 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
ZIF-8 metal–organic frameworks and their hybrid materials: emerging photocatalysts for energy and environmental applications
Diptirani
Behera
,
Priyanka
Priyadarshini
and
Kulamani
Parida
 *
*
Centre for Nanoscience and Nanotechnology, Siksha ‘O’ Anusnadhan (Deemed to be University), Bhubaneswar, Odisha 751030, India. E-mail: kulamaniparida@soa.ac.in; paridakulamani@yahoo.com; Fax: +91-674-235064; Tel: +91-674-2351777
First published on 15th January 2025
Abstract
In the face of escalating environmental challenges such as fossil fuel dependence and water pollution, innovative solutions are essential for sustainable development. In this regard, zeolitic imidazolate frameworks (ZIFs), specifically ZIF-8, act as promising photocatalysts for environmental remediation and renewable energy applications. ZIF-8, a subclass of metal–organic frameworks (MOFs), is renowned for its large specific surface area, high porosity, rapid electron transfer ability, abundant functionalities, ease of designing, controllable properties, and remarkable chemical and thermal stability. However, its application as a standalone photocatalyst is limited by issues such as particle aggregation, poor water stability, and insufficient visible light absorption. By integrating ZIF-8 with various photoactive materials to form composite catalysts, these drawbacks can be mitigated, leading to enhanced photocatalytic efficiency. The review discusses the synthesis, properties, and applications of ZIF-8-based photocatalysts in light-driven H2 evolution, H2O2 evolution, CO2 reduction, and dye and drug degradation. It also highlights the challenges and future research directions in developing cost-effective, scalable, and environmentally friendly ZIF-8 composites for industrial applications. The potential of ZIF-8 composites to contribute to sustainable global energy solutions and environmental cleanup is significant, yet further exploration is required to harness their capabilities thoroughly.
1. Introduction
The world faces growing challenges related to environmental pollution, particularly water contamination, and the need for sustainable energy solutions. According to recent estimates, over 1.2 billion people lack access to sufficient drinking water, and increasing carbon emissions from fossil fuel use exacerbate global environmental concerns, leading to environmental issues that necessitate the search for an alternative sustainable energy supply.1–5 Additionally, agricultural runoff containing harmful chemicals such as pesticides, organic dyes, and industrial waste further pollutes water bodies, causing significant risks to human welfare and ecosystems.6–9 In response to these challenges, solar-light-driven photocatalysis has emerged as a promising, eco-friendly technology.6 This process utilizes semiconductors to produce reactive oxygen species (ROS) such as superoxide (˙O2−), peroxide (O2˙), and hydroxyl radicals (˙OH), which can degrade pollutants, reduce CO2 into hydrocarbon fuels, and address various environmental issues.10–13 These charge carriers facilitate the production of ROSs, which are involved in the degradation of inorganic and organic pollutants, and the reduction of CO2 to hydrocarbon fuels.14–16 Research has extensively explored semiconductor-based heterogeneous photocatalysis driven by direct solar energy or other light sources using materials such as g-C3N4,17–19 perovskites,20 quantum dots,21 TiO2,22 Bi2WO6,23 CuO,24 ZnO,25 CdS,26 ZnS27 and Fe3O4.28 However, photocatalytic applications face challenges such as large bandgaps, fast recombination of photogenerated charge carrier pairs, photocorrosion, limited solar energy application, and low recyclability, all of which restrict their photocatalytic performance.29–31 Therefore, it is essential to develop innovative photocatalysts with inclusive applications.Over the last several decades, there has been an extensive surge in attention to MOFs as encouraging photocatalysts attributed to their extensive surface area, elevated porosity, and adjustable structures, and they have emerged as promising microporous crystalline materials within the coordination polymer family.32–34 MOFs consist of inorganic metal components linked by organic materials to create one-dimensional, two-dimensional, or three-dimensional micro- or nanoporous systems. With their unique structures and properties, MOFs have found applications across various fields, including photocatalysis, adsorption, clean energy generation, electronics, energy storage, and medical applications.35–38 MOF-templated photocatalysts are particularly valued for their high surface area, which enhances active site exposure, mass transfer, and charge carrier transport.39–41 Their tunable properties, superior adsorption capacity, flexible frameworks, and multifunctionality make MOFs highly promising as porous photocatalysts.42,43 MOFs are characterized by their crystalline nature and exceptional porosity.44 With impressive surface areas exceeding 6000 m2 g−1 and pore diameters ranging from 5 to 25 angstroms, MOFs exhibit nearly 90% of free volumes.45 Additionally, their band gaps render them responsive to light, enabling them to utilize solar energy effectively.46 Their sizeable specific surface area facilitates an even and extensive dispersion of active species, benefiting the catalyst's performance.47 Moreover, MOFs’ elevated porosity enhances their ability to adsorb and absorb gases, and the pores can house active species, protecting them from deactivation due to agglomeration.48
By modifying synthesis conditions, various functional MOFs can be created due to their highly adaptable structures. ZIFs are attracting considerable attention because of their outstanding chemical and thermal resistance, maximum yield, modifiable micropore shapes and sizes, and flexible design and functionality.49,50 One notable example of ZIFs is ZIF-8, renowned for its excellent chemical and thermal stability. ZIF-8 comprises Zn(II) ions and 2-methylimidazole ligands. Based on the Hard and Soft Acids and Bases (HSAB) theory, Zn(II) ions create robust coordination bonds with azolate ligands, resulting in the bond between Zn(II) and 2-methylimidazole being among the most stable coordination interactions.51,52 ZIF-8 offers advantages such as an extensive surface area, adjustable pore sizes, active channels, flexible chemical structures, and intense hydrophobicity. ZIF-8's synergistic interaction between the Zn2+ metal center and imidazolate linker provides abundant active sites for photocatalysis, surpassing traditional semiconductors like TiO2 and ZnO, which rely on fixed material structures.53 The Zn2+ center serves as a redox site, facilitating electron transfer, charge stabilization, and reduced recombination, while the organic linker enhances photocatalytic interactions via coordination bonds, π–π stacking, and charge transfer. This hybrid system enables broader light absorption, tunable band gaps, and higher reactivity compared with conventional materials. The porous, high-surface-area framework of ZIF-8 supports guest molecule encapsulation, doping, and multifunctional catalysis, enhancing catalytic efficiency. Its structural flexibility and selective interactions make it ideal for targeted photocatalytic applications like CO2 reduction and pollutant degradation, while its stability under irradiation resists photodegradation, unlike TiO2 or CdS.54 Additionally, ZIF-8 can be prepared at RT with minimal expense. Nonetheless, it has certain limitations: (i) ZIF-8 often clumps together in water, leading to the growth of particle dimensions and resistance to transfer, which diminishes their adsorption capacity; (ii) extracting ZIF-8 nanomaterials from aqueous solutions is demanding, hindering their ability to be regenerated and reused; (iii) ZIF-8 has a limited response to visible light due to its broadband gap, which reduces its effectiveness under solar irradiation. Consequently, the application of ZIF-8 monomers is significantly limited.55 To address these issues, photoactive molecules or semiconductors are combined with ZIF-8 to create ZIF-8-based binary or ternary photocatalysts. The formation of these novel multifunctional composites or hybrids is achieved by carefully combining ZIF-8 with functional materials. Unlike single-component photocatalysts, while rapid electron–hole recombination is common, thoughtfully engineered composite photocatalysts can leverage the combined advantages of each individual component while mitigating their drawbacks, leading to enhanced photocatalytic efficiency.56 Various ZIF-8-based composites, including oxides,57 sulfides,46 C3N4,58 and metals,48 have been developed to date. These hybrids have demonstrated outstanding photocatalytic performance compared with pristine ZIF-8.
This review article offers a comprehensive analysis of ZIF-8 and its hybrid materials, highlighting their dual role in energy generation and environmental remediation. Unlike existing studies that often focus on either adsorption or specific photocatalytic applications, this work uniquely integrates insights across both domains. It demonstrates how ZIF-8-based materials can address clean energy needs, such as hydrogen and hydrogen peroxide production, while simultaneously tackling pollutant degradation and wastewater treatment. This dual approach positions ZIF-8 as a versatile platform for sustainable energy solutions and environmental protection. By bridging these critical areas, the review presents a unified framework for designing multifunctional photocatalytic systems that meet global energy and environmental challenges, marking a significant contribution to the field. The urgency of this work is underscored by the increasing need for sustainable solutions to global challenges like energy shortages and water pollution, making this review a timely and valuable contribution to the field. So this review aims to fill these gaps by providing an integrative analysis of the dual functionality of ZIF-8-based materials in energy generation and environmental remediation. It systematically summarizes recent progress in ZIF-8's structural engineering, highlighting strategies such as doping with metals and composite formation with semiconductors to enhance photocatalytic efficiency. The review further delves into the mechanisms driving ZIF-8's photocatalytic activities, offering a holistic understanding of its role in addressing global challenges in sustainability. Here we have outlined various preparation techniques for ZIF-8 and ZIF-8-based hybrid synthesis in photocatalysis, offering theoretical guidance and promoting the development of ZIF-8-based photocatalysts and summarizing recent advances in ZIF-8 and their hybrids for energy and environmental applications, with a focus on synergistic effects that enhance the stability and photocatalytic activity of these materials. Lastly, it discusses the current challenges and future directions. Overall, this review not only establishes a foundational database for ZIF-8 MOFs and their photocatalytic properties but also provides strategic insights for designing and synthesizing ZIF-based MOFs with possible applications within the discipline of photocatalysis (Fig. 1).
2. Overview of ZIF-8 metal–organic framework
ZIFs are a subclass of MOFs that have garnered widespread attention because of their combination of zeolite and MOF properties, such as extensive surface area, crystallinity, microporosity, and notable chemical and thermal stability.59,60 They are named for their zeolite-like structure, where the typical tetrahedral aluminum or silicon with bridging oxygen is replaced by zinc or silicon bridged by imidazolate. Both structures share a bond angle of 145°, as illustrated in Fig. 3a. Numerous ZIFs have been documented, typically featuring metal ions like cobalt or zinc connected through nitrogen to imidazolate linkers, forming adjustable nanopores.61 These pores are created by a CoN4 or ZnN4 tetrahedral complex, which allows guest molecules to enter for applications in catalysis and gas storage and enable molecular-level differentiation for gas separation. Imidazole is part of the heterocyclic compound family, characterized by a 5-membered ring structure. Its chemical formula is C3N2H4, consisting of 3 carbon atoms and 2 nitrogen atoms positioned at the 1st and 3rd locations in the ring (Fig. 3b). Due to the hydrogen atom's ability to link with either nitrogen atom, imidazole exists in two tautomeric states.62 Also referred to as 1,3-azole, imidazole is a strongly polar compound that dissolves easily in water and displays amphoteric properties, meaning it can act as both an acid and a base.63 In its solid form, imidazole is a white, colorless substance with a boiling point of 267.8 °C and a melting point of 88.9 °C.64Compared with zeolites, ZIFs possess greater flexibility owing to their inorganic–organic framework and functionalized imidazolate linkers. This flexibility allows easier control over their structures and pore sizes, enabling greater ease in modifying their properties. ZIF-8, one of the most extensively researched ZIFs, consists of zinc tetrahedral clusters linked by 2-methyl imidazolate. It is synthesized using zinc salt and 2-methyl imidazole, forming a sodalite (SOD) type zeolite structure that takes the shape of a truncated octahedron.59 These octahedrons interconnect to form a 3D framework, as shown in Fig. 3c. Initially synthesized by Yaghi et al. in 2006 through solvothermal methods, ZIF-8 and other ZIFs demonstrate significant chemical resistance in heated organic solvents, water, and alkaline aqueous solutions, a trait not observed in other MOFs (Fig. 3d).45,59
ZIF-8 possesses extremely high surface areas. In Yaghi's initial study, ZIF-8 demonstrated a Brunauer–Emmett–Teller (BET) surface area of 1630 m2 g−1, significantly exceeding any zeolite, providing abundant active sites that enhance the adsorption of reactant molecules like dyes, drugs, and water, thereby improving photocatalytic efficiency.59,65,66
Its microporous structure, with pore openings of 3.4 Å and pores with a diameter of 11.6 Å, is attributed to the methyl groups on the imidazolate linkers. As a result, pore sizes are roughly twice those of zeolites, while the apertures are analogous to the minimal molecular sieves, enabling selective adsorption and diffusion of target molecules, facilitating the trapping of reactants and intermediates to boost degradation and energy conversion processes.59
ZIF-8 is also thermally and chemically stable, enduring a wide range of temperatures and pH conditions, making it ideal for harsh photocatalytic environments, such as acidic or basic systems used in pollutant degradation.67
Although its wide bandgap (∼5.1 eV) limits direct photocatalysis under visible light, ZIF-8 serves as an excellent support material for narrow-bandgap semiconductors, for facilitating charge transfer when doping with metals (Cu, Ag) or combined with other photocatalytic materials like (TiO2, Cu2O, g-C3N4), improving light absorption and charge separation, and reducing the recombination of charge carriers.65,66
ZIF-8 is stable and insoluble in water and methanol, even in reflux conditions, and resists hydrolysis, which is uncommon among zinc-based MOFs. This exceptional stability is attributed to two factors: the strength of the bond between the imidazolate nitrogen and the metal ion, which is analogous to covalent solids.68 The hydrophobic nature of ZIF-8 pores, which deters water molecules and prevents their attack on the MIm4 (M = Zn) tetrahedral units, which contributes to ZIF-8's exceptional stability, is highly advantageous for various applications, allowing it to withstand harsh chemical and thermal environments.69
The structural tunability of ZIF-8, achieved through ligand modification or metal ion substitution, optimizes its electronic properties, surface chemistry, and light-harvesting abilities for targeted applications. Moreover, its metal center (Zn2+) facilitates electron transfer, enhancing the generation of ROSs such as hydroxyl radicals and superoxide anions, crucial for photocatalytic oxidation.70
Finally, ZIF-8 acts as a stabilizer or protective layer for embedded semiconductors, reducing photocorrosion and ensuring long-term activity, particularly for sensitive materials like Cu2O. These characteristics collectively make ZIF-8 a versatile and robust platform for photocatalytic applications in energy generation and environmental remediation (Fig. 2).71
3. Synthesis procedures
The synthesis strategies for creating ZIF-8 structures with desirable and valuable properties are influenced by various synthetic factors. The primary precursors for ZIF-8 fabrication are zinc metal and 2-methylimidazole. Several parameters need to be fine-tuned during the crystallization procedure, including the concentration of starting materials, temperature, solvent choice, and agitation rate. In this discussion, we explore several methods for synthesizing ZIF-8, including solvothermal, hydrothermal, ionothermal, sonochemical, mechanochemical, and electrochemical synthesis.3.1 Solvothermal
In the pioneering work by Yaghi et al., solvothermal synthesis was used to create twelve distinct ZIF crystals, designated as ZIF-1 to ZIF-12, which were successfully synthesized using a range of organic solvents, including methanol, N,N-dimethylformamide (DMF), N-methyl pyrrolidine (NMP) and N,N-diethylformamide (DEF).59,72 To aid the formation, organic amines like pyridine and triethylamine (TEA) were introduced as deprotonating agents into the DMF or DEF solvent. The development and architecture of ZIF-8 feature large spherical cages called sodalite cages connected by six-ring windows.73Methanol's versatility as a solvent in organic synthesis prompted various research groups to explore its use in ZIF synthesis, with additives such as high molecular weight, which was found to enhance ZIF-8 formation.74 Chen et al. later achieved the synthesis of ZIF-8 (initially referred to as MAF-4) using methanol as the reaction medium. This involved a meticulous layering process where a methanol solution containing 2-methylimidazole was gradually coated onto an aqueous ammonia solution, including Zn(OH)2, over the course of one month.75 Following this, Venna et al. introduced an approach for synthesizing ZIF-8 via the solvothermal method, with methanol as the solvent. They initiated the process by stirring the mixture for 5 minutes at room temperature before transferring it into a Teflon-lined autoclave. Subsequently, the solution was heated for 5 hours at 150 °C under autogenous pressure. This approach, while still utilizing comparatively elevated temperatures, significantly reduced the time required for synthesis and benefited from the cost-effectiveness of methanol as a solvent. The structure of ZIF-8 crystals was also examined. The average crystal size of ZIF-8 was approximately 45 nm, determined using Scherrer's equation from the amplifying of the X-ray diffraction (XRD) peaks shown in Fig. 3e. TEM analysis confirmed the formation of ZIF-8, with diffraction rings matching XRD peaks and indicating a body-centered cubic arrangement with a unit cell dimension measuring 16.48. TEM images revealed sharp, hexagonal crystals around 55 nm, with lattice fringes corresponding to the (222) planes, captured with minimal TEM exposure to prevent crystal amorphization.76 Further nanoscale ZIF-8 was synthesized using methanol by combining a solution of methanol containing Zn(NO3)2 and 2-methylimidazole under ambient conditions.77
 | ||
| Fig. 3 (a) The bridging angle in both ZIFs and zeolites is 145° [reproduced from ref. 78 with the permission from The University of Liverpool]. (b) Imidazole and certain derivatives of it are utilized as linkers [reproduced from ref. 79 with the permission from Elsevier, copyright 2023]. (c) The structure of ZIF-8, with yellow spheres representing the pore volume [reproduced from ref. 78 with the permission from The University of Liverpool]. (d) Sodalite structure of ZIF-8 formed from zinc metal and 2-methylimidazole [reproduced from ref. 45 with the permission from Elsevier, copyright 2017]. (e) XRD pattern of ZIF-8 crystals used for membrane fabrication [reproduced from ref. 76 with the permission from American Chemical Society, copyright 2010]. (f) FTIR spectra of 2-methylimidazole (1) and ZIF-8 sample (ILs-R1) (2) synthesized via the ionothermal method in [emim]Br [reproduced from ref. 80 with the permission from Elsevier, copyright 2015]. (g) Photocatalytic hydrogen generation through water splitting [reproduced from ref. 81 with the permission from Elsevier, copyright 2020]. (h) Solar-powered photocatalytic hydrogen generation performance, including hydrogen production rates (i), for various ZIF-8/modified g-C3N4 nanocomposites ZCN X [reproduced from ref. 82 with the permission from Elsevier copyright 2018]. | ||
To refine the synthesis process, Wiebcke et al. introduced sodium 1-methylimidazole/formate and n-butylamine as modulating ligands into methanol solutions, allowing for the regulation of ZIF-8 crystal size. This adjustment enabled the tuning of crystal sizes ranging from 10 to 65 nm for nanocrystals or approximately 1 mm for microcrystals. Through the utilization of ex situ scanning electron microscopy (SEM) and in situ energy dispersive X-ray diffraction (EDXRD), we have demonstrated that the solvothermal crystallization of ZIF-8, modulated by sodium formate, proceeds rapidly, resulting in the formation of large, high-quality single crystals within a brief timeframe (<4 hours).83,84 Alternative alcohols like isopropyl alcohol and ethanol also demonstrated effectiveness in this context. However, concerns over the costly, toxic, and flammable nature of organic solvents prompted a shift towards hydrothermal synthesis methods during this period.85
3.2 Hydrothermal
Researchers have opted for water, the most economical solvent available, in their endeavors, wherein a solution of zinc(II) nitrate is immersed in an excess amount of 2-methylimidazole (Hmim/Zn = 70).86 This approach aligns with the growing trend of adopting greener and simpler methods, reducing reliance on toxic organic solvents. Additionally, water-based processes for ZIF production have been optimized with additives. Under ambient temperature, ZIF-8 nanoparticles can be synthesized via the hydrothermal method, which involves three main stages: (a) dissolving zinc sources in demineralized water, (b) dissolving organic ligands in demineralized water, and (c) combining both the solutions to initiate the reaction and generate ZIF-8 particles.87 Ding and his co-workers reported that the hierarchically structured ZIF assembled through the hydrothermal technique exhibited a surface area of 304 m2 g−1, with dimensions of several micrometers in length, 1–2 micrometers in width, and around 300 nm in thickness.88 Song et al. capitalized on the benefits of the hydrothermal process and the unique properties of ZIF-8. The XRD pattern indicates that the product possesses a dense diamond (Zn) structure. As depicted in the SEM image, the product exhibits a characteristic plate-like morphology, with dimensions typically ranging from 3 to 5 μm. It is plausible to infer that an excess of water diminishes the overall solution's basicity, thereby retarding the deprotonation rate of Hmim.85Butova et al. (2017) demonstrated a streamlined approach in utilizing triethylamine (TEA) to synthesize a ZIF-8 catalyst in a single phase with remarkable properties: a large specific surface area of 1340 m2 g−1, and excellent thermal stability up to 470 °C in N2 and 400 °C in air, all achieved in water without the need for organic solvents.89 In a separate study, Oozeerally et al., in 2019, compared two synthesis methods for ZIF-8: hydrothermal synthesis at 140 °C and room temperature synthesis. The hydrothermal method yielded a compound with an exceptional specific surface area of 1967 m2 g−1, rendering it highly applicable.90 ZIFs synthesized via the hydrothermal method typically exhibit a particle size of about 100 nm, while those made using the solvothermal approach have a particle size closer to 30 nm. Several composites and heterojunctions incorporating ZIF-8, such as Zn2SnO4/ZIF-8,91 ZIF-8/NiFe2O4,92 and ZIF-8@TiO2 core–shell micron composites,93 have been effectively fabricated using the hydrothermal process.
