Linker substitution influences succinimide ring hydrolysis equilibrium impacting the stability of attachment to antibody–drug conjugates†
Lu
Wang
 *a,
Adrian D.
Hobson
*a,
Adrian D.
Hobson
 a,
Paulin L.
Salomon
a,
Julia
Fitzgibbons
a,
Paulin L.
Salomon
a,
Julia
Fitzgibbons
 a,
Jianwen
Xu
a,
Sean
McCarthy
a,
Kan
Wu
a,
Ying
Jia
a,
Axel
Hernandez
Jr
a,
Xiang
Li
b,
Zhou
Xu
b,
Zhongyuan
Wang
b,
Yajie
Yu
b,
Junxian
Li
b and
Lin
Tao
b
a,
Jianwen
Xu
a,
Sean
McCarthy
a,
Kan
Wu
a,
Ying
Jia
a,
Axel
Hernandez
Jr
a,
Xiang
Li
b,
Zhou
Xu
b,
Zhongyuan
Wang
b,
Yajie
Yu
b,
Junxian
Li
b and
Lin
Tao
b
aAbbVie Bioresearch Center, 381 Plantation Street, Worcester, Massachusetts 01605, USA
bWuXi AppTec, 168 Nanhai Road, Tianjin Economic-Technological Development Area TEDA, TJS 300457, China
First published on 27th November 2023
Abstract
Maleimide chemistry is widely used in antibody–drug conjugate (ADC) generation to connect drugs to antibodies through a succinimide linker. The resulting ADC is prone to payload loss via a reverse Michael reaction, leading to premature drug release in vivo. Complete succinimide hydrolysis is an effective strategy to overcome the instability of ADC. However, we discovered through previous work that hydrolysed succinimide rings can close again in a liquid formulation during storage and under thermal stress conditions. In this work, a set of maleimide linkers with hydrolysis–prone groups were designed. The corresponding ADCs were prepared and subjected to thermal stress conditions. The extent of succinimide hydrolysis and drug release was measured, and ADC properties such as SEC, DAR, pI and clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of linkers were calculated. Our results demonstrated that even though all these groups increased the hydrolysis rate, they have different impacts on maintaining the hydrolysed succinimide ring in an open conformation and ADC stability in a liquid formulation.
P of linkers were calculated. Our results demonstrated that even though all these groups increased the hydrolysis rate, they have different impacts on maintaining the hydrolysed succinimide ring in an open conformation and ADC stability in a liquid formulation.
Introduction
Antibody–drug conjugates (ADCs) combining the selectivity of an antibody with the potency of a small molecule through a cleavable or non-cleavable linker have become a successful technology utilized in the field of drug discovery. Thirteen ADCs have been approved1 by the FDA – all for the treatment of cancers and all must be stored as lyophilized powders and administered by IV infusion.2At AbbVie, we have been working on the design and development of ADCs for immunology (iADC) indications.3 We have developed ABBV-3373, an iADC comprising a glucocorticoid receptor modulator (GRM) drug-linker conjugated to an anti-tumor necrosis factor (TNF) antibody. The α-TNF GRM ADC demonstrated improved efficacy in a mouse contact hypersensitivity model compared to the parent α-TNF antibody.4 Due to the chronic nature of immunological disorders and frequent treatment needs, ADCs stable in a liquid formulation are desired to enable subcutaneous dosing by self-administration to enhance patient convenience and provide optimal cost effectiveness for the healthcare system.5
Maleimide chemistry is widely used in the preparation of ADCs, as it provides a homogeneous drug-to-antibody ratio (DAR) due to the limited number of cysteines accessible for conjugation in each antibody. In fact, 10 out of the 13 commercial ADCs are constructed by linking potent small molecules with cysteines on antibodies through Michael addition with maleimides. A major shortcoming of ADCs that incorporate maleimide chemistry is premature release of the potent small molecule through reverse Michael reaction of the succinimide.6 As is well documented in the literature, when an antibody is linked with a drug through a succinimide ring, the ring can be subsequently hydrolysed and this open conformation provides the desired properties, including improved stability, exposure, and efficacy, compared to the nonhydrolysed (closed form) counterpart.7 Succinimide ring opening occurs at basic pH and elevated temperatures8 and multiple strategies have been developed to successfully achieve rapid ring opening of ADC succinimides. Various functional groups, including PEG,9 basic amines,10 aryl rings,11 dioxane,12 and different length carbon chains between the maleimide and dipeptide, which are adjacent to the succinimide increase the rate of hydrolysis under mild conditions after conjugation.13 In addition, some modifications to the structure of the succinimide allow conjugation to occur directly to the ring opened form.14 Although many methods to favour the ring opened succinimide have been identified, little attention has been paid to the long-term solution stability of ADCs with these hydrolysed succinimide rings. Conjugation to form ABBV-3373 was accomplished via Michael addition of a maleimide to α-TNF interchain cysteines, followed by hydrolysis of the succinimide ring. During investigation of the long-term liquid stability of ABBV-3373, the conversion of the succinimide attachment from the open conformation to the closed conformation was observed in greater than 15% at 25 °C after 6 months.15
In this study, we set out to interrogate if introducing chemical groups prone to hydrolysis into ADC linkers would help maintain the hydrolysed succinimide ring in an open conformation. The ultimate goal is to improve the long-term stability in liquid formulations under accelerated thermal stress conditions and in different pH environments. To this end, we designed a set of maleimide linkers containing functional groups known to accelerate the hydrolysis of the succinimide ring after conjugation, as reported in the literature (basic nitrogen: DL6, DL10, DL13, and DL17; aryl ring: DL11 and DL12; PEG: DL19; carbon chain length: DL15 and DL18) along with novel linkers that incorporated a basic nitrogen within the linker (DL1, DL2, DL3, DL4, DL5, DL7, DL8, DL9, and DL16). In addition, DL14 was included that incorporated two fluorines to DL18 to increase the electron withdrawing effect on the succinimide ring. Lastly, DL20 was included as a succinimide ring that was incapable of undergoing succinimide ring hydrolysis. This linker that incorporates caproic acid between the maleimide ring and dipeptide is known as “MC” and used in many ADCs including brentuximab vedotin.16 The same GRM payload and Ala-Ala dipeptide (Table 1) was attached to all 20 drug-linkers, so that any variation in the properties of the ADC could be directly attributed to the maleimide modification. All these drug-linkers were conjugated via the maleimide to an hTNF antibody to generate ADCs for subsequent analyses. After treatment under accelerated stress conditions, the percentage of ring-opened and ring-closed succinimides was quantified by mass spectroscopy (MS). For each ADC, the following were also assessed: payload loss using MS, isoelectric point using imaged capillary isoelectric focusing (icIEF), and aggregation using size exclusion chromatography (SEC). All ADCs were tested in a GRM reporter assay to monitor the possible structural impacts on in vitro potency. The results of this study provide valuable information to guide the design of potent ADCs with optimized stability in liquid formulations.
Results and discussion
Maleimide linker design
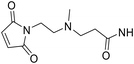
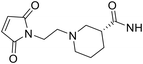
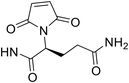
According to our previous research,3 maleimide propionic acid (MP) linkers upon conjugation can be completely hydrolysed in a basic environment to afford stable attachment. However, the opened succinimide ring can partially close under weakly acidic conditions (pH 6.0) and reach equilibrium over time. The resulting ADCs are then vulnerable to premature payload release due to the reverse Michael reaction. To solve this problem, we tried to incorporate hydrolysis facilitating groups into the ADC linkers to drive the equilibrium towards the succinimide ring open conformation while in liquid formulations. Scientists at Seattle Genetics discovered that N-aminoethyl maleimide provided substantial acceleration of the hydrolysis of the succinimide formed after conjugation.8 Inspired by this finding, we designed a set of maleimides (Table 1), keeping the GRM and dipeptide Ala-Ala constant, while inserting different spacer R groups (Table 1) between the maleimide and the dipeptide. Out of the 20 R groups, 11 contained a basic nitrogen approximately two carbons away from the maleimide ring. We included a variety of not only alkyl amines (DL2, DL3, DL6, DL16, and DL17), but also cyclized amines such as piperidine (DL4, DL5, and DL7), pyrrolidine (DL1, DL9, and DL16) and azetidine (DL8). In addition, electron-withdrawing groups (EWG) (DL10, DL13, and DL14) were included in the list. For comparison purposes, literature documented spacers such as glycine (DL15), propionic acid (DL18), ethoxypropanoic acid (DL19), caproic acid (DL20) and phenyl (DL11, DL12) were also included in this set. The maleimide ID (DL number) was assigned based on the hydrolysis rate of the corresponding succinimide with a larger DL number correlating to a longer time for the succinimide to reach complete hydrolysis. To understand how the hydrophobicity of the linker correlated with the results, the clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P was calculated using the hydrolysed form of the succinimide for each linker (Table 1). As the dipeptide (alanine–alanine) and steroid structure remained constant, they were removed from the calculation. clog
P was calculated using the hydrolysed form of the succinimide for each linker (Table 1). As the dipeptide (alanine–alanine) and steroid structure remained constant, they were removed from the calculation. clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of most ADCs with a neutral linker ranged from 0.4 to 2.1. Two terminal amide substitutions, DL10 (−0.41) and DL13 (−1.04), were able to lower the clog
P of most ADCs with a neutral linker ranged from 0.4 to 2.1. Two terminal amide substitutions, DL10 (−0.41) and DL13 (−1.04), were able to lower the clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P to below zero. The introduction of a basic amine effectively decreased the hydrophobicity of the DL linker. As a result, all hydrolysed maleimide linkers containing a basic nitrogen in this set have a negative clog
P to below zero. The introduction of a basic amine effectively decreased the hydrophobicity of the DL linker. As a result, all hydrolysed maleimide linkers containing a basic nitrogen in this set have a negative clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P.
P.
Maleimide preparation
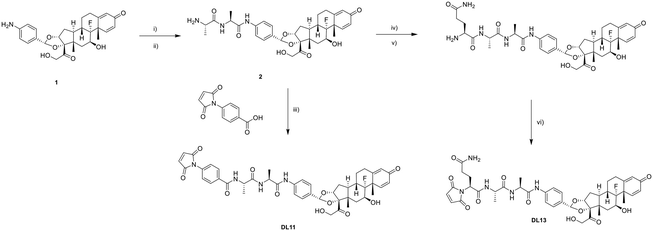
Maleimide linker drugs in the set were prepared in two ways. A representative synthetic route is presented in Scheme 1. A previously identified active GRM compound 1 was reacted with commercially available Boc-Ala-Ala, followed by Boc deprotection to give the common intermediate 2. Intermediate 2 could either react to a maleimide pre-attached linker to afford compound DL11 directly or form an amide bond with the linker alone before the addition of a maleimide molecule to give DL13.ADC preparation
The drug-linkers were conjugated to a partially reduced anti-TNF hIgG1 antibody at pH 6.0 and purified by hydrophobic interaction chromatography (HIC) at pH 7.0. The DAR4 fractions were combined, concentrated, and buffer exchanged into PBS at pH 7.4. A small portion of each ADC was set aside for the hydrolysis kinetics study. ADC21–ADC36 were incubated in 100 mM pH 8.0 borate buffer, while ADC37–40 were incubated in 50 mM pH 9.0 arginine buffer at RT for 72 hours. Hydrolysed ADCs were buffer exchanged to 15 mM pH 6.0 histidine buffer, and the ADC properties (DAR, aggregation, and Isoelectric point) were measured (Table 1).ADC hydrolysis kinetics study results
The extent of succinimide hydrolysis for each ADC was measured at 1 h, 2 h, 4 h, 8 h and 24 h timepoints using MS (Table 2). Based on our experience,3 the succinimide ring opens slightly faster when conjugated to cysteine on the light chain (LC) compared to the heavy chain (HC), which is probably due to LC cysteine being more solvent exposed. Additionally, the MS peaks in the LC region have a much higher signal-to-noise ratio than peaks in the HC region due to less overlap of charge state envelopes, less ionization suppression effects and reduced heterogenicity compared to the HC, which has different glycoforms. For more accurate results and to facilitate direct comparison, only LC succinimide hydrolysis was measured in this study.| ADC ID | pI | % succinimide ring hydrolysis/h | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 24 | ||
| ADC21 | 8.44 | 100 | ||||
| ADC22 | 8.52 | 100 | ||||
| ADC23 | 8.39 | 100 | ||||
| ADC24 | 8.41 | 100 | ||||
| ADC25 | 8.45 | 100 | ||||
| ADC26 | 8.33 | 100 | ||||
| ADC27 | 8.40 | 100 | ||||
| ADC28 | 8.42 | 98 | 100 | |||
| ADC29 | 8.43 | 98 | 90 | 100 | ||
| ADC30 | 8.30 | 59 | 80 | 95 | 100 | |
| ADC31 | 8.30 | 70 | 82 | 97 | 100 | |
| ADC32 | 8.29 | 73 | 89 | 99 | 100 | |
| ADC33 | 8.30 | 67 | 86 | 99 | 100 | |
| ADC34 | 8.30 | 61 | 71 | 85 | 96 | 100 |
| ADC35 | 8.30 | 79 | 85 | 93 | 98 | 100 |
| ADC36 | 8.46 | 65 | 72 | 88 | 97 | 100 |
| ADC37 | 8.55 | 72 | 76 | 80 | 89.4 | |
| ADC38 | 8.29 | 13 | 14 | 16 | 23 | 48 |
| ADC39 | 8.28 | 11 | 10 | 13 | 27 | |
| ADC40 | 8.30 | 8 | 8 | 10 | 11 | |
At pH 8.0, ADC40 containing the 5 carbon chain between the succinimide and the dipeptide was only hydrolysed around 10% even after 24 h, while ADCs with a shorter carbon chain, ADC38 (2 carbons, 48%) and ADC35 (1 carbon, 100%), showed significantly more hydrolysis over the same time frame. The observation of rising hydrolysis rate with a shorter length of carbon spacer is consistent with the literature findings and probably caused by multiple factors including EWG, hydrophilicity and steric effect. Faster hydrolysis of M-Gly-containing linkers is a consequence of having a proximal amide (EWG) in the dipeptide and better ability to draw water closer to the succinimide ring due to higher hydrophilicity and less steric hindrance. The insertion of a phenyl ring into ADC31 and ADC32 effectively reduced the electron density of the nitrogen in the succinimide ring through resonance structures and resulted in accelerated ring opening reactions. ADCs (ADC30, ADC33, and ADC34) with an EWG positioned between the maleimide and the dipeptide all had faster hydrolysis rates (fully hydrolysed within 24 hours at pH 8.0) than their unsubstituted counterparts (see ADC35 and ADC38) most probably due to reduced electron density of the carbonyl group in the succinimide ring. The time to reach complete hydrolysis increases from 8 to 24 h when the EWG was moved further away from the maleimide group, for example, ADC30vs.ADC33. ADC22, ADC23, and ADC40 all have a 5-atom chain between the maleimide and the dipeptide, with ADC40 having a pentyl chain present in 7 marketed ADCs. However, in ADC22 and ADC23, the central carbon was replaced by nitrogen, and this increased hydrolysis from the 10% observed with ADC40 after 24 h to 100% for both ADC22 and ADC23 within 1 hour. Similarly, piperidines (ADC24, ADC25, ADC27), pyrrolidines (ADC21, ADC29), azetidine (ADC28) and primary ethylamine (ADC26) which all have a 2-carbon spacer between the succinimide and a basic nitrogen were able to facilitate succinimide hydrolysis to completion within an hour. In contrast, when the basic nitrogen was placed further away from the succinimide, like in pyrrolidine ADC36 succinimide hydrolysis required 4 hours to reach completion, while butylamine ADC37 did not reach complete hydrolysis at pH 8.0 even after 3 days. The hydrolysis rate dropped significantly from 100% to 27% (ADC22vs.ADC39) when the basic nitrogen was replaced with oxygen. However, succinimide ring hydrolysis of the oxygen analogue ADC39 was still far faster than the carbon analogue ADC40.
Isoelectric point (pI) is the pH at which a biologic has no net electrical charge, and it is determined by charged components. Since the rate of succinimide hydrolysis is elevated in a basic environment, we surmised that the pI value might be useful to predict the rate of succinimide hydrolysis and so the time taken for each ADC to reach complete hydrolysis was plotted against pI measured by icIEF (Fig. 1, Table 2). Even though there was only subtle difference in pI (8.28–8.30), ADCs without a basic nitrogen took longer time to reach 100% hydrolysis with relative lower pI. ADCs with a basic nitrogen had a substantial increase in pI in the set. Most ADCs containing a basic nitrogen demonstrated very fast succinimide hydrolysis (less than 4 hours). The only exceptions were ADC36 and ADC37, in which the basic nitrogen was placed further away from the succinimide and less able to provide a local basic environment.
