DOI:
10.1039/D4EN01041H
(Critical Review)
Environ. Sci.: Nano, 2025, Advance Article
Nanomaterials for the removal and detection of heavy metals: a review
Received
7th November 2024
, Accepted 5th February 2025
First published on 7th February 2025
Abstract
Heavy metal pollution in our water systems has become a global concern. Pollution of heavy metals in water arises from anthropogenic activities such as industrial processes, agricultural runoff, mining, and improper waste disposal. The persistent accumulation of heavy metals in aquatic environments necessitates innovative approaches for remediation. Furthermore, accurate detection and characterization of heavy metals is crucial for proper assessment when evaluating various challenges on water pollution. Emerging applications from the field of nanoscience provide promising developments for both remediation and analytical techniques required for both detection and quantification of heavy metal contaminants. This review provides a comprehensive overview on the current applications of nano-based approaches for heavy metal remediation in water and various analytical techniques based on nanomaterial-based technologies. Important advancement in both removal and characterization of heavy metals provides a holistic outlook on nanomaterials, as well as providing a comprehensive perspective on how nanotechnology can facilitate innovation in water remediation and detection of pollutants.
Environmental significance
The review paper provides a critical review on the utilization of nanomaterials, particularly materials from natural sources, such as cellulosic materials and chitin. It provides insights on how nanomaterials can be utilized to remediate heavy metal pollution, and also to enhance the analysis of trace amounts of heavy metals. The remediation of heavy metals will impact the ecosystem and the health of living systems on planet earth.
|
1. Introduction
1.1 Background on water pollution
Heavy metal contamination in water is a pressing concern due to pollution from both natural processes and human and industrial activities. Naturally driven processes such as weathering of metal rich rocks, wildfires, volcanic and geothermal activities all lead to the release of heavy metals to water bodies.1–3 Erosion of metal rich rocks releases metals such as arsenic, cadmium, and lead into both soil and larger bodies of water such as lakes or rivers.4–6 Additionally, volcanic eruptions and forest fires can release large quantities of metal-laden gases into nearby water bodies.6 Similarly, there is significant contribution of heavy metal pollution from anthropogenic activities. Many industrial processes are contributors to pollution, such as mining, smelting, and manufacturing activities.4,7 These processes directly release metals such as lead, mercury, or arsenic into water bodies through effluents, hazardous waste disposal, and atmospheric emissions.3,5,8–10 Gold mining in particular is known to release significant amounts of mercury, whereas production of steel leads to significant chromium pollution.9–11 Furthermore, agricultural runoff is also a significant source of pollutants. Specifically, fertilizers and pesticides that contain metallic ions can introduce metallic pollutants into soil which then leach into the groundwater.12–14 Arsenic and cadmium were historically used in different fertilizers leading to significant leaching of these metals into groundwater and soil.12–14 Furthermore, increasing urbanization has led to more sources of heavy metal pollution due to improper waste disposal.15 Lead, mercury, and cadmium in batteries contribute to heavy metal pollution when they are improperly disposed.15,16 It is important to note that various sources of heavy metal pollution will affect the water bodies. Rivers and lakes are commonly the recipients of industrial discharge and agricultural runoff, and lakes can act as sinks for atmospheric deposition.13 Sedimentation of heavy metals can lead to long term pollution and is a significant hazard to aquatic life.13 Furthermore, groundwater contamination can occur due to heavy metal filtration through soil, typically caused by agricultural and industrial activities.1,2,16 Groundwater pollution, in particular, is a critical issue in regions where groundwater is the primary source of potable water.12,17
1.2 Background on heavy metals
There is a comprehensive body of studies on the different heavy metals found in water bodies, thus for the purposes of this review, we will focus on the dominant heavy metals of concern, namely arsenic, cadmium, lead, mercury, and chromium. Heavy metals are described as metallic elements that are denser than water and have a large atomic radius.1,18 These include metalloid elements like arsenic that are typically characterized as severely hazardous for human health even at low concentrations in the parts per billion (ppb) or μg L−1.1,18 The dangers of heavy metals are made more prevalent due to their persistent half-life.1,5 Common organic pollutants can be degraded over time, however heavy metals remain in the environment indefinitely and are difficult to be decomposed.1,5 Lead in particular has been widely studied as a pollutant due to its high toxicity and widespread environmental presence.16 Lead contamination is widespread due to common contamination sources of lead from plumbing infrastructure and batteries in automobiles.8,16 Furthermore, lead is highly toxic, with neurodevelopmental, cardiovascular, renal and reproductive health issues at low blood levels of 1–2 μg dL−1.8 Similarly, chromium is a well-documented pollutant with significant sources from industrial processes such as electroplating.19 Chromium has been widely reported in the literature, and is known to be highly toxic and is a carcinogen, with the US Environmental Protection Agency (EPA) setting a maximum contaminant level of 100 ppb.19,20 Furthermore, cadmium is also a common pollutant from industrial activities, with a maximum contaminant level of 5 ppb set by US EPA.14,20 Mercury is another highly hazardous heavy metal that has been released into the environment by various industrial processes.10 In aquatic environments, mercury exists in the form of methylmercury, which can be absorbed by aquatic organisms, and this poses a significant risk for human that consumes these contaminated seafood.10 Lastly, arsenic is a naturally occurring metalloid with severe health risks such as skin lesions, cancer, and various cardiovascular diseases.2,12,13 It is a common pollutant from agricultural activities since it has been historically used in pesticides, which makes it a severe pollutant for groundwater and in regions that use groundwater for consumption.2,12,13 The concentration limit of these heavy metals set by the US EPA and their known health hazards are summarized in Table 1. Key takeaways from these summarized findings underscore the severity of potential toxic effects from exposure to these heavy metals as well as the wide range of possible health effects ranging from respiratory, neurological and carcinogenic. Furthermore, the table highlights how each metal has a very low threshold for potential damage emphasizing the critical need for effective remediation strategies given that all five metals are toxic even in the ppb range.
Table 1 Heavy metal health effects and U.S EPA set limits (MCL) in water
| Heavy metal |
Health hazards17 |
Concentration limit21 |
| Arsenic |
- Carcinogenic |
10 ppb |
| - Skin lesions |
| - Circulatory system damage |
| Chromium |
- Known carcinogen |
100 ppb |
| - Severe respiratory effects |
| Cadmium |
- Kidney damage |
5 ppb |
| - Severe gastrointestinal effects |
| Lead |
- Neurodevelopmental effects |
15 ppb |
| - Kidney damage |
| - Hypertension and heart issues |
| Mercury |
- Kidney damage |
2 ppb |
| - Fatal at low concentrations |
| - Neurotoxin |
1.3 Current filtration technologies
Due to the prevalence of heavy metal pollution, there has been an abundance of research exploring different technologies for remediation. These techniques include adsorption-based methods, membrane filtration, ion-exchange resins, chemical precipitation, photocatalytic separation, and electrochemical separation.2,3,6,7 Each of these techniques faces specific challenges and disadvantages. Adsorption and membrane-based methods both require regeneration and reuse of the adsorptive material and the membrane.2,3,6,7 The cost of regeneration and the loss in efficiency over time are challenges that inhibit widespread use of these methods. Similarly, ion-exchange resins require regeneration in order to maintain efficacy, a process that is costly.2,3,6,7 Chemical precipitation methods are commonly used for large scale treatment but they face severe challenges due to the generation of hazardous chemical sludge.2,3,6,7 Electrochemical methods face issues in terms of energy consumption which create a significant barrier to large scale commercialization.2,3,6,7 Finally photocatalytic methods show promise in terms of effectiveness and environmentally friendly remediation but they face drawbacks in terms of scalability.2,3,6,7 Furthermore, selective removal of different heavy metals is a challenging issue given that wastewater contains a variety of different pollutants that reduce the removal efficiency.6,18,21 Similarly, different remediation methods face issues in terms of efficiency under different pH conditions requiring an optimal pH to achieve high filtration efficacy.6,18,21 From these methods, nanomaterial-based methods are emerging as attractive candidates for remediation. The desirable properties of nanomaterials include their high surface area and large amounts of reactive sites together with tunable surface chemistry that gives nanomaterial-based methods a high degree of efficiency and versatility.22–24 Furthermore, the use of nanomaterials offers a more cost-effective approach for regeneration, a characteristic that is crucial for scalability.22–24 The versatility of nanomaterials allows for a variety of mechanisms for heavy metal remediation, such as adsorption, ion-exchange, and photochemical separation.22–24 Additionally, there is a comprehensive body of literature describing the different functional groups that can be grafted onto nanomaterials such as hydroxyl, thiol, and amino groups, among others that facilitate a wide range of possible adsorption mechanisms.22–24 There is an abundance of versatile applications and variety of nanomaterials for heavy metal filtration of different types of heavy metals.
1.4 Analysis and detection methods for heavy metals
A crucial aspect in addressing water pollution is the accurate quantification and characterization of heavy metal concentrations in water. Three common areas of analysis are spectroscopic, optical and electrochemical methods. Spectroscopic techniques such as atomic absorption spectroscopy and inductively coupled plasma mass spectrometry are commonly used for the analysis of heavy metals in water.13,18,21 Similarly, optical strategies such as colorimetric sensors or fluorescence-based spectroscopy are popular due to their ease of use which relies on the quantitative analysis of visible color changes for the detection of different heavy metals.13,18,21 Finally, electrochemical methods are widely used for on-site heavy metal characterization and detection due to their advantages in terms of rapid response and high sensitivity.13,18,21 Given the severity of heavy metals on human health in trace amounts, there is strong interest in the improvement and development of methods for heavy metal analysis.13,18,21 Spectroscopic methods suffer from interference from competing heavy metal ions that diminish their ability to selectively identify heavy metals in highly polluted environmental samples.13,18,21 Additionally, spectroscopic methods suffer from difficulties in sample preparation and cost of equipment.13,18,21 Optical methods, while easy to use, suffer from low sensitivity when compared to more expensive analytical techniques.13,18,21 Furthermore, optical based strategies have limitations in terms of selectivity, for example colorimetric dyes can often bind multiple competing heavy metal ions.13,18,21 Finally, electrochemical methods suffer from similar issues in terms of detection limits depending on the electrode material and they are sensitive to interference from competing heavy metal ions.13,18,21 Given that heavy metal pollution is widespread and diverse, there is a significant need for more advanced analytical technology such as the emerging interest in the incorporation of nanomaterials to enhance the sensitivity of different analytical techniques.
2. Nanomaterials for heavy metal remediation
2.1 Carbon based nanomaterials
2.1.1 Carbon nanotubes. Carbon nanotubes (CNTs) have been shown to be effective in removing toxic organic contaminants and heavy metals.25–29 CNTs have lengths ranging from a few micrometers to several millimeters, while their diameters are typically in the nanometer range, often between 1 and 100 nanometers, resulting in a larger specific surface area and aspect ratio. It resembles a cylindrical tube with a hollow core, featuring carbon atoms that are sp2-hybridized and arranged in a hexagonal pattern. These carbon atoms are arranged to form a surface consisting of one or more layers of graphene sheets. CNTs formed by one layer of graphene sheet are single-walled carbon nanotubes (SWCNTs), while those with more layers of graphene sheets form multi-walled carbon nanotubes (MWCNTs), as depicted in Fig. 1. The high surface area, abundant mesopores, layered and hollow structure provide a large surface area for heavy metal adsorption. In addition, CNTs possess a modifiable surface, where it is possible to optimize the removal efficiency of heavy metals via modification that increases the surface area and introduces more active adsorption sites.30–32 The process of removing heavy metals using CNTs is complex and involves several mechanisms. These include electrostatic interactions, formation of surface complexes, ligand exchange and sorption–precipitation between the heavy metal ions and the functional groups on the CNT surface.33
 |
| | Fig. 1 Structure of single-walled carbon nanotubes (a) and multi-walled carbon nanotubes (b). | |
Compared to multi-walled carbon nanotubes (MWCNTs), single-walled carbon nanotubes (SWCNTs) display higher removal efficiency for heavy metal ions. Non-modified CNTs exhibit low efficiency in removing heavy metal ions from wastewater due to the aggregation of CNTs driven by weak van der Waals interaction between the CNTs.34,35 The accessible surface area is greater in bundled CNTs compared to individual nanotubes. There are four potential sites for the adsorption of contaminants on the SWCNT bundles as illustrated in Fig. 2. These four adsorption sites consist of the external and internal surface of nanotubes, channels between nanotubes known as the interstitial sites, and outer grooves between the nanotubes.36 MWCNTs differ from SWCNTs in that they do not form bundles, hence, the active sites for adsorption are present on both the exterior and interior surfaces of the nanotubes, as well as within the aggregated pores formed by the merging of adjacent tubes.29,37 Although the aggregation of CNTs increases the accessible binding sites for contaminants, it can also contribute to poor dispersibility which has a negative impact on the removal efficiency.35
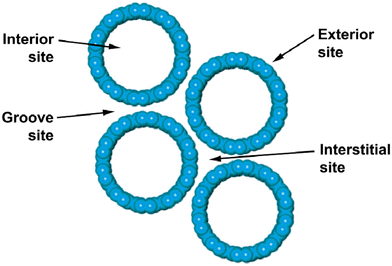 |
| | Fig. 2 Schematic model illustrating the potential adsorption sites within a SWCNT bundle.36 | |
Therefore, modification of CNTs is a necessary strategy to improve the adsorption performance. One of the common modification methods is to introduce functional groups such as –COOH, –CONH2, –CO and OH onto CNTs to improve the dispersibility and hydrophilicity for the adsorption of heavy metals.31,38–40 For instance, CNTs with carboxylic functional groups displayed 100% removal efficiency for lead (Pb2+) at neutral pH.38 The removal efficiency of heavy metal ions such as cadmium (Cd2+), copper (Cu2+), lead (Pb2+) and mercury (Hg+) on SWCNTs is enhanced by the functional groups, such as –COO−, –OH and –CONH2. In the case of CNT-COO−, an adsorption of 120–230% was reported.39
In comparison to pristine CNTs, CNT-OH and CNT-CONH2 displayed an adsorption efficiency of 10–47%.39 The excellent adsorption capacity towards metal ions by CNT-COO− was also reported by several research groups.31,41,42 The governing adsorption mechanism of metal ions on CNT-COOH is ion exchange, as illustrated in Fig. 3.43 Therefore, the adsorption behavior is pH-dependent and metal concentration dependent.44 At higher pH and initial metal ion concentration, CNTs with –COOH and –OH groups display greater adsorption capacity.44 Acid treatment or oxidation is one approach to introduce –COO− and –OH groups, while grafting functional groups onto CNTs is another method to increase oxygen-containing functional groups.45–48 To enhance the removal of metal ions, multiple functional groups were introduced to CNTs, including grafting ethylenediamine tetraacetic acid (EDTA), amino and carboxyl groups.49 Functionalization of CNTs by oxidation and ethylenediamine can introduce functional groups, such as –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –OSO3, OH and –CONH–(CH2)2–NH2 to the CNTs.50 This can be seen in the recent research by Elghamry et al. on the production of acid functionalized multiwalled carbon nanotubes.32 Their work utilized benzimidazole to produce a novel functionalized MWCNT that displayed strong adsorption for both lead and cadmium ions, confirming the versatility of surface modifications of CNTs for heavy metal remediation.32
O, –OSO3, OH and –CONH–(CH2)2–NH2 to the CNTs.50 This can be seen in the recent research by Elghamry et al. on the production of acid functionalized multiwalled carbon nanotubes.32 Their work utilized benzimidazole to produce a novel functionalized MWCNT that displayed strong adsorption for both lead and cadmium ions, confirming the versatility of surface modifications of CNTs for heavy metal remediation.32
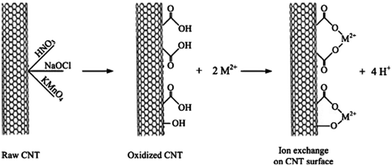 |
| | Fig. 3 Adsorption mechanism of heavy metals on CNTs with COOH and OH groups.43 | |
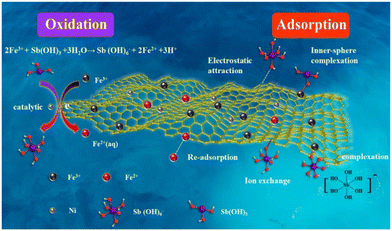
In addition to the above modification of introducing active functional groups to the CNTs, the combination of metal oxides and CNTs has generated considerable attention. The enhanced removal of metal ions by the composites of oxidized CNTs and metal oxides is primarily attributed to several mechanisms, such as adsorption, ion exchange, surface complexation and electrostatic interactions, as shown in Fig. 4.30 The presence of metal oxides on the CNT surface increases the number of active sites for metal ion binding. Specifically, metal oxides such as iron oxide (Fe3O4), manganese dioxide (MnO2), titanium dioxide (TiO2), aluminum oxide (Al2O3) and zinc oxide (ZnO) have a high affinity for certain metal ions due to their ability to form strong bonds with metal cations.51–56 The combination of magnetic nanoparticles such as Fe3O4 and MnO2 and CNTs makes it easier to separate the adsorbent from the liquid.56 Moreover, the composites exhibit good stability and reusability, making them suitable for practical applications in removing heavy metal ions. However, challenges remain in optimizing the synthesis methods to ensure uniform distribution of metal oxides.
