T. Lee and W. S. Liao, Efficient hydroxylation of BaTiO3 nanoparticles by using hydrogen peroxide, Colloids Surf., A, 2010, 361(1–3), 143–149 CrossRef CAS.
- S. Parida, S. K. Rout, P. K. Barhai and J. Bera, Influence of ball milling parameters on the crystallite size of Ba(Ti 1-xZr x)O3, Ferroelectrics, 2012, 429(1), 22–30 CrossRef CAS.
- M. S. Chauhan, R. Kumar, A. Umar, S. Chauhan, G. Kumar and M. Faisal, et al., Utilization of ZnO nanocones for the photocatalytic degradation of acridine orange, J. Nanosci. Nanotechnol., 2011, 11(5), 4061–4066 CrossRef CAS PubMed.
- J. Oliva, E. de La Rosa, L. A. Diaz-Torres, P. Salas and C. Ángeles-Chavez, Annealing effect on the luminescence properties of BaZrO3:Yb3+ microcrystals, J. Appl. Phys., 2008, 104, 023505 CrossRef.
- E. C. Aguiar, A. Z. Simões, C. A. Paskocimas, M. Cilense, E. Longo and J. A. Varela, Photoluminescence of BaZrO3 explained by a order/disorded transformation, J. Mater. Sci.: Mater. Electron., Springer Science and Business Media, LLC, 2015, vol. 26(4), pp. 1993–2001 Search PubMed.
- A. Bisen, A. Satapathy, S. Parida, E. Sinha, S. K. Rout and M. Kar, Structural, optical band gap, microwave dielectric properties and dielectric resonant antenna studies of Ba(1-x)La(2x/3)ZrO3 (0 ≤ x ≤ 0.1) ceramics, J. Alloys Compd., 2014, 615, 1006–1012 CrossRef CAS.
- P. Rosander, E. Fransson, C. Milesi-Brault, C. Toulouse, F. Bourdarot and A. Piovano, et al., Anharmonicity of the antiferrodistortive soft mode in barium zirconate BaZrO3, Phys. Rev. B, 2023, 108, 014309 CrossRef CAS.
- E. Fransson, P. Rosander, P. Erhart and G. Wahnström, Understanding Correlations in BaZrO3: Structure and Dynamics on the Nanoscale, Chem. Mater., 2024, 36, 514–523 CrossRef CAS.
- I. Charrier-Cougoulic, T. Pagnier and G. Lucazeau, Raman Spectroscopy of Perovskite-Type BaCexZr1−xO3(0≤x≤1), J. Solid State Chem., 1999, 142(1), 220–227 CrossRef CAS.
- C. Xin, P. Veber, M. Guennou, C. Toulouse, N. Valle and M. Ciomaga Hatnean, et al., Single crystal growth of BaZrO3 from the melt at 2700 °C using optical floating zone technique and growth prospects from BaB2O4 flux at 1350 °C, CrystEngComm, 2019, 21(3), 502–512 RSC.
- S. Agrawal, Effect of rare earth doping on optical and spectroscopic characteristics of BaZrO3:Eu3+,Tb3+ perovskites, Methods Appl. Fluoresc., 2018, 6(3), 035002 CrossRef PubMed.
- T. L. Sun and X. M. Chen, Raman spectra analysis for Ba[(Mg1- xNix)1/3Nb2/3]O3 microwave dielectric ceramics, AIP Adv., 2015, 5, 017106 CrossRef.
- C. Toulouse, D. Amoroso, R. Oliva, C. Xin, P. Bouvier and P. Fertey, et al., Stability of the tetragonal phase of BaZrO3 under high pressure, Phys. Rev. B, 2022, 106, 134102 CrossRef.
- L. R. MacArio, M. L. Moreira, J. Andrés and E. Longo, An efficient microwave-assisted hydrothermal synthesis of BaZrO3 microcrystals: Growth mechanism and photoluminescence emissions, CrystEngComm, 2010, 12(11), 3612–3619 RSC.
