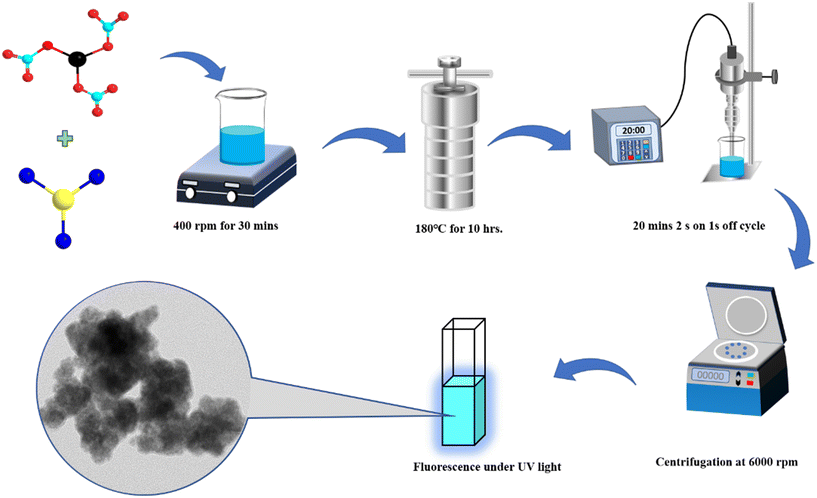
Synthesis of two-dimensional bismuth molybdenum oxide (2D-BMO) nanosheets and their application as fluorescent probes for the detection of explosive nitroaromatic compounds†
Shams Ur
Rehman
a,
Sivakumar Musuvadhi
Babulal
a,
Muhammad
Mustafa
a and
Hui-Fen
Wu
 *abcdef
*abcdef
aDepartment of Chemistry, National Sun Yat-Sen University, Kaohsiung, 70, Lien-Hai Road, Kaohsiung, 80424, Taiwan. E-mail: hwu@faculty.nsysu.edu.tw; Fax: +886 7 5253909; Tel: +886 7 5252000 3955
bSchool of Pharmacy, College of Pharmacy, Kaohsiung Medical University, Kaohsiung, 807, Taiwan
cInstitute of Medical Science and Technology, National Sun Yat-Sen University, Kaohsiung, 80424, Taiwan
dSchool of Medicine, College of Medicine, National Sun Yat-Sen University, Kaohsiung, 80424, Taiwan
eInstitute of Precision Medicine, National Sun Yat-Sen University, Kaohsiung, 80424, Taiwan
fInstitute of BioPharmaceutical Sciences, National Sun Yat-Sen University, 80424, Taiwan
First published on 9th October 2024
Abstract
2D-BMO fluorescent nanosheets were synthesized using a solvothermal method followed by probe sonication. These nanosheets were then employed as fluorescent probes for detecting nitroaromatic compounds picric acid (PA) and 2,4-dinitrophenylhydrazine (2,4-DNPH), which have recently garnered significant attention due to their explosive nature and environmental impact. The fluorescent nanoplatform exhibited stable fluorescence emission at a wavelength of 400 nm (λem) when excited at 340 nm (λex). The fluorescence quenching response towards PA and 2,4-DNPH was assessed within a concentration range of 50 to 2000 nM, showing linear responses within the ranges of 50–1100 nM and 50–1400 nM, respectively. The limits of detection were determined to be 2.21 nM for PA and 2.30 nM for 2,4-DNPH, with corresponding R2 values of 0.999 and 0.994. The interaction between the 2D-BMO nanosheets and nitroaromatics was the synergistic combination of the FRET, IFE, and electrostatic interaction, which was studied and explained by UV-visible absorption spectroscopy and zeta potential analysis. This 2D-BMO fluorescent sensor could pave the way for replacing traditional dyes that pose significant harm to the environment in the foreseeable future.
Environmental significanceFluorescence-based nanosensors are considered low-cost, fast, and environmentally friendly for monitoring environmental pollution. We have prepared 2D-BMO fluorescent nanosheets and utilized them as fluorescent probes for the detection and monitoring of PA and 2,4-DNPH. The widespread application of PA and 2,4-DNPH in producing rocket fuel, fireworks, and dyes results in the release of significant quantities of NO2 pollutants into the surrounding water and soil. PA's structural properties contribute to its slow natural degradation rate, while its potent biological toxicity can induce headaches, weakness, anemia, and liver damage in humans. Similarly, prolonged exposure to 2,4-DNPH can result in liver issues, skin disorders, and disruptions to the central nervous and cardiovascular systems. |
1. Introduction
PA and 2,4-DNPH are common nitroaromatic compounds recognized as significant environmental pollutants and hazardous explosives posing threats to homeland security. Their widespread application in producing rocket fuel, fireworks, and dyes results in the release of significant quantities of NO2 pollutants into the surrounding water and soil.1–4 Trinitrophenol's structural properties contribute to its slow natural degradation rate, while its potent biological toxicity can induce headaches, weakness, anemia, and liver damage in humans.4–7 Similarly, 2,4-DNPH exhibits potential carcinogenic and mutagenic effects, making it a hazardous substance due to its highly toxic impact on cellular DNA. Prolonged exposure to 2,4-DNPH can result in liver issues, skin disorders, and disruptions to the central nervous and cardiovascular systems.8–11 The potent explosive capabilities and environmental pollution associated with PA/2,4-DNPH11–15 reveal the urgent need for rapid, practical, and reliable detection methods, critical for ensuring societal stability and environmental protection.Several detection methods have been investigated for identifying nitroaromatic compounds, including gas chromatography-mass spectrometry (GC/MS),16 ion mobility spectroscopy,17 surface-enhanced Raman spectroscopy (SERS),18 and electrochemical detectors.19 However, these methods often entail expensive equipment, intricate pre-processing, lower selectivity, and prolonged testing times, limiting their practical applications.20,21 In contrast, fluorescent probes offer many advantages such as low cost, rapid response, simplicity, high sensitivity, and excellent selectivity, making them an ideal tool for detection purposes, and are widely adopted in the field of chemistry.22
