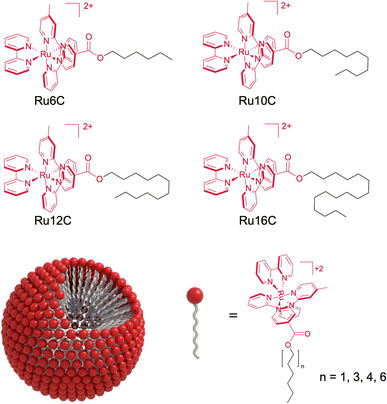
Open Access Article
Open Access ArticleSupramolecular self-assembly of metal complex surfactants (MeCS) into micellar nanoscale reactors in aqueous solution†
Ying
Chen
 a,
Asia
Matatyaho Ya'akobi
a,
Asia
Matatyaho Ya'akobi
 b,
Thao Vy
Nguyen
c,
Shih-Chieh
Kao
a,
Julian G.
West
b,
Thao Vy
Nguyen
c,
Shih-Chieh
Kao
a,
Julian G.
West
 a,
Sibani Lisa
Biswal
a,
Sibani Lisa
Biswal
 c,
Yeshayahu
Talmon
c,
Yeshayahu
Talmon
 b and
Angel A.
Martí
b and
Angel A.
Martí
 *ad
*ad
aDepartment of Chemistry, Rice University, Houston, Texas 77005, USA. E-mail: amarti@rice.edu
bDepartment of Chemical Engineering, The Russell Berrie Nanotechnology Institute, Technion-Israel Institute of Technology, Haifa 3200003, Israel
cDepartment of Chemical and Biomolecular Engineering, Rice University, Houston, TX 77005, USA
dDepartment of Materials Science & Nanoengineering, Department of Bioengineering, Rice University, Houston, TX. 77005, USA
First published on 10th February 2025
Abstract
Surfactants are amphiphilic molecules that can form micellar structures with a hydrophobic core and a hydrophilic corona in water. In this work, we combine the remarkable properties of photoactive metal complexes with the supramolecular organization of surfactants to create photoactive vessels that support photocatalytic processes in aqueous media, even for starting materials that are insoluble in water. Herein, we report a library of photoactive metal complex surfactants (MeCSs) and their photophysical and photochemical properties. These properties are modulated by the length of an alkyl chain attached to the polypyridyl ligand of the metal complex. Finally, an alkene hydroxytrifluoromethylation photocatalytic reaction was demonstrated in aqueous solution, suggesting the usefulness of metal complex surfactants for the development of green aqueous photoreactions.
Introduction
Metal polypyridyl complexes have been used for many applications over the past century due to their excellent photoredox properties.1–6 In particular, metal complexes can form stable, long-lived excited states upon photoexcitation, which facilitates the interactions with substrates during the excited state, enabling a variety of novel reactions under mild conditions.1 For example, the use of tris(2,2′-bipyridine)ruthenium(II) ([Ru(bpy)3]2+) as a visible light photoredox catalyst for organic synthesis has been well studied.4,7–12 However, most of these reactions are diffusion-controlled and typically performed in organic solvents due to solubility constraints of substrates and catalysts. Recent efforts have focused on the use of aqueous media to improve sustainability in organic synthesis. Nevertheless, the use of water as a medium for visible-light photocatalytic transformations has not been thoroughly explored, partly due to the solubility challenges of reactants.13Over the past several decades, many surfactants with novel properties have been designed and synthesized for various applications.14–18 Previously, we have shown that fluorescent surfactants, such as rhodamine-B and eosin-Y attached to aliphatic chains, can self-assemble into micelles.17 These fluorescent surfactants were used for applications including cellular imaging, labeling, and diffusion studies of nanotubes and nanosheets.19,20 Recently, amphiphilic metal complexes with hydrophobic moieties on their ligands have been studied for their stability in both ground and photoexcited states and their rich redox/photophysical properties.21–25 Many of these studies have primarily focused on double or multi-chain metal complex surfactants, where the number of chains attached to the surfactant leads to the formation of vesicles of micrometer dimensions.26–33 Pioneer work by the Lipshitz group in 2018 showed the use of amphiphilic metal complexes in photoredox transformations in water, where they use a double-chain iridium complex (attached to a modified CoQ10 group with an aliphatic chain of 50 carbon atoms and a PEG group) forming micelles around 50 nm in diameter for the sulfonation of alkanes and enol acetates.34
In this work, we used a simple esterification reaction to synthesize a single-chain metal complex surfactant (MeCS) with a ruthenium polypyridyl cationic head and aliphatic groups of different lengths (6, 10, 12, and 16 carbons; Scheme 1). These ruthenium MeCSs were thoroughly characterized, allowing us to correlate their photophysical properties (photoluminescence quantum yield and lifetime) and their critical micelle concentration (CMC) with the chain length. Optimized ruthenium MeCSs were used as a proof-of-concept for the photocatalytic hydroxytrifluoromethylation of aryl alkenes in aqueous solution. To the best of our knowledge, no single study has comprehensively examined single-chain ruthenium amphiphiles, including their synthesis, spectroscopic properties, self-aggregation behavior, and photocatalytic applications in aqueous media. This work fills that gap by providing a detailed investigation of these amphiphiles, highlighting their nanoscale micelle formation (5–6 nm), simple synthesis, and exceptional photophysical properties. In the context of sustainable chemistry, MeCSs offer a promising platform for photocatalytic reactions in water, enabling sustainable applications in pharmaceuticals and materials science.
