Colossal barocaloric effect in fatty acid methyl esters†
Diyi
Fu‡
a,
Xiu
Su‡
ab,
Haoyu
Wang‡
cd,
Zhenxing
Li
b,
Qiang
Zheng
 *a,
Jun
Shen
b,
Bing
Li
*a,
Jun
Shen
b,
Bing
Li
 cd and
Juan
Du
cd and
Juan
Du
 *a
*a
aInstitute of Materials, School of Materials Science and Engineering, Shanghai University, Shanghai, 200444, People's Republic of China. E-mail: qiangzheng616@hotmail.com; jdu-case@hotmail.com
bDepartment of Energy and Power Engineering, School of Mechanical Engineering, Beijing Institute of Technology, Beijing 100081, People's Republic of China
cShenyang National Laboratory for Materials Science, Institute of Metal Research, Chinese Academy of Sciences, 72 Wenhua Road, Shenyang, Liaoning 110016, People's Republic of China
dSchool of Materials Science and Engineering, University of Science and Technology of China, 72 Wenhua Road, Shenyang, Liaoning 110016, People's Republic of China
First published on 20th November 2024
Abstract
The barocaloric effect is a green refrigeration technology, offering a more environmentally friendly alternative to conventional gas compression refrigeration. The barocaloric effect is induced by pressure in phase change materials, and in which high entropy change and adiabatic temperature change were obtained. Here, a colossal barocaloric effect was discovered in solid–liquid transition of fatty acid methyl esters (FAMEs). In methyl palmitate, the isothermal entropy change can reach as high as 707 J kg−1 K−1 under a low pressure of 80 MPa at 311 K; in methyl stearate, the isothermal entropy change can reach up to 680 J kg−1 K−1 under a pressure of 60 MPa at 308 K. These values are comparable to those of traditional refrigeration materials, such as R314a. The calculated adiabatic temperature change is 22 K for methyl palmitate and 13 K for methyl stearate. Raman spectroscopy indicates that unloading pressure to the liquid phase facilitates the formation of gauche bonds and the formation of solid–liquid phase transformation, which results in a larger configuration entropy change. This work provides a new candidate for solid–liquid transition materials in the practical application of barocaloric effect refrigeration.
Introduction
Refrigeration technology is becoming an increasingly important part of our global village, including food manufacture, air conditioning, aerospace, etc.1 Due to the increasing demands for human refrigeration, it is estimated that 20–25% of the world's electricity is consumed in refrigeration technology.2,3 Nowadays, thousands of refrigeration applications mainly utilize gas compression refrigeration technology, which brings huge convenience to our modern life.4,5 However, this technology has many drawbacks, for instance, the greenhouse effect and high energy consumption.6,7 Significantly, emissions from gas compression technology in which hydrochlorofluorocarbons (HCFCs) were applied as refrigerants are thousands of times more harmful to the ozone layer than CO2, thus aggravating global warming.8,9 Therefore, in order to reduce greenhouse gas emissions and protect our environment, it is an urgent task to find an environmentally friendly cooling technology to replace high-emission technology.10 Luckily, caloric effect refrigeration technology is developing rapidly, which is the most promising refrigeration technology to replace traditional ones.11,12Caloric effects can be categorized as magnetocaloric effects,13–15 elastocaloric effects,16 electrocaloric effects17–19 and barocaloric effects (BCEs),20–24 which are driven by a magnetic field, uniaxial stress field, electric field and hydrostatic pressure, respectively. For the barocaloric effect, temperature change and entropy change can be obtained during phase transition under external pressure, which is not system-selective unlike the other caloric effects.25 So far, BCE has been observed in many kinds of materials including hybrid organic–inorganic compounds,26 ferroelectrics,27 ferroelastics,28 thermoelectrics,29 plastic crystals, frustrated antiferromagnets, spin-crossover complexes,30 and so forth. Recently, a colossal barocaloric effect was found in plastic crystals, which was considered as a remarkable milestone in barocaloric materials because of the increase in entropy change from dozens of J kg−1 K−1 for conventional barocaloric materials to several hundreds of J kg−1 K−1 in plastic crystals. For instance, the entropy change of plastic crystal neopentyl glycol (NPG) can reach 389 J kg−1 K−1, which is approximately one order higher than conventional barocaloric materials, and even comparable to the commercial refrigerants (such as R134a).31 Recently, it was shown that metal–organic frameworks (MOFs) with breathing transitions have huge temperature variations under low pressure. For example, Zn2(bdc)2(bpy) can expand the operating temperature range from 273 to 333 K under ultra low pressure (26 bar), and can be explored as a promising material in the future.32 However, the biggest challenge for most BCE materials is to overcome the high driving pressure before the real barocaloric refrigeration application.
The materials will undergo a significant configurational change with a tremendous entropy change when their states were changed, such as from solid state to liquid state. And this is similar to gas–liquid transition, which has a huge entropy change.33,34 Moreover, the liquid–solid transition (L–S-T) materials have a giant volume change caused by density variation. Therefore, the L–S-T materials are sensitive to pressure and suitable for the barocaloric effect.35,36
Fatty acid methyl esters (FAMEs) as a kind of typical L–S-T material are commonly applied for phase change energy storage. Methyl palmitate (MP) and methyl stearate (MS) have many advantages, including high latent heat of phase transition, strong chemical stability and low cost. And the high latent heat of the phase transition means better barocaloric effect. Here, the colossal barocaloric effect associated with methyl palmitate (MP) and methyl stearate (MS) was reported. Both of them undergo a solid-to-liquid phase transition near room temperature. The maximum entropy changes ΔSP0→P are 707 J kg−1 K−1 at 80 MPa for MP and 680 J kg−1 K−1 at 60 MPa for MS, respectively. And an effective refrigeration capacity of 1921 J kg−1 for MP and 4760 J kg−1 for MS can also be obtained. Moreover, these outstanding BCEs of FAMEs make them excellent candidate materials for barocaloric refrigeration applications.
