Development of polyoxometalate-loaded MOFs for heterogeneous catalysis and enhanced dye adsorption†
Ketan
Maru
,
Sarita
Kalla
and
Ritambhara
Jangir
 *
*
Sardar Vallabhbhai National Institute of Technology, Ichchanath, Surat-395 007, Gujarat, India. E-mail: ritambhara.jangir@chem.svnit.ac.in
First published on 8th November 2024
Abstract
This study focuses on the enhancement of MIL-117 functionality by incorporating a well-known polyoxometalate (POM), tetrabutylammonium octamolybdate [(n-C4H9)4N]4[Mo8O26]. Using an encapsulation method with conventional heating, Mo8O264− anions were for the first time successfully integrated into MIL-117 tubular channels (Mo8O26@MIL-117). Comprehensive characterization of the material through FTIR, XRD, BET, FE-SEM, EDX, and XPS confirmed the uniform distribution of Mo8O264− within MIL-117 without compromising its structural integrity. The Mo8O26@MIL-117 composite demonstrates exceptional catalytic performance in oxidative C–N bond formation and Paal–Knorr pyrrole synthesis, achieving high yields under optimized conditions with diverse amine substrates. Characterization and stability assessments confirm Mo8O26@MIL-117 as a robust and recyclable catalyst, maintaining structural integrity and catalytic activity over multiple cycles, highlighting its potential for sustainable applications in synthetic chemistry. The composite material was also evaluated for its efficacy in dye removal, specifically targeting methylene blue (MB) and Rhodamine B (RHB) from aqueous solutions. Mo8O26@MIL-117 exhibited superior adsorption capacity for MB compared to MIL-117 alone, demonstrating high efficiency even at elevated concentrations. The composite showed improved selectivity towards MB over RHB, highlighting its potential for selective dye removal in wastewater treatment applications.
1. Introduction
Metal–organic frameworks (MOFs) are crystalline porous materials defined by metal ions or clusters connected to bi- or multipodal organic linkers.1,2 Their large surface areas, low framework densities, and high pore volumes make them attractive for various applications, such as gas storage,3 wastewater treatment,4 and heterogeneous catalysis.5,6 The externally accessible nanosized cavities and channels within MOFs facilitate the incorporation of substrates, enhancing their catalytic action.7,8 Despite the discovery of over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MOF structures, relatively few studies focus on functionalizing MOFs by incorporating metal ions, organic–inorganic molecules through host–guest interactions (doping), or nanoparticles.9–11
000 MOF structures, relatively few studies focus on functionalizing MOFs by incorporating metal ions, organic–inorganic molecules through host–guest interactions (doping), or nanoparticles.9–11
Polyoxometalates (POMs) are clusters of anionic metal oxides primarily consisting of high-valent early transition-metal ions interconnected by oxygen atoms.12–14 Their modular composition and variable size result in a wide range of POM structures.15 Due to their versatile acid–base and redox properties, along with their strong abilities for electrostatic interactions, hydrogen bonding, and van der Waals forces, POMs have been found suitable for numerous applications in chemical catalysis and wastewater treatment.16,17 More specifically, these applications include catalytic oxidation,18 water oxidation,19 alkene epoxidation,20 and phosphodiester hydrolysis,21 and serving as adsorbents for both cationic and anionic dyes.22 However, the practical use of POMs in catalytic systems is often limited by their high solubility, low specific surface area, and poor stability under catalytic conditions.23,24 Additionally, their chemical and thermal instability, as well as their poor stability in water, make them unsuitable for dye removal applications.25 To address these challenges, various supports have been explored to anchor POMs and create hybrid heterogeneous materials.26 These supports include mesoporous silica,27 polymers,28 covalent organic frameworks (COFs),29,30 and MOFs.31,32 The tunability, porosity, and thermal and chemical stability, along with water stability, of MOFs make them appropriate as heterogeneous catalysts, supports for anchored homogeneous catalysts, and dye adsorbents.33,34 Two main techniques for incorporating POMs into MOFs, known as POM@MOF, are impregnation and encapsulation (Fig. S1†).35,36 Immobilizing POMs onto MOF supports enhances their stability and reusability in catalytic processes and improves their effectiveness as dye adsorbents.37 This enhances their performance and expands their practical application across a wider range of chemical reactions and wastewater treatment.38
The first POM@MOF was synthesised by Férey and colleagues using the impregnation method where MIL-101 was chosen due to its larger apertures and mesopores.39 The impregnation method involves directly adding a synthesized POM to a MOF, which requires the MOF to have large apertures to allow POM diffusion.40 On the other hand, the encapsulation method, which is also known as the “bottle around the ship” method, involves constructing a MOF in the presence of a POM.41 This technique has been used effectively for MOFs like HKUST-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 and other Cu-BTC frameworks (BTC = 1,3,5-benzene-tricarboxylate),43 the NENU44 series, MIL-100/101 (Fe, Cr, and Al),45 and UiO-67.46 These MOFs have cavities large enough to surround the POM while having apertures of appropriate size that prevent the POM from leaching out. Hatton et al. developed an MIL-101/PTA composite and evaluated its catalytic performance across several reactions. This composite showed remarkable efficiency in the Baeyer condensation of benzaldehyde and 2-naphthol, in three-component condensation involving benzaldehyde, 2-naphthol, and acetamide, and in the epoxidation of caryophyllene using hydrogen peroxide. Under microwave-assisted heating, the MIL-101/PTA composites achieved impressive reactant conversion rates, exceeding 80–90%.47 Recently, Kortz et al. successfully synthesized a Pd13Se8@MIL-101 composite material. This innovative composite was evaluated and demonstrated to be an effective, stable, and recyclable heterogeneous pre-catalyst for the Suzuki–Miyaura cross-coupling reaction. The catalytic reaction proceeded efficiently at room temperature, using eco-friendly solvents such as water and methanol.48
42 and other Cu-BTC frameworks (BTC = 1,3,5-benzene-tricarboxylate),43 the NENU44 series, MIL-100/101 (Fe, Cr, and Al),45 and UiO-67.46 These MOFs have cavities large enough to surround the POM while having apertures of appropriate size that prevent the POM from leaching out. Hatton et al. developed an MIL-101/PTA composite and evaluated its catalytic performance across several reactions. This composite showed remarkable efficiency in the Baeyer condensation of benzaldehyde and 2-naphthol, in three-component condensation involving benzaldehyde, 2-naphthol, and acetamide, and in the epoxidation of caryophyllene using hydrogen peroxide. Under microwave-assisted heating, the MIL-101/PTA composites achieved impressive reactant conversion rates, exceeding 80–90%.47 Recently, Kortz et al. successfully synthesized a Pd13Se8@MIL-101 composite material. This innovative composite was evaluated and demonstrated to be an effective, stable, and recyclable heterogeneous pre-catalyst for the Suzuki–Miyaura cross-coupling reaction. The catalytic reaction proceeded efficiently at room temperature, using eco-friendly solvents such as water and methanol.48
Here, we explore the functional applications of MOFs modified by doping with inorganic guest molecules, specifically highlighting MIL-117. MIL-117 stands out due to its strong thermal and chemical stability, along with its spacious tubular channels (∼12 Å) that facilitate efficient mass transport.49 The large tubular channels within MIL-117 also facilitate functionalization with POM anions, enabling the resulting composite materials to be highly effective for various applications. A well-known POM precursor, i.e., tetrabutylammonium octamolybdate [(n-C4H9)4N]4[Mo8O26], has been incorporated which is known as a potent oxidizing agent with good thermal stability in the solid state. The POM@MOF composite, i.e., Mo8O26@MIL-117, has been developed using the encapsulation method in a round bottom flask (RBF) and conventional heating. A complete characterization study confirms the successful synthesis and encapsulation of Mo8O264− anions within the MIL-117 tubular channels. These comprehensive findings underscore that the Mo8O264− anions are tightly encapsulated within the MIL-117 channels, enhancing the composite's stability and preventing leaching. The Mo8O26@MIL-117 composite exhibits superior catalytic performance in key reactions, including C–N bond formation (aldehydes and amines (1° and 2°)) and pyrrole synthesis (2,5-hexadione and amines), highlighting its versatility and potential for advanced catalytic applications in chemical processes. This composite material was also tested for the adsorption of methylene blue (MB) and Rhodamine B (RHB), and it demonstrated a strong affinity towards MB in the MB/RHB mixture, showing selective dye adsorption.
2. Results and discussion
2.1 Synthesis and characterization of Mo8O26@MIL-117
Traditionally, the synthesis of MIL-117 involves the hydrothermal method.49 The Mo8O26@MIL-117 composite was developed using a standard heating and stirring process conducted in an RBF. As part of the synthesis strategy, both the direct sonication (or impregnation) technique and the in situ addition method, also referred to as the “bottle around the ship” or encapsulation method, were employed (Fig. 1 and Fig. S2†). ICP-OES results indicate that the impregnation method achieves only 0.005% Mo loading. In contrast, the encapsulation method, using 1 mmol of [(n-C4H9)4N]4[Mo8O26], results in 1.8% Mo loading, and using 2 mmol leads to 4.4% Mo loading within the tubular cavities of MIL-117 (Table S1†). These results suggest that the encapsulation method is more effective and efficient for trapping Mo8O264− anions in the tubular channels of MIL-117. Furthermore, during the post-synthesis process, washing Mo8O26@MIL-117 with CH3CN and DMF confirms the removal of excess and un-trapped [(n-C4H9)4N]4[Mo8O26], as this compound is highly soluble in these solvents.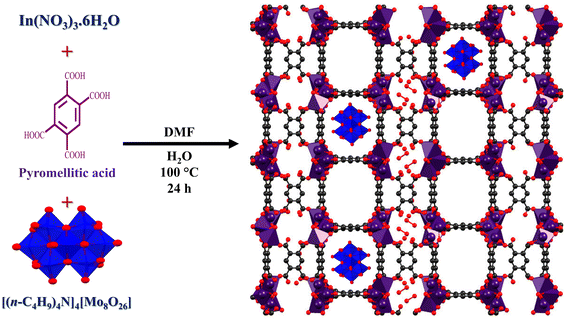 | ||
| Fig. 1 Synthesis of Mo8O26@MIL-117 via the encapsulation method utilizing conventional stirring and heating, and proposed structure of the Mo8O26@MIL-117 composite (grey: carbon, red: oxygen, purple: indium, and blue: Mo, and the image was created using the .cif file from ref. 49). | ||
The MIL-117 structure features tubular channels with a diameter of 1.2 nm, as reported in the literature (Fig. 2(A)).49 These channels are quite long due to the overall structure of the MOF and can accommodate Mo8O264− anions (Fig. 2(B)). The Mo8O264− anions measure 0.9 nm in length and 0.6 nm in width, making them a suitable fit for the tubular channels (Fig. 2(C)).50 Thus, the tubular channels in MIL-117 allow the encapsulation of Mo8O264− anions. The physical confinement enhances stability and prevents the POM anions from leaching out of the MOF. Even in the absence of strong electrostatic or coordination bonds, anions can be held within the channels through van der Waals forces. This is particularly relevant in this case because the channels match the POM dimensions well, ensuring close packing and maximizing these weak interactions. Additionally, the oxygen atoms from Mo8O264− can interact with the indium atoms in MIL-117. Such interactions help in stabilizing the POMs within the channels. The interaction between the MIL-117 tubular channels and Mo8O264− anions is multifaceted and may involve electrostatic interactions, coordination chemistry, hydrogen bonding, van der Waals forces, and physical confinement.
