Rare-earth chalcogenidotetrachloride clusters (RE3ECl4, RE = Dy, Gd, Y; E = S, Se, Te): syntheses and materials properties†
Lei
Li
ab,
Tian-Jiao
Xue
ab,
You-Song
Ding
 *ab and
Zhiping
Zheng
*ab and
Zhiping
Zheng
 *ab
*ab
aDepartment of Chemistry, Southern University of Science and Technology, Shenzhen, Guangdong 518055, China. E-mail: dingys@sustech.edu.cn; zhengzp@sustech.edu.cn
bKey University Laboratory of Rare Earth Chemistry of Guangdong, Southern University of Science and Technology, Shenzhen, Guangdong 518055, China
First published on 6th September 2024
Abstract
Rare-earth-containing materials featuring rare-earth–chalcogen (RE–E) coordination are of interest as optical and magnetic materials with enhanced properties over their counterparts with O-coordination. Herein, a series of rare-earth chalcogenidotetrachloride complexes of the general formula [η5-Cp*RE(μ-Cl)(THF)]3(μ3-Cl)(μ3-E) (RE3ECl4: RE = Dy, Gd, Y; E = S, Se, Te; Cp* = pentamethylcyclopentadienide) were reported. Apart from for the identity of the rare-earth and chalcogen elements, these clusters share a common core motif composed of triangularly arranged RE atoms with one chloro ligand bridging each neighboring pair of RE atoms; the trimetallic plane is additionally capped by triply bridging chloro and chalcogenide ligands, one for each kind. Each RE atom is further coordinated with one Cp* and one THF ligand. Comparative magnetic and luminescence studies revealed fine but definitive differences due to using different metal and chalcogen elements. Experimental and computational studies have been used to gain insights into the dipolar and super-exchange interactions for the magnetically active Dy- and Gd-based clusters.
Introduction
Rare earth (RE) chalcogenides represent a class of advanced functional materials known for their unique optical, electronic, magnetic, and catalytic properties.1–4 For instance, EuS and Ln2S3 (Ln = Gd, Ce) are exemplary magnetic semiconductors and photocatalytic materials, respectively.5–12 EuSe and LaSe2 are notable magneto-optical and transparent conductive materials;13–18 EuTe2, Pr2.74Te4, and RETe3 (RE = Y, La–Dy) are exemplary quantum materials utilized in magnetic semiconductors, magnetic superconductivity, thermoelectricity, and charge density waves, respectively.19–26In this context, atomically precise rare-earth chalcogenide complexes, with their definitive molecular compositions and structures, are propitious for establishing the structure–property relationship. More importantly, they may serve as precursors for the rational design of solid-state rare-earth chalcogenide materials.27–33 Thus, the diversity in composition, structure, interesting materials properties, and ultimately the potential applications of rare-earth chalcogenides provide a strong impetus for the research of rare-earth chalcogenide complexes.
However, this branch of rare-earth coordination chemistry has lagged far behind the chemistry of O-based ligands. As an interesting and revealing note, a search into the database of current Cambridge Crystallographic Data Centre produced less than 1500 hits of RE–E (E = sulfur (S), selenium (Se), and tellurium (Te)) complexes versus more than 53,283 hits for complexes featuring RE–O bonds.34 On one hand, the mismatch between the hard Lewis acidic RE ions and the soft chalcogenide ligands is primarily responsible for the challenges in the preparation of this particular family of rare-earth complexes. On the other hand, the strong oxophilicity of RE metal ions presents a real technical challenge as any rare-earth chalcogenide complexes are naturally air- and moisture-sensitive. Yet another challenge in making rare-earth chalcogenides is forming the chalcogenide ligands; alkali-metal chalcogenide salts are insoluble in common organic solvents and thus, synthetically inapplicable. The most widely adopted approach to obtaining RE–E complexes is by the reduction of elemental sulfur, selenium, or tellurium with divalent RE complexes.35–43 However, most divalent RE complexes, with the exception of those of Sm(II), Eu(II), and Yb(II), are highly unstable and hard to be isolated. As such, in general, this method is hard to apply.
We have recently developed a generally applicable method for the production of rare-earth telluride cluster complexes.44 The multifarious tellurido ligands present in the cluster core are produced in situ by the reduction of element Te with KC8. More recently, we showed that together with NaBH4, rare-earth pentamethylcyclopentadienyl borohydrides (Cp*RE(BH4)2(THF)2) – produced in situ by the reaction between Cp*RECl2 and NaBH4 – is capable of reducing Te powder, and the resulting Te2− ligands organize five Dy(III) ions into a trigonal bipyramidal core of Dy5Te6 in the pentanuclear cluster anion of [(η5-Cp*Dy)5(μ3-Te)6]2− (Cp* = pentamethylcyclopentadienyl).45 In this work, the reduction of elemental S, Se, and Te with NaBH4 has been extended for the formation of a series of rare-earth chalcogenidotetrachlorides of the general formula [η5-Cp*RE(μ-Cl)(THF)]3(μ3-Cl)(μ3-E) (RE3ECl4: RE = Dy, Gd, Y; E = S, Se, Te) (Scheme 1). All nine cluster complexes were fully characterized including structure determination by crystallographic studies. Comparative studies of these isostructural clusters reveal fine yet definitive differences in their photoluminescence and magnetic properties; such disparities can be correlated with the systematically altered structures arising from the different RE and/or E elements used.
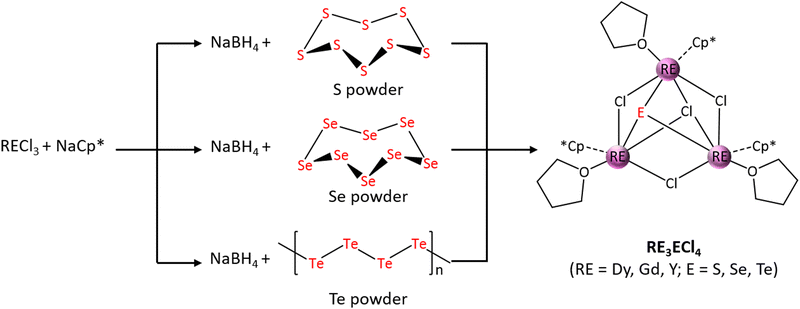 | ||
| Scheme 1 Syntheses of [η5-Cp*RE(μ-Cl)(THF)]3(μ3-Cl)(μ3-E) (RE3ECl4; Cp* = pentamethylcyclopentadienyl; RE = Dy, Gd, Y; E = S, Se, Te). | ||
Experimental
Materials and physical measurements
All manipulations (unless otherwise stated) were carried out under an atmosphere of argon using standard Schlenk techniques on a dual vacuum/inlet manifold or in a glovebox due to the air and moisture sensitivity of the samples. Glassware was dried overnight at 120 °C before use. Anhydrous RECl3 (RE = Dy, Gd, Y)46 and NaCp*![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 were synthesized by following previously published procedures. NaBH4 was purchased from Sigma-Aldrich, while all other reagents, including solvents, were obtained from Energy-Chemical and used as received. Elemental analyses (CHN) were performed on a Carlo Erba EA1110 automatic analyzer. 1H NMR spectra were obtained in CDCl3 solutions at room temperature using an AVANCE III HD 400 MHz spectrometer.
47 were synthesized by following previously published procedures. NaBH4 was purchased from Sigma-Aldrich, while all other reagents, including solvents, were obtained from Energy-Chemical and used as received. Elemental analyses (CHN) were performed on a Carlo Erba EA1110 automatic analyzer. 1H NMR spectra were obtained in CDCl3 solutions at room temperature using an AVANCE III HD 400 MHz spectrometer.
Dy3SCl4 . In an argon-filled glovebox, solution A containing DyCl3 (403.3 mg, 1.500 mmol) and NaCp* (237.3 mg, 1.500 mmol) in 2.5 mL of THF was mixed with solution B obtained by dissolving sulfur (16.0 mg, 0.50 mmol) and NaBH4 (37.8 mg, 1.00 mmol) in 2.5 mL of THF. The resulting mixture was stirred at room temperature for 24 h and then centrifuged (5000 rpm, 5 min) to obtain a bright yellow filtrate. Bright yellow single crystals of Dy3SCl4 were obtained upon standing of the concentrated filtrate (ca. 3 mL) at room temperature (80 mg, 12% based on DyCl3). Anal. Calc. for C42H69Cl4Dy3O3S: C, 39.31; H, 5.42. Found: C, 39.19; H, 5.56.
Dy3SeCl4 . The synthesis followed a similar procedure to that of Dy3SCl4 with the use of Se powder (39.5 mg, 0.50 mmol) in place of S. The desired product was obtained as a polycrystalline solid (75 mg, 11% based on DyCl3). Anal. Calc. for C42H69Cl4Dy3O3Se: C, 37.92; H, 5.23. Found: C, 37.48; H, 5.63.
Dy3TeCl4 . The synthesis followed a similar procedure to that of Dy3SCl4 using Te powder (63.8 mg, 0.50 mmol) instead of S. However, the Te powder did not dissolve like S and Se powder in preparing the S- and Se-containing congeners. The reaction mixture was stirred at 80 °C for 24 hours, during which the color of the mixture changed gradually from silver-gray to black-red. Centrifugation of the cooled reaction mixture followed by the concentration and standing of the filtrate afforded orange single crystals of Dy3TeCl4 (65 mg, 9.0% based on DyCl3). Anal. Calc. for C42H69Cl4Dy3O3Te: C, 36.58; H, 5.04. Found: C, 36.61; H, 5.14.
Gd3SCl4 . The synthesis followed a similar procedure to that of Dy3SCl4, using GdCl3 (395.4 mg, 1.500 mmol) instead of DyCl3. The desired product was obtained as a colorless polycrystalline solid (78 mg, 11% based on GdCl3). Anal. Calc. for C42H69Cl4Gd3O3S: C, 39.80; H, 5.49. Found: C, 39.45; H, 5.80.
Gd3SeCl4 . The synthesis followed a similar procedure to that of Dy3SeCl4, using GdCl3 (395.4 mg, 1.500 mmol) instead of DyCl3. The desired product was obtained as a colorless polycrystalline solid (70 mg, 10% based on GdCl3). Anal. Calc. for C42H69Cl4Gd3O3Se: C, 38.38; H, 5.29. Found: C, 38.18; H, 5.38.
Gd3TeCl4 . The synthesis followed a similar procedure to that of Dy3TeCl4, using GdCl3 (395.4 mg, 1.500 mmol) instead of DyCl3. The desired product was obtained as an orange polycrystalline solid (68 mg, 10% based on GdCl3). Anal. Calc. for C42H69Cl4Gd3O3Te: C, 37.01; H, 5.10. Found: C, 36.98; H, 5.10.
Y3SCl4 . The synthesis followed a similar procedure to that of Dy3SCl4, using YCl3 (292.9 mg, 1.500 mmol) instead of DyCl3. The desired product was obtained as a colorless polycrystalline solid (76 mg, 14% based on YCl3). Anal. Calc. for C42H69Cl4Y3O3S: C, 47.47; H, 6.55. Found: C, 47.15; H, 6.88. 1H NMR: δ 2.05 (s, 45H, C5Me5), δ 1.87 (m, 12H, THF), δ 3.87 (m, 12H, THF).
Y3SeCl4 . The synthesis followed a similar procedure to that of Dy3SeCl4, using YCl3 (292.9 mg, 1.500 mmol) instead of DyCl3. The desired product was obtained as a colorless polycrystalline solid (70 mg, 12% based on YCl3). Anal. Calc. for C42H69Cl4Y3O3Se: C, 45.47; H, 6.27. Found: C, 45.11; H, 6.67. 1H NMR: δ 2.05 (s, 45H, C5Me5), δ 1.87 (m, 12H, THF), δ 3.87 (m, 12H, THF).
Y3TeCl4 . The synthesis followed a similar procedure to that of Dy3TeCl4, using YCl3 (292.9 mg, 1.500 mmol) instead of DyCl3. The desired product was obtained as an orange polycrystalline solid (60 mg, 10% based on YCl3). Anal. Calc. for C42H69Cl4Y3O3Te: C, 43.56; H, 6.01. Found: C, 43.10; H, 6.49. 1H NMR: δ 2.06 (s, 45H, C5Me5), δ 1.88 (m, 12H, THF), δ 3.86 (m, 12H, THF).
Structural characterization
Single-crystal X-ray intensity data were collected on a Bruker D8 VENTURE diffractometer with Mo-Kα radiation (λ = 0.71073 Å) at 100/200 K. Using Olex2,48,49 the structures were solved with the SHELXT structure solution program using Intrinsic Phasing and refined with the SHELXL refinement package using least squares minimization.50 All hydrogen atoms were placed in calculated, ideal positions and refined as riding on their respective carbon atoms, with displacement parameters also dependent on the parent carbon atom Ueq value. Cambridge Crystallographic Data Centre contains the crystal structure with the following CCDC numbers: 2344964 (Dy3SCl4), 2344965 (Dy3SeCl4), 2344954 (Dy3TeCl4), 2344974 (Gd3SCl4), 2344970 (Gd3SeCl4), 2344975 (Gd3TeCl4), 2344958 (Y3SCl4), 2344969 (Y3SeCl4), and 2344973 (Y3TeCl4).†Optical measurements
The absorption spectra of the title compounds were recorded using a computer-controlled UV-vis-NIR 3600i Plus spectrometer in the wavelength range of 300–2000 nm. Emission spectra were acquired at room temperature by using a steady-state spectrometer (FLS-1000, Edinburgh) with a xenon lamp. Time-resolved photoluminescence decay curves were obtained on the same spectrometer but with a 405 nm band of a laser diode.Magnetic measurements
Magnetic susceptibility measurements were obtained using a Quantum Design MPMS-3 SQUID magnetometer between 2 and 300 K. A crushed polycrystalline sample was sealed with melted eicosane in an NMR tube under vacuum.Ab initio calculations
OpenMolcas51 was used to perform the CASSCF-SO calculation of the electronic structures of Dy3ECl4 (E = S, Se, Te) with the molecular geometries taken from the crystallographic analyses; no optimization was made except for taking the largest disorder component. Relativistic effects have been accounted for by using the 2nd order Douglas–Kroll Hess Hamiltonian, and the basis sets from ANO-RCC library52,53 were accordingly employed, with VTZP quality for Dy atoms, VDZP quality for the coordinating C, S, Se, and Te atoms, and VDZ quality for the remaining atoms. Cholesky decomposition of the two-electron integrals with a threshold of 10−8 was performed to save disk space and to reduce computational demand. The state-averaged CASSCF orbitals of the sextets, quartets, and doublets were optimized with 21, 224, and 490 states, respectively, with the RASSCF module. 21, 128, and 130 sextets, quartets, and doublets were chosen to construct and diagonalize in spin–orbit (SO) coupling Hamiltonian with the RASSI module. The resulting spin–orbit wavefunctions were decomposed into their CF wavefunctions, and the magnetic susceptibility was calculated using SINGLE_ANISO.54 The program POLY_ANISO was used to calculate the dipolar interaction and fit the super-exchange coupling in Dy3ECl4 (E = S, Se, Te).55,56Results and discussion
Syntheses and structural characterization
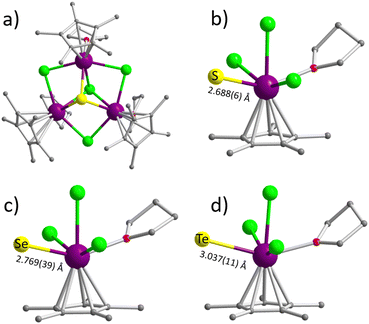
Compounds RE3ECl4 (RE = Dy, Gd, Y; E = S, Se) were prepared using similar procedures. The pre-formed Cp*LnCl2 complex was reacted with the formally doubly negatively charged E2− (E = S or Se) ligand generated by the reduction of elemental E (E = S or Se) with NaBH4; the formation of a bright yellow (S) or an orange-red (Se) solution, accompanied by gas bubbling when NaBH4 and respectively elemental S and Se were dissolved completely in THF, is a good indication of the formation of E2−. However, stirring Te powder and NaBH4 in THF, even with heating, only yielded a suspension instead of a clear solution as in the case of S or Se. As such, the syntheses of the Te-containing clusters RE3TeCl4 (RE = Dy, Gd, Y) were achieved in a one-pot reaction using solution A and the suspension of Te/NaBH4 in THF. It is believed that Cp*RE(BH4)2(THF)2 was produced in situ by the reaction between the pre-formed Cp*LnCl2 complex in solution A with NaBH4; this species is presumably soluble in the reaction mixture, enabling the reduction of Te to afford Te2− for subsequent RE coordination.45 All nine clusters are soluble in THF and CHCl3. Single crystals suitable for X-ray diffraction studies (vide infra) were obtained by slow evaporation of the corresponding saturated THF solution at room temperature in a glovebox.The title clusters are isostructural and crystallize in the monoclinic space group P21/n with comparable unit cells (Tables S1–S3, ESI†). The core motif features triangularly disposed RE atoms with one chloro ligand bridging two neighboring RE atoms, resulting in a crown-like structure (Fig. S1, ESI†). The trimetallic plane is capped on one side by a μ3-Cl− and a μ3-E2− on the other side. Each of the RE atoms is terminally coordinated by one Cp* and one THF. The metal center is formally deca-coordinate but can be viewed as situated in a distorted octahedron if the η5-Cp* is treated as a single-site ligand. It is interesting to note that all the η5-Cp* ligands are on the same side of the triply bridging E2− ligand, while the THF molecules are coordinated on the other side along with the μ3-Cl− ligand. The μ3-E2− are essentially equidistant to the three RE atoms, with an average Dy–E length of 2.688(6) Å, 2.769(39) Å, and 3.037(11) Å for the S-, Se-, and Te-containing clusters, respectively (Fig. 1); the Dy–E distances fall in the range reported for compounds containing the same Dy–E bonds,57–60 and the differences correspond to the different ionic radius of the E2− ions (S2−, 1.84 Å; Se2−, 1.98 Å; Te2−, 2.21 Å).61 It is also of note that the different chalcogenide ligands have a small yet definitive impact on the cluster core structures. Specifically, the average Dy⋯Dy separations increase from 3.8033(11) Å in Dy3SCl4 to 3.8450(9) Å in Dy3SeCl4, and to 3.9287(9) Å in Dy3TeCl4 (Table 1 and Fig. S2a, ESI†). Furthermore, the E2− ions shift progressively away from the Dy3 plane with the use of heavier chalcogenide anions (Fig. S2b, ESI†), with the distance between the E2− ion and the center of the Dy3 plane increasing from 1.552 Å (Dy3SCl4) to 1.722 Å (Dy3SeCl4), and to 2.019 Å (Dy3TeCl4) (Table 1). It is interesting to note that the μ-Cl–Dy bond lengths range from 2.739(3) to 2.775 (5) Å and the μ3-Cl–Dy bond lengths are between 2.821(4) and 2.874(3) Å (Tables S6–S8, ESI†); these metric values are in the range reported for Dy(III) complexes with bridging chloro ligands.62–64 With the gradual size expansion of the trimetallic plane from the S- to Te-containing cluster, the μ3-Cl− ions are “pressed” to be closer to the plane, with the distance between the μ3-Cl− ion and the center of the triangular plane being reduced from 1.838 Å (Dy3SCl4) to 1.795 Å (Dy3SeCl4), and then to 1.704 Å (Dy3TeCl4).
| Bond distance (Å) | Dy3SCl4 | Dy3SeCl4 | Dy3TeCl4 |
|---|---|---|---|
| Dy1–S/Se/Te | 2.682(3) | 2.731(19) | 3.0479(15) |
| Dy2–S/Se/Te | 2.691(3) | 2.808(2) | 3.0346(15) |
| Dy3–S/Se/Te | 2.692(3) | 2.767(9) | 3.0271(14) |
| Average | 2.688(6) | 2.769(39) | 3.037(11) |
| Dy1–Dy2 | 3.7937(8) | 3.8440(14) | 3.9332(12) |
| Dy2–Dy3 | 3.8147(9) | 3.8550(13) | 3.9174(12) |
| Dy3–Dy1 | 3.8006(9) | 3.8360(13) | 3.9354(12) |
| Average | 3.8030(11) | 3.8450(9) | 3.9287(9) |
| E2−-Dy3 plane | 1.552 | 1.722 | 2.019 |
| μ3-Cl−-Dy3 plane | 1.838 | 1.795 | 1.704 |
| Cl3 plane -Dy3 plane | 0.368 | 0.363 | 0.353 |
The nine isostructural clusters with different metal ions and/or E2− ligands form a valuable set of molecules for meaningful comparative studies aiming at the understanding of how the identity of the chalcogen elements and the structural variations due to their size difference may lead to the different photophysical and magnetic properties observed for the clusters of a particular RE element in this series of novel clusters.65–67
Photophysical properties
The solid-state UV-vis-NIR adsorption spectra of RE3ECl4 (RE = Dy, Gd, Y, E = S, Se, Te) were recorded at room temperature from 300 nm to 2000 nm (Fig. S3 and S4, ESI†). The three clusters exhibit essentially the same profile, with six distinct absorption peaks in the visible and near-infrared regions, characteristic of Dy(III), in addition to the absorption in the ultraviolet region (Fig. S3, ESI†).68 Room-temperature solid-state photoluminescence studies were also conducted. As shown in Fig. 2, the excitation spectra indicate that effective energy adsorption occurs in the ultraviolet region and extends slightly into the visible window at 310–420 nm. The emission spectra show two intense emission bands under excitation of 396 nm. The emission peaks at 487, 577, and 587 nm are attributed to transitions from 4F9/2 to 6H15/2 (487 nm) and 6H13/2 (577–587 nm) states of Dy(III), respectively.69,70 The luminescence lifetimes (λem = 577 nm) were obtained by fitting the decay curves with the single exponential mode (Fig. S5, ESI†), producing 69.19 ns, 77.03 ns, and 70.25 ns for the S-. Se-, and Te-containing clusters, respectively.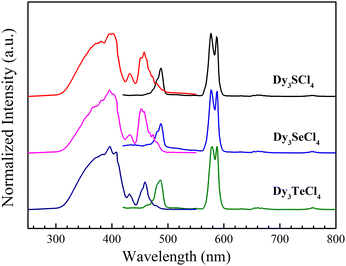 | ||
| Fig. 2 Room-temperature excitation (λex = 396 nm) and emission (λem = 577 nm) spectra of Dy3ECl4 (E = S, Se, Te). | ||
Magnetic properties
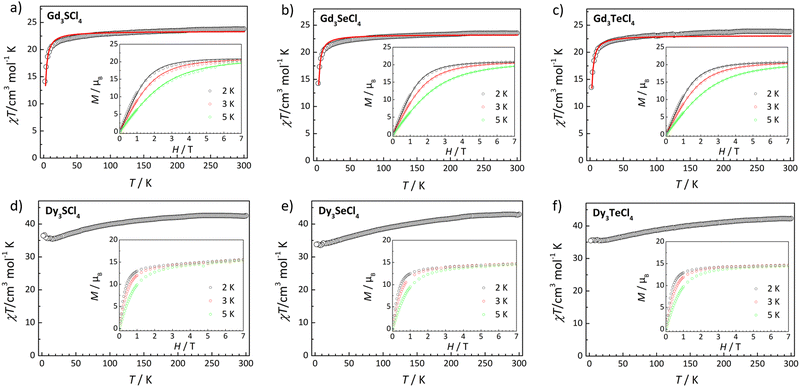
Using polycrystalline samples wrapped in eicosane, the static-field magnetic susceptibilities of RE3ECl4 (RE = Dy, Gd; E = S, Se, Te) were measured under an applied DC field of 1000 Oe and in the 2–300 K temperature range. At 300 K, the χT values for Gd3SCl4, Gd3SeCl4, and Gd3TeCl4 were 23.80, 23.55, and 23.83 cm3 K mol−1, respectively; these values are in good agreement with the expected value of 23.61 cm3 K mol−1 (S = 7/2, g = 2.0) for three uncorrelated Gd(III) ions (Fig. 3).71–73 As the temperature was lowered, the χT values decreased gradually to ca. 20 K, below which an abrupt decrease occurred, reaching 14.14, 14.28, and 13.55 cm3 K mol−1 at 2 K for Gd3SCl4, Gd3SeCl4, and Gd3TeCl4, respectively. The magnetic susceptibilities at low temperatures increased slowly upon the increase of the magnetic field, producing magnetic moments of 20.41, 20.79, and 20.68μB at 2 K and 7 T for the S-, Se-, and Te-containing clusters, respectively; these values are in good agreement with the saturation value of 21.00μB calculated for three Gd(III) ions.73 Both the temperature- and field-dependent magnetic susceptibilities were fitted using the PHI program74 with the equilateral Hamiltonian (H = −2J(SGd1 × SGd2+ SGd2 × SGd3+ SGd1 × SGd3)) in order to evaluate any intramolecular magnetic interactions in these clusters. For all three clusters, the obtained g values (Table 2) are all close to the value of 2 for isotropic ions, and the super-exchange constants (J) are all around −0.04 cm−1. The weak antiferromagnetic interactions are not too surprising as superexchange interactions between 4f ions mediated by a chloro bridging ligand are generally weak as previously observed.75,76| Cluster | g | J/cm−1 |
|---|---|---|
| Gd3SCl4 | 1.9857(9) | −0.0403(4) |
| Gd3SeCl4 | 1.9799(5) | −0.0357(1) |
| Gd3TeCl4 | 1.9731(8) | −0.0391(1) |
For Dy3ECl4 (E = S, Se, Te), the χT values at 300 K were found to be 42.51, 42.91, and 42.27 cm3 K mol−1 for the S-, Se-, and Te-containing clusters, respectively; these values are in close agreement with the value of 42.51 cm3 K mol−1 (6H15/2, S = 5/2, g = 4/3, L = 5) expected for three uncorrelated Dy(III) ions (Fig. 3).77–79 The field-dependent magnetization at 2, 3, and 5 K showed a rapid increase under a low magnetic field, followed by a more gradual increase, producing maximum values at 2 K and 7 T for the three clusters at 15.57, 14.72, and 14.63μB, respectively. These values are significantly smaller than the expected saturation value of 30μB, indicating the presence of a sizable magnetic anisotropy and/or low-lying excited states.80–83 In contrast to the temperature-dependent changes of magnetic susceptibilities of Gd3ECl4 (E = S, Se, Te), the χT values of Dy3SeCl4 and Dy3TeCl4 decreased only very slowly upon lowering of temperature, to 33.81 and 35.45 cm3 K mol−1 at 2 K, respectively. Interestingly for Dy3SCl4, the χT values decreased first to 35.42 cm3 K mol−1 at 16 K, but then increased to 36.45 cm3 K mol−1 with further lowering of temperature to 2 K. These observations indicate the presence of ferromagnetic coupling between the Dy(III) ions, which can attribute to dipolar interactions.84,85
Ab initio calculations
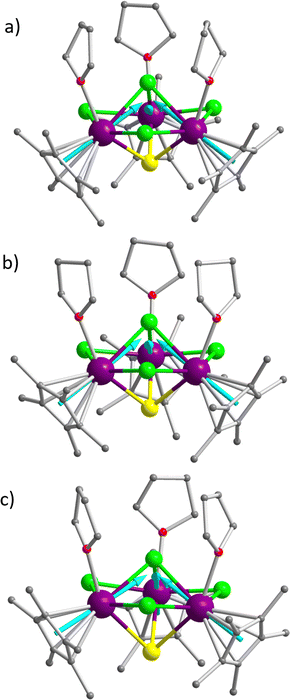
The dipolar interactions in Dy3ECl4 (E = S, Se, Te) were studied by using complete active space self-consistent field spin–orbit (CASSCF-SO) calculations performed using OpenMolcas.51 It has been found that the ground states of the Dy(III) ions are dominated by the most magnetic component of mJ = ±15/2 (>85%), whereas the excited states are mixed with a combination of different magnetic components (Tables S15–S23, ESI†). Such electronic structures are believed to be responsible for the absence of slow magnetic relaxation above 2 K in these clusters. The principal magnetic axes of the ground Kramers’ doublets obtained are shown in Fig. 4; these magnetic axes are different in the orientation of their magnetic axes (either out-of-plane or in-plane type) when compared with the reported Dy3 triangles that display characteristic spin frustration or single-molecule toroidal behaviors.86–89 As indicated by the angles between the axes and the trimetallic plane are comparable (Table 3), the individual axes in the newly formed cluster complexes are essentially in line with the connection between the centroid – of a Cp* ring and the specific Dy atom it coordinates. In comparison, the magnetic axes of the literature precedents are in a toroidal arrangement.86–89| Angles between the principal magnetic axes and the Dy3 planes/deg | J dip/cm−1 | |||
|---|---|---|---|---|
| Dy3SCl4 | Dy1 | 37.38 | Dy1–Dy2 | 1.1281 |
| Dy2 | 20.20 | Dy1–Dy3 | 1.1840 | |
| Dy3 | 27.44 | Dy2–Dy3 | 1.1901 | |
| Average | 28.34 | 1.1674 | ||
| Dy3SeCl4 | Dy1 | 25.51 | Dy1–Dy2 | 1.1603 |
| Dy2 | 30.46 | Dy1–Dy3 | 1.1853 | |
| Dy3 | 31.15 | Dy2–Dy3 | 1.1233 | |
| Average | 29.04 | 1.1563 | ||
| Dy3TeCl4 | Dy1 | 28.83 | Dy1–Dy2 | 1.1345 |
| Dy2 | 29.74 | Dy1–Dy3 | 1.0866 | |
| Dy3 | 30.94 | Dy2–Dy3 | 1.0690 | |
| Average | 29.84 | 1.0967 | ||
The dipolar interaction parameters were calculated by using the program POLY_ANISO.55,56 As expected, all dipolar interactions are ferromagnetic in nature, with those in Dy3SCl4 being the strongest among the three congener clusters, as revealed by its largest average value (1.1674 cm−1) of the coupling parameters (Table 3); the corresponding values are 1.1563 cm−1 and 1.0967 cm−1 for Dy3SeCl4 and Dy3TeCl4, respectively. This difference in dipolar interactions, albeit small, is consistent with the difference in the Dy⋯Dy distances (Table 1) as the dipolar interactions vary inversely with the cube of the separation between the Dy ions.90,91 The stronger ferromagnetic coupling in Dy3SCl4 led to the above-mentioned increase of the temperature-dependent magnetic susceptibilities at low temperatures, while a similar observation was not made above 2 K in the case of Dy3SeCl4 or Dy3TeCl4 due to the presence of antiferromagnetic super-exchange and intermolecular interactions.92,93
Conclusions
A series of novel rare-earth chalcogenidotetrachloride clusters of the general formula [η5-Cp*RE(μ-Cl)(THF)]3(μ3-Cl)(μ3-E) (RE3ECl4: RE = Dy, Gd, Y; E = S, Se, Te) were synthesized and fully characterized, including structural determinations. Magnetic and photophysical properties of these isostructural clusters produced results that can be correlated with the structural variations due to the use of different rare-earth and/or chalcogen elements. Of note are the super-exchange interactions in clusters, antiferromagnetic in nature, being comparable in Gd3ECl4, but the dipolar interactions in these clusters are invariably ferromagnetic and the dipolar coupling parameters decrease with the use of heavier chalcogenide ligands. In addition, ferromagnetic behavior was observed in Dy3SCl4 due to the strongest dipolar interactions among the three Dy3ECl4 (E = S, Se, Te) congeners. The clusters presented in this work provide a family of new rare-earth-containing cluster compounds featuring the coordination with chalcogenido ligands – a class of substances with important magnetic and optical applications but more difficult to make than their counterparts with O-based ligands. More importantly, the chalcogen-dependent properties exhibited by the title clusters are expected to stimulate more future research on the preparation and property studies of this type of rare-earth-containing materials, phosphors with much-enhanced luminescence (due to the suppression of vibronic luminescence by virtue of RE–E coordination) and novel magneto-optic materials, for example.Data availability
Cambridge Crystallographic Data Centre contains the crystal structure with the following CCDC numbers: 2344964 (Dy3SCl4), 2344965 (Dy3SeCl4), 2344954 (Dy3TeCl4), 2344974 (Gd3SCl4), 2344970 (Gd3SeCl4), 2344975 (Gd3TeCl4), 2344958 (Y3SCl4), 2344969 (Y3SeCl4), and 2344973 (Y3TeCl4). Other data supporting the findings of this study are available within the article and its ESI.† Raw experimental and computational data are available upon request to the corresponding author.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support of this work by the National Natural Science Foundation of China (92261203, 22101116, and 21971106), the Key Laboratory of Rare Earth Chemistry of Guangdong Higher Education Institutes (2022KSYS006), the Stable Support Plan Program of Shenzhen Natural Science Fund (20200925161141006), and the Shenzhen Fundamental Research Program (JCYJ20220530115001002 and JCYJ20220818100417037).Notes and references
- L. Dong, S. Zhang, P. Gong, L. Kang and Z. Lin, Coord. Chem. Rev., 2024, 509, 215805 CrossRef CAS.
- P. Feng, J.-X. Zhang, M.-Y. Ran, X.-T. Wu, H. Lin and Q.-L. Zhu, Chem. Sci., 2024, 15, 5869 RSC.
- W. Boncher, H. Dalafu, N. Rosa and S. Stoll, Coord. Chem. Rev., 2015, 289–290, 279 CrossRef CAS.
- J. Zhou, Coord. Chem. Rev., 2016, 315, 112 CrossRef CAS.
- M. D. Regulacio, K. Bussmann, B. Lewis and S. L. Stoll, J. Am. Chem. Soc., 2006, 128, 11173 CrossRef CAS PubMed.
- F. Zhao, H.-L. Sun, S. Gao and G. Su, J. Mater. Chem., 2005, 15, 4209 RSC.
- F. Zhao, H.-L. Sun, G. Su and S. Gao, Small, 2006, 2, 244 CrossRef CAS PubMed.
- R. S. Selinsky, J. H. Han, E. A. M. Pérez, I. A. Guzei and S. Jin, J. Am. Chem. Soc., 2010, 132(45), 15997 CrossRef CAS PubMed.
- S. Kar, W. L. Boncher, D. Olszewski, N. Dollahon, R. Ash and S. L. Stoll, J. Am. Chem. Soc., 2010, 132(40), 13960 CrossRef CAS PubMed.
- A. S. Pereira, P. Rauwel, M. S. Reis, N. J. O. Silva, A. B. Timmonsa and T. Trindade, J. Mater. Chem., 2008, 18, 4572 RSC.
- Y. Hasegawa, T. Adachi, A. Tanaka, M. Afzaal, P. O’Brien, T. Doi, Y. Hinatsu, K. Fujita, K. Tanaka and T. Kawai, J. Am. Chem. Soc., 2008, 130, 5710 CrossRef CAS.
- N. Li, L. Qin, H. Y. Zhao, Z. A. Liu, X. Y. Zhang, Y. Z. Zheng and Y. P. Du, Chem. Mater., 2016, 28(8), 2507 CrossRef CAS.
- C. Wang, D. Zhang, L. Xu, Y. Jiang, F. Dong, B. Yang, K. Yu and Q. Lin, Angew. Chem., Int. Ed., 2011, 50, 7587 CrossRef CAS PubMed.
- W. L. Boncher, N. Rosa, S. Kar and S. L. Stoll, Chem. Mater., 2014, 26(10), 3144 CrossRef CAS.
- P. T. Yang, Z. Y. Liu, K. Y. Chen, X. L. Liu, X. Zhang, Z. H. Yu, H. Zhang, J. P. Sun, Y. Uwatoko, X. L. Dong, K. Jiang, J. P. Hu, Y. F. Guo, B. S. Wang and J.-G. Cheng, Nat. Commun., 2022, 13, 2975 CrossRef CAS PubMed.
- H. Yang, Q. Liu, Z. Liao, L. Si, P. Jiang, X. Liu, Y. Guo, J. Yin, M. Wang, Z. Sheng, Y. Zhao, Z. Wang, Z. Zhong and R.-W. Li, Phys. Rev. B, 2021, 104, 214419 CrossRef CAS.
- S. Dev and M. Singh, Solid State Sci., 2022, 134, 107045 CrossRef CAS.
- F. Y. Xu, K. Meng, S. Cao, C. H. Jiang, T. Chen, J. S. Xu and J. G. Yu, ACS Catal., 2022, 12(1), 164 CrossRef CAS.
- G. Gao, L. Tong, L. Yang, C. Sun, L. Xu, F. Xia, F. Geng, J. Xue, H. Gong and J. Zhu, Appl. Phys. Lett., 2021, 118, 261602 CrossRef CAS.
- G. Gao, L. Yang, B. Dai, S. Guo, Z. Yang, P. Wang, F. Geng, L. Xu, F. Xia, P. Min and J. Zhu, Mater. Lett., 2019, 254, 250 CrossRef CAS.
- M. G. Kanatzidis, Joule, 2018, 2, 583 CrossRef.
- D. Cheikh, B. E. Hogan, T. Vo, P. V. Allmen, K. Lee, D. M. Smiadak, A. Zevalkink, B. S. Dunn, J.-P. Fleurial and S. K. Bux, Joule, 2021, 5, 1168 CrossRef.
- V. Brouet, W. L. Yang, X. J. Zhou, Z. Hussain, R. G. Moore, R. He, D. H. Lu, Z. X. Shen, J. Laverock, S. B. Dugdale, N. Ru and I. R. Fisher, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 235104 CrossRef.
- C. Malliakas, S. J. L. Billinge, H. J. Kim and M. G. Kanatzidis, J. Am. Chem. Soc., 2005, 127(18), 6510 CrossRef CAS PubMed.
- A. Kogar, A. Zong, P. E. Dolgirev, X. Shen, J. Straquadine, Y.-Q. Bie, X. Wang, T. Rohwer, I.-C. Tung, Y. Yang, R. Li, J. Yang, S. Weathersby, S. Park, M. E. Kozina, E. J. Sie, H. Wen, P. Jarillo-Herrero, I. R. Fisher, X. Wang and N. Gedik, Nat. Phys., 2020, 16, 159 Search PubMed.
- H. Komoda, T. Sato, S. Souma, T. Takahashi, Y. Ito and K. Suzuki, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 195101 CrossRef.
- T. Mirkovic, M. A. Hines, P. S. Nair and G. D. Scholes, Chem. Mater., 2005, 17, 3451 CrossRef CAS.
- H. A. Dalafu, N. Rosa, D. James, D. Romar, C. Asuigui, M. McNamara, A. Kawashima, S. Omagari, T. Nakanishi, Y. Hasegawa and S. L. Stoll, Chem. Mater., 2018, 30, 2954 CrossRef CAS.
- F. Zhao and S. Gao, J. Mater. Chem., 2008, 18, 949 RSC.
- L. Tian, T. O. Yang, K. P. Loh and J. J. Vittal, J. Mater. Chem., 2006, 16, 272 RSC.
- M. D. Regulacio, N. Tomson and S. L. Stoll, Chem. Mater., 2005, 17(12), 311 CrossRef.
