 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evaluating the path to sustainability: SWOT analysis of safe and sustainable by design approaches for metal–organic frameworks
Pankti Dhumal†
a,
Prathmesh Bhadane† b,
Bashiru Ibrahima and
Swaroop Chakraborty
b,
Bashiru Ibrahima and
Swaroop Chakraborty *a
*a
aSchool of Geography, Earth & Environmental Sciences, University of Birmingham, Edgbaston, B15 2TT, UK. E-mail: s.chakraborty@bham.ac.uk
bMaterials Engineering, Indian Institute of Technology Gandhinagar, India 382355
First published on 6th March 2025
Abstract
In this review, we conduct a comprehensive SWOT (Strengths, Weaknesses, Opportunities, Threats) analysis of Metal–Organic Frameworks (MOFs) through the lens of Safe and Sustainable by Design (SSbD) guidelines, evaluating their potential to meet environmental, industrial, and societal needs. Renowned for their structural tunability, high surface area, and versatile applications—from gas storage to catalysis and environmental remediation—MOFs offer the strength of customisability through the selection of diverse metal nodes and organic linkers, allowing tailored functionalities that align with SSbD framework. This adaptability supports the development of MOFs with enhanced stability, selectivity, and safety, catering to a broad spectrum of applications. However, concerns remain about their environmental and health impacts across the material lifecycle. This review highlights the adaptability of MOFs, enabled by the strategic selection of metal nodes and organic linkers, allowing tailored functionalities that align with SSbD framework. The weaknesses section addresses the high environmental cost and limited stability associated with traditional MOF synthesis, emphasising the need for greener, scalable methods using benign solvents and renewable resources. The opportunities section explores advances in biocompatible and recyclable MOFs, aligning these materials with circular economy goals and sustainable material cycles that support the Sustainable Development Goals (SDGs). In assessing potential threats, we discuss the emergence of alternative materials, such as carbon nanomaterials and Covalent Organic Frameworks (COFs), which underline the urgency for SSbD-driven innovation within MOFs research. By advocating for a balanced SSbD approach, this review outlines strategies to reduce the environmental footprint of MOFs and enhance their industrial viability, providing a roadmap for the responsible large-scale adoption of MOFs that aligns with global sustainability objectives.
Green foundation1. Advances in Green Chemistry: this tutorial review showcases transformative progress in Metal–Organic Frameworks (MOFs) through green synthesis innovations such as water-based methods, solvent-free approaches etc. These advancements, grounded in the Safe and Sustainable by Design (SSbD) framework, significantly reduce environmental footprints and integrate circular economy ideals.2. Significant Wider Interest: the immense versatility of MOFs in water purification, pollutant capture, and drug delivery positions them as vital tools to address pressing global challenges. This review identifies and addresses critical gaps, including toxicological data, life cycle assessments, and scalability, ensuring MOFs can transition from lab to impactful real-world applications. 3. Future Outlook: by employing a novel SWOT analysis, this review sets a forward-looking agenda for integrating SSbD into MOF research. It envisions a future where green chemistry drives the development of scalable, safe, and environmentally responsible materials to meet industrial and societal needs. |
1. Introduction
Metal–Organic Frameworks (MOFs) are emerging as versatile materials with potential in gas storage, catalysis, and environmental remediation due to their tuneable structures, high surface areas, and porosity.1–3 However, the environmental and health impacts of MOFs across their life cycle remain poorly understood. “Safe and Sustainable by Design” (SSbD) is an approach that integrates safety, environmental sustainability, and functionality throughout the entire lifecycle of chemicals and materials, from conception to disposal. In the context of MOFs, applying SSbD framework ensures that these materials are developed with minimal environmental impact and maximum safety. This is crucial for large-scale adoption, as it addresses potential regulatory concerns and enhances public trust. By adhering to SSbD guidelines, MOFs manufacturers can meet stringent safety and sustainability standards, facilitating broader acceptance and application across various industries.4 The SSbD framework offers a pathway to address these gaps, focusing on materials designed to be safe and sustainable throughout their life cycle. While previous studies primarily explore MOFs synthesis and specific applications,5 few have comprehensively assessed MOFs from an SSbD perspective.5 This review uniquely applies a SWOT (Strengths, Weaknesses, Opportunities, Threats) analysis to evaluate MOFs within the SSbD framework, providing a holistic view that guides the development of MOFs meeting environmental, industrial, and societal standards.Our approach goes beyond traditional reviews, which typically limit their scope to applications or synthesis methods, by identifying both the strengths and challenges of integrating SSbD framework into MOFs research. Such a framework is crucial for balancing MOFs high performance with the demands for environmental responsibility, safety, and economic viability, especially as applications of these materials expand across sectors. Importantly, this SWOT analysis aligns with sustainable development goals (SDGs), as it evaluates MOFs not only on functionality but also on their broader implications for safety, environmental impact, economic feasibility, and social value.
In the Strengths section, this review highlights the adaptability of MOFs, which can be customised through different metal nodes and organic linkers to improve stability, functionality, and safety. Modifications like doping and post-synthetic transformations enable MOFs to perform reliably in challenging conditions, supporting SSbD by reducing replacement frequency and minimising environmental impact.6,7 This adaptability facilitates MOFs application in diverse fields, from water purification to controlled drug delivery, while adhering to sustainable design principles. In Weaknesses, we examine the significant resource demands and environmental impacts associated with traditional MOFs and MOFs composite synthesis for different applications. Inadequate performance, high-temperature processes and hazardous solvents, such as dimethylformamide (DMF), contribute to pollution and energy consumption.8–10 These limitations emphasise the urgent need for greener synthesis methods, including low-energy alternatives, safer solvents, and post-synthetic modifications. Aligning synthesis and applications with SSbD framework can reduce pollution and energy demands, allowing for scalable, less environmentally damaging production that meets regulatory and sustainability standards.
The Opportunities section explores advancements in biocompatible and recyclable MOFs that support circular economy goals. Recent progress in biocompatible MOFs offers promise for medical applications, while recyclable composites incorporating polymers or carbon materials enhance durability and reusability.11–15 Such innovations are crucial to aligning MOFs with SSbD values, reducing environmental toxicity and advancing sustainable material cycles that support SDGs. The potential for MOFs to bridge sustainability with advanced applications makes this field ripe for future-focused development. In threats, we discuss competitive materials like carbon based nanomaterials {Graphene Oxide (GO), reduced graphene oxide (rGO) etc.}, Covalent Organic Frameworks (COFs) and MXenes, which offer enhanced thermal stability and conductivity, making them suitable for high-stress applications.16–19 This competition highlights the importance of SSbD-based innovation within the MOFs field, ensuring that MOFs remain viable by prioritising safety, durability, and reduced environmental impact to meet evolving market and environmental demands.
This review stands out for its comprehensive evaluation of MOFs through the SSbD framework, spotlighting areas of improvement and promoting sustainable development pathways (Fig. 1). By addressing the safety, environmental, economic, and social aspects of MOFs, this SWOT analysis provides a structured approach to SSbD that aligns with next-generation industry standards and SDGs. It highlights the importance of MOFs’ evolution towards safety and sustainability, offering insights to drive their responsible adoption at scale and supporting their role in a sustainable future.
2. SWOT analysis
SWOT analysis is a strategic planning tool that assesses the strengths, weaknesses, opportunities, and threats related to a project or business venture. Applying SWOT in the context of SSbD of MOFs allows researchers and industries to evaluate how well these materials meet safety and sustainability criteria while identifying areas for innovation and potential challenges. It helps in balancing performance with eco-friendliness, anticipating regulatory challenges, and exploiting new applications and markets. This analysis is crucial for enhancing the design, application, and lifecycle management of MOFs to align with environmental and health safety standards. A similar SWOT analysis was performed by our group on the “Green Synthesis” approaches of carbon nanodots.20 The summary of SWOT analysis of SSbD approaches for MOFs is presented in Table 1.| Sr. no | SWOT | Key points | Highlights |
|---|---|---|---|
| 1 | Strength (S) | High customisability through component selection | Customisability and stability: MOFs can be tailored for specific functions through selective use of metal nodes and linkers, and doping with elements like Fe and Ni significantly improves hydrolytic stability, making them suitable for water and environmental applications |
| Enhanced hydrophobicity via modifications: post-synthetic modifications, including fluorinated or alkyl linkers, increase hydrophobicity and durability, aligning MOFs with SSbD framework by reducing degradation and environmental impact | |||
| Effective heavy metal adsorption and reusability: MOFs demonstrate high heavy metal adsorption efficiency with minimal leaching and regeneration capabilities, supporting long-term, sustainable water purification solutions | |||
| Versatility of MOFs in SSbD applications | Environmental remediation and SSbD: MOFs are used in applications like oil–water separation, pesticide degradation, and dye removal, utilising SSbD approaches to reduce environmental impact. | ||
| Scalable and sustainable production: green synthesis methods, such as water-based production, enable large-scale manufacturing of MOFs without compromising their performance, making them suitable for applications like water harvesting and pollutant capture | |||
| Agricultural and biomedical potential: MOFs composites show promise in agriculture for soil improvement and heavy metal removal, and in biomedicine for applications like sepsis treatment, emphasising reusability and low environmental impact | |||
| Advances in Green synthesis techniques | Green synthesis methods: researchers are advancing sustainable MOFs production using water-based and renewable solvents, bio-based ligands, and recyclable materials like PET waste. Notably, Yang et al.'s work on CAU-17-CTAB synthesis in water demonstrates significant environmental advantages, achieving an 86.29% yield, a faster reaction time, and potential applications in targeted drug delivery | ||
| Waste-to-MOFs strategy: Li H. et al. demonstrated a sustainable approach to MOFs production by upcycling PET waste, providing an affordable and scalable alternative to traditional synthesis using expensive ligands. This strategy not only reduces plastic pollution but also supports the development of high-performance, functional materials from waste | |||
| Environmentally friendly techniques: innovative techniques like ball milling and the use of DES are emerging as green alternatives to conventional, chemical-intensive methods. Zhang et al.'s study highlights ball milling for synthesising graphene@Cu-MOFs hybrids, reducing environmental impact by eliminating toxic reagents and leveraging mechanical forces for synthesis. | |||
| 2 | Weakness (W) | Synthesis and stability MOFs and MOFs composites under varied environments | Stability vs. functionality trade-offs: achieving stability in MOFs, especially in aqueous and reactive environments, often compromises adsorption efficiency. Stable frameworks like UiO-66 lack the adsorption capacity needed for environmental applications compared to less stable MOFs (e.g., ZIF-8 or Cu-BTC), which offer more active sites and higher pore volume. This stability-functionality trade-off is a major challenge in using MOFs for environmental applications |
| Impact of structural modifications: strategies to enhance MOFs stability—such as functionalisation, core–shell structures, and composite formations—sometimes reduce adsorption efficiency and add preparation complexity. While modifications can shield MOFs from degradation, they may also lower the performance required for real-world applications, with notable declines in adsorption capacity and reusability | |||
| Need for SSbD integration: MOFs synthesis processes are often resource-intensive and environmentally taxing, involving high temperatures, pressures, and toxic solvents. Integrating SSbD framework is essential to produce MOFs that balance stability, functionality, and environmental compatibility, meeting practical demands without ecological harm | |||
| Insufficient toxicological, Life Cycle Assessment (LCA), Environmental Footprint Data | Toxicological data gaps and need for standardisation: comprehensive toxicological data on MOFs are lacking, especially concerning their transformations in real-world conditions. This gap hinders accurate risk assessment, as MOFs can degrade and release Reactive oxygen species (ROS) and metal ions, causing oxidative stress and toxicity. Standardised toxicity testing is crucial to establishing safe exposure limits and supporting (SSbD) practices in MOFs synthesis | ||
| Environmental impact of solvents: limited life cycle assessments (LCA) data shows significant environmental impacts of MOFs synthesis, especially from solvothermal methods, affecting ozone and ecotoxicity. Solvents like DMSO and DMF are concerning for health, as residual molecules can remain in MOFs pores, releasing toxic byproducts. Addressing solvent-related toxicity is vital, particularly for biomedical and environmental uses | |||
| Need for proactive LCA approaches: despite rapid MOFs development, comprehensive LCA covering the full environmental footprint from synthesis to disposal are rare. Standardising LCA and prioritising representative assessments for key MOF types could support sustainable production and regulatory approval, advancing SSbD integration in MOFs development | |||
| Cost, scalability and reproducibility constraints | Economic and material constraints: high costs associated with eco-friendly materials, organic linkers, and energy-intensive synthesis are major barriers to SSbD for MOFs. Approaches like low-cost linker sources, mechanochemistry, and flow chemistry are being explored to lower costs | ||
| Reproducibility challenges: consistency in MOFs production is difficult due to factors like solvent purity and reaction vessel choice. Variability in yield, particle size, and crystallinity across labs highlights the need for standardised protocols to improve reproducibility | |||
| Scalability and sustainability: scaling up MOFs production is challenged by resource inefficiencies and quality control at industrial scales. Advances in continuous production and reactor design are underway, but achieving uniformity, purity, and crystallinity requires further innovation for sustainable, scalable manufacturing | |||
| Complexity of balancing safety with performance | Innovative green synthesis: researchers are adopting sustainable methods for MOFs production, such as using industrial wastewater for Cr(III) ions and recycled PET as linkers. These methods reuse waste and avoid toxic solvents, aligning with SSbD framework, while achieving high performance—like MIL-101(Cr)'s notable adsorption capacity for acid blue dye | ||
| Performance vs. safety trade-offs: green synthesis can enhance MOFs functionality but introduces challenges in consistency, reproducibility, and cost-effectiveness. Additional purification steps may be necessary, raising production costs. Multi-layered composites, like Fe3O4@SiO2@HKUST-1, face challenges in structural stability after reuse and maintaining eco-friendly qualities | |||
| Assessing metal ion release: a critical but often overlooked aspect of SSbD MOFs is evaluating metal ion release during adsorption. For instance, introducing defects (as in UiO-66) enhances adsorption but may increase metal ion leaching, impacting safety. Testing for ion release is essential to ensure performance improvements don't compromise environmental or human safety | |||
| 3 | Opportunities (O) | Advancements in biocompatible and degradable MOFs | Safe and sustainable drug delivery with BioMOFs: advances in BioMOFs, particularly those with benign metals like Zn, Mg, and Ca, emphasise biocompatibility and degradability for safe biomedical use. A Cu-based BioMOFs using protocatechuic acid as a linker showed high drug loading capacity, proving effective for TB treatment and highlighting BioMOFs’ potential for targeted, low-toxicity drug delivery |
| Cancer treatment innovations with SSbD BioMOFs: SSbD BioMOFs offer novel cancer treatments by combining low systemic toxicity, specific targeting, and multifunctional features. Examples include Fe-based BioMOFs, coated with PEG and functionalised with aptamers for colorectal cancer, that enable drug delivery, imaging, and minimal toxicity, aligning with sustainable cancer therapy solutions | |||
| MOF-based scaffolds in regenerative medicine: BioMOFs also support regenerative medicine applications, such as bone repair, by releasing metal ions to stimulate osteogenesis and angiogenesis. This aligns with health-focused sustainability goals, addressing challenges like stability, degradation, and controlled release for more effective and targeted therapies | |||
| Emerging MOFs applications through machine learning-driven innovation | Predictive design in environmental and biomedical applications: ML enhances MOFs applications by predicting adsorption efficiencies, cytotoxicity, and stability. For instance, Random Forest models identify effective MOFs for water purification (e.g., Pb2+ removal) and predict biocompatibility for drug delivery, supporting SSbD. | ||
| Hydrogen storage optimization: ML, combined with topological data analysis, enables high-throughput screening for hydrogen storage by rapidly assessing MOFs with high hydrogen affinity, reducing costly simulations and improving accuracy for optimal storage capacities | |||
| Untapped potential in agriculture: ML's integration into agricultural MOFs research remains limited. However, it could optimise MOFs formulations for micronutrient delivery and pollutant remediation, enhancing crop resilience and promoting sustainable agricultural practices | |||
| Circular economy potential | Eco-Friendly MOFs synthesis via circular economy principles: Sustainable synthesis methods, like reusable Pd@MOFs catalysts and DES, cut reliance on toxic chemicals and reduce waste. DES functions as both solvent and catalyst, aligning with the circular economy's “Reduce” principle | ||
| Reusability in pollution remediation: MOFs materials, such as the graphene oxide-ZIF-67 composite, maintain high adsorption efficiency over multiple cycles, reducing material waste and costs. This reusability supports resource efficiency, essential for large-scale pollutant removal | |||
| Transformative MOFs recycling processes: converting used MOFs, like MIL-100(Fe), into high-value materials (e.g., graphene/Fe@N-doped carbon hybrid) enables resource recovery, turning waste into reusable resources and aligning with circular economy goals | |||
| Safe and sustainable composites | Enhanced functionality and reusability via composite formation: MOFs composites with polymers, carbon nanomaterials, and inorganic nanoparticles improve stability, durability, and reusability. For instance, MOF-polymer composites support air filters and gas masks, while graphene-MOFs composites enhance water treatment, achieving over 88% retention in adsorption capacity after multiple uses | ||
| Sustainable synthesis aligned with SSbD: these composites employ eco-friendly processes, like recycling PET plastic for MOFs linkers and using non-toxic carbon dots for dye removal. This approach enhances performance (e.g., increased adsorption and durability) and supports waste reduction, advancing sustainability in energy storage, water treatment, and catalysis | |||
| Selective cancer treatment potential: biocompatible MOFs-AuNP composites show promise in cancer treatment, demonstrating high selectivity for cancer cells over normal cells with a green synthesis method. This aligns with SSbD by reducing health risks while providing targeted biomedical applications, underscoring MOFs’ versatility | |||
| 4 | Threat (T) | Alternative materials | Competitive emerging materials: carbon-based nanomaterials (GO, rGO), COFs, and MXenes offer stability, durability, and conductivity, aligning with SSbD framework. These properties make them strong contenders in areas where MOFs have limitations, such as toxic metal adsorption, energy storage, and biomedical applications |
| COFs’ superior stability: COFs demonstrate higher thermal and chemical stability than MOFs, allowing them to withstand harsh environments. This resilience makes COFs effective for applications in catalysis, environmental remediation, and pharmaceutical waste adsorption, where MOFs often fall short | |||
| MXenes’ conductivity and versatility: MXenes excel in applications like catalysis, electronics, and sensors due to their high thermal and electrical conductivity. They offer greater adsorption capacities in REE and dye recovery, outperforming MOFs, with fewer modifications needed, aligning with SSbD framework. | |||
| Regulatory uncertainty and potential of greenwashing | Regulatory gaps and Safety concerns: the rapid development of MOFs has outpaced regulatory frameworks, creating uncertainties around safety and environmental impact. Without standardised regulations, compliance challenges arise, potentially hindering innovation and industry investment. A harmonised approach is essential to support sustainable commercialisation | ||
| Risk of greenwashing: with MOFs marketed as “green” materials, there's a risk of greenwashing, where sustainability claims are exaggerated or unsupported. This can mislead stakeholders and erode consumer trust, slowing genuine environmental progress. Weak regulations allow unverified claims, undermining the credibility of SSbD framework. | |||
| Need for comprehensive tools and industry collaboration: effective MOFs regulations need robust tools like LCA, risk assessments, and collaboration between industry and regulatory bodies. These frameworks can set standardised guidelines for safe production, use, and disposal, ensuring responsible development of this promising technology | |||
| Environmental risks from transformation products | Transformation-induced toxicity: environmental factors like pH, humidity, and chemical exposure can cause MOFs to release toxic metal ions (e.g., Zn2+ from ZIF-8), which accumulate in biological systems, posing ecological and health risks. Prolonged bioavailability, observed in organisms like C. elegans, emphasises the need for toxicity assessments under SSbD framework. | ||
| Structural and Functional Impact of Degradation: MOFs degradation under environmental conditions leads to a loss of essential properties, such as surface area and pore volume, which affects their application in catalysis and CO2 sequestration. The nanoscale nature of MOFs makes them prone to rapid dissolution and ion release, adding challenges to safe application | |||
| Data gaps and need for standardized testing: limited toxicology and lifecycle data hinder understanding of MOFs transformations in real-world conditions. Standardised testing for transformation products, ecotoxicity, and lifecycle impacts is crucial to meet SSbD standards, reducing risks and promoting environmentally safe MOFs designs | |||
| Toxicological implications | SSbD in MOFS design: SSbD framework are essential for creating MOFs with lower toxicity. Choosing safe(r) components and optimised structures helps minimise risks, aligning with EU guidelines on nanomaterials | ||
| Size-dependent toxicity: smaller MOFs nanoparticles (nMOFs) show increased toxicity due to higher cellular uptake, as they can penetrate biological barriers. Studies confirm that particles under 400 nm often pose greater risks to human and environmental health | |||
| Need for standardized testing: standardised toxicity protocols are crucial to assess MOFs safety across their lifecycle. Addressing data gaps will enable safer applications, supporting regulatory and public acceptance |
2.1. Strengths
 | ||
| Fig. 2 Strength of MOFs (A) doping of MOFs improves their efficiency. (F) SEM images show that doping of HKUST-1 MOFs with Fe ions improved its hydrolytic stability.21 (B) The Zr-MOF@Fe3O4 composite shows high adsorption capacity with only a slight reduction over 5 cycles22. (Prepared using Biorender Software). | ||
MOFs exhibit remarkable structural versatility due to the diverse combinations of metal nodes and organic ligands, allowing for the development of customized functionalities for environmental remediation and energy storage applications.28 The choice of metal centers is pivotal in determining the electrochemical performance of MOFs, as different metals contribute unique redox activities, conductivity levels, and charge storage capabilities. For instance, transition metals such as Mn, Co, and Ni demonstrate a synergistic effect in enhancing pseudocapacitance and conductivity, as seen in MnCoNi-MOF, where the presence of multiple redox-active sites significantly improves electron transfer compared to Ni-MOF alone.29 Notably, the energy storage capacity of MnCoNi-MOF was found to be nearly six times higher than that of its monometallic counterpart, highlighting the effectiveness of incorporating multiple metal nodes in boosting energy storage performance. The organic ligand plays a crucial role in shaping the overall structure of a MOF, influencing its porosity, functionality, and crystal morphology.30 Changes in ligand identity can significantly alter the properties of the MOF, impacting its metal ion adsorption capacity, stability in aqueous environments, as well as its performance in gas adsorption and energy storage applications.31 The influence of ligand variation on MOF performance is evident in Zn–Co MOFs synthesized with different ligands. The Zn–Co MOF with 2,5-dihydroxybenzoic acid (DBA) exhibited a significantly higher specific capacitance of approximately 1775 F g−1 and a lower HER overpotential of 186 mV. In contrast, the Zn–Co MOF with benzene-1,2,4,5-tetracarboxylic acid (BTC) showed a much lower specific capacitance of around 137 F g−1 and a higher overpotential of 279 mV.32 DBA, with its hydroxyl groups, enhances electron density around the metal centres, facilitating redox reactions and improving charge transfer, whereas BTC, with carboxyl (–COOH) groups, provides a more rigid and less conductive framework. This highlights the crucial role of ligand selection in tailoring MOF properties for energy and environmental applications. In addition to these synthesis modifications, the potential for post-synthetic modifications in this MOF is a significant advantage. Drache et al.33 improved the stability of DUT-67 MOFs through post-synthetic modification (PSM) by exchanging the formic acid modulator with fluorinated monocarboxylates. These modifications of organic ligands increased the hydrophobicity of MOFs internal surfaces as before modifications, the DUT-67 MOFs exhibited a 34% loss of porosity after water exposure and desorption which indicates a reduction in stability. Post modifications, the MOFs showed significantly less porosity loss ranging from 13% for DUT-67-Tfmba to just 0.3% for DUT-67-Pfoa after water exposure, demonstrating much better water stability compared to the pristine MOFs. Another research34 on PSM of MOFs to improve their stability and hydrophobicity involved the incorporation of long alkyl chains onto their surface. Before modification, ZrTz-68 was hydrophilic with no measurable water contact angle (WCA) due to water absorption. After modification (ZrTz-68-C18), the MOFs became superhydrophobic with a WCA of 152.1°, indicating a successful transition from hydrophilic to superhydrophobic properties. Improving the stability of MOFs using fluorinated and alkyl linkers can align with SSbD framework by reducing degradation products and extending their lifespan, which minimises waste and resource use. However, care must be taken with fluorinated linkers to ensure they don't persist in the environment or pose toxicity risks, ensuring sustainability and safety throughout the material's lifecycle. Long alkyl chain linkers are typically more benign and can offer increased hydrophobicity and durability without introducing persistent or toxic substances. Another area where MOFs’ customisability contributes to safety and sustainability is by its ability to adsorb heavy metals from the environment. Bhadane et al.35 synthesised CuIm MOFs that are both in alignment with green chemistry principles and SSbD frameworks and explored its potential to remove multiple toxic heavy metals such as Pb(II), Cd(II), Mn(II), and Ni(II) from water. A key concern in SSbD is the material's behaviour during and after use. The CuIm MOFs demonstrates low leaching of Cu ions into water, minimising the potential for secondary pollution while retaining its structural stability up to 48 hours and to harsh conditions such as different pH levels and temperatures.
A research group,22 tailored Zr-MOFs by embedding magnetic nanoparticles (NPs) like Fe3O4 into their structure and showed effective adsorption of multiple toxic elements (Hg(II), Pb(II), and Cd(II)) in water in a single process. Functionalising the MOFs with –COOH and Zr–OH improved its removal efficiency for Hg(II), 97.01% while Fe3O4 and Zr-MOFs showed a removal efficiency of 39.35% and 69.71% respectively. After adsorption, the MOFs were regenerated using NaOH or HCl solutions to desorb the metals, enabling reuse. NaOH proved more effective, achieving 94–97% desorption efficiency over five cycles, while HCl had a lower efficiency of 63–71%. This regeneration process allowed the MOFs to maintain a high adsorption capacity, with only a slight drop from 49.2 mg g−1 to 44.7 mg g−1 over five cycles (Fig. 2B), highlighting their sustainability through reduced material waste and extended operational life, making them a cost-effective and environmentally friendly option for water purification. Through these deliberate choices of metal nodes and organic linkers, MOFs can be customised for use in challenging environments, ensuring long-term durability and making them more sustainable and safer for a wide range of applications.
From a SSbD perspective, the customisability of MOFs through targeted selection of metal nodes and organic linkers represents a critical strength for creating materials that meet stringent environmental and safety standards. By tailoring MOFs to exhibit specific properties like enhanced hydrolytic stability, increased hydrophobicity, or effective heavy metal adsorption, researchers can design materials that not only fulfil functional requirements but also minimise ecological and human health risks. Doping and PSM strategies have demonstrated the ability to significantly enhance the stability of MOFs in water and across variable pH conditions, thereby extending their lifespan and reducing degradation into potentially harmful byproducts. In addition, MOFs capacity to adsorb and sequester toxic metals aligns with green chemistry principles, providing a recyclable, low-leaching solution that mitigates secondary pollution risks. By designing MOFs with SSbD in mind, researchers can achieve multifunctional materials that address environmental challenges while prioritising safety, longevity, and environmental compatibility, paving the way for their broader, responsible deployment in industrial, medical, and environmental applications.
| NMOFs and MOFs composite | SSbD dimensions | Sustainable development goals | Recommendations | Ref. | |
|---|---|---|---|---|---|
| 1 | MIL-100(Fe) | Safety: used well controlled pyrolysis process ensuring no occupational hazard | Affordable clean energy, climate action | Further investigation on the use of renewable iron sources or bio-derived ligands in the MOFs synthesis to further align with sustainability goals and reduce the environmental footprint of catalyst production | 98 |
| Social: supports clean fuel production | |||||
| Environmental: green synthesis of MOFS at room temperature | |||||
| Economic: cost effective, durable catalyst with over 120 hours of stability | |||||
| 2 | BD-MOF(Ti)@CS/Fe3O4 | Safety: utilises non-toxic chitosan and titanium-based MOFs | Clean Water and Sanitation, Responsible Consumption and Production | Further investigation on potential applications in treating other heavy metals or contaminants in diverse water sources | 99 |
| Social: efficient solution for heavy metal removal from water contributing to pollution control in aquatic ecosystems | |||||
| Environmental: pH stability with maintaining adsorption capacity | |||||
| Economic: high adsorption capacity (944.9 mg g−1) and impressive reusability across multiple cycles make the material a cost-effective option for large-scale water treatment applications | |||||
| 3 | CrNiFe-MOFs | Safety: utilises waste materials (PET and stainless steel) and non-toxic reagents, minimising environmental and health risks during synthesis | Clean Water and Sanitation, Climate action, Life on land, Life below water | Acid leaching is a highly toxic residue-generating method. Management of residues generated during industrial scale MOFs generation can be difficult. In addition to phosphate removal, investigate the potential for large-scale implementation of this MOFs in various water treatment scenarios such as industrial wastewater containing complex system with organic and inorganic pollutants | 100 |
| Social: provides an effective solution for phosphate removal from water, addressing environmental issues related to eutrophication and enhancing community health | |||||
| Environmental: the MOFs exhibits high stability and efficient phosphate adsorption across various pH levels, contributing to improved water quality and ecosystem health | |||||
| Economic: low-cost synthesis process (using waste materials) and high space–time yield (5760 g m−3 day−1) make it a viable option for large-scale water treatment applications | |||||
| 4 | NHC–Co complex (Co2@MOFs) | Safety: avoids the use of toxic reagents and noble metals, creating a safer catalytic process for CO2 conversion | Climate action | Further explore scale-up potential and investigate applications for other carbon-containing products to maximize CO2 recycling benefits across industries | 101 |
| Social: supports efforts to reduce atmospheric CO2 levels, addressing public concern over carbon emissions and climate change | |||||
| Environmental: provides an efficient method for CO2 recycling, contributing to sustainable practices in carbon capture and utilisation | |||||
| Economic: utilizes a noble-metal-free catalytic system with high reusability and efficiency under ambient conditions, lowering operational costs for CO2 conversion processes | |||||
| 5 | Multiuse Al-MOFs | Safety: ensures in situ detection of toxic mercury levels (below WHO standards), enhancing safety in drinking water and cosmetic products; however, handling of DMF as solvent may pose toxicity risks during synthesis | Good Health and Well-being, Clean Water and Sanitation | Improve safety by exploring greener solvents in synthesis and consider scaling production to support broader application in cosmetic and environmental industries | 102 |
| Social impact: supporting consumer health by addressing mercury contamination concerns in drinking water and skin products | |||||
| Environmental: reduces mercury exposure risks in ecosystems if deployed for water monitoring, though careful disposal of DMF solvent and mercury-loaded sensors is necessary | |||||
| Economic: cost-effective approach using low amounts of sensor material (20 mg) and simple colorimetric changes visible to the naked eye, making it affordable for widespread usage | |||||
| 6 | PS@HKUST-1 | Safety: demonstrated non-genotoxicity in plant safety tests, supporting its application in crops without adverse genetic effects on plants or potential harm to the environment | Life on land, Zero hunger | Field studies are encouraged to validate efficacy and durability in larger agricultural settings | 103 |
| Social: helping agricultural industry | |||||
| Environmental: provides an eco-friendly alternative to agrichemicals | |||||
| Economic: one pot synthesis method | |||||
| 7 | Cu-MOFs loaded chitosan/gelatin film | Safety: strong antibacterial performance against both positive and negative bacterial strains, enhances food safety | Responsible Consumption and Production, Good health and well being | Only effectiveness against bacteria may not be the reason to use MOF-based nanoparticles, Gas adsorption or gas detection applications for these films can be explored | 104 |
| Social: real-time indication of food quality empowers consumers to verify food quality | |||||
| Enviromental: made with biodegradable chitosan and gelatin, the films are environmentally friendly and contribute to reducing plastic waste. However, DMF, used in the synthesis, is environmentally hazardous and should ideally be replaced with a greener alternative | |||||
| Economic: while effective, the use of copper and DMF could raise production costs, potentially limiting scalability | |||||
| 8 | ATP@UiO-66-NH2-CMC | Safety: composite synthesis is not aligned with green synthesis (use of DMF) but the proposed pesticide delivery system is useful for sustainable agriculture | Responsible Consumption and production, Life on land | Alternative eco-friendly solvents would be beneficial. Field tests on various crops could validate its effectiveness | 105 |
| Social: this system can reduce pesticide overuse, benefiting both farmers and consumers by lowering pesticide residues in food and providing a safer pest control method | |||||
| Environmental: with a high loading efficiency (90.79%) and controlled release mechanism, it minimizes pesticide runoff, reducing environmental contamination. However, DMF as a solvent is environmentally hazardous | |||||
| Economic: although effective, the synthesis process may incur higher costs due to materials like zirconium chloride and the use of DMF, which could impact economic scalability | |||||
| 9 | FeAl(BDC) MOFs ceramic filter | Safety: the use of FeAl(BDC) and sand mix in filters appears safe, and no toxic reagents are reported in the final water output. However, handling DMF during FeAl(BDC) synthesis requires caution, and appropriate disposal of sludge/spent materials is critical to avoid contamination | Clean water and sanitation, Responsible consumption and production | For enhanced affordability, efforts could focus on optimizing FeAl(BDC) synthesis with low-cost alternatives and reducing DMF dependency | 106 |
| Social: this system provides a feasible and localized solution for small-scale units with limited resources, directly benefiting communities by improving water quality | |||||
| Environmental: this approach minimizes pollution in aquatic systems by capturing dye pollutants at the source | |||||
| Economic Consideration: Gravity mode operation is more economical and reduces ongoing energy costs. However, the setup, including materials like FeAl(BDC), may still be costly for widespread adoption | |||||
| 10 | PET@UiO-66 | Safety: the hydrolysis process involves HNO3 and DMF, which are hazardous chemicals | Responsible Consumption and production, Life on land | Scale-up studies would be beneficial to assess economic feasibility and environmental safety at a larger scale, making the technology accessible for sustainable waste and water management | 107 |
| Social: this approach supports waste PET recycling and provides an effective way to remove toxic insecticides, which benefits public health by reducing contaminants in water sources. The reuse of PET promotes a circular economy, which aligns with sustainable development goals | |||||
| Environment: utilizing waste PET reduces plastic pollution and contributes to sustainable waste management | |||||
| Economic: while this method provides a cost-effective approach by using recycled PET, the process requires control over chemical use and disposal, which may add costs | |||||
| 11 | Cu-BTC MOF-based hybrid nanocomposites | Safety: the overall process aims to reduce waste generation and toxic by-products, promoting a safer approach to material synthesis | Clean water and sanitation, Responsible consumption and production, Life below water | Recyclability and renewability of these composites needs to be checked | 108 |
| Social: these nanocomposites support water purification efforts, especially in removing organic dye pollutants, thereby promoting safer, cleaner water sources for communities affected by industrial pollution | |||||
| Environment: the green synthesis method minimizes harmful byproducts, aligning with eco-friendly practices | |||||
| Economic: the synthesis involves accessible materials and relatively inexpensive procedures, but the requirement for certain chemicals and energy inputs during solvothermal synthesis may add to operational costs | |||||
| 12 | MOF-801 | Safety: instead of conventional solvothermal synthesis processes, use of ultrasonication at room temperature | Climate action, Responsible consumption and production | The reusability and stability of this MOFs for longer durations need to be checked. For cooling system applications stability with consistent performance is a major criterion | 109 |
| Social: energy efficient method for synthesis of MOFs, with high heat adsorption capacity, providing alternative solution to energy-demanding air-cooling system | |||||
| Environmental: room temperature green synthesis of MOFs with non-hazardous solvents such as water and formic acid | |||||
| Economic: potential solution for energy efficient method for adsorption-based air-cooling system | |||||
| 13 | Sn(II)-BDC MOFS | Safety: green synthesis method using water and NaOH as solvent | Climate action, clean water and sanitation, Life below water | Adsorption capacity still lags compared to other reported MOFs, post-synthetic modification or composite preparation can be done using this MOFs | 110 |
| Social: potential to tackle important issue of industrial wastewater consisting of organic dyes | |||||
| Environmental: the ability to adsorb multiple dyes at a time and non-hazardous synthesis method | |||||
| Economic: high adsorption capacity with simultaneous adsorption of multiple organic dyes can be best fit as a cost-effective method for an adsorption-based water purification system | |||||
| 14 | ZIF-8/CNC nanohybrids | Safety: sustainable process with sustainable materials (cotton waste) | Life on land, climate action, Responsible consumption and production | After adsorption, separation of this material from water is always a challenging task that needs a centrifugation or filtration system. To avoid this filtration system can be build using this composite material | 111 |
| Social: nanocomposite for water purification using waste material from the agricultural industry helps in maintaining a circular economy | |||||
| Environmental: simultaneous removal of multiple heavy metals and reusability with >90% adsorption efficiency up to 5 cycles | |||||
| Economics: reusability and simultaneous removal of multiple toxic ions with use of waste from agricultural industry helps to put this composite as a potential adsorbent industrial scale | |||||
| 15 | Cello-MOF filter | Safety: synthesis of MOFs involves toxic chemicals but the use of natural fib res (Saccharum officinarum bagasse-SBF) as template material for MOFs growth gives and safer solution with increased stability for water decontamination applications | Climate action, clean water and sanitation, Responsible consumption and production | Industrial scale production of this filtration system, with studies on MOFs stability considering transformation of MOFs in aquatic system | 112 |
| Social: fabricated device using natural fiber deposited with MOFs is an innovative idea to use potential of MOFs for human benefits | |||||
| Environment: high adsorption capacity (MB-602 mg g−1) with reusability, use of cellulosic biomass as template gives additional edge to this filtration system compared to other | |||||
| Economic: highly scalable and cost-effective solution in the field of wastewater purification system |
Zheng et al.44 showed versatility of MOFs by developing a green and scalable synthesis method for MOF-303, utilising water as the solvent, which has potential to be used as adsorbent material for water harvesting. This approach enabled the production of 3.5 kg of MOFs per batch with a 91% yield. The resultant MOFs maintained a crystalline and stable structure, with functionalities consistent with those prepared on a smaller scale, with very high efficacy for water harvesting applications. This study highlights that MOFs can be adapted for sustainable, large-scale production without compromising performance, thereby advancing SSbD-aligned innovations in environmental applications. In another study, Shaghaleh et al.45 demonstrated the incorporation of MIL-100(Fe) MOF into aminated cellulose nanofibres to create a nanocomposite material capable of removing Cr(VI) from agricultural soil. Despite the traditional methods used in the MOF preparation, the application of this MOF-based composite was aligned with the SDGs such as life on land. It effectively reduced toxic secondary pollution, was reusable, and enhanced soil quality by adsorbing 98% of Cr(VI). Furthermore, this approach alleviated physiological phytotoxicity symptoms in cultivated wheat plants and promoted their growth, contributing to food safety and sustainable agricultural practices. This study highlights the potential of MOF-based nanocomposites in agriculture to improve soil quality, enhance plant health, and ensure sustainable food production while also addressing environmental contaminants effectively.
Li et al.46 present a dynamically mediated synthesis strategy for simultaneously engineering the crystal, defect, and nanostructures of MOFs, demonstrating the versatility of MOFs in catalytic reactions. Through a one-pot synthesis, the researchers created various Zr-ODB-hz (ODB = 4,4′-oxalyldibenzoate, hz = hydrazine) structures with tailored properties, from amorphous NPs to crystalline and defective nanosheets, each optimised for specific catalytic functions. The self-reduction capability of hydrazine moieties allowed for in situ Pd NPs integration, yielding structure-specific selectivity in catalytic reactions. This innovative approach underscores MOFs potential by SSbD and its versatility in structural modifications to meet diverse requirements in an environmentally concise way. Liu et al.43 reported a novel and sustainable approach using chitin-based MOF-919 composite microspheres (Fig. 3A), with MOFs crystals grown in situ, for effective sepsis treatment. While the composite synthesis may not fully align with green chemistry standards, these microspheres emphasise safety and sustainability, offering reusability and high endotoxin adsorption capacity. A key feature of this method is the recovery of MOFs; by dissolving used microspheres in NaOH/urea, chitin and MOF can be easily separated, underscoring the material's renewability. In Fig. 3B, SEM images confirm that the MOFs crystals maintain their original morphology, showcasing robust acid and base stability. FTIR (Fig. 3C) and BET (Fig. 3D) analyses reveal that the recycled chitin/MOFs microspheres retain the characteristic absorption peaks of MOF-919(Sc) and exhibit a high specific surface area (1309.2 m2 g−1), approximately 93% of the original, demonstrating successful structural preservation and reusability. Such well-designed materials prioritise safety, reusability, and renewability, enhancing their potential for industrial scalability. Such newly developed MOFs, synthesised through green, safe, and sustainable methods, along with their SSbD-compliant applications, hold significant promise for industrial-scale deployment and commercialisation without causing environmental harm. The adaptability of MOFs through SSbD applications underscores their potential to meet urgent environmental and industrial needs. By focusing on eco-friendly synthesis techniques and scalable performance improvements, MOFs are well-positioned to play a pivotal role in the next generation of sustainable solutions across multiple sectors, including environmental (water treatment, pollutant capture, energy storage, and catalysis) and biomedical (drug delivery, imaging etc.).
 | ||
| Fig. 3 Strength of MOFs (A) the recycling process for biomass-derived LPS adsorbent material and the environmental limitations of conventional adsorbent substrates sourced from fossil-based materials (B) scanning electron microscopy (SEM), (C) Fourier transform infrared (FTIR) spectroscopy, and surface area measurement using (D) Brunauer–Emmett–Teller (BET) theory confirm that the recycled chitin/MOFs microspheres retain the original morphology of the MOFs crystals, demonstrating robust acid and base stability along with successful structural preservation and reusability.44 | ||
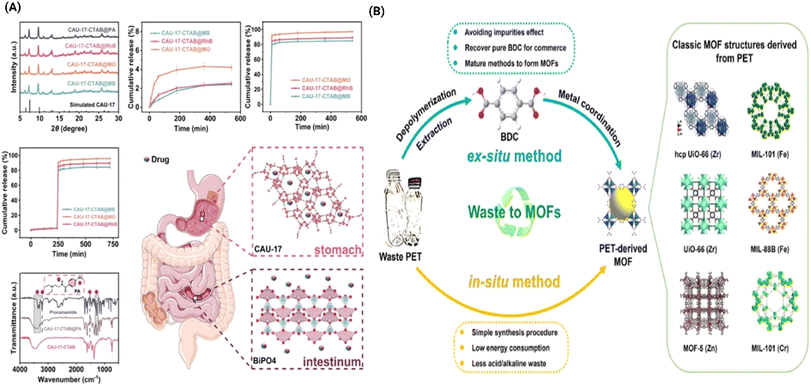 | ||
| Fig. 4 (A) An advancement in green synthesis is demonstrated by utilising PET waste to create PET-derived MOFs, offering an eco-friendly and economically viable alternative that mitigates plastic pollution while enabling scalable and cost-effective production of high-performance materials47 (B) CAU-17-CTAB, synthesised using green chemistry was used as a carrier in targeted drug delivery.48 | ||
Furthermore, researchers are focused on synthesising green MOFs by using renewable and biodegradable materials, such as lignin and cellulose from waste resources, enabling the upcycling of discarded materials into valuable, functional products and prioritising sustainability at every stage.49 Moreover, deep eutectic solvents (DES) are being used as eco-friendly solvents, in contrast to traditional DMF and dimethyl sulfoxide (DMSO) enhancing the green chemistry approach to MOFs production.50 By leveraging these sustainable materials and methods, scientists are advancing the development of MOFs that align with environmental objectives. Ball milling is another green approach to chemically intensive synthesis which often involve toxic reagents and produce hazardous waste. A study by Zhang et al.51 highlights the ball milling technique as a green, environmentally friendly approach to synthesise a graphene@Cu-MOFs hybrid. Ball milling mechanically peels graphite layers without the need for harsh chemicals or waste-producing processes. This method reduces waste, cost, and environmental impact by leveraging mechanical forces and π–π interactions between ligands and graphite, enhancing the efficiency of graphene layer separation. Further, the paper mentions other green methods, such as the use of ligands containing nitrogen-rich flame-retardant elements, which facilitate safer thermal decomposition. Further, the use of ligands containing nitrogen-rich flame-retardant elements facilitates safer thermal decomposition. Therefore, the advancements in green synthesis methods, such as water-based solvents, PET upcycling, and ball milling, are transforming MOFs into more sustainable materials. These eco-friendly approaches reduce environmental impact and increase scalability, enabling MOFs to support sustainable manufacturing in applications ranging from pollution control to advanced materials. As the field progresses, MOFs are set to play a vital role in promoting sustainable industrial practices and environmental responsibility.
2.2. Weaknesses
Many MOFs exhibit significant vulnerability when exposed to aqueous environments or reactive adsorbates, with stability often depending on the specific metal ions and bonding characteristics of the framework. MOFs containing ions such as Zn2+, Cu2+, and Co2+, for instance, tend to degrade when in contact with water or other reactive environments. In contrast, frameworks containing more stable metal ions, including Zr4+, Cr3+, and Ti4+, exhibit improved hydrolytic stability due to stronger coordination bonds. Notable examples include UiO-66, MIL-125, and MIL-101(Cr), which have demonstrated enhanced water stability and are therefore more suitable for specific applications. However, while hydrolytically stable MOFs offer resilience, they often lack the adsorption capacities required for applications like water purification, gas adsorption, and dye removal. For instance, stable frameworks like UiO-66 generally underperform in adsorption capacity compared to their less stable counterparts, such as ZIF-8 and Cu-BTC, due to fewer available active sites and reduced pore volume57–60
To bridge this gap between stability and functionality, researchers have pursued various strategies. Some of the most notable approaches include: (a) post-synthetic functionalisation of MOFs with hydrophobic or reactive groups to shield metal–ligand bonds from aqueous degradation;34 (b) development of MOF-on-MOF (core–shell) hybrid structures to enhance stability while potentially preserving or improving functionality; (c) creation of diverse MOFs composites, such as polymer-MOFs, graphitic, and nanomaterial composites, to introduce synergistic properties;13 (d) doping with stabilising agents;61 and (e) optimisation of synthesis parameters, such as changing linkers, solvents, or reaction conditions to target specific performance traits. For example, amine-functionalised UiO-66 exhibits a lead (Pb2+) adsorption capacity of 92.18 mg g−1, whereas Cu-BTC a less stable MOF achieves an adsorption capacity of 662.2 mg g−1.31,62 Goyal et al.31 further demonstrated that replacing the linker in Cu-BTC with 2-methyl imidazolate extended water stability from 2 hours to 48 hours, though Pb(II) adsorption capacity fell by 25.7% as a trade-off.
Studies also highlight that not all modifications yield improved functionality. As shown in Fig. 5A, Zhang Mengmeng and colleagues introduced a composite structure, U6N@ZIF-8, to enhance REE adsorption, achieving a Nd3+ adsorption capacity of 249.9 mg g−1. Although effective, this value is substantially lower than the 517 mg g−1 Nd3+ capacity achieved by MXene, revealing that the MOF-on-MOF strategy did not substantially improve adsorption capacity due to reductions in surface area and porosity upon modification Fig. 5C, D. Whereas Fig. 5B shows the variation in adsorption efficiency with respect to change in pH of the aqueous solution. It can be seen that, adsorption efficiency was affected by the variation in pH of the solution. This results also highlights that even after preparing such complex composite getting similar performance at broad pH range is difficult. In addition, as shown in Fig. 5E and F the adsorption efficiency of this MOF-on-MOF structure was dropped below 80% within first 5 cycles for both UiO-66@ZIF-8 and ZIF-8@UiO-66.55,63 Such findings suggest that while certain structural modifications can increase stability, they may also compromise the adsorptive performance critical for real-world applications, with increase in complexity in preparation of such multilayer composites.
 | ||
| Fig. 5 Weakness associated with SSbD of MOFs. (A) Schematic representation of the preparation of MOF-on-MOF composites, UiO-66@ZIF-8 and ZIF-8@UiO-66 and its application in REE adsorption. (B) Stability of UiO-66@ZIF-8-x and ZIF-8@UiO-66 under varying pH conditions, which indicates limitations in maintaining structural integrity across a broad pH range. (C) Nitrogen adsorption–desorption isotherms for UiO-66-NH2, ZIF-8, U6N@ZIF-8-x, and ZIF-8@U6N, suggesting constraints in surface area and pore characteristics that may affect adsorption performance. (D) Pore width distribution for UiO-66-NH2, ZIF-8, U6N@ZIF-8-x, and ZIF-8@U6N, which reveals limited porosity adjustments across composites. (E and F) Adsorption and reusability performance of U6N@ZIF-8–20 and ZIF-8@U6N toward Nd(III) ions over five cycles, showing reduced efficiency in adsorption capacity over repeated use, potentially impacting long-term viability in REEs recovery18,55 (G and H) impact of temperature variation on mechanical properties of HKUST-1 MOF, showing how MOFs can behave under different dynamic conditions.56 | ||
Despite notable advances in MOFs and MOFs composite, challenges remain. The CALF-20 MOF, for instance, demonstrates robust hydrolytic stability due to the use of a 1,2,4-triazole linker, yet it falls short in adsorption, with a CO2 capacity of 2.61 mmol g−1 compared to Cu-BTCs 3.55 mmol g−1.64 This highlights the often-inverse relationship between stability and adsorption efficiency in MOFs structures. Strong coordination between the triazole linker and metal nodes enhances stability, but the resulting structural rigidity can restrict access to pores, limiting adsorption potential.65,66 Despite extensive research into linker modifications, MOFs doping, and composite synthesis with polymers or nanoparticles, the combined constraints of reduced adsorption capacity, limited reusability, and compromised structural resilience under varied environmental conditions still impede the widespread industrial application of MOFs.
For environmental remediation, MOFs appeal is tempered by limitations such as pH-dependent adsorption, reduced reusability, and poor long-term stability. Many MOFs face issues like metal ion leaching, structural degradation, and pore clogging, which restrict consistent performance across multiple use cycles.67 This variability hinders their applicability in large-scale water or air purification efforts. Researchers have attempted to address these issues by creating MOF-based monoliths, membranes, and composites tailored for industrial use, yet converting powdered MOFs materials into hierarchically porous structures remains challenging. Key limitations include the complexity of reaction conditions, low catalyst loading, and poor accessibility to active sites, which significantly reduce the efficiency of MOFs in practical settings.68 As shown in Fig. 5G and H, Wang et al.56 examined the mechanical stability of HKUST-1 MOFs under elevated temperatures, finding significant thermal susceptibility. Their results revealed that at 100 °C, HKUST-1 displayed a 51% reduction in Young's modulus and a 53% decrease in yield strength, with behaviour shifting from elastic-plastic to hyperelastic-plastic. Given that many MOFs are employed in environments where temperature fluctuations and mechanical stresses are common, it is crucial to thoroughly study how these factors affect their mechanical properties. Investigating such behaviour across different MOFs is essential to ensure their stability and performance in diverse practical applications, such as catalysis, gas separation, and sensing, where temperature and stress variations are inevitable.
Some other challenges associated with MOFs are highlighted by recent articles, where Nikseresht et al.69 synthesised a Fe-MOFs composite using phosphotungstic acid, which served as a reusable catalyst for oleic acid esterification. Although initially efficient, catalytic activity dropped by 30% after seven cycles, reflecting typical issues with reusability. Zeyadi et al.70 immobilised horseradish peroxidase (HRP) on an amino-functionalised Zr-MOFs for phenol removal yet observed a 40% decline in removal efficiency over ten cycles, further underscoring the difficulties of maintaining performance under repetitive use. In another study, Modak et al.71 reported Cr-MOFs with an exceptionally high adsorption capacity of 800 mg g−1 for polystyrene microplastics. However, the adsorption efficiency was highly pH-sensitive, indicating that environmental variables critically impact MOFs functionality.
While MOFs and MOFs composites hold promise as adsorbents, their scalability and long-term application are hindered by current limitations. Optimising MOFs stability often results in reduced adsorptive capacities or functional deficits, while addressing performance issues like reusability and pH sensitivity remains challenging as discussed above. This balance between stability and functionality is a critical area of research, with significant implications for the future development of MOFs for real-world environmental and industrial applications. Overcoming these barriers requires a nuanced approach to synthesis, modification, and material design, ensuring that MOFs desirable traits are enhanced without sacrificing their operational efficiency across varied environmental conditions. To realise the full potential of MOFs in environmental and industrial applications, an SSbD approach is essential for balancing stability, functionality, and sustainability. Addressing these limitations with a structured, safety-oriented design approach will enable the development of MOFs that can deliver long-term performance and environmental compatibility, ensuring their viability in the face of real-world challenges.
More recently, Far et al.54 have shown that strong acids used in the productions of MOFs can have negative environmental effects and emits toxic byproducts if not well controlled. In support of this findings Sultana et al. have also shown that the metal ions presence in MOFs and functional groups in the organic ligands might also lead to toxicological impacts. Additionally, in a recent paper by Chakraborty et al.75 have also revealed that exposure to nano MOFs could potentially alter conformational structure of the enzymes, resulting in changes in the percentage composition of alpha helices and beta sheets. However, these studies are not comprehensive and requires further detailed toxicological studies to identify the mechanisms of toxicity. Having these crucial toxicity data will support in adoption of SSbD approach into material synthesis and experimental design.72,76
The current body of toxicological data on MOFs is sparse, leaving a critical need for extensive toxicity testing and transparent data to ensure safety and environmental compatibility.77 The primary components in MOFs synthesis—metal ions, organic linkers, and solvents—strongly impact toxicity profiles. Optimising these elements is crucial to reducing harmful health and environmental effects, yet effective combinations that minimise toxicity remain underexplored. Although extensive research has focused on MOFs’ metal ions and organic linkers, the role of solvents in toxicity is often overlooked. Solvents such as ethanol, DMSO, and DMF are commonly used in MOFs synthesis, and residual solvent molecules can remain trapped in MOFs pores, posing potential short- or long-term health and environmental hazards. Such solvents may be gradually released during MOFs application, leading to toxic exposure, particularly in biomedical contexts where MOFs could directly contact tissues or organs.78 Addressing solvent-related toxicity is thus essential to advancing the design of MOFs for safe applications, particularly in biomedical and environmental remediation fields. Another critical concern with MOFs is their potential to accumulate in the environment, leading to the release of ROS and, consequently, oxidative stress, inflammation, cytotoxicity, harmful metal ions, apoptosis, and necrosis. This oxidative stress and metal ion release can detrimentally affect various organisms, leading to cellular damage, genetic mutations, and impaired growth in organisms ranging from microbial life to human cells and plants79. Such effects highlight the urgent need for more detailed, standardised toxicity testing to establish guidelines for MOFs exposure limits, focusing on evaluating their cumulative environmental and health impacts.
A further limitation of current toxicity assessments is their focus on the pristine, unaltered forms of MOFs, which often fail to represent the complex transformations these materials undergo in real-world applications.80 MOFs are particularly susceptible to transformations in response to environmental factors like humidity, temperature, and pH, especially given their porous structure, active sites, and large surface area (discussed in detail in section 2.4.3). These characteristics lead to rapid hydration, metal ion leaching, and unpredictable stability in various fluids, contributing to the toxicological complexity of MOFs.81 However, most toxicity studies neglect these transformation-induced alterations, overlooking the broader environmental impact of MOFs degradation products and the potential increase in toxicity over time. Understanding MOFs behaviour under different environmental conditions is fundamental to mitigating potential health and ecological risks. In many cases, the effects of MOFs transformation on toxicity remain underexplored, limiting the ability to accurately assess the safety of MOFs in applications where they may degrade or interact dynamically with other materials. As these transformations can lead to increased metal ion release and potential interactions with biological tissues, further research is essential to characterise the long-term effects and safety of MOFs in various practical contexts.81 Currently, there is limited data on the comprehensive environmental footprints of MOFs, hindering a full assessment of their long-term sustainability. Most LCA on MOFs focus on synthesis costs, energy consumption, or specific environmental aspects (like solvent use or emissions) without a holistic cradle-to-grave analysis. Given the broad variety of MOFs structures and synthesis methods, this gap limits understanding of their complete environmental impact, especially in large-scale or industrial applications. Addressing this gap would require standardised LCA methodologies for assessing diverse MOFs across all life cycle stages. The increasing application of MOFs is tempered by significant gaps in ecotoxicity data, particularly with respect to the formation of protein and eco-coronas. These coronas critically influence MOFs’ environmental behaviour and biological interactions. The insufficient data on the transformation processes of MOFs, such as homoaggregation, heteroaggregation, dissolution, and degradation, constrains our understanding of their likelihood to release potentially harmful metal ions (e.g., Zn2+) and ROS. Such emissions have the potential to induce oxidative stress, DNA damage, mitochondrial dysfunction, and other deleterious effects in organisms, which may disrupt ecological processes and bioaccumulate. These unknowns represent a significant weakness within the SSbD framework for MOFs, posing challenges to obtaining regulatory approval and limiting broader adoption. However, addressing these data gaps offers a critical opportunity to refine the design and application of MOFs to ensure they are both effective and environmentally safe, thereby enhancing their market viability and regulatory acceptance.
LCA of MOFs is a critical aspect of SSbD approaches. However, despite the existence of over 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 reported MOFs, LCA studies on these materials are limited, leaving gaps in understanding their full sustainability profiles. For example, Grande et al.82 pioneered an LCA for the CPO-27-Ni MOFs, identifying solvent recycling and elimination as key strategies to reduce environmental impact. While this work lays an important foundation, more comprehensive and proactive approaches to LCA are needed to fully assess MOFs’ environmental impacts, particularly as their range of applications expands. Escobar-Hernandez et al.74 further explored the economic feasibility of MOFs production but provided limited insight into the environmental impacts across the synthesis life cycle, including emissions and waste production. Similarly, Ntouros et al.83 conducted an LCA of various ZIF-8 MOFs synthesis routes, exploring different methods but lacking detailed data on emissions, energy consumption, and byproducts. Without this data, it is difficult to assess the environmental sustainability of each method fully, especially given the extensive diversity in synthesis protocols used across MOFs.
000 reported MOFs, LCA studies on these materials are limited, leaving gaps in understanding their full sustainability profiles. For example, Grande et al.82 pioneered an LCA for the CPO-27-Ni MOFs, identifying solvent recycling and elimination as key strategies to reduce environmental impact. While this work lays an important foundation, more comprehensive and proactive approaches to LCA are needed to fully assess MOFs’ environmental impacts, particularly as their range of applications expands. Escobar-Hernandez et al.74 further explored the economic feasibility of MOFs production but provided limited insight into the environmental impacts across the synthesis life cycle, including emissions and waste production. Similarly, Ntouros et al.83 conducted an LCA of various ZIF-8 MOFs synthesis routes, exploring different methods but lacking detailed data on emissions, energy consumption, and byproducts. Without this data, it is difficult to assess the environmental sustainability of each method fully, especially given the extensive diversity in synthesis protocols used across MOFs.
Maklavany et al.84 conducted an LCA for MIL-53 MOFs, yet did not explore the long-term impacts of disposal or recycling options for MIL-53 (Fe). This represents a broader challenge in the field—while there are significant initial insights, gaps remain regarding the full life cycle and end-of-life environmental impact. Addressing these gaps is essential for effective SSbD in MOFs development, particularly as researchers continue to design new MOFs with unique properties and applications. While researchers are increasingly addressing these challenges, a more structured, proactive approach to LCA is essential to progress SSbD framework. Developing a comprehensive LCA framework specific to MOFs would promote consistent, detailed environmental impact assessments across different types and synthesis methods. This would not only enhance sustainability but also make it easier to integrate eco-design considerations in the early development phase. The rapid expansion of the MOFs field, with over 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 unique structures and new ones emerging continuously, presents practical challenges to conducting detailed LCAs for every MOFs variant. Given the volume and diversity of MOFs, performing comprehensive LCAs on each would be complex. A practical solution could involve focusing on representative LCAs for key categories of MOFs or typical synthetic routes, alongside standardised sustainability metrics. This would streamline the assessment process while still providing useful insights to guide safer, more sustainable MOFs development. Therefore, initial LCA efforts are commendable, advancing SSbD in the field of MOFs will require a more extensive, proactive approach to LCA. Standardising LCA criteria and prioritising assessments for key MOFs categories could pave the way for more sustainable, scalable, and environmentally friendly MOFs production processes, meeting the growing demands of diverse applications while minimising ecological impact.
000 unique structures and new ones emerging continuously, presents practical challenges to conducting detailed LCAs for every MOFs variant. Given the volume and diversity of MOFs, performing comprehensive LCAs on each would be complex. A practical solution could involve focusing on representative LCAs for key categories of MOFs or typical synthetic routes, alongside standardised sustainability metrics. This would streamline the assessment process while still providing useful insights to guide safer, more sustainable MOFs development. Therefore, initial LCA efforts are commendable, advancing SSbD in the field of MOFs will require a more extensive, proactive approach to LCA. Standardising LCA criteria and prioritising assessments for key MOFs categories could pave the way for more sustainable, scalable, and environmentally friendly MOFs production processes, meeting the growing demands of diverse applications while minimising ecological impact.
The cost factor remains a major obstacle for scaling up MOFs production, with the high expense of organic linkers presenting a key challenge. In a seminal paper, Paul et al.88 discuss these economic barriers, noting the stark price difference between organic linkers and inorganic precursors, with linkers often costing up to 100 times more. Additionally, they highlight incomplete optimisation of synthesis routes, which hinders consistent quality and drives up production costs, making large-scale manufacturing difficult. Severino et al.89 reinforce these concerns in their comprehensive assessment of MOFs industrialisation, identifying the high cost of organic linkers and the energy-intensive nature of synthesis as primary economic bottlenecks. Their review suggests that using alternative, low-cost linker sources, improving energy efficiency, and optimising methods like flow chemistry and mechanochemistry could make MOFs production more viable. Nonetheless, these high costs and resource demands create a weak link in the scalability chain, making MOFs less practical for industries focused on cost-effective and sustainable materials. Similarly, in “Toward Scalable and Sustainable Synthesis of Metal–Organic Frameworks”, Xie et al.90 explore greener, scalable production pathways, such as solvent-free mechanochemistry and recycling-based strategies. While these methods offer potential sustainability benefits, significant limitations remain in achieving consistent structural quality and cost-efficiency on an industrial scale. The high costs, energy requirements, and production challenges associated with scaling up MOFs highlight the pressing need for innovation within the SSbD framework. These hurdles present new opportunities for research into alternative, affordable linker sources, streamlined synthesis routes, and energy-efficient methods. By addressing these weaknesses, researchers have the chance to reshape the field towards more sustainable, scalable MOFs production, setting new standards in industrial sustainability and enabling broader MOFs applications across diverse sectors.
In a related study,92 the green synthesis of a core–shell magnetic MOFs composite (Fe3O4@SiO2@HKUST-1) demonstrated significant advancements in dye adsorption applications. This composite exhibited a 130% increase in adsorption capacity compared to pristine HKUST-1, showcasing the ability of green synthesis to enhance functionality without sacrificing performance. These studies highlight the potential of eco-friendly synthesis methods in MOFs design, demonstrating that sustainable approaches can preserve or even enhance the functional properties essential for applications like environmental remediation. However, the increased adsorption capacity seen in composites like Fe3O4@SiO2@HKUST-1 also presents challenges. The complexity of this multi-layered composite may lead to economic and structural drawbacks, particularly with potential instability after repeated use. Moreover, maintaining consistent performance without compromising the composite's eco-friendly qualities poses a challenge. It is crucial to rigorously evaluate the safety of these green-synthesised materials alongside their enhanced functionality to ensure they effectively balance performance with safety in environmental applications.
Most of the research articles highlighted the improved adsorption capacities for different dyes, toxic metal ions, and pharmaceutical wastes using MOFs designed with SSbD framework, but the concentration of metal ions released from the MOFs are rarely reported.93,94 Li et al.95 reported that introducing defects in UiO-66 MOFs can enhance its adsorption capacity for tetracycline. However, with the increased adsorption capacity resulting from these defects, it is essential to investigate the release of metal ions from the MOFs into the aqueous solution during the adsorption process. Considering the framework of SSbD, this is a critical parameter to assess for the industrial application of such MOFs. In these methods, while the functionality and properties of MOFs are enhanced for applications such as selectivity and reusability, there can be trade-offs in other structural features. For instance, when synthesising MOFs using green synthesis, non-toxic organic ligands replace conventional ones, resulting in altered bonding between the metal centre and the ligand, which affects the number of active sites on the MOFs. Abhari et al.96 reported using a green activated carbon-HKUST-1 composite for lead removal from wastewater, achieving an adsorption capacity of 249.4 mg g−1. This capacity is four times lower than that of pristine HKUST-1 synthesised through a traditional method, for which Wang et al.97 reported an adsorption capacity of 819.2 mg g−1. Additionally, the need for post-modification synthesis and composite preparations introduces changes that are essential for achieving the desired properties in green and sustainable MOFs production, which may not be required in traditional MOFs. Therefore, striking a balance between performance and safety represents a genuine challenge.
Self-explanatory Table 2 highlights recent research findings on MOFs and MOFs composites, emphasising their alignment with SSbD dimensions and relevant SDGs. It highlights how MOFs are engineered to support sustainability targets across various applications, presenting a concise overview of their benefits, challenges, and recommendations for future advancements.
2.3. Opportunities
In another study,124 researchers developed a scalable method for synthesising a bio-based Cu-MOFs, providing a cost-effective and sustainable approach to bone defect repair. This novel Cu-based MOFs addresses toxicity concerns by gradually releasing Cu2+ ions, which ensures biocompatibility while promoting osteogenesis and angiogenesis, addressing SDGs related to good health and well-being and innovation in sustainable materials. Mechanistic studies indicate that the effectiveness of this MOFs stems from its ability to activate the transforming growth factor-β/bone morphogenetic protein signalling pathway, highlighting its potential and opportunity for MOFs and MOFs based materials as a therapeutic tool in regenerative medicine.125 Babaei et al.122 reported safe and sustainable Fe-based Bio-MOFs prepared using curcumin and green chemistry method, which does not involve use of toxic chemicals and strong acids. This BioMOF used as a system for loading of doxorubicin (DOX), coated with polyethylene glycol (PEG), and targeted with an EpCAM aptamer for Colorectal Cancer cells (CRC) specificity. This BioMOF demonstrated (Fig. 6A) effective tumour regression, low systemic toxicity, and MRI imaging capabilities, highlighting its potential as a sustainable, multifunctional system for CRC treatment and monitoring. In another study,126 porphyrin used as linker to prepare Zr-bio-MOF (using green, rapid and facile synthesis method), which is photosensitive and releases oxygen reactive species when exposed to visible light. Considering this, study prepared porphyrin based Zr-MOFs gold nanoparticle composite for anticancer treatment of colorectal cancer cells assay. By integrating targeted drug delivery, low systemic toxicity, and imaging or photodynamic capabilities, these MOFs align with SDGs aimed at promoting good health and well-being and supporting innovation in sustainable medical technologies. This advancement underscores the potential of BioMOFs to deliver more sustainable, effective, and targeted cancer treatments. However, in case of MOFs and MOFs based scaffold for applications in biomedical industry, challenges such as stability of MOFs, controlled drug release, compatibility with various tissue types, and degradation rate optimisation remain. By integrating drug-loaded MOFs with biocompatible scaffolds, there is a promising pathway for targeted therapies, such as localized pain relief following surgeries or injury, that provide sustained release while minimising systemic side effects. The SSbD alignment of MOFs further enhances their suitability for clinical applications, as it could streamline regulatory approval processes and pave the way for clinical trials. The stability and low toxicity of these MOFs meet regulatory expectations, reducing the time to market for potential treatments. Overcoming current limitations, such as fine-tuning the degradation profile and ensuring uniform drug distribution, could therefore offer substantial commercial opportunities and accelerate their acceptance in medical fields.
 | ||
| Fig. 6 Opportunities associated with MOFs. (A) Targeted in vivo efficacy of Apt-PEG-MOF@DOX MOFs demonstrating minimal toxicity and antitumor efficacy indicating advancement in biocompatible MOFs.122 (B) ML unlocks innovation in MOFs research by quickly screening the MOFs database with high accuracy, enabling precise design, optimising synthesis, and accelerating the discovery of functional materials for various applications. (C) Applying circular economy principles to MOFs offers an opportunity to enhance sustainability by reducing toxic chemical use, reusing solvents, and recycling spent MOFs, thus conserving energy and resources throughout the MOFs lifecycle. (D) The use of PIPS in 3D printing to control MOFs dispersion within resin enables the creation of catalytically accessible, high-resolution printed structures, expanding possibilities for functional materials in applications like catalysis and protective devices.123 (Prepared using Biorender Software). | ||
In one of the recent work, modified version of MOFs was used for X-ray imaging by embedding perovskite material in a Bio-MOF-100, this study introduces a safe and sustainable design for perovskite-MOF nanocomposite used in X-ray imaging. Researchers developed a stable and efficient scintillator that maintains its performance under challenging conditions like heat, air, and UV exposure.127 This approach enhances the brightness and stability of the material, allowing for clear and precise X-ray imaging with excellent resolution. This work marks a significant step toward sustainable, high-performance materials for solid-state imaging and other optoelectronic applications. These advancements highlight the transformative potential of MOFs and MOF composite designed with a SSbD approach, especially in biomedical applications where biocompatibility, degradability, and safety are critical. By integrating green chemistry principles and utilising non-toxic metals and bio-based linkers, such MOFs not only provide effective therapeutic solutions but also address environmental and safety concerns. The progress in designing biocompatible, degradable MOFs like Bio-MOFs with Cu and other non-toxic metals offers a promising pathway toward sustainable, high-performance materials that can revolutionize healthcare applications, from drug delivery to regenerative medicine.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 205 MOFs based on chemical and structural properties, the researchers identified 50 high-performing MOFs, further refining the selection to 26 that are stable in water. RF regression was chosen for its accuracy and stability, effectively predicting MOF adsorption capacities and making high throughput screening feasible. The study highlights how features like larger cavities and carboxyl groups enhance Pb(II) ions adsorption, providing valuable design insights for water-purifying MOFs and demonstrating the efficiency of ML over traditional methods. As discussed earlier, recent advancements have introduced the use of MOFs in biomedical applications119 marking an emerging frontier for biocompatible and degradable MOFs. Menon et al.134 explained the role of ML in designing safe and biocompatible MOFs for drug delivery by predicting the cytotoxicity of metal centres and organic linkers, categorising them as “safe”, “toxic”, or “fatal”. By leveraging toxicity data (e.g., LD50 values) and chemical properties, the ML framework identifies structural features linked to biocompatibility, like porosity and stability, which help select safe(r) MOF structures. This ML-driven screening aligns with SSbD framework by predicting MOFs properties for tailored drug delivery, minimising experimental testing and resource use while lowering environmental risks, ultimately reducing time-to-market for biomedical innovations.
205 MOFs based on chemical and structural properties, the researchers identified 50 high-performing MOFs, further refining the selection to 26 that are stable in water. RF regression was chosen for its accuracy and stability, effectively predicting MOF adsorption capacities and making high throughput screening feasible. The study highlights how features like larger cavities and carboxyl groups enhance Pb(II) ions adsorption, providing valuable design insights for water-purifying MOFs and demonstrating the efficiency of ML over traditional methods. As discussed earlier, recent advancements have introduced the use of MOFs in biomedical applications119 marking an emerging frontier for biocompatible and degradable MOFs. Menon et al.134 explained the role of ML in designing safe and biocompatible MOFs for drug delivery by predicting the cytotoxicity of metal centres and organic linkers, categorising them as “safe”, “toxic”, or “fatal”. By leveraging toxicity data (e.g., LD50 values) and chemical properties, the ML framework identifies structural features linked to biocompatibility, like porosity and stability, which help select safe(r) MOF structures. This ML-driven screening aligns with SSbD framework by predicting MOFs properties for tailored drug delivery, minimising experimental testing and resource use while lowering environmental risks, ultimately reducing time-to-market for biomedical innovations.Further, MOFs offer an excellent balance between thermal stability and storage efficiency, making them ideal for applications requiring safe, reliable hydrogen storage under varying temperature and pressure conditions. A study performed by Shekhar et al.135 ML plays a transformative role in accelerating hydrogen storage research, allowing for rapid screening and analysis of 4000 MOFs to identify structures with high hydrogen affinity. In particular, topological data analysis (TDA) combined with ML models, such as the ResNet-18 model used in this study, enables scientists to assess and predict the performance of MOFs more efficiently than ever before. By incorporating both topological descriptors and conventional structural features, this approach enhances prediction accuracy, minimising the need for costly and time-intensive simulations. This ML-driven method opens new avenues for the discovery of MOFs with optimised storage capacities and contributes to a growing database of sustainable, high-performance hydrogen storage materials. MOFs hold immense potential in sustainable agriculture, particularly in enhancing fertiliser delivery, increasing crop resilience, and remediating pollutants.136 However, there is a notable gap in the integration of ML with MOF applications in this field. ML can significantly enhance the predictability and efficiency of MOF-based interventions by optimising synthesis parameters, predicting adsorption capacities, and evaluating interactions with soil and crop systems. For instance, ML models could predict the best MOF formulations for micronutrient delivery under varying soil pH and moisture levels, optimising the use of MOFs for different crop types and climates. ML integration in MOFs research enhances predictive capabilities, reducing experimental dependency, advancing sustainability while allowing rapid advancements in applications from environmental remediation to biomedical and hydrogen storage. Going forward, the expansion of ML applications within MOF research, particularly in underexplored areas like agriculture, will be crucial for unlocking the full potential of MOFs as sustainable solutions across industry and environmental sectors.
Reducing toxic solvents and materials further advances the sustainability of MOFs synthesis. Dang et al.138 emphasises the “Reduce” principle of the circular economy by employing a reusable Pd@MOF (palladium-based MOF) catalyst and DES in C–C coupling reactions. This reusable catalytic system minimises the need for traditional solvents and non-recyclable catalysts, thus reducing waste. Additionally, the reusability of both the Pd@MOF catalyst and DES decreases the input of fresh resources for each reaction cycle, aligning with circular economy principles aimed at conserving materials. The DES not only functions as a solvent but also as a catalyst, further reducing the need for auxiliary chemicals and contributing to an eco-friendlier process. Solvent-free synthesis of MOFs presents another alternative; however, it often demands substantial energy input.139 Microwave-assisted synthesis offers a green alternative for producing MOFs by minimising solvent use and enhancing energy efficiency through direct coupling with reacting molecules, drastically reducing power consumption. Unlike conventional methods, it can complete reactions in minutes rather than days, saving time and reducing waste. This approach also enhances reaction rates, selectivity, and yields, making it an ideal choice for sustainable chemistry.140
Reusing resources in MOFs synthesis not only reduces the demand for fresh materials but also minimises waste, aligning with sustainable practices. As discussed in further sections, retaining 88% of its adsorption capacity after five cycles, the GO-ZIF-67 composite141 exemplifies this principle. Its high reusability means that less material is required over time for pollutant removal, minimising environmental impact and material waste. This efficiency is particularly valuable in large-scale applications, where the ability to reuse adsorbent materials decreases operational costs, conserves resources, and limits the ecological footprint associated with constant replacement. Integrating such materials into pollutant remediation strategies thus directly supports sustainable practices, contributing to a circular economy where materials are continually repurposed rather than discarded.
Recycling processes can close the material loop, enabling repeated use of MOF-derived products in various applications, such as catalysis, rather than disposing of them after single use. To give an example, Yang et al.142 highlights the recycling of MOFs, specifically MIL-100(Fe) saturated with tetracycline hydrochloride (TCH), by converting it into a graphene/Fe@N-doped carbon hybrid (FexNC) via pyrolysis. This transformation provides a dual benefit: it enables effective antibiotic degradation and recovers resources, creating high-value catalytic applications instead of landfill waste. By repurposing spent MOFs, the study aligns with circular economy principles, emphasizing both environmental and resource sustainability. This approach demonstrates how recycling can convert hazardous waste into valuable, reusable materials.
Further supporting this idea, Zhong et al.143 demonstrated that MOFs can be synthesised using recycled stainless steel as a metal source, creating MOF composites for efficient electrocatalysis in water oxidation. The study highlights how spent stainless steel can serve as a self-sacrificial template, transforming waste materials into functional MOFs with high catalytic activity. This provides a promising route to integrate MOFs into sustainable energy systems while ensuring the reuse of metal-containing waste. Similarly, Chu et al.144 discussed strategies to enhance the sustainability of MOF production emphasising scalable synthesis and resource-efficient recovery. Their work shows the importance of minimising chemical waste, optimising separation techniques, and developing strategies for the reuse of spent MOFs in new applications. The integration of these approaches aligns with circular economy principles, ensuring that MOFs remain viable beyond their initial use.
Future opportunities for integrating a circular economy framework into the field of MOFs offer a promising route to achieve more sustainable production, use, and end-of-life management. Adopting circularity principles would shift the focus from linear resource consumption and disposal to creating closed-loop systems that maximise resource efficiency and minimise environmental impact. For MOFs, this could include innovative approaches to reuse and recycle materials, optimise synthesis to reduce waste and energy use, and develop scalable methods for recycling MOFs components, such as metal ions and organic linkers, from spent materials. On a larger scale, establishing standardised protocols for MOF synthesis, use, and recovery could streamline LCA and make it easier to evaluate sustainability metrics across different applications. As discussed earlier in section 2.2.2, detailed LCA frameworks tailored specifically for MOFs are needed to accurately evaluate their environmental impacts from cradle to grave. A focus on representative LCA studies for key MOFs categories would help in developing robust circular strategies, identifying opportunities for resource recovery, and fostering sustainability within the SSbD approach. By moving toward circularity, the field of MOFs could not only reduce its ecological footprint but also support sustainable industrial applications that align with broader goals of environmental responsibility and resource conservation.
Further, Dubey et al.145 synthesised an organic linker 1,4-benzenedicarboxylic acid (BDC) by breaking down recycled PET plastic bottles through alkaline hydrolysis, transforming waste into a valuable building block for Cu-MOFs. Adding conducting polymers like polyaniline (PANI) was essential to boost the composite's conductivity and stability, as it created efficient pathways for ion and electron transport that pure MOFs lack. As a result, the Cu-MOF/PANI composite achieved a 53% increase in specific capacitance, reaching 160.5 F g−1 at a current density of 0.5 A g−1 compared to 104.8 F g−1 for pristine Cu-MOF. The cyclic stability also improved, with Cu-MOF/PANI retaining 93.4% of its initial capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles, outperforming the pristine Cu-MOF, which retained 89%. This sustainable, high-performance composite aligns with SSbD framework, effectively recycling plastic waste and minimising environmental impact while enhancing energy storage efficiency. Furthermore, incorporating carbon-based materials like graphene, carbon nanotubes (CNTs), and carbon dots (CDs) into MOFs enhances their electrical conductivity, chemical stability, and surface area, expanding their utility in energy storage, catalysis, and sensing applications.141,146 Bano et al.141 added 3D porous graphene to ZIF-67 MOFs which significantly boosted its stability, durability, and reusability. With graphene, the composite achieved an adsorption capacity of 589.46 mg g−1 for Congo Red and 350.79 mg g−1 for Methylene Blue, compared to much lower capacities for ZIF-67 alone. The graphene-MOF composite retained over 88% of its adsorption capacity after five reuse cycles, demonstrating improved durability, whereas pristine ZIF-67 typically suffers structural degradation after fewer cycles. This enhanced stability and reusability make the graphene-MOF composite far more effective and sustainable for repeated water treatment applications. Another research group,147 synthesised UiO-66-NDC/GO composite which significantly outperformed pristine UiO-66-NDC MOF in Pb(II) adsorption, reaching a maximum capacity of 254.45 mg g−1 compared to around 80–150 mg g−1 for the MOF alone. The addition of GO increased the material's surface area by approximately 20–30%, creating more active sites and enhancing stability, as evidenced by simulation studies (−45.459 eV vs. −42.978 eV). This enhanced stability allowed the composite to be reused for up to four cycles with minimal efficiency loss, showcasing its durability and effectiveness for sustainable heavy metal removal from water. These simulations also highlighted the dynamic interactions facilitated by delocalised Zr atoms, confirming the composite's ability to efficiently capture Pb(II) ions at a molecular level. From an SSbD perspective, GO not only enhanced the material's adsorption capacity but also improved its reusability and regeneration potential. The MOF-GO hybrid can be used for multiple cycles with minimal loss in effectiveness, supporting waste reduction and long-term environmental sustainability in water treatment.
000 charge–discharge cycles, outperforming the pristine Cu-MOF, which retained 89%. This sustainable, high-performance composite aligns with SSbD framework, effectively recycling plastic waste and minimising environmental impact while enhancing energy storage efficiency. Furthermore, incorporating carbon-based materials like graphene, carbon nanotubes (CNTs), and carbon dots (CDs) into MOFs enhances their electrical conductivity, chemical stability, and surface area, expanding their utility in energy storage, catalysis, and sensing applications.141,146 Bano et al.141 added 3D porous graphene to ZIF-67 MOFs which significantly boosted its stability, durability, and reusability. With graphene, the composite achieved an adsorption capacity of 589.46 mg g−1 for Congo Red and 350.79 mg g−1 for Methylene Blue, compared to much lower capacities for ZIF-67 alone. The graphene-MOF composite retained over 88% of its adsorption capacity after five reuse cycles, demonstrating improved durability, whereas pristine ZIF-67 typically suffers structural degradation after fewer cycles. This enhanced stability and reusability make the graphene-MOF composite far more effective and sustainable for repeated water treatment applications. Another research group,147 synthesised UiO-66-NDC/GO composite which significantly outperformed pristine UiO-66-NDC MOF in Pb(II) adsorption, reaching a maximum capacity of 254.45 mg g−1 compared to around 80–150 mg g−1 for the MOF alone. The addition of GO increased the material's surface area by approximately 20–30%, creating more active sites and enhancing stability, as evidenced by simulation studies (−45.459 eV vs. −42.978 eV). This enhanced stability allowed the composite to be reused for up to four cycles with minimal efficiency loss, showcasing its durability and effectiveness for sustainable heavy metal removal from water. These simulations also highlighted the dynamic interactions facilitated by delocalised Zr atoms, confirming the composite's ability to efficiently capture Pb(II) ions at a molecular level. From an SSbD perspective, GO not only enhanced the material's adsorption capacity but also improved its reusability and regeneration potential. The MOF-GO hybrid can be used for multiple cycles with minimal loss in effectiveness, supporting waste reduction and long-term environmental sustainability in water treatment.
Qu et al.146 showed that by adding green CDs to the pristine MOFs significantly boosted its effectiveness for dye removal. While Keratin/Ce-MOF148 had an adsorption capacity of around 469 mg g−1 for trypan blue and Ce-UiO-66149 794 mg g−1 for Congo red, the composite MOF with CDs (Ce-UiO-66-F4) achieved 773 mg g−1 for trypan blue and 1429 mg g−1 for Congo red, marking improvements of about 65% and 80%, respectively. This enhancement came from the additional functional groups on the CDs, which strengthened hydrogen bonding and electrostatic interactions, making the composite versatile across different pH levels and salt concentrations. Moreover, the composite retained over 90% of its capacity even after multiple reuse cycles, indicating greater durability compared to the pristine MOF. The composite's use of non-toxic, renewable carbon dots, high reusability, and stable performance across conditions makes it a safer, more sustainable option for water purification. Finally, with deep learning support, the system can assess dye concentrations with 96% accuracy, enabling real-time, reliable monitoring of purified water.
In another study,126 noble metal like gold nanoparticles (AuNPs) and MOF (PCN-224) composites were synthesised and the IC50 values of the composites were 703 μg mL−1 for normal HEK-293 cells and 300 μg mL−1 for cancerous SW-480 cells after 24 hours, showing the composite's ability to target cancer cells more effectively, requiring less than half the concentration needed for normal cells. After 24 hours, 33.84% of the treated cancer cells were in early apoptosis, 27.34% in late apoptosis, and 3.18% showed necrosis, underscoring the composite's potential as an effective anticancer agent. From an SSbD perspective, the biocompatibility with normal cells, combined with high selectivity for cancer cells and green synthesis, highlights a safe(r), more sustainable approach to cancer treatment by minimising health risks and environmental impacts. Shi et al.150 synthesised two MXene-MOF composites (CoSe2@C/MX-B and CoSe2@C/MX-S). CoSe2@C/MX-B uses a solvent-free ball-milling approach, while CoSe2@C/MX-S is created via a solution-based process that requires freeze-drying to maintain MXene stability. This difference results in CoSe2@C/MX-S having a higher surface area (75.6 m2 g−1) and larger pore volume (0.11 cm3 g−1) compared to CoSe2@C/MX-B (38.5 m2 g−1 surface area and 0.03 cm3 g−1 pore volume), though CoSe2@C/MX-B demonstrates better structural stability. For lithium-ion storage, CoSe2@C/MX-B maintains a specific capacity of 669.2 mA h g−1 over 1000 cycles at 1 A g−1, whereas CoSe2@C/MX-S, despite its initial high capacity, experiences faster capacity decay due to structural degradation. The solvent-free synthesis of CoSe2@C/MX-B minimizes environmental impact and health risks by eliminating toxic solvents and reducing energy use. This method also enhances structural stability, potentially prolonging the material's lifespan and reducing waste, aligning with sustainable design principles.
MOF-based composites offer versatile, sustainable solutions across diverse applications, from water treatment to energy storage and cancer treatment, by integrating with polymers, carbon-based nanomaterials, and nanoparticles to enhance stability, reusability, and efficiency. These composites, developed in line with SSbD framework, demonstrate improved performance and durability, which are crucial for real-world, large-scale applications. By advancing the field with eco-friendly innovations, MOF-based composites present a promising path toward safer, high-performance materials that align with environmental and health safety standards, ultimately contributing to a more sustainable future in material science.
2.4. Threats
Carbon based nanomaterials such as GO and rGO are majorly used in applications such as adsorption of toxic metal ions, reinforcements in polymer composite for food packaging and biomedical applications, flexible electronics and energy storage applications because of their well-known properties such as high effective surface area, presence of oxygenated functional groups, and exceptional thermal and electrical conductivity. One notable study by Bhadane et al.35 demonstrated the effectiveness of a biodegradable and sustainable starch-GO composite as an adsorbent for Pb(II) removal from industrial wastewater within just 15 minutes. This approach eliminates the need for centrifugation or filtration, addressing a significant challenge associated with MOFs, which often require complex separation processes after adsorption, to isolate the powdered material from the solution. As shown in Fig. 7A and B, Ge et al.,151 demonstrated application of rGO in efficient energy storage applications. The study highlights the cation-dependent electrochemical behaviours of rGO, revealing distinct charge storage mechanisms influenced by solvated cation adsorption and dehydration. It underscores the potential for tailored electrolyte and electrode designs to optimise energy storage performance in supercapacitors and batteries. Apart from these, numerous other carbon-based materials have been reported for environmental remediation and resource recovery applications.152,153. However, compared to MOFs, these materials exhibit lower selectivity, as metal adsorption primarily relies on oxygen functional groups. As a result, post-functionalization or composite formation is often necessary to enhance their selectivity. Whereas, as discussed in strengths section MOFs have abundant/specific reactive groups which can provide selective adsorption.
 | ||
| Fig. 7 Threats for MOFs (A) synthesis and characterisation of 2D reduced graphene oxide (rGO) through a two-step reduction process involving hydrothermal and thermal reduction methods.151 (B) Electrochemical performance of prepared rGO sheets in different aqueous electrolytes, illustrated through integrated capacitance and cyclic voltammetry, showing the impact of various cations (Li+, Mg2+, Zn2+) on capacitance. The enhancement is highest for zinc ions, followed by magnesium ions, and lowest for lithium ions, aligning with the expected interaction strength between the cations and rGO.151 | ||
Following carbon-based materials, COFs have gained attention as potential alternatives to MOFs.
Theseis COFs, an emerging class of two- or three-dimensional crystalline materials, are formed through reactions between organic precursors, resulting in strong covalent bonds. Like MOFs, COFs exhibit high porosity and structural tunability, but their covalent bonding confers significantly higher thermal and solvent stability, enabling them to withstand harsher environments.154,155 This stability has made COFs increasingly viable in applications demanding resilience to thermal or chemical stress, such as in boiling acids or bases. Although early COF designs faced stability concerns due to reversible covalent bond formation, extensive research has since improved their structural robustness. Today, many COFs surpass MOFs in stability, expanding their suitability for applications where temperature or chemical durability is crucial, like catalysis, energy storage, and environmental remediation.156 For example, compared to MOFs in pharmaceutical waste adsorption, COFs demonstrated comparable adsorption capacities for sulfamethoxazole. Akpe et al.157 reported an adsorption capacity of 483 mg g−1 using a microporous triazine polymer (MCTP), while Cheng et al.158 achieved 283.6 mg g−1 with a polymer-UiO-66 composite. COFs, with their higher structural tunability and stability in harsh conditions, often outperform MOFs in adsorption applications, making them particularly effective for pharmaceutical waste management. While pristine COFs exhibit high porosity and stability, they often lack sufficient functional groups for strong metal coordination, leading to lower selectivity compared to MOFs. Additionally, achieving consistent crystallinity and uniform pore distribution in COFs can be challenging, which may impact their performance in applications requiring precise structural control. The choice between MOFs and COFs will largely depend on the specific application, as each material offers distinct advantages and limitations.
Further as shown in Fig. 8A and B a superior performance of COFs (like Fe-COF nanorods (NRs) in applications such as antibacterial and nitric oxide (NO)-based gas therapy highlights a significant threat to MOFs) as preferred materials. COFs are proving effective in treating complex medical conditions, such as diabetic ulcers, through their biocompatibility and targeted therapeutic properties. The Fe-COF NRs showcased here demonstrate enhanced antibacterial efficacy, reducing bacterial survival rates of S. aureus, which indicates their superior functionality in biomedical applications.159 This level of effectiveness in COFs can potentially overshadow MOFs, particularly in applications requiring high stability, biocompatibility, and efficient therapeutic delivery. Unlike MOFs, COFs offer greater chemical robustness due to their covalent bonding, enhancing their durability under biological conditions without the risk of metal ion leaching, which can pose toxicity concerns. Additionally, COFs often exhibit better structural stability and resistance to degradation, making them attractive alternatives for applications where MOFs might face stability and toxicity limitations. If COFs continue to outperform MOFs in such biomedical applications, particularly in terms of safety and effectiveness, they could become the preferred choice for sustainable design in healthcare, further challenging the role of MOFs in this domain. This competitive edge in safety, stability, and therapeutic efficacy positions COFs as a threat to the wider adoption of MOFs, especially in applications where patient safety and long-term biocompatibility are paramount.
 | ||
| Fig. 8 Threats for MOFs associated with alternative materials (A) schematic of Fe-COF nanorods (NRs) for antibacterial and nitric oxide (NO)-based gas therapy, demonstrating a synergistic approach to treating diabetic ulcers, with steps 1–3 showing the sequential addition in the presence of EDC/NHS.159 (B) In vitro evaluation of bacterial survival rates, showing the antibacterial efficacy of Fe-COF NRs against S. aureus.159 (C) Application of MXene materials (Li+-intercalated Ti3C2Tx) for selective recovery of Nd(III) ions from electronic waste, showing SEM and TEM micrographs with a 2D sheet-like structure of TCF-1 and TCF-2.64 (D) Comparison of Nd(III) adsorption capacity across various adsorbents, highlighting the performance of MXene-based materials. | ||
In addition to stability, the thermal properties of COFs make them a compelling alternative to MOFs. While both COFs and MOFs offer high surface area and permanent porosity, COFs are more resistant to thermal degradation, an area where MOFs often fall short. Further, in energy storage and electronic applications, MOFs show promise but encounter major limitations in terms of thermal and electrical conductivity. Although modifications, such as incorporating guest molecules or post-synthetic treatments, can enhance electrical conductivity in conductive MOFs (EC-MOFs), these adjustments often introduce complexity and compromise key features like porosity and stability. Nonetheless, the limitations of MOFs in electrical and thermal conductivity contrast sharply with the properties of other SSbD-aligned materials, such as Graphene, COFs and MXenes.160,161 As discussed by Soltan et al.162 in their research article, the thermal conductivity of graphene, carbon nanotubes and MXene increased by 64, 64 and 30.6% respectively when dispersed in water. However, when compared to these nanomaterials, the results for MOFs were not as impressive due to their larger surface area and pore size. This implies that while MOFs hold potential in heat transfer applications, they may not perform as effectively as some other nanoparticles, particularly in terms of heat transfer efficiency. Apart from these materials, MOF-derived carbon nanomaterials (CNMs) are gaining attention as strong alternatives to traditional MOFs in environmental remediation, energy storage, and catalysis. A major advantage of these materials is their stability, reusability, and recyclability, as the pyrolysis process transforms MOFs into highly porous carbon structures while retaining key functional properties.163,164 For example, MOF-derived carbon composites containing CNTs and cobalt nanoparticles have shown high adsorption efficiency for contaminants like quinolone antibiotics.165 These materials remain effective across a wide pH range and ionic strengths, making them well-suited for wastewater treatment. Additionally, their magnetic properties allow easy separation and recovery, addressing a common challenge in adsorbent reuse. The composites also maintain their performance over multiple cycles after regeneration, reinforcing their potential as durable and efficient alternatives for environmental and catalytic applications. MXenes, a class of 2D transition metal carbides, nitrides, and carbonitrides, exhibit unique thermal and electrical properties comparable to graphene, making them highly stable under extreme conditions. Their electrical conductivity, combined with thermal resilience, makes MXenes highly suited for applications where MOFs struggle, especially in catalysis, electronics, and sensors. MXenes inherent conductivity and stability present fewer modifications and environmental risks than MOFs, aligning well with SSbD framework and providing an attractive, sustainable option for high-performance applications.166 Recent work published by Cho et al.167 showed use of MOF based composite beads for the recovery of Nd ions. This polymeric microcapsule (PMC) attached with ZIF-8 showed adsorption capacity of 463.6 mg g−1, which is lower than the reported adsorption capacity of MXene shown in Fig. 8C. Yan et al.168 reported use of Nb2CTx for dye removal studies and reported the adsorption capacity of 500 mg g−1, whereas in case of pristine MOFs as well as modified MOFs reported dye adsorption capacity is exceptionally low.169,170 As shown in Fig. 8D, MXene used for the recovery of REEs showed adsorption capacity better than all other adsorbents which included pristine MOFs as well as MOF based monoliths and composite Although MXene-based adsorbents exhibit higher adsorption capacities compared to other materials, their synthesis process is highly complex and energy-intensive171,172 This contradicts the principles of SSbD and the goals of SDGs. In addition, their susceptibility to oxidation and potential issues with long-term stability under ambient conditions can be significant drawbacks when compared to MOFs. As a result, the practicality of MXenes in environmental remediation remains challenging, making other adsorbents more viable alternatives. The distinct advantages offered by GO, rGO, COFs, MXenes, and other emerging materials in terms of stability, thermal resistance, and conductivity highlight potential replacements for MOFs, particularly in applications that require durability and SSbD compliance. While MOFs continue to evolve, these alternative materials offer pathways for sustainable innovation, where structural robustness, reduced need for complex modifications, and fewer environmental risks contribute to safer, more sustainable design. MOFs must offer exceptional properties to justify their selection over alternative options. This necessitates that MOFs not only meet but exceed the performance and functional benchmarks set by other materials in similar applications. In addition, to remain competitive, future MOFs research must integrate SSbD strategies, focusing on inherent stability and reduced toxicity to fulfil their potential across various applications while addressing environmental and health concerns.
Parallels can be drawn from nanomaterials research and their widespread adoption in several application. The regulatory landscape for nanomaterials, including MOFs, remains fragmented. As SSbD framework become more widely adopted, the lack of clear, harmonised regulations for MOFs poses a threat to their sustainable commercialisation. Regulatory hurdles could slow down the adoption of SSbD-aligned MOFs, especially in regions with stringent but unclear nanomaterial legislation. A recent article by Chavez-Hernandez et al.,174 despite their benefits, nanomaterials pose potential environmental and health risks, such as improper disposal leading to ecosystem accumulation and occupational exposure resulting in health issues. It took a significant effort by regulatory bodies internationally including efforts in the United States, European Union, and international organisations like the OECD Working Party on nanomaterials. This article showed the importance of implementing global regulations to promote MOFs research responsibly, adopting a precautionary principle focused on environmental and health protection to ensure the safe use and application of MOFs.
To develop effective regulatory frameworks, tools and strategies such as LCA, risk assessments, and technical tools are required to be employed in MOFs. Given the number of MOFs that are already been developed, it would be challenging and equally a big opportunity to unveil new tools that could help in regulatory approvals of MOFs for large scale commercial applications. With a similar perspective, Wright et al.175 highlighted the absence of specific regulations for MOFs, leading to uncertainties in safety, environmental impact, and long-term effects. The authors emphasised the need for comprehensive regulatory frameworks to ensure safe production, application, and disposal of MOFs. It also highlighted the importance of industry collaboration and transparency in developing standardised guidelines and best practices. Addressing these regulatory challenges is crucial for the responsible development and commercialisation of MOFs. With increasing demand for sustainable materials, there is a risk of greenwashing, where MOFs are marketed as “green” without fulfilling true SSbD criteria. Greenwashing involves making misleading claims about the environmental benefits of a product or technology. In the context of MOFs, companies might overstate their sustainability credentials without substantial evidence. This practice can mislead consumers and stakeholders, undermining trust and potentially delaying genuine environmental progress. The lack of stringent regulations exacerbates this issue, as companies may exploit these gaps to make unverified claims. For instance, the World Economic Forum176 highlights that as international awareness and regulations increasingly call for consumption and investments to be more sustainable, the potential for greenwashing also grows. Superficial sustainability claims can undermine the credibility of the SSbD framework, leading to consumer scepticism and slowing the adoption of genuinely safe and sustainable MOFs.
As MOFs degrade, they lose structural integrity and functional capabilities, leading to a loss of properties like high surface area and pore volume that are essential for applications in gas storage, catalysis, and CO2 sequestration. The transformation of MOFs affects not only their safety profile but also their performance efficiency, highlighting the need for comprehensive assessments of MOFs behaviour under environmental conditions.181 Specifically, MOFs exhibit unique, size-dependent properties due to quantum confinement effects and increased surface reactivity, which make them susceptible to complex and often unpredictable transformation pathways. These nanoscale behaviours mean that MOFs do not simply mirror the properties of bulk materials; rather, they respond dynamically, often leading to rapid dissolution and ion release when exposed to biological fluids or natural waters. Such ion release poses acute risks for aquatic and terrestrial organisms and complicates their safe use in diverse applications.76
From an SSbD perspective, understanding these transformations is critical for developing nMOFs with safe, predictable profiles. A significant component of SSbD involves controlling the material lifecycle to prevent environmental or health hazards. By studying MOFs transformation mechanisms, researchers can predict potential environmental impacts and adjust MOFs synthesis or composition to reduce toxic byproducts. For instance, incorporating stability-focused synthesis strategies or post-synthetic modifications can reduce the likelihood of degradation and ion leaching, aligning MOFs design with SSbD objectives. The molecular-level interactions of nMOFs in biological systems also require thorough investigation to assess how transformations might disrupt critical cellular processes. In particular, exposure to ZIF-8 and ZIF-90 led to significant gut microbiota alterations in zebrafish, increasing the abundance of inflammation-associated bacteria such as Proteobacteria (Aeromonas, Plesiomonas, and Legionella).179 Chakraborty et al. indicates that nMOFs can perturb enzyme structures upon interaction, altering or inhibiting enzyme functionality.76 These transformations, which include surface modifications and reactive species generation, may disrupt biological pathways, potentially leading to toxic effects in organisms. Such molecular transformations underscore the need to assess nMOFs safety not only from a materials perspective but also from a biological and ecological standpoint, as transformations may introduce toxicity through mechanisms unanticipated in their original design.
For SSbD, these insights call for lifecycle assessments and environmental testing that reflect the real-world conditions MOFs will encounter. Evaluating how nMOFs behave and transform in biological systems and the environment can guide safer design choices that minimise toxic transformation products while retaining functionality. As discussed in the section 2.2.2, there is a significant lack of toxicology data, and this gives an opportunity to establish standardised protocols for testing and lifecycle analysis is essential for advancing MOFs within SSbD frameworks. Despite the growing use of MOFs, there is a notable gap in ecotoxicity data, particularly concerning the formation of protein and eco-coronas (Fig. 9), which significantly influence their environmental behaviour and biological interactions. The lack of comprehensive data on how MOFs undergo transformation processes such as homoaggregation, heteroaggregation, dissolution, and degradation, limits our understanding of their potential to release harmful metal ions (e.g., Zn2+) and ROS. These emissions can cause oxidative stress, DNA damage, mitochondrial dysfunction, and other toxic effects in organisms, potentially disrupting natural processes and accumulating through the food web. This could act as a potential threat towards regulatory approval and a widespread adoption of MOFs. Addressing these gaps is crucial for enhancing the SSbD framework to ensure that MOFs designed for environmental applications are truly safe and minimise long-term ecological risks. By incorporating robust testing for transformation behaviours and toxicological impacts, the SSbD approach can support the development of MOFs that are not only high-performing but also environmentally responsible, meeting safety standards for diverse application.
As discussed in weaknesses section, MOFs offer significant potential across multiple fields, the scarcity of comprehensive toxicity data could hinder their advancement. Effective MOF design must incorporate in-depth toxicological evaluations addressing potential transformation effects and long-term environmental impacts. Expanding this dataset and aligning it with SSbD frameworks will be critical for realising MOFs benefits in a safe, sustainable manner. Factors including chemical composition, particle size, and behaviour in biological and environmental systems may raise concerns about the potential toxicity.184 The chemical composition of the MOFs is a critical factor in determining toxicity, as it reflects properties like dissolution, redox capability, ionisation, and affinity to biomolecules, all of which shape the toxicity potential and underlying mechanisms. For instance, Ruyra et al.185 performed a comprehensive in vitro and in vivo toxicity of metal series of MOF-74 composed of the following metals Zn(II), Cu(II), Co(II), Mn(II), and Mg(II) metal ions in liver (HepG2) and Breast (MCF-7) cancer via cell viability assay. The toxicity ranking shows that Cu(II), and Mn(II) exhibits the highest rate of toxicity. In contrast, Co-, Ni-, and Mg-based counterparts have shown promising biocompatibility and Zn-MOF-74 was found to exhibit a moderate level of cytotoxicity. In in vivo studies on selected MOF structures, shows that zebrafish embryos exposed to Co-MOF-74 developed yolk sac edema, whereas embryos incubated with Mg-MOF-74 did not exhibit such adverse effects. Another comparative study of two MOFs, MIL-100(Fe) and HKUST-1(Cu), both of which incorporate 1,3,5-benzenetricarboxylic acid in their structures, revealed notable differences in toxicity. In vitro analysis indicated that both MOF exhibit some toxicity, with the Cu-based MOF showing a significantly higher toxicity level. Like their study, the toxicological screening of two prevalent MOFs (ZIF-8 and MIL-160) for therapeutic use in human lung epithelial cells (BEAS-2B) were considered. The dose–response results demonstrate that lower toxicity of MIL-160 compared to ZIF-8.79
Similar assessment of the toxicity was performed in freshwater alga (Chlamydomonas reinhardtii) using six typical nMOFs, namely, transition metal incorporated aluminium-based porphyrin MOFs [pristine Al-PMOF, Al-PMOF (Cu), Al-PMOF (Ni), Al-PMOF (Co)], an amine functionalised Ti MOF [NH2-MIL-125 (Ti)], and a bimetallic Hofmann MOF (NiCo-PYZ).186 The study demonstrated that all the MOFs concentrations higher than 10 mg L−1 pose a threat to the algae and the effects is MOF specific. Conversely, certain properties of MOFs, including their larger surface area volume ratio, increased chemical reactivity, and enhanced penetration capabilities compared to other materials, raise potential concerns regarding safety in living organisms. Due to their small size, nMOFs can cross biological cell membranes and enter the bloodstream via inhalation or ingestion—something that larger particles typically cannot do and potentially lead to toxicological effects.177 Recently, several reports have shown that size may influence the cellular uptake as well cytotoxicity of MOFs. For instance, Tarasi et al., investigated the size-dependent therapeutic efficacy of Zn-MOF in human breast cancer (SKBR3). The study suggested that varying amounts of MOFs entered the cells, indicating that different particle sizes led to distinct cellular responses. Among these three sizes, 200 nm showed the best efficacy of 82%. This shows that a size of 200 nm with the highest amount of uptake in cells can have more effects. While 300 nm and bulk scale with a lower uptake in cells showed efficacies of 68% and 57%. The size of 100 nm shows the lowest effectiveness of 10%, which is probably due to the small spherical nanostructures and the tendency to agglomerate, which have the lowest amount of efficacy. Wang et al.187 also observed that the toxicity of cobalt-based MOFs (specifically ZIF-67) varies with particle size. The study assessed the biocompatibility of ZIF-67 particles sized 100, 200, 400, 700, and 1200 nm on the Photobacterium phosphoreum T3 strain. The results showed that for particles smaller than 400 nm, toxicity increased as particle size decreased. However, no consistent trend was noted for particles larger than 400 nm. This increased toxicity of smaller particles (100 and 200 nm) was likely due to their ability to penetrate and accumulate within the cytoplasm, leading to heightened cellular damage. In contrast, other studies revealed that n-MOFs are safer for living organisms than their micron-sized counterparts (m-MOFs).188,189 These factors influence the MOFs interaction with biological systems, potentially accounting for the differential toxicological effects observed in the study. Despite the advantages MOFs bring to various applications, the lack of detailed toxicological data across their lifecycle poses significant challenges to their SSbD credentials. Preliminary studies, such as those highlighting the size-dependent toxicity of certain MOFs, underscore potential risks to human health and environmental safety. For instance, smaller nMOFs demonstrate higher cytotoxicity and can penetrate biological barriers, raising concerns about their safe integration into consumer products and industrial applications. Addressing these data gaps is crucial for developing MOFs that are truly benign and align with the framework of SSbD, ensuring that their innovative applications do not compromise safety and sustainability.
3. Outlook and recommendations
The adoption of SSbD framework in MOFs development represents a pivotal step toward creating advanced materials with minimised environmental and health risks while ensuring their long-term societal benefits. This review has demonstrated the multifaceted potential of MOFs across diverse applications, including biomedical, environmental, and energy sectors, highlighting their adaptability, high porosity, and customisability. However, challenges remain, particularly in enhancing stability, reducing toxicity, and addressing scalability concerns for large-scale applications. Emerging solutions such as green synthesis, ML-guided material design, and the incorporation of eco-friendly linkers and metal centres exemplify how sustainability can be seamlessly integrated into MOF design. For MOFs to fulfil their potential as sustainable materials, it is important to align their design with the United Nations SDGs, ensuring that innovation supports human health, environmental protection, and long-term economic viability. As the SSbD framework gains traction, the ongoing evolution of MOFs toward higher stability, biocompatibility, and eco-friendliness will likely accelerate. Advances in biocompatible and degradable MOFs for medical applications, supported by predictive models and high-throughput screening, are expected to reduce resource-intensive experimental methods. Additionally, the integration of ML and AI-assisted predictive modelling will enable rapid identification and optimisation of MOFs for specific applications, from pollutant adsorption to sustainable agriculture. To broaden their adoption, MOF composites, particularly those involving carbon-based nanomaterials, offer pathways to enhance electrical conductivity, chemical stability, and reusability, meeting the requirements for real-world, large-scale implementations.Future roadmap
1. Standardisation and regulatory framework development: establishment of globally harmonised regulatory standards for MOF synthesis, usage, and disposal is crucial. A robust SSbD framework must integrate risk assessment tools, LCA, and hazard profiling to address toxicity, environmental transformation, and long-term ecological impacts. To ensure responsible innovation, it is essential to draw from existing nanomaterial safety regulations (e.g., OECD, REACH) and tailor them to MOFs, with specific attention to bioaccumulation, degradation products, and material persistence.• SDG 3 (Good health and well-being) – ensuring MOFs are designed to minimise human toxicity and unintended health hazards.
• SDG 6 (clean water and sanitation) – regulating MOFs used for water purification and pollutant adsorption to avoid secondary contamination.
• SDG 9 (industry, innovation, and infrastructure) – establishing clear safety guidelines for industrial MOF applications.
• SDG 12 (responsible consumption and production) – promoting sustainable production and end-of-life strategies for MOFs.
• SDG 14 (life below water) & SDG 15 (life on land) – ensuring MOF degradation products do not contribute to bioaccumulation or ecosystem toxicity.
• SDG 17 (partnerships for the goals) – encouraging interdisciplinary collaboration between industry, policymakers, and researchers to develop a unified regulatory framework.
2. Green synthesis and scalability: future research should focus on scaling green synthesis methods to industrial levels, using renewable resources and eco-friendly procedures to align with circular economy objectives. Strategies include biomimetic synthesis, mechanochemistry, and solvent-free or aqueous-phase fabrication, minimising hazardous byproducts. Integration with waste valorisation strategies, such as upcycling industrial byproducts into MOF precursors, will further enhance sustainability.
• SDG 7 (affordable and clean energy) – promoting energy-efficient, green synthetic pathways.
• SDG 9 (industry, innovation, and infrastructure) – developing scalable, eco-friendly manufacturing techniques.
• SDG 12 (responsible consumption and production) – minimising waste generation and ensuring sustainable resource use.
• SDG 13 (climate action) – reducing CO2 footprint in MOF production by adopting low-energy synthetic approaches.
3. Integration of machine learning for design optimisation: expanding the application of ML and AI in MOF design and prediction can significantly improve performance and reduce trial-and-error methods. Developing comprehensive databases that compile the properties, stability, and toxicity profiles of MOFs will enhance ML's role in rapidly screening materials for specific applications.
• SDG 4 (quality education) – enhancing open-access AI-driven MOF databases for training scientists globally.
• SDG 9 (industry, innovation, and infrastructure) – fostering innovation through AI-driven material design.
• SDG 12 (responsible consumption and production) – minimising resource use by optimizing MOF properties before synthesis.
• SDG 17 (partnerships for the goals) – encouraging cross-disciplinary collaborations in AI-driven MOF research.
4. Environmental and toxicological testing protocols: developing standardised testing protocols for environmental transformation, bioaccumulation and toxicological impacts, especially at the nanoscale, is essential. These protocols should include long-term degradation studies, nanoscale toxicity evaluations, and ecosystem-based risk assessments to ensure MOFs do not pose unforeseen environmental hazards. Advanced analytical tools, including high-resolution mass spectrometry and in situ environmental microscopy, should be employed to track MOF degradation pathways and potential ecotoxicological effects.
• SDG 3 (good health and well-being) – ensuring safety assessments for human exposure.
• SDG 6 (clean water and sanitation) – monitoring MOF interactions in water treatment applications.
• SDG 14 (life below water) & SDG 15 (life on land) – studying MOF bioaccumulation in aquatic and terrestrial ecosystems.
5. Exploration of composites for enhanced functionality: composite materials that incorporate polymers, carbon nanomaterials, or other nanoparticles can address the stability, conductivity, and functional limitations of standalone MOFs. Expanding research in MOF-based composites, will be particularly valuable in energy storage, sustainable agriculture, and environmental remediation.
• SDG 7 (affordable and clean energy) – enhancing MOF composites for energy storage and conversion.
• SDG 9 (industry, innovation, and infrastructure) – developing multi-functional MOF-based materials for industrial use.
• SDG 11 (sustainable cities and communities) – improving MOF applications in air purification and urban sustainability.
• SDG 12 (responsible consumption and production) – promoting recyclable and reusable MOF materials.
• SDG 13 (climate action) – developing MOFs for CO2 capture and greenhouse gas reduction.
The integration of SSbD framework offers an exciting direction for future MOFs research (Fig. 10), advancing the development of materials that are not only high-performance but also align with global sustainability goals. By continuing to address these challenges through interdisciplinary collaboration regulatory foresight, and data-driven innovation, MOFs could revolutionise fields such as medicine, environmental remediation, and energy, contributing to a more sustainable future in materials science.
Author contributions
P.D. – conceptualisation, literature curation, original draft, visualisation; writing P.B. conceptualisation, literature curation, original draft, visualisation; writing; BI – literature curation, original draft, visualisation, writing; S.C. conceptualisation, literature curation, original draft, visualisation; writing – review & editing, funding acquisition.Data availability
Data are available upon request from the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
SC acknowledges UKRI NERC Independent Research Fellowship (Grant Number- UKRI187), and Engineering and Physical Sciences Research Council Co-Fund [grant number EP/X525662/1], Birmingham Cross Council Impact Acceleration Account for supporting this work.References
- L. Chen, X. Zhang, X. Cheng, Z. Xie, Q. Kuang and L. Zheng, Nanoscale Adv., 2020, 2, 2628–2647 Search PubMed.
- S. Mallakpour, E. Nikkhoo and C. M. Hussain, Coord. Chem. Rev., 2022, 451, 214262 Search PubMed.
- A. Radwan, H. Jin, D. He and S. Mu, Nano-Micro Lett., 2021, 13, 1–32 CrossRef PubMed.
- Safe and sustainable by design - European Commission, https://research-and-innovation.ec.europa.eu/research-area/industrial-research-and-innovation/chemicals-and-advanced-materials/safe-and-sustainable-design_en, (accessed 26 February 2025).
- P. A. Julien, C. Mottillo and T. Friščić, Green Chem., 2017, 19, 2729–2747 RSC.
- S. Mandal, S. Natarajan, P. Mani, A. Pankajakshan, S. Mandal, P. Mani, A. Pankajakshan and S. Natarajan, Adv. Funct. Mater., 2021, 31, 2006291 Search PubMed.
- C. Verma, T. Rasheed, M. T. Anwar and M. A. Quraishi, Microchem. J., 2023, 192, 108954 CrossRef CAS.
- P. Kumar, B. Anand, Y. F. Tsang, K. H. Kim, S. Khullar and B. Wang, Environ. Res., 2019, 176, 108488 Search PubMed.
- K. Pobłocki, J. Drzeżdżon, B. Gawdzik and D. Jacewicz, Green Chem., 2022, 24, 9402–9427 RSC.
- H. Reinsch, Eur. J. Inorg. Chem., 2016, 2016, 4290–4299 CrossRef CAS.
- R. Abazari, A. R. Mahjoub, F. Ataei, A. Morsali, C. L. Carpenter-Warren, K. Mehdizadeh and A. M. Z. Slawin, Inorg. Chem., 2018, 57, 13364–13379 CrossRef CAS PubMed.
- S. Dong, D. Zhang, H. Cui and T. Huang, Sens. Actuators, B, 2019, 284, 354–361 CrossRef CAS.
- S. Gautam, S. Rialach, S. Paul and N. Goyal, RSC Adv., 2024, 14, 14311–14339 Search PubMed.
- L. B. Vaidya, S. S. Nadar and V. K. Rathod, Colloids Surf., B, 2020, 193, 111052 Search PubMed.
- J. Zeng, J. Su, N. Sun, L. Han, M. Yuan, H. Deng, Y. Zhuang, J. Wang and Y. Zhang, Sep. Purif. Technol., 2024, 345, 127327 CrossRef CAS.
- H. A. Alhadlaq, M. J. Akhtar and M. Ahamed, Toxicology, 2019, 411, 71–80 CrossRef CAS PubMed.
- Y. Ibrahim, M. Meslam, K. Eid, B. Salah, A. M. Abdullah, K. I. Ozoemena, A. Elzatahry, M. A. Sharaf and M. Sillanpää, Sep. Purif. Technol., 2022, 282, 120083 Search PubMed.
- M. Lu, H. Li, W. Han, J. Chen, W. Shi, J. Wang, X. M. Meng, J. Qi, H. Li, B. Zhang, W. Zhang and W. Zheng, J. Energy Chem., 2019, 31, 148–153 CrossRef.
- Y. Zhu, L. Chen, J. Pan, S. Jiang, J. Wang, G. Zhang and K. Zhang, Prog. Mater. Sci., 2025, 148, 101373 Search PubMed.
- P. Dhumal, S. Chakraborty, B. Ibrahim, M. Kaur and E. Valsami-Jones, J. Cleaner Prod., 2024, 480, 144115 CrossRef CAS.
- P. Goyal, A. Paruthi, D. Menon, R. Behara, A. Jaiswal, K. V., A. Kumar, V. Krishnan and S. K. Misra, Chem. Eng. J., 2022, 430, 133088 CrossRef CAS.
- R. Paz, H. Viltres, N. K. Gupta, A. Romero-Galarza and C. Leyva, Environ. Sci.:Adv., 2022, 1, 182–191 CAS.
- V. F. Yusuf, N. I. Malek and S. K. Kailasa, ACS Omega, 2022, 7, 44507–44531 Search PubMed.
- S. Li, Y. Gao, N. Li, L. Ge, X. Bu and P. Feng, Energy Environ. Sci., 2021, 14, 1897–1927 RSC.
- F. Saraci, V. Quezada-Novoa, P. R. Donnarumma and A. J. Howarth, Chem. Soc. Rev., 2020, 49, 7949–7977 RSC.
- A. Bhuyan and M. Ahmaruzzaman, Inorg. Chem. Commun., 2022, 140, 109436 CrossRef CAS.
- M. Ding and H. L. Jiang, CCS Chem., 2021, 3, 2740–2748 CrossRef CAS.
- Q. Zhang, B. Li and L. Chen, Inorg. Chem., 2013, 52, 9356–9362 CrossRef CAS PubMed.
- X. G. Han, P. F. Wang, Y. H. Zhang, H. Y. Liu, J. J. Tang, G. Yang and F. N. Shi, Inorg. Chim. Acta, 2022, 536, 120916 CrossRef CAS.
- N. C. Burtch and K. S. Walton, Acc. Chem. Res., 2015, 48, 2850–2857 Search PubMed.
- P. Goyal, D. Menon, P. Jain, P. Prakash and S. K. Misra, Sep. Purif. Technol., 2023, 318, 123941 CrossRef CAS.
- N. K. Daniel, A. Varghese, K. R. Sunaja Devi, S. J. Chundattu and A. Sreekanth, Colloids Surf., A, 2024, 703, 135177 Search PubMed.
- F. Drache, V. Bon, I. Senkovska, C. Marschelke, A. Synytska and S. Kaskel, Inorg. Chem., 2016, 55, 7206–7213 CrossRef CAS PubMed.
- K. Jayaramulu, F. Geyer, A. Schneemann, Š. Kment, M. Otyepka, R. Zboril, D. Vollmer and R. A. Fischer, Adv. Mater., 2019, 31(32), 1900820 Search PubMed.
- P. Bhadane, P. Mahato, D. Menon, B. K. Satpathy, L. Wu, S. Chakraborty, P. Goyal, I. Lynch and S. K. Misra, Environ. Sci.: Nano, 2024, 11, 2385–2396 RSC.
- H. E. Emam, R. M. Abdelhameed and H. B. Ahmed, J Environ. Chem. Eng., 2020, 8(5), 104386 Search PubMed.
- S. Sangam, A. Gupta, A. Shakeel, R. Bhattacharya, A. K. Sharma, D. Suhag, S. Chakrabarti, S. K. Garg, S. Chattopadhyay, B. Basu, V. Kumar, S. K. Rajput, M. K. Dutta and M. Mukherjee, Green Chem., 2018, 20, 4245–4259 RSC.
- M. Woellner, S. Hausdorf, N. Klein, P. Mueller, M. W. Smith and S. Kaskel, Adv. Mater., 2018, 30(37), 1704679 CrossRef PubMed.
- Y. Zhang, X. Yu, Y. Hou, C. Liu, G. Xie and X. Chen, Mol. Catal., 2024, 555, 113851 Search PubMed.
- J. Gu, H. Fan, C. Li, J. Caro, H. Meng, J. Gu, D. An, C. Li, H. Meng, D. F. An and D. Aro, Angew. Chem., Int. Ed., 2019, 58, 5297–5301 CrossRef CAS PubMed.
- S. E. Morgan, M. L. Willis, G. W. Peterson, J. J. Mahle and G. N. Parsons, ACS Sustainable Chem. Eng., 2022, 10, 2699–2707 CrossRef CAS.
- B. N. Socha, B. Patankar, A. Raj, R. B. Palan, J. Valand, R. H. Patel and S. Krishn Jha, Chem. Eng. J., 2024, 493, 152566 CrossRef CAS.
- A. Liu, L. Chen, L. Qi, J. Huang, Y. Zou, Z. Hu, L. Yu, Z. Zhong, Q. Ye and C. Chen, SusMat, 2024, 4, e235 CrossRef CAS.
- Z. Zheng, H. L. Nguyen, N. Hanikel, K. K. Y. Li, Z. Zhou, T. Ma and O. M. Yaghi, Nat. Protoc., 2023, 18, 136–156 CrossRef CAS PubMed.
- H. Shaghaleh, Y. Alhaj Hamoud, Q. Sun, M. S. Sheteiwy and H. AbdElgawad, Sep. Purif. Technol., 2025, 353, 128440 CrossRef CAS.
- Z. Li, B. Yao, C. Cheng, M. Song, Y. Qin, Y. Wan, J. Du, C. Zheng, L. Xiao, S. Li, P. F. Yin, J. Guo, Z. Liu, M. Zhao and W. Huang, Adv. Mater., 2024, 36(13), 2308427 Search PubMed.
- H. Li, J. Lei, L. Zhu, Y. Yao, Y. Li, T. Li and C. Qiu, Green Energy Environ., 2024, 9, 1650–1665 Search PubMed.
- H. Yang, Y. Zhao, Y. Guo, B. Wu, Y. Ying, Z. Sofer and S. Wang, Small, 2024, 20(15), 2307484, DOI:10.1002/SMLL.202307484.
- F. Mahmoudi and L. G. Bachas, Water, 2024, 16, 3051 Search PubMed.
- Q. Salamat and M. Soylak, J. Food Compos. Anal., 2024, 128, 105997 Search PubMed.
- Z. Y. Zhang, F. S. Zhang and T. Q. Yao, Waste Manage., 2017, 68, 490–497 CrossRef CAS PubMed.
- Y. An, X. Lv, W. Jiang, L. Wang, Y. Shi, X. Hang and H. Pang, Green Chem. Eng., 2024, 5, 187–204 Search PubMed.
- L. Feng, K. Y. Wang, G. S. Day, M. R. Ryder and H. C. Zhou, Chem. Rev., 2020, 120, 13087–13133 CrossRef CAS PubMed.
- B. Farasati Far, N. Rabiee and S. Iravani, RSC Adv., 2023, 13, 34562–34575 Search PubMed.
- M. Zhang, K. Yang, J. Cui, H. Yu, Y. Wang, W. Shan, Z. Lou and Y. Xiong, Chem. Eng. J., 2020, 386, 124023 Search PubMed.
- B. Wang, J. Ke and J. Zhang, J. Mater. Chem. A, 2024, 12, 15071–15081 Search PubMed.
- F. Ahmadijokani, R. Mohammadkhani, S. Ahmadipouya, A. Shokrgozar, M. Rezakazemi, H. Molavi, T. M. Aminabhavi and M. Arjmand, Chem. Eng. J., 2020, 399, 125346 CrossRef CAS.
- R. Ediati, L. L. Zulfa, I. Maulidah, D. O. Sulistiono, H. Fansuri, A. Rosyidah, F. Martak, D. Hartanto, M. A. B. Abdullah, W. P. Utomo, E. N. Kusumawati and M. Shirai, S. Afr. J. Chem. Eng., 2023, 46, 132–142 Search PubMed.
- L. Li, X. Lv, L. Jin, K. Du, J. Jiang, X. Zhao, H. Liang, Y. Guo and X. Wang, Appl. Catal., B, 2024, 344, 123586 CrossRef CAS.
- S. Navalón, A. Dhakshinamoorthy, M. Álvaro, B. Ferrer and H. García, Chem. Rev., 2023, 123, 445–490 CrossRef PubMed.
- D. Yue, P. Rosaiah, S. V. P. Vattikuti, H. P. K. Sudhani, J. Shim, L. T. Huynh and N. Nguyen Dang, Scr. Mater., 2024, 252, 116266 Search PubMed.
- K. Wang, J. Gu and N. Yin, Ind. Eng. Chem. Res., 2017, 56, 1880–1887 Search PubMed.
- H. Cai, M. Rong, Q. Meng, Z. Liu, Y. Zhao, C. Chen and L. Yang, Sep. Purif. Technol., 2024, 331, 125612 CrossRef CAS.
- M. Sedighi, M. J. Azarhoosh, H. Alamgholiloo and N. N. Pesyan, Process Saf. Environ. Prot., 2024, 190, 1481–1493 Search PubMed.
- A. Demessence, D. M. D'Alessandro, M. L. Foo and J. R. Long, J. Am. Chem. Soc., 2009, 131, 8784–8786 CrossRef CAS PubMed.
- R. Oktavian, R. Goeminne, L. T. Glasby, P. Song, R. Huynh, O. T. Qazvini, O. Ghaffari-Nik, N. Masoumifard, J. L. Cordiner, P. Hovington, V. Van Speybroeck and P. Z. Moghadam, Nat. Commun., 2024, 15, 1–10 Search PubMed.
- P. O. Oladoye, S. A. Adegboyega, A.-R. A. Giwa, P. O. Oladoye, S. A. Adegboyega and A.-R. A. Giwa, Environ. Nanotechnol., Monit. Manage., 2021, 16, 100568 Search PubMed.
- K. Ma, Y. H. Cheung, K. O. Kirlikovali, H. Xie, K. B. Idrees, X. Wang, T. Islamoglu, J. H. Xin and O. K. Farha, Adv. Mater., 2024, 36(10), 2300951 Search PubMed.
- A. Nikseresht, A. Daniyali, M. Ali-Mohammadi, A. Afzalinia and A. Mirzaie, Ultrason. Sonochem., 2017, 37, 203–207 Search PubMed.
- M. Zeyadi, Y. Q. Almulaiky, M. Zeyadi and Y. Q. Almulaiky, BioCB, 2024, 14, 25823–25835 CAS.
- S. Modak, M. Kasula and M. R. Esfahani, ACS Appl. Eng. Mater., 2023, 1, 744–755 Search PubMed.
- M. Al Sharabati, R. Sabouni and G. A. Husseini, Nanomaterials, 2022, 12(2), 277 CrossRef CAS PubMed.
- Q. Zhang, S. Yan, X. Yan and Y. Lv, Sci Total Environ, 2023, 902, 165944 CrossRef CAS PubMed.
- H. U. Escobar-Hernandez, Y. Quan, M. I. Papadaki and Q. Wang, ACS Sustainable Chem. Eng., 2023, 11, 4219–4225 CrossRef CAS.
- S. Chakraborty, B. Ibrahim, P. Dhumal, N. Langford, L. Garbett and E. Valsami-Jones, J. Hazard. Mater. Lett., 2024, 5, 100127 Search PubMed.
- F. R. Cassee, E. A. J. Bleeker, C. Durand, T. Exner, A. Falk, S. Friedrichs, E. Heunisch, M. Himly, S. Hofer, N. Hofstätter, D. Hristozov, P. Nymark, A. Pohl, L. G. Soeteman-Hernández, B. Suarez-Merino, E. Valsami-Jones and M. Groenewold, Comput. Struct. Biotechnol. J., 2024, 25, 105–126 Search PubMed.
- Y. Ye, Y. Zhao, Y. Sun and J. Cao, Int. J. Nanomed., 2022, 17, 2367 Search PubMed.
- A. Wagner, Q. Liu, O. L. Rose, A. Eden, A. Vijay, Y. Rojanasakul and C. Z. Dinu, Int. J. Nanomed., 2019, 14, 7583–7591 Search PubMed.
- Y. C. Chen, K. Y. A. Lin, Y. C. Chen, Y. Y. Hong, Y. F. Hsu and C. H. Lin, J. Hazard. Mater., 2024, 479, 135536 Search PubMed.
- S. Chakraborty, D. Menon, I. Mikulska, C. Pfrang, D. Fairen-Jimenez, S. K. Misra and I. Lynch, Nat. Rev. Mater., 2025, 2025, 1–3 Search PubMed.
- R. V. Pinto, C. C. Cao, P. Lyu, I. Dovgaliuk, W. Shepard, E. Rivière, C. Y. Su, G. Maurin, F. Antunes, J. Pires, V. André, C. Henriques, A. Tissot, M. L. Pinto and C. Serre, Small, 2024, 20(48), 2405649 CrossRef CAS PubMed.
- C. A. Grande, R. Blom, A. Spjelkavik, V. Moreau and J. Payet, Sustainable Mater. Technol., 2017, 14, 11–18 CrossRef CAS.
- V. Ntouros, I. Kousis, D. Papadaki, A. L. Pisello and M. N. Assimakopoulos, Energies, 2021, 14, 4998 Search PubMed.
- D. M. Maklavany, Z. Rouzitalab, M. Bazmi, M. Askarieh and A. Nabavi-Pelesaraei, ACS Sustainable Chem. Eng., 2023, 11, 9816–9832 CrossRef CAS.
- H. Chu, Z. Liu, C. C. Wang and P. Wang, Chem. Commun., 2024, 60, 8350–8359 RSC.
- H. L. B. Boström, S. Emmerling, F. Heck, C. Koschnick, A. J. Jones, M. J. Cliffe, R. Al Natour, M. Bonneau, V. Guillerm, O. Shekhah, M. Eddaoudi, J. Lopez-Cabrelles, S. Furukawa, M. Romero-Angel, C. Martí-Gastaldo, M. Yan, A. J. Morris, I. Romero-Muñiz, Y. Xiong, A. E. Platero-Prats, J. Roth, W. L. Queen, K. S. Mertin, D. E. Schier, N. R. Champness, H. H. M. Yeung and B. V. Lotsch, Adv. Mater., 2024, 36(15), 2304832 CrossRef PubMed.
- J. Ren, X. Dyosiba, N. M. Musyoka, H. W. Langmi, M. Mathe and S. Liao, Coord. Chem. Rev., 2017, 352, 187–219 CrossRef CAS.
- T. Paul, A. Juma, R. Alqerem, G. Karanikolos, H. A. Arafat and L. F. Dumée, J. Environ. Chem. Eng., 2023, 11, 111112 Search PubMed.
- M. I. Severino, E. Gkaniatsou, F. Nouar, M. L. Pinto and C. Serre, Faraday Discuss., 2021, 231, 326–341 RSC.
- F. Xie and J. Li, ACS Mater. Lett., 2024, 6, 2400–2408 CrossRef CAS.
- B. E. Keshta, H. Yu, L. Wang and A. H. Gemeay, Sep. Purif. Technol., 2024, 332, 125744 Search PubMed.
- N. Faaizatunnisa, R. Ediati, H. Fansuri, H. Juwono, S. Suprapto, A. R. P. Hidayat and L. L. Zulfa, Nano-Struct. Nano-Objects, 2023, 34, 100968 Search PubMed.
- R. Kaur, A. Kaur, A. Umar, W. A. Anderson and S. K. Kansal, Mater. Res. Bull., 2019, 109, 124–133 Search PubMed.
- C. Petit, Curr. Opin. Chem. Eng., 2018, 20, 132–142 Search PubMed.
- G. Li, Y. Zhang, X. Hu, W. Tan, J. Li, D. Su, H. Wang and M. Yang, Chemosphere, 2023, 338, 139399 Search PubMed.
- P. S. Abhari, F. Manteghi and Z. Tehrani, Nanomaterials, 2020, 10, 1647 Search PubMed.
- Y. Wang, M. Li, J. Hu, W. Feng, J. Li and Z. You, Colloids Surf., A, 2022, 633, 127852 CrossRef CAS.
- H. E. Ahmed, A. E. Rashed, M. E. El-Khouly, M. K. Albolkany and A. A. El-Moneim, J. Environ. Chem. Eng., 2023, 11, 111071 CrossRef CAS.
- J. Li, G. Lin, Z. Zhong, Z. Wang, S. Wang, L. Fu and T. Hu, Int. J. Biol. Macromol., 2024, 258, 129170 CrossRef CAS PubMed.
- K. Boukayouht, L. Bazzi, A. Daouli, G. Maurin and S. El Hankari, ACS Appl. Mater. Interfaces, 2024, 16, 2497–2508 CrossRef CAS PubMed.
- Z. Zhou, X. Liu, J. G. Ma and P. Cheng, ChemSusChem, 2022, 15(19), e202201386 CrossRef CAS PubMed.
- A. Radwan, I. M. El-Sewify, A. Shahat, H. M. E. Azzazy, M. M. H. Khalil and M. F. El-Shahat, ACS Sustainable Chem. Eng., 2020, 8, 15097–15107 CrossRef CAS.
- J. Tang, G. Tang, J. Niu, J. Yang, Z. Zhou, Y. Gao, X. Chen, Y. Tian, Y. Li, J. Li and Y. Cao, J. Agric. Food Chem., 2021, 69, 2382–2391 CrossRef CAS PubMed.
- P. Li, Y. Deng, W. Zou, Z. Ma, X. Yang and Q. Zhao, Food Hydrocolloids, 2025, 160, 110721 CrossRef CAS.
- S. Song, M. Wan, W. Feng, Y. Tian, X. Jiang, Y. Luo and J. Shen, Langmuir, 2022, 38, 10867–10874 CrossRef CAS PubMed.
- H. Singh, S. Raj and J. Bhattacharya, J. Water Process Eng., 2023, 56, 104381 CrossRef.
- O. Semyonov, S. Chaemchuen, A. Ivanov, F. Verpoort, Z. Kolska, M. Syrtanov, V. Svorcik, M. S. Yusubov, O. Lyutakov, O. Guselnikova and P. S. Postnikov, Appl. Mater. Today, 2021, 22, 100910 CrossRef.
- V. Jabbari, J. M. Veleta, M. Zarei-Chaleshtori, J. Gardea-Torresdey and D. Villagrán, Chem. Eng. J., 2016, 304, 774–783 CrossRef CAS.
- I. Jahan, T. H. Rupam, M. L. Palash, K. A. Rocky and B. B. Saha, J. Mol. Liq., 2022, 345, 117760 Search PubMed.
- A. Ghosh and G. Das, Microporous Mesoporous Mater., 2020, 297, 110039 CrossRef CAS.
- A. Mohammadi, E. Jafarpour, K. Mirzaei, A. Shojaei, P. Jafarpour, M. Beikmohammadi Eyni, S. Mirzaei and H. Molavi, ACS Appl. Mater. Interfaces, 2024, 16, 3862–3875 Search PubMed.
- S. V. Kamath, J. R. Attokkaran, A. S. Maraddi, A. Samage, G. B. D'Souza, H. Yoon and S. K. Nataraj, Chem. Eng. J., 2024, 479, 147805 CrossRef CAS.
- M. X. Wu and Y. W. Yang, Adv. Mater., 2017, 29(23), 1606134 Search PubMed.
- A. Bigham, N. Islami, A. Khosravi, A. Zarepour, S. Iravani and A. Zarrabi, Small, 2024, 20(30), 2311903 CrossRef CAS PubMed.
- M. Shyngys, J. Ren, X. Liang, J. Miao, A. Blocki and S. Beyer, Front. Bioeng. Biotechnol., 2021, 9, 603608 Search PubMed.
- P. Wiśniewska, J. Haponiuk, M. R. Saeb, N. Rabiee and S. A. Bencherif, Chem. Eng. J., 2023, 471, 144400 CrossRef PubMed.
- P. Bhakat, A. Nigam and S. Jagtap, Nanotechnol. Environ. Eng., 2023, 8, 815–827 CrossRef CAS.
- J. Chen, K. Shen and Y. Li, ChemSusChem, 2017, 10, 3165–3187 CrossRef CAS PubMed.
- A. E. Angkawijaya, V. Bundjaja, S. P. Santoso, A. W. Go, S. P. Lin, K. C. Cheng, F. E. Soetaredjo and S. Ismadji, Biomater. Adv., 2023, 146, 213269 Search PubMed.
- A. Bieniek, A. P. Terzyk, M. Wiśniewski, K. Roszek, P. Kowalczyk, L. Sarkisov, S. Keskin and K. Kaneko, Prog. Mater. Sci., 2021, 117, 100743 Search PubMed.
- M. Moharramnejad, A. Ehsani, M. Shahi, S. Gharanli, H. Saremi, R. E. Malekshah, Z. S. Basmenj, S. Salmani and M. Mohammadi, J. Drug Delivery Sci. Technol., 2023, 81, 104285 Search PubMed.
- M. Babaei, A. Abrishami, S. Iranpour, A. S. Saljooghi and M. M. Matin, Drug Delivery Transl. Res., 2024, 120 Search PubMed.
- S. D. Perera, R. M. Johnson, R. Pawle, J. Elliott, T. M. Tran, J. Gonzalez, J. Huffstetler, L. C. Ayers, V. Ganesh, M. C. Senarathna, K. P. Cortés-Guzmán, S. Dube, S. Springfield, L. F. Hancock, B. R. Lund and R. A. Smaldone, ACS Appl. Mater. Interfaces, 2024, 16, 10795–10804 CrossRef CAS PubMed.
- X. Zhang, Y. Wang, J. Liu, J. Shi, D. Mao, A. C. Midgley, X. Leng, D. Kong, Z. Wang, B. Liu and S. Wang, Chem. Eng. J., 2021, 421, 129577 CrossRef CAS.
- D. Ma, G. Wang, J. Lu, X. Zeng, Y. Cheng, Z. Zhang, N. Lin and Q. Chen, Eur. J. Med. Chem., 2023, 261, 115884 CrossRef CAS PubMed.
- S. Tehrani Nejad, R. Rahimi, M. Najafi and S. Rostamnia, ACS Appl. Mater. Interfaces, 2024, 16, 3162–3170 Search PubMed.
- H. Wu, P. Ran, L. Yao, H. Cai, W. Cao, Y. Cui, Y. Michael Yang, D. Yang and G. Qian, Chem. Eng. J., 2024, 491, 152098 CrossRef CAS.
- C. Li, L. Bao, Y. Ji, Z. Tian, M. Cui, Y. Shi, Z. Zhao and X. Wang, Coord. Chem. Rev., 2024, 514, 215888 CrossRef CAS.
- M. S. Khan, Y. Li, D. S. Li, J. Qiu, X. Xu and H. Y. Yang, Nanoscale Adv., 2023, 5, 6318–6348 RSC.
- H. Karimi-Maleh, M. Ghalkhani, Z. Saberi Dehkordi, M. Mohsenpour Tehran, J. Singh, Y. Wen, M. Baghayeri, J. Rouhi, L. Fu and S. Rajendran, J. Ind. Eng. Chem., 2024, 129, 105–123 CrossRef CAS.
- X. Wang, T. Ma, J. G. Ma and P. Cheng, Coord. Chem. Rev., 2024, 518, 216067 CrossRef CAS.
- R. Du, R. Xin, H. Wang, W. Zhu, R. Li and W. Liu, Chem. Eng. J., 2024, 490, 151828 CrossRef CAS.
- K. Saini, J. Singh, S. Malik, Y. Saharan, R. Goyat, A. Umar, S. Akbar, A. A. Ibrahim and S. Baskoutas, J. Mol. Liq., 2024, 399, 124365 CrossRef CAS.
- D. Menon and D. Fairen-Jimenez, Matter, 2025, 101958 CrossRef CAS.
- S. Shekhar and C. Chowdhury, Mater. Adv., 2024, 5, 820–830 RSC.
- Z. Cui, Y. Li, O. V. Tsyusko, J. Wang, J. M. Unrine, G. Wei and C. Chen, J. Agric. Food Chem., 2024, 72(16), 8890–8905 Search PubMed.
- E. S. M. El-Sayed and D. Yuan, Green Chem., 2020, 22, 4082–4104 RSC.
- M. H. D. Dang, T. T. T. Nguyen, B. Q. G. Le, L. H. T. Nguyen, N. X. D. Mai, M. Van Nguyen, P. H. Tran and T. L. H. Doan, J. Ind. Eng. Chem., 2022, 111, 111–120 CrossRef CAS.
- J. L. Woodliffe, R. S. Ferrari, I. Ahmed and A. Laybourn, Coord. Chem. Rev., 2021, 428, 213578 CrossRef CAS.
- J. Klinowski, F. A. Almeida Paz, P. Silva and J. Rocha, Dalton Trans., 2010, 40, 321–330 Search PubMed.
- Z. Bano, M. Akram, N. Z. Ali, M. U. Khan, F. Wang, L. Li and M. Xia, J. Water Process Eng., 2024, 59, 104982 CrossRef.
- C. Yang, Y. Zhu, J. Chen, T. Wu, J. Wang, X. Zhao, W. Sun, H. Lin and S. Lv, Chem. Eng. J., 2022, 431, 133443 CrossRef CAS.
- L. Zhong, L. He, N. Wang, Y. Chen, X. Xie, B. Sun, J. Qian, S. Komarneni and W. Hu, Appl. Catal., B, 2023, 325, 122343 CrossRef CAS.
- H. Chu, Z. Liu, C. C. Wang and P. Wang, Chem. Commun., 2024, 60, 8350–8359 RSC.
- P. Dubey, V. Shrivastav, S. Sundriyal and P. H. Maheshwari, ACS Appl. Nano Mater., 2024, 7, 18554–18565 CrossRef CAS.
- M. Qu, H. Yu, Y. He, W. Xu, D. Liu and F. Cheng, Chem. Eng. J., 2024, 486, 150266 CrossRef CAS.
- S. Singh, B. U., T. S. S. Kumar Naik, S. K. Behera, N. A. Khan, J. Singh, L. Singh and P. C. Ramamurthy, Environ. Res., 2023, 216, 114750 CrossRef CAS PubMed.
- V. Hegde, U. T. Uthappa, M. Suneetha, T. Altalhi, S. Soo Han and M. D. Kurkuri, Chem. Eng. J., 2023, 461, 142103 CrossRef CAS.
- R. M. Rego, G. Sriram, K. V. Ajeya, H. Y. Jung, M. D. Kurkuri and M. Kigga, J. Hazard. Mater., 2021, 416, 125941 CrossRef CAS PubMed.
- X. Shi, W. Liang, G. Liu, B. Chen, L. Shao, Y. Wu, Z. Sun and F. García, Chem. Eng. J., 2023, 462, 142271 CrossRef CAS.
- K. Ge, H. Shao, E. Raymundo-Piñero, P. L. Taberna and P. Simon, Nat. Commun., 2024, 15, 1–10 Search PubMed.
- I. A. Shah, M. Bilal, I. W. Almanassra and I. Ihsanullah, Sep. Purif. Technol., 2024, 330, 125277 CrossRef CAS.
- S. Singh, A. G. Anil, B. Uppara, S. K. Behera, B. Nath, N. Pavithra, S. Bhati, J. Singh, N. A. Khan and P. C. Ramamurthy, npj Clean Water, 2024, 7, 1–13 Search PubMed.
- Y. N. Gong, X. Guan and H. L. Jiang, Coord. Chem. Rev., 2023, 475, 214889 CrossRef CAS.
- M. Tong, Y. Lan, Q. Yang and C. Zhong, Chem. Eng. Sci., 2017, 168, 456–464 CAS.
- V. D. da Silva, K. Zalewska, Z. Petrovski, C. D. Buarque, L. C. Branco and P. M. Esteves, Mater. Today Sustainability, 2023, 21, 100279 Search PubMed.
- S. G. Akpe, I. Ahmed, P. Puthiaraj, K. Yu and W. S. Ahn, Microporous Mesoporous Mater., 2020, 296, 109979 CAS.
- G. Cheng, X. Li, X. Li, J. Chen, Y. Liu, G. Zhao and G. Zhu, J. Hazard. Mater., 2022, 423, 127087 CrossRef CAS PubMed.
- X. Wu, T. Feng, X. Zhu, D. Dong, Q. Gao, S. Huang, R. Huang, D. Wang, H. Xiong, Z. Wei, Y. Chen and J. Liang, Chem. Eng. J., 2024, 496, 154179 CrossRef CAS.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902–907 CrossRef CAS PubMed.
- M. Safarkhani, B. F. Far, Y. S. Huh and N. Rabiee, ACS Biomater. Sci. Eng., 2023, 9, 6516–6530 CrossRef CAS PubMed.
- Y. I. Soltan, M. S. Nasser, F. Almomani, K. A. Mahmoud and S. A. Onaizi, J. Mater. Res. Technol., 2024, 31, 2723–2761 CrossRef CAS.
- X. Wang, A. Dong, Y. Hu, J. Qian and S. Huang, Chem. Commun., 2020, 56, 10809–10823 RSC.
- S. Xu, A. Dong, Y. Hu, Z. Yang, S. Huang and J. Qian, J. Mater. Chem. A, 2023, 11, 9721–9747 RSC.
- Y. Liang, Q. Zhang, S. Li, J. Fei, J. Zhou, S. Shan, Z. Li, H. Li and S. Chen, J. Hazard. Mater., 2022, 423, 127181 CrossRef CAS PubMed.
- A. E. Mathew, S. Jose, A. M. Babu and A. Varghese, Mater. Today Chem., 2024, 36, 101927 CrossRef CAS.
- E. Cho, Y. Jung, J. W. Choi, C. Lee and K. W. Jung, Adv. Funct. Mater., 2024, 34, 2407018 CrossRef CAS.
- Y. Yan, H. Han, Y. Dai, H. Zhu, W. Liu, X. Tang, W. Gan and H. Li, ACS Appl. Nano Mater., 2021, 4, 11763–11769 CrossRef CAS.
- H. Jiang, M. Xu, C. Leng, Q. Ma, J. Dai, S. Feng, N. Wang, J. Wei and L. Wang, Colloids Surf., A, 2024, 683, 133021 CrossRef CAS.
- W. Xiang, Q. Wang, Z. Li, J. Dong, J. Liu, L. Zhang, T. Xia, Y. He and D. Zhao, Sep. Purif. Technol., 2024, 330, 125268 CrossRef CAS.
- K. R. G. Lim, M. Shekhirev, B. C. Wyatt, B. Anasori, Y. Gogotsi and Z. W. Seh, Nat. Synth., 2022, 1, 601–614 CrossRef CAS.
- S. Kumar, N. Kumari, T. Singh and Y. Seo, J. Mater. Chem. C, 2024, 12, 8243–8281 RSC.
- Metal Organic Frameworks Market Size & Forecast [Latest], https://www.marketsandmarkets.com/Market-Reports/metal-organic-frameworks-market-67821376.html, (accessed 26 February 2025).
- J. A. Chávez-Hernández, A. J. Velarde-Salcedo, G. Navarro-Tovar and C. Gonzalez, Nanoscale Adv., 2024, 6, 1583–1610 Search PubMed.
- A. M. Wright, M. T. Kapelewski, S. Marx, O. K. Farha and W. Morris, Nat. Mater., 2024, 1–10 Search PubMed.
- A spotter's guide to greenwashing - and what to do about it | World Economic Forum, https://www.weforum.org/stories/2021/05/how-spot-greenwashing/, (accessed 26 February 2025).
- D. Menon and S. Chakraborty, Front. Toxicol., 2023, 5, 1233854 CrossRef PubMed.
- P. Goyal, P. Soppina, S. K. Misra, E. Valsami-Jones, V. Soppina and S. Chakraborty, Front. Toxicol., 2022, 4, 917749 CrossRef PubMed.
- L. Yang, H. Chen, A. E. Kaziem, X. Miao, S. Huang, D. Cheng, H. H. Xu and Z. Zhang, ACS Nano, 2024, 18(37), 25425–25445 CrossRef CAS PubMed.
- C. Yang, J. Wen, Z. Xue, X. Yin, Y. Li and L. Yuan, J. Environ. Sci., 2023, 127, 91–101 CrossRef CAS PubMed.
- Y. Xiang, S. Wei, T. Wang, H. Li, Y. Luo, B. Shao, N. Wu, Y. Su, L. Jiang and J. Huang, Coord. Chem. Rev., 2025, 523, 216263 CrossRef CAS.
- J. W. Bridges, H. Greim, K. van Leeuwen, R. Stegmann, T. Vermeire and K. den Haan, Regul. Toxicol. Pharmacol., 2023, 139, 105356 CrossRef CAS PubMed.
- A. Mech, S. Gottardo, V. Amenta, A. Amodio, S. Belz, S. Bøwadt, J. Drbohlavová, L. Farcal, P. Jantunen, A. Małyska, K. Rasmussen, J. Riego Sintes and H. Rauscher, Regul. Toxicol. Pharmacol., 2022, 128, 105093 CrossRef PubMed.
- S. A. E. Naser, K. O. Badmus and L. Khotseng, Coatings, 2023, 13, 1456 CrossRef CAS.
- A. Ruyra, A. Yazdi, J. Espín, A. Carné-Sánchez, N. Roher, J. Lorenzo, I. Imaz and D. Maspoch, Chemistry, 2015, 21, 2508–2518 CrossRef CAS PubMed.
- Y. Li, S. Shang, J. Shang and W. X. Wang, Environ. Pollut., 2021, 291, 118199 CrossRef CAS PubMed.
- D. Wang, L. Bai, X. Huang, W. Yan and S. Li, Sci. Total Environ., 2022, 851, 158317 CrossRef CAS PubMed.
- P. Chen, M. He, B. Chen and B. Hu, Ecotoxicol. Environ. Saf., 2020, 205, 111110 CrossRef CAS PubMed.
- Z. Zhu, S. Jiang, Y. Liu, X. Gao, S. Hu, X. Zhang, C. Huang, Q. Wan, J. Wang and X. Pei, Nano Res., 2020, 13, 511–526 CrossRef CAS.
Footnote |
| † Authors with equal contribution. |
| This journal is © The Royal Society of Chemistry 2025 |