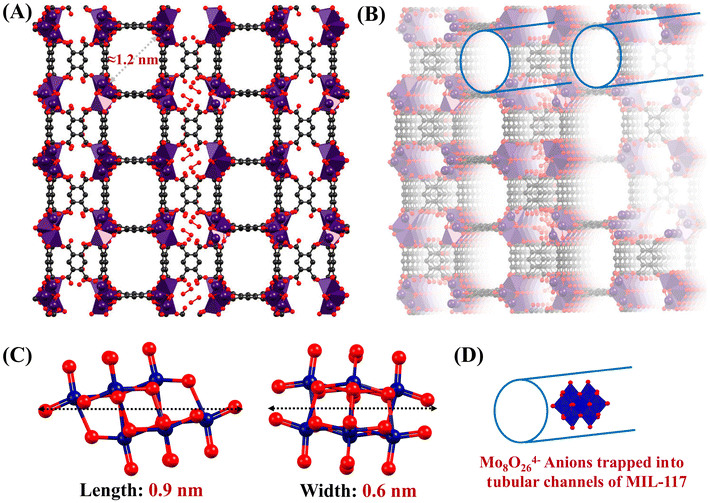 | ||
| Fig. 2 (A and B) Crystal structure of MIL-117 highlighting its tubular channels with a diameter of 1.2 nm (grey: carbon, red: oxygen, purple: indium, and blue: Mo). (C) Structural views of the Mo8O264− anion depicting its X-axis and Y-axis orientations (red: oxygen and blue: Mo) (D). Visual depiction of Mo8O264− confined within the tubular channels of MIL-117 (red: oxygen and blue: Mo and the image was created using the .cif file from ref. 49). | ||
The Fourier transform infrared (FTIR) data of the Mo8O26@MIL-117 material provide valuable insights into the stretching modes present in its structure. The spectra of Mo8O26@MIL-117 at different Mo8O264− loadings (1 mmol and 2 mmol) exhibit almost similar peak positions. Specifically, the characteristic peaks of the carboxylate group in the MOF are observed at 1573 cm−1 and 1583 cm−1 (Fig. 3). Interestingly, the spectra do not show the carboxylic acid vC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak of the ligand, which typically appears at 1710 cm−1. This absence is likely attributed to the formation of carboxylate anions, leading to the establishment of a delocalized electronic cloud and the manifestation of typical carboxylate peaks in the range of 1610 to 1550 cm−1 and 1420 to 1300 cm−1. The MIL-117 parent moiety exhibits similar behaviour in its FTIR spectrum. Furthermore, peaks below 730 cm−1, observed in both Mo8O26@MIL-117 and MIL-117 spectra, suggest the presence of In–O bonding. In the 500–1000 cm−1 region, the observed bands are attributed to the Mo–O stretching modes. The absorption bands at 909 cm−1 and 912 cm−1 are associated with the stretching vibration of Mo
O peak of the ligand, which typically appears at 1710 cm−1. This absence is likely attributed to the formation of carboxylate anions, leading to the establishment of a delocalized electronic cloud and the manifestation of typical carboxylate peaks in the range of 1610 to 1550 cm−1 and 1420 to 1300 cm−1. The MIL-117 parent moiety exhibits similar behaviour in its FTIR spectrum. Furthermore, peaks below 730 cm−1, observed in both Mo8O26@MIL-117 and MIL-117 spectra, suggest the presence of In–O bonding. In the 500–1000 cm−1 region, the observed bands are attributed to the Mo–O stretching modes. The absorption bands at 909 cm−1 and 912 cm−1 are associated with the stretching vibration of Mo![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. These peaks appear slightly higher in frequency compared to the parent moiety [(n-C4H9)4N]4[Mo8O26], which exhibits a peak at 906 cm−1. The absorption bands at 851 cm−1 in [(n-C4H9)4N]4[Mo8O26] and 866 cm−1 and 868 cm−1 in Mo8O26@MIL-117 correspond to the stretching mode of doubly coordinated oxygen (Mo2–O). This mode arises from the oxygen atoms shared between two MoO6 octahedra. Additionally, the bands at 557 cm−1 in [(n-C4H9)4N]4[Mo8O26] and 555 cm−1 in Mo8O26@MIL-117 correspond to the stretching mode of triply coordinated oxygen (Mo3–O), resulting from the oxygen atoms shared between three MoO6 octahedra. The C–H stretching peaks at 2900 cm−1 in [(n-C4H9)4N]4[Mo8O26] are attributed to the tetrabutyl group of this compound. However, in Mo8O26@MIL-117 (1 and 2 mmol), these C–H stretching vibrations are not observed. This absence confirms that the tubular channels of MIL-117 selectively trap the Mo8O264−anions, while the [(n-C4H9)4N]4+ cations are washed away. These findings confirm that Mo8O264− is trapped within the MIL-117 structure, akin to a “ship in a bottle” analogy, where the Mo8O264− species is effectively incorporated and immobilized within MIL-117 tubular channels.
O groups. These peaks appear slightly higher in frequency compared to the parent moiety [(n-C4H9)4N]4[Mo8O26], which exhibits a peak at 906 cm−1. The absorption bands at 851 cm−1 in [(n-C4H9)4N]4[Mo8O26] and 866 cm−1 and 868 cm−1 in Mo8O26@MIL-117 correspond to the stretching mode of doubly coordinated oxygen (Mo2–O). This mode arises from the oxygen atoms shared between two MoO6 octahedra. Additionally, the bands at 557 cm−1 in [(n-C4H9)4N]4[Mo8O26] and 555 cm−1 in Mo8O26@MIL-117 correspond to the stretching mode of triply coordinated oxygen (Mo3–O), resulting from the oxygen atoms shared between three MoO6 octahedra. The C–H stretching peaks at 2900 cm−1 in [(n-C4H9)4N]4[Mo8O26] are attributed to the tetrabutyl group of this compound. However, in Mo8O26@MIL-117 (1 and 2 mmol), these C–H stretching vibrations are not observed. This absence confirms that the tubular channels of MIL-117 selectively trap the Mo8O264−anions, while the [(n-C4H9)4N]4+ cations are washed away. These findings confirm that Mo8O264− is trapped within the MIL-117 structure, akin to a “ship in a bottle” analogy, where the Mo8O264− species is effectively incorporated and immobilized within MIL-117 tubular channels.
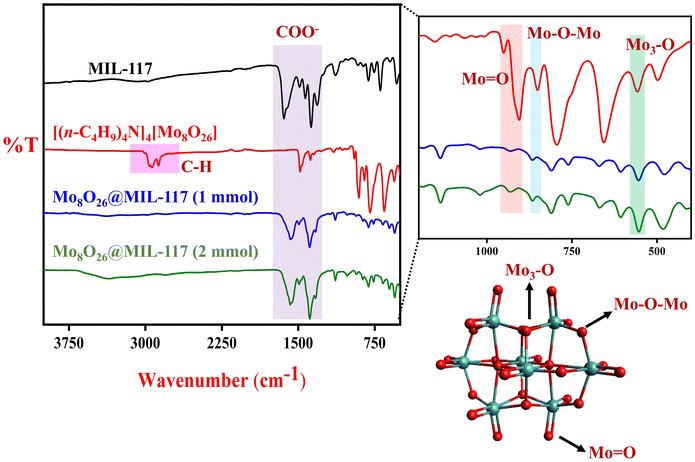 | ||
| Fig. 3 FTIR spectra of the Mo8O26@MIL-117 composite and [(n-C4H9)4N]4[Mo8O26] (red: oxygen and blue: Mo). | ||
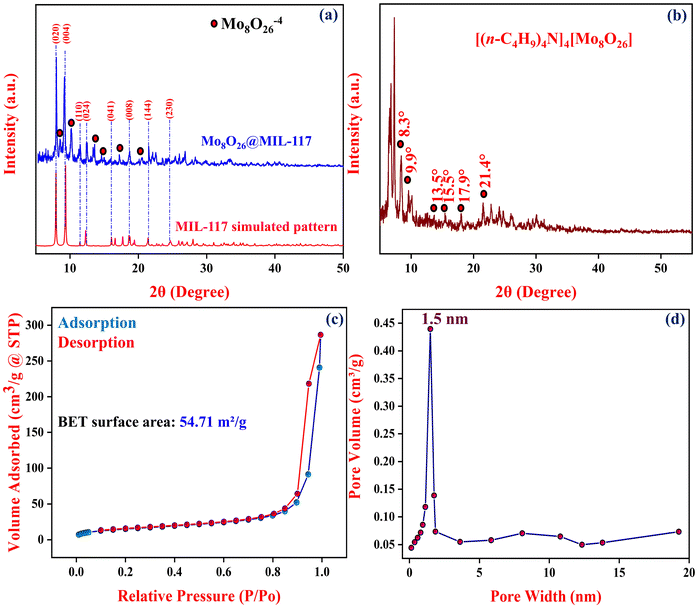
The PXRD patterns were recorded to investigate the phase and crystalline structures of the fabricated Mo8O26@MIL-117 sample, as elucidated in Fig. 4(a). In agreement with the simulated pattern with MIL-117, the PXRD patterns of Mo8O26@MIL-117 demonstrate the normal characterization peaks of MIL-117 at 7.9° and 9.2° with high intensity, indicating the good crystallinity of the synthesized Mo8O26@MIL-117. The prominent peaks observed at 7.9°, 9.2°, 11.4°, 12.4°, 16.0°, 18.7°, 21.5°, and 24.5° correspond to the (020), (004), (110), (024), (041), (008), (144), and (230) planes of MIL-117, which were also found in the synthesized composite (Fig. 4(a)). The main diffraction peaks of [(n-C4H9)4N]4[Mo8O26] are observed at 2θ of 8.3°, 9.9°, 13.5°, 15.5°, 17.9°, and 21.4° (Fig. 4(b)). The existence of these diffraction bands attributed to the POM anions in the Mo8O26@MIL-117 pattern, though with very low intensities, indicates the homogeneous distribution of the POM anions in the porous structure of MIL-117. However, peaks assigned to the POM in the composite material were hardly observed, possibly due to the low content and high dispersion of POM molecules.
The N2 desorption/adsorption isotherms of the Mo8O26@MIL-117 material are displayed in Fig. 4(c). Based on the IUPAC classification, the N2 adsorption–desorption isotherms of these samples reveal type III isotherms with an H3 hysteresis loop, similar to their parent MIL-117. The specific surface area and total pore volume of this material are presented in Table 1. Mo8O26@MIL-117 exhibited a lower surface area and pore volume, compared to MIL117 (Table 1). This significant difference is attributed to the insertion of POM anions into the pores of MIL-117, which occupy most of the pore space.
| Samples | BET surface area (m2 g−1) | Total pore volume (cm3 g−1) |
|---|---|---|
| MIL-117 | 63 m2 g−1 | 0.77 |
| Mo8O26@MIL-117 | 54.71 m2 g−1 | 0.44 |
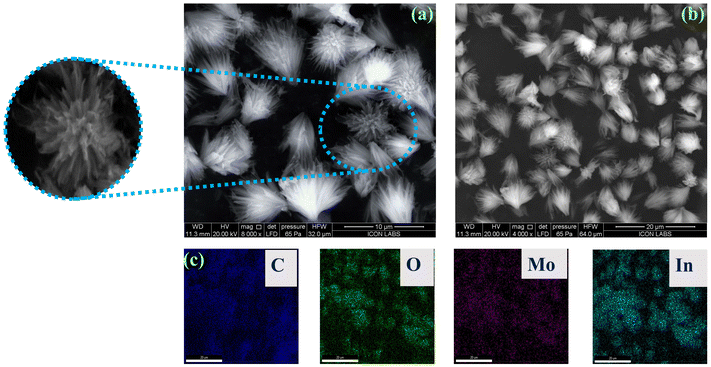
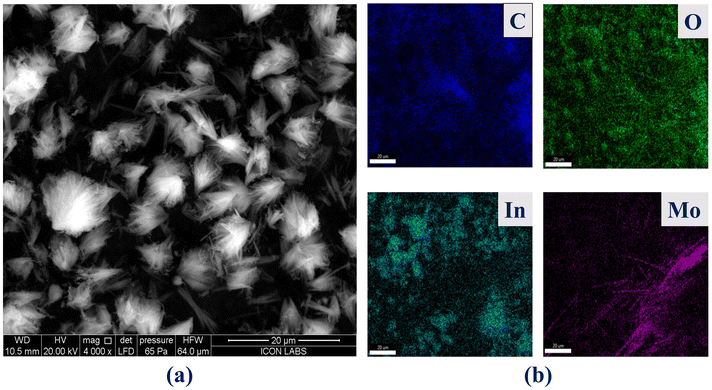
Furthermore, the morphology of the composite material was investigated using FE-SEM analysis, as illustrated in Fig. 5. Pristine MIL-117 particles exhibit an orderly plate-like morphology. However, comparing the FE-SEM images of Mo8O26@MIL-117 with those of MIL-117 shows no specific morphology of MIL-117, indicating that the presence of the POM alters the formation mechanism. The SEM images reveal that Mo8O26@MIL-117 exhibits a flower-like morphology, which is significantly different from its parent compound, MIL-117 (Fig. 4). Despite this morphological change, the PXRD, BET, and FT-IR results confirm that the framework of MIL-117 did not collapse or degrade after encapsulation with Mo8O264− anions. This change in the morphology is attributed to the encapsulation of Mo8O264− anions within the MOF channels. These anions are tightly trapped, causing some distortion in the structure. This structural distortion, along with the interpenetration of anions into the channels, leads to the observed morphological changes. To further investigate the distribution and chemical composition of the Mo8O26@MIL-117 composite, EDX elemental mapping was performed (Fig. 5(c)). A representative SEM image with the corresponding EDX elemental mappings in Fig. 5(c) shows the uniform distribution of C, O, In, and Mo elements in the flower-like Mo8O26@MIL-117 particles, emphasizing the homogeneity of the prepared sample.
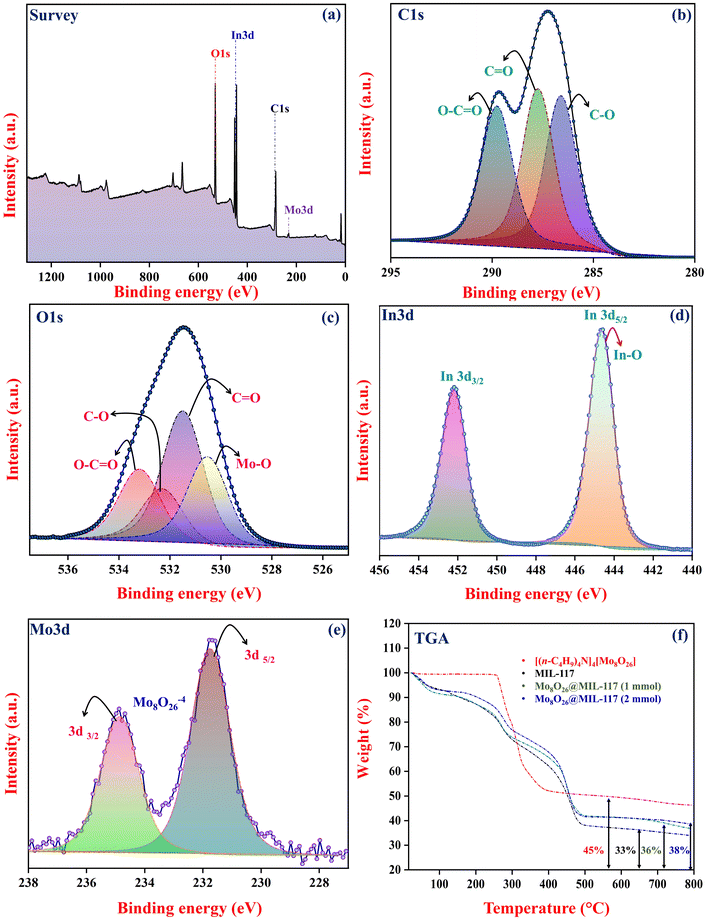
X-ray photoelectron spectroscopy (XPS) analysis was conducted on Mo8O26@MIL-117 to provide a detailed understanding of the bonding interactions. The results, presented in Fig. 6, provide significant insights into the chemical states and bonding environment of the elements within the composite. Notable peaks were observed at specific binding energies (BEs), including 285 eV (C 1s), 530 eV (O 1s), 443 eV (In 3d), and 232 eV (Mo 3d) (Fig. 6a). The C 1s and O 1s elements were identified in the pyromellitic acid linker. The high-resolution C 1s peak exhibited four prominent peaks at 286.3 eV, 287.8 eV, and 288.8 eV BEs, corresponding to C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and O–C
O, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, respectively (Fig. 6b). The O 1s spectra displayed distinct peaks at BEs of 531.5 eV, 532.3 eV, 533.2 eV, and 530.5 eV, which are associated with C
O groups, respectively (Fig. 6b). The O 1s spectra displayed distinct peaks at BEs of 531.5 eV, 532.3 eV, 533.2 eV, and 530.5 eV, which are associated with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, O–C
O, C–O, O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and Mo–O bonds, respectively (Fig. 6c). The peaks observed at 232 eV and 235 eV binding energies (BEs) in the XPS analysis further confirm the presence of Mo8O264− within the composite (Fig. 6e). The XPS spectra of Mo show identical characteristics to those found in the literature of Mo8O264−, confirming that the oxidation state of molybdenum in the composite is the same as that in Mo8O264−. This BE is consistent with that of the Mo8O264− entity and matches the state found in its parent compound [(n-C4H9)4N]4[Mo8O26]. These findings strongly suggest that Mo8O264− is indeed trapped within the channels of MIL-117. The characteristic peaks of In were observed at BEs between 443 and 445 eV, specifically at 443.8 eV (In 3d5/2) and 445.1 eV (In 3d3/2) (Fig. 6d). Additionally, the peak at 445.1 eV confirmed that In is surrounded by oxygen atoms from the pyromellitic acid linker, like tris(2,4-pentanedionato-O,O′)indium. These XPS results suggest that the MIL-117 structure is formed around Mo8O264− like a ship in the bottle.
O, and Mo–O bonds, respectively (Fig. 6c). The peaks observed at 232 eV and 235 eV binding energies (BEs) in the XPS analysis further confirm the presence of Mo8O264− within the composite (Fig. 6e). The XPS spectra of Mo show identical characteristics to those found in the literature of Mo8O264−, confirming that the oxidation state of molybdenum in the composite is the same as that in Mo8O264−. This BE is consistent with that of the Mo8O264− entity and matches the state found in its parent compound [(n-C4H9)4N]4[Mo8O26]. These findings strongly suggest that Mo8O264− is indeed trapped within the channels of MIL-117. The characteristic peaks of In were observed at BEs between 443 and 445 eV, specifically at 443.8 eV (In 3d5/2) and 445.1 eV (In 3d3/2) (Fig. 6d). Additionally, the peak at 445.1 eV confirmed that In is surrounded by oxygen atoms from the pyromellitic acid linker, like tris(2,4-pentanedionato-O,O′)indium. These XPS results suggest that the MIL-117 structure is formed around Mo8O264− like a ship in the bottle.
