 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The cyclic 48-tungsto-8-phosphate [H7P8W48O184]33− Contant–Tézé polyanion and its derivatives [H6P4W24O94]18− and [H2P2W12O48]12−: structural aspects and reactivity
Sib Sankar Mal
 *a,
Abhishek Banerjee
*a,
Abhishek Banerjee
 b and
Ulrich Kortz
b and
Ulrich Kortz
 *c
*c
aMaterials and Catalysis Lab, Department of Chemistry, National Institute of Technology Karnataka, 575025, Surathkal, India. E-mail: malss@nitk.edu.in
bDepartment of Chemistry, Visvesvaraya National Institute of Technology, Nagpur, 440010, India. E-mail: abhishekbanerjee@chm.vnit.ac.in
cSchool of Science, Constructor University, Campus Ring 1, 28759 Bremen, Germany. E-mail: ukortz@constructor.university
First published on 3rd February 2025
Abstract
Polyoxometalates (POMs) are discrete, anionic metal-oxo clusters of early transition metals in high oxidation states (e.g., WVI, MoVI, VV) usually comprised of edge- and corner-shared MO6 octahedra. Lacunary POMs are defect heteropolyanions mainly of the Keggin or Dawson type, and they can be formed by the loss of one or more MO6 octahedra by controlled base hydrolysis. The largest subclass of POMs are tungstophosphates, and several lacunary derivatives are known, such as the Keggin-based [PW11O39]7− and [PW9O34]9−, and the Dawson-based [P2W17O61]10− and [P2W15O56]12−. This review is based on the cyclic 48-tungsto-8-phosphate [H7P8W48O184]33− (P8W48) as well as its smaller derivatives [H6P4W24O94]18− (P4W24), and [H2P2W12O48]12− (P2W12), with a focus on structural aspects, solution stability and reactivity. All three polyanions can be considered as inorganic multidentate O-donor ligands that coordinate with d, f or p-block metal ions. Here we provide a comprehensive overview of guest metal-containing derivatives of the P8W48 wheel, the P4W24 half-wheel and the P2W12 quarter wheel. The structures containing P2W12 as a building unit are presented in a sequence of increasing number of POM units in the resulting assembly. Transition metal-containing POMs have been of interest for decades due to their remarkable capability of forming novel and unexpected structures associated with interesting and relevant physicochemical properties (e.g., catalysis, magnetism, biomedicine, electrochemistry), and this also applies for derivatives containing P8W48, P4W24 and P2W12.
1. Introduction
The field of polyoxometalate (POM) chemistry has seen increased interest in recent decades due to the extensive range of chemical and physical properties of such compounds. Berzelius reported the formation of a molydophosphate polyoxoanion in 1826, and more than a decade later, Keggin identified the crystal structure of H3[PW12O40] using powder X-ray diffraction.1 As such, POMs were elucidated as discrete anionic metal-oxo clusters formed by condensation of simple oxoanions (e.g., WO42−) in aqueous media upon acidification. POMs consist of oxo-bridged early transition metal atoms of groups V and VI in high oxidation states, such as VV, NbV, TaV, MoVI, or WVI.2 For the formation of stable polyanions, the metal addendum atom should possess vacant d orbitals, which allows for dπ–pπ back bonding with terminal oxygen atoms. This phenomenon helps to terminate the condensation process at the level of discrete polyanions and disfavors the formation of extended metal oxide lattices.Based on their chemical composition, POMs can be broadly subdivided into two main subclasses: (i) isopolyanions, which are composed exclusively of metal addenda M and oxo ligands, represented as [MmOy]n−, and (ii) heteropolyanions, which contain one or more hetero atoms inside the polyanion, represented as [XxMmOy]q− (x ≤ m), where X is usually a main group element such as P, Si, Ge, or As. Due to their high thermal and redox stability as well as their radiation-resistant nature, isopolyanions have attracted increasing attention for applications involving the separation and sequestration of radioactive species.3 It is important to note that the formation of polyoxotungstates is accompanied by very slow equilibration of the reaction system, compared to molybdates and vanadates. Prime examples of heteropolyanions are the Keggin ion (e.g. [SiW12O40]4−) and the Wells–Dawson ion (e.g. [P2Mo18O62]6−), incorporating one or two tetrahedral XO4 hetero groups, respectively. Pope and Müller reviewed the synthesis and characterization of POMs in 1991. Subsequently, Hill, as guest editor, highlighted popular research themes within the field of POM chemistry in a thematic issue of Chemical Reviews in 1998.4–9
POMs have generated considerable interest across all areas of chemistry due to their versatile nature with regard to shape, size, composition, redox activity, solubility, photochemistry, and charge distribution.7 Contemporary areas that have emerged in the multidisciplinary field of POM chemistry are mainly associated with various applications of POMs corresponding to developing new materials.10 These have shown promise in the fields of nanotechnology,11 biology,12–15 surfaces,14–17 catalysis,18,19 supramolecular materials,20,21 colloid science,22 and electronic materials,23 sensors,24,25 molecular materials26,27 and magnetism.28 Crucial to the fast development of structural POM chemistry are advances in single-crystal X-ray diffraction (XRD), which allows for faster measurements on smaller crystals, allowing the characterization of large (with several hundred addenda atoms) polyanions.29,30 Polyoxotungstates, in particular, have been observed to be effective homogeneous photocatalysts for the mineralization of organic pollutants.31–35 As such, this review will focus on recent developments of the large, cyclic 48-tungsto-8-phosphate [H7P8W48O184]40− (abbreviated as P8W48) and its smaller fragments [H2P2W12O48]12− (abbreviated as P2W12) and [H6P4W24O94]18− (abbreviated as P4W24). Emphasis will be placed on the synthesis and structure of these polyanions.
In 1945, A. F. Wells suggested a detailed structure for the dimeric (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) tungstophosphate ion,36 based on Pauling's principles and the structure Keggin had shown for the 12-tungstophosphate. In 1952, the formula [P2M18O62]6− (M = Mo, W) proposed by Wells was experimentally established for the molybdo analogue by Tsigdinos. In 1953, Dawson investigated this polyanion using single-crystal X-ray diffraction,37 and demonstrated that the positions of the W atoms in [P2W18O62]6− coincided with what was postulated by Wells. In 1975 Strandberg,38 and in 1976 D'Amour,39 reported complete and accurate X-ray crystal structures of [α-P2Mo18O62]6− and [α-P2W18O62]6−, respectively.
2) tungstophosphate ion,36 based on Pauling's principles and the structure Keggin had shown for the 12-tungstophosphate. In 1952, the formula [P2M18O62]6− (M = Mo, W) proposed by Wells was experimentally established for the molybdo analogue by Tsigdinos. In 1953, Dawson investigated this polyanion using single-crystal X-ray diffraction,37 and demonstrated that the positions of the W atoms in [P2W18O62]6− coincided with what was postulated by Wells. In 1975 Strandberg,38 and in 1976 D'Amour,39 reported complete and accurate X-ray crystal structures of [α-P2Mo18O62]6− and [α-P2W18O62]6−, respectively.
2. The cyclic [H7P8W48O184]33− (P8W48) and its derivatives [H6P4W24W94]18− (P4W24) and [α-H2P2W12O48]12− (P2W12)
The single-crystal X-ray structure for the [P2W18O62]6− polyanion indicated that the eighteen metal centers are not equivalent. Thus, the distinction was made between the α2 positions for the cap tungstens and the α1 positions for the belt tungstens. The two caps in this Wells–Dawson structure are composed of three edge-shared MO6 (M = W, Mo) octahedra, whereas the two equatorial belts are formed via alternating corner- and edge-shared MO6 units. In 1979 Acerete showed by 183W solution NMR that the β geometrical isomer of [P2W18O62]6− differs from the α isomer by a 60° rotation of one W3O13 cap.40,41 The Wells–Dawson derivative can be seen as a derivative of the Keggin structure; the removal of a corner-shared W3O13 triad from [α-PW12O40]3− produces a lacunary structure formulated as [A-α-PW9O34]9−, which combines with another equivalent moiety to form the [α-P2W18O62]6− assembly.Careful solution studies by Contant and Tézé on the chemistry of the complete (plenary) [α-P2W18O62]6− polyanion showed that upon basification a hydrolytic cleavage of W–O(W) bonds occurs, resulting in a mixture of the monovacant (lacunary) species [α1-P2W17O61]10− and [α2-P2W17O61]10−, respectively. Further, if the final pH is kept between 4 and 6, the [α1-P2W17O61]10− anion is observed to transform to the [α2-P2W17O61]10− anion readily.42 At about pH 10, the trilacunary polyanion [P2W15O56]12− is formed.42 Alternatively, in the presence of tris(hydroxymethyl)aminomethane (tris base), the plenary [α-P2W18O62]6− transforms to the hexavacant polyanion [α-H2P2W12O48]12− (P2W12).42 This P2W12 is labile and transforms quickly in aqueous, acidic medium to either the unstable, monolacunary polyanion [α1-P2W17O61]10−, which successively rearranges to either the more stable [α2-P2W17O61]10−,42 or the large cyclic polyanion [H7P8W48O184]40− (P8W48).43 Additionally, Contant and Tézé have revealed that two P2W12 can be connected end-on to form the dimeric [H6P4W24O94]18− (P4W24) species in aqueous solution.43 However, the exact linkage of the two P2W12 units is still unknown.44
Lacunary derivatives of the plenary Wells–Dawson ion [α-P2W18O62]6− and their related terminology (α, α1, α2) have been thoroughly investigated by Contant.42 More recently, Poblet and Cronin have studied the different rotational isomerisms of non-classical Wells–Dawson anions with different heteroatoms based on theoretical and mass spectrometry techniques.45
2.1 The metastable, hexalacunary [α-H2P2W12O48]12− (P2W12)
The hexalacunary 12-tungsto-2-phosphate P2W12 is generated by the treatment of [α-P2W18O62]6− by tris(hydroxymethyl)aminomethane (tris base). The formation of P2W12 can be visualized by removing six tungsten-oxo groups (W-Ot), one from each of the two caps and two from each of the two belts. The P2W12 was first identified by Contant in 1985, who proved its existence by 31P NMR (−8.6 ppm) in lithium chloride solution. The polyanion structure can be inferred from that of the cyclic tetramer P8W48. This is due to the fact that P2W12 is labile and readily undergoes rearrangement in aqueous, acidic medium to the more stable monolacunary [α2-P2W17O61]10−,42 or cyclic P8W48,43 as stated earlier. As such, the 183W NMR spectrum of this polyanion is not clean due to the presence of one or more transformation products in solution simultaneously, as Baker reported.46 Very recently, the polyanion P2W12 was characterized by single-crystal X-ray diffraction studies, as well as a W-bridged dimer and trimer.47,48 Due to the presence of six lacunary sites in P2W12, the reactivity of this polyanion with guest metal ions has been explored in some detail, as will be discussed subsequently (vide infra, section 3).2.2 The dimeric, multilacunary [H6P4W24O94]18− (P4W24)
The P4W24 polyanion has been known since 1985,43 and very likely it comprises of two P2W12 units, which are linked in a C- or S-shaped fashion, resulting in structures with C2v or C2h symmetry in solution (Fig. 1).43 The 31P and 183W NMR spectra have proven the coexistence of both such forms in solution.40,41,46,49 Only a few polyanions have been reported based on the dimeric P4W24 unit till date. In 1998, Roussel et al. investigated the structure of P4W24 by single-crystal X-ray diffraction studies and electron microscopy, which suggested P4W24 to be a phosphate-containing derivative of a polyoxotungstate with the chemical formula (PO4)4(WO3)2m (m = 12).50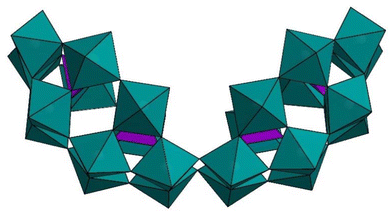 | ||
| Fig. 1 Polyhedral representation of the C-shaped isomer of P4W24. Color code: WO6 octahedra (green), PO4 tetrahedra (pink). | ||
2.3 The crown-shaped, superlacunary [H7P8W48O184]33− (P8W48)
The crown-shaped polyanion [H7P8W48O184]33− (P8W48) comprises of four P2W12 subunits, which are linked via the caps in a cyclic fashion, resulting in a structure with idealized D4h symmetry (Fig. 2). The structural elucidation of the polyanion by single-crystal XRD showed the presence of eight potassium ions within the central cavity. This suggests a templating effect of the alkali ions, which may play an important role in the formation process of this cyclic polyanion. The P8W48 is stable over a broad pH range (ca. 1–8), which represents a remarkable stability in POM chemistry. The presence of potassium ions inside the polyanion crown decreases the solubility of the salt and increases the tendency towards aggregation in solution, causing precipitation. Cronin reported two K-free salts of P8W48.51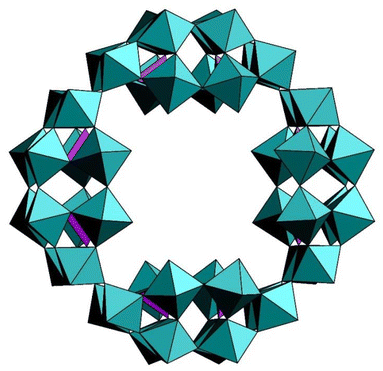 | ||
| Fig. 2 Polyhedral representation of P8W48. Color code: WO6 octahedra (green), PO4 tetrahedra (pink).51 | ||
3. Metal complexes of [α-H2P2W12O48]12−
3.1 Monomeric structures
To date, only a limited number of transition metal-containing derivatives of P2W12 have been reported.52 Hill reported the peroxo-niobium-containing derivative [P2W12(NbO2)6O56]12− (P2W12(NbO2)6), see Fig. 3.53 This peroxo-polyanion was reported to be unstable both in solution and in the solid-state in the absence of hydrogen peroxide. However, the formation of P2W12(NbO2)6 in the presence of H2O2 has been demonstrated using several spectroscopic methods, such as infrared spectroscopy (IR), fast atom bombardment-mass spectroscopy (FAB-MS), and 31P and 183W NMR spectroscopy. The P2W12(NbO2)6 polyanion was prepared by the reaction of P2W12 with the Lindqvist-type isopolyanion [Nb6O19]8− in the presence of aqueous H2O2 as an oxidant. The six Nb atoms in P2W12(NbO2)6 are connected by η2-O ligands, and each has a terminal, side-on peroxo ligand.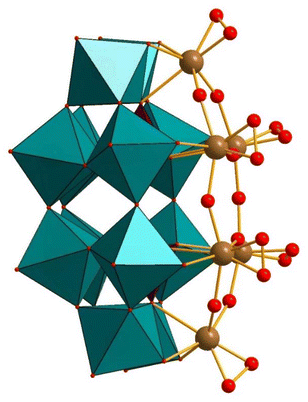 | ||
| Fig. 3 Combined polyhedral/ball-and-stick representation of the peroxo-Nb polyanion [P2W12(NbO2)6O56]12− (P2W12(NbO2)6). Color code: WO6 octahedra (green), PO4 tetrahedra (pink), O (red), Nb (brown).53 | ||
In 2002, Nadjo reported the synthesis and characterization of five mixed 3d (Fe, Cu)-4d (Mo) metal-containing P2W12-based polyanions: α2-[P2W12Fe(OH2)Mo5O61]7− (P2W12FeMo5) and α2-[P2W13Fe(OH2)Mo4O61]7− (P2W13FeMo4), α1- and α2-[P2W12Cu(OH2)Mo5O61]8− (P2W12CuMo5) and α2-[P2W13Cu(OH2)Mo4O61]8− (P2W13CuMo4).54 These compounds were characterized by IR, UV-vis, and 31P NMR spectroscopy. No XRD structures could be obtained, but in particular, IR and 31P NMR spectroscopy were useful tools for purifying the Fe3+ and Cu2+-substituted derivatives. The authors have also studied the electrocatalytic properties of these polyanions, and the Cu and Fe-substituted derivatives showed good activity for the electrocatalytic reduction of nitrate.55–57
Gouzerh reported dimeric 3d transition metal (Fe3+, Co2+, Mn2+, Ni2+) containing derivatives of P2W12. The iron(III)-containing polyanion [H4P2W12Fe9O56(CH3COO)7]6− (Fe9P2W12), see Fig. 4a, was prepared by a simple one-pot reaction of P2W12 with an excess of iron(III) chloride in aqueous solution containing lithium chloride and lithium acetate, at room temperature.58 The monomeric Fe9P2W12 structure is derived from P2W12 by filling the six vacant sites with iron atoms, with the now-formed plenary Wells–Dawson type species {P2W12Fe6} having three additional iron(III) atoms grafted onto the hexa-iron-oxo face of the polyanion, resulting in Fe9P2W12, which has idealized C2 symmetry. Magnetic susceptibility measurements on Fe9P2W12 showed intramolecular antiferromagnetic coupling between the two high-spin Fe3+ centers.
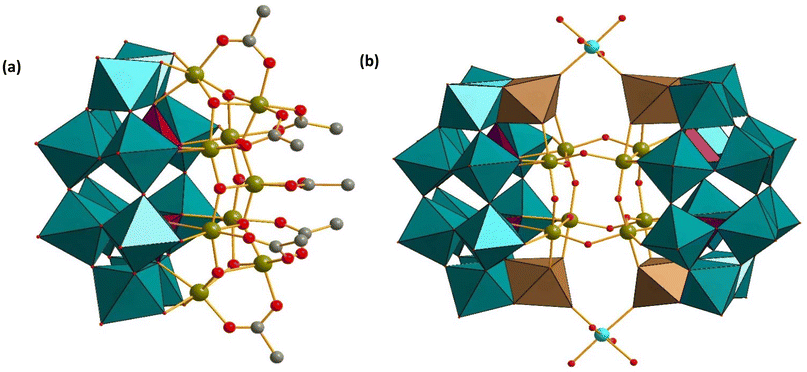 | ||
| Fig. 4 Combined polyhedral/ball-and-stick representations of (a) [H4P2W12Fe9O56(CH3COO)7]6− (Fe9P2W12), and (b) [{M(H2O)4}2{H12P4W28Fe8O120}]12− (M = Co2+, Mn2+, Ni2+). Color code: WO6 octahedra (green and brown), PO4 tetrahedra (pink), Fe (green), M (turquoise), O (red), C (grey). H atoms on carbon have been omitted for clarity.58,59 | ||
3.2 Dimeric structures
Upon further investigation of the Fe3+ and P2W12 system by Gouzerh, it was observed that lowering the Fe3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2W12 ratio and the solution pH, as well as prolonged heating, led to the formation of dimeric polyanions with the general formula [HyP4W28+xFe8−xO120](28−y−3x)−.59 These species have extra tungsten atoms in the new polyanions (with slight variations in the value of x), which have been isolated as different salts. The polyanion [H12P4W28Fe8O120]16− (Fe8P4W28) comprises two {P2W14Fe4} units linked via Fe–O–Fe′ bonds, resulting in a cubic Fe8 topology. The Fe8P4W28 is formed by reaction of P2W12 with hydrous iron(III) oxide or iron(III) acetate [Fe3O(OAc)6(H2O)3]Cl·5H2O.58
P2W12 ratio and the solution pH, as well as prolonged heating, led to the formation of dimeric polyanions with the general formula [HyP4W28+xFe8−xO120](28−y−3x)−.59 These species have extra tungsten atoms in the new polyanions (with slight variations in the value of x), which have been isolated as different salts. The polyanion [H12P4W28Fe8O120]16− (Fe8P4W28) comprises two {P2W14Fe4} units linked via Fe–O–Fe′ bonds, resulting in a cubic Fe8 topology. The Fe8P4W28 is formed by reaction of P2W12 with hydrous iron(III) oxide or iron(III) acetate [Fe3O(OAc)6(H2O)3]Cl·5H2O.58
An extra tungsten atom arising from the decomposition of P2W12 in situ or upon deliberate addition of tungstate to the reaction mixture competes with the added metal ions, as has been observed in the reactions with lanthanide ions.60,61 Frequently, adding of extra tungstate to the reaction mixture is not essential for the formation of such structures.61 The cyclic voltammogram (CV) of the polyanion Fe8P4W28 displays four reduction waves. The first one at −0.32 V vs. SCE is attributed to the reduction of Fe3+ to Fe2+.
A polyanion with the composition [HyP4W28+xFe8−xO120](28−y−3x)− (x ≈ 2) was characterized among the products obtained from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 aqueous mixture of P2W12, sodium tungstate, and iron(III) chloride at pH 4.2.59 When P2W12 is reacted with the mixed-metal complexes [FeIII2MIIO(OAc)6(H2O)3] (M = Co2+, Mn2+, Ni2+), the polyanions [{M(H2O)4}2{H12P4W28Fe8O120}]12− (M2Fe8P4W28) (Fig. 4b) (M = Co2+, Mn2+, Ni2+) are obtained, together with Fe8P4W28.59 These compounds were isolated as potassium salts. Magnetic susceptibility measurements on Co2Fe8P4W28 are consistent with two non-interacting Co2+ centers, suggesting that the ground state of the Fe8P4W28 unit is diamagnetic, along with the intramolecular antiferromagnetic coupling of the eight Fe3+ centers. In contrast, the parent polyanion Fe8P4W28 showed weak magnetization, arising either from paramagnetic impurities or a partial substitution of Fe for W.59 The cyclic voltammogram indicated that the Co2+ centers are not electrochemically active in the potential range investigated.
2 aqueous mixture of P2W12, sodium tungstate, and iron(III) chloride at pH 4.2.59 When P2W12 is reacted with the mixed-metal complexes [FeIII2MIIO(OAc)6(H2O)3] (M = Co2+, Mn2+, Ni2+), the polyanions [{M(H2O)4}2{H12P4W28Fe8O120}]12− (M2Fe8P4W28) (Fig. 4b) (M = Co2+, Mn2+, Ni2+) are obtained, together with Fe8P4W28.59 These compounds were isolated as potassium salts. Magnetic susceptibility measurements on Co2Fe8P4W28 are consistent with two non-interacting Co2+ centers, suggesting that the ground state of the Fe8P4W28 unit is diamagnetic, along with the intramolecular antiferromagnetic coupling of the eight Fe3+ centers. In contrast, the parent polyanion Fe8P4W28 showed weak magnetization, arising either from paramagnetic impurities or a partial substitution of Fe for W.59 The cyclic voltammogram indicated that the Co2+ centers are not electrochemically active in the potential range investigated.
Wang and coworkers investigated the reactivity of P2W12 with Co2+ ions in an aqueous acidic medium. A new heart-shaped dimeric Co2+-containing polyanion [{W2Co2O8(H2O)2}(P2W12O46)2]20− (W2Co2(P2W12)2) was synthesized and structurally characterized by IR spectroscopy, thermogravimetry, electrochemistry, and single-crystal X-ray diffraction.62 The polyanion W2Co2(P2W12)2 comprises two subunits of P2W12, which are fused via four W–O–W bonds and grafting of two W and two Co atoms in the vacant positions. This type of W–O–W connectivity was observed for the first time by Kortz and coworkers in the dimethyletin-containing tungstophosphate [{Sn(CH3)2}4(H2P4W24O92)2]28− (vide infra, section 4). Notably, all the addenda positions in W2Co2(P2W12)2 are disordered in the cluster.
Very recently, a dimeric mixed-addenda Nb/W polyanion, [H6P2W12Nb4O59(NbO2)2]28− (P2W12Nb4(NbO2)2)2, has been isolated under acidic condition by Yue, He, and coworkers.63 This Nb-peroxo-containing polyanion was synthesized by the reaction of K7H[Nb6O19]·13H2O and P2W12 in sodium formate buffer in the presence of H2O2. The polyanion was characterized by single-crystal X-ray diffraction and elemental analysis. The structure revealed that six Nb atoms occupy the vacant sites of the hexalacunary P2W12 precursor to form a {P2W12Nb6(O2)2} mixed-addenda subunit. Two such {P2W12Nb6(O2)2} subunits are joined by two Nb–O–Nb bridges at the belt positions to form a dimeric unit. This polyanion, (P2W12Nb4(NbO2)2)2, is structurally different from the Nb-peroxo polyanions reported by Hill and coworkers, for example, P2W12(NbO2)6, as discussed in section 3.1 (vide supra).53 In P2W12(NbO2)6 structure, all niobium atoms have terminal peroxo ligands and exist as a six-membered Nb-peroxo monomer; whereas in (P2W12Nb4(NbO2)2)2, only two niobium ions have peroxo ligands, and the polyanion is a dimer. A common feature for both polyanions was that the polyanions aggregated to larger species when the peroxo groups in either P2W12Nb4(NbO2)2 or P2W12(NbO2)6 were removed by heating or chemical reduction.64–66
In 2015, Niu, Wang, and coworkers reported two new multi-Nb-containing POM structures, [H13{Nb6(O2)4P2W12O57}2]7− (P2W12Nb6(O2)4)2 (Fig. 5a) and [H14{P2W12Nb7O63(H2O)2}4{Nb4O4(OH)6}]16− (P2W12Nb7)4Nb4 (Fig. 5b).67 Interestingly, (P2W12Nb7)4Nb4 has the highest nuclearity of all niobium-containing heteropolyanions to date. The polyanion {P2W12Nb6(O2)4}2 is a di-Nb–O–Nb-linked dimer of two P2W12 units with the six vacant sites of each P2W12 unit occupied by four niobium-peroxo groups (NbO2) and two niobium-oxo groups (Nb–O). Further, (P2W12Nb7)4Nb4 comprises dimeric [P4W24Nb14O126(H2O)4]18− subunits and an adamantane-like Nb4O6 core. The [P4W24Nb14O126(H2O)4]18− subunit in (P2W12Nb7)4Nb4 comprises two [Nb6P2W12O61]10− units without any niobium-peroxo groups. The [Nb6P2W12O61]10− units are connected through Nb–O–Nb bridges. The adamantane-like {Nb4O6} core is composed of four niobium metal atoms connected by oxo ligands. The authors have demonstrated that careful synthetic control can stabilize the (Nb6P2W12) fragment in both polyanions as a dimer or a tetramer. Moreover, 31P and 183W NMR spectra indicated that (P2W12Nb7)4Nb4 is stable in solution. The photocatalytic activity for H2 evolution has been studied for both polyanions, and (P2W12Nb7)4Nb4 exhibited good activity.
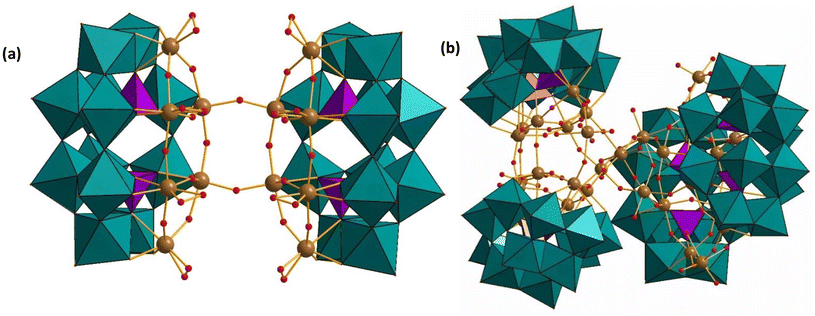 | ||
| Fig. 5 Combined polyhedral, ball-and-stick representations of (a) [H13{Nb6(O2)4P2W12O57}2]7− and (b) [H14{P2W12Nb7O63(H2O)2}4{Nb4O4(OH)6}]16−, respectively. Color code: WO6 octahedra (green), PO4 tetrahedra (pink), Nb (brown), O (red).67 | ||
In 2018, Kögerler and coworkers reported an open dimeric structure based on the P4W24 unit with {P4W24O29} being capped by two phenyl phosphonate or -arsonate ligands, [(PhXO)2P4W24O92]16− (X = P, As) (PhXOP4W24).68 In 2006, Kortz and coworkers have also reported P4W24 units in the dimethyltin-grafted polyanion [{Sn(CH3)2}4(H2P4W24O92)2]28− (Sn(CH3)2P4W24) (vide infra).44 For PhXOP4W24, the 31P NMR spectra confirm a stabilizing effect on the P4W24 unit due to the presence of the two phoshonate/arsonate ligands. Further studies on the incorporation of Co2+ ions into the central cavity of PhXOP4W24 resulted in the formation of [{(H2O)4Co}(PhXO)2P4W24O92]14− units linked into an infinite 1D chain in the solid state via additional “outer” Co2+ centers.
Very recently, the same group reported an unprecedented discrete dimeric polyanion [(o-H2N-C6H4-AsO3)4P4W24O85]14− ((o-NH2-C6H6-AsO)4P4W24O92), by the condensation reaction of o-aminophenylarsonic and P2W12 in acidic media. The polyanion (o-NH2-C6H6-AsO)4P4W24O92 was further reacted with divalent transition metal ions Mn2+, Co2+, Ni2+. The introduction of the divalent transition metal ions in the reaction mixture resulted in V-shaped one-dimensional (1D) coordination polymeric polyanions [{M(H2O)4}P4W24O92(C6H6AsNO)2]14− (M = Mn2+, Co2+, Ni2+) (M(o-NH2-C6H6-AsO)2P4W24),69,70 where a similar type of structure was first observed by Kortz group (vide infra). Each monomeric unit of (o-NH2-C6H6-AsO)4P4W24O92 consists of P2W12, comprising two PW4O21 belts capped with two W2O10 units linked through oxygen atoms. The transition metal complex M(o-NH2-C6H6-AsO)2P4W24 exhibits dimeric anionic assembly and coordinates one oxygen atom from the upper PW4O21 belt of each P2W12L subunits.
3.3 Trimeric structures
In 2008, Wang and coworkers were able to isolate three trimeric polyoxotungstates upon the reaction of 3d metal ions with P2W12 at different pH, [K3⊂{Mn(H2O)4}2{WO2(H2O)2}2{WO(H2O)}3(P2W12O48)3]19− (Mn2(P2W12)3W5) (Fig. 6), K3Na7Li5.5Ni0.25[Na3⊂{Ni3.5(H2O)13}{WO2(H2O)2}2{WO(H2O)}3(P2W12O48)3]·64H2O (Ni3.5(P2W12)3W5), and [Na3⊂{Cu3(H2O)9}{WO2(H2O)2}2{WO(H2O)}3(P2W12O48)3]17− (Cu3(P2W12)3W5).71 These polyanions were prepared by reaction of the corresponding transition metal salts (Mn2+, Ni2+, and Cu2+) with P2W12 and Na2WO4 in aqueous acidic solutions. The polyanion Mn2(P2W12)3W5 was obtained in a pH 4.0 solution, whereas the other two polyanions Ni3.5(P2W12)3W5 and Cu3(P2W12)3W5 were obtained in the pH range 1.5–2.5.71 As based on the formula, the Ni-containing polyanion appears to be a mixture of at least two species.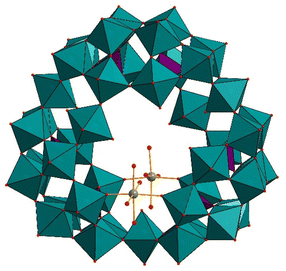 | ||
| Fig. 6 Combined polyhedral/ball-and-stick representation of [K3⊂{Mn(H2O)4}2{WO2(H2O)2}2{WO(H2O)}3(P2W12O48)3]19−. Color code: WO6 octahedra (green), PO4 tetrahedra (pink), Mn (teal), O (red).71 | ||
Yao et al. suggested that the pH plays an important role in forming these P2W12-based heteropolytungstates.72 Upon further decreasing the pH to 1.0, they were able to isolate two new compounds, [Na3⊂{Co(H2O)4}6{WO(H2O)}3(P2W12O48)3]15− (Co6(P2W12)3W3), and [Na3⊂{Ni(H2O)4}6{WO(H2O)}3(P2W12O48)3]15− (Ni6(P2W12)3W3).72 It is worth mentioning that all the vacant sites in Co6(P2W12)3W3 and Ni6(P2W12)3W3 are occupied by the 3d metal ions. Therefore, it is observed that a lower pH facilitates the combination of more transition metal atoms encapsulated within the {(P2W12)3W3} shell. All these polyanions have crown-type structures, comprising three P2W12 subunits linked by three {WO(H2O)} fragments in a corner-sharing arrangement, resulting in the trimeric cluster [P6W39O147(H2O)3]30− (abbreviated as P6W39). In such crown-shaped polyanions, the three W atoms in the hinges are all in a hexa-coordinated environment, with all transition metal atoms also fully coordinated. The six vacant sites on the polyanion Mn2(P2W12)3{WO2(H2O)2}{WO(H2O)}3 are occupied by two WVI atoms, two Mn2+ ions, and two potassium counter cations, respectively. For the polyanion Ni3.5(P2W12)3{WO2(H2O)2}2{WO(H2O)}3, two WVI and four Ni2+ atoms fill up the six vacant sites of the P6W39 shell, with all the Ni2+ centers fully occupied, except the Ni5 position which exhibits a crystallographic site-occupancy disorder with a Na atom, resulting in a total of two WVI and 3.5 Ni2+ ions being incorporated in the structure. In a similar sense, for the polyanion Cu3(P2W12)3{WO2(H2O)2}2{WO(H2O)}3, two WVI and four Cu2+ guest atoms occupy the six vacant sites of the P6W39 unit. However, all these metal sites, except W40, also have site-occupancy disorder, resulting in two WVI and 2.75 Cu2+ atoms as guests in the host structure.
More recently, Wang and coworkers have reported mixed 3d/4f metal ion-based P6W39 structures, [K3⊂{GdMn(H2O)10}{HMnGd2(tart)O2(H2O)15}{P6W42O151(H2O)7}]11− ({MnGd)(HMnGd2P6W42}∞) and [K3⊂{GdCo(H2O)11}2{P6W41O148(H2O)7}]13− (CoGdP6W41)∞, with organic guests (tartrate and (CH3)2NH·HCl respectively).73 According to the authors, the introduction of such secondary organic ligands stabilizes the lanthanide atoms and/or reduces the reactivity of the lanthanide atoms with the polyanions. The crown-shaped P6W39 is formed by the encapsulation of transition metal and lanthanide atoms inside the cavity of the polyanion. The polyanion (MnGd)(HMnGd2P6W42)∞ forms two-dimensional porous frameworks through the Gd and Mn linkers, whereas the polyanion (CoGdP6W41)∞ forms a one-dimensional chain linked through Gd ions, which exhibit a square-antiprismatic geometrical environment. These are the first POM structures comprising mixed lanthanide and transition metal atoms. Wang, Niu, and coworkers have isolated a trimeric assembly of P2W12 units joined by Cr-atoms, [H23(Cr(H2O)2)3(H2P2W12O48)3]4− (Cr3(P2W12)3).74 The structure bears similarity with a trimeric {P6W39} unit, except that Cr has replaced the W-atoms.
3.4 Tetrameric structures
Following Gouzerh's report of the metastable polyanion Fe9(OAc)7P2W12 (section 3.1, vide supra), which was isolated as a lithium–potassium salt,58 subsequent heating of this polyanion in aqueous sodium acetate solution transformed it into different compounds, which were successively isolated as sodium–potassium salts, such as Na16K12[H56P8W48Fe28O248] (Fe28P8W48) and Na16K10[H55P8W49Fe27O248] (Fe27P8W49).58 The polyanion Fe28P8W48 was characterized by electrochemistry and magnetic measurements and by determination of the unit cell parameters. Subsequently, the formula of the polyanion Fe27P8W49 was proposed on the basis of an X-ray diffraction study. Godin et al. hinted at other novel compounds’ formation by lowering either the pH or the Fe3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P2W12 ratio. However, IR spectroscopy and chemical analysis were insufficient to determine the composition and purity of these species. The polyanion Fe27P8W49 comprises four {P2W12Fe6} units, each bridged through Fe–O–Fe linkages to an {Fe4O6} cluster core. The linkage is through pairs of three Fe–O–Fe bridges involving the three outer iron atoms. According to crystallographic refinement, the polyanion Fe27P8W49 seems to have 25% W and 75% Fe occupancy, suggesting a mixture of polyanions. The polyanion exhibits antiferromagnetic coupling between the Fe3+ centers (Fig. 7).
P2W12 ratio. However, IR spectroscopy and chemical analysis were insufficient to determine the composition and purity of these species. The polyanion Fe27P8W49 comprises four {P2W12Fe6} units, each bridged through Fe–O–Fe linkages to an {Fe4O6} cluster core. The linkage is through pairs of three Fe–O–Fe bridges involving the three outer iron atoms. According to crystallographic refinement, the polyanion Fe27P8W49 seems to have 25% W and 75% Fe occupancy, suggesting a mixture of polyanions. The polyanion exhibits antiferromagnetic coupling between the Fe3+ centers (Fig. 7).
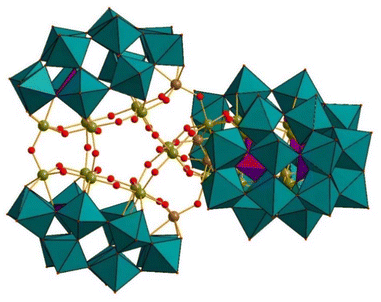 | ||
| Fig. 7 Combined polyhedral/ball-and-stick representation of [H55P8W49Fe27O248]27−. Color code: WO6 octahedra (green), PO4 tetrahedra (pink), O (red), Fe (green-yellow), Fe/W (brown).58 | ||
Wang and coworkers have reported the 3d transition metal (Co2+, Ni2+) modified {P8W49} polyanions {[Co(H2O)2Cl][Co(H2O)3]2[Co(H2O)5]1.5[Co(H2O)3H4P8W49O187(H2O)]}26− ([Co(H2O)2][Co(H2O)3]2[Co(H2O)5]1.5P8W49) and {[Ni(H2O)3]2[{Ni(H2O)3}1.5H3P8W49O187(H2O)]}30− ([Ni(H2O)3]2[Ni(H2O)3]1.5P8W49), respectively, by the reaction of P2W12 with the respective transition metal salt.75 The transformation from P2W12 to P8W49 happens during the reaction performed in an aqueous acidic medium. The structural arrangements of Co2+ and Ni2+ in [Co(H2O)2][Co(H2O)3]2[Co(H2O)5]1.5P8W49 and [Ni(H2O)3]2[Ni(H2O)3]1.5P8W49 are different from that of several other Mn2+ complexes reported by Cronin's and Proust's groups (see section 5).21,76,77
In 2014, Niu and coworkers reported the gigantic Nb28 cluster encapsulated in a hexa-lacunary {P2W12} precursor, [{Nb4O6(OH)4}{Nb6P2W12O61}4]36− ((Nb4O6)(Nb6P2W12)4).78 This polyanion was formed directly by controlling the reaction parameters (e.g., pH, concentration, temperature) and isolated as a sodium salt. The salt was characterized by single-crystal X-ray diffraction, IR spectroscopy, and elemental analysis. The structure of (Nb4O6)(Nb6P2W12)4 reveals that the polyanion comprises two [P4W24Nb12O122]20− dimeric units, rotated 180° with respect to each other and connected by four Nb–O–Nb bridges, resulting in an adamantane-type {Nb4O6} core.
Very recently, Zhang and coworkers have reported the formation of Na24[Mn8(H2O)32P8W48O184]·58H2O, K4Na16H4[Co8(H2O)32P8W48O184]·76H2O, and Na20H4[Ni8(H2O)32P8W48O184]·72H2O (M8P8W48, M = Mn2+, Co2+, Ni2+) by reaction of the respective divalent metal ion with the hexavalent P2W12 at room temperature in aqueous solution.79 The direct reaction of the metal ions with P8W48 was not successful.
3.5 Hexameric structures
In 2015, Niu, Wang, and coworkers reported a hexameric {Nb6P2W12}-based Mn15-oxo cluster, [H123Nb36P12W72MnIII12MnII3NaO424]10− (Mn15(Nb6P2W12)6), with single-molecule magnet (SMM) properties.80 The polyanion structure consists of three main parts: six peroxo-free {Nb6P2W12} units, four {MnIII3} trinuclear cores, and four {MnII} hinges. The {MnIII3} unit is made of three mutually corner-bridged {MnIIIO6} octahedra (Fig. 8). Two of the Mn⋯Mn distances in the trinuclear {MnIII3} unit are observed to be identical and slightly longer or shorter than the third Mn⋯Mn distance, having an overall shape of an equivalent isosceles triangle. Each {MnIII3} unit is surrounded by three Wells–Dawson {Nb6P2W12} units and six Mn–O–Nb bridges. Further, each {MnII} hinge shows crystallographic positional disorder of Mn2+ and Na+ (0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25), being coordinated by three μ2-oxo groups and four-terminal oxygen atoms (water molecules), thus leading to a distorted octahedral geometry. Therefore, the authors suggest that the disordered metal centre should be formulated as [MnII0.75Na0.25(H2O)4]1.75+.
0.25), being coordinated by three μ2-oxo groups and four-terminal oxygen atoms (water molecules), thus leading to a distorted octahedral geometry. Therefore, the authors suggest that the disordered metal centre should be formulated as [MnII0.75Na0.25(H2O)4]1.75+.
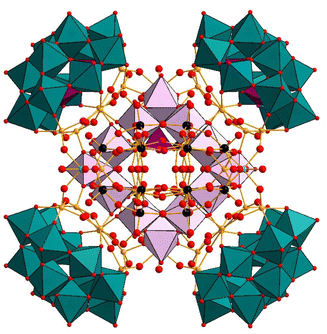 | ||
| Fig. 8 Combined polyhedral/ball-and-stick representation of [H123Nb36P12W72MnIII12MnII3NaO424]10−. Color code: WO6 octahedra (green), W (black), Nb (yellow), MnO6 octahedra (light pink), PO4 tetrahedra (pink), O (red), Na (turquoise).80 | ||
In 2016, Mizuno and coworkers isolated a giant hexameric ring-shaped manganese-substituted polyanion, [{γ-P2W12O48Mn4(acac)2(OAc)}6]42− (acac = acetylacetonate, OAc = acetate) {P2W12O48Mn4}6 by reaction of the hexavacant lacunary P2W12 precursor with Mn(acac)3 in organic medium.81 The polyanion {P2W12O48Mn4}6 is composed of six manganese-substituted monomeric {P2W12O48Mn4} units, overall resulting in a cyclic, hexameric structure. Each {P2W12Mn4} monomeric unit consists of two types of Mn-coordinated ligands acetyl acetonate (acac) and acetate, respectively, so the polyanion has two different active sites. Interestingly, the hexameric polyanion transforms to the tetrameric polyanion, [{γ-P2W12O48Mn4(H2O)6}4(H2O)4]24− {P2W12O48Mn4}4 in the absence of the organic ligands capping the manganese ions.
