Open Access Article
Open Access ArticlePhosphorus recovery from animal manures through pyrolysis: phosphorus transformations, release mechanisms, and applications of manure biochars in agriculture
Jesper T. N.
Knijnenburg
 a,
Siraprapa
Suwanree
a,
Siraprapa
Suwanree
 b,
Duncan
Macquarrie
b,
Duncan
Macquarrie
 c,
Pornnapa
Kasemsiri
c,
Pornnapa
Kasemsiri
 b and
Kaewta
Jetsrisuparb
b and
Kaewta
Jetsrisuparb
 *b
*b
aInternational College, Khon Kaen University, Khon Kaen 40002, Thailand
bDepartment of Chemical Engineering, Khon Kaen University, Khon Kaen 40002, Thailand. E-mail: kaewta@kku.ac.th; Fax: +6643362142; Tel: +6643362240
cDepartment of Chemistry, University of York, York YO10 5DD, UK
First published on 4th February 2025
Abstract
Phosphorus (P) is a vital element to enhance crop growth, but the excessive application of water-soluble P fertilizers has led to dwindling global P resources and elevated P levels in surface and ground waters. At the same time, high levels of P are excreted by livestock and poultry industries. These animal manures present an attractive source of secondary P, but the direct application of manures to farmlands may cause issues with P losses and environmental and health risks. To overcome this, pyrolysis (the thermal conversion of a biomass in oxygen-poor conditions) has been used in some situations without a full understanding of the impacts of the pyrolysis process on P forms and availability in the manure. This article critically reviews the use of pyrolysis to recover P from three types of animal manures (cow, swine, and poultry) in the form of biochars for applications in agriculture. Specific emphasis is paid to the P species in manures and their transformations during the pyrolysis process with the help of spectroscopic techniques (e.g., 31P NMR and XANES) and P fractionation schemes. The P concentrations, species, and availability are highly dependent on manure composition and especially pyrolysis conditions. During pyrolysis, the P is concentrated in the solid phase (biochar) and transformed into more inorganic (orthophosphate) and more crystalline forms as the pyrolysis temperature increases. Higher pyrolysis temperatures reduce the P extractability, which lowers the risk for P losses but may also affect plant P uptake. Strategies to modify P availability are presented and critical perspectives are given on the risks and limitations of manure-derived biochar application in agriculture.
Sustainability spotlightThe pyrolysis of animal manures to recover phosphorus in the form of biochars addresses sustainability issues by contributing to both waste management (i.e., the transformation of manures into clean, phosphorus-rich materials), agriculture (i.e., enhancing soil fertility and crop growth by returning phosphorus to the soil), and reduced dependence on phosphorus fertilizers derived from increasingly scarce phosphorus deposits. The P concentrations and species transformation in the manure biochars and their agronomic effectiveness are highly dependent on the manure composition and pyrolysis conditions, which are reviewed in this work. This aligns with the UN sustainable development goals on zero hunger (SDG 2), clean water and sanitation (SDG 6), and responsible consumption and production (SDG 12). |
1. Introduction
Phosphorus (P) is an indispensable element for all life, and large amounts of P are required in agriculture to meet crop demands to feed the growing world population.1 Nearly all P in fertilizers originates from non-renewable phosphate rock, of which the largest deposits are in Morocco, Western Sahara,2 and (as recently discovered) in Norway.3 Some estimates suggest that phosphate rock may be depleted within the next 30–300 years.4 Furthermore, the use of P in agriculture is largely a linear process (from phosphate rock into P sinks). It is estimated that only around 20% of the mined P is consumed by humans,5 and approximately 3–10% of the P applied to croplands is lost through runoff, erosion, and leaching.6–8 In the former EU15 countries, these losses have been estimated at approximately 0.1 Tg P per year,7,8 while globally, combined losses from runoff, erosion, and leaching are estimated at 3.0 Tg P per year.6 Although P losses from agricultural lands have greatly decreased in the last decades, agriculture still heavily relies on synthetic P fertilizers from non-renewable P sources like phosphate rock.5,6 The agricultural P flows urgently need to be converted into a fully circular system by using P from secondary raw materials,9,10 which can only be done if P in waste streams is effectively fed back to the soil into the food chain.At the same time, P-rich waste streams are produced in large quantities. Animal manures are comprised of animal excreta (feces and urine) mixed with bedding materials and wasted feed that is used for fertilization of land. Manure presents an ideal secondary P source because it is renewable, has high P concentration, and is available in large quantities on livestock farms. In the United States alone it was estimated that approximately 1.4 × 1012 kg (1.5 billion tons) of manure was produced in 2017, the large majority of which is from beef cattle (78%) followed by dairy (19%), horse (3%), poultry (0.07%), and swine (0.04%).11 The excreted P in these manures sums up to a total of 2.3 × 109 kg per year in 2017.11,12 Phosphorus excretion rates per animal have been estimated at 9–28 kg P per individual per year for cows, 3 kg P per individual per year for swine, and 0.12–0.35 kg P per individual per year for poultry.13 Manure thus presents a considerable source of P that is cycled back into the soil and enhances crop yields. Historically, there was little reliance on external P sources and all P from both farm and domestic wastes was recycled back onto the farmlands. But with the emergence of P fertilizers, the fraction of such recycled P has decreased; approximately 50% of P on agricultural lands originated from animal manures in 1961, which dropped to 32% in 2013.6 At the same time, livestock farming has become significantly more intensive during the past few decades, leading to increased manure production. The manure is often applied to relatively small agricultural areas, typically with the aim to meet N requirements of the crops, which results in nutrient surpluses in the soil.11,14–16 This overapplication of manures to farmlands may lead to environmental issues and the subsequent leaching of P into rivers by both surface and subsurface pathways, causing significant deterioration in water quality and biodiversity.17,18 In addition, animal manures may contain heavy metals, pathogenic bacteria, hormones and antibiotics, and the direct disposal of manure to the environment could result in the release of such pollutants to soil, air, and water bodies.19 Moreover, the improper storage and handling of manure presents a risk to human and animal life due to the potential transmission of zoonotic pathogens to food and water.20
Biochars are produced by the thermochemical conversion (pyrolysis) of a biomass under oxygen-limited conditions. During pyrolysis, the organic matrix is largely thermally decomposed and a carbonaceous material rich in inorganics is left behind. In the case of manure, when manure undergoes pyrolysis the solid material has a greatly reduced volume compared to the unpyrolyzed manure, resulting in higher P concentrations and easier storage and transportation. Pyrolysis can eliminate pathogens, antibiotics, steroids, and other micropollutants,14,21 which reduces the environmental and health risks of biochars compared to the raw manure. When the thermal treatment is carried out in a closed system under wet conditions, the process is known as hydrothermal carbonization (HTC), which produces a hydrochar.22 The HTC process has distinct advantages over pyrolysis when wet biomasses are used since drying of the feedstock is not necessary, and a lower energy input is required because of the lower operating temperatures (typically 180–250 °C) than pyrolysis.22 Compared to (hydro)thermal treatment, alternative P recovery approaches from manure such as pelleting or composting have high transportation cost, whereas precipitation of P through the formation of Ca phosphates and/or Mg phosphates is limited by the high organic matter contents of manure.18 Pyrolysis has some advantages over incineration in that pyrolysis results in the generation of combustible gases that can be used for energy generation, and P availability from biochars is generally higher than from ashes.18,23 Moreover, compared to other manure management techniques, pyrolysis leads to lower greenhouse gas emissions.14
Several authors have reviewed the P transformations in thermal conversion of biomasses.14,24–29 Most notably, the article by Huang et al.27 provides an excellent review of P speciation and transformation in (hydro)thermal treatment of a wide range of solid biowastes, and the extensive article by Lidman Olsson et al.28 gives a very comprehensive insight into the P chemistry in thermal conversion of biomasses. Rathnayake et al.14 provide a holistic view of the potential and limitations of manure pyrolysis to provide energy and biochar. Other reviews have focused specifically on the P recovery from sewage sludges through thermal processes.9,30 Recent review articles on livestock manures present P recovery techniques31 and a bibliometric review on the established literature,32 or focus on manure pyrolysis for bioenergy33 or soil remediation.34 The P transformations in manures and other biomasses during HTC and comparisons with pyrolysis have been discussed in several recent review articles.25,27,30,35
This critical review provides specific insight into the P recovery from manures through pyrolysis for applications in agriculture. The article focuses on P species in primarily three types of manures (cow, swine, and poultry). Transformations of the P species during pyrolysis are discussed with the focus on spectroscopic techniques, fractionation schemes, solubility/extractability, and availability to plants. Furthermore, process steps to enhance the P availability are briefly discussed and the article finishes with a critical discussion on limitations and provides future perspectives.
2. Phosphorus content and species in manures
2.1 Concentrations of phosphorus and metal cations in manures
Fig. 1 provides an overview of the typical P concentrations together with major cations in manures of different animals. Generally, poultry and swine manures contain higher P contents than cow manure. Phytate, the main P storage form in plant seeds, cannot be broken down by monogastric animals like swine and poultry because of the low activity of phytase enzymes in the gut.71 To overcome this, the feed has been traditionally supplemented with inorganic P sources, which has led to higher P excretion.72,73 Nowadays it is more common to add phytase in the diet of monogastric animals to break down the organic P, which substantially increases the P availability and utilization and in turn reduces P excretion.73–75 Even though ruminant animals (like cows and sheep) can digest phytate, the P use efficiency is only about 40%, and consequently a large proportion of the P is excreted.73 Within species, manure P contents may differ due to, e.g., diet, growth stage, physiology, size, and manure handling.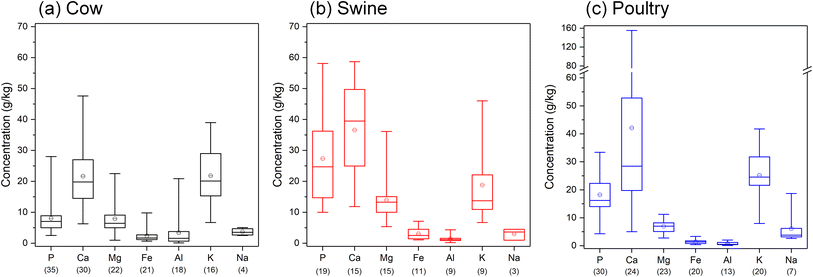 | ||
| Fig. 1 Typical concentrations of P and major cations in manures of (a) cow, (b) swine, and (c) poultry. The values in the boxes represent the median (middle line) with the first (25%) and third (75%) quartiles, and whiskers represent the minimum and maximum values. Circles indicate the mean values. The values in brackets below each element indicate the number of data points used for that element. Figures are compiled from various literature sources for cow,36–54 swine,21,36,38–40,43,45–48,54–60 and poultry manures.36,38,40,43–45,47,51,53–55,59,61–70 | ||
The P in manures does not exist as free (ortho)phosphate ions but is frequently bound to metal cations. When considering the recovery and availability of P, the presence of such cations should be considered as well, because the binding ion may (greatly) affect the P release. In all manures, the major cation is calcium (Ca), which is typically present in the order of 10–50 g kg−1 but may be as high 160 g kg−1. The presence of Ca and magnesium (Mg) is especially important; several studies have demonstrated that the P mobility is related to the Ca and Mg concentrations in manures, and that high Ca contents decrease the P release.36,76–78 After Ca, the second most abundant metal is potassium (K, 6–46 g kg−1) followed by Mg (1–36 g kg−1). Concentrations of iron (Fe), aluminum (Al), and sodium (Na) are relatively minor and are generally below 5 g kg−1.