3.3 Ionothermal
A novel synthesis approach known as ionothermal synthesis has emerged for the production of ZIF materials.94,95 Cooper et al. suggested an alternative solvothermal method for preparing ZIFs, utilizing an ionic liquid as the medium.96 This novel method, known as the ionothermal technique, is gaining significant interest among researchers working with various ZIFs. In ionothermal synthesis, the ionic liquid acts as a salt capable of melting at temperatures between 150 °C and 200 °C, the range typically used for synthesizing zeolites.94 This method utilizes environmentally friendly solvents such as eutectic mixtures and ionic liquids, which offer several advantages and exhibit fascinating properties that make them exceptional solvents. These properties include stability at elevated temperatures, high solubility for both non-polar and polar inorganic and organic compounds, low melting point, and suitable viscosity, along with the advantages of having minimal vapor pressure and being non-flammable. Additionally, a significant advantage of ionic liquids is their ability to be recycled and reused, contributing to sustainable practices.Morris and his team were the first to recognize the feasibility of employing ionic liquids in the synthesis of ZIFs. They documented the generation of four ZIFs, with structures either known or novel, under ionothermal parameters utilizing the ionic liquid 1-ethyl-3-methylimidazolium bis[(trifluoromethyl)sulfonyl]imide.97 Martins and coworkers introduced an ionic liquid synthesis approach utilizing the commercially available IL 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide.97 This synthesis methodology has garnered considerable interest among researchers due to its adaptability in an open system.
Wang and colleagues detailed the production of various ZIFs, notably ZIF-8, through the ionothermal method. The XRD spectra for the ZIF-8 and ZIF-67 samples corresponded closely with the simulated patterns derived from the sod-type single-crystal data. The FTIR spectrum of the ZIF-8 sample shows that the vibrational frequency for the N–H bond of 2-MIm at 3335–2500 and 1820 cm−1 has vanished, indicating complete deprotonation of 2-MIm upon coordination with Zn (Fig. 3f). This finding indicates that the samples produced by both rapid and controlled cooling methods were pure-phase sodalite-type ZIFs. The SEM micrographs of ZIF-8 and ZIF-67 samples created through rapid cooling had an aggregated spherical shape with average particle sizes of 0.5 μm and 0.35 μm, respectively. Conversely, samples cooled through programmed cooling displayed truncated rhombic dodecahedral shapes, with dimensions of 50 μm and 5 μm for ZIF-8 and ZIF-67, respectively. These findings underscored that samples cooled via programmed methods yielded larger and more uniform structures compared with those cooled rapidly.80
3.4 Sonochemical
The sonochemical method involves using ultrasound waves to facilitate chemical reactions, leading to reduced reaction times compared with solvothermal synthesis. It can also be called the sono-crystallization process. This synthesis offers an alternative approach to producing ZIFs, complementing conventional oven heating methods. This method not only facilitates nucleation but also ensures homogeneous dispersion of nucleation.98 The sonochemical method involves generating and collapsing bubbles in solutions, known as acoustic cavitation, which generates high pressures, temperatures, and rapid cooling and heating rates.99Research by Seoane et al. indicates that under ultrasound radiation at a frequency of 47 kHz and a power of 110 W, pure ZIF-8 frameworks were formed at very low temperatures (45–60 °C) and briefer durations (6–9 hours) compared with traditional solvothermal synthesis methods. By incorporating a synthetic activator and enhancing the substrate concentration (Zn2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF = 1
DMF = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.3), an 85% yield was achieved. Consequently, the sonochemical method proves more favorable for rapidly synthesizing smaller-sized ZIF-8 particles. SEM images of the ZIFs were prepared through sonocrystallization. By analyzing these SEM images, cumulative crystal size distributions were derived. Moreover, these crystals were observed to be smaller and exhibited a narrower particle size distribution compared with conventionally synthesized materials.100
9.3), an 85% yield was achieved. Consequently, the sonochemical method proves more favorable for rapidly synthesizing smaller-sized ZIF-8 particles. SEM images of the ZIFs were prepared through sonocrystallization. By analyzing these SEM images, cumulative crystal size distributions were derived. Moreover, these crystals were observed to be smaller and exhibited a narrower particle size distribution compared with conventionally synthesized materials.100
Recent work by Cho and his colleagues demonstrated the successful synthesis of abundant amounts of ZIF-8 employing a direct ultrasonic method in dimethylformamide in the midst of NaOH and triethylamine as a modulating agent. It exhibited comparable physiochemical properties, thermal stability, and chemical stability to traditional synthesized ZIF-8 but with a significantly reduced reaction time of 24 hours.101 Furthermore, they achieved scale-up to a 1-liter synthesis of ZIF-8 through this sonochemical synthesis process, showing potential for industrial applications.
3.5 Mechanochemical
Mechanochemistry, as an environmentally friendly and efficient approach, has exhibited remarkable potential, and it involves ball milling for the fabrication of ZIFs.102 By eliminating the need for solvents and salts during the synthesis method, the mechanochemical synthesis method can significantly decrease environmental pollution and minimize the incorporation of impurities within the crystal lattice. In 2006, the initial step towards obtaining nonporous Zn(IM)2 was achieved through manual milling of ZnO with a substantial excess of IM.103,104 Adams et al. demonstrated the generation of nonporous Zn(IM)2 products through a two-step mechanochemical process initiated from ZnCl2.104,105Recognizing the constraints associated with oxide-based precursors in direct grinding for ZIF synthesis, Friščić et al. introduced advanced mechanochemical approaches known as liquid-assisted grinding (LAG) and ion- and liquid-assisted grinding (ILAG). These techniques allowed the formation of ZIFs from a balanced 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of ZnO and various ligands, including imidazole, 2-ethylimidazole, and 2-methylimidazole, at ambient temperature.106,107 It was observed that adding a small-volume liquid phase enhanced molecular mobility, while salt additives accelerated ZIF formation.108 Moreover, the topology of the resulting ZIFs could be adjusted by varying the milling liquids (such as ethanol, DMF, and DEF) and salt enhancers (like NH4NO3, NH4CH3SO3, and (NH4)2SO4). Friščić et al. also monitored the ball mill grinding process, the synthesis of intermediates, and the transition of ZIF topologies through in situ high-energy synchrotron X-ray diffraction.109
2 molar ratio of ZnO and various ligands, including imidazole, 2-ethylimidazole, and 2-methylimidazole, at ambient temperature.106,107 It was observed that adding a small-volume liquid phase enhanced molecular mobility, while salt additives accelerated ZIF formation.108 Moreover, the topology of the resulting ZIFs could be adjusted by varying the milling liquids (such as ethanol, DMF, and DEF) and salt enhancers (like NH4NO3, NH4CH3SO3, and (NH4)2SO4). Friščić et al. also monitored the ball mill grinding process, the synthesis of intermediates, and the transition of ZIF topologies through in situ high-energy synchrotron X-ray diffraction.109
Lately, Tanaka and co-authors showcased a completely solvent-free mechanical transformation of ZnO to ZIF-8 free from any additional substances. They accomplished this by adding nano-sized ZnO powders (averaging 24 nm in particle size) along with MIm into a ball mill operating at 100 rpm.110 It was observed that larger nanoparticles retained untransformed ZnO, whereas smaller nanoparticles did not, indicating that nano-sized ZnO particles were more prone to converting into ZIF-8. The suggested mechanism involves the breaking down of nanoparticle agglomerates into smaller pieces, followed by the formation of complexes via the reaction between ZnO and MIm. The resulting ZIF-8 agglomerates into nanoparticles, which subsequently coat the ZnO. Besides crystalline ZIFs, Cheetham and his team demonstrated the synthesis of amorphous ZIFs through ball milling. Through grinding, various amorphous ZIF products can be synthesized.
3.6 Electrochemical
For more than a decade, researchers have employed the electrochemical synthesis method to produce MOFs.111 This method is categorized into cathode synthesis and anode synthesis based on the underlying reaction mechanisms. In the solution, anode synthesis involves the dissolution of metals at the anode, forming metal ions that subsequently self-assemble with organic linkers. On the other hand, cathode synthesis utilizes oxoacidates such as NO3− or ClO4− to create an alkaline gradient near the cathode. This gradient aids in the deprotonation of organic linkers, facilitating their self-assembly with incorporated metal ions.112Researchers have increasingly turned to electrochemical synthesis methods to produce ZIF-8 crystals, drawn by their numerous advantages: low energy requirements, mild operational conditions, rapid kinetics, significant yields, and simplicity of operation and equipment.113 Martinez Joaristi and co-authors utilized an anode synthesis method to fabricate ZIF-8 using solvents such as water, ethanol, methanol, and DMF. Notably, ZIF-8 synthesis exhibited flexibility in electrolyte choice (Na2SO4, KCl, or KNO3) and achieved reduced synthesis durations (<20 min).114
Worrall et al. successfully synthesized 5 ZIF coatings using the anodic method, incorporating 2 metals, zinc and cobalt, along with 4 different linkers: imidazole (IM), benzimidazole (bIM), 2-methylimidazole (mIM), and 2-ethylimidazole (eIM). PXRD confirmed the composition of the coatings which verified the synthesis of ZIF-8, that shares the stable SOD structure, when zinc and mIM were used as precursors. SEM was employed to analyze the quality and morphology of the ZIF coatings, and the SEM images suggested tiny crystals with unclear morphology constituting the coating. However, upon in-depth examination, the expected morphology of rhombic dodecahedral characteristic of ZIF-8 crystals became evident.115
The research group of Li proposed a three-electrode setup for an easy and adaptable electrochemical approach to synthesize ZIF-8, ZIF-67, as well as hybrid ZIF-67/ZIF-8 films. These films displayed coherent and consistent properties when deposited on conductive substrates (such as FTO, carbon cloth, and Ni foam) and nanostructured materials in just seconds at standard ambient temperature. An especially significant benefit was the ability to precisely regulate film thickness at the micron scale by adjusting the electrosynthesis time.116
The synthesis of ZIF-8 materials demonstrates the versatility and adaptability of different methods, each offering unique benefits and limitations. From traditional solvothermal and hydrothermal approaches to more novel ionothermal, sonochemical, mechanochemical, and electrochemical methods, advancements have been made to optimize crystal size, morphology, and yield while promoting environmental sustainability and scalability. Each method fine-tunes parameters such as solvents, temperatures, and additives, showcasing the diverse strategies available for ZIF-8 production, making it a promising material across various applications. Table 1 summarizes the advantages and disadvantages of the abovementioned synthetic procedures.99,117–123
| Methods | Advantages | Disadvantages |
|---|---|---|
| Solvothermal | • Readily obtained single crystals | • Requires soluble precursor reagents |
| • Allows controlled tuning of particle morphology | • Large amounts of solvent waste generated | |
| • Potentially hazardous handling of corrosive/explosive metal salts (chlorides, nitrates) in the presence of organic liquids | ||
| Hydrothermal | • Simple, easy and low-cost synthesis method | • The reaction takes a long time for high quality crystals |
| • Production of high quality 1D nanostructures | • Produces relatively large particle sizes, limiting surface area | |
| • The morphology is controlled by synthesis parameters | ||
| Ionothermal | • Eliminates the need for toxic organic solvents by using ionic liquids | • High cost of ionic liquids limits large-scale application |
| Sonochemical | • Rapid synthesis, significantly reducing reaction time | • High-energy ultrasound equipment is necessary |
| • Produces smaller particles with narrow size distributions | • Limited scalability for large-scale production | |
| Mechanochemical | • Solvent-free, eco-friendly, and low-cost synthesis | • Limited control over morphology and size of particles |
| • Rapid and energy-efficient process | • Not suitable for producing large quantities with uniform characteristics | |
| • High yield | ||
| Electrochemical | • Less time required, morphology can be controlled through the control of synthesis parameters such as time, temperature etc. | • Unsuitable for large-scale production |
4. Photocatalytic applications
ZIF-8 demonstrates photocatalytic potential due to its extensive surface area, high porosity, and exceptional chemical stability, which enhance the adsorption and interaction of reactant species. Although its direct light-harvesting ability is constrained by a broad bandgap, the incorporation of ZIF-8 with photoactive semiconductors augments its photocatalytic efficacy by promoting superior charge separation, efficient electron transport, and prolonged carrier lifetimes, thus driving enhanced photocatalytic processes.4.1 H2 evolution
Photocatalytic H2 production from water is seen as a prominent solution to the global energy scarcity.124,125 This process involves three key stages: (1) photons with energy exceeding the bandgap excite electrons in the valence band (VB) to the conduction band (CB), resulting holes in the VB; (2) the resulting charge carriers separate and emigrate to the material's surface; (3) electrons in the CB reduce adsorbed H+ to H2, while holes in the VB oxidize water into oxygen. These steps are crucial for the redox reactions required for H2 production.126| Oxidation: 2H2O → O2 + 4H+ + 4e− E0 = 1.23 eV | (1) |
| Reduction: 2H+ + 2e− → H2 E0 = 0.00 eV | (2) |
| Overall: 2H2O → O2 + 2H2 E0 = 1.23 eV | (3) |
For the H2 evolution reaction (HER) to occur, the CB energy level must be lower than 0 V (E(H+/H2)) under the normal hydrogen electrode (NHE). Additionally, the VB energy level must be higher than 1.23 V (E(O2/H2O)) for the water oxidation reaction to proceed (Fig. 3g).81
The development of numerous photocatalysts has gradually made photocatalytic hydrogen production an ideal and feasible technology. Among them, g-C3N4 has garnered significant attention due to its appropriate energy band (2.8 eV), stable structure, cost-effectiveness, and easy availability. Since 2009, g-C3N4 has been extensively utilized in photocatalytic H2 generation. Thus, Tian and his team effectively incorporated ZIF-8 nanoparticles onto a modified rod-like g-C3N4 structure. The ZIF-8 nanoparticles display a sharply defined polyhedral crystal shape around 200 nm in average size, while the modified g-C3N4 materials form rod-like structures with irregular surface pores. This ZIF-8/g-C3N4 photocatalyst was used for H2 evolution via solar-driven water splitting. Relative to pristine ZIF-8, the ZIF-8/g-C3N4 hybrid photocatalysts demonstrated a marked increase in hydrogen generation activity when exposed to light. Additionally, they investigated the impact of varying amounts of g-C3N4 on the photocatalytic efficiency of the ZIF-8/g-C3N4 hybrid, finding that 400 mg of g-C3N4 generated the highest H2 production rate of 309.5 μmol L−1 g−1 h−1 (Fig. 3h and i). This improved efficiency was supplied to the combined effects of the hybrid, which promoted excellent charge separation and light absorption.82
Nitrogen (N) vacancies on the interface of g-C3N4 create strong electronegativity, resulting in hydrogen adsorption and microbubble formation that obstructs reactions. In high-salinity seawater, charged ions occupy these N vacancies, competing with hydrogen protons for photogenerated electrons and further hindering hydrogen generation. For this, Xing et al. revealed that incorporating ZIF-8 into g-C3N4 eliminated the nitrogen-induced vacancies, enhancing hydrogen generation through photocatalytic seawater splitting. TEM images revealed numerous pores, about 32 nm in diameter, within the sheet structure of ZCNQ40. Nitrogen doping enhanced light absorption in all samples, leading to a decreased band gap. As shown in Fig. 4a, by DRS curves, the band gap of ZCNQ40 reduced from 2.66 eV to 2.58 eV, and the Mott–Schottky arcs of pristine g-C3N4 and ZCNQ40 both showed an ascending line, confirming their n-type semiconductor nature. The band alignment potential of ZCNQ40 shifted negatively by 0.09 V, from −1.33 V for pure g-C3N4 to −1.42 V (vs. SCE), indicating a shift to lower energy in the conduction band potential. The composite containing 40 mg of ZIF-8 (ZCNQ40) produced the most H2 in seawater, reaching 116.95 μmol L−1 g−1 h−1, and in distilled water, achieving 218.4 μmol L−1 g−1 h−1, with these rates being 5.6 times and 3.1 times higher, respectively, compared with those achieved with pure g-C3N4.127 Suda et al. (2020)128 developed an innovative hybrid Z-scheme photoactive catalyst by integrating copper-doped ZIF-8 with g-C3N4 for hydrogen production. The inclusion of g-C3N4 notably boosted the H2 production rate from 32.2 μmol L−1 g−1 h−1 to 213.3 μmol L−1 g−1 h−1. This enhancement was due to the creation of a Z-scheme charge carrier separation mechanism resulting from the combination of copper-ZIF-8 with g-C3N4. Additionally, g-C3N4 optimized the absorption in the 480 nm range, which ZIF-8 alone could not capture.128 Chang et al. developed a nitrogen-doped carbon (C-ZIF) decorated g-C3N4 heterostructure through thermal treatment. This C-ZIF@g-C3N4 heterostructure, with an optimal ratio of C-ZIF and g-C3N4, displayed outstanding photocatalytic performance in H2 generation compared with pure g-C3N4. The SEM images show that the C-ZIF maintains a rhombic dodecahedral structure with roughened surfaces after annealing and cleaning and is well-integrated with g-C3N4, forming a heterostructure with intense interfacial contact (Fig. 4b). The composite demonstrates outstanding H2 evolution activity, achieving 1238 μmol g−1 h−1, which is 1.95 times greater than pure g-C3N4 and 1.57 times greater than 10-C-ZIF + g-C3N4. The increased results were attributed to effective charge transfer at the C-ZIF@g-C3N4 interface, which significantly restrained the recombination of photoexcited electron–hole pairs, thereby enhancing photocatalytic efficiency.129
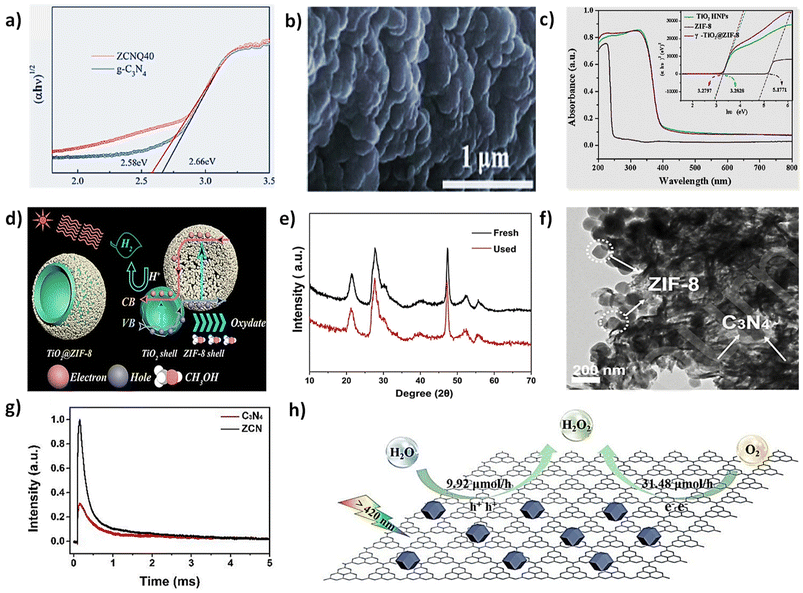 | ||
| Fig. 4 (a) DRS spectra of g-C3N4 and ZCNQx [reproduced from ref. 127 with the permission from Elsevier, copyright 2021]. (b) SEM images of 10-C-ZIF@g-C3N4 [reproduced from ref. 129 with the permission from The Royal Society of Chemistry, copyright 2020]. (c) UV-Vis absorption spectra of TiO2 HNPs, ZIF-8, and γ-TiO2@ZIF-8; fitted curves of the Tauc plot (inset). (d) The hydrogen evolution reaction mechanism of TiO2@ZIF-8 under simulated solar light irradiation [reproduced from ref. 130 with the permission of copyright 2015]. (e) XRD patterns of ZnS/ZIS-20 before and after repeated photocatalytic hydrogen production [reproduced from ref. 86 with the permission from The Royal Society of Chemistry, copyright 2020]. (f) TEM morphology of ZCN catalyst. (g) TPV of C3N4 (red line) and ZCN (black line). (h) The mechanism diagram for photocatalytic water splitting to produce H2O2 using ZCN [reproduced from ref. 131 with the permission from Elsevier copyright 2020]. | ||
Nanomaterials based on carbon, including carbon nanotubes, graphene, and graphitic PAN, can greatly enhance electron–hole separation in g-C3N4 through the formation of carbon/g-C3N4 composites, thereby boosting photocatalytic activity and serving as efficient HER catalysts. Inspired by this idea, He et al. developed C-ZIF-8/g-C3N4, derived from ZIF-8 carbon. Their study on photocatalytic H2 evolution revealed that pure g-C3N4 produced hydrogen at a slow rate of 0.9 μmol L−1 g−1 h−1, attributed to its inert surface for HER. The composite displays an IV-type isotherm, which suggests the presence of mesopores. As the carbon-ZIF content increases in the C-ZIF/g-C3N4 composites, the PL intensity significantly decreases, suggesting enhanced electron–hole separation due to the movement of photoexcited electrons from g-C3N4 to C-ZIF, which acts as an efficient electron receptor. Time-resolved fluorescence decay spectroscopy was used to determine the charge carrier lifetimes of g-C3N4 and the 1 wt% C-ZIF/g-C3N4 composite, both of which exhibited an exponential decay in their charge carrier lifetimes. The short lifetime increases from 1.27 ns in g-C3N4 to 2.39 ns in the composite, while the long lifetime extends from 9.33 ns to 13.93 ns. This increase in charge carrier lifetimes, due to the addition of C-ZIF, enhances photocatalytic efficiency. Remarkably, the inclusion of C-ZIF to g-C3N4 led to a notable increase in the photocatalytic hydrogen generation performance of the composite, reaching 32.6 μmol L−1 g−1 h−1. This rate is 2.8 times greater than that of Pt/g-C3N4 and 36.2 times greater than pristine g-C3N4, indicating enhanced charge separation.132
Loading a photosensitizer like AgBr enhances the visible light response in materials. Upon decomposition, Ag+ ions create a hybrid heterostructure that improves charge transfer and, due to their unique SPR effect, significantly boosts light absorption. Recently, to study this, Dai and colleagues successfully developed a three-component photocatalyst comprising Ag–AgBr/ZIF-8/g-C3N4 utilizing an ionic liquid-assisted in situ growth method. The HRTEM analysis of A(1.1)/g/Z(13) revealed a lattice spacing of 0.282 nm, associated with the (200) plane of AgBr, and the SAED pattern verified its crystalline structure. This newly formed system demonstrated significantly enhanced photocatalytic efficiency for hydrogen generation compared with ZIF-8, AgBr and g-C3N4. The enhancement was largely due to the role of Ag+, which served as a linking bridge, facilitating the creation of multiple electronic transmission pathways. XRD spectra confirmed Ag+ within the composite, while AgBr increased the photocurrent density and acted as a photosensitizer. The AgBr/g-C3N4 and AgBr/ZIF-8 composites demonstrate higher photocurrent densities compared with their individual components. The A(1.1)/g/Z(6.5) composite achieves the maximum density, highlighting it as the optimal ratio. AgBr enhanced light absorption and formed a p–n heterojunction with g-C3N4, suppressing exciton recombination. The composite achieved a H2 generation rate of 2058 μmol L−1 g−1 h−1, which was 49 times more than that of unmodified g-C3N4 alone, maintaining 91% efficiency after 5 cycles.133
Transition metal chalcogenides, with their layered structure, direct band gap, and high absorption coefficient, are promising for hydrogen production.134 For example, TiO2 is highly effective in photocatalytic applications owing to its suitable band gap (∼3.2 eV), strong oxidative power, high chemical stability, non-toxicity, cost-effectiveness, and photostability, ensuring its widespread use in solar energy applications. In 2019, Zhang and co-authors fabricated TiO2@ZIF-8 by coordinative incorporation, employing TiO2 hollow nanospheres (HNPs) coated with ZIF-8 through a sonochemical technique, and significantly enhanced HER under solar irradiation. The process involved a two-step approach: first modifying TiO2's surface to increase its area, followed by hybridizing it, the composite achieved a hydrogen production rate 3.5 times greater than pristine TiO2 HNPs, with a quantum efficiency of up to 50.89% and notable stability. Optoelectronic measurements and photoluminescence indicated that ZIF-8's porous structure and effective charge separation improved the photocatalytic activity. The band gaps for ZIF-8, γ-TiO2@ZIF-8, and TiO2 HNPs were 5.18 eV, 3.28 eV, and 3.28 eV, respectively (Fig. 4c). The reduction in the Eg values of the samples contributed to improved photocatalytic efficiency. The conduction band edges were −0.31 eV for TiO2 and −4.73 eV for ZIF-8. Under artificial sunlight with methanol as the sacrificial reagent, bare ZIF-8 showed no H2 evolution, while TiO2 HNPs had an HER of 74.6 μmol g−1 h−1, limited by low surface area and fast charge carrier recombination. A physical mixture of ZIF-8 and TiO2 HNPs displayed a reduced rate of 4.63 μmol g−1 h−1. However, the γ-TiO2@ZIF-8 composite significantly enhanced the HER to 254.2 μmol g−1 h−1, 3.4 times greater than the pristine TiO2 HNPs. The α-and β-TiO2@ZIF-8 composites also improved the HER, to 186.1 μmol g−1 h−1 and 105.0 μmol g−1 h−1, respectively. The proposed mechanism for photocatalytic H2 evolution is presented in Fig. 4d. Five successive photocatalytic tests were conducted under simulated sunlight, and the amount of H2 produced consistently enhanced with irradiation time during every cycle. The system exhibited excellent stability over a prolonged period of 30 hours, with the hydrogen evolution rate in the fifth cycle.130
Bulk MoS2, with a 1.2 eV band gap, is generally unsuitable as a photocatalyst due to its insufficient oxidation and reduction capabilities. However, exfoliated MoS2 shows a direct band gap ranging from 1.89 to 3.96 eV as a result of quantum confinement effects, enhancing its photocatalytic potential. For example, Ren and associates exfoliated MoS2@ZIF-8 hybrid for the HER through sonication, showing improved absorption of light and hydrogen evolution reaction efficiency in comparison with pristine ZIF-8. The exfoliated photocatalyst revealed an absorption edge at 415 nm, suggesting an appropriate band structure for HER applications. The MoS2@ZIF-8 composite had a measured band gap of 3.41 eV, which is shorter than the 5.15 eV band gap of pure ZIF-8. This composite structure obtained an H2 generation rate of 68.4 μmol L−1 g−1 h−1. The activity of MZ was analyzed by measuring hydrogen generation under using a Xe lamp. The H2 generation rate for ZIF-8 was 0.01 μmol h−1 g−1, and bulk MoS2 showed no activity. In contrast, the exfoliated MoS2 (EM) in the MZ composite significantly enhanced the average H2 evolution rate to 29.9 μmol h−1 g−1.135
ZIF-8 has been extensively utilized in the development of core–shell or core–shell-like catalysts. Zeng and co-authors created a novel core–shell structure, with ZIF-8 forming the shell and CdS NPs as the core. Initially, CdS nanoparticles were solidified using PVP and formed ZIF-8 shells on the CdS cores. Despite the limited hydrogen production rate of the resulting samples, the core–shell CdS@ZIF-8 structures exhibited enhanced specificity for hydrogen synthesis compared with pristine CdS NPs. The photocatalytic breakdown of formic acid was carried out to examine the harmonious interaction between CdS and ZIF-8. The photocatalysts used for formic acid decomposition included pristine CdS nanoparticles, MCS, SCS-20, and SCS-40. The photocatalytic kinetic profiles were obtained. In comparison with pure CdS NPs, the MCSs demonstrate greater photocatalytic performance for H2 production throughout the entire photocatalytic process. However, SCS-20 exhibits enhanced photocatalytic efficiency for H2 production only during the initial 3.5 hours, and SCS-40 displays improved efficiency only during the first 2.5 hours.136
ZnS, a traditional metal sulfide, is widely recognized as a photocatalyst because of its high electron–hole pair generation rate and non-toxic nature. Song et al. aimed to develop bimetallic sulfides that integrate ZIF-derived sulfide structures with the capability for visible light absorption and effectively create bimetallic sulfides that integrate the structural benefits of ZIF-derived sulfides. In this study, a series of ZnS/ZnIn2S4 hybrid materials derived from ZIF-8 were successfully prepared by changing the amount of ZIF-8 to optimize the ratios of ZnS and ZnIn2S4. The hydrogen evolution performance of these materials was tested using TEOA as a hole scavenger. As shown in Fig. 4e, pure ZnS, owing to its wide band gap of 3.55 eV, showed no hydrogen production. Pure ZnIn2S4 achieved a H2 generation rate of 118.4 μmol L−1 g−1 h−1, which is significantly lower than the composites, indicating reduced charge transfer efficiency and faster electron–hole recombination. The ZnS/ZIS-x composite exhibited improved hydrogen generation rates of 332 μmol L−1 g−1 h−1, 453.4 μmol L−1 g−1 h−1, and 286.3 μmol L−1 g−1 h−1 as x varied from 10 to 30, with ZnS/ZIS-20 delivering the maximum output, demonstrating an optimal ratio maximizing HER performance. The hydrogen generation rates achieved by the ZnS/ZIS composites in this study were considerably greater than those of ZnIn2S4-based photocatalysts, highlighting a promising method for developing efficient HER catalysts.86
Transition metal phosphides (TMPs) are promising HER catalysts due to their excellent catalytic and electronic properties. Due to phosphorus sharing similar chemical properties with nitrogen, TMPs retain significant characteristics from multi-electron orbitals.137,138 For instance, Jin and colleagues formulated a ternary novel photocatalyst comprising WO3/NiP2/ZIF-8, utilizing eosin-Y (EY) as a sensitizer, and 30% TEOA was used as a sacrificial agent. XRD, SEM, and TEM characterization revealed that WO3 forms nanowires and ZIF-8 exhibits a well-defined dodecahedron morphology with good crystallinity. XPS confirmed the valence states of the elements, and UV–Vis DRS data indicated that the modified catalyst has a broader visible light absorption range than pure WO3. The hybrid catalysts showed improved electron transfer, better charge separation, and longer lifetimes than the individual catalysts, enhancing H2 production. The photocatalytic HER achieved an emission rate of 341.2 μmol L−1 g−1 h−1 over 5 hours, which was 11.3 times higher than the pure WO3. This improvement was attributed to the increased active sites and surface area presented by the NiP2 co-catalyst in conjunction with ZIF-8. Additionally, NiP2 aided in capturing photogenerated electrons, which then reacted with protons in the solution to produce hydrogen.139
ZIF-8-based nanomaterials have demonstrated significant potential in enhancing H2 generation when integrated with materials like g-C3N4, carbon nanostructures, and other semiconductors. These hybrid systems improve charge separation, light absorption, and stability, leading to higher hydrogen evolution rates compared with pristine materials. By optimizing band gaps and enhancing interfacial interactions, these composites achieve impressive photocatalytic efficiency under light irradiation, highlighting their potential for sustainable hydrogen production. Table 2 summarizes the photocatalytic activity of ZIF-8 MOF-based nanocomposites in H2 and H2O2 evolution.
| Sl. no. | Photocatalytic application | Composite | Sacrificial agent | Light source | Activity | Quantum yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | H2 evolution | TiO2 @ZIF-8 | Methanol | 300 W Xe lamp | 254.2 μmol g−1 h−1 | 50.89 | 130 |
| 2 | CdS/ZIF-8 | Na2S and Na2SO3 | 300 W Xe lamp | 2.10 mmol g−1 h−1 | — | 136 | |
| 3 | MoS2@ZIF-8 | Methanol | — | 29.9 μmol h−1 g−1 | — | 135 | |
| 4 | ZIF-8 derived ZnCdS | Na2S and Na2SO3 | 300 W Xe lamp | 5680 μmol g−1 h−1 | — | 140 | |
| 5 | ZIF-8 derived ZnS | TEOA | 300 W Xe lamp | 3709.23 μmol g−1 h−1 | 0.014 | 141 | |
| 6 | CdS–ZnNi–ZIF-8 | Na2S and Na2SO3 | 300 W Xe lamp | 45.6 mmol g−1 h−1 | — | 142 | |
| 7 | Ag–AgBr/g-C3N4/ZIF-8 | TEOA | 300 W Xe lamp | 2058 μmol g−1 h−1 | 7.91 | 133 | |
| 8 | ZIF-8 derived Co3O4/ZnO | Glycerol | 500 W Xe lamp | 2241 μmol g−1 h−1 | — | 143 | |
| 9 | ZIF-8 derived ZnS/ZnIn2S4 | TEOA | 300 W Xe lamp | 453.4 μmol g−1 h−1 | — | 86 | |
| 10 | Co@ZIF-8/TiO2 | Methanol | — | 13 mmol g−1 h−1 | — | 144 | |
| 11 | C-ZIF@g-C3N4 | TEOA | 300 W Xe lamp | 1238 μmol g−1 h−1 | — | 129 | |
| 12 | WO3/NiP2@ZIF-8 | TEOA | 300 W Xe lamp | 295.7 μmol g−1 h−1 | 50.8 | 139 | |
| 13 | ZIF-8/modified g-C3N4 | TEOA | 300 W Xe-lamp | 309.5 μmol L−1 g−1 h−1 | — | 82 | |
| 14 | H2O2 evolution | BiOBr/ZIF-8/ZnO | — | LED light | 116 mmol L−1 g−1 | — | 145 |
| 15 | ZIF-8/C3N4 | — | Visible light (λ ≥ 420 nm) | 2641 μmol h−1 g−1 | 19.54 | 131 |
4.2 H2O2 generation
Hydrogen peroxide (H2O2) is a clean oxidant and reductant commonly used in industrial chemistry and is also a promising high-energy fuel for fuel cells.146 H2O2 can be produced through either a two-step single-electron reduction or a one-step two-electron reduction. In photocatalytic systems, holes in the VB oxidize H2O to O2 and H+, while e− in the CB reduce adsorbed O2 to H2O2. The one-step, two-electron reduction is considered more efficient for producing H2O2 from a photoelectrochemical standpoint.147–149The two-step single-electron pathway mechanism involves the reactions discussed in eqn (4)–(8). Alternatively, the one-step, two-electron pathway mechanism involves the direct reaction of O2 with two H+ to produce H2O2 (eqn (9)). The overall photocatalytic reaction can be summarized as follows (eqn (10)):
| 2H2O + 4h+ → O2 + 4H+ | (4) |
| O2 + e− → O2˙− | (5) |
| O2˙− + H+ → HO2˙ | (6) |
| HO2˙ + e− → HO2− | (7) |
| HO2− + H+ → H2O2 | (8) |
| O2 + 2H+ + 2e− → H2O2 | (9) |
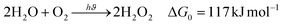 | (10) |
The photocatalytic production of H2O2 from H2O and O2 is an uphill reaction with a standard Gibbs free energy change (ΔG0) of 117 kJ mol−1.
Zhao et al. showed that ZIF-8/C3N4 composite is a highly effective photocatalyst for producing H2O2 by a two-channel pathway, i.e., both oxygen reduction and water oxidation reaction. The SEM image of C3N4 displays a sheet-like structure, while the ZIF-8 (Fig. 4f) display a rhombic dodecahedral shape with an approximate size of 100 nm. ZIF-8 exhibits significant nitrogen adsorption at minimal relative pressures, suggesting the existence of numerous micropores, which is further supported by the pore size distribution analysis. Transient photovoltage (TPV) measurements provide insight into the photocatalytic water-splitting mechanism for H2O2 production using the ZIF-8/C3N4 composite. Fig. 4g illustrates that the ZCN sample (black line) exhibits a notably higher photocurrent response, signifying an increased accumulation of photoinduced charges on the surface. This implies improved electron–hole separation efficiency in ZCN. Under an O2 atmosphere, ZCN's photocurrent decreases more than under H2, indicating rapid oxygen reduction and water oxidation as the rate-determining step. The composite facilitates H2O2 production through simultaneous O2 reduction and H2O oxidation. The proposed reaction mechanism suggests that in the ZCN composite, visible light excites C3N4 to separate electron–hole pairs, where ZIF-8 enhances visible light absorption, and facilitates O2 adsorption for efficient H2O2 production, and its porous structure aids in mass transfer and prevents catalyst poisoning (Fig. 4h). Unlike ZIF-8 alone, which shows no activity, and C3N4, which produces only 67.50 μmol h−1 g−1, the composite significantly enhances efficiency by achieving 2461 μmol h−1 g−1. It demonstrates excellent stability, with reusability for at least five cycles under visible light, with only a slight performance decline due to catalyst loss, and the composite maintains good morphological stability throughout the cycles.131
Bismuth-based materials are commonly employed in photocatalytic studies due to their open structural framework and favorable band gap. Among them, BiOBr is considered an excellent catalyst support because of its strong stability, affordability, responsiveness to visible light, and diverse microstructural properties. To address issues like the agglomeration of ZIF-8, Yang et al.150 employed ZnO/ZIF-8 hybrid photocatalyst under UV light irradiation, which can decompose 98.17% RhB in 12 min. Building on this approach, Han et al. synthesized BiOBr/ZIF-8/ZnO ternary composite and demonstrated effective H2O2 generation under LED lamp irradiation, reducing ZIF-8 aggregation and enhancing charge carrier separation. The DRS spectra reveal the UV light responsiveness of BiOBr, ZIF-8, ZZ-110, and BZ-9, with the latter showing a redshift in absorption edges. BZ-9's edge extended to ∼500 nm, indicating a broader light response and visible light interaction. Tauc plot analysis calculated the band gaps as 2.70 eV for BiOBr and 2.91 eV for ZZ-110. Electrochemical tests (Fig. 5a) showed that BZ-9 produced an enhanced photocurrent compared with the other catalysts, suggesting better optical absorption and enhanced electron–hole separation efficiency. The LSV curve demonstrated that BZ-9 had the lowest potential, implying a reduced electron–hole recombination rate (Fig. 5b). Mott–Schottky analysis showed flat band potentials of −0.117 for BiOBr and −1.007 for ZZ-110. BiOBr and the composites generated significant amounts of H2O2, with BZ-9 exhibiting the greatest production of 116 mmol L−1 g−1 within two hours, 1.4 times greater than pristine BiOBr (Fig. 5c). The photocatalytic mechanism proposed that BiOBr and ZZ-110 establish a conventional type II heterojunction, as illustrated in Fig. 5d. After four cycles, the H2O2 evolution of BZ-9 decreased to 109 mmol L−1 g−1, representing good stability. HCOOH influenced H2O2 generation by capturing holes, oxidizing to HCOO˙, which further reacted to form ˙OH and H2O2, with CB electrons at ZZ-110 to reduce O2 to H2O2.145
 | ||
| Fig. 5 (a) H2O2 production capacity, (b) the band gaps and (c) Nyquist plots of BiOBr, ZIF-8, ZZ-110, and BZ-9. (d) The photocatalytic mechanisms of BiOBr and ZZ-110 involve two distinct heterojunction types: type II heterojunction and an S-scheme heterojunction [reproduced from ref. 145 with the permission from MDPI, copyright 2023]. (e) The total pathways for production of the main products of photocatalysis of CO2 [reproduced from ref. 151 with the permission from Elsevier, copyright 2016]. (f) Time–yield plots of CO and H2 over TiO2/ZIF-8-G2 composites [reproduced from ref. 152 with the permission from The Royal Society of Chemistry, copyright 2021]. (g) TGA of ZIF-8, TiO2@ZIF-8 and TiO2 NPs in air [reproduced from ref. 153 with the permission from Elsevier, copyright 2015]. (h) The synthetic procedure of ZnO/ZIF-8 hybrid photocatalysts [reproduced from ref. 154 with the permission from Elsevier, copyright 2016]. | ||
By integrating ZIF-8 with materials like C3N4, BiOBr, and ZnO, the overall photocatalytic performance is greatly enhanced compared with the individual components, showcasing excellent stability, reusability, and scalability for practical applications. These findings underscore the importance of material design in optimizing photocatalytic processes for energy and environmental applications.
4.3 CO2 reduction
CO2 is a highly stable molecule (ΔG° = −400 kJ mol−1) with a linear structure comprising two double bonds between oxygen and carbon atoms. This stability and its large LUMO–HOMO energy gap of 13.7 eV and high electron affinity of −0.6 ± 0.2 eV make it difficult to convert CO2 into valuable chemicals without catalysts and energy input. The conversion process is hindered by thermodynamic and kinetic limitations.155,156 However, when CO2 adsorbs onto a photocatalyst surface, its linear structure bends, lowering the energy barrier and increasing reactivity by reducing the LUMO level. This adsorption initiates sequential reactions through one-electron transfers involving electron or proton transfers that break C–O bonds and form new C–H bonds. A reducing agent, such as H2O, H2, CH4, or CH3OH, is needed to supply hydrogen for producing hydrocarbons through photocatalysis. The pathways for photocatalytic CO2 reduction depend on the reductant used. Carbon-free reductants like H2O and H2 typically yield C1 products, whereas carbon-containing reductants like CH4 and CH3OH can produce C2 and C3 products. Common products from CO2 photoreduction include CO, HCOOH, HCHO, CH3OH, CH4, and C2H5OH.157–159Fig. 5e illustrates the electrons, oxidizing holes, and protons (H+) required for forming these main products.151As TiO2 has strong oxidative power, high chemical stability, and non-toxicity, Yan et al. synthesized a composite using ZIF-8 and TiO2 through grinding and solid-synthesis methods. These composites were employed for the photocatalytic reduction of CO2. XPS confirmed the appearance of Zn 2p, N 1s, C 1s, Ti 2p, and O 1s in TiO2/ZIF-8-G2. Peaks at 458.6 eV and 464.3 eV correspond to Ti4+ (Ti 2p3/2 and Ti 2p1/2), while peaks at 1022.1 eV and 1045.1 eV are allotted to Zn 2p3/2 and Zn 2p1/2. The CO2 photoreduction efficiency is attributed to TiO2/ZIF-8-G2, with its photocatalytic efficiency at specific time intervals reaching 5.04, 13.38, 31.85, and 43.49 mmol g−1, as depicted in Fig. 5f. The UV-DRS spectra indicate that TiO2/ZIF-8-G2 exhibits a broader absorption band and an extended absorption edge near 400 nm, suggesting enhanced light absorption. The EIS results reveal that TiO2/ZIF-8-G2 has the shortest radius within the TiO2/ZIF-8-GX photocatalysts, indicating superior conductivity and more efficient photogenerated electron transfer. This aligns with the photocurrent and CO2 photoreduction performance. Consequently, a CO production rate of 21.74 mmol g−1 h−1 with a remarkable 99% selectivity was attained in the solid-gas system without the use of sacrificial agents or photosensitizers. The group's previous highest CO generation rate was 10.67 mmol g−1 h−1, achieving 90% selectivity.152
SnO2, known for its high oxidation potential and electron–hole capture, has been widely used in various fields. Wang et al. synthesized T-ZIF-8/SnO2 spherical photocatalysts via the sol–gel method for CO2 reduction, forming type-II heterojunctions. HRTEM showed that T-ZIF-8 retained its framework with spherical deformation, while SnO2 appeared as ∼9 μm particles, covered by T-ZIF-8. The materials exhibited type-IV isotherms with H3 hysteresis loops, indicating slit pores from nanoparticle aggregation. A reduced PL intensity in T-ZIF-8/SnO2 compared with T-ZIF-8 indicated slower electron–hole recombination, attributed to the heterojunction's multiple charge transfer pathways. This enhanced charge separation, reduced recombination, and improved charge carrier transport, increasing active sites and reducing the bandgap. Under simulated sunlight, T-ZIF-8/SnO2 demonstrates exceptional photocatalytic performance, achieving a CO2 generation rate of 16.6 μmol g−1 h−1, which is 1.4 and 4.9 times greater than the pristine SnO2 and T-ZIF-8, respectively. Furthermore, it attains a CH4 generation rate of 14.7 μmol g−1 h−1, which is 1.9 and 6.1 times higher than that of pure SnO2 and T-ZIF-8. These findings verify that the heterojunction significantly enhances both photocatalytic CO2 reduction efficiency and CH4 selectivity.160
CO2 reduction using ZIF-8-based photocatalysts highlights significant advancements in overcoming the inherent stability of CO2 and promoting its conversion into valuable chemicals. The formation of heterojunctions in these composites plays a major role in increasing photocatalytic activity by facilitating charge transfer and enhancing the overall conversion of CO2 into useful products such as CO and CH4 without the need for sacrificial agents. The photocatalytic performance of ZIF-8 MOF-based nanocomposites in CO2 reduction and dye and drug degradation has been summarized in Table 3.
| Photocatalytic application | Composite | Band gap (eV) | Light source | Activity | Ref. | |
|---|---|---|---|---|---|---|
| 1 | CO2 reduction | TiO2/ZIF-8 | 1.97 | LED lamp, 40 W | CO = 21.74 μmol g−1 h−1 | 161 |
| 2 | ZnO/ZIF-8 | 3.23 | Hg lamp, 125 W | CH3OH = 6843.12 μmol g−1 | 162 | |
| 3 | Pt/ZIF-8 | 4.21 | Hg lamp, 125 W | CH3OH = 5365.01 μmol g−1 | 162 | |
| 4 | Cu/ZIF-8 | 2.09 | Hg lamp, 125 W | CH3OH = 426.02 μmol g−1 | 162 | |
| 5 | Au/ZIF-8 | 1.69 | Hg lamp, 125 W | CH3OH = 232.20 μmol g−1 | 162 | |
| 6 | Cu–TiO2/ZIF-8 | — | UV lamp, 320–480 nm | CH3OH = 4.88 μmol g−1 | 163 | |
| 7 | TiO2/ZIF-8 | — | UV lamp, 8 W | CO = 0.53 mmol g−1 h−1, CH4 = 0.18 mmol g−1 h−1 | 164 | |
| 8 | CdS/ZIF-8 | 2.41 | Xe lamp, 300 W | CO = 32.13 μmol h−1 | 161 | |
| 9 | CdSe QD@ZIF-8 | — | Xe lamp, 300 W | CO = 3.526 μmol g−1 h−1, CH4 = 0.102 μmol g−1 h−1 | 165 | |
| 10 | Drug degradation | ZIF-8/TiO2 | 2.98 | 300 W Xe lamp | TC = 90% in 40 min | 93 |
| 11 | ZIF-8/Cu2O | 4.31 | Solar light | TC = 84.1% in 120 min | 166 | |
| 12 | ZIF-8 derived CuO/ZnO | — | 300 W Xe lamp | TC = 87% in 60 min | 167 | |
| 13 | ZIF-8/MoS2 | 1.05 | 300 W Xe lamp | Ciprofloxacin = 93.2% in 180 min | 168 | |
| 14 | ZIF-8/g-C3N4 | 2.73 | — | TC = 87.6% in 40 min | 143 | |
| 15 | Ag/AgCl@ZIF-8/g-C3N4 | — | Visible light | Levofloxacin = 94.5% in 60 min | 169 | |
| 16 | Dye degradation | CuONPs/ZIF-8 | 4.93 | Sunlight | Rh6G = 96.5% in 105 min | 57 |
| 17 | MnO2@ZIF-8 | 5.46 | 300 W xenon lamp | RhB > 96.0% | 170 | |
| 18 | ZIF-8/NiFe2O4 | — | 500 W halogen lamp | MB = 94% in 120 min | 92 | |
| 19 | ZIF-8/Fe2O3 | — | 500 W Xe lamp | Reactive red = 94% | 171 | |
| 20 | ZnO@ZIF8 | 3.31 | Metal halide lamp/575 W | MB = 97.65% in 45 min | 154 | |
| 21 | ZIF-8 derived ZnO | — | UV lamp/18 W | MB = 76% in 60 min | 172 | |
| 22 | RGO/black TiO2/2D-ZIF-8 | — | Visible light | MB = 0.0232 min, DH = 0.0118 min | 173 | |
| 23 | MoO3@ZIF-8 | — | PL-XQ 350 W xenon | MB = 95% in 300 min | 174 | |
| 24 | CuO–ZnO/ZiF-8 | 1.96 | Solar light | AO7 = 98.1% in 100 min | 175 | |
| 25 | ZIF-8/g-C3N4 | 2.80 | Xe lamp/300 W | RhB = 99.8% in 60 min | 58 | |
| 26 | Ag-doped ZIF-8 | 3.2 | Visible LED light | MO = 100%, RhB = 93% in 30 min | 176 | |
| 27 | ZIF-8@AgNPS | 5.13 | UV–vis light | CR = 100% | 177 | |
| 28 | Ag/AgCl@ZIF-8 | 3.3 | Hg lamp/175 W | MB = 100% in 90 min | 178 | |
| 29 | AgCl/Ag@ZIF-8 | — | Xe lamp/210 W | RhB = 86% in 60 min | 179 | |
| 30 | ZIF-8/Ag2S | 3.24 | 100 W Xe and 100 W LED | RhB = 100% in 75 min | 180 | |
| 31 | CdSNPs@ZIF-8 | 2.95 | UV–visible | MB = 83.2% in 120 min | 181 | |
| 32 | ZIF-8/Zn-Al LDH | — | UV light/70 W | MB = 58% in 180 min | 182 | |
| 33 | ZIF-8@CQD | 4.95 | 300 W Xe lamp | MB = 91% | 183 | |
| 34 | TiO2@ZIF-8 | 3.25 | Solar light | MB = 87.5%, RhB = 64.85%. 120 min | 153 |
4.4 Dye degradation
The elimination of dyes from industrial wastewater is essential due to their harmful effects on aquatic life and their potential carcinogenic risks to humans. Traditional dye removal methods often fail to achieve complete degradation and produce significant amounts of suspended solids. Therefore, an efficient process that can fully degrade dyes with minimal waste is critically needed. Photocatalysis is a promising approach for degrading various toxic and organic pollutants in wastewater. The detailed mechanism of photocatalysis is explained in the following reactions.184–186| H2O + hϑ → HO˙ + H˙ | (11) |
| HO˙ + O2 → HO2˙ | (12) |
| O2 + e− → O2˙− | (13) |
| O2˙− + H+ → HO2˙ | (14) |
| HO2˙ + H+ → H2O2 | (15) |
 | (16) |
Finally, the dye reacts with an HO˙ radical to form intermediate products and end products:
| HO˙ + dye → Intermediates → CO2 + H2O |
TiO2 is commonly implemented as a photocatalyst because of its excellent activity and robust chemical and photochemical stability.150 Chandra et al. embedded TiO2 nanotubes within ZIF-8 to fabricate a TiO2@ZIF-8 for the degradation of RhB and MB dyes. FESEM and HRTEM images show that the composite retains the hexagonal morphology of ZIF-8, where TiO2 nanoparticles appear as dark spots within the ZIF-8 cages. According to the UV-DRS spectral analysis, the composite exhibited a flat band structure, identical to what is seen with TiO2 nanoparticles, with a slight reduction in the band gap from about 3.35 eV to 3.25 eV, due to changes in the microenvironment within the ZIF-8 framework, and amplified the photocatalytic performance of the TiO2 NPs encapsulated within the ZIF-8 framework. Surface analysis using BET adsorption measurement revealed a decrease in surface area ranges from 1432.91 m2 g−1 to 253.882 m2 g−1 when comparing the isotherms of the ZIF-8 framework and the composite. This observation confirmed the coating of TiO2 NPs within the framework pores, resulting in reduced void space accessible to N2 gas molecules. Thermal gravimetric analysis indicated high stability of both ZIF-8 and TiO2@ZIF-8 up to 236 °C and 254 °C, respectively, in atmospheric air and up to 240 °C and 270 °C under an inert N2 atmosphere. Beyond these temperatures, the frameworks gradually decomposed, with a gradual decline observed until approximately 670 °C (Fig. 5g). After 2 hours of catalysis, complete elimination of dye color was achieved, and the removal rate of RhB and MB by TiO2@ZIF-8 was 64.85% and 87.5%, respectively. In comparison, TiO2 and ZIF-8 alone exhibited lower degradation rates, with methylene blue degradation at 29% and 86.25% and rhodamine-B degradation at 53.6% and 50%, respectively. Notably, TiO2@ZIF-8 maintained a high recycling rate, demonstrating almost the same efficiency even in the fifth catalytic cycle. In contrast, TiO2 nanotubes exhibited significantly reduced catalytic efficiency after just the second degradation cycle.153
TiO2 thin films doped with Fe, Cu, Ce, or Zn were designed to enhance the photocatalytic efficiency of the nanocomposite.187 Considering this, Yurtsever et al. examined the effects of crystallization time and the amount of ZIF-8 on the photocatalytic performance of Cu–TiO2/ZIF-8. These films exhibited superior light absorption compared with other ZIF-8-coated TiO2 films and bare ZIF-8. This enhanced light absorption likely boosts photocatalytic activity, suggesting that using undoped or doped TiO2 thin films as supports can enhance the photocatalytic efficiency of ZIF-8. They observed that decreasing the ZIF-8 loading and shortening the development time led to enhanced photocatalytic performance. Specifically, nanocomposites with 5% ZIF-8 loading and a growth time of 15 minutes exhibited the greatest photocatalytic efficiency, with a first-order rate constant 4 times and 1.4 times greater than that of pristine ZIF-8 and Cu–TiO2, respectively. However, increasing the ZIF-8 content to 46% led to aggregate clumping, which decreased the photocatalytic efficiency. The ZIF-8/TiO2 nanocomposite thin films, which were synthesized using 1% copper or 1% cerium-doped TiO2 as substrates, displayed the highest activity for MB degradation. Both films removed 19% of the dye under 254 nm LED light in 1 hour and achieved 36% and 29% dye removal, respectively, under 365 nm LED light in the same time frame.188
He et al. developed a ternary system comprising RGO/black TiO2/2D-ZIF-8 for the degradation of MB. The integration of 2D-ZIF-8 into the system enhanced the capacity of dye adsorption and facilitated in situ accumulation. The three-component composite exhibited dual diverse interfaces, which were crucial for the movement and segregation of charge carriers. The rate constant for MB photocatalytic decomposition was found to be 0.0232 min−1, which is 3.3 times higher than black TiO2 alone. The ternary GO/B-TiO2/2D-ZIF-8 composites showed the smallest semicircle diameter, indicating that RGO/B-TiO2/2D-ZIF-8 has the maximum charge carrier mobility. The photocatalytic degradation mechanism explains that photogenerated electrons on black TiO2 surfaces struggle to react with O2 to form O2˙−, as the redox potential of the O2/O2˙− couple (−0.33 eV) is lower than the CB energy of black TiO2 (−0.27 eV). Furthermore, the redox potential of the H2O/OH− couple (2.53 eV) exceeds the VB energy of ZIF-8 (0.34 eV), making it inefficient for photogenerated holes to convert H2O into OH˙. Consequently, the holes directly interact with MB during the photodegradation process. The excellent electrical conductivity of RGO also contributes to the efficient separation of electron–hole pairs and lowers the recombination rate, which in turn improves the photodegradation of MB.173