2D nanomaterials, composed of just one or a few atomic layers, have attracted considerable interest due to their wide-ranging applications across fields such as electronics, optics, medicine, magnetics, catalysis, and sensing. Their appeal stems from their nanoscale dimensions, customizable thickness, robust quantum confinement, significant specific surface area, optical clarity, mechanical pliability, and abundant unmasked surface atoms with exposed active sites.23–29 Graphene, a pioneering single-atom nanomaterial, has achieved remarkable success, prompting the exploration of novel ultrathin 2D nanomaterials surpassing graphene. These materials are poised to broaden our horizons in engineering and design possibilities.30–32
The synthesis of nanomaterials and their properties are greatly influenced by the combination of metals and their oxygen stoichiometry. Metal oxides with MxMyOz nanostructures exhibit superior properties compared to single-metal oxides (MxOy) because they contain two or more metal atoms, offering a greater range of oxidation states.33 Moreover, 2D transition metal oxide (2D-TMO) and bimetallic oxide nanosheets represent a significant category of 2D materials with adjustable chromatic properties, making them suitable candidates for a variety of applications within the 2D material realm.34–38 Recently, researchers have turned their attention to 2D-TMO nanosheets featuring oxygen defects and a combination of metals for a synergistic effect, due to their expanded surface area, which serves as active sites for bio-molecules, and their optical attributes. Through defect engineering, the physical and optoelectronic behaviors of 2D-TMO nanosheets can be modified, leading to their utilization in diverse fields such as catalysis, energy, and notably, sensor technology.39–41 Studies have shown that oxygen defects in metal oxides can enhance their nanosensing capabilities. Moreover, the choice of a suitable synthesis method, synthesis media, surface modification, and selective doping can enhance the fluorimetric biosensing properties of TMOs.42 The hydrothermal chemical route is a highly effective method for preparing high-quality nanostructures due to its numerous advantages, including high crystallinity, increased yield, ease of processing, precise control, minimal pollution, and low energy consumption.43 Our research focuses on the development of fluorescent 2D-TMOD nanoprobes and their utilization as chemosensors and biosensors for detecting various environmental and disease biomarkers.40,41,44,45
Herein, we synthesized two-dimensional bismuth molybdenum oxide (Bi–MoO3) nanosheets by a solvothermal method followed by probe sonication. The prepared 2D-BMO nanosheets have a stable fluorescence emission at λem 400 nm at an excitation λex of 340 nm; the fluorescence characteristics of the nanosheets are due to the synergistic effect of bimetallic and oxygen defects. Subsequently, the fluorescent 2D-BMO was used as a low-cost, label-free, and rapid biosensor for the detection of nitroaromatic compounds PA and 2,4-DNPH. The fluorescence of 2D-BMO was turned off with increasing PA and 2,4-DNPH concentrations. The 2D-BMO nanosheets exhibited very sensitive and specific quenching behaviors towards PA and 2,4-DNPH. The limit of detection (LOD) calculated for PA was 2.21 nM, while the LOD for 2,4-DNPH was 2.30 nM. The PA and 2,4-DNPH detection experiments were also performed in the environmental sample (river and lake water) to investigate the practicability of our prepared chemosensor.
2. Materials and methods
2.1. Materials
Molybdenum trichloride (MoCl3, 98 + 0%) was purchased from Alfa Aesar, United States. Bismuth(III) nitrate pentahydrate (Bi(NO3)3·5H2O, 98.0%) and tetrahydrofuran (C4H8O, 99.9%) were obtained from Sigma Aldrich, United States and all the obtained chemicals were utilized without further purification.2.2. Synthesis of 2D-BMO nanosheets
A solution was prepared by dissolving 0.0972 grams of bismuth(III) nitrate pentahydrate in 25 mL of THF with continuous stirring. Subsequently, 0.02 grams of molybdenum trichloride was added and continuously stirred for 30 minutes to achieve a homogeneous mixture. The solution was subsequently transferred to a Teflon-lined autoclave and kept in an oven at 180 °C for 10 hours. Afterwards, the autoclave was cooled to room temperature. The mixture was subjected to 20 minutes of probe sonication, with intervals of 2 seconds on and 1 second off, followed by centrifugation twice. The supernatant was utilized as a fluorescent probe for detecting nitroaromatic compounds. The synthesis scheme is given in Fig. 1.3. Results and discussion
3.1. Characterization of the 2D nanosheets
The morphology and structural characteristics of the 2D-BMO nanosheets were analyzed using transmission electron microscopy (TEM). A nanosheet morphology is demonstrated by 2D-BMO as depicted in the TEM image in Fig. 2a and S1a,† which show an average size of around 150 nm with a thin layers structure. An atomic-level investigation was conducted using high-resolution TEM (HRTEM), revealing various fringe patterns on crystalline planes and porous structures depicted in Fig. 2b and S1b,† respectively. The d-spacing of the fringe pattern on the 2D-BMO nanosheets was calculated using ImageJ software from the HRTEM image; the calculated marked d-spacing values were 0.33 nm and 0.32 nm, corresponding to Bi and MoO3, respectively. The diffraction patterns of 2D-BMO were investigated with selected area electron diffraction (SAED) as given in Fig. 2c, which demonstrates the polycrystalline nature of the nanosheets. The elemental analysis of the 2D-BMO nanosheets was performed by utilizing High-Angle Annular Dark-Field Scanning Transmission Electron Microscopy (HAADF-STEM) and is depicted in Fig. 2d, which demonstrates that all the three elements Bi, Mo, and O are homogeneously distributed in the nanosheets, where the Bi element is shown by red color, Mo by green color and O by blue color. Furthermore, the elemental composition in the nanosheets was evaluated by Energy Dispersive X-Ray Spectroscopy (EDX) depicted in Fig. S2a.† Dynamic Light Scattering (DLS) was utilized to measure the average size of the nanosheets as shown in Fig. S2b,† which supported the TEM images and demonstrated an average size of 150 nm. All these characterization methods confirm the successful synthesis of 2D-BMO and its polycrystalline nature and nanosheet morphology.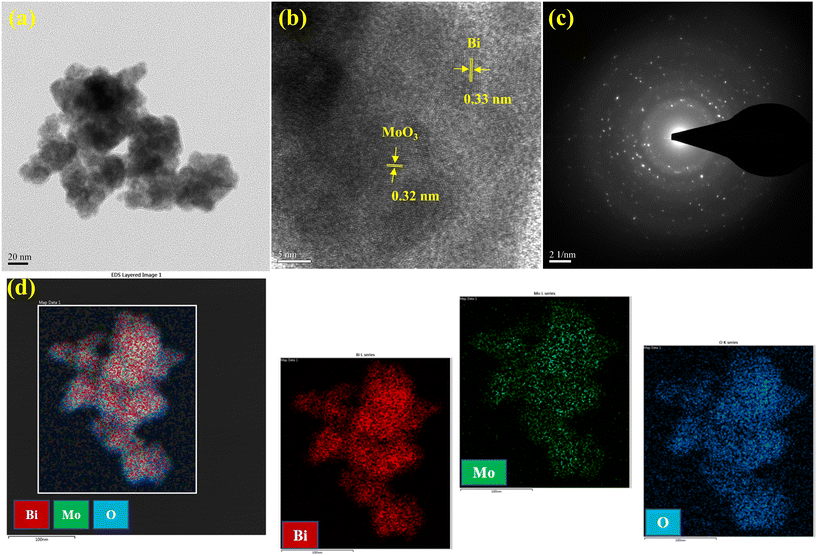 | ||
| Fig. 2 (a) TEM image, (b) HRTEM image, (c) SAED image, and (d) elemental mapping of 2D-BMO nanosheets. | ||
To further investigate the 2D nanosheet morphology of the 2D-BMO nanosheets, AFM characterization was performed. Fig. 3a shows the AFM topography of the 2D-BMO nanosheets, which demonstrated the small size of the nanosheets with an average diameter of 150 nm, while Fig. 3b shows the 3D image of the same scanned area of the nanosheets. In Fig. 3c, the height profile of the AFM image for 2D-BMO is given, which indicates the thickness of the 2D nanosheets, and the observed thickness is 25 nm. All these results support the TEM and DLS characterization of 2D-BMO, regarding its 2D nanosheet morphology.
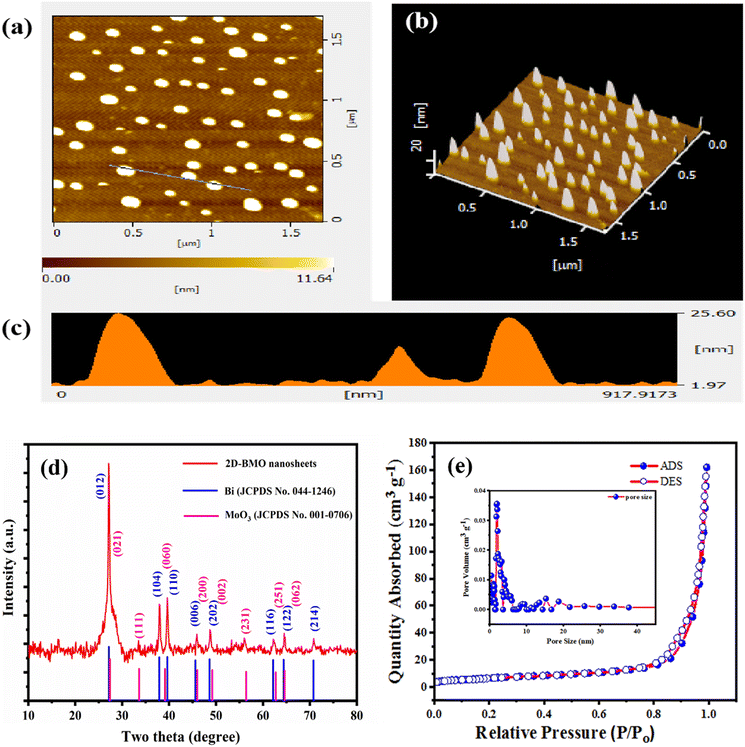 | ||
| Fig. 3 (a) AFM topography, (b) AFM 3D image, (c) height profile, (d) XRD spectra and (e) BET surface analysis and pore size of 2D-BMO nanosheets. | ||
The crystallinity of the 2D-BMO nanosheets was further investigated by XRD analysis (Fig. 3d), which revealed the well-crystalline structure of the 2D-BMO nanosheets. The XRD diffraction peaks of Bi at 27.16°, 37.9°, 39.6°, 45.6°, 48.6°, 62.17°, 64.4°, and 70.76° well matched with the (012), (104), (110), (006), (202), (116), (122), and (214) crystal planes respectively with reference JCPDS No. 044-1246, and the diffraction peaks of MoO3 at 27.3°, 33.6°, 39.13°, 46.0°, 49.2°, 56.4°, 62.7°, and 64.6° matched with the (021), (111), (060), (200), (002), (231), (251), and (062) crystal planes respectively with the reference JCPDS No. 001-0706, which represent the orthorhombic phase of Bi, and MoO3 respectively.46,47 Moreover, the XRD results support the HRTEM fringe pattern d-spacing for both Bi and MoO3 present in the 2D-BMO nanosheets.
The porosities and specific surface areas of the 2D-BMO nanosheets were investigated by N2 adsorption/desorption analysis as shown in Fig. 3e. The isothermal curve of the 2D-BMO nanosheets showed type II isotherms, which experienced rapid N2 uptake at higher pressure suggesting the existence of micropores. The specific Brunauer–Emmett–Teller (BET) surface area observed was 24 m2 g−1 for the 2D-BMO nanosheets with a pore volume of 0.20 cm3 g−1. The pore sizes of the nanosheets were investigated through nonlocal density functional theory (NLDFT). This revealed that 2D-BMO has two micropores at 0.6 nm and 1.8 nm as shown in the inset in Fig. 3b. The porous structure of the 2D-BMO nanosheets, with their significant surface area, provides more catalytic active sites48 and enhances the adsorption of PA and 2,4-DNPH, which subsequently quenches the fluorescence of the 2D-BMO nanosheets.