Experimental details
The methyl palmitate (MP) (99% purity) and methyl stearate (MS) (99% purity) fatty acid esters were commercially available from Aladdin Inc. The raw materials were used as received from suppliers without any further purification. A high-pressure differential scanning calorimeter (μDSC7, Setaram) was used to collect heat flow data as a function of temperature at a constant hydrostatic pressure. The sample, with a mass of 18.8 mg for methyl palmitate and 12.6 mg for methyl stearate, respectively, was enclosed in a high-pressure Hastelloy vessel, and an empty vessel served as a standard. The heat flow of FAMEs was measured between 271 K and 333 K for hydrostatic pressures of 0.1, 20, 40, 60, 80 and 100 MPa. The heating and cooling rates were set to 1 K min−1. The phase transition temperature was determined as the temperature at which the heat flow reached its maximum value. Subsequently, the entropy change at a constant pressure was calculated by integrating the thermal flow within a specific temperature interval. After subtracting the baseline, the entropy change at constant pressure ΔSP was calculated by integrating the thermal flow Q (P, T) within the temperature interval between T1 and T2. The specific heat capacity data at atmospheric pressure was measured between 270 and 330 K by DSC at a heating rate of 1 K min−1. The Cp(T) value at the corresponding phase transition temperature was used to calculate the adiabatic temperature.Raman spectra were acquired utilizing a commercial Raman system (Horiba Labram HR Evolution) with a helium-neon laser (λ = 532 nm) incident at a normal angle. The samples were irradiated by the laser beam through a ×50 objective (numerical aperture 0.6), producing a beam diameter of approximately 1 μm. To maintain a controlled temperature environment, the sample was placed within a continuous-flow liquid-nitrogen cryostat, and temperature regulation was achieved using a calibrated Linkam heating–cooling stage equipped with a thermocouple attached to the sample holder. Raman spectra of MP and MS were measured at different temperatures. The typical frequency range was 50–3000 cm−1, and LabSpec 5 was used to analyze all of the raw data.
Results and discussion
High-pressure differential scanning calorimetry
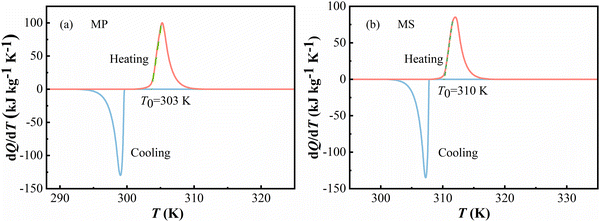
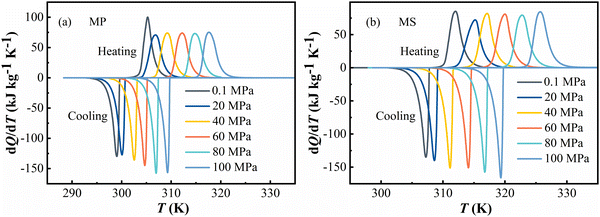
The solid–liquid phase transitions were also investigated by differential scanning calorimetry, and the heat flow peak changes were recorded in dQ/dT (Q is heat and T is temperature) during heating and cooling under ambient pressure. The heat flow data of MP and MS are plotted in Fig. 1, respectively. T0 is defined as the starting transition temperature. Under atmospheric pressure, these FAMEs exhibit a solid–liquid transition at T0 = 303 K for MP and at T0 = 310 K for MS on heating. By integrating the DSC curves, the latent heat Q0 during the heating and cooling process can be determined to be 216 J kg−1 K−1 and 207 J kg−1 K−1 for MP and 206 J kg−1 K−1 and 209 J kg−1 K−1 for MS, respectively. Generally, the temperature of the transition finish on heating and the start and finish temperature for transition on cooling can be influenced by the temperature ramp rate. However, the temperature ramp rate has nominally no effect on the starting transition temperature T0 on heating and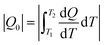 across the solid–liquid transition (see Fig. S1 in the ESI†).20,23
across the solid–liquid transition (see Fig. S1 in the ESI†).20,23
As shown in Fig. 2, according to the determination of dQ/dT at different pressures, the transition temperatures of both FAMEs obviously shifted to higher temperatures with the increase of pressures. The phase transition temperature can be elevated by 10–15 K when the pressure reaches 100 MPa for MP and MS.
