DOI:
10.1039/D4DT03344B
(Paper)
Dalton Trans., 2025,
54, 3759-3773
Controlling pseudopolymorphism via robust and repetitive solvent-containing supramolecular interactions in urea-based isostructural coordination polymers†
Received
1st December 2024
, Accepted 9th January 2025
First published on 10th January 2025
Abstract
In a systematic study, six pseudopolymorphic coordination polymers containing the ditopic 1,3-di(pyridin-4-yl)urea ligand (4bpu) constructed with d10 metal cations, possessing the formula {[M(4bpu)I2]S}n [(M = Zn, Cd and Hg), (S = MeOH or EtOH)], namely Zn-MeOH, Zn-EtOH, Cd-MeOH, Cd-EtOH, Hg-MeOH and Hg-EtOH were obtained. The title compounds were characterized by single-crystal X-ray diffraction analysis (SC-XRD), elemental analysis (CHN), FT-IR spectroscopy, thermogravimetric analysis (TGA), and powder X-ray diffraction (PXRD). The diffraction studies show that these compounds are isostructural 1D zig-zag chain coordination polymers which is also confirmed using XPac 2.0.2 software and the occurrence of three- and one-dimensional (3D and 1D) isostructurality was investigated in terms of their molecular assembly in solid state structures. These comparisons were performed by extracting the dissimilarity index and stretching parameters, providing quantitative insights into the structural similarity across the pseudopolymorphic coordination polymers, which exhibit robust 3D isostructurality. Additionally, the geometry of the zig-zag chain structures was thoroughly analyzed, highlighting the subtle variations and common features that contribute to isostructurality. The supramolecular architecture of these pseudopolymorphs is stabilized by robust and repetitive hydrogen bonding motifs involving N–H⋯O, O–H⋯I and C–H⋯I interactions between the framework and guest solvent molecules (MeOH or EtOH). These interactions replace the commonly observed bifurcated hydrogen bonds (α-tape motif) between urea moieties, emphasizing the pivotal role of solvent molecules in controlling pseudopolymorphism and defining the final structural assembly. This detailed understanding of hydrogen bonding provides valuable insights into tailoring intermolecular interactions, enabling the design of materials with enhanced functionalities for diverse applications. A detailed investigation of urea–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy interactions in urea-based compounds highlights the classification of these interactions as semilocalized (η2-SL) based on geometric parameters and reveals their significance through crystallographic and database studies. Hirshfeld surface analysis has been performed for all compounds to determine the percentage contribution of intermolecular interactions.
O⋯πpy interactions in urea-based compounds highlights the classification of these interactions as semilocalized (η2-SL) based on geometric parameters and reveals their significance through crystallographic and database studies. Hirshfeld surface analysis has been performed for all compounds to determine the percentage contribution of intermolecular interactions.
Introduction
The rapid progress of coordination polymers (CPs) is attributed to their construction from versatile organic linkers and metal nodes, enabling tailored assembly.1–3 The design of functional CPs has emerged as a hot research topic, driven by the extensive range of potential applications for these compounds and their derived materials in gas storage and separation,4,5 catalysis,6,7 sensing,8–10 magnetism,11–13 bioimaging, and drug delivery.14–16 From the crystal engineering point of view, the urea moiety as an important functional group could contribute as a donor and acceptor to form strong and directional multiple hydrogen bonds with a diverse range of organic and inorganic anions, solvents, and also the urea group (self-hydrogen bonding) which make it a potential building block for designing new supramolecular structures. A comprehensive examination of these interactions reveals that six membered cyclic R21(6), eight membered cyclic R22(8), and discrete type D(2) H-bonding interactions are the most abundant in compounds containing the urea functional group.17–19 The analysis of hydrogen bonding in urea-based frameworks plays a crucial role in enabling more effective and diverse applications. Hydrogen bonds provide structural flexibility and enhanced interaction capabilities with guest molecules, allowing these frameworks to achieve tailored functionalities.17 For example, in gas adsorption, the hydrogen bonding sites improve the capture and separation of gases like SO2 and NH3.20 In catalytic activities, these interactions facilitate reaction pathways by stabilizing intermediates,21 while in sensing applications, hydrogen bonds enhance the selective recognition of specific molecules, such as heavy metals or nitroaromatic compounds.22,23 This targeted functionality demonstrates how hydrogen bonding analysis is essential for optimizing these frameworks for practical uses across various scientific and industrial fields.17
The concept of “supramolecular isomerism” is an important aspect of crystal engineering because it illuminates the self-organization of supramolecular assemblies in crystalline materials.24,25 Following a review by Moulton and Zaworotko in 2001, this term gained prominence.26 It refers to a phenomenon similar to molecular isomerism, but occurring in more extended systems (organic or metal–organic systems), in which the building blocks are held together by non-covalent interactions such as hydrogen bonding, halogen bonding, π–π interactions and coordination bonding.27A variety of parameters such as the solvent system,28–33 temperature,34–36 pH value,37,38 molar ratio,39 additive,28 ligand flexibility,40,41 and so on, might possibly influence the construction of supramolecular isomers. It should be mentioned that the terms “supramolecular isomerism” and “polymorphism” in organic and metal–organic networks are quite similar and, in some cases, difficult to distinguish.42–44 In this context, “polymorphism” refers to the presence of several superstructures formed from the same reactant's molecular building blocks.26,45 The term “pseudopolymorphism” also known as “solvatomorphism” represents significant categories of supramolecular compounds, exhibiting variations in the structure concerning the number or nature of guest solvent(s).46,47 Supramolecular isomers, on the other hand, have various network configurations or architectures that have similar chemical compositions but differ in their superstructures. Consequently, “polymorphism” or “pseudopolymorphism” might be considered sort of supramolecular isomerism because it can be explained by supramolecular interactions.45,48 Polymorphism and pseudopolymorphism are prevalent in solid-state chemistry, especially in the pharmaceutical field, where variations in crystal forms significantly affect the material performance.49 For active pharmaceutical ingredients (APIs), different polymorphs or solvatomorphs can exhibit unique physical and chemical properties, such as solubility, stability, and reactivity, which influence drug efficacy, bioavailability, and shelf life.50 Understanding and characterization of solvatomorph forms are therefore essential in drug development to ensure consistent drug performance and optimize formulation conditions.49,50 For instance, the solvatomorphs of naproxen sodium with solvents such as ethanol, n-propanol, isopropanol, n-butanol and isobutanol have been characterized, revealing that the molecular size of each solvent significantly affects the desolvation properties of each crystal form of these solvatomorphs.51 Although non-covalent interactions are crucial in controlling pseudopolymorphism, systematic studies specifically examining their precise influence on stabilizing various pseudopolymorphic forms of inorganic systems are relatively scarce.28,45 For example, in 1D pseudopolymorphic Cu(I) CPs synthesized through self-assembly in different solvents, supramolecular interactions such as hydrogen bonding, π–π stacking, and halogen bonding (notably I−⋯π and S⋯π interactions) stabilize distinct structural conformations, highlighting their essential role in maintaining diverse pseudopolymorphic forms.52
One of the successful methodologies in the construction of supramolecular assemblies is isostructurality which refers to the identical or nearly identical crystal structures displayed by different chemical compounds.53–61 The degree of similarity among crystals of small molecules can mainly be ascribed to directional interactions, which are characterized as supramolecular synthons.62–65 There are a limited number of systematic studies on the construction of isostructural supramolecular architectures in coordination compounds using diverse building units, such as organic linkers or metal ions.66–68 Isostructural CPs and MOFs with the same metals but distinct organic linkers are produced, allowing for a detailed examination into how different organic linkers affect sensing and gas sorption or separation capabilities.69–72 Nevertheless, metal ions, which act as the nodes of coordination polymers, have a substantial impact on their physical and chemical properties such as catalysis73 and gas adsorption.74,75 The exploration and discussion of the impact of central metals on different behaviors have been limited.76–79 To expand our understanding of the relationship between the identity of metal centers and isostructurality, the synthesis of pseudoholomorphic CPs containing ditopic urea-based linkers and different d10 metal ions was carried out. Among these linkers, 1,3-di(pyridin-4-yl)urea (4bpu) is a versatile ligand with pyridyl groups that serve as primary coordination sites, particularly for d10 metals like ZnII, CdII, and HgII. The nitrogen atoms of the pyridyl groups strongly bind to these metals due to their lone pairs and the high Lewis acidity of metals. This ligand can act as a bridge, forming one-dimensional, two-dimensional, or three-dimensional frameworks based on the coordination geometry of the metal and reaction conditions.17
Herein, we report the syntheses and structural characterization of six isostructural urea-based pseudopolymorph CPs of divalent d10 metal ions of group 12, having the {[M(4bpu)I2]S}n general formula: S = MeOH or EtOH, M = Zn (Zn-MeOH and Zn-EtOH), Cd (Cd-MeOH and Cd-EtOH), and Hg (Hg-MeOH and Hg-EtOH). This study allowed us to find robust and repetitive supramolecular synthons responsible for construction of MeOH and EtOH pseudopolymorphs in this family of coordination polymers.
Experimental section
Materials and instruments
All reagents and solvents were obtained from commercial sources and were used directly without further purification. The ligand 1,3-di(pyridin-4-yl)urea (4bpu) was synthesized following the literature procedure80 and was characterized by (1H & 13C) NMR and FTIR spectroscopy (Fig. S1 and S2, ESI†). NMR spectra were recorded on a Bruker DRX-300 Avance NMR spectrometer. Infrared spectra were recorded on an MB102 Bomem spectrometer with KBr pellets in the 400–4000 cm−1 region. A Thermo Nicolet Nexus 470 was utilized for recording attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectra in the region of 650 to 4000 cm−1. Melting points were determined on an Electrothermal 9100 melting point apparatus and were uncorrected. The X-ray powder diffraction (PXRD) patterns were collected on a STADIP STOE apparatus. Elemental analyses (CHN) were performed on a Vario EL III elemental analyzer. TGA analyses were carried out using a Mettler Toledo Star SW 10.00 instrument under a flowing N2 atmosphere at a heating rate of 10 °C min−1.
Caution: Cadmium(II) and mercury(II) compounds should be handled with care due to their high toxicity.
Synthesis of pseudopolymorphic coordination polymers
{[Zn(4bpu)I2]MeOH}n (Zn-MeOH).
Compound Zn-MeOH was synthesized by treatment of the organic ligand and zinc iodide in MeOH under thermal gradient conditions (the convection technique81). For this purpose, 1,3-di(pyridin-4-yl)urea (4bpu) (0.0428 g, 0.2 mmol) and ZnI2 (0.0638 g, 0.2 mmol) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol ratio were placed in the main arm of a branched tube. MeOH was carefully added to fill both arms completely. The tube was sealed and the main arm was immersed in an oil bath at 60 °C while the other arm was kept at ambient temperature for several days and colorless needle shaped single crystals of Zn-MeOH were formed (Scheme 1). Yield: 74%. m.p: 240 °C. Elemental analysis for C11H10I2N4OZn: calculated: C, 24.77; H, 1.89; N, 10.50%; found: C, 23.62; H, 2.22; N, 9.95%. FTIR (KBr pellet, cm−1) (Fig. S3†): 3476(w), 3373(w), 3292(w), 3091(w), 3033(w), 2360(w), 1742(m), 1589(s), 1507(s), 1431(m), 1333(m), 1288(s), 1257(w), 1180(s), 1063(m), 1024(s), 833(s), 745(w), 666(w), 552(m), 534(m), 418(w).
1 mol ratio were placed in the main arm of a branched tube. MeOH was carefully added to fill both arms completely. The tube was sealed and the main arm was immersed in an oil bath at 60 °C while the other arm was kept at ambient temperature for several days and colorless needle shaped single crystals of Zn-MeOH were formed (Scheme 1). Yield: 74%. m.p: 240 °C. Elemental analysis for C11H10I2N4OZn: calculated: C, 24.77; H, 1.89; N, 10.50%; found: C, 23.62; H, 2.22; N, 9.95%. FTIR (KBr pellet, cm−1) (Fig. S3†): 3476(w), 3373(w), 3292(w), 3091(w), 3033(w), 2360(w), 1742(m), 1589(s), 1507(s), 1431(m), 1333(m), 1288(s), 1257(w), 1180(s), 1063(m), 1024(s), 833(s), 745(w), 666(w), 552(m), 534(m), 418(w).
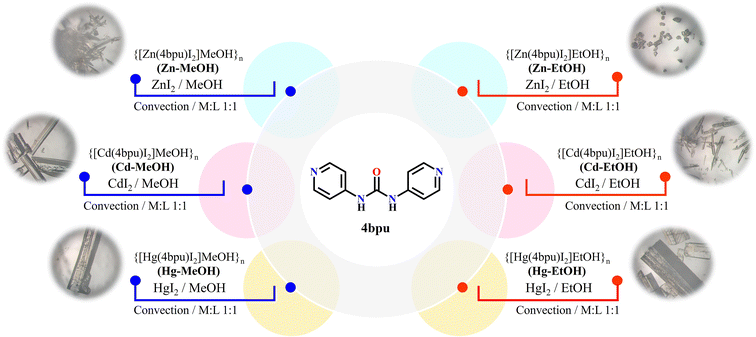 |
| | Scheme 1 Synthesis method and optical microscopic images of compounds M-MeOH and M-EtOH (M = Zn, Cd and Hg). | |
{[Zn(4bpu)I2]EtOH}n (Zn-EtOH).
The synthesis procedure and thermal gradient conditions were similar to the synthesis of Zn-MeOH except that EtOH was used instead of MeOH. Colorless plate shaped single crystals of Zn-EtOH were collected after several days (Scheme 1). Yield: 71%. m.p: 248 °C. Elemental analysis for C11H10I2N4OZn: calculated: C, 24.77; H, 1.89; N, 10.50%; found: C, 23.58; H, 2.28; N, 9.95%. FTIR (KBr pellet, cm−1) (Fig. S4†): 3476(w), 3373(m), 3280(m), 3183(w), 3091(w), 3022(w), 2359(w), 1742(m), 1590(s), 1508(s), 1431(m), 1333(m), 1289(s), 1257(w), 1180(s), 1063(m), 1025(s), 909(w), 833(s), 745(m), 666(w), 552(w), 534(s).
{[Cd(4bpu)I2]MeOH}n (Cd-MeOH).
The synthesis procedure and thermal gradient conditions were similar to the synthesis of Zn-MeOH except that CdI2 (0.0739 g, 0.2 mmol) was used instead of ZnI2 (Scheme 1). After several days, colorless needle shaped single crystals of Cd-MeOH were collected (yield: 75%. m.p: 235 °C). Elemental analysis for C11H10I2N4OCd: calculated: C, 22.76; H, 1.74; N, 9.65%; found: C, 21.71; H, 2.09; N, 9.22%. FTIR (KBr pellet, cm−1) (Fig. S5†): 3489(w), 3298(w), 3205(w), 3091(w), 2359(w), 1739(m), 1590(s), 1521(s), 1506(s), 1429(m), 1333(m), 1287(s), 1254(w), 1183(s), 1061(m), 1017(s), 960(w), 910(w), 830(s), 744(m), 659(w), 545(m), 534(s).
{[Cd(4bpu)I2]EtOH}n (Cd-EtOH).
Under the same thermal gradient conditions, colorless needle shaped single crystals of Cd-EtOH were collected (Scheme 1). The procedure was similar to the synthesis of Cd-MeOH except that EtOH was used instead of MeOH. After several days, colorless needle shaped single crystals of Cd-EtOH were collected (yield: 74%. m.p: 242 °C). Elemental analysis for C11H10I2N4OCd: calculated: C, 22.76; H, 1.74; N, 9.65%; found: C, 21.68; H, 2.09; N, 9.22%. FTIR (KBr pellet, cm−1) (Fig. S6†): 3307(w), 3200(w), 3091(w), 1741(w), 1711(m), 1588(s), 1517(s), 1430(m), 1334(m), 1291(m), 1251(w), 1187(s), 1062(w), 1016(m), 830(s), 743(w), 668(w), 658(w), 586(w), 544(w), 530(s).
{[Hg(4bpu)I2]MeOH}n (Hg-MeOH).
The synthesis procedure and thermal gradient conditions were similar to the synthesis of Zn-MeOH except that HgI2 (0.073 g, 0.2 mmol) was used instead of ZnI2 (Scheme 1). After several days, colorless needle shaped single crystals of Hg-MeOH were collected (yield: 73%. m.p: 215 °C). Elemental analysis for C11H10I2N4OHg: calculated: C, 19.76; H, 1.51; N, 8.38%; found: C, 18.79; H, 1.80; N, 8.03%. FTIR (KBr pellet, cm−1) (Fig. S7†): 3497(w), 3359(w), 3326(w), 3192(w), 3101(w), 3051(w), 3017(w), 1941(w), 1837(w), 1735(s), 1691(s), 1593(m), 1526(m), 1424(m), 1362(w), 1330(m), 1284(m), 1253(w), 1239(w), 1177(m), 1061(m), 1009(s), 908(w), 861(m), 827(s), 797(w), 744(m), 730(w), 688(m), 650(m), 531(s).
{[Hg(4bpu)I2]EtOH}n (Hg-EtOH).
The synthesis and thermal gradient conditions were the same as those for Hg-MeOH except that EtOH was used instead of MeOH (Scheme 1). After several days, colorless needle shaped single crystals of Hg-EtOH were collected (yield: 71%. m.p: 220 °C). Elemental analysis for C11H10I2N4OHg: calculated: C, 19.76; H, 1.51; N, 8.38%; found: C, 18.82; H, 2.00; N, 8.03%. IR (KBr pellet, cm−1) (Fig. S8†): 3523(w), 3359(w), 3326(w), 3304(w), 3206(w), 3114(w), 3045(w), 1942(w), 1837(w), 1735(s), 1691(s), 1596(s), 1526(m), 1425(m), 1363(w), 1330(m), 1284(m), 1253(w), 1233(w), 1176(m), 1085(w), 1061(m), 1009(s), 908(w), 855(m), 827(s), 797(m), 745(m), 727(m), 689(m), 650(m), 530(s).
Single-crystal X-ray diffraction studies
Details of the crystal structure determination for M-MeOH and M-EtOH (M = Zn, Cd and Hg) are presented in the ESI.† The crystal data and refinement details for all compounds are summarized in Table 1. The selected bond distances and angles for all compounds are given in Table S1.†
Table 1 Crystallographic and structure refinement data for compounds M-MeOH and M-EtOH (M = Zn, Cd and Hg)
| |
Zn-MeOH
|
Zn-EtOH
|
Cd-MeOH
|
Cd-EtOH
|
Hg-MeOH
|
Hg-EtOH
|
|
R
1 = ∑||Fo| − |Fc||/∑|Fo|.
wR2 = [∑(w(Fo2 − Fc2)2)/∑w(Fo2)2]1/2.
|
| Formula |
C12H14I2N4O2Zn |
C13H16I2N4O2Zn |
C12H14I2N4O2Cd |
C13H16I2N4O2Cd |
C12H14I2N4O2Hg |
C13H16I2N4O2Hg |
| Formula weight |
565.46 |
579.49 |
612.47 |
626.51 |
700.66 |
714.69 |
| Crystal color, habit |
Colorless, needle |
Colorless, plate |
Colorless, needle |
Colorless, needle |
Colorless, needle |
Colorless, needle |
|
T (K) |
298(2) |
298(2) |
298(2) |
298(2) |
298(2) |
298(2) |
|
λ (Å) |
0.71073 |
0.71073 |
0.71073 |
0.71073 |
0.71073 |
0.71073 |
| Crystal system |
Orthorhombic |
Orthorhombic |
Orthorhombic |
Orthorhombic |
Orthorhombic |
Orthorhombic |
| Space group |
Pna21 |
Pna21 |
Pna21 |
Pna21 |
Pna21 |
Pna21 |
| Crystal size (mm) |
0.50 × 0.20 × 0.20 |
0.25 × 0.20 × 0.10 |
0.30 × 0.20 × 0.15 |
0.30 × 0.20 × 0.10 |
0.20 × 0.15 × 0.10 |
0.25 × 0.15 × 0.10 |
|
a (Å) |
9.1201(18) |
8.9539(18) |
9.1508(18) |
9.0475(18) |
9.1437(18) |
9.0388(18) |
|
b (Å) |
10.018(2) |
10.616(2) |
10.063(2) |
10.378(2) |
10.043(2) |
10.366(2) |
|
c (Å) |
19.490(4) |
19.707(4) |
19.884(4) |
20.096(4) |
19.926(4) |
20.154(4) |
|
V (Å3) |
1780.8(6) |
1873.2(6) |
1831.0(6) |
1886.9(6) |
1829.8(6) |
1888.5(7) |
|
Z
|
4 |
4 |
4 |
4 |
4 |
4 |
|
D
calc. (g cm−1) |
2.109 |
2.055 |
2.222 |
2.205 |
2.543 |
2.514 |
|
θ
min, θmax (°) |
2.286–24.977 |
2.179–24.999 |
2.268–24.996 |
2.209–24.998 |
2.271–24.996 |
2.021–24.494 |
|
F
000
|
1064 |
1096 |
1136 |
1168 |
1264 |
1296 |
|
μ (mm−1) |
4.854 |
4.617 |
4.572 |
4.440 |
11.794 |
11.430 |
| Index ranges |
−9 ≤ h ≤ 10 |
−10 ≤ h ≤ 9 |
−9 ≤ h ≤ 10 |
−9 ≤ h ≤ 10 |
−9 ≤ h ≤ 10 |
−10 ≤ h ≤ 9 |
| −11 ≤ k ≤ 11 |
−12 ≤ k ≤ 11 |
−11 ≤ k ≤ 10 |
−12 ≤ k ≤ 10 |
−11 ≤ k ≤ 10 |
−10 ≤ k ≤ 12 |
| −23 ≤ l ≤ 20 |
−23 ≤ l ≤ 20 |
−20 ≤ l ≤ 23 |
−23 ≤ l ≤ 20 |
−23 ≤ l ≤ 20 |
−20 ≤ l ≤ 23 |
| Data collected |
5320 |
5358 |
5229 |
5383 |
5204 |
5218 |
| Unique data, (Rint) |
2699, (0.1182) |
3064, (0.1101) |
2852, (0.0858) |
3054, (0.0902) |
2987, (0.1319) |
2940, (0.1214) |
|
R
1
, wR2b (I > 2σ(I)) |
0.0681, 0.1642 |
0.0689, 0.1599 |
0.0589, 0.1330 |
0.0604, 0.1387 |
0.0777, 0.1576 |
0.0648, 0.1300 |
|
R
1
, wR2b (all data) |
0.0888, 0.1757 |
0.1084, 0.1766 |
0.0826, 0.1445 |
0.0865, 0.1500 |
0.1596, 0.1946 |
0.1461, 0.1596 |
| GOF on F2 (S) |
0.974 |
0.979 |
0.886 |
0.918 |
0.916 |
0.895 |
| Largest diff. peak, hole (e Å−3) |
0.971, −1.278 |
0.813, −0.572 |
1.071, −1.357 |
0.864, −0.661 |
1.335, −0.842 |
0.980, −1.179 |
| CCDC No. |
2391447
|
2391448
|
2391449
|
2391450
|
2391451
|
2391452
|
Results and discussion
Synthesis
The 4bpu ligand was prepared by using the reported method80 and confirmed by spectroscopic methods. All coordination polymers were prepared in high yield by the self-assembly process of 4bup and MI2 (M = Zn, Cd and Hg) at a mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under thermal gradient conditions (convection technique). Distinguishingly, the single crystals of these compounds were obtained from the MeOH or EtOH solvent system during the synthesis procedure (Scheme 1).
1 under thermal gradient conditions (convection technique). Distinguishingly, the single crystals of these compounds were obtained from the MeOH or EtOH solvent system during the synthesis procedure (Scheme 1).
Structural description of isostructural pseudopolymorph CPs
The main skeleton of all six synthesized M-MeOH and M-EtOH (M = Zn, Cd and Hg) compounds were the same and isostructural with the orthorhombic Pna21 space group. So, the structures of Zn-MeOH and Zn-EtOH are discussed as representatives. The structures of Cd-MeOH, Cd-EtOH, Hg-MeOH and Hg-EtOH will not be described in detail. Fig. S9† depicts the ORTEP diagram and atomic labeling of all compounds and details of crystallographic data and parameters are given in Table 1. Unfortunately, despite many efforts, we were unable to grow higher quality crystals of these compounds and this was the highest resolution possible from the available data (Fig. S10–S12†).
The asymmetric unit of Zn-MeOH and Zn-EtOH contained one ZnII ion, one 4bpu ligand, two coordinated iodide anions and one MeOH molecule for Zn-MeOH, as well as one EtOH molecule for Zn-EtOH (Fig. 1 and Fig. S9†). Each ZnII center is four-coordinate with two nitrogen atoms from two different 4bpu ligands and two iodide anions in Zn-MeOH and Zn-EtOH.
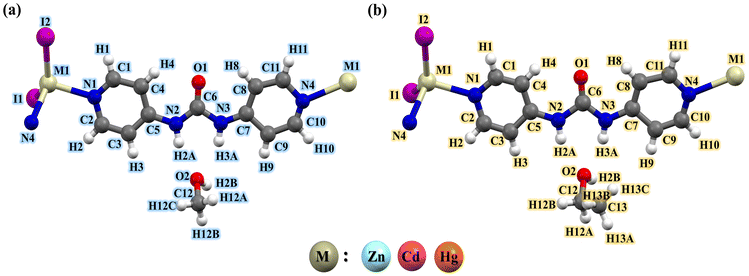 |
| | Fig. 1 View of the coordination environment around the metal center with the atom labeling scheme in (a) compounds Zn-MeOH, Cd-MeOH and Hg-MeOH and (b) compounds Zn-EtOH, Cd-EtOH and Hg-EtOH. Color code: metal (M), beige; O, red; N, blue; C, grey; I, purple and H, white. | |
The geometry of the four-coordinate metal centers can be described by an angular index (τ4) parameter, which was defined by Houser and co-workers as follows (eqn (1)):
| | 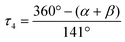 | (1) |
where
α and
β are the two largest angles around a four-coordinate metal center. A
τ4 value of 0 would correspond to a square planar geometry,
τ4 values of 0.07, 0.18, 0.5 and 0.64 correspond to a seesaw geometry, a
τ4 value of 0.85 corresponds to a trigonal pyramidal geometry, and a
τ4 value of 1 corresponds to a tetrahedral geometry.
82 These isostructural CPs have distorted trigonal-pyramidal coordination geometry around Zn
II, Cd
II and Hg
II metal centers. Geometry and angular parameter
τ4 for all compounds are listed in Table S2.
† The Zn–N/I, Cd–N/I and Hg–N/I bond lengths (Table S1, ESI
†) are all located in the normal accepted range of Zn
II, Cd
II and Hg
II coordination compounds, respectively.
30,83–86 The molecular geometry of the
4bpu ligand is found to be slightly nonplanar in all compounds, as evident from the corresponding dihedral angles involving pyridyl-urea and pyridyl–pyridyl planes (Fig. S13, ESI
†). In
Zn-MeOH and
Zn-EtOH the ligand displayed nonplanarity with the dihedral angles of 6.29 and 6.64° involving py1-urea planes, dihedral angles of 5.37 and 5.63° involving py2-urea planes and dihedral angles of 7.67 and 9.25° involving the terminal pyridyl rings, respectively.
In all compounds, each 4bpu links two metal (M) centers to afford an M2L unit that propagates into a one-dimensional (1D) zig-zag chain in which the distance between two adjacent ZnII ions is 14.145 and 14.221 Å for Zn-MeOH and Zn-EtOH, respectively (Fig. 2, Fig. S14 and S15†).
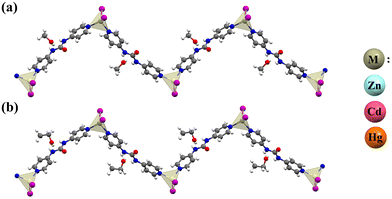 |
| | Fig. 2 A section of the 1D chain structure of (a) compounds M-MeOH (M = Zn, Cd and Hg) and (b) compounds M-EtOH (M = Zn, Cd and Hg). Metal polyhedra are shown in light beige for the metal coordination environment. | |
Comparing overlay images of these isostructural compounds, subtle differences in atomic positions, bond lengths, and dihedral angles become apparent. Understanding these distinctions is crucial for comprehending the implications of chemical substitutions in crystal structures (Fig. 3a). In all compounds, the formed 1D zig-zag chains can be described by the M⋯M distances and M⋯M⋯M angles (Fig. 3b, Fig. S14, S15 ESI,† and Table 2). The values in Table 2 show how close are architectures of the one-dimensional zig-zag chains in all cases.
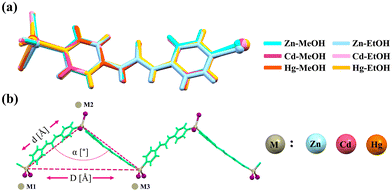 |
| | Fig. 3 (a) Structural overlay image of ligands in M-MeOH and M-EtOH (M = Zn, Cd and Hg). Metal ions are shown as balls. (b) M⋯M distances (D, d/Å) and M⋯M⋯M angles (α/°) in zig-zag chains. | |
Table 2 Geometric characteristics for zig-zag chains of M-MeOH and M-EtOH (M = Zn, Cd and Hg)
| |
Zn-MeOH
|
Zn-EtOH
|
Cd-MeOH
|
Cd-EtOH
|
Hg-MeOH
|
Hg-EtOH
|
| M1⋯M2, d [Å] |
14.145 |
14.221 |
14.542 |
14.595 |
14.760 |
14.804 |
| M1⋯M3 (length of the zig-zag chain period), D [Å] |
21.914 |
22.384 |
22.285 |
22.618 |
22.314 |
22.664 |
| M1⋯M2⋯M3 angle, α [°] |
101.55 |
103.82 |
100.03 |
101.58 |
98.21 |
99.90 |
Interestingly, the typical bifurcated hydrogen bonding interaction between urea moieties (interaction between the carbonyl oxygen atom and the two N–H of the adjacent urea groups) that lead to the formation of 1D hydrogen bonded α-tape motif was absent in these structures. Instead, in a competition between the common α-tape hydrogen bonding and the interaction with the molecules of the alcoholic solvents used, the 1D chains are connected by the hydrogen bonding of the urea group with the guest solvents MeOH or EtOH molecules. As illustrated in Fig. 4, one such a connection is built by a set of bifurcated N–H⋯O and one C–H⋯I hydrogen bonded paths: ∼(ligand)N–H⋯O(MeOH or EtOH)C–H⋯I(MIIIN2)∼. According to graph set notation87,88 this sequence can be written as R12(6)D(2). In these supramolecular structures, the oxygen atom (O2) of MeOH and EtOH guest molecules and also coordinated iodide anions (I1) act as bifurcated hydrogen bond acceptors. In summary, (aromatic)C–H⋯I, (MeOH or EtOH)C–H⋯I, (MeOH or EtOH)O–H⋯I hydrogen bonds and also urea–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy play a crucial role in the crystal packing of these pseudopolymorphs. There are two intramolecular C–H⋯O hydrogen bonding interactions in 4bpu ligands. A more detailed description of the hydrogen bond interactions for Zn-MeOH/Zn-EtOH, Cd-MeOH/Cd-EtOH and Hg-MeOH/Hg-EtOH is provided in Tables 3–5 and Fig. S16 and S17,† respectively.
O⋯πpy play a crucial role in the crystal packing of these pseudopolymorphs. There are two intramolecular C–H⋯O hydrogen bonding interactions in 4bpu ligands. A more detailed description of the hydrogen bond interactions for Zn-MeOH/Zn-EtOH, Cd-MeOH/Cd-EtOH and Hg-MeOH/Hg-EtOH is provided in Tables 3–5 and Fig. S16 and S17,† respectively.
