Elizabeth M. Dauncey, Shashikant U. Dighe, James J. Douglas and Daniele Leonori
Chem. Sci., 2019,10, 7728-7733
DOI:
10.1039/C9SC02616A,
Edge Article
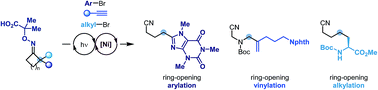
A divergent strategy for the remote arylation, vinylation and alkylation of nitriles is described. These processes proceed through the photoredox generation of a cyclic iminyl radical and its following ring-opening reaction. The distal nitrile radical is then engaged in nickel-based catalytic cycles to form C–C bonds with aryl bromides, alkynes and alkyl bromides.