Open Access Article
Open Access ArticleOptimising reaction conditions in flasks for performances in organic light-emitting devices†
Koki
Ikemoto‡
 *a,
Misato
Akiyoshi‡
a,
Ayano
Kobayashi
a,
Hiroshi
Kita
b,
Hideo
Taka
b and
Hiroyuki
Isobe
*a,
Misato
Akiyoshi‡
a,
Ayano
Kobayashi
a,
Hiroshi
Kita
b,
Hideo
Taka
b and
Hiroyuki
Isobe
 *a
*a
aDepartment of Chemistry, The University of Tokyo, Hongo 7-3-1, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: kikemoto@chem.s.u-tokyo.ac.jp; isobe@chem.s.u-tokyo.ac.jp
bKonica Minolta, Ishikawacho 2970, Hachioji, Tokyo 192-8505, Japan
First published on 23rd December 2024
Abstract
A method for correlating reaction conditions with device performance was developed by combining Design-of-Experiments and machine-learning strategies in multistep device fabrication processes. This method allowed the “from-flask-to-device” optimisation of a macrocyclisation reaction yielding a mixture of methylated [n]cyclo-meta-phenylenes, and a crude raw material was directly applied to the fabrication of Ir-doped organic light-emitting devices via spin-coating. The method succeeded in eliminating energy-consuming and waste-producing separation and purification steps during device fabrication. The device using the optimal raw mixture material recorded a high external quantum efficiency of 9.6%, which surpassed the performance of purified materials. The raw material method was also found to be applicable to screen-printing processes, and image-transferred OLEDs were fabricated using the low-cost, environmentally benign materials.
Introduction
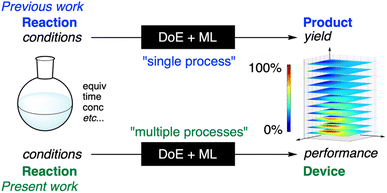
Optimising reaction conditions in flasks for high-yield synthesis is a common process that is performed ubiquitously in synthetic laboratories. The most popular method of optimisation is known as one-factor-at-a-time (OFAT) optimisation,1 where each factor is examined one at a time. The OFAT optimisation is particularly useful for reactions with simple pathways, which can also lead to mechanistic insights.1,2 For reactions with complicated pathways, however, Design-of-Experiments (DoE) optimisation can often be superior because it more effectively covers the parameter space for optimisation.3 Recently, we augmented the DoE optimization data with machine-learning (ML) predictions, which allowed us to find an optimal condition for a reaction passing through complicated pathways with a heatmap representation of the yields in the parameter space (Fig. 1).4 Observing that the “DoE + ML” method was able to correlate the reaction conditions with the yields (outcome of a single experimental process, i.e., reaction) despite the complicated pathways, we came up with the idea of correlating the reaction conditions with the outcome to be derived after multiple experimental processes using the DoE + ML method. Thus, using the DoE + ML method, we correlated the reaction conditions with the performance in organic light-emitting devices (OLEDs) and optimised the device performance by searching for the optimal reaction condition. As a result, the present study allowed us to optimise the performance of OLEDs, following some important principles of green and sustainable chemistry.5Results and discussion
DoE + ML method
Previously, we developed a method for the one-pot synthesis of [n]cyclo-meta-phenylenes ([n]CMPs) by adopting the Ni-mediated Yamamoto coupling reaction for macrocyclisation.6,7 The method was used to synthesise unique nanocarbon molecules,8,9 which also allowed us to develop multipotent OLED materials.10 For example, using dihalotoluene (1) as a starting material, we synthesised a series of [n]CMPs to fabricate single-layer OLEDs doped with Ir emitters (Fig. 2a).10a The highest performance of external quantum yield (EQE) = 22.8% was achieved with the methylated [5]CMP congener, while the performance of other congeners was inferior.11 Considering the growing importance of developing low-cost, energy-saving and environmentally benign OLEDs for low-end consumables, we envisioned that the [n]CMP-based OLEDs can provide an interesting test case for development. Thus, the Yamamoto macrocyclisation afforded a series of [n]CMP congeners from n ≥ 5 (cf.Fig. 2a for a MALDI mass spectrum) without any other noticeable byproducts, and, albeit with a range of EQE performance, the methylated [n]CMP derivatives generally acted as host materials for the Ir emitter.10a Therefore, we reasoned that we could directly use the crude raw material from the reaction for the Ir-doped OLEDs, skip energy-consuming and waste-producing processes such as separation and purification and thus optimise the conditions of the macrocyclisation reaction for the high-performance devices with the DoE + ML method (Fig. 2b).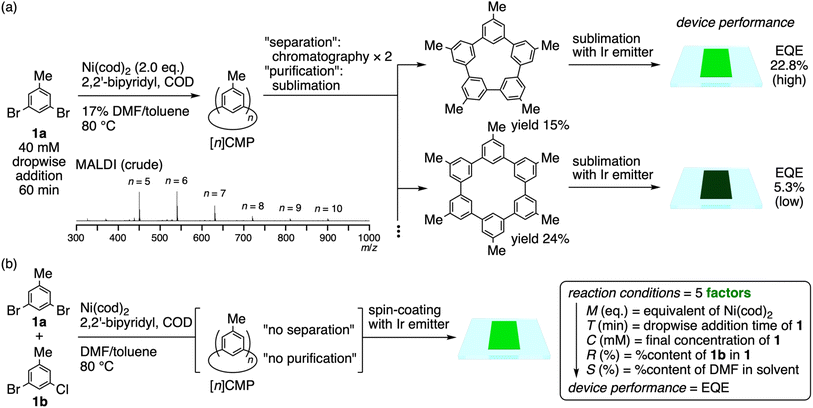 | ||
| Fig. 2 Fabrication of [n]CMP-based OLEDs. (a) Multistep, stepwise processes used in traditional device fabrication protocols.10a (b) Simplified multistep processes used in the present study, which eliminated energy-consuming and waste-producing separation and purification steps. | ||
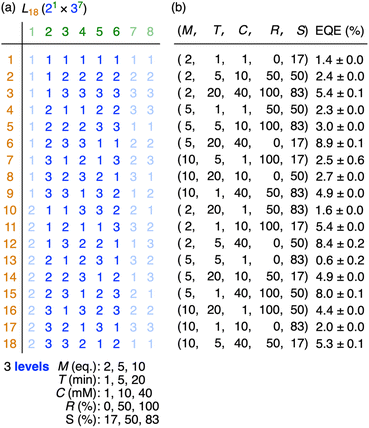
Our DoE + ML method starts by deciding factors and levels for the optimisation to select an appropriate table from Taguchi's orthogonal arrays.3,4 Three factors, i.e., equivalent of Ni(cod)2 (M), dropwise addition time of 1 (T) and final concentration of 1 (C), which were previously found to be influential in Yamamoto macrocyclisation, were also adopted in this study,4,12 and additional two factors, i.e., % content of bromochlorotoluene (1b) in 1 (R) and % content of DMF in solvent (S), were adopted to tweak the product distribution by changing the kinetics at the oxidative addition step and the disproportionation step, respectively.6 Since we decided to examine three levels for each factor, there were 5 factors and 3 levels for the optimisation, and the DoE parameter space was set by referring to the “L18 (21 × 37)” table of Taguchi's orthogonal arrays (Fig. 3a).3 Among the 8 × 18 cells of the L18 (21 × 37) table, 5 × 18 cells were selected to cover the 5 factors, and the 3 levels for each factor were set as shown in Fig. 3a to complete the experimental design. We carried out the 18 reactions of Yamamoto macrocyclisation of 1 under the designed conditions, and 18 sets of crude raw materials were obtained after aqueous workup and passing the mixture through a short-path silica gel column to remove metal and polar residues (see ESI† for details).
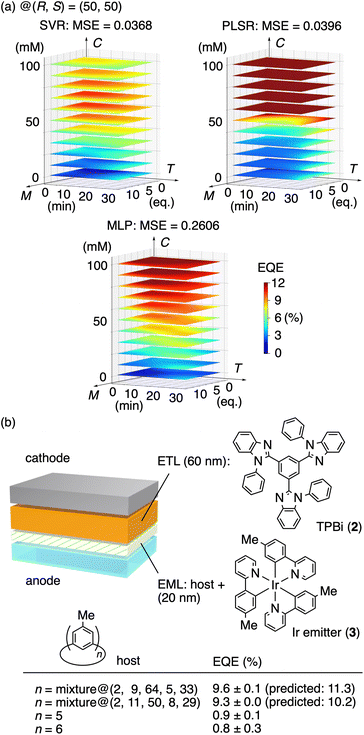
The device performance of each of the 18 raw materials was evaluated by fabricating double-layer OLEDs, which was further supplemented by ML predictions.13 Thus, the double-layer OLED was fabricated by first spin-coating a solution of a crude raw mixture of methylated [n]CMPs mixed with the Ir emitter (3; 14 wt% in the layer)14 as the emission layer (EML; 20 nm) and then sublimating 1,3,5-tris(1-phenyl-1H-benzimidazol-2-yl)benzene (TPBi, 2) as the overlaid electron transport layer (ETL; 60 nm) (cf.Fig. 4b). The device performance was evaluated by EQE in quadruplicate to correlate the five reaction factors of (M, T, C, R, S) with the EQE performance (Fig. 3b). For the ML predictions based on the EQE data, we investigated three ML methods, i.e., support vector regression (SVR), partial least squares regression (PLSR) and multilayer perceptron (MLP), to obtain the EQE heatmaps to fill the five-dimensional parameter space of (M, T, C, R, S) (Fig. S6†).4 As representative heatmaps, the (M, T, C) heatmaps at (R, S) = (50, 50) are shown in Fig. 4a. The ML predictions of the three methods were then evaluated by the mean square errors (MSE) obtained via leave-one-out cross-validations (LOOCV)15,16 to decide the SVR model as the most appropriate predictor (MSE: SVR = 0.0368, PLSR = 0.0396, MLP = 0.2606). Finally, the SVR model was validated by running two test runs (Fig. 4b). The grid search of the five-dimensional SVR model found the highest EQE spot of 11.3% at (M, T, C, R, S) = (2, 9, 64, 5, 33), and the actual test run at this spot yielded a comparable EQE value of 9.6 ± 0.1%. Another high EQE spot at (2, 11, 50, 8, 29) was arbitrarily selected to record the experimental EQE value of 9.3 ± 0.0%, which also agreed well with 10.2% of the SVR model. The results confirmed that the SVR model, which successfully correlated the reaction conditions with the device performance, was credible.
An advantage of the raw-material OLEDs was found by comparing the EQE performance with that of the pure-material OLEDs. When the double-layer OLED was fabricated using the methylated [n]CMP congener after separation and purification as a single compound, the device performance was much inferior to record EQE = 0.9 ± 0.1% and 0.8 ± 0.3% with n = 5 and 6, respectively (Fig. 4b). As found with the single-layer OLEDs using methylated [n]CMPs,12 the device performance is dramatically affected by the well-ordered packing of the host molecules, and the pure-material OLED would be affected by the crystalline character facilitated by the spin-coating process. On the other hand, the raw material is composed of a mixture of the methylated congeners and should maintain the amorphous character to retain the performance as a host material even through the solution process of the spin coating. When we analysed the “hit” raw material obtained at (M, T, C, R, S) = (2, 9, 64, 5, 33), the population of methylated [n]CMP congeners were found as n = 5: 19%, n = 6: 26%, n = 7: 18%, n = 8: 8%, n = 9: 11%, n = 10: 5%, n = 11: 5%, n = 12: 4%, n = 13: 2%, n = 14: 1% and n = 15: 0.4% by using the signal intensities of the MALDI spectrum (Fig. S3†).17 Formulation of such an elaborate mixture of the [n]CMP congeners after standard separation/purification processes should not be easy,18 which was succinctly achieved by a one-pot reaction without purification via ML-assisted design of the raw mixture material. Additional datasets of the [n]CMP population were also obtained by MALDI analysis of the raw materials from other (M, T, C, R, S) coordinates (Fig. S2†), which showed that the EQE changes of the device were originated from subtle changes in the [n]CMP population. The NMR spectra of the raw materials were also obtained, which allowed us to determine the yield of [n]CMP with n = 5, 6 and 7 by using an internal standard. The comparison of the MS and NMR populations of the congeners then confirmed (Fig. S4†), to a certain extent, that the MS population qualitatively reflected the [n]CMP population.
Screen-printed OLED
Finally, we applied the raw-material OLEDs via the flask-to-device processes to the screen-printing fabrication19 (Fig. 5). Thus, the Yamamoto macrocyclisation of 1 was performed at the hit spot of (M, T, C, R, S) = (2, 9, 64, 5, 33) to afford the crude raw material consisting of the mixture of methylated [n]CMPs. The mixture was dissolved in chlorobenzene at 0.34 wt% together with 0.06 wt% of 3. A glass coated with anode materials was covered with a metal mask bearing an image, and the EML solution was drop cast on the substrate. After removing the metal mask, the device was finished by coating with ETL (2; 60 nm) and cathode materials. The screen-printed device fabricated with raw-material [n]CMPs emitted light with images of kanji characters as shown in Fig. 5, demonstrating the applicability of the present strategy for the development of low-cost, environmentally benign OLEDs. | ||
| Fig. 5 Processes and images of screen-printed OLEDs using the raw-material mixture of methylated [n]CMPs from (M, T, C, R, S) = (2, 9, 64, 5, 33). The screen-printed images were created by copying kanji characters ((left): moon, (right): dream) written by a Japanese poet, Basho Matsuo.20 | ||
Conclusions
In summary, we have successfully demonstrated that the reaction conditions in the flask can be correlated with the performance in devices using the DoE + ML method. The crude, raw material from the reaction was found to be applicable as a host material in double-layer OLEDs, which succeeded in eliminating waste-producing, energy-consuming processes of separation and purification of small molecules. Although this “from-flask-to-device” optimisation strategy could not provide information on structure-performance relationships, the ML method provided a unique model that represented “reaction condition-performance relationships” in the five-dimensional parameter space. Importantly, the raw-material mixture was found to be superior to the pure material in terms of the device performance in the double-layer OLEDs, demonstrating the uniqueness and advantages of the “from-flask-to-device” strategy in generating elaborate mixture for materials. The development of small-molecule materials usually considers structure-performance relationships to a considerable extent, which indeed deepens our understanding of molecular materials,21 but the direct connections made by the from-flask-to-device strategy with the help of growing ML technologies can also lead to the development of low-cost, environmentally benign organic devices in general. Design of a house-of-cards assembly of graphene-like molecules by using a mixture from the DoE + ML method, for instance, could be of interest to be applied for battery electrodes.22Data availability
The data that support the findings of this study are available in the ESI.†Author contributions
KI: conceptualization, data curation, formal analysis, funding acquisition, methodology, writing – original draft, writing – review & editing. MA: data curation, formal analysis, investigation, methodology, writing – original draft, writing – review & editing. AK: data curation, formal analysis, investigation. HK: data curation, formal analysis, investigation. HT: data curation, formal analysis, investigation. HI: conceptualization, data curation, formal analysis, funding acquisition, methodology, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
H. I. thanks Professor Roald Hoffmann for sharing a draft manuscript of their stimulative, important essay before the publication (ref. 21), which was one of the important triggers for him to step forward in this unfamiliar field of machine learning. We thank undergraduate students who performed preliminary experiments at the early stage of this study during the May festival of the University of Tokyo. This work was partly supported by KAKENHI (20H05672, 22H02059) and A-STEP (JPMJTR223C). M. A. thanks the JSPS and SPRING GX program for predoctoral fellowships.Notes and references
- C. Daniel, One-at-a-Time Plans, J. Am. Stat. Assoc., 1973, 68, 353–360 CrossRef.
- C. K. Ingold, Structure and Mechanism in Organic Chemistry, Cornell University Press, Ithaka and London, 1969 Search PubMed.
- G. Taguchi, S. Chowdhury and Y. Wu, Taguchi's Quality Engineering Handbook, Wiley, Hoboken, 2005 Search PubMed.
- (a) K. Ikemoto, M. Akiyoshi, T. Mio, K. Nishioka, S. Sato and H. Isobe, Synthesis of a Negatively Curved Nanocarbon Molecule with an Octagonal Omphalos via Design-of-Experiments Optimizations Supplemented by Machine Learning, Angew. Chem., Int. Ed., 2022, 61, e202204035 CrossRef CAS PubMed; (b) M. Akiyoshi, K. Ikemoto and H. Isobe, Tier-grown Expansion of Design-of-Experiments Parameter Spaces for Synthesis of a Nanometer-scale Macrocycle, Chem.–Asian J., 2023, 18, e202201141 CrossRef CAS PubMed.
- M. Doble and A. K. Kruthiventi, Green Chemistry and Engineering, Academic Press, Burlington 2007 Search PubMed.
- (a) T. Yamamoto, Y. Hayashi and A. Yamamoto, A Novel Type of Polycondensation Utilizing Transition Metal-Catalyzed C-C Coupling. I. Preparation of Thermostable Polyphenylene Type Polymers, Bull. Chem. Soc. Jpn., 1978, 51, 2091–2097 CrossRef CAS; (b) T. Yamamoto, S. Wakabayashi and K. Osakada, Mechanism of C-C Coupling Reactions of Aromatic Halides, Promoted by Ni (COD)2 in the Presence of 2,2'-Bipyridine and PPh3, to Give Biaryls, J. Organomet. Chem., 1992, 428, 223–237 CrossRef CAS.
- (a) J. Y. Xue, K. Ikemoto, N. Takahashi, T. Izumi, H. Taka, H. Kita, S. Sato and H. Isobe, Cyclo-meta-phenylene Revisited: Nickel-Mediated Synthesis, Molecular Structures, and Device Applications, J. Org. Chem., 2014, 79, 9735–9739 CrossRef CAS PubMed; (b) A. Yoshii, K. Ikemoto, T. Izumi, H. Taka, H. Kita, S. Sato and H. Isobe, Periphery Design of Macrocyclic Materials for Organic Light-Emitting Devices with a Blue Phosphorescent Emitter, Org. Lett., 2019, 21, 2759–2762 CrossRef CAS PubMed.
- (a) K. Ikemoto, T. M. Fukunaga and H. Isobe, Phenine Design for Nanocarbon Molecules, Proc. Jpn. Acad., Ser. B, 2022, 98, 379–400 CrossRef PubMed; (b) K. Ikemoto, R. Kobayashi, S. Sato and H. and Isobe, Synthesis and Bowl-in-Bowl Assembly of a Geodesic Phenylene Bowl, Angew. Chem., Int. Ed., 2017, 56, 6511–6514 CrossRef CAS PubMed; (c) K. Ikemoto, J. Lin, R. Kobayashi, S. Sato and H. and Isobe, Fluctuating Carbonaceous Networks with a Persistent Molecular Shape: A Saddle-Shaped Geodesic Framework of 1,3,5-Trisubstituted Benzene (Phenine), Angew. Chem., Int. Ed., 2018, 57, 8555–8559 CrossRef CAS PubMed; (d) T. Mio, K. Ikemoto, S. Sato and H. Isobe, Synthesis of a Hemispherical Geodesic Phenine Framework by a Polygon Assembling Strategy, Angew. Chem., Int. Ed., 2020, 59, 6567–6571 CrossRef CAS.
- (a) U. Beser, M. Kastler, A. Maghsoumi, M. Wagner, C. Castiglioni, M. Tommasini, A. Narita, X. Feng and K. Müllen, A C216-Nanographene Molecule with Defined Cavity as Extended Coronoid, J. Am. Chem. Soc., 2016, 138, 4322–4325 CrossRef CAS PubMed; (b) H. Hou, X.-J. Zhao, C. Tang, Y.-Y. Ju, Z. Y. Deng, X.-R. Wang, L.-B. Feng, D.-H. Lin, X. Hou, A. Narita, K. Müllen and Y.-Z. Tan, Synthesis and Assembly of Extended Quintulene, Nat. Commun., 2020, 11, 3976 CrossRef CAS PubMed; (c) S.-H. Liu, H. Hou, Z.-Y. Deng, X.-R. Wang, C. Tang, Y.-Y. Ju, L.-B. Feng and Y.-Z. Tan, Three-Dimensional Conjugated Macrocycle with Large Polyaromatic Blocks Constructed by Post-π-Extension, Sci. China:Chem., 2020, 63, 1626–1631 CrossRef CAS; (d) X.-J. Zhao, H. Hou, P.-P. Ding, Z.-Y. Deng, Y.-Y. Ju, S.-H. Liu, Y.-M. Liu, C. Tang, L.-B. Feng and Y.-Z. Tan, Molecular Defect-Containing Bilayer Graphene Exhibiting Brightened Luminescence, Sci. Adv., 2020, 6, 8541–8569 CrossRef PubMed.
- (a) J. Y. Xue, T. Izumi, A. Yoshii, K. Ikemoto, T. Koretsune, R. Akashi, R. Arita, H. Taka, H. Kita, S. Sota and H. Isobe, Aromatic Hydrocarbon Macrocycles for Highly Efficient Organic Light-Emitting Devices with Single-Layer Architectures, Chem. Sci., 2016, 7, 896–904 RSC; (b) K. Ikemoto, A. Yoshii, T. Izumi, H. Taka, H. Kita, J. Y. Xue, R. Kobayashi, S. Sato and H. Isobe, Modular Synthesis of Aromatic Hydrocarbon Macrocycles for Simplified, Single-Layer Organic Light-Emitting Devices, J. Org. Chem., 2016, 81, 662–666 CrossRef CAS PubMed; (c) T. Izumi, Y. Tian, K. Ikemoto, A. Yoshii, T. Koretsune, R. Arita, H. Kita, H. Taka, S. Sato and H. Isobe, Efficient Blue Electroluminescence from a Single-layer Organic Device Composed Solely of Hydrocarbons, Chem.–Asian J., 2017, 12, 730–733 CrossRef CAS PubMed; (d) A. Yoshii, Y. Onaka, K. Ikemoto, T. Izumi, S. Sato, H. Kita, H. Taka and H. Isobe, Acyclic, Linear Oligo-meta-phenylenes as Multipotent Base Materials for Highly Efficient Single-layer Organic Light-emitting Devices, Chem.–Asian J., 2020, 15, 2181–2186 CrossRef CAS.
- With internal quantum efficiency (IQE) of 100%, OLED can achieve EQE of 20–30%, as a part of emitted light is confined within the device. See also ref. 10a..
- The three factors, (M, T, C), were chosen, as we believed that these could be important factors that could influence intramolecular cyclization and intermolecular oligomerization. See also ref. 4a..
- Our initial attempts to fabricate single-layer OLEDs by the spin-coating method were not successful (ref. 10a), as the method could not achieve necessary thickness to avoid the current leakage..
- K. Dedeian, P. I. Djurovich, F. O. Garces, G. Carlson and R. J. Watts, A New Synthetic Route to the Preparation of a Series of Strong Photoreducing Agents: fac Tris-Ortho-Metalated Complexes of Iridium(III) with Substituted 2-Phenylpyridines, Inorg. Chem., 1991, 30, 1685–1687 CrossRef CAS.
- (a) A. C. Müller and S. Guido, Introduction to Machine Learning with Python, O'Reilly, Sebastopol, 2017 Search PubMed; (b) D. M. Hawkins, S. C. Basak and D. Mills, Assessing Model Fit by Cross-Validation, J. Chem. Inf. Comput. Sci., 2003, 43, 579–586 CrossRef CAS.
- Z.-H. Zhou, Machine Learning, Springer, Singapore, 2021 Search PubMed.
- Careful examination of the isotope distributions of MALDI MS peaks showed that n-mer linear oligo-meta-phenylenes, [n]LOMP, were not included to any significant extent, although [n]LOMP could also serve as the host. See also ref. 10d..
- In previous studies, an elaborate mixture of small molecules was manually formulated for devices, which also requires another set of optimizations besides the reaction conditions. For example, see: (a) Y. Hino, H. Kajii and Y. Ohmori, Efficient Low-Molecule Phosphorescent Organic Light-Emitting Diodes Fabricated by Wet-Processing, Org. Electron., 2004, 5, 265–270 CrossRef CAS; (b) M. Cai, T. Xiao, E. Hellerich, Y. Chen, R. Shinar and J. Shinar, High-Efficiency Solution-Processed Small Molecule Electrophosphorescent Organic Light-Emitting Diodes, Adv. Mater., 2011, 23, 3590–3596 CrossRef CAS PubMed; (c) T.-H. Han, M.-R. Choi, C.-W. Jeon, Y.-H. Kim, S.-K. Kwon and T.-W. Lee, Ultrahigh-Efficiency Solution-Processed Simplified Small-Molecule Organic Light-Emitting Diodes Using Universal Host Materials, Sci. Adv., 2016, 2, e1601428 CrossRef PubMed; (d) R. Kumaresan, A. Maheshwaran, H.-Y. Park, K. Sung, J. Choi, W. Cho, M. Song, S. I. Ahn and S.-H. Jin, Non-Halogenated Solvent-Processed Highly Efficient Green Ir(III) Complexes with an External Quantum Efficiency Exceeding 23% for Phosphorescent Organic Light-Emitting Diodes, J. Mater. Chem. C, 2020, 8, 12959–12967 RSC.
- D. A. Pardo, G. E. Jabbour and N. Peyghambarian, Application of Screen Printing in the Fabrication of Organic Light-Emitting Devices, Adv. Mater., 2000, 12, 1249–1252 CrossRef CAS.
- B. Matsuo, The Narrow Road to the Deep North (Oku no Hosomichi), Handwritten, Iwanami, Tokyo, 1997 Search PubMed.
- This study should also stimulate our discussion related to the phrase, “To predict is not to explain”. See (a) R. Hoffmann and J.-P. Malrieu, Simulation vs. Understanding: A Tension, in Quantum Chemistry and Beyond. Part A. Stage Setting, Angew. Chem., Int. Ed., 2020, 59, 12590–12610 CrossRef CAS PubMed; (b) R. Hoffmann and J.-P. Malrieu, Simulation vs. Understanding: A Tension, in Quantum Chemistry and Beyond. Part B. The March of Simulation, for Better or Worse, Angew. Chem., Int. Ed., 2020, 59, 13156–13178 CrossRef CAS PubMed; (c) R. Hoffmann and J.-P. Malrieu, Simulation vs. Understanding: A Tension, in Quantum Chemistry and Beyond. Part C. Toward Consilience, Angew. Chem., Int. Ed., 2020, 59, 13694–13710 CrossRef PubMed.
- (a) S. Sato, A. Unemoto, T. Ikeda, S. Orimo and H. Isobe, Carbon-Rich Active Materials with Macrocyclic Nanochannels for High-Capacity Negative Electrodes in All-Solid-State Lithium Rechargeable Batteries, Small, 2016, 12, 3381–3387 CrossRef CAS; (b) N. A. Kaskhedikar and J. Maier, Lithium Storage in Carbon Nanostructures, Adv. Mater., 2009, 21, 2664–2680 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc07039a |
| ‡ K. Ikemoto and M. Akiyoshi have equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2025 |