To obtain a deeper understanding of the surface chemical composition of the microporous 2D-BMO nanosheet samples, X-ray photoelectron spectroscopy (XPS) characterization was carried out, as illustrated in the survey scan shown in Fig. 4a, which demonstrated that Bi, Mo, and O are present in the nanosheets; there is one small peak of carbon C 1s in the survey scan which may be from the outside environment or the organic solvent (THF) utilized for the synthesis. The high resolution XPS spectra of Bi 4f are given in Fig. 4b, which splits into two peaks Bi 4f7/2 and Bi 4f5/2 at a binding energy of 157.6 and 162.9 eV, respectively. The relatively low binding energy of Bi 4f indicates the lower oxidation state of Bi(3−x)+ due to localized electrons trapped in oxygen vacancies.49–51 The high-resolution XPS spectra of Mo 3d in Fig. 4c show two characteristic peaks of Mo6+ 3d5/2 and Mo6+ 3d3/2 at a binding energy of 231.8 eV and 235.0 eV, respectively.51 The high-resolution XPS spectra of O 1s in Fig. 4d exhibit a characteristic peak at 529.8 eV, which further split into two peaks at 529.7 eV and 531.0 eV, which indicates the lattice oxygen and oxygen deficiency, respectively.50–52
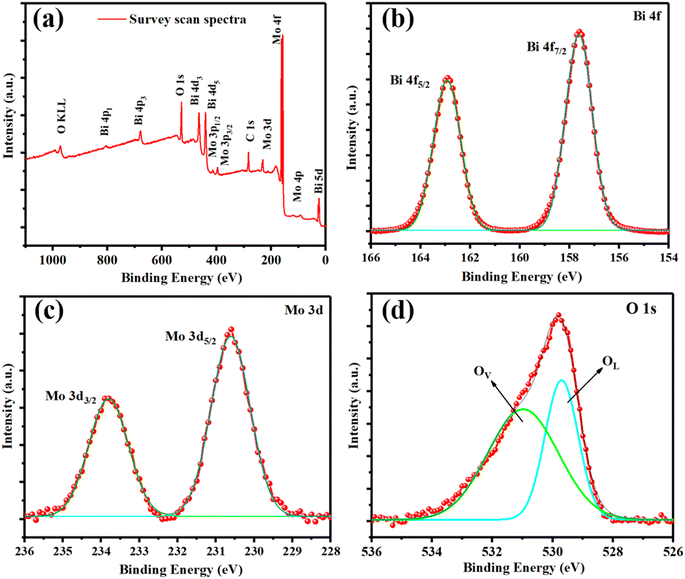 | ||
| Fig. 4 (a) XPS survey scan and (b) deconvoluted Bi 4f, (c) Mo 3d, and (d) O 1s spectra of 2D-BMO nanosheets. | ||
3.2. Optical properties of 2D-BMO nanosheets
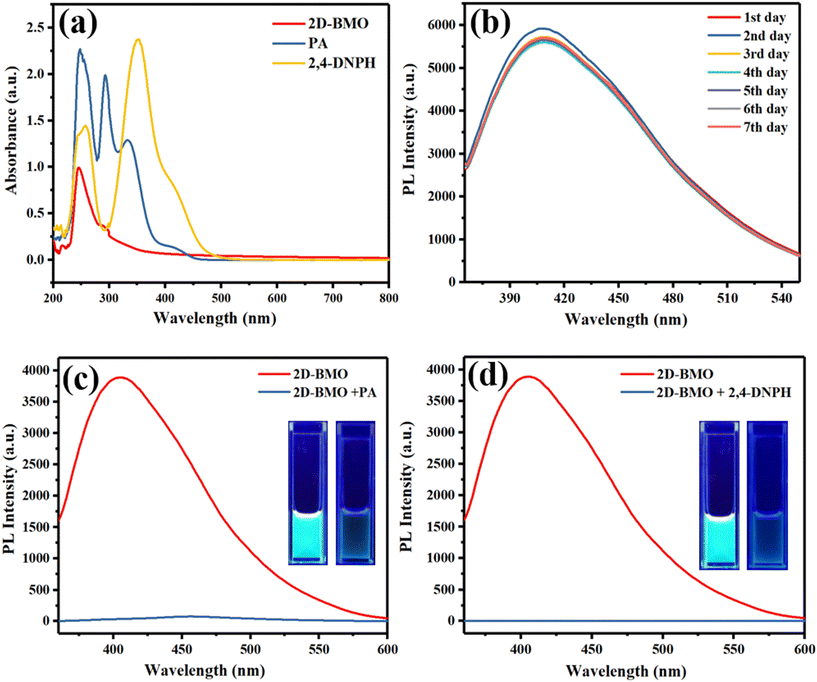
The initial verification of the fluorescent 2D-BMO nanosheets was evaluated by recording their UV-visible absorption spectra as depicted in Fig. 5a, which demonstrated the successful synthesis of the 2D-BMO nanosheets. The sharp peak at 247 nm represents the Bi while the broad peak starting from 250 nm to 400 nm represents the MoO3 of the 2D-BMO nanosheets. The UV-visible absorption spectra of the detected analytes are also provided in Fig. 5a. Thereafter, the fluorescence properties were examined by photoluminescence spectrometry (PL). The PL spectra demonstrated that the synthesized 2D-BMO nanosheets have excellent fluorescence, which were optimized at different excitation wavelengths to obtain the optimized emission intensity. The recorded emission at different excitation wavelengths showed that the intensity enhances from 310 to 340 nm and after that, it starts decreasing as depicted in Fig. S3a.† The excitation wavelength at 340 nm was chosen as the optimized excitation wavelength (340 nm λem) which shows a bluish-green emission spectrum at 400 nm (400 nm λem) on a UV lamp as shown in Fig. S3b† inset. The fluorescence quantum yield of 2D-BMO was calculated, which was around 42.93% by the formula given in the ESI† section S1, where quinine sulfate was utilized as the standard material. Furthermore, the fluorescence stability of 2D-BMO was continuously checked for seven days as given in Fig. 5b, which demonstrated very stable fluorescence for seven days.The synthesized 2D-BMO nanosheets were utilized as a nanosensing platform for the detection of PA and 2,4-DNPH. By the addition of PA to 2D-BMO, the bluish-green fluorescence was quenched as shown in Fig. 5c; in the inset, the two figures represent the bluish-green fluorescence of the 2D-BMO nanosheets on the UV lamp before the addition of PA and after the addition of PA. Similarly, by the addition of 2,4-DNPH, the fluorescence of the 2D-BMO nanosheets was quenched as shown in Fig. 5d, while the visualized quenching of the bluish-green fluorescence of the 2D-BMO nanosheets is given in the inset on the UV lamp.
3.3. Probing the sensing performance of the 2D-BMO nanosheets
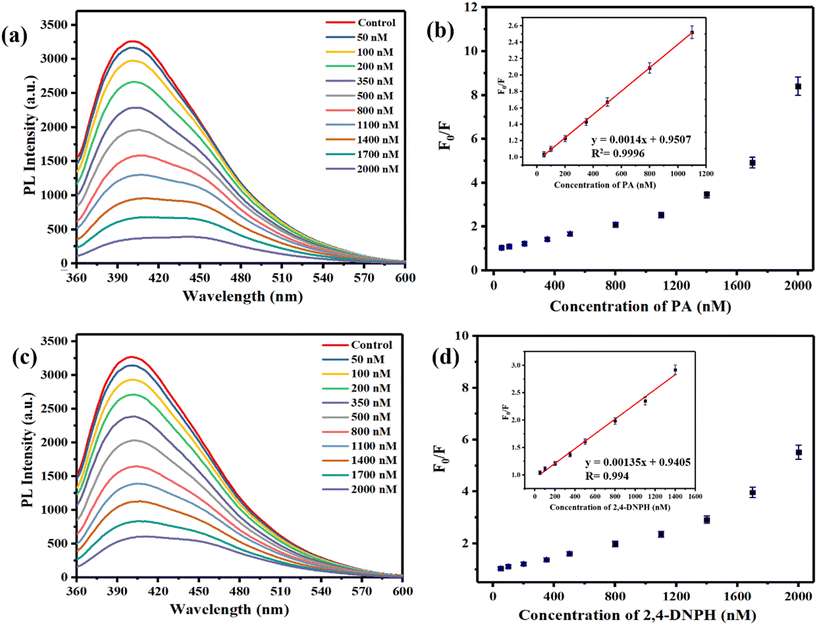
To evaluate the performance of the 2D-BMO nanosheets against PA and perform quantification, the fluorescence intensity was monitored against the PA concentration from 50 nM to 2000 nM keeping the 2D-BMO nanosheet concentration constant, as shown in Fig. 6a. It was shown that with the addition of the PA solution to the 2D-BMO nanosheets, fluorescence quenching occurred, which increased with the increase of the PA concentration. To investigate the effectiveness of quenching, we employed the Stern–Volmer equation: F0/F = 1 + Ksv [Q]. Here, F0 and F indicate the fluorescence intensities before and after introducing the quencher, respectively, Ksv is the quenching constant, and [Q] indicates the quencher concentration.22 The Ksv values for PA and 2,4-DNPH are calculated and given in the ESI† S4. To calculate the detection limit, we used the formula LOD = 3.3 SD/S, where SD represents the standard deviation and S is the slope given in the ESI† S3, based on the intensity–concentration data shown in Fig. 6a. The detection limit was determined to be 2.21 nM. The linear range observed for PA detection was 50 nM to 1100 nM which is given in the inset of Fig. 6b. Following the same protocol utilized for PA quantification, the interaction between 2,4-DNPH and 2D-BMO nanosheets proceeded. When the concentration of 2,4-DNPH was increased from 50 nM to 2000 nM, the intensity of fluorescence decreased accordingly as shown in Fig. 6c, which follows a linear range from 50 nM to 1400 nM with an R2 value of 0.993 as shown in Fig. 6d and S3† and the LOD observed for 2,4-DNPH was 2.30 nM. The Ksv values for PA and 2,4-DNPH are calculated and given in the ESI† S4. The limit of detection calculated was almost the same as that of PA, but the linear range was higher than that of the PA quantification study as given in the inset of Fig. 6d. Therefore, the performance of our proposed fluorescent sensor was compared with previously published literature chemosensors for the detection of PA and 2,4-DNPH, as detailed in Table S1 of the ESI.†3.4. Mechanism of the fluorescence quenching
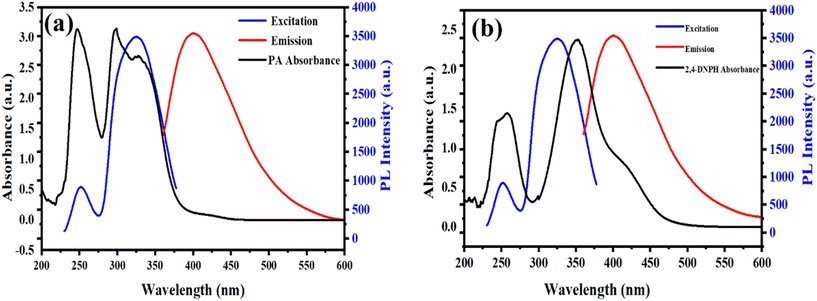
The potential mechanisms involving the 2D-BMO nanosheets and the nitroaromatics (PA and 2,4-DNPH) are outlined as follows: (1) the inner filter effect (IFE) or fluorescence resonance energy transfer (FRET) occurring between the 2D-BMO nanosheets and PA and 2,4-DNPH; (2) the formation of complexes by a donor–acceptor charge-transfer relationship between the catalyst (the 2D-BMO nanosheets) and analytes (PA and 2,4-DNPH); (3) molecular interactions, including π–π, hydrogen bonding and electrostatic interactions, between the 2D-BMO nanosheets and PA and 2,4-DNPH.In both FRET and IFE processes, it is typically essential to have a significant spectral overlap between the excitation and emission spectra of the fluorescent agent and the absorption spectrum of the quencher. In Fig. 7a, the PA UV-visible absorption spectra demonstrated a broad absorption range from 280 to 460 nm, which covered the whole excitation range and most of the emission range of the 2D-BMO nanosheets. Similarly, in Fig. 7b, the UV-visible absorption spectra of 2,4-DNPH completely overlap the excitation and emission range of the 2D-BMO nanosheets. The UV-visible absorption spectra of both nitroaromatics PA and 2,4-DNPH overlap well and they quenched the fluorescence of the 2D-BMO nanosheets possibly by the FRET and IFE combination. The UV-visible absorption spectra of PA and 2,4-DNPH were similar after the addition of the 2D-BMO nanosheets; there was a minor change in the intensity as shown in Fig. S4.† This observation suggests that the interaction between PA, 2,4-DNPH, and the 2D-BMO nanosheets was feeble, and no Meisenheimer complex formation occurred between them, which excluded the chances of the electron transfer mechanism.53,54 Moreover, the fluorescence quenching can be explained by the electrostatic interaction between the 2D-BMO nanosheets and PA and 2,4-DNPH. The 2D-BMO nanosheets have an oxygen vacancy-rich surface area, while PA and 2,4-DNPH have electron deficient –OH and –NO2 functional groups on the benzene ring, which create a strong electrostatic interaction between them. The electrostatic interaction between the 2D-BMO nanosheets was studied by zeta potential, AFM and DLS; the 2D-BMO nanosheets show a zeta potential value of −14.3 mV, while by adding PA and 2,4-DNPH, the zeta values changed into 2.14 mV and 1.34 mV respectively as given in Fig. S5.† The AFM characterization for 2D-BMO before and after the addition of PA and 2,4-DNPH was performed as given in Fig. 3(a–c) and S6.† The thickness (height profile of the AFM image) of the 2D-BMO nanosheets increased to 47 nm and 39.57 nm respectively after the addition of PA and 2,4-DNPH as given in Fig. S6a and b,† which demonstrated their successful adsorption on the surface of the 2D-BMO nanosheets. In the same way, the 2D-BMO average size measured by DLS also increased to average sizes of 400 nm and 350 nm after the addition of PA and 2,4-DNPH as given in Fig. S6c and d† respectively. So, the possible mechanism of the 2D-BMO nanosheet's fluorescence quenching is the synergistic effect of the combination of the FRET, IFE, and electrostatic interaction with PA and 2,4-DNPH.
3.5. Determination of PA and 2,4-DNPH in lake water and river water
The fluorescence-based 2D-BMO nanosheet sensor was utilized to detect PA and 2,4-DNPH in lake water and river water to investigate its real-world application. The lake water samples were taken from Lotus Lake and the river water was taken from Love River (Kaohsiung, Taiwan). The stock solutions of PA and 2,4-DNPH were prepared in the environmental water samples after the centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) and filtration of the water samples through a 0.22 μm syringe to remove any micro-sized particles from the environmental samples. To conduct the sensing experiments, five different concentrations (50, 100, 200, 350, and 500 nM) of PA stock solution, prepared from both lake and river water, were titrated against the 2D-BMO nanosheets, as shown in Fig. S7a and b.† The regression constant (R2) values obtained for the real lake water and river water samples with PA were 0.996 and 0.997, respectively; the findings demonstrated a linear relationship for both real lake and river water samples from 50 nM to 500 nM as given in the inset of Fig. S7a and b† respectively. Following the same protocol as PA, various concentrations of 2,4-DNPH ranging from 50 to 500 nM (from lake and river water) were titrated against the 2D-BMO as shown in Fig. S7c and d.† The regression constant (R2) values obtained for the real lake water and river water samples with 2,4-DNPH were 0.992 and 0.993, respectively, which demonstrated a linear relationship behavior. The 2D-BMO nanosheets demonstrated a great recovery range from 96 to 101%, given in Table 1.
000 rpm) and filtration of the water samples through a 0.22 μm syringe to remove any micro-sized particles from the environmental samples. To conduct the sensing experiments, five different concentrations (50, 100, 200, 350, and 500 nM) of PA stock solution, prepared from both lake and river water, were titrated against the 2D-BMO nanosheets, as shown in Fig. S7a and b.† The regression constant (R2) values obtained for the real lake water and river water samples with PA were 0.996 and 0.997, respectively; the findings demonstrated a linear relationship for both real lake and river water samples from 50 nM to 500 nM as given in the inset of Fig. S7a and b† respectively. Following the same protocol as PA, various concentrations of 2,4-DNPH ranging from 50 to 500 nM (from lake and river water) were titrated against the 2D-BMO as shown in Fig. S7c and d.† The regression constant (R2) values obtained for the real lake water and river water samples with 2,4-DNPH were 0.992 and 0.993, respectively, which demonstrated a linear relationship behavior. The 2D-BMO nanosheets demonstrated a great recovery range from 96 to 101%, given in Table 1.
| Detected nitroaromatic | Real sample source | Concentration added (nM) | Concentration detected (nM) | Recovery (%) |
|---|---|---|---|---|
| PA | Lak water | |||
| 50 | 49.20 | 98.40 | ||
| 100 | 100.03 | 100.03 | ||
| 200 | 200.07 | 100.03 | ||
| 350 | 336.36 | 96.10 | ||
| 500 | 490.36 | 98.07 | ||
| River water | ||||
| 50 | 49.89 | 99.78 | ||
| 100 | 98.92 | 98.92 | ||
| 200 | 200.51 | 100.25 | ||
| 350 | 346.67 | 99.05 | ||
| 500 | 491.88 | 98.37 | ||
| 2,4-DNPH | Lake water | |||
| 50 | 50.53 | 101.07 | ||
| 100 | 100.25 | 100.25 | ||
| 200 | 201.46 | 100.73 | ||
| 350 | 351.15 | 100.32 | ||
| 500 | 498.57 | 99.71 | ||
| River water | ||||
| 50 | 50.50 | 101.00 | ||
| 100 | 99.74 | 99.74 | ||
| 200 | 197.65 | 98.82 | ||
| 350 | 348.85 | 99.67 | ||
| 500 | 501.42 | 100.28 |
3.6. Selectivity of the catalyst towards PA and 2,4-DNPH
After the successful detection of PA and 2,4-DNPH, the selectivity of the prepared fluorescent chemosensor was evaluated with various nitroaromatic and some phenolic compounds like 1,2-dinitrobenzene (1,2-DNB), 2,4-dinitrotoluene (2,4-DNT), 1,4-dinitrobenzene (1,4-DNB), 4-nitrophenol (4-NP), 1,3-dinitrobenzene (1,3-DNB), 1,2-dichloro-4-nitrobenzene (1,2-DC-4-NB), 2,6-dichlorophenol (2,6-DCP), 2,3-dichlorophenol (2,3-DCP), and 2,6-dichloronicotonic acid (2,6-DCNA). As shown in Fig. 8, the other nitroaromatic compound shows a lower percent quenching response compared to PA and 2,4-DNPH, and the chlorophenols and 2,6-DCNA further enhanced the fluorescence of the materials. The lower quenching response of the 2D-BMO nanosheets to another nitroaromatic was because of the less or no overlapping of the absorption spectra to the excitation and emission spectra of the 2D-BMO nanosheets as given in ESI Fig. S8.† PA and 2,4-DNPH demonstrated excellent quenching response when they were added to the 2D-BMO nanosheets because the UV-visible absorption spectra of PA and 2,4-DNPH overlap well with the excitation and emission spectra of the 2D-BMO nanosheets.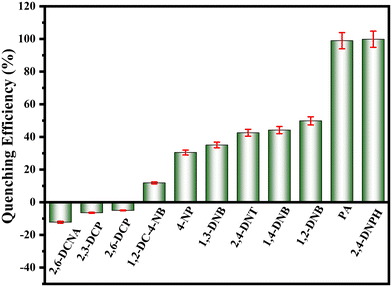 | ||
| Fig. 8 The quenching percentage for different nitroaromatic compounds in the presence of the 2D-BMO nanosheets. | ||
4. Conclusion
Nitroaromatic compounds are dangerous pollutants that contaminate the environment and pose significant health risks. Additionally, their use in explosives presents serious security threats, necessitating stringent monitoring and detection. In this study, we prepared a high fluorescence two-dimensional bismuth molybdenum oxide (2D-BMO) nanosheets for highly selective and sensitive detection of nitroaromatic compounds like picric acid (PA) and 2,4-dinitrophenylhydrazine (2,4-DNPH). The possible mechanism of fluorescence quenching of 2D-BMO after adding nitroaromatic analytes is the synergistic effect of the fluorescence resonance energy transfer (FRET), inner filter effect (IFE), and electrostatic interaction. The limit of detection (LOD) calculated for PA and 2,4-DNPH was 2.21 nM and 2.3 nM, respectively. This 2D-BMO fluorescent sensor offers a promising platform for artificial fluorescence generation, with potential applications across environmental monitoring, medical diagnostics, biological research, and life sciences.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There is no conflict to declare.Acknowledgements
We acknowledge financial support from the National Science and Technology Council (NSTC) of Taiwan with the grant number NSTC 113-2113-M-110-008.References
- H. A. Alidagi, S. O. Tümay, A. Şenocak, Ö. F. Çiftbudak, B. Çoşut and S. Yeşilot, New J. Chem., 2019, 43, 16738–16747 RSC.
- S. O. Tümay and S. Yeşilot, Sens. Actuators, B, 2021, 343, 130088 CrossRef.
- D. Dabur, Y.-T. Chan and H.-F. Wu, Environ. Sci.: Nano, 2023, 10, 3208–3219 RSC.
- X. Jiao, L. Marin and X. Cheng, J. Photochem. Photobiol., A, 2022, 424, 113632 CrossRef CAS.
- S. Nath, S. K. Pathak, B. Pradhan, R. K. Gupta, K. A. Reddy, G. Krishnamoorthy and A. S. Achalkumar, New J. Chem., 2018, 42, 5382–5394 RSC.
- B. BinChen, Z. X. Liu, H. Y. Zou and C. Z. Huang, Analyst, 2016, 141, 2676–2681 RSC.
- T.-P. Huynh, A. Wojnarowicz, A. Kelm, P. Woznicki, P. Borowicz, A. Majka, F. D'Souza and W. Kutner, ACS Sens., 2016, 1, 636–639 CrossRef CAS.
- T. B. Devi and M. Ahmaruzzaman, Chem. Eng. J., 2017, 317, 726–741 CrossRef CAS.
- S. Zhang, L. Ding, F. Lü, T. Liu and Y. Fang, Spectrochim. Acta, Part A, 2012, 97, 31–37 CrossRef CAS.
- J. V. Goodpaster and V. L. McGuffin, Anal. Chem., 2001, 73, 2004–2011 CrossRef CAS.
- K. Ahmad, A. Mohammad, P. Mathur and S. M. Mobin, Electrochim. Acta, 2016, 215, 435–446 CrossRef CAS.
- U. Chandra, B. E. K. Swamy, O. Gilbert, S. S. Shankar, K. R. Mahanthesha and B. S. Sherigara, Int. J. Electrochem. Sci., 2010, 5, 1–9 CrossRef CAS.
- K. Tachibana, M. Yamamoto and Y. Y. Maruo, in 2016 IEEE 16th International Conference on Nanotechnology (IEEE-NANO), IEEE, 2016, pp. 196–198 Search PubMed.
- S. Jabeen, M. R. Shah, A. Rauf, S. Rauf, A. Jabbar and M. I. Bhanger, Curr. Anal. Chem., 2017, 13, 532–539 CrossRef CAS.
- T. Verma, U. P. Singh, P. Verma, R. J. Butcher, C. Ghosh and P. Roy, J. Mol. Struct., 2024, 1302, 137467 CrossRef CAS.
- M. E. Walsh, Talanta, 2001, 54, 427–438 CrossRef CAS PubMed.
- R. G. Ewing, D. A. Atkinson, G. A. Eiceman and G. J. Ewing, Talanta, 2001, 54, 515–529 CrossRef CAS PubMed.
- J. M. Sylvia, J. A. Janni, J. D. Klein and K. M. Spencer, Anal. Chem., 2000, 72, 5834–5840 CrossRef CAS PubMed.
- Z. Gu, H.-X. Liu, Y.-L. Ying, G. Xiu and Y.-T. Long, Analyst, 2018, 143, 2760–2764 RSC.
- B. Liu, C. Tong, L. Feng, C. Wang, Y. He and C. Lü, Chem. – Eur. J., 2014, 20, 2132–2137 CrossRef CAS PubMed.
- J. E. Park, T. Anand, V. Bharadwaj, S. K. Sahoo and H.-J. Choi, J. Photochem. Photobiol., A, 2019, 383, 111990 CrossRef CAS.
- E. Zhang, P. Ju, Z. Zhang, H. Yang, L. Tang, X. Hou, J. You and J. Wang, Spectrochim. Acta, Part A, 2019, 222, 117207 CrossRef CAS PubMed.
- C. Anichini, W. Czepa, D. Pakulski, A. Aliprandi, A. Ciesielski and P. Samorì, Chem. Soc. Rev., 2018, 47, 4860–4908 RSC.
- V. K. Sangwan and M. C. Hersam, Annu. Rev. Phys. Chem., 2018, 69, 299–325 CrossRef CAS PubMed.
- H. Jin, C. Guo, X. Liu, J. Liu, A. Vasileff, Y. Jiao, Y. Zheng and S.-Z. Qiao, Chem. Rev., 2018, 118, 6337–6408 CrossRef CAS PubMed.
- H. Zhang, Chem. Rev., 2018, 118, 6089–6090 CrossRef CAS PubMed.
- S. Campuzano, M. Pedrero, G.-P. Nikoleli, J. M. Pingarrón and D. P. Nikolelis, Biosens. Bioelectron., 2017, 89, 269–279 CrossRef CAS.
- C. Zhu, D. Du and Y. Lin, Biosens. Bioelectron., 2017, 89, 43–55 CrossRef CAS.
- A. Khan, S. Musuvadhi Babulal, S. Ur Rehman and H.-F. Wu, Microchem. J., 2024, 204, 111041 CrossRef CAS.
- R. Mas-Balleste, C. Gomez-Navarro, J. Gomez-Herrero and F. Zamora, Nanoscale, 2011, 3, 20–30 RSC.
- C. Tan, X. Cao, X.-J. Wu, Q. He, J. Yang, X. Zhang, J. Chen, W. Zhao, S. Han and G.-H. Nam, Chem. Rev., 2017, 117, 6225–6331 CrossRef CAS PubMed.
- F. Wang, Z. Wang, L. Yin, R. Cheng, J. Wang, Y. Wen, T. A. Shifa, F. Wang, Y. Zhang and X. Zhan, Chem. Soc. Rev., 2018, 47, 6296–6341 RSC.
- T. Yu, Z. Li, S. Chen, Y. Ding, W. Chen, X. Liu, Y. Huang and F. Kong, ACS Sustainable Chem. Eng., 2018, 6, 7355–7361 CrossRef CAS.
- T. Yang, T. T. Song, M. Callsen, J. Zhou, J. W. Chai, Y. P. Feng, S. J. Wang and M. Yang, Adv. Mater. Interfaces, 2019, 6, 1801160 CrossRef.
- A. B. A. Kayani, S. Kuriakose, M. Monshipouri, F. A. Khalid, S. Walia, S. Sriram and M. Bhaskaran, Small, 2021, 17, 2100621 CrossRef CAS PubMed.
- Y. Jia, X. Yi, Z. Li, L. Zhang, B. Yu, J. Zhang, X. Wang and X. Jia, Talanta, 2020, 219, 121308 CrossRef CAS PubMed.
- C. Qin, B. Wang, N. Wu, C. Han and Y. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 26318–26329 CrossRef CAS.
- S. U. Rehman, S. Musuvadhi Babulal, D. Dabur and H.-F. Wu, ACS ES&T Water, 2023, 4, 134–145 Search PubMed.
- J. Zhao, C. Liu, J. Li, R. Wu, J. Wang, H. Qian, H. Guo, J. Li and K. Ibrahim, AIP Adv., 2019, 9, 55208 CrossRef.
- A. K. Sharma, W.-S. Huang, S. Pandey and H.-F. Wu, Sens. Actuators, B, 2022, 362, 131685 CrossRef CAS.
- Y. Nerthigan, A. K. Sharma, S. Pandey, K. H. Sharma, M. Shahnawaz Khan, D.-R. Hang and H.-F. Wu, Microchim. Acta, 2018, 185, 1–8 CrossRef CAS PubMed.
- M. Jain, S. Madas, P. Vashishtha, P. Rajput, G. Gupta, M. U. Kahaly, K. Özdoğan, A. Vij and A. Thakur, Sci. Rep., 2020, 10, 1–10 CrossRef.
- W. Shi, S. Song and H. Zhang, Chem. Soc. Rev., 2013, 42, 5714–5743 RSC.
- N. Sharma, S. Pandey, A. K. Sharma and H.-F. Wu, ACS Sustainable Chem. Eng., 2019, 7, 7479–7485 CrossRef CAS.
- S. Ur Rehman, S. Musuvadhi Babulal, A. Khan and H.-F. Wu, Appl. Surf. Sci., 2024, 669, 160491 CrossRef CAS.
- Y. Shitrit, M. Duraiyarasu, J. Kumar, S. Reddy, A. Ya'akobovitz, Y. S. Cohen and E. Edri, ACS Appl. Nano Mater., 2022, 5, 16354–16364 CrossRef CAS.
- N. Kumar and R. Kumar, Mater. Chem. Phys., 2022, 275, 125211 CrossRef CAS.
- S. Liu, X. He, X. Hu, Y. Pu and X. Mao, Mater. Adv., 2024, 5, 453–474 RSC.
- Z. Hao, L. Xu, B. Wei, L. Fan, Y. Liu, M. Zhang and H. Gao, RSC Adv., 2015, 5, 12346–12353 RSC.
- Y. Zheng, T. Zhou, X. Zhao, W. K. Pang, H. Gao, S. Li, Z. Zhou, H. Liu and Z. Guo, Adv. Mater., 2017, 29, 1700396 CrossRef.
- G. Li, W. Yang, S. Gao, Q. Shen, J. Xue, K. Chen and Q. Li, Chem. Eng. J., 2021, 404, 127115 CrossRef CAS.
- D. Chen, F. Niu, L. Qin, S. Wang, N. Zhang and Y. Huang, Sol. Energy Mater. Sol. Cells, 2017, 171, 24–32 CrossRef CAS.
- M. Rong, L. Lin, X. Song, T. Zhao, Y. Zhong, J. Yan, Y. Wang and X. Chen, Anal. Chem., 2015, 87, 1288–1296 CrossRef CAS PubMed.
- Y. Wang and Y. Ni, Anal. Chem., 2014, 86, 7463–7470 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00517a |
| This journal is © The Royal Society of Chemistry 2025 |