Following the report of Mizuno's 2016 work, Yamaguchi and coworkers in 2019 have reported the synthesis of the tetra-n-butylammonium (TBA) salts of a series of new isostructural divalent transition metal substituted polyanion family, (TBA5[γ-P2W12O44M2(OAc)(CH3CONH)2]·nH2O·mCH3CN; M = Mn2+, Co2+, Ni2+, Cu2+, or Zn2+; OAc = acetate) (γ-P2W12O44M2(OAc)) including unique edge-shared bis (square-pyramidal) {O2M(μ3-O)2(μ-OAc)MO2} core. The metal ions occupy the vacant belt positions of the γ-P2W12 precursor, whereas the two acetamide (CH3CONH2) groups stabilize the γ-P2W12 unit.82
3.6 Rearrangement of [H2P2W12O48]12− in acidic medium
Lanthanide-containing POMs are interesting due to potentially attractive photoluminescence, Lewis acid catalysis, electrochemistry, and magnetic properties. Due to the larger size and resulting higher coordination number of lanthanide ions as compared to d-block metal ions, they cannot be fully incorporated into the vacant sites of lacunary POMs and hence tend to link two or more polyanions, as observed in one of the largest polyanions, [AsIII12CeIII16(H2O)36W148O524]76− (W148), reported by Pope and coworkers.83In 2000, Pope's group reported the polyanion [Ce4(OH2)9(OH)2(α1,α1-P2W16O59)2]14− (Ce4(α1α1-P2W16)2) in which the P2W12 precursor picks up extra tungsten atoms and then incorporates four cerium(III) ions.60 The polyanion Ce4(α1α1-P2W16)2 exhibits a dimeric structure with C2v symmetry, comprising two {α1,α1-P2W16O59} (abbreviated as P2W16) units connected via a central core of four cerium atoms. There are two structural types of cerium in this polyanion, the first one has 10-coordination and the other has 9-coordination. The 31P and 183W NMR studies in aqueous solution suggested that the polyanion is stable in solution.
In 2003, Kortz reported the lanthanum-substituted polyanion [{La(CH3COO)(H2O)2(α2-P2W17O61)}2]16− (La(OAc)-(α2-P2W17)2), which was synthesized by reaction of La3+ ions with the hexalacunary polyanion P2W12.84 It was observed that P2W12 rearranges quickly in an aqueous, acidic medium to the monolacunary [α1-P2W17O61]10− which in turn rearranges to [α2-P2W17O61]10−. The polyanion La(OAc)-(α2-P2W17)2 is composed of two [α2-P2W17O61]10− fragments connected by two lanthanum acetato dimers (La2(CH3COO)2(H2O)4)4+, resulting in a head-on, transoid dimer with C2h symmetry. Each La3+ atom exhibits a nine-coordinatied geometry.61 The monolacunary Wells–Dawson ion [α2-P2W17O61]10− is known to react with lanthanide ions to form Pope's “head-on dimers” or Francesconi's “side-on dimers”.61,85
The Enbo Wang group has shown considerable interest in synthesizing metal–organic frameworks containing the Wells–Dawson ion. In 2008, they reported three transition metal-containing Wells–Dawson-based assemblies, using the P2W12 anion as a reagent, K4Na10[α1-CuP2W17O60(OH)]2·58H2O (Cu2P4W34), Na2[H2en][H2hn]0.5[Cu(en)2]4.5[α1-CuP2W17O60(OH)]2·43H2O {Cu(en)2α1-CuP2W17}∞, and Na3[H2hn]2.5[P2W17O60Cu(OH)2]·14H2O ((H2hn(CuP2W17)2)∞, where en = 1,2-ethylenediamine; hn = 1,6-hexamethylene diamine).86 The Cu2P4W34 polyanion consists of two α1-P2W17 units with the vacant α1 (belt) position being occupied by a Cu2+ atom and the two Wells–Dawson units are connected by two W-OH-Cu groups resulting in a dimeric [α1-CuP2W17O60(OH)]214− (Cu2P4W34) assembly.
The (Cu(en)2α1-CuP2W17)∞ constitutes the first 2-D organic–inorganic hybrid network based on such double-Dawson-type polyanion (DDTP) building blocks and (Cu(en)2)2+ bridging units, representing a large polyoxotungstate building block for the construction of extended organic–inorganic hybrid materials. Subsequently, {(H2hn)(CuP2W17)2}∞ possesses a 3-D hybrid supramolecular framework with 1-D channels that are constructed from the half-unit of the ‘DDTP’ and ‘hn’ cations. A magnetic study of (Cu(en)2α1-CuP2W17)∞ indicated weak antiferromagnetic interactions and that the two types of Cu2+ centers are well separated.
All the above examples consist of mono-Cu2+ substituted Wells–Dawson-type polyanions. Further exploration by the Wang group focused on feasible synthetic routes by adjusting the composition of Wells–Dawson polyanions to capture more transition metal ions. Subsequently, they reported a 1-D inorganic polymer formed by the reaction of P2W12 with Mn2+ ions, Na8H2L(H2enMe)4[Mn(H2O)2 (W4Mn4O12)(P2W14O54)2]·17H2O, {W4Mn4(MnP2W14)2}∞ (L = pyromellitic dianhydride PMDA).87 The structure of this compound consists of two tetravacant Dawson moieties [P2W14O54]14− (P2W14) sandwiching an eight-metal cluster with W–O–W(Mn) and P–O–W(Mn) connecting modes. The metal centers in this eight-metal cluster form an almost regular cubane-like (W4Mn4) cluster. The solid-state network is completed by multi-Mn2+-substituted DDTP and Mn2+ linkers with idealized C2 symmetry, which is further connected into a 3-D supramolecular network via extensive hydrogen-bonding interactions.
Fang, Kögerler, and coworkers have isolated potassium and lithium salts of a 40-manganese(III)-containing polyanion, [(P8W48O184){(P2W14Mn4O60)(P2W15Mn3O58)2}4]144− ((P8W48)(Mn4P2W14)4(Mn3P2W15)8), using hexavacant P2W12 as a reagent.88 Single-crystal XRD revealed that the polyanion ((P8W48)(Mn4P2W14)4(Mn3P2W15)8) consists of P2W14, P2W15, and P8W48 units. The polyanion was synthesized by reacting P2W12 with [Mn12O12(OAc)16(H2O)4]·4H2O·2HOAc in lithium acetate/acetic acid medium. Interestingly, although the cyclic P8W48 is present in the pocket of the polyanion, no Mn3+ ions are found inside the cavity of the P8W48 unit. It appears that P8W48 acts as a template, reducing the steric repulsion between the tri-Dawson ({P2W14Mn4}{P2W15Mn3}2) units. Magnetic measurements revealed intramolecular antiferromagnetic coupling between the Mn3+ ions.
In 2013, Yang and coworkers reported three lanthanide derivatives of P8W48, {[Ln2(μ-OH)4(H2O)x]2(H24P8W48O184)}12− (Ln = Nd, Sm, Tb) (Ln4P8W48), and a manganese derivative, [K(H2O)2]4[K4(μ-H2O)8]2[K(H2O)]8{[Mn8(H2O)16](H4P8W48O184)} (K8Mn8P8W48), starting from P2W12 as precursor and working under hydrothermal conditions.89 The cavities of P8W48 in Ln4P8W48 are occupied by lanthanide ions bridged by hydroxyl groups, and the 8 lanthanide ions have a 50% occupancy each. The K8Mn8P8W48 polyanion is formed by incorporating eight Mn2+ atoms inside the eight vacant sites within the cavity of the cyclic P8W48. These Mn2+ atoms are observed to be disordered over eight positions with K+ atoms. The compounds were characterized by single-crystal XRD, FTIR, elemental analysis, thermogravimetry, and powder XRD.
In 2020, Abramov and coworkers reported the dimeric tri-niobium-substituted P2W15 polyanion, [cis-(P2W15Nb3O61)2]14− (P2W15Nb3), and two phases of the disordered derivative [trans-(P2W14.7Nb3.3O61)2]14.6− using P2W12 as a precursor.90 The main structural unit consists of the dimeric [(P2W16Nb4O60)2(μ-O)2]n− archetype anion based on two Wells–Dawson-type subunits, connected by two Nb–O–Nb bridges. Interestingly, out of the 12 niobium positions, only four are fully occupied. The addition of Me2NH2Cl to the reaction media yields a mixture of triclinic and orthorhombic crystalline phases. The dimeric units are comprised of two Dawson anions connected via Nb–O–Nb bridges. In 2020, Kortz and coworkers reported the gigantic, macrocyclic 48-FeIII-96-tungsto-16-phosphate, [Fe48(OH)76(H2O)16(HP2W12O48)8]36− (Fe48(P2W12)8), which was prepared by reaction of P2W12 and the 22-iron(III)-containing coordination complex [Fe22O14(OH)3(O2CMe)21(mda)6]·(ClO4)2 (mdaH2 = N-methyldiethanolamine) and isolated as a potassium salt, K36[Fe48(OH)76(H2O)16(HP2W12O48)8].91 The crystal structure of Fe48(P2W12)8 revealed that there are eight equivalent {Fe6P2W12} Dawson-type subunits linked to each other via Fe–O–Fe/W bonds, resulting in a cyclic assembly with idealized D2 point group symmetry and a cavity of ca. 24 Å × 13 Å. Magnetic studies indicated that the 48 Fe3+ centers in Fe48(P2W12)8 share several exchange pathways. The averaged exchange coupling constant was estimated to be Jav = −7.07 K. The electrochemical study of Fe48(P2W12)8 exhibited redox transitions, suggesting the electroactivity of the Fe3+ and WVI ionic states.
4. Metal complexes of [H6P4W24O94]18−
Kortz and co-workers first showed that the super-lacunary Preyssler–Jeannin–Pope ion [H2P4W24O94]22− (P4W24) can react with electrophiles. The dimethyltin-containing hybrid organic–inorganic polyanion [{Sn(CH3)2}4(H2P4W24O92)2]28− ({Sn(CH3)2}4(P4W24)2) (Fig. 9) was prepared by reaction of (CH3)2SnCl2 with P4W24 in an aqueous, acidic medium at ambient temperature and isolated as a potassium salt.44 The polyanion comprises two P4W24 units linked through four dimethyltin groups, leading to a structure with D2d point group symmetry. The two P4W24 units of the polyanion {Sn(CH3)2}4(P4W24)2 are oriented orthogonally to each other and held together by four dimethyltin groups. Room temperature 119Sn [δ (ppm) = −243.2 ppm], 31P [δ (ppm) = −7.1, −8.7 ppm], 13C [δ (ppm) = 8.6 ppm], and 1H [δ (ppm) = 0.7 ppm] NMR studies in aqueous medium proved the integrity of the solid-state structure in solution.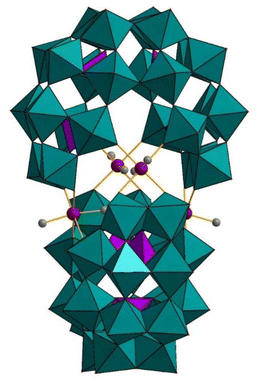 | ||
| Fig. 9 Combined polyhedral/ball-and-stick representation of [{Sn(CH3)2}4(H2P4W24O92)2]28−. Color code: WO6 octahedra (green), PO4 tetrahedra (pink), C (grey), Sn (red). No hydrogen atoms are shown for clarity.44 | ||
The continued study of Kortz and coworkers of the lacunary precursor P4W24 has produced interesting new compounds with interesting physical and chemical properties. Kortz and coworkers studied the interaction of phosphotungstates with actinide ions, especially uranyl, as these complexes can potentially have rich structural, magnetic, and electrochemical properties. In 2008, the uranyl-peroxo-containing 36-tungsto-8-phosphate [Li(H2O)K4(H2O)3{(UO2)4(O2)4(H2O)2}2(PO3OH)2P6W36O136]25− (Li(UO2)4(O2)4(PO3OH)2P6W36) (Fig. 10) was synthesized and structurally characterized using P4W24 as precursor.92 The structure comprises three P2W12 units encapsulating two independent, neutral [(UO2)(O2)]4 units in the central cavity, resulting in a U-shaped (P2W12)3 assembly. Notably, the resulting polyanion could not be isolated using P2W12 as a reagent instead of P4W24. Notably, from the single-crystal X-ray diffraction data, a lithium atom embedded in the structure was observed, which is rare in polyoxotungstate chemistry, given that the high electron density of the W atoms generally tends to obscure such a low electron-dense atom. The coordination of the central uranium-peroxo unit {(UO2)4(O2)4(H2O)2}2 comprises eight uranyl-peroxo units subdivided in two [(UO2)(O2)]4 squares, the η2-peroxo ions being bound side-on to each pair of uranium atoms. The room temperature 31P NMR spectrum of the polyanion in water was fully consistent with its solid-state structure.
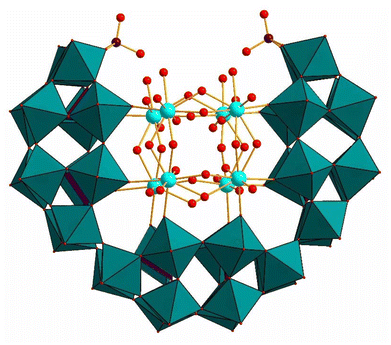 | ||
| Fig. 10 Combined polyhedral/ball-and-stick representation of [Li(H2O)K4(H2O)3{(UO2)4(O2)4(H2O)2}2(PO3OH)2P6W36O136]25−. Color code: WO6 octahedra (green), PO4 tetrahedra (pink), U (turquoise), P (pink), O (red).92 | ||
A mixed-valent vanadium derivative [Rb3⊂{VVVIV3O7(H2O)6}2{H6P6W39O147(H2O)3}]15− ((VVVIV3O7)2(P6W39)) (Fig. 11) was also reported by Kortz and coworkers.93 This polyanion was synthesized by the reaction of vanadium(IV) and vanadium(V) with P4W24 in an acidic aqueous medium (pH 3.2–3.7). A mixed rubidium/potassium salt was isolated and characterized by single-crystal XRD, elemental analysis, TGA, IR, and 31P NMR spectroscopy. The polyanion is composed of three P2W12 subunits, which form a macrocyclic template of P6W39, capped by two mixed-valent {(VV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(VIV
O)(VIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)3(μ2-O)3(H2O)6}3+ ({VVVIV3}) groups. Each {VVVIV3} cap comprises three octahedrally-coordinated VIV atoms and one tetrahedrally-coordinated VV atom. Each of the P2W12 units is linked through {WO(H2O)} groups to form a cyclic P6W39 assembly in this polyanion. The connectivity mode of the P2W12 units in this polyanion resembles what Wang and coworkers had seen previously.58 In the polyanion (VVVIV3O7)2P6W39, the tetrahedral Vv
O)3(μ2-O)3(H2O)6}3+ ({VVVIV3}) groups. Each {VVVIV3} cap comprises three octahedrally-coordinated VIV atoms and one tetrahedrally-coordinated VV atom. Each of the P2W12 units is linked through {WO(H2O)} groups to form a cyclic P6W39 assembly in this polyanion. The connectivity mode of the P2W12 units in this polyanion resembles what Wang and coworkers had seen previously.58 In the polyanion (VVVIV3O7)2P6W39, the tetrahedral Vv![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O oxo group of each {VVVIV3} cap is bridged by three VIVO6 octahedra, which occupy the hexavacant positions in the P6W39 unit. Notably, the terminal oxo-ligands of both the VIV and VV metal centers are directed towards the interior of the polyanion. The solid-state structure of the polyanion (VVVIV3O7)2P6W39 was maintained in solution, as confirmed by 31P NMR. The same type of connectivity and geometry of vanadium(IV/V) has been observed before for Müller's/Pope's mixed-valent vanadium-containing polyanion [K8⊂{VV4VIV2O12(H2O)2}2{P8W48O184}]24− ((VV4VIV2O12)2P8W48) (vide infra, section 6).94
O oxo group of each {VVVIV3} cap is bridged by three VIVO6 octahedra, which occupy the hexavacant positions in the P6W39 unit. Notably, the terminal oxo-ligands of both the VIV and VV metal centers are directed towards the interior of the polyanion. The solid-state structure of the polyanion (VVVIV3O7)2P6W39 was maintained in solution, as confirmed by 31P NMR. The same type of connectivity and geometry of vanadium(IV/V) has been observed before for Müller's/Pope's mixed-valent vanadium-containing polyanion [K8⊂{VV4VIV2O12(H2O)2}2{P8W48O184}]24− ((VV4VIV2O12)2P8W48) (vide infra, section 6).94
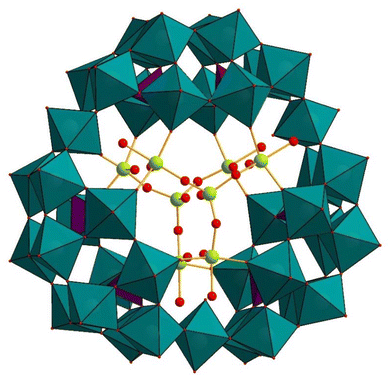 | ||
| Fig. 11 Combined polyhedral/ball-and-stick representation of [Rb3⊂{VVVIV3O7(H2O)6}2{H6P6W39O147(H2O)3}]15−. Color code: WO6 octahedra (green), PO4 tetrahedra (pink), O (red), V (yellow).93 | ||
5. Metal complexes of [H7P8W48O184]33−
Since the pioneering discovery of the Cu2+-containing polyanion [Cu20Cl(OH)24(H2O)12(P8W48O184)]25− (Cu20ClP8W48) (Fig. 12) in 2005, the chemistry of P8W48 has continued to inspire chemists to study this unique cyclic, multilacunary polyanion. Since then, numerous compounds have been reported using the wheel-shaped P8W48 polyanion as a precursor, which includes distinct anionic species as well as zeolitic frameworks.95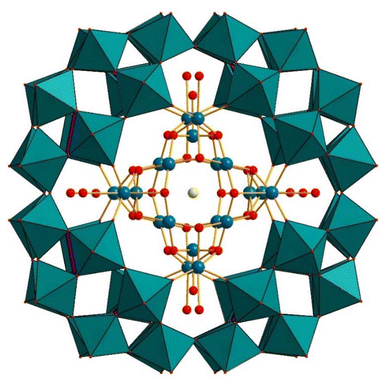 | ||
| Fig. 12 Combined polyhedral/ball-and-stick representation of [Cu20X(OH)24(H2O)12(P8W48O184)]25− (X = Cl, Br, I). Color code: WO6 octahedra (green), PO4 tetrahedra (pink), O (red), Cu (blue), X (yellow).96 | ||
The first transition metal-containing P8W48 polyanion, Cu20ClP8W48, was synthesized by Kortz and coworkers by reacting Cu2+ ions with P8W48 in an aqueous medium, and the product was fully characterized by IR, 31P NMR, single-crystal X-ray diffraction and magnetic studies.96 The 20-copper-oxo cluster in Cu20ClP8W48 comprises three structurally unique types of copper(II) atoms with respect to their coordination geometry, namely octahedral, square-pyramidal, and square-planar, with a central chloride ion acting as a template. The copper ions are connected by μ3-hydroxo-ligands, resulting in a highly symmetrical, cage-like copper-hydroxo cluster assembly, {Cu20(OH)24}16+. In order to study the variation of the magnetic properties of the cluster in the presence of different halide ions, Mal et al. prepared derivatives of Cu20ClP8W48, with the central chloride guest being replaced by a bromide and an iodide ion, [Cu20Br(OH)24(H2O)12(P8W48O184)]25− (Cu20BrP8W48) and [Cu20I(OH)24(H2O)12(P8W48O184)]25− (Cu20IP8W48).97
DFT calculations were performed on the Cu20ClP8W48 polyanion in order to obtain additional information on the properties of the anionic guest inside the cavity created by the 20-copper-hydroxo cage, related to its electronic structure and energies of encapsulation.98 The DFT calculations indicated the central halide ion to be extremely stable inside the polyanion cavity and cannot be released even at higher temperatures, without the destruction of the POM framework. Magnetic measurements showed that the Cu2+ atoms in all halide-derivatives are antiferromagnetically coupled, leading to an overall diamagnetic ground state.97
The solution stability of the polyanion Cu20ClP8W48 was investigated by electrochemical studies at varying pH.98 It was observed that the resolution of the reduction wave for Cu2+ to Cu0 through Cu+ was much better at pH 5.0, than compared with measurements performed at lower or higher pH values. It was also observed that all 20 Cu2+ centers within the polyanionic complex remain electroactive, as observed using controlled potential coulometry measurements and strong electrocatalytic reduction behavior towards NOx.98,99
Scanning tunneling microscopic (STM) and scanning tunneling spectroscopic (STS) studies were performed on a highly oriented pyrolytic graphite (HOPG) surface at room temperature to visualize the 20-copper cluster.100 STS measurements were done especially to better understand the STM results of Cu20ClP8W48, such as the Cu⋯Cu distances and the orientation of the polyanion after deposition. The STM and STS images show a regular assembly of the polyanions and the regularly separated single copper atoms in the organic matrix. Several types of polyanion arrangements were observed by STM measurements on the HOPG surface, ostensibly due to the different concentration levels of Cu20ClP8W48.
Most POMs exhibit hydrophilicity accompanied by high solubility of the corresponding salt in polar solvents, mainly due to a substantial negative charge and oxo/hydroxo/aqua ligands on the surface. Based on such a notion, Kortz and coworkers investigated several solution properties of the Cu20ClP8W48 polyanion, for example, the investigation of supramolecular interaction resulting in “blackberry-type” structures, where the highly soluble homogeneous electrolytes tend to self-assemble into single layer, spherical, vesical-like structures in dilute solution.101,102 Earlier reports of such studies were limited to polyoxomolybdate structures,103–106 until Liu, Kortz, and coworkers showed that polyoxotungstates could also form “blackberry-type” arrangements. Such phenomena were mainly studied by dynamic light scattering (DLS), static light scattering (SLS), and zeta potential measurements.103–106 The DLS studies showed that a slow supramolecular assembly formation in an aqueous Cu20ClP8W48 solution starts spontaneously on the 11th day of the experiment, accompanied by continuous growth until 40 days before finally becoming stable.101,102 Further studies using SLS indicated that the “blackberry-type” structure solution is relatively stable over prolonged periods, as evidenced by no change in scattering intensity for a month-old solution. It should be noted that the “blackberry-type” structure for Cu20ClP8W48 only forms at 50 °C. DLS and SLS also provide important information regarding the mechanism of formation of such “blackberry-type” structures. The individual Cu20ClP8W48 polyanions first overcome the high kinetic energy barrier,107–110 and then slowly nucleate together, quickly forming a “blackberry-type” structure with an average radius of 38 nm. It was also observed that the counter cations play a major role in forming the blackberry solution.111
The Cu20ClP8W48 polyanion was also investigated for the fabrication of organized thin films by the Langmuir–Blodgett (LB) technique. The polyanions can be introduced into organic–inorganic hybrid films using different LB techniques, resulting in well-defined layered structures. Dimethyldioctadecylammonium bromide (DODA) was observed to react with Cu20ClP8W48 to form a surfactant-encapsulated Cu20ClP8W48, which was characterized by different analytical techniques, such as NMR, FT-IR, TGA, powder X-ray diffraction (XRD), and elemental analysis. XRD studies indicated that two different types of DODA-Cu20ClP8W48 structures are present (DODA/Cu20ClP8W48 and DODA-Cu20ClP8W48) based on the diameter and thickness of the layer spacing. The two types of LB films were successfully fabricated onto the substrate by using different deposition methods. It was also observed that Cu20ClP8W48 exhibits different packing modes in the two LB films, depending on the deposition strategy used.112,113 Further studies on Cu20ClP8W48 include electrocatalytic reduction of NOx.98 The polyanion Cu20ClP8W48 has also been shown to be a very efficient heterogeneous catalyst for the solvent-free aerobic oxidation of n-hexadecane.114 In such a study, Cu20ClP8W48 was first supported on 3-aminopropyltriethoxysilane (apts)-modified SBA-15 and then subsequently used for the aerobic oxidation of n-hexadecane, showing an exceptionally high turnover frequency (TOF) of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 and resistance to CS2 poisoning. The efficiency of the polyanion catalyst was observed to increase dramatically upon immobilization on mesoporous support due to a large increase in the surface area, which enhanced the oxidation of n-hexadecane into ketones and alcohols. Moreover, it was found that the supported catalyst can be reused at least five times, retaining almost the same catalytic activity as the fresh catalyst.
000 h−1 and resistance to CS2 poisoning. The efficiency of the polyanion catalyst was observed to increase dramatically upon immobilization on mesoporous support due to a large increase in the surface area, which enhanced the oxidation of n-hexadecane into ketones and alcohols. Moreover, it was found that the supported catalyst can be reused at least five times, retaining almost the same catalytic activity as the fresh catalyst.
Mialane and coworkers have been exploring POMs containing azido ligands since 2003.115 The azido ligands can act as connectors between the 3d metal centers embedded in a POM unit and also act as intermolecular linkers between different POM subunits, leading to high-nuclearity POM complexes. Pichon et al. have shown that azido groups can also function as ligands in P8W48 by preparing the large azido-POM [P8W48O184Cu20(N3)6(OH)18]24− (Cu20(N3)6P8W48).116 The polyanion Cu20(N3)6P8W48 has two {Cu5(OH)4}6+ and two {Cu5(OH)2(μ1,1,3,3-N3)}7+ subunits encapsulated in the crown-shaped P8W48.
In each of the four subunits {Cu5(OH)4}6+ and {Cu5(OH)2(μ1,1,3,3-N3)}7+, the five Cu2+ atoms form a square pyramid with two μ3-hydroxo ligands connecting the apical Cu2+ center to the four basal copper atoms. Interestingly, in each {Cu5(OH)4}6+ fragment, the apical copper atom has an axially distorted coordination geometry, and the four remaining Cu centers exhibit a distorted trigonal–bipyramidal geometry. Moreover, the {Cu5(OH)4}6+ fragments in Cu20(N3)6P8W48 are crystallographically disordered between the hydroxo and azido ligands connecting the Cu2+ atoms. Hence, the polyanion is a mixture of species containing two different {Cu5(OH)4}6+ subunits.
Kortz, Müller, and coworkers have synthesized the 16-Fe3+ containing polyanion [P8W48O184Fe16(OH)28(H2O)4]20− (Fe16P8W48) (Fig. 13a), which was prepared independently.117 The polyanion contains a cationic 16-iron(III)-hydroxo nanocluster {Fe16(OH)28(H2O)4}20+ in the cavity of the crown-shaped P8W48. The {Fe16(OH)28(H2O)4}20+ cluster comprises eight pairs of structurally equivalent, edge-shared {Fe2O12} octahedra, which are connected to each other via corners. Notably, the binding mode of the 16 Fe3+ centers in Fe16P8W48 differs from the 20 Cu2+ centers in Cu20ClP8W48. In Fe16P8W48, each of the 16 equivalent Fe3+ centers is connected to P8W48 by Fe–O(W) and a Fe–O(P) bonds, resulting in a tight anchoring of the 16-iron-hydroxo core, {Fe16(OH)28(H2O)4}20+, to the wheel-shaped POM host. In Cu20ClP8W48, only eight of the 20 Cu2+ ions form two Cu–O(W) bonds each and hence are bound to the P8W48 host. On the other hand, the eight phosphate hetero groups of P8W48 are not directly bonded to the cationic {Cu20(OH)24}16+ cluster. Nevertheless, Cu20ClP8W48 is quite stable in solution. In fact, Fe16P8W48 is structurally more closely related to Mialane's Cu20-azide derivative, Cu20(N3)6P8W48.116 In Cu20(N3)6P8W48, 16 of the 20 Cu2+ ions are connected to the inner cavity of P8W48 in the same fashion as the Fe3+ centers in Fe16P8W48. The sites of the remaining four unique Jahn–Teller distorted Cu2+ atoms in Cu20(N3)6P8W48 are observed to remain empty in Fe16P8W48. Kortz and coworkers further investigated the Fe16P8W48 polyanion toward incorporating lanthanide ions in the remaining vacancies. They successfully prepared an unprecedented, horseshoe-shaped 16-iron(III)-containing polyanion [Fe16O2(OH)23(H2O)9P8W49O189Ln4(H2O)19]11− (Ln = Eu, Gd) (Fe16Ln4P8W49) (Fig. 13b) with a central [Fe16(OH)28(H2O)4]20+ guest.118 These Fe16Ln4P8W49 polyanions can be called open derivatives of Fe16P8W48, where the P8W48 template wheel does not remain intact and is cut open. The {Fe16} ring in Fe16Ln4P8W49 is cleaved between four Fe atoms (Fe1/Fe2 on one side and Fe15/Fe16 on the other). Interestingly, an extra tungsten atom is incorporated into the P8W48 framework, resulting in an open {P8W49} unit. The extra W atom occupies the cap of the P2W12 Wells–Dawson fragment and is connected to the novel open P8W48 fragment through one μ4-oxo and two μ2-oxo bridges.
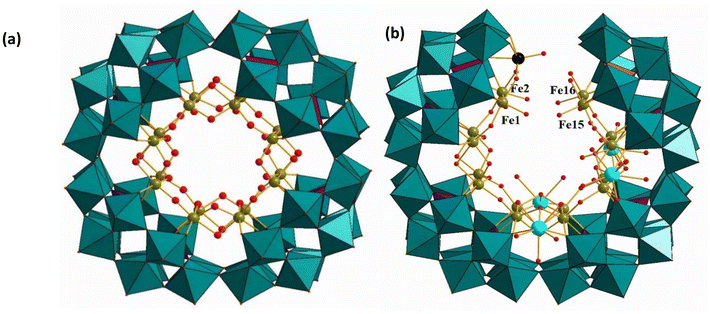 | ||
| Fig. 13 Combined polyhedral/ball-and-stick representations of (a) [P8W48O184Fe16(OH)28(H2O)4]20− and (b) [Fe16O2(OH)23(H2O)9P8W49O189Ln4(H2O)19]11− (Ln = Eu, Gd). Color code: WO6 octahedra (green), PO4 tetrahedra (pink), O (red), Fe (green), Ln (turquoise), W (black).118,119 | ||
Kortz and coworkers have also reported Co2+, Mn2+, Ni2+, and VV-containing derivatives based on the P8W48 wheel. For the Mn and Ni derivatives, the tungsten-oxo wheel has accumulated two extra tungsten atoms, resulting in the unprecedented P8W50 unit.120 All four compounds, K12Li16Co2[Co4(H2O)16P8W48O184] (Co4P8W48),121 (Fig. 14a) K12Li10Mn3[Mn4(H2O)16(P8W48O184)(WO2(H2O)2)2] (Mn4P8W50),121 K14Li8Ni3[Ni4(H2O)16(P8W48O184)(WO2(H2O)2)2] (Ni4P8W50) (Fig. 14b),121 and K20Li16[(VO2)4(P8W48O184)] ((VO2)4P8W48),121 were synthesized and characterized by single-crystal XRD, FTIR, elemental analysis, electrochemistry, magnetic susceptibility, and EPR techniques.
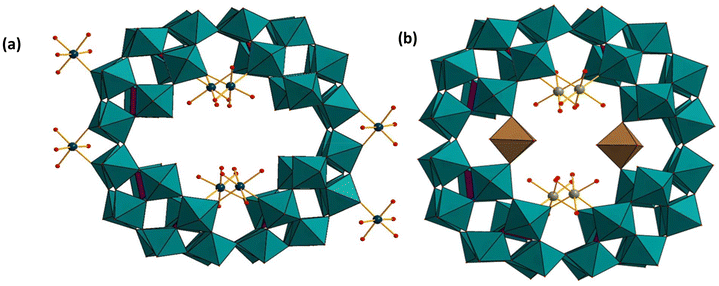 | ||
| Fig. 14 (a) Combined polyhedral/ball-and-stick representations of (a) [Co4(H2O)16P8W48O184]32− and (b) [Ni4(H2O)16(P8W48O184)(WO2(H2O)2)2]28−. Color code: WO6 octahedra (green and brown), PO4 tetrahedra (pink), O (red), Co (deep blue), Ni (green). The brown WO6 octahedra have an occupancy of 50% each.121 | ||
The Co4P8W48 and (VO2)4P8W48 were prepared by reacting Co2+ and VO2+ with P8W48 in aqueous solution, respectively. Bassil et al. have isolated the manganese(II) derivative Mn4P8W50 and its nickel(II) analogue Ni4P8W50, using similar synthetic procedures but in the presence of small amounts of H2O2.121
The solid-state structure of Co4P8W48 consists of four Co2+ atoms coordinated to the hinge-oxygens at the inner rim of P8W48. As perceived from the structures of similar transition metal derivatives of P8W48,71,72 the terminal W–O oxygen atoms are observed to point towards the center of the polyanion. Interestingly, in Co4P8W48 only half of the eight equivalent hinge sites are occupied by the four Co2+ atoms that are coordinated in a cis fashion to two oxo(W) ligands from two adjacent P2W12 subunits. Aqua ligands occupy the remaining four terminal coordination sites. In addition to the four inner Co2+ ions, two outer Co2+ ions were found in Co4P8W48, linking adjacent polyanions and forming a one-dimensional chain in the solid state. Magnetic studies indicated that the two types of Co2+ centers are non-interacting with each other.
Cronin and coworkers have reported a cobalt(II) salt of a cobalt(II)-containing P8W48 assembly {Co4[Co6(P8W48O184)]}∞ (Co4(Co6P8W48)∞) with six internal and four external Co2+ ions bridging neighboring polyanions, resulting in a 1D chain or 3D network, respectively.122 Kortz's group reported the tetra-cobalt(II)-containing polyanion Co4P8W48 (which crystallized with two extra cobalt(II) counter cations). The coordination of two pairs of Co2+ ions in diagonally related positions of Co4P8W48 led to a subtle distortion of the P8W48 assembly, as reflected by a difference of ca. 1.6 Å between the polyanion diameter of opposite W centers where the Co2+ ions are bound and the diameter of opposite W centers perpendicular to the previous one. Wang, Su, and coworkers have synthesized three Co2+ linked derivatives of P8W48, Na8Li8Co5[Co5.5(H2O)19P8W48O184]·60H2O (Co5(Co5.5P8W48)∞), K2Na4Li11Co5[Co7(H2O)28P8W48O184]Cl·59H2O (Co5(Co7P8W48)∞), and K2Na4LiCo11[Co8(H2O)32P8W48O184](CH3COO)4Cl·47H2O (Co11(Co8P8W48)∞), which were characterized by FTIR, thermogravimetric analysis, elemental analysis, and magnetic measurements.123 In Co5{Co5.5P8W48}∞ and Co5{Co7P8W48}∞, four external cobalt(II) ions are observed to link adjacent polyanions, resulting in two-dimensional networks, while Co11{Co8P8W48}∞ is observed to form three-dimensional networks. Co11{Co8P8W48}∞ exhibits the largest cobalt(II) containing P8W48 to date when counterions are also considered.
Kortz's group reported the polyanions Mn4P8W50 and Ni4P8W50 with four Mn2+/Ni2+ ions bound in the cavity of P8W48 as the Co2+ ions in Co4P8W48, and two additional {WO6} octahedral units disordered over the four equivalent positions perpendicular to the plane of the Mn2+/Ni2+ ions, resulting in a polyanion with C2h point group symmetry. The “extra” tungsten centers in Mn4P8W50 and Ni4P8W50 are coordinated to oxygens of the P8W48 wheel just like the Mn2+/Ni2+ ions, but in a trans-related fashion. The average M–O(W) distance for the Mn2+ centers in Mn4P8W50 and for the Ni2+ centers in Ni4P8W50 is 2.13(2) Å and 2.02(2) Å, respectively. The average Mn2+–O(aqua) bond in Mn4P8W50 and the Ni2+–O(aqua) bond in Ni4P8W50 are 2.20(2) and 2.06(2) Å, respectively.
Cronin and coworkers, as well as Proust and coworkers, have reported Mn-based P8W48 derivatives, which differ in the number and location of the Mn2+ ions and their network arrays. The Cronin group reported an open framework nanocube-based, [Mn8(H2O)48P8W48O184]24− ({Mn8(H2O)48P8W48}∞) and a multidimensional framework [Mn14(H2O)30P8W48O184]12− ({Mn14P8W48}∞) polyanion.21,76 Each P8W48 fragment is linked by Mn–O–W coordination bonds, which form a higher-order packing arrangement. Proust and coworkers have reported two new MnII derivatives of P8W48: [Mn8(H2O)26(P8W48O184)]24− (Mn8(H2O)26P8W48) and [Mn6(H2O)22(P8W48O184){WO2(H2O)2}1.5]25− (Mn6{WO2(H2O)2}1.5P8W48).77 In Mn8(H2O)26P8W48, six Mn2+ centers are observed to be located inside the P8W48 cavity, while two other Mn2+ centers are coordinated to the outer rim of P8W48. The internal six Mn2+ ions are distributed among the eight hinges between the {P2W12} subunits. Four of the sites are fully occupied by the four Mn2+, which is the orthogonal plain to the main {P8W48}, and the remaining two Mn2+ centers are disordered over the four other positions, which resembles the Co2+ complex reported by the Cronin group.122 In Mn6{WO2(H2O)2}1.5P8W48, four Mn2+ centers are located inside the P8W48 cavity, while two other Mn2+ centers are coordinated to the outer rim of P8W48, as in the former structure.
Müller and coworkers have also studied the interaction of P8W48 with VO2+ and MoVI in acetate buffer. They have successfully isolated the mixed-valent vanadium(IV/V) containing polyanion [P8W48O184{VV4VIV2O12(H2O)12}2]32− ((VV4VIV2)2P8W48) (Fig. 15a),94 and the mixed-valent molybdenum(V/VI) [{P8W48O184}{MoVIO2}4{(H2O)(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )MoV(μ2-O)2(O
)MoV(μ2-O)2(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )MoV(μ2-H2O)(μ2-O)2MoV(
)MoV(μ2-H2O)(μ2-O)2MoV(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(μ2-O)2MoV(
O)(μ2-O)2MoV(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(H2O)}2]32− (MoVI4MoV4P8W48), the first examples of mixed-valent complexes incorporated in P8W48.124 In (VV4VIV2)2P8W48, two {VV4VIV2O12(H2O)2}4+ units are observed to be trapped inside the cavity of the polyanion. The {VV4VIV2O12(H2O)2}4+ unit consists of two octahedrally-coordinated VIV and four tetrahedrally-coordinated VV centers. The oxidation of VIV to VV occurs in situ due to air. Such type of oxidation has also been observed for (VO2)4P8W48, as reported by Kortz and coworkers.121 Wu's and Bi's groups have reported the reduction of Au3+ in the presence of (VV4VIV2)2P8W48, acting as a stabilizing and reducing agent, forming the bamboo joint-like gold microstructure in aqueous medium at ambient temperature.125
O)(H2O)}2]32− (MoVI4MoV4P8W48), the first examples of mixed-valent complexes incorporated in P8W48.124 In (VV4VIV2)2P8W48, two {VV4VIV2O12(H2O)2}4+ units are observed to be trapped inside the cavity of the polyanion. The {VV4VIV2O12(H2O)2}4+ unit consists of two octahedrally-coordinated VIV and four tetrahedrally-coordinated VV centers. The oxidation of VIV to VV occurs in situ due to air. Such type of oxidation has also been observed for (VO2)4P8W48, as reported by Kortz and coworkers.121 Wu's and Bi's groups have reported the reduction of Au3+ in the presence of (VV4VIV2)2P8W48, acting as a stabilizing and reducing agent, forming the bamboo joint-like gold microstructure in aqueous medium at ambient temperature.125
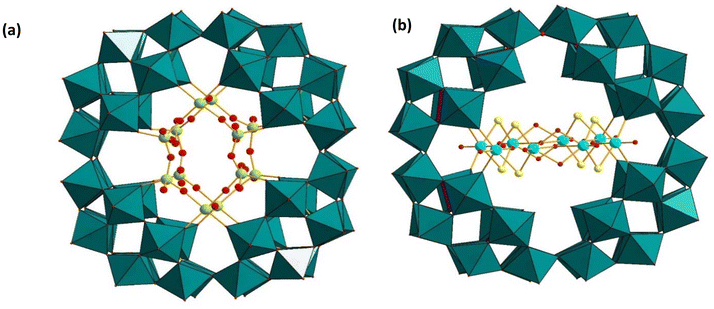 | ||
| Fig. 15 Combined polyhedral/ball-and-stick representations of (a) [P8W48O184{VV4VIV2O12(H2O)12}2]32−, and (b) [{Mo4O4S4(H2O)3(OH)2}2(P8W48O184)]36−. Color code: WO6 octahedra (green), PO4 tetrahedra (pink), Mo (turquoise), V (green), S (yellow), O (red).94,126 | ||
MoVI4MoV4P8W48 was observed to consist of two neutral tetranuclear {MoV4O10(H2O)3} and four {MoVIO2}2+ units connected to the P8W48 ring via Mo–O–W bonds. Furthermore, the {MoV4O10(H2O)3} unit contains two of the well-known diamagnetic {MoV2O4}2+-type units. The four trapped {MoVIO2}2+ units bind to two oxygen atoms of adjacent P2W12 units, resulting in tetrahedral coordination of the Mo atoms. The 31P and 183W NMR data fully support the solid-state structure.
Cadot and coworkers have reported molybdenum oxothiocation complexes with the cyclic P8W48.126 The reaction of the [Mo2S2O2(H2O)6]2+ oxothiocation with P8W48 in an aqueous acidic medium resulted in two new molybdenum oxothiocation based compounds: [K4{Mo4O4S4(H2O)3(OH)2}2(WO2)(P8W48O184)]30− ((Mo4O4S4)2(WO2)P8W48) and [{Mo4O4S4(H2O)3(OH)2}2(P8W48O184)]36− ((Mo4O4S4)2P8W48) (Fig. 15b). In (Mo4O4S4)2(WO2)P8W48, the two disordered [Mo4O4S4(OH)2(H2O)3]2+ oxothiomolybdenum clusters are observed to be grafted on both sides of the cyclic P8W48 surface, resulting in two geometrical isomers where the two {Mo4O4S4(H2O)3(OH)2}2+ groups are arranged either in a perpendicular or parallel mode. The structure also comprises of a {WO2}2+ group, which is disordered over four positions in P8W48. The polyanion (Mo4O4S4)2(WO2)P8W48 is closely related to the compound MoVI4MoV4P8W48,124 where the oxocation {Mo2O4}2+ exhibits a similar mode of connectivity as is usually observed for oxothio {Mo2O2S2}-based polyanions. In MoVI4MoV4P8W48, the neutral core [MoV4O10(H2O)3] is observed to be formed by connections of two dinuclear units through a double oxo-bridge. In contrast, in the oxothio derivative (Mo4O4S4)2WP8W48, such connections are through a double hydroxo bridge. The polyanion (Mo4O4S4)2P8W48 is observed to be composed of the same two disordered [Mo4O4S4(OH)2(H2O)3]2+ oxothiomolybdenum clusters but without the extra {WO2}2+ group. Both compounds were characterized in the solid-state by XRD and solution by NMR.
Kortz and coworkers have further investigated the reactivity of the cyclic P8W48 with 4d transition metal ions in a buffer solution. Interaction of [Ru(p-cymene)Cl2]2 with P8W48 in lithium buffer solution at pH 6.0 resulted in the polyanion [{K(H2O)}3{Ru(p-cymene)(H2O)}4P8W49O186(H2O)2]27− (Ru4P8W49) (Fig. 16).127 The structure of the polyanion Ru4P8W49 reveals that it has four {Ru(p-cymene)(H2O)}2+ groups covalently attached to the inner rim of the cyclic P8W48 unit, resulting in a structure with Ci symmetry. Each organoruthenium group is bound to P8W48 via two Ru–O(W) bonds involving belt oxygens of each of two adjacent, hexalacunary P2W12 building blocks and the extra tungsten atom, resulting in Ru4P8W49, which has been observed previously for other related polyanions.121
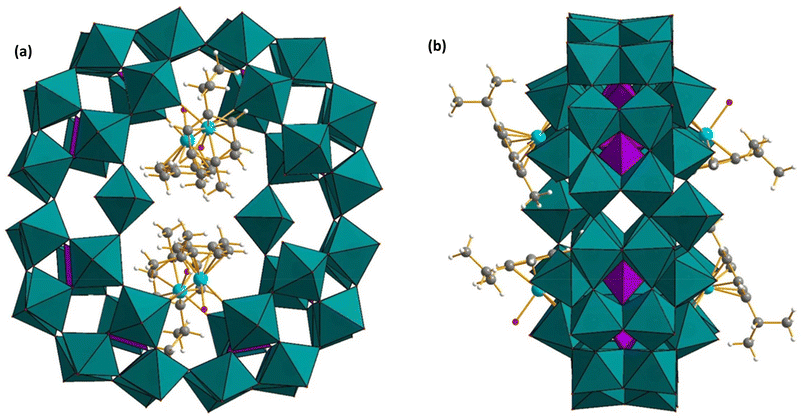 | ||
| Fig. 16 Combined polyhedral/ball-and-stick representation of [{K(H2O)}3{Ru(p-cymene)(H2O)}4P8W49O186(H2O)2]27−. (a) Front-view, (b) side-view. Color code: WO6 octahedra (green), PO4 tetrahedra (pink), Ru (sky blue), O (red), C (grey), H (white).127 | ||
Pope and coworkers have investigated the interaction of the P8W48 ion with early lanthanide metal ions. They have synthesized and structurally characterized a family of four new lanthanide-substituted polyoxotungstates, [K⊂P8W48O184(H4W4O12)2Ln2(H2O)10]25− (Ln2(W4O12)2P8W48) (Ln = La, Ce, Pr, Nd) (Fig. 17) and all polyanions were characterized by infrared spectroscopy, 31P NMR, and X-ray crystallography.128 The structural elucidation of the polyanions reveals that the central cavity of P8W48 is occupied by two additional {W4O12} groups, along with four lanthanides and two potassium ions, each with an occupancy of 50%. Therefore, the polyoxotungstate shell comprises two P2W16 and two P2W12 subunits, with equivalent ones facing each other.
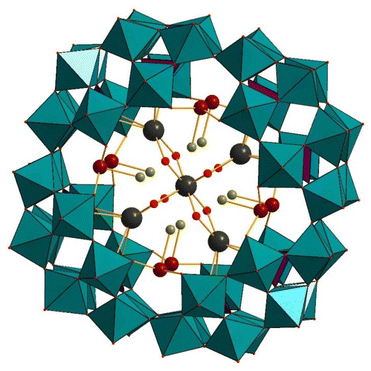 | ||
| Fig. 17 Combined polyhedral/ball-and-stick representation of [K⊂P8W48O184(H4W4O12)2Ln2(H2O)10]25−. Color code: WO6 octahedra (green and brown), PO4 tetrahedra (pink), O (red), Ce (turquoise), K (grey).128 | ||
In 2015, Kögerler and coworkers reported the reactivity of the main group element Sn2+ with P8W48 in aqueous solution and isolated the Sn2+-containing [K4.5⊂(ClSn)8P8W48O184]17.5− ((ClSn)8P8W48) (Fig. 18).129 Interestingly, a color change from bright-orange (reduction of WVI to WV) to brown and then to dark-green (air oxidation of WV to WVI) was observed during the reaction. In the polyanion (ClSn)8P8W48, all eight Sn2+ atoms are incorporated in the central cavity of P8W48, occupying the eight equivalent vacant hinge-positions of the P8W48 moiety. The rate of evaporation of the reaction solution, temperature, and concentration of Sn2+ play a crucial role in the formation of this polyanion. The structure of (ClSn)8P8W48 comprises eight {ClSn} groups, with each Sn2+ atom in a trigonal-pyramidal coordination geometry and the chloride ligand of {ClSn} pointing towards the center of the P8W48 cavity. The bond distance of Sn–Cl in (ClSn)8P8W48 is 2.515(6) Å, which is comparable with Cs[SnCl3] (2.523 Å) and [SnCl2(H2O)]·H2O (2.595 Å). Similarly, the Sn–O bond is in good agreement with the Sn–O bond lengths in [SnCl2(H2O)]·H2O (2.169 Å) and other Sn2+-containing polyoxotungstates.130
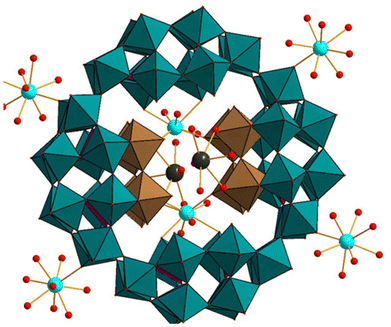 | ||
| Fig. 18 Combined polyhedral/ball-and-stick representation of [K4.5⊂(ClSn)8P8W48O184]17.5−. Color code: WO6 octahedra (green), PO4 tetrahedra (pink), O (red), Sn (brown), K (grey), Cl (green).129 | ||
Recently, Kögerler and coworkers have reported aromatic organoarsenate-functionalized P8W48, [(RAsVO)4PV8WVI48O184]32− [R = C6H5 or p-(H2N)C6H4] ((RAsO)4P8W48, R = C6H5 or p-(H2N)C6H4).131 Recrystallization of the K+/Li+/dimethylammonium salt of ((p-(H2N)C6H4AsO)4P8W48) from 4 M LiCl solution was observed to yield a further functionalized product, [(H3NC6H4AsO)3P8W48O184Hx{WO2(H2O)2}0.4](30.2−x)−, revealing dissociation of the organoarsonate groups in slightly acidic aqueous solution followed by their rearrangement within the inner polyanion cavity.131
In 2018, Khashab and coworkers isolated two main group 3 metal-substituted P8W48 in aqueous acidic solution, {[Na(NO3)(H2O)]4[Al16(OH)24(H2O)8(P8W48O184)]}16− (Al16P8W48) and its Ga analogue [Ga16(OH)32(P8W48O184)]24− (Ga16P8W48).132 The connectivity of Al3+/Ga3+ in Al16P8W48 and Ga16P8W48 is similar to that of Fe16P8W48 reported by Kortz and coworkers (Fig. 13a).117 The incorporated “{Al16} ring” comprises eight pairs of structurally equivalent, edge-shared AlO6 octahedra that are interconnected via corners. The degree of protonation is different in the cationic aluminum-hydroxo core {Al16(OH)24(H2O)8}24+ compared to the isostructural Ga3+ analogue {Ga16(OH)32}16+. This is due to different reaction pH (pH 4.0 for Al16P8W48 vs. pH 5.0 for Ga16P8W48). The bond distances of AlIII–O, GaIII–O, and FeIII–O fall in the range of 1.796(10)–2.044(9), 1.896(5)–2.068(5), and 1.895(12)–2.153(12) Å, respectively, and the corresponding Al–O–Al, Ga–O–Ga, and Fe–O–Fe angles are similar within the respective range of 92.7(4)°–148.0(5)°, 96.4(2)°–143.9(3)°, and 94.2(5)°–139.6(7)°, respectively.
In 2019, Wang and coworkers introduced selenium into the cavity of P8W48 and isolated a mixed potassium–lithium salt, K26Li6[(SeO)4P8W48O184]·98H2O (Se4P8W48).133 Four [SeO3]2− ions were grafted in the cavity of the crown-shaped P8W48, like for Co4P8W48, resulting in a structure with D2h point group symmetry.
Sokolov and coworkers reported the incorporation of {NbO}3+ units into the cyclic P8W48 ion via reaction with the Nb–Ox reagent in different ratios (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 16
1, and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and different concentrations. They have isolated mixed salts of different compounds with differing numbers of coordinated {NbO(H2O)}3+ groups, disordered over the eight equivalent binding sites. The formulas suggest that the compounds are likely mixtures of two or more polyanions: K25.7Li5(NH4)5[(HP8W48O184)(NbO(C2O4)(H2O))3.3]·73H2O ((NbO(C2O4)(H2O))3.3P8W48), K30.8Li3.5(NH4)3[(P8W48O184)(NbO(C2O4)(H2O))1.7]·74.5H2O ((NbO(C2O4)(H2O))1.7P8W48), K21.6Li5(NH4)8H6.8[(P8W48O184)(NbO(H2O))4.4(C2O4)1.5]·66H2O ((NbO(C2O4)1.5(H2O))4.4P8W48), K24.4Li5(NH4)5.5[(HP8W48O184)(NbO(C2O4)(H2O))3.1]·59H2O ((NbO(C2O4)(H2O))3.1P8W48), K26.7Li4(NH4)5.5H2.6[(P8W48O184)(NbO(H2O))3.8(C2O4)2.5]·55.5H2O ((NbO(C2O4)2.5(H2O))3.8P8W48).134
1) and different concentrations. They have isolated mixed salts of different compounds with differing numbers of coordinated {NbO(H2O)}3+ groups, disordered over the eight equivalent binding sites. The formulas suggest that the compounds are likely mixtures of two or more polyanions: K25.7Li5(NH4)5[(HP8W48O184)(NbO(C2O4)(H2O))3.3]·73H2O ((NbO(C2O4)(H2O))3.3P8W48), K30.8Li3.5(NH4)3[(P8W48O184)(NbO(C2O4)(H2O))1.7]·74.5H2O ((NbO(C2O4)(H2O))1.7P8W48), K21.6Li5(NH4)8H6.8[(P8W48O184)(NbO(H2O))4.4(C2O4)1.5]·66H2O ((NbO(C2O4)1.5(H2O))4.4P8W48), K24.4Li5(NH4)5.5[(HP8W48O184)(NbO(C2O4)(H2O))3.1]·59H2O ((NbO(C2O4)(H2O))3.1P8W48), K26.7Li4(NH4)5.5H2.6[(P8W48O184)(NbO(H2O))3.8(C2O4)2.5]·55.5H2O ((NbO(C2O4)2.5(H2O))3.8P8W48).134
Duval and coworkers have introduced the uranyl cation into the cavity of the cyclic P8W48 polyanion, resulting in the salt K11.3Li8.1Na22[(UO2)7.2(HCOO)7.8(P8W48O184)Cl8]·89H2O ((UO2)7.2(HCOO)7.2P8W48) (Fig. 17).135 This is the first time that actinide elements were incorporated in the cavity of P8W48. The structure of (UO2)7.2(HCOO)7.2P8W48 revealed that the 7.2 uranyl cations are disordered over eight positions, suggesting the presence of mixtures of two or more polyanions in the material.126 Interestingly, Kortz and coworkers first introduced the peroxouranium containing wheel-shaped P8W48, resulting in peroxouranium complex K18Li22[(UO2)8(O2)8(P8W48O184)]·133H2O ((UO2)4(O2)4P8W48).136 The polyanion ((UO2)4(O2)4P8W48) consists of four peroxo groups, and each one is connected to two uranium cations. The {(UO2)4(O2)4} unit comprises neutral four uranyl atoms and four peroxo groups, connecting to each other by side-on peroxo bridges, which is similar to previously reported (Li(UO2)4(O2)4(PO3OH)2P6W36) by the same group.92
In 2019, Ibrahim et al. reported an isopoly tetratungsten-oxo cluster incorporated inside the rim of P8W48 along with six internal and four external Mn2+ ions, resulting in [(P8W48O184)(W4O16)K10Li4Mn10Na(H2O)50Cl2]15− (Mn10W4P8W48).137 The Mn–O bond lengths are in the range of 2.087–2.315 Å, while Mn–Cl is 2.365 Å. The oxidation states of the Mn ions were checked by bond valence sum analysis,138–140 and all were shown to be Mn2+. The outer Mn2+ ions assist the formation of a 3D network through intermolecular Mn–O(W) bonding together with potassium ions. Interestingly, the origin of the one Na+ ion in the compound is ambiguous.
Kögerler and coworkers studied the isomerization of the four {α-P2W12O48} units comprising the P8W48 wheel in the presence of Cu2+ ions in 0.66 M acetate buffer at pH 5.2. They were able to isolate K7Li2Na27[αγαγ-P8W48O184{Cu(H2O)}2]·78H2O (Cu2-αγαγ-P8W48), K7.5Na17Cu2.425(WO2)1.325[γγγγ-P8W48O184{Cu(H2O)0.5}4]·102H2O (Cu4-γγγγ-P8W48), and K7Li2Na19.5Cu1.75(WO2)[αγγγ-P8W48O184{Cu(H2O)}3]·72H2O (Cu3-αγγγ-P8W48).141 The molar ratio of Cu2+ to P8W48, temperature, and reaction time played a crucial role when trying to prepare the three compounds. The synthesis of Kortz's Cu20P8W48,78 and Mialane's Cu20(N3)6P8W48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 took 1 h and 15 min, respectively, at 80 °C, whereas Cu2-αγαγ-P8W48 was isolated after 2 h reaction time at 95 °C, in order to transform two P2W12 units from α to γ in the P8W48 wheel. According to the authors, Li+ ions also play a crucial role in such isomeric transformation.
90 took 1 h and 15 min, respectively, at 80 °C, whereas Cu2-αγαγ-P8W48 was isolated after 2 h reaction time at 95 °C, in order to transform two P2W12 units from α to γ in the P8W48 wheel. According to the authors, Li+ ions also play a crucial role in such isomeric transformation.
In the same year, Suzuki, Yamaguchi, and coworkers synthesized a series of P8W48 ions with eight incorporated 3d metal ions from mixed organic solvent. The products were isolated as tetra-n-butylammonium (TBA) salts, TBA14H2-[{MII2(OH2)2}2{MII(OH2)2}4P8W48O176(OCH3)8]·nH2O·mCH3CN, where MII = Mn, Co, Ni, Cu, Zn (M8P8W48O176).142 Edge-shared bis(square pyramidal) metal-aqua sites {(μ-O)2(M-OH2)(μ3O)2(M-OH2)(μ-O)2} were incorporated in the cavity of P8W48. Interestingly, a new type of α,γ,α,γ-type P8W48 was observed after the reaction with the 3d metal ions, with two of the four α-P2W12 units having been transformed to γ-P2W12. The Co2+ ions are disordered over two of the four P2W12 units. However, with increased methanol concentration in the reaction, the Co2+ ions fully occupied each of the four P2W12 units. The Mn2+ and Ni2+-containing P8W48 polyanions exhibit the first examples of edge-shared bis(square pyramidal)manganese-aqua and nickel-aqua complexes. The M–O axial bond length increased for the metal ion M from left to right in the periodic table. For example, in the Mn2+ derivative Mn8P8W48O176, the axial Mn–O bond lengths of 2.17–2.22 Å (Mn3–O13, Mn3–O60, Mn4–O14, Mn4–O59) were similar to the equatorial bond lengths of 2.10–2.24 Å (Mn3–O3, Mn3–O4, Mn3–O39, Mn3–O42; Mn4–O5, Mn4–O6, Mn4–O40, Mn4–O41). On the other hand, for the Co2+ and Ni2+ derivatives, the axial bonds (2.13–2.18 Å) are longer than the equatorial ones (1.99–2.07 Å). As expected, for the Cu2+ derivative, the Cu–O axial bonds (2.22–2.27 Å) are much longer than the equatorial bonds (1.98–2.08 Å). For the Mn2+ and Co2+ derivatives, a decrease in magnetic susceptibility was observed upon cooling, implying antiferromagnetic interactions between the 3d metal ions. However, ferromagnetic interactions were observed for the Ni2+ and Cu2+ derivatives. The same authors have also reported two high-nuclear manganese derivatives of P8W48, (C24PH20)17H37[Mn18P8W48O214]·16H2O·4CH3CN (Mn18P8W48O214) and (C16H36N)12H16[Mn20P8W48O216]·4C2H3N·C2Cl2H4 (Mn20P8W48O216).143 The Mn18P8W48O214 polyanion is a mixed-valent species with 18 Mn2+/3+ ions in the cavity of P8W48. Bond valence sum calculations revealed the presence of 8 Mn2+ and 10 Mn3+ ions. The four P2W12 units isomerized from α- to γ-type in situ in the presence of the transition metal ions, which was observed previously.98,138 The Mn20P8W48O216 polyanion was obtained by reacting P8W48 with 20 equivalents of Mn(OAc)3 in acetonitrile medium. This polyanion was also found to be mixed-valent as per bond valence sum calculations, showing the presence of 12 Mn3+ and 8 Mn4+ ions. All 20 Mn ions are bound in the cavity of P8W48 and all four units of P2W12 remained as α-type. The connectivity of the Mn ions in Mn20P8W48O216 is different from Mn18P8W48O214. In the former, four Mn3+ ions are bound to each α-P2W12 unit, and four Mn ions are bound at the hinges of the four α-P2W12 units. Eight Mn ions of oxidation state either +3 or +4 are bound to the vacant sites within the cavity of the cyclic P8W48, which are disordered over eight positions together with K+ ions.88 Two Mn ions occupy the middle part of opposing P2W12 units, and four Mn ions are at the hinges of the four P2W12 units. Very recently, Suzuki, Yamaguchi, and coworkers have reported a series of multi-nuclear copper(II)-containing P8W48 derivatives, which were synthesized in organic solvent. The four compounds thus reported were formulated as TBA11H13[Cu4(H2O)4P8W48O176(OCH3)8]·28H2O·3CH3NO2 (Cu4P8W48), TBA14H2[Cu8(H2O)12P8W48O176(OCH3)8]·24H2O·CH3CN (Cu8P8W48), TBA14H2[Cu12(H2O)16P8W48O184]·4H2O (Cu12P8W48), and TBA16H8[Cu16(OH)16(H2O)4P8W48O184]·12H2O·C3H6O (Cu16P8W48), respectively.144 Interestingly, the authors were able to obtain the high nuclearity polyanions from the reaction of low nuclearity polyanions with copper(II) salt, for example, Cu8P8W48 from Cu4P8W48, Cu12P8W48 from Cu8P8W48, and Cu16P8W48 from Cu12P8W48, respectively. Moreover, in the case of Cu4P8W48 and Cu8P8W48, two P2W12 units with copper ions connected transformed from α to γ-type isomers by 60° rotation of the central {PO4} hetero groups. The reactive sites of the remaining two {α-P2W12} units are occupied by methoxy groups. This result was similar to the previously reported cobalt-containing P8W48 work by the same group where eight cobalt(II) ions were introduced without affecting the presence of the methoxy groups,145 suggesting that they act as a protecting organic ligands being essential for metal incorporation inside the cavity of P8W48 without disorder. In Cu12P8W48 and Cu16P8W48 comprising the same γ,γ,γ,γ-type P8W48 framework, the copper(II) coordination geometry differs from each other. The arrangements and connectivity of the copper(II) ions in these structures are also different from the Cu20P8W48 polyanion.94 Very recently, the same group has demonstrated the H2-based reduction of copper(II) ions in the cavity of P8W48, resulting in a catalyst which is active for the catalytic hydrogenation of several organic substrates, such as alkenes, alkynes, as well as carbonyl- and nitro-containing compounds.146
In 2019, Cronin and coworkers reported that P8W48 self-assembled into inorganic frameworks in the presence of silver ions, enabling interaction with the POM wheel and linking them together. It was observed that P8W48 was highly reactive towards silver ions, resulting in the formation of fragments as seen in the compounds Li8K9.5Ag21[H16P10W66O251]0.5[H14P9W63O235]·0.5Cl2·50H2O (Ag21P9W63O235), Li8K13Ag13[H12P8W51O196]·50H2O (Ag13P8W51O196), and Li10K12Ag4[H14P8W48O184]·170H2O (Ag4P8W48O148), respectively.147 The species Ag21P9W63O235 revealed two cocrystallized P8W48 units connected by 10 Ag+ ions, forming a “POMzite” framework. In Ag13P8W51O196, the P8W48 units are linked forming a framework with 9 Ag+ ions per formula unit. Further tuning of the reaction conditions yields Ag4P8W48O148, where 4 Ag+ ions are linked to P8W48, resulting in a cubic array, and surprisingly, no Ag+ ions were detected in the cavity of P8W48. Very recently, Suzuki, Yamaguchi, and coworkers have reported a (Ag30) cluster within P8W48, TBA17H[Ag30(P8W48O184)]·10DMF·30H2O (Ag30P8W48), which was synthesized from a {Ag16} cluster-containing P8W48 derivative.145 The Ag30P8W48 nanocluster possesses an additional 14 silver atoms that were introduced into the Ag16 cavity, resulting in an Ag30 nanocluster with distorted body-centered-cubic atom arrangements inside the P8W48 polyanion, revealed by single crystal X-ray crystallography. The Ag30P8W48 exhibits high and selective catalytic activity towards reducing nitrobenzene and aromatic aldehydes under mild conditions. Very recently, Suzuki and coworkers have reported the mixed-metal silver–gold derivative of P8W48, wherein a [Au8Ag26] cluster is embedded within the P8W48 core, (Bu4N)16H8[Au8Ag26(P8W48O184)] (Au8Ag26P8W48).148 In the silver–gold cluster, six out of eight Au atoms form an octahedral {Au6} assembly, while in another one Au atom replaces the Ag site at the center of a hexagonal {Ag7}, forming the {Ag6Au} assembly. The 26 Ag atoms were thus observed to surround Au atoms to form the nano-cluster Au8Ag26P8W48.
Recently, Yang and coworkers were able to incorporate AsIIIO3 and SbIIIO3 in the cavity of P8W48, resulting in [{AsIII5O4(OH)3}2(P8W48O184)]32− (As10P8W48), [(SbIIIOH)4(P8W48O184)]32− (Sb4P8W48), and [(SbIIIOH)8(P8W48O184)]24− (Sb8P8W48).149 Very recently, the same group has reported the 3d–4f iron(III)-cerium(III) derivative [{FeIII8CeIII4O2(OH)12(H2O)8(PO4)2}(P8W48O184)]26− (Fe8Ce4P8W48), comprising an iron(III)–cerium(III)–phosphato moiety {FeIII8CeIII4O2(OH)12(H2O)8(PO4)2} encapsulated in the P8W48 cavity, resulting in a polyanion with idealized D2h symmetry and it exhibited high sensitivity and specificity to detect ascorbic acid (Table 1).150 In Table 1, the structural characteristics and component building blocks of the compounds presented in this review are summarized.
| Sl. no. | Formula | Abbreviation | Brief description | Ref. |
|---|---|---|---|---|
| 1 | Li5.5K3H3.5[P2W12(NbO2)6O56]·H2O | P2W12(Nb-O2)6 | Six {Nb–O2} occupy six vacant positions of {P2W12}. The six Nb atoms are connected by four η2-O atoms, one η4-bridging O atom (cap sites or triply-bridging O atom on belt sites), and one terminal η2-coordinated peroxo unit. | 53 |
| 2 | K7[Fe(OH2)P2W12Mo5O61] | FeP2W12 | All six-vacant positions of {P2W12} are filled up by five MoVI ions in the belt and one cap position and one cap position by a Fe3+ ion. | 54 |
| 3 | K7[α1-Fe(OH2)P2W13Mo4O61] and K8[α2-Cu(OH2)-P2W13Mo4O61] | α1-FeP2W13Mo4, α2-CuP2W13Mo4 | Four Mo6+, one Fe3+/Cu2+ ion, and one extra tungsten atom fill the six vacancies of {P2W12}, generating a {P2W13} moiety. | 54 |
| 4 | K8[α2-Cu(OH2)P2W12Mo5O61] | α2-CuP2W12Mo5 | The vacant positions of {P2W12} are occupied by five MoVI ions in the two belts and a cap and a Cu2+ ion in the other cap. | 54 |
| 5 | Li2K4[H4P2W12Fe9O56(OAc)7]·34H2O | Fe9(OAc)7P2W12 | The six vacancies of {P2W12} are occupied by Fe3+ ions, forming a {P2W12Fe6} unit to which three additional Fe3+ atoms are coordinated. | 58 |
| 6 | K6Na10[H12P4W28Fe8O120]·34H2O | Fe8P4W28 | Dimeric clusters with four extra tungsten atoms in the cap position and eight iron atoms occupying the belt position. | 59 |
| 7 | K12[{M(H2O)4}2{H12P4W28Fe8O120}]·30H2O (M = Co2+, Mn2+, Ni2+) | M2Fe8P4W28 (M = Co2+, Mn2+, Ni2+) | Two {P2W12} units with four extra tungsten atoms in the cap positions and eight Fe3+ atoms in the belt positions. Two such {Fe4P2W14} units are bridged by Co2+, Ni2+, or Mn2+ ions. | 59 |
| 8 | K3Na17[{W2Co2O8(H2O)2}(P2W12O46)2]·30H2O | W2Co2O8(P2W12)2 | The dimeric structure consists of two {P2W12} units fused via four W–O–W bonds and two WVI and Co2+ atoms are bound in the vacant positions. | 62 |
| 9 | K4Na4[H6P2W12Nb4O59(NbO2)2]2·48H2O | {P2W12Nb4(NbO2)2}2 | Six Nb atoms occupy two cap and four belt sites of the lacunary {P2W12} precursor. Two such {P2W12Nb4(NbO2)2} units are dimerized via Nb–O–Nb bonds. | 63 |
| 10 | K7[H13{Nb6(O2)4P2W12O57}2]·31H2O | {P2W12Nb6(O2)4}2 | The Wells–Dawson dimer consists of two {P2W12} units linked by two Nb–O–Nb bridges and the six vacant sites of each {P2W12} unit are filled by a Nb6(O2)4 group. | 67 |
| 11 | (NH4)16[H14{P2W12Nb7O63(H2O)2}4{Nb4O4(OH)6}]·16H2O | {(P2W12)Nb7}4Nb4 | This polyanion comprises an adamantine-like {Nb4O6} core encapsulated by two [Nb6P2W12O61]10− units (without any Nb-peroxo groups). | 67 |
| 12 | K3.5Li8[(CH3)2NH2]4.5[(PhXO)2P4W24O92]·nH2O (X = P, n = 35 and X = As, n = 40) | PhXOP4W24, X = P, As | The structure consists of a {P4W24O29} unit capped by two phenyl-phosphonate or -arsonate ligands. | 68 |
| 13 | KLi3[(CH3)2NH2]10[(o-H2N-C6H4AsO3)4P4W24O85]·17H2O·LiCl, KHLi2[(CH3)2NH2]6[Mn(H2O)4]2[{Mn(H2O)4}(o-H2N-C6H4-AsO)2P4W24O92]·25H2O·0.2KCl·0.4LiCl, K1.5Li2[(CH3)2NH2]6.5-[Co(H2O)4]2[{Co(H2O)4}(o-H2N-C6H4-AsO)2P4W24O92]·24H2O·0.5LiCl, and K1.5Li2[(CH3)2NH2]6.5[Ni(H2O)4]2[{Ni(H2O)4}(o-H2N-C6H4-AsO)2P4W24O92]·24H2O·0.2LiCl. | (o-NH2-C6H6-AsO)4P4W24O92M(o-NH2-C6H6-AsO)2P4W24 (M = Co2+, Mn2+, Ni2+) | The introduction of divalent transition metal ions (Mn2+, Co2+, Ni2+) in the reaction mixture containing (o-NH2-C6H6-AsO)4P4W24O92 resulted in 1D coordination polymers [{M(H2O)4}P4W24O92(C6H6AsNO)2]14− (M = Mn2+, Co2+, Ni2+) (M(o-NH2-C6H6-AsO)2P4W24). | 69 |
| 14 | K4Na15[K3⊂{Mn(H2O)4}2{WO2(H2O)2}2{WO(H2O)}3(P2W12O48)3]·77H2O | Mn2(P2W12)3{WO2(H2O)2}{WO(H2O)}3 | Three {P2W12} units are connected by three WO(H2O) hinges forming a cyclic P6W39 assembly which accommodates two WVI and two Mn2+ guest atoms. | 71 |
| 15 | K3Na7Li5.5Ni0.25[Na3⊂{Ni3.5(H2O)13}{WO2(H2O)2}2{WO(H2O)}3(P2W12O48)3]·64H2O | Ni3.5(P2W12)3{WO2(H2O)2}2{WO(H2O)}3 | Two WVI and four Ni2+ ions are incorporated in the cyclic P6W39 host. One of the Ni2+ ions is disordered with a Na ion. | 71 |
| 16 | K6Na11[Na3⊂{Cu3(H2O)9}{WO2(H2O)2}2{WO(H2O)}3(P2W12O48)3]·47H2O | Cu3(P2W12)3{WO2(H2O)2}2{WO(H2O)}3 | Two WVI and three Cu2+ ions are incorporated in the cyclic P6W39 host. | 71 |
| 17 | Na15[Na3⊂{Co(H2O)4}6{WO(H2O)}3(P2W12O48)3]·109H2O and Na15[Na3⊂{Ni(H2O)4}6{WO(H2O)}3(P2W12O48)3]·110H2O | Co6(P2W12)3{WO(H2O)} and Ni6(P2W12)3{WO(H2O)}3 | Six Co2+ or Ni2+ ions are incorporated in the cyclic P6W39 assembly. | 72 |
| 18 | K3Na8[K3⊂{GdMn(H2O)10}{HMnGd2(tart)O2(H2O)15}{P6W42O151(H2O)7}]∞·44H2O | {(MnGd)(HMnGd2P6W42)}∞ | The trimeric crown-shaped P6W39 encapsulates a Mn2+ and two Gd3+ ions in the cavity. In addition, a Mn2+ and Gd3+ ion are present outside the polyanion, resulting in a two-dimensional solid-state framework. | 73 |
| 19 | K3Na10[K3⊂{GdCo(H2O)11}2{P6W41O148(H2O)7}]·43H2O | (GdCoP6W41)∞ | The structure comprises a trimeric, crown-shaped P6W39 ion encapsulating two Co2+ and a Gd3+ ion as well as two WVI atoms. The second Gd3+ ion is outside P6W39, linking polyanions to a one-dimensional chain, where this Gd3+ ion shows a square-antiprismatic coordination geometry. | 73 |
| 20 | K4[H23(Cr(H2O)2)3(H2P2W12O48)3]·34H2O | Cr3(P2W12)3 | Trimeric, cyclic assembly of {P2W12} units joined by three CrIII ions. | 74 |
| 21 | Na16K12[H56P8W48Fe28O248]·20H2O | Fe28P8W48 | The tetrameric polyanion comprises four {P2W12Fe6} units, and each is bridged through Fe–O–Fe linkages to a {Fe4O6} cluster core. The linking is through pairs of three Fe–O–Fe bridges, which involve the three outer Fe atoms. | 55–57 |
| 22 | Na16K10[H55P8W49Fe27O248]·yH2O | Fe27P8W49 | Similar structure as Fe28P8W48 with one of the sites having an occupancy of 25% W and 75% Fe. | 55–57 |
| 23 | K4Na22{[Co(H2O)2Cl][Co(H2O)3]2[Co(H2O)5]1.5[Co(H2O)3H4P8W49O187(H2O)]}·2NaCl·41.5H2O, and, Na30{[Ni(H2O)3]2[{Ni(H2O)3}1.5H3P8W49O187(H2O)]}·41.5H2O | Co5.5P8W49, and Ni3.5P8W49 | The transformation of four {P2W12} ions to the cyclic P8W49 happens in situ via self-condensation, performed in aqueous acidic medium in the presence of Co2+/Ni2+ ions. The extra W atom originates from partial decomposition of {P2W12}. | 75 |
| 24 | (NH4)36[{Nb4O6(OH)4}{Nb6P2W12O61}4]·16H2O | (Nb4O6)(Nb6P2W12)4 | The polyanion comprises a {Nb4O6} core which is surrounded by four Nb6P2W12 units. | 78 |
| 25 | Na24[Mn8(H2O)32P8W48O184]·58H2O, K4Na16H4[Co8(H2O)32P8W48O184]·76H2O, and Na20H4[Ni8(H2O)32P8W48O184]·72H2O | (M8P8W48, M = Mn, Co, Ni) | Eight divalent 3d metal ions are incorporated in the cyclic P8W48 host. | 79 |
| 26 | K10[H123Nb36P12W72MnIII12MnII3NaO424]·26H2O | Mn15(Nb6P2W12)6 | The structure consists of six {Nb6P2W12} units which are connected alternately by four Mn2+ ions and four trinuclear {MnIII3} moieties. | 80 |
| 27 | [(n-C4H9)4N]20·5H21.5[{γ-P2W12O48Mn4(C5H7O2)2(CH3CO2)}6]·35H2O | {P2W12O48Mn4}6 | The polyanion P2W12 reacts with Mn(acac), forming a hexameric polyanion in an organic medium. Two types of manganese coordination sites are present in each unit of manganese-substituted {P2W12}, and each unit is connected to the other unit of manganese-substituted P2W12 unit. | 81 |
| 28 | [(n-C4H9)4N]16.6H7.4[{γ-P2W12O48Mn4(H2O)6}4(H2O)4]·8H2O | {P2W12O48Mn4}4 | The tetrameric polyanion forms after removing the organic capping in the manganese cation from the hexameric complex. | 81 |
| 29 | ([(n-C4H9)4N]5[γ-P2W12O44M2(OAc)(CH3CONH)2]·nH2O·mCH3CN; M = Mn2+, Co2+, Ni2+, Cu2+, or Zn2+; OAc = acetate) | (γ-P2W12O44M2(OAc)) | The polyanion comprises a central edge-shared bis(square-pyramidal) {O2M(μ3-O)2(μ-OAc)MO2} group bound to the belt area of {γ-P2W12} and two acetamide (CH3CONH2) groups are coordinated to the vacant cap positions. | 82 |
| 30 | K12H2[Ce4(OH2)9(OH)2(α1,α1-P2W16O59)2]·48H2O | Ce4(α1α1-P2W16)2 | The polyanion has a dimeric structure composed of two {α1,α1-P2W16O59} units connected by four cerium(III) ions. | 60 |
| 31 | K16[{La(CH3COO)(H2O)2(α2-P2W17O61)}2]·36H2O | La(OAc)(α2-P2W17)2 | The polyanion consists of two [α2-P2W17O61]10− units connected by two lanthanum acetate dimers (La2(CH3COO)2(H2O)4)4+, resulting in a head-on transoid dimer. In an acidic medium, P2W12 quickly transforms into the monovacant [α2-P2W17O61]10−. | 84 |
| 32 | K4Na10[α1-CuP2W17O60(OH)]2·∼58H2O | Cu2P4W34 | The dimeric polyanion cluster is formed from two units each of α1-{CuP2W17}, connected through the W-OH-Cu bonds resulting in the dimeric cluster. | 86 |
| 33 | Na2[H2en][H2hn]0.5[Cu(en)2]4.5[α1-CuP2W17O60(OH)]2·∼43H2O | {Cu(en)2CuP2W17}∞ | The dimeric polyanion assembly [α1-CuP2W17O60(OH)]2 is linked to an extended network by {Cu(en)2}2+ units. | 86 |
| 34 | Na3[H2hn]2.5[P2W17O60Cu(OH)2]·∼14H2O | {(H2hn)(CuP2W17)2}∞ | The polyanion possesses a 3-D hybrid supramolecular framework with 1-D tunnels. | 86 |
| 35 | Na8H2L(H2enMe)4[Mn(H2O)2(W4Mn4O12)(P2W14O54)2]·17H2O | {W4Mn4(MnP2W14)2}∞ | The 1-D inorganic polymer building blocks comprise multi Mn2+-substituted Wells–Dawson ions and Mn2+ linkers with the idealized C2 symmetry, further connected into a 3-D supramolecular network via extensive hydrogen-bonding interactions. | 87 |
| 36 | K56Li74H14[Mn40P32W224O888]·ca. 680 H2O | (P8W48)(Mn4P2W14)4(Mn3P2W15)8 | The polyanion consists of {P2W14}, {P2W15} and {P8W48} corner-sharing Wells–Dawson type units with no Mn2+ ions found inside the cavity of P8W48. | 88 |
| 37 | [K(H2O)2]4[K4(μ-H2O)4(H2O)4]2{[Ln2(μ-OH)4(H2O)x]2(H24P8W48O184)}·yH2O (Ln = Nd, Sm, Tb) | Ln4P8W48 | Eight lanthanide ions occupy the cavities of P8W48, with each lanthanide ion being bridged through hydroxyl groups and having a 50% occupancy in each position. | 89 |
| 38 | [K(H2O)2]4[K4(μ-H2O)8]2[K(H2O)]8{[Mn8(H2O)16](H4P8W48O184)} | K8Mn8P8W48 | Eight Mn2+ and eight K+ ions are incorporated in the cavity of P8W48. | 89 |
| 39 | [cis-(P2W15Nb3O61)2]14− and two phases of [trans-(P2W14.7Nb3.3O61)2]14.6− | P2W15Nb3 | NbV ions are taken up by P2W12 in acidic media resulting in the dimer [(P2W15Nb3O61)2]14− and a mixture of derivatives with an average formula [(P2W14.7Nb3.3O61)2]14.6−, indicating the coexistence of species with different composition, such as {(P2W15Nb3)2} (70%) and {(P2W14Nb4)2}(30%). | 90 |
| 40 | [Fe48(OH)76(H2O)16(HP2W12O48)8]36− | Fe48(P2W12)8 | This structure contains 48 Fe3+ ions surrounded by eight P2W12 ions, comprising eight equivalent {Fe6P2W12} subunits, linked to each other via Fe–O–Fe/W bonds. | 91 |
| 41 | K17Li11[{Sn(CH3)2}4(H2P4W24O92)2]·51H2O | {Sn(CH3)2}4(P4W24)2 | The structure consists of two P4W24 units linked through four dimethyltin groups, resulting in a dimeric hybrid inorganic–organic polyanion cluster. | 44 |
| 42 | K6Li19[Li(H2O)K4(H2O)3{(UO2)4(O2)4(H2O)2}2(PO3OH)2P6W36O136]·74H2O | Li(UO2)4(O2)4(PO3OH)2P6W36 | The structure consists of three P2W12 units encapsulating two independent, neutral symmetrical uranium-peroxo [(UO2)(O2)]4 units in the central cavity, resulting in a U-shaped {P2W12}3 assembly for the first time. | 92 |
| 43 | K12Rb3[Rb3⊂{VVVIV3O7(H2O)6}2{H6P6W39O147(H2O)3}]·63H2O | (VVVIV3O7)2P6W39 | The polyanion cluster is composed of three α-{P2W12} subunits, which form a macrocyclic template of P6W39, capped by two mixed-valent {(VV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(VIV O)(VIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)3(μ2-O)3(H2O)6}3+ ({VVVIV3}) groups. O)3(μ2-O)3(H2O)6}3+ ({VVVIV3}) groups. |
93 |
| 44 | Na12K8H4[K8⊂{VV4VIV2O12(H2O)2}2{P8W48O184}]·80H2O | (VV4VIV2O12)2P8W48 | In this polyanion, two {VV4VIV2O12(H2O)2}4+ units are observed to be trapped inside the cavity of P8W48. The {VV4VIV2O12(H2O)2}4+ unit consists of two octahedral VIV and four tetrahedral VV centers. The oxidation of VIV to VV occurs due to air. | 94 |
| 45 | K12Li13[Cu20Cl(OH)24(H2O)12(P8W48O184)]·22H2O | Cu20ClP8W48 | This structure was the first example demonstrating that d-metal ions can be incorporated in the cavity of P8W48. Here, twenty Cu2+ ions are grafted in P8W48, and the coordination geometry of Cu2+ ranges from octahedral to square-pyramidal and square-planar, with a chloride ion encapsulated in the center of the structure. | 96 |
| 46 | K12Li13[Cu20Br(OH)24(H2O)12(P8W48O184)]·60H2O and K12Li13[Cu20I(OH)24(H2O)12(P8W48O184)]·50H2O | Cu20BrP8W48, and Cu20IP8W48 | Identical structure to Cu20ClP8W48 but with bromide or iodide acting as the central template rather than instead of chloride. | 97 |
| 47 | LiK14Na9[P8W48O184Cu20(N3)6(OH)18]·60H2O | Cu20(N3)6P8W48 | The polyanion comprises two {Cu5(OH)4}6+ and two {Cu5(OH)2(μ1,1,3,3-N3)}7+ units incorporated in the cavity of P8W48. | 116 |
| 48 | Li4K16[P8W48O184Fe16(OH)28(H2O)4]·66H2O·2KCl | Fe16P8W48 | The polyanion contains a cationic {Fe16(OH)28(H2O)4}20+ cluster incorporated in the cavity of P8W48. The 16-iron(III)-hydroxo core consists of eight pairs of structurally equivalent, edge-shared {Fe2O12} units. | 117 |
| 49 | K9LiNa[Fe16O2(OH)23(H2O)9P8W49O189Gd4(H2O)19]·50H2O and K8.5Na0.5Li0.5Eu0.5[Fe16O2(OH)23(H2O)9P8W49O189Eu4(H2O)19]·70H2O | Fe16Ln4P8W49 (Ln = Gd3+, Eu3+) | The Fe16Ln4P8W49 (Ln = Gd3+, Eu3+) polyanion can be described as a derivative of Fe16P8W48 with four Ln3+ ions grafted on the iron-oxo core and the P8W48 wheel is cleaved with an extra tungsten atom being bound right there. | 118 |
| 50 | K12Li16Co2[Co4(H2O)16P8W48O184]·60H2O | Co4P8W48 | The structure has four Co2+ ions bound at two opposite hinge sites of P8W48. | 121 |
| 51 | K12Li10Mn3[Mn4(H2O)16(P8W48O184)(WO2(H2O)2)2]·67H2O and K14Li8Ni3[Ni4(H2O)16(P8W48O184)(WO2(H2O)2)2]·44H2O | Mn4P8W50 and Ni4P8W50 | This structure is identical to Co4P8W48, except that two additional WVI ions in the form of {WO2(H2O)2} groups are grafted in the cavity, one each at the two hinges which are not occupied by M2+ (M = Mn, Ni) ions, resulting in a cyclic P8W50 unit. | 121 |
| 52 | K20Li16[(VO2)4(P8W48O184)]·48H2O | (VO2)4P8W48 | Four VVO groups are coordinated in the cavity of P8W48. | 121 |
| 53 | K15Li5[{Co10(H2O)34(P8W48O184)}]·54 H2O | {Co10(H2O)34P8W48}∞ | This polyanion structure is similar to that of Co4P8W48, except that an additional four external Co2+ ions link adjacent polyanions resulting in 1D chains. | 122 |
| 54 | K8Li12[{Co10(H2O)44(P8W48O184)}]·60H2O | {Co10(H2O)44P8W48}∞ | Similar to Co4P8W48, except an additional four external Co2+ ions link the adjacent polyanions, resulting in 3D networks. | 122 |
| 55 | Na8Li8Co5[Co5.5(H2O)19P8W48O184]·60H2O | Co5{Co5.5P8W48}∞ | Five external Co2+ ions are observed to link adjacent polyanions of Co5.5P8W48, resulting in two-dimensional chains. | 123 |
| 56 | K2Na4Li11Co5[Co7(H2O)28P8W48O184]Cl·59H2O | Co5{Co7P8W48}∞ | Co7P8W48 is linked via 5 external Co2+ ions are observed to link adjacent polyanions, resulting in two-dimensional chains. | 123 |
| 57 | K2Na4LiCo11[Co8(H2O)32P8W48O184](CH3COO)4Cl·47H2O | Co11{Co8P8W48}∞ | Co8P8W48 is linked via 11 external Co2+ ions into a three-dimensional network. | 123 |
| 58 | K18Li6[Mn8(H2O)48P8W48O184]·108H2O | {Mn8(H2O)48P8W48}∞ | The Mn8(H2O)48P8W48 polyanion forms an open framework nanocube structure with each P8W48 fragment linked by Mn–O–W coordination bonds, which forms the higher order packing arrangement. | 21 and 76 |
| 59 | K12[Mn14(H2O)30P8W48O184]·111H2O | {Mn14P8W48}∞ | A solid-state framework formed by P8W48 units linked by external Mn2+ ions. | 21 and 76 |
| 60 | K13Li11[Mn8(H2O)26(P8W48O184)]·60H2O | Mn8(H2O)26P8W48 | In this polyanion, six Mn2+ ions are observed to be located inside the P8W48 cavity, while two other Mn2+ ions are coordinated to the outer rim of P8W48. | 77 |
| 61 | K12Li13[Mn6(H2O)22(P8W48O184){WO2(H2O)2}1.5]·75H2O | Mn6{WO2(H2O)2}1.5P8W48 | Four Mn2+ ions are located in the cavity of P8W48, whereas two other Mn2+ centers are attached to the surface of the wheel. In addition, one or two {WO2(H2O)2} groups are grafted in the cavity, leading to a mixture of products. | 77 |
| 62 | K10Na14[{P8W48O184}{MoVIO2}4{(H2O)(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )MoV(μ2-O)2(O )MoV(μ2-O)2(O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )MoV(μ2-H2O)(μ2-O)2MoV( )MoV(μ2-H2O)(μ2-O)2MoV(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(μ2-O)2MoV( O)(μ2-O)2MoV(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(H2O)}2]·80H2O O)(H2O)}2]·80H2O |
MoVI4MoV4P8W48 | Observed to consist of two unprecedented neutral tetranuclear {MoV4O10(H2O)3} and four {MoVIO2}2+ units connected to the P8W48 ring via Mo–O–W bonds. | 124 |
| 63 | K20Li6H4[K4{Mo4O4S4(H2O)3(OH)2}2(WO2)(P8W48O184)]·95H2O | (Mo4O4S4)2(WO2)P8W48 | Two cationic [Mo4O4S4(OH)2(H2O)3]2+ groups are grafted on both sides of P8W48. Different bonding modes of the oxothiomolybdenum groups result in two geometrical isomers. | 126 |
| 64 | K26Li2H8[{Mo4O4S4(H2O)3(OH)2}2(P8W48O184)]·90H2O | (Mo4O4S4)2P8W48 | This polyanion is composed of the same two disordered {Mo4O4S4} oxothiomolybdenum clusters observed in (Mo4O4S4)2(WO2)P8W48 but without the extra {WO2}2+ group. | 126 |
| 65 | K16Li11[{K(H2O)}3{Ru(p-cymene)(H2O)}4P8W49O186(H2O)2]·87H2O | Ru4P8W49 | Four {Ru(p-cymene)(H2O)}2+ groups are covalently grafted to the cavity of P8W48 via two Ru–O(W) bonds. | 127 |
| 66 | Ln4(H2O)28K6Li7[K⊂P8W48O184(H4W4O12)2Ln2(H2O)10]·87H2O (Ln = La3+, Ce3+, Pr3+, Nd3+) | Ln2(W4O12)2P8W48 | The cavity of P8W48 contains four lanthanide ions and two {W4O12} groups, along with two potassium ions. | 128 |
| 67 | K10Li17.5[K4.5⊂(ClSn)8P8W48O184]·50H2O | (ClSn)8P8W48 | Eight SnIICl groups are incorporated in the cavity of P8W48. | 129 |
| 68 | K8Li17[(CH3)2NH2]7[(C6H5AsO)4P8W48O184]·130H2O, K10.5Li14[(CH3)2NH2]3.5[(H3NC6H4AsO)4P8W48O184]·92H2O [R = C6H5 or p-(H2N)C6H4] | (RAsO)4P8W48, R = C6H5 or p-(H2N)C6H4 | Four {RAsO3} units are bound covalently to the cavity of P8W48 through As–O(W) bonds. | 131 |
| 69 | K7.2Li23−x[(H3NC6H4AsO)3P8W48O184Hx{WO2(H2O)2}0.4]·nH2O | (H3NC6H4AsO)3P8W48(WO2)0.4 | This compound has the (RAsO)4P8W48 structure, but in addition 0.4 equivalents of tungsten atoms are incorporated in the cavity, which indicates the presence of a compound mixture. | 131 |
| 70 | K8Na3Li5{[Na(NO3)(H2O)]4[Al16(OH)24(H2O)8(P8W48O184)]}·66H2O | Al16P8W48 | The polyanion contains a cationic {Al16(OH)24(H2O)8}24+ hydroxo-cluster inside the cavity of P8W48. The 16-aluminium-hydroxo unit comprises eight pairs of edge-shared AlO6 units connected via corners. | 132 |
| 71 | K11Li9(NH4)4[Ga16(OH)32(P8W48O184)]·112H2O | Ge16P8W48 | The polyanion contains the cationic nanocluster {Ga16(OH)32}16+ incorporated in the cavity of the crown-shaped P8W48. The 16-gallium-hydroxo core {Ga16(OH)32}16+ comprises eight pairs of structurally equivalent, edge-shared GaO6 units interconnected via corners, and all bridging oxygens are monoprotonated. | 132 |
| 72 | K26Li6[(SeO)4P8W48O184]·98H2O | Se4P8W48 | The crown-shaped P8W48 polyanion has four [SeO3]2− ions inside the cavity in such a way that each Se atom is located inside the cavity perpendicular to the main plan of P8W48. | 133 |
| 73 | K25.7Li5(NH4)5[(HP8W48O184)(NbO(C2O4)(H2O))3.3]·73H2O | [(NbO(C2O4)(H2O))3.3P8W48] | The {NbO(H2O)}3+ groups in the cavity of P8W48 have two different types of coordination environments. | 134 |
| 74 | K30.8Li3.5(NH4)3[(P8W48O184)(NbO(C2O4)(H2O))1.7]·74.5H2O | [(NbO(C2O4)(H2O))1.7P8W48] | The {NbO(H2O)}3+ groups in the cavity of P8W48 have two different types of coordination environments. | 134 |
| 75 | K21.6Li5(NH4)8[(P8W48O184)(NbO(C2O4)1.5(H2O))4.4]·66H2O | [(NbO(C2O4)1.5(H2O))4.4P8W48] | The {NbO(H2O)}3+ groups in the cavity of P8W48 have two different types of coordination environments. | 134 |
| 76 | K24.4Li5(NH4)5.5[(HP8W48O184)(NbO(C2O4)(H2O))3.1]·59H2O | [(NbO(C2O4)(H2O))3.1P8W48] | The {NbO(H2O)}3+ groups in the cavity of P8W48 have two different types of coordination environments. | 134 |
| 77 | K26.7Li4(NH4)5.5H2.6[(P8W48O184)(NbO(C2O4)2.5(H2O))3.8]·55.5H2O | [(NbO(C2O4)2.5(H2O))3.8P8W48] | The {NbO(H2O)}3+ groups in the cavity of P8W48 have two different types of coordination environments. | 134 |
| 78 | K11.3Li8.1Na22[(UO2)7.2(HCOOH)7.8(P8W48O184)Cl8]·89H2O | [(UO2)7.2(HCOO)7.2P8W48] | The 7.2 uranyl groups are disordered over eight positions, suggesting a mixture of compounds. | 135 |
| 79 | K18Li22[(UO2)8(O2)8(P8W48O184)]·133H2O | (UO2)4(O2)4P8W48 | The polyanion contains four peroxo groups connected to two uranium ions. The connectivity of each peroxo-group is similar to the previously reported polyanion (Li(UO2)4(O2)4(PO3OH)2P6W36). | 136 |
| 80 | K3Li8Mn2[(P8W48O184)(W4O16)K10Li4Mn10Na(H2O)50Cl2]·62H2O | Mn10W4P8W48 | The cavity of P8W48 contains six Mn2+ ions and a tetratungstate unit {W4O16}8−, and four additional, external Mn2+ ions acting as linkers of the polyanions resulting in an extended network. | 137 |
| 81 | K7Li2Na27[αγαγ-P8W48O184{Cu(H2O)}2]·78H2O | (Cu2-αγαγ-P8W48) | The molar ratio of Cu2+ and P8W48, temperature, and reaction time were crucial for obtaining this compound. | 141 |
| 82 | K7.5Na17Cu2.425(WO2)1.325[γγγγ-P8W48O184{Cu(H2O)0.5}4]·102H2O | (Cu4-γγγγ-P8W48) | The molar ratio of Cu2+ and P8W48, temperature, and reaction time were crucial for obtaining this compound. | 141 |
| 83 | K7Li2Na19.5Cu1.75(WO2)[αγγγ-P8W48O184{Cu(H2O)}3]·72H2O | (Cu3-αγγγ-P8W48) | The molar ratio of Cu2+ and P8W48, temperature, and reaction time were crucial for obtaining this compound. | 141 |
| 84 | [(n-C4H9)4N]14H2[{M2(OH2)2}2{M(OH2)2}4P8W48O176(OCH3)8]·nH2O·mCH3CN | M8P8W48O176 (MII = Mn, Co, Ni, Cu, Zn) | Reaction of divalent 3d metal ions with α-P2W12 resulted in a partial transformation to the γ-P2W12 isomer, yielding a new type of α,γ,α,γ-type P8W48 ring, and the incorporation of eight metal ions MII. | 142 |
| 85 | Li8K9.5Ag21[H16P10W66O251]0.5[H14P9W63O235]0.5Cl2·50H2O and Li8K13Ag13[H12P8W51O196]·50H2O and Li10K12Ag4[H14P8W48O184]·170H2O | Ag21P9W63O235, Ag13P8W51O196, Ag4P8W48O148 | Heating P8W48 in the presence of Ag+ ions at a high concentration (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30). Ag13P8W51O196 forms in a similar procedure with a lower concentration of silver ions (1 30). Ag13P8W51O196 forms in a similar procedure with a lower concentration of silver ions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12) and at a lower temperature. The Ag4P8W48O148 formed at even lower concentration of silver ions at room temperature. 12) and at a lower temperature. The Ag4P8W48O148 formed at even lower concentration of silver ions at room temperature. |
147 |
| 86 | (C24PH20)17H37[Mn18P8W48O214]·16H2O·4CH3CN and (C16H36N)12H16[Mn20P8W48O216]·4C2H3N·C2Cl2H4 | Mn18P8W48O214, Mn20P8W48O216 | The Mn18P8W48O214 polyanion contains 18 Mn ions of oxidation state +2 or +3 in the cavity of P8W48 and the four P2W12 units were transformed from α- to γ-isomer in the course of the reaction. In the Mn20P8W48O216 ion, the 20 Mn ions have an oxidation state either +3 or +4 and are aligned at the inner rim of P8W48. | 143 |
| 87 | [(n-C4H9)4N]11H13[Cu4(H2O)4P8W48O176(OCH3)8]·28H2O·3CH3NO2, and [(n-C4H9)4N]14H2, [Cu8(H2O)12P8W48O176(OCH3)8]·24H2O·CH3CN, and [(n-C4H9)4N]14H2[Cu12(H2O)16P8W48O184]·4H2O, and [(n-C4H9)4N]16H8[Cu16(OH)16(H2O)4P8W48O184]·12H2O·C3H6O | Cu4P8W48, Cu8P8W48, Cu12P8W48, Cu16P8W48 | In Cu4P8W48 and Cu8P8W48 the two middle P2W12 units to which the copper(II) ions are connected, had transformed from from α- to γ-isomer with a 60° rotation of the PO4 hetero groups. In Cu12P8W48 and Cu16P8W48 the same γ,γ,γ,γ-type P8W48 framework is present, but the copper coordination geometry differs from each other. | 144 |
| 88 | [(n-C4H9)4N]17H[Ag30(P8W48O184)]·10DMF·30H2O | Ag30P8W48 | This nanocluster material has exposed silver surfaces and interfaces with metal oxides, and it is highly stable despite the exposed silver surfaces, acting as a catalytically active sites for the selective reduction of organic substrates using H2 under mild reaction conditions. | 145 |
| 89 | (Bu4N)16H8[Au8Ag26(P8W48O184)] | Au8Ag26P8W48 | The polyanion comprises {Au6} as well as {Ag6Au} clusters embedded in the cavity of P8W48. | 148 |
| 90 | (Me2NH2)13K7Na2Li10[{AsIII5O4(OH)3}2(P8W48O184)]·32H2O, K20Li12[(SbIIIOH)4(P8W48O184)]·52H2O, and (Me2NH2)8K6Na5Li5[(SbIIIOH)8(P8W48O184)]·65H2O | As10P8W48, Sb4P8W48, Sb8P8W48 | Ten AsIII ions or four/eight SbIII ions are grafted in the cavity of P8W48. | 149 |
| 91 | K22Li4[{FeIII8CeIII4O2(OH)12(H2O)8(PO4)2}(P8W48O184)]·106H2O | Fe8Ce4P8W48 | The polyanion comprises an iron(III)–cerium(III)–phosphato moiety {FeIII8CeIII4O2(OH)12(H2O)8(PO4)2} in the cavity of P8W48. | 150 |
6. Summary and outlook
Over the years, many tungstophosphates have been synthesized and structurally characterized. This review focuses on the structural family P2W12, P4W24, and P8W48 and their interaction with metal ions during the last 20 years emphasizing synthetic and structural aspects. We have discussed the formation and stability of these polyanions in different reaction conditions, including pH, temperature, solvent, concentration, counterions, and ionic strength. Although P2W12, P4W24, and P8W48 all contain the P2W12 unit as a key building block, their stability and reactivity with metal ions is vastly different, and hence, unique products are observed. While the monomeric P2W12 is the least stable amongst the three in solution, the cyclic P8W48 (comprising four P8W48 units) is the most stable.The reactivity, particularly the large, crown-shaped P8W48 with transition metal ions, has been systematically developed only since 2005. All three derivatives, P8W48, P4W24, and P2W12, are multilacunary, containing six or more vacant sites, which in principle can accommodate multiple transition metal ions. The literature in this area has expanded dramatically in the last two decades, reflecting the synthesis and property studies of a wide variety of compounds.
Subsequently, we discussed the versatile nature of these three polyanions, and their chemistry with an emphasis on their reactivity towards d and f-block metal ions, including mixed d/f derivatives, leading to discrete monomeric, dimeric, trimeric, and tetrameric structures, or extended solid state frameworks. Furthermore, gaining extra tungsten atoms in situ (arising from trace decomposition of the parent polyanion) provides additional degrees of structural flexibility. Other interesting features of P2W12, P4W24, and P8W48 are the multi-functional ways to accommodate high nuclearity transition metal oxo/hydroxo/aqua clusters according to their needs. Several novel and unexpected compounds have been isolated depending on the reaction conditions (e.g., type of transition metal, reaction temperature, solution pH, solvent, ionic strength, ratio and concentration of reagents, and countercations), which are all important parameters in synthetic POM chemistry. Several compounds have shown attractive properties in homogeneous and heterogeneous catalysis, as well as in magnetic studies. Researchers are still searching for new materials based on P2W12, P4W24, and P8W48, which are yet to be explored and examined and can benefit their associated properties and potential applications.
Data availability
This is a review paper and hence no new data is presented.Only scientific publications that can be accessed via the usual academic routes have been cited.
Conflicts of interest
The authors declare no competing conflicts of interest.Acknowledgements
S. M. thanks the Alexander von Humboldt Foundation for a renewed research stipend. A. B. thanks VNIT, Nagpur for research support. U. K. thanks the German Science Foundation (DFG) and Jacobs University (now Constructor University) for continuous support over the years. The polyanion structure figures were prepared using Diamond, version 3.2 (copyright Crystal Impact GbR).References
- J. J. Berzelius, Ann. Phys., 1826, 82, 369 CrossRef.
- M. T. Pope, Heteropoly and Isopoly Oxometalates, 1983 Search PubMed.
- D. A. Malikov, M. S. Milyuokova and B. F. Myasoedov, Radiokhimiya, 1993, 35, 105 CAS.
- M. T. Pope and A. Müller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34 CrossRef.
- A. Müller and S. Roy, in The Chemistry of Nanomaterials, Wiley-VCH Verlag GmbH & Co. KGaA, 2005, p. 452 Search PubMed.
- C. L. Hill, Chem. Rev., 1998, 98, 1 CrossRef CAS PubMed.
- D. L. Long, E. Burkholder and L. Cronin, Chem. Soc. Rev., 2007, 36, 105 RSC.
- D. L. Long, R. Tsunashima and L. Cronin, Angew. Chem., Int. Ed., 2010, 49, 1736 CrossRef CAS PubMed.
- L. Cronin and A. Müller, Chem. Soc. Rev., 2012, 41, 7333 RSC.
- M. I. Khan, J. Solid State Chem., 2000, 152, 105 CrossRef CAS.
- D. L. Long and L. Cronin, Chem. – Eur. J., 2006, 12, 3699 CrossRef PubMed.
- B. Hasenknopf, Front. Biosci.-Landmark, 2005, 10, 275 CrossRef CAS PubMed.
- T. Yamase, J. Mater. Chem., 2005, 15, 4773 RSC.
- H. Y. Ma, J. Peng, Z. G. Han, X. Yu and B. X. Dong, J. Solid State Chem., 2005, 178, 3735 CrossRef CAS.
- K. Nomiya, H. Torii, T. Hasegawa, Y. Nemoto, K. Nomura, K. Hashino, M. Uchida, Y. Kato, K. Shimizu and M. Oda, J. Inorg. Biochem., 2001, 86, 657 CrossRef CAS PubMed.
- R. J. Errington, S. S. Petkar, B. R. Horrocks, A. Houlton, L. H. Lie and S. N. Patole, Angew. Chem., Int. Ed., 2005, 44, 1254 CrossRef CAS PubMed.
- J. T. Rhule, C. L. Hill and D. A. Judd, Chem. Rev., 1998, 98, 327 CrossRef CAS PubMed.
- M. V. Vasylyev and R. Neumann, J. Am. Chem. Soc., 2004, 126, 884 CrossRef CAS PubMed.
- I. M. Mbomekalle, B. Keita, L. Nadjo, P. Berthet, K. I. Hardcastle, C. L. Hill and T. M. Anderson, Inorg. Chem., 2003, 42, 1163 CrossRef CAS PubMed.
- D. Volkmer, B. Bredenkotter, J. Tellenbroker, P. Kögerler, D. G. Kurth, P. Lehmann, H. Schnablegger, D. Schwahn, M. Piepenbrink and B. Krebs, J. Am. Chem. Soc., 2002, 124, 10489 CrossRef CAS PubMed.
- S. G. Mitchell, C. Streb, H. N. Miras, T. Boyd, D. L. Long and L. Cronin, Nat. Chem., 2010, 2, 308 CrossRef CAS PubMed.
- T. B. Liu, E. Diemann, H. L. Li, A. W. M. Dress and A. Müller, Nature, 2003, 426, 59 CrossRef CAS PubMed.
- G. Chaidogiannos, D. Velessiotis, P. Argitis, P. Koutsolelos, C. D. Diakoumakos, D. Tsamakis and N. Glezos, Microelectron. Eng., 2004, 73–4, 746 CrossRef.
- S. Q. Liu, D. Volkmer and D. G. Kurth, Anal. Chem., 2004, 76, 4579 CrossRef CAS PubMed.
- S. Q. Liu, D. G. Kurth and D. Volkmer, Chem. Commun., 2002, 976 RSC.
- E. Coronado, C. Gimenez-Saiz and C. J. Gomez-Garcia, Coord. Chem. Rev., 2005, 249, 1776 CrossRef CAS.
- L. Xu, E. B. Wang, Z. Li, D. G. Kurth, X. G. Du, H. Y. Zhang and C. Qin, New J. Chem., 2002, 26, 782 RSC.
- M. Luban, F. Borsa, S. Bud'ko, P. C. Canfield, S. Jun, J. K. Jung, P. Kögerler, D. Mentrup, A. Müller, R. Modler, D. Procissi, B. J. Suh and M. Torikachvili, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 054407 CrossRef.
- A. Müller, E. Krickemeyer, J. Meyer, H. Bögge, F. Peters, W. Plass, E. Diemann, S. Dillinger, F. Nonnenbruch, M. Randerath and C. Menke, Angew. Chem., Int. Ed. Engl., 1995, 34, 2122 CrossRef.
- A. Müller, E. Beckmann, H. Bögge, M. Schmidtmann and A. Dress, Angew. Chem., Int. Ed., 2002, 41, 1162 CrossRef.
- A. Mylonas, A. Hiskia and E. Papaconstantinou, J. Mol. Catal. A: Chem., 1996, 114, 191 CrossRef CAS.
- A. Hiskia, A. Troupis and E. Papaconstantinou, Int. J. Photoenergy, 2002, 4, 35 CrossRef CAS.
- E. Gkika, P. Kormali, S. Antonaraki, D. Dimoticali, E. Papaconstantinou and A. Hiskia, Int. J. Photoenergy, 2004, 6, 227 CrossRef CAS.
- A. Troupis, E. Gkika, A. Hiskia and E. Papaconstantinou, C. R. Chim., 2006, 9, 851 CrossRef CAS.
- P. Kormali, A. Troupis, T. Triantis, A. Hiskia and E. Papaconstantinou, Catal. Today, 2007, 124, 149 CrossRef CAS.
- A. F. Wells, Structural Inorganic Chemistry, Oxford University Press, 2012 Search PubMed.
- B. Dawson, Acta Crystallogr., 1953, 6, 113 CrossRef CAS.
- R. Strandberg, Acta Chem. Scand., Ser. A, 1975, 29, 350 CrossRef.
- H. d'Amour, Acta Crystallogr., Sect. B, 1976, 32, 729 CrossRef.
- R. Acerete, C. F. Hammer and L. C. W. Baker, J. Am. Chem. Soc., 1979, 101, 267 CrossRef CAS.
- R. Acerete, S. Harmalker, C. F. Hammer, M. T. Pope and L. C. W. Baker, J. Chem. Soc., Chem. Commun., 1979, 777 RSC.
- R. Contant, W. G. Klemperer and O. M. Yaghi, in Inorg Syn, John Wiley & Sons, Inc., 2007, p. 104 Search PubMed.
- R. Contant and A. Tézé, Inorg. Chem., 1985, 24, 4610 CrossRef CAS.
- F. Hussain, U. Kortz, B. Keita, L. Nadjo and M. T. Pope, Inorg. Chem., 2006, 45, 761 CrossRef CAS PubMed.
- L. Vila-Nadal, S. G. Mitchell, D. L. Long, A. Rodriguez-Fortea, X. Lopez, J. M. Poblet and L. Cronin, Dalton Trans., 2012, 41, 2264 RSC.
- R. Acerete, C. F. Hammer and L. C. W. Baker, Inorg. Chem., 1984, 23, 1478 CrossRef CAS.
- Sugiarto and M. Sadakane, Chem. – Eur. J., 2023, 29, e202301051 CrossRef CAS PubMed.
- S. Take, T. Minato and M. Sadakane, Chem. Lett., 2024, 53, upae118 CrossRef.
- R. Acerete, C. F. Hammer and L. C. W. Baker, J. Am. Chem. Soc., 1982, 104, 5384 CrossRef CAS.
- P. Roussel, G. Mather, B. Domenges, D. Groult and P. Labbe, Acta Crystallogr., Sect. B: Struct. Sci., 1998, 54, 365 CrossRef.
- T. Boyd, S. G. Mitchell, D. Gabb, D. L. Long and L. Cronin, Chem. – Eur. J., 2011, 17, 12010 CrossRef CAS PubMed.
- X. Xin and H. Lv, Sci. Sin.: Chim., 2020, 50, 1015 Search PubMed.
- D. A. Judd, Q. Chen, C. F. Campana and C. L. Hill, J. Am. Chem. Soc., 1997, 119, 5461 CrossRef CAS.
- R. Belghiche, R. Contant, Y. W. Lu, B. Keita, M. Abbessi, L. Nadjo and J. Mahuteau, Eur. J. Inorg. Chem., 2002, 1410 CrossRef CAS.
- J. E. Toth and F. C. Anson, J. Am. Chem. Soc., 1989, 111, 2444 CrossRef CAS.
- B. Keita, A. Belhouari, L. Nadjo and R. Contant, J. Electroanal. Chem., 1995, 381, 243 CrossRef.
- M. Sadakane and E. Steckhan, Chem. Rev., 1998, 98, 219 CrossRef CAS PubMed.
- B. Godin, Y.-G. Chen, J. Vaissermann, L. Ruhlmann, M. Verdaguer and P. Gouzerh, Angew. Chem., Int. Ed., 2005, 44, 3072 CrossRef CAS PubMed.
- B. Godin, J. Vaissermann, P. Herson, L. Ruhlmann, M. Verdaguer and P. Gouzerh, Chem. Commun., 2005, 5624 RSC.
- A. Ostuni and M. T. Pope, C. R. Acad. Sci., Ser IIc, 2000, 3, 199 CrossRef CAS.
- U. Kortz, J. Cluster Sci., 2003, 14, 205 CrossRef CAS.
- Z. M. Zhang, S. Yao, Y. F. Qi, Y. G. Li, Y. H. Wang and E. Wang, Dalton Trans., 2008, 3051 RSC.
- Y. H. Ren, Y. C. Hu, Y. C. Shan, Z. P. Kong, M. Gu, B. Yue and H. Y. He, Inorg. Chem. Commun., 2014, 40, 108 CrossRef CAS.
- G. S. Kim, H. D. Zeng, D. VanDerveer and C. L. Hill, Angew. Chem., Int. Ed., 1999, 38, 3205 CrossRef CAS PubMed.
- C. C. Li, S. X. Liu, S. J. Li, Y. Yang, H. Y. Jin and F. J. Ma, Eur. J. Inorg. Chem., 2012, 3229 CrossRef CAS.
- S. J. Li, S. X. Liu, N. N. Ma, Y. Q. Qiu, J. Miao, C. C. Li, Q. Tang and L. Xu, CrystEngComm, 2012, 14, 1397 RSC.
- D. D. Zhang, C. Zhang, P. T. Ma, B. S. Bassil, R. Al-Oweini, U. Kortz, J. P. Wang and J. Y. Niu, Inorg. Chem. Front., 2015, 2, 254 RSC.
- X. F. Yi, N. V. Izarova and P. Kögerler, Chem. Commun., 2018, 54, 2216 RSC.
- T. Iftikhar, N. V. Izarova and P. Kögerler, Inorg. Chem., 2024, 63, 99 CrossRef CAS PubMed.
- T. Iftikhar, N. V. Izarova, J. van Leusen and P. Kögerler, Chem. – Eur. J., 2021, 27, 13376 CrossRef CAS PubMed.
- Z. M. Zhang, S. Yao, Y. G. Li, Y. H. Wang, Y. F. Qi and E. B. Wang, Chem. Commun., 2008, 1650 RSC.
- S. Yao, Z. M. Zhang, Y. G. Li and E. B. Wang, Dalton Trans., 2010, 39, 3884 RSC.
- S. Yao, Z. M. Zhang, Y. G. Li, Y. Lu, E. B. Wang and Z. M. Su, Cryst. Growth Des., 2010, 10, 135 CrossRef CAS.
- J. P. Guo, Y. Q. Zhao, C. Zhang, P. T. Ma, D. D. Zhang, J. Y. Niu and J. P. Wang, Inorg. Chem. Commun., 2017, 75, 5 CrossRef CAS.
- L. C. Zhang, H. Xue, Z. M. Zhu, Z. M. Zhang, Y. G. Li and E. B. Wang, J. Cluster Sci., 2010, 21, 679 CrossRef CAS.
- S. G. Mitchell, T. Boyd, H. N. Miras, D. L. Long and L. Cronin, Inorg. Chem., 2011, 50, 136 CrossRef CAS PubMed.
- S. W. Chen, K. Boubekeur, P. Gouzerh and A. Proust, J. Mol. Struct., 2011, 994, 104 CrossRef CAS.
- D. D. Zhang, Z. J. Liang, S. Q. Xie, P. T. Ma, C. Zhang, J. P. Wang and J. Y. Niu, Inorg. Chem., 2014, 53, 9917 CrossRef CAS PubMed.
- W.-D. Liu, H.-T. Zhu, X. Zhang, F. Su, X.-J. Sang, X.-L. Zhang and L.-C. Zhang, Polyoxometalates, 2025, 4, 9140073 CrossRef.
- D. D. Zhang, F. Cao, P. T. Ma, C. Zhang, Y. Song, Z. J. Liang, X. J. Hu, J. P. Wang and J. Y. Niu, Chem. – Eur. J., 2015, 21, 17683 CrossRef CAS PubMed.
- T. Minato, K. Suzuki, K. Yamaguchi and N. Mizuno, Angew. Chem., Int. Ed., 2016, 55, 9630 CrossRef CAS PubMed.
- K. Suzuki, T. Minato, N. Tominaga, L. Okumo, K. Yonesato, N. Mizuno and K. Yamaguchi, Dalton Trans., 2019, 48, 7281 RSC.
- K. Wassermann, M. H. Dickman and M. T. Pope, Angew. Chem., Int. Ed. Engl., 1997, 36, 1445 CrossRef CAS.
- Q. H. Luo, R. C. Howell, J. Bartis, M. Dankova, W. D. Horrocks, A. L. Rheingold and L. C. Francesconi, Inorg. Chem., 2002, 41, 6112 CrossRef CAS PubMed.
- M. Sadakane, A. Ostuni and M. T. Pope, J. Chem. Soc., Dalton Trans., 2002, 63 RSC.
- Z. M. Zhang, Y. G. Li, Y. H. Wang, Y. F. Qi and E. B. Wang, Inorg. Chem., 2008, 47, 7615 CrossRef CAS PubMed.
- S. Yao, Z. M. Zhang, Y. G. Li and E. Wang, Dalton Trans., 2009, 1786 RSC.
- X. K. Fang, P. Kögerler, Y. Furukawa, M. Speldrich and M. Luban, Angew. Chem., Int. Ed., 2011, 50, 5212 CrossRef CAS PubMed.
- L. Huang, L. Cheng, W. H. Fang, S. S. Wang and G. Y. Yang, Eur. J. Inorg. Chem., 2013, 1693 CrossRef CAS.
- A. A. Shmakova, T. S. Sukhikh, V. V. Volchek, V. Yanshole, D. V. Stass, E. Y. Filatov, E. M. Glebov, P. A. Abramov and M. N. Sokolov, Inorg. Chim. Acta, 2020, 502, 119319 CrossRef CAS.
- J. Goura, B. S. Bassil, J. K. Bindra, I. A. Rutkowska, P. J. Kulesza, N. S. Dalal and U. Kortz, Chem. – Eur. J., 2020, 26, 15821 CrossRef CAS PubMed.
- S. S. Mal, M. H. Dickman and U. Kortz, Chem. – Eur. J., 2008, 14, 9851 CrossRef CAS PubMed.
- A. S. Assran, N. V. Izarova and U. Kortz, CrystEngComm, 2010, 12, 2684 RSC.
- A. Müller, M. T. Pope, A. M. Todea, H. Bögge, J. van Slageren, M. Dressel, P. Gouzerh, R. Thouvenot, B. Tsukerblat and A. Bell, Angew. Chem., Int. Ed., 2007, 46, 4477 CrossRef PubMed.
- T. Boyd, S. G. Mitchell, D. Gabb, D.-L. Long, Y.-F. Song and L. Cronin, J. Am. Chem. Soc., 2017, 139, 5930 CrossRef CAS PubMed.
- S. S. Mal and U. Kortz, Angew. Chem., Int. Ed., 2005, 44, 3777 CrossRef CAS PubMed.
- S. S. Mal, B. S. Bassil, M. Ibrahim, S. Nellutla, J. van Tol, N. S. Dalal, J. A. Fernandez, X. Lopez, J. M. Poblet, R. Ngo Biboum, B. Keita and U. Kortz, Inorg. Chem., 2009, 48, 11636 CrossRef CAS PubMed.
- D. Jabbour, B. Keita, L. Nadjo, U. Kortz and S. S. Mal, Electrochem. Commun., 2005, 7, 841 CrossRef CAS.
- B. Keita, L. Nadjo, R. Contant, M. Fournier and G. Hervé, France Pat, 89/1, 728, 1989 Search PubMed.
- M. S. Alam, V. Dremov, P. Müller, A. V. Postnikov, S. S. Mal, F. Hussain and U. Kortz, Inorg. Chem., 2006, 45, 2866 CrossRef CAS PubMed.
- G. Liu, T. B. Liu, S. S. Mal and U. Kortz, J. Am. Chem. Soc., 2006, 128, 10103 CrossRef CAS PubMed.
- G. Liu, T. B. Liu, S. S. Mal and U. Kortz, J. Am. Chem. Soc., 2007, 129, 2408 CrossRef CAS.
- A. Müller, E. Krickemeyer, H. Bögge, M. Schmidtmann and F. Peters, Angew. Chem., Int. Ed., 1998, 37, 3360 Search PubMed.
- A. Müller, S. K. Das, V. P. Fedin, E. Krickemeyer, C. Beugholt, H. BöggeBögge, M. Schmidtmann and B. Hauptfleisch, Z. Anorg. Allg. Chem., 1999, 625, 1187 CrossRef.
- R. Wang, Z. Zheng, F. W. Koknat, D. J. Marko, A. Müller, S. K. Das, E. Krickemeyer, C. Kuhlmann, B. Therrien, L. Plasseraud, G. Süss-Fink, A. D. Pasquale, X. Lei, T. P. Fehlner, E. L. Diz, S. Haak, E. Cariati, C. Dragonetti, E. Lucenti, D. Roberto, C. Y. Lee, H. Song, K. Lee, B. K. Park, J. T. Park, J. E. Hutchison, E. W. Foster, M. G. Warner, S. M. Reed and W. W. Weare, in Inorg Syn, John Wiley & Sons, Inc., 2004, p. 184 Search PubMed.
- B. L. Chen, H. J. Jiang, Y. Zhu, A. Cammers and J. P. Selegue, J. Am. Chem. Soc., 2005, 127, 4166 CrossRef CAS PubMed.
- A. Müller, H. Bögge, F. L. Sousa, M. Schmidtmann, D. G. Kurth, D. Volkmer, J. van Slageren, M. Dressel, M. L. Kistler and T. Liu, Small, 2007, 3, 986 CrossRef PubMed.
- M. L. Kistler, T. B. Liu, P. Gouzerh, A. M. Todea and A. Müller, Dalton Trans., 2009, 5094 RSC.
- C. Schaffer, A. Merca, H. Bögge, A. M. Todea, M. L. Kistler, T. B. Liu, R. Thouvenot, P. Gouzerh and A. Müller, Angew. Chem., Int. Ed., 2009, 48, 149 CrossRef PubMed.
- T. B. Liu, M. L. K. Langston, D. Li, J. M. Pigga, C. Pichon, A. M. Todea and A. Müller, Science, 2011, 331, 1590 CrossRef CAS PubMed.
- G. Liu and T. B. Liu, Langmuir, 2005, 21, 2713 CrossRef CAS PubMed.
- Y. Y. Bao, L. H. Bi, L. X. Wu, S. S. Mal and U. Kortz, Langmuir, 2009, 25, 13000 CrossRef CAS PubMed.
- J. M. Pigga, M. L. Kistler, C. Y. Shew, M. R. Antonio and T. B. Liu, Angew. Chem., Int. Ed., 2009, 48, 6538 CrossRef CAS PubMed.
- L. F. Chen, J. C. Hu, S. S. Mal, U. Kortz, H. Jaensch, G. Mathys and R. M. Richards, Chem. – Eur. J., 2009, 15, 7490 CrossRef CAS PubMed.
- P. Mialane, A. Dolbecq and F. SécheresseSécheresse, Chem. Commun., 2006, 3477 RSC.
- C. Pichon, P. Mialane, A. Dolbecq, J. Marrot, E. Riviere, B. Keita, L. Nadjo and F. Sécheresse, Inorg. Chem., 2007, 46, 5292 CrossRef CAS PubMed.
- S. S. Mal, M. H. Dickman, U. Kortz, A. M. Todea, A. Merca, H. Bögge, T. Glaser, A. Müller, S. Nellutla, N. Kaur, J. van Tol, N. S. Dalal, B. Keita and L. Nadjo, Chem. – Eur. J., 2008, 14, 1186 CrossRef CAS PubMed.
- A. H. Ismail, B. S. Bassil, G. H. Yassin, B. Keita and U. Kortz, Chem. – Eur. J., 2012, 18, 6163 CrossRef CAS PubMed.
- S. S. Mal, M. H. Dickman, U. Kortz, A. M. Todea, A. Merca, H. Bögge, T. Glaser, A. Müller, S. Nellutla, N. Kaur, J. van Tol, N. S. Dalal, B. Keita and L. Nadjo, Chem. – Eur. J., 2008, 14, 1186 CrossRef CAS PubMed.
- S. S. Mal, Ph.D. thesis, Jacobs University, Bremen, Germany, 2008, mentor U. Kortz.
- B. S. Bassil, M. Ibrahim, S. S. Mal, A. Suchopar, R. Ngo Biboum, B. Keita, L. Nadjo, S. Nellutla, J. van Tol, N. S. Dalal and U. Kortz, Inorg. Chem., 2010, 49, 4949 CrossRef CAS PubMed.
- S. G. Mitchell, D. Gabb, C. Ritchie, N. Hazel, D. L. Long and L. Cronin, CrystEngComm, 2009, 11, 36 RSC.
- Y. Q. Jiao, C. Qin, X. L. Wang, C. G. Wang, C. Y. Sun, H. N. Wang, K. Z. Shao and Z. M. Su, Chem. – Asian J., 2014, 9, 470 CrossRef CAS PubMed.
- F. L. Sousa, H. Bögge, A. Merca, P. Gouzerh, R. Thouvenot and A. Müller, Chem. Commun., 2009, 7491 RSC.
- Y. Y. Bao, B. Wang, R. Q. Meng, L. H. Bi and L. X. Wu, CrystEngComm, 2012, 14, 1550 RSC.
- V. S. Korenev, S. Floquet, J. Marrot, M. Haouas, I. M. Mbomekalle, F. Taulelle, M. N. Sokolov, V. P. Fedin and E. Cadot, Inorg. Chem., 2012, 51, 2349 CrossRef CAS PubMed.
- S. S. Mal, N. H. Nsouli, M. H. Dickman and U. Kortz, Dalton Trans., 2007, 2627 RSC.
- M. Zimmermann, N. Belai, R. J. Butcher, M. T. Pope, E. V. Chubarova, M. H. Dickman and U. Kortz, Inorg. Chem., 2007, 46, 1737 Search PubMed.
- N. V. Izarova, L. Klass, P. de Oliveira, I. M. Mbomekalle, V. Peters, F. Haarmann and P. Kögerler, Dalton Trans., 2015, 44, 19200 RSC.
- B. Kamenar and D. Grdenic, J. Chem. Soc. (Resumed), 1961, 3954 RSC.
- X. F. Yi, N. V. Izarova and P. Kögerler, Inorg. Chem., 2017, 56, 13822 CrossRef CAS PubMed.
- P. Yang, M. Alsufyani, A. H. Emwas, C. Q. Chen and N. M. Khashab, Angew. Chem., Int. Ed., 2018, 57, 13046 CrossRef CAS PubMed.
- K. Y. Wang, S. Zhang, D. Ding, T. Ma, U. Kortz and C. Wang, Eur. J. Inorg. Chem., 2019, 512 CrossRef CAS.
- A. A. Shmakova, V. V. Volchek, V. Yanshole, N. B. Kompankov, N. P. Martin, M. Nyman, P. A. Abramov and M. N. Sokolov, New J. Chem., 2019, 43, 9943 RSC.
- M. Dufaye, S. Duval, G. Stoclet, X. Trivelli, M. Huve, A. Moissette and T. Loiseau, Inorg. Chem., 2019, 58, 1091 CrossRef CAS PubMed.
- J. Goura, A. Sundar, B. S. Bassil, G. Ćirić-Marjanović, D. Bajuk-Bogdanović and U. Kortz, Inorg. Chem., 2020, 59, 16789 CrossRef CAS PubMed.
- M. Ibrahim, I. M. Mbomekalle, P. De Oliveira, G. E. Kostakis and C. Anson, Dalton Trans., 2019, 48, 15545 RSC.
- I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B:Struct. Sci., 1985, 41, 244 CrossRef.
- N. E. Brese and M. O'Keeffe, Acta Crystallogr., Sect. B:Struct. Sci., 1991, 47, 192 CrossRef.
- W. Liu and H. H. Thorp, Inorg. Chem., 1993, 32, 4102 CrossRef CAS.
- X. Yi, N. V. Izarova, T. Iftikhar, J. van Leusen and P. Kögerler, Inorg. Chem., 2019, 58, 9378 CrossRef CAS PubMed.
- S. Sasaki, K. Yonesato, N. Mizuno, K. Yamaguchi and K. Suzuki, Inorg. Chem., 2019, 58, 7722 CrossRef CAS PubMed.
- K. Sato, K. Yonesato, T. Yatabe, K. Yamaguchi and K. Suzuki, Chem. – Eur. J., 2022, 28, e202104051 CrossRef CAS PubMed.
- Y. Koizumi, K. Yonesato, K. Yamaguchi and K. Suzuki, Inorg. Chem., 2022, 61, 9841 CrossRef CAS PubMed.
- K. Yonesato, D. Yanai, S. Yamazoe, D. Yokogawa, T. Kikuchi, K. Yamaguchi and K. Suzuki, Nat. Chem., 2023, 15, 940 CrossRef CAS PubMed.
- Y. Koizumi, K. Yonesato, S. Kikkawa, S. Yamazoe, K. Yamaguchi and K. Suzuki, J. Am. Chem. Soc., 2024, 146, 14610 CrossRef CAS PubMed.
- C.-H. Zhan, Q. Zheng, D.-L. Long, L. Vilà-Nadal and L. Cronin, Angew. Chem., Int. Ed., 2019, 58, 17282 Search PubMed.
- M. Kamachi, K. Yonesato, T. Okazaki, D. Yanai, S. Kikkawa, S. Yamazoe, R. Ishikawa, N. Shibata, Y. Ikuhara, K. Yamaguchi and K. Suzuki, Angew. Chem., Int. Ed., 2024, 63, e202408358 Search PubMed.
- Y. Niu, Y. Ding, H. Sheng, S. Sun, C. Chen, J. Du, H.-Y. Zang and P. Yang, Inorg. Chem., 2022, 61, 21024 CrossRef CAS PubMed.
- H.-X. Sheng, B.-Y. Lin, C.-Q. Chen, J. Du and P. Yang, Polyoxometalates, 2024, 3, 9140060 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |



