Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
SnO2 modified CsH2PO4 (CDP) protonic electrolyte for an electrochemical hydrogen pump†
Minal Guptaa,
Kangkang Zhangab and
Kevin Huang *a
*a
aDepartment of Mechanical Engineering, University of South Carolina, Columbia, South Carolina 29208, USA. E-mail: huang46@cec.sc.edu
bEngineering Directorate, Lawrence Livermore National Laboratory, Livermore, CA 94550, USA
First published on 13th January 2025
Abstract
CsH2PO4 (CDP) is a well-known super-protonic conductor. However, it must operate under high humidity conditions to prevent dehydration and fast conductivity decay. Herein, we report that adding hydrophilic SnO2 into CDP can suppress the rate of dehydration of CDP, thus stabilizing protonic conductivity over a broader range of water partial pressures (pH2O). A total of seven compositions of (1 − x)CDP/(x)SnO2 were prepared, where 5 ≤ x ≤ 40 (wt%), and examined for their phasal, microstructural, and vibrational properties using X-ray diffraction, field emission scanning electron microscopy, and Raman spectroscopy. The signature of H2O retained in SnO2-added CPD was confirmed by Fourier transform infrared (FTIR) spectroscopy. Among these samples, 18 wt% SnO2 in CDP stood out, showing a stable protonic conductivity of 0.6 × 10−2 S cm−1 at 250 °C, even at 10% H2O. We also provide data from pre- and post-test characterization to facilitate the understanding of the observed stability improvement and degradation mechanisms. Finally, we show stable H2 pumping performance of electrochemical cells with pure CDP and 18 wt% SnO2–CDP electrolyte and Pt/C electrode. Overall, 18 wt% SnO2–CDP is the best composition, showing stable conductivity under reduced H2O conditions and 18 wt% SnO2–CDP electrolyte with Pt/C electrode is the best membrane electrode assembly (MEA) for electrochemical H2 pumping for lower water partial pressure applications.
1. Introduction
Solid acid electrolytes (SAEs) exhibiting high protonic conductivity are of great interest for electrochemical devices such as fuel cells, electrolyzers, and H2 pumps.1–4 A representative family of SAEs has a general formula of MxHy(AO4)z, where M = Cs, Na, Rb, NH4, K, and Li; A = P, S, As, and Se, and x, y, and z are numerals.5,6 Among these solid acids, CsH2PO4 (CDP) containing PO4 tetrahedra exhibits the highest proton conductivity.7–9 At 230 °C, CDP transforms from a monoclinic to a cubic phase (also known as the super-protonic phase), triggering gigantic increases in proton conductivity from 10−6 S cm−1 to 10−2 S cm−1.9 This super-protonic phase is stable up to 250 °C, after which it starts to gradually lose conductivity due to dehydration causing the phase change to less conductive Cs2H2P2O7.10,11 Maintaining sufficiently high humidity to prevent dehydration is crucial for practical applications of CDP. The higher the operating temperature, the higher humidity is required. Within 230–250 °C, a minimum of 35–38% H2O (or partial pressure of H2O, pH2O) is needed to prevent dehydration of CDP.12–14 In many practical applications, however, such a high H2O requirement limits the utility of CDP-based electrochemical devices.To improve the stability of CDP under lower pH2O, mixing CDP with oxides or other protonic conductors has been attempted. For example, enhanced stability and conductivity of CDP were reported by adding 10 wt% cerium pyrophosphate (CeP2O7).15 The addition of tin pyrophosphate (SnP2O7) to CDP was found to improve the low-temperature conductivity of CDP.16 Similarly, the SiP2O7/CDP composite has also been used as an electrolyte for steam electrolysis,11 H2 separation of ammonia,17 and fuel cells18 with pH2O = 0.30–0.47 atm. Apart from metal pyrophosphates (MP2O7), neodymium phosphate hydrate (NdPO4·xH2O) as a dopant was also found to improve both conductivity and stability of CDP by thermally stable hydrate water in NdPO4·xH2O.7
On the other hand, adding simple metal oxides into CDP has also been studied, but with conflicting results. For example, TiO2 and SiO2 have been found to stabilize CDP in sealed containers,12,13,15 whereas, in another study, no stabilizing effect was observed by adding SiO2 into CDP in either dry or humid environments.19 It has been reported that adding ZrO2 into CDP in a molar ratio of CDP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZrO2 = 2
ZrO2 = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 improved the fuel cell performance at 275 °C and pH2O = 0.12 atm.20
1 improved the fuel cell performance at 275 °C and pH2O = 0.12 atm.20
Despite the prior efforts to stabilize CDP at higher temperatures or at lower pH2O, none of them have been practically demonstrated. Searching for solutions/materials to stabilize the CDP phase and conductivity for practical applications is still in high demand. Here in this work, we report on the feasibility of using hydrophilic SnO2 as a stabilizer for conductivity.21–23 A total of seven compositions of (1 − x)CDP/xSnO2 (x = 0 ≤ x ≤ 40 wt%) were prepared and characterized using X-ray diffraction, field emission scanning electron microscopy, energy dispersive X-ray spectroscopy, and Raman spectroscopy, followed by electrochemical impedance spectroscopy (EIS) analysis on CDP–SnO2 conductivity at 250 °C over a broad range of pH2O. The CDP–SnO2 conductivity was particularly evaluated as a function of time at low pH2O = 0.10 atm, at which pure CDP is known to be unstable. To understand the electrochemical behaviors and degradation mechanisms, post-tested samples were further analyzed by XRD, SEM, FTIR, and Raman spectroscopy. Subsequently, we performed chronoamperometry at 1 V under low pH2O conditions to acquire the electrochemical H2 pumping performance. The results of this study support the use of SnO2-modified CDP-based membranes for hydrogen separation from various H2-containing gas mixtures such as the products of water gas shift reaction as well as promoting hydrogenation/dehydrogenation reactions.
2. Experimental section
2.1. Sample preparation
Pure CDP was prepared using the co-precipitation method24 where cesium carbonate (Cs2CO3, Alfa Aesar) and phosphoric acid (H3PO4, 85% purity, Sigma Aldrich) were used as a precursor in stoichiometric amounts. Initially, Cs2CO3 and H3PO4 were dissolved in 100 and 50 mL of methanol, respectively, and stirred in separate beakers for 2 hours to form transparent solutions. The solution of H3PO4 was then poured into the Cs2CO3 solution and stirred together for 5 hours to initiate the reaction 2H3PO4 + Cs2CO3 → 2CsH2PO4↓ + H2O + CO2↑. Since the formed CDP is insoluble in methanol, it precipitates out as a solid. The precipitate was then filtered out and dried at 80 °C in an oven for 20 hours. The dried powder was mixed with methanol and then ball-milled for 6 hours. After drying, the final product was ready for use in characterization and testing. To prepare the composite of CDP and SnO2, we simply mixed commercially available SnO2 powder (Fisher Scientific, mean size 2.71 μm) in the desired ratio as listed in Table 1 with the as-synthesized CDP in an agate mortar for 1 hour.| Composite | Symbol | Weight% of SnO2 | Molar ratio (CDP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SnO2) SnO2) |
|---|---|---|---|
| Pure CDP | CDP | 0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
95CDP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05SnO2 05SnO2 |
CS-5 | 5 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
90CDP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10SnO2 10SnO2 |
CS-10 | 10 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
86CDP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14SnO2 14SnO2 |
CS-14 | 14 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
82CDP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18SnO2 18SnO2 |
CS-18 | 18 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75CDP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25SnO2 25SnO2 |
CS-25 | 25 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60CDP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40SnO2 40SnO2 |
CS-40 | 40 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.2. X-ray diffraction (XRD)
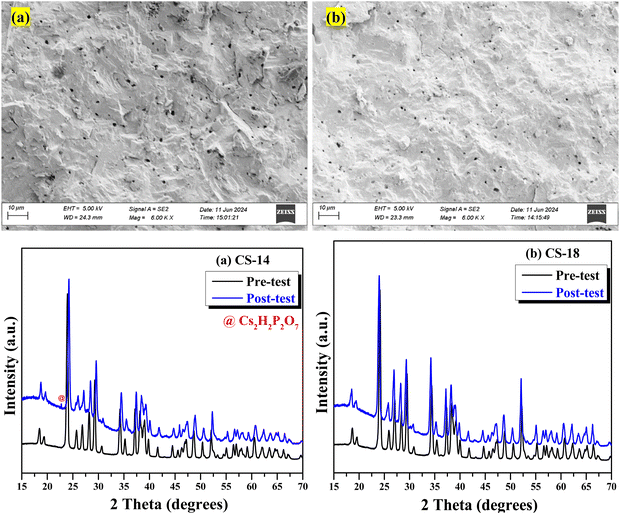
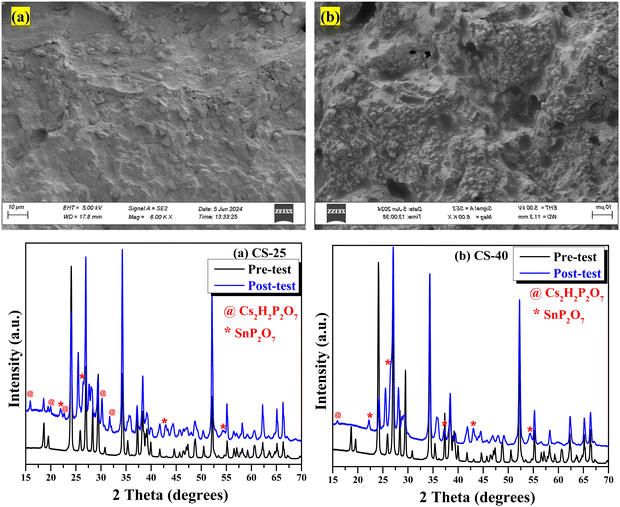
The phase compositions in the powder form of the pre- and post-tested electrolytes were examined with an X-ray diffractometer (Rigaku MiniFlex II) equipped with Cu Kα radiation (λ = 1.5418 Å) over a 2θ = 15–75° range with a step size of 0.02° and a scanning rate of 2° min−1.252.3. Field emission scanning electron microscopy (FESEM) and energy dispersive X-ray spectroscopy (EDX)
Microstructures of fresh and post-tested composite electrolytes were characterized by a field emission scanning electron microscope (Zeiss FESEM with EDX) and elemental mapping was studied by energy-disperse X-ray spectroscopy (EDX) to analyze chemical composition.262.4. Raman spectroscopy
Raman spectroscopy was conducted using Horiba Jobin-Yvon LabRAM HR800 with a 532 nm excitation laser to investigate the vibrational properties of pure and composite pre- and post-test electrolyte samples.272.5. Electrical conductivity measurements
For the conductivity measurements, the symmetrical cell was constructed by sandwiching a uniaxially co-pressing CDP/SnO2 pellet with two identical carbon paper electrodes (Toray Carbon Paper 060 Value Pack, Wet Proofed, Fuel Cell Stores) at 300 MPa for 20 minutes. The area of each electrolyte was 3.14 cm2 (diameter = 20 mm) and thickness was maintained between 1.38 and 1.42 mm. Silver mesh and copper foam were used as current collectors. The experimental setup is illustrated in Fig. S1 (ESI†) and the actual parts used are shown in Fig. S2 (ESI†). EIS spectra were then gathered using a Solartron 1260/1287 electrochemical station within a frequency range of 106–1 Hz and an AC signal amplitude of 10 mV under open-circuit voltage (OCV) conditions. The highest-frequency intersection of the collected spectra with the real axis is the bulk resistance of the sample, from which the ionic conductivity was calculated. The experimental conditions include a constant temperature of 250 °C and a time of 12 hours in which pH2O started from 0.30 atm and decreased by 0.05 atm each time up to pH2O = 0.10 atm. The H2O partial pressure was controlled by a water bubbler at a specific temperature (see Table S1, ESI†), through which steam was provided using pure H2 as a carrier gas. To avoid steam condensation, all gas lines were covered with heating tapes at 100 °C.2.6. Electrochemical pumping performance evaluation
For the electrochemical hydrogen separation, the membrane electrode assemblies (MEAs) were prepared by a co-pressing method at 300 MPa for 20 minutes, which uses Toray Carbon Paper 060 Value Pack, Wet Proofed (Fuel Cell stores) as a support; Pt/C was 10% platinum on Vulcan 72 (Fuel Cell stores) as a catalyst, and naphthalene (Sigma Aldrich) in the electrode to make the electrode porous. The symmetrical MEA composition is as follows:The experimental setup for H2 pump performance testing is shown in Fig. S3 (ESI†). Although we used the same cell fixture as the conductivity measurement with MEA as mentioned above, the gas supplies are different. In this case, one side of the electrolyte membrane was fed with 5%H2–N2 as the H2 source and another side of the electrolyte membrane was supplied with pure Ar to sweep out H2. The composition of H2 + Ar was analyzed by online gas chromatography (Agilent MicroGC 490) for H2 content, from which the H2 rate, was derived. To ensure electrolyte stability, both 5%H2–N2 and pure Ar were humidified to pH2O = 0.10 atm levels through a water bubbler set at a specific temperature (47 °C). The constant DC voltage (1 V) was provided by a Solartron 1260/1287 electrochemical workstation. The rate of H2 as a performance indicator was evaluated as a function of time at 250 °C and pH2O = 0.10 atm for pure CDP and CS-18.
3. Results and discussion
3.1. Phase composition of CDP/SnO2 composites
The two-phase nature of CDP and SnO2 was confirmed by XRD patterns as shown in Fig. 1(a). No extra impurity in the composite was observed. Two-phase Rietveld refinement (details for refined parameters are attached in Table S2, ESI†)28 was also performed to confirm the mixture of CDP and SnO2. For this analysis, we considered P21/m (11) and P42/mnm (136) space groups for CDP and SnO2, respectively, and the refined pattern is shown in Fig. 1(b).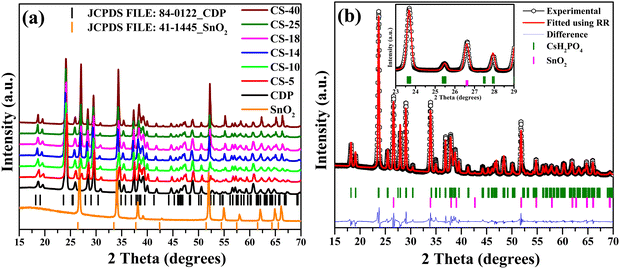 | ||
Fig. 1 (a) X-ray diffraction patterns of pure CDP and composite CDP/SnO2 electrolyte. (b) Rietveld refinement patterns of CS-25 (75CDP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25SnO2). 25SnO2). | ||
3.2. SEM/EDX results
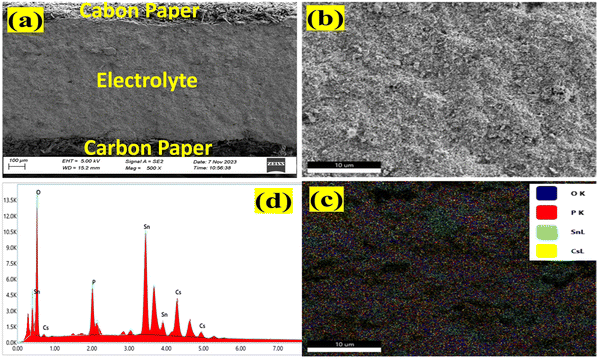
The cross-sectional view of a symmetrical cell consisting of a CDP/SnO2 electrolyte and porous carbon paper-supported electrode is shown in Fig. 2(a). The bonding between the CDP/SnO2 composite electrolyte and the electrode is reasonably good, showing no physical cracks or gaps between the two. In addition, Fig. 2(b) and (c) show that the CDP/SnO2 electrolyte layer is dense in microstructure and uniform in elemental distribution. Fig. 2(d) further indicates the strong presence of Cs, Sn, and P in the composite membrane without other elements.3.3. Raman spectra
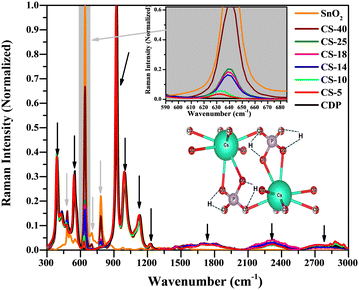
Raman spectroscopy is a powerful technique to detect lattice vibrations in chemical bonds.29–31 To exploit this technique, we studied all the compositions; the results are shown in Fig. 3, in which the black and grey arrows indicate the Raman shifts for CDP and SnO2, respectively. All the shifts seen in pure and composite CDP and SnO2 match well with those reported in the literature; no shifts other than those for CDP and SnO2 were observed, which agrees with the XRD results.The specification and assignment of each Raman shift in CDP and SnO2 are listed in Table 2.32–34 In the case of CDP, the wavenumber from 300 to 1250 cm−1 is mostly related to the lattice vibration of O–P–O and P–O vibrations, whereas higher wavenumber lattice vibrations are dominated by O–H–O and O–H lattice vibrations. There are three types of O–H vibration in CDP: out-of-plan, in-plane, and stretching vibrations. Note that these lattice vibrational shifts may be affected during the test, which can be detected in post-test samples.
| Cesium dihydrogen phosphate (CsH2PO4) (black arrow ↓ in figure) | ||
|---|---|---|
| Wavenumber (cm−1) | Assignment | Description |
| 389, 471, 561 | Ag | (OPO) skeleton bending vibrations |
| 428, 550 | Bg | (OPO) skeleton bending vibrations |
| 921, 1000, 1130, 1230 | Ag | (PO) skeleton stretching vibrations |
| 1700 | Ag | Out-of-plane OH vibrations |
| 2332 | Ag | In-plane OH vibrations |
| 2750 | Ag | (OH) stretching vibrations |
| Tin oxide (SnO2) (grey arrow ↓ in figure) | ||
|---|---|---|
| Wavenumber (cm−1) | Assignment | Description |
| 473 | Eg | Vibration of oxygen |
| 631 | Ag | Symmetric Sn–O stretching |
| 769 | B2g | Asymmetric Sn–O stretching |
| 510, 550, 700 | New | Presence of nano-size SnO2 particles |
For the case of SnO2 vibrations, the most intense peak belongs to Sn–O symmetric vibration at 631 cm−1 and the intensity of this shift aligns linearly with SnO2 addition in the CDP matrix, see the enlarged view in the inset of Fig. 3. Higher SnO2 content leads to higher Raman intensity whereas lower intensity in the case of CS-5 and no SnO2 shift in pure CDP. This indicates that, even with mechanical mixing, SnO2 particles were properly incorporated into the CDP matrix forming a well-dispersed composite. Interestingly, some of the new shifts are observed in SnO2, implying the presence of nano-SnO2 particles, see Table 2.
3.4. EIS analysis
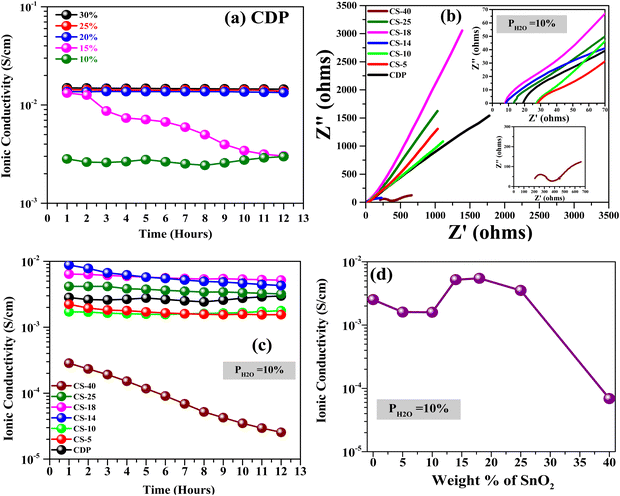
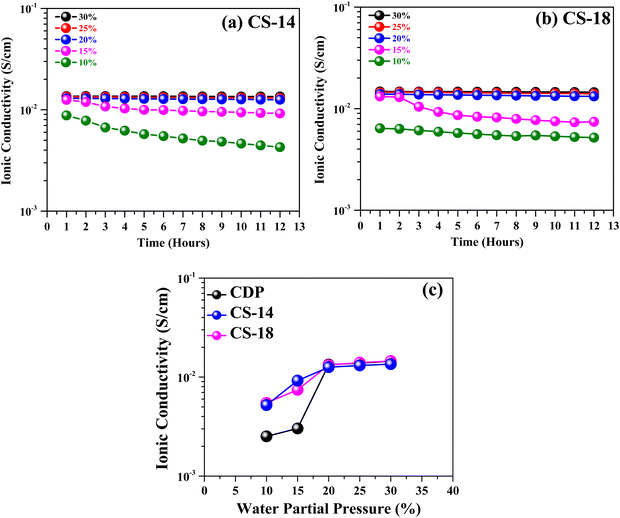
The evolution of ionic conductivity of pure CDP as a function of steam content at 250 °C is shown in Fig. 4(a). Up to 260 °C, CDP remains in the superionic phase and does not undergo phase decomposition.14 At a high steam content of 30%, CDP exhibits a stable conductivity of 1.5 × 10−2 S cm−1 for 12 hours. At a further decrease in steam content to 20%, only a small decrease is observed. However, a significant decrease in conductivity is seen at ≤15% steam by approximately one order of magnitude. These results suggest that at least 20% steam is needed to ensure stable conductivity. A conductivity degradation mechanism for CDP under low steam content is dehydration, i.e. the material loses its ability to withhold H2O that is essential to conduct protons.To minimize the steam dependence of CDP's conductivity, we selected SnO2 as an additive to CDP; the former is known as a wide-bandgap super-hydrophilic material,21 and its presence is expected to enhance the water-holding ability within CDP. The conductivity results of different CDP/SnO2 compositions measured at 250 °C and 10% H2O are depicted in Fig. 4(b). A comparison with Fig. 4(a) indicates that CDP/SnO2 composite electrolytes are more stable than pure CDP but with a lowered ionic conductivity to the magnitude of 10−3 S cm−1. Taking the 7th-hour data in Fig. 4(b) for comparison purposes, Fig. 4(c) shows a peak conductivity at CS-18 composition. This is clearly the net result of the retained H2O and blocked proton conduction in CDP by the presence of SnO2.
Overall, we have observed that SnO2 helps in stabilizing the CDP electrolyte at lower pH2O, and all compositions show stable conductivity greater than 10−3 S cm−1 at 250 °C and 10%H2O, except for CS-40. Among all the composites, for the CDP/SnO2 electrolytes discussed above, moderate SnO2 addition to CDP shows the best performance. Therefore, in the next section, we will further discuss the conductivities of CS-14 and CS-18 samples at 250 °C as a function of pH2O.
3.5. Conductivities of CS-14 and CS-18 versus pH2O
Fig. 8(a) and (b) shows the measured conductivity evolution with time at 250 °C under different H2O contents. The conductivities of both the moderate SnO2 samples are higher at higher H2O, but in general, become lower and less stable under lower H2O. A close comparison of the conductivity values between the two samples in Fig. 8(c) at the 7th-hour marker shows a similar level at <20% H2O but higher than that of pure CDP. At higher H2O content, the differences among CS-14, CS-18 and pure CDP becomes indiscernible. Therefore, for fuel cell and H2 pump applications with low H2O feedstock, CS-18 is a better choice than pure CDP.3.6. FTIR evidence of H2O and SnO2 interaction
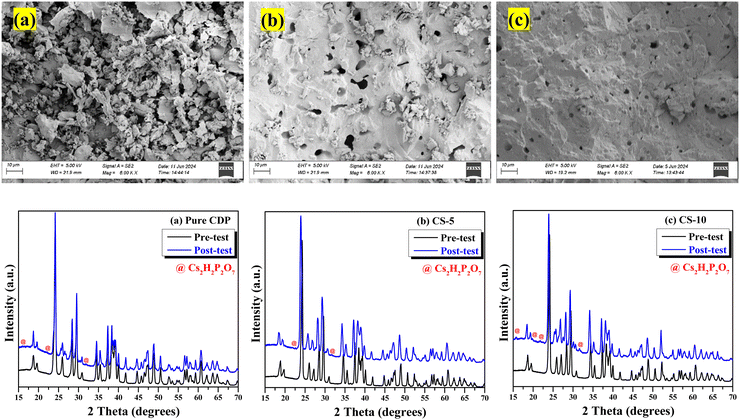
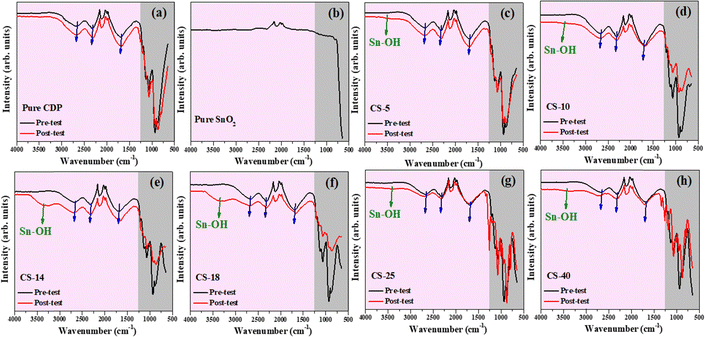
To understand the beneficial effect of SnO2 on retaining the stability of CDP, FTIR was carried out on pre- and post-test samples to probe the H and OH-bonding information; the results are shown in Fig. 9(a)–(h). For pure CDP, the vibrations related to the P–O are seen in the wavenumber range from 700–1250 cm−1 (highlighted by grey color), whereas long and short O–H vibrations are observed above 1500 cm−1 as indicated by the blue color arrow in all figures. For CDP/SnO2 samples, the FTIR spectra of the pre-test samples matched well with the pure CDP, and no new functional group was found. However, for the post-test samples, a new peak was observed between 3000 to 3500 cm−1. Based on the literature data, this peak can be assigned to water molecules bonded with SnO2 particles, generally denoted as Sn–OH.35 Interestingly, the sample with moderate SnO2 loading (CS-14 and CS-18) exhibits the broadest peak compared to other compositions, implying the strongest Sn–OH interaction. The finding of the Sn–OH peak supports the assumption that hydrophilic SnO2 helps retain local water to stabilize CPD even at lower pH2O.3.7. Electrochemical hydrogen pump performance
The CS-18 composition was selected as the electrolyte for our H2 pump testing as it showed the best stability compared to all the other electrolyte compositions. To avoid the splitting of water that can occur above 1.23 V (ideally) due to the presence of external steam, we performed chronoamperometry at 1 volt with pH2O = 0.10 atm to examine electrochemical H2 pumping performance at 250 °C for CS-18 and CDP electrolytes. Fig. 10(a) and (b) show variations in current densities and H2 flux with time. For the CS-18 electrolyte, the pump current density and H2 flux exhibit a slight decline before becoming flattened, while for the CDP electrolyte, the pump current density and H2 flux show a continuous decrease with time. Fig. 10(c) and (d) of EIS spectra suggest that the current/flux decline could be due to the decrease in the ionic conductivity of the electrolyte and electrode resistance for initial hours. Overall, we have demonstrated stable electrochemical H2 pumping performance using CDP/SnO2 electrolytes at lower pH2O.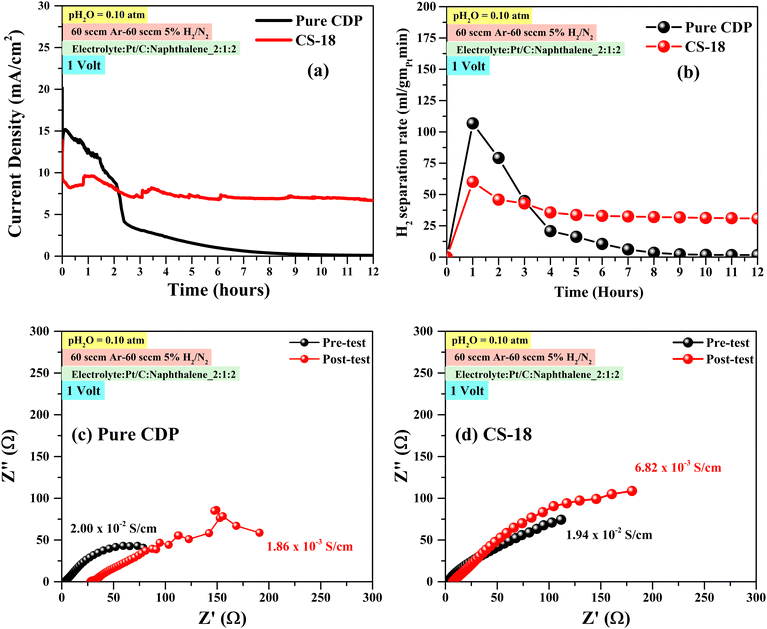 | ||
| Fig. 10 (a) Chronoamperometry at 1 volt, 250 °C and pH2O = 0.10 for CS-18 and CDP membrane. (b) The rate of H2 pumped at 1 volt. (c) and (d) EIS spectra of the pre- and post-test samples. | ||
4. Conclusions
In this study, a range of hydrophilic SnO2 mass was added into CDP to improve the conductivity stability of the latter. The conductivity results show that 18 wt% SnO2 is the optimal content to achieve balanced conductivity and stability in CDP over a wider range of pH2O. At too low SnO2 content, it is insufficient to suppress the dehydration of CDP at lower pH2O, thus causing conductivity decay. At too high SnO2 content, on the other hand, it blocks the CDP protonic pathway by the formation of a secondary phase, thus significantly lowering the protonic conductivity. The signature of Sn–OH as an indicator of water retaining ability of SnO2 is confirmed by FTIR spectra in the post-tested SnO2-added CDPs. Overall, 18 wt% SnO2-added CDP represents a stability-conductivity balanced proton conductor that can potentially find applications in fuel cells, electrolyzers and H2 pumps. Subsequently, stable electrochemical H2 pumping performance is demonstrated with 18 wt% SnO2-added CDP electrolyte and Pt/C electrode at pH2O = 0.10 atm. Overall, 18 wt% SnO2–CDP is the best composition with stable conductivity under reduced H2O conditions and 18 wt% SnO2–CDP electrolyte with Pt/C electrode as the best membrane electrode assembly (MEA) for electrochemical H2 pumping for lower water partial pressure applications.Data availability
The data supporting this article are included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the U.S. Department of Energy, Basic Energy Science division for the financial support of the work through award DE-SC0023376. This work was supported by Lawrence Livermore National Laboratory under the auspices of the U.S. Department of Energy under Contract DE-AC52-07NA27344. The LLNL information management release number is LLNL-JRNL-866536.References
- S. M. Haile, D. A. Boysen, C. R. I. Chisholm and R. B. Merle, Solid acids as fuel cell electrolytes, Nature, 2001, 410(6831), 910–913 CrossRef CAS PubMed.
- D. A. Boysen, T. Uda, C. R. I. Chisholm and S. M. Haile, High-Performance Solid Acid Fuel Cells Through Humidity Stabilization, Science, 2004, 303(5654), 68–70 CrossRef CAS.
- S. M. Haile, C. R. I. Chisholm, K. Sasaki, D. A. Boysen and T. Uda, Solid acid proton conductors: from laboratory curiosities to fuel cell electrolytes, Faraday Discuss., 2006, 134(0), 17–39 Search PubMed.
- A. Goñi-Urtiaga, D. Presvytes and K. Scott, Solid acids as electrolyte materials for proton exchange membrane (PEM) electrolysis: Review, Int. J. Hydrogen Energy, 2012, 37(4), 3358–3372 CrossRef.
- A. V. Belushkin, I. Natkaniec, N. M. Pakida, L. A. Shuvalov and J. Wasicki, Neutron scattering studies of vibrational spectra and structural transformations in the superionic conductors CsHSO4 and CsHSeO4, J. Phys. C: Solid State Phys., 1987, 20(5), 671 CrossRef CAS.
- P. Žguns, K. Klyukin, L. S. Wang, G. Xiong, J. Li and S. M. Haile, et al., Uncovering fast solid-acid proton conductors based on dynamics of polyanion groups and proton bonding strength, Energy Environ. Sci., 2024, 17(15), 5730–5742 RSC.
- T. Anfimova, A. H. Jensen, E. Christensen, J. O. Jensen, N. J. Bjerrum and Q. Li, CsH2PO4/NdPO4 Composites as Proton Conducting Electrolytes for Intermediate Temperature Fuel Cells, J. Electrochem. Soc., 2015, 162(4), F436 CrossRef CAS.
- V. G. Ponomareva and E. S. Shutova, High-temperature behavior of CsH2PO4 and CsH2PO4–SiO2 composites, Solid State Ionics, 2007, 178(7), 729–734 CrossRef CAS.
- E. Ortiz, R. A. Vargas and B. E. Mellander, On the high-temperature phase transitions of CsH2PO4: A polymorphic transition? A transition to a superprotonic conducting phase?, J. Chem. Phys., 1999, 110(10), 4847–4853 CrossRef CAS.
- D. Veer, P. Kumar, D. Singh, D. Kumar, A. Kumar and R. S. Katiyar, Phase Behavior and Ionic Conduction in the Composite Electrolytes CsH2PO4/SDP·2H2O, Russ. J. Inorg. Chem., 2021, 66(14), 2059–2067 CrossRef CAS.
- N. Fujiwara, H. Nagase, S. Tada and R. Kikuchi, Hydrogen Production by Steam Electrolysis in Solid Acid Electrolysis Cells, ChemSusChem, 2021, 14(1), 417–427 CrossRef CAS PubMed.
- D. Singh, J. Singh, P. Kumar, D. Veer, D. Kumar and R. S. Katiyar, et al., The Influence of TiO2 on the Proton Conduction and Thermal Stability of CsH2PO4 Composite Electrolytes, S. Afr. J. Chem. Eng., 2021, 37, 227–236 Search PubMed.
- D. Singh, J. Singh, D. Veer, P. Kumar and R. S. Katiyar, Synergistic effect of SiO2 on proton conduction and thermal behavior for nanocomposite electrolyte CsH2PO4 fuel cells, J. Mater. Sci.: Mater. Electron., 2022, 33(9), 6524–6535 CrossRef CAS.
- C. E. Botez, I. Martinez, A. Price, H. Martinez and J. H. Leal, Superprotonic CsH2PO4 in dry air, J. Phys. Chem. Solids, 2019, 129, 324–328 CrossRef CAS.
- P. Kumar, D. Veer, D. Singh and S. L. Meena, A parametric study of crystal structure, phase stability, and conductivity of the novel phosphate-based composite electrolyte, Appl. Phys. A., 2024, 130(4), 249 CrossRef CAS.
- V. G. Ponomareva and G. V. Lavrova, New type of composite proton electrolytes based on CsH2PO4 synthesized by mechanical activation, Mater. Today: Proc., 2019, 12, 9–12 CAS.
- J. Kim, D. Jang, J. Choi, J. Maeng, H. H. Shin and T. Park, et al., Pt-Based Electrocatalyst Modified by CsH2PO4/SiP2O7 for Electrochemical Oxidation of NH3 to H2 in Solid Acid Electrolysis Cell, Catalysts, 2023, 13(4), 707 CrossRef CAS.
- T. Matsui, T. Kukino, R. Kikuchi and K. Eguchi, Intermediate-Temperature Fuel Cell Employing CsH2PO4/SiP2O7-Based Composite Electrolytes, J. Electrochem. Soc., 2005, 153(2), A339 CrossRef.
- J. H. Leal, H. Martinez, I. Martinez, A. D. Price, A. G. Goos and C. E. Botez, Stability of the superprotonic conduction of (1-x)CsH2PO4/xSiO2 (0 ≤ x ≤ 0.3) composites under dry and humid environments., Mater. Today Commun., 2018, 15, 11–17 CrossRef CAS.
- A. H. Jensen, Q. Li, E. Christensen and N. J. Bjerrum, Intermediate Temperature Fuel Cell Using CsH2PO4/ZrO2-Based Composite Electrolytes, J. Electrochem. Soc., 2013, 161(1), F72 CrossRef.
- Q. Liu, X. Wu, B. Wang and Q. Liu, Preparation and super-hydrophilic properties of TiO2/SnO2 composite thin films, Mater. Res. Bull., 2002, 37(14), 2255–2262 CrossRef CAS.
- Talinungsang, D. D. Purkayastha and M. G. Krishna, Dopant controlled photoinduced hydrophilicity and photocatalytic activity of SnO2 thin films, Appl. Surf. Sci., 2018, 447, 724–731 CrossRef CAS.
- S. N. Pusawale, P. R. Deshmukh and C. D. Lokhande, Chemical synthesis of nanocrystalline SnO2 thin films for supercapacitor application, Appl. Surf. Sci., 2011, 257(22), 9498–9502 CrossRef CAS.
- D. Dang, B. Zhao, D. Chen, S. Yoo, S. Y. Lai and B. Doyle, et al., A durable polyvinyl butyral-CsH2PO4 composite electrolyte for solid acid fuel cells, J. Power Sources, 2017, 359, 1–6 CrossRef CAS.
- Y. Wen and K. Huang, Predicting the Rate of Degradation Related to Oxygen Electrode Delamination in Solid Oxide-Ion Electrolyzers, J. Electrochem. Soc., 2024, 171(3), 034510 CrossRef CAS.
- C. Morey, Q. Tang, S. Sun and K. Huang, A Kinetic Study on H2 Reduction of Fe3O4 for Long-Duration Energy-Storage-Compatible Solid Oxide Iron Air Batteries, J. Electrochem. Soc., 2023, 170(10), 104504 CrossRef CAS.
- K. Zhang, S. Sun and K. Huang, Combined carbon capture and catalytic oxidative dehydrogenation of propane to propylene conversion through a plug-flow dual-phase membrane reactor, Chem. Eng. J., 2024, 481, 148395 CrossRef CAS.
- H. M. Rietveld, A profile refinement method for nuclear and magnetic structures, J. Appl. Crystallogr., 1969, 2(2), 65–71 CrossRef CAS.
- M. Gupta, O. V. Rambadey, S. C. Shirbhate, S. Acharya, A. Sagdeo and P. R. Sagdeo, Probing the Signature of Disordering and Delocalization of Oxygen Vacancies and Anti-site Defects in Doped LaAlO3 Solid Electrolytes, J. Phys. Chem. C, 2022, 126(48), 20251–20262 CrossRef CAS.
- M. Gupta, O. V. Rambadey and P. R. Sagdeo, Probing the effect of R-cation radii on structural, vibrational, optical, and dielectric properties of rare earth (R = La, Pr, Nd) aluminates, Ceram. Int., 2022, 48(16), 23072–23080 CrossRef CAS.
- M. Gupta, O. V. Rambadey, A. Sagdeo and P. R. Sagdeo, Investigating the structural, vibrational, optical, and dielectric properties in Mg-substituted LaAlO3, J. Mater. Sci.: Mater. Electron., 2022, 33(16), 13352–13366 CrossRef CAS.
- B. Marchon and A. Novak, Vibrational study of CsH2PO4 and CsD2PO4 single crystals, J. Chem. Phys., 1983, 78(5), 2105–2120 CrossRef CAS.
- K. K. Singha, P. P. Singh, R. Narzary, A. Mondal, M. Gupta and V. G. Sathe, et al., Crystal Structure, Raman Spectroscopy and Optical Property Study of Mg-Doped SnO2 Compounds for Optoelectronic Devices, Crystals, 2023, 13(6), 932 CrossRef CAS.
- R. N. Mariammal, K. Ramachandran, B. Renganathan and D. Sastikumar, On the enhancement of ethanol sensing by CuO modified SnO2 nanoparticles using fiber-optic sensor, Sens. Actuators, B, 2012, 169, 199–207 CrossRef CAS.
- P. A. Luque, O. Nava, C. A. Soto-Robles, H. E. Garrafa-Galvez, M. E. Martínez-Rosas and M. J. Chinchillas-Chinchillas, et al., SnO2 nanoparticles synthesized with Citrus aurantifolia and their performance in photocatalysis, J. Mater. Sci.: Mater. Electron., 2020, 31(19), 16859–16866 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ya00606b |
| This journal is © The Royal Society of Chemistry 2025 |