- B. Gulen, P. Demircivi and E. B. Simsek, UV-A light irradiated photocatalytic performance of hydrothermally obtained W doped BaZrO3 catalyst against the degradation of levofloxacin and tetracycline antibiotic, J. Photochem. Photobiol., A, 2021, 404, 112869 CrossRef CAS.
- D. Briggs, Handbook of X-ray Photoelectron Spectroscopy, ed. C. D. Wanger, W. M. Riggs, L. E. Davis, J. F. Moulder and G. E. Muilenberg, Perkin-Elmer Corp., Physical Electronics Division, Eden Prairie, Minnesota, USA, 1979, pp. 190–195, Surf. Interf. Anal., 1981, 3(4), v–v Search PubMed.
- R. Chatterjee, S. Saha, D. Sen, K. Panigrahi, U. K. Ghorai and G. C. Das, et al., Neutralizing the charge imbalance problem in Eu3+-Activated BaAl2O4Nanophosphors: Theoretical insights and experimental validation considering K+ Codoping, ACS Omega, 2018, 3(1), 788–800 CrossRef CAS PubMed.
- M. Miodyńska, B. Bajorowicz, P. Mazierski, W. Lisowski, T. Klimczuk and M. J. Winiarski, et al., Preparation and photocatalytic properties of BaZrO3 and SrZrO3 modified with Cu2O/Bi2O3 quantum dots, Solid State Sci., 2017, 74, 13–23 CrossRef.
- P. J. Schmitz, Characterization of the Surface of BaCO3 Powder by XPS, Surf. Sci. Spectra, 2001, 8(3), 190–194 CrossRef CAS.
- H. Zhang, J. Qiao, G. Li, S. Li, G. Wang and J. Wang, et al., Preparation of Ce4+-doped BaZrO3 by hydrothermal method and application in dual-frequent sonocatalytic degradation of norfloxacin in aqueous solution, Ultrason. Sonochem., 2018, 42, 356–367 CrossRef CAS PubMed.
- J. Meng, X. Fu, K. Du, X. Chen, Q. Lin and X. Wei, et al., BaZrO3 hollow nanostructure with Fe(III) doping for photocatalytic hydrogen evolution under visible light, Int. J. Hydrogen Energy, 2018, 43(19), 9224–9232 CrossRef CAS.
- H. Zhou, Y. Mao and S. S. Wong, Shape control and spectroscopy of crystalline BaZrO3 perovskite particles, J. Mater. Chem., 2007, 17(17), 1707–1713 RSC.
- J. Meng, Z. Lan, Q. Lin, T. Chen, X. Chen and X. Wei, et al., Cubic-like BaZrO3 nanocrystals with exposed {001}/{011} facets and tuned electronic band structure for enhanced photocatalytic hydrogen production, J. Mater. Sci., 2019, 54(3), 1967–1976 CrossRef CAS.
- H. Tan, Z. Zhao, W. b. Zhu, E. N. Coker, B. Li and M. Zheng, et al., Oxygen vacancy enhanced photocatalytic activity of pervoskite SrTiO3, ACS Appl. Mater. Interfaces, 2014, 6(21), 19184–19190 CrossRef CAS PubMed.
- Y. Yuan, Z. Zhao, J. Zheng, M. Yang, L. Qiu and Z. Li, et al., Polymerizable complex synthesis of BaZr1-xSnxO 3 photocatalysts: Role of Sn4+ in the band structure and their photocatalytic water splitting activities, J. Mater. Chem., 2010, 20(32), 6772–6779 RSC.
- I. Zeba, R. Jabeen, R. Ahmad, M. Shakil, M. Rafique and M. Rizwan, et al., Effect of anomalous behavior of Be-doping on structural stability, bandgap and optical properties in comparison with Mg-doped BaZrO3 perovskite: insights from DFT calculations, Opt. Quantum Electron., 2020, 52, 234 CrossRef CAS.
- A. S. Patra, G. Gogoi and M. Qureshi, Ordered-Disordered BaZrO3-δ Hollow Nanosphere/Carbon Dot Hybrid Nanocomposite: A New Visible-Light-Driven Efficient Composite Photocatalyst for Hydrogen Production and Dye Degradation, ACS Omega, 2018, 3(9), 10980–10991 CrossRef CAS PubMed.