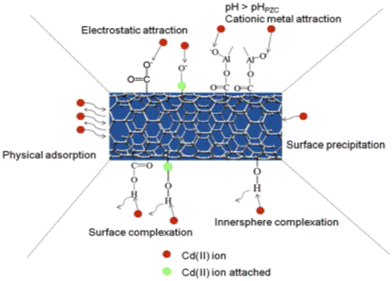 |
| | Fig. 4 Schematic showing the interaction of Cd(II) ions with Al2O3/MWCNTs.30 | |
2.1.2 Graphene and graphene oxide. Graphene has been shown to have great potential in removing heavy metal ions from wastewater due to its large surface area and high stability, however it displays several disadvantages. For CNTs, the van der Waals interactions and π–π stacking between graphene sheets make it difficult to be dispersed in a polar solvent.55 Graphene oxide, a modified variant of graphene, exhibits good adsorption towards cations like copper and lead.57 The pronounced hydrophilic nature of the oxygen-containing reactive groups on graphene oxide, including –COOH, –OH and epoxy groups, enables its dispersion in solvents and provides binding sites for the complexation of metal ions, facilitating its adsorption towards metal ions.58In addition, graphene and graphene oxide could be combined with magnetic metal oxide to address the separation problem.59 The adsorption of TiO2-graphene oxide toward zinc (Zn2+), Cd2+, and Pb2+ approached 88.9 ± 3.3 mg g−1, 72.8 ± 1.6 mg g−1 and 65.6 ± 2.7 mg g−1 after 12 h, respectively, while graphene oxide exhibited a rather low adsorption capacity for these metal ions of only 30.1 ± 2.5 mg g−1 for Zn2+, 14.9 ± 1.5 mg g−1 for Cd2+, and 35.6 ± 1.3 mg g−1 for Pb2+.60 In addition to TiO2, many types of metals or metal oxides can be used to modify graphene oxide, such as Fe3O4, MnO2, silver (Ag), γ-Fe2O3, copper oxide (CuO), zinc oxide (ZnO), zirconium dioxide (ZrO2), iron (Fe), manganese (Mn), nickel oxide (NiO) and gadolinium(III) oxide (Gd2O3).59,61–67 The removal mechanism of graphene/graphene oxide includes adsorption by cation–π interaction, complexation with functional groups containing oxygen, electrostatic interactions between metal ions and ionized oxygen-containing functional groups, ion exchange and reduction of metal ions. The composite of metal oxide and graphene/graphene oxide benefits from the synergistic effects of both components. Aside from the mechanisms described above, it also includes the complexation with metal oxides, ion exchange between the heavy metal ions and ions in the metal oxide lattice and catalysis as summarized in Fig. 5.68 There are also other materials used in the modification of graphene/graphene oxides for the removal of heavy metal ions, such as silica, β-cyclodextrin, polymethacrylamide, polyethylenimine, CNTs, 3-aminopyrazole, thiosemicarbazide and alginate.69–77
 |
| | Fig. 5 Removal mechanism of heavy metal ions by graphene and graphene oxide.68 | |
2.2 Metal nanoparticles such as titanium dioxide and iron oxide
Nanosized metal oxides (NMOs) have significant potential for use as alternative adsorbents in wastewater treatment processes. Their large surface area, well-defined pore structures, substantial pore volume, and high adsorption capacity make them particularly effective. Additionally, the ability to readily alter their surfaces adds to their distinct advantages as adsorbents.78 The mechanisms by which metal oxides remove heavy metals are influenced by the functional groups on the NMOs or the type of heavy metal ion being adsorbed and the composition of the NMO itself. For example, it has been reported that the adsorption performance of magnesium oxide (MgO), Al2O3, and TiO2 towards multiple component solutions of Cd2+, Cu2+, nickel (Ni2+), and Pb2+ was 594.9, 114.6, and 49.4 mg g−1, respectively.79 The removal mechanism of MgO is adsorption and precipitation, whereas adsorption is the main mechanism for TiO2 and Al2O3.79 The adsorption of metal ions on NMOs is achieved by adsorption, ion exchange, complexation with surface hydroxyl groups or other functional groups on the metal oxides, co-precipitation, redox reactions, photocatalysis (in the case of semiconductor oxides, like titanium dioxide, zinc oxide, and cerium oxide), and electrostatic attraction between metal ions and the charged groups on metal oxides, as shown in Fig. 6.79–84 Iron oxide nanoparticles are one of the most popular NMOs (manganese oxides, aluminum oxides, titanium oxides, zinc oxides, magnesium oxides and cerium oxides) used for the removal of metal ions since they are nontoxic, magnetic, display fast adsorption and are widely found in nature.85–87 To further enhance the removal of metal ions by iron oxide nanoparticles, some modifications were performed. For instance, Fe3O4 coated with ascorbic acid exhibited a maximum adsorption capacity of 16.56 mg g−1 for arsenate, As(V), and 46.06 mg g−1 for arsenite, As(III), which is due to the increased surface area and improved dispersibility.88
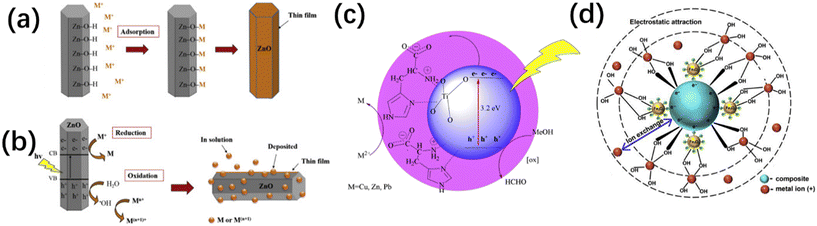 |
| | Fig. 6 Mechanisms of heavy metal ion removal using ZnO or TiO2 particles via (a) adsorption on ZnO (ref. 83), (b) reduction/oxidation process on ZnO (ref. 83) and (c) photocatalysis on TiO2 (ref. 82). d) Potential reactions involved in the adsorption of metal ions by magnetic iron oxide nanoparticles.84 | |
Besides NMOs, pure metal nanoparticles could also be used for the removal of heavy metal ions, such as nano zerovalent iron and silver. Typically, iron is highly reactive when exposed to oxygen and water, and this reactivity becomes even more pronounced when its size is reduced to the nanoscale. The surface of nano zerovalent iron comprises of FeO, Fe2O3, Fe3O4, and FeOOH.89 The removal mechanism of metal ions by nano zerovalent iron is similar to iron oxides, as shown in Fig. 7.90 However, the removal efficiency of nano zerovalent iron is significantly impacted by the mass ratio between the nano zerovalent iron and the heavy metal ions and solution pH. When the ratio is 7–9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the removal amounts of Cd2+, Cu2+, Ni2+ and Pb2+ are 79.33–102 mg g−1, 111.11–142.85 mg g−1, 107.30–137.69 mg g−1 and 110.97–142.68 mg g−1, respectively. However, the removal efficiency decreased to very low levels when the ratio was increased to 140–180
1, the removal amounts of Cd2+, Cu2+, Ni2+ and Pb2+ are 79.33–102 mg g−1, 111.11–142.85 mg g−1, 107.30–137.69 mg g−1 and 110.97–142.68 mg g−1, respectively. However, the removal efficiency decreased to very low levels when the ratio was increased to 140–180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the heavy metal ions were acidified. This is related to the deposition of iron and the subsequent release of heavy metals.91 More recent research into nano zerovalent iron (nZVI) focuses on addressing the aggregation of nZVI as this reduces the potential binding sites for heavy metal sorption. A recent study by Awang et al. utilized biochar to improve stabilization and reduce aggregation behaviour, which allowed for enhanced filtration of heavy metals such as chromium, cadmium, and lead.89
1, and the heavy metal ions were acidified. This is related to the deposition of iron and the subsequent release of heavy metals.91 More recent research into nano zerovalent iron (nZVI) focuses on addressing the aggregation of nZVI as this reduces the potential binding sites for heavy metal sorption. A recent study by Awang et al. utilized biochar to improve stabilization and reduce aggregation behaviour, which allowed for enhanced filtration of heavy metals such as chromium, cadmium, and lead.89
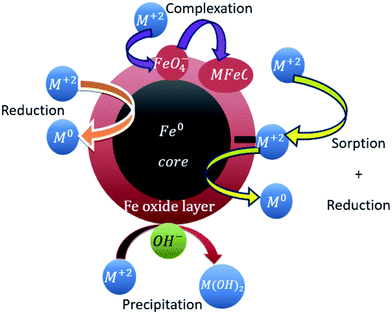 |
| | Fig. 7 Removal mechanism of metal ions by nano zerovalent iron.90 | |
2.3 Biobased nanomaterials
Biobased nanomaterials are derived from natural and renewable sources such as plants, bacteria and algae.22,92,93 They confer strong advantages in terms of sustainability, environmental friendliness, biocompatibility, and low cost.22,92,93 Specifically, biobased materials such as cellulose nanocrystals can be extracted from plant sources at a relatively low cost as detailed in Fig. 8.22,92,93 Additionally, these materials confer specific advantages offered by other nanomaterials such as high surface area and versatile surface chemistry.22,92,93 There is a significant body of literature describing biobased nanomaterials such as cellulose nanocrystals, cellulose nanofibers, and different nanocomposites based on chitosan and chitin for heavy metal removal with the primary mechanism for remediation being adsorption.22,92,94–97 Additionally, the abundance of literature in modifying these materials has articulated a comprehensive body of work on how the same biobased nanomaterials can be modified with different functional groups for different applications.22,92,93
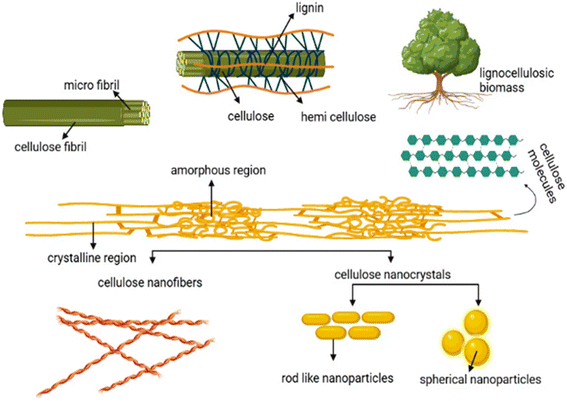 |
| | Fig. 8 Extraction and preparation sequences for cellulose nanocrystals (CNC) and cellulose nanofibers (CNF).22 | |
Cellulose nanocrystals and cellulose nanofibers are two distinct forms of cellulose based nanomaterials.92,94 Both materials are derived from natural cellulosic materials, the most abundant biopolymer on Earth, with each offering distinct properties for different applications.92,94,95 Cellulose nanocrystals (CNC) are rod-like nanoparticles with highly crystalline domains and are produced via acid hydrolysis of cellulosic sources.89,92,94 Cellulose nanofibers (CNF) are long flexible nanofibrils produced through mechanical disintegration of cellulose pulp.22 There is a significant body of work in modifying nanocellulose materials for adsorption of different heavy metals. The high versatility of these modifications includes grafting thiol groups, amino groups, and various types of carboxylic acid moieties onto the surface of nanocellulose.98–101 This comprehensive body of work presents a wide variety of options for tackling different heavy metal pollutants. For example, Li et al. described the use of L-cysteine modified cellulose by chemical grafting of L-cysteine moieties onto the hydroxyl groups of CNC via periodate oxidation followed by reductive amination reactions as detailed in Fig. 9.98 The L-cysteine modified CNC exhibited a high removal efficiency of 93% for mercury (Hg(II) ions) and displayed selective removal of Hg(II) ions with other competing heavy metal ions.98 The work detailed the importance of using L-cysteine as a green and naturally occurring biosorbent alongside CNC.98 Furthermore, it is important to highlight the strong binding affinity between the thiol, amino, and carboxyl groups of L-cysteine and mercury ions.98 Specifically, the lone pair of electrons on each of the sulfur (S), nitrogen (N) and oxygen (O) elements can rapidly complex mercury ions, where there is a high adsorption affinity between thiol groups and mercury ions.98 Note that the affinity between thiol groups and mercury ions as defined by hard–soft acid base theory facilitates the selective removal of mercury ions in the presence of other heavy metals such as lead, cadmium, zinc and copper. Furthermore, Kardam et al. described the use of cellulose nanofibers for the adsorption of cadmium, nickel, and lead via appropriate modification of the hydroxyl groups on CNF.93 Furthermore, acid hydrolysis imparts a negative surface charge that facilitates electrostatic attraction to positively charged heavy metal ions.93 Also, pH was found to impact the metal binding due to van der Waals forces and ion exchange between CNF and metal cations.93 The results were promising with high removal efficiencies and regeneration capabilities.93 Specifically, a high mass loading efficiency for cadmium of 9.7 mg g−1, 9.42 mg g−1 for lead, and 8.55 mg g−1 for nickel has been reported.93 Additionally, regeneration studies were performed via desorption using acid mixtures, such as mixtures of HCl/HNO3 and HNO3 to remove metals from the surface of CNF.93 Adsorption efficiencies above 80% were maintained for cadmium, nickel and lead for three cycles following desorption, indicating the reusability of the material up to three adsorption cycles.93 Similarly, Najib and Chrisodoulatos detailed the use of CNF modified with quaternary amine groups to remove arsenic in the form of arsenate As(V).102 Specifically, glycidyl trimethylammonium chloride was grafted onto CNF, which could then bind As(V) ions via electrostatic interactions between the positively charged quaternary ammonium groups and negatively charged arsenate ions.102 The work reported a high removal efficiency using this process, with removal efficiency of As(V) ions greater than 97% in a pH range of 4–8 and 88% below pH 4. However, there was a marked reduction in removal efficiency to 49% above a pH of 8.102 It described the importance of pH on the speciation of As(V) displaying varying affinities of As(V) for the CNF depending on the dominant species.102 Specifically, H3AsO4, H2AsO4−, HAsO42−, and AsO43− are different species of arsenate ions with the protonated forms existing at lower pH values and with smaller electrostatic attraction compared to the more deprotonated forms at higher pH values.102 Furthermore, at higher pH values there is competition for the affinity of the quaternary amine with hydroxyl ions, thereby lowering the efficacy of arsenate adsorption. These results are consistent across different studies with promising results for removal of different heavy metals that depended on the types of functionalization. Many studies have reported the removal of chromium, mercury, lead, arsenic, cadmium, nickel, and copper with high efficiencies across each pollutant.93,103–105
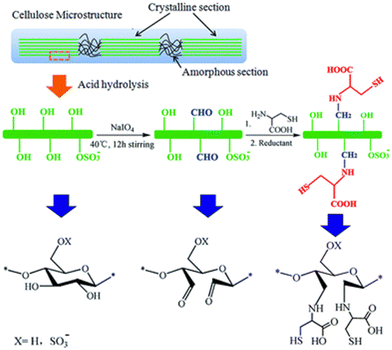 |
| | Fig. 9 Example of surface modification of CNC to produce L-cysteine grafted CNC, with dialdehyde cellulose nanocrystals as an intermediate.98 | |
Key takeaways from a comprehensive review of the literature suggest the importance of tunable surface chemistry for selective heavy metal removal. The versatility in removing different heavy metals and the benefits of using biological materials for heavy metal removal are gaining popularity. It is important to note that cellulosic nanomaterials can be derived from different sources including rice husks, coconut coir and various types of pulp fibers, and there is an abundance of sources that can be deployed for the production of heavy metal adsorbents.106–108 It is also important to note that different nanocomposites can be enhanced via the surface chemistry of biobased polymers such as chitosan and chitin.97,109 Current research focuses heavily on different media in encasing biobased nanomaterials for heavy metal remediation. For example, Kim et al. showcased the remediation of chromium ions via carboxymethyl cellulose nanofibrils embedded within the macrobeads.105 Similar advances were reported by Dou et al. on utilizing nano calcium carbonate doped chitin hydrogel for the removal of copper and cadmium ions.94 Given the promising results of biobased nanomaterials for heavy metal removal, there is strong interest in developing the field for commercialization.
2.4 Nanocomposites
Nanocomposites are advanced polyphasic materials made up of two or more types of nanomaterials.24,110,111 These materials are defined by a structure consisting of multiple phases, with at least one phase having nanoscale dimension.24,110,111 The nanoscale phase introduces a distinctive high surface area to volume ratio to the nanocomposites, which is crucial in developing adsorption based technologies.24,110,111 Nanocomposites can be divided into three groups, characterized by the different types of nanomaterials used. Ceramic matrix nanocomposites are materials where one or more ceramic phases are mixed to enhance different properties such as thermal resistance, chemical stability, or mechanical resistance.111–113 Polymer matrix nanocomposites are nanocomposites containing a polymer matrix mixed with nanofillers and are often used for energy-based applications.24,110,111 Finally, metal matrix nanocomposites are nanocomposites with different embedded metal materials such as metal nanoparticles and are widely utilized in the automotive industry.24,110,111
There is a comprehensive body of literature detailing the various advantages and applications of nanocomposites for heavy metal remediation of a wide range of heavy metals. Nanocomposites possess several desirable qualities that are being exploited for different types of remediation applications. These qualities include high surface area for adsorption and versatile surface modifications to enhance their performance for a specific application.24,110,111 Nanocomposites also possess desirable physical properties such as enhanced mechanical, thermal & chemical stability and durability.23,114,115 Furthermore, the combinations of nanomaterials often lead to synergistic effects that enhance their performance.112,113,116–118 For example, Musico et al. described the improvement of lead removal via a polymer-based graphene oxide nanocomposite.119 Specifically, the binding capacity of lead ions was enhanced with increasing amounts of graphene oxide (GO) incorporated into a polymer matrix of poly(N-vinylcarbazole) (PVK) as outlined in Fig. 10b.119 The work attributes the increasing amounts of oxygen containing functional groups with increasing adsorption for lead, achieving a maximum adsorption capacity of 887.98 mg g−1.119 These results were elucidated by the FT-IR spectra as shown in Fig. 10a.119 There is a broad peak for the PVK-GO and GO spectra at approximately 3200 cm−1 which defines the hydroxyl groups and a sharper peak at 1200 cm−1 that represents the carbonyl stretching of the carboxylic acid groups.119 Subsequent characterization of the PVK-GO nanocomposite before and after exposure to lead (Pb2+) ions via ATR-IR shows strong evidence of the hydroxyl groups and carboxylic acid groups in the binding and removal of heavy metal ions.119 Specifically, there is a marked reduction in the absorbance spectra at 1200 and 3200 cm−1 following the exposure to Pb2+, confirming the binding of lead to the hydroxyl and carboxylic acid groups.119 Furthermore, PVK-GO possesses the common advantages of polymer matrix nanocomposites, such as the versatility of fabrication, polymerization methods, and dispersion.119,120 The synergistic effects between nanomaterials were also articulated by Pitoniak et al. where they reported the use of silica–titania nanocomposites for mercury removal.121 Specifically, titanium dioxide (TiO2) possesses photocatalytic properties that facilitate the conversion of elemental mercury (Hg0) into mercuric oxide (HgO), which is less volatile and therefore less likely to be released to the atmosphere.121 Silica on the other hand possesses a high surface area that functions as a concentrator for the adsorption of elemental mercury.121 Once a specific amount of mercury is accumulated on the silica surface, UV light activates the titanium dioxide which photocatalytically oxidizes Hg0 to HgO.121 The photocatalytic property of TiO2 and the high surface area of silica promoted the efficient removal of mercury greater 90% for elemental mercury.121 Furthermore, both silica and titanium dioxide are natural occurring materials derived from fly ash, making the nanocomposite environmentally friendly.121 There is a significant body of work detailing how different nanomaterial compositions in the nanocomposites could be extended for the removal of different heavy metals, such as chromium, cadmium, arsenic, mercury, lead and others.112,118,122–125
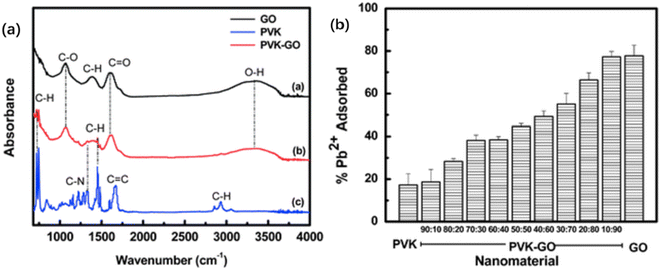 |
| | Fig. 10 a) ATR-IR spectra of graphene oxide (GO), poly(N-vinylcarbazole) (PVK), and PVK-GO polymer nanocomposite. b) Lead (Pb2+) adsorption at varying PVK-GO concentration ratios.119 | |
In addition, there are several articles detailing the regeneration of nanocomposites, a feature vital for sustainable and cost-effective approaches for remediation. Charpentier et al. described the design of magnetic chitosan and carboxymethyl chitosan (CMC) nanoparticles for the removal of lead, copper, and zinc ions.110 These composite nanoparticles possess unique advantages in terms of the high metal binding capacity of carboxymethylchitosan alongside the desirable quality of being a magnetic adsorbent allowing for easy separation.110 These qualities facilitated the strong binding of the heavy metals together with the possibility of regenerating the nanoparticles using ethylenediaminetetraacetic acid (EDTA) solution under acidic conditions allowing for their reuse in other water treatment applications.110 Specifically, EDTA chelation can strip the adsorbed metal ions, whereas the nanoparticles could be removed from water via magnetic separation for further use.110 The magnetic property is imparted via the incorporation of iron oxide, with the appearance of the final colloidal CMC nanoparticles being characterized by high resolution TEM.110 Similarly, Zubair et al. outlined the mechanism of arsenic filtration for both As(III) and As(V) via mesoporous zerovalent iron-magnetite nanocomposites.126 Similar to the magnetic based materials developed by Charpentier et al., Zubair et al. reported the preparation of unique nanocomposites that possessed many advantageous magnetic properties.126 Bimetallic oxides exhibited better removal performance for the removal of arsenic due to the synergetic effect between zerovalent iron (Fe0) and magnetite (Fe3O4), as described in Fig. 11.126 Specifically, there is electron transfer from Fe0 to Fe3O4 via the formation of Fe2+ on the surface which is believed to improve arsenic remediation.126 The arsenic uptake via iron oxides has been reported to form via the inner sphere complexes but there is additional evidence to suggest that a surface redox mechanism partially contributes to the arsenic uptake as shown by the partial redox transformation of As(V) to As(III) on the surface of the nanoparticles.126 Furthermore, regeneration could be performed via a simple procedure with the nanocomposite being dispersed in 0.1 M NaOH and then separated with an external magnet.126 The composite exhibited excellent adsorption capacity for both As(III) and As(V) at 62.6 and 1000 μmol g−1, respectively, and exhibited exceptional regeneration performance with only a 10 and 6% reduction for As(III) and As(V), respectively, after four repeated uses.126
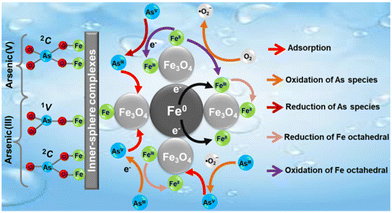 |
| | Fig. 11 Mechanism of arsenic removal via bimetal oxide materials.126 | |
By reviewing the significant body of work on nanocomposites, several key observations on the value of these materials are evident. Firstly, there are several designed nanocomposites that can be regenerated and reused through multiple cycles of adsorption of different heavy metals with minimal loss in efficiency. These materials exhibited promising results for larger scale applications where the cost of materials could be optimized for scalability. Secondly, nanocomposites possess a high degree of versatility in terms of their intended application for heavy metal adsorption, with different types of nanocomposites incorporating different nanomaterials for selective adsorption. Thirdly, they are highly efficient for the selective adsorption of ions due to the synergy of different types of nanomaterials. For example, some nanomaterials could catalyze speciation or photocatalytic conversion of heavy metal ions while other nanomaterials could be used for adsorption. Finally, it is important to note that many nanocomposites have incorporated biobased materials such as chitosan, chitin, or nanocellulose based materials.109,127–129 For example a recent study by Baruah et al. on the utilization of a nanocomposite containing nanocellulose and iron oxide for the remediation of arsenic in groundwater showed promising results.120 Biobased materials are advantageous in terms of renewability and biodegradability, but they can sometimes be limited in terms of efficiency. The incorporation of biobased materials into nanocomposites has yielded promising results in terms of higher removal efficiency together with the use of more environmentally friendly materials. Table 2 provides an overview of key examples, highlighting their chemical/structural descriptions and mechanisms of action for carbon-based nanomaterials, metal nanoparticles, biobased nanomaterials, and nanocomposites. These nanomaterials share common structural and functional advantages, including high surface area, tunable surface chemistry, and versatile structural morphologies. The summarized mechanisms of action reveal that physical adsorption is the predominant method by which nanomaterials bind heavy metal contaminants. Additionally, other binding mechanisms, such as photocatalysis, precipitation, and ion exchange, are also viable remediation processes. However, the emphasis on physical adsorption underscores the critical role of high surface area for effective remediation.
Table 2 Summary of nanomaterials used towards remediation
| Nanomaterial type |
Examples |
Chemical/structural description |
Typical mechanism of action |
| Carbon based nanomaterials |
Carbon nanotubes (multi walled or single walled) |
Composed of carbon atoms in different lattice structures25–29 |
Physical and chemical adsorption of different heavy metals via van der Waals forces, π–π stacking, electrostatic interactions, formation of surface complexes, ligand and ligand exchange25–29 |
| Graphene |
High surface area, electrical conductivity, and mechanical structure25–29 |
Surface functionalization can enhance affinity for different heavy metals25–29 |
| Graphene oxide |
Strong covalent bonding arising from sp2 hybridized structures25–29 |
| Metal nanoparticles |
Titanium dioxide |
Composed of single metals or metal alloys in crystallized structures such as spheres, rods or plates79–84 |
Typical remediation mechanisms through adsorption and precipitation depending on the nanoparticle79–84 |
| Iron oxide |
Versatile electric, optical and catalytic properties from quantum size effects79–84 |
Highly dependent on functional groups present79–84 |
| Nano zerovalent iron |
Photocatalytic capabilities of different metals allow for different binding via conversion of heavy metal species79–84 |
| Biobased nanomaterials |
Cellulose nanocrystals |
Derived from biopolymers including chitin, cellulose, and chitosan22,92,93 |
Remediation typically through physical adsorption alongside ion exchange mechanisms22,92,93 |
| Cellulose nanofibers |
Versatile structures ranging from nanofibers to nanocrystals depending on extraction procedure22,92,93 |
Highly versatile in terms of surface chemistry, with an abundance of literature modifications available for different functional group grafting22,92,93 |
| Chitosan nanoparticles |
Possess excellent biocompatible and biodegradable properties22,92,93 |
| Nanocomposites |
Ceramic matrix nanocomposites |
Polyphasic materials that are a combination of matrix materials such as polymers, metals or ceramic with nanoscale fillers112,118,122–125 |
Versatile binding mechanisms and interactions depending on the type of nanofiller including electrostatic attraction, covalent bonding, and ligand exchange112,118,122–125 |
| Polymer matrix nanocomposites |
Possess versatile mechanical, thermal, and chemical properties due to synergy between matrix and nanofiller112,118,122–125 |
Additionally, possess tunable surface chemistry allowing for different preferential binding mechanisms112,118,122–125 |
| Metal matrix nanocomposites |
Can be designed to possess catalytic properties enabling oxidation or reduction of heavy metal species which could more readily be adsorbed112,118,122–125 |
3. Nanomaterial enhancements for heavy metal analysis
3.1 Spectroscopic based enhancements
Spectroscopic methods are a commonly used analytical tool for heavy metal detection including techniques such as atomic absorption spectroscopy, laser ablation inductively coupled plasma mass spectrometry, and surface-enhanced Raman spectroscopy.130–132 Spectroscopic methods confer advantages in metal ion analysis in terms of their high sensitivity in both the limit of detection (LOD) and limit of quantification (LOQ).130–132 However, there are significant drawbacks in using spectroscopic methods such as sample preparation and matrix interference of different species in the wastewater filtrate.130–132 To address these issues, nanomaterials are emerging as promising candidates to improve and enhance the differentiation between different metal species as well as improving the efficacy of sample preparation. Preconcentration is a vital step in sample preparation for spectroscopic analysis. When analyzing wastewater, the desired analyte can often be below the limit of detection and preconcentration of the analyte is required to bring the analyte sample to a detectable limit.131,133,134 Due to their high surface area and tunable surface chemistry, nanomaterials have been widely exploited for the preconcentration of analyte.131,133–135 The mechanism for preconcentration by nanomaterials is identical to the mechanisms by which nanomaterials are used for remediation. Specifically, nanomaterials such as carbon nanotubes can be used to selectively adsorb and bind different heavy metals within a given sample for preconcentration, improving the sensitivity of detection and quantification by a preconcentration factor.131,133,134 For example, Krawcyzk et al. utilized multiwalled carbon nanotubes (MWCNT) for the preconcentration of cadmium and lead in water samples for analysis via graphite furnace atomic absorption spectrometry, and promising results were obtained.134 Carbon nanotubes (CNTs) are commonly used as sorbents in solid phase extraction and dispersive micro solid-phase extraction as a mode of separation prior to their use in different types of spectroscopic analyses. The binding via electrostatic interaction between positively charged heavy metals and negatively charged CNTs can improve and enhance the preconcentration protocols.134 Krawcyzk et al. described the dispersion of MWCNT in water containing trace amounts of lead and cadmium and following an incubation period, they were introduced into the atomizer for analysis.134 The analysis could achieve detection limits as low as 0.001 and 0.03 μg L−1 and a preconcentration factor of 30 and 15 for cadmium and lead respectively.134 Furthermore, there is an instantaneous interaction between metal ions and MWCNTs which drastically improves the sample preparation time when compared to the classical solid phase extraction.134 Similarly, promising results were reported by Yalcinkaya et al. on novel hybrid nanomaterials incorporating zirconium dioxide and boron trioxide (ZrO2/B2O3) for the preconcentration of cobalt, copper, and cadmium prior to quantification by flame atomic absorption spectrometry (FAAS).133 The synthesized composite was characterized via TEM and SEM as seen in Fig. 12 to verify the particle size as nanoscale, given the importance of a higher surface area for increased preconcentration performance.133 The preconcentration procedure utilized a column prepared with the sorbent nanomaterial, with sample solutions of cobalt, copper, and cadmium at concentrations of 5 μg mL−1 flowing through the column. Following this, the retained analytes adsorbed onto the nanocomposite were eluted using nitric acid and the analytes were aspirated into air–acetylene flame for quantification via FAAS.133 The methodology was capable of achieving detection limits in the 3 μg L−1 range for cobalt, copper, and cadmium.133
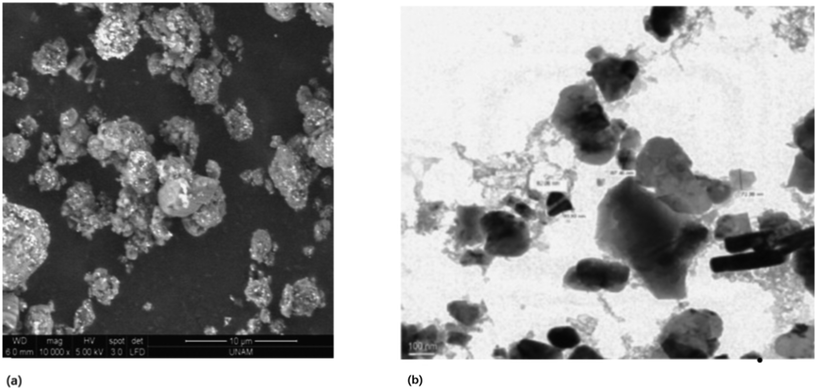 |
| | Fig. 12 a) SEM micrograph and b) TEM micrograph of nanocomposite ZrO2/B2O3.133 | |
Another key component of heavy metal analysis in spectroscopy is the speciation of different heavy metals. Metal speciation is the process of distinguishing between different chemical species of a specific metal, with different species having varying toxicity, mobility, and detection limits.136–138 Nanomaterials are strong candidates for use in various speciation methodologies due to their versatility in terms of engineering different nanomaterials for selective adsorption of varying species of heavy metals.136–138 For example, Gao et al. reported on the synthesis of molybdenum disulfide nanosheets for mercury speciation, which showed promise for the mercury-based detection in wastewater.139 When coupled with other types of spectroscopic techniques such as high-performance liquid chromatography and atomic fluorescence spectroscopy, researchers discussed various efficient detection and separation methodologies for mercury ions (Hg2+), methyl mercury (MeHg+), and ethyl mercury (EtHg+).139 The utilization of these nanosheets allowed for the detection of limits in the ng mL−1 range for Hg2+, MeHg+ and EtHg+.139 This study is important given that mercury in the organic form is far more toxic compared to the inorganic forms, therefore speciation is vital for pollutant analysis.136,139 The application of nanomaterials for speciation is especially prominent, where the use of these nanoparticles for separation through photooxidation and photoreduction mechanisms could be exploited.136,137 In particular, nano-TiO2 as a photocatalyst has seen widespread use in the speciation of specific oxidation states for elements such as arsenic or chromium.136
Another key component of spectroscopy that can be enhanced via nanomaterials is photochemical vapor generation, which is an advanced technique for sample introduction in spectroscopy analysis such as atomic absorption spectroscopy or inductively coupled plasma mass spectroscopy.140–143 There are significant advantages in using this technique for its high selectivity in terms of speciation, high analyte transport efficiency, and ease of operation.140,141 Nanomaterials play a crucial role in this process by acting as catalysts in vapor generation via different nanomaterials possessing photocatalytic capabilities or via enhancement of the efficiency of converting metal ions into more volatile species.140,141 For example Lin et al. described the use of a nanocomposite consisting of gold (Au) nanoparticles deposited on TiO2 nanoparticles for the photocatalytic reduction of arsenic species in the vapor phase.144 The importance of gold nanoparticles in enhancing the photocatalytic reaction extended the detection limits in the sub μg L−1 range for both As(III) and As(V) as shown in Fig. 13.144 The nanocomposite photocatalytically reduces the arsenic into volatile arsenic hydride species that could be introduced into an inductively coupled plasma-mass spectrometer.144 The use of titanium dioxide nanoparticles as effective photocatalysts has been extensively exploited where there is an increasing use of this nanomaterial for both speciation and quantification via photochemical vapor generation.144
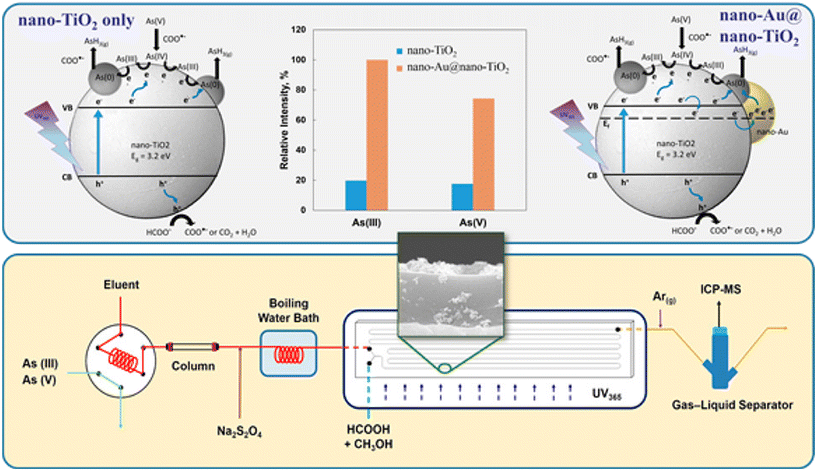 |
| | Fig. 13 Nano-TiO2 and nano-TiO2–Au composite particles for arsenic reduction.144 | |
3.2 Colorimetric and fluorescence enhancements
Colorimetric sensors are analytical tools used for the detection of specific analytes by measuring the visible color changes. The principle behind these sensors is that the target analytes interact with colorimetric reagents, which produces a color change that can be quantified using different spectrophotometric methods.145,146 These sensors are often deployed due to the simplicity of the reagent and ease of operation, however they often suffer in terms of sensitivity and selectivity.145,146 Nanomaterials are rapidly emerging as strong contributors to the field of colorimetric analysis. Nanoparticles in particular possess an optical characteristic known as localized surface plasmon resonance, where conduction electrons on the surface of nanoparticles can resonate with incident light at measurable and specific wavelengths.146–150 For example, Seth prepared L-cysteine functionalized gold nanoparticles and incorporated them into different nanoparticles, where she elucidated their potential for use in the detection of cadmium.151 Specifically, L-cysteine gold nanoparticles exhibited a dark red colour when individual nanoparticles were dispersed in solution, and when the particles were aggregated in the presence of cadmium, where the color changed from dark red to deep blue.151 This method allows for an accurate and measurable detection of cadmium that leverages on the unique optical properties of the nanoparticles.151 Similarly, promising results for the detection of arsenic were reported by Shalvi et al. on polyethylene glycol capped gold nanoparticle (PEG-AuNP) composites.152 Their study described the electrostatic interactions between the PEG-capped gold nanoparticles and As(III), which induced the aggregation of arsenite ions that could be quantified by a hand held colorimetric device.152 PEG-AuNPs were prepared using gold chloride and PEG solution.152 With the addition of PEG, trisodium citrate was added to the solution mixture and an observable transition in colour from light yellow to black was observed, which then transitioned to red indicating the successful capping of AuNPs with PEG as shown in Fig. 14.152 Following this, As(III) ions would aggregate in the presence of hydroxyl groups on the surface of PEG that resulted in the transition from the initial red color to blue that could now be quantified using an integrated hand-held device.152 This work is especially promising given that the results suggest that it is a highly efficient method for the on-site quantification of arsenic, which is extremely relevant in water pollution analysis.152 There is a comprehensive set of literature detailing how colorimetric sensors can be constructed using nanomaterial-based technology with good versatility in terms of detection of different heavy metals such as copper, arsenic, mercury, lead, chromium, cadmium and others.148,149,153–157 These methods are gaining popularity due to the increased sensitivity of detection alongside the fundamental advantages of colorimetric methods such as ease of operation, low cost and flexibility.148,149,153–157
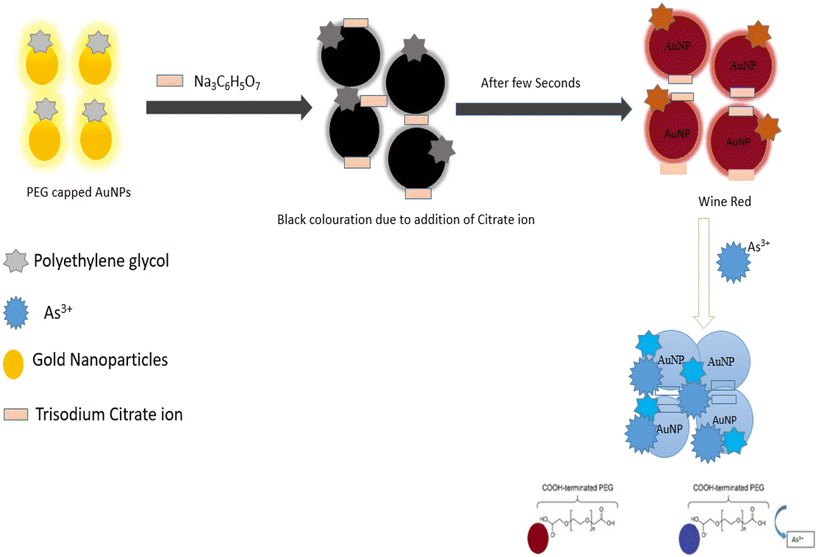 |
| | Fig. 14 Mechanism of PEG-AuNP formation and subsequent interaction with As(III) ions for the formation of an observable complex.152 | |
Fluorescence spectroscopy is a powerful tool used in the field of analytical chemistry. The technique measures the intensity of light emitted by a given fluorophore following the excitation of the fluorophore by light at a specific wavelength.154,156,158,159 The intensity of this fluorescence could be measured quantitatively and then correlated to the concentration of the given analyte.154,156,158,159 Fluorescence sensors have a very high sensitivity and good limits of detection and quantification making these tools extremely attractive for analyzing heavy metal pollution.154,156,158,159 Different nanomaterials such as quantum dots, carbon-based nanomaterials, and nanoparticles have the potential to dramatically enhance the analytical capabilities of fluorescence-based methods.154,156,158,159 For example, Zhang and Chen reported on nitrogen doped carbon quantum dots (N-CQDs), which could be used for the detection of mercury ions.160 Promising results on analysing mercury ions in lake water samples utilizing N-doped carbon quantum dots as a fluorescent probe were reported.160 Nitrogen-doped carbon quantum dots possessed high luminescence properties, and they exhibited excitation wavelength-dependent fluorescence.160 These properties can be leveraged to detect Hg2+ ions since Hg2+ ions can quench the fluorescence of the N-CQDs.160 Utilizing these materials to measure the observable reduction in fluorescence with increasing concentrations of Hg2+ facilitates the detection of Hg2+ at a 0.23 μM detection limit.160 Furthermore, the sensor demonstrated excellent sensitivity and selectivity even with interference from high concentrations of other metal ions.160 Similarly, Wang et al. demonstrated the capabilities of a quantum dot nanohybrid for cadmium ion detection in water samples.158 The nanohybrid consisted of a combination of quantum dots which were covalently linked to silica nanoparticles embedded with red emitting quantum dots.158 The specific mechanism of this composite utilized green-emitting cadmium selenide (CdSe) quantum dots covalently linked to the surface of silica nanoparticles that were covered with red-emitting cadmium telluride (CdTE) quantum dots.158 Subsequently, EDTA solution was etched onto the surface to quench the green fluorescence characteristics of CdSE quantum dots.158 This fluorescence quenching effect could be restored via the binding of cadmium to the surface of quantum dots, producing a green fluorescence that could be quantified and correlated to the concentration of cadmium ions as shown in Fig. 15.158 The study provided a strong rationale for the use of these nanohybrids for on-site detection of cadmium ions in lake water, achieving an exceptional detection limit of 25 nM for Cd2+.158
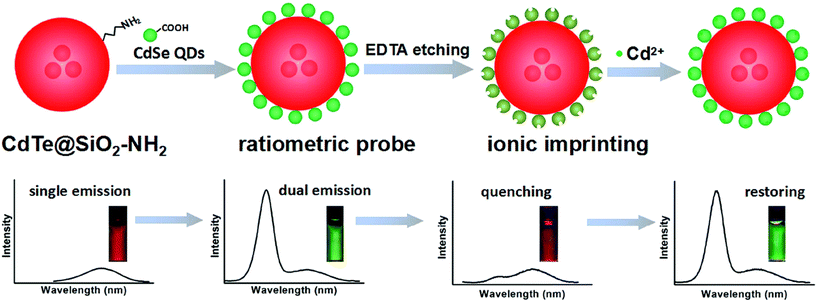 |
| | Fig. 15 Schematic description of cadmium absorption and fluorescence measurement via a dual emission quantum dot nanohybrid.158 | |
3.3 Nanomaterials for electrochemical sensor enhancements
Electrochemical sensors are indispensable tools for the detection and quantification of heavy metal concentrations. These sensors confer significant advantages in terms of high sensitivity, rapid response times, and operating capability under complex environmental conditions such as various types of contaminated wastewater.161–166 The emergence of nanomaterials has generated an abundance of methods for the enhancement of electrochemical sensor applications.161–166 Specific properties that are useful to electrochemical sensors include higher surface area, catalytic function, and selective binding to different heavy metals.161,164,167,168 The utility of nanomaterial-based enhancements can be observed in multiple applications in different types of electrochemical sensors. For example, Mao et al. described the application of titanium dioxide nanoparticles and multi-walled carbon nanotubes in glassy carbon electrodes for the analysis of mercury.169 Glassy carbon electrodes can be used for the determination of trace mercury via linear anodic stripping voltammetry which can be enhanced using titanium dioxide MWCNT materials based on the electrocatalytic behaviour of this nanocomposite.169 Carbon nanotubes, in particular, are extremely useful for electrochemical applications due to characteristics such as strong electrical conductivity, electrocatalytic capabilities, and strong adsorptive properties.169 CNTs are known to possess unique electronic properties facilitating more effective electron transfer.169 Additionally, nano TiO2 has seen widespread application in electrode applications due to its ability to provide more active sites on the surface of the electrode.169 The proposed enhancement of glassy carbon electrodes via nano TiO2 and MWCNTs can attain high selectivity and determination of mercury even in the presence of common potential interfering metal ions with a detection limit of 0.025 μmol L−1.169 Singh et al. described the use of copper metal organic framework based nanoparticles for use in the detection of mercury via differential pulse voltammetry and cyclic voltammetry.170 They found similar beneficial qualities using these nanoparticles and when combined with copper metal organic framework (Cu-MOF) nanoparticles, they possess beneficial electrochemical properties with enhanced sensitivity for the detection of metal ions, such as mercury.170 The specific mechanism of action in the Cu-MOF is detailed in Fig. 16.170 In detail, cuprous oxide (Cu2O) nanoparticles are oxidized to form Cu2+ and the subsequent electron release aids in the reduction of Hg2+ (ref. 170). A subsequent cyclic voltammetry analysis of the Cu-MOF modified electrode can be used to quantify the amount of Hg2+ ions as shown in Fig. 16.171 The sensor was successfully applied for the detection of mercury ions in tap water with a limit of detection of 0.0633 nM.170
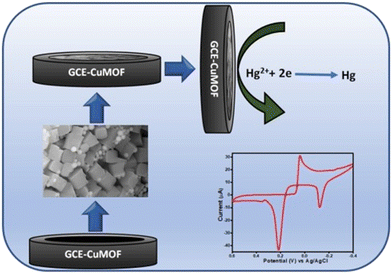 |
| | Fig. 16 Schematic diagram of copper metal organic framework nanoparticles for electrochemical detection of mercury (Hg2+).170 | |
The versatility of nanomaterial-based enhancement is demonstrated in various types of heavy metal sensors constructed using nanomaterials. For example, Liu et al. described the use of a nanocomposite for the development of a highly selective sensor for lead(II) detection.171 They reported the development of a complex aptasensor using a nanocomposite built from nitrogen doped graphene gold nanoparticles functionalized with 2,2′-((1E)-((4-((2-mercaptoethyl)thio)-1,2-phenylene)bis(azanylylidene))bis(methanylylidene))diphenol (ETBD) incorporated alongside iron oxide titanium dioxide core–shell nanoparticles.171 Liu et al. demonstrated the findings in terms of synergistic effects of the iron oxide titanium dioxide nanoparticles alongside the enhanced electrical conductivity of gold nanoparticles.171 They demonstrated an application in the selective detection of lead ions at detection and quantification limits of between 4 × 10−13 and 2 × 10−8 mol L−1.171 A similar study was demonstrated by Ma et al. on a portable microfluidic electrochemical sensor for lead detection using a nanocomposite consisting of silver graphene oxide nanoparticles.172 The system possessed advantages in terms of portable analysis while still maintaining a high detection of limit of 0.00464 μg L−1.172 The body of work reported in the literature provides a strong foundation for research on how nanomaterials can enhance the electrochemical methods for sensing and quantification. Different research groups were able to leverage different nanomaterials and nanocomposites in a wide variety of sensor applications with the literature elucidating the various electrochemical properties possessed by different nanomaterials.173–175 Table 3 summarizes the various characterization techniques and the potential roles nanomaterials can play in enhancing their detection performance. The review of nanomaterial enhancements highlights their versatility and the distinct mechanistic advantages they bring to each of the three techniques discussed above. For spectroscopic methods, properties such as high surface area and adaptable surface chemistry enable the selective physical adsorption of heavy metals, addressing challenges like preconcentration and matrix interference. In fluorescence and colorimetric techniques, the unique optical properties of different nanomaterials can be leveraged for a broad range of sensor applications. For electrochemical techniques, many nanomaterials exhibit properties like enhanced conductivity and electrocatalytic activity, which improve the electrode performance in electrochemical sensors. Overall, Table 3 underscores the broad potential of nanomaterials in enhancing the characterization techniques through various mechanisms.
Table 3 Summary of analytical methods and nanomaterial enhancements
| Analytical method |
Description of technique |
Nanomaterial applications |
| Spectroscopic methods |
Detection of specific wavelengths of light or mass signal emission, absorbed, or reflected by different heavy metal ions130–132 |
Spectroscopic methods often require preconcentration of heavy metal samples to a quantifiable limit. Nanomaterials via adsorption and tunable surface chemistry of intended materials have strong applications for preconcentration130–132 |
| Common techniques include atomic absorption spectroscopy (AAS) and inductively coupled plasma mass spectrometry (ICP MS)130–132 |
Speciation of heavy metals and different competing heavy metal effects can be challenging for spectroscopic identification. Nanomaterials have versatile surface chemistry allowing for the selective binding of different species of heavy metal, allowing for improved speciation130–132 |
| Advanced spectroscopic techniques such as photochemical vapor generation can be facilitated using the photocatalytic capabilities of different nanomaterials130–132 |
| Colorimetric and fluorescence enhancements |
For colorimetric methods, a visible color change is quantified when heavy metals interact with specific reagents148,149,153–157 |
Nanoparticles possess localized surface plasmon resonance where conduction electrons on the surface of nanoparticles can resonate with light at wavelengths which are quantifiable. This facilitates versatile colorimetric methods148,149,153–157 |
| Fluorescence methods utilize different fluorescent dyes or probes that emit a quantifiable amount of light upon their interaction with heavy metals154,156,158,159 |
Different nanomaterials such as quantum dots, carbon-based nanomaterials and nanoparticles possess luminescence properties and fluorescence capabilities, which allow for dramatic enhancements of current fluorescence based spectroscopic methods154,156,158,159 |
| Electrochemical sensors |
Operates via quantification of heavy metal species as either an oxidation or a reduction current161–166 |
Nanomaterials possess beneficial properties for electrochemical sensors such as catalytic function, electric conductivity, and selective binding of different heavy metals161–166 |
| Additionally, quantification of heavy metal ions via electrical potential or conductivity changes161–166 |
Carbon nanotubes in particular possess unique electronic properties for facilitating more efficient electron transfer161–166 |
| Includes techniques such as anodic stripping voltammetry via electrodes, as well as potentiometric or conductometric sensors161–166 |
Nanomaterials such as titanium dioxide can often be incorporated onto electrodes due to the electrocatalytic capabilities of the nanomaterial161–166 |
4. Outlook and perspectives
4.1 Nanomaterial based remediation methods
Nanomaterials are increasingly being incorporated into systems for the remediation of heavy metals in water. They offer many beneficial properties such as high surface area, tunable surface chemistry and interactions with contaminants revealing their effectiveness at the nanoscale. There are many advantages and disadvantages for each type of nanomaterial, which were discussed and elucidated in the literature. As discussed earlier, Table 2 provides a comprehensive overview of the shared mechanistic advantages of nanomaterials in facilitating heavy metal remediation. It particularly highlights the critical role of high surface area, as physical adsorption is the predominant mechanism for heavy metal removal. While all nanomaterials share these common advantages, each type offers unique properties that enhance their effectiveness in remediation. Carbon based nanomaterials such as carbon nanotubes and graphene oxides display strong adsorption capacities and high efficiency in terms of heavy metal removal due to their broad functionalities. However, carbon-based nanotechnology has limitations in terms of scalability and production costs, which limit its potential for commercialization. Metal nanoparticles possess a wide range of remediation of metallic pollutants in terms of adsorption-based methods and photocatalytic characteristics. The regeneration and reuse of metal nanoparticles extend and enhance their lifespan for many applications. However, metal nanoparticles have some limitations in terms of synthesis, which can hinder their long-term use. Specifically, metal nanoparticle synthesis can often be energy intensive and involves hazardous chemicals, which can be counter intuitive for pollutant-based applications. Biobased nanomaterials such as CNC and CNF are environmentally friendly since they are extracted from renewable sources. Additionally, CNC and CNF possess a wide array of surface modifications allowing for different types of heavy metal remediation. However, limitations for biobased nanomaterials are associated with their relatively low removal efficiency compared to other nanomaterial-based filtration materials. Nanocomposites offer several advantages in terms of their enhanced performance for water treatment applications due to the synergistic effects of different nanomaterials being considered. Additionally, nanocomposites have shown remarkable regeneration capabilities offering promising results for sustainable materials for long-term water treatment. However, these regeneration capabilities are limited by the intensive and complex synthesis of the nanocomposites. Additionally, nanocomposites have high production costs due to their more complex preparation procedures, thus the major limitation of nanomaterials used for water treatment applications is the production of the nanomaterials. Given that heavy metal pollution is a widespread phenomenon, there is interest in developing sustainable and scalable solutions for water remediation. Water pollution tends to have a greater impact on developing countries necessitating cost efficient methods for their treatment. As the field continues to develop, special consideration should be given to the scalability and production of the nanomaterials as well as the more potent development of biobased nanomaterials for remediation, given that biobased nanomaterials offer a more sustainable long-term solution.
4.2 Nanomaterial based analysis methods
Analysis and detection are a fundamental aspect of water remediation. Given that different countries have very stringent rules for the heavy metal present in water, thus special focus needs to be devoted to the different methodologies for the characterization of heavy metals in water. This is especially prevalent given that heavy metals are hazardous at extremely low concentrations and therefore the detection needs to be precise for low levels of contaminants. Nanomaterials are rapidly emerging as important systems for the analytical determination of heavy metals, with nanomaterials offering key enhancements in terms of sensitivity, selectivity, and efficiency. Special consideration was given to nanomaterial-based enhancements for spectroscopic tools, colorimetric and fluorescence based methods, and electrochemical sensor applications. Table 3 provides a summary of each technique, and its applications associated with nanomaterial-based enhancements. The summary highlights the versatility of nanomaterials in wastewater analysis, showcasing how each characterization method leverages distinct properties of nanoscale materials. The current field of spectroscopic based methods for heavy metals focuses heavily on reliable methods for preconcentration, speciation of different heavy metals, and photochemical vapor generation for precise injection into different spectroscopic instruments. Nanomaterials have application in the detection and analysis of heavy metals due to their exceptional adsorption capabilities for preconcentration, highly tunable selectivity to enhance speciation, and different photooxidative and photoreductive capabilities that facilitate photochemical vapor generation. Furthermore, given the high selectivity of different fluorescence methods for heavy metal detection and the ease in using colorimetric methods, there is increasing interest in enhancing these methods for higher selectivity and sensitivity. Nanomaterials play a crucial role in this development due to their unique optical properties such as localized surface plasmon resonance by different nanoparticles, or fluorescence based properties of quantum dots. These properties facilitate enhanced detection and sensitivity for different colorimetric and fluorescence based methods when the nanomaterials are incorporated into different sensor applications. Finally, nanomaterials are finding increasing application in different electrochemical based sensors. Electrochemical sensors are widely sought after for heavy metal analysis given their rapid response and potential for on-site use. In this regard, nanomaterial-based enhancements are becoming more popular due to the different catalytic properties, selective adsorption capacities, and electrochemical activity. There is a significant body of literature describing how nanomaterial-based applications can enhance both the selectivity and sensitivity of different electrochemical sensors and electrodes. In terms of the limitations for nanomaterials for heavy metal remediation, there are significant drawbacks in the use of nanomaterials for analysis due to issues with scalability and production costs of these materials. As the field of nanoscience continues to develop, the interest in nanomaterials will continue to increase, and advancements made in this field will drive down cost and increase the sensitivity of detection.
5. Conclusions
Nanoscience is an emerging field with an endless capacity of untapped potential. Current research into heavy metal remediation in water describes several key mechanisms and applications of nanomaterials used in these applications. As heavy metal pollution is a critical global issue, thus there is an increased need for sustainable, effective solutions for both remediation and analysis. Once the production and scalability of nanomaterials are addressed, we should expect a higher degree of interest in applying nanomaterials for heavy metal remediation on a large scale. Its notable that studying nanomaterials for analysis as well as remediation offers a holistic approach to understand the mechanisms of the nanomaterials with heavy metals. The application and review of both remediation and analysis offer vital insight into different prospective applications for nanomaterial utilization, with their being a great deal of potential utility in the field.
Data availability
No new data were generated or analysed as part of this review. All data discussed within the text of the manuscript, included in figures or presented in tables, are sourced from previously published articles, which are cited accordingly. All figures are referenced accordingly and appropriate permissions have been granted.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
KCT acknowledges the funding from NSERC, and GC acknowledges the support from the Chinese Scholarship Council for the funding to pursue her PhD at the University of Waterloo.
References
- M. K. Abd Elnabi, N. E. Elkaliny, M. M. Elyazied, S. H. Azab, S. A. Elkhalifa, S. Elmasry, M. S. Mouhamed, E. M. Shalamesh, N. A. Alhorieny and A. E. Abd Elaty, Toxicity of heavy metals and recent advances in their removal: a review, Toxics, 2023, 11, 580 CrossRef CAS PubMed.
- S. Rajendran, V. Rathinam, A. Sharma, S. Vallinayagam and M. Muthusamy, Arsenic and Environment: A Systematic Review on Arsenic Sources, Uptake Mechanism in Plants, Health Hazards and Remediation Strategies, Top. Catal., 2024, 67, 325–341 CrossRef CAS.
- K. H. H. Aziz, F. S. Mustafa, K. M. Omer, S. Hama, R. F. Hamarawf and K. O. Rahman, Heavy metal pollution in the aquatic environment: efficient and low-cost removal approaches to eliminate their toxicity: a review, RSC Adv., 2023, 13, 17595–17610 RSC.
- S. Hembrom, B. Singh, S. K. Gupta and A. K. Nema, A Comprehensive Evaluation of Heavy Metal Contamination in Foodstuff and Associated Human Health Risk: A Global Perspective, Contemporary Environmental Issues and Challenges in Era of Climate Change, 2020, vol. 1, pp. 33–63 Search PubMed.
- M. I. Ahamad, Impact of heavy metals on aquatic life and human health: a case study of River Ravi Pakistan, Front. Mar. Sci., 2024, 11, 1374835 CrossRef.
- D. H. Maithani, H. Dasila, R. Saxena, A. Tiwari, D. Bhatt, K. Rawat and D. C. Suyal, Heavy Metal Pollution in Water: Cause and Remediation Strategies, in Current Status of Fresh Water Microbiology, 2023, vol. 1, pp. 181–204 Search PubMed.
- M. S. Manna and C. Bhaumik, Opportunities and Challenges in Heavy Metal Removal from Water, Remediation of Heavy Metals, 2021, vol. 1, pp. 347–366 Search PubMed.
- P. Levallois, P. Barn, M. Valcke, D. Gauvin and T. Kosatsky, Public Health Consequences of Lead in Drinking Water, Curr. Environ. Health Rep., 2018, 5, 255–262 CrossRef CAS PubMed.
- B. I. Musah, L. Peng and Y. Xu, Evaluation of chromium application in the steel industry in China: Implications on environmental quality, IOP Conf. Ser. Earth Environ. Sci., 2021, 728, 012011 CrossRef.
- R. Pant, N. Mathpal, R. Chauhan, A. Singh and A. Gupta, A Review of Mercury Contamination in Water and Its Impact on Public Health, Mercury Toxicity Mitigation: Sustainable Nexus Approach, (Earth and Environmental Sciences Library), 2024, vol. 1, pp. 93–115 Search PubMed.
- M. O. Fashola, V. M. Ngole-Jeme and O. O. Babalola, Heavy Metal Pollution from Gold Mines: Environmental Effects and Bacterial Strategies for Resistance, Int. J. Environ. Res. Public Health, 2016, 13, 1047 CrossRef PubMed.
- M. K. Yadav, D. Saidulu, A. K. Gupta, P. S. Ghosal and A. Mukherjee, Status and management of arsenic pollution in groundwater: A comprehensive appraisal of recent global scenario, human health impacts, sustainable field-scale treatment technologies, J. Environ. Chem. Eng., 2021, 9, 105203 CrossRef CAS.
- A. Bhat, T. O. Hara, F. Tian and B. Singh, Review of analytical techniques for arsenic detection and determination in drinking water, Environ. Sci.: Adv., 2023, 2, 171–195 CAS.
- N. A. Suciu, R. Vivo, N. Rizzati and E. Capri, Cd content in phosphate fertilizer: Which potential risk for the environment and human health?, Curr. Opin. Environ. Sci. Health., 2022, 30, 100932 Search PubMed.
- B. H. Robinson, E-waste: an assessment of global production and environmental impacts, Sci. Total Environ., 2009, 408, 183–191 CrossRef CAS PubMed.
- P. Karrari, O. Mehrpour and M. Abdollahi, A systematic review on status of lead pollution and toxicity in Iran; Guidance for preventive measures, Daru, J. Pharm. Sci., 2012, 20, 1–17 CrossRef PubMed.
- M. Jaishankar, T. Tseten, N. Anbalagan, B. B. Mathew and K. N. Beeregowda, Toxicity, mechanism and health effects of some heavy metals, Interdiscip. Toxicol., 2014, 7, 60–72 CrossRef PubMed.
- L. A. Malik, A. Bashir, A. Qureashi and A.
H. Pandith, Detection and removal of heavy metal ions: a review, Environ. Chem. Lett., 2019, 17, 1495–1521 CrossRef CAS.
- M. Tumolo, Chromium Pollution in European Water, Sources, Health Risk, and Remediation Strategies: An Overview, Int. J. Environ. Res. Public Health, 2020, 17, 5438 CrossRef CAS PubMed.
- D. Water, National Primary Drinking Water Regulations, Technical Fact, 2019 Search PubMed.
- M. Llaver, M. N. Oviedo, P. Y. Quintas and R. G. Wuilloud, Analytical Methods for the Determination of Heavy Metals in Water, Remediation of Heavy Metals, 2021, vol. 1, pp. 1–50 Search PubMed.
- M. Gobi, A. Kumar, J. Singh, S. Singh and P. C. Ramamurthy, Nanocellulose-Based Adsorption for the Removal of Heavy Metal from Wastewater—A Review, Water Conserv. Sci. Eng., 2024, 9, 24 CrossRef.
- E. Omanović-Mikličanin, A. Badnjević, A. Kazlagić and M. Hajlovac, Nanocomposites: a brief review, Health Technol., 2019, 10, 51–59 CrossRef.
- A. Karnwal and T. Malik, Nano-revolution in heavy metal removal: engineered nanomaterials for cleaner water, Front. Environ. Sci., 2024, 12, 1393694 CrossRef.
- N. Agasti, Carbon nanotube based magnetic composites for decontamination of organic chemical pollutants in water: A review, Appl. Surf. Sci., 2022, 10, 100270 CrossRef.
- J. Chen, Microwave-induced carbon nanotubes catalytic degradation of organic pollutants in aqueous solution, J. Hazard. Mater., 2016, 310, 226–234 CrossRef CAS PubMed.
- Z. Wang, W. Xu, F. Jie, Z. Zhao, K. Zhou and H. Liu, The selective adsorption performance and mechanism of multiwall magnetic carbon nanotubes for heavy metals in wastewater, Sci. Rep., 2021, 11, 16878 CrossRef CAS PubMed.
- C. Rodriguez, S. Briano and E. Leiva, Increased Adsorption of Heavy Metal Ions in Multi-Walled Carbon Nanotubes with Improved Dispersion Stability, Molecules, 2020, 25, 3106 CrossRef CAS PubMed.
- A. T. Hoang, Heavy metal removal by biomass-derived carbon nanotubes as a greener environmental remediation: A comprehensive review, Chemosphere, 2022, 287, 131959 CrossRef CAS PubMed.
- J. Liang, Facile synthesis of alumina-decorated multi-walled carbon nanotubes for simultaneous adsorption of cadmium ion and trichloroethylene, Chem. Eng. J., 2015, 273, 101–110 CrossRef CAS.
- T. C. Egbosiuba, A. S. Abdulkareem, A. S. Kovo, E. A. Afolabi, J. O. Tijani and W. D. Roos, Enhanced adsorption of As(V) and Mn(VII) from industrial wastewater using multi-walled carbon nanotubes and carboxylated multi-walled carbon nanotubes, Chemosphere, 2020, 254, 126780 CrossRef CAS PubMed.
- I. Elghamry, M. Gouda and Y. S. S. Al-Fayiz, Synthesis of Chemically Modified Acid-Functionalized Multiwall Carbon Nanotubes with Benzimidazole for Removal of Lead and Cadmium Ions from Wastewater, Polymer, 2023, 15, 1421 CAS.
- A. Jawed, V. Saxena and L. M. Pandey, Engineered nanomaterials and their surface functionalization for the removal of heavy metals: A review, J. Water Process Eng., 2020, 33, 101009 CrossRef.
- H. Alijani and Z. Shariatinia, Synthesis of high growth rate SWCNTs and their magnetite cobalt sulfide nanohybrid as super-adsorbent for mercury removal, Chem. Eng. Res. Des., 2018, 129, 132–149 CrossRef CAS.
- S. Mallakpour and S. Soltanian, Surface functionalization of carbon nanotubes: fabrication and applications, RSC Adv., 2016, 6, 109916–109935 RSC.
- P. Kondratyuk and J. T. Yates Jr, Molecular views of physical adsorption inside and outside of single-wall carbon nanotubes, Acc. Chem. Res., 2007, 40, 995–1004 CrossRef CAS PubMed.
- S. B. Mishra and A. K. Mishra, Bio-and Nanosorbents from Natural Resources, Springer, 2018 Search PubMed.
- M. A. Atieh, Effect of carboxylic functional group functionalized on carbon nanotubes surface on the removal of lead from water, Bioinorg. Chem. Appl., 2010, 1, 603978 CrossRef PubMed.
- K. Anitha, S. Namsani and J. K. Singh, Removal of Heavy Metal Ions Using a Functionalized Single-Walled Carbon Nanotube: A Molecular Dynamics Study, J. Phys. Chem. A, 2015, 119, 8349–8358 CrossRef CAS PubMed.
- H. J. Wang, A. L. Zhou, F. Peng, H. Yu and L. F. Chen, Adsorption characteristic of
acidified carbon nanotubes for heavy metal Pb(II) in aqueous solution, Mater. Sci. Eng., A, 2007, 466, 201–206 CrossRef.
- C. Lu and C. Liu, Removal of nickel(II) from aqueous solution by carbon nanotubes, J. Chem. Technol. Biotechnol., 2006, 81, 1932–1940 CrossRef CAS.
- Z. Gao, T. J. Bandosz, Z. Zhao, M. Han and J. Qiu, Investigation of factors affecting adsorption of transition metals on oxidized carbon nanotubes, J. Hazard. Mater., 2009, 167, 357–365 CrossRef CAS PubMed.
- G. Rao, C. Lu and F. Su, Sorption of divalent metal ions from aqueous solution by carbon nanotubes: A review, Sep. Purif. Technol., 2007, 58, 224–231 CrossRef CAS.
- A. Stafiej and K. Pyrzynska, Adsorption of heavy metal ions with carbon nanotubes, Sep. Purif. Technol., 2007, 58, 49–52 CrossRef CAS.
- R. Kumar, M. A. Khan and N. Haq, Application of Carbon Nanotubes in Heavy Metals Remediation, Crit. Rev. Environ. Sci. Technol., 2014, 44, 1000–1035 CrossRef CAS.
- Y. Peng and H. Liu, Effects of oxidation by hydrogen peroxide on the structures of multiwalled carbon nanotubes, Ind. Eng. Chem. Res., 2006, 45, 6483–6488 CrossRef CAS.
- H. Chen, J. Li, D. Shao, X. Ren and X. Wang, Poly(acrylic acid) grafted multiwall carbon nanotubes by plasma techniques for Co(II) removal from aqueous solution, Chem. Eng. J., 2012, 210, 475–481 CrossRef CAS.
- B. Zhao, H. Hu, A. Yu, D. Perea and R. C. Haddon, Synthesis and characterization of water soluble single-walled carbon nanotube graft copolymers, J. Am. Chem. Soc., 2005, 127, 8197–8203 CrossRef CAS PubMed.
- A. M. Desouky, Remove heavy metals from groundwater using carbon nanotubes grafted with amino compound, Sep. Sci. Technol., 2018, 53, 1698–1702 CrossRef CAS.
- G. D. Vuković, Removal of cadmium from aqueous solutions by oxidized and ethylenediamine-functionalized multi-walled carbon nanotubes, Chem. Eng. J., 2010, 157, 238–248 CrossRef.
- J. Feng, S. Xiong, L. Ren and Y. Wang, Atomic layer deposition of TiO2 on carbon-nanotubes membrane for capacitive deionization removal of chromium from water, Chin. J. Chem. Eng., 2022, 45, 15–21 CrossRef CAS.
- W. B. Elsharkawy, Isotherm and kinetics study of Ni(II) ions adsorption from wastewater using carbon nanotubes decorated with ZnO nanoparticles, Fullerenes, Nanotubes, Carbon Nanostruct., 2024, 32, 1005–1016 CrossRef CAS.
- S.-H. Hsieh, J.-J. Horng and C.-K. Tsai, Growth of carbon nanotube on micro-sized Al2O3 particle and its application to adsorption of metal ions, J. Mater. Res., 2006, 21, 1269–1273 CrossRef CAS.
- M. A. Hussein, Porous Al(2)O(3)-CNT Nanocomposite Membrane Produced by Spark Plasma Sintering with Tailored Microstructure and Properties for Water Treatment, Nanomaterials, 2020, 10, 845 CrossRef CAS PubMed.
- D. Li, M. B. Muller, S. Gilje, R. B. Kaner and G. G. Wallace, Processable aqueous dispersions of graphene nanosheets, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS PubMed.
- C. Luo, Z. Tian, B. Yang, L. Zhang and S. Yan, Manganese dioxide/iron oxide/acid oxidized multi-walled carbon nanotube magnetic nanocomposite for enhanced hexavalent chromium removal, Chem. Eng. J., 2013, 234, 256–265 CrossRef CAS.
- R. Sitko, Adsorption of divalent metal ions from aqueous solutions using graphene oxide, Dalton Trans., 2013, 42, 5682–5689 RSC.
- M. A. Kausor, S. S. Gupta and D. Chakrabortty, Graphene-based nanocomposites as scavenger of heavy metal ions from water: a review on synthesis, characterizations and application as adsorbent, Chem. Pap., 2024, 78, 1–27 Search PubMed.
- Q.-U. Ain, M. U. Farooq and M. I. Jalees, Application of Magnetic Graphene Oxide for Water Purification: Heavy Metals Removal and Disinfection, J. Water Process Eng., 2020, 33, 101044 CrossRef.
- Y.-C. Lee and J.-W. Yang, Self-assembled flower-like TiO2 on exfoliated graphite oxide for heavy metal removal, J. Ind. Eng. Chem., 2012, 18, 1178–1185 CrossRef CAS.
- T. S. Sreeprasad, S. M. Maliyekkal, K. P. Lisha and T. Pradeep, Reduced graphene oxide-metal/metal
oxide composites: facile synthesis and application in water purification, J. Hazard. Mater., 2011, 186, 921–931 CrossRef CAS PubMed.
- D. D. Mai, Simultaneous adsorption of heavy metals on mesoporous reduced graphene oxide/γ-Fe2O3 nanocomposites, J. Porous Mater., 2022, 29, 1947–1956 CrossRef CAS.
- Y. Liu, G. Chen, C. Tian, R. Gupta, X. Wang and H. Zeng, Metal oxide nanoparticle-modified graphene oxide for removal of elemental mercury, Environ. Technol., 2019, 40, 3602–3610 CrossRef CAS PubMed.
- S. R. Al-Mhyawi, Zirconium oxide with graphene oxide anchoring for improved heavy metal ions adsorption: Isotherm and kinetic study, J. Mater. Res. Technol., 2023, 22, 3058–3074 CrossRef CAS.
- J. Tang, Y. Huang, Y. Gong, H. Lyu, Q. Wang and J. Ma, Preparation of a novel graphene oxide/Fe-Mn composite and its application for aqueous Hg(II) removal, J. Hazard. Mater., 2016, 316, 151–158 CrossRef CAS PubMed.
- S. B. Shivangi and T. Sarkar, Simultaneous removal of cadmium and lead ions from aqueous solutions by nickel oxide-decorated reduced graphene oxides, Int. J. Environ. Sci. Technol., 2021, 19, 5595–5610 CrossRef.
- S. Lee, L. P. Lingamdinne, J.-K. Yang, Y.-Y. Chang and J. R. Koduru, Potential electromagnetic column treatment of heavy metal contaminated water using porous Gd2O3-doped graphene oxide nanocomposite: Characterization and surface interaction mechanisms, J. Water Process Eng., 2021, 41, 102083 CrossRef.
- W. Chen, Z. Lin, Z. Chen, X. Weng, G. Owens and Z. Chen, Simultaneous removal of Sb(III) and Sb(V) from mining wastewater by reduced graphene oxide/bimetallic nanoparticles, Sci. Total Environ., 2022, 836, 155704 CrossRef CAS PubMed.
- B. Barik, A. Kumar, P. S. Nayak, L. S. K. Achary, L. Rout and P. Dash, Ionic liquid assisted mesoporous silica-graphene oxide nanocomposite synthesis and its application for removal of heavy metal ions from water, Mater. Chem. Phys., 2020, 239, 122028 CrossRef CAS.
- F. Arshad, M. Selvaraj, J. Zain, F. Banat and M. A. Haija, Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions, Sep. Purif. Technol., 2019, 209, 870–880 CrossRef CAS.
- I. Sheet, A. Kabbani and H. Holail, Removal of Heavy Metals Using Nanostructured Graphite Oxide, Silica Nanoparticles and Silica/ Graphite Oxide Composite, Energy Procedia, 2014, 50, 130–138 CrossRef CAS.
- Y. Zhang, Modified graphene oxide composite aerogels for enhanced adsorption behavior to heavy metal ions, J. Environ. Chem. Eng., 2021, 9, 106008 CrossRef CAS.
- M. S. Amini-Fazl, M. Barzegarzadeh and R. Mohammadi, Surface Modification of Graphene Oxide with Crosslinked Polymethacrylamide via RAFT Polymerization Strategy: Effective Removal of Heavy Metals from Aqueous Solutions, J. Inorg. Organomet. Polym. Mater., 2021, 31, 2959–2970 CrossRef CAS.
- W. Zhan, L. Gao, X. Fu, S. H. Siyal, G. Sui and X. Yang, Green synthesis of amino-functionalized carbon nanotube-graphene hybrid aerogels for high performance heavy metal ions removal, Appl. Surf. Sci., 2019, 467–468, 1122–1133 CrossRef CAS.
- A. I. Abd-Elhamid, E. M. A. Elgoud and H. F. Aly, Alginate modified graphene oxide for rapid and effective sorption of some heavy metal ions from an aqueous solution, Cellulose, 2022, 29, 6231–6245 CrossRef CAS.
- M. Alimohammady, M. Jahangiri, F. Kiani and H. Tahermansouri, Highly efficient simultaneous adsorption of Cd(ii), Hg(ii) and As(iii) ions from aqueous solutions by modification of graphene oxide with 3-aminopyrazole: central composite design optimization, New J. Chem., 2017, 41, 8905–8919 RSC.
- H. A. Abubshait, A. A. Farag, M. A. El-Raouf, N. A. Negm and E. A. Mohamed, Graphene oxide modified thiosemicarbazide nanocomposite as an effective eliminator for heavy metal ions, J. Mol. Liq., 2021, 327, 114790 CrossRef CAS.
- H. Borji, G. M. Ayoub, R. Bilbeisi, N. Nassar and L. Malaeb, How Effective Are Nanomaterials for the Removal of Heavy Metals from Water and Wastewater?, Water, Air, Soil Pollut., 2020, 231, 1–35 CrossRef.
- S. Mahdavi, M. Jalali and A. Afkhami, Heavy Metals Removal From Aqueous Solutions Using TiO2, MgO, and Al2O3 Nanoparticles, Chem. Eng. Commun., 2013, 200, 448–470 CrossRef CAS.
- K. Gupta, P. Joshi, R. Gusain and O. P. Khatri, Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials, Coord. Chem. Rev., 2021, 445, 214100 CrossRef CAS.
- X. H. Feng, L. M. Zhai, W. F. Tan, F. Liu and J. Z. He, Adsorption and redox reactions of heavy metals on synthesized Mn oxide minerals, Environ. Pollut., 2007, 147, 366–373 CrossRef CAS PubMed.
- F. Hosseini and S. Mohebbi, High efficient photocatalytic reduction of aqueous Zn2+, Pb2+ and Cu2+ ions using modified titanium dioxide nanoparticles with amino acids, J. Ind. Eng. Chem., 2020, 85, 190–195 CrossRef CAS.
- A. T. Le, S. Y. Pung, S. Sreekantan, A. Matsuda and D. P. Huynh, Mechanisms of removal of heavy metal ions by ZnO particles, Heliyon, 2019, 5, e01440 CrossRef CAS PubMed.
- N. H. Abdullah, K. Shameli, E. C. Abdullah and L. C. Abdullah, Solid matrices for fabrication of magnetic iron oxide nanocomposites: Synthesis, properties, and application for the adsorption of heavy metal ions and dyes, Composites, Part B, 2019, 162, 538–568 CrossRef CAS.
- T. Luo, C. Yang, X. Tian, W. Luo, Y. Nie and Y. Wang, Application of Iron Oxide Nanomaterials for the Removal of Heavy Metals, Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications, 2021, vol. 1, pp. 1–25 Search PubMed.
- P. N. Dave and L. V. Chopda, Application of Iron Oxide Nanomaterials for the Removal of Heavy Metals, J. Nanotechnol., 2014, 1, 398569 Search PubMed.
- M. Hua, S. Zhang, B. Pan, W. Zhang, L. Lv and Q. Zhang, Heavy metal removal from water/wastewater by nanosized metal oxides: a review, J. Hazard. Mater., 2012, 211–212, 317–331 CrossRef CAS PubMed.
- L. Feng, M. Cao, X. Ma, Y. Zhu and C. Hu, Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal, J. Hazard. Mater., 2012, 217–218, 439–446 CrossRef CAS PubMed.
- N. A. Awang, W. N. W. Salleh, F. Aziz, N. Yusof and A. F. Ismail, A review on preparation, surface enhancement and adsorption mechanism of biochar-supported nano zero-valent iron adsorbent for hazardous heavy metals, J. Chem. Technol. Biotechnol., 2022, 98, 22–44 CrossRef.
- M. M. Tarekegn, A. M. Hiruy and A. H. Dekebo, Nano zero valent iron (nZVI) particles for the removal of heavy metals (Cd(2+), Cu(2+) and Pb(2+)) from aqueous solutions, RSC Adv., 2021, 11, 18539–18551 RSC.
- V. Danila, S. Vasarevicius and V. Valskys, Batch removal of Cd(II), Cu(II), Ni(II), and Pb(II) ions using stabilized zero-valent iron nanoparticles, Energy Procedia, 2018, 147, 214–219 CrossRef CAS.
- R. Si, J. Pu, H. Luo, C. Wu and G. Duan, Nanocellulose-Based Adsorbents for Heavy Metal Ion, Polymer, 2022, 14, 5479 CAS.
- A. Kardam, K. R. Raj, S. Srivastava and M. M. Srivastava, Nanocellulose fibers for biosorption of cadmium, nickel, and lead ions from aqueous solution, Clean Technol. Environ. Policy, 2013, 16, 385–393 CrossRef.
- D. Dou, Adsorption of copper (II) and cadmium (II) ions by in situ doped nano-calcium carbonate high-intensity chitin hydrogels, J. Hazard. Mater., 2022, 423, 127137 CrossRef CAS PubMed.
- L. Huang, S. Yuan, L. Lv, G. Tan, B. Liang and S. O. Pehkonen, Poly(methacrylic acid)-grafted chitosan microspheres via surface-initiated ATRP for enhanced removal of Cd(II) ions from aqueous solution, J. Colloid Interface Sci., 2013, 405, 171–182 CrossRef CAS PubMed.
- K. Wang, F. Zhang, K. Xu, Y. Che, M. Qi and C. Song, Modified magnetic chitosan materials for heavy metal adsorption: a review, RSC Adv., 2023, 13, 6713–6736 RSC.
- M.-T. Wu, Y.-L. Tsai, C.-W. Chiu and C.-C. Cheng, Synthesis, characterization, and highly acid-resistant properties of crosslinking β-chitosan with polyamines for heavy metal ion adsorption, RSC Adv., 2016, 6, 104754–104762 RSC.
- W. Li, B. Ju and S. Zhang, A green l-cysteine modified cellulose nanocrystals biosorbent for adsorption of mercury ions from aqueous solutions, RSC Adv., 2019, 9, 6986–6994 RSC.
- B. Geng, Surface-Tailored Nanocellulose Aerogels with Thiol-Functional Moieties for Highly Efficient and Selective Removal of Hg(II) Ions from Water, ACS Sustainable Chem. Eng., 2017, 5, 11715–11726 CrossRef CAS.
- F. H. A. Rodrigues, C. E. C. Magalhães, A. L. Medina and A. R. Fajardo, Hydrogel composites containing nanocellulose as adsorbents for aqueous removal of heavy metals: design, optimization, and application, Cellulose, 2019, 26, 9119–9133 CrossRef CAS.
- S. Hokkanen, A. Bhatnagar, E. Repo, S. Lou and M. Sillanpää, Calcium hydroxyapatite microfibrillated cellulose composite as a potential adsorbent for the removal of Cr(VI) from aqueous solution, Chem. Eng. J., 2016, 283, 445–452 CrossRef CAS.
- N. Najib and C. Christodoulatos, Removal of arsenic using functionalized cellulose nanofibrils from aqueous solutions, J. Hazard. Mater., 2019, 367, 256–266 CrossRef CAS PubMed.
- M. Gouda and A. Aljaafari, Removal of Heavy Metal Ions from Wastewater Using Hydroxyethyl Methacrylate-Modified Cellulose Nanofibers: Kinetic, Equilibrium, and Thermodynamic Analysis, Int. J. Environ. Res. Public Health, 2021, 18, 6581 CrossRef CAS PubMed.
- J. Li, Shape memory aerogels from nanocellulose and polyethyleneimine as a novel adsorbent for removal of Cu(II) and Pb(II), Carbohydr. Polym., 2018, 196, 376–384 CrossRef CAS PubMed.
- Y. Kim, J. Park, J. Bang, J. Kim, H. J. Jin and H. W. Kwak, Highly efficient Cr(VI) remediation by cationic functionalized nanocellulose beads, J. Hazard. Mater., 2022, 426, 128078 CrossRef CAS PubMed.
- C. Zhan, Rice husk based nanocellulose scaffolds for highly efficient removal of heavy metal ions from contaminated water, Environ. Sci.: Water Res. Technol., 2020, 6, 3080–3090 RSC.
- Q. Xu, Y. Wang, L. Jin, Y. Wang and M. Qin, Adsorption of Cu (II), Pb (II) and Cr (VI) from aqueous solutions using black wattle tannin-immobilized nanocellulose, J. Hazard. Mater., 2017, 339, 91–99 CrossRef CAS PubMed.
- A. B. Daniel, E. Zahir and M. A. Asghar, Remediation of vanadium (V) and chromium (III) ions from aqueous media by modified nanocellulose obtained from coconut coir, J. Macromol. Sci., Part B: Phys., 2021, 60, 500–520 CrossRef CAS.
- M. Vakili, Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review, Sep. Purif. Technol., 2019, 224, 373–387 CrossRef CAS.
- T. V. J. Charpentier, A. Neville, J. L. Lanigan, R. Barker, M. J. Smith and T. Richardson, Preparation of Magnetic Carboxymethylchitosan Nanoparticles for Adsorption of Heavy Metal Ions, ACS Omega, 2016, 1, 77–83 CrossRef CAS PubMed.
- H. Hajjaoui, A. Soufi, W. Boumya, M. Abdennouri and N. Barka, Polyaniline/nanomaterial composites for the removal of heavy metals by adsorption: A Review, J. Compos. Sci., 2021, 5, 233 CrossRef CAS.
- C. H. Khong, G. B. Teh and S. W. Phang, Effect of Titanium Dioxide and Carbon Nanotubes on Polyaniline Nanocomposites for Heavy Metals Removal, Macromol. Symp., 2018, 382, 1800087 CrossRef.
- S. Ashraf, A. Siddiqa, S. Shahida and S. Qaisar, Titanium-based nanocomposite materials for arsenic removal from water: A review, Heliyon, 2019, 5, 01577 Search PubMed.
- E. Vunain, A. K. Mishra and R. W. Krause, Fabrication, Characterization and Application of Polymer Nanocomposites for Arsenic(III) Removal from Water, J. Inorg. Organomet. Polym. Mater., 2012, 23, 293–305 CrossRef.
- H. Su, Z. Ye and N. Hmidi, High-performance iron oxide–graphene oxide nanocomposite adsorbents for arsenic removal, Colloids Surf., A, 2017, 522, 161–172 CrossRef CAS.
- L. Cui, EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: Adsorption mechanism and separation property, Chem. Eng. J., 2015, 281, 1–10 CrossRef CAS.
- R. Hasanzadeh, P. N. Moghadam, N. Bahri-Laleh and M. Sillanpaa, Effective removal of toxic metal ions from aqueous solutions: 2-Bifunctional magnetic nanocomposite base on novel reactive PGMA-MAn copolymer@Fe(3)O(4) nanoparticles, J. Colloid Interface Sci., 2017, 490, 727–746 CrossRef CAS PubMed.
- J. Zhu, One-pot synthesis of magnetic graphene nanocomposites decorated with core@double-shell nanoparticles for fast chromium removal, Environ. Sci. Technol., 2012, 46, 977–985 CrossRef CAS PubMed.
- Y. L. F. Musico, C. M. Santos, M. L. P. Dalida and D. F. Rodrigues, Improved removal of lead(ii) from water using a polymer-based graphene oxide nanocomposite, J. Mater. Chem. A, 2013, 1, 3789–3796 RSC.
- J. Baruah, C. Chaliha, E. Kalita, B. K. Nath, R. A. Field and P. Deb, Modelling and optimization of factors influencing adsorptive performance of agrowaste-derived Nanocellulose Iron Oxide Nanobiocomposites during remediation of Arsenic contaminated groundwater, Int. J. Biol. Macromol., 2020, 164, 53–65 CrossRef CAS PubMed.
- E. Pitoniak, C.-Y. Wu, D. W. Mazyck, K. W. Powers and W. Sigmund, Adsorption enhancement mechanisms of silica− titania nanocomposites for elemental mercury vapor removal, Environ. Sci. Technol., 2005, 39, 1269–1274 CrossRef CAS PubMed.
- K. Brungesh, B. Nagabhushana, M. Harish and R. H. Krishna, An efficient removal of toxic Cr (VI) from aqueous solution by MnO2 coated polyaniline nanofibers: kinetic and thermodynamic study, J. Environ. Anal. Toxicol., 2017, 7, 1000442 Search PubMed.
- M. R. Awual, Novel nanocomposite materials for efficient and selective mercury ions capturing from wastewater, Chem. Eng. J., 2017, 307, 456–465 CrossRef CAS.
- W. Liu, Efficient removal of hexavalent chromium from water by an adsorption-reduction mechanism with sandwiched nanocomposites, RSC Adv., 2018, 8, 15087–15093 RSC.
- C. Ianăşi, Removal of cadmium from aqueous solutions using inorganic porous nanocomposites, Korean J. Chem. Eng., 2019, 36, 688–700 CrossRef.
- Y. O. Zubair, S. Fuchida and C. Tokoro, Insight into the Mechanism of Arsenic(III/V) Uptake on Mesoporous Zerovalent Iron-Magnetite Nanocomposites: Adsorption and Microscopic Studies, ACS Appl. Mater. Interfaces, 2020, 12, 49755–49767 CrossRef CAS PubMed.
- X. Xu, X.-k. Ouyang and L.-Y. Yang, Adsorption of Pb(II) from aqueous solutions using crosslinked carboxylated chitosan/carboxylated nanocellulose hydrogel beads, J. Mol. Liq., 2021, 322, 114523 CrossRef CAS.
- B. K. Nath, C. Chaliha, E. Kalita and M. C. Kalita, Synthesis and characterization of ZnO:CeO2:nanocellulose:PANI bionanocomposite. A bimodal agent for arsenic adsorption and antibacterial action, Carbohydr. Polym., 2016, 148, 397–405 CrossRef CAS PubMed.
- S. Zarei, M. Niad and H. Raanaei, The removal of mercury ion pollution by using Fe(3)O(4)-nanocellulose: Synthesis, characterizations and DFT studies, J. Hazard. Mater., 2018, 344, 258–273 CrossRef CAS PubMed.
- M. Krawczyk-Coda, Determination of Selenium in Food Samples by High-Resolution Continuum Source Atomic Absorption Spectrometry After Preconcentration on Halloysite Nanotubes Using Ultrasound-Assisted Dispersive Micro Solid-Phase Extraction, Food Anal. Methods, 2018, 12, 128–135 CrossRef.
- K. Pyrzynska, Recent Applications of Carbon Nanotubes for Separation and Enrichment of Lead Ions, Separations, 2023, 10, 152 CrossRef CAS.
- J. Choi, J. H. Kim, J. W. Oh and J. M. Nam, Surface-enhanced Raman scattering-based detection of hazardous chemicals in various phases and matrices with plasmonic nanostructures, Nanoscale, 2019, 11, 20379–20391 RSC.
- O. Yalcinkaya, O. M. Kalfa and A. R. Turker, Chelating agent free-solid phase extraction (CAF-SPE) of Co(II), Cu(II) and Cd(II) by new nano hybrid material (ZrO2/B2O3), J. Hazard. Mater., 2011, 195, 332–339 CrossRef CAS PubMed.
- M. Krawczyk and M. Jeszka-Skowron, Multiwalled carbon nanotubes as solid sorbent in dispersive micro solid-phase extraction for the sequential determination of cadmium and lead in water samples, Microchem. J., 2016, 126, 296–301 CrossRef CAS.
- L. Torkian, M. M. Amini, T. Gorji and O. Sadeghi, A simple, rapid and sensitive method based on modified multiwalled carbon nanotube for preconcentration and determination of lead ions in aqueous media in natural pHs, Arabian J. Chem., 2019, 12, 1315–1321 CrossRef CAS.
- C. Bendicho, C. Bendicho-Lavilla and I. Lavilla, Nanoparticle-assisted chemical speciation of trace elements, TrAC, Trends Anal. Chem., 2016, 77, 109–121 CrossRef CAS.
- F. Xu, J. Hu, J. Zhang, X. Hou and X. Jiang, Nanomaterials in speciation analysis of mercury, arsenic, selenium, and chromium by analytical atomic/molecular spectrometry, Appl. Spectrosc. Rev., 2018, 53, 333–348 CrossRef CAS.
- K. J. Chen, I. H. Hsu and Y. C. Sun, Determination of methylmercury and inorganic mercury by coupling short-column ion chromatographic separation, on-line photocatalyst-assisted vapor generation, and inductively coupled plasma mass spectrometry, J. Chromatogr. A, 2009, 1216, 8933–8938 CrossRef CAS PubMed.
- X. Gao, Synthesis of MoS(2) nanosheets for mercury speciation analysis by HPLC-UV-HG-AFS, RSC Adv., 2018, 8, 18364–18371 RSC.
- Z. Zou, J. Hu, F. Xu, X. Hou and X. Jiang, Nanomaterials for photochemical vapor generation-analytical atomic spectrometry, TrAC, Trends Anal. Chem., 2019, 114, 242–250 CrossRef CAS.
- C. Li, R. Liu, Y. Lü, X. Hou and P. Wu, Exploration of nano-surface chemistry for spectral analysis, Chin. Sci. Bull., 2013, 58, 2017–2026 CrossRef CAS.
- Y. Hu, Q. Wang, C. Zheng, L. Wu, X. Hou and Y. Lv, Recyclable decoration of amine-functionalized magnetic nanoparticles with Ni(2+) for determination of histidine by photochemical vapor generation atomic spectrometry, Anal. Chem., 2014, 86, 842–848 CrossRef CAS PubMed.
- C. Zheng, L. Wu, Q. Ma, Y. Lv and X. Hou, Temperature and nano-TiO2 controlled photochemical vapor generation for inorganic selenium speciation analysis by AFS or ICP-MS without chromatographic separation, J. Anal. At. Spectrom., 2008, 23, 514–520 RSC.
- C. H. Lin, Y. Chen, Y. A. Su, Y. T. Luo, T. T. Shih and Y. C. Sun, Nanocomposite-Coated Microfluidic-Based Photocatalyst-Assisted Reduction Device To Couple High-Performance Liquid Chromatography and Inductively Coupled Plasma-Mass Spectrometry for Online Determination of Inorganic Arsenic Species in Natural Water, Anal. Chem., 2017, 89, 5891–5899 CrossRef CAS PubMed.
- N. Ullah, M. Mansha, I. Khan and A. Qurashi, Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges, TrAC, Trends Anal. Chem., 2018, 100, 155–166 CrossRef CAS.
- S. O. Fakayode, C. Walgama, V. E. F. Narcisse and C. Grant, Electrochemical and Colorimetric Nanosensors for Detection of Heavy Metal Ions: A Review, Sensors, 2023, 23, 9080 CrossRef CAS PubMed.
- D. Zhang, S. Chu, L. Wang, X. Zhan, P. Zhou and D. Zhang, Dual-mode colorimetric determination of As(III) based on negatively-charged aptamer-mediated aggregation of positively-charged AuNPs, Anal. Chim. Acta, 2022, 1221, 340111 CrossRef CAS PubMed.
- Q. Liu, Covalent organic framework-loaded silver nanoparticles as robust mimetic oxidase for highly sensitive and selective colorimetric detection of mercury in blood, J. Mater. Chem. B, 2022, 10, 10075–10082 RSC.
- G. S. Geleta, A colorimetric aptasensor based on two dimensional (2D) nanomaterial and gold nanoparticles for detection of toxic heavy metal ions: A review, Food Chem. Adv., 2023, 2, 100184 CrossRef.
- S. Sharma, A. Jaiswal and K. Uttam, Colorimetric and surface enhanced Raman scattering (SERS) detection of metal ions in aqueous medium using sensitive, robust and novel pectin functionalized silver nanoparticles, Anal. Lett., 2020, 53, 2355–2378 CrossRef CAS.
- R. Seth, L-cysteine functionalized gold nanoparticles as a colorimetric sensor for ultrasensitive detection of toxic metal ion cadmium, Mater. Today: Proc., 2020, 24, 2375–2382 Search PubMed.
- N. K. Shalvi, K. L. Verma, V. K. Jain and S. Nagpal, Integrated device for colorimetric detection of arsenite using polyethylene glycol capped gold nanoparticles—Lab-on-chip, Toxicology and Environmental Health Sciences, 2021, 13, 351–362 CrossRef.
- F. Hua, Quantitative colorimetric sensing of heavy metal ions via analyte-promoted growth of Au nanoparticles with timer or smartphone readout, Anal. Bioanal. Chem., 2023, 415, 2705–2713 CrossRef CAS PubMed.
- J. Li and J. J. Zhu, Quantum dots for fluorescent biosensing and bio-imaging applications, Analyst, 2013, 138, 1–25 RSC.
- J. M. Vonnie, K. Rovina, K. H. Erna, S. Mantihal, N. Huda and R. A. Wahab, Development of a colorimetric sensor based on tapioca starch and gold nanoparticles for the detection of cadmium residues in fish products, J. Food Nutr. Res., 2022, 61, 315–322 CAS.
- Z. Wang, B. Yao, Y. Xiao, X. Tian and Y. Wang, Fluorescent Quantum Dots and Its Composites for Highly Sensitive Detection of Heavy Metal Ions and Pesticide Residues: A Review, Chemosensors, 2023, 11, 405 CrossRef CAS.
- R. Chadha, A. Das, A. K. Debnath, S. Kapoor and N. Maiti, 2-thiazoline-2-thiol functionalized gold nanoparticles for detection of heavy metals, Hg(II) and Pb(II) and probing their competitive surface reactivity: A colorimetric, surface enhanced Raman scattering (SERS) and x-ray photoelectron spectroscopic (XPS) study, Colloids Surf., A, 2021, 615, 126279 CrossRef CAS.
- J. Wang, Fabrication of an “ion-imprinting” dual-emission quantum dot nanohybrid for selective fluorescence turn-on and ratiometric detection of cadmium ions, Analyst, 2016, 141, 5886–5892 RSC.
- J. Liu, G. Lv, W. Gu, Z. Li, A. Tang and L. Mei, A novel luminescence probe based on layered double hydroxides loaded with quantum dots for simultaneous detection of heavy metal ions in water, J. Mater. Chem. C, 2017, 5, 5024–5030 RSC.
- R. Zhang and W. Chen, Nitrogen-doped carbon quantum dots: facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions, Biosens. Bioelectron., 2014, 55, 83–90 CrossRef CAS PubMed.
- S. P. Akanji, O. M. Ama, S. S. Ray and P. O. Osifo, Metal Oxide Nanomaterials for Electrochemical Detection of Heavy Metals in Water, Nanostructured Metal-Oxide Electrode Materials for Water Purification, 2020, vol. 1, pp. 113–126 Search PubMed.
- X. Liu, Y. Yao, Y. Ying and J. Ping, Recent advances in nanomaterial-enabled screen-printed electrochemical sensors for heavy metal detection, TrAC, Trends Anal. Chem., 2019, 115, 187–202 CrossRef CAS.
- M. D. Shirsat and T. Hianik, Electrochemical Detection of Heavy Metal Ions Based on Nanocomposite Materials, J. Compos. Sci., 2023, 7, 473 CrossRef CAS.
- G. Aragay and A. Merkoçi, Nanomaterials application in electrochemical detection of heavy metals, Electrochim. Acta, 2012, 84, 49–61 CrossRef CAS.
- A. Waheed, M. Mansha and N. Ullah, Nanomaterials-based electrochemical detection of heavy metals in water: Current status, challenges and future direction, TrAC, Trends Anal. Chem., 2018, 105, 37–51 CrossRef CAS.
- L. Yu, Nanomaterials-Based Ion-Imprinted Electrochemical Sensors for Heavy Metal Ions Detection: A Review, Biosensors, 2022, 12, 1096 CrossRef CAS PubMed.
- G. Maduraiveeran and W. Jin, Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications, Trends Environ. Anal. Chem., 2017, 13, 10–23 CrossRef CAS.
- Y. GadelHak, S. H. M. Hafez, H. F. M. Mohamed, E. E. Abdel-Hady and R. Mahmoud, Nanomaterials-modified disposable electrodes and portable electrochemical systems for heavy metals detection in wastewater streams: A review, Microchem. J., 2023, 193, 109043 CrossRef CAS.
- A. Mao, H. Li, Z. Cai and X. Hu, Determination of mercury using a glassy carbon electrode modified with nano TiO2 and multi-walled carbon nanotubes composites dispersed in a novel cationic surfactant, J. Electroanal. Chem., 2015, 751, 23–29 CrossRef CAS.
- S. Singh, A. Numan, Y. Zhan, V. Singh, T. Hung and N. D. Nam, A novel highly efficient and ultrasensitive electrochemical detection of toxic mercury (II) ions in canned tuna fish and tap water based on a copper metal-organic framework, J. Hazard. Mater., 2020, 399, 123042 CrossRef CAS PubMed.
- F.-m. Liu, A high–selectivity electrochemical sensor for ultra-trace lead (II) detection based on a nanocomposite consisting of nitrogen-doped graphene/gold nanoparticles functionalized with ETBD and Fe3O4@TiO2 core–shell nanoparticles, Sens. Actuators, B, 2017, 242, 889–896 CrossRef CAS.
- S. Ma, A portable microfluidic electrochemical sensing platform for rapid detection of hazardous metal Pb2+ based on thermocapillary convection using 3D Ag-rGO-f-Ni (OH) 2/NF as a signal amplifying element, J. Hazard. Mater., 2023, 448, 130923 CrossRef CAS PubMed.
- P. W. Sayyad, Sensitive and selective detection of Cu2+ and Pb2+ ions using Field Effect
Transistor (FET) based on L-Cysteine anchored PEDOT:PSS/rGO composite, Chem. Phys. Lett., 2020, 761, 138056 CrossRef CAS.
- Y. M. Sabri, S. J. Ippolito, J. Tardio, V. Bansal, A. P. O'Mullane and S. K. Bhargava, Gold nanospikes based microsensor as a highly accurate mercury emission monitoring system, Sci. Rep., 2014, 4, 6741 CrossRef CAS PubMed.
- R. R. Silva, Microbial nanocellulose adherent to human skin used in electrochemical sensors to detect metal ions and biomarkers in sweat, Talanta, 2020, 218, 121153 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –OSO3, OH and –CONH–(CH2)2–NH2 to the CNTs.50 This can be seen in the recent research by Elghamry et al. on the production of acid functionalized multiwalled carbon nanotubes.32 Their work utilized benzimidazole to produce a novel functionalized MWCNT that displayed strong adsorption for both lead and cadmium ions, confirming the versatility of surface modifications of CNTs for heavy metal remediation.32
O, –OSO3, OH and –CONH–(CH2)2–NH2 to the CNTs.50 This can be seen in the recent research by Elghamry et al. on the production of acid functionalized multiwalled carbon nanotubes.32 Their work utilized benzimidazole to produce a novel functionalized MWCNT that displayed strong adsorption for both lead and cadmium ions, confirming the versatility of surface modifications of CNTs for heavy metal remediation.32



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the removal amounts of Cd2+, Cu2+, Ni2+ and Pb2+ are 79.33–102 mg g−1, 111.11–142.85 mg g−1, 107.30–137.69 mg g−1 and 110.97–142.68 mg g−1, respectively. However, the removal efficiency decreased to very low levels when the ratio was increased to 140–180
1, the removal amounts of Cd2+, Cu2+, Ni2+ and Pb2+ are 79.33–102 mg g−1, 111.11–142.85 mg g−1, 107.30–137.69 mg g−1 and 110.97–142.68 mg g−1, respectively. However, the removal efficiency decreased to very low levels when the ratio was increased to 140–180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the heavy metal ions were acidified. This is related to the deposition of iron and the subsequent release of heavy metals.91 More recent research into nano zerovalent iron (nZVI) focuses on addressing the aggregation of nZVI as this reduces the potential binding sites for heavy metal sorption. A recent study by Awang et al. utilized biochar to improve stabilization and reduce aggregation behaviour, which allowed for enhanced filtration of heavy metals such as chromium, cadmium, and lead.89
1, and the heavy metal ions were acidified. This is related to the deposition of iron and the subsequent release of heavy metals.91 More recent research into nano zerovalent iron (nZVI) focuses on addressing the aggregation of nZVI as this reduces the potential binding sites for heavy metal sorption. A recent study by Awang et al. utilized biochar to improve stabilization and reduce aggregation behaviour, which allowed for enhanced filtration of heavy metals such as chromium, cadmium, and lead.89