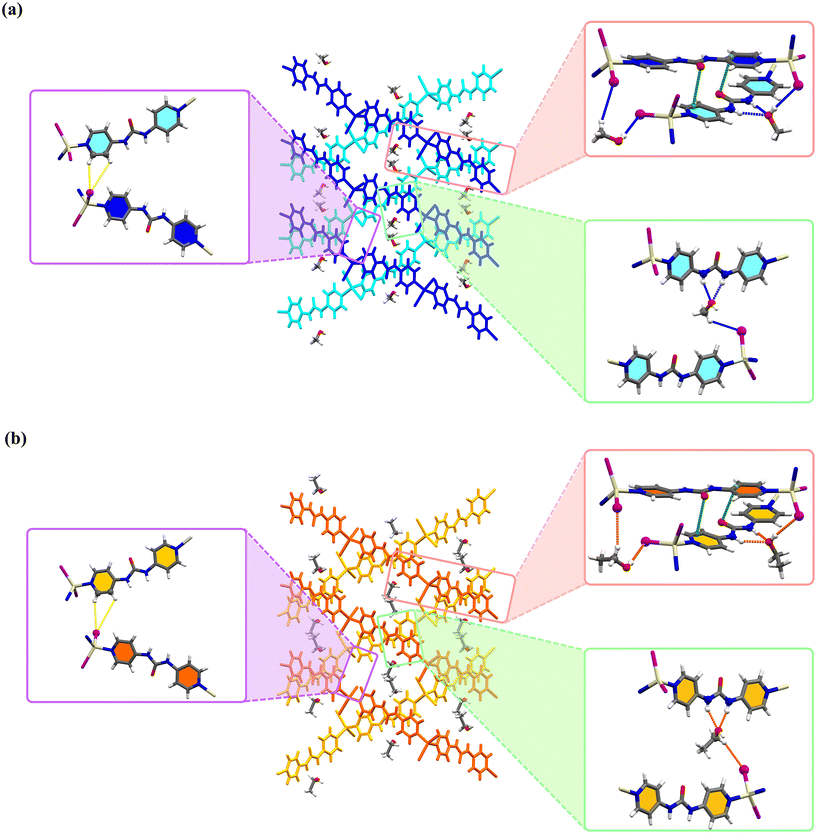 |
| | Fig. 4 The 2D supramolecular layer assembled by hydrogen bond and lone pair–π interactions. These interactions are represented by yellow and green dashed lines. Blue and orange dashed lines denote hydrogen bonds between the MeOH/EtOH and the framework in (a) compounds Zn-MeOH, Cd-MeOH and Hg-MeOH and (b) compounds Zn-EtOH, Cd-EtOH and Hg-EtOH. | |
Table 3 Geometry of hydrogen bonds (D–H⋯A) for Zn-MeOH and Zn-EtOH
| Compounds |
D–H⋯A |
d(D–H)/Å |
d(H⋯A)/Å |
d(D⋯A)/Å |
∠D–H⋯A/° |
|
Zn-MeOH
|
N2–H2A⋯O2#1 |
0.78(7) |
2.20(13) |
2.93(4) |
157(16) |
| N3–H3A⋯O2#1 |
0.78(4) |
2.09(5) |
2.84(4) |
163(6) |
| O2–H2B#1⋯I2#2 |
0.78(6) |
2.78(5) |
3.56(3) |
175(9) |
| C12–H12A#1⋯I2#3 |
0.96 |
3.40 |
4.17 |
138.2 |
| C2–H2⋯I1#4 |
0.93 |
3.96 |
3.15 |
146.9 |
| C3–H3⋯I1#4 |
0.93 |
3.79 |
4.27 |
115.3 |
| C4–H4⋯O1 |
0.93 |
2.23 |
2.82(3) |
121.0 |
| C8–H8⋯O1 |
0.93 |
2.24 |
2.82(3) |
120.0 |
| Symmetry codes: #1: x, y − 1, z − 1; #2: −x + 1, −y, z − 1/2; #3: –x + 1/2, y − 1/2, z − 1/2; #4: x − 1/2, −y − 1/2, z |
|
Zn-EtOH
|
N2–H2A⋯O2#1 |
0.86 |
2.21 |
2.99(4) |
151.0 |
| N3–H3A…O2#1 |
0.86 |
2.02 |
2.85(4) |
160.0 |
| O2–H2B#1⋯I2#2 |
0.9(4) |
2.7(4) |
3.56(3) |
169.0 |
| C12–H12B⋯I2#3 |
0.97 |
3.18 |
4.07 |
153.5 |
| C2–H2⋯I1#4 |
0.93 |
3.47 |
4.32 |
151.8 |
| C3–H3⋯I1#4 |
0.92 |
4.28 |
4.72 |
112.7 |
| C4–H4⋯O1 |
0.93 |
2.22 |
2.82(3) |
122.0 |
| C8–H8⋯O1 |
0.93 |
2.30 |
2.87(3) |
119.0 |
| Symmetry codes: #1: x + 1/2, −y + 1/2, z + 1; #2: −x + 1, −y, z + 1/2; #3: −x + 3/2, y − 1/2, z + 1/2; #4: x + 1/2, −y − 1/2, z |
Table 4 Geometry of hydrogen bonds (D–H⋯A) for Cd-MeOH and Cd-EtOH
| Compounds |
D–H⋯A |
d(D–H)/Å |
d(H⋯A)/Å |
d(D⋯A)/Å |
∠D–H⋯A/° |
|
Cd-MeOH
|
N2–H2A⋯O2 |
0.86 |
2.16 |
2.96(4) |
154.0 |
| N3–H3A⋯O2 |
0.86 |
2.11 |
2.94(4) |
160.0 |
| O2–H2B⋯I2#1 |
0.9(2) |
2.7(3) |
3.61(3) |
170.0 |
| C12–H12A⋯I2#2 |
0.96 |
3.46 |
4.24 |
140.2 |
| C2–H2⋯I1#3 |
0.93 |
3.12 |
3.96 |
150.7 |
| C3–H3⋯I1#3 |
0.92 |
3.87 |
4.33 |
114.2 |
| C4–H4⋯O1 |
0.93 |
2.22 |
2.82(3) |
121.0 |
| C8–H8⋯O1 |
0.93 |
2.22 |
2.82 |
121.0 |
| Symmetry codes: #1: −x + 1, −y + 1, z + 1/2; #2: −x + 3/2, y − 1/2, z + 1/2; #3: x + 1/2, −y + 1/2, z |
|
Cd-EtOH
|
N2–H2A⋯O2#1 |
0.86 |
2.18 |
2.98(3) |
153.0 |
| N3–H3A⋯O2#1 |
0.86 |
2.10 |
2.92(3) |
159.0 |
| O2–H2B#1⋯I2#2 |
0.86(7) |
2.77(7) |
3.61(2) |
164(7) |
| C12–H12B#1⋯I2#3 |
0.97 |
3.53 |
4.18 |
126.3 |
| C2–H2⋯I1#4 |
0.92 |
3.27 |
4.13 |
153.7 |
| C3–H3⋯I1#4 |
0.93 |
4.10 |
4.54 |
112.4 |
| C4–H4⋯O1 |
0.93 |
2.25 |
2.83(3) |
120.0 |
| C8–H8⋯O1 |
0.93 |
2.18 |
2.80(3) |
123.0 |
| Symmetry codes: #1: x, y − 1, z + 1; #2: −x, −y, z + 1/2; #3: –x + 1/2, y − 1/2, z + 1/2; #4: x + 1/2, −y − 1/2, z |
Table 5 Geometry of hydrogen bonds (D–H⋯A) for Hg-MeOH and Hg-EtOH
| Compounds |
D–H⋯A |
d(D–H)/Å |
d(H⋯A)/Å |
d(D⋯A)/Å |
∠D–H⋯A/° |
|
Hg-MeOH
|
N2–H2A⋯O2 |
0.86 |
2.09 |
2.90(7) |
157.0 |
| N3–H3A⋯O2 |
0.86 |
2.17 |
2.98(7) |
156.1 |
| O2–H2B⋯I2#1 |
0.87(3) |
2.86(15) |
3.65(5) |
151(25) |
| C12–H12B⋯I2#2 |
0.95 |
3.78 |
4.24 |
111.9 |
| C2–H2⋯I1#3 |
0.92 |
3.14 |
3.95 |
147.0 |
| C3–H3⋯I1#3 |
0.92 |
3.82 |
4.30 |
115.6 |
| C4–H4⋯O1 |
0.93 |
2.24 |
2.83(6) |
120.6 |
| C8–H8⋯O1 |
0.93 |
2.27 |
2.82(6) |
117.0 |
| Symmetry codes: #1: −x + 1, −y + 1, z + 1/2; #2: −x + 1/2, y − 1/2, z + 1/2; #3: x − 1/2, –y + 1/2, z |
|
Hg-EtOH
|
N2–H2A⋯O2#1 |
0.86 |
2.20 |
3.00(6) |
153.0 |
| N3–H3A⋯O2#1 |
0.86 |
2.04 |
2.86(7) |
160.0 |
| O2–H2B#1⋯I2#2 |
0.9(4) |
2.9(4) |
3.66(4) |
158.0 |
| C12–H12A#1⋯I2#3 |
0.97 |
3.75 |
4.26 |
115.1 |
| C2–H2⋯I1#4 |
0.92 |
3.28 |
4.13 |
153.4 |
| C3–H3⋯I1#4 |
0.92 |
4.03 |
4.48 |
113.3 |
| C4–H4⋯O1 |
0.93 |
2.25 |
2.86(5) |
122.0 |
| C8–H8⋯O1 |
0.93 |
2.25 |
2.84(5) |
121.0 |
| Symmetry codes: #1: x, y, z + 1; #2: −x + 1, −y + 1, z + 1/2; #3: −x + 3/2, y + 1/2, z + 1/2; #4: x + 1/2, −y + 3/2, z |
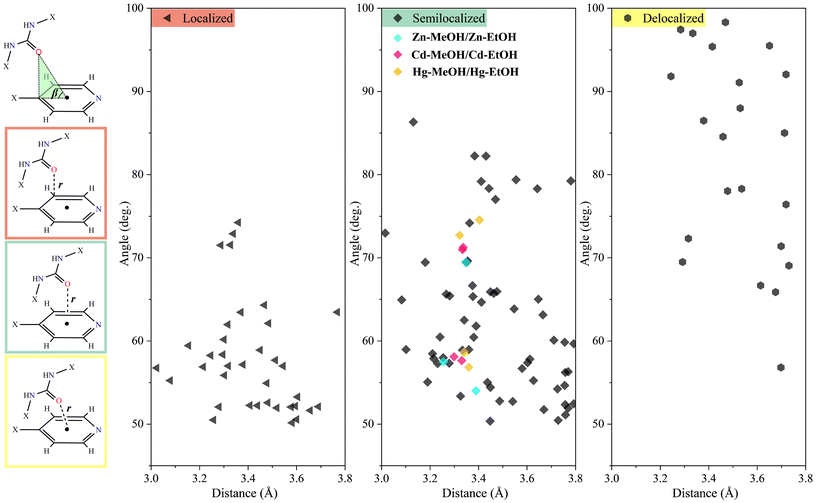
Scheme 2 illustrates the geometric parameters of urea–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy interactions and provides an overview of the classified lone pair–π interactions as localized (η1), semilocalized (η2) and delocalized (η6).89 In the case of Cg(1) and Cg(2) in all titled compounds, according to the urea–C
O⋯πpy interactions and provides an overview of the classified lone pair–π interactions as localized (η1), semilocalized (η2) and delocalized (η6).89 In the case of Cg(1) and Cg(2) in all titled compounds, according to the urea–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy geometric parameters (Table 6), particularly the r value which is slightly longer than the sum of the Bondi's van der Waals radii of the oxygen and carbon atoms (∑RvdW = 3.22 Å),90 these interactions can be classified as semilocalized (η2-SL) interaction.91 In order to study of the urea–C
O⋯πpy geometric parameters (Table 6), particularly the r value which is slightly longer than the sum of the Bondi's van der Waals radii of the oxygen and carbon atoms (∑RvdW = 3.22 Å),90 these interactions can be classified as semilocalized (η2-SL) interaction.91 In order to study of the urea–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy interactions in more detail, we have conducted an extensive single-crystal X-ray crystallographic study on the Cambridge Structural Database (CSD)92 with the help of ConQuest version 2024.1.0. This search focused on urea–C
O⋯πpy interactions in more detail, we have conducted an extensive single-crystal X-ray crystallographic study on the Cambridge Structural Database (CSD)92 with the help of ConQuest version 2024.1.0. This search focused on urea–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯π interactions and C⋯O contacts with a 4-substituted pyridyl ring in urea-based compounds. The processing of the obtained data allowed the recognition of several more examples of these intermolecular interactions (Fig. 5). In the CSD, 278 structures containing at least one urea–C
O⋯π interactions and C⋯O contacts with a 4-substituted pyridyl ring in urea-based compounds. The processing of the obtained data allowed the recognition of several more examples of these intermolecular interactions (Fig. 5). In the CSD, 278 structures containing at least one urea–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and a 4-substituted pyridyl ring have been identified. A detailed examination of the corresponding CIF files reveals that 47 structures (16.9% of the total) exhibit at least one lone pair–π interaction. In total, 118 lone pair–π contacts were identified.
O group and a 4-substituted pyridyl ring have been identified. A detailed examination of the corresponding CIF files reveals that 47 structures (16.9% of the total) exhibit at least one lone pair–π interaction. In total, 118 lone pair–π contacts were identified.