- F. Zhao, M. Yuan, W. Zhang and S. Gao, J. Am. Chem. Soc., 2006, 128(36), 11758 CrossRef CAS PubMed.
- Y. K. Jung, J. L. Kim and J. K. Lee, J. Am. Chem. Soc., 2010, 132(1), 178 CrossRef CAS.
- Note: Data is collected from the Cambridge Crystallographic Data Centre (CCDC Version 2024.1.0).
- M. Kuhling, R. McDonald, P. Liebing, L. Hilfert, M. J. Ferguson, J. Takats and F. T. Edelmann, Dalton Trans., 2016, 45, 10118 RSC.
- D. Werner, G. B. Deacon and P. C. Junk, Inorg. Chem., 2019, 58, 1912 CrossRef CAS.
- Y. Z. Ma, S. Bestgen, M. T. Gamer, S. N. Konchenko and P. W. Roesky, Angew. Chem., Int. Ed., 2017, 56, 13249 CrossRef CAS PubMed.
- W. J. Evans, G. W. Rabe, M. A. Ansari and J. W. Ziller, Angew. Chem., 2006, 106, 2200 CrossRef.
- W. J. Evans, G. W. Rabe, J. W. Ziller and R. J. Doedens, Inorg. Chem., 2002, 33, 2719 CrossRef.
- D. Freedman, T. J. Emge and J. G. Brennan, J. Am. Chem. Soc., 1997, 119, 11112 CrossRef CAS.
- D. Freedman, J. H. Melman, T. J. Emge and J. G. Brennan, Inorg. Chem., 1998, 37, 4162 CrossRef CAS PubMed.
- A. Y. Kornienko, T. J. Emge and J. G. Brennan, J. Am. Chem. Soc., 2001, 123, 11933 CrossRef CAS PubMed.
- J. H. Melman, M. Fitzgerald, D. Freedman, T. J. Emge and J. G. Brennan, J. Am. Chem. Soc., 1999, 121, 10247 CrossRef CAS.
- Y.-S. Ding, X.-L. Jiang, L. Li, C.-Q. Xu, J. Li and Z. P. Zheng, Nat. Synth., 2024, 3, 655 CrossRef.
- L. Li, Y.-S. Ding and Z. P. Zheng, Angew. Chem., Int. Ed., 2024, e202410019 Search PubMed.
- Y.-L. Liu, L.-X. Luo, N. Liu, B.-L. Yao, K. Liu, L. Y. Yuan, Z.-F. Chai and W.-Q. Shi, J. Nucl. Mater., 2018, 508, 63 CrossRef CAS.
- R. K. Thomson, M. J. Monreal, J. D. Masuda, B. L. Scott and J. L. Kiplinger, J. Organomet. Chem., 2011, 696, 3966 CrossRef CAS.
- SCALE3 ABSPACK: Empirical Absorption Correction. 2020, CrysAlis Pro - Software Package, Rigaku Corporation, Oxford Search PubMed.
- O. V. Olomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. J. Puschmann, Appl. Crystallogr., 2009, 42, 339 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, A71, 3 CrossRef PubMed.
- G. Li Manni, I. Fdez. Galván, A. Alavi, F. Aleotti, F. Aquilante, J. Autschbach, D. Avagliano, A. Baiardi, J. J. Bao, S. Battaglia, L. Birnoschi, A. Blanco-González, S. I. Bokarev, R. Broer, R. Cacciari, P. B. Calio, R. K. Carlson, R. Carvalho Couto, L. Cerdán, L. F. Chibotaru, N. F. Chilton, J. R. Church, I. Conti, S. Coriani, J. Cuéllar-Zuquin, R. E. Daoud, N. Dattani, P. Decleva, C. de Graaf, M. G. Delcey, L. De Vico, W. Dobrautz, S. S. Dong, R. Feng, N. Ferré, M. Filatov, L. Gagliardi, M. Garavelli, L. González, Y. Guan, M. Guo, M. R. Hennefarth, M. R. Hermes, C. E. Hoyer, M. Huix-Rotllant, V. K. Jaiswal, A. Kaiser, D. S. Kaliakin, M. Khamesian, D. S. King, V. Kochetov, M. Krośnicki, A. A. Kumaar, E. D. Larsson, S. Lehtola, M.-B. Lepetit, H. Lischka, P. López Ríos, M. Lundberg, D. Ma, S. Mai, P. Marquetand, I. C. D. Merritt, F. Montorsi, M. Mörchen, A. Nenov, V. H. A. Nguyen, Y. Nishimoto, M. S. Oakley, M. Olivucci, M. Oppel, D. Padula, R. Pandharkar, Q. M. Phung, F. Plasser, G. Raggi, E. Rebolini, M. Reiher, I. Rivalta, D. Roca-Sanjuán, T. Romig, A. A. Safari, A. Sánchez-Mansilla, A. M. Sand, I. Schapiro, T. R. Scott, J. Segarra-Martí, F. Segatta, D.-C. Sergentu, P. Sharma, R. Shepard, Y. Shu, J. K. Staab, T. P. Straatsma, L. K. Sørensen, B. N. C. Tenorio, D. G. Truhlar, L. Ungur, M. Vacher, V. Veryazov, T. A. Voß, O. Weser, D. Wu, X. Yang, D. Yarkony, C. Zhou, J. P. Zobel and R. Lindh, J. Chem. Theory Comput., 2023, 19, 6933 CrossRef CAS.
- B. O. Roos, R. Lindh, P.-Å. Malmqvist, V. Veryazov and P.-O. Widmark, J. Phys. Chem. A, 2004, 108, 2851 CrossRef CAS.
- B. O. Roos, R. Lindh, P.-Å. Malmqvist, V. Veryazov and P.-O. Widmark, J. Phys. Chem. A, 2005, 109, 6575 CrossRef CAS PubMed.
- L. Ungur and L. F. Chibotaru, Chem. – Eur. J., 2017, 23, 3708 CrossRef CAS PubMed.
- L. F. Chibotaru, L. Ungur and A. Soncini, Angew. Chem., Int. Ed., 2008, 47, 4126 CrossRef CAS.
- L. Ungur, W. V. d Heuvela and L. F. Chibotaru, New J. Chem., 2009, 33, 1224 RSC.
- J. H. Melman, T. J. Emge and J. G. Brennan, Chem. Commun., 1997, 2269 RSC.
- L. Huebner, A. Kornienko, T. J. Emge and J. G. Brennan, Inorg. Chem., 2005, 44(14), 5118 CrossRef CAS.
- G.-X. Jin, Y. Cheng and Y. Lin, Organometallics, 1999, 18(6), 947 CrossRef CAS.
- D. Freedman, T. J. Emge and J. G. Brennan, Inorg. Chem., 2002, 41(3), 492 CrossRef CAS.
- https://abulafia.mt.ic.ac.uk/shannon/ptable.php .
- J. Wu, S. Demeshko, S. Dechert and F. Meyer, Chem. Commun., 2020, 56, 3887 RSC.
- S. Wang, Q. Yang, T. C. W. Mak and Z. Xie, Organometallics, 1999, 18(26), 5511 CrossRef CAS.
- Z. Lv, Z. Chai, M. Zhu, J. Wei and W. Zhang, J. Am. Chem. Soc., 2021, 143(24), 9151 CrossRef CAS PubMed.
- W. J. Evans, K. A. Miller, D. S. Lee and J. W. Ziller, Inorg. Chem., 2005, 44(12), 4326 CrossRef CAS PubMed.
- W. J. Evans, G. W. Rabe, J. W. Ziller and R. J. Doedens, Inorg. Chem., 1994, 33(13), 2719 CrossRef CAS.
- J. G. Brennan, Lanthanides: sulfur, selenium, and tellurium compounds, Encyclopedia of Inorganic and Bioinorganic Chemistry, Wiley, 2012 Search PubMed.
- M. Runowski, N. Stopikowska and S. Lis, Dalton Trans., 2020, 49, 2129 RSC.
- Z.-F. Li, L. Zhou, J.-B. Yu, H.-J. Zhang, R.-P. Deng, Z.-P. Peng and Z.-Y. Guo, J. Phys. Chem. C, 2007, 111, 2295 CrossRef CAS.
- A. Zhang, J. Zhang, Q. Pan, S. Wang, H. Jia and B. Xu, J. Lumin., 2012, 132, 965 CrossRef CAS.
- K. R. McClain, H. Kwon, K. Chakarawet, R. Nabi, J. G. C. Kragskow, N. F. Chilton, R. D. Britt, J. R. Long and B. G. Harvey, J. Am. Chem. Soc., 2023, 145, 8996 CrossRef CAS PubMed.
- Y.-X. Wang, Q. Xu, P. Ren, W. Shi and P. Cheng, Dalton Trans., 2019, 48, 2228 RSC.
- J.-P. Costes, F. Dahan and F. Nicodème, Inorg. Chem., 2001, 40, 5285 CrossRef CAS PubMed.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, J. Comput. Chem., 2013, 34, 1164 CrossRef CAS PubMed.
- T. Han, Y.-S. Ding, Z.-H. Li, K.-X. Yu, Y.-Q. Zhai, N. F. Chilton and Y.-Z. Zheng, Chem. Commun., 2019, 55, 7930 RSC.
- D. Lüert, A.-K. Kreyenschmidt, C. M. Legendre, R. Herbst-Irmer and D. Stalke, Inorg. Chem., 2022, 61(13), 5234 CrossRef.
- J. Wu, F. Zhang, G. L. Wang, S. Demeshko, S. Dechert, Y. Q. Zhang and F. Meyer, Chem. – Eur. J., 2023, 29, 2458 Search PubMed.
- T. Pugh, V. Vieru, L. F. Chibotaru and R. A. Layfield, Chem. Sci., 2016, 7, 2128 RSC.
- H.-L. Wang, Q.-X. Yu, Z.-H. Zhu, X.-L. Lu, Y.-L. Li, F.-P. Liang and H.-H. Zou, Inorg. Chem., 2023, 62, 5863 CrossRef CAS PubMed.
- H.-L. Wang, Y.-L. Li, Z.-H. Zhu, X.-L. Lu, F.-P. Liang and H.-H. Zou, Inorg. Chem., 2022, 61, 20169 CrossRef CAS PubMed.
- W. Wang, T. Shang, J. Wang, B.-L. Yao, L.-C. Li, Y. Ma, Q.-L. Wang, Y.-Z. Zhang, Y.-Q. Zhang and B. Zhao, Dalton Trans., 2022, 51, 9404 RSC.
- D. Chauhan, K. R. Vignesh, A. Swain, S. K. Langley, K. S. Murray, M. Shanmugam and G. Rajaraman, Cryst. Growth Des., 2022, 23, 197 CrossRef.
- Q. Yuan, Y.-S. Meng, Y.-Q. Zhang, C. Gao, S.-S. Liu, B.-W. Wang and S. Gao, Inorg. Chem. Front., 2022, 9, 2336 RSC.
- C. Jin, X.-L. Li, Z. Liu, A. Mansikkamäki and J. Tang, Dalton Trans., 2020, 49, 10477 RSC.
- J. Wu, X.-L. Li, L. L. Droitte, O. Cador, B. L. Guennic and J. Tang, Dalton Trans., 2021, 50, 15027 RSC.
- J. Tang, I. Hewitt, N. T. Madhu, G. Chastanet, W. Wernsdorfer, C. E. Anson, C. Benelli, R. Sessoli and A. K. Powell, Angew. Chem., Int. Ed., 2006, 45, 1729 CrossRef CAS.
- L. Ungur, S.-Y. Lin, J. Tang and L. F. Chibotaru, Chem. Soc. Rev., 2014, 43, 6894 RSC.
- Y.-S. Meng, Y.-S. Qiao, M.-W. Yang, J. Xiong, T. Liu, Y.-Q. Zhang, S.-D. Jiang, B.-W. Wang and S. Gao, Inorg. Chem. Front., 2020, 7, 447 RSC.
- Y.-X. Wang, Y. Ma, J.-S. Wang, Y. Yang, Y.-N. Guo, Y.-Q. Zhang, K.-J. Jin, Y. Sun and P. Cheng, Adv. Sci., 2022, 9, 2202979 CrossRef CAS.
- M. Kong, X. Feng, J. Wang, Y.-Q. Zhang and Y. Song, Dalton Trans., 2021, 50, 568 RSC.
- X. Liu, X. Ma, W. Yuan, P. Cen, Y.-Q. Zhang, J. Ferrando-Soria, G. Xie, S. Chen and E. Pardo, Inorg. Chem., 2018, 57(23), 14843 CrossRef CAS.
- K. Zhang, V. Montigaud, O. Cador, G.-P. Li, B. L. Guennic, J.-K. Tang and Y.-Y. Wang, Inorg. Chem., 2018, 57(14), 8550 CrossRef CAS PubMed.
- K. Zhang, D. Liu, V. Vieru, L. Hou, B. Cui, F.-S. Guo, L. F. Chibotaru and Y.-Y. Wang, Dalton Trans., 2017, 46, 638 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2344964, 2344965, 2344954, 2344974, 2344970, 2344975, 2344958, 2344969 and 2344973. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4tc02778g |
| This journal is © The Royal Society of Chemistry 2024 |