Open Access Article
Open Access ArticleVisible-light responsive hydrogen production from formate with a photoredox system using enzymes and colloidal platinum nanoparticles†
Shintaro
Yoshikawa
a and
Yutaka
Amao
 *ab
*ab
aGraduate School of Science, Osaka Metropolitan University, 3-3-138 Sugimoto, Sumiyoshi-ku, Osaka 558-8585, Japan. E-mail: amao@omu.ac.jp
bResearch Centre of Artificial Photosynthesis (ReCAP), Osaka Metropolitan University, 3-3-138 Sugimoto, Sumiyoshi-ku, Osaka 558-8585, Japan
First published on 8th January 2025
Abstract
Formic acid and formate are among the most promising candidates for hydrogen energy carriers that can be produced from carbon dioxide. As previously reported, H2 production based on catalytic formate decomposition involves two major issues: the use of strong acidic formate with relatively low pH and reaction control after catalyst addition. To address these two issues, visible-light controlled H2 production from formate with the combination system of a biocatalytic process with formate dehydrogenase from Candida boidinii (CbFDH) and a photoredox reaction of water-soluble zinc porphyrin, methylviologen and colloidal platinum nanoparticles dispersed in polyvinylpyrrolidone (Pt-PVP) was developed. By using this system, the yield for formate to H2 was estimated to be ca. 92% after 25 h irradiation.
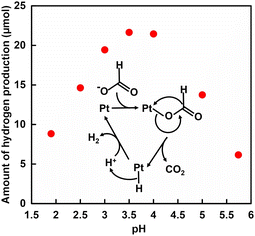
Formic acid has a hydrogen capacity of 4.3 wt% (53 g L−1) and is of growing interest as a promising hydrogen carrier.1,2 The carbon dioxide–formic acid cycle is expected to be a simple and environmentally friendly hydrogen storage and release process.3 To decompose formate into H2 and CO2 selectively, various heterogeneous or homogeneous catalysts containing metals have been studied. For examples of metal-based heterogeneous catalysts, palladium nanoparticles immobilised on inorganic supports and alloy materials containing palladium have been widely studied as heterogeneous catalysts for H2 production based on formate decomposition.4–9 Homogeneous molecular catalysts based on coordination complexes containing metal ions such as Ir3+, Rh3+, and Ru3+ have also been widely investigated as highly active catalysts for H2 production based on formate decomposition.10–16 In contrast, we devoted to colloidal platinum nanoparticles dispersed by a water-soluble polymer with the H2 production catalytic function as a homogeneous catalyst. It was found that colloidal platinum nanoparticles dispersed in polyvinylpyrrolidone (Pt-PVP) catalyse H2 production based on formate decomposition.17–21Fig. 1 shows the pH dependence of H2 production based on formate decomposition with Pt-PVP18 and the mechanism for H2 production.21 The H2 production shown in Fig. 1 is the amount after 3 h incubation.
 | ||
| Fig. 1 The pH dependence of H2 production based on formate decomposition with Pt-PVP. Inset: possible mechanism for H2 production from formate with Pt-PVP. | ||
As shown in Fig. 1, H2 production reaches a maximum around the pKa (3.75) of formic acid. In other words, in formate decomposition with Pt-PVP, H2 production decreases with increasing pH. When formic acid is used safely as a hydrogen carrier, it is more preferable to use it in a formate solution near neutral pH. In addition, the availability of neutral to weakly basic pH solutions allows the simultaneous production of CO2 based on formate decomposition to be fixed in solution as bicarbonate. For example, a study on the practical application of a cycling system for H2 production from potassium formate and hydrogenation of potassium bicarbonate has been reported.22 The volumetric energy density of formate is lower compared to formic acid. However, the safety and toxicological aspects of formate, as well as its sustainability, make it ideal as a hydrogen storage system. Formate is non-corrosive, non-irritant, non-toxic, and easy to handle as a hydrogen energy carrier. Another problem with the thermal H2 production system based on formate decomposition is that H2 production cannot be controlled once the reaction is initiated by the addition of a catalyst. Therefore, we focused on formate dehydrogenase catalysed formate oxidation in the presence of NAD+ under conditions in the neutral pH range.23,24 In particular, FDH from Candida boidinii (CbFDH) is commercially available and widely used as a catalyst for the reduction of CO2.25–27 On the other hand, one approach for controlling hydrogen production is the use of a photoredox system consisting of an electron-donating molecule, visible photosensitising molecule, electron mediator molecule and catalysts. Visible-light driven H2 production with the system of triethanolamine (TEOA) as an electron donor, water-soluble zinc porphyrin, zinc meso-tetra(4-sulfonatophenyl)porphyrin tetrasodium salt (ZnTPPS4−) as a visible photosensitizer, methylviologen (MV2+) as an electron mediator and colloidal platinum nanoparticles or hydrogenase as a catalyst has been reported.28–30 NADH, as well as TEOA, has been reported to act as an electron donor in this system.28 Thus, by using redox coupling between NAD+ and NADH, visible light-driven H2 production from formate can be achieved as shown in Fig. 2.
