Open Access Article
Open Access ArticleExploration of biodegradable polymeric particles in agriculture: a holistic approach for sustainable farming
Kunal
Verma
,
Chandrani
Sarkar
and
Sampa
Saha
 *
*
Department of Materials Science and Engineering, Indian Institute of Technology, Delhi, New Delhi-110016, India. E-mail: ssaha@mse.iitd.ac.in
First published on 15th January 2025
Abstract
Conventional agricultural methodologies often rely on excessive application of fertilizers, pesticides, and water, resulting in adverse environmental consequences such as air/water/soil pollution, soil degradation, etc., thereby diminishing farming efficiency and profitability. The growing demand for sustainable agricultural practices has intensified researchers' interest in exploring biodegradable polymeric particles (BPPs) due to their ability to improve agrochemical delivery, enhance soil health, and mitigate environmental impacts. This review critically examines the state of the art in the design, fabrication, and application of BPPs for agriculture to accomplish sustainable farming. It highlights their significance in enabling controlled release systems, soil improvement, and plant stress tolerance. Key fabrication techniques such as emulsion solvent evaporation, anti-solvent nanoprecipitation, ionotropic gelation, and spray drying are compared based on their scalability, cost-efficiency, and suitability for producing particles with tailored properties. The influence of particle size, shape, and morphology on application efficiency and their biological interactions are thoroughly analyzed, emphasizing the importance of design in optimizing performance. This review also explores the challenges associated with adopting BPPs, including scalability, cost, regulatory compliance, etc., and proposes future directions for advancing their development. By addressing critical gaps and presenting innovative strategies, this review provides a comprehensive framework for integrating biodegradable polymeric particles into sustainable agricultural practices.
Environmental significanceThe adoption of biodegradable polymeric particles (BPPs) in agriculture presents a significant advancement toward sustainable farming practices. By enabling controlled release of agrochemicals such as fertilizers and pesticides, BPPs reduce environmental pollution, enhance soil health, and minimize chemical runoff into water bodies. These particles decompose naturally, thus eliminating the long-term environmental hazards posed by conventional plastics and synthetic materials. The use of BPPs aligns with the global push for reducing reliance on non-renewable resources and limiting the ecological impact of traditional agricultural methods. This holistic approach supports the goal of maintaining biodiversity and soil fertility while ensuring efficient resource use in farming systems. |
1. Introduction
The increasing demand for sustainable agricultural practices has driven significant interest in the development of innovative biodegradable polymeric particles for the agricultural sector.1,2 In recent years, the use of biodegradable polymeric particles in agriculture has been expanded, encompassing various applications such as controlled-release formulations, soil improvement, and crop protection.3–7 For instance, these particles are designed to deliver agrochemicals, such as pesticides and fertilizers, in a controlled manner, thus enhancing their efficacy and mitigating their adverse effects on the environment.8 The controlled release of agrochemicals not only improves crop yield and quality but also reduces the frequency of applications, thereby lowering the overall chemical load on agricultural fields and their toxic side effects.9Traditional farming methods, characterized by intensive chemical inputs and indiscriminate resource consumption, have proven unsustainable, polluting waterways and contributing to climate change.10–12 Moreover, the use of conventional non-degradable plastics or polymers in the agricultural sector has been associated with a multitude of issues. The intensive use of plastics can impair soil fertility and lead to dwindling crop yields.13 There is also the undesirable prospect of toxic additives leaching from plastics seeping into our food chain. In China, agricultural films are found to release 91.5 tons of PAEs (phthalic acid esters) from mulching and greenhouse films, contributing to soil and vegetable contamination, respectively.14 One of the most significant problems is the accumulation of these materials in soils, which can have diverse and long-term effects on crop production and quality.15 Conventional mulch films (mainly polyethylene) accumulate leftover fragments from agricultural soils, which may negatively impact soil productivity and ecology (Fig. 1). Moreover, there is evidence that “non-degradable” mulches pose threats to human health and are found to be carcinogenic, mutagenic, endocrine disrupting and bioaccumulative.16 Many experts have shown concerns about managing conventional plastics and replaced them with biodegradable polymers that decompose in soil through microbial activity, thus minimizing the accumulation.17,18
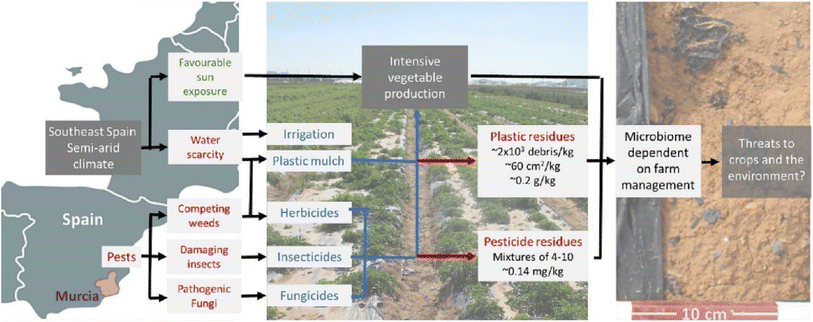 | ||
| Fig. 1 Low-density polyethylene plastic mulch following kohlrabi harvest. The sides of the mulch film are buried in the ground, full removal is not feasible and over time debris builds up. Reused with permission from the author (CC BY).19 | ||
The integration of biodegradable polymeric particles into agricultural practices aligns with the principles of sustainable agriculture, which emphasize the efficient use of resources, reduction of waste, and protection of the environment. By leveraging these materials, farmers can achieve higher productivity while maintaining soil health and biodiversity. Moreover, the biodegradability of these polymers ensures that they do not accumulate in the environment, thus mitigating the long-term ecological impact associated with conventional plastics.20
Biodegradable polymers emerge as a promising sustainable platform in this endeavor, offering a multifaceted approach to addressing agricultural challenges.12,21–24 These materials offer a promising alternative to conventional synthetic polymers, which are often associated with environmental pollution and long-term ecological damage. Biodegradable polymers, mainly derived from renewable resources, decompose naturally into non-toxic byproducts.25 Biopolymers like cellulose and chitosan show promising results, when used for agricultural applications.26 They are often procured from renewable resources or agricultural byproducts and offer economical and eco-friendly solutions.23,27–30 Recent studies have also confirmed the soil biodegradability of poly(butylene adipate-co-terephthalate) (PBAT), a synthetic polyester used in agriculture.31–34 However, recent advancements in biodegradable polymeric materials in agriculture predominantly center on mulching applications.3,33,35,36 Some studies explore their potential as super-absorbent polymers for soil remediation and agrochemical delivery.22,30,37,38
The adoption of biodegradable polymeric particles represents a significant step forward in addressing pressing agricultural challenges,39 including nutrient inefficiency, environmental pollution,31,40 and climate-induced stresses on crops. While numerous reviews focus on specific polymers2,3,41–43 such as chitosan,44–47 starch,30,46,48–50 cellulose,51,52 polylactic acid (PLA),53 and their agricultural uses, others delve into individual applications like controlled-release fertilizers (CRFs),6,54,55 hydrogels,56 controlled-release pesticides,57–60 and mulches.9,33,61 However, there is a lack of comprehensive reviews that integrate all these aspects into a holistic framework. This review bridges that gap by providing an extensive analysis of the fabrication methods, polymers employed, and applications of biodegradable polymeric particles in agriculture.
This review systematically examines the diverse polymers used—ranging from naturally derived materials like cellulose and alginate to synthetic options like PLA and PCL—and their tailored applications. Each application, including nutrient delivery, pesticide encapsulation, soil enhancement, and plant growth regulation, is discussed with an emphasis on the active ingredients and the polymers used to formulate them. Additionally, the review incorporates environmental and economic considerations, highlighting how these materials can enhance agricultural sustainability while minimizing ecological impact. By discussing various fabrication techniques, application-oriented studies, and broader implications, this review offers a unified perspective on the potential of biodegradable polymeric particles to transform agricultural practices.
It provides a critical analysis of the state of the art, recent advancements, and challenges in utilizing biodegradable polymeric particles for agricultural purposes. Unlike previous reviews, which broadly cover polymer development, this work emphasizes the practical implementation of BPPs in agriculture, assessing their effectiveness, scalability, and environmental benefits. By identifying research gaps and providing actionable recommendations, this review aims to guide future innovations and promote the adoption of biodegradable polymeric particle based technologies for sustainable farming practices.
2. Significance of biodegradable polymeric particles in the agricultural sector
Biodegradable polymeric particles offer innovative solutions to overcome the limitations of conventional agricultural practices by enhancing efficiency, sustainability, and environmental stewardship.62 Traditional fertilizer applications often lead to nutrient runoff by leaching and uneven distribution, causing environmental pollution and inefficient nutrient use. Encapsulating fertilizers in biodegradable polymeric particles allows for controlled release, reducing leaching and runoff while ensuring prolonged nutrient availability and improved plant uptake.63Polymeric particles are always found to be better in terms of accuracy and effectiveness of agrochemicals' delivery than other forms of polymers.64 Similarly, conventional pesticide applications face challenges such as drift, non-target effects, and the need for frequent reapplication due to rapid degradation or wash-off. Biodegradable polymeric particles with encapsulated pesticides reduce drift, provide target applications more accurately, and produce slow-release formulations that extend efficacy without compromising the environment by lowering overall pesticide use.65–67 Moreover, the release profile and responsiveness of particles can be tailored to meet the specific requirements of different plants.66,68,69
In large-scale irrigation systems, water and nutrient wastage is a common issue, particularly in water-scarce regions. Biodegradable polymeric particles enhance soil moisture retention70 and deliver nutrients directly to the root zone, reducing water usage and nutrient wastage. While organic farming primarily relies on natural amendments like compost and manure, it could potentially benefit from biodegradable particles made from natural or biobased polymers, provided these particles contain only organic-compliant actives (without synthetic agrochemicals), such as seaweed extract,71 spinosad72 and other organic agrochemicals.73,74 Since the majority of biodegradable polymers (resourced synthetically or naturally) are biocompatible and FDA approved,75 they can be thought to bring about benefits in sustainable farming without harming the soil health in the long run.42 These particles provide controlled water and nutrient release, enhancing the efficiency of organic fertilizers while supporting the soil health and microbial activity.76
Mechanical weed control methods can be labor-intensive and less effective against certain weed types. However, biodegradable polymeric particles can deliver herbicides in a controlled manner, reducing the need for mechanical intervention without compromising the soil structure.60,77 It is observed that polymeric particles have wide scope in agricultural fields ranging from agrochemical delivery to moisture retention capability of soils.
3. Classification of polymeric particles
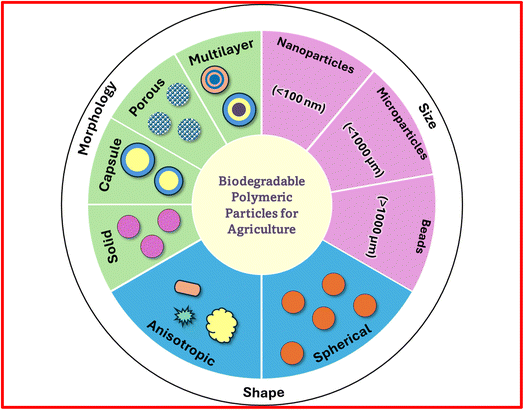
The size, shape, and morphology of polymeric particles are critical factors that influence their behavior in agricultural practices, including how they interact with plants and soils, their degradation rate, and the release profile of the active ingredients they contain. In this review, we have classified the biodegradable polymeric particles based on their size, shape and morphology (Fig. 2).3.1 Size
The size of the polymeric particles significantly impacts their function. For efficient uptake by plants or interaction with soil microbes, particles typically range from nanometers to micrometers in size and their action changes with their sizes. For instance, Lou et al. developed phoxim-loaded polyurethane microcapsules (MCs) with three different average diameters: 1.39 μm (MC-S), 5.78 μm (MC-M), and 23.60 μm (MC-L). In the greenhouse experiments, MC-S and MC-M showed insecticidal activity primarily within the first three days, while MC-L maintained its effectiveness from days three to ten post-application. This study also defined direct and secondary pesticide distributions to assess how particle size influences insecticidal activity in the field. The results showed that MC-S's excellent initial activity was due to its wider distribution on organisms' surfaces, greater adherence to pests, and increased resistance to rain washing. MC-L exhibited superior long-term activity due to its light stability, which caused the shell to crack slowly and release phoxim, when exposed to light. Larger MCs increased the pesticide intake by insects and their movement within their digestive systems. Consequently, increasing MC size can enhance pesticide utilization, if a chemical group responsive to alkaline conditions is integrated into the capsule shell. It was observed that by adjusting particle size, the transfer and release behavior of pesticide from MCs can be regulated in the field.78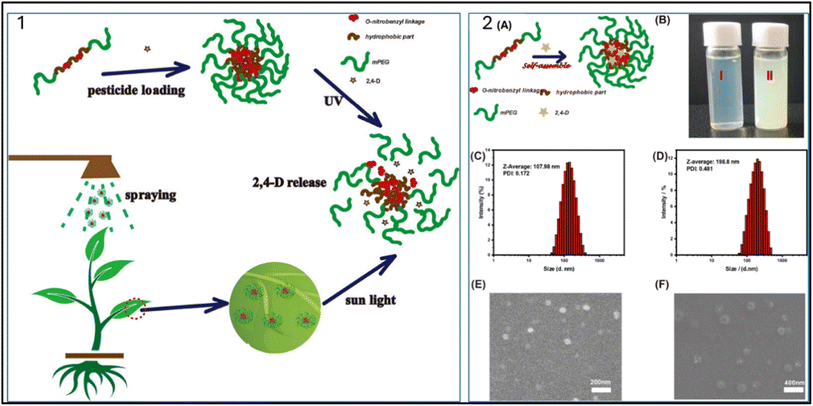 | ||
| Fig. 3 (1) Application of UV-responsive nanoparticles for 2,4-D release and (2) (A) self-assembly of dextran and cellulose to form nanoparticles, (B) formulation of blank and loaded nanoparticles respectively, (C) and (D) size distribution of nanoparticles and (E) and (F) SEM images of blank and loaded nanoparticles, respectively.68 | ||
A study by Wang et al. investigated the utilization of nano-delivery methods for avermectin (Av) with regulated particle sizes, showcasing enhanced controllable release, photostability, and biological activity. Reduced particle sizes led to higher rates of Av release and enhanced biological activity because of greater surface area exposure. These nano-delivery methods exhibited superior penetration and a more gradual and regulated release on target crops in comparison to traditional microcapsules. In addition, they demonstrated excellent storage stability and improved effectiveness, which might potentially lead to a decrease in the need for frequent pesticide application.79
In another study conducted by Carvalho et al., they developed zein nanoparticles loaded with the herbicide atrazine, to demonstrate their high efficiency in pre-emergence weed control. The nano-formulation proved effective against the target weed (Brassica juncea) at a dose 80 times lower than recommended, without harming the crop species (Zea mays). Encapsulation of atrazine in nanoparticles did not increase its mobility in the soil, indicating a minimized environmental impact. The nanoherbicide remained in the upper soil layers, effectively targeting the seed bank. The results of the study showed that the nanoparticles were absorbed and accumulated in the roots of both target and non-target plants, with limited transport to the shoots.80
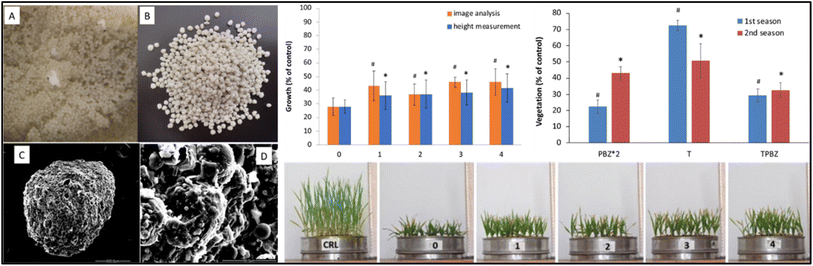 | ||
| Fig. 4 The impact of alginate/clay beads with PCL-PBZ microparticles on oatmeal growth, showing the beads pre- and post-lyophilization (A and B), SEM details (C and D), vegetation growth in treated soil over up to four rainy seasons, and sprout development on sieves after water exposure simulating up to five rainy seasons.64 | ||
In summary, the size of polymeric particles plays a critical role in determining their application efficiency and biological performance in agricultural systems. Nanoscale particles (<100 nm) exhibit enhanced reactivity, penetration, and targeted delivery capabilities, making them ideal for applications requiring precision and controlled release, as demonstrated by nanoherbicide and nanopesticide formulations.79 Microscale particles (<1000 μm) offer a balance between sustained release and scalability, particularly for applications like soil-targeted herbicides. Beads (>1000 μm), with their ability to provide multi-year-controlled release, are well-suited for long-term nutrient delivery and crop growth regulation. The choice of particle size must therefore align with the specific agricultural objective, ensuring optimal efficacy while minimizing environmental impact.
3.2 Shape
Apart from size, the shape of polymeric particles too has a significant impact on their action in the agricultural field.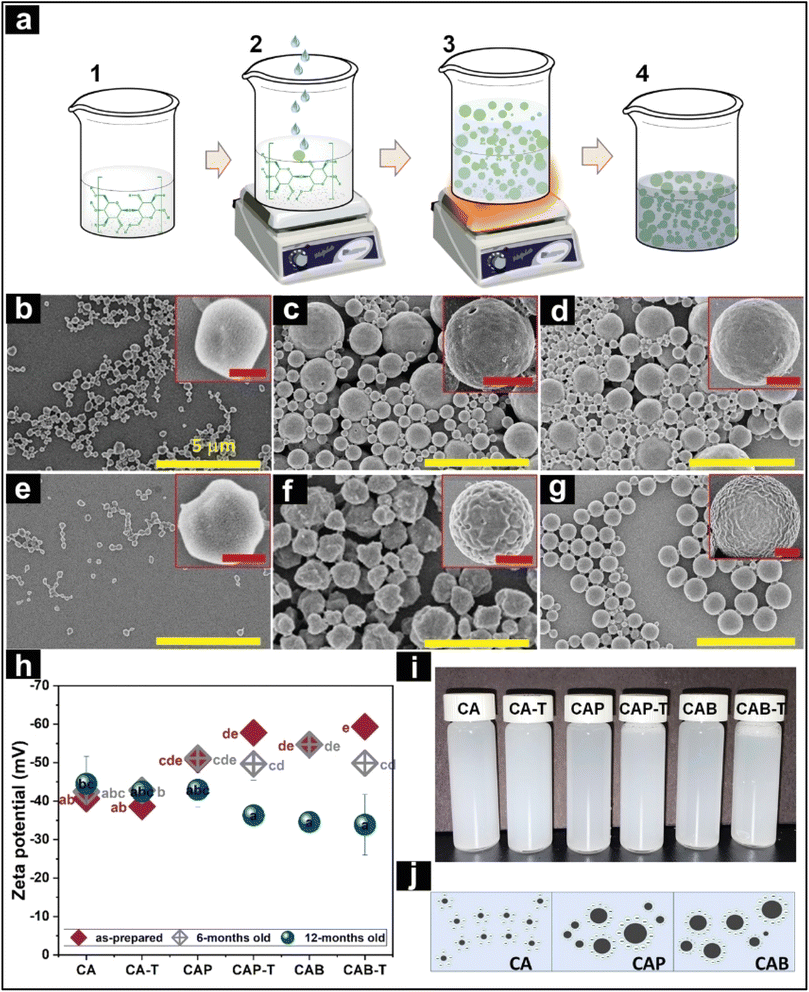 | ||
| Fig. 5 (a) Fabrication of cellulose based microparticles, (b–g) SEM images of various cellulose based microparticle systems, (h) zeta potential of different systems, (i) aqueous dispersion of different microparticle systems, and (j) schematic representation to depict the dispersion stability of CA (cellulose acetate), CAP, and CAB particles.83 Reused with permission from the publisher. | ||
3.3 Morphologies
The surface morphology of the polymeric particles predominantly governs their action in delivering agrochemicals, as discussed below.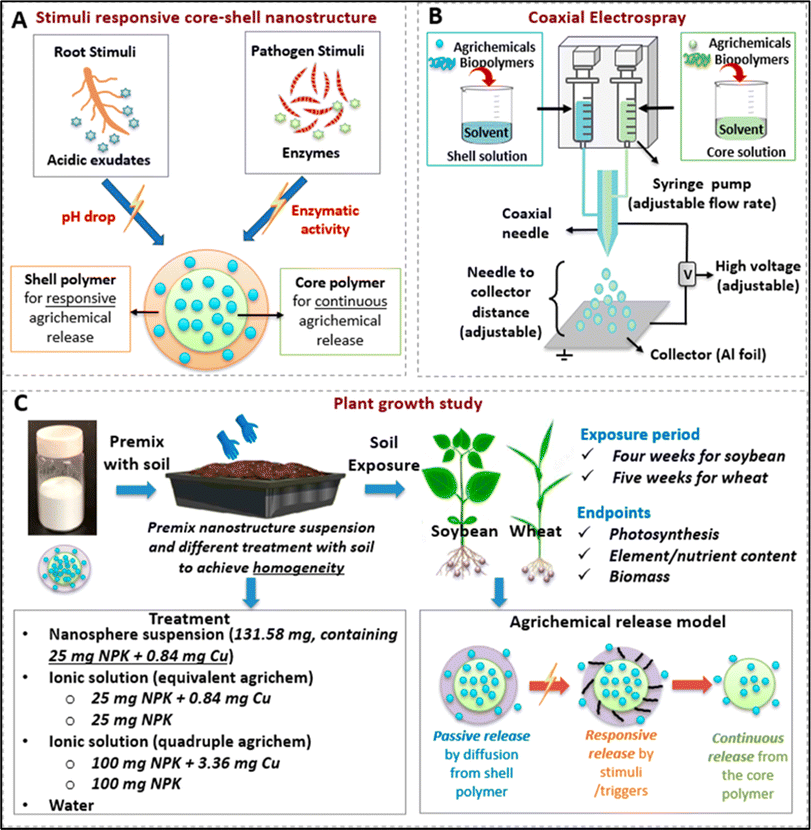 | ||
| Fig. 6 (A) pH and enzyme responsive activity of nanoparticles, (B) fabrication of core–shell nanoparticles using a coaxial needle setup, and (C) soil study and results of treatment with nanoparticles.90 Reused with permission from the publisher. | ||
The findings validated the specificity of the plants; in the case of soybean, the nanostructures led to a 34.3% increase in relative chlorophyll content and a 41.2% increase in the value of photosystem 1 (PS1) centers in photosystem I, as compared to the ionic control with an identical quantity of agrochemicals. The nanostructures resulted in a 37.6% increase in the LEF (linear electron flow) value of wheat as compared to ionic agrichemicals administered at a concentration four times higher. This shows that the responsive core–shell nanostructure is an efficient method for delivering agrichemicals with precision while limiting the amount needed. Moreover, the presence of the nanostructure amendment led to a substantial rise in the zinc (Zn) and sodium (Na) levels in the leaves of four-week-old soybean seedlings. Therefore, the created nanostructures have the potential to improve the accumulation of additional vital micronutrients, indicating a feasible approach for biofortification.90
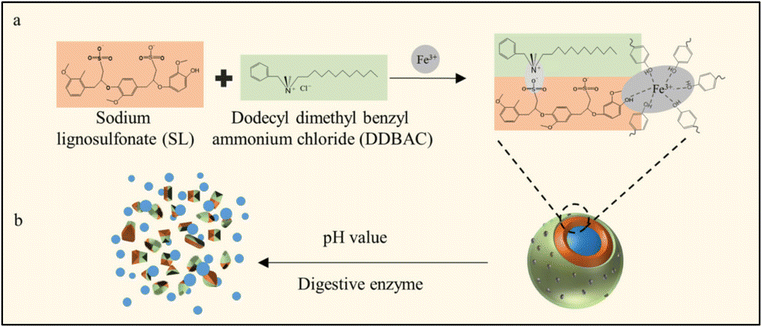 | ||
| Fig. 7 (a) Synthesis of multilayer microparticles. (b) Degradation of microparticles under the influence of pH change and enzymes.93 Reused with permission from the publisher. | ||
The shape and morphology of polymeric particles significantly influence their performance in agricultural applications, dictating the release profiles, environmental stability, and overall efficacy of the active ingredients. Spherical particles, with their ease of manufacture and uniformity, are well-suited for controlled and sustained release applications, while anisotropic shapes demonstrate superior adhesion properties, particularly for foliar applications.
Morphological innovations, such as porous structures, core–shell designs, and multilayer particles, offer advanced capabilities for precision delivery and responsiveness to environmental stimuli. By tailoring shape and morphology to specific agricultural needs, these polymeric particles hold immense potential to enhance agrochemical efficiency, reduce environmental impact, and improve crop resilience under varying conditions. However, the efficacy of biodegradable polymeric particles in agricultural applications depends largely on the efficiency and precision of their fabrication techniques. Some common fabrication techniques of biodegradable polymeric particles are discussed next.
4. Fabrication techniques of biodegradable polymeric particles
Several methods are employed to produce biodegradable polymeric particles as per the requirements based on the end application. These include scalable and customizable production techniques, materials' versatility with diverse sources and functionalization capabilities, and economic benefits from cost-effective production, regulatory compliance, and improved marketability.4.1 Emulsion solvent evaporation method
This widely utilized approach involves the creation of an emulsion, either oil-in-water or water-in-oil, depending on the nature of agrochemicals and polymers used. The polymer is usually dissolved in the organic phase (oil), while the aqueous phase contains a stabilizer. Through solvent evaporation, the organic solvent is removed, leading to the solidification of the polymer and the formation of particles (Fig. 8).3,66,94–97 Taverna et al. investigated the potential of PLA/lignin based microparticles for encapsulating azadirachtin (AZD) (hydrophilic pesticide) and protecting it from photodegradation. The encapsulation efficiency of microparticles with a 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 ratio was 23.25%, demonstrating that the higher lignin content enhances the bio-pesticide's encapsulation efficiency. Release assays in water indicated a slower release rate for formulations with higher PLA content. Moreover, AZD showed greater photodegradation as a free compound as compared to when encapsulated in microparticles, indicating the enhanced stability of actives in the encapsulated form.94
45 ratio was 23.25%, demonstrating that the higher lignin content enhances the bio-pesticide's encapsulation efficiency. Release assays in water indicated a slower release rate for formulations with higher PLA content. Moreover, AZD showed greater photodegradation as a free compound as compared to when encapsulated in microparticles, indicating the enhanced stability of actives in the encapsulated form.94
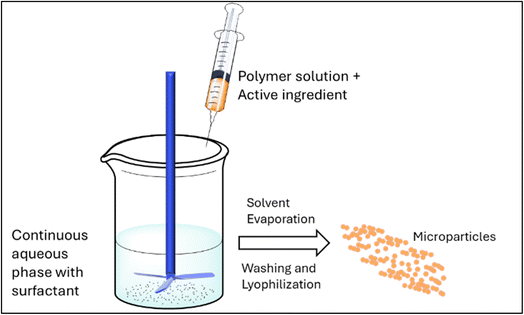 | ||
| Fig. 8 Illustration of the solvent evaporation technique for fabrication of active ingredient-loaded microparticles. | ||
Lopes et al. developed an emulsion/cross-linking method to enclose Bacillus megaterium in starch/PVA microparticles. STMP (trisodium trimetaphosphate) was used as a cross-linking agent. Both types of microparticles retained high cell viability after encapsulation, with controlled release profiles. The bacteria that were enclosed did a better job of retaining their cell viability under heat and fungicide stress than free bacteria. The efficacy of particles was well preserved during storage.49
4.2 Anti-solvent nanoprecipitation
This technique involves the precipitation of a solute from a solution using an antisolvent in which the solute is insoluble. The process begins with the solute, such as a drug or polymer, dissolved in a solvent like ethanol or acetone. This solute solution is then rapidly introduced into an antisolvent, typically water or an aqueous solution, under vigorous stirring. The rapid mixing creates a supersaturated environment, causing the solute to precipitate out of the solution as small nuclei. These nuclei grow into nanoparticles as additional solute molecules aggregate onto them. To prevent these nanoparticles from clustering together, stabilizers or surfactants are added; these molecules adsorb onto the nanoparticles' surface, providing a barrier against aggregation. The sudden change in the environment triggers the rapid precipitation of the polymer into nanoparticles (Fig. 9).96,98 This technique offers good control over particle size by adjusting polymer concentration and mixing ratios. Encapsulation efficiency for hydrophobic agents may be limited; scaling up to produce large quantities can be challenging.80 Researchers in Poland used this method to fabricate particles with porous architectures and dimensions ranging from 2 to 6 μm. Subsequently, the efficacy of the quercetin-loaded microparticles in fertilizing Pisum sativum L. (green peas) was assessed. The study found that using biocompatible and biodegradable carriers that are loaded with flavonoids (hydrophobic) improves plant growth.88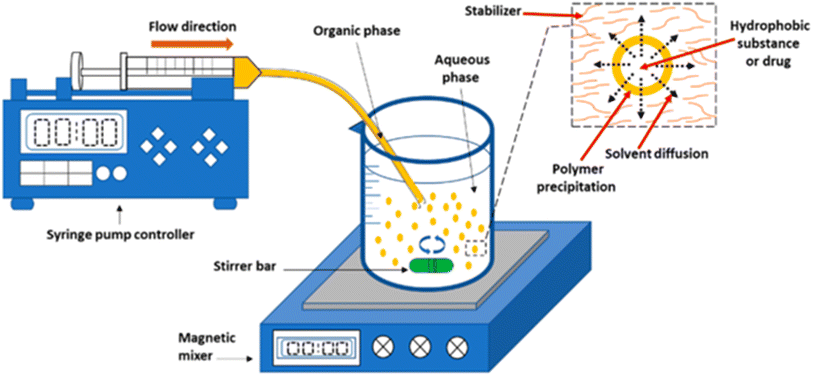 | ||
| Fig. 9 Schematic representation of the anti-solvent nanoprecipitation method.99 Adapted from an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). | ||
4.3 Ionotropic gelation
This technique is used to synthesize nanoparticles, particularly those based on polysaccharides. This method is based on an electrostatic interaction between oppositely charged types that contain at least one polymer under mechanical stirring conditions (Fig. 10).100 Chitosan, a polysaccharide obtained from the shells of crustaceans, is often used in this method. The process involves dissolving polysaccharides such as alginate, gellan, and pectin in water or in a weakly acidic medium (for chitosan). The ionotropic gelation method can also be used with chitosan and negatively charged polyanion groups of sodium tripolyphosphate (TPP) to create chitosan nanoparticles.101 The chitosan/S-nitrosoglutathione nanoparticles (CS-GSNONPs) exhibited prolonged and controlled release of nitric oxide (NO) gas, in contrast to free GSNO. This suggests that the inclusion of GSNO in CSNPs safeguards the donor of NO from rapid degradation and enables optimal release of NO. The resilience of soybean plants to drought was significantly improved by the application of CS-GSNONPs, evidenced by the substantial increase in plant height, biomass, root length, root volume, root surface area, and the number of root tips, forks, and nodules. Follow-up analyses revealed a significant reduction in electrolyte leakage, an increase in proline content, heightened catalase and ascorbate peroxidase activities, and lower levels of malondialdehyde (MDA) and hydrogen peroxide (H2O2) after treatment with 50 μM CS-GSNONPs during drought stress. Further investigations using quantitative real-time PCR indicated that CS-GSNONPs alleviated drought-induced stress by controlling the expression of drought stress marker genes, such as GmDREB1a, GmP5CS, and GmDEFENSIN, as well as NO-related genes GmGSNOR1 and GmNOX1.101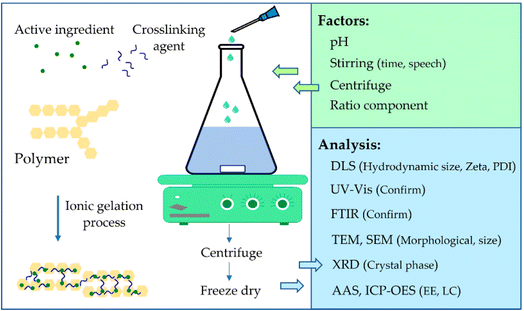 | ||
| Fig. 10 Illustration of the ionotropic gelation method.102 Adapted from an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). | ||
Zheng et al. fabricated Ca-alginate/poly(N-isopropylacrylamide)@polydopamine (Ca-alginate/PNIPAm@PDA) microparticles designed for controlled agrochemical release. These multi-responsive microspheres respond to pH, temperature, and sunlight, making them ideal for agricultural applications. The resulting microspheres were strengthened by a semi-interpenetrating network, composed of a hybrid combination of physically cross-linked Ca-alginate and chemically cross-linked long-chain PNIPAm, which is then coated with PDA. The composite microspheres exhibit sensitivity to sunlight because of the remarkable photothermal conversion of the PDA shell. The Ca-alginate chain, which is sensitive to changes in pH, and the PNIPAm chain, which is sensitive to changes in temperature, were responsible for the effects of the external environment on their water absorbency behavior.103
4.4 Spray drying
In this method, a polymer solution is atomized into a hot drying chamber. Rapid solvent evaporation results in the formation of dry particles. This technique is easily scalable and suitable for continuous production on a large scale (Fig. 11). It provides good control over particle size and morphology.72,104 Perez et al. developed a method for encapsulating the photosensitive spinosad (SP) (hydrophobic) into chitosan (CH) and sodium lignosulfonate (SL) microparticles. The microparticles were acquired through the process of spray drying and were assessed for their shape using scanning electron microscopy (SEM) and particle size. The in vitro release assays exhibited an initial rapid release of the bioinsecticide, which was followed by a gradual and sustained release. The findings of this study indicate that the bioinsecticide carrier system, made from natural polymeric materials, has excellent resistance to photodegradation and effectively releases insecticide. This system may be employed as a viable approach to mitigate the adverse environmental effects associated with agronomic activities.72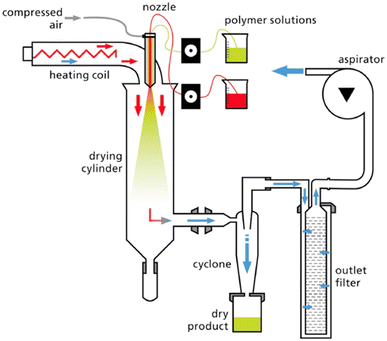 | ||
| Fig. 11 Schematic of the spray drying process.105 Reused with permission from the author. | ||
Saberi-Rise and Moradi-Pour fabricated chitosan-based microcapsules loaded with bacterial inoculants using the spray drying method. They found that the quantity of Streptomyces fulvissimus Uts22 bacteria in chitosan-gellan gum microcapsules, generated using this technique, was around 108 CFU g−1 after being stored for a duration of 2 months.106
The fabrication techniques for biodegradable polymeric particles play a pivotal role in tailoring their properties for diverse agricultural applications. Methods like emulsion solvent evaporation and spray drying offer scalability and versatility, making them suitable for large-scale production with controlled particle sizes and morphologies. Advanced techniques like anti-solvent nanoprecipitation and ionotropic gelation enable the design of specialized nanoparticles with enhanced delivery efficiency and environmental responsiveness. These fabrication approaches not only ensure the stability and efficacy of the active ingredients but also contribute to sustainable agricultural practices by minimizing environmental impact. The choice of the fabrication method depends on the specific application requirements, such as particle size, release profile, and compatibility with active ingredients, highlighting the importance of method selection in optimizing performance and scalability for agricultural innovations (Tables 1 and 2).
| Technique | Advantages | Limitations | Scalability | Particle size control | Encapsulation efficiency | References |
|---|---|---|---|---|---|---|
| Emulsion/solvent evaporation | Simple and versatile, good control over particle size, allows encapsulation of various agents | Moderate encapsulation efficiency, potential for residual organic solvents, difficulty in achieving very small particle sizes | Moderate | Good | High | 49 and 107 |
| Spray drying | Scalable and continuous process, suitable for large-scale production, good control over particle size and morphology | High temperatures may degrade sensitive biomolecules, potential for irregular encapsulation efficiency | High | Good | Moderate | 72 and 108 |
| Antisolvent nano-precipitation | Simple and efficient method for producing small nanoparticles, good control over particle size through adjustments in polymer concentration and mixing ratios | Limited encapsulation efficiency for hydrophobic agents, potential challenges in scaling up production for large quantities | Moderate | Good | Low | 93 |
| Ionotropic gelation | Simple and biocompatible, a wide range of polysaccharides can be used, excellent encapsulation of biomolecules | Limited control over size and morphology (especially after drying), particle properties can be influenced by ionic strength | High | Moderate | Moderate-high | 109 |
| Polymer | Technique | Particle morphology | Active ingredient | Effect | References |
|---|---|---|---|---|---|
| Alginate + starch | Ionotropic gelation | Microbeads | L-tryptophan and B. pumilus (bacteria) | Plant growth promoter by accelerating auxin production and enhancing the drought tolerance of plants | 76 |
| Cellulose nanofibrils (CNFs) | Spray drying | Microcapsules and microspheres (spherical) | KNO3 | Enhanced nutrient uptake and controlled release of the fertilizer for improved plant growth efficiency | 104 |
| Chitosan | Ionotropic gelation | Nanoparticles | S-Nitrosoglutathione | Enhanced root development under drought stress conditions | 101 |
| Chitosan/sodium lignosulfonate | Spray drying | Microparticles | Spinosad | Sustained release of photosensitive insecticides | 72 |
| Chitosan/gum Arabic | Emulsion and ionotropic gelation | Nanoparticles | Geraniol | Modulated release of geraniol to attract whiteflies (pest management) and UV protection of active material | 155 |
| Cellulose-alginate complex | Ionotropic gelation | Beads | Imazethapyr | Sustained release of the herbicide in the field | 156 |
| Lignin/PLA blend | Solvent evaporation method | Microparticles | Azadirachtin | Tunable particles for sustained release of biopesticides | 94 |
| Polylactic acid (PLA) | Solvent evaporation method | Microparticles | Di(t-butanol) dithiophosphate phenethylamine | Sustained release of H2S for enhanced growth of radish plants | 95 |
| Polylactic acid (PLA) | Solvent evaporation method | Microparticles | Buprofezin | Protection against insects and pests like mealybugs, whiteflies and leafhoppers | 66 |
| Poly(salicylic acid) | Nanoprecipitation | Nanoparticles (spherical) | Acifluorfen | Enhanced herbicidal activity and lower environmental pollution | 157 |
| Poly(ε-caprolactone) (PCL) | Solvent evaporation method | Microparticles | Paclobutrazol | Plant growth retardation | 64 |
| Polydopamine (PDA) | Self-assembly | Microcapsules (spherical) | Pyraclostrobin | Controlled release of the pesticide, photostability under UV light | 158 |
| Polyhydroxyalkanoates (PHAs)–PHB and PHBV | Solvent evaporation method | Microparticles | Metribuzin, tribenuron-methyl, fenoxaprop-P-ethyl | Sustained release of herbicides studied on the Elsholtzia ciliata plant | 96 |
| Zein | Nanoprecipitation | Nanoparticles | Atrazine | Targeted and sustained release of the herbicide | 159 |
5. Tailoring properties of polymeric particles for desired applications
The effectiveness of biodegradable polymeric particles in agriculture hinges on a delicate balance between their material properties and the specific application for which they have been designed. Controlling the size, shape, and surface topography of the particles influences their uptake by plants, interaction with soil, and release characteristics. Herein, we delve into the key properties and characteristics that influence the suitability of these particles for various agricultural uses.5.1 Physical properties
A critical characteristic of a particle is the rate at which the encapsulated agent (nutrients, pesticides, etc.) is released from the particle. Ideally, the release profile should be tailored in such a manner that it fulfills the specific needs of the plant at different stages of its growth cycle.64 Factors like polymer type, particle size, and surface modifications can be used to control the release rate. Varona et al. fabricated particles through PGSS (particles from gas-saturated solutions) drying, which typically exhibited two distinct morphologies: spherical particles and small irregularly shaped crystals and needles.110 The PGSS and PGSS-drying methods differed significantly in their encapsulation processes, materials used, operating conditions, and particle characteristics. PGSS mixed molten polyethylene glycol (PEG) and essential oil with CO2 under high pressure, rapidly expanding the mixture to form microcapsules. In contrast, PGSS-drying employed an oil-in-water emulsion with n-octenyl succinic (OSA)-starch as a surfactant, mixed with CO2, and then expanded to remove water and form microcapsules. While PGSS used PEG as the encapsulating material, PGSS-drying uses OSA-modified starches. Operating conditions for PGSS were milder, as it did not require water removal, unlike PGSS-drying, which involved higher temperatures and pressures for water removal. As a result, PGSS produced spherical particles with higher encapsulation efficiency (14–66%), whereas PGSS-drying resulted in both spherical and needle-like particles with lower encapsulation efficiency (6–52%). These differences affected the efficiency, particle size, and morphology of the encapsulated essential oils. Overall findings from the emulsion study suggest that particle morphology, size distribution, and encapsulation efficiency are influenced not only by the precipitation process, but also by the solvent evaporation process. Consequently, it can be inferred that the PGSS-drying technique has its own limitations, particularly when needle morphology is formed, which is unsuitable for achieving controlled oil release. In contrast, PGSS yielded dispersed spherical particles with some degree of agglomeration. This, coupled with the higher encapsulation efficiency observed in PEG encapsulation via PGSS, leads to the conclusion that among the two processes investigated, PGSS is better suited for lavandin type essential oil encapsulation.110Next-generation particles are being developed to incorporate stimuli-responsive features. These particles can release their cargo in response to environmental cues like temperature, pH, or moisture levels. The targeted release ensures that the encapsulated agent is delivered, when it is most needed by the plant, minimizing waste and maximizing efficiency. A novel, scalable, and environmentally friendly approach was developed for creating multi-stimuli responsive nanostructures from biopolymers, aimed at delivering agrichemicals effectively. Using a coaxial electrospray method, these core/shell nanostructures were fabricated with meticulous parameter analysis and optimization, resulting in spherical structures around 160 nm in diameter. These structures demonstrated responsiveness to pH and enzymes, as evidenced by the release kinetics of model agrichemicals (Fig. 12). The core and shell compositions of the nanoparticles were carefully selected to optimize their agricultural performance. The core primarily consists of polycaprolactone (PCL), chitosan, and cellulose acetate, along with a higher concentration of agrichemicals like CuSO4 and NPK fertilizer. PCL was chosen for its biodegradability and ability to improve the processing stability and morphology of the nanostructures. Chitosan provides pH-responsive properties, transitioning between soluble and insoluble forms around pH 6.0–6.5, while cellulose acetate adds hydrophobicity to control the release of agrichemicals. The shell includes starch, PCL, chitosan, cellulose acetate, and zein, with varying concentrations of CuSO4 and NPK depending on the desired release rate. Starch and zein were selected for their enzyme responsiveness, as they can be degraded by soil fungi. The shell's composition was adjusted to be more hydrophilic or hydrophobic to achieve different release profiles, ensuring efficient delivery and utilization of nutrients while minimizing the environmental impact. Field trials with soybean and wheat plants indicated that these responsive nanostructures outperformed conventional agrichemicals, even when applied at higher concentrations. Furthermore, soybean seedlings treated with nanostructures showed significant increases in zinc and sodium contents in their leaves, highlighting the precision and efficacy of agrichemical delivery.90
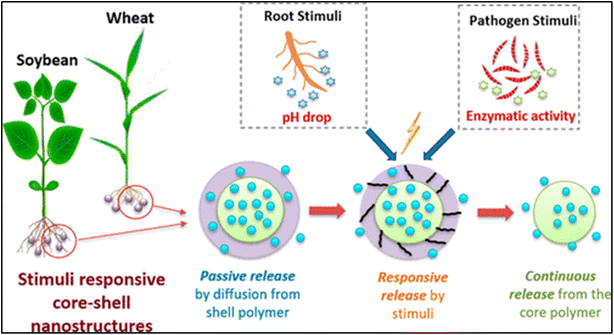 | ||
| Fig. 12 Stimuli responsive nanoparticles.90 | ||
Responsive microgel particles based on octyl-functionalized alginate were fabricated to control the release of agrochemicals and capture heavy metal ions in agriculture. These microgel particles demonstrated efficient loading of the hydrophobic herbicide such as diuron, with its release controlled by adjusting the glutathione (GSH) levels and pH of the medium. Extended release of diuron was achieved, with approximately 100% released after 380 h in 2 mM GSH and about 72% released after 240 h at pH 5. These microgel particles were found to be non-toxic to HEK293T cells up to concentrations of 150 μg mL−1. Moreover, GSH-reduced microgels were also effective in capturing Cu2+ and Hg2+ heavy metal ions.69
5.2 Surface modifications
The surface properties influence the release behavior of the encapsulated agent (nutrients, pesticides, etc.) from the particle. Particles with a hydrophilic surface readily absorb water. This promotes water diffusion into the particle matrix, facilitating the dissolution and release of the encapsulated agent.111 This is advantageous for delivering water-soluble nutrients or beneficial microbes, while those with a hydrophobic surface will repel water. The hydrophobic surface hinders water diffusion into the particle and slows down the release of the encapsulated agent. This can be beneficial for hydrophobic pesticides or herbicides, as it prevents premature release and potential environmental contamination.66 Modifying the surface properties of the particle can influence its interaction with other molecules and its behavior in the soil environment. For instance, hydrophilic surfaces promote water absorption and improve interaction with plant roots, while hydrophobic surfaces can be used for controlled release of hydrophobic pesticides.112 Specific functional groups can be attached to the particle surface to enhance targeted delivery.113,114 These groups can bind to receptors on plant roots or specific types of soil particles, ensuring that the particles reach the desired location for optimal effectiveness.115 Nanoparticles, which are responsive to plant phloem's elevated pH, were engineered to create a targeted delivery system for plants. Photosystem 1 (PS1) is one of the two protein complexes embedded in the thylakoid membrane within chloroplasts of plant cells. It is responsible for light capture, electron transfer, proton pumping and production of NADPH (Nicotinamide Adenine Dinucleotide Phosphate Hydrogen) molecules. Amphiphilic copolymers derived from PS1 were synthesized by incorporating various amines, allowing for flexible adjustment of the hydrophilic–hydrophobic balance, essential for nanoparticle formation. Modulating the level of incorporation and the conditions of nanoprecipitation emerged as effective strategies for controlling the nanoparticles' size, which could prove advantageous in developing novel delivery systems. These nanoparticles, loaded with a hydrophobic model compound, exhibited controlled release under alkaline conditions, with release rates increasing with higher solution pH and lower degrees of incorporation. Additionally, the toxicity of the polymers on plant tissue was evaluated, revealing minimal toxicity at reasonable polymer concentrations.1166. Applications of biodegradable polymeric particles in agriculture
Biodegradable polymeric particles are revolutionizing agriculture by offering a targeted and controlled approach to delivering essential elements to plants and soil. This section delves into the exciting applications of these particles in various agricultural aspects, highlighting their potential for sustainable farming practices.6.1 Controlled release systems for agrochemicals
One of the most promising applications of biodegradable polymeric particles involves the controlled release of agrochemicals like fertilizers, pesticides, and herbicides. Traditional application methods often lead to significant losses due to factors like leaching, volatilization, and runoff. These losses not only reduce efficiency but also contribute to environmental pollution.Biodegradable polymeric particles act as carriers, encapsulating agrochemicals and releasing them at a controlled rate over a predetermined period. This targeted delivery offers several advantages:
• Reduced environmental pollution. By minimizing excess uses, these particles significantly reduce the risk of groundwater contamination and soil pollution from agrochemicals.43
• Improved nutrient efficiency. Controlled release ensures that nutrients are available to plants throughout their growth cycle, maximizing uptake and minimizing waste.69,92,117
• Enhanced efficacy of pesticides/herbicides. Targeted delivery to specific areas or pests improves the effectiveness of these chemicals while reducing the risk of harming beneficial insects and non-target plants.67,69,118,119
A few researchers at the University of Massachusetts combined biodegradable polymers with urea phosphate to create “smart fertilizers” to promote sustainable agricultural practices. Urea phosphate (UP) is used as a water-soluble fertilizer to treat the deficiency of phosphorus in alkaline soils. Slow diffusion of phosphate through coatings of slow-release fertilizers has been identified as a bottleneck in the supply of nitrogen–phosphorus–potassium (NPK) nutrients. They investigated how the structure of the polymer matrix affects release kinetics using biodegradable polyesters and their copolymers such as poly(hexamethylene succinate) (PHS), PBHS 30/70, and PBHS 70/30 (where 30/70 and 70/30 indicate the w/w composition of butylene succinate and hexamethylene succinate, respectively). Composite materials comprising UP and polyester, manufactured through melt processing, were studied to evaluate UP loading efficiency, as well as the distribution and dispersion of the phosphate salt within the polymer matrix. The study showed that the loading levels of urea phosphate in the composites were consistent and this method to develop controlled-release fertilizers can be used, not only in agriculture but also in other controlled-release applications.124
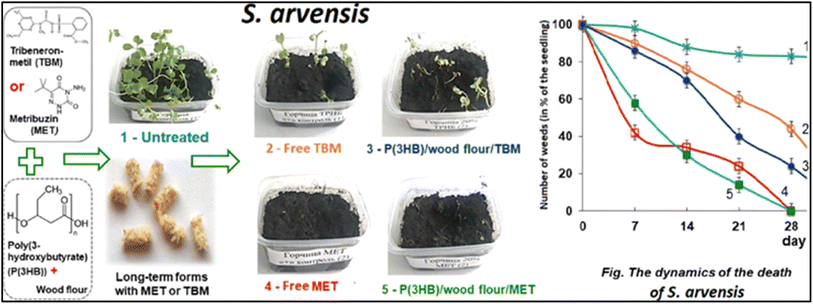 | ||
| Fig. 13 Studying the effect of metribuzin and tribenuron methyl on controlling weed growth.127 | ||
The formulations of poly-3-hydroxybutyrate (PHB) encapsulated with two herbicides, metribuzin (MET) and tribenuron-methyl (TBM), presented as films and microgranules, were tested against weed species with “wheat” (T. aestivum) as the main crop. Both the film and microgranules exhibited substantial efficacy in controlling weed infestation when compared to market formulations. The study concluded that degradable PHB could be a promising material for making slow-release herbicide formulations for pre-emergence weed treatment. Additionally, wheat treated with the experimental formulations exhibited notably higher biomass as compared to commercial formulations.127
Pereira et al. have demonstrated that the encapsulating atrazine (herbicides) proved to be efficient, resulting in stable formulations with modified release profiles governed by anomalous transport. The encapsulated herbicides showed enhanced efficacy against target organisms without harming non-target ones, suggesting improved bioavailability. Additionally, they increased atrazine's mobility in soil, enhancing effectiveness against target organisms while being less genotoxic as compared to the free herbicide.128
6.2 Soil improvement and crop protection
Beyond the controlled release of agrochemicals, biodegradable polymeric particles offer additional benefits for soil health and crop protection.For instance, Kathi et al. evaluated the efficacy of corn starch-based SAP (super-absorbent polymer) particles in decreasing leachate and enhancing water accessibility for plants. Findings indicated that SAP particles derived from cornstarch augmented the soil's water-holding capacity, retaining more water, which helped to preserve nitrogen in the soil. Consequently, this led to increased water and nitrate availability for tomato plants, surpassing treatments with soil alone or soil with fertilizer only. Furthermore, the water and nutrient retention rates, which vary from 35% to 91% depending on the application rate of SAPs, show significant potential for conserving water and nutrients.70
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14). This encapsulation protected the bacteria during the spray drying process and aided the activation of bacteria upon addition in the soil.108
14). This encapsulation protected the bacteria during the spray drying process and aided the activation of bacteria upon addition in the soil.108
By facilitating the targeted delivery of essential nutrients, bioactive compounds, and beneficial microbes, biodegradable polymeric particles hold immense potential for enhancing crop yield and quality. Improved nutrient efficiency, increased plant growth, and enhanced stress tolerance can lead to higher yields with optimal quality parameters.63,129,133–135 Additionally, the targeted delivery of pesticides and herbicides minimizes damage to beneficial organisms, promoting a healthier agricultural ecosystem.58,112,136–138
6.3 Critical analysis of application areas
The application of BPPs in agriculture has progressed unevenly across different domains, namely controlled agrochemical release, soil improvement, plant growth and stress tolerance. Below, we critically analyze the state of the art in these areas, highlighting their advancements, delays, and recommendations for future development.Despite these advancements, cost and scalability still remain as challenges. The use of biodegradable polymers, such as polylactic acid (PLA), increases production costs, and achieving consistent particle quality on an industrial scale is an unmet need.141,142
Nevertheless, Controlled-Release Fertilizers (CRFs), such as controlled-release urea (CRU), have demonstrated significant improvements in nitrogen's use efficiency (NUE) and grain yield as compared to conventional urea. For example, incorporating CRU at 300 kg N ha−1 increased NUE by 27.6% and 22.9% in rice as compared to conventional urea over two consecutive years. Additionally, CRU applied at 200 kg N ha−1 (i.e., one-third less nitrogen) produced 3–5.9% higher grain yield than conventional urea applied at 300 kg N ha−1, highlighting CRU's efficiency in meeting the crop nitrogen demand while reducing fertilizer use.5
Field studies in wheat and maize crops have shown that blended CRU formulations increased wheat yields by 7.9–10.3% and maize yields by 9.1–21.0% as compared to normal urea at equivalent application rates. The NUE was enhanced by 33.7–56.4% for wheat and 16.7–48.5% for maize, and the average annual net profit rose by 14.5–19.9%. These results indicate that CRU fulfills the crop nitrogen demand throughout the growth period, minimizes nitrogen losses, and reduces the need for multiple fertilizer applications, lowering both labor and environmental costs.143
Interestingly, CRU closely matched the nitrogen requirements of rice, thus enhancing the root zone's N concentration and leaf enzyme activity (GS, GOGAT, and NR), resulting in reduced fertilizer use and higher yields.144 CRU's environmental benefits are profound. Studies show that blending CRU with conventional urea in bulk blending urea (BBU) systems decreased reactive nitrogen (Nr) losses by 35.6–54.5% and reduced greenhouse gas (GHG) emissions by 34.1–44.7%. Moreover, N and C footprints were lowered by 41.1–60.8% and 41.8–42.3%, respectively, significantly mitigating ecological impacts. These outcomes clearly demonstrate that CRU-based systems effectively balance productivity and sustainability in agricultural practices.5
To bridge this gap, research should focus on field validation of these technologies and evaluating the ecological compatibility of biodegradable polymeric residues.
Advancing this area will require interdisciplinary research to optimize the compatibility of biodegradable particles with plants and soils, as well as the development of cost-effective production methods for scaling up.
Controlled agrochemical release systems should focus on reducing production costs and optimizing scalability to achieve wider adoption. Soil improvement technologies need thorough field testing and ecological impact assessments. Plant growth and stress tolerance applications require scalable encapsulation techniques and comprehensive field validation. The challenges identified—high costs, scalability issues, and regulatory hurdles—are further explored in the next section, where potential solutions to advance these technologies are discussed.
7. Challenges
Despite their immense potential, biodegradable polymeric particles face some significant challenges that need to be addressed prior to their widespread adoption in agricultural fields.• Cost-effectiveness. Currently, the production cost of biodegradable polymeric particles containing actives is significantly higher as compared to conventional fertilizers and pesticides. This can be a barrier for small-scale farmers and limit their widespread applicability. Research efforts focusing on cost-reduction strategies through material selection, production techniques, and large-scale manufacturing are crucial.
• Delivery system optimization. Fine-tuning the delivery system of biodegradable polymeric particles is essential for maximizing their effectiveness. This involves optimizing the particle size, surface properties, and release profiles for specific applications. For instance, particle size needs to be tailored for efficient uptake by plants or soil microbes, while release profiles should match the specific nutrient's requirements at different stages of plant growth. Sometimes, it is difficult to achieve multiple requisites from a single particular system.
• Long-term impact assessment. While biodegradable polymeric particles are designed to decompose naturally, to understand the long-term effects of their degradation products on soil health, detailed investigations are required. Prior to their application, the materials' toxicity and their interactions with biological entities should be evaluated using various techniques such as SAR (structure activity relationship), molecular modelling, predictions based on available data reported in the literature, etc. A thorough evaluation of potential impacts of the released actives as well as the employed polymers on soil microbial communities and overall soil fertility is necessary for ensuring the long-term sustainability of this technology.
8. Future directions
The field of biodegradable polymeric particles in agriculture is an emerging and rapidly evolving area. Current research is moving beyond single-purpose particles towards the development of multifunctional particles that can address multiple agricultural needs simultaneously. Imagine particles that encapsulate both essential nutrients and pesticides and simultaneously deliver a controlled dose of pest control agents and nutrients at a desired location. Multifunctional particles streamline application processes, reducing the need for multiple treatments and saving time and resources for farmers, in addition to improving the overall health and economy. Combining functionalities within a single particle can lead to synergistic effects. Some nutrients can enhance the efficacy of certain pesticides, leading to improved pest control with lower doses, thereby providing environmental and economic benefits. The development of multi-functional particles offers another frontier for exploration. For instance, encapsulating multiple agrochemicals within a single particulate system may further enhance agricultural efficiency and sustainability. For example, combining a pre-emergent herbicide with a nitrogen fertilizer in one polymer matrix controls weeds while supplying essential nutrients to the desired plant, reducing the need for multiple doses of various actives. These particles may simultaneously deliver nutrients, control pests/unwanted herbs and improve soil health, thus providing comprehensive solutions for sustainable farming practices. Moreover, by minimizing the number of requisite doses, multifunctional particles can further reduce the environmental footprint of agricultural practices, thereby enhancing agricultural sustainability. Dual delivery of insecticides and fungicides in nanocapsules can protect crops like tomatoes from pests and diseases simultaneously. Similarly, a nematicide combined with beneficial microbes in a single carrier may improve root health, while controlling nematodes. In this regard, few studies have already been conducted that showed that dual loading of pesticides in a single polymeric carrier can provide synergistic effects and be more efficient than commercially available formulations.160,161 For instance, Dhiman et al. prepared dextrin-based microgels for slow release of dual fertilizers.162 Other combinations may include pesticides with plant growth regulators, herbicides with soil conditioners and fungicides with bio-stimulants; all may be tailored to enhance the crop health and productivity, while minimizing environmental impact.One more key area for advancement lies in the development of smart and stimuli-responsive particles that release agrochemicals in response to specific environmental triggers such as soil pH, moisture, or temperature. These systems can ensure precise delivery and minimize wastage as well as environmental contamination. Additionally, the potential for biodegradable polymeric particles in biofortification remains largely untapped. By delivering essential micronutrients such as zinc and iron directly to crops, these systems can enhance nutritional quality while maintaining environmental balance. Moreover, most of the degraded fragments of biodegradable polymers may act as nutrients for growing plants, thus further enhancing the soil health and contributing positively to the environment.
To accelerate adoption, large-scale field trials and long-term impact assessments are essential. Such studies would validate the ecological and agronomic benefits of these particles under diverse agricultural conditions. Finally, advancing cost-effective and sustainable fabrication methods such as using agricultural waste as raw materials will make these technologies more sustainable and accessible to small-scale and resource-constrained farmers, driving widespread adoption. By addressing these areas, future research can unlock the full potential of biodegradable polymeric particles, transforming them into essential tools for sustainable and resilient agriculture.
9. Conclusions
Applications of biodegradable polymeric particles in agriculture present a transformative approach to addressing some of the most pressing environmental and sustainable challenges faced by traditional farming. This review highlights the significant potential and versatility of biodegradable particles in providing controlled release of fertilizers, pesticides, and herbicides, as well as their role in soil remediation and plant growth enhancement. Biodegradable polymers offer an eco-friendly alternative to conventional plastics, mitigating the adverse impacts associated with plastic pollution. Their ability to decompose naturally in the soil environment through abiotic and biotic processes ensures minimal accumulation and toxicity, thereby promoting soil health and reducing environmental contamination.While the advancements in active containing polymeric particle fabrication techniques, such as emulsion-based methods, spray drying, coacervation, and nanoprecipitation, have enabled the precise control of particle size, loading efficiency, and release kinetics, challenges remain. These include the high cost and scalability of the production, potential long-term environmental impact of degraded products, regulatory hurdles, and the need for performance optimization. Nevertheless, the future of biodegradable polymeric particles in agriculture still holds immense potential for innovation and impactful applications.
This review highlights the transformative potential of BPPs in advancing sustainable agriculture. By focusing on key applications such as controlled agrochemical release, soil improvement, and plant stress tolerance, it provides a targeted analysis of their utility and addresses the barriers to their adoption, including cost, scalability, and regulatory challenges. Unlike prior studies, this review aligns its discussion closely with agricultural applications, offering a practical framework for researchers and practitioners to develop field-ready solutions. Addressing the identified gaps will not only drive innovation but also contribute to achieving global agricultural sustainability goals.
Ultimately, future research should focus on developing cost-effective and scalable production methods, after thoroughly evaluating the environmental safety of degradation products, and optimizing the performance of active loaded biodegradable polymeric particles. Collaboration between scientists, industry stakeholders, and regulatory bodies will be crucial in establishing clear guidelines and standards to facilitate the adoption of these materials in agricultural practices. In conclusion, biodegradable polymeric particles hold great promise for enhancing agricultural productivity, sustainability, and environmental protection. Continued innovation and research in this field are essential to fully harness their potential and pave the way for a more sustainable and resilient agricultural future.
Data availability
This review article is based on previously published research and does not involve the generation of new datasets. All data supporting the findings and discussions presented are available within the cited references. No new data were created or analyzed in this study. Further information regarding specific studies referenced can be obtained from the corresponding publications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors express their gratitude to the Department of Material Science and Engineering (DMSE), Indian Institute of Technology (IIT), Delhi, and the Department of Science and Technology, India, for the funding and research facility. The authors would like to thank the Central Research Facility (CRF), IIT Delhi, for providing necessary facilities. The authors would like to express their sincere gratitude to the Science and Engineering Research Board (SERB) for funding the research work under the POWER (Promoting Opportunities for Women in Exploratory Research) Scheme (Reference No. SPF/2023/00021). Author Chandrani Sarkar would like to express her deep gratitude to the Science and Engineering Research Board (SERB), India, for funding her research work under the National Post-Doctoral Fellowship Scheme (Reference No. PDF/2022/000679). Authors would also like to acknowledge the use of Quillbot writing tool.References
- R. Phiri, S. Mavinkere Rangappa, S. Siengchin, O. P. Oladijo and H. N. Dhakal, Adv. Ind. Eng. Polym. Res., 2023, 6, 436–450 CAS.
- A. Samir, F. H. Ashour, A. A. A. Hakim and M. Bassyouni, npj Mater. Degrad., 2022, 6, 1–28 CrossRef.
- A. Sikder, A. K. Pearce, S. J. Parkinson, R. Napier and R. K. O'Reilly, ACS Appl. Polym. Mater., 2021, 3, 1203–1217 CrossRef CAS.
- Y. V. Tertyshnaya, M. V. Podzorova, I. A. Varyan, V. V. Tcherdyntsev, M. Y. Zadorozhnyy and E. V. Medvedeva, Polymers, 2023, 15, 1029 CrossRef CAS PubMed.
- X. Xu, F. Ma, J. Zhou and C. Du, Field Crops Res., 2022, 278, 108445 CrossRef.
- H. Jariwala, R. M. Santos, J. D. Lauzon, A. Dutta and Y. Wai Chiang, Environ. Sci. Pollut. Res., 2022, 29, 53967–53995 CrossRef CAS PubMed.
- C. An, C. Sun, N. Li, B. Huang, J. Jiang, Y. Shen, C. Wang, X. Zhao, B. Cui, C. Wang, X. Li, S. Zhan, F. Gao, Z. Zeng, H. Cui and Y. Wang, J. Nanobiotechnol., 2022, 20, 1–19 CrossRef PubMed.
- K. Verma, C. Sarkar and S. Saha, Biodegradable Polymers for Agriculture, in Biodegradable Polymers and Their Emerging Applications, Springer Nature Singapore, Singapore, 2023, pp. 191–212 Search PubMed.
- C. Abbate, A. Scavo, G. R. Pesce, S. Fontanazza, A. Restuccia and G. Mauromicale, Agriculture, 2023, 13, 197 CrossRef CAS.
- B. M. Campbell, P. Thornton, R. Zougmoré, P. van Asten and L. Lipper, Curr. Opin. Environ. Sustain., 2014, 8, 39–43 CrossRef.
- M. W. Aktar, D. Sengupta and A. Chowdhury, Interdiscip. Toxicol., 2009, 2, 1–12 Search PubMed.
- A. Devlet, Frontiers in Life Sciences and Related Technologies, 2021, 2, 21–29 Search PubMed.
- D. Briassoulis, Sci. Total Environ., 2023, 892, 164533 CrossRef CAS PubMed.
- Q. Q. Zhang, Z. R. Ma, Y. Y. Cai, H. R. Li and G. G. Ying, Environ. Sci. Technol., 2021, 55, 12459–12470 CrossRef CAS PubMed.
- I. A. Lakhiar, H. Yan, J. Zhang, G. Wang, S. Deng, R. Bao, C. Zhang, T. N. Syed, B. Wang, R. Zhou and X. Wang, Agronomy, 2024, 14, 548 CrossRef CAS.
- X. Cao, Y. Liang, J. Jiang, A. Mo and D. He, TrAC, Trends Anal. Chem., 2023, 166, 117212 CrossRef CAS.
- M. Sander, Environ. Sci. Technol., 2019, 53, 2304–2315 CrossRef CAS PubMed.
- S. S. Pratibha and P. Hariprasad, J. Cleaner Prod., 2022, 337, 130588 CrossRef CAS.
- N. Beriot, R. Zornoza, E. H. Lwanga, P. Zomer, B. van Schothorst, O. Ozbolat, E. Lloret, R. Ortega, I. Miralles, P. Harkes, J. van Steenbrugge and V. Geissen, Sci. Total Environ., 2023, 900, 165179 CrossRef CAS PubMed.
- C. Maraveas, Agriculture, 2020, 10, 310 CrossRef CAS.
- L. O. Ekebafe, D. E. Ogbeifun and F. E. Okieimen, Biokemistri, 2011, 23(2), 81–89 Search PubMed.
- L. Chang, L. Xu, Y. Liu and D. Qiu, Polym. Test., 2021, 94, 107021 CrossRef CAS.
- A. Dhiman, A. K. Sharma and G. Agrawal, ACS Agric. Sci. Technol., 2022, 2, 693–711 CrossRef CAS.
- M. J. Comstock, Agricultural and Synthetic Polymers, ACS Symposium Series, Foreword: Biodegradability and Utilization, 1990, pp. i–vi Search PubMed.
- M. Sarkar, A. Priya, C. Sarkar and S. Saha, Materials Horizons: From Nature to Nanomaterials, Part F1245, 2023, pp. 1–25 Search PubMed.
- Y. Akbulut and M. Oktav Bulut, Yekarum, 2015, 3(1), 35–44 Search PubMed.
- Y.-R. Seo, J.-W. Kim, S. Hoon, J. Kim, J. H. Chung and K.-T. Lim, J. Biosyst. Eng., 2018, 43, 59–71 Search PubMed.
- K. Sampathkumar, K. X. Tan and S. C. J. Loo, iScience, 2020, 23, 101055 CrossRef CAS PubMed.
- H. Das and S. K. Singh, Crit. Rev. Food Sci. Nutr., 2004, 44(2), 77–89 CrossRef PubMed.
- K. Supare and P. A. Mahanwar, Polym. Bull., 2021, 79, 5795–5824 CrossRef.
- M. Zumstein, G. Battagliarin, A. Kuenkel and M. Sander, Acc. Chem. Res., 2022, 55, 2163–2167 CrossRef CAS PubMed.
- M. T. Zumstein, A. Schintlmeister, T. F. Nelson, R. Baumgartner, D. Woebken, M. Wagner, H. P. E. Kohler, K. McNeill and M. Sander, Sci. Adv., 2018, 4(7), eaas9024 CrossRef CAS PubMed.
- Z. Mansoor, F. Tchuenbou-Magaia, M. Kowalczuk, G. Adamus, G. Manning, M. Parati, I. Radecka and H. Khan, Polymers, 2022, 14(23), 5062 CrossRef CAS PubMed.
- Y. Qi, N. Beriot, G. Gort, E. Huerta Lwanga, H. Gooren, X. Yang and V. Geissen, Environ. Pollut., 2020, 266, 115097 CrossRef CAS PubMed.
- M. Menossi, M. Cisneros, V. A. Alvarez and C. Casalongué, Agron. Sustainable Dev., 2021, 41, 1–27 CrossRef.
- View of Use of Biodegradable Plastic Films in Agriculture and their Fate in Soil, https://www.agronomy.it/agro/article/view/2155/1419, (accessed 15 April 2024) Search PubMed.
- J. Chen, J. Wu, P. Raffa, F. Picchioni and C. E. Koning, Prog. Polym. Sci., 2022, 125, 101475 CrossRef CAS.
- D. Venkatachalam and S. Kaliappa, De Gruyter Open Ltd, 2023, DOI:10.1515/revce-2020-0102.
- M. Vemula and A. V. B. Reddy, Nanotechnology for Environmental Engineering, DOI:10.1007/S41204-023-00319-8.
- C. Maraveas, Agriculture, 2020, 10, 310 CrossRef CAS.
- T. Pirzada, B. V. de Farias, R. Mathew, R. H. Guenther, M. V. Byrd, T. L. Sit, L. Pal, C. H. Opperman and S. A. Khan, Curr. Opin. Colloid Interface Sci., 2020, 48, 121–136 CrossRef CAS PubMed.
- K. Lewicka, I. Szymanek, D. Rogacz, M. Wrzalik, J. Łagiewka, A. Nowik-Zając, I. Zawierucha, S. Coseri, I. Puiu, H. Falfushynska and P. Rychter, Sustainability, 2024, 16, 8439 CrossRef.
- K. Tian and M. Bilal, Abatement of Environmental Pollutants: Trends and Strategies, 2020, pp. 313–330 Search PubMed.
- B. Cheng, B. Pei, Z. Wang and Q. Hu, RSC Adv., 2017, 7, 42036–42046 RSC.
- R. Saberi Riseh, M. Vatankhah, M. Hassanisaadi and R. S. Varma, Int. J. Biol. Macromol., 2024, 260, 129522 CrossRef CAS PubMed.
- K. Supare and P. Mahanwar, J. Polym. Environ., 2022, 30, 2448–2461 CrossRef CAS.
- M. Mujtaba, K. M. Khawar, M. C. Camara, L. B. Carvalho, L. F. Fraceto, R. E. Morsi, M. Z. Elsabee, M. Kaya, J. Labidi, H. Ullah and D. Wang, Int. J. Biol. Macromol., 2020, 154, 683–697 CrossRef CAS PubMed.
- B. E. Channab, A. El Idrissi, M. Zahouily, Y. Essamlali and J. C. White, Int. J. Biol. Macromol., 2023, 238, 124075 CrossRef CAS PubMed.
- M. M. Lopes, L. A. Lodi, C. A. de Oliveira-Paiva and C. S. Farinas, ACS Agric. Sci. Technol., 2024, 2024, 499 Search PubMed.
- A. Gamage, A. Liyanapathiranage, A. Manamperi, C. Gunathilake, S. Mani, O. Merah and T. Madhujith, Sustainability, 2022, 14(10), 6085 CrossRef CAS.
- L. Pang, Z. Gao, H. Feng, S. Wang and Q. Wang, J. Controlled Release, 2019, 316, 105–115 CrossRef CAS PubMed.
- R. Saberi Riseh, Int. J. Biol. Macromol., 2024, 255, 128006 CrossRef CAS PubMed.
- N. A. A. B. Taib, M. R. Rahman, D. Huda, K. K. Kuok, S. Hamdan, M. K. Bin Bakri, M. R. M. Bin Julaihi and A. Khan, Springer Science and Business Media, Deutschland GmbH, 2022, DOI:10.1007/s00289-022-04160-y.
- P. Vejan, T. Khadiran, R. Abdullah and N. Ahmad, J. Controlled Release, 2021, 339, 321–334 CrossRef CAS PubMed.
- D. Lawrencia, S. K. Wong, D. Y. S. Low, B. H. Goh, J. K. Goh, U. R. Ruktanonchai, A. Soottitantawat, L. H. Lee and S. Y. Tang, Plants, 2021, 10, 238 CrossRef CAS PubMed.
- M. Klein and E. Poverenov, J. Sci. Food Agric., 2020, 100, 2337–2347 CrossRef CAS PubMed.
- N. Li, C. Sun, J. Jiang, A. Wang, C. Wang, Y. Shen, B. Huang, C. An, B. Cui, X. Zhao, C. Wang, F. Gao, S. Zhan, L. Guo, Z. Zeng, L. Zhang, H. Cui and Y. Wang, J. Agric. Food Chem., 2021, 69, 12579–12597 CrossRef CAS PubMed.
- A. Singh, N. Dhiman, A. K. Kar, D. Singh, M. P. Purohit, D. Ghosh and S. Patnaik, J. Hazard. Mater., 2020, 385, 121525 CrossRef CAS PubMed.
- K. Ramadhan Makame, M. Sherif, L. Östlundh, J. Sándor, B. Ádám and K. Nagy, Environ. Int., 2023, 174, 107924 CrossRef CAS PubMed.
- S. Marimuthu, P. Pavithran, G. Gowtham, S. Marimuthu, P. Pavithran and G. Gowtham, Pesticides - Updates on Toxicity, Efficacy and Risk Assessment, DOI:10.5772/INTECHOPEN.104629.
- K. Salama and M. Geyer, Environments, 2023, 10, 179 CrossRef.
- A. Dhiman, D. Bhardwaj, K. Goswami and G. Agrawal, Carbohydr. Polym., 2023, 313, 120893 CrossRef CAS PubMed.
- Y. Xiang, C. Li, H. Hao, Y. Tong, W. Chen, G. Zhao and Y. Liu, J. Cleaner Prod., 2021, 294, 126278 CrossRef CAS.
- A. Mun, H. Simaan Yameen, G. Edelbaum and D. Seliktar, Sci. Rep., 2021, 11, 1–12 CrossRef PubMed.
- C. You, L. Ning, Y. Jia, P. Xu, J. Lu, C. Huang and F. Wang, Ind. Crops Prod., 2022, 183, 114938 CrossRef CAS.
- S. Hayashida, R. Saito, K. Watanabe, C. Allard, E. Gaufres, P. Desjardins, R. Ihly, S. van Bezouw, D. Arias, Y. Li, J. Dai, D. Cao, Y. Ma, L. Zhen and F. Chang, IOP Conf. Ser.:Mater. Sci. Eng., 2020, 711, 012026 Search PubMed.
- A. Gupta and A. Dhiman, Polymer Science - Series A, 2023, 65, 744–754 CrossRef.
- P. Shan, Y. Lu, W. Lu, X. Yin, H. Liu, D. Li, X. Lian, W. Wang, Z. Li and Z. Li, ACS Appl. Mater. Interfaces, 2022, 14, 43759–43770 CrossRef CAS PubMed.
- A. Dhiman, A. K. Sharma, D. Bhardwaj and G. Agrawal, Int. J. Biol. Macromol., 2023, 228, 323–332 CrossRef CAS PubMed.
- S. Kathi, C. Simpson, A. Umphres and G. Schuster, HortScience, 2021, 56, 1486–1493 CAS.
- A. Mohamad Jaafar, M. S. Samah and A. S. Abdul Sukor, EDUCATUM Journal Of Science, Mathematics And Technology, 2021, 8, 28–35 CrossRef.
- I. D. Pérez-Landa, I. Bonilla-Landa, J. L. Monribot-Villanueva, M. Ramírez-Vázquez, R. Lasa, W. Ramos-Torres, J. L. Olivares-Romero and F. Barrera-Méndez, Powder Technol., 2021, 377, 514–522 CrossRef.
- R. S. Riseh, Y. A. Skorik, V. K. Thakur, M. M. Pour, E. Tamanadar and S. S. Noghabi, Int. J. Mol. Sci., 2021, 22, 11165 CrossRef PubMed.
- A. Cesari, M. V. Loureiro, M. Vale, E. I. Yslas, M. Dardanelli and A. C. Marques, Sci. Total Environ., 2020, 703, 135548 CrossRef CAS PubMed.
- E. Marin, M. I. Briceño and C. Caballero-George, Int. J. Nanomed., 2013, 8, 3071 Search PubMed.
- S. V. Benítez, R. Carrasco, J. D. Giraldo and M. Schoebitz, J. Microencapsulation, 2024, 41, 170–189 CrossRef PubMed.
- E. Castro, C. Pucci, S. Duarte, N. R. Burgos and T. M. Tseng, Weed Technol., 2020, 34, 647–651 CrossRef.
- J. Luo, X. P. Huang, T. F. Jing, D. X. Zhang, B. Li and F. Liu, ACS Sustain. Chem. Eng., 2018, 6, 17194–17203 CrossRef CAS.
- A. Wang, Y. Wang, C. Sun, C. Wang, B. Cui, X. Zhao, Z. Zeng, J. Yao, D. Yang, G. Liu and H. Cui, Nanoscale Res. Lett., 2018, 13, 2 CrossRef PubMed.
- L. B. Carvalho, I. S. Godoy, A. C. Preisler, P. L. de Freitas Proença, T. Saraiva-Santos, W. A. Verri, H. C. Oliveira, G. Dalazen and L. F. Fraceto, Environ. Sci.:Nano, 2023, 10, 1629–1643 RSC.
- P. Stloukal, P. Kucharczyk, V. Sedlarik, P. Bazant and M. Koutny, J. Agric. Food Chem., 2012, 60, 4111–4119 CrossRef CAS PubMed.
- C. Callaghan, D. Califano, M. H. Feresin Gomes, H. W. Pereira de Carvalho, K. J. Edler and D. Mattia, ACS Sustain. Chem. Eng., 2023, 11, 4749–4758 CrossRef CAS PubMed.
- T. Pirzada, M. Sohail, A. Tripathi, B. V Farias, R. Mathew, C. Li, C. H. Opperman, S. A. Khan, T. Pirzada, M. Sohail, A. Tripathi, B. V Farias, S. A. Khan, R. Mathew, C. Li and C. H. Opperman, Adv. Funct. Mater., 2022, 32, 2108046 CrossRef CAS.
- S. S. Pradhan, C. Sarkar and S. Saha, Materials Horizons: From Nature to Nanomaterials, Part, 2023, F1245, 235–257 Search PubMed.
- K. Pandey and S. Saha, J. Environ. Chem. Eng., 2023, 11, 110493 CrossRef CAS.
- S. Sivalingam, J. S. S. D, G. Golla, L. Arunachalam, T. Singh, K. G, S. A and K. Malaichamy, Colloids Surf., A, 2024, 681, 132681 CrossRef CAS.
- A. Mummasani, S. Marimuthu, D. Balachandar, S. Radhamani, C. Bharathi, G. Gowtham and S. Shanmugapriya, Int. J. Environ. Clim. Change, 2022, 1811–1824 CrossRef.
- A. Kobylińska, B. Kost, K. Cichoń, I. I. Bąk-Sypień and M. Brzeziński, J. Polym. Environ., 2022, 31, 1209–1220 CrossRef.
- A. K. Biswal, A. T. Thodikayil and S. Saha, ACS Appl. Bio Mater., 2021, 4, 2429–2441 CrossRef CAS PubMed.
- T. Xu, Y. Wang, Z. Aytac, N. Zuverza-Mena, Z. Zhao, X. Hu, K. W. Ng, J. C. White and P. Demokritou, ACS Nano, 2022, 16, 6034–6048 CrossRef CAS PubMed.
- A. K. Biswal and S. Saha, Colloids Surf., B, 2019, 175, 281–290 CrossRef CAS PubMed.
- M. M. Iftime, G. L. Ailiesei, E. Ungureanu and L. Marin, Carbohydr. Polym., 2019, 223, 115040 CrossRef CAS PubMed.
- D. Zhang, J. Du, R. Wang, J. Luo, T. Jing, B. Li, W. Mu, F. Liu, Y. Hou, D. Zhang, Y. M. Hou, J. Du, R. Wang, J. Luo, T. Jing, B. Li, W. Mu and F. Liu, Adv. Funct. Mater., 2021, 31, 2102027 CrossRef CAS.
- M. E. Taverna, L. B. Bressan, C. A. Busatto, M. R. Lescano and D. A. Estenoz, J. Polym. Environ., 2024, 32, 1811–1820 CrossRef CAS.
- N. P. R. Ranasinghe Arachchige, E. M. Brown and N. B. Bowden, ACS Agricultural Science and Technology, 2022, 2, 1052–1062 CrossRef CAS PubMed.
- A. V. Samrot, S. K. Samanvitha, N. Shobana, E. R. Renitta, P. Senthilkumar, S. S. Kumar, S. Abirami, S. Dhiva, M. Bavanilatha, P. Prakash, S. Saigeetha, K. S. Shree and R. Thirumurugan, Polymers, 2021, 13, 3302 CrossRef CAS PubMed.
- A. K. Biswal and S. Saha, J. Colloid Interface Sci., 2020, 566, 120–134 CrossRef CAS PubMed.
- C. F. Okey-Onyesolu, M. Hassanisaadi, M. Bilal, M. Barani, A. Rahdar, J. Iqbal and G. Z. Kyzas, John Wiley and Sons Inc, 2021, DOI:10.1002/slct.202102379.
- T. Pulingam, P. Foroozandeh, J. A. Chuah and K. Sudesh, Nanomaterials, 2022, 12, 576 CrossRef CAS PubMed.
- Y. Wang, P. Li, T. T. D. Tran, J. Zhang and L. Kong, Nanomaterials, 2016, 6, 26 CrossRef PubMed.
- N. J. Methela, A. Pande, M. S. Islam, W. Rahim, A. Hussain, D. S. Lee, B. G. Mun, N. P. Maria Joseph Raj, S. J. Kim, Y. Kim and B. W. Yun, BMC Plant Biol., 2023, 23, 1–15 CrossRef PubMed.
- N. H. Hoang, T. Le Thanh, R. Sangpueak, J. Treekoon, C. Saengchan, W. Thepbandit, N. K. Papathoti, A. Kamkaew and N. Buensanteai, Polymers, 2022, 14, 662 CrossRef CAS PubMed.
- D. Zheng, B. Bai, Y. He, N. Hu and H. Wang, Int. J. Biol. Macromol., 2020, 160, 518–530 CrossRef CAS PubMed.
- D. França, J. R. S. de Barros and R. Faez, Cellulose, 2021, 28, 1571–1585 CrossRef.
- S. Chopde, R. Datir, G. Deshmukh, A. Dhotre and M. Patil, J. Agric. Food Res., 2020, 2, 100085 Search PubMed.
- R. Saberi-Riseh and M. Moradi-Pour, Pest Manage. Sci., 2021, 77, 4357–4364 CrossRef CAS PubMed.
- A. K. Biswal and S. Saha, J. Appl. Polym. Sci., 2019, 136, 48009 CrossRef.
- D. C. Campos, F. Acevedo, E. Morales, J. Aravena, V. Amiard, M. A. Jorquera, N. G. Inostroza and M. Rubilar, World J. Microbiol. Biotechnol., 2014, 30(9), 2371–2378 CrossRef CAS PubMed.
- N. H. Hoang, T. Le Thanh, R. Sangpueak, J. Treekoon, C. Saengchan, W. Thepbandit, N. K. Papathoti, A. Kamkaew and N. Buensanteai, Polymers, 2022, 14, 662 CrossRef CAS PubMed.
- S. Varona, S. Kareth, Á. Martín and M. J. Cocero, J. Supercrit. Fluids, 2010, 54, 369–377 CrossRef CAS.
- D. França, Â. F. Medina, L. L. Messa, C. F. Souza and R. Faez, Carbohydr. Polym., 2018, 196, 47–55 CrossRef PubMed.
- B. Liu, Y. Wang, F. Yang, X. Wang, H. Shen, H. Cui and D. Wu, Colloids Surf., B, 2016, 144, 38–45 CrossRef CAS PubMed.
- S. Saha, Mater. Sci. Eng., C, 2019, 104, 109894 CrossRef PubMed.
- A. T. Thodikayil, S. Sharma and S. Saha, ACS Appl. Bio Mater., 2021, 4, 2907–2940 CrossRef CAS PubMed.
- Y. H. Su and Y. C. Liang, Pestic. Biochem. Physiol., 2011, 100, 284–288 CrossRef CAS.
- M. R. Hill, E. J. MacKrell, C. P. Forsthoefel, S. P. Jensen, M. Chen, G. A. Moore, Z. L. He and B. S. Sumerlin, Biomacromolecules, 2015, 16, 1276–1282 CrossRef CAS PubMed.
- K. Sitthisuwannakul, K. Boonpavanitchakul, T. Wirunmongkol, P. Muthitamongkol and W. Kangwansupamonkon, J. Coat. Technol. Res., 2023, 20, 635–646 CAS.
- P. Stloukal, P. Kucharczyk, V. Sedlarik, P. Bazant and M. Koutny, J. Agric. Food Chem., 2012, 60, 4111–4119 CrossRef CAS PubMed.
- B. Liu, Y. Wang, F. Yang, X. Wang, H. Shen, H. Cui and D. Wu, Colloids Surf., B, 2016, 144, 38–45 CrossRef CAS PubMed.
- R. Gil-Ortiz, M. Á. Naranjo, A. Ruiz-Navarro, S. Atares, C. García, L. Zotarelli, A. S. Bautista and O. Vicente, Plants, 2020, 9, 1183 CrossRef CAS PubMed.
- Y. Lyu, X. Yang, H. Pan, X. Zhang, H. Cao, S. Ulgiati, J. Wu, Y. Zhang, G. Wang and Y. Xiao, J. Cleaner Prod., 2021, 293, 126198 CrossRef CAS.
- Z. Majeed, N. K. Ramli, N. Mansor and Z. Man, Rev. Chem. Eng., 2015, 31, 69–95 CrossRef CAS.
- V. Angra, R. Sehgal and R. Gupta, Microb. Ecol., 2023, 85, 572–585 CrossRef CAS PubMed.
- S. Bi, V. Barinelli and M. J. Sobkowicz, Polymers, 2020, 12, 301 CrossRef CAS PubMed.
- C. Sun, K. Shu, W. Wang, Z. Ye, T. Liu, Y. Gao, H. Zheng, G. He and Y. Yin, Int. J. Pharm., 2014, 463, 108–114 CrossRef CAS PubMed.
- Z. Yu, X. Sun, H. Song, W. Wang, Z. Ye, L. Shi, K. Ding, Z. Yu, X. Sun, H. Song, W. Wang, Z. Ye, L. Shi and K. Ding, Mater. Sci. Appl., 2015, 6, 591–604 CAS.
- T. Volova, A. Shumilova, N. Zhila, A. Sukovatyi, E. Shishatskaya and S. Thomas, ACS Omega, 2020, 5, 25135–25147 CrossRef CAS PubMed.
- A. E. S. Pereira, R. Grillo, N. F. S. Mello, A. H. Rosa and L. F. Fraceto, J. Hazard. Mater., 2014, 268, 207–215 CrossRef CAS PubMed.
- T. Arioli, S. W. Mattner, G. Hepworth, D. McClintock and R. McClinock, J. Appl. Phycol., 2021, 33, 1883–1891 CrossRef CAS.
- H. Balasundaram, M. Suba Sri, M. Durai Murugan, P. Monisha, S. Sindhu Sivan, G. Vijay Sree, S. Subbiah, M. Shunmugiah, V. Sakthivel and R. Dineshkumar, Biomass Convers. Biorefin., 2024, 14(12), 13195–13219 CrossRef.
- S. Peil, S. J. Beckers, J. Fischer and F. R. Wurm, Mater. Today Bio, 2020, 7, 100061 CrossRef CAS PubMed.
- S. J. Beckers, L. Wetherbee, J. Fischer and F. R. Wurm, Biopolymers, 2020, 111(12), e23413 CrossRef CAS PubMed.
- T. A. Mueller, M. R. Miles, W. Morel, J. J. Marois, D. L. Wright, R. C. Kemerait, C. Levy and G. L. Hartman, Plant Dis., 2009, 93, 243–248 CrossRef CAS PubMed.
- M. Weih, K. Hamnér and F. Pourazari, Plant Soil, 2018, 430, 7–21 CrossRef CAS.
- Y. Fawzi, Y. Alqasim, S. Abdulla, Y. Al-Ghazal, T. A. Hassan, H. Nahi and K. Al-Barkat, IOP Conf. Ser. Earth Environ. Sci., 2023, 1158, 022016 CrossRef.
- N. Li, C. Sun, J. Jiang, A. Wang, C. Wang, Y. Shen, B. Huang, C. An, B. Cui, X. Zhao, C. Wang, F. Gao, S. Zhan, L. Guo, Z. Zeng, L. Zhang, H. Cui and Y. Wang, J. Agric. Food Chem., 2021, 69, 12579–12597 CrossRef CAS PubMed.
- K. Zhao, J. Hu, Y. Ma, T. Wu, Y. Gao and F. Du, ACS Sustain. Chem. Eng., 2019, 7, 13148–13156 CrossRef CAS.
- A. Zanino, F. Pizzetti, M. Masi and F. Rossi, Eur. Polym. J., 2024, 203, 112665 CrossRef CAS.
- S. Pardeshi, M. More, P. Patil, C. Pardeshi, P. Deshmukh, A. Mujumdar and J. Naik, Drying Technol., 2021, 39, 1447–1491 CrossRef CAS.
- I. Kassem, E. H. Ablouh, F. Z. El Bouchtaoui, M. Jaouahar and M. El Achaby, Prog. Mater. Sci., 2024, 144, 101269 CrossRef CAS.
- B. Debnath, A. B. M. M. Bari, S. M. Ali, T. Ahmed, I. Ali and G. Kabir, Sustainability Analytics and Modeling, 2023, 100017 CrossRef.
- X. Ren, J. Cleaner Prod., 2003, 11, 27–40 CrossRef.
- W. Zheng, M. Zhang, Z. Liu, H. Zhou, H. Lu, W. Zhang, Y. Yang, C. Li and B. Chen, Field Crops Res., 2016, 197, 52–62 CrossRef.
- Y. Yang, M. Zhang, Y. C. Li, X. Fan and Y. Geng, Soil Sci. Soc. Am. J., 2012, 76, 2307–2317 CrossRef CAS.
- M. C. Dozier, S. A. Senseman, D. W. Hoffman and P. A. Baumann, Arch. Environ. Contam. Toxicol., 2002, 43, 292–295 CrossRef CAS PubMed.
- S. Silva Mdos, D. S. Cocenza, R. Grillo, N. F. de Melo, P. S. Tonello, L. C. de Oliveira, D. L. Cassimiro, A. H. Rosa and L. F. Fraceto, J. Hazard. Mater., 2011, 190, 366–374 CrossRef PubMed.
- N. A. Rosli, M. Karamanlioglu, H. Kargarzadeh and I. Ahmad, Int. J. Biol. Macromol., 2021, 187, 732–741 CrossRef CAS PubMed.
- N. Yadav and M. Hakkarainen, Chemosphere, 2021, 265, 128731 CrossRef CAS PubMed.
- T. A. Swetha, V. Ananthi, A. Bora, N. Sengottuvelan, K. Ponnuchamy, G. Muthusamy and A. Arun, Int. J. Biol. Macromol., 2023, 234, 123703 CrossRef CAS PubMed.
- T. Sun, D. Zhan, X. Wang, Q. Guo, M. Wu, P. Shen and M. Wu, Polymers, 2024, 16, 1041 CrossRef CAS PubMed.
- S. Qiu, J. Sun, Y. Li, T. Zhu, H. Li, X. Gu, B. Fei and S. Zhang, J. Cleaner Prod., 2022, 360, 132165 CrossRef CAS.
- A. M. Abdallah, Int. Soil Water Conserv. Res., 2019, 7, 275–285 CrossRef.
- R. Saberi-Rise and M. Moradi-Pour, Int. J. Biol. Macromol., 2020, 152, 1089–1097 CrossRef PubMed.
- T. V. Pinto, C. A. Silva, S. Siquenique and D. A. Learmonth, ACS Agric. Sci. Technol., 2022, 2, 838–857 CrossRef CAS.
- J. L. De Oliveira, E. V. R. Campos, A. E. S. Pereira, L. E. S. Nunes, C. C. L. Da Silva, T. Pasquoto, R. Lima, G. Smaniotto, R. A. Polanczyk and L. F. Fraceto, J. Agric. Food Chem., 2018, 66, 5325–5334 CrossRef PubMed.
- A. B. Nörnberg, V. R. Gehrke, H. P. Mota, E. R. Camargo and A. R. Fajardo, Colloids Surf., A, 2019, 583, 123970 CrossRef.
- H. Wang, G. Tang, Z. Zhou, X. Chen, Y. Liu, G. Yan, X. Zhang, X. Li, Y. Huang, J. Wang and Y. Cao, ACS Appl. Mater. Interfaces, 2023, 15, 4303–4314 CrossRef CAS PubMed.
- Y. Wang, C. Li, X. Zhang, W. Chen and X. Li, Journal of Environmental Science and Health, Part B, 2021, 56, 512–521 CrossRef CAS PubMed.
- R. A. Monteiro, M. C. Camara, J. L. de Oliveira, E. V. R. Campos, L. B. Carvalho, P. L. de F. Proença, M. Guilger-Casagrande, R. Lima, J. do Nascimento, K. C. Gonçalves, R. A. Polanczyk and L. F. Fraceto, J. Hazard. Mater., 2021, 417, 126004 CrossRef CAS PubMed.
- J. Cui, C. Sun, A. Wang, Y. Wang, H. Zhu, Y. Shen, N. Li, X. Zhao, B. Cui, C. Wang, F. Gao, Z. Zeng and H. Cui, Nanomaterials, 2020, 10, 220 CrossRef CAS PubMed.
- X. Xu, B. Bai, H. Wang and Y. Suo, ACS Appl. Mater. Interfaces, 2017, 9, 6424–6432 CrossRef CAS PubMed.
- A. Dhiman, P. Thaper, D. Bhardwaj and G. Agrawal, ACS Appl. Mater. Interfaces, 2024, 16, 11860–11871 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |