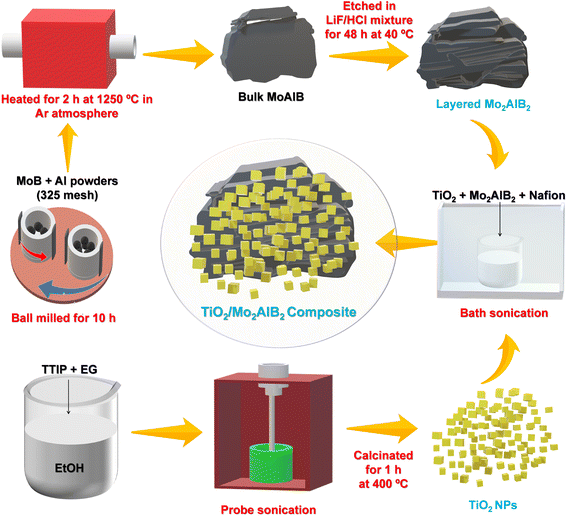
2D Mo2AlB2 transition-metal-aluminum-boride-phase-integrated TiO2 nanoparticles enable accelerated carbendazim photodegradation: impact of ohmic junctions and electric fields†
Moorthy Gnanasekar
Narendran
 and
Aruljothy
John Bosco
and
Aruljothy
John Bosco
 *
*
Advanced Materials Chemistry Laboratory, Department of Chemistry, Faculty of Engineering and Technology, SRM Institute of Science and Technology, Kattankulathur – 603 203, Chengalpattu, Tamil Nadu, India. E-mail: mgnaren13@gmail.com; nm8015@srmist.edu.in; johnbosca@srmist.edu.in; sambosco@gmail.com; Tel: +91 98407 57430
First published on 29th October 2024
Abstract
In the quest for highly efficient nanomaterials to overcome the inherent challenges associated with fungicide elimination from water, herein, a novel ohmic junction was engineered by integrating layered 2D Mo2AlB2 with TiO2 nanoparticles using ultrasound self-assembly technique. A comprehensive array of characterization methods was employed to probe the photophysical properties of the optimized composite (TO/15-MAB). The innovative design of the ohmic junction, facilitated by its internal electric field, significantly reduced the surface charge in the TO/15-MAB composite by transferring free electrons from Mo2AlB2 to TiO2. This charge reduction enhanced the ability of the composite to attract carbendazim because of their opposing charges, promoting its swift adsorption under neutral pH conditions. Upon light irradiation, the junction accelerated the seamless transition of electrons from TiO2 to Mo2AlB2 over a curved energy band, reducing the recombination of photogenerated electrons and holes and converting them into ˙O2− and ˙OH. This culminated in the rapid degradation of 15 ppm carbendazim to ∼1 ppm with an efficiency of 93.4% and an enhanced rate of k = 0.0415 min−1, which is 4 times higher than that of bare TiO2. This assertion was supported by combined experimental and theoretical evaluation. This work showcases the excellent potential of MAB phase materials in harnessing ohmic junctions and electric fields for enhanced photocatalysis, paving the way for a highly efficient and sustainable approach to eliminating fungicides from water.
Environmental significanceThis research demonstrates that an innovative photocatalytic system using the 2D Mo2AlB2 transition-metal-aluminum-boride phase integrated with TiO2 nanoparticles significantly improves the degradation of carbendazim, which is a persistent and widely used fungicide. Carbendazim poses significant environmental risks due to its ability to contaminate soil and water bodies, resulting in negative effects on aquatic life and ecosystems. This system facilitates accelerated photodegradation, offering a promising solution for mitigating the environmental impact of carbendazim. By improving the efficiency of pollutant breakdown through the synergistic effects of ohmic junctions and electric fields, the system reduces the time required for detoxification and minimizes the potential for secondary pollution. This research paves the way for the development of more effective and sustainable photocatalytic technologies that can be applied to a broader range of environmental contaminants, ultimately contributing to cleaner water resources and healthier ecosystems. |
Introduction
The pressing environmental concern of water pollution is a significant consequence of modernization, driven by pesticides, heavy metals, industrial waste, and pharmaceutical remnants.1–3 Among the pesticides, fungicides play a vital role in agricultural productivity but tend to accumulate in water bodies, posing risks to food safety, human health, and aquatic ecosystems.4 Carbendazim has been identified as an imminent hazard material by the Water Framework Directive of the European Commission and a possible human carcinogen by the US EPA owing to its mutagenicity, recalcitrance, and bioaccumulation.5 Prior studies have discovered concentrations of CBZ ranging from 0.003–156 μg L−1 in different water environments, such as surface water, groundwater, oceans, municipal sewage, and drinking water.6 Therefore, it is imperative to eliminate carbendazim from effluents. Current treatment methods for CBZ in water and soil include adsorption,7 thermal degradation,8 biodegradation,9 and photocatalytic degradation.10,11The use of photocatalytic technology based on semiconductors has emerged as a crucial solution for leveraging clean energy to address environmental pollution effectively.12,13 In this case, advanced materials such as metal–organic frameworks (MOFs), covalent organic frameworks (COFs), and metal nanocomposites have attracted considerable attention for their versatile applications in nanoparticle synthesis. To tackle the challenges faced by society, creating materials capable of overcoming pathogenic obstacles is essential. Nanotechnology presents a promising solution for addressing the threats encountered in daily life. Among the diverse materials available in this field, nanocomposites (NCs) have emerged as more efficient compared to individual nanoparticles (NPs). The primary objective in selecting nanoscale materials is that they must exhibit exceptional physicochemical properties, surpassing that of bulk materials. These properties include high porosity, durability, tunable structures, increased surface area, and unique optoelectronic characteristics. In the current era, the advancement of nanocomposites has reached its peak due to the synergistic effects imparted by the precursors incorporated during material preparation.14,15
In metal-oxide and sulfide-based nanocomposites, nanomaterials such as titanium dioxide (TiO2), ZnO, and CdS, have been widely utilized due to their cost-effectiveness, efficacy, and environmentally friendly nature.16 The efficacy of a material as a potential photocatalyst hinges on several factors, including appropriate band gap, enhanced stability, and efficient charge separation and transfer mechanisms. In this field, titanium dioxide represents the ideal photocatalyst, which is well-known for its exceptional optoelectronic properties, abundance in nature, resistance to photo-corrosion, non-toxic nature, and affordability.17 However, despite these merits, the practical application of TiO2 encounters challenges due to its fast recombination rate and limited absorbance capabilities. Thus, to surmount these hurdles, researchers have explored various avenues, such as doping TiO2 with different metal or metal oxide compounds and its surface sensitization.18 Notably, the construction of semiconductor heterojunctions with suitable band structures has emerged as a prevalent and effective strategy to address these limitations.
Furthermore, the integration of a cocatalyst to establish an ohmic junction represents another promising approach to enhance the functionality of TiO2. An ohmic junction is formed when a metal interfaces with a semiconductor, where the metal is characterized by a lower work function than that of the semiconductor. This configuration facilitates the seamless transfer of electrons, given that the absence of a barrier height promotes efficient electron movement. Additionally, the band bending induced by the internal electric field allows electrons to transition directly to the metal surface, fostering effective electron–hole separation.19 In contrast, a Schottky junction is formed when a metal with a higher work function interfaces with a semiconductor, creating a potential energy barrier that can impede electron transfer. Although Schottky junctions offer the advantage of a built-in electric field, which promotes charge separation, their performance can be constrained by their barrier height, hindering effective charge transfer.20–22 Notably, researchers have made significant progress in the development of ohmic junctions, with studies showcasing the potential of cocatalysts in enhancing the photocatalytic activity of semiconductors. For instance, Chunxue Li et al. synthesized a Co9S8/CdIn2S4 ohmic junction for photocatalytic H2 production, yielding significantly improved efficiency compared to bare CdIn2S4.23 Similarly, Qinyi Gu et al. demonstrated the efficacy of NiCoP-modified g-C3N4 ohmic junctions, achieving exceptional degradation rates for tetracycline through the synergistic effects of the ohmic junction and porous structure.24 However, the lack of a built-in electric field in ohmic junctions leads to higher recombination rates. In this case, a metal cocatalyst with a large surface area can mitigate this issue by providing more active sites for electron transfer, enhancing the separation of electron from holes. This facilitates faster electron consumption in reactions, reducing the recombination and improving the photocatalytic efficiency.
In the quest for a metal cocatalyst with a larger surface area and suitable work function to establish an ohmic junction with TiO2, the Mo2AlB2 MAB phase has emerged as a promising candidate. MAB phases, boride analogs of the MAX phases, combine ceramic and metallic properties similar to MAX phases, while offering enhanced mechanical and catalytic attributes.25 Due to their superior hardness and compressive strength compared to MAX phases, MAB phases present compelling alternatives for diverse engineering applications, showcasing their potential to drive innovation in materials science and photocatalysis.26 The versatility of MAB phases is underscored by their utilization in diverse fields. For instance, Amir A. Rezaie and colleagues explored the application of the Nin+1ZnB MAB phase in Li-ion electrodes.27 Lucas T. Alameda et al. demonstrated the enhanced hydrogen evolution reaction (HER) activity of the partially etched MoAlB MAB phase compared to the pure MoAlB.28
In this work, 2D Mo2AlB2 was synthesized by etching bulk MoAlB and integrating it with TiO2 nanoparticles (NPs) using an ultrasound self-assembly method to construct a novel TiO2/Mo2AlB2 ohmic junction, as schematically illustrated in Fig. 1. The optimized catalyst, TO/15-MAB, which incorporated 15 wt% of layered 2D Mo2AlB2 with TiO2 NPs, exhibited a remarkable four-fold increase in photodegradation efficiency towards the carbendazim fungicide compared to bare TiO2. This acceleration was attributed to the transfer of free electrons from Mo2AlB2 to TiO2 through the ohmic junction, reducing the surface charge of the TO/15-MAB composite and favoring the interaction of catalyst and carbendazim under neutral pH conditions. Under light irradiation, the seamless electron transfer from TiO2 to Mo2AlB2 through downward band bending suppressed the charge recombination, promoted redox reactions, and revealed a plausible photocatalytic degradation mechanism. This research underscores the collaborative potential of MAB phase metals with semiconductors to construct ohmic junction photocatalysts, highlighting their promising contributions to the advancement of photocatalysis.
Results and discussion
The photocatalytic performance of the prepared catalyst
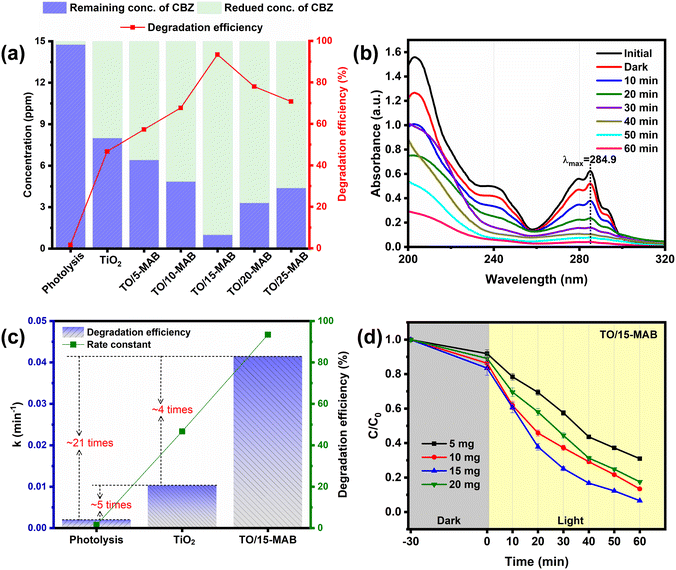
The photocatalytic performance of the as-prepared samples was investigated by monitoring the degradation of CBZ in an aqueous solution under simulated light irradiation (300 W Xenon lamp). The details are given in the ESI.† All the Mo2AlB2 MAB-based nanocomposite photocatalysts showed enhanced photocatalytic activities compared to the reaction without catalyst (photolysis) and bare TiO2 under light illumination. Among the various amounts of Mo2AlB2 in TiO2, the composite with 15 wt% Mo2AlB2 (TO/15-MAB) reduced the initial concentration of CBZ (15 ppm) to ∼1 ppm and showed the maximum degradation efficiency of 93.4% (Fig. 2a and S2a†), while other catalysts such as TiO2, TO/5-MAB, TO/10-MAB, TO/20-MAB, and TO/25-MAB reduced up to 8 ppm, 6.5 ppm, 4.8 ppm, 3.3 ppm, and 4.38 ppm with the degradation efficiency of 47%, 57%, 67%, 78% and 71%, respectively. Even without a catalyst (photolysis), the initial CBZ concentration decreased slightly (15 ppm to 14.76 ppm). This negligible reduction indicated that light irradiation alone had a minimal impact, highlighting the crucial role of the photocatalyst in the degradation process. The UV-vis absorbance of CBZ was monitored as a function of time using TO/15-MAB composite, as shown in Fig. 2b. Subsequently, the reaction rate constant (k) was calculated by fitting the degradation data with the Langmuir–Hinshelwood pseudo-first-order reaction model. Among the samples, the degradation rate constant of TO/15-MAB (k = 0.0415 min−1) is the highest, which is 4 times that of bare TiO2 (k = 0.0103 min−1) and 21 times higher than that of photolysis (Fig. 2c and S2b†). The impact of catalyst dose on the degradation of CBZ was examined using different concentrations of photocatalysts, such as 5, 10, 15, and 20 mg (Fig. 2d). The resultant degradation efficiencies were 69%, 86.6%, 93.4%, and 82.4%, respectively. Notably, the study determined that 15 mg of catalyst was the most effective level for CBZ degradation. Table S1† summarizes the photocatalytic carbendazim degradation rates across various catalysts, showing that the TiO2/Mo2AlB2 (TO/15-MAB) catalyst achieved a competitive 93.4% degradation in 60 min. This significant improvement in speed and efficiency underscores its potential for environmental remediation applications.Exploring the catalytic characteristics of samples
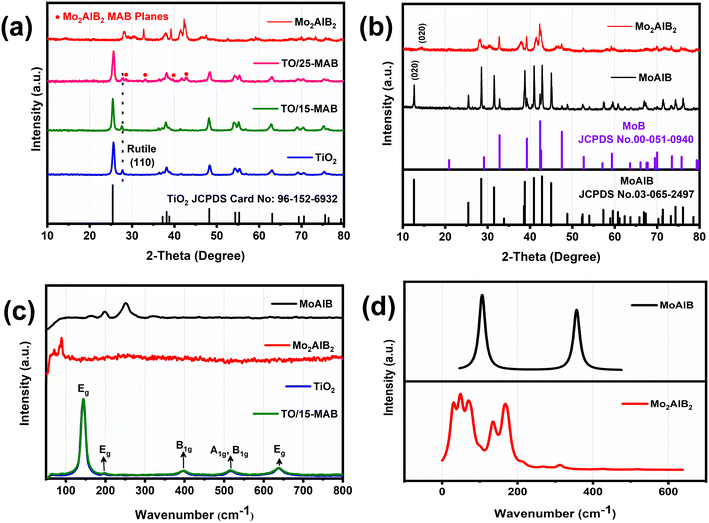
The XRD patterns of the TiO2 nanoparticles, as shown in Fig. 3a, display highly crystalline diffraction peaks. The distinct peaks of TiO2 are well matched to the tetragonal phase with the lattice constant of a = b = 3.771 Å and c = 9.43 Å (space group I41/amd, JCPDS Card No. 96-152-6932). Furthermore, the peak at the 2θ value of 27.5° signifies the presence of the rutile phase of TiO2.29Fig. 3b shows the XRD patterns of MoAlB, exhibiting a well-fitted orthorhombic phase characterized by lattice constants of a = 3.2120 Å, b: 13.9850 Å, c: 3.1020 Å (space group Cmcm, Card No. 03-055-2497). Notably, the XRD pattern of Mo2AlB2 revealed an intriguing shift in the 020-diffraction peak from 12.6° to 14.2° after undergoing partial extraction of the Al layers. Moreover, the d-interlayer distance decreased from 0.69 nm to 0.64 nm, aligning seamlessly with the simulated XRD pattern28,30 (Fig. S3†). This provides compelling evidence that the extraction of the Al layers induced a reduction in both the structure and crystallinity of the MAB phase. Notably, the XRD patterns of TO/15-MAB are similar to that of TiO2, while TO/25-MAB shows the characteristic peaks of Mo2AlB2. This is probably due to the relatively low diffraction and loading amount of Mo2AlB2. In addition, comparing the XRD peaks of the TO/15-MAB composite to bare TiO2, the composite has narrower peaks (Fig. 3a), indicating lattice distortion caused by interaction with Mo2AlB2. This distortion, which was calculated using the Debye–Scherrer and Williamson–Hall equations (Table S2†), shows a smaller crystallite size (16.9 nm) and higher tensile strain (0.709%) for the composite compared to bare TiO2 (34.4 nm and 0.471%, respectively). The smaller crystallite size and higher strain in the TO/15-MAB composite led to a high surface area and defects, enhancing its photocatalytic performance by providing more active sites, improving the charge separation, and promoting light absorption.31,32Fig. 3c depicts the Raman spectra of the bare TiO2 and TO/15-MAB composite, which show strong intense peaks attributed to the vibrational phonon modes in the tetragonal anatase TiO2. The strong peak at 143.6 cm−1 conforms to the Eg mode, whereas the small peaks at 194.8, 395, 515.4, and 638 cm−1 are due to the Eg, B1g, A1g–B1g, and Eg modes, respectively.33 The Raman peaks of MoAlB at 165, 197, and 250 cm−1 correspond to the vibrational orientations of the Mo atoms along the 100, 001, and 010 directions, respectively.26 The Raman-active mode at 325 cm−1 indicates the vibration of Al in the 001 direction.34 Mo2AlB2 shows Raman bands at 70 and 89 cm−1.35 To understand the Raman spectra of Mo2AlB2 after Al extraction from MoAlB, the preliminary calculations were computed for a non-periodic system of both. The results in Fig. 3d show that the Raman disorder and redshift of peaks are similar to the experimental results. The TO/15-MAB composite shows all the characteristic peaks of bare TiO2 and the peak at 89 cm−1 of Mo2AlB2, indicating the successful synthesis of the composite. Furthermore, the noticeable peak broadening, blue shift, and intensity variation in the Raman peak of both bare TiO2 and TO/15-MAB provide concrete evidence of the increased disorder within the TO/15-MAB composite, a result of the integration of Mo2AlB2 onto the TiO2 surface. To delve deeper into the phonon dynamics of the samples, the following time–energy uncertainty relation was employed to determine the phonon lifetime of the samples.36
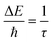 | (1) |
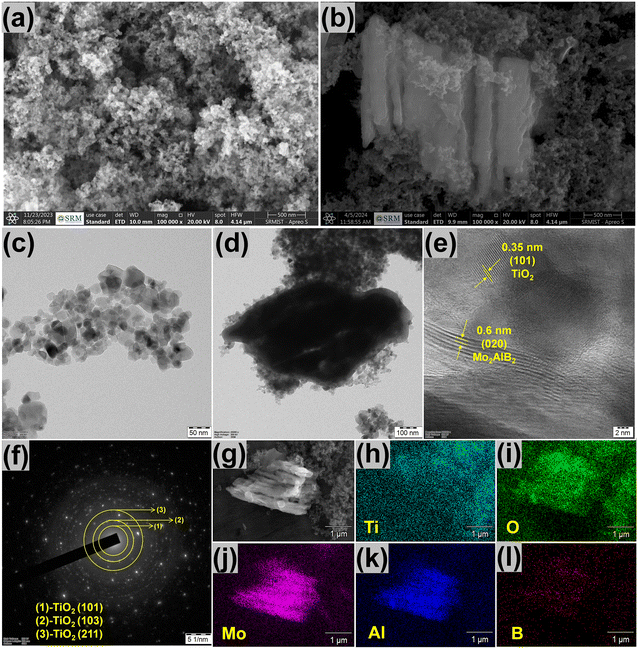
The intricate morphological traits of the catalyst were analyzed via HRSEM and HRTEM. Fig. 4(a and c), respectively, and Fig. S5a† reveals the nanoparticle morphology of TiO2, with an average size of 32.4 nm. The HRSEM images of MoAlB in Fig. S4† exhibit its bulk structure. After the partial removal of the Al layers, the transformed Mo2AlB2 exhibited a stacking layered morphology (Fig. S4b†). The formation of Mo2AlB2 by removing the Al layers is schematically represented in Fig. S4c.† As shown in Fig. 4(b and d), the TO/15-MAB composite exhibited successful integration, with the 2D Mo2AlB2 layers surrounded by TiO2 NPs. Fig. S5b† demonstrates the TiO2 NPs densely packed and evenly distributed onto the surface of the layered Mo2AlB2, creating a favorable arrangement for efficient electron transport within the composite. Fig. 4e presents the calculated d-interlayer distances of TiO2 and Mo2AlB2, confirming their 101 and 020 planes, respectively. Fig. 4h shows the SAED pattern of TO/15-MAB, with circles indicating the tetragonal TiO2 phase. The dispersed dots within the circles are attributed to Mo2AlB2, highlighting its polycrystalline nature. The HRSEM elemental mapping analysis, as depicted in Fig. 4(g–l), reveals the wide distribution of Ti, O, Mo, Al, and B elements throughout the TO/15-MAB composite.
The optical band gaps and band structures are determined through various analyses such as UV-DRS spectra, Tauc plot, Mott–Schottky plot, and XPS valence band spectra. As shown in Fig. 5a, the absorption edge of TiO2 appears in the ultraviolet region at around 410 nm, indicating the intrinsic absorption of anatase TiO2. Conversely, Mo2AlB2 displays a broad optical response in the range of 200–800 nm due to its black color and metallic properties, lacking a distinct absorption edge.35 When combined with Mo2AlB2 as a co-catalyst, TO/15-MAB exhibited a redshift in the visible light region, with an absorption edge at 430 nm, introducing sub-band states and altering its light absorption characteristics. Fig. 5b depicts the bandgap energies of bare TiO2 and TO/15-MAB composite, as determined by the intersection of two fitting lines on the Tauc plot applying the Kubelka–Munk function. This methodology, as outlined by Makuła et al., involves a linear fit to sub-bandgap absorption, providing insight into the bandgap characteristics of the materials.38
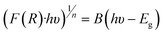 | (2) |
| ENHE = 0.0591 × pH + E0Ag–AgCl | (3) |
| EVB = Eg + ECB | (4) |
 | ||
| Fig. 5 UV DRS spectra (a), Kubelka–Munk plot (b), Mott–Schottky plot (c), and XPS VB-spectra (d) of bare TiO2 and TO/15-MAB composites. | ||
Based on these values, the conduction band potential (ECB) of bare TiO2 and TO/15-MAB composite can be calculated with respect to the normal hydrogen electrode (NHE) using eqn (3).39,40 The ECB values for the bare TiO2 and TO/15-MAB composite were determined to be −1.17 V and −1.25 V, respectively. Additionally, based on eqn (4), the valence band potential of bare TiO2 and TO/15-MAB composite was determined to be 1.98 V and 1.85 V respectively. Moreover, these values are very close to the values obtained from the XPS-VB spectra in Fig. 5d.
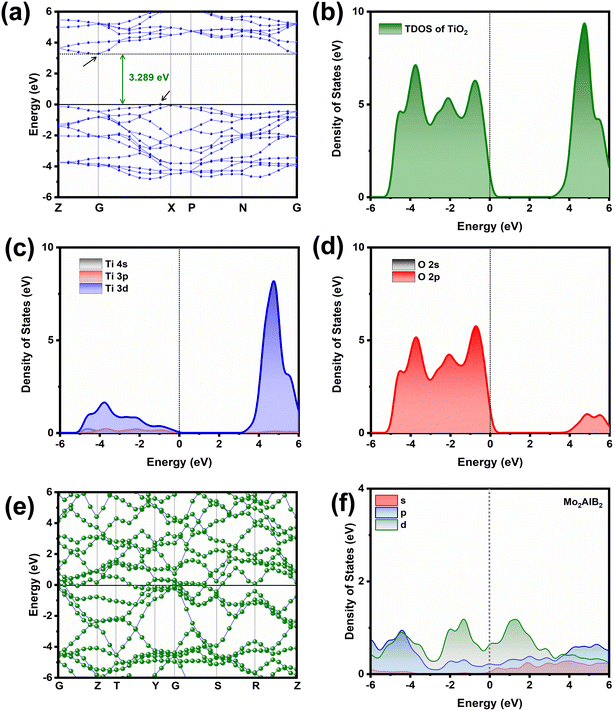
The calculated band structure of TiO2 in Fig. 6a shows a band gap of 3.289 eV, which is closer to the experimental results. The Fermi level in the band structure, as depicted by a black line at 0 eV energy, was set to zero for reference. The conduction band is located at the G point, whereas the valence band is located at the X point, indicating the indirect-type band gap structure of TiO2.41 Moreover, the results of the total density of states and partial density of states, as shown in Fig. 6(b–d), indicate that the conduction band and valence band of TiO2 are composed of Ti 3d and O 2p orbitals, respectively. The calculated band structure and DOS of Mo2AlB2 in Fig. 6(e and f), respectively, show the electronic states near the Fermi level, demonstrating its metallic nature.35
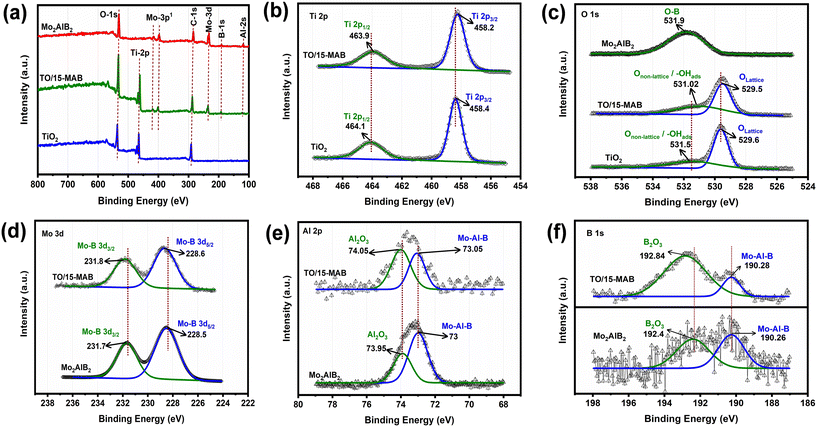
The surface chemical composition and electronic state of the as-prepared samples were studied by XPS analysis. Interestingly, despite the absence of carbon-containing materials in the synthesis process, noticeable carbon peaks were evident in all the survey spectra, as depicted in Fig. 7a. This is due to the fact that the sample was coated on a carbon tape substrate to facilitate the analysis. The C 1s binding energy of 284.8 eV was used as a calibration reference. As shown Fig. 7b, the HR-XPS spectra of the bare TiO2 revealed two well-defined peaks in the Ti 2p region. These peaks, centered at around binding energies (BEs) of ≈464.1 and 458.4 eV, are assigned to the Ti 2p1/2 and Ti 2p3/2 components, respectively, of the Ti4+ oxidation state.42 Moving to the HR-XPS spectra in the O 1s region for the bare TiO2 sample (Fig. 7c), two peaks were observed at the BEs of ≈529.6 and 531.5 eV, which are attributed to the lattice oxygen (Ti–O) and non-lattice origin such as surface-adsorbed –OH, respectively.43,44 As shown in Fig. 7d, the HR-XPS spectra of Mo 3d in Mo2AlB2 displayed peaks of 3d5/2 and 3d3/2 at the BEs 231.7 eV and 228.5 eV, corresponding to the Mo–B bonds, respectively.45,46 The Al 2p spectrum of Mo2AlB2 exhibited peaks at two distinct BEs of 73.95 eV and 73 eV, which are attributed to the Al2O3 and Mo–Al–B components, respectively45,47 (Fig. 7e). The increased peak area corresponds to the binding energy (BE) area of Al2O3, indicating that significant corrosion occurred during the etching process. In the case of the HR-XPS of B 1s for Mo2AlB2, as shown in Fig. 7f, the peaks at the BEs of 192.43 eV and 190.26 eV were identified to be B2O3 and Mo–Al–B, respectively.48 Additionally, the presence of an O 1s peak at the BE of 531.9 eV in the Mo2AlB2 sample indicates the presence of O–B bonds.49
Notably, as shown in Fig. 7(d–f), a positive binding energy shift was observed in the Mo 3d, Al 2p, and B 1s peaks of TO/15-MAB compared to Mo2AlB2. However, as shown in Fig. 7(a and b), the Ti 2p and O 1s peaks of TO/15-MAB moved to a negative position compared with bare TiO2. Generally, the binding energy is negatively related to a lower surface electron density.50 This XPS measurements results confirmed that the electrons migrated from Mo2AlB2 to the TiO2 surface upon contact between Mo2AlB2 and TiO2, resulting in the creation of an ohmic junction between the TiO2/Mo2AlB2 interface.
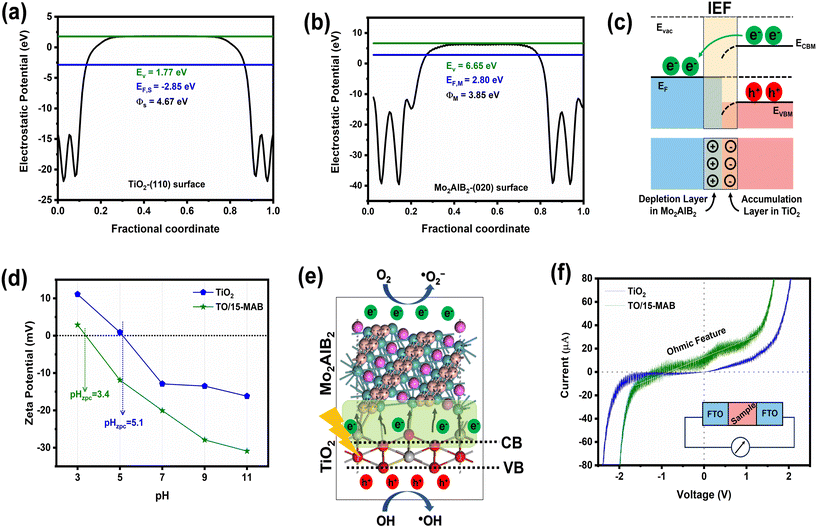
The work function calculations were conducted for the 110 surface of TiO2 and the 020 surface of Mo2AlB2 based on the HRTEM observations (Fig. 8(a and b)), respectively. The work functions (Φ) of TiO2 and Mo2AlB2 were calculated to be 4.67 eV and 3.85 eV, respectively. Upon forming an intimate heterojunction, the free electrons in Mo2AlB2 migrate to TiO2, leading to a decrease in the Fermi level (EF) in Mo2AlB2 and an increase in the Fermi level of TiO2 until equilibrium is reached. This electron transfer results in a positive charge on the surface of Mo2AlB2 due to electron depletion and a negative charge on TiO2 due to electron accumulation. This charge distribution establishes an internal electric field (IEF) from Mo2AlB2 to TiO2, facilitating the separation and migration of photogenerated carriers. The accumulated electrons induce a downward band bending in TiO2, forming an ohmic junction with Mo2AlB2 (Fig. 8c).51
To understand the effects of an ohmic junction and IEF on the surface charge of the catalyst, zeta potential analysis was performed for the bare TiO2 and TO/15-MAB composite (Fig. 8d). The zero point charge (pHZPC) of the bare TiO2 was found to be 5.1, which is consistent with previous findings.52,53 In the case of the TO/15-MAB composite, a decrease in pHZPC to 3.4 was observed. This is because the transfer of free electrons from Mo2AlB2 to TiO2 reduces its surface charge.54 Due to the enhanced negative surface charge of the composite TO/15-MAB, the pHZPC shifted to a lower pH value.55 This shift indicates that a more acidic environment (lower pH) is required to neutralize the additional negative charges and balance the net surface charge. Upon light irradiation, the photogenerated electrons in the conduction band of TiO2 rapidly move towards Mo2AlB2 through the ohmic junction (Fig. 8e). The lack of an interfacial barrier in the ohmic contact enables the IEF to drive charge transfer, enhancing the separation and facilitating the accumulation of electrons in Mo2AlB2 for active participation in catalytic reactions. Despite this, the positively charged carriers (holes) remain in the valence band of TiO2, actively participating in the degradation process.56
To confirm the formation of an ohmic junction, current–voltage (I–V) measurements were carried out for the bare TiO2 and TO/15-MAB composite. As shown in Fig. 8f, the I–V curve for the bare TiO2 exhibits a non-linear characteristic, indicating a typical semiconductor feature. Conversely, the TO/15-MAB composite displayed a linear I–V characteristic, demonstrating behavior that follows Ohm's law. Specifically, the current increases proportionally with voltage, showing a linear relationship between the two variables. This behavior is characteristic of materials with ohmic features.57
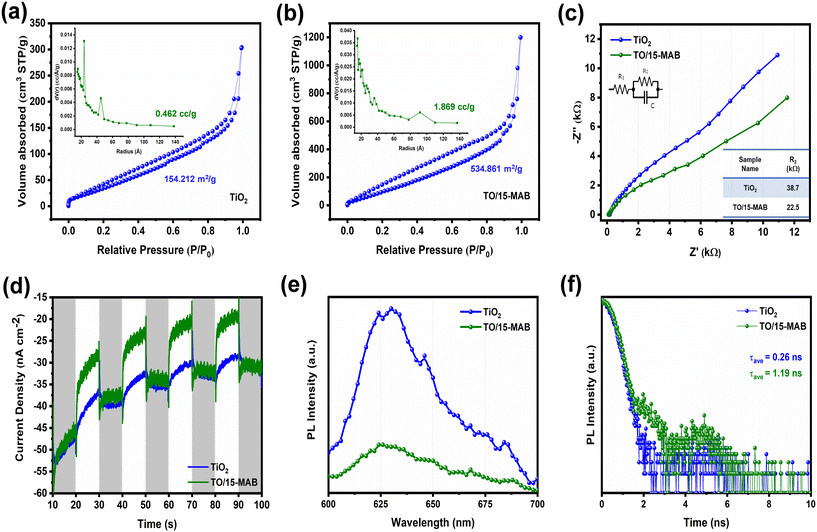
Photocatalytic activity is dependent on several factors, including surface area, light absorption capability, and charge separation efficiency. To determine the surface area, the N2-sorption isotherms were examined. Fig. 9(a and b) illustrate that both the bare TiO2 and TO/15-MAB composite display type IV adsorption isotherms with distinct type-H3 hysteresis loop shapes, indicating their mesoporous nature. Typically, type IV isotherms exhibit an initial rise at lower relative pressures, followed by a sharp increase at higher relative pressures, often accompanied by a hysteresis loop. In contrast to bare TiO2, the TO/15-MAB composite demonstrated a more rapid initial rise in its isotherm, suggesting a higher surface area and greater availability of active adsorption sites. Subsequent BET analysis showed a surface area of 154.212 m2 g−1 for the bare TiO2 and a significantly higher value of 534.861 m2 g−1 for the TO/15-MAB composite. This difference highlights the impact of Mo2AlB2 in enhancing the adsorbate interactions within the composite. The increased surface area of TO/15-MAB composite not only offers more active sites for light absorption but also enhances its pollutant adsorption and photodegradation capabilities, thereby improving its overall photocatalytic performance.58 Additionally, the BJH pore volume measurements indicate a value of 0.462 cc g−1 for the bare TiO2 and a notably higher value of 1.869 cc g−1 for the TO/15-MAB composite (inset of Fig. 9(a and b)). The BJH analyses of TO/15-MAB composite revealed broader and more irregular pore distributions, implying a more prominent mesoporous structure compared to bare TiO2. This enhanced surface area and pore volume in the TO/15-MAB composite are crucial for providing additional active sites, which are essential for promoting redox reactions during photocatalytic processes, ultimately enhancing the efficiency of the photocatalyst.
The charge transfer properties of the bare TiO2 and TO/15-MAB composite were studied using the EIS Nyquist plot, transient photocurrent responses, and photoluminescence spectra. As shown in Fig. 9c, the examination of the resistivity in the bare TiO2 and TO/15-MAB composite was conducted through electrochemical impedance spectroscopy (EIS). The Nyquist diagrams from the EIS demonstrated a semi-circle at higher frequencies, indicating the electron transfer characteristics of the utilized material. The size of the semi-circle exhibits a direct correlation with the charge transfer resistance (R2) of the electrode. A greater diameter of the semi-circle suggests greater charge transfer resistance.59 The EIS Nyquist plot unveiled that the TO/15-MAB composite, with an R2 of 22.5 kΩ, displayed reduced resistivity in comparison to bare TiO2 (R2 = 38.7 kΩ). This outcome highlights that the TO/15-MAB composite is a material with enhanced conductivity, as evidenced by its lower R2 values compared with the examined bare TiO2.
To better understand the behavior of photogenerated electrons and holes, the transient photocurrent responses of bare TiO2 and TO/15-MAB composite electrodes were measured over several on–off cycles, as illustrated in Fig. 9d. Typically, higher photocurrent levels suggest more charge carriers, together with improved transport and separation efficiency.35 As anticipated, coating the GC electrode with TO/15-MAB composite significantly enhanced the photocurrent compared to bare TiO2. This suggests that the TO/15-MAB composite facilitates more efficient separation of photogenerated carriers than bare TiO2.
The intensity of the PL emission is typically influenced by the recombination of electrons and holes. A decrease in PL emission intensity suggests a lower potential for electron–hole pair recombination, leading to improved efficiency in charge carrier migration.60Fig. 9e shows the obtained PL emission maxima of the bare TiO2 and TO/15-MAB composite at 635 nm, indicating that the TO/15-MAB composite has a lower PL emission intensity than the bare TiO2 sample. As a result, the TO/15-MAB composite shows less photoinduced electron–hole pair recombination and can produce more photogenerated charge carriers to participate in photochemical transformations, which increases its photocatalytic activity. Additionally, the time-resolved photoluminescence (TRPL) decay spectra of the bare TiO2 and TO/15-MAB composite, as shown in Fig. 9f, were analyzed to investigate their charge transfer dynamics. The average lifetimes of the photogenerated charge carriers in the bare TiO2 and TO/15-MAB composite were found to be 0.26 ns and 1.19 ns, respectively. This indicates that the Mo2AlB2 loading extended the lifetime of the photogenerated charge carriers in TiO2. It is well-established that a longer lifetime signifies greater separation efficiency and is advantageous for the photocatalysis process.18
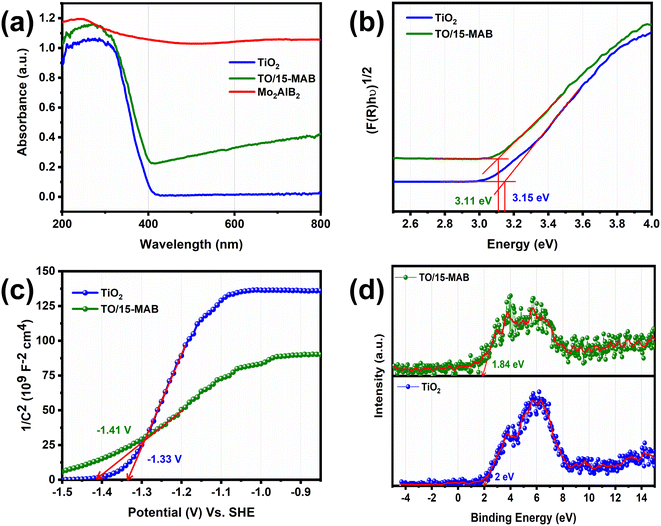
The influence of pH on the degradation of carbendazim was studied to understand the adsorption mechanism. The degradation efficiency was lower at pH 3 (71%) and 11 (80.2%), and highest at the pH of 7 (93.4%) (Fig. 10a). To understand the underlying reason for this, the protonated and deprotonated functionals depending on the pH were predicted by determining the pKa values of carbendazim using the Chemaxon tool,61 as shown in Fig. 10b. The pHZPC of the TO/15-MAB composite was observed to be 3.4 (Fig. 8d). The pHZPC typically signifies the absence of repulsive forces. When the pH is lower than the pHzpc value, the surface of the catalyst becomes protonated, resulting in a positive charge. Alternatively, when the pH exceeds the pHZPC, the surface of the catalyst is deprotonated and carries a negative charge.62 A list of the protonated and deprotonated positions is presented in Table 1. According to these findings (Fig. 10a), it was noted that at a pH of 7, the carbendazim degradation reached a peak of 93.4%. At this neutral pH level, the TO/15-MAB composite is negatively charged and more pKa functionals are protonated (pKa2 and pKa4). This charge interaction between the oppositely charged species plays a crucial role in driving the degradation process forward. At other pH levels, such as 3 and 11, more repulsive forces may occur due to the higher number of similar charges.
 | ||
| Fig. 10 Influence of pH on CBZ degradation (a). Determined pKa values of CBZ corresponding to the functional group (b). | ||
| pH | Charge on the TO/15-MAB surface | Carbendazim | |
|---|---|---|---|
| Protonated positions | Deprotonated positions | ||
| pH 3 | Positive | pKa1, pKa2, and pKa4 | pKa3 |
| pH 7 | Negative | pKa2 and pKa4 | pKa1, and pKa3 |
| pH 11 | Negative | pKa4 | pKa1, pKa2, and pKa3 |
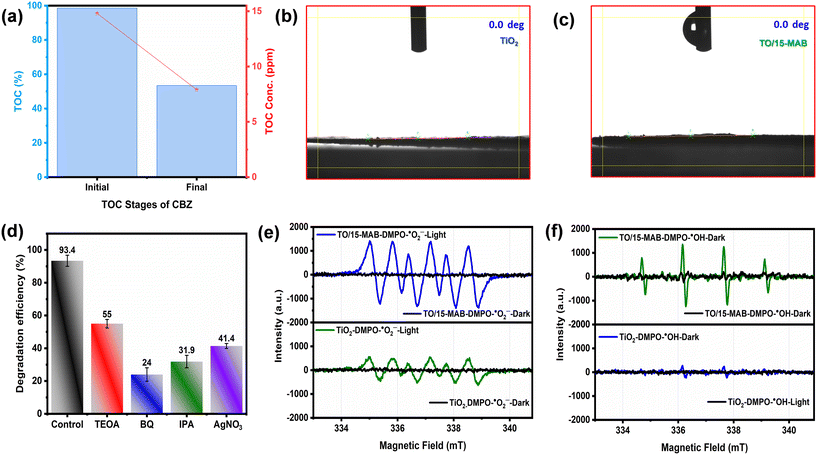
Following the photocatalytic experiment, the catalyst was subjected to recycling tests. The degradation efficiency of CBZ using TO/15-MAB composite decreased by less than 5% after five cycles (Fig. S6a†). As shown in Fig. S6(b and c),† the XRD and HRSEM analyses of TO/15-MAB composite before and after five consecutive photocatalytic cycles reveal that its crystal structure and morphology remained unchanged, respectively. These findings highlight the outstanding structural stability of the TO/15-MAB composite throughout the repeated photocatalytic trials. Fig. 11a and S7† show the relative TOC removal values of carbendazim using the optimized photocatalyst under the optimized conditions. It is evident from the results that after 60 min of photocatalytic treatment, almost half of TOC was removed from the sample, which showed the promising capability of TO/15-MAB composite for the mineralization of carbendazim.
Reactive oxygen species (ROS) are known to be generated on the photocatalyst surface, with their concentrations increasing closer to it. Previous research by Zhang et al. demonstrated that the ˙OH concentration decreases significantly with distance, dropping below 1% at over 38 nm from the catalyst.63 Therefore, when the distance between a pollutant and a photocatalyst is shorter, the greater degradation of the pollutant can be achieved, thus necessitating research on water contact and surface effects on organic pollutant degradation. As shown in Fig. 11(b and c), both the bare TiO2 and composite TO/15-MAB exhibit super hydrophilic characteristics, demonstrating that this trait remained unchanged following the fabrication of the composite, respectively. Additionally, this figure validates that both TiO2 and the TO/15-MAB composite possess the ability to generate ROS, further supporting their efficacy in driving the pollutant degradation process.
For a better understanding of the underlying ROS mechanism for the photocatalytic degradation of CBZ in the presence of the TO/15-MAB composite, which showed the highest photocatalytic activity among the as-prepared photocatalysts, trapping experiments were carried out to detect the involved active species in the presence of this photocatalyst by adding four different scavengers. As shown in Fig. 11d, the addition of TEOA (a hole scavenger) and AgNO3 (an electron scavenger) caused a slight decrease in photocatalytic activity, indicating that both holes and electrons contribute to the degradation process, but to a lesser extent. However, a significant decrease occurred with the addition of BQ (a scavenger for ˙O2−) and IPA (a scavenger for ˙OH). Thus, it can be concluded that ˙O2− and ˙OH played more important roles in the degradation of CBZ under light irradiation.
To further verify the major reactive species (superoxide (˙O2−) and hydroxyl (˙OH) radicals), EPR spectra were acquired for the bare TiO2 and TO/15-MAB composite using 5,5-dimethyl-1-pyrroline-n-oxide (DMPO) under dark and light conditions. As shown in Fig. 11(e and f), no distinct signals were observed under dark conditions, respectively. However, upon light irradiation, signals corresponding to DMPO–˙O2− and DMPO–˙OH were detected in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 intensity ratio, respectively. This indicates that light plays a crucial role in generating these radicals in both the bare TiO2 and TO/15-MAB composite. Furthermore, the intensity of the ESR peaks suggests that radical species generation in the TO/15-MAB composite is improved compared to bare TiO2. The higher intensity of DMPO–˙O2− in the TO/15-MAB composite is due to its ohmic junction, which facilitates easier electron transfer from TiO2 to Mo2AlB2. The absence of a barrier height between the metal and the semiconductor makes this electron transfer more efficient. Additionally, the internal electric field causes band bending, allowing electrons to slide directly to the metal surface, which promotes the efficient separation of electron–hole pairs.19 Subsequently, these electrons play a crucial role in reducing the dissolved O2 into ˙O2− radicals at the Fermi level of Mo2AlB2. The effective electron transfer from TiO2 to Mo2AlB2 through the ohmic junction also decreases the recombination of excited electrons and holes within TiO2 in the TO/15-MAB composite. This reduction in recombination enhances the generation of hydroxyl radicals compared to the bare TiO2, as observed in Fig. 11f.
1 intensity ratio, respectively. This indicates that light plays a crucial role in generating these radicals in both the bare TiO2 and TO/15-MAB composite. Furthermore, the intensity of the ESR peaks suggests that radical species generation in the TO/15-MAB composite is improved compared to bare TiO2. The higher intensity of DMPO–˙O2− in the TO/15-MAB composite is due to its ohmic junction, which facilitates easier electron transfer from TiO2 to Mo2AlB2. The absence of a barrier height between the metal and the semiconductor makes this electron transfer more efficient. Additionally, the internal electric field causes band bending, allowing electrons to slide directly to the metal surface, which promotes the efficient separation of electron–hole pairs.19 Subsequently, these electrons play a crucial role in reducing the dissolved O2 into ˙O2− radicals at the Fermi level of Mo2AlB2. The effective electron transfer from TiO2 to Mo2AlB2 through the ohmic junction also decreases the recombination of excited electrons and holes within TiO2 in the TO/15-MAB composite. This reduction in recombination enhances the generation of hydroxyl radicals compared to the bare TiO2, as observed in Fig. 11f.
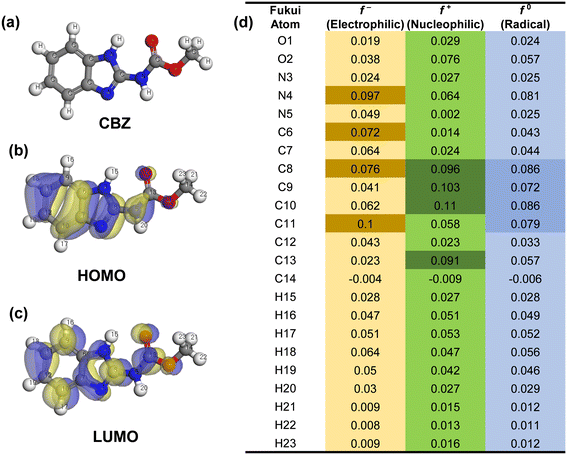
The fragile sites on the CBZ molecules were identified through DFT calculations. In Fig. 12(a–d), the Fukui electrophilic index (f−) and the highest occupied molecular orbital (HOMO) at the C11, N4, C8, and C6 sites of CBZ were found to act as electron donors, making them more prone to attack by electrophilic reagents (h+). Conversely, the nucleophilic index (f+) and the lowest unoccupied molecular orbital (LUMO) reflected the electron-accepting ability, suggesting susceptibility to ˙O2− attack, particularly in the region at the C10, C9, C8, and C13 sites. Moreover, the sites with high radical index values (f0) such as C8, C10, C11, and C9 were observed to be vulnerable to ˙OH attack. This detailed analysis provides valuable insights into the chemical reactivity and susceptibility of the specific sites within the CBZ molecule, shedding light on its potential reactions and interactions with electrophilic and nucleophilic reagents.64
Degradation pathway of carbendazim and its toxicology assessment
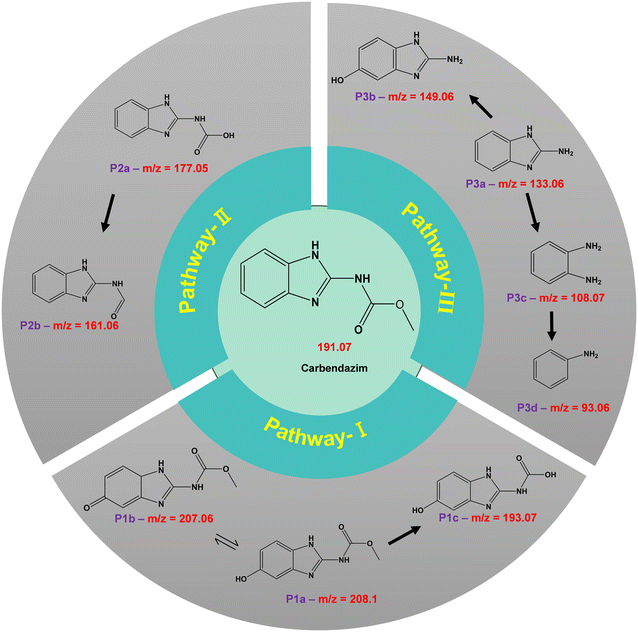
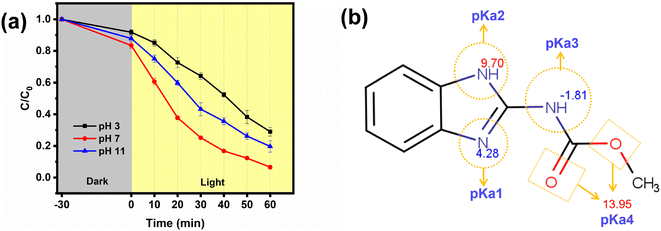
The intermediates formed during the photodegradation of carbendazim were identified using LC-MS analysis. Based on the favorable m/z values obtained (Fig. S8†) and the results of prior investigations, plausible paths were hypothesized, as illustrated in Fig. 13. In the first pathway, the product corresponding to m/z = 208.1, P1a, is formed due to the aromatic hydroxylation in the carbendazim molecule forming a hydroxy carbendazim. This derivative can be either oxidized to form a quinone imine (P1b) or demethylation to produce P1c with m/z = 193.07. In the second pathway, the P2a and P2b products are attributed to the demethylation followed by dehydroxylation of carbendazim, respectively. In the third pathway, the methyl methanoate group is removed from CBZ, resulting in product P3a, which is aromatically hydroxylated to form product P3b. Product P3c is the result of either the removal of the N-methyl carbamate group from carbendazim or the removal of methylamine via hydrolysis of product P3a. This product P3c becomes P3d after the further elimination of ammonia.65–68The Toxicity Estimation Software Tool (T.E.S.T) was utilized to evaluate the toxicity of CBZ and its degradation intermediates. The fathead minnow LC50 90 h acute toxicity measurement signifies the concentration of a substance that proves lethal to 50% of the fathead minnow population following 90 h of exposure. As depicted in Fig. 14a, the anticipated acute toxicity from CBZ due to its degradation intermediates exhibits a decrease from higher harmfulness to lower levels, indicating that the degradation route diminished the toxicity to fathead minnows. Developmental toxicants are substances capable of causing harm to a developing fetus or embryo, potentially resulting in birth defects or other developmental irregularities. Fig. 14b illustrates the developmental toxicity analysis, revealing that post degradation of CBZ, intermediate products P2b, P3b, and P3d transition into developmental non-toxicants. Additionally, certain intermediates such as P1c, P2a, P3c, and P3d show lower developmental toxicity compared to CBZ. The bioconcentration factor (BCF) serves as an indicator of the propensity of a chemical to accumulate in the tissues of organisms through environmental exposure, with higher BCF values suggesting an increased potential for tissue accumulation. In contrast to P1b and P1c, the bioconcentration factor of the CBZ intermediates displayed a significant increase (Fig. 14c). Although the degradation rates may alleviate the toxicity of the intermediates, the projected values imply that a consistent degradation process can lead to heightened bioaccumulation. Mutagenicity testing in toxicology aims to evaluate the capacity of a substance to induce mutations in living organisms. Positive mutagenicity values indicate that the substance exhibits mutagenic activity, signifying its potential to cause mutations and subsequent adverse health effects.69 The outcomes in Fig. 14d reveal that besides CBZ, P1a, P2b, and P3c, the remaining degradation intermediates exhibit negative mutagenicity, suggesting that its photodegradation substantially diminishes its genotoxicity.
 | ||
| Fig. 14 Acute toxicity of CBZ and its degraded products to fathead minnow LC50 90 h (a). Developmental toxicity (b), bioconcentration factor (c), and mutagenicity of degraded products (d). | ||
Conclusion and outlook
Herein, we demonstrated the successful synthesis of a composite material by integrating 2D Mo2AlB2 with TiO2 NPs through an ultrasound self-assembly approach to achieve an ohmic junction. The morphological analysis revealed a wide dispersion of layered Mo2AlB2, with TiO2 nanoparticles densely packed and evenly distributed on its surface. Further investigations utilizing XRD, Raman spectroscopy, and BET analysis unveiled higher structural disorder and an increased number of active sites, which are essential for photocatalytic processes, in the composite material. The DFT studies confirmed the formation of an ohmic junction at the interface between TiO2 and Mo2AlB2 due to their differing work functions, which was further verified by I–V measurements. The zeta potential revealed a reduction in the surface charge of the TO/15-MAB composite, which was facilitated by the transfer of free electrons from Mo2AlB2 to TiO2 at this junction, and further confirmed by the XPS analysis. The reduced surface charge intensified the attraction of carbendazim molecules to the TO/15-MAB composite, expediting their photodegradation. Moreover, this composite showed enhanced light absorption, reduced resistivity, and minimized recombination of photogenerated electrons and holes, promoting the redox reactions, as confirmed by UV DRS, EIS, photocurrent and PL analysis. Meanwhile, the scavenger capture experiments and ESR spectroscopy demonstrated that ˙O2− and ˙OH played key roles in the whole system, which contributed to the breakdown of CBZ into smaller compounds and exhibited remarkable efficiency, achieving a 93.4% degradation rate under solar light exposure. Moreover, the susceptibility of carbendazim to degradation was examined using the Fukui index to identify the vulnerable sites. The degradation intermediates were predicted through LC-MS analysis, while the toxicity of these intermediates was evaluated with T.E.S.T., revealing that the toxicity was reduced compared to carbendazim. Overall, this work will facilitate the understanding of utilizing MAB phase materials in ohmic junctions and the mechanistic insights of applying an electric field in photocatalytic degradation.Experimental
Synthesis of TiO2 NPs
The process for the preparation of the TiO2 nanoparticles involved utilizing the ultrasonic technique. To elaborate, 10 mL of titanium tetraisopropoxide (TTIP) and 4 mL of ethylene glycol (EG) were slowly added to 50 mL of ethanol, while stirring vigorously. The temperature of the solution was maintained at 80 °C. Subsequently, after 1 h of probe sonication in pulse mode (3 s on and 3 s off) at a power of 500 W and frequency of 20 kHz, a white precipitate was obtained. Subsequently, this precipitate was washed with deionized water. The resulting product was dehydrated for 12 h at 80 °C, and then calcined at 400 °C for 1 h.Synthesis of bulk MoAlB and layered Mo2AlB2
The bulk MoAlB was synthesized using a combination of top-down ball milling and an atmosphere-controlled heating process. Initially, a mixture of MoB (Alfa Aesar) and Al (SRL Chemicals) powders was ball-milled for 10 h before being pelletized under a pressure of 30 MPa. The resulting mixture was heated at 1250 °C for 2 h under an argon atmosphere using a tube furnace. Once heated, the pellets were finely pulverized using a mortar and pestle (images of MoAlB pellets before and after heating are shown in Fig. S9†).Next, 2 g of the bulk MoAlB powder was slowly poured into 30 mL of a 3 M LiF and 10 M HCl solution, and the mixture was constantly stirred at 40 °C for 48 h. After this period, the precipitate was stirred with a 1 M HCl solution before being filtered and washed with deionized water until the pH reached neutral. Finally, the separated powder was vacuum-dried for 12 h at 60 °C.
Synthesis of TiO2/Mo2AlB2 nanocomposites
The TiO2/Mo2AlB2 composites were synthesized using the ultrasound method. Initially, a specific amount of Mo2AlB2 powder (5, 10, 15, 20, and 50 wt%) was added to a 10 mL ethanol solution. Then, the mixture was subjected to ultrasonic treatment for 10 min to ensure its proper dispersion. Following this, 100 mg of TiO2 and 20 μL of 5% Nafion solution were introduced into the combined solution. The entire solution was subjected to continuous sonication for 20 min. This step aimed to achieve a uniform distribution of components within the composite. After the sonication process, the resulting samples were centrifuged and washed multiple times with deionized water and ethanol. This washing procedure was crucial to remove any impurities and excess reagents. Subsequently, the samples were dried under a vacuum to ensure the complete removal of any remaining solvent. To differentiate the samples based on the weight percentage of the Mo2AlB2 MAB phase, they were labeled accordingly. The labels included TO/5-MAB, TO/10-MAB, TO/15-MAB, TO/20-MAB, and TO/25-MAB, representing the different weight percentages used in the synthesis process.Data availability
The data used to support the findings of this study are available from the corresponding author (Assistant Professor Aruljothy John Bosco) upon request. The data are not publicly available owing to privacy or ethical restrictions.Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.Acknowledgements
MGN's sincere gratitude to the SRM Institute of Science and Technology (SRMIST), Kattankulathur, for providing a University Research Fellowship. Also, he extended his gratitude to the Department of Chemistry, Energy and Environmental Remediation Lab (EERL), Nanotechnology Research Centre (NRC), SRM Central Instrumentation Facility (SCIF), SRMIST, Sophisticated Analytical Instrument Facility (SAIF), IITM, and Sophisticated Analytical Instrument Facility (SAIF), CSIR-CECRI, Karaikudi, for providing the research facilities. AJB thanks the High-Performance Computing Centre, SRMIST for providing the computational facility.References
- S. Li, M. Sun, X. Huang, H. Chen, J. Zhao and Z. Li, In Situ Sonochemical Synthesis of Flower-like N-Graphyne/BiOCl0.5Br0.5 Microspheres for Efficient Removal of Levofloxacin, Dalton Trans., 2024, 53, 917–931 RSC.
- X. Huang, M. Sun, M. Humayun, S. Li, J. Zhao, H. Chen and Z. Li, In-Situ Synthesis of Efficient N-Graphyne/Bi/BiOBr Photocatalysts for Contaminants Removal and Nitrogen Fixation, J. Alloys Compd., 2024, 976, 173025 CrossRef CAS.
- Y. Zheng, M. Sun, W. Sun, X. Meng, X. Huang and Z. Li, Nitrogen-Doped Graphyne/BiOBr Nanocomposites: In-Situ Sonochemical Synthesis and Boosted Photocatalytic Performance, Sep. Purif. Technol., 2022, 301, 122062 CrossRef CAS.
- I. Nazir, A. Qureashi, A. Bashir, Z. U. Haq, F. A. Ganaie, G. N. Dar and A. H. Pandith, Synergistic Sb2S3-NiS2 Heterostructure: A Robust Electrocatalyst for Electrochemical Sensing of Hg (II), As (III) Ions and Carbendazim Fungicide, J. Environ. Chem. Eng., 2024, 12, 112793 CrossRef CAS.
- W. Liu, Y. Li, Y. Wang, Y. Zhao, Y. Xu and X. Liu, DFT Insights into the Degradation Mechanism of Carbendazim by Hydroxyl Radicals in Aqueous Solution, J. Hazard. Mater., 2022, 431, 128577 CrossRef CAS.
- Y. Wang, J. Miao, M. Saleem, Y. Yang and Q. Zhang, Enhanced Adsorptive Removal of Carbendazim from Water by FeCl3-Modified Corn Straw Biochar as Compared with Pristine, HCl and NaOH Modification, J. Environ. Chem. Eng., 2022, 10, 107024 CrossRef CAS.
- G. Li, J. Li, W. Tan, M. Yang, H. Wang and X. Wang, Effectiveness and Mechanisms of the Adsorption of Carbendazim from Wastewater onto Commercial Activated Carbon, Chemosphere, 2022, 304, 135231 CrossRef CAS PubMed.
- O. Senneca, F. Scherillo and A. Nunziata, Thermal Degradation of Pesticides under Oxidative Conditions, J. Anal. Appl. Pyrolysis, 2007, 80, 61–76 CrossRef CAS.
- Z. Long, X. Wang, Y. Wang, H. Dai, C. Li, Y. Xue, Y. Deng, H. Zhang, Y. Yu and H. Fang, Characterization of a Novel Carbendazim-Degrading Strain Rhodococcus Sp. CX-1 Revealed by Genome and Transcriptome Analyses, Sci. Total Environ., 2021, 754, 142137 CrossRef CAS PubMed.
- Y. P. Bhoi, A. K. Nayak, S. K. Gouda and B. G. Mishra, Photocatalytic Mineralization of Carbendazim Pesticide by a Visible Light Active Novel Type-II Bi2S3/BiFeO3 Heterojunction Photocatalyst, Catal. Commun., 2018, 114, 114–119 CrossRef CAS.
- T. Kaur, A. Sraw, R. K. Wanchoo and A. P. Toor, Solar Assisted Degradation of Carbendazim in Water Using Clay Beads Immobilized with TiO2 & Fe Doped TiO2, Sol. Energy, 2018, 162, 45–56 CrossRef CAS.
- X. Huang, M. Sun, W. Sun, Z. Li, H. Chen and J. Zhao, One-Step Hydrothermal Formation of Porous N-Graphyne Decorated TiO2/Ti3C2 Composites with Enhanced Photocatalytic Activity, Int. J. Hydrogen Energy, 2024, 55, 581–591 CrossRef CAS.
- W. Liu, M. Sun, Z. Ding, Q. Zeng, Y. Zheng, W. Sun and X. Meng, Ball Milling Synthesis of Porous g-C3N4 Ultrathin Nanosheets Functionalized with Alkynyl Groups for Strengthened Photocatalytic Activity, Sep. Purif. Technol., 2022, 282, 120097 CrossRef CAS.
- R. Ravichandran, A. Annamalai, K. Annamalai, A. Jeevarathinam, S. Ranganathan and S. Elumalai, Hand-Crafted Potent Hydroxyl-Rich Husk Succoured Fe3O4 @ Cu, Mn, Ni, Co – Tetra-Metallic Heterogenous Nanocomposite as a Catalytic Accelerant, Nanoscale, 2024, 16, 12081–12094 RSC.
- P. Bhatt and A. Shrivastav, Removal of Pesticides from Water and Waste Water by Microbes, in Development in Wastewater Treatment Research and Processes, ed. M. P. Shah, S. Rodriguez-Couto and R. T. Kapoor, Elsevier, 2022, ch. 17, pp. 371–399 Search PubMed.
- Y. Tao, Z. Ma, W. Wang, C. Zhang, L. Fu, Q. Zhu, Y. Li, G. Li and D. Zhang, Nickel Phosphide Clusters Sensitized TiO2 Nanotube Arrays as Highly Efficient Photoanode for Photoelectrocatalytic Urea Oxidation, Adv. Funct. Mater., 2023, 33, 2211169 CrossRef CAS.
- K. Ham, M. Salman, S. Chung, M. Choi, H. Ju, H. J. Lee and J. Lee, Selective Conversion of N2 to NH3 on Highly Dispersed RuO2 Using Amphiphilic Ionic Liquid-Anchored Fibrous Carbon Structure, J. Energy Chem., 2022, 67, 474–482 CrossRef CAS.
- J. S. Jeyaprakash, M. Rajamani, P. Mani, C. Yazhini, S. H. Sonawane and B. Neppolian, Highly Efficient Ultrasound-Driven Ti3+-Doped TiO2 with Oxygen Vacancies: A Promising Visible-Light Catalyst for Complete Sonophotocatalytic Degradation of Tetracycline, Ind. Eng. Chem. Res., 2024, 63, 5135–5147 CrossRef CAS.
- R. Shen, L. Zhang, X. Chen, M. Jaroniec, N. Li and X. Li, Integrating 2D/2D CdS/α-Fe2O3 Ultrathin Bilayer Z-Scheme Heterojunction with Metallic β-NiS Nanosheet-Based Ohmic-Junction for Efficient Photocatalytic H2 Evolution, Appl. Catal., B, 2020, 266, 118619 CrossRef CAS.
- X. Ren, J. Wang, S. Yuan, C. Zhao, L. Yue, Z. Zeng and Y. He, Decoration of CdMoO4 Micron Polyhedron with Pt Nanoparticle and Their Enhanced Photocatalytic Performance in N2 Fixation and Water Purification, Front. Chem. Sci. Eng., 2023, 17, 1949–1961 CrossRef CAS.
- C. Zhao, L. Yue, S. Yuan, X. Ren, Z. Zeng, X. Hu, L. Zhao, Y. Wu and Y. He, Enhanced Photocatalytic N2 Fixation and Water Purification Using Bi/ZnSnO3 Composite: Mechanistic Insights and Novel Applications, J. Ind. Eng. Chem., 2024, 132, 135–147 CrossRef CAS.
- J. Zhang, L. Yue, Z. Zeng, C. Zhao, L. Fang, X. Hu, H. Lin, L. Zhao and Y. He, Preparation of NaNbO3 Microcube with Abundant Oxygen Vacancies and Its High Photocatalytic N2 Fixation Activity in the Help of Pt Nanoparticles, J. Colloid Interface Sci., 2023, 636, 480–491 CrossRef CAS.
- C. Li, Y. Zhao, X. Liu, P. Huo, Y. Yan, L. Wang, G. Liao and C. Liu, Interface Engineering of Co9S8/CdIn2S4 Ohmic Junction for Efficient Photocatalytic H2 Evolution under Visible Light, J. Colloid Interface Sci., 2021, 600, 794–803 CrossRef CAS PubMed.
- Q. Gu, C. Feng, J. Rong, Y. Zhang, X. Zheng, J. Mei, Z. Li and S. Xu, NiCoP Cocatalyst Modified g-C3N4 as Ohmic Junction Photocatalyst for Efficient Degradation of Tetracycline under Visible Light, Environ. Res., 2024, 249, 118358 CrossRef CAS PubMed.
- L. T. Alameda, R. W. Lord, J. A. Barr, P. Moradifar, Z. P. Metzger, B. C. Steimle, C. F. Holder, N. Alem, S. B. Sinnott and R. E. Schaak, Multi-Step Topochemical Pathway to Metastable Mo2AlB2 and Related Two-Dimensional Nanosheet Heterostructures, J. Am. Chem. Soc., 2019, 141, 10852–10861 CrossRef CAS.
- S. Wang, Y. Xu, Z. Yu, H. Tan, S. Du, Y. Zhang, J. Yang and W. Liu, Synthesis, Microstructure and Mechanical Properties of a MoAlB Ceramic Prepared by Spark Plasma Sintering from Elemental Powders, Ceram. Int., 2019, 45, 23515–23521 CrossRef.
- A. A. Rezaie, Z. Yan, J. P. Scheifers, J. Zhang, J. Guo and B. P. T. Fokwa, Synthesis and Li-Ion Electrode Properties of Layered MAB Phases Ni: N+1ZnBn (n = 1, 2), J. Mater. Chem. A, 2020, 8, 1646–1651 RSC.
- L. T. Alameda, C. F. Holder, J. L. Fenton and R. E. Schaak, Partial Etching of Al from MoAlB Single Crystals to Expose Catalytically Active Basal Planes for the Hydrogen Evolution Reaction, Chem. Mater., 2017, 29, 8953–8957 CrossRef CAS.
- P. Kar, S. Zeng, Y. Zhang, E. Vahidzadeh, A. Manuel, R. Kisslinger, K. M. Alam, U. K. Thakur, N. Mahdi, P. Kumar and K. Shankar, High Rate CO2 Photoreduction Using Flame Annealed TiO2 Nanotubes, Appl. Catal., B, 2019, 243, 522–536 CrossRef CAS.
- K. Kim, C. Chen, D. Nishio-Hamane, M. Okubo and A. Yamada, Topochemical Synthesis of Phase-Pure Mo2AlB2 through Staging Mechanism, Chem. Commun., 2019, 55, 9295–9298 RSC.
- X. Wang, L. Sø, R. Su, S. Wendt, P. Hald, A. Mamakhel, C. Yang, Y. Huang, B. B. Iversen and F. Besenbacher, The Influence of Crystallite Size and Crystallinity of Anatase Nanoparticles on the Photo-Degradation of Phenol, J. Catal., 2014, 310, 100–108 CrossRef CAS.
- A. C. Dodd, A. J. McKinley, M. Saunders and T. Tsuzuki, Effect of Particle Size on the Photocatalytic Activity of Nanoparticulate Zinc Oxide, Catalytic Activity of Strontium Modified TiO2 Nanotubes for Hydrogen Evolution Reaction, J. Nanopart. Res., 2006, 8, 43–51 CrossRef CAS.
- K. M. Emran, Catalytic Activity of Strontium Modified TiO2 Nanotubes for Hydrogen Evolution Reaction, Int. J. Electrochem. Sci., 2020, 15, 4218–4231 CrossRef CAS.
- Y. Bai, X. Qi, A. Duff, N. Li, F. Kong, X. He, R. Wang and W. E. Lee, Density Functional Theory Insights into Ternary Layered Boride MoAlB, Acta Mater., 2017, 132, 69–81 CrossRef CAS.
- M. G. Narendran, S. Peters, A. John Bosco, G. Keerthiga, B. Neppolian, S. M. Senthil Kumar and T. Xiaoteng Liu, Pioneering Exploration of Mo2AlB2-Transition-Metal-Aluminum-Boron-Phase-Supported Hydrophobic SrTiO3/Mo2AlB2 Nanocomposite for Improved Photocatalytic Carbendazim Degradation and CO2 Reduction to Ethanol through the Schottky Junction, Sol. RRL, 2024, 8, 2301043 CrossRef CAS.
- D. Vrushabendrakumar, K. M. Alam, N. Kumar, H. Rajashekhar, N. Chaulagain, S. Riddell, A. E. Kobryn, S. Gusarov and K. Shankar, Cathodically Inserted, Widely Dispersed Sr2+ Surface Dopants Produce a Threefold Improvement in the CO2 Photoreduction Activity of TiO2 Nanotube Arrays, ACS Appl. Energy Mater., 2024, 7, 1–12 CrossRef CAS.
- Y. Zhang, S. Farsinezhad, B. D. Wiltshire, R. Kisslinger, P. Kar and K. Shankar, Optical Anisotropy in Vertically Oriented TiO2 Nanotube Arrays, Nanotechnology, 2017, 28, 374001 CrossRef.
- P. Makuła, M. Pacia and W. Macyk, How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra, J. Phys. Chem. Lett., 2018, 9, 6814–6817 CrossRef.
- L. Wang, Z. Wang, D. Wang, X. Shi, H. Song and X. Gao, The Photocatalysis and Mechanism of New SrTiO3/TiO2, Solid State Sci., 2014, 31, 85–90 CrossRef CAS.
- Y. Luo, B. Deng, Y. Pu, A. Liu, J. Wang, K. Ma, F. Gao, B. Gao, W. Zou and L. Dong, Interfacial Coupling Effects in g-C3N4/SrTiO3 Nanocomposites with Enhanced H2 Evolution under Visible Light Irradiation, Appl. Catal., B, 2019, 247, 1–9 CrossRef CAS.
- M. G. Narendran, E. Vijayakumar, M. Govinda Raj, R. Preetha, J. J. Alphin, R. Mahaan, B. Neppolian and A. John Bosco, Shining a Light on Fungicide- Water: Enhanced Photocatalytic Degradation Using the CoTiO3/CaTiO3 Nanocomposite and Experimental and Theoretical Viewpoints on Improved Intervalence Charge Transfer from O2− to Ti4+ and from Co2+ to Ti4+ Ions and Spatial Charge Transfer, New J. Chem., 2024, 48, 6109–6123 RSC.
- X. Zhu, G. Wen, H. Liu, S. Han, S. Chen, Q. Kong and W. Feng, One-Step Hydrothermal Synthesis and Characterization of Cu-Doped TiO2 Nanoparticles/Nanobucks/Nanorods with Enhanced Photocatalytic Performance under Simulated Solar Light, J. Mater. Sci.: Mater. Electron., 2019, 30, 13826–13834 CrossRef CAS.
- R. Bashiri, M. S. Irfan, N. M. Mohamed, S. Sufian, L. Y. Ling, N. A. Suhaimi and M. F. R. Samsudin, Hierarchically SrTiO3@TiO2@Fe2O3 Nanorod Heterostructures for Enhanced Photoelectrochemical Water Splitting, Int. J. Hydrogen Energy, 2021, 46, 24607–24619 CrossRef CAS.
- P. Kumar, U. K. Thakur, K. Alam, P. Kar, R. Kisslinger, S. Zeng, S. Patel and K. Shankar, Arrays of TiO2 Nanorods Embedded with Fluorine Doped Carbon Nitride Quantum Dots (CNFQDs) for Visible Light Driven Water Splitting, Carbon, 2018, 137, 174–187 CrossRef CAS.
- V. Natu, S. S. Kota and M. W. Barsoum, X-Ray Photoelectron Spectroscopy of the MAB Phases, MoAlB, M2AlB2 (M = Cr, Fe), Cr3AlB4 and Their Binary Monoborides, J. Eur. Ceram. Soc., 2020, 40, 305–314 CrossRef CAS.
- H. Park, A. Encinas, J. P. Scheifers, Y. Zhang and B. P. T. Fokwa, Boron-Dependency of Molybdenum Boride Electrocatalysts for the Hydrogen Evolution Reaction, Angew. Chem., Int. Ed., 2017, 56, 5575–5578 CrossRef CAS.
- A. V. Naumkin, A. 'Kraut-Vass, S. W. Gaarenstroom and C. J. Powell, NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database 20, Version 4.1, Gaithersburg MD, 2012 Search PubMed.
- A. Talapatra, S. K. Bandyopadhyay, P. Sen, P. Barat, S. Mukherjee and M. Mukherjee, X-Ray Photoelectron Spectroscopy Studies of MgB2 for Valence State of Mg, Phys. C, 2005, 419, 141–147 CrossRef CAS.
- Y. P. Xie, G. Liu, G. Q. (Max) Lu and H.-M. Cheng, Boron Oxynitride Nanoclusters on Tungsten Trioxide as a Metal-Free Cocatalyst for Photocatalytic Oxygen Evolution from Water Splitting, Nanoscale, 2012, 4, 1267–1270 RSC.
- Z. Zhuang, Y. Li, Z. Li, F. Lv, Z. Lang, K. Zhao, L. Zhou, L. Moskaleva, S. Guo and L. Mai, MoB/g-C3N4 Interface Materials as a Schottky Catalyst to Boost Hydrogen Evolution, Angew. Chem., Int. Ed., 2018, 57, 496–500 CrossRef CAS.
- H. Wang, H. Jiang, H. Wang, Q. Liu and P. Huo, NiS Cocatalyst–Modified ZnIn2S4 as Ohmic-Junction Photocatalyst for Efficient Conversion of CO2, Energy Technol., 2022, 10, 2101158 CrossRef CAS.
- M. Kosmulski, The Significance of the Difference in the Point of Zero Charge between Rutile and Anatase, Adv. Colloid Interface Sci., 2002, 99, 255–264 CrossRef CAS PubMed.
- J. W. Bullard and M. J. Cima, Orientation Dependence of the Isoelectric Point of TiO2 (Rutile) Surfaces, Langmuir, 2006, 22, 10264–10271 CrossRef CAS PubMed.
- L. Zheng, J. Zhang, Y. H. Hu and M. Long, Enhanced Photocatalytic Production of H2O2 by Nafion Coatings on S,N-Codoped Graphene-Quantum-Dots-Modified TiO2, J. Phys. Chem. C, 2019, 123, 13693–13701 CrossRef CAS.
- J. Wan, Y. Wang, J. Liu, R. Song, L. Liu, Y. Li, J. Li, J. Low, F. Fu and Y. Xiong, Full-Space Electric Field in Mo-Decorated Zn2In2S5 Polarization Photocatalyst for Oriented Charge Flow and Efficient Hydrogen Production, Adv. Mater., 2024, 36, 2405060 CrossRef CAS PubMed.
- J. Li, J. Yao, Q. Yu, X. Zhang, S. A. C. Carabineiro, X. Xiong, C. Wu and K. Lv, Understanding the Unique Ohmic-Junction for Enhancing the Photocatalytic Activity of CoS2/MgIn2S4 towards Hydrogen Production, Appl. Catal., B, 2024, 351, 123950 CrossRef CAS.
- W. Sun, H. Cheng, J. Zhang, X. Fang, W. Chen, J. Zhu and Y. Zheng, Allochroic Platinum/Carbon Nitride with Photoactivated Ohmic Contact for Efficient Visible-Light Photocatalytic Hydrogen Evolution, Chem. Eng. J., 2023, 462, 142337 CrossRef CAS.
- M. Preeyanghaa, E. S. Erakulan, R. Thapa, M. Ashokkumar and B. Neppolian, Scrutinizing the Role of Tunable Carbon Vacancies in g-C3N4 Nanosheets for Efficient Sonophotocatalytic Degradation of Tetracycline in Diverse Water Matrices: Experimental Study and Theoretical Calculation, Chem. Eng. J., 2023, 452, 139437 CrossRef CAS.
- R. Preetha, M. Govinda raj, E. Vijayakumar, M. G. Narendran, E. Varathan, B. Neppolian, U. Jeyapaul and A. J. Bosco, Promoting Photocatalytic Interaction of Boron Doped Reduced Graphene Oxide Supported BiFeO3 Nanocomposite for Visible-Light-Induced Organic Pollutant Degradation, J. Alloys Compd., 2022, 904, 164038 CrossRef CAS.
- C. Zhu, Y. Wang, Z. Jiang, F. Xu, Q. Xian, C. Sun, Q. Tong, W. Zou, X. Duan and S. Wang, CeO2 Nanocrystal-Modified Layered MoS2/g-C3N4 as 0D/2D Ternary Composite for Visible-Light Photocatalytic Hydrogen Evolution: Interfacial Consecutive Multi-Step Electron Transfer and Enhanced H2O Reactant Adsorption, Appl. Catal., B, 2019, 259, 118072 CrossRef CAS.
- Marvin was used for drawing, displaying and characterizing chemical structures, substructures and reactions, Marvin 17.21.0, Chemaxon (https://www.chemaxon.com).
- C. Mungsuk, S. Yommee, S. Supothina and P. Chuaybamroong, Solar Photocatalytic Degradation of Carbendazim in Water Using TiO2 Particle- and Sol-Gel Dip-Coating Filters, Results Eng., 2023, 19, 101348 CrossRef CAS.
- S. Zhang, M. Sun, T. Hedtke, A. Deshmukh, X. Zhou, S. Weon, M. Elimelech and J.-H. Kim, Mechanism of Heterogeneous Fenton Reaction Kinetics Enhancement under Nanoscale Spatial Confinement, Environ. Sci. Technol., 2020, 54, 10868–10875 CrossRef CAS PubMed.
- A. Wang, R. Wang, Y. Pan, J. Ni, X. Liang, M. Du, J. Zhang, D. Liu, J. Ma, J. Wang and W. Wang, Tandem Hybrid Catalysis System by Coupling Photocatalysis and PMS Activation Technology, Appl. Catal., B, 2024, 344, 123621 CrossRef CAS.
- A. Kiss and D. Virág, Photostability and Photodegradation Pathways of Distinctive Pesticides, J. Environ. Qual., 2009, 38, 157–163 CrossRef CAS.
- K. Y. Kumar, M. K. Prashanth, L. Parashuram, B. Palanivel, F. A. Alharti, B. H. Jeon and M. S. Raghu, Gadolinium Sesquisulfide Anchored N-Doped Reduced Graphene Oxide for Sensitive Detection and Degradation of Carbendazim, Chemosphere, 2022, 296, 134030 CrossRef.
- S. Periyasamy, J. V. Kumar, S. M. Chen, Y. Annamalai, R. Karthik and N. Erumaipatty Rajagounder, Structural Insights on 2D Gadolinium Tungstate Nanoflake: A Promising Electrocatalyst for Sensor and Photocatalyst for the Degradation of Postharvest Fungicide (Carbendazim), ACS Appl. Mater. Interfaces, 2019, 11, 37172–37183 CrossRef CAS PubMed.
- R. C. T. Temgoua, U. Bussy, D. Alvarez-Dorta, N. Galland, J. Hémez, C. Thobie-Gautier, I. K. Tonlé and M. Boujtita, Using Electrochemistry Coupled to High Resolution Mass Spectrometry for the Simulation of the Environmental Degradation of the Recalcitrant Fungicide Carbendazim, Talanta, 2021, 221, 121448 CrossRef CAS.
- R. Preetha, M. Govinda raj, E. Vijayakumar, M. G. Narendran, B. Neppolian and A. J. Bosco, Quasi-In Situ Synthesis of Oxygen Vacancy-Enriched Strontium Iron Oxide Supported on Boron-Doped Reduced Graphene Oxide to Elevate the Photocatalytic Destruction of Tetracycline, Langmuir, 2023, 39, 7091–7108 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4en00727a |
| This journal is © The Royal Society of Chemistry 2025 |