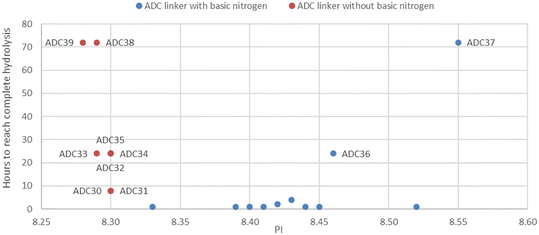 | ||
| Fig. 1 Time to reach complete succinimide hydrolysis vs. pI of ADCs without (red) or with (blue) a basic nitrogen in linkers in a histidine buffer at pH 6.0. | ||
ADC thermal stress test
To assess the chemical stability, all DAR purified ADCs were subjected to a stress test of incubation at 40 °C at pH 6.0 for 28 days. A subset of ADCs were selected for additional tests with different buffer pH values (5.5, 6.0 and 6.5). An aliquot of some ADCs stored at 4 °C for 6 months were also included in the study.Percent change of the succinimide ring closed form
Based on the above-mentioned reasons, only peaks related to the LC were analysed for a more accurate assessment. Peptide mapping analysis on the light chain of 18 ADCs after 28 days of stress test was completed to confirm that the observed mass shift in subunit measurements was not due to orthogonal post translational modifications including dehydration, ammonia loss or cyclization of the N-terminus. The data indicated that each sample contained baseline levels of these modifications and they did not change as a function of the stresses applied. To determine the extent of the succinimide ring closed form after accelerated stress, we first analysed the nonreduced ADC samples collected on day 0, day 14 at 40 °C, day 28 at 40 °C and day 180 at 4 °C when the buffer pH was 6.0 by MS. The peak intensity of the ring closed form was divided by the sum of both the ring open and closed forms to yield the percentage of succinimide ring closed forms for each ADC. Percent changes were calculated by comparing the calculated value in samples from day 0 (Fig. 2, Table 3). | ||
Fig. 2 Percent change of the succinimide ring closed form on LC at pH 6.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 days at 40 °C (blue), 28 days at 40 °C (red), and 180 days at 4 °C (green). 14 days at 40 °C (blue), 28 days at 40 °C (red), and 180 days at 4 °C (green). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 days at 40 °C, 28 days at 40 °C, and 180 days at 4 °C
14 days at 40 °C, 28 days at 40 °C, and 180 days at 4 °C
| ADC | % change of ring closed form at pH 6.0/days and temperature | ||
|---|---|---|---|
| 180 days at 4 °C | 14 days at 40 °C | 28 days at 40 °C | |
| ADC21 | 0.12 | 4.34 | 11.4 |
| ADC22 | 0 | 3.15 | 9.8 |
| ADC23 | 0.51 | 3.38 | 11.8 |
| ADC24 | 0 | 1.67 | 6.3 |
| ADC25 | 0.27 | 1.00 | 6.0 |
| ADC26 | 0 | 0 | 0 |
| ADC27 | 0.65 | 3.15 | 9.4 |
| ADC28 | −0.34 | 5.66 | 13.4 |
| ADC29 | 0.05 | 6.49 | 11.6 |
| ADC30 | 0 | 0 | 5.3 |
| ADC31 | 0 | 0 | 0 |
| ADC32 | NA | 0 | 1.4 |
| ADC33 | NA | 0.69 | 3.9 |
| ADC34 | 0 | 0 | 1.1 |
| ADC35 | 0.20 | 11.45 | 15.8 |
| ADC36 | 0 | 2.41 | 6.5 |
| ADC37 | NA | 6.45 | 11.2 |
| ADC38 | −0.50 | 3.90 | 8.4 |
| ADC39 | 0.89 | 12.96 | 20.9 |
From Fig. 2, it was encouraging to see that all ADCs showed <2% succinimide ring closed form stored at 4 °C after 180 days compared to samples immediately after hydrolysis. This implies that the succinimide ring closing reaction in ADCs with linkers in our set could be minimized during storage in liquid formulations at reduced temperatures. However, most ADCs had more ring closed forms when subjected to the accelerated stress conditions. The percentage of the succinimide ring closed form increased over time from day 0 to day 14 and further increased by day 28. Consistent with our previous findings, ADC35 (M-Gly) and ADC38 (MP) had about 16% and 9% of the succinimide ring closed form after 28 days at 40 °C, respectively. Interestingly, when an EWG was added, ADC34 (difluoro added to MP), ADC30 and ADC33 (amides added to M-Gly) the percent change reduced to only 5% or less. Our data also demonstrated that ADC31 and ADC32, both with phenyl between the maleimide and the dipeptide, were able to maintain <2% of the succinimide ring closed form. The effects of the basic nitrogen group shifting the equilibrium between the open and closed forms were wide ranging. While ADC28 (azetidine) had the largest increase in the ring closed form (>13%), ADC26 (primary ethylamine) showed no presence of the ring closed form before or after the accelerated stress test.
Besides analysis of structural differences, we investigated if the above-mentioned results were associated with the rate of hydrolysis by graphing the percent change of the ring closed form after 28 days of stress at pH 6.0 against the time taken for ADCs to reach complete hydrolysis (Fig. 3). ADCs with a neutral linker are shown in red and ADCs with a linker that contained a basic moiety are shown in blue. ADCs with a neutral linker started to reach complete hydrolysis after 4 hours and the ones that reached 100% hydrolysis within 24 hours had a relatively small percent change of the succinimide ring closed form (<6%), except ADC35. Most of the ADCs with a basic nitrogen reached full hydrolysis within two hours. However, the change in ring closed form after the stress test varied significantly from 0 to 14%. One possible rationale could be the catalytic nature of the succinimide hydrolysis. Neutral linkers speed up hydrolysis by reducing the electron density of the carbonyl group in succinimide rings through either an EWG or a phenyl group, providing more resonance structures, which will be less impacted by pH. While linkers containing a basic moiety will have accelerated succinimide hydrolysis through local base catalysis, the basic nitrogen cannot enable base catalysis if it is protonated in acidic pH 6.0 environment. Since kinetic hydrolysis studies were conducted at pH 8.0, while the stress test was performed at pH 6.0, it is not surprising then that data aligned better for ADCs with a neutral linker compared to ADCs with a basic linker.
Percent change of LC peak intensity
To further investigate the impact of different linkers, stressed ADC samples were reduced and analysed using MS. We noticed a substantial increase in the LC peak intensity, compared to LC plus one drug in some ADCs. This change could be caused by either partial succinimide ring closure, which has a decreased ionisation efficiency, or a small amount of drug loss, or a combination of both. Representative reduced MS spectra for ADC26 are shown in Fig. 4. The peak intensity ratio of LC (MW = 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 409) over LC plus one drug (MW = 24
409) over LC plus one drug (MW = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 233) started at 27/100 at day 0, increased to 84/100 by day 14 and increased further to 100/52 by day 28. We hypothesized that the drug loss could result from a combination of multiple routes including the reverse Michael reaction and Michael addition, succinimide ring opening and closing reactions, and the maleimide ring opening reaction under stress conditions (Scheme 2). The succinimide ring closed form could afford maleimide via the reverse Michael reaction and the newly generated maleimide either covalently bind to antibody via Michael addition or gets hydrolysed to maleamic acid. Linkers that catalyse succinimide ring hydrolysis could also promote the maleimide ring opening reaction. As more maleamic acid was formed, there would be less maleimide available for Michael addition, resulting in drug-linker loss from the ADC over time. The fact that ADCs showed a different percent change in the LC intensity clearly indicated the different impacts that the various linkers had.
233) started at 27/100 at day 0, increased to 84/100 by day 14 and increased further to 100/52 by day 28. We hypothesized that the drug loss could result from a combination of multiple routes including the reverse Michael reaction and Michael addition, succinimide ring opening and closing reactions, and the maleimide ring opening reaction under stress conditions (Scheme 2). The succinimide ring closed form could afford maleimide via the reverse Michael reaction and the newly generated maleimide either covalently bind to antibody via Michael addition or gets hydrolysed to maleamic acid. Linkers that catalyse succinimide ring hydrolysis could also promote the maleimide ring opening reaction. As more maleamic acid was formed, there would be less maleimide available for Michael addition, resulting in drug-linker loss from the ADC over time. The fact that ADCs showed a different percent change in the LC intensity clearly indicated the different impacts that the various linkers had.
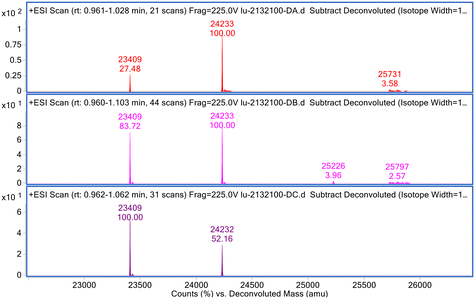 | ||
| Fig. 4 Reduced MS of ADC26: 0 days at 4 °C (top), 14 days at 40 °C (middle), and 28 days at 40 °C (bottom). | ||
For better comparison, we first calculated the percentage of LC using peak intensity of LC divided by the sum of LC and LC plus one drug, and then calculated the percent change between day 0 and day 28 (Table 4). Next, the percent change of the LC peak intensity after 28 days of stress conditions was plotted against linker clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, which represents the hydrophobicity of the linker (Fig. 5). No correlation was observed. Interestingly, ADC22, ADC26 and ADC37 which undergo the most LC peak intensity changes (>15%) all contain either a primary or secondary amine that are not constrained in a ring. Additionally, their corresponding clog
P, which represents the hydrophobicity of the linker (Fig. 5). No correlation was observed. Interestingly, ADC22, ADC26 and ADC37 which undergo the most LC peak intensity changes (>15%) all contain either a primary or secondary amine that are not constrained in a ring. Additionally, their corresponding clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values were on the lower side. Since not all ADCs with low clog
P values were on the lower side. Since not all ADCs with low clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P showed comparable drug loss in the set, it is plausible to assume that the LC peak intensity percentage change was caused by the structural similarity of the three ADC linkers, instead of low clog
P showed comparable drug loss in the set, it is plausible to assume that the LC peak intensity percentage change was caused by the structural similarity of the three ADC linkers, instead of low clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values. For the neutral linkers (Fig. 5, red), most of the ADCs had low LC peak intensity changes (<5%) regardless of clog
P values. For the neutral linkers (Fig. 5, red), most of the ADCs had low LC peak intensity changes (<5%) regardless of clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, except for ADC34 and ADC35. Surprisingly ADC35 (M-Gly) had about 14% LC peak intensity change while ADC38 (MP) had none. The structural difference between these two ADCs is only 1 carbon in the chain between the succinimide and dipeptide. We do not know the exact reason but suspect that the formation of corresponding maleamic acid under the stressed condition could contribute to the higher percentage LC intensity change of ADC34 and ADC35, as we hypothesized earlier. Additionally, the effect of an EWG on the LC peak intensity changes varied with the structure. ADC34 (difluoro) showed a 10% LC change, while ADC30 (amide) did not have any measurable percentage change of LC peak intensity.
P, except for ADC34 and ADC35. Surprisingly ADC35 (M-Gly) had about 14% LC peak intensity change while ADC38 (MP) had none. The structural difference between these two ADCs is only 1 carbon in the chain between the succinimide and dipeptide. We do not know the exact reason but suspect that the formation of corresponding maleamic acid under the stressed condition could contribute to the higher percentage LC intensity change of ADC34 and ADC35, as we hypothesized earlier. Additionally, the effect of an EWG on the LC peak intensity changes varied with the structure. ADC34 (difluoro) showed a 10% LC change, while ADC30 (amide) did not have any measurable percentage change of LC peak intensity.
| ADC ID | % change of LC peak intensity at pH 6.0/days | |
|---|---|---|
| 14 | 28 | |
| ADC21 | 1.8 | −0.9 |
| ADC22 | 8.6 | 18.3 |
| ADC23 | 1.0 | 3.3 |
| ADC24 | 3.0 | 2.8 |
| ADC25 | 4.1 | 6.5 |
| ADC26 | 24.0 | 44.2 |
| ADC27 | 0.9 | 3.0 |
| ADC28 | 0.8 | 4.4 |
| ADC29 | 4.0 | 6.3 |
| ADC30 | 0.0 | 0.0 |
| ADC31 | 0.7 | 0.0 |
| ADC32 | −0.7 | −1.2 |
| ADC33 | −0.4 | 0.6 |
| ADC34 | 14.4 | 14.5 |
| ADC35 | 8.9 | 13.9 |
| ADC36 | 1.4 | 3.9 |
| ADC37 | 7.2 | 16.4 |
| ADC38 | 1.6 | 1.5 |
| ADC39 | 1.9 | 2.1 |
 | ||
Fig. 5 Percent change of the LC peak intensity vs. clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of ADCs with (blue) and without (red) a basic nitrogen in linkers after 28 days at pH 6.0 at 40 °C. P of ADCs with (blue) and without (red) a basic nitrogen in linkers after 28 days at pH 6.0 at 40 °C. | ||
To track both changes in the same sample, we plotted percent change of LC peak intensity against the succinimide ring closed form on day 28 (Fig. 6). ADC21, ADC24, ADC30, ADC36, and ADC38 showed no LC peak intensity changes, while having different levels of changes in the ring closed form after thermal stress (11.4%, 6.3%, 5.2%, 6.5%, and 8.4%, respectively). However, ADC26 suffered the most LC peak intensity percent change (43%), while having little of the ring closed form even after 28 days. The lack of association between these two measurements is consistent with our earlier hypothesis that the percent change of LC peak intensity is affected by the extent of both succinimide ring closure and payload loss. The payload loss is influenced more by the relative rate of maleamic formation, which could be affected by, but not directly correlated with, the level of succinimide ring closed form. From Fig. 6, it can be observed that the introduction of a phenyl ring (ADC31 and ADC32) or amide (ADC30 and ADC33) resulted in ADCs with minimal succinimide ring closing changes and LC peak intensity changes around or less than 5%, which means a lower risk of instability.
 | ||
| Fig. 6 Percent change of the LC peak intensity vs. succinimide ring closed form of ADCs with (blue) and without (red) a basic nitrogen after 28 days at pH 6.0 at 40 °C. | ||
Since all the possible reactions impacting the ADC stability under stress conditions are pH sensitive, we considered if the stability of ADCs could be improved by pH adjustment of the formulation buffer. A small subset of ADCs were subjected to the same stress test at pH 5.5, pH 6.0 and pH 6.5 (Table 5, Fig. 7). When the pH was reduced from 6.0 to 5.5, the LC peak intensity percentage change of ADCs with linkers containing a basic nitrogen (in circles) downshifted slightly from less than 20% to less than 10%, with small fluctuations in ring closed form. When the pH was increased from 6.0 to 6.5, all ADCs had elevated changes of either ring closed form or LC peak intensity. The stability of ADC31 and ADC32 was least affected by pH changes and stayed in <5% region in all measurements.
| ADC ID | % change at pH 5.5 | % change at pH 6.0 | % change at pH 6.5 | |||
|---|---|---|---|---|---|---|
| Ring closed form | LC peak intensity | Ring closed form | LC peak intensity | Ring closed form | LC peak intensity | |
| ADC21 | 10.83 | 2.24 | 12.18 | 1.73 | 16.44 | 6.20 |
| ADC22 | 13.25 | 8.09 | 14.39 | 19.96 | 16.49 | 37.69 |
| ADC23 | 14.67 | 0.40 | 13.15 | 10.41 | 17.98 | 6.50 |
| ADC28 | 16.25 | 3.52 | 13.40 | 4.42 | 19.05 | 8.65 |
| ADC31 | 0.96 | 0.83 | 0.00 | 0.00 | 4.04 | 0.00 |
| ADC32 | 0.00 | −0.11 | 1.43 | 1.25 | 1.48 | 2.27 |
| ADC33 | 5.42 | 1.70 | 3.92 | 0.55 | 7.64 | 2.20 |
In vitro study results
A GRE reporter assay in K562 cells that stably express GRE and human TNF (full length TNF delta 1-12) was developed to measure the potency from TNF-targeted payload delivery. Percent response was normalized to 100 nM dexamethasone and potency of each ADC normalized based on the DAR.All the tested ADCs had a potency range between 0.3 and 3.8 μg mL−1 (Table 6). ADCs with an EWG (difluoro or amide) were equipotent with ADCs without an EWG (ADC30vs.ADC35, ADC13vs.ADC38). Most satisfying was that all ADCs had similar GRE reporter potencies compared to ADC38 (0.94 μg mL−1), the linker from ABBV-3373, clearly demonstrating that incorporation of the succinimide hydrolysis enhancing groups into the ADC linker did not negatively impact ADC catabolism and/or payload release.
| ID | DAR | GRE hTNF/mg mL−1 |
|---|---|---|
| ADC21 | 4.1 | 0.64 |
| ADC22 | 3.9 | 2.29 |
| ADC23 | 4.1 | 3.30 |
| ADC24 | 4.1 | 0.31 |
| ADC25 | 3.9 | 0.84 |
| ADC26 | 4.0 | 0.89 |
| ADC27 | 4.0 | 0.88 |
| ADC28 | 4.1 | 0.81 |
| ADC29 | 4.0 | 1.43 |
| ADC30 | 4.0 | 0.48 |
| ADC31 | 4.0 | 0.52 |
| ADC32 | 3.9 | 0.78 |
| ADC33 | 3.9 | 0.43 |
| ADC34 | 4.0 | 1.62 |
| ADC35 | 3.8 | 2.41 |
| ADC36 | 3.9 | 1.04 |
| ADC37 | 3.8 | 3.73 |
| ADC38 | 4.0 | 0.94 |
| ADC39 | 4.0 | 2.42 |
| ADC40 | 3.9 | 0.81 |
Conclusion
In this study, a set of maleimide linkers with groups designed to enhance succinimide hydrolysis after conjugation have been designed. The corresponding ADCs were prepared and DAR4 purified, and the rate of hydrolysis of all the ADCs was measured. After succinimide ring hydrolysis, ADCs were subjected to accelerated stress conditions to explore the linker modification impact on the stability of the ADC, this being evaluated by analysing the changes of both succinimide ring closed form and LC peak intensity. For ADCs with neutral linkers, the addition of a less hydrophobic linker, a phenyl ring, or an electron withdrawing group close to the succinimide was found to be beneficial at both increasing the rate of succinimide ring hydrolysis and reducing the extent of succinimide ring closed form after the stress test. Relatively, a faster rate of succinimide hydrolysis was associated with a smaller percent change in the ring closed form. For ADCs with a basic nitrogen, rapid hydrolysis occurred when the nitrogen was in close proximity to the succinimide. However, the extent of succinimide ring closed form after the stress test varied, regardless of the rate of hydrolysis. ADCs with an unconstrained primary or secondary amine in linkers exhibited the most percent change in LC peak intensity. ADCs in the set were more stable in a more acidic buffer (pH 5.5 > pH 6.0 > pH 6.5). Our in vitro cellular assay results indicated that the incorporation of groups to enhance succinimide hydrolysis into the ADC linker did not negatively impact the ADC catabolism and/or payload release. However, not all these groups will have a positive impact on ADC stability in liquid formulations. The insertion of a phenyl ring (para- or meta-substituted) into the linker showed the best stability under stress conditions, while linkers incorporating an unconstrained primary or secondary amine exhibited high percent change in LC peak intensity. These results highlight the importance of profiling ADCs during the project optimization stage to ensure stable attachment of the linker to the antibody. Optimized linkers can minimize drug loss in liquid formulations during storage at cold temperatures, allowing for the development of self-administered ADCs.Abbreviations
| ADC | Antibody–drug conjugate |
clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P | Calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
| DAR | Drug-to-antibody ratio |
| DCM | Dichloromethane |
| DiPhPEA | Diphenylphosphino ethylamine |
| DMSO | Dimethyl sulfoxide |
| EWG | Electron withdrawing group |
| GR | Glucocorticoid receptor |
| GRE | Glucocorticoid response element |
| GRM | Glucocorticoid receptor modulator |
| HIC | Hydrophobic interaction chromatography |
| HPLC | High performance liquid chromatography |
| iADC | Immunology antibody–drug conjugate |
| LCMS | Liquid chromatography mass spectrometry |
| mAb | Monoclonal antibody |
| MeCN | Acetonitrile |
| NMR | Nuclear magnetic resonance |
| pI | Isoelectric point |
| TFA | Trifluoro acetic acid |
| TNF | Tumor necrosis factor |
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors LW, ADH, PLS, JF, JX, SM, KW, YJ, AH are employees of AbbVie and XL, ZX, ZW, YY, JL, LT are employees of WuXi. The design, study conduct, and financial support for this research were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication. We thank Abbvie employee Dawn Bennett for their help in writing the manuscript.References
- C. Dumontet, J. M. Reichert, P. D. Senter, J. M. Lambert and A. Beck, Antibody-drug conjugates come of age in oncology, Nat. Rev. Drug Discovery, 2023, 22, 641–661 CrossRef CAS PubMed.
- C. Duerr and W. Friess, Antibody-drug conjugates-stability and formulation, Eur. J. Pharm. Biopharm., 2019, 139, 168–176 CrossRef CAS PubMed.
- M. J. Mcpherson, A. D. Hobson, M. E. Hayes, C. C. Marvin, D. Schmidt, W. Waegell, C. Goess, J. Z. Oh, A. Hernandez Jr. and J. T. Randolph, Glucocorticoid receptor agonist and immunoconjugates thereof, PCT Int. Appl, WO2017210471 A1, 2017 Search PubMed.
- A. D. Hobson, M. J. McPherson, M. E. Hayes, C. Goess, X. Li, J. Zhou, Z. Wang, Y. Yu, J. Yang, L. Sun, Q. Zhang, P. Qu, S. Yang, A. Hernandez, S. H. Bryant, S. L. Mathieu, A. K. Bischoff, J. Fitzgibbons, L. C. Santora, L. Wang, L. Wang, M. M. Fettis, X. Li, C. C. Marvin, Z. Wang, M. V. Patel, D. L. Schmidt, T. Li, J. T. Randolph, R. F. Henry, C. Graff, Y. Tian, A. L. Aguirre and A. Shrestha, Discovery of ABBV-3373, an Anti-TNF Glucocorticoid Receptor Modulator Immunology Antibody Drug Conjugate, J. Med. Chem., 2022, 65, 15893–15934 CrossRef CAS PubMed.
- S. S. Wang, Y. S. Yan and K. Ho, US FDA-approved therapeutic antibodies with high-concentration formulation: summaries and perspectives, Antibody Ther., 2021, 4, 262–272 CrossRef CAS PubMed.
- B. Q. Shen, K. Xu, L. Liu, H. Raab, S. Bhakta, M. Kenrick, K. L. Parsons-Reponte, J. Tien, S. F. Yu, E. Mai, D. Li, J. Tibbitts, J. Baudys, O. M. Saad, S. J. Scales, P. J. McDonald, P. E. Hass, C. Eigenbrot, T. Nguyen, W. A. Solis, R. N. Fuji, K. M. Flagella, D. Patel, S. D. Spencer, L. A. Khawli, A. Ebens, W. L. Wong, R. Vandlen, S. Kaur, M. X. Sliwkowski, R. H. Scheller, P. Polakis and J. R. Junutula, Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates, Nat. Biotechnol., 2012, 30, 184–189 CrossRef CAS PubMed.
- P. Strop, S. H. Liu, M. Dorywalska, K. Delaria, R. G. Dushin, T. T. Tran, W. H. Ho, S. Farias, M. G. Casas, Y. Abdiche, D. Zhou, R. Chandrasekaran, C. Samain, C. Loo, A. Rossi, M. Rickert, S. Krimm, T. Wong, S. M. Chin, J. Yu, J. Dilley, J. Chaparro-Riggers, G. F. Filzen, C. J. O'Donnell, F. Wang, J. S. Myers, J. Pons, D. L. Shelton and A. Rajpal, Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates, Chem. Biol., 2013, 20, 161–167 CrossRef CAS PubMed.
- S. D. Fontaine, R. Reid, L. Robinson, G. W. Ashley and D. V. Santi, Long-Term Stabilization of Maleimide–Thiol Conjugates, Bioconjugate Chem., 2015, 26, 145–152 CrossRef CAS PubMed.
- L. N. Tumey, M. Charati, T. He, E. Sousa, D. Ma, X. Han, T. Clark, J. Casavant, F. Loganzo, F. Barletta, J. Lucas and E. I. Graziani, Mild method for succinimide hydrolysis on ADCs: impact on ADC potency, stability, exposure, and efficacy, Bioconjugate Chem., 2014, 25, 1871–1880 CrossRef CAS PubMed.
- R. P. Lyon, J. R. Setter, T. D. Bovee, S. O. Doronina, J. H. Hunter, M. E. Anderson, C. L. Balasubramanian, S. M. Duniho, C. I. Leiske, F. Li and P. D. Senter, Self-hydrolysing maleimides improve the stability and pharmacological properties of antibody-drug conjugates, Nat. Biotechnol., 2014, 32, 1059–1062 CrossRef CAS PubMed.
- R. J. Christie, R. Fleming, B. Bezabeh, R. Woods, S. Mao, J. Harper, A. Joseph, Q. Wang, Z. Q. Xu, H. Wu, C. Gao and N. Dimasi, Stabilization of cysteine-linked antibody drug conjugates with N-aryl maleimides, J. Controlled Release, 2015, 220, 660–670 CrossRef CAS PubMed.
- I. Dovgan, S. Kolodych, O. Koniev and A. Wagner, 2-(Maleimidomethyl)-1,3-Dioxanes (MD): a Serum-Stable Self-hydrolysable Hydrophilic Alternative to Classical Maleimide Conjugation, Sci. Rep., 2016, 6, 30835 CrossRef CAS PubMed.
- A. D. Hobson, M. J. McPherson, W. Waegell, C. A. Goess, R. H. Stoffel, X. Li, J. Zhou, Z. Wang, Y. Yu, A. Hernandez Jr., S. H. Bryant, S. L. Mathieu, A. K. Bischoff, J. Fitzgibbons, M. Pawlikowska, S. Puthenveetil, L. C. Santora, L. Wang, L. Wang, C. C. Marvin, M. E. Hayes, A. Shrestha, K. A. Sarris and B. Li, Design and Development of Glucocorticoid Receptor Modulators as Immunology Antibody-Drug Conjugate Payloads, J. Med. Chem., 2022, 65, 4500–4533 CrossRef CAS PubMed.
- Y. Wang, F. Xie, L. Liu, X. Xu, S. Fan, W. Zhong and X. Zhou, Development of applicable thiol-linked antibody–drug conjugates with improved stability and therapeutic index, Drug Delivery, 2022, 29, 754–756 CrossRef CAS PubMed.
- A. D. Hobson, J. Xu, C. M. Marvin, D. Welch, M. J. McPherson, B. Gates, X. Liao, M. Hollmann, M. Gattner, K. Dzeyk, H. Sarvaiya, V. Shenoy, M. M. Fettis, A. K. Bischoff, L. Wang, L. C. Santora, L. Wang, J. Fitzgibbons, P. Salomon, A. Hernandez Jr., Y. Jia, C. A. Goess, S. L. Mathieu, S. H. Bryant, M. E. Larsen, B. Cui and Y. Tian, Discovery of ABBV-154 an anti-TNF Glucocorticoid Receptor Modulator Immunology Antibody Drug Conjugate (iADC), J. Med. Chem., 2023, 66, 12544–12558 CrossRef CAS PubMed.
- P. D. Senter and E. L. Sievers, The discovery and development of brentuximab vedotin for use in relapsed Hodgkin lymphoma and systemic anaplastic large cell lymphoma, Nat. Biotechnol., 2012, 30, 631–637 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3md00569k |
| This journal is © The Royal Society of Chemistry 2024 |