Europium ions modulated room temperature phosphorescence in dye-encapsulated MOFs for dual-modal fluorescence-afterglow†
Jiabo
Chen
a,
Renrui
Sun
a,
Wanjun
Yang
a,
Feifei
Xing
a,
Xiaolin
Yu
b and
Lining
Sun
 *a
*a
aDepartment of Chemistry, College of Sciences, Shanghai University, Shanghai 200444, China. E-mail: lnsun@shu.edu.cn
bNikolaev Institute of Inorganic Chemistry, Siberian Branch of the Russian Academy of Sciences, 3 Lavrentiev Ave., 630090, Novosibirsk, Russia
First published on 12th November 2024
Abstract
In recent years, room temperature phosphorescence (RTP) materials have attracted widespread attention in the field of materials science due to their exceptional optical properties. In this study, we explore a strategy for RTP by designing and synthesizing metal–organic frameworks (MOFs) based on lutetium (Lu) and finely modulating the photophysical properties of the materials through introduction of europium (Eu). Utilizing the property that formamide generates formic acid under heating conditions, a type of Lu-MOF with formic acid as the sole ligand was successfully synthesized, which opens up a new pathway for the synthesis of MOFs. The phosphorescence intensity and lifetime of the dye 4,4′-bipyridine are significantly enhanced by being encapsulated in the 1D channels of Lu-MOFs. By partially substituting Lu3+ with Eu3+, we not only adjusted the emission color but also achieved gradient control of fluorescence and phosphorescence intensity, providing precise multilevel optical encoding capabilities for information encryption technologies. This dual-modal fluorescent/phosphorescent MOF system demonstrates high potential for applications in the security field, particularly in the development of advanced anti-counterfeiting and data storage technologies.
1. Introduction
Luminescent materials can absorb energy and re-emit it as light, including fluorescence and phosphorescence.1,2 Fluorescence happens when electrons are excited from the ground state (S0) to the singlet excited state (S1) and quickly return to S0 through radiative decay, lasting from nanoseconds to microseconds. In contrast, phosphorescence involves intersystem crossing (ISC) from S1 to the triplet state (T1) until producing emission through slower radiative decay, lasting milliseconds or longer.3–5 Phosphorescent materials, valued for their long emission time and stability, are widely used in displays, lighting, bioimaging, sensors, and anti-counterfeiting.6–8 Room temperature phosphorescence (RTP) has recently become a research focus due to its promising potential for stable, long-lasting emission at room temperature.9–11To enhance the performance of RTP, scientists have proposed two main strategies: promoting the ISC process and reducing non-radiative relaxation processes. By introducing elements such as halogens, carbonyls, and heavy atoms, the spin–orbit coupling process can be improved, thereby facilitating the ISC process. This enhancement allows transfer of more energy from the excited state to the triplet state, thus boosting phosphorescence.12–14 Additionally, through techniques such as crystal engineering,15,16 polymer co-assembly,17,18 and the use of porous organic framework materials such as metal–organic frameworks (MOFs),19–21 covalent organic frameworks (COFs),22 and hydrogen-bonded organic frameworks (HOFs),23 the luminescent molecules can be fixed within a rigid microenvironment. This effectively suppresses non-radiative decay and energy loss, further enhancing phosphorescence.
As the field of RTP progresses, MOFs have shown significant advantages in dye encapsulation due to their unique structures and excellent properties.24–26 MOFs are constructed through the coordination of metal ions or clusters (inorganic secondary building units) with one or more organic linkers.27–30 Featuring highly tunable pore sizes and abundant functional sites,31–34 MOFs allow for precise control over their pore dimensions and chemical environment by selecting appropriate metal ions and organic ligands, facilitating accurate encapsulation of dye molecules. Specifically, the environment within the pores of MOFs can restrict the movement of dye molecules, reducing non-radiative energy losses and mitigating the negative effects of quenchers on phosphorescence.35–37 This leads to a significant enhancement in phosphorescent properties. Such capabilities make MOFs particularly effective for applications requiring stable and intense phosphorescence, including in sensing, data storage, and anti-counterfeiting technologies.38–40
Although MOFs possess significant advantages in enhancing phosphorescence, current research still faces several challenges. For instance, precisely controlling the intensity of room temperature phosphorescence and constructing material systems with afterglow gradients remain difficult tasks. To address this challenge, we propose a new strategy of modulating the luminescence of dye-encapsulated MOFs by introducing metal ions. Specifically, the molecular fluorescent dye can act as organic antennas to enhance the luminescence of lanthanide (Ln) ions, a phenomenon known as the “antenna effect”.41,42 Energy can be transferred from the T1 of the molecular fluorescent dye to the excited energy levels of Ln3+, resulting in effective sensitized luminescence. Initially, the rigid environment provided by the channels of MOFs leads to phosphorescence emission from the molecular fluorescent dye.43 On the other hand, by introducing Ln3+, the phosphorescence emission of molecular fluorescent dye can be further precisely regulated, providing multicolor fluorescence and afterglow gradients as required.
To validate this strategy, we first designed and synthesized a new lutetium-based MOF (Lu-MOF) for encapsulating the dye 4,4′-bipyridine. In its solid state or when dissolved in formamide, 4,4′-bipyridine did not exhibit visible phosphorescence at room temperature. However, when encapsulated within the 1D channels of Lu-MOFs, the green afterglow of 4,4′-bipyridine was successfully achieved at room temperature. By introducing trivalent europium ions (Eu3+) to partially replace trivalent Lu3+, not only was the luminescence color of the MOFs altered, but also a gradient change in the intensity of the room temperature afterglow was realized. Based on this strategy, we successfully constructed a dual-modal information encryption and anti-counterfeiting system that utilizes both fluorescence and afterglow. This research not only validates the effectiveness of the strategy of introducing metal ions to modulate dye-encapsulated MOFs but also demonstrates its great potential in practical applications.
2. Experimental section
2.1. Synthesis of Lu-MOFs
LuCl3·6H2O (0.30 g, 0.80 mmol) was dissolved in 10 mL formamide and 0.10 mL deionized water, then sealed in a glass bottle (25 mL). The glass bottle was gradually heated to 95 °C at a rate of 5 °C per minute and maintained at 95 °C for 12 hours in an oven. Following the heating process, the mixture was allowed to cool naturally to room temperature. The resulting colorless small block crystals of Lu-MOFs were obtained and washed three times with ethanol. Yield of Lu-MOFs: 81% (based on LuCl3·6H2O). Fourier transform infrared data of Lu-MOFs: 3322(m), 2954(w), 2854(m), 2736(w), 1726(m), 1606(s), 1384(s), 1367(s), 1334(s), 1124(w), 1066(w), 796(m), 734(m), 534(w).2.2. Synthesis of Lu-MOFs-bipy
LuCl3·6H2O (0.30g, 0.80 mmol) and 4,4′-bipyridine (0.19 g, 1.2 mmol) were added to a 50 mL beaker, along with 10 mL formamide and 0.10 mL deionized water. This mixture was then sonicated for 30 minutes. Subsequently, it was transferred to a glass bottle (25 mL). The glass bottle was sealed and gradually heated to 95 °C at a rate of 5 °C per minute and was maintained at 95 °C for 12 hours in an oven. Following the heating process, the mixture was allowed to cool naturally to room temperature. The resulting colorless small block crystals of Lu-MOFs-bipy were filtered and washed thoroughly with formamide and ethanol until no emission of 4,4′-bipyridine was observed in the filtrate under 365 nm excitation.2.3. Synthesis of Lu–x%Eu-MOFs-bipy (x = 1, 5, 10, 25, and 50)
The synthetic procedure for Lu–x%Eu-MOFs-bipy (x = 1, 5, 10, 25, and 50) is similar to that for Lu-MOFs-bipy except that the total amount of doped LuCl3 and EuCl3 is 0.80 mmol. And the ratios of EuCl3 are 1%, 5%, 10%, 25%, and 50% for Lu–x%Eu-MOFs-bipy (x = 1, 5, 10, 25, and 50), respectively.3. Results and discussion
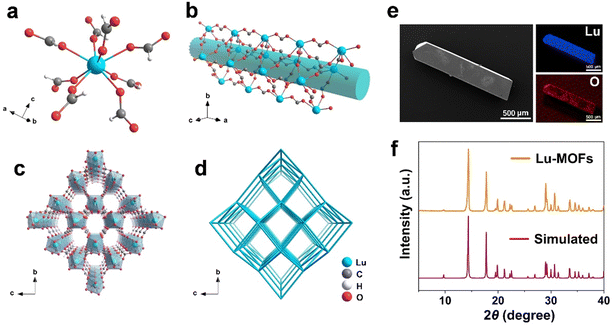
The Lu-MOFs were designed and synthesized by reaction of formamide and Lu3+ through a solvothermal method. As formic acid (HCOOH) is highly volatile and hygroscopic, there has been no previous report of formic acid being used as the sole ligand with lanthanide ions to form MOFs. Our method is to effectively utilize the property that formamide gradually reacts with water during heating to generate ammonia (NH3) and formic acid (Fig. S1, ESI†). Under the conditions of heating and self-generated pressure, deprotonated formic acid and Lu3+ ions self-assembled to form Lu-MOFs. The colorless and transparent chunky crystals were successfully collected from the bottom of the reaction vessel, and named Lu-MOFs. This strategy offers a new approach to the synthesis of MOFs.Single crystal X-ray diffraction analysis indicates that Lu-MOFs crystallize in the orthorhombic space group C2221 (Table S1, CCDC 2356792, ESI†).44 The asymmetric unit of Lu-MOFs contains one crystallographically independent Lu3+ ion, three coordinated bridging HCOO– ligands, and a free formic acid molecule. Each Lu3+ ion is coordinated to eight oxygen atoms, all of which belong to the deprotonated formic acid molecules (Fig. 1a and Fig. S2–S4, ESI†). The Lu–O distances range from 2.253(5) Å to 2.374(6) Å, which are typical values for such coordination modes. Subsequently, the Lu3+ ions are bridged with HCOO– ligands to form a three-dimensional framework structure. Furthermore, it is worth mentioning that, along the crystallographic axis a, the Lu-MOF framework exhibits a one-dimensional open pore channel, with an available volume of 28%, calculated by the PLATON VOID algorithm (Fig. 1b, c and Fig. S5, ESI†). In the pristine sample, uncoordinated HCOOH molecules occupy those channels. According to the ToposPro program, the framework reduces to an 8-connected uninodal network with point symbols 36.415.57, belonging to the ecu topological type (Fig. 1d).
Subsequently, the microstructure of the synthesized Lu-MOF crystals is characterized using scanning electron microscopy (SEM). The observations reveal that the crystal is blocky with a smooth surface, indicating high-quality crystalline characteristics (Fig. 1e). Furthermore, energy dispersive X-ray spectroscopy (EDS) analysis confirms the uniform distribution of Lu and O elements within the Lu-MOF crystals. The powder X-ray diffraction (PXRD) analysis shows that the pattern of Lu-MOFs is highly consistent with the simulated one, thus verifying the phase purity (Fig. 1f). Additionally, thermogravimetric analysis of Lu-MOFs reveals good thermal stability, which indicates that the initial weight loss observed from room temperature to 210 °C was primarily due to the evaporation of solvent and moisture. While the second weight loss observed around 300 °C was mainly due to the decomposition of organic ligands, marking the beginning of structural breakdown (Fig. S6, ESI†).
In the structure of Lu-MOFs, 1D channels along the a-axis were revealed, with dimensions of approximately 6.1 × 5.4 Å2 (Fig. S7a, ESI†). Considering that the width of the 4,4′-bipyridine molecule is about 4.00 Å, this makes it well suited for being embedded in the 1D channels of Lu-MOFs (Fig. S7b, ESI†). In order to introduce 4,4′-bipyridine into the channels of Lu-MOFs, we directly synthesized this MOF using a one-pot method through the addition of lutetium ions to a formamide solution containing 4,4′-bipyridine. The synthesized crystals were filtered and washed thoroughly with formamide and ethanol until no emission of 4,4′-bipyridine was observed in the filtrate under 365 nm excitation. The crystals were then dried in a vacuum oven at 75 °C to obtain the final product, named Lu-MOFs-bipy. PXRD analysis shows that the pattern of Lu-MOFs-bipy closely matched that of Lu-MOFs, indicating that the introduction of 4,4′-bipyridine does not alter the original crystal structure (Fig. S7c, ESI†). The SEM image reveals that the crystal of Lu-MOFs-bipy maintains the chunky shape and smooth surface, indicating high crystalline quality (Fig. S8, ESI†). And EDS shows a uniform distribution of Lu, O, and N (from 4,4′-bipyridine) within the Lu-MOFs-bipy crystals.
The UV-vis absorption spectra of 4,4′-bipyridine, Lu-MOFs, and Lu-MOFs-bipy are displayed in Fig. S9 (ESI†). In comparison with Lu-MOFs, the Lu-MOFs-bipy exhibits a redshift absorption and a new absorption band in the 250–300 nm region attributed to the absorption of 4,4′-bipyridine, indicating the formation of a host–guest system between Lu-MOFs and 4,4′-bipyridine. Photographs of the Lu-MOF crystal and Lu-MOFs-bipy crystal were taken under natural light and 365 nm excitation using an optical camera, respectively (Fig. S10, ESI†). The pictures show that blue light emitted by 4,4′-bipyridine is observed throughout the Lu-MOFs-bipy crystal but not in the Lu-MOFs crystal upon 365 nm excitation, suggesting that the dye was encapsulated in the channels of Lu-MOFs-bipy rather than adsorbed on the surface. The Fourier-transform infrared (FTIR) spectrum of Lu-MOFs-bipy is almost identical to that of Lu-MOFs (Fig. S11, ESI†), displaying similar coordination structures, further indicating that the host MOF structure is Lu-MOFs and 4,4′-bipyridine does not participate in the coordination of Lu-MOFs.45 Thermogravimetric analysis shows that Lu-MOFs-bipy also displays good thermal stability around 300 °C (Fig. S12, ESI†).
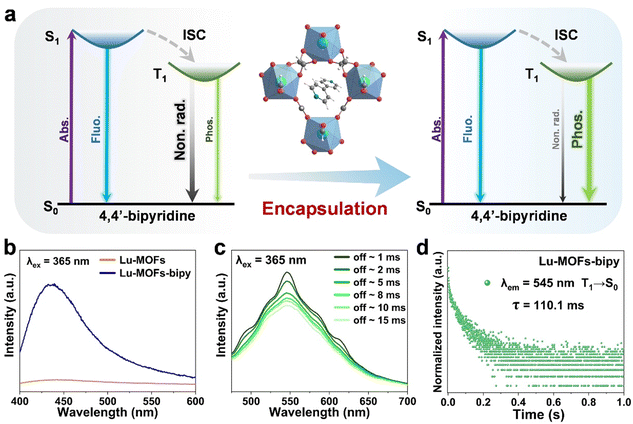
The free rotation of dye molecules around single bonds inevitably increases non-radiative exciton transitions, which is unfavorable for producing room temperature phosphorescence.46 By exploiting the rich topological structure of Lu-MOFs, we introduced the dye into the channels of Lu-MOFs and effectively suppressed molecular vibrations and rotations through spatial effects, thereby reducing non-radiative transitions and endowing the guest dye with efficient room temperature phosphorescence (Fig. 2a). The solid-state emission spectrum of 4,4′-bipyridine shows a band at around 480 nm instead of a single sharp peak under 365 nm excitation at room temperature (Fig. S13, ESI†), which is attributed to the S1 → S0 transition at different energy levels in the aromatic system, and has a luminescence lifetime of 2.6 ns (Fig. S14a, ESI†). Under excitation at 365 nm, free 4,4′-bipyridine in formamide solution emits blue fluorescence centered at 470 nm, with a blue-shift compared to that in the solid-state (Fig. S13, ESI†), possibly influenced by the polarity of formamide solvent (Fig. S15, ESI†). After removal of UV excitation, 4,4′-bipyridine in the solid phase and formamide solution had no RTP that could be seen with the naked eye because of the strong non-radiative relaxation and the inefficient ISC.
Then the fluorescence properties of Lu-MOFs and Lu-MOFs-bipy solid powders were further investigated at room temperature. As expected, under 365 nm excitation, the fluorescence spectrum of Lu-MOFs-bipy displays a band at 435 nm, while Lu-MOFs show almost no luminescence, proving that the blue fluorescence of Lu-MOFs-bipy originates from 4,4′-bipyridine encapsulated within the channels (Fig. 2b and Fig. S16, ESI†). The emission peak of Lu-MOFs-bipy exhibits a blue-shift compared with that of 4,4′-bipyridine in the solid state (Fig. S13, ESI†). This blue-shift may result from the limiting effect of Lu-MOFs-bipy channels on 4,4′-bipyridine, which affects its electron-vibrational coupling. The fluorescence lifetime of Lu-MOFs-bipy is 2.9 ns, which is longer than that of solid-state 4,4′-bipyridine (Fig. S14, ESI†), suggesting that the channels of Lu-MOFs have a limiting effect on the dye.
While upon turning off 365 nm excitation light, the Lu-MOFs-bipy exhibited a broad band emission centered at 545 nm with a slow decay over 0–15 ms (Fig. 2c), which is room temperature phosphorescence. This can be attributed to the radiative transition of 4,4′-bipyridine from the triplet state T1 to S0 at different vibrational energy levels, with a luminescence lifetime of 110.1 ms (Fig. 2d). After removing the 365 nm excitation, the green phosphorescence emitted by Lu-MOFs-bipy was observable by the naked eye, lasting approximately 0.7 seconds (Fig. S17, ESI†). The phosphorescence spectra and lasting green phosphorescence of Lu-MOFs-bipy indicate that 4,4′-bipyridine was successfully encapsulated into Lu-MOFs, since 4,4′-bipyridine in the solid phase and formamide solution had no room temperature phosphorescence as mentioned above. Moreover, the 1D channels of Lu-MOFs effectively suppressed the vibrations and rotations of 4,4′-bipyridine through spatial effects, thereby reducing non-radiative transitions and achieving prolonged phosphorescence emission at room temperature. The significant change in emission color due to the fluorescence/phosphorescence conversion in Lu-MOFs-bipy (Fig. S17, ESI†), could offer a potential opportunity for optical anti-counterfeiting applications in MOF systems, with time-dependent emission changes.
In subsequent research, we explored a new strategy for adjusting the emission of MOFs encapsulated with dye by introducing another lanthanide ion. Considering that both Eu3+ (an ideal red-light emitter) and Lu3+ (optically inert) belong to the lanthanide series and have similar radii due to lanthanide contraction,47 we enriched the luminescent properties of Lu-MOFs-bipy by introducing Eu3+ to replace Lu3+ in Lu-MOFs-bipy, resulting in Lu–Eu-MOFs-bipy. Using the solvothermal method, we synthesized Lu–x%Eu-MOFs-bipy with different concentrations of Eu3+ (x = 1, 5, 10, 25, and 50) using a mixture of Lu3+ and Eu3+ ions in a mixed solvent of formamide and water.
The molar ratios of Lu and Eu were analyzed using inductively coupled plasma atomic emission spectroscopy, and the results show that the compositions of synthesized materials closely match the molar fractions of the reactants (Table S2, ESI†). PXRD analysis reveals that the patterns of Lu–x%Eu-MOFs-bipy match closely with the simulated one of Lu-MOFs, confirming phase purity and indicating that the introduction of Eu3+ did not destroy the original crystal structure (Fig. S18, ESI†). The SEM image shows that even with partial substitution of Eu3+, the crystal morphology of Lu–25%Eu-MOFs-bipy remains unchanged, with good crystal quality, and smooth surfaces (Fig. S19, ESI†). Furthermore, the EDS mapping results indicate that the elemental distribution of Eu3+ and Lu3+ was uniform in Lu–25%Eu-MOFs-bipy. In addition, the UV-visible absorption spectrum of Lu–25%Eu-MOFs-bipy was measured as well (Fig. S9, ESI†), and notably in comparison with that of Lu-MOFs-bipy, new absorption peaks (around 350–400 nm) appeared, corresponding to the f–f transitions of Eu3+. Thermogravimetric analysis indicates that Lu–25%Eu-MOFs-bipy exhibits good thermal stability even at high temperatures around 300 °C (Fig. S20, ESI†).
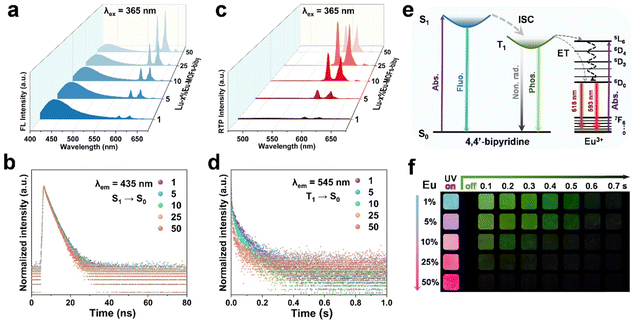
The Lu–x%Eu-MOFs-bipy (x = 1, 5, 10, 25, and 50) materials were prepared into dry powders and measured under identical room temperature conditions. As shown in Fig. 3a, under 365 nm excitation Lu–x%Eu-MOFs-bipy not only exhibits the characteristic broad band emission of 4,4′-bipyridine around 435 nm, but also displays characteristic emissions of Eu3+ at 593 nm and 618 nm corresponding to the 5D0 → 7F1 and 5D0 → 7F2 transitions,48 respectively. It is worth noting that as the concentration of Eu3+ rises, the emission intensity of 4,4′-bipyridine decreases gradually, while that of Eu3+ increases gradually. Photographs of Lu–x%Eu-MOFs-bipy crystals (x = 1, 5, 10, 25, and 50) visually display that the luminescence color of the crystals changes from blue to a strong red as the concentration of Eu3+ increases (Fig. S21, ESI†).
To investigate the performance emissions of 4,4′-bipyridine and Eu3+ of Lu–x%Eu-MOFs-bipy (x = 1, 5, 10, 25, and 50), the excitation spectra were obtained in the solid state monitored at 435 nm (Fig. S22a, ESI†), all showing a broad band ranging from 280 to 400 nm. Meanwhile, the excitation spectra monitored at 618 nm show sharp peaks approximately distributed at 288, 300, 319, 365, 378, 386, and 396 nm, respectively (Fig. S22b, ESI†), attributed to the characteristic absorption of Eu3+ f–f transitions.48,49 Therefore, there is an energy competition between 4,4′-bipyridine and Eu3+ under 365 nm excitation. As the concentration of Eu3+ increases, more energy is absorbed by Eu3+, leading to stronger emissions of Eu3+. Conversely, since the amount of encapsulated 4,4′-bipyridine remains constant, the energy it receives gradually decreases, resulting in a reduction in emission intensity. Lifetime characterization reveals that, under 375 nm excitation, the luminescence lifetime at 435 nm of 4,4′-bipyridine gradually decreases with the increase of Eu3+ concentration, indicating the quenching effect from Eu3+(Fig. 3b and Table S3, ESI†). The luminescence lifetime of Eu3+ at 618 nm also decreased (Fig. S23, ESI†). This is because as the concentration of Eu3+ increases, the distance between the excited Eu3+ and neighboring unexcited Eu3+ reduces, making it easier for energy to be transferred to surrounding Eu3+via non-radiative pathways. This energy transfer results in the dissipation of energy in the form of heat rather than luminescence.48
After the removal of 365 nm excitation, we collected the phosphorescence spectra of Lu–x%Eu-MOFs-bipy at room temperature after a delay of 1 ms. As shown in Fig. 3c, the broad band around 545 nm corresponds to the RTP of 4,4′-bipyridine. With the increase in Eu3+ concentration, the phosphorescence of 4,4′-bipyridine is significantly quenched, making it less pronounced in the spectra. By monitoring at 545 nm, we found that the phosphorescence lifetime of 4,4′-bipyridine also gradually decreased from 108.0 ms to 86.8 ms (Fig. 3d and Table S3, ESI†), indicating a possible energy transfer process from 4,4′-bipyridine to Eu3+, known as the “antenna effect”.50Fig. 3e illustrates the photophysical processes of 4,4′-bipyridine modulated by Eu3+ in Lu–Eu-MOFs-bipy. The reduction in 4,4′-bipyridine phosphorescence is primarily due to two factors: (i) the energy competition between 4,4′-bipyridine and Eu3+ under 365 nm excitation; (ii) the energy transfer from the T1 state of 4,4′-bipyridine to the energy levels of Eu3+. However, the triplet energy level of the 4,4′-bipyridine dye is much higher than the 5D0 excited state of Eu3+ (Fig. 3e). As a result, energy has been lost through non-radiative relaxation during the transfer from the T1 energy level to the 5D0 level of Eu3+, thus the long-lived phosphorescence of Eu3+ is not observed clearly. Due to the modulation effect of Eu3+, the luminescence color changes from the initial blue to a strong red upon UV irradiation, and the afterglow intensity decreases from 0.7 s to 0.1 s after the UV irradiation is turned off (Fig. 3f). By adjusting the doping ratio of Eu3+, we can not only regulate the luminescence intensity and lifetime of the intrinsic emission of Eu3+ ions but also effectively control the intensity and lifetime of the guest 4,4′-bipyridine, achieving a fine afterglow gradient for time-resolved anti-counterfeiting applications. Thus, we have successfully developed optical materials with an illusion emission phenomenon, where the fluorescence is located in the long-wavelength region but displays short-wavelength afterglow. This makes it highly suitable for developing advanced dual-modal encryption technology for fluorescence/afterglow.
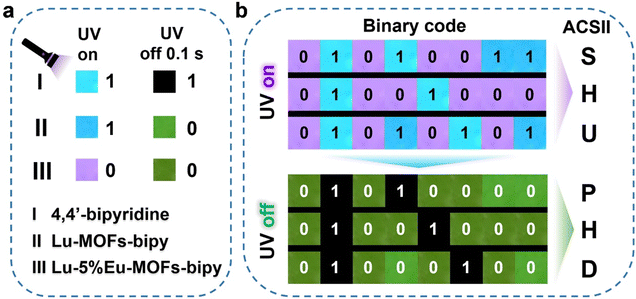
Inspired by the excellent dual-modal luminescent properties of these lanthanide-doped MOFs, we would like to investigate their applications in advanced information storage and encryption. The 4,4′-bipyridine, Lu-MOFs-bipy, and Lu–5%Eu-MOFs-bipy were selected to explore the suitability in this field. As shown in Fig. 4a, under 365 nm excitation, the three different materials produce distinct emission colors. We define the blue emission of 4,4′-bipyridine and Lu-MOFs-bipy as “1”, and the purple emission of Lu–5%Eu-MOFs-bipy as “0”. After removing the 365 nm excitation for 0.1 s, 4,4′-bipyridine which has no phosphorescence, is defined as “1”, while Lu-MOFs-bipy and Lu–5%Eu-MOFs-bipy with their noticeable green phosphorescence, are defined as “0”. By digitally arranging the emission photos of these three materials, we were able to convert the photoluminescence signals into 8-bit ASCII codes, thereby generating different types of optical information storage. This allows different information to be read under the “on” and “off” states of 365 nm excitation (when on, it reads “SHU”; when off, it reads “PHD”, as shown in Fig. 4b). This dual-modal information loading greatly enhances the concealment and security of the information.
4. Conclusions
In summary, MOFs with Lu3+ as the metal center and formic acid as the ligand have been successfully synthesized by a new method using the hydrolysis reaction of formamide to formic acid under heating conditions. Systematic characterizations demonstrate that 4,4′-bipyridine is successfully encapsulated within the 1D channels of Lu-MOFs, and the spatial effects of channels restrict the vibration of 4,4′-bipyridine, enabling it to emit green phosphorescence at room temperature. Subsequently, we propose a strategy based on the introduction of Eu3+ to modulate the optical properties of dye-encapsulated MOFs materials. Through the energy competition and energy transfer between 4,4′-bipyridine and Eu3+, the fluorescence color and phosphorescence intensity of Lu–Eu-MOFs-bipy can be precisely controlled. This strategy provides new directions for the design of long-lived luminescent materials as well as advanced information encryption and anti-counterfeiting systems.Data availability
The data that support the findings of this study are available in the ESI† of this article.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (Grant No. 22275120), and the Science and Technology Commission of Shanghai Municipality (22520711600).References
- Y. Cui, Y. Yue, G. Qian and B. Chen, Chem. Rev., 2012, 112, 1126–1162 CrossRef CAS PubMed.
- J. Rocha, L. D. Carlos, F. A. A. Paza and D. Ananias, Chem. Soc. Rev., 2011, 40, 926–940 RSC.
- S. Cai, X. Yao, H. Ma, H. Shi and Z. An, Aggregate, 2023, 4, e320 CrossRef CAS.
- M. Baroncini, G. Bergamini and P. Ceroni, Chem. Commun., 2017, 53, 2081–2093 RSC.
- Z. Ajoyan, H. A. Bicalho, P. R. Donnarumma, A. Antanovichc and A. J. Howarth, J. Mater. Chem. C, 2023, 11, 8929–8934 RSC.
- J. Qiao, G. Zhou, Y. Zhou, Q. Zhang and Z. Xia, Nat. Commun., 2019, 10, 5267 CrossRef PubMed.
- W. Cheng, X. Tang, Y. Zhang, D. Wu and W. Yang, Trends Food Sci. Technol., 2021, 112, 268–282 CrossRef CAS.
- X. Han, E. Song, Y. Zhou, T. Hu, Z. Xia and Q. Zhang, J. Mater. Chem. C, 2020, 8, 3678–3687 RSC.
- X. Yu, A. A. Ryadun, D. I. Pavlov, T. Y. Guselnikova, A. S. Potapov and V. P. Fedin, Adv. Mater., 2024, 2311939 CrossRef CAS PubMed.
- P. Gao, K. Zhang, D. Ren, H. Liu, H. Zhang, H. Fu, L. Ma and D. Li, Adv. Funct. Mater., 2023, 33, 2300105 CrossRef CAS.
- H. Zhou, J. Han, J. Cuan and Y. Zhou, Chem. Eng. J., 2022, 431, 134170 CrossRef CAS.
- Z. Yang, C. Xu, W. Li, Z. Mao, X. Ge, Q. Huang, H. Deng, J. Zhao, F. L. Gu, Y. Zhang and Z. Chi, Angew. Chem., Int. Ed., 2020, 59, 17451–17455 CrossRef CAS PubMed.
- J. Wang, X. Gu, H. Ma, Q. Peng, X. Huang, X. Zheng, S. H. P. Sung, G. Shan, J. W. Y. Lam, Z. Shuai and B. Z. Tang, Nat. Commun., 2018, 9, 2963 CrossRef PubMed.
- Y. Zhou, L. Qu, S. Yi, C. Wang, X. Chen, S. Tang, H. Tang, Y. Li, K. Wang, Y. Zhao and C. Yang, Adv. Opt. Mater., 2023, 11, 2201904 CrossRef CAS.
- E. Hamzehpoor and D. F. Perepichka, Angew. Chem., Int. Ed., 2020, 59, 9977–9981 CrossRef CAS PubMed.
- O. Bolton, K. Lee, H.-J. Kim, K. Y. Lin and J. Kim, Nat. Chem., 2011, 3, 207–212 CrossRef.
- G. Yin, G. Huo, M. Qi, D. Liu, L. Li, J. Zhou, X. Le, Y. Wang and T. Chen, Adv. Funct. Mater., 2023, 2310043 Search PubMed.
- E. Lucenti, A. Forni, C. Botta, L. Carlucci, C. Giannini, D. Marinotto, A. Previtali, S. Righetto and E. Cariati, J. Phys. Chem. Lett., 2017, 8, 1894–1898 CrossRef CAS PubMed.
- H. Mieno, R. Kabe, N. Notsuka, M. D. Allendorf and C. Adachi, Adv. Opt. Mater., 2016, 4, 1015–1021 CrossRef CAS.
- H. He, Y. Cui, H. Li, K. Shao, B. Chen and G. Qian, Light: Sci. Appl., 2020, 9, 138 CrossRef CAS PubMed.
- Y.-J. Ma, X. Fang, G. Xiao and D. Yan, Angew. Chem., Int. Ed., 2022, 61, e202114100 CrossRef CAS PubMed.
- M.-X. Wu, Y. Wang, G. Zhou and X. Liu, Coord. Chem. Rev., 2021, 430, 213735 CrossRef CAS.
- Z. Zhang, Y. Ye, S. Xiang and B. Chen, Acc. Chem. Res., 2022, 55, 3752–3766 CrossRef CAS PubMed.
- F. Saraci, V. Quezada-Novoa, P. R. Donnarumma and A. J. Howarth, Chem. Soc. Rev., 2020, 49, 7949 RSC.
- J. Yuan, J. Dong, S. Lei and W. Hu, Mater. Chem. Front., 2021, 5, 6824 RSC.
- H. Liu, W. Ye, Y. Mu, H. Ma, A. Lv, S. Han, H. Shi, J. Li, Z. An, G. Wang and W. Huang, Adv. Mater., 2022, 34, 2107612 CrossRef CAS PubMed.
- J. Liu, B. Li, G. Lu, G. Wang, J. Zheng, L. Huang, Y. Feng, S. Xu, Y. Jiang and N. Liu, ACS Appl. Mater. Interfaces, 2024, 16, 26634–26642 CrossRef CAS PubMed.
- E. Djanffar, H. A. Bicalho, Z. Ajoyan, A. J. Howarth and H. Serier-Brault, J. Mater. Chem. C, 2024, 12, 8024–8029 RSC.
- Y. Xie, G. Sun, G. A. Mandl, S. L. Maurizio, J. Chen, J. A. Capobianco and L. Sun, Angew. Chem., Int. Ed., 2023, 62, e202216269 CrossRef CAS PubMed.
- T. L. Easun, F. Moreau, Y. Yan, S. Yang and M. Schröder, Chem. Soc. Rev., 2017, 46, 239–274 RSC.
- S. A. Younis, N. Bhardwaj, S. K. Bhardwaj, K.-H. Kim and A. Deep, Coord. Chem. Rev., 2021, 429, 213620 CrossRef CAS.
- Y. Xie, G. Sun, J. Li and L. Sun, Adv. Funct. Mater., 2023, 33, 2303663 CrossRef CAS.
- D. Zhao, K. Yu, X. Han, Y. He and B. Chen, Chem. Commun., 2022, 58, 747–770 RSC.
- X. Yu, A. A. Ryadun, D. I. Pavlov, T. Y. Guselnikova, A. S. Potapov and V. P. Fedin, Angew. Chem., Int. Ed., 2023, 62, e202306680 CrossRef CAS PubMed.
- S. Lin, Z. Liao, H. Zheng, C. Li, Y. Cui, Z. Wang and G. Qian, J. Mater. Chem. C, 2024, 12, 2391 RSC.
- X. Xu and B. Yan, CrystEngComm, 2022, 24, 5821 RSC.
- H. A. Schwartz, M. Atar, M. Spilles, M. Fill, M. Ott, F. R. S. Purtscher, J. M. Gallmetzer, B. Öcal, S. Olthof, A. Griesbeck, K. Meerholz, T. S. Hofer and U. Ruschewitz, J. Mater. Chem. C, 2024, 12, 8759 RSC.
- J. W. Oh, S. Lee, H. Han, O. Allam, J. I. Choi, H. Lee, W. Jiang, J. Jang, G. Kim, S. Mun, K. Lee, Y. Kim, J. W. Park, S. Lee, S. S. Jang and C. Park, Light: Sci. Appl., 2023, 12, 226 CrossRef CAS PubMed.
- Q. Yu, J. Zhang, J. W. Y. Lam, D. Yang, J. Sun and B. Z. Tang, ACS Mater. Lett., 2023, 5, 2691–2699 CrossRef CAS.
- M.-Y. Zheng, Z.-B. Jin, Z.-Z. Ma, Z.-G. Gu and J. Zhang, Adv. Mater., 2024, 36, 2313749 CrossRef CAS PubMed.
- H.-Q. Zheng, Y. Cui and G. Qian, Acc. Mater. Res., 2023, 4, 982–994 CrossRef CAS.
- J. Chen, M. Li, R. Sun, Y. Xie, J. R. Reimers and L. Sun, Adv. Funct. Mater., 2024, 2315276 CrossRef CAS.
- Y.-Q. Zhu, X.-H. Wang and M.-X. Wu, Adv. Funct. Mater., 2023, 2308096 CrossRef CAS.
- Deposition number 2356792 contains the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- J. Wang, T. Zhang, Z. Gao, M. Wei, Y. Jia, X. Tong, F. Hu and Y. S. Zhao, ACS Appl. Mater. Interfaces, 2024, 16, 23576–23584 CAS.
- H.-Q. Zheng, Y. Yang, Z. Wang, D. Yang, G. Qian and Y. Cui, Adv. Mater., 2023, 35, 2300177 CrossRef CAS PubMed.
- J. Chen, H. Gao, Z. Tao, L. Wang, R. Li and G. Wang, Coord. Chem. Rev., 2023, 485, 215121 CrossRef CAS.
- K. Binnemans, Coord. Chem. Rev., 2015, 295, 1–45 CrossRef CAS.
- Q. Su, S. Han, X. Xie, H. Zhu, H. Chen, C.-K. Chen, R.-S. Liu, X. Chen, F. Wang and X. Liu, J. Am. Chem. Soc., 2012, 134, 20849–20857 CrossRef CAS PubMed.
- P. R. Matthes, J. Nitsch, A. Kuzmanoski, C. Feldmann, A. Steffen, T. B. Marder and K. Mller-Buschbaum, Chem. – Eur. J., 2013, 19, 17369–17378 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Crystal data of Lu-MOFs. PXRD patterns. FT-IR spectra. Luminescence spectra. UV-vis absorption spectra. Excitation spectrum. Decay curves of the luminescence. Thermogravimetric analysis. SEM images. EDS mappings. Elemental analyses by ICP-AES. Photographs of Lu-MOFs, Lu-MOFs-bipy and Lu–Eu-MOFs-bipy. CCDC 2356792. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4tc03221g |
| This journal is © The Royal Society of Chemistry 2025 |