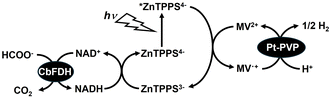 | ||
| Fig. 2 Visible-light driven H2 production from formate with the system of CbFDH, NAD+, ZnTPPS4−, MV2+ and Pt-PVP. | ||
In this study, visible-light controlled H2 production from formate with the combination system of a biocatalytic process with CbFDH and a photoredox reaction of ZnTPPS4−, MV2+ and Pt-PVP was investigated.
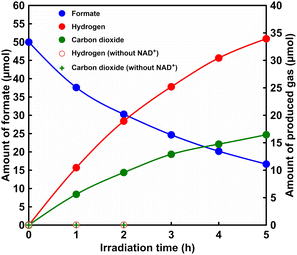
First, visible-light driven H2 production with the system of sodium formate, CbFDH, NAD+, ZnTPPS4−, MV2+ and Pt-PVP in phosphate buffer (pH 7.0) was investigated. Details of the materials used and all experimental procedures are described in the ESI (Fig. S1(a) and (b)†). The amount of formate was estimated by ion chromatography with an ion exclusion column. Fig. S2† shows the chromatogram of sodium formate (0–100 mM) in 500 mM-HEPES buffer (pH 7.0). The inset of Fig. S2† shows the relationship between the sodium formate concentration and the detection peak area. The amount of H2 and CO2 production was determined using a gas chromatograph (GC-2014, SHIMADZU Corporation) with a thermal conductivity detector (TCD). The calibration curve for the determination of the amount of H2 and CO2 by gas chromatograph is shown in Fig. S3, S4, eqn S2 and S3,† respectively. Fig. 3 shows the time dependence of the amount of formate, H2 and CO2 in the sample solution with irradiation. As shown in Fig. 3, H2 and CO2 were produced with irradiation time. On the other hand, formate was decreased with reaction time. After 5 h irradiation, 33.9 μmol of H2 was produced and the yield for formate to hydrogen was estimated to be ca. 68%. In addition, formate consumption was estimated to be ca. 33.3 μmol. The amount of formate consumed was equivalently related to the amount of H2 produced.
In contrast, 16.5 μmol of CO2 was produced after 5 h reaction time. CO2 was detected at about half the amount of H2 produced. It is important to note that the detected amount of carbon dioxide is present in the gas phase of the reaction vessel. The mole fractions of CO2, bicarbonate and carbonate in the solution at pH 7.0 are estimated to be 11.3, 88.5 and 0.2% respectively. After 25 h irradiation, 9.0% hydrochloric acid solution was added to the sample solution and stirred under dark conditions for 1 h to adjust the pH to 1.5. After this procedure, the gas phase was analysed by gas chromatography. 45 and 37 μmol of H2 and CO2 were detected in the gas phase, respectively. The results support that by lowering the pH of the sample solution to the acidic side, bicarbonate was converted to CO2 and transferred to the gas phase. Thus, this suggests that the remaining CO2 dissolves as bicarbonate in solution. After 25 h irradiation, moreover, 45.8 μmol of H2 was produced and the yield for formate to H2 was estimated to be ca. 92%. After 25 h of visible-light irradiation, thus, the initial formate was completely converted to H2. Next let us discuss the tolerance of this system. The change in the UV-visible absorption spectra of the reaction solution during irradiation was measured. Fig. S5† shows the time dependence of difference spectra in the sample solution from before to after light irradiation (inset: UV-vis absorption spectrum of ZnTPPS4−). As shown in Fig. S5,† the absorption bands of ZnTPPS4− (420, 555 and 595 nm) decreased with light irradiation. The reduced concentration of ZnTPPS4− was calculated to be approximately 4.8 μM (24 nmol), and it is estimated that approximately half of the ZnTPPS4− was decomposed after 5 h irradiation. Next, let us discuss the tolerance of CbFDH in this system. After 25 h irradiation, the formate was almost consumed, thus, 50 μmol of sodium formate was added to evaluate the remaining activity of CbFDH. After 5 h of re-addition of sodium formate, approximately 8 μmol was consumed during irradiation as shown in Fig. S6.† Thus, CbFDH-catalysed formate oxidation proceeds, but the catalytic activity is predicted to decrease over time. Let us compare this system with H2 production by photolysis of water using a visible light-responsive photocatalyst such as poly(heptazine imides),31 CoOx and Rh/Cr2O3 on poly(triazine imide).32 It is difficult to make a simple comparison between H2 production using water as an electron source and based on formate decomposition because the purposes are different; however, the wavelength range of visible light that can be used by these photocatalysts is often at the long wavelength end of 500 nm. The advantage of this system is that by using ZnTPPS4− as a sensitizer, it is expected that H2 production can be achieved using visible light more than 500 nm. Fig. S7(a)† shows the time dependence of H2 and CO2 production with irradiation through the optical filter Y52 (the characteristic of the optical filter Y52 is shown in Fig. S7(b)†), which transmits wavelengths above 520 nm. As shown in Fig. S7(a),† H2 and CO2 were produced with irradiation time. After 5 h irradiation, 17.0 and 10.1 μmol of H2 and CO2 were produced. Although the amount of hydrogen produced was less than that without the optical filter, H2 production was achieved even with irradiation with visible light more than 520 nm. Next, it was confirmed that H2 and CO2 were produced from formate in the system shown in Fig. 2. In the presence of NADH, CO2 production proceeds based on formate oxidation catalysed by CbFDH. In addition, it has been reported that visible light-driven H2 production proceeds using a system composed of NADH, ZnTPPS4−, MV2+ and Pt-PVP.28 It has also been reported that H2 production based on formate decomposition catalysed by Pt-PVP does not proceed under pH 7 conditions.18 Because the CbFDH-catalysed formate oxidation and visible-light-driven H2 production are linked by the NAD+/NADH redox cycle, removing NAD+ from the system should result in neither CO2 nor H2 being produced. As shown in Fig. 3, when the system consisting of sodium formate, CbFDH, ZnTPPS4−, MV2+ and Pt-PVP, in other words, excluding NAD+ was irradiated with visible light, no H2 and CO2 were produced. In other words, the CbFDH-catalysed formate oxidation and the visible light-driven H2 production via the NAD+/NADH redox cycle as shown in Fig. 2 were accomplished.
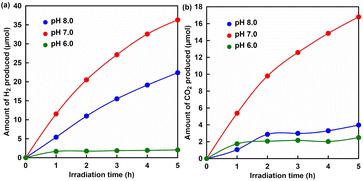
Next, pH dependence of the visible-light driven H2 production with the system of sodium formate, CbFDH, NAD+, ZnTPPS4−, MV2+ and Pt-PVP in phosphate buffer was investigated. Fig. 4 shows the time dependence of H2 (a) and CO2 (b) in the sample solution of sodium formate, CbFDH, NAD+, ZnTPPS4−, MV2+ and Pt-PVP with the irradiation under pH conditions of 6.0, 7.0 and 8.0. As shown in Fig. 4, 2.06, 33.9 and 22.4 μmol of H2 were produced under pH conditions of 6.0, 7.0 and 8.0 after 5 h irradiation, respectively. In contrast, 2.50, 16.8 and 3.98 μmol of CO2 were produced under pH conditions of 6.0, 7.0 and 8.0 after 5 h irradiation, respectively. The production of H2 and CO2 was found to be dependent on the pH of the sample solution. In terms of H2 production, the highest hydrogen production occurred under pH 7.0 conditions of the sample solution, while H2 production decreased under pH 8.0 and 6.0 conditions. The lowest H2 production was observed under pH 6 conditions with a high concentration of H+ compared to other conditions. Also, it was found that CO2 production was lower than that under other pH conditions. Here, the reduction of NAD+ to NADH based on the oxidation of formate catalysed by CbFDH proceeds irrespective of visible light irradiation or non-irradiation. Fig. S9† shows the relationship between the initial concentration of NAD+ and the reaction rate for NADH production due to formate oxidation with CbFDH under various pH conditions.
As shown in Fig. S9,† the rate for formate oxidation was significantly decreased at pH 6 and the optimum pH for CbFDH-catalysed formate oxidation was around 7, so under pH 6 conditions, NADH production was decreased due to the reduced catalytic activity of CbFDH for formate oxidation. In the visible light-driven H2 production system comprising ZnTPPS4−, MV2+ and Pt-PVP, NADH acts as an electron donor, suggesting that under pH 6 conditions, H2 production was reduced due to lower NADH production following CbFDH-catalysed formate oxidation. Under pH 8 conditions, on the other hand, the amount of H2 produced was about 0.66 times lower than that under pH 7 conditions. This is predicted to be due to a decrease in the amount of H+ with increasing pH. On the other hand, a significant decrease in CO2 production was observed compared to hydrogen production under pH 8.0 conditions. It has been reported that the catalytic activity of CbFDH for the oxidation of formate is not significantly reduced under pH 8 conditions. It is predicted that most of the CO2 produced is dissolved as bicarbonate in a buffer solution at pH 8. The amount of CO2 production shown in Fig. 4(b) is the result of quantifying the gas phase of the reaction vessel by gas chromatography. This means that under pH 8 conditions, it was suggested that more than 80% of the CO2 produced was fixed as bicarbonate in the buffer solution. These results show that the system can efficiently produce H2 under pH 7 conditions, and that neutral formate can be used as a raw material instead of strong acidic formic acid.
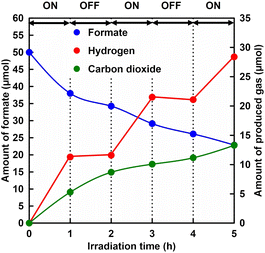
Finally, the visible light control of H2 production using the system of sodium formate, CbFDH, NAD+, ZnTPPS4−, MV2+ and Pt-PVP in phosphate buffer (pH 7) was investigated. Fig. 5 shows the time dependence of the amount of formate, H2 and CO2 in the sample solution during the light-on/off cycle. As shown in Fig. 5, formate was consumed with reaction time irrespective of irradiation or non-irradiation. Also, CO2 was produced with reaction time irrespective of irradiation or non-irradiation. In contrast, H2 was produced in response to irradiation and stopped completely under non-irradiation conditions. Thus, NADH produced during CbFDH-catalysed formate oxidation accumulates under dark conditions, but direct electron transfer from the produced NADH to Pt-PVP does not proceed and H2 production is suppressed. In addition, H2 production based on formate decomposition catalysed by Pt-PVP is also suppressed under neutral pH conditions. Finally, let us discuss the catalytic activity of Pt-PVP in this reaction system. As colloidal Pt-PVP consists of platinum atoms on the surface and in the bulk, the catalytically active sites are likely to be platinum atoms on the surface of Pt-PVP. According to previous reports, the amount of platinum atoms on the surface is calculated to be 0.11 μmol.33,34 Thus, the turnover number (TONs) of Pt-PVP for H2 production from formate solution in this system was determined to be 255. Furthermore, NAD+/NADH recycling was also achieved, as the amount of H2 production was observed above the initial amount of NAD+.
In conclusion, visible-light controlled H2 production from formate with the combination system of a biocatalytic process with CbFDH and a photoredox reaction of ZnTPPS4−, MV2+ and Pt-PVP was developed and formate was completely converted to H2 after 25 h irradiation under pH 7 conditions. On the other hand, it was also shown that approximately half of the CO2 produced during formate decomposition is fixed as bicarbonate in a buffer solution at pH 7.
Data availability
The authors confirm that the data supporting the findings of this manuscript are available within the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by a grant-in-aid for specially promoted Research (23H05404), and Scientific Research (B) (22H01872), (22H01871).Notes and references
- J. Yang, A. Sudik, C. Wolverton and D. J. Siegel, Chem. Soc. Rev., 2010, 39, 656 RSC.
- A. K. Singh, S. Singh and A. Kumar, Catal. Sci. Technol., 2016, 6, 12 Search PubMed.
- H. Zhong, M. Iguchi, M. Chatterjee, Y. Himeda, Q. Xu and H. Kawanami, Adv. Sustainable Syst., 2018, 2, 1700161 CrossRef.
- D. A. Bulushev, L. J. Jia, S. Beloshapkin and J. R. H. Ross, Chem. Commun., 2012, 48, 4184 Search PubMed.
- D. A. Bulushev, S. Beloshapkin, P. E. Plyusnin, Y. V. Shubin, V. I. Bukhtiyarov, S. V. Korenev and J. R. H. Ross, J. Catal., 2013, 299, 171 CrossRef CAS.
- Y. Zhao, L. Deng, S. Y. Tang, D. M. Lai, B. Liao, Y. Fu and O. X. Guo, Energy Fuels, 2011, 25, 3693 CrossRef CAS.
- B. J. O'Neill, E. I. Gürbüz and J. A. Dumesic, J. Catal., 2012, 290, 193 CrossRef.
- O. Metin, X. L. Sun and S. H. Sun, Nanoscale, 2013, 5, 910 RSC.
- K. Mori, M. Dojo and H. Yamashita, ACS Catal., 2013, 3, 1114 CrossRef CAS.
- Y. Himeda, S. Miyazawa and T. Hirose, ChemSusChem, 2011, 4, 487 Search PubMed.
- Y. Himeda, Green Chem., 2009, 11, 2018 RSC.
- Y. Himeda, N. Onozawa-Komatsuzaki, H. Sugihara and K. Kasuga, Organometallics, 2007, 26, 702 Search PubMed.
- Y. Himeda, N. Onozawa-Komatsuzaki, H. Sugihara, H. Arakawa and K. Kasuga, Organometallics, 2004, 23, 1480 CrossRef CAS.
- J. F. Hull, Y. Himeda, W. H. Wang, B. Hashiguchi, R. Periana, D. J. Szalda, J. T. Muckerman and E. Fujita, Nature Chem., 2012, 4, 383 CrossRef CAS PubMed.
- S. Fukuzumi, T. Kobayashi and T. Suenobu, ChemSusChem, 2008, 1, 827 Search PubMed.
- A. Boddien, B. Loges, H. Junge and M. Beller, ChemSusChem, 2008, 1, 751 Search PubMed.
- Y. Minami, Y. Muroga, T. Yoshida and Y. Amao, Chem. Lett., 2019, 48, 775 Search PubMed.
- Y. Minami and Y. Amao, Sustainable Energy Fuels, 2020, 4, 3458 Search PubMed.
- Y. Minami, Y. Muroga and Y. Amao, New J. Chem., 2020, 44, 14334 RSC.
- Y. Minami and Y. Amao, New J. Chem., 2021, 45, 11461 RSC.
- Y. Minami and Y. Amao, J. Jpn. Petrol. Inst., 2021, 64, 203 Search PubMed.
- R. Sang, C. A. M. Stein, T. Schareina, Y. Hu, A. Léval, J. Massa, V. Turan, P. Sponholz, D. Wei, R. Jackstell, H. Junge and M. Beller, Nature Commun., 2024, 15, 7268 Search PubMed.
- W. Hummel and M. R. Kula, Eur. J. Biochem., 1989, 184, 1 CrossRef CAS PubMed.
- J. G. Ferry, FEMS Microbiol. Rev., 1990, 7, 377 CrossRef CAS PubMed.
- J. H. Kim, D. H. Nam and C. B. Park, Curr. Opin. Biotechnol., 2014, 28, 1 CrossRef CAS PubMed.
- S. K. Kuk, R. K. Singh, D. H. Nam, R. Singh, J. K. Lee and C. B. Park, Angew. Chem., Int. Ed., 2017, 56, 3827 CrossRef CAS PubMed.
- H. Wu, C. Tian, X. Song, C. Liu, D. Yang and Z. Jiang, Green Chem., 2013, 15, 1773 RSC.
- Y. Amao, ChemCatChem, 2011, 3, 458 CrossRef CAS.
- Y. Amao and I. Okura, J. Mol. Catal. A:Chem., 1996, 105, 125 CrossRef CAS.
- N. Kaji, S. Aono and I. Okura, J. Mol. Catal., 1986, 36, 201–203 Search PubMed.
- Q. Wang, S. Li, D. Zheng, S. Wang, Y. Hou and G. Zhang, ACS Appl. Energy Mater., 2024, 7, 6090 CrossRef CAS.
- M. Liu, G. Zhang, X. Liang, Z. Pan, D. Zheng, S. Wang, Z. Yu, Yi. Hou and X. Wang, Angew. Chem., Int. Ed., 2023, 62, e202304694 CrossRef CAS PubMed.
- H. Kotani, R. Hanazaki, K. Ohkubo, Y. Yamada and S. Fukuzumi, Chem. Eur. J., 2011, 17, 2777 CrossRef CAS PubMed.
- Y. Matsubara, Y. Muroga, M. Kuwata and Y. Amao, Sustainable Energy Fuels, 2022, 6, 3717 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4se01245c |
| This journal is © The Royal Society of Chemistry 2025 |