- D. Kong, Y. Zheng, M. Kobielusz, Y. Wang, Z. Bai and W. Macyk, et al., Recent advances in visible light-driven water oxidation and reduction in suspension systems, Mater. Today, 2018, 21(8), 97–924 CrossRef.
- K. Dubey, S. Dubey, V. Sahu, A. Modi, J. Bamne, F. Z. Haque and N. K. Gaur, Defects and oxygen vacancies modified properties of transition metal doped Ce0.95X0.05O2 (X = Fe, Co, Ni) nanoparticles, Mater. Sci. Eng., B, 2023, 288, 116154 CrossRef CAS.
- M. B. Sahana, C. Sudakar, A. Dixit, J. S. Thakur, R. Naik and V. M. Naik, Quantum confinement effects and band gap engineering of SnO 2 nanocrystals in a MgO matrix, Acta Mater., 2012, 60(3), 1072–1078 CrossRef CAS.
- S. K. Paswan, S. Kumari, M. Kar, A. Singh, H. Pathak and J. P. Borah, et al., Optimization of structure-property relationships in nickel ferrite nanoparticles annealed at different temperature, J. Phys. Chem. Solids, 2021, 151, 109928 CrossRef CAS.
- K. Telmani, H. Lahmar, M. Benamira, L. Messaadia and M. Trari, Synthesis, optical and photo-electrochemical properties of NiBi2O4 and its photocatalytic activity under solar light irradiation, Optik, 2020, 207, 163762 CrossRef CAS.
- L. L. Chen, B. G. Zhai and Y. M. Huang, Rendering visible-light photocatalytic activity to undoped ZnO via intrinsic defects engineering, Catalysts, 2020, 10(10), 1–16 Search PubMed.
- A. V. Mohod, M. Momotko, N. S. Shah, M. Marchel, M. Imran, L. Kong and G. Boczkaj, Degradation of Rhodamine dyes by Advanced Oxidation Processes (AOPs) - Focus on cavitation and photocatalysis - A critical review, Water Resources and Industry, 2023, 30, 100220 CrossRef CAS.
- D. P. Aarti, R. B. Basavaraj, M. B. Madhusudana Reddy, S. S. Majani, G. R. Navyashree and T. Chandrasekhar, et al., Enhanced photocatalytic, photoluminescence and fingerprint properties of Dy3+ ions doped BaZrO3 nanopowders for multifunctional applications, Inorg. Chem. Commun., 2024, 162, 112255 CrossRef CAS.
- R. Jana, P. M. Rajaitha, S. Hajra and H. J. Kim, Advancements in visible-light-driven double perovskite nanoparticles for photodegradation, Micro and Nano Systems Letters, Soc. Micro Nano Syst., 2023, 11(1), 1–8 CrossRef.
- R. Terki, H. Feraoun, G. Bertrand and H. Aourag, Full potential calculation of structural, elastic and electronic properties of BaZrO3 and SrZrO3, Phys. Status Solidi B, 2005, 242(5), 1054–1062 CrossRef CAS.
- R. Khenata, M. Sahnoun, H. Baltache, M. Rérat, A. H. Rashek, N. Illes and B. Bouhafs, First-principle calculations of structural, electronic and optical properties of BaTiO3 and BaZrO3 under hydrostatic pressure, Solid State Commun., 2005, 136(2), 120–125, DOI:10.1016/j.ssc.2005.04.004.
- R. Khenata, M. Sahnoun, H. Baltache, M. Rérat, A. H. Rashek and N. Illes, et al., First-principle calculations of structural, electronic and optical properties of BaTiO3 and BaZrO3 under hydrostatic pressure, Solid State Commun., 2005, 136(2), 120–125 CrossRef CAS.
- S. M. Alay-E-Abbas, F. Javed, G. Abbas, N. Amin and A. Laref, Density Functional Theory Evaluation of Ceramics Suitable for Hybrid Advanced Oxidation Processes: A Case Study for Ce4+-Doped BaZrO3, J. Phys. Chem. C, 2019, 123(10), 6044–6053 CrossRef CAS.
- S. Muhammad Alay-E-Abbas, S. Nazir and A. Shaukat, Formation energies and electronic structure of intrinsic vacancy defects and oxygen vacancy clustering in BaZrO3, Phys. Chem. Chem. Phys., 2016, 18(34), 23737–23745 RSC.
- V. Mishra, A. Sagdeo, V. Kumar, M. K. Warshi, H. M. Rai and S. K. Saxena, et al., Electronic and optical properties of BaTiO3 across tetragonal to cubic phase transition: An experimental and theoretical investigation, J. Appl. Phys., 2017, 122, 065105 CrossRef.
- N. Kitamura, J. Akola, S. Kohara, K. Fujimoto and Y. Idemoto, Proton distribution and dynamics in Y- and Zn-doped BaZrO3, J. Phys. Chem. C, 2014, 118(33), 18846–18852 CrossRef CAS.
- V. Kumar and J. Jung, Enhancement of gas sensing by doping of transition metal in two-dimensional As2C3 nanosheet: A density functional theory investigation, Appl. Surf. Sci., 2022, 599, 153941 CrossRef CAS.
- M. Rizwan, S. Aleena, M. Shakil, T. Mahmood, A. A. Zafar and T. Hussain, et al., A computational insight of electronic and optical properties of Cd-doped BaZrO3, Chin. J. Phys., 2020, 66, 318–326 CrossRef CAS.
- N. Erum, M. A. Iqbal and I. Bashir, A DFT study of structural, electronic and optical properties of pristine and intrinsic vacancy defects containing barium zarconate (BaZrO3) using mBJ potential, Phys. Scr., 2020, 96, 025807 CrossRef.
- G. Lopez-Candales, Z. Tang, G. J. Cruz, W. Xia, F. Jia and P. Zhang, Quasiparticle band structures of the 4d perovskite oxides SrZr O3 and BaZr O3, Phys. Rev. B, 2021, 104, 195129 CrossRef CAS.
- W. Zulfiqar, S. M. Alay-E-Abbas, G. Abbas, A. Laref, J. A. Larsson and A. Shaukat, Revisiting the structural, electronic and photocatalytic properties of Ti and Zr based perovskites with meta-GGA functionals of DFT, J. Mater. Chem. C, 2021, 9(14), 4862–4876 RSC.
- B. Favelukis, S. Chakrabartty, V. Kumar, S. H. Kim, A. El-Zoka and M. Krämer, et al., Improved Durability of Ti3C2Tz at Potentials above the Reversible Hydrogen Electrode by Tantalum Substitution, Adv. Funct. Mater., 2023, 34, 2309749 CrossRef.
- N. Raja, D. Murali, S. V. M. Satyanarayana and M. Posselt, Y doping of BaZrO3 may lead to optimum conditions for proton conduction at operating temperature of solid oxide fuel cells: a first principles study, Mater. Res. Express, 2023, 10, 065504 CrossRef.
- Y. Parganiha, J. Kaur, V. Dubey, R. Shrivastava and S. J. Dhoble, Synthesis and luminescence study of BaZrO3:Eu3+ phosphor, Superlattices Microstruct., 2015, 88, 262–270 CrossRef CAS.
- P. P. Khirade, S. D. Birajdar, A. B. Shinde and K. M. Jadhav, Room temperature ferromagnetism and photoluminescence of multifunctional Fe doped BaZrO3nanoceramics, J. Alloys Compd., 2017, 691, 287–298 CrossRef CAS.
- C. Yi, Q. Lu, Y. Wang, Y. Wang and B. Yang, Degradation of organic wastewater by hydrodynamic cavitation combined with acoustic cavitation, Ultrason. Sonochem., 2018, 43, 156–165 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nr00517a VK Current Address- Surface Science Laboratory, Department of Materials and Earth Sciences, Technical University of Darmstadt, Otto-Berndt-Straße 3, 64287 Darmstadt, Germany. E-mail: vkumar@surface.tu-darmstadt.de |
| This journal is © The Royal Society of Chemistry 2024 |