Results and discussion
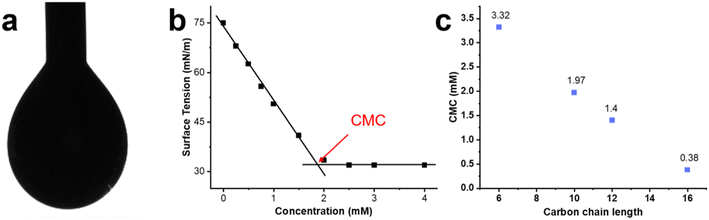
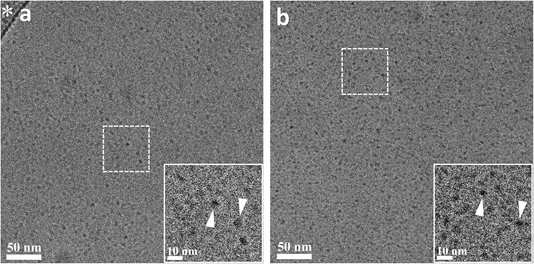
The minimum concentration needed for surfactant monomers to assemble into micelles is called the CMC.35 Once the micelles form, their hydrophobic cores behave as nanoscale lipophilic vessels, which is convenient for photophysical and photocatalytic applications. Here, the pendant drop method was used to determine the interfacial tension of aqueous solutions with different surfactant concentrations.36 Increasing the surfactant concentration decreases the surface tension of a pendant drop due to the migration of the surfactant to the interface between the drop and air (Fig. 1a). Once the CMC is reached, the concentration of monomers at the interface (and the surface tension) reaches a steady state, and any additional surfactant assembles into micelles (Fig. 1b).37 Interestingly, a decrease in the CMC is observed as the alkyl chain length increases (Fig. 1c and S1†). The increase in alkyl chain length causes the surfactant to become more hydrophobic, promoting the aggregation of MeCSs into micelles at lower concentrations and decreasing the CMC.38Cryo-TEM studies show that ruthenium surfactant Ru16C forms spheroidal micelles in aqueous solution (Fig. 2a). Likewise, round spheroidal structures are observed for a 4 mM solution of Ru12C, which is above the CMC. Images for Ru12C are shown in Fig. S3.† The micelles are readily visible given the contrast of the ruthenium complex in the cryo-TEM and have a mean diameter of 5.9 and 5.5 nm for Ru12C and Ru16C, respectively (Fig. S4†). The average hydrodynamic diameter of Ru12C and Ru16C were also determined as 16.2 nm and 25.0 nm by using dynamic light scattering (DLS) (Fig. S5†). Mixed micelles with 20 mM cetrimonium bromide (CTAB) and 1 mM Ru16C can also be formed, as seen in Fig. 2b. Similarly to Ru16C, CTAB is a cationic surfactant with a trimethylammonium ionic head and an aliphatic tail of 16 carbon atoms, similar in structure to Ru16C. The interfacial tension of the mixture of Ru16C and CTAB with different dilutions was obtained (Fig. S2†). Here, only one CMC value was observed, which is consistent with the formation of mixed micelles containing CTAB and Ru16C.
The absorption and emission spectra, photoluminescence quantum yield, and luminescence lifetimes of MeCSs were investigated in aqueous solution and compared to [Ru(bpy)3]2+.39–41 All photophysical experiments of pure MeCS in solution were performed in nitrogen-purged solutions to avoid quenching by molecular oxygen42 and below their CMC to prevent potential self-quenching.43,44 CTAB was added to study the effect of micelle formation on the MeCS properties. CTAB is optically transparent in the visible range and forms mixed micelles with MeCSs, allowing a low MeCS concentration in the micellar environment.
Spectroscopic studies show that ruthenium MeCSs present similar absorption spectra to that reported for [Ru(bpy)3]2+ with a broad metal-to-ligand charge transfer (MLCT) band around 460 nm.39 Furthermore, it was also observed that the length of the alkyl chain did not change the shape of the photoluminescence spectra of the various MeCSs. The absorption and emission spectra of Ru16C are shown in Fig. S6.† Quantum yields for the different ruthenium MeCS are included in Table 1. As expected, the quantum yield for ruthenium MeCS in nitrogen-purged solutions is remarkably higher than in air and consistently increases with the number of carbon atoms in the aliphatic chain. Photoluminescence lifetime experiments also show an increase in the lifetime for all nitrogen-purged solutions (Table 1), but not a strong dependance with alkyl chain length. It is important to note that ruthenium MeCS in micelles does not seem to suffer from self-quenching. Actually, the quantum yield and photoluminescence lifetimes seem to be relatively independent of whether the ruthenium MeCS is monomeric or incorporated in CTAB micelles. This observation contrasts with ruthenium complexes with two alkyl chains in liposomes, where both the quantum yield and lifetimes are significantly reduced, indicating a strong deactivation of the excited state.27 These contrasting behaviors might be related to the difference in size between micelles and liposomes, which will affect the packing and interaction of the ruthenium complexes in these environments. A more complete spectroscopic characterization of these systems would be necessary to clarify these observations.
| Sample | λ max (nm) | λ emmax (nm) | ϕ em (air) | ϕ em (N2) | τ em (ns) (air) | τ em (ns) (N2) |
|---|---|---|---|---|---|---|
a The absorbance and emission spectra were obtained with ruthenium surfactant that was dissolved in water, and nitrogen-purged water at 298 K (λex = 460 nm).
b All the errors are less than 3%.
c Unless otherwise indicated, the photoluminescence decays are monoexponential.
d The luminescence decays are biexponential. The weighted mean lifetimes (τem) were calculated according to 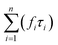 , where fi is the fractional contribution and τi is the decay time.45 , where fi is the fractional contribution and τi is the decay time.45
|
||||||
| Ru6C | 466 | 684 | 0.012 | 0.057 | 168.2 ± 0.5 | 484 ± 4d |
| Ru6C-CTAB | 0.056 | 524 ± 4d | ||||
| Ru10C | 464 | 681 | 0.019 | 0.058 | 203.0 ± 0.6 | 516 ± 6d |
| Ru10C-CTAB | 0.093 | 490 ± 5d | ||||
| Ru12C | 462 | 684 | 0.023 | 0.124 | 231.6 ± 0.6 | 532 ± 1 |
| Ru12C-CTAB | 0.097 | 546 ± 1 | ||||
| Ru16C | 462 | 677 | 0.016 | 0.134 | 264.7 ± 0.6 | 539 ± 1 |
| Ru16C-CTAB | 0.149 | 494 ± 1 | ||||
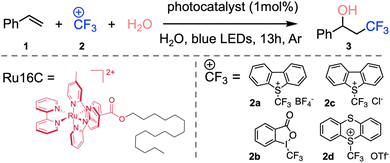
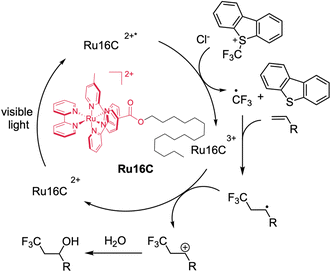
In the last 10 years, groundbreaking research has shown the application of metallosurfactants in the production of solar fuels,27,33,46,47 however, efforts to use metallosurfactants in aqueous photocatalytic transformations of organic products are less common.34 Given their self-assembly, core hydrophobicity, and outstanding photophysical properties, we studied the use of MeCSs as nanoscale photoreactors for chemical reactions in aqueous solution. As a proof-of-concept, we studied the hydroxytrifluoromethylation of alkenes to evaluate the catalytic efficiency of the ruthenium MeCSs, given the importance of trifluoromethylation of small molecules in the pharmaceutical48,49 and agrochemical industries.50
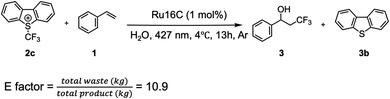
In 2012, the Akita group reported the use of photoactive metal complexes as photoredox catalysts for the hydroxytrifluoromethylation of alkenes and further studies have now been reported.51–54 However, these reactions were carried out in organic solvents to fully solubilize the substrates. Since ruthenium MeCSs have a ruthenium polypyridyl head group, as well as good water solubility, these photoactive micellar structures were used to initiate the desired hydroxytrifluoromethylation. Ru16C was chosen as a photocatalyst due to its lowest CMC and best photophysical properties (e.g., τem and ϕem) among the other synthesized surfactants.