2.2 Phosphorus species in manures
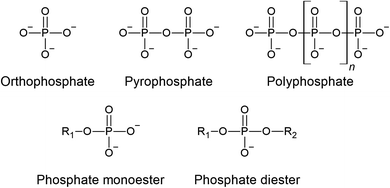
In addition to the P content, the form in which the P is present in manures is also crucial to understand because the species greatly influences its release, mobility, and bioavailability, and may also affect the P recovery approach. The P forms and their availability may be affected by a number of factors such as manure storage,79–81 drying conditions,39,82 and animal diet.42 The most common P forms in manures are highlighted in Fig. 2.83,84 The simplest form is orthophosphate, which can be present as deprotonated (PO43−) or protonated (HPO42− and H2PO4−) forms. Two orthophosphate molecules can condense to form a pyrophosphate (P4O74−) molecule. Further condensation leads to the formation of polyphosphates, the length of which may reach up to several thousand units.85 Inorganic P accounts typically for 50–75% of the total P in manures,43,45 but also higher values have been reported.36 Organic P in biomasses is where the P is bound to carbon in the form of phosphoester (P–O–C) linkages. Examples of phosphate monoesters include phytate (inositol hexakisphosphate), which is the main P storage form in seeds. Phosphate diesters include DNA, RNA, and phospholipids. Examples of organic pyrophosphates and tripolyphosphates are ADP and ATP, respectively.The identification and quantification of the P species in the manures is challenging because of the complex and varying nature of the manure composition. A powerful tool that can be used to provide insight into the P species is 31P nuclear magnetic resonance (NMR) spectroscopy. Compared to solid state NMR spectroscopy, solution 31P NMR has the advantages of narrow peaks that can be easily defined, but a disadvantage is that the P needs to be extracted from the matrix first. Among extractants, most commonly a NaOH-EDTA solution has been used.86 However, the use of improper extraction conditions could affect the P extractability and the disappearance of peaks for specific P forms.61,87,88 Other extractants like DI water may be preferred in some situations.89Fig. 3 provides an overview of the P species that have been identified in manures with solution 31P NMR spectroscopy. By far, the most common P form in manures is orthophosphate, which in most manures accounts for more than half of the total P present. Small amounts of condensed phosphates like pyrophosphate or polyphosphate may be present, but these usually contribute only a small fraction of the P. Monoester phosphates make up another large fraction, primarily in the form of phytate. When comparing manures of different animals, poultry manure tends to have higher organic P concentrations than swine and cow manures. Unlike ruminant animals like cows and sheep, monogastric animals like swine and poultry have insufficient phytase activity in the gut, so the phytic acid cannot be digested and dietary phytate will be excreted if no phytase is included in the diet.71
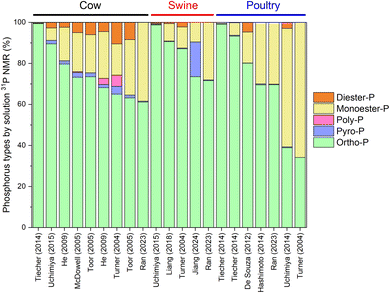 | ||
| Fig. 3 Overview of phosphorus types in cow, swine, and poultry manures as analyzed by liquid phase 31P NMR spectroscopy using data from various sources.21,36,42,45,57,61,77,90–94 | ||
A different approach to specify the P species in manures is by fractionation based on the different solubilities of the different P forms.79,95–98 Hedley et al.99 developed a protocol to separate P forms in soils, which was later adapted for manures,91,95,100 digestates,97 and biochars.101,102 In this procedure, the solid is sequentially extracted with extractants of increasing strength, typically DI water, 0.5 M NaHCO3, 0.1 M NaOH, and 1 M HCl. Durations of each step and solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratios may vary between laboratories. The obtained P fractions can be described as readily water-soluble P (H2O-P), labile P that is readily available to plants (NaHCO3-P), lesser available inorganic P that is associated with Al and Fe compounds (NaOH-P), and inorganic P associated with or trapped in acid-soluble minerals such as apatites (HCl-P).103,104 Any P that is not extracted in these combined steps is the residual P (Res-P). Higher H2O-P values may suggest a higher risk for short-term P losses.102,105,106 The inorganic P (i.e., orthophosphate) concentrations in each fraction are usually measured with UV-vis spectroscopy via either molybdenum blue method or malachite green method. To determine the total P in each fraction, each liquid extract undergoes digestion to convert all organic and condensed P into orthophosphate.107 The total P (now in orthophosphate form) is measured spectroscopically, and the organic P (which also includes condensed P forms) is taken as the difference between total P and inorganic P. Fractionation procedures have been employed in combination with 31P NMR measurements38,46,108 or enzymatic hydrolysis40,63,81,109 to get further insight into the P species.
liquid ratios may vary between laboratories. The obtained P fractions can be described as readily water-soluble P (H2O-P), labile P that is readily available to plants (NaHCO3-P), lesser available inorganic P that is associated with Al and Fe compounds (NaOH-P), and inorganic P associated with or trapped in acid-soluble minerals such as apatites (HCl-P).103,104 Any P that is not extracted in these combined steps is the residual P (Res-P). Higher H2O-P values may suggest a higher risk for short-term P losses.102,105,106 The inorganic P (i.e., orthophosphate) concentrations in each fraction are usually measured with UV-vis spectroscopy via either molybdenum blue method or malachite green method. To determine the total P in each fraction, each liquid extract undergoes digestion to convert all organic and condensed P into orthophosphate.107 The total P (now in orthophosphate form) is measured spectroscopically, and the organic P (which also includes condensed P forms) is taken as the difference between total P and inorganic P. Fractionation procedures have been employed in combination with 31P NMR measurements38,46,108 or enzymatic hydrolysis40,63,81,109 to get further insight into the P species.
Fig. 4a provides an overview of typical distributions of sequential P extracts from cow, swine, and poultry manures. Generally, the P in cow manures is highly labile: the majority (50–87%) of the P is found in the labile P fractions (H2O-P + NaHCO3-P), suggesting a high P availability. Because water-extractable P concentrations can estimate the P in runoff from soil-applied livestock manures,106 highly labile P may thus also indicate a high risk for P dissolution into runoff. The NaOH-P fraction of cow manure contains 6–21% of total P, and the HCl-P and Res-P together may contain 5–37% of total P. Similar P distributions are found in swine manure, with H2O-P + NaHCO3-P (labile P) values of 38–78%, NaOH-P of 5–51%, and HCl-P + Res-P ranging from 11 to 46%. Compared with cow manure, swine manure tends to have less labile P and more NaOH-P. The P in poultry manures, on the other hand, is less readily extractable with a large fraction in HCl-P. The labile P (H2O-P + NaHCO3-P) accounts for 31–69%, NaOH-P is around 3–20% and HCl-P + Res-P is 17–59%. Similar distributions in P species were found with other fractionation procedures.36
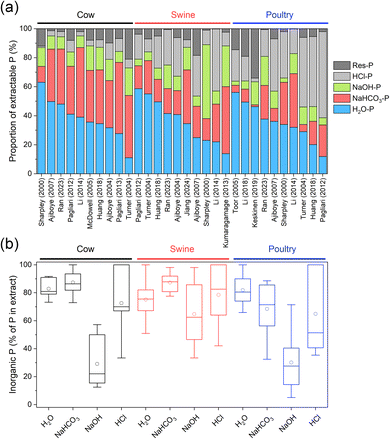 | ||
| Fig. 4 (a) Phosphorus distribution in sequential extracts of cow, swine, and poultry manures based on data from various sources.38–41,43,45,57,61,67,77,90,100,110–112 (b) Percentage of inorganic P in each extract for cow (n = 7), swine (n = 6), and poultry manures (n = 8), based on data from various sources.38,40,43,45,63,100,111 | ||
The extracted P fractions can be further separated into inorganic and organic P. The H2O-P and NaHCO3-P fractions of cow, swine, and poultry manures consist mostly of inorganic P (Fig. 4b), whereas the NaOH fractions are richest in organic P. As confirmed by 31P NMR measurements, the labile P fractions (H2O-P and NaHCO3-P) contain mainly orthophosphate whereas organic P (mainly phytate) is primarily extracted in the NaOH fraction.38,46,108 Others have used P K-edge X-ray absorption near edge spectroscopy (XANES) spectroscopy to identify the P species in fractional extracts of various manures.38,77 Pagliari and Laboski40 analyzed manure samples from 7 species (beef and dairy cattle, swine, chicken, turkey, dairy goat, horse, and sheep) by sequential fractionation. They found a clear division between ruminant + horse and non-ruminant animals. For manures from ruminants and horse, most inorganic P was extracted with H2O and NaHCO3, whereas in non-ruminant species a large inorganic P fraction was also extracted with HCl. Non-ruminant animals had much higher total P and inorganic P contents in manure than ruminant + horse.
The Standards, Measurements, and Testing (SMT) harmonized protocol was developed by Ruban et al.113 for extractable P contents in freshwater sediments. This non-sequential procedure divides the P forms into five fractions: NaOH-extractable P (NaOH-P, which is P bound to Al, Fe, and Mn oxides and hydroxides), HCl-extractable P (HCl-P, which is P associated with Ca), inorganic P (IP), organic P (OP), and total P (TP). The NaOH-P and HCl-P are frequently referred to as non-apatite inorganic P (NAIP) and apatite P (AP), respectively.96 While developed for sediments, this protocol has been occasionally used for P speciation in manures. García-Albacete et al.96 found that pig slurry consisted mainly of inorganic P (95% of total P), which in turn consisted largely of AP (51% of total P) followed by NAIP (35% of total P). Tuszynska et al.98 found that the P fractions in livestock manures decreased in order AP > NAIP > OP. Similarly, Zuo et al.114 measured that chicken manure consisted of 16% OP, 41% NAIP, and 43% AP, and dairy manure contained 7% OP, 60% NAIP, and 33% AP.
Solid state characterization techniques have been applied mainly to provide a qualitative indication of the P species. For example, using solid state 31P NMR analysis, different P species have been identified in poultry manure,115 dairy manure,42 swine feces,56 and sheep feces.116 Other researchers have used P K-edge XANES to differentiate between P forms.21,38,57,77,91,110,117 X-ray diffraction (XRD) has been used to analyze crystalline P forms of manures.45,56,57,116,117 However, due to the limited resolution of solid-state techniques,118 researchers have primarily employed liquid-phase techniques to quantify different P species with higher specificity.
3. Phosphorus transformations during pyrolysis of manures
3.1 Phosphorus concentration in manure biochars
Pyrolysis is the thermochemical conversion of a carbonaceous feedstock in an oxygen-limited environment at temperatures usually ranging from 300 to 800 °C. As the temperature is increased during pyrolysis, weight loss takes place by moisture loss followed by decomposition of hemicellulose, cellulose, and lignin.119 Different products are generated, namely gases (CO2, CO, CH4, and small hydrocarbons), liquid (bio-oil), and solid (biochar), with their relative proportions depending on the operating conditions. For example, fast pyrolysis has high heating rates (∼10–200 K s−1 but can be up to 2500 K s−1 for flash pyrolysis) and short residence times (∼0.5–10 s) and is used for the maximization of bio-oil production. Slow pyrolysis, on the other hand, uses extended residence times (order of minutes to hours) with modest heating rates (∼0.1–2 K s−1) and maximizes the formation of biochar.120,121 Most studies have used slow pyrolysis for the recovery of P from animal manures due to the simple and cost-effective setup, although fast pyrolysis has been investigated as well.122,123Phosphorus retention in manure biochars is high, and on average 89.9% (ranging from 68.5 to 118.6%) of the P is retained (Fig. 5). These values are based on measurements of biochar yield and P concentrations in the manure and biochars, and values deviating from 100% are likely due to measurement uncertainties and possibly losses due to particle entrainment in the gas flow.28 Measurements on pyrolysis of wetland plants have shown that small amounts of P may escape the solid phase and end up in the gaseous or liquid products.125,126 The transfer of P to the gas phase via carbothermic reduction and possibly decomposition of organically bound phosphorus may take place but is generally neglectable below 900 °C.28,127 Thus, practically all P is retained in the biochar.
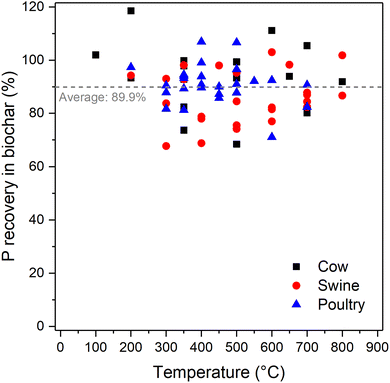 | ||
| Fig. 5 Phosphorus recovery in biochars produced from cow (n = 15), swine (n = 25), and poultry manure (n = 25). The average value of 89.9% is calculated over all temperatures and manure types. Values are based on data from various sources.47–50,56,57,59,65,68–70,124 | ||
The main factors that affect the biochar properties (including P content, species, and availability) are the pyrolysis temperature, raw material, and the duration of the pyrolysis process (also called the holding time or residence time).24 Compared to other parameters, the holding time tends to be less influential on the biochar properties,52,128–130 and the effect of holding time is greater at low temperatures than at high temperatures.131 In biochars prepared from agricultural residues, longer pyrolysis times (1, 2, and 4 h) increased or did not affect the total P and water-soluble P concentrations, depending on the feedstock composition and pyrolysis temperature.129 Across all pyrolysis temperatures and biomass types, Zhang et al.24 concluded that pyrolysis processes that are longer than 2 h increase the proportion of stable P when compared to holding times of 2 hours or less.
Because of the thermal decomposition of the organic matrix and retention of P in the solid material, the biochar yield decreases132 and P concentration in biochars increases with pyrolysis temperature (Fig. 6a–c). Total P concentrations up to 79 mg g−1 have been reported,56 depending on the manure and the pyrolysis temperature. Raw materials with a higher innate P concentration will result in more P-rich biochars. For example, Liu et al.48 reported that the P content in cow manure biochar increased from 13.7 mg g−1 (raw manure) to 15.6, 26.5, 29.4, 32.3, and 32.5 mg g−1 when pyrolyzed at 200, 350, 500, 650, and 800 °C, respectively. At the same temperatures, the P concentration of pig manure biochars were higher and increased from 20.8 mg g−1 to 23.3, 37.5, 43.1, 44.8, and 44.5, respectively, whereas P concentrations in sheep manure biochars were lower (from 8.0 mg g−1 to 8.9, 13.9, 16.0, 17.4, and 17.9 mg g−1, respectively).48 When normalized by the P concentration, a common trend is observed across all manure types (Fig. 6d–f). The P concentration is approximately doubled at around 400–500 °C and in some cases may reach triple the initial concentration at higher temperatures. This is a good prospect for agriculture and waste management, providing a solid material with a high P concentration and low volume.
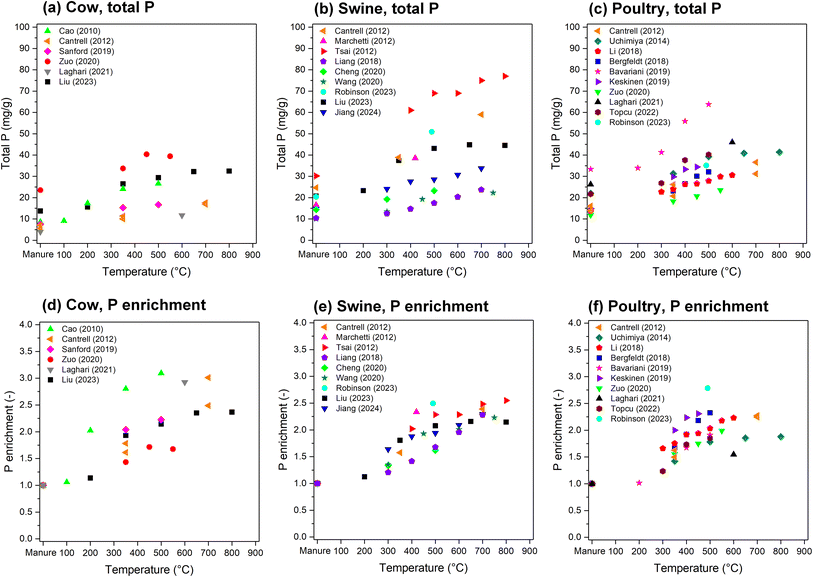 | ||
| Fig. 6 (a–c) Total P and (d–f) P enrichment (P concentration in biochar divided by P concentration in the manure) of biochars prepared from (a and d) cow, (b and e) swine, and (c and f) poultry manures as function of pyrolysis temperature. Data were taken from various sources and references are indicated in the figures.21,47–51,56–59,65,67,69,70,92,101,112,114 | ||
3.2 Transformation of P species during pyrolysis
To track the P species during the thermal conversion process, various authors have used 31P NMR spectroscopy on alkaline (NaOH-EDTA) extracts of the manure biochars.21,56,57,92,93 Example of liquid phase 31P NMR spectra of NaOH-EDTA extracts of swine manure and its derived biochars prepared at 300–700 °C are shown in Fig. 7a from the work of Liang et al.21 Swine manure presented peaks of orthophosphate, pyrophosphate, monoester-P, and diester-P. The monoester-P and diester-P signals have disappeared upon pyrolysis >400 °C. At the same time, the pyrophosphate signal increased at 300 °C and then gradually decreased with higher temperatures, and at 700 °C only orthophosphate was detected.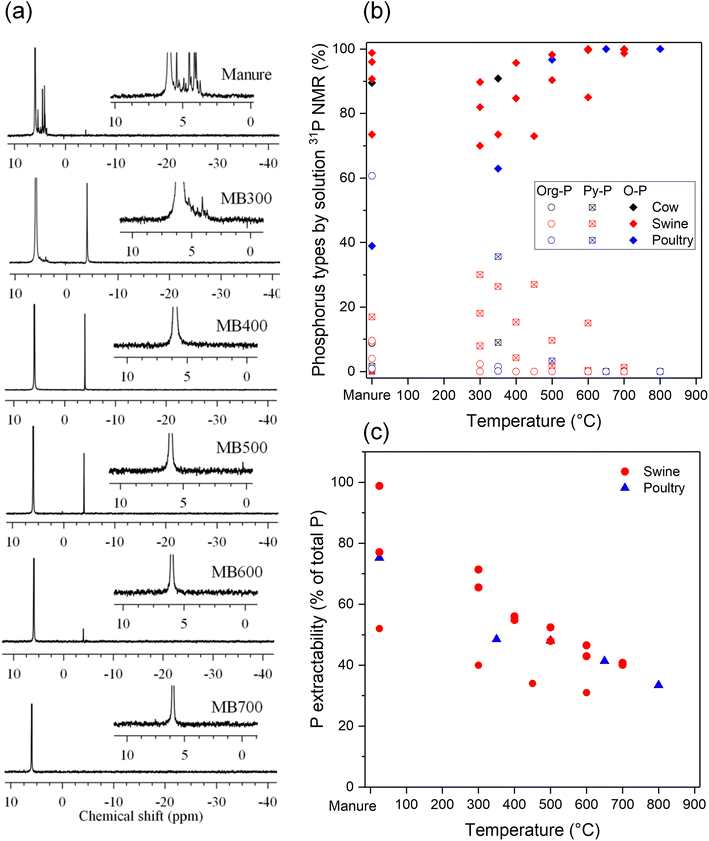 | ||
| Fig. 7 (a) Liquid phase 31P NMR spectra of NaOH-EDTA extracts of swine manure and its derived biochars prepared at 300–700 °C. Peaks were assigned to orthophosphate (δ = 6.0 ppm), pyrophosphate (−4.12 ppm), monoester-P (approx. 5.8 to 4.0 ppm), and diester-P (approx. 1.41 to −0.71 ppm). Reproduced from ref. 21 with permission from Springer Nature, copyright 2017; (b) quantification of P species from 31P NMR spectra of NaOH-EDTA extracts of manure biochars produced at different temperatures. Data taken from various sources.21,56,57,92,93 (c) Extractability of P in NaOH-EDTA decreases with increasing pyrolysis temperatures. Data taken from various sources.21,56,57 | ||
Fig. 7b gives an overview of the concentrations of organic P, orthophosphate and pyrophosphate as function of pyrolysis temperatures based on 31P NMR measurements. These P transformations during biochar pyrolysis can be generalized as follows. At relatively low temperatures, organic P species are degraded first; thermal decomposition of phytic acid may start at 150 °C.133 Uchimiya and Hiradate92 reported that the organic P concentration of broiler litter decreased from 61% to 1% after pyrolysis at 350 °C and was not found at 500 °C and higher. At the same time, pyrophosphate is formed as an intermediate species with a maximum concentration at 300–400 °C, where up to 36% of the P may be in pyrophosphate form.92 At higher temperatures, pyrophosphate concentrations gradually decrease and orthophosphate becomes more abundant. Throughout the pyrolysis process the orthophosphate concentrations increase and at >700 °C only orthophosphate is detected.
However, these results from liquid phase 31P NMR analysis should be interpreted with care because only a fraction of P can be extracted from the biochar which decreases with pyrolysis temperature (Fig. 7c), resulting in the under- or over-estimation of some species. Furthermore, as mentioned previously, the extraction solution may alter the P species, so care should be taken as the NMR spectra may not be fully representative of the P forms in the biochars.
Phosphates in biochars tend to crystallize with Ca because of the high Ca contents in manures, and the most frequently detected crystalline phosphate phases in manure biochars are whitlockite (including (Ca,Mg)3(PO4)2 and similar compounds) and apatites (mainly hydroxyapatite, Ca5(PO4)3(OH)).28 Additionally, different orthophosphates (e.g., KCaPO4, MgNH4PO4·6H2O) and pyrophosphates (e.g., Ca2P2O7, K2CaP2O7, K4P2O7) are sometimes observed,56,70,134 and the XRD patterns commonly contain non-phosphate phases such as SiO2 (quartz), KCl (sylvite), and CaCO3 (calcite). For example, whitlockite was formed when dairy manure was pyrolyzed at 450 °C (ref. 135) and 500 °C,49 and whitlockite and hydroxyapatite were found in swine manure biochars produced at 600 and 700 °C.57 Poultry manures may already contain some crystalline hydroxyapatite, which is further crystallized upon pyrolysis.136 Nevertheless, XRD can only detect crystalline P phases and may thus provide an incomplete picture of the P species present.
To provide a more comprehensive insight into the P transformations taking place within the solid, spectroscopic techniques like P K-edge XANES and solid state 31P NMR spectroscopy have been employed. For example, Jiang et al.136 pyrolyzed poultry litter at 200–600 °C and used solid state 31P NMR, XRD, and FTIR spectroscopy to monitor P transformations (Fig. 8). The XRD patterns confirmed the presence of hydroxyapatite in the poultry litter and all biochars, which became more crystalline at higher pyrolysis temperatures. Solid state 31P NMR measurements indicated that poultry litter contained orthophosphate, phytate, and hydroxyapatite groups (Fig. 8a). Increasing pyrolysis temperatures converted water-bound HPO42− and phytates into hydroxyapatite. Phytates were decomposed above 300 °C, and farringtonite (Mg3(PO4)2) formed above 500 °C. According to the solid state 31P NMR analysis, the P in the raw poultry litter was 21% in the form of hydroxyapatite, which increased to more than 70% at 600 °C (Fig. 8b). At the same time, water-extractable P concentrations greatly decreased from 2.9 g kg−1 in raw poultry litter to less than 0.2 g kg−1 at 400–600 °C (Fig. 8c). Li et al.112 made similar observations in the solid state 31P NMR spectra of pyrolyzed poultry litter at 300–600 °C. Liang et al.21 monitored the transformation of organic P (inositol hexaphosphate) to Ca3(PO4)2 during pyrolysis of swine manure by P XANES analysis supported by liquid phase 31P NMR measurements. The authors also identified small amounts of hydroxyapatite (10–15%) and pyrophosphates (3–8%) in the biochars.
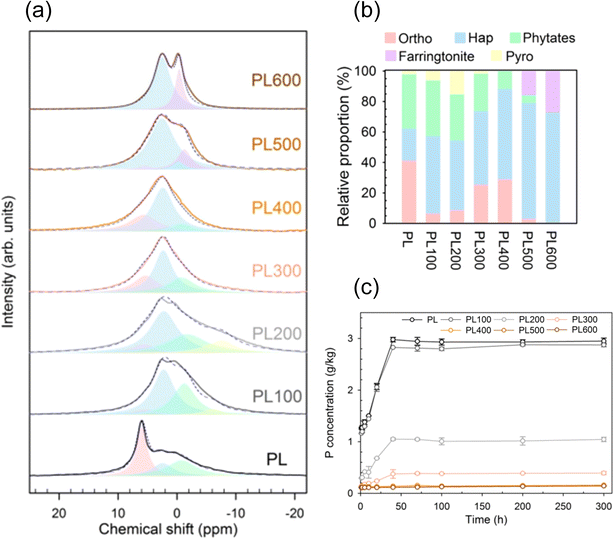 | ||
| Fig. 8 (a) 31P SP/MAS NMR spectra and the deconvoluted forms and (b) relative proportion (%) of the main P species in raw poultry litter (PL) and PL pyrolyzed at 200–600 °C. Ortho, hap, phytates, farringtonite, and pyro refer to the deconvoluted peaks of protonated sodium orthophosphate (δ = 6.1 ppm), hydroxyapatite (δ = 2.6 ppm), phytates (δ = 0.6 ppm), farringtonite (δ = −0.4 ppm), and pyrophosphates (δ = −7 ppm), respectively; (c) phosphorus release from raw and pyrolyzed poultry litter in deionized water. Reproduced (adapted) from ref. 136 with permission from the American Chemical Society, copyright 2019. | ||
Huang et al.110 performed solid state P XANES analysis of cow, swine, and poultry manures. The H2O-extractable P fraction rapidly decreased and the HCl-extractable P fraction gradually increased when the pyrolysis temperature was raised, which was ascribed to the degradation of organic phosphates and gradual crystallization of Ca phosphates. The authors found a similar speciation between manure types because all manures had Ca as major P-binding element. Robinson et al.55 also found that pyrolysis increased the hydroxyapatite content in poultry manure from 34 to 48% and a small amount of pyrophosphate (Mg2P2O7) was identified in the biochar. The P species in pig slurry, however, were hardly altered after pyrolysis.
4. Phosphorus availability from biochars
4.1 Phosphorus extractability and release
The P extractability has been evaluated in various aqueous media in order to understand the potential risk losses and predict the potential availability to crops. Water-soluble P generally decreases with increasing pyrolysis temperatures,23,47,49,64,70,112,134,136–139 suggesting that the P release risk from biochars is lower than from manures. For example, the water-soluble P in fresh and pelletized chicken manure decreased from 50–60% to 3–9% after pyrolysis at 500–700 °C.64 Cantrell et al.47 measured a decrease in soluble P after pyrolysis of 5 types of manure biochars prepared at 350 and 700 °C. Among manures, swine solids had highest soluble P in manure but the lowest soluble P in the biochars. According to Cao and Harris,49 when dairy manure was pyrolyzed at 100–500 °C, the water-soluble P first increased up to 200 °C and then decreased with higher temperatures. The initial increase in P solubility was ascribed to an increase in total P by biomass reduction but may also be due to the increased fraction of P in orthophosphate form (the form that is detected with the molybdenum blue method).Different extractants have been used to get an indication for the P availability to crops. The P extractability (both absolute and relative to the total P content in the biochar) in organic acids like 2% citric acid, 2% formic acid, or acidic ammonium oxalate is generally high23,70,92,101,134,140,141 and tends to be higher from manure biochars than from plant biochars.92,140 Increasing pyrolysis temperatures result in decreased P extractability in 0.01 M HCl from poultry litter biochars.68 The P fraction that was extractable in 1 M HCl was 68% for poultry litter manure and increased to 87–99.8% after pyrolysis at 300–600 °C, with the highest extractability at 450 °C.112 The P extractable in neutral ammonium citrate (NAC) tends to be around 70–90% of total P,70,101,141 but also lower values have been reported.134
A number of studies have adapted soil tests for P to measure extractability in biochars, such as Olsen P,48,65,112,137,142 Bray-1,112 Bray-2,140 Mehlich-3,92,112,143 and Colwell.140 Higher pyrolysis temperatures may decrease48,65 or increase Olsen P,137 or may go through a maximum at intermediate pyrolysis temperatures.112,142 The Mehlich-3 extractable P values were low (<4%) for poultry litter and its biochars,92 but were >75% for poultry litter biochars produced at pyrolysis temperatures up to 450 °C and only dropped at higher temperatures.112 Tsai and Chang143 observed a behavior in between, with high fraction of total P extracted in Mehlich-3 at low temperatures and decreasing values with increasing temperature.
However, there is no clear consensus on which test is best to predict the P phytoavailability from biochars. Li et al.112 suggested that Bray-1 was appropriate for predicting medium-term available P and Mehlich-3 for long-term available P. Rose et al.140 concluded that Bray-2 and water extractable P correlated best with P uptake in ryegrass, whereas Wang et al.141 proposed 2% formic acid as best extractant to predict P availability from high ash biochars, and others have obtained good results with 0.5 M NaHCO3 extraction to predict shoot biomass and P uptake from secondary raw materials and wastes.144,145 Because biochars contain a large diversity in phosphate phases with widely varying solubilities, standard extractants cannot reliably predict plant P uptake.146 Alternative methods have been proposed, such as sink-based P extraction with ion exchange membranes147 or iron bags,145,148 and diffusive gradient in thin films (DGT),148 but such methods have not been evaluated in detail on the P availability from biochars.23,149,150 Hernandez-Mora et al.151 compared 6 P extraction methods (H2O, NAC, electro-ultrafiltration, iron bags, NaHCO3, and DGT) for compliance testing of 30 recycled P fertilizers that were chemically diverse. The DGT procedure provided the most accurate prediction of fertilization efficiency. However, due to the complexity of the DGT procedure, the authors recommend NAC as a routine extractant because of the simplicity, high throughput, and low cost, despite its limitations to predict fertilization efficiency of Fe phosphate-containing fertilizers.
Fractionations of P from biochars have been widely used to understand transformations of P species. Overall, pyrolysis tends to decrease the available P forms and increase the stable P.24 Examples of results from sequential (Hedley) fractionations are shown in Fig. 9 for swine57 and poultry biochars112 produced at different temperatures. It can be seen that generally the labile (combined H2O-P + NaHCO3-P, which are similar to Olsen P152) and NaOH-P fractions decrease, while the HCl-P and Res-P tend to increase, which was also confirmed in other studies.67,101,110
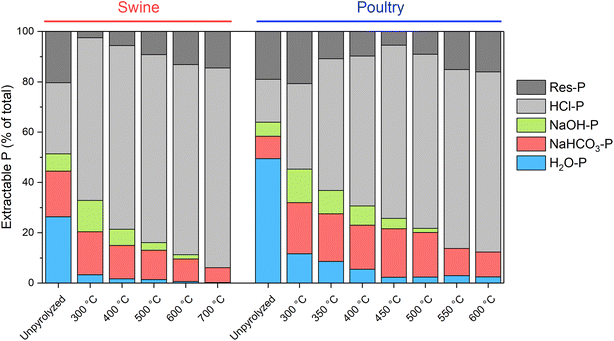 | ||
| Fig. 9 Sequential fractionation of biochars produced at different temperatures from swine manure (data taken from Jiang et al.57) and poultry litter (data taken from Li et al.112). Increasing the pyrolysis temperature decreases the H2O-P fraction and increases the HCl-P fraction. | ||
Using a modified extraction procedure based on soil tests, Liu et al.48 found that the more soluble (i.e., soluble and loosely bound inorganic P, aluminum-bound inorganic P, and organic P) in manure decreased with increasing pyrolysis temperature and transformed into less soluble P forms (i.e., calcium-bound inorganic P and oxide-occluded inorganic P). The Fe-bound inorganic P concentrations were higher for cow manure than for pig and sheep manure.
The SMT protocol has occasionally been used to provide an indication for availability transformations of P in pyrolysis. For example, in the pyrolysis of chicken manure and dairy manure, Zuo et al.114 found that higher pyrolysis temperatures (350 to 550 °C) resulted in transformation from NAIP to AP, suggesting lower availability. Chicken manure biochars contained consistently higher AP concentrations than dairy manure biochars. Simbolon et al.64 also measured a higher AP content in biochars compared to fresh and pelletized chicken litter. However, conclusions about P availability from these transformations should be interpreted with great care. Becker and Kruse152 have recently highlighted that the SMT protocol may not accurately capture the solubility transformations taking place during (hydro)thermal treatment of sewage sludges, which may lead to misleading conclusions about P availability.
The continuous P release in water has been evaluated by suspending the biochar in DI water which is kept under continuous shaking for a number of days. This procedure has been used to measure the P release from biochars produced from different manures21,101,135,137,138,153–156 and also other biochars.157–161 Fitting of the experimental data to kinetic equations (e.g., (pseudo-)first order, (pseudo-)second order, intraparticle diffusion, or Elovich equation) can provide insight into the P release mechanism. An overview of measured P release from manures and their biochars is shown in Fig. 10. The raw manures rapidly release P and between 10 and 60% of P has been dissolved within the first 120 h. Pyrolysis greatly decreases the rate of P dissolution, the effect being stronger for higher temperatures. For example, Liang et al.135 showed that dairy manure pyrolyzed at 450 °C presented a slow, continuous P release over 240 h in water compared to raw dairy manure, which had a rapid initial release and was constant after 24 h. The inhibition of P leaching after pyrolysis was ascribed to the formation of poorly soluble whitlockite ((Ca,Mg)3(PO4)2).135 A similar characteristic was found in the release of P from swine21,101 and poultry manure biochars.101,153 Sun et al.56 have studied the P release from swine manure biochar produced at 450 °C. After 120 hours, 19% of the total P in the biochar (consisting of 11% orthophosphate and 8% pyrophosphate) was released, which was lower than the release from corn stover biochar (45% of total P). The authors suggested that the P dissolution from the swine manure biochar was limited by the formation of crystalline phases like (Ca,Mg)3(PO4)2 and Mg2P2O7/Ca2P2O7. The cation to which the P is bound in the biochar has a strong effect on the P release. For example, Ca-rich biochars present a lower kinetic P release in water than Mg-rich biochars, which is related to the higher solubility of Mg-containing phosphates.158 Using a series of batch experiments, Hadroug et al.162 found that the P release kinetics in water (pH 5.6) from poultry litter decreased at higher pyrolysis temperatures. The authors also studied the effect of pH and dose.
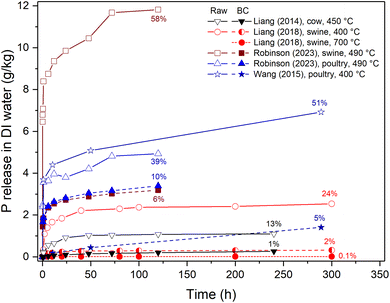 | ||
| Fig. 10 Phosphorus release from manures and their biochars in DI water. Open symbols indicate the raw (unpyrolyzed) manure, and filled and semi-filled symbols indicate the biochars (BC) produced at the indicated temperatures. The values at the end points give the percentage of the total P present in the sample that has been dissolved at that point. Figure was produced using data from various sources.21,101,135,153 | ||
The long-term P release in soil from biochars has been evaluated by a number of authors. Compared to raw dairy manure, manure biochar showed the slow release of P in soil (pH 6.85) controlled by the stable P form (Ca,Mg)3(PO4)2 in biochar over 210 days.135 A similar behavior was reported by Wang et al.,153 where the P release from the soil (sandy loam, pH 6.5) along with poultry litter biochar (400 °C) was significantly slower than the P release from poultry litter. Piash et al.163 studied the P release in soil over 120 days from dairy manure and chicken manure biochar produced at 300 and 500 °C, applied at 2 wt% on a dry basis. The P release in DI water was affected by various factors such as ambient temperature, pH, and anions. The P release from biochars produced at 500 °C was 1.38 times (chicken manure) and 1.10 times (dairy manure) greater than the biochars produced at 300 °C. Sanford et al.50 pyrolyzed dairy manure at 350 and 500 °C and found that the available (Bray-1) P decreased with higher pyrolysis temperature after incubation in two types of soil (loam and sandy loam) over 182 days. At a low application rate (34.5 mg P per kg) there was no difference between the biochars and manure treatments, but at a high rate (172.4 mg P per kg) the 500 °C biochar resulted in lower P availability than 350 °C biochar and manure. In a 98 days incubation study, swine manure biochar application (0.5 and 1.5 wt%) in clay loam and silt loam soils increased available (Olsen) P, increased orthophosphate and pyrophosphate concentrations, and enhanced alkaline phosphomonoesterase activity in a dose-dependent manner.164 In a column experiment, alkaline sandy soil (pH 8.1) amended with 5 and 8 wt% poultry manure biochar presented a slow but continuous P release over 40 days.165
4.2 Plant studies
The conversion of animal manure to biochar not only retains large amounts of P but also prolongs the release of P for plants in the form of P available for plants. However, the available P in animal manure biochar depends on various factors such as pyrolysis temperature and type of manure. Organic P in manures such as phytate, lipids, and DNA are slowly available for plant uptake due to the strong binding of phytate to soils.166 Plants primarily take up P in the form of orthophosphate and condensed phosphates undergo hydrolysis into orthophosphate before being absorbed.167In a recent systematic review of 26 meta-analyses covering more than 1600 articles, Schmidt et al.168 concluded that biochar application has overall beneficial effects on agronomic indicators like plant yield, root development, and soil microbial activity. While biochars have higher P concentrations than the raw manures, a high P application to soil and high P content in the biochar may not necessarily translate into a higher P uptake in crops. Two meta-analyses concluded that biochar application to soil can significantly increase P availability in soils169,170 and also increase P uptake in crops compared to unamended soils.170 For example, yak manure biochars (300 kg ha−1) increased growth of barley compared to an unfertilized control, even though there was no effect on soil N and P levels.171 Both meta-analyses169,170 concluded that the extent of the P efficacy depends on the pyrolysis conditions and soil properties. Soil available P greatly decreases with pyrolysis temperature, and low temperature biochars were more effective in increasing available P in the soil compared to high temperature biochars. Subedi et al.172 studied the P availability from biochars produced from swine manure and poultry litter in two different soil types (silt-loam with pH 6.1 and sandy with pH 8.3). When applied at 2 wt% per dry soil, the low temperature (400 °C) biochars significantly increased both shoot and root dry matter yield of ryegrass compared to an unfertilized control in the silt-loam soil, while biochars produced at high temperature (600 °C) had no effect on either shoot or root dry matter yield. The low temperature biochars had approximately 2.0–3.5 times higher P uptake efficiencies than the high temperature biochars in both soils. When applied at 7.5 t ha−1 (but not at 5 t ha−1), Wang et al.141 found that a biochar produced from a dairy manure–wood mixture at 450 °C resulted in a higher yield of ryegrass compared to pyrolysis at 250 and 550 °C. Overall, Tesfaye et al.170 concluded that there was no apparent effect of pyrolysis temperature on plant P uptake, suggesting that low P availability may not necessarily mean low P uptake.
Concerning soil properties, biochar application tends to be more effective in soils with low pH, fine texture and low available P concentrations.169,170 Subedi et al.172 measured a higher P uptake in ryegrass from swine manure and poultry litter biochars application in acidic silt-loam soil compared to alkaline sandy soil. On the other hand, some authors have reported good efficacy in alkaline soil: in a clay loam soil with pH 7.98, Gunes et al.173 reported that poultry litter biochar (10 g biochar per kg soil) increased dry weight and P uptake of lettuce compared to unfertilized control, to a similar level as the unpyrolyzed poultry litter at an equivalent P application (20 g poultry litter per kg soil).
There is an effect of crop type and experiment type (laboratory versus field),170 but the duration of the experiment does not influence the efficacy.169 Whereas Glaser and Lehr169 did not find any influence of the feedstock, Tesfaye et al.170 concluded that manure biochars were more effective in increasing soil available P and plant P uptake compared to other feedstocks (i.e., crop residues, wood, and sewage sludge). This was possibly due to the higher P concentration and more favorable pH of manure biochars. In both the absence and presence of a commercial P fertilizer, chicken manure biochar was more effective in increasing P uptake in wheat than wheat chaff biochar, but also increased P leaching.174
While most studies have demonstrated positive effects of biochar compared to unamended soils, manure biochars often produced similar or lower results compared to unpyrolyzed manure or commercial fertilizers. Both chicken manure and its biochar (prepared at 400 °C) were as effective as commercial superphosphate in increasing shoot dry matter of rye when applied at 100 mg P per kg soil.148 Both poultry litter biochar and cow manure biochar were equally effective as water-soluble KH2PO4 in increasing shoot biomass of ryegrass grown in a neutral sandy soil at 50 mg total P per kg soil. Cow manure biochar, however, resulted in lower shoot P concentrations compared to poultry litter biochar and KH2PO4.140 After 6 harvests, biochars produced from a dairy manure–wood mixture at 5 and 7.5 t ha−1 were as effective as commercial fertilizers at comparable or lower P applications in increasing yield, but not P uptake in ryegrass.141 Sheep manure biochar was more effective in increasing barley yield than sheep manure and vermicompost when applied at 2 wt% per dry soil.175 However, the authors did not control the application rates of N, P, or any other nutrients, and these differences in treatment composition may have been at least partially responsible for the observed differences.
Recently, Hernandez-Mora et al.176 compared the fertilizer efficiency of 30 recycled P sources to triple superphosphate in pot trials across 3 European locations in wheat, barley, and ryegrass. The fertilizers, including a pelletized product containing chicken manure and grape residue, a poultry litter biochar, and a pyrolyzed pig slurry digestate, were applied at 50 mg P per kg growing media, and the P species in each fertilizer were identified using P K-edge and L2,3-edge XANES. At two of the three locations, all three manure-based materials had equivalent mineral replacement values (MRV) and agronomic mineral replacement values (aMRV), but at the third location the performance decreased in the following order: chicken manure and grape residue pellets > poultry litter biochar > pyrolyzed pig slurry digestate. All fertilizers that contained hydroxyapatite/tricalcium phosphate (such as pyrolyzed pig slurry digestate) had significantly lower MRV and aMRV values than triple superphosphate, while those containing dicalcium phosphate (like chicken manure and grape residue and poultry litter biochar) had a comparable performance.176 The authors classified all three fertilizers as ‘effective’ based on the MRV values above 60%.151
A meta-analysis on biochar applications in European soils did not find sufficient supporting evidence for the agronomic efficiency of biochars compared to mined and synthetic P fertilizers, primarily due to a lack of data.10 Also Sarvi et al.177 demonstrated that in sandy soil (pH 6.5), pyrolyzed broiler manure was less effective than granulated broiler manure in ryegrass when applied at 100 mg P per kg soil. Over 4 consecutive harvests the yield of granulated broiler manure was 36% higher and total P uptake was 53% higher. This was related to the lower P extractability from the biochar in the sequential extraction procedure. The authors stated that the pyrolyzed broiler litter may be suitable as slow release P fertilizer in acidic soils. Vanden Nest et al.178 compared several organic fertilizers applied at equivalent P rates of 39.3 kg P per ha in sandy loam soil, and found that the P use efficiency in ryegrass decreased in order animal manure > digestate > compost > biochar, which was ascribed to the presence of apatite present in the biochar and compost.
In summary, most plant studies have compared biochar application to unamended soils and generally find positive responses, and various studies concluded that biochars can have beneficial effects that are not different from (but in most cases do not exceed) the raw manure. The availability of P from manure biochars depends on the pyrolysis conditions, feedstock, and soil and crop conditions, but is often lower than that of the raw manure. However, there is a lack of studies that directly compared manure biochar to raw manure at equivalent total P application rates on P-responsive soils, while also accounting for the confounding effects of biochar application on crop growth (for example, changes in soil pH and salinity, concentrations and availability of other nutrients, soil organic matter, soil structure, and soil microbial activity).
4.3 Strategies to modify P availability
In order to tailor the P species and its availability, several studies have performed co-pyrolysis of manures with other compounds and some examples are given in Table 1. Whereas the co-pyrolysis of manure with K compounds increases the P extractability,180,181 co-pyrolysis with Mg compounds may either increase138 or decrease the P extractability,137,183 and Ca generally reduced P extractability.137,182 To enhance the total/available P in manure biochars, several authors have enriched manure biochars by co-pyrolysis with P-containing compounds.154–156,184 Other researchers have explored biochar activation through different means. For example, poultry litter biochar was activated with methanesulfonic acid at 400–700 °C, which resulted in higher total P and lower water-extractable P (except at 400 °C), lower pH and electrical conductivity, and a higher water holding capacity.66 Post-treatment of biochars has also been done by char oxidation and steam gasification to increase P availability in soils.51| Manure type | Additive | Pyrolysis conditions | Key findings on manure P | Reference |
|---|---|---|---|---|
| Swine | CaCl2·2H2O, MgCl2·6H2O, KCl, NaCl, polyvinyl chloride (PVC) | 700 °C, 2 h | SMT protocol: higher AP in order CaCl2 (93%) > MgCl2 (92%) > PVC (91%) > unmodified (84%) > NaCl (82%) > KCl (79%) | Xu et al. (2023)179 |
| CaCl2, MgCl2, and PVC: Formation of chlorapatite, stanfieldite (only MgCl2) and possibly Ca3(PO4)2 | ||||
| Swine | CH3COOK | 500–700 °C, 27 min | Higher water-extractable and NAC-extractable P | Buss et al. (2022)180 |
| Swine | KOH, K2CO3, CH3COOK, C6H5K3O7 | 700 °C, 2 h | Slightly lower total P | Liu et al. (2024)181 |
| No effect on water-extractable P | ||||
| Higher P extractability in NaHCO3, NAC, and FA | ||||
| Hedley extraction: higher fraction in NaHCO3 and NaOH, lower fraction in HCl | ||||
| Stronger effect for organic K than inorganic K | ||||
| Formation of CaKPO4 | ||||
| Poultry | CaCl2, MgCl2·6H2O, FeCl3·6H2O | 250–550 °C, 1 h | Total P hardly/not affected | Xiao et al. (2018)137 |
| Lower extractable (Olsen) P in order unmodified > MgCl2 > CaCl2 > FeCl3 | ||||
| Lower P release in water | ||||
| Poultry | Ca-bentonite | 300 °C, 1 h | Lower P release | Piash et al. (2022)182 |
| Poultry | MgCl2 | 500–900 °C, 4 h | Lower extractable P at pH 5.0 | Padilla et al. (2023)183 |
| Formation of Mg3(PO4)2 | ||||
| Poultry | Mg(OH)2 | 300–700 °C, 30 min | Total P increased at high temperature but decreased at low temperature | Leite et al. (2023)138 |
| Lower water-soluble P | ||||
| Lower kinetic P release in water | ||||
| Higher extractable P in 2% citric acid and 2% formic acid (but not at 700 °C) | ||||
| Formation of MgNH4PO4 and Mg3(PO4)2·8H2O |
Most of the modification methods have been carried out on sewage sludges and their biochars, and such approaches may also be used for manure biochars. For example, treatment of sludge-derived biochar with H2SO4 and NaOH increased H2O-P, NaHCO3-P and NaOH-P and decreased HCl-P, which resulted in an increase in shoot dry matter and P uptake of maize, whereas treatment with Ca(OH)2 had little effect.185 Li et al.186 carried out the co-pyrolysis of sewage sludge with CaO or MgO. Using the SMT protocol, the authors found that CaO increased the total P concentration, enhanced XRD peaks of hydroxyapatite and Ca3(PO4)2, and resulted in transformation of NAIP to AP (suggesting a higher P stability). When MgO was added in the pyrolysis process, the total P levels in the biochar were increased, peaks of Mg3(PO4)2 were observed, but the NAIP and AP concentrations were hardly affected. Other authors observed similar effects when sewage sludge was co-pyrolyzed with Ca compounds (CaO, Ca(OH)2, Ca3(PO4)2).187,188
5. Challenges and future perspectives
Pyrolysis of animal manures has various benefits, such as volume reduction (meaning easier storage and transportation), reduced nutrient leaching, reduction of greenhouse gas emissions, energy recovery in the form of combustible gases, and elimination of pathogens, organic contaminants, and odor.14,18,33 And despite the generally good prospects of P recovery from manures through pyrolysis for direct applications in agriculture, this approach still presents several concerns. Recent review articles have highlighted the negative impact of biochar applications in soil.189–192 For example, animal manures may contain significant levels of heavy metals (e.g., Zn, Cu, Ni, Cr, Pb, Cd, As, Cr, and Hg).193 During pyrolysis, heavy metals are largely retained in the biochar and their concentrations generally increase with higher treatment temperatures.53,66,142,194–196 Small (usually <5%) fractions of some more volatile elements (like Cd, Pb, and As) may be lost in the gas phase,193 and their concentrations in biochars may decrease at ≥600 °C.66,194,195 Most biochars contain heavy metal concentrations within permissible levels,53,66 although elevated concentrations of for example Zn, Ni, and Cr have been reported.64,196 Pyrolysis converts the heavy metals from more available into less available forms, and the environmental risk is reduced compared to the raw manure, but not completely eliminated; these may still present a potential for long-term ecotoxicity.193–195Secondly, there are concerns about the toxicity of biochars. Pyrolysis may result in the generation of volatile organic compounds (VOCs) and polycyclic aromatic hydrocarbons (PAHs),14 and several studies have confirmed the potentially toxic effects of VOCs and PAHs to different organisms.192,197–200 The concentrations of both VOCs and PAHs (as well as the toxicity) are strongly dependent on the feedstock and pyrolysis conditions.14,192
In addition to P, the value of other nutrients such as N and K should be considered as well. Pyrolysis at low temperatures (<400 °C) may initially increase the N concentrations of biochars, but losses at higher temperatures decrease the total N concentrations.68,70,201,202 At the same time, extractable N concentrations in biochars decrease with increasing pyrolysis temperatures130,137 as N is converted into organic N species like pyridinic-N and pyrrolic-N.201 As a consequence, the N availability from biochars is lower than that of unpyrolyzed manure.14,203 To reduce N losses during pyrolysis, the separation of water-soluble N from the manure prior to pyrolysis is necessary, which can then be combined with the biochar to form a biochar-based fertilizer.14 Compared to N and P, the aqueous release of K from biochars is generally high and may even increase at higher pyrolysis temperatures.68,70,163 Manure is also an excellent source of soil organic matter,204,205 and the C losses during pyrolysis and the increased aromaticity and stability of C in the biochar may affect soil properties in both short- and long-term.206–209
While most plant trials have demonstrated that P from manure biochars is phytoavailable, further agricultural trials are needed to demonstrate whether manure pyrolysis is beneficial from an agronomic and economic point of view. Such trials should directly compare the performance of manure biochar to that of raw manure at equivalent total P application rates under carefully controlled conditions. Several studies have estimated that animal manure pyrolysis is financially beneficial (including the P value in the char as well as energy value from char, oil, and fuel).123,210 For example, Azuara et al.123 estimated that fast pyrolysis of pig manure would cost around 0.4–4.4 € per ton with an estimated overall benefit of 3.98–5.13 € per ton, which is financially more beneficial than transportation of the manure. Other studies have much higher cost estimates (e.g., 218–274 $ per ton (ref. 211)). There is a very high variability and uncertainty in estimating the cost and benefits of pyrolysis,212–214 so careful consideration through a thorough cost-benefits analysis is required to decide whether pyrolysis is a viable option.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank Vince Knijnenburg for his assistance with the preparation of the figures. This research was supported by the Fundamental Fund of Khon Kaen University. The research on “Product development and investment feasibility study of innovations from waste and by-product from agricultural production” by the Faculty of Engineering and International College at Khon Kaen University has received funding support from the National Science, Research and Innovation Fund (NSRF), Thailand.References
- M. R. Hart, B. F. Quin and M. L. Nguyen, J. Environ. Qual., 2004, 33, 1954–1972 CrossRef CAS PubMed
.
- C. E. Nedelciu, K. V. Ragnarsdottir, P. Schlyter and I. Stjernquist, Global Food Secur., 2020, 26, 100426 CrossRef CAS PubMed
.
-
D. Jones and A. Deuss, OECD Food, Agriculture and Fisheries Papers, No. 208, 2024, DOI:10.1787/18156797
.
- D. Cordell and S. White, Sustainability, 2011, 3, 2027–2049 CrossRef
.
- D. Cordell, J. O. Drangert and S. White, Global Environ. Change, 2009, 19, 292–305 CrossRef
.
- M. Chen and T. E. Graedel, Global Environ. Change, 2016, 36, 139–152 CrossRef
.
- P. J. A. Withers, K. C. van Dijk, T. S. S. Neset, T. Nesme, O. Oenema, G. H. Rubæk, O. F. Schoumans, B. Smit and S. Pellerin, Ambio, 2015, 44, 193–206 CrossRef CAS PubMed
.
- C. Ott and H. Rechberger, Resour., Conserv. Recycl., 2012, 60, 159–172 CrossRef
.
- X. Meng, Q. Huang, J. Xu, H. Gao and J. Yan, Waste Disposal Sustainable Energy, 2019, 1, 99–115 CrossRef
.
- D. Huygens and H. G. M. Saveyn, Agron. Sustainable Dev., 2018, 38, 52 CrossRef
.
-
P. Pagliari, M. Wilson and Z. He, in Animal Manure: Production, Characteristics, Environmental Concerns, and Management, ed. H. M. Waldrip, P. Pagliari and Z. He, 2020, pp. 1–14 Search PubMed
.
- Z. Bian, H. Tian, Q. Yang, R. Xu, S. Pan and B. Zhang, Earth Syst. Sci. Data, 2021, 13, 515–527 CrossRef
.
- M. J. Russell, D. E. Weller, T. E. Jordan, K. J. Sigwart and K. J. Sullivan, Biogeochemistry, 2008, 88, 285–304 CrossRef CAS
.
- D. Rathnayake, H. P. Schmidt, J. Leifeld, J. Mayer, C. A. Epper, T. D. Bucheli and N. Hagemann, GCB Bioenergy, 2023, 15, 1078–1104 CrossRef CAS
.
- J. K. Whalen and C. Chang, J. Environ. Qual., 2001, 30, 229–237 CrossRef CAS PubMed
.
- H. M. Waldrip, P. H. Pagliari, Z. He, R. D. Harmel, N. A. Cole and M. Zhang, Soil Sci. Soc. Am. J., 2015, 79, 1601–1614 CrossRef CAS
.
- M. Kacprzak, K. Malińska, A. Grosser, J. Sobik-Szołtysek, K. Wystalska, D. Dróżdż, A. Jasińska and E. Meers, Crit. Rev. Environ. Sci. Technol., 2023, 53, 914–938 CrossRef CAS
.
- O. F. Schoumans, F. Bouraoui, C. Kabbe, O. Oenema and K. C. van Dijk, Ambio, 2015, 44, 180–192 CrossRef CAS PubMed
.
- P. R. Rout, D. S. Pandey, M. Haynes-Parry, C. Briggs, H. L. C. Manuel, R. Umapathi, S. Mukherjee, S. Panigrahi and M. Goel, Waste Biomass Valorization, 2023, 14, 553–582 CrossRef CAS
.
- G. Ström, A. Albihn, T. Jinnerot, S. Boqvist, A. Andersson-Djurfeldt, S. Sokerya, K. Osbjer, S. San, H. Davun and U. Magnusson, Sci. Total Environ., 2018, 621, 193–200 CrossRef PubMed
.
- X. Liang, Y. Jin, M. He, C. Niyungeko, J. Zhang, C. Liu, G. Tian and Y. Arai, Environ. Sci. Pollut. Res., 2018, 25, 25780–25788 CrossRef CAS PubMed
.
- D. Lachos-Perez, P. César Torres-Mayanga, E. R. Abaide, G. L. Zabot and F. De Castilhos, Bioresour. Technol., 2022, 343, 126084 CrossRef CAS PubMed
.
- W. Christel, S. Bruun, J. Magid and L. S. Jensen, Bioresour. Technol., 2014, 169, 543–551 CrossRef CAS PubMed
.
- S. Zhang, L. Wei, L. Trakal, S. Wang, S. M. Shaheen, J. Rinklebe and Q. Chen, Sci. Total Environ., 2024, 906, 167418 CrossRef CAS PubMed
.
- G. Chen, J. Wang, F. Yu, X. Wang, H. Xiao, B. Yan and X. Cui, Chemosphere, 2022, 301, uc134646 CrossRef PubMed
.
- D. Sun, L. Hale, G. Kar, R. Soolanayakanahally and S. Adl, Chemosphere, 2018, 194, 682–691 CrossRef CAS PubMed
.
- R. Huang, C. Fang, X. Lu, R. Jiang and Y. Tang, Environ. Sci. Technol., 2017, 51, 10284–10298 CrossRef CAS PubMed
.
- E. O. Lidman Olsson, P. Glarborg, K. Dam-Johansen and H. Wu, Energy Fuels, 2023, 37, 6907–6998 CrossRef CAS
.
-
M. Uchimiya, in Applied Manure and Nutrient Chemistry for Sustainable Agriculture and Environment, ed. Z. He and H. Zhang, 2014, pp. 53–68 Search PubMed
.
- Z. Fang, X. Zhuang, X. Zhang, Y. Li, R. Li and L. Ma, Fuel, 2023, 333, 126544 CrossRef CAS
.
- X. He, Y. Wang, Y. Zhang, C. Wang, J. Yu, H. Ohtake and T. Zhang, Mater. Sci. Energy Technol., 2023, 6, 94–104 CAS
.
- X. Ran, Y. Deng, N. S. T. Uppuluri, B. Li, Y. Zheng, P. Chen, R. Dong, J. Müller, J. Guo and H. Oechsner, Sci. Total Environ., 2023, 892, 164346 CrossRef CAS PubMed
.
- G. Su, H. C. Ong, N. W. Mohd Zulkifli, S. Ibrahim, W. H. Chen, C. T. Chong and Y. S. Ok, J. Cleaner Prod., 2022, 343, 130965 CrossRef CAS
.
- S. Tan, G. Zhou, Q. Yang, S. Ge, J. Liu, Y. W. Cheng, P. N. Y. Yek, W. A. Wan Mahari, S. H. Kong, J. S. Chang, C. Sonne, W. W. F. Chong and S. S. Lam, Sci. Total Environ., 2023, 864, 160990 CrossRef CAS PubMed
.
- C. I. Aragón-Briceño, A. K. Pozarlik, E. A. Bramer, L. Niedzwiecki, H. Pawlak-Kruczek and G. Brem, Renewable Energy, 2021, 171, 401–415 CrossRef
.
- T. Tiecher, M. Zafar, F. J. K. Mallmann, E. C. Bortoluzzi, M. A. Bender, L. H. Ciotti and D. R. d. Santos, Rev. Bras. Cienc. Solo, 2014, 38, 1506–1514 CrossRef
.
- R. McDowell, Z. Dou, J. Toth, B. Cade-Menun, P. Kleinman, K. Soder and L. Saporito, J. Environ. Qual., 2008, 37, 741–752 CrossRef CAS PubMed
.
- B. Ajiboye, O. O. Akinremi, Y. Hu and D. N. Flaten, J. Environ. Qual., 2007, 36, 1563–1576 CrossRef CAS PubMed
.
- B. Ajiboye, O. O. Akinremi and G. J. Racz, J. Environ. Qual., 2004, 33, 1062–1069 CrossRef CAS PubMed
.
- P. H. Pagliari and C. A. M. Laboski, J. Environ. Qual., 2012, 41, 901–910 CrossRef CAS PubMed
.
- P. H. Pagliari and C. A. M. Laboski, Biol. Fertil. Soils, 2013, 49, 987–999 CrossRef
.
- Z. He, C. W. Honeycutt, T. S. Griffin, B. J. Cade-Menun, P. J. Pellechia and Z. Dou, J. Environ. Qual., 2009, 38, 1909–1918 CrossRef CAS PubMed
.
- G. Li, H. Li, P. A. Leffelaar, J. Shen and F. Zhang, PLoS One, 2014, 9, e102698 CrossRef PubMed
.
- A. L. Shober, D. L. Hesterberg, J. T. Sims and S. Gardner, J. Environ. Qual., 2006, 35, 1983–1993 CrossRef CAS PubMed
.
- X. Ran, N. S. T. Uppuluri, Y. Deng, Y. Zheng, R. Dong, J. Müller, H. Oechsner, B. Li and J. Guo, Sci. Total Environ., 2023, 874, 162547 CrossRef CAS PubMed
.
- B. L. Turner and A. B. Leytem, Environ. Sci. Technol., 2004, 38, 6101–6108 CrossRef CAS PubMed
.
- K. B. Cantrell, P. G. Hunt, M. Uchimiya, J. M. Novak and K. S. Ro, Bioresour. Technol., 2012, 107, 419–428 CrossRef CAS PubMed
.
- F. Liu, Z. Xiao, J. Fang and H. Li, Sustainability, 2023, 15, 9215 CrossRef CAS
.
- X. Cao and W. Harris, Bioresour. Technol., 2010, 101, 5222–5228 CrossRef CAS PubMed
.
- J. R. Sanford, D. Johnstone and R. A. Larson, Bioresour. Technol. Rep., 2022, 19, 101169 CrossRef CAS
.
- M. Laghari, D. S. Müller-Stöver, M. Puig-Arnavat, T. P. Thomsen and U. B. Henriksen, Waste Biomass Valorization, 2021, 12, 5517–5532 CrossRef
.
- J. Guo, L. Zheng, Z. Li, X. Zhou, S. Cheng, L. Zhang and Q. Zhang, J. Cleaner Prod., 2021, 311, 127458 CrossRef
.
- M. Z. Hossain, M. M. Bahar, B. Sarkar, S. W. Donne, P. Wade and N. Bolan, J. Anal. Appl. Pyrolysis, 2021, 155, 105043 CrossRef CAS
.
- T. Komiyama, T. Ito and M. Saigusa, Soil Sci. Plant Nutr., 2014, 60, 196–207 CrossRef
.
- J. S. Robinson, K. Baumann, Y. Hu, P. Hagemann, L. Kebelmann and P. Leinweber, Ambio, 2018, 47, 73–82 CrossRef CAS PubMed
.
- K. Sun, M. Qiu, L. Han, J. Jin, Z. Wang, Z. Pan and B. Xing, Sci. Total Environ., 2018, 634, 1300–1307 CrossRef CAS PubMed
.
- R. Q. Jiang, G. W. Yu, L. H. Yu, Y. Wang, C. J. Li, Z. J. Xing, X. M. Xue, Y. Wang and C. Yu, Sci. Total Environ., 2024, 915, 170116 CrossRef CAS PubMed
.
- Y. Cheng, L. Luo, J. Lv, G. Li, B. Wen, Y. Ma and R. Huang, Environ. Sci. Technol., 2020, 54, 9008–9014 CrossRef CAS PubMed
.
- W. T. Tsai, S. C. Liu, H. R. Chen, Y. M. Chang and Y. L. Tsai, Chemosphere, 2012, 89, 198–203 CrossRef CAS PubMed
.
- K. Wang, N. Peng, G. Lu and Z. Dang, Waste Biomass Valorization, 2020, 11, 613–624 CrossRef CAS
.
- B. L. Turner, J. Environ. Qual., 2004, 33, 757–766 CAS
.
- E. E. Codling, Soil Sci., 2006, 171, 858–864 CrossRef CAS
.
- Z. He, Z. N. Senwo, R. N. Mankolo and C. W. Honeycutt, J. Food, Agric. Environ., 2006, 4, 304–312 Search PubMed
.
- L. M. Simbolon, D. S. Pandey, A. Horvat, J. J. Leahy, S. A. Tassou and M. Kwapinska, Biomass Convers. Biorefin., 2023, 14, 26443–26457 CrossRef
.
- N. S. Topcu, G. Duman, H. Olgun and J. Yanik, ACS Omega, 2022, 7, 20710–20718 CrossRef CAS PubMed
.
- S. Katuwal, A. J. Ashworth, N.-A.-S. Rafsan and P. Kolar, Biomass, 2022, 2, 209–223 CrossRef
.
- R. Keskinen, J. Hyväluoma, L. Sohlo, H. Help and K. Rasa, Biochar, 2019, 1, 259–270 CrossRef
.
- W. Song and M. Guo, J. Anal. Appl. Pyrolysis, 2012, 94, 138–145 CrossRef CAS
.
- M. Zolfi Bavariani, A. Ronaghi and R. Ghasemi, Commun. Soil Sci. Plant Anal., 2019, 50, 402–411 CrossRef CAS
.
- B. Bergfeldt, M. T. Morgano, H. Leibold, F. Richter and D. Stapf, Agriculture, 2018, 8, 187 CrossRef CAS
.
- E. Humer and Q. Zebeli, Anim. Feed Sci. Technol., 2015, 209, 1–15 CrossRef CAS
.
- M. R. Bedford, Anim. Feed Sci. Technol., 2000, 86, 1–13 CrossRef CAS
.
-
E. Kebreab and A. B. Leytem, in Clinical Aspects of Natural and Added Phosphorus in Foods, ed. O. M. Gutierrez, K. Kalantar-Zadeh and R. Mehrota, 2017, pp. 123–131 Search PubMed
.
- A. Bougouin, J. A. D. R. N. Appuhamy, E. Kebreab, J. Dijkstra, R. P. Kwakkel and J. France, Poult. Sci., 2014, 93, 1981–1992 CrossRef CAS PubMed
.
- P. Rosenfelder-Kuon, W. Siegert and M. Rodehutscord, Arch. Anim. Nutr., 2020, 74, 1–18 CrossRef CAS PubMed
.
- V. D. Nair, D. A. Graetz and D. O. Dooley, Food Agric. Environ., 2003, 1, 217–223 CAS
.
- G. S. Toor, J. D. Peak and J. T. Sims, J. Environ. Qual., 2005, 34, 687–697 CrossRef CAS PubMed
.
- A. B. Leytem, P. W. Plumstead, R. O. Maguire, P. Kwanyuen and J. Brake, J. Environ. Qual., 2007, 36, 453–463 CrossRef CAS PubMed
.
- P. Peperzak, A. Caldwell, R. Hunziker and C. Black, Soil Sci., 1959, 87, 293–302 CrossRef CAS
.
- C. A. E. Peirce, R. J. Smernik and T. M. McBeath, Plant Soil, 2013, 373, 359–372 CrossRef CAS
.
- Z. He, C. W. Honeycutt and T. S. Griffin, Biol. Fertil. Soils, 2003, 38, 78–83 CrossRef
.
- H. W. Dail, Z. He, M. S. Erich and C. W. Honeycutt, Commun. Soil Sci. Plant Anal., 2007, 38, 1879–1895 CrossRef CAS
.
-
P. H. Pagliari, in Applied Manure and Nutrient Chemistry for Sustainable Agriculture and Environment, ed. Z. He and H. Zhang, 2014, pp. 141–161 Search PubMed
.
- Z. He, P. H. Pagliari and H. M. Waldrip, Pedosphere, 2016, 26, 779–816 CrossRef CAS
.
- M. J. Seufferheld and M. J. Curzi, Plant Mol. Biol. Rep., 2010, 28, 549–559 CrossRef CAS
.
- B. J. Cade-Menun, Talanta, 2005, 66, 359–371 CrossRef CAS PubMed
.
- P. Leinweber, L. Haumaier and W. Zech, Biol. Fertil. Soils, 1997, 25, 89–94 CrossRef CAS
.
- B. Cade-Menun and C. W. Liu, Soil Sci. Soc. Am. J., 2014, 78, 19–37 CrossRef
.
- B. J. Cade-Menun, Z. He and Z. Dou, Commun. Soil Sci. Plant Anal., 2015, 46, 1698–1712 CrossRef CAS
.
- R. W. McDowell and I. Stewart, J. Environ. Qual., 2005, 34, 598–607 CrossRef CAS PubMed
.
- Y. Hashimoto, A. Takamoto, R. Kikkawa, K. Murakami and N. Yamaguchi, Environ. Sci. Technol., 2014, 48, 5486–5492 CrossRef CAS PubMed
.
- M. Uchimiya and S. Hiradate, J. Agric. Food Chem., 2014, 62, 1802–1809 CrossRef CAS PubMed
.
- M. Uchimiya, S. Hiradate and M. J. Antal, ACS Sustainable Chem. Eng., 2015, 3, 1642–1649 CrossRef CAS
.
- C. R. de Souza, A. K. Ghosh, I. R. da Silva, E. S. de Alvarenga, R. F. Novais and G. L. de Jesus, Rev. Bras. Cienc. Solo, 2012, 36, 1516–1527 CrossRef
.
- Z. Dou, J. D. Toth, D. T. Galligan, C. F. Ramberg Jr and J. D. Ferguson, J. Environ. Qual., 2000, 29, 508–514 CrossRef CAS
.
- M. García-Albacete, A. Martín and M. C. Cartagena, Waste Manage., 2012, 32, 1061–1068 CrossRef PubMed
.
- K. Dinkler, B. Li, J. Guo, B. Hülsemann, G. C. Becker, J. Müller and H. Oechsner, Bioresour. Technol., 2021, 331, 125038 CrossRef CAS PubMed
.
- A. Tuszynska, K. Czerwionka and H. Obarska-Pempkowiak, J. Environ. Manage., 2021, 278, 111468 CrossRef CAS PubMed
.
- M. Hedley, J. Stewart and B. Chauhan, Soil Sci. Soc. Am. J., 1982, 46, 970–976 CrossRef CAS
.
- A. Sharpley and B. Moyer, J. Environ. Qual., 2000, 29, 1462–1469 CrossRef CAS
.
- J. S. Robinson and P. Leinweber, Waste Manage., 2023, 172, 358–367 CrossRef CAS PubMed
.
- G. Xu, Y. Zhang, H. Shao and J. Sun, Sci. Total Environ., 2016, 569–570, 65–72 CrossRef CAS PubMed
.
- S. Buehler, A. Oberson, I. M. Rao, D. K. Friesen and E. Frossard, Soil Sci. Soc. Am. J., 2002, 66, 868–877 CrossRef CAS
.
- T. T. Qian and H. Jiang, ACS Sustainable Chem. Eng., 2014, 2, 1411–1419 CrossRef CAS
.
- X. Cui, X. Yang, K. Sheng, Z. He and G. Chen, ACS Sustainable Chem. Eng., 2019, 7, 16520–16528 CrossRef CAS
.
- P. J. A. Kleinman, A. N. Sharpley, A. M. Wolf, D. B. Beegle and P. A. Moore Jr, Soil Sci. Soc. Am. J., 2002, 66, 2009–2015 CrossRef CAS
.
- P. J. Worsfold, L. J. Gimbert, U. Mankasingh, O. N. Omaka, G. Hanrahan, P. C. Gardolinski, P. M. Haygarth, B. L. Turner, M. J. Keith-Roach and I. D. McKelvie, Talanta, 2005, 66, 273–293 CrossRef CAS PubMed
.
- Z. He, C. W. Honeycutt, B. J. Cade-Menun, Z. N. Senwo and I. A. Tazisong, Soil Sci. Soc. Am. J., 2008, 72, 1425–1433 CrossRef CAS
.
- Z. He, T. S. Griffin and C. W. Honeycutt, J. Environ. Qual., 2004, 33, 1528–1534 CrossRef CAS PubMed
.
- R. Huang, C. Fang, B. Zhang and Y. Tang, Environ. Sci. Technol., 2018, 52, 3016–3026 CrossRef CAS PubMed
.
- D. Kumaragamage, O. O. Akinremi and L. Grieger, J. Environ. Qual., 2013, 42, 1863–1871 CrossRef CAS PubMed
.
- W. Li, X. Feng, W. Song and M. Guo, Front. Sustainable Food Syst., 2018, 2, 20 CrossRef
.
- V. Ruban, J. López-Sánchez, P. Pardo, G. Rauret, H. Muntau and P. Quevauviller, Fresenius' J. Anal. Chem., 2001, 370, 224–228 CrossRef CAS PubMed
.
- L. Zuo, R. Lin, Q. Shi and S. Xu, Water, Air, Soil Pollut., 2020, 231, ac553 CrossRef
.
- S. Hunger, H. Cho, J. T. Sims and D. L. Sparks, Environ. Sci. Technol., 2004, 38, 674–681 CrossRef CAS PubMed
.
- C. A. Shand, G. Coutts, S. Hillier, D. G. Lumsdon, A. Chudek and J. Eubeler, Environ. Sci. Technol., 2005, 39, 9205–9210 CrossRef CAS PubMed
.
- K. Güngör, A. Jürgensen and K. G. Karthikeyan, J. Environ. Qual., 2007, 36, 1856–1863 CrossRef PubMed
.
- F. Kizewski, Y. T. Liu, A. Morris and D. Hesterberg, J. Environ. Qual., 2011, 40, 751–766 CrossRef CAS PubMed
.
- H. Yang, R. Yan, H. Chen, D. H. Lee and C. Zheng, Fuel, 2007, 86, 1781–1788 CrossRef CAS
.
- T. R. Brown, M. M. Wright and R. C. Brown, Biofuels, Bioprod. Biorefin., 2011, 5, 54–68 CrossRef CAS
.
- T. Y. A. Fahmy, Y. Fahmy, F. Mobarak, M. El-Sakhawy and R. E. Abou-Zeid, Environ. Dev. Sustainability, 2020, 22, 17–32 CrossRef
.
- D. S. Pandey, G. Katsaros, C. Lindfors, J. J. Leahy and S. A. Tassou, Sustainability, 2019, 11, 2533 CrossRef
.
- M. Azuara, S. R. A. Kersten and A. M. J. Kootstra, Biomass Bioenergy, 2013, 49, 171–180 CrossRef CAS
.
- R. Keskinen, J. Hyväluoma, J. Nikama, T. Sainio and K. Ylivainio, Environ. Technol. Innovation, 2023, 32, r103401 CrossRef
.
- A. C. Acosta, C. A. Arias, P. Biller, N. K. Wittig, I. A. Baragau, M. J. Alhnidi, G. Ravenni, Z. Sárossy, L. Benedini, L. E. Abramiuc, D. G. Popescu, W. Negassa, V. F. Marulanda, D. S. Müller-Stöver and H. Brix, Chem. Eng. J., 2024, 492, 151916 CrossRef CAS
.
- W. J. Liu, F. X. Zeng, H. Jiang and H. Q. Yu, Bioresour. Technol., 2011, 102, 3471–3479 CrossRef CAS PubMed
.
- Q. Zhang, H. Liu, W. Li, J. Xu and Q. Liang, Energy Fuels, 2012, 26, 2830–2836 CrossRef CAS
.
- N. Touray, W. T. Tsai and M. H. Li, Waste Biomass Valorization, 2014, 5, 1029–1033 CrossRef CAS
.
- S. Wang, H. Zhang, H. Huang, R. Xiao, R. Li and Z. Zhang, Process Saf. Environ. Prot., 2020, 139, 218–229 CrossRef CAS
.
- Y. Yue, Q. Lin, Y. Xu, G. Li and X. Zhao, J. Anal. Appl. Pyrolysis, 2017, 124, 355–361 CrossRef CAS
.
- J. Sun, F. He, Y. Pan and Z. Zhang, Acta Agric. Scand., Sect. B, 2017, 67, 12–22 CAS
.
- S. Li, S. Harris, A. Anandhi and G. Chen, J. Cleaner Prod., 2019, 215, 890–902 CrossRef CAS
.
- A. L. M. Daneluti and J. R. Matos, Braz. J. Pharm. Sci., 2013, 49, 275–283 CrossRef CAS
.
- M. T. Morgano, B. Bergfeldt, H. Leibold, F. Richter and D. Stapf, Chem. Eng. Trans., 2018, 65, 649–654 Search PubMed
.
- Y. Liang, X. Cao, L. Zhao, X. Xu and W. Harris, J. Environ. Qual., 2014, 43, 1504–1509 CrossRef PubMed
.
- Y. Jiang, C. Ren, H. Guo, M. Guo and W. Li, Environ. Sci. Technol., 2019, 53, 13841–13849 CrossRef CAS PubMed
.
- R. Xiao, J. J. Wang, L. A. Gaston, B. Zhou, J. H. Park, R. Li, S. K. Dodla and Z. Zhang, Waste Manage., 2018, 78, 802–810 CrossRef CAS PubMed
.
- A. D. A. Leite, L. C. A. Melo, L. C. C. Hurtarte, L. Zuin, C. D. Piccolla, D. Werder, I. Shabtai and J. Lehmann, Chemosphere, 2023, 331, 138759 CrossRef CAS PubMed
.
- J. A. Rodriguez, J. F. Lustosa Filho, L. C. A. Melo, I. R. de Assis and T. S. de Oliveira, J. Anal. Appl. Pyrolysis, 2021, 155, 105036 CrossRef CAS
.
- T. J. Rose, C. Schefe, Z. H. Weng, M. T. Rose, L. van Zwieten, L. Liu and A. L. Rose, Plant Soil, 2019, 443, 233–244 CrossRef CAS
.
- T. Wang, M. Camps-Arbestain, M. Hedley and P. Bishop, Plant Soil, 2012, 357, 173–187 CrossRef CAS
.
- G. Gascó, J. Paz-Ferreiro, M. L. Álvarez, A. Saa and A. Méndez, Waste Manage., 2018, 79, 395–403 CrossRef PubMed
.
- C.-C. Tsai and Y.-F. Chang, Agronomy, 2021, 11, 1692 CrossRef CAS
.
- N. H. Christiansen, P. Sørensen, R. Labouriau, B. T. Christensen and G. H. Rubæk, J. Plant Nutr. Soil Sci., 2020, 183, 416–428 CrossRef CAS
.
- O. Duboc, A. Hernandez-Mora, W. W. Wenzel and J. Santner, Sci. Total Environ., 2022, 806, 150486 CrossRef CAS PubMed
.
- S. Kratz, C. Vogel and C. Adam, Nutr. Cycling Agroecosyst., 2019, 115, 1–39 CrossRef CAS
.
- S. Nanzer, A. Oberson, U. Eggenberger and E. Frossard, J. Environ. Qual., 2019, 48, 746–754 CrossRef CAS PubMed
.
- O. Duboc, J. Santner, A. Golestani Fard, F. Zehetner, J. Tacconi and W. W. Wenzel, Sci. Total Environ., 2017, 599–600, 1160–1170 CrossRef CAS PubMed
.
- S. Bruun, S. L. Harmer, G. Bekiaris, W. Christel, L. Zuin, Y. Hu, L. S. Jensen and E. Lombi, Chemosphere, 2017, 169, 377–386 CrossRef CAS PubMed
.
- C. Hong, Y. Su and S. Lu, Environ. Sci. Pollut. Res., 2018, 25, 30547–30556 CrossRef CAS PubMed
.
- A. Hernandez-Mora, O. Duboc, E. K. Bünemann, K. Ylivainio, E. Lombi, S. Symanczik, D. Horn, A. Delgado, N. Abu Zahra, L. Zuin, C. L. Doolette, H. Eigner and J. Santner, Environ. Technol. Innovation, 2025, 37, 103913 CrossRef CAS
.
- G. C. Becker and A. Kruse, ACS Sustainable Chem. Eng., 2023, 11, 15841–15850 CrossRef CAS
.
- Y. Wang, Y. Lin, P. C. Chiu, P. T. Imhoff and M. Guo, Sci. Total Environ., 2015, 512–513, 454–463 CrossRef CAS PubMed
.
- J. F. Lustosa Filho, E. S. Penido, P. P. Castro, C. A. Silva and L. C. A. Melo, ACS Sustainable Chem. Eng., 2017, 5, 9043–9052 CrossRef CAS
.
- J. S. D. S. Carneiro, I. C. A. Ribeiro, B. O. Nardis, C. F. Barbosa, J. F. Lustosa Filho and L. C. A. Melo, Sci. Total Environ., 2021, 760, 143955 CrossRef CAS PubMed
.
- V. Vimal, A. A. Karim, M. Kumar, A. Ray, K. Biswas, S. Maurya, D. Subudhi and N. K. Dhal, Chemosphere, 2022, 300, 134512 CrossRef CAS PubMed
.
- F. Yu, J. Wang, X. Wang, Y. Wang, Q. Guo, Z. Wang, X. Cui, Y. Hu, B. Yan and G. Chen, Biochar, 2023, 5, 82 CrossRef CAS
.
- K. Jetsrisuparb, T. Jeejaila, C. Saengthip, P. Kasemsiri, Y. Ngernyen, P. Chindaprasirt and J. T. N. Knijnenburg, RSC Adv., 2022, 12, 30539–30548 RSC
.
- J. T. N. Knijnenburg, P. Kasemsiri, W. Kaewpradit, T. Tarinta, W. Jantapa, T. Jeejaila, C. Saengthip and K. Jetsrisuparb, Biomass Convers. Biorefin., 2024, 14, 15351–15361 CrossRef CAS
.
- J. T. N. Knijnenburg, K. Laohhasurayotin, P. Khemthong and W. Kangwansupamonkon, Chemosphere, 2019, 223, 310–318 CrossRef CAS PubMed
.
- S. Suwanree, J. T. N. Knijnenburg, P. Kasemsiri, W. Kraithong, P. Chindaprasirt and K. Jetsrisuparb, Biomass Bioenergy, 2022, 156, 106304 CrossRef CAS
.
- S. Hadroug, S. Jellali, J. J. Leahy, M. Kwapinska, M. Jeguirim, H. Hamdi and W. Kwapinski, Water, 2019, 11, 2271 CrossRef CAS
.
- M. I. Piash, K. Iwabuchi, T. Itoh and K. Uemura, Geoderma, 2021, 397, 115100 CrossRef CAS
.
- Y. Jin, X. Liang, M. He, Y. Liu, G. Tian and J. Shi, Chemosphere, 2016, 142, 128–135 CrossRef CAS PubMed
.
- S. Hadroug, S. Jellali, M. Jeguirim, M. Kwapinska, H. Hamdi, J. J. Leahy and W. Kwapinski, Sustainability, 2021, 13, 1–26 CrossRef
.
- S. C. Lung and B. L. Lim, Plant Soil, 2006, 279, 187–199 CrossRef CAS
.
- L. O. Torres-Dorante, N. Claassen, B. Steingrobe and H. W. Olfs, J. Plant Nutr. Soil Sci., 2005, 168, 352–358 CrossRef CAS
.
- H. P. Schmidt, C. Kammann, N. Hagemann, J. Leifeld, T. D. Bucheli, M. A. Sánchez Monedero and M. L. Cayuela, GCB Bioenergy, 2021, 13, 1708–1730 CrossRef CAS
.
- B. Glaser and V. I. Lehr, Sci. Rep., 2019, 9, 9338 CrossRef PubMed
.
- F. Tesfaye, X. Liu, J. Zheng, K. Cheng, R. Bian, X. Zhang, L. Li, M. Drosos, S. Joseph and G. Pan, Environ. Sci. Pollut. Res., 2021, 28, 34108–34120 CrossRef CAS PubMed
.
- J. Zhang, B. Huang, L. Chen, Y. Li, W. Li and Z. Luo, Chem. Speciation Bioavailability, 2018, 30, 57–67 CrossRef CAS
.
- R. Subedi, N. Taupe, I. Ikoyi, C. Bertora, L. Zavattaro, A. Schmalenberger, J. J. Leahy and C. Grignani, Sci. Total Environ., 2016, 550, 924–933 CrossRef CAS PubMed
.
- A. Gunes, A. Inal, M. Taskin, O. Sahin, E. Kaya and A. Atakol, Soil Use Manage., 2014, 30, 182–188 CrossRef
.
- O. F. Madiba, Z. M. Solaiman, J. K. Carson and D. V. Murphy, Biol. Fertil. Soils, 2016, 52, 439–446 CrossRef CAS
.
- M. Najafi-Ghiri, T. Razeghizadeh, M. S. Taghizadeh and H. R. Boostani, Commun. Soil Sci. Plant Anal., 2019, 50, 2610–2625 CrossRef CAS
.
- A. Hernandez-Mora, O. Duboc, E. Lombi, E. K. Bünemann, K. Ylivainio, S. Symanczik, A. Delgado, N. Abu Zahra, J. Nikama, L. Zuin, C. L. Doolette, H. Eigner and J. Santner, J. Cleaner Prod., 2024, 467, 142957 CrossRef CAS
.
- M. Sarvi, M. Hagner, S. Velmala, H. Soinne, R. Uusitalo, R. Keskinen, K. Ylivainio, J. Kaseva and K. Rasa, Environ. Technol. Innovation, 2021, 23, 101584 CrossRef CAS
.
- T. Vanden Nest, F. Amery, L. Fryda, C. Boogaerts, J. Bilbao and B. Vandecasteele, Sci. Total Environ., 2021, 750, 141699 CrossRef CAS PubMed
.
- Y. Xu, F. Qi, Y. Yan, W. Sun, T. Bai, N. Lu, H. Luo, C. Liu, B. Yuan and Z. Sheng, Waste Manage., 2023, 169, 52–61 CrossRef CAS PubMed
.
- W. Buss, C. Wurzer, M. Bach, J. Heberling, T. Appel, H. Gerber and O. Mašek, J. Environ. Manage., 2022, 314, 115035 CrossRef CAS PubMed
.
- T. Liu, T. Shao, J. Jiang, W. Ma, R. Feng, D. Dong, Y. Wang, T. Bai and Y. Xu, Sci. Rep., 2024, 14, 21069 CrossRef CAS PubMed
.
- M. I. Piash, K. Iwabuchi and T. Itoh, Sci. Total Environ., 2022, 822, 153509 CrossRef CAS PubMed
.
- J. T. Padilla, D. W. Watts, J. M. Novak, V. Cerven, J. A. Ippolito, A. A. Szogi and M. G. Johnson, Biochar, 2023, 5, 64 CrossRef CAS PubMed
.
- W. Jantapa, K. Jetsrisuparb, D. Macquarrie, P. Kasemsiri, P. Chindaprasirt and J. T. N. Knijnenburg, Waste Biomass Valorization, 2024 DOI:10.1007/s12649-024-02824-6
.
- C. Kopp, P. Sica, A. G. Eising, D. E. Madsen, J. Magid and D. S. Müller-Stöver, Waste Biomass Valorization, 2024, 15, 4291–4307 CrossRef CAS
.
- J. Li, Y. Li, F. Liu, X. Zhang, M. Song and R. Li, J. Anal. Appl. Pyrolysis, 2023, 173, 106065 CrossRef CAS
.
- S. Tang, F. Yan, C. Zheng and Z. Zhang, ACS Sustainable Chem. Eng., 2018, 6, 9167–9177 CrossRef CAS
.
- J. Chen, S. Tang, F. Yan and Z. Zhang, Water Res., 2020, 171, 115450 CrossRef CAS PubMed
.
- M. Brtnicky, R. Datta, J. Holatko, L. Bielska, Z. M. Gusiatin, J. Kucerik, T. Hammerschmiedt, S. Danish, M. Radziemska, L. Mravcova, S. Fahad, A. Kintl, M. Sudoma, N. Ahmed and V. Pecina, Sci. Total Environ., 2021, 796, 148756 CrossRef CAS PubMed
.
- P. Godlewska, Y. S. Ok and P. Oleszczuk, J. Hazard. Mater., 2021, 403, 123833 CrossRef CAS PubMed
.
- L. Xiang, S. Liu, S. Ye, H. Yang, B. Song, F. Qin, M. Shen, C. Tan, G. Zeng and X. Tan, J. Hazard. Mater., 2021, 420, 126611 CrossRef CAS PubMed
.
-
H. Zheng, B. Liu, G. Liu, Z. Cai and C. Zhang, in Biochar from Biomass and Waste: Fundamentals and Applications, 2018, pp. 349–384 Search PubMed
.
- S. Li, D. Zou, L. Li, L. Wu, F. Liu, X. Zeng, H. Wang, Y. Zhu and Z. Xiao, Chemosphere, 2020, 247, 125962 CrossRef CAS PubMed
.
- T. Bai, W. Qu, Y. Yan, K. Ma, Y. Xu, X. Zhou, Y. Chen and Y. Xu, J. Environ. Sci. Health, Part B, 2020, 55, 941–950 CrossRef CAS PubMed
.
- F. Li, X. Wu, W. Ji, X. Gui, Y. Chen, J. Zhao, C. Zhou and T. Ren, J. Anal. Appl. Pyrolysis, 2020, 152, 104945 CrossRef CAS
.
- K. Wystalska, K. Malińska, J. Sobik-Szołtysek, D. Dróżdż and E. Meers, Materials, 2023, 16, 6392 CrossRef CAS PubMed
.
- A. G. Rombolà, G. Marisi, C. Torri, D. Fabbri, A. Buscaroli, M. Ghidotti and A. Hornung, J. Agric. Food Chem., 2015, 63, 6660–6667 CrossRef PubMed
.
- C. R. Smith, E. M. Buzan and J. W. Lee, ACS Sustainable Chem. Eng., 2013, 1, 118–126 CrossRef CAS
.
- Y. Y. Wang, X. R. Jing, L. L. Li, W. J. Liu, Z. H. Tong and H. Jiang, ACS Sustainable Chem. Eng., 2017, 5, 481–488 CrossRef CAS
.
- P. Oleszczuk, I. Jośko and M. Kuśmierz, J. Hazard. Mater., 2013, 260, 375–382 CrossRef CAS PubMed
.
- L. Leng, S. Xu, R. Liu, T. Yu, X. Zhuo, S. Leng, Q. Xiong and H. Huang, Bioresour. Technol., 2020, 298, 122286 CrossRef CAS PubMed
.
- P. Cely, G. Gascó, J. Paz-Ferreiro and A. Méndez, J. Anal. Appl. Pyrolysis, 2015, 111, 173–182 CrossRef CAS
.
- T. Wang, M. Camps Arbestain, M. Hedley and P. Bishop, Org. Geochem., 2012, 51, 45–54 CrossRef CAS
.
- A. Gross and B. Glaser, Sci. Rep., 2021, 11, 5516 CrossRef CAS PubMed
.
- E. Maillard and D. A. Angers, Global Change Biol., 2014, 20, 666–679 CrossRef PubMed
.
- M. Uchimiya, T. Ohno and Z. He, J. Anal. Appl. Pyrolysis, 2013, 104, 84–94 CrossRef CAS
.
- S. Schouten, J. W. van Groenigen, O. Oenema and M. L. Cayuela, GCB Bioenergy, 2012, 4, 751–760 CrossRef CAS
.
- A. Gross, T. Bromm, S. Polifka, D. Fischer and B. Glaser, Sci. Total Environ., 2024, 954, 176340 CrossRef CAS PubMed
.
- A. Gross, T. Bromm and B. Glaser, Agronomy, 2021, 11, 2474 CrossRef CAS
.
- M. Fernandez-Lopez, M. Puig-Gamero, D. Lopez-Gonzalez, A. Avalos-Ramirez, J. Valverde and L. Sanchez-Silva, Bioresour. Technol., 2015, 182, 184–192 CrossRef CAS PubMed
.
- E. Struhs, A. Mirkouei, Y. You and A. Mohajeri, Appl. Energy, 2020, 279, 115782 CrossRef CAS
.
- R. R. Bora, Y. Tao, J. Lehmann, J. W. Tester, R. E. Richardson and F. You, ACS Sustainable Chem. Eng., 2020, 8, 5763–5775 CrossRef CAS
.
- R. R. Bora, M. Lei, J. W. Tester, J. Lehmann and F. You, ACS Sustainable Chem. Eng., 2020, 8, 8436–8447 CrossRef CAS
.
- L. Campion, M. Bekchanova, R. Malina and T. Kuppens, J. Cleaner Prod., 2023, 408, 137138 CrossRef
.
| This journal is © The Royal Society of Chemistry 2025 |