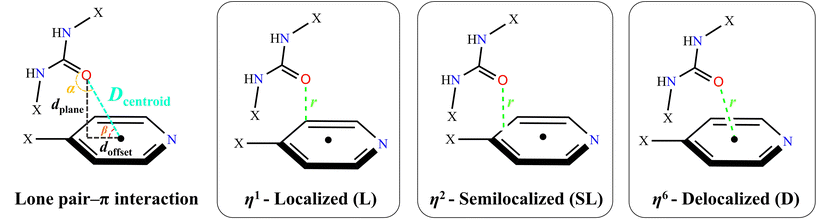 |
| | Scheme 2 Geometrical parametersa (left panel) and urea–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy classification (right panel)b.89aDcentroid is the oxygen distance from the centroid of ring atoms. dplane is the normal line from the oxygen to the ring plane. doffset is the parameter which determines the displacement of oxygen from the center of the aromatic ring [doffset = (Dcentroid2 − dplane2)1/2]. br is the shortest distance between the oxygen and ring member atom, centroid of bond or centroid of the ring atoms. O⋯πpy classification (right panel)b.89aDcentroid is the oxygen distance from the centroid of ring atoms. dplane is the normal line from the oxygen to the ring plane. doffset is the parameter which determines the displacement of oxygen from the center of the aromatic ring [doffset = (Dcentroid2 − dplane2)1/2]. br is the shortest distance between the oxygen and ring member atom, centroid of bond or centroid of the ring atoms. | |
 |
| | Fig. 5 An illustration of the search protocols. Scatter plots of the plane-centroid⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C angle versus the shortest atom⋯O C angle versus the shortest atom⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C distance, bond-centroid⋯O C distance, bond-centroid⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C distance and ring centroid⋯O C distance and ring centroid⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C distance that are in the acceptable range and extracted from the CSD data. C distance that are in the acceptable range and extracted from the CSD data. | |
Table 6 Distances (Dcentroid, dplane, doffset, r/Å) and angles (α, β/°) for the description of the lone pair–π interactions in M-MeOH and M-EtOH (M = Zn, Cd and Hg)a
| Compounds |
Interaction |
D
centroid
|
d
plane
|
d
offset
|
α
|
β
|
r/atom(s) |
Classification |
|
πpy1: N1–C1–C4–C5–C3–C2, πpy2: N4–C10–C9–C7–C8–C11.
|
|
Zn-MeOH
|
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy1 O⋯πpy1 |
3.565 |
3.338 |
1.25 |
97.11 |
69.44 |
3.349/C1–C4 |
η
2-SL |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy2 O⋯πpy2 |
3.750 |
3.161 |
2.01 |
69.93 |
57.45 |
3.256/C7–C9 |
η
2-SL |
|
Zn-EtOH
|
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy1 O⋯πpy1 |
3.565 |
3.338 |
1.25 |
97.50 |
69.44 |
3.349/C1–C4 |
η
2-SL |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy2 O⋯πpy2 |
3.944 |
3.190 |
2.31 |
64.18 |
53.98 |
3.390/C7–C9 |
η
2-SL |
|
Cd-MeOH
|
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy1 O⋯πpy1 |
3.507 |
3.315 |
1.14 |
97.67 |
70.95 |
3.335/C1–C4 |
η
2-SL |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy2 O⋯πpy2 |
3.766 |
3.196 |
1.99 |
67.59 |
58.06 |
3.300/C7–C9 |
η
2-SL |
|
Cd-EtOH
|
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy1 O⋯πpy1 |
3.516 |
3.329 |
1.13 |
98.16 |
71.22 |
3.338/C1–C4 |
η
2-SL |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy2 O⋯πpy2 |
3.814 |
3.220 |
2.04 |
68.30 |
57.59 |
3.332/C7–C9 |
η
2-SL |
|
Hg-MeOH
|
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy1 O⋯πpy1 |
3.462 |
3.305 |
1.03 |
96.27 |
72.67 |
3.323/C1–C4 |
η
2-SL |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy2 O⋯πpy2 |
3.793 |
3.239 |
1.97 |
67.77 |
58.64 |
3.342/C7–C9 |
η
2-SL |
|
Hg-EtOH
|
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy1 O⋯πpy1 |
3.503 |
3.376 |
0.93 |
95.58 |
74.52 |
3.405/C1–C4 |
η
2-SL |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy2 O⋯πpy2 |
3.857 |
3.227 |
2.11 |
66.09 |
56.78 |
3.360/C7–C9 |
η
2-SL |
Isostructurality of compounds
The similarity between all compounds with respect to their subassemblies or supramolecular constructs (SC) was examined using XPac 2.0.2 software introduced by Gelbrich and co-workers.93–95 All the atomic coordinates (in crystal geometry) except the hydrogen atoms, were considered for the XPac analysis. Table 7 and Fig. 6 show the overall structural similarity among these six crystal structures. Supramolecular constructs are the subcomponents of crystal structures that have geometrical closeness with respect to the conformation and position of molecules. The comparison of the structures is merely based on the geometrical basis that takes account of angular, planar, and distance relationships between the kernel molecule (central molecule) and the cluster molecules (surrounding molecules) of a given coordination sphere of molecules generated around a central molecule by relative symmetry operations of the space group of each structure considered for comparison. The XPac dissimilarity index (X)95 calculated for a coordination sphere comprising a kernel (central molecule) and other shell molecules shows 3D similarly between Zn-MeOH and Zn-EtOH with an (X) value of 3.3. The pertinent values of geometrical parameters i.e., angles [a] and planes [p] used to calculate (X) for Zn-MeOH and Zn-EtOH are 1.7 and 2.8, respectively. Moreover, intriguing insights can be extracted from the Fig. S18 and S19.† In the plot of δp (°) vs. δa (°), more data points close to the origin (i.e., data points at lower angles [a]) signify more similarity among the crystal structures. Thus, compound Zn-MeOH is more similar to compound Zn-EtOH than other compounds. Furthermore, when plotting (X) against δd (Å), it provides the stretching parameter ‘D’, which indicates the degree of elongation in one structure compared to another. A lower ‘D’ value signifies greater similarity, reinforcing the notion that compounds Cd-MeOH and Hg-MeOH exhibit significant structural resemblance, as illustrated in Table 7, Fig. S18 and S19.†
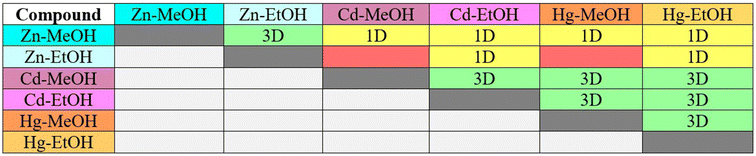 |
| | Fig. 6 Map showing the structural similarity (3D, 1D, and no similarity) for the six crystal structures. The gray color indicates a similar combination. | |
Table 7 Structural similarity (3D and 1D) parameters for the isostructural CPs
| Combination |
Dissimilarity index (X) |
δa (°) |
δp (°) |
D (Å) |
| 3D isostructurality |
Zn-MeOH & Zn-EtOH |
3.3 |
1.7 |
2.8 |
0.23 |
|
Cd-MeOH & Cd-EtOH |
2.1 |
1.0 |
1.8 |
0.13 |
|
Hg-MeOH & Hg-EtOH |
2.8 |
1.2 |
2.5 |
0.15 |
|
Cd-MeOH & Hg-MeOH |
2.2 |
0.8 |
2.0 |
0.03 |
|
Cd-EtOH & Hg-EtOH |
2.5 |
0.9 |
2.3 |
0.05 |
|
Cd-MeOH & Hg-EtOH |
2.9 |
1.2 |
2.7 |
0.17 |
|
Cd-EtOH & Hg-MeOH |
2.8 |
1.3 |
2.5 |
0.11 |
| 1D isostructurality |
Zn-MeOH & Cd-MeOH |
16.1 |
8.6 |
13.6 |
0.18 |
|
Zn-MeOH & Hg-MeOH |
16.8 |
8.9 |
14.1 |
0.20 |
|
Zn-EtOH & Cd-EtOH |
16.5 |
8.8 |
14.0 |
0.11 |
|
Zn-EtOH & Hg-EtOH |
17.4 |
9.1 |
14.8 |
0.14 |
|
Zn-MeOH & Cd-EtOH |
15.7 |
8.5 |
13.2 |
0.35 |
|
Zn-MeOH & Hg-EtOH |
16.5 |
8.8 |
13.9 |
0.37 |
Analysis of Hirshfeld surfaces
In order to gain a comprehensive understanding of the strength and role of hydrogen bonds and other intermolecular interactions, and to assess their significance in maintaining the stability of the crystal lattice in all described crystals, we employed Hirshfeld surface analysis. These analyses serve to highlight the similarities and distinctions in the intermolecular interactions of the studied isostructural coordination polymers, shedding light on their structural relationships. Hirshfeld surface analysis and 2D fingerprint calculations were performed using Crystal Explorer96 software, by importing the atomic coordinates from the CIF files. The distance from the nearest nucleus inside and outside the surface was measured and represented by the di and de, respectively, while a normalized contact distance was represented as dnorm. The white, red, and blue colors are selected for the visualization of the dnorm function with very high resolution. The Hirshfeld surface analysis of compounds is illustrated in Fig. 7, showing surfaces that have been mapped over the dnorm range of −0.7 to 1.6 Å, shape index (−1.0 to 1.0 Å) and curvedness (−4.0 to 0.4 Å). The deep red circular depressions on the dnorm surfaces indicate hydrogen bond interactions, which are notably present in the crystal structures of all compounds. The analysis reveals that in the crystal structure of all compounds the I⋯H hydrogen bonds are the dominant intermolecular interactions (the highest contributor to the Hirshfeld surface area). Also, in agreement with the geometrical analysis, it is found that the iodine atoms in all compounds are involved in X–H⋯I (X = C and O) hydrogen bonding interactions (Tables 3–5 and Fig. 4). The H⋯H intermolecular interaction is observed as a small spike, which makes the second most significant contribution to the total Hirshfeld surfaces. In addition, the presence of relatively weak C⋯H/H⋯C interactions is observed (Fig. 8 and 9).
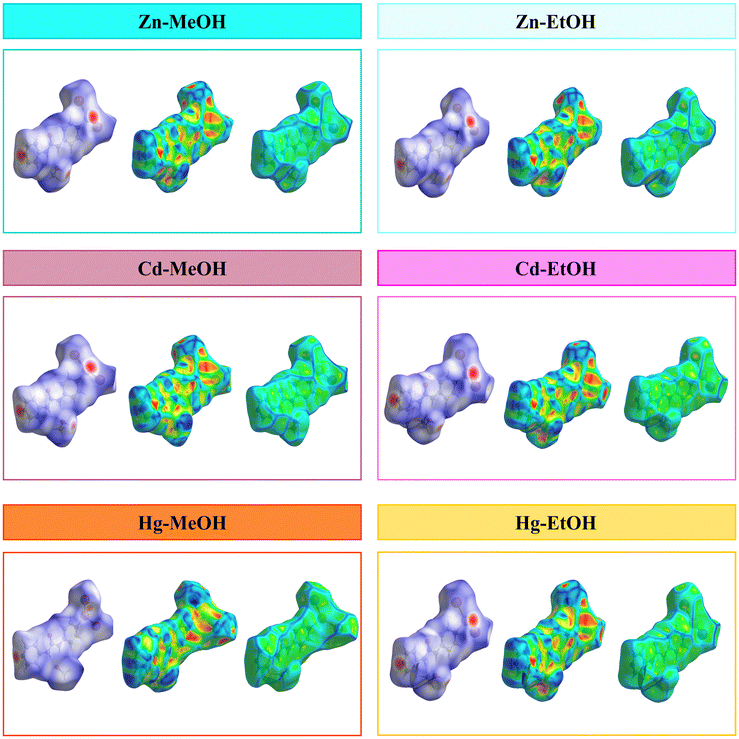 |
| | Fig. 7 Hirshfeld surfaces mapped with dnorm (left panel), shape index (middle panel) and curvedness (right panel) for the presented compounds. | |
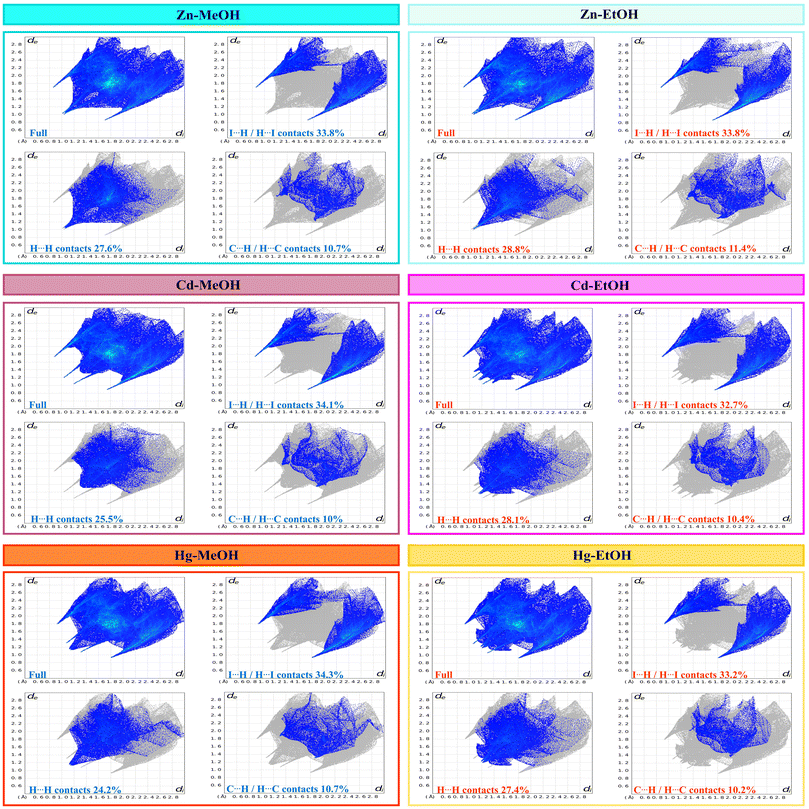 |
| | Fig. 8 Fingerprint plots of compounds, full and resolved into I⋯H/H⋯I, H⋯H, and C⋯H/H⋯C contacts showing the percentages of contacts contributing to the total Hirshfeld surface area of the molecules. | |
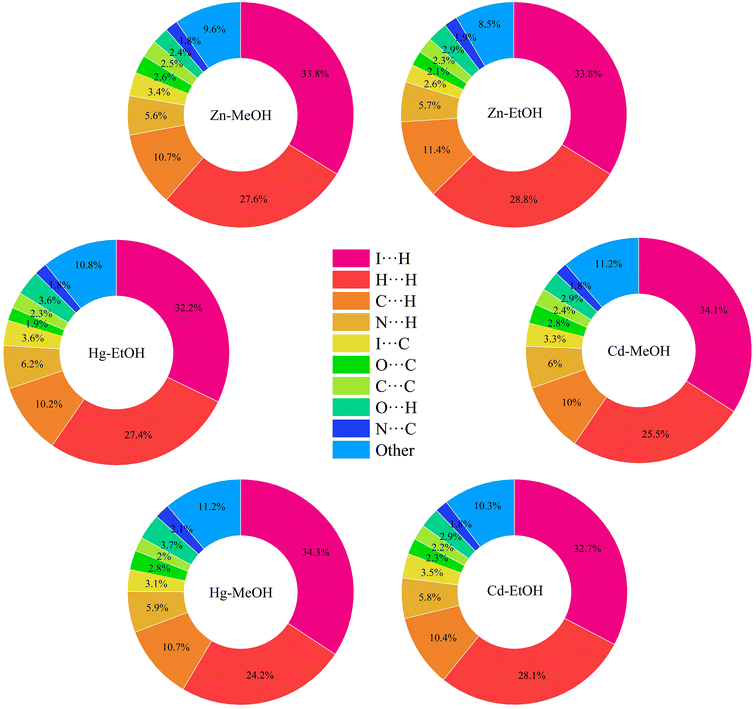 |
| | Fig. 9 The relative contribution of different intermolecular interactions to the Hirshfeld surface area in the presented compounds. | |
FT-IR spectra
The infrared absorption (FT-IR) spectra of the 4bpu ligand and M-MeOH and M-EtOH compounds (M = Zn, Cd and Hg) were obtained using the KBr pellet (Fig. S2–S8, ESI†). All compounds exhibit vibrations related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic, C
C aromatic, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O urea, N–H urea and C–H groups. Upon examining the FT-IR spectrum, we find that the stretching vibrational frequency of the C
O urea, N–H urea and C–H groups. Upon examining the FT-IR spectrum, we find that the stretching vibrational frequency of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in all compounds is sharp and changes from 1735 to 1742 cm−1. The absorption peaks at 1588–1596 cm−1 belong to the bending vibration peak of urea N–H groups. Above 3000 cm−1, stretching vibrational frequencies of the N–H of the urea group fall between 3200 and 3523 cm−1 and those of C–H aromatic range from 3045 to 3091 cm−1, appearing as a weak peak.18,97,98 Since MeOH and EtOH molecules are removed from the structure of these compounds after grinding, the ATR-FTIR spectra were analyzed to enable sample preparation without the grinding step. As shown in Fig. S20,† the differences in the stretching vibrational frequencies of the C–O bonds in MeOH and EtOH clearly distinguish between the FT-IR spectrum of M-MeOH and M-EtOH compounds (M = Zn, Cd and Hg).99
O group in all compounds is sharp and changes from 1735 to 1742 cm−1. The absorption peaks at 1588–1596 cm−1 belong to the bending vibration peak of urea N–H groups. Above 3000 cm−1, stretching vibrational frequencies of the N–H of the urea group fall between 3200 and 3523 cm−1 and those of C–H aromatic range from 3045 to 3091 cm−1, appearing as a weak peak.18,97,98 Since MeOH and EtOH molecules are removed from the structure of these compounds after grinding, the ATR-FTIR spectra were analyzed to enable sample preparation without the grinding step. As shown in Fig. S20,† the differences in the stretching vibrational frequencies of the C–O bonds in MeOH and EtOH clearly distinguish between the FT-IR spectrum of M-MeOH and M-EtOH compounds (M = Zn, Cd and Hg).99
Powder X-ray diffraction (PXRD) and thermogravimetric analysis (TGA)
To confirm the phase purity of all compounds, the PXRD patterns have been recorded at room temperature. The diffraction peaks of the as-synthesized samples are in agreement with the simulated data, indicating the phase purity of the synthesized compounds (Fig. S21 and S22†). Moreover, as shown in Fig. S23,† the isostructurality of these compounds is confirmed based on comparison of the simulated PXRD patterns from the X-ray single-crystal data. The thermal stability of compounds has been investigated through thermogravimetric analysis (TGA), exhibiting similar decomposition patterns (Fig. S24†). The comparable weight loss patterns observed in the TGA curves of the Zn-MeOH/Zn-EtOH, Cd-MeOH/Cd-EtOH and Hg-MeOH/Hg-EtOH imply similar decomposition pathways. However, the distinct percentage of weight loss for each compound indicates a difference in the stability of their frameworks. It is plausible that variations in the nature of bonding or the strength of intermolecular forces within the structures may contribute to the dissimilar thermal behaviors. Table S3† presents the temperature range, along with the calculated and observed weight loss percentages for methanol and ethanol guest molecules.
Conclusion
In summary, this work demonstrates a systematic investigation of the synthesis of six structurally identical ZnII, CdII and HgII pseudopolymorphic urea-based coordination polymers. The crystal structure determination by SC-XRD of these isostructural coordination polymers revealed one-dimensional zig-zag chains. The expected bifurcated hydrogen bonds between urea groups, typically forming an α-tape motif, were absent in these structures. Instead, the coordination polymers exhibit a unique arrangement where robust and repetitive hydrogen bonds between the urea groups and solvent molecules (MeOH or EtOH) connect the chains, forming a distinctive supramolecular network. Additional interactions, including urea–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy (lone pair–π) and C–H⋯I hydrogen bonding, contribute to stabilizing the crystal structure. This work is one of the few systematic studies that comprehensively examines the aspect of controlling pseudopolymorphism via supramolecular interactions. These findings highlight the significant role of solvent interactions in supramolecular assemblies, extend the understanding of pseudopolymorphism and provide insights for designing materials with tailored intermolecular interactions.
O⋯πpy (lone pair–π) and C–H⋯I hydrogen bonding, contribute to stabilizing the crystal structure. This work is one of the few systematic studies that comprehensively examines the aspect of controlling pseudopolymorphism via supramolecular interactions. These findings highlight the significant role of solvent interactions in supramolecular assemblies, extend the understanding of pseudopolymorphism and provide insights for designing materials with tailored intermolecular interactions.
Author contributions
Ghazale Khorshidi: investigation, formal analysis, writing – original draft, software, and writing – review & editing. Behrouz Notash: conceptualization, supervision, funding acquisition, project administration, writing – review & editing, and software.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors thank the Graduate Study Councils of Shahid Beheshti University, G. C. for financial support. We are thankful to Dr Alireza Salimi (Ferdowsi University of Mashhad) for his help in crystallographic part.
References
- S. Bhattacharyya and T. K. Maji, Coord. Chem. Rev., 2022, 469, 214645 CrossRef CAS.
- S. A. A. Razavi and A. Morsali, Coord. Chem. Rev., 2019, 399, 213023 CrossRef.
- N. Li, R. Feng, J. Zhu, Z. Chang and X.-H. Bu, Coord. Chem. Rev., 2018, 375, 558–586 CrossRef CAS.
- Y. Li, Y. Wang, W. Fan and D. Sun, Dalton Trans., 2022, 51, 4608–4618 RSC.
- D.-X. Xue, Q. Wang and J. Bai, Coord. Chem. Rev., 2019, 378, 2–16 CrossRef CAS.
- A. Bavykina, N. Kolobov, I. S. Khan, J. A. Bau, A. Ramirez and J. Gascon, Chem. Rev., 2020, 120, 8468–8535 CrossRef CAS PubMed.
- K. Biradha, A. Goswami and R. Moi, Chem. Commun., 2020, 56, 10824–10842 RSC.
- J.-Q. Liu, Z.-D. Luo, Y. Pan, A. K. Singh, M. Trivedi and A. Kumar, Coord. Chem. Rev., 2020, 406, 213145 CrossRef CAS.
- L. Chen, D. Liu, J. Peng, Q. Du and H. He, Coord. Chem. Rev., 2020, 404, 213113 CrossRef CAS.
- Y. Feng, Y. Wang and Y. Ying, Coord. Chem. Rev., 2021, 446, 214102 CrossRef CAS.
- A. E. Thorarinsdottir and T. D. Harris, Chem. Rev., 2020, 120, 8716–8789 CrossRef CAS PubMed.
- Q. Zhao, Q. Y. Li and J. Li, CrystEngComm, 2022, 24, 6751–6761 RSC.
- J. Wang, N. N. Chen, C. Zhang, L. Y. Jia and L. Fan, CrystEngComm, 2020, 22, 811–820 RSC.
- Z. Zhang, W. Sang, L. Xie and Y. Dai, Coord. Chem. Rev., 2019, 399, 213022 CrossRef CAS.
- R. M. Safdar, H. Ali and Z. Li, Molecules, 2022, 27, 100 CrossRef PubMed.
- S. Mondal and P. Dastidar, Cryst. Growth Des., 2020, 20, 7411–7420 CrossRef CAS.
- A. Karmakar, S. Hazra and A. J. L. Pombeiro, Coord. Chem. Rev., 2022, 453, 214314 CrossRef CAS.
- P. Biswas and P. Dastidar, Inorg. Chem., 2021, 60, 3218–3231 CrossRef CAS PubMed.
- R. Brahma and J. B. Baruah, CrystEngComm, 2021, 23, 3812–3827 RSC.
- S. Glomb, D. Woschko, G. Makhloufi and C. Janiak, ACS Appl. Mater. Interfaces, 2017, 9, 37419–37434 CrossRef CAS PubMed.
- J. M. Roberts, B. M. Fini, A. A. Sarjeant, O. K. Farha, J. T. Hupp and K. A. Scheidt, J. Am. Chem. Soc., 2012, 134, 3334–3337 CrossRef CAS PubMed.
- A. Azhdari Tehrani, L. Esrafili, S. Abedi, A. Morsali, L. Carlucci, D. M. Proserpio, J. Wang, P. C. Junk and T. Liu, Inorg. Chem., 2017, 56, 1446–1454 CrossRef CAS PubMed.
- L. Esrafili, M. Gharib and A. Morsali, Dalton Trans., 2019, 48, 17831–17839 RSC.
- A. M. Cheplakova, D. G. Samsonenko, V. A. Lazarenko, P. V. Dorovatovskii, Y. V. Zubavichus, V. N. Khrustalev, M. I. Rakhmanova and V. P. Fedin, CrystEngComm, 2022, 24, 2057–2071 RSC.
- H. Moon, S. W. Lim, D. Kim, O.-S. Jung and Y.-A. Lee, CrystEngComm, 2021, 23, 1272–1280 RSC.
- B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629–1658 CrossRef CAS PubMed.
- A. Karmakar, A. Paul and A. J. L. Pombeiro, CrystEngComm, 2017, 19, 4666–4695 RSC.
- G. Khorshidi and B. Notash, Sci. Rep., 2024, 14, 27586 CrossRef CAS PubMed.
- P. Cui, J. Wu, X. Zhao, D. Sun, L. Zhang, J. Guo and D. Sun, Cryst. Growth Des., 2011, 11, 5182–5187 CrossRef CAS.
- B. Notash and B. Rezaei Kheirkhah, New J. Chem., 2018, 42, 15014–15021 RSC.
- W.-W. Dong, D.-S. Li, J. Zhao, L.-F. Ma, Y.-P. Wu and Y.-P. Duan, CrystEngComm, 2013, 15, 5412–5416 RSC.
- B. Notash, J. Mol. Struct., 2018, 1156, 534–543 CrossRef CAS.
- B. Notash, B. Rezaei Kheirkhah and G. Khorshidi, J. Mol. Struct., 2023, 1294, 136324 CrossRef CAS.
- S. S. Nagarkar, A. K. Chaudhari and S. K. Ghosh, Cryst. Growth Des., 2012, 12, 572–576 Search PubMed.
- H. Ju, Y. Habata and S. S. Lee, Cryst. Growth Des., 2020, 20, 4640–4648 CrossRef CAS.
- J. J. Wu, W. Xue, M. L. Cao, Z. P. Qiao and B. H. Ye, CrystEngComm, 2011, 13, 5495–5501 RSC.
- A. B. Lago, R. Carballo, S. Rodríguez-Hermida and E. M. Vázquez-López, CrystEngComm, 2013, 15, 1563–1570 Search PubMed.
- M.-L. Han, X.-H. Chang, X. Feng, L.-F. Ma and L.-Y. Wang, CrystEngComm, 2014, 16, 1687–1695 RSC.
- E. Lee, J.-Y. Kim, S. S. Lee and K.-M. Park, Chem. – Eur. J., 2013, 19, 13638–13645 Search PubMed.
- L. Dobrzańska, Inorg. Chem. Commun., 2015, 55, 21–24 Search PubMed.
- H. Wang, J. Yang and T. C. W. Mak, New J. Chem., 2014, 38, 4690–4695 RSC.
- L. K. Das, A. M. Kirillov and A. Ghosh, CrystEngComm, 2014, 16, 3029–3039 RSC.
- K. M. Fromm, J. L. S. Doimeadios and A. Y. Robin, Chem. Commun., 2005, 4548–4550 RSC.
- V. S. S. Kumar, F. Christopher Pigge and N. P. Rath, New J. Chem., 2003, 27, 1554–1556 RSC.
- J.-P. Zhang, X.-C. Huang and X.-M. Chen, Chem. Rev., 2009, 38, 2385–2396 CAS.
- B. Notash, M. Farhadi Rodbari and M. Kubicki, ACS Omega, 2023, 8, 13140–13152 CrossRef CAS PubMed.
- B. Notash, M. Farhadi Rodbari, G. Gallo and R. Dinnebier, Inorg. Chem., 2021, 60, 9212–9223 CrossRef CAS PubMed.
-
J. L. Atwood and J. W. Steed, Encyclopedia of Supramolecular Chemistry, M. Dekker, 2004 Search PubMed.
- H. G. Brittain, J. Pharm. Sci., 2012, 101, 464–484 CrossRef CAS PubMed.
- P. Lu and S. Rohani, Curr. Med. Chem., 2009, 16, 884–905 CrossRef PubMed.
- K. J. Chavez, M. Guevara and R. W. Rousseau, Cryst. Growth Des., 2010, 10, 3372–3377 CrossRef CAS.
- G. Kang, Y. Jeon, K. Y. Lee, J. Kim and T. H. Kim, Cryst. Growth Des., 2015, 15, 5183–5187 Search PubMed.
- Z. Li, J. Zhou, K. Zhang, Y. Zhang, S. Wu and J. Gong, Cryst. Growth Des., 2022, 22, 5322–5334 CrossRef CAS.
- H. R. Khavasi and B. Mir Mohammad Sadegh, Cryst. Growth Des., 2012, 12, 4798–4804 CrossRef CAS.
- R. Thakuria, N. K. Nath, S. Roy and A. Nangia, CrystEngComm, 2014, 16, 4681–4690 RSC.
- J. Galcera, T. Friščić, E. Molins and W. Jones, CrystEngComm, 2013, 15, 1332–1338 Search PubMed.
- J. Galcera, T. Friščić, K. E. Hejczyk, L. Fábián, S. M. Clarke, G. M. Day, E. Molins and W. Jones, CrystEngComm, 2012, 14, 7898–7906 Search PubMed.
- B. K. Saha and A. Nangia, Heteroat. Chem., 2007, 18, 185–194 CrossRef CAS.
- G. Khorshidi, B. Notash and M. Kubicki, CrystEngComm, 2024, 26, 4082–4097 RSC.
- A. Kalman, Acta Crystallogr., Sect. B:Struct. Sci., 2005, 61, 536–547 CrossRef PubMed.
- L. Fábián and A. Kámán, Acta Crystallogr., Sect. B:Struct. Sci., 2004, 60, 547–558 CrossRef PubMed.
- J. Kendrick, R. Montis, M. B. Hursthouse and F. J. J. Leusen, Cryst. Growth Des., 2013, 13, 2906–2915 CrossRef CAS.
- G. R. Desiraju, Angew. Chem., Int. Ed. Engl., 1995, 34, 2311–2327 CrossRef CAS.
- S. K. Nayak, M. K. Reddy, T. N. Guru Row and D. Chopra, Cryst. Growth Des., 2011, 11, 1578–1596 CrossRef CAS.
- A. Singh, A. Ramanan and D. Bandyopadhyay, Cryst. Growth Des., 2011, 11, 2743–2754 CrossRef CAS.
- Y. Zhao, H. Wu, T. J. Emge, Q. Gong, N. Nijem, Y. J. Chabal, L. Kong, D. C. Langreth, H. Liu, H. Zeng and J. Li, Chem. – Eur. J., 2011, 17, 5101–5109 Search PubMed.
- Y.-F. Han, W.-G. Jia, W.-B. Yu and G.-X. Jin, Chem. Soc. Rev., 2009, 38, 3419–3434 Search PubMed.
- Y.-F. Han, W.-G. Jia, Y.-J. Lin and G.-X. Jin, Angew. Chem., Int. Ed., 2009, 48, 6234–6238 Search PubMed.
- J. S. Geng, W. Feng, J. Li, X. Y. Tang, L. Meng, J. P. Yu, K. Q. Hu, L. H. Yuan, L. Mei and W. Q. Shi, Inorg. Chem., 2022, 61, 10694–10704 Search PubMed.
- H. H. Wang, Y. Zhang, D. B. Yang, L. Hou, Z. Y. Li and Y. Y. Wang, Cryst. Growth Des., 2021, 21, 2488–2497 Search PubMed.
- S. Chand, S. Hota, S. M. Elahi and M. C. Das, Polyhedron, 2018, 153, 115–121 CrossRef CAS.
- B. Bhattacharya, D. K. Maity, R. Mondal, E. Colacio and D. Ghoshal, Cryst. Growth Des., 2015, 15, 4427–4437 Search PubMed.
- A. H. Chughtai, N. Ahmad, H. A. Younus, A. Laypkov and F. Verpoort, Chem. Soc. Rev., 2015, 44, 6804–6849 RSC.
- W. Zhou, H. Wu and T. Yildirim, J. Am. Chem. Soc., 2008, 130, 15268–15269 Search PubMed.
- S. R. Caskey, A. G. Wong-Foy and A. J. Matzger, J. Am. Chem. Soc., 2008, 130, 10870–10871 CrossRef CAS PubMed.
- X. Y. Tian, H. L. Zhou, X. W. Zhang, C. Wang, Z. H. Qiu, D. D. Zhou and J. P. Zhang, Inorg. Chem., 2020, 59, 6047–6052 Search PubMed.
- Z. U. Nisa, L. Tashi, C. Sen, N. A. Ashashi, S. C. Sahoo and H. N. Sheikh, New J. Chem., 2020, 44, 8125–8137 RSC.
- G. Liu, Y. K. Lu, Y. Y. Ma, X. Q. Wang, L. Hou and Y. Y. Wang, Dalton Trans., 2019, 48, 13607–13613 RSC.
- B. Dutta, A. Dey, K. Naskar, S. Maity, F. Ahmed, S. Islam, C. Sinha, P. Ghosh, P. P. Ray and M. H. Mir, New J. Chem., 2018, 42, 10309–10316 RSC.
- S. K. Chandran, N. K. Nath, S. Cherukuvada and A. Nangia, J. Mol. Struct., 2010, 968, 99–107 Search PubMed.
- G. Mahmoudi, A. Morsali, A. D. Hunter and M. Zeller, CrystEngComm, 2007, 9, 704–714 Search PubMed.
- L. Yang, D. R. Powell and R. P. Houser, Dalton Trans., 2007, 955–964 RSC.
- J.-Y. Cheng, F.-W. Ding, P. Wang, C.-W. Zhao and Y.-B. Dong, ChemPlusChem, 2016, 81, 743–751 CrossRef CAS PubMed.
- B. Notash, N. Safari and H. R. Khavasi, CrystEngComm, 2012, 14, 6788–6796 Search PubMed.
- B. Notash, N. Safari and H. R. Khavasi, Inorg. Chem., 2010, 49, 11415–11420 CrossRef CAS PubMed.
- X. F. Wang, Y. Lv, T. A. Okamura, H. Kawaguchi, G. Wu, W. Y. Sun and N. Ueyama, Cryst. Growth Des., 2007, 7, 1125–1133 Search PubMed.
- M. C. Etter, Acc. Chem. Res., 1990, 23, 120–126 Search PubMed.
- M. C. Etter, J. C. MacDonald and J. Bernstein, Acta Crystallogr., Sect. B:Struct. Sci., 1990, 46, 256–262 Search PubMed.
- B. Ebrahimi, B. Notash, T. Matar and R. Dinnebier, Inorg. Chem., 2024, 63, 983–999 CrossRef CAS PubMed.
- A. Bondi, J. Phys. Chem. C, 1964, 68, 441–451 CrossRef CAS.
- T. J. Mooibroek, P. Gamez and J. Reedijk, CrystEngComm, 2008, 10, 1501–1515 RSC.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B:Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS PubMed.
- T. Gelbrich and M. B. Hursthouse, CrystEngComm, 2005, 7, 324–336 RSC.
- T. Gelbrich and M. B. Hursthouse, CrystEngComm, 2006, 8, 448–460 RSC.
- F. P. A. Fabbiani, B. Dittrich, A. J. Florence, T. Gelbrich, M. B. Hursthouse, W. F. Kuhs, N. Shankland and H. Sowa, CrystEngComm, 2009, 11, 1396–1406 RSC.
-
S. K. Wolff, D. J. Grimwood, J. J. McKinnon, M. J. Turner, D. Jayatilaka and M. A. Spackman, CrystalExplorer, Version 3.1, University of Western Australia, Australia, 2012 Search PubMed.
- S. Banerjee, D. P. Kumar, S. Bandyopadhay, N. N. Adarsh and P. Dastidar, Cryst. Growth Des., 2012, 12, 5546–5554 Search PubMed.
- U. Manna, S. Kayal, B. Nayak and G. Das, Dalton Trans., 2017, 46, 11956–11969 RSC.
- T. Coldea, C. Socaciu, F. Fetea, F. Ranga, R. Pop and M. Florea, Not. Bot. Horti Agrobot., 2013, 41, 143–149 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Selected bond lengths and angles, PXRD pattern and TGA of compounds Zn-MeOH, Zn-EtOH, Cd-MeOH, Cd-EtOH, Hg-MeOH, and Hg-EtOH. CCDC 2391447 (Zn-MeOH), 2391448 (Zn-EtOH), 2391449 (Cd-MeOH), 2391450 (Cd-EtOH), 2391451 (Hg-MeOH) and 2391452 (Hg-EtOH). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4dt03344b |
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  *
*
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy interactions in urea-based compounds highlights the classification of these interactions as semilocalized (η2-SL) based on geometric parameters and reveals their significance through crystallographic and database studies. Hirshfeld surface analysis has been performed for all compounds to determine the percentage contribution of intermolecular interactions.
O⋯πpy interactions in urea-based compounds highlights the classification of these interactions as semilocalized (η2-SL) based on geometric parameters and reveals their significance through crystallographic and database studies. Hirshfeld surface analysis has been performed for all compounds to determine the percentage contribution of intermolecular interactions.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mol ratio were placed in the main arm of a branched tube. MeOH was carefully added to fill both arms completely. The tube was sealed and the main arm was immersed in an oil bath at 60 °C while the other arm was kept at ambient temperature for several days and colorless needle shaped single crystals of Zn-MeOH were formed (Scheme 1). Yield: 74%. m.p: 240 °C. Elemental analysis for C11H10I2N4OZn: calculated: C, 24.77; H, 1.89; N, 10.50%; found: C, 23.62; H, 2.22; N, 9.95%. FTIR (KBr pellet, cm−1) (Fig. S3†): 3476(w), 3373(w), 3292(w), 3091(w), 3033(w), 2360(w), 1742(m), 1589(s), 1507(s), 1431(m), 1333(m), 1288(s), 1257(w), 1180(s), 1063(m), 1024(s), 833(s), 745(w), 666(w), 552(m), 534(m), 418(w).
1 mol ratio were placed in the main arm of a branched tube. MeOH was carefully added to fill both arms completely. The tube was sealed and the main arm was immersed in an oil bath at 60 °C while the other arm was kept at ambient temperature for several days and colorless needle shaped single crystals of Zn-MeOH were formed (Scheme 1). Yield: 74%. m.p: 240 °C. Elemental analysis for C11H10I2N4OZn: calculated: C, 24.77; H, 1.89; N, 10.50%; found: C, 23.62; H, 2.22; N, 9.95%. FTIR (KBr pellet, cm−1) (Fig. S3†): 3476(w), 3373(w), 3292(w), 3091(w), 3033(w), 2360(w), 1742(m), 1589(s), 1507(s), 1431(m), 1333(m), 1288(s), 1257(w), 1180(s), 1063(m), 1024(s), 833(s), 745(w), 666(w), 552(m), 534(m), 418(w).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under thermal gradient conditions (convection technique). Distinguishingly, the single crystals of these compounds were obtained from the MeOH or EtOH solvent system during the synthesis procedure (Scheme 1).
1 under thermal gradient conditions (convection technique). Distinguishingly, the single crystals of these compounds were obtained from the MeOH or EtOH solvent system during the synthesis procedure (Scheme 1).
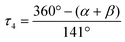

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy play a crucial role in the crystal packing of these pseudopolymorphs. There are two intramolecular C–H⋯O hydrogen bonding interactions in 4bpu ligands. A more detailed description of the hydrogen bond interactions for Zn-MeOH/Zn-EtOH, Cd-MeOH/Cd-EtOH and Hg-MeOH/Hg-EtOH is provided in Tables 3–5 and Fig. S16 and S17,† respectively.
O⋯πpy play a crucial role in the crystal packing of these pseudopolymorphs. There are two intramolecular C–H⋯O hydrogen bonding interactions in 4bpu ligands. A more detailed description of the hydrogen bond interactions for Zn-MeOH/Zn-EtOH, Cd-MeOH/Cd-EtOH and Hg-MeOH/Hg-EtOH is provided in Tables 3–5 and Fig. S16 and S17,† respectively.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy interactions and provides an overview of the classified lone pair–π interactions as localized (η1), semilocalized (η2) and delocalized (η6).89 In the case of Cg(1) and Cg(2) in all titled compounds, according to the urea–C
O⋯πpy interactions and provides an overview of the classified lone pair–π interactions as localized (η1), semilocalized (η2) and delocalized (η6).89 In the case of Cg(1) and Cg(2) in all titled compounds, according to the urea–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy geometric parameters (Table 6), particularly the r value which is slightly longer than the sum of the Bondi's van der Waals radii of the oxygen and carbon atoms (∑RvdW = 3.22 Å),90 these interactions can be classified as semilocalized (η2-SL) interaction.91 In order to study of the urea–C
O⋯πpy geometric parameters (Table 6), particularly the r value which is slightly longer than the sum of the Bondi's van der Waals radii of the oxygen and carbon atoms (∑RvdW = 3.22 Å),90 these interactions can be classified as semilocalized (η2-SL) interaction.91 In order to study of the urea–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy interactions in more detail, we have conducted an extensive single-crystal X-ray crystallographic study on the Cambridge Structural Database (CSD)92 with the help of ConQuest version 2024.1.0. This search focused on urea–C
O⋯πpy interactions in more detail, we have conducted an extensive single-crystal X-ray crystallographic study on the Cambridge Structural Database (CSD)92 with the help of ConQuest version 2024.1.0. This search focused on urea–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯π interactions and C⋯O contacts with a 4-substituted pyridyl ring in urea-based compounds. The processing of the obtained data allowed the recognition of several more examples of these intermolecular interactions (Fig. 5). In the CSD, 278 structures containing at least one urea–C
O⋯π interactions and C⋯O contacts with a 4-substituted pyridyl ring in urea-based compounds. The processing of the obtained data allowed the recognition of several more examples of these intermolecular interactions (Fig. 5). In the CSD, 278 structures containing at least one urea–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group and a 4-substituted pyridyl ring have been identified. A detailed examination of the corresponding CIF files reveals that 47 structures (16.9% of the total) exhibit at least one lone pair–π interaction. In total, 118 lone pair–π contacts were identified.
O group and a 4-substituted pyridyl ring have been identified. A detailed examination of the corresponding CIF files reveals that 47 structures (16.9% of the total) exhibit at least one lone pair–π interaction. In total, 118 lone pair–π contacts were identified.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy classification (right panel)b.89aDcentroid is the oxygen distance from the centroid of ring atoms. dplane is the normal line from the oxygen to the ring plane. doffset is the parameter which determines the displacement of oxygen from the center of the aromatic ring [doffset = (Dcentroid2 − dplane2)1/2]. br is the shortest distance between the oxygen and ring member atom, centroid of bond or centroid of the ring atoms.
O⋯πpy classification (right panel)b.89aDcentroid is the oxygen distance from the centroid of ring atoms. dplane is the normal line from the oxygen to the ring plane. doffset is the parameter which determines the displacement of oxygen from the center of the aromatic ring [doffset = (Dcentroid2 − dplane2)1/2]. br is the shortest distance between the oxygen and ring member atom, centroid of bond or centroid of the ring atoms.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy1
O⋯πpy1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy2
O⋯πpy2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy1
O⋯πpy1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy2
O⋯πpy2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy1
O⋯πpy1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy2
O⋯πpy2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy1
O⋯πpy1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy2
O⋯πpy2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy1
O⋯πpy1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy2
O⋯πpy2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy1
O⋯πpy1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy2
O⋯πpy2



![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic, C
C aromatic, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O urea, N–H urea and C–H groups. Upon examining the FT-IR spectrum, we find that the stretching vibrational frequency of the C
O urea, N–H urea and C–H groups. Upon examining the FT-IR spectrum, we find that the stretching vibrational frequency of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group in all compounds is sharp and changes from 1735 to 1742 cm−1. The absorption peaks at 1588–1596 cm−1 belong to the bending vibration peak of urea N–H groups. Above 3000 cm−1, stretching vibrational frequencies of the N–H of the urea group fall between 3200 and 3523 cm−1 and those of C–H aromatic range from 3045 to 3091 cm−1, appearing as a weak peak.18,97,98 Since MeOH and EtOH molecules are removed from the structure of these compounds after grinding, the ATR-FTIR spectra were analyzed to enable sample preparation without the grinding step. As shown in Fig. S20,† the differences in the stretching vibrational frequencies of the C–O bonds in MeOH and EtOH clearly distinguish between the FT-IR spectrum of M-MeOH and M-EtOH compounds (M = Zn, Cd and Hg).99
O group in all compounds is sharp and changes from 1735 to 1742 cm−1. The absorption peaks at 1588–1596 cm−1 belong to the bending vibration peak of urea N–H groups. Above 3000 cm−1, stretching vibrational frequencies of the N–H of the urea group fall between 3200 and 3523 cm−1 and those of C–H aromatic range from 3045 to 3091 cm−1, appearing as a weak peak.18,97,98 Since MeOH and EtOH molecules are removed from the structure of these compounds after grinding, the ATR-FTIR spectra were analyzed to enable sample preparation without the grinding step. As shown in Fig. S20,† the differences in the stretching vibrational frequencies of the C–O bonds in MeOH and EtOH clearly distinguish between the FT-IR spectrum of M-MeOH and M-EtOH compounds (M = Zn, Cd and Hg).99
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯πpy (lone pair–π) and C–H⋯I hydrogen bonding, contribute to stabilizing the crystal structure. This work is one of the few systematic studies that comprehensively examines the aspect of controlling pseudopolymorphism via supramolecular interactions. These findings highlight the significant role of solvent interactions in supramolecular assemblies, extend the understanding of pseudopolymorphism and provide insights for designing materials with tailored intermolecular interactions.
O⋯πpy (lone pair–π) and C–H⋯I hydrogen bonding, contribute to stabilizing the crystal structure. This work is one of the few systematic studies that comprehensively examines the aspect of controlling pseudopolymorphism via supramolecular interactions. These findings highlight the significant role of solvent interactions in supramolecular assemblies, extend the understanding of pseudopolymorphism and provide insights for designing materials with tailored intermolecular interactions.