Recently, ZnO photocatalysts have attracted significant attention owing to their non-toxicity, stability, cost-effectiveness, and efficient photocatalytic activity. Yang et al. introduced an innovative method for producing ZIF-8/ZnO composite photocatalysts via a micro-catalysis approach, utilizing ZIF-8 as the reactant and AgNO3 as the catalyst, varying the concentration of AgNO3 (Fig. 5h). The treatment with a 25 mM AgNO3 solution resulted in the edges of the ZIF-8 surface becoming spherical, leading to a reduction in particle size. Increasing the AgNO3 concentration to 50 mM further decreased the particle size to approximately 200 nm, with a more pronounced spherical morphology. The composite synthesized with a 50 mM AgNO3 solution (designated as ZIF-8/ZnO (50)) achieved supreme decomposition performance at approximately 98.17% compared with its counterparts synthesized with 100 mM and 25 mM of AgNO3. Additionally, the composite demonstrated robust photostability, retaining a photocatalytic activity of 95.54% after reuse for four cycles as ZnO/ZIF-8(50) showcased high photocatalytic stability, with only a 2.63% reduction in activity after four cycles, attributed primarily to inevitable catalyst loss. Consequently, ZnO/ZIF-8(50) demonstrates commendable preparation and reusability properties.154
ZnO, an n-type semiconductor possessing conductivity between 10−7 and 10−3 S cm−1 and a binding energy of 60 meV, pairs effectively with CuO, a p-type semiconductor possessing a narrow band gap and conductivity around 10−4 S cm−1. Saravanan et al. synthesized CuO/ZnO nanocomposites, followed by Abdollahi and collaborators, used a precipitation method to create a CuO–ZnO/ZIF-8 photocatalyst, which was tested for the photocatalytic decomposition of methylene blue, malachite green and Acid Orange 7. The sample with 20 wt% ZIF-8 was found to have a reduced band gap energy of 1.96 eV, compared with the band gap energies of ZIF-8 and CuO–ZnO, as determined by DRS analysis. Additionally, incorporating ZIF-8 into the CuO–ZnO heterojunction increased the specific surface area from 12.3 m2 g−1 to 22.2 m2 g−1. This increase allowed the photocatalyst to adsorb a larger amount of dye, which was then decomposed under visible light, and the study found that increasing photocatalyst loading from 0.5 to 1 g L−1 improved the photodecomposition rate, attributed to the increased number of active sites. The CuO–ZnO/ZIF-8-20 photocatalyst demonstrated a photocatalytic activity of 51%, 98.1%, and 59% for removing malachite green, Acid Orange 7, and Methylene blue at pH 7.175
Cao et al. effectively coated MnO2 onto ZIF-8 through a reaction process. The incorporation of MnO2 NPs onto ZIF-8 ensured their even dispersion, enhancing the active surface area (Fig. 6a). The MnO2@ZIF-8 nanoparticles display a notable size enlargement as compared with the original ZIF-8. The sizes of the MnO2@ZIF-8 NPs are 605 ± 60 nm, 410 ± 45 nm, and 205 ± 30 nm at I6R4 concentrations of 0.1 mM, 0.5 mM, and 1.0 mM, respectively (Fig. 6b). ZIF-8 and the composite analyzed via N2 adsorption/desorption exhibited type I nitrogen isotherms, indicating micropores at low pressures and mesopores at relative pressures above 0.9. Consequently, the synthesis of the ZIF-8@MnO2 heterojunction facilitated optimized charge carrier separation, leading to the generation of abundant free radicals like OH. Additionally, the MnO2@ZIF-8 NPs have a zeta potential (ζ) value of 15–23 mV, which is noticeably lower than that of the ZIF-8 crystals. In a Fenton-like process, the resulting ZIF-8@MnO2 hybrid served as a photocatalyst for the decomposition of RhB and reached over 96.0% within 120 minutes, significantly outperforming the 63% degradation rate achieved with pristine ZIF-8. The recyclability of MnO2@ZIF-8 nanoparticles in the photocatalytic degradation of RhB indicates that the ratio remained above 80% after five cycles, demonstrating excellent recycling stability and reusability. These promising outcomes stemmed from the porous structure of ZIF-8s, facilitating the adsorption of more RhB molecules.170
 | ||
| Fig. 6 (a) Fabrication of MnO2@ZIF-8 core–shell nanocomposites. (b) Size (hydrodiameter) distribution of the ZIF-8 crystals and the MnO2@ZIF-8 nanocomposites prepared at varied peptide concentrations [reproduced from ref. 170 with the permission from Elsevier, copyright 2021]. (c) Magnetic hysteresis curve of NiFe2O4 nanoparticles and Z/NFO-30 composite [reproduced from ref. 92 with the permission from Elsevier, copyright 2020]. (d) FE-SEM of ZIF-8/Ag2S. (e) Optical bandgab for ZIF-8, Ag2S, and ZIF-8/Ag2S by using Tauc plots. (f) S-scheme mechanism of ZIF-8/Ag2S photocatalyst under vis-light irradiation [reproduced from ref. 180 with the permission from Elsevier, copyright 2016]. (g) TG and DTG curves of MoS2/ZIF-8 composite (a and a′); MZ-5@Carboxyl cotton (b and b′); pristine cotton fabric (c and c′). (h) EIS plots of MZ-5@Carboxyl cotton, MoS2 and ZIF-8 (i) transient photocurrent responses of MoS2, ZIF-8 and MZ-5@Carboxyl cotton [reproduced from ref. 189 with the permission from Elsevier, copyright 2016]. | ||
Faraji and associates harnessed the magnetic characteristics of NiFe2O4 to develop a ZIF-8/NiFe2O4 composite, which allows for convenient separation from the reaction mixture. This composite photocatalyst was scrutinized for its efficacy in decomposing MB under a 500 W halogen lamp, with visible light (≥400 nm) as the irradiation source. The magnetic performance of NiFe2O4 and the Z/NFO-30 composite was analyzed under an external magnetic field of ±1.5T at ambient temperature. Pure NiFe2O4 nanoparticles exhibited superparamagnetic behavior with a saturation magnetization (Ms) of approximately 38.54 emu g−1. In contrast, the composite, which includes ZIF-8 integrated with NiFe2O4, showed a lower Ms of about 10 emu g−1, as shown in Fig. 6c. This reduction in Ms is attributed to the integrating of ZIF-8 into the NiFe2O4 matrix, affecting the total mass and magnetic properties. Among the samples, Z/NFO-30 displayed the weakest emission peak intensity, indicating a reduced recombination rate of photoexcited charge carriers. Their research revealed that the photocatalyst, formulated with 30 wt% NiFe2O4, exhibited the most robust photocatalytic activity, achieving a degradation efficiency of 94% within 120 minutes. Additionally, its magnetic nature enabled the composite to be renewed and reutilized for four cycles while maintaining a degradation efficiency of 90%.92
Metal sulfides are highly effective photocatalysts because of their strong charge carrier migration capabilities, excellent light absorption properties, suitable band structure, and abundance of exposed active sites. Jabbar and co-authors examined the photocatalytic degradation of RhB using a ZIF-8/Ag2S nanocomposite photocatalyst, where Ag2S nanoparticles were effectively dispersed on the ZIF-8 surface, creating a high-efficiency heterogeneous catalyst through the co-precipitation method. The morphology of the composite, as shown in Fig. 6d, revealed semicubic particles with a slight shape change from pure ZIF-8, indicating successful Ag2S nanoparticle precipitation. UV–vis absorption spectra demonstrated improved visible light absorption in the ZIF-8/Ag2S composite due to its reduced band gap, as further confirmed by the band gap values of 5.58 eV for ZIF-8, 0.93 eV for Ag2S, and 3.24 eV for the composite (Fig. 6e). This narrowed band gap is attributed to the effective dispersion of Ag2S within the ZIF-8 structure. In RhB degradation tests, the ZIF-8/Ag2S nanocomposite achieved complete photodegradation within 75 minutes in an airlift reactor, compared with 120 minutes in a batch reactor, with enhanced performance due to improved mixing and increased dissolved oxygen. The study also explored the impact of catalyst dosage, with the composite showing 100% degradation efficiency at optimal loading compared with 51.6% for ZIF-8 and 39.7% for Ag2S alone, and it follows an S-scheme heterojunction photocatalytic mechanism (Fig. 6f). Finally, the composite demonstrated excellent stability and reusability, maintaining high photodegradation efficiency over five cycles, due to the dispersion of Ag2S aggregates on the ZIF-8 surface.180
MoS2, with its low band gap and reactive edge, features a “sandwich” S–Mo–S structure that enhances the visible light response, making it effective for boosting the photocatalytic efficiency of ZIF-8.190 Chen and his team effectively fabricated ZIF-8 on carboxylated cotton cloth and then grew MoS2 on the ZIF-8 surface, creating an MoS2/ZIF-8/carboxyl cotton composite. Fig. 6g shows TG and DTG curves, where MoS2/ZIF-8 powder and MZ-5@Carboxyl cotton display distinct weight losses due to decomposition, leaving final residues of ∼76.5% and ∼34.1%, respectively. XPS analysis revealed that S, Mo, Zn, C, O, and N are the key elements in MZ-5@Carboxyl cotton, with strong peaks indicating the presence of S 2p, Mo 3d, and Zn 2p, confirming that MoS2 is closely integrated with the ZIF-8 surface. The EIS spectra for the composite (Fig. 6h) displayed a smaller arc radius, indicating enhanced light-induced charge transfer due to MoS2/ZIF-8 heterostructures. This was supported by the highest electron–hole separation efficiency observed in the photocurrent response (Fig. 6i) and confirmed by Mott–Schottky analysis (Fig. 7a), identifying MZ-5@Carboxyl cotton as an n-type semiconductor. This composite efficiently degraded methylene blue (MB), achieving approximately 99.8% degradation of the aqueous solution after 21 minutes of visible-light exposure. The fabric with five layers of ZIF-8 demonstrated the highest MB degradation efficiency and retained approximately 94.8% of its photocatalytic activity after six recycling cycles.189
 | ||
| Fig. 7 (a) Mott–Schottky plots of MZ-5@Carboxyl cotton [reproduced from ref. 189 with the permission from Elsevier, copyright 2016]. (b) FTIR spectra of the prepared samples (Ag@ZIF-8 denotes 15% Ag+ doped ZIF-8). (c) TEM images of Ag-15%@ ZIF-8 crystals. (d) Photodegradation performance of pure ZIF-8 and different amounts of Ag-doped ZIF-8 for eliminating RhB. (e) The proposed dye degradation mechanism of Ag@ZIF-8 photo-catalyst under visible-light irradiation [reproduced from ref. 176 with the permission from Elsevier, copyright 2020]. (f) Photodegradation first order rate constant of dyes by different samples in visible LED light: (1) without catalyst (only LED), (2) LED/H2O2, (3) pristine ZIF-8, (4) physical mixture of prior AgNO3 and ZIF-8, (5) Ag-10%@ZIF-8, and (6) Ag-15%@ZIF-8 [reproduced from ref. 176 with the permission from Elsevier, copyright 2020]. (g) SEAD pattern of SZ2 [reproduced from ref. 177 with the permission from Elsevier, copyright 2020]. (h) LSV curves of ZIF-8, AgBr, and 40 wt% AgBr/ZIF-8 photocatalysts in 0.5 M Na2SO4 electrolyte [reproduced from ref. 191 with the permission from Elsevier, copyright 2019]. (i) PL spectra of ZIF-8, Ag3PO4, and Ag3PO4@ZIF-8 nanocomposite [reproduced from ref. 192 with the permission from Elsevier, copyright 2023]. | ||
Doping MOFs with active agents enhances photocatalytic efficiency. The challenge lies in developing cost-effective, stable doped nanomaterials with precisely-controlled properties for efficient visible light absorption. In light of this, Thanh et al. synthesized Fe-doped ZIF-8 with varying molar ratios of Zn/Fe and evaluated the photoinduced photocatalytic activity of the resulting materials for decomposing Remazol deep black B (RDB). The XRD analysis of Fe-doped ZIF-8 displayed the typical peaks of ZIF-8, with no detectable peaks for iron oxide. Nonetheless, the intensity of these peaks diminished as the iron content was increased. The peaks for Fe 2p3/2 were observed at 710 and 709 eV, suggesting the presence of Fe(II) and Fe(III). The N2 adsorption/desorption isotherms for ZIF-8 and Fe-ZIF-8, both exhibiting type IV with H4 hysteresis, are typical of mesoporous materials. The Fe-ZIF-8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) sample displayed a slightly altered shape at high relative pressure, indicating structural distortion due to iron oxide incorporation. ZIF-8 had a specific surface area of 1484 m2 g−1, which decreased with iron addition to 1469, 1104, and 735 m2 g−1 for Fe-ZIF-8 (1
9) sample displayed a slightly altered shape at high relative pressure, indicating structural distortion due to iron oxide incorporation. ZIF-8 had a specific surface area of 1484 m2 g−1, which decreased with iron addition to 1469, 1104, and 735 m2 g−1 for Fe-ZIF-8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9), (2
9), (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8), and (3
8), and (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7), respectively, confirming that iron oxide encapsulation reduced the accessible void space. To assess the sustainability of Fe-ZIF-8 for RDB removal, the used Fe-ZIF-8 was renewed by drying at 100 °C and then used again for photocatalytic decomposition of the RDB dye. After three cycles, the decolorization efficiency of Fe-ZIF-8 catalysts was 90–95% of that observed with the fresh catalysts.193
7), respectively, confirming that iron oxide encapsulation reduced the accessible void space. To assess the sustainability of Fe-ZIF-8 for RDB removal, the used Fe-ZIF-8 was renewed by drying at 100 °C and then used again for photocatalytic decomposition of the RDB dye. After three cycles, the decolorization efficiency of Fe-ZIF-8 catalysts was 90–95% of that observed with the fresh catalysts.193
Jafar Abdi and team synthesized a porous Ag-doped ZIF-8 photocatalyst at ambient temperature using a facile and rapid synthesis. This catalyst was employed for the degradation of both MO and RhB dyes. The FTIR spectrum of Ag@ZIF-8 confirms that the ZIF-8 framework remains intact, with key absorption bands preserved. The broad band at 3400 cm−1 and the band at 1633 cm−1 correspond to the O–H stretching and bending vibrations, respectively. These hydroxyl groups, linked to adsorbed water, are essential for photocatalytic activity (Fig. 7b). ZIF-8 crystals possess a rhombic dodecahedron shape with a mesoporous structure. Fig. 7c demonstrates that Ag nanoparticles are well-dispersed throughout the ZIF-8 framework with little aggregation. The XRD spectrum of Ag@ZIF-8 shows preserved ZIF-8 peaks with no contaminants, while decreased intensity indicates Ag NP integration. EIS experiments revealed that the Ag-15%@ZIF-8 exhibited a narrower Nyquist plot arc radius compared with pure ZIF-8, reflecting enhanced light-induced charge migration and better exciton separation. This sample also illustrates the maximum photocatalytic efficiency, with first-order kinetic rate constants of 0.1016 min−1 for RhB and 0.2102 min−1 for MO. Photodegradation data (Fig. 7d) indicated that Ag NP enhanced dye removal. Increasing Ag content to 20 wt% improved performance, but higher doping did not yield further benefits. The rate constant (k) for decolorization was calculated using a first-order kinetic model (Fig. 7e). The Ag-15%@ZIF-8 photocatalyst mineralized over 93% of the dyes after 4 cycles (Fig. 7f). The photocatalytic efficiency of Ag-doped ZIF-8 was superior to that of a physical mixture of pure ZIF-8 and AgNO3, because of the enhanced performance of the heterojunction formed between them.176
Silver (Ag) has garnered significant attention as a coupling material. Silver nanoparticles and compounds, including Ag3VO4, Ag2O, Ag2S, Ag3PO4, Ag2CO3, and particularly silver halides (AgX, where X = I, Br, and Cl), are extensively used for purifying organically contaminated wastewater through photocatalytic degradation.194–198 The combination of elemental silver with silver halides enhances their activity in the visible light spectrum. Chandra et al. introduced a novel composite, ZIF8/AgNPs, designed for the photocatalytic degradation of MB. With its remarkable surface area of 1370.91 m2 g−1 and distinctive synergistic properties, the composites, labeled as SZ, SZ1, SZ2, and SZ3, varied in silver content. The size of the encapsulated silver NPs increased proportionally with their dosing amount. The crystalline nature of AgNPs, SZ2, and SZ composites was verified through the SEAD pattern depicted in Fig. 7g, respectively. Using the Tauc method, the band gap in all composites has been determined to be 5.27 eV and 5.13 eV, respectively, attributed to ZIF-8. However, the SZ composite, which contains a significant amount of Ag NPs, shows a broad band at 2.16 eV and 1.62 eV. This is because of the Ag and the imidazole ring interactions. Notably, SZ2, synthesized using a 300 mL suspension of Ag NPs, exhibited exceptional photocatalytic efficiency, achieving a 97.25% degradation rate for MB and complete degradation for CR at pH ≥ 7, while SZ, SZ1, SZ3, and ZIF-8 can decompose off 85.56, 90.56, 86.43 and 84.18% MB and 98.09, 96.18, 97.7 and 95.80% CR. This clearly illustrates the greatly improved photocatalytic performance of SZ2 and the superior activity of SZ1 compared with ZIF-8.177
Silver halide photocatalysts, recognized for their strong visible light response and exceptional photoactivity, have attracted considerable interest from researchers. AgX (X = Cl, Br, I) has been confirmed as an effective co-catalyst. In this context, He and colleagues described the synthesis of a ZIF-8/AgBr composite through the solvothermal method, utilized for the light-activated degradation of MB under light exposure. The SEM image of 40 wt% AgBr/ZIF-8 reveals AgBr particles randomly distributed on the ZIF-8 surface, a finding confirmed by EDS analysis. Optical analysis demonstrated enhanced light absorption capacity due to the combination of ZIF-8 and AgBr. Moreover, a bathochromatic shift in the spectral absorption band was observed with higher AgBr content. The LSV curves in 0.5 M Na2SO4 show that AgBr exhibits a greater electrical density than ZIF-8, aligning with the PC results. The 40 wt% of the composite further enhances the electrical density, highlighting the synergistic impact of AgBr and ZIF-8 on electron transfer (Fig. 7h). The improved efficiency in charge separation stemmed from the synergistic impact of ZIF-8 and AgBr coupling. The recorded rate of MB degradation was 0.0273 min−1, and the degradation performance achieved 99.5% under visible light exposure for 60 minutes. Stability and recyclability tests revealed that the 40 wt% of AgBr/ZIF-8 photocatalyst demonstrated reliable photocatalytic performance, maintaining its effectiveness through six repeated cycles.191
A study by Liu and his group demonstrated that the effect of incorporating ZIF-8 on the photocatalytic activity of Ag/AgCl was discussed. Initially, Ag/AgCl NPs were incorporated on the ZIF-8 surface with a 50 wt% loading, resulting in the synthesis of a (50%) Ag/AgCl/ZIF-8 composite. This hybrid displayed significant absorption of 420–800 nm in the wavelength range due to the SPR phenomenon of Ag particles generated in situ on the surface of AgCl nanoparticles. In comparison with Ag/AgCl alone, the composite demonstrated excellent photocatalytic performance, attributed to the bifunctional activity of ZIF-8: adsorption of RhB and production of superoxide species, contributing to RhB mineralization. The photocatalytic efficiency of the hybrid in degrading MB was calculated without catalyst, with ZIF-8 alone, and with Ag/AgCl@ZIF-8 under UV light. Without a catalyst, MB degradation reached 67.31% over 90 min, while ZIF-8 alone exhibited limited improvement, achieving 51.53% degradation after 60 min of UV irradiation. In contrast, Ag/AgCl@ZIF-8 showed remarkable performance, achieving nearly 100% degradation within 20 min of UV exposure, despite minimal removal (9.25%) in the dark. The study concluded that while ZIF-8 alone had marginal effects, Ag/AgCl@ZIF-8 significantly increased photocatalytic performance, primarily attributed to improved photocatalysis.178
Guan et al. prepared a composite material composed of Ag/AgCl/ZIF-8/TiO2/C on cotton fabric using a straightforward method. The Ag/AgCl/ZIF-8/TiO2/C photocatalyst achieved a methylene blue degradation activity of 98.5% in 1 h 45 minutes under visible light, exhibiting a first-order kinetic rate constant of 0.0332 min−1. Additionally, the photocatalyst maintained an 85% degradation efficiency of MB after three cycles, indicating its excellent stability. They demonstrated that the ZIF-8 in the composite promoted oxygen molecules throughout the photocatalytic decomposition, and photogenerated holes in the photocatalyst's VB oxidized water molecules to OH, resulting in the formation of hydroxyl OH˙ and O2˙−, which were the active species liable for the photodegradation of MB during this process.199
Silver orthophosphate (Ag3PO4), a p-type semiconductor, has garnered significant attention in organic pollutant remediation through impressive visible light capture.200 Considering this, Motora et al. synthesized a novel Ag3PO4@ZIF-8 p–n heterojunction using the chemical precipitation method at ambient temperature. The photocatalytic efficiency of this new photocatalyst was assessed for organic pollutants, including Congo Red, Crystal violet, and rhodamine B. The Ag3PO4@ZIF-8 heterojunction displayed distinct peaks for both Ag3PO4 and ZIF-8, with no extra peaks detected, verifying that the heterojunction was successfully synthesized without any impurities. The PL spectra in Fig. 7i demonstrated that the Ag3PO4@ZIF-8 heterojunction had lower PL intensity, indicating reduced charge carrier recombination. Furthermore, the photoinduced current demonstrated that the Ag3PO4@ZIF-8 heterojunction revealed the greatest photocurrent among ZIF-8 and Ag3PO4, indicating superior light absorption. This suggests remarkable electron–hole separation and reduced electron–hole recombination, which improves the photocatalytic activity of the heterojunction. Furthermore, the EIS semicircle radius for the Ag3PO4@ZIF-8 heterojunction was lower than Ag3PO4 and ZIF-8, reflecting enhanced current-carrying capacity, which is critical for effective photocatalysis. The developed photocatalyst showed exceptional photocatalytic activity, degrading 90.7% of CV, 94.7% of CR, and 99.0% of RhB within 2 hours. The reusability of the photocatalyst, which is crucial for its practical application, was also examined. The Ag3PO4@ZIF-8-5% heterojunction demonstrated superior cyclic stability over five cycles, verifying its strong potential for real-world wastewater treatment applications.192
CQDs are fluorescent nanomaterials with a carbon framework and nanocrystals sized 2–10 nm. They are valued for low toxicity, high biocompatibility, tunable luminescence, and easy modification, making them a promising candidate for photocatalysis due to their fluorescence emission across the near-infrared to blue spectrum. Taking these aspects, Si et al. employed an impregnation method in which ZIF-8 structures were modified with carbon quantum dots and evaluated for their ability to remove methylene blue from water. The ultra-high resolution confocal laser scanning microscope (CLSM) image reveals the external morphology of CQDs@ZIF-8. CQDs emit fluorescence between 450 and 470 nm. It shows their distribution within ZIF-8, confirming the successful construction of the core–shell structure. The ZIF-8@CQD hybrid demonstrated a higher absorption capacity and improved responsiveness to visible light due to its core–shell structure, achieving a 91% removal activity for MB and a rate constant that was six times higher than that of ZIF-8 alone. Furthermore, the ZIF-8@CQD photocatalyst maintained excellent structural stability and catalytic activity over three reuse cycles without any loss of performance.183
Studies indicate that combining ZIF-8 with materials such as graphitic carbon nitride, and zinc oxide not only boosts the degradation rates but also enhances the reusability and durability of the photocatalysts. Investigating the underlying mechanisms of charge transfer and degradation pathways in these composites could also provide deeper insights and lead to the improvement of more efficient and versatile photocatalytic systems for various environmental applications.
4.5 Drug degradation
The photocatalytic degradation of drugs involves three key steps: photon absorption, excitation, and reaction. Photogenerated carriers separate and migrate to the photocatalyst surface, where they trigger secondary reactions with adsorbed substances. Photogenerated holes can directly degrade drugs. There are two primary degradation pathways: a reductive pathway, which occurs if the conduction band (CB) potential is negative relative to the O2/O2˙− redox potential (−0.13 eV vs. RHE), and in this pathway, photoexcited electrons react with O2 to produce superoxide radicals (O2˙−); and an oxidative pathway, where holes on the photocatalyst surface generate hydroxyl radicals (˙OH) from H2O/OH−, depending on the pH of the medium. Hydrogen ions can recombine with electrons, producing heat and decreasing photodegradation efficiency. The photocatalyst's redox potential needs to be greater than ˙OH/OH− (+1.99 eV vs. RHE). Reactive radicals (˙OH and O2˙−) can mineralize antibiotics and their intermediates into carbon dioxide and water under extended UV exposure. Both pathways work together to prevent electron accumulation in the CB and reduce electron–hole recombination more effectively than direct interaction pathways.201–204 | (17) |
| h+ + Drug → H2O + CO2 + Degradation products | (18) |
| O2 + e− → O2˙− | (19) |
| H2O/OH− + h+ → OH˙ + H+ | (20) |
| H+ + e− → energy | (21) |
| Drugs + OH˙/O2˙− → CO2 + H2O + Degradation products | (22) |
Metal oxides are widely used in photocatalysis due to their metal content. TiO2 is considered an excellent photocatalyst for its non-toxic nature, cost-effectiveness, and high efficiency. Li et al. synthesized ZIF-8@TiO2 microcomposites via a hydrothermal method. The composite retains a dodecahedron shape with textured surfaces. Vibrational peaks at 565 cm−1 and 486 cm−1 correspond to N–Ti–O and Ti–O–Ti bonds, indicating TiO2 deposition on ZIF-8 through chemical bonding rather than physical mixing (Fig. 8a). XPS confirms Ti4+ binding with ZIF-8's imidazole groups to form N–Ti–O bonds, while EDX mapping shows Ti and O elements dispersed on ZIF-8@TiO2. Under 40 minutes of irradiation, ZIF-8@TiO2 achieves a TC degradation rate of ∼90%, surpassing TiO2 and ZIF-8 (<70%). Trapping experiments revealed O2˙− as the key reactive species, with benzoquinone (BQ) reducing degradation from 95% to 59%. Other species, such as OH and e−, played minor roles. The effectiveness order was: no scavenger > IPA > CCl4 > AO > BQ, confirming O2˙− as the most crucial active species. The reusability and stability of the ZIF-8@TiO2 composite across five degradation cycles, the TC removal rate decreased slightly from 95% to 86%, likely due to the loss of photocatalyst during recovery. This outcome shows that the ZIF-8@TiO2 composite exhibits strong stability and reusability, with its internal interactions ensuring sustained high efficiency.93
 | ||
| Fig. 8 (a) FTIR spectra of ZIF-8@TiO2 [reproduced from ref. 93 with the permission from Elsevier, copyright 2020]. (b) XPS survey spectra of C0.7/Z0.3 sample. (c) ESR spectra of DMPO-O2 of the Cu2O, ZIF-8 and C0.7/Z0.3 [reproduced from ref. 166 with the permission from Elsevier, copyright 2021]. (d) N2 adsorption–desorption isotherm of ZIF-8, g-C3N4, ZIF-8 and 10%-AZCN samples [reproduced from ref. 169 with the permission from Elsevier, copyright 2019]. | ||
Cu2O, with a bandgap of 1.8–2.2 eV, is affordable, eco-friendly, and effective under visible light. Zhou et al. developed Cu2O/ZIF-8 (C/Z) via an in situ growth method. XRD confirmed the synthesis of Cu2O and ZIF-8, while XPS identified key elements: N 1s (398.7 eV) confirmed the N–C bond, with Cu+ peaks at 951.8 eV and 932 eV, and Zn 2p peaks at 1021.5 eV and 1044.7 eV (Fig. 8b). The C/Z composite demonstrated improved photocatalytic performance for tetracycline (TC) degradation because of the narrow bandgap of Cu2O and the increased surface area of ZIF-8, which is 3.3 times greater than Cu2O alone, offering increased adsorption sites. This led to a higher response to visible light and greater charge carrier density compared with individual components. The composite also showed superior photocurrent density and charge transfer efficiency, as indicated by a smaller semicircle diameter in the Nyquist plot. ESR analysis confirmed the generation of free radicals during photocatalysis, with O2˙− being the primary radical, supported by weaker ˙OH signals (Fig. 8c). The C/Z composite achieved over 84.1% TC degradation under visible light within 2 hours, with efficiency slightly decreasing to 75.4% after four cycles. Additionally, the removal rates of TC, oxytetracycline, and tetracycline hydrochloride under visible light were 84.1%, 52.2%, and 73.5%, respectively.166
Graphitic carbon nitride, having a band gap of 2.7 eV, enhanced the reactive sites and the performance of carrier separation in the photocatalyst. The combination of Ag/AgCl@ZIF-8 with g-C3N4 successfully addressed both the extensive band gap of ZIF-8 and the limited surface area of g-C3N4. As previously mentioned, Fan et al. prepared an Ag/AgCl@ZIF-8 composite that demonstrated superb photocatalytic performance.178 Furthermore, Zhou et al. modified this composite by loading g-C3N4 (AZCN) onto its surface to remove levofloxacin (LVFX) by using the facile stirring method. The homogeneous distribution of Ag, Zn, C, N, and Cl validates the successful synthesis of the composite by TEM EDX mapping. The optical characteristics of the photocatalysts were evaluated by UV–vis DRS (Fig. 8d), where g-C3N4 shows an absorption edge around 440 nm in the visible region, while ZIF-8's absorption edge is at 246 nm. The Ag/AgCl@ZIF-8 sample exhibits an absorption range from 300 to 800 nm. The PL spectra, where the 10%-AZCN sample exhibits the smallest peak intensity, indicate that the optimal ratio of Ag/AgCl@ZIF-8 to g-C3N4 enhances photoelectron transfer effectiveness and maximizes photocatalytic activity. LC-MS measurement revealed that the AZCN composite, in conjunction with PMS, effectively breaks down LVFX into smaller molecules by sequentially eliminating diverse functional groups, ultimately oxidizing it to CO2 and H2O. In addition to photocatalysis, advanced oxidation processes (AOPs) were used in drug wastewater purification. Peroxymonosulfate (PMS) can produce SO4˙− radicals when activated by other reactive species. These radicals oxidize most contaminants and assist other reactive species in the synergistic degradation of LVFX. Without PMS, AZCN achieved only a 53.6% degradation rate for LVFX.169
The photocatalytic degradation of drugs using ZIF-8-based composites reveals their potential through effective photon absorption and reactive radical generation. Integrating ZIF-8 with various materials enhances photocatalytic performance by improving charge carrier dynamics and radical production, leading to higher degradation rates for various drugs, such as tetracycline and levofloxacin. Future work should focus on refining these composites and exploring new materials to address diverse drug contaminants and improve practical applications in wastewater treatment.
5. Conclusion
Despite their relatively brief development history, ZIFs have garnered significant attention, leading to numerous achievements in recent years. ZIF-8-based materials are increasingly utilized in energy generation and wastewater treatment due to their catalytic activity and high porosity. This review provides in-depth overviews and analysis of the synthesis and applications of ZIF-8-based photocatalysts and their composites in light-mediated H2 evolution, H2O2 evolution, CO2 reduction, and pollutant removal. ZIF-8 has unique potential for developing alternative photocatalysts due to their exceptional properties, including effective charge separation, abundant active sites, and persistent porosity. Constructing catalyst composites from ZIF-8 and other functional materials offers several advantages for photocatalytic implementations over traditional single catalyst methods. Photoactive semiconductors or molecules in the composite enhance ZIF-8 solar light utilization. ZIF-8-based composites, with their high surface areas, provide active sites, prevent nanoparticle aggregation, and act as electron acceptors for charge carrier separation. Strong interfacial connections between ZIF-8 and semiconductors harvest visible light enhance electron transfer. As a result, ZIF-8-based composites typically demonstrate much greater photocatalytic efficiency compared with catalysts that consist of only one component. Currently, there is substantial interest in developing ZIF-8-based photocatalysts for environmental remediation and energy production that remain stable in the complex while being cost-effective and environmentally friendly. Although this field has made significant progress, much work remains before these photocatalysts can be widely applied (Fig. 9). However, more research is necessary to make ZIF-8 composite components viable for extensive photocatalytic application, as they are currently expensive, and we believe that current studies only scratch the surface of their potential. Our reasoning is based on the following points:I. Most ZIF-based materials are microporous, with pore diameters less than 2 nm. This can limit their effectiveness, particularly when dealing with larger pollutant molecules due to steric hindrance. Therefore, it is beneficial to synthesize hierarchically porous materials from ZIFs that incorporate a range of pore sizes, including mesopores, macropores, and micropores.
II. Numerous synthetic techniques have been developed for both research and large-scale production of ZIF-8, yet achieving controlled synthesis with high crystallinity and uniform size remains difficult. Uniform size and crystallinity are crucial for application performance, especially in small-scale devices and electron conduction applications.
III. ZIF-8 has relatively low water stability due to the susceptibility of its coordination bonds to water. Enhancing the long-term water stability of ZIF-8 in a cost-effective manner continues to be a significant challenge.
IV. The chemical and thermal stability of ZIF-8-based composites must be carefully addressed for industrial implementations. Additionally, the catalytic sites and porosity should be managed skilfully.
V. Solar-active ZIF-8-based composites for hydrogen evolution could significantly contribute to renewable global energy solutions. However, ZIF-8 has a broadband gap, restricting its potential for solar energy utilization. Therefore, an adjustment approach to enhance sensitivity to visible light in both pristine ZIF-8 and its composites is crucial. Incorporating plasmonic materials can be advantageous, but it is essential to keep the cost-effectiveness in check.
VI. Multiple reaction parameters, such as temperature, ionic strength, and pH, are analyzed to assess their impact on pollutant degradation performance. Exploring the relationship between these variables and the effectiveness of ZIF-8 composites is essential to provide valuable references for the most effective synthesis and reaction parameters. This is crucial for the rational fabrication of adsorbents and photocatalysts. Furthermore, ZIF-8 composites must maintain long-term stability in various environmental conditions and be produced cost-effectively for industrial applications. Research should aim to improve solar light absorption efficiency, lower the rate of electron–hole pair recombination, and consider the recyclability of these materials as also important for practical applications.
VII. Photodegradation of organic contaminants using ZIF-8 and its composites is a highly emerging technology for pollution control owing to its low cost and eco-friendly nature. Currently, most research focuses on dye degradation, like RB, MB, and MO. However, there is a need to broaden the scope to include a wider range of organic impurities, such as perfluorinated organic chemicals (PFCs), along with pharmaceutical and personal care products (PPCPs) to better evaluate the photocatalytic decomposition capabilities of these components. It has been recommended to use actual polluted water rather than simulated wastewater and to measure the degradation efficiency by assessing the total organic carbon (TOC) removal percentage along with the highest absorbance of the targeted pollutant. Additionally, to advance toward industrial applications, extensive research is needed to understand the factors influencing photocatalytic efficiency, including co-existing ions, dissolved organic matter, and pH levels.
VIII. Numerous questions concerning the underlying mechanisms still require thorough clarification. In particular, the photocatalytic processes, including the separation of charge carriers generated by light and charge migration within multi-constituent systems, remain insufficiently understood for certain synthesized materials. Gaining a deeper insight into these catalytic mechanisms is crucial for developing next-generation photocatalysts with superior photocatalytic performance. To achieve this, integrating in situ characterization methods combined with theoretical simulations might be essential to clarify these mechanisms.
IX. The reclaimability of photocatalysts from aqueous solutions is vital for practical applications. Most ZIF-8/ZIF-8-based composite photocatalysts are solid powders that, despite being stable in aqueous solutions, have a diffusive nature that makes them challenging to separate from the reaction solution for recycling. It is significant to enhance novel ZIF-8/ZIF-8-based composite materials that can be more easily separated from solutions, such as forming films/membranes or shaping them into pellets/monoliths. Additionally, toxicity analyses have not yet been undertaken, which is vital for their resilient use.
X. A wide range of ZIF-8 composites have been developed, and even small variations in synthesis conditions can lead to significantly different properties. Despite continuous advancements in characterization technologies, examining the interface between ZIF-8 and the incorporated materials remains essential. In situ techniques like SEM, TEM, FTIR, and XPS are commonly employed to study the formation of ZIF-8 composites. Future research should, therefore, aim to explore the synergistic mechanisms of these composites using advanced analytical methods.
XI. Lastly, eco-friendly ZIF-8-based materials are essential for widespread practical applications in energy production and water treatment. The environmentally friendly properties are crucial from a pragmatic standpoint. Therefore, greater emphasis should be placed on achieving a low production-cost ratio while using biocompatible constituents (Fig. 10).
 | ||
| Fig. 10 Schematic representation of challenges and future research directions toward ZIF-8-based nanomaterials for photocatalytic applications. | ||
Author contributions
Diptirani Behera: conceptualization, writing – original draft, visualization. Priyanka Priyadarshini: conceptualization, writing – original draft editing and review. Kulamani Parida: conceptualization, visualization, supervision.Data availability
The submitted review article synthesizes and discusses data that have already been published and are publicly available from the sources cited in the manuscript. No new data were generated during the preparation time frame of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors express their profound gratitude toward Siksha ‘O’ Anusandhan (Deemed to be University) for giving all the necessary facilities and financial support to carry out this immense research work. I am thankful to Anshumika Mishra for her technical support.References
- P. Raizada, P. Shandilya, P. Singh and P. Thakur, J. Taibah Univ. Sci., 2017, 11, 689–699 CrossRef.
- P. Singh, K. Sharma, V. Hasija, V. Sharma, S. Sharma, P. Raizada, M. Singh, A. K. Saini, A. Hosseini-Bandegharaei and V. K. Thakur, Elsevier Ltd, 2019, preprint, DOI:10.1016/j.mtchem.2019.08.005.
- V. Hasija, A. Sudhaik, P. Raizada, A. Hosseini-Bandegharaei and P. Singh, J. Environ. Chem. Eng., 2019, 7, 103272 CrossRef CAS.
- S. Bolisetty, M. Peydayesh and R. Mezzenga, Royal Society of Chemistry, 2019, preprint, 10.1039/c8cs00493e.
- M. S. Rahaman, H. Thérien-Aubin, M. Ben-Sasson, C. K. Ober, M. Nielsen and M. Elimelech, J. Mater. Chem. B, 2014, 2, 1724–1732 RSC.
- S. Patial, V. Hasija, P. Raizada, P. Singh, A. A. P. Khan Singh and A. M. Asiri, J. Environ. Chem. Eng., 2020, 8, 103791 CrossRef CAS.
- P. Thakur, P. Raizada, P. Singh, A. Kumar, A. A. P. Khan and A. M. Asiri, Arabian J. Chem., 2020, 13, 8271–8300 CrossRef CAS.
- K. Sharma, P. Raizada, A. Hosseini-Bandegharaei, P. Thakur, R. Kumar, V. K. Thakur, V. H. Nguyen and S. Pardeep, Process Saf. Environ. Prot., 2020, 142, 63–75 CrossRef CAS.
- L. Biswal, S. P. Tripathy, S. Dash, S. Das, S. Subudhi and K. Parida, Mater. Adv., 2024, 5, 4452–4466 RSC.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Mariñas and A. M. Mayes, Science and technology for water purification in the coming decades, 2009 Search PubMed.
- A. Rahmani-Sani, P. Singh, P. Raizada, E. Claudio Lima, I. Anastopoulos, D. A. Giannakoudakis, S. Sivamani, T. A. Dontsova and A. Hosseini-Bandegharaei, Bioresour. Technol., 2020, 297, 122452 CrossRef CAS PubMed.
- P. Raizada, A. Sudhaik and P. Singh, KeAi Communications Co., 2019, preprint, DOI:10.1016/j.mset.2019.04.007.
- P. Raizada, S. Sharma, A. Kumar, P. Singh, A. A. P. Khan and A. M. Asiri, J. Environ. Chem. Eng., 2020, 8, 104230 CrossRef CAS.
- S. Malato, P. Fernández-Ibáñez, M. I. Maldonado, J. Blanco and W. Gernjak, 2009, preprint, DOI:10.1016/j.cattod.2009.06.018.
- H. Choi, M. G. Antoniou, A. A. de la Cruz, E. Stathatos and D. D. Dionysiou, Desalination, 2007, 202, 199–206 CrossRef CAS.
- S. Nayak, K. Kumar Das and K. Parida, J. Colloid Interface Sci., 2023, 634, 121–137 CrossRef CAS PubMed.
- S. K. Sahoo, L. Acharya, L. Biswal, P. Priyadarshini and K. Parida, Royal Society of Chemistry, 2024, preprint, 10.1039/d4qi00950a.
- H. Huang, L. Jiang, J. Yang, S. Zhou, X. Yuan, J. Liang, H. Wang, H. Wang, Y. Bu and H. Li, Renewable Sustainable Energy Rev., 2023, 173, 113110 CrossRef CAS.
- J. Yang, H. Wang, L. Jiang, H. Yu, Y. Zhao, H. Chen, X. Yuan, J. Liang, H. Li and Z. Wu, Chem. Eng. J., 2022, 427, 130991 CrossRef CAS.
- A. Mishra, N. Priyadarshini, S. Mansingh and K. Parida, Adv. Colloid Interface Sci., 2024, 103300 Search PubMed.
- J. Sahu, D. Prusty, S. Mansingh and K. Parida, Elsevier Ltd, 2023, preprint, DOI:10.1016/j.ijhydene.2023.04.109.
- S. Tuckute, S. Varnagiris, M. Urbonavicius, M. Lelis and S. Sakalauskaite, Appl. Surf. Sci., 2019, 489, 576–583 CrossRef CAS.
- H. Yi, M. Yan, D. Huang, G. Zeng, C. Lai, M. Li, X. Huo, L. Qin, S. Liu, X. Liu, B. Li, H. Wang, M. Shen, Y. Fu and X. Guo, Appl. Catal., B, 2019, 250, 52–62 CrossRef CAS.
- M. W. Kadi, R. M. Mohamed and A. A. Ismail, Ceram. Int., 2020, 46, 8819–8826 CrossRef CAS.
- S. Sa-nguanprang, A. Phuruangrat, T. Thongtem and S. Thongtem, Inorg. Chem. Commun., 2020, 117, 107944 CrossRef CAS.
- K. S. Bhavsar, P. K. Labhane, R. B. Dhake and G. H. Sonawane, Chem. Phys. Lett., 2020, 744, 137202 CrossRef CAS.
- G. J. Lee and J. J. Wu, Elsevier B.V., 2017, preprint, DOI:10.1016/j.powtec.2017.05.022.
- S. B. Atla, W. R. Lin, T. C. Chien, M. J. Tseng, J. C. Shu, C. C. Chen and C. Y. Chen, Mater. Chem. Phys., 2018, 216, 380–386 CrossRef CAS.
- J. Carbajo, M. Jiménez, S. Miralles, S. Malato, M. Faraldos and A. Bahamonde, Chem. Eng. J., 2016, 291, 64–73 CrossRef CAS.
- Sonu, V. Dutta, S. Sharma, P. Raizada, A. Hosseini-Bandegharaei, V. Kumar Gupta and P. Singh, Elsevier B.V., 2019, preprint, DOI:10.1016/j.jscs.2019.07.003.
- J. Sahu, S. Mansingh, B. P. Mishra, D. Prusty and K. Parida, Dalton Trans., 2023, 52, 16525–16537 RSC.
- W. K. Fan and M. Tahir, Elsevier Ltd, 2022, preprint, DOI:10.1016/j.enconman.2021.115180.
- Y. Li, H. Xu, S. Ouyang and J. Ye, Royal Society of Chemistry, 2016, preprint, 10.1039/c5cp05885f.
- S. Dash, S. P. Tripathy, S. Subudhi, L. Acharya, A. Ray, P. Behera and K. Parida, Energy Adv., 2024, 3, 1073–1086 RSC.
- A. Phan, C. J. Doonan, F. J. Uribe-Romo, C. B. Knobler, M. Okeeffe and O. M. Yaghi, Acc. Chem. Res., 2010, 43, 58–67 CrossRef CAS PubMed.
- O. M. Yaghi, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Reticular synthesis and the design of new materials, 2003 Search PubMed.
- K. Li, Y. Zhang, Y. Z. Lin, K. Wang and F. T. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 28918–28927 CrossRef CAS PubMed.
- P. Priyadarshini and K. Parida, Elsevier Ltd, 2024, preprint, DOI:10.1016/j.est.2024.111379.
- J. Ran, H. Zeng, J. Cai, P. Jiang, P. Yan, L. Zheng, Y. Bai, X. Shen, B. Shi and H. Tong, Chem. Eng. J., 2018, 333, 20–33 CrossRef CAS.
- Y. Liu, Z. Zhu, X. Pei, X. Zhang, X. Cheng, S. Hu, X. Gao, J. Wang, J. Chen and Q. Wan, ACS Appl. Mater. Interfaces, 2020, 12, 36978–36995 CrossRef CAS PubMed.
- M. Ahmad, M. Yousaf, W. Cai and Z. P. Zhao, Chem. Eng. J., 2023, 453, 139846 CrossRef CAS.
- X. Tang, J. H. Zhao, Y. H. Li, Z. J. Zhou, K. Li, F. T. Liu and Y. Q. Lan, Dalton Trans., 2017, 46, 10553–10557 RSC.
- A. Karakeçili, B. Topuz, S. Korpayev and M. Erdek, Mater. Sci. Eng., C, 2019, 105, 110098 CrossRef PubMed.
- L. Jiao, J. Y. R. Seow, W. S. Skinner, Z. U. Wang and H. L. Jiang, Elsevier B.V., 2019, preprint, DOI:10.1016/j.mattod.2018.10.038.
- H. Kaur, G. C. Mohanta, V. Gupta, D. Kukkar and S. Tyagi, J. Drug Delivery Sci. Technol., 2017, 41, 106–112 CrossRef CAS.
- A. Dhakshinamoorthy, Z. Li and H. Garcia, Royal Society of Chemistry, 2018, preprint, 10.1039/c8cs00256h.
- W. Zhen, B. Li, G. Lu and J. Ma, Chem. Commun., 2015, 51, 1728–1731 RSC.
- V. Pascanu, G. González Miera, A. K. Inge and B. Martín-Matute, American Chemical Society, 2019, preprint, DOI:10.1021/jacs.9b00733.
- S. R. Batten, B. Chen and J. J. Vittal, ChemPubSoc., 2016, 81, 669–670 CAS.
- Y. V. Kaneti, S. Dutta, M. S. A. Hossain, M. J. A. Shiddiky, K. L. Tung, F. K. Shieh, C. K. Tsung, K. C. W. Wu and Y. Yamauchi, Adv. Mater., 2017, 29, 1700213 CrossRef PubMed.
- T. He, Z. Huang, S. Yuan, X. L. Lv, X. J. Kong, X. Zou, H. C. Zhou and J. R. Li, J. Am. Chem. Soc., 2020, 142, 13491–13499 CrossRef CAS PubMed.
- P. Behera, A. Ray, S. P. Tripathy, L. Acharya, S. Subudhi and K. Parida, J. Photochem. Photobiol., A, 2023, 436, 114415 CrossRef CAS.
- L. Jiang, S. Zhou, J. Yang, H. Wang, H. Yu, H. Chen, Y. Zhao, X. Yuan, W. Chu and H. Li, Adv. Funct. Mater., 2022, 32, 2108977 CrossRef CAS.
- P. A. Desario and K. A. Gray, in Metropolitan Sustainability: Understanding and Improving the Urban Environment, Elsevier Ltd, 2012, pp. 292–316 Search PubMed.
- M. P. M. Dicker, P. F. Duckworth, A. B. Baker, G. Francois, M. K. Hazzard and P. M. Weaver, Elsevier Ltd, 2014, preprint, DOI:10.1016/j.compositesa.2013.10.014.
- Z. C. Kong, J. F. Liao, Y. J. Dong, Y. F. Xu, H. Y. Chen, D. Bin Kuang and C. Y. Su, ACS Energy Lett., 2018, 3, 2656–2662 CrossRef CAS.
- A. Chakraborty, D. A. Islam and H. Acharya, J. Solid State Chem., 2019, 269, 566–574 CrossRef CAS.
- X. Liu, J. Zhang, Y. Dong, H. Li, Y. Xia and H. Wang, New J. Chem., 2018, 42, 12180–12187 RSC.
- K. S. Park, Z. Ni, A. P. Cô, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O′keeffe and O. M. Yaghi, Exceptional chemical and thermal stability of zeolitic imidazolate frameworks, 2006 Search PubMed.
- Y. Pan, Y. Liu, G. Zeng, L. Zhao and Z. Lai, Chem. Commun., 2011, 47, 2071–2073 RSC.
- Y. Song, C. Yu, D. Ma and K. Liu, Elsevier B.V., 2024, preprint, DOI:10.1016/j.ccr.2023.215492.
- K. Shalini, K. Sharma and N. Kumar, Der Chemica Sinica, 2011, 7, 668–677 CAS.
- P. Molina, A. Tárraga and F. Otón, 2012, preprint, 10.1039/c2ob06808g.
- A. Jayashree, B. Narayana, S. M. Kumar, K. R. Raghi, B. K. Sarojini and T. K. M. Kumar, Chem. Data Collect., 2019, 21, 100237 CrossRef CAS.
- H. Bux, F. Liang, Y. Li, J. Cravillon, M. Wiebcke and J. Caro, J. Am. Chem. Soc., 2009, 131, 16000–16001 CrossRef CAS PubMed.
- H. Wu, W. Zhou and T. Yildirim, J. Am. Chem. Soc., 2007, 129, 5314–5315 CrossRef CAS PubMed.
- J. B. James and Y. S. Lin, J. Membr. Sci., 2017, 532, 9–19 CrossRef CAS.
- H. Yin, H. Kim, J. Choi and A. C. K. Yip, Chem. Eng. J., 2015, 278, 293–300 CrossRef CAS.
- W. Zhou, H. Wu, M. R. Hartman and T. Yildirim, J. Phys. Chem. C, 2007, 111, 16131–16137 CrossRef CAS.
- Y. Song, M. He, J. Zhao and W. Jin, Elsevier B.V., 2021, preprint, DOI:10.1016/j.seppur.2021.118722.
- S. Naghdi, M. M. Shahrestani, M. Zendehbad, H. Djahaniani, H. Kazemian and D. Eder, Elsevier B.V., 2023, preprint, DOI:10.1016/j.jhazmat.2022.130127.
- B. Chen, Z. Yang, Y. Zhu and Y. Xia, Royal Society of Chemistry, 2014, preprint, 10.1039/c4ta02984d.
- A. U. Ortiz, A. P. Freitas, A. Boutin, A. H. Fuchs and F. X. Coudert, Phys. Chem. Chem. Phys., 2014, 16, 9940–9949 RSC.
- S. K. Nune, P. K. Thallapally, A. Dohnalkova, C. Wang, J. Liu and G. J. Exarhos, Chem. Commun., 2010, 46, 4878–4880 RSC.
- X. C. Huang, Y. Y. Lin, J. P. Zhang and X. M. Chen, Angew. Chem., Int. Ed., 2006, 45, 1557–1559 CrossRef CAS PubMed.
- S. R. Venna and M. A. Carreon, J. Am. Chem. Soc., 2010, 132, 76–78 CrossRef CAS PubMed.
- A. X. Zhu, R. B. Lin, X. L. Qi, Y. Liu, Y. Y. Lin, J. P. Zhang and X. M. Chen, Microporous Mesoporous Mater., 2012, 157, 42–49 CrossRef CAS.
- K. Milburn and H. Zhang, Synthesis and Characterization of ZIF-8 and ZIF-8/polymer composites, 2014 Search PubMed.
- A. Kumar, S. Rana, G. Sharma, P. Dhiman, M. I. Shekh and F. J. Stadler, Elsevier Ltd, 2023, preprint, DOI:10.1016/j.jece.2023.110770.
- Y. Wang, Y. Xu, D. Li, H. Liu, X. Li, S. Tao and Z. Tian, Chin. J. Catal., 2015, 36, 855–865 CrossRef.
- Y. Liu, H. Cheng, M. Cheng, Z. Liu, D. Huang, G. Zhang, B. Shao, Q. Liang, S. Luo, T. Wu and S. Xiao, Elsevier B.V., 2021, preprint, DOI:10.1016/j.cej.2020.127914.
- L. Tian, X. Yang, Q. Liu, F. Qu and H. Tang, Appl. Surf. Sci., 2018, 455, 403–409 CrossRef CAS.
- J. Cravillon, C. A. Schröder, H. Bux, A. Rothkirch, J. Caro and M. Wiebcke, CrystEngComm, 2012, 14, 492–498 RSC.
- J. Cravillon, R. Nayuk, S. Springer, A. Feldhoff, K. Huber and M. Wiebcke, Chem. Mater., 2011, 23, 2130–2141 CrossRef CAS.
- M. He, J. Yao, L. Li, K. Wang, F. Chen and H. Wang, ChemPlusChem, 2013, 78, 1222–1225 CrossRef CAS PubMed.
- H. Song, N. Wang, H. Meng, Y. Han, J. Wu, J. Xu, Y. Xu, X. Zhang and T. Sun, Dalton Trans., 2020, 49, 10816–10823 RSC.
- Y. Pan and Z. Lai, Chem. Commun., 2011, 47, 10275–10277 RSC.
- B. Ding, X. Wang, Y. Xu, S. Feng, Y. Ding, Y. Pan, W. Xu and H. Wang, J. Colloid Interface Sci., 2018, 519, 38–43 CrossRef CAS PubMed.
- V. V. Butova, A. P. Budnyk, E. A. Bulanova, C. Lamberti and A. V. Soldatov, Solid State Sci., 2017, 69, 13–21 CrossRef CAS.
- R. Oozeerally, S. D. K. Ramkhelawan, D. L. Burnett, C. H. L. Tempelman and V. Degirmenci, Catalysts, 2019, 9, 100812 CrossRef.
- Y. Hong, B. Wang, S. Hu, S. Lu, Q. Wu, M. Fu, C. Gu and Y. Wang, Ceram. Int., 2023, 49, 11027–11037 CrossRef CAS.
- A. Faraji, N. Mehrdadi, N. M. Mahmoodi, M. Baghdadi and A. Pardakhti, J. Mol. Struct., 2021, 1223, 129028 CrossRef CAS.
- R. Li, W. Li, C. Jin, Q. He and Y. Wang, J. Alloys Compd., 2020, 825, 154008 CrossRef CAS.
- E. R. Parnham and R. E. Morris, Acc. Chem. Res., 2007, 40, 1005–1013 CrossRef CAS PubMed.
- R. E. Morris, 2008, preprint, DOI:10.1002/anie.200704888.
- E. R. Cooper, C. D. Andrews, P. S. Wheatley, P. B. Webb, P. Wormald and R. E. Morris, Nature, 2004, 430, 1012–1016 CrossRef CAS PubMed.
- G. A. V. Martins, P. J. Byrne, P. Allan, S. J. Teat, A. M. Z. Slawin, Y. Li and R. E. Morris, Dalton Trans., 2010, 39, 1758–1762 RSC.
- W. J. Son, J. Kim, J. Kim and W. S. Ahn, Chem. Commun., 2008, 6336–6338 RSC.
- K. S. Suslick, P. F. Schubert, R. E. J. Johnson, Amer, W. Tsang, A. Lifshitz, E. Lewis, N. F. Golden, D. M. Smith, G. P. Fujikawa, S. Akamatsu, T. Chendke and P. K. Fogler, 1980, 97, 481. E 2i, McGraw-Hill, 1986, vol. 108 Search PubMed.
- B. Seoane, J. M. Zamaro, C. Tellez and J. Coronas, CrystEngComm, 2012, 14, 3103–3107 RSC.
- H. Y. Cho, J. Kim, S. N. Kim and W. S. Ahn, Microporous Mesoporous Mater., 2013, 169, 180–184 CrossRef CAS.
- M. Schlesinger, S. Schulze, M. Hietschold and M. Mehring, Microporous Mesoporous Mater., 2010, 132, 121–127 CrossRef CAS.
- P. da and F. FRANCESCO Coordinatore Dottorato Relatore Aldo Roda Dario Braga, Alma Mater Studiorum-Università di Bologna DOTTORATO DI RICERCA IN CHIMICA Ciclo XXIX Synthesis and characterization of new luminescent complexes based on Copper(I) Iodide.
- C. J. Adams, M. A. Kurawa and A. G. Orpen, Dalton Trans., 2010, 39, 6974–6984 RSC.
- C. J. Adams, H. M. Colquhoun, P. C. Crawford, M. Lusi and A. G. Orpen, Angew. Chem., Int. Ed., 2007, 46, 1124–1128 CrossRef CAS PubMed.
- P. J. Beldon, L. Fábián, R. S. Stein, A. Thirumurugan, A. K. Cheetham and T. Friščić, Angew. Chem., 2010, 122, 9834–9837 CrossRef.
- D. Braga, M. Curzi, A. Johansson, M. Polito, K. Rubini and F. Grepioni, Angew. Chem., Int. Ed., 2005, 45, 142–146 CrossRef PubMed.
- T. Friščić, D. G. Reid, I. Halasz, R. S. Stein, R. E. Dinnebier and M. J. Duer, Angew. Chem., Int. Ed., 2010, 49, 712–715 CrossRef PubMed.
- T. Friščić, I. Halasz, P. J. Beldon, A. M. Belenguer, F. Adams, S. A. J. Kimber, V. Honkimäki and R. E. Dinnebier, Nat. Chem., 2013, 5, 66–73 CrossRef PubMed.
- S. Tanaka, K. Kida, T. Nagaoka, T. Ota and Y. Miyake, Chem. Commun., 2013, 49, 7884–7886 RSC.
- M. Li and M. Dincǎ, J. Am. Chem. Soc., 2011, 133, 12926–12929 CrossRef CAS PubMed.
- T. Zhang, J. Z. Wei, X. J. Sun, X. J. Zhao, H. Liang Tang, H. Yan and F. M. Zhang, Inorg. Chem. Commun., 2020, 111, 107671 CrossRef CAS.
- O. J. De Lima Neto, A. C. de O. Frós, B. S. Barros, A. F. De Farias Monteiro and J. Kulesza, New J. Chem., 2019, 43, 5518–5524 RSC.
- A. Martinez Joaristi, J. Juan-Alcañiz, P. Serra-Crespo, F. Kapteijn and J. Gascon, Cryst. Growth Des., 2012, 12, 3489–3498 CrossRef CAS.
- S. D. Worrall, H. Mann, A. Rogers, M. A. Bissett, M. P. Attfield and R. A. W. Dryfe, Electrochim. Acta, 2016, 197, 228–240 CrossRef CAS.
- Z. Li, J. Cui, Y. Liu, J. Li, K. Liu and M. Shao, ACS Appl. Mater. Interfaces, 2018, 10, 34494–34501 CrossRef CAS PubMed.
- L. Jiang, D. Zhou, J. Yang, S. Zhou, H. Wang, X. Yuan, J. Liang, X. Li, Y. Chen and H. Li, J. Mater. Chem. A, 2022, 10, 13651–13672 RSC.
- R. Wei, N. Tang, L. Jiang, J. Yang, J. Guo, X. Yuan, J. Liang, Y. Zhu, Z. Wu and H. Li, Elsevier B.V., 2022, preprint, DOI:10.1016/j.ccr.2022.214500.
- C. Mottillo and T. Friščić, MDPI AG, 2017, preprint, DOI:10.3390/molecules22010144.
- N. Kaur, M. Singh, A. Moumen, G. Duina and E. Comini, MDPI AG, 2020, preprint, DOI:10.3390/ma13132974.
- H.-C. Oh, S. Jung, I.-J. Ko and E.-Y. Choi, Ionothermal Synthesis of Metal–Organic Framework, 2016 Search PubMed.
- B. Kołodziej, Pol. J. Chem. Technol., 2024, 4, 39–55 Search PubMed.
- P. E. Lokhande, U. S. Chavan and A. Pandey, Springer Science and Business Media B.V., 2020, preprint, DOI:10.1007/s41918-019-00057-z.
- S. Subudhi, S. P. Tripathy and K. Parida, Royal Society of Chemistry, 2021, preprint, 10.1039/d0qi01117g.
- X. Fang, Q. Shang, Y. Wang, L. Jiao, T. Yao, Y. Li, Q. Zhang, Y. Luo and H. L. Jiang, Adv. Mater., 2018, 30, 1705112 CrossRef PubMed.
- C. Acar, I. Dincer and G. F. Naterer, John Wiley and Sons Ltd, 2016, preprint, DOI:10.1002/er.3549.
- P. Xing, F. Zhou and S. Zhan, Environ. Res., 2021, 197, 111167 CrossRef CAS PubMed.
- K. Suda and M. Nagata, in ECS Meeting Abstracts, 2020, vol. MA2020-02, pp. 3115–3115.
- X. Chang, Y. Wang, X. Zhou, Y. Song and M. Zhang, Dalton Trans., 2021, 50, 17618–17624 RSC.
- M. Zhang, Q. Shang, Y. Wan, Q. Cheng, G. Liao and Z. Pan, Appl. Catal., B, 2019, 241, 149–158 CrossRef CAS.
- Y. Zhao, Y. Liu, J. Cao, H. Wang, M. Shao, H. Huang, Y. Liu and Z. Kang, Appl. Catal., B, 2020, 278, 119289 CrossRef CAS.
- F. He, G. Chen, Y. Zhou, Y. Yu, L. Li, S. Hao and B. Liu, J. Mater. Chem. A, 2016, 4, 3822–3827 RSC.
- X. Dai, S. Feng, W. Zheng, W. Wu, Y. Zhou, Z. Ye, X. Cao and Y. Wang, Int. J. Hydrogen Energy, 2022, 47, 10603–10615 CrossRef CAS.
- M. U. Shahid, T. Najam, M. H. Helal, I. Hossain, S. M. El-Bahy, Z. M. El-Bahy, A. ur Rehman, S. S. A. Shah and M. A. Nazir, Elsevier Ltd, 2024, preprint, DOI:10.1016/j.ijhydene.2024.03.139.
- R. Ren, H. Zhao, X. Sui, X. Guo, X. Huang, Y. Wang, Q. Dong and J. Chen, Catalysts, 2019, 9, 89 CrossRef.
- M. Zeng, Z. Chai, X. Deng, Q. Li, S. Feng, J. Wang and D. Xu, Nano Res., 2016, 9, 2729–2734 CrossRef CAS.
- S. Feng, Y. Yu, J. Li, J. Luo, P. Deng, C. Jia, Y. Shen and X. Tian, Catal. Commun., 2022, 162, 106382 CrossRef CAS.
- J. Joo, T. Kim, J. Lee, S. Il Choi and K. Lee, Wiley-VCH Verlag, 2019, preprint, DOI:10.1002/adma.201806682.
- Z. Jin and Y. Zhang, Catal. Surv. Asia, 2020, 24, 59–69 CrossRef CAS.
- J. Chen, J. Chen and Y. Li, J. Mater. Chem. A, 2017, 5, 24116–24125 RSC.
- J. Zhou, J. Zhao and R. Liu, Appl. Catal., B, 2020, 278, 119265 CrossRef CAS.
- J. H. Zhao, Y. Wang, X. Tang, Y. H. Li, F. T. Liu, Y. Zhang and K. Li, Dalton Trans., 2019, 48, 3560–3565 RSC.
- X. Yuan, S. Qu, X. Huang, X. Xue, C. Yuan, S. Wang, L. Wei and P. Cai, Chem. Eng. J., 2021, 416, 129148 CrossRef CAS.
- M. R. Saleh, H. M. El-Bery and H. N. Abdelhamid, Appl. Organomet. Chem., 2023, 37, e6995 CrossRef CAS.
- X. Han, T. Zhang, Y. Cui, Z. Wang, R. Dong, Y. Wu, C. Du, R. Chen, C. Yu, J. Feng, J. Sun and S. Dong, Molecules, 2023, 28, 2422 CrossRef CAS PubMed.
- S. Kato, J. Jung, T. Suenobu and S. Fukuzumi, Energy Environ. Sci., 2013, 6, 3756–3764 RSC.
- D. Tsukamoto, A. Shiro, Y. Shiraishi, Y. Sugano, S. Ichikawa, S. Tanaka and T. Hirai, ACS Catal., 2012, 2, 599–603 CrossRef CAS.
- H. Hirakawa, S. Shiota, Y. Shiraishi, H. Sakamoto, S. Ichikawa and T. Hirai, ACS Catal., 2016, 6, 4976–4982 CrossRef CAS.
- Y. Xie, Y. Li, Z. Huang, J. Zhang, X. Jia, X. S. Wang and J. Ye, Appl. Catal., B, 2020, 265, 118581 CrossRef CAS.
- X. Yang, Z. Wen, Z. Wu and X. Luo, Inorg. Chem. Front, 2018, 5, 687–693 RSC.
- E. Karamian and S. Sharifnia, J. CO2 Util., 2016, 16, 194–203 CrossRef CAS.
- Y. H. Zou, H. N. Wang, X. Meng, H. X. Sun and Z. Y. Zhou, Nanoscale Adv., 2021, 3, 1455–1463 RSC.
- R. Chandra, S. Mukhopadhyay and M. Nath, Mater. Lett., 2016, 164, 571–574 CrossRef CAS.
- X. Yang, Z. Wen, Z. Wu and X. Luo, Inorg. Chem. Front., 2018, 5, 687–693 RSC.
- R. N. Compton, P. W. Reinhardt and C. D. Cooper, J. Chem. Phys., 1975, 63, 3821–3827 CrossRef CAS.
- I. Ganesh, Elsevier Ltd, 2014, preprint, DOI:10.1016/j.rser.2013.11.045.
- A. Corma and H. Garcia, J. Catal., 2013, 308, 168–175 CrossRef CAS.
- C. C. Yang, J. Vernimmen, V. Meynen, P. Cool and G. Mul, J. Catal., 2011, 284, 1–8 CrossRef CAS.
- J. Mao, T. Peng, X. Zhang, K. Li and L. Zan, Catal. Commun., 2012, 28, 38–41 CrossRef CAS.
- Z. Wang, X. Liu, A. Zhang, H. Zhao, H. Dong, H. Jia and B. Xu, J. Inorg. Organomet. Polym. Mater., 2024, 34, 4594–4608 CrossRef CAS.
- Y. Liu, L. Deng, J. Sheng, F. Tang, K. Zeng, L. Wang, K. Liang, H. Hu and Y. N. Liu, Appl. Surf. Sci., 2019, 498, 143899 CrossRef CAS.
- M. Izadpanah Ostad, M. Niknam Shahrak and F. Galli, J. CO2 Util., 2021, 43, 101373 CrossRef CAS.
- J. W. Maina, J. A. Schütz, L. Grundy, E. Des Ligneris, Z. Yi, L. Kong, C. Pozo-Gonzalo, M. Ionescu and L. F. Dumée, ACS Appl. Mater. Interfaces, 2017, 9, 35010–35017 CrossRef CAS PubMed.
- Z. Huang, P. Dong, Y. Zhang, X. Nie, X. Wang and X. Zhang, J. CO2 Util., 2018, 24, 369–375 CrossRef CAS.
- H. J. Peng, P. Q. Zheng, H. Y. Chao, L. Jiang and Z. P. Qiao, RSC Adv., 2019, 10, 551–555 RSC.
- Y. Zhou, S. Feng, X. Duan, W. Wu, Z. Ye, X. Dai, Y. Wang and X. Cao, J. Solid State Chem., 2022, 305, 122628 CrossRef CAS.
- X. Lei, Y. Cao, Q. Chen, X. Ao, Y. Fang and B. Liu, Colloids Surf., A, 2019, 568, 1–10 CrossRef CAS.
- W. Q. Chen, L. Y. Li, L. Li, W. H. Qiu, L. Tang, L. Xu, K. J. Xu and M. H. Wu, Engineering, 2019, 5, 755–767 CrossRef CAS.
- J. Zhou, W. Liu and W. Cai, Sci. Total Environ., 2019, 696, 133962 CrossRef CAS PubMed.
- M. Cao, Z. Zhuang, Y. Liu, Z. Zhang, J. Xuan, Q. Zhang and W. Wang, J. Colloid Interface Sci., 2022, 608, 2779–2790 CrossRef CAS PubMed.
- N. M. Mahmoodi, S. Keshavarzi, M. Oveisi, S. Rahimi and B. Hayati, J. Mol. Liq., 2019, 291, 111333 CrossRef CAS.
- Y. Du, R. Z. Chen, J. F. Yao and H. T. Wang, J. Alloys Compd., 2013, 551, 125–130 CrossRef CAS.
- J. He, J. Ye, Y. Zhang, L. Kong, X. Zhou, Y. Ma and Y. Yang, ChemistrySelect, 2020, 5, 3746–3755 CrossRef CAS.
- M. Ciprian, P. Xu, S. Chaemchuen, R. Tu, S. Zhuiykov, P. M. Heynderickx and F. Verpoort, Microporous Mesoporous Mater., 2018, 267, 185–191 CrossRef CAS.
- B. Abdollahi, A. Najafidoust, E. Abbasi Asl and M. Sillanpaa, Arabian J. Chem., 2021, 14, 103444 CrossRef CAS.
- J. Abdi, Colloids Surf., A, 2020, 604, 125330 CrossRef CAS.
- R. Chandra and M. Nath, J. Water Process Eng., 2020, 36, 101266 CrossRef.
- G. Fan, J. Luo, L. Guo, R. Lin, X. Zheng and S. A. Snyder, Chemosphere, 2018, 209, 44–52 CrossRef CAS PubMed.
- N. Chang, Y. R. Chen, F. Xie, Y. P. Liu and H. T. Wang, Colloids Surf., A, 2021, 616, 126351 CrossRef CAS.
- L. R. Jabbar and A. Al-Farraji, Environ. Nanotechnol., Monit. Manage., 2022, 18, 100701 CAS.
- A. Malik, M. Nath, S. Mohiyuddin and G. Packirisamy, ACS Omega, 2018, 3, 8288–8308 CrossRef CAS PubMed.
- M. Hu, H. Lou, X. Yan, X. Hu, R. Feng and M. Zhou, Microporous Mesoporous Mater., 2018, 271, 68–72 CrossRef CAS.
- Y. Si, X. Li, G. Yang, X. Mie and L. Ge, J. Mater. Sci., 2020, 55, 13049–13061 CrossRef CAS.
- I. B. S. Will, J. E. F. Moraes, A. C. S. C. Teixeira, R. Guardani and C. A. O. Nascimento, Sep. Purif. Technol., 2004, 34, 51–57 CrossRef CAS.
- M. H. Habibi and H. Vosooghian, J. Photochem. Photobiol., A, 2005, 174, 45–52 CrossRef CAS.
- S. Bustos-Guadarrama, A. Nieto-Maldonado, L. Z. Flores-López, H. Espinoza-Gomez and G. Alonso-Nuñez, J. Taiwan Inst. Chem. Eng., 2023, 142, 104663 CrossRef CAS.
- C. Karunakaran, G. Abiramasundari, P. Gomathisankar, G. Manikandan and V. Anandi, J. Colloid Interface Sci., 2010, 352, 68–74 CrossRef CAS PubMed.
- O. İloğlu and H. A. Yurtsever, Bitlis Eren Univ. Fen Bilimleri Derg., 2023, 12, 764–772 Search PubMed.
- H. Chen, Y. Q. Wang, F. Huang, C. Tu and Li Cui, Appl. Surf. Sci., 2021, 565, 150458 CrossRef CAS.
- S. Das, L. Acharya, L. Biswal and K. Parida, Nanoscale Adv., 2024, 6, 934–946 RSC.
- Y. He, L. Zeng, Z. Feng, Q. Zhang, X. Zhao, S. Ge, X. Hu and H. Lin, Adv. Powder Technol., 2020, 31, 439–447 CrossRef CAS.
- K. G. Motora, C. M. Wu and S. T. Lin, J. Water Process Eng., 2023, 52, 103586 CrossRef.
- M. T. Thanh, T. V. Thien, P. D. Du, N. P. Hung and D. Q. Khieu, J. Porous Mater., 2018, 25, 857–869 CrossRef CAS.
- X. Hu and C. Hu, J. Solid State Chem., 2007, 180, 725–732 CrossRef CAS.
- T. Li, X. Hu, C. Liu, C. Tang, X. Wang and S. Luo, J. Mol. Catal. A: Chem., 2016, 425, 124–135 CrossRef CAS.
- S. Mohanty, P. Babu, K. Parida and B. Naik, Inorg. Chem., 2019, 58, 9643–9654 CrossRef CAS PubMed.
- H. Yu, W. Liu, X. Wang and F. Wang, Appl. Catal., B, 2018, 225, 415–423 CrossRef CAS.
- W. M. Shume, H. C. A. Murthy and E. A. Zereffa, Hindawi Limited, 2020, preprint, DOI:10.1155/2020/5039479.
- X. Guan, S. Lin, J. Lan, J. Shang, W. Li, Y. Zhan, H. Xiao and Q. Song, Cellulose, 2019, 26, 7437–7450 CrossRef CAS.
- G. F. Huang, Z. L. Ma, W. Q. Huang, Y. Tian, C. Jiao, Z. M. Yang, Z. Wan and A. Pan, 2013, preprint, DOI:10.1155/2013/371356.
- T. Velempini, E. Prabakaran and K. Pillay, Elsevier Ltd, 2021, preprint, DOI:10.1016/j.mtchem.2020.100380.
- K. Qin, Q. Zhao, H. Yu, X. Xia, J. Li, S. He, L. Wei and T. An, Environ. Res., 2021, 199, 111360 CrossRef CAS PubMed.
- Y. Li, Y. Fu and M. Zhu, Appl. Catal., B, 2020, 260, 118149 CrossRef CAS.
- X. Chen, J. Yao, B. Xia, J. Gan, N. Gao and Z. Zhang, J. Hazard. Mater., 2020, 383, 121220 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |