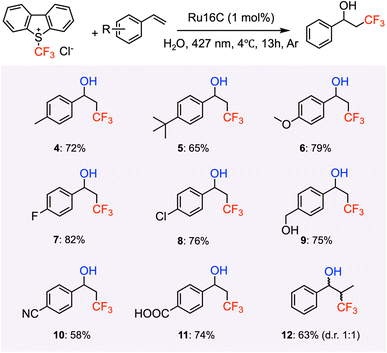
We set out to use styrene (1.1 equiv.) as the model substrate, Umemoto's reagent (2a, 2c) and Togni's reagent (2b), trifluoromethyl thianthrenium triflate (TT-CF3+OTf−, 2d) as the CF3 source, and 1 mol% of Ru16C as the photoredox catalyst in water (Table 2 and Fig. S7†). All these experiments confirmed the formation of 3,3,3-trifluoro-1-propan-1-ol (3) products. Umemoto's reagent affords 3 with a higher yield (39%) compared with the other trifluoromethylating reagents (Table 2, entries 1, 2, and 4). Using chloride as the counterion of Umemoto's reagent further improved the yield to 54% as determined by 19F NMR, which might be related to its higher water solubility (Table 2, entry 3). Tuning the irradiation wavelength (from 456 nm to 427 nm) further increases the yield of 3 to 63% (Table 2, entry 5). Interestingly, a yield of 84% was obtained when the reaction was carried out at 4 °C, which indicates that 3 is favored at lower reaction temperatures (Table 2, entry 6), likely due to the inhibition of the thermal polymerization of styrene.55 A control experiment was run with [Ru(bpy)3]Cl2 as a photocatalyst and compared with the photocatalytic efficiency of Ru16C. Under the same reaction conditions, [Ru(bpy)3]Cl2 resulted in a 14% yield (Table 2, entry 7). Furthermore, even with the combination of 1 mol% [Ru(bpy)3]Cl2 or Ru(bpy)3](PF6)2 and 1 mol% CTAB, the yield is still relatively low (55% and 20%, Table 2, entries 8 and 9). This established that the ruthenium micellar system is necessary for obtaining an efficient reaction in aqueous solution. Mixing [Ru(bpy)3]2+ with CTAB likely sequesters the metal complex inside the micelle, limiting the number of photoactive centers in contact with the reactants and reducing the effective volume of the nanovessel. The photoactive surfactant assembling ensures a high concentration of photoactive group at the surface of the micelle and does not occupy space in the interior. Reactions using Ru10C show only 35% conversion. At 0.5 mM concentration (1 mol%), Ru10C is below the CMC and does not form micelles, supporting the importance of micelle formation for efficient catalysis. Other control experiments performed without light (Table 2, entry 10), without Ru16C (Table 2, entry 11), and without Umemoto's reagent (Table 2, entry 12) did not produce 3, confirming the importance of light, the Umemoto's reagent, and Ru16C for the reaction to proceed. To broaden the substrate scope of aryl alkenes in the context of photocatalytic hydroxytrifluoromethylation employing Ru16C MeCs, various styrene derivatives were tested and summarized in Scheme 2. Styrenes bearing electron-donating groups at the para position, such as methyl (4), tert-butyl (5), methoxyl (6), and hydroxymethyl (9), all resulted in the corresponding products in good yields (65% to 80%). Furthermore, substrates bearing electron-withdrawing groups at the para position, including fluoro (7), chloro (8), nitrile group (10), and carboxylic group (11), were tolerated under our conditions, giving the corresponding products in moderate to good yields ranging 58% to 82%. Trans-β-methylstyrene was also examined, giving only one regioisomer (12) as a mixture of two diastereomers (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 d.r.) in 63% yield. To further illustrate the substrate scope and limitations of this photocatalytic hydroxytrifluoromethylation, details on unreactive and low-conversion substrates (S1–S6) have been included in the ESI.†
1 d.r.) in 63% yield. To further illustrate the substrate scope and limitations of this photocatalytic hydroxytrifluoromethylation, details on unreactive and low-conversion substrates (S1–S6) have been included in the ESI.†
| Entrya | CF3 reagent | Photocatalyst | Wavelength (nm) | Yieldc (%) |
|---|---|---|---|---|
| a Reactions were conducted on a 0.1 mmol scale using Umemoto's reagent (1.0 equiv.), styrene (1.1 equiv.), photocatalyst Ru16C or [Ru(bpy)3]Cl2 (1.0 mol%), 13 h, rt, and LED light (427 nm and 456 nm) (350 mW cm−2). b Reactions were run in a 4 °C fridge, see the details at ESI. c Yields were determined from 19F NMR using fluorobenzene as an NMR standard. d Isolated yield shown in parentheses. | ||||
| 1 | 2a | Ru16C | 456 | 39 |
| 2 | 2b | Ru16C | 456 | 14 |
| 3 | 2c | Ru16C | 456 | 54 |
| 4 | 2d | Ru16C | 456 | 36 |
| 5 | 2c | Ru16C | 427 | 63 |
| 6b | 2c | Ru16C | 427 | 84(80)d |
| 7b | 2c | Ru(bpy)3Cl2·6H2O | 427 | 14 |
| 8b | 2c | Ru(bpy)3Cl2·6H2O + CTAB | 427 | 55 |
| 9b | 2c | Ru(bpy)3(PF6)2 + CTAB | 427 | 20 |
| 10 | 2c | Ru16C | None | 0 |
| 11 | 2c | None | 456 | 0 |
| 12 | None | Ru16C | 456 | 0 |
 | ||
| Scheme 2 Substrate scope studies of the photocatalytic hydroxytrifluoromethylation of various styrene derivatives under the default reaction condition. Yields are given as isolated products. | ||
Mechanistically, the MeCS photocatalyst (e.g., Ru16C) reaches a triplet MLCT excited state upon light absorption, which is quenched by the Umemoto's reagent to generate a CF3 radical. The following addition of the CF3 radical to the alkenes can result in a transient radical intermediate, which can be oxidized by the Ru(III) species, regenerating the photocatalyst and producing a carbocation intermediate. Subsequently, nucleophilic attack by water to the carbocation intermediate affords the final product (Scheme 3).51,54 In order to confirm the formation of the CF3 radical, one equivalent of the radical scavenger 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) was added to the system under the default condition. The detection of the TEMPO-CF3 adduct on the 19F NMR (Fig. S8†) corroborates the presence of CF3 radical, which is consistent with the radical reaction pathway in Scheme 3.
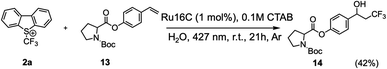
To further demonstrate the synthetic capability and adaptability of MeCS photocatalyst in aqueous solution, Ru16C was used to synthesize more structurally intricate molecules derived from commercially available active pharmaceutical targets. Since research has shown that L-proline (and derivatives) exhibit anticonvulsant properties,56 an n-(tert-butoxycarbonyl)-L-proline derivative (13, Scheme 4), was chosen as substrate. In our initial experiments, we found that the ruthenium MeCS concentration used in the synthesis of 3 (1 mol% of Ru16C with respect to the starting material) was insufficient to solubilize 13 effectively, leading to a solubility challenge with increasing substrate complexity. While increasing the concentration of MeCS could address the solubility problem, it will also reduce light penetration into the solution. To address this, we added 0.1 M of CTAB, which is transparent to visible light, while maintaining a relatively low photocatalyst concentration (ruthenium MeCS = 0.5 mM).57 This system afforded product 14 with 42% isolated yield in aqueous solution.
To assess the sustainability of MeCS in the hydroxytrifloromethylation of vinylbenzene derivatives we calculated the E factor as described by Bu et al. (Scheme 5).34 The E factor58 for this photocatalytic reaction in water was calculated by considering not only the use of organic solvents for the reaction and subsequent product extraction but also the by-products generated during the transformation. This more comprehensive approach provides a more accurate measure of the process's environmental impact. The E factor was determined to be 10.9, which is much lower compared to traditional reactions performed in organic solvents51 or alternative synthetic routes.59 A detailed calculation of the E factor for other synthetic strategies applied to the same transformation is provided in the ESI.† Furthermore, we show that the catalyst can be recovered via simple mini pipette column purification (Fig. S9†) and reused in a second reaction cycle, achieving a comparable yield (82% vs. the initial 84%).
In addition to the hydroxytrifluoromethylation of styrene derivatives, Ru16C MeCS was found to be effective in promoting the hydroxylation of aryl boronic acids (Scheme 6).60 This transformation was achieved under mild conditions with good yields, demonstrating the broad applicability of the catalyst. Detailed reaction conditions and product characterization are provided in the ESI.†
In conclusion, we report here a photoactive MeCS family that uses ruthenium metal complexes as a headgroup. The hydrophobicity of each MeCS was modulated by varying the length of the alkyl chain attached to the metal complex, yet all the synthesized MeCS remained highly soluble in water. The length of the aliphatic chain directly modulates the formation of the micelles, with CMC values as low as 380 μM for the ruthenium MeCS with the longest aliphatic chain. Our results show that the length of the alkyl chain has no significant effect on the absorption spectra, emission spectra, and photoluminescence lifetimes of the MeCSs. However, the quantum yield seems to increase with the length of the alkyl chain and appears to be unaffected by their incorporations in CTAB micelles. This contrasts with previous reports of double-chain ruthenium amphiphiles, where the quantum yield and lifetimes are significantly affected by their incorporation into vesicles. After evaluating the photophysical and CMC properties of each surfactant, Ru16C was selected for the photocatalytic hydroxytrifluoromethylation of aromatic olefins in aqueous solution due to its superior photophysical properties and lower CMC, with yields up to 84%. Furthermore, Ru16C can be applied in other transformation reactions in water, such as the hydroxylation of aryl boronic acids, demonstrating the versatility of MeCS. In water, the micelles' hydrophobic core serves as reaction vessels for substrates, and the hydrophilic surface functions as the photocatalyst. Our MeCS forms micelles in the range of 6 nm in diameter, which highlights the remarkable proximity effects that can occur in this micellar catalytic system. This is expected to enhance the interactions between the metal complex head group (photocatalyst) and the substrate. Finally, the recyclability of Ru16C and the use of water as a solvent significantly improve the system's sustainability.
Data availability
The data supporting this article have been included as part of the ESI.† Specific data files (such as instrument files or data spreadsheets) related to this study are available upon request from the corresponding author.Author contributions
Conceptualization, Y. C. and A. A. M.; methodology, Y. C., A. M. Y., T. V. N., S. C. K., J. G. W., S. L. B., Y. T., and A. A. M.; investigation, Y. C., A. M. Y., T. V. N., and S. C. K.; writing – original draft, Y. C., A. M. Y., Y. T., and A. A. M.; writing – review & editing, Y. C., A. M. Y., T. V. N., S. C. K., J. G. W., S. L. B., Y. T., and A. A. M.; funding acquisition, A. A. M.; resources, J. G. W., S. L. B., Y, T., and A. A. M.; supervision, J. G. W., S. L. B., Y. T., and A. A. M.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support of the Welch Foundation, grant C-2152, and the Rice InterDisciplinary Excellence Award (IDEA) Award. This work was conducted in part using resources of the Shared Equipment Authority at Rice University.References
- K. Zeitler, Angew. Chem., Int. Ed., 2009, 48, 9785–9789 CrossRef CAS PubMed.
- T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527–532 CrossRef CAS PubMed.
- J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC.
- C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed.
- M. H. Shaw, J. Twilton and D. W. C. MacMillan, J. Org. Chem., 2016, 81, 6898–6926 CrossRef CAS PubMed.
- K. Teegardin, J. I. Day, J. Chan and J. Weaver, Org. Process Res. Dev., 2016, 20, 1156–1163 CrossRef CAS PubMed.
- K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035–10074 CrossRef CAS PubMed.
- M. A. Cismesia and T. P. Yoon, Chem. Sci., 2015, 6, 5426–5434 RSC.
- T. Koike and M. Akita, Inorg. Chem. Front., 2014, 1, 562–576 RSC.
- T. P. Yoon, Acc. Chem. Res., 2016, 49, 2307–2315 CrossRef CAS PubMed.
- D. A. Nagib and D. W. C. MacMillan, Nature, 2011, 480, 224–228 CrossRef CAS PubMed.
- D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS PubMed.
- C. Russo, F. Brunelli, G. C. Tron and M. Giustiniano, J. Org. Chem., 2023, 88, 6284–6293 CrossRef CAS.
- M. J. Lawrence, Chem. Soc. Rev., 1994, 23, 417 RSC.
- S. Le Guenic, L. Chaveriat, V. Lequart, N. Joly and P. Martin, J. Surfactants Deterg., 2019, 22, 5–21 CrossRef CAS.
- T. Lorenzetto, G. Berton, F. Fabris and A. Scarso, Catal. Sci. Technol., 2020, 10, 4492–4502 RSC.
- A. D. Smith McWilliams, S. Ergülen, M. M. Ogle, C. A. de los Reyes, M. Pasquali and A. A. Martí, Pure Appl. Chem., 2020, 92, 265–274 CrossRef CAS.
- O. Massarweh and A. S. Abushaikha, Energy Rep., 2020, 6, 3150–3178 CrossRef.
- A. D. Smith McWilliams, Z. Tang, S. Ergülen, C. A. De Los Reyes, A. A. Martí and M. Pasquali, J. Phys. Chem. B, 2020, 124, 4185–4192 CrossRef CAS PubMed.
- U. Umezaki, A. D. Smith McWillams, Z. Tang, Z. M. S. He, I. R. Siqueira, S. J. Corr, H. Ryu, A. B. Kolomeisky, M. Pasquali and A. A. Martí, ACS Nano, 2024, 18, 2446–2454 CrossRef CAS PubMed.
- F. Mancin, P. Scrimin, P. Tecilla and U. Tonellato, Coord. Chem. Rev., 2009, 253, 2150–2165 CrossRef CAS.
- C. Schattschneider, S. Doniz Kettenmann, S. Hinojosa, J. Heinrich and N. Kulak, Coord. Chem. Rev., 2019, 385, 191–207 CrossRef CAS.
- T. Taira, J. Oleo Sci., 2022, 71, 167–175 CrossRef CAS PubMed.
- J. A. Lebrón, F. J. Ostos, M. L. Moyá, M. López-López, C. J. Carrasco and P. López-Cornejo, Colloids Surf., B, 2015, 135, 817–824 CrossRef.
- P. Mahato, S. Saha, S. Choudhury and A. Das, Chem. Commun., 2011, 47, 11074 RSC.
- M. F. Ryan, R. A. Metcalfe, A. B. P. Lever and M. Haga, J. Chem. Soc., Dalton Trans., 2000, 2357–2366 RSC.
- N. Ikuta, S. Takizawa and S. Murata, Photochem. Photobiol. Sci., 2014, 13, 691–702 CrossRef CAS PubMed.
- D. Patra, A. H. Chaaban, S. Darwish, H. A. Saad, A. S. Nehme and T. H. Ghaddar, Colloids Surf., B, 2016, 138, 32–40 CrossRef CAS PubMed.
- S. Nehru, S. Veeralakshmi and S. Arunachalam, New J. Chem., 2017, 41, 13830–13837 RSC.
- S. Bhand, D. N. Lande, E. Pereira, S. P. Gejji, T. Weyhermüller, D. Chakravarty, V. G. Puranik and S. Salunke-Gawali, Polyhedron, 2019, 164, 96–107 CrossRef CAS.
- I. Karakaya, ChemistrySelect, 2021, 6, 11551–11556 CrossRef CAS.
- Y. Timounay, A. Pannwitz, D. M. Klein, A.-L. Biance, M. E. Hoefnagel, I. Sen, A. Cagna, M. Le Merrer and S. Bonnet, Langmuir, 2022, 38, 9697–9707 CrossRef CAS PubMed.
- S. A. Bonke, G. Trezza, L. Bergamasco, H. Song, S. Rodríguez-Jiménez, L. Hammarström, E. Chiavazzo and E. Reisner, J. Am. Chem. Soc., 2024, 146, 15648–15658 CrossRef CAS PubMed.
- M. Bu, C. Cai, F. Gallou and B. H. Lipshutz, Green Chem., 2018, 20, 1233–1237 RSC.
- E. Y. Arashiro and N. R. Demarquette, Mater. Res., 1999, 2, 23–32 CrossRef CAS.
- J. D. Berry, M. J. Neeson, R. R. Dagastine, D. Y. C. Chan and R. F. Tabor, J. Colloid Interface Sci., 2015, 454, 226–237 CrossRef CAS PubMed.
- H. Luo, C.-Y. Sun, Q. Huang, B.-Z. Peng and G.-J. Chen, J. Colloid Interface Sci., 2006, 297, 266–270 CrossRef CAS.
- X. Wang and Y. Gao, Food Chem., 2018, 246, 242–248 CrossRef CAS PubMed.
- K. Suzuki, A. Kobayashi, S. Kaneko, K. Takehira, T. Yoshihara, H. Ishida, Y. Shiina, S. Oishi and S. Tobita, Phys. Chem. Chem. Phys., 2009, 11, 9850 RSC.
- F. Felix, J. Ferguson, H. U. Guedel and A. Ludi, J. Am. Chem. Soc., 1980, 102, 4096–4102 CrossRef CAS.
- D. W. Thompson, A. Ito and T. J. Meyer, Pure Appl. Chem., 2013, 85, 1257–1305 CrossRef CAS.
- P. Kubát and J. Mosinger, J. Photochem. Photobiol. A: Chem., 1996, 96, 93–97 CrossRef.
- H. Sternlicht, G. C. Nieman and G. W. Robinson, J. Chem. Phys., 1963, 38, 1326–1335 CrossRef CAS.
- R. Knoesel, D. Markovitsi, J. Simon and G. Duportail, J. Photochem., 1983, 22, 275–283 CrossRef CAS.
- A. Sillen and Y. Engelborghs, Photochem. Photobiol., 1998, 67, 475–486 CrossRef CAS.
- M. Hansen, S. Troppmann and B. König, Chem.–Eur. J., 2016, 22, 58–72 CrossRef CAS PubMed.
- A. Pannwitz, D. M. Klein, S. Rodríguez-Jiménez, C. Casadevall, H. Song, E. Reisner, L. Hammarström and S. Bonnet, Chem. Soc. Rev., 2021, 50, 4833–4855 RSC.
- W. Zhu, J. Wang, S. Wang, Z. Gu, J. L. Aceña, K. Izawa, H. Liu, V. A. Soloshonok and J. Fluor, Chem, 2014, 167, 37–54 CAS.
- A. S. Nair, A. K. Singh, A. Kumar, S. Kumar, S. Sukumaran, V. P. Koyiparambath, L. K. Pappachen, T. M. Rangarajan, H. Kim and B. Mathew, Processes, 2022, 10, 2054 CrossRef CAS.
- Y. Ogawa, E. Tokunaga, O. Kobayashi, K. Hirai and N. Shibata, iScience, 2020, 23, 101467 CrossRef CAS PubMed.
- Y. Yasu, T. Koike and M. Akita, Angew. Chem., Int. Ed., 2012, 51, 9567–9571 CrossRef CAS PubMed.
- J. W. Beatty, J. J. Douglas, K. P. Cole and C. R. J. Stephenson, Nat. Commun., 2015, 6, 7919 CrossRef PubMed.
- Y. Quan, W. Shi, Y. Song, X. Jiang, C. Wang and W. Lin, J. Am. Chem. Soc., 2021, 143, 3075–3080 CrossRef CAS PubMed.
- B. K. Kundu, C. Han, P. Srivastava, S. Nagar, K. E. White, J. A. Krause, C. G. Elles and Y. Sun, ACS Catal., 2023, 13, 8119–8127 CrossRef CAS.
- W. I. Bengough and G. B. Park, Eur. Polym. J., 1978, 14, 889–894 CrossRef CAS.
- S. Sarhan and N. Seiler, Gen. Pharmacol. Vasc. Syst., 1989, 20, 53–60 CrossRef CAS PubMed.
- C.-C. Chien, S.-C. Kao, C.-J. Chen and Y.-K. Wu, Chem. Commun., 2020, 56, 15470–15472 RSC.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- Q. Li, W. Fan, D. Peng, B. Meng, S. Wang, R. Huang, S. Liu and S. Li, ACS Catal., 2020, 10, 4012–4018 CrossRef CAS.
- Y. Q. Zou, J. R. Chen, X. P. Liu, L. Q. Lu, R. L. Davis, K. A. Jørgensen and W. J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 784–788 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc07623k |
| This journal is © The Royal Society of Chemistry 2025 |