The thermal behaviour exhibited by Mo8O26@MIL-117 closely resembles that of MIL-117, as illustrated in Fig. 6f. The similarity observed implies a lack of strong covalent bonding between the MIL-117 and Mo8O264− anions. This suggests that the Mo8O264− is trapped within the channels and is not part of the building block of the composite structure, nor is it directly attached to the structure. Instead, the interaction is likely mediated by electrostatic interactions, hydrogen bonding, and van der Waals forces, and physical confinement within the composite structure is possible. However, an increase in the amount of metal oxide residue can be observed in Mo8O26@MIL-117 (1 mmol: 36% and 2 mmol: 38%), which suggests that the Mo8O264− species is effectively trapped inside the MIL-117 structure. The TGA curves of MIL-117 and Mo8O26@MIL-117 show two major weight losses, and the first weight loss up to 100 °C is attributed to the removal of physisorbed water. The second weight loss, performed in two steps, between 250 °C and 500 °C, indicates the decomposition of the benzenetetracarboxylate ligand and removal of its fragments. The final residue corresponds to a mixture of indium oxide and molybdenum oxide. In comparison, [(n-C4H9)4N]4[Mo8O26] remains stable up to 257 °C. At this temperature, Mo8O26@MIL-117 also begins to degrade, and due to the very low concentration of anions, it becomes challenging to distinguish a clear signature for [(n-C4H9)4N]4[Mo8O26] within the composite. This overlap in degradation behavior suggests that the presence of the anions does not significantly alter the thermal profile of the composite.
In conclusion, the FTIR data confirm the presence of Mo8O264− in its original form. PXRD analysis indicates that the structure or channels did not collapse and that the material maintained good crystallinity. BET measurements show that the channels are filled, leading to a reduced surface area. Additionally, XPS data confirm that the oxidation state of Mo remains unchanged from its parent moiety. These combined results confirm that MIL-117 successfully traps Mo8O264− anions within its hollow channels without any structural degradation.
2.2 Catalytic study
The synthesis of the Mo8O26@MIL-117 composite material represents a significant leap in catalysis, as it demonstrates remarkable efficacy in two pivotal reactions: C–N bond formation using primary and secondary amines and Pall Knorr pyrrole synthesis. Harnessing the synergistic properties of MIL-117 and Mo8O264−, this composite catalyst exhibits exceptional versatility. Its ability to catalyse oxidative C–N bond formation highlights its role in enabling crucial synthetic pathways. At the same time, its effectiveness in pyrrole synthesis underscores its relevance in producing valuable compounds that have diverse applications in pharmaceuticals and materials science. This multifunctional catalyst showcases promising potential for broadening synthetic possibilities and advancing catalytic methodologies.In the pursuit of efficient oxidative C–N bond formation, a meticulous optimization process was embarked upon, aiming to enhance both yield and selectivity while elucidating critical reaction parameters. Initially, a model reaction between benzaldehyde (1) and aniline (2) was selected, employing TBHP (tert-butyl hydroperoxide) as the oxidant to synthesize benzanilide (3) and to identify the most effective catalytic system (Table S2†). The catalyst screening experiment, conducted in acetonitrile at 75 °C for 1 hour, entailed evaluating the Mo8O26@MIL-117 catalyst with 1 mmol and 2 mmol Mo8O264− loadings, alongside other catalysts such as MIL-117, [(n-C4H9)4N]4[Mo8O26], and In(NO3)3·xH2O. Remarkably, Mo8O26@MIL-117 (2 mmol) at 10 mg loading exhibited the highest yield of 74%, outperforming the other catalysts and loadings, signifying its superior catalytic activity. The subsequent optimization steps delved into various factors influencing the reaction efficiency. Temperature screening (Table S3†) identified 85 °C as the optimal temperature, maximizing the efficiency of the catalyst. Solvent screening (Table S4†) provided critical insights, with acetonitrile emerging as the optimal solvent, yielding 84% product formation, attributed to its polar aprotic nature facilitating effective solvation of reactants and the oxidant. Further investigation into the reaction time (Table S5†) revealed 60 minutes as the optimal duration, yielding 84% product formation, beyond which prolonged reaction times exhibited diminishing returns, indicative of completion of the reaction. Furthermore, in oxidant screening, TBHP exhibited the highest yield of 84%, attributed to its efficient generation of radical species essential for oxidation. Other oxidants, such as benzoyl peroxide (BZ2O2), yielded a moderate 74%, while di-tert-butyl peroxide (DTBP) and di-cumyl peroxide (DCP) showed lower reactivity, yielding 54% and 51% of the product, respectively (Table S6†). Additionally, oxidant loading screening (Table S7†) demonstrated that an oxidant loading of 2.0 equivalents maximized the yield (94%), ensuring a balanced efficiency in oxidant utilization. Substrate ratio screening (Table S8†) further elucidated the significance of stoichiometry, with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 between the amine and aldehyde providing optimal conditions for maximizing product formation, with yields reaching 95%. Collectively, these optimization endeavours underscore the intricate interplay of various parameters in oxidative C–N bond formation. A systematic exploration of catalysts, temperature, solvent, reaction time, oxidant type and loading, and substrate ratio was undertaken to optimize the reaction conditions. This investigation not only refined the parameters but also provided critical insights into the individual and combined effects of these variables, elucidating the synergistic interaction between MIL-117 and [(n-C4H9)4N]4[Mo8O26] in the composite. Notably, this study highlights a significant improvement in the C–N bond formation yield when both components are employed in tandem. While MIL-117 and [(n-C4H9)4N]4[Mo8O26] individually achieve yields of 13% and 39%, respectively, their combination in the composite results in a remarkable yield of 95%. This dramatic enhancement underscores the synergistic interaction, demonstrating that the cooperative behavior between MIL-117 and [(n-C4H9)4N]4[Mo8O26] significantly boosts catalytic performance, far surpassing the efficacy of either.
5 between the amine and aldehyde providing optimal conditions for maximizing product formation, with yields reaching 95%. Collectively, these optimization endeavours underscore the intricate interplay of various parameters in oxidative C–N bond formation. A systematic exploration of catalysts, temperature, solvent, reaction time, oxidant type and loading, and substrate ratio was undertaken to optimize the reaction conditions. This investigation not only refined the parameters but also provided critical insights into the individual and combined effects of these variables, elucidating the synergistic interaction between MIL-117 and [(n-C4H9)4N]4[Mo8O26] in the composite. Notably, this study highlights a significant improvement in the C–N bond formation yield when both components are employed in tandem. While MIL-117 and [(n-C4H9)4N]4[Mo8O26] individually achieve yields of 13% and 39%, respectively, their combination in the composite results in a remarkable yield of 95%. This dramatic enhancement underscores the synergistic interaction, demonstrating that the cooperative behavior between MIL-117 and [(n-C4H9)4N]4[Mo8O26] significantly boosts catalytic performance, far surpassing the efficacy of either.
After optimizing the model reaction, the substrate scope was explored using a variety of primary aromatic, poly-aromatic, benzyl, and cyclic amines with benzaldehyde under the established conditions. The reaction proved versatile, yielding primary amides (Scheme 1) in good to excellent yields, regardless of the electronic nature of substituents on the aromatic amines. For instance, amines with electron-withdrawing groups (–Cl (6), –Br (7), and –NO2 (8)) resulted in products in high yields of 92%, 82%, and 81%, respectively, demonstrating the reaction's robustness against different electronic effects. Furthermore, the reaction conditions with the Mo8O26@MIL-117 catalyst performed well with sterically hindered amines. 2,6-Diisopropyl amine (11), which features bulky substituents, produced a slightly lower yield (64%) than less sterically hindered substrates due to the increased steric hindrance. Additionally, benzylamine (13) and cyclic aliphatic amines, such as cyclohexylamine (14), delivered yields of 75% and 62%, respectively, showcasing the catalyst's efficacy with various amine structures. Lastly, the catalytic system efficiently facilitated reactions with polyaromatic amines. Specifically, naphthylamine (12) combined with benzaldehyde to yield 78%, demonstrating the catalyst's capability to handle more complex aromatic systems effectively. This underscores the versatility and robustness of the Mo8O26@MIL-117 catalyst in promoting oxidative C–N bond formation across a wide range of substrates.
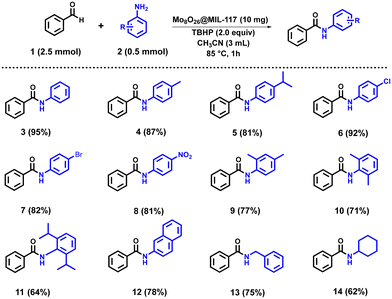 | ||
| Scheme 1 Oxidative C–N bond formation with benzaldehyde and primary amines catalysed by Mo8O26@MIL-117 (2 mmol Mo8O264− loading). Isolated yield in brackets. | ||
Furthermore, the Mo8O26@MIL-117 catalyst demonstrated remarkable efficiency not only with primary amines but also with secondary amines. Under the same optimized conditions, secondary amines such as piperidine, morpholine, and piperazine yielded secondary amides in good yields. Specifically, piperidine (15) yielded 74%, morpholine (16) gave 77%, and piperazine (17) achieved a 75% yield. These results indicate the catalyst's versatility and durability across different types of amines, successfully facilitating oxidative C–N bond formation with both primary and secondary amines (Scheme 2). Moreover, when comparing the performance of Mo8O26@MIL-117 to that of other MOFs or MOF-based composites (Table 2), our catalyst consistently shows superior yields in oxidative C–N bond formation. These results highlight the unique catalytic properties of the Mo8O26@MIL-117 composite, making it a highly effective catalyst for useful organic transformations. The potential of the material as a benchmark catalyst for oxidative C–N bond formation reactions offers a stout and versatile solution.
 | ||
| Scheme 2 Oxidative C–N bond formation with benzaldehyde and secondary amines catalysed by Mo8O26@MIL-117 (2 mmol Mo8O264− loading). Isolated yield in brackets. | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Mo[Mo5O18] composite, trapping Mo5O182− anions, and used it as a nano-catalyst in the Paal–Knorr reaction for pyrrole synthesis. Despite its good catalytic activity, the application of the catalyst is hindered by a complicated, multi-step synthesis process and high costs of preparation.59 These challenges could be effectively addressed by employing the Mo8O26@MIL-117 catalyst.
Mo[Mo5O18] composite, trapping Mo5O182− anions, and used it as a nano-catalyst in the Paal–Knorr reaction for pyrrole synthesis. Despite its good catalytic activity, the application of the catalyst is hindered by a complicated, multi-step synthesis process and high costs of preparation.59 These challenges could be effectively addressed by employing the Mo8O26@MIL-117 catalyst.
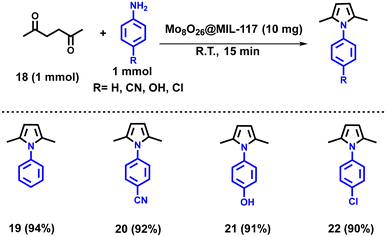
Here, we have utilized the Mo8O26@MIL-117 composite material for the Paal–Knorr synthesis. In the quest for optimal synthesis of pyrrole derivatives via the Paal–Knorr reaction, a systematic optimization endeavour was undertaken. This rigorous process aimed to elevate yield, while unravelling pivotal reaction parameters essential for fine-tuning the reaction conditions. Catalyst screening (Table S9†) revealed that Mo8O26@MIL-117 catalysts, particularly with 2 mmol Mo8O264− loading, exhibited superior performance, yielding up to 94% at a loading of 10 mg. Solvent screening (Table S10†) highlighted the solvent-free condition as optimal, achieving a remarkable yield of 94%, indicating enhanced efficiency without solvent interference. Time screening (Table S11†) further refined the reaction conditions, demonstrating that a reaction time of 15 minutes yielded optimal results with a consistent 94% yield. Substrate ratio screening (Table S12†) reinforced the versatility of the reaction, showcasing high yields (93–94%) across various aniline to 2,5-hexadione ratios. These systematic optimization studies provide valuable insights into enhancing the efficiency and reliability of pyrrole synthesis.
After meticulously optimizing the model reaction, an extensive exploration of the substrate scope was conducted, employing various primary aromatic amines alongside 2,5-hexazinone under the established conditions (Scheme 3). Notably, the catalyst exhibited exceptional performance across a spectrum of electron-releasing (–OH (22)) and electron-withdrawing groups (–CN (21) and –Cl (23)), yielding 92%, 91%, and 92%, respectively. This comprehensive investigation demonstrated the versatility of the reaction, consistently delivering well to excellent yields irrespective of the electronic properties of the substituents on the aromatic amines. Furthermore, upon comparing the efficacy of Mo8O26@MIL-117 with that of other MOFs or MOF-based composites and other composites as listed in Table 3, our catalyst consistently demonstrates superior performance in Paal–Knorr synthesis, yielding higher product yields. This underscores the exceptional catalytic capabilities of the Mo8O26@MIL-117 composite, establishing it as a highly efficient catalyst for such transformations in recent investigations.
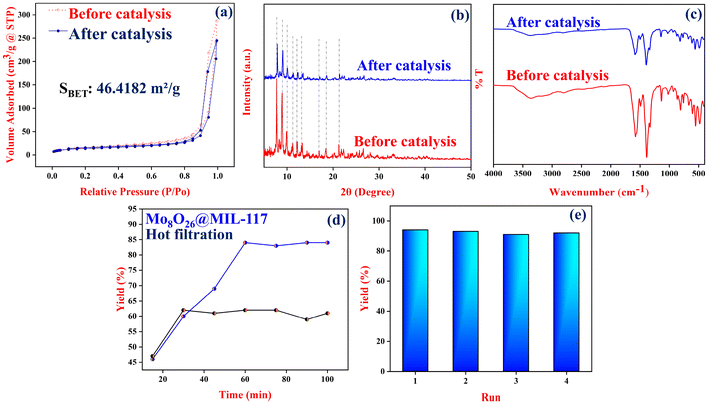
2.3 Catalyst reusability, stability and leaching study
The Mo8O26@MIL-117 catalyst exhibits exceptional stability and reusability, as evidenced by its performance in amide bond formation reactions. A series of recycling experiments were conducted to assess the catalyst's strength. After each catalytic run, the material was separated by centrifugation, thoroughly washed with ethanol, and dried at 80 °C before being reused in subsequent reactions with fresh substrates. Remarkably, as shown in Fig. 7e, the catalyst consistently achieved high yields of the amide product over four consecutive cycles without any significant decline in catalytic activity. This indicates the durable performance and resilience of the catalyst under the reaction conditions. Further structural analysis of the catalyst was carried out to confirm post-catalysis integrity. PXRD patterns demonstrated that the characteristic peaks of the MIL-117 framework in the Mo8O26@MIL-117 structure remained largely unchanged after catalysis, as illustrated in Fig. 7b. This finding underscores that the MIL-117 framework maintains its structural integrity even after the catalytic process, indicating the stability of the composite. Additionally, the N2 adsorption–desorption isotherm revealed a decrease in the surface area of the Mo8O26@MIL-117 catalyst after the catalytic cycles (Fig. 7a). Despite this reduction, the isothermal behaviour classified by the IUPAC remained consistent with that of the fresh composite material even after the fourth catalytic cycle. This consistency suggests that the tubular channels of the catalyst are preserved, and the overall structure of Mo8O26@MIL-117 does not collapse during the catalytic process. SEM analysis further supported the stability of the catalyst, and SEM images showed that the morphology of the microcrystallites of Mo8O26@MIL-117 remained unchanged post-catalysis (Fig. 8a). This observation corroborates that the catalytic reaction does not impact the physical structure of the composite, maintaining the integrity of both the MIL-117 framework and the overall composite throughout the catalytic process. Fig. 8b displays EDX mapping, revealing the consistent dispersion of essential elements throughout the catalyst after catalysis. FT-IR analysis was performed on the Mo8O26@MIL-117 composite after the fourth cycle to validate the structural stability further. The FT-IR spectra of the used catalyst closely matched those of the fresh catalyst, as depicted in Fig. 7c. In summary, the Mo8O26@MIL-1177 catalyst not only demonstrates high catalytic efficiency but also maintains its structural and morphological stability under repeated catalytic cycles. This robust performance makes it a highly effective and reliable catalyst for amide bond formation and potentially other catalytic applications.To evaluate the potential leaching of Mo8O264− from MIL-117 during catalysis, the reaction mixture of amide synthesis with Mo8O26@MIL-117 was filtered after 30 minutes, and the reaction progress was monitored thereafter (Fig. 7d). There was no observable progress in the reaction, suggesting no solid catalyst leakage into the reaction mixture. This confirms the heterogeneous nature of Mo8O26@MIL-117. Furthermore, post-filtration ICP-OES analysis revealed no molybdenum presence in the reaction mix (Table S13†), indicating effective trapping of Mo8O264− within the composite and the preservation of its structural integrity.
2.4 Dye removal
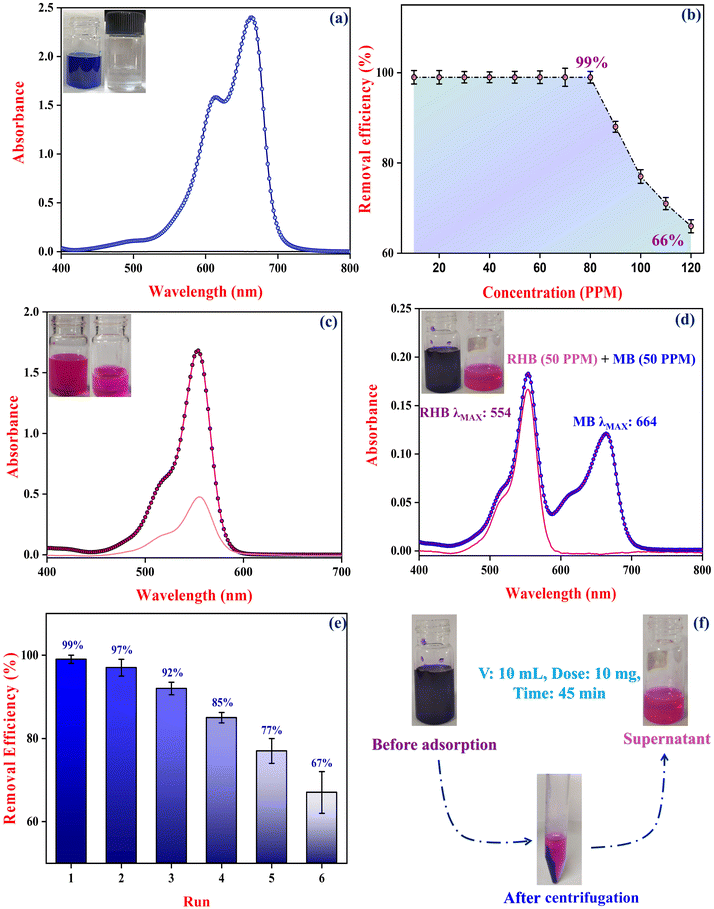
POMs have shown potential in dye removal applications, but their practical utility is often compromised due to several inherent limitations.62 These include poor water stability, limited surface areas (typically less than 10 m2 g−1), and high solubility in aqueous solutions, which complicate their recycling and reuse.24 MOFs often face challenges such as poor water stability and pore blockage, which can hinder their effectiveness in dye removal. However, integrating POMs into MOFs to create POM@MOF composites helps to address these issues, resulting in a material with superior dye removal efficiency.23 This synergy makes POM@MOF composites a potent solution for overcoming the limitations of their components.24 For instance, while MOFs like MIL-117 are effective in adsorption, their performance can be significantly enhanced when combined with Mo8O264−, leading to improved functionality and stability. We investigated the adsorption properties of the Mo8O26@MIL-117 composite and compared its performance to that of the parent MIL-117 framework. Our study specifically focused on the removal of cationic dyes, MB and RHB, from aqueous solutions. The studies also focused on the selective removal efficiency of MB over RHB, providing insights into the composite's preferential adsorption behaviour for these dyes.For the adsorption experiments, solutions were prepared with MB concentrations varying from 10 to 120 ppm. The adsorbent, either 10 milligrams of MIL-117 or Mo8O26@MIL-117, was introduced into 10 mL of these dye solutions (Fig. S6†). The mixtures were then thoroughly stirred to ensure adequate interaction between the dye molecules and the adsorbent. After a set contact time, the mixtures were centrifuged to separate the adsorbent from the supernatant, which contained any unabsorbed dye. To quantify the residual MB dye concentration in the supernatant post-adsorption, UV-visible spectroscopy was used (Fig. 9(a)). This method allowed for precise measurement of the reduction in dye concentration compared to the initial levels. These findings reveal a notable difference in performance between the MIL-117 framework and the Mo8O26@MIL-117 composite, underscoring the advantages of incorporating Mo8O264− anions into the MIL-117 structure. The incorporation of Mo8O264− anions significantly enhances the surface charge of the Mo8O26@MIL-117 composite. The zeta potential measurement for the composite with a 2 mmol loading of Mo8O264− is −60.3 mV, indicating a highly negative surface charge (Fig. S7†). This highly negative charge enhances electrostatic interactions with positively charged MB molecules, thereby promoting adsorption. In contrast, the composite with a lower Mo8O264− loading (1 mmol) exhibits a zeta potential of −30.4 mV. The Mo8O26@MIL-117 (2 mmol) composite achieves an impressive 99% dye removal efficiency for 80 ppm. In comparison, the parent MIL-117 framework also attains 99% removal efficiency for MB, but only at concentrations up to 50 ppm (Fig. S8(a)†). The Mo8O26@MIL-117 composite, however, demonstrates superior performance by maintaining this high efficiency even at elevated dye concentrations of up to 80 ppm. This ability to effectively adsorb MB at higher concentrations highlights the significant enhancement in adsorption capacity provided by the incorporation of Mo8O264− anions. To assess the adsorption of RHB, 10 milligrams of each adsorbent, MIL-117 and the Mo8O26@MIL-117 composite, were tested in 10 mL of a 50 ppm RHB dye solution. The results showed that MIL-117 removed 52% of RHB within 45 minutes (Fig. S8(c)†), whereas the Mo8O26@MIL-117 composite achieved an enhanced removal efficiency of 72% in the same duration (Fig. 9(c)). This study highlights the significant advantages of incorporating Mo8O264− anions into MIL-117 structures for MB and RHB removal.
2.5 Effect of pH, salts, and solvent on MB adsorption onto Mo8O26@MIL-117
The influence of pH, salts, and solvent on MB adsorption by the Mo8O26@MIL-117 composite was systematically investigated using a batch adsorption process (MB: 80 ppm, Mo8O26@MIL-117: 10 mg, and V: 10 mL).2.6 Kinetics and thermodynamics of MB adsorption onto Mo8O26@MIL-117
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k vs. 1/T (Fig. 11(b)). The enthalpy change (ΔH = 23.94 kJ mol−1) suggests chemisorption, as this value is typically associated with the energy range for chemical adsorption, indicating the formation of bonds between the adsorbate and adsorbent. The positive entropy change (ΔS° = 110.46 J mol−1 K−1) reflects increased randomness at the solid–liquid interface, likely due to the displacement of water molecules and a less ordered arrangement of MB molecules on the composite. Collectively, these thermodynamic findings indicate that MB adsorption onto Mo8O26@MIL-117 is spontaneous, endothermic, and predominantly chemisorptive, involving strong interactions between the dye molecules and the composite material.
k vs. 1/T (Fig. 11(b)). The enthalpy change (ΔH = 23.94 kJ mol−1) suggests chemisorption, as this value is typically associated with the energy range for chemical adsorption, indicating the formation of bonds between the adsorbate and adsorbent. The positive entropy change (ΔS° = 110.46 J mol−1 K−1) reflects increased randomness at the solid–liquid interface, likely due to the displacement of water molecules and a less ordered arrangement of MB molecules on the composite. Collectively, these thermodynamic findings indicate that MB adsorption onto Mo8O26@MIL-117 is spontaneous, endothermic, and predominantly chemisorptive, involving strong interactions between the dye molecules and the composite material.
2.7 Adsorbent stability
The stability of the composite material in water is important, particularly considering the toxicity of indium (In)66 and molybdenum (Mo)67 to aquatic ecosystems. Prolonged exposure of these elements to water bodies can harm flora and fauna. In this study, the composite was immersed in water and stirred for 72 hours to evaluate its stability. Following the stirring process, the supernatant was separated by centrifugation and analysed using ICP-OES to detect any leaching of Mo and In (Fig. S10†). The results revealed no Mo and In leaching (Table S16†). Furthermore, the MIL-117 component exhibited remarkable water stability, with negligible signs of degradation or leaching for 72 hours. This finding underscores the resilience of the MIL-117 component in aquatic environments, making it highly suitable for applications in wastewater treatment where high-water stability and minimal ion leaching are crucial. This stability is critical to prevent the release of toxic metals into water systems, thereby safeguarding environmental health.2.8 Adsorbent reusability
The reusability of the Mo8O26@MIL-117 composite was rigorously evaluated by examining its ability to remove MB (10 mL, 80 PPM) dye over multiple cycles. After each adsorption cycle, desorption of MB was performed using 0.2 M NaOH. The composite was then recovered through centrifugation and reactivated under vacuum at 100 °C (Fig. S11†). This process was repeated across five cycles to determine the durability and efficiency of the composite in reusability scenarios. Initially, the Mo8O26@MIL-117 composite exhibited a high removal efficiency of 99% in the first cycle. Remarkably, its performance decreased in efficiency, reaching 67% by the sixth cycle (Fig. 9(e), S9, and S10†). Such good performance over multiple cycles underscores the composite's strength and highlights its potential for sustained use in adsorption processes. Even after several cycles, the observed high removal efficiencies indicate that the Mo8O26@MIL-117 composite is well-suited for repeated applications in wastewater treatment and other scenarios where effective reusability is critical.2.9 Adsorbent dye removal mechanism
The removal mechanism of MB by Mo8O26@MIL-117 has been investigated using XPS. Mo8O26@MIL-117 follows a similar adsorption behaviour to the MIL-117 solid in batch adsorption processes. The MB removal mechanism involves several robust potential pathways. One plausible explanation is the pivotal role played by electrostatic interactions between the cationic dyes MB and the MIL-117 and Mo8O264− within the structure. Additionally, other interactions, such as hydrogen bonding and π–π stacking between the benzene ring of MIL-117 and the dye molecules, cannot be disregarded. The overall adsorption mechanism is intricate, demanding a more comprehensive understanding of the interaction between MB and Mo8O26@MIL-117. The XPS survey scan verified the presence of a sulphur 2p peak, indicating the presence of MB (Fig. 12(a)). The high-resolution C 1s spectra of Mo8O26@MIL-117 obtained before and after MB adsorption exhibit the same three peaks at 286.3 eV, 287.8 eV, and 288.8 eV BEs, corresponding to C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and O–C
O, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. In the spectra obtained after adsorption, two additional peaks appear at 286.5 eV and 286.0 eV, indicating the presence of C–S and C–N bonds of MB (Fig. 12(b)). The observed slight increase in BE is likely attributed to alterations in the chemical environment stemming from the adsorption of MB onto the tubular channels of the composite. The XPS spectra for both O 1s (Fig. 12(c)) and Mo 3d (Fig. 12(d)), obtained before and after adsorption, show distinctive peaks at varying BEs. Before adsorption, the XPS analysis of Mo8O26@MIL-117 reveals the O 1s peak at 530.6 eV. However, after the adsorption of MB, this peak shifts to 531.3 eV. Similarly, the Mo 3d peaks, initially at 531.6 eV and 534.9 eV, shift to 532.5 eV and 535.7 eV after adsorption. These slight shifts in BEs suggest that oxygen and molybdenum interact with MB molecules. The changes indicate a reduction in electron density and modifications in the chemical environment within the tubular channels of the composite, facilitating the adsorption process. Also, the XPS data for In 3d reveal a shift in BEs from 444.6 eV and 452.2 eV to 446.7 eV and 454.3 (Fig. 12(e)), indicative of a change in the chemical environment and a reduction in electron density from the indium nucleus due to interactions with guest molecules.
O, respectively. In the spectra obtained after adsorption, two additional peaks appear at 286.5 eV and 286.0 eV, indicating the presence of C–S and C–N bonds of MB (Fig. 12(b)). The observed slight increase in BE is likely attributed to alterations in the chemical environment stemming from the adsorption of MB onto the tubular channels of the composite. The XPS spectra for both O 1s (Fig. 12(c)) and Mo 3d (Fig. 12(d)), obtained before and after adsorption, show distinctive peaks at varying BEs. Before adsorption, the XPS analysis of Mo8O26@MIL-117 reveals the O 1s peak at 530.6 eV. However, after the adsorption of MB, this peak shifts to 531.3 eV. Similarly, the Mo 3d peaks, initially at 531.6 eV and 534.9 eV, shift to 532.5 eV and 535.7 eV after adsorption. These slight shifts in BEs suggest that oxygen and molybdenum interact with MB molecules. The changes indicate a reduction in electron density and modifications in the chemical environment within the tubular channels of the composite, facilitating the adsorption process. Also, the XPS data for In 3d reveal a shift in BEs from 444.6 eV and 452.2 eV to 446.7 eV and 454.3 (Fig. 12(e)), indicative of a change in the chemical environment and a reduction in electron density from the indium nucleus due to interactions with guest molecules.
3. Conclusion
A polyoxometalate-loaded MOF composite material, i.e. Mo8O26@MIL-117, has been developed successfully via an encapsulation method and a systematic study on its structural and chemical properties has been done. The confirmation of Mo8O264− anion encapsulation within MIL-117 tubular channels was attained through various spectral techniques and analytical methods like FTIR, XRD, FE-SEM, N2 adsorption–desorption isotherms, EDX, XPS, and TGA. The analyses collectively confirmed the integrity of the MIL-117 framework post-encapsulation, highlighting the effective trapping of Mo8O264− anions. Furthermore, Mo8O26@MIL-117 demonstrated efficient catalytic activity in oxidative C–N bond formation and Paal–Knorr pyrrole synthesis across diverse substrates, including aromatic and sterically hindered amines, yielding excellent product yields. The catalyst exhibited robust stability and recyclability over multiple cycles. The Mo8O26@MIL-117 composite significantly improves dye removal efficiency, particularly for MB, demonstrating higher capacity, stability, and reusability compared to MIL-117 alone. Its selective adsorption for MB over RHB, minimal Mo8O264− leaching, and robust performance over multiple cycles make it a promising material for sustainable and efficient wastewater treatment applications. Detailed XPS of the composite after MB adsorption elucidated intricate changes in chemical environments upon MB adsorption, revealing the formation of C–S and C–N bonds. These insights deepen our understanding of MB adsorption mechanisms within the tubular channels of the composite material, reinforcing its efficacy in environmental remediation contexts.Author contributions
K. M. meticulously researched and crafted the comprehensive draft. Additionally, R. J. and S. K. provided guidance as mentors, offering their expertise and granting final approval for the manuscript's final version.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. J. thanks Gujarat Council on Science & Technology (Project No. GUJCOST/STI/2021-22/3877) for providing financial support. K. M. is thankful to the SVNIT Surat for providing a fellowship for his research work. We also extend our gratitude and acknowledge M.Sc. dissertation student, Mr Amarendra Singh for assisting in catalytic work.References
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Reticular synthesis and the design of new materials, Nature, 2003, 423(6941), 705–714 CrossRef CAS PubMed
.
- D. J. Tranchemontagne, J. L. Mendoza-Cortés, M. O'Keeffe and O. M. Yaghi, Secondary building units, nets and bonding in the chemistry of metal–organic frameworks, Chem. Soc. Rev., 2009, 38(5), 1257–1283, 10.1039/B817735J
.
- L. Yan, H.-T. Zheng, L. Song, Z.-W. Wei, J.-J. Jiang and C.-Y. Su, Microporous Fluorinated MOF with Multiple Adsorption Sites for Efficient Recovery of C2H6 and C3H8 from Natural Gas, ACS Appl. Mater. Interfaces, 2024, 16(5), 6579–6588 CrossRef CAS
.
- K. Maru, S. Kalla and R. Jangir, Efficient Dye Extraction from Wastewater Using Indium-MOF-Immobilized Polyvinylidene Fluoride Membranes with Selective Filtration for Enhanced Remediation, Langmuir, 2024, 40(15), 8144–8161 CrossRef CAS PubMed
.
- K. Maru, S. Kalla and R. Jangir, MOF/POM hybrids as catalysts for organic transformations, Dalton Trans., 2022, 51(32), 11952–11986 RSC
.
- J. Juan-Alcañiz, E. V. Ramos-Fernandez, U. Lafont, J. Gascon and F. Kapteijn, Building MOF bottles around phosphotungstic acid ships: One-pot synthesis of bi-functional polyoxometalate-MIL-101 catalysts, J. Catal., 2010, 269(1), 229–241 CrossRef
.
- C. Xiao, X. Guo and J. Li, From nano- to macroarchitectures: designing and constructing MOF-derived porous materials for persulfate-based advanced oxidation processes, Chem. Commun., 2024, 60(33), 4395–4418 RSC
.
- L. Nie, Y. Yang, C. Fang, H. Chen and S. Xin, Co-doped metal–organic framework MIL-125(Ti) derivate for efficient adsorption of tetracycline hydrochloride from water, Appl. Surf. Sci., 2023, 640, 158390 CrossRef CAS
.
- G. Cai, P. Yan, L. Zhang, H.-C. Zhou and H.-L. Jiang, Metal–Organic Framework-Based Hierarchically Porous Materials: Synthesis and Applications, Chem. Rev., 2021, 121(20), 12278–12326 CrossRef CAS PubMed
.
- E. M. Abd El-Monaem, A. S. Eltaweil, G. M. El-Subruiti, M. S. Mohy-Eldin and A. M. Omer, Adsorption of nitrophenol onto a novel Fe3O4-κ-carrageenan/MIL-125(Ti) composite: process optimization, isotherms, kinetics, and mechanism, Environ. Sci. Pollut. Res., 2023, 30(17), 49301–49313 CrossRef CAS PubMed
.
- Q. Hu, J. Dong, Y. Chen, J. Yi, J. Xia, S. Yin and H. Li,
In situ construction of bifunctional MIL-125(Ti)/BiOI reactive adsorbent/photocatalyst with enhanced removal efficiency of organic contaminants, Appl. Surf. Sci., 2022, 583, 152423 CrossRef CAS
.
- M. R. Horn, A. Singh, S. Alomari, S. Goberna-Ferrón, R. Benages-Vilau, N. Chodankar, N. Motta, K. Ostrikov, J. MacLeod and P. Sonar,
et al., Polyoxometalates (POMs): from electroactive clusters to energy materials, Energy Environ. Sci., 2021, 14(4), 1652–1700 RSC
.
- Y. Zhang, Y. Li, H. Guo, Y. Guo and R. Song, Recent advances in polyoxometalate-based materials and their derivatives for electrocatalysis and energy storage, Mater. Chem. Front., 2024, 8(3), 732–768 RSC
.
- L. Vilà-Nadal and L. Cronin, Design and synthesis of polyoxometalate-framework materials from cluster precursors, Nat. Rev. Mater., 2017, 2(10), 17054 CrossRef
.
- H. N. Miras, L. Vilà-Nadal and L. Cronin, Polyoxometalate based open-frameworks (POM-OFs), Chem. Soc. Rev., 2014, 43(16), 5679–5699 RSC
.
- X. López, J. J. Carbó, C. Bo and J. M. Poblet, Structure, properties and reactivity of polyoxometalates: a theoretical perspective, Chem. Soc. Rev., 2012, 41(22), 7537–7571 RSC
.
- V. I. Parvulescu and H. García, Heterogeneous catalysis based on supramolecular association, Catal. Sci. Technol., 2018, 8(19), 4834–4857 RSC
.
- X. Zhong, Y. Lu, F. Luo, Y. Liu, X. Li and S. Liu, A Nanocrystalline POM@MOFs Catalyst for the Degradation of Phenol: Effective Cooperative Catalysis by Metal Nodes and POM Guests, Chem. – Eur. J., 2018, 24(12), 3045–3051 CrossRef CAS
.
- G. Paille, M. Gomez-Mingot, C. Roch-Marchal, M. Haouas, Y. Benseghir, T. Pino, M.-H. Ha-Thi, G. Landrot, P. Mialane and M. Fontecave,
et al., Thin Films of Fully Noble Metal-Free POM@MOF for Photocatalytic Water Oxidation, ACS Appl. Mater. Interfaces, 2019, 11(51), 47837–47845 CrossRef CAS
.
- N. V. Maksimchuk, K. A. Kovalenko, S. S. Arzumanov, Y. A. Chesalov, M. S. Melgunov, A. G. Stepanov, V. P. Fedin and O. A. Kholdeeva, Hybrid Polyoxotungstate/MIL-101 Materials: Synthesis, Characterization, and Catalysis of H2O2-Based Alkene Epoxidation, Inorg. Chem., 2010, 49(6), 2920–2930 CrossRef CAS PubMed
.
- Q. Han, L. Zhang, C. He, J. Niu and C. Duan, Metal–Organic Frameworks with Phosphotungstate Incorporated for Hydrolytic Cleavage of a DNA-Model Phosphodiester, Inorg. Chem., 2012, 51(9), 5118–5127 CrossRef CAS PubMed
.
- X. Tian, L. Hou, J. Wang, X. Xin, H. Zhang, Y. Ma, Y. Wang, L. Zhang and Z. Han, Novel fully reduced phosphomolybdates for highly efficient removal of inorganic hexavalent chromium and the organic dye methylene blue, Dalton Trans., 2018, 47(42), 15121–15130 RSC
.
- S. Zhang, F. Ou, S. Ning and P. Cheng, Polyoxometalate-based metal–organic frameworks for heterogeneous catalysis, Inorg. Chem. Front., 2021, 8(7), 1865–1899 RSC
.
- Y. Liu, C. Tang, M. Cheng, M. Chen, S. Chen, L. Lei, Y. Chen, H. Yi, Y. Fu and L. Li, Polyoxometalate@Metal–Organic Framework Composites as Effective Photocatalysts, ACS Catal., 2021, 11(21), 13374–13396 CrossRef CAS
.
- N. Aramesh, A. Reza Bagheri, Z. Zhang, B. Yadollahi and H. Kee Lee, Polyoxometalate-based materials against environmental pollutants: A review, Coord. Chem. Rev., 2024, 507, 215767 CrossRef CAS
.
- A. S. Cherevan, S. P. Nandan, I. Roger, R. Liu, C. Streb and D. Eder, Polyoxometalates on Functional Substrates: Concepts, Synergies, and Future Perspectives, Adv. Sci., 2020, 7(8), 1903511 CrossRef CAS
.
- B. J. S. Johnson and A. Stein, Surface Modification of Mesoporous, Macroporous, and Amorphous Silica with Catalytically Active Polyoxometalate Clusters, Inorg. Chem., 2001, 40(4), 801–808 CrossRef CAS PubMed
.
- W. Qi and L. Wu, Polyoxometalate/polymer hybrid materials: fabrication and properties, Polym. Int., 2009, 58(11), 1217–1225 CrossRef CAS
.
- R. Xue, Y.-S. Liu, M.-Y. Wang, H. Guo, W. Yang and G.-Y. Yang, Combination of covalent organic frameworks (COFs) and polyoxometalates (POMs): the preparation strategy and potential application of COF–POM hybrids, Mater. Horiz., 2023, 10(11), 4710–4723 RSC
.
- M. Lu, M. Zhang, J. Liu, T.-Y. Yu, J.-N. Chang, L.-J. Shang, S.-L. Li and Y.-Q. Lan, Confining and Highly Dispersing Single Polyoxometalate Clusters in Covalent Organic Frameworks by Covalent Linkages for CO2 Photoreduction, J. Am. Chem. Soc., 2022, 144(4), 1861–1871 CrossRef CAS PubMed
.
- X. Luo, F. Li, F. Peng, L. Huang, X. Lang and M. Shi, Strategies for Perfect Confinement of POM@MOF and Its Applications in Producing Defect-Rich
Electrocatalyst, ACS Appl. Mater. Interfaces, 2021, 13(48), 57803–57813 CrossRef CAS
.
- X. Li, L. Yang, Q. Liu, W. Bai, H. Li, M. Wang, Q. Qian, Q. Yang, C. Xiao and Y. Xie, Directional Shunting of Photogenerated Carriers in POM@MOF for Promoting Nitrogen Adsorption and Oxidation, Adv. Mater., 2023, 35(44), 2304532 CrossRef CAS
.
- T. Islamoglu, S. Goswami, Z. Li, A. J. Howarth, O. K. Farha and J. T. Hupp, Postsynthetic Tuning of Metal–Organic Frameworks for Targeted Applications, Acc. Chem. Res., 2017, 50(4), 805–813 CrossRef CAS PubMed
.
- K.-Y. Wang, J. Zhang, Y.-C. Hsu, H. Lin, Z. Han, J. Pang, Z. Yang, R.-R. Liang, W. Shi and H.-C. Zhou, Bioinspired Framework Catalysts: From Enzyme Immobilization to Biomimetic Catalysis, Chem. Rev., 2023, 123(9), 5347–5420 CrossRef CAS PubMed
.
- C. T. Buru and O. K. Farha, Strategies for Incorporating Catalytically Active Polyoxometalates in Metal–Organic Frameworks for Organic Transformations, ACS Appl. Mater. Interfaces, 2020, 12(5), 5345–5360 CrossRef CAS
.
- M. Samaniyan, M. Mirzaei, R. Khajavian, H. Eshtiagh-Hosseini and C. Streb, Heterogeneous Catalysis by Polyoxometalates in Metal–Organic Frameworks, ACS Catal., 2019, 9(11), 10174–10191 CrossRef CAS
.
- Q. An, Z. Xu, W. Shang, Y. Wang, X. Liu, D. Guo, M. Zeng and Z. Jia, Polyoxometalate-Based Metal–Organic Frameworks as the Solid Support to Immobilize MP-11 Enzyme for Enhancing Thermal and Recyclable Stability, ACS Appl. Bio Mater., 2022, 5(3), 1222–1229 CrossRef CAS PubMed
.
- B. D'Cruz, M. O. Amin and E. Al-Hetlani, Polyoxometalate-Based Materials for the Removal of Contaminants from Wastewater: A Review, Ind. Eng. Chem. Res., 2021, 60(30), 10960–10977 CrossRef
.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area, Science, 2005, 309(5743), 2040–2042 CrossRef
.
- P. Mialane, C. Mellot-Draznieks, P. Gairola, M. Duguet, Y. Benseghir, O. Oms and A. Dolbecq, Heterogenisation of polyoxometalates and other metal-based complexes in metal–organic frameworks: from synthesis to characterisation and applications in catalysis, Chem. Soc. Rev., 2021, 50(10), 6152–6220 RSC
.
- X.-S. Wang, L. Li, J. Liang, Y.-B. Huang and R. Cao, Boosting Oxidative Desulfurization of Model and Real Gasoline over Phosphotungstic Acid Encapsulated in Metal–Organic Frameworks: The Window Size Matters, ChemCatChem, 2017, 9(6), 971–979 CrossRef CAS
.
- H. Liu, L.-G. Gong, C.-X. Wang, C.-M. Wang, K. Yu and B.-B. Zhou, {Cu2SiW12O40}@HKUST-1 synthesized by a one-step solution method with efficient bifunctional activity for supercapacitors and the oxygen evolution reaction, J. Mater. Chem. A, 2021, 9(22), 13161–13169 RSC
.
- Y. Dong, J. Zhang, Y. Yang, L. Qiu, D. Xia, K. Lin, J. Wang, X. Fan and R. Fan, Self-Assembly of Hybrid Oxidant POM@Cu-BTC for Enhanced Efficiency and Long-Term Stability of Perovskite Solar Cells, Angew. Chem., Int. Ed., 2019, 58(49), 17610–17615 CrossRef CAS PubMed
.
- B.-H. Wang and B. Yan, Polyoxometalate-based metal–organic framework NENU-5 hybrid materials for photoluminescence tuning by introducing lanthanide ions and their functionalized soft ionogel/thin film, CrystEngComm, 2019, 21(7), 1186–1192 RSC
.
- C. T. Buru, P. Li, B. L. Mehdi, A. Dohnalkova, A. E. Platero-Prats, N. D. Browning, K.
W. Chapman, J. T. Hupp and O. K. Farha, Adsorption of a Catalytically Accessible Polyoxometalate in a Mesoporous Channel-type Metal–Organic Framework, Chem. Mater., 2017, 29(12), 5174–5181 CrossRef CAS
.
- Y. Benseghir, A. Lemarchand, M. Duguet, P. Mialane, M. Gomez-Mingot, C. Roch-Marchal, T. Pino, M.-H. Ha-Thi, M. Haouas and M. Fontecave,
et al. Co-immobilization of a Rh Catalyst and a Keggin Polyoxometalate in the UiO-67 Zr-Based Metal–Organic Framework: In Depth Structural Characterization and Photocatalytic Properties for CO2 Reduction, J. Am. Chem. Soc., 2020, 142(20), 9428–9438 CrossRef CAS PubMed
.
- L. Bromberg, Y. Diao, H. Wu, S. A. Speakman and T. A. Hatton, Chromium(III) Terephthalate Metal Organic Framework (MIL-101): HF-Free Synthesis, Structure, Polyoxometalate Composites, and Catalytic Properties, Chem. Mater., 2012, 24(9), 1664–1675 CrossRef CAS
.
- S. Bhattacharya, W. W. Ayass, D. H. Taffa, T. Nisar, T. Balster, A. Hartwig, V. Wagner, M. Wark and U. Kortz, Polyoxopalladate-Loaded Metal–Organic Framework (POP@MOF): Synthesis and Heterogeneous Catalysis, Inorg. Chem., 2020, 59(15), 10512–10521 CrossRef CAS PubMed
.
- M. Mazaj, C. Volkringer, T. Loiseau, V. Kaučič and G. Férey, Synthesis and crystal structure of a new MOF-type indium pyromellitate (MIL-117) with infinite chains of unusual cis connection of octahedra InO4(OH)2, Solid State Sci., 2011, 13(8), 1488–1493 CrossRef CAS
.
- M. Ayed, S. Thabet and A. Haddad, Crystal Structure and Physicochemical Properties of Two Supramolecular Compounds: (C4H8NH2)4[Mo8O26] and (NH4)Na2[AsIIIMo6O21(O2CCH2NH3)3]·8H2O, J. Inorg. Organomet. Polym. Mater., 2014, 24(2), 291–301 CrossRef CAS
.
- H. Jiang, H. Cheng, C. Zang, J. Tan, B. Sun and F. Bian, Photocatalytic aldehydes/alcohols/toluenes oxidative amidation over bifunctional Pd/MOFs: Effect of Fe-O clusters and Lewis acid sites, J. Catal., 2021, 401, 279–287 CrossRef CAS
.
- S. Jamalifard, J. Mokhtari and Z. Mirjafary, One-pot synthesis of amides via the oxidative amidation of aldehydes and amines catalyzed by a copper-MOF, RSC Adv., 2019, 9(39), 22749–22754 RSC
.
- J. Li, J. Jiao, J. Chang, M. Li and Q. Han, Visible-Light-Driven C–N Bond Formation by a Hexanickel Cluster Substituted Polyoxometalate-Based Photocatalyst, Inorg. Chem., 2021, 60(13), 10022–10029 CrossRef CAS PubMed
.
- B. Goel, V. Vyas, N. Tripathi, A. Kumar Singh, P. W. Menezes, A. Indra and S. K. Jain, Amidation of Aldehydes with Amines under Mild Conditions Using Metal-Organic Framework Derived NiO@Ni Mott-Schottky Catalyst, ChemCatChem, 2020, 12(22), 5743–5749 CrossRef CAS
.
- A. Dhakshinamoorthy and H. Garcia, Metal–organic frameworks as solid catalysts for the synthesis of nitrogen-containing heterocycles, Chem. Soc. Rev., 2014, 43(16), 5750–5765 RSC
.
- R. Khajuria, S. Dham and K. K. Kapoor, Active methylenes in the synthesis of a pyrrole motif: an imperative structural unit of pharmaceuticals, natural products and optoelectronic materials, RSC Adv., 2016, 6(43), 37039–37066 RSC
.
- K. Mezgebe, Y. Melaku and E. Mulugeta, Synthesis and Pharmacological Activities of Chalcone and Its Derivatives Bearing N-Heterocyclic Scaffolds: A Review, ACS Omega, 2023, 8(22), 19194–19211 CrossRef CAS PubMed
.
- H. Sudibyo, B. Budhijanto, D. V. Cabrera, A. Mahannada, L. Marbelia, D. J. Prasetyo and M. Anwar, Kinetic and Thermodynamic Evidence of the Paal–Knorr and Debus–Radziszewski Reactions Underlying Formation of Pyrroles and Imidazoles in Hydrothermal Liquefaction of Glucose–Glycine Mixtures, Energy Fuels, 2024, 38(4), 3343–3356 CrossRef CAS
.
- R. Keshavarz, M. Farahi, B. Karami, P. Gheibipour and A. Zarnegaryan, TiO2-coated graphene oxide-molybdate complex as a new separable
nanocatalyst for the synthesis of pyrrole derivatives by Paal-Knorr reaction, Arabian J. Chem., 2022, 15(5), 103736 CrossRef CAS
.
- N. T. S. Phan, T. T. Nguyen, Q. H. Luu and L. T. L. Nguyen, Paal–Knorr reaction catalyzed by metal–organic framework IRMOF-3 as an efficient and reusable heterogeneous catalyst, J. Mol. Catal. A: Chem., 2012, 363–364, 178–185 CrossRef CAS
.
- W. Zhang, M. Kauer, O. Halbherr, K. Epp, P. Guo, M. I. Gonzalez, D. J. Xiao, C. Wiktor, F. X. Liabrés i Xamena and C. Wöll,
et al. Ruthenium Metal–Organic Frameworks with Different Defect Types: Influence on Porosity, Sorption, and Catalytic Properties, Chem. – Eur. J., 2016, 22(40), 14297–14307 CrossRef CAS PubMed
.
- L. Qi, Y. Gong, M. Fang, Z. Jia, N. Cheng and L. Yu, Surface-Active Ionic-Liquid-Encapsulated Polyoxometalate Nanospheres: Construction, Self-Assembly, Adsorption Behavior, and Application for Dye Removal, ACS Appl. Nano Mater., 2020, 3(1), 375–383 CrossRef CAS
.
- X. Zhang, Z. Li, S. Lin and P. Théato, Fibrous Materials Based on Polymeric Salicyl Active Esters as Efficient Adsorbents for Selective Removal of Anionic Dye, ACS Appl. Mater. Interfaces, 2020, 12(18), 21100–21113 CrossRef CAS PubMed
.
- H. Zhao, Q. Xia, H. Xing, D. Chen and H. Wang, Construction of Pillared-Layer MOF as Efficient Visible-Light Photocatalysts for Aqueous Cr(VI) Reduction and Dye Degradation, ACS Sustainable Chem. Eng., 2017, 5(5), 4449–4456 CrossRef CAS
.
- M. Chethana, L. G. Sorokhaibam, V. M. Bhandari, S. Raja and V. V. Ranade, Green Approach to Dye Wastewater Treatment Using Biocoagulants, ACS Sustainable Chem. Eng., 2016, 4(5), 2495–2507 CrossRef CAS
.
- H.-F. Chang, P.-T. Yang, H.-W. Lin, K.-C. Yeh, M.-N. Chen and S.-L. Wang, Indium Uptake and Accumulation by Rice and Wheat and Health Risk Associated with Their Consumption, Environ. Sci. Technol., 2020, 54(23), 14946–14954 CrossRef CAS PubMed
.
- J. Yang, Z. Song, J. Ma and H. Han, Toxicity of Molybdenum-Based Nanomaterials on the Soybean–Rhizobia Symbiotic System: Implications for Nutrition, ACS Appl. Nano Mater., 2020, 3(6), 5773–5782 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Optimization of the reaction parameters of the catalytic reactions, tabulated comparison of the heterogeneous catalytic activity of Mo8O26@MIL-117 with other catalysts, PXRD pattern of MIL-117, reusability UV graph, and NMR spectrum (PDF). See DOI: https://doi.org/10.1039/d4dt02645d |
| This journal is © The Royal Society of Chemistry 2025 |