 | ||
| Fig. 2 dQ/dT curves of FAMEs at different pressures P = 0.1, 20, 40, 60, and 80 MPa. (a) Methyl palmitate (MP) and (b) methyl stearate (MS). | ||
Barocaloric effect in fatty acid methyl esters
The entropy change of solid–liquid phase transitions of MP and MS under different pressures was obtained by eqn (S1) in the ESI.†Fig. 3 gives the entropy changes ΔSLS in L–S-T during heating and cooling at P = 0.1, 20, 40, 60, 80 and 100 MPa. It can be seen in Fig. 3a and c that during the heating process both FAMEs showed a tendency of maximum entropy change ΔSLS as the pressure increased, except MP at 20 MPa. It can be seen that the maximum entropy changes are about 726 J kg−1 K−1 and 714 J kg−1 K−1 for MP and MS under 100 MPa, respectively.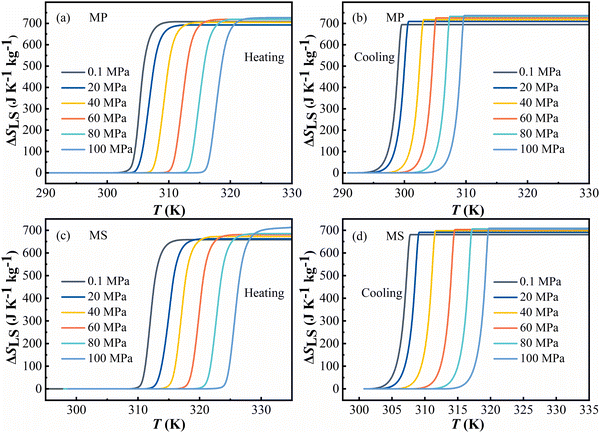 | ||
| Fig. 3 Entropy changes ΔSLS in L–S-T during heating and cooling processes at P = 0.1, 20, 40, 60, 80 and 100 MPa. (a) and (b) Methyl palmitate (MP) and (c) and (d) methyl stearate (MS). | ||
For the cooling process in Fig. 3b and d, the maximum entropy change ΔSLS also increases with the increase of applied pressure. The maximum entropy changes are about 736 J kg−1 K−1 and 708 J kg−1 K−1 for MP and MS under 100 MPa, respectively. In contrast, entropy change was suppressed by pressure in other materials, which might contribute to the weak compressibility of the FAMEs. As usual, the transition temperature shifts to higher temperature with the increase of pressure.
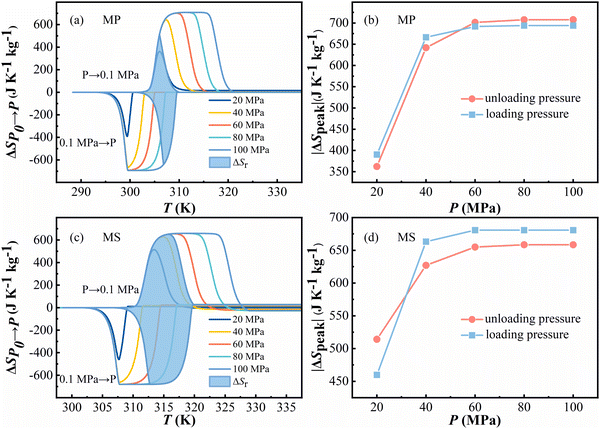
The isothermal entropy change at different pressures can be calculated by eqn (S2) in the ESI.† The pressure-induced entropy changes (ΔSP0→P) are shown in Fig. 4 under different pressures. For MP, the maximum value of ΔSP0→P at 80 MPa is 707 J kg−1 K−1 at around 311 K for the loading pressure process and 694 J kg−1 K−1 at around 300 K for the unloading pressure process. For MS, the maximum value of ΔSP0→P at 60 MPa is 680 J kg−1 K−1 at around 308 K for the loading pressure process and 658 J kg−1 K−1 at around 317 K for the unloading pressure process, respectively. Reversible isothermal entropy change (ΔSrev) is defined as the overlap of the ΔSP0→P curves during cooling and heating. As shown in Fig. 4, the maxima of ΔSrev are about 641 J kg−1 K−1 for MP and 680 J kg−1 K−1 for MS under a pressure of 100 MPa.
Meanwhile, the peak of the isothermal entropy ΔSpeak is shown in Fig. 4b and d and it shows a positive tendency with pressure increasing. With the increase of pressure, the isothermal entropy ΔSpeak also increases and approaches saturation under a pressure of 80 MPa and 60 MPa for MP and MS, respectively.
According to the heat flow data, phase diagrams can be constructed, which were given in Fig. 5. dT/dP is defined as the phase boundary's slope.37 Meanwhile, it's also an important indicator of barocaloric refrigerant materials. For MP, the dT/dP is 0.13 K MPa−1 on heating and 0.11 K MPa−1 on cooling within a pressure range of 0.1–100 MPa. Because of the larger slope while heating, the thermal hysteresis of MP is increased from 6 K at atmospheric pressure to 8 K at 100 MPa. Similarly, for MS, the dT/dP is 0.13 K MPa−1 on both heating and cooling when the pressure changes between 0.1 and 100 MPa, and its hysteresis is kept at 5 K. A few parameters of barocaloric performances of the typical BCE materials are listed in Table 1, including Tt, dTt/dP, 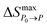 , ΔThys, etc. In summary, both MP and MS have a huge entropy change and a good pressure sensitivity compared with other typical BCE materials, which indicated that they are promising candidate materials for barocaloric refrigeration applications.
, ΔThys, etc. In summary, both MP and MS have a huge entropy change and a good pressure sensitivity compared with other typical BCE materials, which indicated that they are promising candidate materials for barocaloric refrigeration applications.
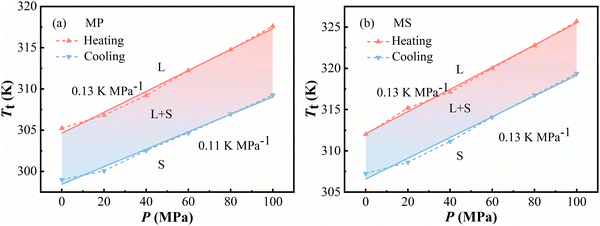 | ||
| Fig. 5 Temperature-pressure diagram of L–S-T. (a) Methyl palmitate (MP) and (b) methyl stearate (MS). | ||
| Material | T t (K) | ΔP (MPa) | dTt/dP (K MPa−1) | (J kg−1 K−1) | ΔTad (K) | T hys (K) | Ref. |
|---|---|---|---|---|---|---|---|
| a Is reversible value and esti is the estimated value. Note: Tt is the transition temperature for cooling, ΔP is the applied pressure, Thys is the thermal hysteresis at atmospheric pressure | |||||||
| NPG (CH3)2C(CH2OH)2 | 300 | 91 | 0.13 | 384 | 16a | 14 | 2 |
| TRIS (NH2)C(CH2OH)3 | 331 | 250 | 0.015 | 600 | 8 | 75 | 38 |
| PG (CH3)C(CH2OH)3 | 350 | 240 | 0.094 | 490a | 10a | 4 | 38 |
| NPA (CH3)3C(CH2OH) | 211 | 260 | 0.12 | 290a | 16a | 20 | 38 |
| C16H34 | 295 | 400 | 0.14 | 730a | 58 | — | 33 |
| C18H38 | 305 | 450 | 0.14 | 700a | 50 | — | 33 |
| Ortho-carborane | 269 | 30 | 0.13 | 88 | — | 8 | 37 |
| Meta-carborane | 277 | 30 | 0.09 | 80.9 | — | 9 | 37 |
| Para-carborane | 297 | 30 | 0.17 | 106.2 | — | 11 | 37 |
| AgI | 407 | 250 | 0.14 | 60a | 18 | 25 | 39 |
| (CH3–(CH2)9)2NH2Cl | 316 | 100 | 0.19 | 400a | 9.4 | 8 | 40 |
| FAI | 339 | 100 | 0.097 | 49.9a | 24esti | 5 | 41 |
| Fe3(pz)2(BH3CN)2 | 318 | 100 | 0.2 | 200 | — | 47 | 30 |
| [Fe(L)2](BF4)2/PVC [L = 2,6 di(pyrazol-1-yl)pyridine] | 255 | 450 | 0.1 | 168 | 49 | — | 42 |
| (CH3–(CH2)8–NH3)2MnCl4 | 289 | 100 | 0.17 | 212a | 10a | 5.2 | 26 |
| (CH3–(CH2)9–NH3)2MnCl4 | 306 | 100 | — | 250a | 12a | 9 | 26 |
| (DA)2MnCl4 (DA = decylammonium) | 308 | 50 | 0.225 | 248a | 7a | 1.4 | 43 |
| (NA)2CuBr4 (NA = nonylammonium) | 303.5 | 50 | 0.267 | 91.3a | 9.4a | 0.4 | 43 |
| C12H26O | 292 | 80 | 0.14 | 748a | 22 | 6.6 | 21 |
| C60 | 257 | 410 | 0.172 | 42 | 16 | 2 | 44 |
| C10H20O2 | 296 | 300 | 0.2 | 600a | 50 | 5 | 45 |
| C12H24O2 | 310 | 300 | 0.2 | 600a | 50 | 4 | 45 |
| C14H28O2 | 321 | 300 | 0.2 | 600a | 50 | 3 | 45 |
| Fe(L)(NCS)2 | 266 | 100 | 0.18 | 114a | 15.9 | 1.5 | 46 |
| MnFe0.8+xNi1.2−xSiGe0.5 | 310 | 100 | — | 43.72 | — | 19 | 47 |
| R134a | 310 | 1 | — | 520a | — | — | 31 |
| CH3(CH2)14CO2CH3 | 299 | 100 | 0.13 | 707 | 22esti | 6 | This work |
| CH3(CH2)16CO2CH3 | 307 | 100 | 0.13 | 680 | 13esti | 5 | This work |
The adiabatic temperature is an important indicator of barocaloric materials, which can be estimated from isothermal entropy change by an indirect method as follows:
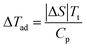 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 313 J kg−1 K−1 (see Fig. S2(b) in the ESI†), and the approximately calculated ΔTad by eqn (1) is 13 K.44 Because the specific heat capacity was measured by DSC at atmospheric pressure, and not under the corresponding high pressures, there should be a certain difference deviated from the actual adiabatic temperature values. Besides ΔSP0→P and ΔTad, another important parameter for barocaloric refrigerant materials is the refrigerant capacity (RC), which can be obtained by two methods in the ESI.† In order to calculate the effective cooling capacity by Wood and Potter's method, the reversible isothermal entropy change (ΔSr) and its δTFWHM are used. In a given caloric effect system, the reversibility is confined to a certain temperature range, which relies heavily on the variation of the applied external field. As usual, the bigger the applied external field, the bigger the reversible entropy change and its full width at half-maximum. These regions can be determined by the overlap of the temperature spans during the cooling and heating processes. In this work, the RC value up to 1921 J kg−1 could be obtained by incorporating ΔSr of 641 J kg−1 K−1 and the corresponding δTFWHM of 3 K at 100 MPa into eqn (S4) (ESI†) for MP. Similarly, the RC value could be up to 4760 J kg−1 obtained by incorporating ΔSr of 680 J kg−1 K−1 and the corresponding δTFWHM of 7 K at 100 MPa into eqn (S4) (ESI†) for MS.
313 J kg−1 K−1 (see Fig. S2(b) in the ESI†), and the approximately calculated ΔTad by eqn (1) is 13 K.44 Because the specific heat capacity was measured by DSC at atmospheric pressure, and not under the corresponding high pressures, there should be a certain difference deviated from the actual adiabatic temperature values. Besides ΔSP0→P and ΔTad, another important parameter for barocaloric refrigerant materials is the refrigerant capacity (RC), which can be obtained by two methods in the ESI.† In order to calculate the effective cooling capacity by Wood and Potter's method, the reversible isothermal entropy change (ΔSr) and its δTFWHM are used. In a given caloric effect system, the reversibility is confined to a certain temperature range, which relies heavily on the variation of the applied external field. As usual, the bigger the applied external field, the bigger the reversible entropy change and its full width at half-maximum. These regions can be determined by the overlap of the temperature spans during the cooling and heating processes. In this work, the RC value up to 1921 J kg−1 could be obtained by incorporating ΔSr of 641 J kg−1 K−1 and the corresponding δTFWHM of 3 K at 100 MPa into eqn (S4) (ESI†) for MP. Similarly, the RC value could be up to 4760 J kg−1 obtained by incorporating ΔSr of 680 J kg−1 K−1 and the corresponding δTFWHM of 7 K at 100 MPa into eqn (S4) (ESI†) for MS.
The other method to obtain RC is called the integration method or Gschneidner method, in which the upper and lower limit of the integration are the temperatures at the half-maximum of ΔSr. Therefore, the RC values of MP and MS are 1442 J kg−1 and 4277 J kg−1, respectively.
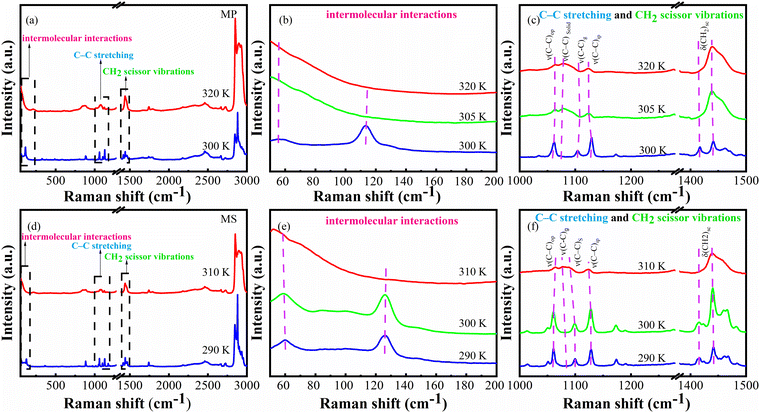
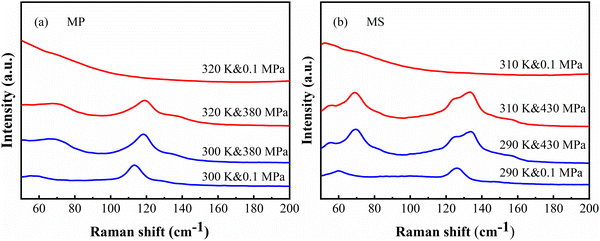
As shown in Fig. 7, it can be seen that the Raman spectra of both MP and MS are similar and both were accompanied by an obvious pressure-induced blue shift of peak positions. And the peak located at 126 cm−1 will be split into two superposed peaks (124.5 and 133.5 cm−1) for the solid state phase of MS at 298 K when the pressure was increased to 430 MPa, indicative of the phase transition in the solid state.50 Also, for the liquid phase at 310 K, well-defined peaks out of the featureless spectrum under ambient pressure will be induced at a certain external pressure just as shown in Fig. 7b, as a verification of the pressure induced phase transitions from liquid state to solid state. This also indicates that the phase transitions can be tuned by both temperature and pressure.
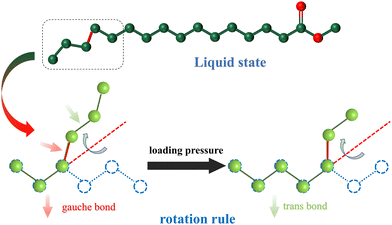
Fig. 8 is the schematic structure of MP and MS under atmospheric pressure. It can be seen that both of them are connected by all-trans bonds. When the temperature increases, trans bonds are converted into gauche bonds. This process does not cause the C–C–C bond to change, but brings about rotations of the C–C bond, resulting in an increase in configurational entropy. In general, the increase of pressure will limit the rotation angles, which will affect the number of gauche bonds and the configuration entropy.
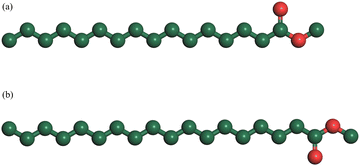 | ||
| Fig. 8 Schematic diagram of the structures of (a) methyl palmitate (MP) and (b) methyl stearate (MS). | ||
Fig. 9 is the schematic structure of MP in the liquid state. It can be seen that it is connected by gauche bonds. When the temperature decreases, the gauche bonds are converted into trans bonds. This process does not cause the C–C–C bond to change, but brings about rotation of the C–C bond, resulting in an decrease in configurational entropy. In organic molecules, the conformations are recorded as innumerable specific images of atoms or groups arranged in space generated by the rotation of single C–C bonds. A complete rotation of a single C–C bond has three potential minima which are a trans form and two gauche forms obtainable from the trans form by internal rotation of ±120°. As usual, the gauche bonds have a higher energy than the trans bond. In general, the increase of pressure will limit the rotation angles, which will affect the number of gauche bonds and the configuration entropy. As shown in Fig. 8, it can be seen that gauche bonds are decreased when pressure increases for the high-temperature phase, which is similar to the situation in pressure-induced liquid–solid phase transition.
Conclusions
The barocaloric effect of MP and MS has been discovered, revealing a dramatic impact with a maximum isothermal entropy change of up to 707 J kg−1 K−1 at 80 MPa for MP, and 680 J kg−1 K−1 at 60 MPa for MS, respectively. Both MP and MS exhibit reversible entropy changes of 641 J kg−1 K−1 and 680 J kg−1 K−1. Raman spectroscopy has confirmed the solid-to-liquid transitions induced by temperature and pressure at transition temperatures. The barocaloric effect, pressure sensitivity, large refrigeration capacity, and minimal thermal hysteresis of a few kelvin position FAMEs as strong contenders for room-temperature barocaloric refrigeration.Author contributions
Diyi Fu: data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); writing – original draft (equal); writing – review & editing (equal). Xiu Su: formal analysis (equal); investigation (equal); methodology (equal). Haoyu Wang: investigation (equal); validation (equal). Zhenxing Li: supervision (equal). Qiang Zheng: conceptualization (equal); supervision (equal); writing – review & editing (equal). Jun Shen: resources (equal). Bing Li: resources (equal). Juan Du: conceptualization (equal); supervision (equal); writing – review & editing (equal).Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (No. 2023YFB3507600) and National Natural Science Foundation of China (No. U23A20549 and 52171184).References
- Low-carbon heating and cooling: Overcoming one of world's most important net zero challenges (climate change: Science and solutions, June 2021), https://royalsociety.org/-/media/policy/projects/climate-change-sciencesolutions/climate-science-solutions-heating–cooling.pdf.
- B. Li, Y. Kawakita, S. Ohira-Kawamura, T. Sugahara, H. Wang, J. Wang, Y. Chen, S. I. Kawaguchi, S. Kawaguchi and K. Ohara, et al., Colossal barocaloric effects in plastic crystals, Nature, 2019, 567(7749), 506–510 CrossRef CAS.
- M. O. McLinden, J. S. Brown, R. Brignoli, A. F. Kazakov and P. A. Domanski, Limited options for low-global-warming-potential refrigerants, Nat. Commun., 2017, 8, 14476 CrossRef CAS PubMed.
- X. She, L. Cong, B. Nie, G. Leng, H. Peng, Y. Chen, X. Zhang, T. Wen, H. Yang and Y. Luo, Energy-efficient and -economic technologies for air conditioning with vapor compression refrigeration: A comprehensive review, Appl. Energy, 2018, 232, 157–186 CrossRef CAS.
- P. C. Stern, K. B. Janda, M. A. Brown, L. Steg, E. L. Vine and L. Lutzenhiser, Opportunities and insights for reducing fossil fuel consumption by households and organizations, Nat. Energy, 2016, 1(5), 16043 CrossRef.
- G. J. Velders, D. W. Fahey, J. S. Daniel, M. McFarland and S. O. Andersen, The large contribution of projected HFC emissions to future climate forcing, Proc. Natl. Acad. Sci. U. S. A., 2009, 106(27), 10949–10954 CrossRef CAS.
- S. A. Montzka, B. D. Hall and J. W. Elkins, Accelerated increases observed for hydrochlorofluorocarbons since 2004 in the global atmosphere, Geophys. Res. Lett., 2009, 36(3), L03804 CrossRef.
- A. Stohl, P. Seibert, J. Arduini, S. Eckhardt, P. Fraser, B. R. Greally, C. Lunder, M. Maione, J. Mühle and S. O'Doherty, et al., An analytical inversion method for determining regional and global emissions of greenhouse gases: Sensitivity studies and application to halocarbons, Atmos. Chem. Phys., 2009, 9(5), 1597–1620 CrossRef CAS.
- P. Purohit, L. Höglund-Isaksson, J. Dulac, N. Shah, M. Wei, P. Rafaj and W. Schöpp, Electricity savings and greenhouse gas emission reductions from global phase-down of hydrofluorocarbons, Atmos. Chem. Phys., 2020, 20(19), 11305–11327 CrossRef CAS.
- A. Henry, R. Prasher and A. Majumdar, Five thermal energy grand challenges for decarbonization, Nat. Energy, 2020, 5(9), 635–637 CrossRef CAS.
- P. Lloveras and J.-L. Tamarit, Advances and obstacles in pressure-driven solid-state cooling: A review of barocaloric materials, MRS Energy Sustainability, 2021, 8, 3–15 Search PubMed.
- L. Mañosa and A. Planes, Solid-state cooling by stress: A perspective, Appl. Phys. Lett., 2020, 116(5), 050501 CrossRef.
- K. A. GschneidnerJr, V. K. Pecharsky and A. O. Tsokol, Recent developments in magnetocaloric materials, Rep. Prog. Phys., 2005, 68(6), 1479–1539 CrossRef.
- V. Franco, J. S. Blázquez, J. J. Ipus, J. Y. Law, L. M. Moreno-Ramírez and A. Conde, Magnetocaloric effect: From materials research to refrigeration devices, Prog. Mater. Sci., 2018, 93, 112–232 CrossRef.
- G. V. Brown, Magnetic heat pumping near room temperature, J. Appl. Phys., 1976, 47(8), 3673–3680 CrossRef CAS.
- D. Cong, W. Xiong, A. Planes, Y. Ren, L. Manosa, P. Cao, Z. Nie, X. Sun, Z. Yang, X. Hong and Y. Wang, Colossal Elastocaloric Effect in Ferroelastic Ni-Mn-Ti Alloys, Phys. Rev. Lett., 2019, 122(25), 255703 CrossRef CAS.
- X. Liu, Z. Wu, T. Guan, H. Jiang, P. Long, X. Li, C. Ji, S. Chen, Z. Sun and J. Luo, Giant room temperature electrocaloric effect in a layered hybrid perovskite ferroelectric: [(CH3) 2CHCH2NH3]2PbCl4, Nat. Commun., 2021, 12(1), 5502 CrossRef CAS PubMed.
- B. Neese, B. Chu, S. G. Lu, Y. Wang, E. Furman and Q. M. Zhang, Large electrocaloric effect in ferroelectric polymers near room temperature, Science, 2008, 321(5890), 821–823 CrossRef CAS PubMed.
- A. S. Mischenko, Q. Zhang, J. F. Scott, R. W. Whatmore and N. D. Mathur, Giant electrocaloric effect in thin-film PbZr0.95Ti0.05O3, Science, 2006, 311(5765), 1270–1271 CrossRef CAS PubMed.
- X. Su, Z. Zhang, J. Liu, Q. Zheng, Z. Li, J. Shen, B. Li and J. Du, Colossal Barocaloric Effect of Binary Fatty Acid Methyl Esters under Low Pressures near Room Temperature, J. Phys. Chem. Lett., 2024, 15(7), 1962–1968 CrossRef CAS.
- Z. Zhang, T. Xiong, Z. Zhang, B. Li, P. Tong, J. Shen, Q. Zheng and J. Du, Colossal Barocaloric Effect near Ambient Temperature in 1-Dodecanol under a Low Pressure, J. Phys. Chem. Lett., 2024, 15(28), 7141–7146 CrossRef CAS PubMed.
- J. Seo, R. Ukani, J. Zheng, J. D. Braun, S. Wang, F. E. Chen, H. K. Kim, S. Zhang, C. Thai and R. D. McGillicuddy, et al., Barocaloric Effects in Dialkylammonium Halide Salts, J. Am. Chem. Soc., 2024, 146(4), 2736–2747 CAS.
- P. Lloveras, A. Aznar, M. Barrio, P. Negrier, C. Popescu, A. Planes, L. Manosa, E. Stern-Taulats, A. Avramenko and N. D. Mathur, et al., Colossal barocaloric effects near room temperature in plastic crystals of neopentylglycol, Nat. Commun., 2019, 10(1), 1803 CAS.
- Y. Kosugi, M. Goto, Z. Tan, A. Fujita, T. Saito, T. Kamiyama, W. T. Chen, Y. C. Chuang, H. S. Sheu, D. Kan and Y. Shimakawa, Colossal Barocaloric Effect by Large Latent Heat Produced by First-Order Intersite-Charge-Transfer Transition, Adv. Funct. Mater., 2021, 31(25), 2009476 CAS.
- D. Boldrin, Fantastic barocalorics and where to find them, Appl. Phys. Lett., 2021, 118(17), 170502 CAS.
- Y. Gao, H. Liu, F. Hu, H. Song, H. Zhang, J. Hao, X. Liu, Z. Yu, F. Shen and Y. Wang, et al., Reversible colossal barocaloric effect dominated by disordering of organic chains in (CH3–(CH2)n−1–NH3)2MnCl4 single crystals, NPG Asia, Materials, 2022, 14(1), 34 CAS.
- M. V. Gorev, E. A. Mikhaleva, I. N. Flerov and E. V. Bogdanov, Conventional and inverse barocaloric effects in ferroelectric NH4HSO4, J. Alloys Compd., 2019, 806, 1047–1051 CAS.
- W. J. Xu, Y. Zeng, W. Yuan, W. X. Zhang and X. M. Chen, A large room-temperature entropy change in a new hybrid ferroelastic with an unconventional bond-switching mechanism, Chem. Commun., 2020, 56(69), 10054–10057 CAS.
- J. Min, A. K. Sagotra and C. Cazorla, Large barocaloric effects in thermoelectric superionic materials. Physical Review, Materials, 2020, 4(1), 015403 CAS.
- R. Li, Z. Zhang and Y. S. Bibik, Gural’skiy, I. y. A.; Zatovsky, I. V.; Liu, Z.; Li, Q.; Li, B.; Levchenko, G.; Liu, B. Colossal barocaloric effect of the spin-crossover compound {Fe(pz)2(BH3CN)2} near room temperature, Appl. Phys. Lett., 2024, 124(12), 122202 CrossRef CAS.
- H. G. Ramírez-Hernández, A. Morales-Fuentes, F. A. Sánchez-Cruz, S. Méndez-Díaz, H. D. García-Lara and S. Martínez-Martínez, Experimental study on the operating characteristics of a display refrigerator phasing out R134a to R1234ze(E) and its binary blends, Int. J. Refrig., 2022, 138, 1–12 CrossRef.
- M. Gelpi, J. Garcia-Ben, S. Rodriguez-Hermida, J. Lopez-Beceiro, R. Artiaga, A. Baalina, M. Romero-Gomez, J. Romero-Gomez, S. Zaragoza and J. Salgado-Beceiro, et al., Empowering CO2 Eco-Refrigeration With Colossal Breathing-Caloric-Like Effects in MOF-508b, Adv. Mater., 2024, 36(16), e2310499 CrossRef.
- J. Lin, P. Tong, K. Zhang, K. Tao, W. Lu, X. Wang, X. Zhang, W. Song and Y. Sun, Colossal and reversible barocaloric effect in liquid-solid-transition materials n-alkanes, Nat. Commun., 2022, 13(1), 596 CrossRef CAS PubMed.
- C. Zhang, D. Wang, S. Qian, Z. Zhang, X. Liang, L. Wu, L. Long, H. Shi and Z. Han, Giant barocaloric effects with a wide refrigeration temperature range in ethylene vinyl acetate copolymers, Mater. Horiz., 2022, 9(4), 1293–1298 RSC.
- M. M. Kenisarin, Thermophysical properties of some organic phase change materials for latent heat storage. A review, Sol. Energy, 2014, 107, 553–575 CrossRef CAS.
- D. G. Atinafu, Y. S. Ok, H. W. Kua and S. Kim, Thermal properties of composite organic phase change materials (PCMs): A critical review on their engineering chemistry, Appl. Therm. Eng., 2020, 181, 115960 CrossRef CAS.
- K. Zhang, R. Song, J. Qi, Z. Zhang, Z. Zhang, C. Yu, K. Li, Z. Zhang and B. Li, Colossal Barocaloric Effect in Carboranes as a Performance Tradeoff, Adv. Funct. Mater., 2022, 32(20), 2112622 CrossRef CAS.
- F. B. Li, M. Li, X. Xu, Z. C. Yang, H. Xu, C. K. Jia, K. Li, J. He, B. Li and H. Wang, Understanding colossal barocaloric effects in plastic crystals, Nat. Commun., 2020, 11(1), 4190 CrossRef CAS.
- A. Aznar, P. Lloveras, M. Romanini, M. Barrio, J. L. Tamarit, C. Cazorla, D. Errandonea, N. D. Mathur, A. Planes, X. Moya and L. Manosa, Giant barocaloric effects over a wide temperature range in superionic conductor AgI, Nat. Commun., 2017, 8(1), 1851 CrossRef PubMed.
- Y. H. Gao, D. H. Wang, F. X. Hu, Q. Z. Huang, Y. T. Song, S. K. Yuan, Z. Y. Tian, B. J. Wang, Z. B. Yu and H. B. Zhou, et al., Low pressure reversibly driving colossal barocaloric effect in two-dimensional vdW alkylammonium halides, Nat. Commun., 2024, 15(1), 1838 CrossRef CAS PubMed.
- C. Yu, J. Huang, J. Qi, P. Liu, D. Li, T. Yang, Z. Zhang and B. Li, Giant barocaloric effects in formamidinium iodide, APL Mater., 2022, 10(1), 011109 CrossRef CAS.
- K. Lunser, E. Kavak, K. Gurpinar, B. Emre, O. Atakol, E. Stern-Taulats, M. Porta, A. Planes, P. Lloveras, J. L. Tamarit and L. Manosa, Elastocaloric, barocaloric and magnetocaloric effects in spin crossover polymer composite films, Nat. Commun., 2024, 15(1), 6171 CrossRef CAS PubMed.
- J. Seo, R. D. McGillicuddy, A. H. Slavney, S. Zhang, R. Ukani, A. A. Yakovenko, S. L. Zheng and J. A. Mason, Colossal barocaloric effects with ultralow hysteresis in two-dimensional metal-halide perovskites, Nat. Commun., 2022, 13(1), 2536 CrossRef CAS PubMed.
- J. Li, D. Dunstan, X. Lou, A. Planes, L. Mañosa, M. Barrio, J.-L. Tamarit and P. Lloveras, Reversible barocaloric effects over a large temperature span in fullerite C60, J. Mater. Chem. A, 2020, 8(39), 20354–20362 RSC.
- T. Xiong, J. Lin, T. Zhou, G. Shi, T. Ye, X. Pan, K. Liu, R. Jiang, R. Zhang and W. Song, Colossal barocaloric effect of phase-change fatty acids, Appl. Phys. Lett., 2024, 125(6), 061903 CrossRef CAS.
- M. Seredyuk, R. Li, K. Znovjyak, Z. Zhang, F. J. Valverde-Muñoz, B. Li, M. C. Muñoz, Q. Li, B. Liu, G. Levchenko and J. A. Real, Reversible Colossal Barocaloric Effect of a New FeII Molecular Complex with Low Hysteretic Spin Crossover Behavior, Adv. Funct. Mater., 2024, 34, 30 CrossRef.
- X. Wang, H. Xuan, M. Ji, F. Chen, Z. Han, P. Han and J. Qiao, Large barocaloric and magnetocaloric effects in MnFe0.8+xNi1.2−xSiGe0.5 high-entropy intermetallics, Appl. Phys. Lett., 2024, 124(9), 092411 CrossRef CAS.
- J. R. Beattie, S. E. Bell and B. W. Moss, A critical evaluation of Raman spectroscopy for the analysis of lipids: fatty acid methyl esters, Lipids, 2004, 39(5), 407–419 CrossRef CAS PubMed.
- A. M. Miranda, E. W. Castilho-Almeida, E. H. Martins Ferreira, G. F. Moreira, C. A. Achete, R. A. S. Z. Armond, H. F. Dos Santos and A. Jorio, Line shape analysis of the Raman spectra from pure and mixed biofuels esters compounds, Fuel, 2014, 115, 118–125 CrossRef CAS.
- A. Francisco-López, B. Charles, O. J. Weber, M. I. Alonso, M. Garriga, M. Campoy-Quiles, M. T. Weller and A. R. Goñi, Pressure-Induced Locking of Methylammonium Cations versus Amorphization in Hybrid Lead Iodide Perovskites, J. Phys. Chem. C, 2018, 122(38), 22073–22082 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc03483j |
| ‡ These three authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |