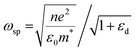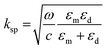Open Access Article
Open Access ArticleAdvancements in surface plasmon resonance sensors for real-time detection of chemical analytes: sensing materials and applications
Sung Hwan Cho†
a,
Seungwon Choi†a,
Jun Min Suh†ab and
Ho Won Jang *ac
*ac
aDepartment of Materials Science and Engineering, Research Institute of Advanced Materials, Seoul National University, Seoul, 08826, Republic of Korea. E-mail: hwjang@snu.ac.kr
bSchool of Transdisciplinary Innovations, Seoul National University, Seoul 08826, Republic of Korea
cAdvanced Institute of Convergence Technology, Seoul National University, Suwon 16229, Republic of Korea
First published on 26th February 2025
Abstract
Chemicals are being used in various fields with the development of industry, but the importance of human safety from chemical exposure is becoming increasingly evident. Traditional detection methods, which analyze analytes through preprocessing, have limitations in real-time applications. In contrast, LSPR and SPR sensors, which can rapidly and accurately detect chemicals in real time, are among the most reliable methods for protecting against chemical threats. LSPR and SPR sensors detect minute interactions between sensing materials and chemicals through changes in absorbance and refractive index, enabling the accurate detection of even the smallest changes. To maximize the performance of LSPR and SPR sensors capable of real-time detection and apply them across various fields, ongoing research has focused on innovative materials, fabrication techniques, and nanostructures. The future perspectives on sensing materials for real-time detection technologies used by LSPR and SPR sensors are discussed. This review presents guidelines for selecting sensing materials for use in LSPR and SPR sensors in real-time applications.
1. Introduction
In modern society, the importance and versatility of chemicals in various industries, including biotechnology, semiconductors, and agriculture, are growing.1,2 Additionally, as various compounds are newly synthesized or discovered, the types of chemicals used in modern society are increasing exponentially. However, with the development of these chemicals, concerns about the risks they pose—especially those that can have fatal effects on the human body even in small amounts—are also increasing.3 For the continued development of humanity, coexistence with various types of chemicals is essential. Therefore, appropriate measures, such as early warning systems for chemicals emitted due to inadequate management, accidental damage, or improper disposal, are necessary.4Developing accurate methods for detecting chemical risks is crucial for ensuring the safe use of chemicals. Various detection technologies have been developed, including inductively coupled plasma mass spectrometry (ICP-MS),5 gas chromatography (GC),6 and nuclear magnetic resonance (NMR).7 However, existing methods have the disadvantages of being expensive and lacking real-time detection capabilities, which are essential for early risk detection. Real-time detection refers to a continuous and immediate monitoring process that provides instantaneous results or feedback as events unfold. It provides fast and accurate information about the presence of chemicals, even at extremely low levels, distinguishing it from traditional methods that need time-consuming laboratory analyses. The capacity to give rapid data is critical in emergency response scenarios, industrial settings, and environmental monitoring, ensuring efficient risk management.8–10
Sensor technology is a popular and promising detection method due to its advantages of excellent accessibility and the ability to detect chemicals in a relatively short period of time.11,12 Sensors detect chemicals and transmit information through various methods, serving as an alarm to alert individuals to potential chemical threats. In addition to addressing chemical threats, constant monitoring of air, water, food, and daily products by sensors is required to gain a basic understanding of chemical exposures and ensure human safety.
Sensor technology has been developed in various forms to detect chemicals, including chemoresistive,13 colorimetric,14 field-effect transistor,15 acoustic,16 and plasmonic sensors.17 In addition, sensors have made significant advances and play an important role in rapidly detecting very low concentrations of analytes. As a proactive safeguard, they allow for the identification, monitoring, and mitigation of possible risks to human health, environmental sustainability, and industrial safety in our complex and chemically saturated modern society. Among the various sensor technologies, researchers have been actively focused on technologies capable of accurate and real-time detection, while localized surface plasmon resonance (LSPR) and surface plasmon resonance (SPR) sensors have been gaining significant attention because of their low-concentration detection limit, instantaneous detection, and exceptional adaptability.18–20 The excellent sensing properties of LSPR sensors at the nanoscale allow them to detect minute amounts of target molecules at the ppb level, increasing the real-time validity of the detection results for trace amounts of hazardous substances.21 Because LSPR sensors detect chemicals through changes in absorbance, they show a color change that can be easily confirmed even with a very small amount of analyte. Based on this easy accessibility, LSPR sensors do not require complex optical systems, making their miniaturization easier.22 Furthermore, they can be integrated with microfluidics or lab-on-a-chip to construct compact systems.23 This enables the development of small and portable sensing devices for real-time and on-site detection in diagnostics, food safety, and environmental monitoring. Alternatively, SPR sensors detect chemicals upon changes in refractive index through the adsorption of the sensing materials. Given that these detection methods are based on the real-time interaction of liquid or gas as it flows, they have the advantages of being able to measure even in flowing fluids.24 Unlike LSPR sensors, they consist of bulky optical components for the accurate alignment of light, making it challenging to miniaturize them into simple devices.22 Thus, to overcome this limitation, recent research has reported various approaches for miniaturizing SPR sensors, such as reducing the probe size by grating- or fiber-based SPR sensors that do not require prisms and optimizing the optical design by minimizing the size of optical components.25,26 Furthermore, SPR sensors give crucial kinetic information, allowing researchers to confirm binding events and comprehend their temporal dynamics. Both LSPR and SPR show great promise for accurate real-time detection technology, promising breakthroughs in on-site monitoring and integration with emerging technologies, underlining their importance in the growth of analytical sciences.
The various advantages of LSRP and SPR sensors originate from the ability of sensing materials to respond to even the slightest interaction with chemicals, and thus they are widely used in both biological and non-biological domains. These properties are vital given that they allow selective interactions with the target analyte, ensuring that the sensor accurately and specifically responds to the desired substance. The tunability of the interactions that occur on the surface of the sensing material can improve both the specificity and sensitivity required for the detection of a wide range of chemicals, the development of which is continuously evolving. In the field of LSPR sensors, where the size and shape of nanoparticles are important, research is in progress through the shape of nanoparticles and the suitability of ligands, such as metal nanoparticles (NPs), bimetallic NPs, and metal–organic materials (MOMs). Alternatively, in the field of SPR sensors, where reactions with chemicals through adsorption are important, research is being conducted on materials that can be manufactured in the form of thin films, such as two-dimensional (2D) materials, metal oxides, and organic composites, as shown in Fig. 1.
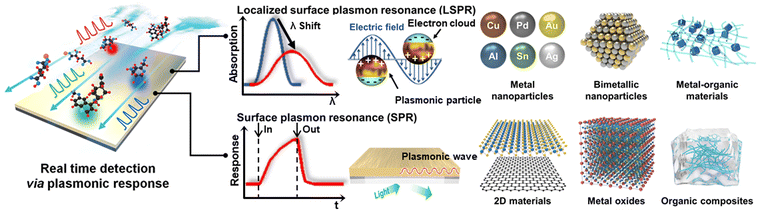 | ||
| Fig. 1 Schematic of two representative technologies (LSPR and SPR) used in real-time detection via plasmonic reactions and the materials used for each technology. | ||
The need for the development of LSPR and SPR sensors is increasing based on their wide applicability in bio and non-bio fields, and accordingly, research is being conducted on sensing materials suitable for each measurement method. In response to these emerging needs, active research is being conducted in LSPR and SPR sensors, as evidenced by the notable rise in the number of published papers and citations over the past decade, as shown in Fig. 2.
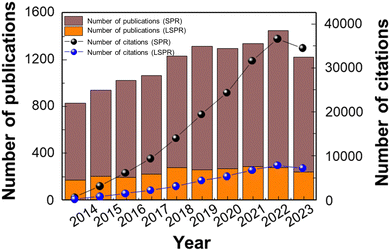 | ||
| Fig. 2 Number of publications and citations on LSPR and SPR sensors. The data were collected using the Web of Science Core Collection, with the keywords “LSPR sensor” or “SPR sensor”. | ||
2. Principles of LSPR and SPR sensors
LSPR and SPR sensors have their roots in the broader fields of plasmonics and nanotechnology. The theoretical foundation for localized plasmons was presented by theorists including Mie in 1908, with early experimental work gaining momentum in the 1980s and 1990s.27 The development of advanced nanofabrication techniques allowed precise control of the size and shape of NPs, influencing their sensing properties. In the late 1990s, researchers recognized the potential of LSPR and SPR for sensing applications due to their sensitivity to changes in the local environment.28,29 This led to the introduction of LSPR- and SPR-based sensors, enabling real-time detection on the nanoscale.The usage of LSPR and SPR sensors has evolved significantly over the years. LSPR sensors have found applications in diverse areas, ranging from biomedicine to environmental monitoring.30,31 The unique optical properties of metal NPs in LSPR sensors allow the highly sensitive and selective detection of analytes at the nanoscale. In addition, research to improve their performance through material-based research based on metal NPs is continuing. Alternatively, SPR sensors, with the ability to monitor interactions of biomolecules and chemicals in real-time, have become indispensable in fields such as biochemistry, pharmaceuticals, and medical diagnostics.32 The miniaturization and integration of SPR systems, together with advancements in SPR imaging techniques, have expanded their utility to portable and point-of-care applications. Ongoing research in both LSPR and SPR continues to explore new materials, configurations, and applications, promising further advancements in the field of optical sensing.
Surface plasmon refers to the collective oscillations of free electrons on the surface of metals, such as gold, silver, and copper. The electric field generated by surface plasmons at the metal–dielectric interface is called surface plasmon polariton (SPP), propagating along the interface and exponentially decaying away from the surface. When the size of a metal particle is comparable to the wavelength of light, the confined surface plasmon is referred to as localized surface plasmon (LSP). An absorption peak appears at the plasmon frequency of LSP (ωsp) when irradiated in the visible and infrared range.33
The position of this absorption peak depends on the size and shape of the metal NP, as well as the refractive index near its surface. When integrated into sensors, LSPR sensors operate by observing changes in absorption wavelength caused by the interaction between the analytes and the surface of NPs. This enhanced sensitivity to minute changes in NPs facilitates precise and real-time detection, positioning LSPR sensors as indispensable instruments in fields such as biotechnology, environmental monitoring, and medical diagnostics. A critical component in maximizing the sensing properties of LSPR-based sensors lies in material research, where investigations into metal NPs strive to tailor their size and shape for enhanced sensitivity and plasmonic effects. Bimetallic NPs offer a means for fine-tuning the LSPR characteristics, ensuring improved stability and durability, while MOMs introduce functionalization, amplifying the selectivity and enabling multifunctionality. This holistic approach to material research, encompassing metal NPs, bimetallic NPs, and MOMs, plays an important role in increasing the versatility and efficiency of LSPR sensors in a variety of applications ranging from biomedical diagnostics to environmental monitoring.
Alternatively, in SPR sensors, polarized light is irradiated on a nanometer-thick metal thin film at a specific incident angle and wavelength. The generated SPP on the metal film has a propagation constant (ksp), which is calculated as follows:
When the propagation constant of SPP and the wavevector of incident polarized light are well matched, SPR occurs on the surface of the metal film.34 The resonance angle and wavelength are sensitively influenced by changes in the refractive index near the metal surface. The adsorption of analytes induces a change in the refractive index near the metal surface and influences the resonance angle and wavelength. Thus, by tracking the resonance conditions, the interaction and kinetics between the analytes and sensing materials can be monitored sensitively in real-time. Accordingly, SPR sensors play an important role in applications such as drug discovery, medical diagnostics, and environmental monitoring due to their exceptional sensitivity and binding kinetics. Concurrently, research focused on the recognition layer, which is a thin functionalized sensing material on the sensor surface and responsible for adsorbing chemicals in SPR sensors, has proven to be indispensable for optimizing the performance of these sensors. Sensing materials in the recognition layer are being researched for their potential application in 2D materials such as graphene, which offer improved biocompatibility and high surface-to-volume ratio for enhanced sensitivity and real-time detection. Metal oxides improve the sensitivity and are valuable in applications such as environmental monitoring owing to their low cost and easy and versatile synthesis methods. Additionally, organic composites with customized functionalization are highly suitable for biomedical and chemical sensing due to their high selectivity, enabling the detection of the desired chemical. The ongoing investigation and refinement of material choices for the recognition layer constitute pivotal strides in advancing SPR sensors for a spectrum of applications, spanning medical diagnostics to environmental analysis.
3. Real-time monitoring properties of LSPR sensors
LSPR sensors offer distinct advantages for the real-time detection of chemicals over other technologies in terms of versatility and accuracy. The real-time monitoring capabilities of LSPR sensors allow continuous observation of chemicals to gain insight into the kinetics and affinity of the interaction between the sensing materials and chemicals. The low detection limit and great sensitivity of LSPR sensors are due to the characteristics of their sensing materials, which change their shape and size in response to even the slightest change, and with the development of optical measurement equipment, even minute changes in absorbance can be accurately measured. Additionally, LSPR sensors have the advantage of being able to detect chemicals with the naked eye without measuring equipment by detecting chemicals through changes in absorbance even with a small amount of sensing material. These various advantages improve the portability and applicability of LSPR sensors, allowing efficient, low-cost detection without restrictions in various fields where chemicals exist. Overall, the comprehensive attributes of LSPR sensors make them powerful tools for diverse applications in chemical sensing. The adaptability and versatility of LSPR sensors extend their utility across a wide range of applications, including biochemistry, environmental monitoring, medical diagnostics, and food safety. Thus, their wide applicability and ability to provide real-time information contribute to their widespread adoption in both research and industrial settings.The advantages of LSPR sensors position them as valuable tools in the field of sensing, offering unique capabilities for precise, efficient, and real-time detection in chemical applications. Thus, diverse sensing materials are required to improve their sensing performance and real-time detection ability. Research is being actively conducted on metal NPs that most easily exhibit the LSRP phenomenon,35–44 LSPR sensors with improved characteristics using various bimetallic NPs,45–54 and the material-based LSPR sensors that show improved sensitivity by using organic particles in metal nanoparticles are summarized in Table 1.55–60
| Material type | Target type | Sensing materialsa | Analyte | Detection range | Limit of detection | Ref. |
|---|---|---|---|---|---|---|
| Metal NPs | Bio | AuNRs | β-Galactosidase | 0.1–10 nM | 128 pM | 35 |
| AgNPs | Melamine | 0–10 μM | 0.099 μM | 36 | ||
| AgNPs | Timolol | 0.1–1000 μM | 1.2 μM | 37 | ||
| AgNPs | Aflatoxin B1 | 1–10 ng mL−1 | 0.36 ng mL−1 | 38 | ||
| AgNPs | Cholic acid | 0–30 μM | 1 μM | 39 | ||
| AgNPs | Omeprazole | 0.05–40 μM | 15 nM | 40 | ||
| Non-bio | AuNRs | Cu2+ | 0–1 mM | 0.5 nM | 41 | |
| AgNPs | Cd2+ | 1–10 μM | 87 nM | 42 | ||
| AgNPs | As3+ | 5–500 μg L−1 | 2 μg L−1 | 43 | ||
| Ag NPR | Hg2+ | 0.005–10 μM | 0.2 nM | 44 | ||
| Bimetallic NPs | Bio | Ag/Au nanoshell | Glucose | 0.002–2 mM | 45 | |
| Au nanocage | Gallic acid | 0.01–5 μM | 46 | |||
| Au nanocage | Vitamin C | 0.05–7.5 μM | 0.024 μM | 47 | ||
| AuNRs | Ellagic acid | 0.2–20 μM | 0.04 μM | 48 | ||
| Non-bio | GSH-s-Au/Ag nanoframe | Co2+ | 1.7–17 μM | 0.04 μM | 49 | |
| Pd/Au/Cu | H2 | 50 | ||||
| Au nanosphere/AgNRs | ClO− | 0.5–30 μM | 0.24 μM | 51 | ||
| Au/Ag nanocage | Hg2+ | 0.03–35 μM | 10 nM | 52 | ||
| Au/Ag/AgCl core–shell NPs | NH3 | 0–5000 μM | 6.4 μM | 53 | ||
| Ag/Cu NPs | Hg2+ | 0.001–50 μM | 0.51 nM | 54 | ||
| MOMs | Bio | Ag NPR/glucose oxidase | Glucose | 0.2–100 μM | 0.2 μM | 55 |
| Sucrose capped AuNPs | Daclatasvir | 0.01–1 μg ml−1 | 0.008 μg ml−1 | 56 | ||
| Amine/POSS-APBAs/AuNPs | Glucose | 1–1000 μM | 25 μM | 57 | ||
| Non-bio | SA–AgNP/PVA nanocomposites | Hg2+ | 0.9–1200 ppb | 0.9 ppb | 58 | |
| AuNPs/PO-EGMA | Pb2+ | 0.1–100 nM | 25 pM | 59 | ||
| Melamine–Au nanostars | Uric acid | 0–100 μM | 8.5 nM | 60 | ||
3.1 Metal NP-based LSPR sensor
Metal NP-based LSPR sensors are now widely used in sensing technology. The high surface-to-volume ratio and adjustable properties of these NPs, regulated by their form, make them ideal for immobilizing target molecules.61,62 The exact geometry of metal NPs has a substantial impact on their plasmonic resonance frequencies, allowing fine tuning to meet the desired wavelength. Metal NPs exhibit a unique characteristic where their light absorption wavelength changes based on variations in their shape and size due to minuscule interactions with chemicals. This property enhances the sensitivity of LSPR sensors to environmental changes, resulting in superior detection capability and precision. The adaptability of structuring metal NPs allows subtle control of the localized electromagnetic field, which affects the detection capabilities of LSPR sensors. With these considerations, LSPR sensors based on metal NPs, with their shape-dependent effects, have found significant use in biomedical diagnostics, environmental monitoring, and chemical sensing. This highlights their ability to provide high-performance and adaptable sensing technologies that meet the specific needs of various areas.The sensing mechanism described involves a multi-colorimetric assay using AuNRs and enzyme-induced metallization. In the absence of β-gal, the unhydrolyzed substrate, p-aminophenyl β-D-galactopyranoside (PAPG), cannot reduce Ag+ to metallic Ag. As a result, the solution retains the initial color of the AuNRs, appearing light pink. However, in the presence of β-galactosidase, it cleaves PAPG into galactoside and a reducing agent (PAP). This reducing agent, in the presence of AuNRs, then reduces Ag+ to metallic Ag. The reduced metallic Ag coats the surface of AuNRs, inducing a multicolor shift in the sample solution. Consequently, the color of the solution transitions from light-green to orange-red, correlating with the concentration of β-gal. This multi-colorimetric assay provides a visual indication of the presence of β-gal, allowing a qualitative and potentially quantitative assessment based on the observed color changes in real-time.
The researchers used the absorbance spectra to monitor the change in AuNRs in varying β-gal concentrations (Fig. 3(a)). As the concentration increased, the absorbance of the AuNRs increased and the wavelength absorbed decreased. A dynamic relationship between Δλmax and β-gal concentration was seen in the range of 0.1 × 10−9 to 10 × 10−9 M, as shown in Fig. 3(b). Fig. 3(b) demonstrates that the Δλmax value increased with an increase in the concentration of β-gal. As the concentration increased, the change in the wavelength decreased, but at low concentrations, the correlation between concentration and Δλmax was observed to be linear. This change in Δλmax could also be observed as a color change in the solution. As shown in Fig. 3(c), the detection solutions exhibited a color shift from light-green to orange-red compared to the control. The distinct multicolor changes at the concentration of 2.0 × 10−9 M could be easily identified visually. As shown in Fig. 3(d) and (e), the transmission electron microscopy (TEM) images showed that the synthesized AuNRs possessed a length of 59 ± 8 nm and width of 11 ± 1 nm. After, the reaction of the Ag shell on the body sides of AuNRs was observed. The deposition of an Ag shell on the surface of AuNRs was further investigated using high-resolution TEM-energy dispersive X-ray spectroscopy (EDS). The corresponding EDS Ag (red color) and Au (green color) mapping images also confirmed the coating of metallic silver on the surface of AuNRs. The Ag peaks in the EDS spectrum were observed after the enzyme-induced metallization. Both an enhancement in absorbance intensity and blue shift in the longitudinal LSPR peak were observed after the enzyme-induced Ag metallization on the surface of AuNRs. The longitudinal LSPR peak shifted from 885 to 520 nm, and the absorbance intensity of the longitudinal LSPR peak likewise increased with an increase in β-gal concentration.
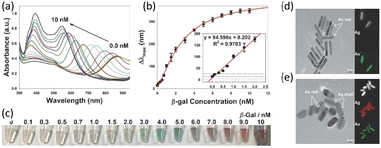 | ||
| Fig. 3 (a) UV-vis absorption spectra and (b) the blue shift in the longitudinal LSPR peak of Au NRs in response to β-gal (0 to 10 nM). (c) Photographs of multi-colorimetric assays of AuNRs toward various concentrations of β-gal. TEM images of AuNRs (d) before and (e) after incubation with β-gal. Reproduced with permission from ref. 35 Copyright©2016, John Wiley & Sons. | ||
Chen et al. reported the fabrication of an LSPR Hg2+ sensor based on the shape change of unmodified Ag nanoprisms (AgNPRs) by adding Hg2+ and S2O32−.44 The proposed sensing mechanism in this study involves apical activation and passivation of triangular AgNPRs through the actions of S2O32− and Hg2+. S2O32− acts as a leaching agent, rapidly truncating the sharp tips of unmodified AgNPRs into round nanodiscs, resulting in a distinct color change and a significant shift in the LSPR wavelength. This change is prevented in the presence of Hg2+, which protects the AgNPRs from etching by forming HgS at their corner sites. Thus, the shape of the AgNPRs is frozen, allowing the quantification of Hg2+ ions in the solution, as shown in Fig. 4(a). The optical properties of the prepared AgNPR colloid, including a blue color and distinct LSPR absorption bands, were highly sensitive to trace reactions, controlling the edge length, thickness, and sharp tips of the AgNPRs. The sculpturing effect of S2O32− and the passivation effect of Hg2+ were illustrated through changes in colloidal color and absorption spectra, as shown in Fig. 4(b). The addition of S2O32− induced facet-selective etching reactions, leading to a blue to yellow color change and a blue-shift in the LSPR peak. In the presence of both S2O32− and Hg2+, the color change and LSPR shift are halted due to the formation of insoluble HgS at the tips of AgNPRs. The changes in the shape of individual AgNPRs in three separate cases were also verified via TEM analysis. TEM imaging revealed the original triangular shapes of AgNPR (Fig. 4(c)), circular nanodisc shapes in the presence of S2O3− (Fig. 4(d)), and additional angles in the presence of both S2O3− and Hg2+ (Fig. 4(e)).
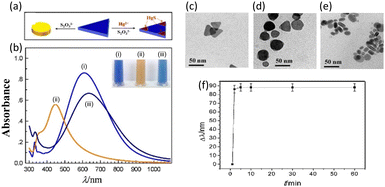 | ||
| Fig. 4 (a) Schematic of the shape evolution of an AgNPR and growth of HgS in the presence of S2O32− and Hg2+. (b) UV-vis spectra and photographs (inset) of AgNPRs under 3 different conditions: (i) as-prepared, (ii) with S2O32−, and (iii) with S2O32− and Hg2+. TEM images of AgNPR: (c) as-prepared and with the addition of (d) Na2S2O3 and (e) Hg2+ and Na2S2O3. (f) Wavelength shift as a function of the reaction time for the AgNPR–Hg2+–Na2S2O3 mixture, pH 2.9. Reproduced with permission from ref. 44 Copyright©2015, Elsevier B. V. | ||
Fig. 4(f) demonstrates that the reaction reached a steady state within 5 min, allowing quick detection and real-time detection. The concentration of Hg2+ can further tune the color change and LSPR wavelength, providing distinctive characteristics for wide-range Hg2+ detection within a short timeframe. The dominant interaction among AgNPR, S2O32−, and Hg2+ was identified as HgS deposition reactions within 5 min, contrasting with the longer etching reactions observed without S2O32−.
Metal NP-based LSPR sensors with a color shift due to agglomeration are dynamic and responsive sensing platforms. Metal NPs, often Au or Ag, are scattered in a solution to form these sensors. When exposed to specific analytes, the NPs aggregate, changing their spatial arrangement and causing a shift in the plasmonic resonance. This agglomeration-induced alteration appears as a distinct color shift in the solution, providing a visible readout. The color change mechanism provides an immediate and understandable indication of the presence and concentration of the target analytes, making these sensors ideal for applications requiring a quick and qualitative response. The issue of regulating agglomeration in these systems emphasizes the significance of careful design and optimization to improve the reliability and sensitivity of color-changing LSPR sensors in a variety of analytical and detecting applications.
3.2 Bimetallic NP-based LSPR sensors
Bimetallic NPs exhibit synergistic effects, where the interplay between the two metals influences their resonant frequency and nanostructure, resulting in an enhanced sensor performance. In addition to improved stability and durability, the distinctive characteristics of bimetallic materials, such as flexible morphological tunability, enable visual detection through multicolor changes and enhance the sensitivity and accuracy in LSPR sensing applications across diverse fields, including biomedical diagnostics, environmental monitoring, and chemical analysis.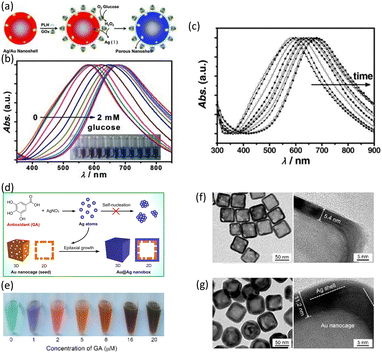 | ||
| Fig. 5 (a) Process for the fabrication of Ag/Au bimetallic NSs and the sensing mechanism. (b) Normalized UV-vis extinction spectra of Ag/Au NSs after incubation with glucose in various concentrations (0 to 2 mM). (c) Time-dependent UV-vis absorption spectra of Ag/Au bimetallic NSs upon incubation with 3 × 10−3 M glucose. Reproduced with permission from ref. 45 Copyright©2012, John Wiley & Sons. (d) Schematic of the fabrication of Au/Ag nanoboxes. (e) Color changes in the creation of Au/Ag nanoboxes at various concentrations of gallic acid. TEM images of (f) Au nanocages in the absence of gallic acid and (g) Ag/Au nanoboxes in the presence of gallic acid. Reproduced with permission from ref. 46 Copyright©2018, Elsevier B. V. | ||
Also, they investigated the sensitivity and applicability of the Ag/Au–GOx NS system for optical glucose sensing. In this measurement, 1 mL aliquots of the as-prepared Ag/Au–GOx NS suspension were incubated with 20 μL glucose solutions with a final concentration in the range of 0.002 × 10−3 to 2 × 10−3 M for 30 min. Fig. 5(b) shows a steady red-shift in the LSPR peak and a clear color change as the glucose concentration increased. A linear association was found between the LSPR peak shift of Δλmax and glucose concentration, ranging from approximately 0.5 × 10−6 M to 0.02 × 10−3 M.
Evaluating the real-time detection capability of a sensor can be effectively done by observing changes over a period of time. Fig. 5(c) displays the UV-vis spectra of the nanocomplex over time when incubated with a glucose concentration of 3 × 10−3 M. The λmax of the nanocomplex progressively shifted to longer wavelengths when glucose was injected. After around 1–2 h, the shift leveled off and reached a saturation value. An observable transition in color, shifting from red-violet to blue, occurred within a time frame of 10 min. This implies that the Ag/Au–GOx NS have the potential to be utilized for the practical colorimetric detection of glucose.
Nanostructures, with their small size and high surface-to-volume ratio, improve the sensing properties in a variety of applications.13 The increased surface area of nanostructured materials creates more active sites, resulting in more significant interactions with the target analytes. The morphological change in Au nanocage to Au@Ag nanobox, and thus the LSPR evolution, was efficiently regulated by trace amounts of antioxidant, which acted as both a reductant in seed-mediated growth and a detecting target. Ag atoms can be produced through the redox reaction between Ag+ and an antioxidant, such as gallic acid, as utilized in this study.46 When Au nanocages (seeds) with a cubic shape, hollow interior, and numerous holes in their wall are present, newly generated Ag atoms deposit uniformly on the surface of the seeds by heterogeneous nucleation rather than self-nucleation. This results in the creation of Au@Ag nanoboxes with a hollow interior and closed wall (Fig. 5(d)). A new colorimetric approach for antioxidant assessment was devised using the morphological changes in nanoprobes and triggered LSPR evolution in the absence and presence of antioxidants.
This technique achieved high sensitivity via the heterogeneous nucleation of metal NPs, which requires a lower energy barrier compared to self-nucleation. Fig. 5(e) shows sample photographs of the color change of the solution at various gallic acid doses (0 to 20 μM). As the gallic acid content increased, the color changed from dark green to blue, red, orange, and brown.
The TEM images in Fig. 5(f) reveal hollow cubic Au nanocages with an outer edge length of 54.2 ± 3.5 nm and a wall thickness of 5.4 ± 0.8 nm. The pores on the side faces of the Au nanocages confirmed the characteristic nanocage morphology. In the presence of 4 μM gallic acid, the cubic nanocages were converted into nanoboxes with a hollow interior and closed walls. Compared to the original nanocages, the nanoboxes had significantly longer outer edges and thicker walls (Fig. 5(g)). The nanoboxes had an average size of 65.8 ± 5.3 nm, indicating an Ag layer of approximately 5.8 nm on the surface of the Au nanocages. The TEM images at a higher magnification revealed the outer layer of Ag and the contained nanocage due to the contrast between Au and Ag.
Recently, Darmadi et al. demonstrated that a ternary PdAuCu alloy for plasmonic optical hydrogen detection could provide a solution to the poisoning effect.50 Initially, they explored ternary PdAuCu nanoparticle arrays created using hole-mask colloidal lithography, which consisted of quasi-random configurations of nanodisks with an average diameter of 190 nm and a height of 25 nm (Fig. 6(a)). Au primarily contributes to the closure of the hysteresis gap, whereas Cu causes an elevation in the plateau region to higher hydrogen partial pressures. In the context of hydrogen sensors, the optimal ternary alloy composition is a balance between eliminating the hysteresis gap and maximizing the magnitude of Δλmax per hydrogen partial pressure change. Criterion (i) requires alloys with at least 25% Au, whereas criterion (ii) limits the Au and Cu concentrations, given that increasing them reduces the total sensor sensitivity due to the reduced peak per hydrogen partial pressure change. As a result of these selection criteria, the Pd65Au25Cu10 ternary alloy system was identified as the best compromise, and hence the champion system. It is worth noting that the tiny gap between the absorption and desorption branches seen in Fig. 6(b) for the Pd65Au25Cu10 alloy is a measurement artifact rather than an intrinsic hydride hysteresis gap. The absence of the α–β phase transition along the curve suggests hysteresis-free hydride production.
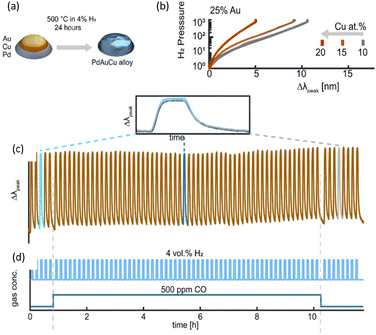 | ||
| Fig. 6 (a) Schematic of the fabrication of PdAuCu alloy NPs. (b) Pressure-Δλpeak for PdAuCu ternary alloys with 25% Au concentration and 10%, 15%, 20% Cu content. (c) Temporal response of Δλpeak for the Pd65Au35Cu10 alloy system to 4 vol% H2 pulses in synthetic air at 30 °C. (d) Response with and without the 500 ppm CO background. Reproduced with permission from ref. 50 Copyright©2021, the American Chemical Society. | ||
Darmadi's group investigated the resistance of Pd65Au25Cu10 to poisoning, as shown in Fig. 6(c). They exposed it to over 50 pulses of 4% H2 for 5 min each, while maintaining a steady background of 500 ppm CO, which is widely known to interfere with the reaction with hydrogen, in synthetic air carrier gas at a temperature of 30 °C.68 This experiment lasted for 12 h, as shown in Fig. 6(d). The LSPR sensor exhibited remarkable stability and resistance to deactivation, as evidenced by its consistent response and recovery times throughout the experiment. Furthermore, the overall morphology and configuration of the nanodiscs on the surface remained unaltered even after the extended duration of the test.
Bimetallic NP-based LSPR sensors are a sophisticated type of sensing platform that exhibit the advantage of the synergistic features of two distinct metals, typically Au and Ag or Au and Cu. This metal combination allows greater tuning and control of the plasmonic sensing properties. Bimetallic NPs have distinct optical characteristics, which provide additional information for sensing applications. The different electrical and catalytic properties of each metal in the bimetallic framework help to improve the sensitivity and selectivity when detecting target analytes. These sensors have found applications in a variety of sectors, including environmental monitoring and biomedical diagnostics, where their customized composition enables their fine tuning for specific detection requirements. The use of bimetallic NPs in LSPR sensors demonstrates a diverse and advanced strategy that improves the capabilities of plasmonic sensing technologies for precise and reliable detection.
3.3 MOM-based LSPR sensors
MOMs have garnered attention for their unique properties in the context of LSPR sensors. MOMs are hybrid materials comprised of metal nodes interconnected by organic ligands, offering a diverse and tunable platform for LSPR applications. By incorporating various ligands into these structures, researchers can precisely control the properties of the resulting MOMs, influencing their electronic and optical characteristics. The surface functionalization of metal NPs provides selective binding sites and prevents the dissociation of metal NPs, leading to reversible measurements. This versatility allows the LSPR response to be tailored to specific applications, enhancing the sensitivity and selectivity of the sensor. The integration of MOMs in LSPR sensors with various ligands opens up possibilities for a wide range of functionalities, making them promising candidates for advanced sensing technologies in fields such as chemical analysis, environmental monitoring, and biomedical diagnostics.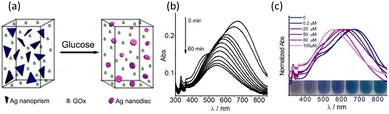 | ||
| Fig. 7 (a) Schematic of the Ag–GOx composite for glucose sensing. (b) Time-dependent LSRP absorption spectra of the Ag NPR upon incubation with 100 μM of glucose (0 to 60 min). (c) Normalized LSRP absorption spectra of Ag–GOx at various concentrations of glucose (0 to 100 μM). Reproduced with permission from ref. 55 Copyright©2013, the American Chemical Society. | ||
The time-dependent LSPR bands of the homogeneous system that was exposed to a concentration of 0.1 mM glucose during incubation are shown in Fig. 7(b). The in-plane dipole band shift was observed to rapidly diminish within the first 10 min (90 nm), followed by a gradual decline until it reached a saturation value after around 30 min. The photograph of the LSPR sensor showed a steady blue shift in the in-plane dipole band and a clear color change in the Ag nanoplates (blue to purple to mauve) with an increase in glucose content, as shown in Fig. 7(c). This change in color is due to a change in the shape of Ag through the reaction. Due to its excellent sensitivity, only 10–20 μL of serum was sufficient for a single measurement. The proposed sensing device was extremely convenient, where the entire process, from Ag nanoprism creation to test completion, took less than an hour.
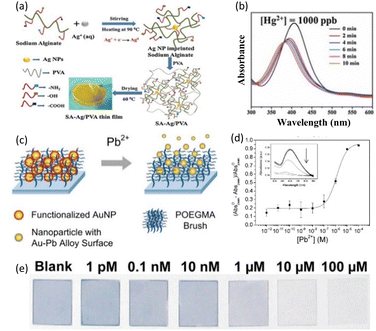 | ||
| Fig. 8 (a) Schematic of the SA–Ag composite thin film for Hg2+ sensing. (b) LSPR peak of the SA–Ag composite in an aqueous solution of 1000 ppb of Hg2+. Reproduced with permission from ref. 58 Copyright©2017, Elsevier B. V. (c) Pb2+ sensing mechanism of functionalized AuNPs. (d) Responses and (e) photograph to various concentrations of Pb2+ ranging from 1 pM to 100 μM. Reproduced with permission from ref. 59 Copyright©2013, the American Chemical Society. | ||
The functional groups containing oxygen in PVA have a binding affinity for Hg and Hg2+, allowing them to diffuse into the nanocomposite film. Because the reduction potential of Hg2+/Hg is higher than that of Ag+/Ag, Ag is oxidized to Ag+ and Hg2+ is reduced to Hg, forming an Ag/Hg amalgam and depositing Hg within the SA–Ag/PVA film simultaneously. This influenced the position and intensity of the LSPR peak for AgNPs within a few minutes. Fig. 8(b) exhibits the UV-vis spectra of the SA–Ag/PVA nanocomposite thin films immersed for 10 min in an aqueous solution with 1000 ppb of Hg2+ ions. Even after 2 min, the inclusion of Hg2+ caused a blue shift in the LSPR peak and decreased its intensity rapidly. The researchers also detected Hg2+ concentrations ranging from 2 to 1200 ppb and showed the good linearity of the absorption intensity, where the correlation coefficient was larger than 0.99. Furthermore, this sensor could detect Hg2+ concentrations ranging from 2 to 1200 ppb. The SA–Ag/PVA nanocomposite thin film sensor had computed LOD and LOQ values of 0.9 ppb and 3.07 ppb for Hg2+, respectively.
An AuNP-poly(oligo-(ethylene glycol)methacrylate) (POEGMA) system was used to detect lead, which is classified as a major environmental pollutant due to its high toxicity.59 Fig. 8(c) depicts the resultant sensing platform, which was developed using a detection chemical based on AuNP leaching. Firstly, AuNPs immobilized in POEGMA were functionalized with thiosulfate (by thiosulfate adsorption on AuNP surface). The thiosulfate-functionalized AuNPs dissolved in the presence of 2-mercaptoethanol (2ME) and Pb2+ ions, forming an Au–Pb alloy on the AuNP surface and soluble Au+–2ME complexes. The leached AuNPs with Au–Pb alloy on their surface no longer strongly interacted with the brush EG chains, allowing them to be released into solution. The AuNPs released from the POEGMA matrix reduced the UV-vis absorbance and color fading in the AuNP-POEGMA coated glass cover slip. This detection technique was chosen primarily because similar chemistry has been shown to detect lead with good sensitivity and specificity in solution. As a result, the performance in solution would be a useful baseline for comparison with solid-phase systems. Furthermore, unlike in-solution detection, which involves mixing thiosulfate and 2ME with the target and AuNPs in a single step, we were able to isolate the thiosulfate adsorption step here. This shows that preloaded AuNPs can be post-functionalized within the polymer brush using alternative detection techniques of interest. The detection capability of AuNPs in POEGMA was studied by immersing the substrates in water containing varying concentrations of Pb2+ from 1.00 pM to 100 μM in the presence of 2ME and calculating the reduction in absorbance, as shown in Fig. 8(d). Fig. 8(e) shows images of the AuNP-POEGMA samples after the detection of Pb2+. The POEGMA-coated samples were easily identifiable by the naked eyes because of their high AuNP loading.
4. Real-time monitoring properties of SPR sensors
SPR sensors exhibit several advantages for the real-time detection of chemicals, distinguishing them from other detection technologies. A variety of analytes on the surface of metals can be sensed through adsorption on the sensing materials, allowing the detection of even minute levels in both liquid and gas. Also, SPR sensors are suitable for real-time measurement, given that they detect the interaction with chemicals in a fluid in motion. This simplifies experimental procedures, reduces potential interferences, and enables the direct and real-time observation of molecular binding events. SPR sensors excel in providing real-time kinetic data, offering insights into the association and dissociation rates of chemicals, which is crucial for understanding the dynamics of the interactions between chemicals and sensing materials. The high sensitivity of SPR sensors allows the detection of molecular interactions at low concentrations, making them particularly valuable for applications requiring the precise and quantitative analysis of chemicals.SPR sensors have the advantage of being adaptable to a wide range of sensing materials that can selectively interact with the specific chemical being studied. SPR sensors possess high sensitivity, enabling the precise monitoring of molecule interactions even at low concentrations, down to the picomolar or femtomolar level. This adaptability applies to a range of sensing materials, allowing their modification based on the specific characteristics of the target chemical and enabling the immediate analysis of complicated materials, such as biological fluids. Additionally, SPR sensors are suitable for studying complex chemicals, offering a real-time approach to analyze the binding kinetics in their native environment. These attributes make SPR sensors powerful tools in chemical sensing, providing researchers and clinicians with a valuable platform for studying molecular interactions with high sensitivity, specificity, and real-time capabilities.
Various strategies can be employed to integrate these advantages with other detection techniques, depending on the type of sensing materials. Table 2 categorizes previously reported SPR sensors based on the types of sensing materials (2D materials,69–78 metal oxides,79–88 and organic composites.89–98) and analytes, and summarizes their performance.
| Material type | Target type | Sensing materialsa | Analyte | Detection range | Limit of Detection | Sensitivity | Ref. |
|---|---|---|---|---|---|---|---|
| 2D materials | Bio | ADP3NSs | Amyloid-beta 1–42 | 1–104 pM | 1 pM | 69 | |
| AuNPs/GeP5NSs | SARS-CoV-2 RNA | 10−2–104 fM | 10 aM | 146° RIU−1 | 70 | ||
| ssDNA/Cu-TCPP | Dopamine | 5–5 × 105 pM | 0.15 pM | 2820.83 nm RIU−1 | 71 | ||
| MoS2NSs | Ferritin | 50–400 ng mL−1 | Ferritin: 12 ng mL−1 | Ferritin: 0.024 nm (ng mL−1)−1 | 72 | ||
| Ti3C2-MXene/AuNPs | Carcinoembryonic antigen | 2 × 10−4–2 × 104 pM | 0.07 fM | 73 | |||
| Peptide/Cu-TCPP NSs | Programmed death ligand-1 exosome | 104–5 × 106 particles per mL | 16.7 particles per mL | 137.67° RIU−1 | 74 | ||
| Graphene/Au | Glucose | 0–300 mg dL−1 | 3113 nm RIU−1 | 75 | |||
| AuNRs-ssDNA/SbNSs | microRNA-21 | 10−2–104 fM | 10 aM | 171° RIU−1 | 76 | ||
| Non-bio | Graphene | 2,4 Dinitrophenol | 100–500 ppb | 3 ppb | 77 | ||
| Ti2C-MXene NSs | Pb2+ | 1–20 μg L−1 | 79.2 ng L−1 | 3.788 nm (μg L−1)−1 | 78 | ||
| Metal oxides | Bio | ZnO NPs/MoSe2 NSs | Glucose | 0–1.2 mg mL−1 | 4.16 μg mL−1 | 72.17 nm (mg mL−1)−1 | 79 |
| Ta2O5NFs | Xanthine | 0–3 μM | 0.0127 μM | 26.204 nm μM−1 | 80 | ||
| Fe3O4 | Glucose | 0.1–10 mM | 19.95 × 10−2 mM | 81 | |||
| ZnO nanowires/CeO2 | Dopamine | 10−3–10 pM | 95° RIU−1 | 82 | |||
| ZnO | Neisseria meningitidis DNA | 10–180 ng μL−1 | 5 ng μL−1 | 0.03° (ng μL−1)−1 | 83 | ||
| Ta2O5 NPs embedded in rGO | Fenitrothion | 0.25–4 μM | 38.2 nM | 24.02 nm μM−1 | 84 | ||
| Non-bio | WO3 | NO2 | 0.5–50 ppm | 85 | |||
| NiO-doped ITO | H2S | 0.1–100 pm | 86 | ||||
| ZnO | CO | 0.5–100 ppm | <500 ppb | 0.091° ppm−1 | 87 | ||
| Fe2H2O4 | As3+ | 0.4–10 ppb | 0.6 ppb | As3+: 1.092° ppb−1 | 88 | ||
| Organic composites | Bio | AgNCs/chitosan | Mouse IgG | 0.6–40 μg mL−1 | 89 | ||
| MIP film | Histamine | 25–103 μg L−1 | 25 μg L−1 | 90 | |||
| Triangular AgNPs/chitosan | Bovine IgG | 7.5 × 10−2–40 μg mL−1 | 0.075 μg mL−1 | 91 | |||
| Graphene/MIP film | L-Tryptophan | 0.15–2.5 mM | 0.105 mM | 92 | |||
| AgNPs-CS-PSS-CS/GO | Beta-amyloid 1-42 | 2–4 × 108 fM | 1.21 fg mL−1 | 93 | |||
| Lipase/polyacrylamide gel | Triacylglyceride | 0.5–7 mM | 3.17 nm mM−1 | 94 | |||
| Non-bio | MIP film | Profenofos | 10−4–10−1 μg L−1 | 2.5 × 10-6 μg L−1 | 12.7 nm (μg L−1)−1 | 95 | |
| GO/MIP film | Benzylpenicillin | 1–100 ppb | 0.021 ppb | 96 | |||
| MIP film | Triclosan | 0.05–1 ng mL−1 | 0.017 ng mL−1 | 97 | |||
| Pyrrole/chitosan/ITO | Cd2+ | 0–200 μg L−1 | 0.129 nM | 1.306 nm (μg L−1)−1 | 98 | ||
4.1 2D material-based SPR sensors
2D materials are substances with layered structures such as graphene, transition metal dichalcogenides (TMDs), and MXenes. 2D materials have been gradually applied in chemical sensors due to their unique properties, such as large specific surface areas, optical properties, and photoelectric properties. The electron transport from 2D materials to Au films is facilitated by the lower work function of 2D materials in comparison to Au films when applied in SPR sensors.99 The enhanced electron density on the Au film surface amplifies the plasmonic electric wave and the evanescent wave penetration depth. Because of its increased penetration depth, the SPR sensor responded more sensitively to changes in the refractive index of the sensing medium, leading to an increase in sensitivity. The large specific surface areas of 2D materials provide many reactive sites for interacting directly with target molecules. Furthermore, for the sensitive and selective detection of target molecules, a variety of receptors, such as antibodies, aptamers, and enzymes, is utilized. 2D materials can serve as immobilization layers for these receptors. These ultra-thin and uniform 2D materials exhibit a low damping effect given that they do not interfere with the plasmonic wave of metal films.100 Ultimately, introducing 2D materials in SPR sensors enables selective and sensitive detection, resulting in fast response times, low cost, and simplicity to use, which are not shown by traditional techniques.Owing to the rapid aging of the global population, the problems associated with aging are attracting increasing attention. Alzheimer's disease (AD) is one of the most common neurological disorders. To achieve its early diagnosis, the need for sensors that can distinguish between AD and amnestic mild cognitive impairment (aMCI) is rising. To diagnose AD, research is concentrating on sensors that can detect biomarkers, especially amyloid-beta 1–42 (Aβ42). Gao et al. applied 2D biocomponents that can selectively bind to Aβ42 to SPR sensors.69 They detected Aβ42 sensitively in the serum and plasma to distinguish between AD and aMCI through Alzheimer's disease peptoid 3 nanosheet (ADP3NS)-based SPR sensors. The amphiphilic peptoids have self-assembly characteristics through hydrophobic and electrostatic properties, making it easy to synthesize ordered ADP3NSs and form Aβ42-binding loops on the surface. The surface-exposed loops on ADP3NSs function like the loops in an antibody that binds to a target antigen. To enhance the adsorption of Aβ42 and restrict the nonspecific binding, Gao's group deposited uniformly distributed ADP3NSs on the surface of an Au layer and demonstrated the high sensitivity of the ADP3NS-based SPR sensor to Aβ42 at the nM level, changing the refractive index near the sensing materials to further induce SPR signals (Fig. 9(a)). Fig. 9(b) shows the real-time SPR response to Aβ42 in the concentration range of 0.14 to 2.20 nM. Significant signals were observed despite the extremely low concentration of Aβ42. After about 300–400 s, the specific binding between the injected Aβ42 and ADP3NSs reached dynamic equilibrium, where the response remained constant. Even at low concentrations of Aβ42, it could be detected through specific binding with ADP3 and Aβ42 in a rapid response time.
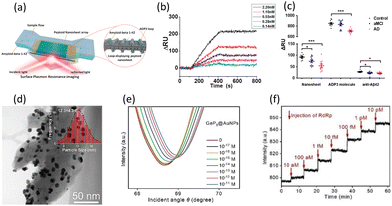 | ||
| Fig. 9 (a) Schematic of the detection of Aβ42 by ADP3NSs. (b) Real-time SPR responses for Aβ42 at different concentrations ranging from 0.14 to 2.2 nM. (c) SPR responses for control individuals (n = 10), aMCI patients (n = 10), and AD patients (n = 10) using ADP3NSs, ADP3 molecules, and anti-Aβ42. Reproduced with permission from ref. 69 Copyright©2020, the American Chemical Society. (d) TEM image of AuNPs-decorated GeP5NSs (inset: the size distribution of AuNPs). (e) SPR spectra and (f) real-time SPR responses for RdRp at different concentrations ranging from 10−17 to 10−11 M. Reproduced with permission from ref. 70 Copyright©2023, Elsevier B. V. | ||
As shown in Fig. 9(c), Aβ42 was detected in serum and plasma extracted from the blood of normal individuals (n = 10) and real patients with AD (n = 10) and aMCI (n = 10) based on different sensing materials, including ADP3NSs, ADP3 molecules, and anti-Aβ42. In the case of these three sensing materials, it was observed that the MCI patients had lower signals than the normal individuals, whereas the AD patients had much lower signals, evaluating the ability of these sensors to distinguish between AD and aMCI and provide an accurate diagnosis. However, the difference in the signals among the groups was greatest in ADP3NSs, indicating that 2D ADP3NSs are most effective in distinguishing aMCI and AD. This research also demonstrated the outstanding sensitivity of ADP3NSs by showing a linear relationship between the SPR signals and Aβ42 concentrations ranging from 1 pM to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pM. As a result, the authors demonstrated a highly sensitive and real-time SPR sensor capable of recognizing Aβ42 at incredibly low Aβ42 concentrations. The 2D ADP3NS SPR sensor can measure the concentration of Aβ42 in the serum and plasma of real patients, and sensitively and accurately diagnose normal individuals, aMCI patients, and AD patients.
000 pM. As a result, the authors demonstrated a highly sensitive and real-time SPR sensor capable of recognizing Aβ42 at incredibly low Aβ42 concentrations. The 2D ADP3NS SPR sensor can measure the concentration of Aβ42 in the serum and plasma of real patients, and sensitively and accurately diagnose normal individuals, aMCI patients, and AD patients.
Covid-19, which emerged in 2019, rapidly spread worldwide, causing epidemic respiratory diseases and resulting in the immense loss of life. Due to the highly contagious nature of this virus, prompting the World Health Organization (WHO) to recommend self-isolation for infected individuals,101 there was a crucial need for accurate and rapid diagnostics. Chang et al. introduced metallic 2D GeP5NSs, which exhibited excellent electron transport properties upon contact with Au, decorated with AuNPs on an Au layer to detect SARS-CoV-2 RNA (RdRp).70 In addition to enhancing the plasmonic wave by transporting electrons to the Au film, GeP5NSs significantly improved the sensitivity of the sensor by strengthening the coupling between the SPR of the Au film and the LSPR of AuNPs. Also, AuNPs immobilized complementary RdRp (cRdRp), which hybridized with RdRp. When RdRp is injected, the cRdRp on AuNPs captures RdRp, causing fluctuations in the refractive index around the sensing materials. Then, GeP5 and AuNPs amplify the SPR signals, leading to highly sensitive detection. Chang's group successfully assembled GeP5NSs on an Au film via the layer-by-layer technique and decorated the NSs with AuNPs facilely to assist the sensitivity enhancement. As shown in Fig. 9(d), the TEM image confirmed the presence of uniformly distributed AuNPs on GeP5NSs and the inset presenting the size distribution of AuNPs shows that the average size of AuNPs is 12.3 ± 4.3 nm.
The SPR spectra for various concentrations of RdRp ranging from 10−17 to 10−11 M are shown in Fig. 9(e). In the SPR spectra, the reflectance curves shifted upward and to the right as the concentration of RdRp increased. The significant increase in resonance angle and minimum reflectance was caused by the hybridization of the injected RdRp with cRdRp. The ability to exhibit linearity across an extensive range and produce a meaningful response even at a very low concentration of 1 pM showed the high sensitivity of this sensor for detecting RdRp. Fig. 9(f) demonstrates that the proposed AuNP/GeP5NS SPR sensor can be applied for the real-time monitoring for RdRp. This sensor showed a linear and real-time increase in response as the concentration of RdRp continuously increased. Moreover, the sensor reached dynamic equilibrium within several minutes for each detection, and the time taken to reach equilibrium was consistent across all concentrations. Thus, this ultrasensitive, real-time, and rapid SPR sensor, which showed a noticeable performance, is applicable for detecting RdRp and diagnosing Covid-19.
2D materials can enhance the electron density on plasmonic metals and further improve the plasmonic electric field, leading to an enhancement in sensitivity. Also, the large surface area of 2D materials provides abundant active binding sites based on the immobilization of biocomponents. As a result, the sensitivity and selectivity of SPR sensors are dramatically enhanced. 2D materials offer the potential to be applied in practical applications such as pharmaceutical research and disease diagnosis using SPR biosensors.
Isopropyl alcohol (IPA) is a solvent used for cleaning in semiconductor processing technology. Thus, maintaining a high purity of IPA is critical for increasing the overall yield of semiconductor manufacturing processes.102 Cho et al. reported the fabrication of a graphene-based SPR sensor that can detect 2,4-dinitrophenol (2,4-DNP), one of the aromatic compounds that may dissolve in IPA.77 Fig. 10(a) illustrates the SPR measurement system, where 2,4-DNP interacts with the recognition layer, graphene. SPR signals are produced by the adsorption/desorption of 2,4-DNP on graphene through pi–pi stacking, which induces fluctuations in the local refractive index on the Au film. The authors demonstrated that 2,4-DNP, containing electron-withdrawing NO2 groups, adsorbs on graphene by pi–pi stacking, causing p-doping in graphene. The shift in the transfer curve of solution-gated field-effect transistors and the peak shift in Raman spectra were used as evidence of this p-doping effect.103,104 The real-time detection of 2,4-DNP was shown in the concentration range of 100 to 500 ppb (Fig. 10(b)). Even at very low concentrations of several hundred ppb, the SPR sensor exhibited substantial responses and outstanding quick response time and recovery time of 31 and 38 s, respectively. Other aromatic and non-aromatic compounds were detected by this SPR sensor at 500 ppb. The aromatic compound phenanthrene, which can strongly interact with graphene through pi–pi stacking, showed a high response, while two non-aromatic compounds, 2-pentanone and n-propanol, exhibited no significant responses (Fig. 10(c)). The binding energies calculated by density functional theory confirmed a similar trend to these results.
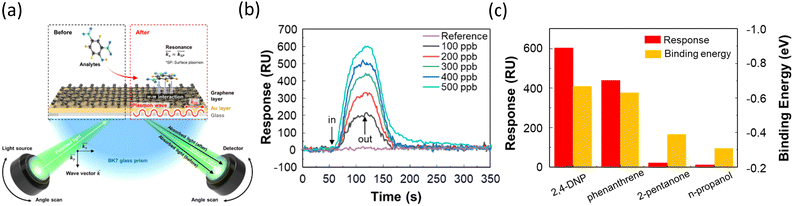 | ||
| Fig. 10 (a) Schematic of the detection of 2,4DNP on a graphene-based SPR sensor. (b) Real-time SPR responses for 2,4 DNP at different concentrations ranging from 100 to 500 ppb. (c) Responses and calculated binding energies for various impurities. Reproduced with permission from ref. 77 Copyright©2024, John Wiley & Sons. | ||
Owing to their substantial surface area and remarkable electrical and optical properties, 2D materials exhibit strong and sensitive binding with chemical substances.105 In particular, graphene and its derivatives, composed of carbon rings in an extensively large surface area, can effectively interact with chemical molecules containing aromatic rings, maximizing the pi–pi stacking. This enables graphene and its derivatives to efficiently adsorb various molecules and produce SPR signals. The ability to enhance the plasmonic electric wave and maximize the binding with chemical molecules enables the use of 2D materials for the real-time detection of chemical molecules via SPR sensors and the observation of the kinetic binding between 2D materials and chemical molecules. The combination of 2D materials and SPR holds broad applications in environmental monitoring, food monitoring, and chemical analysis.
4.2 Metal oxide-based SPR sensors
In recent years, metal oxides have attracted significant interest in the field of bio-chemical sensors, owing to their biocompatibility, low-cost, and facile synthesis. Their versatility enables the development of composites containing 2D materials and organic materials, as well as the fabrication of diverse nanostructures. Metal oxides, when incorporated into SPR sensors, exhibit multifunctionality, including enhancing sensitivity, immobilizing biocomponents, and directly binding with target molecules. Metal oxides applied in guided wave surface plasmon resonance can strengthen the plasmonic wave and improve the sensitivity of the sensor when deposited very thinly on plasmonic metals due to their high refractive index.106 Additionally, metal oxides can directly interact with target molecules through intrinsic oxygen vacancies or electron transfer. Also, they can immobilize biocomponents that selectively bind to target molecules. The binding of target molecules to metal oxides or biocomponents on metal oxides induces changes in the refractive index around the sensing materials, resulting in the generation of SPR signals.Among the metabolites, xanthine is one of the intermediate compounds occurring during the metabolism of purine nucleotides. Given that xanthine is converted into uric acid by the xanthine oxidase (XO) enzyme, the concentration of xanthine serves as an indicator for pathological diseases such as gout, hyperuricemia, cerebral ischemia, perinatal, and tumor hyperthermia.109 Kant et al. reported the fabrication of a sensitive and selective SPR sensor for xanthine by depositing Ta2O5 nanofibers (Ta2O5NFs) and immobilizing XO enzyme on a Ag layer (Fig. 11(a)).80 They enhanced the plasmonic wave and further improved the sensing performance by depositing a thin film of high refractive index Ta2O5 NFs on an Ag film. Additionally, they immobilized XO enzymes on the surface of the sensing material, selectively decomposing only xanthine and inducing changes in the refractive index around the sensing material. The combination of these two effects resulted in the development of a highly sensitive and selective sensor for xanthine.
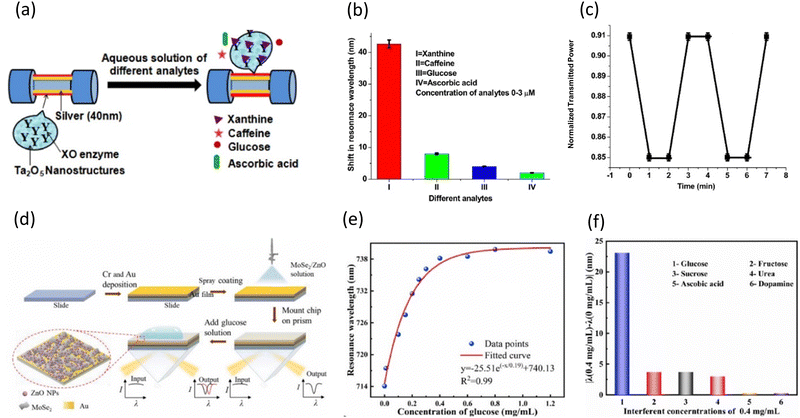 | ||
| Fig. 11 (a) Schematic of the detection of xanthine on an XO enzyme-entrapped Ta2O5 NF-based SPR sensor. (b) Resonance wavelength shifts compared with interferents. (c) Real-time SPR responses for 3 μM xanthine. Reproduced with permission from ref. 80 Copyright©2017, Elsevier B.V. (d) Schematic of the fabrication of MoSe2/ZnO-based SPR chips. (e) Resonance wavelength shifts for glucose at different concentrations ranging from 0 to 1.2 mg mL−1. (f) Selectivity analysis with 0.4 mg mL−1 interferents. Reproduced with permission from ref. 79 Copyright©2023, Elsevier B.V. | ||
As shown in Fig. 11(b), to validate the selectivity of the XO enzyme, three interferents were compared with respect to shifts in the resonance wavelength. Interferents such as caffeine, glucose, and ascorbic acid showed negligible shifts of less than 10 nm, whereas xanthine showed a significant shift of over 40 nm. The specific recognition and decomposition of enzymes are suitable for application in SPR sensors for sensitive and selective detection. Fig. 11(c) illustrates the real-time SPR response of the XO/Ta2O5-based sensor. The change in the transmission intensity (λ = 617.5 nm) before and after the injection of 3 μM xanthine clearly shows that the response decreased and saturated within one minute after the injection. Furthermore, the response recovered to its original value and remained steady one minute after xanthine was removed. This indicated that both the response time and recovery time are less than one minute, implying that the proposed SPR sensor is very fast and can detect in real time.
Kant et al. improved the sensing performance by depositing a high refractive index metal oxide, enhancing the plasmonic electric wave. They also immobilized an enzyme on a metal oxide. As a result, the sensitivity and selectivity of the sensor were remarkable, and its rapid response time and recovery time make it highly suitable for practical application in disease diagnosis.
One of the most significant indicators of health management is glucose, which is especially relevant for diabetes. The real-time measurement of blood glucose levels is necessary for maintaining the blood glucose levels within the normal range and diagnosing diabetes.110 Besides diabetes care, medical and biological research,111 food monitoring,112 and systematic training for athletes113 are all greatly correlated with the prompt and accurate detection of glucose. Chen et al. introduced a ZnO NP/MoSe2 NS composite film in an SPR sensor for the detection of glucose without utilizing enzymes.79 Fig. 11(d) illustrates the simple procedure for preparing the non-enzymatic ZnONP/MoSe2 NS-based sensor. The sensor chip was fabricated by spray coating a ZnO NP/MoSe2 NSs composite solution onto an Au film. The ZnO NPs in the sensing layer can bind to glucose through chemisorption on their surface, inducing fluctuations in the refractive index. Furthermore, ZnONPs can easily form composites with MoSe2 NSs, which have large surface areas and outstanding electrical properties. This can be achieved by simply dissolving them in an organic solvent and heating. The composite film of ZnO NPs and MoSe2 NSs recognized and bound to glucose sensitively and selectively without enzymes and amplified the signals.
Employing the non-enzymatic ZnO NP/MoSe2 NS-based sensor, the authors detected glucose in the concentration range of 0 to 1.2 mg mL−1 (Fig. 11(e)). Under the optimized conditions, this sensor exhibited a total wavelength shift of 25.75 nm for glucose in the range of 0 to 1.2 mg mL−1. Furthermore, this optimized sensor demonstrated linearity within the range of 0 to 0.3 mg mL−1, sensitivity of 72.17 nm (mg mL−1−1), and calculated limit of detection (LOD) of 0.023 mM. This highly sensitive performance is comparable to that of previously reported enzyme-based sensors. Because of their similar structures and properties to glucose, fructose, sucrose, urea, ascorbic acid, and dopamine are significant interferents in actual glucose detection applications. Fig. 11(f) illustrates the resonance wavelength shifts for the interferents and glucose at 0.4 mg mL−1. The resonance wavelength shifts for fructose, sucrose, and urea were 3.75 nm, 3.8 nm, and 2.41 nm, respectively. Also, the shifts for ascorbic acid and dopamine were less than 1 nm. All these shifts were negligible in comparison to the large shift generated by glucose, indicating the excellent selectivity of the non-enzymatic ZnO NPs/MoSe2 NS-based sensor.
A highly sensitive and selective ZnO/MoSe2-based glucose sensor without enzymes was reported by Chen's group. ZnO NPs bind to glucose, and MoSe2 NSs enhanced the sensitivity of the sensor. With an LOD of 0.023 mM and sensitivity of 72.17 nm (mg mL−1)−1 in the linear range of 0 to 0.3 mg mL−1, the sensor performed excellently not only in detecting glucose but also exhibited good selectivity against five interferents. Owing to its performance, this sensor has potential to be applied as a biosensor in medical diagnostics, bio-medical research, and food monitoring.
Depositing a thin layer of high refractive index metal oxide onto a plasmonic metal can enhance the plasmonic wave, thereby improving the sensing performance. Additionally, the characteristics of metal oxides, such as their ability to form composites easily with other substances, together with their intrinsic features such as oxygen vacancies and electron transfer for direct interaction with target substances, contribute to enhancing the binding with target molecules. Owing to these effects, metal oxide-based SPR sensors can rapidly, sensitively, and selectively capture target molecules. These sensors are expected to find widespread use in fields demanding highly sensitive, label-free, and real-time sensing capabilities, such as healthcare devices and medical research.
Among the toxic gases, NO2 is a serious air pollution gas that can cause significant health issues in humans.117 Thus, in the last few decades, various sensors have been developed to detect NO2, including colorimetric sensors,118 chemoresistive sensors,119 and surface acoustic sensors.120 However, all these sensors have certain shortcomings, such as low sensitivity, excessive power consumption, and sluggish response time. Alternatively, because of the high sensitivity, low power consumption and fast response time of SPR sensors incorporating metal oxides, they have become a promising technology for NO2 detection. Most importantly, metal oxide-based SPR sensors are particularly suitable for applications such as industrial safety monitoring and environmental monitoring, where the real-time detection of hazardous gases such as NO2 at room temperature is critical. This has led to ongoing research on SPR sensors for accurate and efficient NO2 monitoring in various environments. Paliwal et al. proposed an SPR sensor with a WO3 thin film as the sensing material for NO2 detection at room temperature.85 Considering the properties of SPR sensors where gas detection occurs on the sensor surface, a WO3 film was deposited onto an Au layer through RF-magnetron sputtering at different growth pressures to control the grain boundaries and surface roughness. Fig. 12(a) illustrates the SPR gas sensing measurement. They suggested the mechanism for NO2 detection based on the change in the refractive index of WO3 due to the adsorption of NO2. The mechanism is related to the refractive index changes of WO3 based on the adsorption of NO2. As an oxidizing gas, NO2 takes electrons from WO3, forming either NO2−, NO, or O−, and then NO2− and O− adsorb on the surface of WO3. The refractive index of WO3 changes as a result of electron loss and the adsorption of NO2− and O−, leading to the generation of an SPR signal.
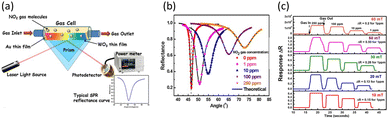 | ||
| Fig. 12 (a) Schematic of the SPR gas sensing measurement. (b) SPR spectra for NO2 gas at different concentrations ranging from 0 to 250 ppm. (c) Real-time SPR responses for sensors with WO3 thin films grown at different pressures, with repetitive exposure to NO2 gas at different concentrations ranging from 1 to 250 ppm. Reproduced with permission from ref. 85 Copyright©2015, Elsevier B.V. | ||
Fig. 12(b) shows the SPR spectra at different concentrations of NO2 from 0 to 250 ppm for the sensor fabricated under the optimized condition. As the concentration of NO2 increased, it was found that the resonance angle and the minimum reflectance increased, and the curves became broader. The huge shift in resonance angle with concentration indicates that the above-mentioned mechanism is valid. Furthermore, the significant changes in resonance angle and minimum reflectance demonstrate that this sensor is highly sensitive and effective for NO2 detection. The pink curve in Fig. 12(c) represents the real-time SPR responses based on the reflectance changes of the sensor fabricated under the optimized condition. The responses saturated in less than 2 s when NO2 was injected for every measured concentration. As air was reinjected for recovery, the responses also returned to their original values within 2 s. The rapid response and recovery times achieved when detecting at room temperature were notably fast and comparable to or even faster than traditional methods measured at elevated temperatures. These advantages make the WO3-based SPR sensor highly suitable for real world application, where the rapid detection of hazardous gases is essential. Furthermore, after the repetitive detection of NO2, there was no discernible change in the baseline. All these results confirm the high sensitivity of the WO3-based SPR sensor reported by Paliwald's group, together with its very fast response and recovery time, real-time detection capability, and good stability, which are attributed to the physical and chemical adsorption of NO2, withdrawing electrons from WO3.
Metal oxides are inexpensive, easy to form nanostructures, and can synergize with other materials, making them suitable for the detection of chemical substances such as gas molecules in SPR sensors. Metal oxides can adsorb chemical molecules physically and chemically either through their intrinsic oxygen vacancies or direct electron transfer. Compared to previously reported sensors, metal oxide-based SPR sensors exhibit significantly faster response and recovery times, together with higher sensitivity. Furthermore, metal oxide-based SPR sensors have exceptional properties for repeatability and long-term stability due to the inherent stability of metal oxides. As a result, metal oxide-based SPR sensors can be widely applied in the fields of industrial safety monitoring, environmental monitoring, and water quality management.
4.3 Organic composite-based SPR sensors
Organic composites refer to materials formed by the combination of organic molecules, which are composed of carbon skeletons and other elements such as H, O, and N, with other substances such as other organic molecules, metals, metal oxides, and nitrides. Organic composites are employed in constructing sensing materials to uniformly distribute metal NPs and reactive materials that interact with target molecules to enhance the plasmonic electric field and increase the number of active sites. Additionally, organic composites are also utilized in the formation of molecular imprinting polymer (MIP) films, which provide specific binding sites for target molecules. The MIP film method is a technique for forming sensing materials by mixing and polymerizing target molecules with other organic molecules. After depositing a uniform polymer film, the target molecules are eliminated, creating imprinted sites on the surface of the sensing material. The created vacant sites can only adsorb the target molecules highly selectively and sensitively, leading to a fluctuation in the refractive index around the sensing material and generation of SPR signals. Due to these capabilities, researchers have employed many organic composites for composing sensing materials in the fields of environmental monitoring, biomedical research, and biosensors.Immunoglobulin G (IgG) is one of the antibodies found in the body fluids of humans and other mammals. IgG binds to specific viruses or bacteria to neutralize their toxicity, and it modulates various immune responses within the immune system.121 Also, it is essential for regulating immunological reactions. Prior to human trials, studies with mice are frequently carried out to evaluate stability and safety.80 As a result, there is a growing interest in the sensitive detection of mouse IgG with the increasing demand for mouse IgG. Zhang et al. reported the fabrication of an SPR sensor using Ag nanocube (AgNCs)/chitosan composites as sensing materials (Fig. 13(a)).89 They used organic molecules to produce composites of AgNCs, glutaraldehyde, and chitosan, which were spin-coated onto an Au layer. This composite involved the binding of 3-mercaptopropinic acid (MPA), an organic molecule with thiol functional groups, to AgNCs, forming Ag–S bonds. This process successfully prevented the oxidation of AgNCs. Additionally, the researchers aimed to enhance the sensitivity by spreading AgNCs very uniformly and thinly onto an Au layer, promoting the interaction between the plasmonic electric fields of AgNCs and Au film. Then, they immobilized antibodies for mouse IgG on the surface of the composite film by flowing a solution containing the antibodies. Through Schiff alkali reaction with the aldehyde groups of glutaraldehyde, the injected antibodies could be bound. Subsequently, when flowing mouse IgG solution, the immobilized antibodies strongly bind to mouse IgG through antigen–antibody interactions, causing a shift in the resonance wavelength.
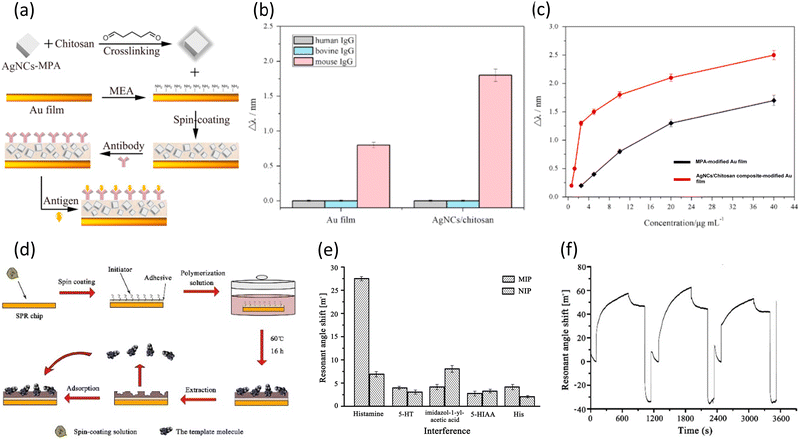 | ||
| Fig. 13 (a) Schematic for the fabrication of TSNP/chitosan composite-based SPR sensor. (b) Resonance wavelength shifts for interferents on different sensing layers. (c) Resonance wavelength shifts for mouse IgG at different concentrations ranging from 0.6 to 40 μg mL−1. Reproduced with permission from ref. 89 Copyright©2015, Elsevier B.V. (d) Schematic of the fabrication of a histamine-imprinted polymer-based SPR sensor. (e) Resonance angle shifts compared with interferents on MIP and NIP. (f) Real-time and repetitive SPR responses for 500 μg L−1 histamine. Reproduced with permission from ref. 90 Copyright© 015, Elsevier B.V. | ||
Fig. 13(b) compares the resonance wavelength shifts for three different IgGs on both the MPA-modified Au film SPR sensor and the AgNC/chitosan-based SPR sensor after immobilizing the same antibodies on their surfaces. Both sensors showed extremely specific binding to mouse IgG, demonstrating the selectivity of the antibodies utilized in mouse IgG detection. Also, the sensitivity of both sensors to mouse IgG was twice as high in the AgNC/chitosan layer compared to the MPA-modified Au layer. This shows the effectiveness of the proposed AgNC/chitosan layer in enhancing the sensitivity of the SPR sensor, emphasizing its impact on improving the sensitivity in mouse IgG detection. Fig. 13(c) illustrates the resonance wavelength shifts for the AgNC/chitosan composite-based sensor and MPA-modified Au film sensor with respect to the mouse IgG concentration in the range of 0.6 to 40 μg mL−1. Before initiating the detection of mouse IgG, ethanolamine hydrochloride was used to ensure specific binding to the surface of the sensing materials. The AgNCs/chitosan based sensor exhibited a resonance wavelength change of 2.50 nm in the detection range, and the calculated minimum detectable concentration was four-times lower compared to the MPA-modified sensor. It has been shown that uniformly dispersing AgNCs on an Au layer via AgNC/chitosan composites improved the plasmonic electric wave of the Au layer, and consequently enhanced the SPR signals.
Histamine, an organic nitrogen compound, serves as a neurotransmitter and actively participates in immune system functions. This small molecule can be found in tissues such as white blood cells and the nervous system. Thus, the accurate detection of histamine is crucial in the fields of medicine, pharmaceuticals, and biotechnology for diagnosing neurological and immunological disorders and developing pharmaceuticals. Jiang et al. prepared an MIP film-based SPR sensor for the highly sensitive, selective, and reliable detection of histamine.90 They used the polymerization of a solution containing histamine and other organic molecules. Subsequently, histamine was removed to form empty sites on the surface (Fig. 13(d)). Histamine could selectively bind to the empty sites during the measurement. Even at a low concentration, histamine could bind to the sites, sufficiently changing the refractive index around the sensing material to generate signals.
The resonance angle shifts were measured for histamine and four interferents to assess the selectivity of the MIP film for histamine using both MIP film-based sensors and non-imprinting polymer (NIP) film-based sensors (Fig. 13(e)). The NIP film-based sensor exhibited resonance angle shifts below 10 m° for all five molecules including histamine, indicating that the NIP film did not sensitively interact with any of the molecules. Alternatively, the MIP film-based sensor showed shifts below 10 m° for four interferents, while histamine showed a significant shift of approximately 27 m°. Considering the concentration differences between the measured histamine (0.1 mg L−1) and interferents (10 mg L−1), it was confirmed that the selectivity and sensitivity of the MIP film for histamine were substantial. The real-time SPR responses for three successive 500 μg L−1 histamine detections are shown in Fig. 13(f). After each histamine detection, a regeneration process with 0.1 M HCl solution was conducted to detach histamine from the sensing material. The small differences in resonance angle shifts for each detection confirm the repeatability of this sensor. The resonance angle reached its maximum point within approximately 600 s for each detection, highlighting the real-time detection capability of the proposed sensor. The histamine MIP film-based sensor, as validated by the authors for its selectivity, sensitivity, real-time monitoring, and repeatability, demonstrated the selective binding of histamine to the imprinted sites. Thus, it is anticipated to have a significant impact on researchers and pharmaceutical companies engaged in studies related to histamine.
Various organic composites, such as well-dispersed metal NP layers and MIPs, are being utilized in SPR sensors to enhance their sensitivity and selectivity. Unlike previous technologies for detecting biomolecules, these sensors do not require labels and can be easily measured with simple equipment, eliminating the need for specialized personnel. Moreover, their rapid response time and real-time measurement capabilities make organic composite-based SPR sensors applicable in various fields, including disease diagnosis, pharmaceutical research, and medical studies.
Organophosphorus pesticides (OPPs) have been used to kill pests when growing cotton, vegetables, cereals, and other crops. However, even at very low concentrations, OPPs can cause serious neurological diseases in humans and are a major problem for environmental and aquatic organisms because of their comparatively high environmental mobility in water.122 Consequently, there is a critical need for the rapid and reliable detection of OPPs. However, previously reported methods for detecting OPPs have difficulties such as insufficient selectivity, requirement for labels, and limitation for real-time monitoring. Thus, to overcome these difficulties, research is ongoing to develop approaches for the selective, real-time and label-free detection of OPPs. Shrivastav et al. presented an SPR sensor that employed an MIP film to detect the hazardous chemical profenofos, one of the most widely used OPPs.95 The MIP film functioned by incorporating the target molecule to be detected into the sensing material using organic composites, and then removing it, consequently leaving imprinted sites on the surface of the sensing material (Fig. 14(a)). Other molecules cannot bind to the imprinted sites and only profenofos binds to the sites strongly.
 | ||
| Fig. 14 (a) Schematic of the highly selective and sensitive profenofos-imprinted sensor. (b) Resonance wavelength shifts compared with interferents. (c) Real-time SPR responses for 10−4 μg L−1 profenofos. Reproduced with permission from ref. 95 Copyright©2015, Elsevier B.V. (d) Real-time SPR responses for benzylpenicillin at different concentrations ranging from 1 to 100 ppb. (e) Repetitive SPR responses for 25 ppb benzylpenicillin. (f) Reflectance changes compared with interferents on different sensing layers. Reproduced with permission from ref. 96 Copyright©2022, Elsevier B.V. | ||
As shown in Fig. 14(b), to validate the effectiveness of the MIP film, the authors observed the resonance wavelength shifts of an Ag-based SPR sensor using the MIP film and an Ag-based SPR sensor using an NIP film in response to 0.1 μg L−1 profenofos and other interferents. The Ag/NIP sensor without imprinted sites for profenofos showed similar small shifts for profenofos and other interferents. This confirmed that the sensor lacked distinct selectivity and did not respond sensitively to profenofos in the absence of the imprinted sites. Alternatively, the profenofos-imprinted Ag/MIP sensor responded to profenofos intensively, exhibiting a notable shift. It showed a clear difference from seven interferents, indicating its good selectivity. In the Ag-based sensor with MIP film, profenofos can attach firmly and selectively, while interferents have minimal binding on the imprinted sites of the film. The real-time SPR response for 0.0001 μg L−1 profenofos is shown in Fig. 14(c). The transmitted intensity at λ = 554 nm was observed with and without profenofos, where profenofos was injected for 2 min, followed by the removal of profenofos for one minute. This process was repeated twice to validate the repeatability of the real-time sensor. Upon the removal of profenofos, there was a significant change in the transmission in about one minute. After one minute of another profenofos injection, the response remained constant and stabilized. These results demonstrated that the sensor could detect profenofos selectively and rapidly in real-time through multiple cycles. The exceptionally fast and selective real-time detection capabilities of the MIP-based SPR sensor reported by Shrivastav's group make it promising for applications in environmental monitoring.
Antibiotics are drugs that support the immune system in both humans and animals by decreasing the activity of pathogens when they are infected. The proper use and disposal of antibiotics can prevent the early invasion of pathogens, allowing the host to overcome the crisis with mild symptoms such as a cold and cough. However, the improper disposal of antibiotics can lead to their discharge into the ecosystem, producing antibiotic-resistant bacteria and causing serious problems.123 Thus, it is essential to develop sensors that can detect antibiotics that are still present in food and water in real-time. Celik et al. reported the fabrication of an SPR sensor with a sensing layer consisting of a GO-based MIP film to detect benzylpenicillin, one of the most widely used β-lactam antibiotics.96 Through the specific binding of benzylpenicillin in MIP film, the sensor can selectively detect benzylpenicillin. Additionally, GO, a 2D material with excellent electrical and optical properties, was used to amplify the signals from the binding of the target molecule on the imprinted sites. GO possesses a lower work function than Au, and the electron transfer from GO to Au improves the sensitivity of the SPR sensor.124
Fig. 14(d) shows the real-time responses of the MIP-GO-based SPR sensor to benzylpenicillin at various concentrations ranging from 1 to 100 ppb. The responses stabilized several hundred seconds after the injection of benzylpenicillin, and the sensor exhibited exceptional sensitivity even at ppb levels. Although saturation was achieved within 100 s up to 50 ppb, a concentration of 100 ppb showed a steady increase in response even after 100 s. Overall, as the concentration increased, more binding events occurred, leading to a longer time to be saturated. However, the comparable fast response time up to 50 ppb confirmed the rapid detection of benzylpenicillin in real-time. After that, the repeatability of the MIP-GO-based sensor was measured (Fig. 14(e)). Four successive detections were conducted for 25 ppb benzylpenicillin, and for each detection, 10 mM NaCl was flowed through to desorb benzylpenicillin from the imprinted site. Because there was no additional stabilizing process after each detection, the reflectance changes began with negative values after the initial detection. However, the reflectance changes remained at approximately 6% for the four detections. These constant responses demonstrated that there was no degradation in the binding capacity between MIP and benzylpenicillin even after several adsorption and desorption cycles, thereby confirming the potential of the proposed sensor. Simultaneously, Celik's group conducted comparative experiments using three types of sensing materials and two interferents to demonstrate the improved sensitivity and selectivity achieved through GO and MIP (Fig. 14(f)). By comparing the GO-NIP-based sensor, which lacked imprinted sites and exhibited low responses for all three substances, with the GO-MIP-based sensor, which possessed imprinted sites and showed exceptionally high response only to benzylpenicillin, the selectivity of the MIP film for benzylpenicillin was confirmed. Furthermore, the comparison between the GO-MIP based sensor and the MIP-based sensor revealed that while both sensors exhibited selectivity for benzylpenicillin, the presence of GO resulted in enhanced sensitivity. Celik's group reported that the GO-MIP-based SPR sensor could detect benzylpenicillin rapidly in real-time by amplifying the selective but weak signals from MIP.
Utilizing molecular imprinting films, organic composites become highly effective when there are numerous interferents in the detection environment that may hinder the detection of the target chemical molecules. MIP films can selectively and sensitively capture target molecules due to the presence of imprinted sites. The requirement to immobilize biocomponents for selective detection is removed, lowering the possibility of the detachment of the biocomponents throughout the procedure. This approach can be easily carried out by simply mixing organic molecules and target molecules, and then removing the target molecules. Thus, combining organic composites with SPR sensors, which enable real-time, label-free, and selective detection, holds great potential for applications in environmental monitoring, disease diagnosis, and pharmaceutical research.
5. Practical applications of LSPR and SPR sensors
The significance of the real-world applications of LSPR and SPR sensors stems from their positive impact on many scientific, industrial, and medical areas. In practical applications, these sensors allow the real-time detection of molecular interactions, providing essential insights into biological, chemical, and environmental processes. In healthcare, LSPR and SPR sensors are crucial in drug discovery, medical diagnostics, and biomolecular interaction monitoring, all contributing to advances in precision medicine. Environmental monitoring benefits from the ability to identify pollutants and toxins with high sensitivity. These sensors are used in industrial settings for quality control, process monitoring, and detecting trace contaminants. The real-world application of LSPR and SPR sensors promotes the development of a variety of sectors by providing efficient and dependable instruments for monitoring and analysis, eventually boosting innovation, improving research outcomes, and improving diagnostic and decision-making processes.Coskun et al. presented a microfluidic-based plasmonic biosensing system that combines plasmonic microarrays with dual color lens-free imaging.125 This system allowed the real-time and multiplexed monitoring of binding events over a large field-of-view (>20 mm2) in low resource settings. This platform uses an optoelectronic sensor (complementary metal-oxide-semiconductor) to record diffraction patterns of plasmonic nanoapertures at the bottom of a microfluidic channel, as shown in Fig. 15(a)–(c). This allows the controlled delivery of the target solution to surface-functionalized nanosensor arrays. Fig. 15(d) shows that ligand proteins functionalize plasmonic pixels to capture the target proteins. The biosensing device employs a plasmonic nanohole array with excellent light confinement and nanoscale field improvements, resulting in high sensitivity to the surface conditions. Changes in the refractive index near the sensor surface cause a shift in the peak wavelength of the plasmonic mode supported by the nanohole array.
 | ||
| Fig. 15 (a) Photograph and (b) illustration of the LSPR sensing device employing (c) a microfluidic channel. (d) Schematic of the sensing mechanism of microfluidic channel. (b) LSPR peak of SA–Ag composite in an aqueous solution of 1000 ppb of Hg2+. Reproduced with permission from ref. 125 Copyright©2014, Springer Nature. (c) Pb2+ sensing mechanism of functionalized AuNPs. Schematic of (e) a smartphone-based SPR sensor and (f) its internal structure. Photograph of (g) SPR sensor installed on a smartphone and (h) the smart phone measurement channel. Reproduced with permission from ref. 126 Copyright©2015, Springer Nature. | ||
Liu et al. presented a flexible, compact dual-channel SPR system.126 Fig. 15(e) and (h) display a schematic diagram and a photograph and the running interface of the detection system, respectively. Given that all components are attached to the phone case on the back of the smartphone (Fig. 15(f)), the touch-screen interface and display remain unaffected during the detecting process, as shown in Fig. 15(g) and (h). The sensor components and the smartphone can be readily combined into an instrument and dismantled after the measurement.
6. Challenges and future perspectives
LSPR sensors rely on morphological changes in plasmonic metal NPs, while SPR sensors monitor changes in the refractive index around metal films. Although both sensors demonstrate high sensitivity to trace concentrations of analytes, they are vulnerable to environmental interferences. Environmental factors, such as temperature changes and vibrations near the sensors, generate background noise, which is further deteriorated by non-specific adsorption. This undesirable noise increases the LOD, reduces the sensitivity and selectivity, and eventually degrades the overall sensor performance. Therefore, addressing these challenges is crucial for improving the reliability and practical applicability of these sensors.In particular, the synthesis of metal NPs with a uniform size and shape at the nanoscale for LSPR sensors is challenging. Given that their detection mechanism is based on morphological changes, ensuring a uniform distribution of homogeneous metal NPs produces a consistent and strong plasmonic resonance frequency, leading to distinct shifts in the absorption peak and noticeable color changes.127,128 A variation in the size, shape, or spatial arrangement of the fabricated NP dispersion can reduce the detection accuracy and reproductivity. Thus, advancements in precise synthesis techniques and complementary strategies are essential for reliable detection.
In addition to background noise, SPR sensors are also limited by sensing materials that are either thicker than the effective detection region or cause optical loss at the resonance wavelength. The evanescent wave, SPP, generated on the metal surface can only penetrate a few hundred nanometers above the metal film.129 Refractive index changes occurring beyond this detection region are difficult to detect. Moreover, the incident light required to excite a plasmonic wave typically falls within the visible or infrared range. If sensing materials that strongly absorb light in this wavelength range are deposited on the metal film, the intensity of the excited SPP decreases, significantly weakening the SPR signals. Thus, to overcome these problems, depositing ultra-thin sensing materials with suitable optical characteristics is one of the most important considerations in developing SPR sensors.
Despite the challenges faced by LSPR and SPR sensors, ongoing research and development indicate their promising future. Advancements in nanomaterial synthesis, the incorporation of novel materials with plasmonic metals, and progress in optical technology serve as key factors for enhancing the performance and versatility of these sensors. By functionalizing metal surfaces with organic molecules, receptors, and novel materials that selectively bind to analytes without weakening the plasmonic signals, the real-world applications of these sensors can be expanded, increasing their importance. Thus, LSPR and SPR technologies are expected to become valuable candidates in disease diagnostics, drug discovery, and environmental monitoring.
7. Conclusion
LSPR and SPR sensors are plasmonic-based sensors that can precisely detect analytes using optics. LSPR sensors, which detect absorbance through changes in the shape of the sensing material due to interactions, and SPR sensors, which detect changes in refractive index that occur when the sensing material interacts by chemical adsorption, detect very low concentrations of chemicals in real time using cutting-edge sensing materials. Sensing materials for LSPR and SPR sensors play important roles in improving their sensing performance and ability for real-time detection based on rapid response. Table 3, which summarizes the general advantages and disadvantages of each sensing material, provides a clear understanding of how these materials enhance the sensor performance in various applications and the roles they play. As a result, sensing materials such as metal NPs, bimetal NPs, MOMs, 2D materials, metal oxides, and organic composites have been studied, and various structures and compositions have been developed. This material-based research enables lower detection limits and improved real-time sensing capabilities by LSPR and SPR sensors, enabling their use in a wide range of applications requiring real-time sensing, including biomedical diagnostics, environmental, and industrial monitoring. In addition, LSPR and SPR sensors can be miniaturized and easily function using a light source and thus are suitable for application in portable measurement equipment, providing excellent accessibility to measurements that require real-time detection. The combined impact of sensing material improvements and practical applicability presents the significant potential of LSPR and SPR sensors in driving precision and real-time sensing technologies across the scientific, medical, and industrial fields, promising new answers to complex analytical challenges regarding the safe coexistence of chemicals and humanity.| Sensor type | Material type | Advantages | Disadvantages |
|---|---|---|---|
| LSPR sensors | Metal NPs | • Even slight morphological changes in size and shape can significantly affect the plasmonic resonance frequency. | • Difficulty in fabricating metal NPs with uniform size and shape. |
| • High surface-to-volume ratio maximizes interactions with analytes. | |||
| Bimetallic NPs | • Improved stability and sensitivity due to the synergistic effect of each metal. | • Difficulty in fabricating bimetallic NPs with uniform size and shape. | |
| • More flexible tunability in morphology. | |||
| • Detection with the naked eye through multicolour changes. | |||
| MOMs | • Selective binding to analytes via surface functionalization of metal NPs. | • Poor reproducibility and long-term stability due to degradation of surface functionalization. | |
| • Reversible detection is possible by capping the surface of metal NPs to prevent their dissociation. | |||
| SPR sensors | 2D materials | • Enhancement of plasmonic waves due to charge transfer from 2D materials to metal films. | • Formation of undesirable defects during the transfer of 2D materials |
| • Abundant adsorption sites for analytes and anchoring sites for various receptors via large specific surface area. | • High cost for large-area and high-quality production. | ||
| • Low damping effect of ultra-thin and uniform surface. | |||
| Metal oxides | • Cost-effective and facile synthesis for nanostructures. | • Deterioration of SPR signals due to optical loss in the visible and infrared region. | |
| • Catalytic effect for various chemical and bioanalytes. | |||
| • Chemical and thermal stability. | |||
| Organic composites | • Feasibility of forming uniform films containing metal NPs or bioreceptors. | • Limitation in controlling the precise thickness at the nanoscale, causing plasmon damping. | |
| • Highly selective and sensitive detection through MIP films. | |||
Data availability
Data availability is not applicable to this article as no new data were created or analysed in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Nano Material Technology Development Program (2022M3H4A1A01011993 & RS-2024-00405016) through the NRF (National Research Foundation of Korea), funded by the Ministry of Science and ICT. Research facilities for this work were provided by the Inter-University Semiconductor Research Center, the Institute of Engineering Research, and Soft Foundry Institute at Seoul National University.References
- T. Biswal, S. K. BadJena and D. Pradhan, Mater. Today: Proc., 2020, 30, 274–282 Search PubMed.
- A. Rahimi and J. M. García, Nat. Rev. Chem., 2017, 1, 46 CrossRef.
- M. Harada, Crit. Rev. Toxicol., 1995, 25, 1–24 CrossRef PubMed.
- A. S. Ayangbenro and O. O. Babalola, Int. J. Environ. Res. Public Health, 2017, 14, 94 CrossRef.
- S. Lee, X. Bi, R. B. Reed, J. F. Ranville, P. Herckes and P. Westerhoff, Environ. Sci. Technol., 2014, 48, 10291–10300 CrossRef PubMed.
- W. B. Dunn, D. Broadhurst, P. Begley, E. Zelena, S. Francis-McIntyre, N. Anderson, M. Brown, J. D. Knowles, A. Halsall and J. N. Haselden, Nat. Protoc., 2011, 6, 1060–1083 CrossRef PubMed.
- N. P. Ivleva, Chem. Rev., 2021, 121, 11886–11936 CrossRef PubMed.
- M. Rana and V. Mittal, IEEE Sens. J., 2020, 21, 1187–1207 Search PubMed.
- Q. Pang, D. Lou, S. Li, G. Wang, B. Qiao, S. Dong, L. Ma, C. Gao and Z. Wu, Adv. Sci., 2020, 7, 1902673 CrossRef PubMed.
- M. Javaid, A. Haleem, R. P. Singh, S. Rab and R. Suman, Sens Int., 2021, 2, 100110 CrossRef.
- M. Jayaweera, H. Perera, B. Gunawardana and J. Manatunge, Environ. Res., 2020, 188, 109819 CrossRef PubMed.
- S. H. Cho, J. M. Suh, T. H. Eom, T. Kim and H. W. Jang, Electron. Mater. Lett., 2021, 17, 1–17 CrossRef.
- S. H. Cho, M.-J. Choi, B. Koo, J. Kim, T. H. Lee, J. M. Suh, T. H. Eom, S. Y. Park, T. Kim and W. Jung, Sens. Actuators, B, 2024, 403, 135137 CrossRef.
- S. Hwan Cho, J. Min Suh, B. Jeong, T. Hyung Lee, K. Soon Choi, T. Hoon Eom, T. Kim and H. Won Jang, Chem. Eng. J., 2022, 446, 136862 CrossRef.
- C. W. Lee, J. M. Suh, S. Choi, S. E. Jun, T. H. Lee, J. W. Yang, S. A. Lee, B. R. Lee, D. Yoo, S. Y. Kim, D. S. Kim and H. W. Jang, npj 2D Mater. Appl., 2021, 5, 1–13 CrossRef.
- A. Kumar and R. Prajesh, Sens. Actuators, A, 2022, 339, 113498 CrossRef.
- Q. Duan, Y. Liu, S. Chang, H. Chen and J. Chen, Sensors, 2021, 21, 5262 CrossRef PubMed.
- J. Homola, S. S. Yee and G. Gauglitz, Sens. Actuators, B, 1999, 54, 3–15 CrossRef.
- J. N. Anker, W. P. Hall, O. Lyandres, N. C. Shah, J. Zhao and R. P. Van Duyne, Nat. Mater., 2008, 7, 442–453 CrossRef PubMed.
- J. Homola, Chem. Rev., 2008, 108, 462–493 CrossRef PubMed.
- K. M. Mayer and J. H. Hafner, Chem. Rev., 2011, 111, 3828–3857 CrossRef PubMed.
- J.-F. Masson, Analyst, 2020, 145, 3776–3800 RSC.
- G. Simone, Crit. Rev. Anal. Chem., 2024, 54, 2183–2208 CrossRef PubMed.
- J. Homola, Anal. Bioanal. Chem., 2003, 377, 528–539 CrossRef PubMed.
- X. Zhang, Z. Li, W. Yan, A. Li, F. Zhang, X. Li, M. Lu and W. Peng, Talanta, 2024, 269, 125440 CrossRef PubMed.
- S. Long, J. Cao, Y. Wang, S. Gao, N. Xu, J. Gao and W. Wan, Sens. Actuators Rep., 2020, 2, 100016 CrossRef.
- G. Mie, Ann. Phys., 1908, 330, 377–445 CrossRef.
- K. Matsubara, S. Kawata and S. Minami, Appl. Spectrosc., 1988, 42, 1375–1379 CrossRef CAS.
- K. Matsubara, S. Kawata and S. Minami, Opt. Lett., 1990, 15, 75–77 CrossRef CAS PubMed.
- G. Cappi, F. M. Spiga, Y. Moncada, A. Ferretti, M. Beyeler, M. Bianchessi, L. Decosterd, T. Buclin and C. Guiducci, Anal. Chem., 2015, 87, 5278–5285 CrossRef CAS PubMed.
- V. G. Kravets, F. Schedin, A. V. Kabashin and A. N. Grigorenko, Opt. Lett., 2010, 35, 956–958 CrossRef CAS PubMed.
- M. Pirzada and Z. Altintas, Micromachines, 2020, 11, 356 CrossRef PubMed.
- K. M. Mayer and J. H. Hafner, Chem. Rev., 2011, 111, 3828–3857 CrossRef CAS PubMed.
- P. Englebienne, A. Van Hoonacker and M. Verhas, J. Spectrosc., 2003, 17, 255–273 CrossRef CAS.
- J. Chen, A. A. Jackson, V. M. Rotello and S. R. Nugen, Small, 2016, 12, 2469–2475 CrossRef PubMed.
- X. Liu, J. Wang, Y. Wang, C. Huang, Z. Wang and L. Liu, ACS Omega, 2021, 6, 23630–23635 CrossRef PubMed.
- A. Amirjani, M. Bagheri, M. Heydari and S. Hesaraki, Nanotechnology, 2016, 27, 375503 CrossRef PubMed.
- J. Lerdsri, W. Chananchana, J. Upan, T. Sridara and J. Jakmunee, Sens. Actuators, B, 2020, 320, 128356 CrossRef.
- Q. Zhu, T. Li, Y. Ma, Z. Wang, J. Huang, R. Liu and Y. Gu, RSC Adv., 2017, 7, 19250–19256 RSC.
- S. Rasheed, N. Ahmad, M. A. ul Haq, W. Ahmad and D. Hussain, J. Ind. Eng. Chem., 2023, 128, 450–458 CrossRef.
- Z. Zhang, Z. Chen, C. Qu and L. Chen, Langmuir, 2014, 30, 3625–3630 CrossRef PubMed.
- P. Huang, B. Liu, W. Jin, F. Wu and Y. Wan, J. Nanopart. Res., 2016, 18, 1–9 CrossRef CAS.
- K. Shrivas, R. Shankar and K. Dewangan, Sens. Actuators, B, 2015, 220, 1376–1383 CrossRef CAS.
- S. Chen, J. Tang, Y. Kuang, L. Fu, F. Ma, Y. Yang, G. Chen and Y. Long, Sens. Actuators, B, 2015, 221, 1182–1187 CrossRef CAS.
- H. He, X. Xu, H. Wu and Y. Jin, Adv. Mater., 2012, 24, 1736–1740 CrossRef CAS PubMed.
- Y. Wang, P. Zhang, W. Fu and Y. Zhao, Biosens. Bioelectron., 2018, 122, 183–188 CrossRef CAS PubMed.
- Y. Wang, P. Zhang, X. Mao, W. Fu and C. Liu, Sens. Actuators, B, 2016, 231, 95–101 CrossRef CAS.
- Y. Wang, Y. Zeng, W. Fu, P. Zhang, L. Li, C. Ye, L. Yu, X. Zhu and S. Zhao, Anal. Chim. Acta, 2018, 1002, 97–104 CrossRef PubMed.
- H. Lee, H. K. Sung, C. Park and Y. Kim, J. Ind. Eng. Chem., 2017, 48, 235–241 CrossRef.
- I. Darmadi, S. Z. Khairunnisa, D. Tomecek and C. Langhammer, ACS Appl. Nano Mater., 2021, 4, 8716–8722 CrossRef.
- X. Li, X. Lin, S. Lin, X. Sun, D. Gao, B. Liu, H. Zhao, J. Zhang, S. Cong and L. Wang, ACS Appl. Nano Mater., 2019, 2, 3161–3168 CrossRef.
- J.-K. Chen, S.-M. Zhao, J. Zhu, J.-J. Li and J.-W. Zhao, J. Alloys Compd., 2020, 828, 154392 CrossRef.
- Z. Qiu, Y. Xue, J. Li, Y. Zhang, X. Liang, C. Wen, H. Gong and J. Zeng, Chin. Chem. Lett., 2021, 32, 2807–2811 CrossRef.
- S. Li, T. Wei, M. Tang, F. Chai, F. Qu and C. Wang, Sens. Actuators, B, 2018, 255, 1471–1481 CrossRef CAS.
- Y. Xia, J. Ye, K. Tan, J. Wang and G. Yang, Anal. Chem., 2013, 85, 6241–6247 CrossRef CAS PubMed.
- Z. Karimzadeh, A. Jouyban, M. Khoubnasabjafari, A. Gharakhani and E. Rahimpour, Plasmonics, 2022, 17, 1999–2008 CrossRef CAS.
- H.-M. Kim, W.-J. Kim, K.-O. Kim, J.-H. Park and S.-K. Lee, J. Alloys Compd., 2021, 884, 161140 CrossRef.
- D. Sahu, N. Sarkar, G. Sahoo, P. Mohapatra and S. K. Swain, Sens. Actuators, B, 2017, 246, 96–107 CrossRef.
- A. R. Ferhan, L. Guo, X. Zhou, P. Chen, S. Hong and D.-H. Kim, Anal. Chem., 2013, 85, 4094–4099 CrossRef PubMed.
- S. S. Dandu, D. J. Joshi, T. J. Park and S. K. Kailasa, Appl. Spectrosc., 2023, 77, 360–370 CrossRef PubMed.
- I. E. Paul, A. Rajeshwari, T. C. Prathna, A. M. Raichur, N. Chandrasekaran and A. Mukherjee, Anal. Methods, 2015, 7, 1453–1462 RSC.
- J. Song, F. Wu, Y. Wan and L. Ma, Food Control, 2015, 50, 356–361 Search PubMed.
- I. Onyido, A. R. Norris and E. Buncel, Chem. Rev., 2004, 104, 5911–5930 CrossRef PubMed.
- F. J. Gifford, R. M. Gifford, M. Eddleston and N. Dhaun, Kidney Int. Rep., 2017, 2, 282–292 CrossRef PubMed.
- M. Fisser, R. A. Badcock, P. D. Teal and A. Hunze, Sens. Actuators, B, 2018, 259, 10–19 CrossRef.
- S. H. Cho, J. M. Suh, B. Jeong, T. H. Lee, K. S. Choi, T. H. Eom, T. Kim and H. W. Jang, Chem. Eng. J., 2022, 446, 136862 CrossRef.
- C. Wadell, S. Syrenova and C. Langhammer, ACS Nano, 2014, 8, 11925–11940 CrossRef PubMed.
- L. Boon-Brett, J. Bousek, G. Black, P. Moretto, P. Castello, T. Hübert and U. Banach, Int. J. Hydrogen Energy, 2010, 35, 373–384 Search PubMed.
- H. Gao, M. Liu, Z. Zhao, C. Yang, L. Zhu, Y. Cai, Y. Yang and Z. Hu, ACS Appl. Mater. Interfaces, 2020, 12, 9693–9700 CrossRef PubMed.
- S. Chang, L. Liu, C. Mu, F. Wen, J. Xiang, K. Zhai, B. Wang, L. Wu, A. Nie and Y. Shu, J. Colloid Interface Sci., 2023, 651, 938–947 CrossRef PubMed.
- J. Feng, J. Gao, W. Yang, Y. Li, J. Shi, C. Liu, M. Jiang and S. Jiang, ACS Appl. Nano Mater., 2023, 6, 12775–12783 CrossRef CAS.
- P. Thawany, A. Khanna, U. K. Tiwari and A. Deep, Sci. Rep., 2023, 13, 5297 CrossRef CAS PubMed.
- Q. Wu, N. Li, Y. Wang, Y. Xu, S. Wei, J. Wu, G. Jia, X. Fang, F. Chen and X. Cui, Biosens. Bioelectron., 2019, 144, 111697 CrossRef CAS PubMed.
- Y. Wang, Z. Mao, Q. Chen, K. Koh, X. Hu and H. Chen, Biosens. Bioelectron., 2022, 201, 113954 CrossRef CAS PubMed.
- J. Ma, B. Lu, P. Zhang, D. Li and K. Xu, Sens. Actuators, A, 2023, 353, 114227 CrossRef CAS.
- T. Xue, W. Liang, Y. Li, Y. Sun, Y. Xiang, Y. Zhang, Z. Dai, Y. Duo, L. Wu and K. Qi, Nat. Commun., 2019, 10, 28 CrossRef CAS PubMed.
- S. H. Cho, J. M. Suh, W. Kim, J. Kim, Y. J. Kim, T. H. Lee, J. Y. Kim, J. Sim, S. W. Choi and B. H. Hong, Energy Environ. Mater., 2025, 8(1), e12801 CrossRef CAS.
- S. Gan, B. Ruan, Y. Xiang and X. Dai, IEEE Sens. J., 2020, 21, 347–352 Search PubMed.
- R. Chen, C. Guo, G. Lan, P. Luo, J. Yi and W. Wei, Biosens. Bioelectron., 2023, 115469 CrossRef CAS PubMed.
- R. Kant, R. Tabassum and B. D. Gupta, Biosens. Bioelectron., 2018, 99, 637–645 Search PubMed.
- T. O. C. Rahayu, N. L. W. Septiani, G. Gumilar, D. R. Adhika and B. Yuliarto, IEEE Sens. J., 2021, 21, 19959–19966 CAS.
- B. Karki, Y. Trabelsi, A. Pal, S. A. Taya and R. B. Yadav, Opt. Mater., 2024, 147, 114555 CrossRef CAS.
- G. Kaur, A. Paliwal, M. Tomar and V. Gupta, Biosens. Bioelectron., 2016, 78, 106–110 CrossRef CAS PubMed.
- R. Kant, Microchim. Acta, 2020, 187, 1–11 CrossRef PubMed.
- A. Paliwal, A. Sharma, M. Tomar and V. Gupta, Sens. Actuators, B, 2015, 216, 497–503 CrossRef CAS.
- S. K. Mishra, S. Rani and B. D. Gupta, Sens. Actuators, B, 2014, 195, 215–222 CrossRef CAS.
- A. Paliwal, A. Sharma, M. Tomar and V. Gupta, Sens. Actuators, B, 2017, 250, 679–685 CrossRef CAS.
- Y. Mustapha Kamil, S. H. Al-Rekabi, M. H. Abu Bakar, Y. W. Fen, H. A. Mohammed, N. H. Mohamed Halip, M. T. Alresheedi and M. A. Mahdi, Photonic Sens., 2022, 12, 220306 CrossRef CAS.
- D. Zhang, Y. Sun, Q. Wu, P. Ma, H. Zhang, Y. Wang and D. Song, Talanta, 2016, 146, 364–368 CrossRef CAS PubMed.
- S. Jiang, Y. Peng, B. Ning, J. Bai, Y. Liu, N. Zhang and Z. Gao, Sens. Actuators, B, 2015, 221, 15–21 CrossRef CAS.
- J. Zhang, Y. Sun, H. Zhang, B. Xu, H. Zhang and D. Song, Anal. Chim. Acta, 2013, 769, 114–120 CrossRef CAS PubMed.
- X. Xu, Y. Zhang, B. Wang, L. Luo, Z. Xu and X. Tian, RSC Adv., 2018, 8, 32538–32544 RSC.
- S. Nangare and P. Patil, Int. J. Biol. Macromol., 2022, 214, 568–582 CrossRef CAS PubMed.
- A. Baliyan, P. Bhatia, B. D. Gupta, E. K. Sharma, A. Kumari and R. Gupta, Sens. Actuators, B, 2013, 188, 917–922 CrossRef CAS.
- A. M. Shrivastav, S. P. Usha and B. D. Gupta, Biosens. Bioelectron., 2016, 79, 150–157 CrossRef CAS PubMed.
- O. Çelik, Y. Saylan, I. Göktürk, F. Yılmaz and A. Denizli, Talanta, 2023, 253, 123939 CrossRef PubMed.
- N. Atar, T. Eren, M. L. Yola and S. Wang, Sens. Actuators, B, 2015, 216, 638–644 CrossRef CAS.
- R. Verma and B. D. Gupta, Food Chem., 2015, 166, 568–575 CrossRef CAS PubMed.
- K. Chung, J. S. Lee, E. Kim, K. Lee, K. Kim, J. Lee, D. Kim, S. O. Kim, S. Jeon and H. Park, Adv. Mater. Interfaces, 2018, 5, 1800433 CrossRef.
- S. H. Choi, Y. L. Kim and K. M. Byun, Opt. Express, 2011, 19, 458–466 CrossRef CAS PubMed.
- G. M. Varghese, R. John, A. Manesh, R. Karthik and O. C. Abraham, Indian J. Med. Res., 2020, 151, 401 CrossRef PubMed.
- T. Ohmi, S. Sudoh and H. Mishima, IEEE Trans. Semicond. Manuf., 1994, 7, 440–446 CrossRef.
- D. Shin, H. R. Kim and B. H. Hong, Nanoscale Adv., 2021, 3, 1404–1412 RSC.
- A. Das, S. Pisana, B. Chakraborty, S. Piscanec, S. K. Saha, U. V. Waghmare, K. S. Novoselov, H. R. Krishnamurthy, A. K. Geim, A. C. Ferrari and A. K. Sood, Nat. Nanotechnol., 2008, 3, 210–215 CrossRef PubMed.
- C. W. Lee, S. E. Jun, S. J. Kim, T. H. Lee, S. A. Lee, J. W. Yang, J. H. Cho, S. Choi, C. Kim, S. Y. Kim and H. W. Jang, InfoMat, 2023, 5, e12427 CrossRef.
- C. Lin and S. Chen, Opt. Commun., 2019, 445, 155–160 CrossRef.
- A. Salinas-Castillo, I. Pastor, R. Mallavia and C. R. Mateo, Biosens. Bioelectron., 2008, 24, 1053–1056 CrossRef PubMed.
- A. Bornø, L. Foged and G. van Hall, J. Mass Spectrom., 2014, 49, 980–988 CrossRef PubMed.
- P. Kalimuthu, S. Leimkühler and P. V. Bernhardt, Anal. Chem., 2012, 84, 10359–10365 CrossRef PubMed.
- J. K. Kirk and J. Stegner, J. Diabetes Sci. Technol., 2010, 4, 435–439 CrossRef PubMed.
- R. M. Anjana, R. Pradeepa, M. Deepa, M. Datta, V. Sudha, R. Unnikrishnan, A. Bhansali, S. R. Joshi, P. P. Joshi and C. S. Yajnik, Diabetologia, 2011, 54, 3022–3027 CrossRef PubMed.
- R. D. Janine Freeman and M. P. H. Lynne Lyons, Diabetes spectrum, 2008, 21, 134 CrossRef.
- M. Flockhart and F. J. Larsen, Sports Med., 2023, 1–9 Search PubMed.
- T. H. Eom, S. H. Cho, J. M. Suh, T. Kim, J. W. Yang, T. H. Lee, S. E. Jun, S. J. Kim, J. Lee, S. H. Hong and H. W. Jang, Small, 2022, 18, 1–12 CrossRef PubMed.
- D. Cho, J. M. Suh, S. Nam, S. Y. Park, M. Park, T. H. Lee, K. S. Choi, J. Lee, C. Ahn and H. W. Jang, Adv. Sci., 2021, 8, 2001883 CrossRef CAS PubMed.
- Y.-S. Shim and H. W. Jang, J. Sens. Sci. Technol., 2016, 25, 178–183 CrossRef.
- G. Weinmayr, E. Romeo, M. de Sario, S. K. Weiland and F. Forastiere, Environ. Health Perspect., 2010, 118, 449–457 CrossRef PubMed.
- Z. Xu, W. Shi, C. Yang, J. Xu, H. Liu, J. Xu and B. Zhu, Luminescence, 2020, 35, 299–304 CrossRef PubMed.
- T. H. Eom, S. H. Cho, J. M. Suh, T. Kim, T. H. Lee, S. E. Jun, J. W. Yang, J. Lee, S. H. Hong and H. W. Jang, J. Mater. Chem. A, 2021, 9, 11168–11178 RSC.
- C. Müller, T. Nirmaier, A. Rügemer and M. V. Schickfus, Sens. Actuators, B, 2000, 68, 69–73 CrossRef.
- K. Baker, T. Rath, W. I. Lencer, E. Fiebiger and R. S. Blumberg, Cell. Mol. Life Sci., 2013, 70, 1319–1334 CrossRef PubMed.
- K. Rekha, M. D. Gouda, M. S. Thakur and N. G. Karanth, Biosens. Bioelectron., 2000, 15, 499–502 CrossRef PubMed.
- F. Baquero, J.-L. Martínez and R. Cantón, Curr. Opin. Biotechnol, 2008, 19, 260–265 CrossRef PubMed.
- S. Ji, B. K. Min, S. K. Kim, S. Myung, M. Kang, H.-S. Shin, W. Song, J. Heo, J. Lim and K.-S. An, Appl. Surf. Sci., 2017, 419, 252–258 CrossRef.
- A. F. Coskun, A. E. Cetin, B. C. Galarreta, D. A. Alvarez, H. Altug and A. Ozcan, Sci. Rep., 2014, 4, 6789 CrossRef PubMed.
- Y. Liu, Q. Liu, S. Chen, F. Cheng, H. Wang and W. Peng, Sci. Rep., 2015, 5, 12864 CrossRef PubMed.
- A. G. M. da Silva, T. S. Rodrigues, J. Wang, L. K. Yamada, T. V. Alves, F. R. Ornellas, R. A. Ando and P. H. C. Camargo, Langmuir, 2015, 31, 10272–10278 CrossRef PubMed.
- A. D. Chowdhury, F. Nasrin, R. Gangopadhyay, A. B. Ganganboina, K. Takemura, I. Kozaki, H. Honda, T. Hara, F. Abe, S. Park, T. Suzuki and E. Y. Park, Biosens. Bioelectron., 2020, 170, 112657 CrossRef CAS PubMed.
- S. J. Yoon and D. Kim, J. Opt. Soc. Am. A, 2007, 24, 2543–2549 CrossRef PubMed.
Footnote |
| † S. H. Cho, S. Choi, and J. M. Suh contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |