Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A pinch of silver salt to enable rac-lactide ring-opening polymerisation at room temperature using Ti-salen complexes†
Justin Koh ,
Chloe A. Baker,
Marianna N. Diamantakis
,
Chloe A. Baker,
Marianna N. Diamantakis ,
Nicholas J. Long
,
Nicholas J. Long * and
Charles Romain
* and
Charles Romain *
*
Department of Chemistry, Imperial College London, MSRH, W12 0BZ, London, UK. E-mail: n.long@imperial.ac.uk; c.romain@imperial.ac.uk
First published on 26th February 2025
Abstract
Exploiting non-covalent interactions (NCIs) has become a powerful tool in catalyst design, including those for polymerisation reactions. Herein we report a simple strategy relying on the addition of silver salt to Ti-salen complexes, leading to Ti/Ag cooperativity via cation-π interactions. Three different Ti-salen complexes were investigated alongside several silver salts. In all cases, these Ti/Ag systems led to good or high activity at room temperature for rac-lactide ROP, a rare feature for Ti-based catalysts. Interestingly, the weakly coordinating anion (WCA) of the silver salt was found not only to affect activity but also stereocontrol in the polymerisation. Heterotactic PLA with Pr up to 0.7 was obtained.
Introduction
Exploiting non-covalent interactions (NCIs) has become a powerful tool in designing catalysts for various organic reactions. Different attractive interactions (e.g. hydrogen bonds, π interactions) and strategies (e.g. inter-ligand, substrate-ligand) have been investigated to control the formation of “supramolecular” catalytic species.1 Such approaches exploiting NCIs have also been applied in polymerisation catalysis, especially for olefin polymerisation using late-transition metal catalysts.2 For example, CArF⋯H interactions between fluorinated ligands and polymeric chains have been demonstrated to reduce β-H elimination and to lead to high molar mass polymers.3 Regarding Lewis acid metal-catalysed polymerisations such as ring-opening polymerisation (ROP) and ring-opening copolymerisation (ROCOP), similar strategies have been investigated but remain underexplored compared to organo-catalysed polymerisations where hydrogen bonding is ubiquitous.4In epoxide/CO2 ROCOP, ionic interactions between the carbonate chain-end of the polymeric chain and cationic species (either exogeneous or ligand-tagged) were found to enhance selectivity towards the formation of carbonate linkages (leading to the development of bifunctional catalysts).5 In ROP, since the pioneering work of Rzepa and co-workers drawing attention to the role of weak attractive C–H⋯π interactions in lactide polymerisation,6 few other studies have highlighted the role of NCIs in metal-catalysed ROP. Comprehensive investigations from Carpentier, Guillaume and co-workers demonstrated that CH⋯π or CH⋯Cl interactions between the ligand and growing polymeric chains were responsible for the stereoselective polymerisation of some functionalised β-lactones using yttrium-bisphenolate complexes.7 Other strategies relying on hydrogen bonding from the NH moieties in reduced Schiff base ligands and amino-phenolate ligands have been proposed to explain high activity or selectivity both in ROP and ROCOP.8 Along these lines, some of us highlighted the role of hydrogen bonds in the catalytic pocket of catam aluminium complexes to orientate and activate monomers.9 More recently, our group exploited cation-π interactions to form in-situ highly active heterobimetallic Ag–Ti species for lactide ROP,10 thus taking advantage of metal cooperativity in lactone ROP.11 The addition of silver tetrakis[3,5-bis(trifluoromethyl)phenyl]borate [Ag][B(ArCF3)4] to a simple Ti-salen complex was found to enable fast and controlled lactide polymerisation at room temperature. Under similar conditions, both components were independently inactive and only the combination of both “switched on” the polymerisation.10 This was attributed to cation-π interactions positioning the silver cation near the Ti-metal centre which allowed for monomer activation and stabilisation of key intermediates during the polymerisation. Similarly, Waymouth and co-workers recently reported the key role of counter-ions in anionic ROP mediated by organocatalysts were two alkali–metal cations where differently positioned through cation-π interactions and showed contrasting roles.12 Herein we report a series of bicomponent catalytic systems for rac-lactide ROP comprising different Ti-salen complexes and various silver salts containing weakly coordinating anions (WCA). Among others, the effect of the WCA on polymerisation activity and stereoselectivity was investigated. Following condition optimisations, the Ti/Ag cooperativity led to a highly active system at room temperature, enabling access to heterotatic PLA with Pr up to 0.7.13
Results and discussion
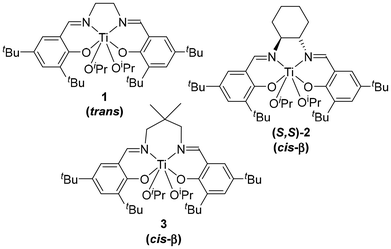
Bis(iso-propoxide) Ti-salen complexes 1–3 with various Schiff-base ligands (and thus different geometry and flexibility) were synthesised (Fig. 1) via standard procedures using the corresponding ligand precursor and Ti(OiPr)4.14,15 Complex 2 was prepared using a racemic mixture of the trans ligand precursor, whereas the complex referred to as (S,S)-2 was prepared using the (S,S)-trans ligand precursor as depicted in Fig. 1. All complexes were obtained in moderate to good yields (∼50%–70%). 1H NMR spectroscopy analyses in C6D6 confirmed that 1 adopts a trans configuration whereas 2 and 3 adopt a cis-β configuration in solution, consistent with previous reports.14,15 Indeed, the 1H NMR spectrum of 1 is in line with a C2-symmetric structure in C6D6 with only two tBu signals, two aromatic proton signals, one imine signal and one isopropyl [i.e. heptet for CH(CH3)] signal. On the contrary, the 1H NMR spectra of 2 and 3 show four tBu signals, four aromatic proton signals, two imine signals and two isopropyl signals [i.e. heptet for CH(CH3)] (see ESI†).In order to develop a straightforward method to gauge the potential cooperativity between silver salts and Ti-salen complexes for rac-LA ROP, we turned our attention toward silver salts [Ag][X] which: (i) were soluble in DCM at room temperature, as is rac-LA, (ii) have a stable or inert counter-anion [X]−; (iii) provide a source of a “naked” silver cation by possessing a weakly coordinating anion (WCA), and (iv) are ideally commercially available. Thus, the following silver salts [Ag][X] were found to be suitable candidates, with [X]− = [OTf]− (triflate),‡ [SbF6]− (hexafluoroantimonate), [B(ArCF3)4]− (tetrakis[3,5-bis(trifluoromethyl)phenyl]borate), and [Al(ORF)4]− (tetrakis[perfluoro tert-butoxy]aluminate, i.e. RF = C(CF3)3).
The combination of 1 with any of the selected silver salts (Table 1) led to efficient rac-LA ROP at room temperature to afford atactic PLA with controlled molar masses and low dispersity (∼1.2), in line with a controlled living polymerisation. For example, about 87% of rac-LA was converted in 20 minutes at room temperature in DCM (Table 1, entry 5) whereas in the absence of the silver salt, only 73% conversion was reached in 24 h at 90 °C in toluene (Fig. 2). Plots of ln([rac-LA]0/[rac-LA]t) against time show linear trends as generally observed for such reactions.10,14
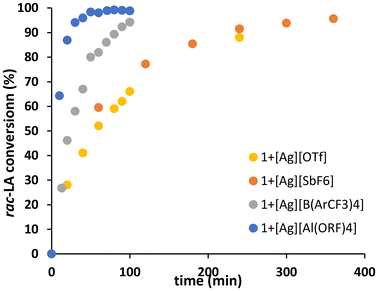 | ||
| Fig. 2 rac-LA conversion versus time for polymerisation initiated by 1 in the presence of different silver salts AgX (conditions: [rac-LA]0 = 1 M, DCM, [1]/[AgX]/[rac-LA]0 = 1/1/100). | ||
| Entry | Cat./[Ag][X]/rac-LA (equiv.) | [X]− | Time (min) | Conversiona (%) | Mn (Đ)b (kg mol−1) | Mn (calc.)c (kg mol−1) |
|---|---|---|---|---|---|---|
| Reaction conditions: cat. = 1, [rac-LA]0 = 1 M, DCM, 25 °C.a Calculated using 1H NMR spectroscopy.b Calculated using SEC calibrated with polystyrene standards and corrected by 0.58.c Calculated considering, two chains per Ti-salen complex (one chain per isopropoxide initiating).d Calculated considering one chain per Ti-salen complex (only one isopropoxide initiating).e Not isolated due to low conversion.f Reaction at 90 °C in toluene. | ||||||
| 1 | 1/1/100 | [OTf]− | 240 | 88.0 | 11.5 (1.2) | 12.7d |
| 2 | 1/1/100 | [SbF6]− | 180 | 56.4 | 3.2 (1.2) | 4.1 |
| 3 | 1/1/100 | [B(ArCF3)4]− | 60 | 81.9 | 6.2 (1.2) | 5.9 |
| 4 | 1/1/100 | [B(ArCF3)4]−·CH3CN | 80 | 7.3 | —e | 0.5 |
| 5 | 1/1/100 | [Al(ORF)4]− | 20 | 86.9 | 6.5 (1.3) | 6.3 |
| 610 | 1/0/100 | — | 240 | 0 | — | — |
| 714f | 1/0/100 | — | 1440 | 73 | 6.6 (1.3) | 5.3 |
| 8 | 0/1/100 | [B(ArCF3)4]− | 180 | 0 | — | — |
| 9 | 0/1/100 | [Al(ORF)4]− | 180 | 0 | — | — |
| 10 | 0/1/100 | [SbF6]− | 180 | 0 | — | — |
Reactions rates were calculated assuming a first order in monomer and using a temporal least squares fit (see ESI†). The molar masses increase linearly versus rac-LA conversion and the polymers feature relatively low dispersity, consistent with a controlled living polymerisation. In all cases (except Table 1, entry 1), the molar masses are in accordance with each isopropoxide group being able to initiate one chain, as confirmed by MALDI-ToF analysis showing OiPr terminated PLA and no evidence of transesterification as peaks are spaced by 144 (see ESI†). In the case of AgOTf as a co-catalyst, only one chain is initiated by the catalyst (only one isopropoxide). This could be attributed to the OTf anion being the most coordinating anion of the series, and thus hampering initiation of the second chain by the other isopropoxide group due to potential interaction between the OTf anion and the OiPr group. It should be noted that in the absence of any silver salt, no activity is observed in the same conditions (i.e. room temperature), and any of the silver salts alone (i.e. without 1) do not initiate ROP of lactide, even in the presence of one equivalent of alcohol as initiator. These results confirm the cooperativity between 1 and Ag+ for rac-LA ROP at room temperature and demonstrate how the simple addition of a silver salt can significantly increase catalyst activity in mild conditions.
Looking at the effect of the counter anion of the different silver salts (Table 1, entries 1–5), reactions using the most weakly coordinating anion such as [B(ArCF3)4]− and [Al(ORF)4]− showed the highest activities, whereas the most coordinating anions such as [OTf]− and [SbF6]− showed the lowest activities, as per Fig. 3. Interestingly, the acetonitrile adduct of [Ag][B(ArCF3)4] (Table 1, entry 4) displayed low activity as compared to the Et2O adduct (Table 1, entry 3). This was attributed to the strong coordination of CH3CN to the silver cation, and thus hindering the interaction of Ag+ with 1 and the resulting cooperativity.10 Overall, these results show that the counter-anion influences the formation of a suitable supramolecular entity between Ag+ and the Ti-salen complex 1 which in turn impacts the subsequent rac-LA polymerization.
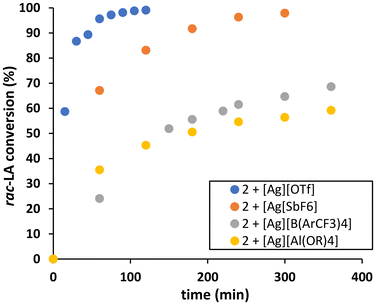 | ||
| Fig. 3 rac-LA conversion versus time for polymerisation initiated by 2 in the presence of different silver salts AgX (conditions: [rac-LA]0 = 1 M, DCM, [2]/[AgX]/[rac-LA]0 = 1/1/100). | ||
In consideration of any potential deactivation of the catalytic system (i.e. 1 in the presence of a silver salt) and to investigate the difference in reactivity between the silver salts, “same excess” experiments were carried out on two catalytic systems (i.e. the most active and least active), i.e. 1 + [Ag][SbF6] and 1 + [Ag][Al(ORF)4], with initial concentration of [rac-LA]0 = 1.5 M and 1.0 M (see details in ESI†).9b,16 In the case of [Ag][Al(ORF)4] as a co-catalyst, the time-shifted curve for the reaction at higher concentration superimposed onto the curve of the reaction at lower concentration with minimal deviation, indicating no deactivation of the catalytic system, in line with [Al(ORF)4] being an inert counter-anion. On the contrary, in the case of [Ag][SbF6] as a co-catalyst, the time-shifted curve for the higher concentration reaction did not overlap with the curve for the reaction at lower concentration showing significantly lower activity, thus suggesting some deactivation. This could be attributed to the SbF6 counter-anion which, while being a WCA, is known to decompose to SbF5 + F− and then can further react with Ti.17 These results highlight the importance of the counter anion, not only being weakly coordinating, but also inert to prevent undesired reactions with the Ti metal centre. MALDI-ToF analysis of complex 1 in the presence of [Ag][SbF6] and [Ag][Al(ORF)4] was performed (Fig. S1 and S2†). In both cases, the adduct corresponding to m/z = [1 + Ag]+ was observed, likely due to cation π interactions. Interestingly, in the case of [Ag][SbF]6, a fluorinated cationic Ti–F salen complex has been observed, in line with potential decomposition of SbF6 providing a source a fluoride.
As [Al(ORF)4]− was confirmed to be a suitable counter-anion (i.e. inert and weakly coordinating), further investigations were carried out to examine the influence of the stoichiometry of the silver salt co-catalyst on the ROP. Thus, rac-LA ROP was found to be successfully initiated by one equivalent of 1 in the presence of half, one and two equivalents of [Ag][Al(ORF)4]. While using one or two equivalents of Ag[Al(ORF)4]− led to comparable activities (86.9% and 92.2% rac-LA conversion in 20 minutes, respectively), using only 0.5 equivalents of [Ag][Al(ORF)4] led to a significantly slower reaction (62% conversion in 60 minutes), as per Fig. S37.† This is in line with the proposed formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 aggregate between 1 and Ag+.10
1 aggregate between 1 and Ag+.10
Having established the best conditions for 1, similar investigations were carried out with Ti-salen complex 2 bearing a more rigid ligand with a cyclohexyl moiety, and complex 3 bearing a more flexible ligand with a dimethyl propyl backbone. Both complexes 2 and 3 were previously reported to afford very slow polymerisation of rac-LA in toluene at 90 °C and 70 °C, respectively (Table 2, entry 6 and 10).14,15 In the presence of any of the aforementioned silver salts, both catalysts 2 and 3 resulted in moderate to very high activity at room temperature for rac-LA ROP, as previously observed with 1. This shows that this strategy could be applied to different Ti-salen complexes adopting either trans configurations (complex 1) or β-cis conformations (complexes 2 and 3), and that for these configurations, the binding of Ag+ via cation-π interactions is possible, as supported by MALDI-ToF analysis (Fig. S3–S6†).
| Entry | Cat. | cat./Ag[X]/rac-LA (equiv.) | [X]− (1 equiv.) | Time (min) | Conversiona (%) | Mn (Đ)b (kg mol−1) | Mn (calc.)c (kg mol−1) | Pr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
|---|---|---|---|---|---|---|---|---|
| Reaction conditions: [rac-LA]0 = 1 M, DCM, 25 °C.a Calculated using 1H NMR spectroscopy.b Calculated using SEC calibrated with polystyrene standards and corrected by 0.58.c Calculated considering two chains per Ti-salen complex (one chain per isopropoxide initiating).d Determined via 1H{1H} decoupled NMR spectrum of isolated PLA.e Calculated considering one chain per Ti-salen complex (only one isopropoxide initiating).f Reaction performed at 70 °C in toluene with [rac-LA]0 = 1 M and [2]/[rac-LA] = 1/100, data from reference.14.g Reaction performed at 100 °C in toluene with [rac-LA]0 = 0.5M and [3]/[rac-LA] = 1/100, data from reference.15 | ||||||||
| 1 | 2 | 1/1/100 | [OTf]− | 30 | 86.7 | 11.2 (1.2) | 12.5e | 0.71 |
| 2 | 2 | 1/1/100 | [SbF6]− | 180 | 91.7 | 6.2 (1.2) | 6.6 | 0.49 |
| 3 | 2 | 1/1/100 | [B(ArCF3)4]− | 150 | 51.9 | 3.4 (1.2) | 3.7 | 0.61 |
| 4 | 2 | 1/1/100 | [Al(ORF)4]− | 180 | 50.6 | 4.3 (1.1) | 3.7 | 0.60 |
| 5 | (S,S)-2 | 1/1/100 | [Al(ORF)4]− | 180 | 83.8 | 5.3 (1.2) | 6.0 | 0.40 |
| 6f | 2 | 1/0/100 | — | 1440 | 57 | 5.3 (1.1) | 8.2 | 0.54 |
| 7 | 3 | 1/1/100 | [OTf]− | 90 | 50.8 | 12.0 (1.4) | 7.3e | 0.71 |
| 8 | 3 | 1/1/100 | [SbF6]− | 20 | 84.8 | 22.4 (1.4) | 6.1 | 0.44 |
| 9 | 3 | 1/1/100 | [Al(ORF)4]− | 5 | 80.3 | 3.6 (1.6) | 5.8 | 0.38 |
| 10g | 3 | 1/0/100 | — | 2880 | 10.9 | 2.7 (1.2) | 1.6e | 0.67 |
All the catalytic systems using 2 show controlled rac-LA ROP to afford polymers with predictable molar masses and low dispersity (Đ ∼ 1.2). For all systems, one polymeric chain is initiated by each isoproproxide (i.e. two chains per complex 2, see Table 2, entries 2–4, and Fig. S83†), except when [Ag][OTf] is used in which only one chain is initiated per Ti-salen complex (Table 2, entry 1), similar to what we observed when using 1 and [Ag][OTf].
Interestingly, the activity trend for catalytic systems using 2 is reciprocal to that for 1. When using 2, catalytic systems with weakly coordinating anions [B(ArCF3)4]− and [Al(ORF)4]− show comparable activities (∼50% rac-LA conversion in 3 hours) and were significantly slower than the most active system using [Ag][OTf] which show 87% conversion in 30 minutes. When using 1, the reverse trend is observed with the [Ag][OTf] system affording 80% conversion in 4 hours as opposed to the system with [Ag][Al(ORF)4] which afforded 80% conversion in 20 minutes. This difference in reactivity could be due to the difference in the trans and β-cis configurations of 1 and 2 respectively. As the β-cis configuration of 2 offers a more open coordination sphere, an Ag+ cation surrounded by a less dissociating counter-anion such as OTf− can thus be accommodated more easily. This is also supported by the kinetic analysis (Fig. S47 and S52†) showing a departure from the assumed first order in monomer when using the [Al(ORF)4]− and [B(ArCF3)4]−, suggesting catalyst saturation with lactide,18 in line with a more open coordination sphere. By extension, this would also suggest that the conformation and the flexibility of the salen ligand would also influence the nature of the supramolecular aggregate formed by the Ti-salen complex and silver salt.
In addition to enabling high activity at room temperature, the use of 2 in the presence of a silver salt as a co-catalyst was found to enable some stereocontrol, affording heterotactic PLA with Pr up to ∼0.7. In the absence of silver salts, elevated temperatures are needed (>70 °C) for ROP and hence, stereocontrol is compromised (Pr ∼ 0.5, Table 2, entry 6). The highest tacticity (Table 2, entry 1) was observed for AgOTf as a co-catalyst, in line with our previous observation that the triflate anion is the least weakly coordinating anion of the investigated series, thus congesting the coordination sphere, with potential interactions involving the OTf−.19 It is worthy to note that whereas zirconium and hafnium complexes were previously reported for stereocontrolled rac-lactide ROP,13c,d,20 their titanium counterparts with the same ligand usually afforded atactic polymers.21 Indeed, Ti complexes usually require higher temperature (usually > 70 °C) to exhibit significant activity for lactide ROP, and thus compromising stereocontrol. These results prompted us to investigate lactide ROP initiated by (S,S)-2 obtained from the corresponding (S,S)-trans ligand precursor (see ESI†). In similar conditions as per 2 (Table 2, entry 5), rac-LA ROP using (S,S)-2 in the presence of [Ag][Al(ORF)4] leads to a slightly isotactic PLA with Pr = 0.4, suggesting an enantiomorphic site control mechanism. This is confirmed by a slight difference in polymerisation rate when using D-lactide and L-lactide, one being the favoured isomer and the other the disfavoured one (Fig. S66, S67 and Table S3†).22 The change from slightly heterotactic PLA (when using 2) to slightly isotactic PLA (when using (S,S)-2) can be attributed to polymeryl chain exchanges which can occur when a racemic mixture of catalysts is used and their dimerization favoured.9b,22b
In the case of 3, the addition of silver salt led to very fast conversion of rac-lactide, in particular using [Ag][Al(ORF)4], with up to 80% conversion in just 5 minutes (Table 2, entry 8). In the absence of silver salts, only 11% conversion was observed in 48 h at 100 °C. However, while high activity was observed, polymerisation control was found to be poorer with significant discrepancy between theoretical and experimental molar masses. Molar masses tended to be higher than expected, suggesting partial or poorly controlled formation of active species. Interestingly, in some cases (Table 2, entries 7 and 8) the polymers show an isotactic bias (Pr ∼ 0.4). Further investigations are ongoing to identify the nature of the active species.
Conclusions
Three bis(iso-propoxide) Ti-salen complexes were investigated for rac-lactide ROP in the presence of different silver salts. In all cases, these Ti/Ag bi-component systems afforded rac-lactide ROP at room temperature, a rather rare feature for Ti-based systems which usually require high temperatures (>70 °C) for significant activity. In the absence of any silver salt, Ti-salen complexes showed no activity at room temperature and only low to moderate activity at higher temperatures (>70 °C). These results highlight the ability of Ti/Ag cooperativity to enable polymerisation under mild conditions.11 As we previously demonstrated with similar systems, this results from cation-π interactions positioning the silver cation near the Ti centre, allowing monomer activation and stabilisation of key intermediates.10 More interestingly, the lower temperature afforded by addition of silver salts, allowed for stereocontrol which was observed with formation of heterotactic polymers with Pr up to 0.7. Finally, we observed that the nature of the counter anion in the silver salts influence both activity and polymer tacticity, depending on the Ti-salen complex. In line with our previous findings, this is likely related to the formation of different supramolecular catalytic species, for which further investigations are ongoing. Overall, this study has highlighted a simple strategy based on Ti/Ag cooperativity using several Ti-salen complexes to enable activity at room temperature for rac-LA ROP along with some stereocontrol. The findings in this work suggest that the same strategy could also be applied to other metal phenolate complexes to enable (stereo-controlled) ROP under mild conditions.Author contributions
Experimental investigations were performed by J. K., C. B. and M. N. D. All authors contributed to the project's conceptualisation. The manuscript was written and edited by all authors. C. R. and N. J. L. supervised the project and acquired the funding.Data availability
The data supporting this article have been included as part of the ESI† and are also available on a data repository at https://doi.org/10.14469/hpc/14821.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. K. thanks the NTU C N Yang Scholarship program and M. N. D. thanks the NERC SSCP DTP for their scholarships. N. J. L. and C. A. B. acknowledge financial support from the Sir Edward Frankland BP Endowment, Imperial College London.References
- (a) J. N. H. Reek, B. de Bruin, S. Pullen, T. J. Mooibroek, A. M. Kluwer and X. Caumes, Chem. Rev., 2022, 122, 12308–12369 Search PubMed; (b) J. Trouvé and R. Gramage-Doria, Chem. Soc. Rev., 2021, 50, 3565–3584 Search PubMed; (c) Y. Lou, J. Wei, M. Li and Y. Zhu, J. Am. Chem. Soc., 2022, 144, 123–129 Search PubMed; (d) M. W. Drover, Chem. Soc. Rev., 2022, 51, 1861–1880 Search PubMed; (e) M. Raynal, P. Ballester, A. Vidal-Ferran and P. W. N. M. van Leeuwen, Chem. Soc. Rev., 2014, 43, 1660–1733 Search PubMed; (f) H. J. Davis and R. J. Phipps, Chem. Sci., 2017, 8, 864–877 Search PubMed.
- H. Zheng and H. Gao, Macromolecules, 2024, 57, 6899–6913 Search PubMed.
- (a) C. Chen, Nat. Rev. Chem., 2018, 2, 6–14 Search PubMed; (b) L. Falivene, L. Cavallo and G. Talarico, Mol. Catal., 2020, 494, 111118 Search PubMed.
- (a) N. E. Kamber, W. Jeong, R. M. Waymouth, R. C. Pratt, B. G. G. Lohmeijer and J. L. Hedrick, Chem. Rev., 2007, 107, 5813–5840 Search PubMed; (b) A. P. Dove, ACS Macro Lett., 2012, 1, 1409–1412 Search PubMed; (c) X. Geng, X. Liu, Q. Yu, C. Zhang and X. Zhang, J. Am. Chem. Soc., 2024, 146, 25852–25859 Search PubMed; (d) J. Xu, X. Wang, J. Liu, X. Feng, Y. Gnanou and N. Hadjichristidis, Prog. Polym. Sci., 2022, 125, 101484 CrossRef.
- (a) G. A. Bhat and D. J. Darensbourg, Green Chem., 2022, 24, 5007–5034 RSC; (b) C. A. L. Lidston, S. M. Severson, B. A. Abel and G. W. Coates, ACS Catal., 2022, 12, 11037–11070 CrossRef.
- E. L. Marshall, V. C. Gibson and H. S. Rzepa, J. Am. Chem. Soc., 2005, 127, 6048–6051 CrossRef PubMed.
- (a) R. Ligny, M. M. Hänninen, S. M. Guillaume and J.-F. Carpentier, Chem. Commun., 2018, 54, 8024–8031 RSC; (b) R. M. Shakaroun, A. Dhaini, R. Ligny, A. Alaaeddine, S. M. Guillaume and J.-F. Carpentier, Polym. Chem., 2023, 14, 720–727 RSC; (c) R. Ligny, M. M. Hänninen, S. M. Guillaume and J.-F. Carpentier, Angew. Chem., Int. Ed., 2017, 56, 10388–10393 CrossRef PubMed; (d) A. Dhaini, R. M. Shakaroun, A. Alaaeddine, J.-F. Carpentier and S. M. Guillaume, Polym. Chem., 2024, 15, 999–1014 RSC; (e) H. Li, R. M. Shakaroun, S. M. Guillaume and J.-F. Carpentier, Chem. – Eur. J., 2020, 26, 128–138 CrossRef PubMed.
- (a) Y. Zhou, Z. Gao, C. Hu, S. Meng, R. Duan, Z. Sun and X. Pang, Macromolecules, 2022, 55, 9951–9959 CrossRef; (b) J. Bruckmoser, S. Pongratz, L. Stieglitz and B. Rieger, J. Am. Chem. Soc., 2023, 145, 11494–11498 CrossRef PubMed; (c) T. Pongpanit, T. Saeteaw, P. Chumsaeng, P. Chasing and K. Phomphrai, Inorg. Chem., 2021, 60, 17114–17122 CrossRef PubMed; (d) K. M. Osten, D. C. Aluthge, B. O. Patrick and P. Mehrkhodavandi, Inorg. Chem., 2014, 53, 9897–9906 CrossRef CAS PubMed; (e) P. McKeown, M. G. Davidson, G. Kociok-Kohn and M. D. Jones, Chem. Commun., 2016, 52, 10431–10434 RSC; (f) B. Zhao, Z. Han and K. Ding, Angew. Chem., Int. Ed., 2013, 52, 4744–4788 CrossRef CAS PubMed.
- (a) S. Gesslbauer, H. Cheek, A. J. P. White and C. Romain, Dalton Trans., 2018, 47, 10410–10414 RSC; (b) S. Gesslbauer, G. Hutchinson, A. J. P. White, J. Burés and C. Romain, ACS Catal., 2021, 11, 4084–4093 Search PubMed; (c) S. Gesslbauer, R. Salvela, Y. Chen, A. J. P. White and C. Romain, ACS Catal., 2019, 9, 7912–7920 CrossRef CAS.
- C. A. Baker, C. Romain and N. J. Long, Chem. Commun., 2021, 57, 12524–12527 RSC.
- (a) W. Gruszka and J. A. Garden, Nat. Commun., 2021, 12, 3252 CrossRef CAS; (b) L.-J. Wu, W. Lee, P. Kumar Ganta, Y.-L. Chang, Y.-C. Chang and H.-Y. Chen, Coord. Chem. Rev., 2023, 475, 214847 CrossRef CAS; (c) W. Gruszka and J. A. Garden, Chem. Commun., 2022, 58, 1609–1612 RSC; (d) U. Yolsal, P. J. Shaw, P. A. Lowy, R. Chambenahalli and J. A. Garden, ACS Catal., 2024, 14, 1050–1074 CrossRef CAS PubMed.
- (a) J. Zhang, K. H. Lui, C. N. Jadrich, Y. Jia, P. L. Arrechea, J. L. Hedrick and R. M. Waymouth, ACS Catal., 2023, 13, 16097–16104 CrossRef; (b) C. N. Jadrich, V. E. Pane, B. Lin, G. O. Jones, J. L. Hedrick, N. H. Park and R. M. Waymouth, J. Am. Chem. Soc., 2022, 144, 8439–8443 CrossRef.
- (a) Y. Jeong, M. Shin, M. Seo and H. Kim, Organometallics, 2022, 41, 328–334 CrossRef; (b) W. Wang, ACS Omega, 2024, 9, 29983–29993 CrossRef PubMed; (c) M. J. Stanford and A. P. Dove, Chem. Soc. Rev., 2010, 39, 486–494 RSC; (d) A. Sauer, A. Kapelski, C. Fliedel, S. Dagorne, M. Kol and J. Okuda, Dalton Trans., 2013, 42, 9007–9023 RSC.
- C. K. Gregson, I. J. Blackmore, V. C. Gibson, N. J. Long, E. L. Marshall and A. J. White, Dalton Trans., 2006, 3134–3140 RSC.
- B. Gao, X. Li, R. L. Duan and X. Pang, New J. Chem., 2015, 39, 2404–2408 RSC.
- C. D. T. Nielsen and J. Burés, Chem. Sci., 2019, 10, 348–353 RSC.
- W. Beck and K. Suenkel, Chem. Rev., 1988, 88, 1405–1421 CrossRef.
- M. O. Miranda, Y. DePorre, H. Vazquez-Lima, M. A. Johnson, D. J. Marell, C. J. Cramer and W. B. Tolman, Inorg. Chem., 2013, 52, 13692–13701 CrossRef PubMed.
- I. M. Riddlestone, A. Kraft, J. Schaefer and I. Krossing, Angew. Chem., Int. Ed., 2018, 57, 13982–14024 CrossRef PubMed.
- (a) A. J. Chmura, D. M. Cousins, M. G. Davidson, M. D. Jones, M. D. Lunn and M. F. Mahon, Dalton Trans., 2008, 1437–1443 RSC; (b) C. Romain, B. Heinrich, S. B. Laponnaz and S. Dagorne, Chem. Commun., 2012, 48, 2213–2215 RSC.
- (a) A. J. Chmura, M. G. Davidson, M. D. Jones, M. D. Lunn, M. F. Mahon, A. F. Johnson, P. Khunkamchoo, S. L. Roberts and S. S. F. Wong, Macromolecules, 2006, 39, 7250–7257 CrossRef; (b) C. Romain, L. Brelot, S. Bellemin-Laponnaz and S. Dagorne, Organometallics, 2010, 29, 1191–1198 CrossRef.
- (a) Z. Zhong, P. J. Dijkstra and J. Feijen, J. Am. Chem. Soc., 2003, 125, 11291–11298 CrossRef; (b) N. Nomura, R. Ishii, Y. Yamamoto and T. Kondo, Chemistry, 2007, 13, 4433–4451 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4py01319k |
| ‡ It should be noted that AgOTf has a limited solubility in DCM and a mixture DCM/toluene as solvent was used instead (see ESI†). |
| This journal is © The Royal Society of Chemistry 2025 |